Allii Macrostemonis Bulbus: A Comprehensive Review of Ethnopharmacology, Phytochemistry and Pharmacology
Abstract
1. Introduction
2. Method
3. Geographical Distribution and Botany
4. Ethnopharmacology
5. Phytochemistry
5.1. Steroids and Steroidal Saponins
5.2. Volatile Oils and Sulfur-Containing Components
5.3. Nitrogen-Containing Components
5.4. Phenylpropanoids
5.5. Flavonoids
5.6. Polysaccharides
5.7. Other Components
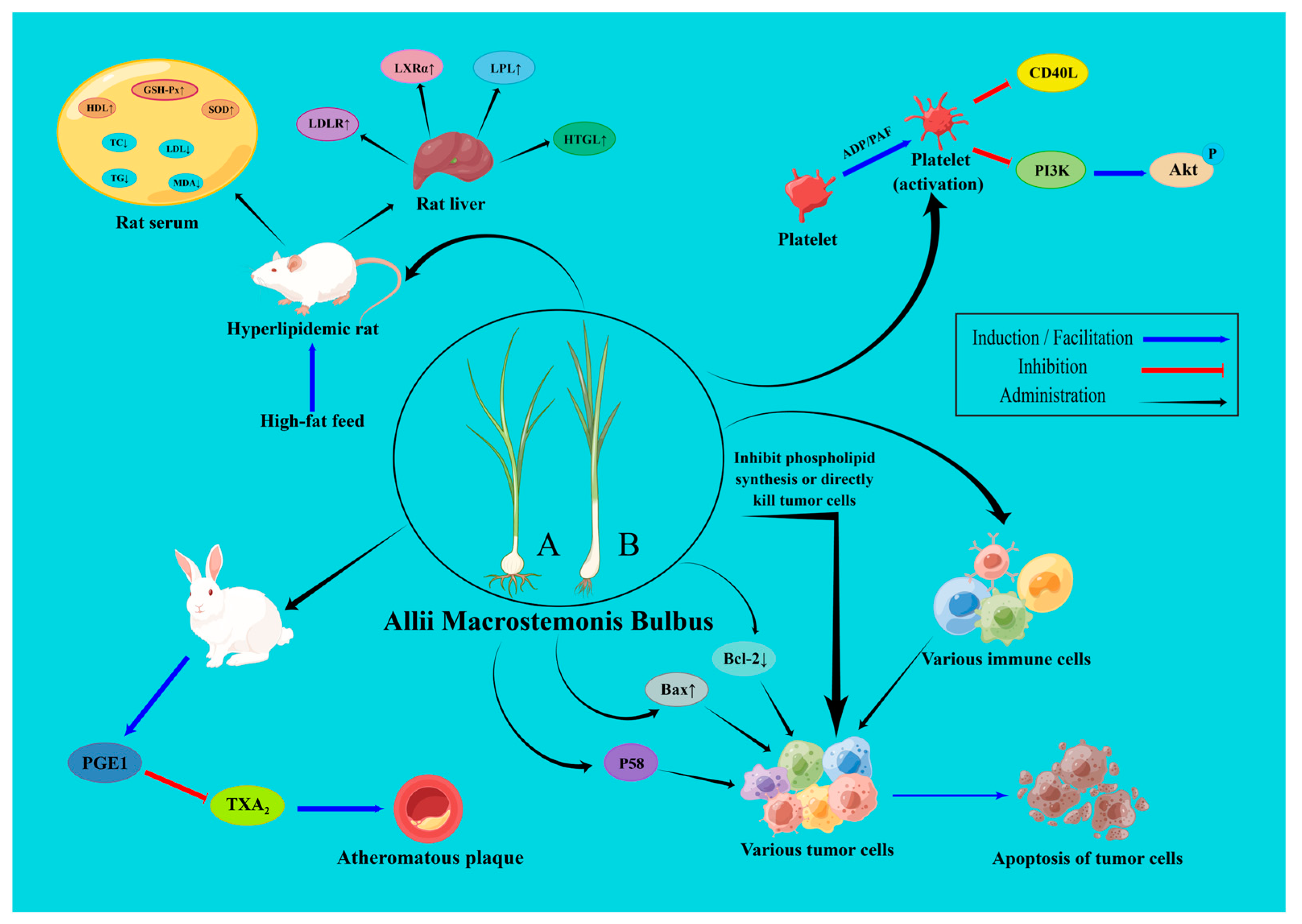
6. Pharmacological Activities
6.1. Anti-Platelet Aggregation Effect
6.2. Hypolipidemic and Anti-Atherosclerotic Effects
6.3. Protection of Cardiomyocytes and Vascular Endothelial Cells
6.4. Anti-Cancer Effect
6.5. Antibacterial Effect
6.6. Anti-Asthmatic Effect
6.7. Antioxidant Effect
6.8. Antidepressant Effect
6.9. Other Pharmacological Effects
7. Quality Control
8. Toxicology
9. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China; 2020 Edition; China Medical Science Press: Beijing, China, 2020; Part 1; pp. 392–393. [Google Scholar]
- Yao, Z.H.; Qin, Z.F.; Dai, Y.; Yao, X.S. Phytochemistry and pharmacology of Allii Macrostemonis Bulbus, a traditional Chinese medicine. Chin. J. Nat. Med. 2016, 14, 481–498. [Google Scholar] [CrossRef] [PubMed]
- Editorial Committee of the Flora of China. Liliaceae. In Flora of China; Science Press: Beijing, China, 2004; Volume 14, pp. 259–260. Available online: http://www.efloras.org/volume_page.aspx?volume_id=2014&flora_id=2 (accessed on 18 April 2022).
- Editorial Committee of the Flora of China. Liliaceae. In Flora of China; Science Press: Beijing, China, 2004; Volume 14, pp. 265–266. Available online: http://flora.huh.harvard.edu/china/mss/volume14/index.htm (accessed on 18 April 2022).
- Donova, M.V.; Egorova, O.V. Microbial steroid transformations: Current state and prospects. Appl. Microbiol. Biotechnol. 2012, 94, 1423–1447. [Google Scholar] [CrossRef] [PubMed]
- Fragkaki, A.G.; Angelis, Y.S.; Koupparis, M.; Tsantili-Kakoulidou, A.; Kokotos, G.; Georgakopoulos, C. Structural characteristics of anabolic androgenic steroids contributing to binding to the androgen receptor and to their anabolic and androgenic activities. Applied modifications in the steroidal structure. Steroids 2009, 74, 172–197. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Ushiroguchi, T.; Itakura, Y.; Fuwa, T. A furostanol glycoside from Allium chinense G. DON. Chem. Pharm. Bull. 1989, 37, 1390–1391. [Google Scholar] [CrossRef]
- Peng, J.P.; Wu, Y.; Yao, X.S.; Okuyama, T.; Narui, T. Two new steroidal saponins from Allium macrostemon. Acta Pharm. Sin. 1992, 27, 918–922. [Google Scholar]
- Ren, G.; Qiao, H.X.; Yang, J.; Zhou, C.X. Protective effects of steroids from Allium chinense against H2O2-induced oxidative stress in rat cardiac H9C2 cells. Phytother. Res. 2010, 24, 404–409. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Wang, N.L.; Yao, X.S.; Kitanaka, S. Steroidal saponins from the bulbs of Allium chinense. Stud. Plant. Sci. 1999, 6, 212–219. [Google Scholar]
- Cheng, S.B. Study on steroidal from the bulbs of Allium macrostemon. Masters Thesis, Zhejiang University, Zhejiang, China, 2013. [Google Scholar]
- He, X.J.; Wang, N.L.; Qiu, F.; Yao, X.S. Study on the active spirostanol saponins of Gualou xiebai baijiutang. Acta Pharm. Sin. 2003, 38, 433–437. [Google Scholar]
- Peng, J.P.; Wang, X.; Yao, X.S. Studies on two new furostanol glycosides from Allium macrostemon Bunge. Acta Pharm. Sin. 1993, 28, 526–531. [Google Scholar]
- Baba, M.; Ohmura, M.; Kishi, N.; Okada, Y.; Shibata, S.; Peng, J.; Yao, S.S.; Nishino, H.; Okuyama, T. Saponins isolated from Allium chinense G. Don and antitumor-promoting activities of isoliquiritigenin and laxogenin from the same drug. Bio. Pharm. Bull. 2000, 23, 660–662. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimaki, Y.; Kameyama, A.; Sashida, Y.; Nikaido, T. Steroidal saponins from Allium chinense and their inhibitory activities on cyclic AMP phosphodiesterase and Na+/K+ ATPase. Phytochemistry 1995, 40, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, N.; Yao, X.; Kitanaka, S. A new spirostanol saponin from Allium Chinense. Chin. Chem. Tetters 1997, 8, 965–966. [Google Scholar]
- Kim, Y.S.; Suh, W.S.; Park, K.J.; Choi, S.U.; Lee, K.R. Allimacrosides A-E, new steroidal glycosides from Allium macrostemon Bunge. Steroids 2017, 118, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R. Study on chemical constituents and biological activity of Allium chinense G. Don. Masters Thesis, Jilin Unversity, Changchun, China, 2021. [Google Scholar]
- Chen, H.F. Further Research of Active Components from a Chinese Medicine Allium macrostemon Bunge. Ph.D. Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2005. [Google Scholar]
- Peng, J.; Yao, X.; Kobayashi, H.; Ma, C. Novel furostanol glycosides from Allium macrostemon. Planta Med. 1995, 61, 58–61. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.; Wang, N.; Yang, M.; Wang, Z.; Wang, X.; Yao, X. New furostanol saponins from the bulbs of Allium macrostemon Bunge and their cytotoxic activity. Pharmazie 2007, 62, 544–548. [Google Scholar]
- Peng, J.; Yao, X.; Okada, Y.; Okuyama, T. Further studies on new furostanol saponins from the bulbs of Allium macrostemon. Chem. Pharm. Bull. 1994, 42, 2180–2182. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.C.; Zhong, Y.; Liu, B.R.; Liu, S.M. Furostanol saponins from Allium Macrostemon Bunge Bulbs inhibit platelet CD40L expression and leukocyte - platelet adhesion. Guangdong Med. J. 2011, 32, 833–835. [Google Scholar]
- He, X.J.; Qiu, F.; Shoyama, Y.; Tanaka, H.; Yao, X.S. Two new steroidal saponins from “Gualou - xiebai - baijiu - tang” consisting of fructus trichosanthis and bulbus allii macrostemi. Chem. Pharm. Bull. 2002, 50, 653–655. [Google Scholar] [CrossRef]
- Chen, H.F.; Wang, N.L.; Yao, X.S. Study on bioactive steroidal saponins of Allium macrostemon Bunge. Chin. J. Med. Chem. 2005, 15, 142–147. [Google Scholar]
- Peng, J.P.; Yao, X.S.; Tezuka, Y.; Kikuchi, T. Furostanol glycosides from bulbs of Allium chinense. Phytochemistry 1996, 41, 283–285. [Google Scholar] [CrossRef]
- Ou, W.C.; Chen, H.F.; Zhong, Y.; Liu, B.R.; Liu, S.M.; Chen, K.J. Inhibition of platelet activation and aggregation by furostanol saponins isolated from the bulbs of Allium macrostemon Bunge. Am. J. Med. Sci. 2012, 344, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, T. Study on Chemical Constituents from Allii Macrostemonis Bulbus. Masters Thesis, Jilin University, Changchun, China, 2017. [Google Scholar]
- Peng, J.; Yao, X.; Tezuka, Y.; Kikuchi, T.; Narui, T. New furostanol glycosides, chinenoside IV and V, from Allium chinense. Planta Med. 1996, 62, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yi, X.; Xiang, L.; Huang, Y.; Wang, Z.; He, X. Furostanol saponins from Chinese onion induce G2/M cell-cycle arrest and apoptosis through mitochondria-mediate pathway in HepG2 cells. Steroids 2019, 148, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Yao, H.; Yan, J.H.; Sun, Z.H.; Zhang, Y.; Fang, X.Q.; Li, X.W.; Jin, Y.R. Chemical constituents of new steroidal saponins from Allium chinense G. Don. Chem. J. Chin. Univ. 2021, 42, 1742–1753. [Google Scholar]
- He, J.X. The Research on Metabolites of Andrographolide in Rats and Active Constituents of Gualou Xiebai Baijiu Decoction. Ph.D. Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2002. [Google Scholar]
- Kang, X.D.; Wu, X.Q.; Zhang, P. Chemical constituents in Allii Macrostemonis Bulbus. Drugs Clin. 2012, 27, 97–99. [Google Scholar]
- Zhang, C.Y.; Si, M.Z.; Li, L.; Zhang, D.Q. Research on volatiles of bulbus Allii Macrostemonis from different areas and different original plants based on headspace and SERS. Spectrosc. Spectral. Anal. 2015, 35, 395–401. [Google Scholar]
- Han, C.H.; Gao, S.N.; Bai, Y.H.; Ma, Y.; Li, C.X. Gas chromatography-mass spectrometry (GC-MS) analysis of volatile oils from bulbs and leaves before and after Allium macrostemon Bge. processing. Lishizhen Med. Mater. Medica. Res. 2017, 28, 111–113. [Google Scholar]
- Pino, J.A.; Fuentes, V.; Correa, M.T. Volatile constituents of Chinese chive (Allium tuberosum Rottl. ex Sprengel) and rakkyo (Allium chinense G. Don). J. Agric. Food Chem. 2001, 49, 1328–1330. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, J.P.; Yao, L.Q.; Yao, X.S. A study on the volatile oils of Allium macrostemon Bunge. J. Shenyang Pharm. Univ. 1993, 10, 45–46+62. [Google Scholar]
- Lin, L.; Jiang, H.Z.; Luo, L.L.; Xu, H.G.; Hu, K.; Geng, Y. GC-MS analysis of the volatile oil from bulbus Allii Macrostemonis extracted by supercritical carbon dioxide. Chin. J. Anal. Lab. 2008, 27, 115–118. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.Y.; Ding, Q.; Zhao, J.; Zhang, Y.Y.; Sun, B.G.; Chen, H.T. Analysis of volatile aroma compounds in Allium macrostemon Bunge. Food Sci. 2015, 36, 117–121. [Google Scholar]
- Huang, F.; Zhou, H.; Yu, S.S. Optimization of extraction of volatile oil from Allium macrostemon Bunge and chemical composition analysis by gas chromatography-mass spectrometry. Food Sci. 2014, 271, 504–522. [Google Scholar]
- Peng, J.P.; Qiao, Y.Q.; Xiao, K.Y.; Yao, X.S. Further study on the volatile oil of Allium chinense G. Don. Chin. J. Med. Chem. 1994, 4, 282–283+288. [Google Scholar]
- Peng, J.P.; Qiao, Y.Q.; Yao, X.S. Nitrogen-containing compounds from Allium macrostemon Bunge and Allium chinense G. Don. Chin. J. Med. Chem. 1995, 5, 134–139. [Google Scholar]
- Okuyama, T.; Shibata, S.; Hoson, M.; Kawada, T.; Osada, H.; Noguchi, T. Effect of oriental plant drugs on platelet aggregation; III. Effect of Chinese drug “xiebai” on human platelet aggregation. Planta Med. 1986, 52, 171–175. [Google Scholar] [CrossRef]
- Goda, Y.; Shibuya, M.; Sankawa, U. Inhibitors of the arachidonate cascade from Allium chinense and their effect on in vitro platelet aggregation. Chem. Pharm. Bull. 1987, 35, 2668–2674. [Google Scholar] [CrossRef]
- Okuyama, T.; Fujita, K.; Shibata, S.; Hoson, M.; Kawada, T.; Masaki, M.; Yamate, N. Effects of Chinese drugs “xiebai” and “dasuan” on human platelet aggregation (Allium bakeri, A. sativum). Planta Med. 1989, 55, 242–244. [Google Scholar] [CrossRef]
- He, Q.; Huang, S.; Wu, Y.; Zhang, W.; Wang, F.; Cao, J.; Sheng, Q.; Liang, Z.; Liu, L.; Ou, W.B. Comparative study on the composition of free amino acids and derivatives in the two botanical origins of an edible Chinese herb “Xiebai”, i.e., Allium chinense G. Don and Allium macrostemon Bunge species. Food Res. Int. 2018, 106, 446–457. [Google Scholar] [CrossRef]
- He, X.J.; Qiu, F.; Yao, X.S. The active constituents research of Gualou xiebai baijiutang (IV): Nitrogen-containing compounds and others. Nat. Prod. Res. Dev. 2003, 15, 9–12. [Google Scholar]
- Wang, Y.H.; Yi, X.M.; Rao, Z.H.; He, X.J. Study on chemical constituents from the bulbs of Allium chinense G. Don. J. Guangdong Pharm. Univ. 2017, 33, 453–456. [Google Scholar]
- Usui, A.; Matsuo, Y.; Tanaka, T.; Ohshima, K.; Fukuda, S.; Mine, T.; Nakayama, H.; Ishimaru, K. Ferulic Acid Esters of Oligo-glucose from Allium macrostemon. Nat. Prod. Commun. 2017, 12, 89–91. [Google Scholar] [CrossRef]
- Usui, A.; Matsuo, Y.; Tanaka, T.; Ohshima, K.; Fukuda, S.; Mine, T.; Yakashiro, I.; Ishimaru, K. Ferulic acid esters of glucosylglucose from Allium macrostemon Bunge. J. Asian Nat. Prod. Res. 2017, 19, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Nakane, R.; Iwashina, T. Flavonol Glycosides from the Leaves of Allium macrostemon. Nat. Prod. Commun. 2015, 10, 1381–1382. [Google Scholar] [CrossRef]
- Xia, X.K. The Study on Extraction, Purification and Antioxidative Activity of Polysaccharides From Allium macrosttemon Bge. Masters Thesis, Northwest A&F University, Shaanxi, China, 2007. [Google Scholar]
- Gan, Y.Z.; Zhong, K.Y.; Huang, L. Study on extraction, isolation and purification of polysaccharides from Allium macrostemon Bunge and its interaction with DNA. Bio. Chem. Eng. 2019, 5, 82–84. [Google Scholar]
- Zhang, Z.J.; Wang, F.H.; Wang, M.C.; Ma, L.P.; Ye, H.; Zeng, X.X. A comparative study of the neutral and acidic polysaccharides from Allium macrostemon Bunge. Carbohydr. Polym. 2015, 117, 980–987. [Google Scholar] [CrossRef]
- Sun, Q.L. Study on prostaglandins in plants III. Isolation and identification of prostaglandin A1 and B1 from Longstamen Onion (Allium Macrostemon). Chin. Tradit. Herb. Drugs. 1991, 22, 150–152+191. [Google Scholar]
- He, X.J.; Qiu, F.; Shoyama, Y.; Tanaka, H.; Yao, X.S. The active constituents from Gualou-xiebai-baijiu-tang part I: Active saponins. J. Asian Nat. Prod. Res. 2002, 4, 189–196. [Google Scholar] [CrossRef]
- Xia, X.K.; Yang, H.X.; Chen, L.J. Analysis of fatty acids composition of Allium macrostemon Bge. by GC-MS. Food Sci. Technol. 2010, 35, 279–280+283. [Google Scholar]
- Jin, R.; Xiao, A.Y.; Song, Z.; Yu, S.; Li, J.; Cui, M.Z.; Li, G. Platelet CD40 mediates leukocyte recruitment and neointima formation after arterial denudation injury in atherosclerosis-prone mice. Am. J. Pathol. 2018, 188, 252–263. [Google Scholar] [CrossRef]
- Lievens, D.; Zernecke, A.; Seijkens, T.; Soehnlein, O.; Beckers, L.; Munnix, I.C.; Wijnands, E.; Goossens, P.; van Kruchten, R.; Thevissen, L.; et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood 2010, 116, 4317–4327. [Google Scholar] [CrossRef]
- Aslam, R.; Speck, E.R.; Kim, M.; Crow, A.R.; Bang, K.W.; Nestel, F.P.; Ni, H.; Lazarus, A.H.; Freedman, J.; Semple, J.W. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood 2006, 107, 637–641. [Google Scholar] [CrossRef]
- Wang, Z.P.; Feng, H.; Guo, M.; Wang, C.S. Effects of saponins from Allium Macrostemon Bunge bulbs on platelet aggregation and interactions between platelets and neutrophils. Chin. J. Inf. Tradit. Chin. Med. 2018, 25, 33–37. [Google Scholar]
- Chen, G.R. Effect of Chinese botanical Allium macrostemon Bunge on human platelet aggregation. Chin. Tradit. Herb. Drugs 1987, 18, 12. [Google Scholar]
- Feng, H.; Wang, Z.; Wang, C.; Zhu, X.; Liu, Z.; Liu, H.; Guo, M.; Hou, Q.; Chu, Z. Effect of furostanol saponins from Allium Macrostemon Bunge bulbs on platelet aggregation rate and PI3K/Akt pathway in the rat model of coronary heart disease. J. Evidence-Based Complementary Altern. Med. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nat. Rev. Drug. Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers. 2019, 5, 56. [Google Scholar] [CrossRef]
- Zhou, M.; Ren, P.; Zhang, Y.; Li, S.; Li, M.; Li, P.; Shang, J.; Liu, W.; Liu, H. Shen-Yuan-Dan capsule attenuates atherosclerosis and foam cell formation by enhancing autophagy and inhibiting the PI3K/Akt/mTORC1 signaling pathway. Front. Pharmacol. 2019, 10, 603. [Google Scholar] [CrossRef]
- Li, G.; Wang, M.; Caulk, A.W.; Cilfone, N.A.; Gujja, S.; Qin, L.; Chen, P.Y.; Chen, Z.; Yousef, S.; Jiao, Y.; et al. Chronic mTOR activation induces a degradative smooth muscle cell phenotype. J. Clin. Invest. 2020, 130, 1233–1251. [Google Scholar] [CrossRef]
- Gisterå, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Tousoulis, D.; Simopoulou, C.; Papageorgiou, N.; Oikonomou, E.; Hatzis, G.; Siasos, G.; Tsiamis, E.; Stefanadis, C. Endothelial dysfunction in conduit arteries and in microcirculation. Novel therapeutic approaches. Pharmacol. Ther. 2014, 144, 253–267. [Google Scholar] [CrossRef]
- Lei, R.J.; Li, J.; Jin, S.X.; Xu, S.Y.; Yan, G.M.; He, Q.J. Hyperlipidemic effect of total steroidal saponins extracted from Allium chinense G. Don in high-fat diet-induced hyperlipidemia rats. Chin. Tradit. Patent. Med. 2013, 35, 1615–1619. [Google Scholar]
- Lin, Y.P.; Lin, L.Y.; Yeh, H.Y.; Chuang, C.H.; Tseng, S.W.; Yen, Y.H. Antihyperlipidemic activity of Allium chinense bulbs. J. Food Drug Anal. 2016, 24, 516–526. [Google Scholar] [CrossRef]
- Gu, Y.Q.; Liu, Y.Y.; Yang, X.H.; Chen, D.; Fu, F.H. Effect of Allium cepa L. var. agrogatum Don and Allium macrostemon Bunge on arachidonic acid metabolism. Acta Pharm. Sin. 1988, 23, 8–11. [Google Scholar]
- Chen, D.; Liu, Y.Y. Effects of Allium macrostemon Bunge on prostaglandin E1 in rabbits. J. Jilin Univ., Med. Ed. 1989, 15, 91. [Google Scholar]
- Ju, K.; Wan, Y.Y.; Zhang, K.L. Study on the effect and mechanism of Allii Macrostemonis Bulbus on blood lipid levels in hyperlipi- demia model rats. China Pharm. 2018, 29, 976–979. [Google Scholar]
- Zhou, H.; Yang, X.; Wang, N.L.; Zhang, Y.O.; Cai, G.P. Macrostemonoside A promotes visfatin expression in 3T3-L1 cells. Biol. Pharm. Bull. 2007, 30, 279–283. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, Y.; Wang, N.; Zhou, H.; Du, L.; Ma, X.; Shi, X.; Cai, G. Novel effects of macrostemonoside A, a compound from Allium macrostemon Bung, on hyperglycemia, hyperlipidemia, and visceral obesity in high-fat diet-fed C57BL/6 mice. Eur. J. Pharm. 2008, 599, 159–165. [Google Scholar] [CrossRef]
- Jia, W.; Li, Y.; Wan, J.; Cui, X.; Lu, J.; Liu, J.; Li, D.; Li, L.; Zou, T.; Ding, J.; et al. Effects of Xuezhitong in patients with hypertriglyceridemia: A multicentre, randomized, double-blind, double simulation, positive drug and placebo parallel control study. Cardiovasc. Drugs Ther. 2020, 34, 525–534. [Google Scholar] [CrossRef]
- Meng, X.B.; Zhu, T.; Yang, D.H.; Liang, W.; Sun, G.B.; Sun, X.B. Xuezhitong capsule, an extract of Allium macrostemon Bunge, exhibits reverse cholesterol transport and accompanies high-density lipoprotein levels to protect against hyperlipidemia in ApoE(-/-) mice. Ann. Transl. Med. 2019, 7, 239. [Google Scholar] [CrossRef]
- Arslan, F.; Bongartz, L.; Ten Berg, J.M.; Jukema, J.W.; Appelman, Y.; Liem, A.H.; de Winter, R.J.; van ’t Hof, A.W.J.; Damman, P. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: Comments from the Dutch ACS working group. Neth. Heart J. 2018, 26, 417–421. [Google Scholar] [CrossRef]
- Heusch, G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef]
- Neri, M.; Riezzo, I.; Pascale, N.; Pomara, C.; Turillazzi, E. Ischemia/reperfusion injury following acute myocardial infarction: A critical issue for clinicians and forensic pathologists. Mediators. Inflamm. 2017, 2017, 7018393. [Google Scholar] [CrossRef]
- González-Montero, J.; Brito, R.; Gajardo, A.I.; Rodrigo, R. Myocardial reperfusion injury and oxidative stress: Therapeutic opportunities. World J. Cardiol. 2018, 10, 74–86. [Google Scholar] [CrossRef]
- Koller, A.; Balasko, M.; Bagi, Z. Endothelial regulation of coronary microcirculation in health and cardiometabolic diseases. Intern. Emerg. Med. 2013, 8, S51–S54. [Google Scholar] [CrossRef]
- Wu, Y.L.; Liu, K.; Qi, J.S.; Jia, Z.H.; Li, Y.N. Effect of bulbus Allii Macrostemi on gene expression profile asociated with vascular endothelium injure of qi stagnation rats. J. Chin. Med. Mater. 2007, 30, 1266–1270. [Google Scholar]
- Ji, Z.S.; Wu, Y.L.; Jia, Z.H.; Qi, J.S. Influence of bulbus Allii Macrostemi on contents and interaction of COX-2 and iNOS in vascular endothelial injury of qi-stagnation type. J. Beijing Univ. Tradit. Chin. Med. 2008, 31, 835–838+867–868. [Google Scholar]
- Wu, X.F.; Li, Z.; Lai, J.; Wu, X.C.; Jia, Z.H.; Wang, H.T.; Wang, L.L. Study on the effect and mechanism of Bulbus Allium Macrostemi on vascular endothelial dysfunction in rats with stagnant energy. J. Basic. Chin. Med. 2013, 19, 505–506+528. [Google Scholar]
- Li, F.; Xu, Q.; Zheng, T.; Huang, F.; Han, L. Metabonomic analysis of Allium macrostemon Bunge as a treatment for acute myocardial ischemia in rats. J. Pharm. Bio. Anal. 2014, 88, 225–234. [Google Scholar] [CrossRef]
- Lei, J.; Duan, G.F. Protective effect of Allium macrostemon extract on acute myocardial ischemia injury in rats. J. Jianghan Univ. Nat. Sci. Ed. 2018, 46, 67–71. [Google Scholar]
- Wei, C.; Zhang, Y.F.; Jia, Z.H.; Yuan, G.Q.; Zhang, Z.H.; Wu, Y.L. The effects of restraint stress on the expression of 5-HT1D and5-HT2A receptors in aorta of rats and the intervention effect of Tongxinluo and Allium extract. Chin. J. Gerontol. 2010, 30, 3668–3671. [Google Scholar]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, E.; Hou, Q.; Chen, W.N.; Zhang, Y.J.; Han, H.R. Influence of the volatile oil extracted from Allium Macrostemon Bunge on immune function of mice bearing S180. Acta Acad. Med. Weifang 2002, 24, 94–95. [Google Scholar]
- Zhang, Q.; Gao, E. The experimental study of the volatile oil extracted from Allium macrostemon Bunge on anti tumor effects. Tumor 2003, 23, 228–231. [Google Scholar]
- Wu, Z.M.; Zhang, Q.F.; Xue, Y.W.; Pang, D.; Zhang, Y.B. Apoptosis of human gastric cancer cells included by bulbus Allii Macrostemi volatile oil. Chin. J. Tissue. Eng. Res. 2006, 10, 115–117. [Google Scholar]
- Chen, H.F.; Wang, G.H.; Luo, Q.; Wang, N.L.; Yao, X.S. Two new steroidal saponins from Allium macrostemon Bunge and their cytotoxity on different cancer cell lines. Molecules 2009, 14, 2246. [Google Scholar] [CrossRef]
- Bai, J.S. Studies on the Isolation, Purification, Identification and Function of Antibacterial and Anticancer Active Components of Allium Plant-Allium chinense. Masters Thesis, Hunan Normal University, Changsha, China, 2004. [Google Scholar]
- Luo, T.; Shi, M.Q.; Liu, X.; Zhou, J.G.; Yang, W.Y.; Yang, H.M. Effect of total saponin from Allium macrostemon Bunge on proliferation and apoptosis of cervix cancer HeLa cells. Chin. J. Difficult Complicat. Cases 2012, 11, 762–765. [Google Scholar]
- Wang, Y.; Tang, Q.; Jiang, S.; Li, M.; Wang, X. Anti-colorectal cancer activity of macrostemonoside A mediated by reactive oxygen species. Biochem. Biophysi. Res. Commun. 2013, 441, 825–830. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, T.; Zhou, F.; Xiao, X.; Ding, X.; He, H.; Rang, J.; Quan, M.; Wang, T.; Zuo, M.; et al. Anticancer activity of saponins from Allium chinense against the B16 melanoma and 4T1 breast carcinoma cell. J. Evid.-Based Complement. Altern. Med. 2015, 2015, 725023. [Google Scholar]
- Xiao, X.; He, H.; Ding, X.; Yang, Q.; Liu, X.; Liu, S.; Rang, J.; Wang, T.; Zuo, M.; Xia, L. Purification and cloning of lectin that induce cell apoptosis from Allium chinense. Phytomedicine 2015, 22, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X. A preliminary study on the bacteriostasis of Allium macrostemon. J. Hangzhou Norm. Univ. Nat. Sci. Ed. 2004, 3, 337–340. [Google Scholar]
- Zhang, C.J.; Liu, C.; Jiang, X.K. Antibacterial effect of ethanol extract from Allium macrostemon Bunge bulbs. Food Sci. 2011, 32, 119–122. [Google Scholar]
- Yu, Z.H.; Ding, X.Z.; Xia, L.Q.; Xiao, X.Q.; Cao, Z.P.; Xu, S.; Liu, S.; Liu, X.M. Antimicrobial activity and mechanism of total saponins from Allium chinense. Food Sci. 2013, 34, 75–80. [Google Scholar]
- Meng, S.; Hu, S.B.; Xie, W.A.; Ding, X.Z.; Sun, Y.J.; Xia, L.Q. Antifungal effects and mechanism of bioactive components of Allium chinense on candida albicans. Food Sci. 2005, 26, 101–105. [Google Scholar]
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma epidemiology and risk factors. Semin. Immunopathol. 2020, 42, 5–15. [Google Scholar] [CrossRef]
- Sockrider, M.; Fussner, L. What Is Asthma? Am. J. Respir. Crit. Care. Med. 2020, 202, P25–P26. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.J.; Xiang, C.; Jiang, G.W.; Xu, Y.D.; Yin, L.M.; Zhou, D.D.; Liu, Y.Y.; Yang, Y.Q. Discovery of potential asthma targets based on the clinical efficacy of traditional Chinese medicine formulas. J. Ethnopharmacol. 2020, 252, 112635. [Google Scholar] [CrossRef]
- Yan, S.F.; Yu, T.; Li, F.S.; Huang, Y.; Wang, M.H. Effectiveness and safety of 3 different traditional Chinese therapies for asthma in minors: A protocol for systematic review and network meta-analysis. Medicine 2020, 99, e23021. [Google Scholar] [CrossRef]
- Doganci, A.; Sauer, K.; Karwot, R.; Finotto, S. Pathological role of IL-6 in the experimental allergic bronchial asthma in mice. Clin. Rev. Allergy. Immunol. 2005, 28, 257–270. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Jo, T.; Takeda, N.; Al Heialy, S.; Siddiqui, S.; Shalaby, K.H.; Risse, P.A.; Maghni, K.; Martin, J.G. EGF receptor activation during allergic sensitization affects IL-6-induced T-cell influx to airways in a rat model of asthma. Eur. J. Immunol. 2010, 40, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Morjaria, J.B.; Babu, K.S.; Vijayanand, P.; Chauhan, A.J.; Davies, D.E.; Holgate, S.T. Sputum IL-6 concentrations in severe asthma and its relationship with FEV1. Thorax 2011, 66, 537. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Ho, C.Y.; Ko, F.W.; Chan, C.H.; Ho, A.S.; Hui, D.S.; Lam, C.W. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin. Exp. Immunol. 2001, 125, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, M.W.; Peden, D.B. Allergen provocation augments endotoxin-induced nasal inflammation in subjects with atopic asthma. J. Allergy. Clin. Immunol. 2000, 105, 475–481. [Google Scholar] [CrossRef]
- Wang, L.; Xue, Y.W.; Li, Y.; Shi, Q.; Wang, S.Z. Release of TXA2, PGI2,,TNFα, IL-8, IL-10 by alveolar macrophages in patients with asthma and modulation of drugs. J. Shandong Univ. Health Sci. 1997, 35, 59–64. [Google Scholar]
- Wasserman, M.A.; Ducharme, D.W.; Wendling, M.G.; Griffin, R.L.; De Graaf, G.L. Bronchodilator effects of prostacyclin (PGI2) in dogs and guinea pigs. Eur. J. Pharm. 1980, 66, 53–63. [Google Scholar] [CrossRef]
- Hoshino, M. Effect of AA-2414, a thromboxane A2 receptor antagonist, on airway inflammation in subjects with asthma. J. Allergy Clin. Immunol. 1999, 103, 1054–1061. [Google Scholar] [CrossRef]
- Zhang, H.T.; Zhang, Y.M.; Wang, C.Y.; Fang, S.C. Effect of xiebai extracts on IL-6,TXB2 and 6-Keto-PGF1α in serum of guinea pig with asthma. J. Radioimmunol. 2012, 25, 154–156. [Google Scholar]
- Qin, L.R.; Wu, S.; Wei, J.B. Effect of extracts of Allium macrostemon bunge on antiasthmaticaction. Guangxi Med. J. 2008, 30, 1844–1845. [Google Scholar]
- Tan, Z.Y.; Zhang, J.H.; Liu, Y.X.; Pan, L.H.; Zhang, Y.Y.; Liu, X.Q. A screening of the effective fraction on antiasthmatic activity of Allii macrostemonis Bulbus. Mod. Chin. Med. 2011, 13, 40–41+47. [Google Scholar]
- Choi, H.Y.; Lee, J.H.; Jegal, K.H.; Cho, I.J.; Kim, Y.W.; Kim, S.C. Oxyresveratrol abrogates oxidative stress by activating ERK-Nrf2 pathway in the liver. Chem. Biol. Interact. 2016, 245, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J.A. Oxidative DNA damage & repair: An introduction. Free Radic. Biol. Med. 2017, 107, 2–12. [Google Scholar] [PubMed]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Taleb, A.; Ahmad, K.A.; Ihsan, A.U.; Qu, J.; Lin, N.; Hezam, K.; Koju, N.; Hui, L.; Qilong, D. Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases. Biomed. Pharmacother. 2018, 102, 689–698. [Google Scholar] [CrossRef]
- Seyhan, N.; Canseven, A.G. In vivo effects of ELF MFs on collagen synthesis, free radical processes, natural antioxidant system, respiratory burst system, immune system activities, and electrolytes in the skin, plasma, spleen, lung, kidney, and brain tissues. Electromagn. Biol. Med. 2006, 25, 291–305. [Google Scholar] [CrossRef]
- Niki, E. Oxidant-specific biomarkers of oxidative stress. Association with atherosclerosis and implication for antioxidant effects. Free Radic. Biol. Med. 2018, 120, 425–440. [Google Scholar] [CrossRef]
- Yuhai, G.U.; Zhen, Z. Significance of the changes occurring in the levels of interleukins, SOD and MDA in rat pulmonary tissue following exposure to different altitudes and exposure times. Exp. Ther. Med. 2015, 10, 915–920. [Google Scholar] [CrossRef]
- Armagan, G.; Sevgili, E.; Gürkan, F.T.; Köse, F.A.; Bilgiç, T.; Dagcı, T.; Saso, L. Regulation of the Nrf2 pathway by glycogen synthase kinase-3β in MPP⁺-induced cell damage. Molecules. 2019, 24, 1377. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. BBA Mol. Basis. Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Q.; Zhou, J.; Tan, G.; Deng, X.; Ci, X. Daphnetin-mediated Nrf2 antioxidant signaling pathways ameliorate tert-butyl hydroperoxide (t-BHP)-induced mitochondrial dysfunction and cell death. Free Radic. Biol. Med. 2017, 106, 38–52. [Google Scholar] [CrossRef]
- Xiao, Q.; Piao, R.; Wang, H.; Li, C.; Song, L. Orientin-mediated Nrf2/HO-1 signal alleviates H2O2-induced oxidative damage via induction of JNK and PI3K/AKT activation. Int. J. Biol. Macromol. 2018, 118, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, T.; Zhou, H.; Zhang, C.; Feng, Y.; Tang, F.; Hoi, M.P.; He, C.; Zheng, Y.; Lee, S.M. Schisantherin A attenuates neuroinflammation in activated microglia: Role of Nrf2 activation through ERK phosphorylation. Cell Physiol. Biochem. 2018, 47, 1769–1784. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Feng, D.; Li, M.; Gao, Y.; Ramirez, T.; Cao, H.; Kim, S.J.; Yang, Y.; Cai, Y.; Ju, C.; et al. Hepatic mitochondrial DNA/Toll-like receptor 9/MicroRNA-223 forms a negative feedback loop to limit neutrophil overactivation and acetaminophen hepatotoxicity in mice. Hepatology 2017, 66, 220–234. [Google Scholar] [CrossRef]
- Chang, S.H.; Mori, D.; Kobayashi, H.; Mori, Y.; Nakamoto, H.; Okada, K.; Taniguchi, Y.; Sugita, S.; Yano, F.; Chung, U.I.; et al. Excessive mechanical loading promotes osteoarthritis through the gremlin-1-NF-κB pathway. Nat. Commun. 2019, 10, 1442. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Hu, L.; Yang, H.; Gao, C.; Zeng, K.; Yu, M.; Feng, J.; Qiu, J.; Liu, C.; Fu, F.; et al. Reduction of SIRT1 blunts the protective effects of ischemic post-conditioning in diabetic mice by impairing the Akt signaling pathway. Biochim. Biophys. Acta Mol. Basis. Dis. 2019, 1865, 1677–1689. [Google Scholar] [CrossRef]
- Kong, X.; Guan, J.; Li, J.; Wei, J.; Wang, R. P66(Shc)-SIRT1 regulation of oxidative stress protects against cardio-cerebral vascular disease. Mol. Neurobiol. 2017, 54, 5277–5285. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, H.; Wu, Y.; Zhang, B.; Wang, N.; Mao, H.; Xing, C. P53 contributes to cisplatin induced renal oxidative damage via regulating P66shc and MnSOD. Cell Physiol. Biochem. 2015, 37, 1240–1256. [Google Scholar] [CrossRef]
- Li, X.H.; Gu, L.Z.; Zhang, B.S.; Wang, A.H.; Duan, S.J. The antioxidation of effects from Allium macrostemon. J. Chin. Med. Mater. 1994, 17, 34–37+56. [Google Scholar]
- Guan, F.; Zhang, F.L.; Hao, L.Z.; Shi, B.; Yang, Z.R. Antioxidant activity of total spaonion of Allium macrostemon. Plant Physiology. J. 2014, 50, 382–388. [Google Scholar]
- Xia, X.K.; Dou, C.L. Sulfated modification of polysaccharides from Allium macrosttemon Bge. and in vitro antioxidant activity. Nat. Prod. Res. Dev. 2015, 27, 881–885. [Google Scholar]
- Xia, X.K.; Dou, C.L. Enzymatic modification and antioxidant activity of polysaccharides from Allium macrosttemon Bge. Food Ind. 2015, 36, 185–188. [Google Scholar]
- Han, Q.J.; Wang, X.L.; Wang, F.; Qi, J.H.; Li, H.Y.; Ran, L.H.; Wang, Z.Y. Extraction of polysaccharide from Allium macrostemon Bunge and their antioxidant activity in vitro. Appl. Chem. Ind. 2018, 47, 1680–1683. [Google Scholar]
- Wu, Z.Q.; Li, K.; Ma, J.K.; Huang, Q.; Tian, X.; Li, Z.J. Antioxidant activity of organic sulfides from fresh Allium macrostemon Bunge and their protective effects against oxidative stress in Caenorhabditis elegans. J. Food Biochem. 2020, 44, e13447. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Primers. 2016, 2, 16065. [Google Scholar] [CrossRef]
- D’Elia, A.; Bawor, M.; Dennis, B.B.; Bhatt, M.; Litke, K.; McCabe, K.; Whattam, J.; Garrick, L.; O’Neill, L.; Simons, S.; et al. Feasibility of behavioral activation group therapy in reducing depressive symptoms and improving quality of life in patients with depression: The BRAVE pilot trial. Pilot Feasibility Stud. 2020, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Liu, J.; Dai, J.; Wu, M.; Wang, W.; Liu, C.; Zhao, D.; Wang, H.; Zhang, J.; Li, M.; et al. Brain-derived neurotrophic factor in 5-HT neurons regulates susceptibility to depression-related behaviors induced by subchronic unpredictable stress. J. Psychiatr. Res. 2020, 126, 55–66. [Google Scholar] [CrossRef]
- Vega-Rivera, N.M.; Ortiz-López, L.; Granados-Juárez, A.; Estrada-Camarena, E.M.; Ramírez-Rodríguez, G.B. Melatonin reverses the depression-associated behaviour and regulates microglia, fractalkine expression and neurogenesis in adult mice exposed to chronic mild stress. Neuroscience 2020, 440, 316–336. [Google Scholar] [CrossRef]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2018, 72, 3–12. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Jiang, M.Q. Study on the Intervention Effect of Allium Macrostemon Saponin on Depression Models in Rats and Mice. Masters Thesis, Henan University of Chinese Medicine, Henan, China, 2014. [Google Scholar]
- Lee, S.; Kim, D.H.; Lee, C.H.; Jung, J.W.; Seo, Y.T.; Jang, Y.P.; Ryu, J.H. Antidepressant-like activity of the aqueous extract of Allium macrostemon in mice. J. Ethnopharmacol. 2010, 131, 386–395. [Google Scholar] [CrossRef]
- Chen, S.; Wei, C.; Gao, P.; Kong, H.; Jia, Z.; Hu, C.; Dai, W.; Wu, Y.; Xu, G. Effect of Allium macrostemon on a rat model of depression studied by using plasma lipid and acylcarnitine profiles from liquid chromatography/mass spectrometry. J. Pharm. Biomed. Anal. 2014, 89, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dai, Y.; Ji, Z.; Zhang, X.; Fu, W.; Han, C.; Xu, Y. Allium macrostemon Bunge. exerts analgesic activity by inhibiting NaV1.7 channel. J. Ethnopharmacol. 2021, 281. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y. Processing of Bulbus Allii Macrostemi. J. Chin. Med. Mater. 1995, 18, 192–194. [Google Scholar]
- Wan, J.H.; Zhang, X.L.; Xin, S.L. The influence of polymeric materials on mouse immunity function. J. Chengde Med. Univ. 2005, 22, 188–190. [Google Scholar]
- Kim, H.J.; Lee, S.H.; Lee, S.H.; Lee, J.; Kim, H.; Chang, G.T.; Lee, D. Longitudinal bone growth stimulating effect of Allium macrostemon in adolescent female rats. Molecules 2020, 25, 5449. [Google Scholar] [CrossRef]
- Wei, J.B.; Zang, L.Q.; Ning, Z.; Li, L.; Zhang, Y.M.; Huang, R.B.; Wang, N.P. Study the effect of aqueous extract of Allium macrostemon bunge on the content of cytchrome P450 of mice. J. Snake 2006, 18, 187–189. [Google Scholar]
- Liu, X.C.; Liu, Q.; Zhou, L.; Liu, Z.L. Evaluation of larvicidal activity of the essential oil of Allium macrostemon Bunge and its selected major constituent compounds against Aedes albopictus (Diptera: Culicidae). Parasites Vectors 2014, 7, 184. [Google Scholar] [CrossRef]
- Peng, J.; Narui, T.; Suzuki, H.; Ishii, R.; Abuki, H.; Okuyama, T. Anti-blood coagulation and cytotoxic effects of compounds from Chinese plants used for thrombosis-like diseases. Nat. Med. 1996, 50, 358–362. [Google Scholar]
- Chen, H.; Ou, W.; Wang, G.; Wang, N.; Zhang, L.; Yao, X. New steroidal glycosides isolated as CD40L inhibitors of activated platelets. Molecules 2010, 15, 4589. [Google Scholar] [CrossRef]
- Ling, S.S.; Zeng, Y.; Li, S.Z.; Ou, W.C. Effect of Allium macrostemon saponin on ADP-induced platelet-derived membrane vesicle inflammation. J. Chin. Med. Mater. 2019, 42, 2157–2162. [Google Scholar]
- Deng, K.; Feng, H.; Wang, Z.P.; Wang, C.S. Study on the effect and mechanism of Allium saponins on platelet aggregation rate in patients with coronary heart disease with cold phlegm blockade syndrome. J. Basic. Chin. Med. 2019, 25, 783–786. [Google Scholar]
- Liu, Z.J.; Wang, Z.P.; Wang, C.S.; Feng, H.; Guo, M.; Hou, Q.; Chu, Z.R. Effects of furostanol saponins from Allium macrostemon bunge on platelet aggregation and coagulation in rats with coronary heart disease. Mod. Med. J. 2019, 47, 381–384. [Google Scholar]
- He, L.H. Applying factorial design to research on the lipid-reducing efficacy of snake-gourd and Allium macrostemon. Guiding J. Tradit. Chin. Med. Pharm. 2002, 8, 205–207. [Google Scholar]
- Wu, B.; Cao, H.; Chen, S.W.; Wang, M.W.; Wang, N.L.; Yao, X.S. Effects of the extract of bulbus Allii Macrostemi on isolated rabbit aortic strips. J. Shenyang Pharm. Univ. 2000, 17, 447–449+455. [Google Scholar]
- Wu, B.; Chen, S.W.; Cao, H.; Wang, M.W. Effects of the extract of Bulbus Allii macrostemi on hypoxia and myocardial ischemia and reperfusion. J. Shenyang Pharm. Univ. 2001, 18, 131–133. [Google Scholar]
- Okuyama, T.; Matsuda, M.; Kishi, N.; Lee, S.N.; Nishino, H. Studies on the cancer chemoprevention of natural resources. XI anti-tumor promoting activities of crude drug ‘Xiebai’ and Kampo prescriptions composed of ‘Xiebai’. Nat. Med. 1995, 49, 261. [Google Scholar]
- Jiang, Y.; Wang, N.; Yao, X.; Susumu, K. Structural elucidation of the anticoagulation and anticancer constituents from Allium chinense. Acta Pharm. Sin. 1998, 33, 355–361. [Google Scholar]
- Bai, J.S.; Wu, Y.J.; Mo, X.T.; Zheng, S.H.; Chen, W.; Xia, L.Q. GC-MS analysis of the antibacterial active components from Allium Chinense and research of its mechanism. Food Sci. 2004, 25, 146–149. [Google Scholar]
- Zhang, X.M.; Liu, H.Y.; Wang, C.X.; Yu, J. Preliminary study on the anti-microbial activity of Allium macrostemon Bunge. J. Anhui Agricultural Sci. 2005, 33, 1676–1677. [Google Scholar]
- Chen, H.F.; Wang, N.L.; Dai, Y.; Yao, X.S. Determination of saponin I in extracts of Allium macrostemnon. China J. Chin. Mater. Med. 2006, 31, 990–992. [Google Scholar]
- Ma, J.; Wang, N.L.; Gao, P.H.; Yao, X.S. The quantitative determination of adenosine in Allium macrostemon Bunge by RP─HPLC. J. Shenyang Pharm. Univ. 1996, 13, 31–34+68. [Google Scholar]
- Ou, W.C.; Qi, L.J.; Feng, J.L.; Liu, N.N. Determination of Allium Mecrostemon saponins in rat plasma and tissues by HPLC-MS. J. Chin. Med. Mater. 2016, 39, 1104–1107. [Google Scholar]
- Qin, Z.; Lin, P.; Dai, Y.; Yao, Z.; Wang, L.; Yao, X.; Liu, L.; Chen, H. Quantification and semiquantification of multiple representative components for the holistic quality control of Allii Macrostemonis Bulbus by ultra high performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J. Sep. Sci. 2016, 39, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; Ma, J.; Qu, G.X.; Wang, N.L.; Yao, X.S. Determination of furostanol saponins in bulbus Allii Macroste. China J. Chin. Mater. Med. 2000, 25, 37–39. [Google Scholar]
- Zhou, H.H. One case of severe diarrhea caused by taking Allium Macrostemon Bunge. China J. Chin. Mater. Med. 1998, 23, 58. [Google Scholar]
- Chao, Z.M.; He, B. Overview of the study of Gualou Xiebai Decoction. Chin. J. Exp. Tradit. Med. Formulae 1999, 5, 59–62. [Google Scholar]

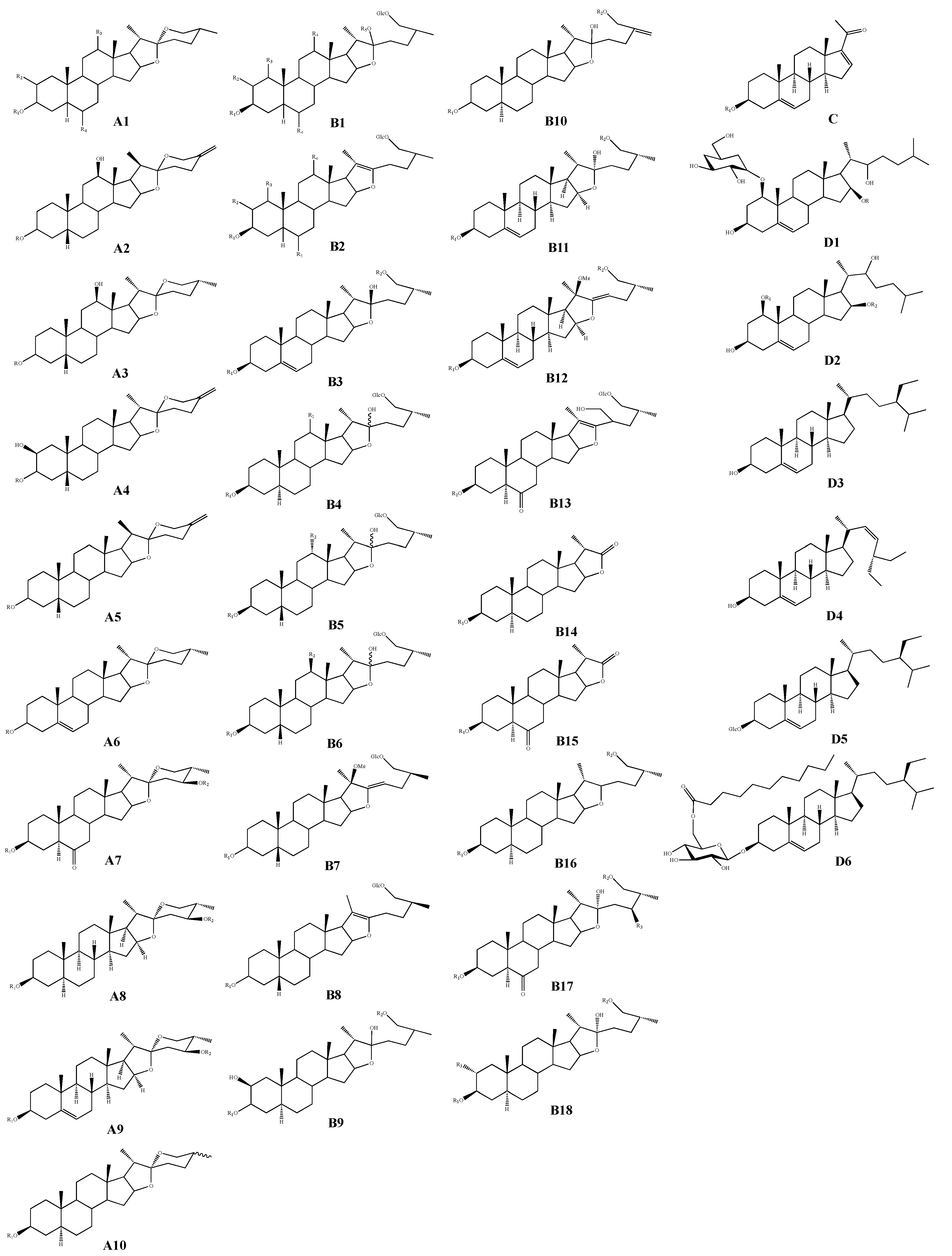

| Distinctions | A. macrostemon | A. chinense |
|---|---|---|
| Bulbs | Subglobose with yellowish papery or membranous exine | Narrowly ovate with white or reddish membranous exine |
| Leaves | Semiterete and grooved, slightly shorter than the scapes | Terete and about as long as the scapes |
| Flowers | Hemispheric to globose, with numerous and crowded flowers, dark purple bulblets and pink or rose-red oblong-ovate to oblong-lanceolate tepals | Subhemispheric, with looser flowers and lavender to bluish-purple broadly elliptic to suborbicular tepals |
| Ovaries | Subglobose | Obovoid |
| Flowering and fruiting period | May–July | October–November |
| Dynasty | Processing Method | Monograph |
|---|---|---|
| Tang Dynasty | Cut into one-inch lengths | Waitai Miyao |
| Song Dynasty | Wash the soil from the surface | Taiping Shenghui Fang |
| Remove the green part, leaving the white part | Bencao Tujing | |
| Stir-fried AMB with the fat of lamb kidney | Zhenglei Bencao | |
| Fry AMB in vinegar to turn it charred black | Shengji Zonglu | |
| Remove the fibrous roots and stems, steam and dry in the sun | Zengding Weiyao Tiaobian | |
| Ming Dynasty | Remove the green part and finely cut | Qixiao Liangfang |
| Modern | 1. Cleaning: Pick impurities and sieve out fibrous roots and debris. 2. Cutting: After cleaning, cut into several sections and dry in the sun. 3. Stir-frying: Put clean AMB into a wok and fry over slow fire until the outer surface shows charred spots, remove and cool. | Chinese medicine sea |
| Stir-fry with baijiu: For every 500 g of AMB, use 50 mL of baijiu, mix the two together, moisten slightly, and then fry in a wok over a slow fire until yellow in color. | Practical Chinese medicine processing | |
| Remove impurities, wash the soil, place it in a suitable container for light steaming, take it out, and dry it in the sun. | General guide to modern Chinese herbal medicine commodities | |
| Wash, remove the bearded root, steam through or put in boiling water and scald through, dry in the sun. | ChP (2020) |
| Dynasty | Preparation/Single Medicine | Main Compositions | Traditional Uses | Monograph |
|---|---|---|---|---|
| Han Dynasty | AMB | AMB | Weapon injury-induced suppuration, anti-fatigue | Shennong Bencao Jing |
| Gualou-Xiebai-Baijiu-Decoction | AMB, Trichosanthes kirilowii, Baijiu | Chest paralysis and heart pain, wheezing and cough, phlegm | Jingui Yaolue | |
| Gualou-Xiebai-Banxia-Decoction | AMB, Trichosanthes kirilowii, Pinellia ternate, Baijiu | Jingui Yaolue | ||
| Zhishi-Xiebai-Guizhi-Decoction | AMB, Trichosanthes kirilowii, Citrus aurantium, Houpoea officinalis, Cassia twig | Jingui Yaolue | ||
| Gualou-Xiebai-Tea | AMB, Trichosanthes kirilowii, Flower tea | Jingui Yaolue | ||
| Jin Dynasty | AMB | AMB | Sudden death | Mingyi Bielu |
| Baizhimo-Ointment | AMB, Angelica dahurica, Glycyrrhiza uralensis, | The carbuncle has been festered | Liu Juanzi Guiyi Fang | |
| Aconitum carmichaeli, Green bamboo bark | ||||
| Tang Dynasty | Xiaobiejia-Decoction | AMB, Trionyx sinensis, Scutellaria baicalensis, Cimicifuga foetida, Ephedra, Antelope horn, Cinnamomum cassia, Almond, Peucedanum praeruptorum, Smoked plum | Physical weakness with edema | Beiji Qianjin Yao Fang |
| Cangmi-Decoction | AMB, Rice, Mutton fat, Fragrant fermented soy beans | Cold dysentery | Beiji Qianjin Yao Fang | |
| Xiebai-Ointment | AMB, Angelica sinensis, Angelica dahurica, Goat spinal cord | Muscle growth and pain relief | Beiji Qianjin Yao Fang | |
| AMB | AMB | Muscle production, fetus settling, heartache | Qianjin Yi Fang | |
| AMB | AMB | Weapon damage | Xinxiu Bencao | |
| AMB | AMB | Stroke | Shiliao Bencao | |
| Chi-Xie-Decoction | AMB, Fermented black beans | Typhoid fever, abdominal pain | Waitai Miyao | |
| Bu-Wei-Decoction | AMB, Poria cocos, Panax ginseng, Pericarpium citri reticulatae, Zingiber officinale, Fermented black beans, Polished glutinous rice | Stomach maintenance | Waitai Miyao | |
| Chen-Tong-Powder | AMB, Achyranthes bidentata, Angelica sinensis, Cinnamomum cassia, Atractylodes macrocephala, Astragalus membranaceus, Radix angelicae tuhuo, Zingiber officinale, Glycyrrhiza uralensis | Benefiting Qi, tonifying blood, warming menstruation and relieving pain | Jingxiao Chanbao | |
| Song Dynasty | Huanglian-Decoction | AMB, Coptis chinensis, Gardenia jasminoides (nuts), Fermented black beans | Dysentery | Taiping Shenghui Fang |
| Xiebai-Renshen-Powder | AMB, Panax ginseng, Atractylodes macrocephala, Houpoea officinalis, Elsholtzia ciliata | Cholera, dry heaving | Taiping Shenghui Fang | |
| Xiebai-Decoction | AMB, Glycyrrhiza uralensis, Angelica sinensis, Sanguisorba officinalis, Polished glutinous rice | Dysentery with abdominal pain in pregnancy | Taiping Shenghui Fang | |
| Jiao-Chi-Decoction | AMB, Collacoriiasini, Fermented black beans, Zingiber officinale | Postpartum cold and dysentery, diarrhea and abdominal pain | Taiping Shenghui Fang | |
| AMB | AMB | Tonic for deficiency and detoxification | Bencao Tujing | |
| Xiebai-Decoction | AMB, Fermented black beans, Gardenia jasminoides (nuts) | Typhoid fever, abdominal pain | Leizheng Huoren Shu | |
| AMB | AMB | Burn and scald | Bencao Yanyi | |
| Cong-Xie-Decoction | AMB, A. fistulosum (white part), Schizonepeta spike, Caulis bambusae, Fermented black beans, Zingiber officinale, Bunge pricklyash seed | Typhoid fever | Sheng Ji Zonglu | |
| Huangqi-Xiebai-Decoction | AMB, Panax ginseng, Poria cocos (white part), Schisandra chinensis, Atractylodes macrocephala, A. fistulosum (white part), Polished glutinous rice, Paeonia lactiflora (white), Zingiber officinale, Goat or Sheep kidney | Weakness after typhoid fever | Sheng Ji Zonglu | |
| Congbai-Decoction | AMB, A. fistulosum (white part), Glycyrrhiza uralensis, Artemisia apiacea, Almond | Night sweats, muscle wasting | Sheng Ji Zonglu | |
| Shexiang-Decoction | AMB, Bupleurum fruticosum, Ferulae resina, Glycyrrhiza uralensis, Artemisia apiacea, Semen persicae, Willow branch, Rosa laevigata (branch), A. fistulosum (white part), Areca catechu | Tuberculosis | Sheng Ji Zonglu | |
| Xiebai-Noodles | AMB, Zingiber officinale, Flour | Post-typhoid dysentery with water and grain | Sheng Ji Zonglu | |
| Goji-Berry-Porridge | AMB, Goji Berry, A. fistulosum (white part), Fermented black beans, Rice | Weakness after typhoid fever and pain in the back | Sheng Ji Zonglu | |
| Ejiao-Pieces | AMB, Collacoriiasini, Dried ginger | Dysentery | Sheng Ji Zonglu | |
| Xiebai-Cake | AMB, Egg yolk, Amber | Watery dysentery, dysentery with purulent and bloody stools | Sheng Ji Zonglu | |
| La-Xie-Cake | AMB, Paraffin, Egg, Flour | Dysentery with purulent and bloody stools | Sheng Ji Zonglu | |
| Yuan Dynasty | AMB | AMB | Dysentery | Tangye Becao |
| AMB | AMB | Long-term dysentery, cholera | Bencao Yuanming Bao | |
| Ming Dynasty | Xiebai-Powder | AMB, Trionyx sinensis, Collacoriiasini, Antler glue | Prolonged cough, vomiting of blood, hemoptysis | Qixiao Liangfang |
| Baishuji-Porridge | AMB, Tremella fuciformis, Rice | Dysentery with purulent and bloody stools | Yifang Leiju | |
| AMB | AMB | Thoracic obstruction and tingling, calming the fetus | Bencao Gangmu | |
| Xiebai-Chen-Tong-Powder | AMB, Astragalus membranaceus, Angelica sinensis, Achyranthes bidentata, Cinnamomum cassia, Atractylodes macrocephala, Radix angelicae tuhuo, Zingiber officinale, Glycyrrhiza uralensis | Postpartum weakness and pain around the body | Chishui Xuanzhu | |
| AMB | AMB | Warming the stomach and removing food stagnation | Bencao Huiyan | |
| Qing Dynasty | AMB | AMB | Cough and asthma | Bencao Beiyao |
| AMB | AMB | Promoting muscle production, dispersing nodules, relieving asthma and calming the fetus | Bencao Yidu | |
| AMB | AMB | Food accumulation, worm accumulation | Benjing Fengyuan | |
| AMB | AMB | Relieving diarrhea, calming the fetus and relieving pain | Cahngsha Yaojie | |
| AMB | AMB | Giving birth, muscle and dysentery | Bencao Congxin | |
| AMB | AMB | Relieving diarrhea, promoting blood circulation, relieving asthma, relieving pain and calming the fetus | Bencao Qiuzhen | |
| AMB | AMB | Cardiothoracic pain, back pain | Yao Zheng | |
| Leng-Xie-Duan-Lou-Pills | AMB, Arcae concha, Chicken’s Gizzard-membrane, Corydalis yanhusuo, Myrrh, Cyperus rotundus, Semen persicae, Trichosanthes kirilowii (nuts), Perilla frutescens (seeds), Sinapis alba (seeds), Raphanus sativus (seeds) | Abdominal mass, stagnation of phlegm and dyspepsia | Yiji Baojian | |
| AMB | AMB | Invigorates the muscles, moves Qi and invigorates blood | Bencao Fenjing | |
| AMB | AMB | Stroke and CHD | Yaoxing Jiyao Bianlan | |
| AMB | AMB | Dispersing nodules, relieving pain, relieving diarrhea and calming the fetus | Suixi Juyin Shipu | |
| JiaWei-Baihe-Decoction | AMB, Lindera aggregata, Lilii Bulbus, Fritillary, Trichosanthes kirilowii, Cardamom | Chest and diaphragm pain | Buzhi Yi Biyao | |
| AMB | AMB | Promoting Qi flow and stopping diarrhea | Benbao Biandu | |
| JiaWei-Si-Ni-Powder | AMB, Bupleurum fruticosum, Citrus aurantium, Paeonia lactiflora (white), Dried ginger, Glycyrrhiza uralensis (fried with honey), Cassia twig, Poria cocos, Radix aconiti lateralis preparata | Deadly cold hand and foot, dry cough, palpitations, abdominal pain | Chongding Tongsu Shanghan Lun | |
| Modern | Qingyi-Pills | AMB, Bupleurum fruticosum, Scutellaria baicalensis, Pinellia ternata, Trichosanthes kirilowii, Citrus aurantium, Szechwan chinaberry fruit, Paeonia lactiflora (white), Chinese rhubarb | Abdominal pain, hypochondriac pain, and back pain in the recovery period of acute pancreatitis | New Acute Abdominology |
| Xinnaoning-Capsules | AMB, Ginkgo leaves, Buxus microphylla, Salvia miltiorrhiza, Litsea lancilimba | CHD, cerebral arteriosclerosis | ChP (2020) | |
| Xuezhitong-Capsules | AMB | Hyperlipidemia | ChP (2020) | |
| Dan-Lou-Tablets | AMB, Trichosanthes kirilowii, Salvia miltiorrhiza, Radix puerariae, Ligusticum chuanxiong, Paeonia lactiflora (red), Alisma plantago-aquatica, Astragalus membranaceus, Davallia mariesii, Radix curcumae | CHD, AP | ChP (2020) | |
| Tongxiening-Granules | AMB, Paeonia lactiflora (white), Pericarpium citri reticulatae viride, Atractylodes macrocephala | Abdominal pain, diarrhea | ChP (2020) | |
| Buxinqi-Oral Liquid | AMB, Astragalus membranaceus, Panax ginseng, Acorus tatarinowii | Thoracic obstruction and heartache | ChP (2020) | |
| Zhenxintong-Oral Liquid | AMB, Codonopsis pilosula, Panax notoginseng, Corydalis yanhusuo, Earthworm, Semen lepidii, Cinnamomum cassia, Borneol, Menthol | CHD, AP | ChP (2020) |
| Classification | No. | Skeleton | Ingredient Name | R1 | R2 | R3 | R4 | R5 | R6 | Sources | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spirostanol saponins | 1 | A1 | Macrostemonoside A | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | H | H | H | - | - | A. macrostemon A. chinense | [8,9] |
| 2 | Macrostemonoside D | Gal(1-4)-Glc-[(1-2)-Glc-(1-6)-Ac]-(1-3)-Glc | H | H | H | - | - | A. macrostemon A. chinense | [8,10] | ||
| 3 | (3β,5β,12β,25R)-12-hydroxyspirostan-3-yl-2-O-β-D-glucopyranosyl-β-D-galactopyranoside | Gal(1-2)-Glc | H | OH | H | - | - | A. macrostemon | [11] | ||
| 4 | (2β,3β,5β,25R)-2-hydroxyspirostan-3-yl-2-O-β-D-glucopyranosyl-β-D-galactopyranoside | Gal(1-2)-Glc | OH | H | H | - | - | A. macrostemon | [12] | ||
| 5 | Timosaponin AII | Gal(1-2)-Glc | OH | H | H | - | - | A. macrostemon | [12] | ||
| 6 | Schidigera saponin C2 | Gal(1-2)-Glc | OH | H | H | - | - | A. macrostemon | [12] | ||
| 7 | (3β, 5β, 25R)-spirostan-3-yl-2-O-β-D-glucopyranosyl-β-D-galactopyranoside | Gal(1-2)-Glc | H | H | H | - | - | A. macrostemon | [13] | ||
| 8 | Smilagenin | H | H | H | H | - | - | A. macrostemon | [13] | ||
| 9 | Laxogenin | H | H | H | O | - | - | A. macrostemon A. chinense | [12,14] | ||
| 10 | Xiebai saponin I | Glc[(1-4)-Xyl]-(1-6)-Ara | H | H | O | - | - | A. macrostemon A. chinense | [9,12] | ||
| 11 | Smilaxin A | Glc-(1-6)-Ara | H | H | O | A. macrostemon A. chinense | [12,14] | ||||
| 12 | (3β,5β)-spirost-25(27)-en-3-yl-2-O-β-D-glucopyranosyl-β-D-galactopyranoside | Gal(1-2)-Glc | H | H | H | - | - | A. macrostemon | [11] | ||
| 13 | Odospiroside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | H | H | H | - | - | A. macrostemon | [11] | ||
| 14 | (25R)-spirostane-5(6)-en-3β-3-O-β-D-glucopyranosyl(1→2)[β-D-glucopyranosyl(l→3)]-β-D-glucopyranosyl-6-acetyl(l→4)-β-D-galactopyranoside | Gal(1-4)-Glc-[(1-2)-Glc-(1-6)-Ac]-(1-3)-Glc | H | H | H | - | - | A. macrostemon | [11] | ||
| 15 | Macrostemonoside S | Gal(1-2)-Glc | H | OH | H | - | - | A. macrostemon | [11] | ||
| 16 | (2α, 3β, 5α, 25S)-2-hydroxyspirostan-3-yl-O-β-D-glucopyranosyl-(1→2)-O-[β-D- glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | OH | H | H | - | - | A. chinense | [15] | ||
| 17 | (2α, 3β, 5α, 25R)-2-hydroxyspirostan-3-yl-O-β-D-glucopyranosyl-(1→2)-O-[β-D- glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | OH | H | H | - | - | A. chinense | [15] | ||
| 18 | (2α, 3β, 5α, 25S)-2-hydroxyspirostan-3-yl-O-β-D-glucopyranosyl-(1→2)-O-β-D- glucopyranosyl- (1→4)-β-D-galactopyranoside | Gal(1-4)-Glc-(1-2)-Glc | OH | H | H | - | - | A. chinense | [15] | ||
| 19 | Petunioside | Gal(1-4)-Glc-(1-2)-Glc | OH | H | H | - | - | A. chinense | [15] | ||
| 20 | A2 | 5β-spirostane-25(27)-en-3β,12β-diol-3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside | Gal(1-2)-Glc | - | - | - | - | - | A. macrostemon | [11] | |
| 21 | A3 | (25R)-5β-spirostane-3β,12β-diol-3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside | Gal(1-2)-Glc | - | - | - | - | - | A. macrostemon | [11] | |
| 22 | A4 | 5β-spirostane-25(27)-en-2β,3β-diol-3-O-β-D-glucopyranosyl(1→2)-β-D-galactopyranoside | Gal(1-2)-Glc | - | - | - | - | - | A. macrostemon | [11] | |
| 23 | A5 | 5β-spirostane-25(27)-en-3β-3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside | Gal(1-2)-Glc | - | - | - | - | - | A. macrostemon | [11] | |
| 24 | A6 | Odospiroside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | - | - | - | - | - | A. macrostemon | [11] | |
| 25 | A7 | Chinenoside VI | Glc(1-6)-Ara | Glc | - | - | - | - | A. chinense | [16] | |
| 26 | A8 | Allimacrosides B | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | Glc | - | - | - | - | A. macrostemon | [17] | |
| 27 | A9 | Allimacrosides C | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | Glc | - | - | - | - | A. macrostemon | [17] | |
| 28 | A10 | (25R,S)-26-O-β-D-glucopyranosyl-5α-spirotane-3β-ol-3-O-β-D-glucopyranosyl-(1→2)-[β-Dglucopyranosyl-(1→3)]-(6-acetyl-β-D-glucopyranosyl-(1→4)-β-D-galacopyranosid | Gal(1-4)-Glc-6-acetyl-[(1-2)-Glc]-(1-3)-Glc | - | - | - | - | - | A. chinense | [18] | |
| Furostanol saponins | 29 | B1 | Macrostemonoside B | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | H | H | H | H | H | A. macrostemon A. chinense | [18,19] |
| 30 | Macrostemonoside C | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | H | H | H | CH3 | H | A. macrostemon | [19] | ||
| 31 | Macrostemonoside G | Gal(1-2)-Glc | H | H | OH | H | H | A. macrostemon | [19] | ||
| 32 | Macrostemonoside H | Gal(1-2)-Glc | H | H | OH | CH3 | H | A. macrostemon | [20] | ||
| 33 | Macrostemonoside I | Gal(1-2)-Glc | H | H | OH | H | H | A. macrostemon | [20] | ||
| 34 | Macrostemonoside J | Gal(1-2)-Glc | OH | H | H | H | H | A. macrostemon | [21] | ||
| 35 | Macrostemonoside K | Gal(1-2)-Glc | OH | H | H | CH3 | H | A. macrostemon | [22] | ||
| 36 | Macrostemonoside M | H | OH | OH | H | H | OH | A. macrostemon | [19] | ||
| 37 | Macrostemonoside N | H | OH | OH | H | H | OH | A. macrostemon | [19] | ||
| 38 | Macrostemonoside O | Gal(1-2)-Glc | H | H | H | H | H | A. macrostemon | [21] | ||
| 39 | Macrostemonoside P | Gal(1-2)-Glc | H | OH | H | H | H | A. macrostemon | [21] | ||
| 40 | Macrostemonoside Q | Gal(1-2)-Glc | OH | OH | H | H | H | A. macrostemon | [21] | ||
| 41 | Macrostemonoside R | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | OH | H | H | H | H | A. macrostemon | [21] | ||
| 42 | (3β,5α,12β,25R)-26-O-β-D-glucopyranosyloxy-12,22-dihydroxyfurostan-3-yl-O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | H | H | OH | H | H | A. macrostemon | [19] | ||
| 43 | (3β,5α,12β)-26-O-β-D-glucopyranosyloxy-12,22-dihydroxyfurost-25-en-3-yl-O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | H | H | OH | H | H | A. macrostemon | [19] | ||
| 44 | (3β,5α,12α,25R)-26-O-β-D-glucopyranosyloxy-12,22-dihydroxyfurostan-3-yl-O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | H | H | OH | H | H | A. macrostemon | [23] | ||
| 45 | (3β,5β,12α,25R)-26-O-β-D-glucopyranosyloxy-12,22-dihydroxyfurostan-3-yl-2-O-β-D-glucopyranosyl-β-D-galactopyranoside | Gal(1-2)-Glc | H | H | OH | H | H | A. macrostemon | [23] | ||
| 46 | Elephanoside E | Gal(1-2)-Glc | H | H | OH | H | H | A. macrostemon | [23] | ||
| 47 | (3β,5β,12β,25R)-26-O-β-D-glucopyranosyloxy-22-methoxy-12-hydroxyfurostan-3-yl-2-O-β-D-glucopyranosyl-β-D-galactopyranoside | Gal(1-2)-Glc | H | H | OH | CH3 | H | A. macrostemon | [19] | ||
| 48 | (3β,5β,12α,25R)-26-O-β-D-glucopyranosyloxy-22-methoxy-12-hydroxyfurostan-3-yl-2-O-β-D-glucopyranosyl-β-D-galactopyranoside | Gal(1-2)-Glc | H | H | OH | CH3 | H | A. macrostemon | [19] | ||
| 49 | (3β,5β)-26-O-β-D-glucopyranosyloxy-22-methoxy-25(27)-en-12-onefurost-3-yl-2-O-β-D-glucopyranosyl-β-D-galactopyranoside | Gal(1-2)-Glc | H | H | OH | CH3 | H | A. macrostemon | [19] | ||
| 50 | (1β,3β,5β,6β,22α)-26-O-β-D-glucopyranosyloxy-1,6,22-trihydroxyfurost-25-en-3-yl-β-D-galactopyranoside | Gal | H | OH | H | H | H | A. macrostemon | [24] | ||
| 51 | Timosaponin B II | Gal(1-2)-Glc | H | H | H | H | H | A. macrostemon | [11] | ||
| 52 | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5β-furost-3β,26-diol-3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside | Gal(1-2)-Glc | H | H | H | H | H | A. macrostemon | [11] | ||
| 53 | (3β,25R)-26-O-β-D-glucopyranosyloxy-22-hydroxyfurost-5-en-3-yl-O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | H | H | H | H | H | A. macrostemon | [25] | ||
| 54 | Chinenoside I | Glc[(1-4)-Xyl]-(1-6)-Ara | H | H | H | H | O | A. chinense | [7] | ||
| 55 | B2 | Macrostemonoside E | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | H | H | H | H | - | A. macrostemon | [19] | |
| 56 | Macrostemonoside F | Gal(1-2)-Glc | H | H | H | H | - | A. macrostemon | [19] | ||
| 57 | Macrostemonoside L | Gal(1-2)-Glc | OH | H | H | H | - | A. macrostemon | [22] | ||
| 58 | (3β,5β,12β)-26-O-β-D-glucopyranosyloxy-5β-furost-20(22)-25(27)-dien-3β,12β,26-triol-3-β-2-O-β-D-glucopyranosyl-β-D-galactopyranoside | Gal(1-2)-Glc | H | H | OH | H | - | A. macrostemon | [19] | ||
| 59 | (3β,5β,12α,25R)-26-O-β-D-glucopyranosyloxy-12-hydroxyfurost-20(22)-en-3-yl-2-O-β-D-glucopyranosyl-β-D-galactopyranoside | Gal(1-2)-Glc | H | H | OH | H | - | A. macrostemon | [19] | ||
| 60 | Chinenoside II | Glc-[(1-4)-Xyl]-(1-6)-Ara | H | H | H | O | - | A. chinense | [26] | ||
| 61 | Chinenoside III | Glc-(1-6)-Ara | H | H | H | O | - | A. chinense | [26] | ||
| 62 | 26-O-β-D-glucopyranosyl-5β-furostane-20(22)-25(27)-dien-3β,26-diol-3-O-β-D-glucopyranosyl-(l→2)-β-D-galactopyranoside | Gal(1-2)-Glc | H | H | H | H | - | A. macrostemon | [11] | ||
| 63 | B3 | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-furost-5(6)-ene-3β,26-diol-3-O-β-D-glucopyranosyl(1→2)[β-D-glucopyranosyl(1→3)]-β-D-glucopyranosyl(1 →4)-β-D-galactopyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | Gal | - | - | - | - | A. macrostemon | [25] | |
| 64 | B4 | (25R)-26-O-β-D-glucopyranosyl-5α-furostane-3β,12β,22,26-tetraol-3-O-β-D-glucopyranosyl(1→2)[β-D-glucopyranosyl(1→3)]-β-D-glucopyranosyl (1→4)-β-D-galactopyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | β-OH | - | - | - | - | A. macrostemon A. chinense | [18,27] | |
| 65 | (25R)-26-O-β-D-glucopyranosyl-5α-furostane-3β,12α,22,26-tetraol-3-O-β-D-glucopyranosyl (1→2) [β-D-glucopyranosyl (1→3)]-β-D-glucopyranosyl (1→4)-β-D-galacto- pyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | α-OH | - | - | - | - | A. macrostemon | [27] | ||
| 66 | B5 | (25R)-26-O-β-D-glucopyranosyl-5β-furostane-3β,12α,22,26-tetraol-3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | Glc(1-2)-Glc | OH | - | - | - | - | A. macrostemon | [27] | |
| 67 | B6 | (25R)-26-O-β-D-glucopyranosyl-5β-furostane-12β,3β,22,26-tetraol-3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | Glc(1-2)-Glc | OH | - | - | - | - | A. macrostemon | [23] | |
| 68 | B7 | (25R)-26-O-β-D-glucopyranosyl-5β-furostane-22(23)-en-20-methoxyl-3β,26-diol-3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | Gal(1-2)-Glc | - | - | - | - | - | A. macrostemon | [11] | |
| 69 | B8 | (25R)-26-O-β-D-glucopyranosyl-5β-furostane-20(22)-en-3β,12α,26-triol-3-O-β-D-glucopyranosyl(l→2)-β-D-galactopyranoside | Gal(1-2)-Glc | - | - | - | - | - | A. macrostemon | [11] | |
| 70 | B9 | (25S)-26-O-β-D-glucopyranosyl-5α-furostane-2α,3β,22,26-tetraol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl(1→4)-β-D-galactopyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | Gal | - | - | - | - | A. macrostemon | [28] | |
| 71 | B10 | 25(27)-ene-26-O-β-D-glucopyranosyl-5α-furostane-3β,22,26-triol-3-O-β-D-glucopyra-nosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl(1→4)-β-D-galact opyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | Gal | - | - | - | - | A. macrostemon | [28] | |
| 72 | B11 | Allimacrosides D | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | Glc | - | - | - | - | A. macrostemon | [17] | |
| 73 | B12 | Allimacrosides E | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | Glc | - | - | - | - | A. macrostemon | [17] | |
| 74 | B13 | Chinenoside IV | Glc-[(1-4)-Xyl]-(1-6)-Ara | - | - | - | - | - | A. chinense | [29] | |
| 75 | Chinenoside V | Glc-(1-6)-Ara | - | - | - | - | - | A. chinense | [29] | ||
| 76 | (25R)-6-one-5α-furostane-3β,26-triol-20(22)-en-26-O-β-D-glucopyranoside | H | - | - | - | - | - | A. chinense | [30] | ||
| 77 | B14 | 5α-cholano-22,16-lactone-3-hydroxyl-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galacopyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | - | - | - | - | - | A. chinense | [31] | |
| 78 | B15 | 6-one-5α-cholano-22,16-lactone-3-hydroxyl-3-O-β-D-xylopyranosyl-(1→4)-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside | Glc[(1-4)-Xyl]-(1-6)-Ara | - | - | - | - | - | A. chinense | [31] | |
| 79 | B16 | (25R)-26-O-β-D-glucopyranosyl-5α-furostane-3β,26-diol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galacopyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | Glc | - | - | - | - | A. chinense | [31] | |
| 80 | B17 | (25R)-6-one-26-O-β-D-glucopyranosyl-5α-furostane-3β,22α,26-triol-3-O-β-D-xylopyranosyl-(1→4)-β-D-glucopyranoside | Glc(1-4)-Xyl | Glc | H | - | - | - | A. chinense | [31] | |
| 81 | (25R)-6-one-5α-furostane-3β,22α,24β,26-tetraol-3-O-β-D-xylopyranosyl-(1→4)-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside | Glc[(1-4)-Xyl]-(1-6)-Ara | H | OH | - | - | - | A. chinense | [31] | ||
| 82 | (25R)-6-one-26-O-β-D-glucopyranosyl-5α-furostane-3β,22,26-triol-3-O-α-L-arabinopyranosyl-(1→6)-β-D-glucopyranoside | Glc(1-6)-Ara | Glc | H | - | - | - | A. chinense | [18] | ||
| 83 | (25R)-6-one-26-O-β-D-glucopyranosyl-5α-furostane-3β,22,26-triol-3-O-β-D-xylopyranosyl-(1→4)-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside | Glc[(1-4)-Xyl]-(1-6)-Ara | Glc | H | - | - | - | A. chinense | [18] | ||
| 84 | (25R)-6-one-5α-furostane-3β,22α,26-triol-26-O-β-D-glucopyranoside | H | Glc | H | - | - | - | A. chinense | [18] | ||
| 85 | (25R)-6-one-26-O-β-D-glucopyranosyl-5α-furostane-3β,22α,26-triol-3-O-β-D-glucopyranoside | Glc | Glc | H | - | - | - | A. chinense | [18] | ||
| 86 | B18 | (25R)-26-O-β-D-glucopyranosyl-5α-furostane-2α,3β,22,26-tetraol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galacopyranoside | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | Glc | OH | - | - | - | A. chinense | [18] | |
| 87 | (25R)-5α-furostane-2β,3β,22α,26-tetraol-26-O-β-D-glucopyranoside | H | Glc | OH | - | - | - | A. chinense | [31] | ||
| 88 | (25R)-26-O-β-D-glucopyranosyl-5α-furostane-3β,26-didyroxy-3-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | Gal(1-4)-Glc | Glc | H | - | - | - | A. chinense | [30] | ||
| 89 | Tomatoside A | Gal(1-4)-Glc-(1-2)-Glc | Glc | H | - | - | - | A. chinense | [30] | ||
| Pregnane glycoside | 90 | C | Allimacrosides A | Gal(1-4)-Glc-[(1-2)-Glc]-(1-3)-Glc | - | - | - | - | - | A. macrostemon | [17] |
| Cholestane glycosides | 91 | D1 | (1β,3β,16β,22S)-1-[(6-deoxy-α-L-mannopyranosyl)oxy]-3,22-dihydroxycholest-5-en-16-O-β-D-glucopyranoside | Glc | - | - | - | - | - | A. macrostemon | [19] |
| 92 | D2 | (22S)-cholest-5-ene-1β,3β,16β,22-tetraol-1-O-α-L-rhamnopyranosyl-16-O-β-D-glucopyranoside | Rha | Glc | - | - | - | - | A. macrostemon | [18] | |
| Sterols | 93 | D3 | Sitosterol | - | - | - | - | - | - | A. macrostemon | [32] |
| 94 | D4 | Stigmasterol | - | - | - | - | - | - | A. macrostemon | [19] | |
| 95 | D5 | Daucosterol | - | - | - | - | - | - | A. macrostemon | [33] | |
| 96 | D6 | Sitosteryl-6’-O-undecane-β-D-glucoside | - | - | - | - | - | - | A. macrostemon | [33] |
| Classification | No. | Skeleton | Ingredient Name | R1 | R2 | Sources | Reference |
|---|---|---|---|---|---|---|---|
| Sulfur-containing compounds | 97 | E1 | Ethyl cis-1-propenyl sulfide | ethyl | cis-1-propenyl | A. chinense | [36] |
| 98 | Diallyl sulfide | allyl | allyl | A. chinense | [36] | ||
| 99 | 3-[(1-methylethy) thio]-1-propene | isopropyl | allyl | A. macrostemon | [37] | ||
| 100 | Methyl allyl sulfide | methyl | allyl | A. macrostemon | [38] | ||
| 101 | E2 | Methanethiol | - | - | A. macrostemon | [39] | |
| 102 | E3 | 1-hydroxyl-2-sulfhydryl-ethane | - | - | A. macrostemon | [38] | |
| 103 | E4 | 2, 4-dimethylthiophene | - | - | A. macrostemon | [37] | |
| 104 | E5 | 1, 3-dimethylthiophene | - | - | A. macrostemon | [38] | |
| 105 | E6 | Dimethyl sulfone | - | - | A. macrostemon | [39] | |
| 106 | E7 | 2,4-dihydro-4,5-dimethyl-3H-1,2,4-triazole-3-thione | - | - | A. macrostemon | [39] | |
| 107 | E8 | 3,4-dimethyl-thiophene | A. macrostemon | [39] | |||
| 108 | E9 | 1, 3-propane sultone | - | - | A. macrostemon | [40] | |
| 109 | E10 | Isobutyl isothiocyanate | - | - | A. macrostemon | [40] | |
| 110 | E11 | 1, 3, 2-dioxathiane-2, 2-dioxide | - | - | A. macrostemon | [40] | |
| 111 | F1 | Dimethyl disulfide | methyl | methyl | A. macrostemon A. chinense | [36,37] | |
| 112 | Methyl ethyl disulfide | methyl | ethyl | A. macrostemon A. chinense | [36,38] | ||
| 113 | Methyl propyl disulfide | methyl | propyl | A. macrostemon A. chinense | [36,37] | ||
| 114 | Methyl allyl disulfide | methyl | allyl | A. macrostemon A. chinense | [36,37] | ||
| 115 | Methyl cis-1-propenyl disulfide | methyl | cis-1-propenyl | A. macrostemon A. chinense | [36,38] | ||
| 116 | Methyl isopropyl disulfide | methyl | isopropyl | A. macrostemon | [38] | ||
| 117 | Methyl butyl disulfide | methyl | butyl | A. chinense | [36] | ||
| 118 | Ethyl propyl disulfide | ethyl | propyl | A. chinense | [36] | ||
| 119 | Ethyl cis-1-propenyl disulfide | ethyl | cis-1-propenyl | A. chinense | [36] | ||
| 120 | Ethyl trans-1-propenyl disulfide | ethyl | trans-1-propenyl | A. chinense | [36] | ||
| 121 | Propyl propenyl disulfide | propyl | propenyl | A. macrostemon | [38] | ||
| 122 | Propyl isopropyl disulfide | propyl | isopropyl | A. macrostemon | [37] | ||
| 123 | Propyl allyl disulfide | propyl | allyl | A. macrostemon A. chinense | [37,41] | ||
| 124 | Diallyl disulfide | allyl | allyl | A. macrostemon A. chinense | [36,38] | ||
| 125 | Allyl isopropyl disulfide | allyl | isopropyl | A. macrostemon A. chinense | [37,41] | ||
| 126 | Allyl cis-1-propenyl disulfide | allyl | cis-1-propenyl | A. chinense | [36] | ||
| 127 | Allyl trans-1-propenyl disulfide | allyl | trans-1-propenyl | A. chinense | [36] | ||
| 128 | bis (1-methylethyl) disulfide | isopropyl | isopropyl | A. macrostemon | [39] | ||
| 129 | F2 | 1, 3-dimercaptopropane | - | - | A. macrostemon | [38] | |
| 130 | F3 | 1,3-dithiane | - | - | A. macrostemon A. chinense | [37,41] | |
| 131 | F4 | 2, 2-bis(methylthio)propane | - | - | A. macrostemon | [37] | |
| 132 | F5 | 3-mercapto-2-(mercaptomethyl)-propanoic acid | - | - | A. macrostemon | [39] | |
| 133 | F6 | 2-ethylidene [1,3]dithiane | - | - | A. macrostemon | [39] | |
| 134 | F7 | S-methyl methanethiosulfinate | - | - | A. macrostemon | [39] | |
| 135 | G1 | Dimethyl trisulfide | methyl | methyl | A. macrostemon A. chinense | [36,37] | |
| 136 | Methyl ethyl trisulfide | methyl | ethyl | A. chinense | [36] | ||
| 137 | Methyl butyl trisulfide | methyl | butyl | A. chinense | [36] | ||
| 138 | Methyl propyl trisulfide | methyl | propyl | A. macrostemon A. chinense | [36,37] | ||
| 139 | Methyl allyl trisulfide | methyl | allyl | A. macrostemon A. chinense | [36,37] | ||
| 140 | Methyl cis-1-propenyl trisulfide | methyl | cis-1-propenyl | A. chinense | [36] | ||
| 141 | Methyl trans-1-propenyl trisulfide | methyl | trans-1-propenyl | A. macrostemon A. chinense | [36,37] | ||
| 142 | Dipropyl trisulfide | propyl | propyl | A. macrostemon | [37] | ||
| 143 | Propyl allyl trisulfide | propyl | allyl | A. macrostemon A. chinense | [37,41] | ||
| 144 | Diallyl trisulfide | allyl | allyl | A. macrostemon A. chinense | [38,41] | ||
| 145 | G2 | 3, 5-dimethyl-1, 2, 4-tridithiane | - | - | A. macrostemon | [37] | |
| 146 | G3 | 4-methyl-1, 2, 3-tridithiane | - | - | A. macrostemon | [37] | |
| 147 | G4 | 3,5-diethyl-1,2,4-trithiolane | - | - | A. macrostemon | [39] | |
| 148 | H1 | Dimethyl tetrasulfide | methyl | methyl | A. macrostemon A. chinense | [36,37] | |
| 149 | Methyl pentyl tetrasulfide | methyl | pentyl | A. chinense | [36] | ||
| 150 | Propyl cis-l-propenyl tetrasulfide | propyl | cis-l-propenyl | A. chinense | [36] | ||
| 151 | Propyl trans-l-propenyl tetrasulfide | propyl | trans-l-propenyl | A. chinense | [36] | ||
| 152 | H2 | 5-methyl-1, 2, 3, 4-tetradithiane | - | - | A. macrostemon | [37] | |
| 153 | I | Methyl propyl pentasulfide | methyl | propyl | A. chinense | [36] | |
| 154 | Propyl cis-l-propenyl pentasulfide | propyl | cis-l-propenyl | A. chinense | [36] |
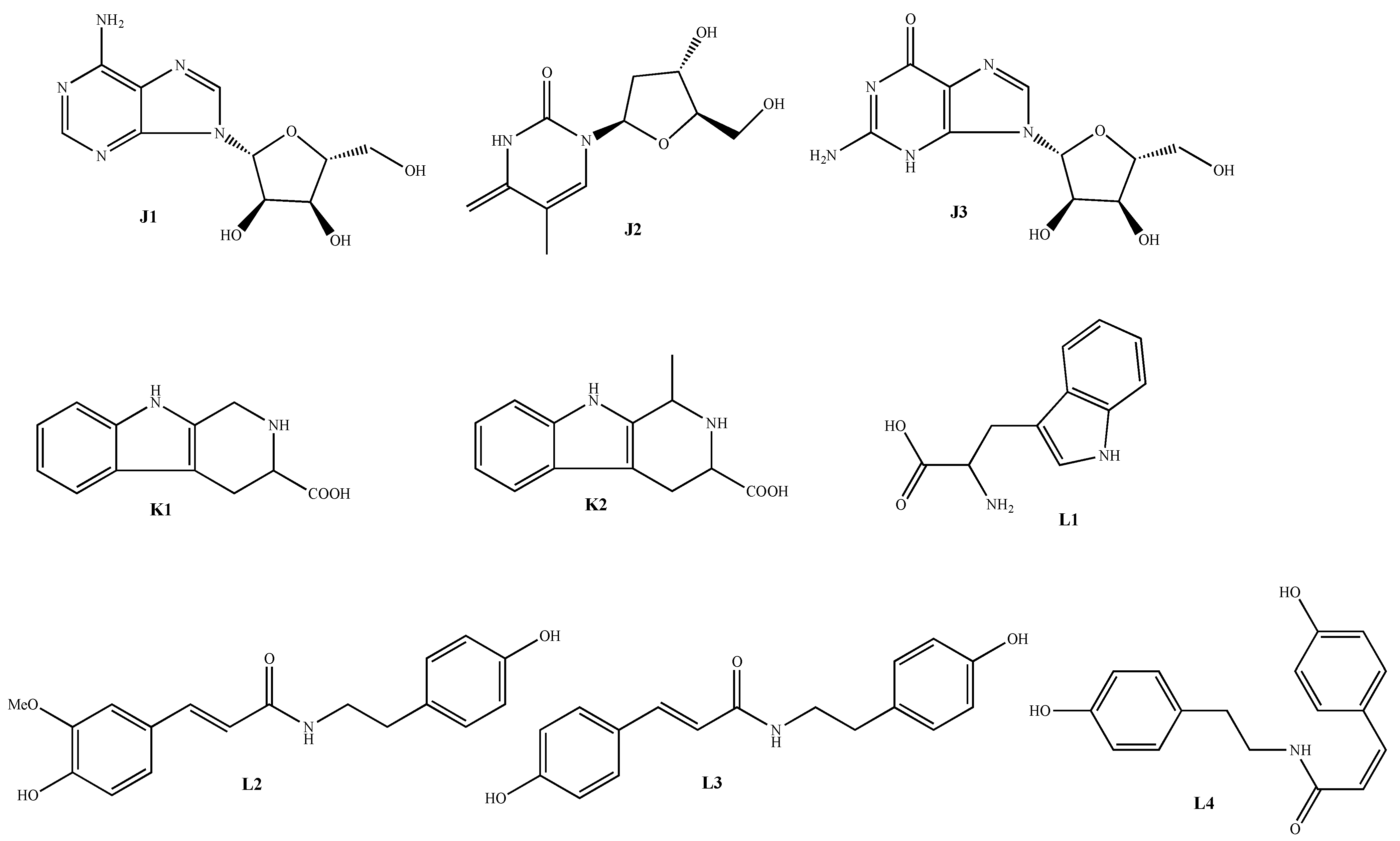
| Classification | No. | Skeleton | Ingredient Name | Sources | Reference |
|---|---|---|---|---|---|
| Nitrogen-containing compounds | 155 | J1 | Adenosine | A. macrostemon A. chinense | [42,47] |
| 156 | J2 | Thymidine | A. macrostemon | [42] | |
| 157 | J3 | Guanosine | A. chinense | [45] | |
| 158 | K1 | 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3- carboxylic acid | A. macrostemon A. chinense | [42,47] | |
| 159 | K2 | 2,3,4,9-tetrahydro-1-methyl-1H-pyrido[3,4-b]indole-3-carboxylic acid | A. macrostemon | [42] | |
| 160 | L1 | Tryptophan | A. macrostemon A. chinense | [42,47] | |
| 161 | L2 | N-trans-feruloyltyramine | A. chinense | [43] | |
| 162 | L3 | N-(p-trans-coumaroyl)-tyramine | A. chinense | [44] | |
| 163 | L4 | N-(p-cis-coumaroyl)-tyramine | A. chinense | [44] |
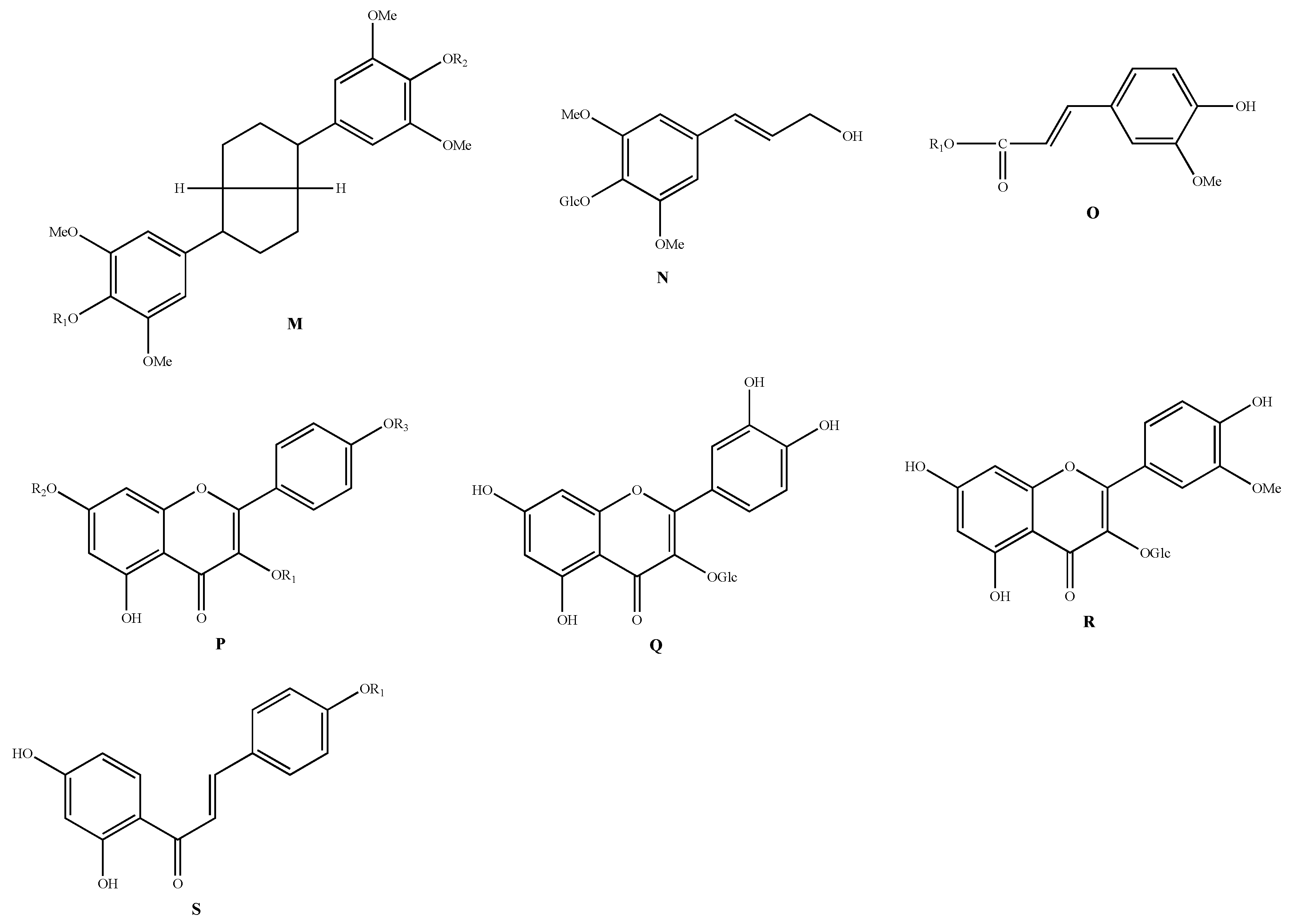
| Classification | No. | Skeleton | Ingredient Name | R1 | R2 | R3 | Sources | Reference |
|---|---|---|---|---|---|---|---|---|
| Phenylpropanoids | 164 | M | Acanthoside D | - | - | - | A. chinense | [48] |
| 165 | N | Syringin | - | - | - | A. macrostemon | [42] | |
| 166 | O | Allimacronoid A | Glc[(1-2)-Glc]-(1-6)-Glc | - | - | A. macrostemon | [50] | |
| 167 | Allimacronoid B | Glc(1-4)-Glc-[(1-2)-Glc]-(1-6)-Glc | - | - | A. macrostemon | [50] | ||
| 168 | Allimacronoid C | Glc(1-2)-Glc-[(1-6)-Glc]-(1-6)-Glc | - | - | A. macrostemon | [50] | ||
| 169 | Allimacronoid D | Glc-(1-2)-Glc-(1-6)-Glc | - | - | A. macrostemon | [49] | ||
| 170 | Tuberonoid A | Glc-(1-2)-Glc | - | - | A. macrostemon | [50] | ||
| 171 | 1-O-(E)-feruloyl-β--D-gentiobioside | Glc-(1-6)-Glc | - | - | A. macrostemon | [49] | ||
| 172 | 1-O-(E)-feruloyl-β-D-glucopyranoside | Glc | - | - | A. macrostemon | [49] | ||
| 173 | trans-Ferulic acid | H | - | - | A. macrostemon | [49] | ||
| Flavonoids | 174 | P | Kaempferol-3-O-β-D-glucoside | Glc | H | H | A. macrostemon | [51] |
| 175 | Kaempferol-3,7-O-β-D-diglucoside | Glc | Glc | H | A. macrostemon | [51] | ||
| 176 | Kaempferol-3,4’-O-β-D-diglucoside | Glc | H | Glc | A. macrostemon | [51] | ||
| 177 | Q | Quercetin-3-O-β-D-glucoside | - | - | - | A. macrostemon | [51] | |
| 178 | R | Isorhamnetin-3-O-β-D-glucoside | - | - | - | A. macrostemon | [51] | |
| 179 | S | Isoliquiritigenin | H | - | - | A. chinense | [14] | |
| 180 | Isoliquiritigenin-4-O-glucoside | Glc | - | - | A. chinense | [14] |
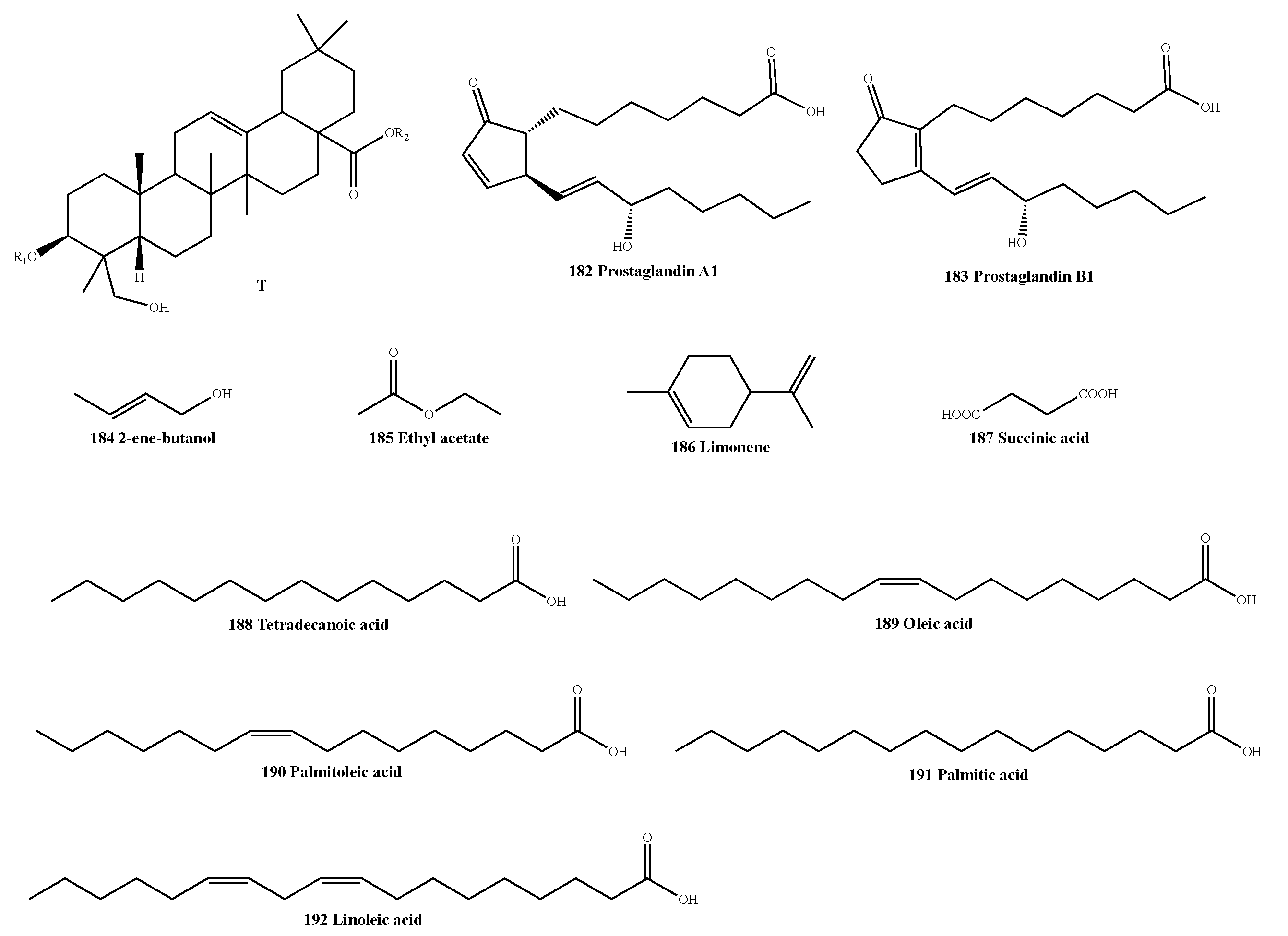
| Classification | No. | Skeleton | Ingredient Name | R1 | R2 | Sources | Reference |
|---|---|---|---|---|---|---|---|
| Others | 181 | T | (3β, 4α)-olean-12-en-28-oic acid-3-O-β-D-galactopyranosyloxy-23-hydroxy-6-O-β-D-xylopyranosyl-β-D-galactopyranosyl ester | Gal(1-4)-Xyl | Gal | A. macrostemon | [56] |
| 182 | - | Prostaglandin A1 | - | - | A. macrostemon | [55] | |
| 183 | - | Prostaglandin B1 | - | - | A. macrostemon | [55] | |
| 184 | - | 2-ene-butanol | - | - | A. chinense | [41] | |
| 185 | - | Ethyl acetate | - | - | A. chinense | [36] | |
| 186 | - | Limonene | - | - | A. chinense | [36] | |
| 187 | - | Succinic acid | - | - | A. macrostemon | [40] | |
| 188 | - | Tetradecanoic acid | - | - | A. macrostemon | [57] | |
| 189 | - | Oleic acid | - | - | A. macrostemon | [37] | |
| 190 | - | Palmitoleic acid | - | - | A. macrostemon | [37] | |
| 191 | - | Palmitic acid | - | - | A. macrostemon | [37] | |
| 192 | - | Linoleic acid | - | - | A. macrostemon | [37] |
| Pharmacological Effects | Source | Extract/Compounds | In Vivo/In Vitro | Mechanism | Models | Results | Reference |
|---|---|---|---|---|---|---|---|
| Anti-platelet aggregation effect | A. macrostemon | 161, 163 | In vitro | - | ADP induces human platelet aggregation | Compound 161 showed significant inhibition of both first-phase and second-phase platelet aggregation, while compound 163 showed inhibition of first-phase aggregation only | [62] |
| A. macrostemon | 1 | In vitro | - | ADP-induced platelet aggregation in rabbits | Strong inhibitory effect on platelet aggregation, IC50 = 0.065 mmol | [8] | |
| A. macrostemon | 55, 56 | In vitro | - | ADP induces human platelet aggregation | All these compounds strongly inhibited platelet aggregation, with IC50 = 0.417 mmol for compound 55 and IC50 = 0.020 mmol for compound 56 | [13] | |
| A. macrostemon A. chinense | 139 | In vitro | - | - | Strong inhibitory effect on platelet aggregation | [37,41] | |
| A. macrostemon A. chinense | 155, 158 | In vitro | - | - | All these compounds strongly inhibited platelet aggregation, with IC50 = 0.085 mmol for compound 155 and IC50 = 0.188 mmol for compound 158 | [42] | |
| A. chinense | 60, 61 | In vitro | - | ADP induces human platelet aggregation | Compounds 60 and 61 both prolong clotting time | [159] | |
| A. macrostemon | 10, 11 | In vitro | - | ADP or PAF induced platelet aggregation in rabbits | All these compounds strongly inhibited platelet aggregation, with IC50 = 0.078 mmol for compound 10 and IC50 = 0.082 mmol for compound 11 | [12] | |
| A. macrostemon | 31 | In vitro | - | ADP or PAF induced platelet aggregation in rabbits | Strong inhibitory effect on platelet aggregation, IC50 = 0.410 mmol | [19] | |
| A. macrostemon | 64, 65 | In vitro | Inhibition of platelet CD40L expression | ADP-induced platelet activation in rats | All these compounds were able to significantly inhibit the expression of platelet CD40L | [160] | |
| A. macrostemon | 59, 64, 65 | In vitro and in vivo | Inhibition of platelet CD40L expression | ADP-induced adhesion between human platelets and neutrophils | All of these compounds showed significant inhibition of platelet CD40L expression at a concentration of 320 μmol/L. Compound 64 at a concentration of 80 μmol/L and compounds 59 and 65 at a concentration of 320 μmol/L significantly inhibited the adhesion between platelets and neutrophils | [23] | |
| A. macrostemon | 64 | In vitro | - | ADP induces human platelet aggregation | Significantly inhibited platelet aggregation and the expression of P-selectin and integrin β-3, significantly reduced the expression of p-Akt in platelets, and inhibited calcium ion mobilization | [27] | |
| A. macrostemon | AMB saponins | In vitro and in vivo | - | AA, ADF and PAF induced platelet aggregation in rats | Inhibits platelet aggregation and reduces the concentration of calcium ions in washed platelets and adhesion between neutrophils and thrombin-activated platelets, and inhibits platelet aggregation induced by neutrophil supernatant | [61] | |
| A. macrostemon | AMB saponins | In vitro | May be related to CD40L/JNK/P38/NF-κB inflammation-related signaling pathway | ADP induces an inflammatory response in human platelet-derived extracellular vesicles | Inhibits ADP-induced inflammatory response in platelet-derived extracellular vesicles and suppresses inflammatory response in endothelial cells | [161] | |
| A. macrostemon | AMB saponins | In vitro | May act on two ADP receptors P2Y1 and P2Y12 on platelet membrane to reduce intracytoplasmic calcium ion concentration and increase CAMP content | ADP induces human platelet aggregation | AMB saponin at medium to high doses significantly inhibited platelet aggregation, and AMB saponin at 4 μmol/L significantly reduced the expression rate of CD62p in activated platelets, and the expression rate of GPIIb/IIIa was lower than that after activation | [162] | |
| A. macrostemon | AMB saponins | In vivo | Inhibition of platelet CD40L expression | Establishment of a rat model of coronary heart disease by high-fat diet feeding and injection of posterior pituitary hormone | It can inhibit platelet aggregation, prolong prothrombin time, and thrombin time, activate partial thromboplastin time and reduce plasma fibrinogen content in the arterial blood of rats | [163] | |
| A. macrostemon | 14, 62, 64, 65, 66, 69 | In vitro and in vivo | Inhibition of platelet PI3K expression and Akt phosphorylation | ADP-induced platelet aggregation in rats | All of these compounds inhibit platelet aggregation and inhibit the expansion of platelets on immobilized fibrinogen | [63] | |
| Hypolipidemic and anti-atherosclerotic effects | A. macrostemon | AMB 95% ethanol extracts | In vivo | Promotes the secretion of PGE1 | Domestic rabbits | Can increase the synthesis of PGE1 in rabbits, thus inhibiting the synthesis of TXA2, and can inhibit the formation of experimental atheromatous plaques | [74] |
| A. macrostemon | AMB aqueous extracts | In vivo | - | High-fat diet and methylthioxypyrimethane-induced hyperlipidemia in rats | Significantly reduced serum levels of TC, TG, and LDL in rats, and reduced atherosclerotic index | [164] | |
| A. macrostemon | 1 | In vitro | Increased visfatin mRNA levels in 3T3-L1 cells and mediated through P38 MAPK | 3T3-L1 cells | Compound 1 increases visfatin mRNA levels in 3T3-L1 adipocytes and significantly enhances visfatin protein expression, partly mediated by the MAPK signaling pathway | [76] | |
| A. macrostemon | 1 | In vivo | Increased total lipase activity in visceral adipocytes | High-fat diet-induced hyperglycemia and hyperlipidemia in C57BL/6 mice | Compound 1 significantly reduced serum levels of TC, TG, and LDL, and lowered blood glucose levels in mice | [77] | |
| A. chinense | AMB saponins | In vivo | - | Construction of hyperlipidemic rat model by high-fat diet feeding | It significantly reduced the levels of TC, TG, LDL, and MDA, and significantly increased the levels of HDL, GSH-Px, and SOD in the serum of rats. At the same time, the levels of LPL and HTGL in rat liver were also significantly increased and the production of fat droplets was significantly reduced | [71] | |
| A. chinense | AMB volatile oils | In vivo | - | Construction of hyperlipidemic rat model by high-fat diet feeding | Significantly reduced TC, TG, and LDL levels in serum and liver, and increased HDL levels in serum in rats, in addition to showing protective effects associated with histopathological changes in the liver | [72] | |
| A. macrostemon | 10% AMB powder | In vivo | Up-regulation of LDLR, LXRα mRNA expression levels in liver tissues | Construction of hyperlipidemic rat model by high-fat diet feeding | Significantly lowered serum TC and LDL levels and significantly increased serum HDL levels in rats | [75] | |
| XZT | AMB extracts | In vivo | Activation of RCT and increase in HDL levels | ApoE−/− mices | Significantly reduced the serum levels of FAS and LDL in mice | [79] | |
| XZT | AMB extracts | In vivo | - | Patients with hyperlipidemia | Significantly reduced TG levels in hyperlipidemic patients | [78] | |
| Protection of cardiomyocytes and vascular endothelial cells | A. macrostemon | AMB 5% ethanol extracts | In vitro | Blockade of calcium channels | Isolated rabbit aortic strips | May exert vasodilatory effects by inhibiting calcium channels activated by high potassium and NA | [165] |
| A. macrostemon | AMB extracts | In vitro | Reduces myocardial oxygen consumption | ISO-induced normoxia model in mice, acute myocardial ischemia model in rats, and myocardial ischemia-reperfusion model in rats caused by the posterior pituitary hormone | It can prolong the survival time of normoxia in mice, counteract acute myocardial ischemia in rats, and significantly protect myocardial injury caused by ischemia-reperfusion in rats | [166] | |
| A. macrostemon | AMB extracts | In vivo | Improvement of abnormal gene expression profiles in vascular lesions | Establishment of qi stagnation vascular endothelial injury model in rats fed with restraint and high methionine diet | Reduces gene expression of COX-2, COX-1, iNOS, ECE, and eNOS, and increases gene expression of antioxidant SOD, thus protecting vascular endothelium | [85] | |
| A. macrostemon | AMB extracts | In vivo | - | Establishment of qi stagnation vascular endothelial injury model in rats fed with restraint and high methionine diet | Reduces COX-2 and iNOS protein content in rat blood vessels, thereby protecting the endothelium from damage | [86] | |
| A. macrostemon | AMB extracts | In vivo | Regulation of 5-HT receptor expression | Stressed rats using restraint method | It can protect vascular endothelial function by enhancing 5-HT1D mRNA and protein expression, which mediates the diastolic effect and inhibiting 5-HT2A mRNA and protein expression, which mediates the vasoconstrictive effect | [90] | |
| A. macrostemon | AMB extracts | In vivo | Inhibition of endoplasmic reticulum stress | Establishment of qi stagnation vascular endothelial injury model in rats fed with restraint and high methionine diet | It can significantly reduce the plasma ET level, increase the serum NO level and inhibit the expression of GRP78 protein in aortic tissues, thus inhibiting the endoplasmic reticulum stress in model rats to improve their vascular endothelial function | [87] | |
| A. macrostemon | AMB ethanol extracts | In vivo | Branched-chain amino acids such as leucine, isoleucine, valine and threonine protect the heart from myocardial infarction damage | Open-chest ligation of the anterior descending branch of the left coronary artery in rats | It can regulate the balance of lipid and protein metabolism and reduce the damage caused by acute myocardial ischemia in the rat organism | [88] | |
| A. macrostemon | AMB extracts | In vivo | - | Open-chest ligation of the anterior descending branch of the left coronary artery in rats | It can increase serum GSH-Px activity, decrease TChE activity, NEFA and MDA content, and reduce the extent of myocardial damage in rats | [89] | |
| Anti-cancer effect | A. macrostemon | AMB methanol extracts | In vitro | Associated with its regulation of the EGFR/PI3K/m TOR and RAF/MAPK signaling pathways | Human non-small cell lung cancer A549 and human lung cancer cells PC-9 | Ability to significantly inhibit the proliferation of A549 and PC-9 | [13] |
| A. chinense | AMB 20% ethanol extracts | In vivo | - | Tetradecanoyl phorbol acetate (TPA) and dihydroxy methyl butyric acid induced skin cancer model and 5% glycerol and 4-Nitroquinoline-1-oxide (4NQO) induced lung cancer model in mice | It can significantly inhibit the activity of cancer cells in two models of mice | [167] | |
| A. chinense | 25, 60 | In vitro | - | - | All of these compounds have antitumor activity | [168] | |
| A. chinense | 9, 10, 11 | In vitro | Inhibition of TPA-induced phospholipid synthesis in Hela cell membranes | TPA-stimulated 32Pi-incorporation into phospholipids of HeLa cells | All of these compounds inhibited Hela cell proliferation, and in addition, compound 9 showed strong inhibitory activity against lung tumor formation induced by both 4-NQO and glycerol in an in vitro lung cancer stage 2 carcinogenesis assay | [14] | |
| A. macrostemon | AMB volatile oils | In vitro and in vivo | Enhance the immune function of tumor-bearing mice, especially the cellular immune function, which is the dominant part of tumor immunity | Mice xenograft model inoculated with mice sarcoma cells S180 | It can significantly inhibit tumor growth and increase splenic index, macrophage phagocytosis rate, and splenocyte proliferation index | [93] | |
| A. macrostemon | AMB volatile oils | In vitro and in vivo | Directly kill tumor cells by destroying nucleus and organelles, and promote the expression of cellular wtp53 gene mRNA | A mice xenograft model inoculated with mice sarcoma cells S180 and mice liver cancer cells H22 | Inhibits both S180 and H22 in vitro and in vivo, directly kills tumor cells, and induces apoptosis | [94] | |
| A. chinense | AMB extracts | In vitro | Altering the G2/M cell cycle of tumor cells | Human hepatocellular carcinoma cells HepG2 and human cervical carcinoma HeLa cells | Strong inhibitory activity against HepG2 and HeLa cells | [97] | |
| A. macrostemon | 30, 52, 63 | In vitro | - | Human neural carcinoma cells SF-268 and human large cell lung cancer cells NCI-H460 | These compounds showed good inhibition of SF-268 and NCI-H460 cell growth at 25 mg·L−1 mass concentration | [25] | |
| A. macrostemon | AMB volatile oils | In vitro | Promote the expression of P53 protein | Human gastric cancer cells SGC-7901 | Able to increase the expression of p53 protein and thus induce apoptosis in SGC-7901 cells | [95] | |
| A. macrostemon | 34, 38, 40, 52 | In vitro | - | Human neural carcinoma cell SF-268, human large cell lung cancer cell NCI-H460, human breast cancer MCF-7, human liver cancer cell HepG2 | Compounds 38 and 52 showed significant cytotoxic effects on SF-268, NCI-H460, MCF-7, and HepG2 cells, while compounds 34 and 40 had cytotoxic effects only on NCI-H460 and HepG2 cells | [21] | |
| A. macrostemon | 58, 71 | In vitro | - | Human neural carcinoma cells SF-268 and human large cell lung cancer cells NCI-H460 | Compound 58 had cytotoxic effects on both SF-268 and NCI-H460 cells, while compound 71 had cytotoxic effects on SF-268 cells only | [96] | |
| A. macrostemon | AMB saponins | In vitro | It can reduce the mitochondrial membrane potential of HeLa cells, up-regulate Bax mRNA expression, down-regulate Bcl-2 mRNA expression and Bcl-2/Bax ratio, and enhance the activity of Caspase-9 and Caspase-3 | Human cervical cancer HeLa cells | It can significantly reduce the mitochondrial membrane potential of HeLa cells, inhibit the proliferation of HeLa cells and promote their apoptosis | [98] | |
| A. macrostemon | 1 | In vitro and in vivo | Induces apoptosis by activating caspase activity, decreasing Bcl-2 expression, and inducing ROS production | A BALB/c nude mice xenograft model inoculated with human colon cancer cells SW-480 | Significantly inhibits the proliferation of SW480 cells and induces apoptosis | [99] | |
| A. chinense | AMB saponins | In vitro and in vivo | By protecting the liver and spleen of mice, thus improving their immunity and inhibiting tumor cells | C57 BL/6 mice xenograft model inoculated with mice melanoma cells B16 and mice breast cancer cells 4T1 | Inhibits the proliferation and induces apoptosis of B16 and 4T1 cells, and effectively protects the liver and spleen of mice | [100] | |
| A. chinense | A. chinense lectin | In vitro | Induced apoptosis in Hep-3B cells by upregulating the expression of caspase-3 and Bax | Human hepatocellular carcinoma cells Hep-3B | A. chinense lectin alters the morphological structure of Hep-3B and induces apoptosis | [101] | |
| A. chinense | 30, 84, 86, 88, 89 | In vitro | Induction of G2/M cell cycle arrest and apoptosis in HepG2 cells via a mitochondria-mediated pathway | Human hepatocellular carcinoma cell HepG2, human non-small cell lung cancer A549, human lung adenocarcinoma cell SPC-A-1, human gastric cancer cell MGC80-3, human breast cancer cell MDA-MB-231, human colon cancer cell SW620 and human nasopharyngeal cancer cell CNE-1 | Inhibited all 7 types of cancer cells, but compound 84 only weakly inhibited HepG2 and CNE-1 | [30] | |
| Antibacterial effect | A. macrostemon | AMB aqueous extracts | In vitro | - | Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, Escherichia coli, Salmomella sp, Pseudomonas aeruginosa | Inhibition ability in the order of Staphylococcus aureus > Bacillus subtilis > Bacillus cereus > Escherichia coli > Pseudomonas aeruginosa > Salmomella sp | [102] |
| A. chinense | AMB extracts | In vitro | - | Candida albicans | Dimethyl trisulfide (135) 25.46% and methyl cis-1-propenyl disulfide (115) 14.69% higher content and better bacterial inhibitory effect | [169] | |
| A. chinense | AMB extracts | In vitro | Altered cell wall structure by disrupting the glycosidic bond of β-(1-3)-D glucan in the cell wall of Candida albicans | Candida albicans | It can inhibit the acidification of Candida albicans medium and cause the leakage of cellular OD260nm substance, thus inhibiting its reproduction | [105] | |
| A. macrostemon | AMB fresh juice | In vitro | - | Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Proteus vulgaris, Enterobacter aerogenes, Alicrococcus tetragenus, Sarcina, Brewer’s yeast, Ranunculus repens, Aspergillus oryzae, Penicillium citrinum, Trichoderma viride | The activity of antibacterial substances in the bulbs was higher than that of the above-ground parts, and in addition, the fresh juice of AMB had a significant inhibitory effect on both Gram-negative and positive bacteria, and on the spore germination of the test mycobacteria | [170] | |
| A. macrostemon | AMB 75% ethanol extracts | In vitro | - | Staphylococcus aureus, Escherichia coli, Penicillium sp, Aspergillus niger, Saccharomyces cerevisiae | The order of inhibition effect: Escherichia coli > Staphylococcus aureus > Penicillium sp > Saccharomyces cerevisiae > Aspergillus niger | [103] | |
| A. chinense | AMB saponins, AMB 30% and 60% ethanol extracts | In vitro | By reducing the utilization of glucose by bacteria, it affects the growth and reproduction of bacteria, reduces the activity of some key enzymes required for physiological metabolism, and thus inhibits the synthesis of related proteins. | Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, Tritirachium album, Saccharomycete | AMB saponins inhibited Saccharomycete, Tritirachium album, and Staphylococcus aureus, while the utilization of glucose by the above three bacteria treated with AMB saponins and AMB alcohol extracts was reduced, peroxidase activity was inhibited, and the total protein content of the bacteria decreased or even disappeared | [104] | |
| Anti-asthmatic effect | A. macrostemon | AMB extracts | In vivo | - | Asthma model in guinea pigs by phosphate-histamine spray | AMB extract prolonged the latency period of asthma in guinea pigs, and the panting effect was enhanced with an increasing dose | [119] |
| A. macrostemon | AMB saponins | In vitro | - | Histamine-induced constriction of isolated guinea pig tracheal lamellae model | Significantly relaxed histamine-induced spasm in isolated guinea pig bronchial smooth muscle | [120] | |
| A. macrostemon | AMB extracts | In vivo | Relieves chronic inflammation by suppressing the inflammatory response, which in turn relieves the spasticity of bronchial smooth muscle | Ultrasonic nebulization with 1% ovalbumin solution to produce an asthma model in guinea pigs | It can reduce the expression level of IL-6 and TXB2 and up-regulate the expression level of 6-Keto-PGF1α in the serum of asthmatic guinea pigs, thus achieving the effect of calming asthma | [118] | |
| Antioxidant effect | A. macrostemon | AMB extracts | In vivo | Increase the activity of antioxidant enzymes and promote the scavenging of free radicals | Rat model of liquor-induced oxidative stress | It can increase the activity of serum SOD and CAT in rats, has a protective effect on T lymphocytes, and significantly inhibits the formation of serum lipid peroxide | [138] |
| A. macrostemon | AMB saponins | In vitro | - | - | It can effectively scavenge DPPH, O2- and ·OH, and the antioxidant capacity of saponin components in AMB leaves is stronger than that of saponin components in bulbs | [139] | |
| A. macrostemon | AMB polysaccharides | In vitro | - | - | Sulfation modification of AMB polysaccharides by chlorosulfate-pyridine method can improve their in vitro antioxidant activity | [140] | |
| A. macrostemon | AMB polysaccharides | In vitro | - | - | Modification of AMB polysaccharides with α-amylase enhances their in vitro antioxidant activity | [141] | |
| A. macrostemon | AMB polysaccharides | In vitro | - | - | Relatively strong scavenging ability of AMB polysaccharide for ·OH | [142] | |
| A. macrostemon | Sulfur-containing compounds in AMB | In vitro and in vivo | Increase the activity of antioxidant enzymes and promote the scavenging of free radicals | Paraquat-methyl-14C induces oxidative stress in Cryptobacterium hidradenum | In vitro, the sulfur-containing compounds in AMB can effectively scavenge DPPH and ·OH and prevent the oxidation of Fe2+; in vivo, these sulfur-containing compounds can enhance the activity of SOD, GSH-Px, and CAT, thus promoting the scavenging of free radicals | [143] | |
| Antidepressant effect | A. macrostemon | AMB aqueous extracts | In vivo | Promotes neurogenesis and BDNF release | Construction of a mice depression model using the behavioral desperation method of tail suspension and forced swimming | Ability to reduce immobility time and promote neurogenesis and BDNF expression levels in forced swim test and hanging tail test model mice | [151] |
| A. macrostemon | AMB saponins | In vivo | Regulate the balance of the internal environment of depression model animals, such as hormone levels, at the same time, can significantly improve the pathological changes of related organs and tissues | Construction of a mice depression model using the behavioral desperation method of tail suspension and forced swimming; a mice depression model induced by intraperitoneal injection of reserpine; a rat model of chronic unpredictable depression by a 21-day chronic mild stimulation method | It can improve the tail suspension and swimming immobility time in mice with behavioral despair depression model, and also improve the body temperature decrease in mice with lisinopril depression model; in rats with chronic unpredictable depression model, it can also significantly improve the content of monoamine neurotransmitters 5-HT, NE, etc. in brain homogenate and serum corticosterone, adrenocorticotropic hormone levels, and improve the body immune function, and thymus, spleen, adrenal gland and hypothalamic nerve cell lesions | [150] | |
| A. macrostemon | AMB aqueous extracts | In vivo | - | Chronic stationary stress constructs a depression model in rats | Restores to normal levels several lysophosphatidylcholines and most medium and long chain acylcarnitines, phosphatidylcholines, and triglycerides that are abnormally altered in the plasma of depressed rats | [152] | |
| Other pharmacological effects | A. macrostemon | AMB aqueous extracts | In vivo | - | Chemically and thermally induced pain mice model, NaNO2 poisoning and ISO-induced hypoxia mice model | Reduces the number and duration of writhing and foot-licking responses in model mice, and prolongs the duration of hypoxia tolerance in mice | [154] |
| A. macrostemon | AMB extracts | In vivo | - | Non-specific and specific immune mice models were constructed by intravenous injection of ink and intraperitoneal injection of sheep red blood cells, respectively | It can increase the weight of the spleen and thymus, increase the carbon particle contouring index K and phagocytosis index α | [155] | |
| A. macrostemon | AMB aqueous extracts | In vivo | - | - | Significantly reduces the content of cytochrome P450 in mice, and has a significant inhibitory effect on hepatic drug enzymes | [157] | |
| A. macrostemon | AMB volatile oils, 113, 135 | In vivo | - | - | All of these have a strong killing effect on Aedes albopictus larvae | [158] | |
| A. macrostemon | AMB 30% ethanol extracts | In vivo | Regulation of bone formation and absorption | - | Increased expression of insulin-like growth factor-1 and bone morphogenetic protein-2, resulting in increased bone growth | [156] | |
| A. macrostemon | AMB aqueous extracts | In vitro and in vivo | Suppression of Nav1.7 channels | Chemically induced and thermally induced pain mice models | Reduces the number and duration of writhing and foot-licking responses in model mice and decreases the excitability of dorsal root ganglia by inhibiting Nav1.7 channels | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wang, L.; Cui, Y.; Liu, F.; Zhang, J. Allii Macrostemonis Bulbus: A Comprehensive Review of Ethnopharmacology, Phytochemistry and Pharmacology. Molecules 2023, 28, 2485. https://doi.org/10.3390/molecules28062485
Wu J, Wang L, Cui Y, Liu F, Zhang J. Allii Macrostemonis Bulbus: A Comprehensive Review of Ethnopharmacology, Phytochemistry and Pharmacology. Molecules. 2023; 28(6):2485. https://doi.org/10.3390/molecules28062485
Chicago/Turabian StyleWu, Jianfa, Lulu Wang, Ying Cui, Fei Liu, and Jing Zhang. 2023. "Allii Macrostemonis Bulbus: A Comprehensive Review of Ethnopharmacology, Phytochemistry and Pharmacology" Molecules 28, no. 6: 2485. https://doi.org/10.3390/molecules28062485
APA StyleWu, J., Wang, L., Cui, Y., Liu, F., & Zhang, J. (2023). Allii Macrostemonis Bulbus: A Comprehensive Review of Ethnopharmacology, Phytochemistry and Pharmacology. Molecules, 28(6), 2485. https://doi.org/10.3390/molecules28062485






