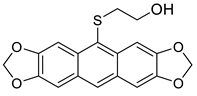
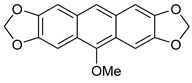
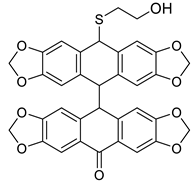
Abstract
This paper presents the use of O,S-acetals in a new modification of the oxo-Friedel–Crafts–Bradsher cyclization. In this reaction, under mild reaction conditions (25 °C), three- and four-ring fused RO-acenes (major) and/or HO(CH2)2S-acenes (minor) are formed, the latter products having never been observed before in this type of cyclization. In this way, two electronically different fluorophores could be obtained in a single cyclization reaction, one of them having strong electron donor properties (+M effect of alkoxy groups) and the other having donor-acceptor properties (+M and −I effects of the HO(CH2)2S-group, Hammett’s constants). Further increasing the reaction temperature, HCl concentration or prolonging reaction time, surprisingly, yielded a 2:1 mixture of cis and trans dimeric isomers, as the only products of this cyclization. The DFT calculations confirmed a greater stability of the cis isomer compared to the trans isomer. The formation of unexpected dimeric products and HO(CH2)2S-acenes sheds light on the mechanism of oxo-Friedel–Crafts–Bradsher cyclization, involving competitive O/S atom protonation in strained O,S-acetals and in strain-free side groups of intermediate species.
1. Introduction
Organic electronics and optoelectronics are relatively new fields of basic knowledge and technology, which have become a subject of interest to chemists, physicists and process engineers [1]. Therefore, a search for organic fluorescent and semiconducting materials for the construction of new-generation electronic devices, such as organic light-emitting diodes (OLEDs), organic field-effect transistors (OFETs), organic solar cells (OPVs), organic solar concentrators (OSCs), organic lasers, etc., has drawn the attention of numerous multidisciplinary joint laboratories [2]. Among aromatic hydrocarbons, linearly fused acenes are being considered as key organic compounds for achieving these goals. Anthracene and its derivatives are particularly attractive due to high thermal stability [3], relatively good solubility, low price, blue photoluminescent [4] and electroluminescent properties [5]. Many blue-light-emitting materials with an anthracene core structure [6,7,8,9,10,11,12,13,14,15,16,17,18] have been developed; however, deep blue is still in demand due to the lack of electrically and photochemically stable light-emitting materials [19,20].
In the literature, examples of intramolecular cyclizations of o-formyl [21], o-acyl [22] and o-carboxy [23] diarylmethanes as well as o-carboxy [24,25] diarylketones, leading to the required fused aromatic systems, have been described. The first two types of reactions and our present modification of the oxo-Friedel–Crafts–Bradsher cyclization, utilizing O,S-acetals, lead directly to fused aromatic hydrocarbons, while the remaining transformations require additional steps involving reductions in intermediate products, i.e., anthrones or anthraquinones, followed by aromatization of the obtained cyclic system. In addition, these reactions require harsh reaction conditions, such as high concentrations of Brønsted acids and high temperatures up to 180 °C or more, which preclude the presence of most substituents on the aromatic system [26,27,28]. Only a few examples of reactions, carried out under milder, non-aqueous reaction conditions, are known [29,30]. Our approach employs a dilute, aqueous methanolic solution (2:1) of hydrochloric acid as a strong carbocation solvating medium and room temperature, being the mildest reaction conditions ever used in these types of intramolecular, electrophilic and aromatic cyclizations [31,32,33]. These mild conditions allow for the installation of thermally and chemically sensitive functional groups on aromatic systems and, thus, the oxo-Friedel–Crafts–Bradsher cyclization gives rise to highly substituted, fused aromatics, as we demonstrated in this study.
Earlier, we obtained hetero (XR = OR, SR)-substituted acenes I via cyclization of diarylmethanol derivatives, i.e., ortho-O,O-acetals III (path a, Scheme 1) via oxo-Friedel–Crafts–Bradsher cyclization [32,33,34] or S,S-dithioacetals IV (path b, Scheme 1) via the thio-Friedel–Crafts–Bradsher cyclization [3]. In both hetero-Friedel–Crafts–Bradsher cyclizations, a new benzene ring, fused to two other (hetero)aromatic moieties, ArI and ArII, is formed in the acene I. It is worth noting that the cyclization of O,O-acetals III took place only in the Brønsted acid aqueous solutions and did not occur under anhydrous conditions in the presence of Lewis acid (FeCl3/KI). On the other hand, the cyclization of S,S-dithioacetals IV proceeded exclusively in the presence of FeCl3/KI in an organic solvent solution.
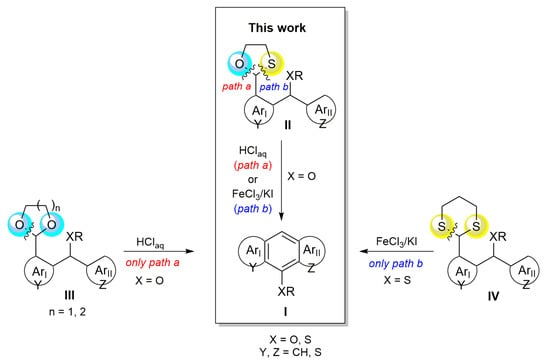
Scheme 1.
The hetero-Friedel–Crafts–Bradsher cyclization of O,O-, O,S- and S,S-acetals.
This differentiated behavior of O,O-acetals and S,S-dithioacetals is due to the greater hydrolytic susceptibility of acetal C-O bonds than dithioacetal C-S bonds towards relatively dilute Brønsted acids and a lack of reactivity of the FeCl3/KI system towards C-O bonds. In this way, suitable reaction conditions can be selected for the preservation of sensitive substituents on the aromatic system.
In the present study, we employed O,S-acetals (1,3-oxathiolanes) II, which possess C-O and C-S bonds, as precursors of three- and four-ring fused aromatics I. Both bonds are cleaved under different reaction conditions, yielding carbocation intermediates that are active in the new oxo-Friedel–Crafts–Bradsher cyclization modification (Scheme 1). The importance of O,S-acetals, as carbocation-equivalent reagents for carbon–carbon bond formation and as protecting groups for carbonyl compounds, has been well documented. Generally, O,S-acetals are prepared via condensation of carbonyl compounds with mercaptoalcohols in the presence of protic acids [35,36,37]. Among O,S-acetals, 1,3-oxathiolanes and 1,3-oxathianes have long been used [38,39]. They are considerably more stable than the O,O-acetals under acidic conditions and easier to remove than S,S-acetals [40]. Mechanistic studies on the rate of acid-catalyzed cleavage show that O,S-acetals have a stability that lies between S,S-dithioacetals and O,O-acetals [41].
2. Results and Discussion
2.1. Synthesis
The synthesis of a series of three- and four-ring fused aromatics 7 and 13 was based on the new modification of the oxo-Friedel–Crafts–Bradsher reaction [3], employing cyclization of O,S-acetals 5 and 12 (Scheme 2). The strategy of the synthesis involved is as follows: (1) protection of the aldehyde group in ortho-bromo aromatic aldehydes 1 and 10 with 1,2-mercaptoethanol to give O,S-acetals 2 and 12, (2) the Br/Li exchange reaction in the latter followed by condensation with aromatic aldehydes 3 to afford diarylmethanols 4, (3) protection of the hydroxyl group in 4 with methyl or benzyl halides to obtain diarylmethyl or benzyl ethers 5 and 6 to avoid the formation of lactones from the reaction of free hydroxyl with aldehyde groups and (4) acid-driven cyclization of 5 and 12 to the corresponding acenes 7 and 13 (Scheme 2).
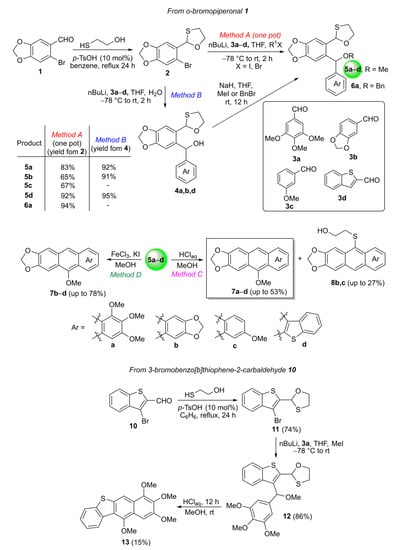
Scheme 2.
Synthesis RO-acenes 7a–d and 13 (major) and HO(CH2)2S-acenes 8b,c (minor) via the oxo-Friedel–Crafts–Bradsher cyclization. The yields given refer to products of the reactions on about a 0.3 g scale and purified by column chromatography.
Turning to a more detailed discussion of this synthesis, the first O,S-acetalization step was performed with 2-mercaptoethanol, catalytic amount of p-TsOH and the corresponding ortho-bromo aromatic aldehydes 1 or 10 in refluxing benzene using the Dean-Stark trap to remove water (24 h). The crude reaction mixture was purified with column chromatography to give a colorless solid of o-bromo O,S-acetals 2 in 80% yield and 11 as colorless oil in a 74% yield (Scheme 2). Ortho-lithiation of 2 in THF at low temperature followed by the reaction with different aromatic aldehydes 3 led to diarylmethanols 4 in up to an 88% yield, which were next converted to the corresponding ethers 5, 6 with methyl or benzyl halides (Method B, Scheme 2). Product 6a was obtained in a 94% yield; however, it decomposed on silica gel during attempts of purification and, therefore, was not used in further transformations. It should be noted that the last two steps can be carried out as a one-pot procedure, which was found to also be effective in the synthesis of ether 12 directly from o-bromo O,S-acetal 11 (Method A, Scheme 2). The synthesized alcohols 4 and ethers 5, 6 and 12 were obtained as inseparable mixtures of two diastereoisomers, which were used in further reactions. However, in the case of o-(O,S-acetalaryl)arylmethyl methyl ether 5d, the major and minor diastereoisomers were successfully separated by column chromatography over silica. In the 1H NMR spectra, characteristic singlets at around 5.5 ppm due to OCHS, multiplets at around 5.3 ppm from 1,3-dioxolane ring and singlets at around 6.5 ppm from the dibenzylic proton were observed for the discussed compounds.
Cyclization reaction. As mentioned, in the case of O,O-acetals, especially five- and six-membered ones, the cyclization to acenes proceeded only with Brønsted acids in an aqueous media (path a, Scheme 1) [31,32,33] with the cleavage of C-O acetal bonds, while with six-membered S,S-acetals, the cyclization occurred only with Lewis acids in anhydrous media with the cleavage of the C-S dithioacetal bonds (path b, Scheme 1). One of the reasons for this differentiated behavior is the lower electron density on the bigger sulfur atom than on the oxygen atom and the lower electronegativity of the former, which means that the 1,3-dithiane sulfur atoms in moderately concentrated mineral acid aqueous solutions at room temperature do not undergo an effective protonation, with the consequence that they also do not undergo apparent hydrolysis through the intermediate benzyl-type carbocations that are required for the thio-Friedel–Crafts–Bradsher cyclization to occur.
We discovered that a different situation exists in strained five-membered O,S-acetals, in which both C-O and C-S bonds can be cleaved in the presence of mineral acids (HCl), especially when the reaction conditions were intensified (higher HCl concentration, higher temperature, longer reaction time). Therefore, in this study, we installed ortho-O,S-acetal moiety on one of the aryl groups in 5 and 12 to benefit from the ability to cleave both C-O and C-S bonds under different reaction conditions (Method C and D, Scheme 2) and to study the mechanism of this electrophilic modification.
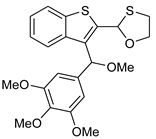
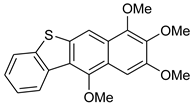
Thus, having in hand o-(O,S-acetalaryl)arylmethyl methyl ethers 5 and 12, we started the investigation of the oxo-Friedel–Crafts–Bradsher cyclization with these substrates (Table 1). The cyclization was performed with aqueous solution of hydrochloric acid in methanol (r.t., 72 h, Method C, Scheme 2) and in the presence of the FeCl3/KI in methanol under anhydrous conditions (65 °C, 12 h, Method D, Scheme 2). The latter conditions gave better yields (up to 78%) of fused RO-acenes 7 while the former ones delivered up to 53% yields and required longer reaction times. Interestingly, the yield of the four-ring acene 13 was 62% when the aldehyde 10 was used while the yield of another four-ring acene 7d was only 15% when the aldehyde 3d was employed, both aldehydes derived from benzothiophene. The structure of 7d was unambiguously confirmed via X-ray analysis (Figure 1).

Table 1.
A comparison of the cyclization results of ethers 5 and 12.
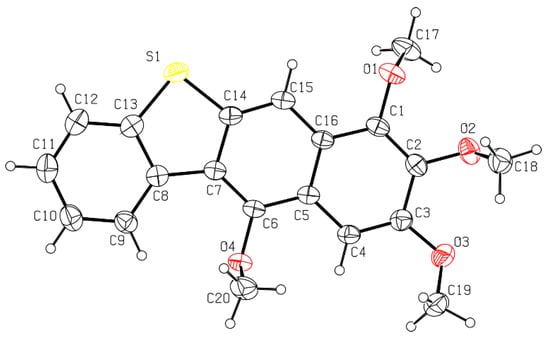
Figure 1.
A view of molecule 13, showing the atom numbering scheme and displacement ellipsoids drawn at the 30% probability level. H atoms are shown as small spheres of arbitrary radii.
Increasing the reaction temperature, HCl concentration or prolonging reaction time resulted in the formation of unexpected HO(CH2)2S-acenes 8 in yields up to 27%, which had never been observed in this type of reaction before.
Surprisingly, neither the acene 7 nor the acene 8 was formed in this case. TLC and HPLC analysis confirmed the presence of the two products (Scheme 3). In both 1H and 13C NMR spectra, doubling signals were observed in a 2:1 ratio, which corresponded to the formation of the two isomers cis-9b/trans-9b. In the 13C NMR spectrum, characteristic signals due to cis-9b/trans-9b carbonyl groups were observed at 195.09 and 194.34 ppm. The presence of the latter was further confirmed by observation of the band at 1697 cm−1 in the IR spectrum and by the DFT calculation.
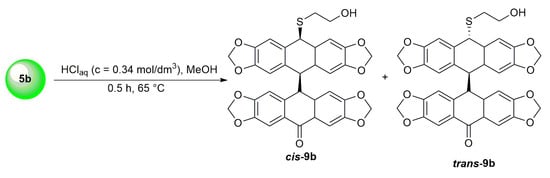
Scheme 3.
Formation of dimeric isomers cis-9b and trans-9b from o-(O,S-acetalaryl)arylmethyl methyl ether 5b.
2.2. DFT Calculations for 9a and 9b
The optimized geometries and electronic structures of both the cis- and trans-isomers of 9b in the gas phase, at the ground state obtained from DFT calculations using the gradient-corrected three-parameter hybrid functional (B3LYP) with the 6-31++G(d,p) basis set, are presented in Figure 2 (see also Tables S1 and S2 in Supplementary Materials). According to the DFT calculations, the cis-9b is more thermodynamically stable than the trans-9b by 4.58 kcal/mol (Figure 2a). It is seen from Figure 2b that cis-9b is also chemically more stable with HOMO-LUMO energy gap (EG) of 3.871 eV compared to the trans isomer with EG = 3.742 eV. The higher thermodynamic and chemical stability of the cis isomer compared to the trans isomer may be due to the presence of an intramolecular non-covalent interaction between the S atom of the substituent attached to the cyclohexadiene ring of the cis isomer and the H atom of the cyclohexanone ring (dashed pink lines in Figure 2a and Figure 3), as revealed by the non-covalent interaction (NCI) analysis (see Supplementary Materials for details). The distance between non-covalently bonded S and H atoms (2.746 Å) is smaller by 0.25 Å than the sum of their van der Waals radii (3.00 Å). The geometrical parameters of this interaction, and especially the C-H···S angle of 144.69° (Figure 3), indicated that it could be treated as a weak unconventional hydrogen bond of the C-H···S type. This interaction forms a closed seven-membered ring S(7) (Figure 2a). The distance between the corresponding S and H atoms in trans-9b is 4.534 Å, so this kind of non-covalent interaction has no possibility to occur in this molecule. It is worth noting that in the case of the trans isomer, the S atom is involved in the formation of short non-covalent contacts with the hydrogen atoms attached to aromatic carbons (Figure 3). Different non-covalent interactions involving the sulfur atom, i.e., C-H···S (in cis-9b) and two C-H···S (in trans-9b) interactions (Figure 2a and Figure 3), led to differences in the molecular configuration of the two isomers. For example, in cis-9b, the distance between the sulfur atom and the cyclohexan-1-one 4-Csp3 atom is only 3.692 Å, while in the trans isomer, it is about 1.5 Å greater. In the cis isomer, the C-H···S interaction forms an intramolecular ring S(7) that prevents free rotation around the Csp3-Csp3 bond connecting the two-ring systems.
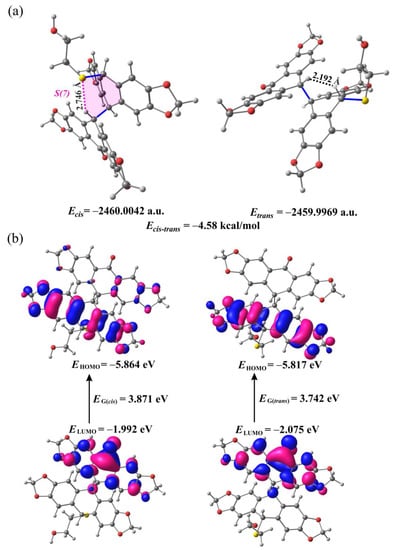
Figure 2.
Molecular structures cis-9b and trans-9b (a) and their HOMO and LUMO orbitals (b) optimized at the DFT theory B3LYP/6-31++G(d,p). The dashed pink and black lines denote non-covalent C-H···S and H···H interactions, respectively. The blue solid lines show cis and trans configurations.
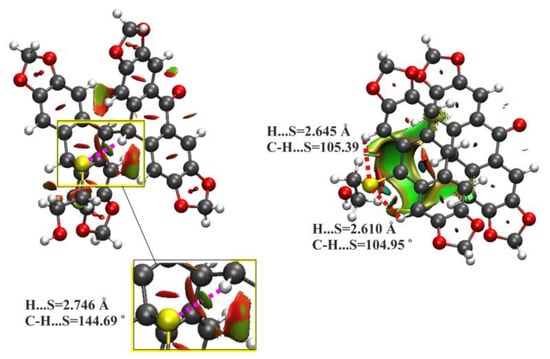
Figure 3.
Color-filled reduced-density gradient (RDG) isosurfaces depicting non-covalent interactions in cis-9b (left) and trans-9b (right). The pink and red dashed lines denote weak C-H···S hydrogen bonds, respectively.
2.3. Mechanistic Considerations and DFT Calculations
The formation of two unexpected types of products 8 and 9 made it possible to explain not only a pathway for obtaining these products but also to propose the overall mechanism of the oxo-Friedel–Crafts–Bradsher cyclization reaction using O,S-acetals (Scheme 4). To make the proposed mechanism credible, we performed DFT calculations (B3LYP 6-311++G(d,p)) in the gas phase in the ground state and the quantitative analysis of molecular surfaces [42,43] for 5b, 16a and 16b. As a result of this analysis, the largest minima of electrostatic potential (ESP) on the van der Waals surfaces of the compounds and more precise ESP values on the local surfaces (surface corresponding to a given atom) were calculated for oxygen and sulfur atoms in the O,S-acetal 5b as well as for the MeO oxygen and X atom (X = O, S) in the XCH2CH2YH side chains in 16a and 16b (Scheme 4, Figure 4). ESP values reflect the electron density in these atoms. As the electron density in a given atom decreases, its affinity for the proton also decreases, making the atom less basic, and vice versa. The atom is red, indicating that it is rich in electrons, and if the color of the atom gradually changes toward yellow and green, then the atom becomes steadily less rich in electrons.
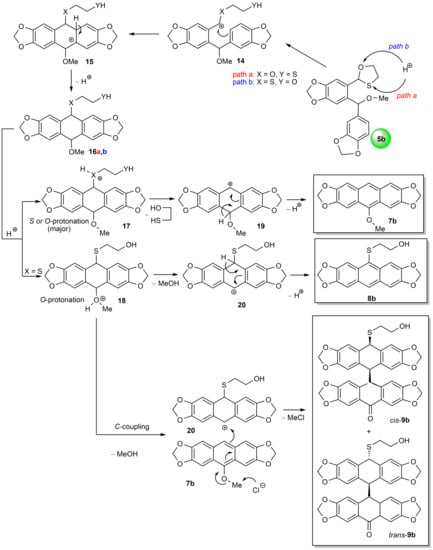
Scheme 4.
General mechanism for the oxo-Friedel–Crafts–Bradsher cyclization reaction using O,S-acetals on the example of the O,S-ether 5b cyclization.
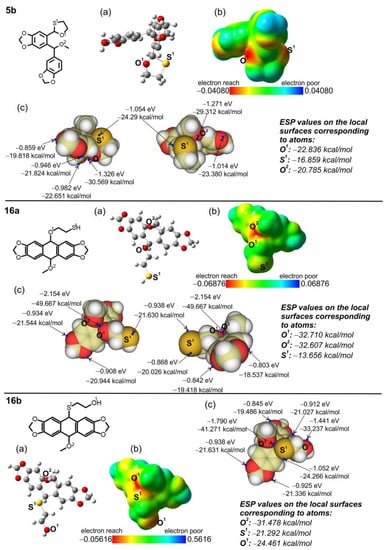
Figure 4.
(a) Molecular structures of 5b, 16a and 16b optimized at the DFT theory B3LYP/6-311++G(d,p) (see also Tables S3–S5 in Supplementary Materials), (b) electron density maps from total SCF B3LYP mapped with ESP (isovalue = 0.02; blue corresponds to low electron densities, whereas red corresponds to high electron densities), (c) the largest minima of ESP (blue points) on the van der Waals surfaces and the ESP values on the local surfaces corresponding to O1, O2 and O3 atoms (italic).
Thus, on the basis of the obtained experimental and calculation data, we assumed that both oxygen and sulfur atoms can be protonated in strained five-membered O,S-acetals systems, with an obvious preference for the O,S-acetal oxygen atom because the difference in the ESP values between oxygen (−22.836 kcal/mol) and sulfur (−16.859 kcal/mol) atoms in 5b is only −5.977 kcal/mol. It means that the difference in electron density in sulfur and oxygen in the cyclic O,S-acetal, and consequently affinity of the latter for the proton, may be regarded as comparable (cf. 16b, vide infra). We also assumed that protonation of the sulfide sulfur atom in the strain-free side groups of intermediates 15–18 (X = S) and also in the final products 9b would be more difficult than in the strained O,S-acetals because the difference in the ESP values between oxygen (−31.478 kcal/mol) and sulfur (−21.292 kcal/mol) atoms in 16b is twice as high as in 5b and equals 10.186 kcal/mol. However, the protonation in 16b to give 17 (X = S) can be possible, to some extent, under harsh reaction conditions, SUCH AS higher HCl concentration, higher temperature and/or longer reaction times. This minor pathway, as in the case of major pathway for 16a, also leads to carbocation 19 and then to anthracene 7b.
Thus, the possible protonation of both heteroatoms leads to O,S-acetal cleavage and formation of the reactive benzylic carbocation 14 followed by the intramolecular SEAr cyclization to give 15 and next aromatization one of the benzene rings to form 16a,b. Preferential protonation of oxygen in 16a or sulfur atoms in 16b (the latter under harsh conditions), as mentioned above, produces 17, which next undergoes aromatization through the intermediate dibenzylic carbocation 19 to give the major cyclization product 7. Protonation of the MeO oxygen atom in 16b gives 18 and, after aromatization through 19, delivers the minor aromatic product 8b of the oxo-Friedel–Crafts–Bradsher reaction. Finally, the obtained product 7b couples with the intermediate dibenzylic carbocation 20 to give the dimeric products cis-9b and trans-9b. This reaction predominates over pathways leading to 7b and 8b at higher temperature (65 °C) and higher HCl concentration (c = 0.34 mol/dm3).
2.4. Electron Character of RO-Acenes 7, 13 and HO(CH2)2S-Acenes 8
The electron nature of the obtained highly substituted acenes 7 and 8 and, in consequence, their photophysical properties are related to the character of the substituents attached to the acene system. The measure of the electron effect exerted by these substituents is the Hammett constants, which were calculated using the ACD/Percepta program [44]. The methoxy and methylene-1,3-dioxa groups with negative σp values of −0.27 and −0.13, respectively, have a strong electron donor character and increase electron density in acene systems 7a–d and 13. On the other hand, the small but positive values of σp = 0.07 constants confirm a weak electron-acceptor character of the HO(CH2)2S- group when attached to electron-rich acenes 8b and 8c substituted by electron-donating alkoxy groups [3,45].
The electron effects operating in the discussed functional groups (MeO, methylene-1,3-dioxa- and HO(CH2)2S-) were further analyzed with the σind and σres Hammett’s components. They show that the electron-donating properties of the methoxy group with σind/σres = 0.30/−0.58 and methylene-1,3-dioxa group with σind/σres = 0.35/−0.48 are connected with a dominance of the positive resonance (+M) effect over the inductive (−I) effect of both alkoxy-type substituents.
On the other hand, in the HO(CH2)2S- group with σind/σres = 0.26/−0.21, the predominantly negative inductive effect (−I) dominates over the resonance effect. It accounts for the electron-withdrawing character of the RS group in electron-rich aromatics 8b and 8c.
2.5. Photopysical Properties
Thus, RO-acenes 7 and 13 belong to a group of highly substituted donor chromophores absorbing UV light in a typical range of 270–395 nm and emitting blue light at 380–445 nm [32]. Sulfur-substituted products, represented here by HO(CH2)2S-acenes 8b and 8c, belong to a group of donor-acceptor chromophores that normally absorb light in a range of 270–425 nm and emit blue light at longer wavelengths of 404–457 nm [3]. UV/Vis absorption and emission spectra of the obtained substituted acene derivatives 7b, 7c, 8c and 13 are shown in Figure 5 and Table 2.
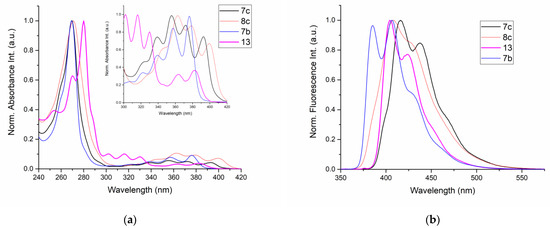
Figure 5.
Normalized absorption (a) and emission (b) spectra of 7b, 7c, 8c and 13 in CH2Cl2 (10−5 mol/dm3, 25 °C).

Table 2.
Absorption and emission maxima (λmax), Stokes shifts in dichloromethane solution (10−5 mol/dm3, 25 °C) for 7b,c, 8c and 13 (underlined values are highest absorption maxima in the lower part of the spectrum in the 300–420 nm range).
In particular, in a range of 240–300 nm electron-donor anthracene 7b, 7c and a weak electron donor-acceptor anthracene 8c due to the presence of the thio group with the −I effect, revealed almost identical absorption maxima at c.a. 269 nm and the same absorption profile. A further comparison of absorption spectra of electron-rich three-ring acenes (7b, 7c), with the four-ring acene 13 of the same electron character, showed a redshift by 11 nm in a range of 240–300 nm, which indicated the effect of a larger aromatic conjugation in 13 (Figure 5a). In a range of 300–420 nm, all investigated compounds exhibited absorption bands of lower intensity in the long-wavelength part of the spectrum. The TD-DFT calculations in the gas phase, made for 7b, revealed, in this part of the spectrum, two strong transitions, i.e., HOMO → LUMO (0.97), corresponding to a band at 380.79 nm and HOMO-1 → LUMO (0.73), HOMO → LUMO+1 (0.26) at 346.56 nm (Figure 6).
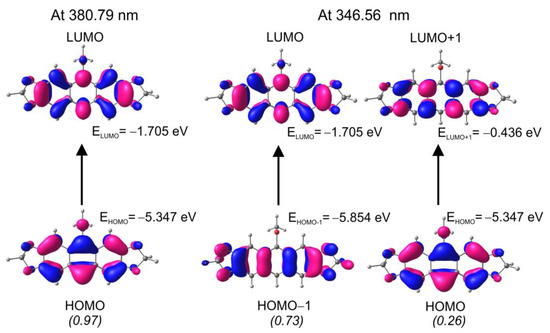
Figure 6.
TD-DFT calculated orbitals involved in the electron transitions for 7b.
The fluorescence spectra of the obtained acenes exhibited blue emission and covered a region from 385 to 438 nm (Figure 5b). A redshift of 19–31 nm was observed for emission maxima of 7c, 8c and 13 relative to 7b.
3. Materials and Methods
Organic solvents were purchased from commercial sources (ChemPur, Piekary Śląskie, Poland) and used as received or dried using standard procedures. Tetrahydrofuran (THF) was purchased from J.T. Baker and purified on Solvent Purification System (MBraun SPS-800). All reagents were from commercial suppliers (Sigma-Aldrich, Merck–USA, Beijing, China) and used without further purification. The 1H NMR and 13C NMR spectra were measured with a Bruker AV 200 or AV 500 spectrometer (Billerica, MA, USA), with chemical shifts given in ppm relative to TMS as an internal standard. High-resolution mass spectrometry (HRMS) measurements were performed using SQ Detector 2 mass spectrometer (Waters, Milford, MA, USA). Melting points were measured using Boetius apparatus. Thin-layer chromatography (TLC) was performed on precoated Merck 60 (F254 60, Darmstad, Germany) silica gel plates with fluorescent indicator, with detection by means of UV light at 254 and 360 nm. Column chromatography was performed on Merck silica gel (Kieselgel 60, Darmstad, Germany, 230–400 mesh) or using Pure FlashPrep 850 Chromatography System (Büchi, Flawil, Switzerland). The UV-Vis absorption spectra were recorded in 1 cm cuvettes on a Shimadzu UV-2700 spectrophotometer (Kioto, Japan) using two types of light source: a D2 64604 deuterium lamp and a Wl L6380 halogen lamp (Kioto, Japan, 220–600 nm). The emission spectra were obtained with the Horiba Jobin Yvon, FluoroMax-4 spectrofluorometer (Glasgow, UK), using a xenon lamp as a light source. The IR absorption spectra were recorded on Nicolet 6700 FT-IR spectrometer (Thermo Scientific, Waltham, MA, USA).
3.1. Synthesis of o-Bromopiperonal O,S-acetal 2
2-Mercaptoethanol (7.50 g, 96.05 mmol, 6.7 mL) and p-TsOH·H2O (10 mol%, 1.83 g) were added to a solution of o-bromopiperonal 1 (20 g, 87.32 mmol) in benzene (200 mL) and refluxed for 24 h using the Dean–Stark trap to remove water. The mixture was concentrated and purified with column chromatography using toluene as an eluent to give a colorless solid of 2 (20 g, 80%). mp = 72–73 °C. (Lit.: 73–74 °C [46])
1H NMR (500 MHz, CDCl3): 7.11 (s, 1H), 6.95 (s, 1H), 6.23 (s, 1H), 5.96 (d, J = 10.1 Hz, 1H), 5.95 (d, J = 10.1 Hz, 1H), 4.64–4.50 (m, 1H), 3.92 (dt, J = 9.0, 6.1 Hz, 1H), 3.28–3.08 (m, 2H) ppm. 13C{1H} NMR (126 MHz, CDCl3): 148.3, 147.7, 132.7, 112.5, 112.4, 107.3, 102.0, 85.8, 72.2, 33.7 ppm. Anal. calcd for C10H9BrO3S: C, 41.54, H, 3.14, S, 11.09; found C, 41.49, H, 3.17, S, 11.03. HRMS (ESI): m/z [M + H]+ calcd for C10H10BrO3S: 288.9534; found 288.9536.
3.2. General Procedure for the Synthesis of o-(O,S-acetalaryl)arylmethanols 4
o-Bromopiperonal O,S-acetal 2 (0.289 g, 1.0 mmol) was placed in the round-bottom flask (50 mL) and dissolved in dry THF (8 mL) under argon atmosphere. The temperature of the resulting solution was lowered to −78 °C and n-BuLi (1.1 mmol, 2.5 M in hexanes) was added. The resulting mixture was stirred under argon for 15 min and then the corresponding aromatic aldehyde 3a–d (1.2 mmol) in dry THF (5 mL) was added. Stirring was continued for 2 h at −78 °C and the reaction mixture was warmed to room temperature. The saturated aqueous NH4Cl solution was added and organic layer was concentrated. The residue was diluted with ethyl acetate (3 × 10 mL), washed with water (15 mL) and dried over anhydrous MgSO4. After filtration, ethyl acetate was removed in vacuum and the crude product 4 was purified by column chromatography over silica gel with a mixture of toluene/ethyl acetate (1:1 v/v) as an eluent.
(6-(1,3)-Oxathiolan-2-yl-benzo[d][1,3]dioxol-5-yl)(3,4,5-trimethoxyphenyl)methanol 4a. 1H NMR (200 MHz, C6D6): 7.44 (s, 1H), 7.13 (s, 1H), 6.84 (s, 2H), 6.34 (s, 1H), 6.24 (d, J = 2.5 Hz, 1H), 5.32 (dd, J = 5.9, 1.2 Hz, 2H), 3.98 (ddd, J = 9.3, 6.1, 2.2 Hz, 1H), 3.88 (s, 3H), 3.46 (s, 6H), 3.29 (ddd, J = 9.3, 6.1, 6.1 Hz, 1H), 2.87–2.67 (m, 2H), 2.58 (ddd, J = 9.3, 6.1, 2.2 Hz, 1H) ppm. 13C{1H} NMR (50 MHz, C6D6): 152.6, 147.0, 146.2, 137.6, 136.9, 135.4, 129.5, 126.8, 107.0, 106.5, 102.9, 102.0, 99.8, 83.3, 70.4, 70.1, 59.0, 54.2, 32.4. Anal. calcd for C20H22O7S: C, 59.10, H, 5.46, S, 7.89; found C, 59.11, H, 5.50, S, 7.82. HRMS (ESI): m/z [M + Na]+ calcd for C20H22O7SNa: 429.0984; found 429.0985. Yield: 68%, colorless oil.
(6-(1,3)-Oxathiolan-2-yl-benzo[d][1,3]dioxol-5-yl)(benzo[d][1,3]dioxol-5-yl)methanol 4b. Two diastereoisomers (A and B, 2:1)—1H NMR (200 MHz, C6D6): 7.39 (s, 1H, B), 7.37 (s, 1H, A), 7.09 (d, J = 1.6 Hz, 1H, B), 7.06 (d, J = 1.6 Hz, 1H, A), 7.01 (s, 1H, A), 6.97 (s, 1H, B), 6.93–6.76 (m, 2H, A+B), 6.71–6.53 (m, 2H), 6.22 (s, 1H, B), 6.17 (s, 1H, A), 6.03 (s, 1H, A), 5.97 (s, 1H, B), 5.38–5.18 (m, 8H), 3.88 (ddd, J = 8.9, 6.4, 2.4 Hz, 2H, A+B), 3.20 (ddd, J = 9.3, 4.7, 4.7 Hz, 2H, A+B), 2.76–2.58 (m, 2H, A+B), 2.56–2.40 (m, 2H, A+B), 2.15 (s br, 2H, A+B) ppm. 13C{1H} NMR (50 MHz, C6D6): 146.8, 146.8, 146.7, 146.7, 146.1, 146.1, 145.7, 136.5, 136.3, 134.9, 129.4, 129.3, 126.8, 119.0, 118.9, 106.7, 106.6, 106.4, 106.3, 106.1, 99.9, 99.6, 82.90, 82.5, 70.2, 70.1, 69.8, 63.2, 32.4, 32.4 ppm. Anal. calcd for C18H16O6S: C, 59.99, H, 4.48, S, 8.90; found C, 59.95, H, 4.50, S, 8.86. HRMS (ESI): m/z [M + Na]+ calcd for C18H16O6SNa: 383.0566; found 383.0565. Yield: 32%, yellowish oil.
6-(1,3-Oxatiolan-2-yl)benzo[d](1,3-dioxol-5-yl)(benzo[b]thien-2-yl)methanol 4d. Two diastereoisomers (A and B, 1:1)—1H NMR (200 MHz, C6D6): 7.67–7.46 (m, 4H, A+B), 7.43 (s, 1H, A), 7.41 (s, 1H, B), 7.20–6.94 (m, 6H, A+B), 6.40 (s, 1H, A), 6.35 (s, 1H, B), 6.31 (s, 2H, A+B), 5.33 (dd, J = 14.5, 5.1 Hz, 4H, A+B), 4.08–3.84 (m, 2H, A+B), 3.50–3.30 (m, 1H, A), 3.27 (dt, J = 15.3, 8.9 Hz, 1H, B), 2.70 (td, J = 16.2, 9.4 Hz, 2H, A+B), 2.60–2.43 (m, 2H, A+B) ppm. 13C{1H} NMR (50 MHz, C6D6): 147.7, 146.9, 146.6, 138.9, 138.6, 133.8, 129.4, 122.9, 122.4, 121.2, 120.2, 106.6, 106.2, 100.0, 82.9, 70.1, 68.0, 32.5 ppm. Anal. calcd for C19H16O4S2: C, 61.27, H, 4.33, S, 17.22; found C, 61.25, H, 4.36, S, 17.22. HRMS (ESI): m/z [M + Na]+ calcd for C19H16O4S2Na: 395.0388; found 395.0387. Yield: 88%, yellowish oil.
3.3. General Procedure for the One-Pot Synthesis of o-(O,S-acetalaryl)arylmethyl Methyl Ethers 5 from o-Bromopiperonal O,S-acetal 2 (Method A)
o-Bromopiperonal O,S-acetal 2 (0.289 g, 1.0 mmol) was placed in the round-bottom flask (50 mL) and dissolved in dry THF (8 mL) at −78 °C under argon atmosphere. Next, n-BuLi (1.1 mmol, 2.5 M in hexanes) was added. The resulting mixture was stirred under argon for 15 min, and then the corresponding aromatic aldehyde 3a–c or 3d (1.2 mmol) was added in dry THF. Stirring was continued for 2 h at −78 °C and 5 equiv. of MeI was added. The reaction mixture was warmed to room temperature. The saturated aqueous NH4Cl solution was added, and organic layer was concentrated. The residue was diluted with ethyl acetate (3 × 10 mL), washed with water (15 mL) and dried over anhydrous MgSO4. After filtration, ethyl acetate was removed in vacuum and the crude product 5 was purified by column chromatography over silica gel with a mixture of toluene/ethyl acetate (1:1 v/v) as an eluent.
5-[Methoxy-(3,4,5-trimethoxyphenyl)methyl]-6-[1,3]oxathiolan-2-ylbenzo[1,3]dioxole 5a. Two diastereoisomers (A and B, 1.4: 1)—1H NMR (200 MHz, C6D6): 7.62 (s, 1H, A), 7.56 (s, 1H, B), 7.11 (s, 1H, B), 7.09 (s, 1H, A), 6.90 (s, 2H, B), 6.82 (s, 2H, A), 6.52 (s, 1H, B), 6.45 (s, 1H, A), 5.59 (2×s, 2H, A+B), 5.36–5.24 (m, 4H, A+B), 4.13–3.94 (m, 2H, A+B), 3.90 (2×s, 6H, A+B), 3.51 (4×s, 18H, A+B), 3.46, 3.36, 3.32, 3.38–3.19 (m, 2H, A+B), 2.86–2.68 (m, 2H, A+B), 2.66–2.51 (m, 2H, A+B) ppm. 13C{1H} NMR (50 MHz, CDCl3): 152.0, 151.8, 146.7, 146.5, 146.3, 146.2, 135.7, 135.0, 131.3, 130.6, 130.1, 106.3, 106.1, 105.6, 105.5, 102.8, 102.5, 100.0, 82.0, 81.9, 79.9, 79.6, 70.7, 70.6, 59.5, 55.8, 54.8, 32.9, 32.7, 28.4 ppm. Anal. calcd for C21H24O7S: C, 59.99, H, 5.75, S, 7.62; found C, 59.91, H, 5.70, S, 7.66. HRMS (ESI): m/z [M + Na]+ calcd for C21H24O7SNa: 443.1140; found 443.1136. Yield: 83%, yellowish crystals, mp: 110–112 °C.
5-((5-(1,3-Oxathiolan-2-yl)benzo[d][1,3]dioxol-6-yl)(methoxy)methyl)benzo[d][1,3]dioxole 5b. Two diastereoisomers (A and B, 1.6: 1)—1H NMR (500 MHz, C6D6): 7.46 (s, 1H, A), 7.41 (s, 1H, B), 7.04 (s, 1H, B), 7.00 (s, 1H, A), 6.97 (s, 1H, A), 6.91 (s, 1H, B), 6.78 (d, J = 8.00 Hz, 1H, B), 6.72 (d, J = 8.00 Hz, 1H, A), 6.58 (d, J = 8.00 Hz, 1H, B), 6.55 (d, J = 8.00 Hz, 1H, A), 6.30 (s, 1H, B), 6.23 (s, 1H, A), 5.35 (s, 1H, A), 5.28 (s, 1H, B), 5.25–5.14 (m, 8H, A+B), 3.93–3.83 (m, 2H, A+B), 3.23–3.14 (m, 2H, A+B), 3.15 (s, 3H, A), 3.11 (s, 3H, B), 2.68–2.56 (m, 2H, A+B), 2.50–2.36 (m, 2H, A+B) ppm. 13C{1H} NMR (126 MHz, C6D6): 149.1, 149.0 (2×s), 148.7 (2×s), 148.2, 137.0, 136.7, 134.2, 133.1, 133.0, 129.0, 121.7, 121.6, 109.0, 108.9, 108.8 (2×s), 108.7, 108.5, 108.0, 101.9, 101.7, 84.4, 84.3, 82.4, 82.2, 72.4, 72.4, 57.3, 34.6, 34.5 ppm. Anal. calcd for C19H18O6S: C, 60.95, H, 4.85, S, 8.56; found C, 60.98, H, 4.80, S, 8.50. HRMS (ESI): m/z [M + Na]+ calcd for C19H18O6SNa: 397.0722; found 397.0723. Yield: 65%, white crystals, mp: 116–117 °C.
5-(Methoxy(3-methoxyphenyl)methyl)-6-(1,3-oxathiolan-2-yl)benzo[d][1,3]dioxole 5c. Two diastereoisomers A and B (5.5:1)—1H NMR (500 MHz, CDCl3): 7.57 (s, 1H, B), 7.52 (s, 1H, A), 7.30 (s, 1H, A), 7.26 (s, 1H, B), 7.15 (d, J = 4.55 Hz, 4H, A+B), 7.05 (s, 1H, B), 7.01 (s, 1H, A), 6.76 (s br, 2H, A+B), 6.45 (s, 1H, A), 6.38 (s, 1H, B), 5.58 (s, 1H, B), 5.52 (s, 1H, A), 5.28 (d, J = 18.56 Hz, 4H, A+B), 4.03–3.92 (m, 2H, A+B), 3.37 (s, 3H, A), 3.34 (s, 3H, B), 3.30 (s, 3H, B), 3.32–3.21 (m, 2H, A+B), 3.26 (s, 3H, A), 2.79–2.68 (m, 2H, A+B), 2.55–2.48 (m, 2H, A+B) ppm. 13C{1H} NMR (126 MHz, CDCl3): 160.1, 148.2, 147.9, 143.8, 143.4, 133.3, 132.3, 129.4, 129.4, 128.1, 119.5, 119.4, 113.1, 112.7, 112.6, 108.3, 107.8, 107.7, 107.2, 101.0, 83.5, 81.4, 71.5, 56.4, 54.5, 33.7 ppm. Anal. calcd for C19H20O5S: C, 63.32, H, 5.59, S, 8.90; found C, 63.29, H, 5.51, S, 8.94. HRMS (ESI): m/z [M + Na]+ calcd for C19H20O5SNa: 383.0929; found 383.0930. Yield: 67%, yellowish oil.
5-((Benzo[b]thien-2-yl)(methoxy)methyl)-6-(1,3-oxathiolan-2-yl)benzo[d][1,3]dioxole 5d. Two diastereoisomers (A and B, 1.3:1 ratio). Major diastereoisomer A—1H NMR (200 MHz, C6D6): 7.65–7.43 (m, 3H), 7.29–6.98 (m, 4H), 6.38 (s, 1H), 5.83 (s, 1H), 5.42–5.30 (m, 2H), 3.99 (dd, J = 11.1, 4.4 Hz, 1H), 3.38–3.19 (m, 1H), 3.31 (s, 3H), 2.75 (ddd, J = 9.6, 6.6, 6.6 Hz, 1H), 2.54 (dd, J = 11.1, 4.4 Hz, 1H) ppm. Anal. Calcd for C20H18O4S2: C, 62.16, H, 4.69, S, 16.59; Found C, 62.13, H, 4.64, S, 16.55. HRMS (ESI): m/z [M + Na]+ calcd for C20H18O4S2Na: 409.0544; found 409.0545. Yield: 43%, yellowish oil. Minor diastereoisomer B—1H NMR (200 MHz, C6D6): 7.66–7.41 (m, 3H), 7.20–6.99 (m, 4H), 6.45 (s, 1H), 5.71 (d, J = 1.1 Hz, 1H), 5.32 (dd, J = 6.6, 1.3 Hz, 2H), 3.93 (ddd, J = 8.7, 6.4, 2.1 Hz, 1H), 3.33–3.11 (m, 1H), 3.27 (s, 3H), 2.71 (ddd, J = 9.7, 6.4, 6.4 Hz, 1H), 2.56–2.41 (m, 1H) ppm. 13C{1H} NMR (50 MHz, C6D6): 148.3, 147.4, 140.4, 139.7, 132.5, 132.2, 128.1, 124.1, 123.7, 122.4, 122.0, 108.3, 108.0, 101.2, 83.6, 79.0, 71.5, 56.5, 33.6 ppm. Anal. calcd for C20H18O4S2: C, 62.16, H, 4.69, S, 16.59; found C, 62.19, H, 4.51, S, 16.50. HRMS (ESI): m/z [M + Na]+ calcd for C20H18O4S2Na: 409.0544; found 409.0546. Yield: 33%, yellowish oil. Yield: 92% (diastereoisomers A+B).
3.4. Procedure for the Synthesis of o-(O,S-acetalaryl)arylmethyl Benzyl Ether 6a
o-Bromopiperonal O,S-acetal 2 (0.289 g, 1.0 mmol) was placed in the round-bottom flask (50 mL) and dissolved in dry THF (8 mL) at −78 °C under argon atmosphere. Next, n-BuLi (1.1 mmol, 2.5 M in hexanes) was added. The resulting mixture was stirred under argon for 15 min and then 3,4,5-trimethoxybenzaldehyde 3a (0.235 g, 1.2 mmol) was added in dry THF. Stirring was continued for 2 h at −78 °C then benzyl bromide (BnBr) (0.171 g, 1.0 mmol) was added. The reaction mixture was warmed to room temperature. The saturated aqueous NH4Cl solution was added, and organic layer was concentrated. The residue was diluted with ethyl acetate (3 × 10 mL), washed with water (15 mL) and dried over anhydrous MgSO4. After filtration, ethyl acetate was removed in vacuum and the crude product 6a was obtained as a yellow oil. The product decomposed on silica gel during purification attempts.
5-((Benzyloxy)(3,4,5-trimethoxyphenyl)methyl)-6-(1,3-oxathiolan-2-yl)benzo[d][1,3]dioxole 6a. Two diastereoisomers (A:B, 2:1)—1H NMR (200 MHz, C6D6): 7.65 (s, 1H, B), 7.58 (s, 1H, A), 7.55–7.36 (m, 4H, A+B), 7.33–7.25 (m, 2H, A+B), 7.20–7.14 (m, 2H, A+B), 7.15–6.98 (m, 4H, A+B), 6.96 (s, 2H, A), 6.87 (s, 2H, B), 6.44 (s, 1H, B), 6.38 (s, 1H, A), 5.90 (s, 1H, B), 5.87 (s, 1H, A), 5.32 (d, J = 1.3 Hz, 2H, A), 5.27 (d, J = 1.3 Hz, 2H, B), 4.95–3.85 (m, 2H, A+B), 4.73 (d, J = 12.0 Hz, 1H, B), 4.58 (d, J = 12.0 Hz, 1H, B), 4.67 (d, J = 12.2 Hz, 1H, A), 4.52 (d, J = 12.2 Hz, 1H, A), 4.02 (s, 3H, B), 3.91 (s, 3H, A), 3.51 (s, 6H, A), 3.44 (s, 6H, B), 3.41–3.16 (m, 2H, A+B), 2.76 (dt, J = 9.6, 6.6 Hz, 2H, A+B), 2.54 (ddd, J = 9.6, 5.0, 2.0 Hz, 2H, A+B) ppm. Yield: 94% (crude product), yellow oil.
3.5. General Procedure for the Synthesis of o-(O,S-acetalaryl)arylmethyl Methyl Ethers 5 from o-(O,S-acetalaryl)arylmethanols 4 (Method B)
o-(O,S-Acetalaryl)arylmethanol 4a,b or 4d (1.0 mmol) and KI (5 mol%) were placed in the round-bottom flask (50 mL) and dissolved in dry THF (8 mL) at room temperature; then, NaH (1.1 mmol) was added and stirred for 30 min under argon atmosphere. Then, the resulting mixture was treated with MeI (1.5 mmol) and was left at room temperature overnight. After 12 h, the residue was diluted with ethyl acetate (3 × 10 mL), washed with water (15 mL) and dried over anhydrous MgSO4. After filtration, ethyl acetate was removed in vacuum and the crude product 5 was purified by column chromatography over silica gel with a mixture of toluene/ethyl acetate (1:1 v/v) as an eluent.
3.6. General Procedure for the Synthesis of MeO-Substituted Acenes 7, 13 and 8 Using HClaq (Method C)
To a solution of o-(O,S-acetalaryl)arylmethyl methyl ether 5 or 11 (0.8 mmol), dissolved in MeOH (20 mL), aqueous solution of 1N or 2N HCl (4 mL) was added and the resulting mixture was stirred at the relevant temperature (see Table 1) until disappearance of the starting material (monitoring by TLC). The reaction mixture was extracted with ethyl acetate (50 mL) and the organic layer was washed with water (30 mL), saturated solution of NaHCO3 (30 mL) and again with water (30 mL). After drying over anhydrous MgSO4 and filtration, the solvent was removed in vacuum and the crude products were purified by column chromatography over silica gel with a mixture of n-hexane/ethyl acetate 10:1 (v/v) to afford corresponding acenes 7 and 13. A mixture of n-hexane/ethyl acetate 3:1 (v/v) was used to purify anthracene 8.
3.7. General Procedure for the Synthesis of MeO-Substituted Acene 7 Using FeCl3/KI in MeOH (Method D)
To a solution of o-(O,S-acetalaryl)arylmethyl methyl ether 5 (1.1 mmol) in dry MeOH (10 mL), FeCl3 (0.195 g, 1.2 mmol) and KI (0.199 g, 1.2 mmol) were added. The mixture was refluxed until disappearance of the starting material (monitoring by TLC, Table 1). After completion of the reaction, the solvent was removed. To the crude product, ethyl acetate (10 mL) was added and the resulting mixture was poured onto the saturated solution of Na2S2O3 (10 mL). The organic layer was dried over anhydrous MgSO4. After filtration, the solvent was evaporated and the crude products were purified with column chromatography (n-hexane/ethyl acetate, 10:1 v/v) to give MeO-acenes 7 in up to 72% yield accompanying by HO(CH2)2S-acenes 8 in up to 27% yields (Table 1).
5,7,8,9-Tetramethoxyanthra[2,3-d][1,3]dioxole 7a [34]. 1H NMR (200 MHz, C6D6): 8.52 (s, 1H), 7.74 (s, 1H), 7.37 (s, 1H), 7.20 (s, 1H), 5.35 (s, 2H), 4.01 (s, 3H), 3.93 (s, 3H), 3.81 (s, 3H), 3.56 (s, 3H) ppm. 13C{1H} NMR (50 MHz, CDCl3): 152.6, 148.0, 147.4, 140.6, 138.5, 128.9, 128.7, 124.0, 122.1, 121.3, 114.9, 103.3, 101.0, 95.8, 95.0, 61.8, 61.4, 55.9 ppm.
HRMS (ESI): m/z [M + Na]+ calcd for C19H18O6Na: 365.1001; found 365.0998. Yield: 53% (Method C), yellow crystals, mp: 130–132 °C.
5-methoxyanthra[2,3-d:6,7-d’]bis[1,3]dioxole 7b. 1H NMR (500 MHz, CDCl3): 7.77 (s, 1H), 7.42 (s, 2H), 7.13 (s, 2H), 6.04 (s, 4H), 4.02 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): 150.5, 147.6, 147.5, 129.5, 121.3, 119.8, 106.4, 102.6, 101.0, 97.1, 62.1 ppm. HRMS (ESI): m/z [M + Na]+ calcd for C17H12O5Na: 319.0582; found 319.0584. Yield: 36% (Method D), yellow crystals, mp: 129–130 °C.
5,7-Dimethoxyanthra[2,3-d][1,3]dioxole 7c [34]. 1H NMR (200 MHz, C6D6): 7.74 (s, 1H), 7.69 (d, J = 9.3 Hz, 1H), 7.54 (d, J = 2.5 Hz, 1H), 7.28 (dd, J = 9.3, 2.5 Hz, 1H), 7.23 (s, 1H), 7.14 (s, 1H), 5.35 (s, 2H), 3.79 (s, 3H), 3.55 (s, 3H) ppm. 13C{1H} NMR (126 MHz, C6D6): 158.3, 151.2, 149.4, 148.5, 130.9, 129.9, 129.5, 126.0, 123.9, 122.2, 120.8, 104.1, 101,7, 99.1, 98.1, 62.0, 55.4 ppm.
HRMS (ESI): m/z [M + Na]+ calcd for C17H14O4Na: 305.0790; found 305.0796. Yield: 78% (Method D), yellowish oil.
5-Methoxy-6H-[1,3]benzodioxolo[3,2-b]dibenzothiophene 7d. 1H NMR (500 MHz, C6D6): 7.90 (s, 1H), 7.83 (d, J = 7.8 Hz, 1H), 7.59 (s, 1H), 7.43 (d, J = 7.9 Hz, 1H), 7.17–7.12 (m, 1H), 7.10–7.06 (m, 2H), 5.25 (s, 2H), 3.70 (s, 3H) ppm. 13C{1H} NMR (126 MHz, C6D6): 149.0, 148.45, 146.1, 140.6, 135.9, 131.0, 128.7, 127.8, 124.9, 123.5, 122.5, 116.7, 115.9, 111.3, 104.7, 101.5, 98.2, 60.3 ppm. Anal. Calcd for C18H12O3S: C, 70.11, H, 3.92, S, 10.40; Found C, 70.09, H, 3.96, S, 10.44. HRMS (ESI): m/z [M + Na]+ calcd for C18H12O3SNa: 331.0405; found 331.0403. Yield: 62% (Method D) yellowish oil.
2-(Anthra[2,3-d:6,7-d’]bis[1,3]dioxol-10-ylthio)ethanol 8b. 1H NMR (500 MHz, DMSO-d6): 8.10 (s, 1H), 7.99 (s, 2H), 7.25 (s, 2H), 6.12 (s, 4H), 4.77 (br s, 1H), 3.33 (m, 2H), 2.79 (t, J = 6.8 Hz, 2H) ppm. 13C{1H} NMR (126 MHz, DMSO-d6): 149.1, 147.4, 132.0, 128.62, 126.6, 125.1, 103.1, 101.9, 101.6, 60.5, 39.1. HRMS (ESI): m/z [M + H]+ calcd for C18H15O5S: 343.0640; found 343.0636. Yield = 25% (Method C), yellowish oil.
2-(7-Methoxyanthra[2,3-d][1,3]dioxol-10-ylthio)ethanol 8c. 1H NMR (500 MHz, C6D6): 8.45 (s, 1H), 8.25 (d, J = 2.7 Hz, 1H), 7.78 (s, 1H), 7.52 (d, J = 9.0 Hz, 1H), 7.18 (dd, J = 9.0, 2.7 Hz, 1H), 6.98 (s, 1H), 5.22 (s, 2H), 3.55 (s, 3H), 3.16 (t, J = 6.3 Hz, 2H), 2.61 (t, J = 6.3 Hz, 2H) ppm. 13C{1H} NMR (126 MHz, C6D6): 158.5, 149.8, 147.2, 135.7, 134.1, 130.3, 128.2, 127.9, 124.4, 120.8, 119.7, 103.3, 103.2, 102.0, 100.8, 61.0, 54.6, 38.9 ppm. 1H NMR (500 MHz, DMSO-d6): 8.30 (s, 1H), 8.06 (s, 1H), 8.03 (s, 1H), 7.88 (d, J = 9.0 Hz, 1H), 7.35 (s, 1H), 7.13 (d, J = 9.0 Hz, 1H), 6.15 (s, 2H), 4.78 (br s, 1H), 3.92 (s, 3H), 3.43–3.25 (m, 2H), 2.84 (t, J = 6.7, Hz, 2H) ppm. 13C{1H} NMR (126 MHz, DMSO-d6): 158.1, 149.9, 147.3, 135.0, 133.8, 130.8, 128.0, 127.9, 127.2, 124.3, 119.6, 103.5, 103.5, 102.0, 101.4, 60.6, 55.6, 38.8 ppm. HRMS (ESI): m/z [M + H]+ calcd for C18H17O4S: 329.0848; found 329.0845. Yield: 27% (Method C), yellow solid, mp: 153–154 °C.
3.8. Synthesis of 3-Bromobenzo[b]thiophene-2-carbaldehyde O,S-acetal 11
2-Mercaptoethanol (2.62 mmol, 205 mg, 184 µL) and p-TsOH·H2O (50 mg, 0.2 mmol, 10 mol%) were added to a solution of 3-bromobenzo[b]thiophene-2-carbaldehyde 10 (2.62 mmol, 0.633 g) in benzene (6 mL), and the resulting mixture was refluxed for 24 h using the Dean–Stark trap to remove water. The mixture was concentrated and purified with column chromatography using toluene as an eluent. Evaporation of the solvent gave a colorless oil of 11 (0.580 g, 74%) [47].
1H NMR (200 MHz, C6D6): 7.67 (d, J = 7.6 Hz, 1H), 7.41 (d, J = 7.8 Hz, 1H), 7.20–7.10 (m, 1H), 7.10–6.99 (m, 1H), 6.55 (s, 1H), 4.10–3.95 (m, 1H), 3.55–3.33 (m, 1H), 2.92–2.72 (m, 1H), 2.57 (ddd, J = 12.6, 8.0, 5.2 Hz, 1H) ppm. 13C{1H} NMR (50 MHz, C6D6): 140.6, 138.2, 137.7, 128.7, 125.6, 125.0, 122.9, 122.6, 82.2, 72.1, 33.7 ppm. Anal. calcd for C11H9BrOS2: C, 43.86, H, 3.01, S, 21.29; found C, 43.83, H, 2.98, S, 21.35. HRMS (ESI): m/z [M + H]+ calcd for C11H10BrOS2: 300.9356; found 300.9347.
3.9. Synthesis of o-(O,S-acetalaryl)arylmethyl Methyl Ether 12
o-Bromothioacetal 11 (500 mg, 1.66 mmol) was placed in the round-bottom flask (50 mL) and dissolved in dry THF (6 mL) at −78 °C under argon atmosphere. Next, n-BuLi (0.7 mL, 2.6 M in hexanes, 1.83 mmol) was added. The resulting mixture was stirred for 15 min under argon and then solution of the aldehyde 3a (326 mg, 1.66 mmol) in dry THF (4 mL) was added. After 2 h, MeI (1.13 g, 8.0 mmol) was added. The reaction mixture was warmed to room temperature and stirred for 12 h. The saturated aqueous NH4Cl solution was added, and organic layer was concentrated. The residue was diluted with ethyl acetate (3 × 10 mL), washed with water (15 mL) and dried over anhydrous MgSO4. After filtration, ethyl acetate was removed in vacuum and the crude product was purified by column chromatography over silica gel with a mixture of toluene/ethyl acetate (10:1 v/v). Fraction with Rf = 0.65 yielded 617 mg of product 12.
2-(3-(Methoxy(3,4,5-trimethoxyphenyl)methyl)benzo[b]thien-2-yl)-1,3-oxathiolane 12. Two diastereoisomers (A and B)—1H NMR (500 MHz, CDCl3): 8.04 (d, J = 8.2 Hz, 1H, A), 8.02 (d, J = 8.2 Hz, 1H, B), 7.58 (d, J = 8.2 Hz, 2H, A+B), 7.24–7.03 (m, 10H, A+B), 6.96 (s, 1H, A), 6.95 (s, 1H, B), 6.83 (s, 1H, A), 6.82 (s, 1H, B), 5.87 (s, 1H, A), 5.82 (s, 1H, B), 4.10 (dddd, J = 15.6, 9.2, 6.2, 2.9 Hz, 2H, A+B), 3.82 (s, 1H, A), 3.81 (s, 1H, B),3.55–3.39 (m, 2H, A+B), 3.49 (s, 6H, A), 3.48 (s, 6H, B), 3.29 (s, 6H, A+B), 3.00–2.84 (m, 2H, A+B), 2.66–2.59 (m, 2H, A+B) ppm. 13C{1H} NMR (126 MHz, CDCl3): 153.8, 143.4, 143.2, 139.7 (2×s), 138.8, 138.7, 138.3, 137.7, 136.3, 136.2, 132.9, 132.5, 129.1, 128.3, 125.4, 125.0, 124.2, 124.1, 123.9, 122.6, 122.5, 104.3, 81.4, 81.3, 79.2, 79.1, 72.2, 71.9, 60.2, 56.6, 55.6, 34.0, 21.2 ppm. Anal. calcd for C22H24O5S2: C, 61.09; H, 5.59; S, 14.82; found C, 61.06, H, 5.52, S, 14.77. HRMS (ESI): m/z [M + Na]+ calcd for C22H24O5S2Na: 455.0963; found 455.0965. Yield: 86%, yellowish oil.
3.10. Synthesis of Acene 13 Using HClaq
To a solution of o-(O,S-acetalaryl)arylmethyl methyl ether 12 (0.073 g, 0.17 mmol), dissolved in MeOH (4 mL), the aqueous solution of 2 N HCl (0.8 mL) was added and the resulting mixture was stirred at room temperature for 12 h. The reaction mixture was extracted with ethyl acetate (20 mL) and the organic layer was washed with water (10 mL), saturated solution of NaHCO3 (15 mL) and again with water (10 mL), then dried over anhydrous MgSO4. After filtration, ethyl acetate was removed in vacuum and the crude product was purified with PLC plate with a mixture of hexane/acetone (3:1 v/v). Fraction with Rf = 0.45 yielded 9 mg of product 13.
7,8,9,11-Tetramethoxybenzo[b]naphtho[2,3-d]tiophene 13. 1H NMR (500 MHz, C6D6): 8.71 (d, J = 7.7 Hz, 1H), 8.47 (s, 1H), 7.49 (d, J = 7.7 Hz, 1H), 7.34 (s, 1H), 7.28 (dd, J = 7.7, 7,7 Hz, 1H), 7.15 (dd, J = 7.7, 7.7 Hz, 1H), 3.79 (s, 6H), 3.66 (s, 3H), 3.44 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): 153.2, 152.4, 147.7, 142.0, 140.0, 136.7, 134.5, 127.0, 126.4, 126.2, 125.8, 124.7, 123.0, 122.5, 111.4, 96.6, 60.9, 60.7, 60.1, 55.1 ppm. Anal. calcd for C20H18O4S: C, 67.78; H, 5.12; S, 9.05; found C, 67.75, H, 5.15, S, 9.09. HRMS (ESI): m/z [M + Na]+ calcd for C20H18O4SNa: 377.0823; found 377.0821. Yield: 15%; yellowish crystals.
Crystal structure data: C20H18O4S, M = 340.40, monoclinic, space group P21/n (No. 14), a = 12.1338(3) Å, b = 7.37769(18) Å, c = 19.7054(6), β = 103.602(3)°, V = 1714.54(8) Å3, Z = 4, T = 293(2) K, Dcalc = 1.373 g∙cm−3, CuKα radiation, 2θmax = 134.912°, 15,804 reflections collected, 3063 reflections unique and 2763 reflections with I > 2σ(I). Final GooF = 1.042, R1 = 0.0388 and wR2 = 0.1095 for 2763 reflections and 231 parameters [48].
3.11. Synthesis of Dimeric Isomers cis-9b and trans-9b from o-(O,S-acetalaryl)arylmethyl Methyl Ether 5b Using HClaq
To a solution of o-(O,S-acetalaryl)arylmethyl methyl ether 5b (0.3 g, 0.8 mmol), dissolved in MeOH (20 mL), aqueous solution of 2 N HCl (4 mL) was added and the resulting mixture was stirred for 0.5 h at 65 °C until disappearance of the starting material (monitoring by TLC). The reaction mixture was extracted with ethyl acetate (50 mL) and the organic layer was washed with water (30 mL), saturated solution of NaHCO3 (30 mL) and again with water (30 mL). After drying over anhydrous MgSO4 and filtration, the solvent was removed in vacuum and the crude products were purified by column chromatography over silica gel with a mixture of n-hexane/ethyl acetate in a 2:1 (v/v) ratio to afford isomeric cis-9b and trans-9b (2:1) as a deep-red solid in 55% yield (0.274 g).
1H NMR (500 MHz, C6D6, A+B): 7.60 (s, 1H, A), 7.55 (d, J = 1.7 Hz, 1H, B), 7.30 (dd, J = 8.0, 1.8 Hz, 1H, B), 7.22 (d, J = 1.7 Hz, 1H, B), 7.14 (s, 1H, B), 7.12 (s, 1H, B), 7.11 (s, 1H, A), 7.04 (dd, J = 8.1, 1.7 Hz, 1H, B), 6.73 (s, 1H, B), 6.67 (d, J = 1.8 Hz, 1H, A), 6.63 (d, J = 8.0 Hz, 1H, A), 6.64 (d, J = 8.0 Hz, 1H, B), 6.58 (s, 1H, A), 6.53 (d, J = 8.1 Hz, 1H, B), 6.48 (s, 1H, A), 6.44 (d, J = 2.1 Hz, 1H, A), 6.42 (s, 1H, A), 6.40 (d, J = 1.7 Hz, 1H, B), 6.31 (d, J = 6.0 Hz, 1H, A), 6.32 (d, J = 3.7 Hz, 1H, A), 5.53 (s, 1H, B), 5.44 (s, 1H, A), 5.29–5.08 (m, 16H, A+B), 3.61 (td, J = 6.3, 2.5 Hz, 2H, A), 3.24 (dtd, J = 16.9, 11.2, 5.7 Hz, 2H, B), 2.67–2.58 (m, 1H, A), 2.51–2.42 (m, 1H, A), 2.22 (dt, J = 13.7, 5.5 Hz, 1H, B), 2.15–2.06 (m, 1H, B) ppm. 13C{1H} NMR (126 MHz, C6D6, A+B): 195.1, 194.3, 155.5, 155.2, 151.5, 151.4, 149.0, 148.7, 148.4, 148.2 (2×s) 148.0, 147.9, 147.8 (2×s) 147.0, 146.9, 146.8, 146.5, 140.9 (2×s) 135.8, 134.7, 134.6 (2×s), 132.4, 132.0, 126.2, 125.8, 125.4, 125.3, 125.2, 124.8, 123.0, 122.7, 122.2, 122.0, 111.0, 110.8, 109.4, 109.1, 109.0, 108.9, 108.6, 108.5, 108.2, 108.0, 107.7, 105.2, 105.0, 104.3, 104.2, 101.8, 101.1 (2×s), 101.0, 100.7 (2×s), 61.2, 60.4, 50.4, 50.3, 35.6, 35.1 ppm. HRMS (ESI): m/z [M + Na]+ calcd for C34H24O10SNa: 647.0988; found 647.0989; m/z [M + K]+ calcd for C34H24O10SK: 663.0727; found 663.0732. FT-IR (vmax/cm−1, neat): 926, 1005, 1239, 1440, 1471, 1500, 1696, 2895. Deep-red solid, mp: 118–119 °C.
4. Conclusions
In summary, in this study, we presented a new modification of the oxo-Friedel–Crafts–Bradsher (F-C-B) cyclization reaction using O,S-acetals, which have not been previously studied in this type of reaction. Unlike other hetero-F-C-B modifications, involving O,O-acetals and S,S-dithioacetals, this reaction is more flexible and can be carried out both in aqueous solutions and under anhydrous conditions, which is important for water-sensitive substrates. Like other hetero-F-C-B cyclizations, oxo-F-C-B cyclization may be a source of highly substituted acenes, the hallmark of this type of reaction. Interestingly, in this modification, two kinds of electron donor and donor-acceptor acenes can be obtained in one reaction step. The formation of two types of products 8 and 9, which have never been observed in this type of cyclization, and the support of DFT calculations made it possible to propose a general reaction mechanism for this new modification of oxo-Friedel–Crafts–Bradsher cyclization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28062474/s1. The 1H and 13C NMR spectra of obtained compounds. Atom coordinates (Å), total energy (Hartree) and the number of imaginary vibrational frequencies for the geometry of cis-9b and trans-9b optimized at the B3LYP/6-31++(d,p) level in the gas phase using Gaussian 09 (Tables S1 and S2). Atom coordinates (Å), total energy (Hartree) and the number of imaginary vibrational frequencies for the geometry of 5b, 16a and 16b optimized at the B3LYP/6-311++(d,p) level in the gas phase using Gaussian 09 (Tables S3–S5). Refs. [42,49,50,51] are cited in the supplementary materials.
Author Contributions
Conceptualization, K.O., E.R.-S. and P.B.; methodology, K.O., E.R.-S. and P.B.; software, E.R.-S. and K.O.; DFT calculations, E.R.-S.; validation, K.O., E.R.-S. and P.B.; formal analysis, K.O., E.R.-S. and P.B.; investigations, K.O. and E.R.-S.; resources K.O. and E.R.-S.; data curation, K.O. and E.R.-S.; writing—original draft preparation, K.O., E.R.-S. and P.B.; writing—review & editing, K.O., E.R.-S. and P.B.; visualization, K.O. and E.R.-S.; supervision, K.O., E.R.-S. and P.B.; project administration, P.B.; funding acquisition, P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Center (Poland), grant number UMO-2013/11/B/ST5/01610.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Experimental details can be found in the Supporting Information. CCDC 1475147 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif,orby (accessed on 15 December 2021), emailing data_request@ccdc.cam.ac.uk or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK; Fax: +44-1223-336033.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Yang, Y.; Börjesson, K. Electroactive covalent organic frameworks: A new choice for organic electronics. Trends Chem. 2022, 4, 60–75. [Google Scholar] [CrossRef]
- Koprowski, M.; Owsianik, K.; Knopik, Ł.; Vivek, V.; Romaniuk, A.; Różycka-Sokołowska, E.; Bałczewski, P. Comprehensive Review on Synthesis, Properties, and Applications of Phosphorus (PIII, PIV, PV) Substituted Acenes with More Than Two Fused Benzene Rings. Molecules 2022, 27, 6611. [Google Scholar] [CrossRef]
- Bałczewski, P.; Kowalska, E.; Różycka-Sokołowska, E.; Skalik, J.; Owsianik, K.; Koprowski, M.; Marciniak, B.; Guziejewski, D.; Ciesielski, W. Mono-Aryl/Alkylthio-Substituted (Hetero)acenes of Exceptional Thermal and Photochemical Stability by the Thio-Friedel–Crafts/Bradsher Cyclization Reaction. Chem.—A Eur. J. 2019, 25, 14148–14161. [Google Scholar] [CrossRef]
- Im, Y.; Byun, S.Y.; Kim, J.H.; Lee, D.R.; Oh, C.S.; Yook, K.S.; Lee, J.Y. Recent Progress in High-Efficiency Blue-Light-Emitting Materials for Organic Light-Emitting Diodes. Adv. Funct. Mater. 2017, 27, 1603007. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, T.; Yu, P.; Zhang, H.; Zhang, H.; Ji, W. A review on the electroluminescence properties of quantum-dot light-emitting diodes. Org. Electron. 2021, 90, 106086. [Google Scholar] [CrossRef]
- Lim, H.; Woo, S.-J.; Ha, Y.H.; Kim, Y.-H.; Kim, J.-J. Breaking the Efficiency Limit of Deep-Blue Fluorescent OLEDs Based on Anthracene Derivatives. Adv. Mater. 2022, 34, 2100161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, T.; Dong, S.; Wen, Z.; Xu, H.; Miao, Y.; Wang, H.; Yu, J. Anthracene and carbazole based asymmetric fluorescent materials for high-efficiency deep-blue non-doped organic light emitting devices with CIEy=0.06. Dye. Pigment. 2022, 199, 110047. [Google Scholar] [CrossRef]
- Haykir, G.; Aydemir, M.; Han, S.H.; Gumus, S.; Hizal, G.; Lee, J.Y.; Turksoy, F. The investigation of sky-blue emitting anthracene-carbazole derivatives: Synthesis, photophysics and OLED applications. Org. Electron. 2018, 59, 319–329. [Google Scholar] [CrossRef]
- Lim, H.; Cheon, H.J.; Lee, G.S.; Kim, M.; Kim, Y.-H.; Kim, J.-J. Enhanced Triplet–Triplet Annihilation of Blue Fluorescent Organic Light-Emitting Diodes by Generating Excitons in Trapped Charge-Free Regions. ACS Appl. Mater. Interfaces 2019, 11, 48121–48127. [Google Scholar] [CrossRef]
- Song, D.; Yu, Y.; Yue, L.; Zhong, D.; Zhang, Y.; Yang, X.; Sun, Y.; Zhou, G.; Wu, Z. Asymmetric thermally activated delayed fluorescence (TADF) emitters with 5,9-dioxa-13b-boranaphtho[3,2,1-de]anthracene (OBA) as the acceptor and highly efficient blue-emitting OLEDs. J. Mater. Chem. C 2019, 7, 11953–11963. [Google Scholar] [CrossRef]
- Park, J.; Jang, B.; Moon, Y.J.; Lee, H.; Kim, Y.K.; Yoon, S.S. Efficient deep blue organic light emitting diodes based on [2,7,7,13,13-pentamethyl-9-(10-phenylanthracen-9-yl)-7,13-dihydrobenzo[5,6]-s-indaceno[1,2-g]quinoline] derivatives. Mol. Cryst. Liq. Cryst. 2020, 705, 120–126. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Ye, S.; Zhang, Q.; Duan, Y.; Guo, R.; Wang, L. Molecular engineering of anthracene-based emitters for highly efficient nondoped deep-blue fluorescent OLEDs. J. Mater. Chem. C 2020, 8, 9678–9687. [Google Scholar] [CrossRef]
- Desai, N.K.; Kolekar, G.B.; Patil, S.R. Fabrication and Characterization of Anthracene Doped P-terphenyl Thin Films By Spin Coating Technique; Investigation of Fluorescence Properties. J. Fluoresc. 2020, 30, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Sun, M.; Xu, L.; Wang, R.; Zhou, H.; Pan, Y.; Zhang, S.; Sun, Q.; Xue, S.; Yang, W. Highly efficient non-doped blue fluorescent OLEDs with low efficiency roll-off based on hybridized local and charge transfer excited state emitters. Chem. Sci. 2020, 11, 5058–5065. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.-S.; Ha, Y.H.; Kwon, S.-K.; Kim, Y.-H.; Kim, J.-J. Design Strategy of Anthracene-Based Fluorophores toward High-Efficiency Deep Blue Organic Light-Emitting Diodes Utilizing Triplet–Triplet Fusion. ACS Appl. Mater. Interfaces 2020, 12, 15422–15429. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, X.; Li, Y.; Chen, H.; Zhuang, Z.; Shen, P.; Zeng, J.; Chi, J.; Ma, D.; Zhao, Z.; et al. High-Performance Hybrid White OLEDs with Ultra-Stable Emission Color and Small Efficiency Roll-Off Achieved by Incorporating a Deep-Blue Fluorescent Neat Film. Adv. Opt. Mater. 2021, 9, 2100298. [Google Scholar] [CrossRef]
- Kang, S.; Kwon, H.; Jeong, J.; Kim, Y.-C.; Park, J. Synthesis and Electroluminescence Properties of New Blue Emitting Polymer Based on Dual-Core Type for Solution Process OLEDs. Macromol. Res. 2022, 30, 454–459. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, G.; Luo, X.; Tian, X.; Zhang, D.; Guo, S.; Zhou, H.; Miao, Y.; Huang, J.; Wang, H. Anthracene-based blue fluorescence materials utilized in non-doped OLEDs with high luminance and a low efficiency roll-off. Dye. Pigment. 2022, 204, 110391. [Google Scholar] [CrossRef]
- Lee, J.H.; Lin, H.-Y.; Chen, C.-H.; Lee, Y.-T.; Chiu, T.-L.; Lee, J.-H.; Chen, C.-T.; Adachi, C. Deep Blue Fluorescent Material with an Extremely High Ratio of Horizontal Orientation to Enhance Light Outcoupling Efficiency (44%) and External Quantum Efficiency in Doped and Non-Doped Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2021, 13, 34605–34615. [Google Scholar] [CrossRef]
- Bałczewski, P.; Kowalska, E.; Różycka-Sokołowska, E.; Uznański, P.; Wilk, J.; Koprowski, M.; Owsianik, K.; Marciniak, B. Organosulfur Materials with High Photo- and Photo-Oxidation Stability: 10-Anthryl Sulfoxides and Sulfones and Their Photophysical Properties Dependent on the Sulfur Oxidation State. Materials 2021, 14, 3506. [Google Scholar] [CrossRef]
- Newman, M.S.; Hussain, N.S. Synthesis of nuclear monobromobenz[a]anthracenes. J. Org. Chem. 1982, 47, 2837–2840. [Google Scholar] [CrossRef]
- Andrus, M.B.; Ye, Z.; Zhang, J. Highly selective glycine phase-transfer catalysis using fluoroanthracenylmethyl cinchonidine catalysts. Tetrahedron Lett. 2005, 46, 3839–3842. [Google Scholar] [CrossRef]
- Harvey, R.G.; Cortez, C.; Sugiyama, T.; Ito, Y.; Sawyer, T.W.; DiGiovanni, J. Biologically active dihydrodiol metabolites of polycyclic aromatic hydrocarbons structurally related to the potent carcinogenic hydrocarbon 7,12-dimethylbenz[a]anthracene. J. Med. Chem. 1988, 31, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Hallman, J.L.; Bartsch, R.A. Synthesis of naphtho[f]ninhydrin. J. Org. Chem. 1991, 56, 6243–6245. [Google Scholar] [CrossRef]
- Platt, K.L.; Oesch, F. Reductive cyclization of keto acids to polycyclic aromatic hydrocarbons by hydroiodic acid-red phosphorus. J. Org. Chem. 1981, 46, 2601–2603. [Google Scholar] [CrossRef]
- Ihmels, H.; Meiswinkel, A.; Mohrschladt, C.J.; Otto, D.; Waidelich, M.; Towler, M.; White, R.; Albrecht, M.; Schnurpfeil, A. Anthryl-Substituted Heterocycles as Acid-Sensitive Fluorescence Probes. J. Org. Chem. 2005, 70, 3929–3938. [Google Scholar] [CrossRef]
- Sangaiah, R.; Gold, A.; Toney, G.E. Synthesis of a series of novel polycyclic aromatic systems: Isomers of benz[a]anthracene containing a cyclopenta-fused ring. J. Org. Chem. 1983, 48, 1632–1638. [Google Scholar] [CrossRef]
- Newman, M.S.; Prabhu, V.S.; Veeraraghavan, S. Synthesis of nuclear monobromobenz[a]anthracenes. J. Org. Chem. 1983, 48, 2926–2928. [Google Scholar] [CrossRef]
- Bradsher, C.; Sinclair, E. Notes—Cyclodehydrations in Liquid Sulfur Dioxide. J. Org. Chem. 1957, 22, 79–81. [Google Scholar] [CrossRef]
- Yamato, T.; Sakaue, N.; Shinoda, N.; Matsuo, K. Selective preparation of polycyclic aromatic hydrocarbons. Part 4.1 New synthetic route to anthracenes from diphenylmethanes using Friedel–Crafts intramolecular cyclization. J. Chem. Soc. Perkin Trans. 1 1997, 8, 1193–1200. [Google Scholar] [CrossRef]
- Bałczewski, P.; Skalik, J.; Uznański, P.; Guziejewski, D.; Ciesielski, W. Use of isomeric, aromatic dialdehydes in the synthesis of photoactive, positional isomers of higher analogs of o-bromo(hetero)acenaldehydes. RSC Adv. 2015, 5, 24700–24704. [Google Scholar] [CrossRef]
- Bodzioch, A.; Marciniak, B.; Różycka-Sokołowska, E.; Jeszka, J.K.; Uznański, P.; Kania, S.; Kuliński, J.; Bałczewski, P. Synthesis and Optoelectronic Properties of Hexahydroxylated 10-O-R-Substituted Anthracenes via a New Modification of the Friedel–Crafts Reaction Using O-Protected ortho-Acetal Diarylmethanols. Chem.—A Eur. J. 2012, 18, 4866–4876. [Google Scholar] [CrossRef] [PubMed]
- Bałczewski, P.; Bodzioch, A.; Różycka-Sokołowska, E.; Marciniak, B.; Uznański, P. First Approach to Nitrogen-Containing Fused Aromatic Hydrocarbons as Targets for Organoelectronics Utilizing a New Transformation of O-Protected Diaryl Methanols. Chem.—A Eur. J. 2010, 16, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Bałczewski, P.; Kowalska, E.; Skalik, J.; Koprowski, M.; Owsianik, K.; Różycka-Sokołowska, E. Ultrasound-assisted synthesis of RO- and RS-substituted (hetero)acenes via oxo- and thio-Friedel-Crafts/Bradsher reactions. Ultrason. Sonochem. 2019, 58, 104640. [Google Scholar] [CrossRef]
- Mondal, E.; Sahu, P.R.; Khan, A.T. A Useful and Catalytic Method for Protection of Carbonyl Compounds into the Corresponding 1,3-Oxathiolanes and Deprotection to the Parent Carbonyl Compounds. Synlett 2002, 2002, 0463–0467. [Google Scholar] [CrossRef]
- Djerassi, C.; Gorman, M. Studies in Organic Sulfur Compounds. VI.1 Cyclic Ethylene and Trimethylene Hemithioketals. J. Am. Chem. Soc. 1953, 75, 3704–3708. [Google Scholar] [CrossRef]
- Liang, X.; Gao, S.; Yang, J.; He, M. Synthesis of a Novel Strong Brønsted Acidic Ionic Liquid and its Catalytic Activities for the Oxathioacetalization. Catal. Lett. 2008, 125, 396–400. [Google Scholar] [CrossRef]
- Yus, M.; Nájera, C.; Foubelo, F. The role of 1,3-dithianes in natural product synthesis. Tetrahedron 2003, 59, 6147–6212. [Google Scholar] [CrossRef]
- Morton, D.R.; Hobbs, S.J. Facile preparation of cyclic ethylene thioketals and thioacetals with 2-phenyl- and 2-chloro-1,3,2-dithiaborolanes. J. Org. Chem. 1979, 44, 656–658. [Google Scholar] [CrossRef]
- Fuji, K.; Ueda, M.; Sumi, K.; Kajiwara, K.; Fujita, E.; Iwashita, T.; Miura, I. Chemistry of carbanions stabilized by sulfur. 1. Chemistry of 1,3-oxathianes. Synthesis and conformation of 2-substituted 1,3-oxathianes. J. Org. Chem. 1985, 50, 657–661. [Google Scholar] [CrossRef]
- Satchell, D.P.N.; Satchell, R.S. Mechanisms of hydrolysis of thioacetals. Chem. Soc. Rev. 1990, 19, 55–81. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm. J. Mol. Graph. Model. 2012, 38, 314–323. [Google Scholar] [CrossRef]
- ACD/Percepta, Version 14.0.0; Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2015.
- Míšek, J.; Vargas Jentzsch, A.; Sakurai, S.; Emery, D.; Mareda, J.; Matile, S. A Chiral and Colorful Redox Switch: Enhanced π Acidity in Action. Angew. Chem. Int. Ed. 2010, 49, 7680–7683. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xing, Z.; Fang, B.; Xie, X.; She, X. Visible light photoredox catalyzed deprotection of 1,3-oxathiolanes. Org. Biom. Chem. 2020, 18, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Björk, M.; Grivas, S. Synthesis of novel 2-aminoimidazo[4,5-b]pyridines, including the thieno analogue of the cooked-food mutagen IFP. J. Heterocycl. Chem. 2006, 43, 101–109. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Chemcraft—Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 1 March 2023).
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).