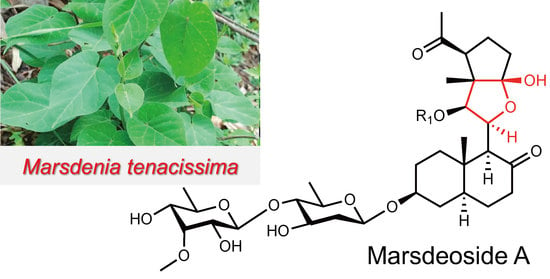
Nine New Pregnane Glycosides from the Cultivated Medicinal Plant Marsdenia tenacissima
Abstract
1. Introduction
2. Results and Discussion
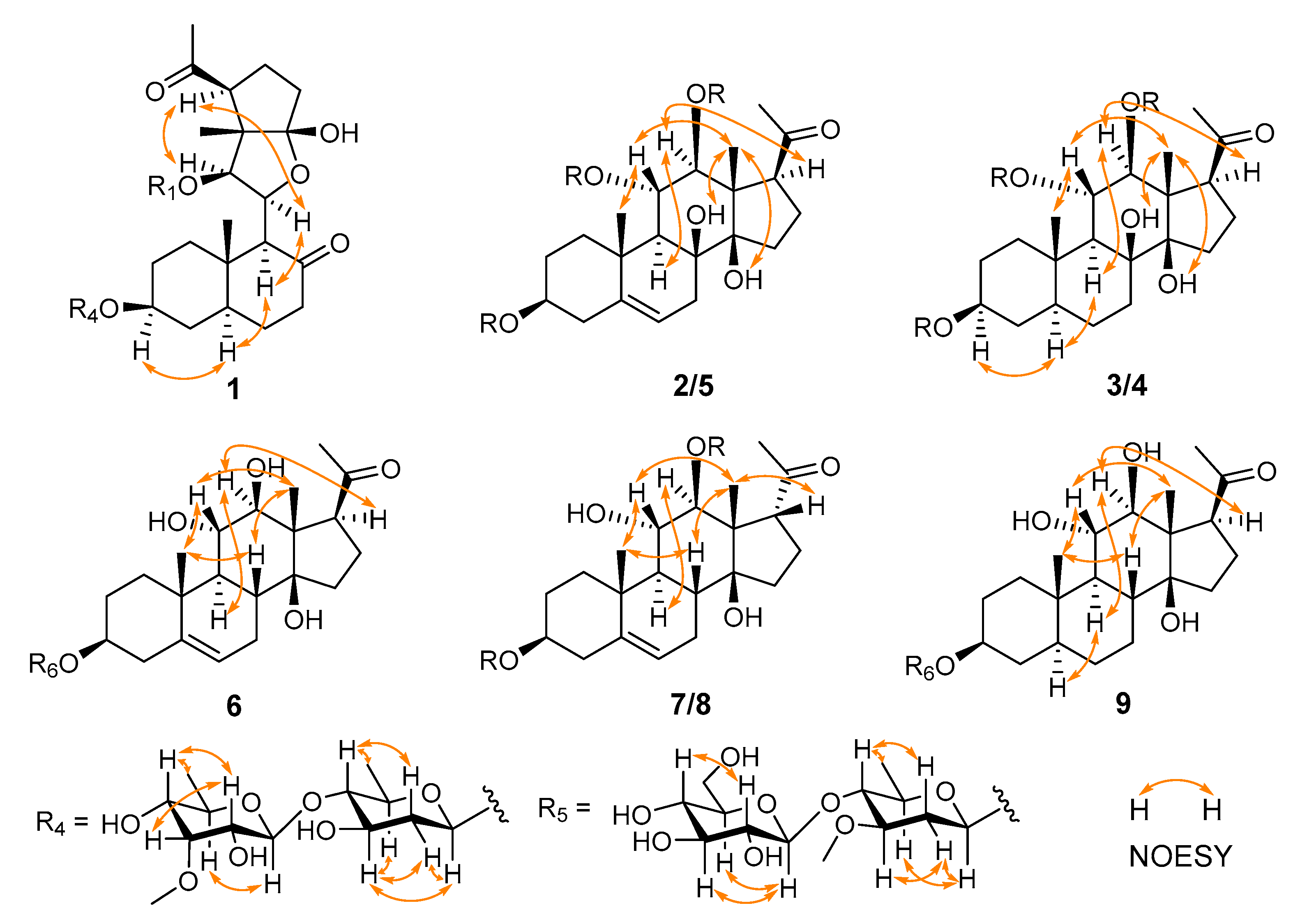
2.1. Structural Elucidation of Compounds 1–9
2.2. Inhibitory Activities of Compounds on NO Production by Lipopolysaccharide-Activated Macrophage (RAW264.7)
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Marsdeoside A (1)
3.3.2. Marsdeoside B (2)
3.3.3. Marsdeoside C (3)
3.3.4. Marsdeoside D (4)
3.3.5. Marsdeoside E (5)
3.3.6. Marsdeoside F (6)
3.3.7. Marsdeoside G (7)
3.3.8. Marsdeoside H (8)
3.3.9. Marsdeoside I (9)
3.4. Acidic Hydrolysis of Compounds 1–9
3.5. Anti-Inflammatory Activity Assays
3.6. Determination of the Absolute Configuration of the Monosaccharides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Flora of China. Flora of China Editorial Committee; Science Press: Beijing, China, 1977; pp. 463–465. [Google Scholar]
- State Pharmacopoeia Commission. Chinese Pharmacopoeia; China Medical Science and Technology Press: Beijing, China, 2020; p. 309. [Google Scholar]
- Zhou, X.Q.; Chang, Y.Z.; Shen, C.Y.; Han, J.; Chang, R.A. Xiaoaiping injection combined with chemotherapy for advanced gastric cancer: An updated systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 1023314. [Google Scholar] [CrossRef] [PubMed]
- Na, Z.; Gongpan, P.C.; Fan, Q.F. Polyoxypregnane glycosides from root of Marsdenia tenacissima and inhibited nitric oxide levels in LPS stimulated RAW 264.7 cells. Molecules 2023, 282, 886. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; To, K.W.; Ma, L.; Yin, C.; Tang, C.; Chai, S.; Ke, C.Q.; Lin, G.; Yang, Y. Polyoxypregnane steroids with an open-chain sugar moiety from Marsdenia tenacissima and their chemoresistance reversal activity. Phytochemistry 2016, 126, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Zhao, M.B.; Zhuang, G.B.; Li, P.P. Marsdenia tenacissima extract restored gefitinib sensitivity in resistant non-small cell lung cancer cells. Lung Cancer. 2012, 75, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, J.; Zhu, Z.; Zhang, X. Marsdenia tenacissima: A review of traditional uses, phytochemistry and pharmacology. Am. J. Chin. Med. 2018, 46, 1449–1480. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Okabe, H.; Yamauchi, T.; Honda, K.; Hayashi, N. Pregnane glycosides from Marsdenia tomentosa. Chem. Pharm. Bull. 1999, 47, 869–875. [Google Scholar] [CrossRef]
- Luo, S.Q.; Lin, L.Z.; Cordell, G.A.; Xue, L.; Johnson, M.E. Polyoxypregnanes from Marsdenia tenacissima. Phytochemistry 1993, 34, 1615–1620. [Google Scholar] [CrossRef]
- Xia, Z.H.; Mao, S.L.; Lao, A.N.; Uzawa, J.; Yoshida, S.; Fujimoto, Y. Five new pregnane glycosides from the stems of Marsdenia tenacissima. J. Asian Nat. Prod. Res. 2011, 13, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Peng, S.L.; Ding, L.S. Further polyoxypregnane glycosides from Marsdenia tenacissima. J. Asian Nat. Prod. Res. 2010, 12, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.H.; Xing, W.X.; Mao, S.L.; Lao, A.N.; Uzawa, J.; Yoshida, S.; Fujimoto, Y. Pregnane glycosides from the stems of Marsdenia tenacissima. J. Asian Nat. Prod. Res. 2004, 6, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, D.; Li, X.Z.; Feng, Y.; Wen, X.Y.; Wang, Y.J.; Renbo, A.; Chen, G.; Li, N. Pregnane glycosides from Adonis amurensis and their bioactivity. Phytochemistry 2022, 194, 113046. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.R.; Peng, X.R.; Lei, S.Y.; Zhou, L.; Qiu, M.H. C21 steroidal glycosides with cytotoxic activities from Cynanchum otophyllum. Bioorg. Med. Chem. Lett. 2018, 96, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Kubo, S.; Masatani, D.; Matsuo, Y.; Sakagami, H.; Mimaki, Y. Aestivalosides A–L, twelve pregnane glycosides from the seeds of Adonis aestivalis. Phytochemistry 2019, 150, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Gag, A.; Joa, A.; Oeo, B.; Apc, D.; Mn, C.; Aos, B.; Asb, E.; Mk, C. Iloneoside: A cytotoxic ditigloylated pregnane glycoside from the leaves of Gongronema latifolium. Benth. Nat. Prod. Res. 2018, 32, 1478–6427. [Google Scholar] [CrossRef]
- Aquino, R.; Peluso, G.; Tommasi, N.D.; Francesco, D.S.; Pizza, C. New polyoxypregnane ester derivatives from Leptadenia hastata. J. Nat. Prod. 1996, 59, 555. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Liu, X.; Dai, R.; Meng, S.H.; Chen, W.; Deng, Y. Six new polyhydroxy steroidal glycosides from Dregea sinensis Hemsl. Phytochem. Lett. 2015, 11, 209–214. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Snene, A.; Mokni, R.E.; Jmii, H.; Jlassi, I.; Jadane, H.; Falconieri, D.; Piras, A.; Dhaouadi, H.; Porcedda, S.; Hammami, S. In vitro antimicrobial, antioxidant and antiviral activities of the essential oil and various extracts of wild (Daucus virgatus (Poir.) Maire) from Tunisia. Ind. Crops. Prod. 2017, 109, 109–115. [Google Scholar] [CrossRef]


| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.51, overlapped 1.69, overlapped | 2.47, dt (13.5, 3.5) 1.43, td (13.5, 3.5) | 2.27, dt (13.5, 3.8) 1.36, td (13.5, 3.8) | 2.24, dt (13.2, 4.0) 1.29, td (13.2, 4.3) | 2.62, overlapped 2.63, overlapped | 2.60, br. d (12.0) 2.46, overlapped | 2.61, br. d (12.0) 2.47, overlapped | 2.58, overlapped 2.40, br. t (12.0) | 3.14, br. d (14.0) 1.46, br. t (14.0) |
| 2 | 1.99, m 1.58, m | 2.12, overlapped 1.92, overlapped | 1.86, overlapped 1.65, ddd (13.5, 13.5, 13.5) | 2.03, m 1.77, overlapped | 2.13, m 1.99, overlapped | 2.11, m 1.86, m | 2.14, m 1.85, overlapped | 2.17, overlapped 1.83, overlapped | 2.10, overlapped 1.74, overlapped |
| 3 | 3.80, m | 3.89, m | 3.91, m | 3.94, m | 3.93, m | 3.86, m | 3.87, m | 3.83, m | 3.91, m |
| 4 | 1.73, m 1.22, br. t (12.2) | 2.63, dd (13.0, 4.5) 2.56, t (13.0) | 1.84, overlapped 1.53, overlapped | 1.83, br. d (12.0) 1.53, overlapped | 3.35, br. d (13.6) 1.44, td (13.6, 3.8) | 3.25, br. d (14.3) 1.43, td (14.8, 3.0) | 3.26, br. d (13.9) 1.48, td (14.3, 3.6) | 2.16, overlapped 1.41, td (12.6, 3.6) | 1.77, overlapped 1.39, overlapped |
| 5 | 1.57, m | 1.23, m | 1.19, m | 1.11, m | |||||
| 6 | 1.46, m 1.41, m | 5.44, d (5.5) | 1.96, ddd (13.0, 13.0, 13.0) 1.19, m | 1.93, m 1.16, overlapped | 5.41, d (5.1) | 5.55, overlapped | 5.57, d (5.6) | 5.55, d (5.4) | 2.50, br .d (12.1) 1.16, overlapped |
| 7 | 2.22, m 2.05, br. d (12.0) | 2.66, dd (18.0, 5.5) 2.39, br. d (18.0) | 2.41, overlapped 1.56, overlapped | 2.37, m 1.52, overlapped | 2.62, m 2.29, m | 2.67, br. d (17.0) 1.97, m | 2.66, overlapped 1.98, overlapped | 2.70, overlapped 2.07, overlapped | 1.28, overlapped 1.21, overlapped |
| 8 | 2.03, overlapped | 2.14, overlapped | 2.21, td (11.7, 4.7) | 1.92, overlapped | |||||
| 9 | 3.29, d (10.0) | 2.27, d (10.5) | 2.11, d (11.0) | 1.97, d (11.0) | 1.99, overlapped | 1.61, t (11.0) | 1.73, t (9.7) | 1.79, overlapped | 1.31, overlapped |
| 11 | 5.06, overlapped | 6.47, br. t (10.5) | 6.82, t (10.6) | 6.63, t (10.7) | 4.89, td (10.7, 6.4) | 4.10, overlapped | 4.19, overlapped | 5.68, t (9.9) | 3.96, t (9.7) |
| 12 | 6.59, d (4.7) | 5.50, d (10.5) | 5.76, d (10.6) | 5.62, d (10.0) | 5.49, d (9.7) | 3.56, overlapped | 5.32, d (9.7) | 3.90, d (9.6) | 3.48, d (9.5) |
| 15 | 2.21, m, 2H | 2.29, overlapped2.09, m | 2.47, overlapped2.17, m | 2.40, overlapped 2.13, m | 2.19, m 2.04, m | 2.06, overlapped 1.94, overlapped | 2.09, overlapped 1.86, overlapped | 2.11, overlapped 1.95, overlapped | 2.07, overlapped 1.96, overlapped |
| 16 | 2.47, m 1.80, m | 2.33, m 2.07, m | 2.33, m 2.17, m | 2.31, m 2.14, m | 2.28, m 2.05, m | 2.22, m 1.86, overlapped | 2.68, overlapped 1.87, overlapped | 2.71, overlapped 1.99, overlapped | 2.26, m 1.86, m |
| 17 | 3.25, br. t (8.0) | 3.22, dd (9.0, 5.0) | 3.37, dd (9.8, 5.7) | 3.36, dd (9.3, 5.5) | 2.21, overlapped | 3.88, overlapped | 3.57, overlapped | 3.49 br. t (8.8) | 3.83, dd (8.8, 5.2) |
| 18 | 1.19, s, 3H | 1.68, s, 3H | 1.79, s, 3H | 1.74, s, 3H | 1.64, s, 3H | 1.35, s | 1.74, s, 3H | 1.79, s, 3H | 1.36, s, 3H |
| 19 | 0.89, s, 3H | 1.72, s, 3H | 1.58, s, 3H | 1.55, s, 3H | 1.82, s, 3H | 1.39, s | 1.33, s, 3H | 1.33, s, 3H | 1.01, s, 3H |
| 21 | 2.56, s, 3H | 2.20, s, 3H | 2.07, s, 3H | 2.06, s, 3H | 2.21, s, 3H | 2.30, s | 2.28, s, 3H | 2.37, s, 3H | 2.31, s, 3H |
| 8-OH | 5.30, s | 4.90, s | 4.77, s | ||||||
| 11-OH | 6.49, d (6.4) | ||||||||
| 14-OH | 8.16, s | 5.82, s | 6.00, s | 5.96, s | 5.60, s |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Substituents | 11-Benzoyl | 11-Tigloyl | 11-Benzoyl | 11-Tigloyl | 11-Acetyl | ||||
| 1′ | |||||||||
| 2′ | 1.96, s, 3H | ||||||||
| 3′ | 8.27, d (8.4) | 7.01, q (7.2) | 8.05, d (8.4) | 6.84, q (7.1) | |||||
| 4′ | 7.41, t (8.4) | 1.61, d (7.2), 3H | 7.23, t (8.4) | 1.39, d (7.1) | |||||
| 5′ | 7.55, t (8.4) | 1.84, s, 3H | 7.31, t (8.4) | 1.55, s, 3H | |||||
| 6′ | 7.41, t (8.4) | 7.23, t (8.4) | |||||||
| 7′ | 8.27, d (8.4) | 8.05, d (8.4) | |||||||
| 12-Tigloyl | 12-Benzoyl | 12-Benzoyl | 12-Tigloyl | 12-Acetyl | |||||
| 1′′ | |||||||||
| 2′′ | 1.98, s, 3H | ||||||||
| 3′′ | 7.10, q (7.1) | 8.10, d (8.4) | 8.28, d (8.4) | 7.03, q (7.1) | |||||
| 4′′ | 1.64, d (7.1), 3H | 7.25, t (8.4) | 7.42, t (8.4) | 1.56, d (7.1) | |||||
| 5′′ | 1.91, s, 3H | 7.36 t (8.4) | 7.52, t (8.4) | 1.85, s, 3H | |||||
| 6′′ | 7.25, t (8.4) | 7.42, t (8.4) | |||||||
| 7′′ | 8.10, d (8.4) | 8.28, d (84) | |||||||
| Sugar moieties | 3-O-deMe-D-Ole | D-Ole | D-Ole | D-Ole | 3-O-deMe-D-Ole | D-Ole | D-Ole | 3-O-deMe-D-Ole | D-Ole |
| 1′′′ | 4.87, br. d (9.3) | 4.81, br. d (9.9) | 4.84, d (9.6) | 4.87, d (9.5) | 4.93, d (9.7) | 4.81, d (9.5) | 4.80, d (9.7) | 4.90, d (9.6) | 4.84, d (9.8) |
| 2′′′ | 2.57, overlapped 2.03, overlapped | 2.42, dd (12.8, 5.4) 1.75, overlapped | 2.42, overlapped 1.75, overlapped | 2.45, dd (12.0, 4.8) 1.77, overlapped | 2.55, dd (12.2, 5.3) 2.02, overlapped | 2.46, overlapped 1.79, overlapped | 2.45, overlapped 1.80, overlapped | 2.58, overlapped 2.04, overlapped | 2.48, br. d (12.5) 1.82, overlapped |
| 3′′′ | 4.07, m | 3.65, m | 3.67, m | 3.68, m | 4.03, ddd (13.6, 8.6, 5.3) | 3.64, overlapped | 3.64, overlapped | 4.04, ddd (13.4, 8.5, 5.2) | 3.66, overlapped |
| 4′′′ | 3.42, t (9.0) | 3.74, t (9.0) | 3.74, t (9.0) | 3.76, t (8.9) | 3.39, t (9.6) | 3.63, overlapped | 3.65, overlapped | 3.40, t (9.0) | 3.64, overlapped |
| 5′′′ | 3.68, dq (9.0, 6.5) | 3.68, dq (15.0, 6.0) | 3.73, overlapped | 3.75, m | 3.64, overlapped | 3.61, overlapped | 3.58, overlapped | 3.66, overlapped | 3.66, overlapped |
| 6′′′ | 1.67, d (6.1), 3H | 1.73, d (6.5), 3H | 1.75, d (6.0), 3H | 1.78, d (5.9), 3H | 1.60, d (6.0), 3H | 1.66, d (5.8), 3H | 1.66, d (6.0), 3H | 1.61, d (6.3), 3H | 1.69, d (4.3), 3H |
| -OMe | 3.52, s, 3H | 3.51, s, 3H | 3.52, s, 3H | 3.53, s, 3H | 3.54, s, 3H | 3.56, s, 3H | |||
| Sugar moieties | 6-deoxy-3-O-deMe-D-Allo | D-Glc | D-Glc | D-Glc | 6-deoxy-3-O-deMe-D-Allo | 6-deoxy-3-O-deMe-D-Allo | 6-deoxy-3-O-deMe-D-Allo | 6-deoxy-3-O-deMe-D-Allo | 6-deoxy-3-O-deMe-D-Allo |
| 1′′′′ | 5.18, d (8.3) | 5.15, d (7.8) | 5.15, d (7.8) | 5.16, d (7.8) | 5.15, d (8.0) | 5.31, d (8.2) | 5.33, d (8.1) | 5.15, d (8.0) | 5.34, d (8.2) |
| 2′′′′ | 3.98, dd (8.3, 3.0) | 4.03, br. t (7.0) | 4.03, br. t (8.0) | 4.04, br. t (7.7) | 3.96, overlapped | 3.89, m | 3.91, m | 3.97, dd (8.1, 3.0) | 3.93, overlapped |
| 3′′′′ | 4.10, t (3.0) | 4.24, overlapped | 4.24, overlapped | 4.25, overlapped | 4.09, t (3.0) | 4.10, overlapped | 4.10, t (2.7) | 4.09, t (3.0) | 4.10, m |
| 4′′′′ | 3.64, m | 4.24, overlapped | 4.24, overlapped | 4.24, overlapped | 3.63, overlapped | 3.63, overlapped | 3.65, overlapped | 3.64, overlapped | 3.65, overlapped |
| 5′′′′ | 4.24, dq (10.0, 6.5) | 3.96, m | 3.95, m | 3.96, m | 4.23, dq (9.7, 6.3) | 4.17, m | 4.19, overlapped | 4.23, m | 4.18, overlapped |
| 6′′′′ | 1.49, d (6.5), 3H | 4.54, br. d (11.0) 4.38, dd (11.0, 6.0) | 4.54, d (11.7) 4.37, dd (11.4, 5.4) | 4.54, d (11.4) 4.38, dd (11.4, 5.5) | 1.49, d (6.0), 3H | 1.55, d (6.2), 3H | 1.56, d (6.2), 3H | 1.49, d (6.0), 3H | 1.56, d (6.3), 3H |
| -OMe | 3.84, s | 3.52, s, 3H | 3.84, s, 3H | 3.84, s, 3H | 3.85, s, 3H | 3.84, s, 3H | 3.85, s, 3H |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 38.5, CH2 | 40.3, CH2 | 39.6, CH2 | 39.4, CH2 | 39.7, CH2 | 40.1, CH2 | 40.2, CH2 | 40.2, CH2 | 40.0, CH2 |
| 2 | 29.4, CH2 | 30.0, CH2 | 29.7, CH2 | 29.8, CH2 | 30.0, CH2 | 30.8, CH2 | 30.8, CH2 | 30.9, CH2 | 30.8, CH2 |
| 3 | 76.2, CH | 77.7, CH | 76.0, CH | 76.2, CH | 78.1, CH | 78.1, CH2 | 78.1, CH2 | 77.8, CH | 77.0, CH |
| 4 | 34.1, CH2 | 39.7, CH2 | 35.0, CH2 | 35.0, CH2 | 40.8, CH2 | 40.0, CH2 | 40.1, CH2 | 39.1, CH2 | 35.9, CH2 |
| 5 | 43.7, CH | 139.6, C | 45.6, CH | 45.6, CH | 140.5, C | 140.8, C | 141.2, C | 140.5, C | 45.6, CH |
| 6 | 30.6, CH2 | 118.8, CH | 25.3, CH2 | 25.3, CH2 | 118.5, CH | 122.6, CH | 122.5, CH | 122.9, CH | 28.9, CH2 |
| 7 | 42.5, CH2 | 35.5, CH2 | 35.6, CH2 | 35.6, CH2 | 35.6, CH2 | 28.5, CH2 | 27.9, CH2 | 28.2, CH2 | 30.1, CH2 |
| 8 | 211.5, C | 76.0, C | 78.3, C | 78.3, C | 75.9, C | 37.6, CH | 38.2, CH | 38.0, CH | 40.4, CH |
| 9 | 62.5, CH | 49.2, CH | 51.4, CH | 51.3, CH | 50.4, CH | 49.9, CH | 50.4, CH | 48.5, CH | 52.5, CH |
| 10 | 42.7, C | 39.2, C | 38.4, C | 38.4, C | 39.5, C | 39.6, C | 40.0, C | 39.7, C | 38.3, C |
| 11 | 76.5, CH | 71.5, CH | 72.0, CH | 71.2, CH | 68.5, CH | 72.0, CH | 71.0, CH | 75.5, CH | 72.3, CH |
| 12 | 81.0, CH | 78.3, CH | 79.6, CH | 79.7, CH | 81.1, CH | 78.5, CH | 76.2, CH | 72.4, CH | 79.4, CH |
| 13 | 55.8, C | 55.5, C | 55.7, C | 55.6, C | 55.8, C | 55.9, C | 55.3, C | 57.2, C | 56.0, C |
| 14 | 115.7, C | 85.5, C | 85.5, C | 85.5, C | 85.6, C | 85.1, C | 85.6, C | 85.5, C | 84.9 C |
| 15 | 36.8, CH2 | 36.6, CH2 | 36.2, CH2 | 36.1, CH2 | 36.6, CH2 | 35.3, CH2 | 31.9, CH2 | 32.4, CH2 | 34.8, CH2 |
| 16 | 23.3, CH2 | 24.2, CH2 | 24.7, CH2 | 24.7, CH2 | 24.3, CH2 | 24.6, CH2 | 21.2, CH2 | 21.5, CH2 | 24.9, CH2 |
| 17 | 58.1, CH | 59.2, CH | 59.4, CH | 59.4, CH | 59.6, CH | 58.9, CH | 61.4, CH | 61.9, CH | 59.1, CH |
| 18 | 12.1, CH3 | 13.5, CH3 | 14.1, CH3 | 14.0, CH3 | 13.8, CH3 | 11.2, CH3 | 15.8, CH3 | 4.9, CH3 | 11.4, CH3 |
| 19 | 12.5, CH3 | 18.1, CH3 | 13.4, CH3 | 13.3, CH3 | 17.4, CH3 | 19.2, CH3 | 19.2, CH3 | 19.8, CH3 | 12.7, CH3 |
| 20 | 207.7, C | 213.1, C | 213.6, C | 213.7, C | 213.9, C | 217.0, C | 208.9 C | 209.9, C | 216.7, C |
| 21 | 31.1, CH3 | 31.2, CH3 | 31.7, CH3 | 31.6, CH3 | 31.4, CH3 | 32.8, CH3 | 31.7, CH3 | 32.0, CH3 | 32.8, CH3 |
| 11-Bz | 11-Tig | 11-Bz | 11-Tig | 11-Ac | |||||
| 1′ | 165.9, C | 166.8, C | 165.9, C | 166.9, C | 170.9, C | ||||
| 2′ | 130.2, C | 129.1, C | 130.5, C | 128.9, C | 22.4, CH3 | ||||
| 3′(-7′) | 129.9, CH | 138.3, CH | 129.8, CH | 138.5, CH | |||||
| 4′(-6′) | 128.9, CH | 14.2, CH3 | 128.4, CH | 14.0, CH3 | |||||
| 5′ | 133.5, CH | 12.0, CH3 | 133.0, CH | 11.6, CH3 | |||||
| 12-Tig | 12-Bz | 12-Bz | 12-Tig | 12-Ac | |||||
| 1′′ | 167.9, C | 166.8, C | 166.8, C | 168.3, C | 170.4, C | ||||
| 2′′ | 128.4, C | 129.9, C | 130.3, C | 129.2, C | 21.3, CH3 | ||||
| 3′′(-7′′) | 138.5, CH | 129.8, CH | 130.0, CH | 137.2, CH | |||||
| 4′′(-6′′) | 14.2, CH3 | 128.6, CH | 128.8, CH | 14.0, CH3 | |||||
| 5′′ | 12.0, CH3 | 133.3, CH | 133.5, CH | 12.2, CH3 | |||||
| 3-O-deMe-D-Ole | D-Ole | D-Ole | D-Ole | 3-O-deMe-D-Ole | D-Ole | D-Ole | 3-O-deMe-D-Ole | D-Ole | |
| 1′′′ | 97.6, CH | 97.9, CH | 97.3, CH | 97.4, CH | 97.8, CH | 98.1, CH | 98.2, CH | 98.5, CH | 97.8, CH |
| 2′′′ | 40.0, CH2 | 37.5, CH2 | 37.5, CH2 | 37.5, CH2 | 40.1, CH2 | 38.1, CH2 | 38.2, CH2 | 40.5, CH2 | 38.2, CH2 |
| 3′′′ | 70.1, CH | 79.5, CH | 79.5, CH | 79.5, CH | 70.1, CH | 79.9, CH | 79.9, CH | 70.6, CH | 80.0, CH |
| 4′′′ | 88.6, CH | 83.4, CH | 83.4, CH | 83.4, CH | 88.6, CH | 83.6, CH | 83.6, CH | 89.0, CH | 83.7, CH |
| 5′′′ | 71.2, CH | 71.9, CH | 71.9, CH | 71.9, CH | 71.1, CH | 72.2, CH | 72.3, CH | 71.6, CH | 72.4, CH |
| 6′′′ | 18.3, CH3 | 18.9, CH3 | 18.9, CH3 | 18.9, CH | 18.2, CH3 | 19.4, CH3 | 19.4, CH3 | 18.7, CH3 | 19.5,CH3 |
| -OMe | 57.0, CH3 | 56.9, CH3 | 57.0, CH3 | 57.5, CH3 | 57.5, CH3 | 57.5, CH3 | |||
| 6-deoxy-3-O-deMe-D-Allo | D-Glc | D-Glc | D-Glc | 6-deoxy-3-O-deMe-D-Allo | 6-deoxy-3-O-deMe-D-Allo | 6-deoxy-3-O-deMe-D-Allo | 6-deoxy-3-O-deMe-D-Allo | 6-deoxy-3-O-deMe-D-Allo | |
| 1′′′′ | 103.2, CH | 104.4, CH | 104.4, CH | 104.4, CH | 103.2, CH | 102.4, CH | 102.5, CH | 103.7, CH | 102.4, CH |
| 2′′′′ | 72.5, CH | 75.6, CH | 75.6, CH | 75.6, CH | 72.5, CH | 73.5, CH | 73.6, CH | 73.0, CH | 73.6, CH |
| 3′′′′ | 83.8, CH | 78.6, CH | 78.6, CH | 78.6, CH | 83.8, CH | 84.3, CH | 84.4, CH | 84.3, CH | 84.4, CH |
| 4′′′′ | 74.1, CH | 71.8, CH | 71.8, CH | 71.8, CH | 74.0, CH | 74.9, CH | 74.9, CH | 74.5, CH | 74.9, CH |
| 5′′′′ | 71.1, CH | 78.0, CH | 78.0, CH | 78.0, CH | 71.1, CH | 71.2, CH | 71.3, CH | 71.5, CH | 71.3, CH |
| 6′′′′ | 18.0, CH3 | 62.9, CH2 | 62.9, CH2 | 62.9, CH2 | 18.0, CH3 | 18.9, CH3 | 19.0, CH3 | 18.5, CH3 | 19.0, CH3 |
| -OMe | 62.1, CH3 | 62.0, CH3 | 62.4, CH3 | 62.4, CH3 | 62.5, CH3 | 62.4, CH3 |
| Compounds | IC50 (μM) |
|---|---|
| L-NMMA | 39.30 ± 1.23 |
| 1 | 37.46 ± 1.91 |
| 8 | 38.80 ± 0.76 |
| 9 | 42.78 ± 1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.-Q.; Tong, S.-Y.; Peng, X.-R.; Zhao, Y.-Q.; Li, Z.-H.; Chen, H.-P.; Liu, J.-K. Nine New Pregnane Glycosides from the Cultivated Medicinal Plant Marsdenia tenacissima. Molecules 2023, 28, 2705. https://doi.org/10.3390/molecules28062705
Meng Q-Q, Tong S-Y, Peng X-R, Zhao Y-Q, Li Z-H, Chen H-P, Liu J-K. Nine New Pregnane Glycosides from the Cultivated Medicinal Plant Marsdenia tenacissima. Molecules. 2023; 28(6):2705. https://doi.org/10.3390/molecules28062705
Chicago/Turabian StyleMeng, Qian-Qian, Shun-Yao Tong, Xing-Rong Peng, Yu-Qing Zhao, Zheng-Hui Li, He-Ping Chen, and Ji-Kai Liu. 2023. "Nine New Pregnane Glycosides from the Cultivated Medicinal Plant Marsdenia tenacissima" Molecules 28, no. 6: 2705. https://doi.org/10.3390/molecules28062705
APA StyleMeng, Q.-Q., Tong, S.-Y., Peng, X.-R., Zhao, Y.-Q., Li, Z.-H., Chen, H.-P., & Liu, J.-K. (2023). Nine New Pregnane Glycosides from the Cultivated Medicinal Plant Marsdenia tenacissima. Molecules, 28(6), 2705. https://doi.org/10.3390/molecules28062705