Abstract
5,6-Dihydrophenanthridines are common aza heterocycle frameworks of natural products and pharmaceuticals. Herein, we reported the first palladium-catalyzed intramolecular C−H/C−H dehydrogenative coupling reaction of two simple arenes to generate 5,6-dihydrophenanthridines. The approach features a broad substrate scope and good tolerance of functional groups, offering an efficient alternative synthesis route for important 5,6-dihydrophenanthridine compounds.
1. Introduction
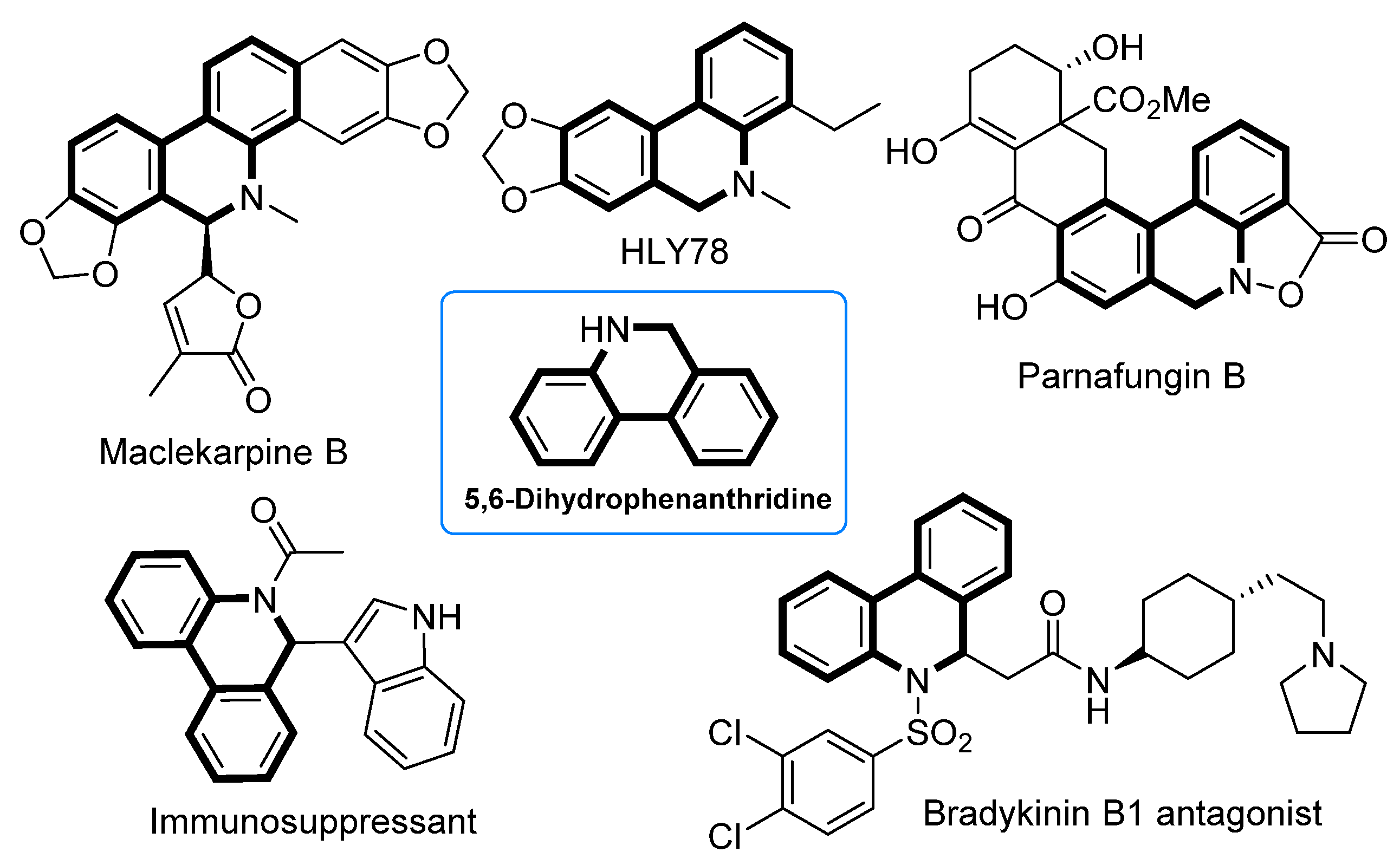
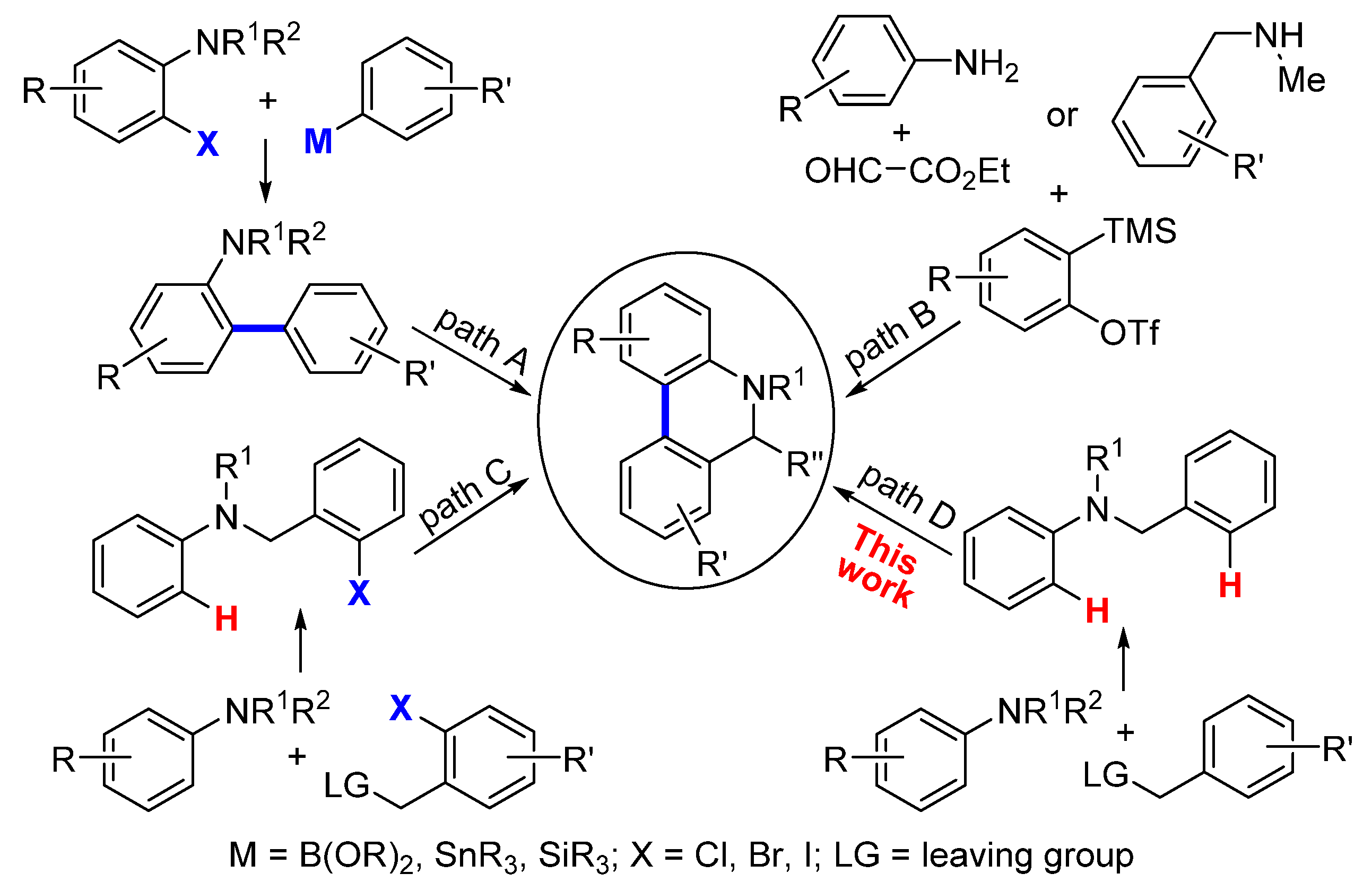
5,6-Dihydrophenanthridines are common aza heterocycle frameworks of natural products and pharmaceuticals [1,2,3,4,5,6,7,8,9,10,11,12], exhibiting various biological activities including antibiotic, anti-inflammatory, and anticancer activity (Figure 1) [13,14,15,16,17,18,19]. More recently, studies have shown that the current COVID-19 pandemic is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and 5,6-dihydrophenanthridine derivatives can interact tightly with SARS-CoV-2 nucleocapsid protein and inhibit the replication of SARS-CoV-2 in vitro [20,21]. Owing to the synthetic challenges in their unique polycyclic skeleton structures, as well as their potential druggability, 5,6-dihydrophenanthridines have aroused considerable interest in synthetic chemists [22,23]. In view of the structural feature of 5,6-dihydrophenanthridines, the formation of their aryl−aryl bond is undoubtedly the key step. To date, there are three main strategies to forge the aryl−aryl bond of 5,6-dihydrophenanthridines (Scheme 1): (1) transition-metal-catalyzed cross-couplings of organometallic aryls with aryl halides (path A) [24,25,26,27,28,29,30]; (2) annulation via benzyne intermediates (path B) [31,32,33]; and (3) the direct arylation of nonactivated aryl C−H bonds with aryl halides (path C) [34,35,36,37,38,39,40,41,42,43,44,45]. Compared to paths A and B, the biggest advantage of path C is that the more expensive and difficult-to-prepare organometallic coupling partner is replaced in this transformation. Nevertheless, the simplest and ideal approach to access 5,6-dihydrophenanthridines is the dehydrogenative coupling of two nonactivated aryl C−H bonds (path D), particularly when aryl halides are not readily available. However, so far there has been no report on using the ideal strategy in the construction of 5,6-dihydrophenanthridine. The major hindrance to the ideal strategy lies in three challenges: (1) the low reactivity of the aryl C−H bond [46]; (2) the regioselectivity issue, especially when there are several reactive sites; and (3) the strong coordinative nitrogen atom of the substrate and product that can easily poison the metal catalyst [23]. In order to accomplish the ideal approach, we hypothesized that an appropriate directing group could be introduced into the substrate to control the regioselectivity whilst at the same time enhancing the reactivity of the aryl C−H bond. Meanwhile, the nitrogen atom of the substrate should be protected by a proper protecting group. In addition, an efficient catalytic system should undoubtedly be sought. Herein, we document the successful execution of this hypothesis to realize the first palladium-catalyzed intramolecular dehydrogenative coupling of two aryl C−H bonds to construct 5,6-dihydrophenanthridines.
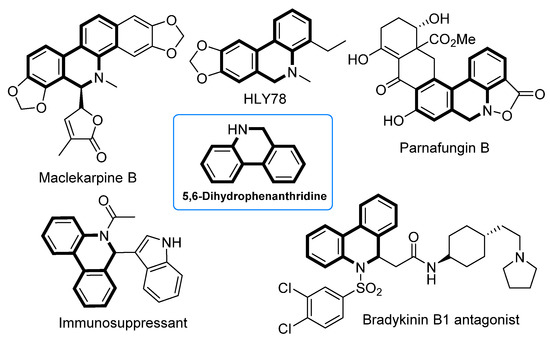
Figure 1.
Natural products and drugs containing 5,6-dihydrophenidine skeleton.
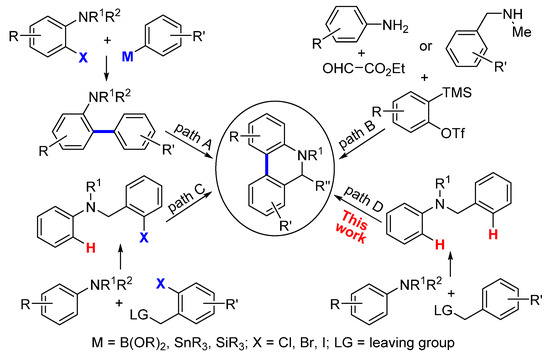
Scheme 1.
The main strategies to forge aryl−aryl bond of 5,6-dihydrophenanthridines.
2. Results and Discussion
2.1. Optimization of Reaction Conditions
We commenced this research by investigating the phenol-protecting group, which had the potential directing feature for the ortho-functionalization of arenes. A series of phenol-protecting groups were tested with 10 mol% Pd(OAc)2 as the catalyst and acetyl as the protecting group of the nitrogen atom in dimethyl sulfoxide (DMSO) under an air atmosphere (Table 1). The reaction did not occur without the phenol-protecting group (entry 1). The protecting groups CONMe2 and O-(2-pyridyl)carbonyl are excellent directing groups in many C-H functionalization reactions of arenes, but they almost did not work in this reaction (entries 2 and 3). Gratifyingly, when the O-(2-pyridyl)sulfonyl group was used as the protecting group, the desired aryl C−H/C−H coupling product 2a was generated with a 9% yield (entry 4). The structure of 2a was confirmed by single-crystal X-ray diffraction. We considered that the reactivity of the O-(2-pyridyl)-sulfonyl group should lie in it being not only a great directing group but also a good activating group to facilitate the formation of a phenyl−Pd complex through the ortho C−H bond activation of phenol [47,48]. Encouraged by the preliminary result, we then carefully examined other parameters of the reaction. The influence of the solvent showed that CF3CH2OH was the best option, providing 2a with a 12% yield (entries 5–7). After screening the oxidant, copper(II) trifluoroacetate hydrate provided the highest yield (31%, entries 8–12). Note that without any oxidant the reaction gave 2a with a 8% yield under an argon atmosphere (entry 13). Next, the reaction temperature was checked (entries 14–17). Increasing the temperature benefitted the reaction, and the yield was increased to 45% at 100 °C. It turned out that the N-protecting group was crucial for this reaction. As speculated, the replacement of the N-protecting group with Ts, Boc, methyl, or phenyl all led to inferior results (entries 18–21). Then, the palladium source was investigated, indicating that Pd(TFA)2 was the best catalyst (entries 22–25). The investigation of the amount of Pd(TFA)2 and Cu(TFA)2·H2O indicated that Pd(TFA)2 (15 mol%) and Cu(TFA)2·H2O (2.2 equiv.) were the best choices (entries 26–31). It is noteworthy that the reaction proceeded similarly under an argon atmosphere (entry 31). Accordingly, the optimized reaction conditions were identified as the following: Pd(TFA)2 (15 mol%) and Cu(TFA)2·H2O (2.2 equiv.) in CF3CH2OH at 100 °C under an air atmosphere for 20 h (entry 29).

Table 1.
Optimization of conditions for the synthesis of 5,6-dihydrophenanthridine a.
2.2. Substrate Scope
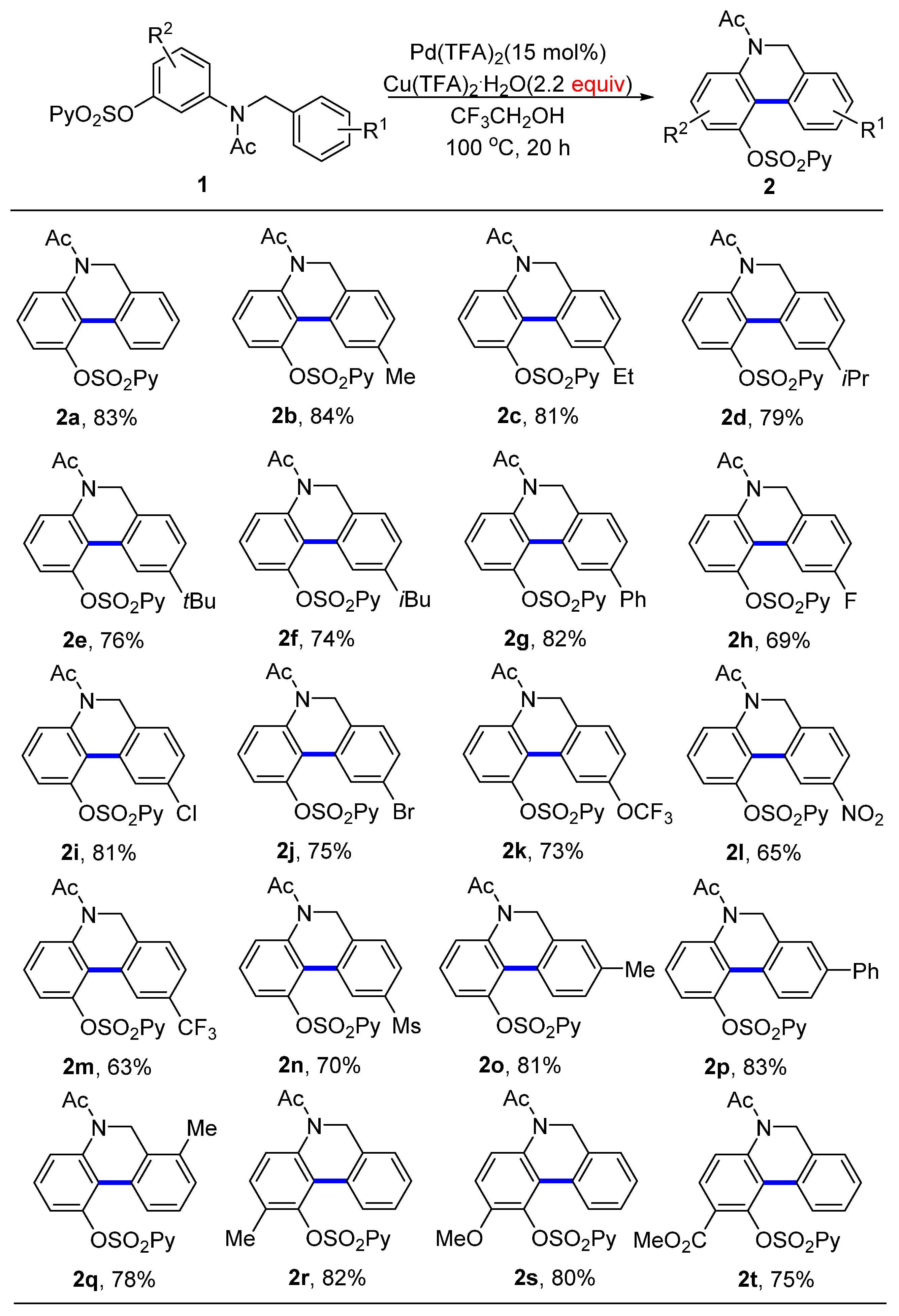
With the optimal conditions in hand, we first examined the effect of the substituents on the right aromatic ring for the aryl C−H/C−H coupling (Scheme 2). A variety of aryls with both electron-donating and electron-withdrawing groups could be engaged in this transformation, providing the desired 5,6-dihydrophenanthridines with good-to-excellent yields. A broad range of functional groups such as alkyls (2b−g), halides (F, Cl, Br, 2h−j), trifluoromethoxy (2k), nitro (2l), trifluoromethyl (2m), and methyl sulfonyl group (Ms, 3n) were compatible with this process. These provided synthetically interesting results because such substituents acted as versatile handles for further transformations. The position of the substituent on the aromatic rings had almost no effect on the reactivity (2o−q). Next, we investigated the substituents on the left phenol ring. Again, the aryl C−H/C−H dehydrogenative coupling reaction was insensitive to the electronic property of the substituent groups such as electron-donating methyl and methoxy, and electron-withdrawing ester groups; all reactions proceeded successfully, affording the desired products with 75–82% yields (2r−t).
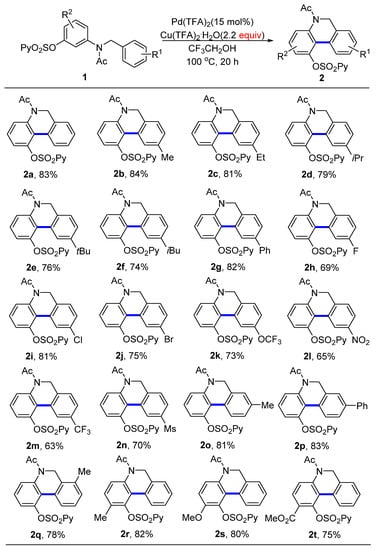
Scheme 2.
Scope of the two-aryl C−H/C−H coupling reaction. Reaction conditions: 1 (0.5 mmol), Pd(TFA)2 (15 mol%) and Cu(TFA)2·H2O (1.1 mmol) in CF3CH2OH (2 mL) under air atmosphere at 100 °C for 20 h.
2.3. Large-Scale Experiment and Synthetic Application
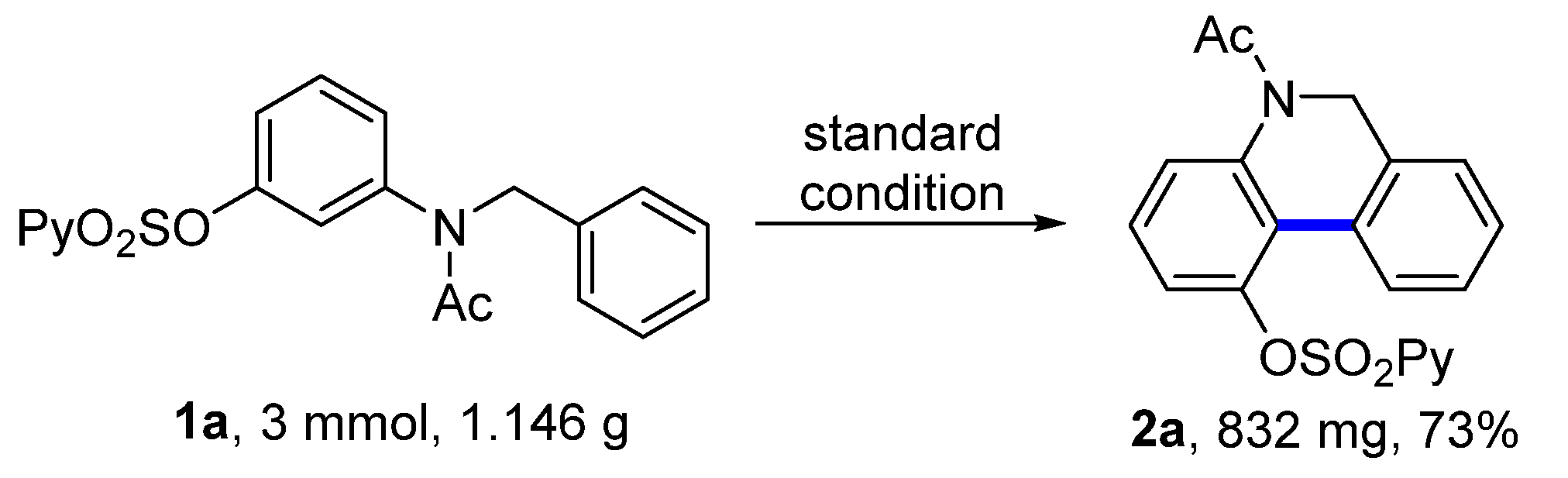
To test the practicality of this dehydrogenative coupling, a large-scale experiment was carried out. With the above standard reaction conditions, 1a (1.146 g, 3.0 mmol) provided 5,6-dihydrophenanthridine 2a (832 mg) with a 73% yield (Figure 2).
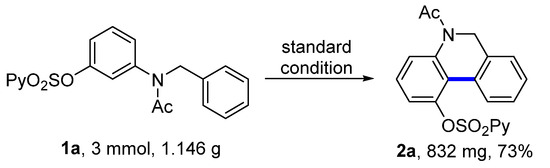
Figure 2.
Large-scale experiment.
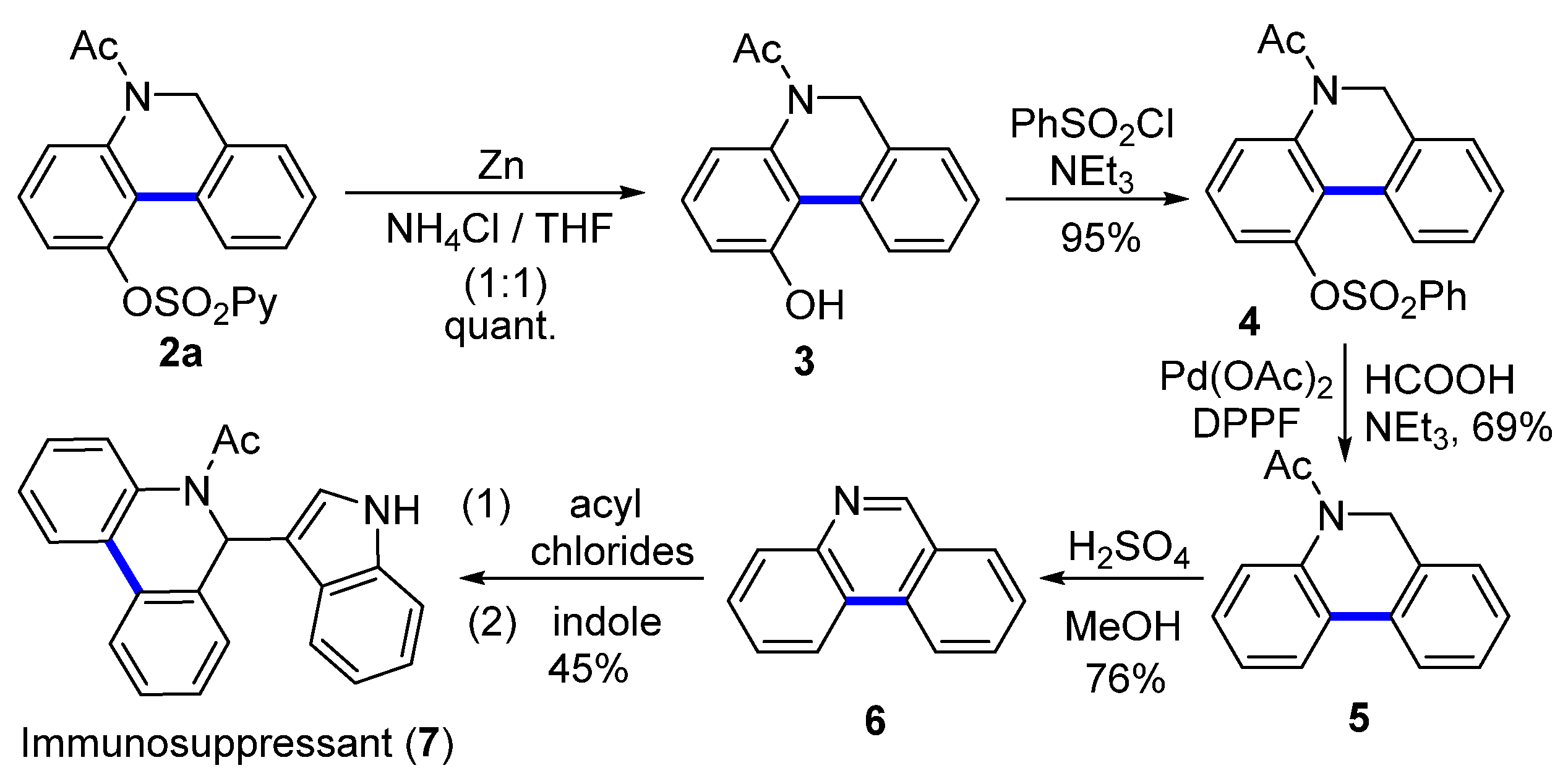
To demonstrate the synthetic application of this methodology, we employed dehydrogenative coupling as the key step to synthesize an inhibitor of potassium channels KV1.3 and IK-1 (7) (Scheme 3) [6]. First, the 2-pyridysulfonyl group was readily removed by zinc in NH4Cl (aq)/THF (1:1) at room temperature, affording 3 in a quantitative yield. Then, the hydroxy group was transformed into a benzenesulfonate group (4), which was further removed to give product 5 [49,50]. Next, the acetyl group in product 5 could be readily eliminated using H2SO4 in MeOH, affording the product phenanthridine 6 with a 76% yield. Finally, the inhibitor dihydrophenanthridine 7 was achieved by the activation of the imine structure in phenanthridine with acyl chlorides to give an intermediary imminium ion followed by in situ nucleophilic attack with indole [6].

Scheme 3.
The synthesis of immunosuppressant 7.
2.4. Mechanistic Investigations
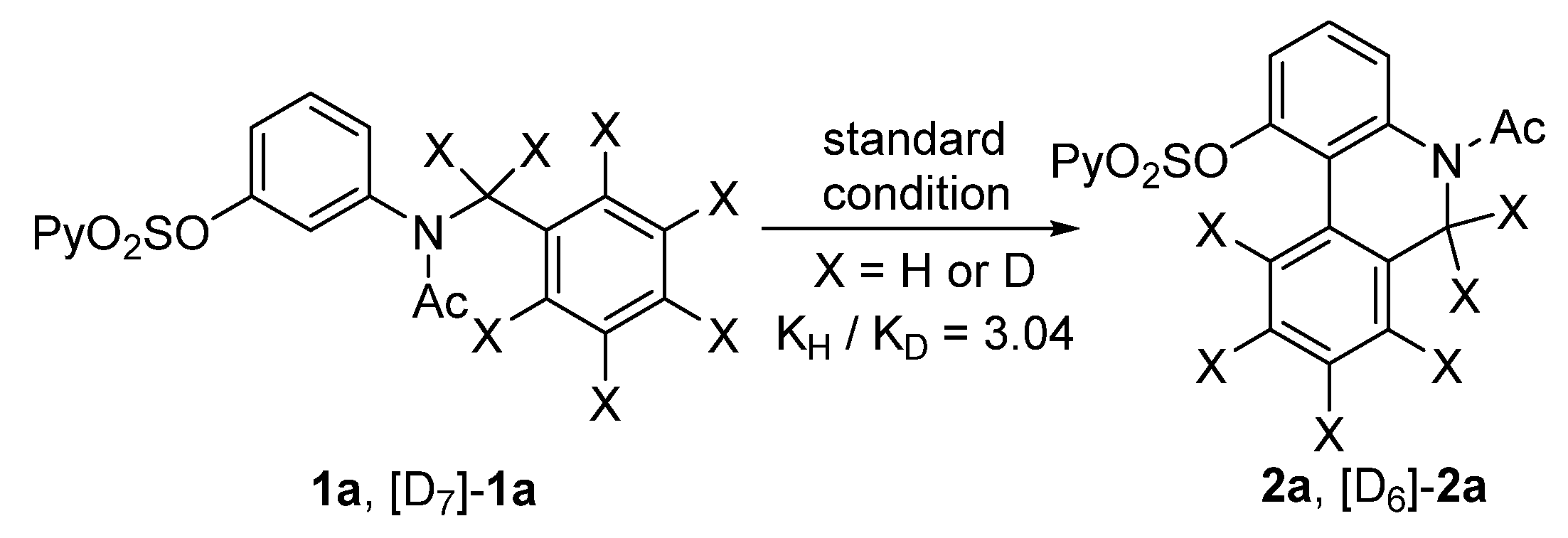
To gain insight into the mechanism of this intramolecular dehydrogenative coupling of the two aryl C−H bonds, a kinetic isotope effect (KIE) experiment was performed. The KIE value of the two parallel competition reactions of 1a and [D7]-1a was found to be 3.04 (Figure 3). This implied that an electrophilic aromatic palladation mechanism was unlikely, and the cleavage of the C−H bond on the right aromatic ring should be involved in the rate-determining step. In addition, a radical-trapping experiment was performed. In the presence of 1.0 equiv. of 2,2,6,6-tetramethylpiperidine oxide (TEMPO), under the above standard conditions, the reaction of 1a proceeded well to give 2a with almost the same yield, illustrating a low possibility for a free radical pathway.
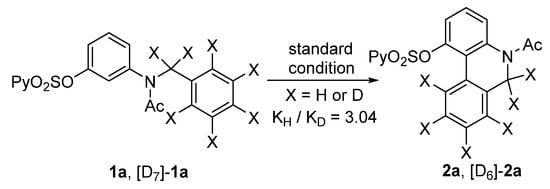
Figure 3.
KIE experiment.
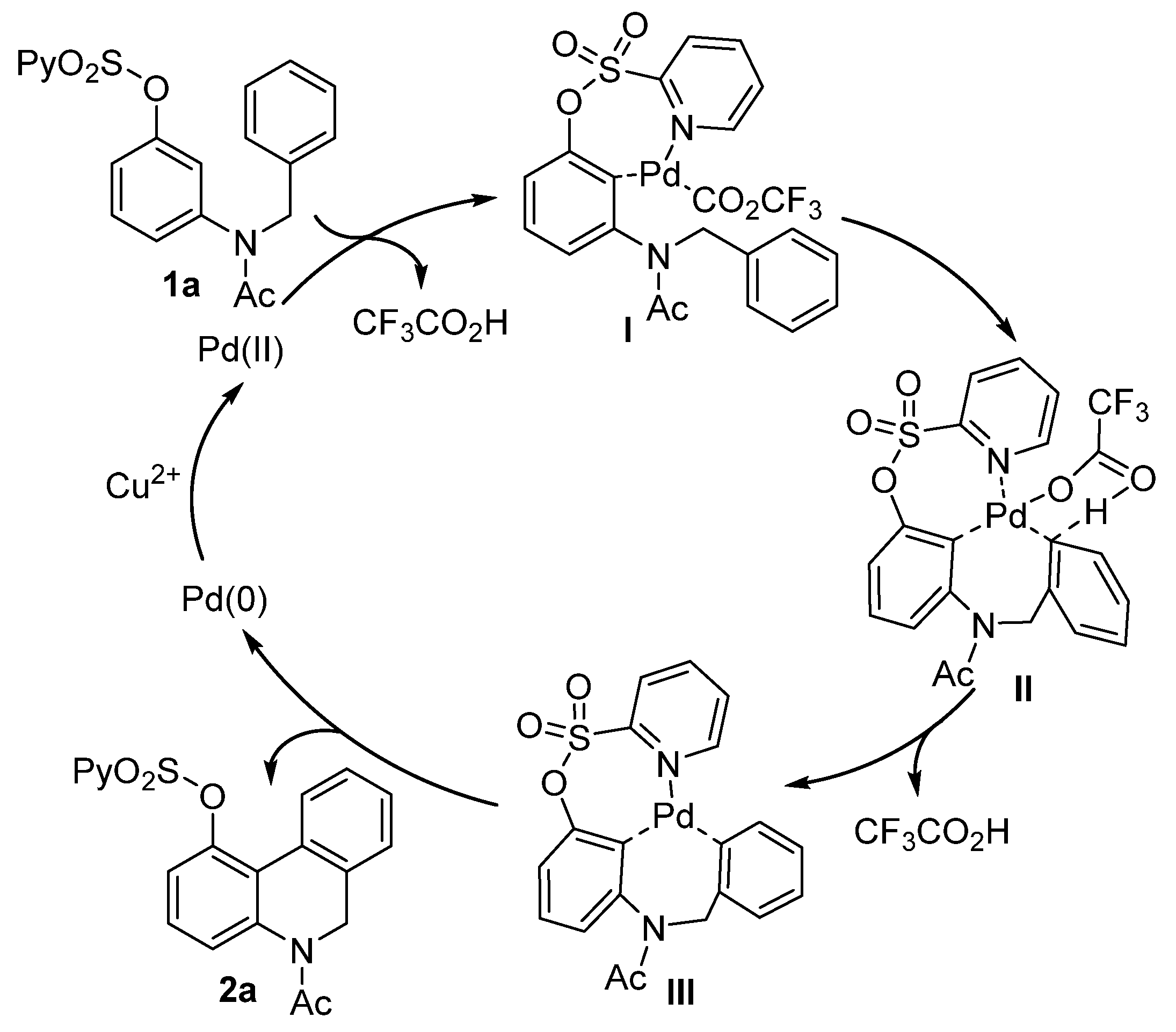
On the basis of the above results and the relevant literature [51,52,53,54,55], a plausible mechanism is proposed in Scheme 4. Initially, the O-(2-pyridyl)sulfonyl group-directed palladation formed complex I, which then underwent an intramolecular concerted metalation−deprotonation (CMD) step assisted by trifluoroacetate via a six-membered transition state (II) to afford intermediate III. Finally, the reductive elimination of intermediate III produced the 5,6-dihydrophenanthridine product along with Pd(0), which was reoxidized by Cu2+ to regenerate the Pd(II) species to complete the catalytic cycle.
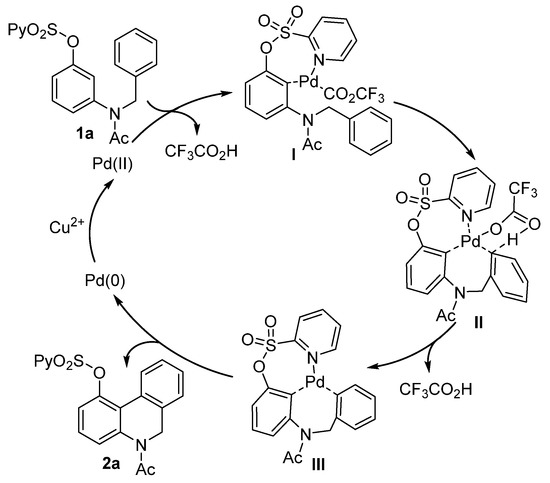
Scheme 4.
Plausible mechanism.
3. Materials and Methods
3.1. General Information
All reactions were carried out under an air atmosphere. Unless noted otherwise, commercially available chemicals were used without further purification. Flash chromatography was performed with silica gel (200–300 mesh). An oil bath served as the heat source. NMR spectra were acquired on either a Bruker 400 MHz (1H at 400 MHz, 13C at 100 MHz) or Jeol 400 MHz (1H at 400 MHz, 13C at 100 MHz) device, and NMR spectra were recorded in CDCl3 (TMS, δ = 0.00 ppm for 1H and δ = 77.10 ppm for 13C), DMSO-d6 (δ = 2.50 ppm for 1H and δ = 39.52 ppm for 13C), or CD3OD (δ = 3.31 ppm for 1H and δ = 49.00 ppm for 13C) using the solvent residue peaks as the internal references. Coupling constants were reported in hertz (Hz). Data for 1H NMR spectra were reported as follows: chemical shift (ppm, referenced to protium, s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublets, td = triplet of doublets, ddd = doublet of doublet of doublets, m = multiplet, coupling constant (Hz), and integration). Infrared (IR) data were acquired on a Bruker Invenio-RFT-IR spectrometer. Absorbance frequencies were reported in reciprocal centimeters (cm−1). Mass spectra were acquired on a BrukerDaltonics S2 MicroTof-Q II mass spectrometer. X-ray crystal structure analyses were measured on a Bruker Smart APEXIICCD instrument using Mo-Kα radiation. The structures were solved and refined using the SHELXTL software package.
3.2. General Procedure A for Preparation of Substrates
To a solution of N-(3-hydroxyphenyl)acetamide (30.0 mmol, 4.51g) in CH2Cl2 (50 mL), Et3N (40.0 mmol, 4.04 g) was added. The mixture was stirred at room temperature for 30 min, and then pyridine-2-sulfonyl chloride (30.0 mmol, 5.31 g) was added. The mixture was stirred overnight. The solvent was removed by distillation, and then EtOAc (50 mL) was added. The resulting solution was washed with water (3 × 10 mL) and brine (3 × 5 mL), dried over MgSO4, and concentrated. The residue was used without further purification in the next step.
To a solution of residue (1 mmol) in anhydrous tetrahydrofuran (10 mL), NaH (60 mg, 1.5 mmol, 60%) was added. The mixture was stirred at room temperature for 30 min, and then benzyl bromide (1.2 mmol) was added. The mixture was stirring at room temperature until completion of the reaction (monitored by TLC). Then, the reaction mixture was filtered and purified by column chromatography (eluent: PET (petroleum ether):EA (ethyl acetate) = 2:1).
3.3. General Procedure B for Preparation of Substrates
To a solution of 3-aminophenol (30.0 mmol, 4.51 g) in CH2Cl2 (50 mL), NEt3 (40.0 mmol, 4.04 g) was added. The mixture was stirred at room temperature for 30 min, and then pyridine-2-sulfonyl chloride (30.0 mmol, 5.31 g) was added. The mixture was stirred overnight. The solvent was removed by distillation, and then EtOAc (50 mL) was added. The resulting solution was washed with water (3 × 10 mL) and brine (3 × 5 mL), dried over MgSO4, and concentrated. The residue was used without further purification in the next step.
To a solution of residue (1 mmol) in CH2Cl2 (10 mL), acetic anhydride (122 mg, 1.2 mmol) was added. The mixture was stirred overnight. The solvent was removed by distillation, and then CH2Cl2 (15 mL) was added. The resulting solution was washed with water (3 × 5 mL) and brine (3 × 5 mL), dried over MgSO4, and concentrated. The residue was purified by column chromatography (eluent: PET:EA = 2:1) to give the product.
To a solution of above product (1 mmol) in anhydrous tetrahydrofuran (10 mL), NaH (60 mg, 1.5 mmol, 60%) was added. The mixture was stirred at room temperature for 30 min, and then benzyl bromide (1.2 mmol) was added. The mixture was stirring at room temperature until completion of the reaction (monitored by TLC). Then, the reaction mixture was filtered and purified by column chromatography (eluent: PET:EA = 2:1).
3.4. General Procedure C for Preparation of Products
3-(N-benzylacetamido)phenyl pyridine-2-sulfonate derivatives 1 (0.2 mmol), Pd(TFA)2 (15 mol %), and Cu(TFA)2·H2O (2.2 equiv.) were added to a 10 mL round-bottom flask in CF3CH2OH, and these were held at 100 °C under an air atmosphere until completion of the reaction (monitored by TLC). Then, purification by flash chromatography afforded product 2.
3.5. The Synthesis of Immunosuppressant 7
1-Hydroxyphenanthridin-5(6H)-yl)ethan-1-one (3): A suspension of 5-acetyl-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate 2 (152mg, 0.4 mmol) and Zn powder (not activated, 1.3 g, 20 mmol) in a 1:1 mixture of THF and saturated NH4Cl solution in water (20 mL) was stirred at 30 °C until consumption of the starting material (monitored by TLC). The mixture was filtered over a pad of celite to remove the Zn. The filtrate was extracted with EA (15 mL) and washed with a saturated aqueous solution of ammonium chloride and brine. The combined organic phase was dried over MgSO4 and concentrated. The residue was purified by flash chromatography to afford the corresponding product 3 (eluent: PET:EA = 1:1, Rf = 0.2) as a white solid. Quant.: m. p. = 212–213 °C; 1H NMR (400 MHz, CD3OD) δ 8.46 (d, J = 7.9 Hz, 1H), 7.33–7.29 (m, 1H), 7.28–7.19 (m, 2H), 7.16–7.12 (m, 1H), 6.86 (d, J = 7.4 Hz, 2H), 4.61 (d, J = 9.8 Hz, 2H), 2.09 (s, 3H); 13C NMR (100 MHz, CD3OD) δ 170.4, 155.3, 139.7, 135.2, 130.5, 128.1, 127.7, 126.9, 126.6, 125.0, 117.0, 115.8, 114.2, 45.3, 20.9; HRMS (ESI) m/z calculated for C15H13NO2Na [M+Na]+: 262.0838; found 262.0830.
5-Acetyl-5,6-dihydrophenanthridin-1-yl benzenesulfonate (4): The compound 3 (0.4 mmol, 96 mg) was dissolved in DCM (10 mL) in a bottle filled under an argon atmosphere. The solution was cooled to 0 °C. Then, NEt3 (0.078 mL, 0.56 mmol, 1.4 equiv) was added dropwise to the solution, which was followed by the addition of benzenesulfonyl chloride (84.5 mg, 0.48 mmol, 1.2 equiv). After 5 min, the ice bath was removed, and the reaction was monitored by TLC. Once the phenol was completely consumed, the reaction was stopped. The solvent was evaporated under a vacuum, and the residue was purified by flash column chromatography to give 4 as a colorless gel (144mg, 95%). 1H NMR (400 MHz, CDCl3) δ 8.01 (dd, J = 7.9, 1.3 Hz, 1H), 7.32–7.41 (m, 5H), 7.29–7.25 (m, 1H), 7.24–7.19 (m, 2H), 7.14–7.07 (m, 3H), 4.27 (s, 2H), 2.14 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.9, 146.39, 140.2, 136.1, 133.9, 129.3, 128.4, 128.3 (3C), 128.2 (2C), 127.8, 127.7, 125.7, 123.5, 122.0, 45.0, 21.7.
1-(Phenanthridin-5(6H)-yl)ethan-1-one (5) [56]: To a bottle were added 4 (144 mg, 0.38 mmol), Pd(OAc)2 (8.84 mg 0.039 mmol), DPPF (7.3 mg, 0.039 mmol), Et3N (119 mg 1.18 mmol), HCOOH (36 mg 0.78 mmol), and DMF (4 mL). The atmosphere was replaced by an argon atmosphere. The mixture was stirred at 80 °C for 6 h. After the usual workup, the residue was purified by flash column chromatography to give 5 as a yellow gel (58.5 mg, 69%). H NMR (400 MHz, CDCl3) δ 7.75 (dd, J = 8.6, 6.1 Hz, 2H), 7.45–7.14 (m, 6H), 4.90 (s, 2H), 2.14 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.3, 137.8, 135.0, 131.6, 129.6, 127.9 (2C), 127.6, 126.2, 126.0, 124.5, 124.3, 123.2, 44.8, 22.0.
Phenanthridin (6) [57]: A stock solution was prepared by dropwise addition of concentrated sulfuric acid (1 mL) to reagent-grade methanol (5 mL) in a scintillation vial (20 mL in volume). The stock solution was stirred for 5 min, and then the solution was cooled to room temperature. A bottle equipped with a spin bar was charged with 1-(phenanthridin-5(6H)-yl)ethan-1-one 5 (0.26 mmol). A fraction of the stock solution (0.5 mL) was added slowly to the solid reagent, and the reaction mixture was stirred at 60 °C for 15 min. Then, the reaction mixture was cooled to room temperature, and distilled water (2 mL), EtOAc (5 mL), and saturated Na2CO3 solution (2 mL) were added dropwise to the reaction mixture. The aqueous phase was extracted with EA (2 × 8 mL), and the combined organic fractions were dried over anhydrous Na2SO4 or MgSO4. The combined organic fractions were filtered through celite, and the filtrate was concentrated. The residue was purified by flash column chromatography to give phenanthridine 6 as a white solid (35.7 mg, 76%). m. p. = 106–109 °C; 1H NMR (400 MHz, CDCl3) δ 9.30 (s, 1H), 8.61 (dd, J = 12.6, 7.4 Hz, 2H), 8.20 (d, J = 9.5 Hz, 1H), 8.06 (d, J = 7.9 Hz, 1H), 7.90–7.85 (m, 1H), 7.79–7.67 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 153.7, 144.5, 132.6, 131.1, 130.2, 128.9, 128.8, 127.6, 127.2, 126.5, 124.1, 122.3, 122.0.
Immunosuppressant (7) [58]: Phenanthridine (0.2 mmol) was dissolved in dry tetrahydrofuran (2.0 mL) under an argon atmosphere. After cooling to 0°C, the appropriate acetyl chloride (1.2 mmol) was added dropwise. The mixture was stirred for 2 h at room temperature and then cooled to 0 °C again. Triethylamine (0.3 mmol) was added followed by 1H-indole (1.5 mmol). After stirring for 3 h at room temperature, water was added, and the mixture was extracted several times with ethyl acetate. After washing the combined organic phases with brine and drying over Na2SO4, the solvent was removed in vacuo. The residue was purified by flash column chromatography to give immunosuppressant 7 as a white solid (35.7 mg, 76%). m. p. = 211–213 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.68 (s, 1H), 7.99 (dd, J = 26.9, 7.7 Hz, 2H), 7.73 (d, J = 7.7 Hz, 1H), 7.52–7.47 (m, 2H), 7.41–7.37 (m, 1H), 7.33 (s, 1H), 7.26 (d, J = 7.8 Hz, 2H), 7.17 (d, J = 4.4 Hz, 2H), 7.05–7.01 (m, 2H), 6.11 (s, 1H), 2.19 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 168.6, 137.4, 136.3, 135.7, 130.8, 128.8, 128.2, 128.0, 127.8, 126.9, 126.00, 125.96, 125.8, 124.6, 124.2, 123.8, 121.4, 119.0 (2C), 113.9, 111.6, 49.7, 22.6; IR (KBr): 3016, 2961, 2927, 1662, 1455, 1426, 1455, 1426, 1378.9, 1213, 1186, 1118, 1049, 1049, 987, 874, 753, 667, 594 cm−1; HRMS (ESI) m/z calculated for C23H18N2ONa [M+Na]+: 361.1311; found 361.1307.
3.6. Characterization Data of Substrates and Products
3-(N-Benzylacetamido)phenyl pyridine-2-sulfonate (1a): The product was synthesized by general method A (eluent: EA:PE = 1:2, Rf = 0.23); 278.9 mg, 73% yield; white solid; m. p. = 78–79 °C; 1H NMR (400 MHz, CDCl3) δ 8.73 (s, 1H), 7.92–7.88 (m, 2H), 7.58 (d, J = 3.0 Hz, 1H), 7.38–7.18 (m, 4H), 7.15–7.07 (m, 3H), 6.99–6.71 (m, 2H), 4.80 (s, 2H), 1.81 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.9, 153.2, 150.6, 149.9, 143.9, 138.3, 136.9, 130.5, 128.5 (2C), 128.3, 127.5, 127.0, 124.2, 122.4, 121.9, 52.7, 22.7; IR (KBr): 3063, 2924, 1658, 1601, 1581, 1485, 1428, 1376, 1196, 1117, 938, 616, 593 cm−1; HRMS (ESI) m/z calculated for C20H18N2O4SNa [M+Na]+: 405.0879; found 405.0871.
3-(N-(4-Methylbenzyl)acetamido)phenyl pyridine-2-sulfonate (1b): The product was synthesized by general method A (eluent: EA:PE = 1:2, Rf = 0.22); 332.7 mg, 84% yield; white solid; m. p. = 83–94 °C; 1H NMR (400 MHz, CDCl3) δ 8.73 (s, 1H), 7.92–7.88 (m, 2H), 7.58 (d, J = 3.3 Hz, 1H), 7.34–7.21 (m, 1H), 7.11 (d, J = 7.0 Hz, 1H), 7.01 (dd, J = 16.4, 7.4 Hz, 4H), 6.88 (d, J = 7.3 Hz, 1H), 6.83 (s, 1H), 4.75 (s, 2H), 2.28 (s, 3H), 1.78 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.8, 153.2, 150.6, 149.9, 143.9, 138.3, 137.1, 133.9, 130.5, 129.2, 128.5, 128.3, 127.1, 124.2, 122.5, 121.9, 52.4, 22.8, 21.1; IR (KBr): 2924, 1731, 1620, 1572, 1490, 1467, 1378, 1195, 1067, 1032, 840 cm−1; HRMS (ESI) m/z calculated for C21H20N2O4SNa [M+Na]+: 419.1036; found 419.1033.
3-(N-(4-Ethylbenzyl)acetamido)phenyl pyridine-2-sulfonate (1c): The product was synthesized by general method A (eluent: EA:PE = 1:1, Rf = 0.25); 369.9 mg, 91% yield; colorless gel; 1H NMR (400 MHz, CDCl3) δ 8.69 (d, J = 4.6 Hz, 1H), 7.85 (d, J = 2.1 Hz, 2H), 7.54 (ddd, J = 6.7, 4.7, 2.4 Hz, 1H), 7.25–7.19 (m, 1H), 7.12–6.94 (m, 5H), 6.86 (d, J = 8.1 Hz, 1H), 6.80 (s, 1H), 4.72 (s, 2H), 2.54 (q, J = 7.6 Hz, 2H), 1.74 (s, 3H), 1.13 (t, J = 7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 169.7, 152.9, 150.4, 149.7, 143.8, 143.2, 138.2, 133.9, 130.3, 128.3, 128.1, 127.8, 126.9, 124.0, 122.3, 121.7, 52.2, 28.3, 22.5, 15.3; IR (KBr): 3057, 2926, 1914, 1854, 1806, 1529, 1428, 1379, 1197, 1139, 1118, 926, 867, 772, 594, 553 cm−1; HRMS (ESI) m/z calculated for C22H23N2O4S [M+H]+: 411.1373; found 411.1384.
3-(N-(4-Isopropylbenzyl)acetamido)phenyl pyridine-2-sulfonate (1d): The product was synthesized by general method A (eluent: EA:PE = 1:1, Rf = 0.25); 361.3 mg, 85% yield; colorless gel; 1H NMR (400 MHz, CDCl3) δ 8.75 (d, J = 4.7 Hz, 1H), 7.93 (d, J = 7.9 Hz, 2H), 7.61 (ddd, J = 6.8, 4.6, 2.2 Hz, 1H), 7.32–7.26 (m, 1H), 7.19–7.03 (m, 5H), 6.94 (d, J = 8.0 Hz, 1H), 6.88 (s, 1H), 4.79 (s, 2H), 2.87 (p, J = 7.0 Hz, 1H), 1.81 (s, 3H), 1.21 (d, J = 2.0 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 13C NMR (101 MHz, CDCl3) δ 169.6, 152.8, 150.4, 149.7, 147.8, 143.8, 138.2, 134.0, 130.3, 128.2, 128.1, 126.9, 126.3, 124.0, 122.2, 121.6, 52.2, 33.5, 23.8, 22.5; IR (KBr): 3551, 2959, 1643, 1601, 1485, 1428, 1380, 1197, 1138, 1117, 1085, 1017, 926, 865, 768, 751, 592, 548 cm−1; HRMS (ESI) m/z calculated for C23H25N2O4S [M+H]+: 425.1530; found 425.1546.
3-(N-(4-(tert-Butyl)benzyl)acetamido)phenyl pyridine-2-sulfonate (1e): The product was synthesized by general method A (eluent: EA:PE = 1:2, Rf = 0.28); 363.7 mg, 83% yield; white solid; m. p. = 82–93 °C; 1H NMR (400 MHz, CDCl3) δ 8.76 (d, J = 4.5 Hz, 1H), 7.93 (d, J = 4.5 Hz, 2H), 7.60 (dd, J = 8.8, 4.6 Hz, 1H), 7.29 (dd, J = 11.3, 5.4 Hz, 3H), 7.15 (d, J = 7.8 Hz, 1H), 7.07 (d, J = 8.2 Hz, 2H), 6.94 (d, J = 7.5 Hz, 1H), 6.89 (s, 1H), 4.78 (s, 2H), 1.81 (s, 3H), 1.29 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 169.9, 153.3, 150.6, 150.4, 149.9, 144.1, 138.2, 133.8, 130.4, 128.2 (2C), 127.1, 125.4, 124.2, 122.5, 121.8, 52.4, 34.5, 31.3, 22.7; IR (KBr): 2960, 1660, 1601, 1513, 1428, 1377, 1295, 1196, 1117, 926, 865, 801, 722, 550 cm−1; HRMS (ESI) m/z calculated for C24H26N2O4SNa [M+Na]+: 461.1505; found 461.1515.
3-(N-(4-Isobutylbenzyl)acetamido)phenyl pyridine-2-sulfonate (1f): The product was synthesized by general method A (eluent: EA:PE = 1:1, Rf = 0.25); 403.9 mg, 92% yield; colorless gel; 1H NMR (400 MHz, CDCl3) δ 8.76 (d, J = 4.6 Hz, 1H), 7.93 (d, J = 3.6 Hz, 2H), 7.63–7.57 (m, 1H), 7.29–7.25 (m, 1H), 7.13 (d, J = 7.7 Hz, 1H), 7.06–7.00 (m, 4H), 6.93–6.84 (m, 2H), 4.79 (s, 2H), 2.43 (d, J = 7.2 Hz, 2H), 1.81 (s, 3H), 0.87 (d, J = 6.6 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 169.7, 153.1, 150.5, 149.8, 143.8, 140.8, 138.2, 134.0, 130.3, 129.1, 128.2, 128.1, 127.0, 124.1, 122.4, 121.7, 52.3, 44.9, 30.0, 22.6, 22.2; IR (KBr): 2955, 2925, 1659, 1601, 1581, 1485, 1428, 1379, 1197, 1138, 1117, 926, 866, 771, 736, 594, 550 cm−1; HRMS (ESI) m/z calculated for C24H27N2O4S [M+H]+: 439.1686; found 439.1683.
3-(N-([1,1’-Biphenyl]-4-ylmethyl)acetamido)phenyl pyridine-2-sulfonate (1g): The product was synthesized by general method A (eluent: EA:PE = 1:2, Rf = 0.24); 348.2 mg, 76% yield; white solid; m. p. = 102–103 °C; 1H NMR (400 MHz, CDCl3) δ 8.69 (d, J = 4.6 Hz, 1H), 7.89 (d, J = 7.8 Hz, 1H), 7.85–7.81 (m, 1H), 7.63–7.54 (m, 2H), 7.50 (dd, J = 8.2, 4.6 Hz, 3H), 7.43–7.39 (m, 2H), 7.33–7.26 (m, 2 H), 7.21 (d, J = 8.1 Hz, 2H), 7.14 (d, J = 7.9 Hz, 1H), 6.96 (d, J = 7.5 Hz, 1H), 6.91 (s, 1H), 4.86 (s, 2H), 1.84 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.8, 153.0, 150.4, 149.8, 143.8, 140.3, 140.0, 138.1, 135.8, 130.4, 128.8, 128.6, 128.1, 127.2, 127.0, 126.9, 126.8, 123.9, 122.3, 121.8, 52.2, 22.6; IR (KBr): 3058, 2926, 1661, 1601, 1581, 1486, 1428, 1379, 1294, 1197, 801, 766, 616, 594 cm−1; HRMS (ESI) m/z calculated for C26H22N2O4SNa [M+Na]+: 481.1192; found 481.1190.
3-(N-(4-Fluorobenzyl)acetamido)phenyl pyridine-2-sulfonate (1h): The product was synthesized by general method A (eluent: EA:PE = 1:2, Rf = 0.27); 336.1 mg, 84% yield; white solid; m. p. = 83–84 °C; 1H NMR (400 MHz, CDCl3) δ 8.81–8.74 (m, 1H), 8.04–7.90 (m, 2H), 7.62 (ddd, J = 6.8, 4.7, 2.1 Hz, 1H), 7.33–7.28 (m, 1H), 7.19 (d, J = 8.2 Hz, 1H), 7.16–7.10 (m, 2H), 6.93 (dd, J = 17.8, 9.0 Hz, 3H), 6.86 (s, 1H), 4.80 (s, 2H), 1.83 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.9, 162.2 (JC-F = 244.0 Hz), 153.6, 150.7, 150.1, 143.8, 138.3, 132.8 (JC-F = 3.0 Hz), 130.62, 130.58, 130.5, 128.2, 127.1, 124.1, 122.4 (JC-F = 45.0 Hz), 115.4 (JC-F = 22.0 Hz), 51.9, 22.7; IR (KBr): 2922, 1660, 1602, 1581, 1509, 1428, 1378, 1303, 1197, 1139, 1086, 945, 773 cm−1; HRMS (ESI) m/z calculated for C20H17FN2O4SNa [M+Na]+: 423.0785; found 423.0795.
3-(N-(4-Chlorobenzyl)acetamido)phenyl pyridine-2-sulfonate (1i): The product was synthesized by general method A (eluent: EA: PE = 1:2, Rf = 0.23); 332.8 mg, 80% yield; white solid; m. p. = 84–85 °C; 1H NMR (400 MHz, CDCl3) δ 8.73 (d, J = 4.3 Hz, 1H), 7.93 (d, J = 3.7 Hz, 2H), 7.60 (dd, J = 8.9, 4.3 Hz, 1H), 7.32–7.28 (m, 1H), 7.20 (d, J = 8.0 Hz, 2H), 7.15 (d, J = 8.1 Hz, 1H), 7.07 (d, J = 8.1 Hz, 2H), 6.91 (d, J = 7.7 Hz, 1H), 6.85 (s, 1H), 4.77 (s, 2H), 1.81 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.9, 153.3, 150.6, 150.0, 143.6, 138.3, 135.5, 133.2, 130.6, 130.0, 128.6, 128.2, 126.9, 124.1, 122.4, 122.0, 51.9, 22.6; IR (KBr): 2923, 1661, 1601, 1486, 1428, 1379, 1291, 1197, 1139, 1117, 925, 802, 773 cm−1; HRMS (ESI) m/z calculated for C20H17ClN2O4SNa [M+Na]+: 439.0490; found 439.0492.
3-(N-(4-Bromobenzyl)acetamido)phenyl pyridine-2-sulfonate (1j): The product was synthesized by general method A (eluent: EA:PE = 1:1, Rf = 0.23); 291.1 mg, 76% yield; yellow gel; 1H NMR (400 MHz, CDCl3) δ 8.75 (d, J = 4.7 Hz, 1H), 7.95 (d, J = 6.5 Hz, 2H), 7.75–7.54 (m, 1H), 7.52–7.26 (m, 3H), 7.16 (d, J = 8.6 Hz, 1H), 7.03 (d, J = 8.1 Hz, 2H), 6.94 (d, J = 8.0 Hz, 1H), 6.87 (s, 1H), 4.78 (s, 2H), 1.83 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.9, 153.1, 150.5, 149.9, 143.5, 138.2, 135.9, 131.4, 130.6, 130.2, 128.2, 126.8, 124.0, 122.2, 121.9, 121.3, 51.9, 22.5; IR (KBr): 2959, 2920, 1660, 1602, 1582, 1486, 1428, 1379, 1197, 1139, 1118, 926, 866, 801, 773, 594, 551 cm−1; HRMS (ESI) m/z calculated for C20H17BrN2O4SNa [M+Na]+: 482.9985; found 482.9979.
3-(N-(4-(Trifluoromethoxy)benzyl)acetamido)phenyl pyridine-2-sulfonate (1k): The product was synthesized by general method A (eluent: EA:PE = 1:2, Rf = 0.24); 358.8 mg, 77% yield; white solid; m. p. = 77–78 °C; 1H NMR (400 MHz, CDCl3) δ 8.75 (d, J = 4.4 Hz, 1H), 8.03–7.84 (m, 2H), 7.67–7.58 (m, 1H), 7.35–7.30 (m, 1H), 7.19 (d, J = 8.5 Hz, 3H), 7.11 (d, J = 8.1 Hz, 2H), 6.92 (d, J = 8.9 Hz, 2H), 4.82 (s, 2H), 1.84 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.1, 153.4, 150.6, 150.1, 148.5, 143.8, 138.3, 135.7, 130.7, 130.1, 128.2, 126.9, 124.1, 122.5, 122.1, 120.9, 120.4 (JC-F = 255.0 Hz), 52.0, 22.7; IR (KBr): 2924, 1662, 1602, 1582, 1429, 1380, 1255, 1227, 1196, 991, 867, 801 cm−1; HRMS (ESI) m/z calculated for C21H17F3N2O5SNa [M+Na]+: 489.0702; found 489.0700.
3-(N-(4-Nitrobenzyl)acetamido)phenyl pyridine-2-sulfonate (1l): The product was synthesized by general method A (eluent: EA:PE = 1:2, Rf = 0.20); 354.5 mg, 83% yield; white solid; m. p. = 100–101 °C; 1H NMR (400 MHz, CDCl3) δ 8.75 (d, J = 4.6 Hz, 1H), 8.11 (d, J = 8.6 Hz, 2H), 8.01–7.93 (m, 2H), 7.66–7.57 (m, 1H), 7.36–7.32 (m, 3H), 7.16 (d, J = 7.8 Hz, 1H), 6.96 (d, J = 6.5 Hz, 2H), 4.92 (s, 2H), 1.87 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.2, 153.4, 150.6, 150.2, 147.3, 144.4, 143.5, 138.4, 130.8, 129.3, 128.3, 126.7, 124.1, 123.8, 122.4, 122.2, 52.2, 22.6; IR (KBr): 3077, 2923, 1662, 1601, 1518, 1428, 1378, 1344, 1197, 1140, 931, 861 cm−1; HRMS (ESI) m/z calculated for C20H17N3O6SNa [M+Na]+: 450.0730; found 450.0731.
3-(N-(4-(Trifluoromethyl)benzyl)acetamido)phenyl pyridine-2-sulfonate (1m): The product was synthesized by general method A (eluent: EA:PE = 1:2, Rf = 0.26); 342.1 mg, 76% yield; white solid; m. p. = 85–86 °C; 1H NMR (400 MHz, CDCl3) δ 8.78–8.65 (m, 1H), 7.99–7.87 (m, 2H), 7.71–7.56 (m, 1H), 7.52 (d, J = 8.1 Hz, 2H), 7.36–7.25 (m, 3H), 7.16 (d, J = 7.7 Hz, 1H), 6.94 (d, J = 8.2 Hz, 2H), 4.88 (s, 2H), 1.85 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.1, 153.4, 150.6, 150.1, 143.7, 141.0, 138.3, 130.7, 129.7 (JC-F = 32.0 Hz), 128.8, 128.2, 126.8, 125.5, 124.08, 124.07 (JC-F = 270.0 Hz), 122.4, 122.1, 52.4, 22.6; IR (KBr): 2925, 1663, 1602, 1486, 1380, 1324, 1198, 1115, 1066, 930, 802, 593 cm−1; HRMS (ESI) m/z calculated for C21H17F3N2O4SNa [M+Na]+: 473.0753; found 473.0750.
3-(N-(4-(Methylsulfonyl)benzyl)acetamido)phenyl pyridine-2-sulfonate (1n): The product was synthesized by general method A (eluent: EA:PE = 1:1, Rf = 0.22); 358.8 mg, 78% yield; yellow solid; m. p. = 132–137 °C; 1H NMR (400 MHz, CDCl3) δ 8.75 (d, J = 4.6 Hz, 1H), 8.00–7.95 (m, 2H), 7.86 (d, J = 8.2 Hz, 2H), 7.67–7.59 (m, 1H), 7.44–7.35 (m, 3H), 7.18 (d, J = 8.9 Hz, 1H), 6.98 (d, J = 7.4 Hz, 1H), 6.91 (s, 1H), 4.93 (s, 2H), 3.06 (s, 3H), 1.89 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 167.0, 152.7, 150.4, 149.7, 143.2, 143.0, 139.3, 138.3, 130.6, 129.0, 128.3, 127.3, 126.5, 123.9, 122.1, 121.9, 52.0, 44.2, 22.3; IR (KBr): 2954, 2923, 1659, 1600, 1548, 1378, 1302, 1197, 1147, 1089, 958, 931, 764, 594, 553, 523 cm−1; HRMS (ESI) m/z calculated for C21H20N2O6S2Na [M+Na]+: 483.0655; found 483.0652.
3-(N-(3-Methylbenzyl)acetamido)phenyl pyridine-2-sulfonate (1o): The product was synthesized by general method A (eluent: EA:PE = 1:2, Rf = 0.22); 281.2 mg, 71% yield; white solid; m. p. = 68–69 °C; 1H NMR (400 MHz, CDCl3) δ 8.78–8.74 (m, 1H), 7.95–7.88 (m, 2H), 7.64–7.56 (m, 1H), 7.29 (dd, J = 9.8, 6.4 Hz, 1H), 7.16–7.12 (m, 2H), 7.05 (d, J = 7.5 Hz, 1H), 6.98 (s, 1H), 6.90 (dd, J = 14.3, 6.7 Hz, 3H), 4.79 (s, 2H), 2.30 (s, 3H), 1.83 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.9, 153.4, 150.6, 149.9, 144.0, 138.2 (2C), 136.8, 130. 4, 129.3, 128.3 (2C), 128.2, 127.1, 125.6, 124.2, 122.5, 121.9, 52.7, 22.7, 21.4; IR (KBr): 2923, 1660, 1602, 1515, 1486, 1428, 1294, 1197, 1139, 1117, 1039, 948, 801, 773cm−1; HRMS (ESI) m/z calculated for C21H20N2O4SNa [M+Na]+: 419.1036; found 419.1026.
3-(N-([1,1’-Biphenyl]-3-ylmethyl)acetamido)phenyl pyridine-2-sulfonate (1p): The product was synthesized by general method A (eluent: EA:PE = 1:1, Rf = 0.24); 394.7 mg, 86% yield; yellow gel; 1H NMR (400 MHz, CDCl3) δ 8.64 (d, J = 4.1 Hz, 1H), 7.84–7.72 (m, 2H), 7.48 (dd, J = 19.3, 7.3 Hz, 4H), 7.42–7.22 (m, 6H), 7.01–6.86 (m, 2H), 7.15–7.07(m, 2H), 4.89 (s, 2H), 1.83 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.0, 153.2, 150.6, 150.0, 143.8, 141.3, 140.7, 138.2, 137.4, 130.6, 129.0, 128.8, 128.2, 127.5, 127.4, 127.3, 127.1(2C), 126.4, 124.2, 122.5, 122.1, 52.7, 22.8; IR (KBr): 2920, 2851, 1660, 1601, 1582, 1484, 1428, 1378, 1261, 1197, 1139, 1117, 936, 761, 737, 699, 593, 550 cm−1; HRMS (ESI) m/z calculated for C26H23N2O4S [M+H]+: 459.1373; found 459.1357.
3-(N-(2-Methylbenzyl)acetamido)phenyl pyridine-2-sulfonate (1q): The product was synthesized by general method A (eluent: EA:PE = 1:2, Rf = 0.24); 308.9 mg, 78% yield; white solid; m. p. = 104–105 °C; 1H NMR (400 MHz, CDCl3) δ 8.75 (d, J = 4.5 Hz, 1H), 7.94–7.87 (m, 2H), 7.59 (dd, J = 8.5, 4.9 Hz, 1H), 7.27–7.23 (m, 1H), 7.18–6.98 (m, 5H), 6.90–6.86 (m, 2H), 4.87 (s, 2H), 2.13 (s, 3H), 1.84 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.7, 153.3, 150.6, 149.9, 143.7, 138.2, 136.3, 134.5, 130.3 (2C), 129.2, 128.2, 127.5, 127.0, 125.9, 124.2, 122.4, 121.9, 49.9, 22.7, 19.0; IR (KBr): 2924, 1662, 1602, 1486, 1379, 1302, 1198, 1140, 1117, 925, 803, 770, 738, 617 cm−1; HRMS (ESI) m/z calculated for C21H20N2O4SNa [M+Na]+: 419.1036; found 419.1046.
5-(N-Benzylacetamido)-2-methylphenyl pyridine-2-sulfonate (1r): The product was synthesized by general method B (eluent: EA:PE = 1:1, Rf = 0.22); 333.5 mg, 84% yield; yellow gel; 1H NMR (400 MHz, CDCl3) δ 8.76 (d, J = 4.6 Hz, 1H), 7.93 (d, J = 3.4 Hz, 2H), 7.64–7.58 (m, 1H), 7.26 (d, J = 6.7 Hz, 3H), 7.19–7.11 (m, 3H), 6.90 (s, 1H), 6.80 (d, J = 8.1 Hz, 1H), 4.82 (s, 2H), 2.22 (s, 3H), 1.87 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.0, 153.4, 150.5, 148.2, 141.1, 138.2, 136.9, 132.0, 131.3, 128.4, 128.3, 128.1, 127.2, 126.6, 123.8, 122.0, 52.4, 22.5, 16.0; IR (KBr): 2955, 2924, 2853, 1659, 15613, 1579, 1504, 1428, 1197, 1104, 1087, 941, 867, 801, 751, 715, 596, 552cm−1; HRMS (ESI) m/z calculated for C21H21N2O4S [M+H]+: 397.1217; found 397.1207.
5-(N-Benzylacetamido)-2-methoxyphenyl pyridine-2-sulfonate (1s): The product was synthesized by general method B (eluent: EA:PE = 1:1, Rf = 0.22); 370.8 mg, 90% yield; yellow gel; 1H NMR (400 MHz, CDCl3) δ 8.75 (d, J = 5.1 Hz, 1H), 8.03–7.86 (m, 2H), 7.62 (dd, J = 4.4, 1.9 Hz, 1H), 7.36–7.11 (m, 5H), 6.96–6.92 (m, 1H), 6.86–6.77 (m, 2H), 4.82 (s, 2H), 3.59 (s, 3H), 1.88 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.4, 154.0, 151.2, 150.2, 138.3, 138.0, 137.0, 135.0, 128.6, 128.3, 127.9, 127.8, 127.3, 124.1, 123.7, 112.7, 55.8, 52.6, 22.6; IR (KBr): 2925, 2850, 1654, 1509, 1380, 1295, 1271, 1197, 1112, 780, 764, 599, 545 cm−1; HRMS (ESI) m/z calculated for C21H20N2O5SNa [M+Na]+: 435.0985; found 435.0984.
Methyl 4-(N-benzylacetamido)-2-((pyridin-2-ylsulfonyl)oxy)benzoate (1t): The product was synthesized by general method B (eluent: EA:PE = 1:1, Rf = 0.22); 295.5 mg, 67% yield; colorless gel; 1H NMR (400 MHz, CDCl3) δ 8.68 (d, J = 4.7 Hz, 1H), 8.03–7.93 (m, 2H), 7.88 (d, J = 8.3 Hz, 1H), 7.63–7.57 (m, 1H), 7.26 (d, J = 6.8 Hz, 3H), 7.18–7.08 (m, 3H), 7.04 (d, J = 8.3 Hz, 1H), 4.89 (s, 2H), 3.80 (s, 3H), 1.96 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.8, 164.3, 154.0, 150.4, 148.8, 147.1, 138.4, 136.6, 132.8, 128.6, 128.3, 128.1, 127.6, 126.4, 124.3, 123.9, 123.8, 52.7, 52.5, 22.9; IR (KBr): 2954, 2924, 2853, 1726, 1665, 1462, 1378, 1264, 1199, 1153, 740, 593 cm−1; HRMS (ESI) m/z calculated for C22H21N2O6S [M+H]+: 441.1115; found 441.1104.
5-Acetyl-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2a): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.22); 63.9 mg, 83% yield; white solid; m. p. = 151–152 °C; 1H NMR (400 MHz, CDCl3) δ 8.37 (d, J = 3.1 Hz, 1H), 8.00 (d, J = 7.4 Hz, 1H), 7.70 (d, J = 7.7 Hz, 1H), 7.66–7.62 (m, 1H), 7.11–7.52 (m, 7H), 4.43 (s, 2H), 2.12 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.8, 153.0, 149.6, 146.8, 140.3, 137.5, 135.9, 131.9, 128.2, 128.1, 127.9, 127.8, 127.8 (2C), 125.7, 123.8, 123.7, 121.9, 45.1, 22.1; IR (KBr): 2924, 2853, 1663, 1444, 1377, 1218, 1187, 1048, 960, 765, 735, 553 cm−1; HRMS (ESI) m/z calculated for C20H16N2O4SNa [M+Na]+: 403.0723; found 403.0720.
5-Acetyl-9-methyl-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2b): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.24); 67.8 mg, 84% yield; white solid; m. p. = 145–146 °C; 1H NMR (400 MHz, CDCl3) δ 8.39 (d, J = 2.4 Hz, 1H), 7.83–7.72 (m, 2H), 7.69–7.65 (m, 1H), 7.46–7.19 (m, 4H), 6.99 (dd, J = 20.2, 7.5 Hz, 2H), 4.40 (s, 2H), 2.28 (s, 3H), 2.11 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.8, 153.3, 149.6, 146.7, 140.4, 137.5, 137.1, 133.1, 128.7, 128.6, 127.8, 127.7, 125.5, 123.8, 123.7, 123.5, 121.9, 120.4, 44.8, 22.1, 21.4; IR (KBr): 2824, 1664, 1608, 1454, 1427, 1378, 1340, 1207, 1190, 1086, 1058, 767 cm−1; HRMS (ESI) m/z calculated for C21H18N2O4SNa [M+Na]+: 417.0879; found 417.0887.
5-Acetyl-9-ethyl-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2c): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.20); 66.3 mg, 81% yield; white solid; m. p. = 123–125 °C; 1H NMR (400 MHz, CDCl3) 8.31 (s, 1H), 7.77 (s, 1H), 7.65 (d, J = 7.8 Hz, 1H), 7.60–7.53 (m, 1H), 7.36–7.12 (m, 4H), 6.99–6.90 (m, 2H), 4.33 (s, 2H), 2.52 (q, J = 7.6 Hz, 2H), 2.04 (s, 3H), 1.17 (t, J = 7.6 Hz, 3H).; 13C NMR (100 MHz, CDCl3) δ 168.8, 153.1, 149.6, 146.8, 143.5, 140.3, 137.4, 133.3, 128.0, 127.8, 127.7, 127.6 (2C), 125.6, 124.0, 123.6 (2C), 121.8, 44.8, 28.8, 22.1, 15.6; IR (KBr): 3055, 2958, 2854, 1662, 1454, 1426, 1375, 1188, 1118, 1044, 948, 902, 878, 826, 810, 765, 724, 593, 564 cm−1; HRMS (ESI) m/z calculated for C22H21N2O4S [M+H]+: 409.1217; found 409.1201
5-Acetyl-9-isopropyl-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2d): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.3); 72.6mg, 79% yield; yellow solid; m. p. = 163–165 °C; 1H NMR (400 MHz, CDCl3) δ 8.39 (s, 1H), 7.91 (s, 1H), 7.71 (d, J = 7.8 Hz, 1H), 7.67–7.59 (m, 1H), 7.46–7.13 (m, 4H), 7.09–7.01 (m, 2H), 4.39 (s, 2H), 2.89 (p, J = 7.1, 5.8 Hz, 1H), 2.12 (s, 3H), 1.27 (d, J = 7.0 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 168.8, 152.9, 149.5, 148.1, 146.7, 140.3, 137.4, 133.4, 127.8, 127.7 (2C), 126.4, 126.3, 125.5, 124.1, 123.7, 123.6, 121.6, 44.8, 33.9, 23.9, 22.1; IR (KBr): 3016, 2961, 2927, 1662, 1455, 1426, 1379, 1213, 1186, 1118, 1049, 987, 949, 753, 667, 594 cm−1; HRMS (ESI) m/z calculated for C22H22N2O4SNa [M+Na]+: 445.1192; found 445.1188.
5-Acetyl-9-(tert-butyl)-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2e): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.21); 73.3 mg, 84% yield; white solid; m. p. = 181–182 °C; 1H NMR (400 MHz, CDCl3) δ 8.37 (s, 1H), 8.11 (s, 1H), 7.68 (d, J = 7.2 Hz, 1H), 7.66–7.56 (m, 1H), 7.48–7.20 (m, 5H), 7.06 (d, J = 7.9 Hz, 1H), 4.37 (s, 2H), 2.12 (s, 3H), 1.36 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 168.8, 152.9, 150.6, 150.5, 149.5, 146.8, 140.3, 138.2, 137.4, 133.1, 127.7 (2C), 125.4, 125.3, 124.4 123.8, 123.6, 121.5, 44.8, 34.8, 31.3, 22.2; IR (KBr): 2960, 1664, 1455, 1427, 1377, 1340, 1207, 1187, 1085, 1055, 882, 766 cm−1; HRMS (ESI) m/z calculated for C24H24N2O4SNa [M+Na]+: 459.1349; found 459.1345.
5-Acetyl-9-isobutyl-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2f): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.22); 64.5 mg, 74% yield; yellow solid; m. p. = 148–152 °C; 1H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 7.80 (s, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.69–7.62 (m, 1H), 7.40–7.29 (m, 3H), 7.22 (s, 1H), 7.04 (d, J = 7.6 Hz, 1H), 6.96 (d, J = 7.7 Hz, 1H), 4.49 (s, 2H), 2.42 (d, J = 7.1 Hz, 2H), 2.13 (s, 3H), 2.01–1.59 (m, 1H), 0.91 (d, J = 6.7 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 168.9, 153.4, 149.7, 146.8, 141.2, 140.4, 137.5, 133.3, 129.0, 128.8, 127.9, 127.7 (2C), 125.4, 124.1, 123.7 (2C), 121.7, 45.3, 44.9, 30.1, 22.4, 22.2; IR (KBr): 2955, 2925, 2868, 1663, 1579, 1455, 1418, 1377, 1340, 1206, 1190, 1118, 1052, 987, 950, 909, 871, 812, 768, 727, 593, 555 cm−1; HRMS (ESI) m/z calculated for C24H24N2O4SNa [M+Na]+: 459.1349; found 459.1345.
5-Acetyl-9-phenyl-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2g): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.20); 76.5 mg, 82% yield; white solid; m. p. = 141–142 °C; 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 3.3 Hz, 1H), 8.24 (d, J = 1.2 Hz, 1H), 7.69 (d, J = 7.8 Hz, 1H), 7.63 (d, J = 7.3 Hz, 2H), 7.55–7.49 (m, 1H), 7.51–7.36 (m, 6H), 7.31–7.19 (m, 3H), 4.52 (s, 2H), 2.16 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.9, 153. 1, 149.7, 146.7, 140.5, 140.3, 137.5, 134.9, 128.9 (2C), 128.5, 128.2, 127.9, 127.7, 127.5, 127.1, 126.9, 126.7, 126.2, 123.8, 123.5, 121.9, 44.8, 22.2; IR (KBr): 3057, 2924, 1664, 1605, 1452, 1376, 1340, 1217, 1205, 1186, 765, 742 cm−1; HRMS (ESI) m/z calculated for C26H20N2O4SNa [M+Na]+: 479.1036; found 479.1030.
5-Acetyl-9-fluoro-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2h): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.26); 53.7 mg, 69% yield; white solid; m. p. = 151–152 °C; 1H NMR (400 MHz, CDCl3) δ 8.46 (d, J = 3.4 Hz, 1H), 7.87 (d, J = 7.8 Hz, 1H), 7.80–7.76 (m, 1H), 7.75–7.64 (m, 1H), 7.52–7.37 (m, 3H), 7.30 (s, 1H), 7.13 (dd, J = 8.0, 5.9 Hz, 1H), 6.90–6.86 (m, 1H), 4.58 (s, 2H), 2.14 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.9, 161.6 (JC-F = 243.0 Hz), 153.2, 149.8, 146.7, 140.2, 137.9, 131.7, 129.7 (JC-F = 5.0 Hz), 128.7, 128.0, 126.9 (JC-F = 4.0 Hz), 123.7 (2C), 122.8, 122.0, 115.0 (JC-F = 16.2 Hz), 114.8 (JC-F = 13.3 Hz), 44.4, 22.0; IR (KBr): 3728, 2361, 2341, 1665, 1606, 1591, 1454, 1420, 1381, 1340, 1219, 1184, 814 cm−1; HRMS (ESI) m/z calculated for C20H15FN2O4SNa [M+Na]+: 421.0629; found 421.0620.
5-Acetyl-9-chloro-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2i): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.22); 63.8 mg, 81% yield; white solid; m. p. = 172–173 °C; 1H NMR (400 MHz, CDCl3) δ 8.44 (d, J = 3.9 Hz, 1H), 7.92 (d, J = 7.7 Hz, 2H), 7.81–7.77 (m, 1H), 7.39 (ddd, J = 12.4, 11.3, 6.3 Hz, 3H), 7.27 (d, J = 7.3 Hz, 1H), 7.18–7.06 (m, 2H), 4.60 (s, 2H), 2.13 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.8, 153.5, 149.9, 146.7, 140.4, 137.9, 134.4, 133.3, 129.8, 128.8, 128.0, 127.9 (2C), 126.9, 123.8, 123.7, 122.5, 122.2, 44.5, 22.1; IR (KBr): 2923, 2852, 2361, 1665, 1606, 1467, 1452, 1403, 1380, 1338, 1218, 1186, 816,764 cm−1; HRMS (ESI) m/z calculated for C20H15ClN2O4SNa [M+Na]+: 437.0333; 437.0330.
5-Acetyl-9-bromo-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2j): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.22); 68.5mg, 75% yield; white solid; m. p. = 167–169 °C; 1H NMR (400 MHz, CDCl3) δ 8.40 (d, J = 5.2 Hz, 1H), 8.01 (d, J = 3.3 Hz, 1H), 7.91 (d, J = 8.0 Hz, 1H), 7.82–7.74 (m, 1H), 7.46–7.30 (m, 3H), 7.29–7.18 (m, 2H), 7.01 (d, J = 8.1 Hz, 1H), 4.42 (s, 2H), 2.10 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.9, 153.7, 149.9, 146.8, 140.4, 138.1, 134.9, 130.9, 130.8, 130.3 128.8, 128.0, 127.2 (2C), 123.8 (2C), 122.3 (2C), 121.4, 44.7, 22.2; IR (KBr): 3055, 2924, 2853, 1661, 1578, 1451, 1373, 1337, 1217, 1185, 1117, 871, 842, 763, 735, 721, 592, 556 cm−1; HRMS (ESI) m/z calculated for C20H15N2O4SBrNa [M+Na]+: 480.9828; found 480.9827.
5-Acetyl-9-(trifluoromethoxy)-5,6-dihydrophenanthridin-1-yl pyridine- 2-sulfonate (2k): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.21); 77.0 mg, 83% yield; white solid; m. p. = 145–146 °C; 1H NMR (400 MHz, CDCl3) δ 8.44 (d, J = 4.1 Hz, 1H), 7.87 (d, J = 7.7 Hz, 2H), 7.78–7.74 (m, 1H), 7.50–7.37 (m, 3H), 7.30 (s, 1H), 7.21 (d, J = 8.3 Hz, 1H), 7.06 (d, J = 8.2 Hz, 1H), 4.59 (s, 2H), 2.16 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.8, 153.2, 149.7, 148.2, 146.8, 140.2, 137.9, 134.5, 134.4, 129.9, 128.9, 127.9, 126.9, 123.7, 122.5, 121.8, 120.8, 120.4, 120.3 (JC-F = 255.0 Hz), 44.5, 22.1; IR (KBr): 2923, 2360, 1666, 1455, 1427, 1382, 1340, 1253, 1215, 1118, 872, 811, 765 cm−1; HRMS (ESI) m/z calculated for C21H15F3N2O5SNa [M+Na]+: 487.0546; found 487.0556.
5-Acetyl-9-nitro-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2l): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.23); 60.9 mg, 68% yield; white solid; m. p. = 167–168 °C; 1H NMR (400 MHz, CDCl3) δ 8.81 (d, J = 1.8 Hz, 1H), 8.47 (d, J = 3.9 Hz, 1H), 8.09–8.03 (m, 1H), 7.99 (d, J = 7.8 Hz, 1H), 7.89–7.77 (m, 1H), 7.51–7.41 (m, 4H), 7.37–7.26 (m, 1H), 4.82 (s, 2H), 2.17 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.9, 153.6, 150.1, 147.4, 146.8, 142.7, 140.4, 138.2, 129.8, 129.5, 128.3, 126.7, 123.9, 123.8, 123.0, 122.8, 122.1, 121.9, 44.9, 22.1; IR (KBr): 2922, 2852, 2361, 1667, 1608, 1524, 1383, 1347, 1219, 1187, 872, 835, 811 cm−1; HRMS (ESI) m/z calculated for C20H15N3O6SNa [M+Na]+: 448.0574; found 448.0581.
5-Acetyl-9-(trifluoromethyl)-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2m): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.22); 65.4 mg, 73% yield; white solid; m. p. = 153–154 °C; 1H NMR (400 MHz, CDCl3) δ 8.41 (d, J = 4.1 Hz, 1H), 8.24 (s, 1H), 7.89 (d, J = 7.9 Hz, 1H), 7.78–7.74 (m, 1H), 7.45 (dd, J = 16.0, 8.3 Hz, 3H), 7.40–7.35 (m, 1H), 7.34–7.27 (m, 2H), 4.70 (s, 2H), 2.16 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.9, 153.4, 149.8, 146.9, 140.4, 139.6, 138.0, 129.8 (JC-F = 32.4 Hz), 129.1, 129.0, 128.0, 126.3 (JC-F = 1.8 Hz), 124.8 (JC-F = 4.2 Hz, 2C), 123.8 (2C), 123.7 (JC-F = 270.8 Hz), 122.4, 122.0, 44.9, 22.1; IR (KBr): 2924, 2361, 1667, 1455, 1422, 1382, 1331, 1254, 1167, 1119, 1078, 810, 764 cm−1; HRMS (ESI) m/z calculated for C21H15F3N2O4SNa [M+Na]+: 471.0597; found 471.0591.
5-Acetyl-9-(methylsulfonyl)-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2n): The product was synthesized by general method C (eluent: EA:PE = 1.5:1, Rf = 0.20); 64.1 mg, 70% yield; yellow solid; m. p. = 132–137 °C; 1H NMR (400 MHz, CDCl3) δ 8.69 (s, 1H), 8.49 (d, J = 4.7 Hz, 1H), 7.98 (d, J = 7.9 Hz, 1H), 7.94–7.76 (m, 2H), 7.51–7.37 (m, 4H), 7.29 (s, 1H), 4.77 (s, 2H), 3.17 (s, 3H), 2.17 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.0, 153.0, 149.9, 146.9, 141.4, 140.4, 140.1, 138.4, 129.7, 129.3, 128.2, 127.2, 126.7 (2C), 124.4, 123.8, 122.5, 121.8, 44.9, 44.3, 22.1; IR (KBr): 3019, 2925, 1664, 1377, 1317, 1214, 1148, 1118, 745, 667, 562 cm−1; HRMS (ESI) m/z calculated for C21H18N2O6S2Na [M+Na]+: 481.0498; found 481.0496.
5-Acetyl-8-methyl-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2o): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.23); 62.3 mg, 79% yield; white solid; m. p. = 190–191 °C; 1H NMR (400 MHz, CDCl3) δ 8.41 (s, 1H), 7.90 (d, J = 8.0 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.68–7.64 (m, 1H), 7.39–7.30 (m, 3H), 7.22 (s, 1H), 7.03 (d, J = 7.6 Hz, 1H), 6.96 (s, 1H), 4.48 (s, 2H), 2.32 (s, 3H), 2.12 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.0, 153.2, 149.7, 146.7, 140.2, 138.4, 137.5, 135.9, 128.5, 128.1, 127.7, 127.5, 126.4, 125.3, 123.9 (2C), 123.7, 121.9, 45.2, 22.2, 21.2; IR (KBr): 2923, 2853, 1664, 1456, 1378, 1212, 1188, 1118, 1054, 986, 959, 866 cm−1; HRMS (ESI) m/z calculated for C21H18N2O4SNa [M+Na]+: 417.0879; found 417.0883.
5-Acetyl-8-phenyl-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2p): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.22); 75.9mg, 83% yield; white solid; m. p. = 209–210 °C; 1H NMR (400 MHz, CDCl3) δ 8.36 (d, J = 4.7 Hz, 1H), 8.05 (d, J = 8.2 Hz, 1H), 7.74 (d, J = 7.9 Hz, 1H), 7.60 (dd, J = 19.8, 7.8 Hz, 3H), 7.42 (ddd, J = 30.2, 13.9, 7.7 Hz, 7H), 7.30–7.21 (m, 2H), 4.48 (s, 2H), 2.15 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.1, 153.1, 149.7, 146.9, 140.8, 140.3, 139.8, 137.5, 136.5, 129.0, 128.7, 128.0, 127.9, 127.8, 127.0, 126.9, 126.3, 124.1, 124.0, 123.7 (2C), 122.2, 45.2, 22.2; IR (KBr): 3010, 2924, 2853, 1660, 1466, 1377, 1336, 1214, 1188, 1118, 986, 957, 866, 809, 748, 699, 594, 557, 541cm−1; HRMS (ESI) m/z calculated for C26H21N2O4S [M+H]+: 457.1217; found 457.1198.
5-Acetyl-7-methyl-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2q): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.25); 63.1 mg, 80% yield; white solid; m. p. = 159–160 °C; 1H NMR (400 MHz, CDCl3) δ 8.39 (s, 1H), 7.84 (d, J = 7.7 Hz, 1H), 7.74–7.56 (m, 2H), 7.48–7.21 (m, 4H), 7.13–7.09 (m, 1H), 7.03 (d, J = 7.3 Hz, 1H), 4.43 (s, 2H), 2.34 (s, 3H), 2.13 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.9, 153.1, 149.5, 146.7, 140.3, 137.4, 135.2, 133.5, 129.9, 127.9, 127.4, 127.1, 125.9 (2C), 124.4, 123.9, 123.5, 122.0, 41.7, 22.1, 19.1; IR (KBr): 2924, 1664, 1480, 1454, 1427, 1377, 1339, 1189, 1118, 813, 766, 621 cm−1; HRMS (ESI) m/z calculated for C21H18N2O4SNa [M+Na]+: 417.0879; found 417.0870.
5-Acetyl-2-methyl-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2r): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.22); 71.7 mg, 91% yield; white solid; m. p. = 206–208 °C; 1H NMR (400 MHz, CDCl3) δ 8.47 (s, 1H), 7.92 (d, J = 7.9 Hz, 1H), 7.80 (d, J = 7.8 Hz, 1H), 7.76–7.69 (m, 1H), 7.47–7.32 (m, 1H), 7.22–7.07 (m, 3H), 7.04–6.97 (m, 1H), 6.95 (d, J = 8.8 Hz, 1H), 4.54 (s, 2H), 3.76 (s, 3H), 2.09 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.0, 153.8, 151.1, 149.6, 145.6, 138.1, 137.4, 135.7, 131.8, 130.3, 128.9, 127.9, 127.7, 127.4, 125.6, 124.1, 123.5, 123.0, 45.3, 22.1, 17.7; IR (KBr): 3019, 2361, 1654, 1481, 1452, 1377, 1214, 1117, 1049, 868, 743, 667, 591, 546 cm−1; HRMS (ESI) m/z calculated for C21H19N2O4S [M+H]+: 395.1060; found 395.1061.
5-Acetyl-2-methoxy-5,6-dihydrophenanthridin-1-yl pyridine-2-sulfonate (2s): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.22); 65.9 mg, 80% yield; yellow solid; m. p. = 210–213 °C; 1H NMR (400 MHz, CDCl3) δ 8.36 (d, J = 4.7 Hz, 1H), 7.75 (d, J = 7.9 Hz, 1H), 7.59 (dd, J = 16.3, 7.7 Hz, 2H), 7.27 (d, J = 7.7 Hz,2H), 7.15 (s, 1H), 7.10–7.00 (m, 2H), 6.94 (d, J = 7.8 Hz, 1H), 4.62 (s, 2H), 2.55 (s, 3H), 2.10 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.0, 153.8, 149.6, 145.7, 138.2, 137.4, 135.8, 131.8, 130.4, 128.9, 127.9, 127.7 (2C), 127.4, 125.7, 124.1, 123.5, 123.0, 45.3, 22.2, 17.8; IR (KBr): 3008, 2926, 2848, 1652, 1481, 1429, 1376, 1236, 1196, 1111, 1049, 1019, 986, 964, 867, 790, 746, 703, 666, 587, 553 cm−1; HRMS (ESI) m/z calculated for C21H20N2O5SNa [M+Na]+: 435.0985; found 435.0984.
Methyl 5-acetyl-1-((pyridin-2-ylsulfonyl)oxy)-5,6-dihydrophenanthridine-2-carb- oxylate (2t): The product was synthesized by general method C (eluent: EA:PE = 1:1, Rf = 0.22); 65.7 mg, 75% yield; white solid; m. p. = 200–203 °C; 1H NMR (400 MHz, CDCl3) δ 8.20 (dd, J = 4.7, 1.9 Hz, 1H), 7.97 (dd, J = 14.3, 8.9 Hz, 2H), 7.68 (d, J = 7.8 Hz, 1H), 7.61–7.57 (m, 1H), 7.29 (s, 1H), 7.20 (ddd, J = 7.6, 4.7, 1.2 Hz, 1H), 7.12 (dd, J = 5.8, 3.3 Hz, 2H), 7.08–7.01 (m, 1H), 4.53 (s, 2H), 4.01 (s, 3H), 2.17 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.7, 165.5, 153.3, 149.6, 145.6, 143.3, 137.5, 135.5, 130.7, 128.5, 128.4, 128.1, 127.9, 127.7, 125.5, 124.9 (2C), 123.6, 123.1, 52.7, 45.0, 22.4; IR (KBr): 2925, 2851, 1656, 1496, 1481, 1452, 1429, 1376, 1237, 1197, 1164, 1112, 1050, 1019, 988, 965, 867, 808, 790, 725, 702, 615, 587, 554 cm−1; HRMS (ESI) m/z calculated for C22H18N2O6SNa [M+Na]+: 461.0778; found 461.0772.
4. Conclusions
In summary, we developed the first palladium-catalyzed intramolecular C−H/C−H dehydrogenative coupling between two simple arenes to construct 5,6-dihydrophenanthridines. The approach featured a broad substrate scope and good tolerance of functional groups, and it offers an efficient alternative synthesis route for the important 5,6-dihydrophenanthridine compounds. Further application and mechanistic studies are currently ongoing in our laboratory.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28062498/s1, Reference [59] is cited in the Supplementary Materials.
Author Contributions
Y.-Q.W. conceived and designed the experiments; M.-Y.W. and X.-Q.Z. performed the experiments; B.-Y.Z. and H.-X.Z. analyzed the data; M.-Y.W., Y.-Q.W. and Q.J. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC-21971206).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stermitz, F.R.; Larson, K.A.; Kim, D.K. Structural relations among cytotoxic and antitumor benzophenanthridine alkaloid derivatives. J. Med. Chem. 1973, 16, 939–940. [Google Scholar] [CrossRef] [PubMed]
- Krane, B.D.; Fagbule, M.O.; Shamma, M.; Gözler, B. The Benzophenanthridine Alkaloids. J. Nat. Prod. 1984, 47, 1–43. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Hsieh, P.-W.; Chang, F.-R.; Wu, R.-R.; Liaw, C.-C.; Lee, K.-H.; Wu, Y.-C. Two New Protopines Argemexicaines A and B and the Anti-HIV Alkaloid 6-Acetonyldihydrochelerythrine from Formosan Argemone Mexicana. Planta Med. 2003, 69, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, O.B.; Morikawa, T.; Ando, S.; Matsuda, H.; Yoshikawa, M. New Crinine-Type Alkaloids with Inhibitory Effect on Induction of Inducible Nitric Oxide Synthase from Crinum yemense. J. Nat. Prod. 2004, 67, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Fotie, J.; Bohle, D.S.; Olivier, M.; Gomez, M.A.; Nzimiro, S. Trypanocidal and Antileishmanial Dihydrochelerythrine Derivatives from Garcinia lucida. J. Nat. Prod. 2007, 70, 1650–1653. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, S.; Lang, M.; Dreker, T.; Kraus, J.; Hamm, S.; Meere, C.; Feurle, J.; Tasler, S.; Prütting, S.; Kuras, Z.; et al. Inhibitors of potassium channels KV1.3 and IK-1 as immunosuppressants. Bioorg. Med. Chem. Lett. 2009, 19, 2299–2304. [Google Scholar] [CrossRef]
- Park, J.E.; Cuong, T.D.; Hung, T.M.; Lee, I.; Na, M.; Kim, J.C.; Ryoo, S.; Lee, J.H.; Choi, J.S.; Woo, M.H.; et al. Alkaloids from Chelidonium majus and their inhibitory effects on LPS-induced NO production in RAW264.7 cells. Bioorg. Med. Chem. Lett. 2011, 21, 6960–6963. [Google Scholar] [CrossRef]
- Yang, X.-J.; Miao, F.; Yao, Y.; Cao, F.-J.; Yang, R.; Ma, Y.-N.; Qin, B.-F.; Zhou, L. In Vitro Antifungal Activity of Sanguinarine and Chelerythrine Derivatives against Phytopathogenic Fungi. Molecules 2012, 17, 13026–13035. [Google Scholar] [CrossRef]
- Éles, J.; Beke, G.; Vágó, I.; Bozó, É.; Huszár, J.; Tarcsay, Á.; Kolok, S.; Schmidt, É.; Vastag, M.; Hornok, K.; et al. Quinolinyl- and phenantridinyl-acetamides as bradykinin B1 receptor antagonists. Bioorg. Med. Chem. Lett. 2012, 22, 3095–3099. [Google Scholar] [CrossRef]
- Lee, S.-S.; Venkatesham, U.; Rao, C.P.; Lam, S.-H.; Lin, J.-H. Preparation of secolycorines against acetylcholinesterase. Bioorg. Med. Chem. 2007, 15, 1034–1043. [Google Scholar] [CrossRef]
- Malhotra, R.; Rarhi, C.; Diveshkumar, K.V.; Barik, R.; D’cunha, R.; Dhar, P.; Kundu, M.; Chattopadhyay, S.; Roy, S.; Basu, S.; et al. Dihydrochelerythrine and its derivatives: Synthesis and their application as potential G-quadruplex DNA stabilizing agents. Bioorg. Med. Chem. 2016, 24, 2887–2896. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Z.; Jing, C.-X.; Cai, J.-Y.; Wu, J.-B.; Wang, S.; Yin, J.-L.; Li, X.-N.; Li, L.; Hao, X.-J. Design, Synthesis, and Structural Optimization of Lycorine-Derived Phenanthridine Derivatives as Wnt/β-Catenin Signaling Pathway Agonists. J. Nat. Prod. 2016, 79, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Suzuki, M.; Saimoto, A.; Kabasawa, T. Structural Considerations of NK109, an Antitumor Benzo[c]phenanthridine Alkaloid. J. Nat. Prod. 1999, 62, 864–867. [Google Scholar] [CrossRef]
- Beuria, T.K.; Santra, M.K.; Panda, D. Sanguinarine Blocks Cytokinesis in Bacteria by Inhibiting FtsZ Assembly and Bundling+. Biochemistry 2005, 44, 16584–16593. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, W.-D.; Liu, R.-H.; Zhang, C.; Shen, Y.-H.; Li, H.-L.; Liang, M.-J.; Xu, X.-K. Benzophenanthridine Alkaloids from Zanthoxylum nitidum (Roxb.) DC, and Their Analgesic and Anti-Inflammatory Activities. Chem. Biodivers. 2006, 3, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Cheng, M.-J.; Chiang, M.Y.; Kuo, Y.-H.; Wang, C.-J.; Chen, I.-S. Dihydrobenzo[c]phenanthridine Alkaloids from Stem Bark of Zanthoxylum nitidum. J. Nat. Prod. 2008, 71, 669–673. [Google Scholar] [CrossRef]
- Parhi, A.; Kelley, C.; Kaul, M.; Pilch, D.S.; LaVoie, E.J. Antibacterial activity of substituted 5-methylbenzo[c]phenanthridinium derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 7080–7083. [Google Scholar] [CrossRef] [PubMed]
- Farrow, S.C.; Facchini, P.J. Dioxygenases Catalyze O-Demethylation and O,O-Demethylenation with Widespread Roles in Benzylisoquinoline Alkaloid Metabolism in Opium Poppy. J. Bio. Chem. 2013, 288, 28997–29012. [Google Scholar] [CrossRef]
- Yu, X.; Gao, X.; Zhu, Z.; Cao, Y.; Zhang, Q.; Tu, P.; Chai, X. Alkaloids from the Tribe Bocconieae (Papaveraceae): A Chemical and Biological Review. Molecules 2014, 19, 13042–13060. [Google Scholar] [CrossRef]
- Chen, D.-Z.; Fan, S.-R.; Yang, B.-J.; Yao, H.-C.; Wang, Y.-T.; Cai, J.-Y.; Jing, C.-X.; Pan, Z.-H.; Luo, M.; Yuze, Y.-Q.; et al. Phenanthridine Derivative Host Heat Shock Cognate 70 DownRegulators as Porcine Epidemic Diarrhea Virus Inhibitors. J. Nat. Prod. 2021, 84, 1175–1184. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Long, X.-Y.; Ding, X.; Fan, S.-R.; Cai, J.-Y.; Yang, B.-J.; Zhang, X.-F.; Luo, R.-h.; Yang, L.; Ruan, T.; et al. Novel nucleocapsid protein-targeting phenanthridine inhibitors of SARS-CoV-2. Eur. J. Med. Chem. 2022, 227, 113966. [Google Scholar] [CrossRef] [PubMed]
- Read, M.L.; Gundersen, L.-L. Synthesis of Phenanthridine Derivatives by Microwave-Mediated Cyclization of o-Furyl(allylamino)arenes. J. Org. Chem. 2013, 78, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, F.; Zhang, S.; He, Y.; Yang, N.; Fan, Q.-H. Ruthenium-Catalyzed Enantioselective Hydrogenation of Phenanthridine Derivatives. Org. Lett. 2017, 19, 1458–1461. [Google Scholar] [CrossRef]
- Intrieri, D.; Mariani, M.; Caselli, A.; Ragaini, F.; Gallo, E. [Ru(TPP)CO]-Catalysed Intramolecular Benzylic C-H Bond Amination, Affording Phenanthridine and Dihydrophenanthridine Derivatives. Chem. Eur. J. 2012, 18, 10487–10490. [Google Scholar] [CrossRef]
- Augustine, J.K.; Bombrun, A.; Alagarsamy, P.; Jothi, A. Selective synthesis of 5,6-dihydrophenanthridines, 5,6-dihydrobenzo[c][1,8]naphthyridines and their fully aromatized analogues via the Pictet-Spengler reaction mediated by peptide coupling agent propylphosphonic anhydride (T3P). Tetrahedron Lett. 2012, 53, 6280–6287. [Google Scholar] [CrossRef]
- Lee, W.-I.; Jung, J.-W.; Jang, J.; Yun, H.; Suh, Y.-G. Synthesis of 5,6-dihydrophenanthridines via N,O-acetal TMS ethers. Tetrahedron Lett. 2013, 54, 5167–5171. [Google Scholar] [CrossRef]
- Bao, X.; Yao, W.; Zhu, Q.; Xu, Y. Synthesis of 6-Substituted Phenanthridine Derivatives by Palladium-Catalysed Domino Suzuki-Miyaura/Aza-Michael Reactions. Eur. J. Org. Chem. 2014, 2014, 7443–7450. [Google Scholar] [CrossRef]
- Wang, C.; An, D.; Guan, X.; Fan, Y.; Liu, G.; Zhang, G.; Zhang, S. Organocatalytic Enantioselective Synthesis of 6-Aryl-5,6-dihydrophenanthridines via a Modified Pictet-Spengler Reaction of Biphenyl-2-amines and Aromatic Aldehydes. Eur. J. Org. Chem. 2017, 2017, 1865–1869. [Google Scholar] [CrossRef]
- Raju, S.; Annamalai, P.; Chen, P.-L.; Liu, Y.-H.; Chuang, S.-C. Palladium-Catalyzed C−H Bond Activation by Using Iminoquinone as a Directing Group and an Internal Oxidant or a Co-oxidant: Production of Dihydrophenanthridines, Phenanthridines, and Carbazoles. Org. Lett. 2017, 19, 4134–4137. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, C.; Zhang, X.; Fan, X. sSelective Synthesis of Dihydrophenanthridine and Phenanthridine Derivatives from the Cascade Reactions of o-Arylanilines with Alkynoates through C−H/N−H/C−C Bond Cleavage. J. Org. Chem. 2021, 86, 5805–5819. [Google Scholar] [CrossRef]
- Barluenga, J.; Fañanás, F.J.; Sanz, R.; Fernández, Y. Synthesis of Functionalized Indole- and Benzo-Fused Heterocyclic Derivatives through Anionic Benzyne Cyclization. Chem. Eur. J. 2002, 8, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.S.; Lagishetti, C.; Chen, S.; Kiran, I.N.C.; He, Y. Synthesis of Dihydrophenanthridines and Oxoimidazolidines from Anilines and Ethylglyoxylate via Aza Diels-Alder Reaction of Arynesand KF-Induced Annulation. Org. Lett. 2016, 18, 4546–4549. [Google Scholar] [CrossRef] [PubMed]
- Asamdi, M.; Chauhan, P.M.; Patel, J.J.; Chikhalia, K.H. Palladium catalyzed annulation of benzylamines and arynes via C-H activation to construct 5,6-dihydrophenanthridine derivatives. Tetrahedron 2019, 75, 3485–3494. [Google Scholar] [CrossRef]
- Campeau, L.-C.; Parisien, M.; Jean, A.; Fagnou, K. Catalytic Direct Arylation with Aryl Chlorides, Bromides, and Iodides: Intramolecular Studies Leading to New Intermolecular Reactions. J. Am. Chem. Soc. 2006, 128, 581–590. [Google Scholar] [CrossRef]
- Cropper, E.L.; White, A.J.P.; Ford, A.; Hii, K.K. Ligand Effects in the Synthesis of N-Heterocycles by Intramolecular Heck Reactions. J. Org. Chem. 2006, 71, 1732–1735. [Google Scholar] [CrossRef]
- Bonnaterre, F.; Bois-Choussy, M.; Zhu, J. Synthesis of dihydrophenanthridines by a sequence of Ugi-4CR and palladium-catalyzed intramolecular C-H functionalization. Beilstein J. Org. Chem. 2008, 4, 10. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Nath, S.; Chattopadhyay, B.; Sinha, B. Palladium-Catalyzed Tethered Intramolecular Arylation: An Unusual Synthesis of Linearly Fused Pyridocoumarin Derivatives. Synthesis 2010, 22, 3918–3926. [Google Scholar] [CrossRef]
- Sun, C.-L.; Gu, Y.-F.; Huang, W.-P.; Shi, Z.-J. Neocuproine–KOtBu promoted intramolecular cross coupling to approach fused rings. Chem. Commun. 2011, 47, 9813–9815. [Google Scholar] [CrossRef]
- Roman, D.S.; Takahashi, Y.; Charette, A.B. Potassium tert-Butoxide Promoted Intramolecular Arylation via a Radical Pathway. Org. Lett. 2011, 13, 3242–3245. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, P.; Singh, H.B.; Butcher, R.J. Intramolecular C-C coupling of 2,6-disubstituted-1-bromoaryls for dihydrophenanthridines. Tetrahedron Lett. 2012, 53, 4591–4594. [Google Scholar] [CrossRef]
- De, S.; Mishra, S.; Kakde, B.N.; Dey, D.; Bisai, A. Expeditious Approach to Pyrrolophenanthridones, Phenanthridines, and Benzo[c]phenanthridines via Organocatalytic Direct Biaryl-Coupling Promoted by Potassium tert-Butoxide. J. Org. Chem. 2013, 78, 7823–7844. [Google Scholar] [CrossRef] [PubMed]
- Laha, J.K.; Dayal, N.; Jain, R.; Patel, K. Palladium-Catalyzed Regiocontrolled Domino Synthesis of N-Sulfonyl Dihydrophenanthridines and Dihydrodibenzo[c,e]azepines: Control over the Formation of Biaryl Sultams in the Intramolecular Direct Arylation. J. Org. Chem. 2014, 79, 10899–10907. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, M.; Sharma, S.; Nayal, O.S.; Kumar, N.; Singh, B.; Sharma, U. Microwave Assisted Synthesis of Phenanthridinones and Dihydrophenanthridines by Vasicine/KOtBu Promoted Intramolecular C-H Arylation. Org. Biomol. Chem. 2016, 14, 8536–8544. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Neuburger, M.; Baudoin, O. Chiral Bifunctional Phosphine-Carboxylate Ligands for Palladium(0)-Catalyzed Enantioselective C-H Arylation. Angew. Chem. Int. Ed. 2018, 57, 1394–1398. [Google Scholar] [CrossRef]
- An, Y.; Zhang, B.-S.; Zhang, Z.; Liu, C.; Gou, X.-Y.; Ding, Y.-N.; Liang, Y.-M. A carboxylate-assisted amination/unactivated C(sp2)-H arylation reaction via a palladium/ norbornene cooperative catalysis. Chem. Commun. 2020, 56, 5933–5936. [Google Scholar] [CrossRef]
- Alberico, D.; Scott, M.E.; Lautens, M. Aryl−Aryl Bond Formation by Transition-Metal-Catalyzed Direct Arylation. Chem. Rev. 2007, 107, 174–238. [Google Scholar] [CrossRef]
- García-Rubia, A.; Arrayás, R.G.; Carretero, J.C. Palladium(II)-Catalyzed Regioselective Direct C2 Alkenylation of Indoles and Pyrroles Assisted by the N-(2-Pyridyl)sulfonyl Protecting Group. Angew. Chem. Int. Ed. 2009, 48, 6511–6515. [Google Scholar] [CrossRef]
- Yan, Z.-L.; Chen, W.-L.; Gao, Y.-R.; Mao, S.; Zhang, Y.-L.; Wang, Y.-Q. Palladium-Catalyzed Intermolecular C-2 Alkenylation of Indoles Using Oxygen as the Oxidant. Adv. Synth. Catal. 2014, 356, 1085–1092. [Google Scholar] [CrossRef]
- Perez, K.A.; Rogers, C.R.; Weiss, E.A. Quantum Dot-Catalyzed Photoreductive Removal of Sulfonyl-Based Protecting Groups. Angew. Chem. Int. Ed. 2020, 59, 14091–14095. [Google Scholar] [CrossRef]
- Cuny, G.D. Synthesis of (±)-Aporphine Utilizing Pictet−Spengler and Intramolecular Phenol ortho-Arylation Reactions. Tetrahedron Lett. 2004, 45, 5167–5170. [Google Scholar] [CrossRef]
- García-Rubia, A.; Urones, B.; Arrayás, R.G.; Carretero, J.C. PdII-Catalysed C-H Functionalisation of Indoles and Pyrroles Assisted by the Removable N-(2-Pyridyl)sulfonyl Group: C2-Alkenylation and Dehydrogenative Homocoupling. Chem. Eur. J. 2010, 16, 9676–9685. [Google Scholar] [CrossRef] [PubMed]
- Pintori, D.G.; Greaney, M.F. Intramolecular Oxidative C-H Coupling for Medium-Ring Synthesis. J. Am. Chem. Soc. 2011, 133, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liang, Z.; Wang, Y.; Zhang, Y. Palladium(II)-Catalyzed Direct Alkenylation and Arylation of Arenes: Removable 2-Pyridylsulfinyl Group Assisted C-H Bond Activation. J. Org. Chem. 2011, 76, 4987–4994. [Google Scholar] [CrossRef] [PubMed]
- Laha, J.K.; Jethava, K.P.; Dayal, N. Palladium-Catalyzed Intramolecular Oxidative Coupling Involving Double C(sp2)-H Bonds for the Synthesis of Annulated Biaryl Sultams. J. Org. Chem. 2014, 79, 8010–8019. [Google Scholar] [CrossRef] [PubMed]
- Laha, J.K.; Dayal, N.; Jethava, K.P.; Prajapati, D.V. Access to Biaryl Sulfonamides by Palladium-Catalyzed Intramolecular Oxidative Coupling and Subsequent Nucleophilic Ring Opening of Heterobiaryl Sultams with Amines. Org. Lett. 2015, 17, 1296–1299. [Google Scholar] [CrossRef]
- Shen, Z.; Ni, Z.; Mo, S.; Wang, J.; Zhu, Y. Palladium-Catalyzed Intramolecular Decarboxylative Coupling of AreneCarboxylic Acids/Esters with Aryl Bromides. Chem. Eur. J. 2012, 18, 4859–4865. [Google Scholar] [CrossRef]
- Laha, J.K.; Gupta, P. Sulfoxylate Anion Radical-Induced Aryl Radical Generation and Intramolecular Arylation for the Synthesis of Biarylsultams. J. Org. Chem. 2022, 87, 4204–4214. [Google Scholar] [CrossRef]
- Pegoraro, S.; Lang, M.; Feurle, J.; Krauss, J. Novel Phenantridine Analogues and Their Uses as Inhibitors of Hyperproliferation of T Cells and/or Keratinocytes. WO 2005/105752 Al., 10 November 2005. [Google Scholar]
- Zhang, X.-S.; Zhang, Y.-F.; Li, Z.-W.; Luo, F.-X.; Shi, Z.-J. Synthesis of Dibenzo[c,e]oxepin-5(7H)-ones from Benzyl Thioethers and Carboxylic Acids: Rhodium-Catalyzed Double C-H Activation Controlled by Different Directing Groups. Angew. Chem., Int. Ed. 2015, 54, 5478–5482. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).