Mercury and Organic Pollutants Removal from Aqueous Solutions by Heterogeneous Photocatalysis with ZnO-Based Materials
Abstract
1. Introduction
2. Results and Discussion
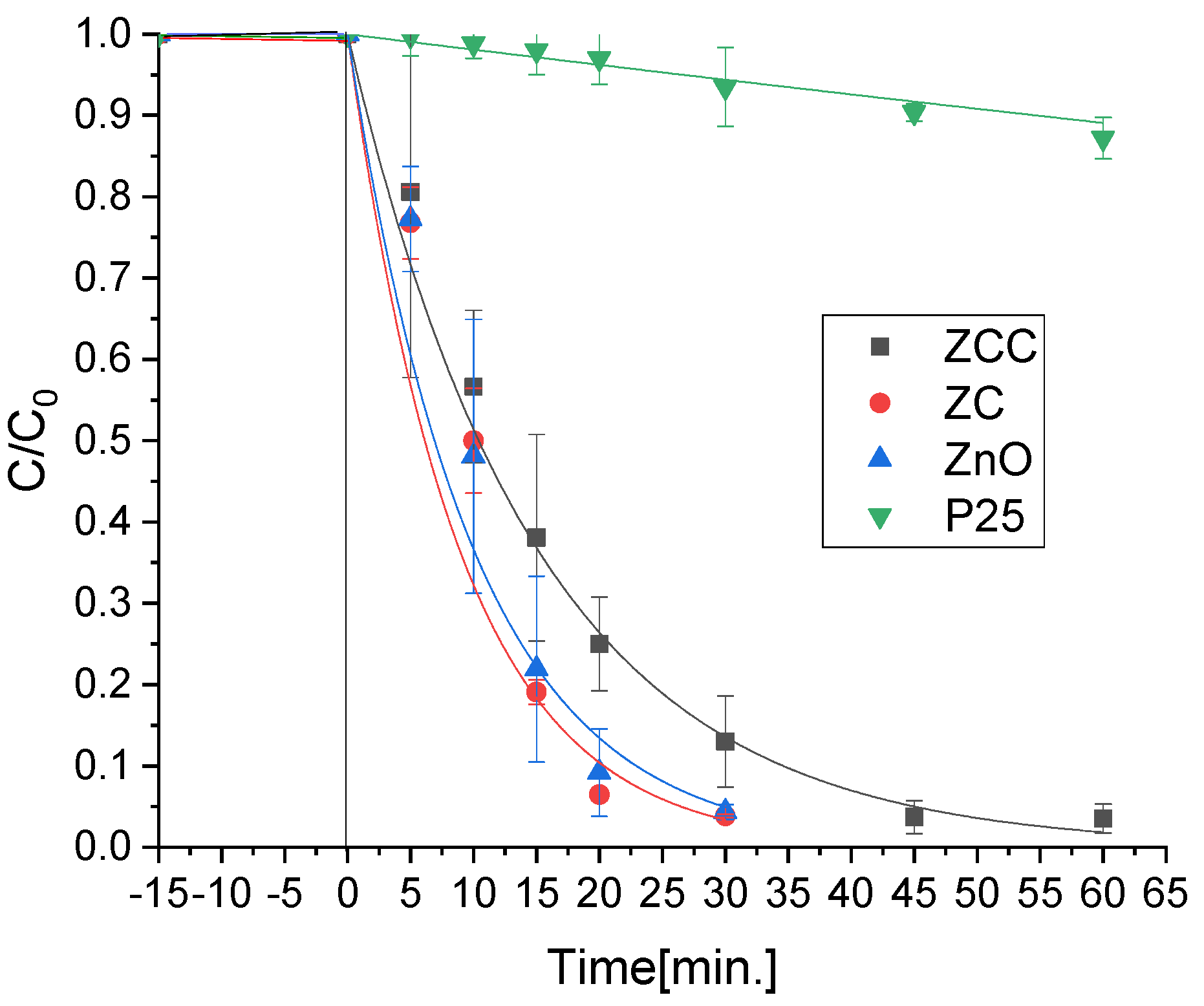
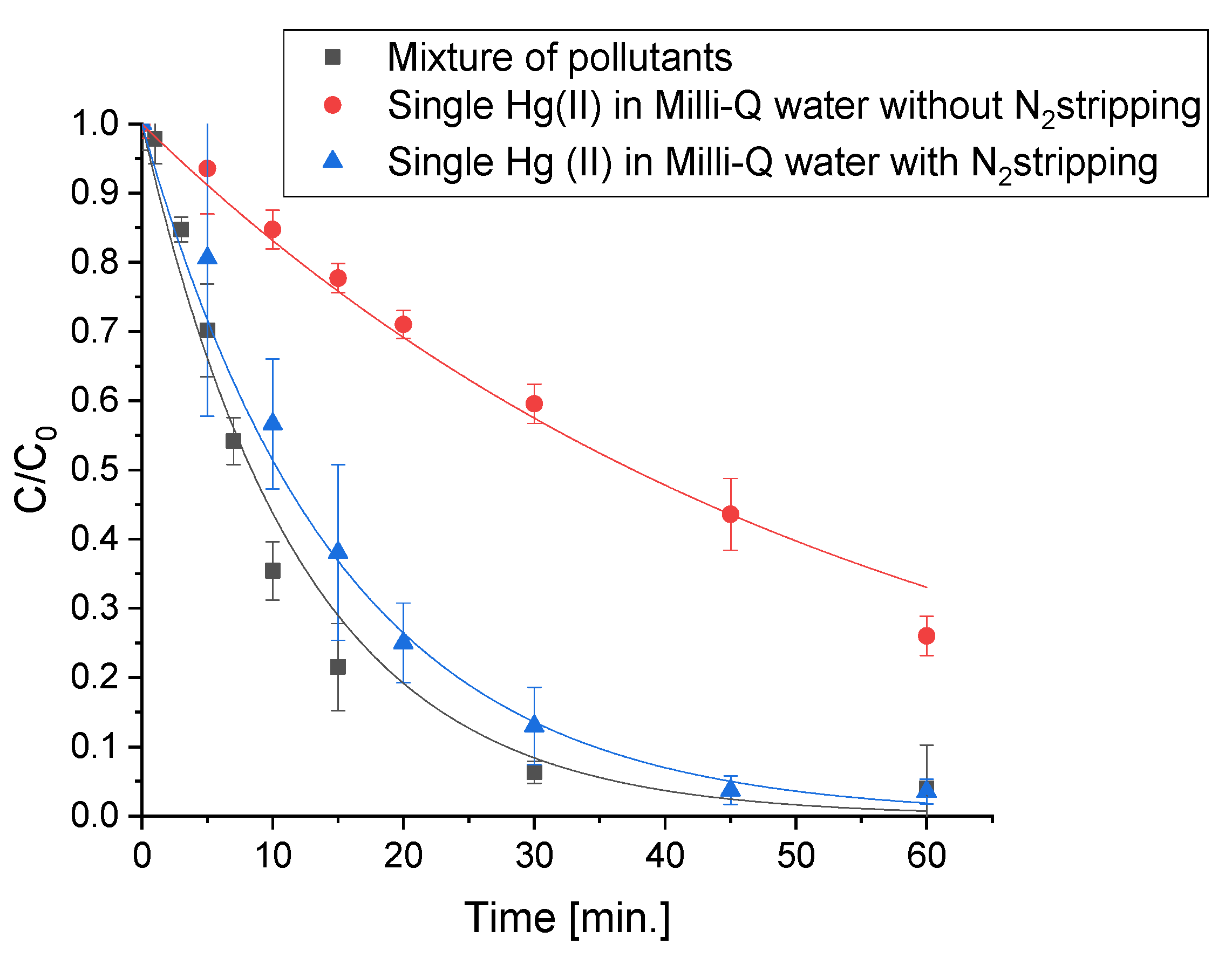
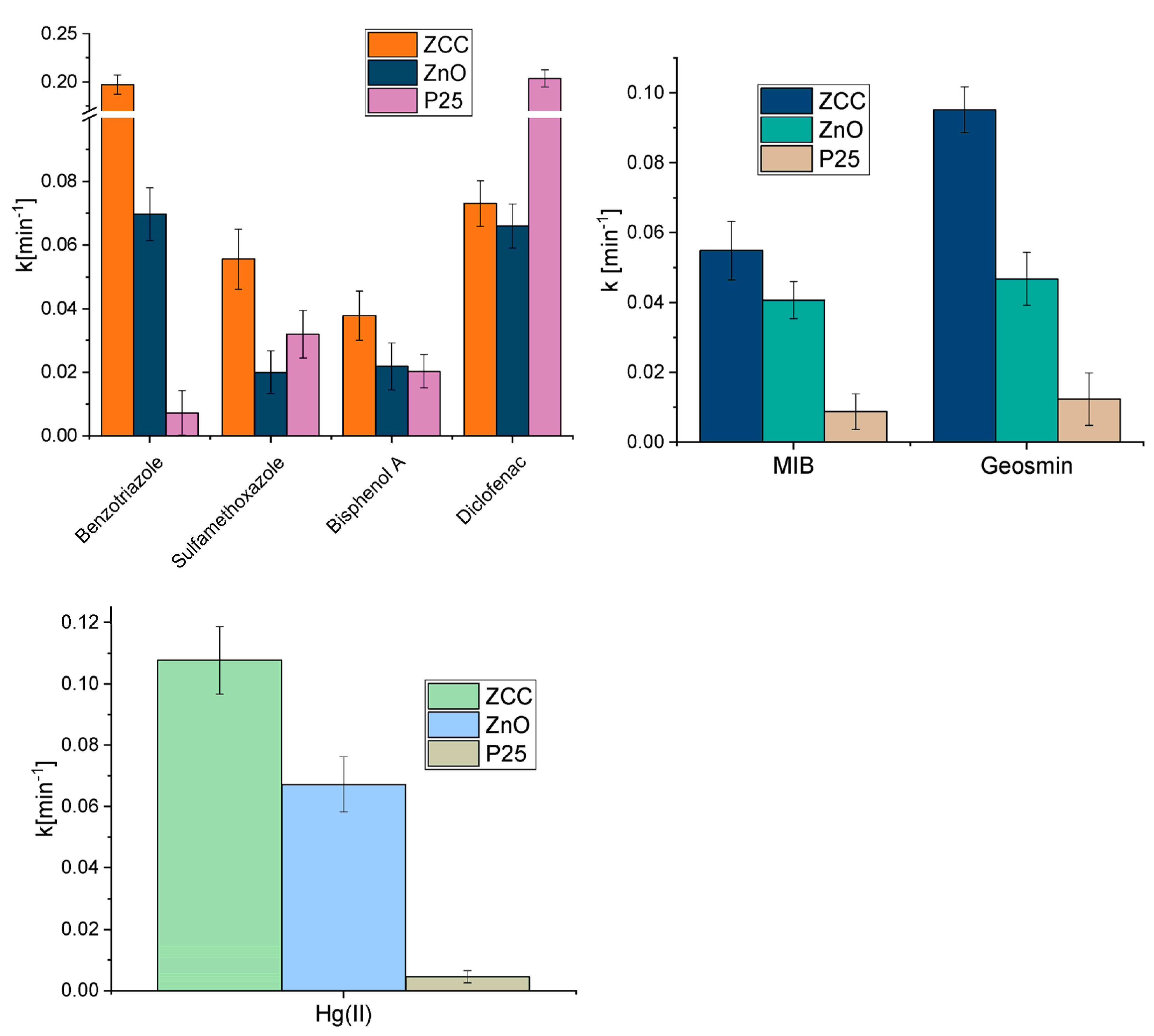
2.1. Mercury Removal
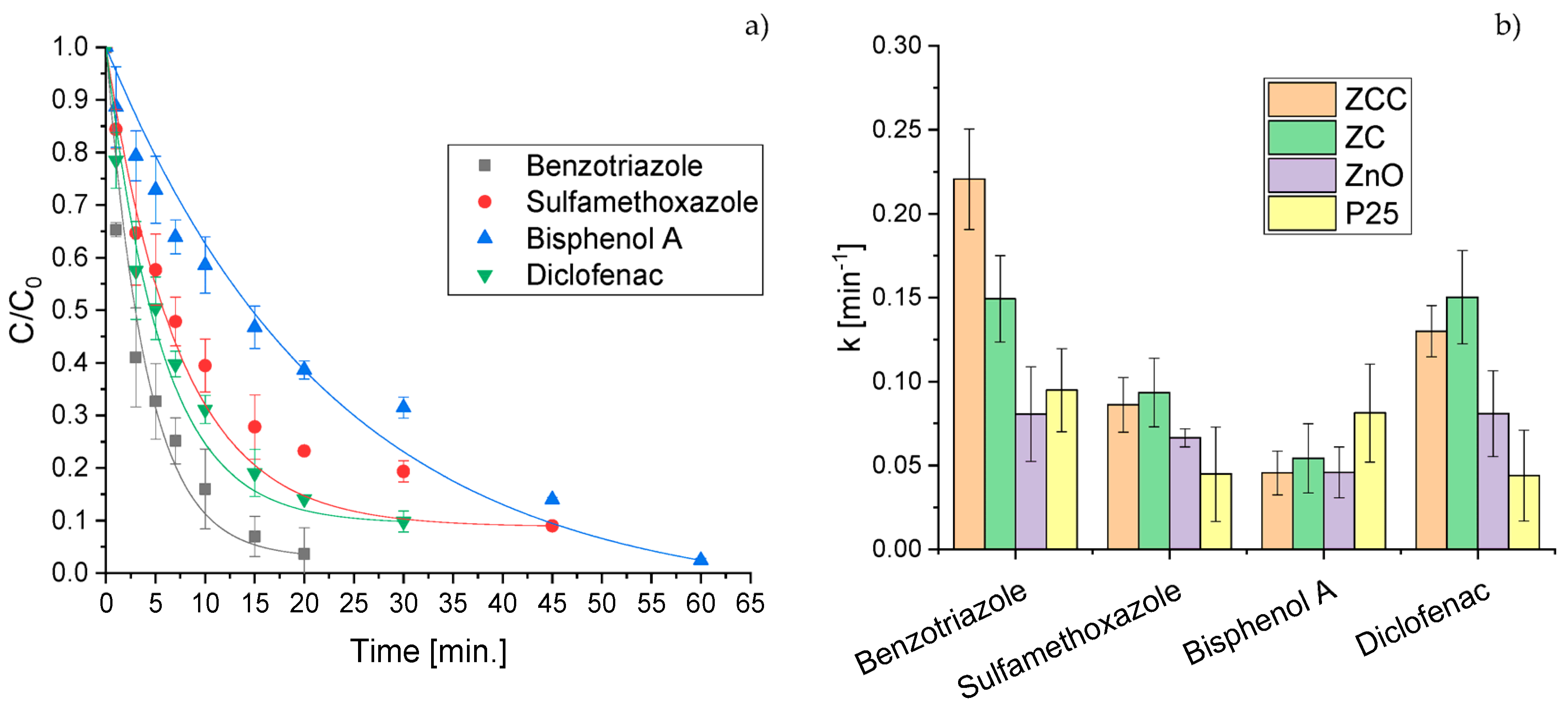
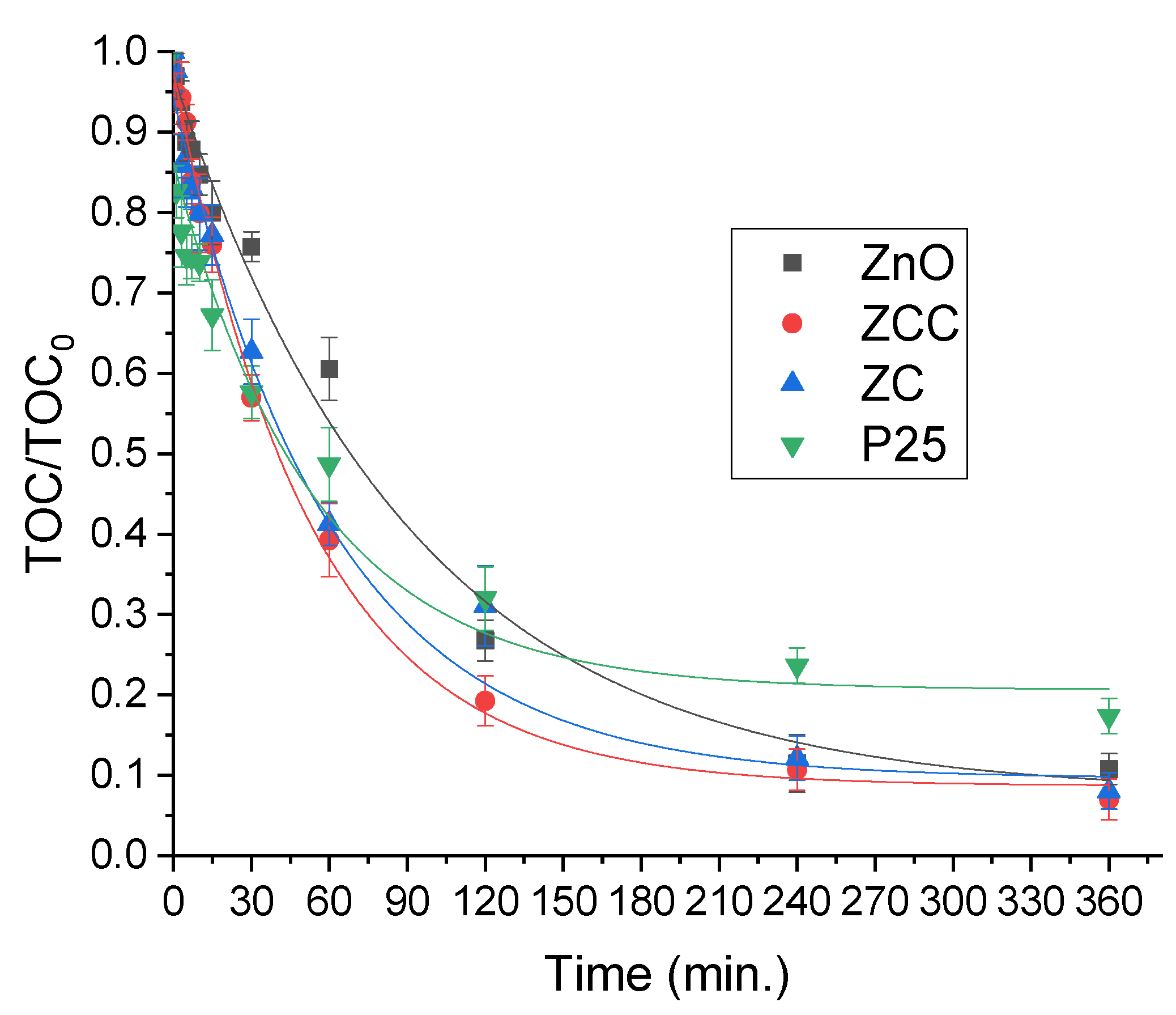
2.2. CECs Degradation
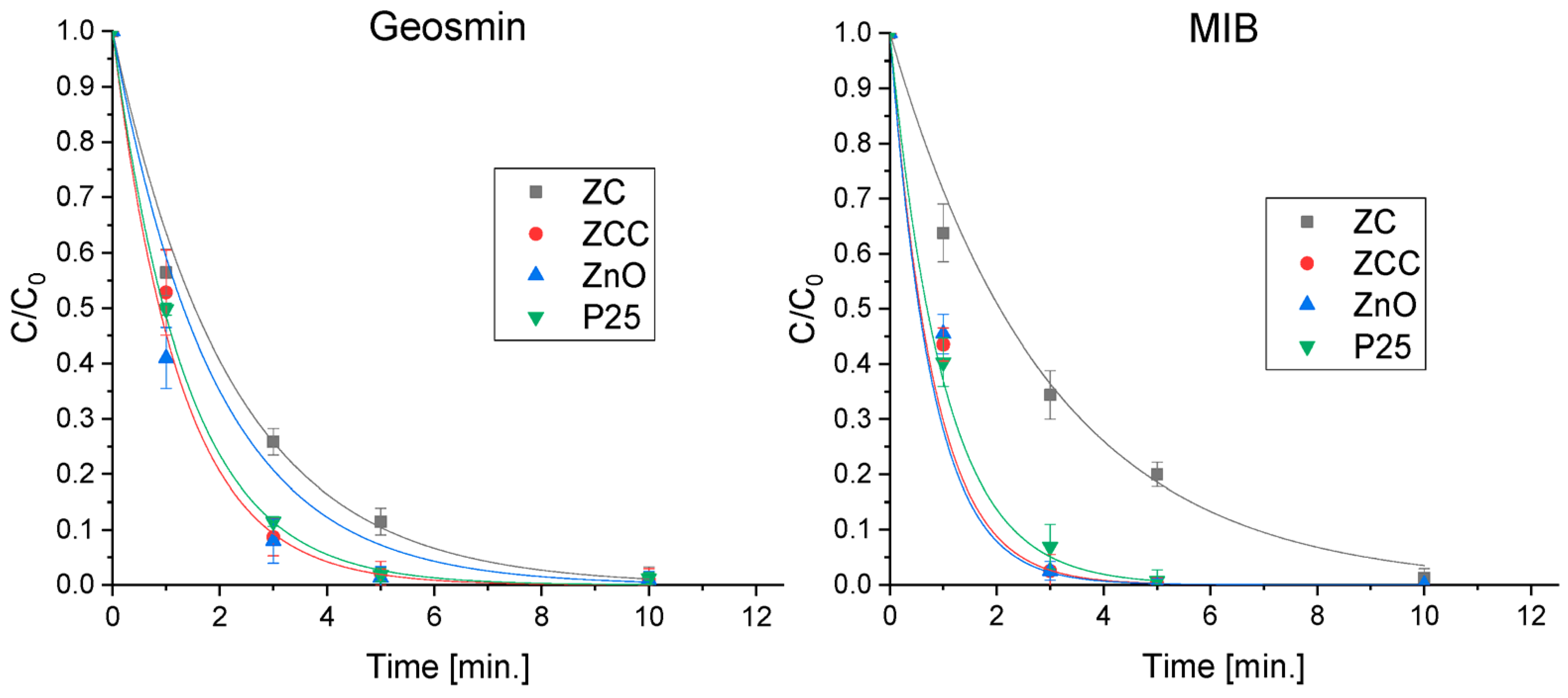
2.3. Geosmin and MIB Degradation
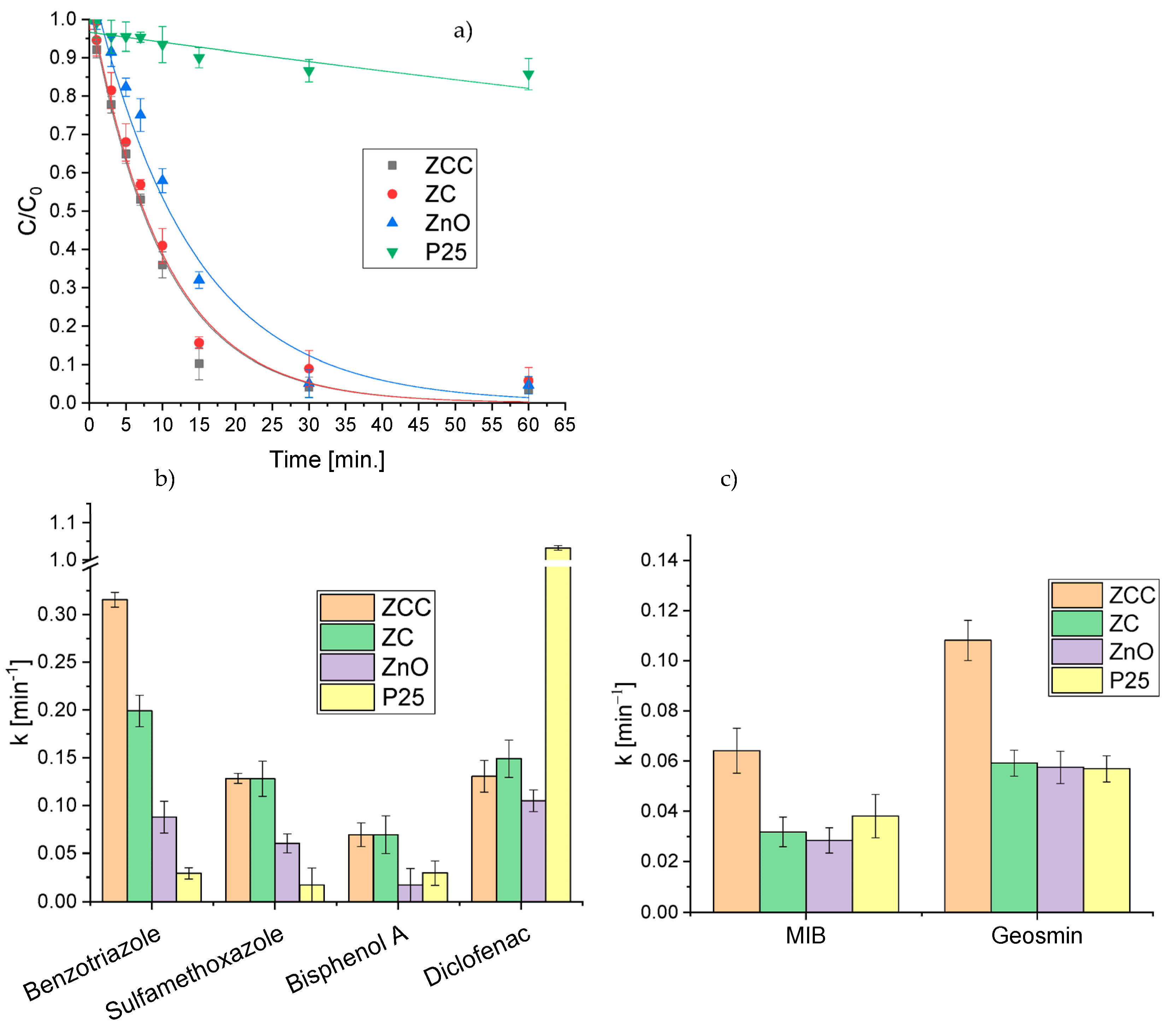
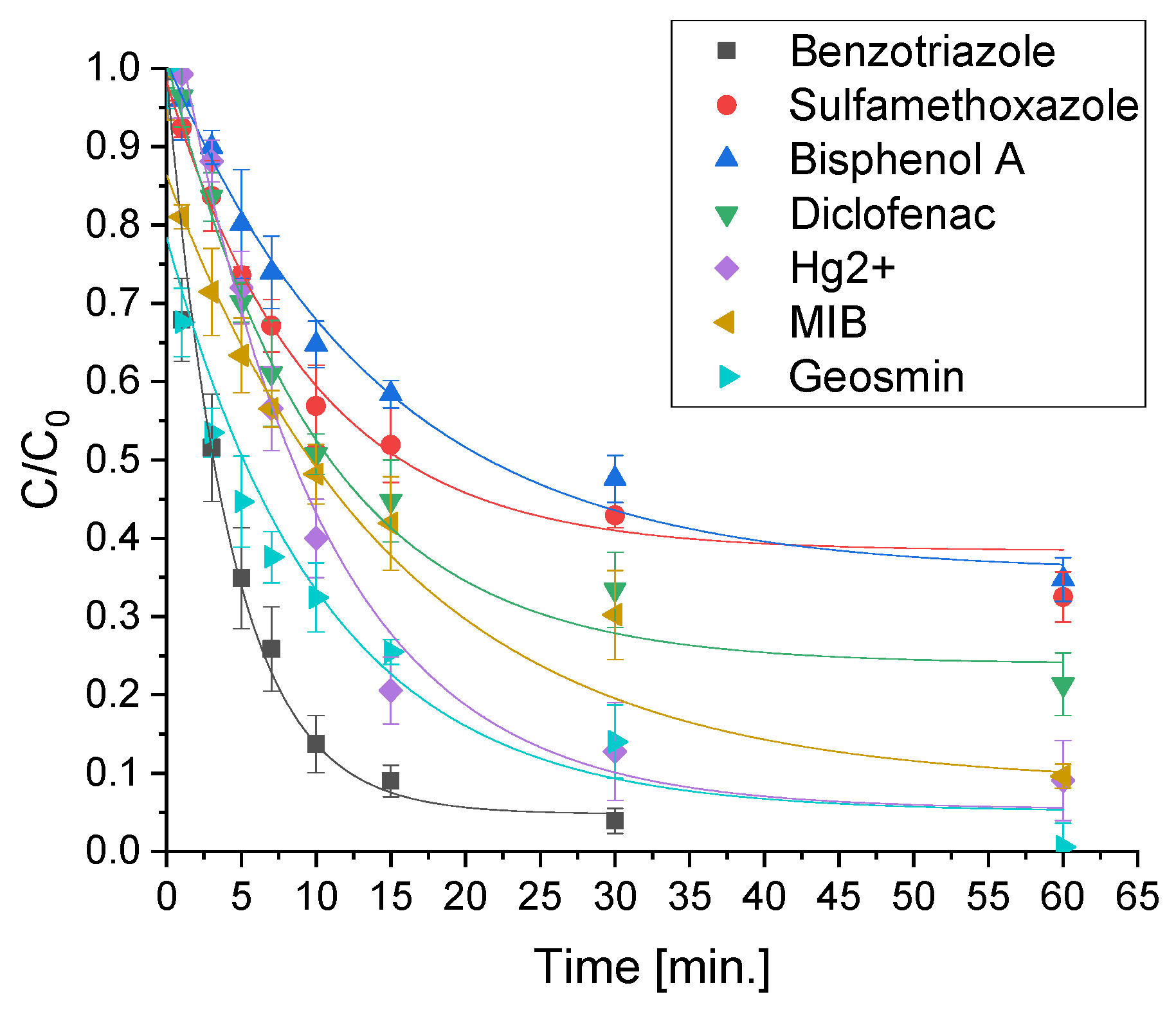
2.4. Removal of the Mixture of Different Classes of Pollutants in Actual Water
3. Materials and Methods
3.1. Synthesis of the Materials
3.2. Pollutants Removal
3.2.1. Mercury Removal
3.2.2. CECs Degradation
3.2.3. Geosmin and MIB Degradation
3.2.4. Removal of the Different Classes of Pollutants in Mixture and in Actual Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization; United Nations Children’s Fund. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2017: Special Focus on Inequalities; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Pörtner, H.-O.; Roberts, D.C.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. IPCC, 2022: Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Sanchez-Salas, J.; Flores, D.; Bandala, E. Water recalcitrant contaminants: Sources, assessment and remediation. In Wastewater and Water Contamination: Sources, Assessment and Remediation; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2018; pp. 1–76. [Google Scholar]
- Warren, N.; Allan, I.J.; Carter, J.E.; House, W.A.; Parker, A. Pesticides and other micro-organic contaminants in freshwater sedimentary environments—A review. Appl. Geochem. 2003, 18, 159–194. [Google Scholar] [CrossRef]
- Alharbi, O.M.L.; Basheer, A.A.; Khattab, R.A.; Ali, I. Health and environmental effects of persistent organic pollutants. J. Mol. Liq. 2018, 263, 442–453. [Google Scholar] [CrossRef]
- Golovko, O.; Örn, S.; Sörengård, M.; Frieberg, K.; Nassazzi, W.; Lai, F.Y.; Ahrens, L. Occurrence and removal of chemicals of emerging concern in wastewater treatment plants and their impact on receiving water systems. Sci. Total Environ. 2021, 754, 142122. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Strungaru, S.-A.; Nicoara, M.; Teodosiu, C.; Baltag, E.; Ciobanu, C.; Plavan, G. Patterns of toxic metals bioaccumulation in a cross-border freshwater reservoir. Chemosphere 2018, 207, 192–202. [Google Scholar] [CrossRef]
- González-Rubio, S.; Ballesteros-Gómez, A.; Asimakopoulos, A.G.; Jaspers, V.L.B. A review on contaminants of emerging concern in European raptors (2002–2020). Sci. Total Environ. 2021, 760, 143337. [Google Scholar] [CrossRef]
- Little, D.C.; Newton, R.W.; Beveridge, M.C.M. Aquaculture: A rapidly growing and significant source of sustainable food? Status, transitions and potential. Proc. Nutr. Soc. 2016, 75, 274–286. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Lindholm-Lehto, P.C.; Vielma, J. Controlling of geosmin and 2-methylisoborneol induced off-flavours in recirculating aquaculture system farmed fish—A review. Aquac. Res. 2018, 50, 9–28. [Google Scholar] [CrossRef]
- Howgate, P. Tainting of farmed fish by geosmin and 2-methyl-iso-borneol: A review of sensory aspects and of uptake/depuration. Aquaculture 2004, 234, 155–181. [Google Scholar] [CrossRef]
- Calza, P.; Sakkas, V.A.; Medana, C.; Baiocchi, C.; Dimou, A.; Pelizzetti, E.; Albanis, T. Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl. Catal. B Environ. 2006, 67, 197–205. [Google Scholar] [CrossRef]
- Ollis, D.F.; Pelizzetti, E.; Serpone, N. Photocatalyzed destruction of water contaminants. Environ. Sci. Technol. 1991, 25, 1522–1529. [Google Scholar] [CrossRef]
- Musial, J.; Mlynarczyk, D.T.; Stanisz, B.J. Photocatalytic degradation of sulfamethoxazole using TiO(2)-based materials—Perspectives for the development of a sustainable water treatment technology. Sci. Total Environ. 2023, 856, 159122. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, Y.; Sun, W.; Hong, Y.; Li, X.; Lin, X.; Guo, F.; Shi, J. Improvement of synergistic effect photocatalytic/peroxymonosulfate activation for degradation of amoxicillin using carbon dots anchored on rod-like CoFe2O4. Chin. J. Chem. Eng. 2022, 52, 136–145. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Z.; Shi, Y.; Cao, L.; Cheng, X.; Shi, W.; Chen, L.; Lin, X. A ragged porous hollow tubular carbon nitride towards boosting visible-light photocatalytic hydrogen production in water and seawater. Renew. Energy 2022, 188, 1–10. [Google Scholar] [CrossRef]
- Shi, W.; Sun, W.; Liu, Y.; Zhang, K.; Sun, H.; Lin, X.; Hong, Y.; Guo, F. A self-sufficient photo-Fenton system with coupling in-situ production H2O2 of ultrathin porous g-C3N4 nanosheets and amorphous FeOOH quantum dots. J. Hazard. Mater. 2022, 436, 129141. [Google Scholar] [CrossRef]
- López-Muñoz, M.J.; Aguado, J.; Arencibia, A.; Pascual, R. Mercury removal from aqueous solutions of HgCl2 by heterogeneous photocatalysis with TiO2. Appl. Catal. B Environ. 2011, 104, 220–228. [Google Scholar] [CrossRef]
- Chowdhury, P.; Elkamel, A.; Ray, A. Photocatalytic processes for the removal of toxic metal ions. In Heavy Metals in Water: Presence, Removal and Safety; Royal Society of Chemistry: London, UK, 2014; pp. 25–43. [Google Scholar]
- Folli, A.; Bloh, J.Z.; Strøm, M.; Pilegaard Madsen, T.; Henriksen, T.; Macphee, D.E. Efficiency of Solar-Light-Driven TiO2 Photocatalysis at Different Latitudes and Seasons. Where and When Does TiO2 Really Work? J. Phys. Chem. Lett. 2014, 5, 830–832. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Film. 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- He, X.; Zhang, C. Recent advances in structure design for enhancing photocatalysis. J. Mater. Sci. 2019, 54, 8831–8851. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef]
- Cerrato, E.; Paganini, M.C. Mechanism of visible photon absorption: Unveiling of the C3N4–ZnO photoactive interface by means of EPR spectroscopy. Mater. Adv. 2020, 1, 2357–2367. [Google Scholar] [CrossRef]
- Cerrato, E.; Calza, P.; Cristina Paganini, M. Photocatalytic reductive and oxidative ability study of pristine ZnO and CeO2-ZnO heterojunction impregnated with Cu2O. J. Photochem. Photobiol. A Chem. 2022, 427, 113775. [Google Scholar] [CrossRef]
- Cerrato, E.; Gionco, C.; Paganini, M.C.; Giamello, E.; Albanese, E.; Pacchioni, G. Origin of Visible Light Photoactivity of the CeO2/ZnO Heterojunction. ACS Appl. Energy Mater. 2018, 1, 4247–4260. [Google Scholar] [CrossRef]
- Calza, P.; Gionco, C.; Giletta, M.; Kalaboka, M.; Sakkas, V.A.; Albanis, T.; Paganini, M.C. Assessment of the abatement of acelsulfame K using cerium doped ZnO as photocatalyst. J. Hazard. Mater. 2017, 323, 471–477. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization. Toxicological and Health Aspects of Bisphenol A: Report of Joint FAO/WHO Expert Meeting 2–5 November 2010 and Report of Stakeholder Meeting on Bisphenol A, 1 November 2010 Ottawa, Canada; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Cantwell, M.G.; Sullivan, J.C.; Burgess, R.M. Chapter 16—Benzotriazoles: History, Environmental Distribution, and Potential Ecological Effects. In Comprehensive Analytical Chemistry; Zeng, E.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 67, pp. 513–545. [Google Scholar]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: A review. Environ. Sci. Pollut. Res. 2015, 22, 5711–5741. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Meena, R.A.A.; Palanisami, T.; Ashokkumar, V.; Palvannan, T.; Gu, F.L. Occurrence, interactive effects and ecological risk of diclofenac in environmental compartments and biota—A review. Sci. Total Environ. 2020, 698, 134057. [Google Scholar] [CrossRef]
- Dantas, R.F.; Contreras, S.; Sans, C.; Esplugas, S. Sulfamethoxazole abatement by means of ozonation. J. Hazard. Mater. 2008, 150, 790–794. [Google Scholar] [CrossRef]
- Cerrato, E.; Rebolini, E.; Fabbri, D.; Calza, P.; Paganini, M.C. Ternary systems based on ZnO/CeO2/Cu2O for the degradation of phenol and carbamazepine. J. Alloys Compd. 2021, 856, 158167. [Google Scholar] [CrossRef]
- Moctezuma, E.; Leyva, E.; Lara-Pérez, C.; Noriega, S.; Martínez-Richa, A. TiO2 Photocatalytic Degradation of Diclofenac: Intermediates and Total Reaction Mechanism. Top. Catal. 2020, 63, 601–615. [Google Scholar] [CrossRef]
| Matrix | Composition | k [min −1] | ||||||
|---|---|---|---|---|---|---|---|---|
| BTA | SMZ | BPA | DIC | Hg(II) | MIB | Geosmin | ||
| Milli-Q water | Single class | 0.22 | 0.09 | 0.05 | 0.13 | 0.07 | 1.68 | 0.80 |
| Milli-Q water | Mixture | 0.32 | 0.13 | 0.07 | 0.13 | 0.10 | 0.06 | 0.11 |
| Aquaculture | Single class | 0.17 | 0.05 | 0.04 | 0.05 | 0.11 | 0.06 | 0.09 |
| Aquaculture | Mixture | 0.20 | 0.06 | 0.04 | 0.07 | 0.10 | 0.05 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaggero, E.; López-Muñoz, M.J.; Paganini, M.C.; Arencibia, A.; Bertinetti, S.; Fernández de Paz, N.; Calza, P. Mercury and Organic Pollutants Removal from Aqueous Solutions by Heterogeneous Photocatalysis with ZnO-Based Materials. Molecules 2023, 28, 2650. https://doi.org/10.3390/molecules28062650
Gaggero E, López-Muñoz MJ, Paganini MC, Arencibia A, Bertinetti S, Fernández de Paz N, Calza P. Mercury and Organic Pollutants Removal from Aqueous Solutions by Heterogeneous Photocatalysis with ZnO-Based Materials. Molecules. 2023; 28(6):2650. https://doi.org/10.3390/molecules28062650
Chicago/Turabian StyleGaggero, Elisa, María José López-Muñoz, Maria Cristina Paganini, Amaya Arencibia, Stefano Bertinetti, Nieves Fernández de Paz, and Paola Calza. 2023. "Mercury and Organic Pollutants Removal from Aqueous Solutions by Heterogeneous Photocatalysis with ZnO-Based Materials" Molecules 28, no. 6: 2650. https://doi.org/10.3390/molecules28062650
APA StyleGaggero, E., López-Muñoz, M. J., Paganini, M. C., Arencibia, A., Bertinetti, S., Fernández de Paz, N., & Calza, P. (2023). Mercury and Organic Pollutants Removal from Aqueous Solutions by Heterogeneous Photocatalysis with ZnO-Based Materials. Molecules, 28(6), 2650. https://doi.org/10.3390/molecules28062650









