Abstract
The investigation of the impact of the Fukushima accident is still going on although more than ten years have passed since the disaster. The main goal of this paper was to summarize the results of tritium and radiocarbon determinations in different environmental samples, possibly connected with the Fukushima Dai-ichi Nuclear Power Plant (FDNPP) accident. A document containing compiled data may serve as a solid basis for further research in the selected fields. To accomplish such effort, we went through dozens of relevant published papers, reporting 3H and 14C activity concentrations in precipitations, groundwater, seawater, river systems, tree rings, and, in some more extraordinary samples, such as herbaceous plants or debris from the damaged reactor buildings. As the referenced results would not be obtainable without adequate analytical techniques, the most common methods for routine measurement of tritium and radiocarbon concentrations are discussed as well. We believe that the correct identification of the affected environmental compartments could help quantify the released 3H and 14C activities and track their following fate, which could be especially important for plans to discharge contaminated water from the FDNPP in the upcoming years.
1. Introduction
The nuclear accident at the Fukushima Dai-ichi Nuclear Power Plant (FDNPP) was one of the most catastrophic events in the last decade. In March 2011, an exceptionally strong earthquake and the consequent tsunami waves struck the eastern coast of Honshu (Japan), causing severe damage to the critical systems of the FDNPP and eventually leading to a loss of ability to control and cool the shutdown reactors. Despite the enormous effort, hydrogen explosions, which emerged from the reaction of heated steam with nuclear fuel cladding, occurred in three out of four reactor buildings [1]. The total amount of released radionuclides from the FDNPP nuclear core inventories into the atmosphere was estimated to be 9.6 ± 3.4 EBq, comprising mainly short-lived fission products, such as 133Xe and 131,133I [2]. However, the cores contained many different radionuclides with various half-lives, including tritium (3H or T) and radiocarbon (14C), which were also emitted as a consequence of the accident, although to a much smaller extent [3,4]. These releases were transported by the vertical movement of air masses and then removed from the atmosphere by dry/wet deposition or other processes, impacting not only areas close to the FDNPP but also regions distant, even regions thousands of kilometers away [5]. In addition to the atmospheric route, radionuclides entered the Pacific Ocean via direct discharges of liquid wastes that were produced by the need to inject water on the melted nuclear reactors. On top of that, heavy rains caused runoffs of contaminated waters from reactor basements and trenches to the coastal region offshore Fukushima [2]. Sporadic releases of increased radioactivity in this area were documented several years after the accident [6]. Horizontal and vertical mixing in the Pacific Ocean allowed for the further spreading of diluted radionuclide concentrations over long distances [7,8,9].
Tritium (T1/2 = 12.32 yr) is a naturally occurring cosmogenic radionuclide that is produced by interactions of cosmic-ray particles with the nuclei of nitrogen and oxygen in the stratosphere. The steady-state worldwide inventory of natural 3H was calculated to be ~2.2 EBq [10], which is less than the nondecayed amount generated during the nuclear weapons testing era in the previous century (~6.0 EBq) [11]. Other sources, such as nuclear accidents, nuclear fuel reprocessing, and operations of nuclear power plants, can be considered minor and may have influence only on the local or regional scale [12,13,14]. Whether it is produced by a natural or anthropogenic process, tritium dominantly enters the environment in the form of water (HTO) or gas (T2). Tritiated water molecules can be transferred by means of the hydrological cycle to all compartments. In plants and animals, tritium can then be metabolized into organically bound tritium (OBT) [15,16,17], which is potentially more hazardous than HTO due to its much longer biological half-life. On the other hand, the radiotoxicity of 3H is rather low (the maximum energy of emitted beta-particles is only 18.6 keV), which is illustrated by international limits for drinking water, ranging from 100 to more than 70,000 Bq L−1, depending on the country [18]. The ingestion dose equivalent for one-year-old children is 4.1 × 10−11 and 1.1 × 10−10 Sv Bq−1 for tritiated water and OBT, respectively [19]. Its unique properties make tritium an excellent tracer for biochemical research [20], groundwater transport measurements [21], and oceanographic studies [22]. Tritium activity is often reported in tritium units (TU), where 1 TU is equivalent to 0.119 Bq L−1.
Like tritium, radiocarbon (T1/2 = 5730 yr) is a cosmogenic radionuclide that is naturally produced by the reaction of neutrons with stratospheric and tropospheric nitrogen and oxygen atoms. After being produced, it is rapidly oxidized to carbon monoxide and consequently to carbon dioxide. Molecules of 14CO2 are then transported to the lower parts of the atmosphere, absorbed in the hydrosphere, and incorporated into the biomass by photosynthesis, becoming part of the global carbon cycle. The largest reservoir can be found in the oceans where ~6.9 EBq of radiocarbon has been accumulated [23]. While nuclear weapons testing increased the total radiocarbon inventory by ~0.2 EBq, the contribution of other anthropogenic sources can be regarded as almost negligible [10]. Depending on the type of reactor, radiocarbon can be emitted from a nuclear power plant in the form of carbon dioxide or hydrocarbons (14CnHn) [24]. Anthropogenic and natural radiocarbon can be reliably distinguished one from another [25,26,27], which may be important for different studies, exploiting 14C as a tracer [28]. From a radiotoxicity point of view, radiocarbon could be relevant because it is contained in all of the organic molecules of living organisms, including DNA, which is susceptible to unrepairable breaks and mutations caused by ionizing radiation [29,30], though the energy of the beta particles it emits is not very high (156 keV). In environmental sciences, the 14C concentration is usually given as Δ14C (‰) which represents an excess of radiocarbon content relative to a standard [31].
The FDNPP operated four boiling water reactors (BWR), whose parameters are summarized in Table 1. In the reactors, tritium was generated mainly by ternary fission of 235U and 239Pu with yields of 0.013% and 0.023%, respectively. Further production options comprised neutron capture on deuterium, reaction on lithium impurities 7Li (n, αn) 3H present in the water coolant, and reaction on boron 10B (n, 2α) 3H from the boron carbide control rods [32,33]. According to calculations [34], each reactor contained ~1.0–1.2 PBq of 3H, meaning that the ~3.4 PBq could be cumulatively released from three damaged reactors at maximum. Regarding radiocarbon, its production in the FDNPP BWRs by ternary fission of uranium was very low (yield of 0.00016%). Therefore, its formation was dominated by neutron capture on a stable carbon isotope (13C) and by reactions 14N (n, p) 14C and 17O (n, α) 14C on impurities in the fuel rods, coolant, and moderator [24] (Figure 1). However, the role of impurities in the fuel was probably less significant than expected due to the fact that most of the air composed of nitrogen and oxygen was removed from the FDNPP fuel rods during their manufacture [3]. The total inventory of 14C in all FDNPP nuclear reactors on the day of the accident was estimated to be ~1.0–1.6 TBq [35].

Table 1.
Basic parameters of the FDNPP boiling water reactors [36].
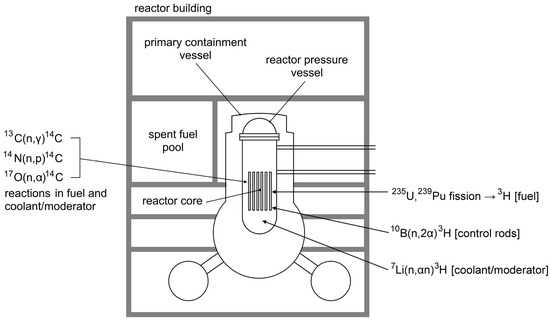
Figure 1.
Simplified scheme of a boiling water reactor (BWR) operated at the FDNPP with the typical production pathways of 3H and 14C.
The aim of this review is to chronologically summarize the results of tritium and radiocarbon determinations in different environmental samples that were presumably connected with the FDNPP accident. A compiled document containing data obtained through more than ten years of investigation may serve as a solid basis for further research in the selected fields. Although 3H and 14C are both pure low-energy beta emitters, they are easily incorporated into biological systems, which makes them potentially important for future radiological studies. Moreover, tritium and radiocarbon are suitable for the tracing of various physical, chemical, and geological processes, which could bring interesting answers to some interesting scientific questions. A correct identification of the impacted environmental compartments affected could help quantify the 3H and 14C activities released from the FDNPP and track their following fate. Since such an effort would not be doable without proper analytical techniques, the most common methods for routine measurement of tritium and radiocarbon concentrations are briefly discussed as well.
2. Methods for the Determination of 3H and 14C
2.1. Liquid Scintillation Counting (LSC)
Radionuclides that emit beta particles are readily determined by liquid scintillation counting (LSC). In this technique, the sample containing separated radionuclides of interest is mixed with an organic scintillator cocktail to form a solution that is then counted by a proper detection system, e.g., TriCarb or Quantulus (PerkinElmer, Waltham, MA, USA). The colorlessness and homogeneity of the solution ensure low self-absorption and high counting efficiency [37]. In the case of 3H, tritiated water is usually purified by distillation to remove possible interfering radiocontaminants before the LSC sample is prepared and measured, as most of them are significantly less volatile (e.g., 40K). The distillation step can be repeated several times if contaminating radionuclides remain in the first distillate. Solid samples are first to be combusted to obtain HTO, which is then measured [38]. Tritium can also be incorporated into benzene molecules that can be synthesized from the original water sample and used as a solvent for a scintillation cocktail [39]. The limit of detection of ~10 mBq for the measurement of 3H activity by LSC has been achieved [38], though if the level of tritium in the sample is beyond this value, it can be increased by a factor of 30–100 with the use of electrolytical enrichment [40].
The determination of radiocarbon by LSC is slightly different compared to 3H. Unlike tritiated water, it is impossible to directly combine 14CO2 with a scintillator cocktail. Regardless of whether it was sampled from the air, obtained by combustion of organic material, or by hydrolysis of an inorganic compound, carbon dioxide is first adsorbed into a suitable chemical medium (e.g., NaOH, BaCO3, and Carbosorb) or converted to benzene. The former option offers a relatively fast and simple procedure. However, the latter possibility leads to a higher precision of measurement due to a much higher carbon density, as more than 90% of the mass of benzene comes from carbon [41,42,43]. A typical three-step conversion includes the reaction of CO2 with molten lithium to produce lithium carbide, the addition of water to obtain acetylene, and finally its catalytic trimerization to benzene, which is stored for a month before LSC measurement to allow radon and its daughter radionuclides to completely decay [44], though the radon problem can be avoided with a modification of the reaction [45]. On top of that, modern detection systems are capable of evaluating radon contamination and applying a correction to the measured radiocarbon activity [46]. The limit of detection for 14C determination by LSC has been reported to be ~15 mBq [38,47].
2.2. 3H-3He in-growth Mass Spectrometry
Since tritium has a very low natural abundance (the isotopic ratio 3H/1H ≈ 10−18), it is quite difficult to determine it directly by mass spectrometry. This problem has been solved by the development of 3H-3He in-growth spectrometry which is based on the measurement of 3He produced by the decay of tritium and the subsequent back calculation of its activity [48,49]. The method is so sensitive that the 3H/1H isotopic ratios can be determined down to ~10−20, corresponding to the tritium activity of 1.19 mBq L−1 or 0.01 TU [50]. The technique is excellent for the analysis of environmental samples (e.g., seawater) whose tritium concentration is too low for LSC or gas counting, even after electrolytic enrichment. It is the same as for LSC, the water sample needs to be distilled prior to analysis to avoid any possible contamination. After the sample is transferred into a hermetically sealed container, tritium is left to decay for several months to accumulate enough 3He. The tritiogenic fraction is purified and spiked with a known amount of the 4He standard [51]. The actual measurement of the 3He/4He isotopic ratio can be conducted with a sector-field noble gas spectrometer [52,53].
2.3. Accelerator Mass Spectrometry (AMS)
Although it is possible to use AMS for the determination of 3H-labelled molecules [54], the tritium concentrations of typical environmental samples are by about a factor of 100 below its limit of detection. In the case of radiocarbon, the high sensitivity of AMS enables measuring it down to the level of 14C/12C ≈ 10−16 [55], which is sufficient for most types of environmental samples. The technique is based on the separation of the radionuclide of interest from interfering monoatomic and polyatomic isobars present in the accelerated ion beam with respect to their masses and energies. In the case of radiocarbon, it is inserted into the ion source, either in the form of graphite or carbon dioxide (see below), where it is sputtered to produce 14C– and other ions. Since 14N does not form negative ions, the main potential interferent is not an issue. The generated ions are then analyzed according to their mass and injected into an accelerator in which they gain much higher energies and a positive charge by stripping electrons, leading to the dissociation of molecular ions, and getting, thus, high sensitivity. In the postacceleration part, 14C ions are selected by mass–charge analyzers and counted by an end detector.
The preparation of 14C samples for AMS is quite complex. The first step is to obtain CO2, e.g., by combustion or acidic dissolution of carbonate precipitate, which is then introduced directly into an ion source, or converted to a graphite target. While the former is especially useful for small samples [56,57,58], the latter is utilized in most laboratories. Graphite targets can be prepared by the catalytic reduction of sample CO2 with the use of hydrogen and a suitable metal, such as iron, which also serves as the agent for the deposition of synthesized graphite. Both gases and the iron catalyst are enclosed in a reactor, which is heated to 500–600 °C [59,60]. Another option is to completely omit H2 and replace it with zinc which can reduce CO2 to CO and then CO to graphite when heated to similar temperatures [60]. In recent years, sealed tube graphitization methods have been developed to accommodate the increasing demand for precise 14C AMS analyses. As an example of such procedures, sample CO2 is cryogenically transferred into a glass tube with iron and titanium hydride which releases hydrogen for reduction after heating in an oven to 500 °C and zinc for reaction with emerging water [61]. The same effect can be achieved with zinc as the sole reducing agent [62,63]. Since graphitization is very sensitive to catalytic poisons (e.g., sulfur and halogens), sample CO2 must be purified which is generally carried out by passing the gas through heated copper and silver columns.
3. FDNPP Impact on Tritium Environmental Levels
3.1. The Concentration of 3H in Atmospheric Precipitation and Water Vapor
Due to the nature of the FDNPP releases, precipitation has been routinely monitored after the nuclear accident, tritium being one of the most interesting radionuclides (Figure 2). The data collected in the southwest direction from the FDNPP site for the period from March to May 2011 showed the highest 3H rainwater concentration of ~160 TU in Tsukuba during the first two weeks, leading to an estimation of 1.5 kBq m−3 for the source tritium atmospheric activity [64], which corresponds to the 3H inventory of 0.6 PBq for a BWR reactor operated for one year [65]. The tritium level decreased steadily in the following days, however, its excess over the background was observed even at the locations 700 km from the damaged nuclear power plant. In the summer of 2011, tritium rainfall activities in Tsukuba were already comparable with pre-Fukushima values [66] which was also true for Chiba, where 3H activity in rainwater peaked at 12.7 TU in March 2011 [67].
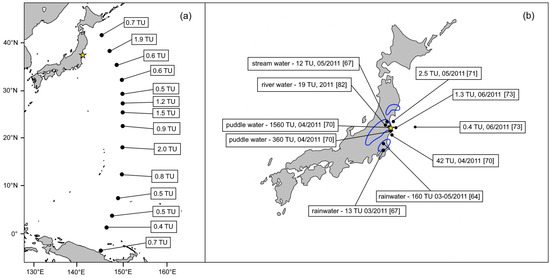
Figure 2.
Tritium activity concentrations in various water samples after the FDNPP accident: (a) surface seawater values of the western North Pacific Ocean in winter 2012 according to [68], and (b) selected 2011 samples from Japan, given with the date of their collection and respective reference [64,67,69,70,71,72]. The yellow star represents the location of the FDNPP. The blue lines show the approximate areas of the significant wet deposition during the second half of March 2011 [2].
In addition to precipitation, scientists have been interested in monitoring the atmospheric concentration of HTO. From October 2016 to March 2021, two locations in the vicinity of the FDNPP have been investigated for this purpose [73,74]. The mean HTO concentration (~55 mBq m−3) was found to be significantly higher than the background level for the location 1 km south of the FDNPP site while the more distant location did not show any quantifiable impact. The results suggested that the elevated atmospheric HTO concentration could originate in the releases from the FDNPP site, which were transported by winds prevailing at the time of sampling. This means that the FDNPP remains a potential source of atmospheric 3H, though the documented levels do not pose an immediate health risk to humans or other living organisms.
3.2. The Concentration of 3H in the Pacific Ocean and Coastal Seawater
Several studies have been conducted with the aim of evaluating the influence of the FDNPP accident on marine tritium levels. The earliest measurement of coastal seawater in April 2011 revealed a large contamination of 42 TU some 30 km south of the FDNPP site [69]. In the first half of May 2011, the coastal area near the damaged power plant was screened to evaluate potential discharges. From the obtained values, it was concluded that only 0.05 PBq of tritium was directly released from the FDNPP at that time. The surface and subsurface seawater activities of 3H varied from 0.08 to 0.29 Bq L−1, which was higher than the estimated pre-Fukushima background of 0.07 Bq L−1 [70]. The peak concentrations, which were caused by the advection of the coastal current, were found north and south of the FDNPP seaport where ~0.2 Pbq of 3H was cumulatively discharged directly into the sea [75]. The extended area offshore Fukushima was investigated again in June 2011. The 3H activity concentrations in these samples were determined to be in the range between 0.4 and 1.3 TU, which was a factor of ~3 above the global fallout background [71]. The water column data showed an expected trend of decreasing values with increasing depth; however, the penetration of FDNPP-derived tritium below 100 m was clearly observed for a sampling station very close to the nuclear power plant. The total amount of 3H activity released and deposited to the studied area was 0.1–0.5 PBq which was calculated using measures of the 3H/137C activity ratios and estimated inventory of released and deposited 137Cs [76,77]. Furthermore, the coastal waters off the Aomori and Iwate prefectures, located north of Fukushima, were also studied; however, there was no obvious impact of the FDNPP accident on 3H surface levels [78].
A situation in the western North Pacific Ocean after the FDNPP accident, which was evidently affected by direct discharges of radioactive water and deposition from the atmosphere [77], was intensively explored in the winter of 2012. The analysis of the radiocesium content (134,137Cs) indicated that the ocean waters were influenced by the FDNPP accident, which was also confirmed by tritium measurements. Both surface and vertical profile samples showed increased levels of 3H, with the highest value (2.0 TU) found in the area closest to the FDNPP. The tropical region of the western North Pacific Ocean was also affected (up to 1.0 TU on the surface), implying that precipitation was important for the spread of tritium released from the FDNPP. At some locations, tritium penetrated down to 400–500 m or even deeper. Based on surface data and water column inventories, 0.4–1.0 PBq of tritium was deposited in the western North Pacific Ocean [68,79,80].
3.3. The Concentration of 3H in Freshwater Systems
Terrestrial waters became a target of investigators right after the FDNPP accident. In April 2011, a very high concentration of 3H activity of ~184 Bq L−1 was determined in a sample of puddle water, collected 1.5 km from the FDNPP site, which is the highest value in any environmental sample documented in the literature. Paddy water was also sampled in the vicinity of the puddle water, yielding a high tritium level of ~68 Bq L−1, which means that the ratio of the 3H concentration between the puddle and the paddy water was only 0.4 [69]. This would suggest that significantly contaminated rainwater was diluted by mixing with stagnant water in rice paddy fields. The second puddle water sample collected during the same campaign at a more distant location also contained a high concentration of tritium (~42 Bq L−1).
The purity of water sources is critical for ecosystems and human lives. Therefore, the need for their monitoring in Japan after the FDNPP events was not surprising at all. Groundwater, stream water, and spring water from two headwater catchments located about 35 km from the FDNPP were analyzed for tritium concentration between May 2011 and June 2012, obtaining mostly background values, with the exception of the May spring water sample (11.7 TU) [67]. In autumn 2012, fifty wells in Fukushima Prefecture were screened that showed a varying 3H distribution (1.1–12.9 TU). Although the highest concentrations were determined at locations close to the FDNPP (<25 km) and at shallow depths (<10 m), the deeper and more distant wells appeared not to be affected [81]. With the use of a simple mixing model, precipitation containing tritium released from damaged FDNPP cores was identified as a probable source of increased groundwater values. However, wastewater stored and treated onsite at FDNPP remained a potential risk for groundwater even several years after the accident, as was documented by a recent study. The sump water collected at the boundary in the 2013–2019 period showed an average 3H concentration of 20 Bq L−1 [82]. Although this is two to four orders of magnitude lower than the 3H concentration in well water from the FDNPP site itself, uncontrolled leakages of any amounts of anthropogenic radioactivity into the environment should be avoided.
From 2011 to 2014, a river system in Fukushima Prefecture was extensively studied during base flow conditions and flood events. The peak tritium concentrations of ~2.2 Bq L−1, which was well above the natural background, were determined in the case of two small rivers analyzed in the first year of the investigation [72]. The influence of 3H released from the FDNPP exponentially decreased to nondistinguishable values over the next three years. The rest of the river system, which was sampled only from 2012, showed similar temporal changes in the tritium content and its unexpectedly good correlation with the 137Cs inventory for the same catchment areas. The screening in the same region continued in October 2014 when samples were collected from four estuaries and respective coastal surface seawaters. However, neither rivers nor marine environments were affected by the potential 3H contamination [83]. Further monitoring of the Fukushima Prefecture rivers brought the very same result [84].
3.4. The Concentration of 3H in Biota and Other Samples
The number of organic samples analyzed for the tritium content in connection with the FDNPP accident was quite low, probably due to the complexity of applied treatment protocols compared to, e.g., water. A survey was carried out from March to August 2011 whose main objective was to measure the concentration of free-water tritium (FWT) in herbaceous plant shoots and evergreen tree leaves, collected in the area around the FDNPP site. The impact of FDNPP releases was clearly visible for the first two months, with the highest FWT concentration of 167 Bq L−1 found just outside the 20 km evacuation zone [85]. In less than four months, the value at the same location decreased by a factor of ~30, although it remained above the background level. An assumed relation between the FTW concentration and the distance from the FDNPP was observed as well. In the summer of 2012, branches and leaves from cut and living trees and rubble were sampled in the vicinity of the FDNPP reactor buildings and treated for multinuclide radiochemical analysis. The average 3H concentrations of the tree and rubble samples were determined to be 0.31 and 0.62 Bq g−1, respectively. Tritium activity was fairly uniform in the case of all samples, though there was no obvious correlation between its 3H and 137Cs content [86]. From 2015 to 2018, the OBT and FWT concentrations were measured in flounders living in the coastal region of Fukushima, reaching only the limits of detection [87].
4. FDNPP Impact on Radiocarbon Environmental Levels
4.1. The Activity of 14C in the North Pacific Ocean and Coastal Seawater
Similar to tritium, radiocarbon was studied in the Fukushima offshore region and western North Pacific Ocean to see if their 14C levels were disturbed by the accident. The first samples were collected in June 2011. The average surface Δ14C level of −55‰ was obtained for the extended coastal region while the value increased to −20‰ at the depth of 100–200 m for the same area. The maximum contribution of the FDNPP was estimated to be 6% and 9% for surface seawater and the subsurface layer, respectively, though the lack of background data made it difficult to calculate the actual impact [76]. The measured negative Δ14C values were somewhat unexpected. However, their origin was explained by the fact that the investigated region was under the influence of the Oyashio current from the North Pacific Ocean. With seasonal variation, the current can create an intrusion that can bring subarctic waters with a much lower radiocarbon concentration southward, reaching even the eastern coast of Japan [88,89].
Recent modeling results suggested that the majority of radiocarbon releases from the FDNPP were transported over the North Pacific Ocean [90] whose surface seawater was first explored several times during the summer months in the 2011–2016 period. However, no elevated radiocarbon concentrations, which could be attributed to the FDNPP impact, were observed in the data set [91]. In the winter of 2012, scientists focused on the western part of the North Pacific Ocean where water columns were also studied, although no clear correlation was found between measured 14C activities and the reported radiocesium signal from the FDNPP [68]. The northern negative Δ14C values (−40‰), which were under the influence of the Oyashio intrusion described in the previous paragraph, changed to the positive values (68‰) in the south. This trend, together with the uniform mixing of radiocarbon in the surface mixed layers (the depth of 100–200 m), was in good agreement with the previous results [92,93]. The anthropogenic (bomb-produced) 14C water column inventories at 35–40°N, which would be noticeably increased in the case of the significant impact of the FDNPP accident, were actually lower. This decline was probably driven by the lowering of bomb-produced radiocarbon to greater depths and its movement along isopycnic layers [80].
4.2. The Activity of 14C in Tree Rings and Other Samples
Tree rings represent a natural reservoir that can annually accumulate atmospheric radiocarbon, emitted, e.g., from a nuclear power plant (Table 2). The Japanese cedars that were cut down in Iwaki and Okuma some 50 km and 1.5 km away from the FDNPP, respectively, showed a typical temporal exponential decrease of the Δ14C values and intensifying Suess effect. In addition, a small 14C peak was found for the 2011 Iwaki tree ring, although it could not be identified in the case of the Okuma sample [94,95]. More detailed results were obtained in a study that comprised six additional cedar tree samples, collected in the north and northwestern directions from the damaged nuclear power plant. The average excess 14C activity of ~40 Bq kg−1 C was measured for two 2011 early wood subring samples from the close vicinity to the FDNPP, followed by a sharp decline to zero in 2012, which would well correspond with the permanent shutdown of the reactors. However, even higher excess 14C activity was reported for the same location in 2010 when the reactors were in routine operation [35]. In order to add more values to the dataset, two cypress and cedar trees from the Namie region were analyzed. The results suggested that the maximum impact of the FDNPP accident could be equivalent to the excess 14C activity of ~10 Bq kg−1 C [96]. Furthermore, a possible southwestern dispersion of radiocarbon from the FDNPP was postulated by the investigation of additional Okuma trees, in which the average late wood Δ14C value reached ~260‰ over the background. The higher contamination of the late wood rather than the early wood was explained by potential post-accidental releases [97]. The most recent study confirmed that no influence on the radiocarbon level in trees or plants can be anticipated beyond the 30 km border from the FDNPP site [90].

Table 2.
Average measured radiocarbon activity in Japanese tree rings from 2011.
Except for already discussed seawater and tree rings, there have been only a few other radiocarbon samples documented in the literature which could be connected to the FDNPP. In November 2011, a leaf litter sample was collected in Kawamata in northeast Japan and divided into two distinct fractions. Despite their quite high content of radiocesium, neither of the fractions yielded an increased 14C concentration which was in concordance with the atmospheric level [98]. The radiocarbon screening of the surrounding of the FDNPP reactor buildings, which was conducted in the summer of 2012, has brought some interesting results. While the 14C activity in the unit one rubble sample, together with tree branches and leaves, was measured to be below the limit of detection, its value for most of the rubble samples of units three and four was in the range of 0.13–2.7 Bq g−1 [86]. This would suggest that no large amount of radiocarbon was dispersed away from the reactor buildings.
5. Summary and Perspectives
Even though more than ten years have passed since the disaster, the investigation of the FDNPP accident’s impact on the environmental tritium and radiocarbon levels is still ongoing. In contrast to radiocesium, tritium and radiocarbon remain fairly understudied. There are some crucial FDNPP-connected aspects that we do not completely understand, e.g., total releases of radiocarbon and its deposition into the marine environment, inhomogeneous distribution of the released radionuclides of interest in different samples and magnitude of their intake by population, their incorporation on the molecular level and its potentially harmful effects, etc. This discrepancy between the radionuclides is mainly caused by the present background, which is almost negligible in the case of radio-cesium, while the nuclear weapon test legacy and the natural production of 3H and 14C are quite significant and can make a fresh anthropogenic signal difficult to detect. The problem has become less critical with the option to apply modern analytical methods. However, even their great capabilities might be insufficient in some cases. Further development of ultrasensitive techniques could be essential in order to push the limits of the research connected with the long-lived radionuclides released from the FDNPP.
The government of Japan has recently agreed to discharge into the Pacific Ocean radioactive wastewater which has been accumulated at the FDNPP site. The water still contains significant amounts of 3H and 14C which cannot be removed in a reasonable way by any physical or chemical process. The current estimations suggest that the total activity of tritium stored in tanks is ~1 PBq while the value is by four to five orders of magnitude lower in the case of radiocarbon [99]. Although the amount of 3H is higher than the calculated activity already released from the FDNPP, a plan to discharge the wastewater over a few decades should minimize its impact on the environment. However, there are several uncertainties that can strongly affect the actual prediction [100], though with more details of the discharge plan revealed, scientists can improve their transport models and obtain more realistic outcomes on the fate of discharged radionuclides [101,102]. The obtained modeled data should then be compared with the values from sample analyses. Therefore, the monitoring of tritium and radiocarbon activity in the Pacific Ocean will be important during and after the release campaign.
Future radioecological and environmental studies will require additional relevant post-FDNPP accident samples, which will help to gather more relevant 3H and 14C data. For the former, such an effort could be complicated due to its shorter half-life. However, the latter does not have this disadvantage and inorganic and organic molecules labeled with the FDNPP-derived radiocarbon will be part of the environment for millennia. As documented in several papers, tree rings have proven to be able to mirror the 14C concentration in the atmosphere around the FDNPP. Corals or other appropriate organisms could play a similar role in the Pacific Ocean. However, the topic of organically bound tritium (OBT) has been barely explored in the literature regarding the FDNPP accident and deserves more attention. In general, the increased number of 3H and 14C determinations will lead to a better evaluation of the released radionuclide inventories and transport pathways. This knowledge would improve the ability to exploit radionuclides as tracers in various physical and chemical processes in the atmosphere–biosphere–hydrosphere (ocean) ecosystems.
Author Contributions
Conceptualization, J.K. and P.P.P.; writing—original draft preparation, J.K. and I.K.; investigation, J.K; writing—review and editing, P.P.P.; visualization, I.K.; supervision, P.P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Support provided by the Slovak Science and Grant Agency (VEGA-1/0625/21), EU project Advancing University Capacity and Competence in Research, Development and Innovation (ACCORD) ITMS2014, no. 313021X329, and by International Atomic Energy Agency’s Technical Cooperation Program (Project RER7014) is highly acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Povinec, P.P.; Hirose, K.; Aoyama, M.; Tateda, Y. Fukushima Accident: 10 Years After; Elsevier: New York, NY, USA, 2021. [Google Scholar]
- IAEA. The Fukushima Daiichi Accident; IAEA: Vienna, Austria, 2015. [Google Scholar]
- Steinhauser, G. Fukushima’s Forgotten Radionuclides: A Review of the Understudied Radioactive Emissions. Environ. Sci. Technol. 2014, 48, 4649–4663. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, G.; Brandl, A.; Johnson, T.E. Comparison of the Chernobyl and Fukushima Nuclear Accidents: A Review of the Environmental Impacts. Sci. Total Environ. 2014, 470–471, 800–817. [Google Scholar] [CrossRef] [PubMed]
- Masson, O.; Baeza, A.; Bieringer, J.; Brudecki, K.; Bucci, S.; Cappai, M.; Carvalho, F.P.; Connan, O.; Cosma, C.; Dalheimer, A.; et al. Tracking of Airborne Radionuclides from the Damaged Fukushima Dai-Ichi Nuclear Reactors by European Networks. Environ. Sci. Technol. 2011, 45, 7670–7677. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, M. Long-Term Behavior of 137Cs and 3H Activities from TEPCO Fukushima NPP1 Accident in the Coastal Region off Fukushima, Japan. J. Radioanal. Nucl. Chem. 2018, 316, 1243–1252. [Google Scholar] [CrossRef]
- Tsumune, D.; Tsubono, T.; Aoyama, M.; Uematsu, M.; Misumi, K.; Maeda, Y.; Yoshida, Y.; Hayami, H. One-Year, Regional-Scale Simulation of 137Cs Radioactivity in the Ocean Following the Fukushima Dai-Ichi Nuclear Power Plant Accident. Biogeosciences 2013, 10, 5601–5617. [Google Scholar] [CrossRef]
- Aoyama, M.; Uematsu, M.; Tsumune, D.; Hamajima, Y. Surface Pathway of Radioactive Plume of TEPCO Fukushima NPP1 Released 134Cs and 137Cs. Biogeosciences 2013, 10, 3067–3078. [Google Scholar] [CrossRef]
- Kumamoto, Y.; Aoyama, M.; Hamajima, Y.; Murata, A.; Kawano, T. Impact of Fukushima-Derived Radiocesium in the Western North Pacific Ocean about Ten Months after the Fukushima Dai-Ichi Nuclear Power Plant Accident. J. Environ. Radioact. 2015, 140, 114–122. [Google Scholar] [CrossRef]
- UNSCEAR. Sources and Effects of Ionizing Radiation. Report of the United Nations Scientific Committee on the Effects of Atomic Radiation to the General Assembly; UNSCEAR: New York, NY, USA, 2008. [Google Scholar]
- IAEA. Worldwide Marine Radioactivity Studies (WOMARS): Radionuclide Levels in Oceans and Seas; IAEA: Vienna, Austria, 2005. [Google Scholar]
- Akata, N.; Kakiuchi, H.; Shima, N.; Iyogi, T.; Momoshima, N.; Hisamatsu, S. Tritium Concentrations in the Atmospheric Environment at Rokkasho, Japan before the Final Testing of the Spent Nuclear Fuel Reprocessing Plant. J. Environ. Radioact. 2011, 102, 837–842. [Google Scholar] [CrossRef]
- Muranaka, T.; Yamashita, J.; Shima, N. Variation of Tritium Concentration in Coastal Seawater Collected along the Pacific Coast in Aomori Prefecture. Fusion Sci. Technol. 2011, 60, 1264–1267. [Google Scholar] [CrossRef]
- Kokubon, Y.; Fujita, H.; Nkano, M.; Sumiya, S. Tritium Concentration and Diffusion in Seawater Discharged from Tokai Reprocessing Plant. Prog. Nucl. Sci. Technol. 2011, 1, 384–387. [Google Scholar] [CrossRef]
- Diabate, S.; Strack, S. Organically Bound Tritium. Health Phys. 1993, 65, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Workman, W.G.; Korolevych, V.; Davis, P.A. Field Measurements of Key Parameters Associated with Nocturnal OBT Formation in Vegetables Grown under Canadian Conditions. J. Environ. Radioact. 2012, 104, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Baglan, N.; Davis, P.A. Current Understanding of Organically Bound Tritium (OBT) in the Environment. J. Environ. Radioact. 2013, 126, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, J.; Bronić, I.K.; Todorović, N.; Stojković, I.; Barešić, J.; Petrović-Pantić, T. Tritium in Water: Hydrology and Health Implications. In Tritium: Advances in Research and Applications; Janković, M.M., Ed.; Nova Science Publishers: New York, NY, USA, 2018; pp. 157–212. [Google Scholar]
- ICRP. Age-Dependent Doses to Members of the Public from Intake of Radionuclides—Part 1. ICRP Publication 56; ICRP: Oxford, UK, 1990. [Google Scholar]
- Saljoughian, M.; Williams, P. Recent Developments in Tritium Incorporation for Radiotracer Studies. Curr. Pharm. Des. 2000, 6, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Eastoe, C.J.; Watts, C.J.; Ploughe, M.; Wright, W.E. Future Use of Tritium in Mapping Pre-Bomb Groundwater Volumes. Ground Water 2012, 50, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Broecker, W.S.; Peng, T.H.; Ostlund, G. The Distribution of Bomb Tritium in the Ocean. J. Geophys. Res. 1986, 91, 14331. [Google Scholar] [CrossRef]
- Key, R.M.; Kozyr, A.; Sabine, C.L.; Lee, K.; Wanninkhof, R.; Bullister, J.L.; Feely, R.A.; Millero, F.J.; Mordy, C.; Peng, T.H. A Global Ocean Carbon Climatology: Results from Global Data Analysis Project (GLODAP). Global Biogeochem. Cycles 2004, 18, GB4031. [Google Scholar] [CrossRef]
- Zazzeri, G.; Yeomans, E.A.; Graven, H.D.; Acuña Yeomans, E.; Graven, H.D. Global and Regional Emissions of Radiocarbon from Nuclear Power Plants from 1972 to 2016. Radiocarbon 2018, 60, 1068–1081. [Google Scholar] [CrossRef]
- Rubin, S.I.; Key, R.M. Separating Natural and Bomb-Produced Radiocarbon in the Ocean: The Potential Alkalinity Method. Glob. Biogeochem. Cycles 2002, 16, 1105. [Google Scholar] [CrossRef]
- Sweeney, C.; Gloor, E.; Jacobson, A.R.; Key, R.M.; McKinley, G.; Sarmiento, J.L.; Wanninkhof, R. Constraining Global Air-Sea Gas Exchange for CO2 with Recent Bomb 14C Measurements. Glob. Biogeochem. Cycles 2007, 21, GB2015. [Google Scholar] [CrossRef]
- Broecker, W.S.; Sutherland, S.; Smethie, W.; Peng, T.-H.; Ostlund, G. Oceanic Radiocarbon: Separation of the Natural and Bomb Components. Glob. Biogeochem. Cycles 1995, 9, 263–288. [Google Scholar] [CrossRef]
- Peacock, S. Debate over the Ocean Bomb Radiocarbon Sink: Closing the Gap. Glob. Biogeochem. Cycles 2004, 18, GB2022. [Google Scholar] [CrossRef]
- Tauchi, H.; Toyoshima-Sasatani, M.; Nagashima, H.; Shimura, T.; Umata, T.; Tachibana, A. Tritium Biology in Japan: A Search for a New Approach. Fusion Eng. Des. 2018, 128, 28–32. [Google Scholar] [CrossRef]
- Mahaney, B.L.; Meek, K.; Lees-Miller, S.P. Repair of Ionizing Radiation-Induced DNA Double-Strand Breaks by Non-Homologous End-Joining. Biochem. J. 2009, 417, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Stuiver, M.; Polach, H.A. Discussion; Reporting of 14C Data. Radiocarbon 1977, 19, 355–363. [Google Scholar] [CrossRef]
- Hou, X. Rapid Analysis of 14C and 3H in Graphite and Concrete for Decommissioning of Nuclear Reactor. Appl. Radiat. Isot. 2005, 62, 871–882. [Google Scholar] [CrossRef]
- Kakiuchi, H.; Momoshima, N.; Okai, T.; Maeda, Y. Tritium Concentration in Ocean. J. Radioanal. Nucl. Chem. 1999, 239, 523–526. [Google Scholar] [CrossRef]
- Nishihara, K.; Yamagishi, I.; Yasuda, K.; Ishimori, K.; Tanaka, K.; Kuno, T.; Inada, S.; Gotoh, Y. Radionuclide Release to Stagnant Water in the Fukushima-1 Nuclear Power Plant1. J. Nucl. Sci. Technol. 2015, 52, 301–307. [Google Scholar] [CrossRef]
- Xu, S.; Cook, G.T.; Cresswell, A.J.; Dunbar, E.; Freeman, S.P.H.T.; Hou, X.; Jacobsson, P.; Kinch, H.R.; Naysmith, P.; Sanderson, D.C.W.; et al. Radiocarbon Releases from the 2011 Fukushima Nuclear Accident. Sci. Rep. 2016, 6, 36947. [Google Scholar] [CrossRef]
- Povinec, P.P.; Hirose, K.; Aoyama, M. Fukushima Accident: Radioactivity Impact on the Environment; Elsevier: New York, NY, USA, 2013. [Google Scholar]
- Ješkovský, M.; Kaizer, J.; Kontuĺ, I.; Lujaniené, G.; Müllerová, M.; Povinec, P.P. Analysis of Environmental Radionuclides. In Handbook of Radioactivity Analysis: Volume 2; L’Annunziata, M.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137–261. [Google Scholar]
- Hou, X.; Roos, P. Critical Comparison of Radiometric and Mass Spectrometric Methods for the Determination of Radionuclides in Environmental, Biological and Nuclear Waste Samples. Anal. Chim. Acta 2008, 608, 105–139. [Google Scholar] [CrossRef]
- Jakonić, I.; Todorović, N.; Nikolov, J.; Bronić, I.K.; Tenjović, B.; Vesković, M. Optimization of Low-Level LS Counter Quantulus 1220 for Tritium Determination in Water Samples. Radiat. Phys. Chem. 2014, 98, 69–76. [Google Scholar] [CrossRef]
- Lehto, J.; Hou, X. Chemistry and Analysis of Radionuclides: Laboratory Techniques and Methodology; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Vokal, B.; Kobal, I. Radiocarbon Releases at the Krško Nuclear Power Plant. Radiocarbon 1997, 39, 285–292. [Google Scholar] [CrossRef]
- Horvatinčić, N.; Barešić, J.; Krajcar Bronić, I.; Obelić, B. Measurement of Low 14 C Activities in a Liquid Scintillation Counter in the Zagreb Radiocarbon Laboratory. Radiocarbon 2004, 46, 105–116. [Google Scholar] [CrossRef]
- Tamers, M.A. Chemical Yield Optimization of the Benzene Synthesis for Radiocarbon Dating. Int. J. Appl. Radiat. Isot. 1975, 26, 676–682. [Google Scholar] [CrossRef]
- Horvatinčić, N.; Obelić, B.; Krajcar Bronić, I.; Srdoč, D.; Bistrović, R. Sources of Radon Contamination in 14C Dating. Radiocarbon 1995, 37, 749–757. [Google Scholar] [CrossRef]
- Hood, D.; Hatfield, R.; Patrick, C.; Stipp, J.; Tamers, M.; Leidl, R.; Lyons, B.; Polach, H.; Robertson, S.; Zhou, W. Radon Elimination During Benzene Preparation for Radiocarbon Dating by Liquid Scintillation Spectrometry. Radiocarbon 1989, 31, 254–259. [Google Scholar] [CrossRef]
- Theodórsson, P. Simultaneously Measuring 14C and Radon in Benzene Dating Samples. Radiocarbon 2005, 47, 231–234. [Google Scholar] [CrossRef]
- Hou, X. Tritium and 14C in the Environment and Nuclear Facilities: Sources and Analytical Methods. J. Nucl. Fuel Cycle Waste Technol. 2018, 16, 11–39. [Google Scholar] [CrossRef]
- Clarke, W.B.; Jenkins, W.J.; Top, Z. Determination of Tritium by Mass Spectrometric Measurement of 3He. Int. J. Appl. Radiat. Isot. 1976, 27, 515–522. [Google Scholar] [CrossRef]
- Surano, K.A.; Hudson, G.B.; Failor, R.A.; Sims, J.M.; Holland, R.C.; MacLean, S.C.; Garrison, J.C. Heliuim-3 Mass Spectrometry for Low-Level Tritium Analysis of Environmental Samples. J. Radioanal. Nucl. Chem. Artic. 1992, 161, 443–453. [Google Scholar] [CrossRef]
- Jean-Baptiste, P.; Mantisi, F.; Dapoigny, A.; Stievenard, M. Design and Performance of a Mass Spectrometric Facility for Measuring Helium Isotopes in Natural Waters and for Low-Level Tritium Determination by the 3He Ingrowth Method. Int. J. Radiat. Appl. Instrum. Part 1992, 43, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Palcsu, L.; Major, Z.; Köllő, Z.; Papp, L. Using an Ultrapure 4He Spike in Tritium Measurements of Environmental Water Samples by the 3He-Ingrowth Method. Rapid Commun. Mass Spectrom. 2010, 24, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.; Palcsu, L.; Major, Z.; Rinyu, L.; Tóth, I. A Mass Spectrometric Line for Tritium Analysis of Water and Noble Gas Measurements from Different Water Amounts in the Range of Microlitres and Millilitres. Isotopes Environ. Health Stud. 2012, 48, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.J.; McElroy, R.G.C.; Surette, R.A.; Brown, R.M. Tritium Sampling and Measurement. Health Phys. 1993, 65, 610–627. [Google Scholar] [CrossRef]
- Love, A.H.; Hunt, J.R.; Vogel, J.S.; Knezovich, J.P. Improving Tritium Exposure Reconstructions Using Accelerator Mass Spectrometry. Anal. Bioanal. Chem. 2004, 379, 198–203. [Google Scholar] [CrossRef]
- Jull, A.J.T.; Burr, G.S. Accelerator Mass Spectrometry: Is the Future Bigger or Smaller? Earth Planet. Sci. Lett. 2006, 243, 305–325. [Google Scholar] [CrossRef]
- Povinec, P.P.; Litherland, A.E.; von Reden, K.F. Developments in Radiocarbon Technologies: From the Libby Counter to Compound-Specific AMS Analyses. Radiocarbon 2009, 51, 45–78. [Google Scholar] [CrossRef]
- Bronk Ramsey, C.; Ditchfield, P.; Humm, M. Using a Gas Ion Source for Radiocarbon AMS and GC-AMS. Radiocarbon 2004, 46, 25–32. [Google Scholar] [CrossRef]
- Fahrni, S.M.; Wacker, L.; Synal, H.A.; Szidat, S. Improving a Gas Ion Source for 14C AMS. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2013, 294, 320–327. [Google Scholar] [CrossRef]
- Povinec, P.P.; Masarik, J.; Ješkovský, M.; Kaizer, J.; Šivo, A.; Breier, R.; Pánik, J.; Staníček, J.; Richtáriková, M.; Zahoran, M.; et al. Development of the Accelerator Mass Spectrometry Technology at the Comenius University in Bratislava. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2015, 361, 87–94. [Google Scholar] [CrossRef]
- Slota, P.J.; Jull, A.J.T.; Linick, T.W.; Toolin, L.J. Preparation of Small Samples for 14C Accelerator Targets by Catalytic Reduction of CO. Radiocarbon 1987, 29, 303–306. [Google Scholar] [CrossRef]
- Xu, X.; Trumbore, S.E.; Zheng, S.; Southon, J.R.; McDuffee, K.E.; Luttgen, M.; Liu, J.C. Modifying a Sealed Tube Zinc Reduction Method for Preparation of AMS Graphite Targets: Reducing Background and Attaining High Precision. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2007, 259, 320–329. [Google Scholar] [CrossRef]
- Orsovszki, G.; Rinyu, L. Flame-Sealed Tube Graphitization Using Zinc as the Sole Reduction Agent: Precision Improvement of EnvironMICADAS 14C Measurements on Graphite Targets. Radiocarbon 2015, 57, 979–990. [Google Scholar] [CrossRef]
- Xu, X.; Gao, P.; Salamanca, E.G. Ultra Small-Mass Graphitization by Sealed Tube Zinc Reduction Method for AMS 14C Measurements. Radiocarbon 2013, 55, 608–616. [Google Scholar] [CrossRef]
- Matsumoto, T.; Maruoka, T.; Shimoda, G.; Obata, H.; Kagi, H.; Suzuki, K.; Yamamoto, K.; Mitsuguchi, T.; Hagino, K.; Tomioka, N.; et al. Tritium in Japanese Precipitation Following the March 2011 Fukushima Daiichi Nuclear Plant Accident. Sci. Total Environ. 2013, 445–446, 365–370. [Google Scholar] [CrossRef]
- Peterson, H.T.; Baker, D.A. Tritium Production, Releases and Population Doses at Nuclear Power Reactors. Fusion Technol. 1985, 8, 2544–2550. [Google Scholar] [CrossRef]
- Maruoka, T.; Kawamuto, T.; Ohno, T.; Muramatsu, Y.; Matsuzaki, H.; Matsumoto, T.; Aggarwal, P. Tritium and Iodine-129 Concentrations in Precipitation at Tsukuba, Japan, after the Fukushima Daiichi Nuclear Power Plant Accident. Geochem. J. 2017, 51, 449–455. [Google Scholar] [CrossRef]
- Sakakibara, K.; Iwagami, S.; Tsujimura, M.; Abe, Y.; Hada, M.; Pun, I.; Onda, Y. Groundwater Age and Mixing Process for Evaluation of Radionuclide Impact on Water Resources Following the Fukushima Dai-Ichi Nuclear Power Plant Accident. J. Contam. Hydrol. 2019, 223, 103474. [Google Scholar] [CrossRef]
- Kaizer, J.; Aoyama, M.; Kumamoto, Y.; Molnár, M.; Palcsu, L.; Povinec, P.P. Tritium and Radiocarbon in the Western North Pacific Waters: Post-Fukushima Situation. J. Environ. Radioact. 2018, 184–185, 83–94. [Google Scholar] [CrossRef]
- Querfeld, R.; Pasi, A.E.; Shozugawa, K.; Vockenhuber, C.; Synal, H.A.; Steier, P.; Steinhauser, G. Radionuclides in Surface Waters around the Damaged Fukushima Daiichi NPP One Month after the Accident: Evidence of Significant Tritium Release into the Environment. Sci. Total Environ. 2019, 689, 451–456. [Google Scholar] [CrossRef]
- Takahata, N.; Tomonaga, Y.; Kumamoto, Y.; Yamada, M.; Sano, Y. Direct Tritium Emissions to the Ocean from the Fukushima Dai-Ichi Nuclear Accident. Geochem. J. 2018, 52, 211–217. [Google Scholar] [CrossRef]
- Povinec, P.P.; Aoyama, M.; Biddulph, D.; Breier, R.; Buesseler, K.; Chang, C.C.; Golser, R.; Hou, X.L.; Ješkovský, M.; Jull, A.J.T.; et al. Cesium, Iodine and Tritium in NW Pacific Waters-a Comparison of the Fukushima Impact with Global Fallout. Biogeosciences 2013, 10, 5481–5496. [Google Scholar] [CrossRef]
- Ueda, S.; Hasegawa, H.; Kakiuchi, H.; Ochiai, S.; Akata, N.; Hisamatsu, S. Nuclear Accident-Derived 3H in River Water of Fukushima Prefecture during 2011–2014. J. Environ. Radioact. 2015, 146, 102–109. [Google Scholar] [CrossRef]
- Hirao, S.; Kakiuchi, H. Investigation of Atmospheric Tritiated Water Vapor Level around the Fukushima Daiichi Nuclear Power Plant. Fusion Eng. Des. 2021, 171, 112556. [Google Scholar] [CrossRef]
- Hirao, S.; Kakiuchi, H.; Akata, N.; Tamari, T.; Sugihara, S.; Shima, N.; Yokoyama, S.; Tanaka, M. Characterization of Atmospheric Tritiated Water Concentration in the Vicinity of the Fukushima Daiichi Nuclear Power Plant. J. Radioanal. Nucl. Chem. 2022, 331, 3077–3083. [Google Scholar] [CrossRef]
- Machida, M.; Iwata, A.; Yamada, S.; Otosaka, S.; Kobayashi, T.; Funasaka, H.; Takami, M. Estimation of Temporal Variation of Discharged Tritium from Port of Fukushima Dai-Ichi Nuclear Power Plant: Analysis of the Temporal Variation and Comparison with Released Tritium from Japan and Major Nuclear Facilities Worldwide. Trans. At. Energy Soc. Japan 2022, 21, J20.036. [Google Scholar] [CrossRef]
- Povinec, P.P.; Liong Wee Kwong, L.; Kaizer, J.; Molnár, M.; Nies, H.; Palcsu, L.; Papp, L.; Pham, M.K.; Jean-Baptiste, P. Impact of the Fukushima Accident on Tritium, Radiocarbon and Radiocesium Levels in Seawater of the Western North Pacific Ocean: A Comparison with Pre-Fukushima Situation. J. Environ. Radioact. 2017, 166, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, M.; Kajino, M.; Tanaka, T.Y.; Sekiyama, T.T.; Tsumune, D.; Tsubono, T.; Hamajima, Y.; Inomata, Y.; Gamo, T. 134Cs and 137Cs in the North Pacific Ocean Derived from the March 2011 TEPCO Fukushima Dai-Ichi Nuclear Power Plant Accident, Japan. Part Two: Estimation of 134Cs and 137Cs Inventories in the North Pacific Ocean. J. Oceanogr. 2016, 72, 67–76. [Google Scholar] [CrossRef]
- Shirotani, Y.; Inatomi, N.; Miyamoto, K.; Yamada, M.; Kusakabe, M. Distributions of Tritium in the Coastal Waters off Aomori and Iwate Prefectures. Fusion Eng. Des. 2021, 172, 112738. [Google Scholar] [CrossRef]
- Kumamoto, Y.; Murata, A.; Kawano, T.; Aoyama, M. Fukushima-Derived Radiocesium in the Northwestern Pacific Ocean in February 2012. Appl. Radiat. Isot. 2013, 81, 335–339. [Google Scholar] [CrossRef]
- Kaizer, J.; Kumamoto, Y.; Molnár, M.; Palcsu, L.; Povinec, P.P. Temporal Changes in Tritium and Radiocarbon Concentrations in the Western North Pacific Ocean (1993–2012). J. Environ. Radioact. 2020, 218, 106238. [Google Scholar] [CrossRef]
- Kashiwaya, K.; Muto, Y.; Kubo, T.; Ikawa, R.; Nakaya, S.; Koike, K.; Marui, A. Spatial Variations of Tritium Concentrations in Groundwater Collected in the Southern Coastal Region of Fukushima, Japan, after the Nuclear Accident. Sci. Rep. 2017, 7, 12578. [Google Scholar] [CrossRef] [PubMed]
- Shozugawa, K.; Hori, M.; Johnson, T.E.; Takahata, N.; Sano, Y.; Kavasi, N.; Sahoo, S.K.; Matsuo, M. Landside Tritium Leakage over through Years from Fukushima Dai-Ichi Nuclear Plant and Relationship between Countermeasures and Contaminated Water. Sci. Rep. 2020, 10, 19925. [Google Scholar] [CrossRef]
- Aoyama, M.; Thébault, H.; Hamajima, Y.; Charmasson, S.; Arnaud, M.; Duffa, C. 137Cs and Tritium Concentrations in Seawater off the Fukushima Prefecture: Results from the SOSO 5 Rivers Cruise (October 2014). In Oceanography Challenges to Future Earth; Springer International Publishing: Cham, Switzerland, 2019; pp. 407–409. [Google Scholar]
- Sakuma, K.; Machida, M.; Kurikami, H.; Iwata, A.; Yamada, S.; Iijima, K. A Modeling Approach to Estimate 3H Discharge from Rivers: Comparison of Discharge from the Fukushima Dai-Ichi and Inventory in Seawater in the Fukushima Coastal Region. Sci. Total Environ. 2022, 806, 151344. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, T.; Kakiuchi, H.; Tauchi, H.; Yamamoto, T.; Yamamoto, I. Discussions on Tritiated Water Treatment for Fukushima Daiichi Nuclear Power Station. Fusion Sci. Technol. 2020, 76, 430–438. [Google Scholar] [CrossRef]
- Tanaka, K.; Shimada, A.; Hoshi, A.; Yasuda, M.; Ozawa, M.; Kameo, Y. Radiochemical Analysis of Rubble and Trees Collected from Fukushima Daiichi Nuclear Power Station. J. Nucl. Sci. Technol. 2014, 51, 1032–1043. [Google Scholar] [CrossRef]
- Kuwata, H.; Misono, T.; Fujiwara, K.; Takeishi, M.; Manabe, S.; Kitamura, A. Rapid Tritium Analysis for Marine Products in the Coastal Area of Fukushima. Radiat. Environ. Med. 2020, 9, 28–34. [Google Scholar]
- Tatebe, H.; Yasuda, I. Oyashio Southward Intrusion and Cross-Gyre Transport Related to Diapycnal Upwelling in the Okhotsk Sea. J. Phys. Oceanogr. 2004, 34, 2327–2341. [Google Scholar] [CrossRef]
- Ding, L.; Ge, T.; Gao, H.; Luo, C.; Xue, Y.; Druffel, E.R.M.; Wang, X. Large Variability of Dissolved Inorganic Radiocarbon in the Kuroshio Extension of the Northwest North Pacific. Radiocarbon 2018, 60, 691–704. [Google Scholar] [CrossRef]
- Xu, S.; Cook, G.T.; Cresswell, A.J.; Dunbar, E.; Freeman, S.P.H.T.; Hastie, H.; Hou, X.; Jacobsson, P.; Naysmith, P.; Sanderson, D.C.W.; et al. 14C Levels in the Vicinity of the Fukushima Dai-Ichi Nuclear Power Plant Prior to the 2011 Accident. J. Environ. Radioact. 2016, 157, 90–96. [Google Scholar] [CrossRef]
- Chen, B.; Xu, S.; Cook, G.T.; Freeman, S.P.H.T.; Hou, X.; Philip, C.L.; Katsuhiko, N. Local Variance of Atmospheric 14C Concentrations around Fukushima Dai-Ichi Nuclear Power Plant from 2010 to 2012. J. Radioanal. Nucl. Chem. 2017, 314, 1001–1007. [Google Scholar] [CrossRef]
- Varga, T.; Palcsu, L.; Ohta, T.; Mahara, Y.; Jull, A.J.T.; Molnár, M. Variation of 14C in Japanese Tree Rings Related to the Fukushima Nuclear Accident. Radiocarbon 2019, 61, 1029–1040. [Google Scholar] [CrossRef]
- Aramaki, T.; Nakaoka, S.; Terao, Y.; Kushibashi, S.; Kobayashi, T.; Osonoi, Y.; Mukai, H.; Tohjima, Y. Variation of Surface Radiocarbon in the North Pacific During Summer Season 2004–2016. Radiocarbon 2019, 61, 1367–1375. [Google Scholar] [CrossRef]
- Key, R.M.; Quay, P.D.; Schlosser, P.; McNichol, A.P.; von Reden, K.; Schneider, R.J.; Elder, K.L.; Stuiver, M.; Göte Östlund, H. Woce Radiocarbon IV: Pacific Ocean Results; P10, P13N, P14C, P18, P19 & S4P. Radiocarbon 2002, 44, 239–392. [Google Scholar]
- Kumamoto, Y.; Murata, A.; Kawano, T.; Watanabe, S.; Fukasawa, M. Decadal Changes in Bomb-Produced Radiocarbon in the Pacific Ocean from the 1990s to 2000s. Radiocarbon 2013, 55, 1641–1650. [Google Scholar] [CrossRef]
- Xu, S.; Cook, G.T.; Cresswell, A.J.; Dunbar, E.; Freeman, S.P.H.T.; Hastie, H.; Hou, X.; Jacobsson, P.; Naysmith, P.; Sanderson, D.C.W. Radiocarbon Concentration in Modern Tree Rings from Fukushima, Japan. J. Environ. Radioact. 2015, 146, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Matsunaka, T.; Sasa, K.; Takahashi, T.; Matsumura, M.; Satou, Y.; Shen, H.; Sueki, K.; Matsuzaki, H. Pre-and Post-Accident 14C Activities in Tree Rings near the Fukushima Dai-Ichi Nuclear Power Plant. Radiocarbon 2019, 61, 1633–1642. [Google Scholar] [CrossRef]
- Paterne, M.; Evrard, O.; Hatté, C.; Laceby, P.J.; Nouet, J.; Onda, Y. Radiocarbon and Radiocesium in Litter Fall at Kawamata, ~45 Km NW from the Fukushima Dai-Ichi Nuclear Power Plant (Japan). J. Radioanal. Nucl. Chem. 2019, 319, 1093–1101. [Google Scholar] [CrossRef]
- Buesseler, K.O. Opening the floodgates at Fukushima. Science 2020, 369, 621–622. [Google Scholar] [CrossRef]
- Men, W. Discharge of contaminated water from the Fukushima Daiichi Nuclear Power Plant Accident into the Northwest Pacific: What is known and what needs to be known. Mar. Pollut. Bull. 2021, 173, 112984. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, G.; Zhang, M.; Wang, G.; de With, G.; Bezhenar, R.; Maderich, V.; Xia, C.; Zhao, B.; Jung, K.T.; et al. Transport and dispersion of tritium from the radioactive water of the Fukushima Daiichi nuclear plant. Mar. Pollut. Bull. 2021, 169, 112515. [Google Scholar] [CrossRef] [PubMed]
- Bezhenar, R.; Takata, H.; de With, G.; Maderich, V. Planned release of contaminated water from the Fukushima storage tanks into the ocean: Simulation scenarios of radiological impact for aquatic biota and human from seafood consumption. Mar. Pollut. Bull. 2021, 173, 112969. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).