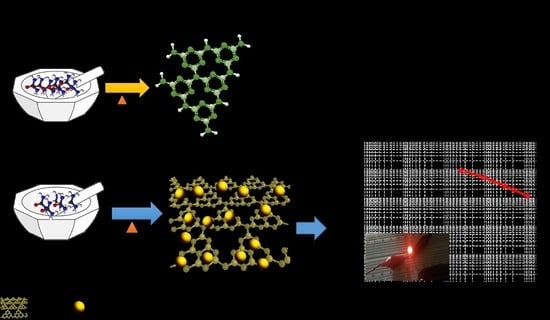
Enhancing Electrochemical Performance with g-C3N4/CeO2 Binary Electrode Material
Abstract
1. Introduction
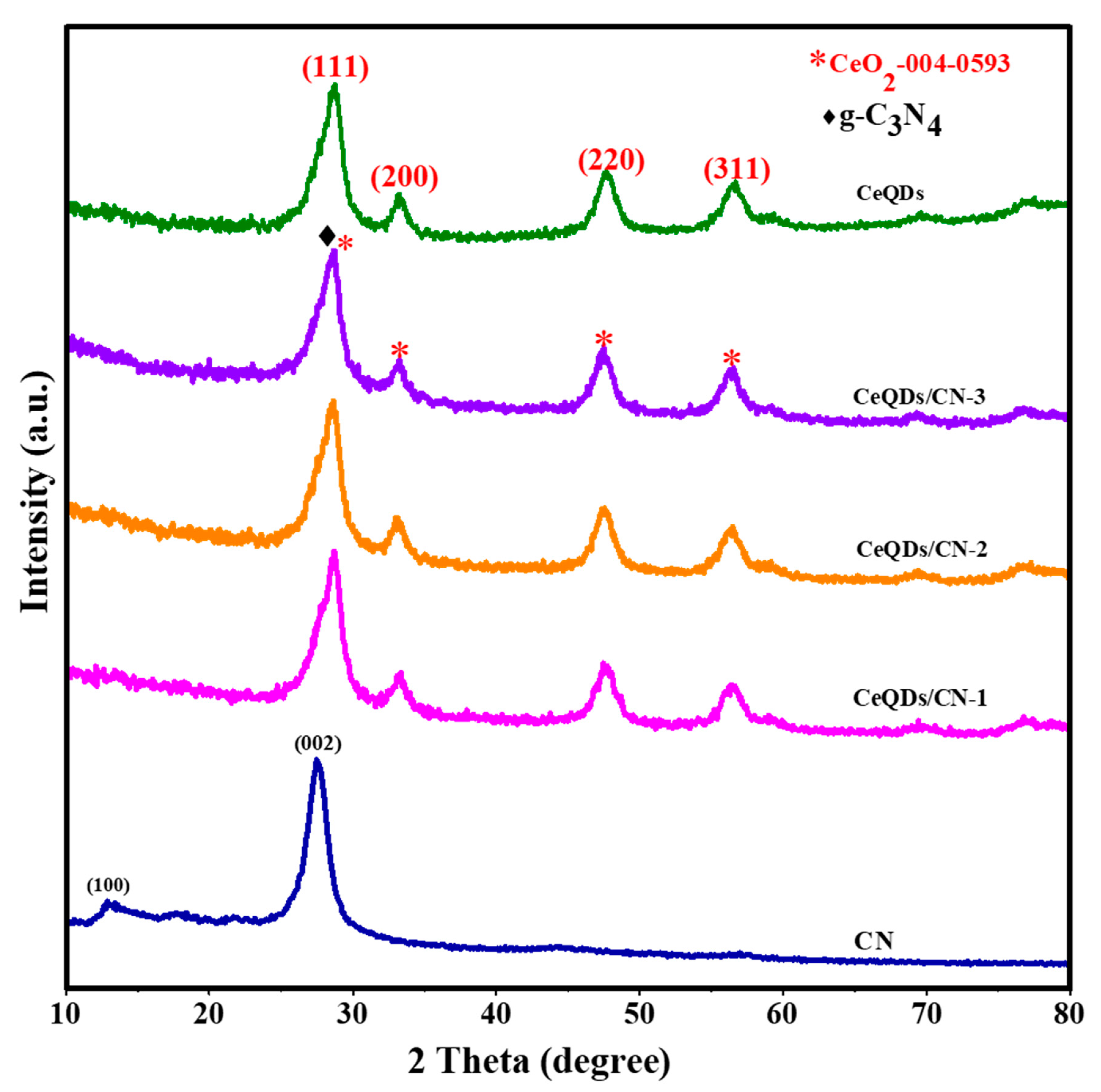
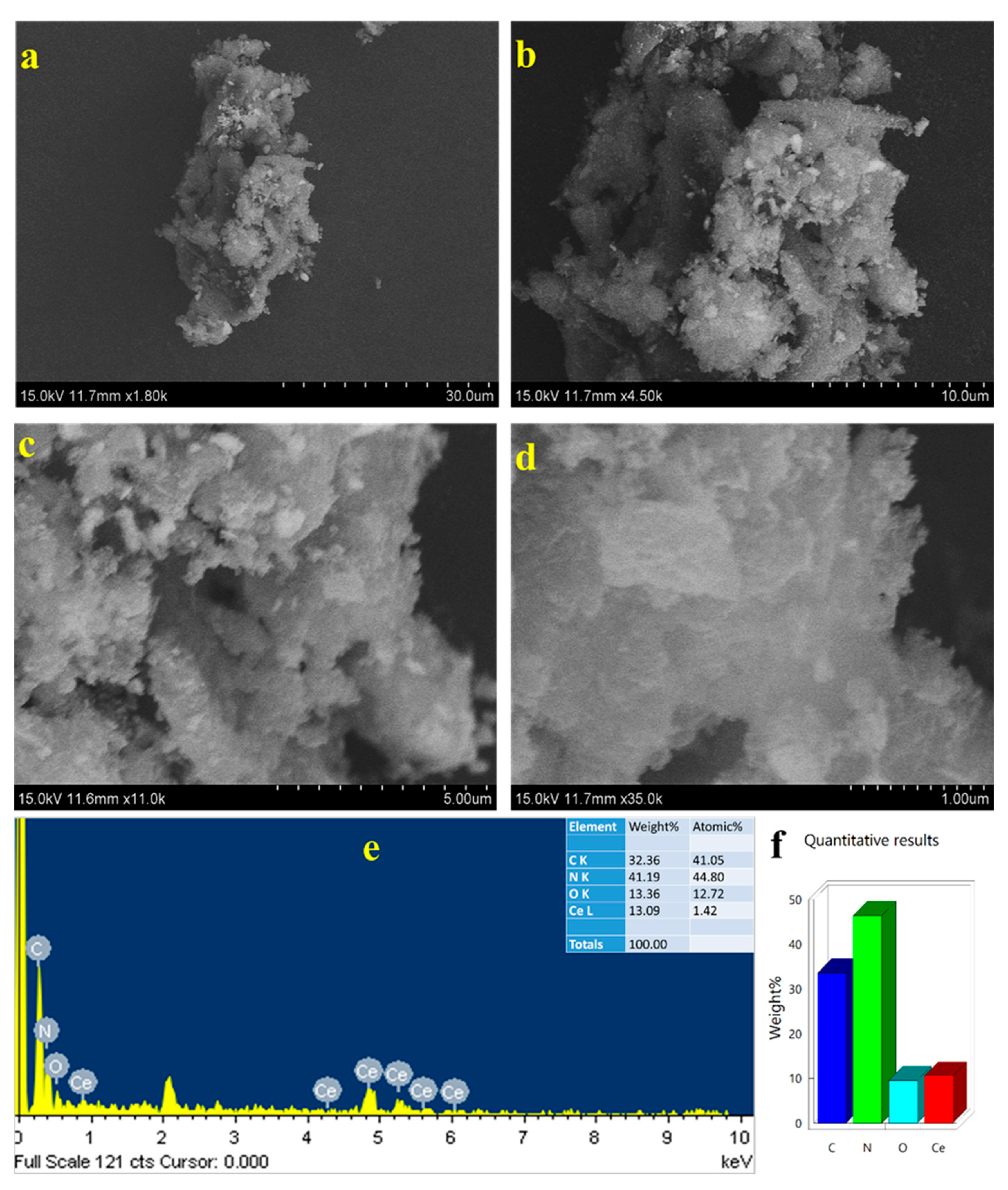
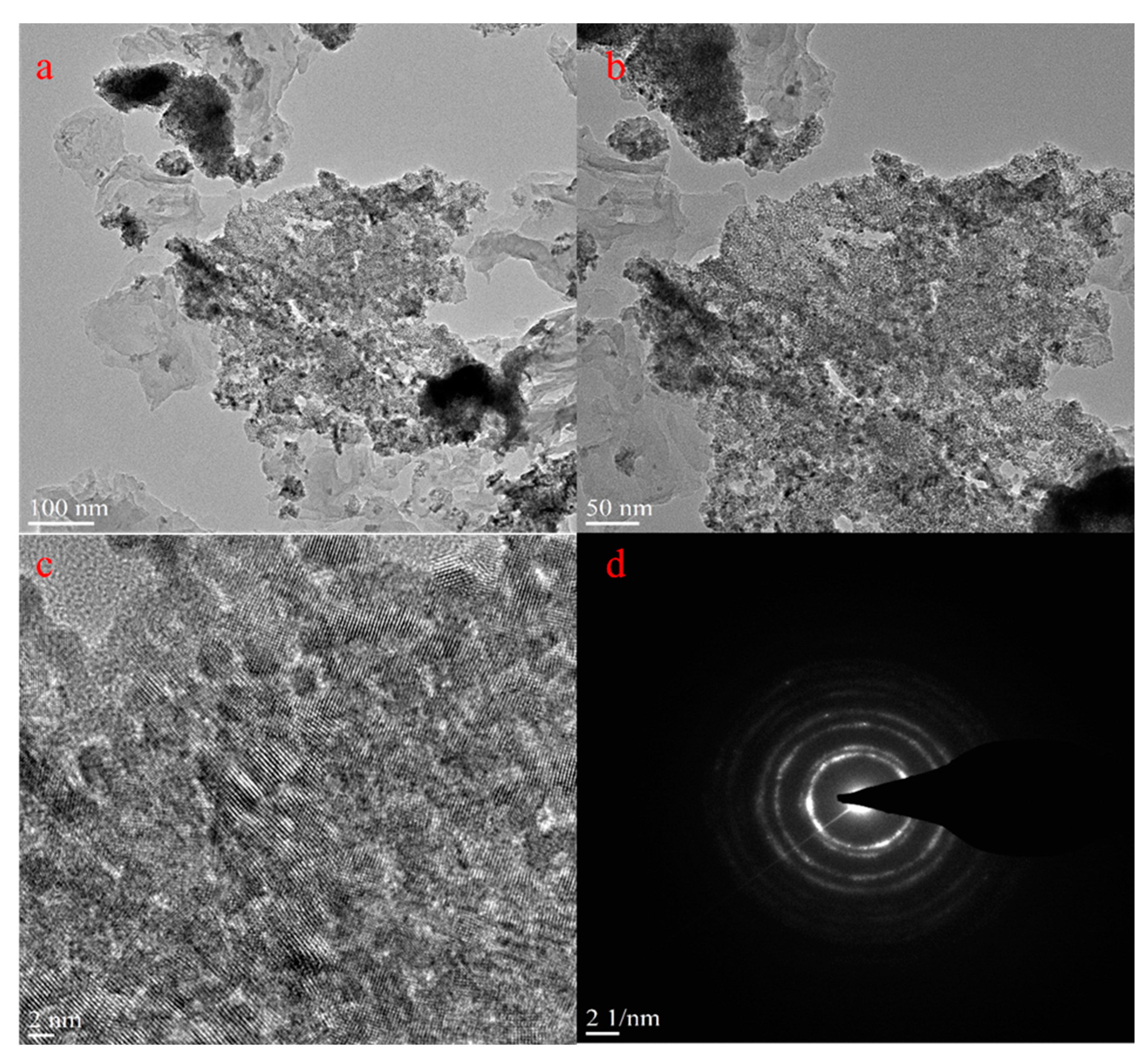
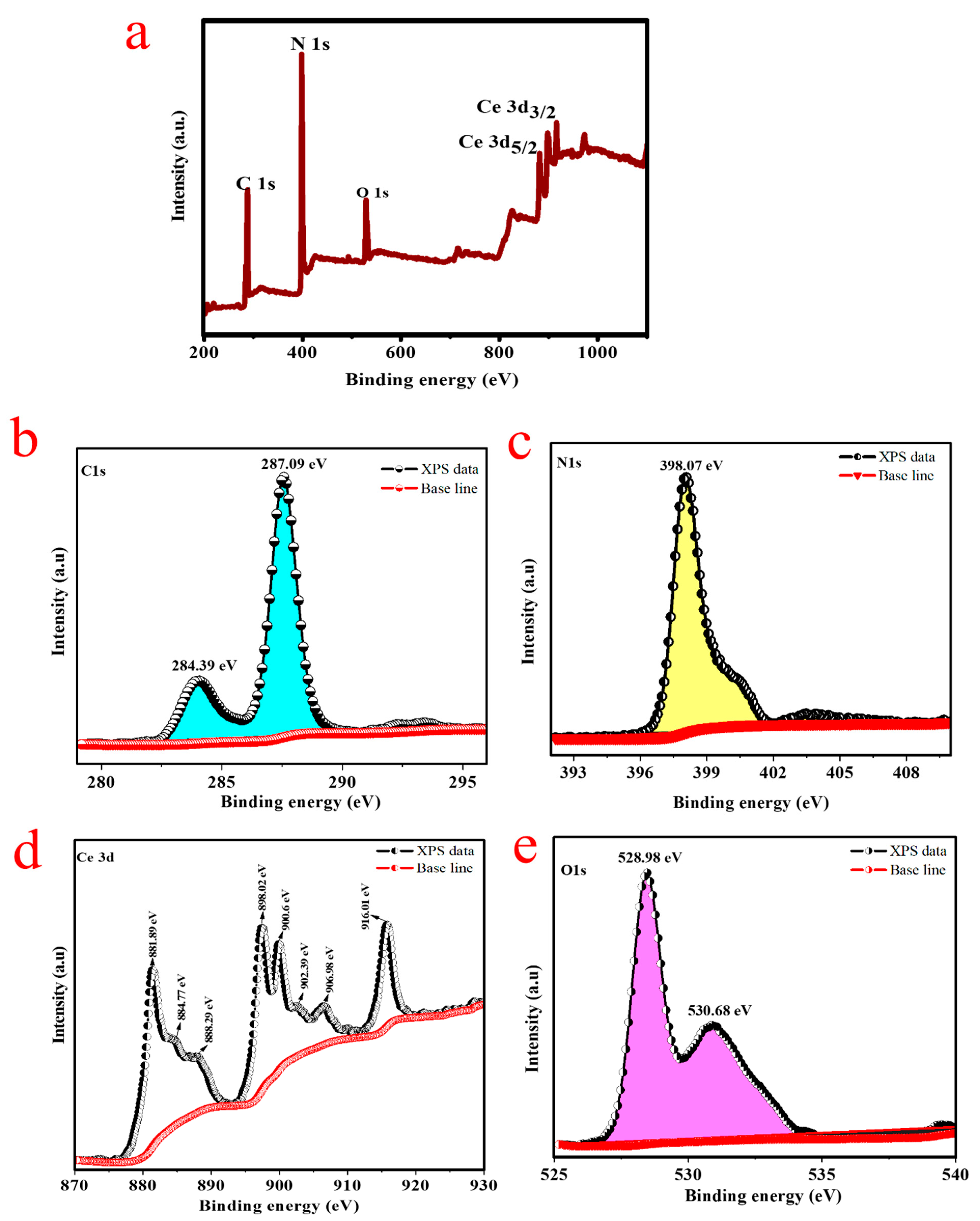
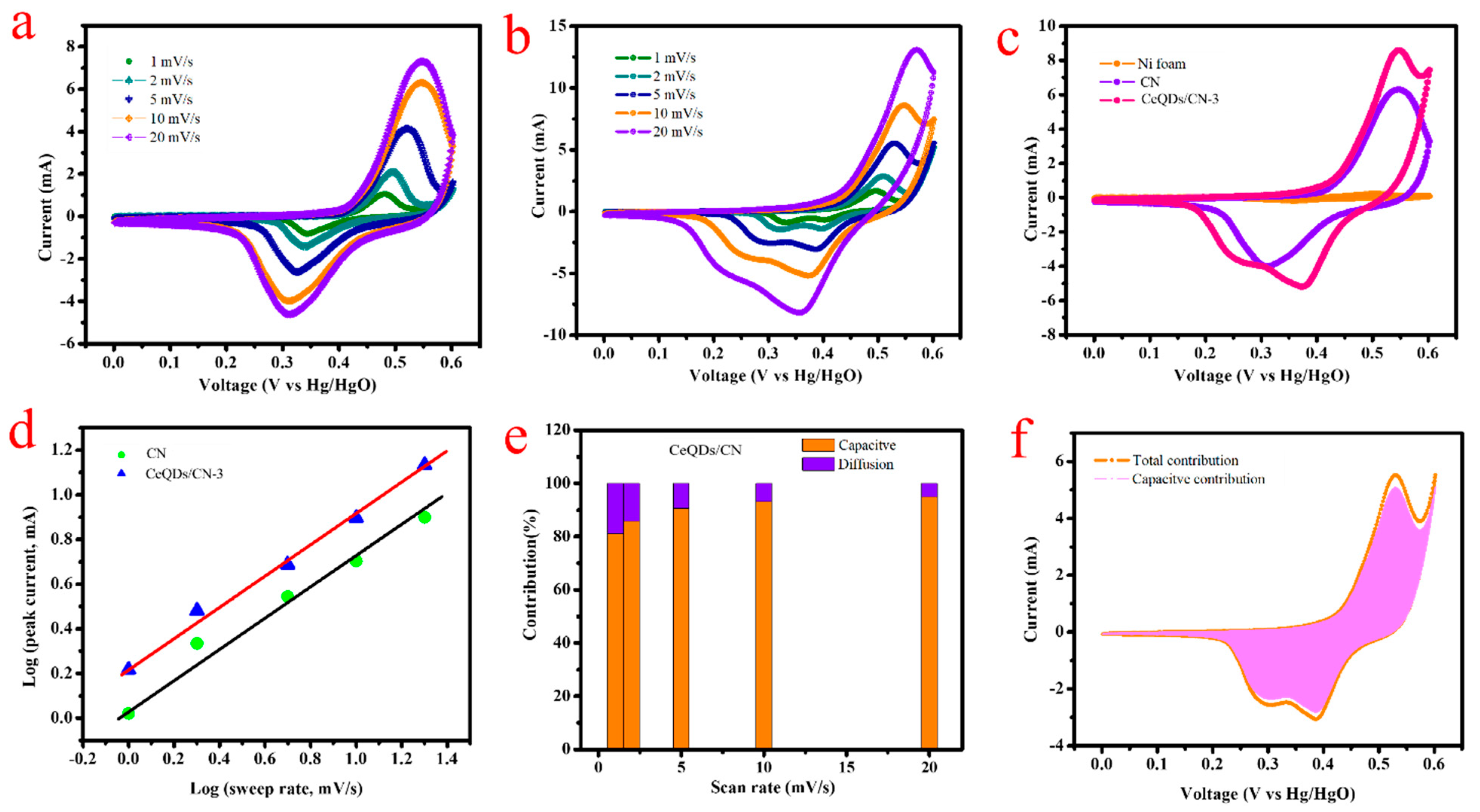
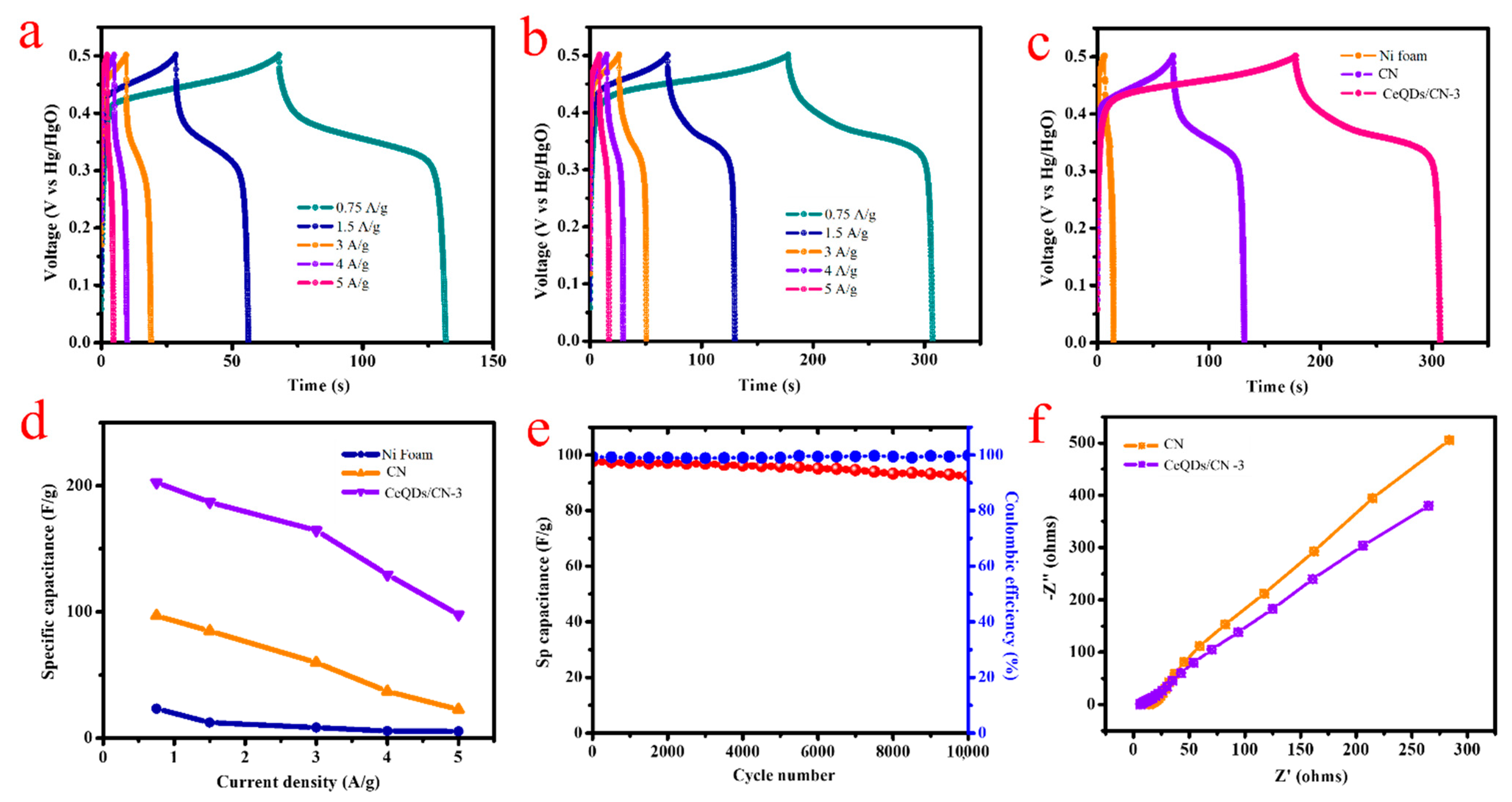
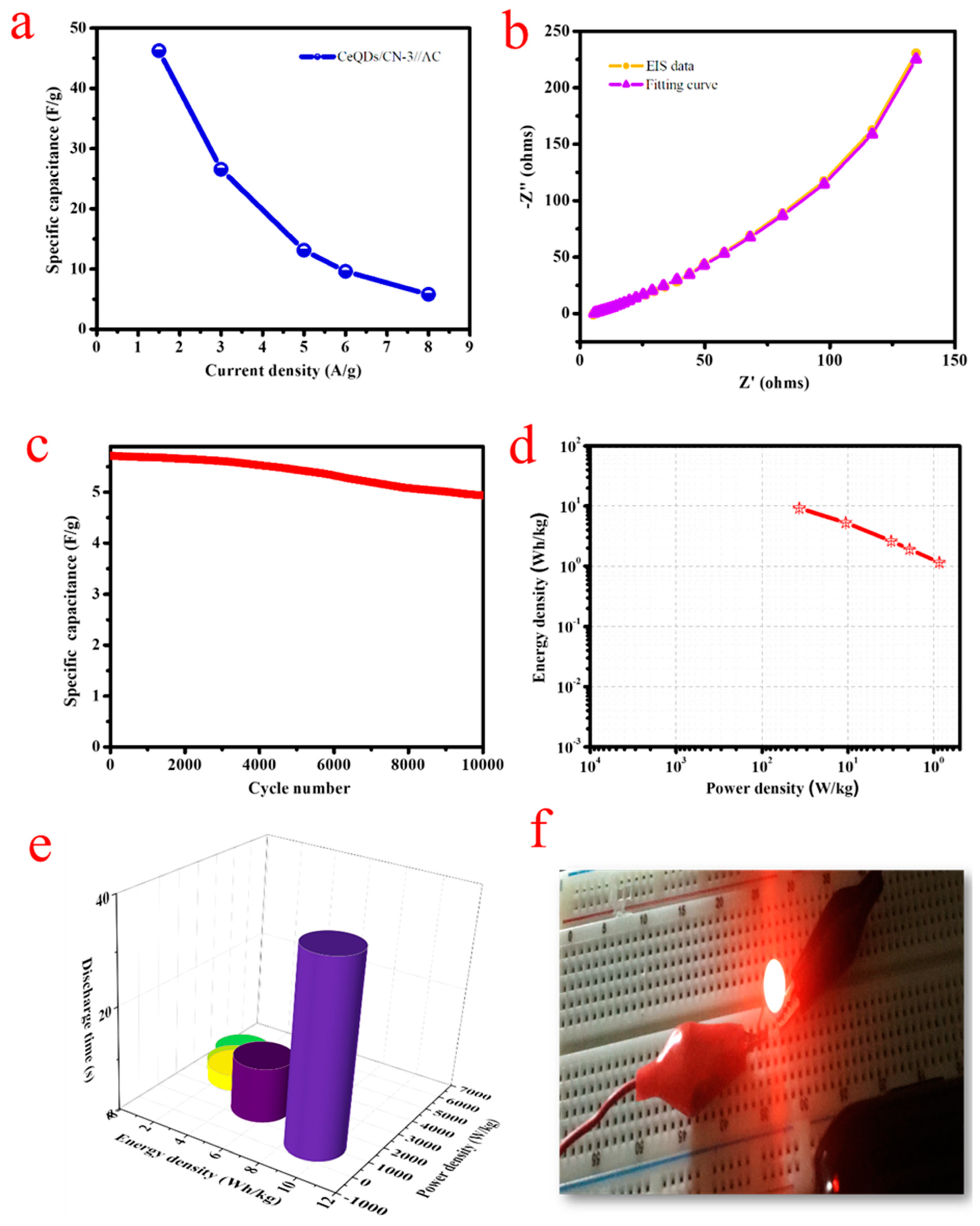
2. Results and Discussion
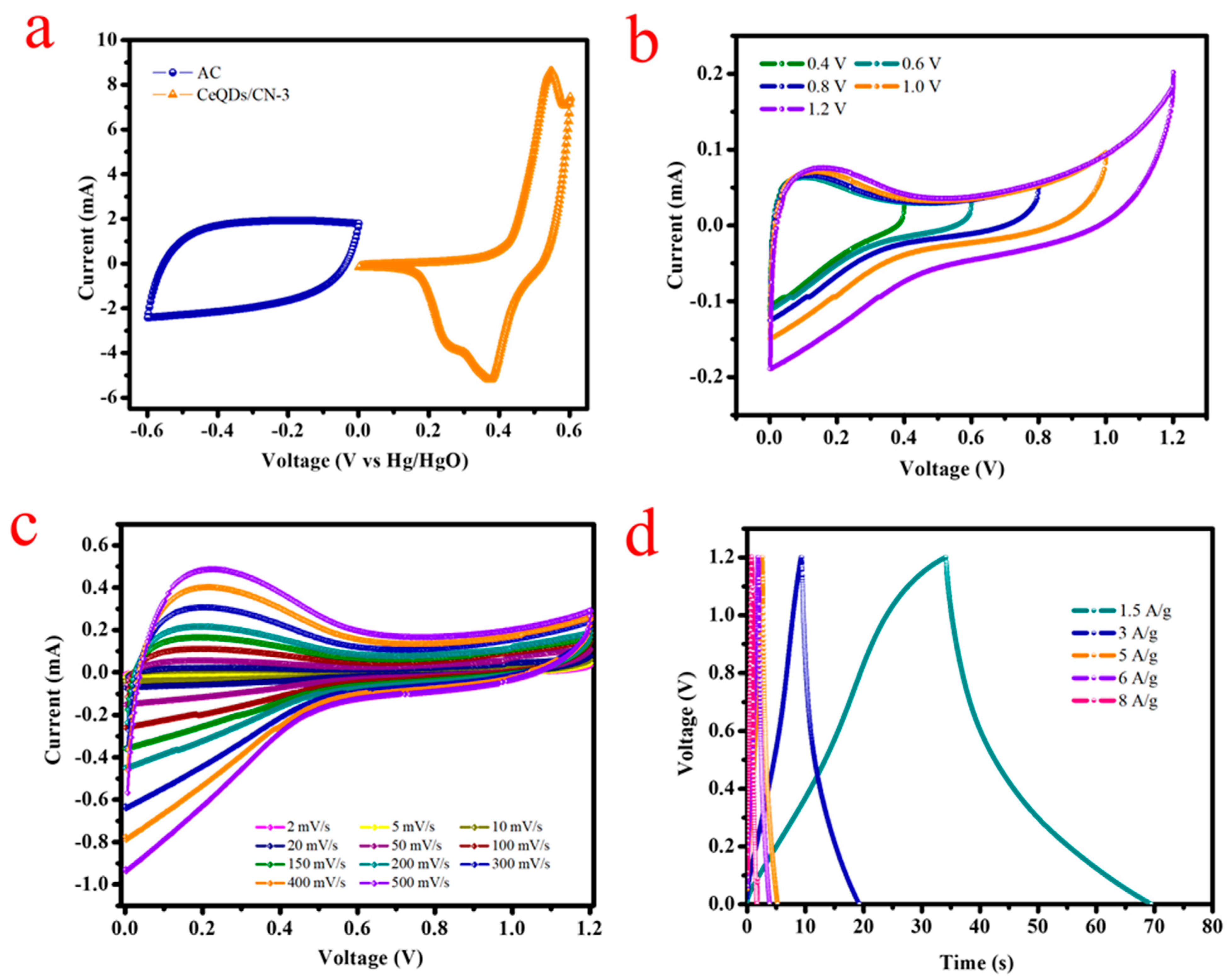
Hybrid Coin Cell-Type Asymmetric Supercapacitor Device (HCASD)
3. Experimental Section
Preparation of g-C3N4/CeO2 Nanostructure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajagopal, S.; Vallikkattil, R.P.; Ibrahim, M.M.; Velev, D.G. Electrode Materials for Supercapacitors in Hybrid Electric Vehicles: Challenges and Current Progress. Condens. Matter 2022, 7, 6. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, L.; Zhang, Q.; Dang, S.; Yu, H.; Zhang, G.; Guo, R.; Zhao, W. Research on electric vehicle-supercapacitor hybrid system participates in the application of tracking PV project output. Energy Sci. Eng. 2022, 10, 120–131. [Google Scholar] [CrossRef]
- Devarayapalli, K.C.; Lee, K.; Do, H.B.; Dang, N.N.; Yoo, K.; Shim, J.; Vattikuti, S.V.P. Mesostructured g-C3N4 nanosheets interconnected with V2O5 nanobelts as electrode for coin-cell-type-asymmetric supercapacitor device. Mater. Today Energy 2021, 21, 100699. [Google Scholar] [CrossRef]
- Tahir, M.; Cao, C.; Mahmood, N.; Butt, F.K.; Mahmood, A.; Idrees, F.; Hussain, S.; Tanveer, M.; Ali, Z.; Aslam, I. Multifunctional g-C3N4 Nanofibers: A Template-Free Fabrication and Enhanced Optical, Electrochemical, and Photocatalyst Properties. ACS Appl. Mater. Interfaces 2014, 6, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode Materials for Supercapacitors: A Review of Recent Advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Pany, S.; Nashim, A.; Parida, K.; Nanda, P.K. Construction of NiCo2O4/O-g-C3N4 Nanocomposites: A Battery-Type Electrode Material for High-Performance Supercapacitor Application. ACS Appl. Nano Mater. 2021, 4, 10173–10184. [Google Scholar] [CrossRef]
- Ghosh, S.; Inta, H.R.; Chakraborty, M.; Tudu, G.; Koppisetti, H.V.; Paliwal, K.S.; Saha, D.; Mahalingam, V. Nanoporous Graphitic Carbon Nitride Nanosheets Decorated with Nickel–Cobalt Oxalate for Battery-Like Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 7246–7258. [Google Scholar] [CrossRef]
- Ji, X.; Xu, B.; Zhang, H.; Xia, X.; Ji, K.; Szymska, A.; Matras-Postolek, K.; Yang, P. Dimensional Nanoarchitectonics of g-C3N4/Co Nanocomposites for Photo- and Electro-Chemical Applications. ACS Appl. Nano Mater. 2022, 5, 11731–11740. [Google Scholar] [CrossRef]
- Xin, X.; Xu, Y.; Wuliji, H.; Sun, F.; Liu, Q.; Wang, Z.; Wei, T.R.; Zhao, X.; Song, X.; Gao, L. Covalently Assembled Black Phosphorus/Conductive C3N4 Hybrid Material for Flexible Supercapacitors Exhibiting a Superlong 30,000 Cycle Durability. ACS Nano 2023, 17, 657–667. [Google Scholar] [CrossRef]
- Yesmin, S.; Hussain, I.; Devi, M.; Dasgupta, R.; Dhar, S.S. Exploration of Cu/g-C3N4 Nanocomposites as a Cost-Effective High-Performance Asymmetric Supercapacitor Electrode Material. IEEE Trans. Nanotechnol. 2022, 21, 474–480. [Google Scholar] [CrossRef]
- Subhash, K.G.; Benoy, M.D.; Duraimurugan, J.; Siranjeevi, R.; Prabhu, S. Synthesis, and characterization of CuO/g-C3N4 nanocomposites for high performances supercapacitor application. Mater. Lett. 2023, 330, 133288. [Google Scholar] [CrossRef]
- Zhang, L.; Ou, M.; Yao, H.; Li, Z.; Qu, D.; Liu, F.; Wang, J.; Wang, J.; Li, Z. Enhanced supercapacitive performance of graphite-like C3N4 assembled with NiAl-layered double hydroxide. Electrochim. Acta 2015, 186, 292–301. [Google Scholar] [CrossRef]
- Meftahi, A.; Shabani-Nooshabadi, M.; Reisi-Vanani, A. AgI/g-C3N4 nanocomposite as electrode material for supercapacitors: Comparative study for its efficiency in three different aqueous electrolytes. Electrochim. Acta 2022, 430, 141052. [Google Scholar] [CrossRef]
- Meftahi, A.; Reisi-Vanani, A.; Shabani-Nooshabadi, M. Comparison of performance of CuI/g-C3N4 nanocomposites synthesized on Ni-foam and graphitic substrates as suitable electrode materials for supercapacitors. Fuel 2023, 331, 125683. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Su, D.; Xu, S.; Cao, S.; Xiao, Y. Constructing a p-n heterojunction in 3D urchin-like CoNixSy/g-C3N4 composite microsphere for high performance asymmetric supercapacitors. J. Alloys Compd. 2022, 902, 163784. [Google Scholar] [CrossRef]
- Vivek, E.; Arulraj, A.; Khalid, M.; Potheher, I.V. Samarium hydroxide nanorolls anchored graphitic carbon nitride nanosheets: An active electrode material for supercapacitors. J. Alloys Compd. 2022, 908, 164541. [Google Scholar] [CrossRef]
- Li, Z.; Yao, M.; Hu, Z.; Zhang, L.; Gou, S.; Feng, H.; Yang, Y.; Lu, X. g-C3N4 promoted NiFe-LDH self-assemble high performance supercapacitor composites. J. Alloys Compd. 2022, 919, 165805. [Google Scholar] [CrossRef]
- Rebekah, A.; Amir, H.; Viswanathan, C.; Ponpandian, N. Enhanced bifunctional aspects of oxygen vacancy rich cation substituted MnCo2O4 intercalated with g-C3N4 as an oxygen evolution and supercapacitor electrode. Int. J. Hydrog. Energy 2023, 48, 6384–6398. [Google Scholar] [CrossRef]
- Li, C.; Li, M.; Yin, S.; Zeng, L.; Zhang, L. Electrochemical performance enhancement by the partial reduction of NiFe2O4@g-C3N4 with core-shell hollow structure. J. Alloys Compd. 2021, 861, 157986. [Google Scholar] [CrossRef]
- Soltani, H.; Bahiraei, H.; Ghasemi, S. Effect of electrodeposition time on the super-capacitive performance of electrodeposited MnO2 on g-C3N4 nanosheets. J. Alloys Compd. 2022, 904, 163565. [Google Scholar] [CrossRef]
- Wang, X.; Hu, A.; Meng, C.; Wu, C.; Yang, S.; Hong, X. Recent Advance in Co3O4 and Co3O4-Containing Electrode Materials for High-Performance Supercapacitors. Molecules 2020, 25, 269. [Google Scholar] [CrossRef]
- Poudel, M.B.; Lohani, P.C.; Kim, A.A. Synthesis of silver nanoparticles decorated tungsten oxide nanorods as high-performance supercapacitor electrode. Chem. Phys. Lett. 2022, 804, 139884. [Google Scholar] [CrossRef]
- Aihemaitituoheti, R.; Alhebshi, N.A.; Abdullah, T. Effects of Precursors and Carbon Nanotubes on Electrochemical Properties of Electrospun Nickel Oxide Nanofibers-Based Supercapacitors. Molecules 2021, 26, 5656. [Google Scholar] [CrossRef]
- Rodriguez-Romero, J.; de Larramendi, I.R.; Goikolea, E. Nanostructured Manganese Dioxide for Hybrid Supercapacitor Electrodes. Batteries 2022, 8, 263. [Google Scholar] [CrossRef]
- Das, H.T.; Dutta, S.; Das, N.; Das, P.; Mondal, A.; Imran, M. Recent trend of CeO2-based nanocomposites electrode in supercapacitor: A review on energy storage applications. J. Energy Storage 2022, 50, 104643. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, A.R.; Ramakrishan, S.; Logeshwaran, N.; Ramasamy, S.K.; Kim, H.J.; Yoo, D.J. Integrating the essence of metal organic framework-derived ZnCoTe–N–C/MoS2 cathode and ZnCo-NPS-N-CNT as anode for high-energy density hybrid supercapacitors. Compos. Part B Eng. 2022, 247, 110339. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, A.A.; Lohani, P.C.; Yoo, D.J.; Kim, H.J. Assembling zinc cobalt hydroxide/ternary sulfides heterostructure and iron oxide nanorods on three-dimensional hollow porous carbon nanofiber as high energy density hybrid supercapacitor. J. Energy Storage 2023, 60, 106713. [Google Scholar] [CrossRef]
- He, G.; Fan, H.; Ma, L.; Wang, K.; Liu, C.; Ding, D.; Chen, L. Dumbbell-like ZnO nanoparticles-CeO2 nanorods composite by one-pot hydrothermal route and their electrochemical charge storage. Appl. Surf. Sci. 2016, 366, 129–138. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Reddy, B.P.; Byon, C.; Shim, J. Carbon/CuO nanosphere-anchored g-C3N4 nanosheets as ternary electrode material for supercapacitors. J. Solid State Chem. 2018, 262, 106–111. [Google Scholar] [CrossRef]
- Chameh, B.; Khosroshahi, N.; Bakhtian, M.; Moradi, M.; Safarifard, V. MOF derived CeO2/CoFe2O4 wrapped by pure and oxidized g-C3N4 sheet as efficient supercapacitor electrode and oxygen reduction reaction electrocatalyst materials. Ceram. Int. 2022, 48, 22254–22265. [Google Scholar] [CrossRef]
- Heydariyan, Z.; Monsef, R.; Salavati-Niasari, M. Insights into impacts of Co3O4-CeO2 nanocomposites on the electrochemical hydrogen storage performance of g-C3N4: Pechini preparation, structural design and comparative study. J. Alloys Compd. 2022, 924, 166564. [Google Scholar] [CrossRef]
- Liang, M.; Borjigin, T.; Zhang, Y.; Liu, B.; Liu, H.; Guo, H. Controlled assemble of hollow heterostructured g-C3N4@CeO2 with rich oxygen vacancies for enhanced photocatalytic CO2 reduction. Appl. Catal. B Environ. 2019, 243, 566–575. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, X.; Wang, Y.; Zhang, W.; Li, Y.; Zhang, Z.; Su, J.; Zhang, J.; Hu, S. CeO2 quantum dots anchored g-C3N4: Synthesis, characterization and photocatalytic performance. Appl. Surf. Sci. 2022, 576, 151901. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.; Li, J.; Li, X.; Wang, H.; Huo, P.; Yan, Y. CeO2/3D g-C3N4 heterojunction deposited with Pt cocatalyst for enhanced photocatalytic CO2 reduction. Appl. Surf. Sci. 2021, 537, 147891. [Google Scholar] [CrossRef]
- Soren, S.; Hota, I.; Debnath, A.K.; Aswal, D.K.; Varadwaj, K.S.K.; Parhi, P. Oxygen Reduction Reaction Activity of Microwave Mediated Solvothermal Synthesized CeO2/g-C3N4 Nanocomposite. Front. Chem. 2019, 7, 403. [Google Scholar] [CrossRef]
- Joseph, N.; Bose, A.C. Metallic MoS2 grown on porous g-C3N4 as an efficient electrode material for supercapattery application. Electrochim. Acta 2019, 301, 401–410. [Google Scholar] [CrossRef]
- Wei, B.; Liang, H.; Wang, R.; Zhang, D.; Qi, Z.; Wang, Z. One-step synthesis of graphitic-C3N4/ZnS composites for enhanced supercapacitor performance. J. Energy Chem. 2018, 27, 472–477. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods Fundamentals of Electrochemistry; Department of Chemistry and Biochemistry, University of Texas at Austin: Austin, TX, USA; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Fang, L.; Qiu, Y.; Zhai, T.; Wang, F.; Lan, M.; Huang, K.; Jing, Q. Flower-like nanoarchitecture assembled from Bi2S3 nanorod/MoS2 nanosheet heterostructures for high-performance supercapacitor electrodes. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 535, 41–48. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Wu, N.; Yao, W.; Xue, R.; Wang, M.; Yang, W. Rational design of nickel-cobalt selenides derived from multivariate bimetal metal-organic frameworks for high-performance asymmetric supercapacitor. J. Alloys Compd. 2020, 856, 156535. [Google Scholar] [CrossRef]
- Liu, S.; Hui, K.S.; Hui, K.N. Flower-like Copper Cobaltite Nanosheets on Graphite Paper as High-Performance Supercapacitor Electrodes and Enzymeless Glucose Sensors. ACS Appl. Mater. Interfaces 2016, 8, 3258–3267. [Google Scholar] [CrossRef]
- Sankar, K.V.; Selvan, R.K. The ternary MnFe2O4/graphene/polyaniline hybrid composite as negative electrode for supercapacitors. J. Power Sources 2015, 275, 399–407. [Google Scholar] [CrossRef]
- Bai, X.; Liu, Q.; Zhang, H.; Liu, J.; Li, Z.; Jing, X.; Yuan, Y.; Liu, L.; Wang, J. Nickel-Cobalt Layered Double Hydroxide Nanowires on Three Dimensional Graphene Nickel Foam for High Performance Asymmetric Supercapacitors. Electrochim. Acta 2016, 215, 492–499. [Google Scholar] [CrossRef]
- Chang, X.; Zhai, X.; Sun, S.; Gu, D.; Dong, L.; Yin, Y.; Zhu, Y. MnO2/g-C3N4 nanocomposite with highly enhanced supercapacitor performance. Nanotechnology 2017, 28, 135705. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.Y.; Guan, B.; Zhang, J.M.; Dong, F.; Liu, X.Y.; Zhang, Y.X. Facile Fabrication of NiCo2O4@g-C3N4(C) Hybrids for High-Performance Supercapacitors. J. Nanosci. Nanotechnol. 2019, 19, 73–80. [Google Scholar] [CrossRef]
- Pathak, M.; Jose, J.R.; Chakraborty, B.; Rout, C.S. High performance supercapacitor electrodes based on spinel NiCo2O4@MWCNT composite with insights from density functional theory simulations. J. Chem. Phys. 2020, 152, 064706. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Xiao, Y.; Yang, X. PEDOT/g-C3N4 binary electrode material for supercapacitors. J. Electroanal. Chem. 2015, 743, 99–104. [Google Scholar] [CrossRef]
- Xu, L.; Xia, J.; Xu, H.; Yin, S.; Wang, K.; Huang, L.; Wang, L.; Li, H. Reactable ionic liquid assisted solvothermal synthesis of graphite-like C3N4 hybridized α-Fe2O3 hollow microspheres with enhanced supercapacitive performance. J. Power Sources 2014, 245, 866–874. [Google Scholar] [CrossRef]
- Tahir, M.; Cao, C.; Butt, F.K.; Idrees, F.; Mahmood, N.; Ali, Z.; Aslam, I.; Tanveer, M.; Rizwan, M.; Mahmood, T. Tubular graphitic-C3N4: A prospective material for energy storage and green photocatalysis. J. Mater. Chem. A 2013, 1, 13949–13955. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Chen, M.; Yan, X.H.; Ren, J.; Dai, Y.; Wang, J.J.; Pan, J.M.; Wang, Y.P.; Cheng, X.N. Hydrothermal synthesis of Fe3O4 nanorods/graphitic C3N4 composite with enhanced supercapacitive performance. Mater. Lett. 2017, 198, 114–117. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Liu, H.; Que, M.; Cai, X.; Tan, S.; Huang, L. Flower-like Ni(OH)2 hybridized g-C3N4 for high-performance supercapacitor electrode material. Mater. Lett. 2015, 145, 150–153. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekhar, M.C.; Kumar, N.S.; Asif, M.; Vattikuti, S.V.P.; Shim, J. Enhancing Electrochemical Performance with g-C3N4/CeO2 Binary Electrode Material. Molecules 2023, 28, 2489. https://doi.org/10.3390/molecules28062489
Sekhar MC, Kumar NS, Asif M, Vattikuti SVP, Shim J. Enhancing Electrochemical Performance with g-C3N4/CeO2 Binary Electrode Material. Molecules. 2023; 28(6):2489. https://doi.org/10.3390/molecules28062489
Chicago/Turabian StyleSekhar, M. Chandra, Nadavala Siva Kumar, Mohammad Asif, Surya Veerendra Prabhakar Vattikuti, and Jaesool Shim. 2023. "Enhancing Electrochemical Performance with g-C3N4/CeO2 Binary Electrode Material" Molecules 28, no. 6: 2489. https://doi.org/10.3390/molecules28062489
APA StyleSekhar, M. C., Kumar, N. S., Asif, M., Vattikuti, S. V. P., & Shim, J. (2023). Enhancing Electrochemical Performance with g-C3N4/CeO2 Binary Electrode Material. Molecules, 28(6), 2489. https://doi.org/10.3390/molecules28062489