Chemical Profiling and Therapeutic Evaluation of Standardized Hydroalcoholic Extracts of Terminalia chebula Fruits Collected from Different Locations in Manipur against Colorectal Cancer
Abstract
1. Introduction
2. Results
2.1. Yield of the Extract
2.2. GC-MS Analysis of Hydroalcoholic Extracts of T. chebula Fruits
2.3. High-Performance Thin-Layer Chromatography (HPTLC)
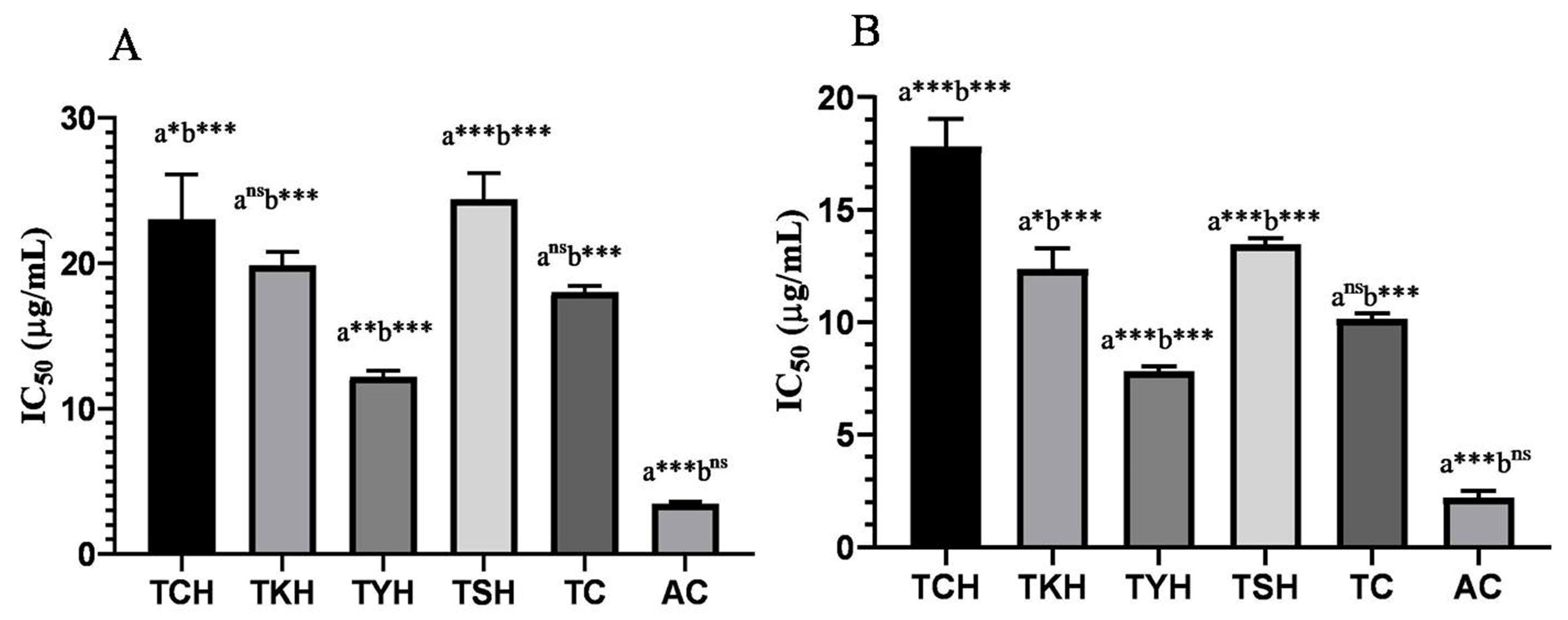
2.4. Total Phenolic, Total Flavonoid, and Antioxidant Activity of T. chebula Extracts
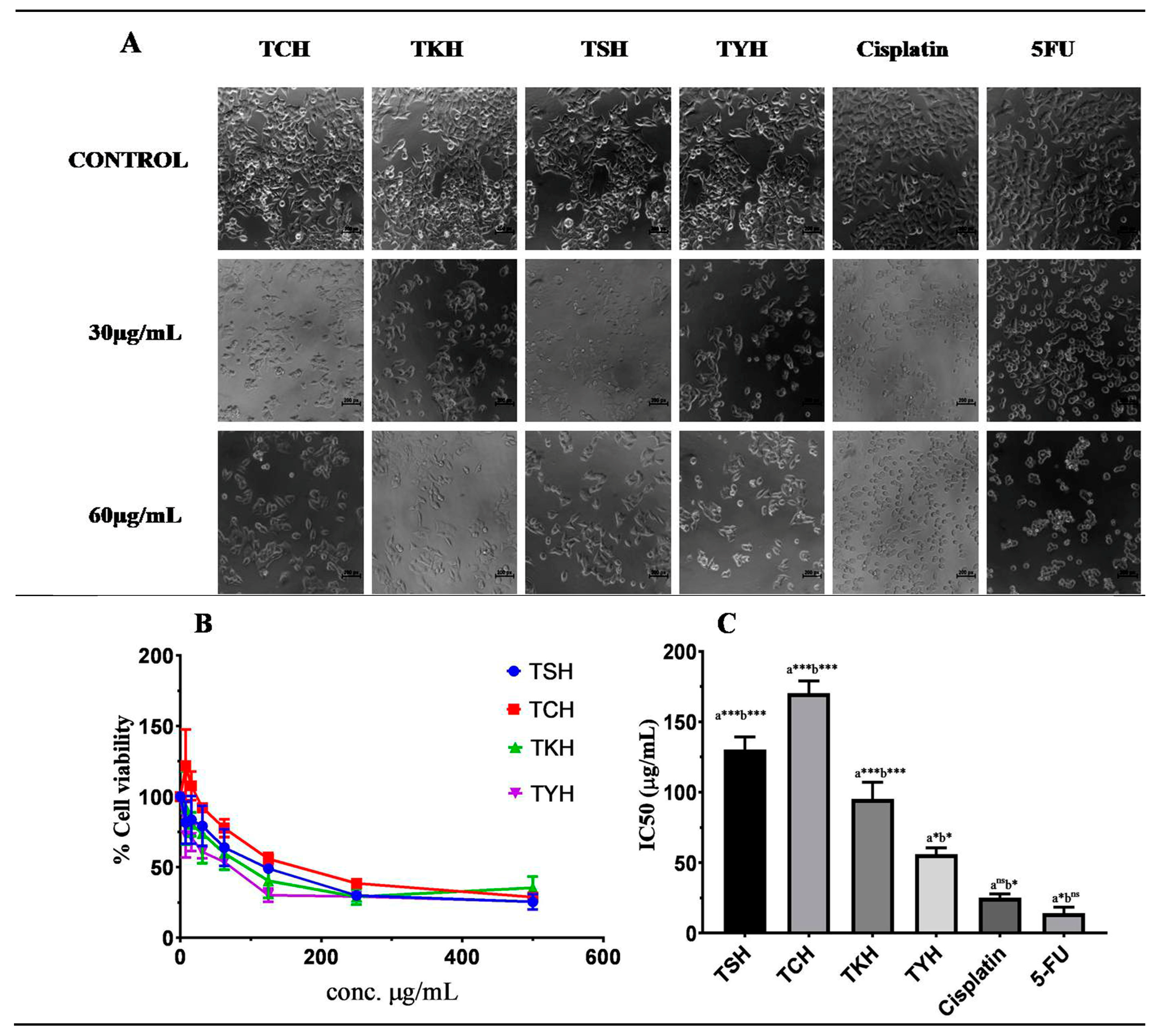
2.5. MTT Assay
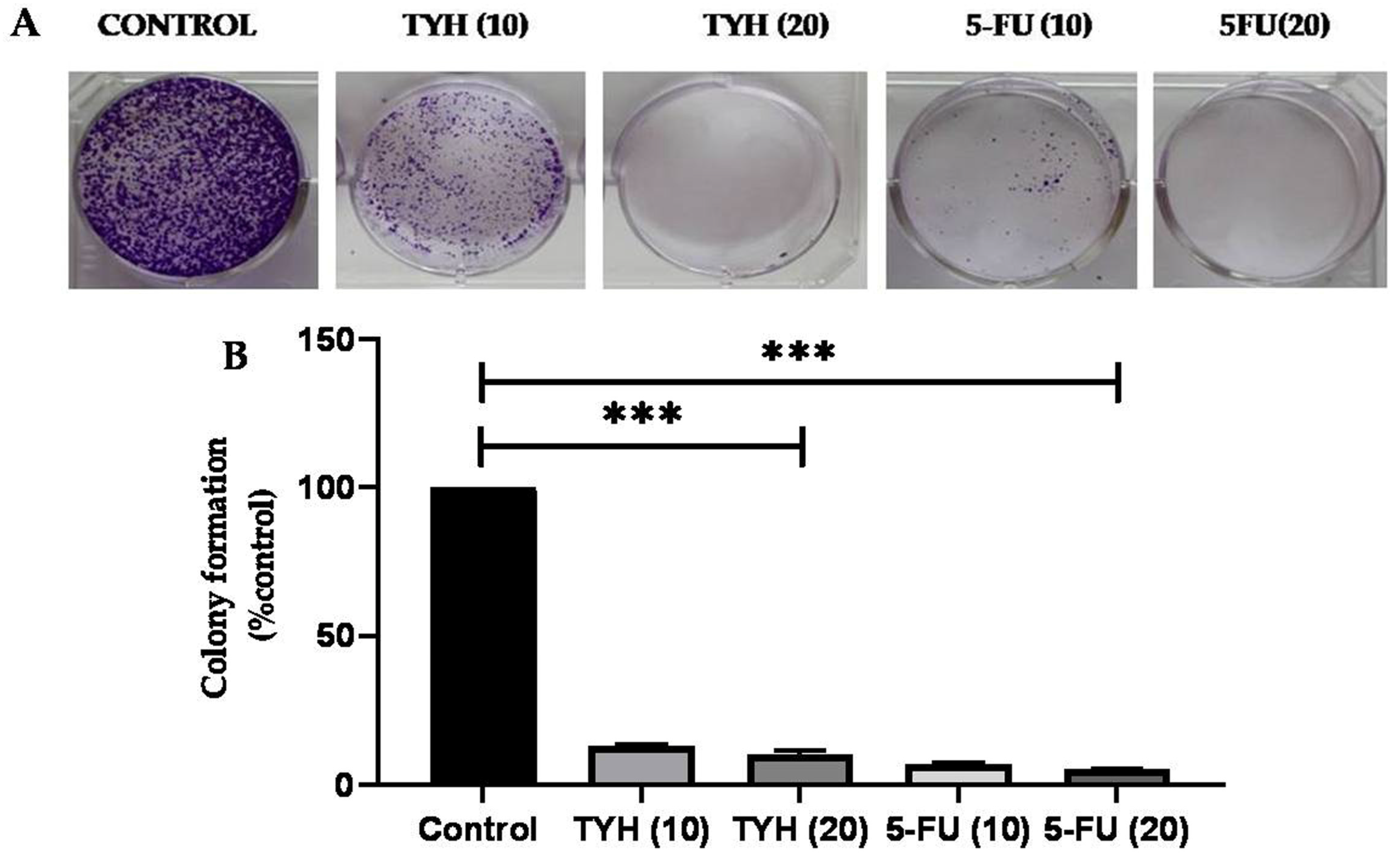
2.6. Effect of T. chebula Extracts on Colony-Forming Capacity of HCT 116 Cells
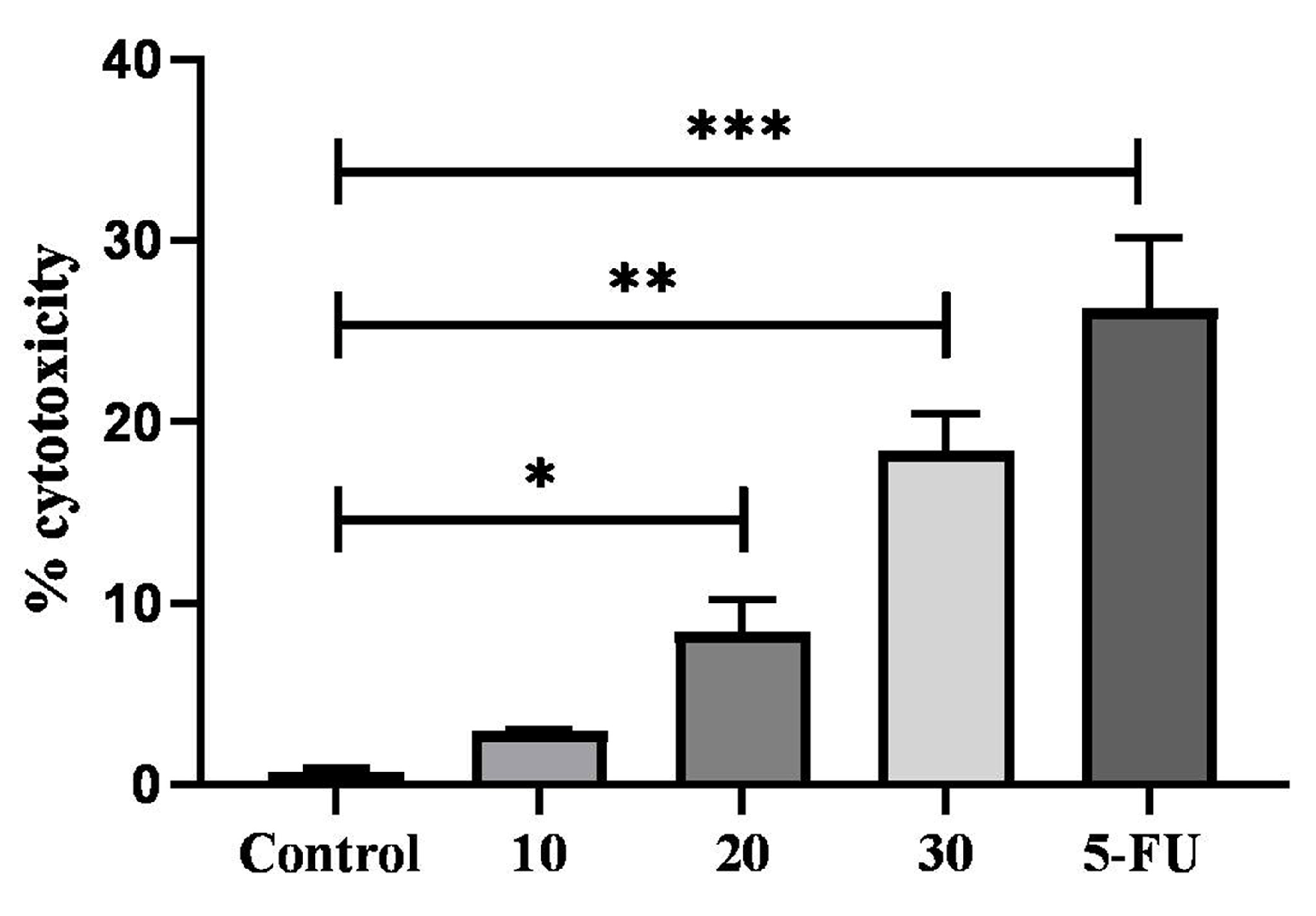
2.7. Cytotoxic Activity of TYH on HCT 116 Colon Cancer Cell
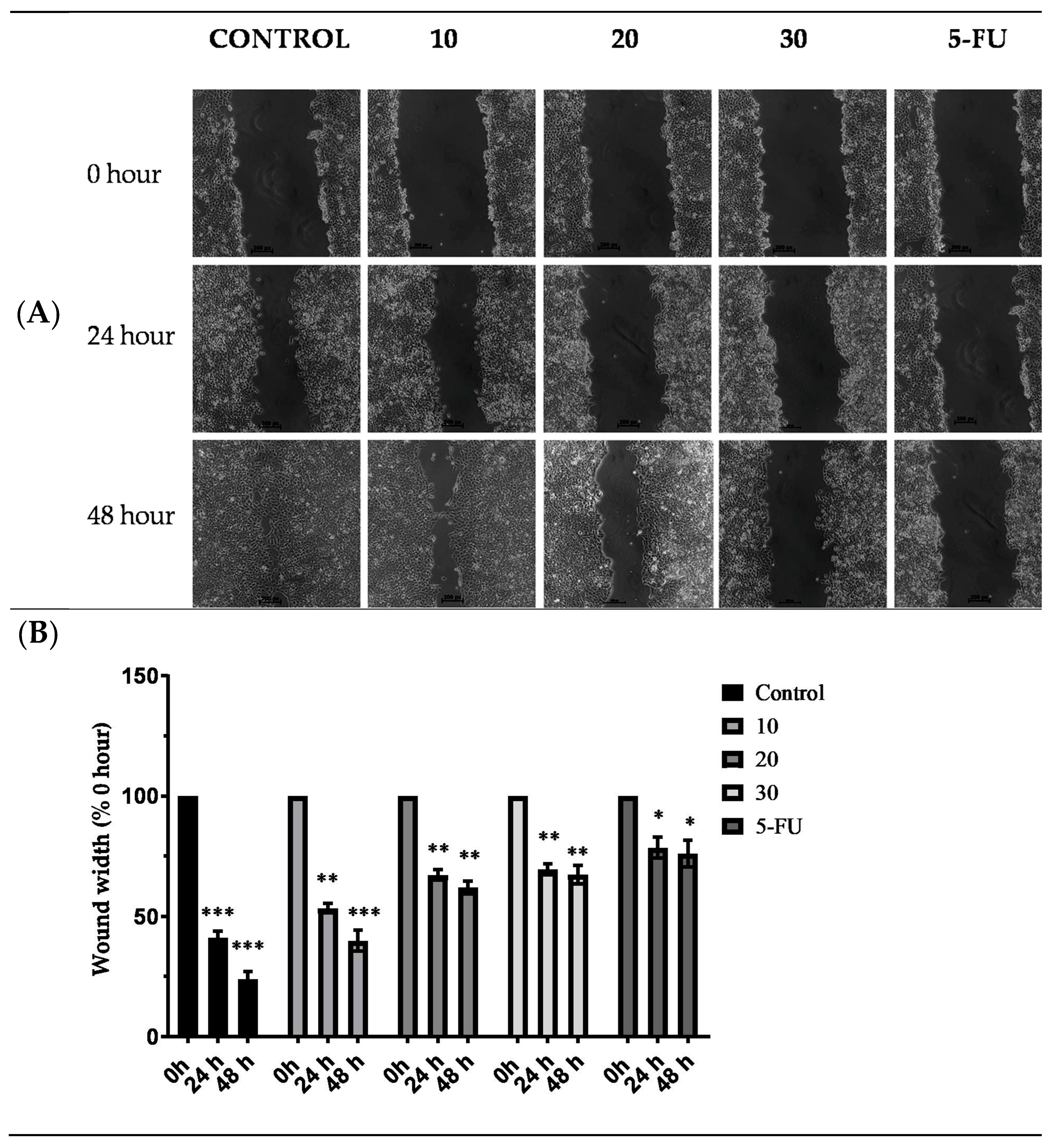
2.8. Effect of TYH on Migration of HCT 116 Cells
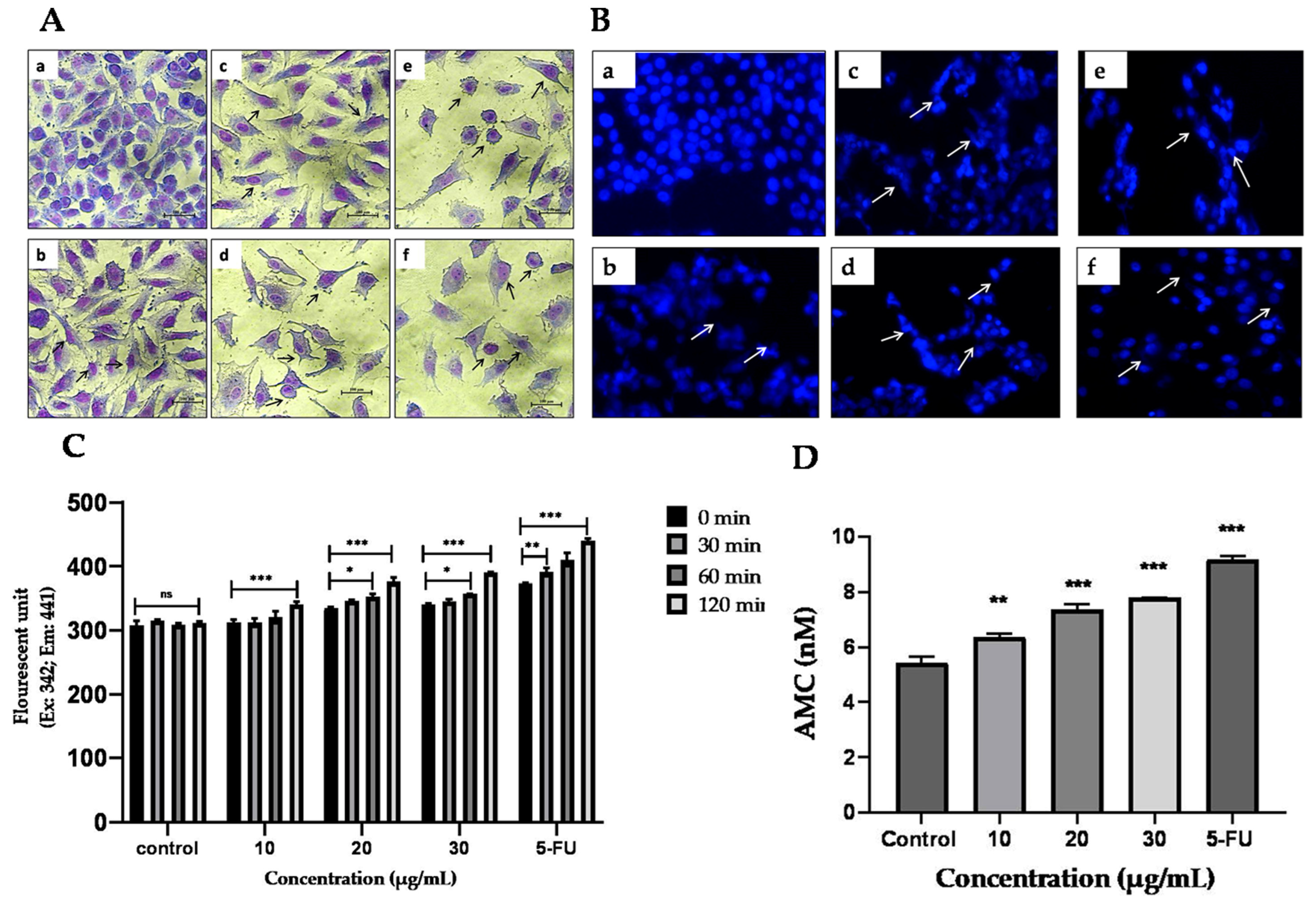
2.9. Analyses for Apoptosis
2.9.1. Effect of TYH on the Morphology of HCT 116 Cells
2.9.2. Apoptotic Effect of TYH Observed through DAPI Staining
2.9.3. TYH Activates Caspase-3 in HCT 116 Cells
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Collection of Samples
4.3. Preparation of Extracts
4.4. Determination of Total Phenolic Content
4.5. Determination of Total Flavonoid Content
4.6. Maintenance of Cell
4.7. Qualitative Phytochemical Screening
4.8. GC-MS Analysis
4.9. Quantitative Estimation of Gallic Acid and Ellagic Acid by HPTLC
4.10. Antioxidant Activity
4.10.1. DPPH Free Radical Scavenging Assay
4.10.2. ABTS Free Radical Scavenging Assay
4.11. Cell Viability Assay
4.12. Colony-Forming Assay
4.13. Test for Cytotoxicity Using LDH Assay
activity/Maximum LDH activity − Spontaneous LDHactivity) × 100
4.14. Effect on Cell Migration
4.15. Giemsa Staining
4.16. DAPI Staining
4.17. Caspase-3 Activity Assay
5. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. The development of Terminalia chebula Retz.. (Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013, 3, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Kusirisin, W.; Srichairatanakool, S.; Lerttrakarnnon, P.; Lailerd, N.; Suttajit, M.; Jaikang, C.; Chaiyasut, C. Antioxidative activity, polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Med. Chem. 2009, 5, 139–147. [Google Scholar] [PubMed]
- Kannan, P.; Ramadevi, S.R.; Hopper, W. Antibacterial activity of Terminalia chebula fruit extract. Afr. J. Microbiol. Res. 2009, 3, 180–184. [Google Scholar]
- Saleem, A.; Ahotupa, M.; Pihlaja, K. Total phenolics concentration and antioxidant potential of extracts of medicinal plants of Pakistan. Z. Naturforsch. C J. Biosci. 2001, 56, 973–978. [Google Scholar] [CrossRef]
- Li, K.; Diao, Y.; Zhang, H.; Wang, S.; Zhang, Z.; Yu, B.; Huang, S.; Yang, H. Tannin extracts from immature fruits of Terminalia chebula Fructus Retz. promote cutaneous wound healing in rats. BMC Complement. Altern. Med. 2011, 11, 86. [Google Scholar] [CrossRef]
- Gupta, P.C. Biological and pharmacological properties of Terminalia chebula Retz.(Haritaki)-An overview. Int. J. Pharm. Pharm. Sci. 2012, 4, 62–68. [Google Scholar]
- Poudel, P.; Thapa, R.; Lamichhane, S.; Paudel, H.R.; Devkota, H.P. Terminalia chebula Retz. In Himalayan Fruits and Berries; Academic Press: Cambridge, MA, USA, 2023; pp. 435–449. [Google Scholar]
- Barthakur, N.N.; Arnold, N.P. Nutritive value of the chebulic myrobalan (Terminalia chebula Retz.) and its potential as a food source. Food Chem. 1991, 40, 213–219. [Google Scholar]
- Manandhar, N.P. Plants and People of Nepal; Timber Press: Portland, OR, USA, 2002; 599p. [Google Scholar]
- Rege, N.N.; Thatte, U.M.; Dahanukar, S.A. Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytother. Res. 1999, 13, 275–291. [Google Scholar] [CrossRef]
- Singh, N.; Nath, R.; Mishra, N.; Kohli, R.P. An experimental evaluation of anti-stress effects of ‘Geriforte’(an Ayurvedic drug). Q. J. Crude Drug Res. 1978, 16, 125–136. [Google Scholar]
- Upadhyay, A.; Singh, D.K. Molluscicidal activity of Sapindusmukorossi and Terminalia chebula against the freshwater snail Lymnaea acuminata. Chemosphere 2011, 83, 468–474. [Google Scholar] [CrossRef]
- Ahmed, S.; Ding, X.; Sharma, A. Exploring Scientific Validation of Triphala Rasayana in Ayurveda as a Source of Rejuvenation for Contemporary Healthcare: An Update. J. Ethnopharm. 2021, 273, 113829. [Google Scholar] [CrossRef]
- Singh, S.R.; Phurailatpam, A.K.; Wangchu, L.; Ngangbam, P.; Chanu, T.M. Traditional medicinal knowledge of underutilized minor fruits as medicine in Manipur. Int. J. Agric. Sci. 2014, 4, 241–247. [Google Scholar]
- Thokchom, A.D.; Ningthoujam, S.S.; Talukdar, A.D.; Singh, B. Ethnobotanical survey of Medicinal Plants used as Hepatoprotective in Imphal east and west district of Manipur, India. IJSRP 2016, 6, 217–224. [Google Scholar]
- Ningthoujam, S.S.; Das Talukdar, A.; Potsangbam, K.S.; Choudhury, M.D. Traditional uses of herbal vapour therapy in Manipur, North East India: An ethnobotanical survey. J. Ethnopharmacol. 2013, 147, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Bahadur, S.; Chaudhary, S.K.; Kar, A.; Mukherjee, K. Quality related safety issue-evidence-based validation of herbal medicine farm to pharma. In Evidence-Based Validation of Herbal Medicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–28. [Google Scholar]
- Singh, A.K.; Rai, S.N.; Maurya, A.; Mishra, G.; Awasthi, R.; Shakya, A.; Chellappan, D.K.; Dua, K.; Vamanu, E.; Chaudhary, S.K.; et al. Therapeutic potential of phytoconstituents in management of Alzheimer’s disease. Evid.-Based Complement. Altern. Med. 2021, 2021, 5578574. [Google Scholar] [CrossRef]
- Kumar, K.J. Effect of geographical variation on contents of tannic acid, gallic acid, chebulinic acid and ethyl gallate in Terminalia chebula. Nat. Prod. 2006, 2, 170–175. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Nandakumar, A.; National Cancer Registry Programme; Indian Council of Medical Research, Consolidated Report of the Population-Based Cancer Registries, New Delhi, India. Consolidated Report of the Population Based Cancer Registries: 1990–96. 1990. Available online: http://ncdirindia.org/All_Reports/PBCR_Suppliment/supplement_pbcr.pdf (accessed on 27 May 2019).
- Obesity and Colorectal Cancer-ICMR Bulletin. Available online: https://icmr.nic.in/sites/default/files/icmr_bulletins/Bul_July_Sept.pdf (accessed on 27 May 2019).
- Ma, J.; Stampfer, M.J.; Giovannucci, E.; Artigas, C.; Hunter, D.J.; Fuchs, C.; Willett, W.C.; Selhub, J.; Hennekens, C.H.; Rozen, R. Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res. 1997, 57, 1098–1102. [Google Scholar]
- Giovannucci, E.; Stampfer, M.J.; Colditz, G.A.; Rimm, E.B.; Trichopoulos, D.; Rosner, B.A.; Speizer, F.E.; Willett, W.C. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J. Natl. Cancer Inst. 1993, 85, 875–884. [Google Scholar] [CrossRef]
- Mathew, A.; Peters, U.; Chatterjee, N.; Kulldorff, M.; Sinha, R. Fat, fiber, fruits, vegetables, and risk of colorectal adenomas. Int. J. Cancer 2004, 108, 287–292. [Google Scholar] [CrossRef]
- Slattery, M.L.; Potter, J.D.; Duncan, D.M.; Berry, T.D. Dietary fats and colon cancer: Assessment of risk associated with specific fatty acids. Int. J. Cancer 1997, 73, 670–677. [Google Scholar] [CrossRef]
- Sarkar, S.; Mukherjee, R.; Paira, S.K.; Roy, B.; Banerjee, S.; Mukherjee, S.K. Profile of colorectal cancer in Eastern India. J. Indian Med. Assoc. 2012, 110, 901–903. [Google Scholar] [PubMed]
- Sankaranarayanan, R.; Mathew, B.; Jacob, B.J.; Thomas, G.; Somanathan, T.; Pisani, P.; Pandey, M.; Ramadas, K.; Najeeb, K.; Abraham, E. Early findings from a community-based, cluster-randomized, controlled oral cancer screening trial in Kerala, India. The Trivandrum Oral Cancer Screening Study Group. Cancer 2000, 88, 664–673. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bhattacharya, S.; Basu, R.; Bera, P.; Halder, A. Colorectal cancer: A study of risk factors in a tertiary care hospital of north bengal. J. Clin. Diagn. Res. 2014, 8, FC08–FC10. [Google Scholar] [CrossRef]
- Raju, S.K.; Sekar, P.; Kumar, S.; Murugesan, M.; Karthikeyan, M.; Elampulakkadu, A. Plant secondary metabolites for the prevention and treatment of colorectal cancer: A review. J. Pharmacogn. Phytochem. 2022, 11, 229–246. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J. Effects of carbon dioxide, water supply, and seasonally on terpene content and emission by Rosmarinus officinalis. J. Chem. Ecol. 1997, 23, 979–993. [Google Scholar] [CrossRef]
- Stoddart, M.J.; Louis, K.S.; Siegel, A.C.; Kupcsik, L.; Czekanska, E.M. Chapters 1–5: Mammalian Cell Viability; Stoddart, M.J., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 1–27. [Google Scholar]
- van Meerlo, J.; Kaspers, G.J.L.; Cloos, J.; Glaysher, S.; Cree, I.A.; Hartley, J.M.; Spanswick, V.J.; Hartley, J.A. Chapters 20–22, 25: Cancer Cell Culture Methods and Protocols, 2nd ed.; Cree, I.A., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 237–259. [Google Scholar]
- Yang, N.; Ray, S.D.; Krafts, K. Cell Proliferation. Encycl. Toxicol. 2014, 1, 761–765. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Chang, C.L.; Lin, C.S. Phytochemical Composition, Antioxidant Activity, and Neuroprotective Effect of Terminalia chebulaRetzius Extracts. Evid. Based Complement. Altern. Med. 2012, 2012, 125247. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, P. Extraction, gas chromatography-mass spectrometry analysis and screening of fruits of Terminalia chebula Retz. for its antimicrobial potential. Pharmacogn. Res. 2013, 5, 162–168. [Google Scholar] [CrossRef]
- Amala, V.E.; Jeyaraj, M. Comparative evaluation of phytocomponents present in the methanolic extract of Terminalia chebula Retz., Terminalia bellirica Roxb., and Phyllanthus emblica L., fruit extracts using GC-MS analysis. IJPBS 2014, 5, B-927–B-934. [Google Scholar]
- Cynthia, I.F.; Hery, S.; Akhmad, D. Antibacterial and antioxidant activities of pyrogallol and synthetic pyrogallol dimer. Res. J. Chem. Environ. 2018, 22, 39–47. [Google Scholar]
- Biskup, I.; Golonka, I.; Gamian, A.; Sroka, Z. Antioxidant activity of selected phenols estimated by ABTS and FRAP methods. Postep. Hig. Med. Dosw. 2013, 67, 958. [Google Scholar] [CrossRef]
- Hirasawa, M.; Takada, K. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. J. Antimicrob. Chemother. 2004, 53, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Ganesh, D.; Panchal, P.; Teimouri, M.; Narayana, V.S. GC-MS analysis of phytocomponents in the methanolic extract of Emblica officinalis Gaertn (Indian Gooseberry). J. Chem. Pharm. Res. 2014, 6, 843–845. [Google Scholar]
- Mendes, V.; Vilaça, R.; de Freitas, V.; Ferreira, P.M.; Mateus, N.; Costa, V. Effect of myricetin, pyrogallol, and phloroglucinol on yeast resistance to oxidative stress. Oxid. Med. Cell. Longev. 2015, 2015, 782504. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Lampronti, I.; Martello, D.; Bianchi, N.; Jabbar, S.; Choudhuri, M.S.K.; Datta, B.K.; Gambari, R. Identification of pyrogallol as an antiproliferative compound present in extracts from the medicinal plant Emblica officinalis: Effects on in vitro cell growth of human tumor cell lines. Int. J. Oncol. 2002, 21, 187–192. [Google Scholar] [CrossRef]
- Park, W.H.; Park, M.N.; Han, Y.H.; Kim, S.W. Pyrogallol inhibits the growth of gastric cancer SNU-484 cells via induction of apoptosis. Int. J. Mol. Med. 2008, 22, 263–268. [Google Scholar]
- Yu, X.; Zhao, M.; Liu, F.; Zeng, S.; Hu, J. Antioxidants in volatile Maillard reaction products: Identification and interaction. LWT-Food Sci. Technol. 2013, 53, 22–28. [Google Scholar] [CrossRef]
- Hsiang, C.H.; Straus, D.S. Cyclopentenone causes cell cycle arrest and represses cyclin D1 promoter activity in MCF-7 breast cancer cells. Oncogene 2002, 21, 2212–2226. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef] [PubMed]
- Kocaçalişkan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. Naturforsch. C J. Biosci. 2006, 61, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Knuth, S.; Abdelsalam, R.; Khayyal, M.; Schweda, F.; Heilmann, J.; Kees, M.; Mair, G.; Kees, F.; Jürgenliemk, G. Catechol is a bioactive metabolite of Willow bark. Planta Med. 2013, 79, SL49. [Google Scholar] [CrossRef]
- Li, R.; Narita, R.; Ouda, R.; Kimura, C.; Nishimura, H.; Yatagai, M.; Fujita, T.; Watanabe, T. Structure-dependent antiviral activity of catechol derivatives in pyroligneous acid against the encephalomycarditis virus. RSC Adv. 2018, 8, 35888–35896. [Google Scholar] [CrossRef] [PubMed]
- Smolyaninov, I.V.; Burmistrova, D.A.; Arsenyev, M.V.; Polovinkina, M.A.; Pomortseva, N.P.; Fukin, G.K.; Poddel’sky, A.I.; Berberova, N.T. Synthesis and Antioxidant Activity of New Catechol Thioethers with the Methylene Linker. Molecules 2022, 27, 3169. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Sharma, R.; Cruz-Martins, N.; Valko, M.; Upadhyay, N.K.; Kuča, K.; Bhardwaj, P. Studies of Phytochemicals, Antioxidant, and Antibacterial Activities of Pinus gerardiana and Pinus roxburghii Seed Extracts. BioMed. Res. Int. 2022, 2022, 5938610. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, H.M.; Jin, C.H.; Kang, S.Y.; Kim, J.B.; Jeon, Y.G.; Park, K.Y.; Lee, I.S.; Han, A.R. Composition and Antioxidant Activities of Volatile Organic Compounds in Radiation-Bred Coreopsis Cultivars. Plants 2020, 9, 717. [Google Scholar] [CrossRef]
- Premathilaka, R.; Silva, M. Bioactive compounds and antioxidant activity of Bunchosiaarmenica. World J. Pharm. Pharm. Sci. 2016, 5, 1237–1247. [Google Scholar]
- Palafox-Carlos, H.; Gil-Chávez, J.; Sotelo-Mundo, R.R.; Namiesnik, J.; Gorinstein, S.; González-Aguilar, G.A. Antioxidant interactions between major phenolic compounds found in ‘Ataulfo’ mango pulp: Chlorogenic, gallic, protocatechuic and vanillic acids. Molecules 2012, 17, 12657. [Google Scholar] [CrossRef]
- Zhao, J.; Li, G.; Bo, W.; Zhou, Y.; Dang, S.; Wei, J.; Li, X.; Liu, M. Multiple effects of ellagic acid on human colorectal carcinoma cells identified by gene expression profile analysis. Int. J. Oncol. 2017, 50, 613–621. [Google Scholar] [CrossRef]
- Ozturk Sarikaya, S.B. Acethylcholinesterase inhibitory potential and antioxidant properties of pyrogallol. J. Enzyme Inhib. Med. Chem. 2015, 30, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Zhao, J.; Muhammad, I.; Zhang, Y. Optimization of total phenolic content from Terminalia chebula Retz. fruits using response surface methodology and evaluation of their antioxidant activities. PloS ONE 2018, 13, e0202368. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Naeini, K.M.; Lorigooini, Z.; Namjoo, R. Oral acute and sub-acute toxic effects of hydroalcoholic Terminalia chebula Retz. and Achillea wilhelmsii extracts in BALB/c mice. BioMedicine 2019, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Verma, R.J. Antioxidant activity of polyphenolic extract of Terminalia chebulaRetzius fruits. J. Taibah Univ. Sci. 2016, 10, 805–812. [Google Scholar] [CrossRef]
- Boik, J. Natural Compounds in Cancer Therapy; Oregon Medical Press: Princeton, MN, USA, 2001. [Google Scholar]
- Lee, C.C.; Houghton, P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J. Ethnopharmacol. 2005, 100, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Sembiring, E.N.; Elya, B.; Sauriasari, R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. J. 2018, 10, 123–127. [Google Scholar] [CrossRef]
- Sandip, P.; Shreewardhan, R.; Sandeephan, M.; Abhay, C.; Debjani, D. Phytochemical evaluation and free radical scavenging potential of HugoniaMystax (L.) leaf extract. Bionano. Front. 2014, 7, 20. [Google Scholar]
- Evans, W.C. Trease and Evans’ pharmacognosy. Gen. Pharmacol. 1997, 2, 291. [Google Scholar]
- Akter, R.; Khan, S.S.; Kabir, M.T.; Halder, S. GC-MS-employed phytochemical characterization, synergistic antioxidant, and cytotoxic potential of Triphala methanol extract at non-equivalent ratios of its constituents. Saudi J. Biol. Sci. 2022, 29, 103287. [Google Scholar] [CrossRef]
- Karthika, R.S.; Hameed, A.S.; Meenu, M.T. HPTLC estimation of gallic acid and ellagic acid in amrtottarakvatha prepared in two ratios. Int. J. Ayurveda Pharma Res. 2019, 7, 1–9. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Antioxidative activity of phenolic composition of commercial extracts of sage and rosemary. Lwt 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Cho, J.; Lee, H.K. Wogonin inhibits excitotoxic and oxidative neuronal damage in primary cultured rat cortical cells. Eur. J. Pharmacol. 2004, 485, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Spectrophotometric determination of antioxidant activity. Redox Rep. 1996, 2, 161–171. [Google Scholar] [CrossRef]
- Rajurkar, N.S.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Gupta, A.; Makabenta, J.M.V.; Park, J.; Amante, J.J.; Chattopadhyay, A.N.; Matuwana, D.; Kearney, C.J.; Rotello, V.M. Ultrasound-Enhanced Antibacterial Activity of Polymeric Nanoparticles for Eradicating Bacterial Biofilms. Adv. Healthc. Mater. 2022, 11, e2201060. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Fang, K.; Zhang, X.-H.; Han, Y.-T.; Wu, G.-R.; Cai, D.-S.; Xue, N.-N.; Guo, W.-B.; Yang, Y.-Q.; Chen, M.; Zhang, X.-Y.; et al. Design, Synthesis, and Cytotoxic Analysis of Novel Hederagenin⁻Pyrazine Derivatives Based on Partial Least Squares Discriminant Analysis. Int. J. Mol. Sci. 2018, 19, 2994. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Oz, E.S.; Aydemir, E.; Fışkın, K. DMSO exhibits similar cytotoxicity effects to thalidomide in mouse breast cancer cells. Oncol. Lett. 2012, 3, 927–929. [Google Scholar] [CrossRef] [PubMed]
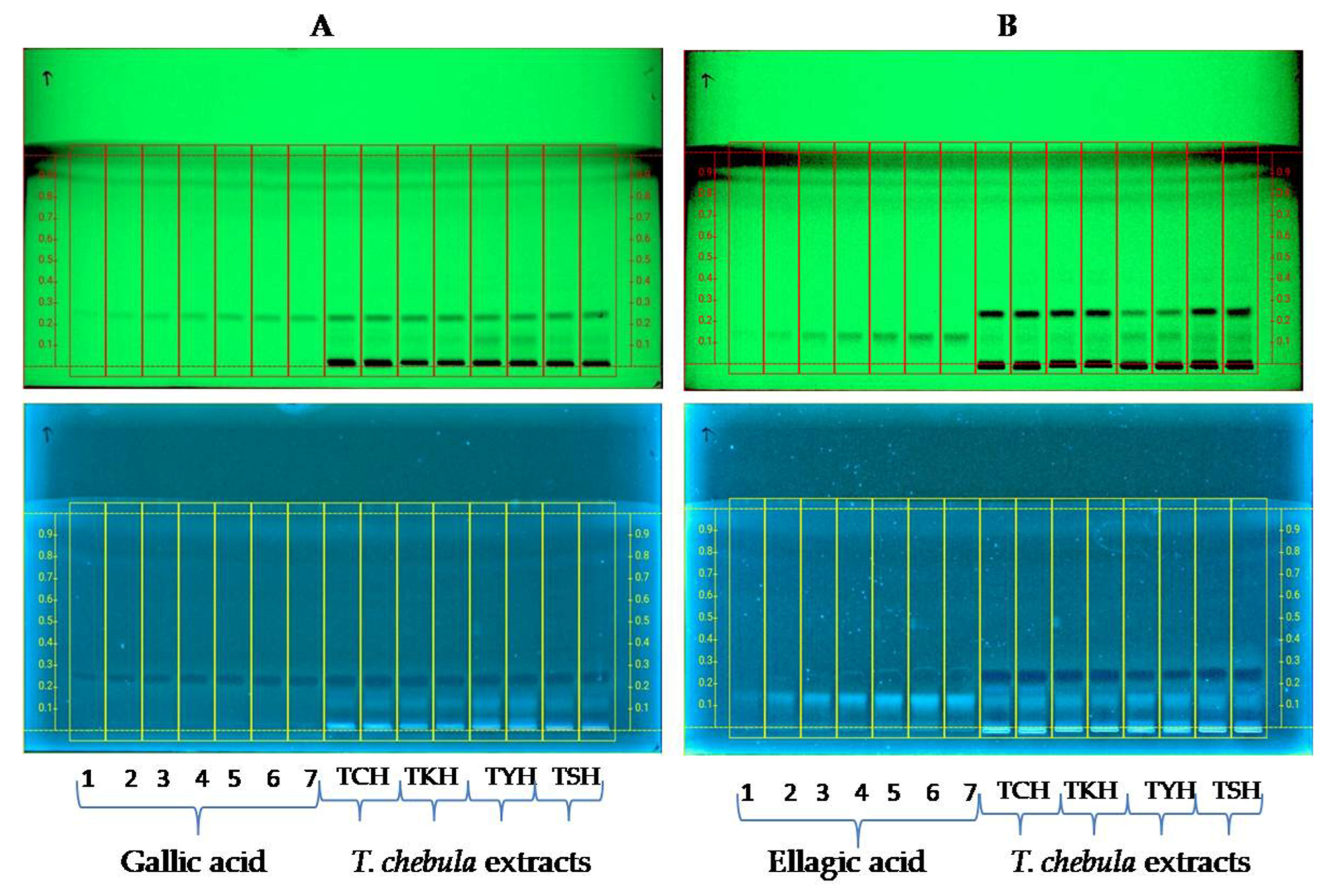
| Sample | Collection Site with GPS Coordinates | Yield Percentage (w/w) | Gallic Acid Content (µg/mg) as Determined by HPTLC | Ellagic Acid Content (µg/mg) as Determined by HPTLC |
|---|---|---|---|---|
| TCH | Chandel, N 24°25′35.7″ E94°00′47.7″ | 25.2 | 22.44 ± 0.056 | 2.652 ± 0.07 |
| TSH | Sagolband, N 24°48′06″ E93°55′16″ | 28.7 | 21.61 ± 0.183 | 3.597 ± 0.145 |
| TKH | Kakching, N 24°30′01.9″ E93°58′27.1″ | 30.3 | 18.275 ± 0.272 | 3.081 ± 0.089 |
| TYH | Yumnam Huidrom, N 24°39′46″ E93°54′14″ | 28.7 | 19.629 ± 0.055 | 11.265 ± 0.089 |
| Sl.No. | Name of the Compounds | % Content in TCH | % Content in TKH | % Content in TYH | % Content in TSH |
|---|---|---|---|---|---|
| 1 | 1,2,3-Benzenetriol | 20.95 | 26.53 | 43.56 | 40.41 |
| 2 | 2-Cyclopenten-1-one | 19.35 | 12.79 | 9.07 | 14.66 |
| 3 | Catechol | 12.82 | 6.85 | NA | 12.47 |
| 4 | 5-Hydroxymethylfurfural | 6.04 | 11.26 | 4.03 | NA |
| 5 | 1-Hexyl-2-nitrocyclohexane | 4.13 | 2.76 | NA | 2.11 |
| 6 | 1-Undecanol | 3.15 | 1.88 | NA | 1.74 |
| 7 | Phenol | 3.27 | 0.37 | 5.40 | NA |
| 8 | Hexadecanamide | 1.95 | 1.31 | NA | 1.56 |
| 7 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl | 1.74 | 1.68 | NA | 0.74 |
| 8 | 2,6-Difluorobenzoic acid, 4-nitrophenyl ester | 1.72 | 3.05 | NA | NA |
| 9 | 1-(1′-Pyrrolidinyl)-2-butanone | 1.33 | NA | NA | NA |
| 10 | Phosphonic acid, (p-hydroxyphenyl)- | NA | 3.82 | NA | 4.64 |
| 11 | Sucrose | 0.22 | 2.27 | NA | NA |
| 12 | 3-Furaldehyde | 0.68 | 1.59 | 0.89 | 0.50 |
| 13 | 2-Cyclohexen-1-one | NA | 1.49 | NA | 1.22 |
| 14 | Cyclobutaneethanol, á-methylene | 0.92 | 1.40 | 0.93 | NA |
| 15 | Benzeneacetaldehyde | 0.89 | 1.13 | 1.56 | NA |
| 16 | 2,3,4,5-Tetrahydropyridazine | NA | NA | 1.65 | NA |
| 17 | 1,2-Benzenediol, mono(methylcarbamate) | NA | 0.19 | 1.57 | NA |
| 18 | 2,5-Furandione, 3-methyl- | NA | NA | 1.18 | 0.18 |
| 19 | 2,6,10,14-Tetramethylpentade can-6-ol | NA | NA | 1.06 | NA |
| 20 | S-Methyl 2-methylpropanethioate | NA | NA | NA | 1.26 |
| Sample | DPPH Assay IC50 (µg/mL) | ABTS Assay IC50 (µg/mL) | TPC (GAEmg/g ext) | TFC (QEmg/g ext.) |
|---|---|---|---|---|
| TCH | 23.00 ± 3.11 | 17.81 ± 1.21 | 286.76 ± 0.35 | 8.98 ± 0.35 |
| TKH | 19.85 ± 0.93 | 12.35 ± 0.92 | 295.35 ± 0.98 | 8.10 ± 0.87 |
| TYH | 12.16 ± 0.42 | 7.80 ± 0.23 | 304.56 ± 1.23 | 7.65 ± 0.45 |
| TSH | 24.42 ± 1.78 | 13.45 ± 0.28 | 270.65 ± 0.78 | 7.95 ± 0.79 |
| Trolox(TC) | 18.01 ± 0.44 | 10.15 ± 0.24 | - | - |
| Ascorbic Acid(AC) | 3.46 ± 0.12 | 2.19 ± 0.003 | - | - |
| Sample/Drugs | IC50 (µg/mL) Values of the T chebula Extracts against HCT 116 Cells |
|---|---|
| TSH | 133.58 ± 2.0 |
| TCH | 172.05 ± 2.0 |
| TYH | 52.42 ± 0.87 |
| TKH | 94.07 ± 1.70 |
| Cisplatin | 24.38 ± 1.26 |
| 5-FU | 12.75 ± 1.03 |
| Sl.No | Sample (Place of Collection) | Voucher Specimen No. | Latitude Longitude | Elevation(m) |
|---|---|---|---|---|
| 1 | TCH (Chandel, Manipur) | IBSD/M-283-A | N 24°25′35.7″ E94°00′47.7″ | 817 |
| 2 | TKH (Kakching Manipur) | IBSD/M-283-C | N 24°30′01.9″ E93°58′27.1″ | 778 |
| 3 | TSH (Sagolband, Imphal, Manipur) | IBSD/M-283-B | N 24°48′06″ E93°55′16′ | 780 |
| 4 | TYH (Yumnam Huidrom, Imphal, Manipur) | IBSD/M-283-D | N 24°39′46″ E93°54′14″ | 784 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thoithoisana Devi, S.; Devika Chanu, K.; Singh, N.B.; Chaudhary, S.K.; Keithellakpam, O.S.; Singh, K.B.; Mukherjee, P.K.; Sharma, N. Chemical Profiling and Therapeutic Evaluation of Standardized Hydroalcoholic Extracts of Terminalia chebula Fruits Collected from Different Locations in Manipur against Colorectal Cancer. Molecules 2023, 28, 2901. https://doi.org/10.3390/molecules28072901
Thoithoisana Devi S, Devika Chanu K, Singh NB, Chaudhary SK, Keithellakpam OS, Singh KB, Mukherjee PK, Sharma N. Chemical Profiling and Therapeutic Evaluation of Standardized Hydroalcoholic Extracts of Terminalia chebula Fruits Collected from Different Locations in Manipur against Colorectal Cancer. Molecules. 2023; 28(7):2901. https://doi.org/10.3390/molecules28072901
Chicago/Turabian StyleThoithoisana Devi, Soibam, Khaidem Devika Chanu, Nameirakpam Bunindro Singh, Sushil Kumar Chaudhary, Ojit Singh Keithellakpam, Kshetrimayum Birla Singh, Pulok K. Mukherjee, and Nanaocha Sharma. 2023. "Chemical Profiling and Therapeutic Evaluation of Standardized Hydroalcoholic Extracts of Terminalia chebula Fruits Collected from Different Locations in Manipur against Colorectal Cancer" Molecules 28, no. 7: 2901. https://doi.org/10.3390/molecules28072901
APA StyleThoithoisana Devi, S., Devika Chanu, K., Singh, N. B., Chaudhary, S. K., Keithellakpam, O. S., Singh, K. B., Mukherjee, P. K., & Sharma, N. (2023). Chemical Profiling and Therapeutic Evaluation of Standardized Hydroalcoholic Extracts of Terminalia chebula Fruits Collected from Different Locations in Manipur against Colorectal Cancer. Molecules, 28(7), 2901. https://doi.org/10.3390/molecules28072901






