The Effect of Sodium Bentonite in the Thermo-Catalytic Reduction of Viscosity of Heavy Oils
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Determination of Physical Parameters
2.3. Initial Crude Oil Reaction, Product Separation, and Analysis
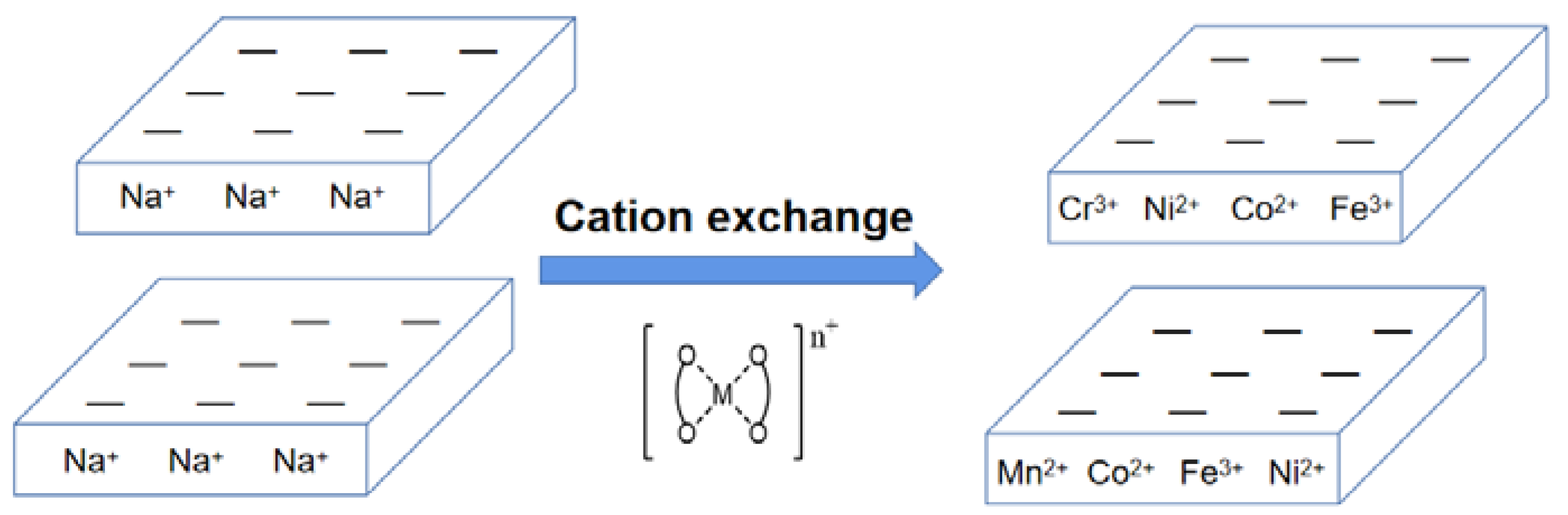
2.4. Catalyst Preparation
2.5. Viscosity Evaluation
2.6. Differential Scanning Calorimetry Analysis (DSC)
2.7. BET-N2 Adsorption
2.8. Gas Chromatography (GC)
2.9. TGA Analysis
2.10. GC-MS
2.11. Elemental Analysis and SARA Analysis
2.12. Scanning Electron Microscopy (SEM)
3. Results and Discussion
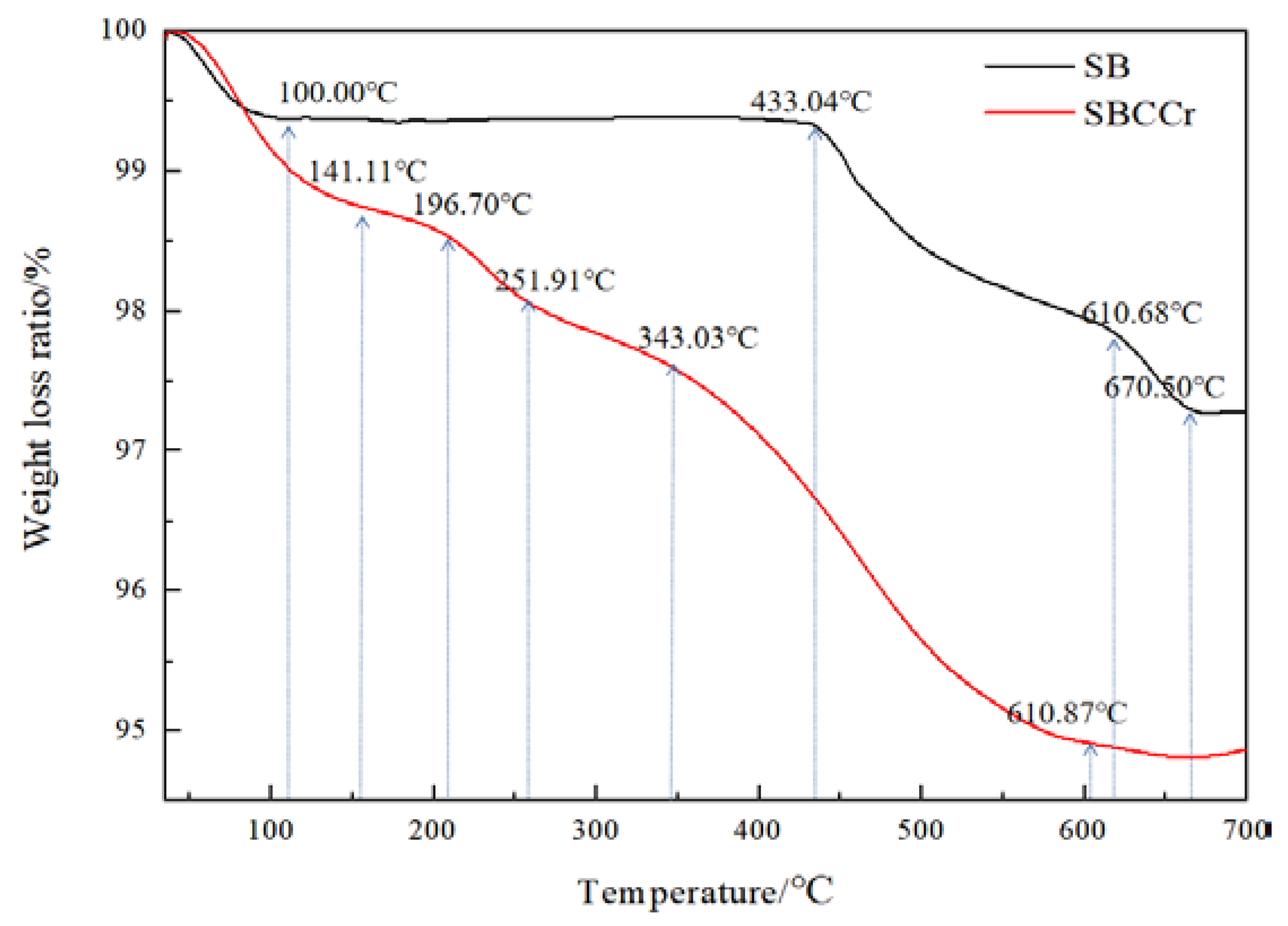
3.1. Thermogravimetric Analysis of the Catalyst
3.2. Adsorption-Desorption Curves
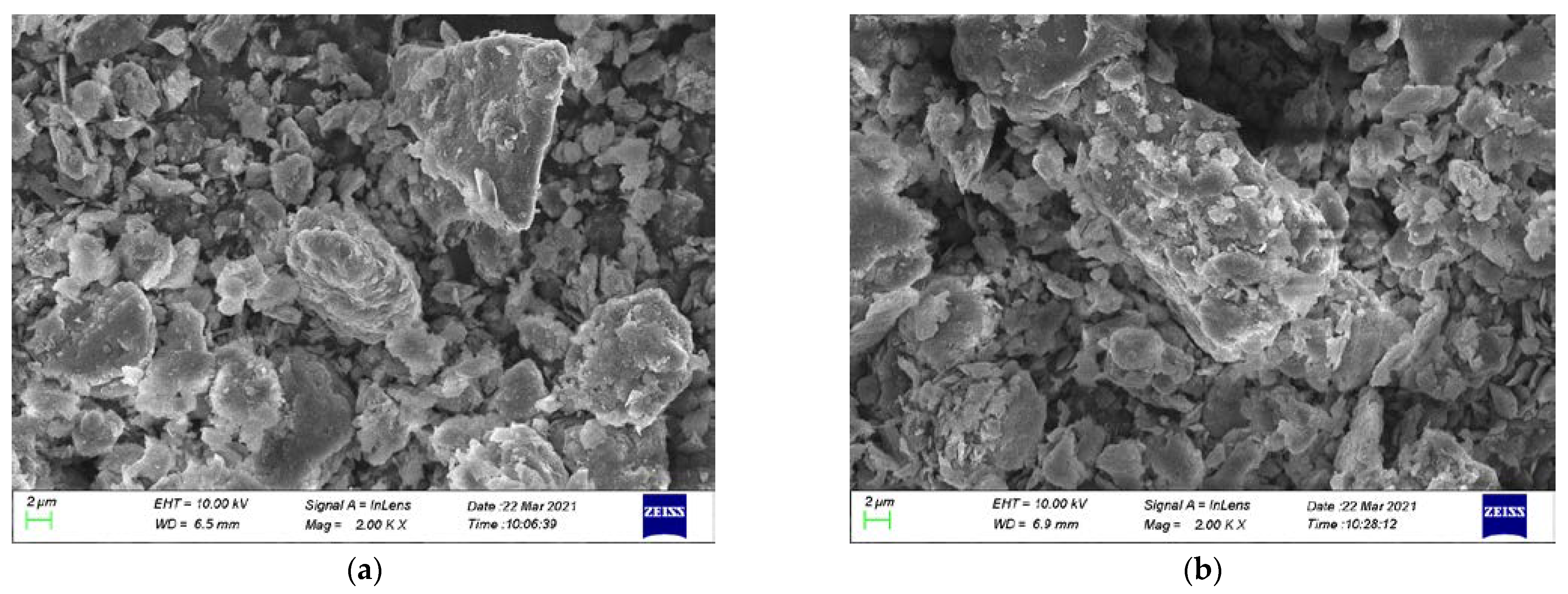
3.3. SEM Analysis
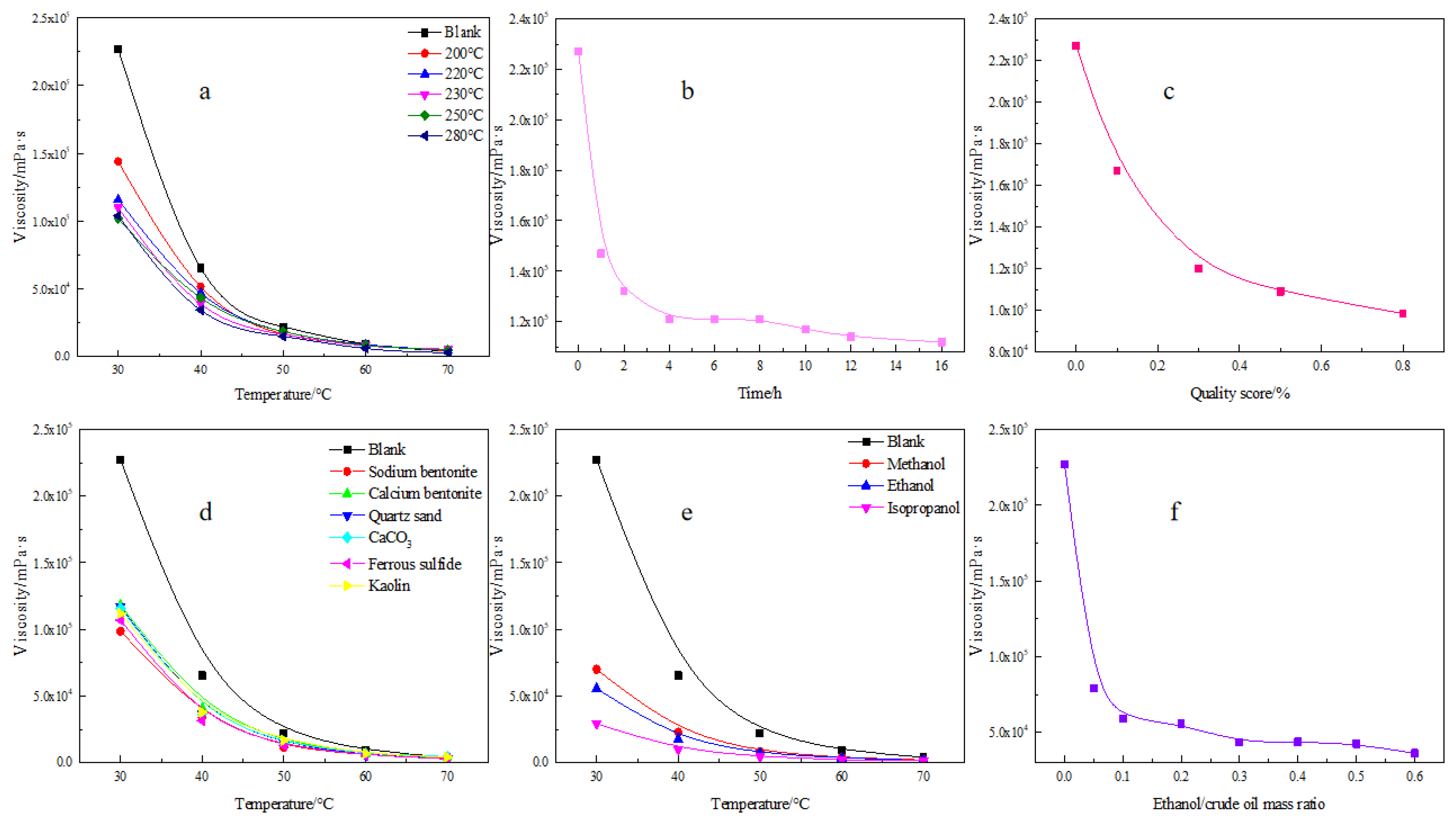
3.4. Optimal Physical and Chemical Conditions
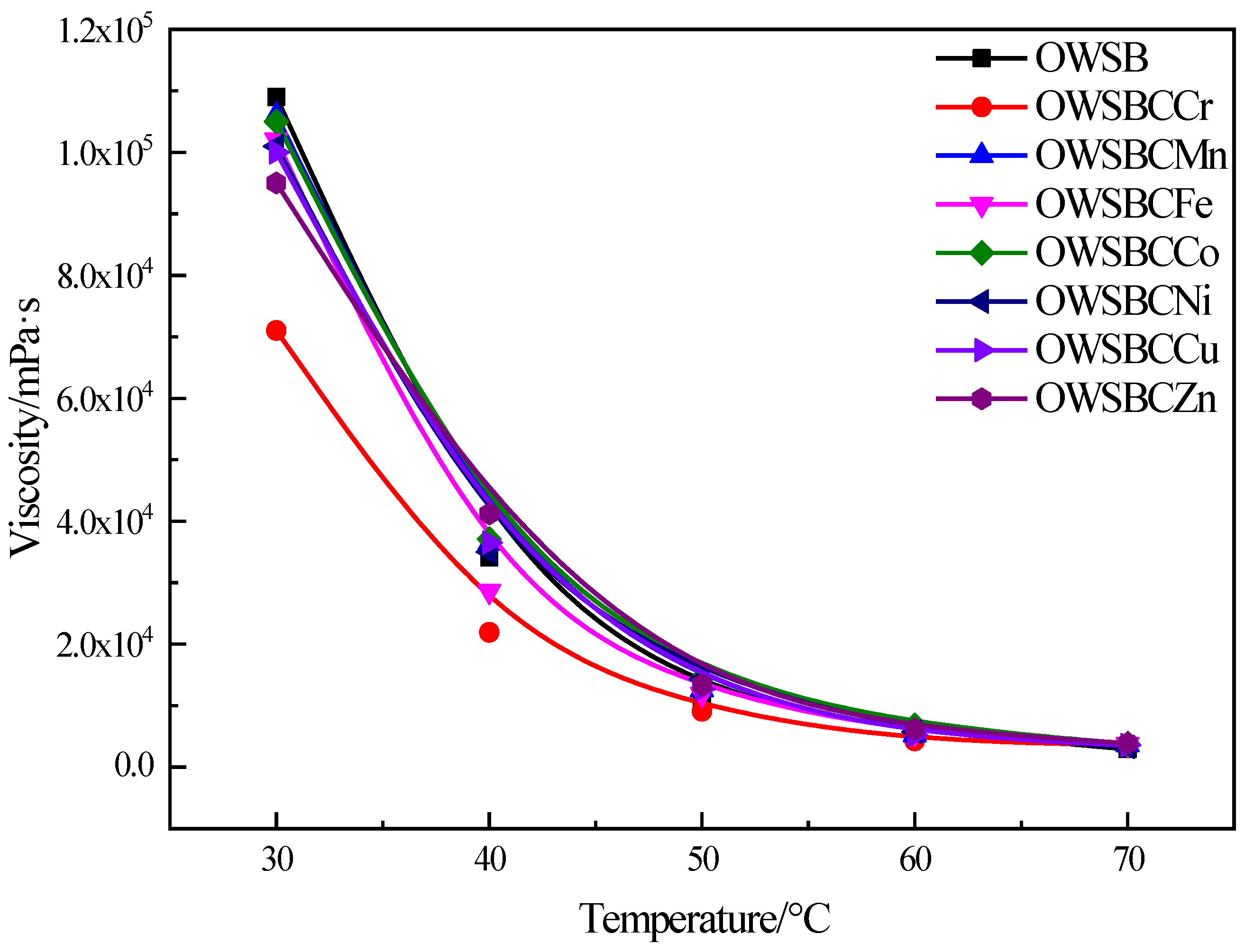
3.5. Viscosity Analysis of Heavy Oil Catalyzed by Different Catalysts
3.6. Pour Point Depression
3.7. Catalyst Stability Evaluation
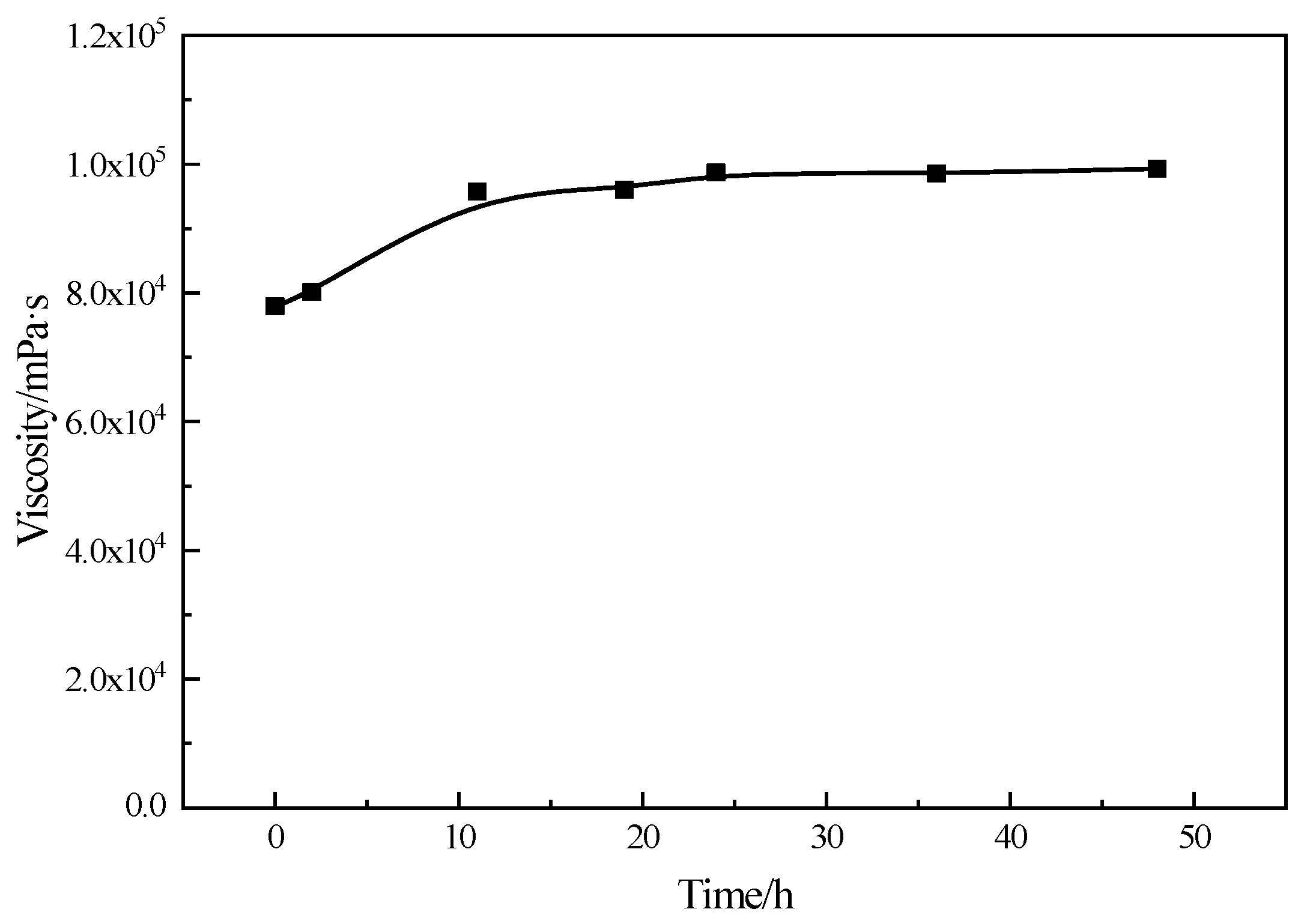
3.8. Viscosity Stability Evaluation
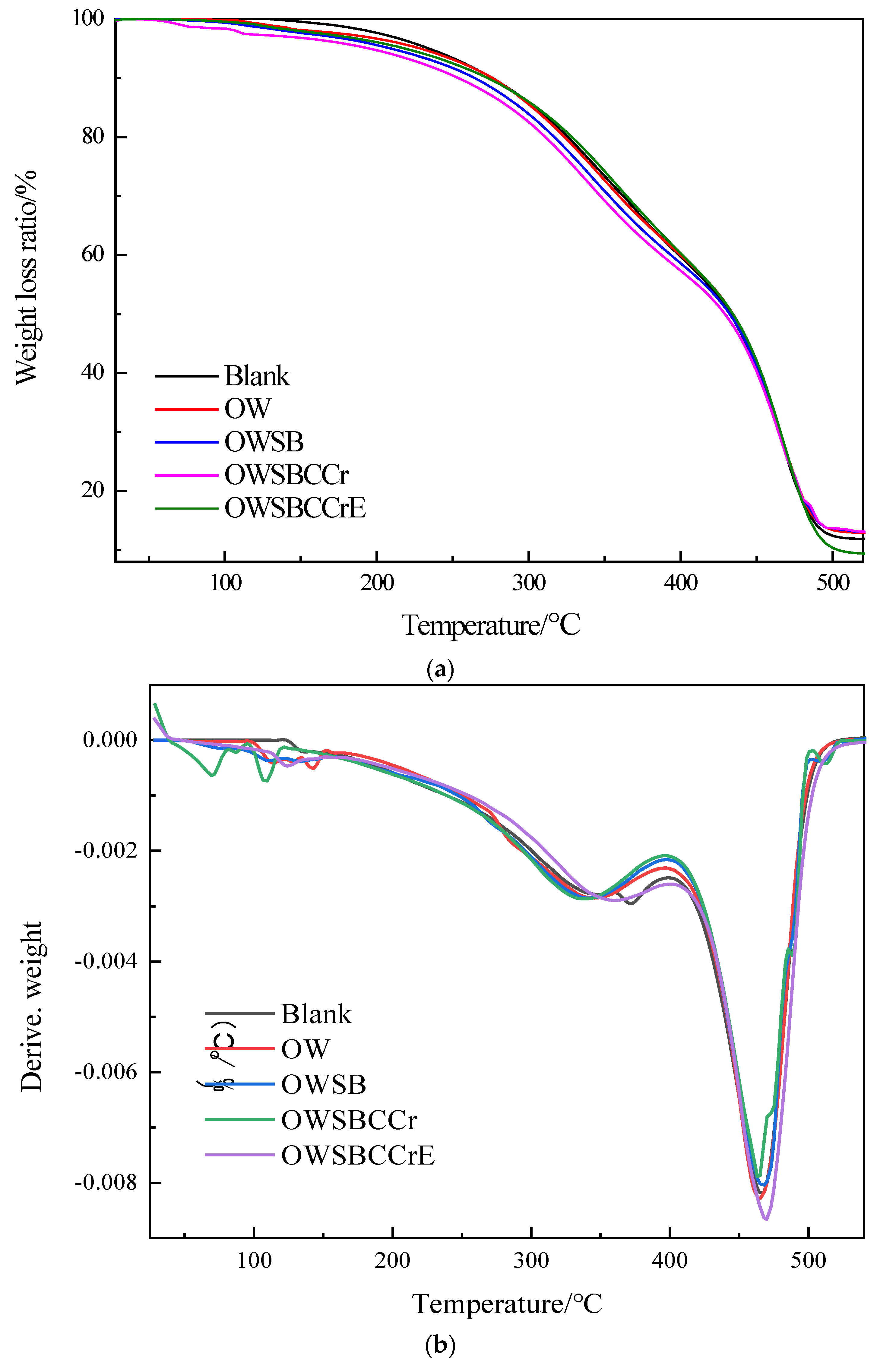
3.9. TGA/DTG Analysis
3.10. Elemental Analysis
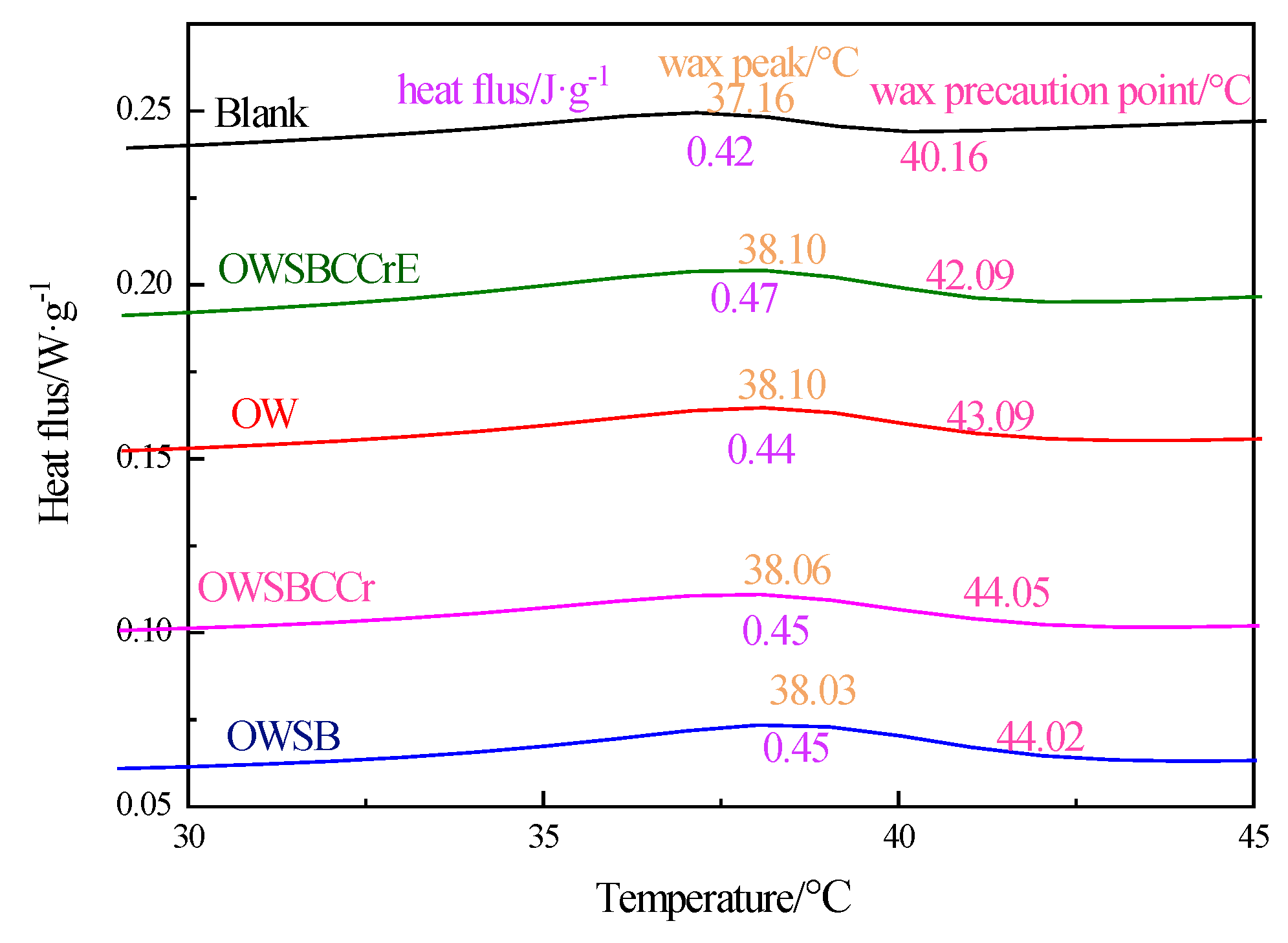
3.11. Differential Scanning Calorimetry (DSC)
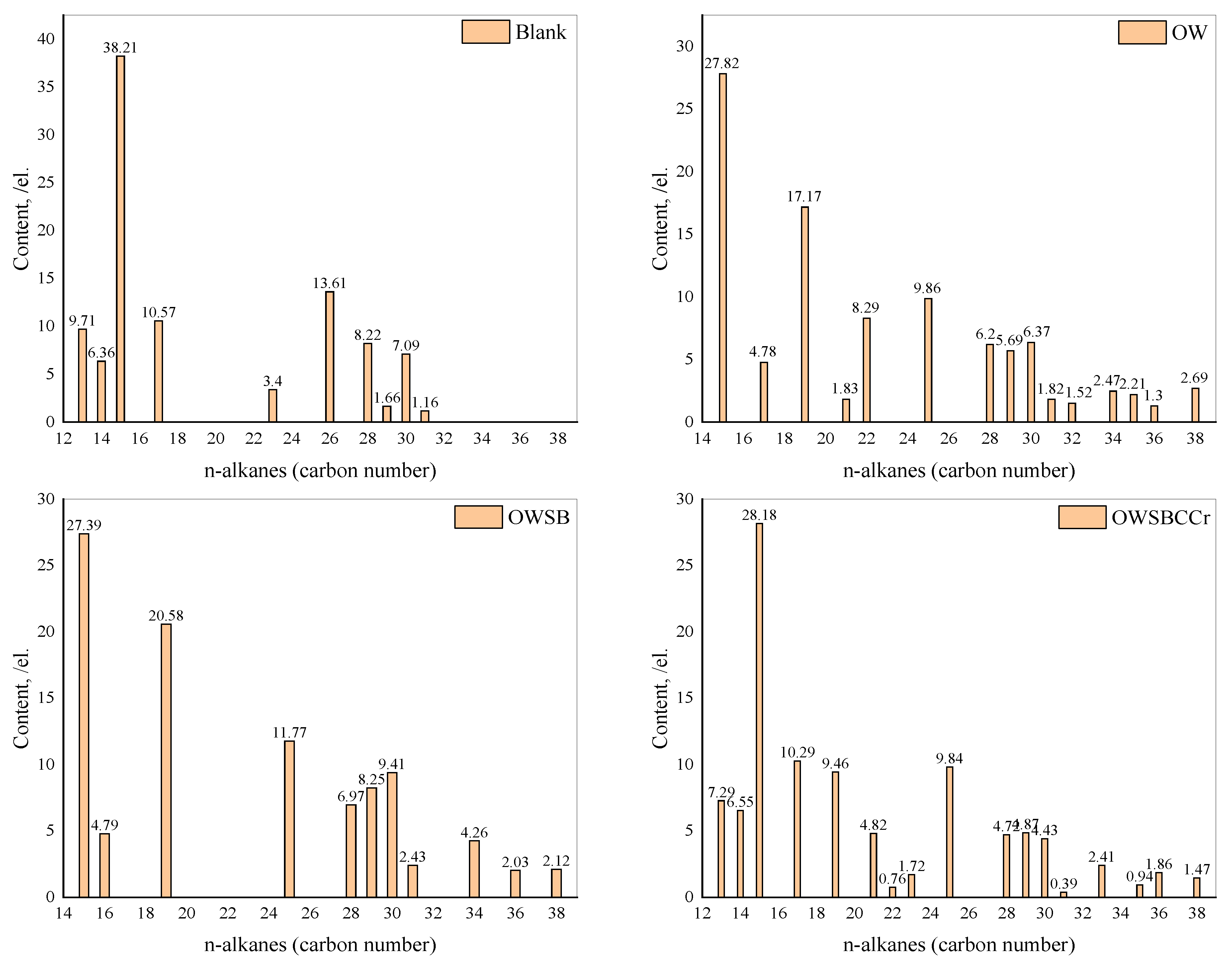
3.12. Gas Chromatography (GC) Analysis of the Saturated HC
4. Mechanism
4.1. Catalytic Aquathermolysis of Model Compounds
4.2. Viscosity Reduction Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, G.; Zhou, Z.C.; Shi, X.D.; Zhang, X.L.; Dong, S.B.; Zhang, J. Synthesis of alkylbenzenesulfonate and its behavior as flow improver in crude oil. Fuel 2021, 288, 119644. [Google Scholar] [CrossRef]
- Mao, J.C.; Liu, J.W.; Wang, H.B.; Yang, X.J.; Zhang, Z.Y.; Yang, B.; Zhao, J.Z. Novel terpolymers as viscosity reducing agent for Tahe super heavy oil. ACS Omega 2017, 7, 19257–19261. [Google Scholar] [CrossRef]
- Ahmadi, M.; Chen, Z.X. Challenges and future of chemical assisted heavy oil recovery processes. Adv. Resin Interface Sci. 2020, 275, 102081. [Google Scholar] [CrossRef] [PubMed]
- Voskov, D.; Zaydullin, R.; Lucia, A. Heavy oil recovery efficiency using SAGD, SAGD with propane co-injection and STRIP-SAGD. Comput. Chem. Eng. 2016, 88, 115–125. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. A review on surfactant retention on rocks: Mechanisms, measurements, and influencing factors. Fuel 2021, 293, 120459. [Google Scholar] [CrossRef]
- Xi, C.F.; Qi, Z.Y.; Zhang, Y.J.; Liu, T.; Shen, D.H.; Mu, H.; Dong, H.; LI, X.; Jiang, Y.W.; Wang, H.Z. CO2 assisted steam flooding in late steam flooding in heavy oil reservoirs. Pet. Explor. Dev. 2019, 46, 1242–1250. [Google Scholar] [CrossRef]
- Kirmani, F.U.D.; Raza, A.; Gholami, R.; Haidar, M.Z.; Fareed, C.S. Analyzing the effect of steam quality and injection temperature on the performance of steam flooding. Energy Geosci. 2021, 2, 83–86. [Google Scholar] [CrossRef]
- Huang, J.J.; Babadagli, T. Efficiency improvement of heavy-oil recovery by steam-assisted gravity drainage injection using new generation chemicals. Energy Fuels 2020, 34, 4433–4447. [Google Scholar] [CrossRef]
- Wang, Y.W.; Liu, H.Q.; Zhou, Y.H. Development of a deep learning-based model for the entire production process of steam-assisted gravity drainage (SAGD). Fuel 2021, 287, 119565. [Google Scholar] [CrossRef]
- Aydar, A.A.; Ilnaz, T.R.; Artashes, A.K.; Artem, A.P.; Sergei, A.S.; Alexander, V.G.; Yury, N.O.; Mikhail, A.V. Thermocatalytic upgrading of heavy oil by iron oxides nanoparticles synthesized by oil-soluble precursors. J. Pet. Sci. Eng. 2018, 169, 200–204. [Google Scholar]
- Clark, P.D.; Hyne, J.B. Chemistry of organosulphur compound types occurring in heavy oil sands: 3. Reaction of thiophene and tetrahydrothiophene with vanadyl and nickel salts. Fuel 1984, 63, 1649–1654. [Google Scholar] [CrossRef]
- Clark, P.D.; Dowling, N.I.; Lesage, K.L.; Hyne, J.B. Chemistry of organosulphur compound types occurring in heavy oil sands: 5. Reaction of thiophene and tetrahydrothiophene with aqueous Group VIIIB metal species at high temperature. Fuel 1987, 66, 1699–1702. [Google Scholar] [CrossRef]
- Li, C.; Huang, W.W.; Zhou, C.G.; Chen, Y.L. Advances on the transition-metal based catalysts for aquathermolysis upgrading of heavy crude oil. Fuel 2019, 257, 115779. [Google Scholar] [CrossRef]
- Shaban, S.; Dessouky, S.; Badawi, A.E.F.; Sabagh, A.E.; Zahran, A.; Mousa, M. Upgrading and viscosity reduction of heavy oil by catalytic ionic liquid. Energy Fuels 2014, 28, 6545–6553. [Google Scholar] [CrossRef]
- Chen, M.; Li, C.; Li, G.; Li, G.R.; Chen, Y.L.; Zhou, C.G. In situ preparation of well-dispersed CuO nanocatalysts in heavy oil for catalytic aquathermolysis. Pet. Sci. 2019, 16, 439–446. [Google Scholar] [CrossRef]
- Chao, K.; Chen, Y.L.; Li, J.; Zhang, X.M.; Dong, B.Y. Upgrading and visbreaking of super-heavy oil by catalytic aquathermolysis with aromatic sulfonic copper. Fuel Process. Technol. 2012, 104, 174–180. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, W.H.; Yan, J.; Meng, M.; Guo, Z.; Gu, X.F.; Zhang, J.; Qu, C.T.; Song, H.; Jeje, A. Zn (II) complex catalyzed coupling aquathermolysis of water-heavy oil-methanol at low temperature. Pet. Chem. 2018, 58, 197–202. [Google Scholar] [CrossRef]
- Suwaid, M.A.; Varfolomeev, M.A.; Al-muntaser, A.A.; Yuan, C.D.; Starshinova, V.L.; Zinnatullin, A.; Vagizov, F.G.; Rakhmatullin, I.Z.; Emelianov, D.A.; Chemodanov, A.E. In-situ catalytic upgrading of heavy oil using oil-soluble transition metal-based catalysts. Fuel 2020, 281, 118753. [Google Scholar] [CrossRef]
- Li, C.; Su, L.; Li, Q.Y.; Wang, X.D.; Li, X.H.; Yang, J.J.; Zhang, Z.J. Enhanced heavy oil recovery in mild conditions by SO42-/TIO2-ZrO2 solid superacid prepared by different methods. J. Nanomater. 2016, 2016, 7436057. [Google Scholar] [CrossRef]
- Deepa, S.; Kathleen, W.; Abbas, F. Ionic liquids as viscosity modifiers for heavy and extra-heavy crude oils. Fuel 2015, 143, 519–526. [Google Scholar]
- Li, H.Y.; Cui, K.X.; Jin, L.; Wang, L.L.; Yu, B. Experimental study on the viscosity reduction of heavy oil with nano-catalyst by microwave heating under low reaction temperature. J. Pet. Sci. Eng. 2018, 170, 374–382. [Google Scholar]
- Wu, L.M.; Zhou, C.H.; Keeling, J.; Tong, D.S.; Yu, W.H. Towards an understanding of the role of clay minerals in crude oil reservoir, migration and accumulation. Earth-Sci. Rev. 2012, 115, 373–386. [Google Scholar] [CrossRef]
- Espitalie, J.; Madec, M.; Tissot, B. Role of Mineral Matrix in Kerogen Pyrolysis: Influence on Petroleum Generation and Migration. Aapg Bull.-Am. Assoc. Pet. Geol. 1980, 64, 59–66. [Google Scholar]
- Ágnes, P.; Imre, D. Ion exchange and molecular adsorption of a cationic surfactant on clay minerals. Resins Surf. A Physicochem. Eng. Asp. 1993, 71, 299–307. [Google Scholar]
- Chen, G.; Yan, J.; Bai, Y.; Gu, X.F.; Zhang, J.; Li, Y.F.; Jeje, A. Clean aquathermolysis of heavy oil catalyzed by Fe(III) complex at relatively low temperature. Pet. Sci. Technol. 2017, 35, 113–119. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, W.H.; Bai, Y.; Zhao, W.; Gu, X.F.; Zhang, J.; Jeje, A. Ethanol enhanced aquathermolysis of heavy oil catalyzed by a simple Co(II) complex at low temperature. Pet. Chem. 2017, 57, 389–394. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, W.H.; Su, H.J.; Zhang, J.; Gu, X.F.; Bai, Y.; Jeje, A. Methanol enhanced catalytic viscosity-reduction of heavy oil by transition metal-Mannich base complex under low temperature. Russ. J. Appl. Chem. 2016, 89, 1853–1860. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, W.H.; Wu, Y.; Zhang, J.; Song, H.; Jeje, A.; Song, S.F.; Qu, C.T. Catalytic aquathermolysis of heavy oil by coordination complex at relatively low temperature. Pet. Chem. 2017, 57, 881–884. [Google Scholar] [CrossRef]
- Song, S.F.; Guo, Z.; Bai, Y.; Gu, X.F.; Chen, G.; Zhang, J.; Li, B.Q.; Zhang, Z.F. The use of a tartaric-Co(II) complex in the catalytic aquathermolysis of heavy oil. Pet. Sci. Technol. 2017, 35, 661–666. [Google Scholar] [CrossRef]
- Gu, X.F.; Zhang, F.; Chen, G.; Zhang, J.; Meng, M.; Li, B.Q.; Zhang, Z.F. Viscosity reduction of citric-Fe(Ⅲ) in catalytic aquathermolysis of heavy oil at low temperature. Russ. J. Appl. Chem. 2016, 89, 2061–2065. [Google Scholar] [CrossRef]
- Hao, H.R.; Su, H.J.; Chen, G.; Zhao, J.R.; Li, H. Viscosity reduction of heavy oil by aquathermolysis with coordination complex at low temperature. Open Fuels Energy Sci. J. 2015, 8, 93–98. [Google Scholar] [CrossRef]
- Adams, J.M.; Mccabe, R.W. Chapter 10.2 clay minerals as catalysts. Dev. Clay Sci. 2006, 1, 541–581. [Google Scholar]
- Zhou, L.; Slaný, M.; Bai, B.B.; Du, W.C.; Qu, C.T.; Zhang, J.; Tang, Y. Enhanced removal of sulfonated lignite from oil wastewater with multidimensional MgAl-LDH nanoparticles. Nanomaterials 2021, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lin, J.; Hu, W.M.; Cheng, C.; Gu, X.F.; Du, W.C.; Zhang, J.; Qu, C.T. Characteristics of a crude oil composition and its in situ waxing inhibition behavior. Fuel 2018, 218, 213–217. [Google Scholar] [CrossRef]

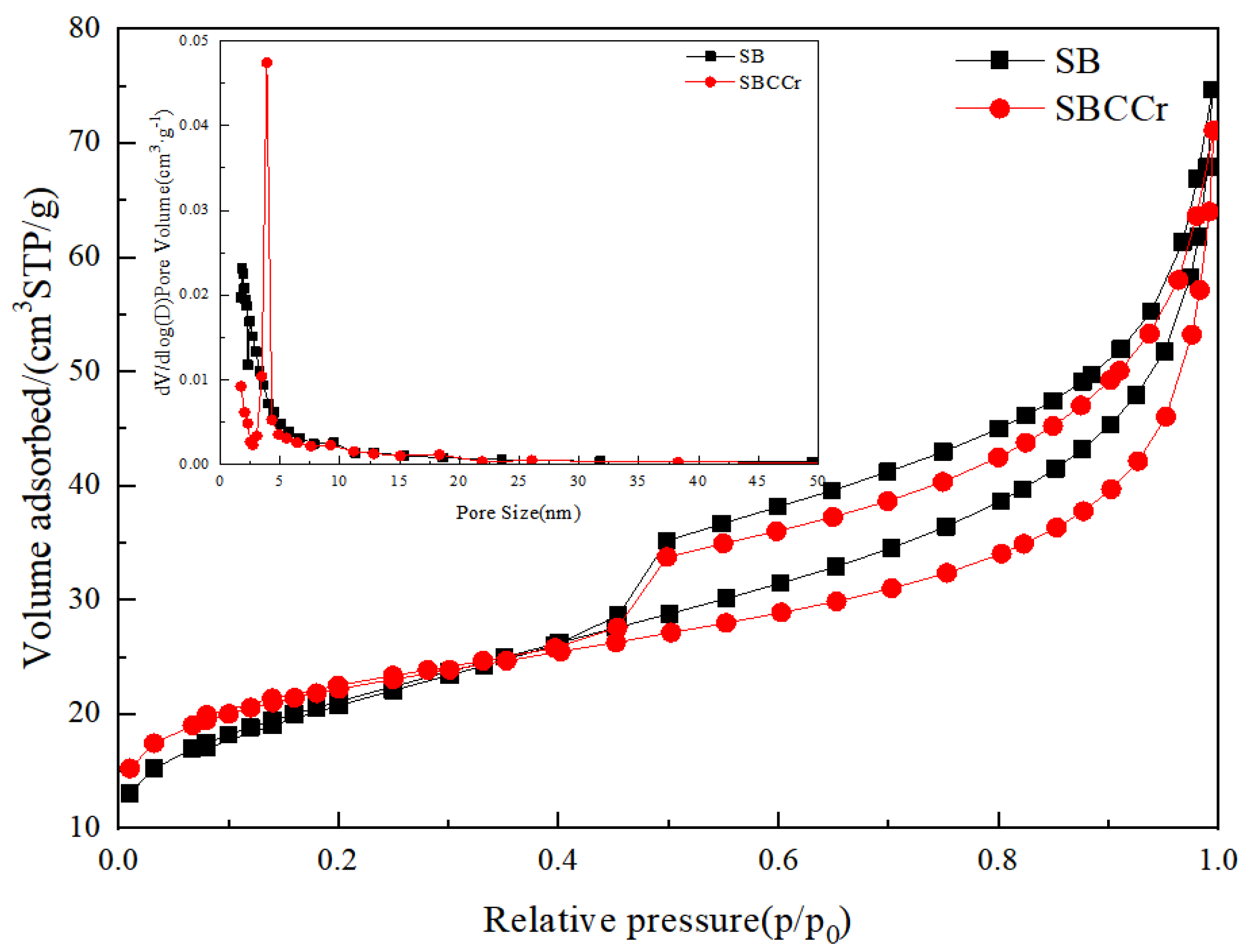


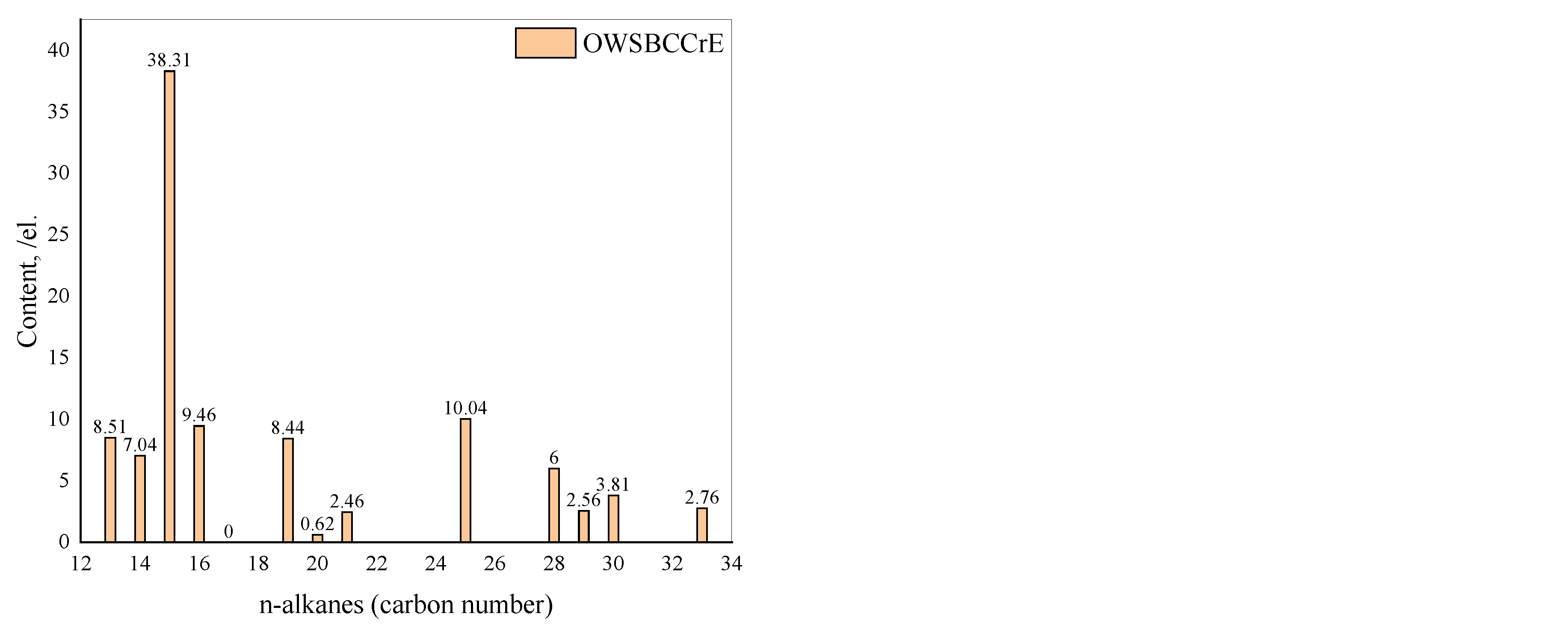

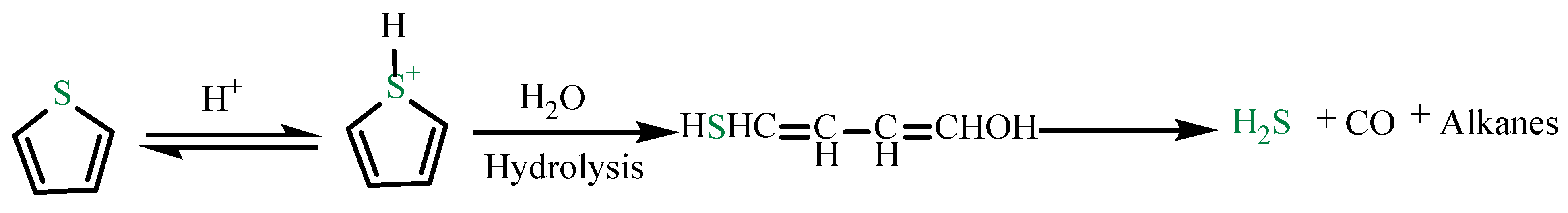


| Sample | Pore Diameter/nm | Pore Volume/cm3·g−1 | Specific Surface Area/m2·g−1 |
|---|---|---|---|
| SB | 6.2834 | 0.1155 | 73.5293 |
| SBCCr | 6.0402 | 0.1099 | 72.8337 |
| Oil Sample | Pour Point/°C |
|---|---|
| Blank | 24.0 |
| OW | 21.0 |
| OWSB | 19.0 |
| OWCCr | 14.0 |
| OWSBCCr | 9.0 |
| OWSBCMn | 9.5 |
| OWSBCFe | 9.5 |
| OWSBCCo | 9.0 |
| OWSBCNi | 9.5 |
| OWSBCCu | 9.0 |
| OWSBCZn | 9.5 |
| OWSBCCrE | 9.0 |
| Cycles | Viscosity/mPa·s | Pour Point/°C | ||||
|---|---|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | 60 °C | 70 °C | ||
| 1 | 72,300 | 25,100 | 9140 | 4010 | 1980 | 9.0 |
| 2 | 84,700 | 31,400 | 10,800 | 5180 | 3250 | 9.0 |
| 3 | 85,500 | 30,300 | 11,000 | 5100 | 3100 | 9.5 |
| 4 | 85,400 | 31,200 | 11,300 | 4530 | 3300 | 10.0 |
| Oil Sample | C/% | H/% | N/% | S/% |
|---|---|---|---|---|
| Blank | 86.12 | 10.48 | 1.51 | 0.50 |
| OW | 88.34 | 7.50 | 1.48 | 0.46 |
| OWSB | 84.20 | 10.09 | 1.35 | 0.43 |
| OWSBCCr | 81.09 | 9.95 | 0.50 | 0.25 |
| OWSBCCrE | 87.43 | 10.13 | 1.47 | 0.43 |
| Reactant | Catalysts | Product |
|---|---|---|
| phenol, H2O | no | Benzene |
| phenol, H2O | SB | |
| phenol, H2O | SBCCr | |
| phenol, H2O, ethanol | SBCCr |
| Reactant | Catalysts | Product |
|---|---|---|
| thiophene, H2O | no | Phenol, toluene |
| thiophene, H2O | SB | |
| thiophene, H2O | SBCCr | |
| thiophene, H2O, ethanol | SBCCr |
| Reactant | Catalysts | Product |
|---|---|---|
| nonylphenol, H2O | no | 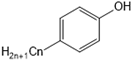 n = 1, 3, 4, 5, 6, 7, 8 n = 1, 3, 4, 5, 6, 7, 8 |
| nonylphenol, H2O | SB | |
| nonylphenol, H2O | SBCCr | 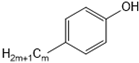 m = 0, 1, 3, 4, 5, 6, 7, 8 m = 0, 1, 3, 4, 5, 6, 7, 8 |
| nonylphenol, H2O, ethanol | SBCCr |
| Reactant | Catalysts | Product |
|---|---|---|
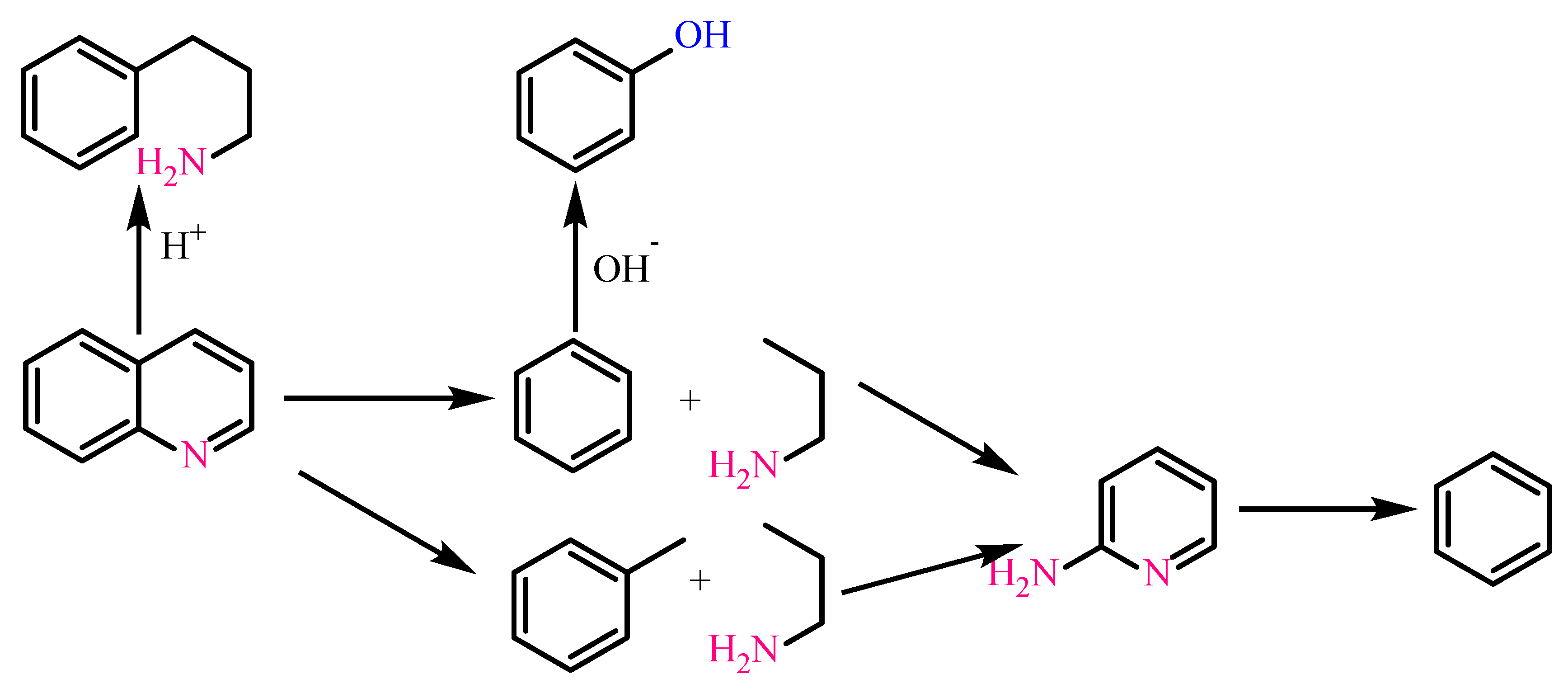
| quinoline, H2O | no | Toluene, 3-phenyl-1-propylamine, propylamine |
| quinoline, H2O | SB | Toluene, pyridine |
| quinoline, H2O | SBCCr | |
| benzothiophene, H2O, ethanol | SBCCr | Toluene |
| Reactant | Catalysts | Product |
|---|---|---|
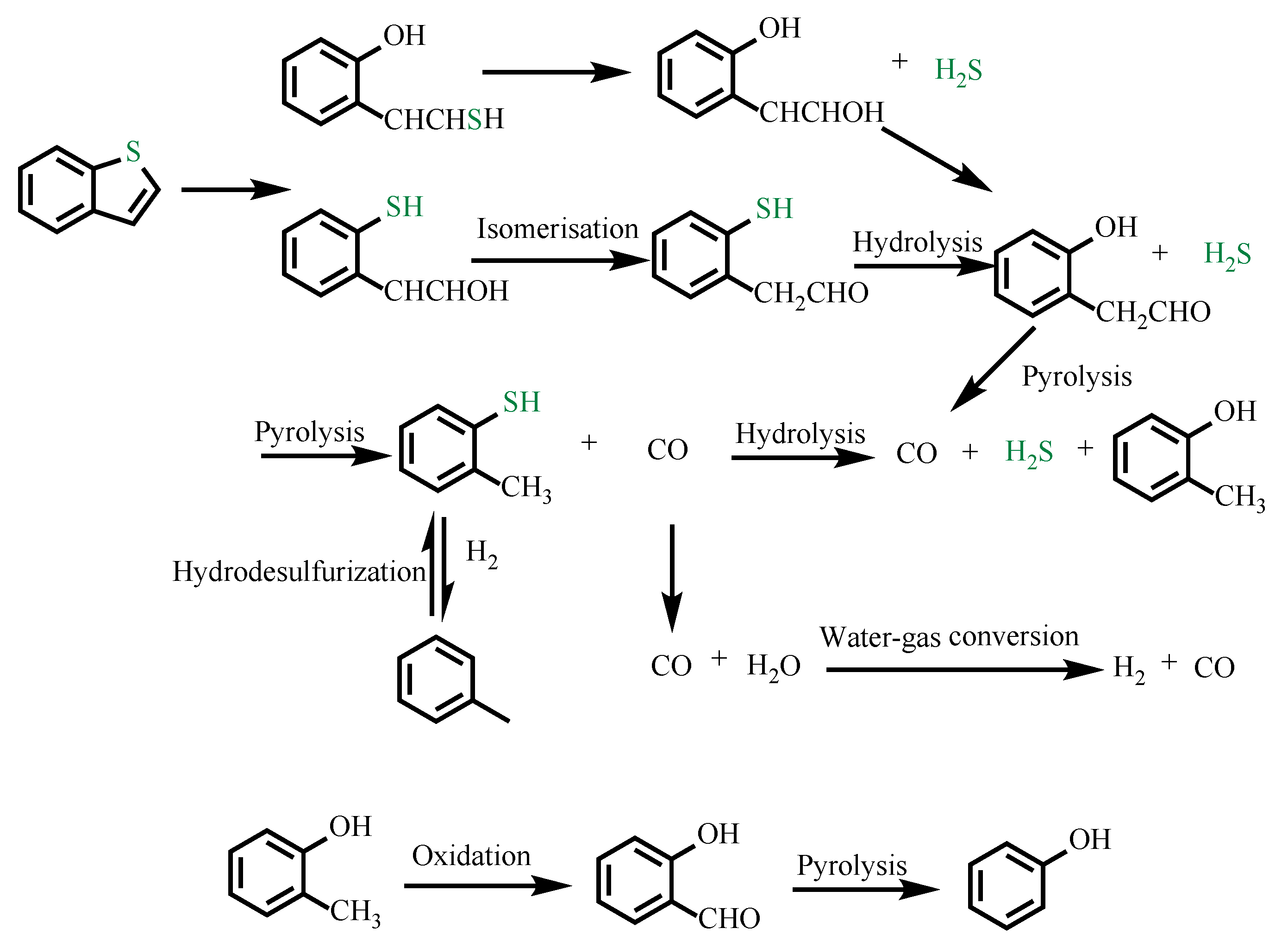
| benzothiophene, H2O | no | Toluene, 2-mercaptotoluene, phenol |
| benzothiophene, H2O | SB | Toluene, phenol |
| benzothiophene, H2O | SBCCr | |
| benzothiophene, H2O, ethanol | SBCCr | Toluene, 2-mercaptotoluene, phenol |
| Reactant | Catalysts | Product |
|---|---|---|
| pyridine, H2O | no | Pyridine |
| pyridine, H2O | SB | |
| pyridine, H2O | SBCCr | |
| pyridine, H2O, ethanol | SBCCr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Zhang, W.; Yu, T.; Li, Y.; Struhárová, A.; Matejdes, M.; Slaný, M.; Chen, G. The Effect of Sodium Bentonite in the Thermo-Catalytic Reduction of Viscosity of Heavy Oils. Molecules 2023, 28, 2651. https://doi.org/10.3390/molecules28062651
Zhou Z, Zhang W, Yu T, Li Y, Struhárová A, Matejdes M, Slaný M, Chen G. The Effect of Sodium Bentonite in the Thermo-Catalytic Reduction of Viscosity of Heavy Oils. Molecules. 2023; 28(6):2651. https://doi.org/10.3390/molecules28062651
Chicago/Turabian StyleZhou, Zhichao, Wangyuan Zhang, Tao Yu, Yongfei Li, Alena Struhárová, Marián Matejdes, Michal Slaný, and Gang Chen. 2023. "The Effect of Sodium Bentonite in the Thermo-Catalytic Reduction of Viscosity of Heavy Oils" Molecules 28, no. 6: 2651. https://doi.org/10.3390/molecules28062651
APA StyleZhou, Z., Zhang, W., Yu, T., Li, Y., Struhárová, A., Matejdes, M., Slaný, M., & Chen, G. (2023). The Effect of Sodium Bentonite in the Thermo-Catalytic Reduction of Viscosity of Heavy Oils. Molecules, 28(6), 2651. https://doi.org/10.3390/molecules28062651







