Monodisperse MoS2/Graphite Composite Anode Materials for Advanced Lithium Ion Batteries
Abstract
1. Introduction
2. Results and Discussion
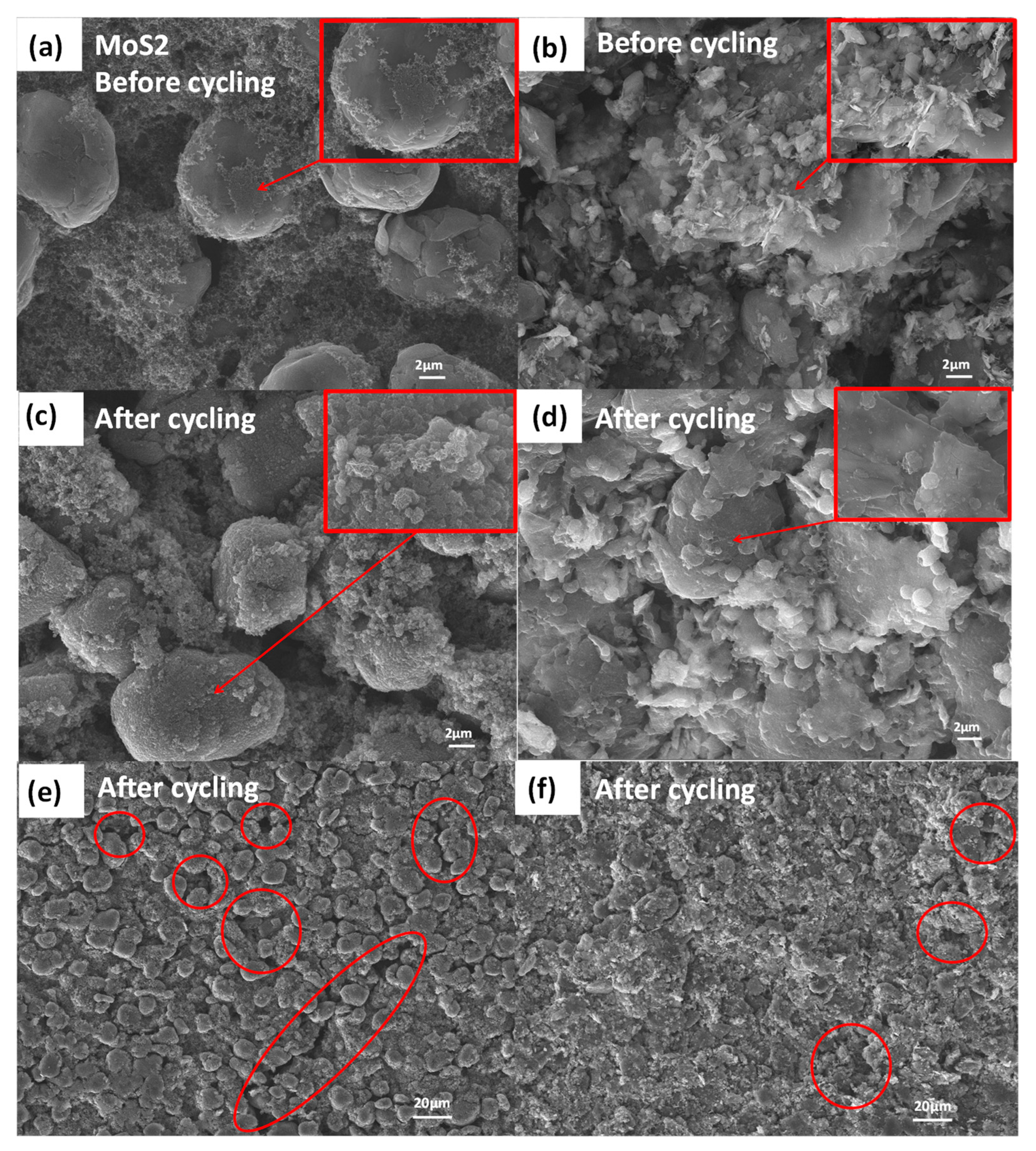
2.1. Structure and Morphology of MoS2/Graphite Composites with Different Ratios
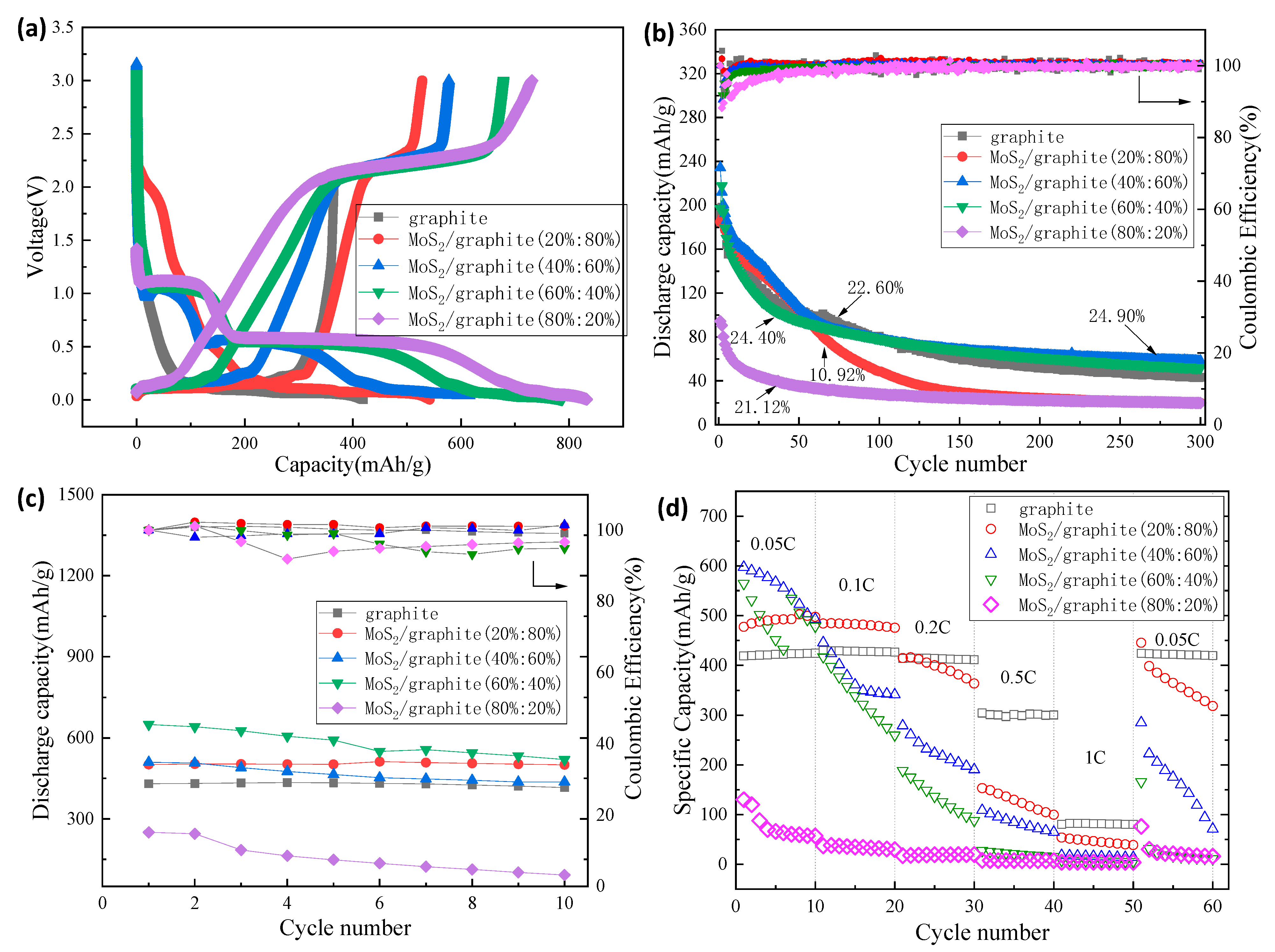
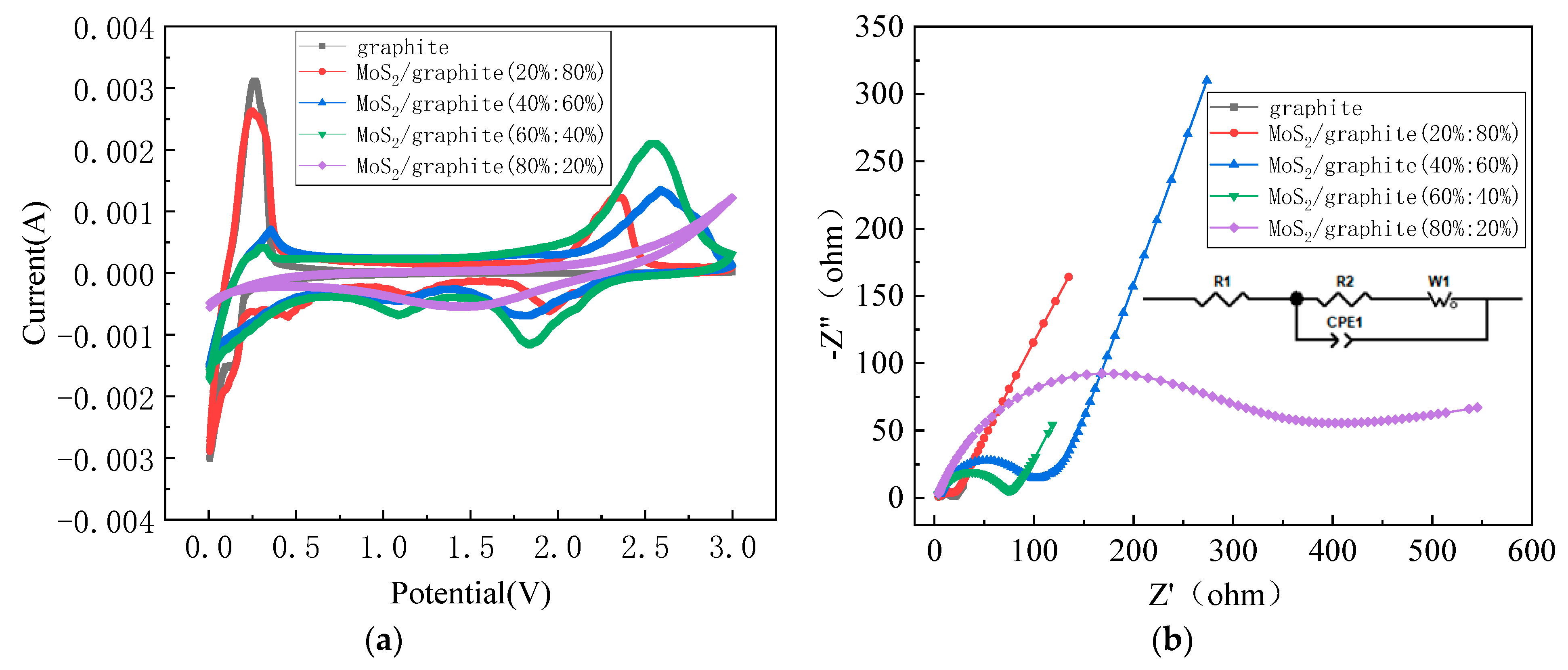
2.2. Electrochemical Properties of MoS2/Graphite Composites with Different Proportions
3. Experiment
3.1. Preparation of Anode Materials
3.2. Preparation of Button Cell
3.3. Material Structure and Morphology Characterization Testing
3.4. Electrochemical Performance Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rojaee, R.; Shahbazian-Yassar, R. Two-Dimensional Materials to Address the Lithium Battery Challenges. ACS Nano 2020, 14, 2628–2658. [Google Scholar] [CrossRef]
- Cha, E.; Kim, D.K.; Choi, W. Advances of 2D MoS2 for High-Energy Lithium Metal Batteries. Front. Energy Res. 2021, 9, 645403. [Google Scholar] [CrossRef]
- Wenelska, K.; Adam, V.; Thauer, E.; Singer, L.; Klingeler, R.; Chen, X.; Mijowska, E. Fabrication of 3D graphene/MoS2 spherical heterostructure as anode material in Li-ion battery. Front. Energy Res. 2022, 10, 960786. [Google Scholar] [CrossRef]
- Wu, J.; Ciucci, F.; Kim, J.-K. Molybdenum Disulfide Based Nanomaterials for Rechargeable Batteries. Chem.-A Eur. J. 2020, 26, 6296–6319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, C.; Lu, F.; Jiang, H.; Wei, F. Si-induced insertion of Li into SiC to form Li-rich SiC twin crystal. Particuology 2023, 74, 56–63. [Google Scholar] [CrossRef]
- Qian, H.; Ren, H.; Zhang, Y.; He, X.; Li, W.; Wang, J.; Hu, J.; Yang, H.; Sari, H.M.K.; Chen, Y.; et al. Surface Doping vs. Bulk Doping of Cathode Materials for Lithium-Ion Batteries: A Review. Electrochem. Energy Rev. 2023, 5, 2. [Google Scholar] [CrossRef]
- Zhang, N.; Deng, T.; Zhang, S.; Wang, C.; Chen, L.; Wang, C.; Fan, X. Critical Review on Low-Temperature Li-Ion/Metal Batteries. Adv. Mater. 2022, 34, 2107899. [Google Scholar] [CrossRef]
- Qiu, Q.; Huang, Z. Photodetectors of 2D Materials from Ultraviolet to Terahertz Waves. Adv. Mater. 2021, 33, e2008126. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wu, Z.-P.; Huang, K.-W.; Ye, J.; Zhang, H. Surface Modification of 2D Photocatalysts for Solar Energy Conversion. Adv. Mater. 2022, 34, 2200180. [Google Scholar] [CrossRef]
- Zhu, K.; Zhu, Z.; Jin, B.; Li, H.; Jin, E.; Jeong, S.; Jiang, Q. 3D flower-Like Co1-xS/MoS2 composite for long-life and high-rate lithium storage. J. Energy Storage 2020, 27, 101135. [Google Scholar] [CrossRef]
- Olsson, E.; Yu, J.; Zhang, H.; Cheng, H.-M.; Cai, Q. Atomic-Scale Design of Anode Materials for Alkali Metal (Li/Na/K)-Ion Batteries: Progress and Perspectives. Adv. Energy Mater. 2022, 12, 2200662. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, X.; Meng, Y.; Zhao, J. Hierarchical carbon/MoS2 composites as anodes for advanced electrochemical performance. Ionics 2022, 28, 5499–5504. [Google Scholar] [CrossRef]
- Hai, N.Q.; Kim, H.; Yoo, I.S.; Hur, J. Facile and Scalable Preparation of a MoS(2)/Carbon Nanotube Nanocomposite Anode for High-Performance Lithium-Ion Batteries: Effects of Carbon Nanotube Content. J. Nanosci. Nanotechnol. 2019, 19, 1494–1499. [Google Scholar] [CrossRef]
- Kaiyu, Q.; Zeming, Y.; Bangwen, Z. Preparation and electrochemical performance of MoS2 nanosheets/graphene as anode material. J. Inn. Mong. Univ. Sci. Technol. 2019, 38, 166–171+176. [Google Scholar] [CrossRef]
- Lee, W.J.; Kim, C.S.; Yang, S.Y.; Lee, D.; Kim, Y.S. Spontaneous Exfoliation of Large-Sized Graphene Oxide with Low Defect Concentration by Simple Wet Chemistry. Carbon 2021, 182, 214–222. [Google Scholar] [CrossRef]
- Xin, J.; Ying, Z.; Wenhao, H.; Chen, W.; Lili, J.; Lizhi, S. In-Situ Carbon-Coaed Molybdenum Disulfide as Anode Material for Lithium-Ion Batteries. Nonferrous Met. Eng. 2021, 11, 12–16. [Google Scholar]
- Jain, R.; Lakhnot, A.S.; Bhimani, K.; Sharma, S.; Mahajani, V.; Panchal, R.A.; Kamble, M.; Han, F.D.; Wang, C.S.; Koratkar, N. Nanostructuring versus microstructuring in battery electrodes. Nat. Rev. Mater. 2022, 7, 736–746. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, D.; Wang, L.-T.; Zhu, H.-G.; Hong, G. Controllable synthesis of hierarchical MoS2 nanotubes with ultra-uniform and superior storage potassium properties. J. Colloid Interface Sci. 2020, 561, 593–600. [Google Scholar] [CrossRef]
- Choi, W.; Choi, Y.S.; Kim, H.; Yoon, J.; Kwon, Y.; Kim, T.; Ryu, J.-H.; Lee, J.H.; Lee, W.; Huh, J.; et al. Evidence for the Coexistence of Polysulfide and Conversion Reactions in the Lithium Storage Mechanism of MoS2 Anode Material. Chem. Mater. 2021, 33, 1935–1945. [Google Scholar] [CrossRef]
- Mateti, S.; Rahman, M.M.; Cizek, P.; Chen, Y. In situ production of a two-dimensional molybdenum disulfide/graphene hybrid nanosheet anode for lithium-ion batteries. RSC Adv. 2020, 10, 12754–12758. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Seo, S.D.; Song, H.J.; Kim, D.W. Free-standing molybdenum disulfides on porous carbon cloth for lithium-ion battery anodes. Int. J. Energy Res. 2021, 45, 11329–11337. [Google Scholar] [CrossRef]
- Yu, X.; Tang, J.; Terabe, K.; Sasaki, T.; Gao, R.; Ito, Y.; Nakura, K.; Asano, K.; Suzuki, M.-a. Fabrication of graphene/MoS2 alternately stacked structure for enhanced lithium storage. Mater. Chem. Phys. 2020, 239, 121987. [Google Scholar] [CrossRef]
- Ju, W.; Zhu, C.; Wei, Z. Intercalated ion tuning of the cross-plane thermal transport properties of graphite. AIP Adv. 2020, 10, 095225. [Google Scholar] [CrossRef]
- Fedoseeva, Y.V.; Makarova, A.A.; Stolyarova, S.G.; Arkhipov, V.E.; Ruehl, E.; Okotrub, A.V.; Bulusheva, L.G. Lithium-induced intralayer rearrangement of molybdenum disulfide: Effect of graphene coating. Appl. Surf. Sci. 2022, 598, 153846. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Wang, Z.; Zhang, C.; Zhang, X.; Zhou, W.; Liu, J.; Wang, W.; Yu, P. Solid-state reaction synthesis of amorphous/nanostructured Si@SiO -Cu3Si composites by mechanical milling for lithium-ion anodes. J. Alloys Compd. 2022, 905, 164207. [Google Scholar] [CrossRef]
- Li, X.L.; Li, T.C.; Huang, S.; Zhang, J.; Pam, M.E.; Yang, H. Y Controllable Synthesis of Two-Dimensional Molybdenum Disulfide (MoS2) for Energy-Storage Applications. ChemSusChem 2020, 13, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Dong, H.; Liang, F.; Zhang, Y.; Ge, M. Interfacial Reinforcement Structure Design towards Ultrastable Lithium Storage in MoS2-based Composited Electrode. Chem. Eng. J. 2021, 416, 129094. [Google Scholar] [CrossRef]
- Wei, X.; Lin, C.-C.; Wu, C.; Qaiser, N.; Cai, Y.; Lu, A.-Y.; Qi, K.; Fu, J.-H.; Chiang, Y.-H.; Yang, Z.; et al. Three-dimensional hierarchically porous MoS2 foam as high-rate and stable lithium-ion battery anode. Nat. Commun. 2022, 13, 6006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shen, S.; Chen, F.; Zhong, M.; Chew, J.W. Preparation and Properties of Mesoporous Carbon Composite as Negative Electrode Materials. Int. J. Electrochem. Sci. 2019, 14, 10058–10069. [Google Scholar] [CrossRef]
- Volkov, A.I.; Eliseeva, S.N.; Tolstopjatova, E.G.; Kondratiev, V.V. Enhanced electrochemical performance of MoS(2)anode material with novel composite binder. J. Solid State Electrochem. 2020, 24, 1607–1614. [Google Scholar] [CrossRef]
- Zhong, W.; Hong, J.; Wang, C.; Li, Z.; Chen, J.; Dmytro, S. MoS2/graphene nanosheet composites prepared by xylitol-assisted ball milling as high-performance anode materials for lithium-ion batteries. Ionics 2022, 29, 917–930. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C. Electrode Materials for Lithium Ion Battery. Prog. Chem. 2011, 23, 275–283. [Google Scholar]
- Lee, Y.K. Effect of transition metal ions on solid electrolyte interphase layer on the graphite electrode in lithium ion battery. J. Power Sources 2021, 484, 229270. [Google Scholar] [CrossRef]
- Jha, V.; Krishnamurthy, B. Modeling the SEI layer formation and its growth in lithium-ion batteries (LiB) during charge-discharge cycling. Ionics 2022, 28, 3661–3670. [Google Scholar] [CrossRef]
- Marriam, I.; Tebyetekerwa, M.; Chen, H.; Chathuranga, H.; Motta, N.; Alarco, J.A.; He, Z.-J.; Zheng, J.-C.; Du, A.; Yan, C. Few-layer MoS2 nanosheets with and without silicon nanoparticles as anodes for lithium-ion batteries. J. Mater. Chem. A 2023, 11, 2670–2678. [Google Scholar] [CrossRef]
- Li, R.; O’Kane, S.; Marinescu, M.; Offer, G.J. Modelling Solvent Consumption from SEI Layer Growth in Lithium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 060516. [Google Scholar] [CrossRef]
- Heidrich, B.; Boerner, M.; Winter, M.; Niehoff, P. Quantitative determination of solid electrolyte interphase and cathode electrolyte interphase homogeneity in multi-layer lithium ion cells. J. Energy Storage 2021, 44, 103208. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Hur, J. MoS2–TiC–C Nanocomposites as New Anode Materials for High-Performance Lithium-Ion Batteries. J. Nanosci. Nanotechnol. 2019, 19, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ottmann, A.; Thauer, E.; Neef, C.; Sai, H.; Sun, Q.; Cendrowski, K.; Meyer, H.P.; Vaynzof, Y.; Mijowsk, E. A facile synthesis method and electrochemical studies of hierarchical structured MoS2/C-nanocomposite. arXiv 2017, arXiv:1701.05894. [Google Scholar] [CrossRef]
- Cong, R.; Park, H.-H.; Jo, M.; Lee, H.; Lee, C.-S. Synthesis and Electrochemical Performance of Electrostatic Self-Assembled Nano-Silicon@N-Doped Reduced Graphene Oxide/Carbon Nanofibers Composite as Anode Material for Lithium-Ion Batteries. Molecules 2021, 26, 4831. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Cui, J.; Wei, W. Graphene enwrapped molybdenum disulfide for long life rechargeable batteries. Mater. Express 2020, 10, 1358–1363. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, S.; Kim, K.; Kang, H.; Ko, J.M.; Choi, W. Preparation of Carbon Nanowall and Carbon Nanotube for Anode Material of Lithium-Ion Battery. Molecules 2021, 26, 6950. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.X.; Wu, C.; Huo, Y.J. Controllable Preparation of Nano Molybdenum Disulfide by Hydrothermal Method. Ceram.-Silik. 2017, 61, 158–162. [Google Scholar] [CrossRef]
- Shelke, N.T.; Karle, S.C.; Karche, B.R. Hydrothermal Growth and Humidity-Dependent Electrical Properties of Molybdenum Disulphide Nanosheets. J. Nanosci. Nanotechnol. 2019, 19, 5158–5166. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, X.; Xu, L.; Xu, X.; Zhang, L. Microwave-Assisted Hydrothermal Preparation of SnO2/MoS2 Composites and their Electrochemical Performance. Nano 2016, 11, 1650023. [Google Scholar] [CrossRef]
- Zhai, Y.-J.; Li, J.-H.; Chu, X.-Y.; Xu, M.-Z.; Li, X.; Fang, X.; Wei, Z.-P.; Wang, X.-H. Preparation and Application of Molybdenum Disulfide Nanostructures. J. Inorg. Mater. 2015, 30, 897–905. [Google Scholar]
- Zhao, Z.; Shen, S.; Li, Y.; Chen, Q.; Yu, X.; Zhong, M.; Chen, X. Preparation and Electrochemical Properties of bowl-shaped carbon/molybdenum disulfide composite as cathode material for Lithium Ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 6290–6301. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nakayasu, Y.; Iwase, K.; Kobayashi, H.; Honma, I. Supercritical hydrothermal synthesis of MoS2 nanosheets with controllable layer number and phase structure. Dalton Trans. 2020, 49, 9377–9384. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, Y.; Meng, X.; Cao, W.; Liu, C.; Huang, Q.; Naik, N.; Murugadoss, V.; Huang, M.; Guo, Z. The impact of electrode with carbon materials on safety performance of lithium-ion batteries: A review. Carbon 2022, 191, 448–470. [Google Scholar] [CrossRef]
- Zhao, L.; Ding, B.; Qin, X.-Y.; Wang, Z.; Lv, W.; He, Y.-B.; Yang, Q.-H.; Kang, F. Revisiting the Roles of Natural Graphite in Ongoing Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2106704. [Google Scholar] [CrossRef]
- Tan, J.; Tan, X.; Kang, W.; Zhang, C. Two Dimensional Molybdenum Disulfide/Graphite Composites Prepared by Direct All-Solid-State Ball-Milling for Lithium-Ion Batteries. Chin. J. Appl. Chem. 2017, 34, 810–817. [Google Scholar]
- Wang, M.; Dang, D.Y.; Meyer, A.; Arsenault, R.; Cheng, Y.T. Effects of the Mixing Sequence on Making Lithium Ion Battery Electrodes. J. Electrochem. Soc. 2020, 167, 100518. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, G.; Ozawa, K.; Cai, Y.; Cheng, Z.; Kimura, H. Nanoporous MoS2/C Composites for High Performance Lithium Ion Battery Anode Material. Electrochim. Acta 2017, 239, 74–83. [Google Scholar] [CrossRef]
- Murray, V.; Hall, D.S.; Dahn, J.R. A Guide to Full Coin Cell Making for Academic Researchers. J. Electrochem. Soc. 2019, 166, A329–A333. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Li, F.; Li, H.; Zhang, S.; Liu, J.; He, X.; Sun, Z.; Yu, Z.; Zhang, Y.; Huang, X.; et al. Monodisperse MoS2/Graphite Composite Anode Materials for Advanced Lithium Ion Batteries. Molecules 2023, 28, 2775. https://doi.org/10.3390/molecules28062775
Liu B, Li F, Li H, Zhang S, Liu J, He X, Sun Z, Yu Z, Zhang Y, Huang X, et al. Monodisperse MoS2/Graphite Composite Anode Materials for Advanced Lithium Ion Batteries. Molecules. 2023; 28(6):2775. https://doi.org/10.3390/molecules28062775
Chicago/Turabian StyleLiu, Baosheng, Feng Li, Hongda Li, Shaohui Zhang, Jinghua Liu, Xiong He, Zijun Sun, Zhiqiang Yu, Yujin Zhang, Xiaoqi Huang, and et al. 2023. "Monodisperse MoS2/Graphite Composite Anode Materials for Advanced Lithium Ion Batteries" Molecules 28, no. 6: 2775. https://doi.org/10.3390/molecules28062775
APA StyleLiu, B., Li, F., Li, H., Zhang, S., Liu, J., He, X., Sun, Z., Yu, Z., Zhang, Y., Huang, X., Guo, F., Wang, G., & Jia, X. (2023). Monodisperse MoS2/Graphite Composite Anode Materials for Advanced Lithium Ion Batteries. Molecules, 28(6), 2775. https://doi.org/10.3390/molecules28062775






