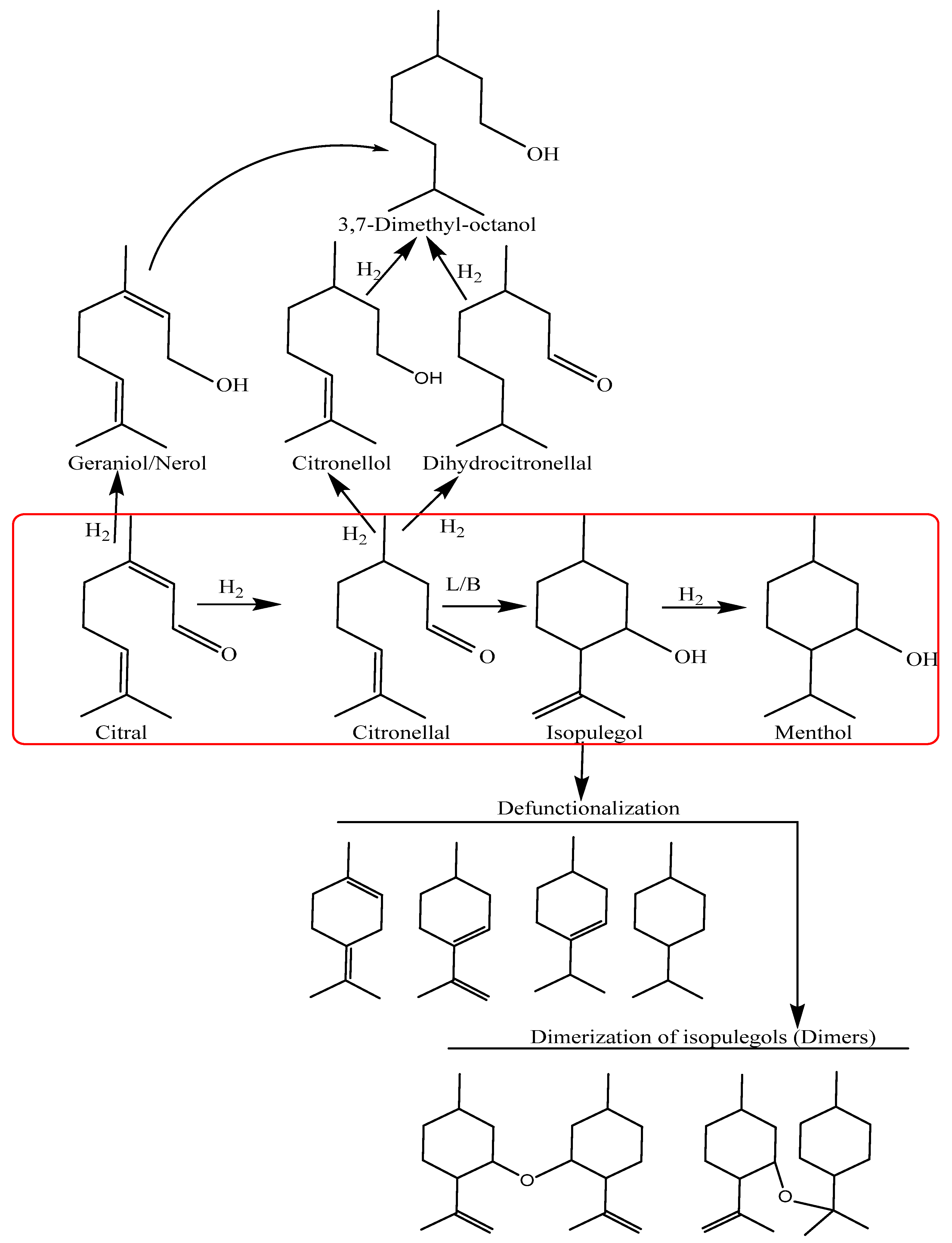
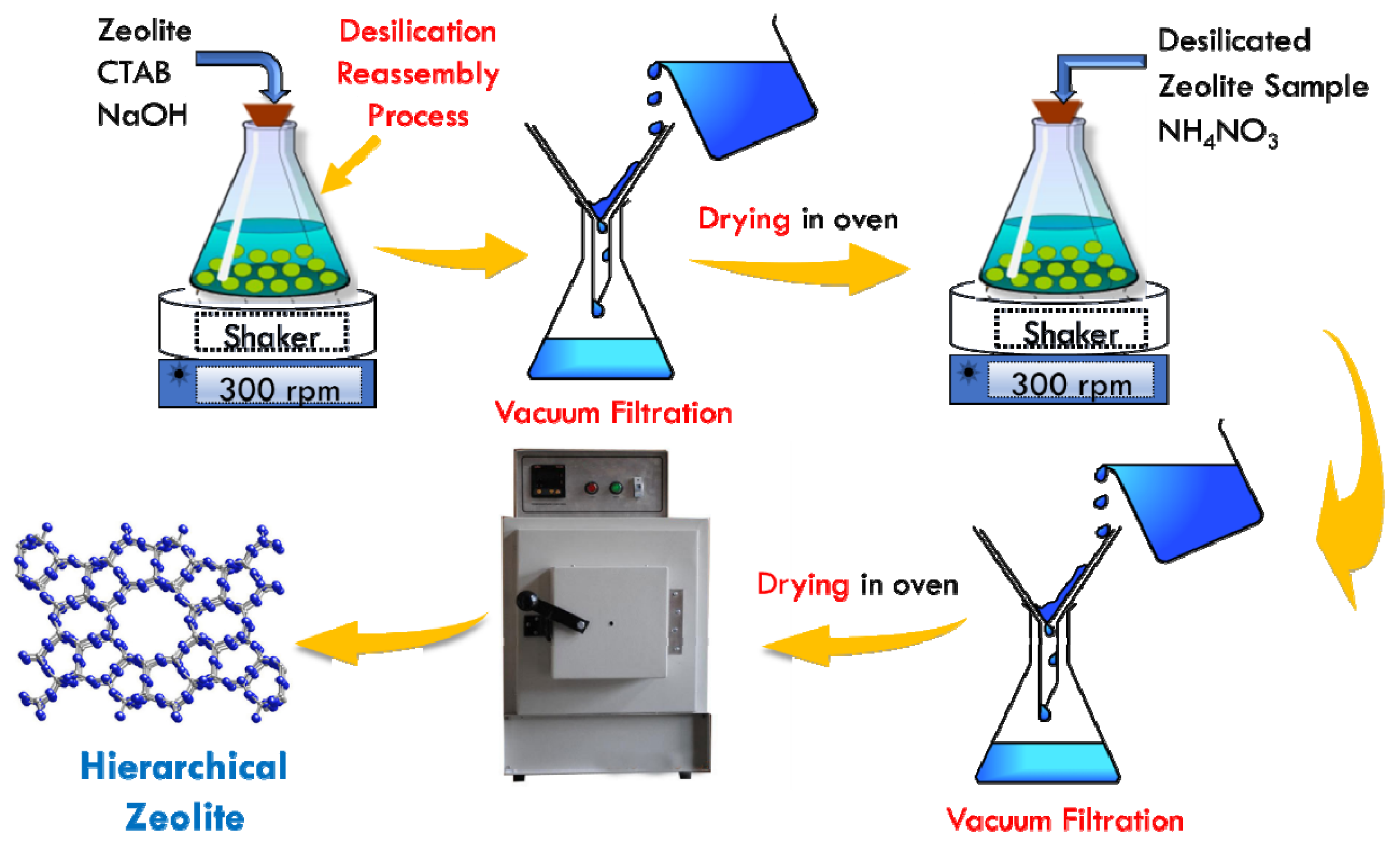
The desilication reassembly process was used to investigate the structural changes and acidic characteristics of zeolites. ZSM-5, as well as USY zeolites, were desilicated with alkali concentrations at optimized conditions. During this alkali treatment, major changes were identified in porosity and structure, as well as acidity. To evaluate the catalyst properties, a number of characterization techniques, such as PXRD, FTIR, pyridine adsorption, NH3-TPD, and H2-TPR, as well as BET sorption, were carried out. We will study and review each characterization in detail to correlate catalyst characterization changes with catalytic activity and menthol synthesis. The detailed characteristics of Ni-supported hierarchical zeolite samples were determined and studied in detail.
2.1. Catalysts Characterization
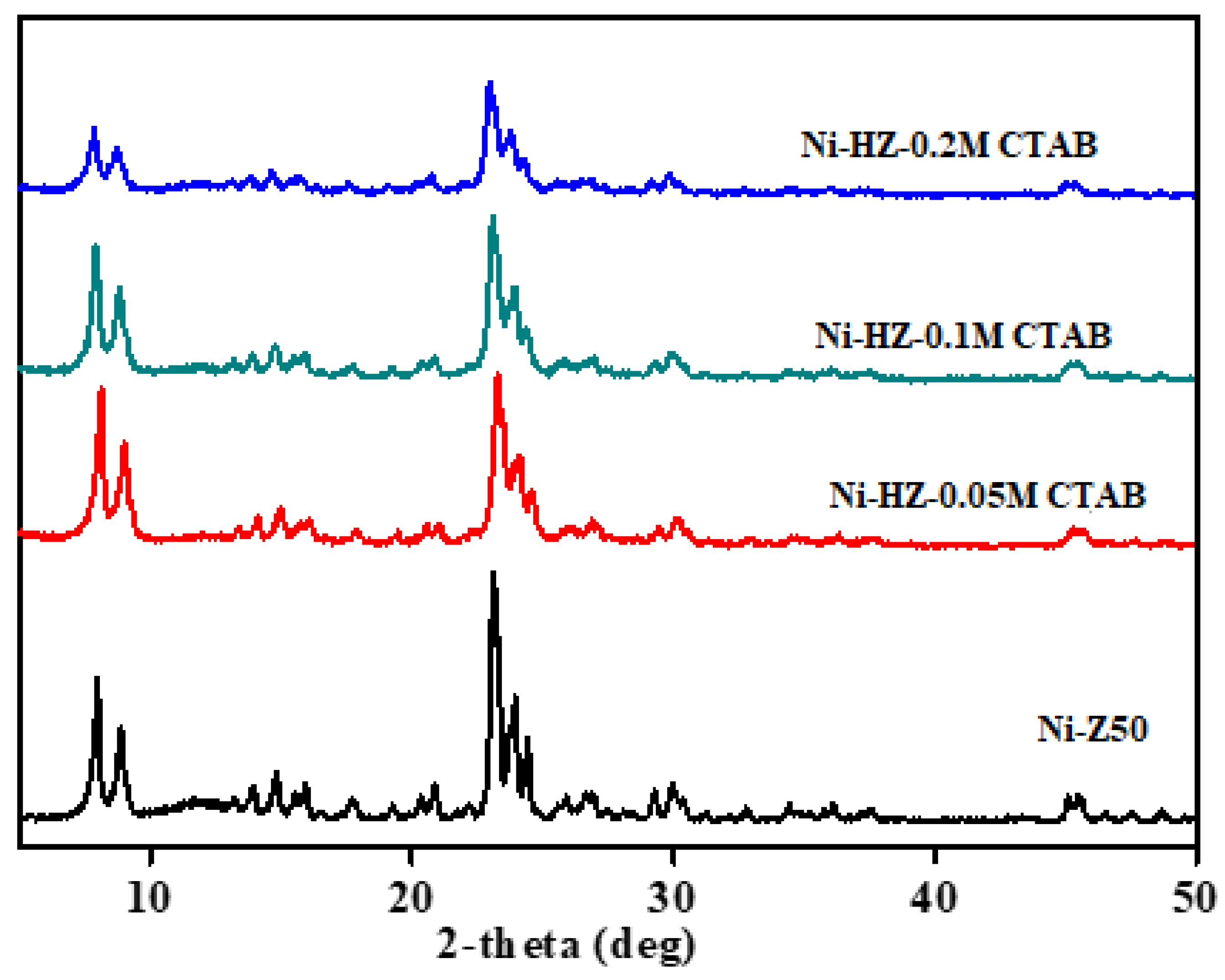
XRD analysis of all prepared catalysts (nickel-supported) was conducted. The high crystal peaks were seen in the Ni/Z50 catalyst. The XRD peak intensity was drastically decreased with an increase in alkali concentrations. This change may indicate the loss of Si ions from the zeolite framework and weaken the zeolite XRD structure. The Ni-HZ-0.5 M catalyst was found as the most stable material after desilication treatment. NiO metal deposition was confirmed from XRD peaks at 44, 55, and 77°. Similarly, the USY80 zeolite was treated with alkali treatment (0.5 M NaOH and 0.05 M CTAB) and then doped with the nickel precursor. Ni-USY80 was found as the most stable material and possessed high-intensity XRD peaks, which were further decreased after alkali treatment. The reduction in XRD peaks confirms the loss of Si atoms and partial structural damage (
Figure 1). Similarly, ZSM-5 zeolites were further desilicated at a fixed amount of NaOH (0.5 M) using different concentrations of the CTAB surfactant (0.05 M, 0.1 M, and 0.2 M). The effects of CTAB concentrations on the zeolite XRD structure are shown in
Figure 2. A higher concentration of CTAB resulted in more dissolution of the zeolite framework and a destroyed crystal structure. The optimum CTAB concentration was 0.05 M. The effects of the CTAB surfactant were observed in the XRD structure, structure stability, and porosity enhancement. The effects of alkali treatment with respect to NaOH and CTAB concentration variations on zeolitic XRD structures were analyzed, as shown in
Figure 1 and
Figure 2. Higher concentrations of NaOH and CTAB have completely dissolved zeolite framework and caused more Si leaching. This effect has destroyed XRD crystal structure of zeolites. During optimization study, 0.5 M NaOH and 0.05 M CTAB concentrations were found optimum for obtaining hierarchical zeolite with a well-maintained XRD structure.
Furthermore, ICP-OES analysis of all nickel-supported catalysts was carried out. The amounts of Ni and Si/Al were analyzed in all zeolite samples. The amount of Si/Al is decreased during desilication reassembly process as shown in
Table 1. Alkali treatment was conducted at different sodium hydroxide concentrations. The reduction in Si/Al means Si ions leached during desilication. The Si reduction amount confirms the desilication process, which was carried out successfully. Nickel loading was improved over the desilicated samples. ICP-OES analysis provided confirmation that nickel precursor particles were almost completely attached to zeolite surfaces.
Furthermore, BET sorption–desorption analysis was conducted for all nickel-supported catalysts, as shown in
Table 1. The parent zeolite possessed a high amount of micropore volume and low mesopore volume. The values of the mesopore volume and surface area were drastically enhanced with alkali treatment. More severe Si leaching from the zeolite framework caused an increase in the mesopore volume and mesopore surface area. The original value of the micropore volume was continuously decreased with alkali treatment (
Table 1).
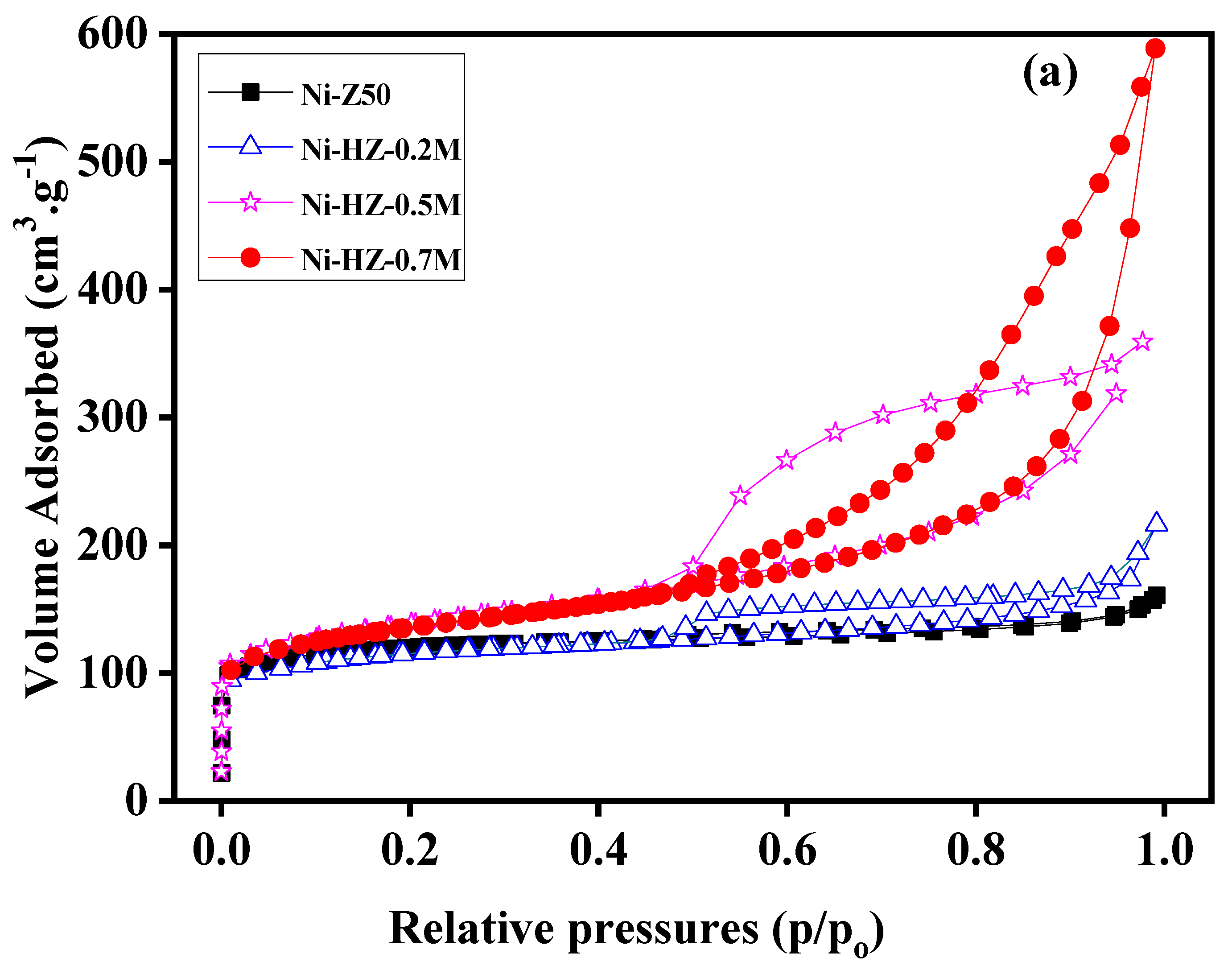
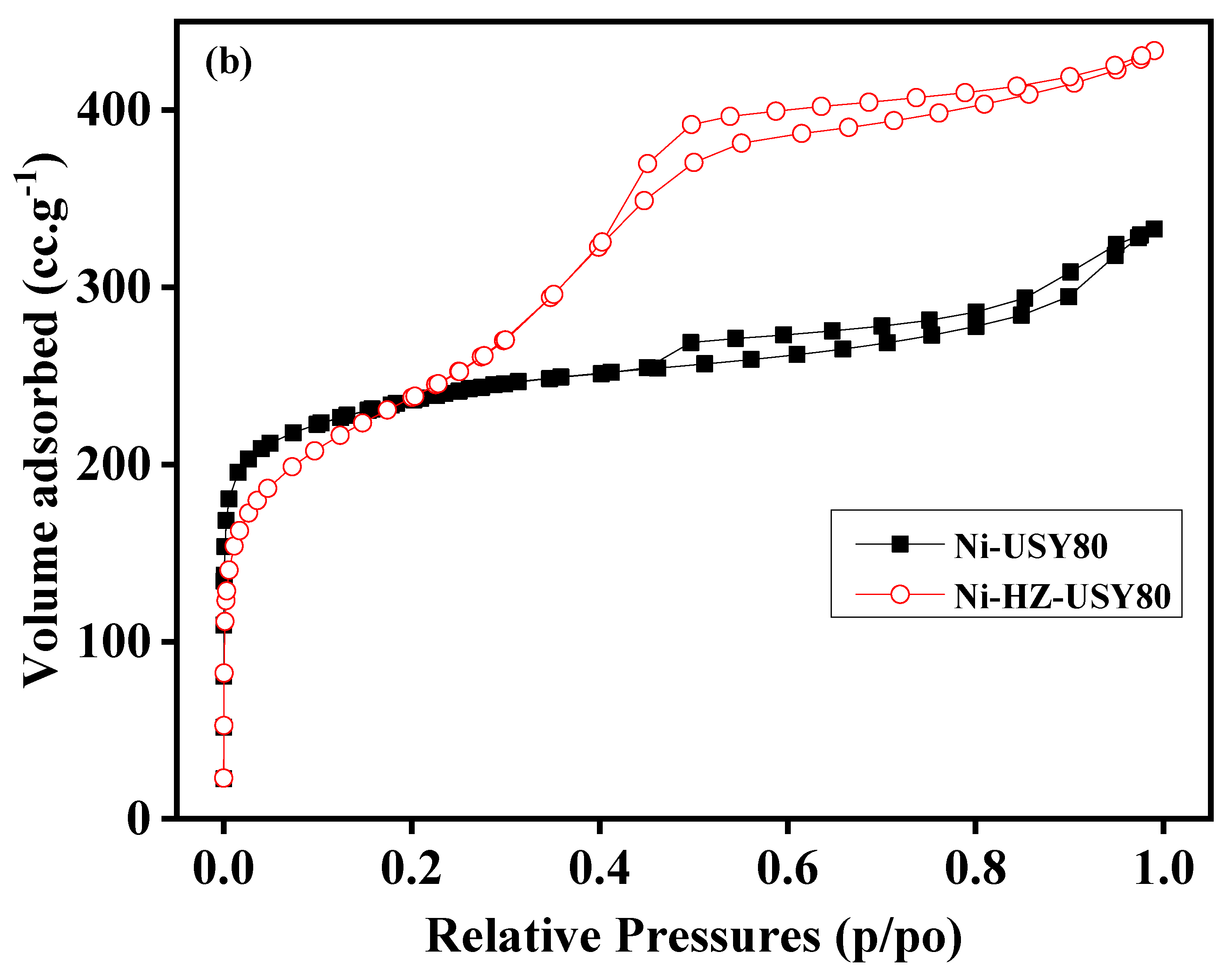
Similarly, BET sorption isotherm data of all samples (ZSM-5 and USY hierarchical zeolites) were revealed, as shown in
Figure 3a,b. The sorption isotherm of Ni-Z50 (ZSM-5) may indicate the presence of micropores in the zeolite structure. With alkali treatment, the zeolite framework structure was reshaped, and its micropore characteristics were decreased, and new mesopores were formed inside the zeolite structure. This desilication process produces a number of wide mesopores in the zeolite framework, which helps in surface area enhancement (
Table 1 and
Figure 3a,b). According to IUPAC classifications, the two isotherms are of the IV shape with a H4 hysteresis loop, which is generally attributed to a hierarchical porous material containing both micropores and mesopores. The strong uptake at low relative pressure certifies the presence of micropores.
Furthermore, it was observed from BET and isotherm data that no more changes in textural properties were found in hierarchical zeolites after nickel doping. The ratio of Si/Al decreased with alkali concentration, which may enhance the mesopore surface area and mesopore volume, as shown in
Table 1. Furthermore, it was observed that the Ni particle size was drastically increased with respect to alkali treatment. This size enhancement might be linked to wide mesopore formations.
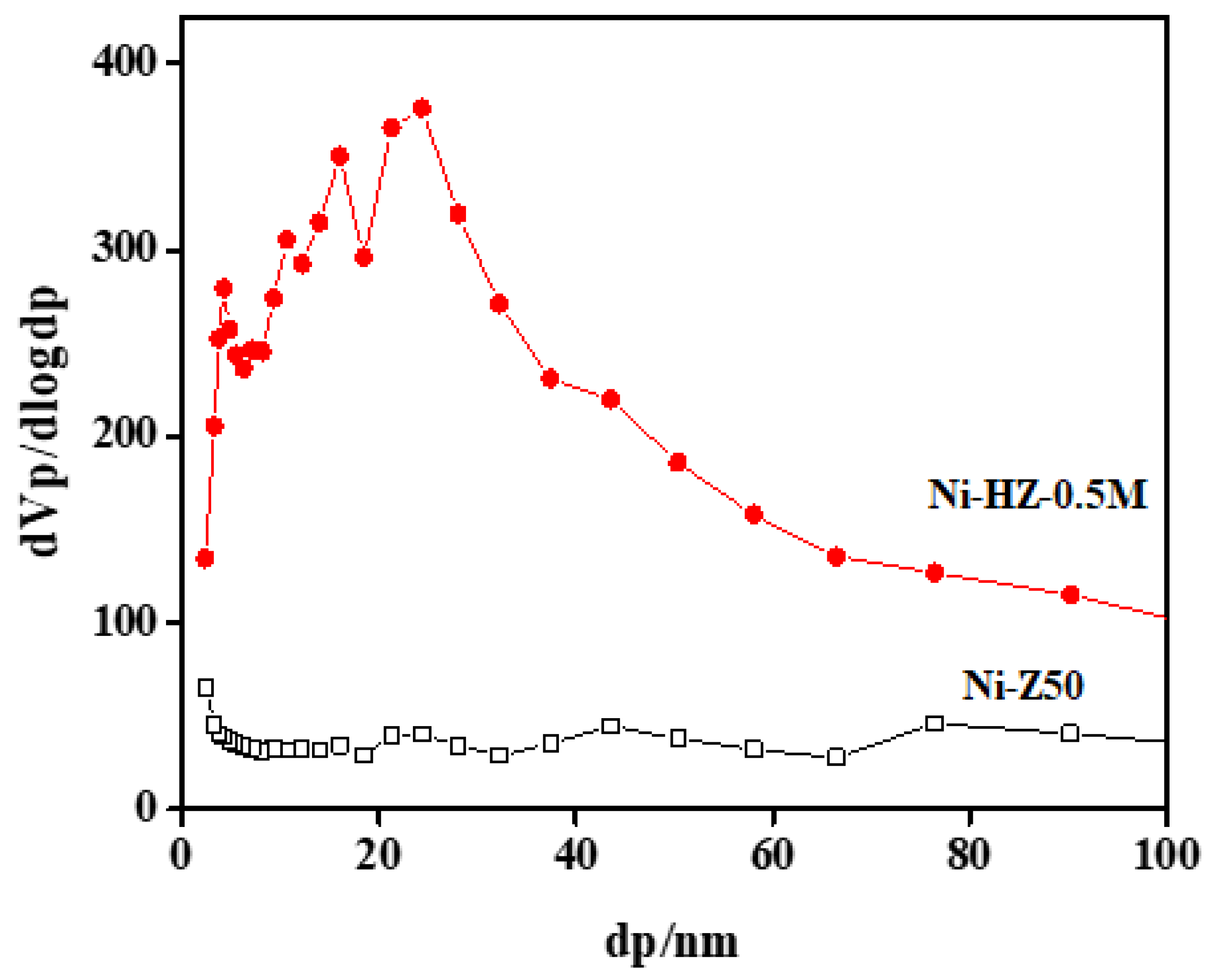
BJH pore size distribution analyses of parent zeolite and hierarchical zeolite samples are given in
Figure 4. The BJH pore size distribution curves of Ni-Z50 and Ni-HZ-0.5 M are shown in
Figure 4. A major difference was observed in both samples. Parent zeolites possessed a purely microporous structure (e.g., a peak appeared at 2 nm), whereas new pore structure peaks appeared between the 5 and 35 nm range, which may confirm the formation of mesopores on the surface of zeolite frameworks after the desilication process, as shown in
Figure 4. There was also a wide distribution of larger pores, stretching into the mesopore range up to 35 nm.
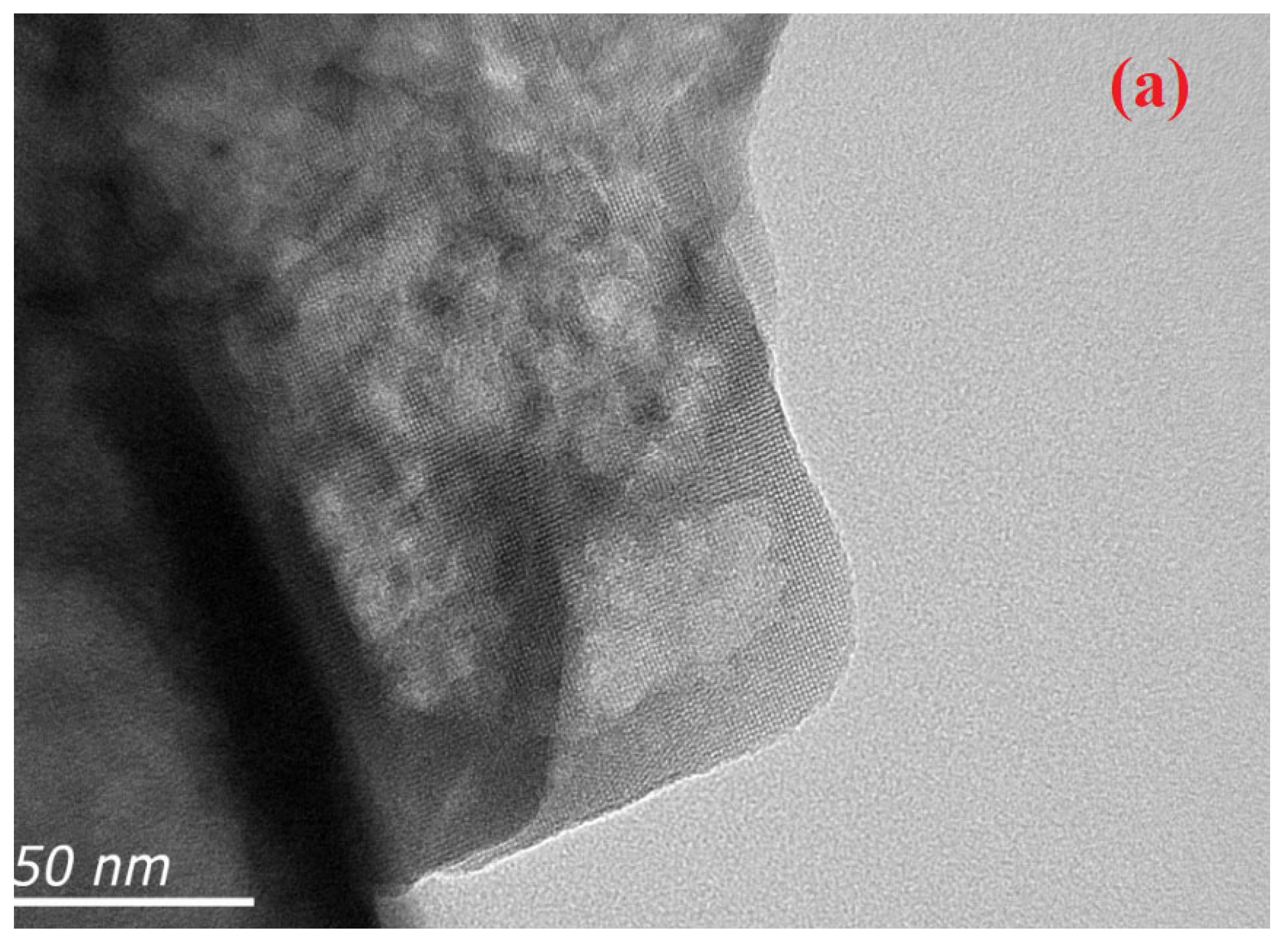
TEM analysis revealed the typical platelet-like morphology of the hierarchical zeolite (
Figure 5). The TEM images show some morphological changes in the zeolite surface morphology. TEM analysis reflects the BET data that confirm mesopore formation. The black spots on the hierarchical zeolite surface may indicate the deposition of nickel particles, as shown in
Figure 5b.
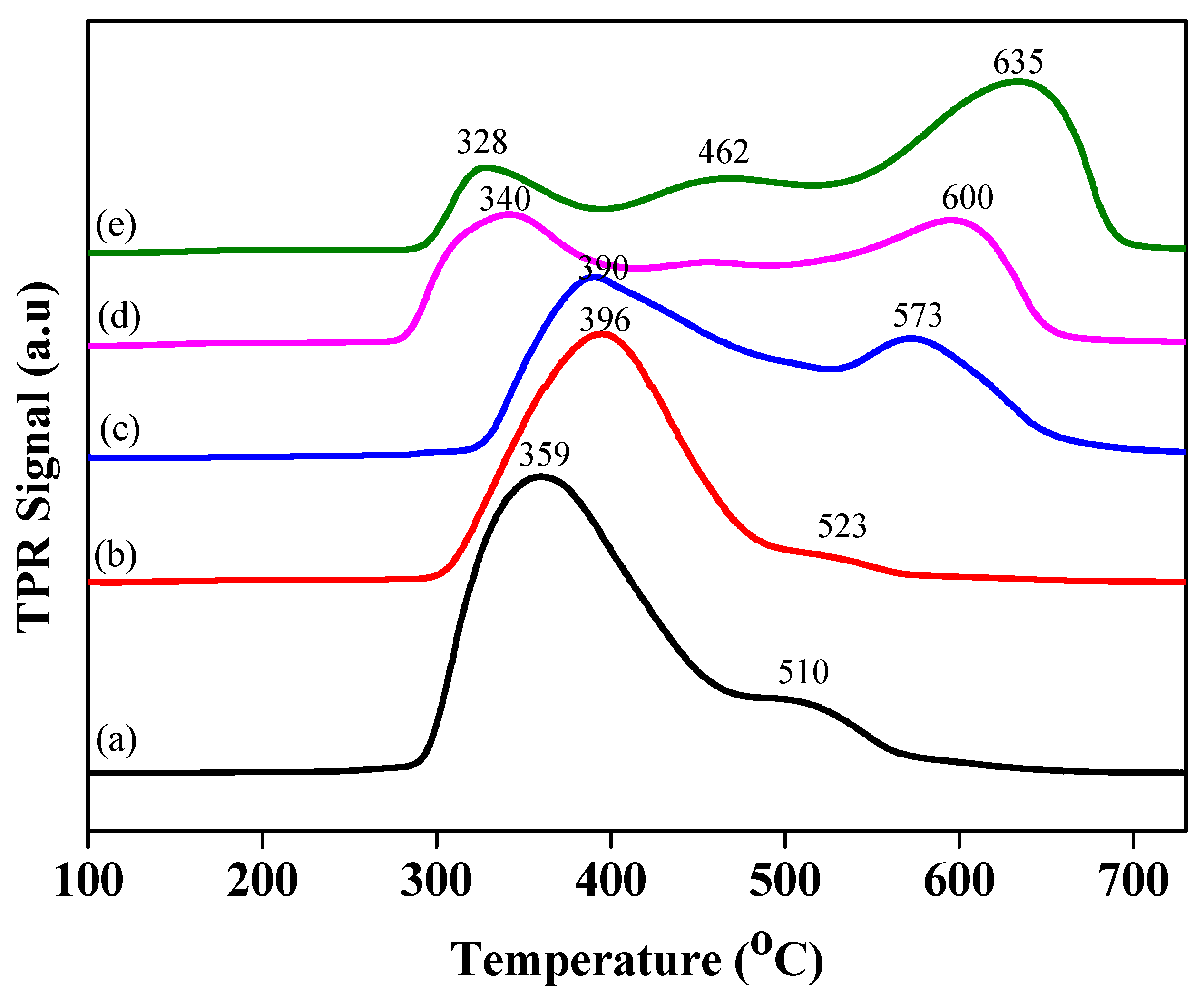
Further, H
2-TPR analysis of all catalysts was conducted to evaluate the thermal changes in catalysts at different temperature ranges. TPR analysis, as well as nickel dispersion and particle size analysis of all samples, are provided in
Figure 6 and
Table 2. Low-temperature (LT) and high-temperature (HT) peaks can be seen in the TPR graph. This analysis reveals the presence of Ni
2+ and NiO ions, as well as their interaction behaviors with hierarchical supports with reduction profiles at LT and HT peaks. The LT and HT peaks changed with alkali treatment. Temperature increase profiles were observed, as shown in
Figure 6. The metal dispersion of Ni-supported hierarchical zeolite catalysts was assessed using the H
2 chemisorption method, and the results are presented in
Table 2. Ni particles were evenly distributed within the catalysts’ mesopores. The amount of Ni was dispersed over hierarchical zeolite surfaces because of the wider mesopores and high surface area (180–301 m
2·g
−1). The nickel metal dispersion rate was increased with the increase in mesoporosity and surface area. Nickel dispersion also had a connection with alkali treatment. It might be presumed that alkali treatment induced changes in the zeolite framework crystal structure and acidic properties.
Nickel dispersion over the parent and desilicated zeolites might have a negligible impact on surface properties. Nickel dispersion may slightly change metal and acid site strengths; its direct effects on catalytic activity and menthol selectivity were not observed. Similarly, nickel particle size variations may not have a direct impact on catalytic activity
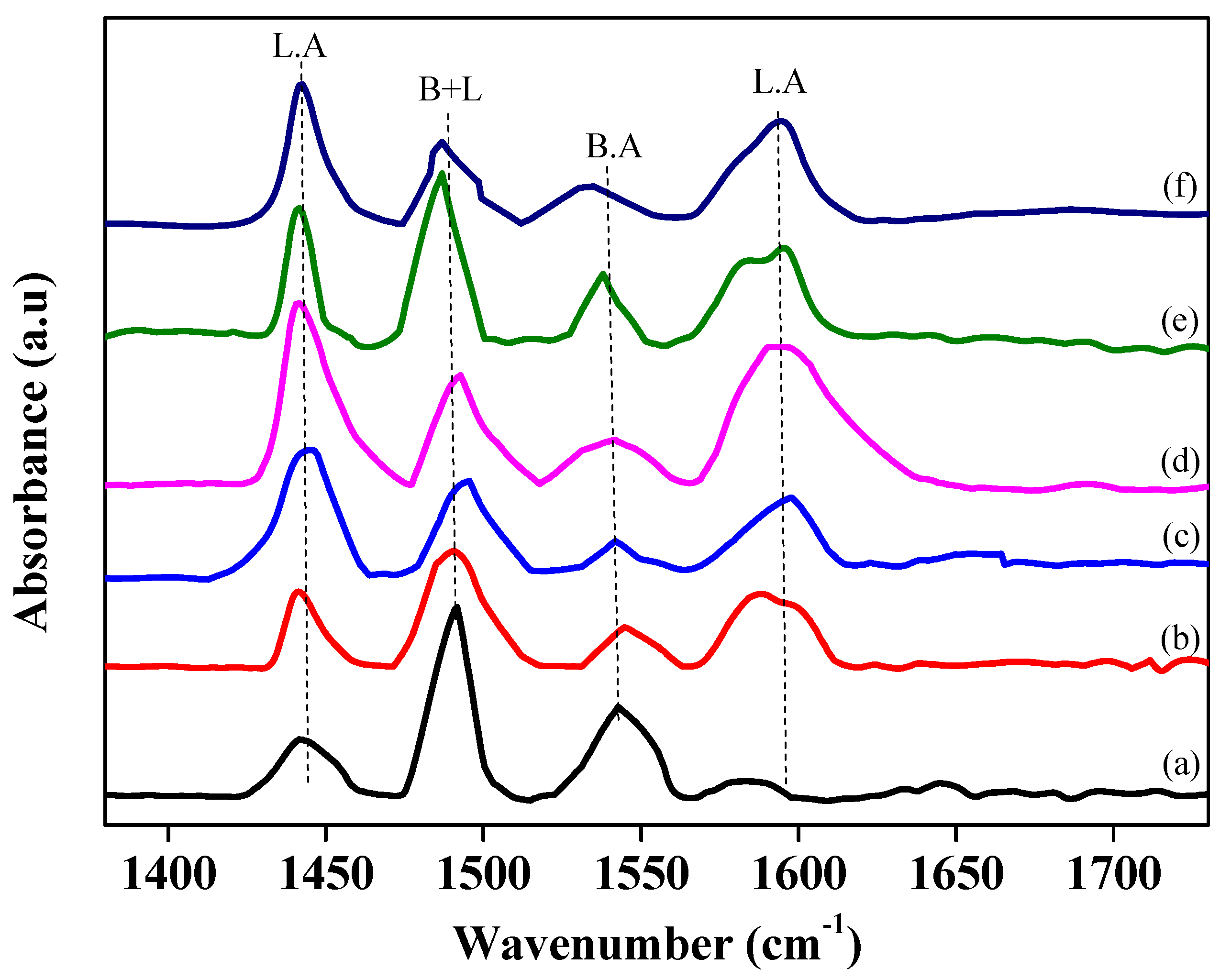
Table 3 and
Figure 7 and
Figure 8 show pyridine adsorption FTIR and NH
3-TPD characterization data of Ni-supported hierarchical zeolite catalysts. The acid strength and the number of acid sites are basic properties of solid acid catalysts/zeolites. The strength is probably measured by ammonia TPD, whereas the acid amount can be determined through the pyridine adsorption technique. The measurement of temperature-programmed desorption (TPD) and the heat of adsorption using a typical base, such as ammonia and pyridine, are the most useful techniques for evaluating the relative acid strength and the number of acid sites. Pyridine adsorption analysis also provided the quantitative and qualitative data of all zeolite samples. In the case of solid acid catalysts/zeolites, it is expected that the number of adsorption molecules will be equal to that of acid sites on the surface. It measures the nature and strength of acid sites. Additionally, we used NH
3-TPD and pyridine adsorption techniques to evaluate the acidity characteristics of the prepared catalysts. The NH
3-TPD profile showed high-temperature peaks as well as lower-temperature peaks. High-temperature peaks indicate strong acidic sites, whereas lower-temperature peaks indicate weak acid sites [
24,
25]. NH
3-TPD and pyridine adsorption data show exceptional changes in acidity and acid site strength (
Table 2 and
Figure 7 and
Figure 8).
Table 3 shows NH
3-TPD and ICP-OES analysis data of catalyst samples. The amount of acidity (at low-temperature adsorption) improved with an increase in Al content. This Al content may be correlated with Si ion removal during the desilication process. Two curve peaks were recorded in the NH
3 TPD spectrum: (a) from the total NH
3 desorption at temperatures higher than 150 °C and (b) from the NH
3 desorbed at temperatures higher than 390 °C, as shown in
Figure 8. According to Hegde et al. [
26], this distinction is necessary since, at lower temperatures, part of ammonia desorbs not from Bronsted sites but from structural defects, which are largely present in the hierarchical zeolites. It was, hence, assumed that the ammonia excess, in relation to the aluminum content, desorbed at temperatures between 150 °C and 390 °C would be adsorbed on structural defects. According to Jansen et al. [
27], these defects are present in hierarchical zeolites due to local silicon unsaturated bonding, thereby introducing potential Lewis acid sites. Since stronger acid sites desorb ammonia at higher temperatures [
28].
Characterization data suggest the presence of strong Bronsted and weak Lewis acid sites on the surface of the parent zeolite sample (Ni-Z50) [
24]. After the desilication reassembly process, the strength of Lewis acid sites of zeolite was increased with Si leaching. The alkali treatment resulted in the creation of new Lewis acid sites, whereas the strength of Bronsted acid sites decreased, as shown in
Figure 8 and
Table 3. This drastic change in acid sites might be due to Si leaching from the zeolite framework and an increase in Al content. This increase in Al content may cause more increases in total acidity, as shown in NH
3-TPD data (
Table 3). This acidity enhancement is correlated with the nature of acid sites and their strength. After the desilication process, the Ni-HZ-0.5 M catalyst exhibited strong Lewis acid sites and medium Bronsted sites as compared to the Ni-Z50 catalyst, whereas the Ni-HZ-0.7 M catalyst exhibited strong Lewis acid sites as well as weaker Bronsted acid sites.
The sudden enhancement in Lewis acid sites is probably interconnected with Si leaching and Al content deposition on the zeolite exterior surface. NH
3-TPD and pyridine adsorption analysis data reveal that total acidity and acid site ratios were increased with alkali treatment. Similarly, the pyridine adsorption characterization of the USY zeolite and its hierarchical zeolite catalysts were carried out to evaluate the nature and strength of the acid sites. The parent USY zeolite contained strong Bronsted and weak Lewis acid sites on its surface. During desilication treatment, there were drastic changes in Lewis/Bronsted sites. The strength of Lewis acid sites improved after alkali treatment, as shown in
Table 3. USY zeolite samples contained a high amount of acidity as compared to ZSM-5 samples. Whereas Bronsted sites drastically decreased during desilication treatment. The quantity of Lewis acid sites were improved as compared to Bronsted acid sites. It can be assumed that this type of radical change in acidity and acid sites may introduce some changes in the overall catalytic performance and menthol synthesis. This reaction might be connected to mesopores and acid sites. This reaction probably took place through physical surface adsorption into mesopores and interaction with active acid sites.
2.2. Synthesis of Menthol from Citral Hydrogenation
Citral was hydrogenated in an autoclave batch reactor at 100 °C and 1.0 MPa hydrogen to investigate the characteristics of Ni-supported hierarchical zeolite catalysts. The reaction performance of all catalysts is reported in
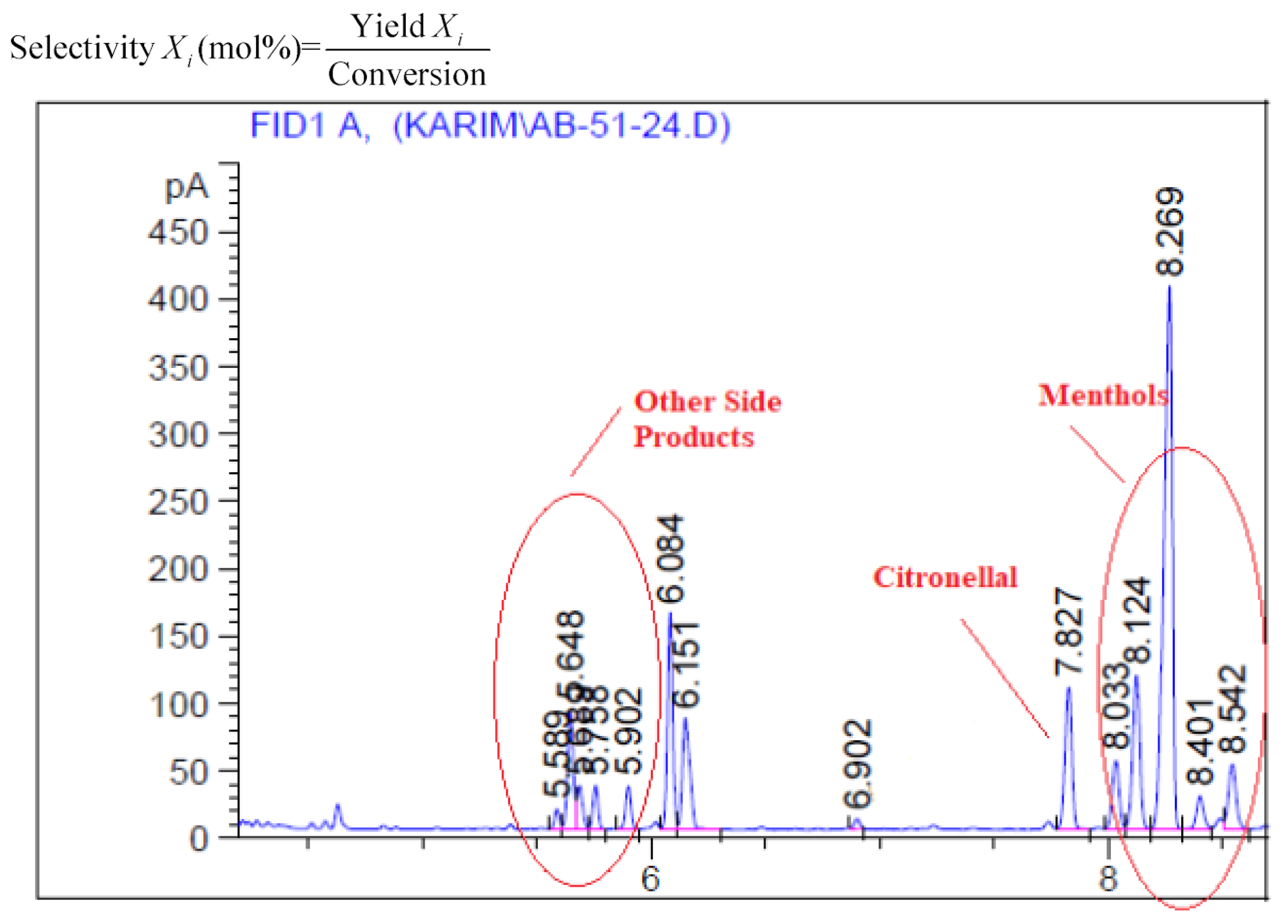
Table 4. Citral hydrogenation produced the primary compounds, including citronellal, isopulegol, menthol, citronellol, and 3,7-dimethyl octanol, with only a minor number of dehydrated products produced.
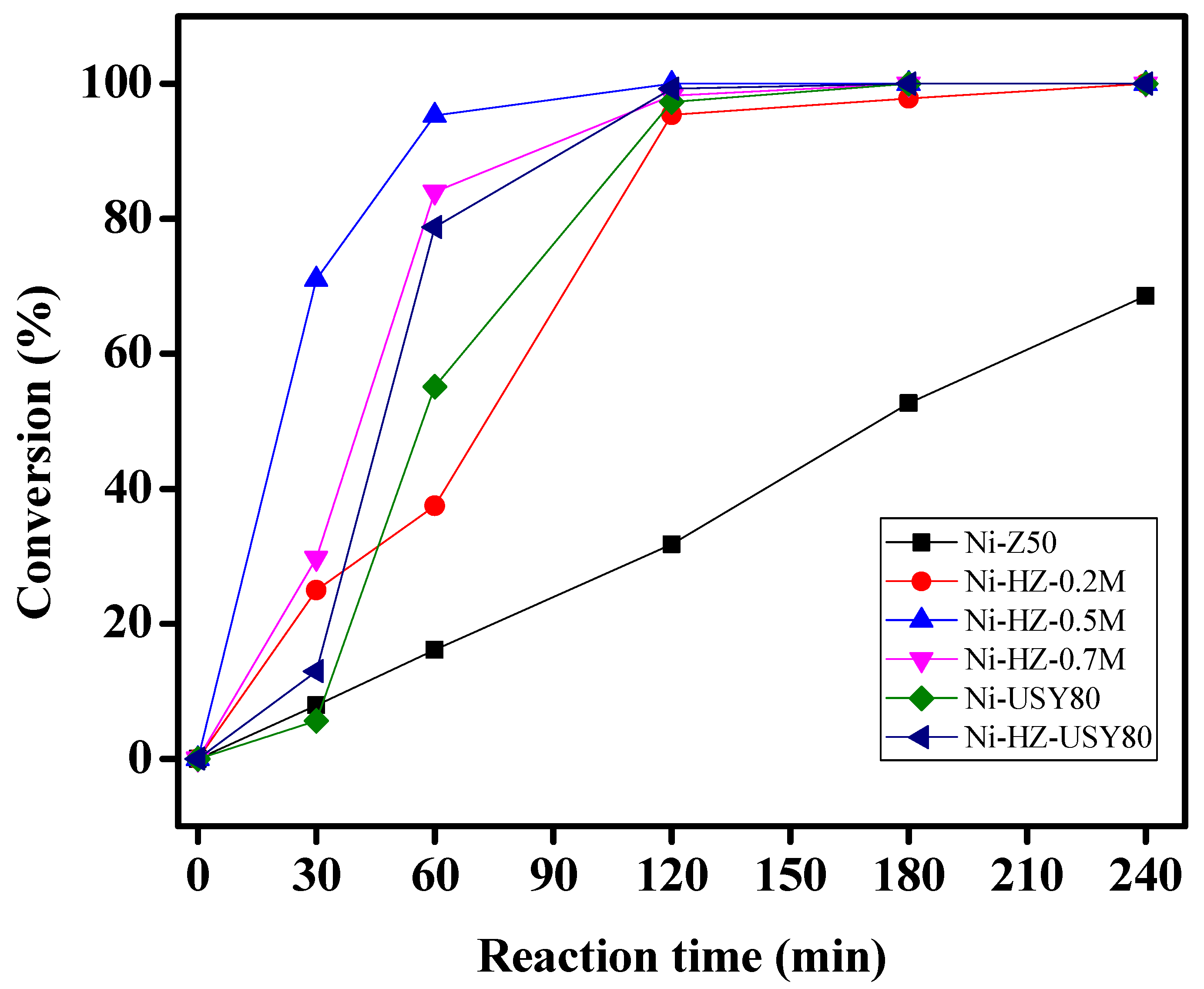
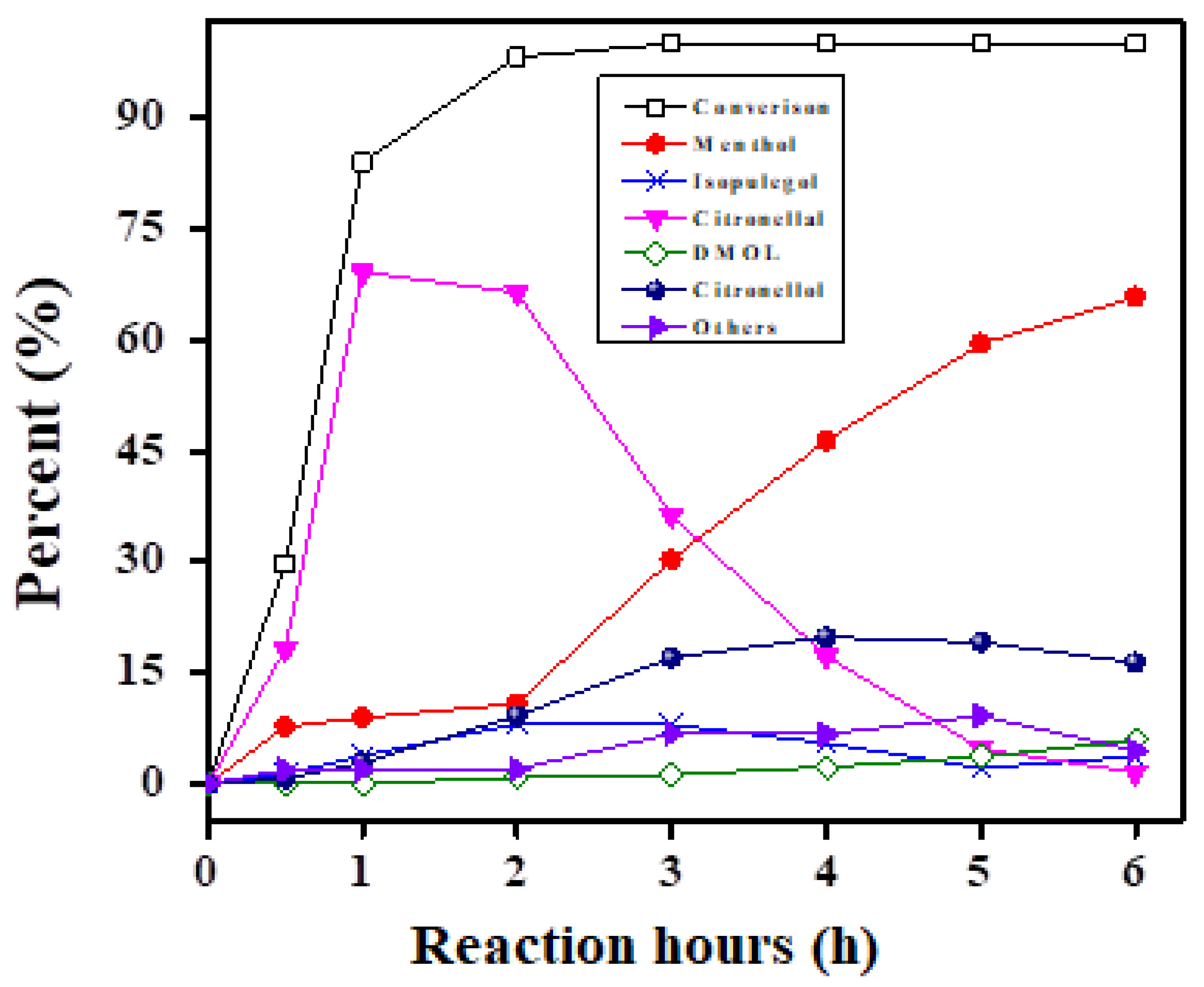
Figure 9 highlight the catalytic activities of various catalysts during the hydrogenation of citral as well as changes in the catalytic activity product distribution and citral conversion over time. It is interesting to note that citronellal and isopulegol were produced at the first stage of hydrogenation before being converted to menthol or citronellol.
Similarly,
Figure 10 shows the catalytic performance of various catalysts for menthol production. All hierarchical zeolites showed different catalytic performance in menthol synthesis. The surface, as well as the acidic properties of all desilicated zeolites, drastically changed with respect to alkali treatment. The Ni-Z50 catalyst was totally microporous in nature; its mass transfer rate was the lowest when compared to the other samples. This catalyst probably could produce menthol less than 8% within 24 h. Specific textural changes were observed in the parent zeolite after alkali treatment; its surface and acidic properties were enhanced, which helped in catalytic activity enhancement and menthol formation, as shown in
Figure 10. Among these catalysts, the Ni-HZ-0.5 M catalyst could produce 83% menthol within 300 min. By comparing the characterization and reaction data, catalytic activity and menthol formation have no direct link to Ni particle size.
Table 4 illustrates that the Ni-supported hierarchical zeolite (Ni/HZ-0.5 M) catalyst outperformed other catalysts in the citral hydrogenation reaction, achieving 100% conversion in 1 h and a TOF of 7.12 h
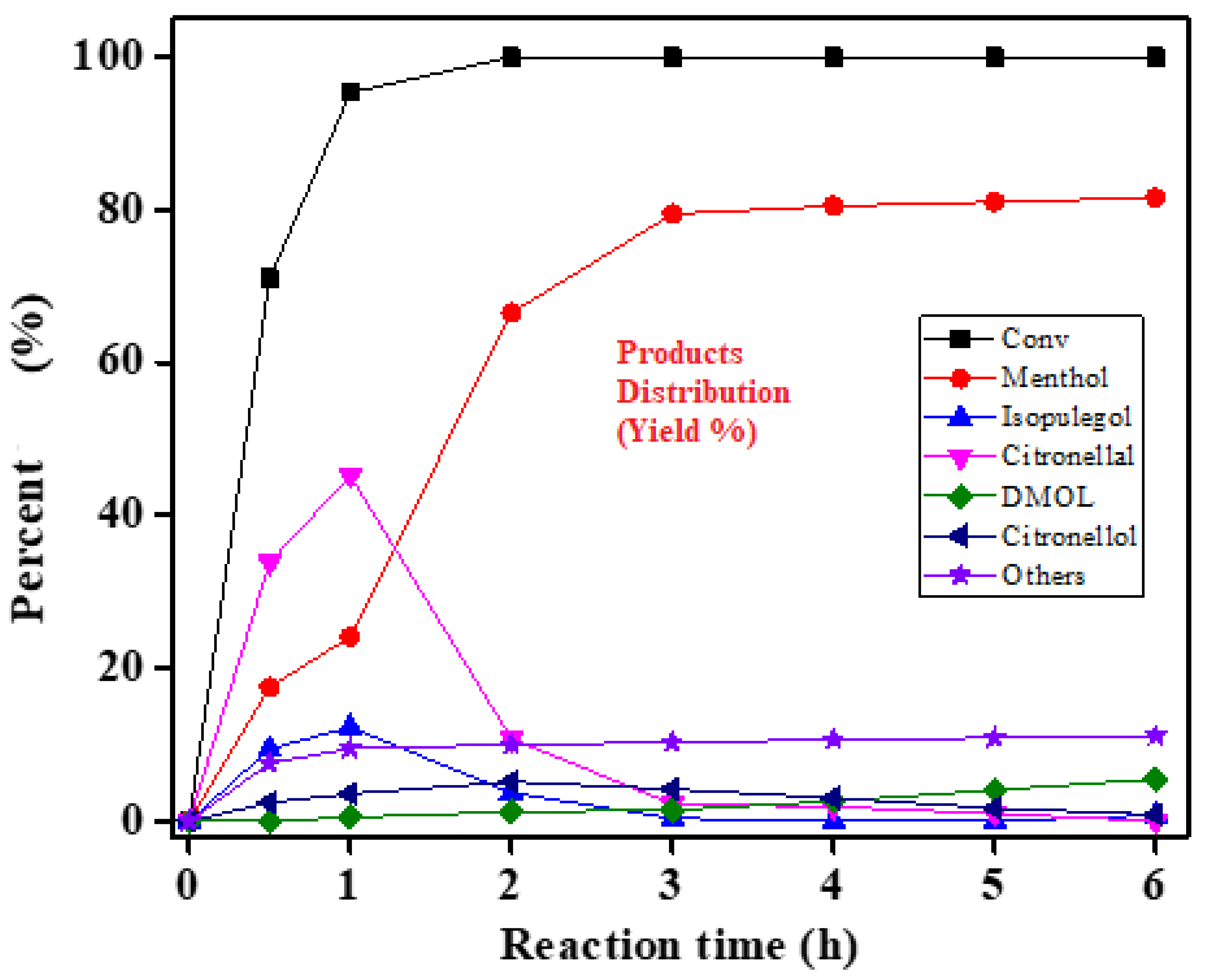
−1. After 1 h, the rate of menthol synthesis increased quickly, yielding 83% of menthol after 3 h. Citronellal converted to isopulegol more quickly than other catalysts, although subsequent hydrogenation to menthol happened more slowly. Mass transfer rates, selectivity, and reaction rates all improved. Without favoring alternative hydrogenation processes, this catalyst enabled the direct conversion of citral to citronellal and, finally, isopulegol (by roughly 94%). (
Figure 11). Due to the dehydration of isopulegol, some amounts of fractured products were produced with reaction time. The catalytic activity was specifically in the range Ni-HZ-0.7 M > Ni-HZ-0.5 M > Ni-HZ-0.2 M > Ni-Z50.
The desilication reassembly process of zeolite may bring structural changes that may affect catalytic activity and selectivity. XRD and BET analyses reveal the formation of micro-mesoporous structures. The narrow pore structures cause the poor mass transfer of molecules, the accumulation of molecules (lower diffusion), and less accessibility to acid sites as well as active sites of catalysts. Purely micro porous nature zeolite, ZMS-5 (Ni-supported Z50) exhibited poor catalytic performance and produced lower amount of menthol in one pot reaction, this poor performance might be connected with zeolite narrow pores that resulted low diffusion. Ni-Z50 has presumed poor catalytic activity (TOF 0.8 h
−1), and menthol formation was 4%. However, the desilication reassembly process might have induced porosity structure changes that have a close relation to catalytic activity and menthol production enhancement. The hierarchical zeolite-supported nickel had enhanced catalytic activity, which was increased from 0.8 TOF h
−1 to 4.75 TOF h
−1. This resulted in full substrate conversion and produced 83% menthol within short time (3 h). The excellent catalytic performance and fast diffusion was achieved in hierarchical zeolite samples; this change might be connected with high mesoporosity and balanced metal-acid sites (
Figure 9,
Figure 10 and
Figure 11 and
Table 4). Catalytic performance and selectivity are majorly connected. This hierarchical zeolite introduced an excellent change in structural and porosity properties, which improved catalytic performance, reaction rate, and product selectivity characteristics.
It was revealed that the desilication reassembly process of zeolite induces novel changes in the microporous structure and acidic properties. The reactant molecules may not have access to active acidic sites for the reactant because of the narrow micropore structure. The molecules’ approach is negligible, which causes lower catalytic activity and product formation. It was revealed from previous studies that the presence of acid sites over the catalyst surface is necessary for the transformation of citronellal to isopulegol, then menthol. The easy access of reactant molecules to active acidic sites enhanced catalytic activity and menthol product formation. The easy molecular access to acid sites is probably because of wide pore formation and an increase in surface area. These structural and acidic changes might have some effects on the reaction performance of one-pot menthol synthesis. It was revealed that the total acidity strength of the catalyst may be closely interconnected to mesoporosity enhancement. Similarly, the enhancement in acidity improved catalytic performance in liquid-phase citral hydrogenation. Textural and reaction performance characteristic data are shown in
Table 1,
Table 2,
Table 3 and
Table 4. Wider mesopores probably provide more accessibility of reactant molecules to active inner and outer acidic sites of the catalyst. It was exposed that these acid sites may develop during aluminum ion deposition on the exterior surface of the zeolite framework, which create new Lewis acid sites (e.g., on Si leaching from the zeolite framework by use of the desilication reassembly process). Experimental data justified the improved catalytic performance (TOF from 0.8 to 7.14 h
−1) and menthol selectivity (~83%), which are directly interlinked with enhanced acidity and mesoporosity characteristics (
Table 1,
Table 2,
Table 3 and
Table 4).
The types and nature of acidic sites (e.g., Lewis and Bronsted) have a great influence on catalytic activity and selectivity enhancement. The strength of acid sites is considered a major problem in catalyst design based on the reaction mechanism. A zeolite is a narrow micropore structure and possesses strong Bronsted acidity. The Si leaching process (desilication reassembly process) changed the acidity strength and L/B ratio. The strength of Lewis acid sites improved during Al deposition on the zeolite extra framework. Similarly, the strength of Bronsted acidity decreased, whereas Lewis acidity strength increased. During the preparation of the hierarchical zeolite, Lewis acidity was stronger than Bronsted acidity, which may promote citronellal cyclisation to isopulegol, then hydrogenation to menthols. An experimental study and previous study predicted that strong Bronsted acidity may cause the cracking or dehydration of isopulegol/menthol-based cyclic compounds, which may result in a reduction in menthol production. However, Lewis acid sites of the hierarchical zeolite were enhanced as compared to Bronsted sites (e.g., total acidity increased from 1.25 to 2.12 mmol·g
−1) (
Figure 8 and
Table 4). The poor performance of the parent zeolite in citronellal cyclisation might be due to the presence of micropores and Bronsted acid sites. Although, the new Lewis acid sites formed due to the creation of wider mesopores inside the zeolite framework.
Experimental data (
Table 4 and
Figure 9 and
Figure 10) indicate that Ni-supported zeolites (Ni/50 and Ni/HZ-0.2 M) possess poor catalytic performance in citral hydrogenation for one-pot menthol synthesis; this might be due to the presence of micropores, less surface area, and weak Lewis acid sites. The menthol production rate improved with an increase in Lewis acidity after alkali treatment. Higher concentration of Lewis acid sites on the hierarchical zeolite surface has been achieved due to Si leaching, However, the Ni/HZ-0.5 M catalyst produced 83% menthol at full citral substrate conversion within a short time (~3 h) in the presence of strong Lewis and weaker Bronsted acid sites (
Table 3 and
Table 4). The formation of wider mesopores results in an increase in Lewis acid sites and provides easy accessibility to substrate molecules for fast and desired reactions [
29].
Similarly, the effects of nickel metal dispersion on the surface of hierarchical zeolites were studied, and their correlation with catalytic activity and menthol selectivity was determined. It was observed from experimental and characterization data that the nickel dispersion rate improved with the formation of mesopores and high surface area. The metal dispersion has a close interaction with the mesoporosity and acidity strength characteristics that may affect (improve) catalytic activity and selectivity. The metal dispersion with mesoporosity and acidity properties showed different catalytic behavior in one-pot menthol synthesis. When the nickel metal was doped over the parent zeolite (Ni/Z50), it showed less metal dispersion on the surface of the zeolite, which resulted in poor catalytic performance in the one-pot menthol synthesis reaction. It may be assumed that this weak catalyst performance might be due to the unavailability of mesopores and weak acidity, which restricts facilitating mass transfer or fast diffusion. In this era, an additional characterization study was carried to find out the effects of nickel metal dispersion on the hierarchical zeolite surface. The nickel dispersion rate was drastically enhanced in the hierarchical zeolite (4.99%) as compared to the parent zeolite (2.95%). This drastic improvement might be related to wide mesopores and high surface area (180~301 m
2·g
−1) and the decrease in crystallite size (34.28~20.25 nm), as shown in
Table 1 and
Table 2. On the utilization of the nickel-dispersed hierarchical zeolite (Ni-HZ-0.5 M) in liquid-phase citral hydrogenation, it gave outstanding catalytic performance and menthol synthesis. Metal particles were widely distributed in the mesopores of the hierarchical zeolites, which may facilitate hydrogenation and cyclisation reactions with fast mass transfer/diffusion. The presence of Ni active particles facilitates citral hydrogenation to citronellal, whereas balanced acid sites (L/B) help citronellal cyclisation to isopulegol. Furthermore, isopulegol molecules were hydrogenated to menthols over Ni metal sites. When specific amounts of nickel metal (8 wt %) were doped over various hierarchical zeolites (HZ-0.2 M, HZ-0.5 M, and HZ-0.7 M), various distribution rates were observed, along with dissimilar reaction behaviors.
It was examined from pyridine adsorption data that the strength of Lewis and Bronsted acid sites varied after the desilication reassembly process of zeolite. The number of Lewis acid sites increased in the formation of the hierarchical zeolite. Furthermore, the high dispersion of Ni metal particles over the surface of the hierarchical zeolite may change the strength of acidic sites, and a balanced ratio of metal–acidic sites was achieved, as shown in
Figure 8 and
Table 3. It was revealed that Ni doping on the prepared hierarchical zeolites (e.g., treated with alkali treatment at different NaOH concentrations) gave a variable Ni dispersion percentage on the hierarchical zeolite. This variable Ni dispersion ratio changed the catalytic behavior and performance. The overall catalytic performance of all prepared Ni-supported catalysts is mentioned in
Table 4. This drastic change is mostly related to the ratio of metal–acid sites. This change has a large impact on citral conversion and menthol yield. The optimum catalytic performance and menthol yield is seen as possible because of balanced metal–acid sites and mesopore formation. The product distribution of citral hydrogenation to menthol is shown in
Figure 11. Ni-HZ-0.5 M hierarchical based catalyst exhibited an excellent catalytic performance and higher menthol yield. A high nickel dispersion rate was found in the Ni-HZ-0.7 M catalyst because of its high mesoporosity, but the XRD structure was partially damaged. The catalytic menthol production (47% menthol yield) was recorded with the use of the Ni-HZ-0.7 M catalyst, which was much lower than the Ni-HZ-0.5 M catalyst performance. From the experimental and characterization data, it was observed that balanced metal–acid sites were maintained, which promotes hydrogenation, as well as cyclisation reactions. This helps in improving menthol selectivity. The rates of cracking and dehydration side reactions are controlled because of balanced acid sites (L/B).
In addition, NH
3-TPD analysis was carried out and estimated the total acidity of all prepared catalysts. The acidity of the Ni/Z50 catalyst was 1.25 mmol·g
−1, which was further enhanced after desilication reassembly treatment (
Table 3). However, more leaching of Si ions from the zeolite framework caused an increase in acidity. The acidity increase may have an interconnection with the Al content. The Ni metal dispersion increased according to the pore size and surface area. The enhancement in surface and porosity properties would have direct/indirect impact on the Ni dispersion rate. Similarly, a H
2-TPR (temperature-programmed reduction) analytical characterization of all prepared catalysts was carried out to examine the surface chemistry of metal oxides under thermal conditions. The low-temperature (LT) and high-temperature (HT) parameters of Ni/Z50 are 359 and 510 °C, respectively. These temperature parameters are increased (e.g., increased with alkali concentration treatment) with an increase in mesoporosity and acidity. It can be examined from the available data that the hierarchical zeolite supported with highly dispersed nickel (HZ highly dispersed Ni catalyst (Ni/HZ-0.7 M)) gave medium menthol selectivity (47%), which assisted the hydrogenation of citronellal to citronellol, as can be seen in
Table 4. It is assumed that active acid sites may be covered by metal sites due to zeolite narrow structure. Due to narrow pore structure, reactant molecules movement might be slow and all molecules may have accumulated into narrow pores and might have negligible access to active acid sites of zeolites that resulted low mass transfer rate and poor catalytic activity. There was no direct access for the reactant molecules to the active acidic sites of zeolites, which might be a due to the partial blockage of active acid sites by nickel ions. Therefore, the rate of cyclisation was found to be lower when using the nickel-supported parent zeolite as compared to the hierarchical zeolites. Moreover, when parent zeolites were transformed into hierarchical zeolites, this introduced wide mesopores and balanced metal–acid sites, along with an improved mass transfer rate. These types of textural changes brought improvements in mass transfer and reaction rates, catalytic activity, and selectivity. The balanced metal–acid site ratios were obtained with the desired dispersion of Ni particles over the surface of the hierarchical zeolites (Ni-HZ-0.5 M). This catalyst exhibited higher catalytic activity (TOF 7.12 h
−1) and menthol production (~83%), which facilitated a cyclisation reaction of around 94% [
30], which is more consistent with the product distribution of citral hydrogenation (
Figure 11). The overall catalytic performance and menthol formation under optimized conditions are majorly connected with improved mesoporosity, acidity, balanced acid site ratios, and nickel dispersion characteristics.
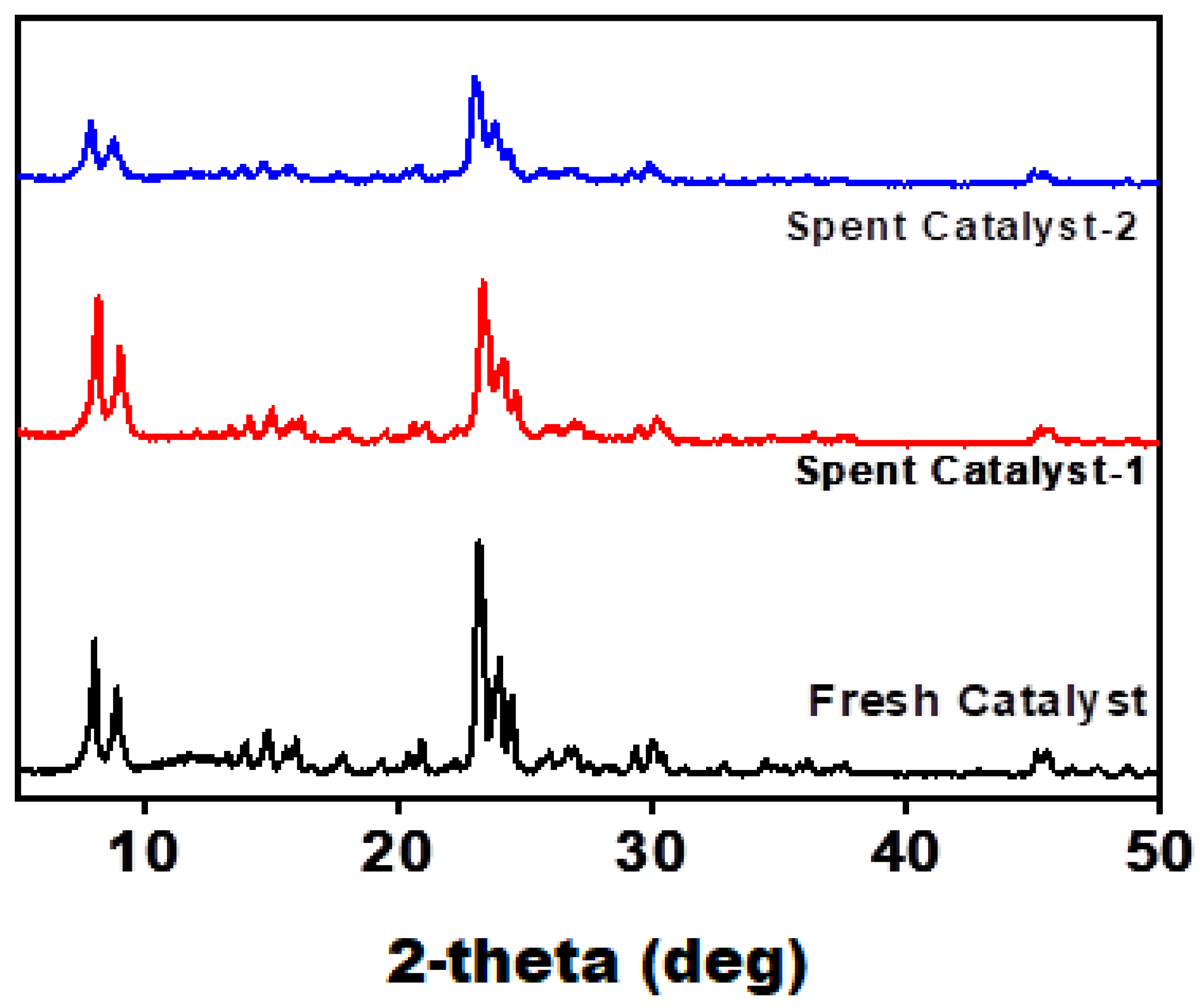
After a detailed experimental and characterization study, the recyclability test of the best catalyst (Ni-HZ-0.5 M) was evaluated. In the first reaction cycle, the catalyst gave 100% conversion and 83% menthol selectivity. After one reaction cycle was complete, the catalyst was filtered and washed with a toluene solvent and dried in an oven for 8 h and then reused in the second reaction cycle. However, the second reaction cycle gave an almost similar performance, as shown in
Table 2. A similar procedure was conducted for catalyst regeneration and reused in the citral hydrogenation reaction. The reaction proceeded for 3 h. The third cycle also gave an almost similar reaction performance. During the recyclability test, the catalyst (Ni-HZ-0.5 M) was found to be the more stable and active catalyst for citral hydrogenation for menthol synthesis (
Table 5). During the recyclability test of the best catalyst, XRD characterization was carried out and then compared with catalytic activity. It was observed that the catalyst remained stable in the three consecutive reaction cycles and gave an almost similar reaction performance. The XRD patterns remained almost similar in the consecutive recycle tests, as shown in
Figure 12.
2.3. Comparative Study with Other Catalysts
For the evaluation of the catalytic performances of other zeolites with ZMS-5 and its hierarchical zeolites, a comparative study was carried out. In this study, USY (Si/Al 80) was chosen as the comparative zeolite catalyst. Similar optimum desilication process parameters were used in preparation of hierarchical zeolites. A similar characterization of USY and its prepared hierarchical zeolite was carried out after nickel impregnation. The characterization data showed an almost similar trend in physicochemical and catalytic properties as compared to Ni-HZ-0.5 M. In addition, the Ni-USY80 and Ni/HZ-USY80 catalysts exhibited good catalytic performances (e.g., the catalytic activity of Ni-USY of TOF 0.56 h
−1 was enhanced to 1.30 h
−1 after the desilication process); these drastic changes might be due to the extra increase in the mesopore surface area (260~730 m
2·g
−1) and total acidity (2.42~2.54 mmol·g
−1), as can be seen in
Table 1,
Table 2,
Table 3 and
Table 4. In the case of USY and the hierarchical-based catalyst, metal dispersion had no direct connection with catalyst activity enhancement. The strength of Bronsted acidity decreased in the preparation of hierarchical zeolites. The metal dispersion did not show any effect on menthol selectivity enhancement when using the USY zeolite, whereas it was improved about 58% due to the decrease in the Bronsted acid sites of the hierarchical USY support (
Figure 8), and the cracking rate decreased. It was observed that around 40% isopulegol was cracked into side products in the presence of stronger Bronsted acid sites (e.g., Ni-USY80), which results in lower menthol production (
Figure 13). No more mass transfer limitation issues were rectified in the USY zeolite reaction study.
Table 6 shows a comparative study of various bifunctional metal–acid catalyst performances in menthol synthesis. The different nature of acidic supports, such as beta zeolites, alumina, ALPO, heteropoly-acid-supported alumina, MCM-41, and montmorillonite clay, were impregnated with metals and applied in citral hydrogenation. All zeolitic-type catalysts have shown good catalytic conversion but these catalysts were limited to menthol synthesis. Menthol selectivity was achieved in the range of 2 to 75%. In our research study, zeolite ZSM-5 was desilicated and transformed into a hierarchical (micro-mesoporous) zeolite at optimum alkali treatment conditions, which showed excellent catalytic activity and the highest menthol yield as compared to other reported catalysts, as shown in
Table 6.
In summary, it can be precisely concluded that the desilication reassembly process was found as the most suitable option for the formation of hierarchical zeolites (micro-mesoporous zeolites) at optimum process conditions (0.5 M NaOH, 0.05 M CTAB, and 12 h reaction). The catalytic performance of purely microporous zeolites was further improved in citral hydrogenation after the desilication step. This process improved the mesoporosity, total acidity, and metal dispersion, which helped the enhancement in catalytic activity and menthol selectivity. After the desilication process and Ni doping, metal and acid sites were improved and balanced, which controlled the citronellal cyclisation and isopulegol hydrogenation reaction rates for the optimum menthol yield. Strong Lewis acid site and weaker Bronsted acid site ratios were found for perfect cyclisation. High surface area, high mesopore volume, high acidity, and strong Lewis acid sites were the main parameters for obtaining the highest menthol selectivity. Among these catalysts, a bifunctional metal–acid (Ni/HZ-0.5 M) catalyst was the more promising catalyst for the liquid-phase citral hydrogenation reaction for one-pot menthol synthesis to obtain a higher yield of menthol with an improved mass transfer rate.