Using Sandwiched Silicon/Reduced Graphene Oxide Composites with Dual Hybridization for Their Stable Lithium Storage Properties
Abstract
1. Introduction
2. Results and Discussion
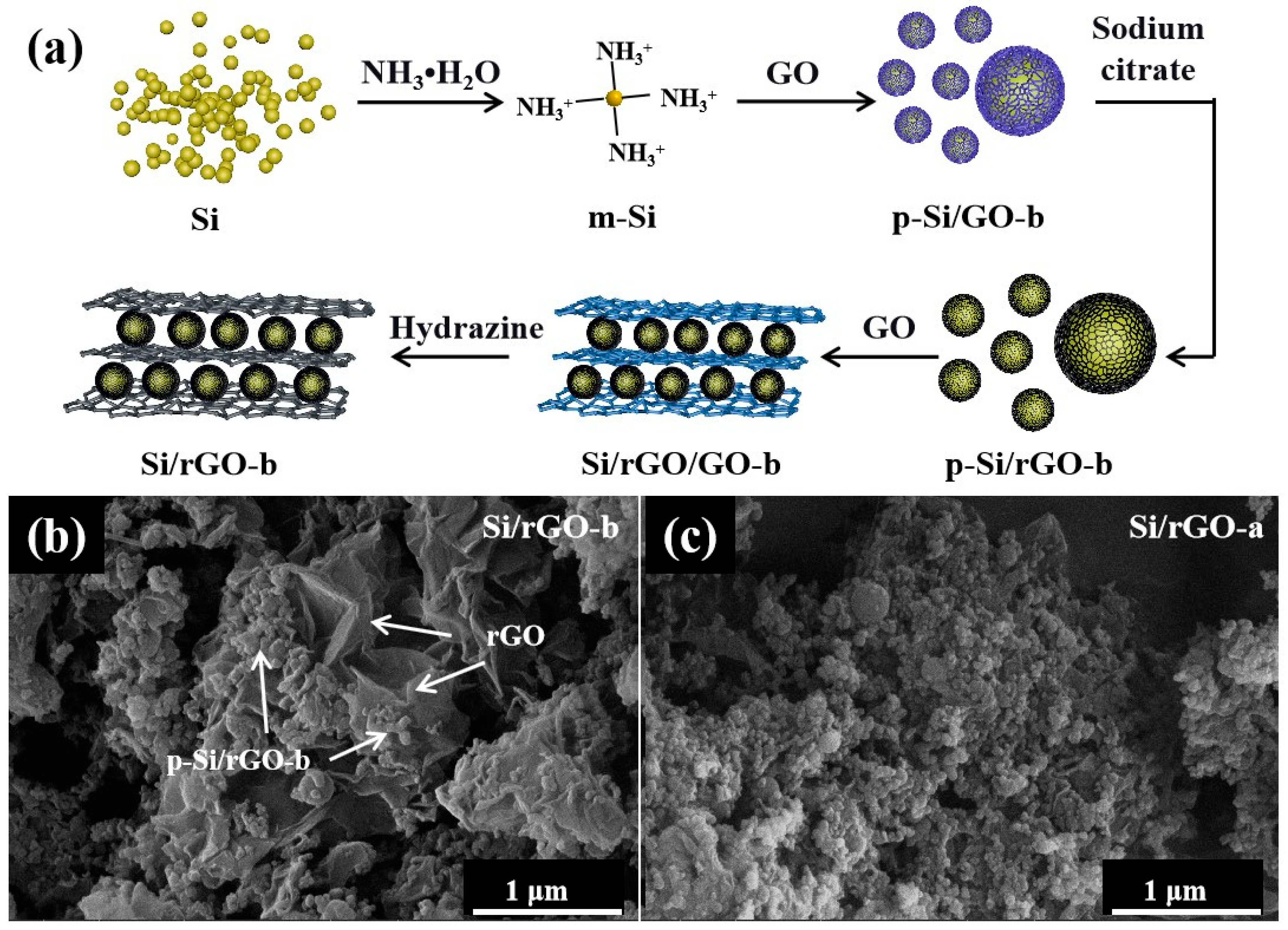
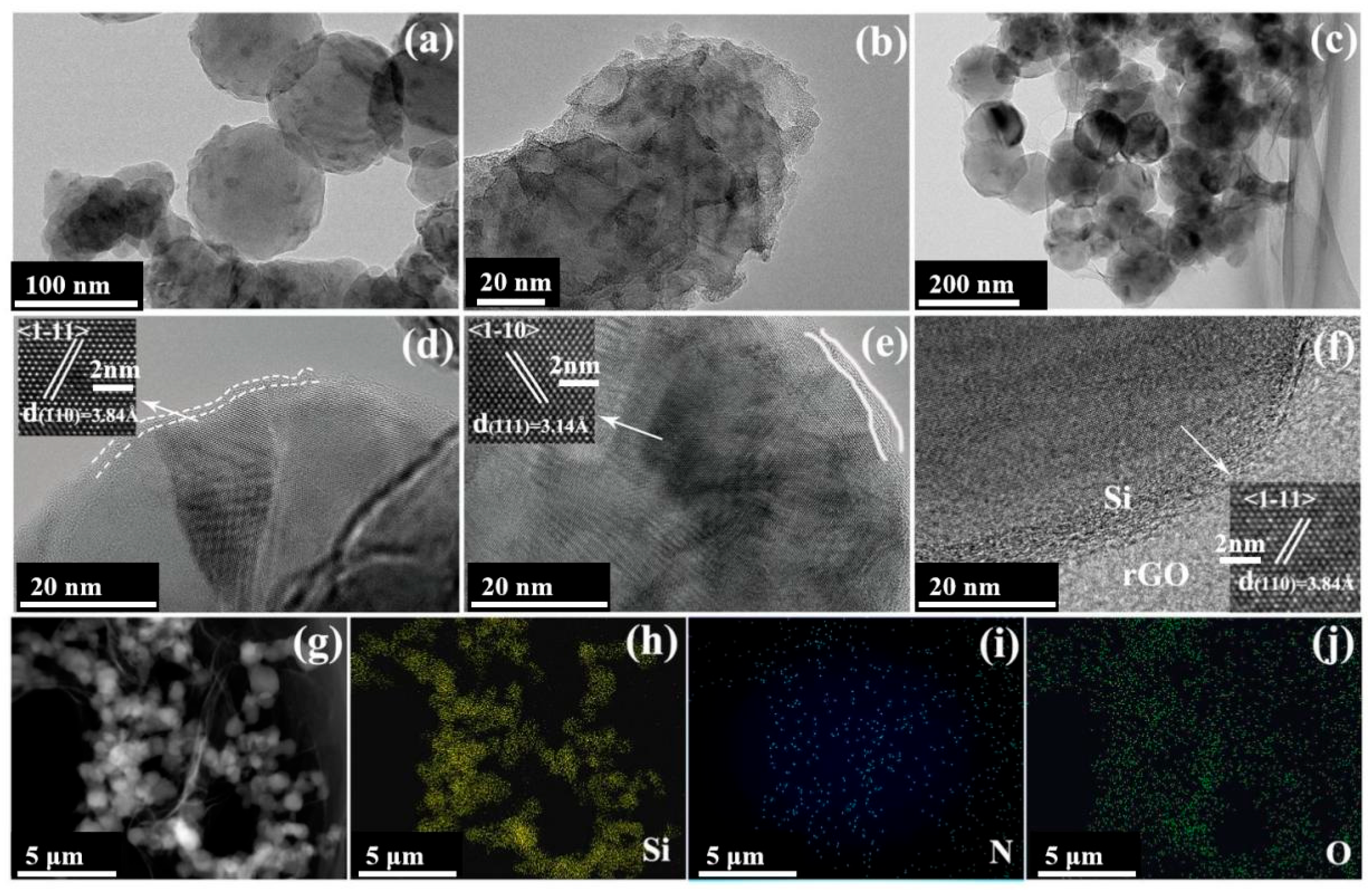
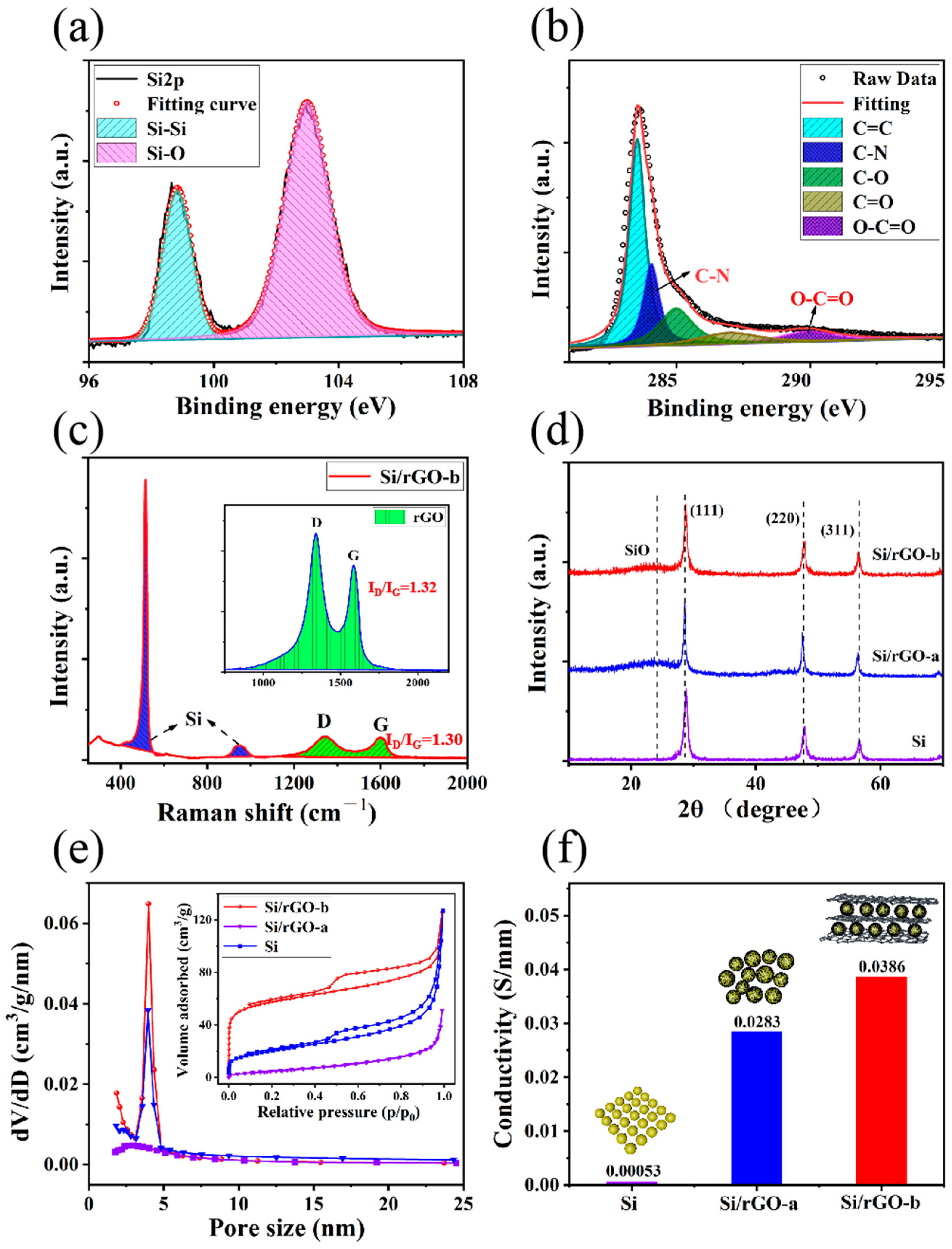
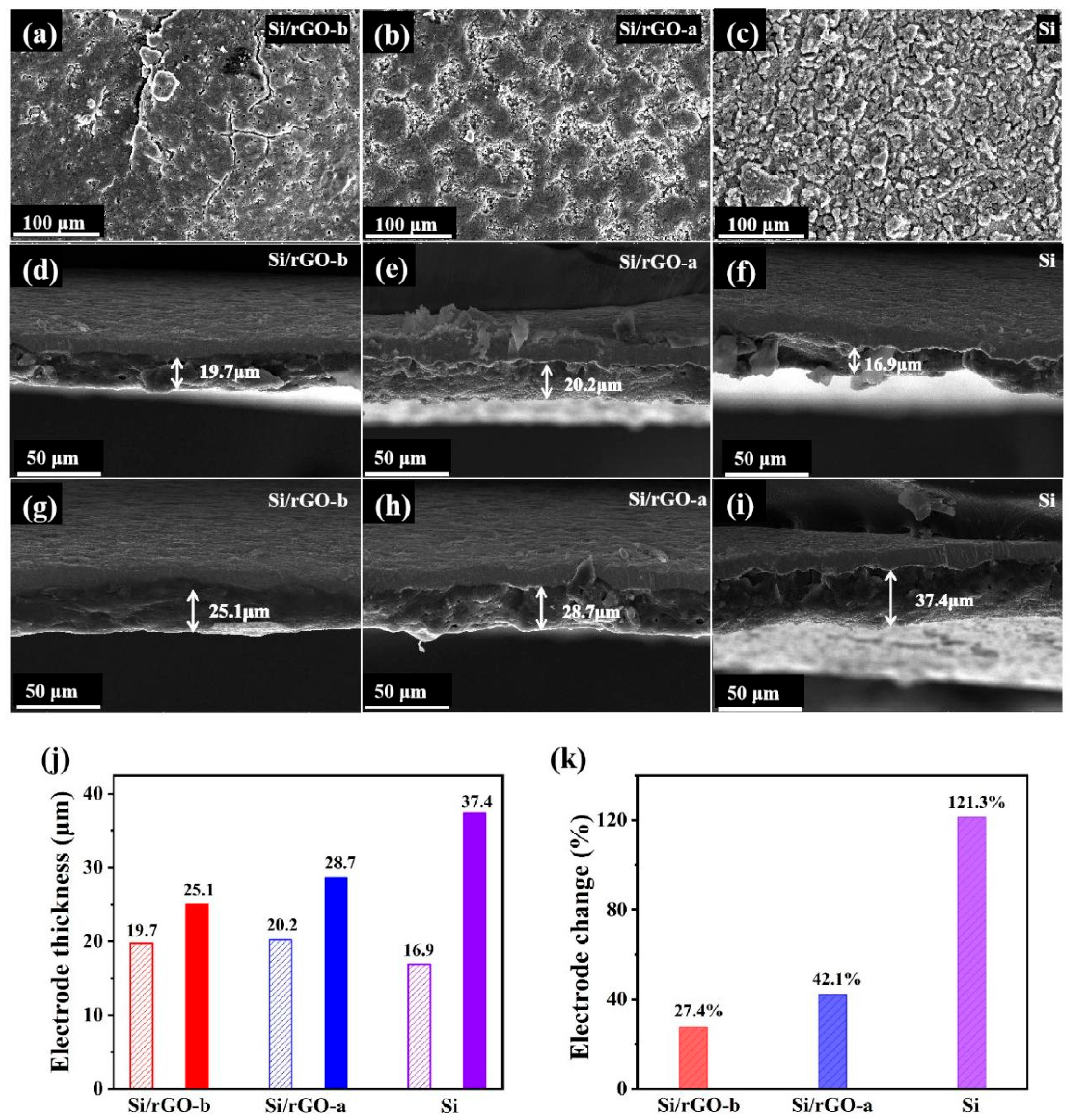
2.1. Structural Characterization
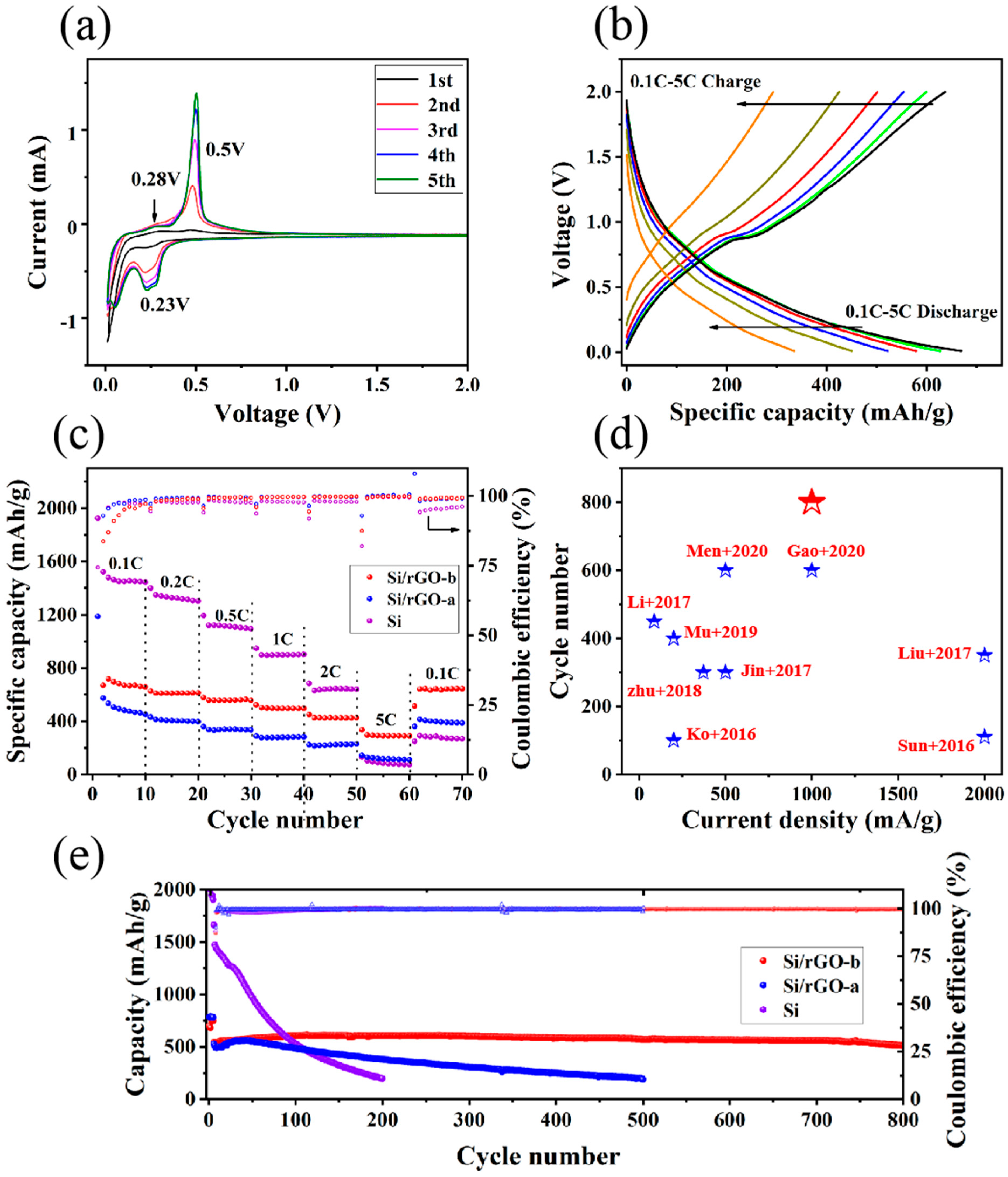
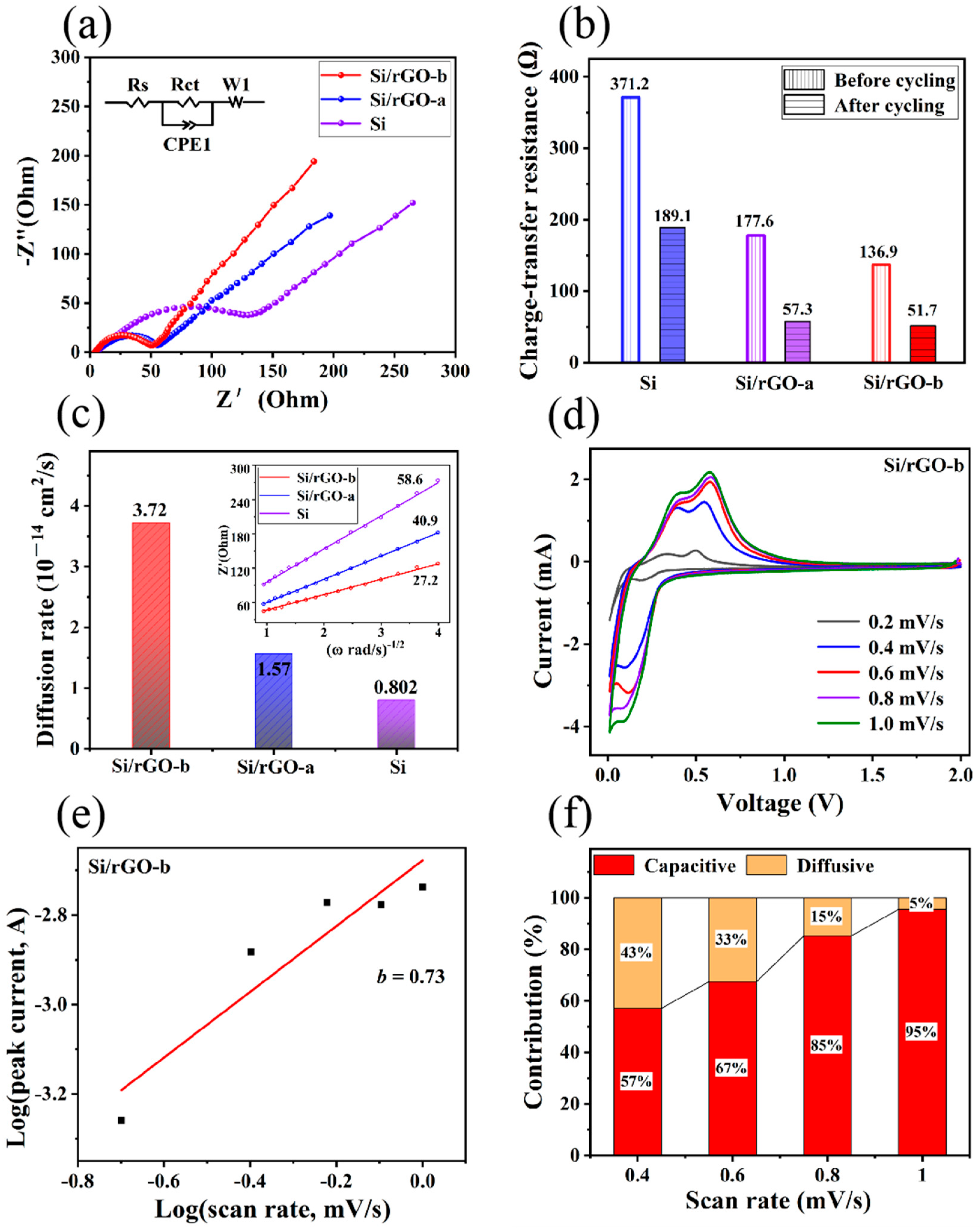
2.2. Electrochemical Characterization
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.; Ding, Z.; Lee, M.H.; Lim, K.; Yoon, G.; Kang, K. Recent progress in electrode materials for sodium-ion batteries. Adv. Energy Mater. 2016, 6, 1600943. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Wang, K.; Yu, F.; Xie, J.; Zhang, Q. Multi-thiol-supported dicarboxylate-based metal–organic framework with excellent performance for lithium-ion battery. Chem. Eng. J. 2022, 431, 133234. [Google Scholar] [CrossRef]
- Son, I.H.; Park, J.H.; Kwon, S.; Park, S.; Rümmeli, M.; Bachmatiuk, A.; Song, H.J.; Ku, J.; Choi, J.W.; Choi, J.M. Silicon carbide-free graphene growth on silicon for lithium-ion battery with high volumetric energy density. Nat. Commun. 2015, 6, 7393. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhou, J.; Guan, Y.; Cai, W.; Zhao, Y.; Zhu, Y.; Zhu, L.; Zhu, Y.; Qian, Y. Hierarchical graphene-scaffolded silicon/graphite composites as high performance anodes for lithium-Ion batteries. Small 2018, 14, 1802457. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Chen, X.; Xia, Y.; Chen, M.; Wang, L.; Wang, Q.; Li, W.; Yang, J. Surface and Interface Engineering of Silicon-Based Anode Materials for Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1701083. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon as a potential anode material for Li-ion batteries: Where size, geometry and structure matter. Nanoscale 2015, 8, 74–103. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yuan, Y.; Yue, M.; Hu, Q.; Ren, X.; Pan, B.; Zhang, C.; Wang, K.; Zhang, Q. Recent advances in pristine iron triad metal–organic framework cathodes for alkali metal-ion batteries. Small 2023, 14, 2310373. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Peng, H.; Liu, G.; Mcilwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2007, 3, 31–35. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; Mcdowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef]
- Kim, H.; Han, B.; Choo, J.; Cho, J. Three-dimensional porous silicon particles for use in high-performance lithium secondary batteries. Angew. Chem. Int. Ed. 2008, 47, 10151–10154. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lu, Z.; Zhao, J.; Mcdowell, M.T.; Lee, H.W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.S.; Liu, J.Y.; Li, H.Y.; Wu, T.S.; Li, F.; Wang, H.Y.; Niu, L. Facile synthesis of reduced graphene oxide-porous silicon composite as superior anode material for lithium-ion battery anodes. J. Power Sources 2016, 315, 9–15. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Chen, J.; Zhang, X.; Li, C.; Huang, H. Preparation of graphene supported porous Si@C ternary composites and their electrochemical performance as high capacity anode materials for Li-ion batteries. Ceram. Int. 2015, 41, 8533–8540. [Google Scholar] [CrossRef]
- Qiu, L.; Shao, Z.; Wang, D.; Wang, F.; Wang, W.; Wang, J. Novel polymer Li-ion binder carboxymethyl cellulose derivative enhanced electrochemical performance for Li-ion batteries. Carbohydr. Polym. 2014, 112, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, X.X.; Huang, Y.; Zhang, X.; Zhao, Q.; Xiang, X.; Li, G.; He, P.; Wen, Z.; Li, J. Enhanced electrochemical performance promoted by monolayer graphene and void space in silicon composite anode materials. Nano Energy 2016, 27, 647–657. [Google Scholar] [CrossRef]
- Luo, Z.; Xiao, Q.; Lei, G.; Li, Z.; Tang, C. Si nanoparticles/graphene composite membrane for high performance silicon anode in lithium ion batteries. Carbon 2016, 98, 373–380. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, H.; Wang, Y.; Xia, L.; Tan, Q.; Li, H.; Zhong, Z.; Su, F.; Zhao, X. Growth of silicon/carbon microrods on graphite microspheres as improved anodes for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 4483–4489. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A major constituent of brown algae for Use in high-capacity Li-ion batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Wu, Z.; Ma, Z.; Guo, X.; Guo, F.; Zhang, J.; Li, Y. Closely packed Si@C and Sn@C nano-particles anchored by reduced graphene oxide sheet boosting anode performance of lithium ion batteries. J. Mater. Sci. Technol. 2021, 87, 18–28. [Google Scholar] [CrossRef]
- Ruan, H.; Zhang, L.; Li, S.; Wang, K.; Huang, W.; Guo, S. Carbon polyhedra encapsulated Si derived from Co-Mo bimetal MOFs as anode materials for lithium-ion batteries. J. Mater. Sci. Technol. 2021, 159, 91–98. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; He, S.; Huang, C.; Gong, Z.; Gan, L.; Long, M. N-doped rGO/C@Sicomposites using sustainable chitosan as the carbon source for lithium-ion batteries. Appl. Surf. Sci. 2020, 501, 144136. [Google Scholar] [CrossRef]
- Wu, X.R.; Yu, C.H.; Li, C.C. Carbon-encapsulated gigaporous microsphere as potential Si anode-active material for lithium-ion batteries. Carbon 2020, 160, 255–264. [Google Scholar] [CrossRef]
- Liu, N.; Wu, H.; Mcdowell, M.T.; Yao, Y.; Wang, C.; Cui, Y. A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett. 2012, 12, 3315. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, W.; Li, X.; Zhao, X.; Yan, X. Bean pod-like Si@dopamine-derived amorphous carbon@N-doped graphene nanosheet scrolls for high performance lithium storage. J. Mater. Chem. A 2016, 4, 10948–10955. [Google Scholar] [CrossRef]
- Huang, W.; Wang, W.; Wang, Y.; Qu, Q.; Jin, C.; Zheng, H. Overcoming the fundamental challenge of PVDF binder use with silicon anodes with a super-molecular nano-layer. J. Mater. Chem. A 2021, 9, 1541–1551. [Google Scholar] [CrossRef]
- Assresahegn, B.D.; Ossonon, B.D.; Bélanger, D. Graphene nanosheets and polyacrylic acid grafted silicon composite anode for lithium ion batteries. J. Power Sources 2018, 391, 41–50. [Google Scholar] [CrossRef]
- Wang, X.; Huang, R.; Niu, S.; Xu, L.; Zhang, Q.; Abbas, A.; Cheng, C. Research progress on graphene-based materials for high-performance lithium-metal batteries. New Carbon Mater. 2021, 36, 711–728. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.; Xing, C.; Guo, M.; Xu, F.; Wang, X.; Gruber, H.J.; Zhang, B.; Tang, J. Sodium citrate: A universal reducing agent for reduction / decoration of graphene oxide with Au nanoparticles. Nano Res. 2011, 4, 599–611. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.; Fan, H.; Feng, L.; Wen, G.; Qin, L.-C. Roles of water in the formation and preparation of graphene oxide. RSC Adv. 2021, 11, 15808–15816. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Tang, J.; Zhang, K.; Ozawa, K.; Qin, L.C. A sandwich-like silicon–carbon composite prepared by surface-polymerization for rapid lithium-ion storage. Nano Energy 2020, 78, 105341. [Google Scholar] [CrossRef]
- Gao, R.; Tang, J.; Tang, S.; Zhang, K.; Ozawa, K.; Qin, L.C. Biomineralization-inspired: Rapid preparation of a silicon-based composite as a high-performance lithium-ion battery anode. J. Mater. Chem. A 2021, 9, 11614–11622. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zhao, J.; Zhang, S. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 2015, 16, 488–515. [Google Scholar] [CrossRef]
- Sehrawat, P.; Shabir, A.; Abid; Julien, C.M.; Islam, S.S. Recent trends in silicon/graphene nanocomposite anodes for lithium-ion batteries. J. Power Sources 2021, 501, 229709. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, S.; Niu, Y.; Song, R.; Song, H.; Chen, X.; Zhou, J.; Hong, S. Towards Si@SiO2 core-shell, yolk-shell, and SiO2 hollow structures from Si nanoparticles through a self-templated etching-deposition process. Procedia Eng. 2015, 102, 1903–1907. [Google Scholar] [CrossRef]
- Han, J.; Tang, X.; Ge, S.; Shi, Y.; Zhang, C.; Li, F.; Bai, S. Si/C particles on graphene sheet as stable anode for lithium-ion batteries. J. Mater. Sci. Technol. 2021, 80, 259–269. [Google Scholar] [CrossRef]
- Jian, Y.; He, X.D.; Yue, S.; Yao, L. Electron beam-physical vapor deposition of SiC/SiO2 high emissivity thin film. Appl. Surf. Sci. 2007, 253, 4361–4366. [Google Scholar]
- Zhu, J.; Wang, T.; Fan, F.; Mei, L.; Lu, B. Atomic scale control of silicon expansion space as ultra-stable battery anodes. ACS Nano 2016, 10, 8243–8251. [Google Scholar] [CrossRef]
- Venugopal, G.; Jung, M.H.; Suemitsu, M.; Kim, S.J. Fabrication of nanoscale three-dimensional graphite stacked junctions by focused-ion-beam and observation of anomalous transport characteristics. Carbon 2011, 49, 2766–2772. [Google Scholar] [CrossRef]
- Mi, H.; Li, F.; Xu, S.; Li, Z.; Chai, X.; He, C.; Li, Y.; Liu, J. A tremella-like nanostructure of silicon@void@graphene-like nanosheets composite as an anode for lithium-ion batteries. Nanoscale Res. Lett. 2016, 11, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, M.C.; Han, S.B.; Lee, G.H.; Choe, H.S.; Kwak, D.H.; Choi, S.Y.; Son, B.G.; Shin, M.S.; Park, K.W. 3D flexible Si based-composite (Si@Si3N4)/CNF electrode with enhanced cyclability and high rate capability for lithium-ion batteries. Nano Energy 2016, 27, 545–553. [Google Scholar] [CrossRef]
- Alem, N.; Erickson, K.; Zettl, A.; Erni, R.; Lee, Z. Determination of the local chemical structure of graphene oxide and reduced graphene oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar]
- Etacheri, V.; Wang, C.; O’Connell, M.J.; Chan, C.K.; Pol, V.G. Porous carbon sphere anodes for enhanced lithium-ion storage. J. Mater. Chem. A 2015, 3, 9861–9868. [Google Scholar] [CrossRef]
- Jeong, M.G.; Du, H.L.; Islam, M.; Lee, J.K.; Sun, Y.K.; Jung, H.G. Self-rearrangement of silicon nanoparticles embedded in micro-carbon sphere framework for high-energy and long-life lithium-ion batteries. Nano Lett. 2017, 17, 5600–5606. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Hou, Z.; Wei, H. Porous sandwiched graphene/silicon anodes for lithium storage. Electrochim. Acta 2017, 229, 445–451. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Ding, F.; Xu, W.; Xiao, J.; Cao, Y.; Meduri, P.; Liu, J.; Graff, G.L.; Zhang, J.-G. Conductive rigid skeleton supported silicon as high-performance Li-Ion battery anodes. Nano Lett. 2012, 12, 4124. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Hong, D.; Choi, S.; Park, S. Synthesis of ultrathin Si nanosheets from natural clays for lithium-ion battery anodes. ACS Nano 2016, 10, 2843. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qin, X.; Miao, C.; He, Y.B.; Liang, G.; Zhou, D.; Liu, M.; Han, C.; Li, B.; Kang, F. A honeycomb-cobweb inspired hierarchical core–shell structure design for electrospun silicon/carbon fibers as lithium-ion battery anodes. Carbon 2016, 98, 582–591. [Google Scholar] [CrossRef]
- Jeena, M.T.; Bok, T.; Si, H.K.; Park, S.; Kim, J.Y.; Park, S.; Ryu, J.H. A siloxane-incorporated copolymer as an in situ cross-linkable binder for high performance silicon anodes in Li-ion batteries. Nanoscale 2016, 8, 9245–9253. [Google Scholar] [CrossRef]
- Gu, Z.; Li, W.; Miao, Y.; Chen, Y.; Xia, X.; Chen, G.; Liu, H. Influencing factors and behavior mechanism of the initial coulombic efficiency of silicon/graphite composites in lithium-ion batteries. Electrochim. Acta 2021, 366, 137424. [Google Scholar] [CrossRef]
- Lim, K.W.; Lee, J.I.; Yang, J.; Kim, Y.K.; Jeong, H.Y.; Park, S.; Shin, H.S. Catalyst-free synthesis of Si-SiOx core-shell nanowire anodes for high-rate and high-capacity lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 6340–6345. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Chae, S.; Ma, J.; Kim, N.; Lee, H.W.; Cui, Y.; Cho, J. Scalable synthesis of silicon-nanolayer-embedded graphite for high-energy lithium-ion batteries. Nat. Energy 2016, 1, 16113. [Google Scholar] [CrossRef]
- Li, X.; Yan, P.; Xiao, X.; Woo, J.H.; Wang, C.; Liua, J.; Zhang, J.G. Design of porous Si/C–graphite electrodes with long cycle stability and controlled swelling. Energy Environ. Sci. 2017, 10, 1427–1434. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, P.; Liu, B.; Xie, W.; Liu, D.; He, D. Carbon-coated Si nanoparticles/reduced graphene oxide multilayer anchored to nanostructured current collector as lithium-ion battery anode. Appl. Surf. Sci. 2017, 396, 41–47. [Google Scholar] [CrossRef]
- Mu, T.; Zuo, P.; Lou, S.; Pan, Q.; Zhang, H.; Du, C.; Gao, Y.; Cheng, X.; Ma, Y.; Huo, H. A three-dimensional silicon/nitrogen-doped graphitized carbon composite as high-performance anode material for lithium ion batteries. J. Alloys Compd. 2019, 777, 190–197. [Google Scholar] [CrossRef]
- Jin, Y.; Tan, Y.; Hu, X.; Zhu, B.; Zheng, Q.; Zhang, Z.; Zhu, G.; Yu, Q.; Jin, Z.; Zhu, J. Scalable production of the silicon-tin yin-yang hybrid structure with graphene coating for high performance lithium-ion battery anodes. ACS Appl. Mater. Interfaces 2017, 9, 15388–15393. [Google Scholar] [CrossRef]
- Men, X.; Kong, X.; Yang, X.; Wang, B.; Wang, Y.; Liu, Y.; Yu, L.; Li, H.; Xu, B. Synthesis of a pomegranate shaped reduced graphene oxide stabilized secondary Si nanoparticles composite anode for lithium ion batteries. Int. J. Hydrogen Energy 2020, 45, 29492–29504. [Google Scholar] [CrossRef]
- Gao, R.; Tang, J.; Yu, X.; Zhang, K.; Ozawa, K.; Qin, L.C. A green strategy for the preparation of a honeycomb-like silicon composite with enhanced lithium storage properties. Nanoscale 2020, 12, 12849–12855. [Google Scholar] [CrossRef]
- Jiang, J.; Lin, Z.; Ju, Q.; Ma, Z.; Zheng, C.; Wang, Z. Electrochemical impedance spectra for lithium-ion battery ageing considering the rate of discharge ability. Energy Procedia 2017, 105, 844–849. [Google Scholar] [CrossRef]
- Du, X.; Wen, H.; Zhang, X.; Yue, Y.; Hong, L.; Zhang, X.; Min, D.; Ge, X.; Yi, D. Enhancing the electrochemical performance of lithium ion batteries using mesoporous Li3V2(PO4)3/C microspheres. J. Mater. Chem. 2012, 22, 5960–5969. [Google Scholar] [CrossRef]
- Gao, R.; Tang, J.; Yu, X.; Tang, S.; Ozawa, K.; Sasaki, T.; Qin, L.-C. In situ synthesis of MOF-derived carbon shells for silicon anode with improved lithium-ion storage. Nano Energy 2020, 70, 104444. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, R.; Zhang, Q.; Feng, L.; Wen, G.; Qin, L.-C.; Wang, D. Using Sandwiched Silicon/Reduced Graphene Oxide Composites with Dual Hybridization for Their Stable Lithium Storage Properties. Molecules 2024, 29, 2178. https://doi.org/10.3390/molecules29102178
Yang Y, Zhang R, Zhang Q, Feng L, Wen G, Qin L-C, Wang D. Using Sandwiched Silicon/Reduced Graphene Oxide Composites with Dual Hybridization for Their Stable Lithium Storage Properties. Molecules. 2024; 29(10):2178. https://doi.org/10.3390/molecules29102178
Chicago/Turabian StyleYang, Yuying, Rui Zhang, Qiang Zhang, Liu Feng, Guangwu Wen, Lu-Chang Qin, and Dong Wang. 2024. "Using Sandwiched Silicon/Reduced Graphene Oxide Composites with Dual Hybridization for Their Stable Lithium Storage Properties" Molecules 29, no. 10: 2178. https://doi.org/10.3390/molecules29102178
APA StyleYang, Y., Zhang, R., Zhang, Q., Feng, L., Wen, G., Qin, L.-C., & Wang, D. (2024). Using Sandwiched Silicon/Reduced Graphene Oxide Composites with Dual Hybridization for Their Stable Lithium Storage Properties. Molecules, 29(10), 2178. https://doi.org/10.3390/molecules29102178





