Effect of Redox Potential on Diiron-Mediated Disproportionation of Hydrogen Peroxide
Abstract
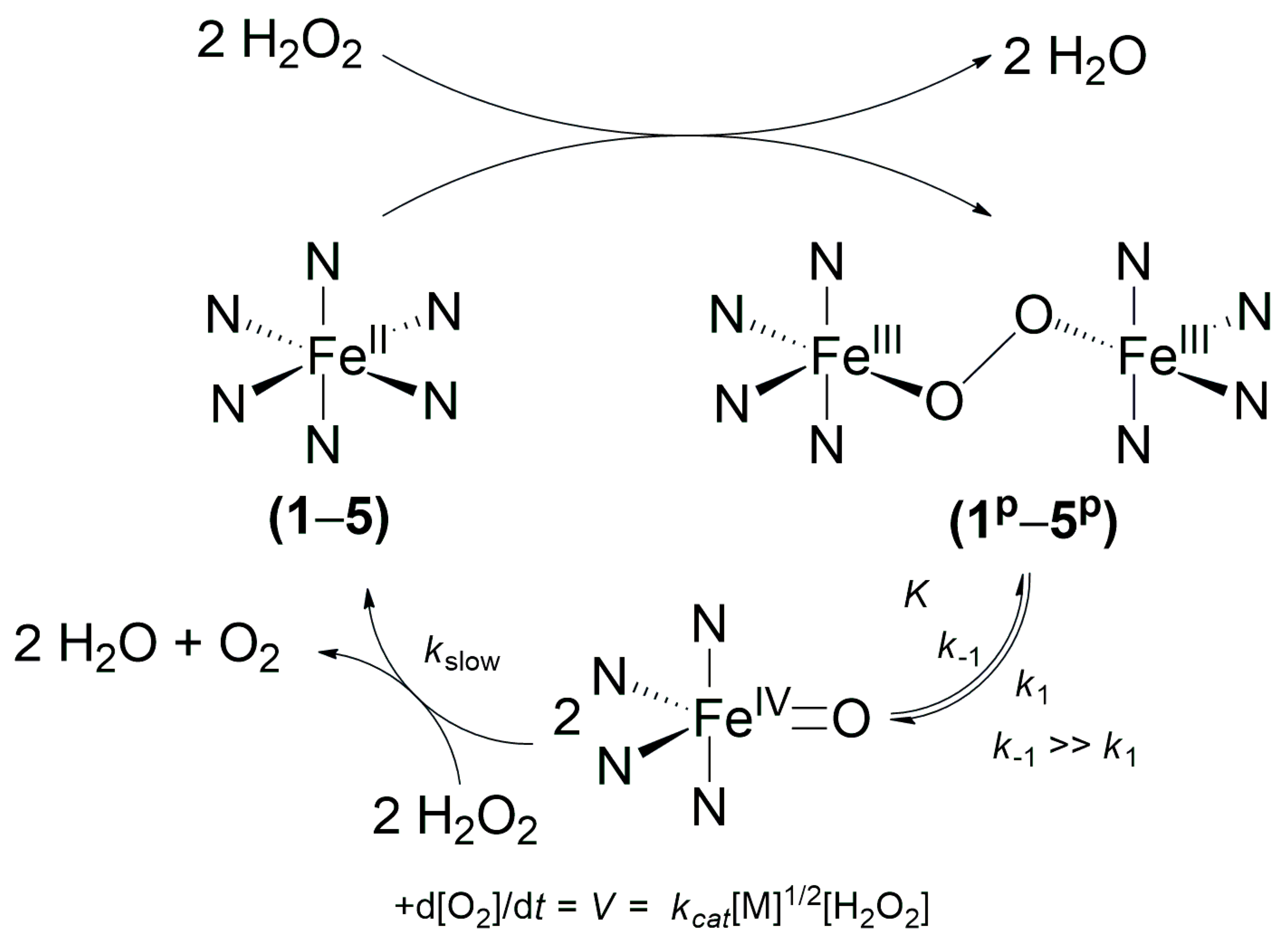
1. Introduction
2. Results and Discussions
3. Experimental
3.1. Materials and Methods
3.2. Synthesis of [FeII(L3)3](CF3SO3)2 (5)
3.3. Gas Volumetric Measurements
3.4. UV–Vis Spectroscopic Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Beyer, W.F.; Fridovich, I. Catalases-with and without heme. Basic Life Sci. 1988, 49, 651–661. [Google Scholar] [PubMed]
- Zamocky, M.; Furtmuller, P.G.; Obinger, C. Evolution of catalases from bacteria to humans. Antioxid. Redox Signal. 2008, 10, 1527–1548. [Google Scholar] [CrossRef] [PubMed]
- Zamocky, M.; Gasselhuber, B.; Furtmüller, P.G.; Obinger, C. Molecular evolution of hydrogen peroxide degrading enzymes. Arch. Biochem. Biophys. 2012, 525, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.P.; Day, J.; Malkin, A.J.; McPherson, A. Structure of orthorhombic crystals of beef liver catalase. Acta Crystallogr. 1999, D55, 1383–1394. [Google Scholar] [CrossRef]
- Ivancich, A.; Jouve, H.M.; Sartor, B.; Gaillard, J. EPR investigation of compound I in Proteus mirabilis and bovin liver catalases: Formation of porphyrin and tyrosyl radical intermediates. Biochemistry 1997, 36, 9356–9364. [Google Scholar] [CrossRef] [PubMed]
- Góth, L. Two cases of acatalasemia in Hungary. Clin. Chim. Acta 1992, 207, 155–158. [Google Scholar] [CrossRef]
- Al-Abrash, A.S.; Al-Quobaili, F.A.; Al-Akhras, G.N. Catalase evaluation in different human diseases associated with oxidative stress. Saudi Med. J. 2000, 21, 826–830. [Google Scholar]
- Habib, L.K.; Lee, M.T.C.; Yang, J. Inhibitors of catalaseamyloid interactions protect cells from β-amyloid induced oxidative stress and toxicity. J. Biol. Chem. 2010, 285, 38933–38943. [Google Scholar] [CrossRef]
- Ogata, M. Acatalasemia. Hum. Gen. 1991, 86, 331–340. [Google Scholar] [CrossRef]
- Tiedge, M.; Lortz, S.; Drinkgern, J.; Lenzen, S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997, 46, 1733–1742. [Google Scholar] [CrossRef]
- Rolo, A.P.; Pameira, C.M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Tox. Appl. Pharm. 2006, 212, 167–178. [Google Scholar] [CrossRef]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S.; Ischiropoulos, H. Reactive oxygen and nitrogen species: Weapons of neuronal destruction in models of Parkinson’s disease. Antioxid. Redox Signal. 2005, 7, 685–693. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen free radicals and iron in relation to biology and medicine: Some problems and concepts. Arch. Biochem. Biophys. 1986, 246, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef]
- Farr, S.B.; Kogoma, T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 1991, 55, 561–585. [Google Scholar] [CrossRef]
- Zuber, P. Management of oxidative stress in Bacillus. Annu. Rev. Microbiol. 2009, 63, 575–597. [Google Scholar] [CrossRef]
- Nicholls, P.; Fita, I.; Loewen, P.C. Enzymology and structure of catalases. Adv. Inorg. Chem. 2001, 51, 51–106. [Google Scholar]
- Kono, Y.; Fridovich, I. Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J. Biol. Chem. 1983, 258, 6015–6019. [Google Scholar] [CrossRef]
- Barynin, V.V.; Whittaker, M.M.; Antonyuk, S.V.; Lamzin, V.S.; Harrison, P.M.; Artymiuk, P.J.; Whittaker, J.W. Crystal Structure of Manganese Catalase from Lactobacillus plantarum. Structure 2001, 9, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Antonyuk, S.V.; Melik-Adman, V.R.; Popov, A.N.; Lamzin, V.S.; Hempstead, P.D.; Harrison, P.M.; Artymyuk, P.J.; Barynin, V.V. Three-dimensional structure of the enzyme dimanganese catalase from Thermus thermophilus at 1 Å resolution. Crystallogr. Rep. 2000, 45, 105–113. [Google Scholar] [CrossRef]
- Barynin, V.V.; Grebenko, A.I. T-catalase is nonheme catalase of the extremely thermophilic bacterium Thermus thermophilus HB8. Dokl. Akad. Nauk SSSR 1986, 286, 461–464. [Google Scholar]
- Allgood, G.S.; Perry, J.J. Characterization of a manganese-containing catalase from the obligate thermophile Thermoleophilum album. J. Bacteriol. 1986, 168, 563–567. [Google Scholar] [CrossRef]
- Amo, T.; Atomi, H.; Imanaka, T. Unique Presence of a Manganese Catalase in a Hyperthermophilic Archaeon, Pyrobaculum calidifontis VA1. J. Bacteriol. 2002, 184, 3305–3312. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.W. Non-heme manganese catalase-the “other” catalase, Arch. Biochem. Biophys. 2012, 525, 111–120. [Google Scholar] [CrossRef]
- Bihani, S.C.; Chakravarty, D.; Ballal, A. KatB, a cyanobacterial Mn-catalase with unique active site configuration: Implications for enzyme function. Free Rad. Biol. Med. 2016, 93, 118–129. [Google Scholar] [CrossRef]
- Cardenas, J.P.; Quatrini, R.; Holmes, D.S. Aerobic Lineage of the Oxidative Stress Response Protein Rubrerythrin Emerged in an Ancient Microaerobic, (Hyper)Thermophilic Environment. Front. Microbiol. 2016, 7, 1822–1831. [Google Scholar] [CrossRef]
- Signorella, S.; Palopoli, C.; Ledesma, G. Rationally designed mimics of antioxidant manganoenzymes: Role of structural features in the quest for catalysts with catalase and superoxide dismutase activity. Coord. Chem. Rev. 2018, 365, 75–102. [Google Scholar] [CrossRef]
- Wu, A.J.; Penner-Hahn, J.E.; Pecoraro, V.L. Structural, Spectroscopic, and Reactivity Models for the Manganese Catalases. Chem. Rev. 2004, 104, 903–938. [Google Scholar] [CrossRef] [PubMed]
- Tovmasyan, A.; Maia, C.G.C.; Weitner, T.; Carballal, S.; Sampaio, R.S.; Lieb, D.; Ghazaryan, R.; Ivanovic-Burmazovic, I.; Radi, R.; Reboucas, J.S.; et al. A comprehensive evaluation of catalase-like activity of different classes of redox-active therapeutics. Free Rad. Biol. Med. 2015, 86, 308–321. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins—From superoxide dismutation to HO-driven pathways. Redox Biol. 2015, 5, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Kaizer, J.; Baráth, G.; Speier, G.; Réglier, M.; Giorgi, M. Synthesis, structure and catalase mimics of novel homoleptic manganese(II) complexes of 1,3-bis(2′-pyridylimino)isoindoline, Mn(4R-ind)2 (R = H, Me). Inorg. Chem. Commun. 2007, 10, 292–294. [Google Scholar] [CrossRef]
- Kaizer, J.; Kripli, B.; Speier, G.; Párkányi, L. Synthesis, structure, and catalase-like activity of a novel manganese(II) complex: Dichloro [1,3-bis(2′-benzimidazolylimino)isoindoline]manganese(II). Polyhedron 2009, 28, 933–936. [Google Scholar] [CrossRef]
- Kaizer, J.; Csay, T.; Kővári, P.; Speier, G.; Párkányi, L. Catalase mimics of a manganese(II) complex: The effect of axial ligands and pH. J. Mol. Catal. A Chem. 2008, 280, 203–209. [Google Scholar] [CrossRef]
- Kripli, B.; Garda, Z.; Sólyom, B.; Tircsó, G.; Kaizer, J. Formation, stability and catalase-like activity of mononuclear manganese(II) and oxomanganese(IV) complexes in protic and aprotic solvents. New J. Chem. 2020, 44, 5545–5555. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Berlett, B.S.; Chock, P.B. Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc. Natl. Acad. Sci. USA 1990, 87, 384–388. [Google Scholar] [CrossRef]
- Kripli, B.; Sólyom, B.; Speier, G.; Kaizer, J. Stability and Catalase-Like Activity of a Mononuclear Non-Heme Oxoiron(IV) Complex in Aqueous Solution. Molecules 2019, 24, 3236. [Google Scholar] [CrossRef] [PubMed]
- Meena, B.I.; Kaizer, J. Design and Fine-Tuning Redox Potentials of Manganese(II) Complexes with Ioindoline-Based Ligands: H2O2 Oxidation and Oxidative Bleaching Performance in Aqueous Solution. Catalysts 2020, 10, 404. [Google Scholar] [CrossRef]
- Pap, J.S.; Draksharapu, A.; Giorgi, M.; Browne, W.R.; Kaizer, J.; Speier, G. Stabilisation of mu-peroxido-bridged Fe(III) intermediates with non-symmetric bidentate N-donor ligands. Chem. Commun. 2014, 50, 1326–1329. [Google Scholar] [CrossRef]
- Szávuly, M.I.; Surducan, M.; Nagy, E.; Surányi, M.; Speier, G.; Silaghi-Dumitrescu, R.; Kaizer, J. Functional models of nonheme enzymes: Kinetic and computational evidence for the formation of oxoiron(IV) species from peroxo-diiron(III) complexes, and their reactivity towards phenols and H2O2. Dalton Trans. 2016, 45, 14709–14718. [Google Scholar] [CrossRef]
- Kripli, B.; Csendes, F.V.; Török, P.; Speier, G.; Kaizer, J. Stoichiometric Aldehyde Deformylation Mediated by Nucleophilic Peroxo-diiron(III) Complex as a Functional Model of Aldehyde Deformylating Oxygenase. Chem. Eur. J. 2019, 25, 14290–14294. [Google Scholar] [CrossRef]
- Pap, J.S.; Cranswick, M.A.; Balogh-Hergovich, É.; Baráth, G.; Giorgi, M.; Rohde, G.T.; Kaizer, J.; Speier, G.; Que, L., Jr. An Iron(II)[1,3-bis(2′-pyridylimino)isoindoline] Complex as a Catalyst for Substrate Oxidation with H2O2—Evidence for a Transient Peroxidodiiron(III) Species. Eur. J. Inorg. Chem. 2013, 2013, 3858–3866. [Google Scholar] [CrossRef]
- Kripli, B.; Szávuly, M.; Csendes, F.V.; Kaizer, J. Functional models of nonheme diiron enzymes: Reactivity of the mu-oxo-mu-1,2-peroxo-diiron(III) intermediate in electrophilic and nucleophilic reactions. Dalton Trans. 2020, 49, 1742–1746. [Google Scholar] [CrossRef] [PubMed]
- Oloo, W.N.; Fielding, A.J.; Que, L., Jr. Rate determining water assisted O-O bond cleavage of a FeIII-OOH intermediate in a bioinspired nonheme iron-catalyzed oxidation. J. Am. Chem. Soc. 2013, 135, 6438–6441. [Google Scholar] [CrossRef] [PubMed]
- Lakk-Bogáth, D.; Török, P.; Csendes, F.V.; Keszei, S.; Gantner, B.; Kaizer, J. Disproportionation of H2O2 Mediated by Diiron-Peroxo Complexes as Catalase Mimics. Molecules 2021, 26, 4501. [Google Scholar] [CrossRef]
- Török, P.; Unjaroen, D.; Csendes, F.V.; Giorgi, M.; Browne, W.R.; Kaizer, J. A nonheme peroxo-diiron(III) complex exhibiting both nucleophilic and electrophilic oxidation of organic substrates. Dalton Trans. 2021, 50, 7181–7185. [Google Scholar] [CrossRef] [PubMed]
- Török, P.; Lakk-Bogáth, D.; Kaizer, J. Stoichiometric Alkane and Aldehyde Hydroxylation Reactions Mediated by In Situ Generated Iron(III)-Iodosylbenzene Adduct. Molecules 2023, 28, 1855. [Google Scholar] [CrossRef]
- Reeder, K.A.; Dose, E.V.; Wilson, L.J. Solution-state spin-equilibrium properties of the tris[2-(2-pyridyl)imidazole]iron(II) and tris[2-(2-pyridyl)benzimidazole]iron(II) cations. Inorg. Chem. 1978, 17, 1071–1075. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; England, J.; White, A.J.P. Non-heme Iron(II) Complexes Containing Tripodal Tetradentate Nitrogen Ligands and Their Application in Alkane Oxidation Catalysis. Inorg. Chem. 2005, 44, 8125–8134. [Google Scholar] [CrossRef]
- Prat, I.; Company, A.; Corona, T.; Parella, T.; Ribas, X.; Costas, M. Assessing the Impact of Electronic and Steric Tuning of the Ligand in the Spin State and Catalytic Oxidation Ability of the FeII(Pytacn) Family of Complexes. Inorg. Chem. 2013, 52, 9229–9244. [Google Scholar] [CrossRef]
- Mialane, P.; Nivorojkine, A.; Pratviel, G.; Azéma, L.; Slany, M.; Godde, F.; Simaan, A.; Banse, F.; Kargar-Grisel, T.; Bouchoux, G.; et al. Structures of Fe(II) complexes with N,N,N′-tris(2-pyridylmethyl)ethane-1,2-diamine type ligands. Bleomycin-like DNA cleavage and enhancement by an alkylammonium substituent on the N′ atom of the ligand. Inorg. Chem. 1999, 38, 1085–1092. [Google Scholar] [CrossRef] [PubMed]

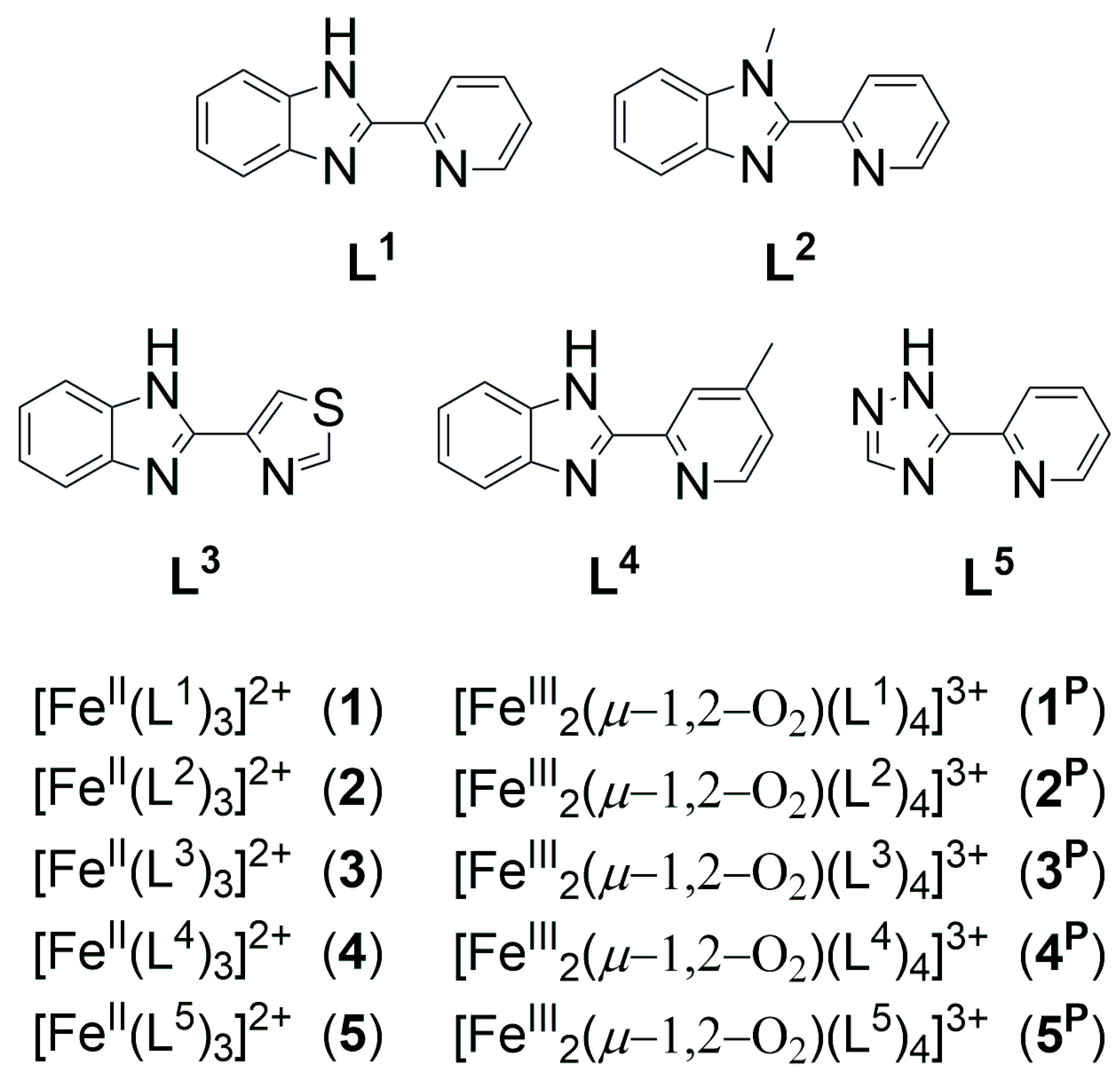
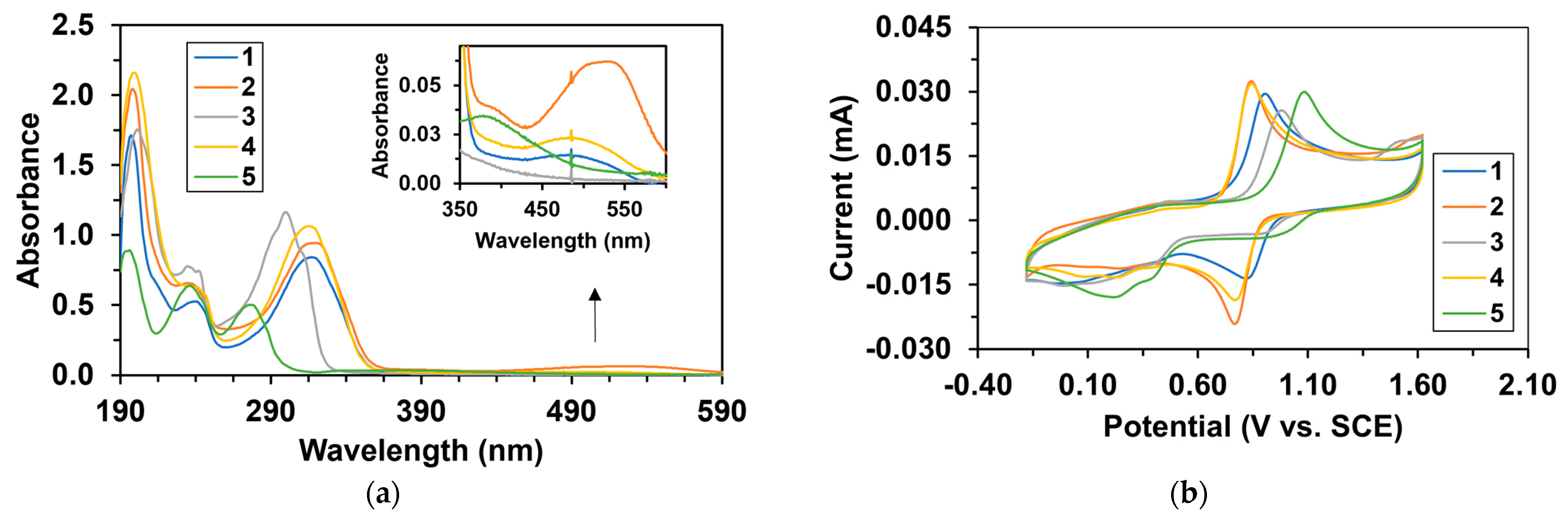

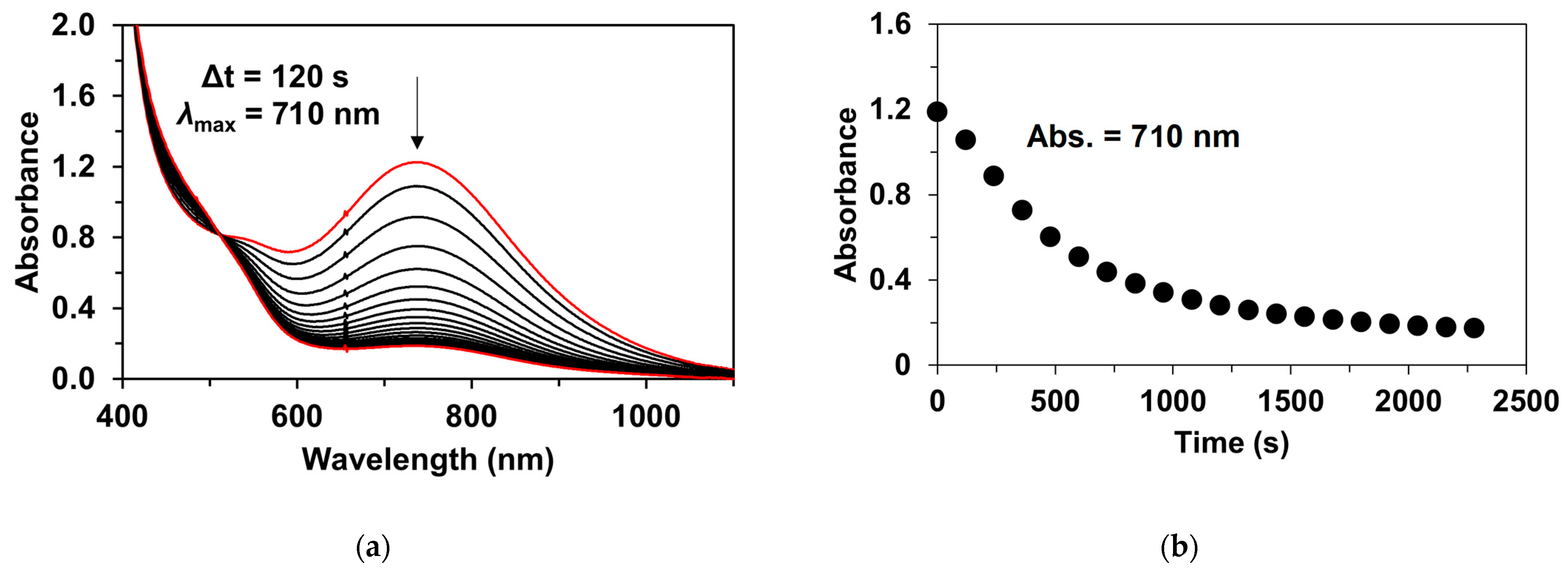
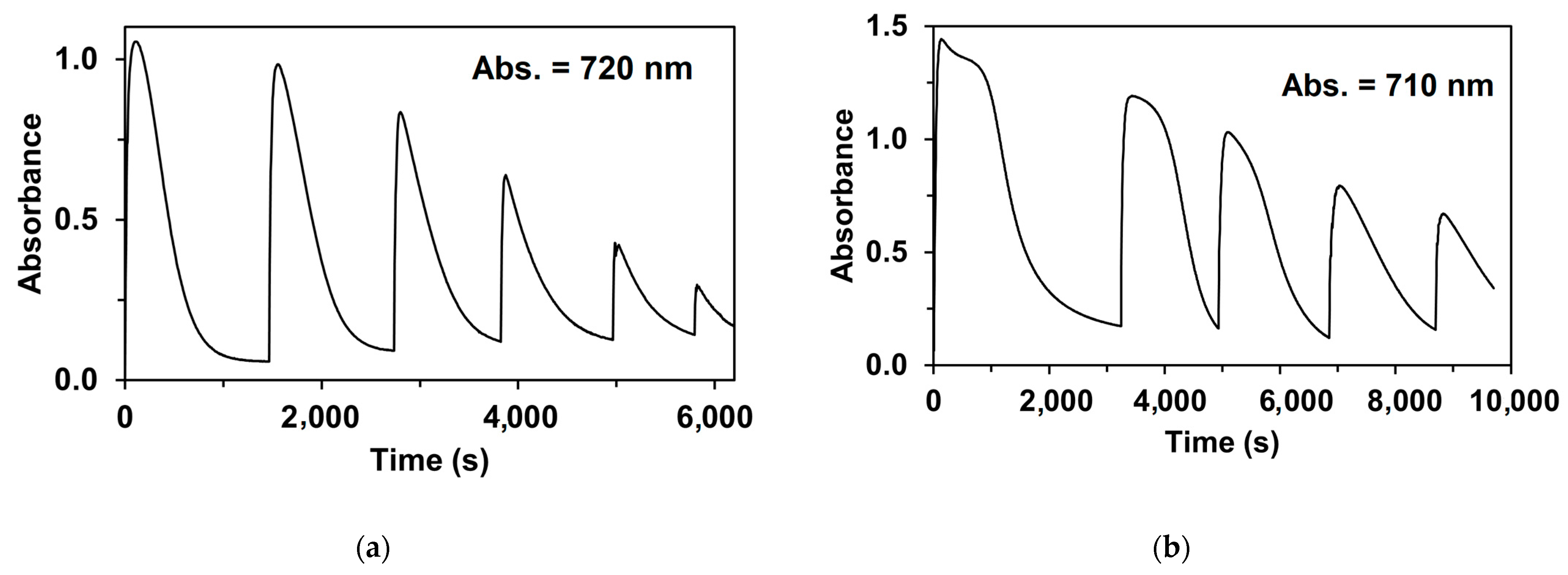

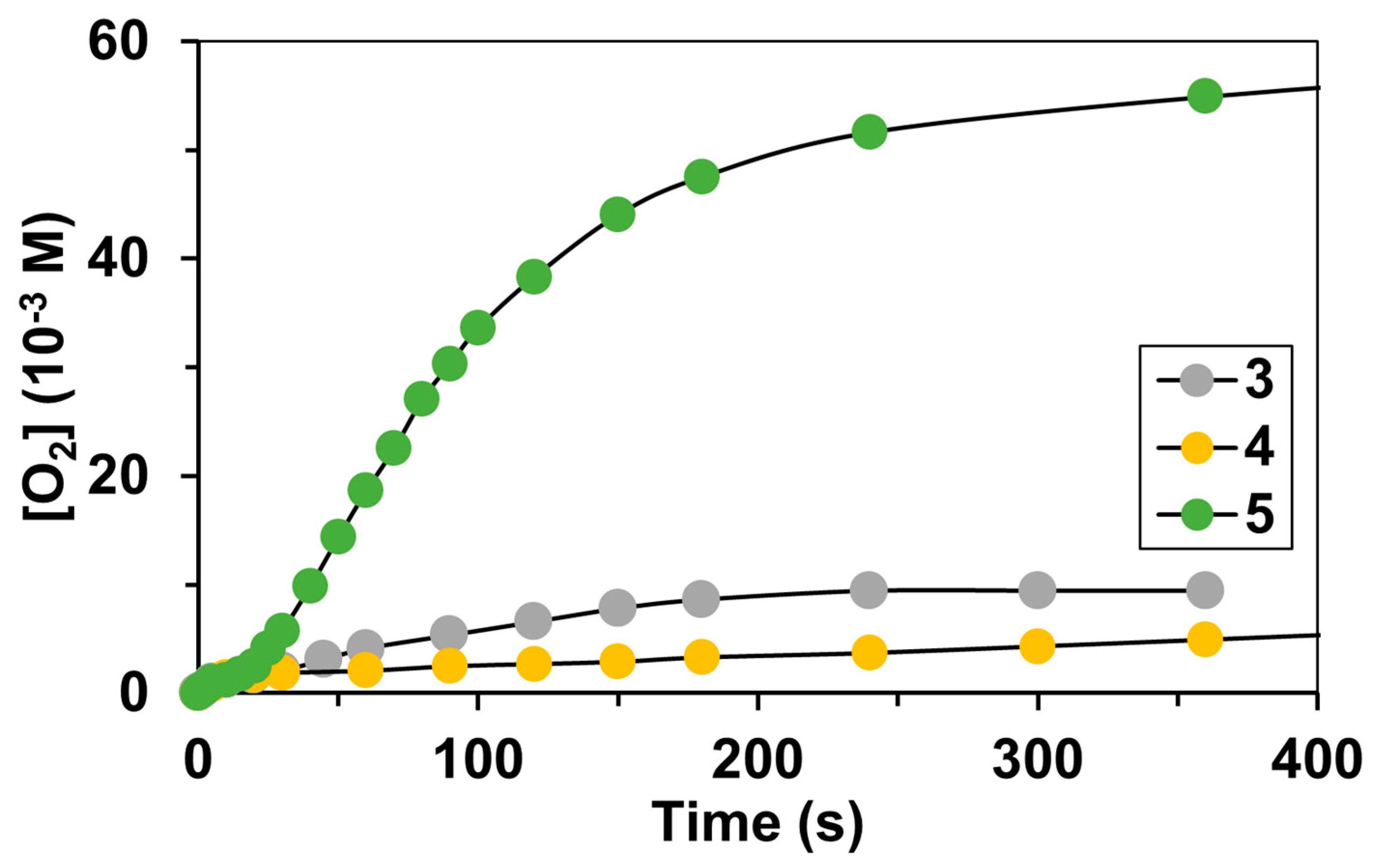


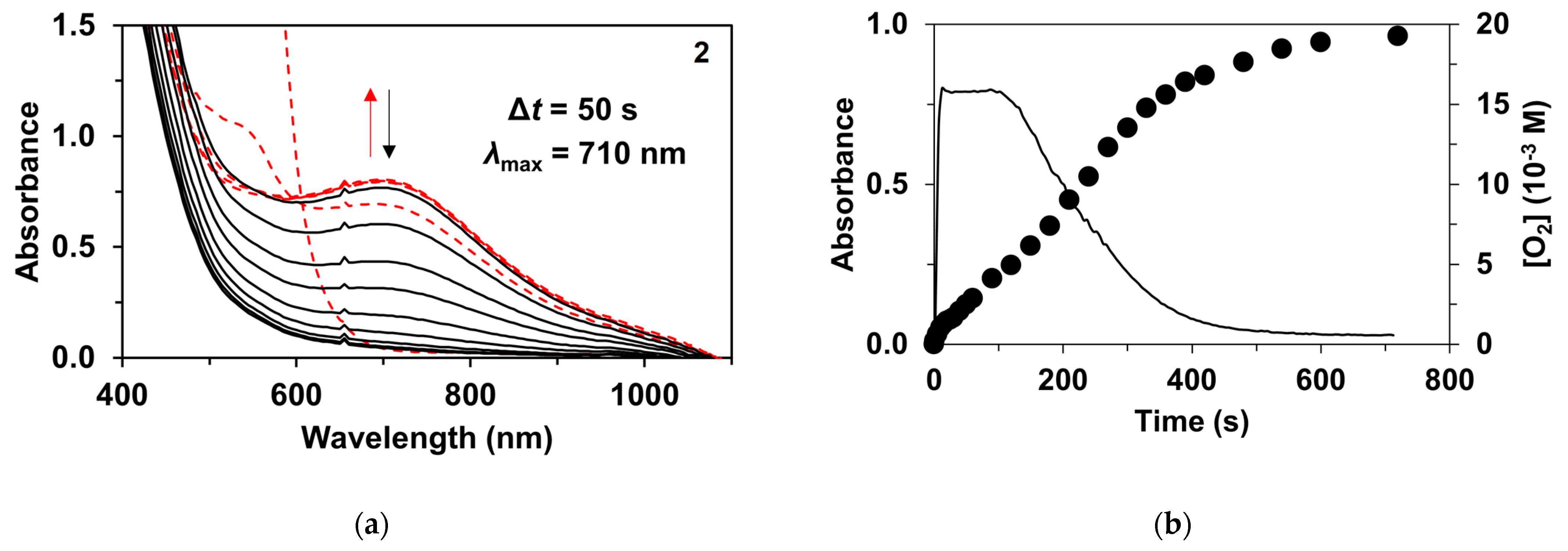



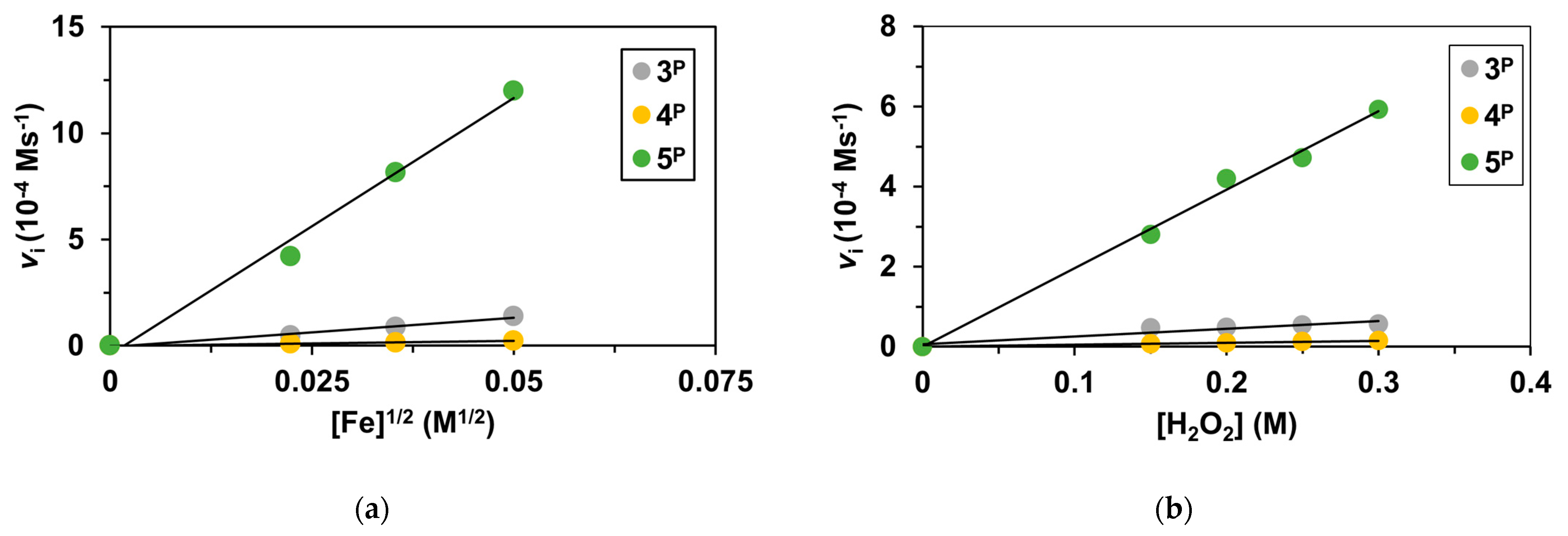
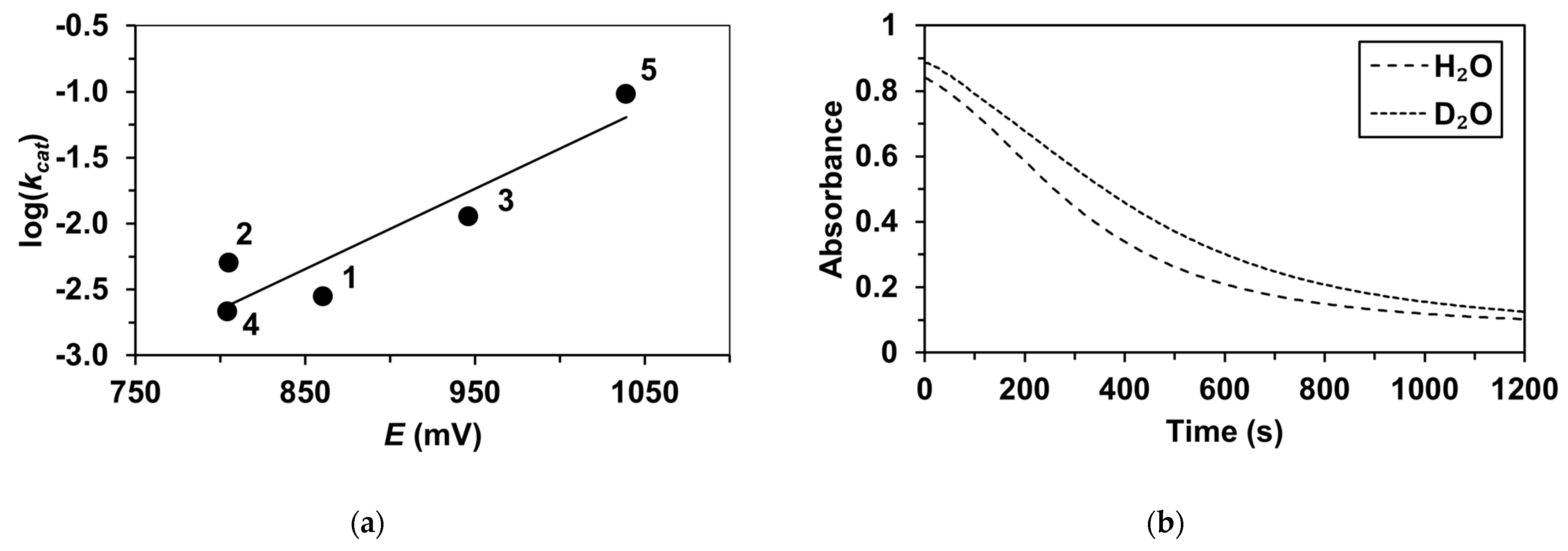
| Catalyst | λ1Fe(II) (nm) | ν1Fe(II) (cm−1) | Epa (mV) | Epc (mV) | E1/2 (mV) | kcat1 (10−3M−1/2s−1) |
|---|---|---|---|---|---|---|
| 1 | 317 | 31,546 | 898 | 823 | 860.5 | 2.81 2 |
| 2 | 319 | 31,348 | 840 | 771 | 805.5 | 5.06 2 |
| 3 | 301 3 | 33,223 | 970 | 922 | 946 | 11.3 |
| 4 | 316 | 31,646 | 840 | 768 | 804 | 2.16 |
| 5 | 277 | 36,101 | 1083 | 996 | 1039 | 96.6 |
| Catalyst | Yield (%) | TON/Fe [H2O2]t/[Catalyst]0 | TOF * [H2O2]t/[Catalyst]0/h |
|---|---|---|---|
| 1 | 9.5 | 28.5 | 53 |
| 2 | 16.3 | 48.9 | 61 |
| 3 | 6.7 | 20.0 | 102 |
| 4 | 6.8 | 20.4 | 27 |
| 5 | 48.4 | 145 | 1065 |
| Catalyst | [Catalyst]0 (10−3M) | [H2O2]0 (M) | Vi1 (10−5Ms−1) | kcat2 (10−3M−1/2s−1) |
|---|---|---|---|---|
| 3P | 0.50 | 0.20 | 4.78 | 10.7 |
| 1.25 | 0.20 | 8.63 | 12.2 | |
| 2.50 | 0.20 | 13.8 | 13.8 | |
| 0.50 | 0.15 | 4.64 | 13.8 | |
| 0.50 | 0.20 | 4.78 | 10.7 | |
| 0.50 | 0.25 | 5.35 | 9.57 | |
| 0.50 | 0.30 | 5.65 | 8.42 | |
| 11.3 | ||||
| 4P | 0.50 | 0.20 | 0.95 | 2.14 |
| 1.25 | 0.20 | 1.50 | 2.12 | |
| 2.50 | 0.20 | 2.39 | 2.39 | |
| 0.50 | 0.15 | 0.65 | 1.94 | |
| 0.50 | 0.20 | 0.95 | 2.14 | |
| 0.50 | 0.25 | 1.21 | 2.16 | |
| 0.50 | 0.30 | 1.51 | 2.25 | |
| 2.16 | ||||
| 5P | 0.50 | 0.20 | 40.4 | 90.3 |
| 1.25 | 0.20 | 81.5 | 115 | |
| 2.50 | 0.20 | 120 | 120 | |
| 0.50 | 0.15 | 28.1 | 83.8 | |
| 0.50 | 0.20 | 40.4 | 90.3 | |
| 0.50 | 0.25 | 47.2 | 84.4 | |
| 0.50 | 0.30 | 59.2 | 88.3 | |
| 96.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Török, P.; Lakk-Bogáth, D.; Kaizer, J. Effect of Redox Potential on Diiron-Mediated Disproportionation of Hydrogen Peroxide. Molecules 2023, 28, 2905. https://doi.org/10.3390/molecules28072905
Török P, Lakk-Bogáth D, Kaizer J. Effect of Redox Potential on Diiron-Mediated Disproportionation of Hydrogen Peroxide. Molecules. 2023; 28(7):2905. https://doi.org/10.3390/molecules28072905
Chicago/Turabian StyleTörök, Patrik, Dóra Lakk-Bogáth, and József Kaizer. 2023. "Effect of Redox Potential on Diiron-Mediated Disproportionation of Hydrogen Peroxide" Molecules 28, no. 7: 2905. https://doi.org/10.3390/molecules28072905
APA StyleTörök, P., Lakk-Bogáth, D., & Kaizer, J. (2023). Effect of Redox Potential on Diiron-Mediated Disproportionation of Hydrogen Peroxide. Molecules, 28(7), 2905. https://doi.org/10.3390/molecules28072905







