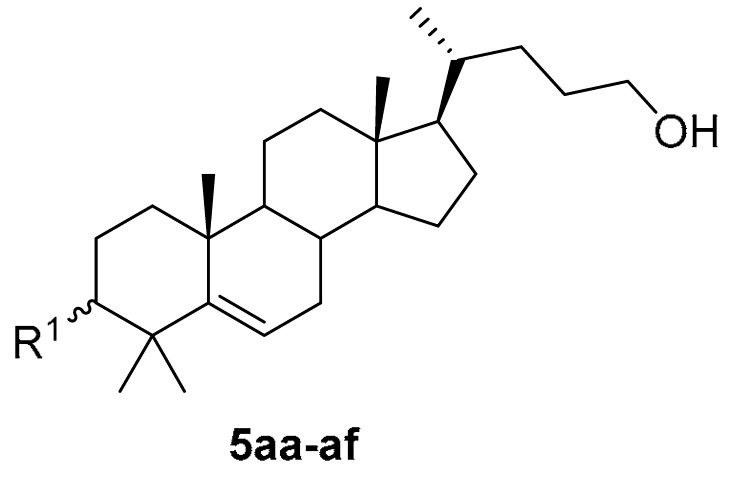
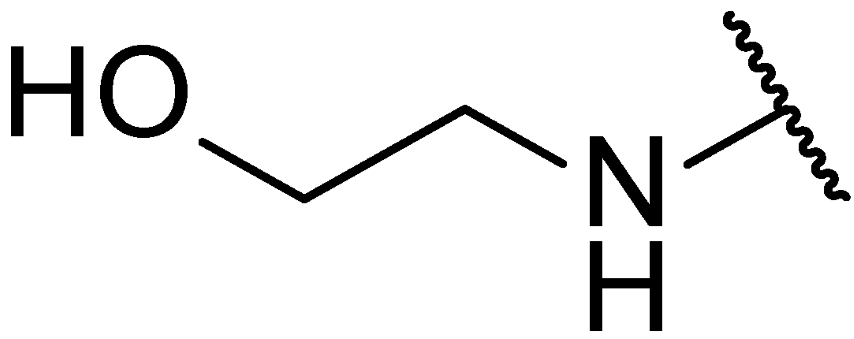
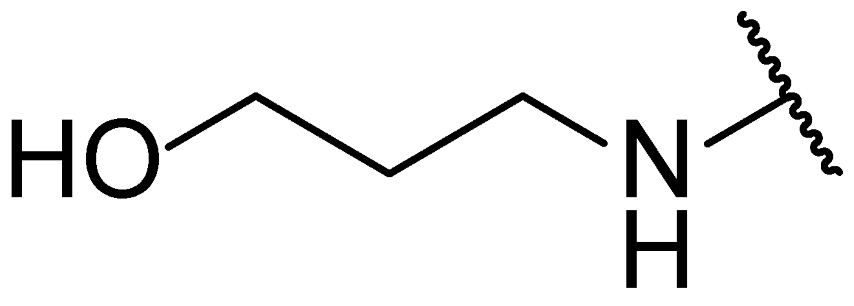
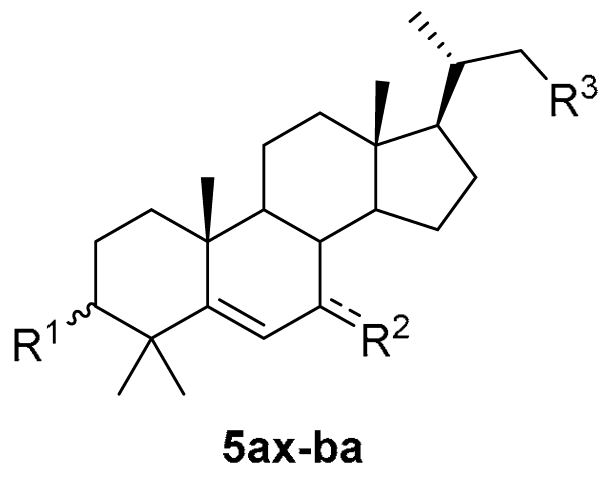
3.1. Chemical Synthesis
All reagents and solvents were obtained from commercial suppliers and used without further purification unless otherwise indicated. Melting points (mps) were taken in open capillaries on a WRS-2 melting point system. The 1H NMR and 13C NMR spectra were recorded using TMS as the internal standard on a Bruker Ascend 400 spectrometer at 400 and 100 MHz, respectively. The 1H NMR chemical shifts were reported in parts per million (ppm) relative to the centerline of the singlet signal of the solvent molecule (7.26 ppm for CDCl3 or 3.31 ppm for CD3OD). The 13C NMR chemical shifts were reported in ppm relative to the centerline at 77.16 ppm for CDCl3 or at 49.00 ppm for CD3OD. The data were reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, dd = doublet of doublets, m = multiplet), coupling constant (Hz), and integration. Evaporation was carried out in vacuo using a rotating evaporator. Silica gel chromatography was performed using silica gel (200–300 mesh). Reactions were monitored by TLC with detection by phosphomolybdic acid visualization or UV light (λ = 254 nm). All reactions involving air- or moisture-sensitive reagents were performed under a nitrogen atmosphere using anhydrous solvents. The purity of the final compounds was >95%, as deduced by 1H NMR spectra. High-resolution mass spectroscopy (HRMS) was performed on a time-of-flight instrument with electrospray ionization (ESI) in the positive ionization mode.
3.1.1. Methyl (4R)-4-((3R,10S,13R,17R)-3-hydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (7)
TsOH.H2O (47.5 mg, 0.25 mmol) was added to a stirred solution of lithocholic acid 6 (1.88 g, 5 mmol) in MeOH (20 mL). The mixture was refluxed for 2 h, and TLC indicated the consumption of the starting material. MeOH was removed in vacuo, and aqueous NaHCO3 solution was added. The mixture was extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated to afford compound 7 (1.95 g, 100% yield) as a white solid. The mp was 105.3–106.5 °C. 1H-NMR (400 MHz, CDCl3) δ 3.65 (s, 3H), 3.62–3.59 (m, 1H), 2.38–2.30 (m, 1H), 2.24–2.17 (m, 1H), 1.96–1.93 (m, 1H), 1.87–0.96 (m, other aliphatic ring protons), 0.90 (s, 3H), 0.89 (d, J = 6.0 Hz, 3H), 0.63 (s, 3H). HRMS (ESI): calcd for C25H42NaO3 [M + Na]+ 413.3026; found 413.3037.
3.1.2. Methyl(4R)-4-((10R,13R,17R)-10,13-dimethyl-3-oxo-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (8)
IBX (3.5 g, 12.5 mmol), TFA (0.2 mL) and a solution of compound 7 (1.95 g, 5 mmol) in DMSO (20 mL) were combined. The mixture was warmed to 50 °C, and all the solids were dissolved. After stirring for 2 h under a N2 atmosphere, the reaction was completed, as indicated by TLC. The reaction mixture was cooled and added to an aqueous NaHSO3 solution (20 mL). The mixture was extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with water, brine, dried over Na2SO4, filtered, and concentrated. The crude material was purified by silica gel column chromatography using PE/EtOAc (10:1, v/v) to afford compound 8 (1.02 g, 53% yield) as a white solid. The mp was 124.2–126.1 °C. 1H-NMR (400 MHz, CDCl3) δ 5.71 (s, 1H), 3.65 (s, 3H), 2.45–2.30 (m, 4H), 2.27–2.17 (m, 2H), 2.02–1.98 (m, 2H), 1.85–0.99 (m, other aliphatic ring protons), 0.90 (d, J = 6.4 Hz, 3H), 0.70 (s, 3H). HRMS (ESI): calcd for C25H38NaO3 [M + Na]+ 409.2713; found 409.2732.
3.1.3. Methyl(4R)-4-((10R,13R,17R)-4,4,10,13-tetramethyl-3-oxo-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (9)
Potassium tert-butoxide (1.18 g, 10.56 mmol) was added in batches to the suspension of compound 8 (1.02 g, 2.64 mmol) in absolute tert-butyl alcohol (50 mL). The mixture was stirred at r.t. for 30 min to form a clear yellow solution and then added to MeI (1.5 g, 10.56 mmol) dropwise. The reaction was stirred at r.t. for 12 h in the dark under a N2 atmosphere, and the starting material disappeared, as monitored by TLC. Upon addition of an aqueous Na2S2O3 solution (5 mL) for quenching the excess MeI, the mixture was evaporated and water (30 mL) was added. The suspension was acidified to pH < 6 with an aqueous HCl solution. The mixture was extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated. The crude material was dissolved in MeOH (20 mL), and TsOH.H2O (47.5 mg, 0.25 mmol) was added. The mixture was refluxed for 2 h, and the MeOH was removed in vacuo. An aqueous NaHCO3 solution was added, and it was extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered, concentrated, and purified by silica gel column chromatography using PE/EtOAc (10:1, v/v) to obtain compound 9 (547 mg, 50% yield) as a white solid. The mp was 133.6–135.8 °C. 1H-NMR (400 MHz, CDCl3) δ 5.55–5.53 (m, 1H), 3.66 (s, 3H), 2.59–2.43 (m, 2H), 2.39–2.31 (m, 1H), 2.26–2.18 (m, 1H), 2.13–2.06 (m, 1H), 2.02–1.97 (m, 2H), 1.90–1.76 (m, 2H), 1.68–1.02 (m, other aliphatic ring protons), 0.91 (d, J = 6.4 Hz, 3H), 0.84 (s, 3H), 0.68 (s, 3H). HRMS (ESI): calcd for C27H42NaO3 [M+Na]+ 437.3026; found 437.3059.
3.1.4. Methyl(4R)-4-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (10)
Compound 9 (547 mg, 1.32 mmol) was dissolved in a saturated NH4OAc/EtOH solution (20 mL). It was added to NaBH3CN (166 mg, 2.64 mmol) and NH3.H2O (0.8 mL). The mixture was refluxed for 12 h under a N2 atmosphere, and TLC indicated the consumption of the starting material. The solvents were removed in vacuo and water (20 mL) was added. The mixture was extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered, concentrated, and purified by silica gel column chromatography using DCM/MeOH/NH3.H2O (100:3:0.5, v/v/v) to obtain compound 10 (412 mg, 75% yield) as a white solid. The mp was 145.0–147.3 °C. 1H-NMR (400 MHz, CDCl3) δ 5.55–5.51 (m, 1H), 3.65 (s, 3H), 2.38–2.31 (m, 2H), 2.25–2.17 (m, 1H), 2.11–2.04 (m, 1H), 1.99–1.96 (m, 1H), 1.86–1.77 (m, 2H), 1.73–1.69 (m, 1H), 1.63–0.95 (m, other aliphatic ring protons), 0.91 (d, J = 6.4 Hz, 3H), 0.66 (s, 3H).
3.1.5. (4R)-4-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentan-1-ol (5aa)
LiAlH4 (11 mg, 0.3 mmol) was added in batches at 0 °C to a dry THF solution (20 mL) of compound 10 (116 mg, 0.28 mmol). The mixture was stirred at r.t. for 12 h under a N2 atmosphere. Water (11 μL) was added to quench the reaction, then a 15% NaOH solution (11 μL) and then water (33 μL). The suspension was filtrated, and the solid residue was washed with THF (10 mL). The solvents were removed in vacuo, and water (30 mL) was added to the residue. The mixture was extracted with DCM (3 × 30 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered, concentrated, and purified by silica gel column chromatography using DCM/MeOH/NH3.H2O (100:5:0.5, v/v/v) to obtain compound 5aa (81 mg, 75% yield) as a white solid. The mp was 163.5–166.3 °C. 1H-NMR (400 MHz, CDCl3) δ 5.55–5.52 (m, 1H), 3.59–3.57 (m, 2H), 2.37–2.33 (m, 1H), 2.10–2.05 (m, 1H), 2.00–1.97 (m, 1H), 1.87–1.80 (m, 1H), 1.73–1.70 (m, 1H), 1.65–0.89 (m, other aliphatic ring protons), 0.66 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 150.52, 119.58, 63.52, 58.37, 57.43, 56.02, 51.22, 42.33, 41.01, 39.91, 37.79, 37.04, 35.71, 32.80, 32.05, 30.93, 29.54, 28.43, 28.35, 27.73, 24.29, 23.76, 21.38, 20.65, 18.81, 12.00. HRMS (ESI) calcd for C26H46NO [M+H]+ 388.3501; found 388.3585.
3.1.6. Methyl(4R)-4-((10R,13R,17R)-3-((2-hydroxyethyl)amino)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (11a)
Potassium carbonate (166 mg, 1.2 mmol) and 2-bromoethanol (82 mg, 0.66 mmol) were added to a solution of compound 10 (249 mg, 0.6 mmol) in DMF (10 mL), and the mixture was heated to 80 °C and stirred for 2 h under a N2 atmosphere. When the reaction was completed, as indicated by TLC, water (30 mL) was added to the residue. The mixture was filtered, concentrated, and purified by silica gel column chromatography using DCM/MeOH/NH3.H2O (100:5:0.5, v/v/v) to obtain compound 11a (168 mg, 61% yield) as a white solid. The mp was 165.4–166.8 °C. 1H-NMR (400 MHz, CDCl3) δ 5.56–5.54 (m, 1H), 3.65 (s, 3H), 3.62–3.51 (m, 2H), 3.00–2.95 (m, 1H), 2.67–2.61 (m, 1H), 2.38–2.31 (m, 1H), 2.24–0.87 (m, other aliphatic ring protons), 0.65 (s, 3H). HRMS (ESI) calcd for C29H50NO3 [M + H]+ 460.3785; found 460.3795.
3.1.7. Methyl(4R)-4-((10R,13R,17R)-3-((3-hydroxypropyl)amino)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (11b)
Compound 11b was synthesized in a 63% yield as a white solid using a similar procedure to that in 3.1.6. 1H-NMR (400 MHz, CDCl3) δ 5.56–5.54 (m, 1H), 3.82 (t, J = 5.2 Hz, 2H), 3.65 (s, 3H), 3.59–3.47 (m, 1H), 3.18–3.13 (m, 1H), 2.69–2.63 (m, 1H), 2.38–2.30 (m, 2H), 2.25–2.17 (m, 2H), 2.10–0.87 (m, other aliphatic ring protons), 0.66 (s, 3H). HRMS (ESI): calcd for C30H52NO3 [M + H]+ 474.3942; found 474.3973.
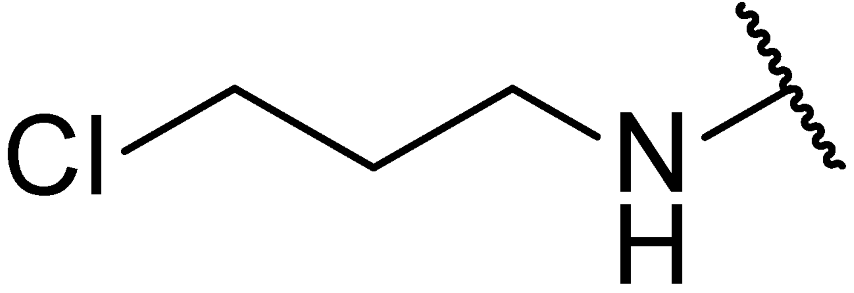
3.1.8. Methyl(4R)-4-((10R,13R,17R)-3-((3-chloropropyl)amino)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (11c)
Compound 11c was synthesized in a 47% yield as a white solid using a similar procedure to that in 3.1.6, and the reaction temperature was r.t. The mp was 119.7–121.3 °C. 1H-NMR (400 MHz, CDCl3) δ 5.56–5.53 (m, 1H), 3.65 (s, 3H), 3.63 (t, J = 6.4 Hz, 2H), 2.95–2.88 (m, 2H), 2.61–2.56 (m, 1H), 2.39–2.31 (m, 1H), 2.25–2.17 (m, 1H), 2.11–2.04 (m, 1H), 1.99–0.87 (m, other aliphatic ring protons), 0.66 (s, 3H). HRMS (ESI): calcd for C30H51ClNO2 [M + H]+ 492.3603; found 492.3646.
3.1.9. Methyl(4R)-4-((10R,13R,17R)-4,4,10,13-tetramethyl-3-(propylamino)-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (11d)
Compound 11d was synthesized in an 86% yield as a white solid using a similar procedure to that in 3.1.6. 1H-NMR (400 MHz, CDCl3) δ 5.57–5.53 (m, 1H), 3.66 (s, 3H), 2.83–2.76 (m, 1H), 2.54–2.48 (m, 1H), 2.39–2.31 (m, 1H), 2.25–2.20 (m, 1H), 2.11–2.05 (m, 2H), 2.00–1.97 (m, 1H), 1.88–0.89 (m, other aliphatic ring protons), 0.66 (s, 3H). HRMS (ESI): calcd for C30H52NO2 [M+H]+ 458.3993; found 458.4004.
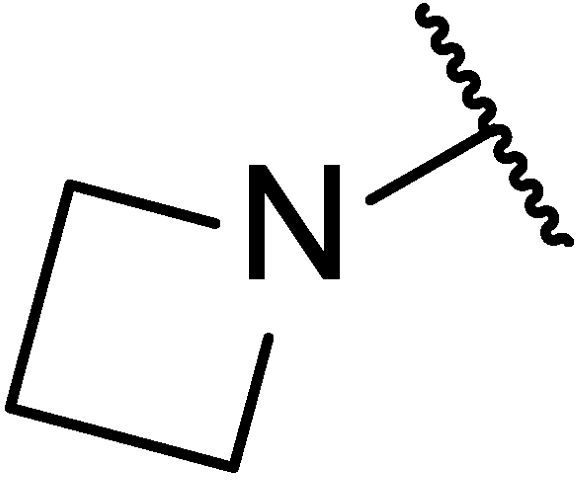
3.1.10. Methyl(4R)-4-((10R,13R,17R)-3-(azetidin-1-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (11e)
Compound 11e was synthesized in a 73% yield as a white solid using a similar procedure to that in 3.1.6. The mp was 173.7–175.5 °C. 1H-NMR (400 MHz, CDCl3) δ 5.56–5.54 (m, 1H), 3.65 (s, 3H), 3.60–3.63 (m, 4H), 2.38–2.31 (m, 1H), 2.25–0.84 (m, other aliphatic ring protons), 0.65 (s, 3H). HRMS (ESI): calcd for C30H50NO2 [M + H]+ 456.3836; found 456.3853.
3.1.11. (4R)-4-((10R,13R,17R)-3-((2-hydroxyethyl)amino)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentan-1-ol (5ab)
Compound 5ab was synthesized in a 68% yield as a white solid using a similar procedure to that in 3.1.5. The mp was 184.3–187.5 °C. 1H-NMR (400 MHz, CDCl3:CD3OD = 3:1) δ 5.54–5.52 (m, 1H), 3.66–3.56 (m, 2H), 3.52–3.48 (m, 2H), 2.87–2.81 (m, 1H), 2.60–2.54 (m, 1H), 2.09–2.01 (m, 2H), 2.00–1.96 (m, 1H), 1.83–1.78 (m, 1H), 1.77–1.71 (m, 2H), 1.62–0.84 (m, other aliphatic ring protons), 0.64 (s, 3H). 13C-NMR (100 MHz, CDCl3:CD3OD = 3:1) δ 150.56, 119.88, 65.41, 63.12, 60.80, 57.54, 56.19, 51.27, 50.24, 42.42, 40.73, 40.00, 37.38, 37.11, 35.87, 32.82, 32.13, 31.03, 29.35, 28.44, 27.52, 24.89, 24.36, 24.33, 21.40, 20.78, 18.76, 12.00. HRMS (ESI): calcd for C28H50NO2 [M + H]+ 432.3836; found 432.3840.
3.1.12. (4R)-4-((10R,13R,17R)-3-((3-hydroxypropyl)amino)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentan-1-ol (5ac)
Compound 5ac was synthesized in a 62% yield as a white solid using a similar procedure to that in 3.1.5. The mp was 183.5–186.8 °C. 1H-NMR (400 MHz, CDCl3) δ 5.57–5.55 (m, 1H), 3.82 (t, J = 5.2 Hz, 2H), 3.60 (t, J = 6.4 Hz, 2H), 3.18–3.13 (m, 1H), 2.69–2.63 (m, 1H), 2.11–2.06 (m, 2H), 2.02–1.98 (m, 1H), 1.88–0.87 (m, other aliphatic ring protons), 0.66 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 150.46, 119.85, 65.74, 64.88, 63.70, 57.44, 56.03, 51.15, 49.39, 42.35, 40.72, 39.91, 37.51, 36.98, 35.71, 32.76, 31.99, 31.36, 30.94, 29.53, 28.42, 27.81, 24.98, 24.27, 21.35, 20.71, 18.81, 12.02. HRMS (ESI): calcd for C29H51NNaO2 [M + Na]+ 468.3812; found 468.3805.
3.1.13. (4R)-4-((10R,13R,17R)-3-((3-chloropropyl)amino)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentan-1-ol (5ad)
Compound 5ad was synthesized in a 79% yield as a white solid using a similar procedure to that in 3.1.5. The mp was 162.7–165.5 °C. 1H-NMR (400 MHz, CDCl3) δ 5.56–5.52 (m, 1H), 3.66–3.60 (m, 4H), 2.96–2.92 (m, 1H), 2.64–2.59 (m, 1H), 2.10–0.91 (m, other aliphatic ring protons), 0.66 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 150.98, 119.55, 64.98, 63.70, 57.45, 56.02, 51.17, 45.44, 43.42, 42.35, 41.02, 39.92, 37.70, 36.99, 35.71, 33.29, 32.76, 31.98, 30.97, 29.52, 28.42, 27.61, 24.90, 24.81, 24.27, 21.40, 20.70, 18.80, 12.02. HRMS (ESI): calcd for C29H51ClNO [M + H]+ 464.3654; found 464.3676.
3.1.14. (4R)-4-((10R,13R,17R)-4,4,10,13-tetramethyl-3-(propylamino)-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentan-1-ol (5ae)
Compound 5ae was synthesized in a 90% yield as a white solid using a similar procedure to that in 3.1.5. The mp was 147.5–150.4 °C. 1H-NMR (400 MHz, CDCl3) δ 5.56–5.53 (m, 1H), 3.62–3.59 (m, 2H), 2.82–2.76 (m, 1H), 2.54–2.47 (m, 1H), 2.10–2.05 (m, 2H), 2.01–1.98 (m, 1H), 1.84–1.80 (m, 2H), 1.79–1.74 (m, 1H), 1.62–0.87 (m, other aliphatic ring protons), 0.66 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 150.71, 119.74, 65.25, 63.68, 57.45, 56.03, 51.16, 50.50, 42.35, 40.77, 39.92, 37.53, 37.00, 35.71, 32.77, 31.99, 30.94, 29.53, 28.42, 27.58, 24.99, 24.32, 24.27, 22.82, 21.39, 20.70, 18.81, 12.02, 11.89. HRMS (ESI): calcd for C29H52NO [M + H]+ 430.4043; found 430.4068.
3.1.15. (4R)-4-((10R,13R,17R)-3-(azetidin-1-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentan-1-ol (5af)
Compound 5af was synthesized in a 77% yield as a white solid using a similar procedure to that in 3.1.5. The mp was 174.9–177.6 °C. 1H-NMR (400 MHz, CDCl3) δ 5.53–5.51 (m, 1H), 3.60 (t, J = 6.4 Hz, 2H), 3.37 (dd, J = 11.2, 4.4 Hz, 2H), 3.27 (dd, J = 11.2, 4.4 Hz, 2H), 2.09–1.97 (m, 4H), 1.87–1.84 (m, 2H), 1.78–1.75 (m, 1H), 1.64–0.86 (m, other aliphatic ring protons), 0.65 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 150.70, 119.22, 72.83, 63.70, 57.46, 56.02, 55.32 (2C), 51.30, 42.32, 41.75, 39.92, 37.68, 37.02, 35.70, 32.87, 31.99, 30.90, 29.53, 28.92, 28.42, 26.06, 24.26, 21.38, 20.70, 20.08, 18.81, 17.99, 12.01. HRMS (ESI): calcd for C29H50NO [M + H]+ 428.3887; found 428.3866.
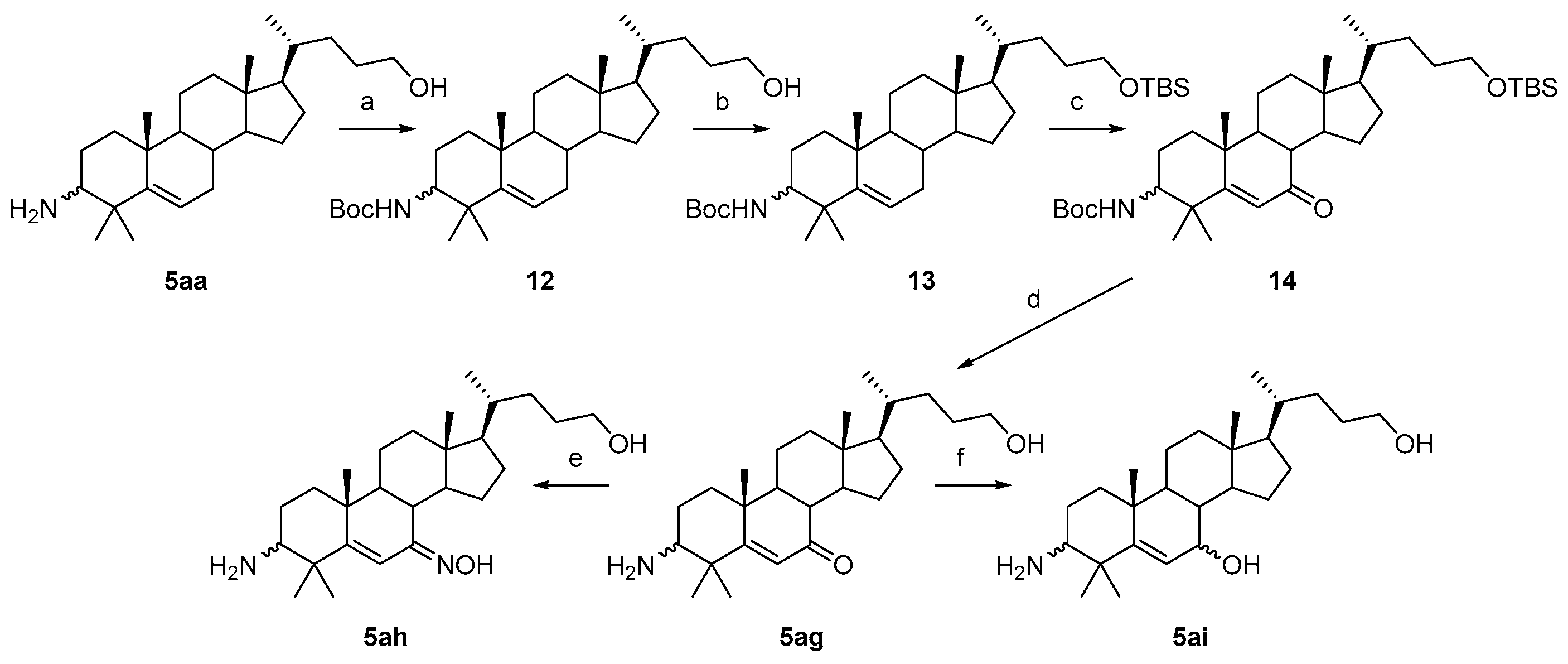
3.1.16. Tert-butyl((10R,13R,17R)-17-((R)-5-hydroxypentan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (12)
Boc2O (524 mg, 2.4 mmol) and Et3N (404 mg, 4 mmol) were added to a solution of compound 5aa (776 mg, 2 mmol) in dry DCM (30 mL), and the reaction was stirred at r.t. for 2 h; TLC indicated the consumption of the starting material. Water (30 mL) was added, and the mixture was extracted with DCM (3 × 30 mL). The combined organic layer was washed with aqueous NaOH solution, brine, dried over Na2SO4, filtered, concentrated, and recrystallized in 5 mL of EtOAc to obtain compound 12 (945 mg, 97% yield) as a white solid. 1H-NMR (400 MHz, CDCl3) δ 5.58–5.55 (m, 1H), 4.44 (d, J = 10.0 Hz, 1H), 3.61 (t, J = 6.0 Hz, 2H), 3.32–3.27 (m, 1H), 2.09–2.04 (m, 1H), 2.00–1.97 (m, 1H), 1.86–1.79 (m, 1H), 1.73–0.92 (m, other aliphatic ring protons), 0.92 (d, J = 6.0 Hz, 3H), 0.66 (s, 3H).
3.1.17. Tert-butyl((10R,13R,17R)-17-((R)-5-((tert-butyldimethylsilyl)oxy)pentan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (13)
TBSCl (226 mg, 1.5 mmol) was added to a solution of compound 12 (366 mg, 0.75 mmol) and imidazole (204 mg, 3 mmol) in dry DMF (8 mL). The reaction mixture was stirred at 80 °C for 6 h under a N2 atmosphere, and the starting material disappeared, as monitored by TLC. The mixture was added with an aqueous NaOH solution (20 mL) and extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with water, brine, dried over Na2SO4, filtered, concentrated, and recrystallized in 5 mL of EtOAc to obtain compound 13 (451 mg, 100% yield) as a white solid. The mp was 183.4–185.1 °C. 1H-NMR (400 MHz, CD3OD) δ 5.56–5.54 (m, 1H), 4.43 (d, J = 10.0 Hz, 1H), 3.56 (t, J = 6.4 Hz, 2H), 3.33–3.27 (m, 1H), 2.09–1.97 (m, 2H), 1.85–1.78 (m, 1H), 1.73–0.86 (m, other aliphatic ring protons), 0.66 (s, 3H), 0.04 (s, 6H).
3.1.18. Tert-butyl((10R,13R,17R)-17-((R)-5-((tert-butyldimethylsilyl)oxy)pentan-2-yl)-4,4,10,13-tetramethyl-7-oxo-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (14)
Na2Cr2O7.2H2O (243 mg, 0.81 mmol) was added to a solution of compound 13 (406 mg, 0.74 mmol), N-hydroxyphthalimide (241 mg, 1.48 mmol), and HOAc (0.2 mL) in acetone (30 mL). The reaction was heated to 50 °C and stirred for 12 h under a N2 atmosphere, and then, another quantity of Na2Cr2O7.2H2O (110 mg, 0.37 mmol) was added. The reaction was continued for 6 h. When the reaction was completed, as indicated by TLC, the mixture was concentrated and purified by silica gel column chromatography using PE/EtOAc (5:1, v/v) to obtain compound 14 (419 mg, 92% yield) as a white solid. mp 190.5–192.6 °C. 1H-NMR (400 MHz, CD3OD) δ 5.95 (s, 1H), 4.48 (d, J = 10.0 Hz, 1H), 3.56 (t, J = 6.4 Hz, 2H), 3.49–3.44 (m, 1H), 2.31–2.29 (m, 1H), 2.22–2.17 (m, 1H), 2.03–1.01 (m, other aliphatic ring protons), 0.91 (d, J = 6.0 Hz, 3H), 0.88 (s, 9H), 0.67 (s, 3H), 0.04 (s, 6H). HRMS (ESI): calcd for C37H65NNaO4Si [M + Na]+ 638.4575; found 638.4576.
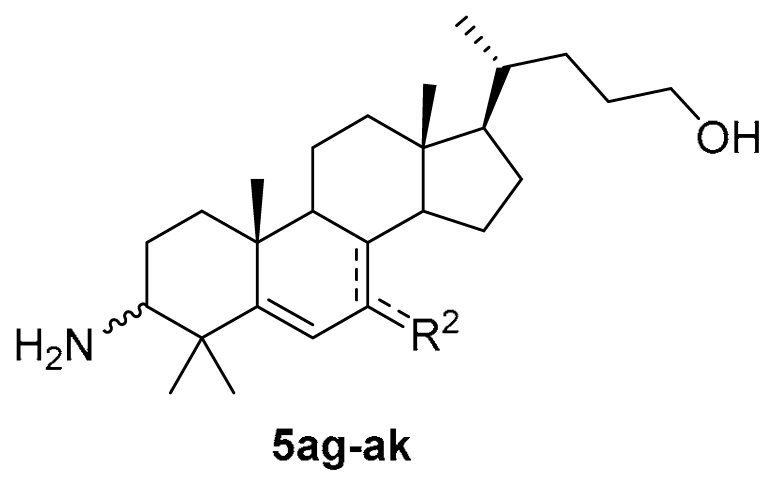
3.1.19. (10R,13R,17R)-3-amino-17-((R)-5-hydroxypentan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-7H-cyclopenta[a]phenanthren-7-one (5ag)
An aqueous 4N HCl solution (5 mL) was added to the solution of compound 14 (167 mg, 0.32 mmol) in THF (5 mL). The reaction was refluxed for 2 h, and TLC indicated the consumption of the starting material. The suspension was basified to pH > 8 with aqueous NaOH solution. The solvent THF was removed in vacuo, and water (30 mL) was added. The mixture was extracted with DCM (3 × 30 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated to obtain compound 5ag (121 mg, 94% yield) as a white solid. The mp was 151.3–153.7 °C. 1H-NMR (400 MHz, CDCl3) δ 5.93 (s, 1H), 3.57 (t, J = 6.4 Hz, 2H), 2.53–2.49 (m, 1H), 2.30–2.26 (m, 1H), 2.22–2.17 (m, 1H), 2.02–1.99 (m, 1H), 1.88–1.85 (m, 1H), 1.80–1.76 (m, 1H), 1.66–1.02 (m, other aliphatic ring protons), 0.91 (d, J = 6.0 Hz, 3H), 0.67 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 203.23, 176.64, 124.91, 63.48, 57.21, 54.82, 52.00, 50.80, 45.20, 43.40, 42.10, 38.95, 38.77, 36.54, 35.67, 32.00, 29.52, 28.67, 27.88, 26.66, 26.48, 23.20, 20.91, 19.59, 18.92, 12.04. HRMS (ESI): calcd for C26H44NO2 [M + H]+ 402.3367; found 402.3372.
3.1.20. (10R,13R,17R)-3-amino-17-((R)-5-hydroxypentan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-7H-cyclopenta[a]phenanthren-7-oxime (5ah)
NH2OH.HCl (35 mg, 0.5 mmol) and NaOAc (136 mg, 1 mmol) was added to a solution of compound 5ag (40 mg, 0.1 mmol) in EtOH (5 mL). The mixture was refluxed for 2 h. When the reaction was completed, as indicated by TLC, the solvent EtOH was removed in vacuo, and an aqueous NaOH solution (10 mL) was added. The mixture was extracted with DCM (3 × 30 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered, concentrated, and recrystallized in MeCN to obtain compound 5ah (39 mg, 93% yield) as a white solid. The mp was 218.3–219.9 °C. 1H-NMR (400 MHz, CDCl3:CD3OD = 10:1) δ 6.86 (s, 1H), 3.53 (t, J = 6.4 Hz, 2H), 2.46–2.42 (m, 1H), 2.31–2.27 (m, 1H), 2.21–2.18 (m, 1H), 2.00–1.97 (m, 1H), 1.80–0.82 (m, other aliphatic ring protons), 0.66 (s, 3H). 13C-NMR (100 MHz, CDCl3:CD3OD = 10:1) δ 163.70, 157.64, 110.97, 63.30, 57.53, 54.83, 51.89, 51.67, 43.17, 41.96, 38.97, 38.57, 37.50, 36.79, 35.59, 31.94, 29.31, 28.42, 27.59, 27.32, 26.95, 23.38, 20.68, 19.91, 18.90, 12.25. HRMS (ESI): calcd for C26H45N2O2 [M + H]+ 417.3476; found 417.3486.
3.1.21. (10R,13R,17R)-3-amino-17-((R)-5-hydroxypentan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-7-ol (5ai)
NaBH4 (19 mg, 0.5 mmol) was added to the solution of compound 5ag (40 mg, 0.1 mmol) in MeOH (10 mL). The mixture was stirred at r.t. for 2 h under a N2 atmosphere. When the reaction was completed, as indicated by TLC, HOAc (0.1 mL) was added, and the solvent was removed in vacuo. An aqueous NaOH solution (10 mL) was added, and the mixture was extracted with DCM (3 × 30 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered, concentrated, and recrystallized in EtOAc to obtain compound 5ai (37 mg, 73% yield) as a white solid. The mp was 171.4–173.2 °C. 1H-NMR (400 MHz, CDCl3) δ 5.52–5.48 (m, 1H), 3.81–3.79 (m, 1H), 3.51 (t, J = 6.4 Hz, 2H), 2.40–2.37 (m, 1H), 2.06–2.03 (m, 1H), 1.88–1.02 (m, other aliphatic ring protons), 0.97 (d, J = 5.6 Hz, 3H), 0.73 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 153.20, 123.49, 73.77, 63.02, 57.80, 56.31, 55.37, 49.98, 42.63, 40.46, 39.84, 39.47, 37.09, 36.96, 35.57, 31.90, 29.16, 28.46, 27.28 (2C), 25.98, 23.23, 21.08, 20.63, 18.63, 11.71. HRMS (ESI): calcd for C26H46NO2 [M + H]+ 404.3523; found 404.3548.
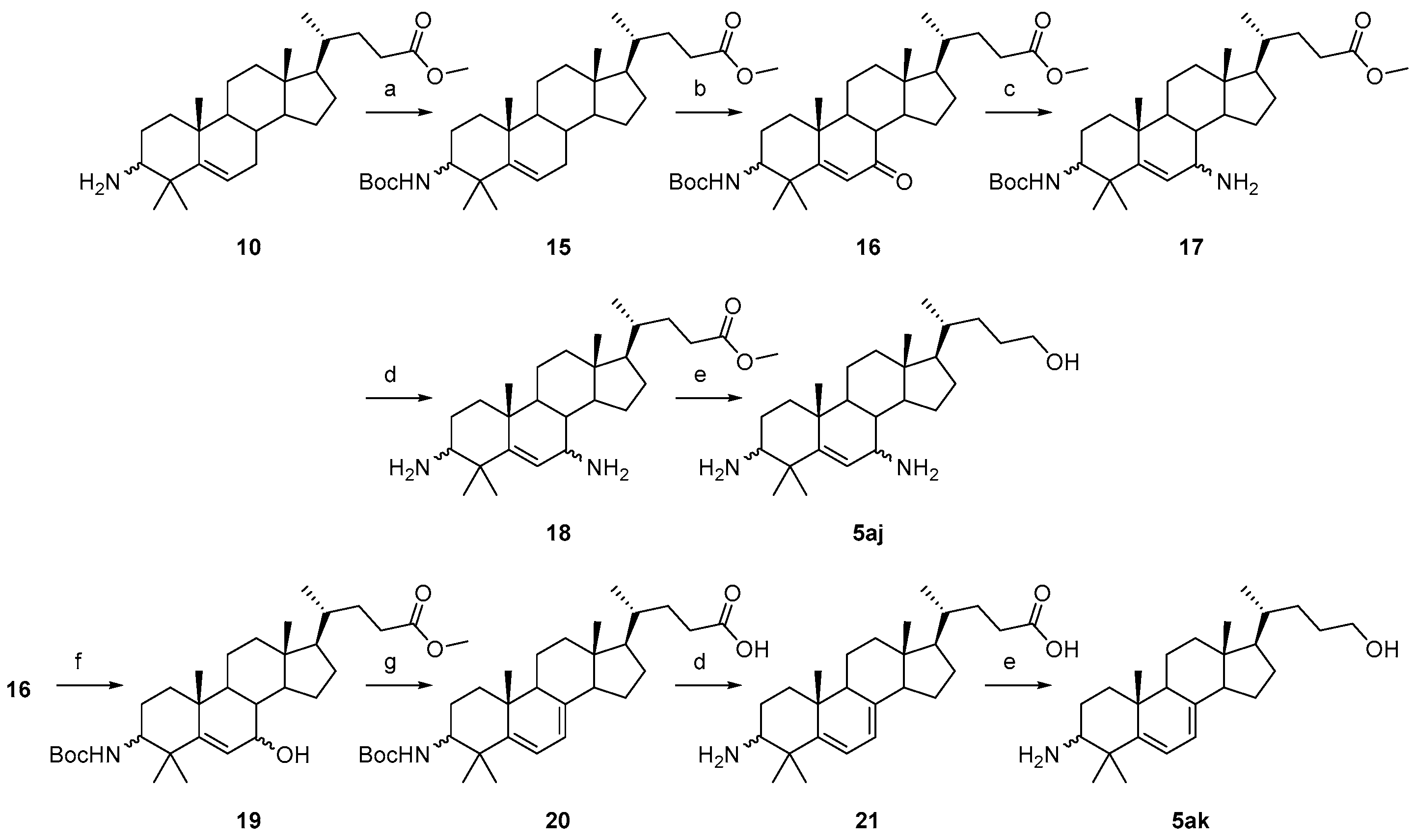
3.1.22. Methyl(4R)-4-((10R,13R,17R)-3-((tert-butoxycarbonyl)amino)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (15)
Compound 15 was synthesized in a 99% yield as a white solid using a similar procedure to that in 3.1.16. The mp was 190.6–191.8 °C. 1H-NMR (400 MHz, CDCl3) δ 5.56–5.53 (m, 1H), 4.43 (d, J = 10.0 Hz, 1H), 3.65 (s, 3H), 3.32–3.27 (m, 1H), 2.39–2.31 (m, 1H), 2.25–2.17 (m, 1H), 2.09–2.04 (m, 1H), 1.88–1.96 (m, 1H), 1.85–0.95 (m, other aliphatic ring protons), 0.91 (d, J = 6.0 Hz, 3H), 0.66 (s, 3H). HRMS (ESI): calcd for C32H53NNaO4 [M + Na]+ 538.3867; found 538.3880.
3.1.23. Methyl(4R)-4-((10R,13R,17R)-3-((tert-butoxycarbonyl)amino)-4,4,10,13-tetramethyl-7-oxo-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (16)
Compound 16 was synthesized in a 90% yield as a white solid using a similar procedure to that in 3.1.18. 1H-NMR (400 MHz, CDCl3) δ 5.95 (s, 1H), 4.48 (d, J = 9.6 Hz, 1H), 3.65 (s, 3H), 3.49–3.44 (m, 1H), 2.38–2.30 (m, 2H), 2.25–2.17 (m, 2H), 2.02–1.99 (m, 1H), 1.91–1.89 (m, 1H), 1.81–1.05 (m, other aliphatic ring protons), 0.91 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). HRMS (ESI): calcd for C32H52NO5 [M + H]+ 530.3840; found 530.3835.
3.1.24. Methyl(4R)-4-((10R,13R,17R)-7-amino-3-((tert-butoxycarbonyl)amino)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (17)
Compound 17 was synthesized in a 74% yield as a white solid using a similar procedure to that in 3.1.4. and was used for the next reaction without further purification.
3.1.25. Methyl(4R)-4-((10R,13R,17R)-3,7-diamino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (18)
Compound 17 (160 mg, 0.3 mmol) was dissolved in an anhydrous EtOAc/HCl solution (10 mL). The mixture was stirred at r.t. for 4 h. Water (20 mL) was added, and the suspension was basified to pH > 8 with an aqueous NaOH solution. The mixture was extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated to obtain compound 18 (118 mg, 92% yield) as a white solid. 1H-NMR (400 MHz, CDCl3) δ 5.42–5.40 (m, 1H), 3.65 (s, 3H), 3.03 (dd, J = 8.0, 2.8 Hz, 1H), 2.40–2.32 (m, 2H), 2.25–2.19 (m, 1H), 1.99–0.98 (m, other aliphatic ring protons), 0.91 (d, J = 6.0 Hz, 3H), 0.68 (s, 3H). HRMS (ESI): calcd for C27H46N2NaO2 [M + Na]+ 453.3451; found 453.3447.
3.1.26. (4R)-4-((10R,13R,17R)-3,7-diamino-4,4,10,13-tetramethyl-tetradeca hydro-1H-cyclopenta[a]phenanthren-17-yl)pentan-1-ol (5aj)
Compound 5aj was synthesized in a 66% yield as a white solid using a similar procedure to that in 3.1.5. The mp was 178.7–180.2 °C. 1H-NMR (400 MHz, CDCl3) δ 5.40–5.35 (m, 1H), 3.55 (t, J = 4.0 Hz, 2H), 3.03–3.00 (m, 1H), 2.38–2.34 (m, 1H), 1.99–1.96 (m, 1H), 1.89–1.82 (m, 1H), 1.77–0.95 (m, other aliphatic ring protons), 0.91 (d, J = 6.0 Hz, 3H), 0.66 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 152.40, 124.81, 63.21, 58.04, 56.93, 55.37, 54.59, 51.07, 43.07, 41.74, 40.76, 39.79, 37.45, 36.80, 35.66, 32.08, 29.55, 28.66, 28.19, 27.39, 26.41, 23.68, 21.82, 20.89, 18.82, 12.02.
3.1.27. Methyl(4R)-4-((10R,13R,17R)-3-((tert-butoxycarbonyl)amino)-7-hydroxy-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate (19)
Compound 19 was synthesized in a 92% yield as a white solid using a similar procedure to that in 3.1.21. The mp was 198.3–200.4 °C. 1H-NMR (400 MHz, CDCl3) δ 5.51 (d, J = 2.0 Hz, 1H), 4.45 (d, J = 10.0 Hz, 1H), 3.91–3.89 (m, 1H), 3.65 (s, 3H), 3.35–3.29 (m, 1H), 2.38–2.31 (m, 1H), 2.25–2.17 (m, 1H), 1.99–1.00 (m, other aliphatic ring protons), 0.91 (d, J = 6.0 Hz, 3H), 0.67 (s, 3H). HRMS (ESI): calcd for C32H53NNaO5 [M + Na]+ 554.3816; found 554.3831.
3.1.28. (4R)-4-((10R,13R,17R)-3-((tert-butoxycarbonyl)amino)-4,4,10,13-tetramethyl-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoic acid (20)
Sixty percent NaH (30 mg, 0.75 mmol) and MeI (107 mg, 0.75 mmol) were added to a solution of compound 19 (133 mg, 0.25 mmol) in dry DMF (10 mL). The mixture was stirred at r.t. for 2 h under a N2 atmosphere, and the starting material disappeared. An aqueous Na2S2O3 solution (5 mL) was added to quench the excess MeI. Water (30 mL) was added, and the mixture was extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with water, brine, dried over Na2SO4, filtered, concentrated, and recrystallized in 5 mL of EtOH to obtain compound 20 (76 mg, 61% yield) as a white solid and used for the next reaction without further purification.
3.1.29. (4R)-4-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-2,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoic acid (21)
Compound 21 was synthesized in a 92% yield as white solid using a similar procedure to that in 3.1.25 and used for the next reaction without further purification.
3.1.30. (4R)-4-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-2,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentan-1-ol (5ak)
Compound 5ak was synthesized in a 92% yield as a white solid using a similar procedure to that in 3.1.5. The mp was 179.5–181.3 °C. 1H-NMR (400 MHz, CDCl3) δ 6.21 (d, J = 10.0 Hz, 1H), 5.63 (d, J = 10.0 Hz, 1H), 3.61 (t, J = 6.4 Hz, 2H),2.47–2.36 (m, 2H), 2.33–2.25 (m, 1H), 2.00–1.97 (m, 1H), 1.89–0.95 (m, other aliphatic ring protons), 0.88 (s, 3H), 0.76 (s, 3H), 0.65 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 146.41, 126.76, 126.66, 125.27, 63.61, 59.86, 55.99, 55.90, 50.35, 43.72, 38.10, 37.15, 36.74, 36.16, 34.74, 31.76, 29.45, 28.78, 28.21, 27.53, 24.94, 19.54, 19.28, 19.00, 16.08, 13.13. HRMS (ESI): calcd for C26H44NO [M + H]+ 386.3417; found 386.3429.
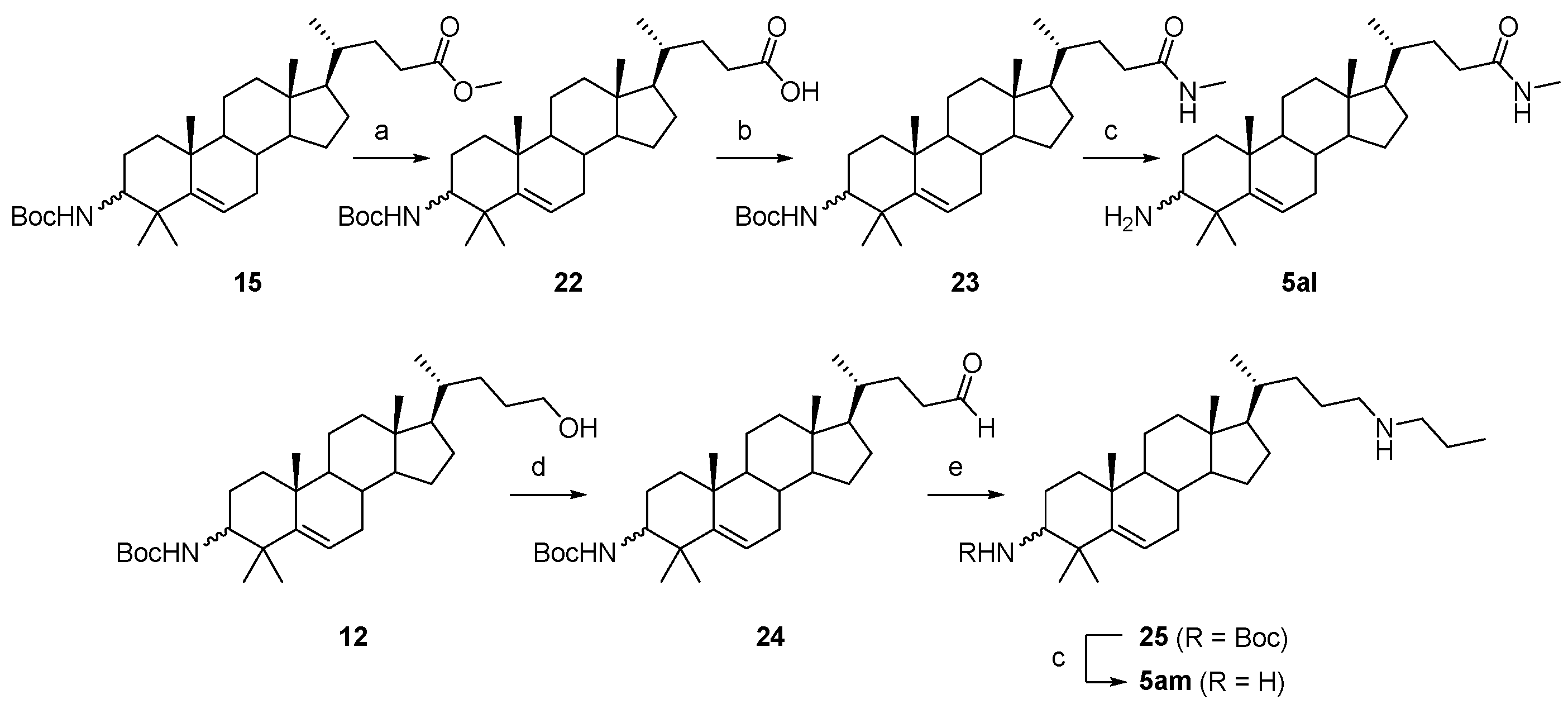
3.1.31. (4R)-4-((10R,13R,17R)-3-((tert-butoxycarbonyl)amino)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoic acid (22)
An aqueous 2N NaOH solution (1 mL) was added to the solution of compound 15 (516 mg, 1.0 mmol) in MeOH (5 mL). The reaction was stirred at r.t. for 2 h, and TLC indicated the consumption of the starting material. Water (20 mL) was added, and the suspension was acidified to pH < 6 with aqueous HCl solution. The solvent MeOH was removed in vacuo. The mixture was extracted with DCM (3 × 30 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated to obtain compound 22 (470 mg, 94% yield) as a white solid. The mp was 243.1–245.5 °C. 1H-NMR (400 MHz, CDCl3) δ 5.57–5.52 (m, 1H), 4.45 (d, J = 10.0 Hz, 1H), 3.33–3.27 (m, 1H), 2.43–2.35 (m, 1H), 2.29–2.21 (m, 1H), 2.09–2.05 (m, 1H), 2.00–1.97 (m, 1H), 1.89–1.77 (m, 2H), 1.73–0.92 (m, other aliphatic ring protons), 0.67 (s, 3H). HRMS (ESI): calcd for C31H51NNaO4 [M + Na]+ 524.3710; found 524.3726.
3.1.32. Tert-butyl((10R,13R,17R)-4,4,10,13-tetramethyl-17-((R)-5-(methylamino)-5-oxopentan-2-yl)-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (23)
MeNH2.HCl (27 mg, 0.4 mmol), HOBt (54 mg, 0.4 mmol), EDCI (77 mg, 0.4 mmol), and DIPEA (52 mg, 0.4 mmol) were added to a solution of compound 22 (100 mg, 0.2 mmol) in DMF (10 mL). The reaction mixture was stirred at r.t. for 12 h under a N2 atmosphere, and the starting material disappeared, as monitored by TLC. Water (30 mL) was added, and the mixture was extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with water, brine, dried over Na2SO4, filtered, concentrated, and purified by silica gel column chromatography using DCM/MeOH (30:1, v/v) to obtain compound 23 (85 mg, 83% yield) as a white solid. The mp was 228.8–230.2 °C. 1H-NMR (400 MHz, CDCl3) δ 5.60–5.58 (m, 1H), 5.54–5.51 (m, 1H), 4.44 (d, J = 10.0 Hz, 1H), 3.30–3.25 (m, 1H), 2.78 (d, J = 4.4 Hz, 3H), 2.26–2.19 (m, 1H), 2.08–2.00 (m, 2H), 1.98–1.95 (m, 1H), 1.87–0.95 (m, other aliphatic ring protons), 0.91 (d, J = 6.4 Hz, 3H), 0.65 (s, 3H). HRMS (ESI): calcd for C32H54N2NaO3 [M + Na]+ 537.4027; found 537.4039.
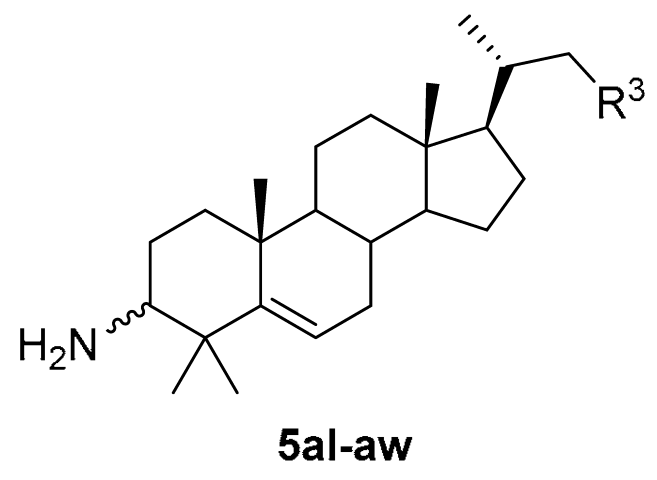
3.1.33. (4R)-4-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-N-methylpentanamide (5al)
Compound 5al was synthesized in a 93% yield as a white solid using a similar procedure to that in 3.1.25. The mp was >300 °C. 1H-NMR (400 MHz, CD3OD) δ 5.69–5.65 (m, 1H), 3.28–3.26 (m, 2H), 2.86–2.83 (m, 1H), 2.66 (s, 3H), 2.19–0.97 (m, other aliphatic ring protons), 0.92 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). 13C-NMR (100 MHz, CD3OD) δ 177.31, 148.94, 122.93, 60.46, 58.55, 57.15, 52.23, 43.39, 40.95, 39.86, 37.84, 37.55, 36.87, 33.99, 33.60, 33.31, 32.01, 29.20, 27.79, 26.33, 25.13, 25.03, 24.58, 21.64, 21.63, 18.86, 12.32. HRMS (ESI): calcd for C27H47N2O [M + H]+ 415.3683; found 415.3668.
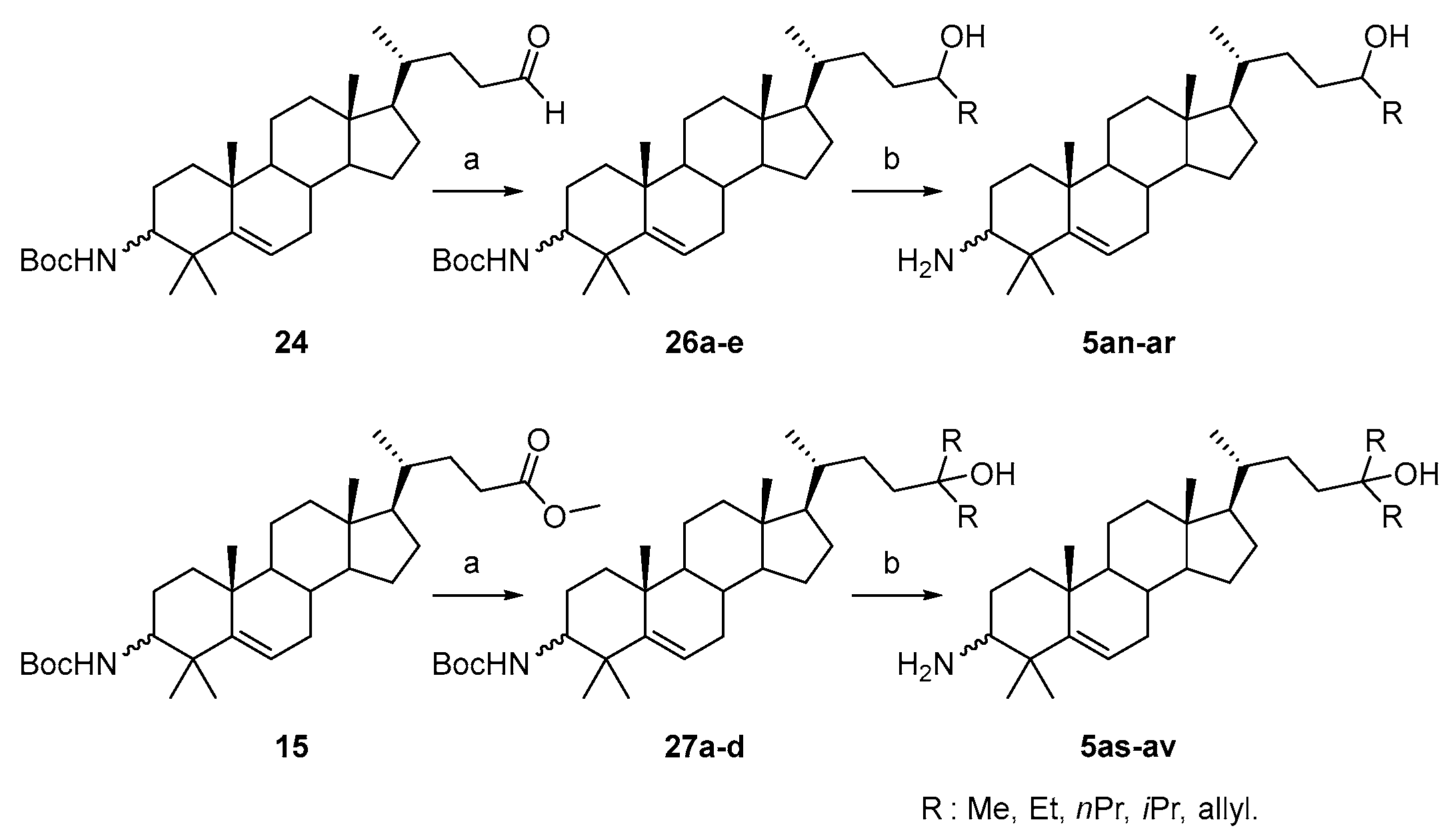
3.1.34. Tert-butyl((10R,13R,17R)-4,4,10,13-tetramethyl-17-((R)-5-oxopentan-2-yl)-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (24)
IBX (616 mg, 2.2 mmol) was added to a solution of compound 12 (976 mg, 2 mmol) in DMSO (10 mL). The mixture was stirred at r.t. for 2 h under a N2 atmosphere. When the reaction was completed, as indicated by TLC, the reaction mixture was added to an aqueous NaHSO3 solution (20 mL). The mixture was extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with water, brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude material was purified by silica gel column chromatography using PE/EtOAc (10:1, v/v) to obtain compound 24 (602 mg, 62% yield) as a white solid. The mp was 173.3–174.8 °C. 1H-NMR (400 MHz, CD3OD) δ 9.75 (s, 1H), 5.56–5.53 (m, 1H), 4.43 (d, J = 9.6 Hz, 1H), 3.31–3.2 (m, 1H), 2.47–2.40 (m, 1H), 2.38–2.29 (m, 1H), 2.09–2.04 (m, 1H), 1.99–1.96 (m, 1H), 1.90–0.95 (m, other aliphatic ring protons), 0.91 (d, J = 6.4 Hz, 3H), 0.66 (s, 3H). HRMS (ESI): calcd for C31H51NNaO3 [M + Na]+ 508.3761; found 508.3770.
3.1.35. Tert-butyl((10R,13R,17R)-4,4,10,13-tetramethyl-17-((R)-5-(propylamino)pentan-2-yl)-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (25)
NaBH(OAc)3 (85 mg, 0.4 mmol) at 0 °C was added to a solution of compound 24 (92 mg, 0.2 mmol) and n-propylamine (18 mg, 0.3 mmol) in DCM (10 mL). The mixture was stirred at r.t. for 2 h under a N2 atmosphere, and the starting material disappeared. Water (30 mL) was added, and the mixture was extracted with DCM (3 × 30 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered, concentrated, and purified by silica gel column chromatography using DCM/MeOH/NH3.H2O (100:5:0.5, v/v/v) to obtain compound 25 (80 mg, 75% yield) as a white solid. 1H-NMR (400 MHz, CD3OD) δ 5.55–5.51 (m, 1H), 4.43 (d, J = 9.6 Hz, 1H), 3.30–3.27 (m, 1H), 2.55 (t, J = 7.2 Hz, 4H), 2.08–2.1.97 (m, 2H), 1.87–1.78 (m, 1H), 1.72–0.88 (m, other aliphatic ring protons), 0.65 (s, 3H). HRMS (ESI): calcd for C34H61N2O2 [M + H]+ 529.4728; found 529.4728.
3.1.36. (10R,13R,17R)-4,4,10,13-tetramethyl-17-((R)-5-(propylamino)pentan-2-yl)-tetradecahydro-1H-cyclopenta[a]phenanthren-3-amine (5am)
The compound 5am was synthesized in a 90% yield as a white solid using a similar procedure to that in 3.1.25. mp >300 °C. 1H-NMR (400 MHz, CD3OD) δ 5.63–5.57 (m, 1H), 2.66 (t, J = 7.2 Hz, 4H), 2.42–2.39 (m, 1H), 2.13–2.08 (m, 1H), 2.07–2.04 (m, 1H), 1.91–1.84 (m, 1H), 1.80–1.77 (m, 1H), 1.69–0.94 (m, other aliphatic ring protons), 0.72 (s, 3H). 13C-NMR (100 MHz, CD3OD) δ 151.19, 121.08, 59.59, 58.71, 57.29, 52.58, 51.96, 50.68, 43.40, 41.50, 41.11, 38.66, 38.01, 36.99, 34.49, 33.76, 32.15, 29.33, 28.05, 27.70, 26.05, 25.19, 24.46, 22.60, 21.77, 21.66, 19.16, 12.35, 11.80. HRMS (ESI): calcd for C29H53N2 [M + H]+ 429.4203; found 429.4237.
3.1.37. Tert-butyl((10R,13R,17R)-17-((2R)-5-hydroxyhexan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (26a)
A 1M MeMgCl solution in THF (1.1 mL, 1.1 mmol) was added dropwise at 0 °C to a dry THF solution (20 mL) of compound 24 (243 mg, 0.5 mmol) under a N2 atmosphere. The mixture was stirred at r.t. for 12 h under a N2 atmosphere. When the reaction was completed, as indicated by TLC, an aqueous NH4Cl solution (2 mL) was added, and the solvents were removed in vacuo. Water (30 mL) was added, and the mixture was extracted with DCM (3 × 30 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered, concentrated, and purified by silica gel column chromatography using DCM/MeOH (30:1, v/v) to obtain compound 26a (130 mg, 52% yield) as a white solid. The mp was 200.8–202.5 °C. 1H-NMR (400 MHz, CDCl3) δ 5.57–5.55 (m, 1H), 4.43 (d, J = 10.0 Hz, 1H), 3.77–3.71 (m, 1H), 3.33–3.27 (m, 1H), 2.10–2.04 (m, 1H), 2.00–1.97 (m, 1H), 1.88–1.81 (m, 1H), 1.73–0.83 (m, other aliphatic ring protons), 0.66 (s, 3H). HRMS (ESI): calcd for C32H55NNaO3 [M + Na]+ 524.4074; found 524.4083.
3.1.38. Tert-butyl((10R,13R,17R)-17-((2R)-5-hydroxyheptan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (26b)
Compound 26b was synthesized in a 57% yield as a white solid using a similar procedure to that in 3.1.37. The mp was 187.3–188.8 °C. 1H-NMR (400 MHz, CDCl3) δ 5.47–5.45 (m, 1H), 4.45 (d, J = 9.2 Hz, 1H), 3.54–3.52 (m, 1H), 3.48–3.46 (m, 1H), 2.09–1.99 (m, 3H), 1.88–1.82 (m, 1H), 1.74–0.82 (m, other aliphatic ring protons), 0.66 (s, 3H). HRMS (ESI): calcd for C33H57NNaO3 [M + Na]+ 538.4231; found 538.4248.
3.1.39. Tert-butyl((10R,13R,17R)-17-((2R)-5-hydroxyoctan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (26c)
Compound 26c was synthesized in a 49% yield as a white solid using a similar procedure to that in 3.1.37. The mp was 173.1–174.5 °C. 1H-NMR (400 MHz, CDCl3) δ 5.47–5.45 (m, 1H), 4.45 (d, J = 9.2 Hz, 1H), 3.54–3.53 (m, 1H), 3.52–3.49 (m, 1H), 2.10–1.99 (m, 3H), 1.88–1.82 (m, 1H), 1.66–0.82 (m, other aliphatic ring protons), 0.66 (s, 3H). HRMS (ESI): calcd for C34H59NNaO3 [M + Na]+ 552.4387; found 552.4389.
3.1.40. Tert-butyl((10R,13R,17R)-17-((2R)-5-hydroxy-6-methylheptan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (26d)
Compound 26d was synthesized in a 45% yield as a white solid using a similar procedure to that in 3.1.37. and was used for the next reaction without further purification.
3.1.41. Tert-butyl((10R,13R,17R)-17-((2R)-5-hydroxyoct-7-en-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (26e)
Compound 26e was synthesized in a 48% yield as a white solid using a similar procedure to that in 3.1.37. The mp was 191.4–192.5 °C. 1H-NMR (400 MHz, CDCl3) δ 5.88–5.78 (m, 1H), 5.56–5.53 (m, 1H), 5.15–5.11 (m, 2H), 4.44 (d, J = 10.0 Hz, 1H), 3.60–3.59 (m, 1H), 3.33–3.27 (m, 1H), 2.35–2.28 (m, 1H), 2.18–0.86 (m, other aliphatic ring protons), 0.66 (s, 3H). HRMS (ESI): calcd for C34H57NNaO3 [M + Na]+ 550.4231; found 550.4247.
3.1.42. (5R)-5-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)hexan-2-ol (5an)
Compound 5an was synthesized in a 92% yield as a white solid using a similar procedure to that in 3.1.25. The mp was >300 °C. 1H-NMR (400 MHz, CD3OD) δ 5.70–5.65 (m, 1H), 3.68–3.62 (m, 1H), 2.78–2.75 (m, 1H), 2.16–2.11 (m, 1H), 2.07–2.04 (m, 1H), 1.93–0.95 (m, other aliphatic ring protons), 0.72 (s, 3H). 13C-NMR (100 MHz, CD3OD) δ 149.56, 122.44, 69.19, 60.25, 58.62, 57.33, 52.35, 43.35, 41.03, 40.30, 37.89, 37.14, 36.70, 33.66, 33.19, 32.06, 29.27, 27.88, 25.42, 25.16, 24.89, 23.65, 23.40, 21.70, 21.65, 19.27, 12.37. HRMS (ESI): calcd for C27H48NO [M + H]+ 402.3730; found 402.3752.
3.1.43. (6R)-6-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)heptan-3-ol (5ao)
Compound 5ao was synthesized in a 91% yield as a white solid using a similar procedure as that in 3.1.25. The mp was >300 °C. 1H-NMR (400 MHz, CD3OD) δ 5.72–5.64 (m, 1H), 3.39–3.35 (m, 1H), 2.89–2.86 (m, 1H), 2.17–2.12 (m, 1H), 2.07–2.04 (m, 1H), 1.96–0.93 (m, other aliphatic ring protons), 0.72 (s, 3H). 13C-NMR (100 MHz, CD3OD) δ 148.87, 122.87, 74.46, 60.44, 58.50, 57.27, 52.17, 43.28, 40.91, 39.81, 37.79, 37.52, 37.15, 34.29, 33.56, 33.04, 31.95, 31.03, 30.72, 29.23, 27.79, 25.09, 24.54, 21.65, 21.58, 19.29, 12.35, 10.42. HRMS (ESI): calcd for C28H49NNaO [M + Na]+ 438.3706; found 438.3692.
3.1.44. (7R)-7-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)octan-4-ol (5ap)
Compound 5ap was synthesized in a 92% yield as a white solid using a similar procedure to that in 3.1.25. The mp was >300 °C. 1H-NMR (400 MHz, CD3OD) δ 5.72–5.65 (m, 1H), 3.48–3.46 (m, 1H), 2.83–2.80 (m, 1H), 2.17–2.12 (m, 1H), 2.07–2.04 (m, 1H), 1.92–0.93 (m, other aliphatic ring protons), 0.72 (s, 3H). 13C-NMR (100 MHz, CD3OD) δ 149.25, 122.72, 72.83, 60.36, 58.62, 57.36, 52.33, 43.35, 41.02, 40.80, 40.50, 40.10, 37.88, 37.73, 37.22, 37.00, 34.93, 33.63, 33.11, 32.05, 29.29, 27.82, 25.15, 24.92, 21.67, 19.97, 19.30, 14.51, 12.34. HRMS (ESI): calcd for C29H51NNaO [M + Na]+ 452.3863; found 452.3856.
3.1.45. (6R)-6-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-2-methylheptan-3-ol (5aq)
Compound 5aq was synthesized in a 90% yield as a white solid using a similar procedure to that in 3.1.25. The mp was >300 °C. 1H-NMR (400 MHz, CD3OD) δ 5.71–5.66 (m, 1H), 3.22–3.20 (m, 1H), 2.79 (d, J = 11.2 Hz, 1H), 2.16–2.12 (m, 1H), 2.08–2.04 (m, 1H), 1.91–0.90 (m, other aliphatic ring protons), 0.72 (s, 3H). 13C-NMR (100 MHz, CD3OD) δ 149.36, 122.62, 78.11, 60.32, 58.63, 57.48, 52.34, 43.37, 41.03, 40.16, 37.88, 37.78, 37.36, 34.87, 33.65, 33.36, 32.06, 31.67, 29.32, 27.84, 25.17, 24.91, 21.68, 21.65, 19.56, 19.36, 18.00, 17.53, 12.37. HRMS (ESI): calcd for C29H52NO [M + H]+ 430.4043; found 430.4068.
3.1.46. (7R)-7-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)oct-1-en-4-ol (5ar)
Compound 5ar was synthesized in a 90% yield as a white solid using a similar procedure to that in 3.1.25. The mp was 250.4–252.6 °C. 1H-NMR (400 MHz, CD3OD) δ 5.91–5.81 (m, 1H), 5.68–5.63 (m, 1H), 5.06 (d, J = 15.6 Hz, 1H), 5.03 (d, J = 8.4 Hz, 1H), 3.54–3.52 (m, 1H), 2.63 (d, J = 11.2 Hz, 1H), 2.27–2.19 (m, 2H), 2.17–2.09 (m, 1H), 2.07–2.04 (m, 1H), 1.91–0.95 (m, other aliphatic ring protons), 0.72 (s, 3H). 13C-NMR (100 MHz, CD3OD) δ 150.11, 136.54, 121.98, 117.16, 72.74, 60.01, 58.66, 57.37, 52.44, 43.36, 43.09, 41.06, 40.72, 38.15, 37.93, 37.23, 34.23, 33.70, 33.01, 32.09, 29.31, 27.93, 26.23, 25.18, 24.72, 21.73, 21.66, 19.32, 12.37. HRMS (ESI): calcd for C29H50NO [M + H]+ 428.3887; found 428.3886.
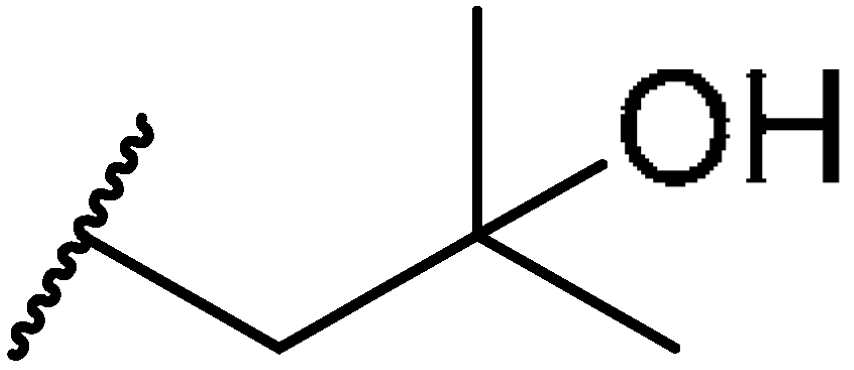
3.1.47. Tert-butyl((10R,13R,17R)-17-((R)-5-hydroxy-5-methylhexan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (27a)
Compound 27a was synthesized in a 68% yield as a white solid using a similar procedure to that in 3.1.37. The mp was 220.4–223.2 °C. 1H-NMR (400 MHz, CDCl3) δ 5.56–5.51 (m, 1H), 4.43 (d, J = 10.0 Hz, 1H), 3.30–3.26 (m, 1H), 2.09–2.04 (m, 1H), 2.00–1.97 (m, 1H), 1.89–1.80 (m, 1H), 1.73–0.95 (m, other aliphatic ring protons), 0.92 (d, J = 6.4 Hz, 3H), 0.66 (s, 3H). HRMS (ESI): calcd for C33H57NNaO3 [M + Na]+ 538.4231; found 538.4234.
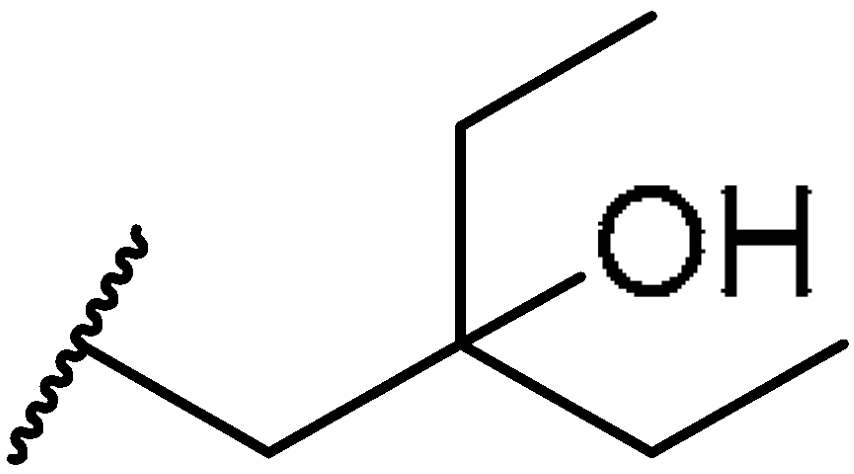
3.1.48. Tert-butyl((10R,13R,17R)-17-((R)-5-ethyl-5-hydroxyheptan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (27b)
Compound 27b was synthesized in a 64% yield as a white solid using a similar procedure to that in 3.1.37. The mp was 242.2–243.7 °C. 1H-NMR (400 MHz, CDCl3) δ 5.56–5.53 (m, 1H), 4.43 (d, J = 10.0 Hz, 1H), 3.33–3.26 (m, 1H), 2.09–2.04 (m, 1H), 2.00–1.97 (m, 1H), 1.90–1.80 (m, 1H), 1.73–0.96 (m, other aliphatic ring protons), 0.92 (d, J = 6.0 Hz, 3H), 0.85 (t, J = 7.2 Hz, 3H), 0.84 (t, J = 7.2 Hz, 3H), 0.66 (s, 3H). HRMS (ESI): calcd for C35H61NNaO3 [M + Na]+ 566.4544; found 566.4538.
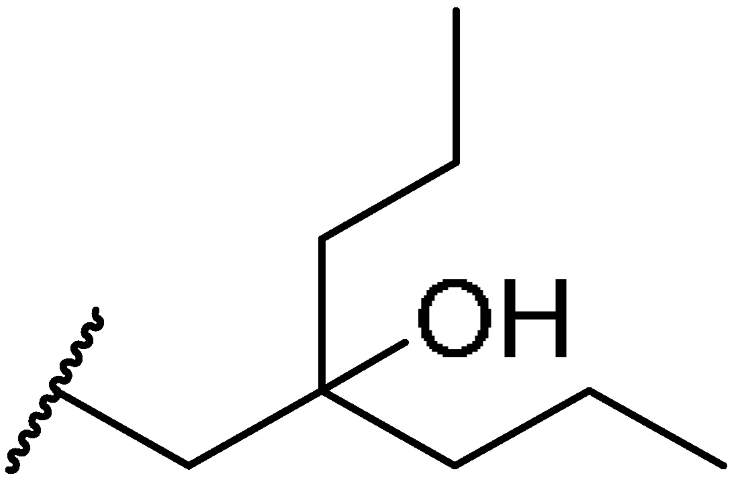
3.1.49. Tert-butyl((10R,13R,17R)-17-((R)-5-hydroxy-5-propyloctan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (27c)
Compound 27c was synthesized in a 55% yield as a white solid using a similar procedure to that in 3.1.37. The mp was 205.2–207.6 °C. 1H-NMR (400 MHz, CDCl3) δ 5.57–5.54 (m, 1H), 5.29 (d, J = 10.0 Hz, 1H), 3.75–3.69 (m, 1H), 2.18–2.14 (m, 1H), 2.10–2.05 (m, 1H), 1.99–1.97 (m, 1H), 1.89–1.81 (m, 1H), 1.70–0.91 (m, other aliphatic ring protons), 0.66 (s, 3H). HRMS (ESI): calcd for C37H65NNaO3 [M + Na]+ 594.4857; found 594.4842.
3.1.50. Tert-butyl((10R,13R,17R)-17-((R)-5-allyl-5-hydroxyoct-7-en-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (27d)
Compound 27d was synthesized in a 61% yield as a white solid using a similar procedure to that in 3.1.37. The mp was 181.5–183.2 °C. 1H-NMR (400 MHz, CDCl3) δ 5.89–5.79 (m, 2H), 5.56–5.53 (m, 1H), 5.13 (d, J = 8.0 Hz, 2H), 5.10 (d, J = 16.0 Hz, 2H), 4.43 (d, J = 10.0 Hz, 1H), 3.33–3.27 (m, 1H), 2.20 (d, J = 7.2 Hz, 4H), 2.09–2.04 (m, 1H), 1.99–1.96 (m, 1H), 1.88–1.81 (m, 1H), 1.73–0.96 (m, other aliphatic ring protons), 0.91 (d, J = 6.4 Hz, 3H), 0.65 (s, 3H).
3.1.51. (5R)-5-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-2-methylhexan-2-ol (5as)
Compound 5as was synthesized in a 93% yield as a white solid using a similar procedure to that in 3.1.25. The mp was 215.2–217.6 °C. 1H-NMR (400 MHz, CDCl3:CD3OD = 5:1) δ 5.47–5.43 (m, 1H), 2.34–2.31 (m, 1H), 2.00–1.96 (m, 1H), 1.90–1.87 (m, 1H), 1.75–1.69 (m, 1H), 1.66–1.63 (m, 1H), 1.53–0.80 (m, other aliphatic ring protons), 0.56 (s, 3H). 13C-NMR (100 MHz, CDCl3:CD3OD = 5:1) δ 149.37, 120.11, 70.95, 58.20, 57.19, 55.61, 50.94, 42.09, 40.20, 39.78, 39.64, 37.23, 36.76, 35.85, 32.54, 30.68, 30.13, 28.77, 28.43, 28.08, 27.29, 26.63, 24.05, 23.58, 21.07, 20.42, 18.58, 11.72. HRMS (ESI): calcd for C28H50NO [M + H]+ 416.3887; found 416.3898.
3.1.52. (6R)-6-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-3-ethylheptan-3-ol (5at)
Compound 5at was synthesized in a 93% yield as a white solid using a similar procedure to that in 3.1.25. The mp was >300 °C. 1H-NMR (400 MHz, CDCl3:CD3OD = 2:1) δ 5.47–5.45 (m, 2H), 2.47–2.43 (m, 1H), 1.99–1.92 (m, 1H), 1.87–1.84 (m, 1H), 1.76–0.74 (m, other aliphatic ring protons), 0.70 (t, J = 7.2 Hz, 3H), 0.69 (t, J = 7.2 Hz, 3H), 0.52 (s, 3H). 13C-NMR (100 MHz, CDCl3:CD3OD = 2:1) δ 148.45, 120.73, 74.68, 58.55, 57.08, 55.59, 50.78, 42.03, 39.52, 39.49, 36.80, 36.63, 36.03, 33.79, 32.42, 30.77, 30.58, 30.47, 29.08, 28.10, 27.08, 25.25, 23.98, 23.71, 20.95, 20.35, 18.56, 11.61, 7.43, 7.32. ESI-MS (m/z): 444.65 (M + H)+.
3.1.53. (7R)-7-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-4-propyloctan-4-ol (5au)
Compound 5au was synthesized in a 92% yield as a white solid using a similar procedure to that in 3.1.25. The mp was 242.6–244.9 °C. 1H-NMR (400 MHz, CDCl3) δ 5.62–5.60 (m, 1H), 2.89–2.86 (m, 1H), 2.11–2.07 (m, 1H), 2.00–1.98 (m, 2H), 1.84–1.78 (m, 2H), 1.66–1.60 (m, 2H), 1.49–0.91 (m, other aliphatic ring protons), 0.66 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 148.14, 121.47, 74.73, 59.83, 57.29, 55.81, 50.88, 42.32, 42.00, 41.73, 39.77, 39.26, 36.89, 36.68, 36.26, 35.50, 32.67, 30.84, 29.85, 29.45, 28.39, 28.07, 25.36, 24.29, 24.08, 21.27, 20.66, 18.94, 16.91, 16.82, 14.89, 11.99. ESI-MS (m/z): 472.71 (M + H)+.
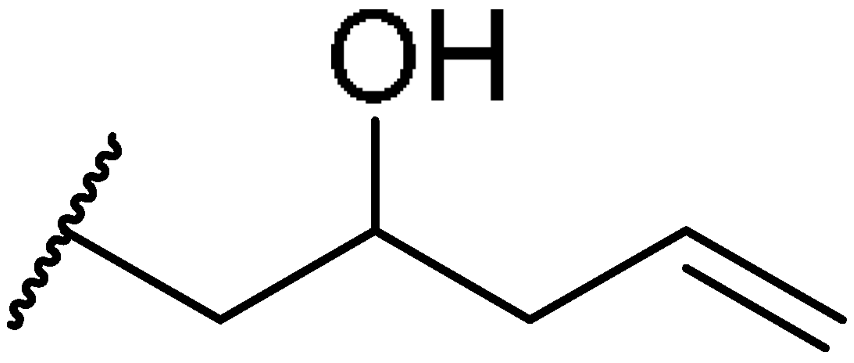
3.1.54. (7R)-4-allyl-7-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)oct-1-en-4-ol (5av)
Compound 5av was synthesized in a 90% yield as a white solid using a similar procedure to that in 3.1.25. The mp was 235.5–237.4 °C. 1H-NMR (400 MHz, CDCl3) δ 5.89–5.79 (m, 2H), 5.56–5.53 (m, 1H), 5.13 (d, J = 8.0 Hz, 2H), 5.15 (d, J = 16.0 Hz, 2H), 2.47–2.43 (m, 1H), 2.20 (d, J = 7.2 Hz, 4H), 2.10–2.05 (m, 1H), 1.99–1.96 (m, 1H), 1.87–1.82 (m, 1H), 1.74–0.94 (m, other aliphatic ring protons), 0.90 (d, J = 6.4 Hz, 3H), 0.65 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 150.07, 133.96, 133.93, 119.91, 118.73, 118.64, 73.74, 58.62, 57.39, 55.80, 51.12, 43.93, 43.71, 42.31, 40.68, 39.84, 37.56, 36.99, 36.21, 35.52, 32.77, 30.90, 29.38, 28.44, 27.78, 27.58, 24.29, 24.06, 21.35, 20.63, 18.92, 11.97. HRMS (ESI): calcd for C32H54NO [M + H]+ 468.4200; found 468.4200.
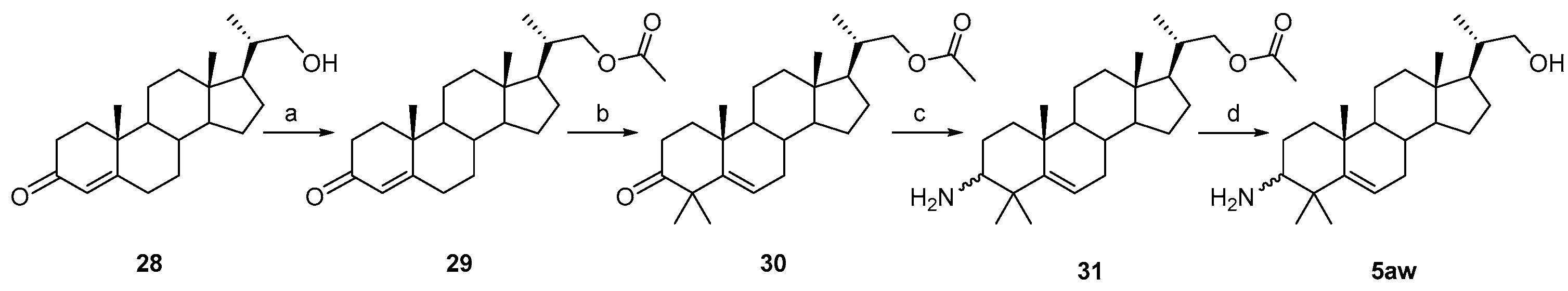
3.1.55. (2S)-2-((10R,13S,17R)-4,4,10,13-tetramethyl-3-oxo-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)propylacetate (29)
Ac2O (286 mg, 2.8 mmol), Et3N (1 g, 10 mmol), and DMAP (12 mg, 0.1 mmol) were added to a solution of 21-hydroxy-20-methylpregn-4-en-3-one 28 (717 mg, 2 mmol) in dry DCM (30 mL), and the mixture was stirred at r.t. for 12 h under a N2 atmosphere; TLC indicated the consumption of the starting material. Water (30 mL) was added, and the mixture was extracted with DCM (3 × 30 mL). The combined organic layer was washed with aqueous NaOH solution, brine, dried over Na2SO4, filtered, concentrated, and recrystallized in 5 mL of EtOAc to obtain compound 29 (780 mg, 97% yield) as a white solid, which was used for the next reaction without further purification.
3.1.56. (10R,13S,17R)-17-((S)-1-hydroxypropan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one (30)
Compound 30 was synthesized in a 67% yield as a white solid using a similar procedure to that in 3.1.3., except for the esterification procedure, and was used for the next reaction without further purification.
3.1.57. (2S)-2-((10R,13S,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)propylacetate (31)
Compound 31 was synthesized in a 76% yield as a white solid using a similar procedure to that in 3.1.4. The mp was 198.3–199.9 °C. 1H-NMR (400 MHz, CDCl3) δ 5.56–5.55 (m, 1H), 4.07 (dd, J = 10.8, 3.2 Hz, 1H), 3.76 (dd, J = 10.8, 3.2 Hz, 1H), 2.42–2.38 (m, 1H), 2.12–2.06 (m, 1H), 2.04 (s, 3H), 2.00–1.97 (m, 1H), 1.83–0.89 (m, other aliphatic ring protons), 0.68 (s, 3H). HRMS (ESI): calcd for C26H43NNaO2 [M + Na]+ 424.3186; found 424.3199.
3.1.58. (2S)-2-((10R,13S,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)propan-1-ol (5aw)
Compound 5aw was synthesized in a 90% yield as a white solid using a similar procedure to that in 3.1.31. The mp was 170.6–173.8 °C. 1H-NMR (400 MHz, CDCl3:CD3OD = 5:1) δ 5.47–5.45 (m, 1H), 3.52–3.49 (m, 1H), 3.21–3.16 (m, 1H), 2.29–2.25 (m, 1H), 2.03–1.96 (m, 1H), 1.93–1.90 (m, 1H), 1.77–1.70 (m, 1H), 1.67–1.63 (m, 1H), 1.57–0.76 (m, other aliphatic ring protons), 0.60 (s, 3H). 13C-NMR (100 MHz, CDCl3:CD3OD = 5:1) δ 149.84, 119.76, 67.37, 58.02, 56.99, 52.35, 51.00, 42.25, 40.56, 39.56, 38.77, 37.44, 36.82, 32.58, 30.75, 27.73, 27.40, 27.31, 24.23, 23.52, 21.14, 20.45, 16.60, 11.82. HRMS (ESI) calcd for C24H42NO [M + H]+ 360.3261; found 360.3267.
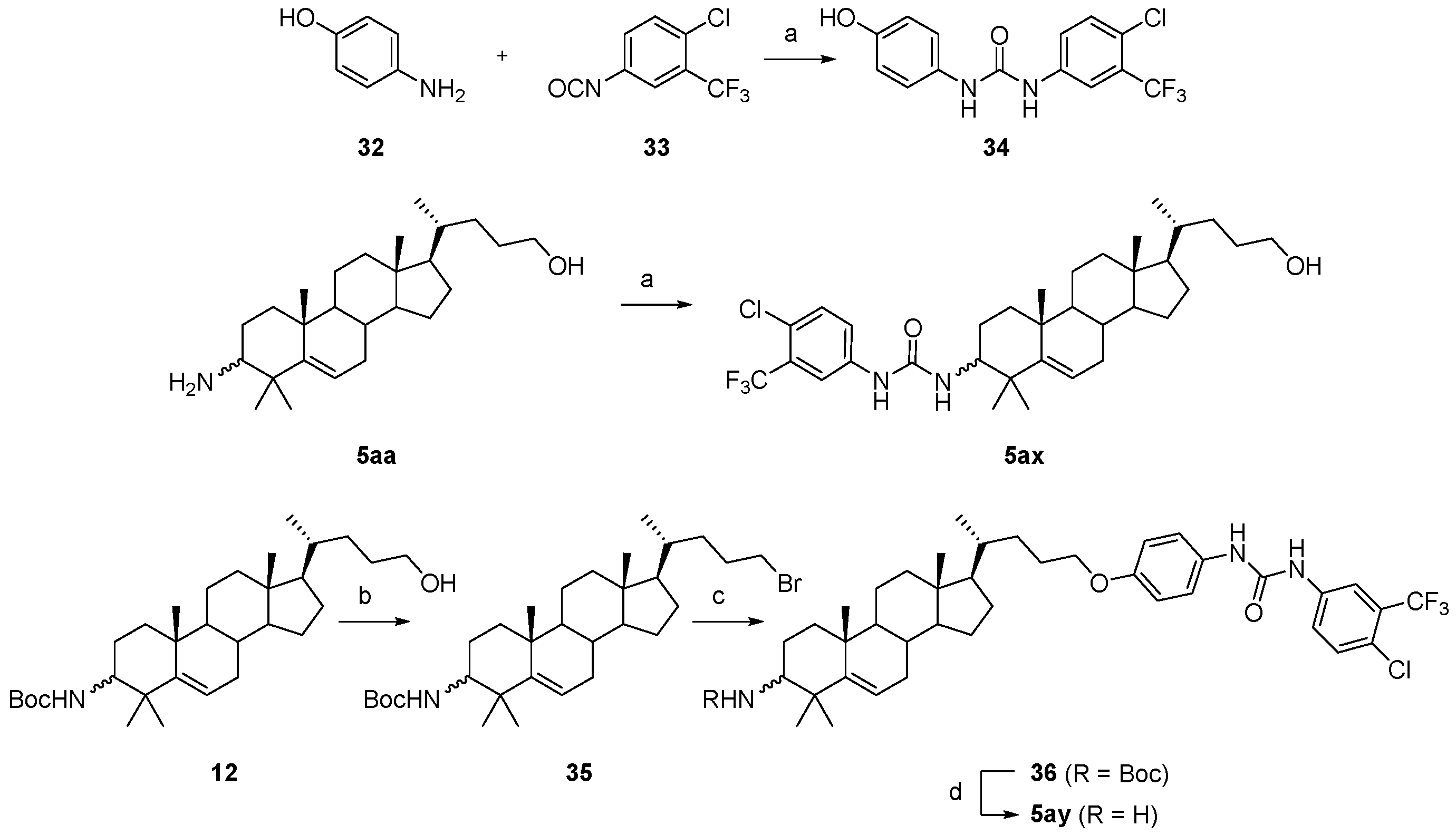
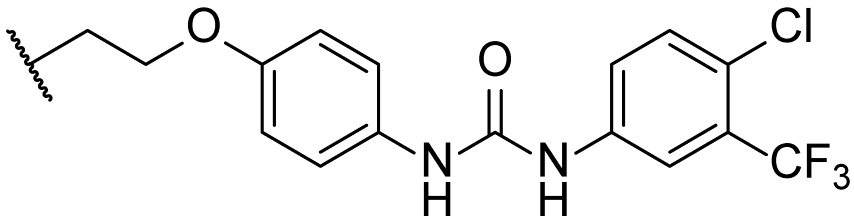
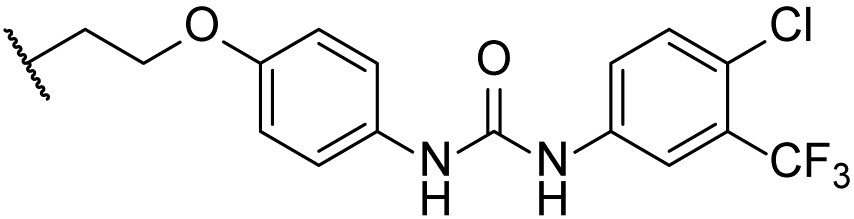
3.1.59. 1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-hydroxyphenyl)urea (34)
Compound 33 (443 mg, 2 mmol) was added to a solution of compound 32 (218 mg, 2 mmol) in dry DCM (10 mL). The reaction was stirred at r.t. for 10 min, and the starting material disappeared. Water (30 mL) was added, and the mixture was extracted with DCM (3 × 30 mL). The combined organic layer was washed with brine, dried over Na2SO4, filtered, concentrated, and recrystallized in 2 mL of MeCN to obtain compound 34 (635 mg, 99% yield) as a brown solid. The mp was 232.1–233.6 °C. 1H-NMR (400 MHz, CDCl3:CD3OD = 5:1) δ 7.74 (d, J = 2.0 Hz, 1H), 7.53 (dd, J = 8.4, 2.0 Hz, 1H), 7.33 (d, J = 8.4 Hz, 1H), 7.13 (d, J = 8.8 Hz, 2H), 6.73 (d, J = 8.8 Hz, 2H). HRMS (ESI): calcd for C14H11ClF3N2O2 [M + H]+ 331.0456; found 331.0451.
3.1.60. 1-(4-chloro-3-(trifluoromethyl)phenyl)-3-((10R,13R,17R)-17-((R)-5-hydroxypentan-2-yl)-4,4,10,13-tetramethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)urea (5ax)
Compound 5ax was synthesized in a 96% yield as a white solid using a similar procedure to that in 3.1.59. The mp was 221.1–223.7 °C. 1H-NMR (400 MHz, CDCl3:CD3OD = 3:1) δ 7.72 (s, 1H), 7.47 (d, J = 8.8 Hz, 1H), 7.29 (d, J = 8.8 Hz, 1H), 5.73 (d, J = 10.0 Hz, 1H), 5.54–5.50 (m, 1H), 3.49 (t, J = 4.8 Hz, 2H), 3.47–3.40 (m, 1H), 2.07–2.00 (m, 1H), 1.98–1.94 (m, 1H), 1.85–1.76 (m, 1H), 1.72–0.86 (m, other aliphatic ring protons), 0.63 (s, 3H). 13C-NMR (100 MHz, CDCl3:CD3OD = 3:1) δ 156.05, 149.73, 139.29, 131.82, 128.50 (q, J = 31.2 Hz), 123.94, 122.81 (q, J = 228.0 Hz), 122.31, 120.48, 117.27 (d, J = 5.8 Hz), 63.13, 57.41, 56.41, 56.18, 51.08, 42.39, 40.54, 39.91, 37.62, 36.87, 35.88, 32.73, 32.11, 31.03, 29.37, 28.43, 27.89, 26.43, 24.80, 24.32, 21.36, 20.72, 18.74, 11.97. 19F-NMR (375 MHz, CDCl3:CD3OD = 3:1) δ−63.05. HRMS (ESI): calcd for C34H48ClF3N2NaO2 [M + Na]+ 631.3249; found 631.3247.
3.1.61. Tert-butyl((10R,13R,17R)-17-((R)-5-bromopentan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (35)
PPh3 (131 mg, 0.5 mmol), imidazole (33 mg, 0.5 mmol), and CBr4 (150 mg, 0.45 mmol) were added to a solution of compound 12 (146 mg, 0.3 mmol) in dry DMF (10 mL). The reaction was stirred at r.t. for 2 h, and TLC indicated the consumption of the starting material. Upon the addition of MeOH (0.2 mL) for quenching, water (30 mL) was added and the mixture was extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with water, brine, dried over Na2SO4, filtered, concentrated, and purified by silica gel column chromatography using PE/EtOAc (50:1, v/v) to obtain compound 35 (126 mg, 76% yield) as a white solid. The mp was 192.3–194.7 °C. 1H-NMR (400 MHz, CDCl3) δ 5.59–5.57 (m, 1H), 4.43 (d, J = 10.0 Hz, 1H), 3.43–3.35 (m, 2H), 3.33–3.27 (m, 1H), 2.11–2.04 (m, 1H), 2.00–1.97 (m, 1H), 1.92–1.83 (m, 2H), 1.80–0.95 (m, other aliphatic ring protons), 0.92 (d, J = 6.4 Hz, 3H), 0.66 (s, 3H).
3.1.62. Tert-butyl((10R,13R,17R)-17-((R)-5-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)phenoxy)pentan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (36)
Compound 36 was synthesized in a 68% yield as a white solid using a similar procedure to that in 3.1.6 and used for the next reaction without further purification.
3.1.63. 1-(4-(((4R)-4-((10R,13R,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentyl)oxy)phenyl)-3-(4-chloro-3-(trifluoromethyl)phenyl)urea (5ay)
Compound 5ay was synthesized in a 94% yield as a white solid using a similar procedure to that in 3.1.25. The mp was 192.3–194.5 °C. 1H-NMR (400 MHz, CDCl3:CD3OD = 5:1) δ 7.65 (s, 1H), 7.54 (d, J = 8.4 Hz, 1H), 7.31 (d, J = 8.4 Hz, 1H), 7.19 (d, J = 8.4 Hz, 2H), 6.79 (d, J = 8.4 Hz, 2H), 5.54–5.50 (m, 1H), 3.84 (t, J = 7.6 Hz, 2H), 2.36–2.32 (m, 1H), 2.00–1.97 (m, 1H), 1.88–1.79 (m, 2H), 1.71–0.86 (m, other aliphatic ring protons), 0.66 (s, 3H). 13C-NMR (100 MHz, CDCl3:CD3OD = 5:1) δ 155.68, 153.57, 149.85, 138.40, 131.76, 131.02, 128.43 (d, J = 31.2 Hz), 124.57, 122.78 (q, J = 271.9 Hz), 122.65, 122.09, 119.89 (2C), 117.59 (d, J = 4.4 Hz), 115.02 (2C), 68.96, 58.15, 57.30, 55.89, 51.06, 42.24, 40.60, 39.77, 37.48, 36.89, 35.59, 32.66, 32.09, 30.80, 28.29, 27.49, 27.38, 25.91, 24.18, 23.62, 21.22, 20.53, 18.65, 11.88. 19F-NMR (375 MHz, CDCl3:CD3OD = 5:1) δ -62.77. HRMS (ESI): calcd for C40H54ClF3N3O2 [M + H]+ 700.3851; found 700.3900.
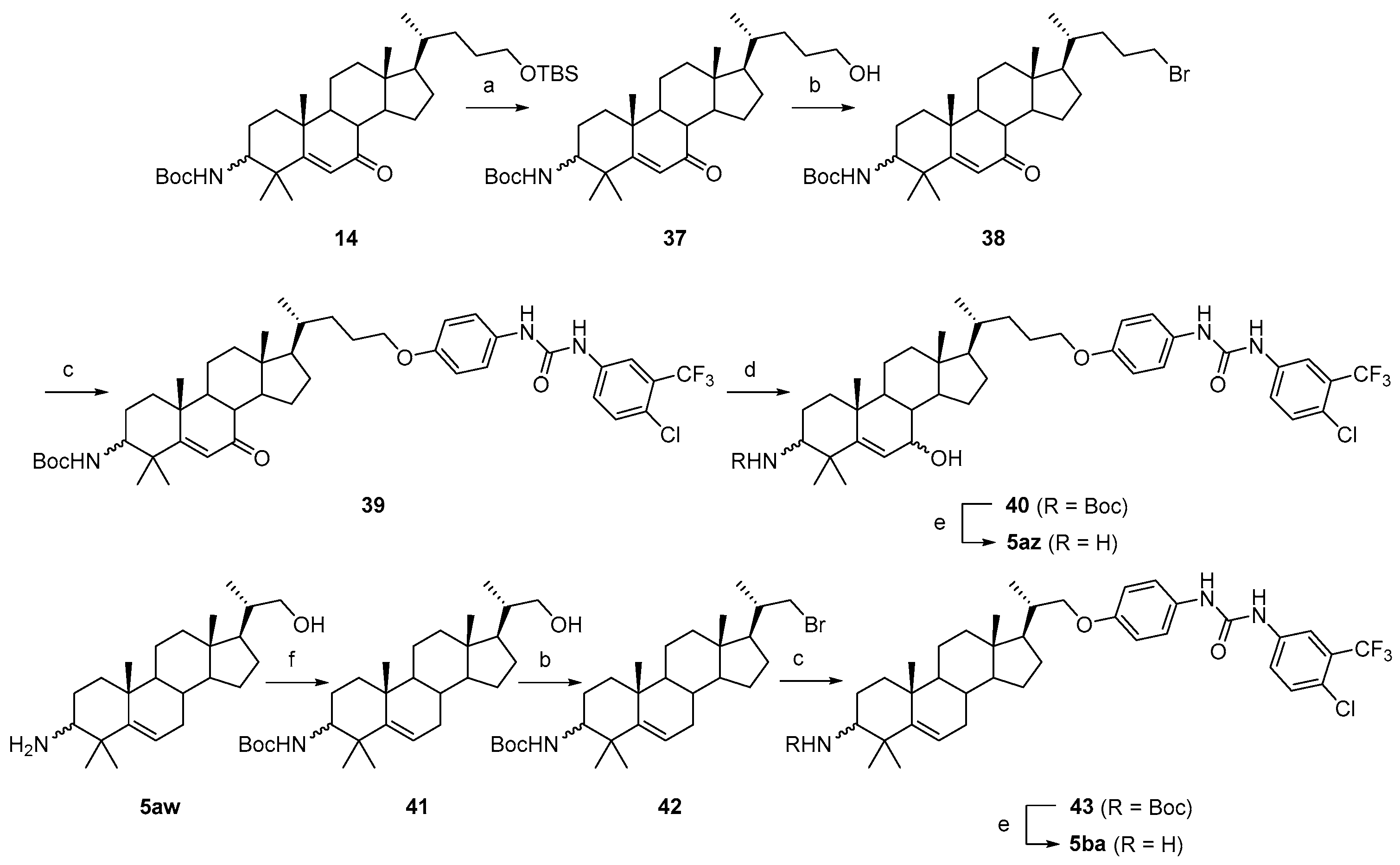
3.1.64. Tert-butyl((10R,13R,17R)-17-((R)-5-hydroxypentan-2-yl)-4,4,10,13-tetramethyl-7-oxo-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (37)
Compound 37 was synthesized in a 96% yield as a white solid using a similar procedure to that in 3.1.19., and the reaction temperature was r.t. The mp was 225.5–227.2 °C. 1H-NMR (400 MHz, CDCl3) δ 5.94 (s, 1H), 4.49 (d, J = 10.0 Hz, 1H), 3.60 (t, J = 6.0 Hz, 2H), 3.49–3.43 (m, 1H), 2.31–2.26 (m, 1H), 2.22–2.17 (m, 1H), 2.03–2.00 (m, 1H), 1.89–1.85 (m, 1H), 1.81–1.00 (m, other aliphatic ring protons), 0.93 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). HRMS (ESI): calcd for C31H51NNaO4 [M + Na]+ 524.3710; found 524.3728.
3.1.65. Tert-butyl((10R,13R,17R)-17-((R)-5-bromopentan-2-yl)-4,4,10,13-tetramethyl-7-oxo-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (38)
Compound 38 was synthesized in a 72% yield as a white solid using a similar procedure to that in 3.1.61. The mp was 226.3–228.8 °C. 1H-NMR (400 MHz, CDCl3) δ 5.95 (s, 1H), 4.48 (d, J = 10.0 Hz, 1H), 3.49–3.42 (m, 1H), 3.40–3.32 (m, 2H), 2.31–2.28 (m, 1H), 2.23–2.17 (m, 1H), 2.02–1.09 (m, other aliphatic ring protons), 0.93 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). HRMS (ESI): calcd for C31H50BrNNaO3 [M + Na]+ 586.2866; found 586.2864.
3.1.66. Tert-butyl((10R,13R,17R)-17-((R)-5-(4-(3-(4-chloro-3-(trifluoromethyl) phenyl)ureido)phenoxy)pentan-2-yl)-4,4,10,13-tetramethyl-7-oxo-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (39)
Compound 39 was synthesized in a 77% yield as a white solid using a similar procedure to that in 3.1.6. The mp was 223.1–224.3 °C. 1H-NMR (400 MHz, CDCl3) δ 7.86 (s, 1H), 7.55 (s, 1H), 7.51 (d, J = 8.8 Hz, 1H), 7.47 (s, 1H), 7.29 (d, J = 8.8 Hz, 1H), 7.16 (d, J = 8.4 Hz, 2H), 6.77 (d, J = 8.4 Hz, 2H), 5.93 (s, 1H), 4.58 (d, J = 10.0 Hz, 1H), 3.81 (t, J = 6.0 Hz, 2H), 3.47–3.41 (m, 1H), 2.28–2.18 (m, 2H), 2.05–1.08 (m, other aliphatic ring protons), 0.95 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). HRMS (ESI): calcd for C45H59ClF3N3NaO5 [M + Na]+ 836.3988; found 836.3964.
3.1.67. Tert-butyl((10R,13R,17R)-17-((R)-5-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)phenoxy)pentan-2-yl)-7-hydroxy-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (40)
Compound 40 was synthesized in a 94% yield as a white solid using a similar procedure to that in 3.1.21. The mp was 248.7–249.4 °C. 1H-NMR (400 MHz, CDCl3) δ 7.84 (s, 1H), 7.55–7.53 (m, 2H), 7.49 (d, J = 8.8 Hz, 1H), 7.28 (d, J = 8.8 Hz, 1H), 7.12 (d, J = 8.4 Hz, 2H), 6.76 (d, J = 8.4 Hz, 2H), 5.46–5.44 (m, 1H), 4.59 (d, J = 10.0 Hz, 1H), 3.85–3.83 (m, 1H), 3.80 (t, J = 6.0 Hz, 2H), 3.28–3.22 (m, 1H), 1.99–0.86 (m, other aliphatic ring protons), 0.66 (s, 3H). HRMS (ESI): calcd for C45H61ClF3N3NaO5 [M + Na]+ 838.4150; found 838.4134.
3.1.68. 1-(4-(((4R)-4-((10R,13R,17R)-3-amino-7-hydroxy-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentyl)oxy)phenyl)-3-(4-chloro-3-(trifluoromethyl)phenyl)urea (5az)
Compound 5az was synthesized in a 96% yield as a white solid using a similar procedure to that in 3.1.25. The mp was 198.1–199.7 °C. 1H-NMR (400 MHz, CDCl3:CD3OD = 20:1) δ 7.65 (s, 1H), 7.52 (d, J = 8.4 Hz, 1H), 7.29 (d, J = 8.4 Hz, 1H), 7.19 (d, J = 8.4 Hz, 2H), 6.77 (d, J = 8.4 Hz, 2H), 5.73 (d, J = 4.4 Hz, 1H), 3.88–3.82 (m, 3H), 2.36–2.32 (m, 1H), 1.96–1.94 (m, 1H), 1.85–0.91 (m, other aliphatic ring protons), 0.93 (d, J = 6.0 Hz, 3H), 0.64 (s, 3H). 13C-NMR (100 MHz, CDCl3:CD3OD = 20:1) δ 155.43, 154.73, 153.56, 138.41, 131.65, 131.07, 128.31 (d, J = 31.3 Hz), 124.38, 122.71 (q, J = 271.1 Hz), 122.54, 122.14, 121.77 (2C), 117.50, 114.90 (2C), 68.85, 65.78, 57.75, 55.57, 49.22, 43.33, 41.88, 40.59, 38.96, 37.89, 37.02, 36.59, 35.53, 32.01, 28.28, 27.45, 27.03, 25.78, 24.15, 23.41, 20.00, 19.69, 18.57, 11.43. 19F-NMR (375 MHz, CDCl3:CD3OD = 20:1) δ -62.73. HRMS (ESI): calcd for C40H54ClF3N3O3 [M + H]+ 716.3800; found 716.3761.
3.1.69. Tert-butyl((10R,13S,17R)-17-((S)-1-hydroxypropan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (41)
Compound 41 was synthesized in a 98% yield as a white solid using a similar procedure to that in 3.1.16. The mp was 213.0–214.3 °C. 1H-NMR (400 MHz, CDCl3) δ 5.56–5.52 (m, 1H), 4.44 (d, J = 9.6 Hz, 1H), 3.65–3.62 (m, 1H), 3.37–3.26 (m, 2H), 2.10–2.05 (m, 1H), 2.00–1.97 (m, 1H), 1.86–1.77 (m, 1H), 1.69–0.85 (m, other aliphatic ring protons), 0.68 (s, 3H). HRMS (ESI): calcd for C29H49NNaO3 [M + Na]+ 482.3605; found 482.3615.
3.1.70. Tert-butyl((10R,13S,17R)-17-((S)-1-bromopropan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (42)
Compound 42 was synthesized in a 78% yield as a white solid using a similar procedure to that in 3.1.61. The mp was 232.2–234.8 °C. 1H-NMR (400 MHz, CDCl3) δ 5.56–5.53 (m, 1H), 4.43 (d, J = 10.0 Hz, 1H), 3.51–3.49 (m, 1H), 3.35–3.31 (m, 2H), 2.10–2.05 (m, 1H), 1.97–1.94 (m, 1H), 1.88–1.86 (m, 1H), 1.73–0.85 (m, other aliphatic ring protons), 0.68 (s, 3H). HRMS (ESI): calcd for C29H48BrNNaO2 [M + Na]+ 544.2761; found 544.2759.
3.1.71. Tert-butyl((10R,13S,17R)-17-((S)-1-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)phenoxy)propan-2-yl)-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl)carbamate (43)
Compound 43 was synthesized in an 86% yield as a white solid using a similar procedure to that in 3.1.6 and used for the next reaction without further purification.
3.1.72. 1-(4-((2S)-2-((10R,13S,17R)-3-amino-4,4,10,13-tetramethyl-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)propoxy)phenyl)-3-(4-chloro-3-(trifluoromethyl)phenyl)urea (5ba)
Compound 5ba was synthesized in an 86% yield as a white solid using a similar procedure to that in 3.1.25. The mp was 197.1–198.6 °C. 1H-NMR (400 MHz, CDCl3:CD3OD = 10:1) δ 7.65 (s, 1H), 7.48 (d, J = 8.4 Hz, 1H), 7.27 (d, J = 8.4 Hz, 1H), 7.17 (d, J = 8.4 Hz, 2H), 6.76 (d, J = 8.4 Hz, 2H), 5.52–5.50 (m, 1H), 3.81 (dd, J = 8.8, 2.4 Hz, 1H), 3.55 (dd, J = 8.8, 8.0 Hz, 1H), 2.35–2.31 (m, 1H), 2.08–1.97 (m, 2H), 1.80–1.77 (m, 2H), 1.70–0.80 (m, other aliphatic ring protons), 0.68 (s, 3H). 13C-NMR (100 MHz, CDCl3:CD3OD = 10:1) δ 155.86, 153.64, 149.71, 138.39 (d, J = 2.5 Hz), 131.64, 130.90, 128.32 (d, J = 31.2 Hz), 124.41, 122.71 (q, J = 271.4 Hz), 122.57, 121.88, 119.81 (2C), 117.51 (d, J = 5.2 Hz), 114.90 (2C), 73.42, 58.05, 56.92, 52.61, 50.97, 42.35, 40.48, 39.53, 37.38, 36.80, 36.46, 32.55, 30.75, 27.78, 27.36, 27.18, 24.21, 23.50, 21.11, 20.44, 17.25, 11.84. 19F-NMR (375 MHz, CDCl3:CD3OD = 10:1) δ -62.78. HRMS (ESI): calcd for C38H50ClF3N3O2 [M + H]+ 672.3538; found 672.3574.