Antiproliferative Activity and DNA Interaction Studies of a Series of N4,N4-Dimethylated Thiosemicarbazone Derivatives
Abstract
1. Introduction
2. Results
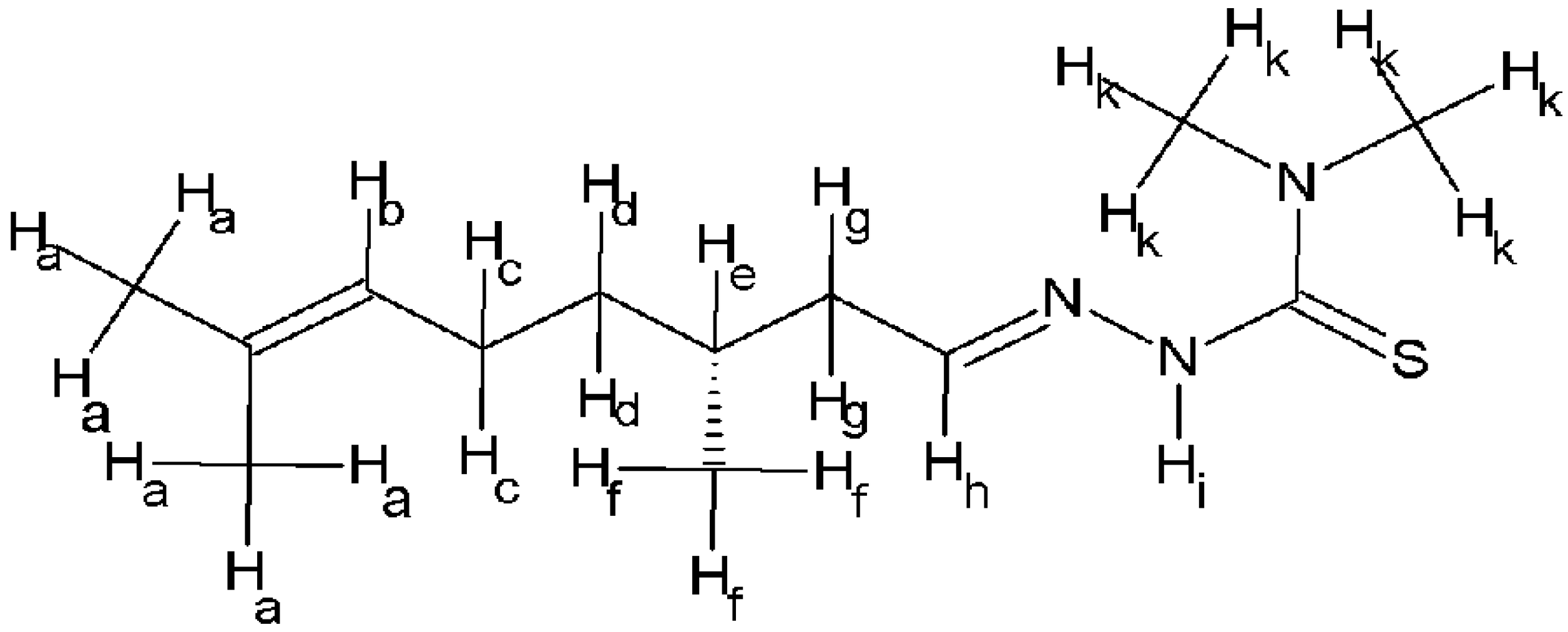
2.1. Chemistry
2.2. Cytotoxicity of Newly Synthesised Molecules on U937 Cells
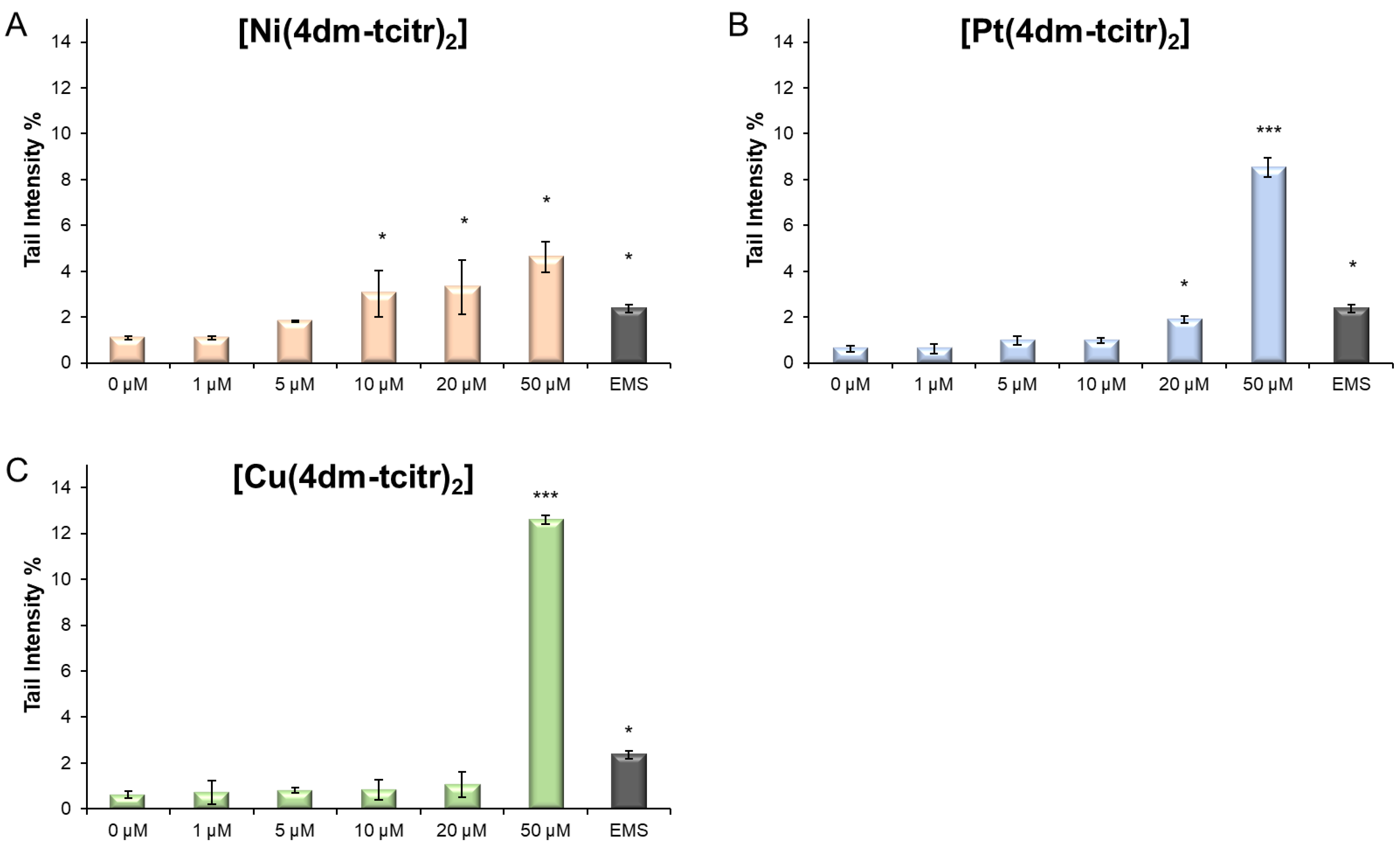
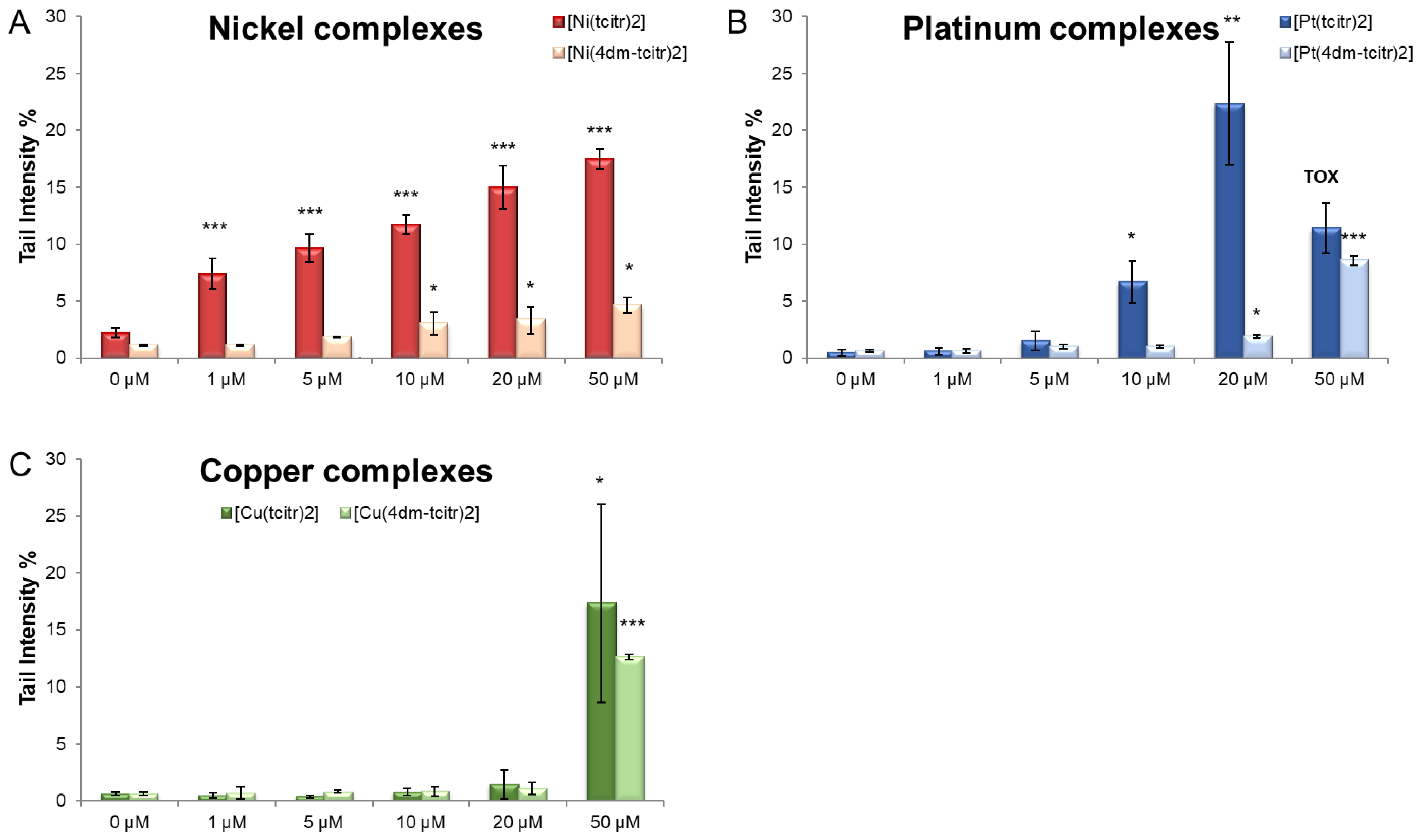
2.3. Genotoxicity of Newly Synthesised Molecules on U937 Cells
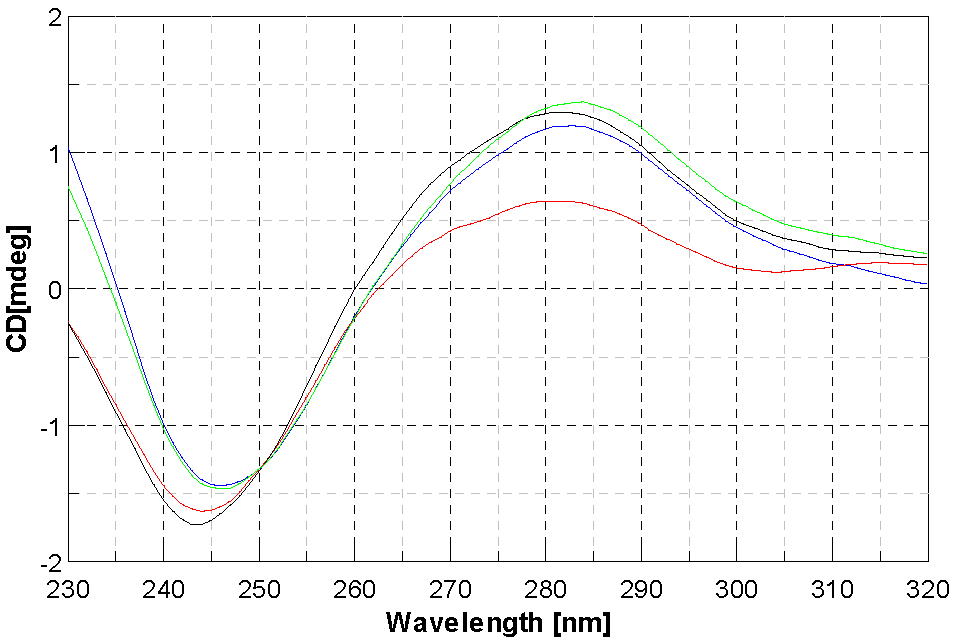
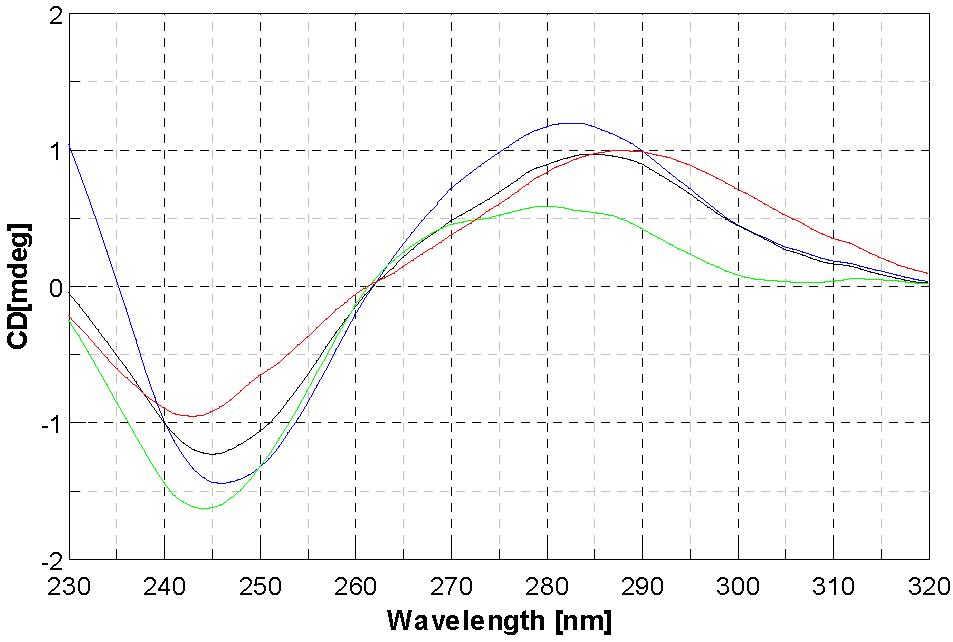
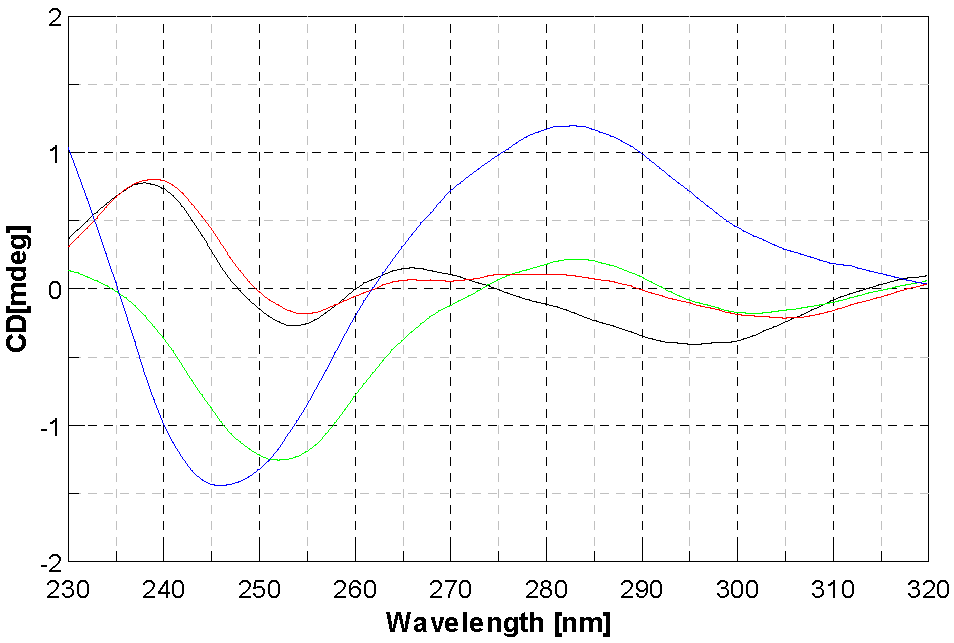
2.4. DNA Interaction Studies
3. Discussion
4. Materials and Methods
4.1. Synthesis and Chemical Characterisation
4.1.1. Synthesis of 4dm-Htcitr
4.1.2. Synthesis of [Ni(4dm-(tcitr)2]
4.1.3. Synthesis of [Pt(4dm-(tcitr)2]
4.1.4. Synthesis of [Cu(4dm-(tcitr)2]
4.2. Cell Lines and Culture Conditions
4.3. In Vitro Antiproliferative Activity of Metal Complexes on Human Cancer Cells by MTS Assay
4.4. In Vitro Genotoxic Activity of Metal Complexes on U937 Cells through Alkaline Comet Assay
4.5. CD Titrations
4.6. Melting Temperature Determination
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bonaccorso, C.; Marzo, T.; La Mendola, D. Biological Applications of Thiocarbohydrazones and Their Metal Complexes: A Perspective Review. Pharmaceuticals 2019, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira, L.R.P.; de Moraes Gomes, P.A.T.; de Lima Ferreira, L.P.; de Melo Rêgo, M.J.B.; Leite, A.C.L. Multi-target compounds acting in cancer progression: Focus on thiosemicarbazone, thiazole and thiazolidinone analogues. Eur. J. Med. Chem. 2019, 170, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Heffeter, P.; Pape, V.F.S.; Enyedy, É.A.; Keppler, B.K.; Szakacs, G.; Kowol, C.R. Anticancer Thiosemicarbazones: Chemical Properties, Interaction with Iron Metabolism, and Resistance Development. Antioxid. Redox Signal. 2019, 30, 1062–1082. [Google Scholar] [CrossRef]
- Chandra, S. Synthesis, spectroscopic, anticancer and antibacterial studies of Ni(II) and Cu(II) complexes with 2-carboxybenzaldehyde thiosemicarbazone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Pósa, V.; Hajdu, B.; Tóth, G.; Dömötör, O.; Kowol, C.R.; Keppler, B.K.; Spengler, G.; Gyurcsik, B.; Enyedy, É.A. The coordination modes of (thio)semicarbazone copper(II) complexes strongly modulate the solution chemical properties and mechanism of anticancer activity. J. Inorg. Biochem. 2022, 231, 111786. [Google Scholar] [CrossRef]
- Pelosi, G. Thiosemicarbazone Metal Complexes: From Structure to Activity. Open Crystallogr. J. 2010, 3, 16–28. [Google Scholar] [CrossRef]
- Hussein, M.A.; Iqbal, M.A.; Umar, M.I.; Haque, R.A.; Guan, T.S. Synthesis, structural elucidation and cytotoxicity of new thiosemicarbazone derivatives. Arab. J. Chem. 2019, 12, 3183–3192. [Google Scholar] [CrossRef]
- Kanso, F.; Khalil, A.; Noureddine, H.; El-Makhour, Y. Therapeutic perspective of thiosemicarbazones derivatives in inflammatory pathologies: A summary of in vitro/in vivo studies. Int. Immunopharmacol. 2021, 96, 107778. [Google Scholar] [CrossRef]
- Milunović, M.N.M.; Palamarciuc, O.; Sirbu, A.; Shova, S.; Dumitrescu, D.; Dvoranová, D.; Rapta, P.; Petrasheuskaya, T.V.; Enyedy, E.A.; Spengler, G.; et al. Insight into the Anticancer Activity of Copper(II) 5-Methylenetrimethylammonium-Thiosemicarbazonates and Their Interaction with Organic Cation Transporters. Biomolecules 2020, 10, 1213. [Google Scholar] [CrossRef]
- Montalbano, S.; Degola, F.; Bartoli, J.; Bisceglie, F.; Buschini, A.; Carcelli, M.; Feretti, D.; Galati, S.; Marchi, L.; Orsoni, N.; et al. The AFLATOX® Project: Approaching the Development of New Generation, Natural-Based Compounds for the Containment of the Mycotoxigenic Phytopathogen Aspergillus flavus and Aflatoxin Contamination. Int. J. Mol. Sci. 2021, 22, 4520. [Google Scholar] [CrossRef]
- Rogolino, D.; Gatti, A.; Carcelli, M.; Pelosi, G.; Bisceglie, F.; Restivo, F.M.; Degola, F.; Buschini, A.; Montalbano, S.; Feretti, D.; et al. Thiosemicarbazone scaffold for the design of antifungal and antiaflatoxigenic agents: Evaluation of ligands and related copper complexes. Sci. Rep. 2017, 7, 11214. [Google Scholar] [CrossRef] [PubMed]
- Pham, E.C.; Truong, T.N.; Dong, N.H.; Vo, D.D.; Hong Do, T.T. Synthesis of a Series of Novel 2-Amino-5-Substituted 1,3,4-oxadiazole and 1,3,4-thiadiazole Derivatives as Potential Anticancer, Antifungal and Antibacterial Agents. Med. Chem. 2021, 18, 558–573. [Google Scholar] [CrossRef] [PubMed]
- Atasever Arslan, B.; Kaya, B.; Şahin, O.; Baday, S.; Saylan, C.C.; Ülküseven, B. The iron(III) and nickel(II) complexes with tetradentate thiosemicarbazones. Synthesis, experimental, theoretical characterization, and antiviral effect against SARS-CoV-2. J. Mol. Struct. 2021, 1246, 131166. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.S.; Jayabalan, K.; Ragavendran, V.; Nityananda Shetty, A. Syntheses, quantum mechanical modeling, biomolecular interaction and in vitro anticancer—Tubulin activity of thiosemicarbazones. Bioorg. Chem. 2020, 102, 104081. [Google Scholar] [CrossRef]
- Sun, D.L.; Poddar, S.; Pan, R.D.; Rosser, E.W.; Abt, E.R.; Van Valkenburgh, J.; Le, T.M.; Lok, V.; Hernandez, S.P.; Song, J.; et al. Isoquinoline thiosemicarbazone displays potent anticancer activity with in vivo efficacy against aggressive leukemias. RSC Med. Chem. 2020, 11, 392–410. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Wu, S.; Ren, S.; Zhu, M.; Luo, G.; Xiang, H. Synthesis and evaluation of novel thiosemicarbazone and semicarbazone analogs with both anti-proliferative and anti-metastatic activities against triple negative breast cancer. Bioorg. Med. Chem. 2021, 37, 116107. [Google Scholar] [CrossRef] [PubMed]
- Dobrova, A.; Platzer, S.; Bacher, F.; Milunovic, M.N.; Dobrov, A.; Spengler, G.; Enyedy, É.A.; Novitchi, G.; Arion, V.B. Structure-antiproliferative activity studies on l-proline- and homoproline-4-N-pyrrolidine-3-thiosemicarbazone hybrids and their nickel(ii), palladium(ii) and copper(ii) complexes. Dalton Trans. 2016, 45, 13427–13439. [Google Scholar] [CrossRef]
- Buschini, A.; Pinelli, S.; Pellacani, C.; Giordani, F.; Ferrari, M.B.; Bisceglie, F.; Giannetto, M.; Pelosi, G.; Tarasconi, P. Synthesis, characterization and deepening in the comprehension of the biological action mechanisms of a new nickel complex with antiproliferative activity. J. Inorg. Biochem. 2009, 103, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Buschini, A.; Pinelli, S.; Alinovi, R.; Mussi, F.; Bisceglie, F.; Rivetti, C.; Doniselli, N.; Pelosi, G. Unravelling mechanisms behind the biological activity of bis(S-citronellalthiosemicarbazonato)nickel(II). Metallomics 2014, 6, 783–792. [Google Scholar] [CrossRef]
- Bisceglie, F.; Orsoni, N.; Pioli, M.; Bonati, B.; Tarasconi, P.; Rivetti, C.; Amidani, D.; Montalbano, S.; Buschini, A.; Pelosi, G. Cytotoxic activity of copper(ii), nickel(ii) and platinum(ii) thiosemicarbazone derivatives: Interaction with DNA and the H2A histone peptide. Metallomics 2019, 11, 1729–1742. [Google Scholar] [CrossRef]
- Baruffini, E.; Ruotolo, R.; Bisceglie, F.; Montalbano, S.; Ottonello, S.; Pelosi, G.; Buschini, A.; Lodi, T. Mechanistic insights on the mode of action of an antiproliferative thiosemicarbazone-nickel complex revealed by an integrated chemogenomic profiling study. Sci. Rep. 2020, 10, 10524. [Google Scholar] [CrossRef] [PubMed]
- Bisceglie, F.; Tavone, M.; Mussi, F.; Azzoni, S.; Montalbano, S.; Franzoni, S.; Tarasconi, P.; Buschini, A.; Pelosi, G. Effects of polar substituents on the biological activity of thiosemicarbazone metal complexes. J. Inorg. Biochem. 2018, 179, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Bisceglie, F.; Pinelli, S.; Alinovi, R.; Goldoni, M.; Mutti, A.; Camerini, A.; Piola, L.; Tarasconi, P.; Pelosi, G. Cinnamaldehyde and cuminaldehyde thiosemicarbazones and their copper(II) and nickel(II) complexes: A study to understand their biological activity. J. Inorg. Biochem. 2014, 140, 111–125. [Google Scholar] [CrossRef]
- Bisceglie, F.; Alinovi, R.; Pinelli, S.; Goldoni, M.; Buschini, A.; Franzoni, S.; Mutti, A.; Tarasconi, P.; Pelosi, G. Ni(II) and Cu(II) N(4)-ethylmorpholine citronellalthiosemicarbazonate: A comparative analysis of cytotoxic effects in malignant human cancer cell lines. Metallomics 2013, 5, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Bisceglie, F.; Pinelli, S.; Alinovi, R.; Tarasconi, P.; Buschini, A.; Mussi, F.; Mutti, A.; Pelosi, G. Copper(II) thiosemicarbazonate molecular modifications modulate apoptotic and oxidative effects on U937 cell line. J. Inorg. Biochem. 2012, 116, 195–203. [Google Scholar] [CrossRef]
- Oliveira, C.G.; Romero-Canelón, I.; Coverdale, J.P.C.; Maia, P.I.S.; Clarkson, G.J.; Deflon, V.M.; Sadler, P.J. Novel tetranuclear PdII and PtII anticancer complexes derived from pyrene thiosemicarbazones. Dalton Trans. 2020, 49, 9595–9604. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Ma, Z.Y.; Li, A.; Liu, Y.H.; Xie, C.Z.; Qiang, Z.Y.; Xu, J.Y. Thiosemicarbazone Cu(II) and Zn(II) complexes as potential anticancer agents: Syntheses, crystal structure, DNA cleavage, cytotoxicity and apoptosis induction activity. J. Inorg. Biochem. 2014, 136, 13–23. [Google Scholar] [CrossRef]
- González-Barcia, L.M.; Fernández-Fariña, S.; Rodríguez-Silva, L.; Bermejo, M.R.; González-Noya, A.M.; Pedrido, R. Comparative study of the antitumoral activity of phosphine-thiosemicarbazone gold(I) complexes obtained by different methodologies. J. Inorg. Biochem. 2020, 203, 110931. [Google Scholar] [CrossRef]
- Beebe, S.J.; Celestine, M.J.; Bullock, J.L.; Sandhaus, S.; Arca, J.F.; Cropek, D.M.; Ludvig, T.A.; Foster, S.R.; Clark, J.S.; Beckford, F.A.; et al. Synthesis, characterization, DNA binding, topoisomerase inhibition, and apoptosis induction studies of a novel cobalt(III) complex with a thiosemicarbazone ligand. J. Inorg. Biochem. 2020, 203, 110907. [Google Scholar] [CrossRef]
- Hałdys, K.; Goldeman, W.; Anger-Góra, N.; Rossowska, J.; Latajka, R. Monosubstituted Acetophenone Thiosemicarbazones as Potent Inhibitors of Tyrosinase: Synthesis, Inhibitory Studies, and Molecular Docking. Pharmaceuticals 2021, 14, 74. [Google Scholar] [CrossRef]
- Ferreira, F.B.; Pereira, T.M.; Souza, D.L.N.; Lopes, D.S.; Freitas, V.; Ávila, V.M.R.; Kümmerle, A.E.; Sant’Anna, C.M.R. Structure-Based Discovery of Thiosemicarbazone Metalloproteinase Inhibitors for Hemorrhage Treatment in Snakebites. ACS Med. Chem. Lett. 2017, 8, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, C.; Haribabu, J.; Jeyalakshmi, K.; Bhuvanesh, N.S.P.; Karvembu, R.; Emi, N.; Awale, S. Nickel(II) bis(isatin thiosemicarbazone) complexes induced apoptosis through mitochondrial signaling pathway and G0/G1 cell cycle arrest in IM-9 cells. J. Inorg. Biochem. 2018, 182, 208–221. [Google Scholar] [CrossRef]
- Finch, R.A.; Liu, M.; Grill, S.P.; Rose, W.C.; Loomis, R.; Vasquez, K.M.; Cheng, Y.; Sartorelli, A.C. Triapine (3-aminopyridine-2-carboxaldehyde- thiosemicarbazone): A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem. Pharmacol. 2000, 59, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Gojo, I.; Tidwell, M.L.; Greer, J.; Takebe, N.; Seiter, K.; Pochron, M.F.; Johnson, B.; Sznol, M.; Karp, J.E. Phase I and pharmacokinetic study of Triapine, a potent ribonucleotide reductase inhibitor, in adults with advanced hematologic malignancies. Leuk. Res. 2007, 31, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Nutting, C.M.; van Herpen, C.M.; Miah, A.B.; Bhide, S.A.; Machiels, J.P.; Buter, J.; Kelly, C.; de Raucourt, D.; Harrington, K.J. Phase II study of 3-AP Triapine in patients with recurrent or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2009, 20, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhou, J.; Xu, B.; Li, W.; Wang, Z. Ribonucleotide reductase subunit M2 as a novel target for clear-cell renal cell carcinoma. Onco Targets Ther. 2019, 12, 3267–3275. [Google Scholar] [CrossRef]
- Kunos, C.A.; Andrews, S.J.; Moore, K.N.; Chon, H.S.; Ivy, S.P. Randomized Phase II Trial of Triapine-Cisplatin-Radiotherapy for Locally Advanced Stage Uterine Cervix or Vaginal Cancers. Front. Oncol. 2019, 9, 1067. [Google Scholar] [CrossRef]
- Mortazavi, A.; Ling, Y.; Martin, L.K.; Wei, L.; Phelps, M.A.; Liu, Z.; Harper, E.J.; Ivy, S.P.; Wu, X.; Zhou, B.S.; et al. A phase I study of prolonged infusion of triapine in combination with fixed dose rate gemcitabine in patients with advanced solid tumors. Invest. New Drugs 2013, 31, 685–695. [Google Scholar] [CrossRef]
- Odenike, O.M.; Larson, R.A.; Gajria, D.; Dolan, M.E.; Delaney, S.M.; Karrison, T.G.; Ratain, M.J.; Stock, W. Phase I study of the ribonucleotide reductase inhibitor 3-aminopyridine-2-carboxaldehyde-thiosemicarbazone (3-AP) in combination with high dose cytarabine in patients with advanced myeloid leukemia. Invest. New Drugs 2008, 26, 233–239. [Google Scholar] [CrossRef]
- Schelman, W.R.; Morgan-Meadows, S.; Marnocha, R.; Lee, F.; Eickhoff, J.; Huang, W.; Pomplun, M.; Jiang, Z.; Alberti, D.; Kolesar, J.M.; et al. A phase I study of Triapine in combination with doxorubicin in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2009, 63, 1147–1156. [Google Scholar] [CrossRef]
- Choi, B.S.; Alberti, D.B.; Schelman, W.R.; Kolesar, J.M.; Thomas, J.P.; Marnocha, R.; Eickhoff, J.C.; Ivy, S.P.; Wilding, G.; Holen, K.D. The maximum tolerated dose and biologic effects of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) in combination with irinotecan for patients with refractory solid tumors. Cancer Chemother. Pharmacol. 2010, 66, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Kunos, C.A.; Sherertz, T.M. Long-Term Disease Control with Triapine-Based Radiochemotherapy for Patients with Stage IB2-IIIB Cervical Cancer. Front. Oncol. 2014, 4, 184. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.; Shimer, G.H., Jr.; Meehan, T. Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry 1987, 26, 6392–6396. [Google Scholar] [CrossRef]
- Kasparkova, J.; Vrana, O.; Farrell, N.; Brabec, V. Effect of the geometry of the central coordination sphere in antitumor trinuclear platinum complexes on DNA binding. J. Inorg. Biochem. 2004, 98, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Brabec, V.; Kleinwächter, V.; Butour, J.L.; Johnson, N.P. Biophysical studies of the modification of DNA by antitumour platinum coordination complexes. Biophys. Chem. 1990, 35, 129–141. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, Y.; Zheng, W.; Luo, Q.; Han, J.; Liu, J.; Zhao, Y.; Jia, F.; Wu, K.; Wang, F. Discovery of Cisplatin Binding to Thymine and Cytosine on a Single-Stranded Oligodeoxynucleotide by High Resolution FT-ICR Mass Spectrometry. Molecules 2019, 24, 1852. [Google Scholar] [CrossRef]
- Rahal, O.N.; Fatfat, M.; Hankache, C.; Osman, B.; Khalife, H.; Machaca, K.; Muhtasib, H.G. Chk1 and DNA-PK mediate TPEN-induced DNA damage in a ROS dependent manner in human colon cancer cells. Cancer Biol. Ther. 2016, 17, 1139–1148. [Google Scholar] [CrossRef]
- Belicchi Ferrari, M.; Bisceglie, F.; Pelosi, G.; Sassi, M.; Tarasconi, P.; Cornia, M.; Capacchi, S.; Albertini, R.; Pinelli, S. Synthesis, characterization and X-ray structures of new antiproliferative and proapoptotic natural aldehyde thiosemicarbazones and their nickel(II) and copper(II) complexes. J. Inorg. Biochem. 2002, 90, 113–126. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- Reichmann, M.E.; Rice, S.A.; Thomas, C.A.; Doty, P. A Further Examination of the Molecular Weight and Size of Desoxypentose Nucleic Acid. J. Am. Chem. Soc. 1954, 76, 3047–3053. [Google Scholar] [CrossRef]
- Wilson, W.D.; Tanious, F.A.; Fernandez-Saiz, M.; Rigl, C.T. Methods in Molecular Biology; Fox, K.R., Ed.; Humana: Clifton, NJ, USA, 1997; Volume 90. [Google Scholar]

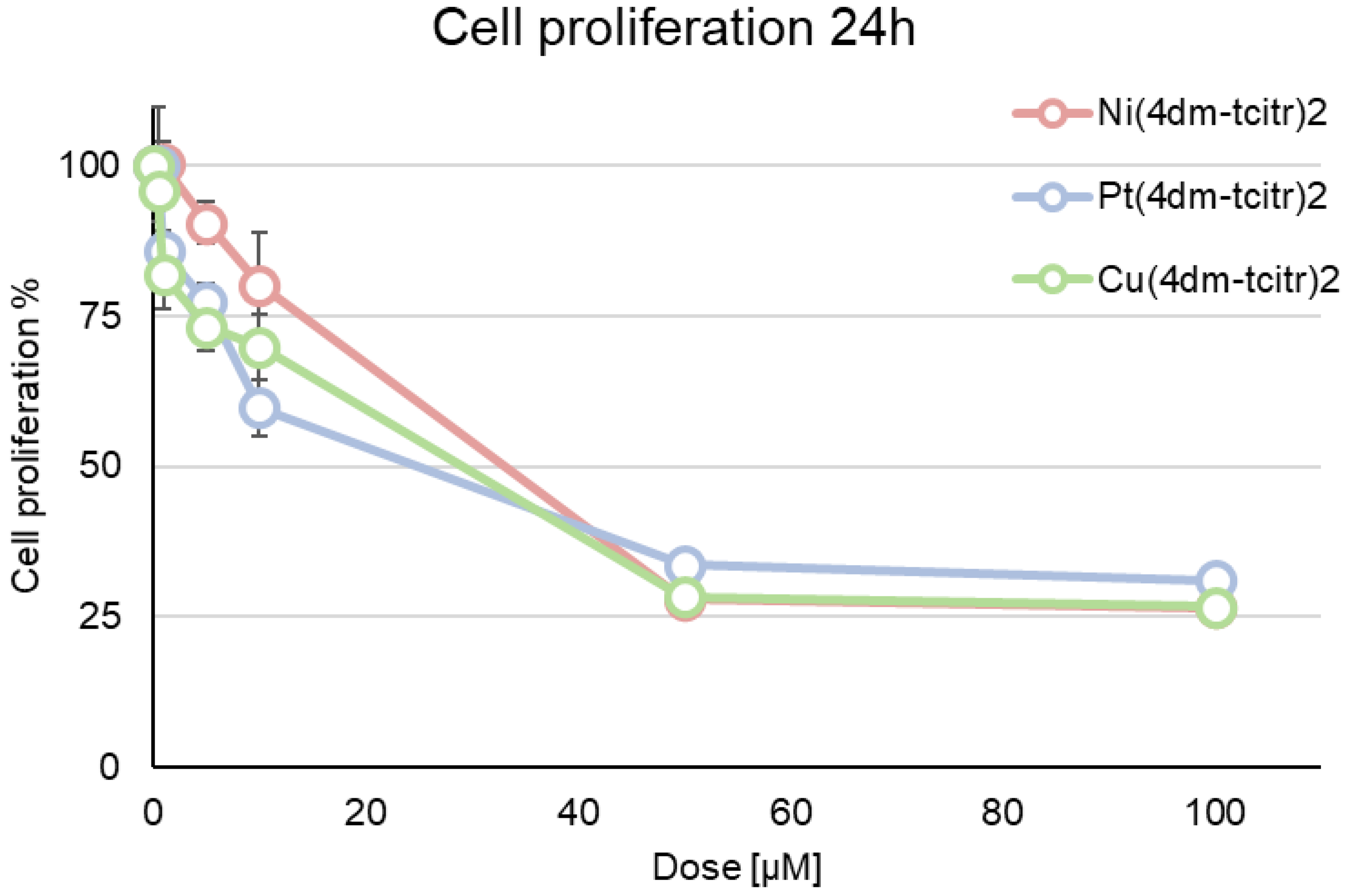
| GI50 Value (µM) ± sd | |
|---|---|
| [Ni(4dm-tcitr)2] | 33.5 ± 2.9 |
| [Pt(4dm-tcitr)2] | 25.3 ± 3.4 |
| [Cu(4dm-tcitr)2] | 30.2 ± 2.3 |
| [Ni(tcitr)2] | 10.0 ± 0.9 |
| [Pt(tcitr)2] | 7.0 ± 0.16 |
| [Cu(tcitr)2] | 33.0 ± 1.2 |
| DMSO | 0.5 µM | 1.0 µM | 5.0 µM | 10.0 µM | 50.0 µM | 100.0 µM | |
|---|---|---|---|---|---|---|---|
| [Ni(4dm-tcitr)2] | - | - | - | - | *** | *** | *** |
| [Pt(4dm-tcitr)2] | - | - | - | *** | *** | *** | *** |
| [Cu(4dm-tcitr)2] | - | - | ** | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalbano, S.; Buschini, A.; Pelosi, G.; Bisceglie, F. Antiproliferative Activity and DNA Interaction Studies of a Series of N4,N4-Dimethylated Thiosemicarbazone Derivatives. Molecules 2023, 28, 2778. https://doi.org/10.3390/molecules28062778
Montalbano S, Buschini A, Pelosi G, Bisceglie F. Antiproliferative Activity and DNA Interaction Studies of a Series of N4,N4-Dimethylated Thiosemicarbazone Derivatives. Molecules. 2023; 28(6):2778. https://doi.org/10.3390/molecules28062778
Chicago/Turabian StyleMontalbano, Serena, Annamaria Buschini, Giorgio Pelosi, and Franco Bisceglie. 2023. "Antiproliferative Activity and DNA Interaction Studies of a Series of N4,N4-Dimethylated Thiosemicarbazone Derivatives" Molecules 28, no. 6: 2778. https://doi.org/10.3390/molecules28062778
APA StyleMontalbano, S., Buschini, A., Pelosi, G., & Bisceglie, F. (2023). Antiproliferative Activity and DNA Interaction Studies of a Series of N4,N4-Dimethylated Thiosemicarbazone Derivatives. Molecules, 28(6), 2778. https://doi.org/10.3390/molecules28062778








