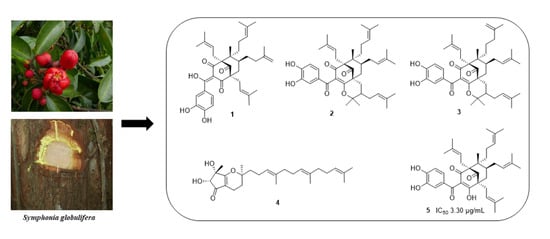
Constituents of the Stem Bark of Symphonia globulifera Linn. f. with Antileishmanial and Antibacterial Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
2.2. Antileishmanial and Antibacterial Activities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material and Identification
3.3. Extraction and Isolation
3.3.1. Guttiferone U (1)
3.3.2. Guttiferone V (2)/Guttiferone W (3)
3.3.3. Globuliferanol (4)
3.4. Antileishmanial and Cytotoxicity Assays
3.5. Antibacterial Bioassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Ngouateu, O.M.; Dondji, B. Leishmaniasis in Cameroon and neighboring countries: An overview of current status and control challenges. Curr. Res. Parasitol. Vector-Borne Dis. 2022, 2, 100077. [Google Scholar] [CrossRef] [PubMed]
- Uliana, S.R.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of leishmaniasis: Present challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global leishmaniasis surveillance, 2017–2018, and first report on five additional indicators. Wkly. Epidemiol. Rec. 2020, 25, 265–280. [Google Scholar]
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where we are and where we are heading? Parasitol. Res. 2021, 120, 151–1554. [Google Scholar] [CrossRef]
- Yimer, M.; Nibret, E.; Yismaw, G. Updates on prevalence and trends status of visceral leishmaniasis at two health facilities in amhara regional state, northwest Ethiopia: A retrospective study. Biochem. Res. Int 2022, 2022, 3603892. [Google Scholar] [CrossRef]
- Kaye, P.M.; Mohan, S.; Mantel, C.; Malhame, M.; Revill, P.; Rutte, E.L.; Parkash, V.; Layton, A.M.; Lacey, C.J.N.; Malvoti, S. Overcoming roadblocks in the development of vaccines for leishmaniasis. Exp. Rev. Vacc. 2021, 20, 1419–1430. [Google Scholar] [CrossRef]
- Kitano, H.; Sanjoba, C.; Goto, Y.; Iwamoto, Y.; Kitagawa, K.; Nomura, T.; Shigemoto, N.; Hide, M.; Matsumoto, Y.; Ohge, H. Complicated cutaneous leishmaniasis caused by an imported case of Leishmania tropica in Japan: A case report. Trop. Med. Health 2021, 49, 20. [Google Scholar] [CrossRef]
- Mann, S.; Frasca, K.; Scherre, S.; Henao-Martinez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A review of leishmaniasis: Current knowledge and future directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Gonzalez, D.; Blanco, O.; Fraga, J.; Capo, V.; Herrera, A.; Montalvo, A.M. Imported cases of cutaneous leishmaniasis in Cuba, 2017: Role of human movement. Trop. Dis. Travel Med. Vaccines. 2022, 8, 15. [Google Scholar] [CrossRef]
- WHO. Ending the Neglected to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021−2030; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Freitas-Junior, L.H.; Chatelain, E.; Kim, H.A.; Siqueira-Neto, J.L. Visceral leishmaniasis treatment: What do we have, what do we need and how to deliver it? Int. J. Parasitol. Drugs Drug. Resist. 2012, 2, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Fotie, J.; Bohle, D.S.; Olivier, M.; Gomez, M.A.; Nzimiro, S. Trypanosomial and antileishmanial dihydrochelerythrine derivatives from Garcinia lucida. J. Nat. Prod. 2007, 70, 1650–1653. [Google Scholar] [CrossRef] [PubMed]
- Lenta, B.N.; Vonthron-Sénécheau, C.; Weniger, B.; Devkota, K.P.; Ngoupayo, J.; Kaiser, M.; Sewald, N. Leishmanicidal and cholinesterase inhibiting activities of phenolic compounds from Allanblackia monticola and Symphonia globulifera. Molecules 2007, 12, 1548–1557. [Google Scholar] [CrossRef]
- Azebaze, A.G.B.; Ouahouo, B.M.W.; Vardamides, J.C.; Valentin, A.; Kuete, V.; Acebey, L.; Meyer, M. Antimicrobial and antileishmanial xanthones from the stem bark of Allanblackia gabonensis (Guttiferae). Nat. Prod. Res. 2008, 22, 333–341. [Google Scholar] [CrossRef]
- Garba, J.K.; Nguengang, R.T.; Youmbi, G.T.; Menache, J.N.; Ngansop, C.A.N.; Bankeu, J.J.K.; Chouna, J.R.; Boyom, F.F.; Sewald, N.; Lenta, B.N. Antileishmanial, antibacterial and cytotoxicity activity of the extracts, fractions, and compounds from the fruits and stem bark of Pentadesma butyraceae Sabine. Z. Naturforsch. B 2022, 77, 9–15. [Google Scholar] [CrossRef]
- Dick, C.W.; Heuertz, M. The complex biogeographic history of widespread tropical tree species. Evolution 2008, 62, 2760–2774. [Google Scholar] [CrossRef]
- Marti, G.; Eparvier, V.; Moretti, C.; Prado, S.; Grellier, P.; Hue, N.; Thoison, O.; Delpech, B.; Gueritte, F.; Litaudon, M. Antiplasmodial benzophenone derivatives from the root barks of Symphonia globulifera (Clusiaceae). Phytochemistry 2010, 71, 964–997. [Google Scholar] [CrossRef]
- Ssegawa, P.; Kasenene, J.M. Medicinal plant diversity and uses in the Sango bay area, southern Uganda. J. Ethnopharmacol. 2007, 113, 521–540. [Google Scholar] [CrossRef]
- Fromentin, Y.; Cottet, K.; Kritsanida, M.; Mihel, S.; Gaboriaud-Kolar, N.; Lallemand, M.C. Symphonia globulifera, a widespread source of complex metabolites with potent biological activities. Planta Med. 2014, 81, 95–107. [Google Scholar] [CrossRef]
- Majekodunmi, S.O.; Aliga, U.L. A systematic study on flow ability and compressibility of Symphonia globulifera stem bark powder for tablet dosage form. Am. J. Biomed. Eng. 2017, 7, 1–8. [Google Scholar]
- Irvine, F.R. Woody Plants of Ghana with Special Reference to Their Uses; Oxford University Press: London, UK, 1961; pp. 143–144. [Google Scholar]
- Gupta, M.P.; Solis, P.N.; Calderon, A.I.; Guinneau-Sinclair, F.; Correa, M.; Galdames, C.; Ocampo, R. Medical ethnobotany of the tribes of bocas del toro Panama. J. Ethnopharmacol. 2005, 96, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Hudson, J.B.; Towers, G.H.N. Antiviral and antimicrobial activities of Colombian medicinal plants. J. Ethnopharmacol. 2001, 77, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Nkengfack, A.E.; Mkounga, P.; Meyer, M.; Fomum, Z.T.; Bodo, B. Globulixanthones C, D and E: Three prenylated xanthones with antimicrobial properties from the root bark of Symphonia globulifera. Phytochemistry 2002, 61, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Mkounga, P.; Fomum, Z.T.; Meyer, M.; Bodo, B.; Nkemgfack, A.E. Globulixanthone F, a new polyoxygenated xanthone with an isoprenoid group and two antimicrobial biflavonoids from the stem bark of Symphonia globulifera. Nat. Prod. Commun. 2009, 4, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Fromentin, Y.; Gaboriaud-Kolar, N.; Lenta, B.N.; Wansi, J.D.; Buisson, D.; Mouray, E.; Michel, S. Synthesis of novel guttiferone A derivative: In-vitro evaluation toward Plasmodium falciparum, Trypanosoma brucei and Leishmania donovani. Eur. J. Med. Chem. 2013, 65, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Brodie, P.J.; Miller, J.S.; Ratovoson, F.; Birkinshaw, C.; Randrianasolo, S.; Kingston, D.G. Guttiferones. K and L, antiproliferative compounds of Rheedia calcicola from the Madagascar rainforest. J. Nat. Prod. 2007, 70, 686–688. [Google Scholar] [CrossRef]
- Ngouela, S.; Lenta, B.N.; Noungoue, D.T.; Ngoupayo, J.; Boyom, F.F.; Tsamo, E.; Connolly, J.D. Antiplasmodial and antioxidant activities of constituents of the seed shells of Symphonia globulifera Linn f. Phytochemistry 2006, 67, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Botta, B.; Monachè, D.F.; Monache, D.G.; Kabangu, K. Acetylvismione D from Psorospermum febrifugum. Phytochemistry 1986, 25, 766. [Google Scholar] [CrossRef]
- Cao, S.G.; Lim, T.B.; Sim, K.Y.; Goh, S.H. A highly prenylated xanthone from the bark of Calophyllum gracilipes (Guttiferae). Nat. Prod. Lett. 1997, 10, 55–58. [Google Scholar] [CrossRef]
- Lenta, B.N.; Ngouela, S.; Noungoue, D.T.; Tsamo, E.; Connolly, J.D. Symphonin: A new prenylated pyranoxanthone with antimicrobial activity from the seeds of Symphonia globulifera (Guttiferae). Bull. Chem. Soc. Ethiop. 2004, 18, 175–180. [Google Scholar] [CrossRef]
- Tosa, H.; Iinuma, M.; Murakami, K.I.; Ito, T.; Tanaka, T.; Chelladurai, V.; Riswan, S. Three xanthones from Poeciloneuron pauciflorum and Mammea acuminate. Phytochemistry 1997, 45, 133–136. [Google Scholar] [CrossRef]
- Ahmed, Y.; Rahman, S.; Akhtar, P.; Islam, F.; Rahman, M.; Yaakob, Z. Isolation of steroids from n-hexane extract of the leaves of Saurauia roxburghii. Int. Food. Res. J. 2013, 20, 2939–2943. [Google Scholar]
- Xiao, Z.P.; Wu, H.K.; Wu, T.; Shi, H.; Hang, B.; Aisa, H.A. Kaempferol and quercetin flavonoids from Rosa rugose. Chem. Nat. Comp. 2006, 42, 736–737. [Google Scholar] [CrossRef]
- Silva, A.T.M.; Magalhaes, C.G.; Duarte, L.P.; Mussel, W.N.; Ruiz, A.L.T.G.; Shiozawa, L.; Carvalho, J.E.; Trindade, C.T.; Filho, S.A.V. Lupeol and its esters: NMR, powder XRD data and in vitro evaluation of cancer cell growth. Braz. J. Pharm. Sci. 2017, 53, e00251. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Anticancer properties and mechanism of action of oblongifolin C, guttiferone K and related poplyprenylated acylphloroglucinols. Nat. Prod. Bioprospect. 2021, 11, 629–641. [Google Scholar] [CrossRef]
- Gustafson, K.R.; Blunt, J.W.; Munro, M.H.; Fuller, R.W.; McKee, T.C.; Cardellina II, J.H.; Boyd, M.R. The guttiferones, HIV-inhibitory benzophenones from Symphonia globulifera, Garcinia livingstonei, Garcinia ovalifolia and Clusia rosea. Tetrahedron 1992, 48, 10093–10102. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Nguyen, T.T.; Duong, T.H.; Tran, N.M.A.; Nguyen, C.H.; Nguyen, T.H.A.; Sichaem, J. α-Glucosidase inhibitory and antimicrobial benzoylphloroglucinols from Garcinia schomburgakiana fruits: In vitro and in silico studies. Molecules 2022, 27, 2574. [Google Scholar] [CrossRef]
- Ohnmacht, S.; West, R.; Simionescu, R.; Atkinson, J. Assignment of the 1H and 13C NMR of tocotrienols. Magn. Reson. Chem. 2008, 46, 287–294. [Google Scholar] [CrossRef]
- Zeutsop, J.F.; Zebaze, N.J.; Nono, R.; Frese, M.; Chouna, J.R.; Lenta, N.B.; Nkeng-Efouet-Alango, P.; Sewald, N. Antioxydant and cytotoxicity activities of δ-tocotrienol from the seed of Allophylus africanus. Nat. Prod. Res. 2022, 36, 4661–4671. [Google Scholar] [CrossRef]
- Collakova, E.; DellaPenna, D. Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol. 2001, 127, 1113–1124. [Google Scholar] [CrossRef]
- Suffredini, I.B.; Paciencia, M.L.B.; Díaz, I.E.; Frana, S.A.; Bernardi, M.M. Mice behavioral phenotype changes after administration of Anani (Symphonia globulifera, Clusiaceae), an alternative Latin American and African medicine. Pharmacogn. Mag. 2017, 13, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Téné, D.G.; Tih, A.E.; Kamdem, M.H.K.; Talla, R.M.; Diboue, P.H.B.; Melongo, Y.K.D.; Ghogomu, R.T. Antibacterial and antioxidant activities of compounds isolated from the leaves of Symphonia globulifera (Clusiaceae) and their chemophenetic significance. Biochem. Syst. Ecol. 2021, 99, 104345. [Google Scholar] [CrossRef]
- Tan, X.; Han, X.; Teng, H.; Li, Q.; Chen, Y.; Lei, X.; Yang, G. Structural elucidation of garcipaucinones A and B from Garcinia paucinervis using quantum chemical calculations. J. Nat. Prod. 2021, 84, 972–978. [Google Scholar] [CrossRef]
- Fuentes, R.G.; Pearce, K.C.; Du, Y.; Rakotondrafara, A.; Valenciano, A.L.; Cassera, M.B.; Kingston, D.G. Phloroglucinols from the roots of Garcinia dauphinensis and their antiproliferative and antiplasmodial activities. J. Nat. Prod. 2018, 82, 431–439. [Google Scholar] [CrossRef]
- Marques, E.D.J.; Ferraz, C.G.; dos Santos, I.B.; dos Santos, I.I.; El-Bachá, R.S.; Ribeiro, P.R.; Cruz, F.G. Chemical constituents isolated from Clusia criuva subsp. Criuva and their chemophenetics significance. Biochem. Sys. Ecol. 2021, 97, 104293. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Kuete, V.; Nguemeving, J.R.; Penlap, B.V.; Nkengfack, A.E.; Meyer, J.M.; Krohn, K. Antimicrobial activity of the extracts and compounds obtained from Vismia guineensis (Guttiferae). Asian J. Tradit. Med. 2008, 3, 211–223. [Google Scholar]
- Iinuma, M.; Tosa, H.; Tanaka, T.; Kanamaru, S.; Asai, F.; Kobayashi, Y.; Shimano, R. Antibacterial activity of some Garcinia benzophenone derivatives against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 1996, 19, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Siqueira-Neto, J.L.; Song, O.R.; Oh, H.; Sohn, J.H.; Yang, G.; Nam, J.; Jang, J.; Cechetto, J.; Lee, C.B.; Moon, S.; et al. Antileishmanial high-throughput drug screening reveals drug candidates with new Scaffolds. PLoS Negl. Trop. Dis. 2010, 4, e675. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]


| Position | (1) | (2) | (3) | |||
|---|---|---|---|---|---|---|
| δC | δH (m, J (Hz)) | δC | δH (m, J (Hz)) | δC | δH (m, J (Hz)) | |
| 1 | 196.5 | 170.3 | 170.5 | |||
| 2 | 116.1 | 125.6 | 125.9 | |||
| 3 | 191.9 | 193.4 | 193.6 | |||
| 4 | 70.3 | 68.9 | 69.0 | |||
| 5 | 51.8 | 48.3 | 48.4 | |||
| 6 | 39.7 | 1.87 (m) | 39.4 | 1.88 (m) | 39.4 | 1.86 (m) |
| 7 | 43.3 | 2.05 (m) | 38.1 | 2.31 (m) | 38.3 | 2.28 (m) |
| 8 | 57.8 | 51.2 | 51.5 | |||
| 9 | 208.8 | 206.2 | 206.3 | |||
| 10 | 193.9 | 191.2 | 191.3 | |||
| 11 | 132.0 | 130.3 | 130.4 | |||
| 12 | 116.6 | 7.23 (d, 2.2) | 114.7 | 7.39 (d, 2.0) | 114.9 | 7.38 (d, 2.0) |
| 13 | 144.8 | 144.8 | 144.9 | |||
| 14 | 150.9 | 150.2 | 150.3 | |||
| 15 | 114.2 | 6.81 (d, 8.3) | 114.4 | 6.85 (d, 8.2) | 114.6 | 6.82 (d, 8.2) |
| 16 | 123.9 | 7.06 (dd, 8.3, 2.1) | 122.7 | 7.14 (dd, 8.2, 2.0) | 123.0 | 7.07 (dd, 8.2, 2.0) |
| 17 | 25.2 | 2.68 (m) | 24.9 | 2.59 (m) | 24.9 | 2.54 (m) |
| 18 | 120.7 | 5.15 (brs) | 120.5 | 4.95 (m) | 120.6 | 4.95 (m) |
| 19 | 134.2 | 133.1 | 133.3 | |||
| 20 | 25.2 | 1.71 (s) | 25.4 | 1.56 (brs) | 25.5 | 1.56 (brs) |
| 21 | 17.5 | 1.65 (s) | 17.3 | 1.70 (brs) | 17.4 | 1.70 (brs) |
| 22 | 18.1 | 1.16 (s) | 17.9 | 1.18 (brs) | 17.9 | 1.18 (brs) |
| 23 | 35.0 | 1.28 (m)/1.31 (m) | 35.1 | 1.21 (m)/1.43 (m) | 35.3 | 1.21 (m)/1.43 (m) |
| 24 | 28.4 | 2.05 (m) | 28.8 | 2.80 (m) | 28.9 | 2.80 (m) |
| 25 | 35.4 | 1.91 (m) | 125.5 | 5.02 (m) | 125.5 | 5.02 (m) |
| 26 | 145.6 | 132.2 | 132.2 | |||
| 27 | 21.9 | 1.70 (brs) | 25.2 | 1.71 (brs) | 25.2 | 1.71 (brs) |
| 28 | 109.4 | 4.66 (brs) | 17.6 | 1.71 (brs) | 17.6 | 1.71 (brs) |
| 29 | 28.4 | 2.05 (m) | 28.3 | 3.00 (d, 3.3)/ 3.05 (dd, 14.0, 3.3) | 28.3 | 3.00 (d, 3.3)/ 3.05 (dd, 14.0, 3.3) |
| 30 | 123.9 | 5.06 (m) | 41.8 | 1.10 (m) | 42.9 | 1.10 (m) |
| 31 | 134.2 | 86.2 | 86.5 | |||
| 32 | 25.1 | 1.65 (brs) | 27.9 | 0.89 (brs) | 28.0 | 0.89 (brs) |
| 33 | 17.2 | 1.53 (brs) | 20.6 | 1.27 (brs) | 20.7 | 1.29 (brs) |
| 34 | 22.7 | 1.91 (m) | 29.2 | 2.10 (brs) | 29.3 | 2.10 (brs) |
| 35 | 124.3 | 5.11 (m) | 122.0 | 5.22 (m) | 122.0 | 5.22 (m) |
| 36 | 131.2 | 132.9 | 132.9 | |||
| 37 | 25.0 | 1.68 (s) | 25.0 | 1.77 (brs) | 25.1 | 1.77 (brs) |
| 38 | 16.9 | 1.60 (brs) | 17.11 | 1.63 (brs) | 17.2 | 1.63 (brs) |
| 39 | 22.4 | 1.94 (m) | 22.4 | 1.94 (m) | ||
| 40 | 124.2 | 5.12 (m) | 35.4 | 1.21 (m) | ||
| 41 | 131.2 | 145.0 | ||||
| 42 | 24.9 | 1.67 (brs) | 21.5 | 1.74 (brs) | ||
| 43 | 16.7 | 1.60 (brs) | 110.4 | 4.79 (brs) |
| Position | 13C | 1H |
|---|---|---|
| δC | δH (m, J (in Hz)) | |
| 2 | 82.2 | |
| 3 | 29.9 | 1.73 (dd, 14.0, 6.2)/1.22 (m) |
| 4 | 13.7 | 2.03 (m) |
| 5 | 198.8 | |
| 6 | 80.9 | 3.90 (d, 6.3) |
| 7 | 75.9 | |
| 8 | 181.6 | |
| 9 | 109.5 | |
| 10 | 21.4 | 1.17 (s) |
| 11 | 24.1 | 1.28 (s) |
| 12 | 38.3 | 1.63 (m)/1.53 (brs) |
| 1′ | 21.9 | 2.03 (m) |
| 2′ | 124.2 | 5.12 (t, 7.1) |
| 3′ | 135.2 | |
| 4′ | 39.5 | 2.03 (m) |
| 5′ | 26.6 | 2.03 (m) |
| 6′ | 124.3 | 5.06 (m) |
| 7′ | 134.8 | |
| 8′ | 39.6 | 2.03 (m)/1.93 (q, 6.3) |
| 9′ | 26.4 | 2.04 (m, 2H) |
| 10′ | 124.5 | 5.06 (m) |
| 11′ | 131.0 | |
| 12′ | 16.22 | 1.58 (m) |
| 13′ | 16.25 | 1.55 (s) |
| 14′ | 18.0 | 1.55 (s) |
| 15′ | 25.9 | 1.63 (s) |
| OH-6 | - | 5.53 (d, 6.5) |
| OH-7 | - | 5.32 (s) |
| Extracts/ Compounds | Antileishmanial Activity IC50 ± SD (μg/mL) | Macrophages CC50 ± SD (μg/mL) | Selectivity Index SI ± SD (=CC50/IC50) |
|---|---|---|---|
| ME | >100 | ||
| HF | 43.11 ± 0.01 | >20 | |
| EF | >100 | ||
| BF | >100 | ||
| 1 | 12.91 ± 1.11 | 28.06 ± 5.72 | 2.17 |
| 2 and 3 | 12.13 ± 1.08 | 9.60 ± 0.26 | 0.79 |
| 4 | 14.03 ± 1.14 | >20 | >1.39 |
| 5 | 3.30 ± 0.51 | 5.20 ± 0.02 | 1.57 |
| 6 | 15.97 ± 1.20 | >20 | >1.25 |
| 7 | 12.91 ± 1.11 | >20 | >1.54 |
| 8 | 12.91 ± 1.11 | >20 | >1.54 |
| 9 | ND | ND | |
| 10 | 47.04 ± 1.67 | >20 | >0.42 |
| Amphotericin B | 0.048 |
| Extracts/ Compounds | Antibacterial Activity (MIC in µg.mL−1) | ||||||
|---|---|---|---|---|---|---|---|
| St | Se | Sa | Sau | Kpc | Kp | Pa | |
| ME | 250 | - | 250 | 250 | 250 | 1000 | 125 |
| HF | 15.7 | 62.5 | 250 | 62.5 | 31.2 | 125 | 31.2 |
| EF | 62.5 | 500 | 1000 | 31.2 | 31.2 | 500 | 31.2 |
| BF | 500 | - | - | 500 | 1000 | 1000 | 500 |
| 1 | 3.9 | 62.5 | 125 | 3.9 | 3.9 | 62.5 | 3.9 |
| 2 and 3 | 15.6 | 62.5 | 500 | 62.5 | 15.6 | 62.5 | 15.6 |
| 4 | 31.2 | 125 | 500 | 62.5 | 31.2 | 62.5 | 31.2 |
| 5 | 3.9 | 125 | 250 | 31.2 | 3.9 | 62.5 | 3.9 |
| Gentamycin | 0.048 | 0.07 | 0.07 | 0.03 | 0.048 | 0.07 | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguengang, R.T.; Tchegnitegni, B.T.; Nono, E.C.N.; Bellier Tabekoueng, G.; Fongang, Y.S.F.; Bankeu, J.J.K.; Chouna, J.R.; Nkenfou, C.N.; Fekam, F.B.; Sewald, N.; et al. Constituents of the Stem Bark of Symphonia globulifera Linn. f. with Antileishmanial and Antibacterial Activities. Molecules 2023, 28, 2473. https://doi.org/10.3390/molecules28062473
Nguengang RT, Tchegnitegni BT, Nono ECN, Bellier Tabekoueng G, Fongang YSF, Bankeu JJK, Chouna JR, Nkenfou CN, Fekam FB, Sewald N, et al. Constituents of the Stem Bark of Symphonia globulifera Linn. f. with Antileishmanial and Antibacterial Activities. Molecules. 2023; 28(6):2473. https://doi.org/10.3390/molecules28062473
Chicago/Turabian StyleNguengang, Ruland Tchuinkeu, Billy Toussie Tchegnitegni, Eric Carly Nono Nono, Georges Bellier Tabekoueng, Yannick Stéphane Fotsing Fongang, Jean Jules Kezetas Bankeu, Jean Rodolphe Chouna, Céline Nguefeu Nkenfou, Fabrice Boyom Fekam, Norbert Sewald, and et al. 2023. "Constituents of the Stem Bark of Symphonia globulifera Linn. f. with Antileishmanial and Antibacterial Activities" Molecules 28, no. 6: 2473. https://doi.org/10.3390/molecules28062473
APA StyleNguengang, R. T., Tchegnitegni, B. T., Nono, E. C. N., Bellier Tabekoueng, G., Fongang, Y. S. F., Bankeu, J. J. K., Chouna, J. R., Nkenfou, C. N., Fekam, F. B., Sewald, N., & Lenta, B. N. (2023). Constituents of the Stem Bark of Symphonia globulifera Linn. f. with Antileishmanial and Antibacterial Activities. Molecules, 28(6), 2473. https://doi.org/10.3390/molecules28062473