Abstract
The present investigation is focused on exploring the possibilities of identifying biomolecules from the fruiting body of the medicinal mushroom Ganoderma lucidum against the mango anthracnose pathogen Colletotrichum gloeosporioides. The fruiting body (cap and stipe portion) of G. lucidum extracted with ethyl acetate solvent at a maximum inhibitory concentration of 1 percent exhibited the maximum mycelial growth inhibition of C. gloeosporioides with 70.10 percent and 40.77 percent, respectively. Furthermore, subjecting the ethyl acetate extracts from the cap portion of G. lucidum through thin layer chromatography (TLC) revealed the presence of two bands with Rf values of 0.38 and 0.35. The compounds eluted from band 1 recorded with the maximum mycelial growth inhibition of C. gloeosporioides by 53.77 percent followed by band 2 (46.33 percent) using an agar well diffusion test. Similarly, the analysis of ethyl acetate extracts from the cap portion of G. lucidum through Gas Chromatography-Mass spectroscopy (GC-MS) revealed the presence of the organoheterocyclic compound benzothiazole, as expressed in the highest peak area at 22.03 RT with the highest probability percentage (97%). Confirmation of the antifungal nature of benzothiazole was obtained by testing the standard sample of benzothiazole which showed a cent percent of inhibition on mycelial growth of C. gloeosporioides at 50 ppm minimum fungicidal concentration. Furthermore, benzothiazole caused abnormality in the mycelial structures, viz., distortion, shrinkage, clumping of mycelium, conidial malformation, and complete arrestment of conidial germination of C. gloeosporioides as observed through Scanning Electron Microscopy. The research on biomolecular extract of G. lucidum could be a novel and interesting concept for the possibility in suppression of plant pathogenic microbes in the natural field.
1. Introduction
Mango is an ancient fruit crop known around the world for its high nutritive value, superb flavor, and delicious taste. Moreover, it is a highly nourishing fruit containing carbohydrates; proteins; fats; minerals such as potassium, magnesium, sodium, and phosphorus [1]; and vitamins including vitamin A (beta carotene), vitamin B1, vitamin B2, and vitamin C (ascorbic acid). Though this fruit crop is affected by various pathogens such as bacteria, fungi, viruses, and phytoplasmas. Mango anthracnose caused by C. gloeosporioides (Penz) is considered to be the most devastating disease given that it can present pre-harvest as well as post-harvest and can result in yield losses of even up to 100 percent [2,3]. Under the current scenario, continuous and judicious application of fungicide against the pathogen has resulted in environmental pollution and health problems. Apart from that, it also leads to buildup of fungicide resistance in the pathogen. This situation has forced scientists to develop a fungicidal formulation from various natural sources that might provide a method to tackle the problem. In this context, macro-basidiomycete fungi are in the limelight and evoked interest globally for their bioactive compounds which have been found to have pharmaceutical and therapeutical values as well as nutritional value [4]. Moreover, mushrooms possess natural compounds exhibiting bioactivities of antifungal, antibacterial, and antiviral properties [5]. Based on the investigation, the current research is focused on the exploitation of antimicrobial compounds from G. lucidum against plant pathogens, as is evidenced by the invention of the fungicide Azoxystrobin from mushroom fungi Strobilurus tenacellus. This fungicide is effective against both downy mildew as well as powdery mildew of grapes. Cordyceps sobolifera has been used as a potential biocontrol agent against Colletotrichum gleosporioides [6]. Similarly, the report showed that a solvent extracted antimicrobial properties of various Ganoderma species, including Ganoderma lucidum, Ganoderma applanatum, and Ganoderma austral, against plant pathogenic fungi [7]. However, the authors reported that the ethyl acetate fraction of the fruiting body of ectomycorrhizal fungus Pisolinthus tinctorius possessed antifungal activity against soil-borne plant pathogens [8]. Similarly, the antimicrobial activity of crude culture filtrate from L. edodes, G. lucidum, C. sinensis, and A. polytricha showed maximum inhibitory activity against the mycelial growth of C. capsici by agar well diffusion assay [9]. Interestingly, in another study [10], the methanolic extract from fruiting bodies of Coprinus comatus inhibited the mycelial growth of Fusarium spp. The chloroform extract of antimicrobials from the fruiting body of G. lucidum exhibited higher antagonistic activity against Colletotrichum capsici [10]. In this aspect, the present study was framed to screen the antimicrobial activity of G. lucidum against C. gloeosporioides, which causes anthracnose disease in mango, and also to characterize the antimicrobial compounds which help us to develop fungicidal formulations from naturally available compounds.
2. Results
2.1. Testing the Ethyl Acetate Extracted Antimicrobial Compound from Fruiting Body (Cap and Stipe) of G. lucidum against the C. gloeosporioides
Perusal of literature revealed that the fruiting body of mushrooms is also known to possess copious antimicrobial properties. The current study was intended to tap the antimicrobial compound from the fruiting body of G. lucidum. For this purpose, the required fruiting bodies of G. lucidum were produced at the Department of Plant Pathology, Coimbatore, (image shown) by following the cultivation technology provided by the Directorate of Mushroom Research, Solan, Himachal Pradesh (www.nrcm.org.in (access on 10 September 2019)) (Figure 1). The cap and stipe portion of G. lucidum fruiting bodies were separated and stored for further studies.
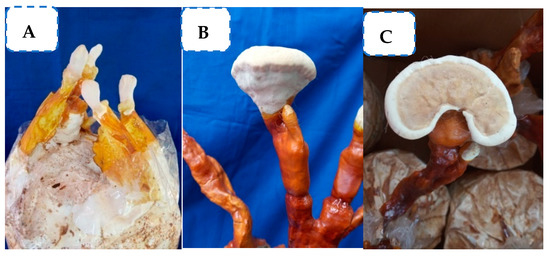
Figure 1.
Production of the fruiting body of G. lucidum. (A) Elongation of stipe; (B) expansion of fruiting body; and (C) bracket-shaped fruiting body.
Using ethyl acetate solvent, the antimicrobial compounds were extracted from both the cap and stipe portion of the fruiting body. Furthermore, the compounds were made into different concentrations, viz., 0.05 percent, 0.1 percent, 0.2 percent, 0.4 percent, 0.6 percent, 0.8 percent, and 1 percent concentrations, to test the appropriate concentration that could inhibit the maximum mycelial growth of C. gloeosporioides. The result showed that the solvent (ethyl acetate) fractions of the cap portion of G. lucidum exhibited antifungal activity at all concentrations, as shown by mycelial inhibition ranging from 42.2 to 70.10 percent. However, maximum mycelial inhibition percentage of 70.10 was observed at a 1 percent concentration of ethyl acetate fraction of the cap portion of G. lucidum (Figure 2; Table 1).
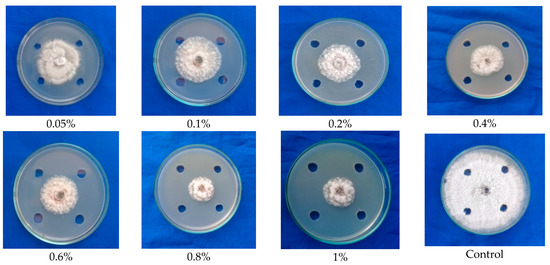
Figure 2.
Testing the ethyl acetate-extracted antimicrobial compounds from fruiting body (cap) of G. lucidum against mycelial growth of C. gloeosporioides.

Table 1.
Testing the ethyl acetate-extracted antimicrobial compound from the fruiting body (cap and stipe) of G. lucidum against C. gloeosporioides.
The solvent fraction of the stipe portion of G. lucidum exhibited an inhibition of mycelial growth of 34.00 percent to 40.77 percent. However, a maximum inhibition of mycelial growth of 40.77 percent was observed at 1 percent concentration (Figure 3), which was comparatively lower than the antifungal activity of fraction from the cap portion of G. lucidum. From the result, it is obvious that the cap portion of the fruiting body possesses maximum antimicrobial activity, and it was further subjected to GC-MS analyses to identify the compound.
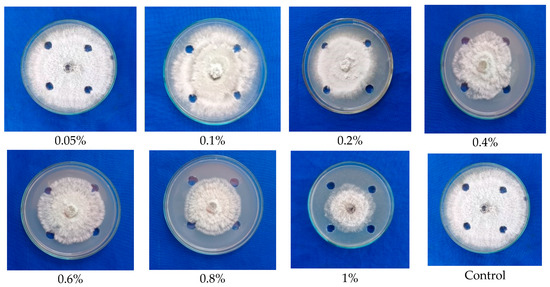
Figure 3.
Testing the ethyl acetate extracted antimicrobial compounds from the fruiting body (Stipe) of G. lucidum against mycelial growth of C. gloeosporioides.
2.2. Identifying the Presence of Antifungal Biomolecules from Ethyl Acetate Solvent Extracts of Fruiting Body (Cap) of G. lucidum through TLC Studies
Detection of specific compounds from ethyl acetate solvent extracts of the fruiting body of G. lucidum culture filtrates was analyzed through TLC. The TLC analysis for the ethyl acetate solvent extracts of the fruiting body (cap) of G. lucidum using the mobile phase Chloroform: Methanol: Water at the proportion 30:4:1 indicated the appearance of two bright bands under UV lamp at the Rf values of 0.38 and 0.35 (Figure 4a).
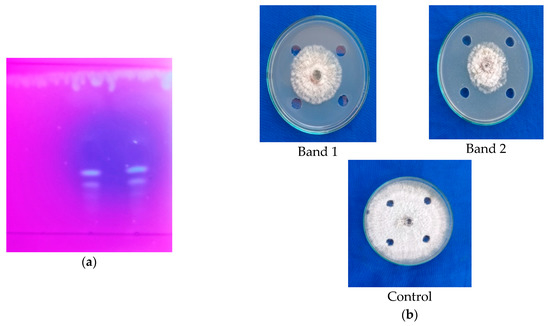
Figure 4.
(a) Detection of antimicrobial compounds by ethyl acetate extracts from the fruiting body of G. lucidum (cap) through TLC; (b) antimicrobial activity of compound eluted from TLC.
Further, the bands (band 1 and band 2) were eluted. The antifungal activity of eluted extract from the bands was tested against C. gloeosporioides using the agar well diffusion technique indicated that bands 1 and 2 (Rf value: 0.38 and 0.35, respectively) showed inhibition of mycelial growth of C. gloeosporioides in the range of 46.33 percent and 53.77 percent, respectively (Figure 4b; Table 2). Based on the result, it was obvious that band 2 possessed maximum antifungal activity against C. gloeosporioides when compared to band 1. This further confirmed that the fruiting bodies (cap) of G. lucidum possess higher antimicrobial activity.

Table 2.
Testing the antimicrobial activity of compounds eluted from TLC band of G. lucidum (extracts from fruiting body) against mycelial growth of C. gloeosporioides.
2.3. Detection of Antimicrobial Compound from Cap Portion of Fruiting Body of G. lucidum through GC-MS
From the previous result, it was predicted that the cap portion contains more antimicrobial compounds when compared to stipe portion as observed by a mycelial inhibition of 70.10 percent. Hence, the antimicrobial compound from the cap portion of the fruiting body was separately characterized through GC-MS analysis. GC-MS analysis of ethyl acetate fraction of the fruiting body (the cap portion) indicated the presence of different compounds, viz., 5H-Cyclopropa [3,4] Benz [1,2-e] azulen-5-one; Diisooctyl phthalate; Docosanoic acid, 1,2,3-propanetriyl ester; Propanoic acid; and 7, 8-Epoxylanostan-11-ol,3-acetoxy, with most of the compounds possessing antimicrobial activity. The benzothiazole compound detected at 22.03 RT showed the highest peak area with antimicrobial activity and was used for further testing (Table 3 and Supplemental Figure S1).

Table 3.
List of antimicrobial compounds from ethyl acetate solvent fractions from fruiting body of G. lucidum using GCMS.
2.4. Testing the Antifungal Activity of Standard Sample of Benzothiazole against C. gloeosporioides
From the GC-MS analysis, the compound benzothiazole from the fruiting body (cap) of G. lucidum was identified with high peak area percentage (22.03), as observed in a previous study. Hence, the standard sample of benzothiazole was purchased from Sigma–Aldrich chemicals (Compound code: 101338-500G) and tested against mycelial inhibition and spore germination activity of C. gloeosporioides.
2.4.1. Testing Antifungal Activity of Benzothiazole (Standard Sample) at Different Concentrations against Mycelial Growth of C. gloeosporioides (Agar Well Diffusion)
Initially, the standard sample benzothiazole was dissolved in acetone, and then it was made into different inhibitory concentrations, viz., 1, 5, 10, 50, 75, 100, and 150 ppm. Furthermore, the different concentrations were tested separately against the mycelial growth of C. gloeosporioides via the agar well diffusion technique.
Based on the result obtained, it was found that the compound benzothiazole completely arrested the mycelial growth at the maximum inhibitory concentration of 50 ppm. The compound showed 50 percent mycelial inhibition at 1 ppm MIC. However, the growth pattern of mycelium in Petri plate was observed as the push back of mycelium near the agar well zone, deformation of mycelium, blackened mycelial growth, and breakage of mycelial disc; whereas the control showed normal mycelial growth with concentric rings (Figure 5; Table 4).
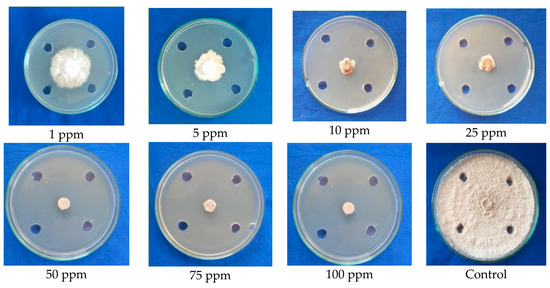
Figure 5.
Conformation of the antifungal efficacy of benzothiazole at different concentrations against mycelial growth of C. gloeosporioides (Agar well test).

Table 4.
Testing the antifungal efficacy of benzothiazole at different concentrations against mycelial growth of C. gloeosporioides.
2.4.2. Confirming the Antifungal Activity of Benzothiazole (Standard Sample) against Mycelial Growth of C. gloeosporioides (Paper Disc Assay)
The antifungal activity of benzothiazole (standard sample) was further cross-checked via a paper disc assay. The result revealed that benzothiazole was also found to exhibit 100 percent inhibition at the maximum fungicidal concentration of 75 ppm in the paper disc assay. It clearly depicted that the compound has remarkably higher antifungal activity against C. gloeosporioides, as observed by the linear mycelium growth towards the paper disc, changes in the growth pattern of mycelium, retardation of mycelium growth, shrinkage of mycelial growth, and complete arresting of mycelial growth when compared to the control with normal mycelial growth (Figure 6; Table 5).
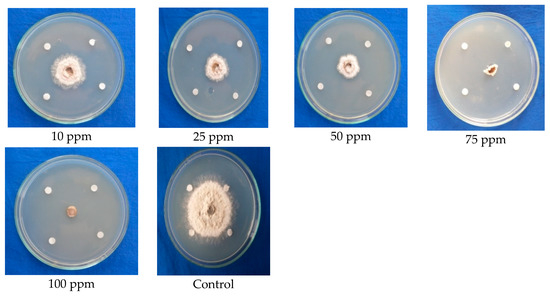
Figure 6.
Confirmation of the antifungal efficacy of benzothiazole at different concentrations against mycelial growth of C. gloeosporioides (paper disc assay).

Table 5.
Testing the antifungal efficacy of different concentrations of benzothiazole against mycelial growth C. gloeosporioides by paper disc assay.
2.4.3. Testing the Effect of Benzothiazole (Standard Sample) at Optimum Concentration (50 ppm) on Conidial Germination of C. gloeosporioides
Antifungal activity of benzothiazole was tested against the conidial germination of the target pathogen by the cavity slide method. The result revealed that no conidial germination was observed at 6 h in both the control and the treated groups. However, the conidial germination was 26.82 numbers in the control at 12 h and 50.34 numbers at 24 h; whereas the benzothiazole completely reduced the conidial germination of C. gloeosporioides, and no germination was observed even at 24 h. The compound showed 100 percent inhibition of conidial germination at 6, 12, and 24 h compared to the control (Table 6).

Table 6.
Testing the effect of benzothiazole (50 ppm) on germination of spores of C. gloeosporioides.
2.5. Antifungal Effect of Benzothiazole (Standard Sample) against Spores and Mycelium of C. gloeosporioides by Scanning Electron Microscopy (SEM)
The antifungal activity of benzothiazole on the morphological changes of mycelium and conidia of C. gloeosporioides was observed under Scanning Electron Microspcopy (SEM). The results showed that the standard sample benzothiazole completely inhibited the conidial germination of C. gloeosporioides when compared to the control (untreated), in which a certain percentage of conidial germination was observed (Figure 7). Further, distortion, shrinkage, clumping of mycelium, and conidial malformations were prominent in the benzothiazole treated samples. The untreated mycelium and conidia were normal without any morphological changes. The results further clearly depicted the antifungal nature of benzothiazole, which proves that G. lucidum possess biomolecules with fungicidal activity.
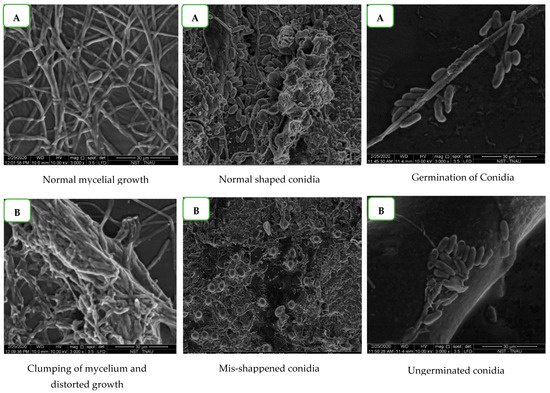
Figure 7.
Effect of benzothiazole on germination of spores of C. gloeosporioides under Scanning Electron Microscopy (SEM). (A) Control. (B) Treatment.
3. Discussion
Mushrooms contain various natural compounds with bioactive potential, viz., antifungal, antibacterial, antiviral, antitumor, anti-nemic, anti-inflammatory, anti-allergic, anti-atherogenic, and anti-diabetic properties. Every species of mushrooms contains various bioactive molecules and pharmacological effects [11]. Hence, it was decided to investigate the antimicrobial compound from the fruiting body of G. lucidum, including both the cap and stipe portions separately against C. gloeosporioides.
The result showed that the solvent (ethyl acetate) fractions of the cap portion of G. lucidum exhibited antifungal activity ranging from 42.2 to 70.10 percent at all concentrations. However, a maximum mycelial inhibition percentage of 70.10 was observed at a 1 percent concentration of ethyl acetate fraction of the cap portion of G. lucidum. The solvent fraction of the stipe portion of G. lucidum which exhibited maximum inhibition of mycelial growth of 40.77 percent was at 1 percent concentration, which was comparatively lower than the antifungal activity of the fraction from the cap portion of G. lucidum. Our findings were in line with the scientist who tested the solvent-extracted antimicrobial property of various Ganoderma species, including G. lucidum, G. applanatum and G. austral, against plant pathogenic fungi and found that the crude extract of G. lucidum showed a greater zone of inhibition (24.3 mm) against Aspergillus niger followed by G. applanatum (23.3 mm) [7].
Detection of specific compounds from ethyl acetate solvent extracts of the fruiting body of G. lucidum culture filtrates was analyzed through TLC, which indicated the appearance of two bright bands under a UV lamp at the Rf values of 0.38 and 0.35. Furthermore, the bands (band 1 and band 2) were eluted, and their antifungal activity was tested against C. gloeosporioides using the agar well diffusion technique. The results indicated that bands 1 and 2 (Rf values of 0.38 and 0.35, respectively) eluted from the TLC plate showed inhibition of mycelial growth of C. gloeosporioides in the range of 46.33 percent and 53.77 percent, respectively. This further confirmed that the fruiting bodies (cap) of G. lucidum possess higher antimicrobial activity. Accordingly, [10] performed the TLC analysis for the chloroform extracts of the fruiting body of G. lucidum, and the results indicated the presence of two bands with Rf values of 0.29 and 0.62. The eluted compounds showed inhibition percentages of 66.67 and 60.42 against the mycelial growth of C. capsici.
In the present study, the GC-MS analysis of the ethyl acetate fraction of the fruiting body (the cap portion) of G. lucidum indicated the presence of different compounds, viz., 5H-Cyclopropa [3,4] Benz[1,2-e] azulen-5-one; Diisooctyl phthalate; Docosanoic acid, 1,2,3-propanetriyl ester; Propanoic acid; and 7,8-Epoxylanostan-11-ol, 3-acetoxy, with most of the compounds possessing antimicrobial activity. H-Cyclopropa [3,4] Benz[1,2-e] azulen-5-one possessed antibacterial property. Propanoic acid and benzothiazole possessed antifungal activity, whereas 7,8-Epoxylanostan-11-ol, 3-acetoxy possessed anti-nemic activity. The organoheterocyclic compound benzothiazole, a compound detected at 22.03 RT, showed the highest peak area with antimicrobial activity and was used for further studies. Accordingly, [12] identified bioactive compounds including triterpenoid and vulpinic acid from the fruiting body of Scleroderma citrinum, which showed antagonistic activity against mycelial growth of Candida albicans. This is in line with the findings [13] from which they also reported that the compounds obtained from the fruiting body of Scleroderms citrinum, including 4,4-Dimethoxy methyl vulpinate (DMV) and 4,4 Dimethoxy vulpinic acid (DMVA), were found to possess antifungal activity against C. gloeosporioides. Another report [8] showed that three olypins such as 9-hydroxy 10, 12-octadecadienoic acid; 13-oxo 10, 12 octadecadienoic acid; and 9-oxo 10, 12 octadecadienoic acid from Pisolithus tinctorius showed antagonistic activity against plant pathogenic fungi such as C. gloeosporioides, C. fragariae, C. acutatum, Botrytis cinerea, F. oxysporum, Phomopsis obscurang, and Phomopsis viticola.
Benzothiazole is an aromatic organoheterocyclic compound and also a potent antimicrobial secondary metabolite and a volatile compound found in plants and bacteria. On the other hand, the mechanism of benzothiazole against plant pathogens is still unclear. Here, in this study, a complete mycelial inhibition was observed at 50 ppm concentration of benzothiazole, and a 50 percent mycelial inhibition was observed at 1 ppm concentration. The growth pattern of mycelium was observed as mycelium pushed back near the agar well zone, distortion in mycelial growth, blackening of mycelial growth, and breakage of the mycelial disc; whereas the control showed normal mycelial growth with concentric rings. Remarkably, the benzothiazole compound possessed antimicrobial activity against Phytophthora capsica, disrupted the cell membrane, and inhibited the hyphal growth of the fungi [14]. More fascinatingly, benzothiazole possessed antifungal activity against Penicillium italicum, which causes postharvest navel orange. Furthermore, it acts as the analog of naturally occurring salicylic acid and is highly effective for induction of systemic acquired resistance (SAR) in plants. These findings line up with our finding, namely that benzothiazole possesses antifungal activity against C. gloeosporioides.
The results observed under SEM showed that the standard sample of benzothiazole completely inhibited the conidial germination of C. gloeosporioides when compared to the control (untreated), in which conidial germination was observed. Furthermore, distortion, shrinkage, clumping of mycelium, and conidial malformations were prominent in the benzothiazole treated samples. The untreated mycelium and conidia were normal without any morphological changes. The results further depicted the antifungal nature of benzothiazole, which proves that G. lucidum possesses biomolecules with fungicidal activity. Furthermore, the results clearly depict the antisporulant nature of benzothiazole. Similar to our study, [14] observed the hyphal morphology of Phytophthora capsici treated with benzothiazole concentration 150 mg/L at different hours of incubation and showed that the hyphae treated with benzothiazole exhibited swelling and contortion at 24 h. The hyphae produced more abnormal branches after exposure for 48 h, and the hyphae began to develop periodical swellings after exposure for 72 h.
4. Material and Methods
4.1. Production of the Fruiting Body of G. lucidum
Based on the protocol (www.dmr.org.in (accessed on 10 September 2019)) provided by the Directorate of Mushroom Research, Solan, the cultivation of the fruiting body of G. lucidum was carried out at the Department of Plant Pathology, Tamil Nadu Agricultural University, Coimbatore, India. Coconut sawdust along with 20% wheat bran was used as a substrate for mushroom production. Optimum moisture of 65% was maintained in sawdust, and it was packed at 1 kg in a polypropylene bag plugged with non-absorbent cotton. The bag was sterilized using autoclave at 121 °C, 15 lbs for 2 h. After autoclaving, the sterilized bags were spawned (2%) with the sorghum-based spawn of G. lucidum, and the bags were incubated at 28 ± 2 °C for the complete run of spawn run up to 35 days. Furthermore, the bags were transferred into the cropping room, and the optimum temperature of 28 °C and 80% RH were maintained for the emergence of a pinhead. Later, the temperature was lowered to 25 °C with 60% RH for cap maturation. Required quantities of fruiting bodies were harvested and kept for further analysis.
4.2. Extraction of Antimicrobial Compounds from Fruiting Body of G. lucidum
The fruiting body of G. lucidum was harvested, and the cap and stipe portion were separated. The cap and stipe portion were ground separately into a fine powder using a pestle and mortar. For 10 g of the powdered sample, 100 mL of ethyl acetate solvent was added. The flasks were kept overnight in a shaker at 150 rpm. Later, they were filtered through double-layered muslin cloth followed by Whatman No. 1 filter paper, and the solvents were evaporated using a vacuum flask evaporator at 40 °C. The residue obtained was dissolved in HPLC grade methanol. The extracts were filtered through a membrane filter (0.2 µm) and stored at 4 °C for further research. The same procedure was followed for the stipe portion of the fruiting body.
In Vitro Antimicrobial Activity of Ethyl Acetate Extracts from Fruiting Body of G. lucidum against C. gloeosporioides
The ethyl acetate solvent extracts from the cap and stipe portions of G. lucidum were tested against the mycelial growth of C. gloeosporioides at different concentrations from 0.1 percent to 1 percent via the agar well diffusion technique. Four wells were made at equal distance, with a margin of 1 cm from the periphery of the Petri plate containing sterilized PDA medium. About 100 µL from the extracts of each concentration was added to the plate. A 9 mm mycelial disc of C. gloeosporioides was placed at the center of the plate. Three replications were maintained for each concentration. Sterile water served as the control. Plates were incubated at room temperature, and the percentage inhibition of mycelial growth in pathogen over the control was calculated by using the formula given in [15].
4.3. Identification of Antimicrobial Compounds Solvent Extracts of the Fruiting Body (Cap) of G. lucidum through TLC
Antimicrobial compounds from the ethyl acetate-extracted fruiting body (the cap portion) extracts of G. lucidum were spotted on the silica-gel-coated TLC plates at the rate of 20 µL/spot. The spots were allowed to dry, and the chromatograph (Thermostat Vertical Autoclave, STE-V 60, MRC lab, India) was developed using a solvent system, viz., Chloroform: Methanol: Distilled water 30:4:1. The plate was allowed to run at the ¾th position of the plate. Then, the plates were observed under a UV transilluminator (UV 25 Transilluminator, Hoefer Scientific, Hollistan, MA, USA) at 254 nm. Bands observed in the UV lamp were marked, and the Rf value was calculated and recorded.
Testing the Antifungal Activity of TLC Eluted Compounds of G. lucidum against C. gloeosporioides
Specific bands obtained through TLC studies were scrapped from the silica plate and suspended in 1mL HPLC grade methanol. The solution was vortexed for 10–15 min and then further centrifuged at 10,000 rpm for 10 min. The methanol along with the compound and the silica were separated and filtered through a membrane filter (0.2 µm), and the antimicrobial activity of the compound was tested against the mycelial growth of C. gloeosporioides via the agar well diffusion technique by following the procedure as described earlier with three replications.
4.4. Profiling of Antimicrobial Compounds from G. lucidum through GC-MS
Based on the results obtained from the previous study, the ethyl acetate-extracted antimicrobial compound from the cap portion of G. lucidum showed a quite higher mycelial inhibition percentage against C. gloeosporioides than the stipe portion. Therefore, the antimicrobial compound from the cap portion of the fruiting body was characterized through GC-MS analysis. In this study, the Trace GC Ultra and DSQII model MS from Thermo Fisher Scientific Limited, Markha, Canada were engaged for analysis [9,16].
4.5. Testing the Antifungal Activity of the Standard Sample (Benzothiazole) against C. gloeosporioides
From the GC-MS analysis, the compound benzothiazole from the fruiting body (cap) of G. lucidum with a high peak area and probability percentage were identified as the antimicrobial compound. Hence, to confirm the antifungal activity, the standard sample benzothiazole was purchased from Sigma-Aldrich chemicals (Sigma Aldrich, Saint Louis, MO, USA) and tested against mycelial inhibition of C. gloeosporioides via the agar well diffusion technique.
Benzothiazole standard sample was tested at lower concentrations because higher concentrations inhibited complete mycelial growth. Thus, the standard sample of benzothiazole was dissolved in acetone, and then it was made into different concentrations, viz., 1, 5, 10, 50, 75, and 100 ppm. Furthermore, the different concentrations were tested separately against the mycelial growth of C. gloeosporioides by agar well diffusion assay, as discussed earlier, with three replications for each treatment.
4.6. Effect of Standard Sample Benzothiazole on C. gloeosporioides under Scanning Electron Microscopy
Scanning Electron Microscope analysis was carried at the Department of Nano Science and Technology, Tamil Nadu Agricultural University, Coimbatore, and was used to assess the changes in mycelium and conidia of C. gloeosporioides due to the fungicidal action of benzothiazole at 50 ppm. The morphostructural changes of the pathogen were viewed under Scanning Electron Microscope (SEM: Quanta 250, FEI, Hillsboro, OR, USA) with Large Field Detector (LFD). The SEM was operated in vacuum 10 KV with a spot size of 3.0 and a pressure of 60 Pa. The sample images were recorded at 5000× magnification. The source of electrons used in the SEM was Tungston Filament, and Thermoionic emission was used for the detection of the samples in SEM.
4.7. Statistical Analysis
The design of experiments (CRBD) and statistical analyses were followed as per the suggestions of (Gomez and Gomez, 1984). Statistical software SPSS (version number 16.0) was used for the analyses of the data. In case of zero values, the data were arcsine-transformed (1/4n) before statistical analysis.
5. Conclusions
The results of the current research overwhelmingly indicated the presence of innate and novel biomolecules in the medicinal mushroom G. lucidum, with antimicrobials which performed well in inhibiting both vegetative and reproductive structures of C. gloeosporioides. The compound benzothiazole showed promising effects against C. gloeosporioides. Future studies are planned towards studying the mechanism of benzothiazole and its role in secretion of defense enzymes against pathogens in plants. Thus, G. lucidum offers a spectrum of opportunity for setting a pathway on the eco-friendly management of anthracnose disease in mango plants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28062476/s1, Figure S1: Characterization of antimicrobial compounds from ethyl acetate solvent fractions from the fruiting body of G. lucidum.
Author Contributions
T.G. planned the concept on antifungal activity of G. lucidum to assess the biological activity against the anthracnose pathogens. P.T., A.S.B., A.P.I., R.S., K.A.S. and H.S. contributed to the experimental analysis, methodologies and resource. G.M. experimented. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are available in the manuscript.
Acknowledgments
The authors thank the university, Department of Plant Pathology and ICAR-AICRP on mushroom for carrying out the research activities.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds benzothiazole are available from the authors.
References
- Leharwan, S.; Malik, V.K.; Rajender, S.; Leharwan, M. Effect of Culture Media and Temperature on Growth and Sporulation Colletotrichum gloeosporioides cause of Anthracnose Disease of Mango. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1835–1842. [Google Scholar] [CrossRef]
- Arauz, L.F. Mango anthracnose: Economic impact and current options for integrated management. Plant Dis. 2000, 84, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.N.; Narsaiah, K.; Sharma, A.D.; Singh, M.; Bansal, S.; Kumar, R. Quality parameters of mango and potential of non-destructive techniques for their measurement—A review. J. Food Sci. Technol. 2010, 47, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Poucheret, P.; Françoise, F.; Sylvie, R. Biological and pharmacological activity of higher fungi: 20-year retrospective analysis. Cryptogam. Mycol. 2006, 27, 311. [Google Scholar]
- Zahid, S.; Chibuike, C.U.; Athar, A.; Michael, O.E.; Edward, P.S.; Paul, H. New bioactive natural products from Coprinus Micaceus. Nat. Prod. Res. 2006, 20, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Imtiaj, A.; Lee, T.S. Screening of antibacterial and antifungal activities from Korean wild mushrooms. World J. Agric. Sci. 2007, 3, 316–321. [Google Scholar]
- Jonathan, S.G.; Awotona, F. Studies on antimicrobial potentials of three Ganoderma species. Afr. J. Biomed. Res. 2010, 13, 131–139. [Google Scholar]
- Ganesh, K. Exploration of Antifungal Bioactive Compounds of Pisolithus tinctorius (Pers) Coker against Some Soil Borne Plant Pathogens. Ph.D. Thesis, Tamil Nadu Agric University, Coimbatore, India, 2014. [Google Scholar]
- Priya, K.; Thiribhuvanamala, G.; Kamalakannan, A.; Krishnamoorthy, A.S. Antimicrobial Activity of Biomolecules from Mushroom Fungi against Colletotrichum capsici (Syd.) Butler and Bisby, the Fruit Rot Pathogen of Chilli. Int. J. Curr. Microbiol. A Sci. 2019, 8, 1172–1186. [Google Scholar] [CrossRef]
- Jeeva, S.; Krishnamoorthy, A.S. Antifungal Potential of Myco-molecules of Coprinopsis cinerea (Schaeff) S. Gray s. lat. against Fusarium spp. Madras Agric. J. 2018, 105, 1. [Google Scholar]
- Priya, K. Characterization of antimicrobial compounds from mushroom fungi and medicinal plants against Colletotrichum capsici (syd.) Butler and Bisby, an inciting agent of chilli anthracnose. Master’s Thesis, Tamil Nadu Agric University, Coimbatore, India, 2019; pp. 36–46. [Google Scholar]
- Lindequist, U.; Niedermeyer, T.H.; Jülich, W.-D. The pharmacological potential of mushrooms. Evid. -Based Complement. Altern. Med. 2005, 2, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Kanokmedhakul, S.; Kwanjai, K.; Thirada, P.; Kasem, S.; Palangpon, K.; Apichart, S. A bioactive triterpenoid and vulpinic acid derivatives from the mushroom Scleroderma citrinum. Planta Med. 2003, 69, 568–571. [Google Scholar] [PubMed]
- Soytong, K.; Phoutthasone, S.; Kwanchai, K.; Somdej, K. Biological active compounds of Scleroderma citrinum that inhibit plant pathogenic fungi. J. Agric. Technol. 2014, 10, 79–86. [Google Scholar]
- Mei, X.; Liu, Y.; Huang, H.; Du, F.; Huang, L.; Wu, J.; Li, Y.; Zhu, S.; Yang, M. Benzothiazole inhibits the growth of Phytophthora capsici through inducing apoptosis and suppressing stress responses and metabolic detoxification. Pestic. Biochem. Physiol. 2019, 154, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, P.; Balamurali, A.S.; Subbiah, K.A.; Sevugapperumal, N.; Gurudevan, T.; Uthandi, S.; Shanmugam, H. Antifungal Volatilomes Mediated Defense Mechanism against Fusarium oxysporum f. sp. lycopersici, the Incitant of Tomato Wilt. Molecules 2022, 27, 3631. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).