Raman Spectroscopy for Early Detection of Cervical Cancer, a Global Women’s Health Issue—A Review
Abstract
1. Introduction
1.1. Cervical
1.2. Cervical Cancer Screening and Diagnosis
1.3. Raman Spectroscopy
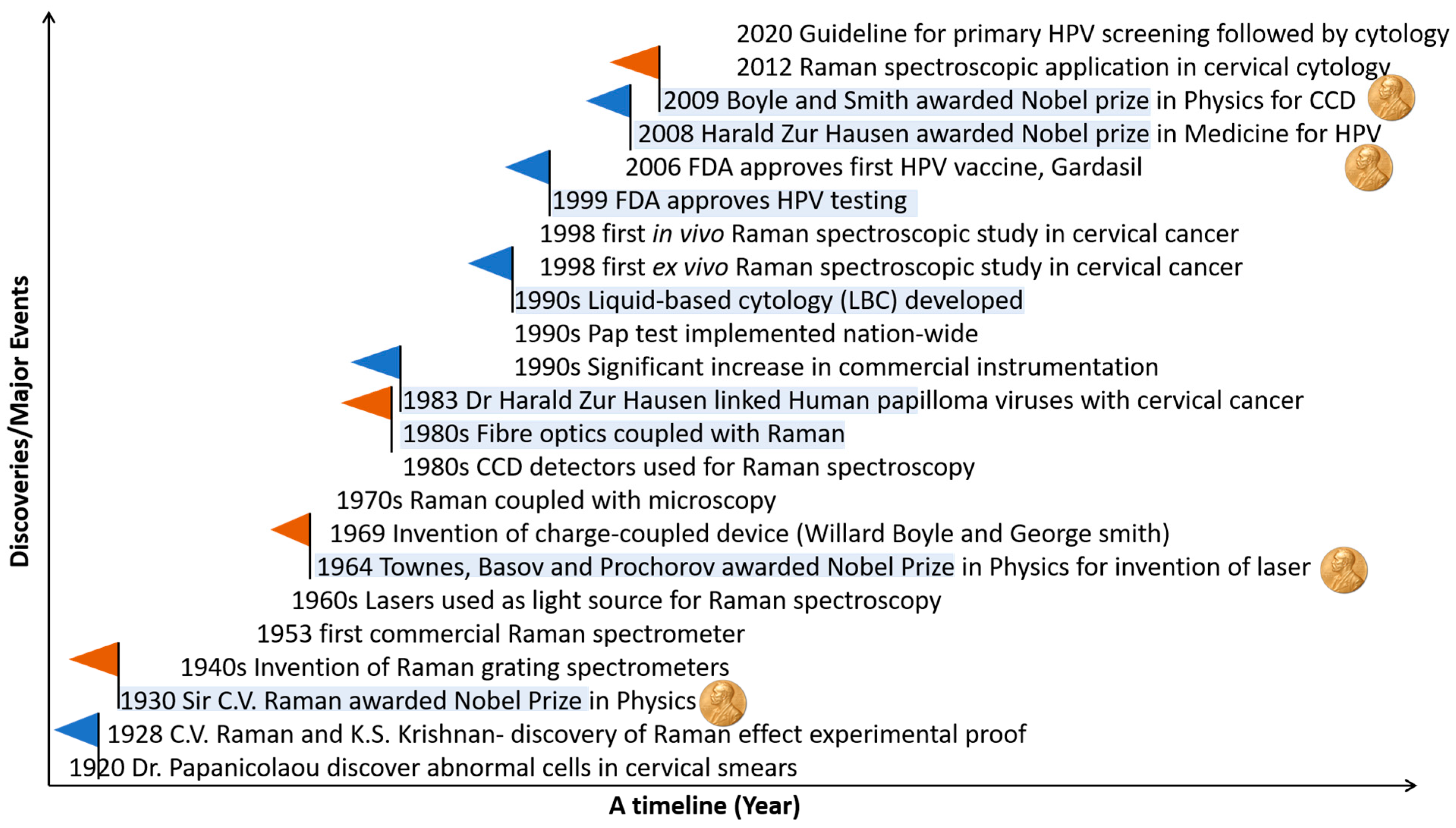
1.4. Evolution of Raman Spectroscopy and Its Application in Cervical Cancer
2. Cytology
2.1. Cell Pellets
| Year | Authors (Research Group) | Sample/Patient Numbers | Sample Type/Sample Prep/Substrate | Raman Parameters | Data Analysis Methodology | Main Findings | Reference |
|---|---|---|---|---|---|---|---|
| 2012 | Vargis et al. (Mahadevan-Jansen group) | 50 patient samples—25 HPV negative and 25 HPV positive | Centrifugation and washing with sterile water Pellets of exfoliated cells on calcium fluoride slides | Renishaw Invia Raman microscope 785 nm laser, ∼30 mW at the sample 50X/0.75 NA objective lens Spectral resolution ∼6 cm−1 45 to 60 s acquisition, 3 accumulations | Sparse multinomial logistic regression (SMLR) | Discrimination of HPV-positive and HPV-negative cytology samples Spectral differences: lipid, amino acid, protein, and DNA content Accuracy: 98.5% | [31] |
| 2013 | Rubina et al. (Krishna group) | 94 patient samples—45 negative cytology and 49 cervical cancer cytology | Centrifugation and washing with saline Pellets of exfoliated cells on calcium fluoride slides Red blood cell (RBC) lysis buffer treatment | Horiba- Jobin-Yvon fibre-optic Raman microprobe system 785 nm laser ∼40 mW at the sample 40X/0.65 NA objective lens Spectral resolution ∼4 cm−1 6 s acquisition, 3 accumulations | Principal Component Analysis Linear Discriminant Analysis (PCA-LDA) | Treatment of cell pellet with RBC lysis buffer to remove blood contamination Discrimination of negative and cancer cytology Spectral differences: protein Accuracy: 80% | [32] |
| 2014 | Bonnier et al. (Lyng group) | 63 patient samples—50 negative cytology and 13 high-grade cytology | ThinPrep method Single exfoliated cells on ThinPrep glass slides Hydrogen peroxide pre-treatment | Horiba- Jobin-Yvon XploRA Raman microscope 532 nm laser ∼8 mW at the sample 100X/0.9 NA objective lens Spectral resolution ∼3 cm−1 10 s acquisition, 3 accumulations | PCA | Pre-treatment of ThinPrep slides with hydrogen peroxide to eliminate variability due to blood contamination Discrimination of negative and high-grade cytology Spectral differences: DNA, RNA | [35] |
| 2016 | Ramos et al. (Lyng group) | 166 patient samples—88 negative cytology, 35 low-grade cytology, and 43 high-grade cytology | Sample preparation as for Bonnier et al. [28] | Raman set up as for Bonnier et al., 30 s acquisition, 2 accumulations | PCA-LDA | Discrimination of negative, low-grade, and high-grade cytology Spectral differences: lipids, nucleic acids, and proteins Sensitivity: 90.91–100% Specificity: 97.24–100% | [36] |
| 2017 | Kearney et al. (Lyng group) | 80 patient samples—30 negative cytology, 50 high-grade cytology | Sample preparation as for Bonnier et al. [28] | Raman set up as for Bonnier et al., 30 s acquisition, 2 accumulations | PCA-Factorial Discriminant Analysis (FDA) | Raman spectral signatures of superficial, intermediate, and parabasal cells High variability in spectra from cytoplasm due to glycogen Discrimination of negative and high-grade cytology Spectral differences: lipids, nucleic acids and proteins Sensitivity: 92%, Specificity: 97% | [37] |
| 2018 | Traynor et al. (Lyng group) | 60 patient samples—45 negative cytology and 15 high-grade cytology | Sample preparation as for Bonnier et al. [28] | Raman set up as for Bonnier et al., 30 s acquisition, 2 accumulations | PLSDA | Biochemical changes due to high-grade cytology more pronounced than hormone related changes Discrimination of negative and high-grade cytology Spectral differences: glycogen, nucleic acids, and proteins Sensitivity: 96–98%, Specificity: 97–98% | [38] |
| 2018 | Duraipandian et al. (Lyng group) | 35 patient samples—18 negative cytology and 17 high-grade cytology | Sample preparation as for Bonnier et al. [28] | Raman set up as for Bonnier et al., 30 s acquisition, 2 accumulations | PCA-LDA and PLSDA | Discrimination of negative and high-grade cytology (morphologically normal superficial and intermediate cells) Spectral differences: glycogen, nucleic acids, and proteins Sensitivity: 75.6% (PCA-LDA), 96.1% (PLSDA) Specificity: 84.5% (PCA-LDA), 93.5% (PLSDA) | [39] |
| 2018 | Traynor et al. (Lyng group) | 30 patient samples—15 negative cytology and 15 high-grade cytology | Sample preparation as for Bonnier et al. [28] | Raman set up as for Bonnier et al. 30 s acquisition, 2 accumulations | PLSDA | Pre-treatment of ThinPrep vial with hydrogen peroxide to remove excessive blood contamination (blood scale index 2–3) Discrimination of negative and high-grade cytology (morphologically normal superficial and intermediate cells) Spectral differences: glycogen, nucleic acids, and proteins Sensitivity: 82–92%, Specificity: 87–93% | [40] |
| 2018 | Hole et al. (Krishna group) | 66 patient samples—28 negative cytology and 38 cervical cancer cytology | Centrifugation and washing with saline Pellets of exfoliated cells on calcium fluoride slides Red blood cell (RBC) lysis buffer treatment | Horiba-Jobin-Yvon fibre-optic Raman microprobe system 785 nm laser ∼40 mW at the sample 40X/0.65 NA objective lens Spectral resolution ∼4 cm−1 15 s acquisition time, 3 accumulations | PCA-LDA | Discrimination of negative and cancer cytology Spectral differences: DNA, protein Accuracy: 84% | [34] |
| 2019 | Aljouch et al. (El-Mashtoly/Gerwert group) | 30 patient samples—10 negative, 10 low-grade and 10 high-grade cytology | Single exfoliated cells prepared using a cytospin centrifuge onto quartz slides | WITec Raman microscope 532 nm laser 60X/1.0 NA objective lens, water immersion | Deep convolutional neural networks (DCNN) | Raman imaging of single exfoliated cells Discrimination of negative, low-grade, and high-grade cytology Spectral differences: lipids, proteins, polysaccharides, and nucleic acids Accuracy: 94–100% | [41] |
| 2020 | X. Zheng et al. (Wu group) | 63 patient samples 33-normal 30- HR-HPV | 5 µL of preserved cell samples on aluminum foil, dried at room temperature | LabRam HR evolution with 532 nm laser source was focused using 50X objective | PCA-LDA | The authors observed the diagnostic accuracy of 99.4% | [42] |
| 2020 | Sitarz et al. (Kaczor group) | 96 patient samples––negative, low-grade, high-grade, and cancer cytology and HPV- and HPV+ | Single exfoliated cells fixed with 2.5% glutaraldehyde, washed in PBS, and placed on calcium fluoride slides | WITec Raman microscope 532 nm laser ∼28 mW at the sample 63X/1.0 NA objective lens, water immersion Spectral resolution ∼3 cm−1 | Cluster analysis (CA) | Increased glycogen metabolism with HPV infection—for cells with large nuclear diameter, glycogen decreased in HPV positive compared to HPV negative samples | [43] |
| 2020 | Karunakaran et al. (Maiti group) | 124 patient samples comprising 47 negative, 41 high-grade, and 36 cancer cytology | Single exfoliated cells, cell pellets and extracted DNA incubated with gold nanoparticles (AuNPs, 40–45 nm) on glass slide | WITec Raman microscope 633 nm laser 20X objective lens | Support vector machines (SVM) | Discrimination of negative, high-grade, and cancer cytology Spectral differences—nucleic acids and amino acids Accuracy: 94.46% (single exfoliated cells), 71.6% (cell pellets) and 97.72% (extracted DNA) | [44] |
| 2021 | Karunakaran et al. (Maiti group) | 9 patient samples comprising 3 negative, 3 high-grade, and 3 cancer cytology | Density gradient centrifugation Single exfoliated cells on glass slide Incubation with SERS nanotag for 45 min | WITec Raman microscope 633 nm laser 10 mW power 20X objective lens Spectral resolution ∼1 cm−1 10 s acquisition, 3 accumulations | n/a | SERS detection of cervical cancer biomarkers p16 and Ki67 in single exfoliated cells | [45] |
| 2021 | Sitarz et al. (Kaczor group) | 63 patient samples comprising negative, low-grade, high-grade, and cancer cytology and HPV- and HPV+ | Sample preparation as for Sitarz et al. [36] | Raman set up as for Sitarz et al. | K means cluster analysis (KMCA) | Dual switch of lipid metabolism—decreased lipid in low-grade cytology and increased lipid in high-grade and cancer cytology compared to negative cytology | [46] |
| 2021 | Traynor et al. (Lyng group) | 60 patient samples for training set—30 HPV DNA positive, mRNA negative and 30 HPV DNA positive, mRNA positive 14 blinded patient samples for test set | Sample preparation as for Bonnier et al. [28] | Raman set up as for Bonnier et al. 30 s acquisition, 2 accumulations | PLSDA | Discrimination of transient and transforming HPV infections (morphologically normal superficial and intermediate cells) Spectral differences: glycogen, nucleic acids and proteins Accuracy: 93% | [47] |
| 2022 | Traynor et al. (Lyng group) | 662 patient samples for training set—326 negative cytology, 200 low-grade cytology and 136 high-grade cytology 69 blinded patient samples for test set | Sample preparation as for Bonnier et al. [28] | Raman set up as for Bonnier et al. 30 s acquisition, 2 accumulations | PLSDA | Discrimination of negative, CIN1, and CIN2+ samples (morphologically normal superficial and intermediate cells) Spectral differences: glycogen, nucleic acids, and proteins Accuracy: 91.3% | [48] |
2.2. Single Exfoliated Cells
2.3. Raman Imaging
2.4. SERS
3. Ex Vivo Studies on Cervical Tissues
| Year | Authors (Research Group) | Sample/Patient Numbers | Sample Type/Sample Prep/Substrate | Raman Parameters | Data Analysis Methodology | Main Findings | Reference |
|---|---|---|---|---|---|---|---|
| 1998 | Mahadevan-Jansen et al. (Rebecca Richard-Kortum’s group) | 36 biopsies from 18 patients | Tissues snap-frozen in liquid nitrogen and stored at −85 ºC, until measurements | A 40 mW GaAlAs diode laser (Diolite 800, LiCONix, Santa Clara, CA, USA) was used to excite samples at 789 nm through a 200 Wm core diameter glass optical fibre | PCA | Discrimination of inflammation and metaplasia from squamous intra-epithelial lesions | [49] |
| 2008 | Vidyasagar et al. (Krishna group) | 25 malignant tissues, 25 samples after radiotherapy and 16 normal tissues | Tissue samples collected in saline. | In house-built Raman setup, which used 785 nm laser source and HR 300 spectrograph coupled to liquid nitrogen-cooled CCD was used. | PCA | Classification between responding and nonresponding tissues. | [50] |
| 2013 | Rubina et al. (Krishna group) | 11 normal, 16 tumor, 14 post radiation | Tissues were collected in PBS and stored in liquid nitrogen. | LabRam equipped with a 785 nm laser source and a spectrograph with 950 groves/mm and CCD as detector was used. | PC-LDA | Differentiation between pre- and post-treated tumor tissues. | [51] |
| 2014 | Rashid et al. (Lyng group) | 5 NILM, 2LSIL, 10 HSIL (5 CIN 2, 5 CIN 3) and 3 carcinomas in-situ | 10-micron thickness formalin fixed paraffin cervical tissue was cut and mounted on a calcium fluoride slide and dewaxed. | Horiba Jobin Yvon HR800 Raman microscope with a 785 nm laser source, 100X air objective. The spot size was ~1 micron on the sample, the confocal hole was 100 microns on a CCD detector for the range between 400–1800 cm−1 using 300 lines/mm diffraction grating | KMCA and PCA | Differentiation of NILM cervical tissue into three layers—stroma, basal, and superficial layers. For HSIL tissue with normal and abnormal regions, the normal region was not normal as in the case of NILM samples. | [52] |
| 2015 | Daniel et al. (Ganesan group) | 36 normal and 25 cervical cancer samples | Fresh frozen tissues sliced to 20-micron thickness were mounted on a quartz slide. | LabRam HR 800, 784.12 nm laser was focused using a 50X objective, Polarized Raman spectra were obtained by placing an analyzer along the parallel and perpendicular | LDA | Polarized Raman spectroscopy provided better classification. | [53] |
| 2019 | Zheng et al. (Yue group) | 95 cases- 45 cervical adenocarcinomas 50 cervical squamous cell carcinomas | 10-micron FFPP tissue sections were dewaxed. | LabRam HR evolution with 532 nm laser source was focused using 50X objective, 100 um step-size | PCA-SVM model | Differentiation between cervical adenocarcinoma and cervical squamous cell carcinoma | [54] |
| 2021 | Zhang et al. (Lv group) | 49 inflammation samples, 29 LSIL samples and 45 HSIL samples. | Formalin-fixed tissue embedded in paraffin, cut into 10-micron thick tissue sections and dewaxed | Labram HR evolution using an excitation wavelength of 532 nm and focused using a 50X objective lens was employed, the laser spot at the sample was 6 microns, and the power on the sample surface was 100 mW. | airPLS-PLS-KNN and airPLS-PLS-ELM; feature fusion-KNN and feature fusion-ELM. | Accuracy of the model increased by 5.38% and 2.7% after feature fusion. | [55] |
| 2021 | Yang et al. (Lv group) | 45 cervicitis samples, 29 LSIL, 44 HSIL, 39 SCC and 38 adenocarcinoma samples. | Formalin-fixed tissue embedded in paraffin, cut into 10-micron thick tissue sections and dewaxed | LabRam HR evolution with an excitation wavelength of 532 nm was used. | KNN, ELM, ABC-SVM, CS-SVM, PSO-SVM and CNN-LSTM | Classification of cervicitis, LSIL, HSIL, SCC and adenocarcinoma | [56] |
| 2021 | Wang et al. (Wu group) | 210 samples 60-Cervicitis, 30-CIN I, 30-CIN II, 30-CIN III, 30-SCC, 30-Adenocarcinoma | Dewaxed formalin-fixed tissue sections | A confocal Raman micro-spectrometer (LabRAM HR Evolution), 532 nm laser source, 25 NM laser power, 50X objective (NA = 0.5) | SVM | Classification of cervicitis, CIN I, CIN II, CIN III, SCC and adenocarcinoma R | [57] |
4. In Vivo Studies on Cervical Tissues
| Year | Authors | Patient Numbers | Raman Parameter | Data Analysis Methodology | Main Findings | Reference |
|---|---|---|---|---|---|---|
| 1998 | Mahadevan-Jansen et al. (Richard-Kortum group) | No details given | Laser-789 nm diode laser, laser power 15 mW, spot size 900 µm, an imaging spectrograph and a CCD camera, Fiber-optic probe | Peak ratios | The Raman probe can be used to measure NIR in-vivo Raman Spectra from the cervix, and it suggested that laser power can be up to 80 mW, via Monte Carlo and thermal modelling prediction | [58] |
| 2001 | Utzinger et al. (Mahadevan-Jansen group) | 13 patients 24 spectra | Laser-789 nm diode laser, laser power 15–16.5 mW, an imaging spectrograph and a CCD camera, Fiber-optic probe-12 mm head, spectral acquisition time-60 to 180 s | Peak ratios | In squamous dysplastic tissue, the ratio of 1454 to 1656 cm−1 is higher and 1330 to 1454 cm−1 is lower, than in other tissue types | [59] |
| 2007 | Robichaux-Viehoever et al. (Mahadevan-Jansen group) | 79 patients | Laser-785 nm diode laser, fibre-optic probe (Visionex Inc., Atlanta, GA), an imaging spectrograph, liquid nitrogen cooled charge-coupled device (CCD) camera all controlled with a laptop computer, laser power-80 mW, spectral acquisition time-5 to 15 s | Peak ratios | Raman spectroscopy can distinguish between high-grade dysplasia and benign tissue with 89% sensitivity and 81% specificity | [60] |
| 2009 | Kanter et al. (Mahadevan-Jansen group) | 43 patients | Laser-785 nm diode laser, fiber optic probe, an imaging spectrograph, thermo-electrically cooled charge-coupled device (CCD) camera, Laser power-80 mW, Penetration depth ~300 um, spectral acquisition time 3 s | MRDF-SMLR | Improvement in the classification efficiency from 88 to 94% by incorporating the hormonal status in the menstrual cycle into the classification algorithm | [61] |
| 2009 | Kanter et al. (Mahadevan-Jansen group) | 122 patients | Laser-785 nm diode laser, fiber optic probe, imaging spectrograph, thermo-electrically cooled charge coupled device (CCD) camera, Laser power-80 mW, spectral acquisition time 3 s | MRDF-SMLR algorithm | Stratifying the menopause data can improve the classification efficiency of LGSIL to 97% (as compared to previous reports- 74%) | [62] |
| 2009 | Kanter et al. (Mahadevan-Jansen group) | 66 patients | Laser-785 nm diode laser, fiber optic probe, an imaging spectrograph, thermo-electrically cooled charge-coupled device (CCD) camera, Laser power-80 mW, spectral acquisition time 5 s | LDA, MRDF-SMLR | High-grade spectra classify with 95%, and low-grade spectra classified with 74% classification efficiency, improving classification sensitivity to 98% and specificity to 96% | [63] |
| 2009 | Mo et al. (Zhiwei Huang group) | 46 patients | Laser-785 nm, a high-throughput spectrometer, equipped with an NIR-enhanced charge-coupled device (CCD) detector, and fiber-optic Raman probe, 100 mW laser power with 1 s acquisition time | PCA-LDA | High wavenumber in vivo Raman Spectroscopy yielded a diagnostic 93.5 % sensitivity and 97.8% specificity. | [64] |
| 2012 | Duraipandian et al. (Zhiwei Huang group) | 44 patients | Laser-785 nm, a high-throughput spectrometer, equipped with a NIR-enhanced charge-coupled device (CCD) detector, and a custom-made fiber-optic Raman probe with NIR-coated Sapphire ball lens, 100 mW laser power with 1 s acquisition time | PLS-DA | Simultaneous fingerprint and high wavenumber in vivo Raman Spectroscopy can classify dysplasia and normal cervix tissue with 82% classification accuracy. | [65] |
| 2013 | Duraipandian et al. (Zhiwei Huang group) | 84 patients | Diode laser-785 nm, a high-throughput spectrometer, equipped with a NIR-enhanced charge-coupled device (CCD) detector, and a custom-made fibre-optic Raman probe, 100 mW laser power with 1 s acquisition time | PCA-LDA | 84.1% classification accuracy (81% sensitivity and 87% specificity) Best classification achieved using confocal Raman spectroscopy compared to composite NIR AF/Raman spectroscopy or NIR AF spectroscopy alone. | [66] |
| 2014 | Rubina et al. (Krishna group) | 93 patients | HE-785 commercial Raman spectrometer, diode laser 785, charge-coupled device (CCD) detector, Custom-made Raman Probe, laser power 80 mW and 5-s acquisition time | PCA-LDA | 97% classification efficiency for normal and tumour tissue Utility of vaginal tissue as a control | [67] |
| 2016 | Rubina et al. (Krishna group) | 46 patients | Raman System: HE-785 commercial Raman spectrometer, diode laser 785, charge-coupled device (CCD) detector, Custom-made Raman Probe, laser power 80 mW and 5-s acquisition time Diffuse-reflectance System: Tungsten Halogen lamp, fibre-optic coupled spectrometer, control via laptop, and bifurcated probe (ZR 400-5 VIS/NIR, ocean optics) | PCA-LDA | Classification efficiency of Raman spectroscopy (sensitivity 91%, and specificity 96%) and diffuse reflectance spectroscopy (sensitivity 85%, and specificity 95%). | [68] |
5. Studies on Biofluids
| Year | Authors (research Group) | Sample/Patient Numbers | Sample Type/Sample Prep/Substrate | Raman Parameters | Data Analysis Methodology | Main Findings | Reference |
|---|---|---|---|---|---|---|---|
| 2013 | Feng et al. (Zeng group) | 110 samples––60 cervical cancer patients, 50 healthy volunteers | After collection and centrifugation, plasma was mixed 1:1 with silver colloidal nanoparticles and incubated for 1.5 h at 4 °C. A drop of plasma on the aluminium plate | Renishaw Raman spectrometer, 785 nm laser 20X objective lens Spectral resolution 2 cm−1 10 s acquisition | Principal Component Analysis Linear Discriminant Analysis (PCA-LDA) | Discrimination of cancer and healthy control serum samples Spectral differences: amino acids, saccharides and esters Sensitivity: 96.7%, Specificity: 92% | [69] |
| 2014 | Gonzalez-Solıs et al. (Palomares-Anda group) | 42 samples—3 precancer and 19 cervical cancer patients, 20 healthy volunteers | The serum was frozen in liquid nitrogen after collection and centrifugation A drop of serum on aluminium substrate | Jobin-Yvon LabRAM HR800 Raman spectrometer, 830 nm laser, ∼17 mW at the sample 50X objective lens Spectral resolution ∼0.6 cm−1 20 to 40 s acquisition | Principal Component Analysis (PCA) | Discrimination of precancer, cancer, and healthy control serum samples Spectral differences: glutathione, tryptophan, β carotene, and amide III | [70] |
| 2019 | Raja et al. (Ganesan group) | 48 samples—18 cervical cancer patients and 30 healthy volunteers | Plasma spectra measured in quartz cuvettes immediately after collection and centrifugation | Jobin-Yvon LabRAM HR800 Raman spectrometer, 785 nm laser, ∼12 mW at the sample 60 s acquisition, 2 accumulations | PCA-LDA | Discrimination of cancer and healthy control plasma samples Spectral differences: nucleic acids, protein, tyrosine, tryptophan, lipids, and β-carotene Sensitivity: 94.4%, Specificity: 96.7% | [72] |
| 2020 | Lu et al. (Cao group) | 150 patient samples—30 healthy subjects, 30 CINI, 30 CINII, 30 CINIII, and 30 cervical cancer patients | Serum frozen at −80 °C after collection and centrifugation SERS tag—Au-Ag nanoshuttles (Au-AgNS) and Capture substrate—hydrophobic filter paper-based Au nanoflowers (AuNF) | No detail given | n/a | SERS-based simultaneous detection of squamous cell carcinoma antigen (SCCA) and osteopontin (OPN) in serum Detection limit: 8.628 pg/mL for SCCA and 4.388 pg/mL for OPN SERS intensities at 1593 cm−1 (SCCA) and 1334 cm−1 (OPN) increased in serum from cervical cancer and precancer patients compared to healthy subjects Good agreement between SCCA and OPN levels measured by SERS and ELISA in serum samples | [73] |
| 2021 | Shrivastava et al. (Singh group) | 93 samples—63 cervical cancer patients and 30 healthy volunteers | Serum frozen at −20 °C after collection and centrifugation | WITec Raman alpha 300R Raman microscope, 532 nm laser, ∼28 mW at the sample 50X/0.8 NA objective lens 5 s acquisition, 5 accumulations | PCA-LDA | Discrimination of cancer and healthy control serum samples and of patients at different stages of chemoradiotherapy (before, during, after treatment) Spectral differences: glycogen, phenylalanine, tryptophan, amide III, nucleic acids, glutathione and β carotene (control vs. cancer); phospholipids, glutathione, proteins, and nucleic acids (during chemoradiotherapy) Sensitivity: 50–92.5%, Specificity: 25–85.7% | [71] |
| 2021 | Xia et al. (Cao group) | 150 patient samples—30 healthy subjects, 30 CINI, 30 CINII, 30 CINIII, and 30 cervical cancer patients | Serum frozen at −80 °C after collection and centrifugation SERS immunoprobes based on monoclonal antibody-coupled and Raman reporter-labeled nano-Ag polydopamine nanospheres (PDA@Ag-NPs) | Renishaw inVia Raman microscope, 785 nm laser 5 mW power 50X objective lens 1 s acquisition | n/a | SERS-based lateral flow assay for simultaneous detection of SCCA and cancer antigen 125 (CA125) in serum Detection limit: 8.093 pg/mL for SCCA and 7.370 pg/mL for CA125 SERS intensities at 1083 cm−1 (SCCA) and 1330 cm−1 (CA125) increased in serum from cervical cancer and precancer patients compared to healthy subjects Good agreement between SCCA and CA125 levels measured by SERS and ELISA in serum samples | [74] |
| 2021 | Liu et al. (Cao/Lu group) | 160 patient samples—40 chronic cervicitis, 40 LSIL, 40 HSIL and 40 cervical cancer patients | Serum frozen at −80 °C after collection and centrifugation SERS tag—Au-Ag nanoshells (Au–AgNS) Capture substrate—Au–Ag nanobox (Au–AgNB) array | Raman spectrometer, 785 nm laser 5 mW power 50X objective lens 10 s acquisition | n/a | SERS-based simultaneous detection of SCCA and survivin in serum Detection limit: 6 pg/mL for SCCA and 5 pg/mL for survivin SERS intensities at 1081 cm−1 (SCCA) and 1327 cm−1 (survivin) increased in serum from cervical cancer and precancer patients compared to patients with cervicitis Good agreement between SCCA and survivin levels measured by SERS and ELISA in serum samples | [75] |
| 2021 | Panikar et al. (Del Toro-Arreola/De La Rosa group) | 10 patient samples— 9 cervical cancer 1 healthy subject | Serum frozen at −80 °C after collection and centrifugation SERS nanoprobe—anti-B7-H6@ATP@AuNPs Capture substrate -zwitterionic L-cysteine substrate | Renishaw inVia Raman Microscope, 785 nm laser, power 2.4 mW 50X objective lens 20 s acquisition, 10 accumulations | n/a | SERS-based detection of B7-H6 in serum Detection limit: 10.8 fg/mL for B7-H6 SERS intensities at 731 cm−1 increased in serum from cervical cancer patients compared to healthy subject Good agreement between B7-H6 levels measured by SERS and ELISA in serum samples and 100-fold increase in SERS detection compared to ELISA for 10−14 M B7-H6 | [76] |
6. Summary and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Walboomers, J.M.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.F.; Peto, J.; Meijer, C.J.L.M.; Muñoz, N. Human Papillomavirus Is a Necessary Cause of Invasive Cervical Cancer Worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.N.; Apter, D.; Kitchener, H.; Castellsague, X.; Teixeira, J.C.; Skinner, S.R.; et al. Efficacy of Human Papillomavirus (HPV)-16/18 AS04-Adjuvanted Vaccine against Cervical Infection and Precancer Caused by Oncogenic HPV Types (PATRICIA): Final Analysis of a Double-Blind, Randomised Study in Young Women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.L.; Schlecht, N.F.; Saslow, D. The Epidemiology of Cervical Cancer. Cancer J. 2003, 9, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Simms, K.T.; Steinberg, J.; Caruana, M.; Smith, M.A.; Lew, J.-B.; Soerjomataram, I.; Castle, P.E.; Bray, F.; Canfell, K. Impact of Scaled up Human Papillomavirus Vaccination and Cervical Screening and the Potential for Global Elimination of Cervical Cancer in 181 Countries, 2020–2099: A Modelling Study. Lancet Oncol. 2019, 20, 394–407. [Google Scholar] [CrossRef]
- Sundström, K.; Miriam Elfström, K. Advances in Cervical Cancer Prevention: Efficacy, Effectiveness, Elimination? PLoS Med. 2020, 17, e1003035. [Google Scholar] [CrossRef]
- Davies-Oliveira, J.C.; Smith, M.A.; Grover, S.; Canfell, K.; Crosbie, E.J. Eliminating Cervical Cancer: Progress and Challenges for High-Income Countries. Clin. Oncol. (R. Coll. Radiol.) 2021, 33, 550–559. [Google Scholar] [CrossRef]
- Bruni, L.; Diaz, M.; Barrionuevo-Rosas, L.; Herrero, R.; Bray, F.; Bosch, F.X.; de Sanjosé, S.; Castellsagué, X. Global Estimates of Human Papillomavirus Vaccination Coverage by Region and Income Level: A Pooled Analysis. Lancet Glob. Health 2016, 4, e453–e463. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Lehtinen, M.; Sparén, P.; Dillner, J.; Elfström, K.M. Impact of HPV Vaccination on Cervical Screening Performance: A Population-Based Cohort Study. Br. J. Cancer 2020, 123, 155–160. [Google Scholar] [CrossRef]
- Tota, J.E.; Bentley, J.; Blake, J.; Coutlée, F.; Duggan, M.A.; Ferenczy, A.; Franco, E.L.; Fung-Kee-Fung, M.; Gotlieb, W.; Mayrand, M.H.; et al. Introduction of Molecular HPV Testing as the Primary Technology in Cervical Cancer Screening: Acting on Evidence to Change the Current Paradigm. Prev. Med. 2017, 98, 5–14. [Google Scholar] [CrossRef]
- Bonde, J.; Floore, A.; Ejegod, D.; Vink, F.J.; Hesselink, A.; Ven, P.M.; Valenčak, A.O.; Pedersen, H.; Doorn, S.; Quint, W.G.; et al. Methylation Markers FAM19A4 and miR124-2 as Triage Strategy for Primary Human Papillomavirus Screen Positive Women: A Large European Multicenter Study. Int. J. Cancer 2020, 148, 396–405. [Google Scholar] [CrossRef]
- Ellis, D.I.; Cowcher, D.P.; Ashton, L.; O’Hagan, S.; Goodacre, R. Illuminating Disease and Enlightening Biomedicine: Raman Spectroscopy as a Diagnostic Tool. Analyst 2013, 138, 3871–3884. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.V. The Colour of the Sea. Nature 1921, 108, 367. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Wells, L.J. Diagnosis of Uterine Cancer by the Vaginal Smear. By George, N. Papanicolaou and Herbert, F. Traut. The Commonwealth Fund, New York. Vii + 46 Pp. 1943 ($5.00). Anat. Rec. 1943, 86, 591–592. [Google Scholar] [CrossRef]
- Swid, M.A.; Monaco, S.E. Should Screening for Cervical Cancer Go to Primary Human Papillomavirus Testing and Eliminate Cytology? Mod. Pathol. 2022, 35, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Javier, R.T.; Butel, J.S. The History of Tumor Virology. Cancer Res. 2008, 68, 7693–7706. [Google Scholar] [CrossRef]
- Bedell, S.L.; Goldstein, L.S.; Goldstein, A.R.; Goldstein, A.T. Cervical Cancer Screening: Past, Present, and Future. Sex. Med. Rev. 2020, 8, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Gibb, R.K.; Martens, M.G. The Impact of Liquid-Based Cytology in Decreasing the Incidence of Cervical Cancer. Rev. Obstet. Gynecol. 2011, 4, S2–S11. [Google Scholar] [PubMed]
- Cheng, L.; Wang, Y.; Du, J. Human Papillomavirus Vaccines: An Updated Review. Vaccines 2020, 8, 391. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Broache, M.; Vaughan, L.; Andrews, J.; Gary, D.; Kaufman, H.W.; Alagia, D.P.; Chen, Z.; Onisko, A.; Austin, R.M. Cotesting in Cervical Cancer Screening. Am. J. Clin. Pathol. 2021, 155, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Maver, P.J.; Poljak, M. Primary HPV-Based Cervical Cancer Screening in Europe: Implementation Status, Challenges, and Future Plans. Clin. Microbiol. Infect. 2020, 26, 579–583. [Google Scholar] [CrossRef] [PubMed]
- What Early Detection and Prevention Measures Are Available? Germany. Available online: https://www.krebsdaten.de/krebs/en/content/cancer_sites/cervical_cancer/cervical_cancer_node.html (accessed on 3 January 2020).
- Rezende, M.T.; Bianchi, A.G.C.; Carneiro, C.M. Cervical Cancer: Automation of Pap Test Screening. Diagn. Cytopathol. 2021, 49, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-W.; Zhang, L.-J.; Shen, Z.-Y.; Zhang, Z.-F.; Xu, F.; Zhang, X.; Li, R.; Xiao, Z. Efficacy of Raman Spectroscopy in the Diagnosis of Uterine Cervical Neoplasms: A Meta-Analysis. Front. Med. 2022, 9, 1277. [Google Scholar] [CrossRef]
- Sitarz, K.; Czamara, K.; Szostek, S.; Kaczor, A. The Impact of HPV Infection on Human Glycogen and Lipid Metabolism—A Review. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188646. [Google Scholar] [CrossRef] [PubMed]
- Traynor, D.; Behl, I.; O’Dea, D.; Bonnier, F.; Nicholson, S.; O’Connell, F.; Maguire, A.; Flint, S.; Galvin, S.; Healy, C.M.; et al. Raman Spectral Cytopathology for Cancer Diagnostic Applications. Nat. Protoc. 2021, 16, 3716–3735. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2015, 50, 46–111. [Google Scholar] [CrossRef]
- Ramos, I.R.M.; Malkin, A.; Lyng, F.M. Current Advances in the Application of Raman Spectroscopy for Molecular Diagnosis of Cervical Cancer. BioMed Res. Int. 2015, 2015, 561242. [Google Scholar] [CrossRef]
- Shaikh, R.; Chilakapati, M. Raman Spectroscopy in Cervical Cancers: An Update. J. Cancer Res. Ther. 2015, 11, 10–17. [Google Scholar]
- Bazant-Hegemark, F.; Edey, K.; Swingler, G.R.; Read, M.D.; Stone, N. Review: Optical Micrometer Resolution Scanning for Non-Invasive Grading of Precancer in the Human Uterine Cervix. Technol. Cancer Res. Treat. 2008, 7, 483–496. [Google Scholar] [CrossRef]
- Chilakapati, M.; Sockalingum, G.; Vidyasagar, M.; Manfait, M.; Fernanades, D.; Vadhiraja, B.; Maheedhar, K. An Overview on Applications of Optical Spectroscopy in Cervical Cancers. J. Cancer Res. Ther. 2008, 4, 26. [Google Scholar] [CrossRef]
- Vargis, E.; Tang, Y.-W.; Khabele, D.; Mahadevan-Jansen, A. Near-Infrared Raman Microspectroscopy Detects High-Risk Human Papillomaviruses. Transl. Oncol. 2012, 5, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Rubina, S.; Amita, M.; Kedar, K.D.; Bharat, R.; Krishna, C.M. Raman Spectroscopic Study on Classification of Cervical Cell Specimens. Vib. Spectrosc. 2013, 68, 115–121. [Google Scholar] [CrossRef]
- Gautam, R.; Vanga, S.; Ariese, F.; Umapathy, S. Review of Multidimensional Data Processing Approaches for Raman and Infrared Spectroscopy. EPJ Tech. Instrum. 2015, 2, 8. [Google Scholar] [CrossRef]
- Hole, A.; Tyagi, G.; Sahu, A.; Shaikh, R.; Chilakapati, M. Exploration of Raman Exfoliated Cytology for Oral and Cervical Cancers. Vib. Spectrosc. 2018, 98, 35–40. [Google Scholar] [CrossRef]
- Bonnier, F.; Traynor, D.; Kearney, P.; Clarke, C.; Knief, P.; Martin, C.; O’Leary, J.J.; Byrne, H.J.; Lyng, F. Processing ThinPrep Cervical Cytological Samples for Raman Spectroscopic Analysis. Anal. Methods 2014, 6, 7831–7841. [Google Scholar] [CrossRef]
- Ramos, I.; Meade, A.D.; Ibrahim, O.; Byrne, H.; McMenamin, M.; McKenna, M.; Malkin, A.; Lyng, F. Raman Spectroscopy for Cytopathology of Exfoliated Cervical Cells. Faraday Discuss. 2015, 187, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.; Traynor, D.; Bonnier, F.; Lyng, F.M.; O’Leary, J.J.; Martin, C.M. Raman Spectral Signatures of Cervical Exfoliated Cells from Liquid-Based Cytology Samples. J. Biomed. Opt. 2017, 22, 1. [Google Scholar] [CrossRef]
- Traynor, D.; Duraipandian, S.; Martin, C.M.; O’Leary, J.J.; Lyng, F.M. Improved Removal of Blood Contamination from ThinPrep Cervical Cytology Samples for Raman Spectroscopic Analysis. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef]
- Duraipandian, S.; Traynor, D.; Kearney, P.; Martin, C.; O’Leary, J.J.; Lyng, F.M. Raman Spectroscopic Detection of High-Grade Cervical Cytology: Using Morphologically Normal Appearing Cells. Sci. Rep. 2018, 8, 15048. [Google Scholar] [CrossRef]
- Traynor, D.; Kearney, P.; Ramos, I.; Martin, C.M.; O’Leary, J.J.; Lyng, F.M. A Study of Hormonal Effects in Cervical Smear Samples Using Raman Spectroscopy. J. Biophotonics 2018, 11, e201700240. [Google Scholar] [CrossRef] [PubMed]
- Aljakouch, K.; Hilal, Z.; Daho, I.; Schuler, M.; Krauß, S.D.; Yosef, H.K.; Dierks, J.; Mosig, A.; Gerwert, K.; El-Mashtoly, S.F. Fast and Noninvasive Diagnosis of Cervical Cancer by Coherent Anti-Stokes Raman Scattering. Anal. Chem. 2019, 91, 13900–13906. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Yin, L.; Luo, B.; Lv, X.; Wu, G. Label-Free Detection of High-Risk Human Papillomaviruses Infection Using Raman Spectroscopy and Multivariate Analysis. Laser Phys. Lett. 2020, 17, 115601. [Google Scholar] [CrossRef]
- Sitarz, K.; Czamara, K.; Bialecka, J.; Klimek, M.; Zawilinska, B.; Szostek, S.; Kaczor, A. HPV Infection Significantly Accelerates Glycogen Metabolism in Cervical Cells with Large Nuclei: Raman Microscopic Study with Subcellular Resolution. Int. J. Mol. Sci. 2020, 21, 2667. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, V.; Saritha, V.N.; Joseph, M.M.; Nair, J.B.; Saranya, G.; Raghu, K.G.; Sujathan, K.; Kumar, K.S.; Maiti, K.K. Diagnostic Spectro-Cytology Revealing Differential Recognition of Cervical Cancer Lesions by Label-Free Surface Enhanced Raman Fingerprints and Chemometrics. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102276. [Google Scholar] [CrossRef]
- Karunakaran, V.; Saritha, V.N.; Ramya, A.N.; Murali, V.P.; Raghu, K.G.; Sujathan, K.; Maiti, K.K. Elucidating Raman Image-Guided Differential Recognition of Clinically Confirmed Grades of Cervical Exfoliated Cells by Dual Biomarker-Appended SERS-Tag. Anal. Chem. 2021, 93, 11140–11150. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, K.; Czamara, K.; Bialecka, J.; Klimek, M.; Szostek, S.; Kaczor, A. Dual Switch in Lipid Metabolism in Cervical Epithelial Cells during Dysplasia Development Observed Using Raman Microscopy and Molecular Methods. Cancers 2021, 13, 1997. [Google Scholar] [CrossRef]
- Traynor, D.; Martin, C.M.; White, C.; Reynolds, S.; D’Arcy, T.; O’Leary, J.J.; Lyng, F.M. Raman Spectroscopy of Liquid-Based Cervical Smear Samples as a Triage to Stratify Women Who Are HPV-Positive on Screening. Cancers 2021, 13, 2008. [Google Scholar] [CrossRef]
- Traynor, D.; Duraipandian, S.; Bhatia, R.; Cuschieri, K.; Tewari, P.; Kearney, P.; D’Arcy, T.; O’Leary, J.J.; Martin, C.M.; Lyng, F.M. Development and Validation of a Raman Spectroscopic Classification Model for Cervical Intraepithelial Neoplasia (CIN). Cancers 2022, 14, 1836. [Google Scholar] [CrossRef]
- Traynor, D.; Duraipandian, S.; Bhatia, R.; Cuschieri, K.; Martin, C.M.; O’Leary, J.J.; Lyng, F.M. The Potential of Biobanked Liquid Based Cytology Samples for Cervical Cancer Screening Using Raman Spectroscopy. J. Biophoton. 2019, 12, e201800377. [Google Scholar] [CrossRef]
- Mahadevan-Jansen, A.; Mitchell, M.F.; Ramanujam, N.; Malpica, A.; Thomsen, S.; Utzinger, U.; Richards-Kortum, R. Near-Infrared Raman Spectroscopy for In Vitro Detection of Cervical Precancers. Photochem. Photobiol. 1998, 68, 123. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, M.S.; Maheedhar, K.; Vadhiraja, B.M.; Fernendes, D.J.; Kartha, V.B.; Krishna, C.M. Prediction of Radiotherapy Response in Cervix Cancer by Raman Spectroscopy: A Pilot Study. Biopolymers 2008, 89, 530–537. [Google Scholar] [CrossRef]
- Shaikh, R.; Vidyasagar, M.; Chilakapati, M. Raman Spectroscopic Study on Prediction of Treatment Response in Cervical Cancers. J. Innov. Opt. Health Sci. 2013, 6, 1350014. [Google Scholar]
- Rashid, N.; Nawaz, H.; Poon, K.W.C.; Bonnier, F.; Bakhiet, S.; Martin, C.; O’Leary, J.J.; Byrne, H.J.; Lyng, F.M. Raman Microspectroscopy for the Early Detection of Pre-Malignant Changes in Cervical Tissue. Exp. Mol. Pathol. 2014, 97, 554–564. [Google Scholar] [CrossRef]
- Daniel, A.; Prakasarao, A.; Dornadula, K.; Ganesan, S. Polarized Raman Spectroscopy Unravels the Biomolecular Structural Changes in Cervical Cancer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 58–63. [Google Scholar] [CrossRef]
- Zheng, C.; Qing, S.; Wang, J.; Lü, G.; Li, H.; Lü, X.; Ma, C.; Tang, J.; Yue, X. Diagnosis of Cervical Squamous Cell Carcinoma and Cervical Adenocarcinoma Based on Raman Spectroscopy and Support Vector Machine. Photodiagn. Photodyn. Ther. 2019, 27, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, C.; Ma, C.; Chen, C.; Zhu, Z.; Yang, B.; Chen, F.; Jia, D.; Li, Y.; Lv, X. Feature Fusion Combined With Raman Spectroscopy for Early Diagnosis of Cervical Cancer. IEEE Photon. J. 2021, 13, 3900311. [Google Scholar] [CrossRef]
- Yang, B.; Chen, C.; Chen, F.; Ma, C.; Chen, C.; Zhang, H.; Gao, R.; Zhang, S.; Lv, X. Feature Fusion Combined with Tissue Raman Spectroscopy to Screen Cervical Cancer. J. Raman Spectrosc. 2021, 52, 1830–1837. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, C.-X.; Ma, C.-L.; Zheng, X.-X.; Lv, X.-Y.; Lv, G.-D.; Tang, J.; Wu, G.-H. Raman Spectroscopic Study of Cervical Precancerous Lesions and Cervical Cancer. Lasers Med. Sci. 2021, 36, 1855–1864. [Google Scholar] [CrossRef]
- Mahadevan-Jansen, A.; Mitchell, M.F.; Ramanujam, N.; Utzinger, U.; Richards-Kortum, R. Development of a Fiber Optic Probe to Measure NIR Raman Spectra of Cervical Tissue In Vivo. Photochem. Photobiol. 1998, 68, 427–431. [Google Scholar] [CrossRef]
- Utzinger, U.; Heintzelman, D.L.; Mahadevan-Jansen, A.; Malpica, A.; Follen, M.; Richards-Kortum, R. Near-Infrared Raman Spectroscopy for in Vivo Detection of Cervical Precancers. Appl. Spectrosc. 2001, 55, 955–959. [Google Scholar] [CrossRef]
- Robichaux-Viehoever, A.; Kanter, E.; Shappell, H.; Billheimer, D.; Jones, H.; Mahadevan-Jansen, A. Characterization of Raman Spectra Measured In Vivo for the Detection of Cervical Dysplasia. Appl. Spectrosc. 2007, 61, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Kanter, E.M.; Vargis, E.; Majumder, S.; Keller, M.D.; Woeste, E.; Rao, G.G.; Mahadevan-Jansen, A. Application of Raman Spectroscopy for Cervical Dysplasia Diagnosis. J. Biophotonics 2009, 2, 81–90. [Google Scholar] [CrossRef]
- Kanter, E.M.; Majumder, S.; Kanter, G.J.; Woeste, E.M.; Mahadevan-Jansen, A. Effect of Hormonal Variation on Raman Spectra for Cervical Disease Detection. Am. J. Obstet. Gynecol. 2009, 200, 512.e1–512.e5. [Google Scholar] [CrossRef]
- Kanter, E.M.; Majumder, S.; Vargis, E.; Robichaux-Viehoever, A.; Kanter, G.J.; Shappell, H.; Jones III, H.W.; Mahadevan-Jansen, A. Multiclass Discrimination of Cervical Precancers Using Raman Spectroscopy. J. Raman Spectrosc. 2009, 40, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Zheng, W.; Low, J.J.H.; Ng, J.; Ilancheran, A.; Huang, Z. High Wavenumber Raman Spectroscopy for in Vivo Detection of Cervical Dysplasia. Anal. Chem. 2009, 81, 8908–8915. [Google Scholar] [CrossRef]
- Duraipandian, S.; Zheng, W.; Ng, J.; Low, J.J.H.; Ilancheran, A.; Huang, Z. Simultaneous Fingerprint and High-Wavenumber Confocal Raman Spectroscopy Enhances Early Detection of Cervical Precancer In Vivo. Anal. Chem. 2012, 84, 5913–5919. [Google Scholar] [CrossRef]
- Duraipandian, S.; Zheng, W.; Ng, J.; Low, J.J.H.; Ilancheran, A.; Huang, Z. Near-Infrared-Excited Confocal Raman Spectroscopy Advances in Vivo Diagnosis of Cervical Precancer. J. Biomed. Opt. 2013, 18, 067007. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.; Dora, T.K.; Chopra, S.; Maheshwari, A.; Kedar, K.D.; Bharat, R.; Krishna, C.M. In Vivo Raman Spectroscopy of Human Uterine Cervix: Exploring the Utility of Vagina as an Internal Control. J. Biomed. Opt. 2014, 19, 087001. [Google Scholar] [CrossRef]
- Shaikh, R.; Prabitha, V.; Tapas, D.; Chopra, S.; Maheshwari, A.; Kedar, D.; Rekhi, B.; Sukumar, N.; Chilakapati, M.; Narayan, S. A Comparative Evaluation of Diffuse Reflectance and Raman Spectroscopy in the Detection of Cervical Cancer. J. Biophotonics 2017, 10, 242–252. [Google Scholar] [CrossRef]
- Feng, S.; Lin, D.; Lin, J.; Li, B.; Huang, Z.; Chen, G.; Zhang, W.; Wang, L.; Pan, J.; Chen, R.; et al. Blood Plasma Surface-Enhanced Raman Spectroscopy for Non-Invasive Optical Detection of Cervical Cancer. Analyst 2013, 138, 3967–3974. [Google Scholar] [CrossRef]
- González-Solís, J.L.; Martínez-Espinosa, J.C.; Torres-González, L.A.; Aguilar-Lemarroy, A.; Jave-Suárez, L.F.; Palomares-Anda, P. Cervical Cancer Detection Based on Serum Sample Raman Spectroscopy. Lasers Med. Sci. 2014, 29, 979–985. [Google Scholar] [CrossRef]
- Shrivastava, A.; Aggarwal, L.M.; Murali Krishna, C.; Pradhan, S.; Mishra, S.P.; Choudhary, S.; Patel, C.B.; Singla, S.; Ashish; Singh, R.K. Diagnostic and Prognostic Application of Raman Spectroscopy in Carcinoma Cervix: A Biomolecular Approach. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 250, 119356. [Google Scholar] [CrossRef] [PubMed]
- Raja, P.; Aruna, P.; Koteeswaran, D.; Ganesan, S. Characterization of Blood Plasma of Normal and Cervical Cancer Patients Using NIR Raman Spectroscopy. Vib. Spectrosc. 2019, 102, 1–7. [Google Scholar] [CrossRef]
- Lu, D.; Ran, M.; Liu, Y.; Xia, J.; Bi, L.; Cao, X. SERS Spectroscopy Using Au-Ag Nanoshuttles and Hydrophobic Paper-Based Au Nanoflower Substrate for Simultaneous Detection of Dual Cervical Cancer–Associated Serum Biomarkers. Anal. Bioanal. Chem. 2020, 412, 7099–7112. [Google Scholar] [CrossRef]
- Xia, J.; Liu, Y.; Ran, M.; Lu, W.; Bi, L.; Wang, Q.; Lu, D.; Cao, X. The Simultaneous Detection of the Squamous Cell Carcinoma Antigen and Cancer Antigen 125 in the Cervical Cancer Serum Using Nano-Ag Polydopamine Nanospheres in an SERS-Based Lateral Flow Immunoassay. RSC Adv. 2020, 10, 29156–29170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ran, M.; Sun, Y.; Fan, Y.; Wang, J.; Cao, X.; Lu, D. A Sandwich SERS Immunoassay Platform Based on a Single-Layer Au–Ag Nanobox Array Substrate for Simultaneous Detection of SCCA and Survivin in Serum of Patients with Cervical Lesions. RSC Adv. 2021, 11, 36734–36747. [Google Scholar] [CrossRef] [PubMed]
- Panikar, S.S.; Banu, N.; Haramati, J.; Gutierrez-Silerio, G.Y.; Bastidas-Ramirez, B.E.; Tellez-Bañuelos, M.C.; Camacho-Villegas, T.A.; Del Toro-Arreola, S.; De la Rosa, E. Anti-Fouling SERS-Based Immunosensor for Point-of-Care Detection of the B7-H6 Tumor Biomarker in Cervical Cancer Patient Serum. Anal. Chim. Acta 2020, 1138, 110–122. [Google Scholar] [CrossRef]
- Hampson, I.N. Effects of the Prophylactic HPV Vaccines on HPV Type Prevalence and Cervical Pathology. Viruses 2022, 14, 757. [Google Scholar] [CrossRef]
- Suresh, A.; Suresh, P.; Biswas, R.; Rajanbabu, A.; Sreedhar, S.; Biswas, L. Prevalence of High-risk HPV and Its Genotypes—Implications in the Choice of Prophylactic HPV Vaccine. J. Med. Virol. 2021, 93, 5188–5192. [Google Scholar] [CrossRef]
- Sundström, K.; Herweijer, E.; Wang, J. Cervical Screening in High-Income Countries: The Need for Quality Assurance, Adjunct Biomarkers and Rational Adaptation to HPV Vaccination. Prev. Med. 2021, 144, 106382. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, R.; Daniel, A.; Lyng, F.M. Raman Spectroscopy for Early Detection of Cervical Cancer, a Global Women’s Health Issue—A Review. Molecules 2023, 28, 2502. https://doi.org/10.3390/molecules28062502
Shaikh R, Daniel A, Lyng FM. Raman Spectroscopy for Early Detection of Cervical Cancer, a Global Women’s Health Issue—A Review. Molecules. 2023; 28(6):2502. https://doi.org/10.3390/molecules28062502
Chicago/Turabian StyleShaikh, Rubina, Amuthachelvi Daniel, and Fiona M. Lyng. 2023. "Raman Spectroscopy for Early Detection of Cervical Cancer, a Global Women’s Health Issue—A Review" Molecules 28, no. 6: 2502. https://doi.org/10.3390/molecules28062502
APA StyleShaikh, R., Daniel, A., & Lyng, F. M. (2023). Raman Spectroscopy for Early Detection of Cervical Cancer, a Global Women’s Health Issue—A Review. Molecules, 28(6), 2502. https://doi.org/10.3390/molecules28062502







