Advances in the Modification of Silane-Based Sol-Gel Coating to Improve the Corrosion Resistance of Magnesium Alloys
Abstract
1. Introduction
- No complicated equipment is needed.
- It can be synthesized at room temperature without vacuum conditions.
- Large-area thin films can be prepared on the surface of Mg.
- Modification of the coating composition and microstructure is simple. It is easy to prepare a uniform multi-component composite coating, and the thickness can be adjusted at the micron level.
2. Bare Sol-Gel Coatings
2.1. Bare Sol-Gel with Corrosion Inhibitor
2.2. Bare Sol-Gel with Nanoparticles
2.3. Hybrid (Inhibitors and Nanoparticles)
2.4. Substrate Pretreatment and Repair Agent
3. Composite Sol-Gel Coatings
3.1. Sol-Gel Coating as Pretreatment
3.2. Sol-Gel Coating as the Surface Layer
3.2.1. Chemical Conversion Coating/Sol-Gel
3.2.2. Anodizing/Sol-Gel
3.3. Multilayer Hybrid Coating
4. Conclusions and Outlooks
- Most of the synthetic methods reviewed in this paper were carried out under laboratory conditions.
- The durability of the coated surface is considered to be the most important aspect that should be further enhanced in future work. Although the corrosion inhibitor/nano-filler silane hybrid coating has improved its protective effect, it is far from enough to be used in the industry. The sol-gel composite coating with long-lasting corrosion protection should be addressed in a future study.
- There is a lack of work considering the mechanical properties of the coating, such as ductility and hardness. These properties are worth paying attention to in the practical application of coatings in industrial applications.
- The versatility of the coating is also very important. Apart from the anti-corrosion aspect, sol-gel coatings also need to provide oxidation resistance, abrasion resistance, water resistance, biocompatibility, and many other useful properties. With the in-depth study of sol-gel technology and related characterization techniques, sol-gel coatings will have wider and more practical applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| |Z|0.01Hz | Low frequency impedance |
| 8-HQ | 8-hydroxyquinoline |
| CNTs | Carbon nanotubes |
| EIS | Electrochemical impedance spectroscopy |
| FATP | Fluorinated attapulgite particles |
| GNPs | Graphene nanoplatelets |
| HA | Hydroxyapatite |
| HA | Hydroxyapatite |
| HNDs | Hydroxylated nanodiamonds |
| icorr | Corrosion current density |
| LEIM | Local electrochemical impedance spectroscopy experiments in the mapping mode |
| MAO | Microarc oxidation |
| Mg | Magnesium |
| N-GQDs | N-doped graphene quantum dots |
| OF | Oxidized fullerene |
| OH-MWCNT | Hydroxylated multi-walled carbon nanotube |
| OIH coating | Organic-inorganic hybrid coating |
| P-B ratio | Pilling-Bedworth ratio |
| PEO | Plasma electrolytic oxidation |
| PP | Polypropylene |
| Rct | The charge transfer resistance |
| Rp | Polarization resistance |
| Rtot: Rf + Rct | values are calculated as the sum of all the faradaic resistance by using the fitted data |
| SBF | Simulated Body Fluid |
| SDS | sodium dodecyl sulfate |
| SVET | Scanning Vibrating Electrode Technique |
| XPS | X-ray spectroscopy |
References
- Xue, S.; Li, B.; Mu, P.; Li, J. Designing attapulgite-based self-healing superhydrophobic coatings for efficient corrosion protection of magnesium alloys. Prog. Org. Coat. 2022, 170, 106966. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloys 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Guo, L.; Wu, W.; Zhou, Y.; Zhang, F.; Zeng, R.; Zeng, J. Layered double hydroxide coatings on magnesium alloys: A review. J. Mater. Sci. Technol. 2018, 34, 1455–1466. [Google Scholar] [CrossRef]
- Salman, S.A.; Okido, M. Self-assembled monolayers formed on AZ31 Mg alloy. J. Phys. Chem. Solids 2012, 73, 863–866. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, X.; Li, S.; Mao, Y.; Feng, Z.; Ke, W.; Liu, F. Study on Strengthening and Toughening of Mechanical Properties of Mg-Li Alloy by Adding Non-Rare-Earth Elements Al and Si. Jom-Us 2022, 74, 2554–2565. [Google Scholar] [CrossRef]
- Hu, R.-G.; Zhang, S.; Bu, J.-F.; Lin, C.-J.; Song, G.-L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2012, 73, 129–141. [Google Scholar] [CrossRef]
- Zhao, M.-C.; Liu, M.; Song, G.-L.; Atrens, A. Influence of pH and chloride ion concentration on the corrosion of Mg alloy ZE41. Corros. Sci. 2008, 50, 3168–3178. [Google Scholar] [CrossRef]
- Atrens, A.; Johnston, S.; Shi, Z.; Dargusch, M.S. Viewpoint-Understanding Mg corrosion in the body for biodegradable medical implants. Scr. Mater. 2018, 154, 92–100. [Google Scholar] [CrossRef]
- Kainer, K.U.; von Buch, F. The Current State of Technology and Potential for Further Development of Magnesium Applications. In Magnesium—Alloys and Technology; WILEY-VCH Verlag GmbH & Co. KG aA: Weinheim, Germany, 2003; pp. 1–22. [Google Scholar]
- Zheng, X.; Liu, Q.; Ma, H.; Das, S.; Gu, Y.; Zhang, L. Probing local corrosion performance of sol-gel/MAO composite coating on Mg alloy. Surf. Coat. Technol. 2018, 347, 286–296. [Google Scholar] [CrossRef]
- Saji, V.S. Organic conversion coatings for magnesium and its alloys. J. Ind. Eng. Chem. 2019, 75, 20–37. [Google Scholar] [CrossRef]
- Cao, F.; Song, G.-L.; Atrens, A. Corrosion and passivation of magnesium alloys. Corros. Sci. 2016, 111, 835–845. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Saji, V.S. Review of rare-earth-based conversion coatings for magnesium and its alloys. J. Mater. Res. Technol. 2019, 8, 5012–5035. [Google Scholar] [CrossRef]
- Ballerini, G.; Bardi, U.; Bignucolo, R.; Ceraolo, G. About some corrosion mechanisms of AZ91D magnesium alloy. Corros. Sci. 2005, 47, 2173–2184. [Google Scholar] [CrossRef]
- Liu, H.; Cao, F.; Song, G.-L.; Zheng, D.; Shi, Z.; Dargusch, M.S.; Atrens, A. Review of the atmospheric corrosion of magnesium alloys. J. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- Xu, W.; Birbilis, N.; Sha, G.; Wang, Y.; Daniels, J.E.; Xiao, Y.; Ferry, M. A high-specific-strength and corrosion-resistant magnesium alloy. Nat Mater 2015, 14, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Imandoust, A.; Barrett, C.D.; Al-Samman, T.; Inal, K.A.; El Kadiri, H. A review on the effect of rare-earth elements on texture evolution during processing of magnesium alloys. J. Mater. Sci. 2016, 52, 1–29. [Google Scholar] [CrossRef]
- Wei, L.; Li, J.; Zhang, Y.; Lai, H. Effects of Zn content on microstructure, mechanical and degradation behaviors of Mg-xZn-0.2Ca-0.1Mn alloys. Mater. Chem. Phys. 2020, 241, 122441. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Cui, Z.; Qi, W.; Wang, J.; Ju, P.; Zhao, Y.; Liu, B.; Zhang, T.; Wang, F. Influence of Rare Earth Element (Y) on Microstructure and Corrosion Behavior of Hot Extrusion AZ91 Magnesium Alloy. Materials 2020, 13, 3651. [Google Scholar] [CrossRef]
- Yao, W.; Liang, W.; Huang, G.; Jiang, B.; Atrens, A.; Pan, F. Superhydrophobic coatings for corrosion protection of magnesium alloys. J. Mater. Sci. Technol. 2020, 52, 100–118. [Google Scholar] [CrossRef]
- Qi, J.; Ye, Z.; Gong, N.; Qu, X.; Mercier, D.; Światowska, J.; Skeldon, P.; Marcus, P. Formation of a trivalent chromium conversion coating on AZ91D magnesium alloy. Corros. Sci. 2021, 186, 109459. [Google Scholar] [CrossRef]
- Rajabalizadeh, Z.; Seifzadeh, D. Strontium phosphate conversion coating as an economical and environmentally-friendly pretreatment for electroless plating on AM60B magnesium alloy. Surf. Coat. Technol. 2016, 304, 450–458. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Sabour Rouhaghdam, A. Microstructural, protective, inhibitory and semiconducting properties of PEO coatings containing CeO2 nanoparticles formed on AZ31 Mg alloy. Surf. Coat. Technol. 2018, 352, 561–580. [Google Scholar] [CrossRef]
- Feng, Z.; Li, J.; Yang, Z.; Buchheit, R. The Effect of Vanadate, Phosphate, Fluoride Compounds on the Aqueous Corrosion of Magnesium Alloy AZ31 in Dilute Chloride Solutions. Materials 2020, 13, 1325. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, T.; Zeng, Y.; Chen, C.; Guo, H.; Lei, B.; Zhang, P.; Feng, Z.; Meng, G. A Novel sol-gel coating via catechol/lysine polymerization for long-lasting corrosion protection of Mg Alloy AZ31. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 656, 130361. [Google Scholar] [CrossRef]
- Yang, S.; Sun, R.; Chen, K. Self-healing performance and corrosion resistance of phytic acid/cerium composite coating on microarc-oxidized magnesium alloy. Chem. Eng. J. 2022, 428, 131198. [Google Scholar] [CrossRef]
- Lu, X.; Feng, X.; Zuo, Y.; Zhang, P.; Zheng, C. Improvement of protection performance of Mg-rich epoxy coating on AZ91D magnesium alloy by DC anodic oxidation. Prog. Org. Coat. 2017, 104, 188–198. [Google Scholar] [CrossRef]
- Kang, Z.; Li, W. Facile and fast fabrication of superhydrophobic surface on magnesium alloy by one-step electrodeposition method. J. Ind. Eng. Chem. 2017, 50, 50–56. [Google Scholar] [CrossRef]
- Talha, M.; Ma, Y.; Xu, M.; Wang, Q.; Lin, Y.; Kong, X. Recent Advancements in Corrosion Protection of Magnesium Alloys by Silane-Based Sol–Gel Coatings. Ind. Eng. Chem. Res. 2020, 59, 19840–19857. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kuznetsova, A.; Kallip, S.; Starykevich, M.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. A novel bilayer system comprising LDH conversion layer and sol-gel coating for active corrosion protection of AA2024. Corros. Sci. 2018, 143, 299–313. [Google Scholar] [CrossRef]
- Zandi Zand, R.; Verbeken, K.; Adriaens, A. Corrosion resistance performance of cerium doped silica sol–gel coatings on 304L stainless steel. Prog. Org. Coat. 2012, 75, 463–473. [Google Scholar] [CrossRef]
- Balaji, J.; Roh, S.-H.; Edison, T.N.J.I.; Jung, H.-Y.; Sethuraman, M.G. Sol-gel based hybrid silane coatings for enhanced corrosion protection of copper in aqueous sodium chloride. J. Mol. Liq. 2020, 302, 112551. [Google Scholar] [CrossRef]
- Castro, Y.; Durán, A.; Moreno, R.; Ferrari, B. Thick Sol-Gel Coatings Produced by Electrophoretic Deposition. Adv. Mater. 2002, 14, 505–508. [Google Scholar] [CrossRef]
- Figueira, R.B. Hybrid Sol-gel Coatings for Corrosion Mitigation: A Critical Review. Polymers 2020, 12, 689. [Google Scholar] [CrossRef] [PubMed]
- Fedel, M.; Callone, E.; Fabbian, M.; Deflorian, F.; Dirè, S. Influence of Ce 3+ doping on molecular organization of Si-based organic/inorganic sol-gel layers for corrosion protection. Appl. Surf. Sci. 2017, 414, 82–91. [Google Scholar] [CrossRef]
- Hernández-Barrios, C.A.; Saavedra, J.A.; Higuera, S.L.; Coy, A.E.; Viejo, F. Effect of cerium on the physicochemical and anticorrosive features of TEOS-GPTMS sol-gel coatings deposited on the AZ31 magnesium alloy. Surf. Interfaces 2020, 21, 100671. [Google Scholar] [CrossRef]
- Gasiorek, J.; Szczurek, A.; Babiarczuk, B.; Kaleta, J.; Jones, W.; Krzak, J. Functionalizable Sol-Gel Silica Coatings for Corrosion Mitigation. Materials 2018, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, M. Sol-gel coatings on metals. J. Sol-Gel Sci. Technol. 1997, 8, 443–449. [Google Scholar] [CrossRef]
- Nair, P.A.K.; Vasconcelos, W.L.; Paine, K.; Calabria-Holley, J. A review on applications of sol-gel science in cement. Constr. Build. Mater. 2021, 291, 123065. [Google Scholar] [CrossRef]
- Zheng, K.; Boccaccini, A.R. Sol-gel processing of bioactive glass nanoparticles: A review. Adv. Colloid. Interface Sci. 2017, 249, 363–373. [Google Scholar] [CrossRef]
- Montemor, M.F.; Ferreira, M.G.S. Analytical and microscopic characterisation of modified bis-[triethoxysilylpropyl] tetrasulphide silane films on magnesium AZ31 substrates. Prog. Org. Coat. 2007, 60, 228–237. [Google Scholar] [CrossRef]
- Zhu, D.; van Ooij, W.J. Corrosion protection of AA 2024-T3 by bis-[3-(triethoxysilyl)propyl]tetrasulfide in sodium chloride solution. Corros. Sci. 2003, 45, 2177–2197. [Google Scholar] [CrossRef]
- Song, J.; Van Ooij, W.J. Bonding and corrosion protection mechanisms of γ-APS and BTSE silane films on aluminum substrates. J. Adhes. Sci. Technol. 2003, 17, 2191–2221. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Calabrese, L.; Caprì, A.; Proverbio, E. Anti-corrosion performances of hybrid silane coatings on AZ31 alloy. Anti-Corros. Methods Mater. 2018, 65, 317–324. [Google Scholar] [CrossRef]
- Metroke, T.L.; Parkhill, R.L.; Knobbe, E.T. Passivation of metal alloys using sol–gel-derived materials—A review. Prog. Org. Coat. 2001, 41, 233–238. [Google Scholar] [CrossRef]
- Banjo, N.; Sasaki, T.T.; Hono, K. Microstructural origin of adhesion and corrosion properties of Ti-based conversion coatings on A6063 alloy. Appl. Surf. Sci. 2022, 604, 154411. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Tallman, D.E.; Levine, K.L.; Siripirom, C.; Gelling, V.G.; Bierwagen, G.P.; Croll, S.G. Nanocomposite of polypyrrole and alumina nanoparticles as a coating filler for the corrosion protection of aluminium alloy 2024-T3. Appl. Surf. Sci. 2008, 254, 5452–5459. [Google Scholar] [CrossRef]
- Yang, W.; Feng, W.; Liao, Z.; Yang, Y.; Miao, G.; Yu, B.; Pei, X. Protection of mild steel with molecular engineered epoxy nanocomposite coatings containing corrosion inhibitor functionalized nanoparticles. Surf. Coat. Technol. 2021, 406, 126639. [Google Scholar] [CrossRef]
- Zheng, S.; Li, J. Inorganic–organic sol gel hybrid coatings for corrosion protection of metals. J. Sol-Gel Sci. Technol. 2010, 54, 174–187. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Zheludkevich, M.L.; Ferreira, M.; Salvado, I.M. Sol-gel coatings for corrosion protection of metals. J. Mater. Chem. Interdiscip. 2005, 15, 48. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Montemor, M.F.; Galio, A.F.; Zheludkevich, M.L.; Trindade, C.; Dick, L.F.; Ferreira, M.G.S. Novel hybrid sol–gel coatings for corrosion protection of AZ31B magnesium alloy. Electrochim. Acta 2008, 53, 4773–4783. [Google Scholar] [CrossRef]
- Hu, J.; Li, Q.; Zhong, X.; Kang, W. Novel anti-corrosion silicon dioxide coating prepared by sol–gel method for AZ91D magnesium alloy. Prog. Org. Coat. 2008, 63, 13–17. [Google Scholar] [CrossRef]
- Khramov, A.N.; Balbyshev, V.N.; Kasten, L.S.; Mantz, R.A. Sol–gel coatings with phosphonate functionalities for surface modification of magnesium alloys. Thin Solid Film. 2006, 514, 174–181. [Google Scholar] [CrossRef]
- Hernández-Barrios, C.A.; Cuao, C.A.; Jaimes, M.A.; Coy, A.E.; Viejo, F. Effect of the catalyst concentration, the immersion time and the aging time on the morphology, composition and corrosion performance of TEOS-GPTMS sol-gel coatings deposited on the AZ31 magnesium alloy. Surf. Coat. Technol. 2017, 325, 257–269. [Google Scholar] [CrossRef]
- Rodriguez-Alonso, L.; Lopez-Sanchez, J.; Serrano, A.; Rodriguez de la Fuente, O.; Galvan, J.C.; Carmona, N. Hybrid Sol-Gel Coatings Doped with Non-Toxic Corrosion Inhibitors for Corrosion Protection on AZ61 Magnesium Alloy. Gels 2022, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, V.; Bergseth, Z.; Kelly, B.; Battocchi, D. Silica-Based Sol-Gel Coating on Magnesium Alloy with Green Inhibitors. Coatings 2017, 7, 86. [Google Scholar] [CrossRef]
- Galio, A.F.; Lamaka, S.V.; Zheludkevich, M.L.; Dick, L.F.P.; Müller, I.L.; Ferreira, M.G.S. Inhibitor-doped sol–gel coatings for corrosion protection of magnesium alloy AZ31. Surf. Coat. Technol. 2010, 204, 1479–1486. [Google Scholar] [CrossRef]
- Ren, P.; Li, J.; Wang, L.; Guo, H.; Lei, B.; Feng, Z.; Meng, G. Organo-Cerium as a Quick Repair Agent for Coating Damage on Carbon Steel. J. Mater. Eng. Perform. 2023, 5, 1–10. [Google Scholar] [CrossRef]
- Zhong, X.; Li, Q.; Hu, J.; Yang, X.; Luo, F.; Dai, Y. Effect of cerium concentration on microstructure, morphology and corrosion resistance of cerium–silica hybrid coatings on magnesium alloy AZ91D. Prog. Org. Coat. 2010, 69, 52–56. [Google Scholar] [CrossRef]
- Murillo-Gutiérrez, N.V.; Ansart, F.; Bonino, J.-P. Hybrid sol-gel coatings doped with cerium to protect magnesium alloys from corrosion. Mater. Tehnol. 2015, 49, 453–456. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, N.; Jain, V.; Rai, B. Amino acids as copper corrosion inhibitors: A density functional theory approach. Appl. Surf. Sci. 2020, 514, 145905. [Google Scholar] [CrossRef]
- Li, W.; Su, Y.; Ma, L.; Zhu, S.; Zheng, Y.; Guan, S. Sol-gel coating loaded with inhibitor on ZE21B Mg alloy for improving corrosion resistance and endothelialization aiming at potential cardiovascular application. Colloids Surf B Biointerfaces 2021, 207, 111993. [Google Scholar] [CrossRef] [PubMed]
- Goni, L.K.M.O.; Jafar Mazumder, M.A.; Quraishi, M.A.; Mizanur Rahman, M. Bioinspired Heterocyclic Compounds as Corrosion Inhibitors: A Comprehensive Review. Chem. Asian J. 2021, 16, 1324–1364. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, M.A.; Chauhan, D.S.; Saji, V.S. Heterocyclic biomolecules as green corrosion inhibitors. J. Mol. Liq. 2021, 341, 117265. [Google Scholar] [CrossRef]
- Shi, H.; Liu, F.; Han, E.-h. Corrosion protection of AZ91D magnesium alloy with sol–gel coating containing 2-methyl piperidine. Prog. Org. Coat. 2009, 66, 183–191. [Google Scholar] [CrossRef]
- Wang, F.; Cai, S.; Shen, S.; Yu, N.; Zhang, F.; Ling, R.; Li, Y.; Xu, G. Preparation of Phytic Acid/Silane Hybrid Coating on Magnesium Alloy and Its Corrosion Resistance in Simulated Body Fluid. J. Mater. Eng. Perform. 2017, 26, 4282–4290. [Google Scholar] [CrossRef]
- Li, Y.; Cai, S.; Shen, S.; Xu, G.; Zhang, F.; Wang, F. Self-healing hybrid coating of phytic acid/silane for improving the corrosion resistance of magnesium alloy. J. Coat. Technol. Res. 2018, 15, 571–581. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Taryba, M.; Salak, A.N.; Kallip, S.; Zheludkevich, M.L.; Ferreira, M.G.; Montemor, M.F. Hydroxyapatite microparticles as feedback-active reservoirs of corrosion inhibitors. ACS Appl. Mater. Interfaces 2010, 2, 3011–3022. [Google Scholar] [CrossRef]
- Nikbakht, A.; Dehghanian, C.; Parichehr, R. Silane coatings modified with hydroxyapatite nanoparticles to enhance the biocompatibility and corrosion resistance of a magnesium alloy. RSC Adv. 2021, 11, 26127–26144. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Vaghefinazari, B.; Mei, D.; Petrauskas, R.P.; Höche, D.; Zheludkevich, M.L. Comprehensive screening of Mg corrosion inhibitors. Corros. Sci. 2017, 128, 224–240. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, X.; Lamaka, S.V.; Ju, P.; Blawert, C.; Zhang, T.; Wang, F.; Zheludkevich, M.L. Active protection of Mg alloy by composite PEO coating loaded with corrosion inhibitors. Appl. Surf. Sci. 2020, 504, 144462. [Google Scholar] [CrossRef]
- Zhang, W.D.; Phang, I.Y.; Liu, T.X. Growth of Carbon Nanotubes on Clay: Unique Nanostructured Filler for High-Performance Polymer Nanocomposites. Adv. Mater. 2006, 18, 73–77. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. PTMS/OH-MWCNT sol-gel nanocomposite for corrosion protection of magnesium alloy. Surf. Coat. Technol. 2018, 335, 228–240. [Google Scholar] [CrossRef]
- Malik, M.U.; Tabish, M.; Yasin, G.; Anjum, M.J.; Jameel, S.; Tang, Y.; Zhang, X.; Manzoor, S.; Ibraheem, S.; Khan, W.Q. Electroless codeposition of GO incorporated silane nanocomposite coating onto AZ91 Mg alloy: Effect of GO content on its morphology, mechanical and corrosion protection properties. J. Alloys Compd. 2021, 883, 160790. [Google Scholar] [CrossRef]
- Samadianfard, R.; Seifzadeh, D.; Habibi-Yangjeh, A.; Jafari-Tarzanagh, Y. Oxidized fullerene/sol-gel nanocomposite for corrosion protection of AM60B magnesium alloy. Surf. Coat. Technol. 2020, 385, 125400. [Google Scholar] [CrossRef]
- Samadianfard, R.; Seifzadeh, D.; Habibi-Yangjeh, A. Sol-gel coating filled with SDS-stabilized fullerene nanoparticles for active corrosion protection of the magnesium alloy. Surf. Coat. Technol. 2021, 419, 127292. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D.; Habibi-Yangjeh, A. Nanodiamond incorporated sol−gel coating for corrosion protection of magnesium alloy. Trans. Nonferrous Met. Soc. China 2020, 30, 1535–1549. [Google Scholar] [CrossRef]
- Wang, H.; Akid, R.; Gobara, M. Scratch-resistant anticorrosion sol–gel coating for the protection of AZ31 magnesium alloy via a low temperature sol–gel route. Corros. Sci. 2010, 52, 2565–2570. [Google Scholar] [CrossRef]
- Ma, Y.; Talha, M.; Wang, Q.; Zhao, Q.; Li, Z.; Lin, Y. Nano-silica/chitosan composite coatings on biodegradable magnesium alloys for enhanced corrosion resistance in simulated body fluid. Mater. Corros. 2021, 73, 436–450. [Google Scholar] [CrossRef]
- Wang, S.; Ye, X.; Zhang, H.; Qian, Z.; Li, Q.; Wu, Z.; Li, S. Superhydrophobic Silane/Fluorinated Attapulgite@SiO 2 Composite Coatings on Magnesium Alloy for Corrosion Protection. ChemistrySelect 2020, 5, 10329–10338. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Liu, Z.; Peng, W.-X.; Yoysefi, N. Amino acid and TiO2 nanoparticles mixture inserted into sol-gel coatings: An efficient corrosion protection system for AZ91 magnesium alloy. Prog. Org. Coat. 2019, 136, 105296. [Google Scholar] [CrossRef]
- Montemor, M.F.; Ferreira, M.G.S. Analytical characterisation and corrosion behaviour of bis-aminosilane coatings modified with carbon nanotubes activated with rare-earth salts applied on AZ31 Magnesium alloy. Surf. Coat. Technol. 2008, 202, 4766–4774. [Google Scholar] [CrossRef]
- Adsul, S.H.; Siva, T.; Sathiyanarayanan, S.; Sonawane, S.H.; Subasri, R. Self-healing ability of nanoclay-based hybrid sol-gel coatings on magnesium alloy AZ91D. Surf. Coat. Technol. 2017, 309, 609–620. [Google Scholar] [CrossRef]
- Adsul, S.H.; Siva, T.; Sathiyanarayanan, S.; Sonawane, S.H.; Subasri, R. Aluminum pillared montmorillonite clay-based self-healing coatings for corrosion protection of magnesium alloy AZ91D. Surf. Coat. Technol. 2018, 352, 445–461. [Google Scholar] [CrossRef]
- Saxena, A.; Raman, R.K.S. Role of Surface Preparation in Corrosion Resistance Due to Silane Coatings on a Magnesium Alloy. Molecules 2021, 26, 6663. [Google Scholar] [CrossRef] [PubMed]
- Supplit, R.; Koch, T.; Schubert, U. Evaluation of the anti-corrosive effect of acid pickling and sol–gel coating on magnesium AZ31 alloy. Corros. Sci. 2007, 49, 3015–3023. [Google Scholar] [CrossRef]
- Dalmoro, V.; Azambuja, D.S.; Alemán, C.; Armelin, E. Hybrid organophosphonic-silane coating for corrosion protection of magnesium alloy AZ91: The influence of acid and alkali pre-treatments. Surf. Coat. Technol. 2019, 357, 728–739. [Google Scholar] [CrossRef]
- Diaz, L.; García-Galván, F.R.; Llorente, I.; Jiménez-Morales, A.; Galván, J.C.; Feliu Jr, S. Effect of heat treatment of magnesium alloy substrates on corrosion resistance of a hybrid organic–inorganic sol–gel film. RSC Adv. 2015, 5, 105735–105746. [Google Scholar] [CrossRef]
- Zhong, X.; Li, Q.; Hu, J.; Zhang, S.; Chen, B.; Xu, S.; Luo, F. A novel approach to heal the sol–gel coating system on magnesium alloy for corrosion protection. Electrochim. Acta 2010, 55, 2424–2429. [Google Scholar] [CrossRef]
- Zhang, L.; Mohammed, E.A.A.; Adriaens, A. Synthesis and electrochemical behavior of a magnesium fluoride-polydopamine-stearic acid composite coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2016, 307, 56–64. [Google Scholar] [CrossRef]
- Lakshmi, L.; Aruna, S.T.; Sampath, S. Ceria nanoparticles vis-à-vis cerium nitrate as corrosion inhibitors for silica-alumina hybrid sol-gel coating. Appl. Surf. Sci. 2017, 393, 397–404. [Google Scholar]
- Lu, X.; Zuo, Y.; Zhao, X.; Tang, Y. The improved performance of a Mg-rich epoxy coating on AZ91D magnesium alloy by silane pretreatment. Corros. Sci. 2012, 60, 165–172. [Google Scholar] [CrossRef]
- Liu, X.; Yue, Z.; Romeo, T.; Weber, J.; Scheuermann, T.; Moulton, S.; Wallace, G. Biofunctionalized anti-corrosive silane coatings for magnesium alloys. Acta Biomater 2013, 9, 8671–8677. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hernán, J.P.; López, A.J.; Torres, B.; Rams, J. Silicon oxide multilayer coatings doped with carbon nanotubes and graphene nanoplatelets for corrosion protection of AZ31B magnesium alloy. Prog. Org. Coat. 2020, 148, 105836. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Zeng, R.-C.; Lin, C.-G.; Wang, L.; Chen, X.-B.; Chen, D.-C. Corrosion resistance of self-cleaning silane/polypropylene composite coatings on magnesium alloy AZ31. J. Mater. Sci. Technol. 2020, 41, 43–55. [Google Scholar] [CrossRef]
- AhadiParsa, M.; Eivaz Mohammadloo, H.; Mirabedini, S.M.; Roshan, S. Bio-corrosion assessment and surface study of hydroxyapatite-coated AZ31 Mg alloy pre-treated with vinyl tri-ethoxy silane. Mater. Chem. Phys. 2022, 287, 126147. [Google Scholar] [CrossRef]
- Tarzanagh, Y.J.; Seifzadeh, D.; Samadianfard, R. Combining the 8-hydroxyquinoline intercalated layered double hydroxide film and sol—Gel coating for active corrosion protection of the magnesium alloy. Int. J. Miner. Metall. Mater. 2022, 29, 536–546. [Google Scholar] [CrossRef]
- Fernandez-Hernan, J.P.; Lopez, A.J.; Torres, B.; Martinez-Campos, E.; Matykina, E.; Rams, J. Anticorrosion and Cytocompatibility Assessment of Graphene-Doped Hybrid Silica and Plasma Electrolytic Oxidation Coatings for Biomedical Applications. ACS Biomater. Sci. Eng. 2021, 7, 5861–5877. [Google Scholar] [CrossRef] [PubMed]
- Ostapiuk, M.; Taryba, M.G.; Calado, L.M.; Bieniaś, J.; Montemor, M.F. A study on the galvanic corrosion of a sol-gel coated PEO Mg-CFRP couple. Corros. Sci. 2021, 186, 109470. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Xiao, G.Y.; Lu, Y.P. Phosphate chemical conversion coatings on metallic substrates for biomedical application: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 47, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Q.; Chen, B.; Yang, X. Preparation and corrosion resistance studies of nanometric sol–gel-based CeO2 film with a chromium-free pretreatment on AZ91D magnesium alloy. Electrochim. Acta 2010, 55, 870–877. [Google Scholar] [CrossRef]
- Hu, J.; Li, Q.; Zhong, X.; Zhang, L.; Chen, B. Composite anticorrosion coatings for AZ91D magnesium alloy with molybdate conversion coating and silicon sol–gel coatings. Prog. Org. Coat. 2009, 66, 199–205. [Google Scholar] [CrossRef]
- Yue, Y.-Y.; Liu, Z.-X.; Wan, T.-T.; Wang, P.-C. Effect of phosphate–silane pretreatment on the corrosion resistance and adhesive-bonded performance of the AZ31 magnesium alloys. Prog. Org. Coat. 2013, 76, 835–843. [Google Scholar] [CrossRef]
- Murillo-Gutiérrez, N.V.; Ansart, F.; Bonino, J.P.; Menu, M.J.; Gressier, M. Protection against corrosion of magnesium alloys with both conversion layer and sol–gel coating. Surf. Coat. Technol. 2013, 232, 606–615. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. Application of novel sol–gel composites on magnesium alloy. J. Magnes. Alloys 2019, 7, 419–432. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D. Application of CeH–V/ sol–gel composite coating for corrosion protection of AM60B magnesium alloy. Trans. Nonferrous Met. Soc. China 2017, 27, 352–362. [Google Scholar] [CrossRef]
- Durán, K.S.; Hernández-Barrios, C.A.; Coy, A.E.; Viejo, F. Effect of fluoride conversion pretreatment time and the microstructure on the corrosion performance of TEOS-GPTMS sol–gel coatings deposited on the WE54 magnesium alloy. J. Mater. Res. Technol. 2021, 15, 4220–4242. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Moradi-Alavian, S.; Esrafili, M.D.; Kazempour, A. Hybrid sol-gel coatings based on silanes-amino acids for corrosion protection of AZ91 magnesium alloy: Electrochemical and DFT insights. Prog. Org. Coat. 2019, 131, 191–202. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Moradi-Alavian, S.; Jafari, R.; Kazempour, A.; Asghari, E. Effect of amino acids and montmorillonite nanoparticles on improving the corrosion protection characteristics of hybrid sol-gel coating applied on AZ91 Mg alloy. Mater. Chem. Phys. 2019, 225, 298–308. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Moradi-Alavian, S.; Kazempour, A. Salt-nanoparticle systems incorporated into sol-gel coatings for corrosion protection of AZ91 magnesium alloy. Prog. Org. Coat. 2019, 135, 475–482. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, F.; Song, L.; Zeng, R.-C.; Li, S.-Q.; Han, E.-H. Corrosion resistance of ceria/polymethyltrimethoxysilane modified magnesium hydroxide coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2017, 328, 121–133. [Google Scholar] [CrossRef]
- Bestetti, M.; Da Forno, A.; Cavallotti, P.L.; Gronchi, P.; Barlassina, F. Anodic oxidation and sol–gel coatings for corrosion and wear protection of AM60B alloy. Trans. IMF 2013, 88, 57–62. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Knörnschild, G.; Snihirova, D.V.; Taryba, M.G.; Zheludkevich, M.L.; Ferreira, M.G.S. Complex anticorrosion coating for ZK30 magnesium alloy. Electrochim. Acta 2009, 55, 131–141. [Google Scholar] [CrossRef]
- Afsharimani, N.; Talimian, A.; Merino, E.; Durán, A.; Castro, Y.; Galusek, D. Improving corrosion protection of Mg alloys (AZ31B) using graphene-based hybrid coatings. Int. J. Appl. Glass Sci. 2021, 13, 143–150. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Babaei, K. Impressive strides in amelioration of corrosion and wear behaviors of Mg alloys using applied polymer coatings on PEO porous coatings: A review. J. Magnes. Alloys 2022, 10, 1171–1190. [Google Scholar] [CrossRef]
- Ivanou, D.K.; Yasakau, K.A.; Kallip, S.; Lisenkov, A.D.; Starykevich, M.; Lamaka, S.V.; Ferreira, M.G.S.; Zheludkevich, M.L. Active corrosion protection coating for a ZE41 magnesium alloy created by combining PEO and sol–gel techniques. RSC Adv. 2016, 6, 12553–12560. [Google Scholar] [CrossRef]
- Shang, W.; Chen, B.; Shi, X.; Chen, Y.; Xiao, X. Electrochemical corrosion behavior of composite MAO/sol–gel coatings on magnesium alloy AZ91D using combined micro-arc oxidation and sol–gel technique. J. Alloys Compd. 2009, 474, 541–545. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Gao, S.-D.; Li, P.-P.; Zeng, R.-C.; Zhang, F.; Li, S.-Q.; Han, E.-H. Corrosion resistance of a self-healing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2017, 118, 84–95. [Google Scholar] [CrossRef]
- Pezzato, L.; Rigon, M.; Martucci, A.; Brunelli, K.; Dabalà, M. Plasma Electrolytic Oxidation (PEO) as pre-treatment for sol-gel coating on aluminum and magnesium alloys. Surf. Coat. Technol. 2019, 366, 114–123. [Google Scholar] [CrossRef]
- Merino, E.; Durán, A.; Castro, Y. Integrated corrosion-resistant system for AZ31B Mg alloy via plasma electrolytic oxidation (PEO) and sol-gel processes. Int. J. Appl. Glass Sci. 2021, 12, 519–530. [Google Scholar] [CrossRef]
- Chen, Z.; Geng, X.; Yong, X.; Chen, X.; Sun, Z. Microstructural Changes of the Peo Coating Induced by Silane-Based Sol–Gel Treatment. Surf. Rev. Lett. 2022, 29, 7. [Google Scholar] [CrossRef]
- Jiang, B.K.; Chen, A.Y.; Gu, J.F.; Fan, J.T.; Liu, Y.; Wang, P.; Li, H.J.; Sun, H.; Yang, J.H.; Wang, X.Y. Corrosion resistance enhancement of magnesium alloy by N-doped graphene quantum dots and polymethyltrimethoxysilane composite coating. Carbon 2020, 157, 537–548. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Mahdavian, M.; Naderi, R. Effective PEO/Silane pretreatment of epoxy coating applied on AZ31B Mg alloy for corrosion protection. Corros. Sci. 2020, 169, 108608. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Mahdavian, M.; Naderi, R. Superior corrosion protection and adhesion strength of epoxy coating applied on AZ31 magnesium alloy pre-treated by PEO/Silane with inorganic and organic corrosion inhibitors. Corros. Sci. 2021, 178, 109065. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Liu, J.; Jiang, J.; Yuan, N.; Pu, J.; Ding, J. An organic/inorganic composite multi-layer coating to improve the corrosion resistance of AZ31B Mg alloy. Surf. Coat. Technol. 2019, 360, 276–284. [Google Scholar] [CrossRef]
- Pereira, G.S.; Prada Ramirez, O.M.; Avila, P.R.T.; Avila, J.A.; Pinto, H.C.; Miyazaki, M.H.; de Melo, H.G.; Bose Filho, W.W. Cerium conversion coating and sol-gel coating for corrosion protection of the WE43 Mg alloy. Corros. Sci. 2022, 206, 110527. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Xie, Y.; Liu, L.; Yang, H.; Zhu, R.; Gong, J.; Peng, L.; Ding, W. Preparation of superhydrophobic silica film on Mg–Nd–Zn–Zr magnesium alloy with enhanced corrosion resistance by combining micro-arc oxidation and sol–gel method. Surf. Coat. Technol. 2012, 213, 192–201. [Google Scholar] [CrossRef]

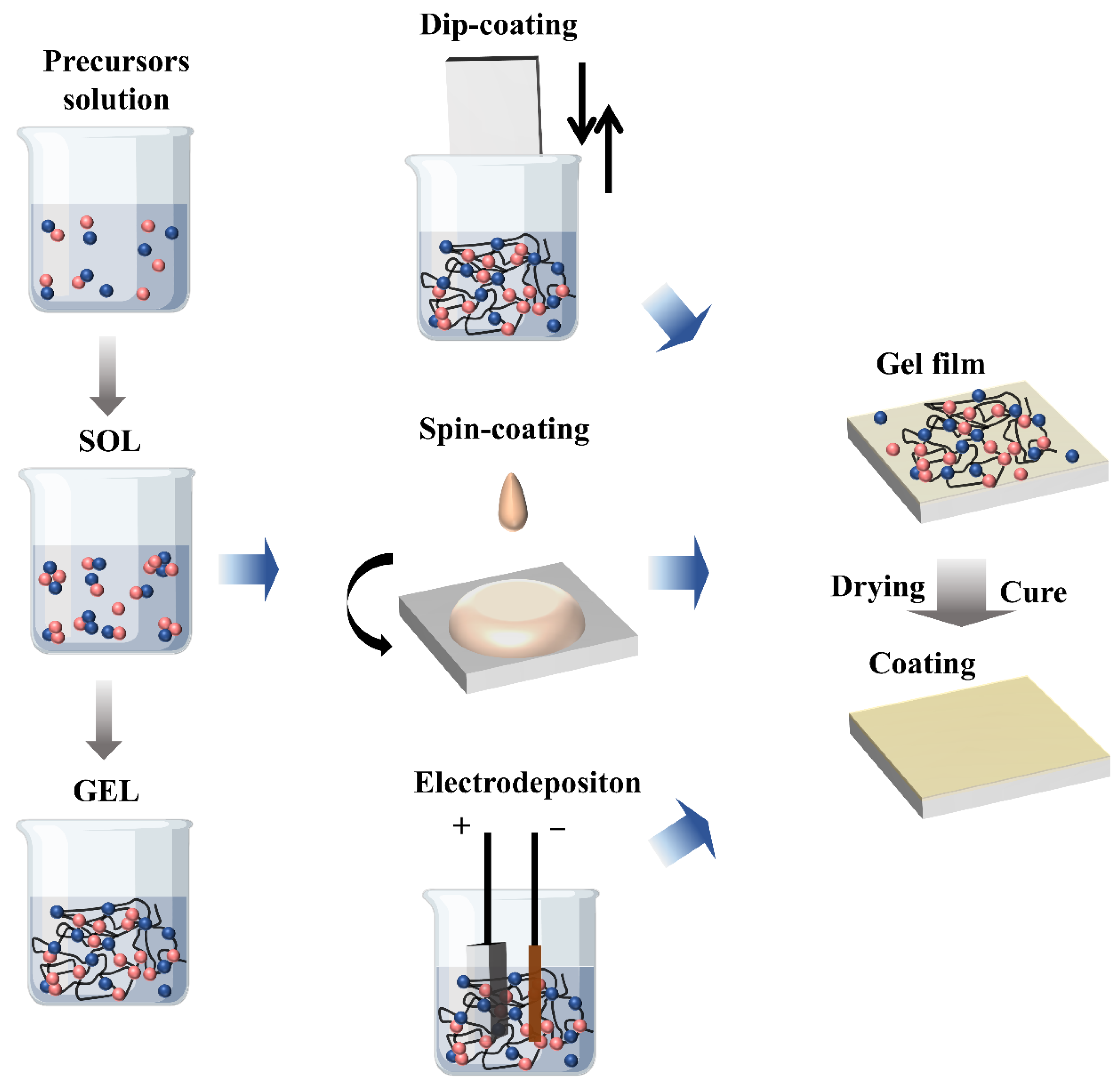
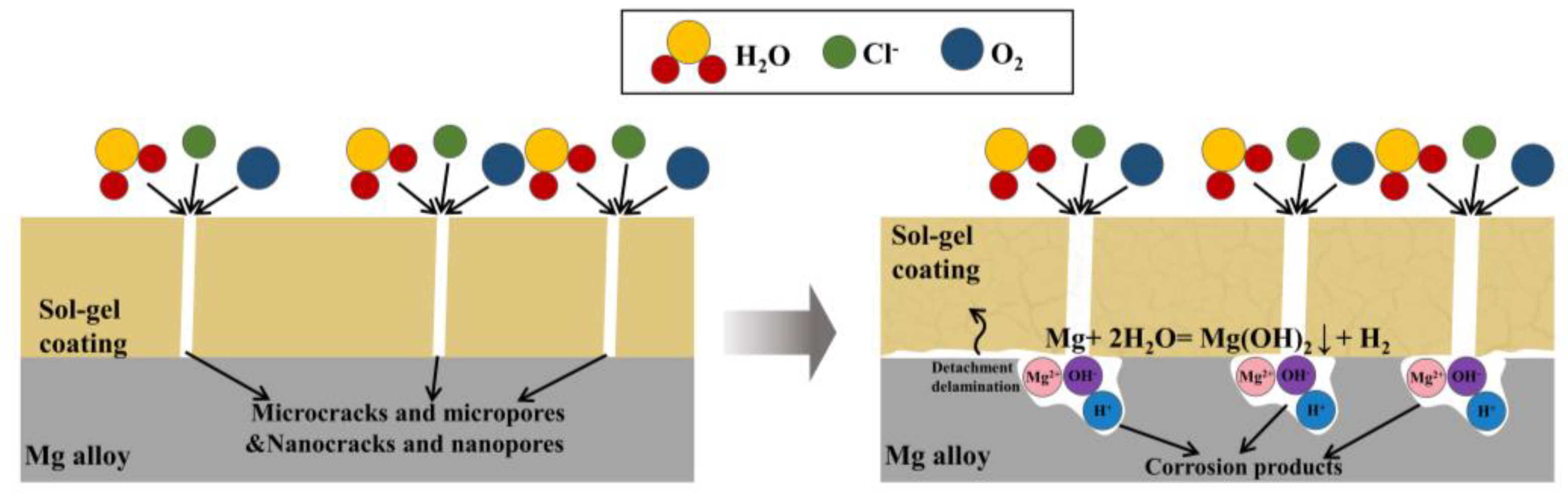
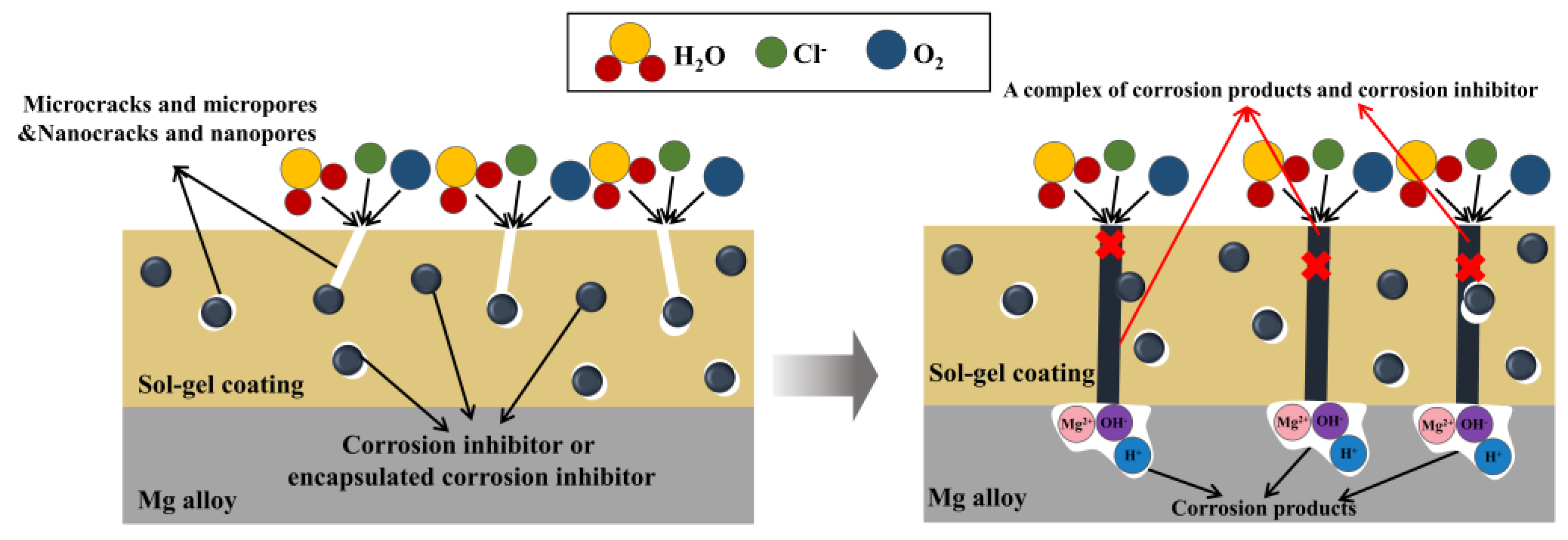


| Chemical Name | Abbreviation | Ref. | |
|---|---|---|---|
| 1 | Tetraethoxysilane | TEOS | [37,56,57,58,63,67,74,80,81,82,85,86,88,89,91,92,94] |
| 2 | Triethoxyvinylsilane | VTEO | [56] |
| 3 | Diethylphosphonatoethyltriethoxy-silane | PHS | [57] |
| 4 | 3-Glycidoxypropyltrimethoxysilane | GPTMS | [37,58,61,67,70,74,79,80,81,82,85,88,89,94] |
| 5 | Vinyltriethoxysilane | VETO | [63] |
| 6 | 3-(Trimethoxysilyl)propylmethacrylate | MAP | [64] |
| 7 | Methyltriethoxysilane | MTES | [74,91] |
| 8 | Tetraethoxysilane | TMOS | [70,93] |
| 9 | 3-Aminopropyltrimethoxysilane | γ-APS | [71,72] |
| 10 | Phenyl-trimethoxysilane | PTMS | [78] |
| 11 | Triethoxyvinylsilane | TEVS | [86] |
| 12 | Bis-[triethoxy amino] silane | BAS | [87] |
| 13 | Bis-1,2-(TriethoxySilyl)Ethane | BTSE | [90] |
| 14 | Methylmethoxysilane | MTMS | [92] |
| 15 | γ-Methacryloyloxypropyltrimethoxysilane | MAPTMS | [93] |
| Chemical Name | Abbreviation | Ref. | |
|---|---|---|---|
| 1 | γ-Glycidoxy propyl trimethoxy silane | γ-GPS | [97,126] |
| 2 | Bistriethoxysilylethane | BTSE | [98] |
| 3 | 3-Amino-propyltrimethox-ysilane | γ-APS | [98,129,130] |
| 4 | Poly(3-aminopropyl)trimethoxysilane | PAPTMS | [100] |
| 5 | Vinyl tri-ethoxy silane | VTES | [101] |
| 6 | Tetraethoxysilane | TEOS | [26,99,107,110,111,112,113,114,115,117,119,122,124,125,126,128,129,131,132] |
| 7 | 3-Glycidoxypropyltrimethoxysilane | GPTMS | [26,76,107,108,109,110,111,112,118,119,121,125,131] |
| 8 | Phenyl-trimethoxysilane | PTMS | [110,121] |
| 9 | 3-Methacryloxypropyl trimethoxysilane | - | [117] |
| 10 | Methyltriethoxysilane | MTES | [99,113,114,115,124,132] |
| 11 | γ-Amino propyltriethoxysilane | APTES | [128] |
| 12 | Bis [3-(triethoxysilyl)propyl]tetrasulfide | BTESPT | [130] |
| Methylmethoxysilane | MTMS | [116,123,127] |
| Substrate | Coatings | Sol-Gel Solution Composition | Thickness | Electrolyte | Anti-Corrosion Effect * | Ref. | |
|---|---|---|---|---|---|---|---|
| 2008 | AZ91D | Bare sol-gel coating | SiO2 (TV) sol: the molar ratio of TEOS:VTEO: ethanol: water: acetic acid is equal to 0.25:0.75:10:4:0.01. SiO2 (T) sol: the molar ratio of TEOS: ethanol: water: acetic acid is equal to 1:10:4:0.01. | - | 3.5 wt.% NaCl | icorr Mg = 1.29 × 10−5 A/cm2 icorr SiO2 (T) = 1.15 × 10−6 A/cm2 icorr SiO2 (TV) = 2 × 10−6.8 A/cm2 | [56] |
| 2006 | AZ31B | Bare sol-gel coating | The molar ratio of the silanes was 1:2 (PHS: TEOS). | 600–700 nm | Harrison’s solution (0.35 wt.% (NH4)2SO4 and 0.05 wt.% NaCl) | |Z|0.01Hz: uncoated≪ silica coated ≪coated with PHS: TEOS film. | [57] |
| 2017 | AZ31 | Bare sol-gel coating | Mixing TEOS and GPTMS precursors in a molar ratio of 3:1 and using ethanol and acetic acid as solvent and catalyst, respectively. | 0.7–2.5 μm | 0.1 M NaCl | icorr is about an order of magnitude lower compared to magnesium alloys, simultaneously, a protection range up to 150 mV. | [58] |
| 2010 | AZ91D | Bare sol-gel coating (Ce3+) | Mixing GPTMS, VETO, distilled water, and ethanol in 1:3:12:30 molar ratios. Ce(NO3)3·6H2O was added to yield 0.01 M of Ce3+. | - | 3.5 wt.% NaCl | icorr Mg = 1.29 × 10−5 A/cm2 icorr sol-gel = 8.64 × 10−7 A/cm2 icorr sol-gel (MPD) = 5.75 × 10−8 A/cm2 | [63] |
| 2015 | Elektron 21 (El21) alloy | Bare sol-gel coating (Ce3+) | Mixing the starting precursors consisting of TEOS and MAP, deionized water, and ethanol with a molar ratio of 11:1:60:80. The production of cerium-doped sols was performed by adding cerium nitrate (Ce(NO3)3 · 6 H2O) at four different concentrations: (0.005, 0.01, 0.05, and 0.1) mol/L. | 1 μm | 0.05 M NaCl | The hybrid film exhibited a high resistive modulus (105–106 Ω cm2) during the first few hours of immersion, and the addition of cerium at a concentration of 0.01 M to the sol significantly increased the durability of the film (2 days). | [64] |
| 2020 | AZ31 | Bare sol-gel coating (Ce3+) | Mixing TEOS and GPTMS in a molar ratio of 3:1 that was dissolved in ethanol. Then use an inhibitor solution of 2.5 mol% Ce(NO3)3 (relative to the precursor) and a catalyst of 2.5 vol% AcOH. Obtain mixed sols by mixing the two solutions at a volume ratio of 4.5:1. The hybrid sol was obtained by mixing both solutions in a volume ratio of 4.5:1. | 0.9–3.3 μm | 0.1 M NaCl | Hybrid coatings achieved a reduction of the corrosion current density by about two and three orders of magnitude with regard to the undoped coated specimen and the AZ31 alloy respectively, also exhibiting a protection range of up to 160 mV. | [37] |
| 2010 | AZ31 | Bare sol-gel coating (8-HQ) | Solution A: zirconium (IV) propoxide (70% solution in 2-propanol) and ethylacetoacetate with volume ratio 1:1. Solution B: GPTMS and 2-propanol with 1:1 volume ratio. The final solution: solutions (A + B) with a volume ratio of 1:1. Inhibitor-doped sol-gel films were prepared adding 0.26 wt.% of 8-HQ. | 3 μm | 0.005 M NaCl | After 14 days immersed, |Z|0.01Hz sol-gel ≈2 × 105 Ω cm2 |Z|0.01Hz sol-gel(8-HQ)≈ 1 MΩ cm2 Rct sol-gel = 687 kΩ cm2 Rct sol-gel (8-HQ) = 1649 kΩ cm2 | [61] |
| 2010 | ZE21B | Sol-gel coating + corrosion inhibitor | Mixing GPTMS and TEOS (molar ratios = 5:1), the solvent is an appropriate amount of distilled water and ethanol. The above solution was doped with corrosion inhibitor (PCTyr Schiff base). | - | Simulated Body Fluid (SBF) | icorr Mg = 1.31 × 10−4 A/cm2 icorr sol-gel = 4.29 × 10−6 A/cm2 icorr sol-gel (PCTyr) = 3.64 × 10−6 A/cm2 | [67] |
| 2009 | AZ91D | Sol-gel coating + corrosion inhibitor | The mixed precursors were GPTMS and TMOS with molar ratio of 3:1 in acetic acid solution of 0.05 mol/L. The molar ratio of GPTMS: acetic acid is 60:1. The above solution was doped with 0.001 mol/L MPD. The inhibitor was pre-resolved in 20 mL of distilled water and then added into sol solution. | - | Harrison’s solution | icorr Mg = 7.10 × 10−3 A/cm2 icorr sol-gel = 2.41 × 10−7 A/cm2 icorr sol-gel (MPD) = 4.5 ×10−10 A/cm2 | [70] |
| 2017 | AZ31 | Sol-gel coating + corrosion inhibitor | Phytic acid and γ-APS (mole ratios were 1:1) were added into 40 mL of mixed solution with water/ethanol volume ratio of 3:2. | - | SBF | icorr Mg = 49.41 μA/cm2 icorr sol-gel (Phytic acid) = 3.57 μA/cm2 | [71] |
| 2021 | AZ31 | Sol-gel coating + corrosion inhibitor | The silane sols consisted of three different precursors: MTES, GPTMS, and TEOS in equal volumes (6.6% V/V) in a combination of 10% distilled water and 70% ethanol. 1000 mg/L hydroxyapatite (HA) nanoparticles were added to the sol. | 3.81 μm | SBF solution | Rf: the overall resistance of the coating response. The Rf of silane coating modified with HA nanoparticles reached 41 kΩ cm2, which was more than 100 times higher than that without modification after being soaked for 4 days. | [74] |
| 2018 | AM60B | Sol-gel coating + nanoparticles | 500 ppm OH-MWCNTs were added to PTMS, and the mixture was ultrasonically agitated for about 20 min. | 1.4–1.5 μm | Harrison’s solution | Rp sol-gel = 207.5 kΩ cm2 Rp sol-gel (OH-MWCNTs) = 368.6 kΩ cm2 1440min later. Rp sol-gel = 22.6 kΩ cm2 Rp sol-gel (OH-MWCNTs) = 44.1 kΩ cm2 | [78] |
| 2021 | AZ91 | Sol-gel coating + nanoparticles | The GPTMS/GO was prepared by mixing 0.25 mL GO, 10 mL ethanol, 10 mL GPTMS, and 79.85 mL deionized water. | 10 μm | 3.5 wt.% NaCl | icorr Mg = 49.90 μA/cm2 icorr sol-gel = 0.25 μA/cm2 icorr sol-gel (GO) = 0.016 μA/cm2 Rct Mg = 0.87 kΩ cm2 Rct sol-gel = 3.9 kΩ cm2 Rct sol-gel (GO) =5.02 kΩ cm2 | [79] |
| 2020 | AM60B | Sol-gel coating + nanoparticles | Mixing 0.02 mol TEOS, 0.02 mol GPTMS 0.14 mol water (the pH was formulated to 1.5 with HCl). Then, 100 mg/L oxidized fullerene was added into the sol. | 1.5–2 μm | 3.5 wt.% NaCl | after being soaked for 1440 min. Rp Mg = 1.405 kΩ cm2 Rp sol-gel (OF) = 500.018 kΩ cm2 | [80] |
| 2021 | AM60B | Sol-gel coating + nanoparticles | Mixing 0.02 mol TEOS, 0.02 mol GPTMS 0.14 mol water (the pH was formulated to 1.5 with HCl). Then, 500 ppm F-SDS (the SDS molecules were stabilized on the fullerene C60 nanoparticles) was added into the sol. | 3 μm | 3.5 wt.% NaCl | After being soaked for 48h. Rp sol-gel = 6 kΩ cm2 Rp sol-gel (F-SDS) = 23 kΩ cm2 | [81] |
| 2020 | AM60B | Sol-gel coating + nanoparticles | Mixing 0.02 mol TEOS and 0.02 mol GPTMS. Acidic water (pH = 1, HCl) was added to the sol with 1:1 alkoxy to H2O molar ratio. Then, 0.01 wt.% of the hydroxylated nanodiamonds was added into the sol. | 0.7–0.8 μm | Harrison’s solution | After 3 h immersion, icorr sol-gel = 2.202 μA/cm2 icorr sol-gel (HND) = 0.476 μA/cm2 | [82] |
| 2020 | AZ31B | Sol-gel coating + nanoparticles | The hydrolysis and polymerization of GPTMS and TEOS were under acidic condition. After a certain amount of F-ATP@SiO2 particles were added to the sol. | - | 3.5 wt.% NaCl | After 3 h immersion, icorr Mg = 7.143 × 10−5 A/cm2 icorr sol-gel (SiO2) = 5.519 × 10−8 μA/cm2 | [85] |
| 2019 | AZ91 | Sol-gel coating + inhibitor + nanoparticles | Adding TEOS and TEVS with a molar ratio of 1:3. 0.5 wt.% cysteine and 1.0 wt.% TiO2 were added to the sol. | 450 nm | 0.05 M NaCl | icorr sol-gel = 1168.1 nA/cm2 icorr sol-gel (cysteine +TiO2) = 25.0 nA/cm2 Rct Mg = 0.238 kΩ cm2 Rct sol-gel = 5.554 kΩ cm2 Rct sol-gel (cysteine +TiO2) = 224.090 kΩ cm2 | [86] |
| 2008 | AZ31 | Sol-gel coating + inhibitor + nanoparticles | The BAS was prepared by dissolving 5% (vol/vol) of silane in a mixture of methanol (10% vol/vol) and 85% (vol/vol) of distilled water. Modified by Ce(NO3)3 or La(NO3)3 CNTs were then added to the sol. | 5.5–6 μm | 0.05 M NaCl | For the silane coating modified with the untreated CNTs, the anodic current densities attained values around 60 µA/cm2 for all the test period (up to 24 h) and the cathodic currents attained values around −60 µA/cm2. The silane coatings modified with the CNTs treated with cerium revealed the lowest corrosion activity. After 24 h of immersion, the activity decreased and both anodic and cathodic current densities ranged between 2 and −2 μA/cm2. | [87] |
| 2017 | AZ91D | Sol-gel coating + inhibitor + nanoparticles | The organic-inorganic hybrid matrix sol was prepared by hydrolysis of GPTMS with TEOS in molar ratio of 3:5:1 with 0.1 N HCl as catalyst. | - | 3.5 wt.% NaCl | After being soaked for 24h, icorr Mg =6.0 × 10−5 A/cm2 icorr sol-gel = 1.4 × 10−6 A/cm2 icorr sol-gel (Ce3+/Zr4 +halloysite nanotubes) = 0.9 × 10−6 A/cm2 | [88] |
| 2018 | AZ91D | Sol-gel coating + inhibitor + nanoparticles | GPTMS and TEOS were taken in molar ratio of 3:5 and hydrolysed in presence of 0.1 N HCl as catalyst, to synthesize the hybrid organic-inorganic matrix sol. | 2.5 ± 0.5 μm | 3.5 wt.% NaCl | After being soaked for 120 h, icorr sol-gel = 1.513 × 10−5 A/cm2 icorr sol-gel (Ce3+/Zr4 +halloysite nanotubes) = 5.602 × 10−7 A/cm2 | [89] |
| 2010 | AZ91D | Bare sol-gel coating | The silane sol was synthesized by mixing GPTMS, TEOS, distilled water, and ethanol in 3:1:13:40 molar ratios. | - | 0.005 M NaCl + zinc nitrate | After the introduction of zinc nitrate for 48 h, the resistance value increased and arrived at about 180 kΩ, which exceeded that of the sample initially immersed in undoped solution for 1 h (about 140 kΩ). | [94] |
| 2012 | AZ91D | Composite sol-gel coating (sol-gel/ Mg-rich epoxy primer) | Adding 10 wt.% γ-GPS to a 1:8 mixture of methanol and distilled water. Glycerol (0.15 vol.% of the total silane solution) was added. | - | 3 wt.% NaCl | |Z|0.01Hz sol-gel/ Mg-rich epoxy primer: higher than 1011 Ω cm2; For magnesium-rich primer of AZ91D alloy without pre-treatment, EIS results show that the alloy substrate is corroded after 840 h immersion; for magnesium rich primer of AZ91D alloy pretreated with silane, the EIS results show that the substrate will corrode after being soaked for 1800 h. | [97] |
| 2013 | AZ31 | Composite sol-gel coating (sol-gel/ sol-gel) | BTSE or γ-APS solution was prepared by mixing 5% silane, 90% ethanol, and 5% Milli-Q water. | - | SBF solution | icorr Mg = 8.32 ± 0.63 μA/cm2 icorr sol-gel = 2.69 ± 0.31 μA/cm2 icorr sol-gel /sol-gel = 0.90 ± 0.24 μA/cm2 Rp Mg = 2650 ± 538 Ω cm2 Rp Mg-B = 7788 ± 2572 Ω cm2 Rp Mg-B-A =13635 ± 2745 Ω cm2 | [98] |
| 2020 | AZ31B | Single sol-gel coating + nanoparticles | TEOS + MTES/Isopropanol/water: 1/5/10. To obtain the initial sols, TEOS and MTES were mixed in molar fraction of 40 %/60 %. 0.005 wt.% COOH-GNPs was added to isopropyl alcohol, and the final concentration of nano charges measured in the coating was 0.046 wt.% of COOH-GNPs. | 2.2 μm | 3.5 wt.% NaCl | After being soaked for 24 h, icorr Mg = 6.6 × 10−6 A/cm2 icorr sol-gel =1.2 × 10−6 A/cm2 icorr sol-gel /sol-gel = 5.2 × 10−7 A/cm2 | [99] |
| 2020 | AZ31 | Composite sol-gel coating (sol-gel/ PP) | PAPTMS: (APTMS: ethanol: deionized water = 3:22:75, V/V/V) | About 60 μm | 3.5 wt.% NaCl | icorr Mg = 4.96 × 10−5 A/cm2 icorr sol-gel = 1.95 × 10−6 A/cm2 icorr sol-gel/ PP = 9.08 × 10−8 A/cm2 Rp Mg = 190.9 Ω cm2 Rp sol-gel = 7578 Ω cm2 Rp sol-gel/ PP = 2.80 × 105 Ω cm2 | [100] |
| 2022 | AZ31 | Composite sol-gel coating (sol-gel/ HA) | 2.5 mL of VTES was added to a mixture of 5 mL DIW, 5 mL of acetone, and 95 mL of ethanol under magnetic stirring. | - | 3.5 wt.% NaCl | icorr Mg= 36.1 ± 0.1 μA/cm2 icorr sol-gel/ HA = 0.9 ± 0.1 μA/cm2 Rp Mg = 253 Ω cm2 Rp sol-gel/ HA = 12155 Ω cm2 | [101] |
| 2023 | AZ31 | Composite sol-gel coating (sol-gel/(CA/Lys)) | Silicon sol was prepared from GPTMS, TEOS, deionized water, and ethanol, mixed in a volume ratio of 3:1:1:5. Then, add Ce(NO3)3 to make the concentration of Ce(NO3)3 reach 0.01 M. | 9 ± 0.5 μm | 0.1 M NaCl | Sol-gel: after being soaked for 4 days, Rct = 1.030e3. CA/Lys @Sol-gel: after being soaked for 4day, Rct = 1.344e6. After 18 days of the test, the value of Rct was still as high as 105 Ohm⋅cm2. | [26] |
| 2009 | AZ91D | Composite sol-gel coating (molybdate/sol-gel) | Silicon sol was prepared from TEOS, GPTMS, and ethanol, which were mixed in a molar ratio of 0.25:0.75:10. | - | 3.5 wt.% NaCl | icorr Mg= 1.29 × 10−5 A/cm2 icorr conversion coating = 1.76 × 10−5 A/cm2 icorr conversion coating/sol-gel = 3.80 × 10−5 A/cm2 Rp conversion coating = 552 Ω cm2 Rp conversion coating/sol-gel = 4.5× 104 Ω cm2 | [107] |
| 2013 | Elektron21 | Composite sol-gel coating (phosphate /sol-gel) | Mixing starting precursors consisting of GPTMS and aluminum-tri-sec-butoxide, deionized water, and propanol in a molar ratio of 2:1:1:10. | 7 μm | 0.05 M NaCl | After being soaked for 192 h, |Z|0.01Hz Mg ≈3 × 103 Ω cm2 |Z|0.01Hz sol-gel≈3 × 103 Ω cm2 |Z|0.01Hz conversion coating /sol-gel ≈ 3 × 104 Ω cm2 | [109] |
| 2013 | AZ31 | Composite sol-gel coating (phosphate /sol-gel) | Silane solution: 10 g/L KH560 | 4.9 μm | 3.5 wt.% NaCl | Rtot conversion coating = 2227 Ω cm2 Rtot conversion coating/sol-gel = 5.6 × 103 Ω cm2 | [108] |
| 2022 | WE43 | Composite sol-gel coating (Cerium/sol-gel) | The inorganic TEOS (10% V/V) and organic GPTMS (20% V/V) precursors were added together to a mixture of ethanol (10% V/V) and distilled water (60% V/V). | 2.06 ± 0.05 μm | 0.1 M NaCl | After being soaked for 24 h, icorr Mg = 10.9 μA/cm2 icorr conversion coating = 3.0 μA/cm2 icorr conversion coating/sol-gel = 0.6 μA/cm2 Rct Mg = 3177 Ω cm2 Rct conversion coating = 4363 Ω cm2 Rct conversion coating /sol-gel = 22485 Ω cm2 | [131] |
| 2017 | AM60B | Composite sol-gel coating (Cerium vanadate /sol-gel) | Mixing 0.04 mol TEOS, 0.02 mol GPTMS, and 1.23 mol acidic water so that the molar ratio of the water molecules to alkoxide groups was about 5:1. | 2 μm | Harrison’s solution | icorr Mg = 310.9 μA/cm2 icorr conversion coating = 145.8 μA/cm2 icorr conversion coating/sol-gel = 4.6 μA/cm2 Rct Mg =21.4 Ω cm2 Rct conversion coating = 114.5 Ω cm2 Rct conversion coating /sol-gel = 3750.0 Ω cm2 | [111] |
| 2019 | AM60B | Composite sol-gel coating (Ti-Zr/sol-gel) | Here, 0.02 mol TEOS and 0.02 mol GPTMS precursors were mixed. | 1.5–2 μm | 0.05 M NaCl | icorr Mg= 9.670 μA/cm2 icorr conversion coating = 5.692 μA/cm2 icorr conversion coating/sol-gel = 0.027 μA/cm2 Rp Mg = 4.7× 103 Ω cm2 Rp conversion coating = 7.9× 103 Ω cm2 Rp conversion coating/sol-gel = 858.5 × 103 Ω cm2 | [110] |
| 2021 | WE54 | Composite sol-gel coating (fluoride/sol-gel) | Hybrid sols were synthesized by mixing TEOS and GPTMS in a molar ratio of 3:1, employing ethanol as solvent and an acidic mixture of acetic acid and nitric acid as catalysts in a volume proportion of 2.5:1. | - | 0.1 M NaCl | icorr Mg = 1.78 × 10−5 A/cm2 icorr conversion coating = 1.84 × 10−6 A/cm2 icorr conversion coating/sol-gel = 1.86 × 10−7 A/cm2 | [112] |
| 2019 | AZ91 | Composite sol-gel coating (CLP/sol-gel) | Adding TEOS and MTES with a molar ratio of 2:3 to an acidic solution of nitric and acetic acids in 1:5 vol ratio. Then, 1 wt.% L-Aspartic was added to the sol as corrosion inhibitors. | 850 nm | 3.5% NaCl | Rtotal Mg = 0.119 kΩ cm2 Rtotal conversion coating =0.681 kΩ cm2 Rtotal conversion coating /sol-gel= 85.417 kΩ cm2 | [113] |
| 2019 | AZ91 | Composite sol-gel coating (CLP/sol-gel) | A mixture of TEOS and MTES with a molar ratio of 2:3 was hydrolyzed in a solution of acetic and nitric acids in a 5:1 vol ratio. Then, 0.5 wt.% of cloisite Na+ and 0.5 wt.% of methionine were added to the sol. | 800 nm | 3.5% NaCl | Rtotal Mg = 0.119 kΩ cm2 Rtotal conversion coating =0.681 kΩ cm2 Rtotal conversion coating /sol-gel= 434.731 kΩ cm2 | [114] |
| 2019 | AZ91 | Composite sol-gel coating (CLP/sol-gel) | A mixture of TEOS and MTES with a molar ratio of 2:3 was hydrolyzed in a solution of acetic and nitric acids in a 5:1 vol ratio. Then, 0.5 wt.% potassium hypophosphite and 0.5 wt.% of cloisite 20A nanoparticle were added to the sol. | - | 3.5% NaCl | Rtotal Mg = 0.119 kΩ cm2 Rtotal conversion coating = 0.681 kΩ cm2 Rtotal conversion coating /sol-gel = 127.382 kΩ cm2 | [115] |
| 2017 | AZ31 | Composite sol-gel coating (Mg (OH)2/sol-gel) | PMTMS/CeO2: a mixture of MTMS, ethanol and water (3:10:20, V/V/V), cerium nitrate (10−3 M). | 12.86 ± 0.01 μm | 3.5 wt.% NaCl | icorr Mg = 1.51 ± 0.08 × 10−5 A/cm2 icorr Mg(OH)2/sol-gel = 2.46 ± 0.07 × 10−8 A/cm2 Rct Mg = 854.4 Ω cm2 Rct Mg(OH)2/sol-gel = 4.03 × 105 Ω cm2 | [116] |
| 2010 | AM60B | Composite sol-gel coating (AO/sol-gel) | Mixing together with TEOS (4.7 g), 3-metacryloxypropyl trimethoxysilane (10.4 g), ethylalcohol (15.8 g), distilled water (4.9 g), and tert-butylhydroperoxide (1.9 g). | 4 μm | 3.5 wt.% NaCl | icorr Mg = 3 × 10−5 A/cm2 icorr AO = 2 × 10−6 A/cm2 icorr AO/sol-gel = 7 × 10−9 A/cm2 | [117] |
| 2009 | AZ91D | Composite sol-gel coating (MAO/sol-gel) | TEOS, zirconyl chloride octahydrate (ZrOCl2·8H2O), and ethanol were mixed together. | 5 μm | 3.5 wt.% NaCl | icorr Mg = 3.395 × 10−5 A/cm2 icorr MAO = 3.921 × 10−7 A/cm2 icorr MAO/sol-gel =1.577 × 10−9 A/cm2 | [122] |
| 2012 | NZ30K | Composite sol-gel coating (MAO/sol-gel) | The desired amounts of TEOS, C2H5OH, NH4OH, and H2O were mixed with a molar ratio of 1:30:1:1. The calculated amount of MTES (molar ratio of MTES/TEOS = 1/2) was added dropwise into the mixed solution. | 3.5–7 μm | 3.5 wt.% NaCl | icorr Mg = 2.2 × 10−5 A/cm2 icorr MAO = 2.5 × 10−7 A/cm2 icorr MAO/sol-gel =2.6 × 10−8 A/cm2 |Z|0.01Hz Mg ≈ 103 Ω cm2 |Z|0.01Hz MAO ≈ 105 Ω cm2 |Z|0.01Hz MAO/sol-gel ≈ 3 × 106 Ω cm2 | [132] |
| 2016 | ZE41 | Composite sol-gel coating (PEO/sol-gel) | Silane sol: mixing GPTMS and PTMS (volume ratio was 1: 1); metal organic: mixing TPOT (70 wt.% in 2-propanol) and acetylacetone in stoichiometric proportion. Both metal organic and silane sols were mixed together. | 7.8–8.4 μm | 3% NaCl | After 7 days of immersion, Sol-gel: about 50% of the coating was exfoliated from the surface. |Z|0.01Hz MAO/sol-gel = 3 × 108 Ω cm2 | [121] |
| 2017 | AZ31 | Composite sol-gel coating (MAO/sol-gel) | Polymethyltrimethoxysilane (PMTMS): (MTMS: ethanol: DI water = 3:10: 20) | 13.65 μm | 3.5 wt.% NaCl | icorr Mg = 1.37 × 10−5 A/cm2 icorr MAO/sol-gel = 2.86 × 10−8 A/cm2 Rct Mg = 275.30 Ω cm2 Rct MAO/sol-gel = 2.24 × 106 Ω cm2 | [123] |
| 2019 | AZ80 | Composite sol-gel coating (PEO/sol-gel) | Ethanol: silica precursors: water: hydrochloric acid (a molar ratio) = 2:1:4:0.01. The ratio between TEOS and MTES were fixed at 30:70. | 22 μm | 0.1 M Na2SO4 + 0.05 M NaCl | PEO/sol-gel that is characterized by currents about two orders of magnitude lower than the untreated one. | [124] |
| 2021 | AZ31B | Composite sol-gel coating (PEO/sol-gel) | Mixing 0.5 mol TEOS, 0.5 mol GPTMS, and 0.54 mol of a colloidal SiO2 nanoparticles suspension. Ethanol containing 0.1 mol of 1-Methylimidazole (MI) were added. | 3.5 μm | 3.5 wt.% NaCl | icorr Mg = 1.61 × 10−5 A/cm2 icorr PEO = 2.64 × 10−7 A/cm2 icorr PEO/sol-gel = 2.80 × 10−8 A/cm2 Rp Mg = 207.3 Ω cm2 Rp PEO =31432.5 Ω cm2 Rp PEO/sol-gel =31,546.8 Ω cm2 PEO/sol-gel includes an additional diffusive resistance (68716 Ωcm2) (non-faradaic resistance) between the sol-gel coating and PEO oxide layer | [125] |
| 2021 | AZ31 | Composite sol-gel coating (PEO/sol-gel) | A molar fraction of 40% TEOS and 60% MTES. Diluting in isopropanol and 0.1 M of HCl acidulated H2O in a molar ratio of 1 mol of the mixture of precursors, 5 mol of isopropanol, and 10 mol of acidulated H2O. In addition, sol was doped with 0.005 wt.% Grade 4 −COOH functionalized GNPs (COOH−GNPs). | 36.7 μm | Hanks’ solution (pH = 7) | After being soaked for 24 h, icorr Mg = 1.5 × 10−6 A/cm2 icorr PEO = 1.6 × 10−7 A/cm2 icorr PEO/sol-gel = 2.50 × 10−8 A/cm2 | [103] |
| 2022 | AM6 | Composite sol-gel coating (PEO/sol-gel) | Mixing two different sols using controllable hydrolysis of γ-GPS and TEOS. | 19.3 μm | 3.5 wt.% NaCl | Rp PEO = 3.37 ×105 Ω cm2 Rp PEO/sol-gel = 3.58 × 109 Ω cm2 | [126] |
| 2020 | AZ91D | Composite sol-gel coating (N-GQDs/sol-gel) | PMTMS: (MTMS: ethanol: DI water = 3: 10: 20) | 19 μm | 3.5 wt.% NaCl | Rct Mg = 78.3 Ω cm2 Rct N-GQDs /sol-gel = 1.7 × 104 Ω cm2 | [127] |
| 2020 | AZ31 | Composite sol-gel coating (PEO/sol-gel/epoxy) | T50/A50: (TEOS: APTES: Water: Ethanol =2.14:2.14:2:8(Volume ratio) | - | 3.5 wt.% NaCl | After being soaked for 28 day, Rcoat PEO/epoxy ≈ 2 × 106 Ω cm2, Rcoat PEO/sol-gel/epoxy ≈ 1× 108 Ω cm2 | [128] |
| 2021 | AZ31 | Composite sol-gel coating (PEO/sol-gel/epoxy) | T50/A50: (TEOS: APTES: Water: Ethanol =2.14:2.14:2:8(Volume ratio) 5 ppm of organic inhibitors 8-HQ, I3C, 2- MBO, and DDTC were added individually to silane solutions. | - | 3.5 wt.% NaCl/0.5 wt.% NaCl | After being soaked for 28 days in 3.5 wt.% NaCl, log |Z|0.01Hz Triplex ≈ 7.5, log |Z|0.01Hz Triplex-Ce-HQ ≈ 8.8; Log |Z|0.01Hz Triplex = 4.88, log |Z|0.01Hz Triplex-Ce-HQ = 6.06 with artificial defects, immersed in 0.5 wt.% NaCl solution for 48 h. | [129] |
| 2019 | AZ31B | Composite sol-gel coating (sol-gel/GO/sol-gel) | The molar ratio of mixed APS/BTESPT was ½, and the volume ratio of mixed silane:deionized water:ethanol = 1:1:8. | 1100 nm | 3.5 wt.% NaCl | icorr Mg = 2.1852e−4 A/cm2 icorr sol-gel/GO/sol-gel = 1.381e−8 A/cm2 | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Bai, H.; Feng, Z. Advances in the Modification of Silane-Based Sol-Gel Coating to Improve the Corrosion Resistance of Magnesium Alloys. Molecules 2023, 28, 2563. https://doi.org/10.3390/molecules28062563
Li J, Bai H, Feng Z. Advances in the Modification of Silane-Based Sol-Gel Coating to Improve the Corrosion Resistance of Magnesium Alloys. Molecules. 2023; 28(6):2563. https://doi.org/10.3390/molecules28062563
Chicago/Turabian StyleLi, Jiao, Huanhuan Bai, and Zhiyuan Feng. 2023. "Advances in the Modification of Silane-Based Sol-Gel Coating to Improve the Corrosion Resistance of Magnesium Alloys" Molecules 28, no. 6: 2563. https://doi.org/10.3390/molecules28062563
APA StyleLi, J., Bai, H., & Feng, Z. (2023). Advances in the Modification of Silane-Based Sol-Gel Coating to Improve the Corrosion Resistance of Magnesium Alloys. Molecules, 28(6), 2563. https://doi.org/10.3390/molecules28062563







