Nitrile Oxide, Alkenes, Dipolar Cycloaddition, Isomerization and Metathesis Involved in the Syntheses of 2-Isoxazolines
Abstract
1. Introduction
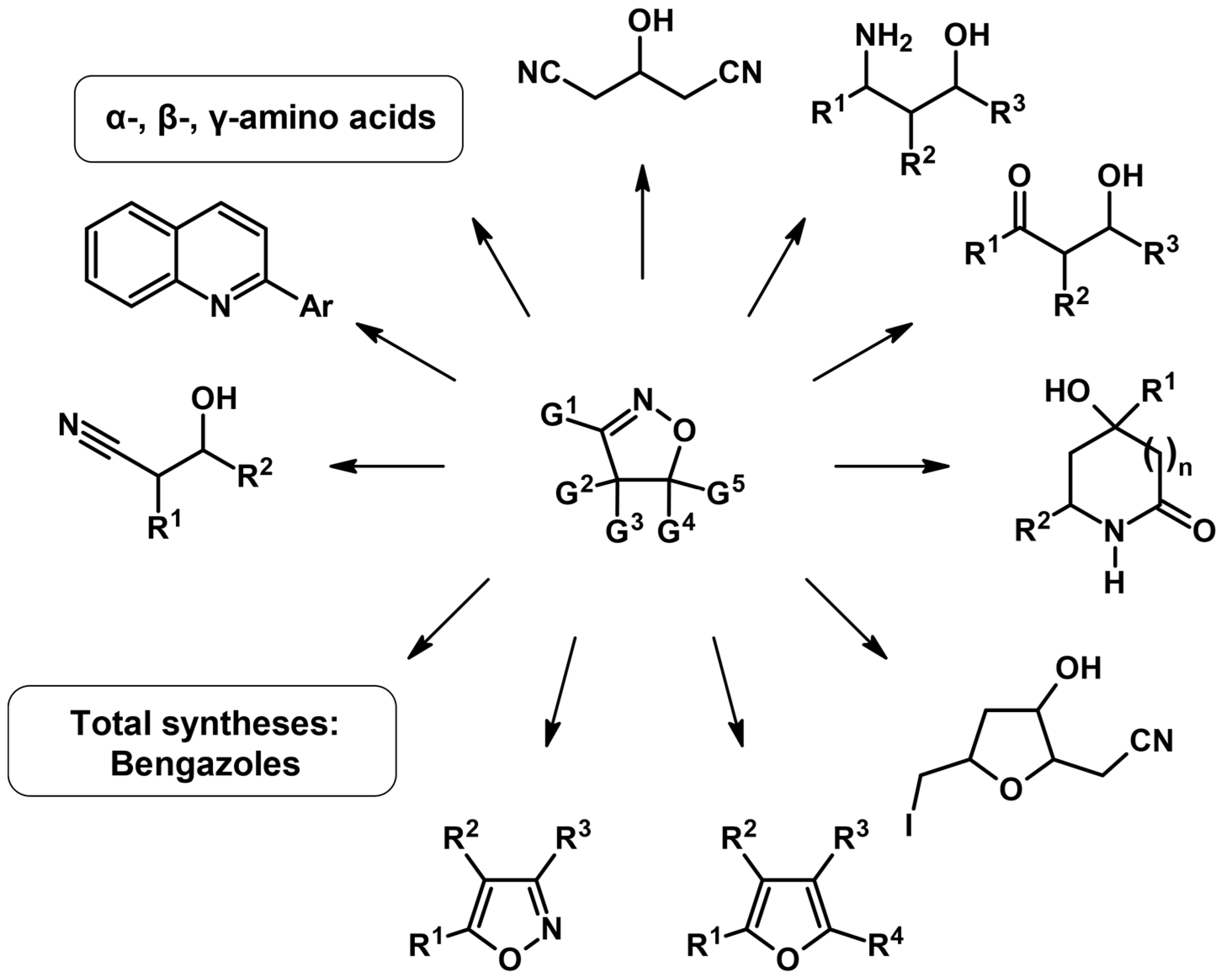
2. Syntheses of Substituted 2-Isoxazolines via 1,3-DP Cycloaddition of Nitrile Oxide to a Carbon–Carbon Double Bond
2.1. 3-Substituted 2-Isoxazolines
2.2. 3,4-Disubstituted 2-Isoxazolines
2.3. 3,5-Disubstituted Isoxazolines
2.4. 5,5-Disubstituted 2-Isoxazolines
2.5. 3,4,5-Trisubstituted 2-Isoxazolines
2.5.1. Syntheses of 3,4,5-Trisubstituted 2-Isoxazolines from Qallyl and RCNO Involving Double Bond Migration, Metathesis, and Dipolar Cycloaddition in Various Sequences
Syntheses of 3,4,5-Trisubstituted 2-Isoxazolines from Qallyl via Qallyl Izomerization to Q(1-Propenyl) Derivatives followed by 1,3-DP Cycloaddition of RCNO into Q(1-PROPENYL) Compounds
Synthesis of 3,4,5-Trisubstituted 2-Isoxazolines from Qallyl by the following Reaction Sequences: Double Bond Migration–Self-Metathesis-1,3-DP Cycloaddition
Syntheses of 3,4,5-Trisubstituted 2-Isoxazolines via Qallyl Self-Metathesis to QCH2CH=CHCH2Q Followed by RCNO Cycloaddition
3,4,5-Trisubstituted 2-Isoxazolines from Qallyl and the following Reaction Sequences: Qallyl Self-Metathesis, Double Bond in Self-Metathesis Products, Dipolar Cycloaddition
Syntheses of 3,4,5-Trisubstituted 2-Isoxazolines Starting from Allyl Substrate of the QCH2CH=CHCH2Q Type Obtained from XCH2CH=CHCH2X
Syntheses of 3,4,5-Trisubstituted 2-Isoxazolines Using High-Pressure Conditions
2.6. 3,5,5-Trisubstituted 2-Isoxazolines
2.7. 3,4,4,5-Tetrasubstituted 2-Isoxazolines
2.8. 3,4,5,5-Tetrasubstituted 2-Isoxazolines
2.9. Multisubstituted Isoxazolines
3. Mechanistic Aspect
4. Side Reaction Accompanying 1,3-Dipolar Cycloaddition
5. Methods of 2-Isoxazolines Synthesis Other Than Dipolar Cycloaddition
5.1. From Nitrocompounds
5.2. From Nitronates
5.3. From β-Carboxy-Substituted α,β-Unsaturated Ketones via Cascade Oxa-Michael-Cyclization
5.4. From Oximes (Simple Oximes, Unsaturated and Oxo-Oximes)
5.5. From Organometallics (Allyl–Metal)
5.6. From Alkenes, In Situ-Generated Carbenes, and Nitroso Radical
5.7. From Alkenes, Diazocompounds and t–BuONO
5.8. From R2–C≡C–CH(R1)O–NH2
5.9. From Isoxazoles
6. 2-Isoxazolines in Organic Synthesis
7. 2-Isoxazolines—Biological Activity
8. Syntheses of Isoxazolines via 1,3-DP Cycloaddition of Nitrile Oxide—Remaining Challenges
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1,3-DP | 1,3-dipolar |
| AcOEt | ethyl acetate |
| ADMET | absorption, distribution, metabolism, excretion, and toxicity |
| AM | alkene-metathesis |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AMPAR | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors |
| ANOC | acyclic nitronate olefin cycloaddition |
| BACE1 | β-site APP cleaving enzyme-1 |
| BARAC | biarylazacyclooctynone |
| BINIM | binaphthyldiimine ligand |
| BINOL | 1,1′-bi-2-naphthol |
| BMIM | 1-butyl-3-meth-ylimidazolium cation |
| BNO | benzoyl hydroxamic acid |
| Boc | tert-butyloxycarbonyl group |
| BODIPY | 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene |
| BTMA | benzyltrimethylammonium group |
| CAN | cerium(IV) ammonium nitrate |
| CD-Dip | dipolarophile tethered to cyclodextrin |
| cod | cyclooctadiene |
| COX | cyclooxygenase |
| CPBA | chloroperoxybenzoic acid |
| CPVBIm | cross-linked poly-1-(4-vinylbenzyl)imidazole |
| CPVP | cross-linked poly-4-vinylpyridine |
| DABCO | 1,4-diazabicyclo[2.2.2]octane |
| DAP | diaminopimelic acid analogous |
| dba | dibenzylideneacetone |
| DBM | double bond migration |
| DBU | 1,8-diazabicyclo(5.4.0)undec-7-ene |
| DCE | dichloroethane |
| DCM | dichloromethane |
| de | diastereomeric excess |
| DFT | density functional theory |
| DIB | (diacetoxyiodo)benzene |
| DIDMH | 1,3-diiodo-5,5-dimethylhydantoin |
| DIPEA | N,N-diisopropylethylamine |
| DIPT | diisopropyl tartrate |
| DMAP | 4-dimethylaminopyridine |
| DME | dimethoxyethane |
| DMF | dimethylformamide |
| DMSO | dimethyl sulfoxide |
| DNA | deoxyribonucleic acid |
| DNMT1 | DNA methyltransferase 1 |
| DPC | dipolar cycloaddition |
| DPPH | 1,1-diphenyl-2-picryl-hydrazyl |
| dr | diastereomeric ratio |
| DVB | divinylbenzene |
| ee | enantiomeric excess |
| EP | european patent |
| er | enantiomeric ratio |
| EWG | electron withdrawing group |
| FMO | frontier molecular orbital |
| Free-Dip | free dipolarophfile |
| FtsZ | filamentous temperature-sensitive mutant Z |
| g-C3N4 | graphitic carbon nitride |
| GABACls | γ-aminobutyric acid-gated chloride channels |
| GP | glycoprotein |
| GPb | glycogen phosphorylase |
| HAP | hydroxyapatite |
| hCA | human carbonic anhydrase |
| HDAC | histone deacetylase |
| HFIP | hexafluoroisopropanol |
| HOMO | highest occupied molecular orbital |
| HPLC | high-performance liquid chromatography |
| HTIB | [hydroxy(tosyloxy)iodo]benzene |
| HTPI | hydroxytelechelic cis-1,4-polyisoprene |
| huMIF | human migration inhibitory factor |
| IC 50 | half-maximal inhibitory concentration |
| ICl | iodine monochloride |
| INOC | intramolecular nitrile oxide 1,3-dipolar cycloaddition |
| iNOS | inducible nitric oxide synthase |
| LA | Lewis acid |
| LDA | lithium diisopropylamide |
| LED | light-emitting diode |
| LO | lipoxygenas |
| LOL | localized orbital localizer |
| LUMO | lowest unoccupied molecular orbital |
| m-Ddh | meso-diaminopimelate dehydrogenase |
| MCD | malonyl-coenzyme A decarboxylase |
| MDR | multiple drug-resistant |
| MEDT | molecular electron density theory |
| MEM | 2-methoxyethoxymethyl group |
| MEP | electrostatic potential map |
| Mes | mesityl |
| MesCNO | 2,4,6-trimethylbenzonitrile |
| MIC | minimal inhibitory concentration |
| MIF | migration inhibitory factor |
| MOM | methoxymethyl group |
| MPM | methoxybenzyl group |
| MS | molecular sieves |
| Mtb | mycobacterium tuberculosis |
| MTBE | methyl tert-butyl ether |
| MW | microwaves |
| MXT | mitoxantrone |
| nAChRs | neuronal nicotinic acetylcholine receptors |
| NBAc | N-bromoacetamide |
| NBS | N-bromosuccinimide |
| NCS | N-chlorosuccinimide |
| NIS | N-iodosuccinimid |
| NMDA | N-methyl-D-aspartic acid |
| NMO | N-methylmorpholine N-oxide |
| NMR | nuclear magnetic resonance |
| NRK | normal rat kidney |
| OTf | triflate group |
| OXONE | potassium peroxymonosulfate |
| PEG | poly(ethylene glycol) |
| PIDA | phenyliodine(III) diacetate |
| Piv | pivaloyl group |
| PMB | p-methoxybenzyl group |
| PMDETA | N,N,N’,N″,N″-pentamethyldiethylenetriamine |
| PMMA | poly(methyl methacrylate) |
| POSS | polyhedral oligomeric silsesquioxane |
| PS | polystyrene |
| PSA | polar surface area |
| PTC | phase-transfer catalysis |
| PTSA | p-toluenesulfonic acid |
| Py | pyridine |
| RCC | radical-carbene coupling |
| SAHA | suberoylanilide hydroxamic acid |
| SAR | structure-activity relationship |
| scCO2 | supercritical carbon dioxide |
| SDS | sodium dodecylsulfate |
| SmA | smectic A mesophase |
| SS | stainless steel electrode |
| STREM | metals scavenging agent |
| t-Am | tert-amyl group |
| TBAF | tetra-n-butylammonium fluoride |
| TBDMS | tert-butyldimethylsilyl group |
| TBDPS | tert-butyldiphenylsilyl group |
| TBN | tert-butyl nitrite |
| TBS | tert-butyldimethylsilyl group |
| TBTO | tributyltin oxide |
| TEMPO | 2,2,6,6-tetramethylpiperidin-1-oxyl |
| TFE | 2,2,2-trifluoroethanol |
| THF | tetrahydrofuran |
| THP | tetrahydropyranyl group |
| TIPS | triisopropylsilyl group |
| TMDS | 1,3,3-tetramethyldisiloxane |
| TMEDA | tetramethylethylenediamine |
| TMS | trimethylsilyl group |
| Tos | tosyl group |
| TS | transition state |
| XP | extra-precision |
References
- Isoxazolines Are the Animal Health Industry’s Most Successful Molecules to Date. Available online: https://www.linkedin.com/pulse/usd-25-billion-2021-isoxazolines-animal-health-industrys-juneja?trk=public (accessed on 25 September 2022).
- Agihara, T.; Yamanaka, H. Novel Bicycloheptene Derivatives and Process for the Preparation Thereof. E.P.O. Patent EP1174409A1, 23 January 2002. [Google Scholar]
- Yagihara, T.; Takebayashi, M.; Yamanaka, H. Process for the Preparation of (e)-3-(1-Propenyl)Isoxazoline. E.P.O. Patent EP1174429A1, 23 January 2002. [Google Scholar]
- Goncharov, T.K.; Dubikhin, V.V.; Ignat’eva, E.L.; Nazin, G.M.; Aliev, Z.G.; Aldoshin, S.M. Structure and Stability of Isoxazoline Compounds. Russ. J. Gen. Chem. 2013, 83, 717–721. [Google Scholar] [CrossRef]
- Shing, T.K.M.; Wong, W.F.; Cheng, H.M.; Kwok, W.S.; So, K.H. Intramolecular Nitrile Oxide−Alkene Cycloaddition of Sugar Derivatives with Unmasked Hydroxyl Group(s). Org. Lett. 2007, 9, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Raihan, M.J.; Kavala, V.; Kuo, C.-W.; Rama Raju, B.; Yao, C.-F. ‘On-water’ synthesis of chromeno-isoxazoles mediated by [hydroxy(tosyloxy)iodo] benzene (HTIB). Green Chem. 2010, 12, 1090–1096. [Google Scholar] [CrossRef]
- Plumet, J. The 1,3-Dipolar Cycloaddition Reactions of Nitrile Oxides in Water Media. Curr. Org. Chem. 2021, 25, 2683–2707. [Google Scholar] [CrossRef]
- Sun, R.; Li, Y.; Xiong, L.; Liu, Y.; Wang, Q. Design, Synthesis, and Insecticidal Evaluation of New Benzoylureas Containing Isoxazoline and Isoxazole Group. J. Agric. Food Chem. 2011, 59, 4851–4859. [Google Scholar] [CrossRef] [PubMed]
- Toker, J.D.; Wentworth, P.; Hu, Y.; Houk, K.N.; Janda, K.D. Antibody-Catalysis of a Bimolecular Asymmetric 1,3-Dipolar Cycloaddition Reaction. J. Am. Chem. Soc. 2000, 122, 3244–3245. [Google Scholar] [CrossRef]
- Kanemasa, S.; Matsuda, H.; Kamimura, A.; Kakinami, T. Synthesis of Hydroximoyl Chlorides from Aldoximes and Benzyltrimethylammonium Tetrachloroiodate (BTMA ICl4). Tetrahedron 2000, 56, 1057–1064. [Google Scholar] [CrossRef]
- Yang, K.-S.; Lain, J.-C.; Lin, C.-H.; Chen, K. Diastereoselective [3+2] cycloadditions of a camphor-derived chiral N-acryloylhydrazide with nitrile oxides: The preparation of optically pure Δ2-isoxazolines. Tetrahedron Lett. 2000, 41, 1453–1456. [Google Scholar] [CrossRef]
- Yamamoto, H.; Sadaka, W.; Kadotani, K.; Hasegawa, M.; Noguchi, M.; Kanemasa, S. Metal ion-mediated diastereoface-selective 1,3-dipolar cycloaddition of nitrile oxides with dipolarophiles bearing an oxazolidinone chiral auxiliary. Tetrahedron Lett. 2000, 41, 3131–3136. [Google Scholar] [CrossRef]
- Pitts, W.J.; Wityak, J.; Smallheer, J.M.; Tobin, E.A.; Jetter, J.W.; Buynitsky, J.S.; Harlow, P.P.; Solomon, K.A.; Corjay, M.H.; Mousa, S.A.; et al. Isoxazolines as Potent Antagonists of the Integrin αvβ3. J. Med. Chem. 2000, 43, 27–40. [Google Scholar] [CrossRef]
- Davies, C.D.; Marsden, S.P.; Stokes, E.S.E. Enhanced asymmetric induction in cycloadditions to bridgehead-chiral vinyl dioxazaborocines. Tetrahedron Lett. 2000, 41, 4229–4233. [Google Scholar] [CrossRef]
- Sandanayaka, V.P.; Yang, Y. Dipolar Cycloaddition of Novel 6-(Nitrileoxidomethyl) Penam Sulfone: An Efficient Route to a New Class of β-Lactamase Inhibitors. Org. Lett. 2000, 2, 3087–3090. [Google Scholar] [CrossRef] [PubMed]
- Bosanac, T.; Yang, J.; Wilcox, C.S. Precipitons-Functional Protecting Groups to Facilitate Product Separation: Applications in Isoxazoline Synthesis. Angew. Chem. Int. Ed. 2001, 40, 1875–1879. [Google Scholar] [CrossRef]
- Simoni, D.; Roberti, M.; Invidiata, F.P.; Rondanin, R.; Baruchello, R.; Malagutti, C.; Mazzali, A.; Rossi, M.; Grimaudo, S.; Capone, F.; et al. Heterocycle-Containing Retinoids. Discovery of a Novel Isoxazole Arotinoid Possessing Potent Apoptotic Activity in Multidrug and Drug-Induced Apoptosis-Resistant Cells. J. Med. Chem. 2001, 44, 2308–2318. [Google Scholar] [CrossRef] [PubMed]
- Dirnens, V.; Slyadevskaya, O.; Lukevics, E. Addition of Nitrile Oxides to Allyl Esters of Aryl(hetaryl)carboxylic Acids. Chem. Heterocycl. Compd. 2002, 38, 434–437. [Google Scholar] [CrossRef]
- Shang, Y.J.; Wang, Y.G. Synthesis of Isoxazolines and Isoxazoles Using Poly(ethylene glycol) as Support. Synthesis 2002, 12, 1663–1668. [Google Scholar]
- Pirrung, M.C.; Tumey, L.N.; Raetz, C.R.H.; Jackman, J.E.; Snehalatha, K.; McClerren, A.L.; Fierke, C.A.; Gantt, S.L.; Rusche, K.M. Inhibition of the Antibacterial Target UDP-(3-O-acyl)-N-acetylglucosamine Deacetylase (LpxC): Isoxazoline Zinc Amidase Inhibitors Bearing Diverse Metal Binding Groups. J. Med. Chem. 2002, 45, 4359–4370. [Google Scholar] [CrossRef] [PubMed]
- Desroses, M.; Chéry, F.; Tatibouët, A.; De Lucchi, O.; Rollin, P. Sugar-based ethenyl ethers: Stereoselective dipolar cycloadditions of nitrile oxides. Tetrahedron Asymmetry 2002, 13, 2535–2539. [Google Scholar] [CrossRef]
- Kim, H.C.; Woo, S.W.; Seo, M.J.; Jeon, D.J.; No, Z.; Kim, H.R. Diastereoselective Synthesis of 2-Cyanomethyl-3-hydroxy-5-iodomethyltetrahydrofuran from Isoxazolines by Iodoetheration. Synlett 2002, 10, 1691–1693. [Google Scholar] [CrossRef]
- Barbachyn, M.R.; Cleek, G.J.; Dolak, L.A.; Garmon, S.A.; Morris, J.; Seest, E.P.; Thomas, R.C.; Toops, D.S.; Watt, W.; Wishka, D.G.; et al. Identification of Phenylisoxazolines as Novel and Viable Antibacterial Agents Active against Gram-Positive Pathogens. J. Med. Chem. 2003, 46, 284–302. [Google Scholar] [CrossRef]
- Sieburth, S.M.; O’Hare, H.K.; Xu, J.; Chen, Y.; Liu, G. Asymmetric Synthesis of α-Amino Allyl, Benzyl, and Propargyl Silanes by Metalation and Rearrangement. Org. Lett. 2003, 5, 1859–1861. [Google Scholar] [CrossRef] [PubMed]
- Conti, D.; Rodriquez, M.; Sega, A.; Taddei, M. 1,3-Cycloaddition of nitrile oxides in ionic liquids. An easier route to 3-carboxy isoxazolines, potential constrained glutamic acid analogues. Tetrahedron Lett. 2003, 44, 5327–5330. [Google Scholar] [CrossRef]
- Minter, A.R.; Fuller, A.A.; Mapp, A.K. A Concise Approach to Structurally Diverse β-Amino Acids. J. Am. Chem. Soc. 2003, 125, 6846–6847. [Google Scholar] [CrossRef] [PubMed]
- Kociolek, M.G.; Hongfa, C. Diastereoselective cycloadditions of chiral homoallylic alcohols with benzonitrile oxide. Tetrahedron Lett. 2003, 44, 1811–1813. [Google Scholar] [CrossRef]
- Tamai, T.; Asano, S.; Totani, K.; Takao, K.; Tadano, K.I. Highly Stereoselective [3 + 2] Cycloadditions of Nitrile Oxides to Methyl 4-O-Acryloyl-6-deoxy-2,3-O-(t-butyldimethylsilyl)-α-D-glucopyranoside. Synlett 2003, 12, 1865–1867. [Google Scholar]
- Rodriquez, M.; Sega, A.; Taddei, M. Ionic Liquid as a Suitable Phase for Multistep Parallel Synthesis of an Array of Isoxazolines. Org. Lett. 2003, 5, 4029–4031. [Google Scholar] [CrossRef]
- Lam, P.Y.S.; Adams, J.J.; Clark, C.G.; Calhoun, W.J.; Luettgen, J.M.; Knabb, R.M.; Wexler, R.R. Discovery of 3-Amino-4-Chlorophenyl P1 as a Novel and Potent Benzamidine Mimic Via Solid-Phase Synthesis of an Isoxazoline Library. Bioorg. Med. Chem. Lett. 2003, 13, 1795–1799. [Google Scholar] [CrossRef]
- Keyes, R.F.; Carter, J.J.; Englund, E.E.; Daly, M.M.; Stone, G.G.; Nilius, A.M.; Ma, Z. Synthesis and Antibacterial Activity of 6-O-Arylbutynyl Ketolides with Improved Activity against Some Key Erythromycin-Resistant Pathogens. J. Med. Chem. 2003, 46, 1795–1798. [Google Scholar] [CrossRef]
- Sheng, S.-R.; Liu, X.-L.; Xu, Q.; Song, C.-S. One-Pot Synthesis of 3-Substituted Isoxazoles from Phenyl Vinylic Selenide. Synthesis 2003, 18, 2763–2764. [Google Scholar] [CrossRef]
- Krompiec, S.; Malarz, J.; Pietraszuk, C.; Powała, B.; Rogalski, S.; Małecki, J.G.; Penkala, M.; Filapek, M.; Musioł, R.; Jampílek, J.; et al. New strategy for the synthesis of 3,4,5-trisubstituted isoxazolines from allyl compounds. Curr. Org. Chem. 2014, 18, 2280–2296. [Google Scholar] [CrossRef]
- Pesti, J.A.; Yin, J.; Zhang, L.-H.; Anzalone, L.; Waltermire, R.E.; Ma, P.; Gorko, E.; Confalone, P.N.; Fortunak, J.; Silverman, C.; et al. Efficient Preparation of a Key Intermediate in the Synthesis of Roxifiban by Enzymatic Dynamic Kinetic Resolution on Large Scale. Org. Process Res. Dev. 2004, 8, 22–27. [Google Scholar] [CrossRef]
- Alksnis, E.; Muravenko, V.; Dirnens, V.; Lukevics, E. Addition of Nitrile Oxides to Aryl Allyl Ethers. Chem. Heterocycl. Compd. 2004, 40, 797–800. [Google Scholar] [CrossRef]
- Bouissane, L.; Kazzouli, S.E.; Rakib, E.M.; Khouili, M.; Hannioui, A.; Benchidmi, M.; Essassi, E.M.; Guillaumet, G. Synthesis and 1,3-Dipolar Cycloaddition Reactions of New Pyrazolo[1,5,4-ef][1,5]bezodiazepines. Heterocycles 2004, 63, 1651–1658. [Google Scholar]
- Weidner-Wells, M.A.; Werblood, H.M.; Goldschmidt, R.; Bush, K.; Foleno, B.D.; Hilliard, J.J.; Melton, J.; Wira, E.; Macielag, M.J. The synthesis and antimicrobial evaluation of a new series of isoxazolinyl oxazolidinones. Bioorg. Med. Chem. Lett. 2004, 14, 3069–3072. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-M.; Wang, Y.-G.; Miao, M.-Z.; Huang, X. A Novel Cleavage for Polystyrene-Supported Selenium Resins: An Efficient Route to 3,5-Disubstituted Isoxazolines and Their Derivatives. Synthesis 2005, 13, 2143–2146. [Google Scholar]
- Xu, W.M.; Tang, E.; Huang, X. Preparation of isoxazol(in)yl substituted selenides and their further deselenenylation reaction to synthesize 3,5-disubstituted isoxazoles. Tetrahedron 2005, 61, 501–506. [Google Scholar] [CrossRef]
- Chiacchio, M.A.; Borello, L.; Di Pasquale, G.; Pollicino, A.; Bottino, F.A.; Rescifina, A. Synthesis of functionalized polyhedral oligomeric silsesquioxane (POSS) macromers by microwave assisted 1,3-dipolar cycloaddition. Tetrahedron 2005, 61, 7986–7993. [Google Scholar] [CrossRef]
- Conti, P.; De Amici, M.; Grazioso, G.; Roda, G.; Pinto, A.; Hansen, K.B.; Nielsen, B.; Madsen, U.; Bräuner-Osborne, H.; Egebjerg, J.; et al. Synthesis, Binding Affinity at Glutamic Acid Receptors, Neuroprotective Effects, and Molecular Modeling Investigation of Novel Dihydroisoxazole Amino Acids. J. Med. Chem. 2005, 48, 6315–6325. [Google Scholar] [CrossRef]
- Trost, B.M.; Shin, S.; Sclafani, J.A. Direct Asymmetric Zn−Aldol Reaction of Methyl Vinyl Ketone and Its Synthetic Applications. J. Am. Chem. Soc. 2005, 127, 8602–8603. [Google Scholar] [CrossRef]
- Dirnens, V.; Belyakov, S.; Lukevics, E. Addition of Nitrile Oxides To N-Allylsaccharin. Chem. Heterocycl. Compd. 2005, 41, 393–399. [Google Scholar] [CrossRef]
- Ros, A.; Alvarez, E.; Dietrich, H.; Fernández, R.; Lassaletta, J.M. A Practical Synthesis of Enantiopure 4,5-Dihydroisoxazole-5-carboxylic Acids. Synlett 2005, 19, 2899–2904. [Google Scholar] [CrossRef]
- Clayton, R.; Ramsden, C.A. A Novel N-Vinyl-Nitroimidazole Cycloadditions: Potential Routes to Nucleoside Analogues N-Vinyl-Nitroimidazole Cycloadditions. Synthesis 2005, 16, 2695–2700. [Google Scholar]
- Toker, J.D.; Tremblay, M.R.; Yli-Kauhaluoma, J.; Wentworth, A.D.; Zhou, B.; Wentworth, P.; Janda, K.D. Exploring the Scope of the 29G12 Antibody Catalyzed 1,3-Dipolar Cycloaddition Reaction. J. Org. Chem. 2005, 70, 7810–7815. [Google Scholar] [CrossRef]
- Son, B.S.; Bo Gan Song, B.G.; Kim, J.K.; Seo, M.J.; No, Z.; Kim, H.R. Double Diastereoselective Synthesis of syn,syn-Bis(1,2-isoxazolin-5-yl)methanol and syn,syn,syn-1,2-Bis(1,2-isoxazol-5-yl)ethane-1,2-diols: Facile Route for the Synthesis of Polyols. Heterocycles 2005, 65, 1289–1294. [Google Scholar]
- Cheng, K.F.; Al-Abed, Y. Critical modifications of the ISO-1 scaffold improve its potent inhibition of macrophage migration inhibitory factor (MIF) tautomerase activity. Bioorg. Med. Chem. Lett. 2006, 16, 3376–3379. [Google Scholar] [CrossRef] [PubMed]
- Dallanoce, C.; Meroni, G.; De Amici, M.; Hoffmann, C.; Klotz, K.-N.; De Micheli, C. Synthesis of enantiopure Δ2-isoxazoline derivatives and evaluation of their affinity and efficacy profiles at human β-adrenergic receptor subtypes. Bioorg. Med. Chem. 2006, 14, 4393–4401. [Google Scholar] [CrossRef] [PubMed]
- Brel, V.K. Synthesis of 4,5-Dihydroisoxazoles Connected by Short Spacers to the Pentafluoro-λ6-sulfanyl Group. Synthesis 2006, 16, 2665–2670. [Google Scholar] [CrossRef]
- Cheng, J.-F.; Huang, Y.; Penuliar, R.; Nishimoto, M.; Liu, L.; Arrhenius, T.; Yang, G.; O’Leary, E.; Barbosa, M.; Barr, R.; et al. Discovery of Potent and Orally Available Malonyl-CoA Decarboxylase Inhibitors as Cardioprotective Agents. J. Med. Chem. 2006, 49, 4055–4058. [Google Scholar] [CrossRef]
- Conti, P.; Caligiuri, A.; Pinto, A.; Roda, G.; Tamborini, L.; Nielsen, B.; Madsen, U.; Frydenvang, K.; Colombo, A.; De Micheli, C. Synthesis and pharmacological evaluation of novel conformationally constrained homologues of glutamic acid. Eur. J. Med. Chem. 2007, 42, 1059–1068. [Google Scholar] [CrossRef]
- Quadrelli, P.; Mella, M.; Assanelli, G.; Piccanello, A. From 1,3-cyclohexadiene through nitrosocarbonyl chemistry, the synthesis of pyrimidine isoxazoline-carbocyclic nucleosides. Tetrahedron 2008, 64, 7312–7317. [Google Scholar] [CrossRef]
- Bull, J.A.; Balskus, E.P.; Horan, R.A.J.; Langner, M.; Ley, S.V. Total Synthesis of Potent Antifungal Marine Bisoxazole Natural Products Bengazoles A and B. Chem. Eur. J. 2007, 13, 5515–5538. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, M.A.; Mahdavinia, G.H.; Jafari, S. The Synthesis of Benzhydroximoyl Chloride and Nitrile Oxides under Solvent Free Conditions. J. Chem. Res. 2007, 1, 26–28. [Google Scholar] [CrossRef]
- Yamamoto, H.; Hayashi, S.; Kubo, M.; Harada, M.; Hasegawa, M.; Noguchi, M.; Sumimoto, M.; Hori, K. Asymmetric 1,3-Dipolar Cycloaddition Reactions of Benzonitrile Oxide Mediated by a Chiral Lewis Acid. Eur. J. Org. Chem. 2007, 2007, 2859–2864. [Google Scholar] [CrossRef]
- Stosic-Grujicic, S.; Cvetkovic, I.; Mangano, K.; Fresta, M.; Maksimovic-Ivanic, D.; Harhaji, L.; Popadic, D.; Momcilovic, M.; Miljkovic, D.; Kim, J.; et al. A Potent Immunomodulatory Compound, (S,R)-3-Phenyl-4,5-dihydro-5-isoxasole Acetic Acid, Prevents Spontaneous and Accelerated Forms of Autoimmune Diabetes in NOD Mice and Inhibits the Immunoinflammatory Diabetes Induced by Multiple Low Doses of Streptozotocin in CBA/H Mice. J. Pharmacol. Exp. Ther. 2007, 320, 1038–1049. [Google Scholar] [PubMed]
- Brel, V.K. Synthesis of 3-Azido-4-(diethoxyphosphoryl)alka-1,3-dienes and Their Transformation to Derivatives of 2H-Azirine. Synthesis 2007, 17, 2674–2680. [Google Scholar] [CrossRef]
- Jeddeloh, M.R.; Holden, J.B.; Nouri, D.H.; Kurth, M.J. A Library of 3-Aryl-4,5-dihydroisoxazole-5-carboxamides. J. Comb. Chem. 2007, 9, 1041–1045. [Google Scholar] [CrossRef]
- Sawant, S.D.; Singh, P.S.; Qazi, N.A.; Sampath Kumar, H.M. Addition of Allylindium Bromide to Nitryle Oxides in Aqueous Media: Convenient Synthesis of 5-Methylisoxazolines. Chem. Lett. 2007, 36, 296–297. [Google Scholar] [CrossRef]
- Dirnens, V.; Skrastina, I.; Popelis, J.; Lukevics, E. Synthesis of isoxazolinylxanthines. Chem. Heterocycl. Compd. 2007, 43, 193–196. [Google Scholar] [CrossRef]
- Yasuhito, K.; Morio, Y.; Toshikazu, T. New Click Chemistry: Click Polymerization via 1,3-Dipolar Addition of Homo-ditopic Aromatic Nitrile Oxides Formed In Situ. Chem. Lett. 2008, 37, 918–919. [Google Scholar]
- Wankhede, K.S.; Vaidya, V.V.; Suriyanarayanan, H.; Salunkhe, M.M.; Trivedi, G.K. Studies on the Intermolecular Cycloaddition of Diphenylacetonitrile Oxide with Substituted Olefins. Synth. Commun. 2008, 38, 2404–2412. [Google Scholar] [CrossRef]
- Yuan, H.; He, R.; Wan, B.; Wang, Y.; Pauli, G.F.; Franzblau, S.G.; Kozikowski, A.P. Modification of the side chain of micromolide, an anti-tuberculosis natural product. Bioorg. Med. Chem. Lett. 2008, 18, 5311–5315. [Google Scholar] [CrossRef] [PubMed]
- Ono, F.; Ohta, Y.; Hasegawa, M.; Kanemasa, S. Molecular sieve 4 Å generates nitrile oxides from hydroximoyl chlorides. Development of catalyzed enantioselective nitrile oxide cycloadditions to monosubstituted alkenes. Tetrahedron Lett. 2009, 50, 2111–2114. [Google Scholar] [CrossRef]
- Rakesh; Sun, D.; Lee, R.B.; Tangallapally, R.P.; Lee, R.E. Synthesis, optimization and structure–activity relationships of 3,5-disubstituted isoxazolines as new anti-tuberculosis agents. Eur. J. Med. Chem. 2009, 44, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Giannini, G.; Marzi, M.; Pezzi, R.; Brunetti, T.; Battistuzzi, G.; Di Marzo, M.; Cabri, W.; Vesci, L.; Pisano, C. N-Hydroxy-(4-oxime)-cinnamide: A versatile scaffold for the synthesis of novel histone deacetilase (HDAC) inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 2346–2349. [Google Scholar] [CrossRef]
- Jayashankar, B.; Lokanath Rai, K.M.; Baskaran, N.; Sathish, H.S. Synthesis and pharmacological evaluation of 1,3,4-oxadiazole bearing bis(heterocycle) derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 3898–3902. [Google Scholar] [CrossRef]
- Lohse-Fraefel, N.; Carreira, E.M. Polyketide Buildings Blocks via Diastereoselective Nitryle Oxide Cycloadditions with Homoallylic Alcohols and Monoprotected Homoallylic Diols. Chem. Eur. J. 2009, 15, 12065–12081. [Google Scholar] [CrossRef]
- Lohse-Fraefel, N.; Carreira, E.M. A Modular Approach to Polyketide Building Blocks: Cycloadditions of Nitrile Oxides and Homoallylic Alcohols. Org. Lett. 2005, 7, 2011–2014. [Google Scholar] [CrossRef]
- Mendelsohn, B.A.; Lee, S.; Kim, S.; Teyssier, F.; Aulakh, V.S.; Ciufolini, M.A. Oxidation of Oximes to Nitryle Oxides with Hipervalent Iodine Reagents. Org. Lett. 2009, 11, 1539–1542. [Google Scholar] [CrossRef]
- Dallanoce, C.; Magrone, P.; Bazza, P.; Grazioso, G.; Rizzi, L.; Riganti, L.; Gotti, C.; Clementi, F.; Frydenvang, K.; De Amici, M. New Analogues of Epiboxidine Incorporating the 4,5-Dihydroisoxazole Nucleus: Synthesis, Binding Affinity at Neuronal Nicotinic Acetylcholine Receptors, and Molecular Modeling Investigations. Chem. Biodivers. 2009, 6, 244–259. [Google Scholar] [CrossRef]
- Thalassitis, A.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Miltiadou, P. Synthesis of modified homo-N-nucleosides from the reactions of mesityl nitrile oxide with 9-allylpurines and their influence on lipid peroxidation and thrombin inhibition. Bioorg. Med. Chem. Lett. 2009, 19, 6433–6436. [Google Scholar] [CrossRef]
- Jewett, J.C.; Sletten, E.M.; Bertozzi, C.R. Rapid Cu-Free Click Chemistry with Readily Synthesized Biarylazacyclooctynones. J. Am. Chem. Soc. 2010, 132, 3688–3690. [Google Scholar] [CrossRef] [PubMed]
- Orth, R.; Böttcher, T.; Sieber, S.A. The biological targets of acivicin inspired 3-chloro- and 3-bromodihydroisoxazole scaffolds. Chem. Commun. 2010, 46, 8475–8477. [Google Scholar] [CrossRef] [PubMed]
- Gucma, M.; Gołębiewski, M. Synthesis and biological activity of 3-substituted isoxazolecarboxamides. Monatsh. Chem. 2010, 141, 461–469. [Google Scholar] [CrossRef]
- Dallanoce, C.; Magrone, P.; Matera, C.; Lo Presti, L.; De Amici, M.; Riganti, L.; Clementi, F.; Gotti, C.; De Micheli, C. Synthesis of novel chiral Δ2-isoxazoline derivatives related to ABT-418 and estimation of their affinity at neuronal nicotinic acetylcholine receptor subtypes. Eur. J. Med. Chem. 2010, 45, 5594–5601. [Google Scholar] [CrossRef]
- Meng, L.; Lorsbach, B.A.; Sparks, T.C.; Fettinger, J.C.; Kurth, M.J. Parallel Synthesis of Bis-heterocyclic Isoxazolylmethyl- and Isoxazolinylmethylpyrazoles. J. Comb. Chem. 2010, 12, 129–136. [Google Scholar] [CrossRef]
- Conti, P.; Tamborini, L.; Pinto, A.; Sola, L.; Ettari, R.; Mercurio, C.; De Micheli, C. Design and Synthesis of novel isoxazole-based HDAC inhibitors. Eur. J. Med. Chem. 2010, 45, 4331–4338. [Google Scholar] [CrossRef]
- Gutsmiedl, K.; Fazio, D.; Carell, T. High-Density DNA Functionalization by a Combination of Cu-Catalyzed and Cu-Free Click Chemistry. Chem. Eur. J. 2010, 16, 6877–6883. [Google Scholar] [CrossRef]
- Sperry, J.; Harris, E.B.J.; Brimble, M.A. Total Synthesis and Absolute Configuration of (–)-Berkeleyamide A. Org. Lett. 2010, 12, 420–423. [Google Scholar] [CrossRef]
- Vitale, P.; Di Nunno, L.; Scilimati, A. A Novel Synthesis of N-Unsubstituted ß-Enamino Thioesters from 3-Arylisoxazoles and 3-Aryl-5-phenylthio-2-isoxazolines. Synthesis 2010, 18, 3195–3203. [Google Scholar]
- Matsumura, T.; Ishiwari, F.; Koyama, Y.; Takata, T. C–C Bond Forming Click Synthesis of Rotaxanes Exploiting Nitryle N-Oxide. Org. Lett. 2010, 12, 3828–3831. [Google Scholar] [CrossRef]
- Kushnir, O.V.; Mel’nichenko, N.V.; Vovk, M.V. Heterocyclizations of functionalized heterocumulenes with C,N-, C,O-, and C,S-binucleophiles: XIII. Synthesis of dialkyl 2-oxo-3-allyl-1,2,3,6-tetrahydropyrimidine-4,5-dicarboxylates and their reaction with arylhydroxymoyl chlorides. Russ. J. Org. Chem. 2011, 47, 1727–1732. [Google Scholar] [CrossRef]
- Shailaja, M.; Manjula, A.; Rao, B.V. Synthesis of novel 3, 5-disubstituted-4,5-dihydroisoxazoles and 3,4,5-trisubstituted isoxazoles and their biological activity. Indian J. Chem. Sec. B 2011, 50B, 214–222. [Google Scholar] [CrossRef]
- Brel, V.K. Synthesis of Alkene, Alcohols, and Heterocycles Containing the Pentafluorosulfanyl (SF5) Grouping. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 1284–1287. [Google Scholar] [CrossRef]
- Vilela, G.D.; Da Rosa, R.R.; Schneider, P.H.; Bechtold, I.H.; Eccher, J.; Merlo, A.A. Expeditious preparation of isoxazoles from Δ2-isoxazolines as advanced intermediates for functional materials. Tetrahedron Lett. 2011, 52, 6569–6572. [Google Scholar] [CrossRef]
- Castellano, S.; Kuck, D.; Viviano, M.; Yoo, J.; López-Vallejo, F.; Conti, P.; Tamborini, L.; Pinto, A.; Medina-Franco, J.L.; Sbardella, G. Synthesis and Biochemical Evaluation of Δ2-Isoxazoline Derivatives as DNA Methyltransferase 1 Inhibitors. J. Med. Chem. 2011, 54, 7663–7677. [Google Scholar] [CrossRef]
- Chronowska, A.; Gallienne, E.; Nicolas, C.; Kato, A.; Adachi, I.; Martin, O.R. An expeditious synthesis of an analogue of (−)-steviamine by way of the 1,3-dipolar cycloaddition of a nitrile oxide with a 1-C-allyl iminosugar. Tetrahedron Lett. 2011, 52, 6399–6402. [Google Scholar] [CrossRef]
- Alam, A.; Pal, C.; Goyal, M.; Kundu, M.K.; Kumar, R.; Iqbal, M.S.; Dey, S.; Bindu, S.; Sarkar, S.; Pal, U.; et al. Synthesis and bio-evaluation of human macrophage migration inhibitory factor inhibitor to develop anti-inflammatory agent. Bioorg. Med. Chem. 2011, 19, 7365–7373. [Google Scholar] [CrossRef]
- Tavares, A.; Vilela, G.D.; Toldo, J.; Gonçalves, P.F.B.; Eccher, J.; Bechtold, I.H.; Sampaio, A.R.; Viscovini, R.C.; Schneider, P.H.; Merlo, A.A. The 2:1 cycloadducts from [3 + 2] 1,3-dipolar cycloaddition of nitrile oxide and vinylacetic acid. Synthesis and liquid crystal behaviour. Liq. Cryst. 2011, 39, 175–184. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, C.; Wang, E.; Wang, W.; Liu, M.; Yin, Q.; Chen, H.; Wang, K.; Li, X.; Zhang, J. Synthesis and biological activities of novel isoxazoline-linked pseudodisaccharide derivatives. Carbohydr. Res. 2012, 351, 7–16. [Google Scholar] [CrossRef]
- Rakesh; Bruhn, D.; Madhura, D.B.; Maddox, M.; Lee, R.B.; Trivedi, A.; Yang, L.; Scherman, M.S.; Gilliland, J.C.; Gruppo, V.; et al. Antitubercular nitrofuran isoxazolines with improved pharmacokinetic properties. Bioorg. Med. Chem. 2012, 20, 6063–6072. [Google Scholar]
- Kikuchi, D.; Yoshida, M.; Shishido, K. Total Synthesis of (±)-3-Hydroxy-β-ionone through a Ring-Closing Enyne Metathesis. Synlett 2012, 23, 577–580. [Google Scholar]
- Dadiboyena, S.; Nefzi, A. Solid phase synthesis of isoxazole and isoxazoline-carboxamides via [2+3]-dipolar cycloaddition using resin-bound alkynes or alkenes. Tetrahedron Lett. 2012, 53, 2096–2099. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Wang, X.; Bao, H.; Cheng, C.; Liu, N.; Hu, Y. Total Syntheses of (−)-Hanishin, (−)-Longmide B, and (−)-Longmide B Methyl Ester via a Novel Preparation of N-Substituted Pyrrole-2-Carboxylates. Org. Lett. 2012, 14, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, Y.; Zhao, W.-T.; Tang, X.-Y. Facile synthesis of 3-aryl-5-(2-oxopyrrolidin-1-yl)- and 5-(piridin-4-yl)-4,5-dihydroisoxazoles via 1,3-dipolar cycloaddition under mild conditions. J. Chem. Res. 2013, 8, 499–502. [Google Scholar] [CrossRef]
- Liu, K.; Wu, X.; Kan, S.B.J.; Shirakawa, S.; Maruoka, K. Phase-Transfer-Catalyzed Asymmetric Synthesis of Axially Chiral Anilides. Chem. Asian J. 2013, 8, 3214–3221. [Google Scholar] [CrossRef]
- Arlt, A.; Benson, S.; Schulthoff, S.; Gabor, B.; Fürstner, A. A Total Synthesis of Spirastrellolide A Methyl Ester. Chem. Eur. J. 2013, 19, 3596–3608. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Xiong, L.; Li, Y.; Yang, N.; Wang, Q. Design, Synthesis, and Insecticidal Evaluation of New Pyrazole Derivatives Containing Imine, Oxime Ether, Oxime Ester, and Dihydroisoxazoline Groups Based on the Inhibitor Binding Pocket of Respiratory Complex I. J. Agric. Food Chem. 2013, 61, 8730–8736. [Google Scholar] [CrossRef]
- Wang, C.-G.; Koyama, Y.; Yonekawa, M.; Uchida, S.; Takata, T. Polymer nitrile N-oxides directed toward catalyst- and solvent-free click grafting. Chem. Commun. 2013, 49, 7723–7725. [Google Scholar] [CrossRef]
- Patel, N.C.; Schwarz, J.; Hou, X.J.; Hoover, D.J.; Xie, L.; Fliri, A.J.; Gallaschun, R.J.; Lazzaro, J.T.; Bryce, D.K.; Hoffmann, W.E.; et al. Discovery and Characterization of a Novel Dihydroisoxazole Class of α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) Receptor Potentiators. J. Med. Chem. 2013, 56, 9180–9191. [Google Scholar] [CrossRef]
- Cheawchan, S.; Koyama, Y.; Uchida, S.; Takata, T. Catalyst-free click cascade functionalization of unsaturated-bond-containing polymers using masked-ketene-tethering nitrile N-oxide. Polymer 2013, 54, 4501–4510. [Google Scholar] [CrossRef]
- Han, L.; Zhang, B.; Xiang, C.; Yan, J. One-Pot Synthesis of Isoxazolines from Aldehydes Catalyzed by Iodobenzene. Synthesis 2014, 46, 503–509. [Google Scholar]
- Rodrigues, G.C.; Feijó, D.F.; Bozza, M.T.; Pan, P.; Vullo, D.; Parkkila, S.; Supuran, C.T.; Capasso, C.; Aguiar, A.P.; Vermelho, A.B. Design, Synthesis, and Evaluation of Hydroxamic Acid Derivatives as Promising Agents for the Management of Chagas Disease. J. Med. Chem. 2014, 57, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, Z.; Yu, Z.; Shang, Y. Novel isoxazoline ligand with ferrocene backbone: Preparation and application in Heck reaction with water as solvent. Appl. Organometal. Chem. 2014, 28, 657–660. [Google Scholar] [CrossRef]
- Xiang, C.; Li, T.; Yan, J. Hypervalent Iodine–Catalyzed Cycloaddition of Nitrile Oxides to Alkenes. Synth. Commun. 2014, 44, 682–688. [Google Scholar] [CrossRef]
- Roßbach, J.; Harms, K.; Koert, U. αCrotyl-α-difluoroboranyloxy-amides: Structure and Reactivity of Isolable Intermediates in Stereospecific αKetol Rearrangements. Org. Lett. 2015, 17, 3122–3125. [Google Scholar] [CrossRef]
- Da Rosa, R.R.; Brose, I.S.; Vilela, G.D.; Merlo, A.A. 3,5-diarylisoxazoles: A New Entry to Soft Crystal Phase. Mol. Cryst. Liq. Cryst. 2015, 612, 158–168. [Google Scholar] [CrossRef]
- Brel, V.K. Synthesis of gem-bisphosphonates with (3-aryl-4,5-dihydroisoxazol-5-yl)methylamino moiety. Mendeleev Commun. 2015, 25, 234–235. [Google Scholar] [CrossRef]
- Bykhovskaya, V.; Aladzheva, I.M.; Brel, V.K. N-Allylsubstituted aminomethylene1,1-bisphosphonates in 1,3-dipolar cycloaddition reaction with aromatic nitrile N-oxides. Russ. Chem. Bull. 2017, 66, 1256–1260. [Google Scholar] [CrossRef]
- Fu, J.; Karur, S.; Lee, P.; Sweeney, Z.K. Isoxazoline Hydroxamic Acid Derivatives as LpxC Inhibitors. International Patent WO2015/164458A1, 29 October 2015. [Google Scholar]
- Filali, I.; Bouajila, J.; Znati, M.; Bousejra-El Garah, F.; Ben Jannet, H. Synthesis of new isoxazoline derivatives from harmine and evaluation of their anti-Alzheimer, anti-cancer and anti-inflammatory activities. J. Enzyme Inhib. Med. Chem. 2015, 30, 371–376. [Google Scholar] [CrossRef]
- Akehi, M.; Kawamoto, M.; Mandai, T. Chiral 1-(1,3-dithian-2-yl) prop-2-en-1-ols: New scaffolds for enantiopure α-hydroxyaldehydes. Tetrahedron 2015, 71, 6488–6498. [Google Scholar] [CrossRef]
- Kumar, K.S.V.; Lingaraju, G.S.; Bommegowda, Y.K.; Vinayaka, A.C.; Bhat, P.; Kumara, C.S.P.; Rangappa, K.S.; Gowda, D.C.; Sadashiva, M.P. Synthesis, antimalarial activity, and target binding of dibenzazepine-tethered isoxazolines. RSC Adv. 2015, 5, 90408–90421. [Google Scholar] [CrossRef]
- Koyama, Y.; Lee, Y.-G.; Kuroki, S.; Takata, T. Synthesis, 13C NMR, and UV spectroscopic study of 13C-labeled nitrile N-oxide. Tetrahedron Lett. 2015, 56, 7038–7042. [Google Scholar] [CrossRef]
- Li, C.; Deng, H.; Li, C.; Jia, X.; Li, J. Palladium-Catalyzed Synthesis of Δ2Isoxazoline from Toluene Derivatives Enabled by the Triple Role of Silver Nitrate. Org. Lett. 2015, 17, 5718–5721. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.M.; Viau, C.M.; dos Santos, J.M.; Bonacorso, H.G.; Martins, M.A.P.; Amaral, S.S.; Saffi, J.; Zanatta, N. Synthesis and cytotoxic activity evaluation of some novel 1-(3-(aryl-4,5-dihydroisoxazol-5-yl)methyl)-4-trihalomethyl-1H-pyrimidin-2-ones in human cancer cells. Eur. J. Med. Chem. 2015, 101, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Gucma, M.; Gołębiewski, W.M.; Michalczyk, A.K. Studies on [2 + 3] cycloaddition reaction of nitrile oxides to linear dipolarophiles bearing multiple double bonds. Monatsh. Chem. 2016, 147, 1809–1818. [Google Scholar] [CrossRef]
- Zayane, M.; Rahmouni, A.; Daami-Remadi, M.; Mansour, M.B.; Romdhane, A.; Jannet, H.B. Design and synthesis of antimicrobial, anticoagulant, and anticholinesterase hybrid molecules from 4-methylumbelliferone. J. Enzyme. Inhib. Med. Chem. 2016, 31, 1566–1575. [Google Scholar] [CrossRef]
- Kulyashova, A.; Krasavin, M. Convenient modular construction of medicinally important 5-acylamino-4,5-dihydroisoxazoles featuring four elements of diversity. Tetrahedron Lett. 2016, 57, 4395–4397. [Google Scholar] [CrossRef]
- Fritsch, L.; Merlo, A.A. An old dog with new tricks: Schiff bases for liquid crystals materials based on isoxazolines and isoxazoles. ChemistrySelect 2016, 1, 23–29. [Google Scholar] [CrossRef]
- Poh, J.-S.; García-Ruiz, C.; Zúñiga, A.; Meroni, F.; Blakemore, D.C.; Browne, D.L.; Ley, S.V. Synthesis of trifluoromethylated isoxazoles and their elaboration through inter- and intra-molecular C–H arylation. Org. Biomol. Chem. 2016, 14, 5983–5991. [Google Scholar] [CrossRef]
- Ismail, T.; Shafi, S.; Singh, S.; Sidiq, T.; Khajuria, A.; Rouf, A.; Yadav, M.; Saikam, V.; Singh, P.P.; Alam, M.S.; et al. Synthesis and immunopotentiating activity of novel isoxazoline functionalized coumarins. Eur. J. Med. Chem. 2016, 123, 90–104. [Google Scholar] [CrossRef]
- Brullo, C.; Ricciarelli, R.; Prickaerts, J.; Arancio, O.; Massa, M.; Rotolo, C.; Romussi, A.; Rebosio, C.; Marengo, B.; Pronzato, M.A.; et al. New insights into selective PDE4D inhibitors: 3-(Cyclopentyloxy)-4-methoxybenzaldehyde O-(2-(2,6-dimethylmorpholino)-2-oxoethyl)oxime (GEBR-7b) structural development and promising activities to restore memory impairment. Eur. J. Med. Chem. 2016, 124, 82–102. [Google Scholar] [CrossRef] [PubMed]
- Tsyganov, D.V.; Krayushkin, M.M.; Konyushkin, L.D.; Strelenko, Y.A.; Semenova, M.N.; Semenov, V.V. Facile Synthesis of Natural Alkoxynaphthalene Analogues from Plant Alkoxybenzenes. J. Nat. Prod. 2016, 79, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, L.; Cullia, G.; Nielsen, B.; De Micheli, C.; Conti, P.; Pinto, A. Synthesis and pharmacological evaluation of conformationally constrained glutamic acid higher homologues. Bioorg. Med. Chem. 2016, 24, 5741–5747. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Zong, K.; Choe, J.C. Regioselectivity of 1,3-Dipolar Cycloadditions of Benzonitrile Oxide to Alkenyl Boronic Esters: An Experimental and Computational Study. J. Heterocycl. Chem. 2016, 54, 1007–1014. [Google Scholar] [CrossRef]
- Efremova, M.M.; Kostikov, R.R.; Larina, A.G.; Molchanov, A.P. Regio- and stereoselective (3 + 2)-cycloaddition of nitrile oxides and nitrones to N-vinylindole. Russ. J. Org. Chem. 2017, 53, 246–250. [Google Scholar] [CrossRef]
- Jakubiec, D.; Przypis, A.; Suwiński, J.W.; Walczak, K.Z. Synthesis of 5-hetaryluracil derivatives via 1,3-dipolar cycloaddition reaction. Arkivoc 2017, 2, 149–161. [Google Scholar] [CrossRef]
- Choe, H.; Cho, H.; Ko, H.-J.; Lee, J. Total Synthesis of (+)-Pochonin D and (+)-Monocillin II via Chemo- and Regioselective Intramolecular Nitrile Oxide Cycloaddition. Org. Lett. 2017, 19, 6004–6007. [Google Scholar] [CrossRef]
- Ma, H.; Stone, V.N.; Wang, H.; Kellog, G.E.; Xu, P.; Zhang, Y. Diaminopimelic acid (DAP) analogs bearing isoxazoline moiety as selective inhibitors against meso-diaminopimelate dehydrogenase (m-Ddh) from Porphyromonas gingivalis. Bioorg. Med. Chem. Lett. 2017, 27, 3840–3844. [Google Scholar] [CrossRef]
- Kamath, P.; Viner, R.C.; Smith, S.C.; Lal, M. A Novel Route to 2-Arylquinolines: Reductive Cleavage of 2′-Nitroaryl-∆2-isoxazolines. Synlett. 2017, 28, 1341–1345. [Google Scholar]
- Takada, T.; Sogawa, H.; Tani, M.; Noguchi, T.; Kambara, T.; Isomura, Y. Rotaxane Compound. Japanese Patent JP2017160164A, 14 September 2017. [Google Scholar]
- Suga, H.; Hashimoto, Y.; Toda, Y.; Fukushima, K.; Esaki, H.; Kikuchi, A. Amine-Urea-Mediated Asymmetric Cycloadditions between Nitrile Oxides and o-Hydroxystyrenes by Dual Activation. Angew. Chem. Int. Ed. 2017, 56, 11936–11939. [Google Scholar] [CrossRef]
- Picconi, P.; Prabaharan, P.; Auer, J.L.; Sandiford, S.; Cascio, F.; Chowdhury, M.; Hind, C.; Wand, M.E.; Sutton, J.M.; Rahman, K.M. Novel pyridyl nitrofuranyl isoxazolines show antibacterial activity against multiple drug resistant Staphylococcus species. Bioorg. Med. Chem. 2017, 25, 3971–3979. [Google Scholar] [CrossRef] [PubMed]
- Korgavkar, N.N.; Samant, S.D. 1,3-Dipolar cycloaddition reaction of aryl nitrile oxides with alkenes using imidazole and pyridine containing reusable polymeric base catalysts. Synth. Commun. 2018, 48, 387–394. [Google Scholar] [CrossRef]
- Mirosław, B.; Babyuk, D.; Łapczuk-Krygier, A.; Kącka-Zych, A.; Demchuk, O.M.; Jasinski, R. Regiospecific formation of the nitromethyl-substituted 3-phenyl-4,5-dihydroisoxazole via [3 + 2] cycloaddition. Monatsh. Chem. 2018, 149, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, Y.; Xu, H. Synthesis of novel isoxazoline-containing podophyllotoxin/2′(2′,6′)-(di)halogenopodophyllotoxin derivatives and their insecticidal/acaricidal activities. Bioorg. Med. Chem. 2018, 28, 1410–1416. [Google Scholar] [CrossRef]
- Toda, Y.; Koyama, M.; Esaki, H.; Fukushima, K.; Suga, H. Enantioselective Cycloadditions between Aliphatic Nitrile Oxides and 2-Hydroxystyrenes Catalyzed by Chiral Amine-Urea. Heterocycles 2018, 97, 147–150. [Google Scholar]
- Sağirli, A.; Dürüst, Y. Regioselective synthesis of some isoxazolines and isoxazolidines bearing caprolactam moiety. Synth. Commun. 2018, 48, 1413–1424. [Google Scholar] [CrossRef]
- Low, J.D.; Bartberger, M.D.; Cheng, Y.; Whittington, D.; Xue, Q.; Wood, S.; Allen, J.R.; Minatti, A.E. Diastereoselective synthesis of fused cyclopropyl-3-amino-2,4-oxazine β-amyloid cleaving enzyme (BACE) inhibitors and their biological evaluation. Bioorg. Med. Chem. Lett. 2018, 28, 1111–1113. [Google Scholar] [CrossRef]
- Lee, P.S.; Lapointe, G.; Madera, A.M.; Simmons, R.L.; Xu, W.; Yifru, A.; Tjandra, M.; Karur, S.; Rico, A.; Thompson, K.; et al. Application of Virtual Screening to the Identification of New LpxC Inhibitor Chemotypes, Oxazolidinone and Isoxazoline. J. Med. Chem. 2018, 61, 9360–9370. [Google Scholar] [CrossRef]
- Adardour, M.; Zaballos-García, E.; Loughzail, M.; Dahaoui, S.; Baouid, A. Synthesis, characterization and X-ray structure of heterocyclic systems prepared via 1,3-dipolar cycloaddition of nitrile oxides with benzimidazolone. J. Mol. Struct. 2018, 1165, 153–161. [Google Scholar] [CrossRef]
- Zhao, G.; Liang, L.; Ethan Wen, C.H.; Tong, R. In Situ Generation of Nitrile Oxides from NaCl−Oxone Oxidation of Various Aldoximes and Their 1,3-Dipolar Cycloaddition. Org. Lett. 2019, 21, 315–319. [Google Scholar] [CrossRef]
- Distante, F.; Collina, S.; Quadrelli, P. A practical synthesis of (3-(phenanthren-9-yl)-4,5-dihydroisoxazol-5-yl)methyl (tert-butoxycarbonyl)-L-alaninate. Arkivoc 2020, 6, 66–72. [Google Scholar] [CrossRef]
- Song, D.; Bi, F.; Zhang, N.; Qin, Y.; Liu, X.; Teng, Y.; Ma, S. Design, synthesis of novel 4,5-dihydroisoxazole-containing benzamide derivatives as highly potent FtsZ inhibitors capable of killing a variety of MDR Staphylococcus aureus. Bioorg. Med. Chem. 2020, 28, 115729. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.L.; Da Rosa, R.R.; Eifler-Lima, V.L.; Merlo, A.A. The use of isoxazoline and isoxazole scaffolding in the design of novel thiourea and amide liquid-crystalline compounds. Beilstein J. Org. Chem. 2020, 16, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Plumet, J. 1,3-Dipolar Cycloaddition Reactions of Nitrile Oxides under “Non-Conventional” Conditions: Green Solvents, Irradiation, and Continuous Flow. ChemPlusChem 2020, 85, 2252–2271. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, N.; Imayoshi, A.; Tsubaki, K. Nitrile oxide cycloaddition reactions of alkenes or alkynes and nitroalkanes substituted with O-alkyloxime groups convertible to various functional groups. Tetrahedron Lett. 2020, 61, 152213. [Google Scholar] [CrossRef]
- D’Ascenzio, M.; Secci, D.; Carradori, S.; Zara, S.; Guglielmi, P.; Cirilli, R.; Pierini, M.; Poli, G.; Tuccinardi, T.; Angeli, A.; et al. 1,3-Dipolar Cycloaddition, HPLC Enantioseparation, and Docking Studies of Saccharin/Isoxazole and Saccharin/Isoxazoline Derivatives as Selective Carbonic Anhydrase IX and XII Inhibitors. J. Med. Chem. 2020, 63, 2470–2488. [Google Scholar] [CrossRef]
- Fráguas, R.M.; Costa, V.A.; Terra, W.C.; Aguiar, A.P.; Martins, S.J.; Campos, V.P.; Oliveira, D.F. Toxicities of 4,5-Dihydroisoxazoles Against Root-Knot Nematodes and in Silico Studies of Their Modes of Action. J. Agric. Food Chem. 2020, 68, 523–529. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, J.; Yan, X.; Cheng, W.; Wang, X.; Yang, R.; Guo, Y. Discovery of Natural Product-Based Fungicides (II): Semisynthesis and Biological Activity of Sarisan Attached 3-Phenylisoxazolines as Antifungal Agents. Chem. Biodivers. 2020, 17, e2000763. [Google Scholar] [CrossRef]
- Sebbar, N.K.; Taha, M.L.; Ellouz, M.; Essassi, E.M.; Zerzouf, A.; Karrouchi, K.; Ouzidan, Y.; Zakaria, M.; Mague, J.T. Synthesis, DFT Study and Antibacterial Activity of some Isoxazoline Derivatives Containing 1,4-benzothiazin-3-one Nucleus Obtained Using 1,3-dipolar Cycloaddition Reaction. Iran. J. Chem. Chem. Eng. 2020, 39, 53–67. [Google Scholar]
- Aarjane, M.; Slassi, S.; Ghaleb, A.; Amine, A. Synthesis, spectroscopic characterization (FT-IR, NMR) and DFT computational studies of new isoxazoline derived from acridone. J. Mol. Struct. 2021, 1231, 129921. [Google Scholar] [CrossRef]
- De Angelis, L.; Crawford, A.M.; Su, Y.-L.; Wherritt, D.; Arman, H.; Doyle, M.P. Catalyst-Free Formation of Nitrile Oxides and Their Further Transformations to Diverse Heterocycles. Org. Lett. 2021, 23, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, Y.-S.; Gu, B.-M.; Yang, W.-L.; Tian, B.-X.; Deng, W.-P. Cooperative Nheterocyclic Carbene and Iridium Catalysis Enables Stereoselective and Regiodivergent [3 + 2] and [3 + 3] Annulation Reactions. ACS Catal. 2021, 11, 3810–3821. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, K.; Lv, M.; Hao, M. Construction of Cholesterol Oxime Ether Derivatives Containing Isoxazoline/Isoxazole Fragments and Their Agricultural Bioactive Properties/Control Efficiency. J. Agric. Food Chem. 2021, 69, 8098–8109. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, N.B.; Afroz, P.; Ahmad, M.N.; Kaul, G.; Shukla, M.; Nanduri, S.; Dasgupta, A.; Chopra, S.; Yaddanapudi, V.M. Design, synthesis, in vitro and in silico evaluation of new 3-phenyl-4,5-dihydroisoxazole-5-carboxamides active against drug-resistant mycobacterium tuberculosis. J. Mol. Struct. 2021, 1227, 129545. [Google Scholar] [CrossRef]
- Endoori, S.; Gulipalli, K.C.; Bodige, S.; Shaikh, A.S.; Vemula, D.; Surapureddi, S.R.; Seelam, N. Design, synthesis, anti-cancer activity and in-silico studies of some novel 4,5-dihydroisoxazole-5-carboxamide derivatives. Synth. Commun. 2021, 51, 3416–3426. [Google Scholar] [CrossRef]
- Pajkert, R.; Koroniak, H.; Kafarski, P.; Röschenthaler, G.-V. Reductive Hypervalent-iodine mediated one-pot synthesis of isoxazolines and isoxazoles bearing a difluoromethyl phosphonate moiety. Org. Biomol. Chem. 2021, 19, 4871–4876. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Hsu, C.-S. Enantioselective Total Synthesis of (+)–EBC–23, a Potent Anticancer Agent from the Australian Rainforest. J. Org. Chem. 2021, 86, 6351–6360. [Google Scholar] [CrossRef]
- Chalyk, B.A.; Khutorianskyi, A.V.; Vashchenko, B.V.; Danyleiko, K.; Grynyova, A.; Osipova, A.O.; Kozytskiy, A.; Syniuchenko, D.; Tsymbaliuk, A.; Gavrilenko, K.S.; et al. Reductive Recyclization of sp3-Enriched Functionalized Isoxazolines into α-Hydroxy Lactams. J. Org. Chem. 2022, 87, 1001–1018. [Google Scholar] [CrossRef]
- Rhazi, Y.; Chalkha, M.; Nakkabi, A.; Hammoudan, I.; Akhazzane, M.; Bakhouch, M.; Chtita, S.; El Yazidi, M. Novel Quinazolinone–Isoxazoline Hybrids: Synthesis, Spectroscopic Characterization, and DFT Mechanistic Study. Chemistry 2022, 4, 969–982. [Google Scholar] [CrossRef]
- Taia, A.; Essaber, M.; Oubella, A.; Aatif, A.; Bodiguel, J.; Jamart-Grégoire, B.; Itto, M.Y.A.; Morjani, H. Synthesis, characterization, and biological evaluation of new heterocyclic systems 1, 2, 3-triazole-isoxazoline from eugenol by the mixed condensation reactions. Synth. Commun. 2022, 50, 2052–2065. [Google Scholar] [CrossRef]
- Oubella, A.; Taia, A.; Byadi, S.; Lahcen, M.A.; Bimoussa, A.; Essaber, M.; Podlipnik, C.; Morjani, H.; Itto, M.Y.A.; Aatif, A. Chemical profiling, cytotoxic activities through apoptosis induction in human fibrosarcoma and carcinoma cells, and molecular docking of some 1,2,3-triazole-isoxazoline hybrids using the eugenol as a precursors. J. Biomol. Struct. Dyn. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Batt, D.G.; Houghton, G.C.; Daneker, W.F.; Jadhav, P.K. Synthesis of Cis and Trans Isomers of an Isoxazoline Ring-Hydroxylated Metabolite of Roxifiban, a Platelet Glycoprotein IIb/IIIa Receptor Antagonist. J. Org. Chem. 2000, 65, 8100–8104. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, J.R.; Pinto, D.J.; Estrella, M.J.; Bostrom, L.L.; Knabb, R.M.; Wong, P.C.; Wright, M.R.; Wexler, R.R. Isoxazolines and Isoxazoles as Factor Xa Inhibitors. Bioorg. Med. Chem. Lett. 2000, 10, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Faita, G.; Paio, A.; Quadrelli, P.; Rancati, F.; Seneci, P. Solid supported chiral auxiliaries in asymmetric synthesis. Part 2: Catalysis of 1,3-dipolar cycloadditions by Mg(II) cation. Tetrahedron 2001, 57, 8313–8322. [Google Scholar] [CrossRef]
- Easton, C.J.; Heath, G.A.; Hughes, C.M.M.; Lee, C.K.Y.; Savage, G.P.; Simpson, G.W.; Tiekink, E.R.T.; Vuckovic, G.J.; Webster, R.D. Electrochemical and yeast-catalyzed ring-opening of isoxazoles in the synthesis of analogues of the herbicide Grasp. J. Chem. Soc. Perkin Trans. 1 2001, 1168–1174. [Google Scholar] [CrossRef]
- Bode, J.W.; Fraefel, N.; Muri, D.; Carreira, E.M. A General Solution to the Modular Synthesis of Polyketide Building Blocks by Kanemasa Hydroxy-Directed Nitrile Oxide Cycloadditions. Angew. Chem. Int. Ed. 2001, 40, 2082–2085. [Google Scholar] [CrossRef]
- Kamimura, A.; Kaneko, Y.; Ohta, A.; Matsuura, K.; Fujimoto, Y.; Kakehi, A.; Kanemasa, S. Enantioselective preparation of 3,4,5-trisubstituted 4,5-dihydroisoxazoles and their stereoselective elaboration of 5-side chain. Tetrahedron 2002, 58, 9613–9620. [Google Scholar] [CrossRef]
- Kai, H.; Tomida, M.; Nakai, T.; Takase, A. A convenient synthesis of 3-benzoylisoxazoles by 1,3-dipolar cycloaddition. Heterocycles 2002, 57, 2299–2308. [Google Scholar] [CrossRef]
- Conti, P.; De Amici, M.; di Ventimiglia, S.J.; Stensbøl, T.B.; Madsen, U.; Bräuner-Osborne, H.; Russo, E.; De Sarro, G.; Bruno, G.; De Micheli, C. Synthesis and Anticonvulsant Activity of Novel Bicyclic Acidic Amino Acids. J. Med. Chem. 2003, 46, 3102–3108. [Google Scholar] [CrossRef]
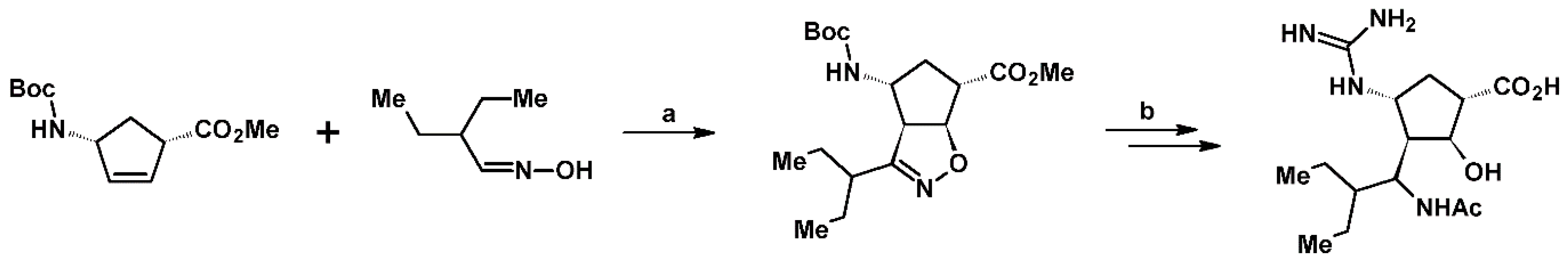
- Kiss, L.; Nonn, M.; Fülöp, F. Syntheses of Isoxazoline-Based Amino Acids by Cycloaddition of Nitrile Oxides and Their Conversion into Highly Functionalized Bioactive Amino Acid Derivatives. Synthesis 2012, 44, 1951–1963. [Google Scholar] [CrossRef]
- Mineno, T.; Miller, M.J. Stereoselective Total Synthesis of Racemic BCX-1812 (RWJ-270201) for the Development of Neuraminidase Inhibitors as Anti-influenza Agents. J. Org. Chem. 2003, 68, 6591–6596. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Watanabe, S.; Hasegawa, M.; Noguchi, M.; Kanemasa, S. Synthesis of chiral isoxazoline derivatives by highly diastereoface-selective 1,3-dipolar cycloaddition of nitrile oxides mediated by magnesium bromide and ytterbium trifluoromethanesulfonate. J. Chem. Res. 2003, 5, 284–286. [Google Scholar] [CrossRef]
- Mincheva, Z.; Courtois, M.; Crèche, J.; Rideau, M.; Viaud-Massuard, M.-C. One-pot synthesis of functionalized 4,5-dihydroisoxazole derivatives via nitrile oxides and biological evaluation with plant cells. Bioorg. Med. Chem. 2004, 12, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Sibi, M.P.; Itoh, K.; Jasperse, C.P. Chiral Lewis Acid Catalysis in Nitrile Oxide Cycloadditions. J. Am. Chem. Soc. 2004, 126, 5366–5367. [Google Scholar] [CrossRef] [PubMed]
- Morozova, Y.V.; Starikova, Z.A.; Maksimov, B.I.; Yashunskii, D.V.; Ponomarev, G.V. 1,3-Cycloaddition of the nickel meso-cyanooctaethylporphyrin N-oxide complex to olefins. Molecular and crystal structure of a double cycloaddition product to 2,5-norbornadiene. Russ. Chem. Bull. 2004, 53, 2192–2195. [Google Scholar] [CrossRef]
- Muri, D.; Lohse-Fraefel, N.; Carreira, E.M. Total Synthesis of Erythronolide A by MgII -Mediated Cycloadditions of Nitrile Oxides. Angew. Chem. Int. Ed. 2005, 44, 4036–4038. [Google Scholar] [CrossRef]
- Litvinovskaya, R.P.; Aver’kova, M.A.; Lyakhov, A.S.; Koval’, N.V.; Baranovskii, A.V.; Khripach, V.A. Crystal and Molecular Structure of (4′R,5′R,22R)-22-Hydroxy-22-(3′,4′-dimethylisoxazolin-5′-yl)-6β-methoxy-3α,5-cyclo-23,24-dinorcholane. Russ. J. Gen. Chem. 2005, 75, 1276–1279. [Google Scholar] [CrossRef]
- Quadrelli, P.; Piccanello, A.; Martinez, N.V.; Bovio, B.; Mella, M.; Caramella, P. Isoxazoline-carbocyclic aminols for nucleoside synthesis through aza-Diels–Alder reactions. Tetrahedron 2006, 62, 7370–7379. [Google Scholar] [CrossRef]
- Quadrelli, P.; Bovio, B.; Piccinini, A.; Caramella, P.; De Sarlo, F.; Machetti, F. Conversion of a nitrosocarbonyl hetero Diels–Alder cycloadduct to useful isoxazoline-carbocyclic aminols. Tetrahedron 2009, 65, 10679–10684. [Google Scholar] [CrossRef]
- Savion, M.; Memeo, M.G.; Bovio, B.; Grazioso, G.; Legnani, L.; Quadrelli, P. Synthesis and molecular modeling of novel dihydroxycyclopentane-carbonitrile nor-nucleosides by bromonitrile oxide 1,3-dipolar cycloaddition. Tetrahedron 2012, 68, 1845–1852. [Google Scholar] [CrossRef]
- Barr, L.; Lincoln, S.F.; Easton, C.J. Reversal of Regioselectivity and Enhancement of Rates of Nitrile Oxide Cycloadditions through Transient Attachment of Dipolarophiles to Cyclodextrins. Chem. Eur. J. 2006, 12, 8571–8580. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.-H.; Li, W.-S.; Chao, I.; Lee, G.-H.; Chung, W.-S. Regioselectivity in the 1,3-dipolar cycloaddition of adamantylidenefulvene and its modification by inclusion in cyclodextrins’ solutions. Tetrahedron 2006, 62, 7380–7389. [Google Scholar] [CrossRef]
- Hayashi, S.; Mori, A.; Nishina, M.; Sumimoto, M.; Hori, K.; Yamamoto, H. Regio- and diastereo-selective formation of isoxazoline derivatives by Lewis acid mediated 1,3-dipolar cycloaddition reactions of nitrile oxide. J. Chem. Res. 2007, 7, 394–396. [Google Scholar] [CrossRef]
- Madapa, S.; Sridhar, D.; Yadav, G.P.; Maulik, P.R.; Batra, S. A General Approach to the Synthesis of Substituted Isoxazolo[4,3-c]quinolines via Chalcones. Eur. J. Org. Chem. 2007, 2007, 4343–4351. [Google Scholar] [CrossRef]
- Pinto, A.; Conti, P.; De Amici, M.; Tamborini, L.; Grazioso, G.; Colleoni, S.; Mennini, T.; Gobbi, M.; De Micheli, C. Synthesis of enantiomerically pure HIP-A and HIP-B and investigation of their activity as inhibitors of excitatory amino acid transporters. Tetrahedron Asymmetry 2008, 19, 867–875. [Google Scholar] [CrossRef]
- Roda, G.; Conti, P.; De Amici, M.; He, J.; Polavarapu, P.L.; De Micheli, C. Enantiopure stereoisomeric homologues of glutamic acid: Chemoenzymatic synthesis and assignment of their absolute configurations. Tetrahedron Asymmetry 2004, 15, 3079–3090. [Google Scholar] [CrossRef]
- Gołębiewski, W.M.; Gucma, M. Enantioselective 1,3-Dipolar Cycloaddition Reactions Using Chiral Lanthanide Catalysts. J. Heterocycl. Chem. 2008, 45, 1687–1693. [Google Scholar] [CrossRef]
- Kleinbeck, F.; Carreira, E.M. Total Synthesis of Bafilomycin A1. Angew. Chem. Int. Ed. 2009, 48, 578–581. [Google Scholar] [CrossRef]
- Singh, V.; Hutait, S.; Yadav, G.P.; Maulik, P.R.; Batra, S. Unusual Retention of Isoxazole Ring under the Influence of 3-(Substituted nitrophenyl)-2-Isoxazoline during Catalytic Hydrogenation of Isoxazoline-Substituted Isoxazole Systems. J. Heterocyclic Chem. 2009, 46, 762–769. [Google Scholar] [CrossRef]
- Rosella, C.E.; Harper, J.B. The effect of ionic liquids on the outcome of nitrile oxide cycloadditions. Tetrahedron Lett. 2009, 50, 992–994. [Google Scholar] [CrossRef]
- Yau, H.M.; Chan, S.J.; George, S.R.D.; Hook, J.M.; Croft, A.K.; Harper, J.B. Ionic Liquids: Just Molten Salts After All? Molecules 2009, 14, 2521–2534. [Google Scholar] [CrossRef]
- Ye, Y.; Ma, L.-B.; Luo, Y.; Wang, W.-H.; Liu, L.-Z.; Zhao, Y.-F. Regioselective Cycloadditions of β-Substituted Vinylphosphonate with Nitrile Oxides. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 135–140. [Google Scholar] [CrossRef]
- Frie, J.L.; Jeffrey, C.S.; Sorensen, E.J. A Hypervalent Iodine-Induced Double Annulation Enables a Concise Synthesis of the Pentacyclic Core Structure of the Cortistatins. Org. Lett. 2009, 11, 5394–5397. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Adachi, Y.; Fujimoto, K.; Furihata, Y.; Tsuchida, T.; Kakehi, A.; Baba, T. Asymmetric 1,3-Dipolar Cycloaddition Reactions of Nitrile Oxides Catalyzed by Chiral Binaphthyldiimine-Ni(II) Complexes. J. Org. Chem. 2009, 74, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Romanski, J.; Chapuis, C.; Jurczak, J. 1,3-Dipolar Cycloadditions of a 2-Oxoethanenitrile Oxide Derived from (2R)-Bornane-10,2-sultam to Electronically Modified 4,4′-Disubstituted Stilbenes. Helv. Chim. Acta 2009, 92, 1056–1069. [Google Scholar] [CrossRef]
- Bujak, P.; Krompiec, S.; Malarz, J.; Krompiec, M.; Filapek, M.; Danikiewicz, W.; Kania, M.; Gębarowska, K.; Grudzka, I. Synthesis of 5-aminoisoxazolines from N-allyl compounds and nitrile oxides via tandem isomerization-1,3-dipolar cycloaddition. Tetrahedron 2010, 66, 5972–5981. [Google Scholar] [CrossRef]
- Zheng, H.; McDonald, R.; Hall, D.G. Boronic Acid Catalysis for Mild and Selective [3+2] Dipolar Cycloadditions to Unsaturated Carboxylic Acids. Chem. Eur. J. 2010, 16, 5454–5460. [Google Scholar] [CrossRef]
- Molteni, G.; Del Buttero, P. Stable nitrile oxide dipolar cycloadditions in pure water. Tetrahedron 2011, 67, 7343–7347. [Google Scholar] [CrossRef]
- Memeo, M.G.; Mantione, D.; Bovio, B.; Quadrelli, P. RuO4-Catalyzed Oxidation Reactions of N-Alkylisoxazolino-2-azanorbornane Derivatives: An Expeditious Route to Tricyclic γ-Lactams. Synthesis 2011, 13, 2165–2174. [Google Scholar]
- Memeo, M.G.; Bovio, B.; Quadrelli, P. RuO4-catalyzed oxidation reactions of isoxazolino-2-azanorbornane derivatives: A short-cut synthesis of tricyclic lactams and peptidomimetic γ-amino acids. Tetrahedron 2011, 67, 1907–1914. [Google Scholar] [CrossRef]
- Pinto, A.; Conti, P.; Grazioso, G.; Tamborini, L.; Madsen, U.; Nielsen, B.; De Micheli, C. Synthesis of new isoxazoline-based acidic amino acids and investigation of their affinity and selectivity profile at ionotropic glutamate receptors. Eur. J. Med. Chem. 2011, 46, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Kiss, L.; Nonn, M.; Forró, E.; Sillanpää, R.; Fülöp, F. Synthesis of novel isoxazoline-fused cispentacin stereoisomers. Tetrahedron Lett. 2009, 50, 2605–2608. [Google Scholar] [CrossRef]
- Nonn, M.; Kiss, L.; Forró, E.; Mucsi, Z.; Fülöp, F. Synthesis of novel isoxazoline-fused cyclic ß-amino esters by regio- and stereoselective 1,3-dipolar cycloaddition. Tetrahedron 2011, 67, 4079–4085. [Google Scholar] [CrossRef]
- Gucma, M.; Gołębiewski, M. Application of chiral ligands: Carbohydrates, alkaloids–lanthanides and other Lewis acid complexes to control regio- and stereoselectivity of the dipolar cycloaddition reactions of nitrile oxides and crotonamides and cinnamides. Catal. Sci. Technol. 2011, 1, 1354–1361. [Google Scholar] [CrossRef]
- Gucma, M.; Gołębiewski, W.M.; Krawczyk, M. Application of chiral ligands: Carbohydrates, nucleoside-lanthanides and other Lewis acid complexes to control regio- and stereoselectivity of the dipolar cycloaddition reactions of nitrile oxides and esters. RSC Adv. 2015, 5, 13112–13124. [Google Scholar] [CrossRef]
- Moggio, Y.; Legnani, L.; Bovio, B.; Memeo, M.G.; Quadrelli, P. Synthesis of novel anthracene derivatives of isoxazolino-carbocyclic nucleoside analogues. Tetrahedron 2012, 68, 1384–1392. [Google Scholar] [CrossRef]
- Yonekawa, M.; Koyama, Y.; Kuwata, S.; Takata, T. Intramolecular 1,3-Dipolar Cycloaddition of Nitrile N-Oxide Accompanied by Dearomatization. Org. Lett. 2012, 14, 1164–1167. [Google Scholar] [CrossRef]
- Vitale, P.; Tacconelli, S.; Perrone, M.G.; Malerba, P.; Simone, L.; Scilimati, A.; Lavecchia, A.; Dovizio, M.; Marcantoni, E.; Bruno, A.; et al. Synthesis, Pharmacological Characterization, and Docking Analysis of a Novel Family of Diarylisoxazoles as Highly Selective Cyclooxygenase-1 (COX-1) Inhibitors. J. Med. Chem. 2013, 56, 4277–4299. [Google Scholar] [CrossRef]
- Jin, J.; Teng, P.; Liu, H.-L.; Wu, J.; Liu, Y.-M.; Xu, Q.; Li, J.-X. Microfluidics assisted synthesis and bioevaluation of sinomenine derivatives as anti-inflammatory agents. Eur. J. Med. Chem. 2013, 62, 280–288. [Google Scholar] [CrossRef]
- Jia, Q.; Benjamin, P.M.S.; Huang, J.; Du, Z.; Zheng, X.; Zhang, K.; Conney, A.H.; Wang, J. Synthesis of 3,4-Disubsituted Isoxazoles via Enamine [3+2] Cycloaddition. Synlett 2013, 24, 79–84. [Google Scholar]
- Gucma, M.; Gołębiewski, W.M. 1,3-Dipolar Cycloaddition Reaction of Nitrile Oxides Revisited—Unusual Side Products Characterized by 2D NMR. J. Heterocycl. Chem. 2014, 51, 572–578. [Google Scholar] [CrossRef]
- Zeng, H.; Zeng, Y.; Geng, L.; Li, X.; Meng, X. Synthesis, characterization, and antioxidant activity of pyrazolic macrocycle. Monatsh. Chem. 2015, 146, 199–206. [Google Scholar] [CrossRef]
- Kara, Y.S. Substituent effect study on experimental 13C NMR chemical shifts of (3-(substituted phenyl)-cis-4,5-dihydroisoxazole-4,5-diyl)-bis(methylene)diacetate derivatives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Vilková, M.; Maľučká, L.U.; Imrich, J. Prediction by 13C NMR of regioselectivity in 1,3-dipolar cycloadditions of acridin-9-yl dipolarophiles. Magn. Reson. Chem. 2016, 54, 8–16. [Google Scholar] [CrossRef]
- Ungvarská Maľučká, L.; Vilková, M.; Kožíšek, J.; Imrich, J. Strong deshielding in aromatic isoxazolines. Magn. Reson. Chem. 2016, 54, 17–27. [Google Scholar] [CrossRef]
- Singh, D.; Devi, N.; Kumar, V.; Malakar, C.C.; Mehra, S.; Rawal, R.K.; Kaitha, B.S.; Singh, V. Metal-free 1,3-dipolar cycloaddition approach towards the regioselective synthesis of β-carboline and isoxazole based molecular hybrids. RSC Adv. 2016, 6, 88066–88076. [Google Scholar] [CrossRef]
- Kesornpun, C.; Aree, T.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Water-Assisted Nitrile Oxide Cycloadditions: Synthesis of Isoxazoles and Stereoselective Syntheses of Isoxazolines and 1,2,4-Oxadiazoles. Angew. Chem. Int. Ed. 2016, 5, 3997–4001. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Liu, M.-C.; Meng, Y.-J.; Xu, L. Two new entries to the ABF tricyclic ring system of 7,17-seco-type C19-diterpenoid alkaloids via free radical cyclization and [3+2] cycloaddition of nitrile oxide. Tetrahedron 2016, 72, 3171–3176. [Google Scholar] [CrossRef]
- Suresh, G.; Nadh, R.V.; Srinivasu, N.; Kaushal, K. Novel coumarin isoxazoline derivatives: Synthesis and study of antibacterial activities. Synth. Commun. 2016, 46, 1972–1980. [Google Scholar] [CrossRef]
- Efimov, I.V.; Shafikov, M.Z.; Beliaev, N.A.; Volkova, N.N.; Beryozkina, T.V.; Dehaen, W.; Fan, Z.; Grishko, V.V.; Lubec, G.; Slepukhin, P.A.; et al. Combined experimental and theoretical studies of regio- and stereoselectivity in reactions of β-isoxazolyl- and β-imidazolyl enamines with nitrile oxides. Beilstein J. Org. Chem. 2016, 12, 2390–2401. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, H.; Wang, B.; Zhang, L.; Xue, L.; Zhao, X. Synthesis and biological evaluation of various new bis-isoxazoline derivatives as potential antioxidant additives. J. Chem. Res. 2016, 40, 558–563. [Google Scholar] [CrossRef]
- Slagbrand, T.; Kervefors, G.; Tinnis, F.; Adolfsson, H. An Efficient One–pot Procedure for the Direct Preparation of 4,5-Dihydroisoxazoles from Amides. Adv. Synth. Catal. 2017, 359, 1990–1995. [Google Scholar] [CrossRef]
- Krompiec, S.; Marcol, B.; Zych, D.; Kurpanik, A.; Danikiewicz, W.; Matussek, M.; Kuźnik, N. Crown Ether Base: Highly Active, Regioselective and Reusable Catalytic Systems for Double Bond Migration in Allylic Compounds. ChemistrySelect 2017, 2, 6717–6727. [Google Scholar] [CrossRef]
- Krompiec, S.; Marcol, B.; Zych, D.; Leszczyńska-Sejda, K.; Benke, G.; Malarz, J. One-Stage Method for Obtaining 3,4,5-Trisubstituted Isoxazolines. Polish Patent PL236066B1, 30 November 2020. [Google Scholar]
- Ou, Z.; Huang, Q.; Kou, Y.-D.; Cheng, F.; Kalita, S.J.; Zhao, Z.-N.; Huang, Y.-Y. 1,3-Dipolar Cycloadditions of β-Fluoroalkyl Vinylsulfones and Nitrile Oxides for Fluoroalkylated Isoxazolines and Isoxazoles. Asian J. Org. Chem. 2019, 8, 2184–2187. [Google Scholar] [CrossRef]
- Liu, X.; Ma, X.; Feng, Y. Introduction of an isoxazoline unit to the β-position of porphyrin via regioselective 1,3-dipolar cycloaddition reaction. Beilstein J. Org. Chem. 2019, 15, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Zatsikha, Y.V.; Didukh, N.O.; Swedin, R.K.; Yakubovskyi, V.P.; Blesener, T.S.; Healy, A.T.; Herbert, D.E.; Blank, D.A.; Nemykin, V.N.; Kovtun, Y.P. Preparation of Viscosity-Sensitive Isoxazoline/Isoxazolyl-Based Molecular Rotors and Directly Linked BODIPY–Fulleroisoxazoline from the Stable meso-(Nitrile Oxide)-Substituted BODIPY. Org. Lett. 2019, 21, 5713–5718. [Google Scholar] [CrossRef]
- Wang, D.-W.; Zhang, R.-B.; Yu, S.-Y.; Liang, L.; Ismail, I.; Li, Y.-H.; Xu, H.; Wen, X.; Xi, Z. Discovery of Novel N-Isoxazolinylphenyltriazinones as Promising Protoporphyrinogen IX Oxidase Inhibitors. J. Agric. Food Chem. 2019, 67, 12382–12392. [Google Scholar] [CrossRef]
- Alshamari, A.; Al-Qudah, M.; Hamadeh, F.; Al-Momani, L.; Abu-Orabi, S. Synthesis, Antimicrobial and Antioxidant Activities of 2-Isoxazoline Derivatives. Molecules 2020, 25, 4271. [Google Scholar] [CrossRef]
- Herrero, M.A.; Kremmer, J.M.; Kappe, C.O. Nonthermal Microwave Effects Revisited: On the Importance of Internal Temperature Monitoring ans Agitation in Mcrowave Chemistry. J. Org. Chem. 2008, 73, 36–47. [Google Scholar] [CrossRef]
- Tang, Z.; Peng, Y.; Liu, F. Design and synthesis of novel quinoline derivatives bearing oxadiazole, isoxazoline, triazolothiadiazole, triazolothiadiazine, and piperazine moieties. J. Heterocycl. Chem. 2020, 57, 2330–2338. [Google Scholar] [CrossRef]
- Pultar, F.; Hansen, M.E.; Wolfrum, S.; Böselt, L.; Fróis-Martins, R.; Bloch, S.; Kravina, A.G.; Pehlivanoglu, D.; Schäffer, C.; LeibundGut-Landmann, S.; et al. Mutanobactin D from the Human Microbiome: Total Synthesis, Configurational Assignment, and Biological Evaluation. J. Am. Chem. Soc. 2021, 143, 10389–10402. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.-K.; Yin, Z.-C.; Chen, J.-S.; Wang, G.-W. [3 + 2] Cycloaddition reactions of nitrile oxides generated in situ from aldoximes with alkenes and alkynes under ball-milling conditions. Green Chem. Lett. Rev. 2022, 15, 519–538. [Google Scholar] [CrossRef]
- Krompiec, S.; Bujak, P.; Szczepankiewicz, W. Convenient synthesis of isoxazolines via tandem isomerization of allylcompounds to vinylic derivatives and 1,3-dipolar cycloaddition of nitrileoxides to the vinylic compounds. Tetrahedron Lett. 2008, 49, 6071–6074. [Google Scholar] [CrossRef]
- Krompiec, S.; Bujak, P.; Malarz, J.; Krompiec, M.; Skórka, Ł.; Pluta, T.; Danikiewicz, W.; Kania, M.; Kusz, J. An isomerization-1,3-dipolar cycloaddition tandem reaction towards the synthesis of 3-aryl-4-methyl-5-O-substituted isoxazolines from O-allyl compounds. Tetrahedron 2012, 68, 6018–6031. [Google Scholar] [CrossRef]
- Krompiec, S.; Filapek, M.; Grudzka-Flak, I.; Słodek, A.; Kula, S.; Malecki, J.G.; Malarz, J.; Szafraniec-Gorol, G.; Penkala, M.; Schab-Balcerzak, E.; et al. Multifaceted strategy for the synthesis of diverse 2,2′-bithiophene derivative. Molecules 2015, 20, 4565–4593. [Google Scholar] [CrossRef]
- Krompiec, S.; Malarz, J.; Pietraszuk, C.; Powała, B.; Rogalski, S.; Filapek, M.; Marcol, B.; Penkala, M.; Kowalska, E.; Polański, J.; et al. Trisubstituted Isoxazolines and a Process for Their Preparation. Polish Patent PL224926B1, 28 February 2017. [Google Scholar]
- Krompiec, S.; Malarz, J.; Pietraszuk, C.; Powała, B.; Rogalski, S.; Filapek, M.; Marcol, B.; Penkala, M.; Kowalska, E.; Polański, J.; et al. Trisubstituted Isoxazolines at Positions 3, 4 and 5 and Their Preparation. Polish Patent PL224381B1, 30 December 2016. [Google Scholar]
- Krompiec, S.; Malarz, J.; Pietraszuk, C.; Powała, B.; Rogalski, S.; Filapek, M.; Marcol, B.; Penkala, M.; Kowalska, E.; Polański, J.; et al. 3,4,5-Tri-Substituted Isoxazolines and a Method for Their Preparation. Polish Patent PL224382B1, 30 December 2016. [Google Scholar]
- Krompiec, S.; Malarz, J.; Filapek, M.; Paluch, M.; Pawlus, S.; Pietraszuk, C.; Powała, B.; Rogalski, S.; Marcol, B.; Penkala, M.; et al. Method for Preparing 3,4,5-Trisubstituted Isoxazolines. Polish Patent PL232036B1, 31 May 2019. [Google Scholar]
- Matsumoto, K.; Hamana, H.; Iida, H. Compendium of Cycloaddition Reactions under High Pressure. Helv. Chim. Acta 2005, 88, 2033–2234. [Google Scholar] [CrossRef]
- Margetic, D. High Pressure Organic Synthesis; De Gruyter: Berlin, Germany, 2019. [Google Scholar]
- Mičúch, P.; Fišera, L.; Cyrański, M.K.; Krygowski, T.M.; Krajčík, J. Reversal of Diastereoselectivity of Nitrile Oxide 1,3-Dipolar Cycloadditions by Mg(II). Acceleration of Cycloaddition by Microwave Irradiation. Tetrahedron 2000, 56, 5465–5472. [Google Scholar] [CrossRef]
- Park, K.-H.; Kurth, M.J. Diastereoselective Synthesis of Hydantoin- and Isoxazoline-Substituted Dispirocyclobutanoids. J. Org. Chem. 2000, 65, 3520–3524. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Button, M.A.C.; Denisenko, S.N. Efficient Synthesis of 3,5-Functionalized Isoxazoles and Isoxazolines via 1,3-Dipolar Cycloaddition Reactions of 1-Propargyl- and 1-Allylbenzotriazoles. J. Heterocycl. Chem. 2000, 37, 1505–1510. [Google Scholar] [CrossRef]
- Di Nunno, L.; Scilimati, A.; Vitale, P. 5-Hydroxy-3-phenyl-5-vinyl-2-isoxazoline and 3-phenyl-5-vinylisoxazole: Synthesis and reactivity. Tetrahedron 2005, 61, 11270–11278. [Google Scholar] [CrossRef]
- Feddouli, A.; Ait Itto, M.Y.; Ait Ali, M.; Hasnaoui, A. Efficient approach for the synthesis of novel functionalized isoxazolines from limonene. Synth. Commun. 2006, 36, 3617–3624. [Google Scholar] [CrossRef]
- Brinkmann, Y.; Madhushaw, R.J.; Jazzar, R.; Bernardinelli, G.; Kündig, E.P. Chiral ruthenium Lewis acid-catalyzed nitrile oxide cycloadditions. Tetrahedron 2007, 63, 8413–8419. [Google Scholar] [CrossRef]
- Shao, P.P.; Ye, F.; Weber, A.E.; Li, X.; Lyons, K.A.; Parsons, W.H.; Garcia, M.L.; Priest, B.T.; Smith, M.M.; Felix, J.P.; et al. Discovery of a novel class of isoxazoline voltage gated sodium channel blockers. Bioorg. Med. Chem. Lett. 2009, 19, 5329–5333. [Google Scholar] [CrossRef]
- Benltifa, M.; Hayes, J.M.; Vidal, S.; Gueyrard, D.; Goekjian, P.G.; Praly, J.-P.; Kizilis, G.; Tiraidis, C.; Alexacou, K.-M.; Chrysina, E.D.; et al. Glucose-based Spiro-isoxazolines: A New Family of Potent Glycogen Phosphorylase Inhibitors. Bioorg. Med. Chem. 2009, 17, 7368–7380. [Google Scholar] [CrossRef]
- Benltifa, M.; Vidal, S.; Gueyrard, D.; Goekjian, P.G.; Msaddek, M.; Pralya, J.-P. 1,3-Dipolar cycloaddition reactions on carbohydrate-based templates: Synthesis of spiro-isoxazolines and 1,2,4-oxadiazoles as glycogen phosphorylase inhibitors. Tetrahedron Lett. 2006, 47, 6143–6147. [Google Scholar] [CrossRef]
- Akama, T.; Balko, T.W.; Defauw, J.M.; Plattner, J.J.; White, W.H.; Winkle, J.R.; Zhang, Y.-K.; Zhou, Y. Boron-Containing Small Molecules. U.S. Patent US20130131017A1, 23 May 2013. [Google Scholar]
- Zhang, Y.-K.; Plattner, J.J.; Easom, E.E.; Akama, T.; Zhou, Y.; White, W.H.; Defauw, J.N.; Winkle, J.R.; Balko, T.W.; Cao, J.; et al. Optimization of isoxazoline amide benzoxaboroles for identification of a development candidate as an oral long acting animal ectoparasiticide. Bioorg. Med. Chem. Lett. 2016, 26, 3182–3186. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wagerle, T.; Long, J.K.; Lahm, G.P.; Barry, J.D.; Smith, R.M. Insecticidal quinoline and isoquinoline isoxazolines. Bioorg. Med. Chem. Lett. 2014, 24, 4026–4030. [Google Scholar] [CrossRef] [PubMed]
- Salomone, A.; Scilimati, A.; Vitale, P. 3-Aryl-5-vinyl-2-isoxazolines and 3-Aryl-5-vinylisoxazoles from Aryl Nitrile Oxides and Methyl Vinyl Ketone Lithium Enolate: Reaction Limits and Synthetic Utility Exploitation. Synthesis 2015, 47, 807–816. [Google Scholar]
- Goyard, D.; Kónya, B.; Chajistamatiou, A.S.; Chrysina, E.D.; Leroy, J.; Balzarin, S.; Tournier, M.; Tousch, D.; Petit, P.; Duret, C.; et al. Glucose-derived spiro-isoxazolines are anti-hyperglycemic agents against type 2 diabetes through glycogen phosphorylase inhibition. Eur. J. Med. Chem. 2016, 108, 444–454. [Google Scholar] [CrossRef]
- Yang, C.; Le Hir de Fallois, L.P.; Meng, C.Q.; Long, A.; Gorter de Vries, R.J.; Baillon, B.; Lafont, S.; Gay de Saint Michel, M.; Kozlovic, S. Process for the Preparation of Isoxazoline Compounds. U.S. Patent US20170311601A1, 2 November 2017. [Google Scholar]
- Yang, C.; Le Hir de Fallois, L.P.; Meng, C.Q.; Long, A.; Gorter de Vries, R.J.; Baillon, B.; Lafont, S.; Gay de Saint Michel, M.; Kozlovic, S. Process for the Preparation of Enantiomerically Enriched Isoxazoline Compounds—Crystalline Toluene Solvate of (s)-Afoxolaner. International Patent WO2017176948A1, 12 October 2017. [Google Scholar]
- Abdelli, A.; Gharsa, H.; Jmaï, M.; Gaucher, A.; Efrit, M.L.; M’rabet, H.; Prim, D. Versatile approach to densely substituted isoxazolines and pyrazolines: Focus on a quaternary carbon centre as a constitutive feature. Tetrahedron Lett. 2020, 61, 151958. [Google Scholar] [CrossRef]
- Hu, Y.; Shi, W.; Zheng, B.; Liao, J.; Wang, W.; Wu, Y.; Guo, H. Organocatalytic Asymmetric C(sp2)–H Allylic Alkylation: Enantioselective Synthesis of Tetrasubstituted Allenoates. Angew. Chem. Int. Ed. 2020, 59, 19820–19824. [Google Scholar] [CrossRef] [PubMed]
- Lijuan, L.; Chang, G.; Shang Zhiqiang, S.; Lijuan, L.; Chang, G.; Zhiqiang, S. Preparation Method of Phosphonyl-Containing Isoxazole. Chinese Patent CN111440214A, 24 July 2020. [Google Scholar]
- Peters, O.; Haaf, K.B.; Bojack, G.; Law, K.R.; Machettira, A.B.; Dietrich, H.; Gatzweiler, E.; Rosinger, C.H.; Asmus, E. Herbicidally Active 3-Phenylisoxazoline-5-Carboxamides of Tetrahydro—And Dihydrofurancarboxamides. U.S. Patent US20200216403A1, 9 July 2020. [Google Scholar]
- Peters, O.; Haaf, K.B.; Lindell, S.D.; Bojack, G.; Law, K.R.; Machettira, A.B.; Dietrich, H.; Gatzweiler, E.; Rosinger, C.H. Herbicidally Active 3-Phenylisoxazoline-5-Carboxamides of Tetrahydro and Dihydrofuran Carboxylic Acids and Esters. U.S. Patent US20210292312A1, 23 September 2021. [Google Scholar]
- Kakuuchi, A.; Umemura, S.; Asada, M.; Ruvinsky, A.; Zhang, Y.; Takahashi, H.; Krilov, G.; Inoyama, D.; Konze, K.; Svensson, M. 3-Azabicyclo(3.1.0)Hexane Derivatives Having kdm5 Inhibitory Activity and Use Thereof. International Patent WO2021/223699A1, 11 November 2021. [Google Scholar]
- Oubella, A.; Byadi, S.; Bimoussa, A.; Fawzi, M.; Auhmani, A.; Podlipnik, C.; Morjani, H.; Riahi, A.; Robert, A.; Itto, M.Y.A. Novel isoxazoline-linked 1,3,4-thiadiazole hybrids as anticancer agents: Design, synthesis, biological evaluation, molecular docking, and molecular dynamics simulation. Arch. Pharm. 2022, 355, 2200066. [Google Scholar] [CrossRef]
- Dhage, Y.D.; Pabba, J.; Badgujar, S.A.; Raju, J.R.; Saxena, R.; Purohit, H.Y.; Saragur, R.S.; Klausener, A.G.M. Isoxazoline Compounds and Their Use as Pest Control Agents. International Patent WO2022/123502A1, 16 June 2022. [Google Scholar]
- Bakthadoss, M.; Vinayagam, V. A novel protocol for the facile construction of tetrahydroquinoline fused tricyclic frameworks via an intramolecular 1,3-dipolar nitrile oxide cycloaddition reaction. Org. Biomol. Chem. 2015, 13, 10007–10014. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; De Amici, M.; Grazioso, G.; Roda, G.; Negra, F.F.B.; Nielsen, B.; Stensbøl, T.B.; Madsen, U.; Brauner-Osborne, H.; Frydenvang, K.; et al. Design, Synthesis, and Pharmacological Characterization of Novel, Potent NMDA Receptor Antagonists. J. Med. Chem. 2004, 47, 6740–6748. [Google Scholar] [CrossRef] [PubMed]
- Sibi, M.P.; Ma, Z.; Itoh, K.; Prabagaran, N.; Jasperse, C.P. Enantioselective Cycloadditions with α,β-Disubstituted Acrylimides. Org. Lett. 2005, 7, 2349–2352. [Google Scholar] [CrossRef]
- Krompiec, S.; Penczek, R.; Bujak, B.; Kubik, E.; Malarz, J.; Penkala, M.; Krompiec, M.; Kuźnik, N.; Maciejewski, H. A new method for the synthesis of mixed orthoesters from O-allyl acetals. Tetrahedron Lett. 2009, 50, 1193–1195. [Google Scholar] [CrossRef]
- Kissane, M.; Lawrence, S.E.; Maguire, A.R. 1,3-Dipolar cycloadditions of 2-thio-3-chloroacrylamides with nitrile oxides and nitrones. Tetrahedron 2010, 66, 4564–4572. [Google Scholar] [CrossRef]
- Gucma, M.; Gołębiewski, M.W.; Michalczyk, A.K. NMR studies on [2+3] cycloaddition of nitrile oxides to cyclohexene derivatives. J. Mol. Struct. 2014, 1060, 223–232. [Google Scholar] [CrossRef]
- Lian, X.; Guo, S.; Wang, G.; Lin, L.; Liu, X.; Feng, X. Asymmetric Synthesis of Spiro[isoxazolin-3,3′-oxindoles] via the Catalytic 1,3-Dipolar Cycloaddition Reaction of Nitrile Oxides. J. Org. Chem. 2014, 79, 7703–7710. [Google Scholar] [CrossRef]
- Bartlett, S.L.; Sohtome, Y.; Hashizume, D.; White, P.S.; Sawamura, M.; Johnson, J.S.; Sodeoka, M. Catalytic Enantioselective [3 + 2] Cycloaddition of α-Keto Ester Enolates and Nitrile Oxides. J. Am. Chem. Soc. 2017, 139, 8661–8666. [Google Scholar] [CrossRef]
- Roscales, S.; Plumet, J. Metal-catalyzed 1,3-dipolar cycloaddition reactions of nitrile oxides. Org. Biomol. Chem. 2018, 16, 8446–8461. [Google Scholar] [CrossRef] [PubMed]
- Kanemasa, S.; Nishiuchi, M.; Wada, E. Regiocontrol of nitrile oxide cycloadditions to allyl alcohols. Synthesis of 4-substituted and 4,4-disubstituted 5-hydroxymethyl-2-isoxazolines. Tetrahedron Lett. 1992, 33, 1357–1360. [Google Scholar] [CrossRef]
- Gucma, M.; Gołębiewski, M.W.; Żelechowski, K.; Krawczyk, M. Studies on the [2+3] cycloaddition reaction of nitrile oxides to abietic acid esters. J. Serb. Chem. Soc. 2019, 84, 1073–1081. [Google Scholar] [CrossRef]
- Liu, Z.; Han, M.; Yan, X.; Cheng, W.; Tang, Z.; Cui, L.; Yang, R.; Guo, Y. Design, Synthesis, and Biological Evaluation of Novel Osthole-Based Isoxazoline Derivatives as Insecticide Candidates. J. Agric. Food Chem. 2022, 70, 7921–7928. [Google Scholar] [CrossRef] [PubMed]
- Baumann, E.; Deyn, W.; Kudis, S.; Langemann, K.; Mayer, G.; Misslitz, U.; Neidlein, U.; Walter, H.; Zagar, C.; Witschel, M. 3-(4,5-Dihydroisoxaxol-5-yl)Benzoylcyclohexenones and the Use Thereof as Herbicides. U. S. Patent US20030199699A1, 23 October 2003. [Google Scholar]
- Kuhn, B.; Willms, L.; Frenzel, T.; Haaf, K.B.; Lindell, S.D.; Dietrich, H.; Schmutzler, D.; Gatzweiler, E.; Rosinger, C.H. 3-Phenylisoxazolin Derivatives with Herbicidal Action. U. S. Patent US20150245615A1, 3 September 2015. [Google Scholar]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the Mysteries of the [3 + 2] Cycloaddition Reactions. J. Eur. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Hashimoto, T.; Maruoka, K. Recent Advances of Catalytic Asymmetric 1,3-Dipolar Cycloadditions. Chem. Rev. 2015, 115, 5366–5412. [Google Scholar] [CrossRef]
- Fukuda, S.; Kamimura, A.; Kanemasa, S.; Horia, K. An Ab initio Molecular Orbital Study on the Magnesium Controlled 1,3-Cycloaddition of Nitrile Oxides and Allylic Alcohols with Regio- and Stereoselectivity. Tetrahedron 2000, 56, 1637–1647. [Google Scholar] [CrossRef]
- Toma, L.; Quadrelli, P.; Perrini, G.; Gandolfi, R.; Di Valentin, C.; Corsaro, A.; Caramella, P. Cycloadditions of Nitrile Oxides to α,ß-Unsaturated Aldehydes. Frontier Orbital Interactions and Secondary Orbital Interactions at Work in Determining Regiochemistry. Tetrahedron 2000, 56, 4299–4309. [Google Scholar] [CrossRef]
- Prakesch, M.; Grÿe, D.; Grÿe, R.; Carter, J.; Washington, I.; Houk, K.N. Stereoselectivity of Nitrile Oxide Cycloadditions to Chiral Allylic Fluorides: Experiment and Theory. Chem. Eur. J. 2003, 9, 5664–5672. [Google Scholar] [CrossRef]
- Luft, J.A.R.; Meleson, K.; Houk, K.N. Transition Structures of Diastereoselective 1,3-Dipolar Cycloadditions of Nitrile Oxides to Chiral Homoallylic Alcohols. Org. Lett. 2007, 9, 555–558. [Google Scholar] [CrossRef]
- Kobychev, V.B.; Pradedova, A.G.; Trofimov, B.A. A one-pot assembly of Δ2-isoxazolines from ketones, aryl acetylenes and hydroxylamine: Revisiting the mechanism in terms of quantum chemistry. J. Mol. Struct. 2021, 1246, 131185. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Ríos-Gutiérrez, M.; Kula, K.; Woliński, P.; Mirosław, B.; Krawczyk, T.; Jasiński, R. The Participation of 3,3,3-Trichloro-1-nitroprop-1-ene in the [3 + 2] Cycloaddition Reaction with Selected Nitrile N-Oxides in the Light of the Experimental and MEDT Quantum Chemical Study. Molecules 2021, 26, 6774. [Google Scholar] [CrossRef]
- Beutick, S.E.; Vermeeren, P.; Hamlin, T.A. The 1,3-Dipolar Cycloaddition: From Conception to Quantum Chemical Design. Chem. Asian J. 2022, 17, e202200553. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, C.; Dean, J.M. Nitrile Oxides. V. Stable Aromatic Nitrile Oxides. J. Org. Chem. 1965, 30, 2809–2812. [Google Scholar] [CrossRef]
- Krivovicheva, V.; Bubyrev, A.; Kalinin, S.; Dar’in, D.; Krasavin, M. Synthetic Exploration of Novel Sulfamoyl Cyanide N-Oxides in Heterocycle Synthesis. Eur. J. Org. Chem. 2022, 2022, e202201162. [Google Scholar] [CrossRef]
- Bhandari, S.V.; Parikh, J.K.; Bothara, K.G.; Chitre, T.S.; Lokwani, D.K.; Devale, T.L. Design, synthesis, and evaluation of anti-inflammatory, analgesic, ulcerogenicity, and nitric oxide releasing studies of novel indomethacin analogs as non-ulcerogenic derivatives. J. Enzym. Inhib. Med. Chem. 2010, 25, 520–530. [Google Scholar] [CrossRef]
- Larin, A.A.; Bystrov, D.M.; Fershtat, L.L.; Konnov, A.A.; Makhova, N.N.; Monogarov, K.A.; Meerov, D.B.; Melnikov, I.N.; Pivkina, A.N.; Kiselev, V.G.; et al. Nitro-, Cyano-, and Methylfuroxans, and Their Bis-Derivatives: From Green Primary to Melt-Cast Explosives. Molecules 2020, 25, 5836. [Google Scholar] [CrossRef]
- Belen’kii, L.I. Nitrile Oxides. In Nitrile Oxides, Nitrones, and Nitronates in Organic Synthesis: Novel Strategies in Synthesis, 2nd ed.; Feuer, H., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–128. [Google Scholar]
- Feuer, H. (Ed.) Nitrile Oxides, Nitrones, and Nitronates in Organic Synthesis: Novel Strategies in Synthesis, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kumar, G.; Shankar, R. 2–Isoxazolines: A Synthetic and Medicinal Overview. ChemMedChem 2021, 16, 430–447. [Google Scholar] [CrossRef]
- Maestri, G.; Cañeque, T.; Della Ca’, N.; Derat, E.; Catellani, M.; Chiusoli, G.P.; Malacria, M. Pd Catalysis in Cyanide–Free Synthesis of Nitriles from Haloarenes via Isoxazolines. Org. Lett. 2016, 18, 6108–6111. [Google Scholar] [CrossRef]
- Ushakov, P.Y.; Khatuntseva, E.A.; Nelyubina, Y.V.; Tabolin, A.A.; Ioffe, S.L.; Sukhorukov, A.Y. Synthesis of Isoxazolines from Nitroalkanes via a [4+1]–Annulation Strategy. Adv. Synth. Catal. 2019, 361, 5322–5327. [Google Scholar] [CrossRef]
- Mitcheltree, M.J.; Stevenson, J.W.; Pisipati, A.; Myers, A.G. A Practical, Component–Based Synthetic Route to Methylthiolincosamine Permitting Facile Northern–Half Diversification of Lincosamide Antibiotics. J. Am. Chem. Soc. 2021, 143, 6829–6835. [Google Scholar] [CrossRef]
- Pan, X.; Xin, X.; Mao, Y.; Li, X.; Zhao, Y.; Liu, Y.; Zhang, K.; Yang, X.; Wang, J. 3–Benzoylisoxazolines by 1,3–Dipolar Cycloaddition: Chloramine–T–Catalyzed Condensation α-Nitroketones with Dipolarophiles. Molecules 2021, 26, 3491. [Google Scholar] [CrossRef] [PubMed]
- Trogu, E.; De Sarlo, F.; Machetti, F. Michael Additions versus Cycloaddition Condensations with Ethyl Nitroacetate and electron-Deficient Olefins. Chem. Eur. J. 2009, 15, 7940–7948. [Google Scholar] [CrossRef] [PubMed]
- Antonova, Y.A.; Nelyubina, Y.V.; Ioffe, S.L.; Sukhorukov, A.Y.; Tabolina, A.A. Ring Closure of Nitroalkylmalonates for the Synthesis of Isoxazolines under the Acylation Conditions. Adv. Synth. Catal. 2022, 364, 2606–2612. [Google Scholar] [CrossRef]
- Fawzi, M.; Oubella, A.; Bimoussa, A.; Bamou, F.Z.; Khdar, Z.A.; Auhmani, A.; Riahi, A.; Robert, A.; Morjani, H.; Ait Itto, M.Y. Design, synthesis, evaluation of new 3–acetylisoxazolines and their hybrid analogous as anticancer agents: In vitro and in silico analysis. Comput. Biol. Chem 2022, 98, 107666. [Google Scholar] [CrossRef] [PubMed]
- Sedenkova, K.N.; Andriasov, K.S.; Eremenko, M.G.; Grishin, Y.K.; Alferova, V.A.; Baranova, A.A.; Zefirov, N.A.; Zefirova, O.N.; Zarubaev, V.V.; Gracheva, Y.A.; et al. Bicyclic Isoxazoline Derivatives: Synthesis and Evaluation of Biological Activity. Molecules 2022, 27, 3546. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Geng, C.; Jiao, P. Silyl Nitronate Cycloadditions Catalyzed by Cu(II)-Bisoxazoline. J. Org. Chem. 2015, 80, 10992–11002. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Dong, L.; Geng, C.; Jiao, P. Catalytic Asymmetric Synthesis of Isoxazolines from Silyl Nitronates. Org. Lett. 2015, 17, 3194–3197. [Google Scholar] [CrossRef]
- Jiang, M.; Feng, L.; Feng, J.; Jiao, P. Catalytic Asymmetric Cycloadditions of Silyl Nitronates Bearing αAryl Group. Org. Lett. 2017, 19, 2210–2213. [Google Scholar] [CrossRef]
- Ma, L.; Kou, L.; Jin, F.; Cheng, X.; Tao, S.; Jiang, G.; Bao, X.; Wan, X. Acyclic nitronate olefin cycloaddition (ANOC): Regio–and stereospecific synthesis of isoxazolines. Chem. Sci. 2021, 12, 774–779. [Google Scholar] [CrossRef]
- Ma, L.; Jin, F.; Cheng, X.; Tao, S.; Jiang, G.; Li, X.; Yang, J.; Bao, X.; Wan, X. [2 + 2 + 1] Cycloaddition of N–tosylhydrazones, tert–butyl nitrite and alkenes: General and practical access to isoxazolines. Chem. Sci. 2021, 12, 9823–9830. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Asano, Y. A Cyanide–free Biocatalytic Process for Synthesis of Complementary Enantiomers of 4-Chloro-3-hydroxybutanenitrile From Allyl Chloride. ChemCatChem 2021, 13, 4237–4242. [Google Scholar] [CrossRef]
- Kacka-Zych, A.; Jasinski, R. Understanding the molecular mechanism of the stereoselective conversion of N–trialkylsilyloxy nitronates into bicyclic isoxazoline derivatives. New J. Chem. 2021, 45, 9491–9500. [Google Scholar] [CrossRef]
- Lee, H.-J.; Eun, B.; Sung, E.; Hwang, G.T.; Kob, Y.K.; Cho, C.-W. Catalytic enantioselective synthesis of carboxy–substituted 2–isoxazolines by cascade oxa–Michael–cyclization. Org. Biomol. Chem. 2018, 16, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Lv, M.; Xu, H. Synthesis of Piperine Analogs Containing Isoxazoline/Pyrazoline Scaffold and Their Pesticidal Bioactivities. J. Agric. Food Chem. 2018, 66, 11254–11264. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Ngaini, Z. Synthesis of Benzalacetophenone–based Isoxazoline and Isoxazole Derivatives. Curr. Org Chem. 2022, 26, 679–692. [Google Scholar] [CrossRef]
- Liao, J.; Ouyang, L.; Jin, Q.; Zhang, J.; Luo, R. Recent advances in the oxime–participating synthesis of isoxazolines. Org. Biomol. Chem. 2020, 18, 4709–4716. [Google Scholar] [CrossRef]
- Tang, S.; He, J.; Sun, Y.; He, L.; She, X. Efficient and Regioselective Synthesis of 5–Hydroxy–2–isoxazolines: Versatile Synthons for Isoxazoles, β–Lactams, and γ–Amino Alcohols. J. Org. Chem. 2010, 75, 1961–1966. [Google Scholar] [CrossRef]
- Pohjakallio, A.; Pihko, P.M.; Liu, J. Base–Catalyzed Isomerization of 2–Isoxazolines Enables a Two–Step Enantioselective Synthesis of β–Hydroxynitriles from Enals. J. Org. Chem. 2010, 75, 6712–6715. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.Y.; Tatarinova, I.V.; Ivanova, E.V.; Zorina, N.V.; Ushakov, I.A.; Trofimov, B.A. A One–Pot Approach to Δ2–Isoxazolines from Ketones and Arylacetylenes. Org. Lett. 2013, 15, 104–107. [Google Scholar] [CrossRef]
- Tripathi, C.B.; Mukherjee, S. Catalytic Enantioselective Iodoetherification of Oximes. Angew. Chem. Int. Ed. 2013, 52, 8450–8453. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, C.B.; Mukherjee, S. Catalytic Enantioselective 1,4–Iodofunctionalizations of Conjugated Dienes. Org. Lett. 2015, 17, 4424–4427. [Google Scholar] [CrossRef] [PubMed]
- Triandafillidi, I.; Kokotos, C.G. Green Organocatalytic Synthesis of Isoxazolines via a One–Pot Oxidation of Allyloximes. Org. Lett. 2017, 19, 106–109. [Google Scholar] [CrossRef]
- Drosik, O.L.; Suwiński, J. The Formation of 2-Isoxazolines in the Reactions of α,ß-unsaturated carbonyl compounds with hydroxylamine and its derivatives—A critical review. Curr. Org. Chem. 2018, 22, 345–361. [Google Scholar] [CrossRef]
- Rouno, T.; Niwa, T.; Nishibashi, K.; Yamamoto, N.; Egami, H.; Hamashima, Y. Enantioselective 5–exo–Fluorocyclization of Ene–Oximes. Molecules 2019, 24, 3464. [Google Scholar] [CrossRef]
- Li, X.-T.; Lv, L.; Wang, T.; Gu, Q.-S.; Xu, G.-X.; Li, Z.-L.; Ye, L.; Zhang, X.; Cheng, G.-J.; Liu, X.-Y. Diastereo– and Enantioselective Catalytic Radical Oxysulfonylation of Alkenes in β,γ–Unsaturated Ketoximes. Chem 2020, 6, 1692–1706. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Zhao, W.; Wang, G. Copper–Catalyzed Oxydifluoroalkylation of β,γ–Unsaturated Oximes for the Construction of Isoxazolines with a Difluoroalkyl Sidechain. Synlett 2021, 32, 1029–1033. [Google Scholar] [CrossRef]
- Wu, X.-B.; Gao, Q.; Fan, J.-J.; Zhao, Z.-Y.; Tu, X.-Q.; Cao, H.-Q.; Yu, J. Anionic Chiral Co(III) Complexes Mediated Asymmetric Halocyclization—Synthesis of 5–Halomethyl Pyrazolines and Isoxazolines. Org. Lett. 2021, 23, 9134–9139. [Google Scholar] [CrossRef]
- Mondal, S.; Biswas, S.; Ghosh, K.G.; Sureshkumar, D. TEMPO–Mediated Selective Synthesis of Isoxazolines, 5–Hydroxy–2–isoxazolines, and Isoxazoles via Aliphatic δ–C(sp3)–H Bond Oxidation of Oximes. Chem. Asian J. 2021, 16, 2439–2446. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Gangani, A.J.; Panja, A. Synthesis of 5-Vinyl-2-isoxazolines by Palladium-Catalyzed Intramolecular O-Allylation of Ketoximes. Org. Lett. 2021, 23, 6227–6231. [Google Scholar] [CrossRef]
- Hu, F.; Zhang, H.; Chu, Y.; Hui, X.-P. Efficient enantioselective synthesis of pyrazolines and isoxazolines enabled by an iridium–catalyzed intramolecular allylic substitution reaction. Org. Chem. Front. 2022, 9, 2734–2738. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Si, Y.-F.; Qiao, L.-P.; Zhao, Y.-F.; Chen, X.-L.; Yu, B. Visible Light–Promoted Recyclable Carbon Nitride–Catalyzed Dioxygenation of β,γ–Unsaturated Oximes. Adv. Synth. Catal. 2022, 364, 574–580. [Google Scholar] [CrossRef]
- Holman, S.D.L.; Wills, A.G.; Fazakerley, N.J.; Poole, D.L.; Coe, D.M.; Berlouis, L.A.; Reid, M. Electrochemical Synthesis of Isoxazolines: Method and Mechanism. Chem. Eur. J. 2022, 28, e2021037. [Google Scholar] [CrossRef] [PubMed]
- Qazi, N.A.; Sampath Kumar, H.M.; Taneja, S.C. Domino addition of allylzinc bromide to nitrile oxides: Synthesis of 5–butenylisoxazolines. Tet. Lett. 2005, 46, 4391–4393. [Google Scholar] [CrossRef]
- Qazi, N.A.; Singh, P.P.; Jan, S.; Sampath Kumar, H.M. Anionic Domino C–O–Heterocyclization Approach for the Synthesis of 5–Vinyl Isoxazolines. Synlett 2007, 9, 1449–1451. [Google Scholar] [CrossRef]
- Li, X.-T.; Gu, Q.-S.; Dong, X.-Y.; Meng, X.; Liu, X.-Y. A Copper Catalyst with a Cinchona–Alkaloid–Based Sulfonamide Ligand for Asymmetric Radical Oxytrifluoromethylation of Alkenyl Oximes. Angew. Chem. Int. Ed. 2018, 57, 7668–7672. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; Wang, Y.; Li, W.; Chen, M.; Zhang, J. Enantioselective Synthesis of Isoxazolines Enabled by Palladium–Catalyzed Carboetherification of Alkenyl Oximes. Angew. Chem. Int. Ed. 2020, 59, 4421–4427. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, Y.; Fang, S.; Long, W.; Sun, H.; Wan, X. Coupling Reaction of Cu–Based Carbene and Nitroso Radical: A Tandem Reaction To Construct Isoxazolines. Org. Lett. 2017, 19, 5896–5899. [Google Scholar] [CrossRef]
- Xiong, M.; Liang, X.; Gao, Z.; Lei, A.; Pan, Y. Synthesis of Isoxazolines and Oxazines by Electrochemical Intermolecular [2 + 1 + n] Annulation: Diazo Compounds Act as Radical Acceptors. Org. Lett. 2019, 21, 9300–9305. [Google Scholar] [CrossRef]
- Escudero, J.; Bellosta, V.; Cossy, J. Synthesis of Isoxazolidines, Isoxazolines and Isoxazoles from O–Unsaturated Alkoxyamines. Lett. Org. Chem. 2018, 15, 365–374. [Google Scholar] [CrossRef]
- Das, P.; Hasan, M.H.; Mitra, D.; Bollavarapu, R.; Valente, E.J.; Tandon, R.; Raucher, D.; Hamme, A.T. Design, Synthesis, and Preliminary Studies of Spiro–isoxazoline–peroxides against Human Cytomegalovirus and Glioblastoma. J. Org. Chem. 2019, 84, 6992–7006. [Google Scholar] [CrossRef] [PubMed]
- Fuller, A.A.; Chen, B.; Minter, A.R.; Mapp, A.K. Succinct Synthesis of β-Amino Acids via Chiral Isoxazolines. J. Am. Chem. Soc. 2005, 127, 5376–5383. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, Y. Reduction of Δ2-Isoxazolines to β-Hydroxy Ketones with Iron and Ammonium Chloride as Reducing Agent. J. Org. Chem. 2008, 73, 9181–9183. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.W.; Carreira, E.M. A Mild and Chemoselective Method for the Reduction of Conjugated Isoxazolines to β-Hydroxy Ketones. Org. Lett. 2001, 3, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Karpavičiene, I.; Lapinskaite, R.; Brukštus, A.; Čikotiene, I. Reductive Ring Cleavage of Nonconjugated Δ2-Isoxazolines to β-Hydroxy Ketones with Aluminum and Copper(II) Chloride. Synlett 2012, 23, 381–384. [Google Scholar] [CrossRef]
- Feng, J.; Li, T.; Zhang, J.; Jiao, P. Application of chiral 2-isoxazoline for the synthesis of syn-1,3-diol analogs. Beilstein J. Org. Chem. 2019, 15, 1840–1847. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, Y. One-Pot Synthesis of Furans from Isoxazolines with Iron as Reducing Agent. Lett. Org. Chem. 2010, 7, 661–665. [Google Scholar] [CrossRef]
- Pinho e Melo, T.M.V.D. 4-Isoxazolines: Scaffolds for Organic Synthesis. Eur. J. Org. Chem. 2010, 2010, 3363–3376. [Google Scholar] [CrossRef]
- Namboothiri, I.; Rastogi, N. Isoxazolines form Nitro Compounds: Synthesis ans Applications. In Synthesis of Heterocycles via Cycloadditions I; Hassner, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 12, pp. 1–44. [Google Scholar]
- Agrawal, N.; Mishra, P. The Synthetic and Therapeutic Expedition of Isoxazole and its Analogs. Med. Chem. Res. 2018, 27, 1309–1344. [Google Scholar] [CrossRef]
- Zimecki, M.; Bąchor, U.; Mączyński, M. Isoxazole Derivatives as Regulators of Immune Functions. Molecules 2018, 23, 2724. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, V.; Sharma, A.K.; Gupta, G.K. Isoxazoline containing natural products as anticancer agents: A review. Eur. J. Med. Chem. 2014, 77, 121–133. [Google Scholar] [CrossRef]
- Zhou, X.; Hohman, A.E.; Hsu, W.H. Current review of isoxazoline ectoparasiticides used in veterinary medicine. J. Vet. Pharmacol. Ther. 2022, 45, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.L.; das Neves, G.M.; Porto Kagami, L.; Eifler-Lima, V.L.; Merlo, A.A. Discovery, development, chemical diversity and design of isoxazoline-based insecticides. Bioorg. Med. Chem. 2021, 30, 115934. [Google Scholar] [CrossRef] [PubMed]
- Lamberth, C. Oxazole and Isoxazole Chemistry in Crop Protection. Heterocyclic Chem. 2018, 55, 2035. [Google Scholar] [CrossRef]
- Zhou, X.; Hohman, A.; Hsu, W.H. Review of extralabel use of isoxazolines for treatment of demodicosis in dogs and cats. J. Am. Vet. Med. Assoc. 2020, 256, 1342–1346. [Google Scholar] [CrossRef]
- Mermans, C.; Dermauw, W.; Geibel, S.; Van Leeuwen, T. Activity, selection response and molecular mode of action of the isoxazoline afoxolaner in Tetranychus urticae. Pest Manag Sci. 2023, 79, 183–193. [Google Scholar] [CrossRef]
- Long, A. Isoxazolines: Preeminent Ectoparasiticides of the Early Twenty-first Century. In Ectoparasites: Drug Discovery against Moving Targets; Meng, C.Q., Sluder, A.E., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 319–351. [Google Scholar]
- Phanumartwiwath, A.; Kesornpun, C.; Sureram, S.; Hongmanee, P.; Pungpo, P.; Kamsri, P.; Punkvang, A.; Eurtivong, C.; Kittakoop, P.; Ruchirawat, S. Antitubercular and antibacterial activities of isoxazolines derived from natural products: Isoxazolines as inhibitors of Mycobacterium tuberculosis InhA. J. Chem. Res. 2021, 45, 1003–1015. [Google Scholar] [CrossRef]
- Margetić, D. High-pressure cycloaddition reactions in the synthesis of biologically relevant heterocycles. In Green Synthetic Approaches for Biologically Relevant Heterocycles, 2nd ed.; Brahmachari, G., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; Volume 1: Advanced Synthetic Techniques, pp. 9–47. [Google Scholar]
- Miyamoto, H.; Sakumoto, C.; Takekoshi, E.; Maeda, Y.; Hiramoto, N.; Itoh, T.; Kato, Y. Effective Method To Remove Metal Elements from Pharmaceutical Intermediates with Polychelated Resin Scavenger. Org. Process Res. Dev. 2015, 19, 1054–1061. [Google Scholar] [CrossRef]
- Urawa, Y.; Miyazawa, M.; Ozeki, N.; Ogura, K. A Novel Methodology for Efficient Removal of Residual Palladium from a Product of the Suzuki-Miyaura Coupling with Polymer-Supported Ethylenediamine Derivatives. Org. Process Res. Dev. 2003, 7, 191–195. [Google Scholar] [CrossRef]
- Welch, C.J.; Albaneze-Walker, J.; Leonard, W.R.; Biba, M.; DaSilva, J.; Henderson, D.; Laing, B.; Mathre, D.J.; Spencer, S.; Bu, X.; et al. Adsorbent Screening for Metal Impurity Removal in Pharmaceutical Process Research. Org. Process Res. Dev. 2005, 9, 198–205. [Google Scholar] [CrossRef]
- Phillips, S.; Kauppinen, P. The Use of Metal Scavengers for Recovery of Palladium Catalyst from Solution. Platin. Met. Rev. 2010, 54, 69–70. [Google Scholar] [CrossRef]
- Stala, Ł.; Ulatowska, J.; Polowczyk, I. A review of polyampholytic ion scavengers for toxic metal ion removal from aqueous systems. Water Res. 2021, 203, 117523. [Google Scholar] [CrossRef] [PubMed]

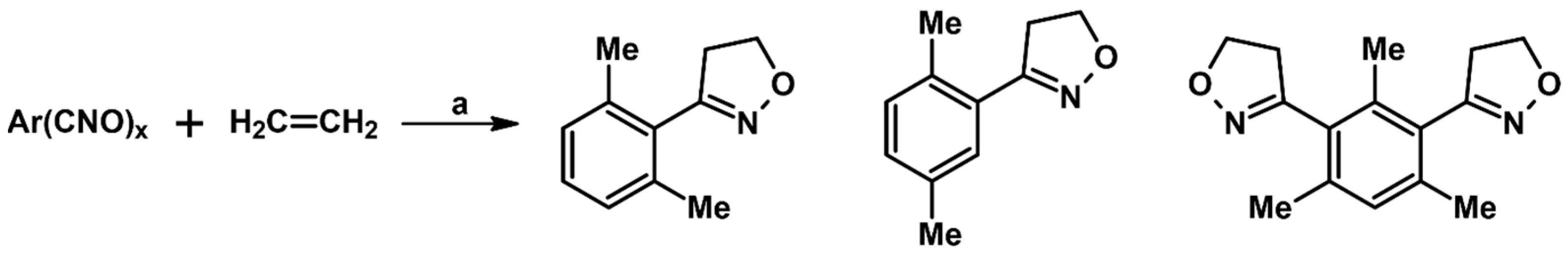

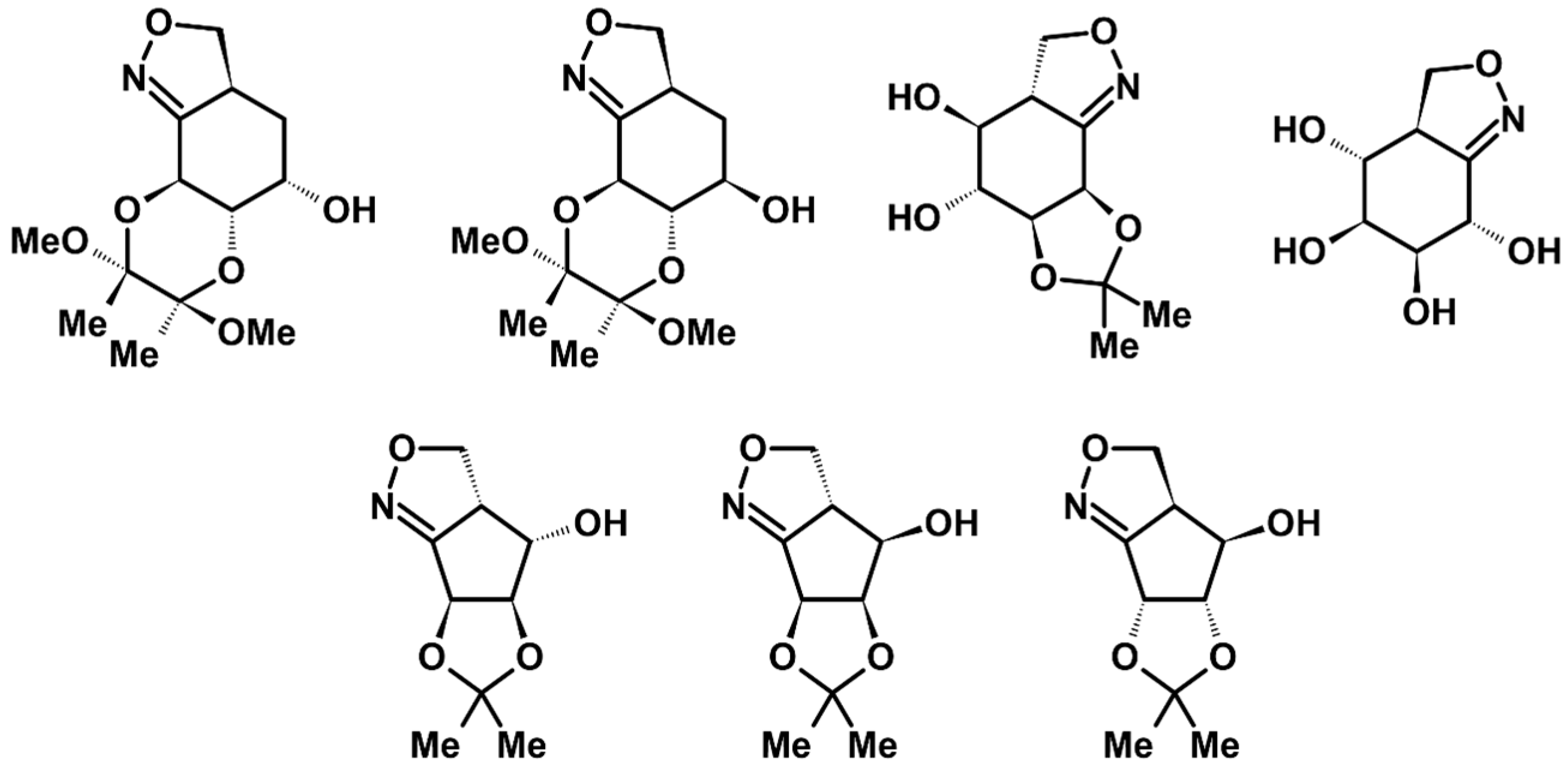

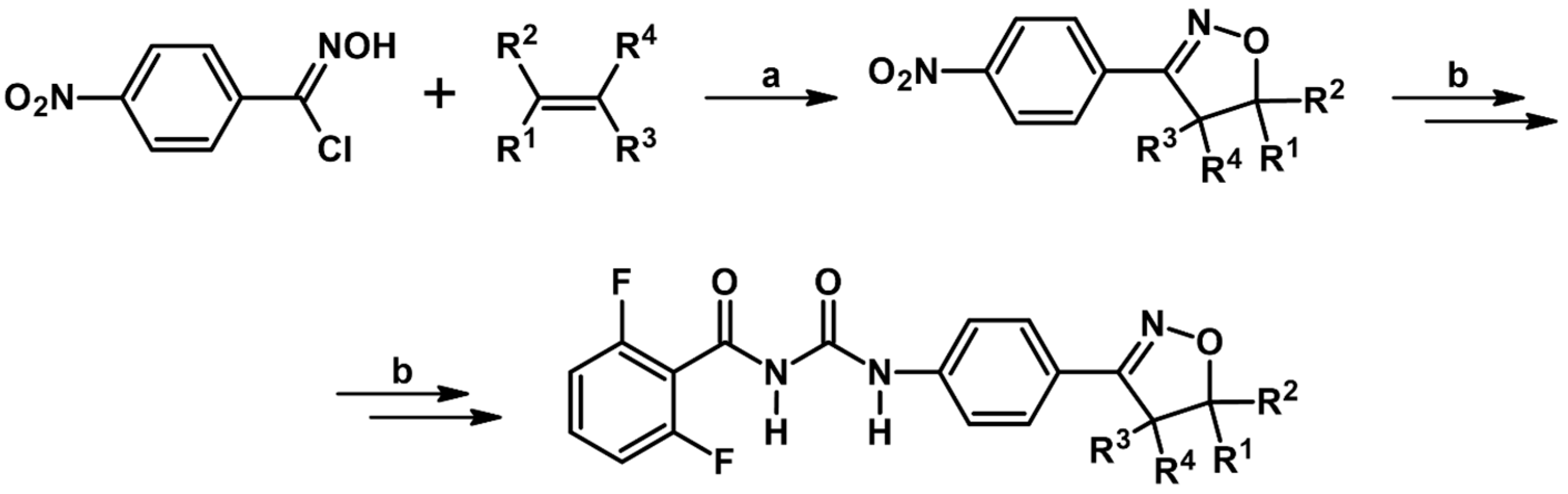
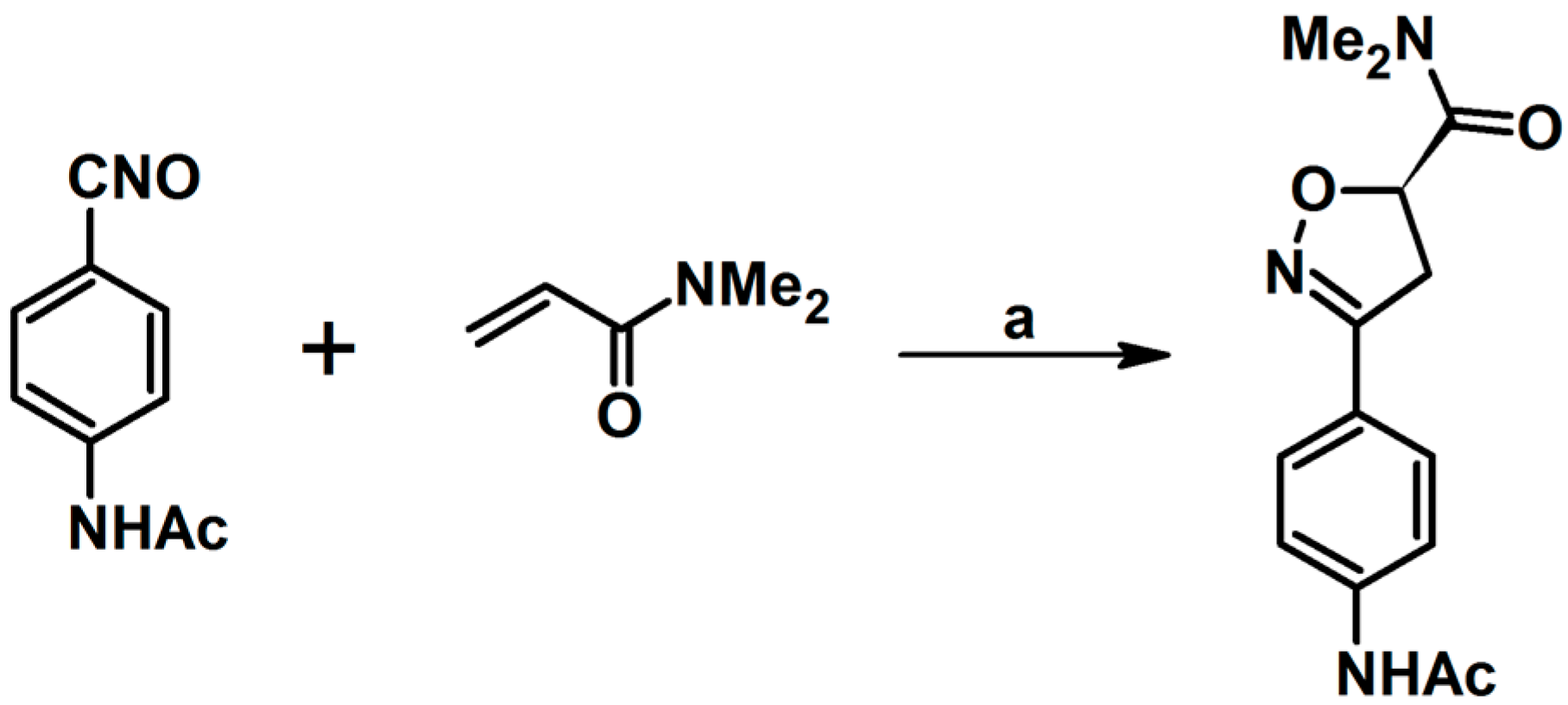
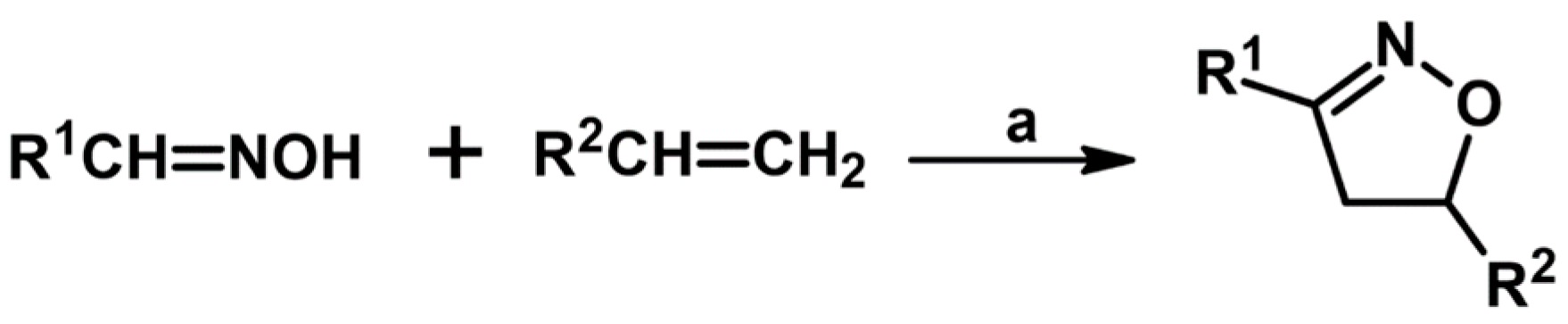
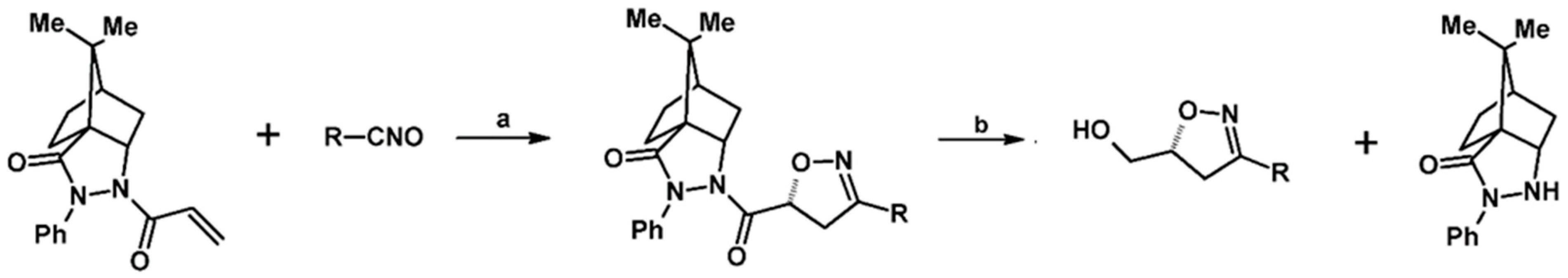
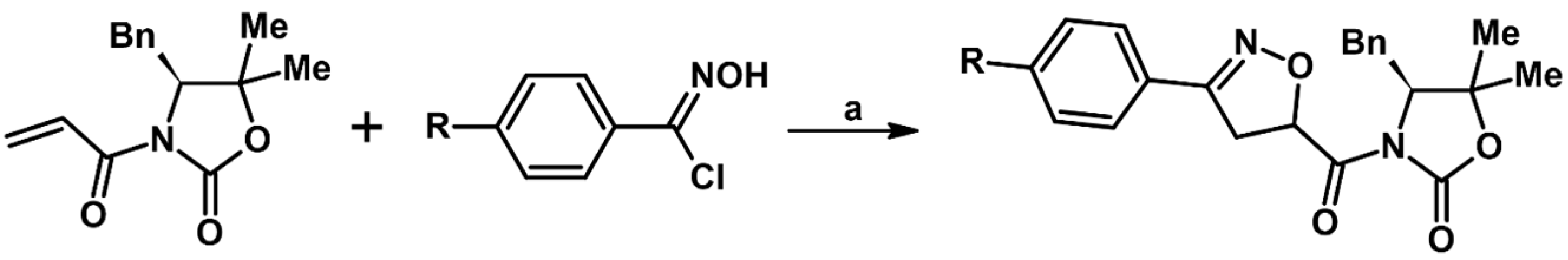
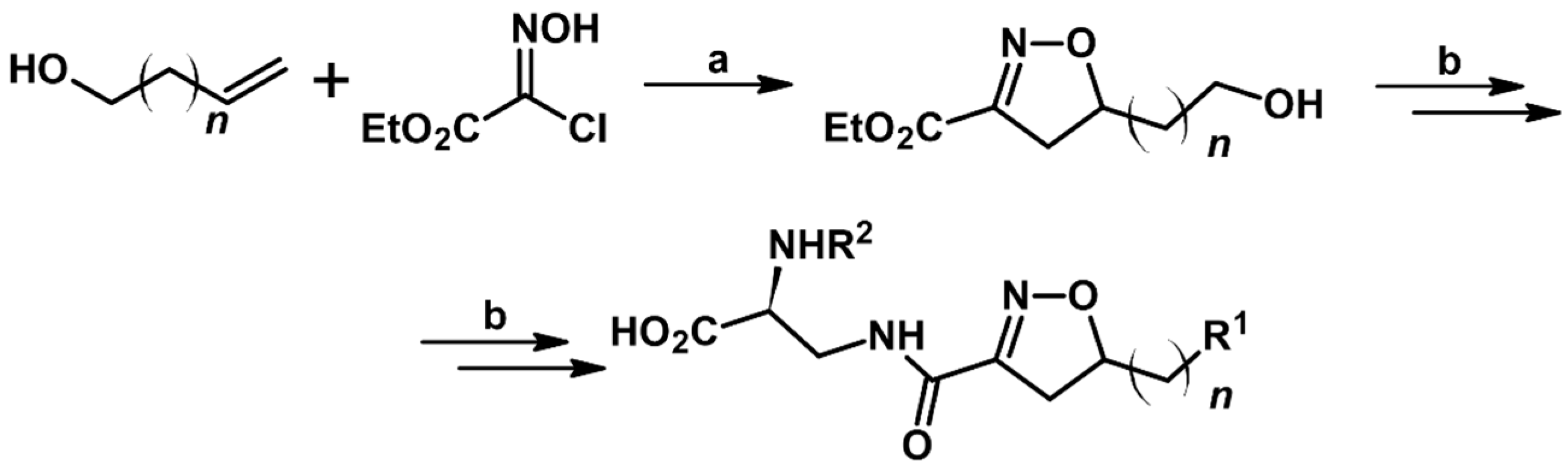

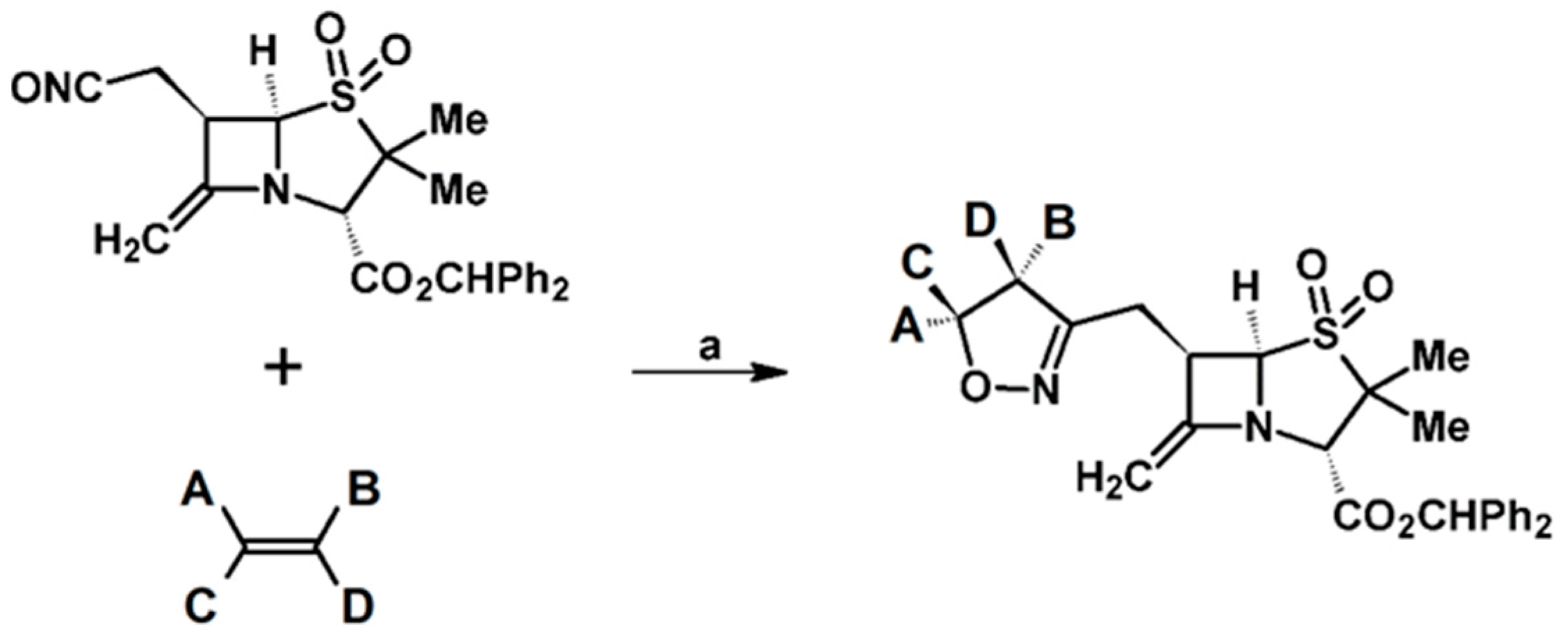
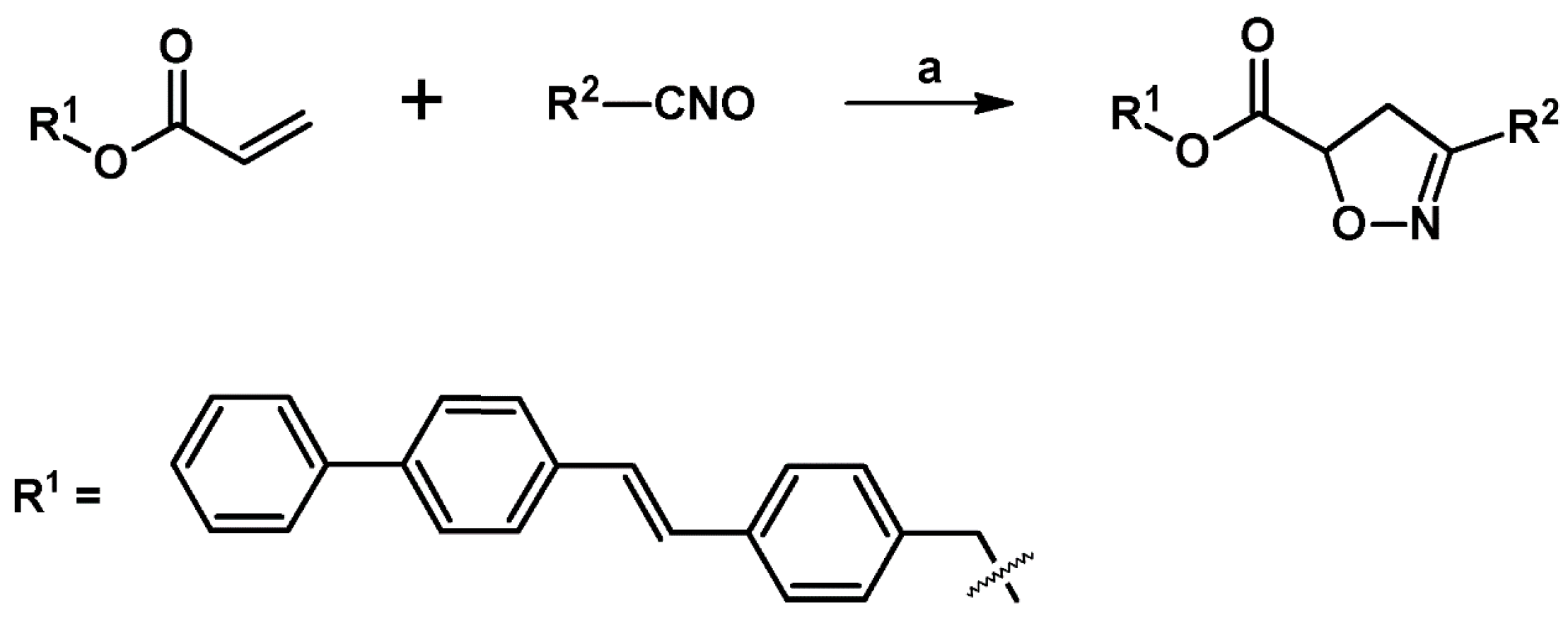
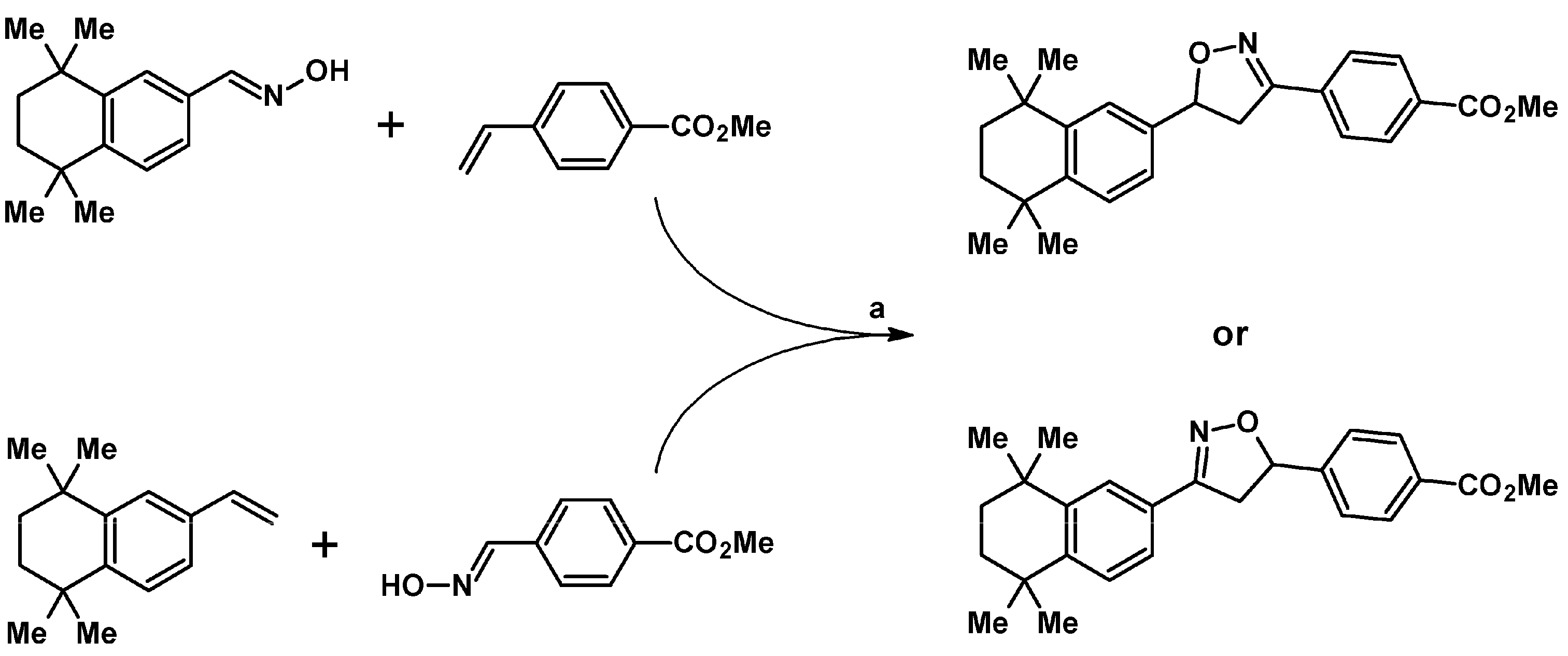
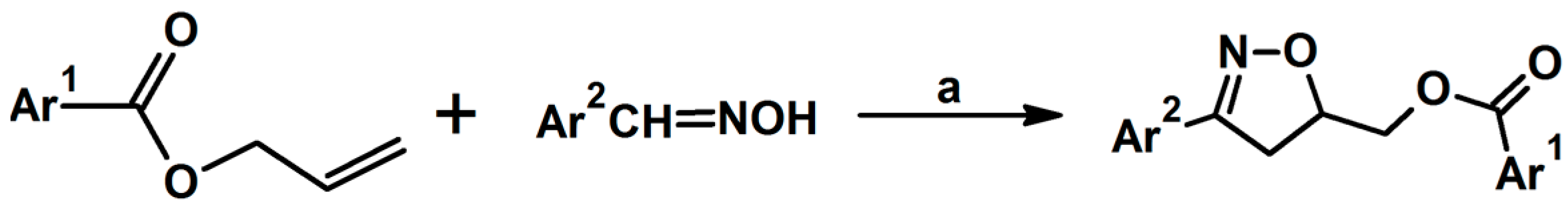
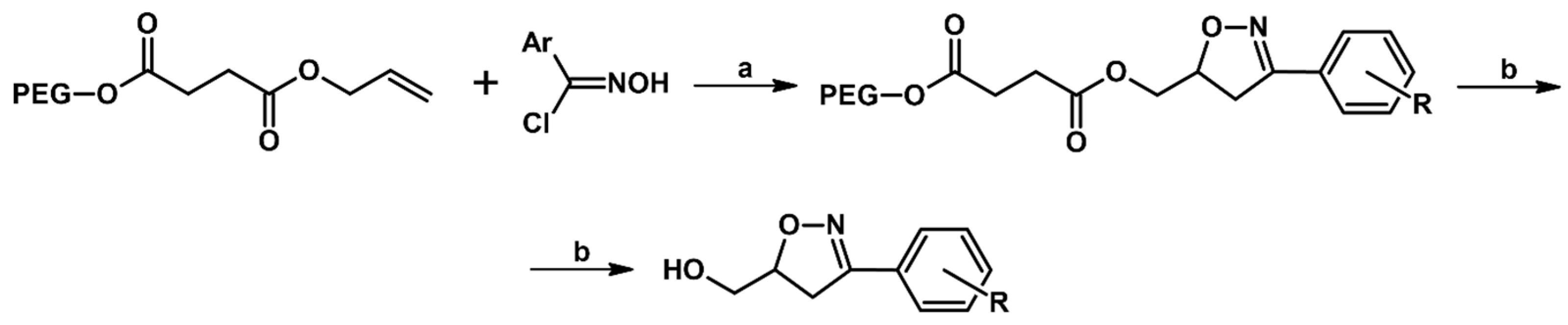
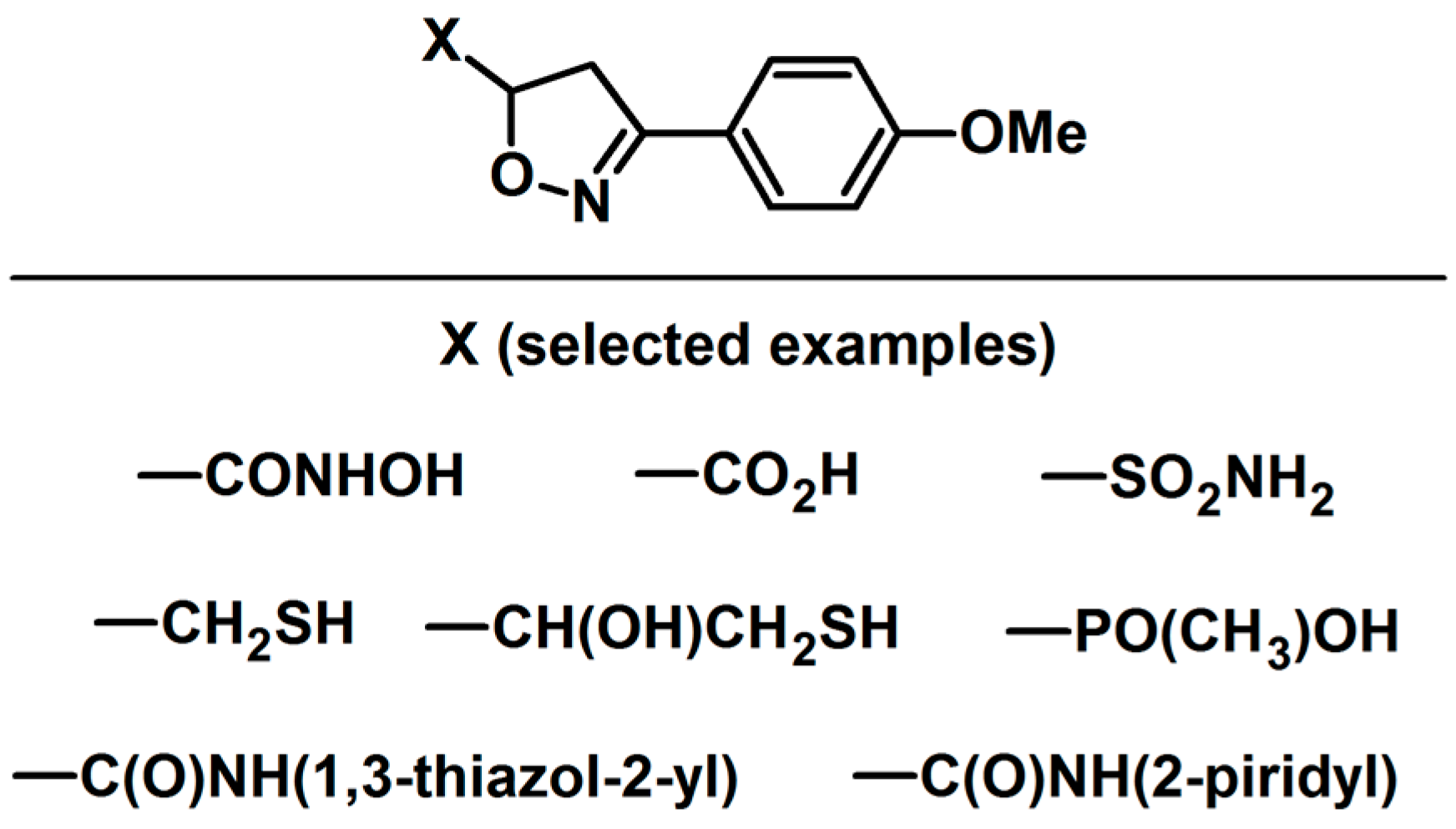
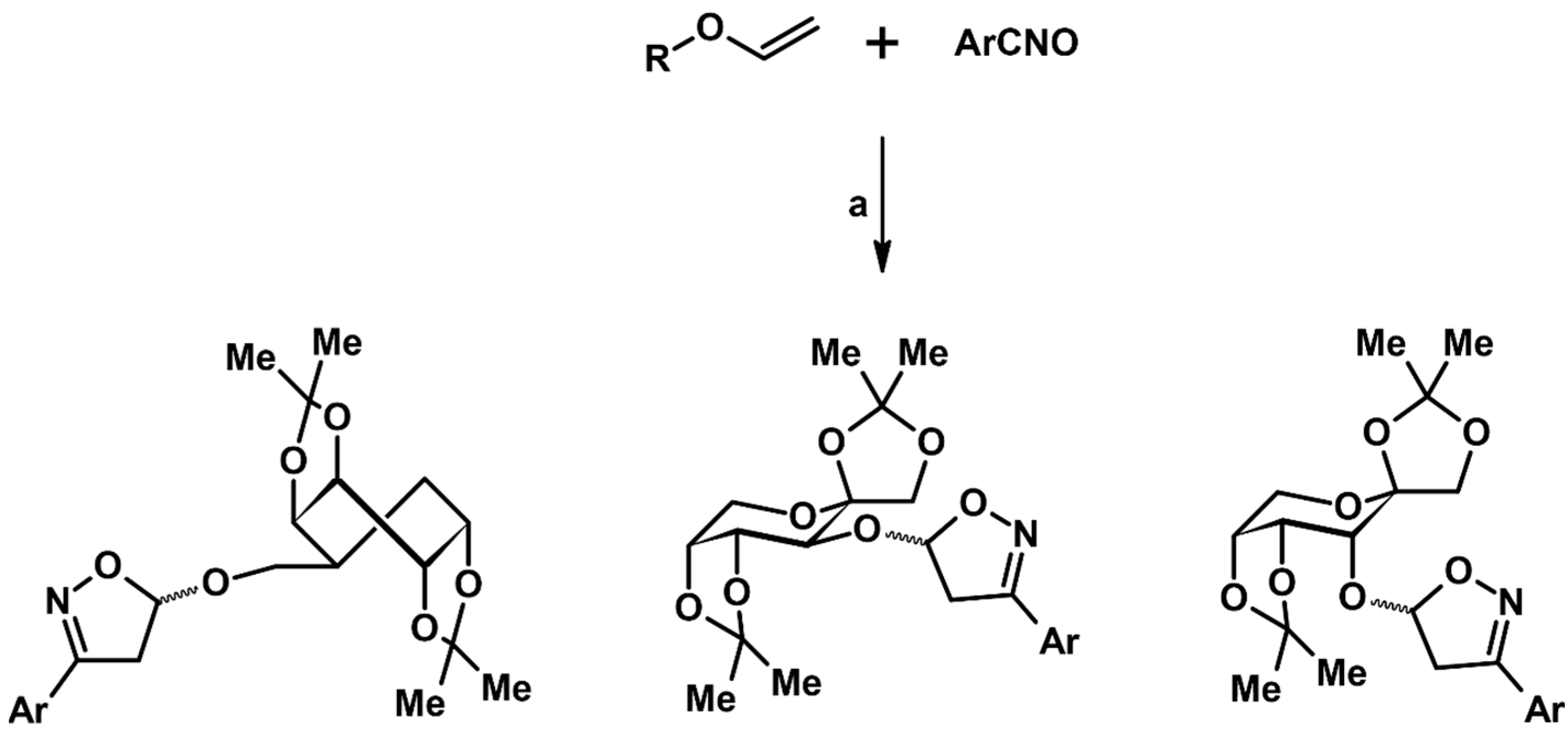
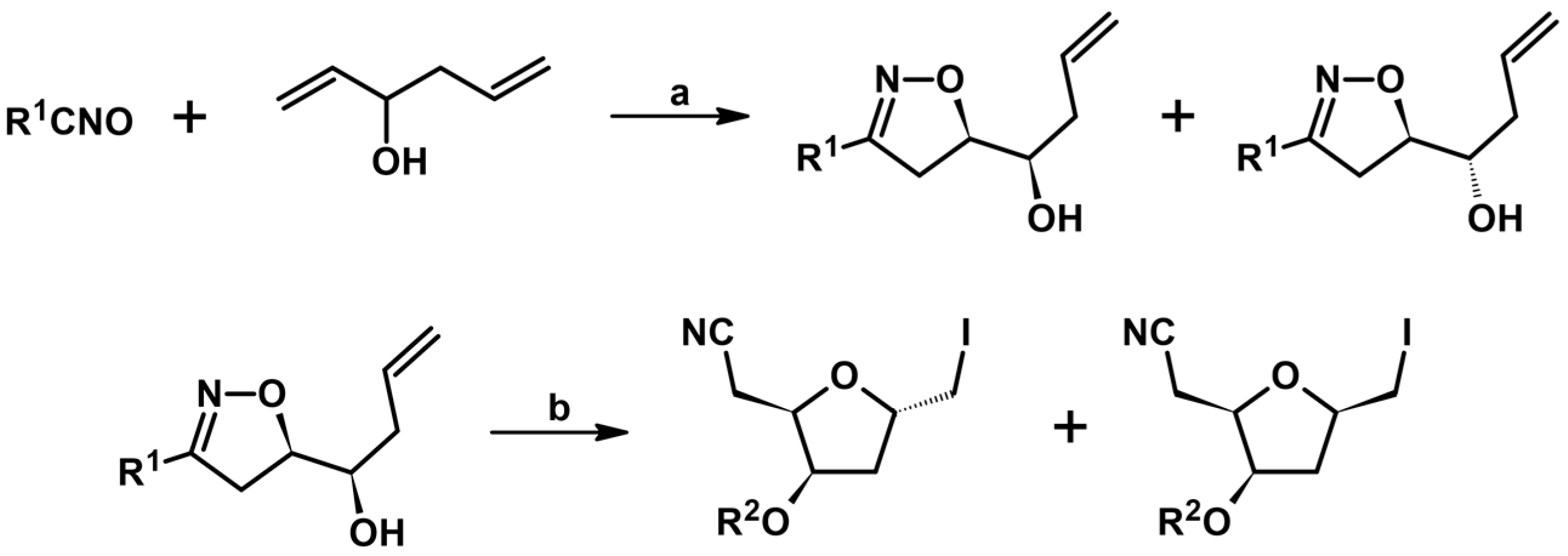
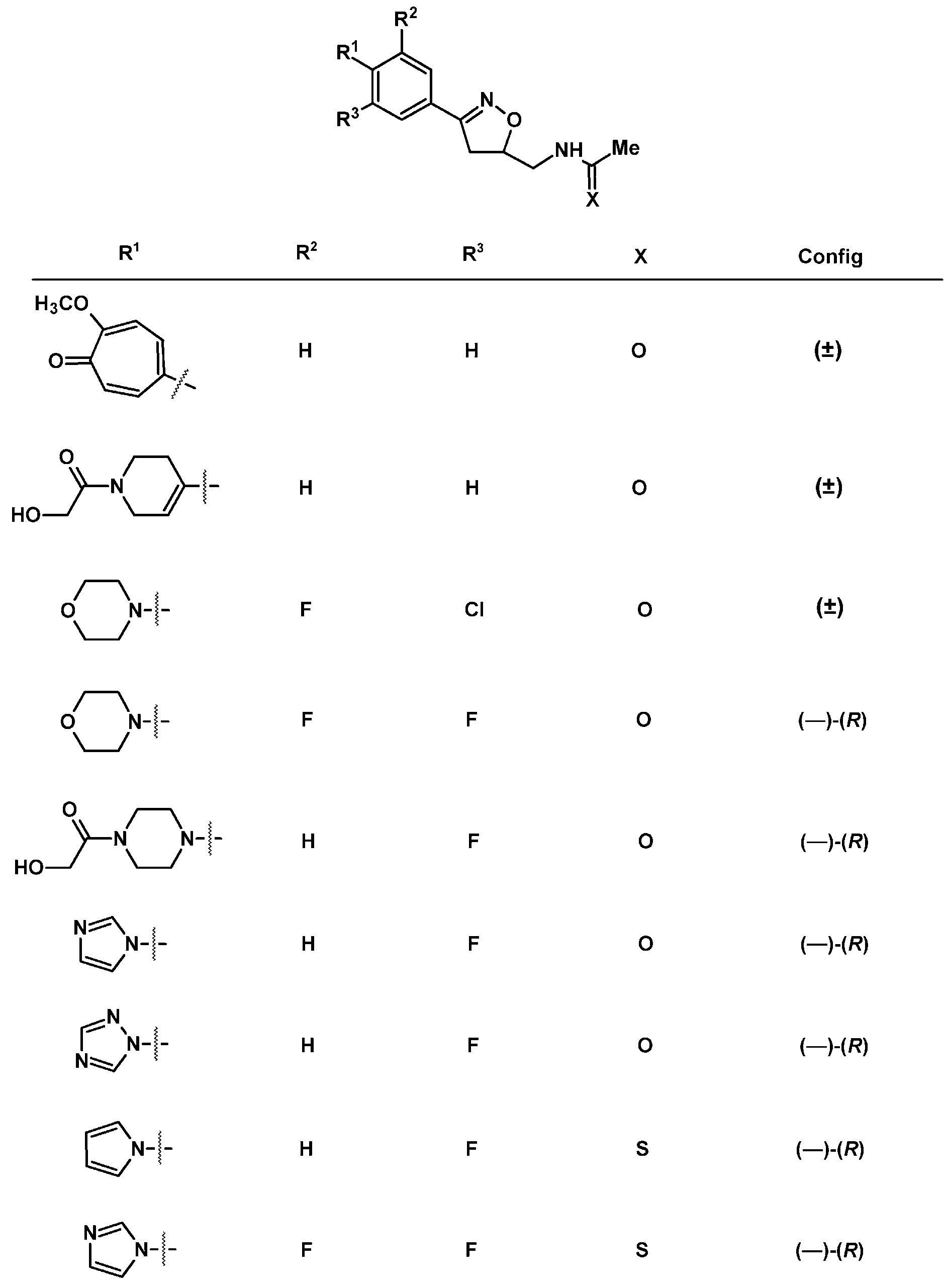
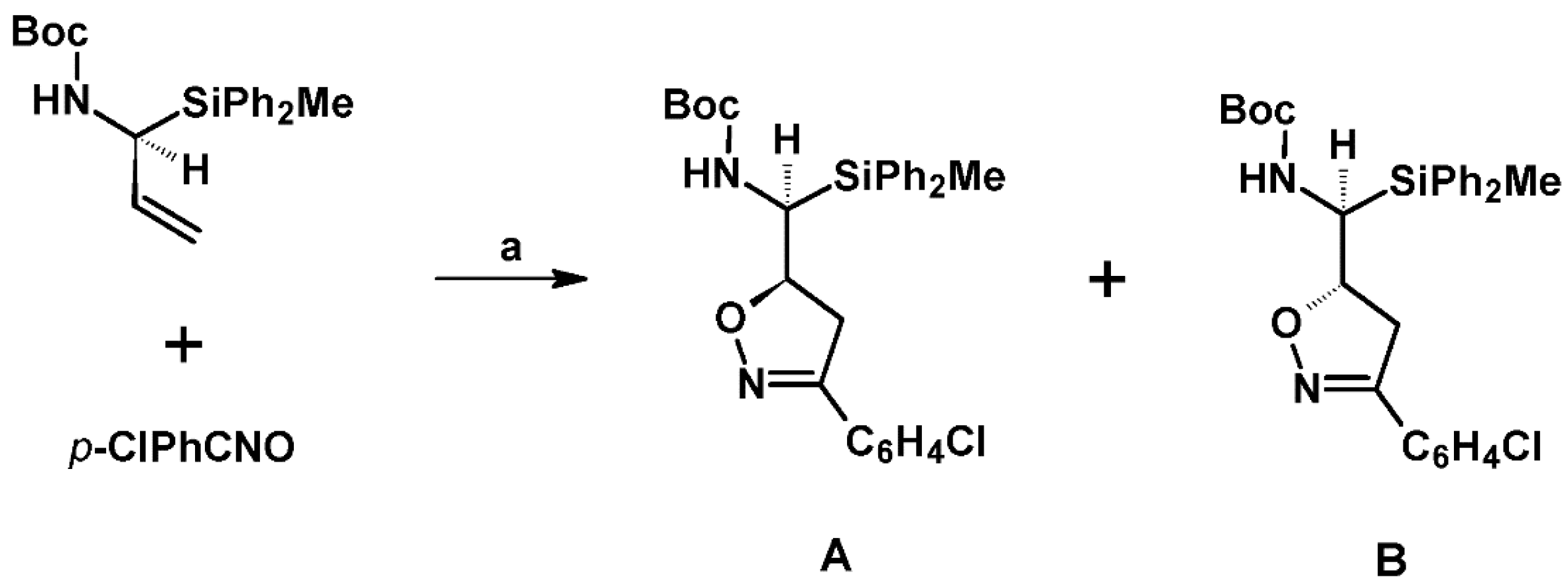
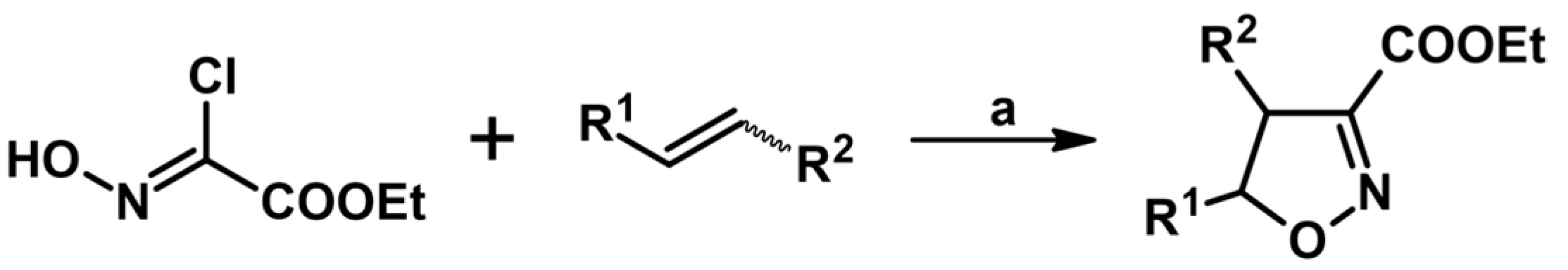
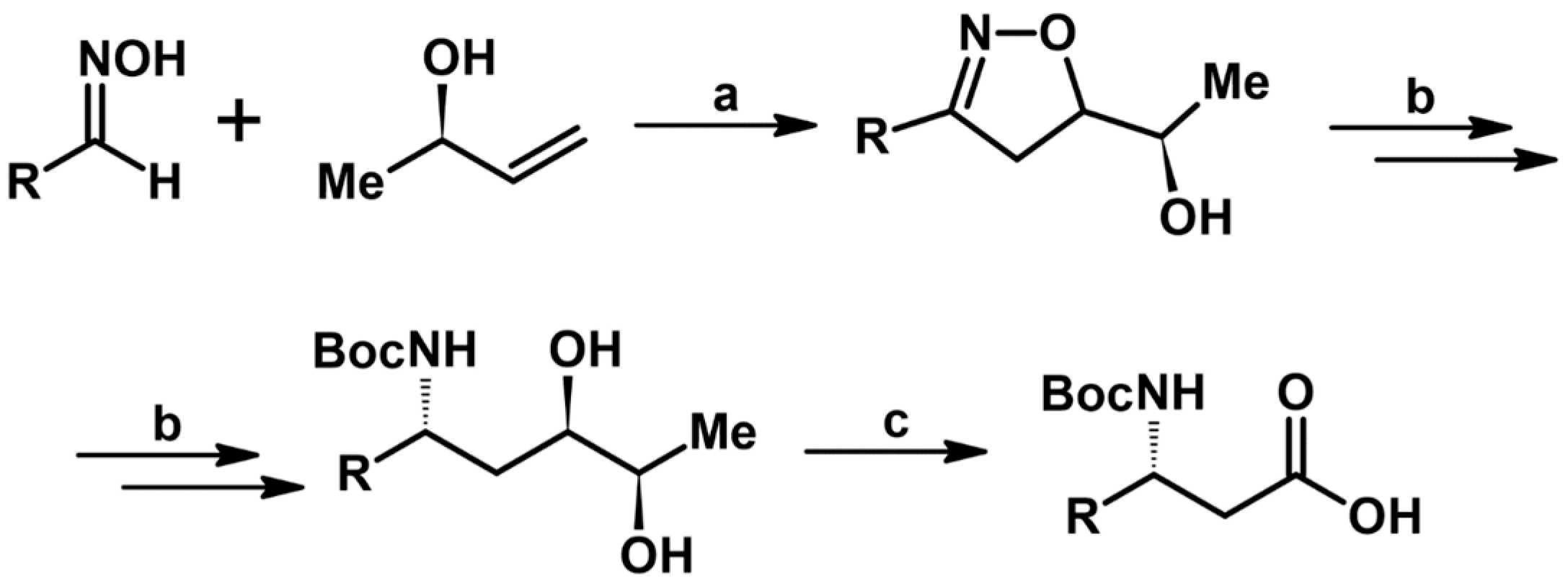
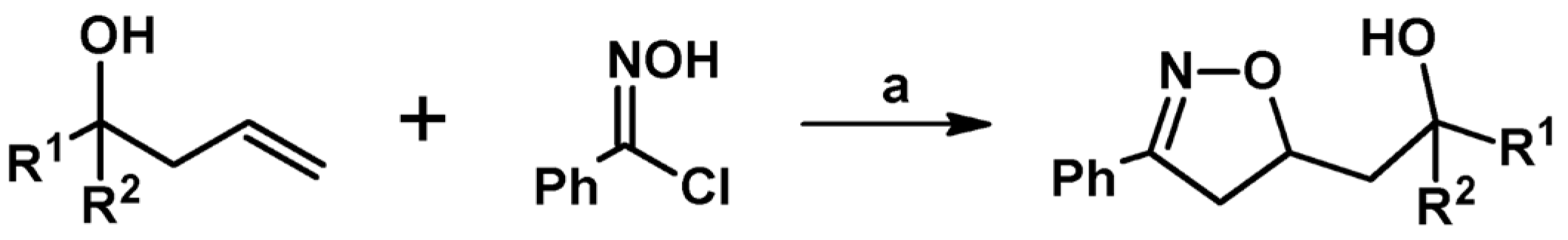
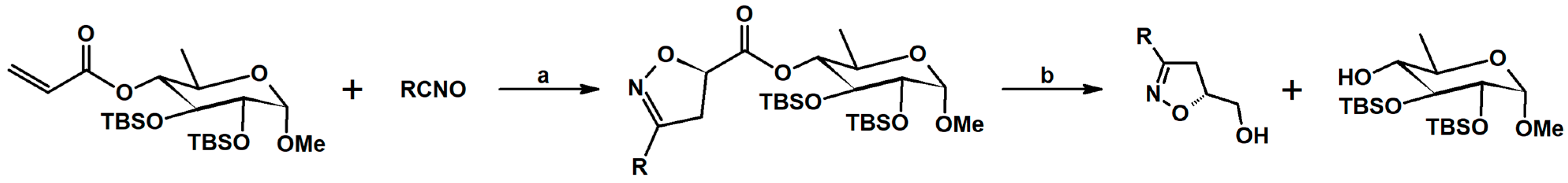
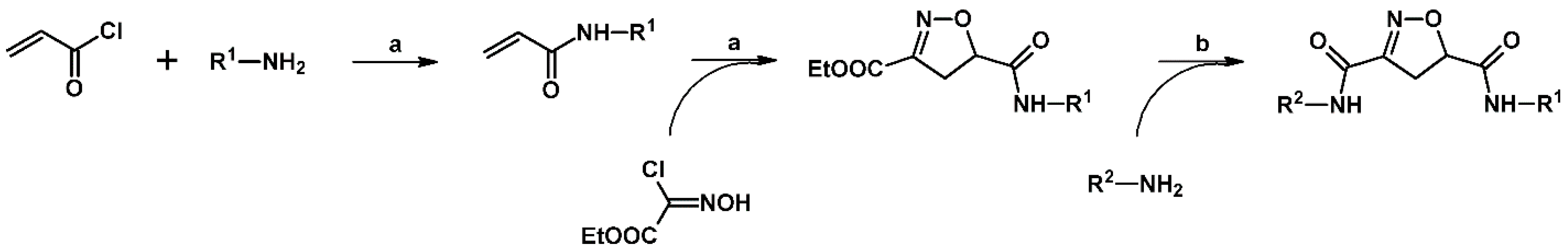
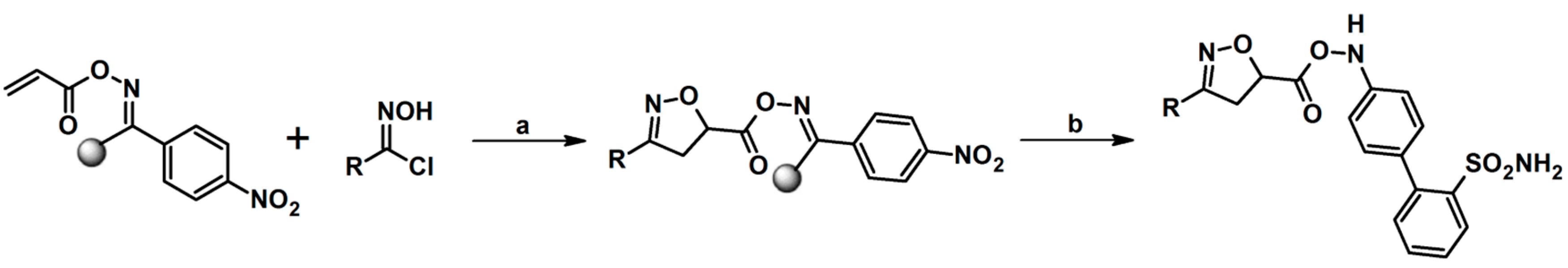
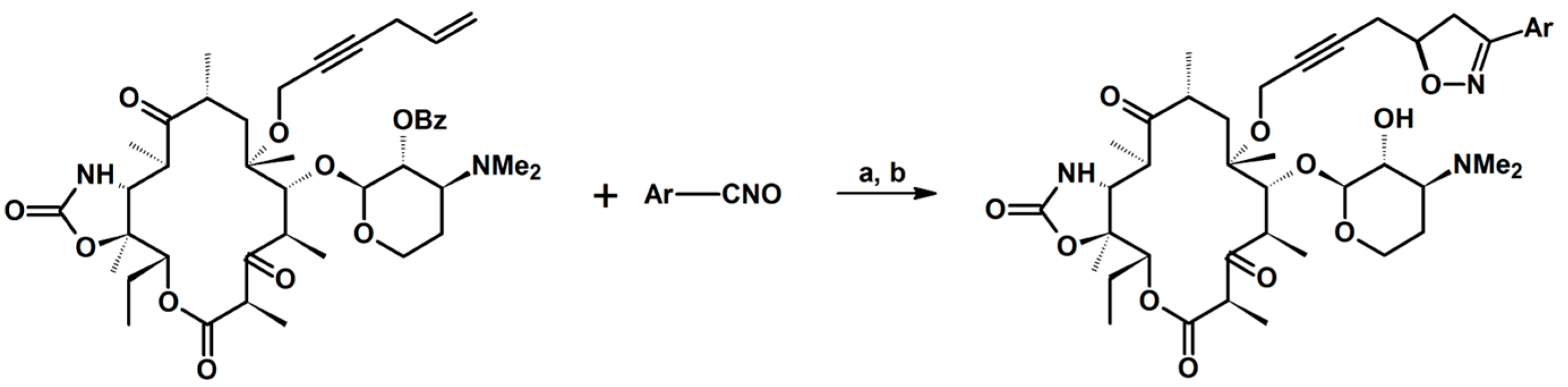
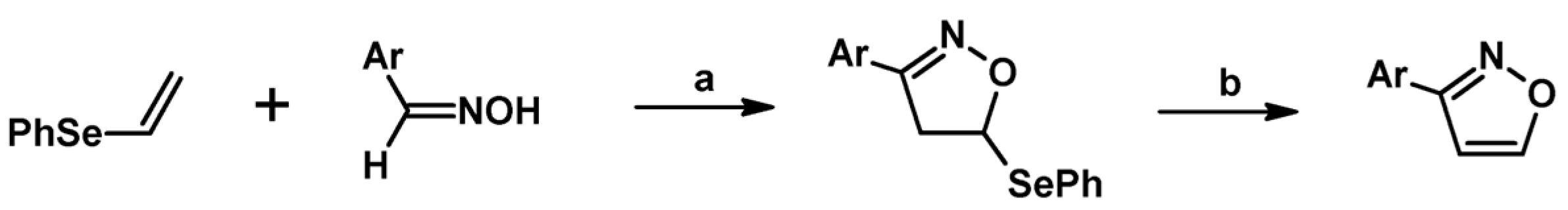


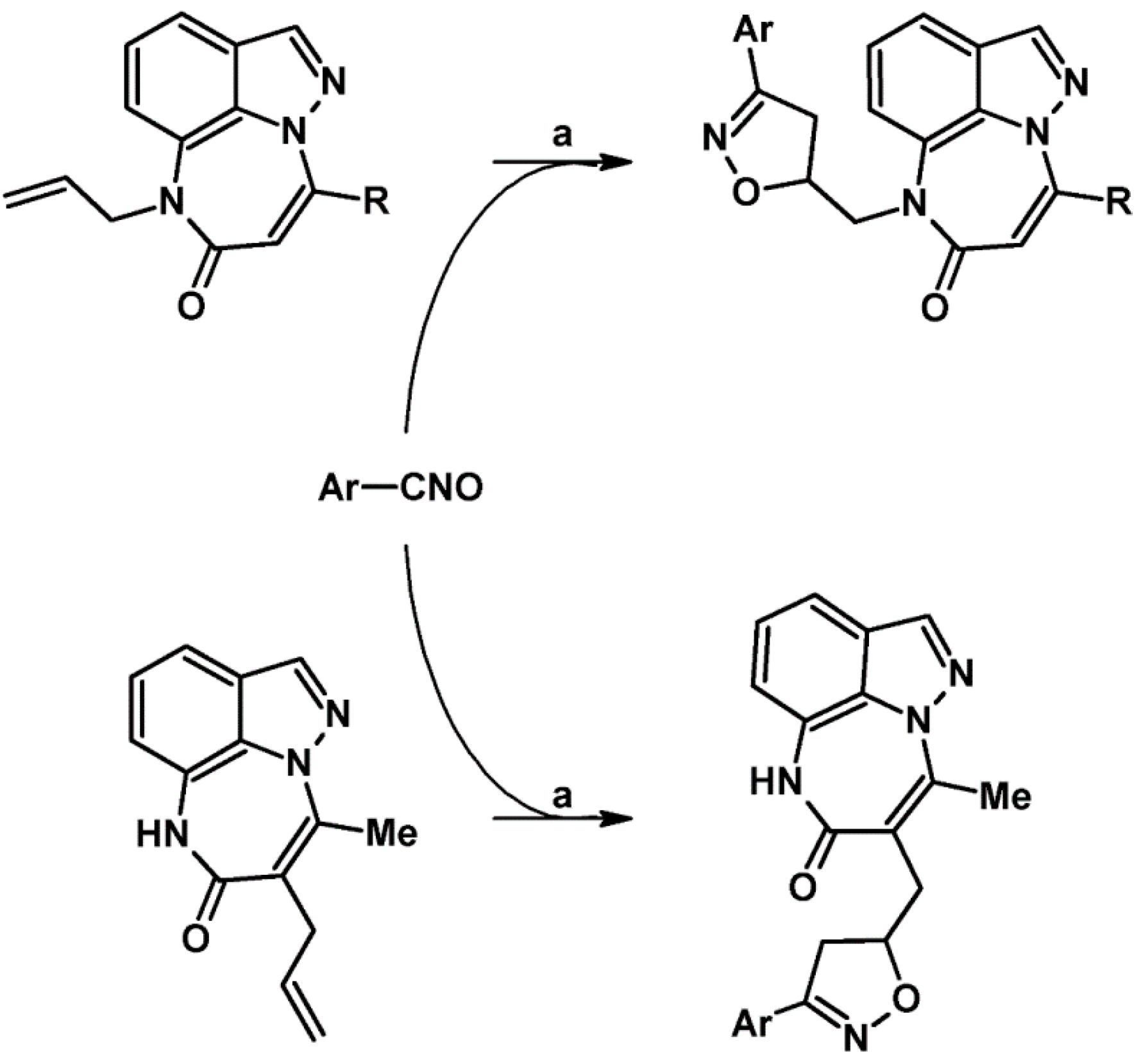
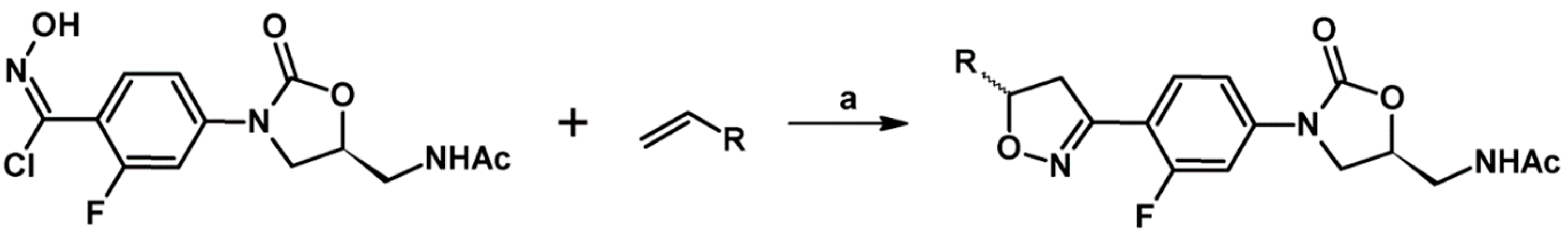

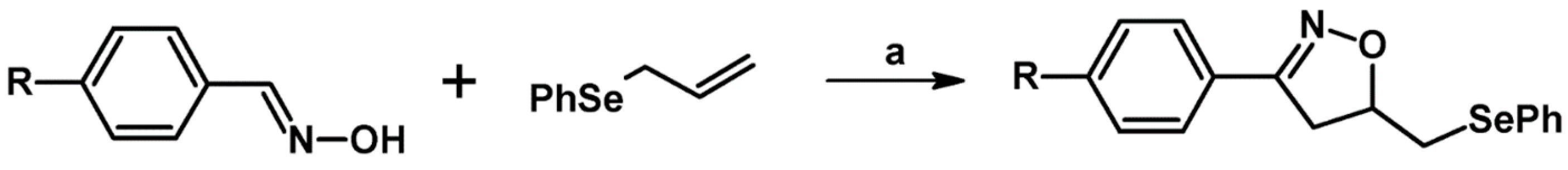
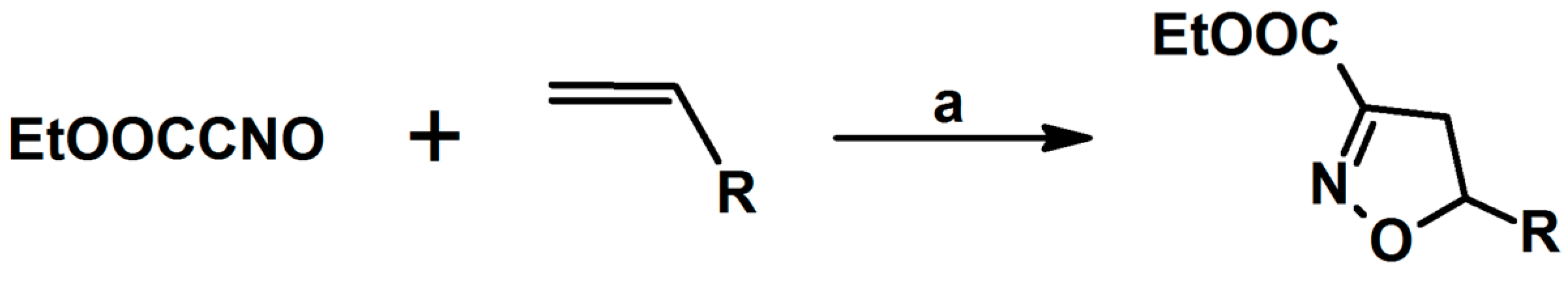
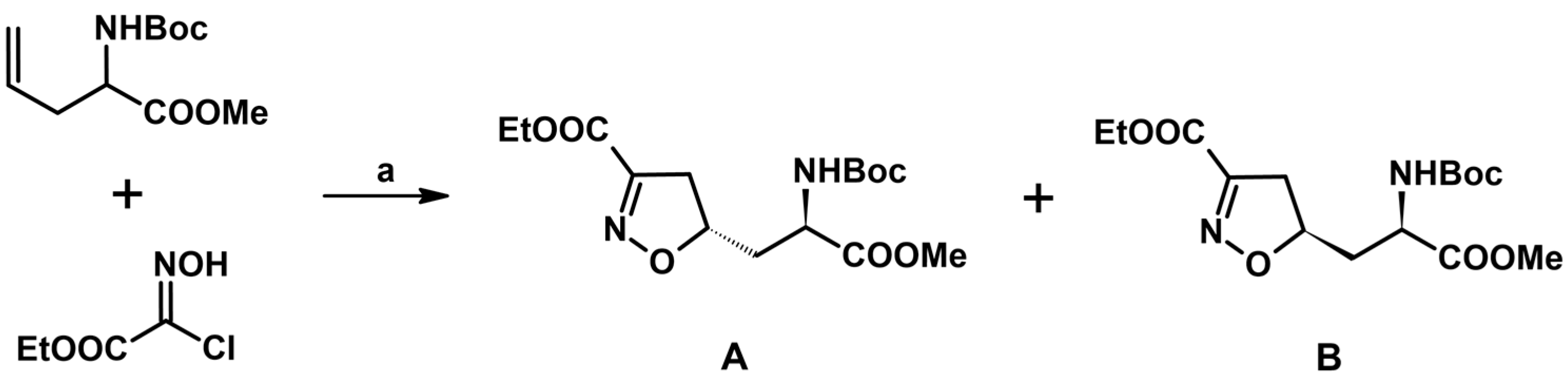
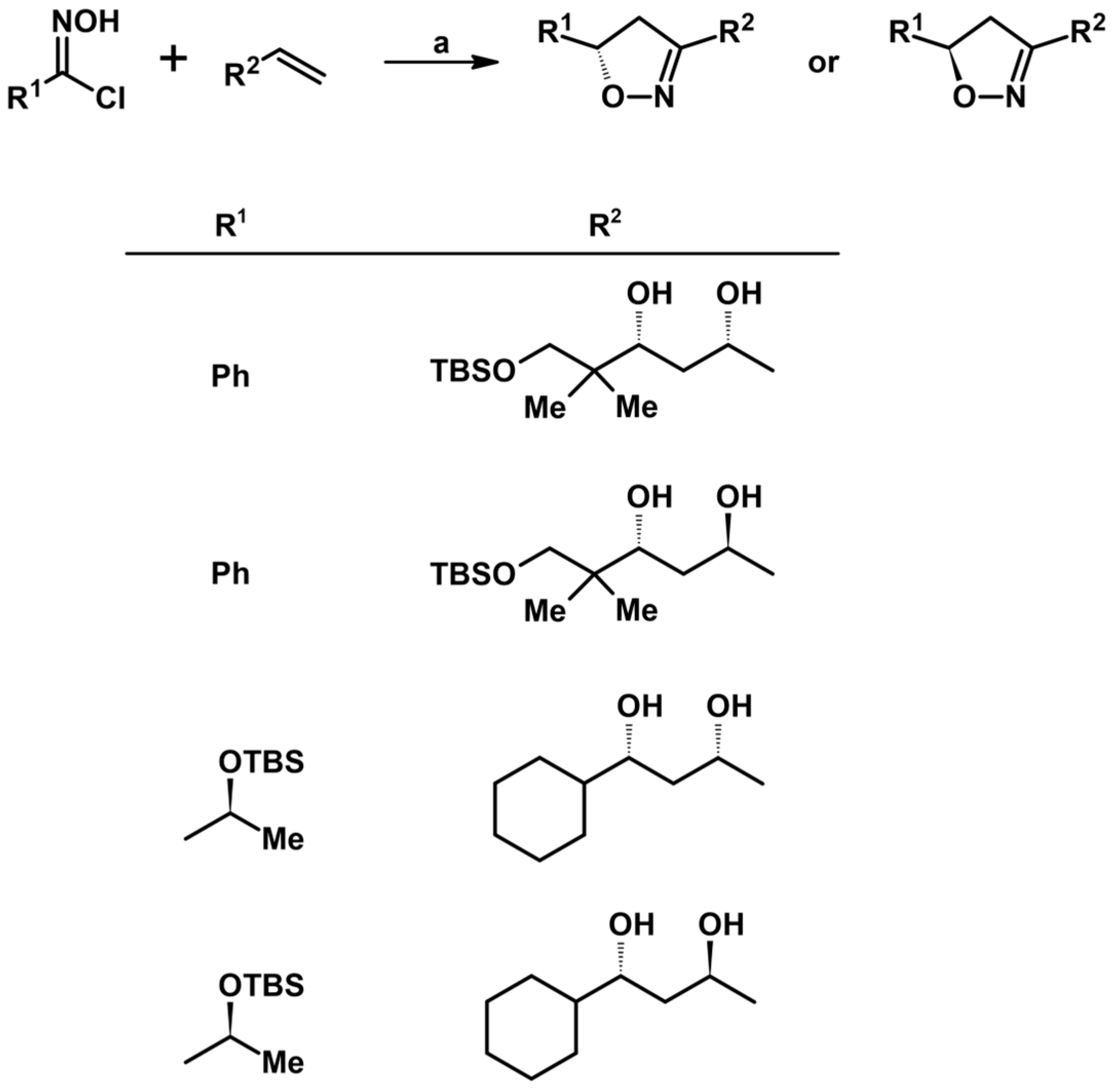
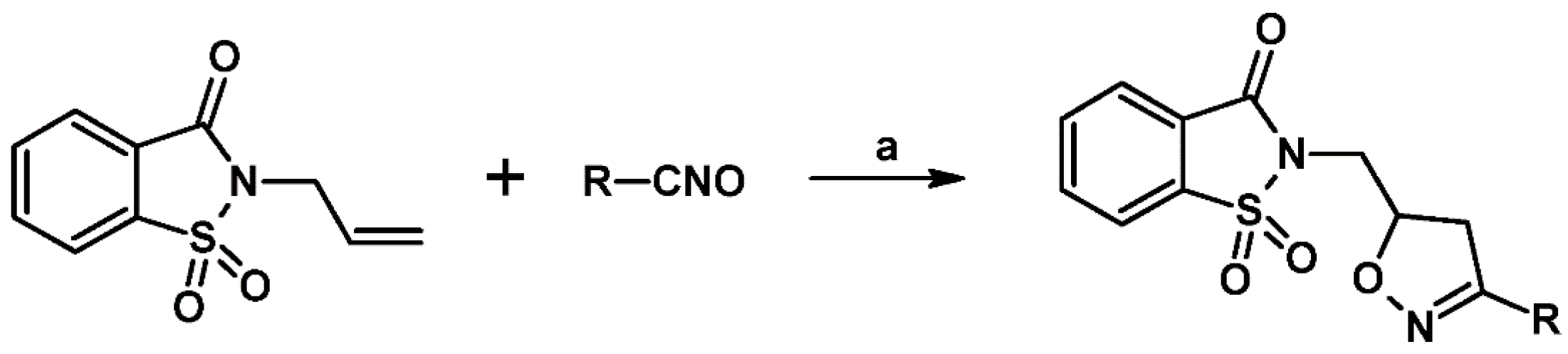

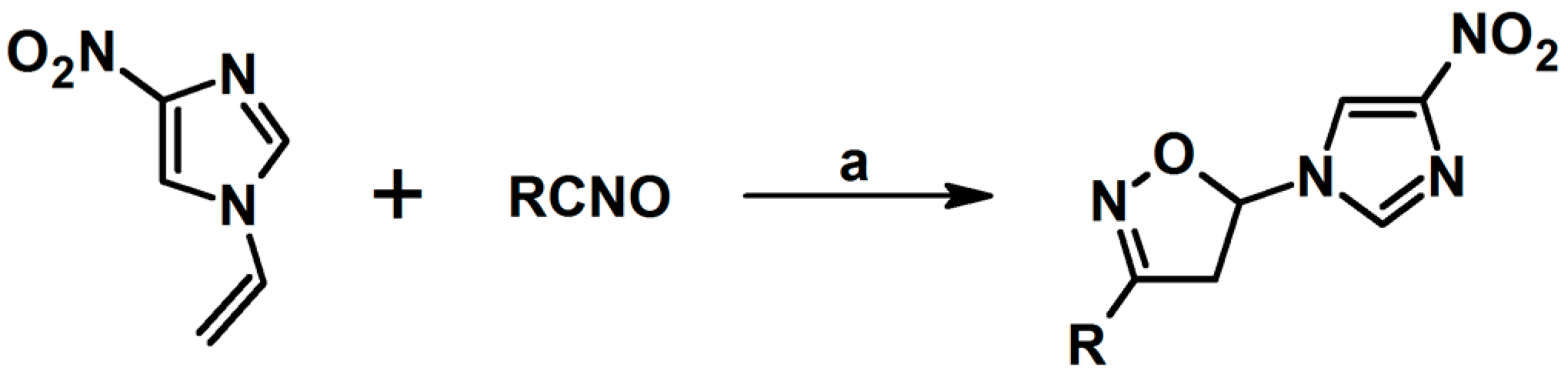
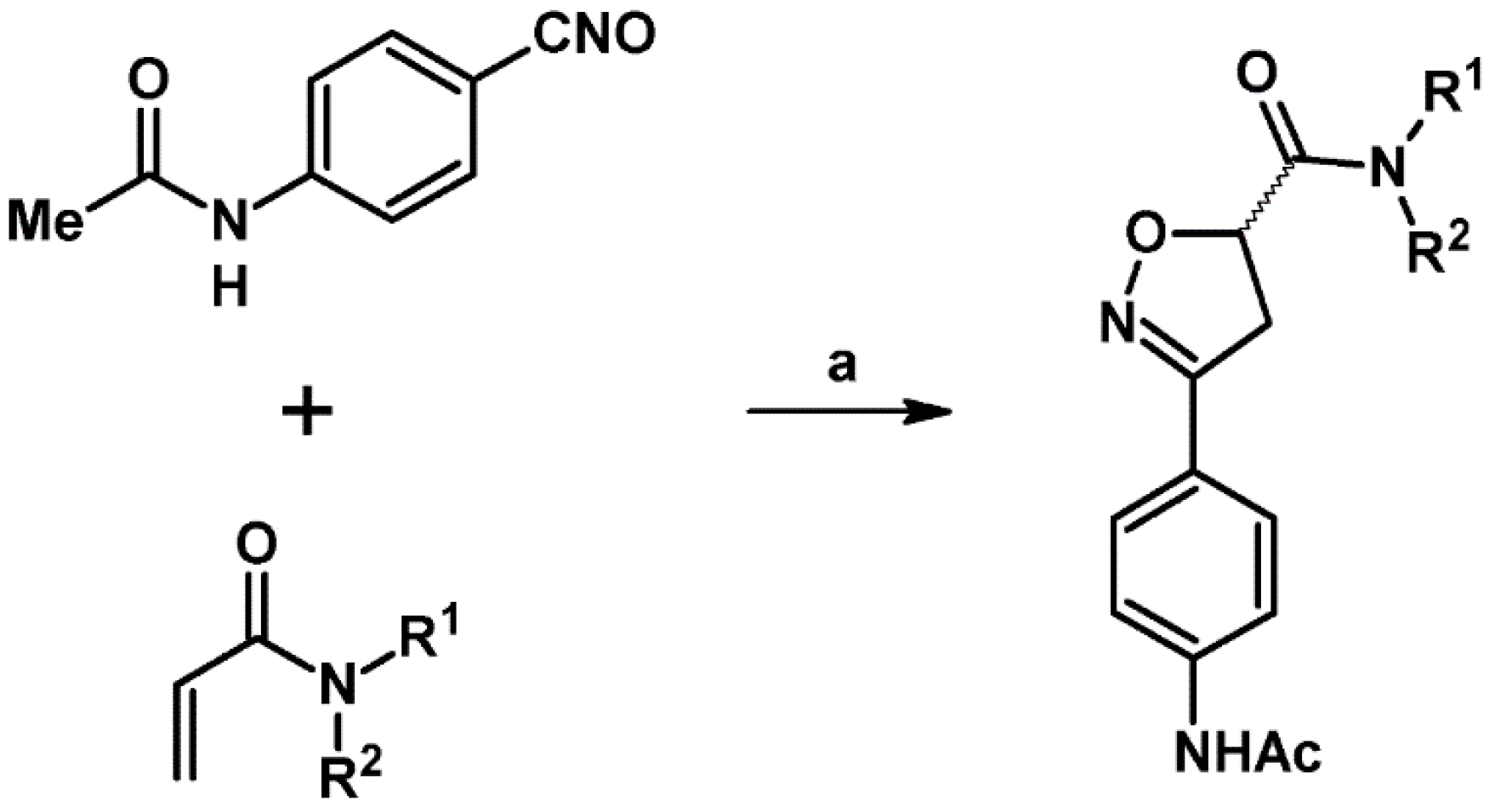

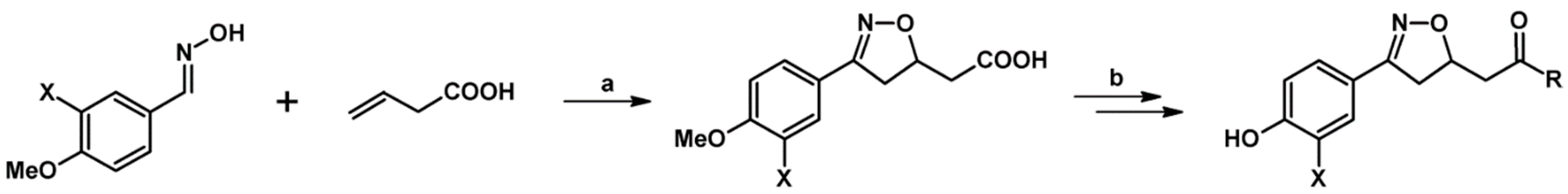

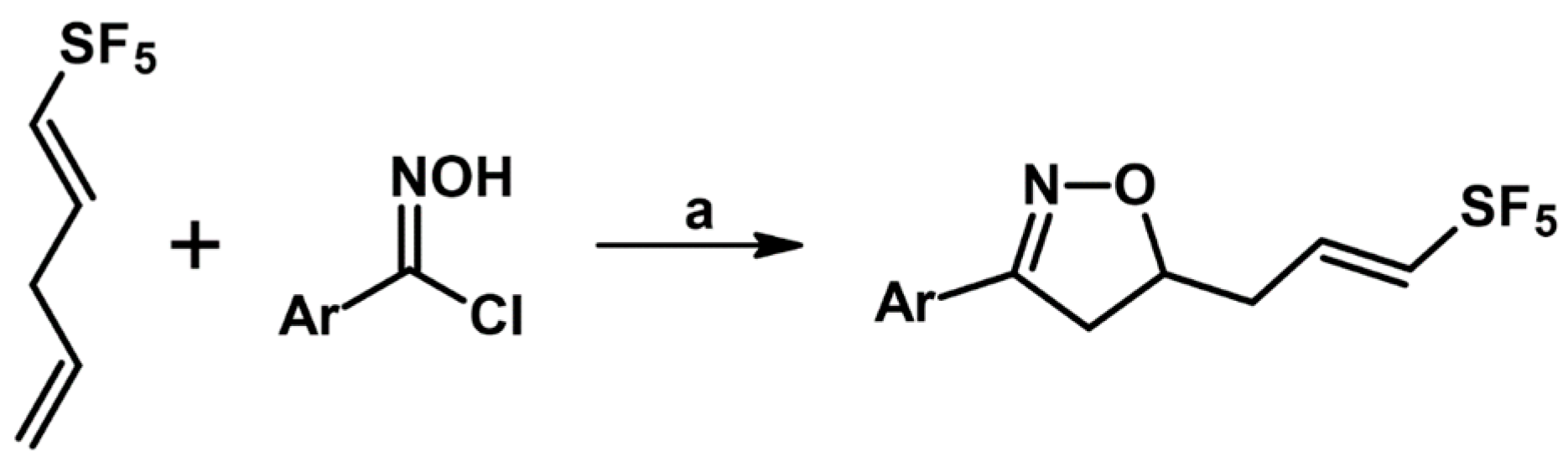
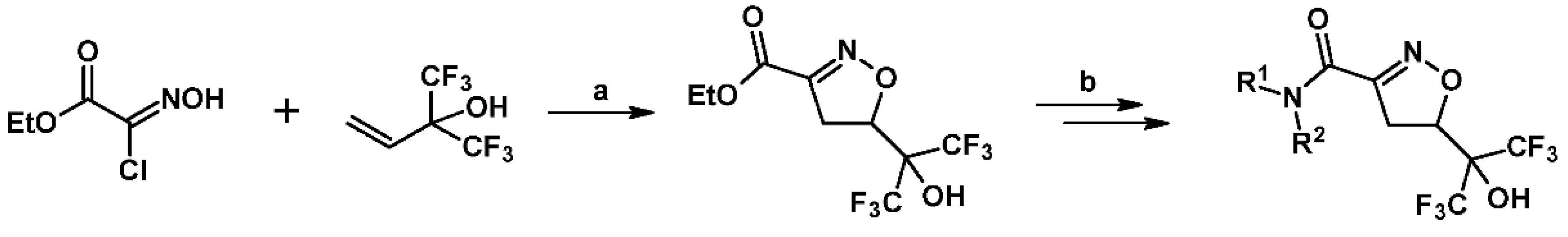
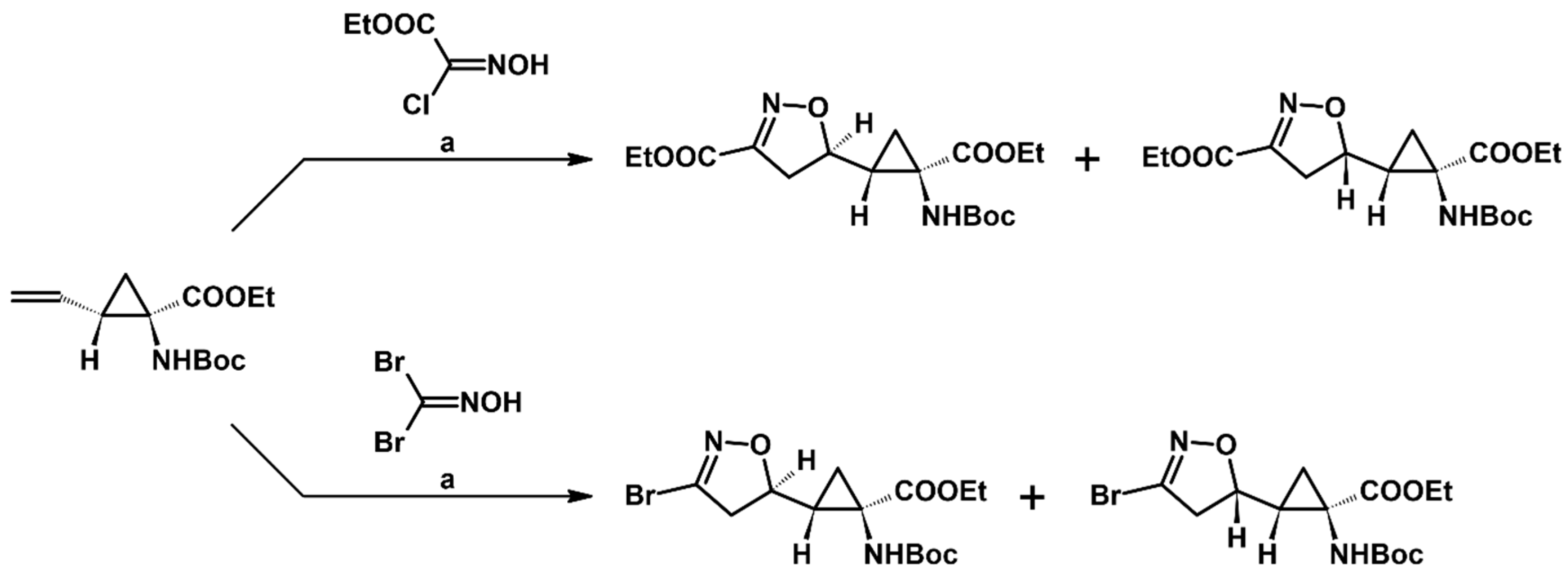
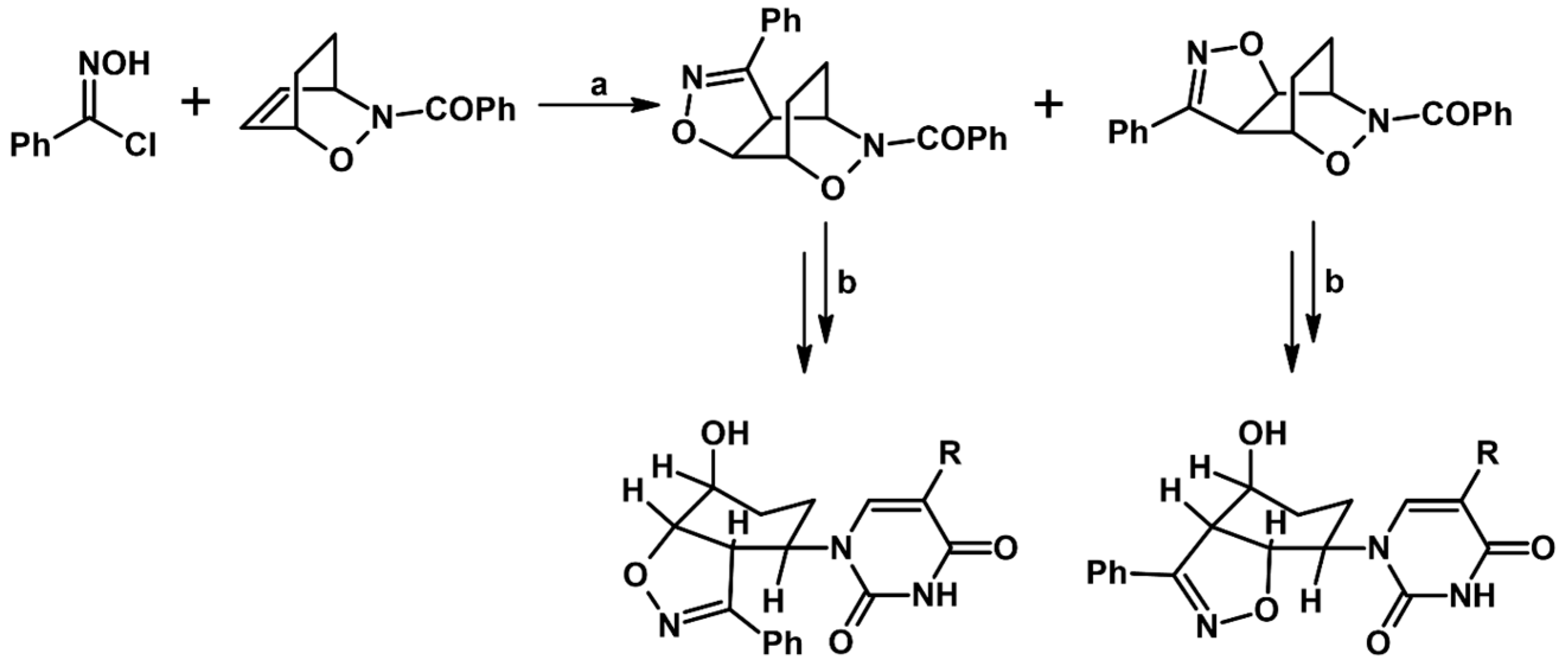
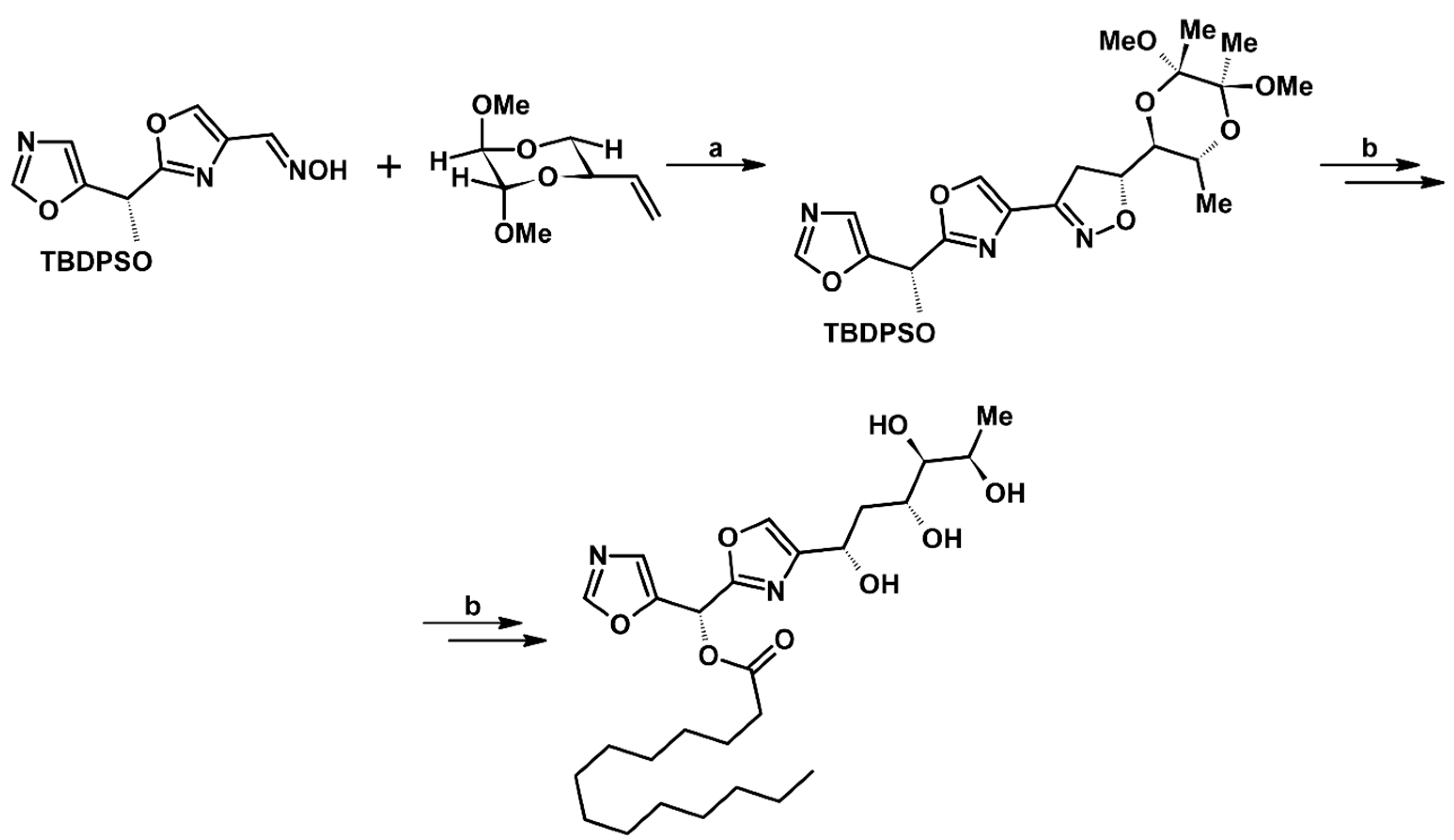
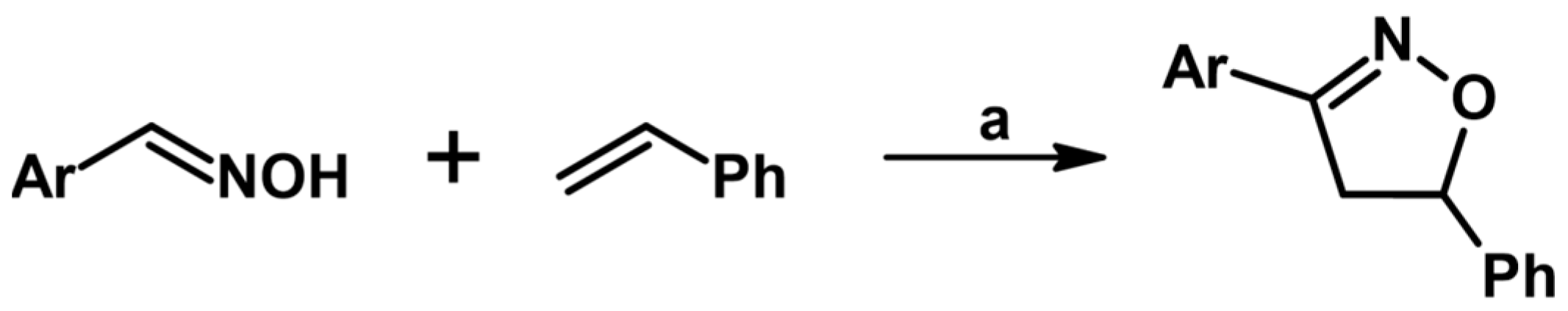

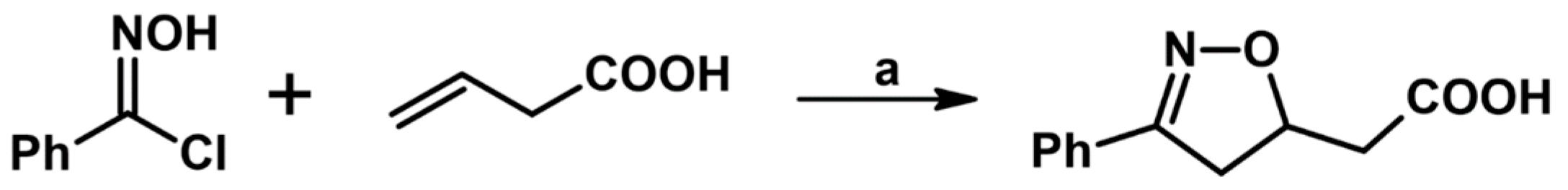
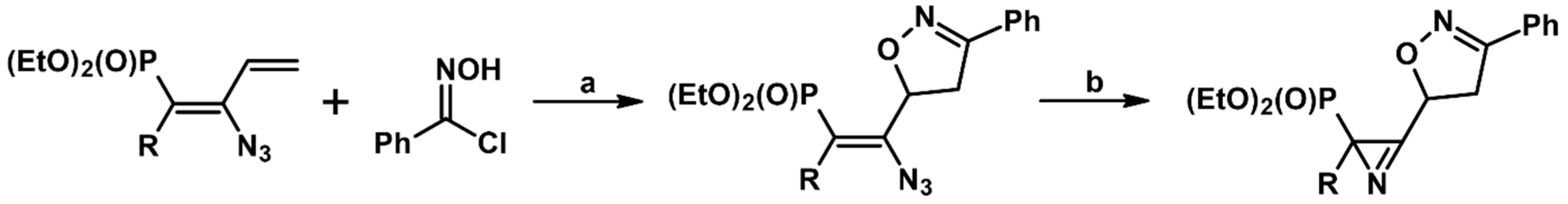
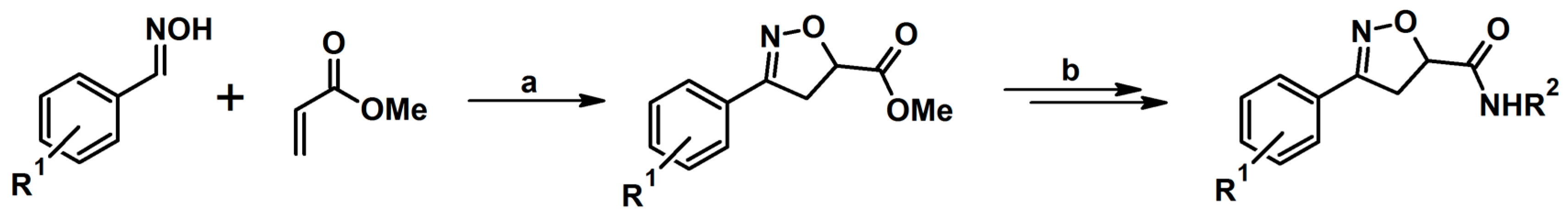
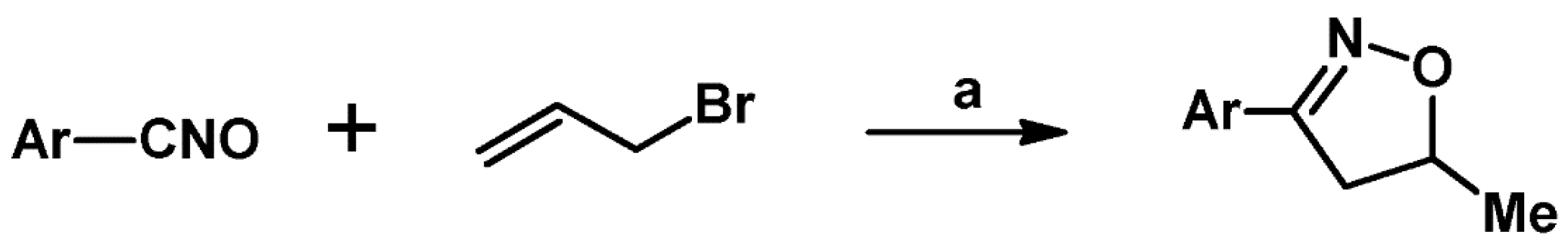
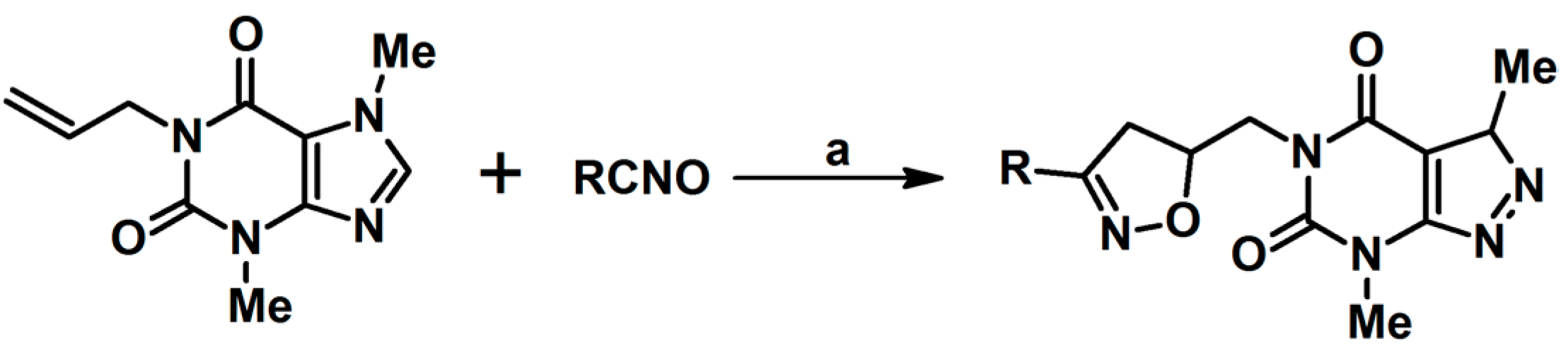
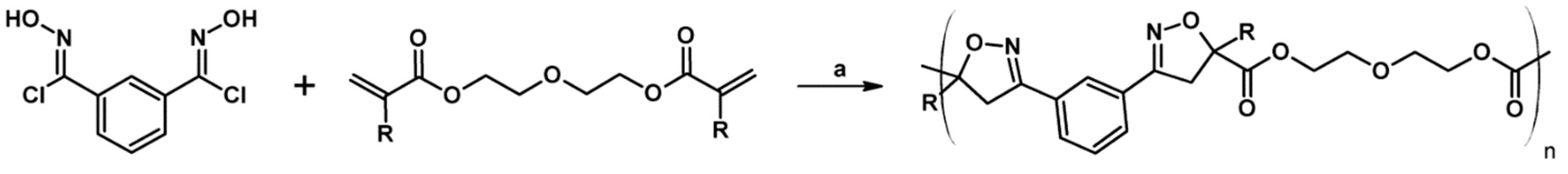

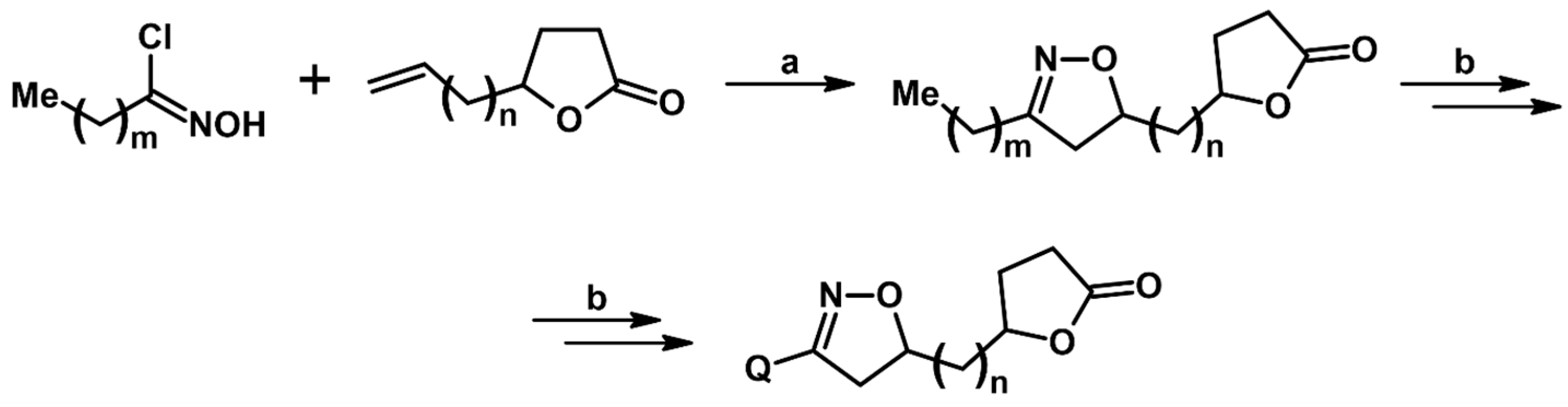

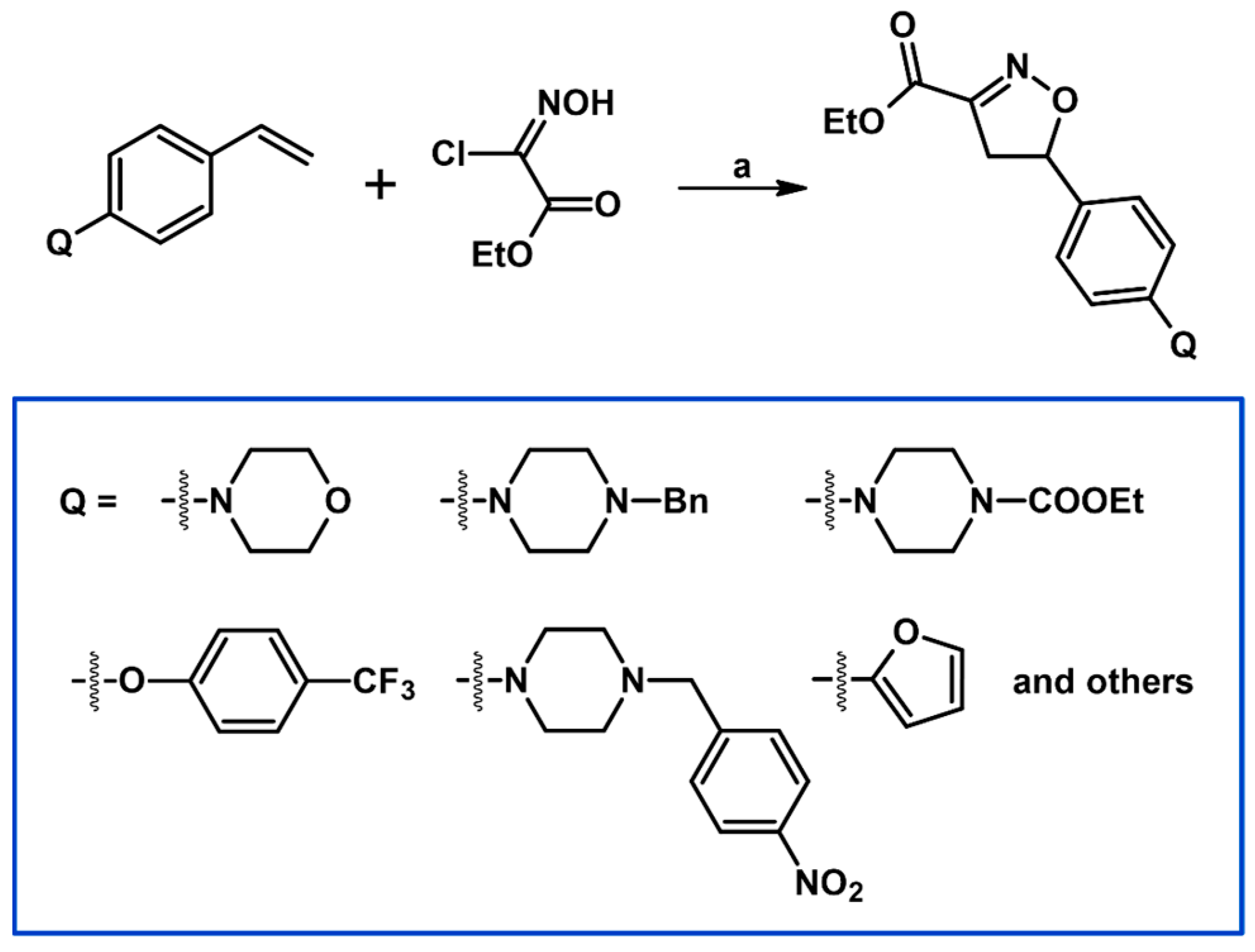
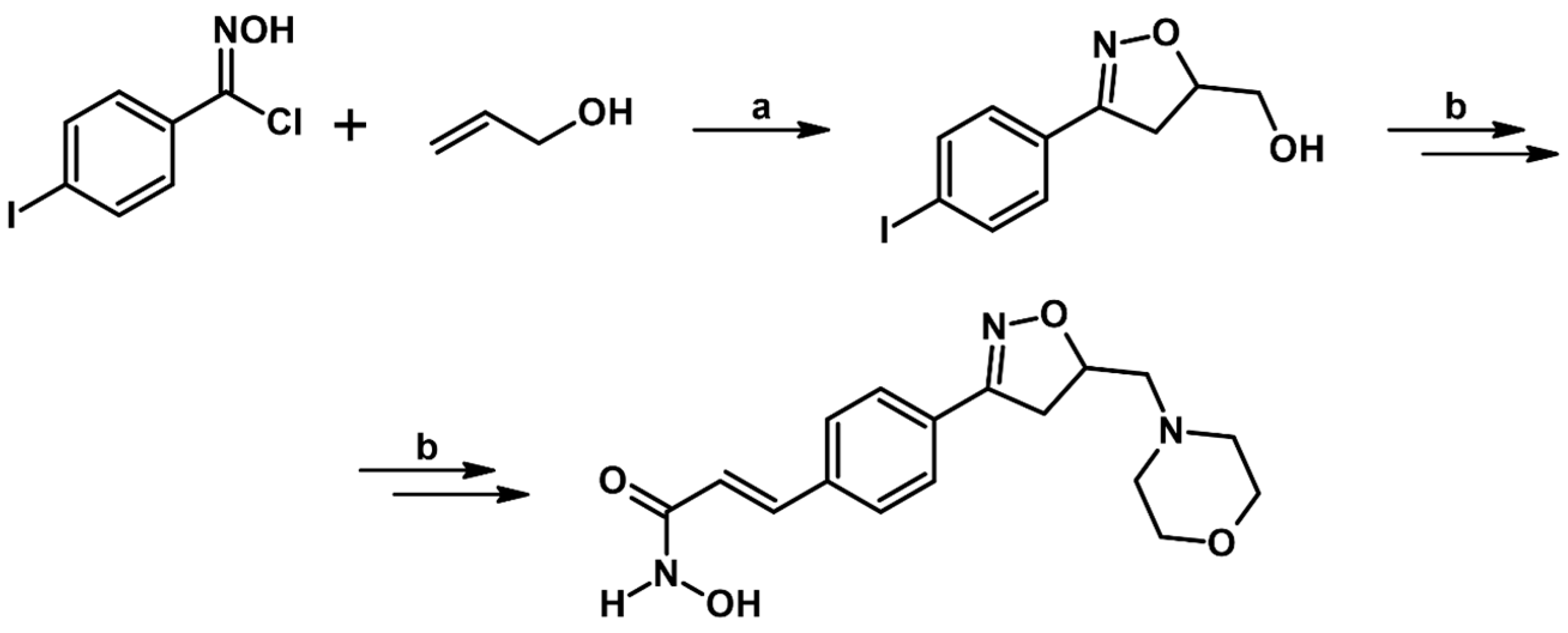
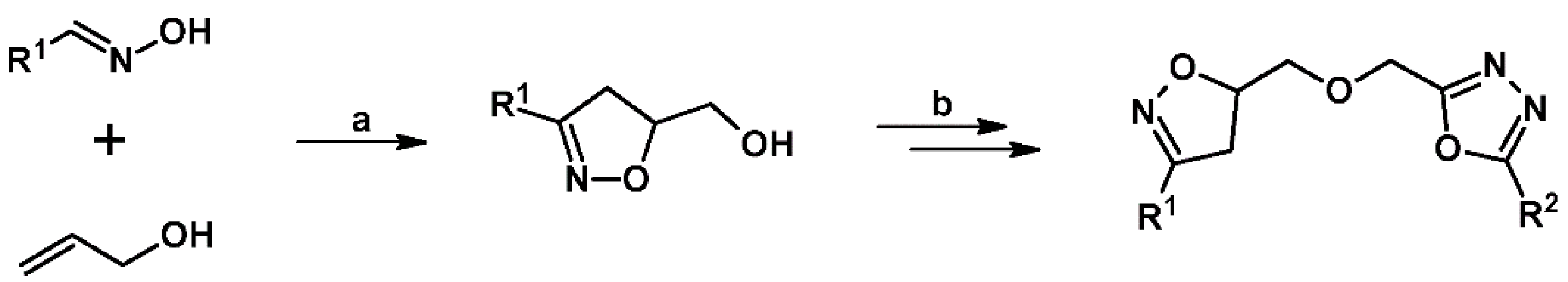
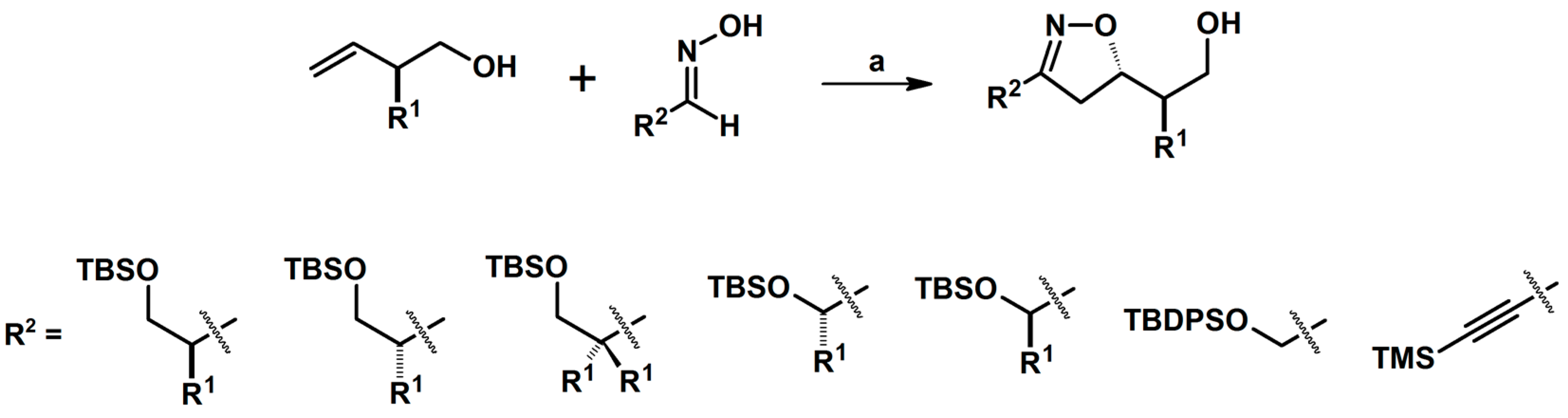
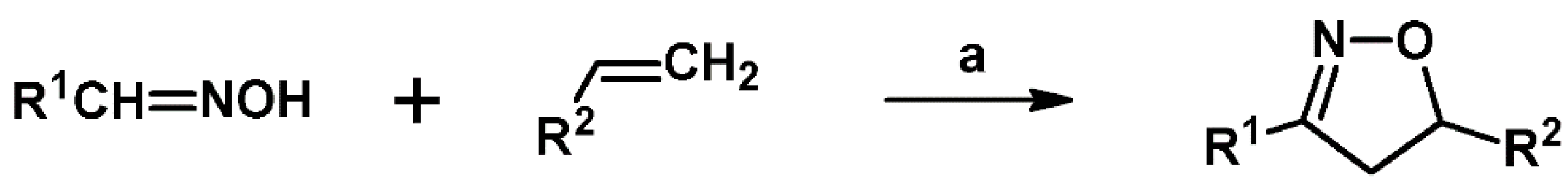
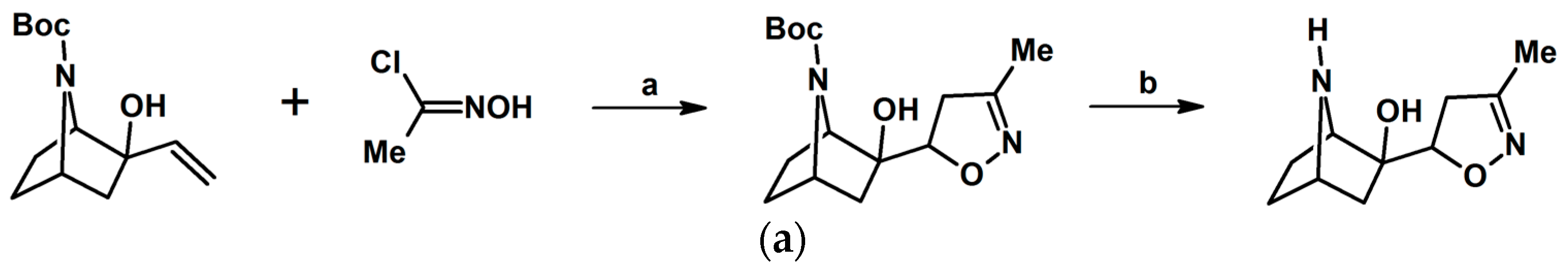

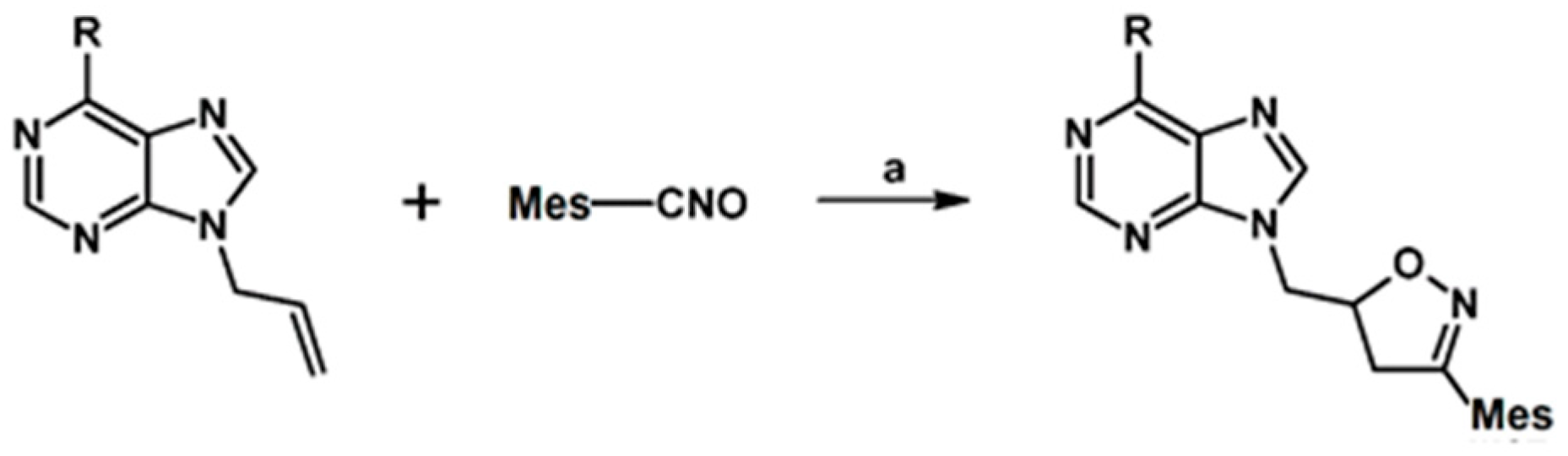
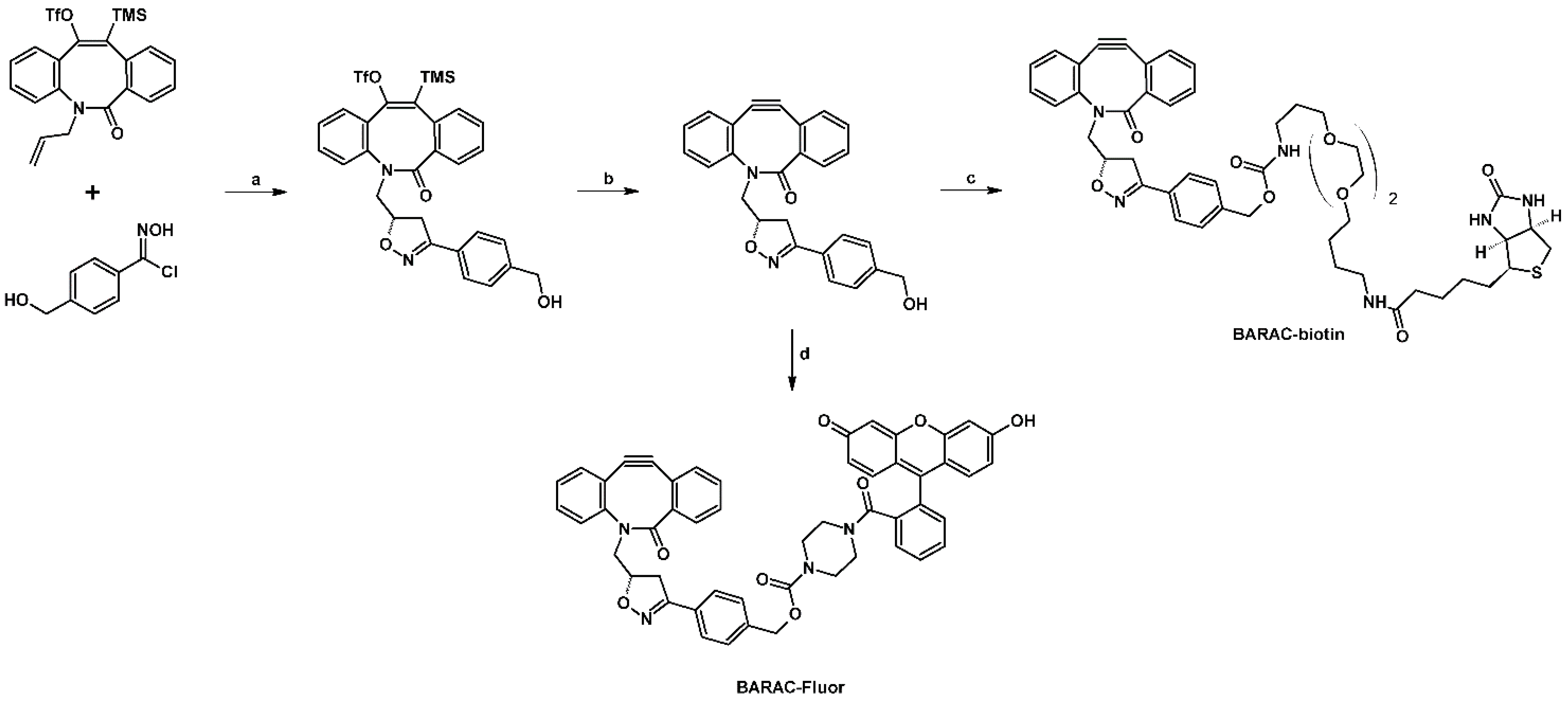
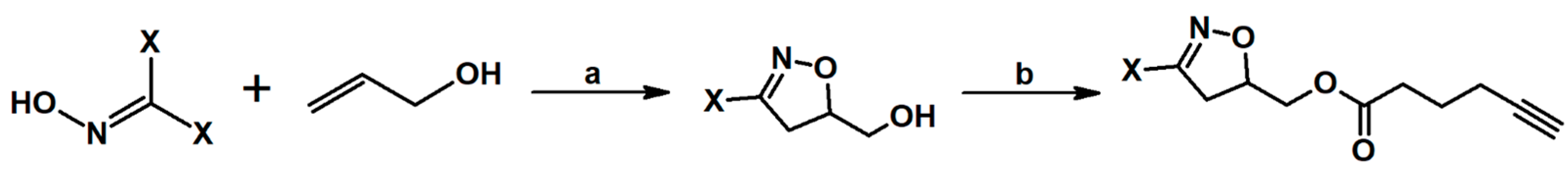
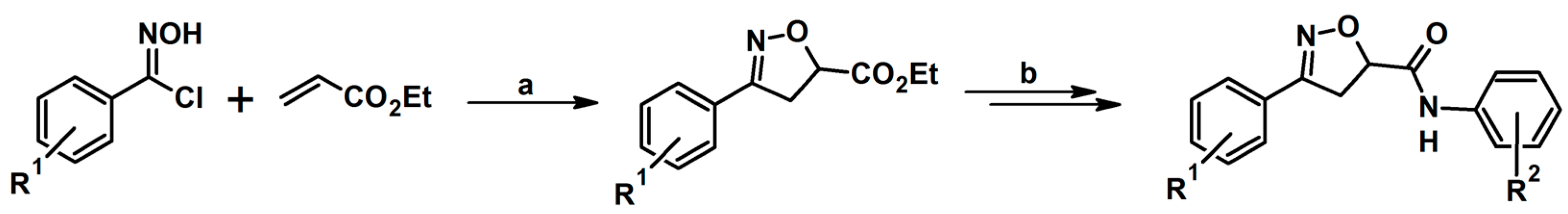
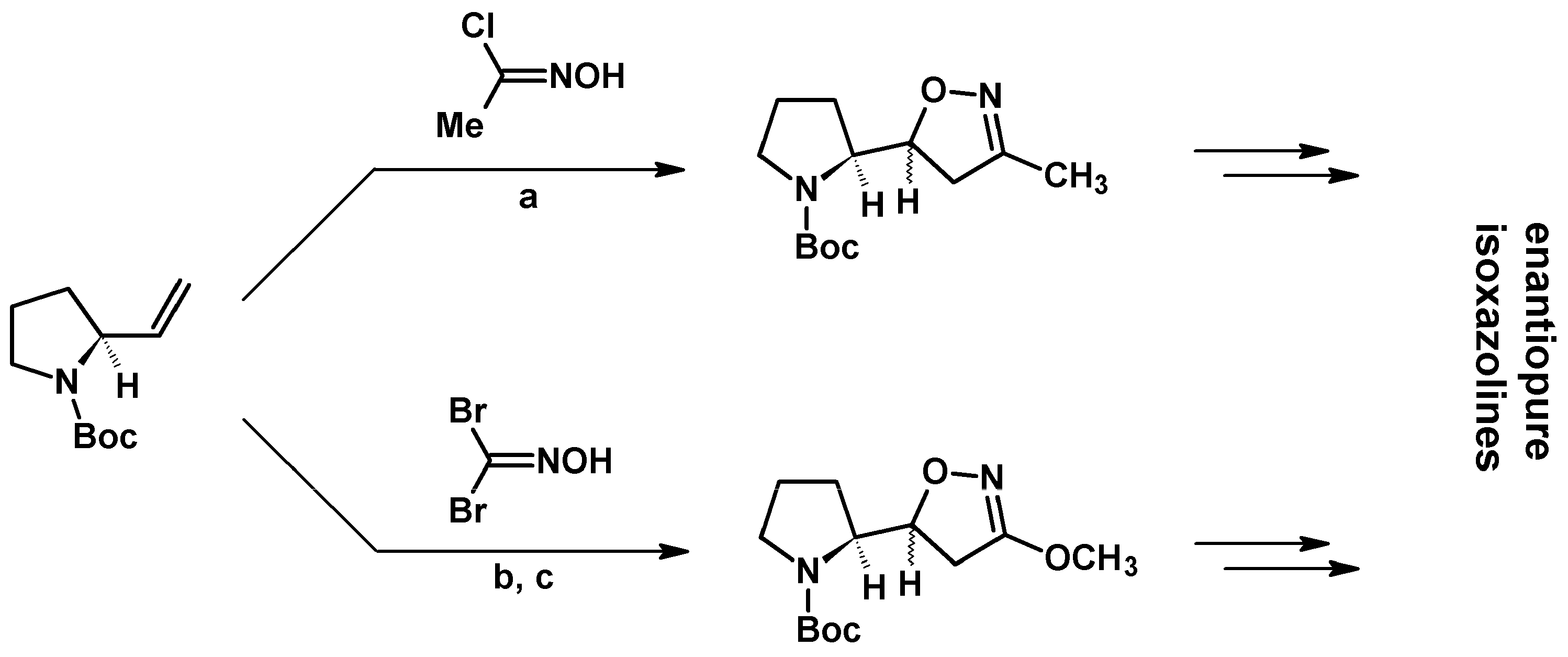


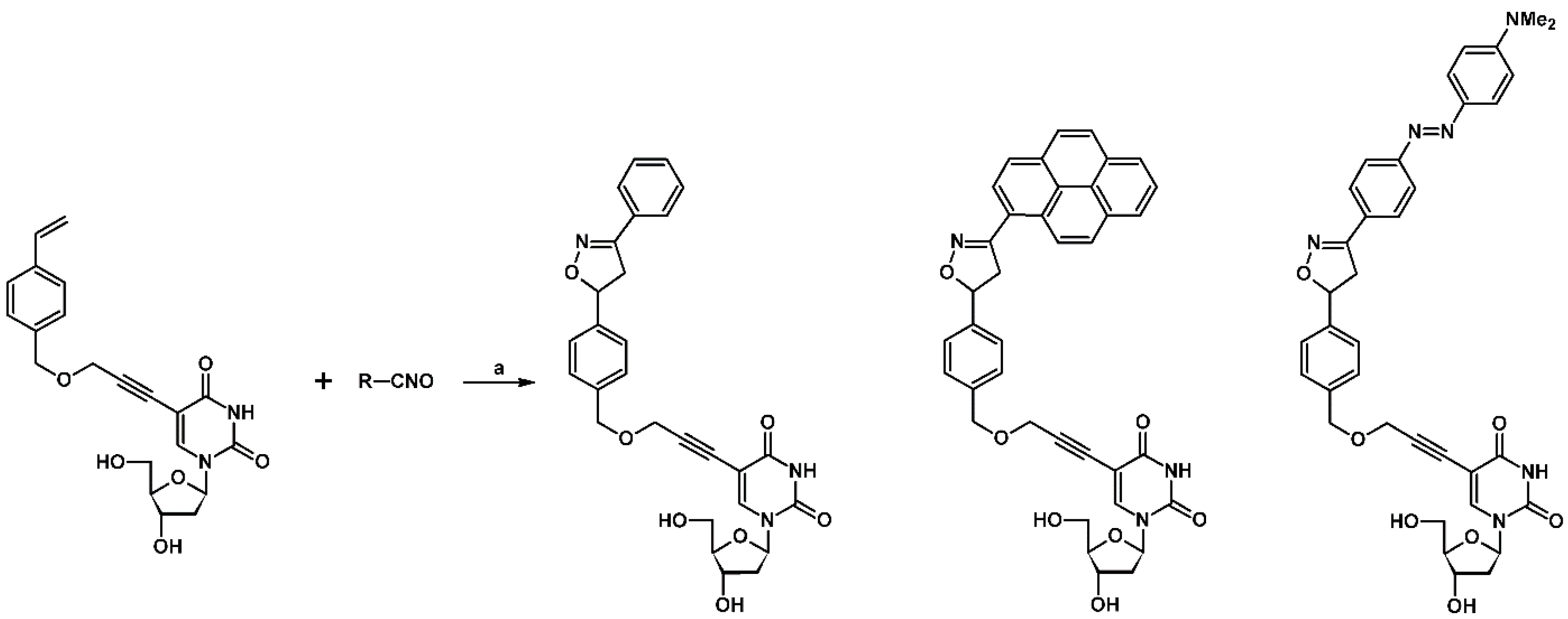
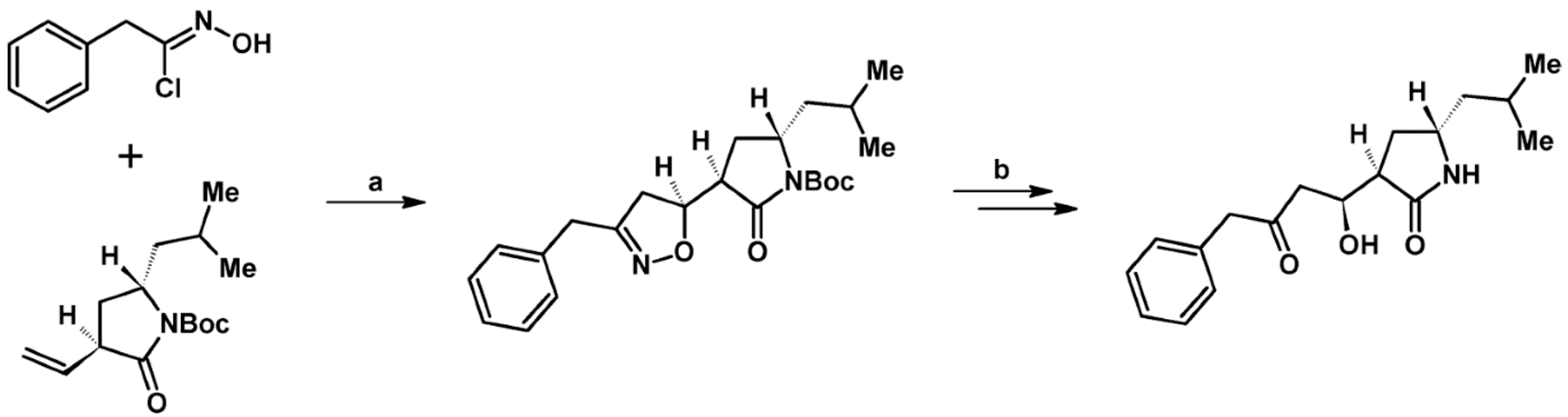
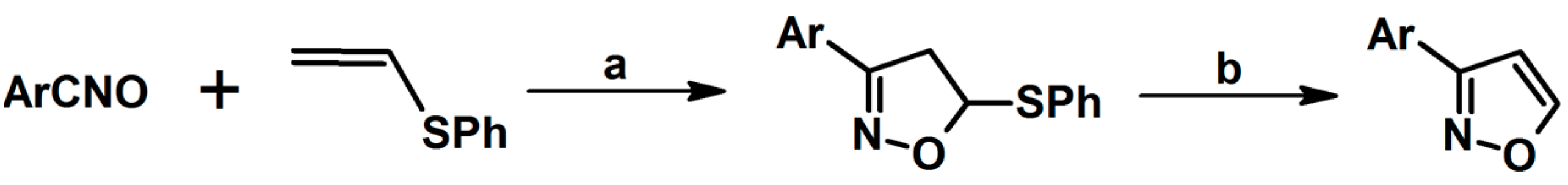
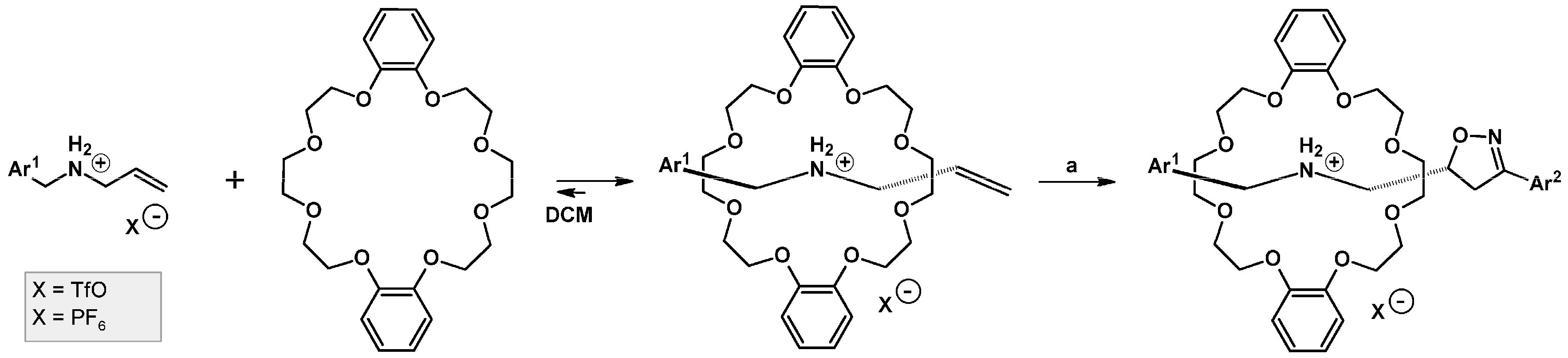
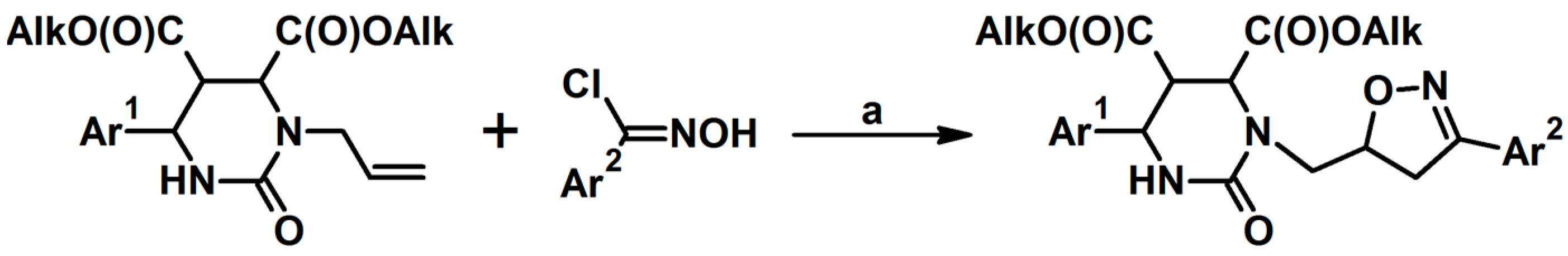
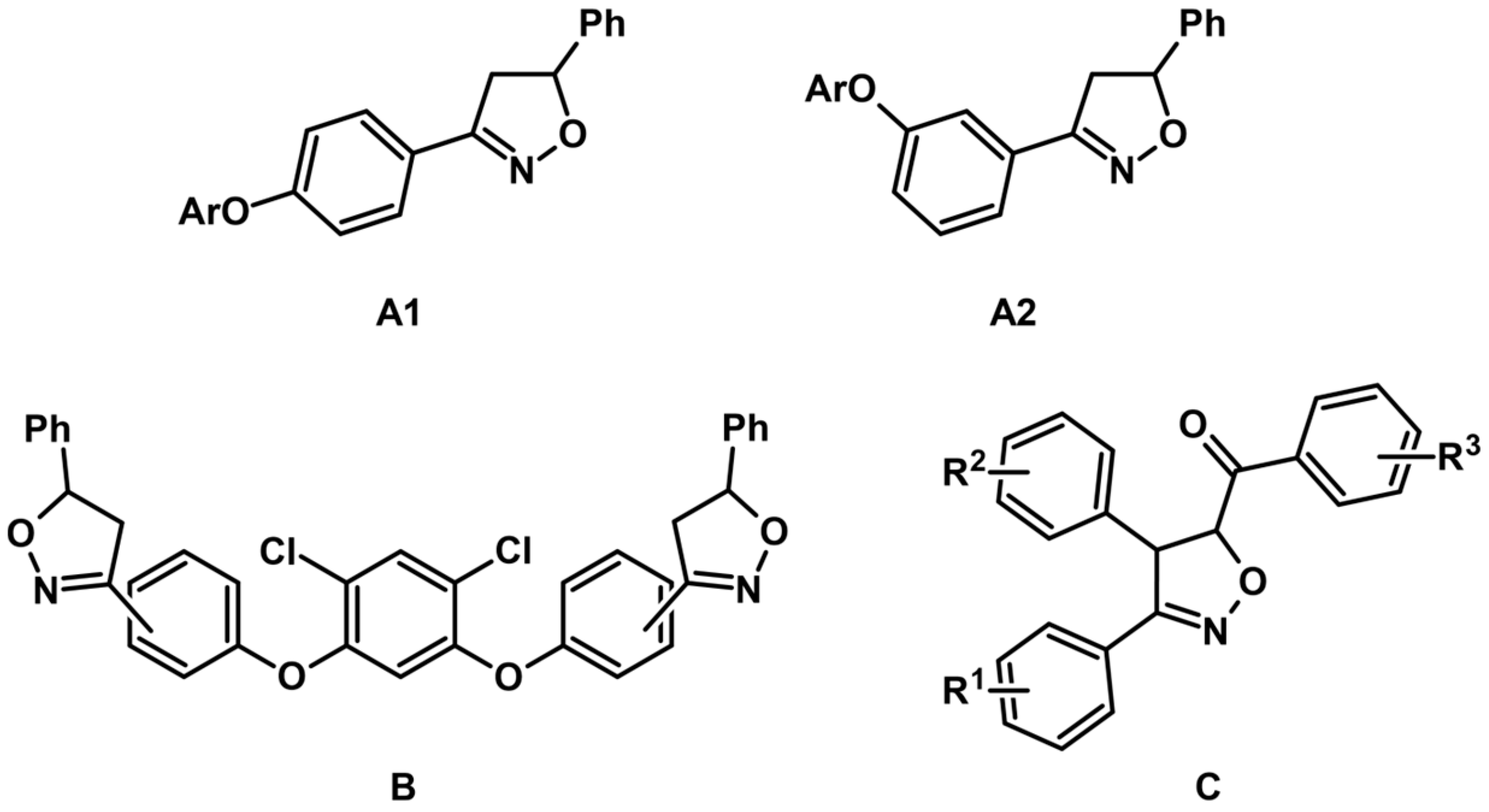
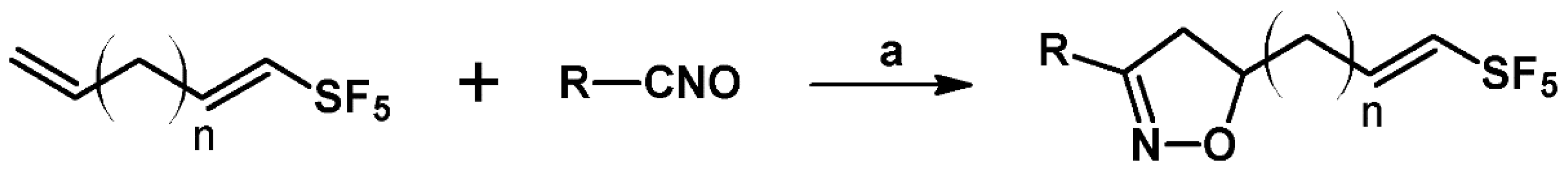
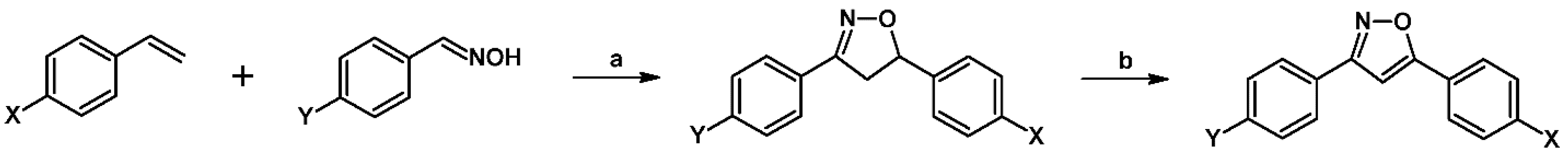
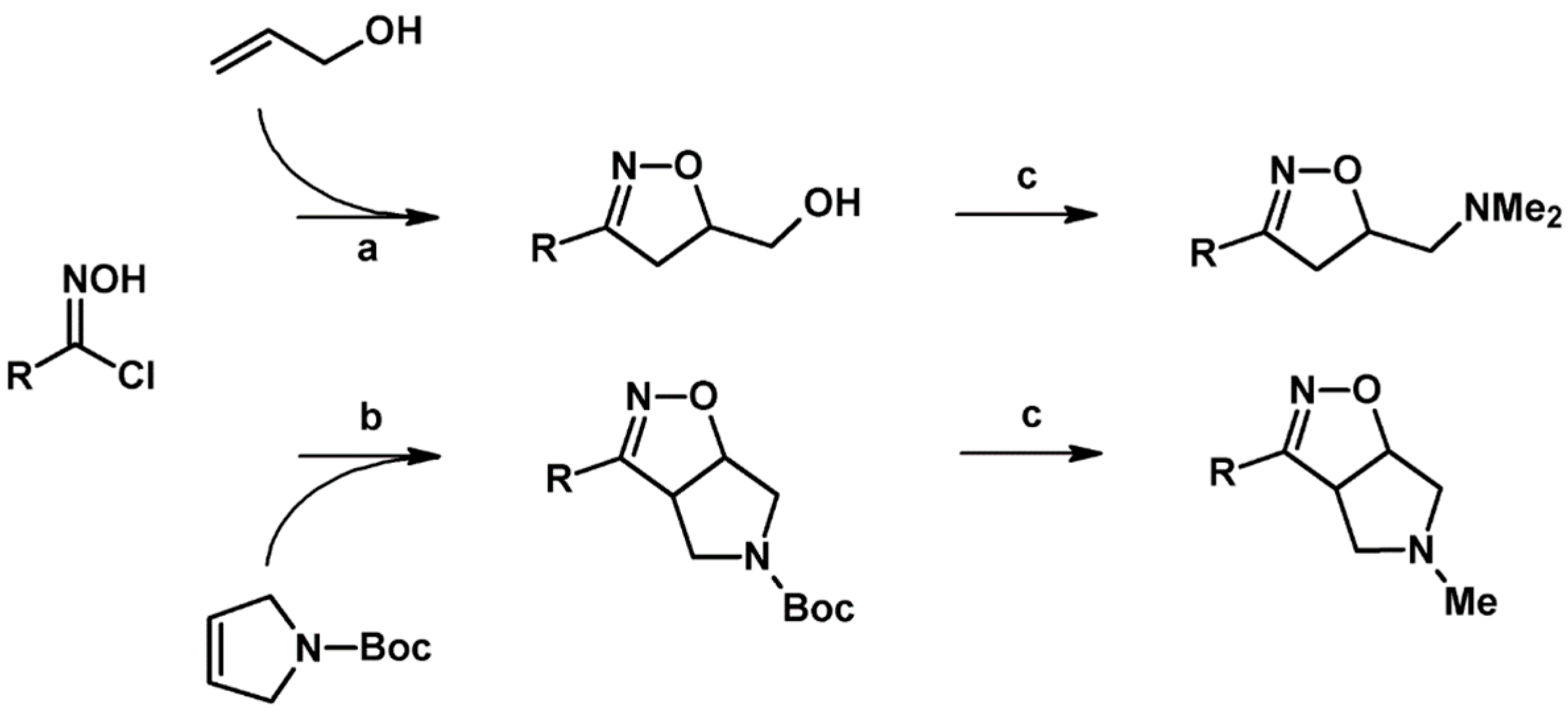

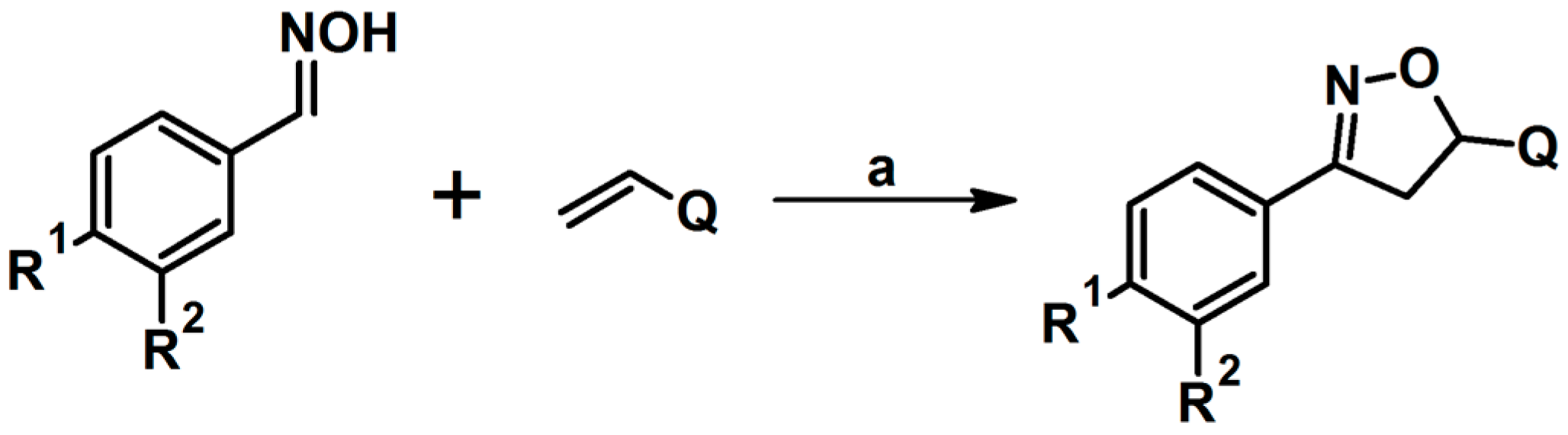
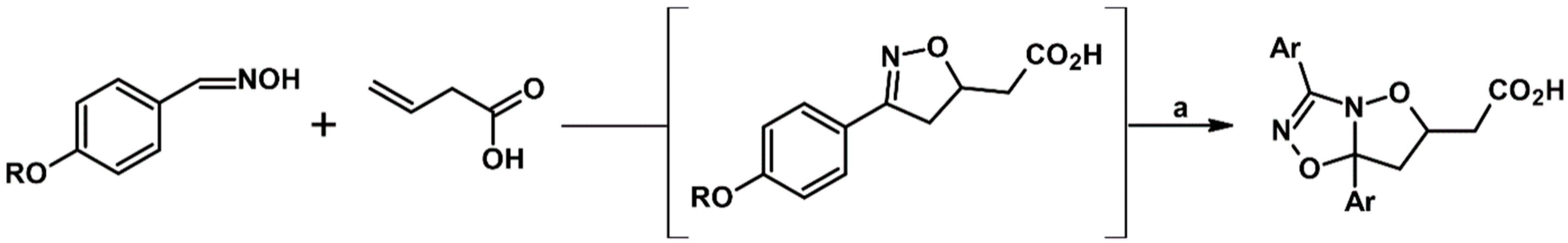
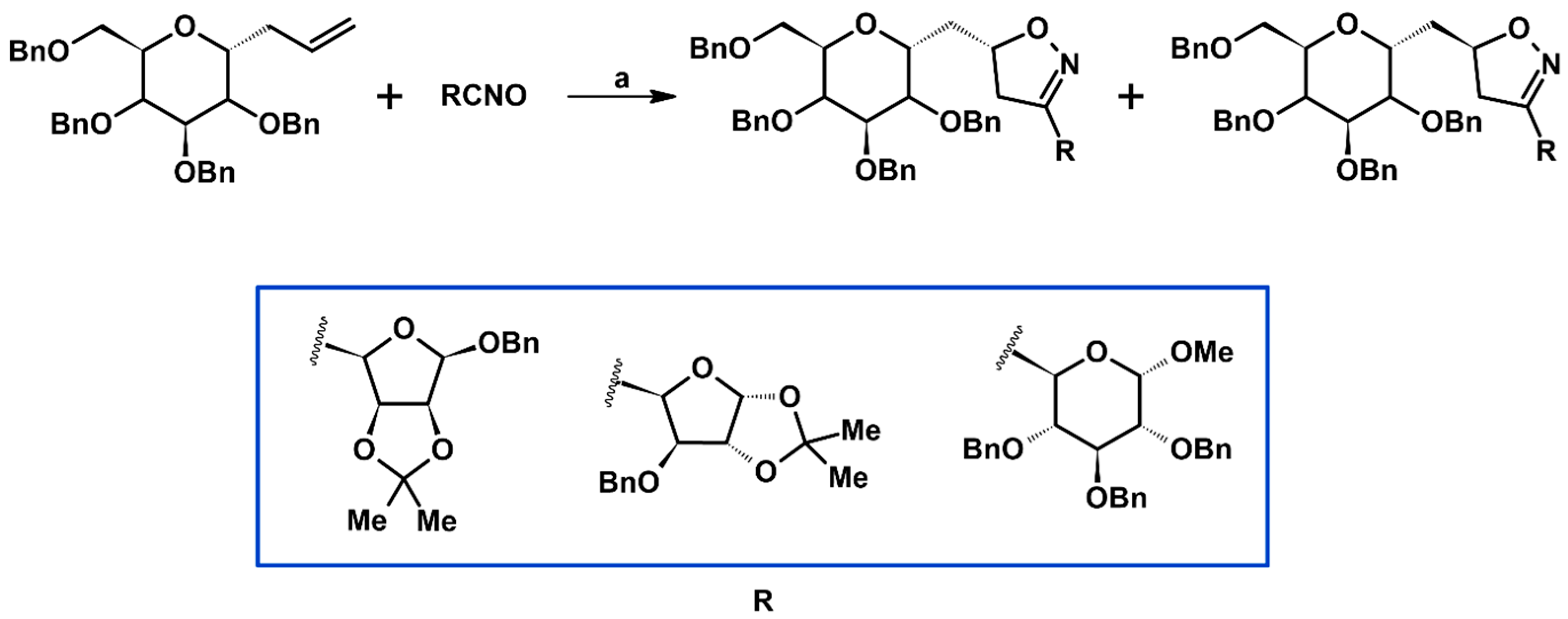

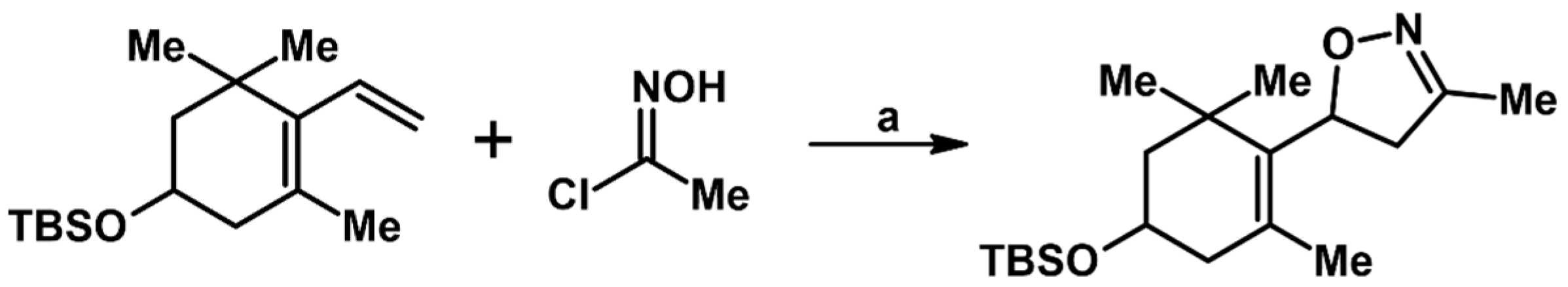
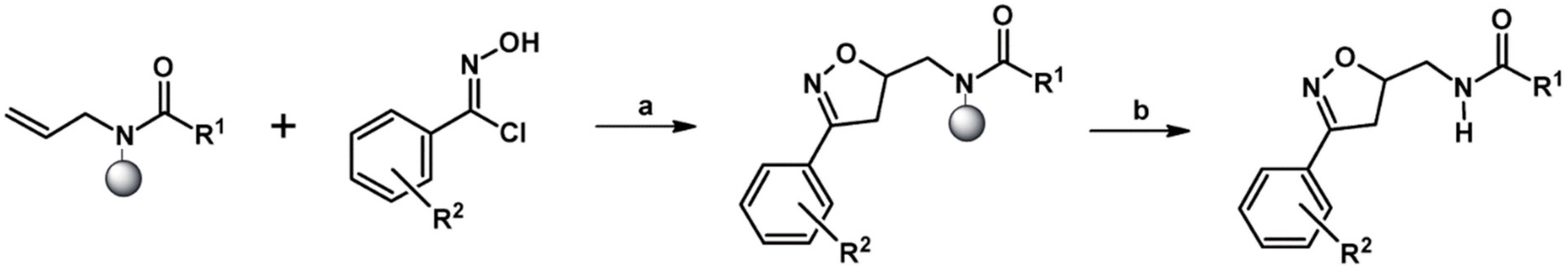
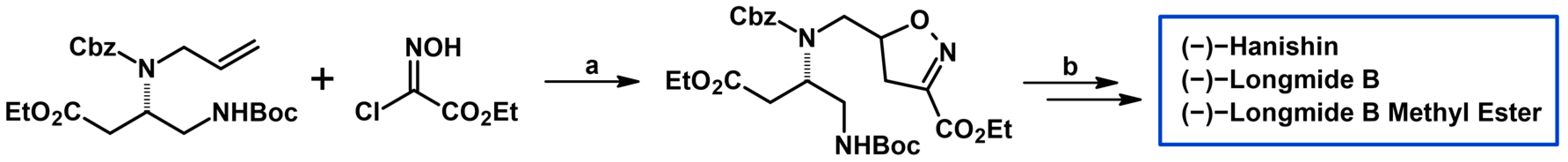
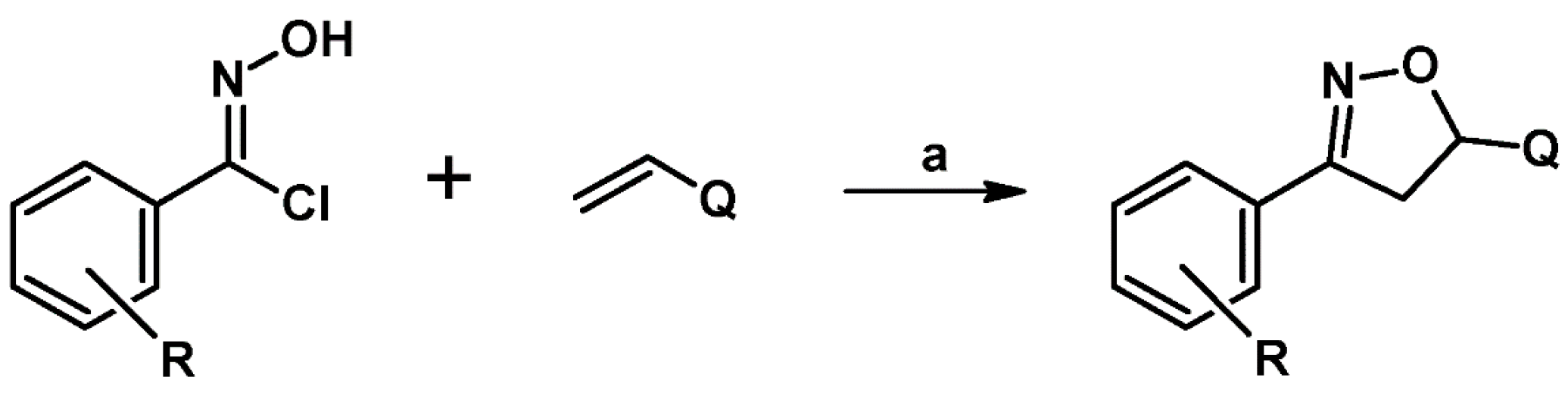
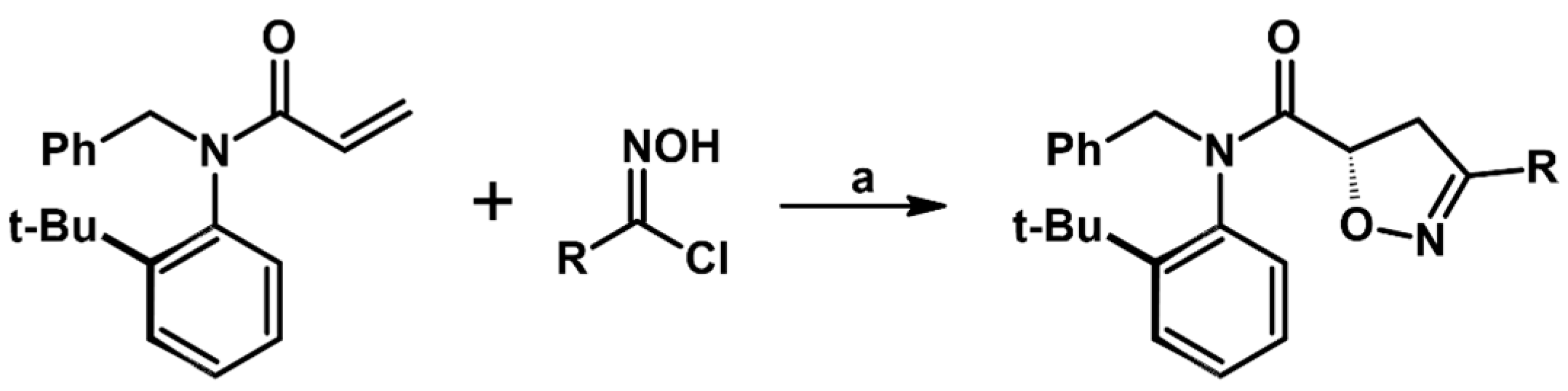

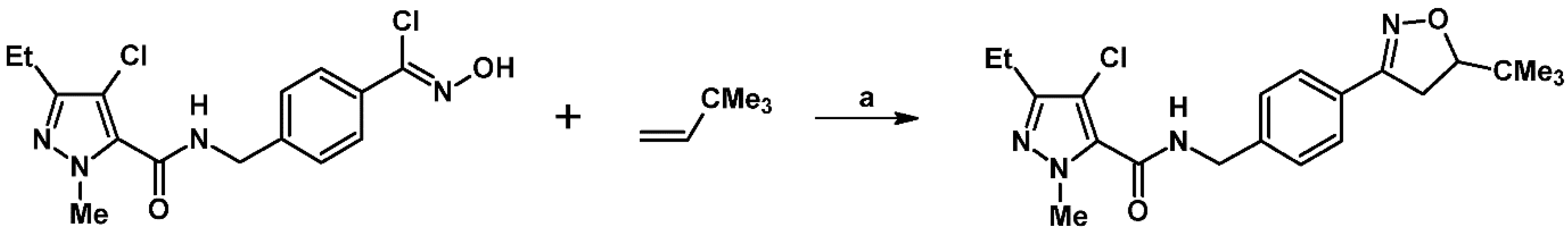
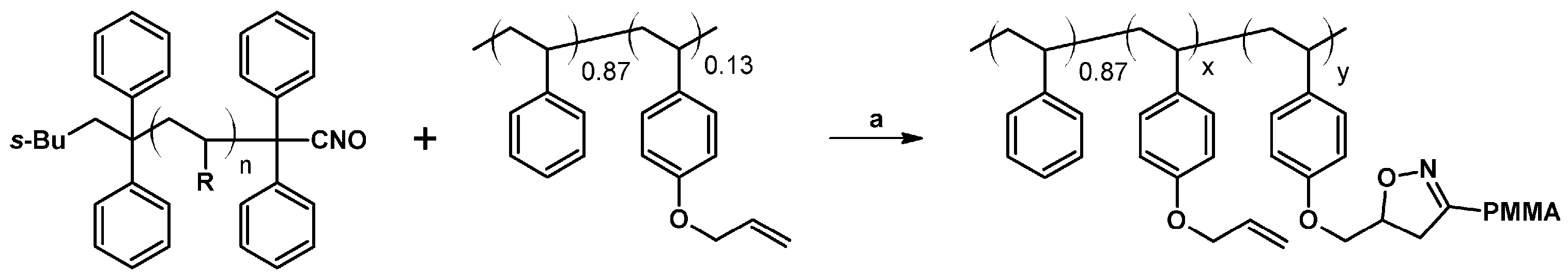

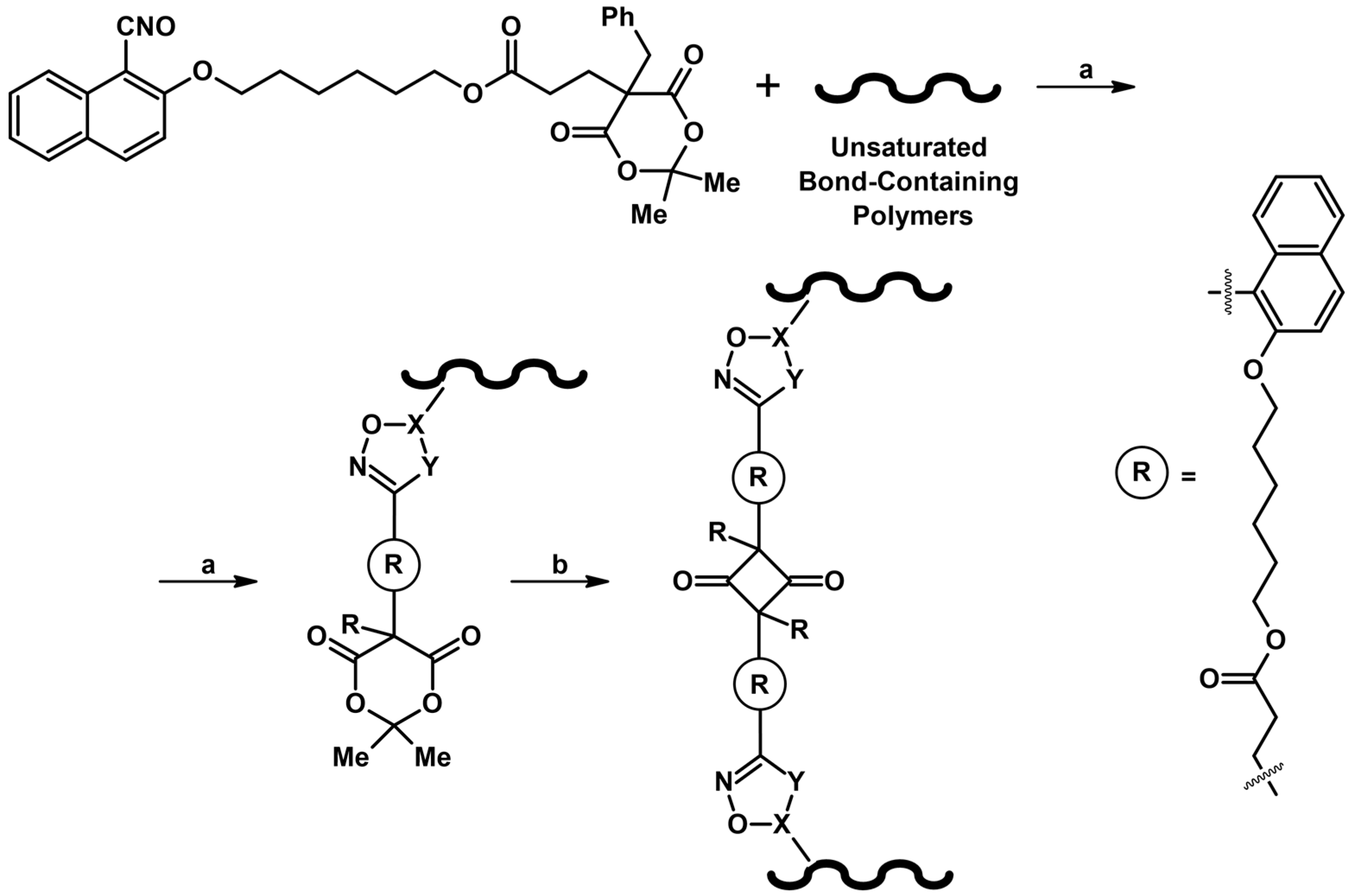
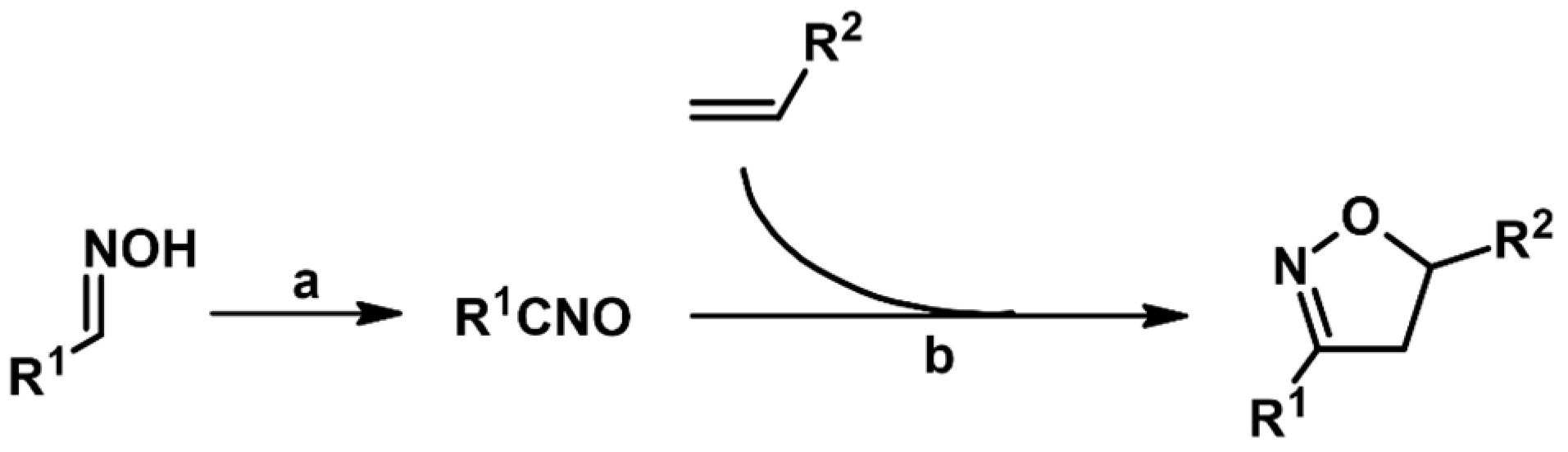
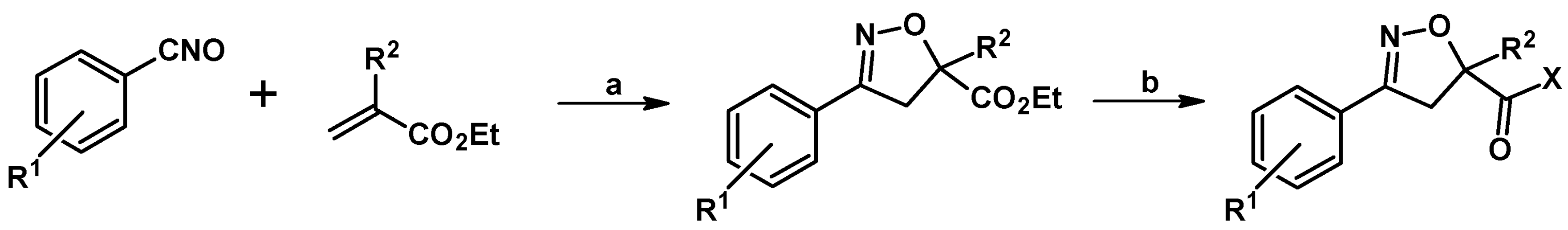
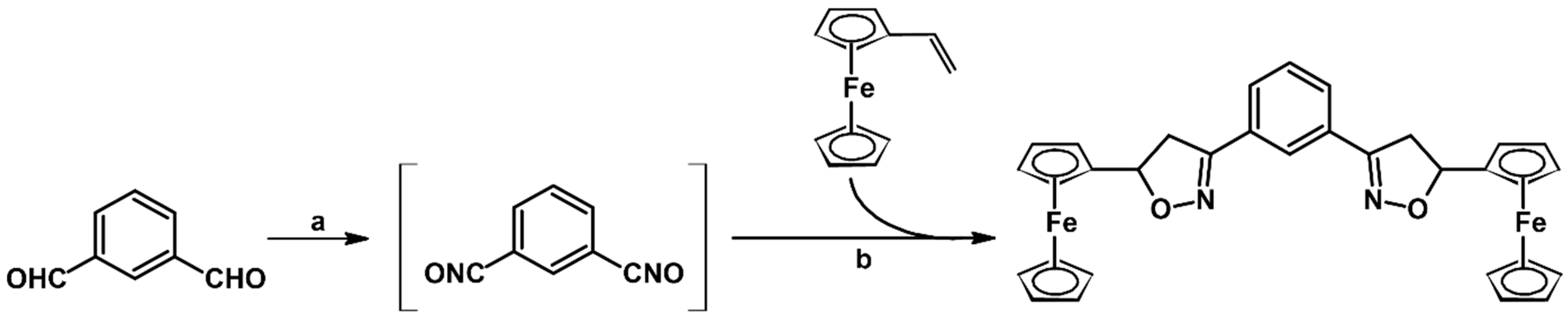
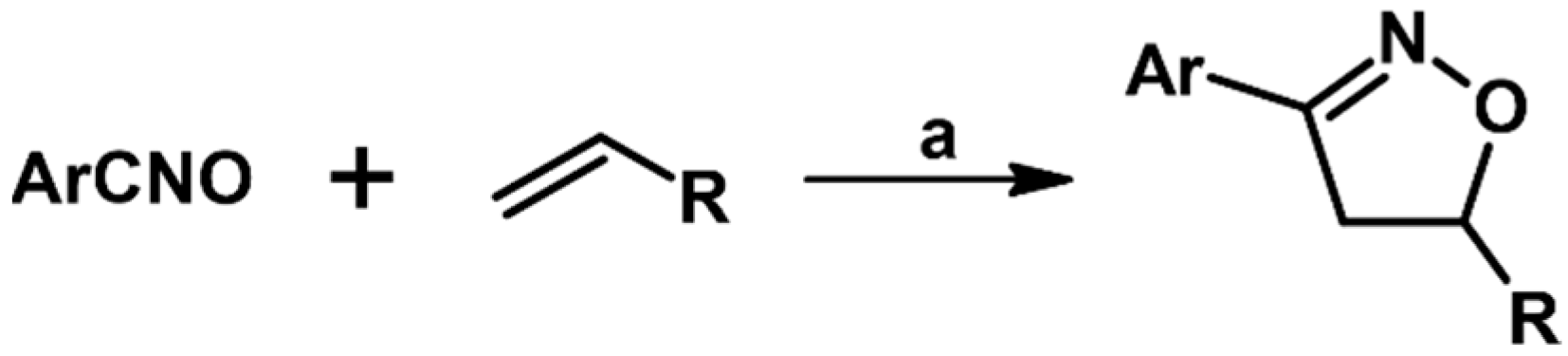
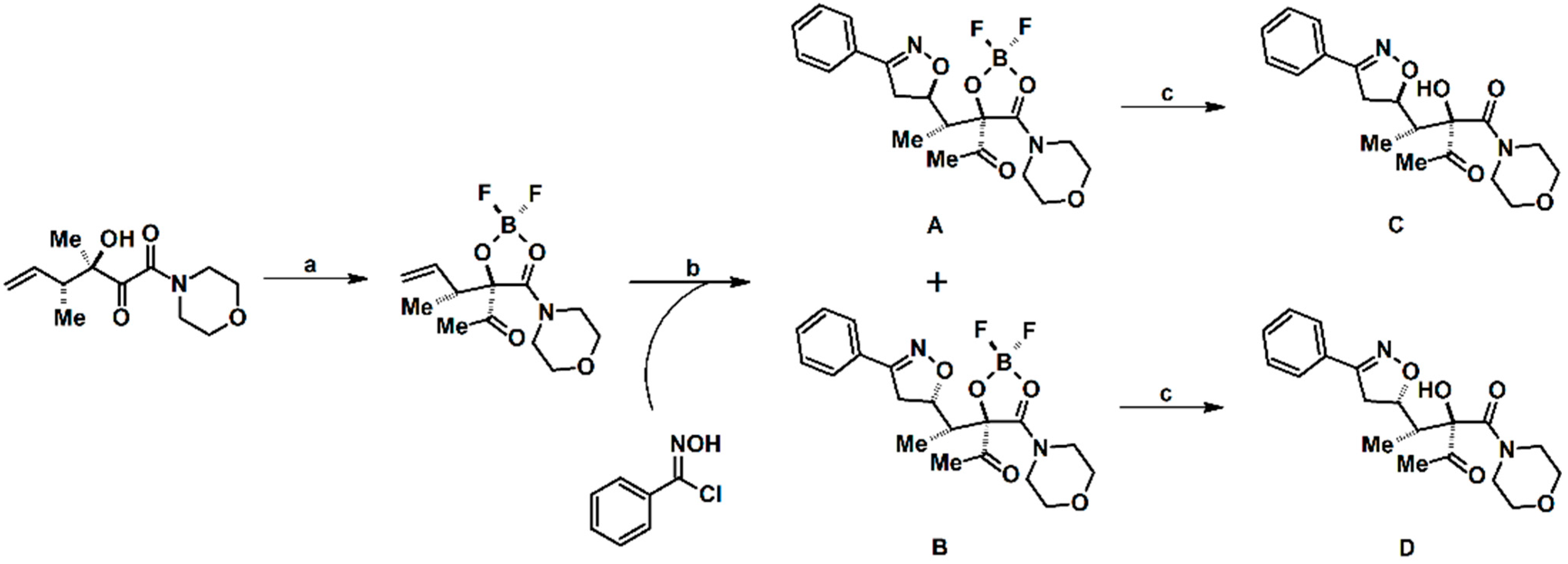

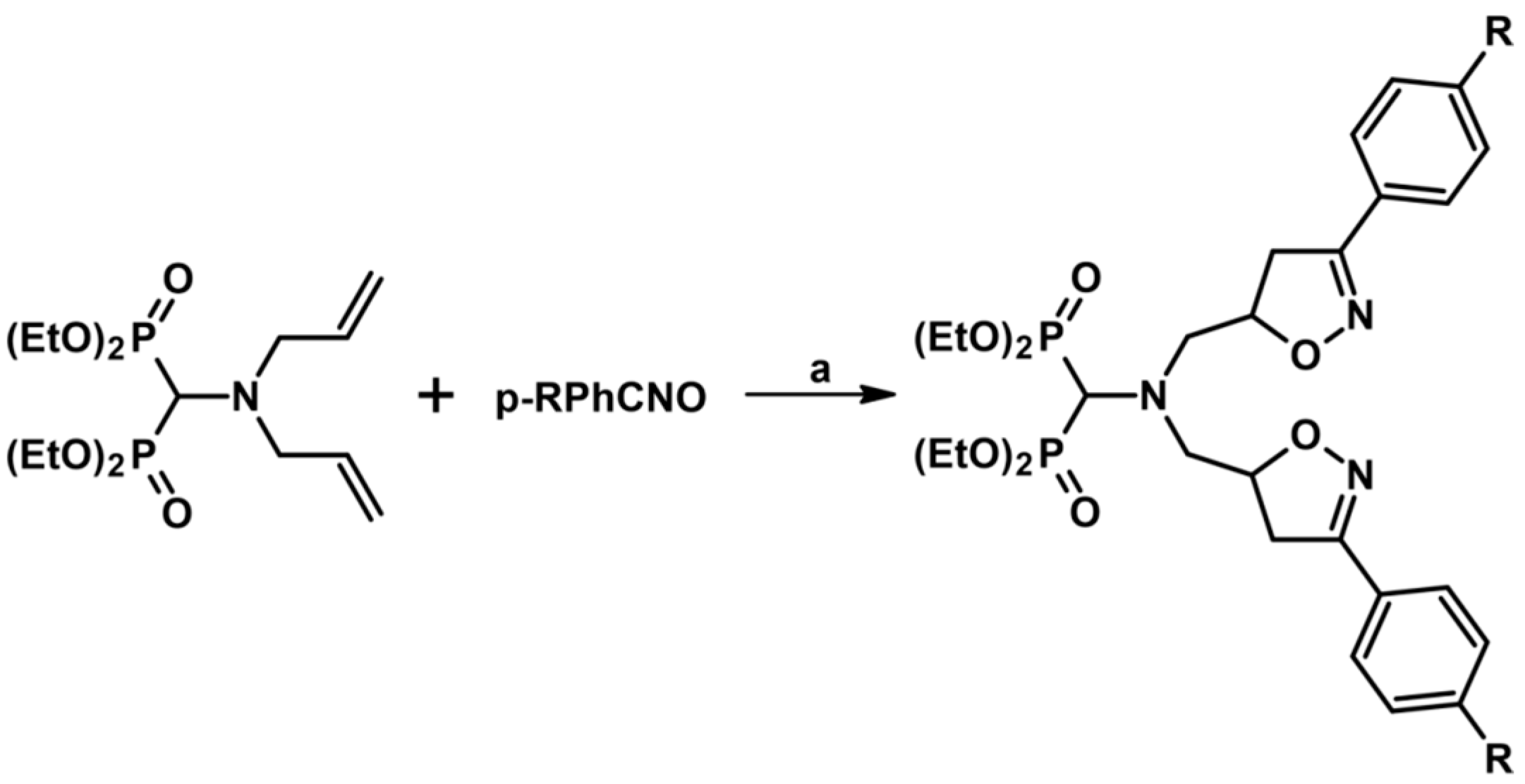
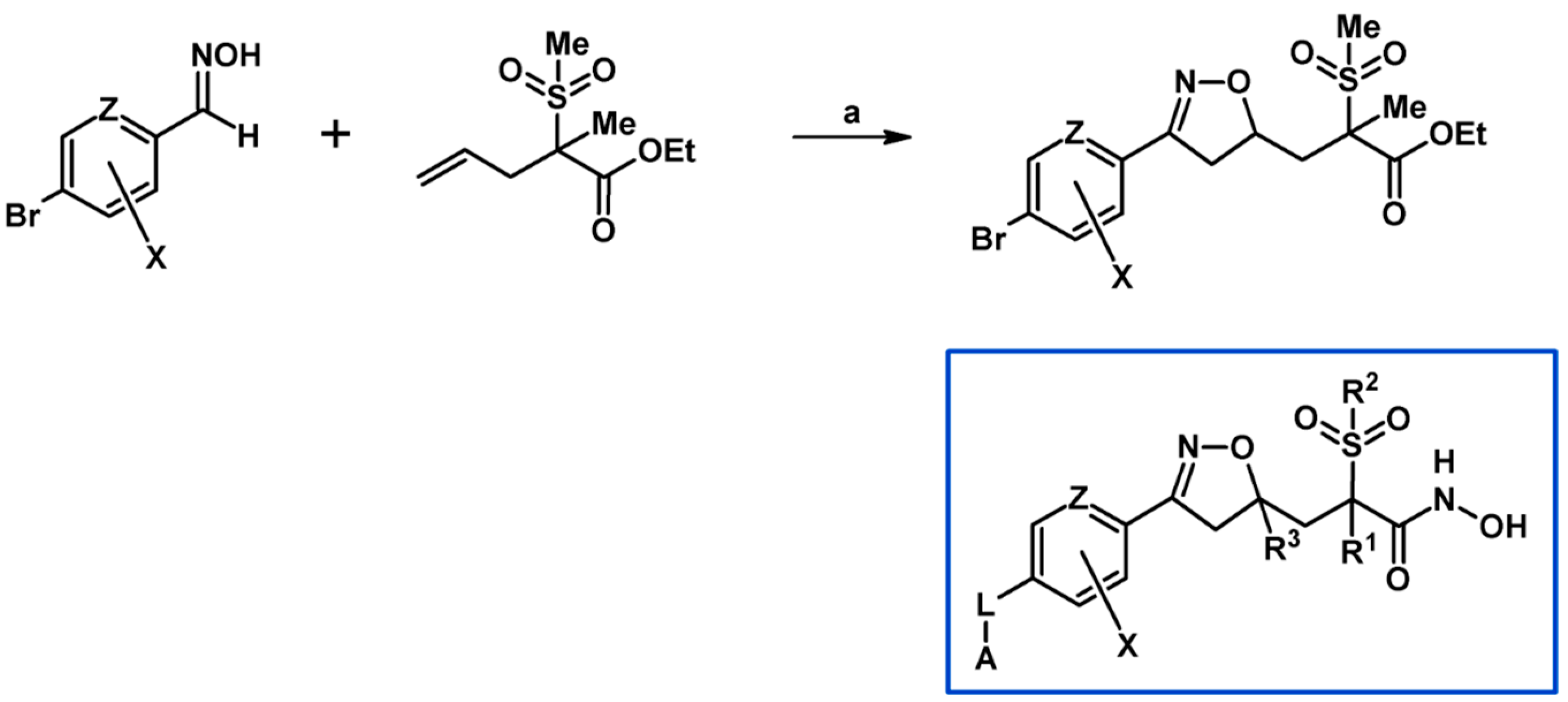
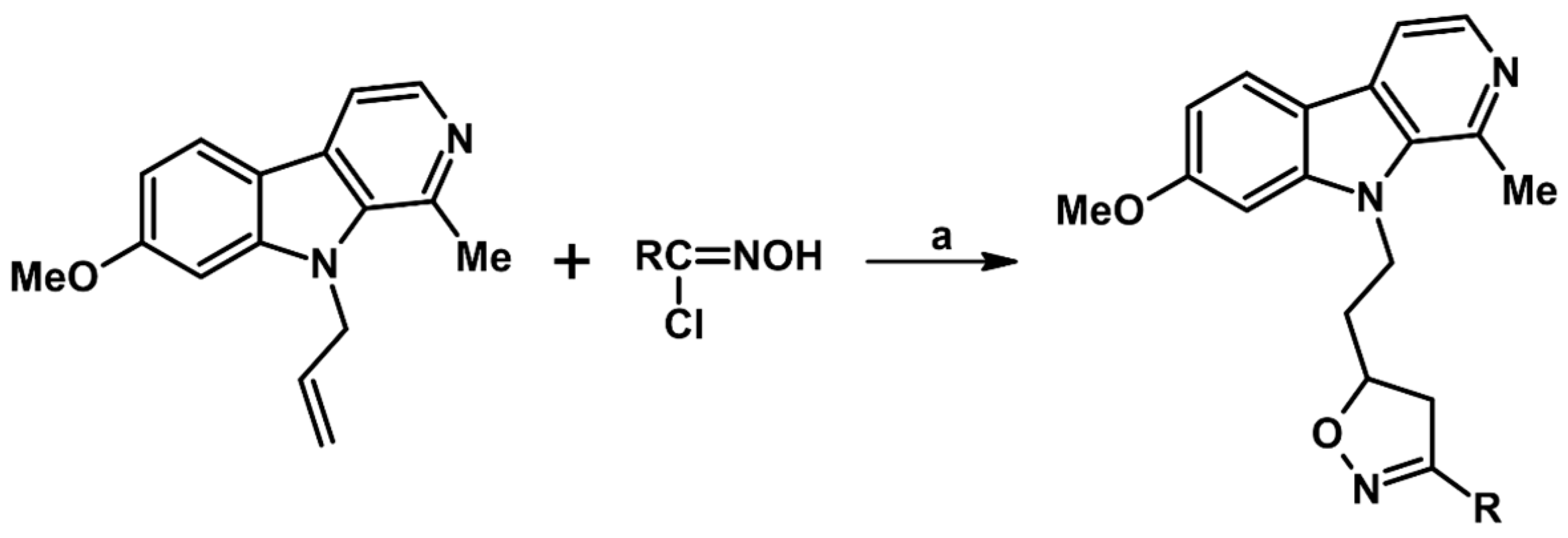

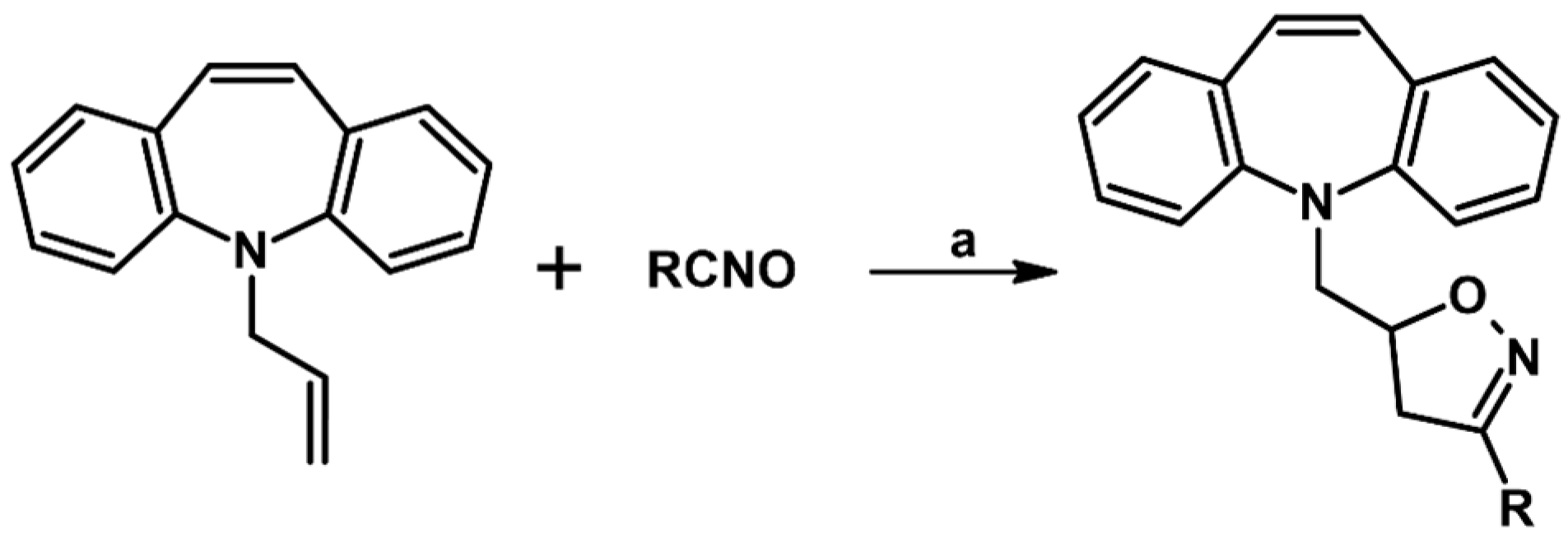
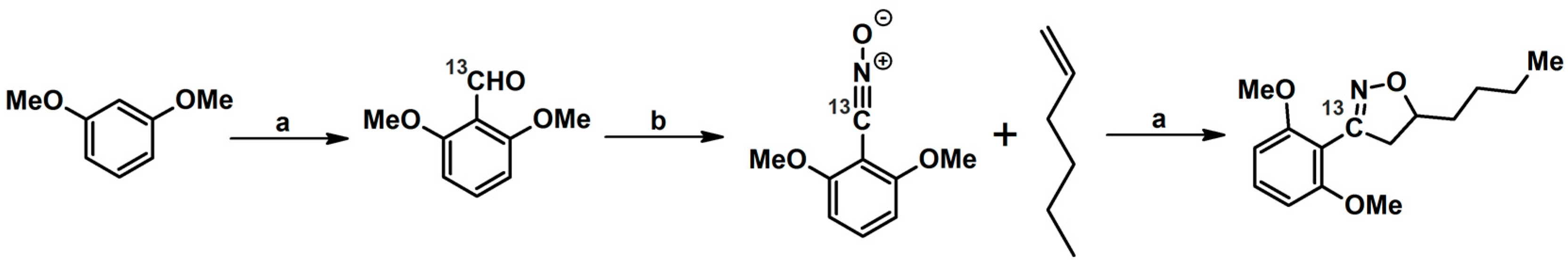
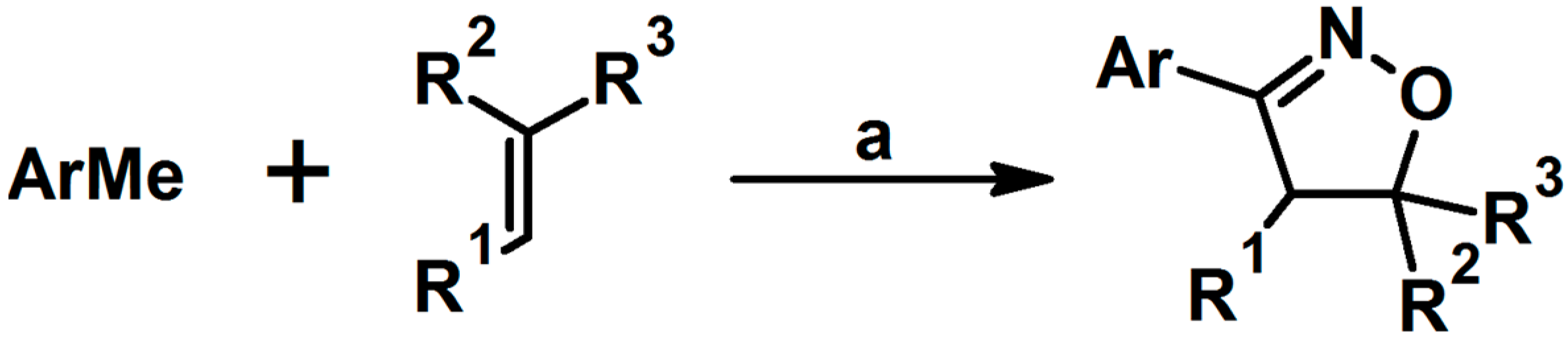

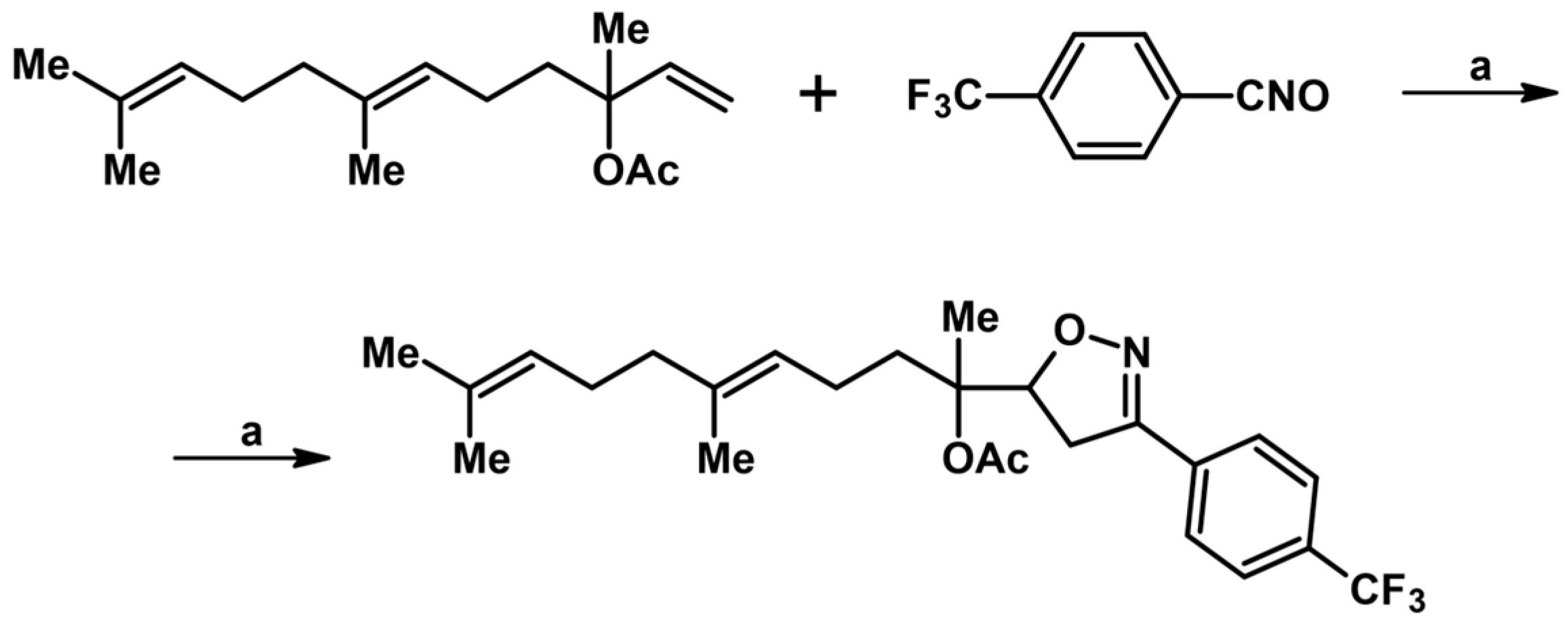
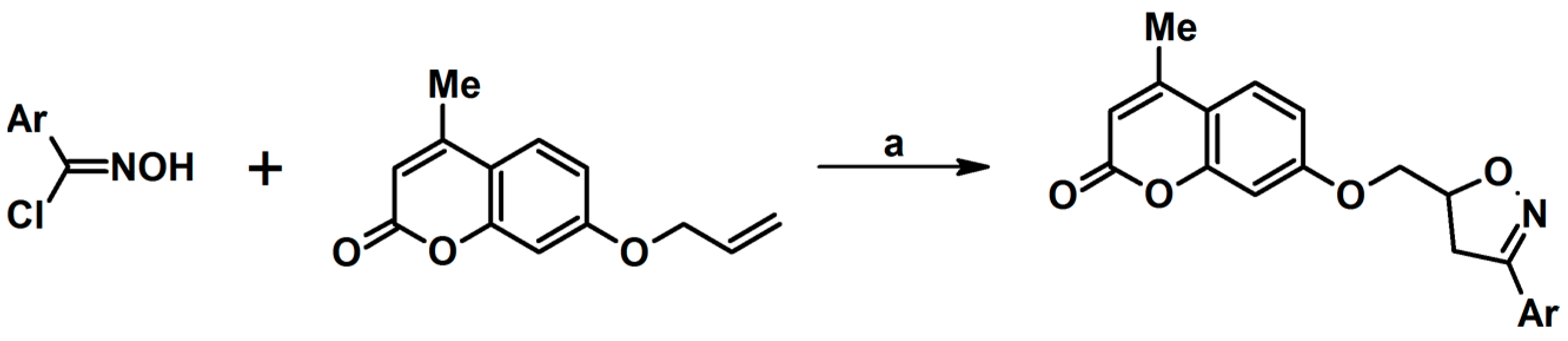
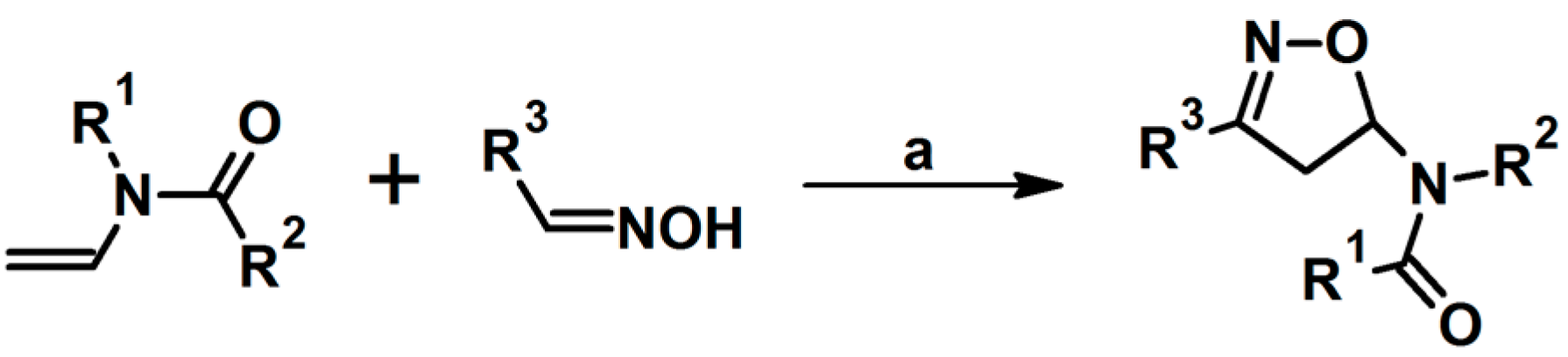

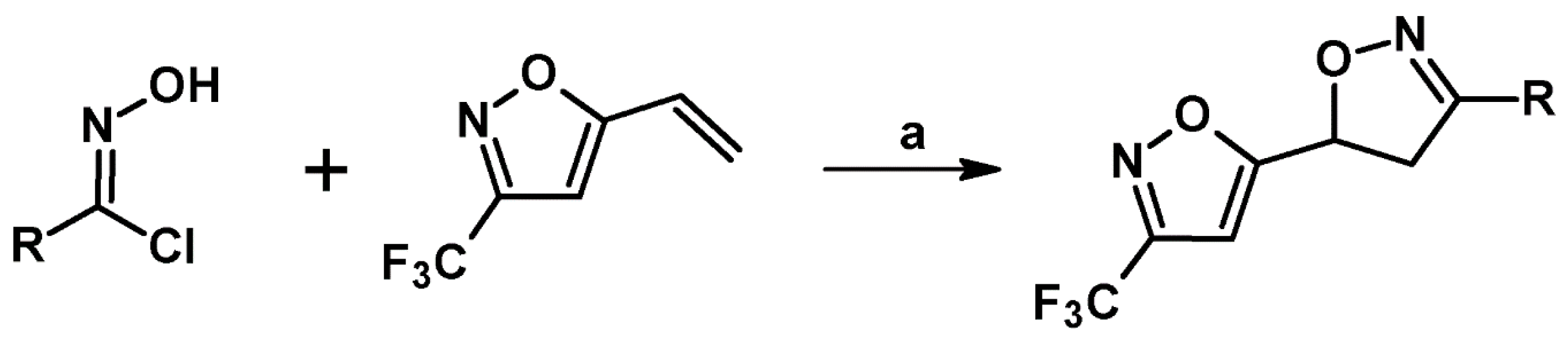
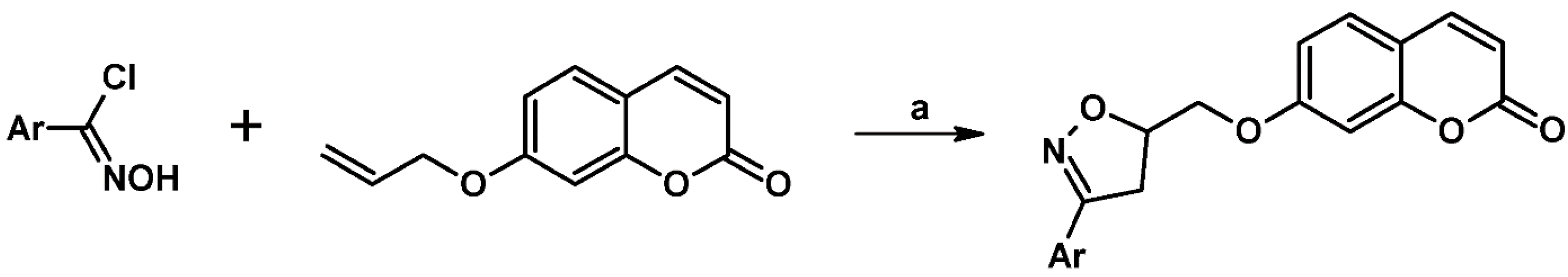
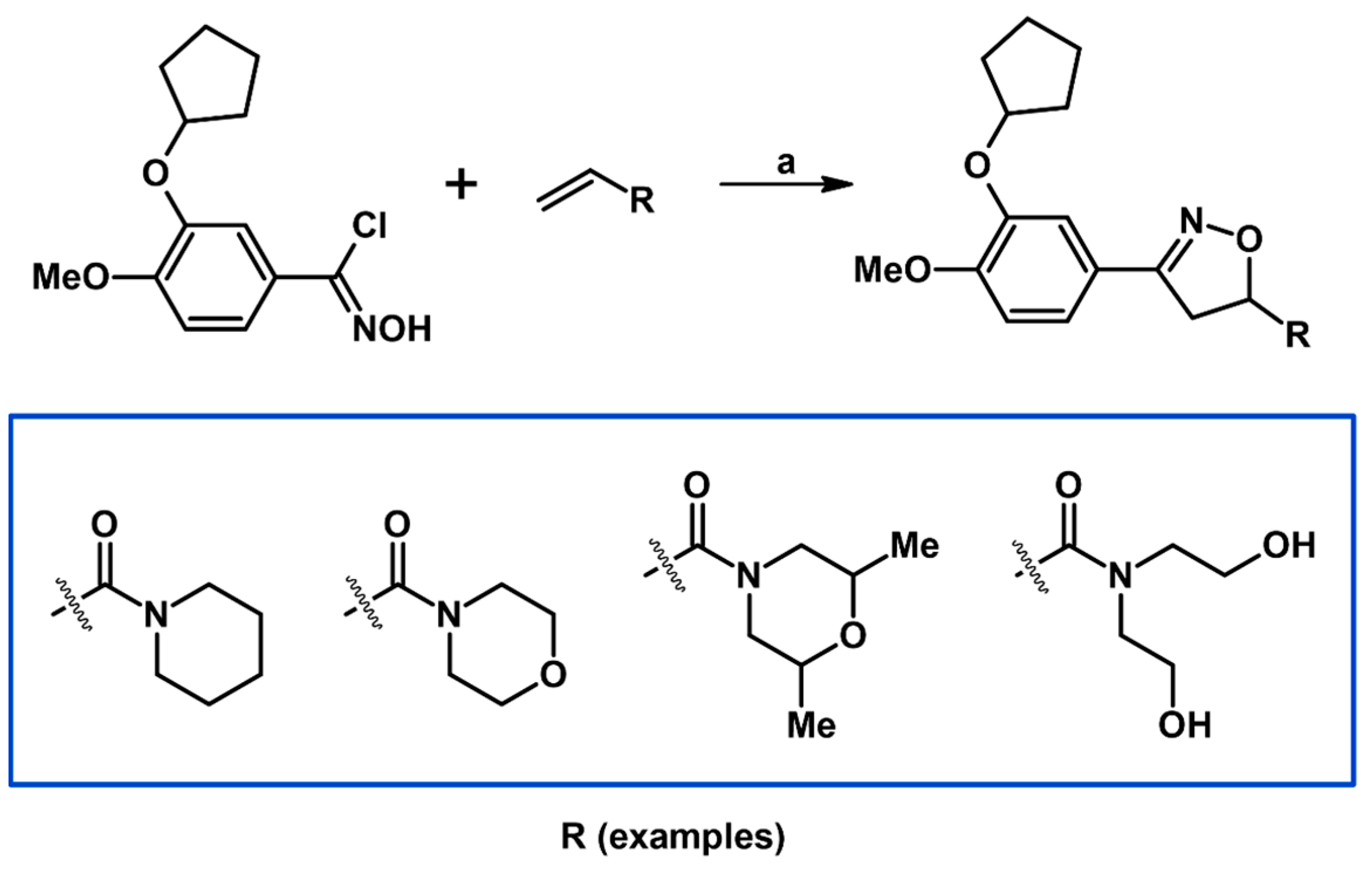
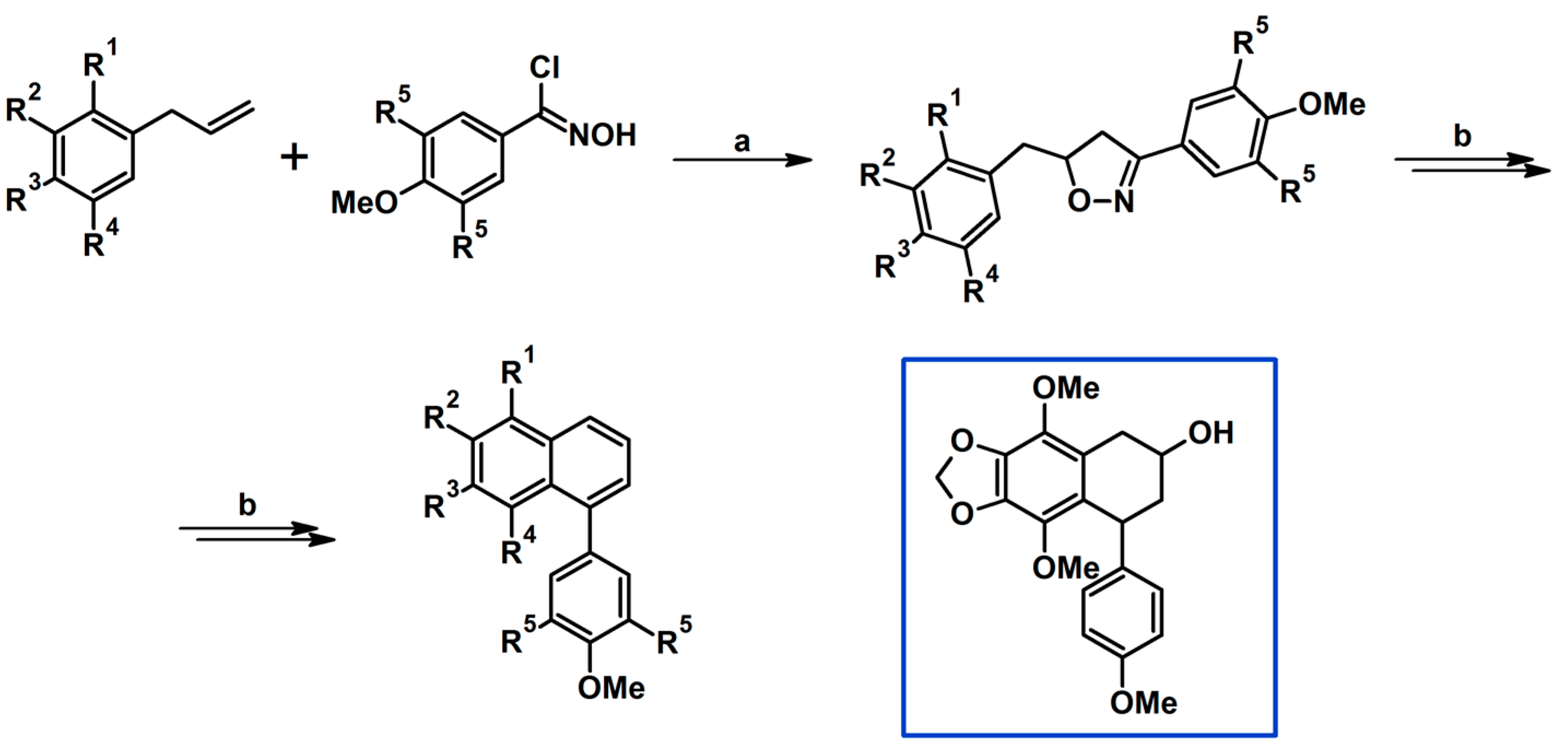
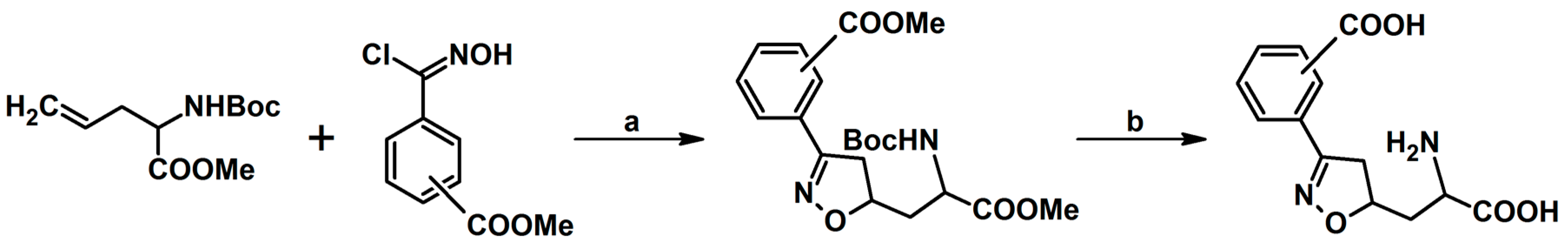
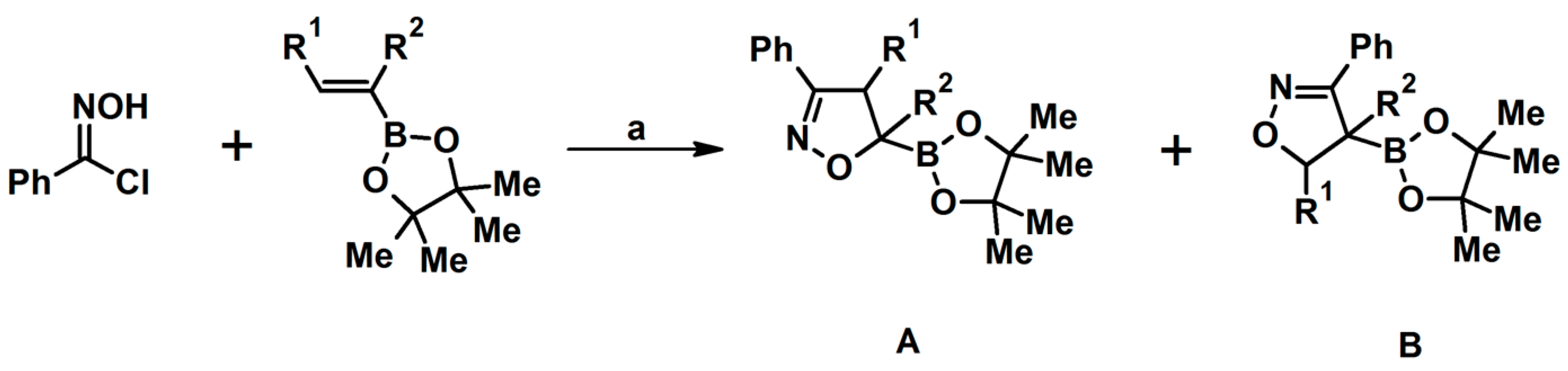

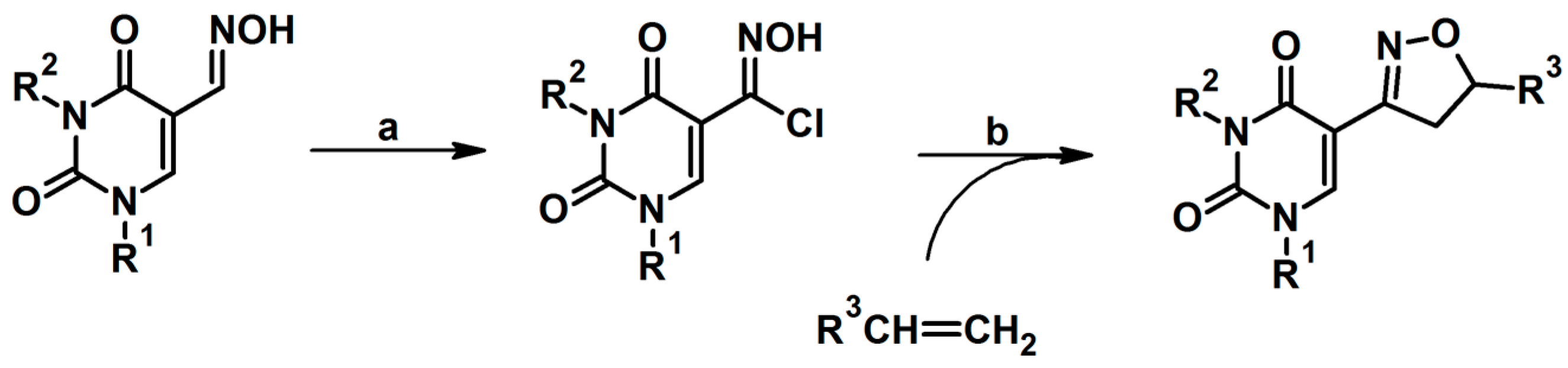

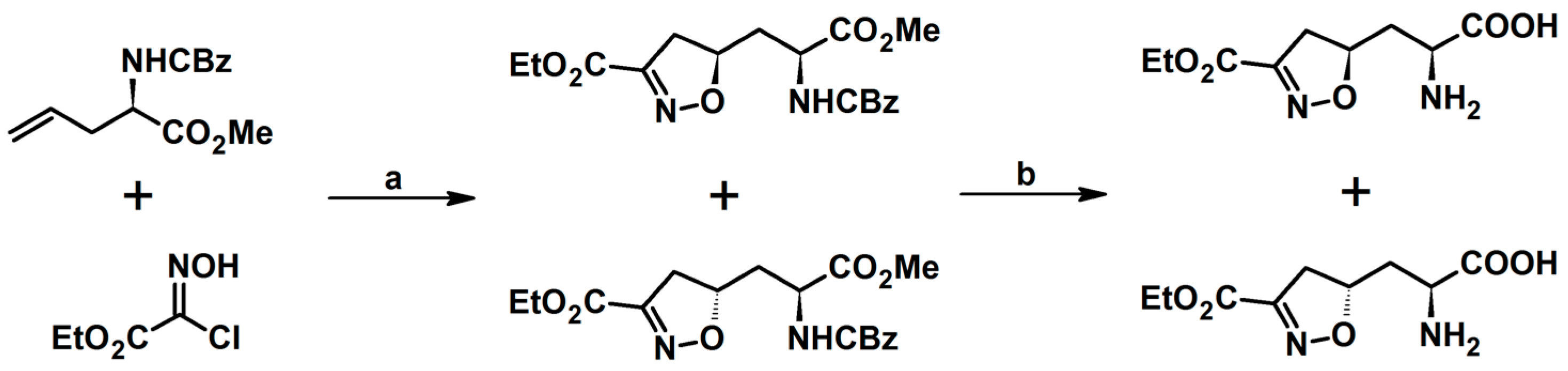
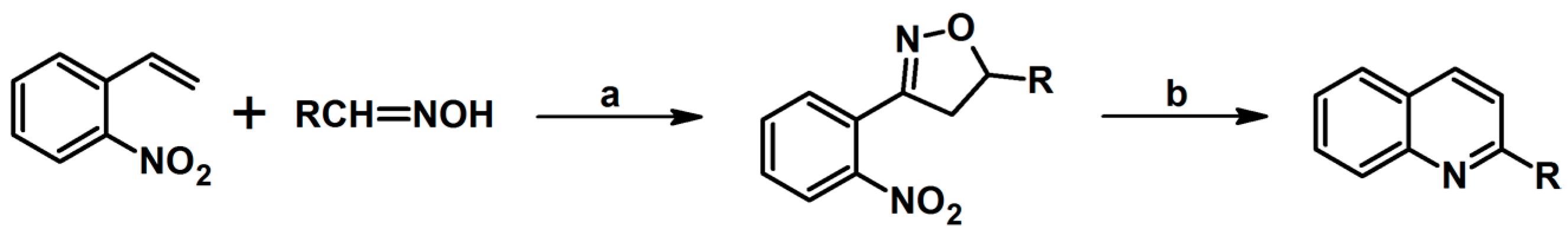

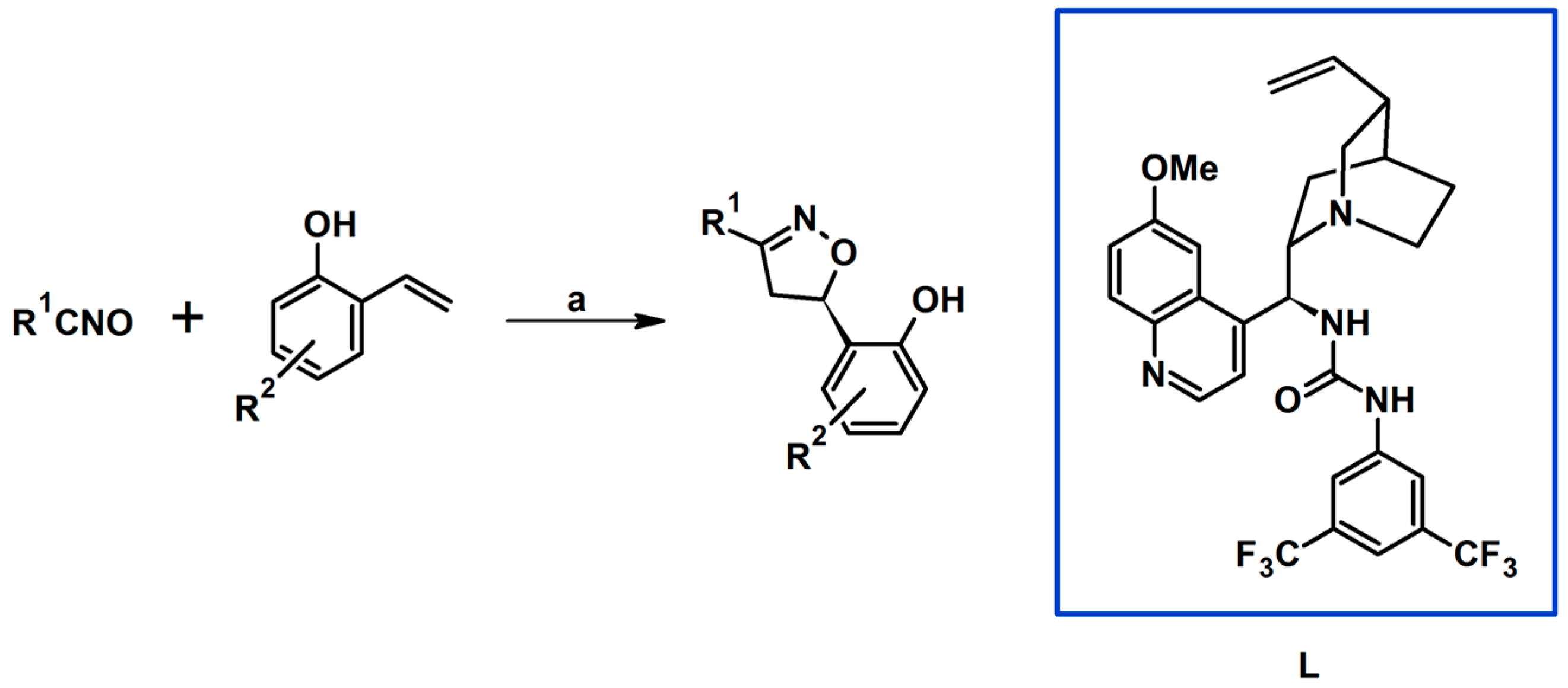
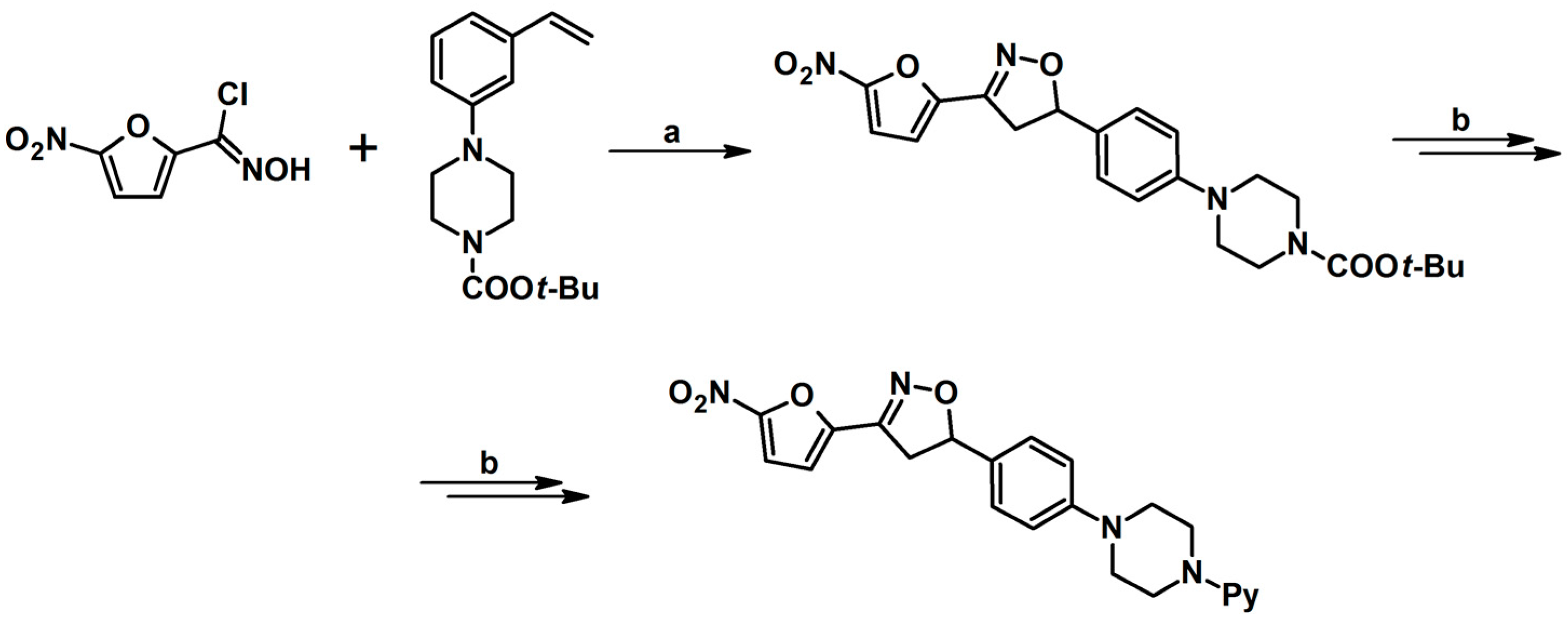

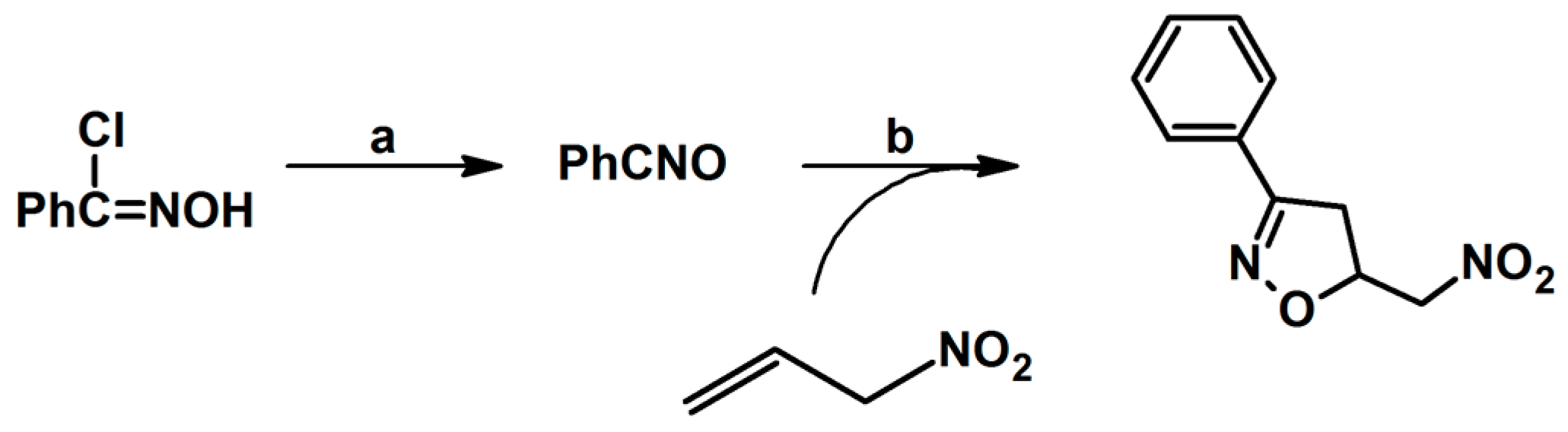
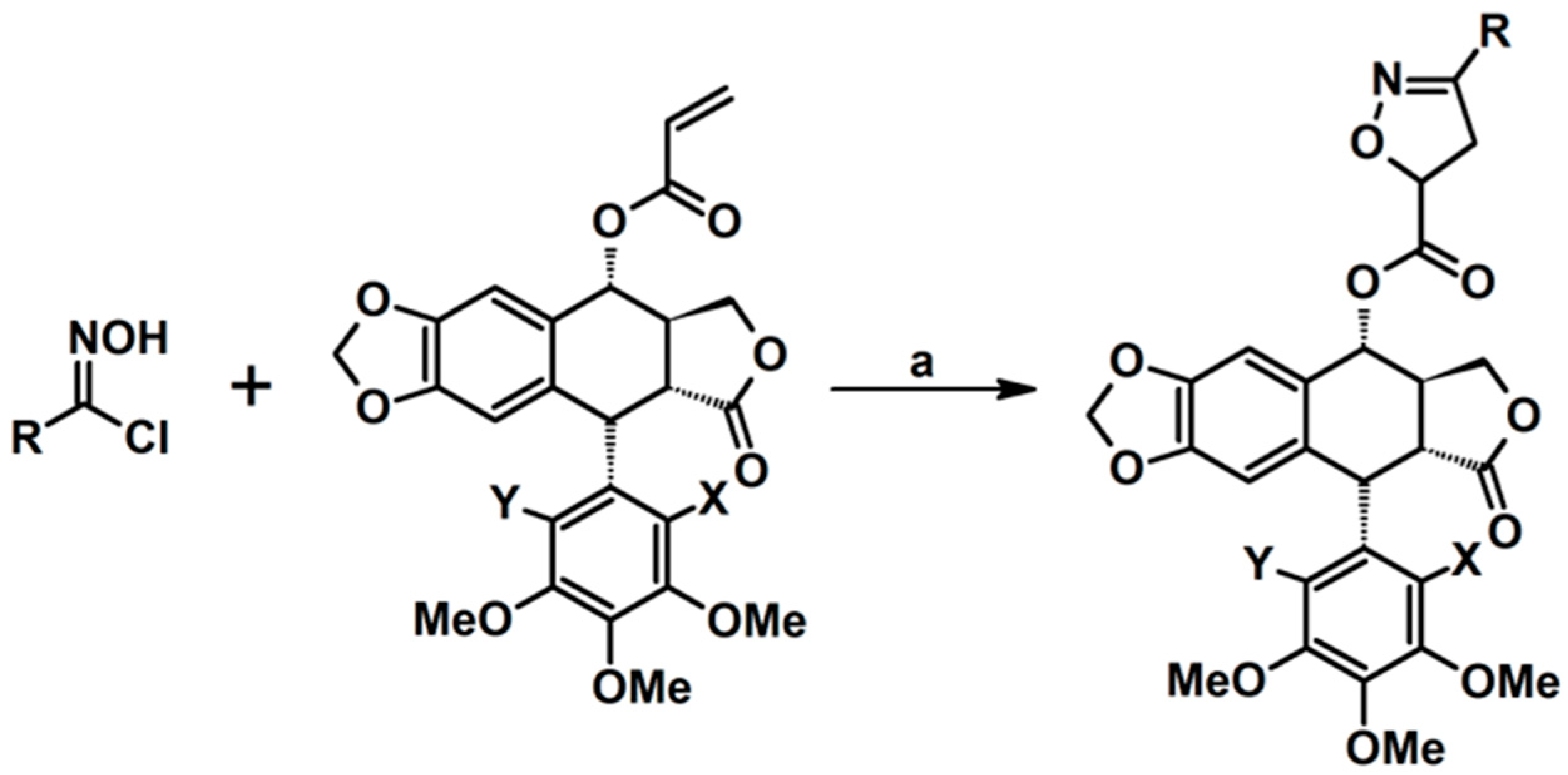
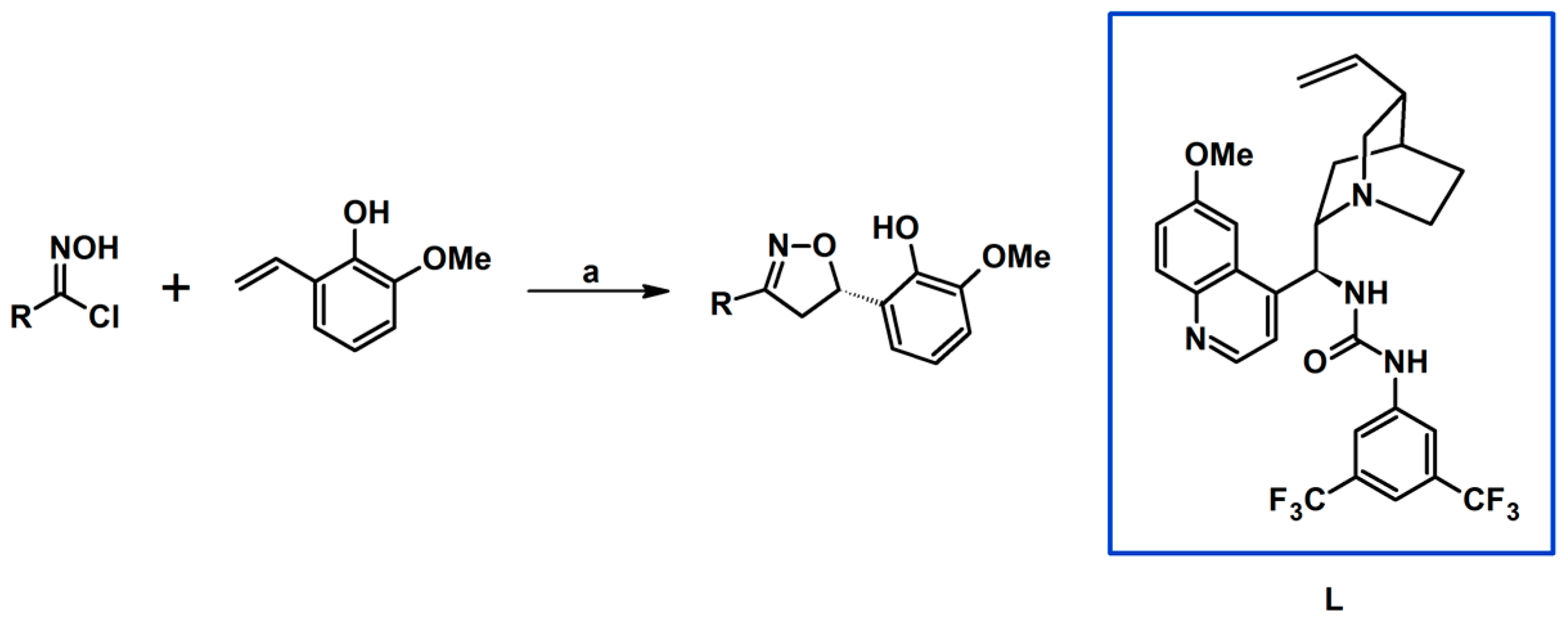
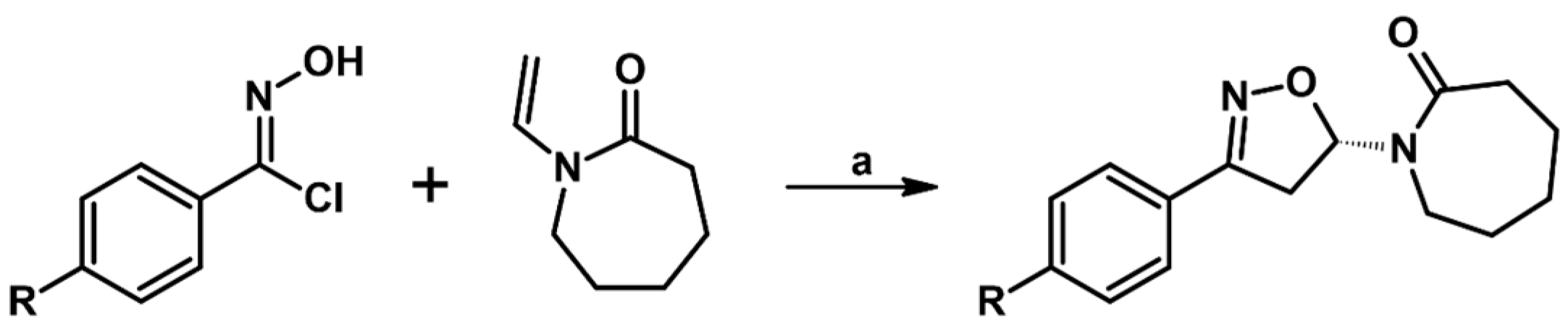
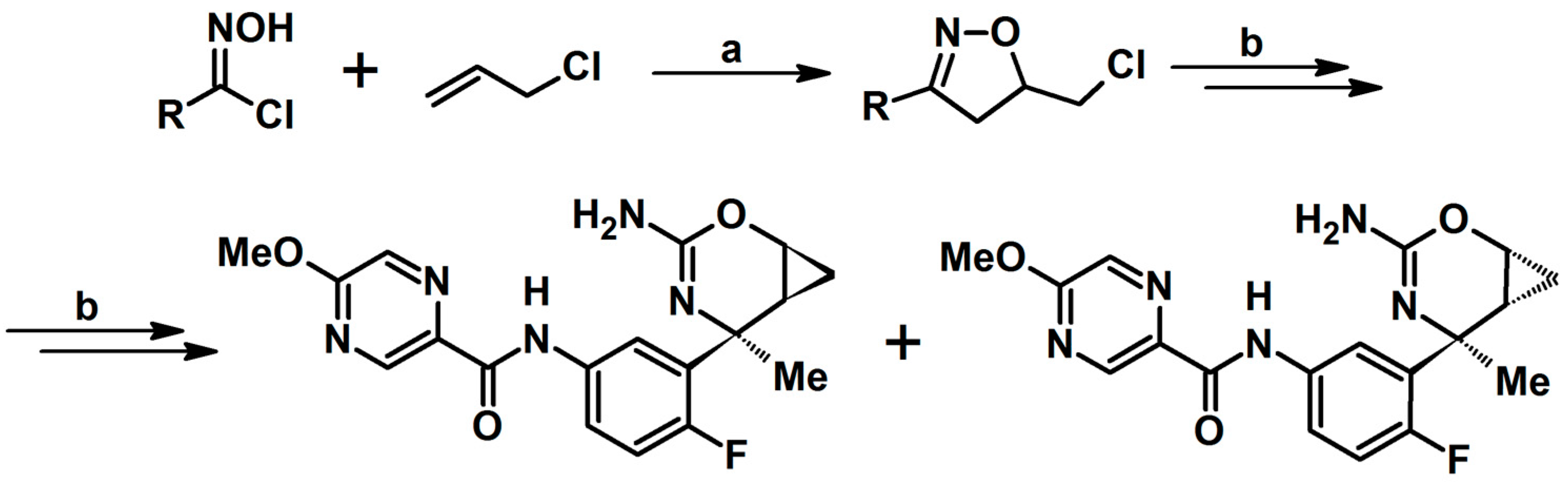
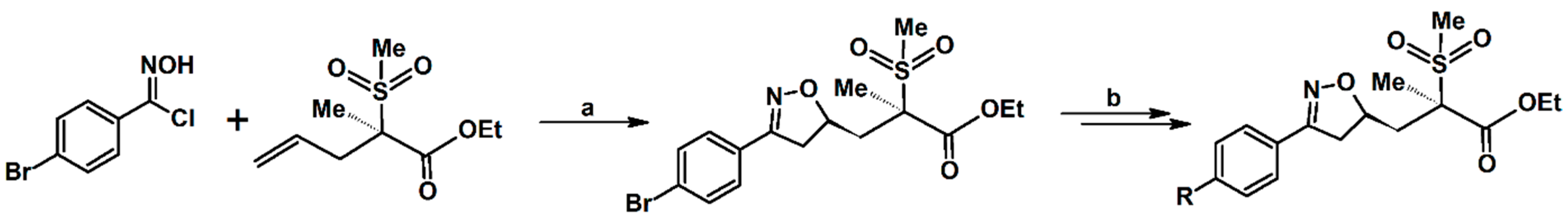

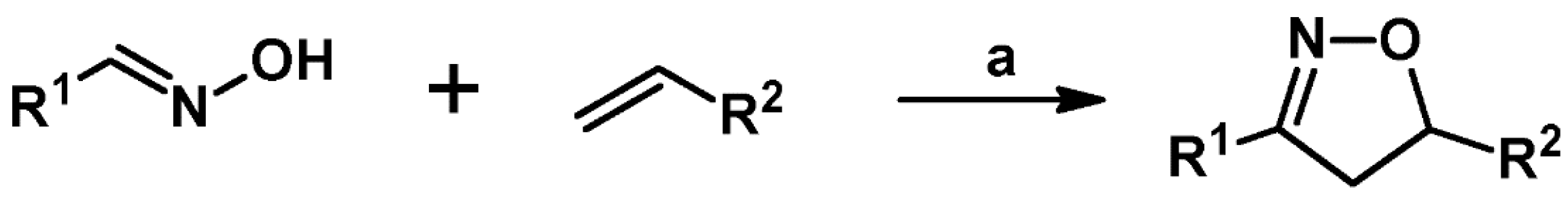
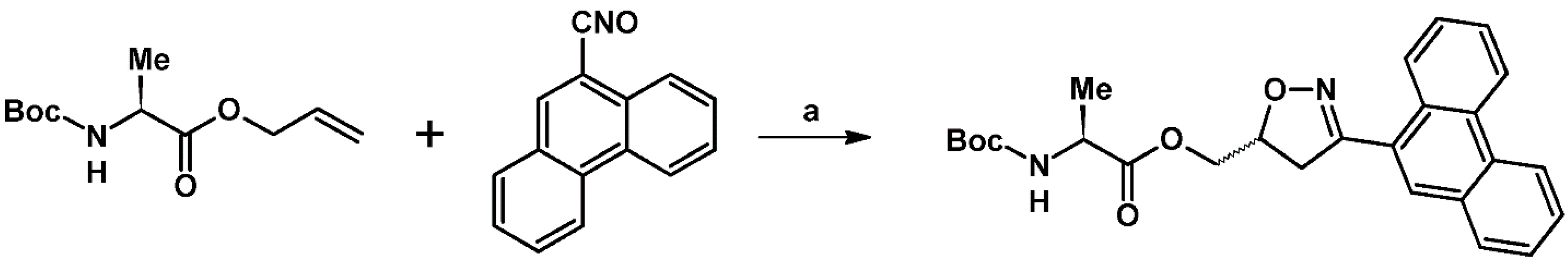
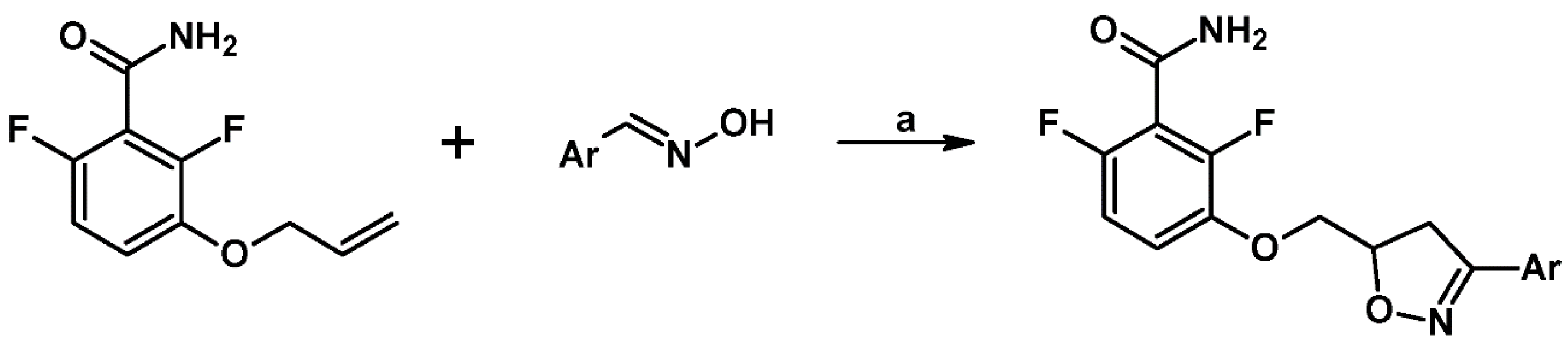
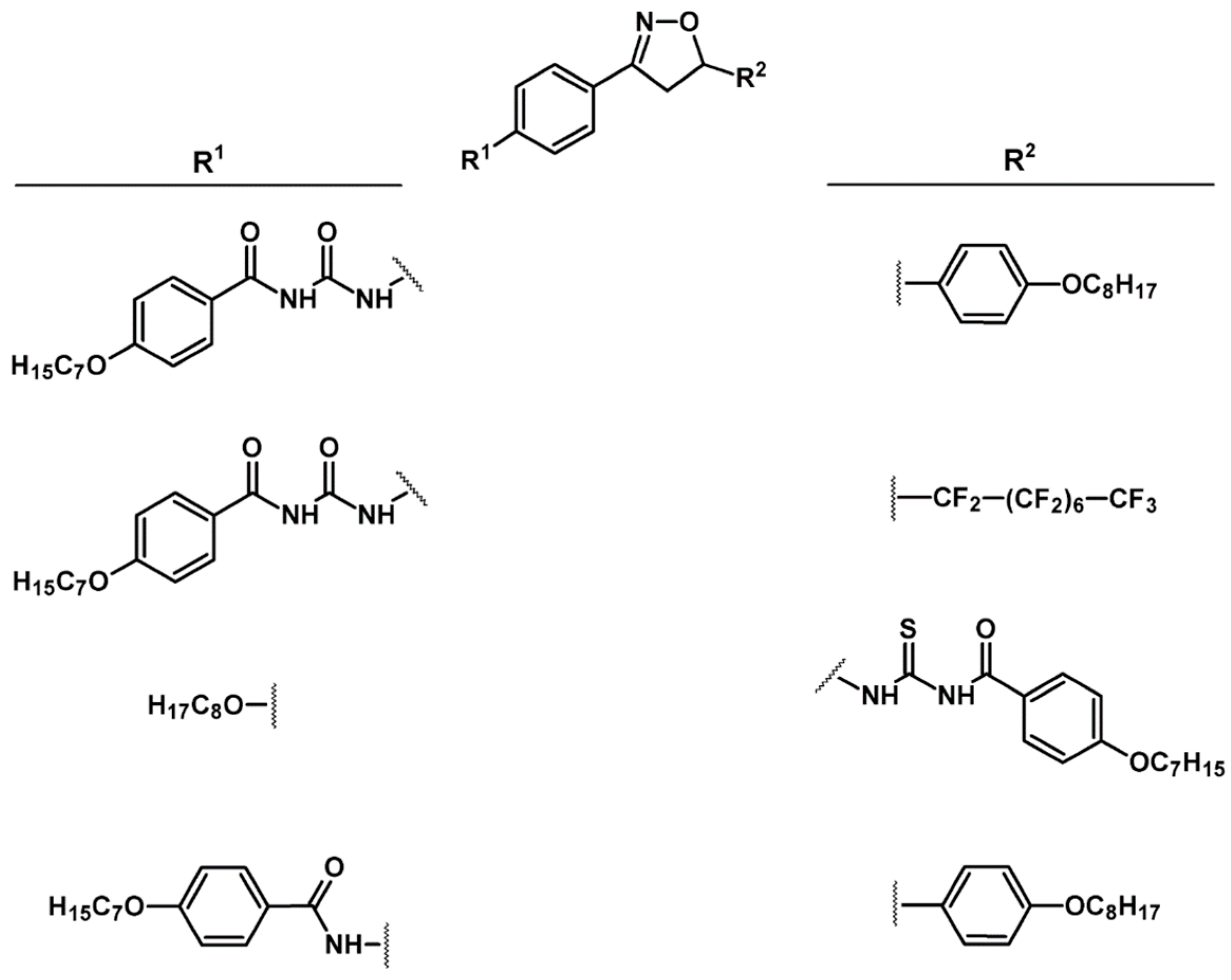
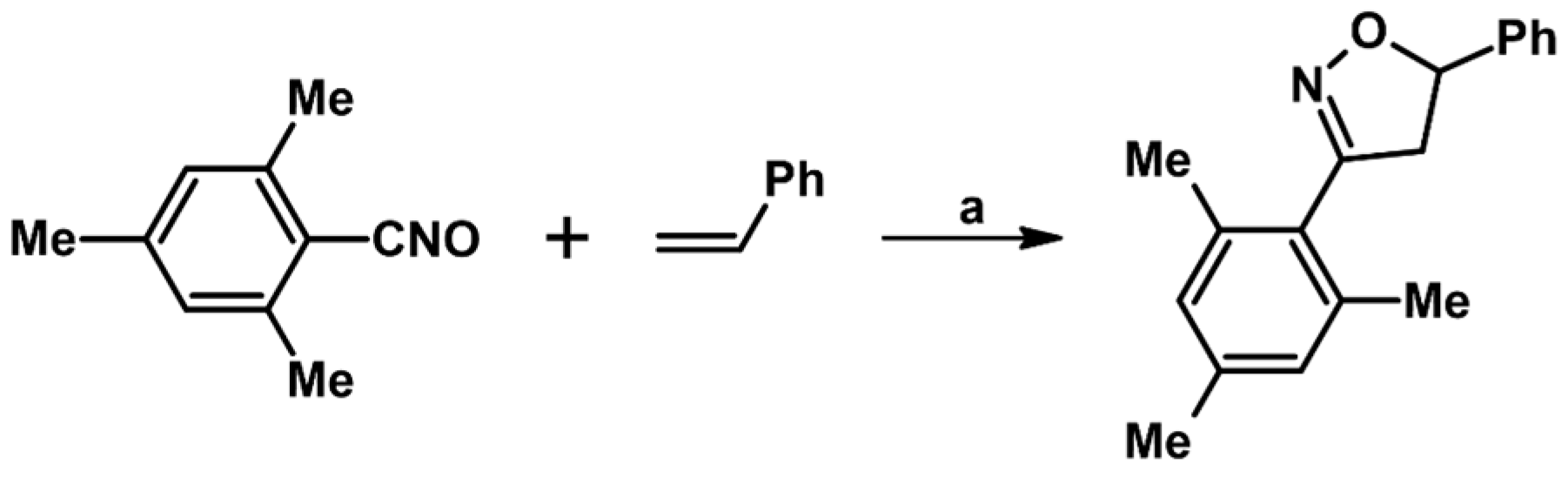

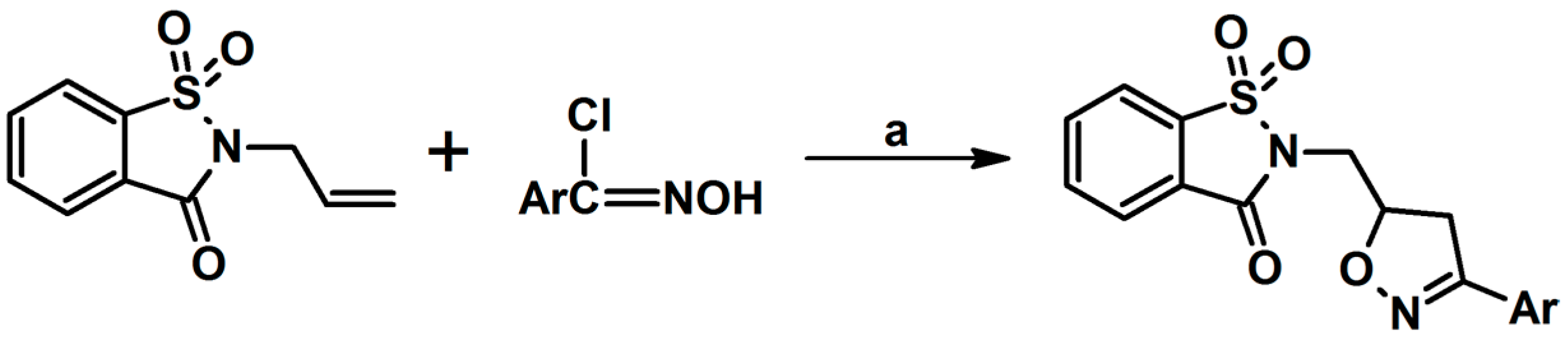
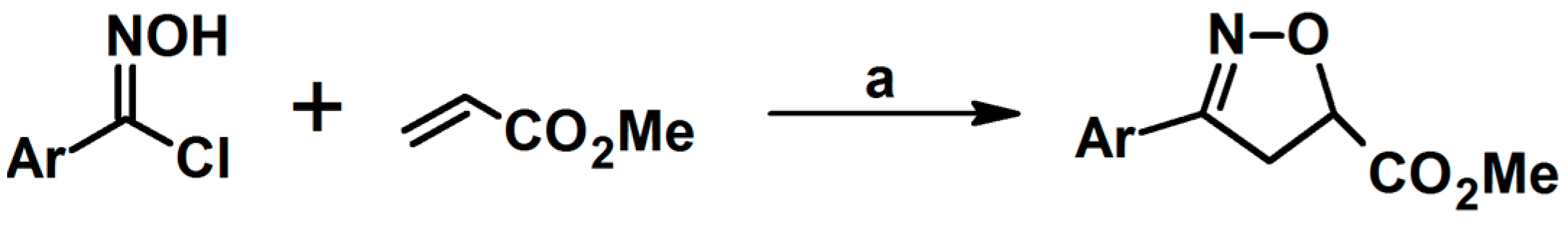
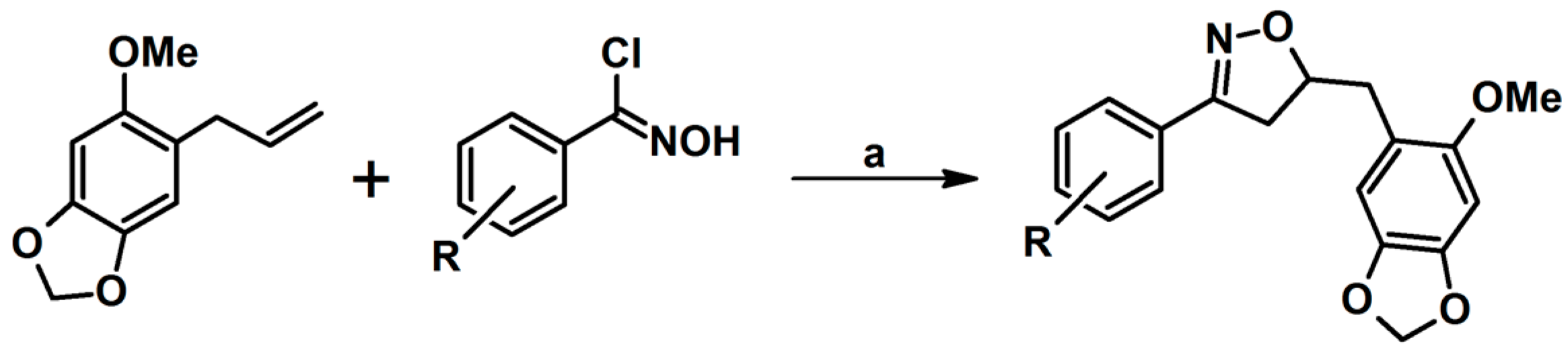
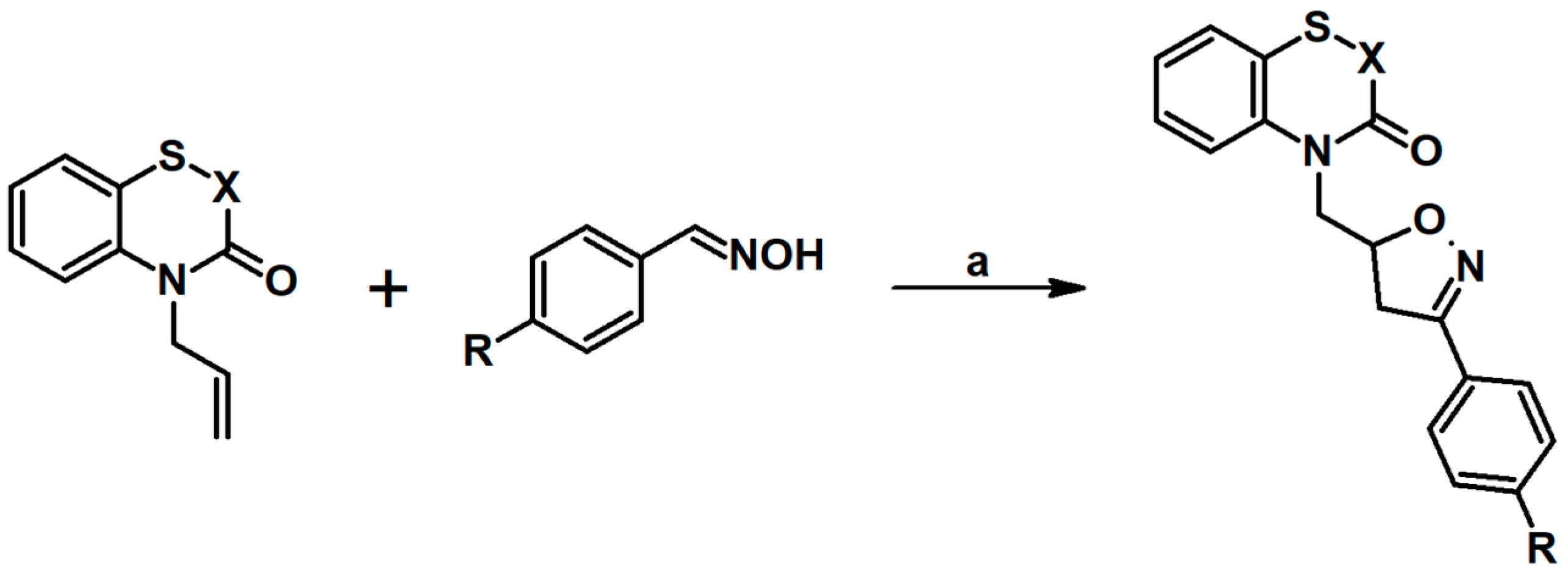
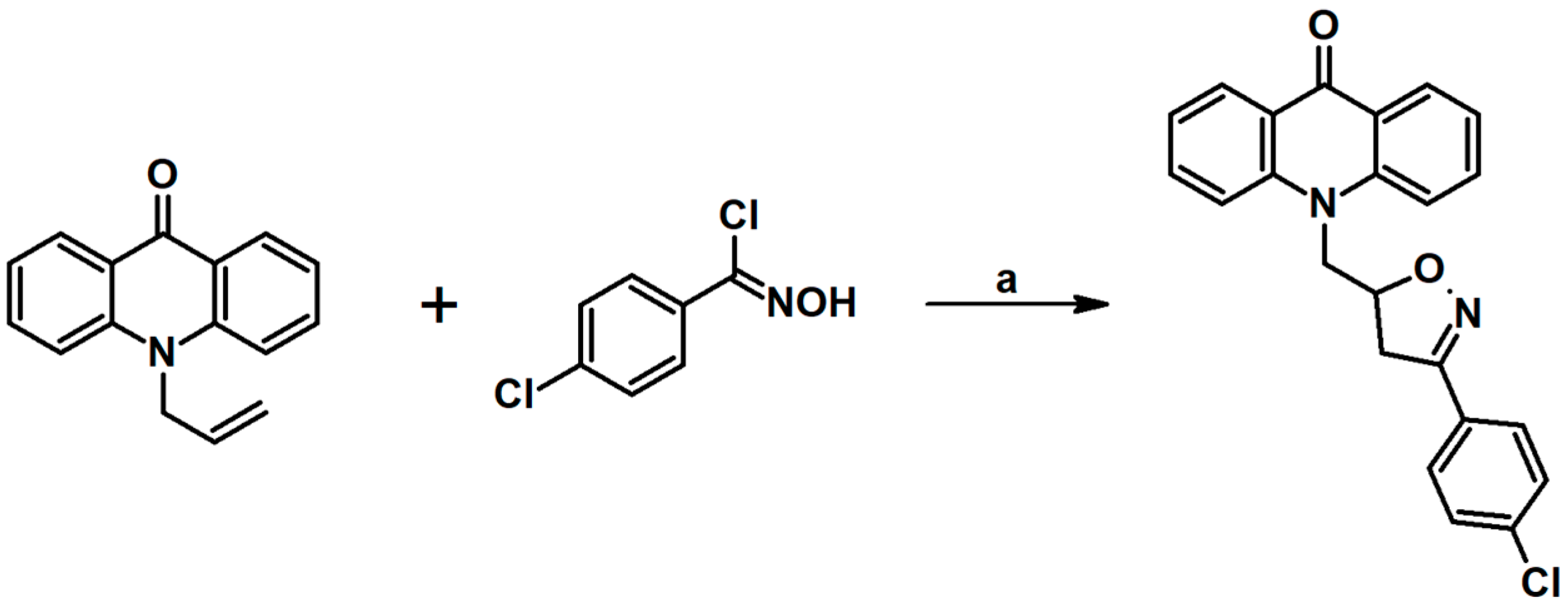
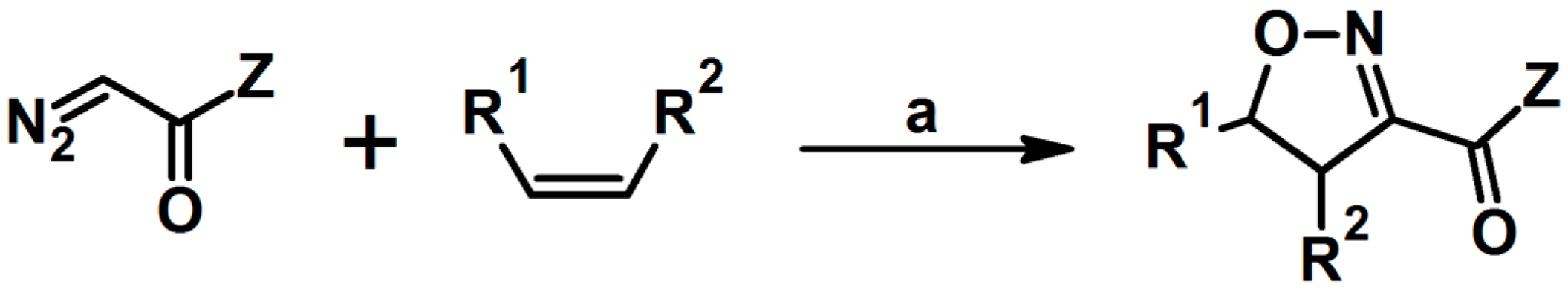
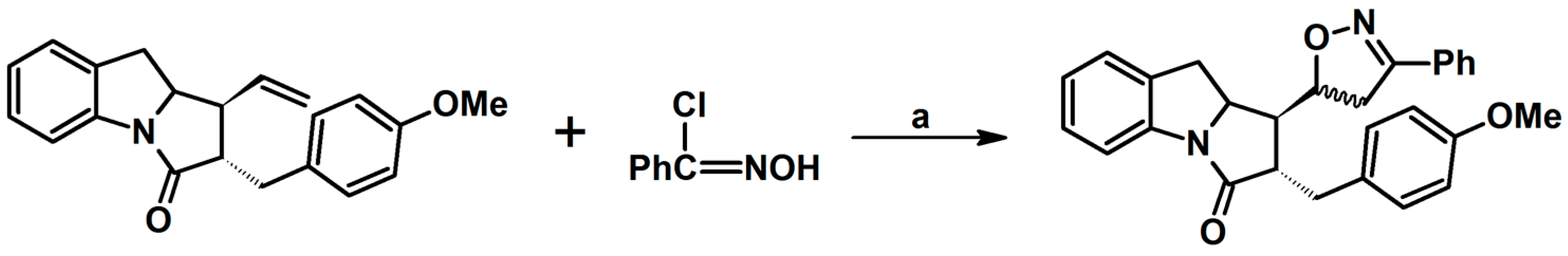

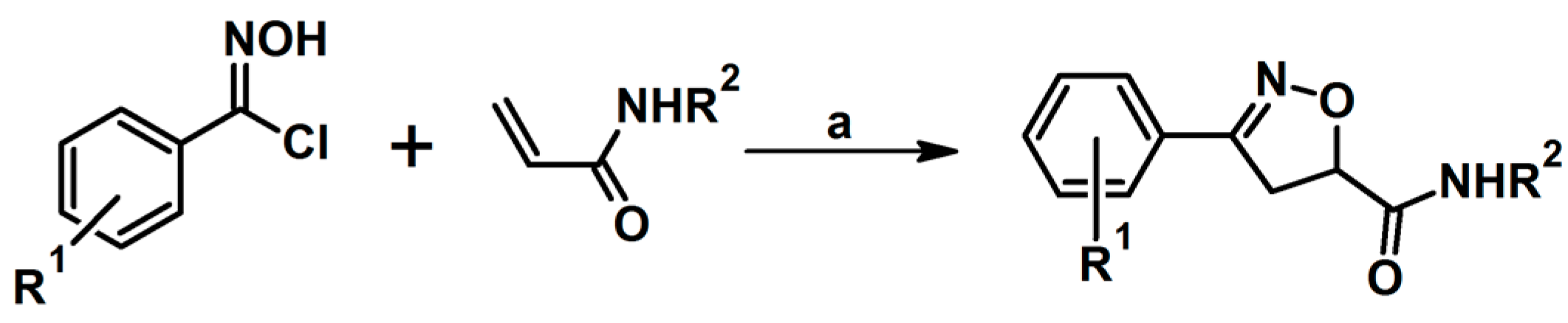
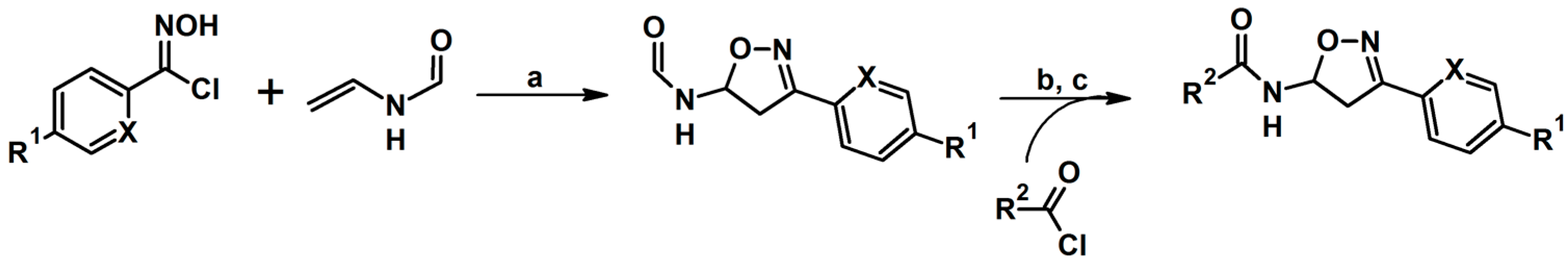
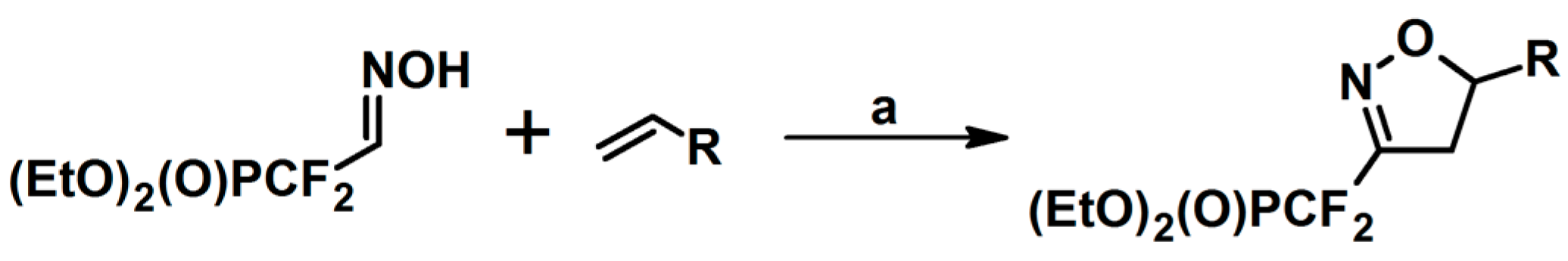
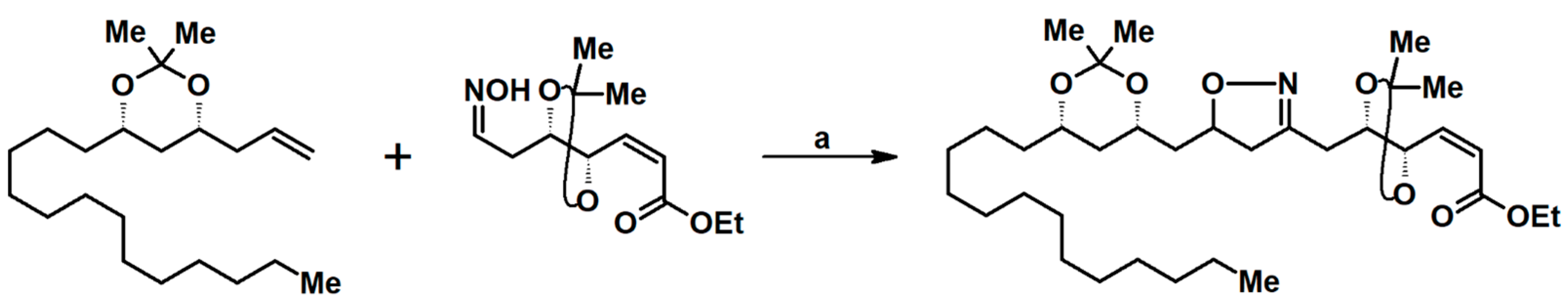
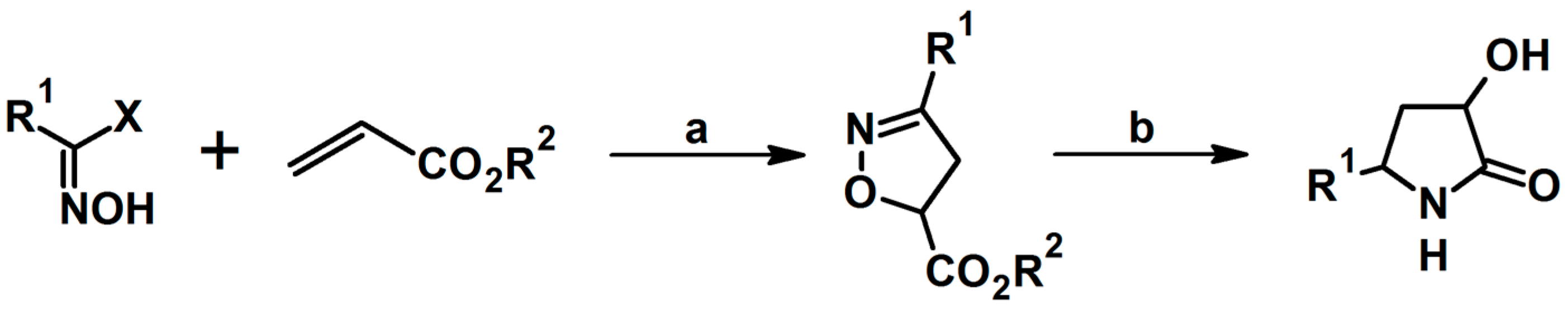
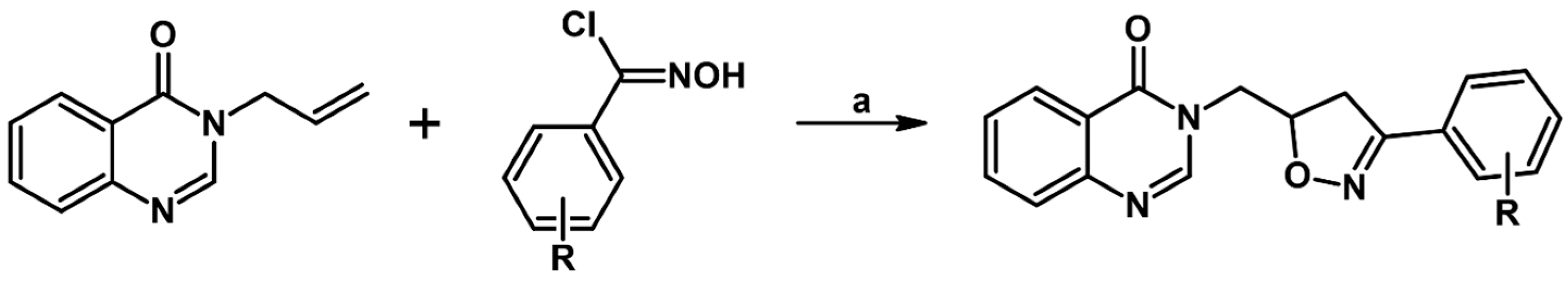
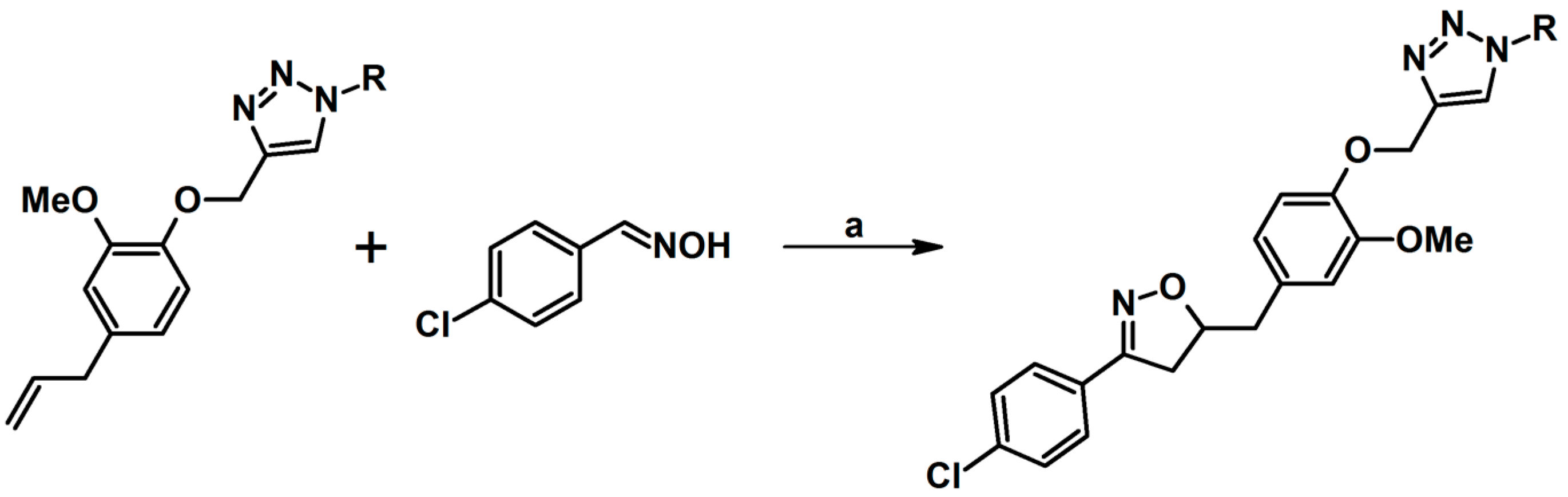
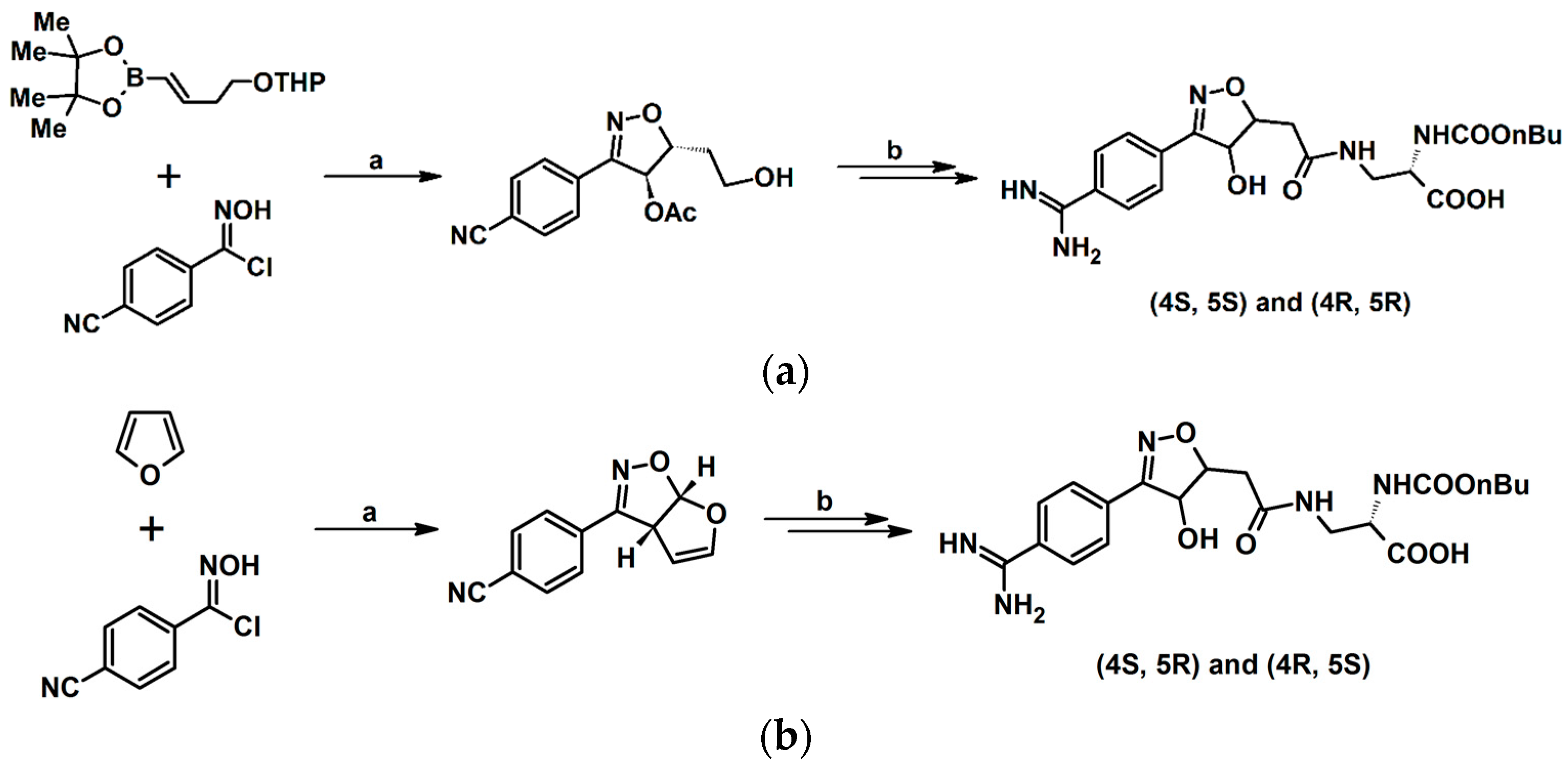
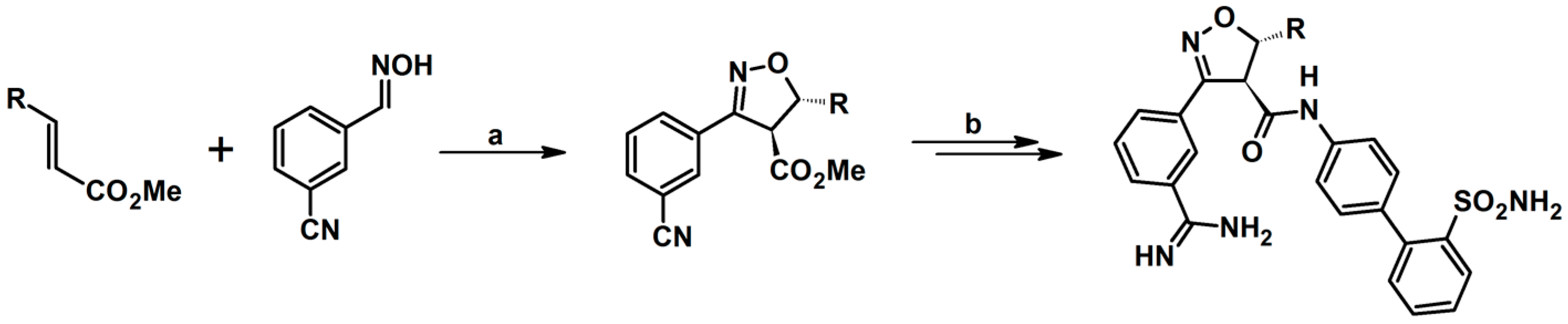
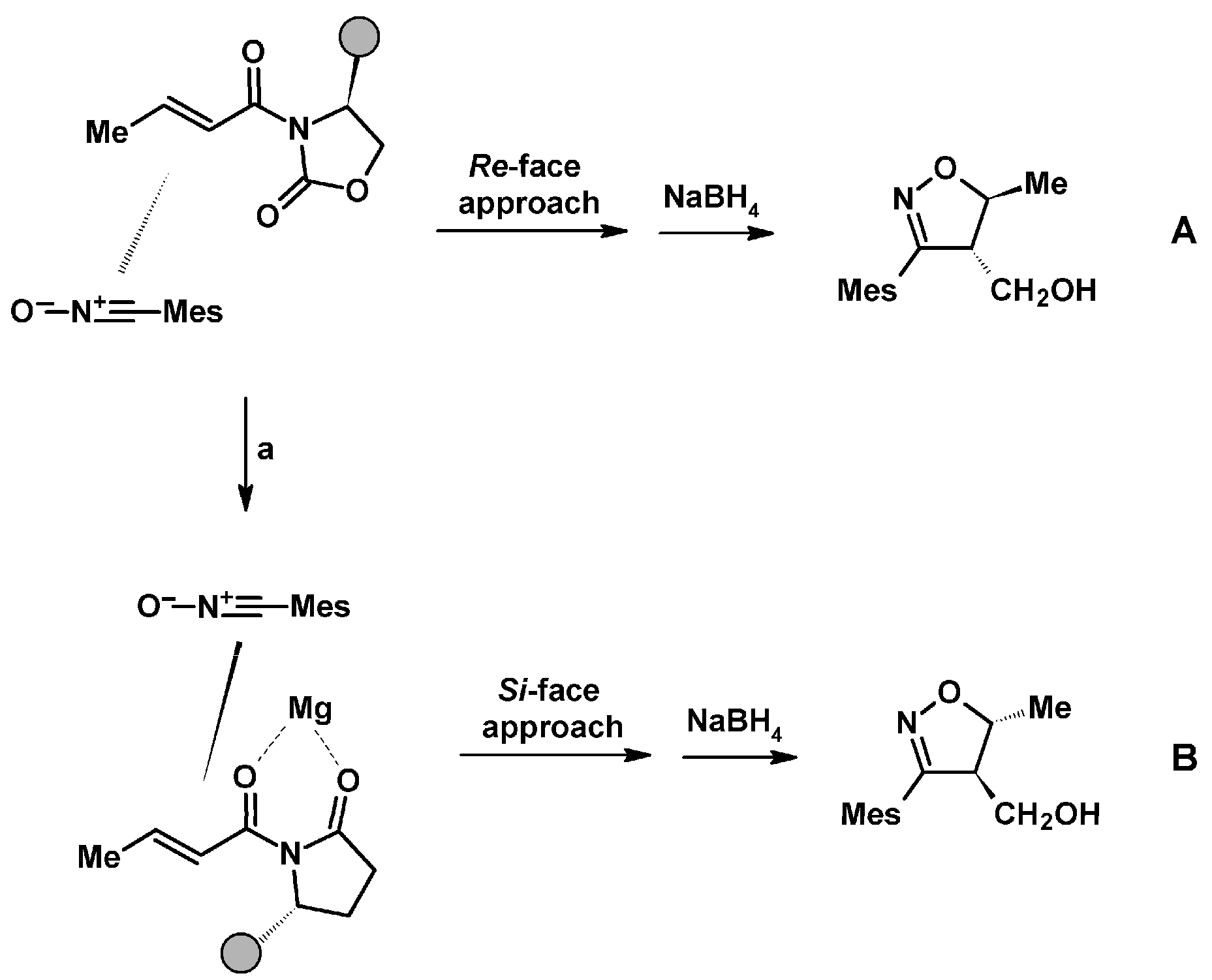
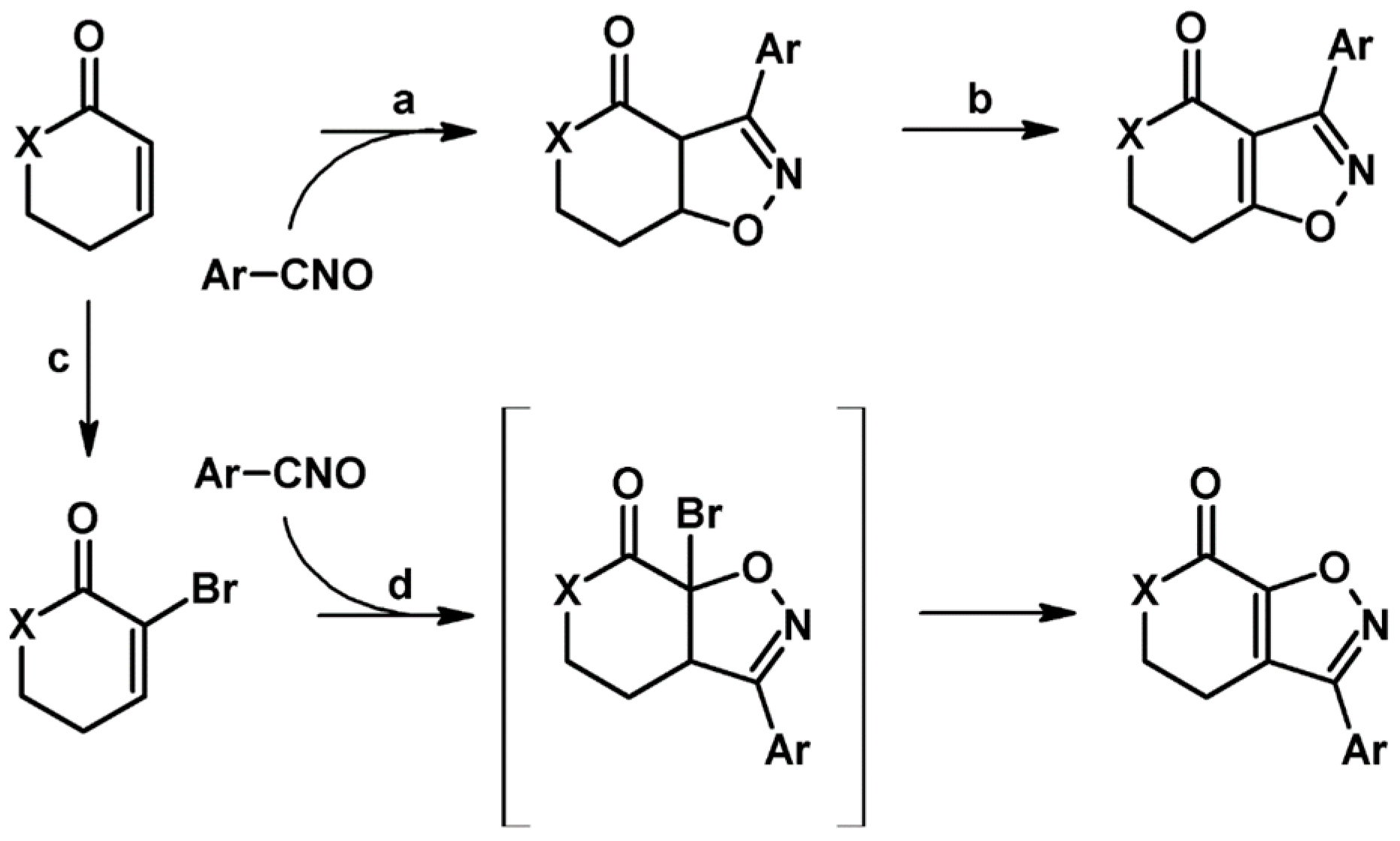
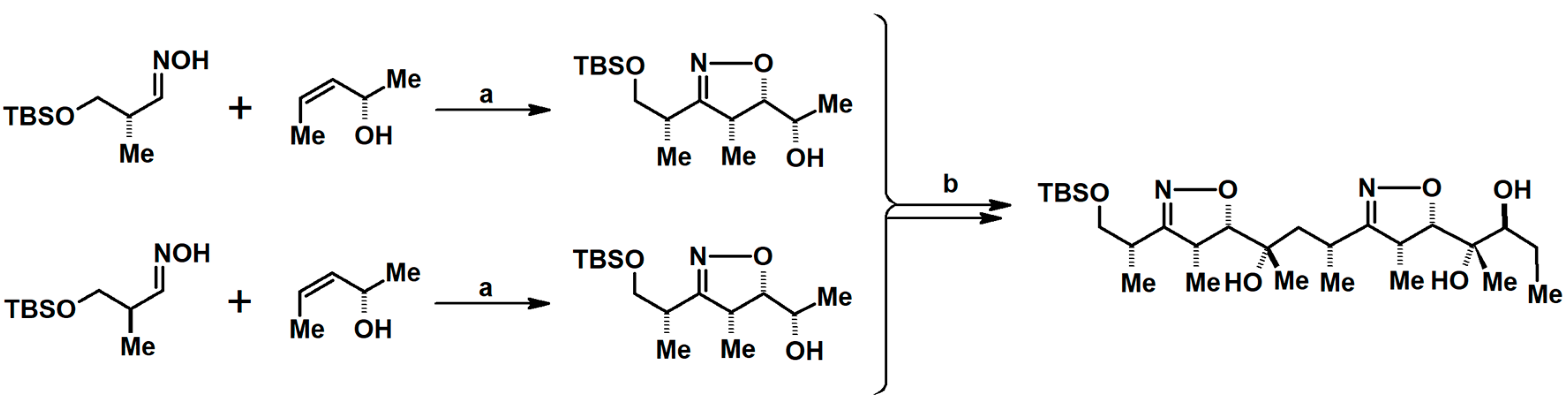
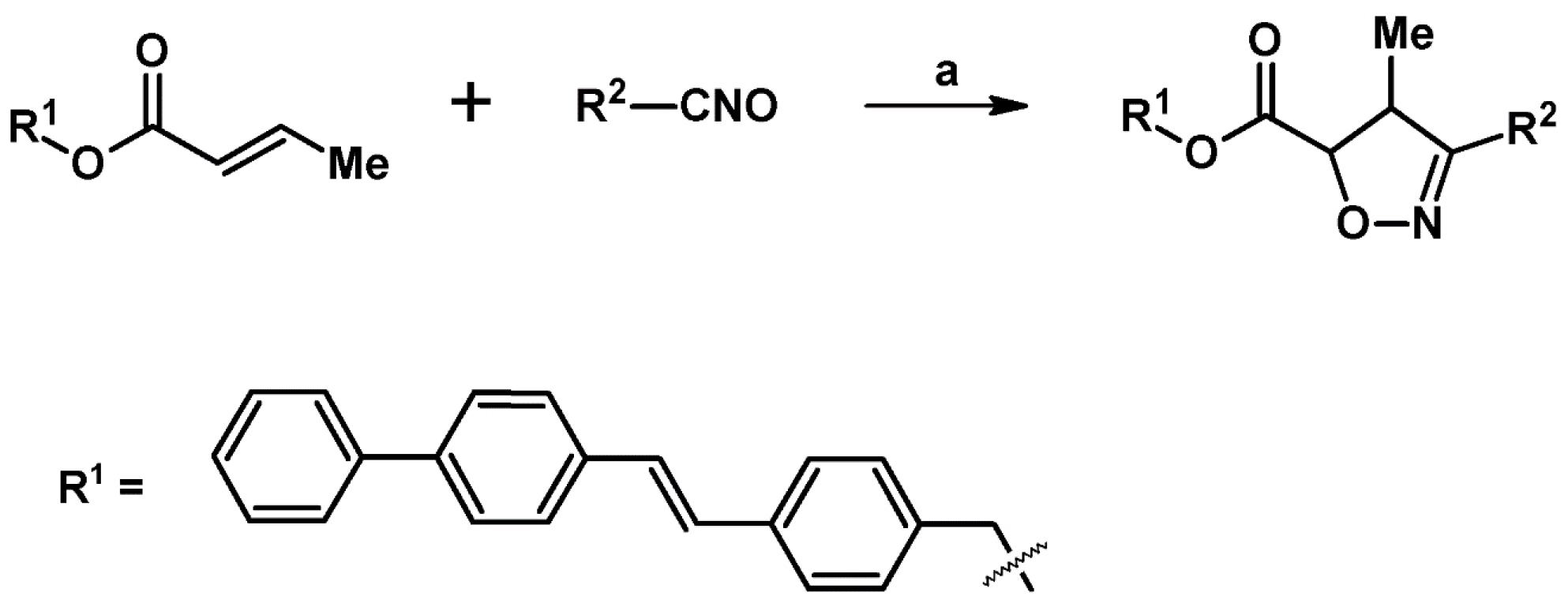
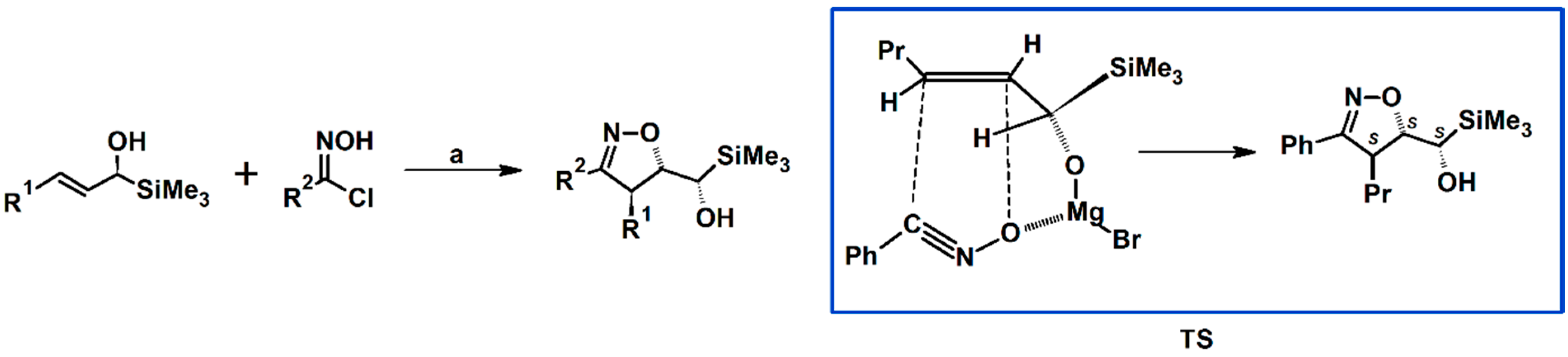
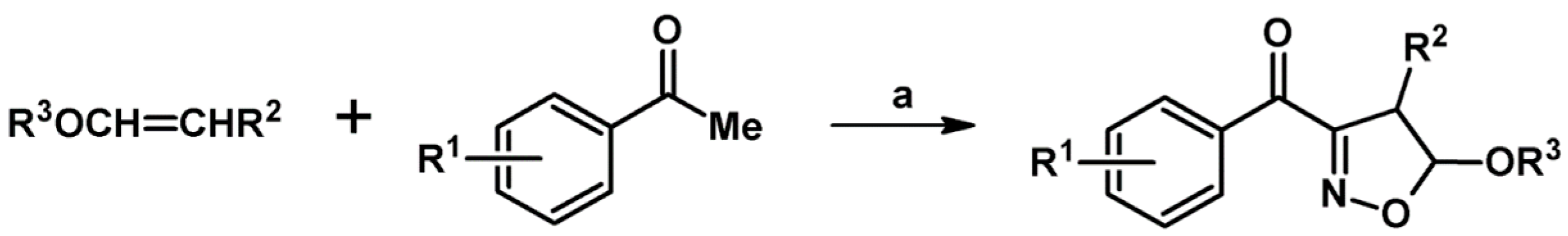
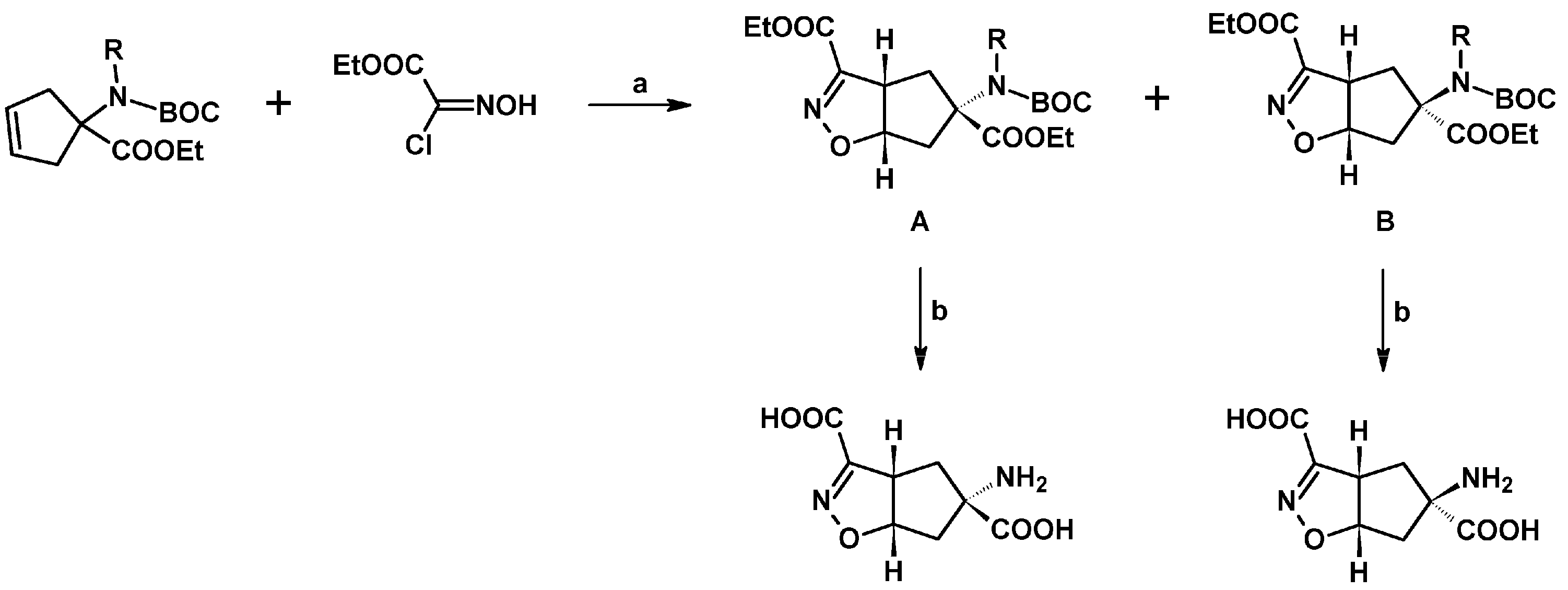
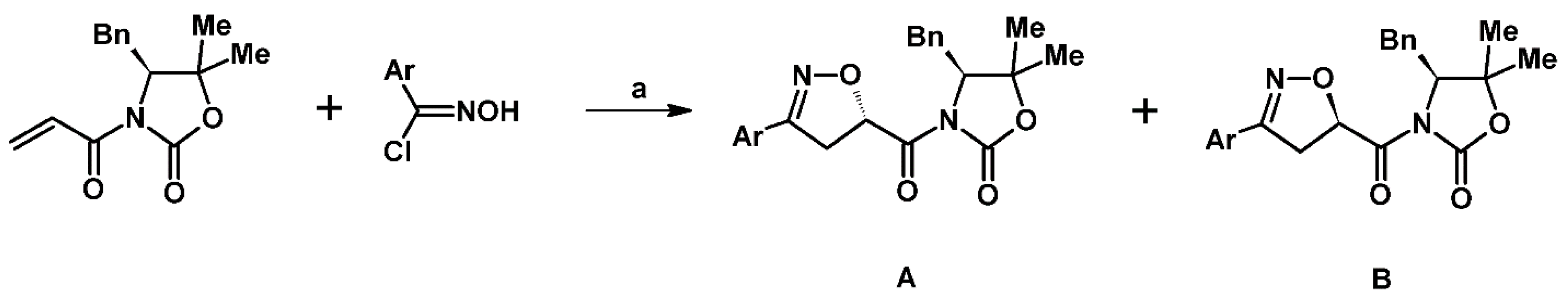
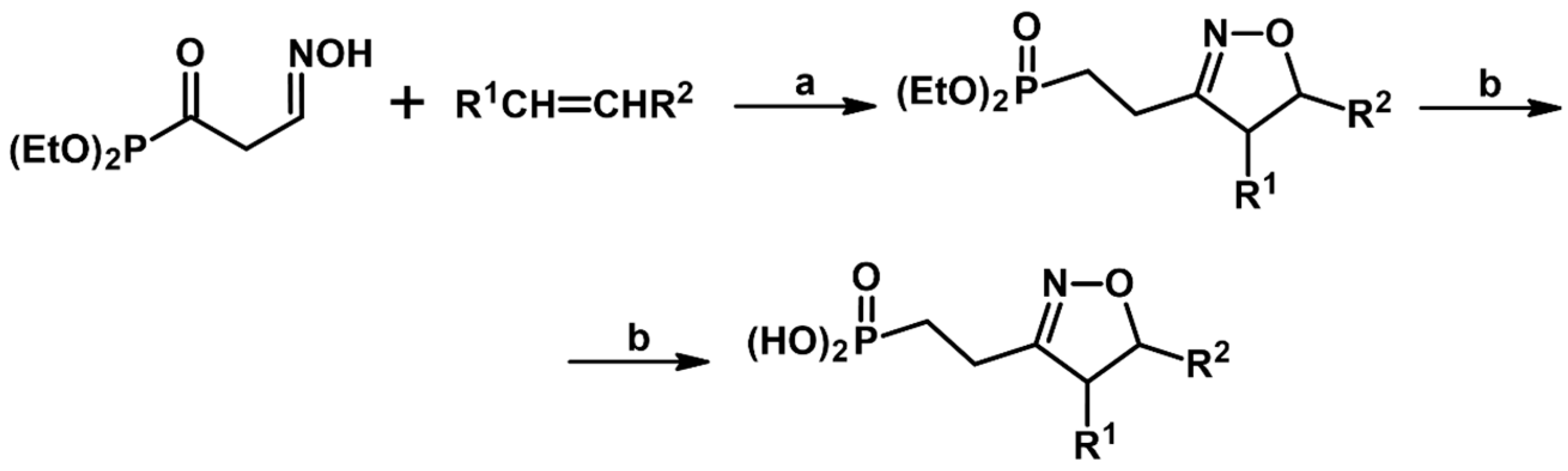
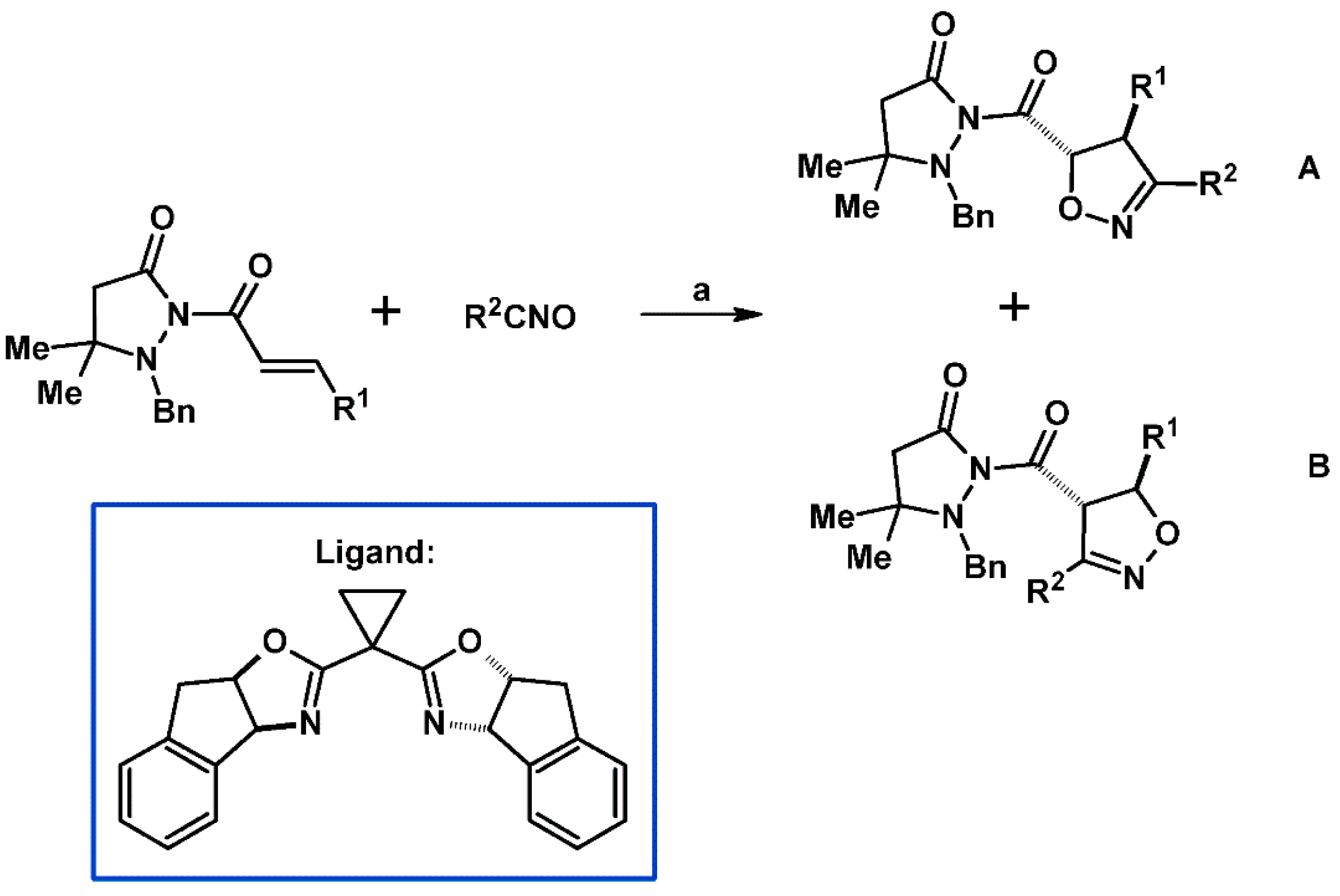
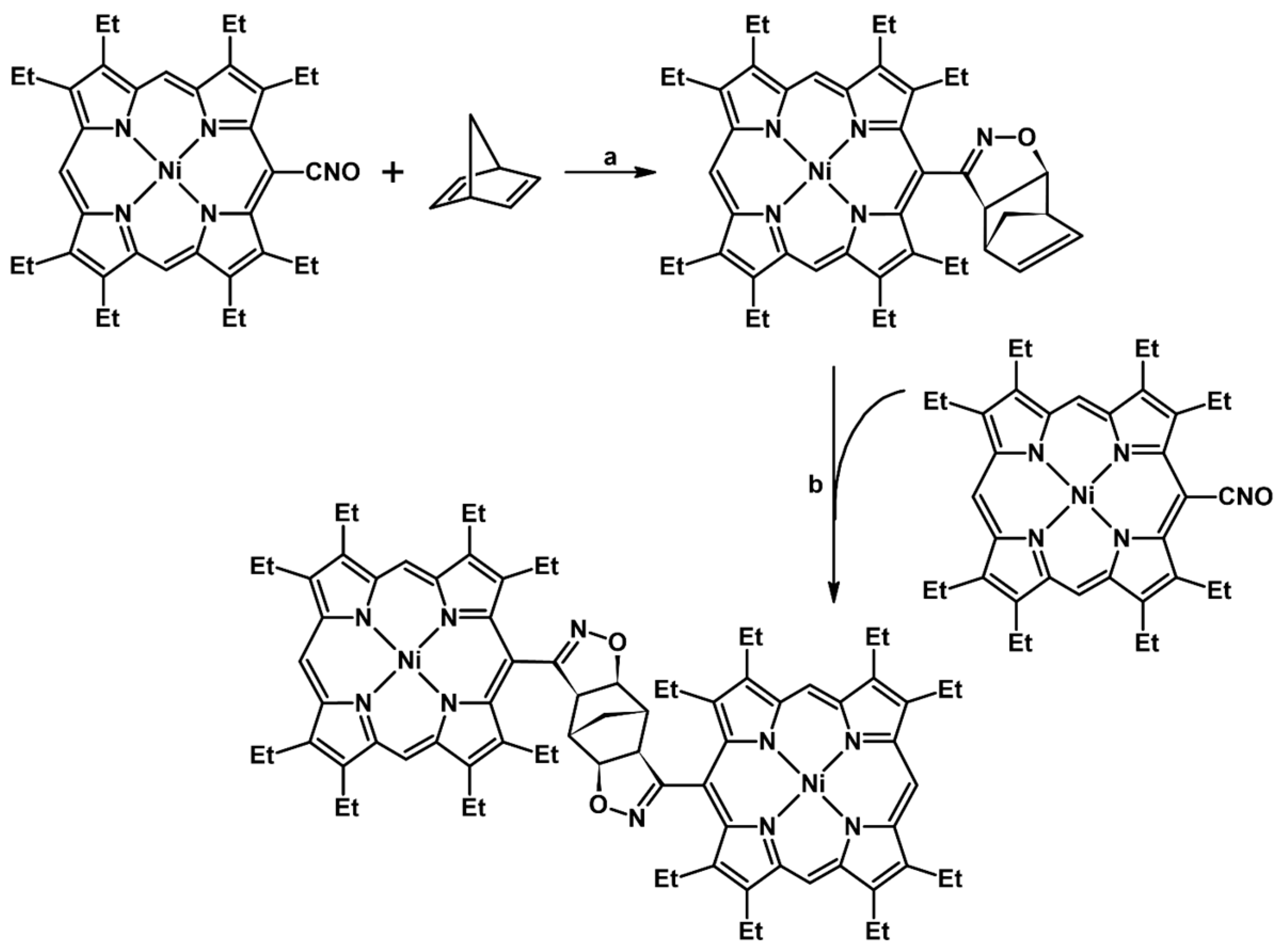
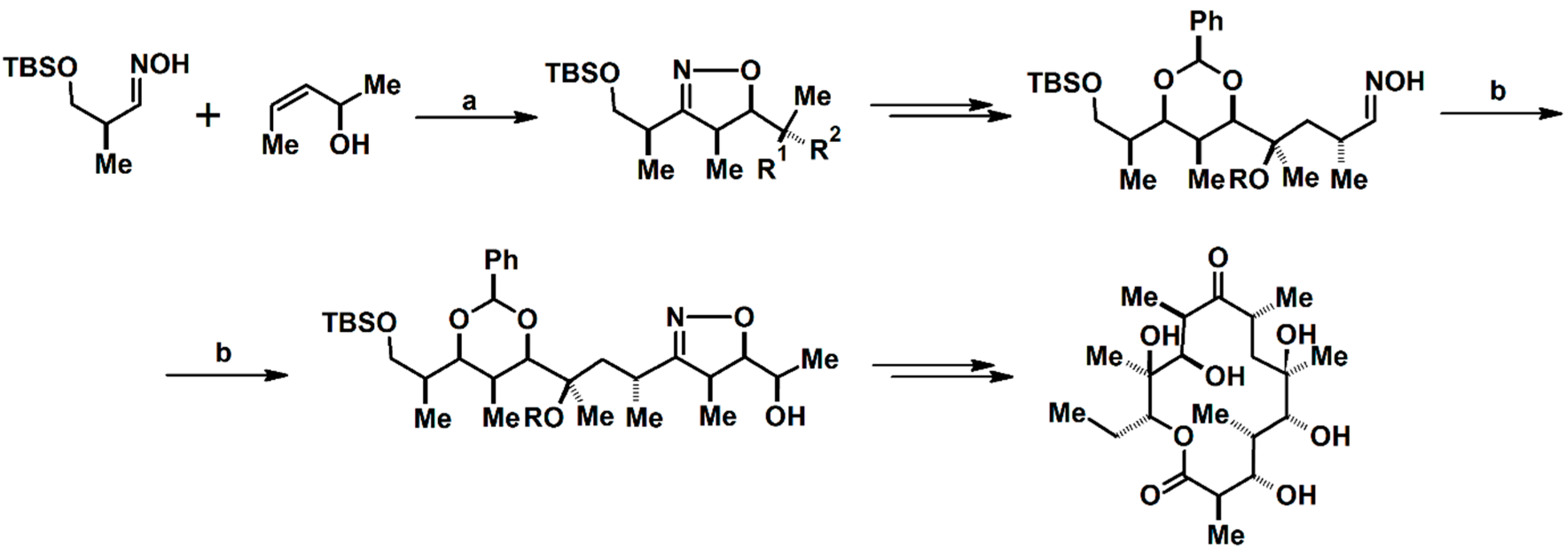
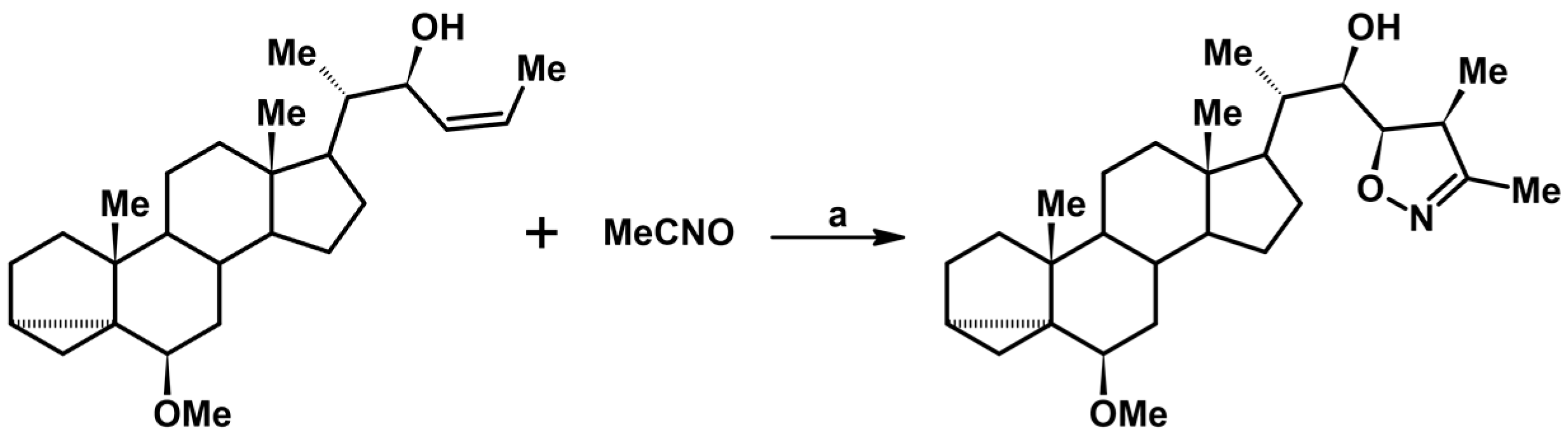
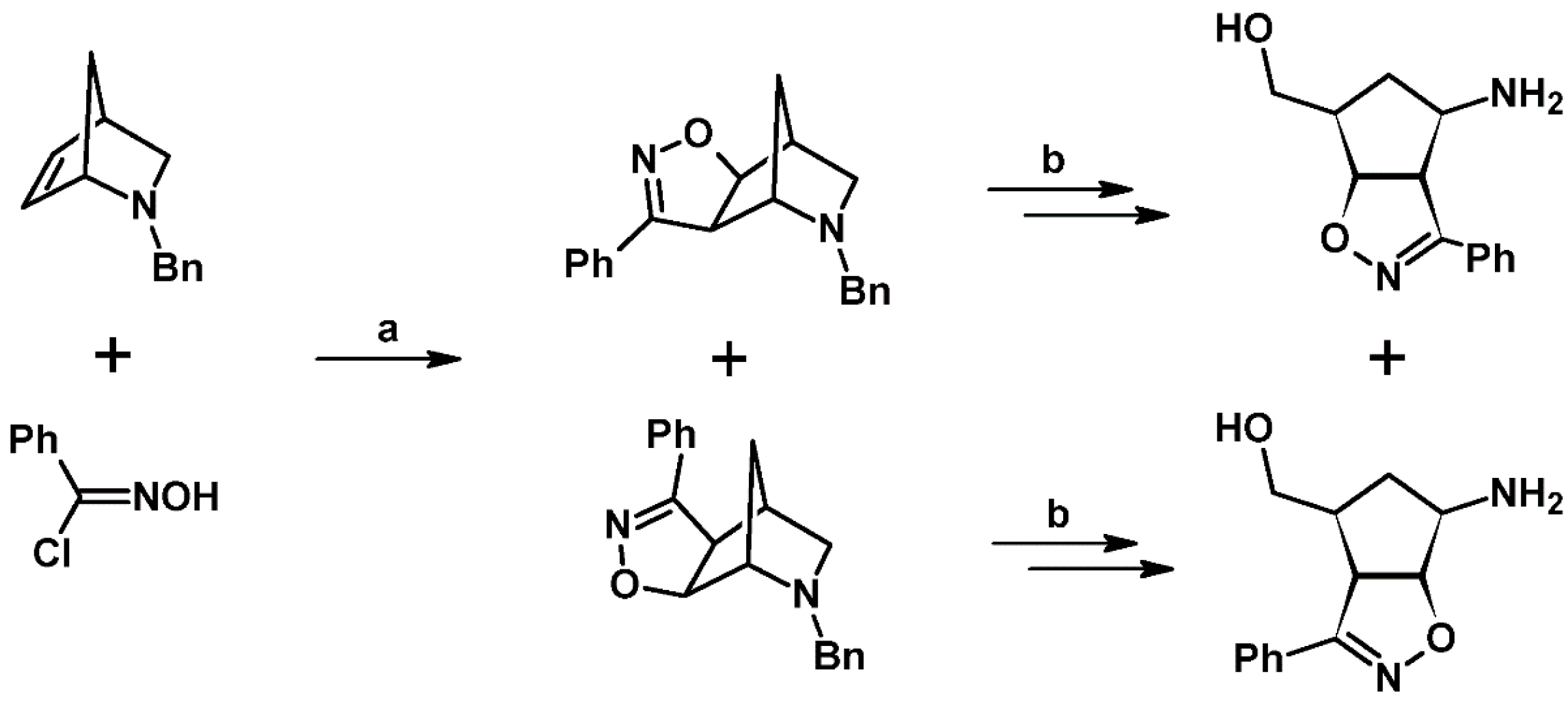
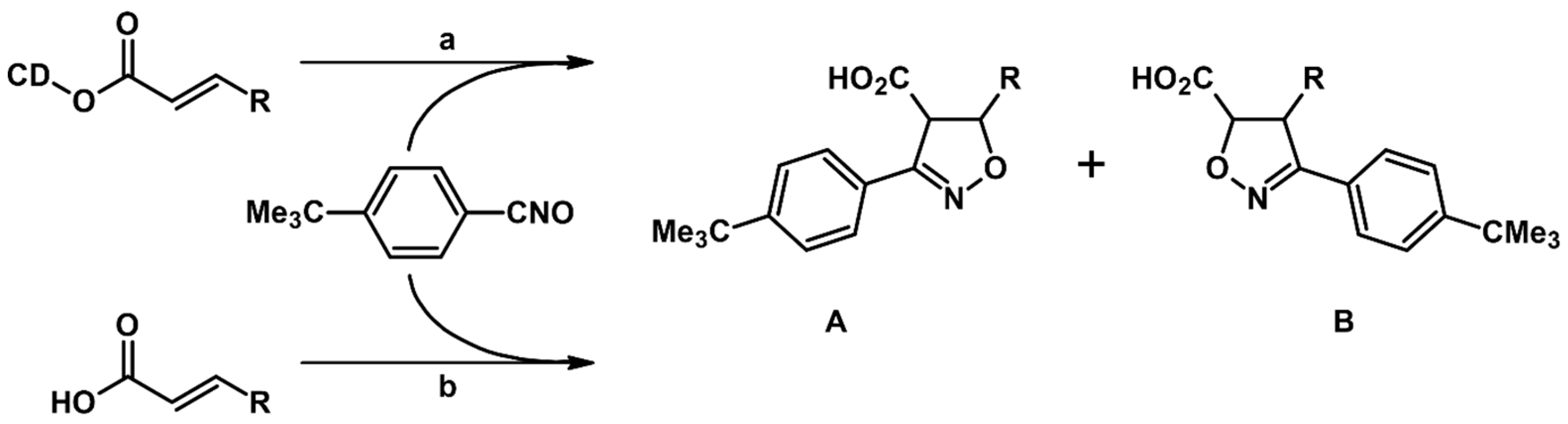
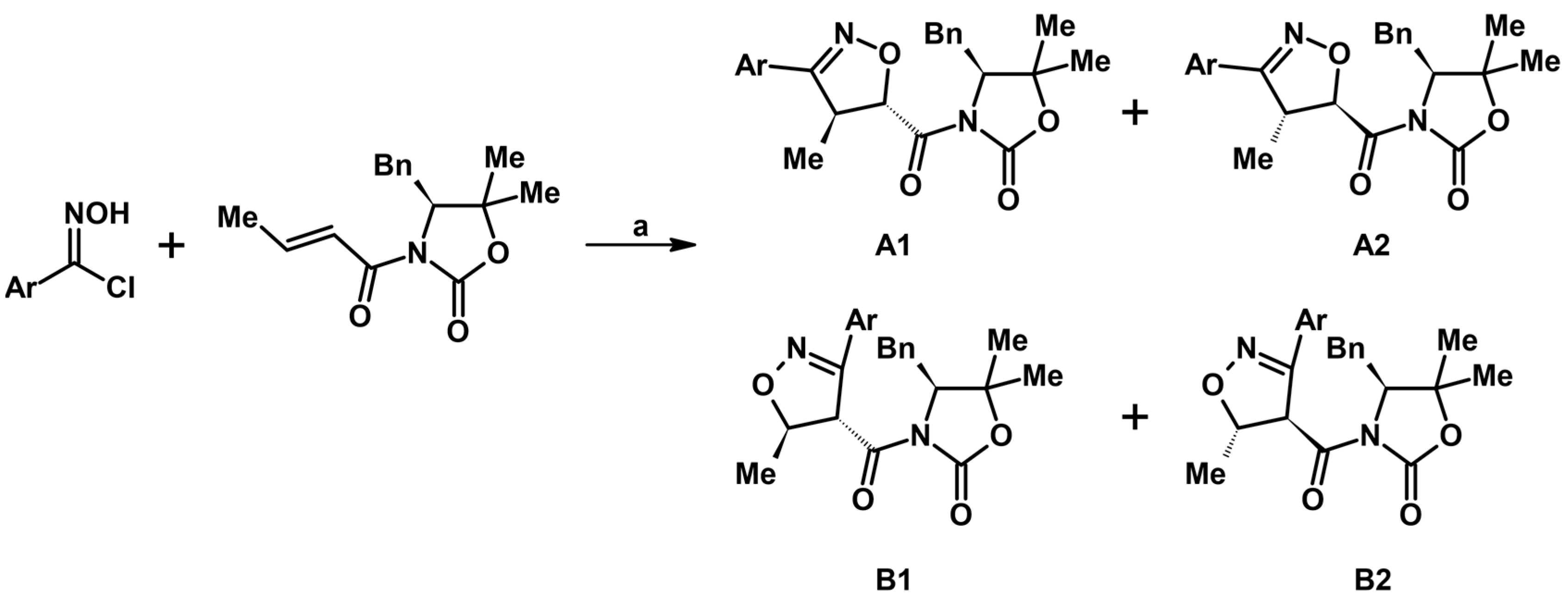
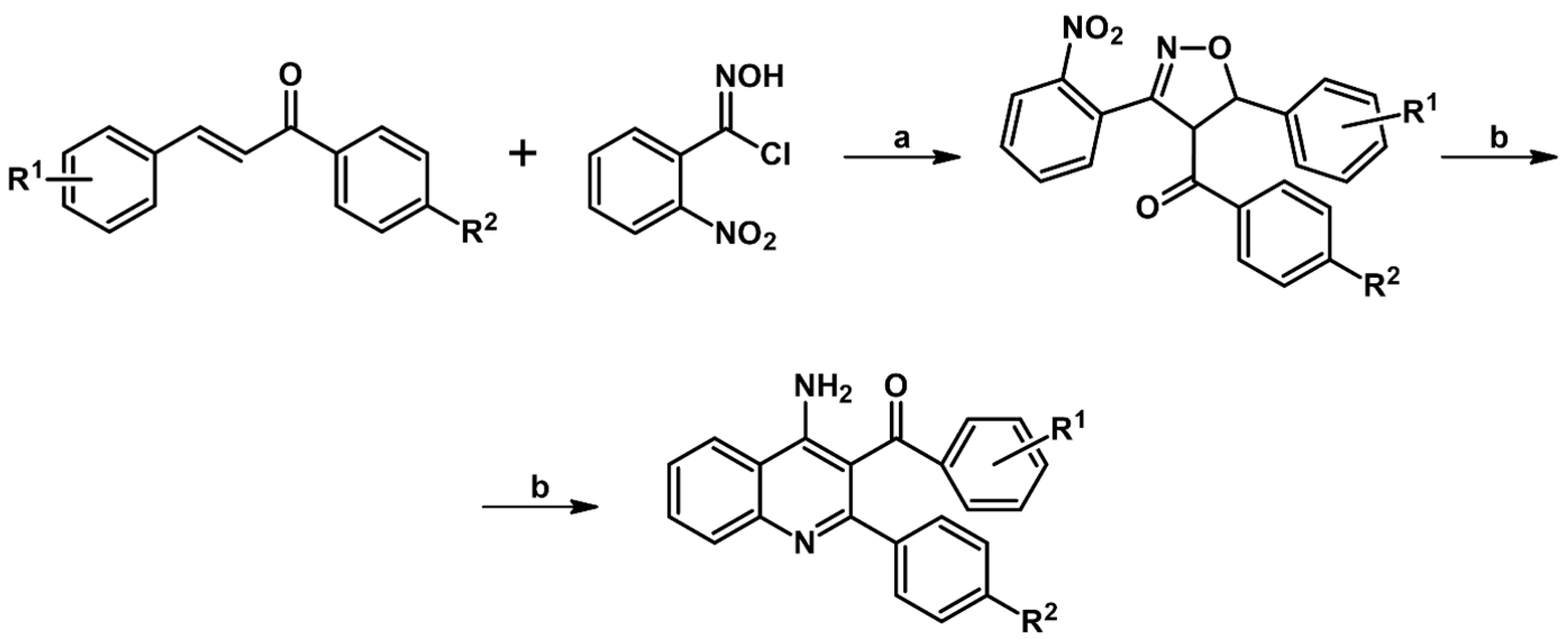
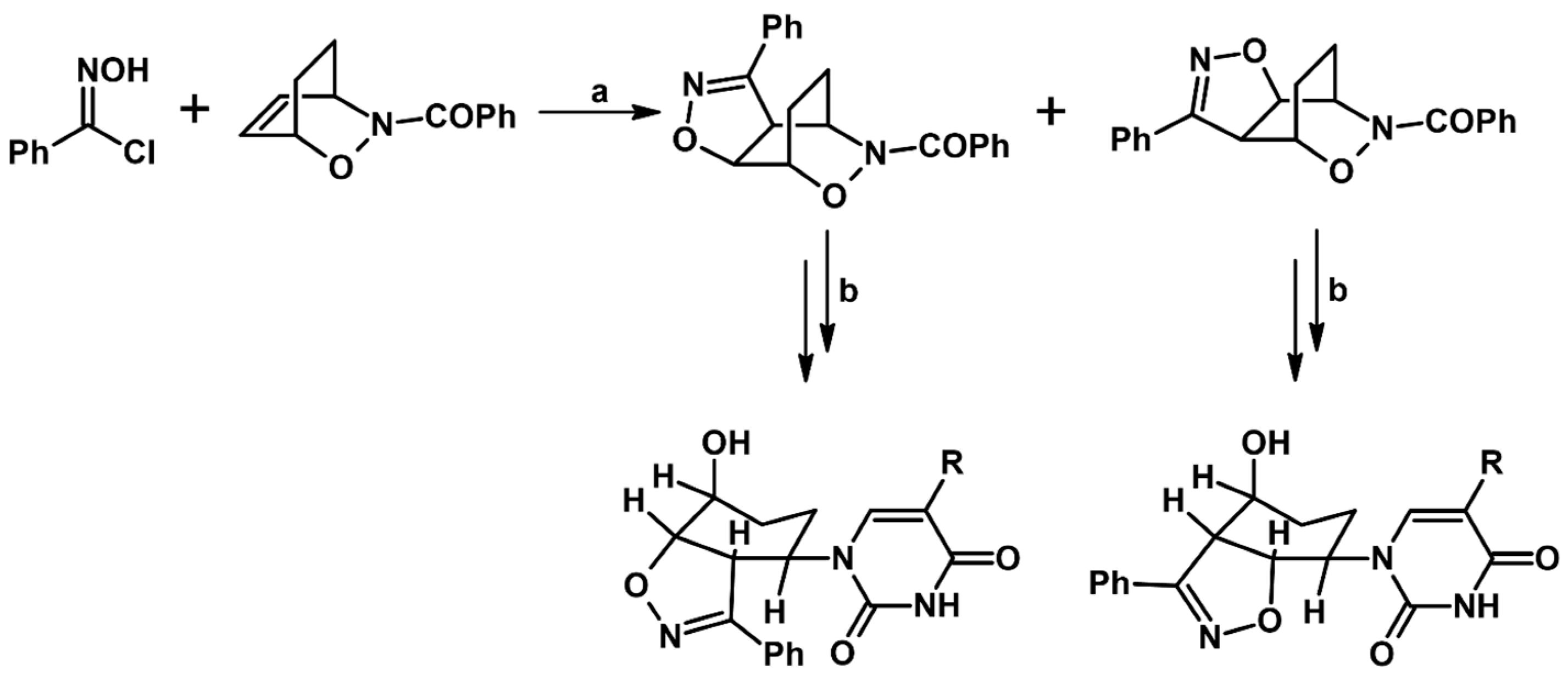
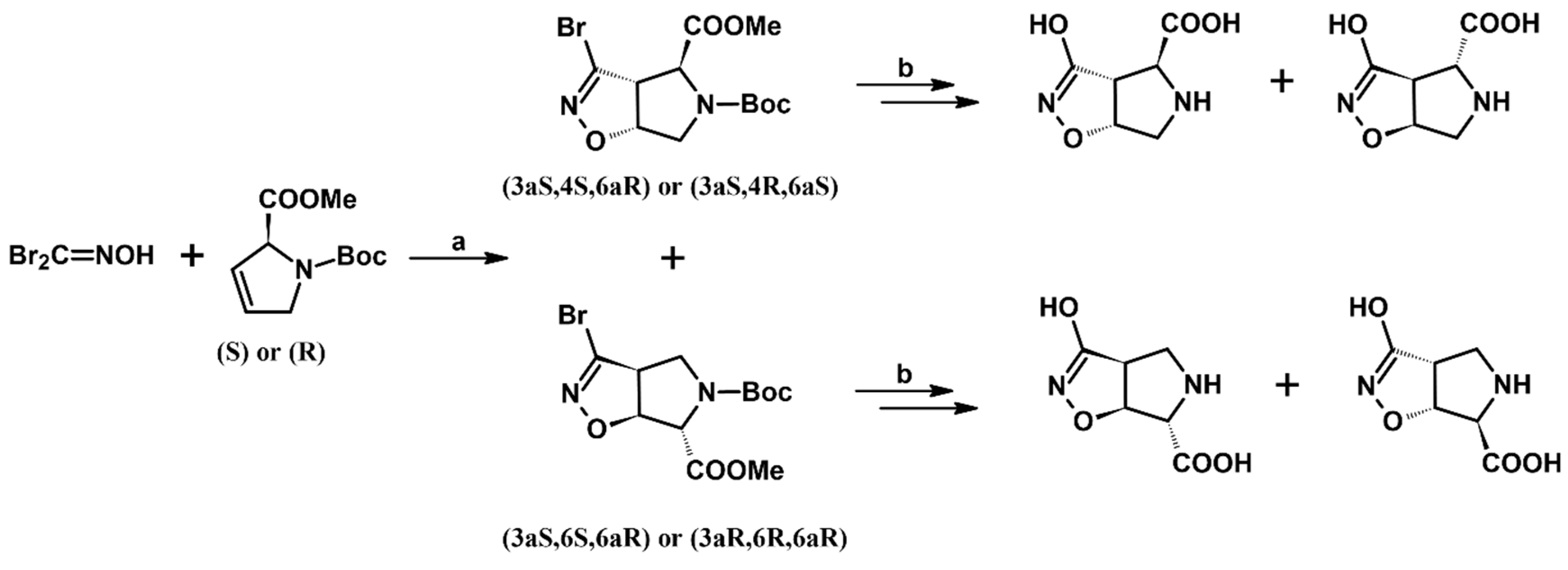
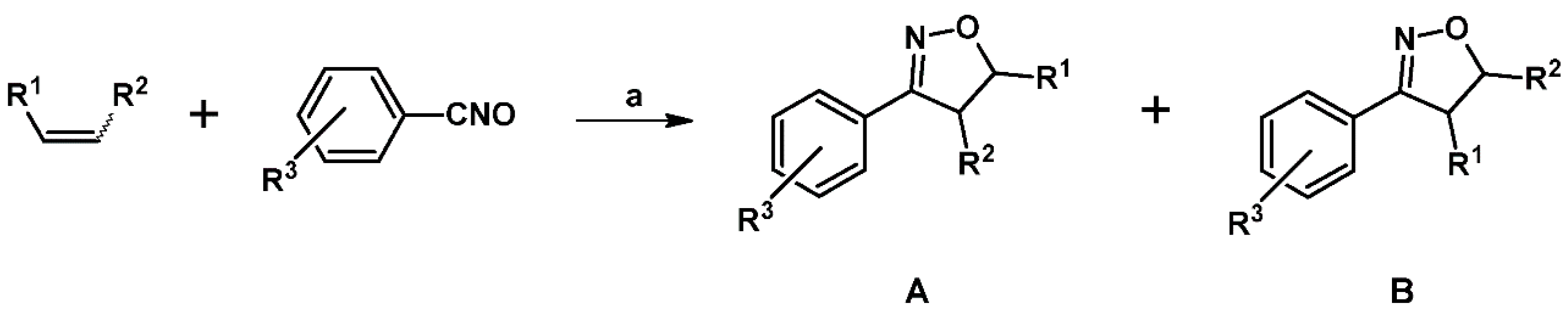
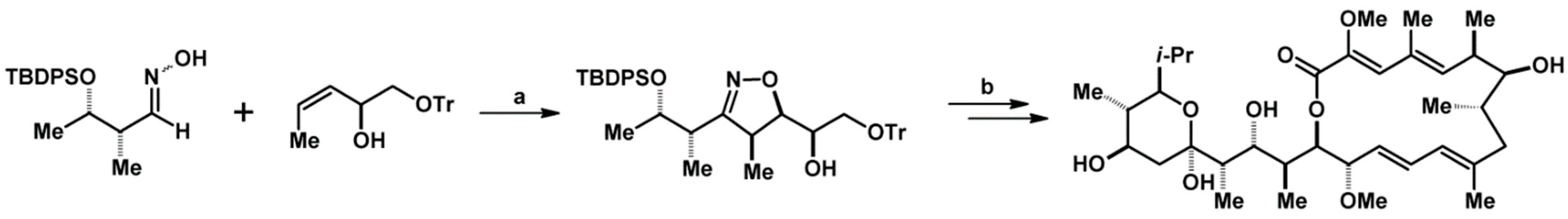
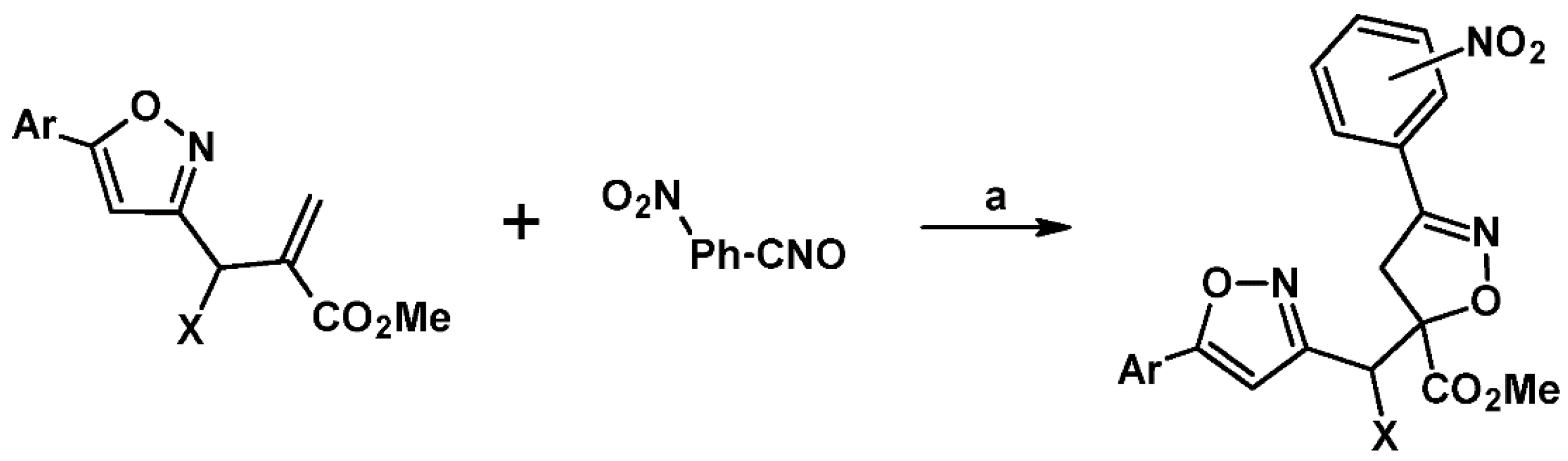
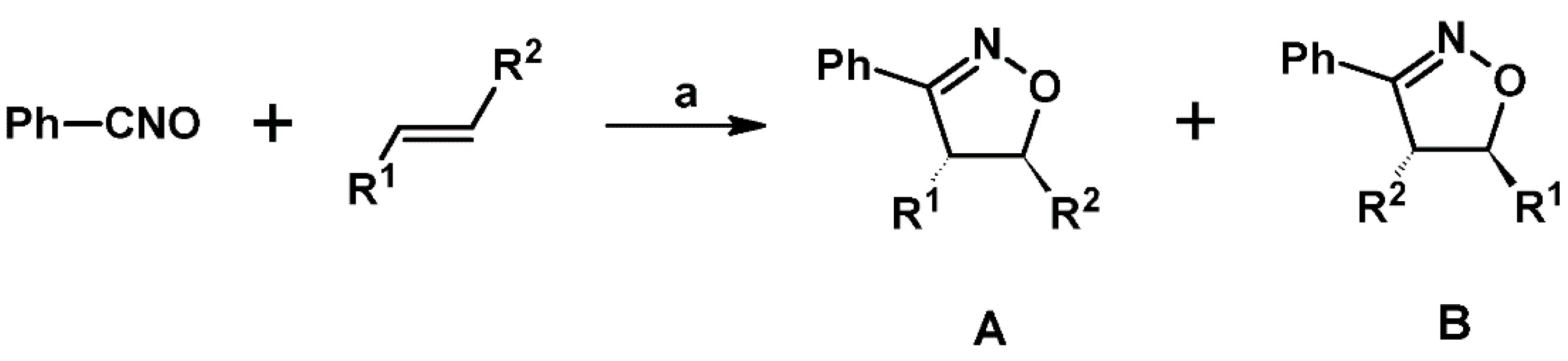
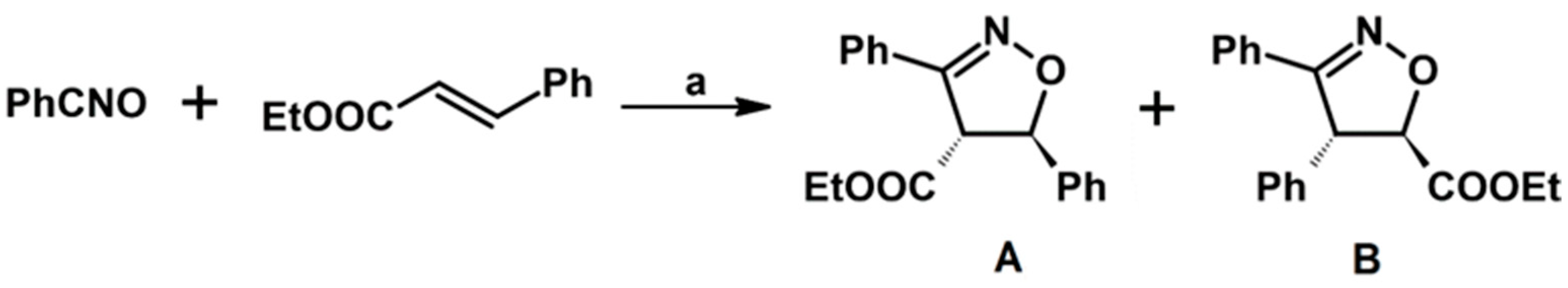

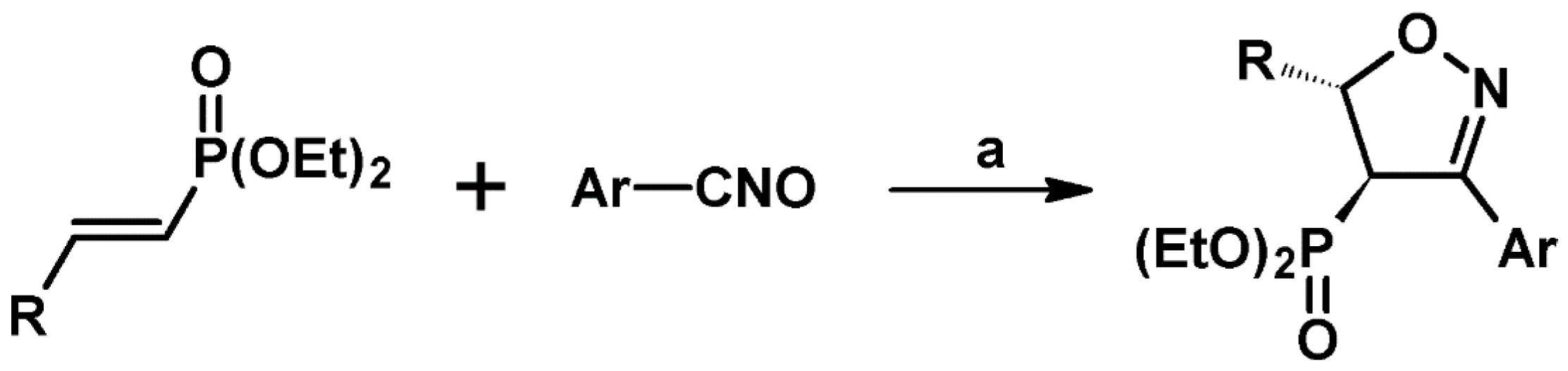
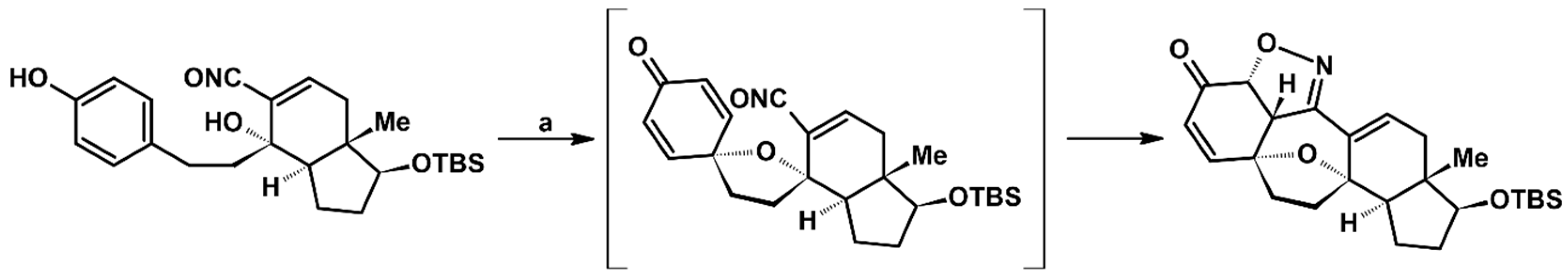
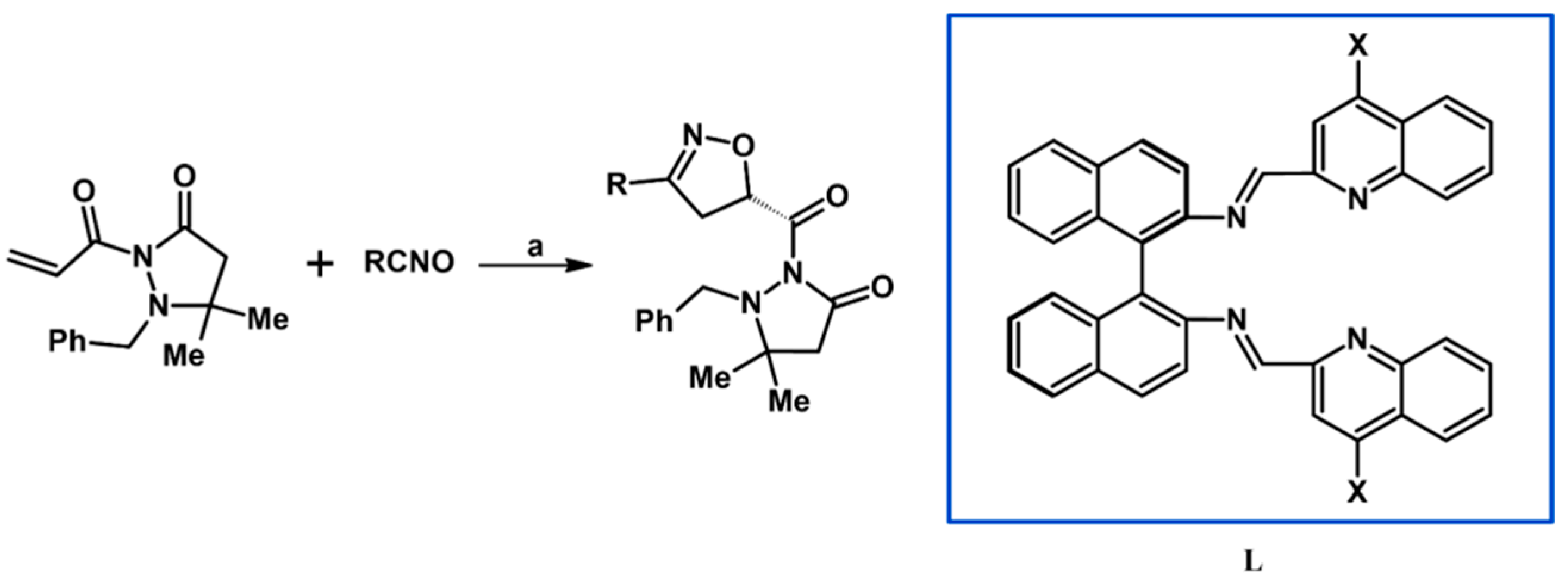
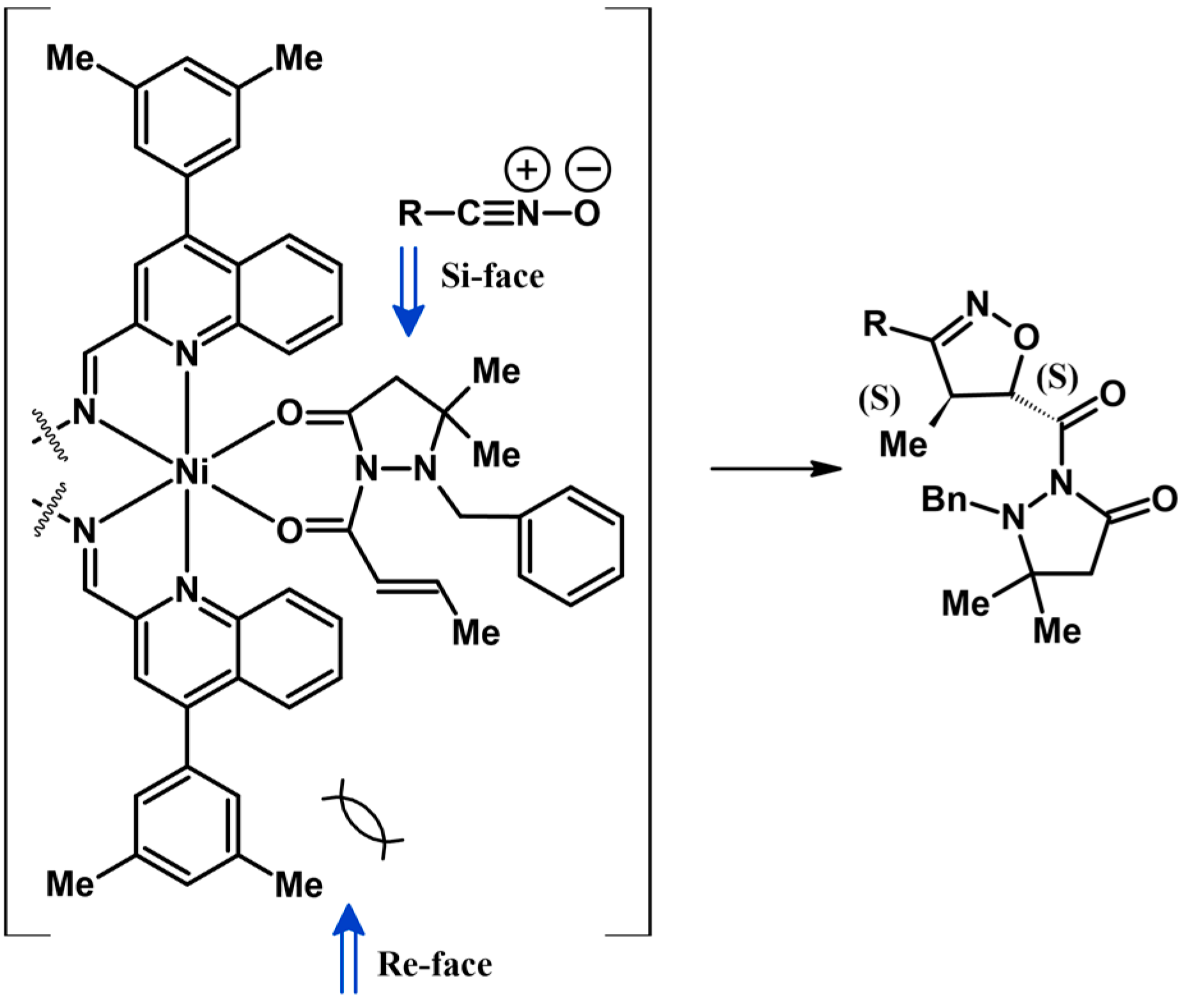

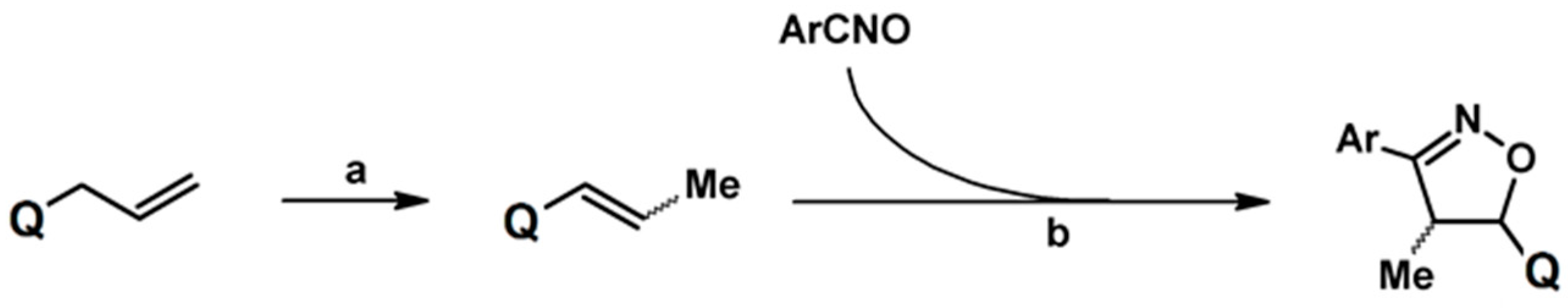
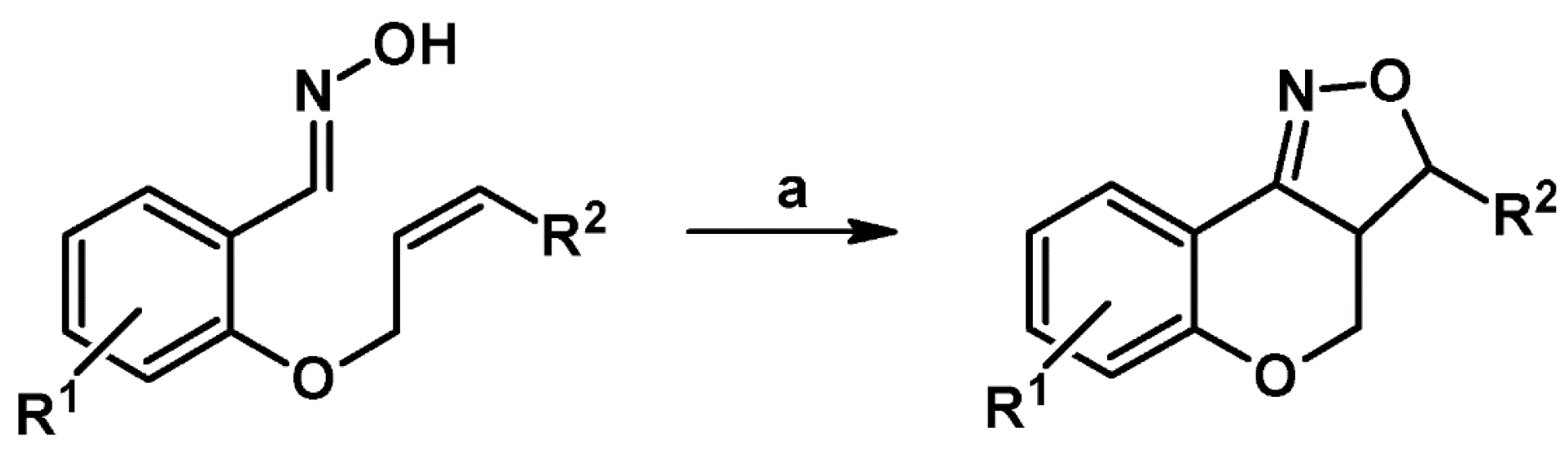
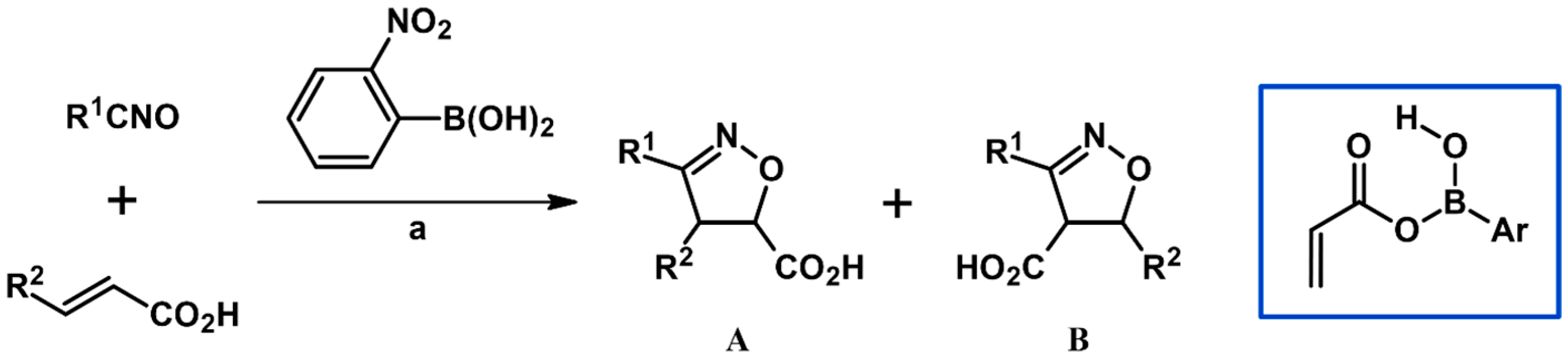
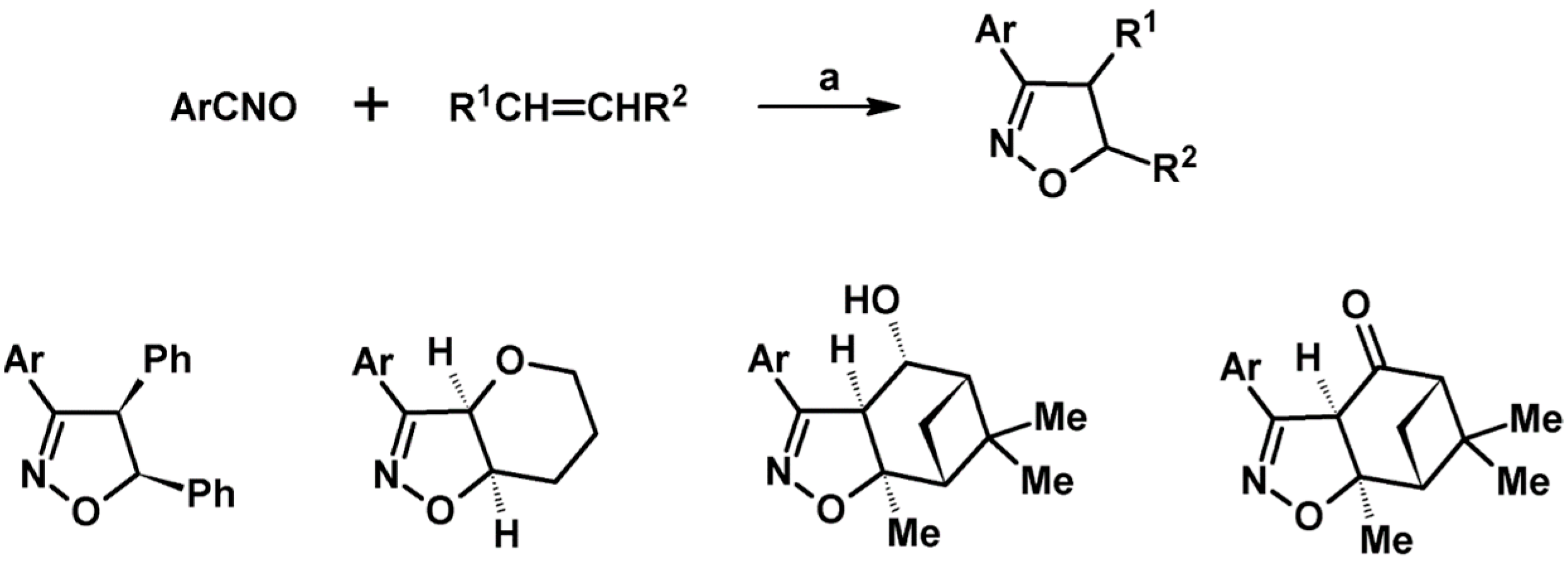
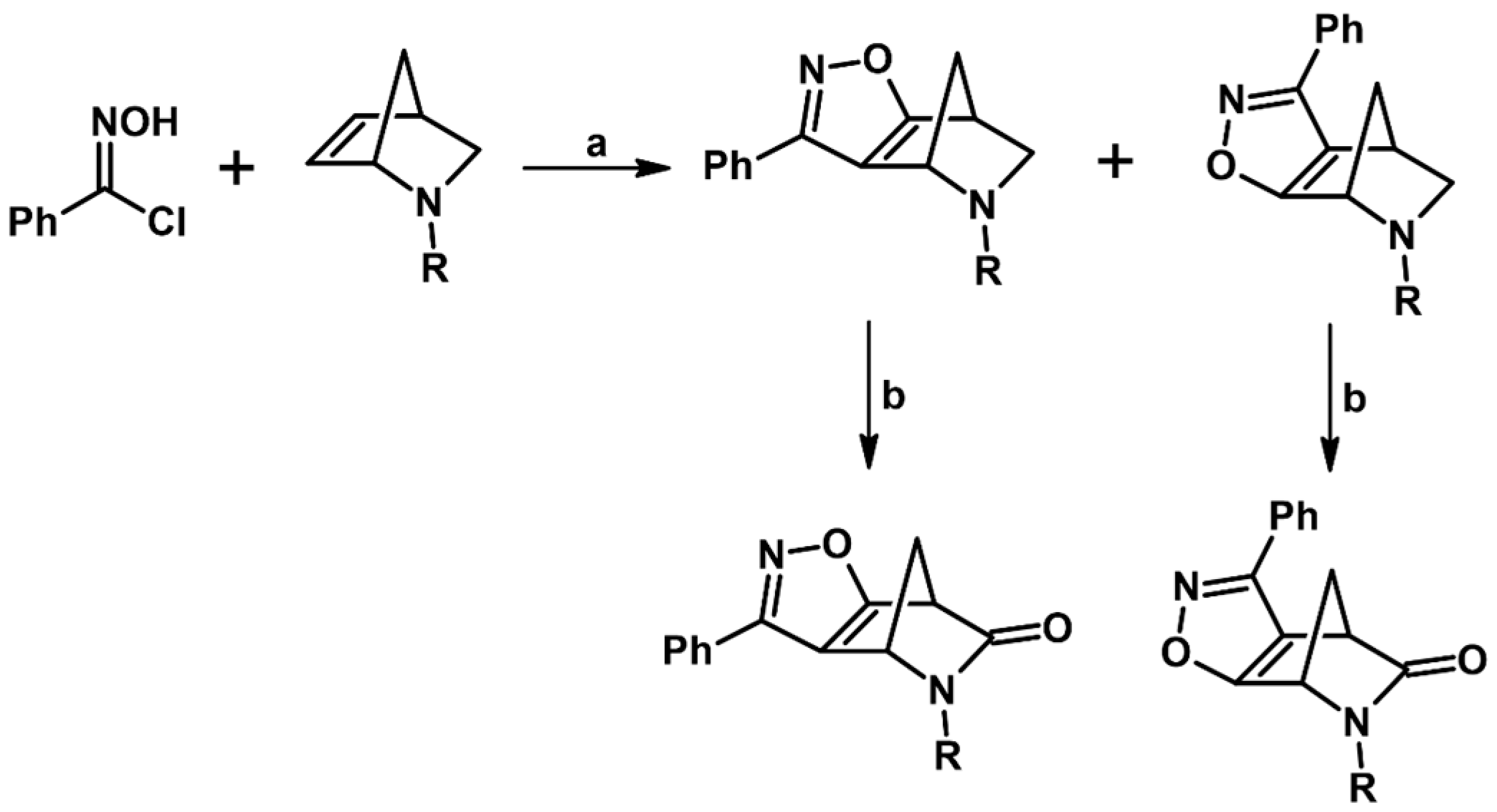
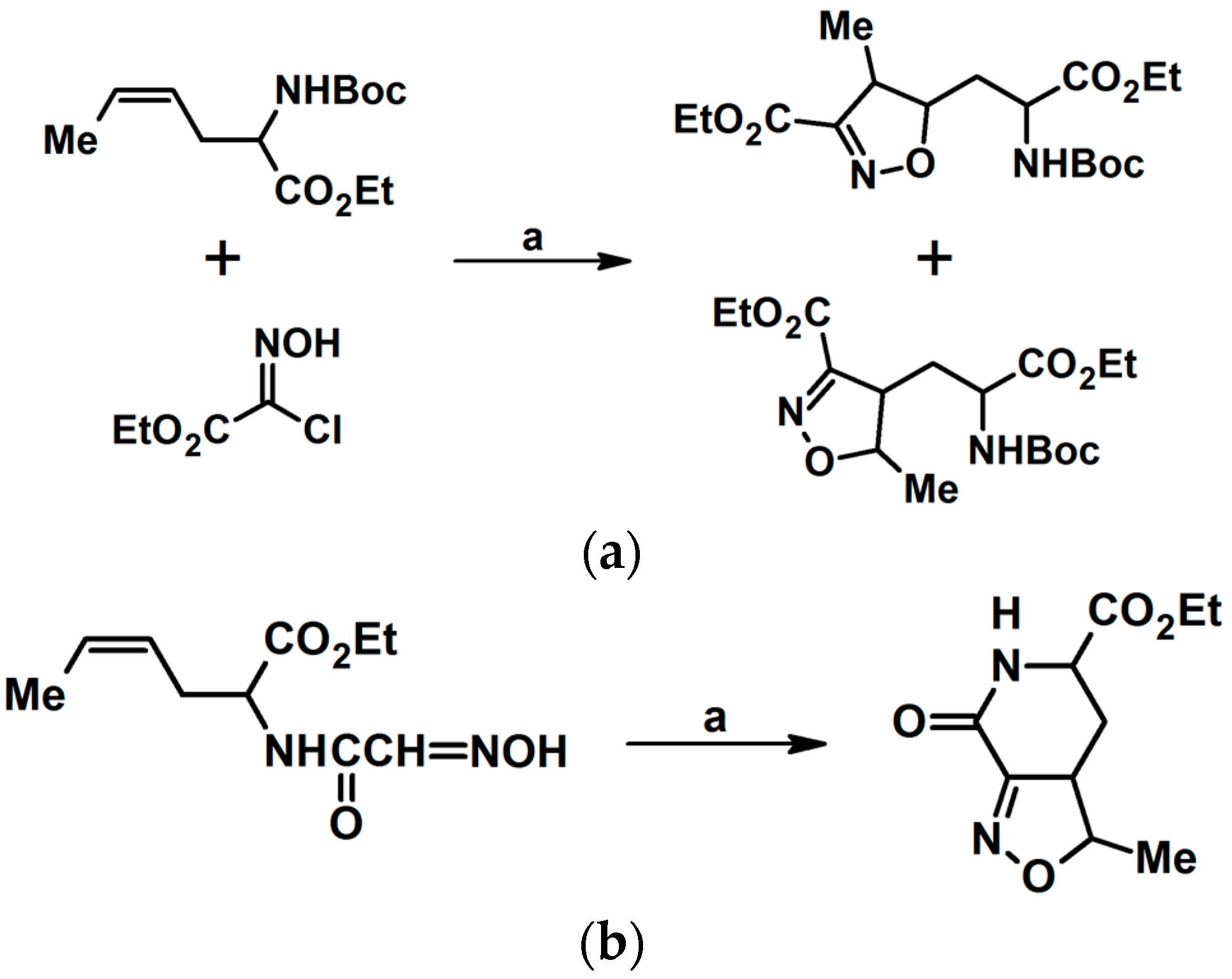
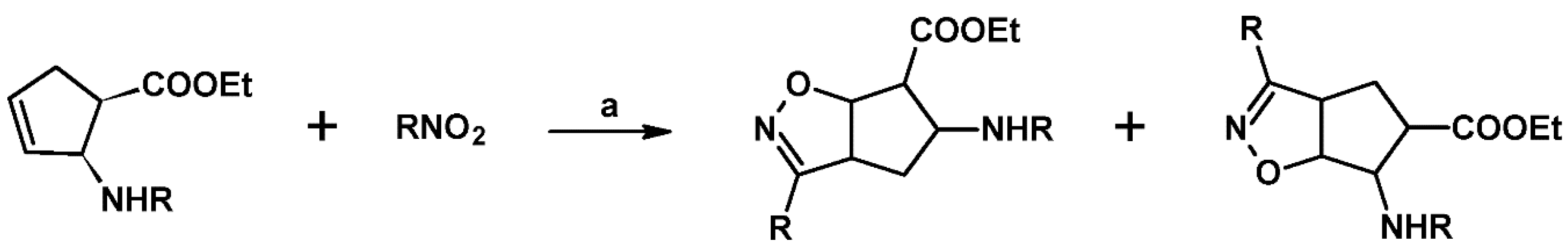
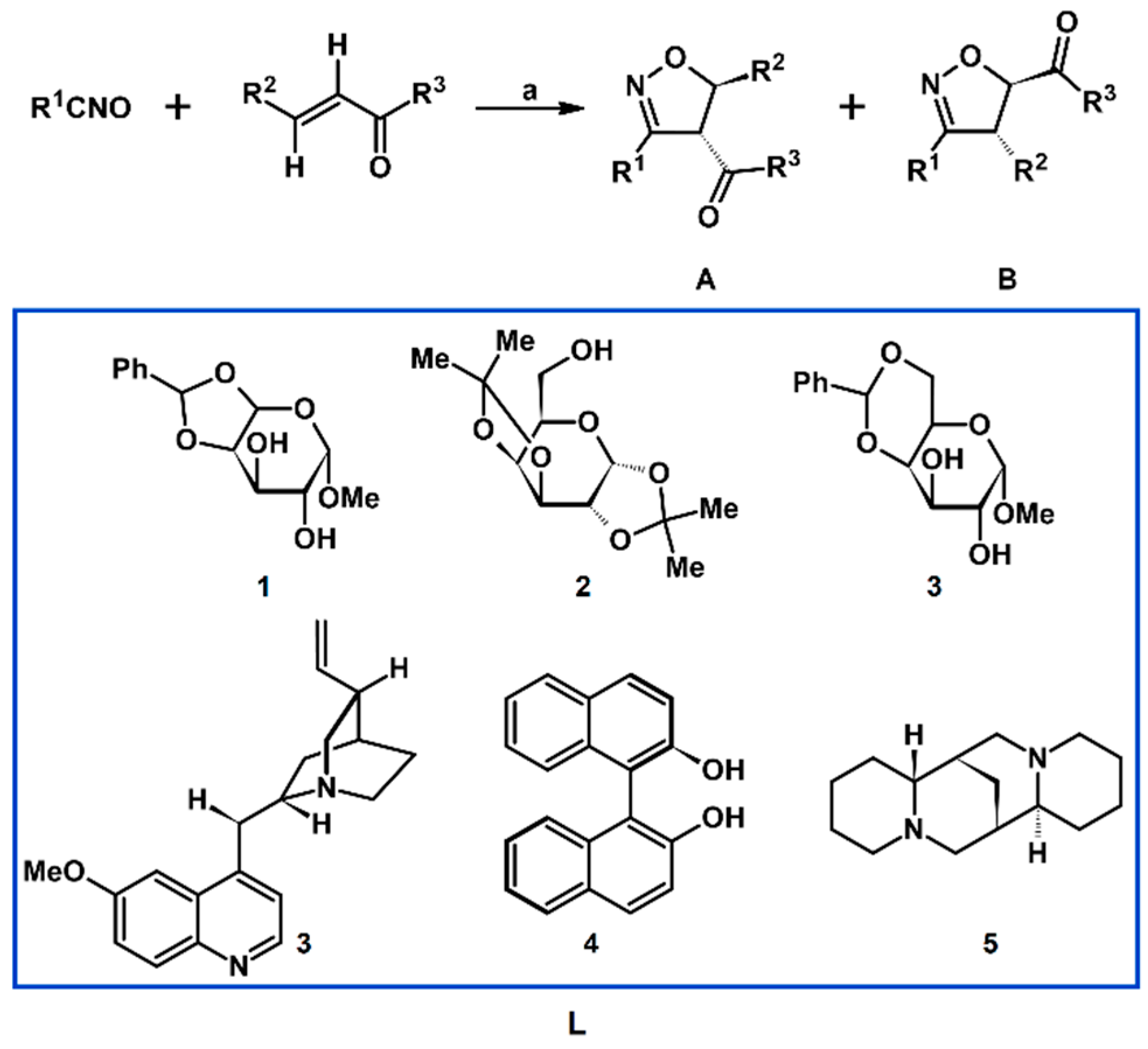
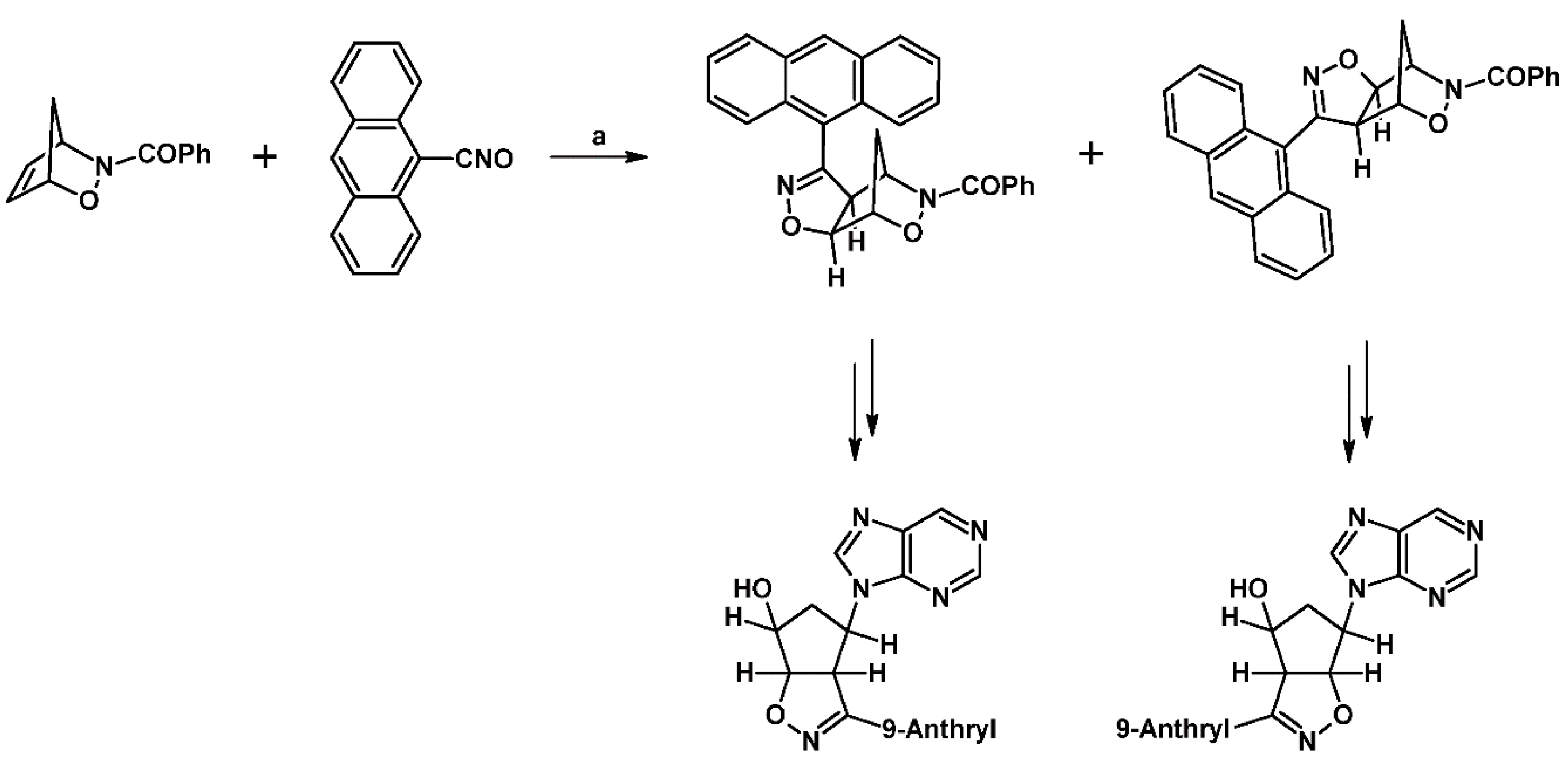
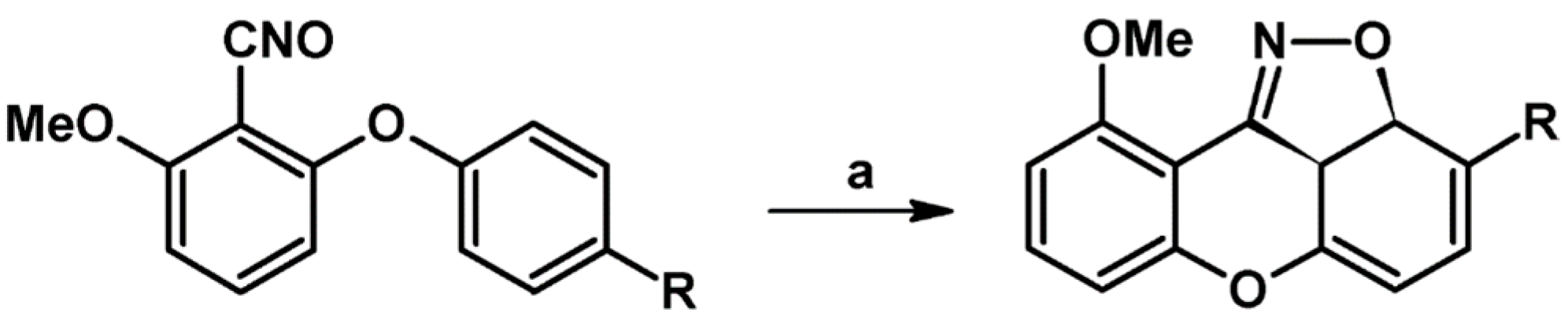

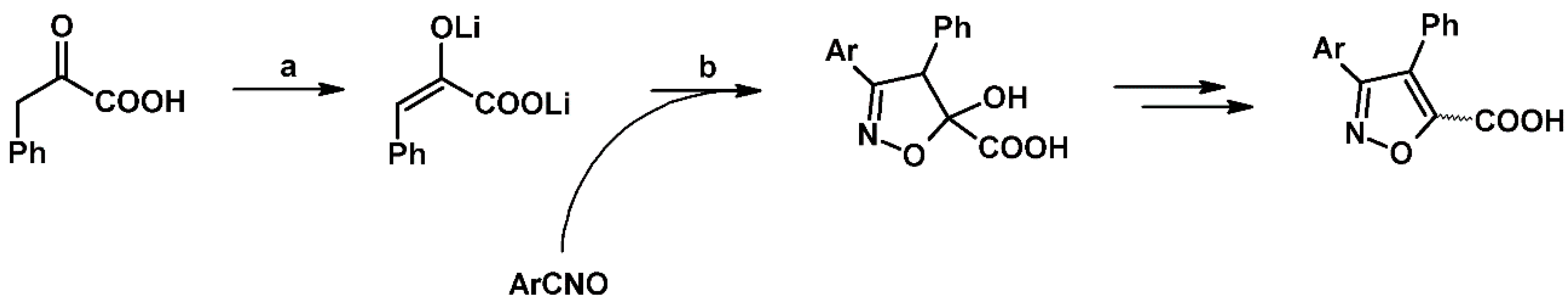
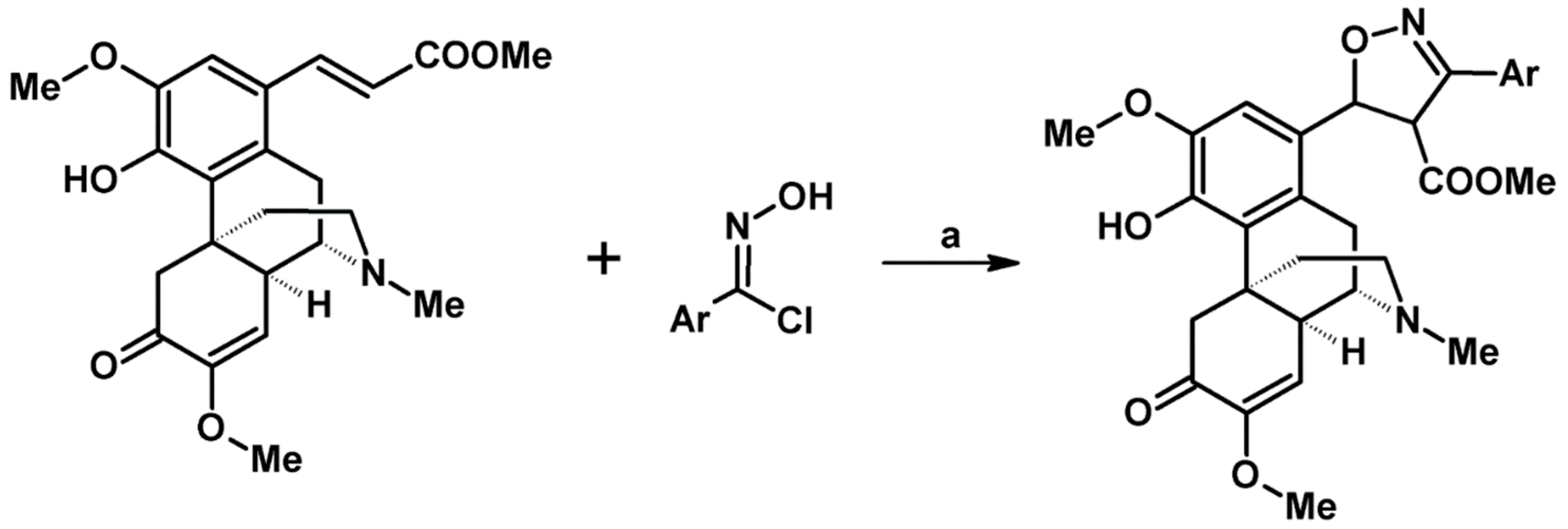
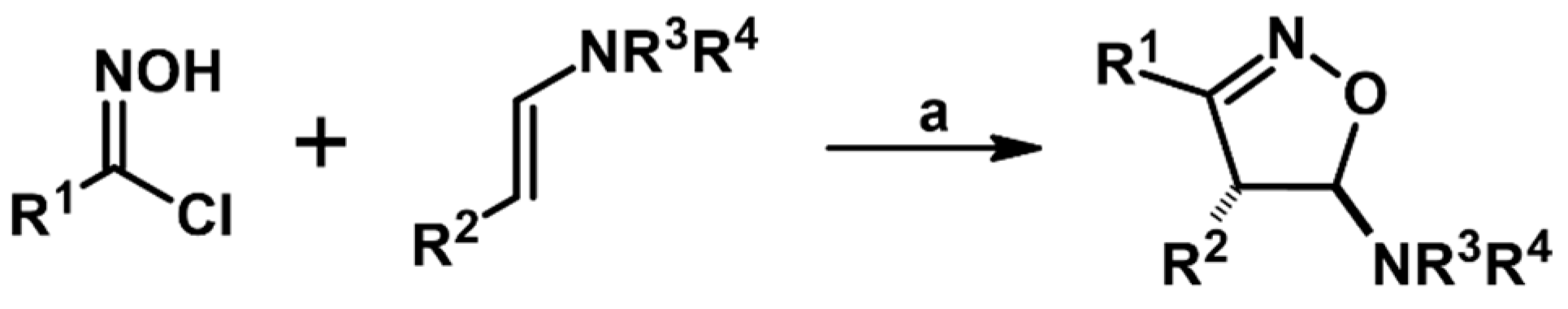
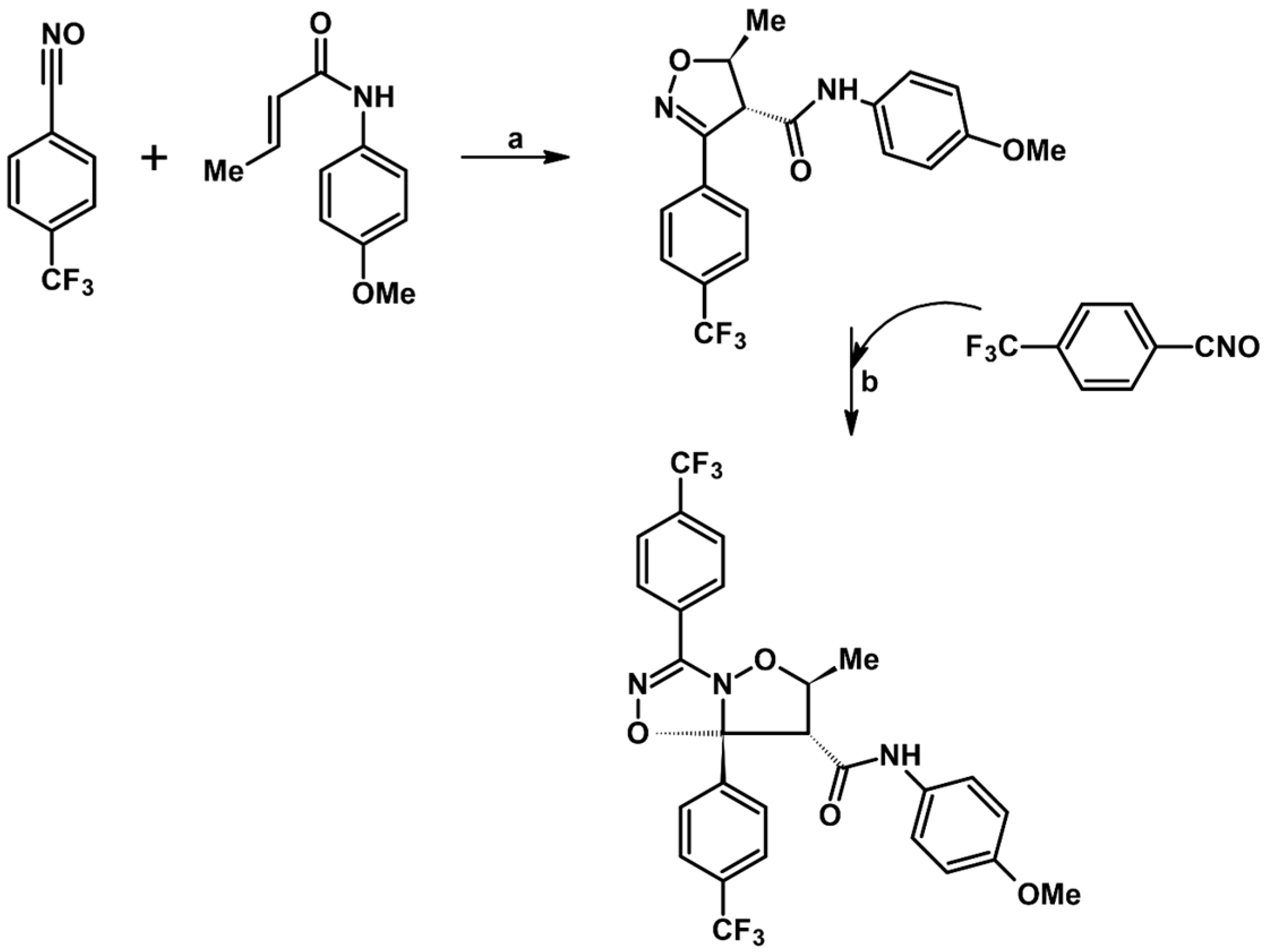
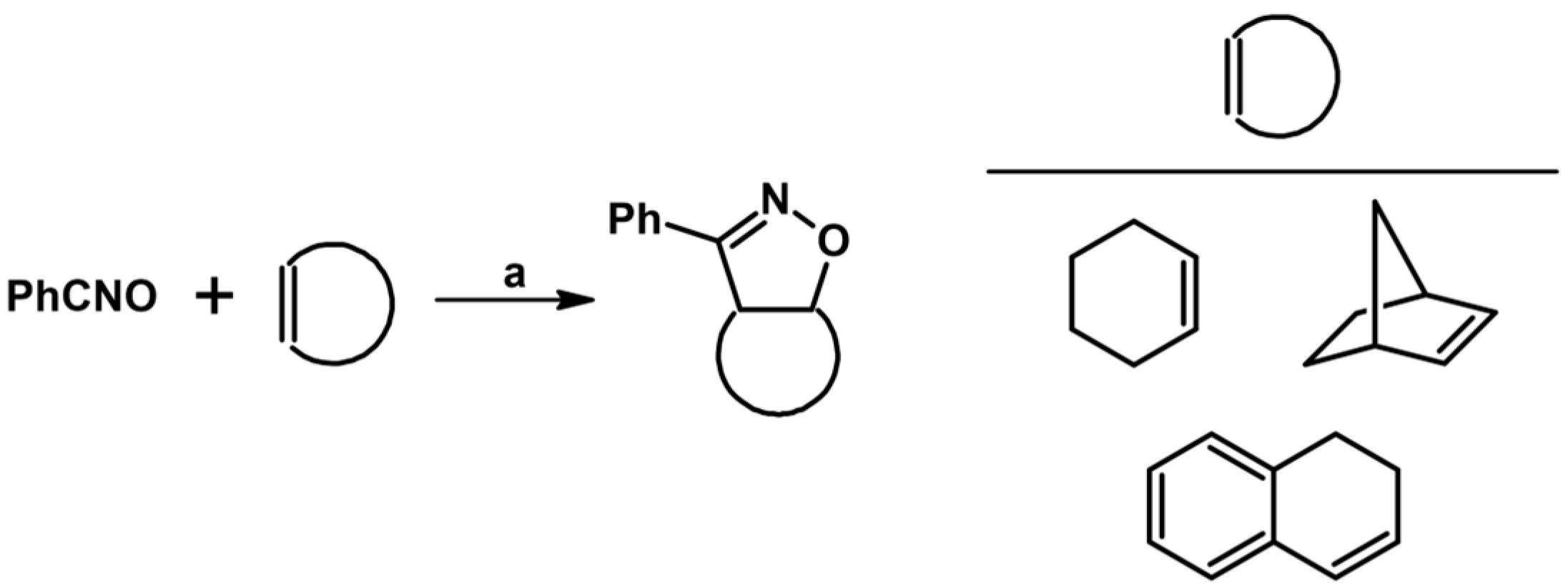
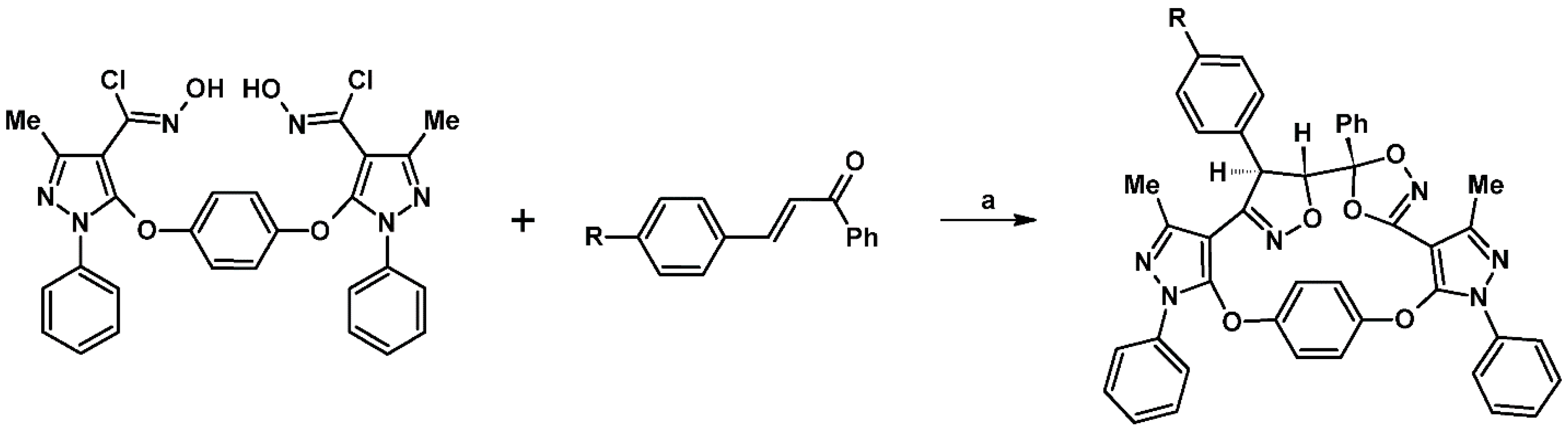
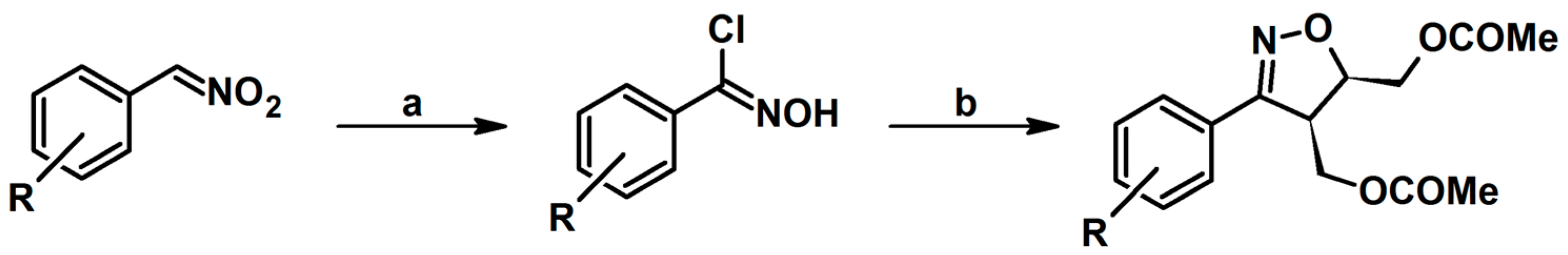
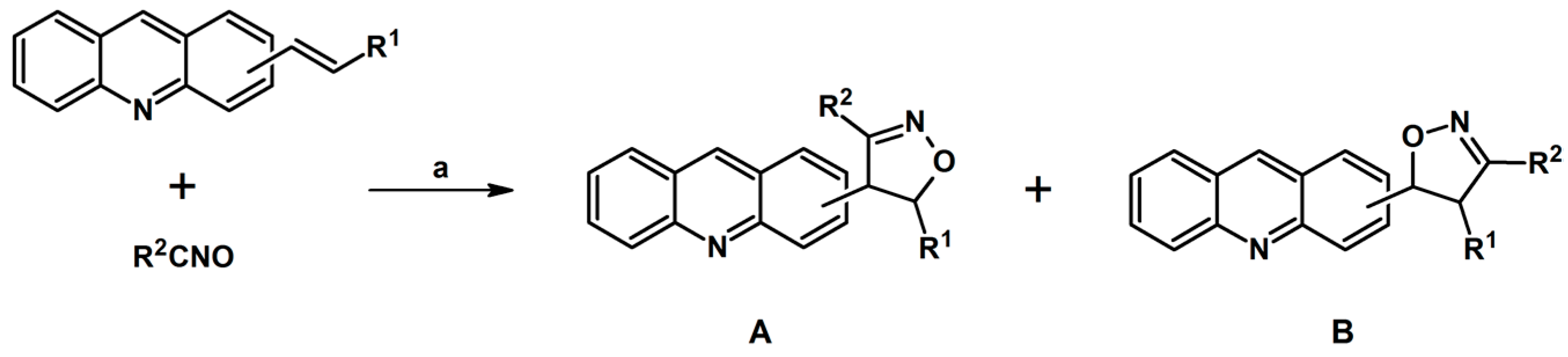


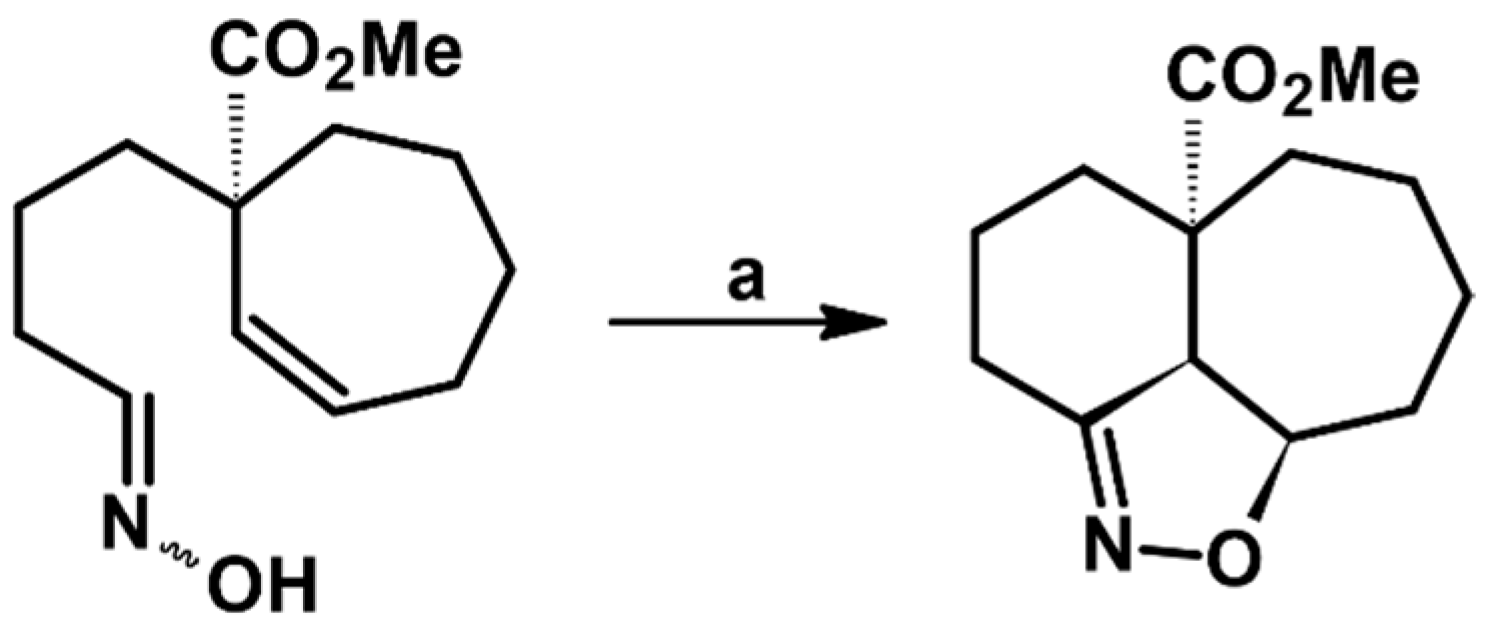
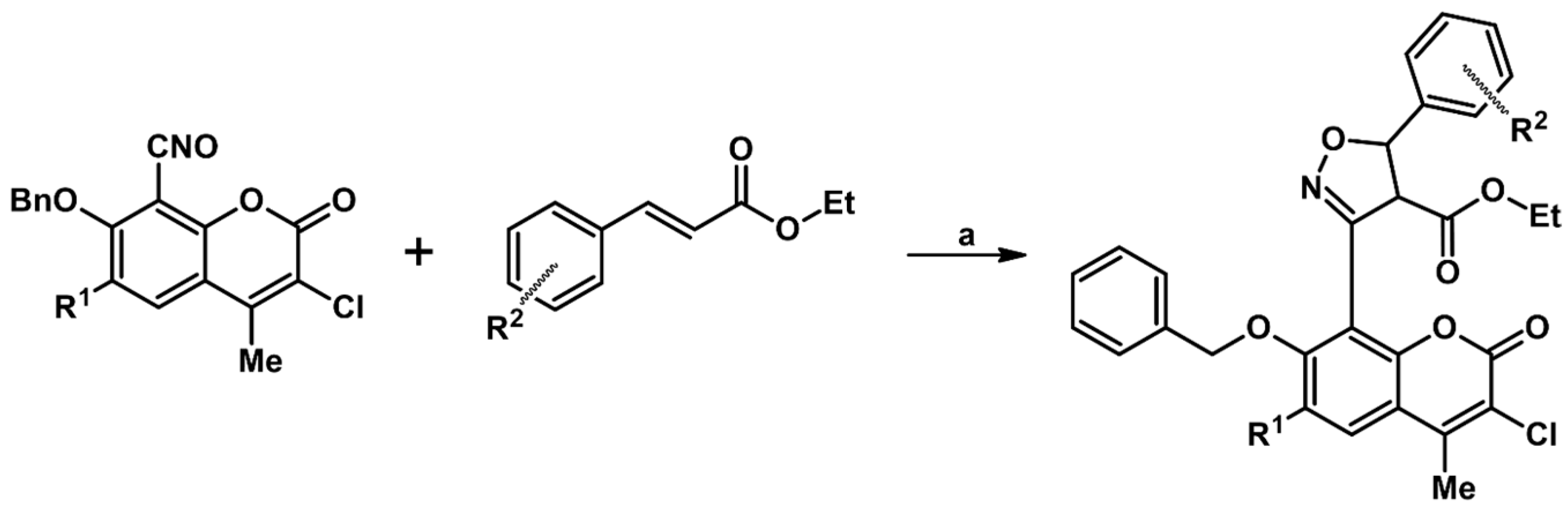
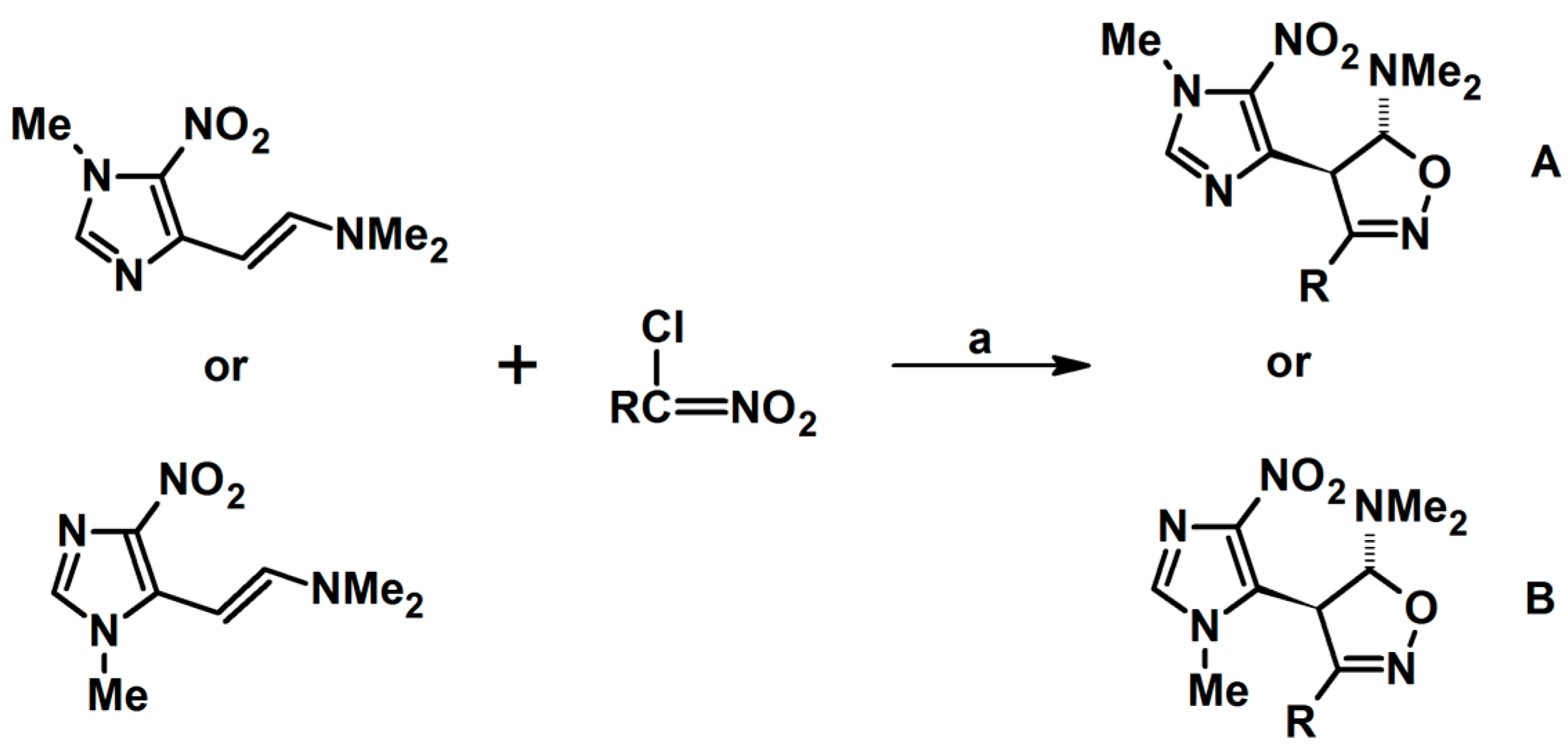
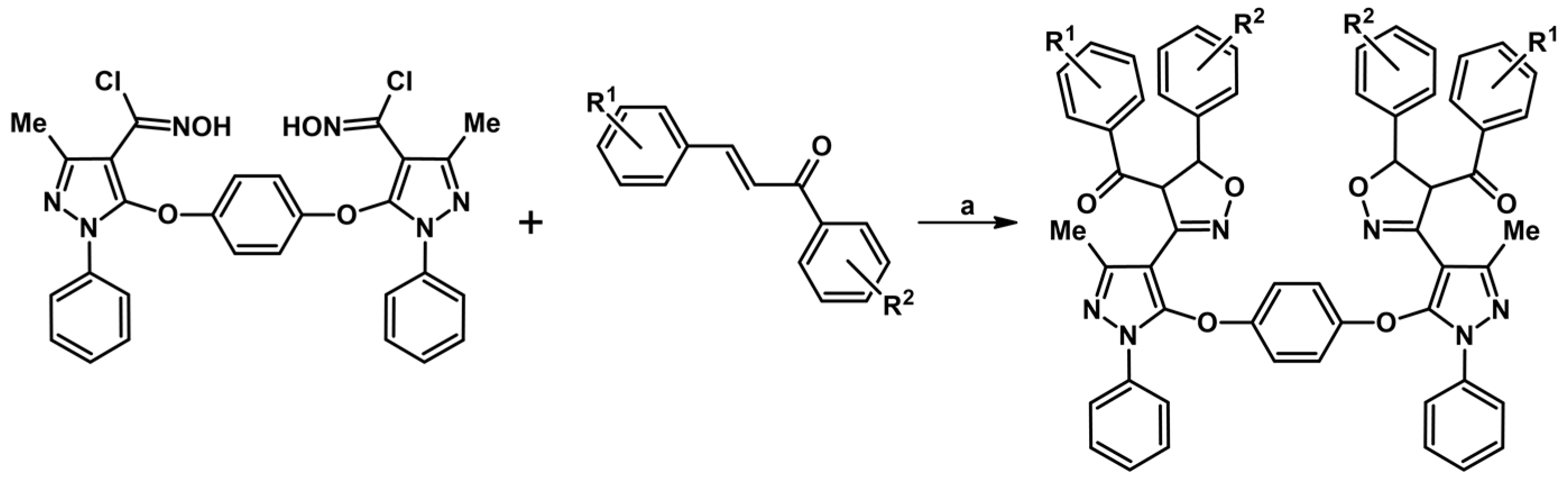
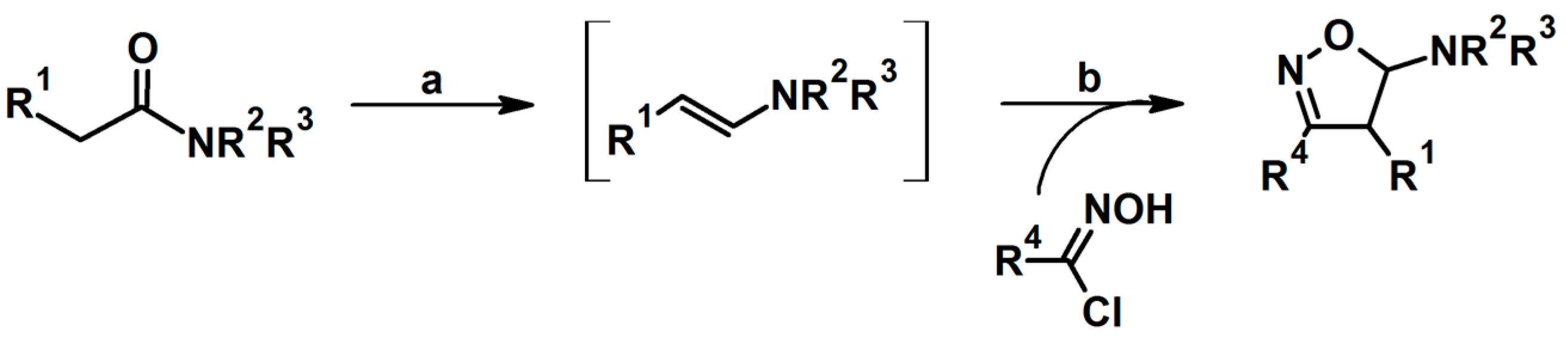
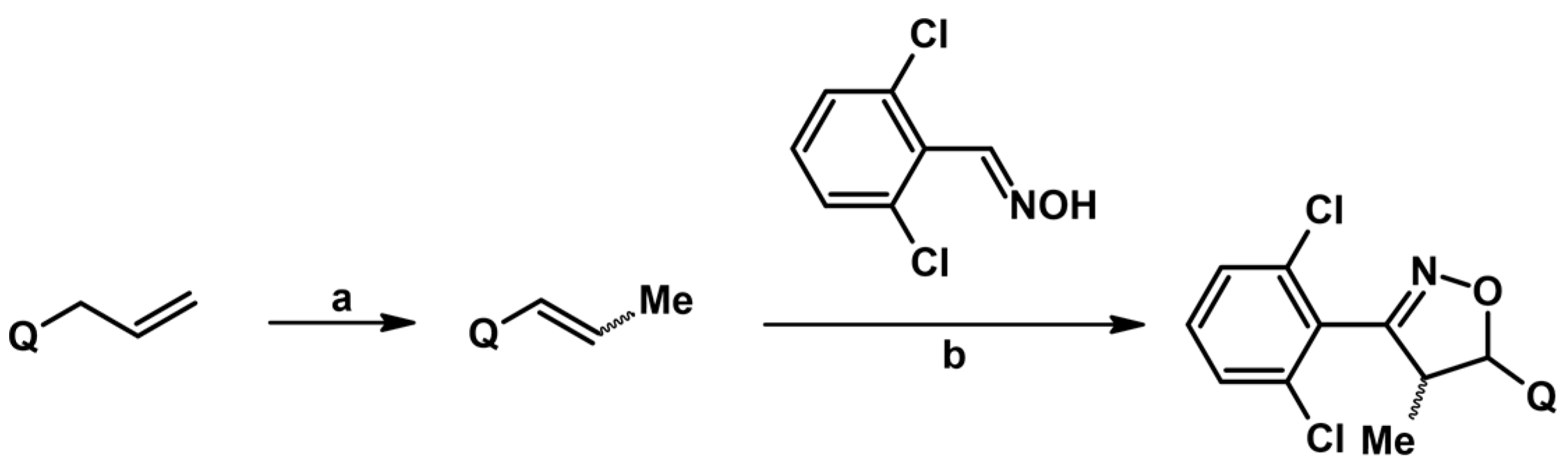
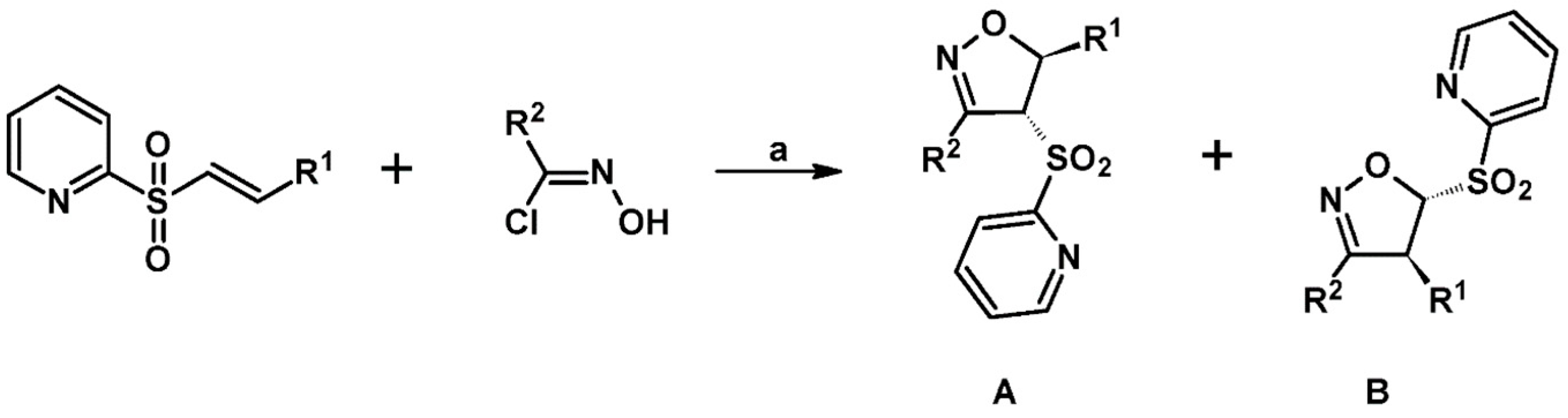
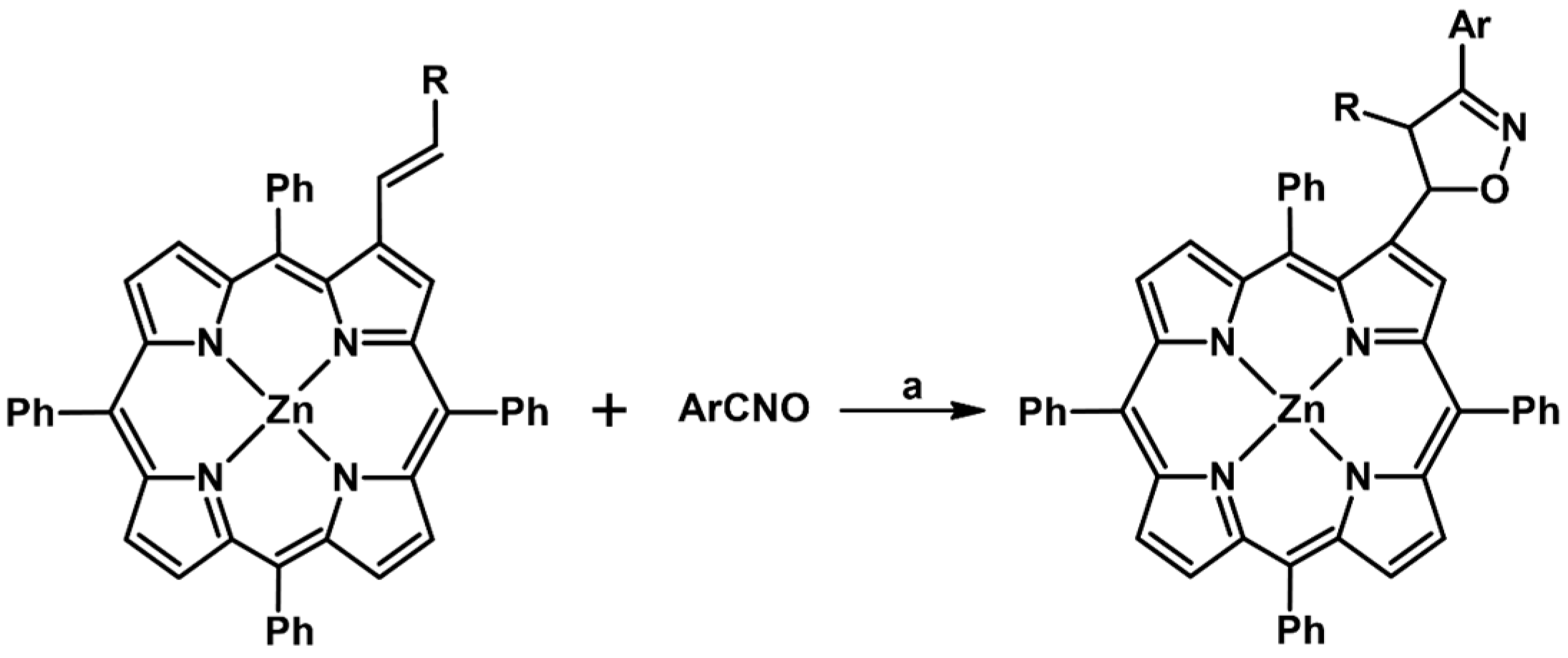
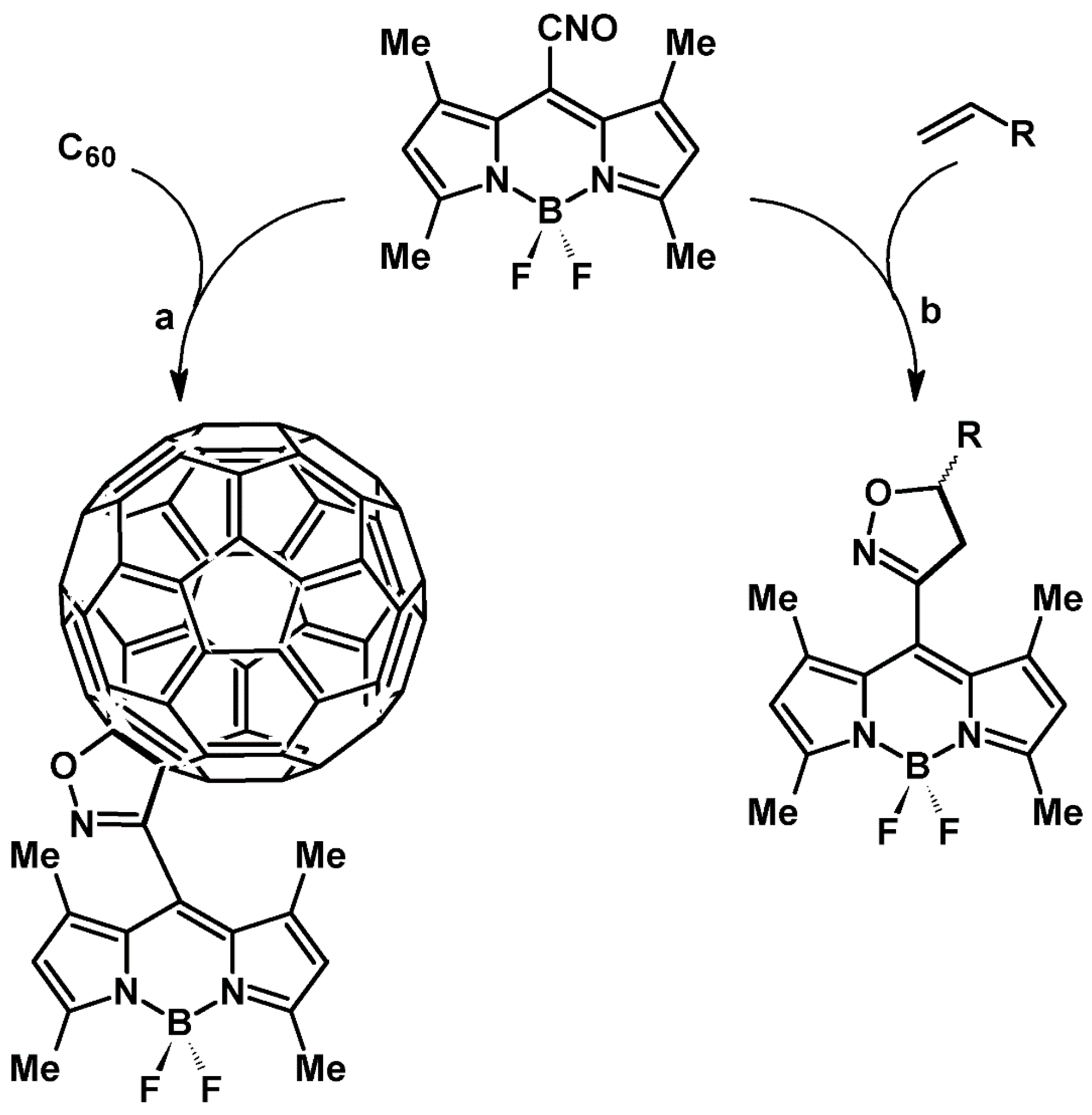
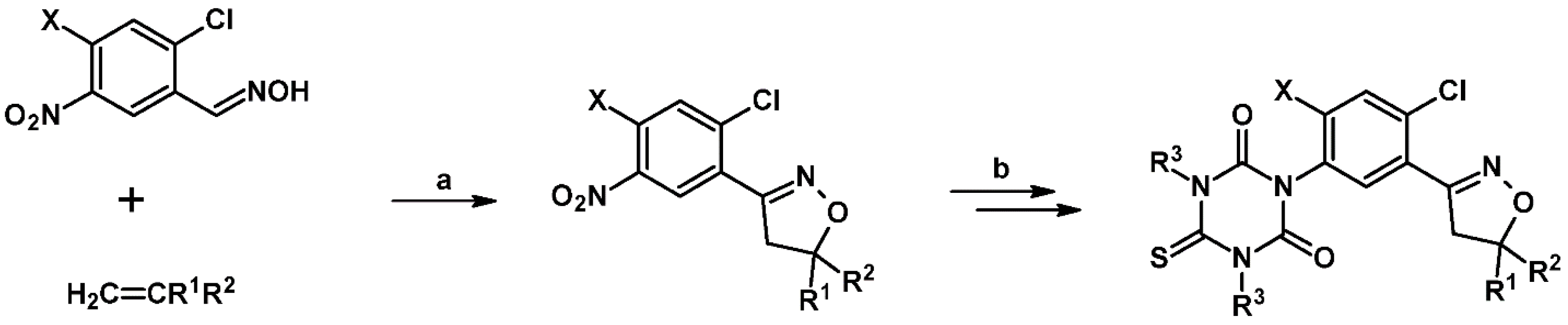
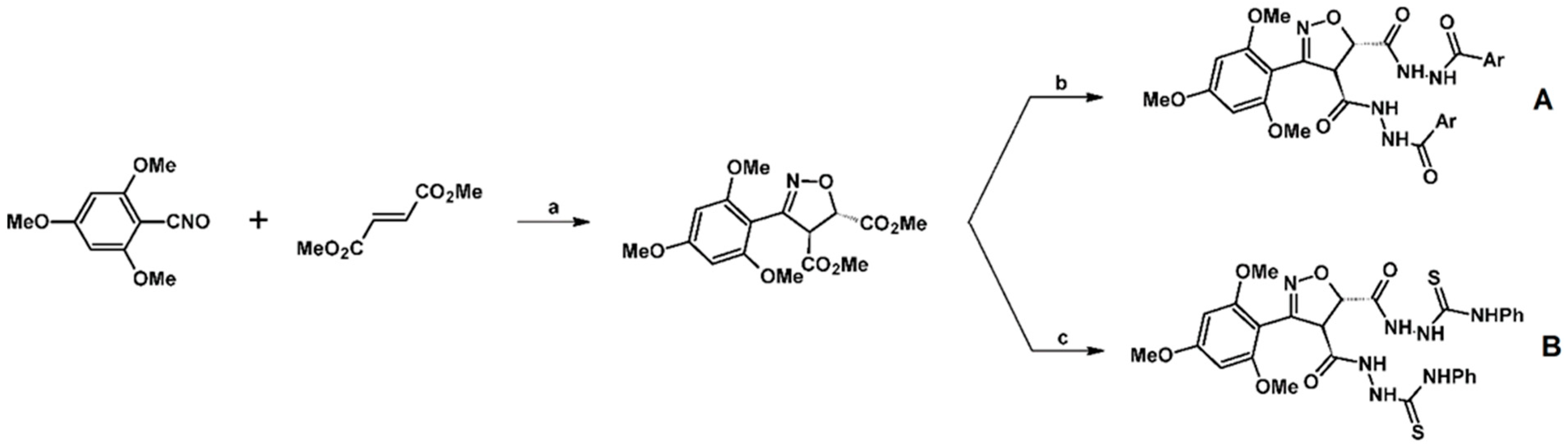
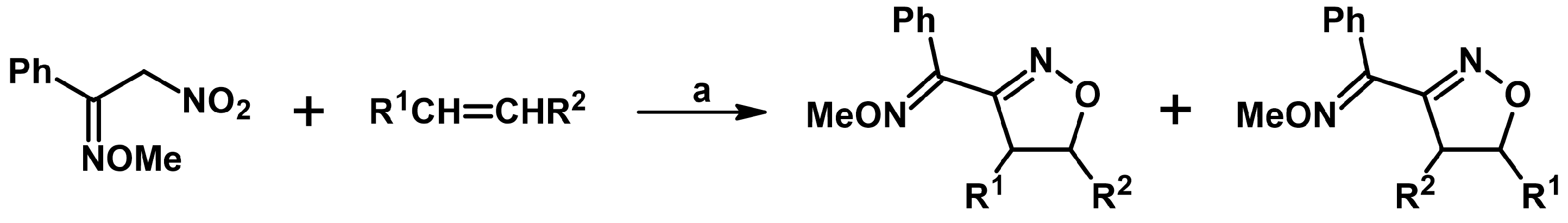
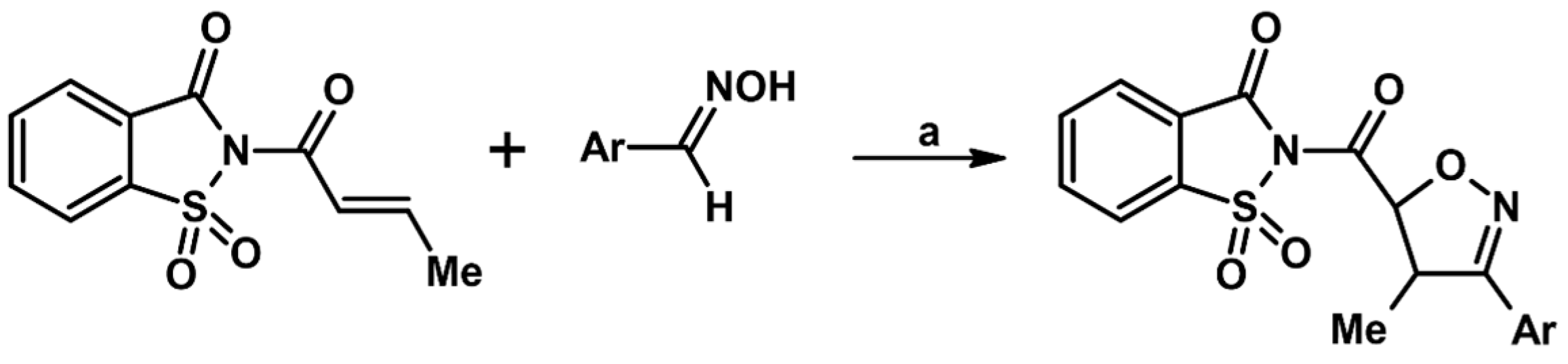
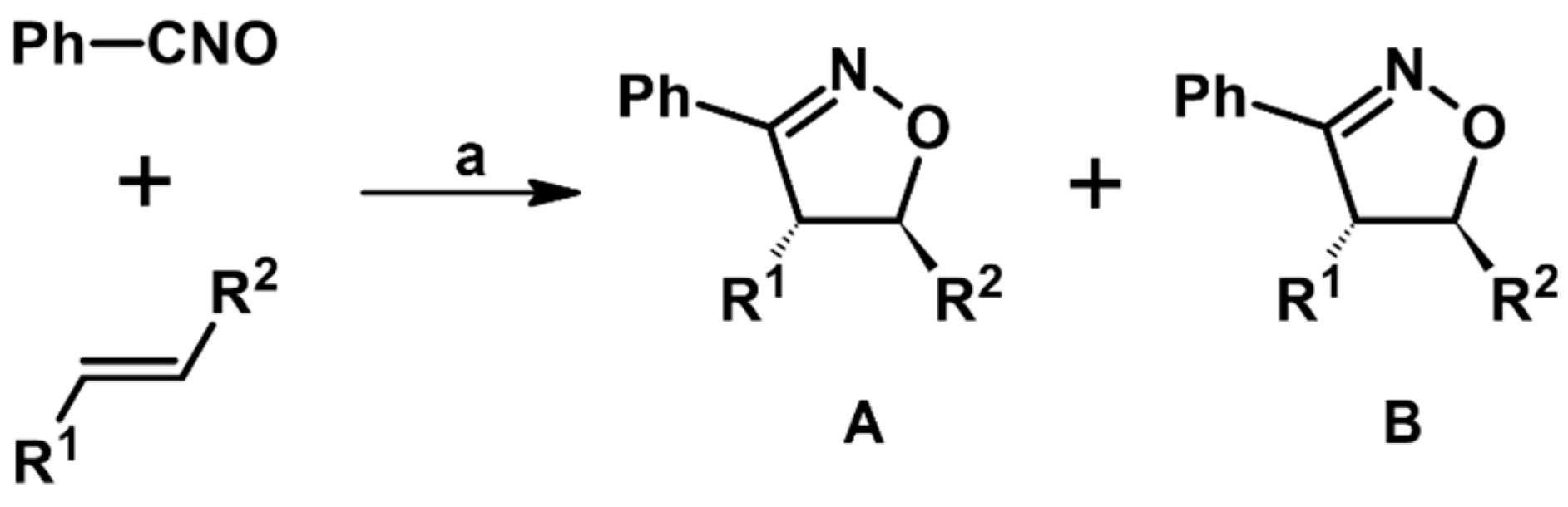
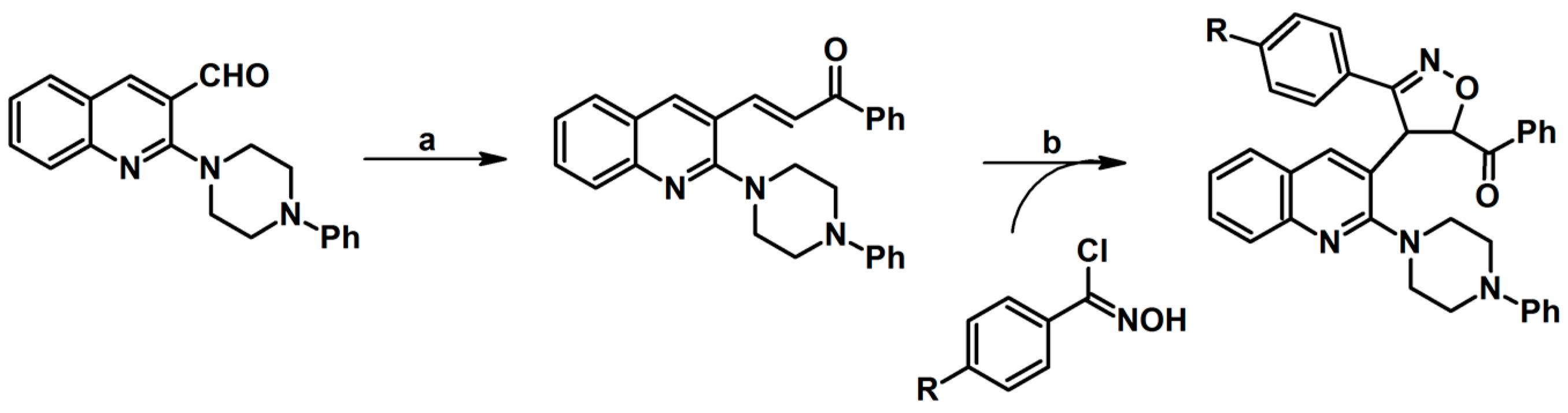
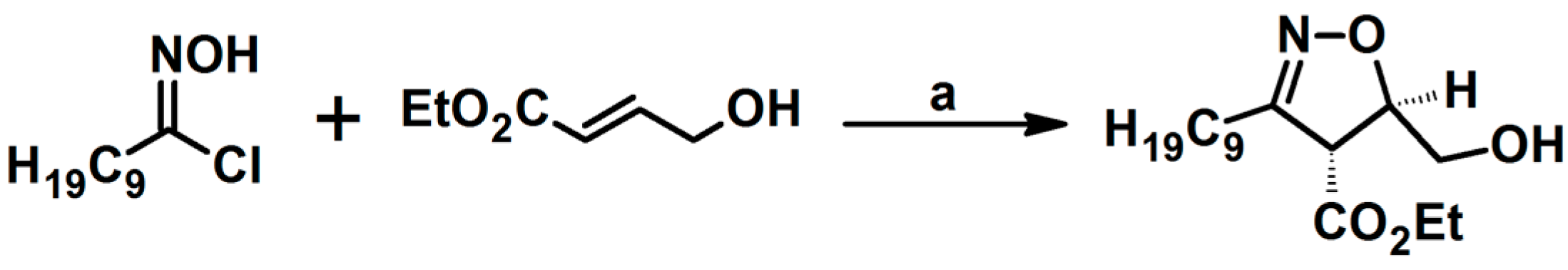
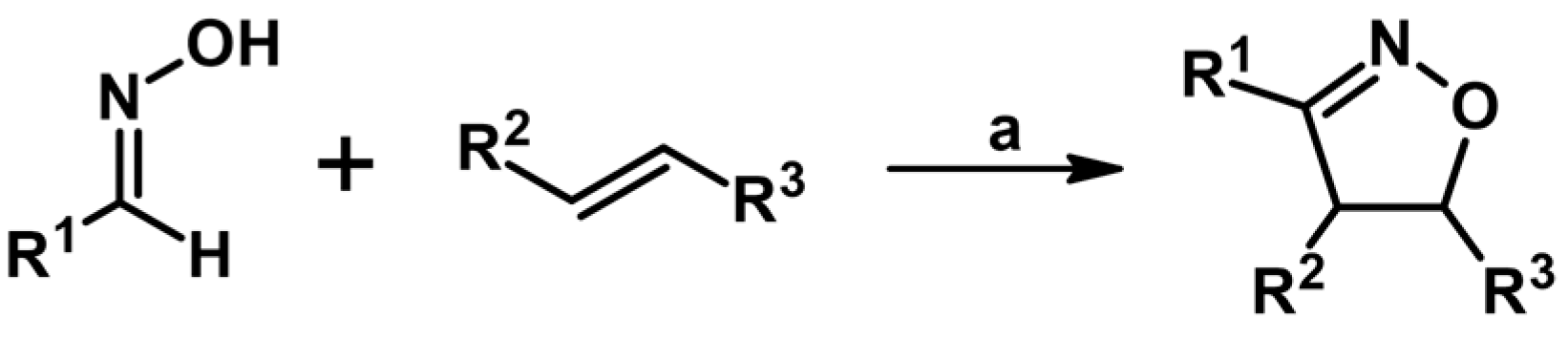
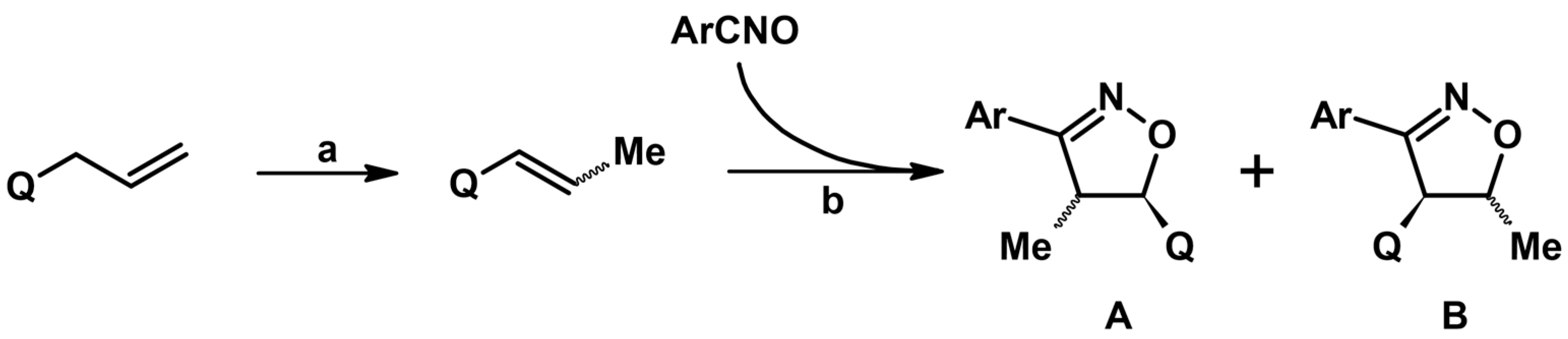
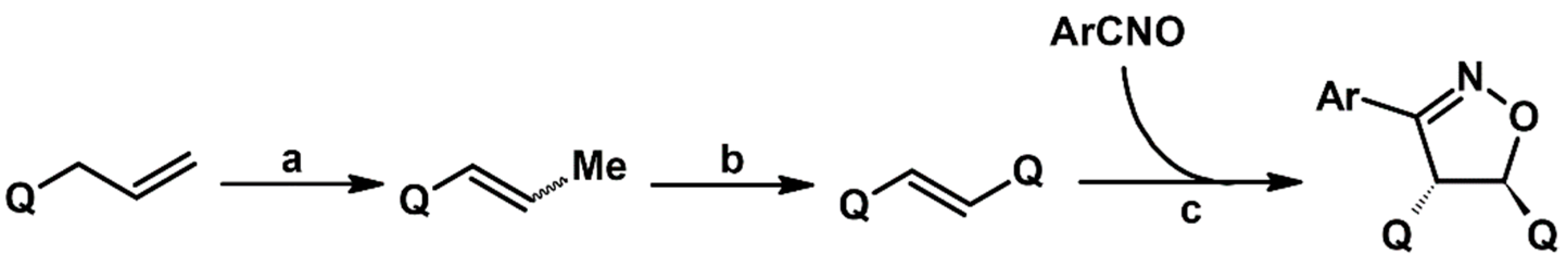
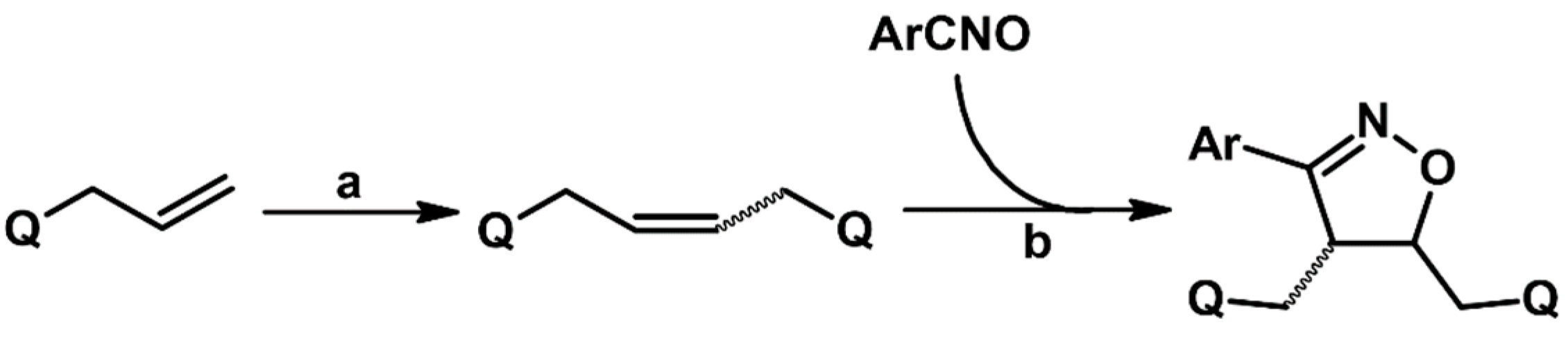
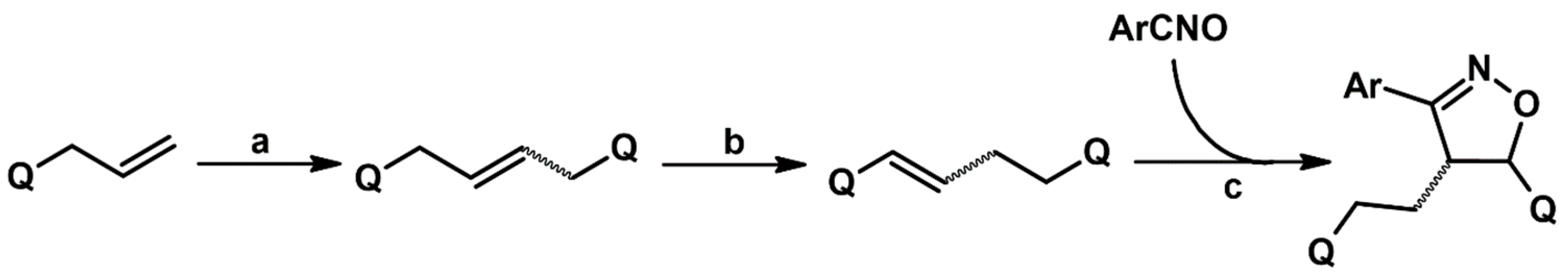
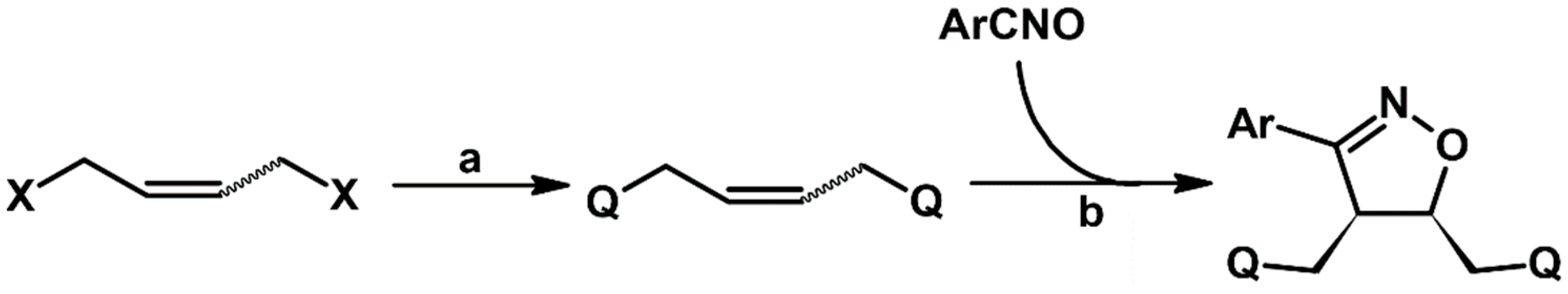
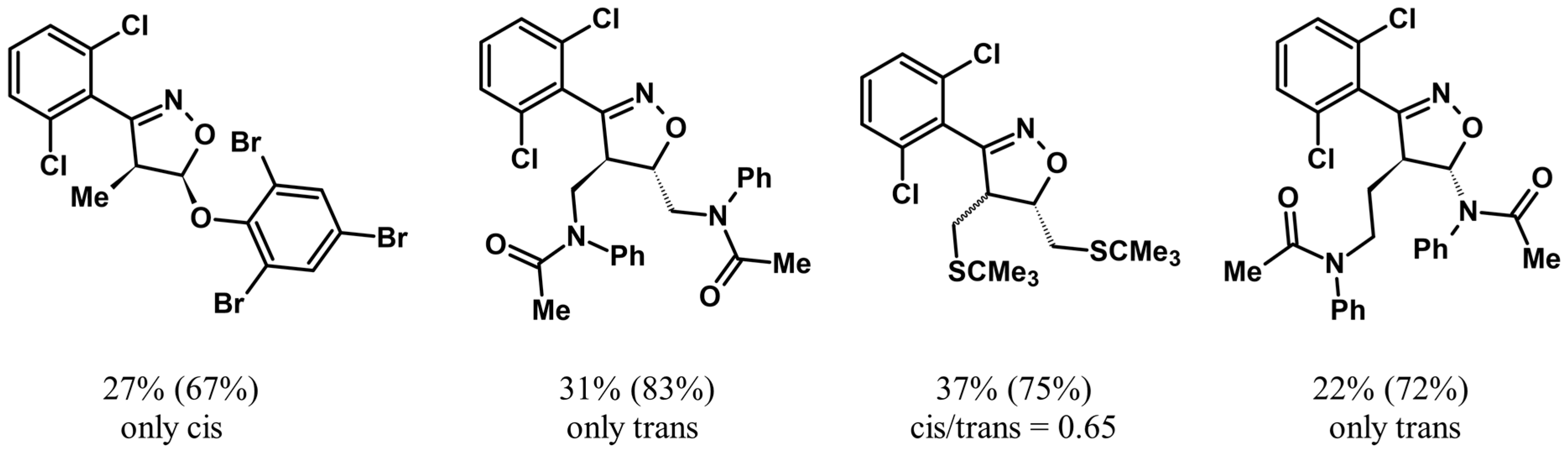

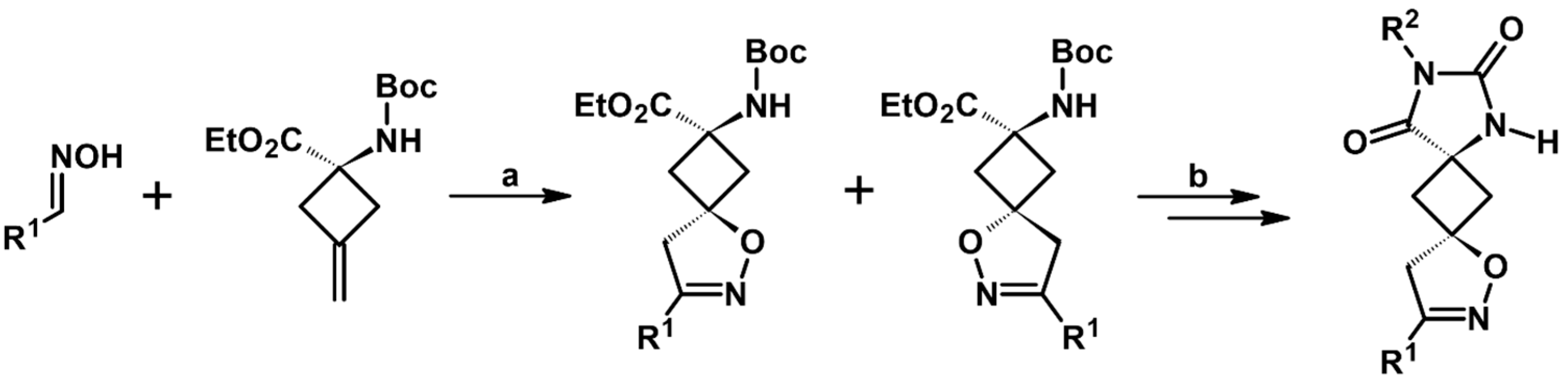
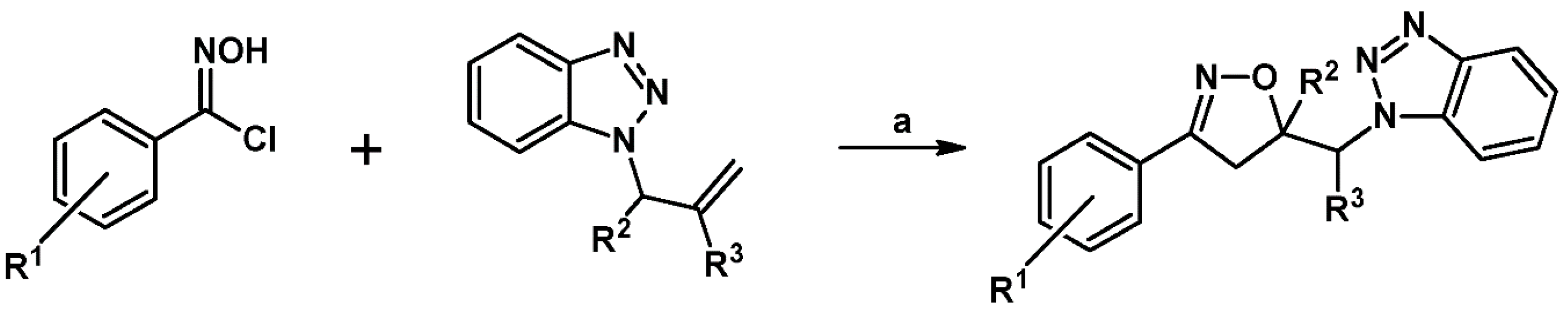
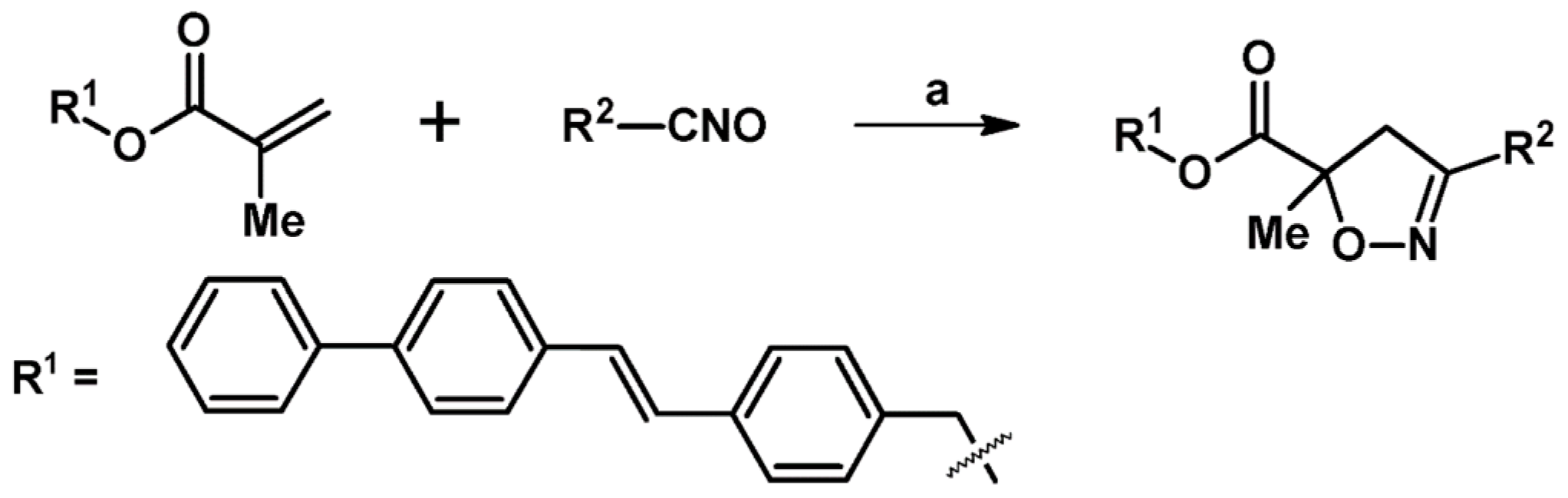
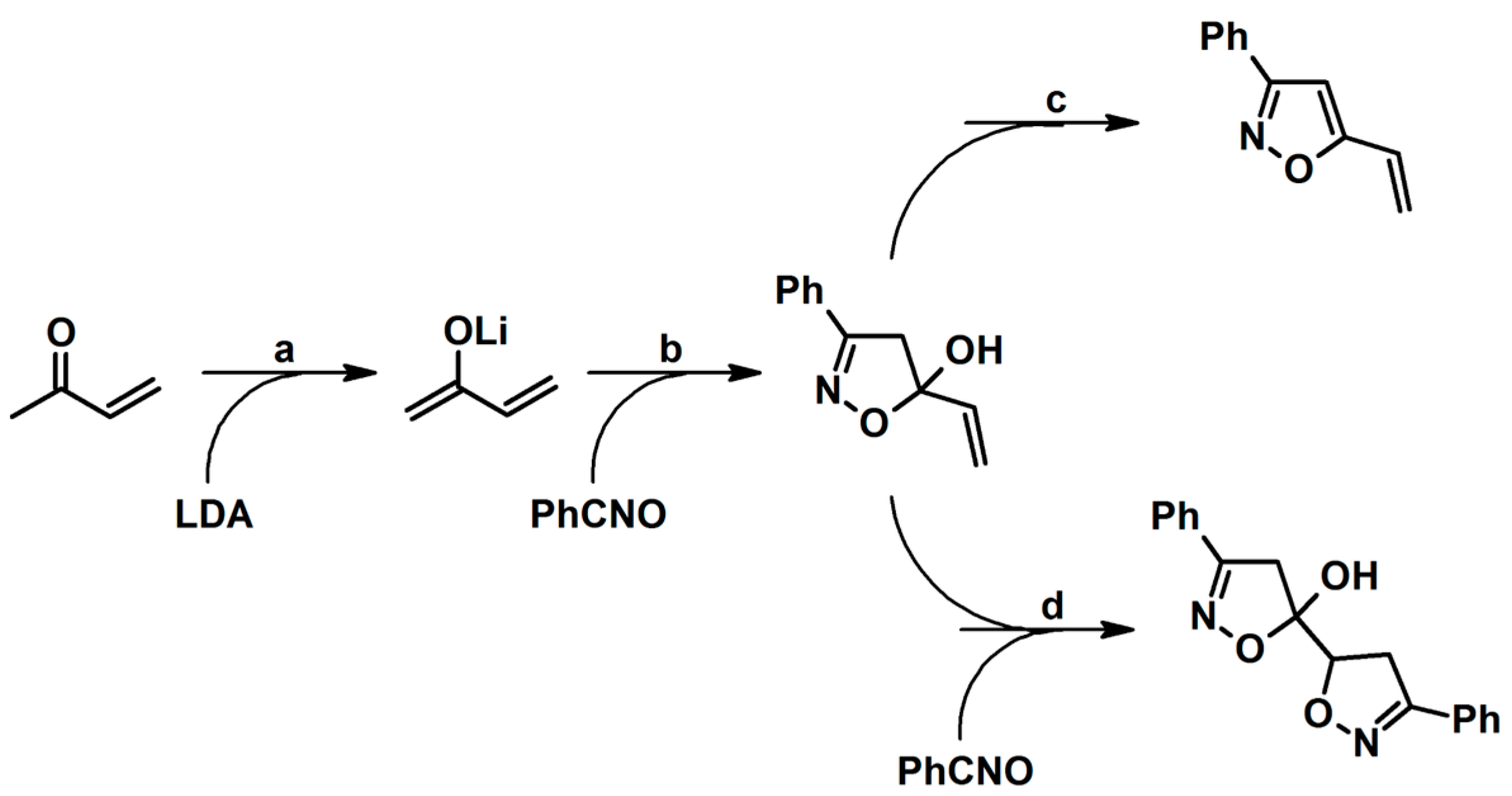
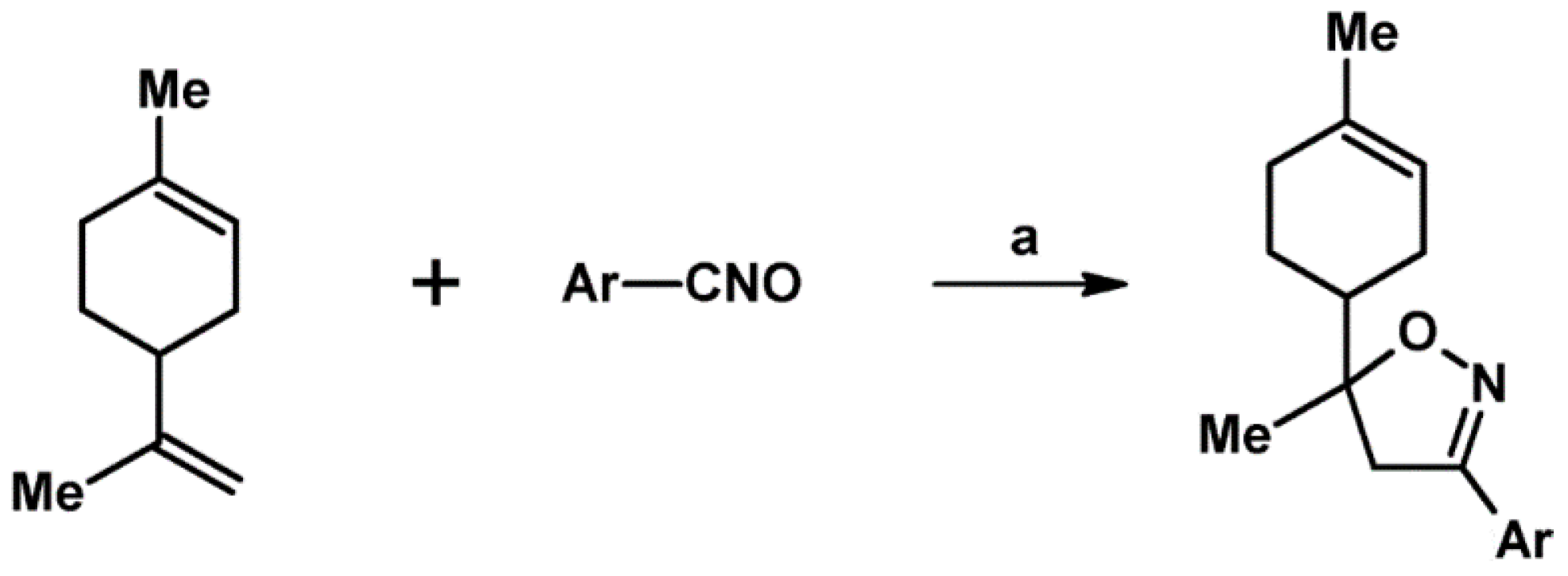
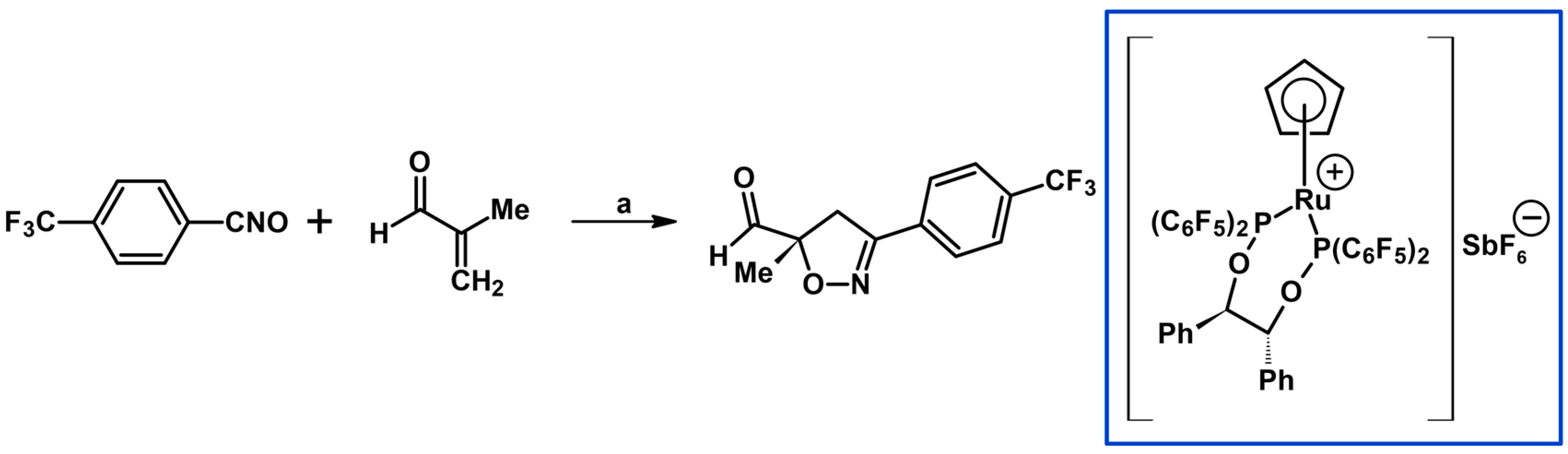
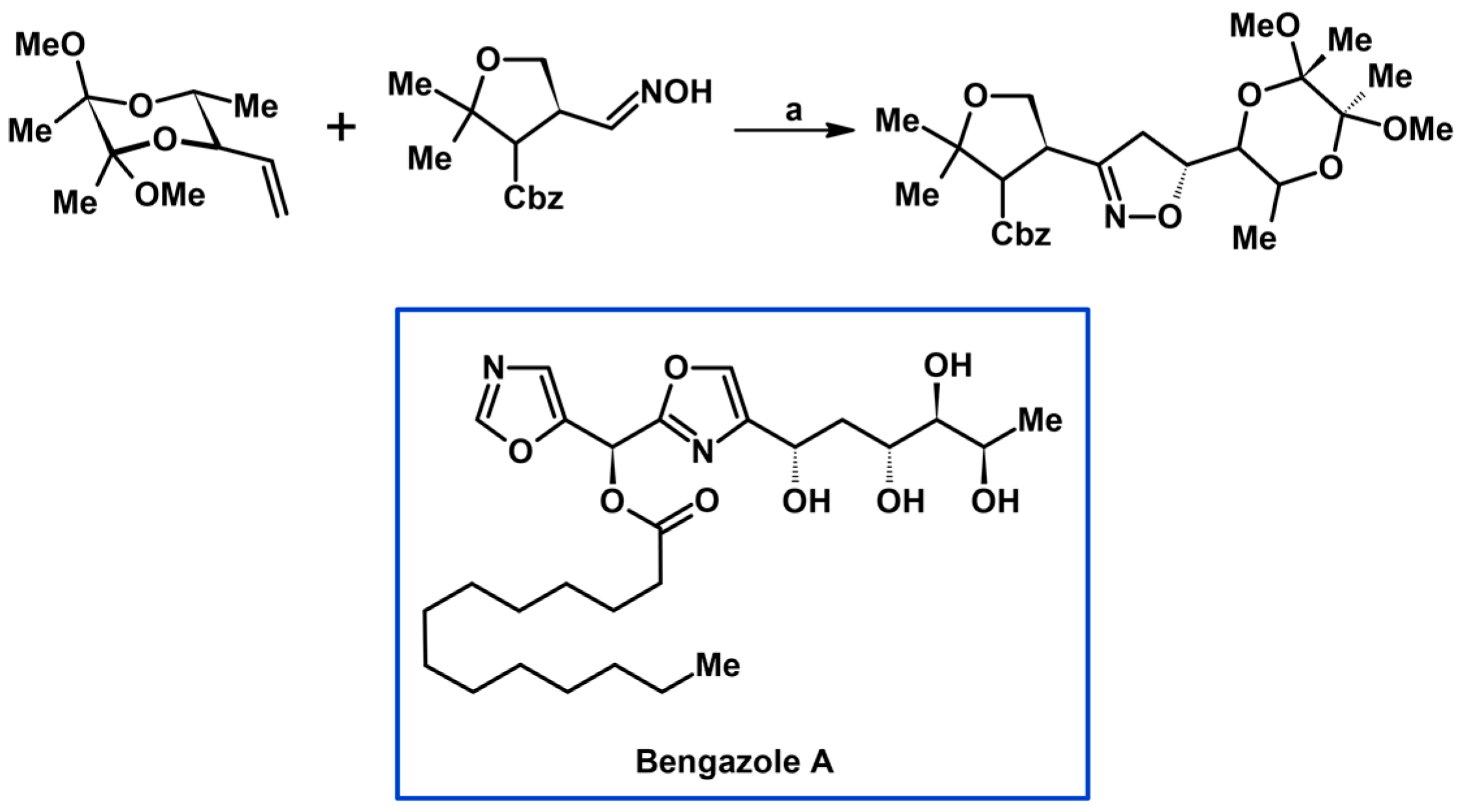
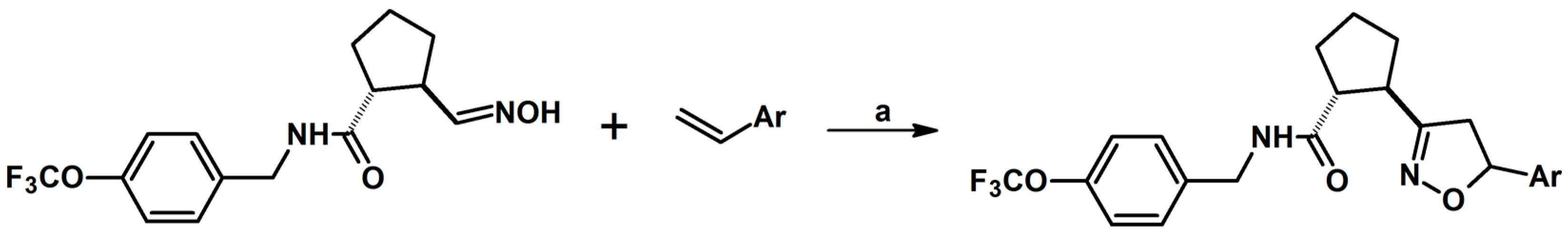
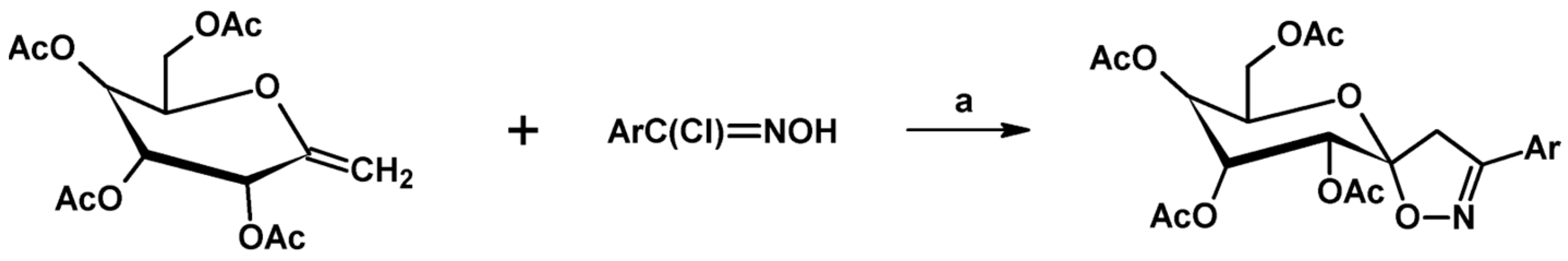
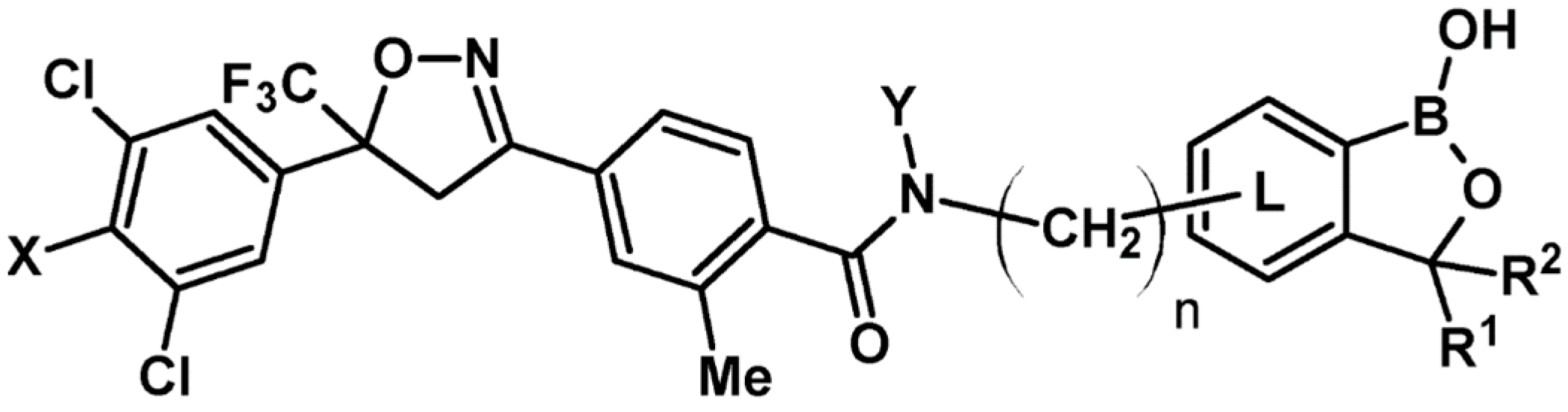

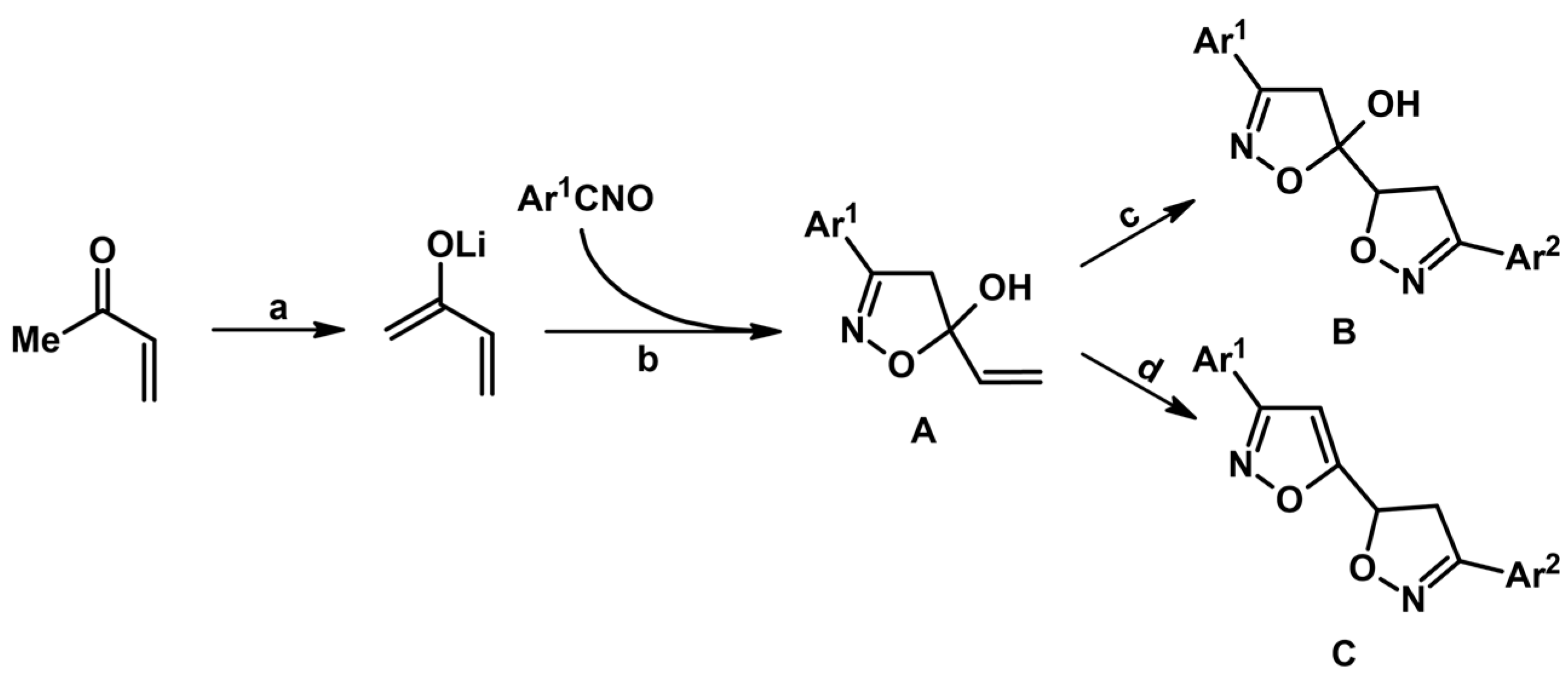


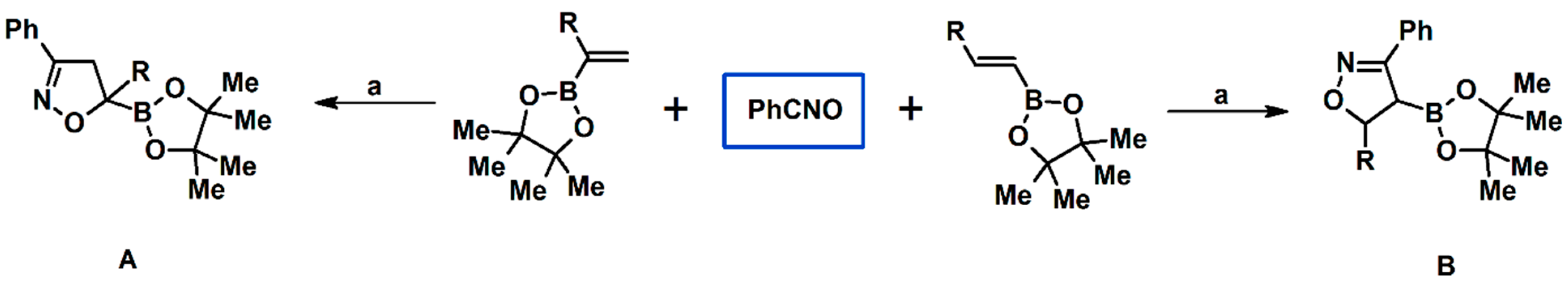
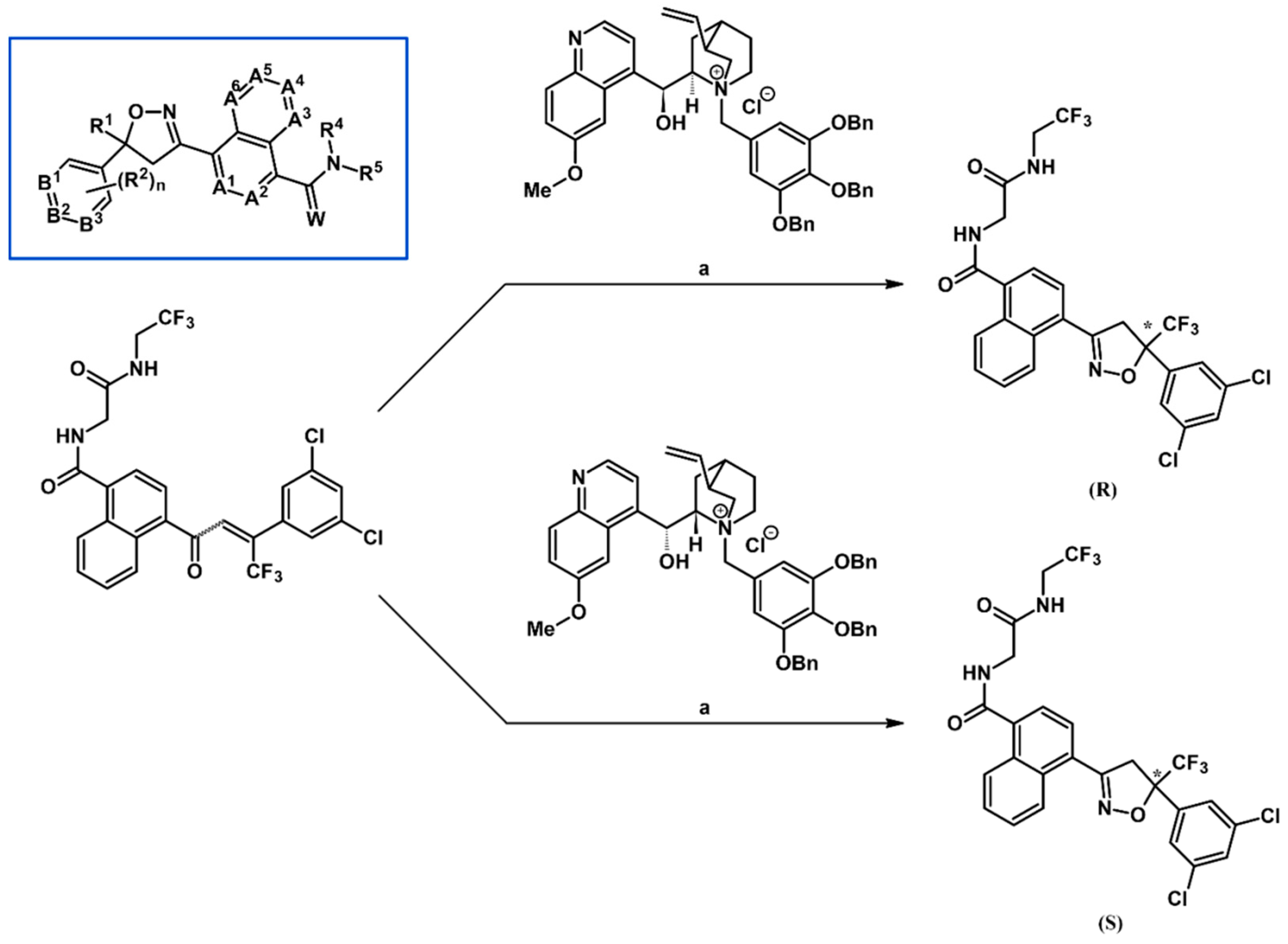
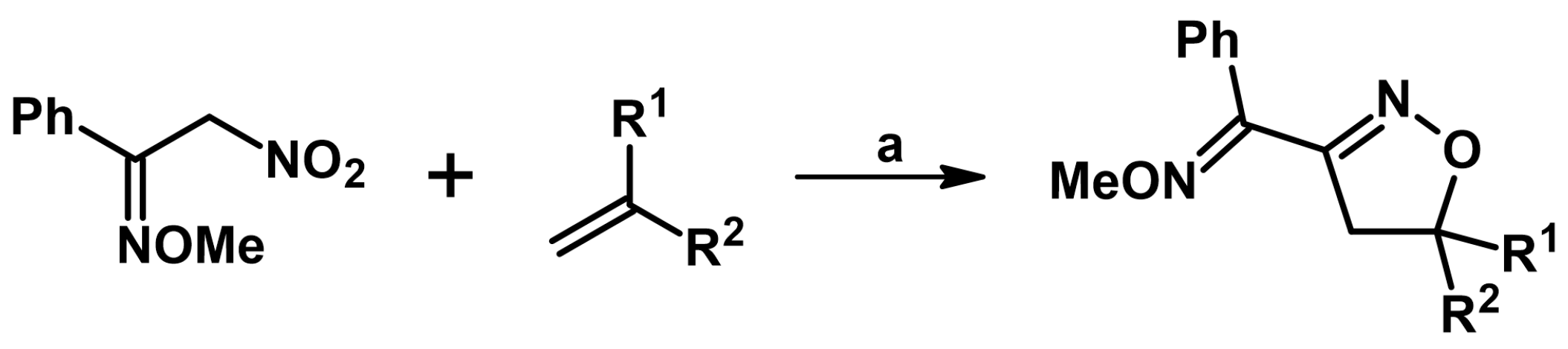
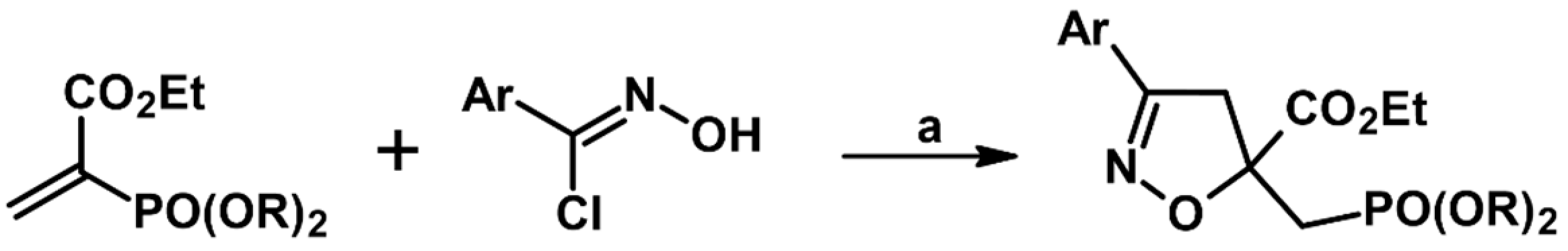
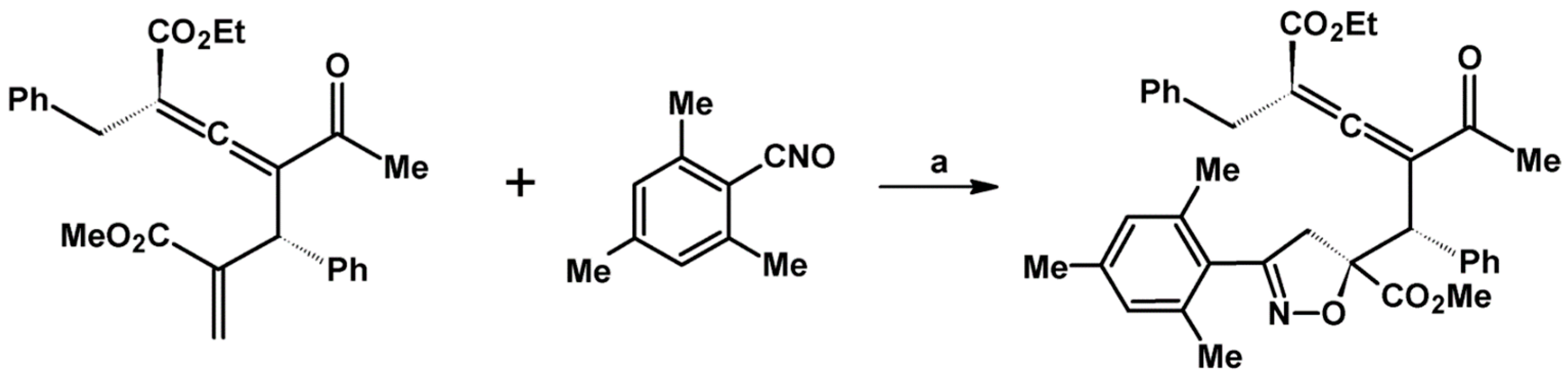
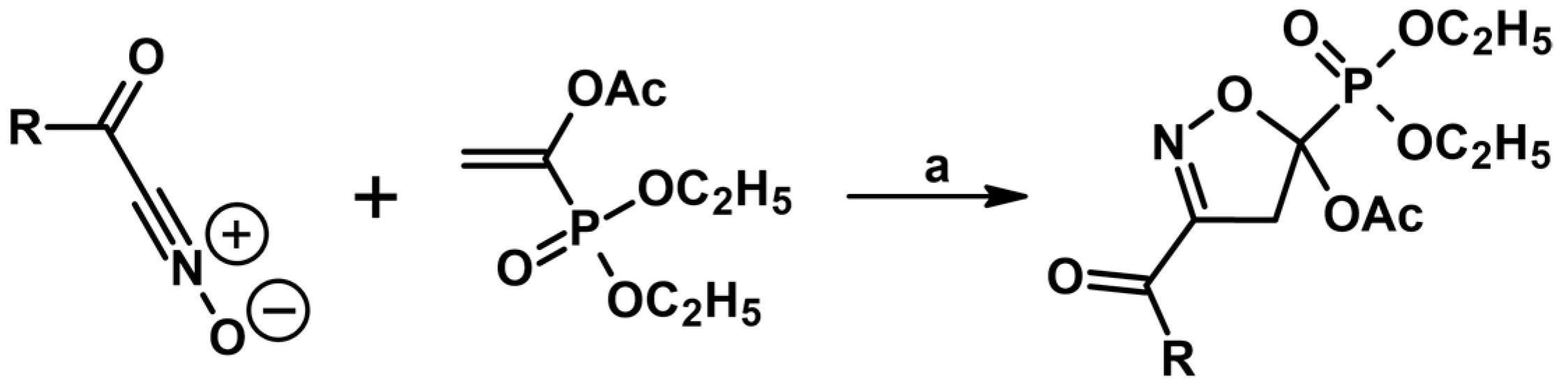
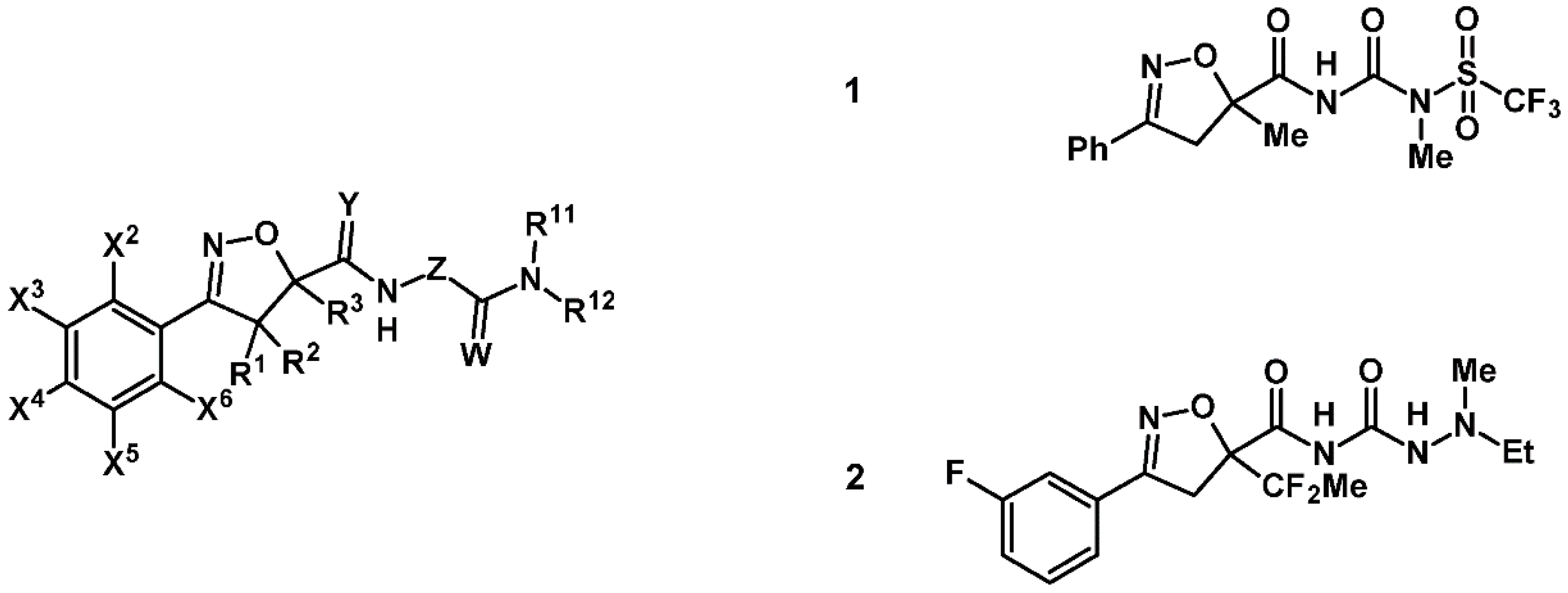
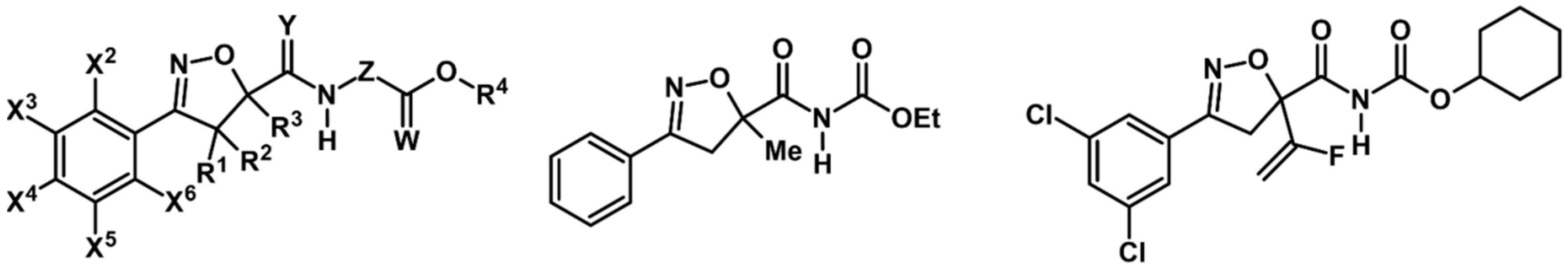
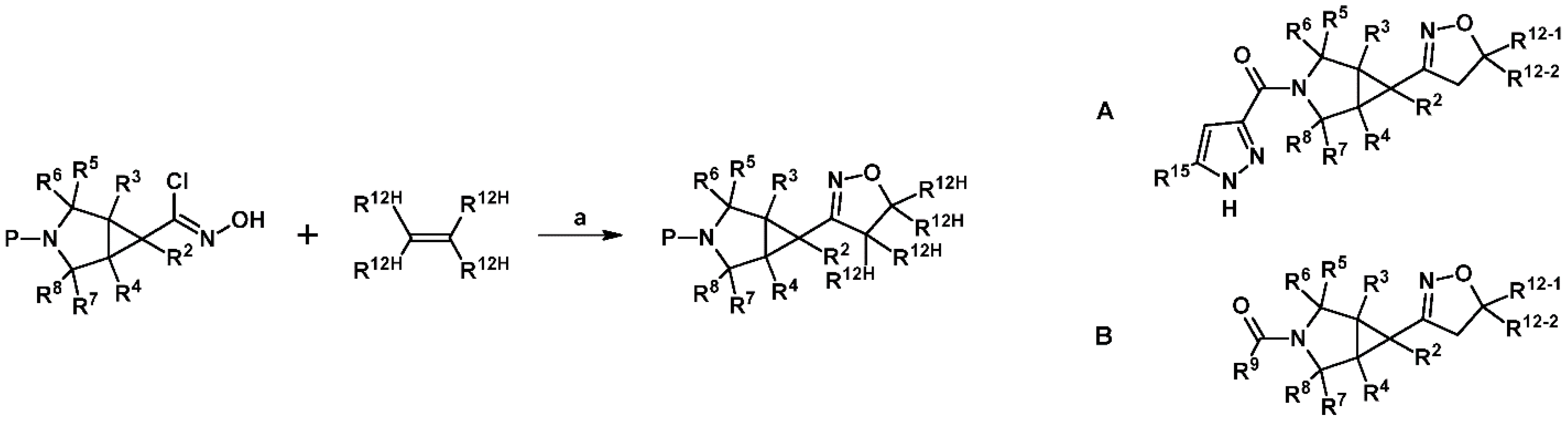
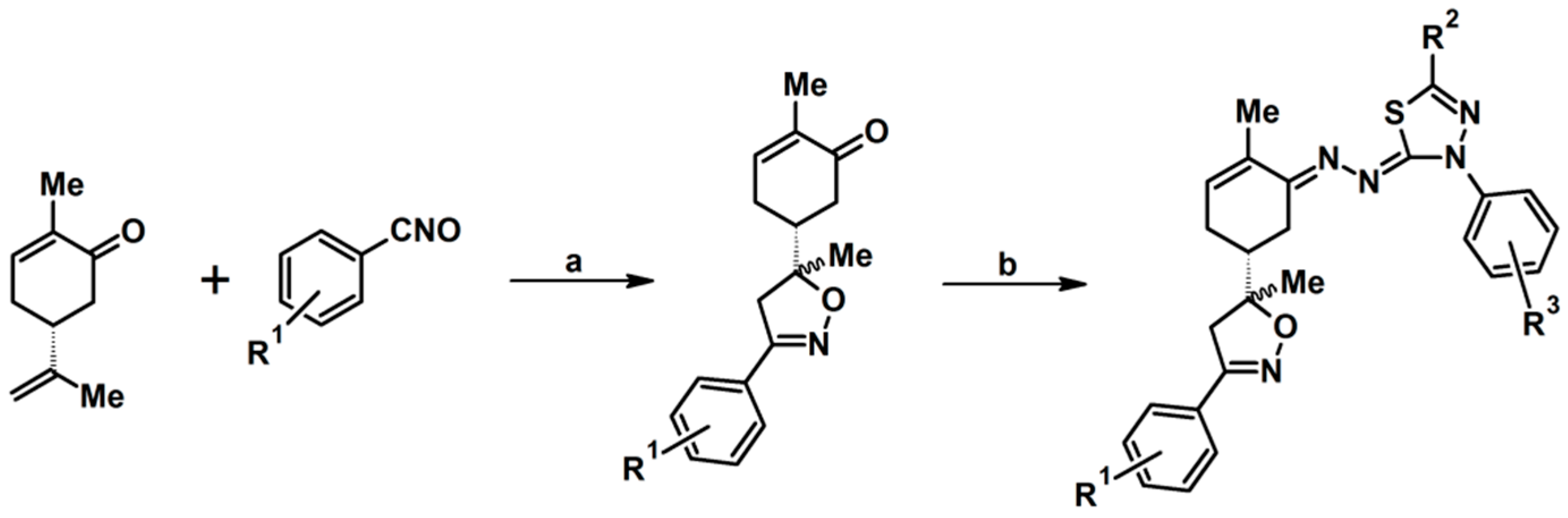
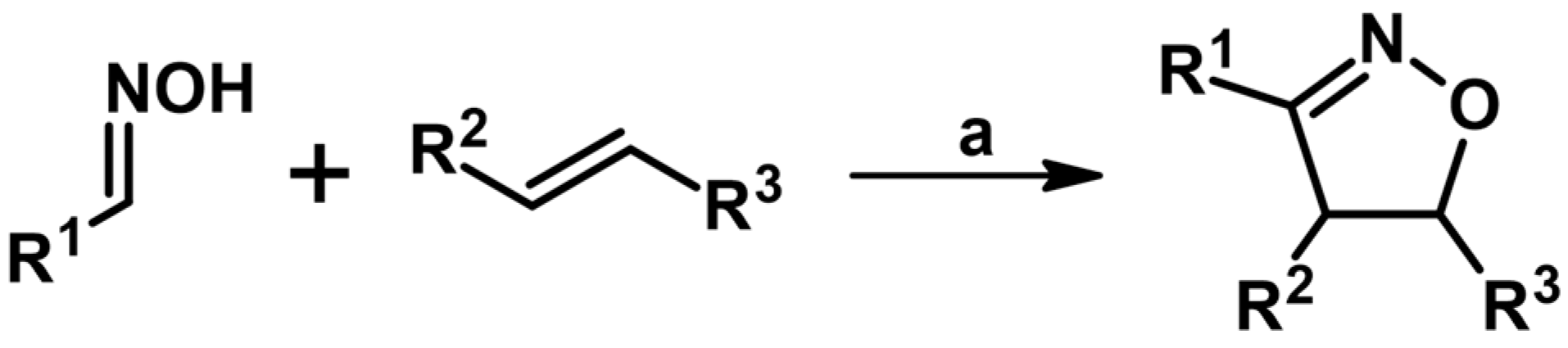
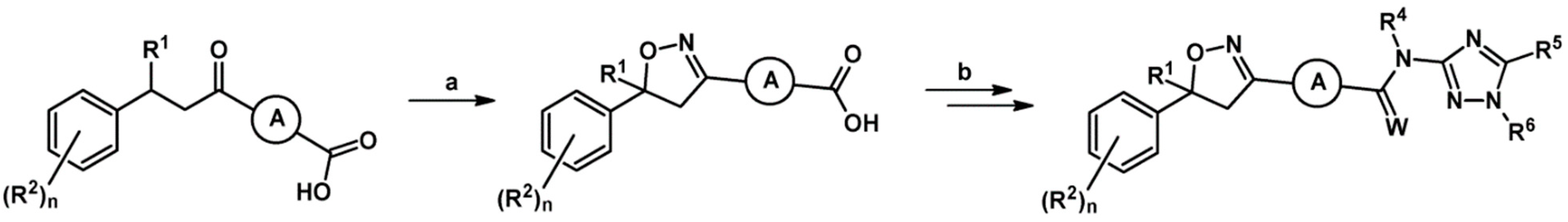
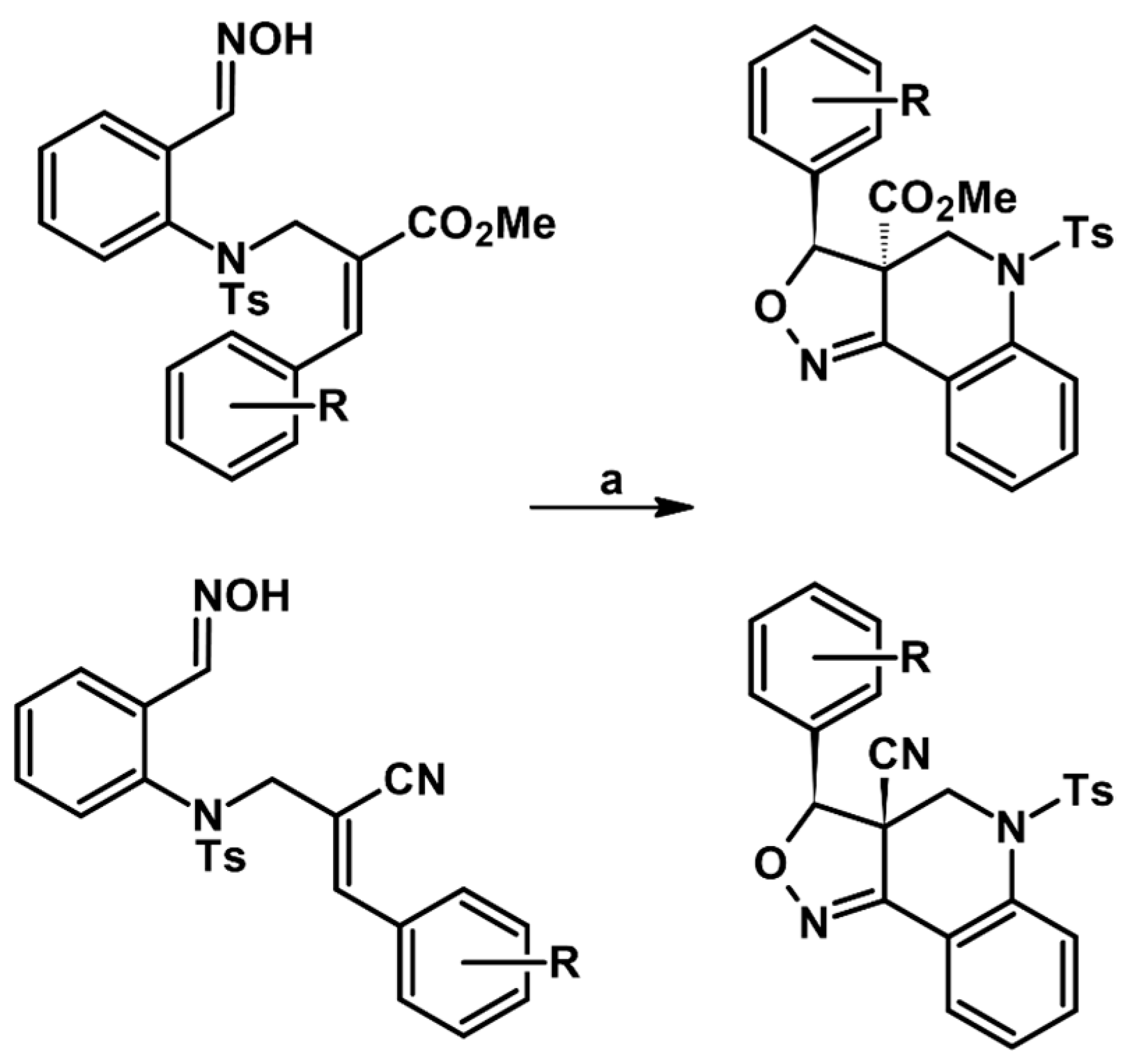
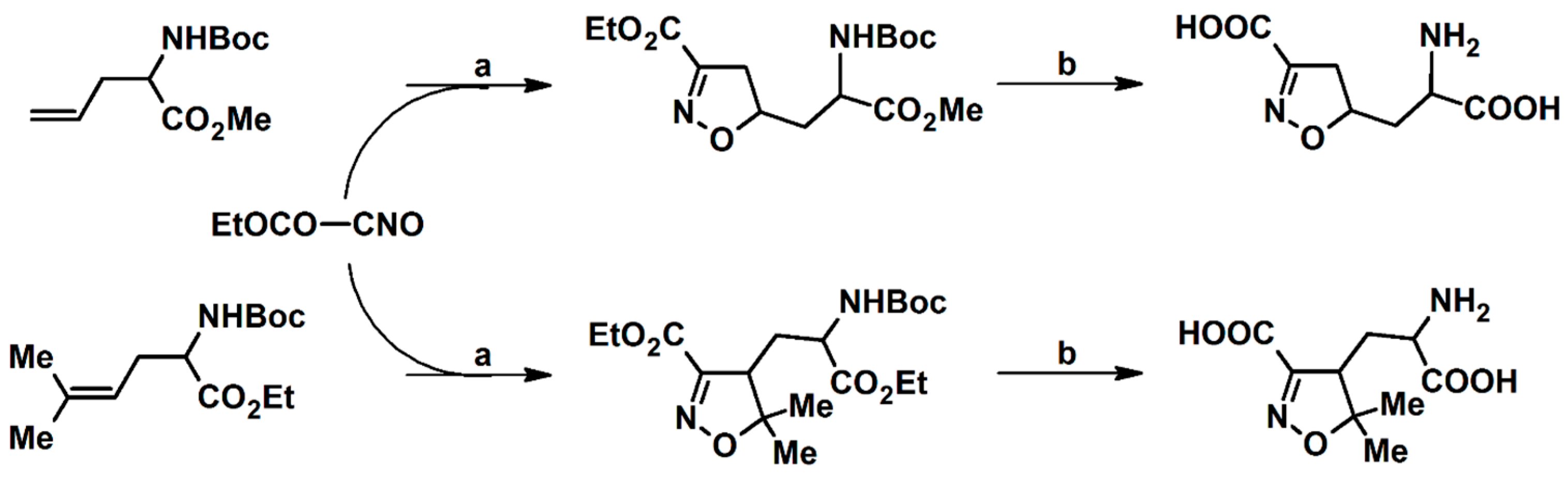

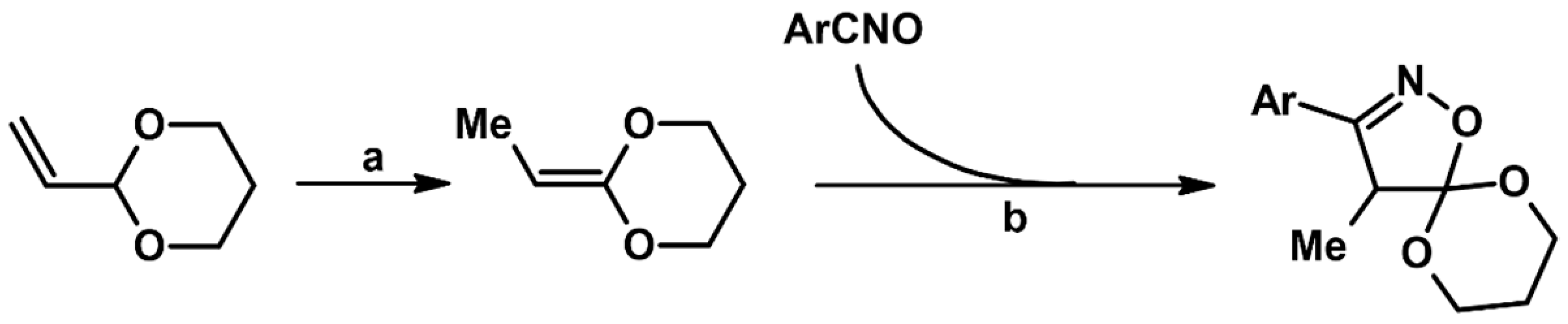
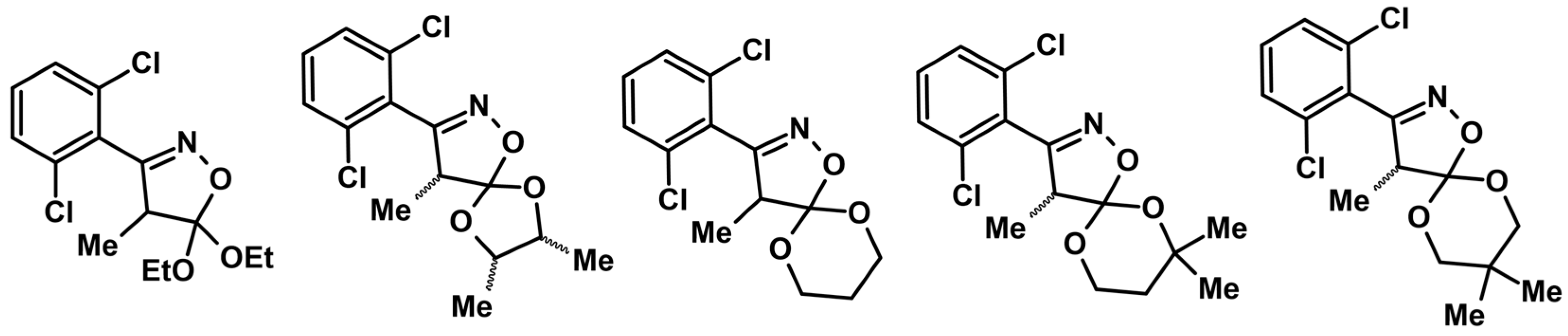
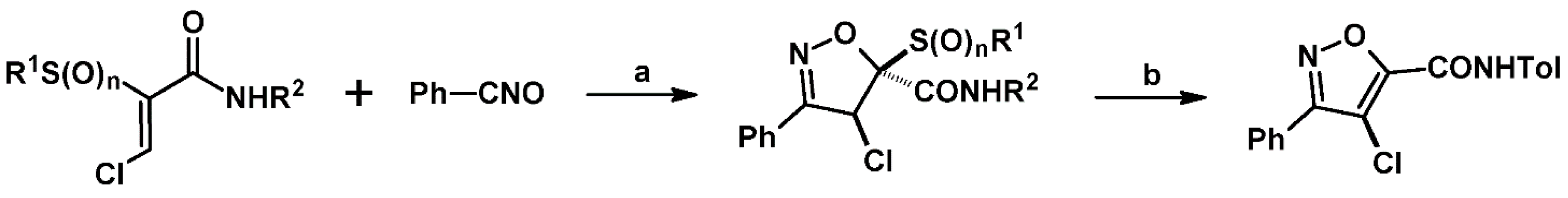
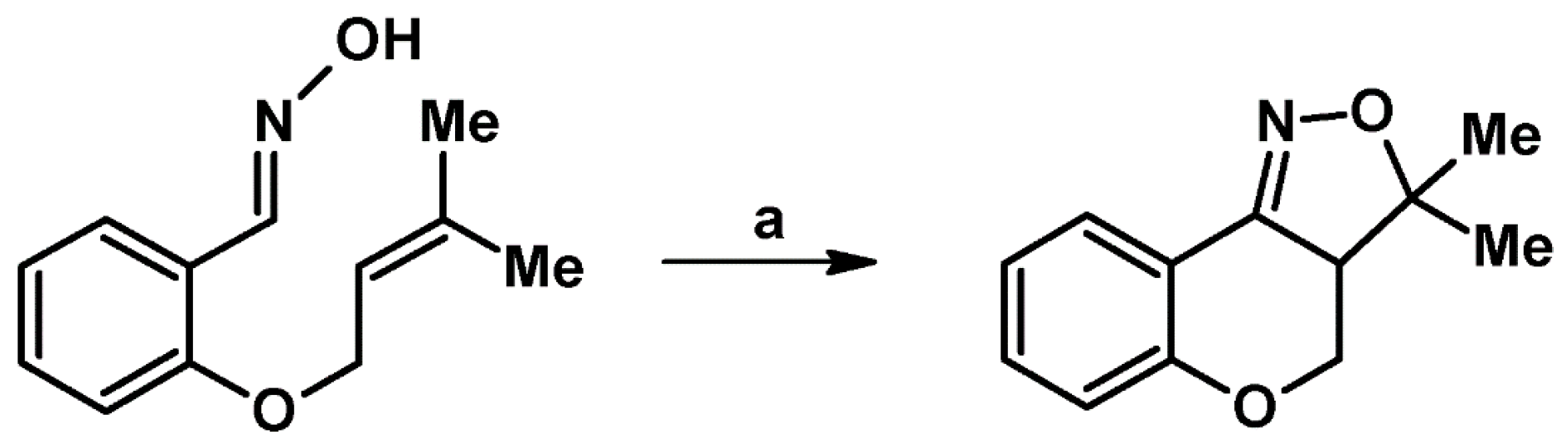
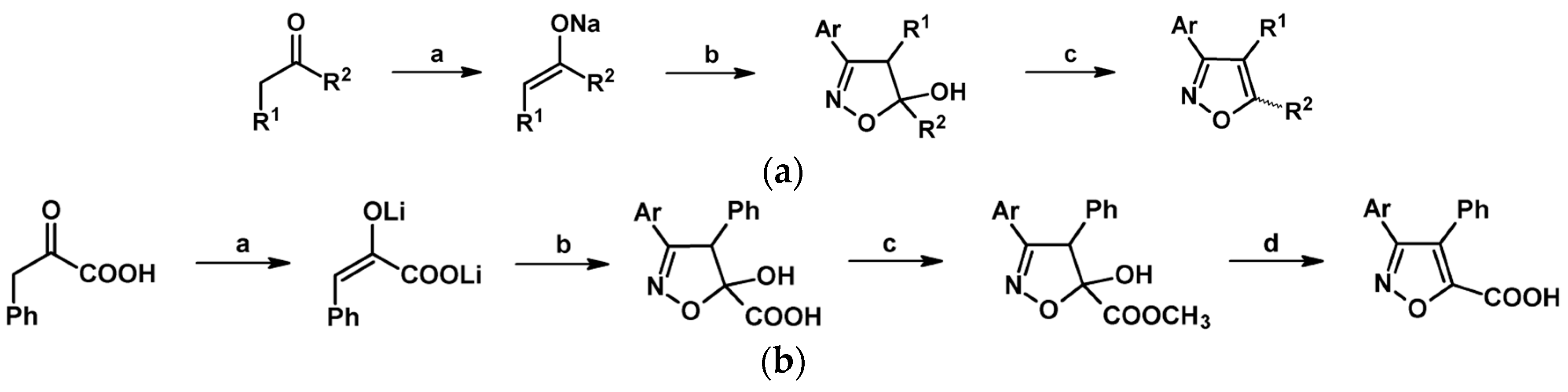
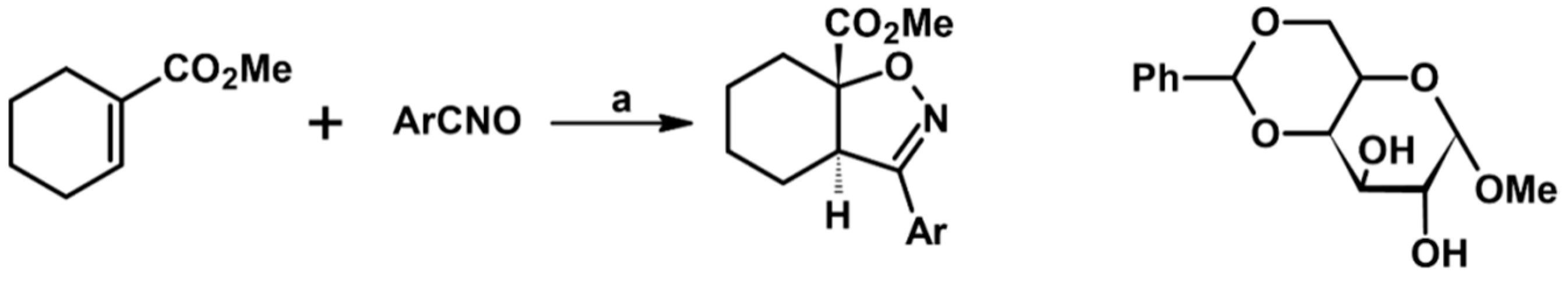
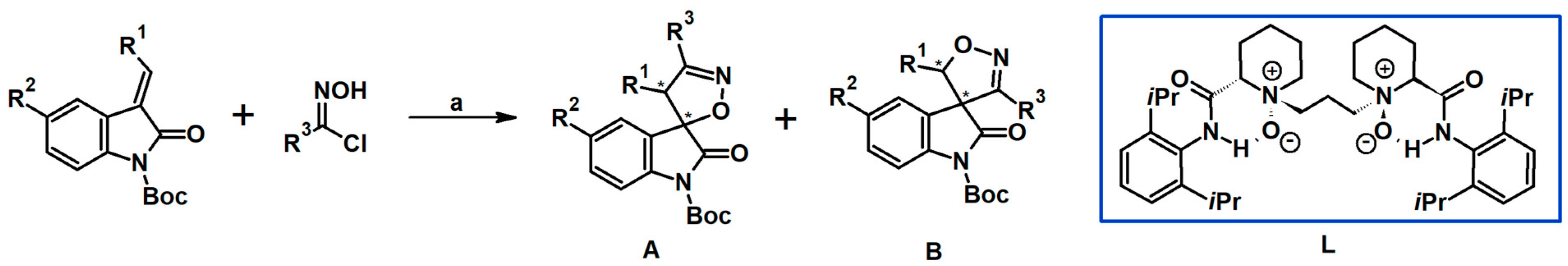
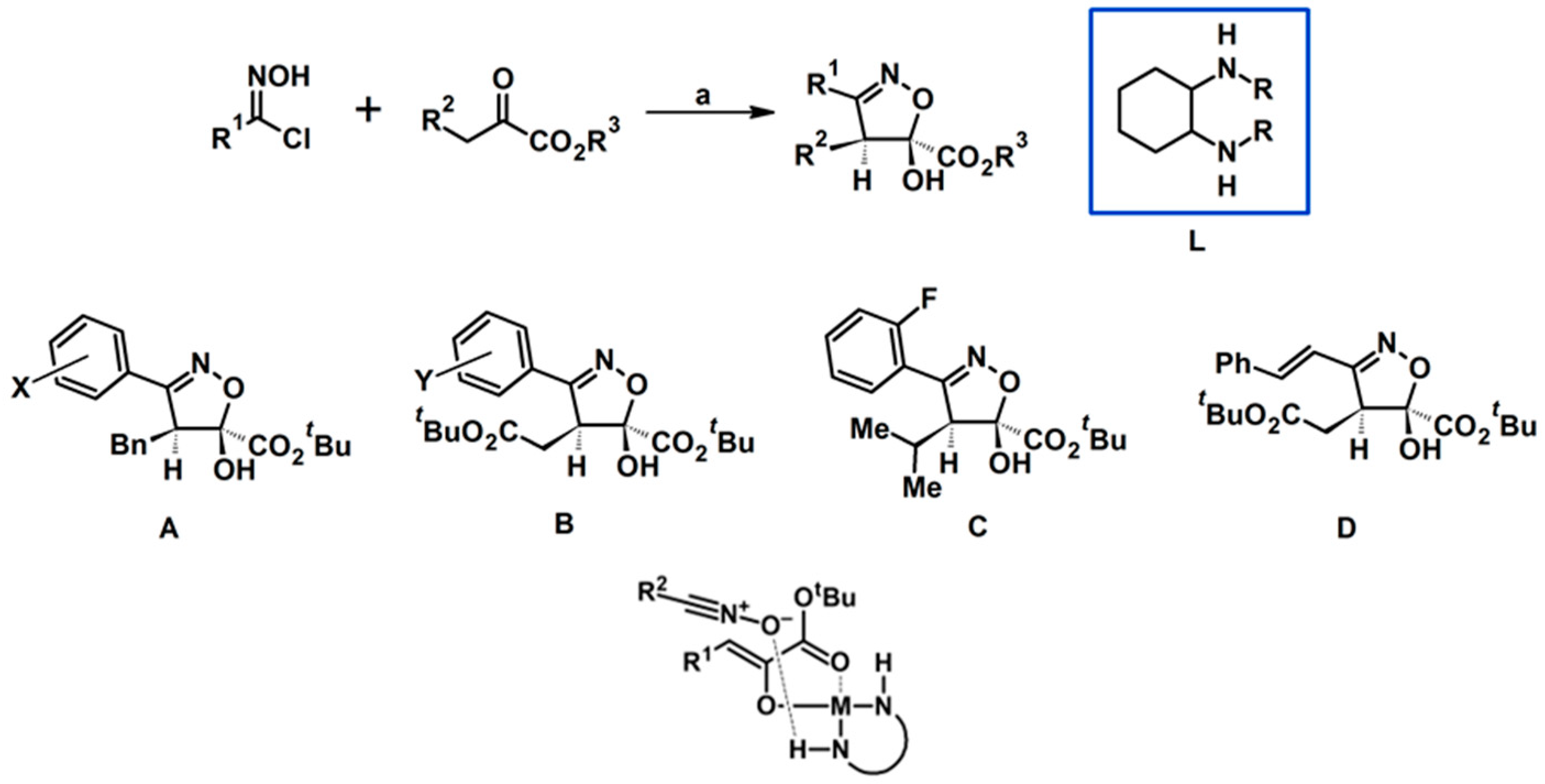

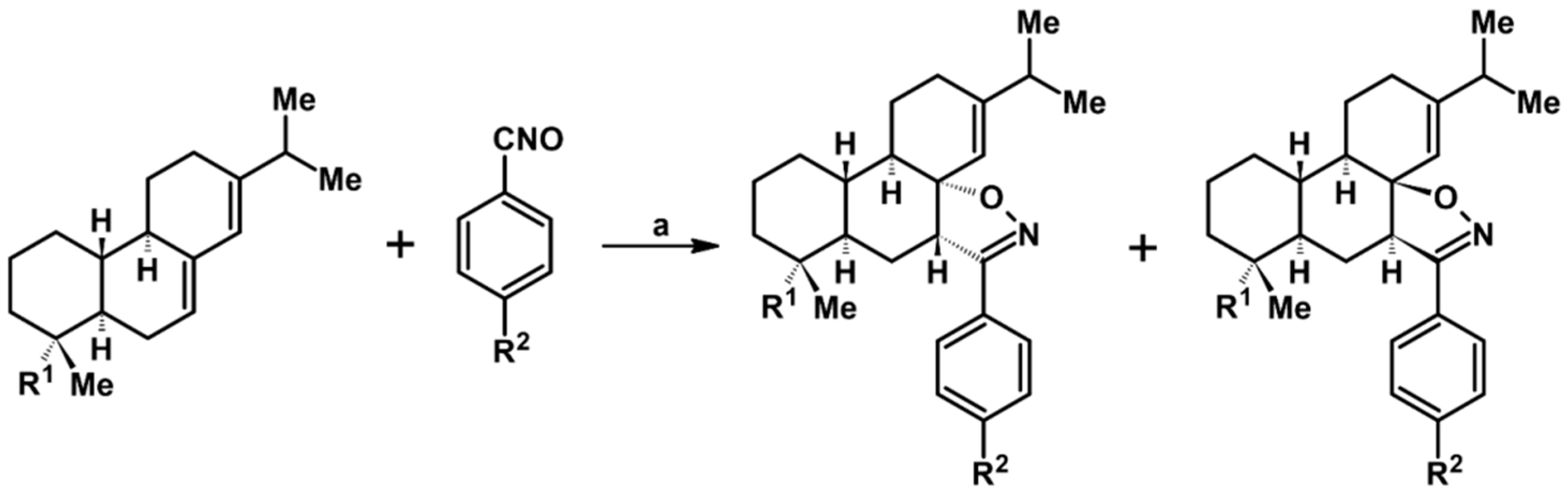

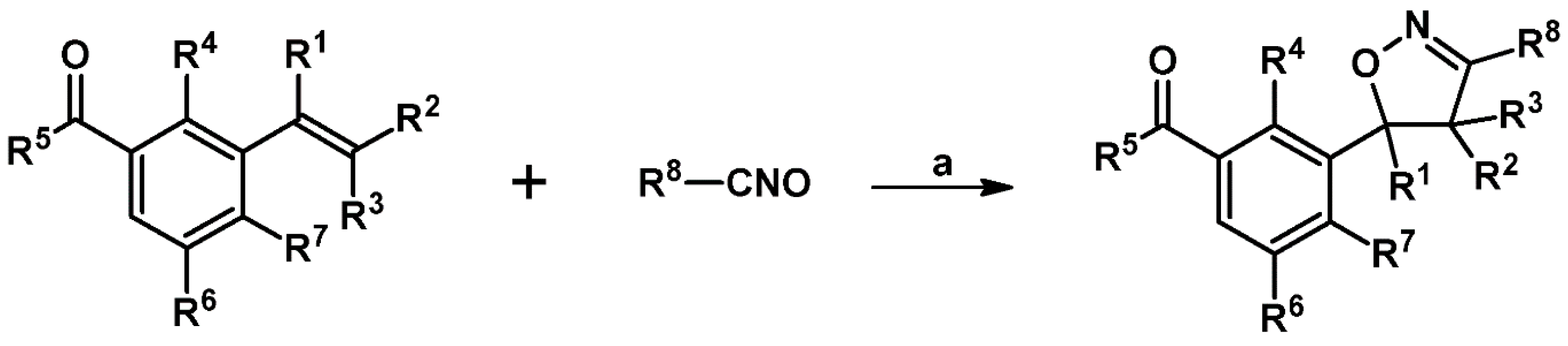
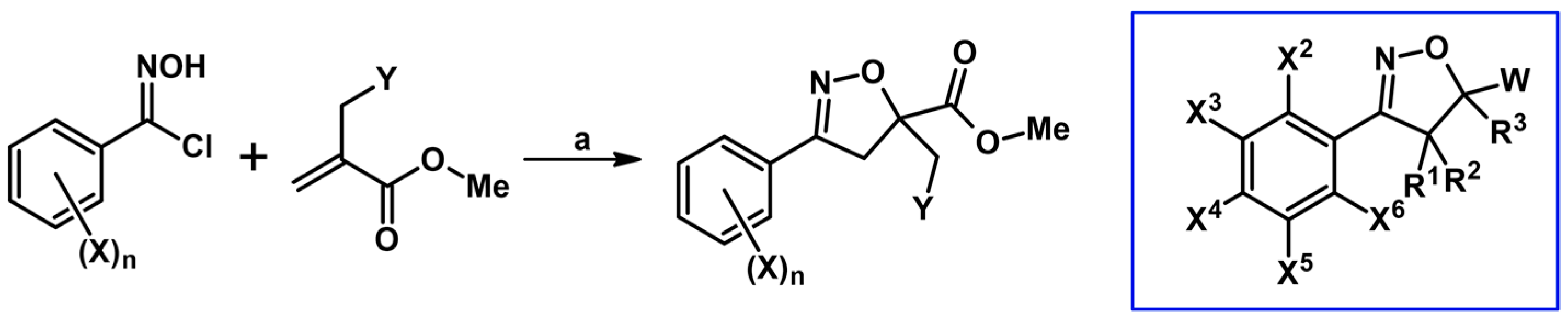
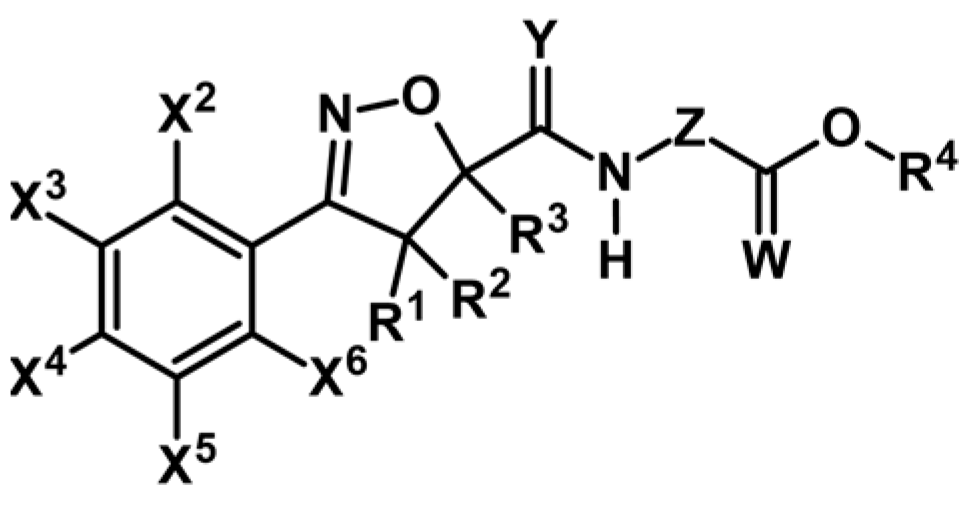
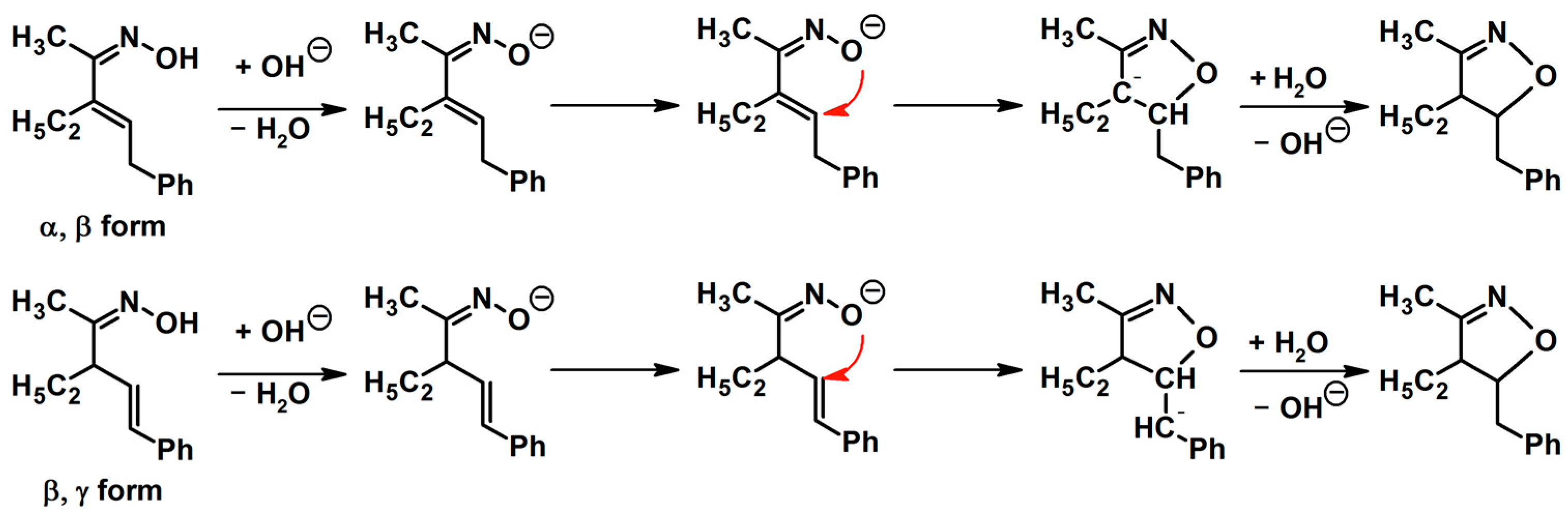
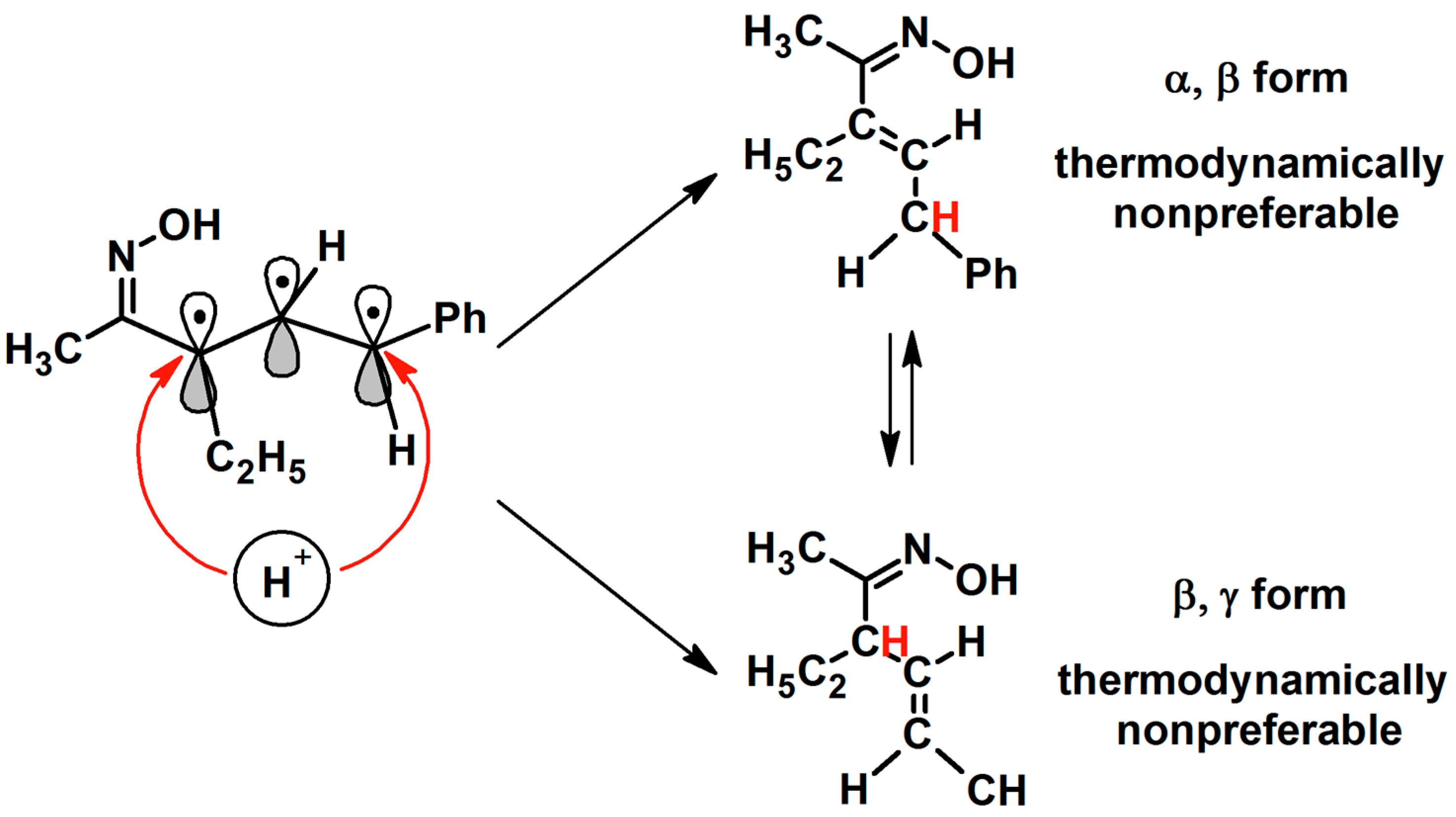
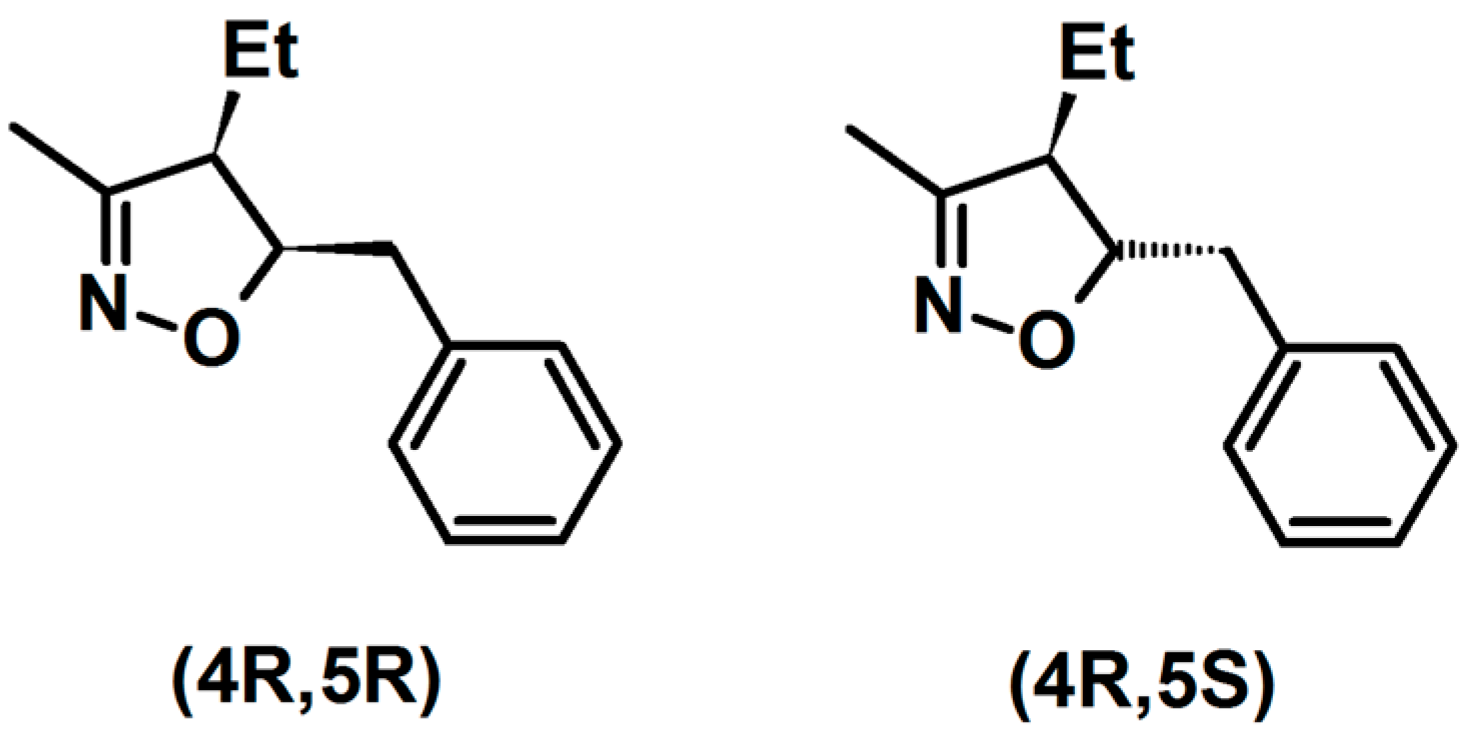

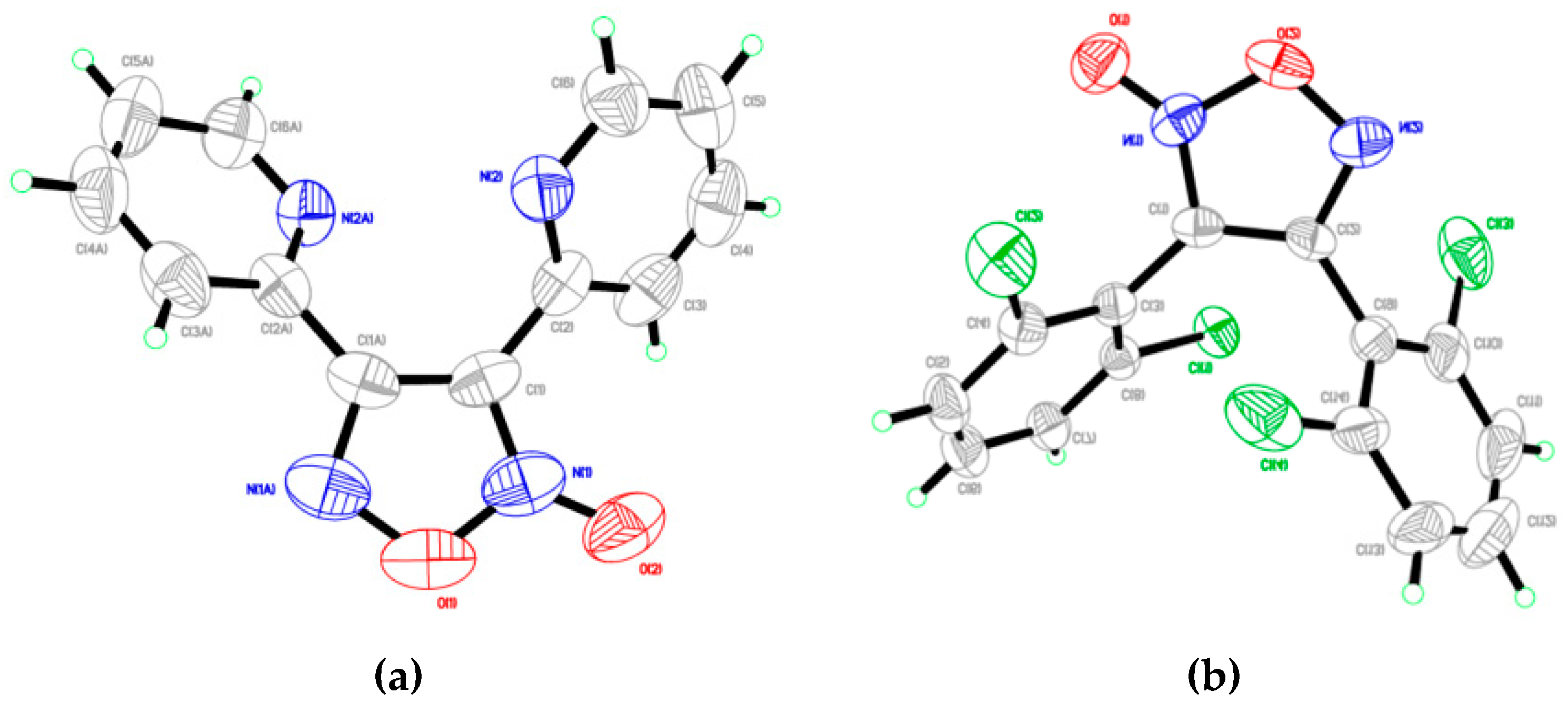

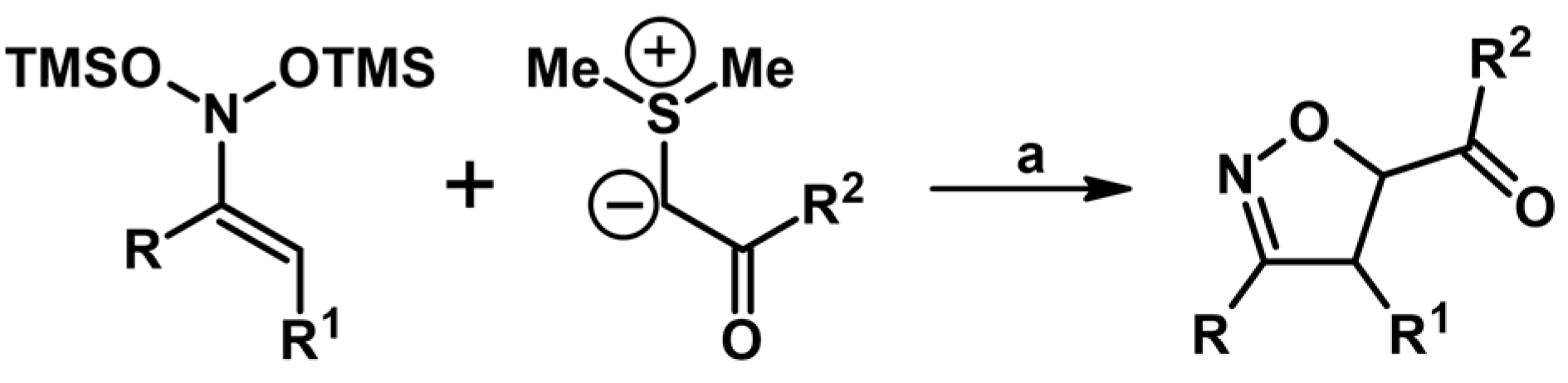
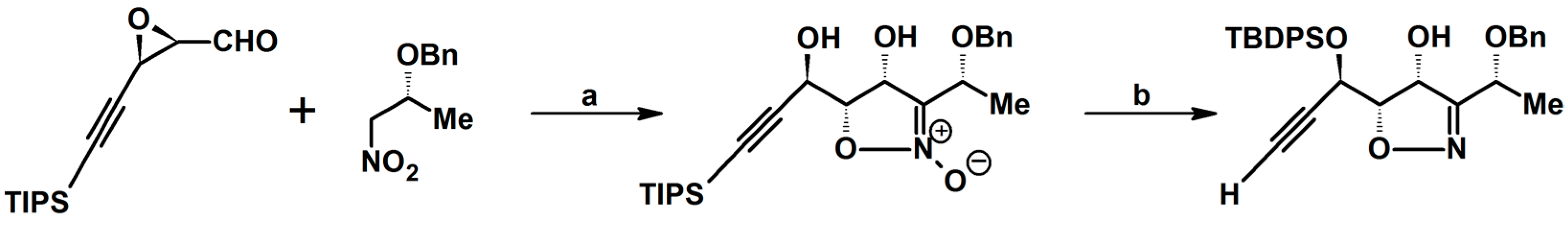
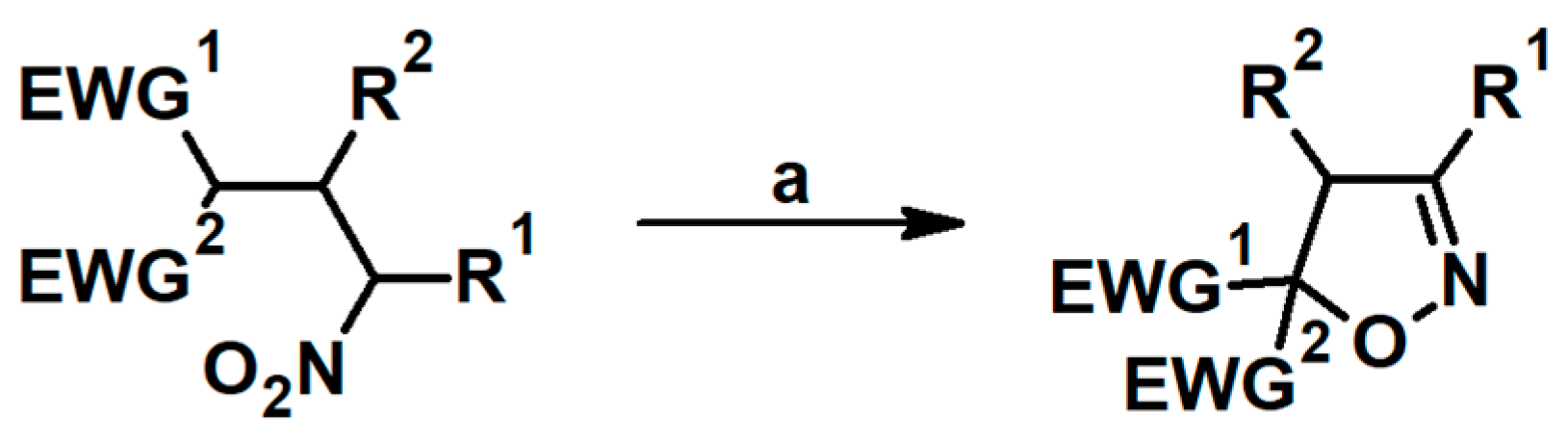
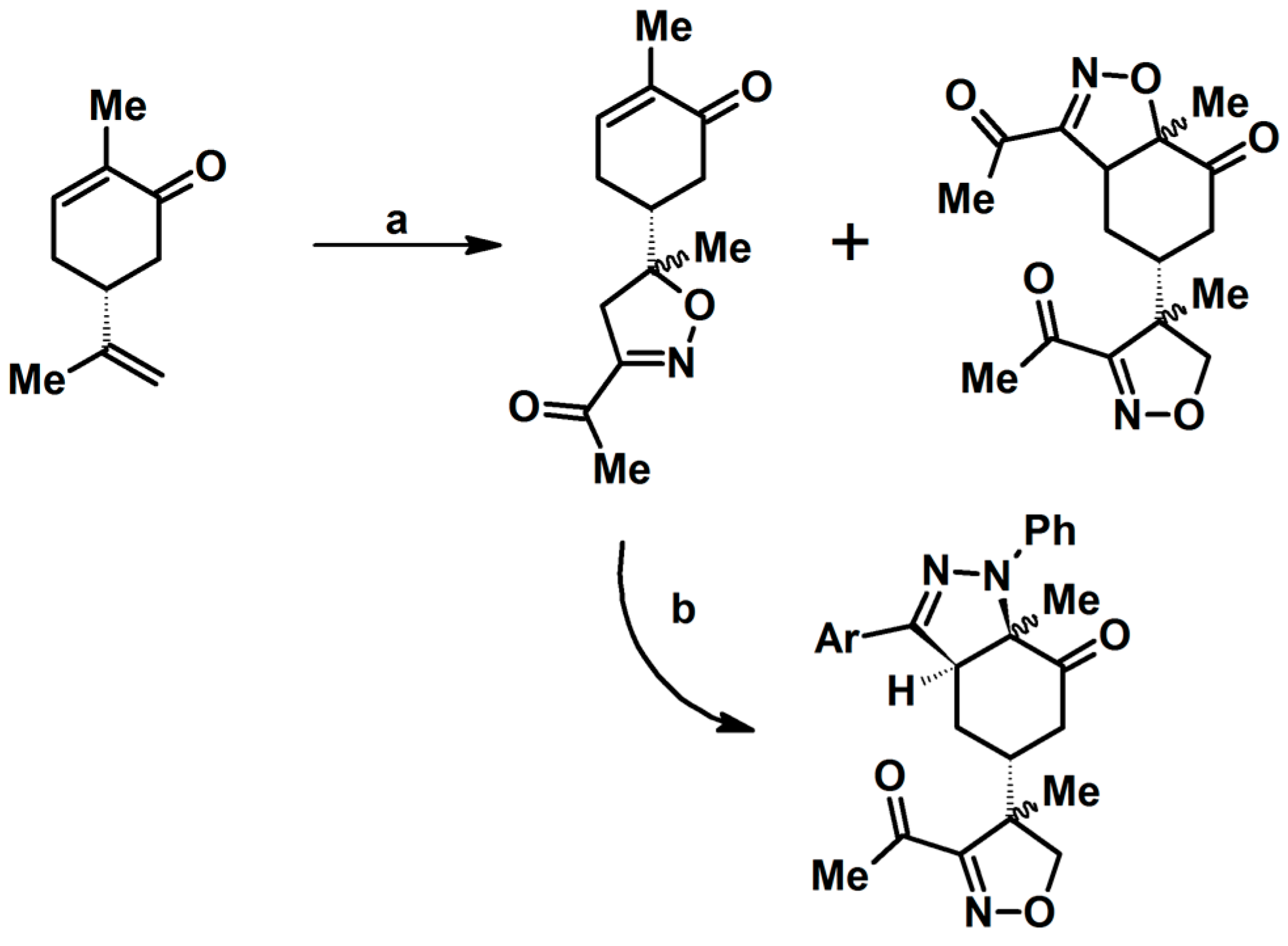
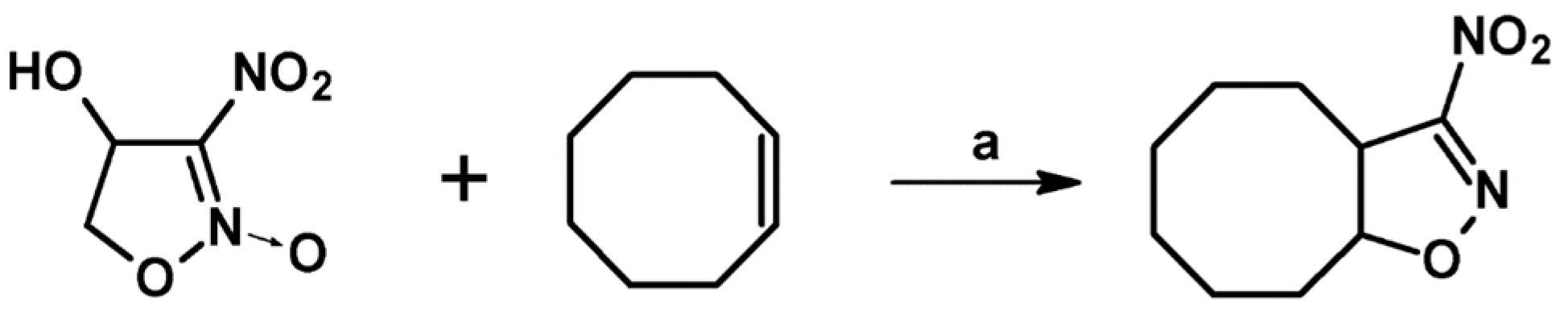
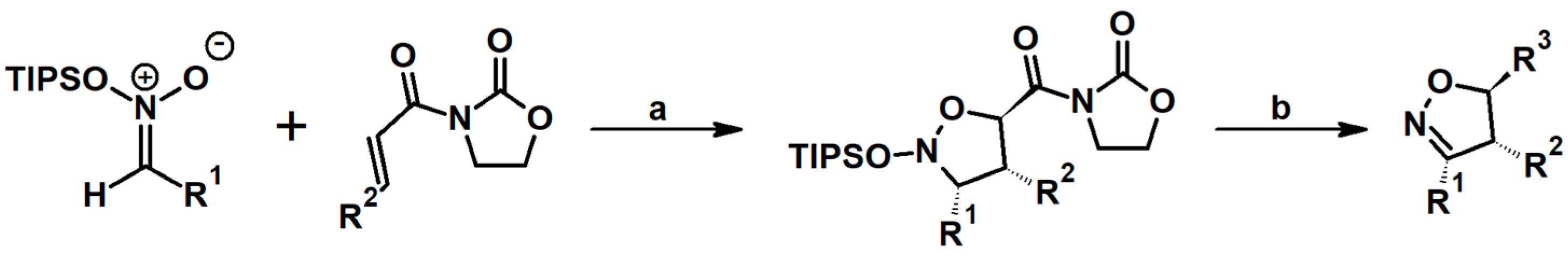
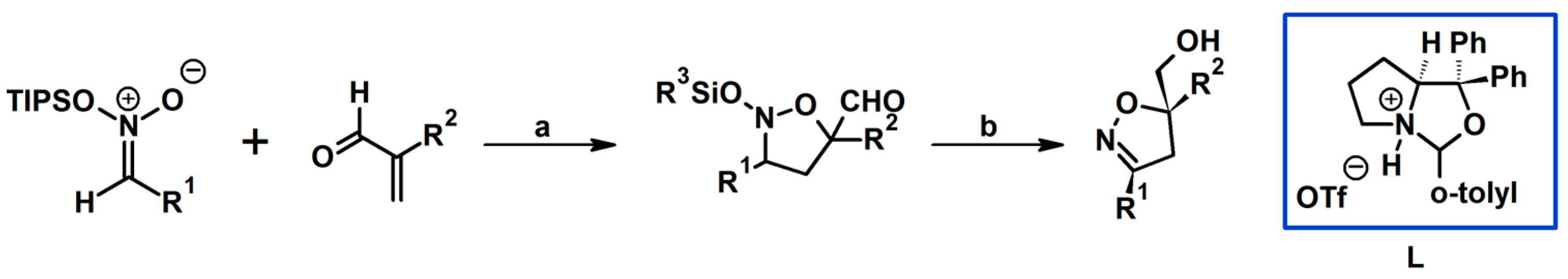
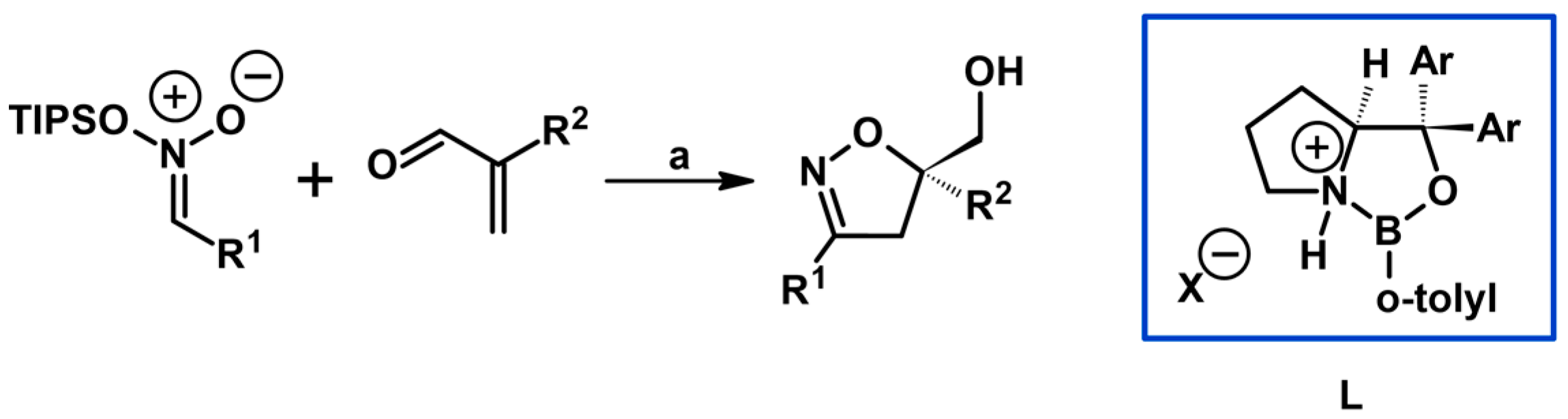
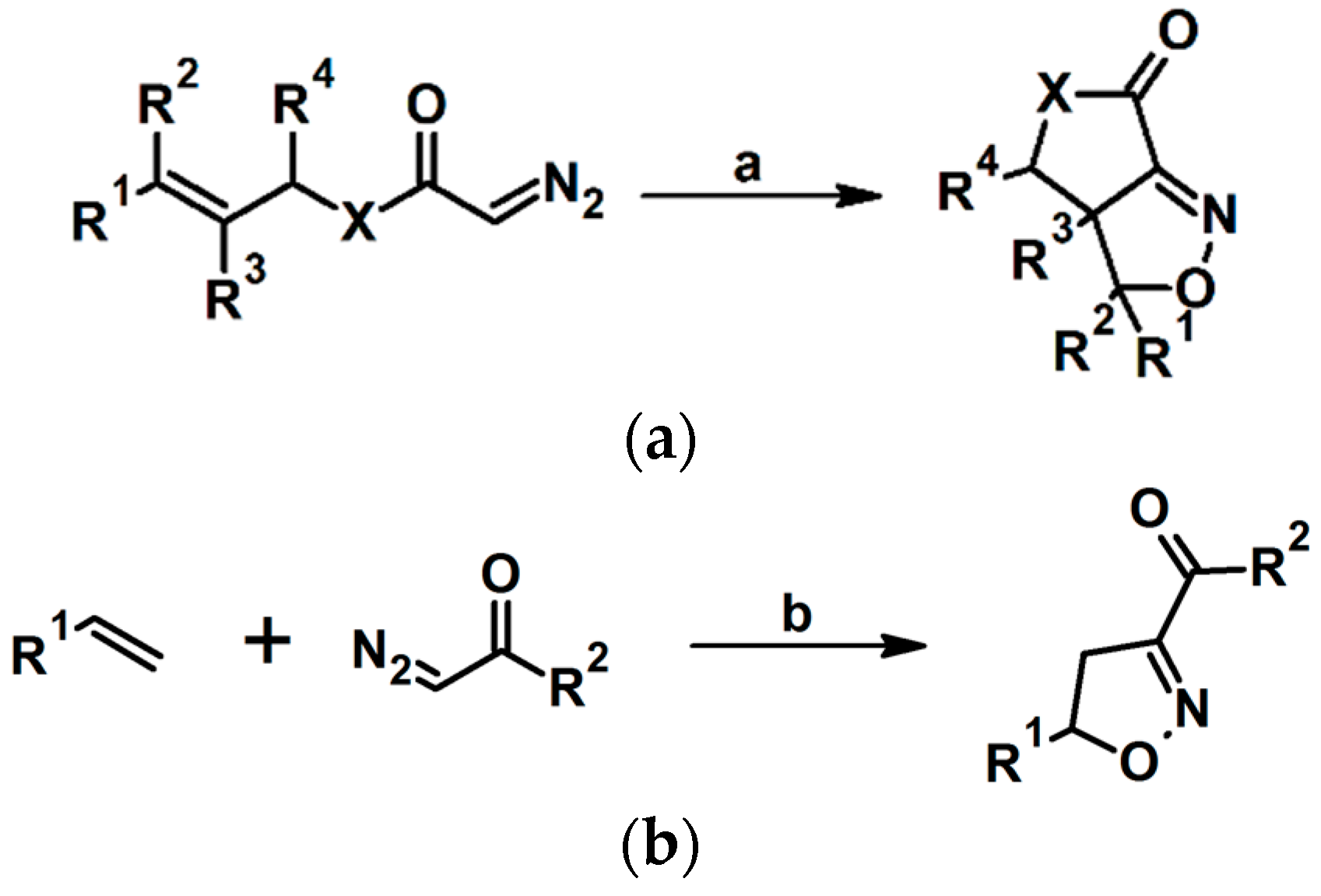
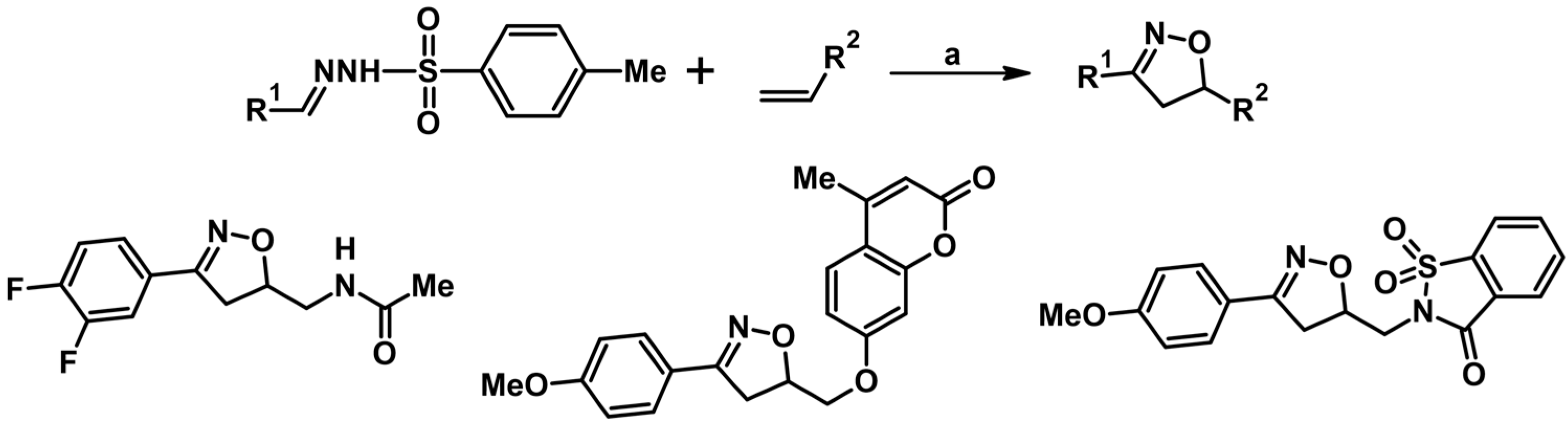
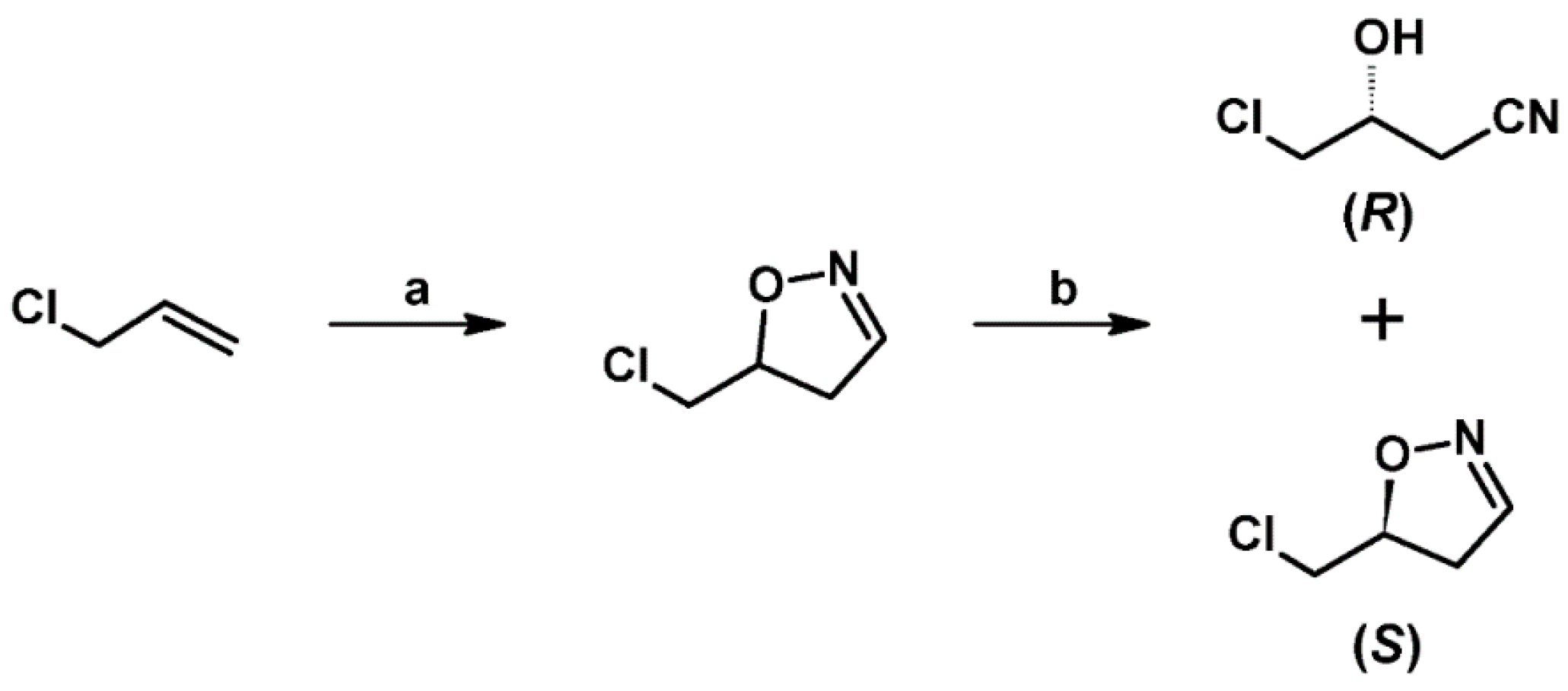
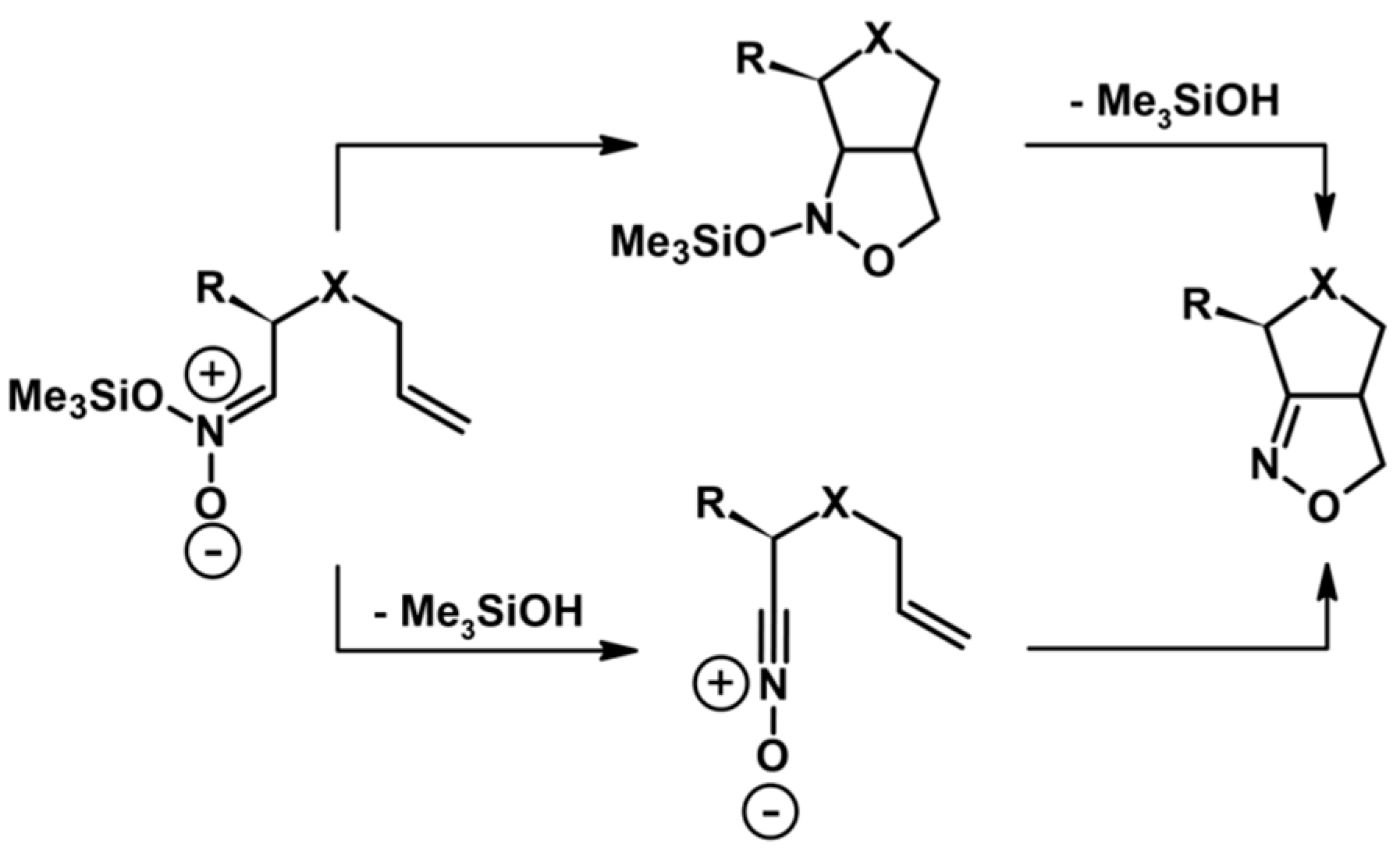
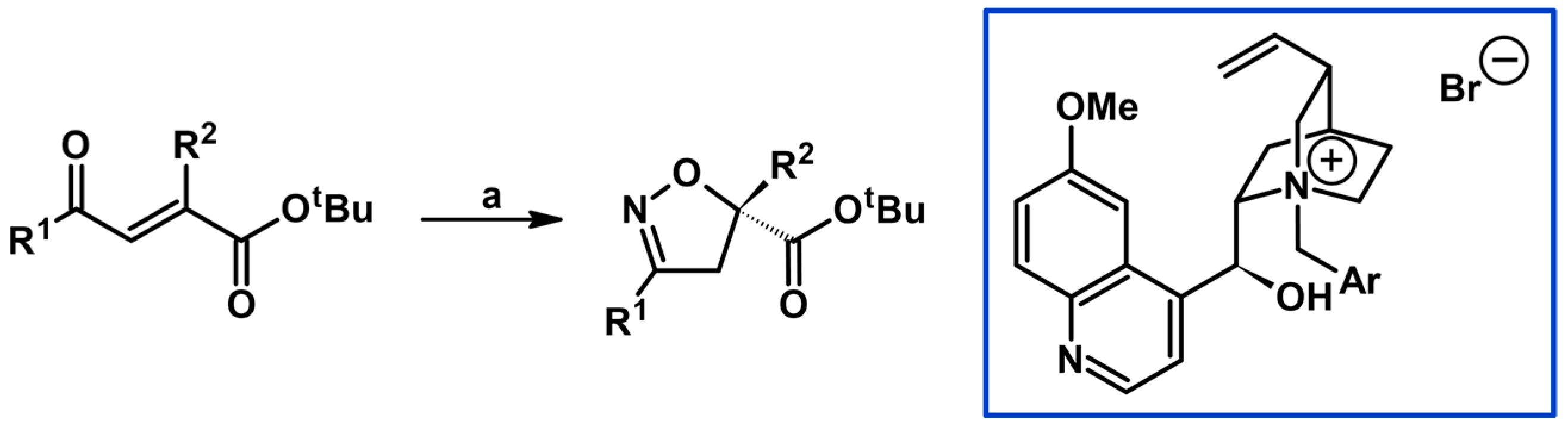
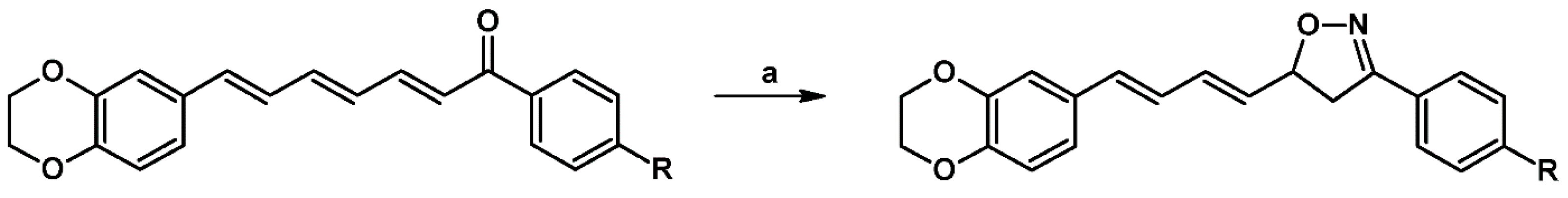
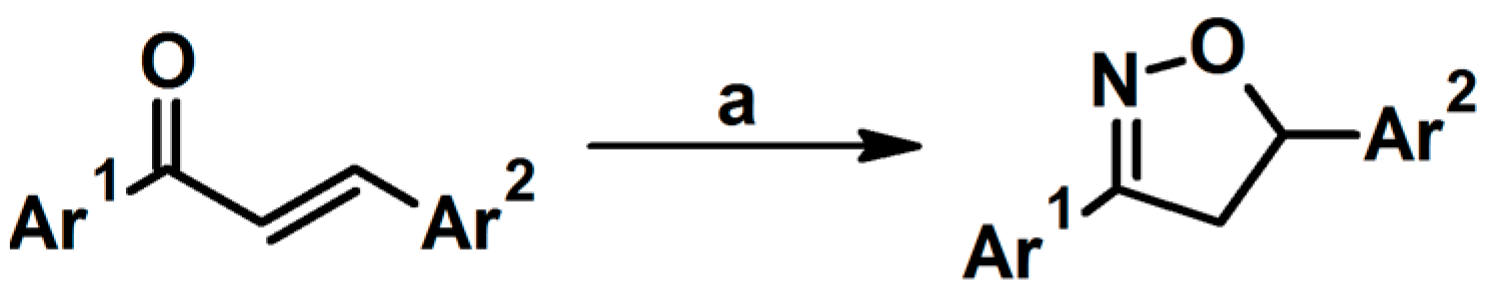
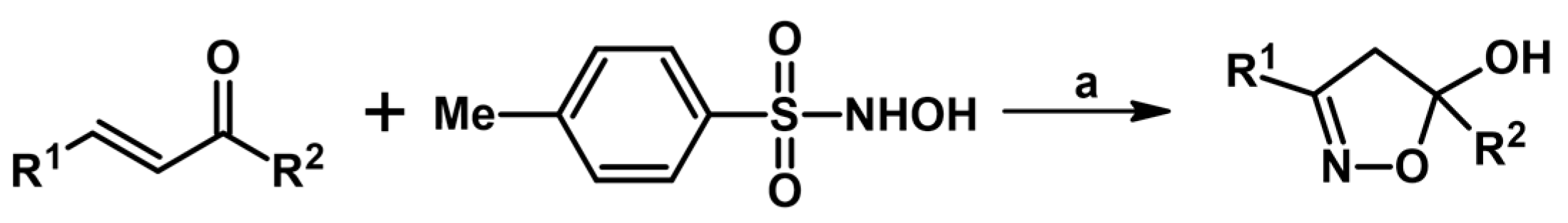

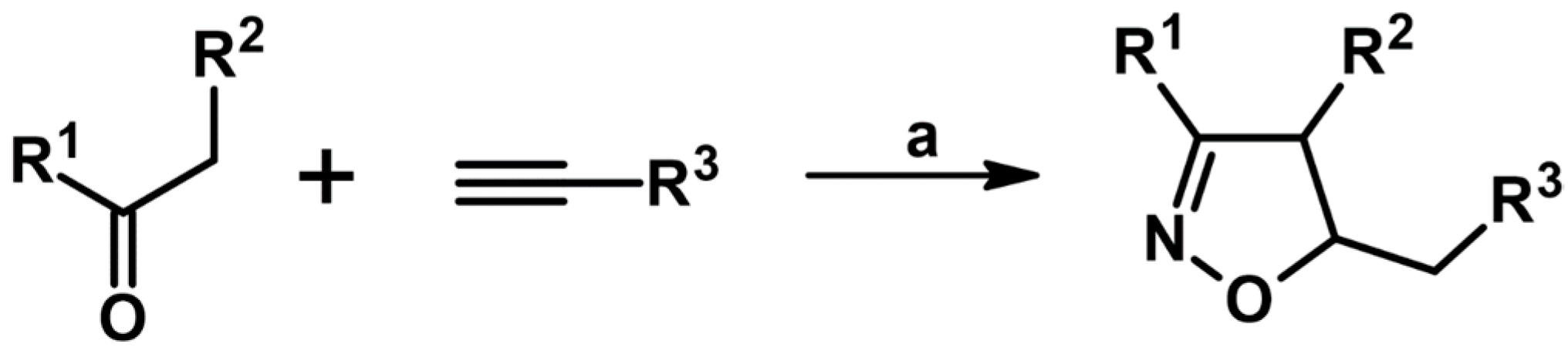
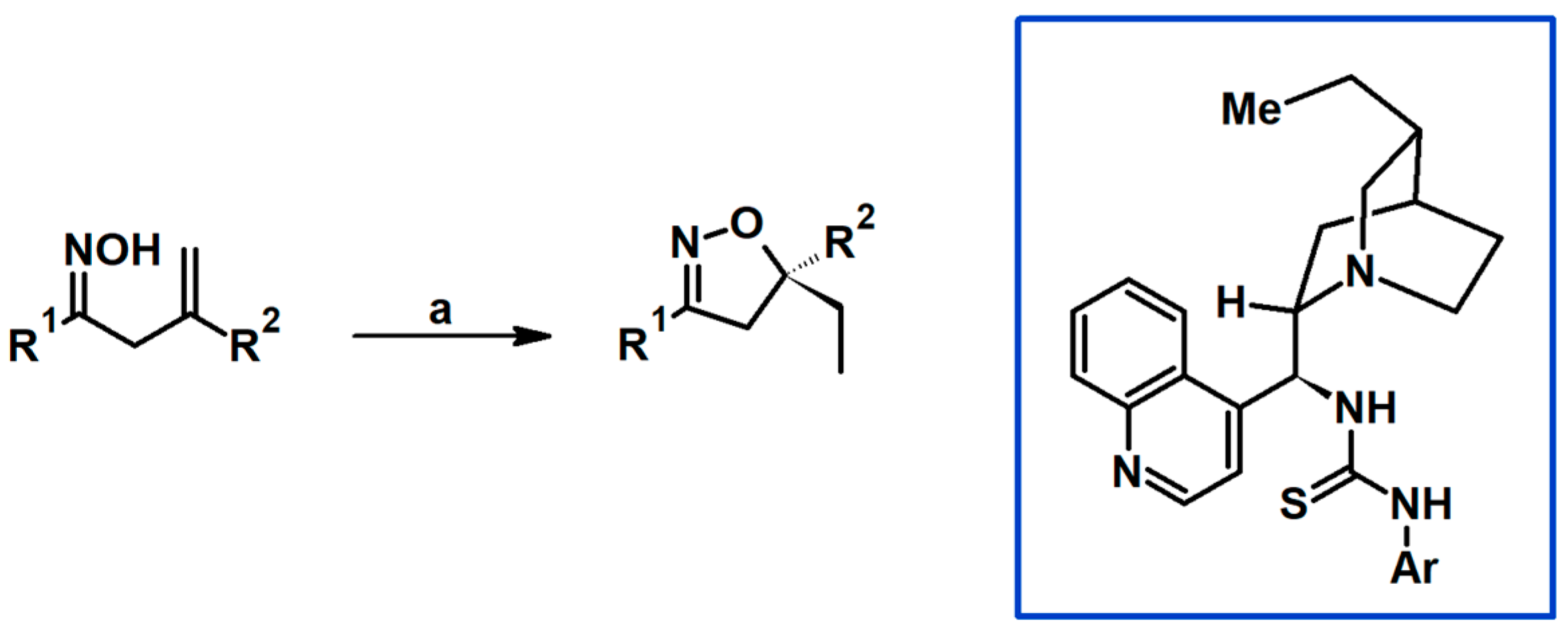

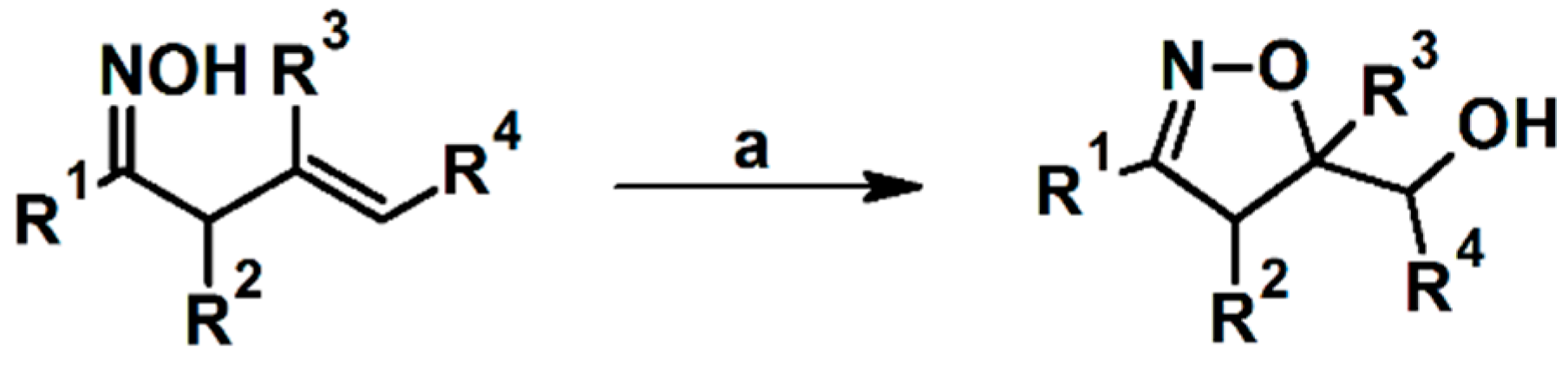

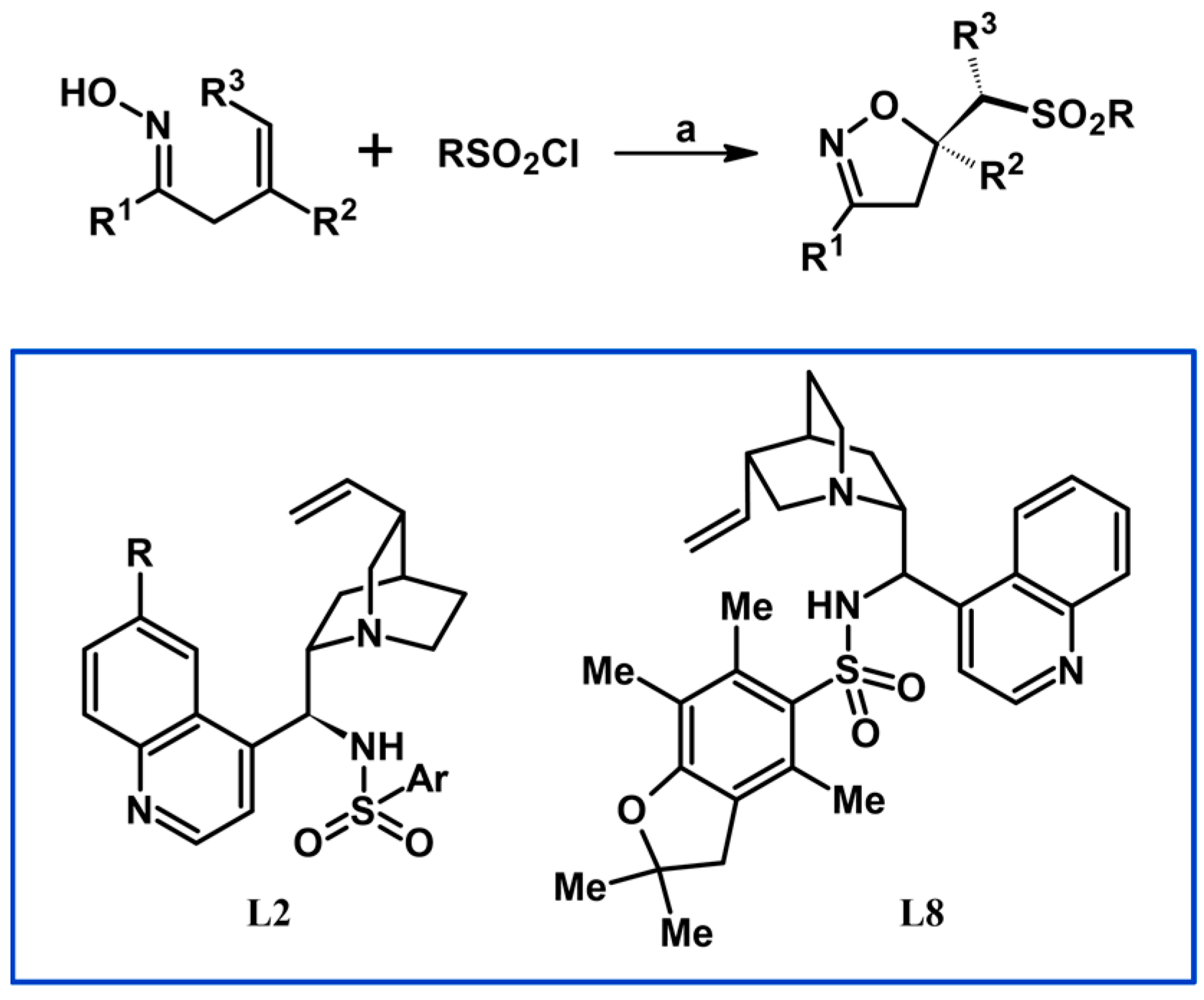
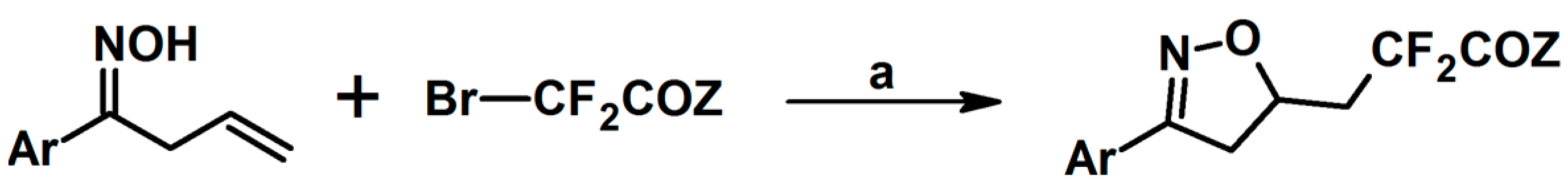
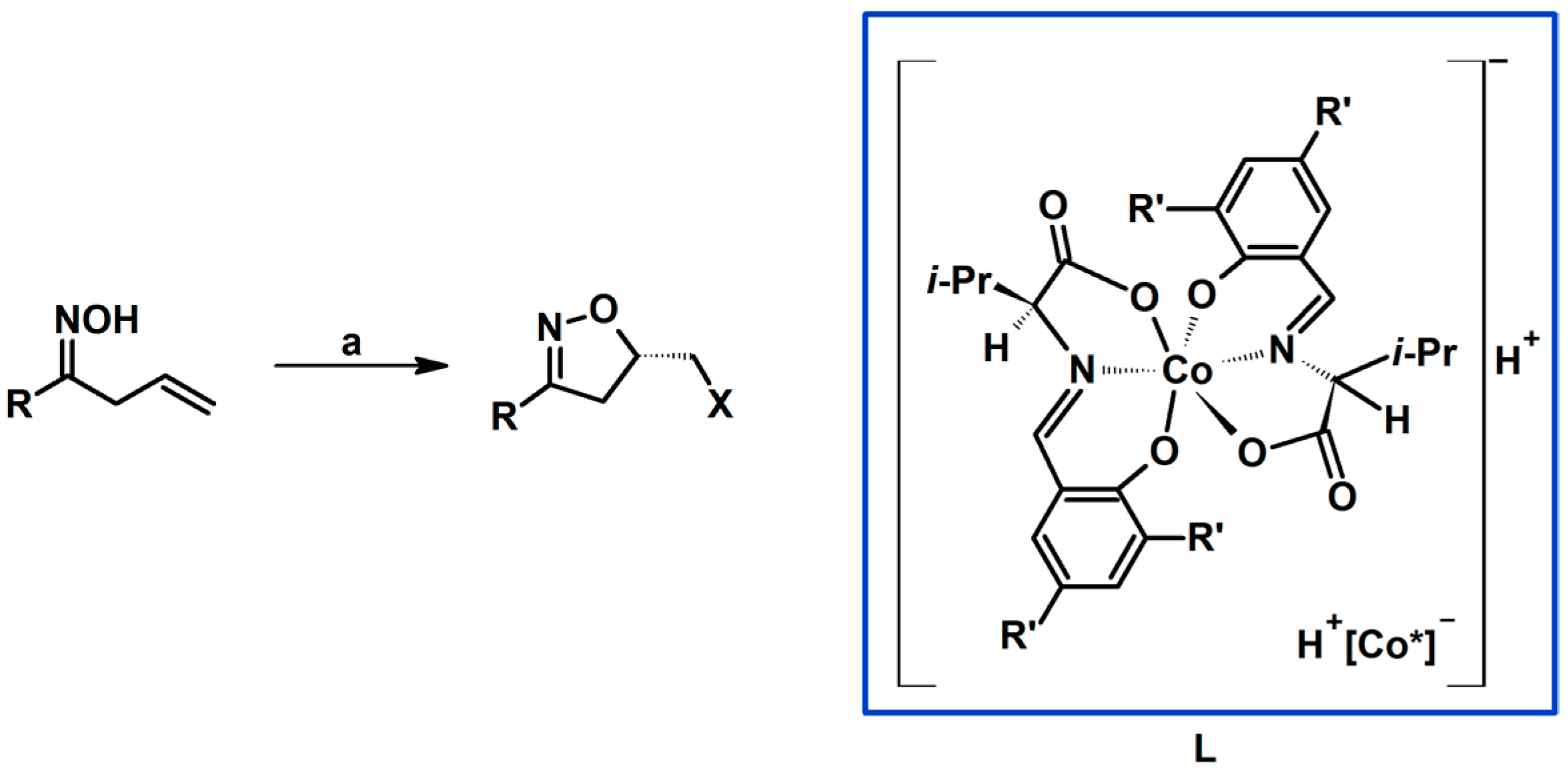
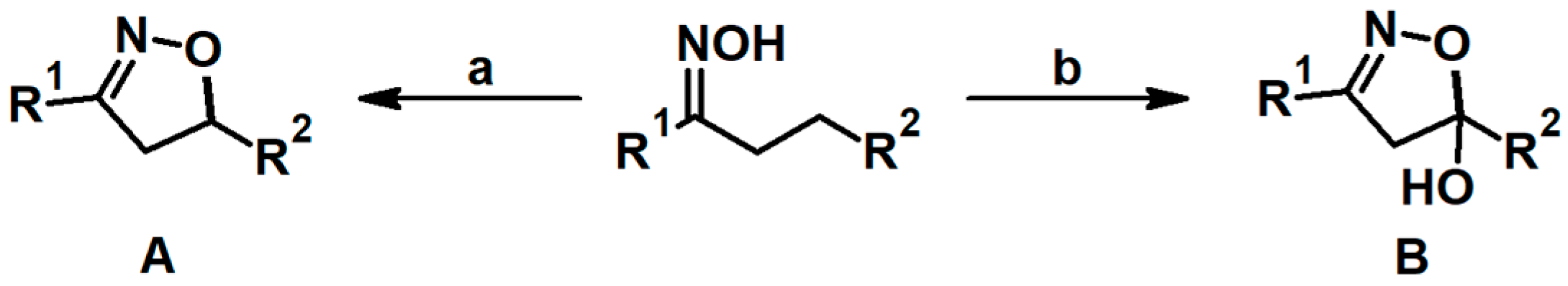

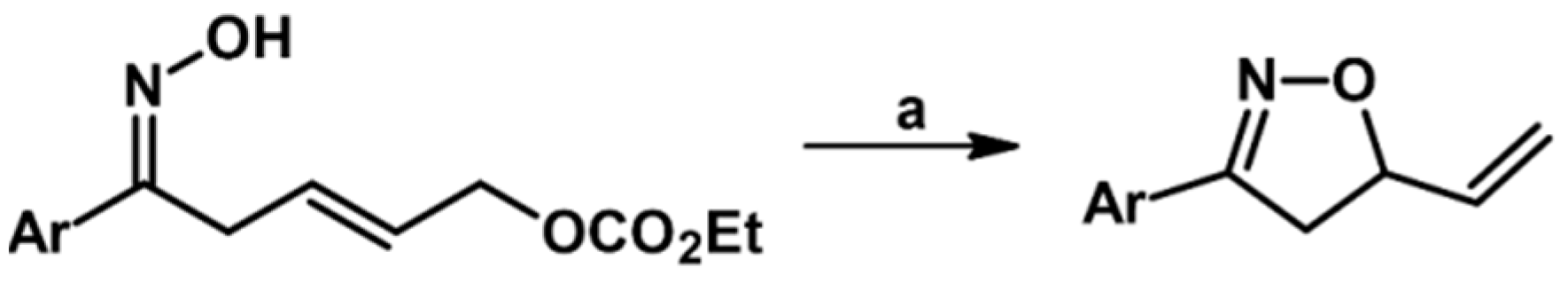
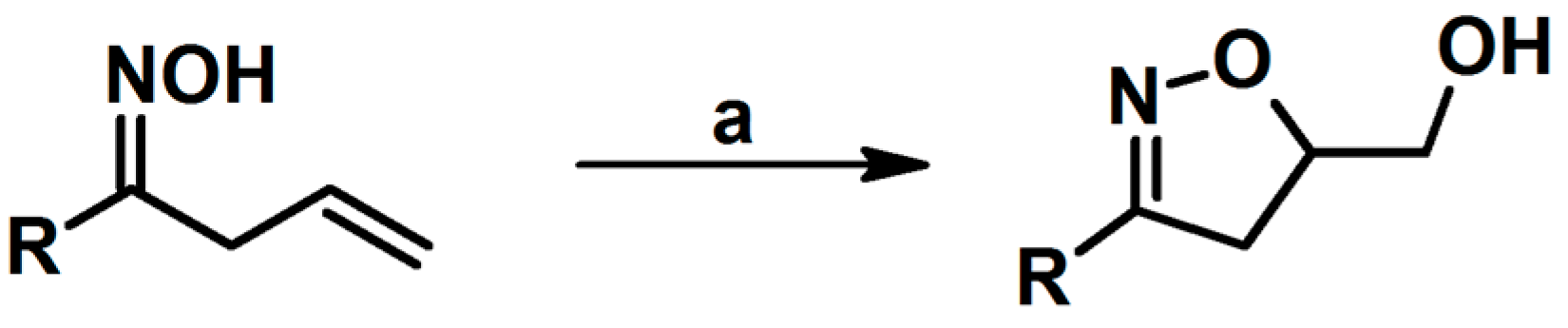
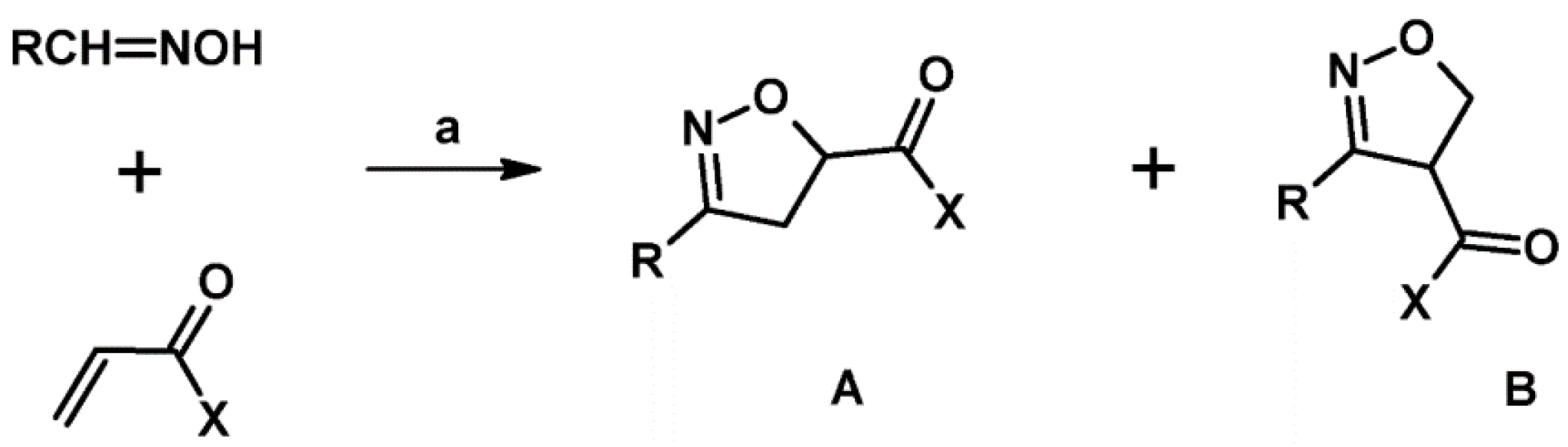
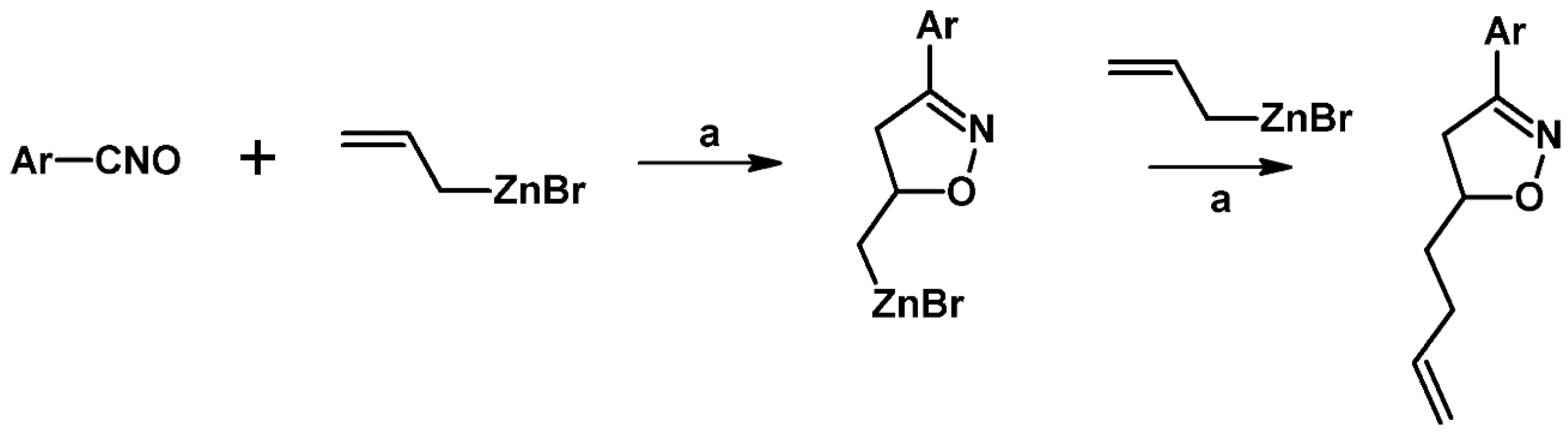


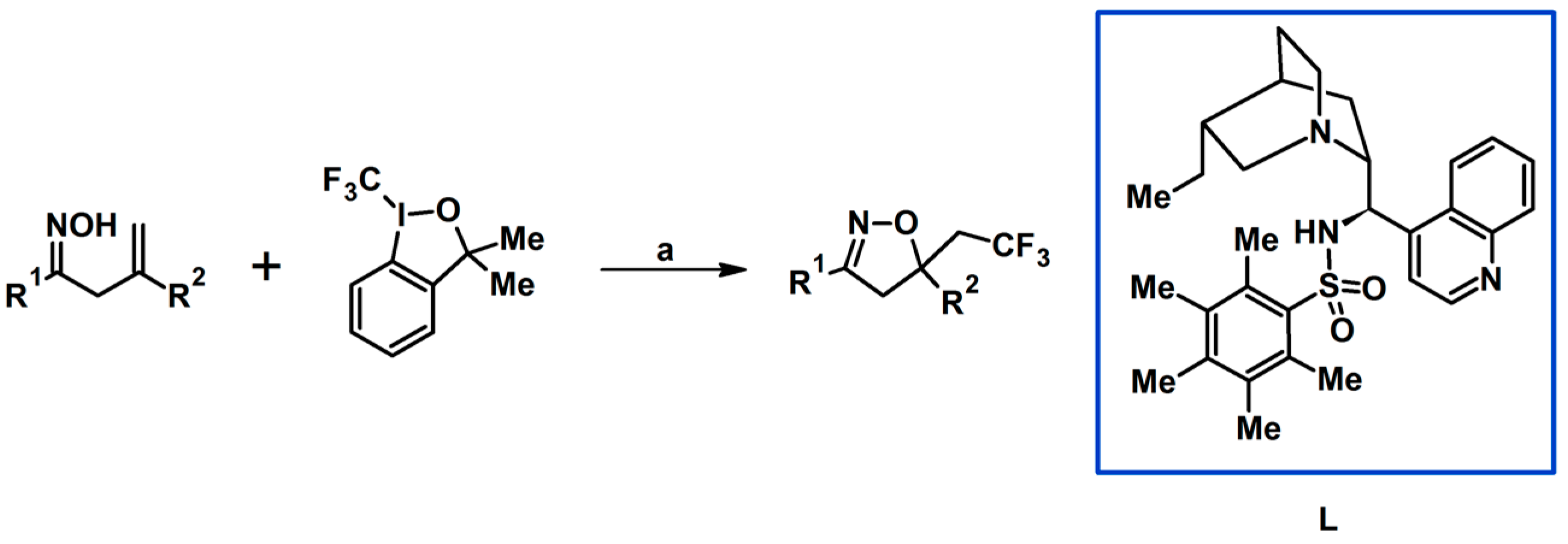


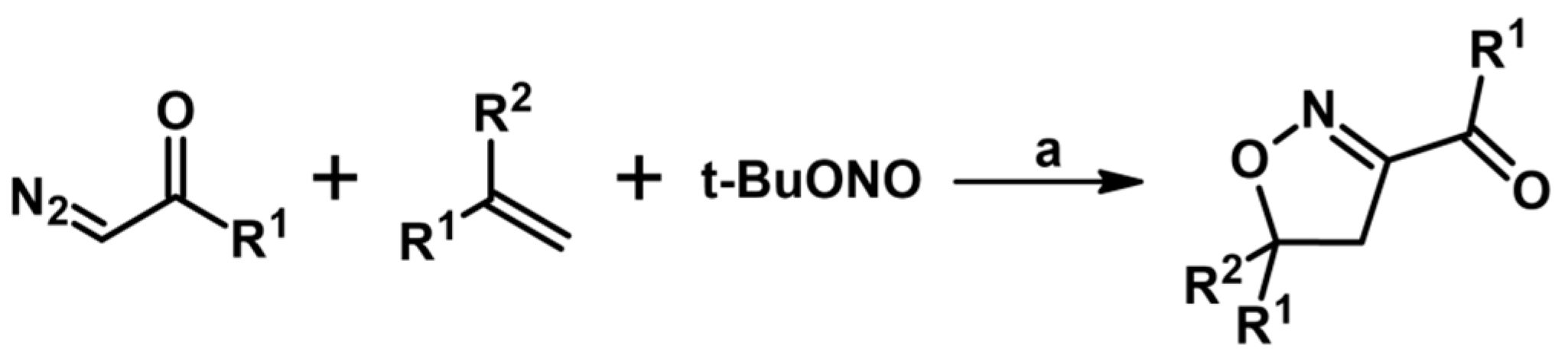
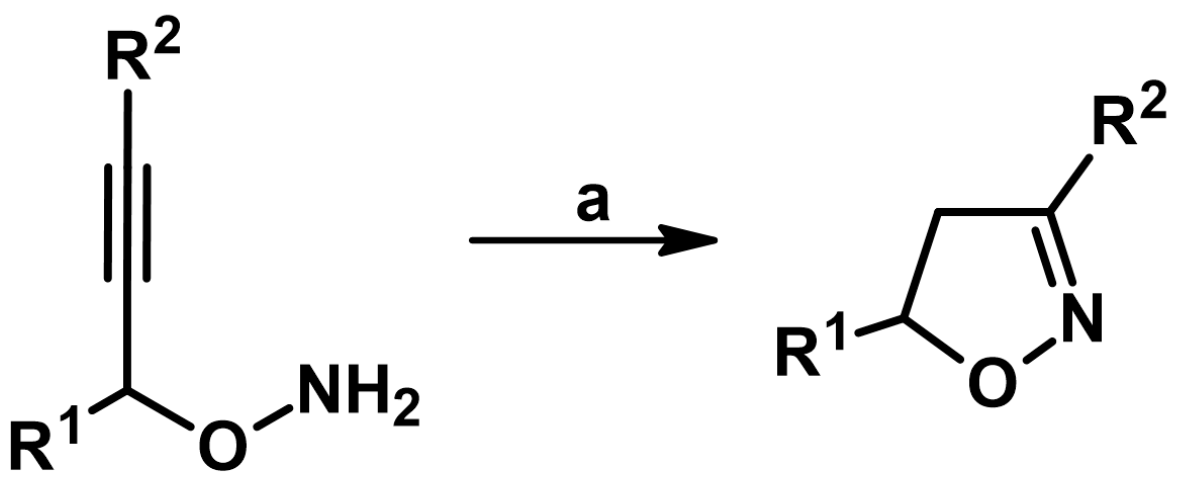
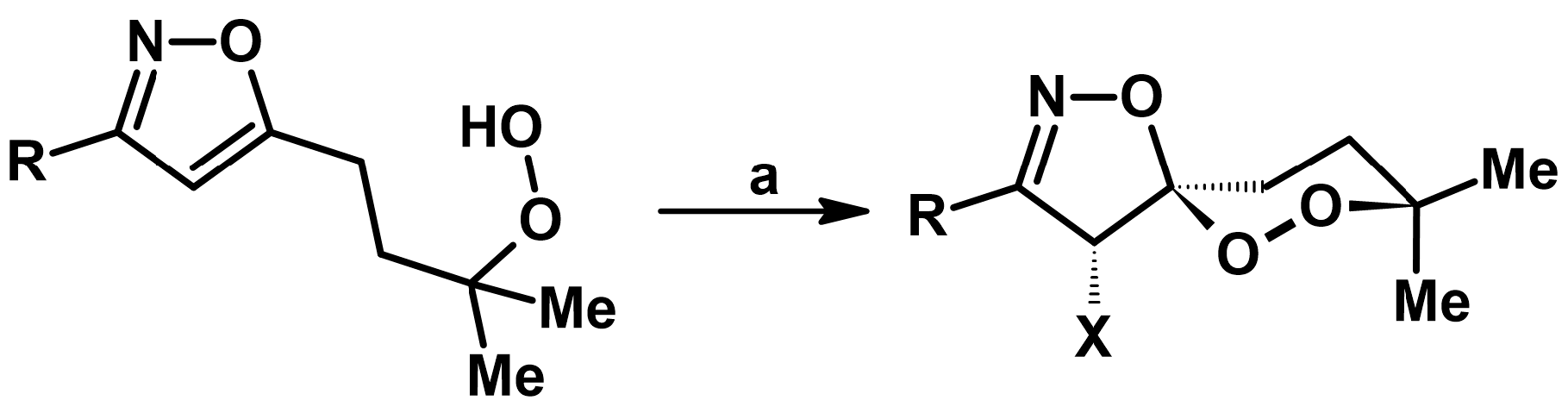
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krompiec, S.; Lodowski, P.; Kurpanik-Wójcik, A.; Gołek, B.; Mieszczanin, A.; Fijołek, A.; Matussek, M.; Kaszuba, K. Nitrile Oxide, Alkenes, Dipolar Cycloaddition, Isomerization and Metathesis Involved in the Syntheses of 2-Isoxazolines. Molecules 2023, 28, 2547. https://doi.org/10.3390/molecules28062547
Krompiec S, Lodowski P, Kurpanik-Wójcik A, Gołek B, Mieszczanin A, Fijołek A, Matussek M, Kaszuba K. Nitrile Oxide, Alkenes, Dipolar Cycloaddition, Isomerization and Metathesis Involved in the Syntheses of 2-Isoxazolines. Molecules. 2023; 28(6):2547. https://doi.org/10.3390/molecules28062547
Chicago/Turabian StyleKrompiec, Stanisław, Piotr Lodowski, Aneta Kurpanik-Wójcik, Bogumiła Gołek, Angelika Mieszczanin, Aleksandra Fijołek, Marek Matussek, and Klaudia Kaszuba. 2023. "Nitrile Oxide, Alkenes, Dipolar Cycloaddition, Isomerization and Metathesis Involved in the Syntheses of 2-Isoxazolines" Molecules 28, no. 6: 2547. https://doi.org/10.3390/molecules28062547
APA StyleKrompiec, S., Lodowski, P., Kurpanik-Wójcik, A., Gołek, B., Mieszczanin, A., Fijołek, A., Matussek, M., & Kaszuba, K. (2023). Nitrile Oxide, Alkenes, Dipolar Cycloaddition, Isomerization and Metathesis Involved in the Syntheses of 2-Isoxazolines. Molecules, 28(6), 2547. https://doi.org/10.3390/molecules28062547