Subcritical Water Extraction of Onosma mutabilis: Process Optimization and Chemical Profile of the Extracts
Abstract
1. Introduction
2. Results and Discussion
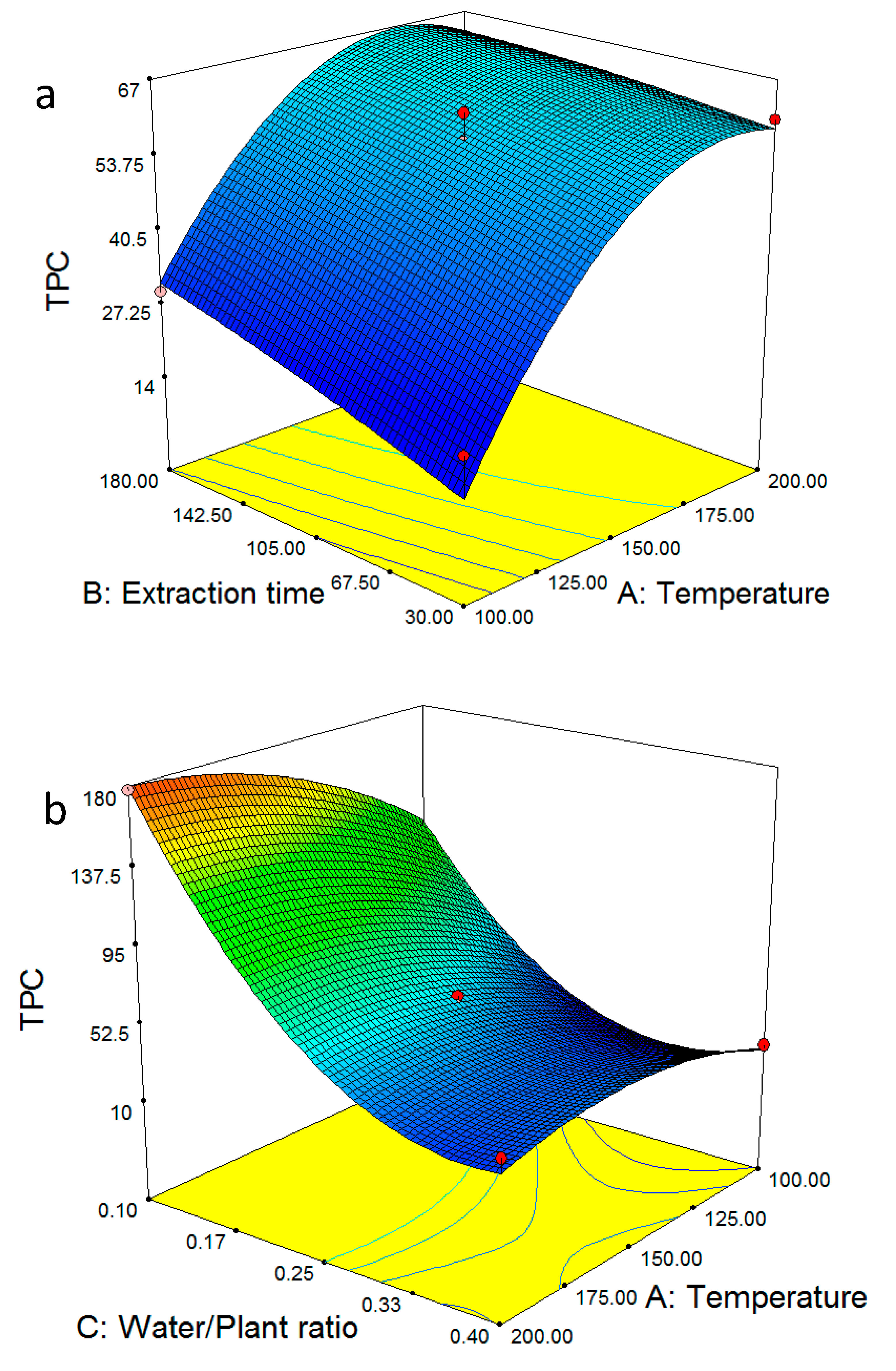
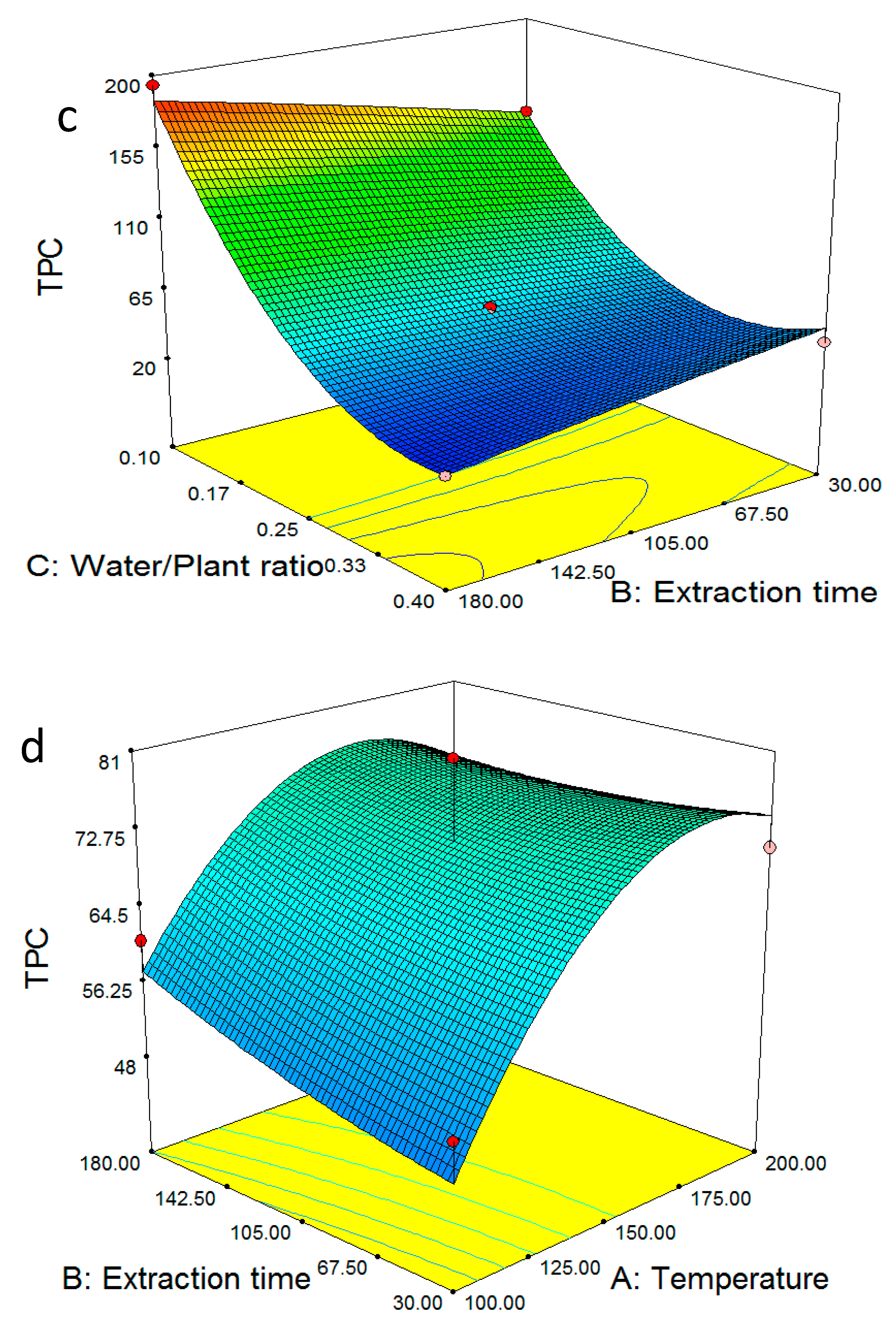
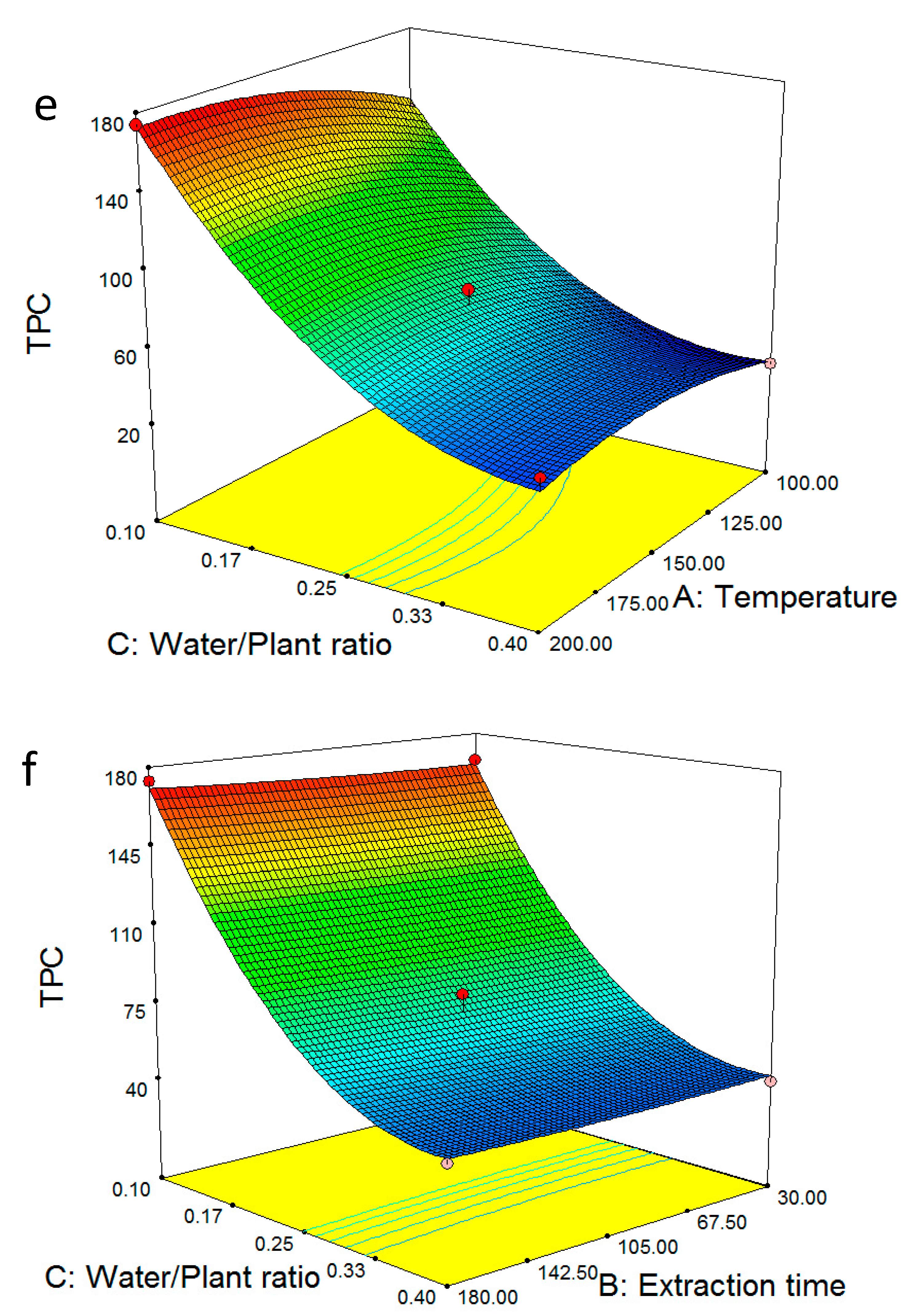
2.1. Optimum Extraction Yield and Total Phenolic Content
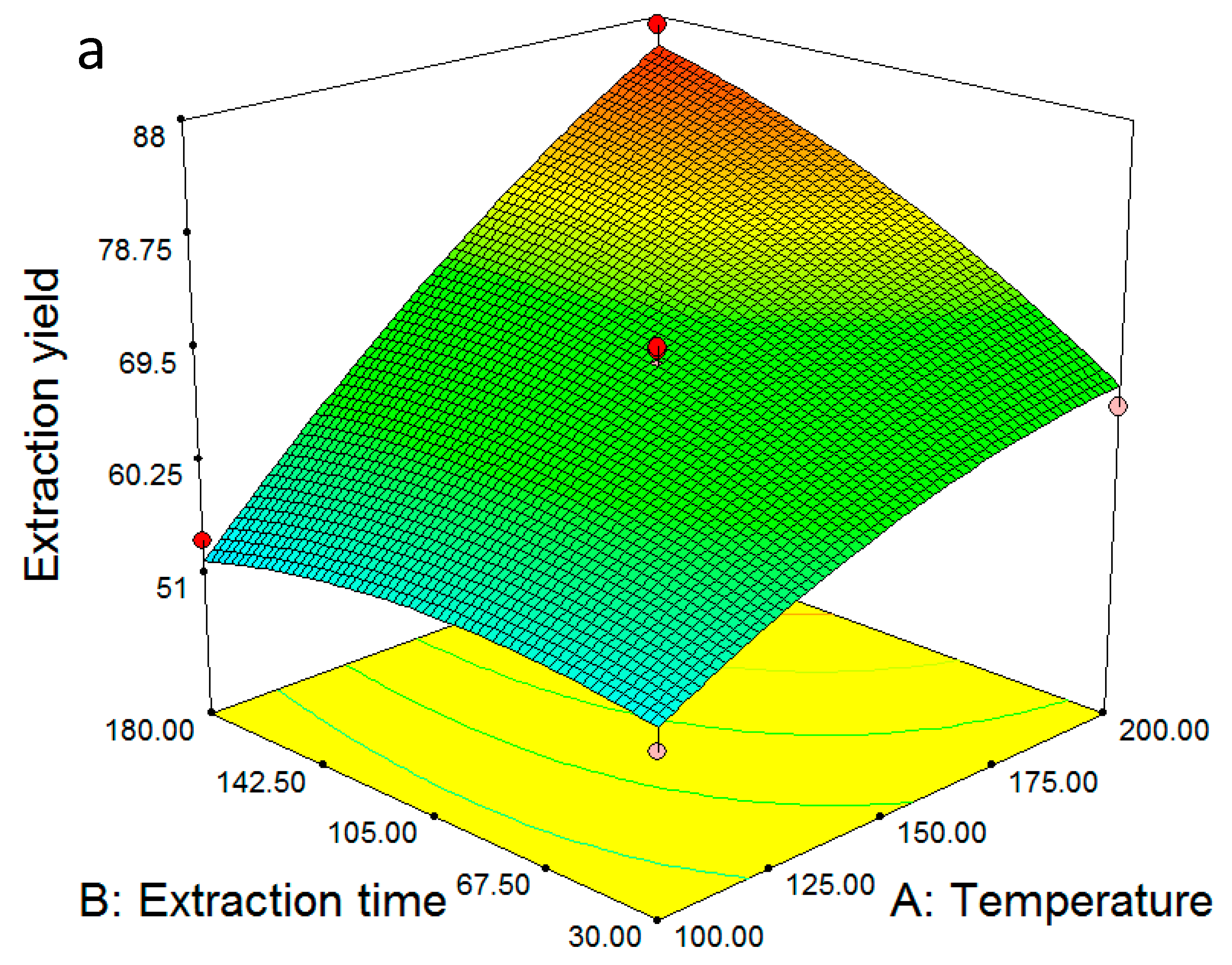
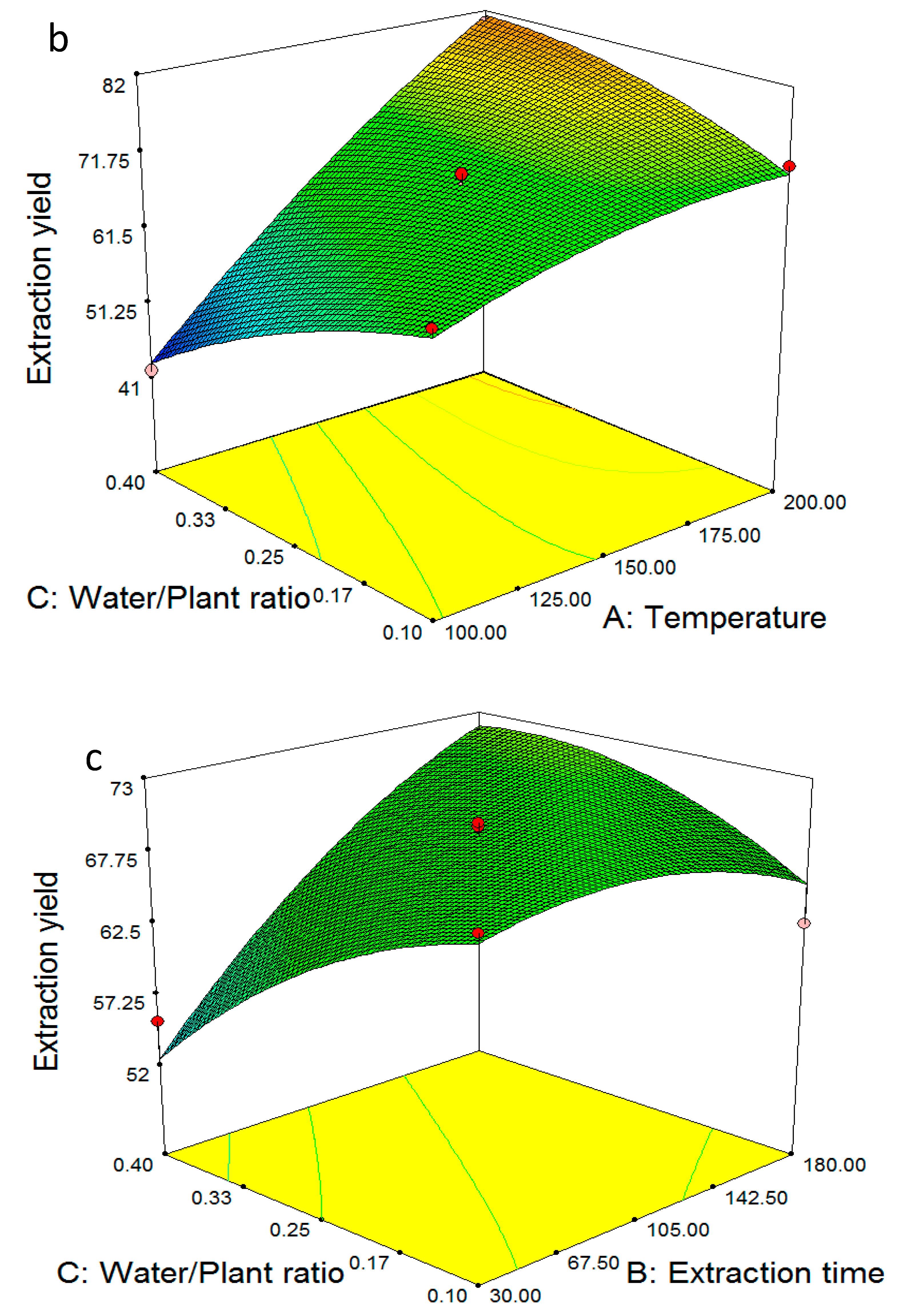
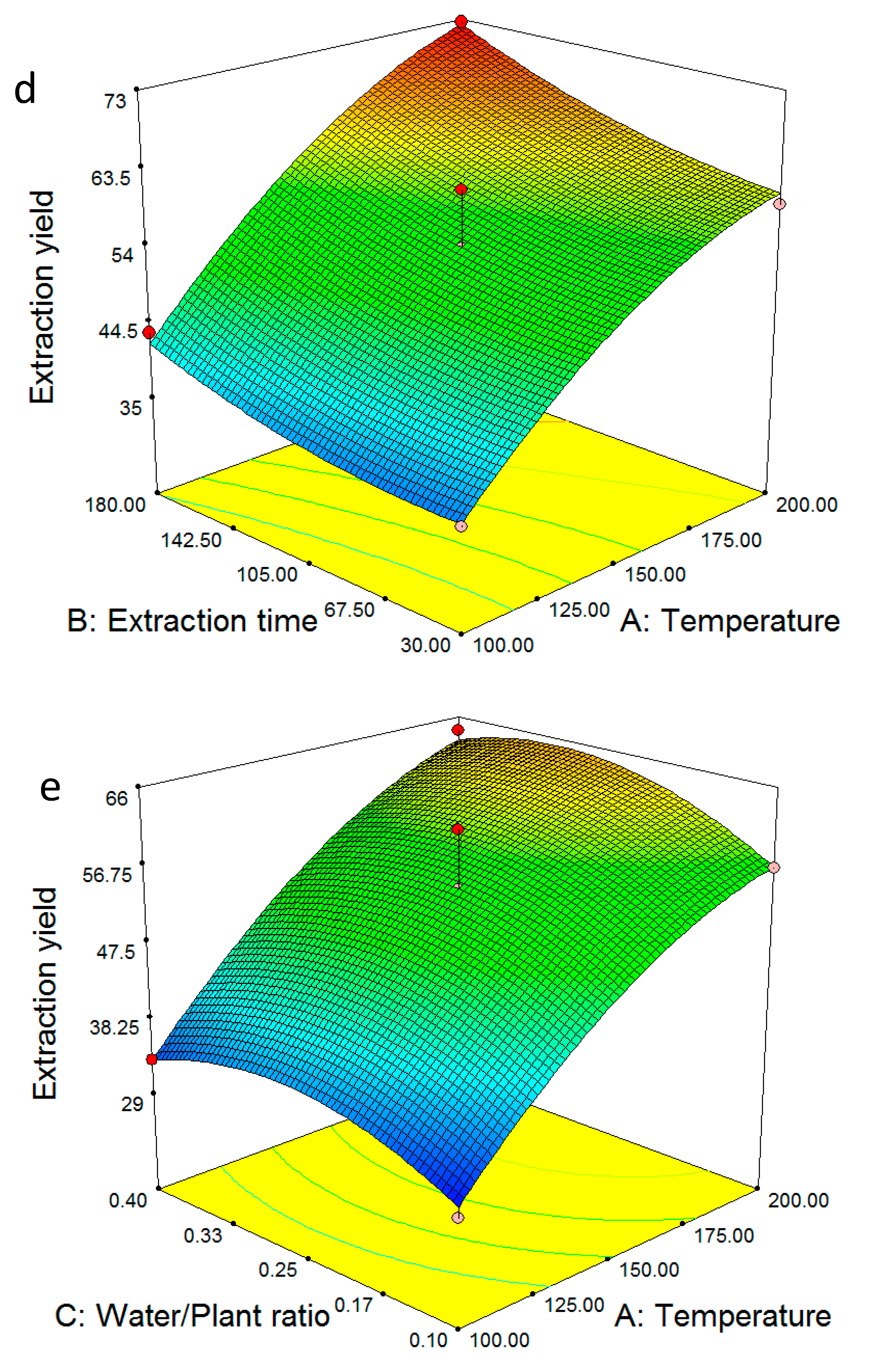
2.2. Multiple Regression Modelling of the Extraction Yields and Analysis of Variance
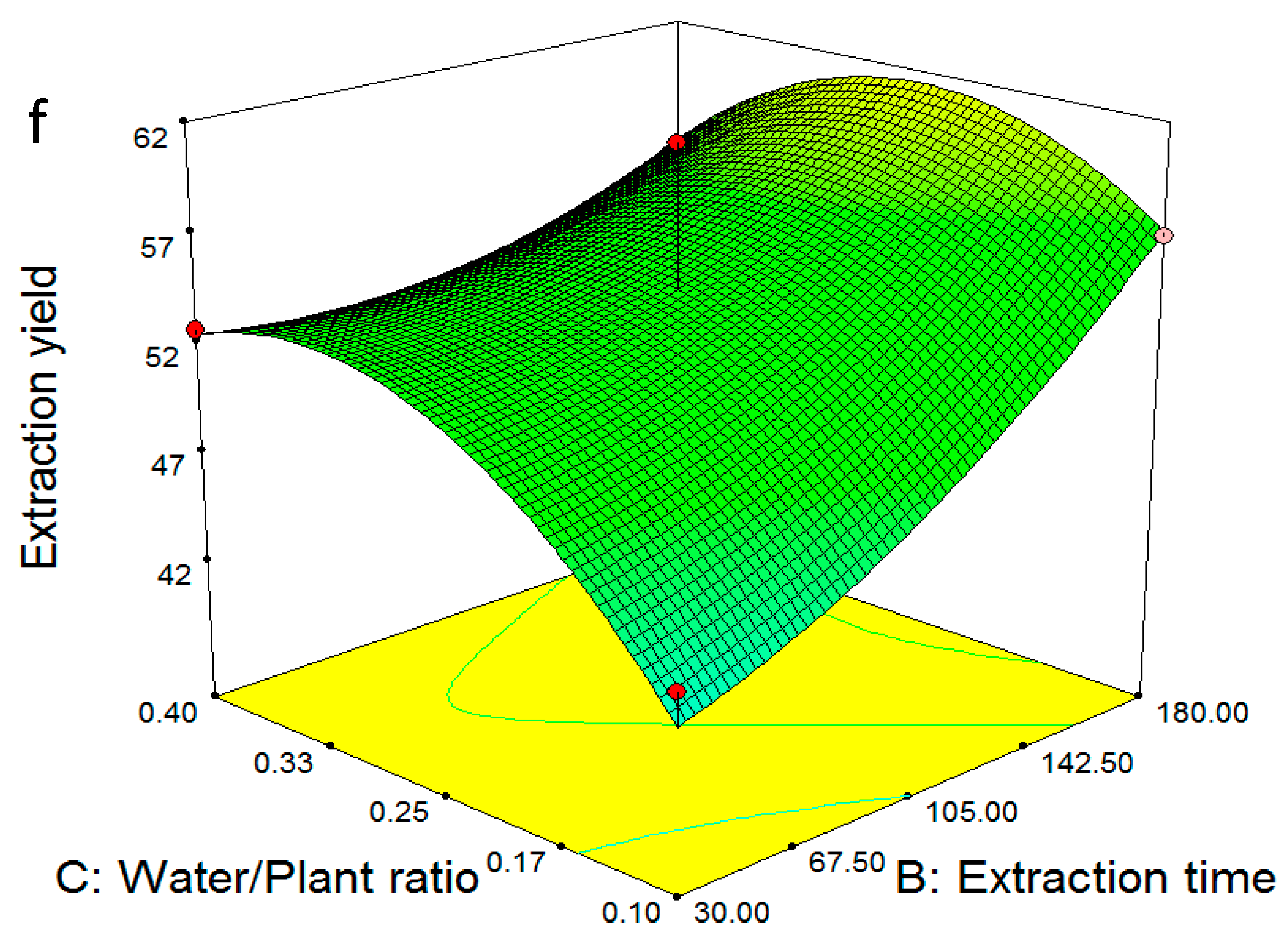
2.3. Multiple Regression Modelling of the Total Phenol Content and Analysis of Variance
2.4. Model Validation
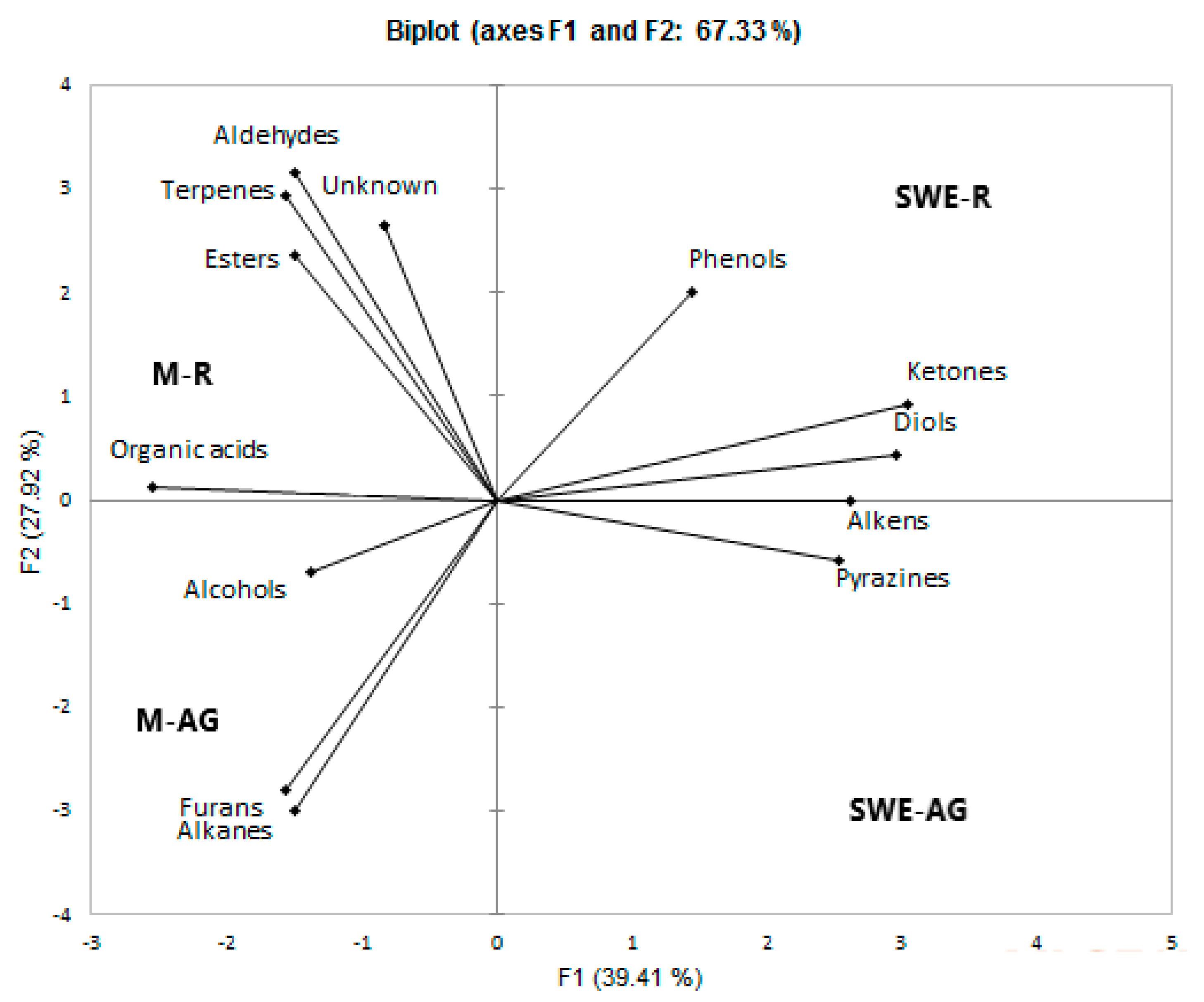
2.5. Chemical Composition of O. mutabilis Extracts
2.6. Method Validation and Quantification of Phenolic Compounds
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation and Extraction Procedures
3.3. Optimization of SWE Process
3.4. Determination of Total Phenolic Content
3.5. Analytical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baltaci, N.; Aydogdu, N.; Sarikurkcu, C.; Tepe, B. Onosma gracilis (Trautv.) and O. oreodoxa (Boiss. & Heldr.): Phytochemistry, in silico docking, antioxidant and enzyme inhibitory activities. S. Afr. J. Bot. 2021, 143, 410–417. [Google Scholar] [CrossRef]
- Benarba, B.; Pandiella, A. Medicinal Plants as Sources of Active Molecules Against COVID-19. Front. Pharmacol. 2020, 11, 1189. [Google Scholar] [CrossRef]
- Xiong, L.; Hu, W.-B.; Yang, Z.-W.; Chen, H.; Ning, W.; Xin, L.; Wang, W.-J. Enzymolysis-ultrasonic assisted extraction of flavanoid from Cyclocarya paliurus (Batal) Iljinskaja: HPLC profile, antimicrobial and antioxidant activity. Ind. Crop. Prod. 2019, 130, 615–626. [Google Scholar] [CrossRef]
- Tian, J.-L.; Liu, T.-L.; Xue, J.-J.; Hong, W.; Zhang, Y.; Zhang, D.-X.; Cui, C.-C.; Liu, M.-C.; Niu, S.-L. Flavanoids derivatives from the root bark of Broussonetia papyrifera as a tyrosinase inhibitor. Ind. Crop. Prod. 2019, 138, 111445. [Google Scholar] [CrossRef]
- Casagrande, M.; Zanela, J.; Wagner, A.; Busso, C.; Wouk, J.; Iurckevicz, G.; Montanher, P.F.; Yamashita, F.; Malfatti, C.R.M. Malfatti, Influence of time, temperature and solvent on the extraction of bioactive compounds of Baccharis dracunculifolia: In vitro antioxidant activity, antimicrobial potential, and phenolic compound quantification. Ind. Crop. Prod. 2018, 125, 207–219. [Google Scholar] [CrossRef]
- Cilla, A.; Bosch, L.; Barberá, R.; Alegría, A. Effect of processing on the bioaccessibility of bioactive compounds—A review focusing on carotenoids, minerals, ascorbic acid, tocopherols and polyphenols. J. Food Compos. Anal. 2018, 68, 3–15. [Google Scholar] [CrossRef]
- Cao, J.-Q.; Pang, X.; Guo, S.-S.; Wang, Y.; Geng, Z.-F.; Sang, Y.-L.; Guo, P.-J.; Du, S.-S. Pinene-rich essential oils from Haplophyllum dauricum (L.) G. Don display anti-insect activity on two stored-product insects. Int. Biodeterior. Biodegradation 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Pavela, R.; Bartolucci, F.; Desneux, N.; Lavoir, A.-V.; Canale, A.; Maggi, F.; Benelli, G. Chemical profiles and insecticidal efficacy of the essential oils from four Thymus taxa growing in central-southern Italy. Ind. Crop. Prod. 2019, 138, 111460. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Zhang, X.; Song, Z.; Liu, F.; Liang, Y.; Zhang, J.; Jin, D.-Q.; Xu, J.; Lee, D.; et al. Bioactive terpenoids from Euonymus verrucosus var. pauciflorus showing NO inhibitory activities. Bioorganic Chem. 2019, 87, 447–456. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E. The effect of microwave roasting on bioactive compounds, antioxidant activity and fatty acid composition of apricot kernel and oils. Food Chem. 2018, 243, 414–419. [Google Scholar] [CrossRef]
- Lessa, O.A.; Reis, N.D.S.; Leite, S.G.F.; Gutarra, M.L.E.; Souza, A.O.; Gualberto, S.A.; de Oliveira, J.R.; Aguiar-Oliveira, E.; Franco, M. Effect of the solid state fermentation of cocoa shell on the secondary metabolites, antioxidant activity, and fatty acids. Food Sci. Biotechnol. 2018, 27, 107–113. [Google Scholar] [CrossRef]
- Belyagoubi-Benhammou, N.; Belyagoubi, L.; Gismondi, A.; Di Marco, G.; Canini, A.; Bekkara, F.A. GC/MS analysis, and antioxidant and antimicrobial activities of alkaloids extracted by polar and apolar solvents from the stems of Anabasis articulata. Med. Chem. Res. 2019, 28, 754–767. [Google Scholar] [CrossRef]
- Jiang, Z.-M.; Wang, L.-J.; Gao, Z.; Zhuang, B.; Yin, Q.; Liu, E.-H. Green and efficient extraction of different types of bioactive alkaloids using deep eutectic solvents. Microchem. J. 2019, 145, 345–353. [Google Scholar] [CrossRef]
- Cappato, L.P.; Ferreira, M.V.S.; Moraes, J.; Pires, R.P.; Rocha, R.S.; Silva, R.; Neto, R.P.; Tavares, M.I.B.; Freitas, M.Q.; Rodrigues, F.N.; et al. Whey acerola-flavoured drink submitted Ohmic Heating: Bioactive compounds, antioxidant capacity, thermal behavior, water mobility, fatty acid profile and volatile compounds. Food Chem. 2018, 263, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Rios-Romero, E.A.; Ochoa-Martínez, L.A.; Morales-Castro, J.; Bello-Perez, L.A.; Quintero-Ramos, A.; Gallegos-Infante, J.A. Ultrasound in orange sweet potato juice: Bioactive compounds, antioxidant activity, and enzymatic inactivation. J. Food Process. Preserv. 2018, 42, e13633. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of fermentation conditions on the production of bioactive compounds with anticancer, anti-inflammatory and antioxidant properties in kombucha tea extracts. Process. Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef]
- Zengin, G.; Stefanucci, A.; Rodrigues, M.J.; Mollica, A.; Custodio, L.; Aumeeruddy, M.Z.; Mahomoodally, M.F. Scrophularia lucida L. as a valuable source of bioactive compounds for pharmaceutical applications: In vitro antioxidant, anti-inflammatory, enzyme inhibitory properties, in silico studies, and HPLC profiles. J. Pharm. Biomed. Anal. 2019, 162, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Kovačević, D.B. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Hernández, Y.; Lobo, M.G.; González, M. Factors affecting sample extraction in the liquid chromatographic determination of organic acids in papaya and pineapple. Food Chem. 2009, 114, 734–741. [Google Scholar] [CrossRef]
- Kleijnen, J.P.C. Response Surface Methodology, International Series in Operations Research and Management Science; Springer: Berlin/Heidelberg, Germany, 2015; Volume 216, pp. 81–104. [Google Scholar] [CrossRef]
- Kutlu, N.; Yeşilören, G.; Işci, A.; Şakıyan, Ö. Alternative to Conventional Extraction: Green Technologies. J. Food 2017, 42, 514–526. [Google Scholar] [CrossRef]
- Liang, X.; Fan, Q. Application of Sub-Critical Water Extraction in Pharmaceutical Industry. J. Mater. Sci. Chem. Eng. 2013, 1, 1–6. [Google Scholar] [CrossRef]
- Wilson, I.D.; Davis, P.; Ruane, R.J. Supercritical Fluid Chromatography and Extraction of Pharmaceuticals, Applications of Supercritical Fluids in Industrial Analysis; Springer: Berlin/Heidelberg, Germany, 1993; pp. 74–103. [Google Scholar] [CrossRef]
- Ozel, M.; Gogus, F.; Lewis, A.C. Subcritical water extraction of essential oils from Thymbra spicata. Food Chem. 2003, 82, 381–386. [Google Scholar] [CrossRef]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern Extraction Methods for Preparation of Bioactive Plant Extracts. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Ibañez, E.; Kubátová, A.; Señoráns, F.J.; Cavero, S.; Reglero, G.; Hawthorne, S.B. Subcritical water extraction of antioxidant compounds from rosemary plants. J. Agric. Food Chem. 2003, 51, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Separation & Purification Reviews Subcritical Water Extraction of Biological Materials Subcritical Water Extraction of Biological Materials, Subcritical Water Extraction of Biological Materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Wen, C.; Zhou, J.; Gu, J.; Duan, Y.; Zhang, H.; Ren, X.; Ma, H. Structural characterization and immunostimulatory activity of a novel polysaccharide isolated with subcritical water from Sagittaria sagittifolia L. Int. J. Biol. Macromol. 2019, 133, 11–20. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Gu, J.; Ji, C.; Duan, Y.; Zhang, H. Effects of subcritical water extraction microenvironment on the structure and biological activities of polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2019, 123, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, C.; Qin, W.; Qin, P.; Zhang, H.; Duan, Y. Ultrasonic-enhanced subcritical water extraction of polysaccharides by two steps and its characterization from Lentinus edodes. Int. J. Biol. Macromol. 2018, 118, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Luebert, F.; Cecchi, L.; Frohlich, M.W.; Gottschling, M.; Guilliams, C.M.; Hasenstab-Lehman, K.E.; Hilger, H.H.; Miller, J.S.; Mittelbach, M.; Nazaire, M.; et al. Familial classification of the boraginales. Taxon 2016, 65, 502–522. [Google Scholar] [CrossRef]
- Davis, P.H. Flora of Turkey and the East Aegean Islands; Edinburgh University Press: Edinburgh, Scotland, 1978. [Google Scholar]
- Yildirim, Ş. The chorology of the Turkish species to Boraginaceae family, Ot Sistematik Botanik Dergisi. Ot Sistematik Botanik Dergisi 2000, 7, 257–272. Available online: https://search.trdizin.gov.tr/tr/yayin/detay/26086/ (accessed on 18 October 2022).
- Binzet, R.; Orcan, N. A New Species of Onosma (Boraginaceae) from Southern Turkey. Novon 2007, 17, 8–10. [Google Scholar] [CrossRef]
- Tosun, A.; Akkol, E.K.; Bahadır, O.; Yeşilada, E. Evaluation of anti-inflammatory and antinociceptive activities of some Onosma L. species growing in Turkey. J. Ethnopharmacol. 2008, 120, 378–381. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, R.; Kishore, K.; Onosma, L. A review of phytochemistry and ethnopharmacology. Pharmacogn. Rev. 2013, 7, 140–151. [Google Scholar] [CrossRef]
- Caponio, F.; Alloggio, V.; Gomes, T. Phenolic compounds of virgin olive oil: Influence of paste preparation techniques. Food Chem. 1999, 64, 203–209. [Google Scholar] [CrossRef]
- Samadi, M.; Abidin, Z.Z.; Yoshida, H.; Yunus, R.; Biak, D.A.; Lee, C.H.; Lok, E.H. Subcritical water extraction of essential oil from Aquilaria malaccensis leaves. Sep. Sci. Technol. 2020, 55, 2779–2798. [Google Scholar] [CrossRef]
- Dias, I.P.; Barbieri, S.F.; Fetzer, D.E.L.; Corazza, M.L.; Silveira, J.L.M. Effects of pressurized hot water extraction on the yield and chemical characterization of pectins from Campomanesia xanthocarpa Berg fruits. Int. J. Biol. Macromol. 2020, 146, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Mikucka, W.; Zielinska, M.; Bulkowska, K.; Witonska, I. Subcritical water extraction of bioactive phenolic compounds from distillery stillage. J. Environ. Manag. 2022, 318, 115548. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of temperature on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Ko, M.-J.; Lee, J.-H.; Nam, H.-H.; Chung, M.-S. Subcritical water extraction of phytochemicals from Phlomis umbrosa Turcz, IFSET. Innov. Food Sci. Emerg. Technol. 2017, 42, 1–7. [Google Scholar] [CrossRef]
- Ho, C.H.; Cacace, J.E.; Mazza, G. Extraction of lignans, proteins and carbohydrates from flaxseed meal with pressurized low polarity water, LWT—Food Science and Technology. LWT 2007, 40, 1637–1647. [Google Scholar] [CrossRef]
- Kumar, M.Y.; Dutta, R.; Prasad, D.; Misra, K. Subcritical water extraction of antioxidant compounds from Seabuckthorn (Hippophae rhamnoides) leaves for the comparative evaluation of antioxidant activity. Food Chem. 2011, 127, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Souza-Silva, É.A.; Gionfriddo, E.; Pawliszyn, J. A critical review of the state of the art of solid-phase microextraction of complex matrices II. Food analysis. TrAC Trends Anal. Chem. 2015, 71, 236–248. [Google Scholar] [CrossRef]
- Jabbar, A.A. Onosma mutabilis: Phytochemical composition, antioxidant, cytotoxicity, and acute oral toxicity. Food Sci. Nutr. 2021, 9, 5755–5764. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Ulbrich, K.; Rehberg, C.; Rohn, S.; Rimbach, G. Thermal stability, antioxidant, and anti-inflammatory activity of curcumin and its degradation product 4-vinyl guaiacol. Food Funct. 2015, 6, 887–893. [Google Scholar] [CrossRef]
- Ihssen, J.; Jankowska, D.; Ramsauer, T.; Reiss, R.; Luchsinger, R.; Wiesli, L.; Schubert, M.; Thöny-Meyer, L.; Faccio, G. Engineered Bacillus pumilus laccase-like multi-copper oxidase for enhanced oxidation of the lignin model compound guaiacol. Protein Eng. Des. Sel. 2017, 30, 449–453. [Google Scholar] [CrossRef]
- Juhás, M.; Zitko, J. Molecular Interactions of Pyrazine-Based Compounds to Proteins. J. Med. Chem. 2020, 63, 8901–8916. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Kobayashi, Y.; Wasaki, J.; Koyama, H. Organic acid excretion from roots: A plant mechanism for enhancing phosphorus acquisition, enhancing aluminum tolerance, and recruiting beneficial rhizobacteria. Soil Sci. Plant Nutr. 2018, 64, 697–704. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Q.; Lv, H.; Wang, F.; Liu, R.; Zeng, N. Effect of pulegone on the NLPR3 inflammasome during inflammatory activation of THP 1 cells. Exp. Ther. Med. 2020, 19, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Sahinler, S.S.; Husunet, M.T.; Istifli, E.S.; Tepe, B. Two endemic Onosma species (O. sieheana and O. stenoloba): A comparative study including docking data on biological activity and phenolic composition. Ind. Crop. Prod. 2020, 154, 112656. [Google Scholar] [CrossRef]
- Ozgen, U.; Ikbal, M.; Hacimuftuoglu, A.; Houghton, P.; Gocer, F.; Dogan, H.; Coskun, M. Fibroblast growth stimulation by extracts and compounds of Onosma argentatum roots. J. Ethnopharmacol. 2006, 104, 100–103. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Nastić, N.; Švarc-Gajić, J.; Delerue-Matos, C.; Barroso, M.F.; Soares, C.; Moreira, M.M.; Morais, S.; Mašković, P.; Srček, V.G.; Slivac, I.; et al. Subcritical water extraction as an environmentally-friendly technique to recover bioactive compounds from traditional Serbian medicinal plants. Ind. Crop. Prod. 2018, 111, 579–589. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Cvetanović, A.; Segura-Carretero, A.; Linares, I.B.; Mašković, P. Characterisation of ginger extracts obtained by subcritical water. J. Supercrit. Fluids 2017, 123, 92–100. [Google Scholar] [CrossRef]
- Ersan, P.; Sönmez, Ö.; Gözmen, B. Microwave-assisted d-pinitol extraction from carob: Application of Box–Behnken design. J. Iran. Chem. Soc. 2020, 17, 871–879. [Google Scholar] [CrossRef]
| Experimental Design Parameters | Aboveground Part | Roots | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Extraction Time (min) | Water/Plant Ratio | Extraction Yield (%) Predicted | Extraction Yield (%) Actual | TPC mg GAE/g | TPC mg GAE/g Actual | Extraction Yield (%) Predicted | Extraction Yield (%) Actual | TPC mg GAE/g Predicted | TPC mg GAE/g Actual |
| 200.00 | 105.00 | 0.4 | 81.28 | 80.13 | 32.42 | 40.06 | 63.00 | 64.21 | 37.68 | 44.01 |
| 100.00 | 105.00 | 0.4 | 42.94 | 41.88 | 24.15 | 26.18 | 33.16 | 33.25 | 30.05 | 28.74 |
| 150.00 | 180.00 | 0.4 | 71.88 | 71.21 | 29.28 | 28.94 | 56.2 | 54.69 | 45.91 | 43.84 |
| 100.00 | 105.00 | 0.1 | 62.66 | 63.81 | 112.37 | 104.73 | 30.72 | 29.51 | 139.83 | 133.50 |
| 100.00 | 180.00 | 0.25 | 51.93 | 53.65 | 31.33 | 29.64 | 41.82 | 43.24 | 57.50 | 60.87 |
| 200.00 | 30.00 | 0.25 | 66.67 | 64.94 | 58.47 | 60.16 | 60.65 | 59.23 | 74.28 | 70.91 |
| 150.00 | 30.00 | 0.4 | 52.43 | 55.30 | 55.90 | 46.57 | 52.51 | 52.72 | 44.77 | 41.82 |
| 100.00 | 30.00 | 0.25 | 53.69 | 51.87 | 14.49 | 21.78 | 35.34 | 35.04 | 48.16 | 52.42 |
| 200.00 | 105.00 | 0.1 | 70.74 | 71.79 | 179.99 | 177.96 | 56.67 | 56.58 | 172.83 | 174.14 |
| 150.00 | 105.00 | 0.25 | 69.16 | 69.61 | 57.84 | 56.07 | 55.00 | 50.00 | 72.33 | 80.24 |
| 150.00 | 30.00 | 0.1 | 68.00 | 68.67 | 136.38 | 136.72 | 42.75 | 44.26 | 164.84 | 166.91 |
| 150.00 | 105.00 | 0.25 | 69.16 | 69.86 | 57.84 | 61.27 | 55.00 | 61.08 | 72.33 | 67.49 |
| 150.00 | 105.00 | 0.25 | 69.16 | 68.02 | 57.84 | 56.18 | 55.00 | 53.91 | 72.33 | 69.27 |
| 200.00 | 180.00 | 0.25 | 85.37 | 87.19 | 63.22 | 55.93 | 72.3 | 72.6 | 72.01 | 67.75 |
| 150.00 | 180.00 | 0.1 | 65.49 | 62.61 | 184.58 | 193.91 | 57.19 | 56.98 | 170.77 | 173.13 |
| Extraction Yield (Aboveground Part) | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1771.50 | 9 | 196.83 | 26.69 | 0.0011 |
| A—temperature | 1077.41 | 1 | 1077.41 | 146.12 | <0.0001 |
| B—extraction time | 143.48 | 1 | 143.48 | 19.46 | 0.0069 |
| C—water/plant ratio | 42.14 | 1 | 42.14 | 5.71 | 0.0624 |
| AB | 104.76 | 1 | 104.76 | 14.21 | 0.0130 |
| AC | 229.07 | 1 | 229.07 | 31.07 | 0.0026 |
| BC | 120.67 | 1 | 120.67 | 16.37 | 0.0099 |
| A2 | 21.23 | 1 | 21.23 | 2.88 | 0.1505 |
| B2 | 20.44 | 1 | 20.44 | 2.77 | 0.1568 |
| C2 | 20.62 | 1 | 20.62 | 2.80 | 0.1554 |
| Residual | 36.87 | 5 | 7.37 | ||
| Cor Total | 1808.37 | 14 | |||
| R2 | 0.9796 | ||||
| CV % | 4.15 | ||||
| Extraction yield (roots) | |||||
| Model | 1965.28 | 9 | 218.36 | 14.57 | 0.0044 |
| A—temperature | 1556.26 | 1 | 1556.26 | 103.81 | 0.0002 |
| B—extraction time | 164.35 | 1 | 164.35 | 10.96 | 0.0212 |
| C—water/plant ratio | 38.46 | 1 | 38.46 | 2.57 | 0.1701 |
| AB | 6.68 | 1 | 6.68 | 0.45 | 0.5339 |
| AC | 3.78 | 1 | 3.78 | 0.25 | 0.6368 |
| BC | 28.89 | 1 | 28.89 | 1.93 | 0.2238 |
| A2 | 70.58 | 1 | 70.58 | 4.71 | 0.0822 |
| B2 | 13.37 | 1 | 13.37 | 0.89 | 0.3883 |
| C2 | 82.86 | 1 | 82.86 | 5.53 | 0.0655 |
| Residual | 74.96 | 5 | 14.99 | ||
| Cor Total | 2040.24 | 14 | |||
| R2 | 0.9633 | ||||
| CV % | 7.57 |
| TPC (Aboveground) | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 41,856.44 | 9 | 4650.72 | 54.20 | 0.0002 |
| A—temperature | 2879.53 | 1 | 2879.53 | 33.56 | 0.0022 |
| B—extraction time | 233.04 | 1 | 233.04 | 2.72 | 0.1603 |
| C—water/plant ratio | 27,796.69 | 1 | 27,796.69 | 323.94 | <0.0001 |
| AB | 36.55 | 1 | 36.55 | 0.43 | 0.5428 |
| AC | 880.66 | 1 | 880.66 | 10.26 | 0.0239 |
| BC | 1399.17 | 1 | 1399.17 | 16.31 | 0.0099 |
| A2 | 845.86 | 1 | 845.86 | 9.86 | 0.0257 |
| B2 | 2.54 | 1 | 2.54 | 0.030 | 0.8701 |
| C2 | 7319.23 | 1 | 7319.23 | 85.30 | 0.0002 |
| Residual | 429.04 | 5 | 85.81 | ||
| Cor Total | 42,285.47 | 14 | |||
| R2 | 0.9899 | ||||
| CV % | 12.68 | ||||
| TPC (Root) | |||||
| Model | 35,735.85 | 9 | 3970.65 | 75.22 | < 0.0001 |
| A—temperature | 825.62 | 1 | 825.62 | 15.64 | 0.0108 |
| B—extraction time | 24.96 | 1 | 24.96 | 0.47 | 0.5222 |
| C—water/plant ratio | 29,995.72 | 1 | 29,995.72 | 568.26 | <0.0001 |
| AB | 33.75 | 1 | 33.75 | 0.64 | 0.4602 |
| AC | 160.83 | 1 | 160.83 | 3.05 | 0.1413 |
| BC | 5.75 | 1 | 5.75 | 0.11 | 0.7548 |
| A2 | 400.28 | 1 | 400.28 | 7.58 | 0.0401 |
| B2 | 4.19 | 1 | 4.19 | 0.079 | 0.7894 |
| C2 | 4063.80 | 1 | 4063.80 | 76.99 | 0.0003 |
| Residual | 263.93 | 5 | 52.79 | ||
| Cor Total | 35,999.78 | 14 | |||
| R2 | 0.9927 | ||||
| CV % | 8.54 |
| Aboveground Part | Roots | |||
|---|---|---|---|---|
| Predicted | Experimental | Predicted | Experimental | |
| Extraction yield (%) | 72.78 | 71.61 ± 0.66 | 67.08 | 64.48 ± 0.78 |
| TPC mg GAE/g | 193.9 | 193.63 ± 0.40 | 173.96 | 173.13 ± 0.91 |
| Compound | R2 | Equation | Linearity (mg/L) | Reproducibility RSD (%) | Repeatability RSD (%) | Sensitivity | |
|---|---|---|---|---|---|---|---|
| LOD (mg/L) | LOQ (mg/L) | ||||||
| Gallic acid | 0.9994 | Y = 69029x − 36592 | 0.5–50 | 0.120 | 4.892 | 0.010 | 0.032 |
| Pyrocatechol | 0.9998 | Y = 32014x − 9886.1 | 0.5–100 | 0.765 | 2.051 | 0.014 | 0.048 |
| Catechin | 0.9959 | Y = 11231x − 7354.7 | 0.5–100 | 0.907 | 2.201 | 0.076 | 0.253 |
| Caffeic acid | 0.9977 | Y = 52828x − 66606 | 0.5–100 | 0.282 | 1.260 | 0.005 | 0.017 |
| Epicatechin | 0.9993 | Y = 14059x − 6188.7 | 0.5–50 | 1.821 | 1.839 | 0.029 | 0.098 |
| p-coumaric acid | 0.9998 | Y = 89753x − 30217 | 0.5–100 | 0.072 | 1.136 | 0.008 | 0.025 |
| Ferulic acid | 0.9998 | Y = 63350x − 22113 | 0.5–100 | 0.260 | 1.443 | 0.002 | 0.006 |
| Quercetin | 0.9999 | Y = 41175x − 19407 | 0.5–100 | 3.394 | 7.219 | 0.051 | 0.171 |
| µg/g Extract | ||||
|---|---|---|---|---|
| SWE Aboveground | SWE Roots | Maceration Aboveground | Maceration Roots | |
| Gallic acid | 39.969 ± 4.40 | 37.02 ± 0.33 | 12.18 ± 2.87 | 9.22 ± 0.24 |
| Pyrocatechol | 544.42 ± 68.41 | 1062.78 ± 114.29 | 28.93 ± 10.37 | 10.22 ± 2.65 |
| Catechin | 163.99 ± 0.42 | 145.08 ± 23.10 | 128.25 ± 2.80 | 31.54 ± 1.12 |
| Caffeic acid | 33.92 ± 10.38 | 33.58 ± 12.21 | 64.05 ± 5.382 | 58.11 ± 4.93 |
| Epicatechin | 513.69 ± 55.57 | 1109.94 ± 37.08 | 72.72 ± 5.93 | 23.41 ± 6.01 |
| p-coumaric acid | 65.57 ± 13.61 | 59.40 ± 15.14 | 63.09 ± 0.56 | 27.04 ± 2.71 |
| Ferulic acid | 14.02 ± 5.20 | 6.68 ± 1.59 | 5.57 ± 1.76 | 95.69 ± 1.43 |
| Quercetin | 67.55 ± 20.95 | 15.87 ± 2.38 | 19.52 ± 3.08 | 6.91 ± 0.21 |
| Variables | Symbols | Codes | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Temperature/°C | A | 100 | 150 | 200 |
| Extraction time/min | B | 30 | 105 | 180 |
| Water/plant ratio | C | 0.1 | 0.25 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çalhan, S.D.; Meryemoğlu, B.; Eroğlu, P.; Saçlı, B.; Kalderis, D. Subcritical Water Extraction of Onosma mutabilis: Process Optimization and Chemical Profile of the Extracts. Molecules 2023, 28, 2314. https://doi.org/10.3390/molecules28052314
Çalhan SD, Meryemoğlu B, Eroğlu P, Saçlı B, Kalderis D. Subcritical Water Extraction of Onosma mutabilis: Process Optimization and Chemical Profile of the Extracts. Molecules. 2023; 28(5):2314. https://doi.org/10.3390/molecules28052314
Chicago/Turabian StyleÇalhan, Selda Doğan, Bahar Meryemoğlu, Pelin Eroğlu, Barış Saçlı, and Dimitrios Kalderis. 2023. "Subcritical Water Extraction of Onosma mutabilis: Process Optimization and Chemical Profile of the Extracts" Molecules 28, no. 5: 2314. https://doi.org/10.3390/molecules28052314
APA StyleÇalhan, S. D., Meryemoğlu, B., Eroğlu, P., Saçlı, B., & Kalderis, D. (2023). Subcritical Water Extraction of Onosma mutabilis: Process Optimization and Chemical Profile of the Extracts. Molecules, 28(5), 2314. https://doi.org/10.3390/molecules28052314







