Research Progress on Neuroprotective Effects of Isoquinoline Alkaloids
Abstract
1. Introduction
2. Isoquinoline Alkaloids with Neuroprotective Effects
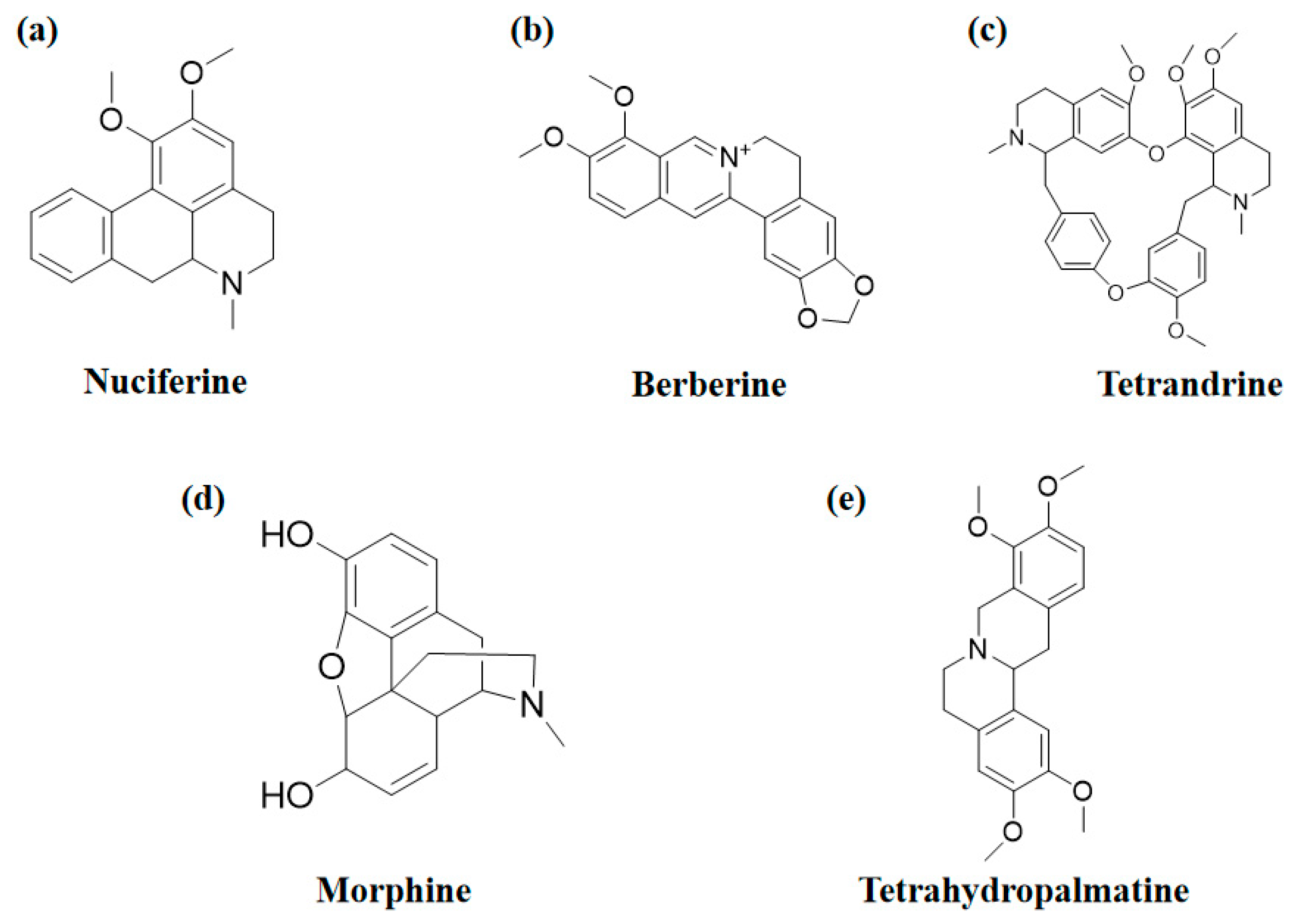
2.1. Nuciferine
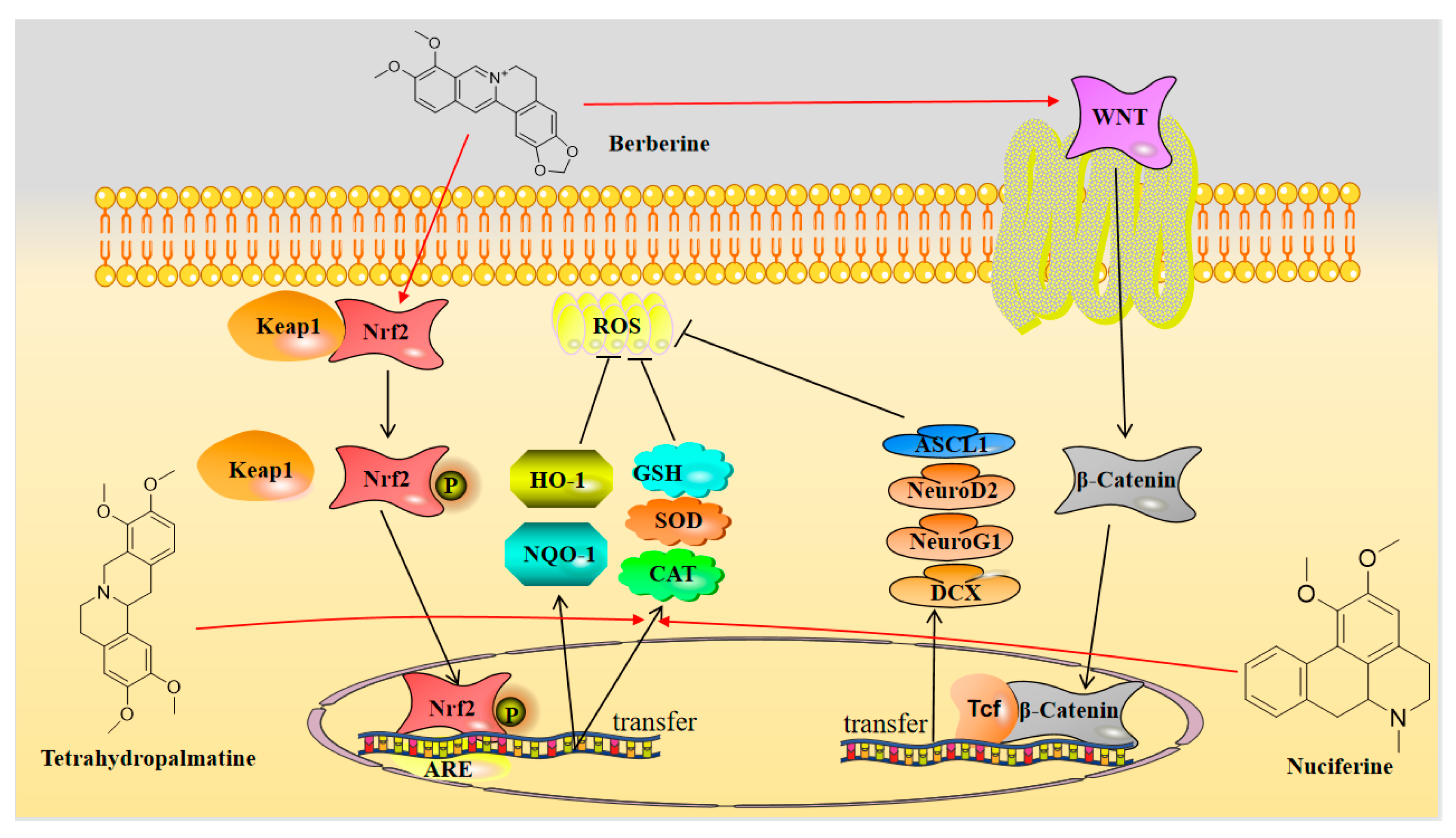
2.2. Berberine
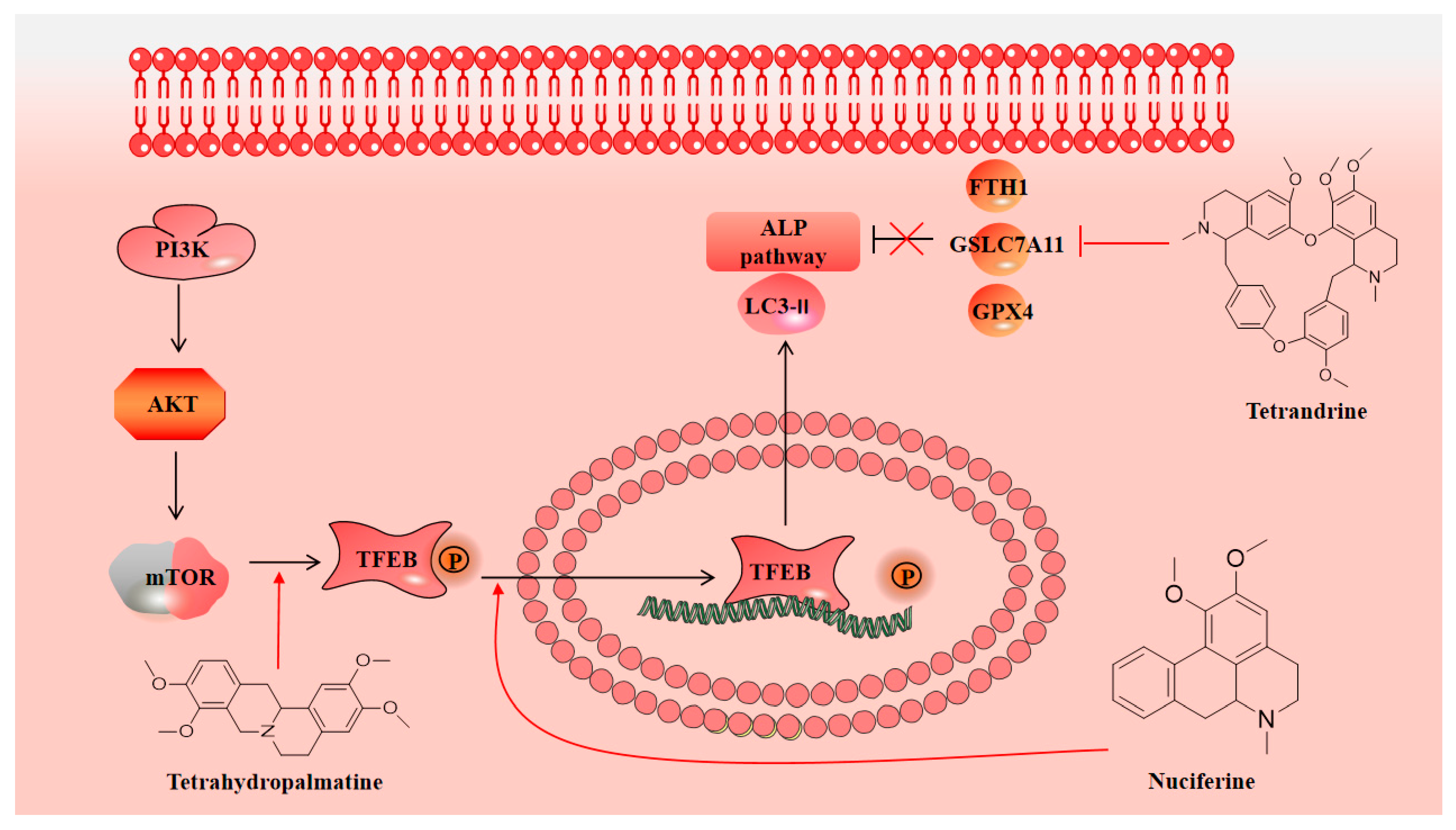
2.3. Tetrandrine
2.4. Morphine
2.5. Tetrahydropalmatine
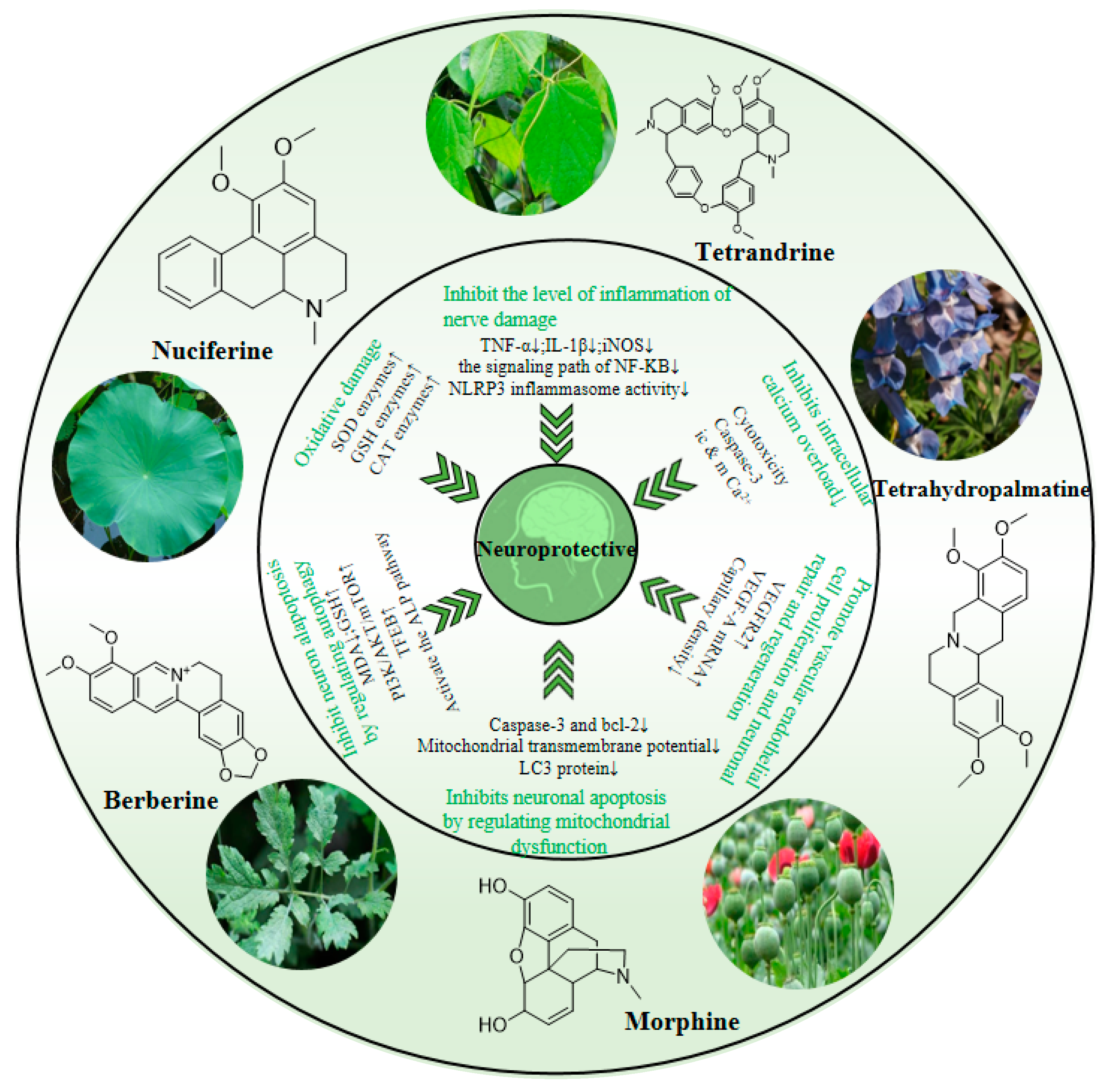
3. Neuroprotective Effect and Mechanism of Isoquinoline Alkaloids
3.1. Neuroprotection towards Inflammatory Injury
3.2. Neuroprotection towards Oxidative Stress
3.3. Neuroprotection towards Regulating Autophagy
3.4. Neuroprotection towards Calcium Overload
3.5. Neuroprotection towards Mitochondrial Dysfunction
3.6. Neuroprotective Effects of Promoting Vascular Endothelial Proliferation and Neuronal Regeneration
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bondy, S.C. Anthropogenic pollutants may increase the incidence of neurodegenerative disease in an aging population. Toxicology 2016, 341–343, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, M.; Hattori, N. Neurodegenerative diseases. Nihon. Rinsho. 2012, 70, 94–98. [Google Scholar] [PubMed]
- Jurcau, A.; Simion, A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int. J. Mol. Sci. 2021, 23, 14. [Google Scholar] [CrossRef]
- Boese, A.C.; Hamblin, M.H.; Lee, J.P. Neural stem cell therapy for neurovascular injury in Alzheimer’s disease. Exp. Neurol. 2020, 324, 113112. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Jahangeer, M.; Maknoon Razia, D.; Ashiq, M.; Ghaffar, A.; Akram, M.; El Allam, A.; Bouyahya, A.; Garipova, L.; Shariati, M.A.; et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta. 2021, 522, 114–126. [Google Scholar] [CrossRef]
- Kins, S.; Schäfer, K.H.; Endres, K. Drug development for neurodegenerative diseases. Biol. Chem. 2021, 403, 1. [Google Scholar] [CrossRef]
- Wang, Q.; Su, C.P.; Zhang, H.M.; Ren, Y.L.; Wang, W.; Guo, S.Z. Anti-inflammatory mechanism of heat-clearing and detoxifying Chinese herbs. Zhongguo Zhong Yao Za Zhi 2018, 43, 3787–3794. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Lou, G.; Zeng, H.-R.; Hu, J.; Huang, Q.; Peng, W.; Yang, X.-B. Coptidis Rhizoma: A comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm. Biol. 2019, 57, 193–225. [Google Scholar] [CrossRef]
- Qi, Y.; Ni, S.; Heng, X.; Qu, S.; Ge, P.; Zhao, X.; Yao, Z.; Guo, R.; Yang, N.; Zhang, Q.; et al. Uncovering the Potential Mechanisms of Coptis chinensis Franch. for Serious Mental Illness by Network Pharmacology and Pharmacology-Based Analysis. Drug Des. Dev. Ther. 2022, 16, 325–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, D.; Gao, Y.; Liang, C.; Zhang, Y.; Ma, Z.; Liu, Y.; Peng, H.; Zhang, Y.; Qin, H.; et al. History of uses, phytochemistry, pharmacological activities, quality control and toxicity of the root of Stephania tetrandra S. Moore: A review. J. Ethnopharmacol. 2020, 260, 112995. [Google Scholar] [CrossRef]
- Wąsik, A.; Antkiewicz-Michaluk, L. The mechanism of neuroprotective action of natural compounds. Pharmacol. Rep. 2017, 69, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Han, L.; Shi, W.; Fang, X.; Hong, Y.; Cao, Y. Research Advances in Lotus Leaf as Chinese Dietary Herbal Medicine. Am. J. Chin. Med. 2022, 50, 1423–1445. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, J.; Sun, L.; Lyu, X.; Chang, X.Y.; Mi, X.; Hu, M.G.; Wu, C.; Chen, X. Akkermansia muciniphila: A potential novel mechanism of nuciferine to improve hyperlipidemia. Biomed. Pharmacother. 2021, 133, 111014. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X.; Mi, Y.; Zhang, B.; Gu, S.; Liu, G.; Li, X. PLGA nanoparticles for the oral delivery of nuciferine: Preparation, physicochemical characterization and in vitro/in vivo studies. Drug Deliv. 2017, 24, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Duan, F.; Xie, Y.; Wan, Q.; Liu, H.; Gong, J.; Huang, L.; Song, Z. Nuciferine attenuates acute ischemic stroke in a rat model: A metabolomic approach for the mechanistic study. Mol. Omics. 2022, 18, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Kong, B.; Yang, N.; Cao, B.; Feng, D.; Yu, X.; Ge, C.; Feng, S.; Fei, F.; Huang, J.; et al. The Hypoglycemic Effect of Berberine and Berberrubine Involves Modulation of Intestinal Farnesoid X Receptor Signaling Pathway and Inhibition of Hepatic Gluconeogenesis. Drug Metab. Dispos. 2021, 49, 276–286. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine and barberry (Berberis vulgaris): A clinical review. Phytother. Res. 2019, 33, 504–523. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Z.; Cai, B.; Chen, Q. Berberine as a Potential Multi-Target Agent for Metabolic Diseases: A Review of Investigations for Berberine. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 971–979. [Google Scholar]
- Baska, A.; Leis, K.; Gałązka, P. Berberine in the Treatment of Diabetes Mellitus: A Review. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 1379–1386. [Google Scholar] [CrossRef]
- Finkbeiner, S. The Autophagy Lysosomal Pathway and Neurodegeneration. Cold Spring Harb. Perspect. Biol. 2020, 12, a033993. [Google Scholar] [CrossRef]
- Habtemariam, S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol. Res. 2020, 155, 104722. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Zhang, J.; Song, H.; Zhu, S.; Zhang, A.; Hua, Y.; Han, S.; Fu, Y. Mechanism of Tetrandrine Against Endometrial Cancer Based on Network Pharmacology. Drug Des. Dev. Ther. 2021, 15, 2907–2919. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.Y.; Zhang, J.; Chen, S.; Chen, Y.; Zhang, Y.; Ma, Z.Y.; Zhang, F.; Xie, W.M.; Fan, Y.F.; Duan, J.S.; et al. Endothelium-derived hydrogen sulfide acts as a hyperpolarizing factor and exerts neuroprotective effects via activation of large-conductance Ca2+-activated K+ channels. Br. J. Pharmacol. 2021, 178, 4155–4175. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, L.; Wang, S.; Tian, X.; Zhang, L.; Li, H.; Li, C.; Xue, Y.; Wang, Q.; Fang, L.; et al. Tetrandrine promotes angiogenesis via transcriptional regulation of VEGF-A. Vascul. Pharmacol. 2021, 141, 106920. [Google Scholar] [CrossRef]
- Li, J.; Shi, M.; Liu, L.; Wang, J.; Zhu, M.; Chen, H. Tetrandrine Inhibits Skeletal Muscle Differentiation by Blocking Autophagic Flux. Int. J. Mol. Sci. 2022, 23, 8148. [Google Scholar] [CrossRef]
- Liu, J.; Yu, P.; Dai, F.; Jiang, H.; Ma, Z. Tetrandrine reduces oxidative stress, apoptosis, and extracellular matrix degradation and improves intervertebral disc degeneration by inducing autophagy. Bioengineered 2022, 13, 3944–3957. [Google Scholar] [CrossRef]
- Zhaleh, H.; Azadbakht, M.; Bidmeshki Pour, A. Low concentrations of morphine enhanced the neuroglia-like differentiation. Bratisl. Lek. Listy 2020, 121, 271–277. [Google Scholar] [CrossRef]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of Plant Derived Alkaloids and Their Mechanism in Neurodegenerative Disorders. Int. J. Biol. Sci. 2018, 14, 341–357. [Google Scholar] [CrossRef]
- Magdy, S.; Gamal, M.; Samir, N.F.; Rashed, L. IκB kinase inhibition remodeled connexins, pannexin-1, and excitatory amino-acid transporters expressions to promote neuroprotection of galantamine and morphine. J. Cell Physiol. 2021, 236, 7516–7532. [Google Scholar] [CrossRef]
- Amini, K.; Zhaleh, H.; Tahvilian, R.; Farnia, V. Low concentration of morphine protects against cell death, oxidative stress and calcium accumulation by nicotine in PC12 cells. Bratisl. Lek. Listy 2019, 120, 256–262. [Google Scholar] [CrossRef]
- Wang, B.; Su, C.J.; Liu, T.T.; Zhou, Y.; Feng, Y.; Huang, Y.; Liu, X.; Wang, Z.H.; Chen, L.H.; Luo, W.F.; et al. The Neuroprotection of Low-Dose Morphine in Cellular and Animal Models of Parkinson’s Disease Through Ameliorating Endoplasmic Reticulum (ER) Stress and Activating Autophagy. Front. Mol. Neurosci. 2018, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Li, J.F.; Li, T.Z.; Pan, C.X.; Xue, F.S.; Wang, G.Y. Morphine pretreatment protects against cerebral ischemic injury via a cPKCγ-mediated anti-apoptosis pathway. Exp. Ther. Med. 2021, 22, 1016. [Google Scholar] [CrossRef] [PubMed]
- Luzzati, M.; Coviello, C.; De Veye, H.S.; Dudink, J.; Lammertink, F.; Dani, C.; Koopmans, C.; Benders, M.; Tataranno, M.L. Morphine exposure and neurodevelopmental outcome in infants born extremely preterm. Dev. Med. Child Neurol. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Xie, W.; Kang, Z.; Jiang, C.; Liu, N. Hydromorphone protects CA1 neurons by activating mTOR pathway. Neurosci. Lett. 2018, 687, 49–54. [Google Scholar] [CrossRef]
- Gupta, S.; Iudicello, J.E.; Shi, C.; Letendre, S.; Knight, A.; Li, J.; Riggs, P.K.; Franklin DR, J.r.; Duarte, N.; Jin, H.; et al. Absence of neurocognitive impairment in a large Chinese sample of HCV-infected injection drug users receiving methadone treatment. Drug Alcohol Depend. 2014, 137, 29–35. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Zhao, W.R.; Shi, W.T.; Xiao, Y.; Ma, Z.L.; Xue, J.G.; Zhang, L.Q.; Ye, Q.; Chen, X.L.; Tang, J.Y. Endothelial-Dependent and Independent Vascular Relaxation Effect of Tetrahydropalmatine on Rat Aorta. Front. Pharmacol. 2019, 10, 336. [Google Scholar] [CrossRef]
- Du, Q.; Meng, X.; Wang, S. A Comprehensive Review on the Chemical Properties, Plant Sources, Pharmacological Activities, Pharmacokinetic and Toxicological Characteristics of Tetrahydropalmatine. Front. Pharmacol. 2022, 13, 890078. [Google Scholar] [CrossRef]
- Zhang, C.L.; Huang, Q.L.; Zhu, Q.; He, J.; Chen, J.; Zhang, F.; Cao, Z.Y. Alkaloids from Corydalis decumbens modulate neuronal excitability. Bioorg. Chem. 2020, 99, 103795. [Google Scholar] [CrossRef]
- Liu, L.; Liu, M.; Zhao, W.; Zhao, Y.L.; Wang, Y. Levo-tetrahydropalmatine: A new potential medication for methamphetamine addiction and neurotoxicity. Exp. Neurol. 2021, 344, 113809. [Google Scholar] [CrossRef]
- Cahlíková, L.; Vrabec, R.; Pidaný, F.; Peřinová, R.; Maafi, N.; Mamun, A.A.; Ritomská, A.; Wijaya, V.; Blunden, G. Recent Progress on Biological Activity of Amaryllidaceae and Further Isoquinoline Alkaloids in Connection with Alzheimer’s Disease. Molecules 2021, 26, 5240. [Google Scholar] [CrossRef]
- Saglam, E.; Zırh, S.; Aktas, C.C.; Muftuoglu, S.F.; Bilginer, B. Papaverine provides neuroprotection by suppressing neuroinflammation and apoptosis in the traumatic brain injury via RAGE- NF-B pathway. J. Neuroimmunol. 2021, 352, 577476. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Park, J.S.; Leem, Y.H.; Park, J.E.; Kim, D.Y.; Choi, Y.H.; Park, E.M.; Kang, J.L.; Kim, H.S. The phosphodiesterase 10 inhibitor papaverine exerts anti-inflammatory and neuroprotective effects via the PKA signaling pathway in neuroinflammation and Parkinson’s disease mouse models. J. Neuroinflammation 2019, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- Leem, Y.H.; Park, J.S.; Park, J.E.; Kim, D.Y.; Kim, H.S. Papaverine Exerts Neuroprotective Effect by Inhibiting NLRP3 Inflammasome Activation in an MPTP-Induced Microglial Priming Mouse Model Challenged with LPS. Biomol. Ther. 2021, 29, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Luhach, K.; Kulkarni, G.T.; Singh, V.P.; Sharma, B. Attenuation of neurobehavioural abnormalities by papaverine in prenatal valproic acid rat model of ASD. Eur. J. Pharmacol. 2021, 890, 173663. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Liu, Q.; Gu, H.; Zhang, Y.Y.; Wei, P.L.; Qi, Y.F.; Liu, J.; Wang, Z. Pluripotent anti-inflammatory immunomodulatory effects of papaverine against cerebral ischemic-reperfusion injury. J. Pharmacol. Sci. 2020, 144, 69–75. [Google Scholar] [CrossRef]
- Leem, Y.H.; Park, J.S.; Park, J.E.; Kim, D.Y.; Kang, J.L.; Kim, H.S. Papaverine inhibits α-synuclein aggregation by modulating neuroinflammation and matrix metalloproteinase-3 expression in the subacute MPTP/P mouse model of Parkinson’s disease. Biomed. Pharmacother. 2020, 130, 110576. [Google Scholar] [CrossRef]
- Yang, X.; Du, W.; Zhang, Y.; Wang, H.; He, M. Neuroprotective Effects of Higenamine Against the Alzheimer’s Disease Via Amelioration of Cognitive Impairment, Aβ Burden, Apoptosis and Regulation of Akt/GSK3β Signaling Pathway. Dose Response 2020, 18, 1559325820972205. [Google Scholar] [CrossRef]
- Wu, M.P.; Zhang, Y.S.; Zhou, Q.M.; Xiong, J.; Dong, Y.R.; Yan, C. Higenamine protects ischemia/reperfusion induced cardiac injury and myocyte apoptosis through activation of β2-AR/PI3K/AKT signaling pathway. Pharmacol. Res. 2016, 104, 115–123. [Google Scholar] [CrossRef]
- Yang, S.; Chu, S.; Ai, Q.; Zhang, Z.; Gao, Y.; Lin, M.; Liu, Y.; Hu, Y.; Li, X.; Peng, Y.; et al. Anti-inflammatory effects of higenamine (Hig) on LPS-activated mouse microglia (BV2) through NF-κB and Nrf2/HO-1 signaling pathways. Int. Immunopharmacol. 2020, 85, 106629. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Li, L.; Li, X.; Wang, Q.; Ding, H.; Wang, X.; Ye, Z.; Wu, L.; Zhang, X.; et al. Sinomenine Provides Neuroprotection in Model of Traumatic Brain Injury via the Nrf2-ARE Pathway. Front. Neurosci. 2016, 10, 580. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, W.; Wang, Y.; Kou, F.; Lyu, C.; Wei, H. Metabolic mechanism and anti-inflammation effects of sinomenine and its major metabolites N-demethylsinomenine and sinomenine-N-oxide. Life Sci. 2020, 261, 118433. [Google Scholar] [CrossRef]
- Bao, X.; He, Y.; Huang, L.; Li, H.; Li, Q.; Huang, Y. Sinomenine exerts a neuroprotective effect on PD mouse model through inhibiting PI3K/AKT/mTOR pathway to enhance autophagy. Int. J. Neurosci. 2022, 1–9. [Google Scholar] [CrossRef]
- Gao, B.; Wu, Y.; Yang, Y.J.; Li, W.Z.; Dong, K.; Zhou, J.; Yin, Y.Y.; Huang, D.K.; Wu, W.N. Sinomenine exerts anticonvulsant profile and neuroprotective activity in pentylenetetrazole kindled rats: Involvement of inhibition of NLRP1 inflammasome. J. Neuroinflammation 2018, 15, 152. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, N.; Ou, Y.; Wei, Q.; Tang, W.; Zheng, M.; Xing, Y.; Li, J.J.; Ling, Y.; Li, J.; et al. N-Demethylsinomenine, an active metabolite of sinomenine, attenuates chronic neuropathic and inflammatory pain in mice. Sci. Rep. 2021, 11, 9300. [Google Scholar] [CrossRef]
- Ramazi, S.; Fahanik-Babaei, J.; Mohamadi-Zarch, S.M.; Tashakori-Miyanroudi, M.; Nourabadi, D.; Nazari-Serenjeh, M.; Roghani, M. Neuroprotective and anticonvulsant effects of sinomenine in kainate rat model of temporal lobe epilepsy: Involvement of oxidative stress, inflammation and pyroptosis. J. Chem. Neuroanat. 2020, 108, 101800. [Google Scholar] [CrossRef]
- Singh, D.; Agrawal, A.; Singal, C.M.S.; Pandey, H.S.; Seth, P.; Sharma, S.K. Sinomenine inhibits amyloid beta-induced astrocyte activation and protects neurons against indirect toxicity. Mol. Brain. 2020, 13, 30. [Google Scholar] [CrossRef]
- Lai, W.D.; Wang, S.; You, W.T.; Chen, S.J.; Wen, J.J.; Yuan, C.R.; Zheng, M.J.; Jin, Y.; Yu, J.; Wen, C.P. Sinomenine regulates immune cell subsets: Potential neuro-immune intervene for precise treatment of chronic pain. Front. Cell Dev. Biol. 2022, 10, 1041006. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.N.; Wu, P.F.; Chen, X.L.; Zhang, Z.; Gu, J.; Yang, Y.J.; Xiong, Q.J.; Ni, L.; Wang, F.; Chen, J.G. Sinomenine protects against ischaemic brain injury: Involvement of co-inhibition of acid-sensing ion channel 1a and L-type calcium channels. Br. J. Pharmacol. 2011, 164, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dai, P.; Bao, H.; Liang, P.; Wang, W.; Xing, A.; Sun, J. Anti-inflammatory and neuroprotective effects of sanguinarine following cerebral ischemia in rats. Exp. Ther. Med. 2017, 13, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, C.M.; Lee, S.J.; Park, G. Involvement of heme oxygenase-1 in neuroprotection by sanguinarine against glutamate-triggered apoptosis in HT22 neuronal cells. Environ. Toxicol. Pharmacol. 2014, 38, 701–710. [Google Scholar] [CrossRef]
- Yu, C.; Li, P.; Wang, Y.X.; Zhang, K.G.; Zheng, Z.C.; Liang, L.S. Sanguinarine Attenuates Neuropathic Pain by Inhibiting P38 MAPK Activated Neuroinflammation in Rat Model. Drug Des. Devel. Ther. 2020, 14, 4725–4733. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, Y.X.; Yang, G.; Zheng, Z.C.; Yu, C. Sanguinarine Attenuates Neuropathic Pain in a Rat Model of Chronic Constriction Injury. Biomed. Res. Int. 2021, 2021, 3689829. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Hung, C.Y.; Chiu, K.M.; Lee, M.Y.; Lu, C.W.; Wang, S.J. Neferine, an Alkaloid from Lotus Seed Embryos, Exerts Antiseizure and Neuroprotective Effects in a Kainic Acid-Induced Seizure Model in Rats. Int. J. Mol. Sci. 2022, 23, 4130. [Google Scholar] [CrossRef]
- Sengking, J.; Oka, C.; Yawoot, N.; Tocharus, J.; Chaichompoo, W.; Suksamrarn, A.; Tocharus, C. Protective Effect of Neferine in Permanent Cerebral Ischemic Rats via Anti-Oxidative and Anti-Apoptotic Mechanisms. Neurotox. Res. 2022, 40, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Poornima, P.; Huang, C.Y.; Padma, V.V. Neferine prevents NF-κB translocation and protects muscle cells from oxidative stress and apoptosis induced by hypoxia. Biofactors 2016, 42, 407–417. [Google Scholar] [CrossRef]
- Chen, J.; Tang, M.; Liu, M.; Jiang, Y.; Liu, B.; Liu, S. Neferine and lianzixin extracts have protective effects on undifferentiated caffeine-damaged PC12 cells. BMC Complement. Med. Ther. 2020, 20, 76. [Google Scholar] [CrossRef]
- Sengking, J.; Oka, C.; Wicha, P.; Yawoot, N.; Tocharus, J.; Chaichompoo, W.; Suksamrarn, A.; Tocharus, C. Neferine Protects Against Brain Damage in Permanent Cerebral Ischemic Rat Associated with Autophagy Suppression and AMPK/mTOR Regulation. Mol. Neurobiol. 2021, 58, 6304–6315. [Google Scholar] [CrossRef]
- Wong, V.K.; Wu, A.G.; Wang, J.R.; Liu, L.; Law, B.Y. Neferine attenuates the protein level and toxicity of mutant huntingtin in PC-12 cells via induction of autophagy. Molecules 2015, 20, 3496–3514. [Google Scholar] [CrossRef]
- Zhong, Y.; He, S.; Huang, K.; Liang, M. Neferine suppresses vascular endothelial inflammation by inhibiting the NF-κB signaling pathway. Arch. Biochem. Biophys. 2020, 696, 108595. [Google Scholar] [CrossRef]
- Wu, C.; Chen, J.; Yang, R.; Duan, F.; Li, S.; Chen, X. Mitochondrial protective effect of neferine through the modulation of nuclear factor erythroid 2-related factor 2 signalling in ischaemic stroke. Br. J. Pharmacol. 2019, 176, 400–415. [Google Scholar] [CrossRef]
- Hao, T.; Yang, Y.; Li, N.; Mi, Y.; Zhang, G.; Song, J.; Liang, Y.; Xiao, J.; Zhou, D.; He, D. Inflammatory mechanism of cerebral ischemia-reperfusion injury with treatment of stepharine in rats. Phytomedicine 2020, 79, 153353. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, Y.; Yang, Y.; Liu, J.; Chen, G.; Lin, B.; Hou, Y.; Li, N. Natural potential neuroinflammatory inhibitors from Stephania epigaea H.S. Lo. Bioorganic Chem. 2020, 107, 104597. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.Y.; Lahiry, A.; Ferdous, R.; Hasan, M.K.; Kader, M.A.; Alam, A.K.; Saud, Z.A.; Sadik, M.G. Stephania japonica Ameliorates Scopolamine-Induced Memory Impairment in Mice through Inhibition of Acetylcholinesterase and Oxidative Stress. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 8305271. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pu, Z.; Li, M.; Wang, K.; Deng, L.; Chen, W. Antioxidative and antiapoptosis: Neuroprotective effects of dauricine in Alzheimer’s disease models. Life Sci. 2020, 243, 117237. [Google Scholar] [CrossRef]
- Pu, Z.; Ma, S.; Wang, L.; Shang, L.; Luo, Y.; Chen, W. Amyloid-beta Degradation and Neuroprotection of Dauricine Mediated by Unfolded Protein Response in a Caenorhabditis elegans Model of Alzheimer’s disease. Neuroscience 2018, 392, 25–37. [Google Scholar] [CrossRef]
- Wang, K.; Wang, L.; Chen, L.; Peng, C.; Luo, B.; Mo, J.; Chen, W. Intranasal administration of dauricine loaded on graphene oxide: Multi-target therapy for Alzheimer’s disease. Drug Deliv. 2021, 28, 580–593. [Google Scholar] [CrossRef]
- Li, M.; Liu, G.; Wang, K.; Wang, L.; Fu, X.; Lim, L.Y.; Chen, W.; Mo, J. Metal ion-responsive nanocarrier derived from phosphonated calix [4]arenes for delivering dauricine specifically to sites of brain injury in a mouse model of intracerebral hemorrhage. J. Nanobiotechnol. 2020, 18, 61. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Wang, J.; Yu, H.; Zhang, Z.; Liu, J.; Chen, X.; Zhu, F.; Yang, X. Dauricine Attenuates Spatial Memory Impairment and Alzheimer-Like Pathologies by Enhancing Mitochondrial Function in a Mouse Model of Alzheimer’s Disease. Front. Cell Dev. Biol. 2021, 8, 624339. [Google Scholar] [CrossRef]
- Omoruyi, S.I.; Ibrakaw, A.S.; Ekpo, O.E.; Boatwright, J.S.; Cupido, C.N.; Hussein, A.A. Neuroprotective Activities of Crossyne flava Bulbs and Amaryllidaceae Alkaloids: Implications for Parkinson’s Disease. Molecules 2021, 26, 3990. [Google Scholar] [CrossRef]
- Cortes, N.; Castañeda, C.; Osorio, E.H.; Cardona-Gomez, G.P.; Osorio, E. Amaryllidaceae alkaloids as agents with protective effects against oxidative neural cell injury. Life Sci. 2018, 203, 54–65. [Google Scholar] [CrossRef]
- Hua, S.; Liu, J.; Zhang, Y.; Li, J.; Zhang, X.; Dong, L.; Zhao, Y.; Fu, X. Piperine as a neuroprotective functional component in rats with cerebral ischemic injury. Food Sci. Nutr. 2019, 7, 3443–3451. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Oboh, G.; Okeke, B.M. Comparative effects of berberine and piperine on the neuroprotective potential of neostigmine. J. Complement. Integr. Med. 2021, 18, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, S.; Jamwal, S. Neuroprotective potential of quercetin in combination with piperine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. Neural Regen. Res. 2017, 12, 1137–1144. [Google Scholar] [CrossRef]
- Kaushik, P.; Ali, M.; Salman, M.; Tabassum, H.; Parvez, S. Harnessing the mitochondrial integrity for neuroprotection: Therapeutic role of piperine against experimental ischemic stroke. Neurochem. Int. 2021, 149, 105138. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.Y.; Chang, Y.; Wang, S.J. Piperine Provides Neuroprotection against Kainic Acid-Induced Neurotoxicity via Maintaining NGF Signalling Pathway. Molecules 2022, 27, 2638. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, P. Neuroprotective potential of curcumin in combination with piperine against 6-hydroxy dopamine induced motor deficit and neurochemical alterations in rats. Inflammopharmacology 2017, 25, 69–79. [Google Scholar] [CrossRef]
- Salman, M.; Tabassum, H.; Parvez, S. Piperine mitigates behavioral impairments and provides neuroprotection against 3-nitropropinoic acid-induced Huntington disease-like symptoms. Nutr. Neurosci. 2022, 25, 100–109. [Google Scholar] [CrossRef]
- Duan, W.; Chen, X. Jatrorrhizine can improve nerve cell injury induced by Aβ 25-35, acting through miR-223-3p/HDAC4 axis. Am. J. Transl. Res. 2021, 13, 4644–4655. [Google Scholar]
- Luo, T.; Shen, X.Y.; Li, S.; Ouyang, T.; Mai, Q.A.; Wang, H.Q. The Protective Effect of Jatrorrhizine Against Oxidative Stress in Primary Rat Cortical Neurons. CNS Neurol. Disord. Drug Targets 2017, 16, 617–623. [Google Scholar] [CrossRef]
- Wu, G.; Mu, T.; Zhang, L.; Chen, X. Jatrorrhizine Hydrochloride alleviates tert-butyl hydroperoxide-induced endothelial cell injury through its anti-inflammatory activity and PPAR-γ activation. Cell Mol. Biol. 2020, 66, 125–129. [Google Scholar] [CrossRef]
- Ramírez Hernández, E.; Alanis Olvera, B.; Carmona González, D.; Guerrero Marín, O.; Pantoja Mercado, D.; Valencia Gil, L.; Hernández-Zimbrón, L.F.; Sánchez Salgado, J.L.; Limón, I.D.; Zenteno, E. Neuroinflammation and galectins: A key relationship in neurodegenerative diseases. Glycoconj. J. 2022, 39, 685–699. [Google Scholar] [CrossRef]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Muthuraju, S.; Zakaria, R.; Karuppan, M.K.M.; Al-Rahbi, B. The Role of Neuroinflammation in Cellular Damage in Neurodegenerative Diseases. Biomed. Res. Int. 2020, 2020, 9231452. [Google Scholar] [CrossRef]
- Wang, J.; Guo, M.; Ma, R.; Wu, M.; Zhang, Y. Tetrandrine alleviates cerebral ischemia/reperfusion injury by suppressing NLRP3 inflammasome activation via Sirt-1. PeerJ 2020, 8, e9042. [Google Scholar] [CrossRef]
- Liu, H.; He, S.; Li, C.; Wang, J.; Zou, Q.; Liao, Y.; Chen, R. Tetrandrine alleviates inflammation and neuron apoptosis in experimental traumatic brain injury by regulating the IRE1α/JNK/CHOP signal pathway. Brain Behav. 2022, 12, e2786. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Fu, Y.; Wang, L.; Liu, J.; Zhong, X.; Yuan, J.; Jiang, C.; Wang, H.; Li, Z. Tetrandrine ameliorated Alzheimer’s disease through suppressing microglial inflammatory activation and neurotoxicity in the 5XFAD mouse. Phytomedicine 2021, 90, 153627. [Google Scholar] [CrossRef]
- Rezaee, R.; Monemi, A.; SadeghiBonjar, M.A.; Hashemzaei, M. Berberine Alleviates Paclitaxel-Induced Neuropathy. J. Pharmacopunct. 2019, 22, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.F.; Yang, C.J.; Morris-Natschke, S.L.; Li, J.C.; Yin, X.D.; Liu, Y.Q.; Guo, X.; Peng, J.W.; Goto, M.; Zhang, J.Y. Biologically active isoquinoline alkaloids covering 2014–2018. Med. Res. Rev. 2020, 40, 2212–2289. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.R.; Tay, K.C.; Su, Y.X.; Wong, C.K.; Tan, W.N.; Khaw, K.Y. Potential of Naturally Derived Alkaloids as Multi-Targeted Therapeutic Agents for Neurodegenerative Diseases. Molecules 2021, 26, 728. [Google Scholar] [CrossRef]
- Aski, M.L.; Rezvani, M.E.; Khaksari, M.; Hafizi, Z.; Pirmoradi, Z.; Niknazar, S.; Mehrjerdi, F.Z. Neuroprotective effect of berberine chloride on cognitive impairment and hippocampal damage in experimental model of vascular dementia. Iran. J. Basic Med. Sci. 2018, 21, 53–58. [Google Scholar]
- Song, C.; Cao, J.; Lei, Y.; Chi, H.; Kong, P.; Chen, G.; Yu, T.; Li, J.; Kumar, P.R.; Xia, J. Nuciferine prevents bone loss by disrupting multinucleated osteoclast formation and promoting type H vessel formation. FASEB J. 2020, 34, 4798–4811. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Di Liberto, V.; Mudò, G. Role of Bioactive Molecules on Neuroprotection, Oxidative Stress, and Neuroinflammation Modulation. Int. J. Mol. Sci. 2022, 23, 15925. [Google Scholar] [CrossRef]
- Ibarrola, J.; Lu, Q.; Zennaro, M.C.; Jaffe, I.Z. Mechanism by Which Inflammation and Oxidative Stress Induce Mineralocorticoid Receptor Gene Expression in Aging Vascular Smooth Muscle Cells. Hypertension 2023, 80, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.W.; Cheung, C.K.; Gao, J.; Shi, W.W.; Shaw, P.C. Berberine Protects C17.2 Neural Stem Cells From Oxidative Damage Followed by Inducing Neuronal Differentiation. Front. Cell Neurosci. 2019, 13, 395. [Google Scholar] [CrossRef]
- Sun, K.; Luo, Z.L.; Hu, C.; Gong, T.L.; Tang, G.H.; Wu, S.P. Protective effect and immune mechanism of berberine on cerebral ischemia/reperfusion injury in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2020, 36, 656–661. [Google Scholar] [PubMed]
- Almalki, D.A.; Alghamdi, S.A. Hepatorenal protective effects of some plant extracts on experimental diabetes in male rats. Int. J. Pharmacol. 2019, 15, 238–247. [Google Scholar]
- Khan, S.; Khan, H.U.; Khan, F.A.; Shah, A.; Wadood, A.; Ahmad, S.; Almehmadi, M.; Alsaiari, A.A.; Shah, F.U.; Kamran, N. Anti-Alzheimer and Antioxidant Effects of Nelumbo nucifera L. Alkaloids, Nuciferine and Norcoclaurine in Alloxan-Induced Diabetic Albino Rats. Pharmaceuticals 2022, 15, 1205. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhang, J.; Yang, H.; Huo, L.; Gao, J.; Chen, H.; Gao, W. Protective effect of tetrahydropalmatine against d-galactose induced memory impairment in rat. Physiol. Behav. 2016, 154, 114–125. [Google Scholar] [CrossRef]
- Liu, Y.; Levine, B. Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ. 2015, 22, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int. J. Mol. Sci. 2020, 21, 2576. [Google Scholar] [CrossRef] [PubMed]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; He, S.; Wang, J.; Li, C.; Liao, Y.; Zou, Q.; Chen, R. Tetrandrine Ameliorates Traumatic Brain Injury by Regulating Autophagy to Reduce Ferroptosis. Neurochem. Res. 2022, 47, 1574–1587. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zhang, H.; Wang, W.; Li, Y. Tetrahydropalmatine protects against acute lung injury induced by limb ischemia/reperfusion through restoring PI3K/AKT/mTOR-mediated autophagy in rats. Pulm. Pharmacol. Ther. 2020, 64, 101947. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, X.; Zhang, H.; Che, X.; Luo, J.; Wang, P.; Xu, J.; Xing, Z.; Yuan, L.; Liu, Y.; et al. Neuronal-targeted TFEB rescues dysfunction of the autophagy-lysosomal pathway and alleviates ischemic injury in permanent cerebral ischemia. Autophagy 2019, 15, 493–509. [Google Scholar] [CrossRef]
- Du, X.; Di Malta, C.; Fang, Z.; Shen, T.; Niu, X.; Chen, M.; Jin, B.; Yu, H.; Lei, L.; Gao, W.; et al. Nuciferine protects against high-fat diet-induced hepatic steatosis and insulin resistance via activating TFEB-mediated autophagy-lysosomal pathway. Acta Pharm. Sin B. 2022, 12, 2869–2886. [Google Scholar] [CrossRef]
- Paez, P.M.; Lyons, D.A. Calcium Signaling in the Oligodendrocyte Lineage: Regulators and Consequences. Annu. Rev. Neurosci. 2020, 43, 163–186. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Hernando-Pérez, E.; López-Vázquez, S.; Núñez, J.; Villalobos, C.; Núñez, L. Remodeling of Intracellular Ca2+ Homeostasis in Rat Hippocampal Neurons Aged In Vitro. Int. J. Mol. Sci. 2020, 21, 1549. [Google Scholar] [CrossRef]
- Xu, D.D.; Hoeven, R.; Rong, R.; Cho, W.C. Rhynchophylline Protects Cultured Rat Neurons against Methamphetamine Cytotoxicity. Evid.-Based Complement. Altern. Med. 2012, 2012, 636091. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Mei, X.; Cao, Y.; Guo, Z.; Yuan, Y.; Zhao, Z.; Song, C.; Guo, Y.; Shen, Z. Berberine attenuated pro-inflammatory factors and protect against neuronal damage via triggering oligodendrocyte autophagy in spinal cord injury. Oncotarget 2017, 8, 98312–98321. [Google Scholar] [CrossRef]
- Yu, S.B.; Pekkurnaz, G. Mechanisms Orchestrating Mitochondrial Dynamics for Energy Homeostasis. J. Mol. Biol. 2018, 430, 3922–3941. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Lu, M.H.; Yuan, D.J.; Xu, D.E.; Yao, P.P.; Ji, W.L.; Chen, H.; Liu, W.L.; Yan, C.X.; Xia, Y.Y.; et al. Mitochondrial Dysfunction in Neural Injury. Front. Neurosci. 2019, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, C.; Lu, J.; Huang, P.; Barnstable, C.J.; Zhang, C.; Zhang, S.S. Tetrandrine protects mouse retinal ganglion cells from ischemic injury. Drug Des. Devel. Ther. 2014, 8, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Kasala, S.; Briyal, S.; Prazad, P.; Ranjan, A.K.; Stefanov, G.; Donovan, R.; Gulati, A. Exposure to Morphine and Caffeine Induces Apoptosis and Mitochondrial Dysfunction in a Neonatal Rat Brain. Front. Pediatr. 2020, 8, 593. [Google Scholar] [CrossRef]
- Biczo, G.; Vegh, E.T.; Shalbueva, N.; Mareninova, O.A.; Elperin, J.; Lotshaw, E.; Gretler, S.; Lugea, A.; Malla, S.R.; Dawson, D.; et al. Mitochondrial Dysfunction, Through Impaired Autophagy, Leads to Endoplasmic Reticulum Stress, Deregulated Lipid Metabolism, and Pancreatitis in Animal Models. Gastroenterology 2018, 154, 689–703. [Google Scholar] [CrossRef]
- Wang, L.; Sheng, W.; Tan, Z.; Ren, Q.; Wang, R.; Stoika, R.; Liu, X.; Liu, K.; Shang, X.; Jin, M. Treatment of Parkinson’s disease in Zebrafish model with a berberine derivative capable of crossing blood brain barrier, targeting mitochondria, and convenient for bioimaging experiments. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2021, 249, 109151. [Google Scholar] [CrossRef]
- Wang, T.; Fang, X.; Yin, Z.S. Endothelial progenitor cell-conditioned medium promotes angiogenesis and is neuroprotective after spinal cord injury. Neural Regen. Res. 2018, 13, 887–895. [Google Scholar]
- Cui, H.; Yang, X.; Wang, Z.; Li, G.; Li, L.; Huo, S.; Zhang, B.; He, R.; Chen, K.; Xu, B.; et al. Tetrahydropalmatine triggers angiogenesis via regulation of arginine biosynthesis. Pharmacol. Res. 2021, 163, 105242. [Google Scholar] [CrossRef]
- Hao, H.F.; Liu, L.M.; Pan, C.S.; Wang, C.S.; Gao, Y.S.; Fan, J.Y.; Han, J.Y. Rhynchophylline Ameliorates Endothelial Dysfunction via Src-PI3K/Akt-eNOS Cascade in the Cultured Intrarenal Arteries of Spontaneous Hypertensive Rats. Front. Physiol. 2017, 8, 928. [Google Scholar] [CrossRef] [PubMed]
- Plazas, E.; Avila, M.M.C.; Muñoz, D.R.; Cuca S, L.E. Natural isoquinoline alkaloids: Pharmacological features and multi-target potential for complex diseases. Pharmacol. Res. 2022, 177, 106126. [Google Scholar] [CrossRef] [PubMed]


| Alkaloid | Structure | Neuroprotective Mechanism | Reference |
|---|---|---|---|
| Papaverine | 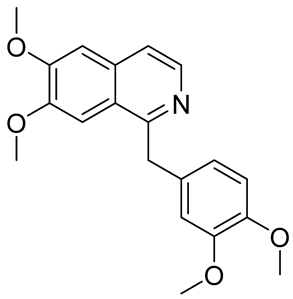 | anti-inflammatory; anti-oxidation; anti-apoptosis; promote neurogenesis; inhibition of α-synuclein aggregation | [41,42,43,44,45,46] |
| Higenamine | 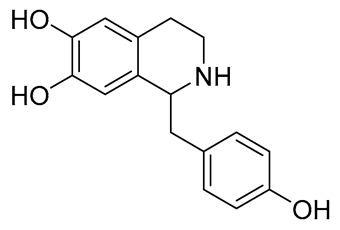 | anti-inflammatory; anti-oxidation; anti-apoptosis; | [47,48,49] |
| Sinomenine | 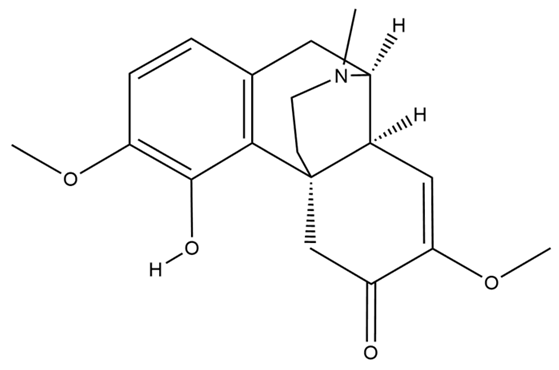 | anti-inflammatory; anti-oxidation; regulating autophagy; anti-pyroptosis; anti-apoptosis; neuroimmune intervention; inhibition of Ca2+ overload | [50,51,52,53,54,55,56,57,58] |
| Sanguinarine |  | anti-inflammatory; anti-apoptosis; mitochondrial protection; inhibition of Ca2+ overload | [59,60,61,62] |
| Neferine |  | anti-inflammatory; anti-oxidation; anti-apoptosis; regulating autophagy; inhibition of Ca2+ overload; mitochondrial protection | [63,64,65,66,67,68,69,70] |
| Stepharine | 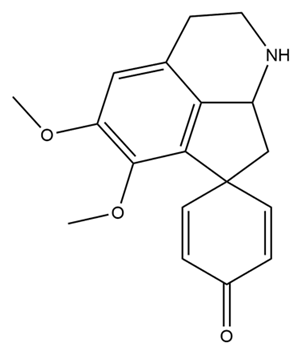 | anti-inflammatory; anti-apoptosis; anti-oxidation | [71,72,73] |
| Dauricine | 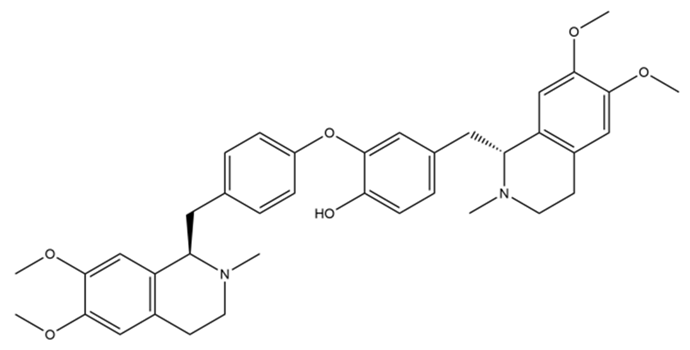 | anti-inflammatory; anti-oxidation; anti-apoptosis; acceleration of Aβ protein degradation; inhibition of ferroptosis; enhance mitochondrial function | [74,75,76,77,78] |
| Lycorine |  | anti-oxidation; anti-apoptosis; | [79,80] |
| Piperine | 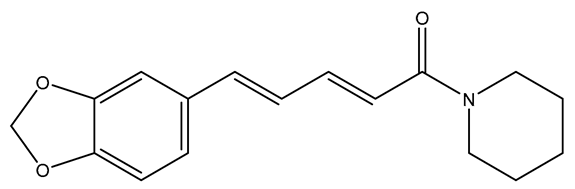 | anti-inflammatory; anti-oxidation; anti-apoptosis; improve mitochondrial dysfunction; reduce the toxicity of excitatory amino acids; up-regulate nerve growth factor | [81,82,83,84,85,86,87] |
| Jatrorrhizine |  | anti-inflammatory; anti-oxidation; anti-apoptosis; improve vascular endothelial dysfunction | [88,89,90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wu, Y.; Dong, S.; Yu, Y.; Wu, Y.; Xiang, B.; Li, Q. Research Progress on Neuroprotective Effects of Isoquinoline Alkaloids. Molecules 2023, 28, 4797. https://doi.org/10.3390/molecules28124797
Li J, Wu Y, Dong S, Yu Y, Wu Y, Xiang B, Li Q. Research Progress on Neuroprotective Effects of Isoquinoline Alkaloids. Molecules. 2023; 28(12):4797. https://doi.org/10.3390/molecules28124797
Chicago/Turabian StyleLi, Jinhua, Yarong Wu, Shuze Dong, Ye Yu, Yuhao Wu, Benhan Xiang, and Qin Li. 2023. "Research Progress on Neuroprotective Effects of Isoquinoline Alkaloids" Molecules 28, no. 12: 4797. https://doi.org/10.3390/molecules28124797
APA StyleLi, J., Wu, Y., Dong, S., Yu, Y., Wu, Y., Xiang, B., & Li, Q. (2023). Research Progress on Neuroprotective Effects of Isoquinoline Alkaloids. Molecules, 28(12), 4797. https://doi.org/10.3390/molecules28124797






