Abstract
This article reports in detail a method for the synthesis of 3-benzoxoxazoline by the reaction of alkenes (alkynes) and a variety of α-nitroketones in the presence of p-TsOH. The scope of alkenes is broad, including different alkenes and the alkyne. This reaction provides a convenient and efficient synthetic method of 3-benzoylisoxazolines.
1. Introduction
Heterocycles are important structural elements, which are present in natural products from all classes and in many biologically active synthetic compounds [1]. Heterocyclic compounds perform an important role in chemical industry, e.g., food fragrance and dyes. Amongst these, isoxazole and its derivatives represent a group of five-element heterocyclic compounds containing oxygen and nitrogen atoms of a valuable class [2]. Additionally, they have performed a vital role in the theoretical development of heterocyclic chemistry and are also extensively used in organic synthesis [3]. Isoxazoles have attracted an increasing research interest, and are widely used and studied in the modern drug discoveries as non-classical amide or ester bioisosteres, and potential pharmacophores endowed, and most isoxazoles have strong biological activity [4,5].
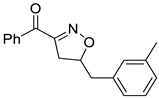
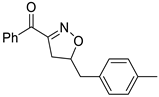
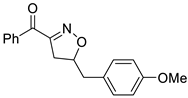
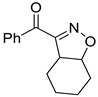
Isoxazolines are partially saturated analogs of isoxazoles as important intermediates for synthesis of varieties of fascinating organic molecules applicable to both basic organic synthesis and life sciences [6]. Isoxazolines can be converted into various synthetic units, such as hydroxy ketones [7], amino alcohols [8], β-hydroxynitrile [9], and masked aldols [10], and be used as synthetic equivalent of 1,3-dicarbonyl structure [11]. Isoxazolines can exhibit a variety of bioactivities, such as anti-inflammatory [12], anticancer [13], hypoglycemic [14], antibacterial [15], anti-HIV [16], anti-Alzheimer’s [17], antifungal [18], antimalarial [19], antioxidant [20], anti-tuberculosis [21], and antinociceptive [22] activities (Figure 1). Isoxazolines can also be good herbicides [23] and insecticides [24]. Therefore, the development of new methods for more efficient synthesis has been always an attractive task.
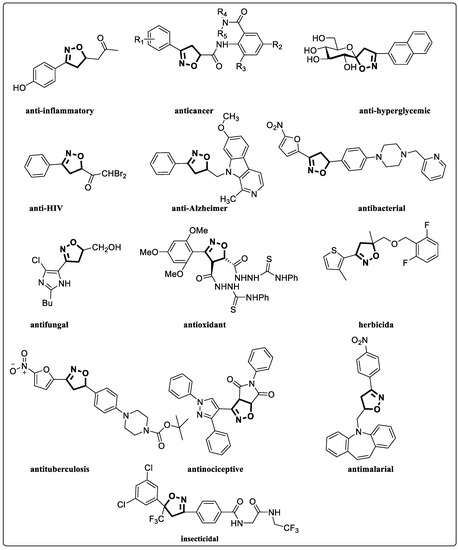
Figure 1.
Structures of isooxazolines with bioactivity.
Based on the characteristics and wide application of isoxazolines derivatives, the research progress of isoxazolines derivatives have progressed rapidly in recent years, and a large number of synthetic methods for isoxazole derivatives are reported in the literature every year. These approaches can be summarized as the following four major types: (1) 1,3-dipolar cycloaddition between nitrile oxide and unsaturated hydrocarbon [25,26,27,28,29], (2) intramolecular addition cyclization reaction of unsaturated hydroxime [30,31,32,33], (3) condensation reactions of 1,3-dicarbonyl derivatives [34], and (4) cycloisomerization [35]. In the past decades, the 1,3-dipole cycloaddition reaction of alkenes with nitrile oxide is the most direct and extensive method for the construction of isoxazoline skeletons [36]. Nitrile oxides are usually derived from aldoximes and nitro compounds [37,38], but the common use of transition metal catalysts, such as Cu(I), Cu(II), and Ru(II), in the reaction makes the products residual metal and cytotoxic [39,40,41], which limits its application in biology and drug development (Scheme 1). Therefore, the development of practical, simple, and cost-effective new methods for synthesis 2-oxazolines would complement current methods.
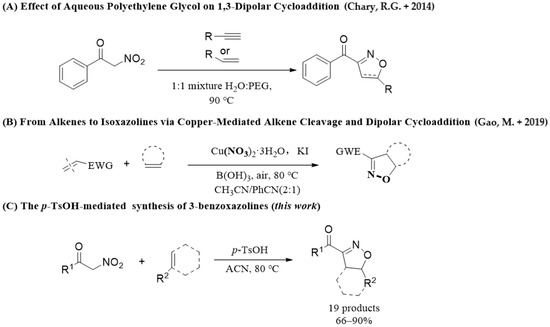
Scheme 1.
Overview of the 1,3-dipolar cycloaddition of hydrocarbons and α-nitro ketones [36,39].
In the previous study, we first used the alkaline catalyst chloramine-T to catalyze the reaction of α-nitroketone and alkene to synthesize isoxazoline with a yield of 77% [42]. In the present work, we report a novel synthesis of dihydroisoxazoles by p-TsOH (anhydrous)-participated 1,3-dipolar cycloaddition of isoxazoline with α-nitroketones. On one hand, compared with the strong acid (H2SO4)-catalyzed synthesis of isoxazole [43], p-TsOH gives a milder reaction condition that avoids carbonization of organic substance, and it is low in toxicity and is inexpensive. On the other hand, Natarajan Arumugam and co-workers [44] reported a good, facile, and efficient method for the rapid synthesis of fused pyrrolidine and indolizinoindole heterocycles through 1,3-dipolar cycloaddition in the presence of p-TsOH. Additionally, Zhenghui Guan and co-workers [45] also demonstrated a p-TsOH mediated 1,3-dipolar cycloaddition approach of nitroolefins and sodium azide for the synthesis of 4-aryl-NH-1,2,3-triazoles, and a slightly higher yield (93%) was isolated. It is an efficient p-TsOH-mediated 1,3-dipole cycloaddition reaction that can tolerate a wide range of functional groups, and quickly and easily obtain the target product under mild conditions. p-TsOH was discovered as a vital additive in this type of 1,3-dipolar cycloaddition. Herein, isoxazolines, given the importance of preparing biologically active molecules, are chosen for validation of the accessibility, operational simplicity, and atom economy of our method.
2. Results and Discussion
2.1. Optimization of the Reaction Conditions
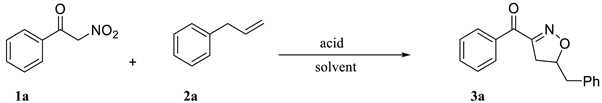
First, we compared the effects of different acids and solvents. The reaction of benzoylnitromethane 1a with allylbenzene 2a to form isoxazoline 3a was performed and the results were summarized in Table 1. It is noted that the reaction without acid did not proceed. In the presence of various acids, i.e., HCl, HNO3, H2SO4, TFA, H3PO4, fluoroboric acid, MsOH, and p-TsOH, the yield was significantly improved. It appears that oxidative acids produced similar good yield (Table 1, entries 3 and 4), but reagents used there are expensive, toxic, and dangerous. It was found that MsOH in i-PrOH at 80 °C effectively promoted the formation desired isooxazoline. However, the yield of 3a was slightly lower than that of p-TsOH (LD50: 1410 mg/kg) (Table 1, entries 8 and 9), and MsOH (LD50: 200 mg/kg) is highly toxic. Therefore, p-TsOH, which is non-oxidizing, corrosive, and low toxic was selected to participate in the synthesis of isoxazoline with a yield of 67%. While among the five tested solvents (ACN, i-PrOH, DMF, DMSO, and H2O), it was found that the yield reduced significantly with the relative polarity of the solvent (Table 1, entries 9–13) and can obviously is the best solvent.

Table 1.
Reaction condition optimization a.
The reaction temperature and amount of p-TsOH were further optimized. The results were shown in Figure 2. Then, the optimal condition was regarded acanACN as solvent, 4 equiv of p-TsOH was involved in the reaction at 80 °C for 22 h, in which a good yield of 90% could be obtained.
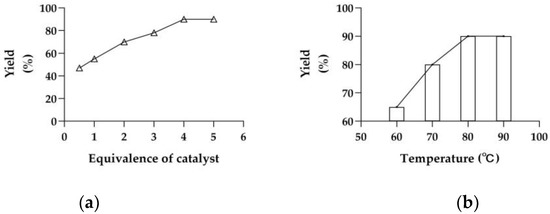
Figure 2.
Optimization of the reaction conditions. (a) The effect of various equivalence of p-TsOH; (b) The effect of various temperature. The amount of p-TsOH was 0.5 mmol. General conditions: 1a (0.125 mmol), 2a (0.625 mmol), ACN (0.2 mL) at 80 °C or indicated temperature for 22 h.
2.2. Substrate Scope Studies
With the optimal reaction conditions, we first tested the 1,3-dipolar addition reaction for benzoyl nitromethane and allylbenzene and had a yield of product of 90%. Then, to examine the generality and scopes of this methodology, we took a variety of benzoylnitromethane derivatives 1 (Scheme 2) and allylbenzene 2a as substrates and representative results were shown in Scheme 2. These results showed that a variety of electronically varied aromatic α-nitroketones were well compatible with the cycloaddition in all the reactions, and reaction generally obtains in moderate to good yields for the synthesis of isoxazoline derivatives. Moreover, in this reaction, we found a good regioselectivity, which was consistent with the work of Ken-ichi Itoh [45].
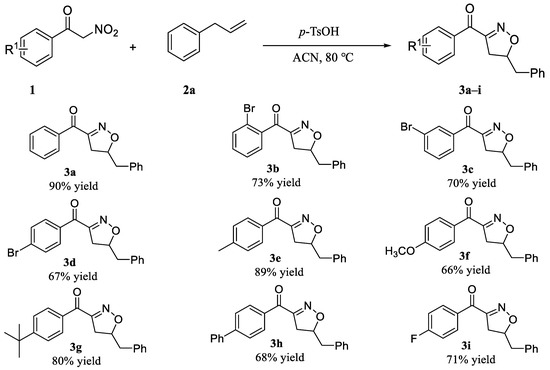
Scheme 2.
Scopes of the substituted phenylnitroketones.
At first, it was found that the R1 substituents would affect the cycloaddition efficiency in these reactions. The electron-rich α-nitroketones (1e, 1g, Scheme 2) provided products (3e, 3g, Scheme 2) in slightly better yields in comparison to the electron-deficient ones (1d, 1f, 1i, Scheme 2). Different electron-withdrawing substituents at the same position of phenyl-α-nitroketone resulted in similar yields (3d, 3i, Scheme 2). Additionally, surprisingly, isoxazoline derivative (3f, Scheme 2) were obtained in moderate yields when electron-donating group(-OMe) were used with a yield of 66%, which was close to the yield of isoxazoline obtained by electron-deficient α-nitroketone. In the case of electron-deficient α-nitroketone, the corresponding were obtained in good yields (3b–3d, Scheme 2), respectively 73%, 70%, and 67%. The results show that the position of the substituent has no effect on the reaction results. Additionally, aromatic substrate, such as benzene, behaved similar to an electron-withdrawing substituents and gave a yield of 68% for product 3h. However, the reaction rate was slower than that of aliphatic substituted substrates.
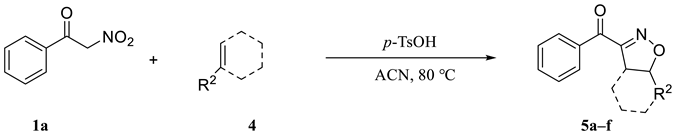
Next, we also investigated the scope of the alkenes (Table 2). The reaction of 1a with alkenes derivatives 4 was carried out under the optimum reaction conditions whose results are shown in Table 2. All the reactions gave 5a–5f as product, respectively, in good to excellent yield, except 5f. The type of reaction substrate alkenes was modified, and it was found that the reaction proceeded well with both aliphatic alkenes and aromatic alkenes affording isoxazolines in good yields from the same α-nitroketone (entries 1–5, Table 2). In addition, cycloaddition of cyclohexene (4f) with benzoylnitromethane (1a) could also be achieved in fairly good yields, the corresponding isoxazoline 5f was obtained in 69% yield (entry 6, 5f, Table 2).

Table 2.
Scopes of alkenes.
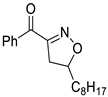
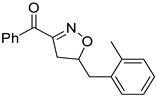
In addition, in order to expand the applicability of the reaction, we further examined the types of reaction materials. Isoxazolines were synthesized using the dipolarophiles 2a (allylbenzene) and alkyl nitroketones 6 (Scheme 3). The results showed that in the presence of p-TsOH, alkyl nitroketones were also able to react with dipolar reagents to obtain isoxazoline derivatives; unfortunately, compared with 3 and 5 phenylisoxazoline, 7a and 7b yields were lower, 23% and 20%, respectively.
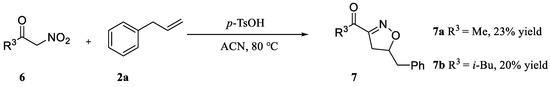
Scheme 3.
Variation of alkyl nitroketones.
Finally, the alkyen 8 was used for the reaction with α-nitroketone (1a) under the optimized reaction conditions, which obtained in excellent yields Isoxazoles. Under the same conditions, the reaction rate with alkyen was quicker than with alkenes. Nevertheless, 9a and 9b were obtained in 85% and 88% yield, respectively (Scheme 4).
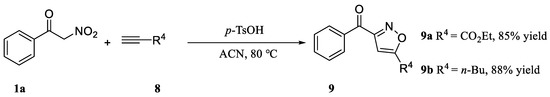
Scheme 4.
Variation of alkyne.
2.3. Mechanistic Studies
After screening the reaction conditions and studying the application of the products, the reaction mechanism was also studied. On the basis of the reaction mechanism reported by Ken-ichi Itoh et al. [46,47]. We proposed the theory of 1,3-dipolar cycloaddition of benzoylnitromethane with allylbenzene was deduced as follows (Scheme 5): In this reaction, α-nitroketones are converted to nitroso cations in the presence of non-aqueous phase protons, then nitrile oxides are formed from nitroso cations. Finally, isoxazolines and their derivatives are obtained by intermolecular the 1,3-dipolar cycloaddition cyclization of dipolarophiles (alkenes or alkynes) and nitrile oxide.
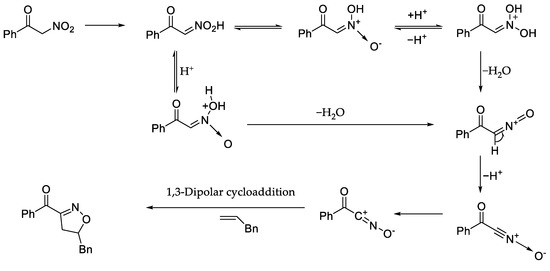
Scheme 5.
Reaction mechanism.
3. Materials and Methods
3.1. General Experimental Methods
The structures of produced compounds were firmly confirmed by 13C NMR and 1HNMR spectra, and supported by HRMS, and IR data (see the Supplementary Materials).
1H NMR (400 MHz) and 13C NMR (101 MHz) were recorded at room temperature on DRX-400 spectrometer (Bruker, Saarbrücken, Saarland, Germany) in CDCl3. The chemical shifts are given in parts per million (ppm) on the delta (δ) scale. The solvent peak was used as a reference value, for 1H NMR: CDCl3 δH 7.26; for 13C NMR: CDCl3 δC 77.16 ppm. IR spectra were recorded using an Avatar 360 FT-IR ESP spectrometer (Nicolet, Madison, Wisconsin, USA) at room temperature. HR-ESI-MS spectra were acquired using an Agilent 6210 ESI/TOF mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). Analytical TLC was conducted on silica gel plates (GF254, Yantai Institute of Chemical Technology, Yantai, China). Spots on the plates were observed under UV light. Column chromatography was performed on silica gel (200~300 mesh and 300~400 mesh; Qingdao Marine Chemical Factory, Qingdao, China). Super-dry solvent i-PrOH, ACN, DMSO and DMF were purchased from Aldrich and used as supplied. The α-nitroketones were synthesized using the same method as reported in the literature [48,49].
3.2. General Procedure for the Cycloaddition of Alkenes and α-Nitroketones
p-TsOH (0.500 mmol, 4 equiv) was added to a solution of 1 (0.125 mmol, 1 equiv) or 6 (0.125 mmol, 1 equiv) and 2 (0.625 mmol, 5 equiv) (or 4 (0.625 mmol, 5 equiv) or 8 (0.625 mmol, 5 equiv)) in ACN (0.2 mL). The mixture was then stirred at 80 °C until the starting material disappeared as monitored by TLC. Subsequently, the mixture was directly purified by flash chromatography (with ethyl acetate/petroleum ether as the eluent) to obtain the desired product (3, 5, 7 or 9).
4. Conclusions
In conclusion, we have developed an efficient cycloaddition of a variety of α-nitroketones with alkenes or alkyne using inexpensive and gentle acid. Among the synthesized compounds, the yield of cycloaddition products of substituted phenylnitroketone is high (66–90 %), while the yield of cycloaddition products of alkyl nitroketones (such as 7a–b) is low (20–23 %). This synthesis is based on cycloaddition 1,3-dipolar in presence of p-TsOH, which is attractive that the low cost, simple synthetic route, and ease of handling of the gentle acid. It is particularly noteworthy that the reaction provides an effective synthesis method for 3-carbonylisoxazolines. The development of other methods for the synthesis of 3-carbonylisoxazoline is currently under investigation and will be disclosed in due course.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28062565/s1, The Supplementary Materials contain experimental protocols, analytical data for products and NMR spectra [29,42,46,48,49,50].
Author Contributions
Conceptualization, X.P., X.Y., and J.W.; methodology, X.P. and X.X., formal analysis, C.Y., X.P.; K.Y.; S.H., K.Z., Q.L., X.X., and J.L.; original manuscript writing, C.Y. and X.P.; revision and supervision, X.P., X.Y., and J.W.; funding acquisition, X.P., X.Y., and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was by National Natural Science Foundation of China (82160651); The Open Project of Stake Key Laboratory of Natural and Biomimetic Drugs, Peking University (K202103); The Open Project of Key Laboratory of Xinjiang Phytomedicine Resource and Utilization, Ministry of Education (XPRU202004); Youth Innovative Talent Cultivation Projects of Shihezi University (CXPY202005); The National Innovation and Entrepreneurship Training Program for Undergraduate (202210759029); and The Open Sharing Fund for the Large-scale Instruments and Equipments of Shihezi University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Acknowledgments
The authors thank Liang Guo for assistance with NMR.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 3a-i, 5a-f, 7a-b and 9a-b are available from the authors.
References
- Hemmerling, F.; Hahn, F. Biosynthesis of oxygen and nitrogen-containing heterocycles in polyketides. Beilstein J. Org. Chem. 2016, 12, 1512–1550. [Google Scholar] [CrossRef] [PubMed]
- Sysak, A.; Obminska-Mrukowicz, B. Isoxazole ring as a useful scaffold in a search for new therapeutic agents. Eur. J. Med. Chem. 2017, 137, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Matus, M.F.; Poblete, T.; Amigo, J.; Vallejos, G.; Astudillo, L. Isoxazoles: Synthesis, evaluation and bioinformatic design as acetylcholinesterase inhibitors. J. Pharm. Pharmacol. 2013, 65, 1796–1804. [Google Scholar] [CrossRef]
- Koufaki, M.; Fotopoulou, T.; Kapetanou, M.; Heropoulos, G.A.; Gonos, E.S.; Chondrogianni, N. Microwave-assisted synthesis of 3,5-disubstituted isoxazoles and evaluation of their anti-ageing activity. Eur. J. Med. Chem. 2014, 83, 508–515. [Google Scholar] [CrossRef]
- Bano, S.; Alam, M.S.; Javed, K.; Dudeja, M.; Das, A.K.; Dhulap, A. Synthesis, biological evaluation and molecular docking of some substituted pyrazolines and isoxazolines as potential antimicrobial agents. Eur. J. Med. Chem. 2015, 95, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Ouyang, L.; Jin, Q.; Zhang, J.; Luo, R. Recent advances in the oxime-participating synthesis of isoxazolines. Org. Biomol. Chem. 2020, 18, 4709–4716. [Google Scholar] [CrossRef] [PubMed]
- Hyean Kim, B.; Jun Chung, Y.; Jung Ryu, E. Synthesis of α-hydroxy ketomethylene dipeptide isosteres. Tetrahedron Lett. 1993, 34, 8465–8468. [Google Scholar] [CrossRef]
- Goncalves, I.L.; Machado das Neves, G.; Porto Kagami, L.; Eifler-Lima, V.L.; Merlo, A.A. Discovery, development, chemical diversity and design of isoxazoline-based insecticides. Bioorg. Med. Chem. 2021, 30, 115934. [Google Scholar] [CrossRef]
- Pohjakallio, A.; Pihko, P.M.; Laitinen, U.M. Synthesis of 2-isoxazolines: Enantioselective and racemic methods based on conjugate additions of oximes. Chemistry 2010, 16, 11325–11339. [Google Scholar] [CrossRef]
- Liu, X.; Ma, X.; Feng, Y. Introduction of an isoxazoline unit to the β-position of porphyrin via regioselective 1,3-dipolar cycloaddition reaction. Beilstein J. Org. Chem. 2019, 15, 1434–1440. [Google Scholar] [CrossRef]
- Akagawa, K.; Kudo, K. Iterative Polyketide Synthesis via a Consecutive Carbonyl-Protecting Strategy. J. Org. Chem. 2018, 83, 4279–4285. [Google Scholar] [CrossRef]
- Lopes, E.F.; Penteado, F.; Thurow, S.; Pinz, M.; Reis, A.S.; Wilhelm, E.A.; Luchese, C.; Barcellos, T.; Dalberto, B.; Alves, D.; et al. Synthesis of Isoxazolines by the Electrophilic Chalcogenation of beta, gamma-Unsaturated Oximes: Fishing Novel Anti-Inflammatory Agents. J. Org. Chem. 2019, 84, 12452–12462. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, V.; Sharma, A.K.; Gupta, G.K. Isoxazoline containing natural products as anticancer agents: A review. Eur. J. Med. Chem. 2014, 77, 121–133. [Google Scholar] [CrossRef]
- Goyard, D.; Konya, B.; Chajistamatiou, A.S.; Chrysina, E.D.; Leroy, J.; Balzarin, S.; Tournier, M.; Tousch, D.; Petit, P.; Duret, C.; et al. Glucose-derived spiro-isoxazolines are anti-hyperglycemic agents against type 2 diabetes through glycogen phosphorylase inhibition. Eur. J. Med. Chem. 2016, 108, 444–454. [Google Scholar] [CrossRef]
- Picconi, P.; Prabaharan, P.; Auer, J.L.; Sandiford, S.; Cascio, F.; Chowdhury, M.; Hind, C.; Wand, M.E.; Sutton, J.M.; Rahman, K.M. Novel pyridyl nitrofuranyl isoxazolines show antibacterial activity against multiple drug resistant Staphylococcus species. Bioorg. Med. Chem. 2017, 25, 3971–3979. [Google Scholar] [CrossRef] [PubMed]
- Dallanoce, C.; Meroni, G.; De Amici, M.; Hoffmann, C.; Klotz, K.N.; De Micheli, C. Synthesis of enantiopure Delta2-isoxazoline derivatives and evaluation of their affinity and efficacy profiles at human beta-adrenergic receptor subtypes. Bioorg. Med. Chem. 2006, 14, 4393–4401. [Google Scholar] [CrossRef]
- Filali, I.; Bouajila, J.; Znati, M.; Bousejra-El Garah, F.; Ben Jannet, H. Synthesis of new isoxazoline derivatives from harmine and evaluation of their anti-Alzheimer, anti-cancer and anti-inflammatory activities. J. Enzyme Inhib. Med. Chem. 2015, 30, 371–376. [Google Scholar] [CrossRef]
- Basappa; Sadashiva, M.P.; Mantelingu, K.; Swamy, S.N.; Rangappa, K.S. Solution-phase synthesis of novel delta2-isoxazoline libraries via 1,3-dipolar cycloaddition and their antifungal properties. Bioorg. Med. Chem. 2003, 11, 4539–4544. [Google Scholar] [CrossRef]
- Vinay Kumar, K.S.; Lingaraju, G.S.; Bommegowda, Y.K.; Vinayaka, A.C.; Bhat, P.; Pradeepa Kumara, C.S.; Rangappa, K.S.; Gowda, D.C.; Sadashiva, M.P. Synthesis, antimalarial activity, and target binding of dibenzazepine-tethered isoxazolines. RSC Adv. 2015, 5, 90408–90421. [Google Scholar] [CrossRef]
- Alshamari, A.; Al-Qudah, M.; Hamadeh, F.; Al-Momani, L.; Abu-Orabi, S. Synthesis, Antimicrobial and Antioxidant Activities of 2-Isoxazoline Derivatives. Molecules 2020, 25, 4271. [Google Scholar] [CrossRef] [PubMed]
- Tangallapally, R.P.; Sun, D.; Rakesh; Budha, N.; Lee, R.E.; Lenaerts, A.J.; Meibohm, B.; Lee, R.E. Discovery of novel isoxazolines as anti-tuberculosis agents. Bioorg. Med. Chem. Lett. 2007, 17, 6638–6642. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Veenus Seelan, T.; Lalitha, K.G.; Perumal, P.T. Synthesis and antinociceptive activity of pyrazolyl isoxazolines and pyrazolyl isoxazoles. Bioorg. Med. Chem. Lett. 2009, 19, 3370–3373. [Google Scholar] [CrossRef]
- Yang, J.; Guan, A.; Wu, Q.; Cui, D.; Liu, C. Design, synthesis and herbicidal evaluation of novel uracil derivatives containing an isoxazoline moiety. Pest Manag. Sci. 2020, 76, 3395–3402. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Kikuchi, T.; Mizukoshi, T.; Yaosaka, M.; Komoda, M. Preparation of Isoxazoline-Substituted Benzamide Derivatives as Insecticides, Acaricides, and Parasiticides. CN1930136; Nissan Chemical Industries Co., Ltd.: Tokyo, Japan, 2007; 2007-03-14. [Google Scholar]
- Slagbrand, T.; Kervefors, G.; Tinnis, F.; Adolfsson, H. An Efficient One-pot Procedure for the Direct Preparation of 4,5-Dihydroisoxazoles from Amides. Adv. Synth. Catal. 2017, 359, 1990–1995. [Google Scholar] [CrossRef]
- Boruah, M.; Konwar, D. KF/Al2O3: Solid-Supported Reagent Used in 1,3-Dipolar Cycloaddition Reaction of Nitrile Oxide. Synth. Commun. 2012, 42, 3261–3268. [Google Scholar] [CrossRef]
- Kesornpun, C.; Aree, T.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Water-Assisted Nitrile Oxide Cycloadditions: Synthesis of Isoxazoles and Stereoselective Syntheses of Isoxazolines and 1,2,4-Oxadiazoles. Angew. Chem. Int. Ed. Engl. 2016, 55, 3997–4001. [Google Scholar] [CrossRef]
- Svejstrup, T.D.; Zawodny, W.; Douglas, J.J.; Bidgeli, D.; Sheikh, N.S.; Leonori, D. Visible-light-mediated generation of nitrile oxides for the photoredox synthesis of isoxazolines and isoxazoles. Chem. Commun. 2016, 52, 12302–12305. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Tan, X.; Luo, Q.; Yu, X.; Zhang, S.-G.; Liu, F.; Zhang, H.-W. Synthesis of 3-Acyl-isoxazoles and Δ2-Isoxazolines from Methyl Ketones, Alkynes or Alkenes, and tert-Butyl Nitrite via a Csp3-H Radical Functionalization/Cycloaddition Cascade. Org lett. 2019, 21, 5096–5100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-W.; Xiao, Z.-F.; Zhuang, Y.-J.; Wang, M.-M.; Kang, Y.-B. Metal-Free Autoxidative Nitrooxylation of Alkenyl Oximes with Molecular Oxygen. Adv. Synth. Catal. 2016, 358, 1942–1945. [Google Scholar] [CrossRef]
- Liu, R.-H.; Wei, D.; Han, B.; Yu, W. Copper-Catalyzed Oxidative Oxyamination/Diamination of Internal Alkenes of Unsaturated Oximes with Simple Amines. ACS Catal. 2016, 6, 6525–6530. [Google Scholar] [CrossRef]
- Wang, L.-J.; Chen, M.; Qi, L.; Xu, Z.; Li, W. Copper-mediated oxysulfonylation of alkenyl oximes with sodium sulfinates: A facile synthesis of isoxazolines featuring a sulfone substituent. Chem. Commun. (Camb). 2017, 53, 2056–2059. [Google Scholar] [CrossRef]
- Triandafillidi, I.; Kokotos, C.G. Green Organocatalytic Synthesis of Isoxazolines via a One-Pot Oxidation of Allyloximes. Org. Lett. 2017, 19, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Tang, Y.; Zhou, Y.; Deng, G.; Liu, Z.; Wu, J.; Li, Y.; Zhang, J.; Xu, S. Recycling Catalyst as Reactant: A Sustainable Strategy To Improve Atom Efficiency of Organocatalytic Tandem Reactions. Org. Lett. 2018, 20, 6559–6563. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.N.; Hirner, J.J.; Blum, S.A. Oxyboration with and without a Catalyst: Borylated Isoxazoles via B-O sigma-Bond Addition. Org. Lett. 2016, 18, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Chary, R.G.; Reddy, G.R.; Ganesh, Y.S.S.; Prasad, K.V.; Raghunadh, A.; Krishna, T.; Mukherjee, S.; Pal, M. Effect of Aqueous Polyethylene Glycol on 1,3-Dipolar Cycloaddition of Benzoylnitromethane/Ethyl 2-Nitroacetate with Dipolarophiles: Green Synthesis of Isoxazoles and Isoxazolines. Adv. Synth. Catal. 2014, 356, 160–164. [Google Scholar] [CrossRef]
- Zhang, X.-W.; He, X.-L.; Yan, N.; Zheng, H.X.; Hu, X.-G. Oxidize Amines to Nitrile Oxides: One Type of Amine Oxidation and Its Application to Directly Construct Isoxazoles and Isoxazolines. J. Org. Chem. 2020, 85, 15726–15735. [Google Scholar] [CrossRef]
- Li, B.-B.; Jiang, Q. Preparation of a novel isoxazoline compound, fluorelamine. Guangzhou Chem. Ind. 2020, 47, 215–216. [Google Scholar]
- Gao, M.; Gan, Y.; Xu, B. From Alkenes to Isoxazolines via Copper-Mediated Alkene Cleavage and Dipolar Cycloaddition. Org. Lett. 2019, 21, 7435–7439. [Google Scholar] [CrossRef]
- Morita, T.; Fukuhara, S.; Fuse, S.; Nakamura, H. Gold(I)-Catalyzed Intramolecular S(E)Ar Reaction: Efficient Synthesis of Isoxazole-Containing Fused Heterocycles. Org. Lett. 2018, 20, 433–436. [Google Scholar] [CrossRef]
- Ueda, M.; Ikeda, Y.; Sato, A.; Ito, Y.; Kakiuchi, M.; Shono, H.; Miyoshi, T.; Naito, T.; Miyata, O. Silver-catalyzed synthesis of disubstituted isoxazoles by cyclization of alkynyl oxime ethers. Tetrahedron 2011, 67, 4612–4615. [Google Scholar] [CrossRef]
- Pan, X.-H.; Xin, X.-B.; Mao, Y.; Li, X.; Zhao, Y.-N.; Liu, Y.-D.; Zhang, K.; Yang, X.-D.; Wang, J.-H. 3-Benzoylisoxazolines by 1,3-Dipolar Cycloaddition: Chloramine-T-Catalyzed Condensation of alpha-Nitroketones with Dipolarophiles. Molecules 2021, 26, 3491. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, K.G.; Shvidenko, K.V.; Pinchuk, A.M.; Tolmachev, A.A. Synthesis of 7-Amino-1-nitro-2-heptanone Derivatives. Synth. Commun. 2003, 33, 4241–4252. [Google Scholar] [CrossRef]
- Arumugam, N.; Raghunathan, R.; Almansour, A.I.; Karama, U. An efficient synthesis of highly functionalized novel chromeno [4,3-b] pyrroles and indolizino [6,7-b] indoles as potent antimicrobial and antioxidant agents. Bioorg. Med. Chem. Lett. 2012, 22, 1375–1379. [Google Scholar] [CrossRef]
- Quan, X.-J.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H. p-Toluenesulfonic acid mediated 1,3-dipolar cycloaddition of nitroolefins with NaN3 for synthesis of 4-aryl-NH-1,2,3-triazoles. Org. Lett. 2014, 16, 5728–5731. [Google Scholar] [CrossRef]
- Itoh, K.-i.; Aoyama, T.; Satoh, H.; Fujii, Y.; Sakamaki, H.; Takido, T.; Kodomari, M. Application of silica gel-supported polyphosphoric acid (PPA/SiO2) as a reusable solid acid catalyst to the synthesis of 3-benzoylisoxazoles and isoxazolines. Tetrahedron Lett. 2011, 52, 6892–6895. [Google Scholar] [CrossRef]
- Itoh, K.; Horiuchi, C.A. Formation of isoxazole derivatives via nitrile oxide using ammonium cerium nitrate (CAN): A novel one-pot synthesis of 3-acetyl- and 3-benzoylisoxazole derivatives. Tetrahedron 2004, 60, 1671–1681. [Google Scholar] [CrossRef]
- June, L.J.; Jihye, K.; Moo, J.Y.; Min, L.B.; Hyo, K.B. Indium-mediated one-pot synthesis of benzoxazoles or oxazoles from 2-nitrophenols or 1-aryl-2-nitroethanones. Tetrahedron 2009, 65, 8821–8831. [Google Scholar]
- Zhang, H.Q.; Pan, X.H.; Zhang, K.; Wang, H.Y.; Wang, J.H. Synthesis of α-nitroketones. J. Shihezi Univ. (Nat. Sci. Ed.) 2017, 35, 602–605. [Google Scholar]
- Lindsay, A.C.; Kilmartin, P.A.; Organic, J.S.J.; Sperry, J. Synthesis of 3-nitroindoles by sequential paired electrolysis. Org. Biomol. Chem. 2021, 19, 7903–7913. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).