Abstract
One new dibenzyltyrolactone lignan dysoslignan A (1), three new arylnaphthalide lignans dysoslignan B–C (2–4), along with fourteen known metabolites (5–18), were isolated from the roots and rhizomes of Dysosma versipellis. Their structures and stereochemistry were determined from analysis of NMR spectroscopic and circular dichroism (CD) data. Compound 2 represents the first report of naturally occurring arylnaphthalide lignan triglycoside. The cytotoxic activities of all isolated compounds were evaluated against A-549 and SMMC-7721 cell lines. Compounds 7–10 and 14–16 were more toxic than cisplatin in two tumor cell lines. This investigation clarifies the potential effective substance basis of D. versipellis in tumor treatment.
1. Introduction
Arylnaphthalide lignans have received much attention due to their potent antiviral, antineoplastic, anti-inflammatory, and immunosuppressive properties [1]. The representative effective component (such as podophyllotoxin) has been the subject of extensive research on new antiviral and antineoplastic drugs. Podophyllotoxin tincture is used clinically to treat condyloma acuminatum. Podophyllotoxin derivatives, for instance etoposide and teniposide, are the frontline chemotherapeutic drugs against various cancers. Since remote times, plants containing podophyllotoxin and its analogues have been used by diverse nationalities as laxatives and for the treatment of gonorrhea, tuberculosis, menstrual disorders, psoriasis, dropsy, cough, syphilis and venereal warts [2,3]. So a medicinal plant rich in arylnaphthalide lignans is an important source of natural anticancer agents.
Dysosma versipellis (Hance) M. Cheng ex Ying, belonging to the family of Berberidaceae, is widely distributed in the central/south regions of China [4]. As an important medicinal plant, it has been described in Shennong’s Herbal Classic. Its dried roots and rhizomes (called “Bajiaolian” in Chinese) are mainly used for the treatment of parotitis [4], sore throat, snake bite, fall injury [5], epidemic encephalitis B [6], epidemic hemorrhagic fever, condyloma accuminata, and esophagus and breast carcinoma [7]. Previous phytochemical and pharmacological investigations revealed that D. versipellis is particularly rich in arylnaphthalide lignans and biflavonoids, and has attracted wide attention due to their cytotoxic and neuraminidase and acetylcholinesterase inhibitory properties [8,9,10,11]. In our search for cytotoxic natural products, one dibenzyltyrolactone lignan dysoslignan A (1), three new arylnaphthalide lignans dysoslignans B–C (2–4), along with fourteen known metabolites (5–18), were isolated from the roots and rhizomes of D. versipellis (Figure 1). Reported herein are their detailed isolation, structure elucidation, and cytotoxic activity.
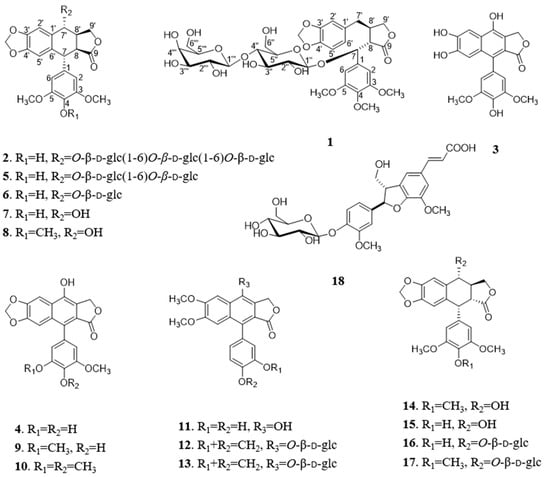
Figure 1.
Chemical structures of compounds 1–18.
2. Results and Discussion
The 95% EtOH and 50% EtOH extract of the roots and rhizomes of D. versipellis were adsorbed by silicious earth, and then fractioned by CH2Cl2, EtOAc, and MeOH, respectively. The MeOH extract was isolated and purified by repeated column chromatography, allowing the isolation of one new dibenzyltyrolactone lignan dysoslignan A (1), three new arylnaphthalide lignans dysoslignan B–C (2–4), along with fourteen known metabolites (5–18). By comparing their physical and spectroscopic data with literature values, the known metabolites were identified as sinolignan B (5) [12], 4-demethylpicropodophyllotoxin 7′-O-β-d-glucopyranoside (6) [13], 4-demethylpicropodophyllotoxin (7) [13], picropodophyllotoxin (8) [13], 4-demethyldehydropodophyllotoxin (9) [12], dehydropodophyllotoxin (10) [12], taiwanin H (11) [14], cleistanthin B (12) [15], arabelline (13) [16], podophyllotoxin (14) [12], 4-demethylpodophyllotoxin (15) [12], 4-demethylpodophyllotoxin 7′-O-β-d-glucopyranoside (16) [12], podophyllotoxin 7′-O-β-d-glucopyranoside (17) [12], and aegineoside (18) [17].
Compound 1 was obtained as a white amorphous powder and its molecular formula was determined as C34H44O18 on the basis of its HR-ESI-MS (m/z 763.2419 [M + Na]+, calcd for 763.2425). The 1H NMR spectrum (Table 1 and Figure S1) showed three methoxy groups at δ 3.73 (6H, s), 3.63 (3H, s); one 1,3,4-tri-substituted benzene ring at δ 6.40 (1H, d, J = 1.0 Hz), 6.64 (1H, d, J = 7.9 Hz), 6.35 (1H, dd, J = 7.9, 1.0 Hz); one 1,3,4,5-tetra-substituted benzene ring at δ 6.76 (2H, s); and one methylenedioxy group at δ 5.92 (1H, s), 5.94 (1H, s). The 13C NMR spectrum (Table 1 and Figure S2) exhibited one carbonyl group at δ 176.7; twelve aromatic carbons and five aliphatic carbons at δ 76.8, 51.7, 38.6, 36.0, 72.1; as well as three methoxy groups at δ 55.5 (×2), 59.9; one methylenedioxy group at δ 100.7; one set of glucopyranosyl group at δ 99.8, 73.5, 76.5, 81.0, 76.3, 61.0; and one set of galactopyranosyl group at δ 103.2, 73.3, 74.6, 70.8, 74.7, 61.1. The aglycone was identified as poporhizol by comparison of its NMR and ECD data with those reported in the literature [18], combined with data observed in the HSQC, HMBC, DEPT, 1H-1H COSY, NOESY, and HR-ESI-MS spectra (Figures S3–S9). The 13C NMR chemical shifts δ 99.8, 103.2, and spin-spin coupling constants (7.8, 7.9 Hz) of two anomeric protons allowed the identification of β-glucopyranosyl and β-galactopyranosyl moieties. The absolute configurations of glucose and galactose were determined by a microhydrolysis method and HPLC analysis [19]. The HMBC cross peaks (Figure 2) of the anomeric proton at δ 4.14 (1H, d, J = 7.8 Hz, H-1″) with C-7 (δ 76.8) and the other anomeric proton at δ 4.26 (1H, d, J = 7.9 Hz, H-1‴) with C-4″ (δ 81.0), respectively, indicated that the sugar sequence was β-d-glucopyranosyl-(1→4)-β-d-galactopyranosyl group and was attached at C-7 of the aglycone.

Table 1.
1H NMR (500 MHz) and 13 C NMR (125 MHz) data (DMSO-d6) of 1–2.
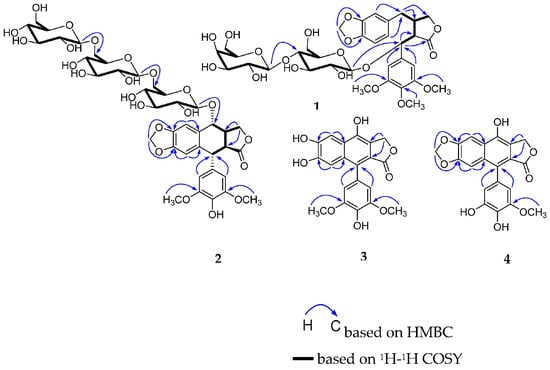
Figure 2.
Key 1H-1H COSY and HMBC correlations of compounds 1–4.
Establishment of the relative configuration was based on the chemical shift of H-9′ and NOESY experiment (Figure S7). NOE correlation of H-7 (δ 5.23) with H-8′ (δ 2.85) indicated that the relationship for H-8/H-8′ was trans. This was also supported by the Δδ Hα-9′-Hβ-9′ value of 0.35 (this value ≥ 0.2 for trans, and ≈ 0 for cis) [20]. The ECD spectrum of 1 (Figure S8) was in good agreement with the ECD spectrum of the 7R,8S,8′R-isomer cleistonkiside B [20]. So the 7R, 8S, and 8′R-configurations were assigned for 1. Thus, compound 1 was identified as poporhizol 7-O-β-d-glucopyranosyl-(1→4)-β-d-galactopyranoside, and named dysoslignan A.
Compound 2 was obtained as a white amorphous powder and its molecular formula was determined as C39H50O23 on the basis of its HR-ESI-MS (m/z 909.2637 [M + Na]+, calcd for 909.2641). The 1H NMR spectrum (Table 1 and Figure S10) showed two methoxy groups at δ 3.73 (6H, s), four aromatic protons at δ 7.23 (1H, s), 5.95 (1H, s), 6.57 (2H, s); and one methylenedioxy group at δ 5.94 (1H, s), 5.86 (1H, s). The 13C NMR spectrum (Table 1 and Figure S11) exhibited one carbonyl group at δ 178.1, twelve aromatic carbons and five aliphatic carbons, as well as two methoxy groups at δ 56.2 (×2), one methylenedioxy group at δ 100.9, and three sets of glucopyranosyl groups at δ 103.6, 73.9, 77.0, 70.4, 76.9, 68.4, 103.4, 73.6, 76.8, 70.1, 76.8, 68.3, 103.2, 73.5, 76.4, 69.8, 75.0, 61.1. The aglycone was identified as picropodophyllotoxin by comparison of its NMR data with those reported in the literature [21], combined with data observed in the HSQC, HMBC, DEPT, 1H-1H COSY, NOESY, and HR-ESI-MS spectra (Figures S12–S18). The 13C NMR chemical shifts δ 103.6, 103.4, 103.2 and spin-spin coupling constants (7.4, 8.3, 8.0 Hz) of three anomeric protons allowed the identification of three β-glucopyranosyl moieties. The absolute configuration of glucose was determined by the same method as compound 2. The HMBC cross peaks (Figure 2) of the anomeric proton at δ 4.51 (1H, d, J = 7.4 Hz, H-1″) with C-7′ (δ 76.6), and the other two anomeric protons at δ 4.12 (1H, d, J = 8.3 Hz, H-1‴) and δ 4.10 (1H, d, J = 8.0 Hz, H-1⁗) with C-6″ (δ 68.4) and C-6‴ (δ 68.3), respectively, indicated that the sugar sequence was β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl (1→6)-β-d-glucopyranosyl group and was attached at C-7′ of the aglycone.
Establishment of the relative configuration was based on the chemical shift of C-9, the 1H coupling constants (J values) and NOESY experiment (Figure S16). For a cis-orientation of lactone at C-8′ and C-8, the signal of C-9 was at around δ 178.0 ppm, while for a trans-orientation, the signal of C-9 upfield shifted to around δ 175.0 ppm [21]. According to a signal of C-9 at δ 178.1, the orientation of H-8′/H-8 of compound 2 was determined to be cis. The JH-7/H-8 (8.1 Hz) and JH-7′/H-8′ (10.0 Hz) values indicated the trans-forms of H-7/H-8 and H-7′/H-8′. The NOE correlation of H-7/H-7′ and H-8/H-8′ also supported the relative configuration of 7,8-trans-7′,8′-trans-8,8′-cis. Studies on the ECD curves of 7-aryltetralin lignans showed that all 7β (S)-aryl compounds gave negative Cotton effects at around 280–290 nm, while all 7α (R)-aryl compounds gave a positive Cotton effect [12]. The ECD spectrum (Figure S17) of compound 2 exhibited a positive Cotton effect at 290 nm. Consequently, the absolute configuration of C-7 was determined to be R. Thus, compound 2 was established as 4-demethylpicropodophyllotoxin 7′-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside, and named dysoslignan B.
Compound 3 was obtained as a white amorphous powder and possessed a molecular formula C20H16O8, as revealed by its HR-ESI-MS analysis (m/z 407.0737 [M + Na]+, calcd for 407.0743). The 1H NMR spectrum (Table 2 and Figure S19) showed two methoxy group at δ 3.72 (6H, s); four aromatic protons at δ 7.54 (1H, s), 7.03 (1H, s), 6.48 (2H, s); and one methylene group at δ 5.30 (2H, s). The 13C NMR spectrum (Table 2 and Figure S20) revealed a skeleton of arylnaphthalide lactone lignan including one carbonyl group at δ 169.9, sixteen aromatic carbons and one aliphatic carbon at δ 66.5, as well as two methoxy groups at δ 56.0 (×2). A careful comparison of the NMR spectra of 3 with 4-demethyl-dehydropodophyllotoxin, combined with data observed in the HSQC, HMBC, and HR-ESI-MS spectra (Figures S21–S23), indicated that compound 3 was a demethylene derivative of 4-demethyl-dehydropodophyllotoxin [12]. The HMBC correlation (Figure 2 and Figure S22) between two methoxy groups at δ 3.72 (6H, s) and δ 147.5 (C-3, 5) indicated that they were located at C-3 and C-5. Thus, compound 3 was identified as 6′,7′-demethylene-4-demethyldehydropodophyllotoxin, and named dysoslignan C.

Table 2.
1H NMR (500 MHz) and 13C NMR (125 MHz) data (DMSO-d6) of 3–4.
Compound 4 was obtained as a white amorphous powder and possessed a molecular formula C20H14O8, as revealed by its HR-ESI-MS analysis (m/z 383.0768 [M + H]+, calcd for 383.0767). The 1H NMR spectrum (Table 2 and Figure S24) showed one methoxy group at δ 3.70 (3H, s); four aromatic protons at δ 7.60 (1H, s), 6.89 (1H, s), 6.30 (1H, d, J = 1.9 Hz), and 6.28 (1H, d, J = 1.9 Hz); one methylenedioxy group at δ 6.151 (1H, s), 6.152 (1H, s); and one methylene group at δ 5.30 (2H, s). The 13C NMR spectrum (Table 2 and Figure S25) revealed a skeleton of arylnaphthalene lactone lignan including one carbonyl group at δ 169.5, sixteen aromatic carbons and one aliphatic carbon at δ 66.5, as well as one methoxyl group at δ 55.9, and one methylenedioxy group at δ 101.9. A careful comparison of the NMR spectra of 4 with 4-demethyl-dehydropodophyllotoxin, combined with data observed in the HSQC, HMBC, and HR-ESI-MS spectra (Figures S26–S28), suggested compound 4 to be a demethylation derivative of 4-demethyldehydropodophyllotoxin [12]. The HMBC correlation (Figure 2 and Figure S27) between the methoxy group at δ 3.70 (3H, s) and δ 148.0 (C-3), indicated that it was located at C-3. Thus, compound 4 was identified as 3,4-di-demethyldehydropodophyllotoxin, and named dysoslignan D.
All isolated compounds were evaluated for their in vitro cytotoxic activities against the A-549 and SMMC-7721 cell lines using the MTS assay [22] with cisplatin and paclitaxel as positive controls, and the IC50 values are summarized in Table 3. Compounds 7–12 and 14–17 showed more potent cytotoxicities against the SMMC-7721 cell line than the A549 cell line. Compounds 7–10 and 14–16 exhibited more potent activities than cisplatin in two tumor cell lines. Compound 14 showed the highest cytotoxicity against the A-549 and SMMC-7721 cell lines, with IC50 values of 0.130 and 0.0088 μM, respectively. The glycosylation of 7′-hydroxy group strongly reduced the activity; for example, comparing 16 to 15, 17 to 14, and 2, 5, and 6 to 7. The cis-fusion compounds (6, 7 and 8) between the tetraline and lactone were more cytotoxic than those corresponding trans-fusion analogues (16, 15, and 14). Compounds 7, 15 and 8, 14 containing a non-aromatized ring C exhibited more cytotoxic activity than aromatized compounds 9 and 10, indicating that the non-aromatized ring C played an important role in the cytotoxicity against A-549 and SMMC-7721 cells lines. The methylenedioxy-bearing compound (9) was found to be more potent than the ring A-opened analogue (3). The preliminary structure-activity relationship investigation suggested that the trans-fusion between the tetraline and lactone, non-aromatized ring C, and a methylenedioxy at ring A, were structurally required for maintaining cytotoxicity for related podophyllotoxin analogues.

Table 3.
Cytotoxicities of compounds 1–18 against A549 and SMMC-7721 cell lines (IC50, μM) a.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations and ECD spectra were determined by a Rudolph AP-IV polarimeter (Rudolph, Hackettstown, NJ, USA) and an Applied Photophysics Chirascanq CD spectropolarimeter (AppliedPhotophysics, Leatherhead, Surrey, UK), respectively. UV and IR spectra were obtained using a Thermo EVO 300 spectrometer (Thermo, Waltham, MA, USA) and a Thermo Nicolet IS 10 spectrometer (Thermo, Waltham, MA, USA), respectively. NMR and mass spectra were performed on a Bruker Avance III 500 spectrometer (Bruker, Rheinstetten, Germany) and a Bruker maXisHD mass spectrometer (Bruker, Bremen, Germany), respectively. Preparative HPLC separations were run on a SEP system (Beijing Sepuruisi scientific Co., Ltd., Beijing, China) equipped with a variable-wavelength UV detector, using a YMC-Pack ODS-A column (250 × 20 mm, 5 μm). ODS (50 μm), sephadex LH-20 (40–70 μm), and silica gel (160–200 mesh) were acquired from YMC Co. Ltd. (Kyoto, Japan), Amersham Pharmacia Biotech AB, (Uppsala, Sweden), and Marine Chemical Industry, (Qingdao, China), respectively. MCI gel CHP-20 and Diaion HP-20 were obtained from Mitsubishi Chemical Corp. (Tokyo, Japan). Chemical reagents for isolation were of analytical grade and purchased from Tianjin Siyou Co., Ltd., Tianjin, China. Biological reagents were from Sigma Company.
3.2. Plant Material
The roots and rhizomes of D. versipellis were collected in Qingzhen, Guizhou Province, China, in July 2019, and identified by Prof. Cheng-Ming Dong at School of Pharmacy, Henan University of Chinese Medicine, where a voucher specimen (DV 20190706) was deposited.
3.3. Extraction and Isolation
The powered roots and rhizomes of D. versipellis (40 kg) were refluxed with 95% EtOH (v/v 120 L × 3, 1.5 h each) and 50% EtOH (v/v 120 L × 1, 1.5 h each) at 95 ℃, respectively. The filtrate was evaporated under reduced pressure to give a dark brown residue (5.4 kg). The residue was adsorbed by silicious earth and eluted by CH2Cl2, EtOAc, and MeOH. The MeOH extract (3.4 kg) was fractioned by silica gel column chromatography (CC), eluting with a gradient of CH2Cl2–MeOH (v/v 100:0, 100:1, 100:3, 100:5, 100:7, 100:10, 100:30, 100:50, 0:100). Nine fractions M1~M9 were obtained on the basis of TLC monitoring results. The white precipitates (3.5 g) from fraction M4 was isolated by preparative HPLC (MeOH:H2O, 66:34) at a flow rate of 3 mL min−1 to give compounds 7 (tR 13.7 min, 3.6 mg), 15 (tR 16.0 min, 12.4 mg), 8 (tR 18.3 min, 2.7 mg), 14 (tR 21.2 min, 2.7 mg), 9 (tR 28.1 min, 5.2 mg), and 10 (tR 44.5 min, 3.0 mg). Fraction M4 (90.2 g) was subjected to sephadex LH-20 CC eluted by methanol to yield subfractions M4–1~M4–3. Subfraction M4–1 (23.7 g) was submitted to ODS CC eluted by MeOH–H2O (10:90, 30:70, 50:50, 70:30, 90:10, 100:0) to afford subfractions M4–1–1~M4–1–6. Subfraction M4–1–2 (4.8 g) was separated by sephadex LH-20 CC eluted by methanol to yield subfractions M4–1–2–1~M4–1–2–6. Subfraction M4–1–2–3 (1.6 g) was isolated by preparative HPLC (MeOH:H2O, 52:48) at a flow rate of 3 mL min−1 to give subfractions M4–1–2–3–1 (tR 7.9 min), M4–1–2–3–2 (tR 8.3 min), M4–1–2–3–3 (tR 10.8 min), M4–1–2–3–4 (tR 12.8 min), M4–1–2–3–5 (tR 16.4 min), and M4–1–2–3–6 (tR 25.1 min). Subfraction M4–1–2–3–2 (22.7 mg) was purified by preparative HPLC (MeOH:H2O, 48:52) at a flow rate of 3 mL min−1 to afford 3 (tR 11.9 min, 2.5 mg). Subfraction M4–1–2–3–5 (19.2 mg) was isolated by preparative HPLC (MeOH:H2O, 42:58) at a flow rate of 3 mL min−1 to afford 11 (tR 54.1 min, 2.1 mg). Subfraction M4–1–2–3–6 (25.3 mg) was purified by preparative HPLC (MeOH:H2O, 50:50) at a flow rate of 3 mL min−1 to afford 4 (tR 32.5 min, 2.2 mg). Fraction M5 (110.0 g) was subjected to sephadex LH-20 CC eluted by methanol to yield subfractions M5–1~M5–8. Subfractions M5–1~M5–5 (22.9 g) were combined and submitted to MCI CC eluted by MeOH–H2O (0:100, 10:90, 30:70, 50:50, 70:30, 90:10, 100:0) to afford subfractions M5–1–1~M5–1–4. Subfraction M5–1–2 (3.7 g) was applied to silica gel CC with a CHCl3-MeOH (100:0, 100:1, 100:3, 100:5 100:7, 100:10, 7:1, 3:1) gradient to give subfractions M5–1–2–1~M5–1–2–8. Subfraction M5–1–2–6 (50 mg) was isolated by preparative HPLC (MeOH:H2O, 54:46) at a flow rate of 3 mL min–1 to give compound 16 (tR 18.5 min, 5.6 mg). Subfraction M5–1–3 (0.25 g) was separated by silica gel CC with a CHCl3-MeOH (100:0, 100:1, 100:3, 100:5 100:7, 100:10, 7:1, 3:1) gradient to give subfractions M5–1–3–1~M5–1–3–9. Subfraction M5–1–3–5 (2.5 g) was purified by preparative HPLC (MeOH:H2O, 55:45) at a flow rate of 3 mL min–1 to give compounds 12 (tR 49.0 min, 4.3 mg) and 17 (tR 32.2 min, 3.3 mg). The precipitates from subfraction M5 were washed repeatedly by MeOH, and then the white powder (compound 6) was obtained. Fraction M6 (130.0 g) was subjected to sephadex LH-20 CC eluted by methanol to yield subfractions M6–1 and M6–2. The subfraction M6–1 (57.3 g) was applied to ODS CC with a MeOH-H2O (10:90, 30:70, 50:50, 70:30, 90:10, 100:0) gradient to give subfractions M6–1–1~M6–1–4. Subfraction M6–1–2 (5.8 g) was separated by silica gel CC with a CH2Cl2-MeOH (100:0, 100:1, 100:3, 100:5, 100:7, 100:10, 100:30) gradient to give subfractions M6–1–2–1~M6–1–2–7. Subfraction M6–1–2–4 (1.03 g) was isolated by preparative HPLC (MeOH:H2O, 45:55) at a flow rate of 3 mL min–1 to give subfraction M6–1–2–4–1 (tR 11.0 min) and 2 (tR 39.1 min, 6.6 mg). Subfraction M6–1–2–4–1 (15.2 mg) was applied to preparative HPLC (MeOH:H2O, 38:62) at a flow rate of 3 mL min−1 to give 18 (tR 17.0 min, 3.2 mg). The subfraction M6–1 (57.3 g) was applied to ODS CC with a MeOH-H2O (10:90, 30:70, 50:50, 70:30, 90:10, 100:0) gradient to give subfractions M6–1–1~M6–1–4. Subfraction M6–2 (65.9 g) was separated by silica gel CC with a CH2Cl2-MeOH (100:1, 100:3, 100:5, 100:7, 100:10, 100:30) gradient to give subfractions M6–2–1~M6–2–6. Subfraction M6–2–5 (1.7 g) was submitted to preparative HPLC (MeOH:H2O, 60:40) at a flow rate of 3 mL min−1 to give 13 (tR 22.9 min, 4.0 mg). The subfractions M7 and M8 were combined and then applied to Diaion HP-20 CC with an EtOH-H2O (10:90, 30:70, 50:50, 70:30, 90:10, 100:0) gradient to give subfractions M7–1~M7–6. The white sticky gum from subfraction M7–2 was washed repeatedly by MeOH and then separated by preparative HPLC (MeOH:H2O, 35:65) at a flow rate of 3 mL min−1 to give compounds 1 (tR 16.5 min, 10.0 mg) and 5 (tR 21.7 min, 3.7 mg).
3.4. Spectroscopic and Physical Data
Dysoslignan A (1): white, amorphous powder; [α–24.6 (c 0.28, MeOH); ECD (MeOH) λmax (Δε) 206 (–15.0), 222 (+0.5), 237 (–2.0), 285 (–0.3) nm; UV (MeOH) λmax (log ε) 204 (4.81), 275 (3.79), 285 (3.66) nm; IR (iTR)νmax 3386, 2931, 2905, 2832, 1759, 1653, 1594, 1506, 1462, 1447, 1422, 1389, 1334, 1244, 1192, 1169, 1127, 1074, 1037 cm−1; HR-ESI-MS (positive): m/z 763.2419 [M + Na]+ (calcd for C34H44O18Na, 763.2425); NMR data (DMSO-d6), see Table 1.
Dysoslignan B (2): white, amorphous powder; [α–39.7 (c 0.25, MeOH); ECD (MeOH) λmax (Δε) 208 (+3.75), 238 (+0.31), 290 (+0.42) nm; UV (MeOH) λmax (log ε) 204 (4.45), 242 (3.62), 284 (3.36) nm; IR (iTR)νmax 3381, 2361, 1764, 1616, 1523, 1475, 1375, 1335, 1264, 1219, 1168, 1121, 1033 cm−1; HR-ESI-MS (positive): m/z 909.2637 [M + Na]+ (calcd for C39H50O23Na, 909.2641); NMR data (DMSO-d6), see Table 1.
Dysoslignan C (3): white, amorphous powder; UV (MeOH) λmax (log ε) 204 (4.49), 225 (4.19), 264 (4.35), 326 (3.76), 363 (3.60) nm; IR (iTR)νmax 3367, 2989, 2946, 2833, 1741, 1608, 1520, 1467, 1420, 1348, 1274, 1213, 1186, 1115, 1090, 1027 cm−1; HR-ESI-MS (positive): m/z 385.0920 [M + H]+ (calcd for C20H17O8, 385.0923), m/z 407.0737 [M + Na]+ (calcd for C20H16O8Na, 407.0743); NMR data (DMSO-d6), see Table 2.
Dysoslignan D (4): white, amorphous powder; UV (MeOH) λmax (log ε) 202 (4.48), 225 (4.29), 263 (4.38), 312 (3.82), 355 (3.60) nm; IR (iTR)νmax 3410, 2939, 2839, 1745, 1605, 1535, 1465, 1352, 1244, 1131, 1094, 1030 cm−1; HR-ESI-MS (positive): m/z 383.0768 [M + H]+ (calcd for C20H15O8, 383.0767); NMR data (DMSO-d6), see Table 2.
3.5. Acid Hydrolysis and Sugar Determination
The absolute configurations of the galatose and glucose moieties were determined by the previously reported method [19]. Compounds 1 (1.0 mg) and 2 (1.0 mg) were dissolved in 1.0 mL of 2M HCl, and then hydrolyzed at 90 °C for 3 h. The HCl in the reaction mixture was removed under reduced pressure. The remaining reaction mixture was extracted with CH2Cl2. The water layers were directly analyzed by HPLC [column: Asahipak NH2P-50 4E (4.6 mm × 250 mm); mobile phase: CH3CN-H2O (17:3), flow rate: 0.7 mL/min]. The peaks at 13.15 and 14.27 min were coincided with D-glucose and D-galatose.
3.6. Cytotoxicity Asssay
By the previously reported MTS method [22], the cytotoxic activities of compounds 1–18 were evaluated against human lung cancer A-549, hepatocellular carcinoma SMMC-7721 cell lines. The cells were cultured in RPMI-1640 medium, supplemented with 10% fetal bovine serum (FBS) at 37 ℃ under 5% CO2 in a humidified atmosphere. Cell viability was assessed by conducting colorimetric measurements of the amount of insoluble formazan formed in living cells based on the reduction of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS). To be brief, 100 µL of cells were seeded into each well in a 96-well cell culture plate in advance. After 24 h, various concentrations of all test compounds were added. After the incubation for 48 h, MTS (20 μL) was added to each well, and the incubation continued for 4 h at 37 ℃. The optical density at 492 nm was determined using a 96-well microtiter plate reader. The IC50 values were calculated by the Reed–Muench method. Statistical analysis were performed by SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). All experiments were performed in triplicate.
4. Conclusions
Further phytochemical studies on D. versipellis resulted in the isolation of one new dibenzyltyrolactone lignan dysoslignan A (1), three new arylnaphthalide lignans dysoslignan B–C (2–4), along with fourteen known metabolites (5–18). Compound 2 is the first reported example of naturally occurring arylnaphthalide lignan triglycoside. All isolated compounds were tested for their in vitro cytotoxic activity against A-549 and SMMC-7721 cell lines using MTS assay. Among them, compounds 7, 14, and 15 were cytotoxic, with IC50 values of less than 1μM. Our research further demonstrated that the arylnaphthalide lignans are mainly responsible for the potent anticancer effect of D. versipellis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28072909/s1: Figures S1–S28: NMR and HR-ESI-MS spectra of compounds 1–4, and ECD spectra of compounds 1–2.
Author Contributions
Y.S. and W.F. designed the research; H.W., R.H., H.B., M.L. and J.W., performed the research and analyzed the data; Y.S. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by supported by Basic Science Foundation of Henan University of Chinese Medicine (No. 2014KYYWF-QN26), Science and Technology Innovation Talent Support Scheme of Henan University of Chinese Medicine (No. 2016XCXRC01), Scientific and Technological Key Project in Henan Province (No.192102310438), and Research Project on Chinese Medicine Science in Henan Province (No. 20-21ZY1039).
Data Availability Statement
Data are contained within the manuscript.
Acknowledgments
The authors thank Qinghua Kong for the technical assistance in MTS assay, and Xuan Zhao for NMR test.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Isolated compounds are not available from the authors.
References
- Guerram, M.; Jiang, Z.Z.; Zhang, L.Y. Podophyllotoxin, A medicinal agent of plant origin: Past, present and future. Chin. J. Nat. Med. 2012, 10, 161–169. [Google Scholar] [CrossRef]
- Desbne, S.; Giorgi-Renault, S. Drugs that inhibit tubulin polymerization: The particular case of podophyllotoxin and analogues. Curr. Med. Chem. 2002, 2, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.; Gohar, U.F.; Jamshed, I.; Mushtaq, A.; Mukhtar, H.; Zia-UI-Haq, M.; Toma, S.I.; Manea, R.; Moga, M.; Popovici, B. Podophyllotoxin: History, recent advances and future prospects. Biomolecules 2021, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.C.; Fu, C.X.; Qiu, Y.X.; Zhou, S.L.; Comes, H.P. Genetic structure and breeding system of a rare understory herb, Dysosma versipellis (Berberidaceae), from temperate deciduous forests in China. Am. J. Bot. 2010, 97, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Zhang, C.N.; He, T.; Sun, L.; Wang, Q.; Han, S.; Wang, W.X.; Kong, J.; Yuan, F.L.; Huang, J.M. Study on potential toxic material base and mechanisms of hepatotoxicity induced by Dysosma versipellis based on toxicological evidence chain (TEC) concept. Ecotox. Environ. Safe 2020, 190, 110073. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Gao, X.H.; Jin, L.H.; Bhadury, P.S.; Yuan, K.; Hu, D.Y.; Song, B.A.; Yang, S. Antiproliferation and cell apoptosis inducing bioactivities of constituents from Dysosma versipellis in PC3 and Bcap-37 cell lines. Cell Div. 2011, 6, 14. [Google Scholar] [CrossRef]
- Shi, Y.C.; Yuan, H.P.; Zou, R.; Liu, B.B. Complete chloroplast genome sequence of Dysosma versipellis (Berberidaceae), a rare and threatened species endemic to China. Mitochondrial DNA Part B 2019, 4, 4218–4219. [Google Scholar] [CrossRef]
- Jiang, F.; Tian, H.Y.; Zhang, J.L.; Ye, Q.M.; Jiang, R.W. Chemical constituents from Dysosma versipellis. Chin. Tradi. Herb. Drugs 2011, 42, 634–639. [Google Scholar]
- Chen, R.D.; Duan, R.G.; Wei, Y.N.; Zou, J.H.; Li, J.W.; Liu, X.Y.; Wang, H.Y.; Guo, Y.; Li, Q.H.; Dai, J.G. Flavonol dimers from callus cultures of Dysosma versipellis and their in vitro neuraminidase inhibitory activities. Fitoterapia 2015, 107, 77–84. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Y.Q.; Zhou, H.; Cao, X.J.; Jiang, X.H.; Wang, K.W.; Wu, S.H. A novel strategy for screening new natural products by a combination of reversed-phase liquid chromatography fractionation and 13C-NMR pattern recognition: The discovery of new anticancer flavone dimers from Dysosma versipellis (Hance). RSC Adv. 2015, 5, 77553–77564. [Google Scholar] [CrossRef]
- Sun, Y.J.; Han, R.J.; Bai, H.Y.; Wang, H.J.; Li, M.; Si, Y.Y.; Wang, J.M.; Gong, J.H.; Chen, H.; Feng, W.S. Structurally diverse biflavonoids from Dysosma versipellis and their bioactivity. RSC Adv. 2022, 12, 34962–34970. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Li, Z.L.; Chen, H.; Liu, X.Q.; Zhou, W.; Hua, H.M. Three new cytotoxic aryltetralin lignans from Sinopodophyllum emodi. Bioorg. Med. Chem. Lett. 2011, 21, 3794–3797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Q.; Huang, J.; Nagatsu, A.; Ogihara, Y. Two new podophyllotoxin glucosides from Sinopodophyllum emodi (Wall). Ying. Chem. Pharm. Bull. 2001, 49, 773–775. [Google Scholar] [CrossRef]
- Al-Abed, Y.; Abu-Zarga, M.; Sabri, S.; Atta-Ur-Rahman; Voelter, W. A arylnaphthalene lignan from Haplophyllum buxbaumii. Phytochemistry 1998, 49, 1779–1781. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xie, Y.G.; Zhang, Y.; Li, T.; Li, H.L.; Yan, S.K.; Jin, H.Z.; Zhang, W.D. New norlignans and flavonoids of Dysosma versipellis. Phytochem. Lett. 2016, 16, 75–81. [Google Scholar] [CrossRef]
- Al-Abed, Y.; Sabri, S.; Zarga, M.A.; Shah, Z.; Atta-ur-Rahman. Chemical constituents of the flora of Jordan, part V-B. Three new arylnaphthalene lignan glucosides from Haplophyllum buxbaumii. J. Nat. Prod. 1990, 53, 1152–1161. [Google Scholar] [CrossRef]
- Chen, Z.X.; Liu, D.L.; Gao, W.Y.; Zhang, T.J. A new macrolide and glycosides from the stem of Sargentodoxa cuneate. Chin. Chem. Lett. 2009, 20, 1339–1341. [Google Scholar] [CrossRef]
- Kuhnt, M.; Rimpler, H.; Heinrich, M. Lignans and other compounds from the Mixe Indian medicinal plant Hyptzs vertucullata. Phytochemistry 1994, 36, 485–489. [Google Scholar] [CrossRef]
- Sugimoto, S.; Yamano, Y.; Desoukey, S.Y.; Katakawa, K.; Wanas, A.S.; Otsuka, H.; Matsunami, K. Isolation of sesquiterpene-amino acid conjugates, Onopornoids A–D, and a flavonoid glucoside from Onopordum alexandrinum. J. Nat. Prod. 2019, 82, 1471–1477. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Vu, V.N.; Thi, D.P.; Tran, V.H.; Litaudon, M.; Roussi, F.; Nguyen, V.H.; Chau, V.M.; Mai, H.D.T.; Pham, V.C. Cytotoxic lignans from fruits of Cleistanthus tonkinensis. Fitoterapia 2020, 140, 104432. [Google Scholar] [CrossRef]
- Fonseca, S.F.; Rúveda, E.A.; Mcchesney, J.D. 13C NMR analysis of podophyllotoxin and some of its derivatives. Phytochemistry 1980, 19, 1527–1530. [Google Scholar] [CrossRef]
- Sun, Y.J.; Pan, R.Y.; Chen, H.J.; Zhao, C.; Han, R.J.; Li, M.; Xue, G.M.; Chen, H.; Du, K.; Wang, J.M.; et al. Cytotoxic polyhydroxylated oleanane triterpenoids from Cissampelos pareira var. hirsuta. Molecules 2022, 27, 1183. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).