Fabrication of Noble-Metal-Free Mo2C/CdIn2S4 Heterojunction Composites with Elevated Carrier Separation for Photocatalytic Hydrogen Production
Abstract
1. Introduction
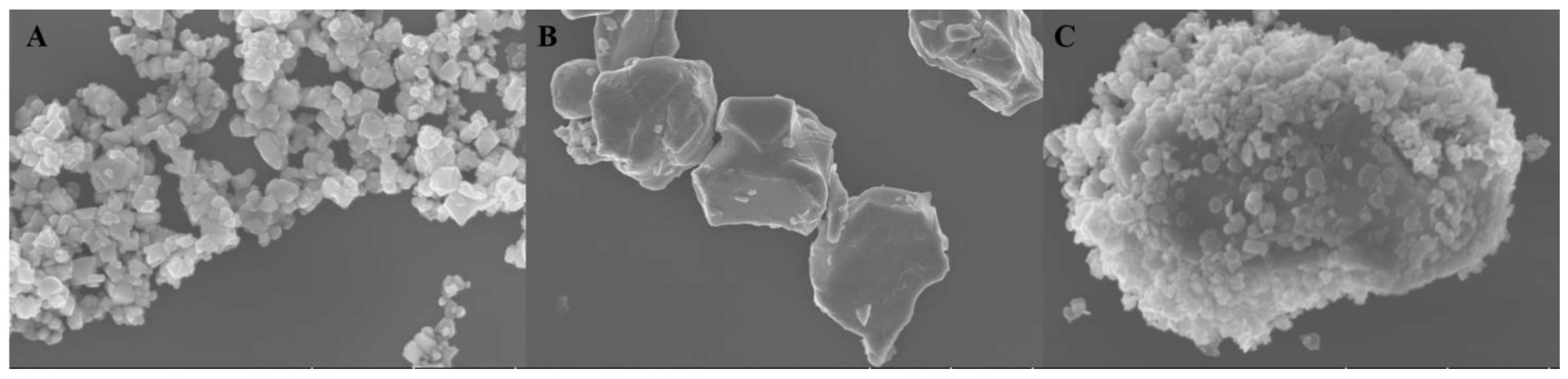
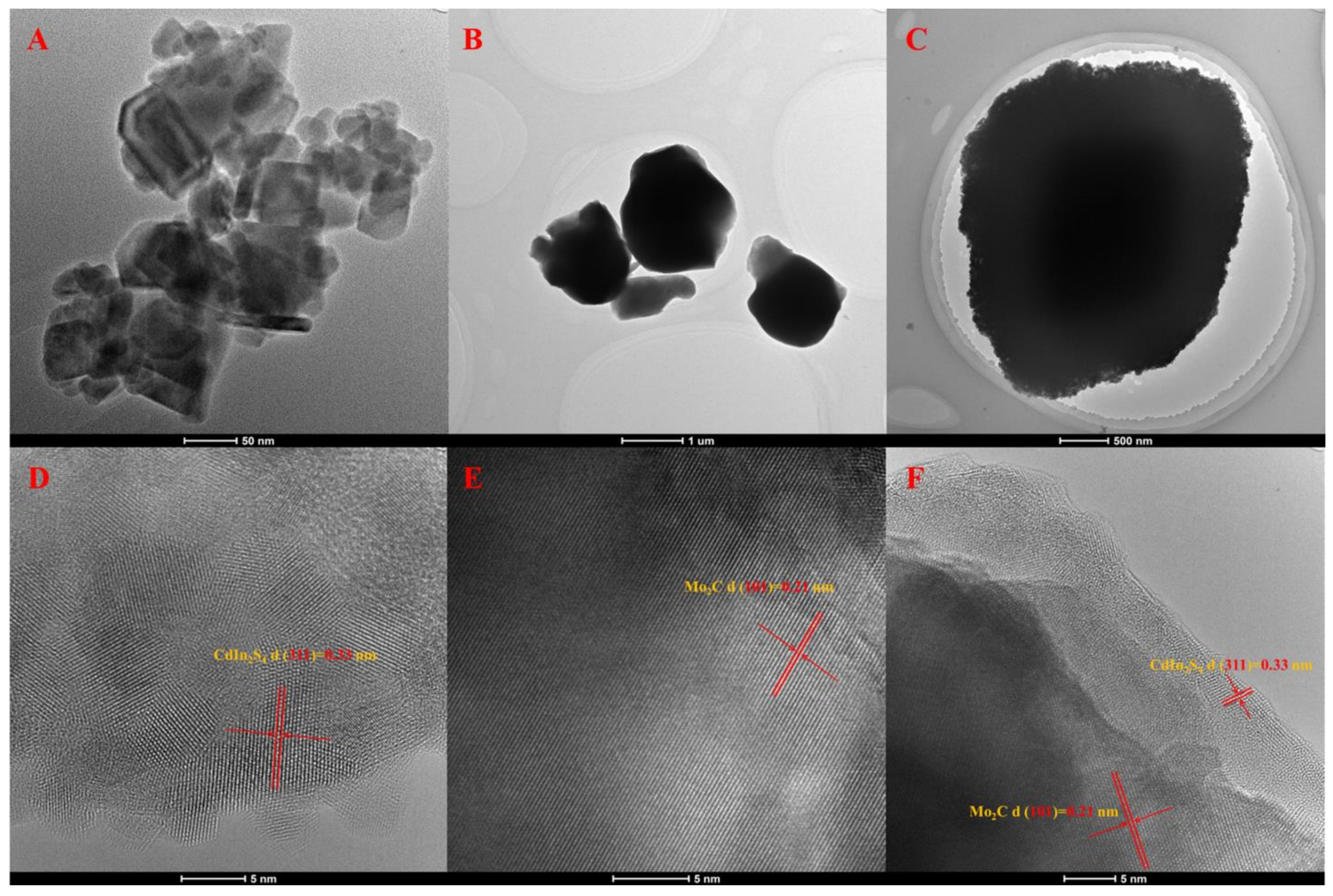
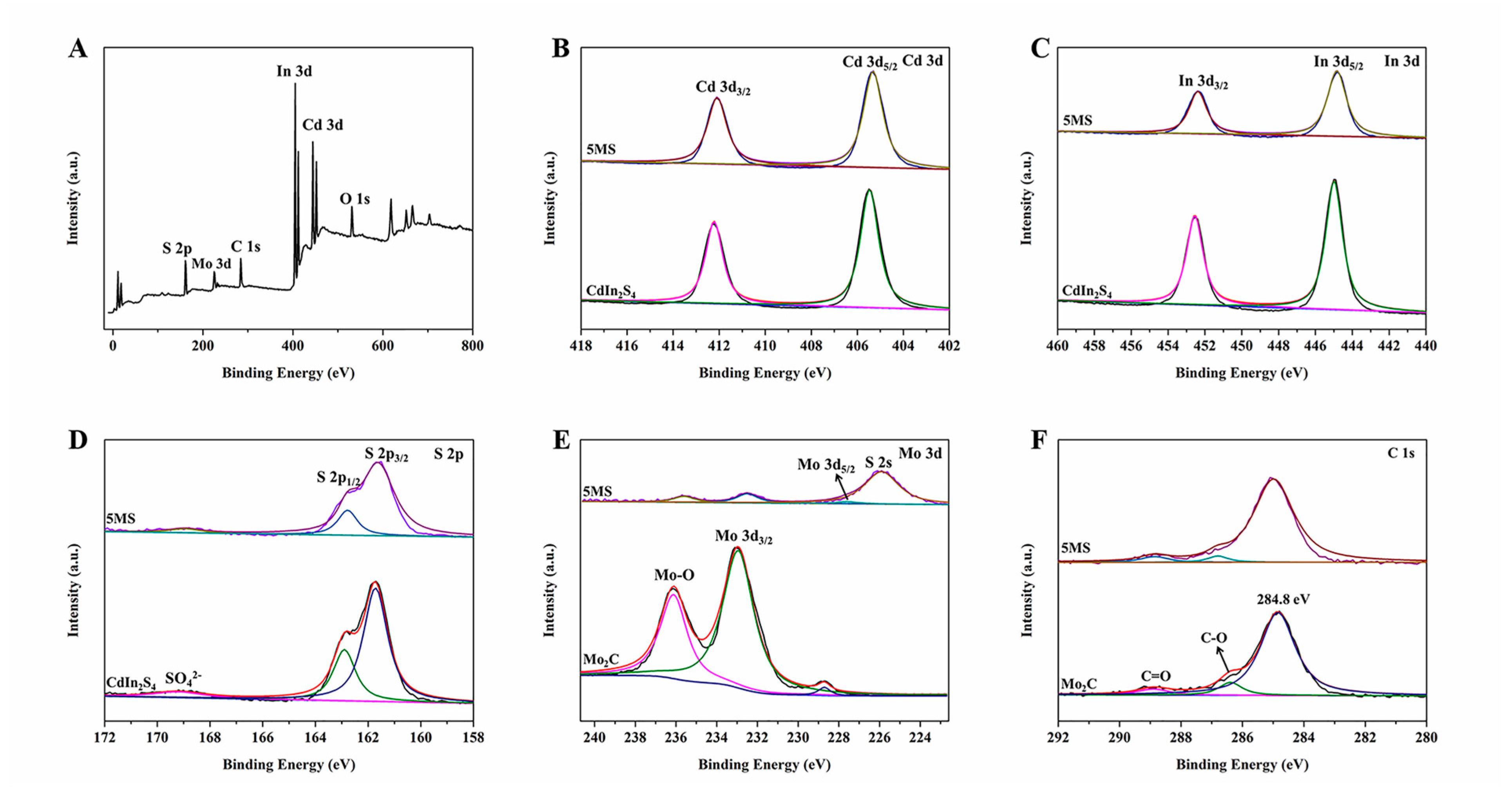
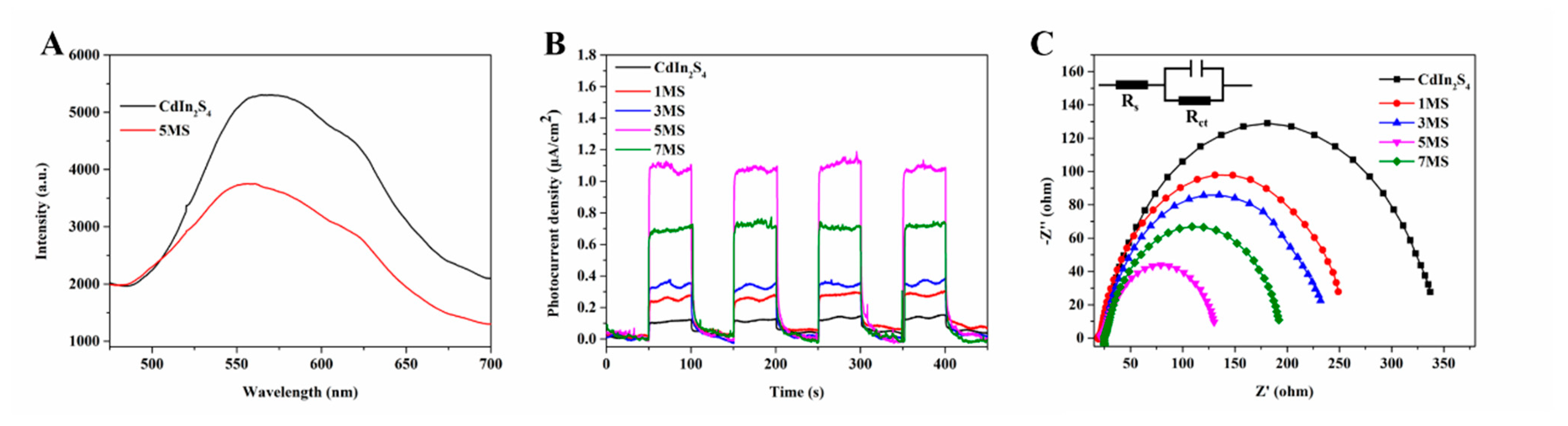
2. Results and Discussion
3. Experiment
3.1. Reagents
3.2. Synthesis of CdIn2S4 and Mo2C-CdIn2S4
3.3. Characterization
3.4. Electrochemical Measurements
3.5. Photocatalytic H2 Evolution Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Wang, M.; Han, K.; Zhang, S.; Sun, L. Integration of organometallic complexes with semiconductors and other nanomaterials for photocatalytic H2 production. Coord. Chem. Rev. 2015, 287, 1–14. [Google Scholar] [CrossRef]
- Li, X.; Li, N.; Gao, Y.; Ge, L. Design and applications of hollow-structured nanomaterials for photocatalytic H2 evolution and CO2 reduction. Chin. J. Catal. 2022, 43, 679–707. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Z.; Arif, N.; Jiang, W.-C.; Zeng, Y.-J. 2D material based heterostructures for solar light driven photocatalytic H2 production. Mater. Adv. 2022, 3, 3389–3417. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063. [Google Scholar] [CrossRef]
- Bie, C.; Zhu, B.; Wang, L.; Yu, H.; Jiang, C.; Chen, T.; Yu, J. A Bifunctional CdS/MoO2/MoS2 Catalyst Enhances Photocatalytic H2 Evolution and Pyruvic Acid Synthesis. Angew. Chem. Int. Ed. Engl. 2022, 61, e202212045. [Google Scholar] [CrossRef]
- Mohite, S.V.; Kim, S.; Lee, C.; Bae, J.; Kim, Y. Z-scheme heterojunction photocatalyst: Deep eutectic solvents-assisted synthesis of Cu2O nanocluster improved hydrogen production of TiO2. J. Alloy. Compd. 2022, 928, 167168. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Shi, J.; Chen, P.; Zong, S.; Cheng, C.; Chen, K.; Chen, Y.; Ma, L. (Oxy)nitride heterojunction-strengthened separation of photogenerated carriers in g-C3N4 towards enhanced photocatalytic H2 evolution. Appl. Catal. A Gen. 2022, 643, 118746. [Google Scholar] [CrossRef]
- Guo, S.; Li, Y.; Xue, C.; Sun, Y.; Wu, C.; Shao, G.; Zhang, P. Controllable construction of hierarchically CdIn2S4/CNFs/Co4S3 nanofiber networks towards photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 419, 129213. [Google Scholar] [CrossRef]
- Dang, X.; Xie, M.; Dai, F.; Guo, J.; Liu, J.; Lu, X. The in situ construction of ZnIn2S4/CdIn2S4 2D/3D nano hetero-structure for an enhanced visible-light-driven hydrogen production. J. Mater. Chem. A 2021, 9, 14888–14896. [Google Scholar] [CrossRef]
- Zhang, H.H.; Zhan, G.P.; Liu, Z.K.; Wu, C.D. Photocatalytic Hydrogen Evolution Coupled with Production of Highly Value-Added Organic Chemicals by a Composite Photocatalyst CdIn2S4@MIL-53-SO3Ni1/2. Chem. Asian J. 2021, 16, 1499–1506. [Google Scholar] [CrossRef]
- Kale, B.B.; Baeg, J.O.; Lee, S.M.; Chang, H.; Moon, S.J.; Lee, C.W. CdIn2S4 Nanotubes and “Marigold” Nanostructures: A Visible-Light Photocatalyst. Adv. Funct. Mater. 2006, 16, 1349–1354. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.W.; Zou, Y.; Fan, Z.; Shi, J.; Cheng, L.; Sun, D.; Wang, Z.; Niu, C. Multiple carrier-transfer pathways in a flower-like In2S3/CdIn2S4/In2O3 ternary heterostructure for enhanced photocatalytic hydrogen production. Nanoscale 2018, 10, 7860–7870. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, G.; Wang, G.; Lv, Z. Visible-light-driven ZnIn2S4/CdIn2S4 composite photocatalyst with enhanced performance for photocatalytic H2 evolution. Int. J. Hydrog. Energy 2013, 38, 1278–1285. [Google Scholar] [CrossRef]
- Bai, X.; Wu, W.; Lv, H. Effects of Cu2+ Doping on Structure, Morphology and Photocatalytic Hydrogen Production Performance of Porous CdIn2S4 Microsphere. IOP Conf. Ser. Mater. Sci. Eng. 2020, 735, 012041. [Google Scholar] [CrossRef]
- Bai, X.F.; Li, J.S. Photocatalytic Hydrogen Evolution over Cr3+ Doped Porous CdIn2S4 Photocatalysts under Visible Light Irradiation. Adv. Mater. Res. 2012, 486, 181–186. [Google Scholar] [CrossRef]
- He, J.; Li, B.; Yu, J.; Qiao, L.; Li, S.; Zu, X.; Xiang, X. Ultra-thin CdIn2S4 nanosheets with nanoholes for efficient photocatalytic hydrogen evolution. Opt. Mater. 2020, 108, 110231. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Q.; Yu, M.; Gong, X.; Li, S.; Wang, S.; Yu, H.; Li, Z. In situ construction of a direct Z-scheme CdIn2S4/TiO2 heterojunction for improving photocatalytic properties. CrystEngComm 2021, 23, 5070–5077. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Zhang, W.; Li, Y.; Song, Q.; Dong, L. Fabricate Globular Flower-like CuS/CdIn2S4/ZnIn2S4 with High Visible Light Response via Microwave-assisted One–step Method and Its Multipathway Photoelectron Migration Properties for Hydrogen Evolution and Pollutant Degradation. ACS Sustain. Chem. Eng. 2016, 4, 6680–6688. [Google Scholar] [CrossRef]
- Qiang, M.; Zhang, X.; Song, H.; Pi, C.; Wang, X.; Gao, B.; Zheng, Y.; Peng, X.; Chu, P.K.; Huo, K. General synthesis of nanostructured Mo2C electrocatalysts using a carbon template for electrocatalytic applications. Carbon 2022, 197, 238–245. [Google Scholar] [CrossRef]
- Huo, L.; Liu, B.; Zhang, G.; Zhang, J. Universal Strategy to Fabricate a Two-Dimensional Layered Mesoporous Mo2C Electrocatalyst Hybridized on Graphene Sheets with High Activity and Durability for Hydrogen Generation. ACS Appl. Mater. Interfaces 2016, 8, 18107–18118. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, X.; Lin, K.; Li, J.; Liu, Y.; Zhan, H.; Liu, W. A novel ultraefficient non-noble metal composite cocatalyst Mo2N/Mo2C/graphene for enhanced photocatalytic H2 evolution. Int. J. Hydrog. Energy 2017, 42, 18977–18984. [Google Scholar] [CrossRef]
- Li, H.; Hong, W.; Cui, Y.; Fan, S.; Zhu, L. Effect of Mo2C content on the structure and photocatalytic property of Mo2C/TiO2 catalysts. J. Alloy. Compd. 2013, 569, 45–51. [Google Scholar] [CrossRef]
- Ma, X.; Ren, C.; Li, H.; Liu, X.; Li, X.; Han, K.; Li, W.; Zhan, Y.; Khan, A.; Chang, Z.; et al. A novel noble-metal-free Mo2C-In2S3 heterojunction photocatalyst with efficient charge separation for enhanced photocatalytic H2 evolution under visible light. J. Colloid Interface Sci. 2021, 582, 488–495. [Google Scholar] [CrossRef]
- Yue, X.; Yi, S.; Wang, R.; Zhang, Z.; Qiu, S. Well-controlled SrTiO3@Mo2C core-shell nanofiber photocatalyst: Boosted photo-generated charge carriers transportation and enhanced catalytic performance for water reduction. Nano Energy 2018, 47, 463–473. [Google Scholar] [CrossRef]
- Yue, X.; Yi, S.; Wang, R.; Zhang, Z.; Qiu, S. A novel architecture of dandelion-like Mo2C/TiO2 heterojunction photocatalysts towards high-performance photocatalytic hydrogen production from water splitting. J. Mater. Chem. A 2017, 5, 10591–10598. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, M.; He, B.; Wang, R.; Wang, H.; Gong, Y. Facile synthesis of rod-like g-C3N4 by decorating Mo2C co-catalyst for enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2019, 470, 565–572. [Google Scholar] [CrossRef]
- Ma, X.; Li, W.; Ren, C.; Dong, M.; Geng, L.; Fan, H.; Li, Y.; Qiu, H.; Wang, T. Construction of novel noble-metal-free MoP/CdIn2S4 heterojunction photocatalysts: Effective carrier separation, accelerating dynamically H2 release and increased active sites for enhanced photocatalytic H2 evolution. J. Colloid Interface Sci. 2022, 628, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Y.; Liu, X.; Huo, P.; Yan, Y.; Wang, L.; Liao, G.; Liu, C. Interface engineering of Co9S8/CdIn2S4 ohmic junction for efficient photocatalytic H2 evolution under visible light. J. Colloid Interface Sci. 2021, 600, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xue, W.; Fan, J.; Liu, E.; Yu, Q. Preparation of Ni12P5-decorated Cd0.5Zn0.5S for efficient photocatalytic H2 evolution. J. Alloy. Compd. 2021, 854, 156951. [Google Scholar] [CrossRef]
- Wen, J.; Li, X.; Li, H.; Ma, S.; He, K.; Xu, Y.; Fang, Y.; Liu, W.; Gao, Q. Enhanced visible-light H2 evolution of g-C3N4 photocatalysts via the synergetic effect of amorphous NiS and cheap metal-free carbon black nanoparticles as co-catalysts. Appl. Surf. Sci. 2015, 358, 204–212. [Google Scholar] [CrossRef]
- Ma, B.; Xu, H.; Lin, K.; Li, J.; Zhan, H.; Liu, W.; Li, C. Mo2C as Non-Noble Metal Co-Catalyst in Mo2C/CdS Composite for Enhanced Photocatalytic H2 Evolution under Visible Light Irradiation. ChemSusChem 2016, 9, 820–824. [Google Scholar] [CrossRef]
- Ma, X.; Li, W.; Ren, C.; Li, H.; Liu, X.; Li, X.; Wang, T.; Dong, M.; Liu, S.; Chen, S. A novel noble-metal-free binary and ternary In2S3 photocatalyst with WC and “W-Mo auxiliary pairs” for highly-efficient visible-light hydrogen evolution. J. Alloy. Compd. 2021, 875, 160058. [Google Scholar] [CrossRef]
- Song, Y.; Xia, K.; Gong, Y.; Chen, H.; Li, L.; Yi, J.; She, X.; Chen, Z.; Wu, J.; Li, H.; et al. Controllable synthesized heterostructure photocatalyst Mo2C@C/2D g-C3N4: Enhanced catalytic performance for hydrogen production. Dalton. Trans. 2018, 47, 14706–14712. [Google Scholar] [CrossRef]
- Xiaohui, M.; Wenjun, L.; Hongda, L.; Mei, D.; Xinyang, L.; Liang, G.; Hongxia, F.; Yanyan, L.; Hong, Q.; Tianyu, W. Fabrication of novel and noble-metal-free MoP/In2S3 Schottky heterojunction photocatalyst with efficient charge separation for enhanced photocatalytic H2 evolution under visible light. J. Colloid Interface Sci. 2022, 617, 284–292. [Google Scholar] [CrossRef]
- Ma, X.; Li, W.; Ren, C.; Li, H.; Li, X.; Dong, M.; Gao, Y.; Wang, T.; Zhou, H.; Li, Y. Fabrication of novel noble-metal-free ZnIn2S4/WC Schottky junction heterojunction photocatalyst: Efficient charge separation, increased active sites and low hydrogen production overpotential for boosting visible-light H2 evolution. J. Alloy. Compd. 2022, 901, 163709. [Google Scholar] [CrossRef]
- Ma, X.; Li, W.; Li, H.; Dong, M.; Geng, L.; Wang, T.; Zhou, H.; Li, Y.; Li, M. Novel noble-metal-free Co2P/CdIn2S4 heterojunction photocatalysts for elevated photocatalytic H2 production: Light absorption, charge separation and active site. J. Colloid Interface Sci. 2023, 639, 87–95. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.X.; Hu, X.Y.; Wang, Y.S.; Liu, E.Z.; Fan, J. A novel S-scheme MoS2/CdIn2S4 flower-like heterojunctions with enhanced photocatalytic degradation and H2 evolution activity. J. Phys. D Appl. Phys. 2020, 53, 205101. [Google Scholar] [CrossRef]
- Li, Q.; Liu, W.L.; Xie, X.J.; Yang, X.L.; Chen, X.F.; Xu, X.G. Synthesis and Characterization of Amorphous Molybdenum Sulfide (MoSx)/CdIn2S4 Composite Photocatalyst: Co-Catalyst Using in the Hydrogen Evolution Reaction. Catalysts 2020, 10, 1455. [Google Scholar] [CrossRef]

 is peaks of Mo2C, the (1 1 1), (2 2 0), (3 1 1), (4 0 0), (5 1 1), (4 4 0), (6 2 2), and (7 3 1) planes corresponds to CdIn2S4, the (1 0 0), (1 0 1), (1 1 0), (0 0 2), (2 0 0), (2 0 1), (1 1 2), and (1 0 2) planes corresponds to Mo2C).
is peaks of Mo2C, the (1 1 1), (2 2 0), (3 1 1), (4 0 0), (5 1 1), (4 4 0), (6 2 2), and (7 3 1) planes corresponds to CdIn2S4, the (1 0 0), (1 0 1), (1 1 0), (0 0 2), (2 0 0), (2 0 1), (1 1 2), and (1 0 2) planes corresponds to Mo2C).
 is peaks of Mo2C, the (1 1 1), (2 2 0), (3 1 1), (4 0 0), (5 1 1), (4 4 0), (6 2 2), and (7 3 1) planes corresponds to CdIn2S4, the (1 0 0), (1 0 1), (1 1 0), (0 0 2), (2 0 0), (2 0 1), (1 1 2), and (1 0 2) planes corresponds to Mo2C).
is peaks of Mo2C, the (1 1 1), (2 2 0), (3 1 1), (4 0 0), (5 1 1), (4 4 0), (6 2 2), and (7 3 1) planes corresponds to CdIn2S4, the (1 0 0), (1 0 1), (1 1 0), (0 0 2), (2 0 0), (2 0 1), (1 1 2), and (1 0 2) planes corresponds to Mo2C).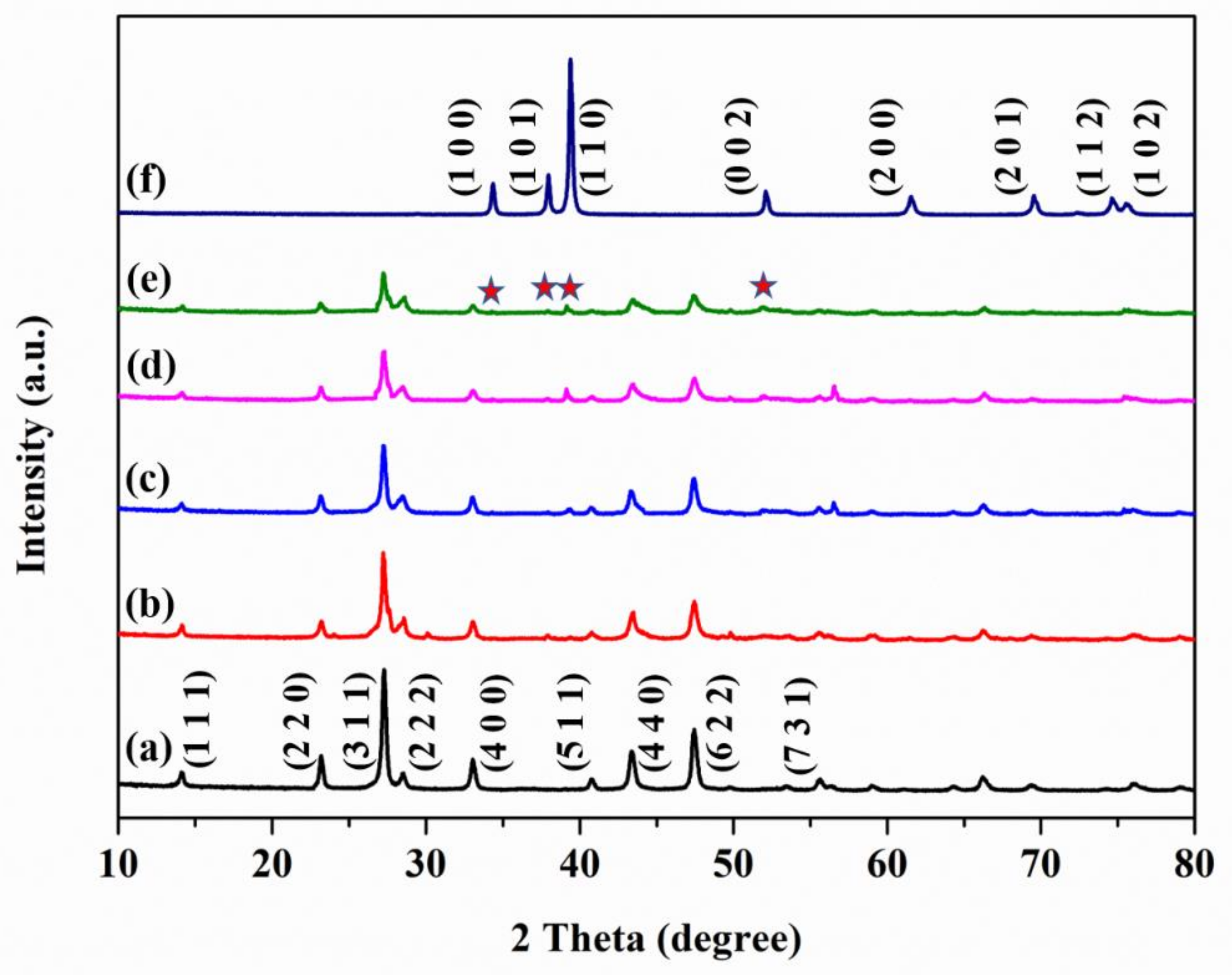


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, H.; Ma, X.; Fan, H.; Fan, Y.; Li, Y.; Zhou, H.; Li, W. Fabrication of Noble-Metal-Free Mo2C/CdIn2S4 Heterojunction Composites with Elevated Carrier Separation for Photocatalytic Hydrogen Production. Molecules 2023, 28, 2508. https://doi.org/10.3390/molecules28062508
Qiu H, Ma X, Fan H, Fan Y, Li Y, Zhou H, Li W. Fabrication of Noble-Metal-Free Mo2C/CdIn2S4 Heterojunction Composites with Elevated Carrier Separation for Photocatalytic Hydrogen Production. Molecules. 2023; 28(6):2508. https://doi.org/10.3390/molecules28062508
Chicago/Turabian StyleQiu, Hong, Xiaohui Ma, Hongxia Fan, Yueyan Fan, Yajie Li, Hualei Zhou, and Wenjun Li. 2023. "Fabrication of Noble-Metal-Free Mo2C/CdIn2S4 Heterojunction Composites with Elevated Carrier Separation for Photocatalytic Hydrogen Production" Molecules 28, no. 6: 2508. https://doi.org/10.3390/molecules28062508
APA StyleQiu, H., Ma, X., Fan, H., Fan, Y., Li, Y., Zhou, H., & Li, W. (2023). Fabrication of Noble-Metal-Free Mo2C/CdIn2S4 Heterojunction Composites with Elevated Carrier Separation for Photocatalytic Hydrogen Production. Molecules, 28(6), 2508. https://doi.org/10.3390/molecules28062508




