A Review on Catalytic Fast Co-Pyrolysis Using Analytical Py-GC/MS
Abstract
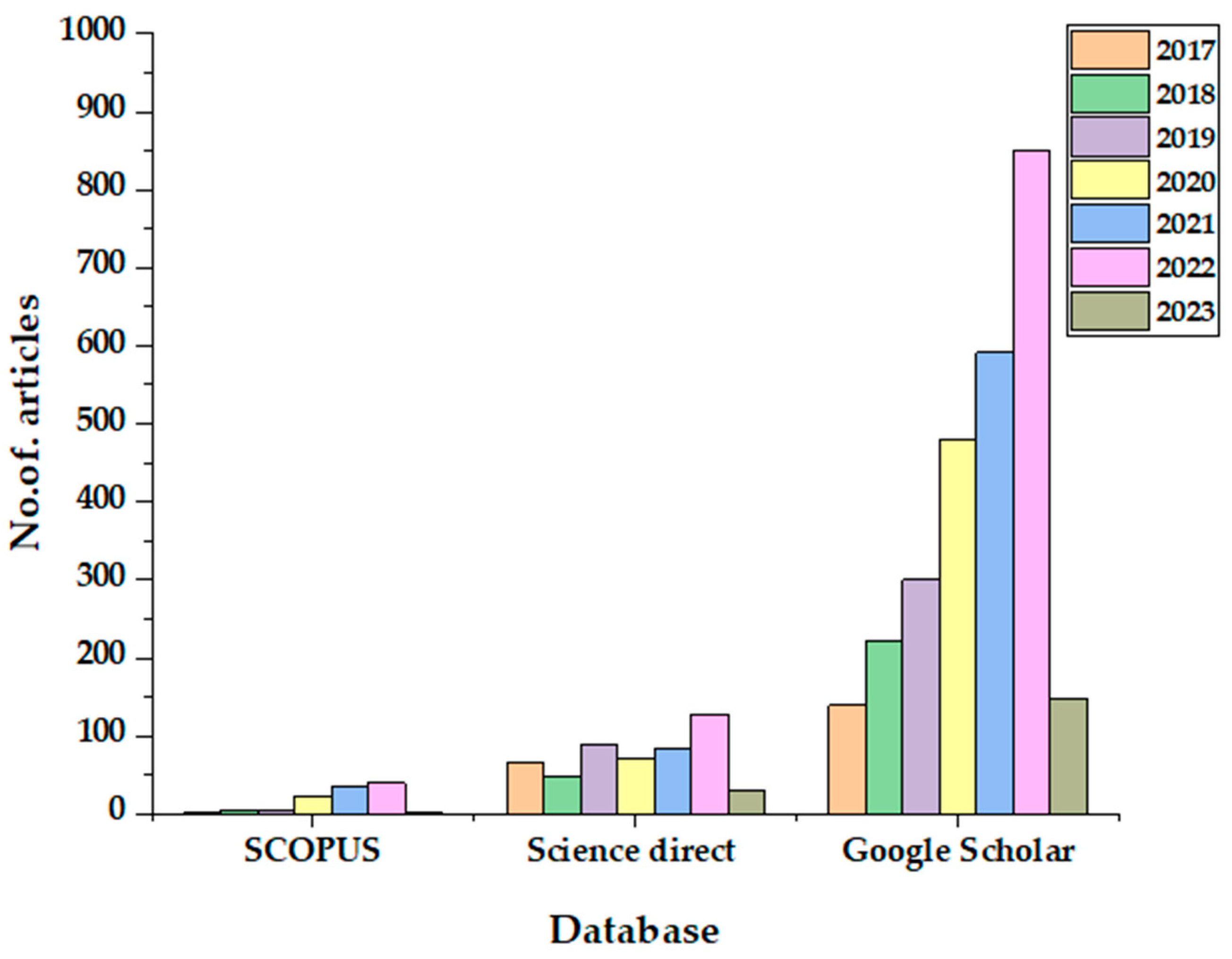
1. Introduction
- To review bio-oil analysis from fast catalytic co-pyrolysis using a micro-pyrolyser attached with GC-MS.
- To discuss the effect of feedstock and catalyst type on product generation of volatiles.
- To understand commonly proposed reaction mechanisms due to catalytic co-pyrolysis of biomass with various feedstocks.
- To discuss how to increase specific volatile product yields by altering feedstock and catalyst ratios.
- To suggest the research gaps and opportunities for future work.
2. Zeolite Catalysts—Single or Comparison
2.1. Biomass-Biomass with Single Zeolites
2.2. Biomass-Plastics with Single Zeolites
2.3. Other Feeds with Single Zeolites
| Feedstock | Catalysts | Pyrolysis Operating Conditions | GC-MS Operating Conditions | Products | Reaction Mechanism | Reference |
|---|---|---|---|---|---|---|
| Names: Mixing/blending ratio: BR | Name: Catalyst to feed ratio/loading: CR Catalyst to catalyst ratio: CCR | Instrument (I): Temperature (T): °C Heating rate (HR): Time (t): Carrier gas and flow rate (CG): mL/min | Instrument (I): Capillary column (CC): Temperature: (T): °C Split ratio (SR): Scan range (SC): m/z | →: yields ↓: decreases ↑: increases | ||
| MSW (PP, PE and humus) –corn stalks BR: 3:1. 1:1, 1:3, 3:1, 1:1, 1:3 | HZSM-5, nMFI | I: CDS5200 (CDS Analytical Co. Ltd., Oxford, PA, USA) T: 600 °C HR: 20 °C/ms t: 20 s CG: He | I: Agilent 7890B-5977A CC: HP-5 ms T: 250 °C SR: 1:50 SC: 33–500 m/z |
| Under ZSM-5, cyclization and aromatization of olefins turn feedstock into AHs. Diels–Alder reactions of C6-C10 olefins with furans were more frequently observed with nMFI with more Lewis acid sites than with ZSM-5. | [25] |
| Chlorella vulgaris–MSW BR: 1:1 | ZSM-5, Hi-ZSM-5, Al-MCM-41, Al-SBA-15 CR: 1:1 | I: CDS5200 (CDS Analytical Co. Ltd., Oxford, PA, USA) T: 600 °C HR: 10 °C/min t: 20 s CG: N2 | I: Agilent 7890B-5977A CC: HP-5 ms T: 250 °C SR: 1:50 SC: 35–300 m/z |
| Co-pyrolysis increased mono-AHs and aliphatic hydrocarbons while decreasing poly-AHs and N2-compounds. | [28] |
| Chili straw–PP BR: 1:1 | HZSM-5 CR: 1:1 | I: Pyroprobe 5200 Pyrolyser (CDS Analytical Co. Ltd.) T: 750 °C HR: 1.2 × 106 °C/min t: 10 min CG: N2 | I: Agilent 7890B-5977A CC: HP-5 ms T: 300 °C |
| The alkane content increased as the micro power level increased, but the oxygenate organics decreased. | [29] |
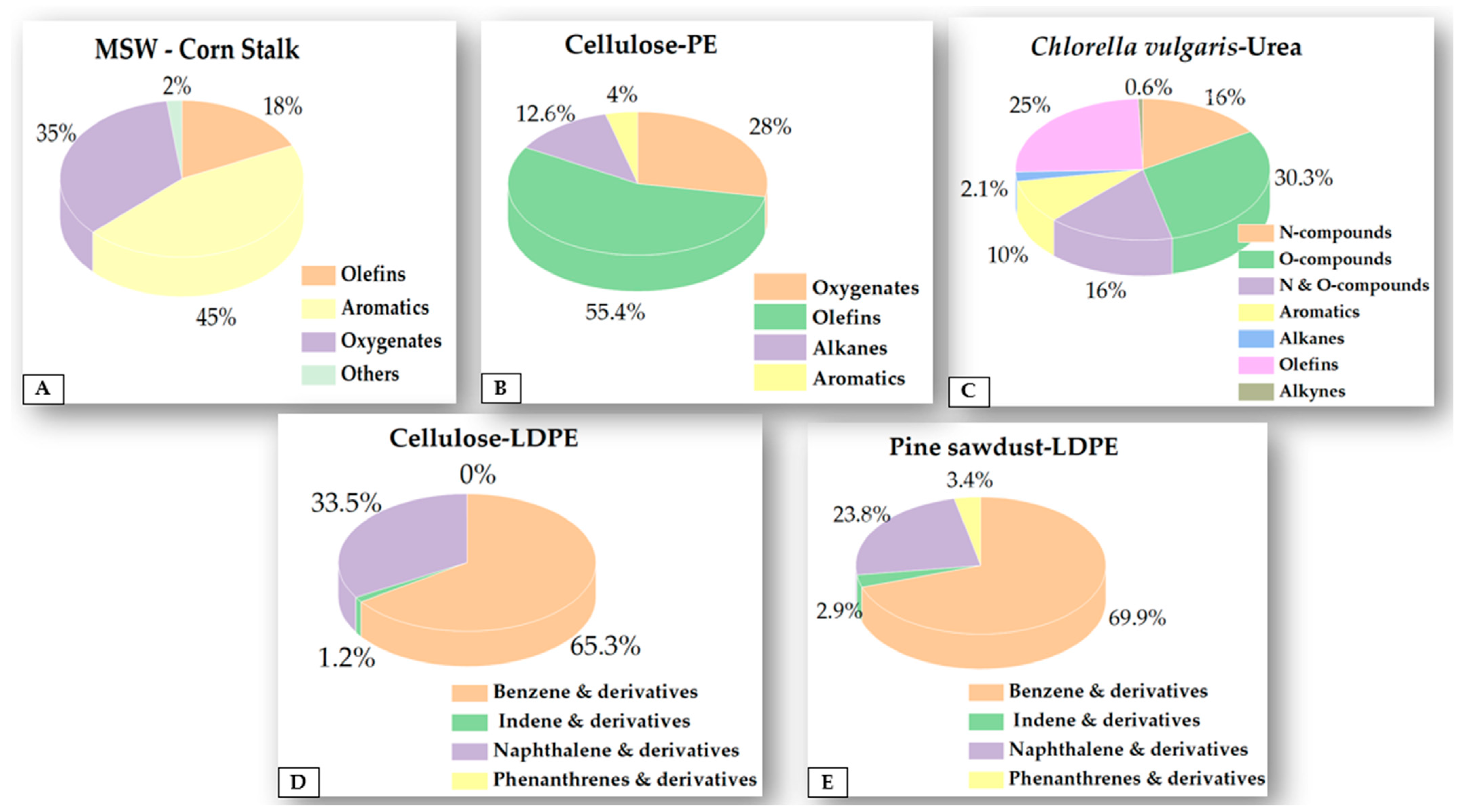
| CE or pine sawdust–LDPE BR: 1:1 | HZSM-5 CR: 1:1 | I: A specially designed fixed-bed reactor T: 500 °C t: 30 min CG: N2 at 150 mL/min | I: ITQ 900 Instrument CC: HP-5 ms T: 280 °C SR: 1:10 SC: 30–500 m/z |
| The yield of AHs is increased by Diels–Alder reactions between oxygenated compounds derived from biomass and olefins derived from plastic. | [34] |
| CE–PE BR: 1:1 | HZSM-5 CR: 1:4 | I: EGA/PY-3030D pyrolyser T: 650 °C HR: 20 °C/ms t: 30 s | I: QP2010Ultra (Shimadzu, Japan) CC: DB-5 ms T: 280 °C SR: 1:80 SC: 33–500 m/z |
| The addition of HZSM-5 advances the Diels–Alder reaction, suppressing the free radical reactions. The amount of the alcohol compound is decreased in this. | [35] |
| Quercus Variabilis–waste plastic film BR: 1:1 | HZSM-5 or HY CR: 1:5 | I: Pyrolyser (Py-3030D, Frontier Laboratories Ltd., Fukushima, Japan) T: 600 °C | I: Agilent 7890A/5975C CC: UA-5 T: 600 °C SR: 1:100 SC: 15–800 m/z |
| The catalytic cracking effects of the mixture produced significantly more light olefins (C2~C4) and then converted them to AHs via a Diels–Alder reaction. | [36] |
| Poplar wood sawdust–HDPE powder BR: 1:1 | ZSM-5 (0.1, 0.2, 0.3, 0.5, 0.7 M sulphuric acid) CR: 1:1 | I: CDS Pyro Probe 5200 HP Pyrolyser (Chemical Data Systems) T: 500 °C | I: Agilent 7890B (GC) Agilent 5977B (MS) CC: HP-5ms T: 300 °C SR: 1:50 |
| Several processes contribute to the formation of AHs at the Bronsted acid site, including dehydrogenation cracking, oligomerization, Diels–Alder reaction, cyclization dehydrogenation, and the hydrocarbon pool mechanism. | [43] |
| Poplar wood–PP BR: 1:1 | HZSM-5, HBeta, HY, HUSY CR: 1:4 | I: Pyrolyser (CDS5200HP-R) T: 600 °C HR: 20 °C/ms t: 60 s | I: Agilent 6890 (GC) and mass detector Agilent 5973 CC: DB-17 ms T: 280 °C SR: 1/50 SC: 40–500 m/z |
| To varying degrees, all synergies increased alkene yield. HBeta demonstrated greater synergistic deoxygenation than HZSM-5. Furthermore, the synergistic effects of HBeta and HY promoted the formation of mono AHs, whereas HZSM-5 increased the selectivity of poly-AHs. | [38] |
| CE–PP BR: 1:1 | MCM-41 and Al-MCM-41 CR: 1:10 | I: 5200HP, CDS, USA T: 300 °C, 400 °C, 500 °C, 650 °C HR: 20 °C/ms t: 18 s CG: He at 1 | I: Clarus 560S, PerkinElmer, Shelton, CT, USA CC: DB-5MS T: First: hold 50 °C for 4 min; second: heat to 280 °C at heating rate of 3 °C/min; final: hold for 20 min at 250 °C SR: 1:20 |
| Catalytic co-pyrolysis using Al-MCM-41 involves acid centers within zeolites, hydrocarbon reactions, and Diels–Alder reaction | [39] |
| CE–PP BR: 1:1 | (Ni)-MCM-41 CR: 1:10 | I: 5000 HP, CDS, Oxford, PA, USA T: 650 °C HR: 20 °C/ms t: 20 s CG: He at 1 | I: Thermo Scientific, Trace DSQII) T: First: hold at 50 °C for 4 min; second: heat to 280 °C at a rate of 3 °C/min; final: maintain at 280 °C for 12 min SR: 1:20 |
| Ni → active metal sites → ↑ bond breaking ability and deoxidation performance of catalysts → promotes reaction pathways → olefins and AHs | [40] |
| Laminaria japonica–PP BR: 1:1 | Zeolite catalyst CRl 1:1 | I: Frontier-Lab Co., Py-2020iD, Fukushima, Japan T: 500 °C | I: Agilent Technology, 7890A/5975i, Santa Clara, CA, USA CC: UA-5 capillary column |
| Acid sites on catalyst → ↑ AHs while the pore size of the catalysts was more important in removing oxygenates and wax species. | [41] |
| Water hyacinth–scrap tire BR: 1:1 | HZSM-5 and multilamellar MFI nanosheets. CR: 2:1, 1:1, 1:2 and 1:4 | I: Py: CDS5200, CDS Analytical Co. Ltd. T: 600 °C HR: 20 °C/ms t: 20 s CG: He at 1. | I: GC/MS: Agilent 7890B-5977A) |
| Multilamellar MFI → multiple pore types and high accessibility of acidic sites → access mono-AHs and BTEXs. | [42] |
| Chlorella vulgaris–urea BR: 2:1, 1:1, 1:2 | HZSM-5 CR: 1:0.25 | I: Pyrolyser (CDS5200, CDS Analytical Co. Ltd.) T: 600 °C HR: 20 °C/ms t: 60 s | Agilent 7890B-5977A CC; HP-5 ms T: 280 °C SR: 1/60 |
| The addition of HZSM-5 accelerated the removal of oxygen-containing groups in acids and esters as well as the cleavage of their long chains, converting acids and esters to olefins. | [47] |
| Biomass carbohydrate–LLDPE BR: 1:1 | Mesoporous ZSM-5 (MZSM-5) and Al-SBA-15 CR: 2% of catalyst | I: Tandem μ-reactor (RX-3050TR, Frontier Laboratories, Fukushima, Japan) T: 500 °C | I: GC/MS (7890A/5975C inert, Agilent Technology, Santa Clara, CA, USA) |
| Catalysts produced micropores and mesopores, causing larger mono-AHs due to shape selectivity to form AHs and diffusion of large molecular pyrolysates. | [37] |
3. Zeolites in Combination
3.1. Biomass-Biomass with Combined Zeolites
3.2. Biomass-Plastics with Combined Zeolites
3.3. Other Feeds with Combined Zeolites
4. Catalysts Other Than Zeolites
5. Summary and Future Work
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAEMs | Alkali and alkaline earth metals |
| AHs | Aromatics |
| BTEX | Benzene, toluene, ethylbenzene, and xylene |
| BTX | Benzene, toluene, and xylene |
| CE | Cellulose |
| e-PCB | Epoxy-printed circuit board |
| FTIR | Fourier-transform infrared spectroscopy |
| HDPE | High-density polyethylene |
| LDPE | Low-density polyethylene |
| LLDPE | Linear low-density polyethylene |
| MSW | Municipal solid waste |
| NMR | Nuclear magnetic resonance |
| PE | Polyethylene |
| PP | Polypropylene |
| PVC | Polyvinyl chloride |
| Py-GC/MS | Gas chromatography–mass spectrometry |
| TGA | Thermogravimetric analysis |
| TMR | Tandem microreactor |
References
- Amin, M.; Shah, H.H.; Fareed, A.G.; Khan, W.U.; Chung, E.; Zia, A.; Rahman Farooqi, Z.U.; Lee, C. Hydrogen Production through Renewable and Non-Renewable Energy Processes and Their Impact on Climate Change. Int. J. Hydrog. Energy 2022, 47, 33112–33134. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Gan, Y.Y.; Chen, W.-H.; Chong, C.T.; Ok, Y.S. Co-Pyrolysis of Microalgae and Other Biomass Wastes for the Production of High-Quality Bio-Oil: Progress and Prospective. Bioresour. Technol. 2022, 344, 126096. [Google Scholar] [CrossRef] [PubMed]
- Fakayode, O.A.; Wahia, H.; Zhang, L.; Zhou, C.; Ma, H. State-of-the-Art Co-Pyrolysis of Lignocellulosic and Macroalgae Biomass Feedstocks for Improved Bio-Oil Production- A Review. Fuel 2023, 332, 126071. [Google Scholar] [CrossRef]
- Hansen, S.; Mirkouei, A.; Diaz, L.A. A Comprehensive State-of-Technology Review for Upgrading Bio-Oil to Renewable or Blended Hydrocarbon Fuels. Renew. Sustain. Energy Rev. 2020, 118, 109548. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Chen, S.; Wu, J. Catalytic Co-Pyrolysis of Lignocellulosic Biomass with Polymers: A Critical Review. Green Chem. 2016, 18, 4145–4169. [Google Scholar] [CrossRef]
- Ryu, H.W.; Kim, D.H.; Jae, J.; Lam, S.S.; Park, E.D.; Park, Y.K. Recent Advances in Catalytic Co-Pyrolysis of Biomass and Plastic Waste for the Production of Petroleum-like Hydrocarbons. Bioresour. Technol. 2020, 310, 123473. [Google Scholar] [CrossRef]
- Zou, R.; Wang, C.; Qian, M.; Huo, E.; Kong, X.; Wang, Y.; Dai, L.; Wang, L.; Zhang, X.; Mateo, W.C.; et al. Catalytic Co-Pyrolysis of Solid Wastes (Low-Density Polyethylene and Lignocellulosic Biomass) over Microwave Assisted Biochar for Bio-Oil Upgrading and Hydrogen Production. J. Clean. Prod. 2022, 374, 133971. [Google Scholar] [CrossRef]
- Mariyam, S.; Alherbawi, M.; Rashid, N.; Al-Ansari, T.; McKay, G. Bio-Oil Production from Multi-Waste Biomass Co-Pyrolysis Using Analytical Py–GC/MS. Energies 2022, 15, 7409. [Google Scholar] [CrossRef]
- Grams, J. Chromatographic Analysis of Bio-Oil Formed in Fast Pyrolysis of Lignocellulosic Biomass. Rev. Anal. Chem. 2020, 39, 65–77. [Google Scholar] [CrossRef]
- Akoueson, F.; Chbib, C.; Monchy, S.; Paul-Pont, I.; Doyen, P.; Dehaut, A.; Duflos, G. Identification and Quantification of Plastic Additives Using Pyrolysis-GC/MS: A Review. Sci. Total Environ. 2021, 773, 145073. [Google Scholar] [CrossRef]
- Qiu, B.; Yang, C.; Shao, Q.; Liu, Y.; Chu, H. Recent Advances on Industrial Solid Waste Catalysts for Improving the Quality of Bio-Oil from Biomass Catalytic Cracking: A Review. Fuel 2022, 315, 123218. [Google Scholar] [CrossRef]
- Bhoi, P.R.; Ouedraogo, A.S.; Soloiu, V.; Quirino, R. Recent Advances on Catalysts for Improving Hydrocarbon Compounds in Bio-Oil of Biomass Catalytic Pyrolysis. Renew. Sustain. Energy Rev. 2020, 121, 109676. [Google Scholar] [CrossRef]
- Xu, S.; Cao, B.; Uzoejinwa, B.B.; Odey, E.A.; Wang, S.; Shang, H.; Li, C.; Hu, Y.; Wang, Q.; Nwakaire, J.N. Synergistic Effects of Catalytic Co-Pyrolysis of Macroalgae with Waste Plastics. Process Saf. Environ. Prot. 2020, 137, 34–48. [Google Scholar] [CrossRef]
- Ma, C.; Kumagai, S.; Saito, Y.; Kameda, T.; Yoshioka, T. An Integrated Utilization Strategy of Printed Circuit Boards and Waste Tire by Fast Co-Pyrolysis: Value-Added Products Recovery and Heteroatoms Transformation. J. Hazard. Mater. 2022, 430, 128420. [Google Scholar] [CrossRef]
- Zhu, J.; Jin, L.; Luo, Y.; Hu, H.; Xiong, Y.; Wei, B.; Wang, D. Fast Co-Pyrolysis of a Massive Naomaohu Coal and Cedar Mixture Using Rapid Infrared Heating. Energy Convers. Manag. 2020, 205, 112442. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, B.; Liu, C.; Mwenya, S.; Zhang, H. A Minireview on Catalytic Fast Co-Pyrolysis of Lignocellulosic Biomass for Bio-Oil Upgrading via Enhancing Monocyclic Aromatics. J. Anal. Appl. Pyrolysis 2022, 164, 105544. [Google Scholar] [CrossRef]
- Mishra, R.K.; Chistie, S.M.; Naika, S.U.; Mohanty, K. Catalytic Pyrolysis of Biomass over Zeolites for Bio-Oil and Chemical Production: A Review on Their Structure, Porosity and Acidity Co-Relation. Bioresour. Technol. 2022, 366, 128189. [Google Scholar] [CrossRef]
- Niu, Q.; Ghysels, S.; Wu, N.; Rousseau, D.P.L.; Pieters, J.; Prins, W.; Ronsse, F. Effects of Demineralization on the Composition of Microalgae Pyrolysis Volatiles in Py-GC–MS. Energy Convers. Manag. 2022, 251, 114979. [Google Scholar] [CrossRef]
- Hassan, H.; Lim, J.K.; Hameed, B.H. Recent Progress on Biomass Co-Pyrolysis Conversion into High-Quality Bio-Oil. Bioresour. Technol. 2016, 221, 645–655. [Google Scholar] [CrossRef]
- Smith, J.V. Origin and Structure of Zeolites. In Zeolite Chemistry and Catalysis; Rabo, J.A., Ed.; American Chemical Society Monograph: Washington, DC, USA, 1976; pp. 3–79. [Google Scholar]
- Bhatia, S. Introduction. In Zeolite Catalysis: Principles and Applications; Bhatia, S., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 1–6. [Google Scholar]
- Huang, M.; Xu, J.; Ma, Z.; Yang, Y.; Zhou, B.; Wu, C.; Ye, J.; Zhao, C.; Liu, X.; Chen, D.; et al. Bio-BTX Production from the Shape Selective Catalytic Fast Pyrolysis of Lignin Using Different Zeolite Catalysts: Relevance between the Chemical Structure and the Yield of Bio-BTX. Fuel Process. Technol. 2021, 216, 106792. [Google Scholar] [CrossRef]
- Ehsan Kianfar, R.A. Zeolite Catalyst Based Selective for the Process MTG: A Review. In Zeolites: Advances in Research and Applications; Mahler, A., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2020. [Google Scholar]
- Ma, W.; Rajput, G.; Pan, M.; Lin, F.; Zhong, L.; Chen, G. Pyrolysis of Typical MSW Components by Py-GC/MS and TG-FTIR. Fuel 2019, 251, 693–708. [Google Scholar] [CrossRef]
- He, H.; Ma, X.; Yu, Z.; Chen, L.; Chen, X. A Study on the Deoxidation Effect of Different Acidic Zeolites during the Co-Pyrolysis of Aged Municipal Solid Waste and Corn Stalk. J. Anal. Appl. Pyrolysis 2021, 159, 105319. [Google Scholar] [CrossRef]
- Chaerusani, V.; Zahra, A.C.A.; Anniwaer, A.; Zhang, P.; Chaihad, N.; Rizkiana, J.; Kusakabe, K.; Kasai, Y.; Abudula, A.; Guan, G. Catalytic Upgrading of Bio-Oils Derived from Terrestrial and Marine Biomass over Various Types of Zeolites. J. Anal. Appl. Pyrolysis 2022, 168, 105735. [Google Scholar] [CrossRef]
- Ling, Q.; He, S.; Babadi, A.A.; Yuan, C.; Pan, C.; Jiang, D.; Cao, B.; Hu, Y.; Wang, S.; Zheng, A. Preparation of Low-Nitrogen Chlorella Based on Proteolysis Technology and Pyrolysis Characteristics of the Products. J. Anal. Appl. Pyrolysis 2022, 167, 105630. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Chen, L.; Tang, F.; Ma, X. Fast Catalytic Co-Pyrolysis Characteristics and Kinetics of Chlorella Vulgaris and Municipal Solid Waste over Hierarchical ZSM-5 Zeolite. Bioenergy Res. 2021, 14, 226–240. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Z.; Lu, X.; Ma, X. Catalytic Co-Pyrolysis of Microwave Pretreated Chili Straw and Polypropylene to Produce Hydrocarbons-Rich Bio-Oil. Bioresour. Technol. 2021, 319, 124191. [Google Scholar] [CrossRef] [PubMed]
- Mullen, C.A.; Dorado, C.; Boateng, A.A. Catalytic Co-Pyrolysis of Switchgrass and Polyethylene over HZSM-5: Catalyst Deactivation and Coke Formation. J. Anal. Appl. Pyrolysis 2018, 129, 195–203. [Google Scholar] [CrossRef]
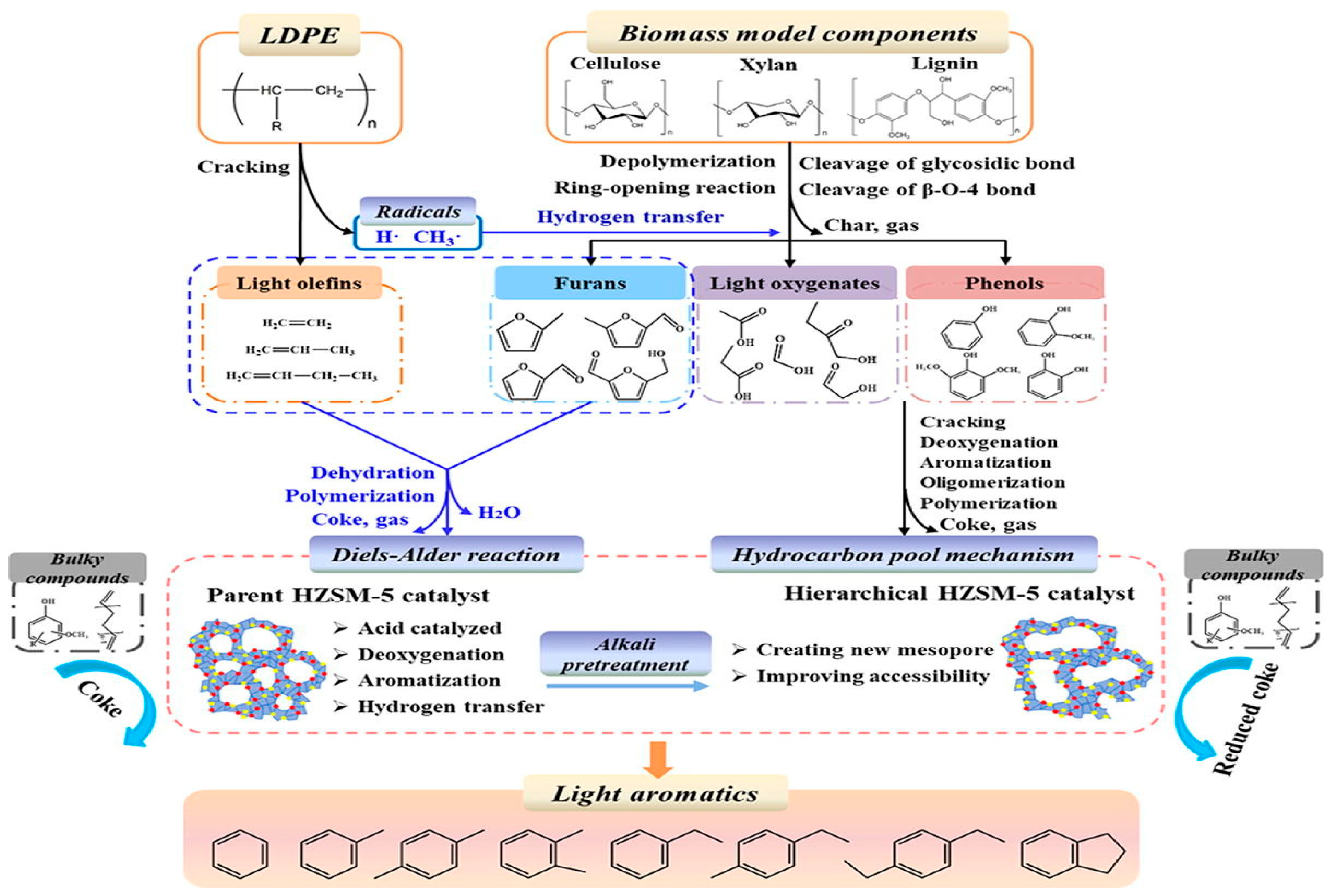
- Huang, M.; Zhu, L.; Zhang, W.; Zhu, L.; Ma, Z.; Chen, D. Insight into the Synergistic Reaction Mechanism of Biomass Pseudo Components and Low-Density Polyethylene for the Production of Light Aromatics through Co-Catalytic Fast Pyrolysis over Hierarchical HZSM-5. Fuel 2022, 324, 124699. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, D.; Cao, B.; Wang, S.; Li, H.; Barati, B.; Xia, Z.; Zhou, J.; Wang, S. Study on ZSM-5 Catalytic Pyrolysis Mechanism of Cellulose Based on the Py-GC/MS and the Density Functional Theory. Combust. Flame 2022, 241, 112131. [Google Scholar] [CrossRef]
- Ke, L.; Wang, Y.; Wu, Q.; Zhou, N.; Dai, L.; Tian, X.; Huang, W.; Peng, Y.; Xu, J.; Zou, R.; et al. Pressurized Ex-Situ Catalytic Co-Pyrolysis of Polyethylene and Lignin: Efficient BTEX Production and Process Mechanism Analysis. Chem. Eng. J. 2022, 431, 134122. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z. Study of the Thermal Behavior, Kinetics, and Product Characterization of Biomass and Low-Density Polyethylene Co-Pyrolysis by Thermogravimetric Analysis and Pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2018, 133, 185–197. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Fu, Z.; Li, R.; Wu, Y. Synergistic Effect of Catalytic Co-Pyrolysis of Cellulose and Polyethylene over HZSM-5. J. Therm. Anal. Calorim. 2020, 140, 363–371. [Google Scholar] [CrossRef]
- Park, Y.K.; Lee, B.; Lee, H.W.; Watanabe, A.; Jae, J.; Tsang, Y.F.; Kim, Y.M. Co-Feeding Effect of Waste Plastic Films on the Catalytic Pyrolysis of Quercus Variabilis over Microporous HZSM-5 and HY Catalysts. Chem. Eng. J. 2019, 378, 122151. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, H.W.; Jae, J.; Jung, K.B.; Jung, S.C.; Watanabe, A.; Park, Y.K. Catalytic Co-Pyrolysis of Biomass Carbohydrates with LLDPE over Al-SBA-15 and Mesoporous ZSM-5. Catal. Today 2017, 298, 46–52. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Z.; Wang, Q. Evaluation of Zeolite Catalysts on Product Distribution and Synergy during Wood-Plastic Composite Catalytic Pyrolysis. Energy 2019, 189, 116174. [Google Scholar] [CrossRef]
- Chi, Y.; Xue, J.; Zhuo, J.; Zhang, D.; Liu, M.; Yao, Q. Catalytic Co-Pyrolysis of Cellulose and Polypropylene over All-Silica Mesoporous Catalyst MCM-41 and Al-MCM-41. Sci. Total Environ. 2018, 633, 1105–1113. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Zhuo, J.; Yao, Q. Investigation of a Ni-Modified MCM-41 Catalyst for the Reduction of Oxygenates and Carbon Deposits during the Co-Pyrolysis of Cellulose and Polypropylene. ACS Omega 2020, 5, 20299–20310. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, H.W.; Choi, S.J.; Jeon, J.K.; Park, S.H.; Jung, S.C.; Kim, S.C.; Park, Y.K. Catalytic Co-Pyrolysis of Polypropylene and Laminaria Japonica over Zeolitic Materials. Int. J. Hydrog. Energy 2017, 42, 18434–18441. [Google Scholar] [CrossRef]
- Chen, L.; Ma, X.; Tang, F.; Li, Y.; Yu, Z.; Chen, X. Comparison of Catalytic Effect on Upgrading Bio-Oil Derived from Co-Pyrolysis of Water Hyacinth and Scrap Tire over Multilamellar MFI Nanosheets and HZSM-5. Bioresour. Technol. 2020, 312, 123592. [Google Scholar] [CrossRef]
- Sarker, M.; Liu, R.; Rahman, M.M.; Li, C.; Chai, M.; Nishu; He, Y. Impact of Acid-Modified ZSM-5 on Hydrocarbon Yield of Catalytic Co-Pyrolysis of Poplar Wood Sawdust and High-Density Polyethylene by Py-GC/MS Analysis. J. Energy Inst. 2020, 93, 2435–2443. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Yu, Z.; Deng, T.; Chen, X.; Chen, L.; Dai, M. A Study on Catalytic Co-Pyrolysis of Kitchen Waste with Tire Waste over ZSM-5 Using TG-FTIR and Py-GC/MS. Bioresour. Technol. 2019, 289, 121585. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Ruan, R.; Li, J.; Ma, L.; Wang, C.; Zhou, W. Aromatics Production from Fast Co-Pyrolysis of Lignin and Waste Cooking Oil Catalyzed by HZSM-5 Zeolite. Appl. Energy 2020, 263, 114629. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, B.; Zong, P.; Tian, Y.; Xu, F.; Li, D.; Li, F.; Tian, Y. Thermal Behavior, Kinetics and Fast Pyrolysis Characteristics of Palm Oil: Analytical TG-FTIR and Py-GC/MS Study. Energy Convers. Manag. 2019, 199, 111964. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, Z.; Li, M.; Bin, Y.; Zhang, X.; Wei, C.; Liao, Y.; Zheng, A.; Ma, X. Catalytic Co-Pyrolysis of Chlorella Vulgaris and Urea: Thermal Behaviors, Product Characteristics, and Reaction Pathways. J. Anal. Appl. Pyrolysis 2022, 167, 105667. [Google Scholar] [CrossRef]
- Kim, Y.M.; Han, T.U.; Kim, S.; Jae, J.; Jeon, J.K.; Jung, S.C.; Park, Y.K. Catalytic Co-Pyrolysis of Epoxy-Printed Circuit Board and Plastics over HZSM-5 and HY. J. Clean. Prod. 2017, 168, 366–374. [Google Scholar] [CrossRef]
- Hou, J.; Zhong, D.; Liu, W. Catalytic Co-Pyrolysis of Oil Sludge and Biomass over ZSM-5 for Production of Aromatic Platform Chemicals. Chemosphere 2022, 291, 132912. [Google Scholar] [CrossRef]
- Park, Y.K.; Siddiqui, M.Z.; Karagöz, S.; Han, T.U.; Watanabe, A.; Kim, Y.M. In-Situ Catalytic Co-Pyrolysis of Kukersite Oil Shale with Black Pine Wood over Acid Zeolites. J. Anal. Appl. Pyrolysis 2021, 155, 105050. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Z.; Zhang, B.; Ding, K.; Xue, Z.; Deng, A.; Ruan, R. Upgraded Bio-Oil Production via Catalytic Fast Co-Pyrolysis of Waste Cooking Oil and Tea Residual. Waste Manag. 2017, 60, 357–362. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K.; Wang, X. Pyrolysis Kinetic Behavior and Py-GC–MS Analysis of Waste Dahlia Flowers into Renewable Fuel and Value-Added Chemicals. Fuel 2020, 260, 116338. [Google Scholar] [CrossRef]
- Deng, T.; Yu, Z.; Zhang, X.; Zhang, Y.; Chen, L.; Ma, X. Catalytic Co-Pyrolysis Behaviors and Kinetics of Camellia Shell and Take-out Solid Waste Using Pyrolyzer—Gas Chromatography/Mass Spectrometry and Thermogravimetric Analyser. Bioresour. Technol. 2020, 297, 122419. [Google Scholar] [CrossRef]
- Haji Morni, N.A.; Yeung, C.M.; Tian, H.; Yang, Y.; Phusunti, N.; Abu Bakar, M.S.; Azad, A.K. Catalytic Fast Co-Pyrolysis of Sewage Sludge−sawdust Using Mixed Metal Oxides Modified with ZSM-5 Catalysts on Dual-Catalysts for Product Upgrading. J. Energy Inst. 2021, 94, 387–397. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, Z.; Zhang, B.; Wang, W.; Seufitelli, G.V.S.; Resende, F.L.P. Catalytic Fast Co-Pyrolysis of Waste Greenhouse Plastic Films and Rice Husk Using Hierarchical Micro-Mesoporous Composite Molecular Sieve. Waste Manag. 2020, 102, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Zhong, Z.; Wang, J.; Zhang, B.; Fan, L.; Liu, S.; Wang, Y.; Liu, Y.; Zhong, D.; Chen, P.; et al. Improving Hydrocarbon Yield from Catalytic Fast Co-Pyrolysis of Hemicellulose and Plastic in the Dual-Catalyst Bed of CaO and HZSM-5. Bioresour. Technol. 2018, 261, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, J.; Zhong, Z.; Wang, K.; Wang, X.; Zhang, B.; Ruan, R.; Li, M.; Ragauskas, A.J. Catalytic Fast Co-Pyrolysis of Bamboo Sawdust and Waste Plastics for Enhanced Aromatic Hydrocarbons Production Using Synthesized CeO2/Γ-Al2O3 and HZSM-5. Energy Convers. Manag. 2019, 196, 759–767. [Google Scholar] [CrossRef]
- Ding, K.; He, A.; Zhong, D.; Fan, L.; Liu, S.; Wang, Y.; Liu, Y.; Chen, P.; Lei, H.; Ruan, R. Improving Hydrocarbon Yield via Catalytic Fast Co-Pyrolysis of Biomass and Plastic over Ceria and HZSM-5: An Analytical Pyrolyzer Analysis. Bioresour. Technol. 2018, 268, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ghorbannezhad, P.; Park, S.; Onwudili, J.A. Co-Pyrolysis of Biomass and Plastic Waste over Zeolite- and Sodium-Based Catalysts for Enhanced Yields of Hydrocarbon Products. Waste Manag. 2020, 102, 909–918. [Google Scholar] [CrossRef]
- Alcazar-Ruiz, A.; Sanchez-Silva, L.; Dorado, F. Enhancement of BTX Production via Catalytic Fast Pyrolysis of Almond Shell, Olive Pomace with Polyvinyl Chloride Mixtures. Process Saf. Environ. Prot. 2022, 163, 218–226. [Google Scholar] [CrossRef]
- Uzoejinwa, B.B.; Cao, B.; Wang, S.; Hu, X.; Hu, Y.; Pan, C.; Li, B.; Anyadike, C.C.; Asoiro, F.U.; Oji, N.A.; et al. Catalytic Co-Pyrolysis of Macroalgal Components with Lignocellulosic Biomass for Enhanced Biofuels and High-Valued Chemicals. Int. J. Energy Res. 2022, 46, 2674–2697. [Google Scholar] [CrossRef]
- Cao, B.; Sun, Y.; Yuan, J.; Wang, S.; Gong, X.; Barati, B.; Zheng, A.; Jiang, D.; Hu, Y.; Yuan, C.; et al. Co-Pyrolysis Characteristics of Polysaccharides-Cellulose and the Co-Pyrolyzed Compound Distributions over Two Kinds of Zeolite Catalysts. Int. J. Energy Res. 2020, 44, 6350–6362. [Google Scholar] [CrossRef]
- Dai, M.; Yu, Z.; Fang, S.; Ma, X. Behaviors, Product Characteristics and Kinetics of Catalytic Co-Pyrolysis Spirulina and Oil Shale. Energy Convers. Manag. 2019, 192, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Z.; Ding, K.; Zhang, B.; Deng, A.; Min, M.; Chen, P.; Ruan, R. Co-Pyrolysis of Bamboo Residual with Waste Tire over Dual Catalytic Stage of CaO and Co-Modified HZSM-5. Energy 2017, 133, 90–98. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Zhong, Z.; Ding, K.; Deng, A.; Min, M.; Chen, P.; Ruan, R. Catalytic Fast Co-Pyrolysis of Bamboo Residual and Waste Lubricating Oil over an Ex-Situ Dual Catalytic Beds of MgO and HZSM-5: Analytical PY-GC/MS Study. Energy Convers. Manag. 2017, 139, 222–231. [Google Scholar] [CrossRef]
- Yao, D.; Li, H.; Dai, Y.; Wang, C.H. Impact of Temperature on the Activity of Fe-Ni Catalysts for Pyrolysis and Decomposition Processing of Plastic Waste. Chem. Eng. J. 2021, 408, 127268. [Google Scholar] [CrossRef]
- Dinh Ngo, S.; Tuong Vi Tran, T.; Kongparakul, S.; Reubroycharoen, P.; Kidkhuntod, P.; Chanlek, N.; Wang, J.; Guan, G.; Samart, C. Catalytic Pyrolysis of Napier Grass with Nickel-Copper Core-Shell Bi-Functional Catalyst. J. Anal. Appl. Pyrolysis 2020, 145, 104745. [Google Scholar] [CrossRef]
- Song, Q.; Zhao, H.; Jia, J.; Yang, L.; Lv, W.; Bao, J.; Shu, X.; Gu, Q.; Zhang, P. Pyrolysis of Municipal Solid Waste with Iron-Based Additives: A Study on the Kinetic, Product Distribution and Catalytic Mechanisms. J. Clean. Prod. 2020, 258, 120682. [Google Scholar] [CrossRef]
- Mahadevan, R.; Adhikari, S.; Shakya, R.; Wang, K.; Dayton, D.; Lehrich, M.; Taylor, S.E. Effect of Alkali and Alkaline Earth Metals on In-Situ Catalytic Fast Pyrolysis of Lignocellulosic Biomass: A Microreactor Study. Energy Fuels 2016, 30, 3045–3056. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Z.; Zhang, Z.; Sun, J.; Wang, Q.; Pittman, C.U. Catalytic Fast Pyrolysis of a Wood-Plastic Composite with Metal Oxides as Catalysts. Waste Manag. 2018, 79, 38–47. [Google Scholar] [CrossRef]
- Jin, L.; Bai, X.; Li, Y.; Dong, C.; Hu, H.; Li, X. In-Situ Catalytic Upgrading of Coal Pyrolysis Tar on Carbon-Based Catalyst in a Fixed-Bed Reactor. Fuel Process. Technol. 2016, 147, 41–46. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Lee, J.W.; Popov, B.N. A Review of the Development of Nitrogen-Modified Carbon-Based Catalysts for Oxygen Reduction at USC. Catal. Sci. Technol. 2011, 1, 207–217. [Google Scholar] [CrossRef]
- Yuan, C.; El-Fatah Abomohra, A.; Wang, S.; Liu, Q.; Zhao, S.; Cao, B.; Hu, X.; Marrakchi, F.; He, Z.; Hu, Y. High-Grade Biofuel Production from Catalytic Pyrolysis of Waste Clay Oil Using Modified Activated Seaweed Carbon-Based Catalyst. J. Clean. Prod. 2021, 313, 127928. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, G.; Song, W.; Zhao, Y.; Miao, Z.; Yao, R.; Gao, J. Catalytic Microwave Pyrolysis of Orange Peel: Effects of Acid and Base Catalysts Mixture on Products Distribution. J. Energy Inst. 2021, 98, 172–178. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Yang, H.; Zhu, Y.; Zhao, L.; Li, M. Effects of Solid Acid and Base Catalysts on Pyrolysis of Rice Straw and Wheat Straw Biomass for Hydrocarbon Production. J. Energy Inst. 2022, 101, 140–148. [Google Scholar] [CrossRef]
- Sawato, T.; Saito, N.; Yamaguchi, M. Chemical Systems Involving Two Competitive Self-Catalytic Reactions. ACS Omega 2019, 4, 5879–5899. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive Utilization Status of Red Mud in China: A Critical Review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Gupta, J.; Papadikis, K.; Kozhevnikov, I.V.; Konysheva, E.Y. Exploring the Potential of Red Mud and Beechwood Co-Processing for the Upgrading of Fast Pyrolysis Vapours. J. Anal. Appl. Pyrolysis 2017, 128, 35–43. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Liu, J.; Evrendilek, F.; Xie, W.; He, Y.; Buyukada, M. Comparative (Co-)Pyrolytic Performances and by-Products of Textile Dyeing Sludge and Cattle Manure: Deeper Insights from Py-GC/MS, TG-FTIR, 2D-COS and PCA Analyses. J. Hazard. Mater. 2021, 401, 123276. [Google Scholar] [CrossRef]
- Yu, Z.; Dai, M.; Huang, M.; Fang, S.; Xu, J.; Lin, Y.; Ma, X. Catalytic Characteristics of the Fast Pyrolysis of Microalgae over Oil Shale: Analytical Py-GC/MS Study. Renew. Energy 2018, 125, 465–471. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Z.; Liang, J.; Liao, Y.; Ma, X. Co-Pyrolysis of Chlorella Vulgaris and Kitchen Waste with Different Additives Using TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2018, 177, 582–591. [Google Scholar] [CrossRef]
- Lin, X.; Kong, L.; Cai, H.; Zhang, Q.; Bi, D.; Yi, W. Effects of Alkali and Alkaline Earth Metals on the Co-Pyrolysis of Cellulose and High Density Polyethylene Using TGA and Py-GC/MS. Fuel Process. Technol. 2019, 191, 71–78. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Gu, J.; Yuan, H.; Chen, Y. Synergistic Effects for Fast Co-Pyrolysis of Strong-Acid Cation Exchange Resin and Cellulose Using Py-GC/MS. Fuel 2021, 302, 121232. [Google Scholar] [CrossRef]
- Fadillah, G.; Fatimah, I.; Sahroni, I.; Musawwa, M.M.; Mahlia, T.M.I.; Muraza, O. Recent Progress in Low-Cost Catalysts for Pyrolysis of Plastic Waste to Fuels. Catalysts 2021, 11, 837. [Google Scholar] [CrossRef]
- Jiang, Y.; Zong, P.; Tian, B.; Xu, F.; Tian, Y.; Qiao, Y.; Zhang, J. Pyrolysis Behaviors and Product Distribution of Shenmu Coal at High Heating Rate: A Study Using TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2019, 179, 72–80. [Google Scholar] [CrossRef]


| Feedstock | Catalysts | Pyrolysis Operating Conditions | GC-MS Operating Conditions | Products | Reaction Mechanism | Reference |
|---|---|---|---|---|---|---|
| Camellia shell–take-out solid waste BR: 0:1, 0.7:1, 0.5:1, 1:0 | HZMS-5, CaO, MgO CR: 1:2 CCR: HZSM-5 with CaO and MgO is 1:1 | I: Pyro probe 5200 Pyrolyser (CDS Analytical Co. Ltd.) T: 500–700 °C HR: 20 °C/ms t: 20 s CG: N2 | I: Agilent 7890B-5977A CC: HP-5 ms, 30 m × 0.25 mm ID 0.25 μm T: 50 to 250 °C HR: 10 °C/min SR: 1:50 SC: 30–500 m/z |
| The takeout solid waste process, which contains more hydrogen, enhances the conversion of oxygenated compounds to hydrocarbon products. | [53] |
| Sewage sludge-–awdust BR: 1:2 | MMOs CCR: 1:1 of NiO and MoO3 to form MMOs. MMOs modified with ZSM-5 refers to MMOs + ZSM-5 (50%) CR: 1:1 | I: CDS pyro probe model 5200 Pyrolyser T: 600 °C HR: 30 °C/min t: 10 s CG: He | I: GC/MS (Shimadzu GC/MS-QP 2010 Ultra) CC: Rtx-5 polar column T: 40 to 270 °C HR: 8 °C/min SR: 1:50 SC: 35 and 500 m/z |
| The use of both prohibited aldehydes and a specific reaction pathway improves the production of phenolic compounds, both light and heavy. The use of MMOs (manganese oxide) increases the yield of light phenols by promoting demethoxylation, decarbonylation, and cracking reactions. | [54] |
| Waste greenhouse plastic films– rice husk | HZSM-5/MCM-41 CR: 1:2 | I: CDS Pyro probe 5200 Pyrolyser HR: 20,000 °C/s t: 20 s CG: He | I: Agilent Technologies, 7890A/5975C, 2010, Santa Clara, CA, USA CC: DB-5 ms capillary column (0.25 mm × 0.25 μm × 30 m) T: from 50 to 290 °C HR: 8 °C/min. |
| HZSM-5 catalyzes deoxygenation reactions by its shape selectivity. When plastic and biomass are used together in co-pyrolysis, oxygenated chemicals are produced, leading to the breakage and fragmentation of long-chain organic molecules. This promotes the production of hydrocarbons, which is the desired outcome for bio-oil production. | [55] |
| Hemicellulose: LLDPE BR: 0 to 100 wt.% | CR: 1:2 CCR: CaO to HZSM-5—1:0, 2:1, 1:1, 1:2 and 0:1 | I: CDS Pyro probe 5200 T: 450–700 °C HR: 20,000 °C/s t: 20 s | I: GC/MS, Agilent 7890A/5975C CC: DB-5 ms capillary column (length = 30 m, I.D. = 0.25 mm, film thickness = 0.25 μm T: 30 to 290 °C HR: 8 °C/min SR: 60:1 SC: 25–550 m/z |
| LLDPE enhances hydrocarbons (alkene) due to Diels–Alder reactions and hydrocarbon pool reactions. Increases AHs from∼27% over sole HZSM-5 to utmost ∼40%. CaO deoxygenated the acids, HZM-5 aromatization of ketones. | [56] |
| Bamboo sawdust– LLDPE | CeO2/γ-Al2O3 and HZSM-5 CCR: HZSM-5 and synthesized CeO2/γ-Al2O3 with 8 wt.% CeO2 loading | I: CDS Pyro probe 5200 Pyrolyser T: 600 °C HR: 2000 C/s t: 30 s CG: He | I: GC/MS, 7890A/5975C, Agilent) CC: HP-5MS, 0.25 mm × 0.25 μm × 30 m T: 40 to 180 °C at a heating rate of 5 °C/min, and then was increased to 280 °C at 10 °C/min SC: 28–350 |
| AHs increased at first and then decreased as the LLDPE percentage was elevated from 20% to 100%. Increasing LLDPE (due to the additive effect of monocyclic AHs) favours the production of xylenes, ethylbenzene, and alkylbenzenes. The olefins from LLDPE pyrolysis favors the Diels–Alder cycloaddition and AH production. | [57] |
| Corn stover: LDPE BR: LDPE to CS was set at 1:5, 1:2, 1:1, 2:1 and 5:1 | CeO2 and HZSM-5 in tandem CRR: CeO2 to HZSM-5—1:5, 1:2, 1:1, 2:1, and 5:1 | I: CDS Pyro probe 2000 Pyrolyser T: 600 °C HR: 10,000 °C/s t: 30 s CG: He | I: Agilent 7890A/5975C GC/MS T: 40 to 280 °C at 5 °C/min to a final temperature of 280 °C SR: 60:1 SC: 30–500 m/z |
| Catalysis of CeO2; aldehydes and acids are converted to ketones. HZSM-5 promotes the Diels–Alder reactions and cyclization and dehydration reactions to form mono-AHs and alkenes. | [58] |
| Sugarcane bagasse pith– PET BR: biomass to plastic ratio—0 to 5. | HZSM-5 and sodium carbonate/gamma-alumina served. CCR: HZSM-5 to Na2CO3—1 to 5 | I: Rx-300 TR, Frontier Laboratories, Japan T: 400 to 800 °C CG: He | I: 7890A, Agilent Technologies, Santa Clara, CA, USA T: 45 to 280 °C HR: 10 °C/min SR: 1:50 |
| Plastics support the hydrocarbon and Diels–Alder pathways, reducing coke formation. Additionally, H2O decreased, increasing AH compounds (ethylbenzene, toluene, and xylenes). The presence of sodium prevented coke formation. | [59] |
| Olive pomace or almond shell–PVC BR: biomass to plastic—1:1.5 | NaZSM-5 and HZSM-5. ZSM-5 in sodium and acid form (NaZSM-5 and HZSM-5) CR and CRR: biomass–PVC mixture varying the biomass/PVC–zeolite mass ratio (2:1, 1:1 and 1:2) | I: CDS Pyro probe 6200 T: 650 °C HR: 20 °C/ms t: 20 s. | I: Agilent Technologies 7890B/5977B GC/MS CC: Elite-35MS capillary column (30 m × 0.25 µm) T: 40 to 280 °C HR: 5 °C/min SR: 1:80 |
| The use of HZMS-5 catalysts during co-pyrolysis leads to a reduction in acid and an increase in AHs through a series of reactions such as deoxygenation, isomerization, and oligomerization. Additionally, the formation of toluene and xylene is enhanced through the processes of dehydration and demethoxylation of phenolics. | [60] |
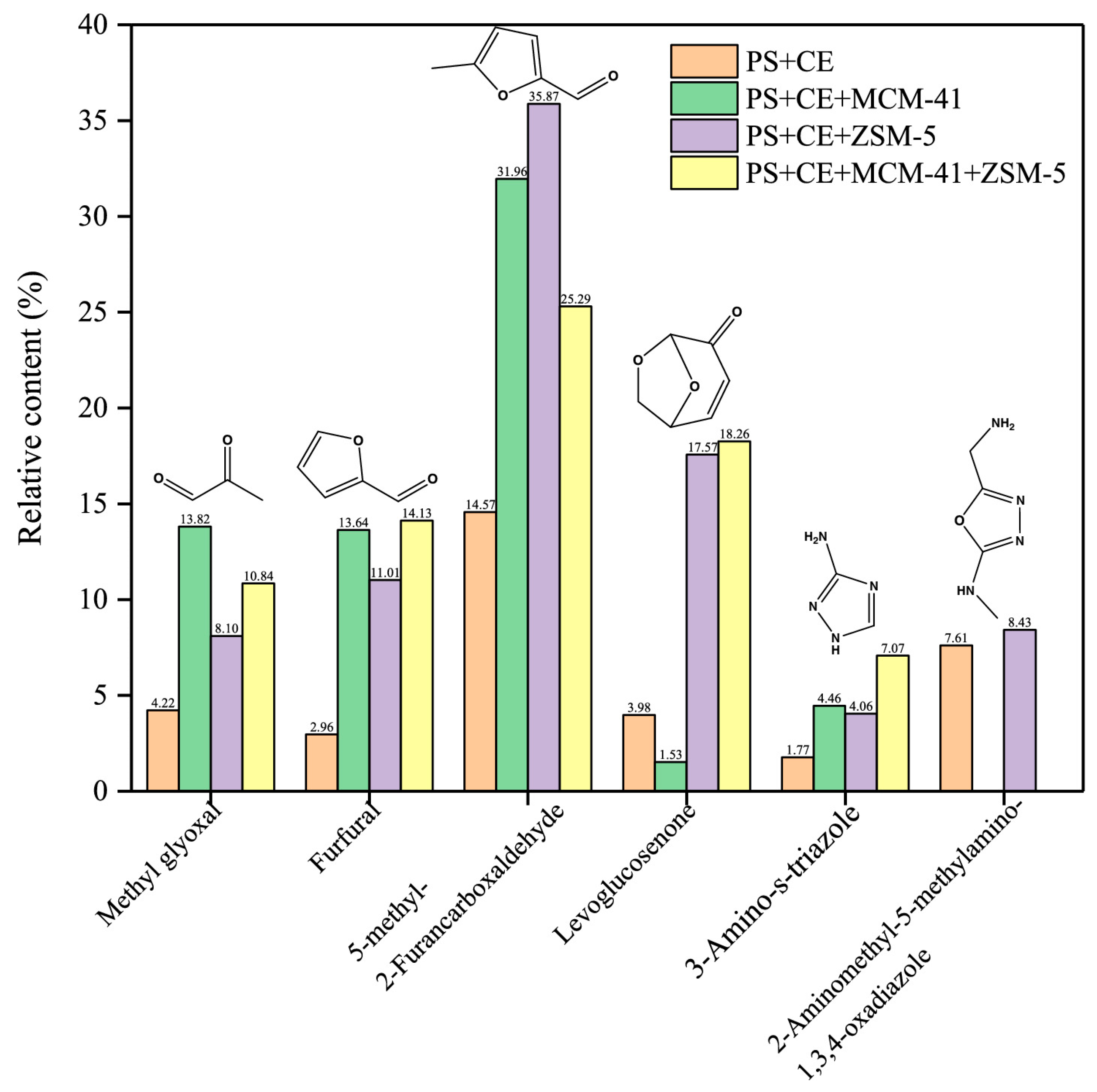
| Rice husk– main components of Enteromorpha clathrata (protein, polysaccharide, and ash) | ZSM-5 and MCM-41 CR and CCR: 1:1:1:1 (rice husk:seaweed component: catalyst) | I: CDS 5250 Pyro Probe, Oxford, PA, USA T: 550 °C HR: 10,000 °C/s CG: Ar at 1 | I: Agilent Technologies 7890 A/5975C CC: HP-5MS—30 m × 250 μm × 0.25 μm T: 40 to 200 °C at 5 °C/min, and raised to 280 °C at 10 °C/min SR: 80:1 SC: 35 to 550 m/z |
| Free radicals in the biomass result in AH compounds due to cracking, reformation, oxidation, and polymerization. ZSM-5 favoured dehydration, decarboxylation, cracking, and aromatization reactions to remove oxygenated compounds. MCM- 41 decreased oxygenates and increased aliphatics and AHs. | [61] |
| Polysaccharides–CE BR: 1:1 | MCM-41, ZSM-5 CR: 1:1 | I: Py: CDS5200, CDS Analytical Co. Ltd. T: 550 °C HR: 20 °C/ms t: 20 s CG: He | I: Agilent Technology, 7890A/5975i CC: HP-5 ms T: 280 and 300 °C SC: 35 and 550 amu |
| DFT calculation → free radicals pyrolysed from soluble polysaccharides → ↓ ring-opening reaction of D-glucopyranose. | [62] |
| Spirulina–oil shale BR: 0, 10, 30, 50, 70, 90 and 100 wt.% of SP | HZSM-5 and CaO CR: 1:1 (BR was set at 1:1) CRR: 1:0, 3:1, 1:1, 1:3 CaO/HZM-5 ratio | I: Pyro probe 5200 Pyrolyser (CDS Analytical) T: 600 °C HR: (20 °C/ms) t: 20 s CG: N2 | I: 7890A/5975C GC/MS analyser T: 50 to 290 °C HR: 10 °C/min SR: 1:50 SC: 35–300 amu CG: He at 3 |
| CaO →↑ secondary cracking reaction, ↓ coke deposition. Combined use of catalysts since HZSM-5 →↑ hydrocarbon production via cracking, dehydration, decarbonylation and aromatization reactions. | [63] |
| Bamboo residual–waste tire | 1 mg each, separated by quartz wool → HZSM-5, CaO, mixture of feed, CaO, HZSM-5. | I: CDS Pyro probe 5200 Pyrolyser T: 600 °C HR: 2000 T: 600 °C/s CG: He at 1 | I: GC/MS, 7890A/ 5975C, Agilent CC: HP-5MS, 0.25 mm × 0.25 μm × 30 m SR: 1:80 |
| Combination → effective removal of acids and improved formation of AHs and olefins through catalytic cracking, neutralization, and thermal cracking. Zeolites provided shape selectivity for deoxygenation and increased hydrocarbon yields. | [64] |
| Bamboo residual–waste lubricating oil (WLO) | Dual catalytic beds of MgO and HZSM-5 CR: 1:2 | I: CDS analytical Pyro probe 5200 Pyrolyser T: 500–700 °C HR: 2000 °C/s CG: He | I: GC/MS, 7890A/5975C, Agilent CC: HP-5MS, 0.25 mm × 0.25 μm × 30 m T: 40 to 180 °C at 5 °C/min, and then to 280 °C at a 10 °C/min SR: 1:80 SC: 28–350 m/z |
| MgO exhibited more deacidification and ketonisation. HZSM-5/MgO highest yields of AHs via Diels–Alder reaction. WLO increases the yields due to hydrocarbon pool reactions. | [65] |
| Feedstock | Catalysts | Pyrolysis Operating Conditions | GC-MS Operating Conditions | Products | Reaction Mechanism | Reference |
|---|---|---|---|---|---|---|
| Beechwood (BW) and red mud (catalyst) (RM) BR: BW: RM 1:1, 1:2, 1:4 | Oxides: α-Al2O3, Fe2O3, and SiO2 in red mud as catalyst | I: 5200 Pyrolyser (CDS Analytical, Oxford, PA, USA T: 500 °C HR: 20 °C/ms t: 20 s CG: He at 30 | I: 7890A/5975C gas chromatograph (GC)/mass spectrometer (MS) (Agilent Technology, Santa Clara, CA, USA) CC: HP5MS T: 50 to 300 °C SR: 1:50 SC: 35–400 amu |
| RM-950, Fe2O3, and TiO2 during depolymerization of CE and hemicellulose → increased furfurals and acetic acid→ increased depolymerization reactions and selectivity towards the products. | [78] |
| Textile dyeing Sludge (catalyst) –cattle manure BR: 0.9:0.1, 0.7:0.3, 1:1, 0.3:0.7, 0.1:0.9 | Iron in the feed as a catalyst | I: Pyrolysis reactor (Frontier Lab PY-2020id, Fukushima, Japan) t: 24 s CG: He | I: Thermo DSQ Ⅱ, USA Capillary column (CC): HP-5MS T: Started at 45 °C for 2 min, increased to 300 °C at 4 °C/min, and remained at 300 °C for 15 min |
| During the co-pyrolysis of the blend, the mechanism of the process changed from a diffusion model to a reaction-order mode. Gases were primarily produced as a result of the diffusion reaction. | [79] |
| Microalgae–oil shale (catalyst) BR: OS of 1%, 3%, 7%, and 10% | OS as a catalyst | I: 5200 Pyrolyser (CDS Analytical, Oxford, PA, USA T: 500, 600, 700 and 800 °C HR: 20 °C/ms t: 20 s CG: Helium at 30 mL/min | I: 7890A/5975C gas chromatograph (GC)/mass spectrometer (MS) (Agilent Technology, Santa Clara, CA, USA) CC: HP5MS T: 50 to 290 °C SC: 50–300 amu |
| Higher temperature—large molecules cleaved and cracked. High H/C ratio in OS → hydrocarbons and olefins → Diels–Alder reaction → AHs through cycloaddition. Chain scission of organic volatile matter during pyrolysis of OS due to high activated energy of the oxygenates in MA. | [80] |
| Chlorella vulgaris—kitchen waste BR: 1:0, 0.8:0.2, 0.5:0.5, 02:08, 0:10 | CaCO3, CaO, SiO2, and permutite. CR: 100, 80, 50, 20 and 0 wt.%. | I: CDS5200, CDS Analytical Co. Ltd. T: 700 °C HR: 20 °C/ms t: 20 s CG: Helium | I: Agilent Technology, 7890A/5975i CC: HP-5 ms T: First: 50 °C for 2 min; second: to 250 °C for 5 min at a heating rate of 10 °C/min SR: 1:50 SC: 33 and 500 amu |
| Potential: chain breaking, group rearrangement, depolymerization, polymerization, dehydration, cyclization, isomerization, etc. | [81] |
| CE and high-density polyethylene BR: 1:1 | AAEMs impregnated with potassium concentrations of 0.1, 0.14, and 0.4 M | I: 5200 Pyrolyser (CDS Analytical, Oxford, PA, USA T: 550 °C HR: 20 °C/ms t: 30 s CG: Helium at 1 | I: 6890N/5973i gas chromatograph (GC)/mass spectrometer (MS) (Agilent Technology, Santa Clara, CA, USA) CC: Agilent DB-17 ms T: First: held at 40 °C for 4 min; second: ramped at 5 °C/min to the temperature of 230 °C; final: 2 min SR: -50:1 SC: 40–500 amu |
| Co-pyrolysis → synergistic effect → promotes glycosidic bond cleavage and hydrogen transfer reactions → enhanced anhydrosugars and hydrocarbons. AAEMs during co-pyrolysis → furans and ketones through ring cracking and dehydration reactions of CE. | [82] |
| Strong-acid cation exchange resin (Amberlyst-15, Check sp A15)—CE | Sulfonic acid groups from A15 as the catalyst | I: 5200 Pyrolyser (CDS Analytical, USA T: 300 °C, 400 °C, 500 °C, 600 °C and 700 °C HR: 20 °C/ms t: 20 s CG: He at 1 | I: GC/MS (7890A/5975C inert, Agilent Technology, USA) CC: Agilent J&W DB-1701 T: First: held at 50 °C for 3 min; second: increased to 280 °C at a rate of 5 °C/min; final: stayed at 280 °C for another 10 min |
| Sulfonic groups improved levoglucosenone and inorganic sulphur-containing molecules at temperatures below 400 °C → promotion of CE pyrolysis. | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariyam, S.; Zuhara, S.; Parthasarathy, P.; McKay, G. A Review on Catalytic Fast Co-Pyrolysis Using Analytical Py-GC/MS. Molecules 2023, 28, 2313. https://doi.org/10.3390/molecules28052313
Mariyam S, Zuhara S, Parthasarathy P, McKay G. A Review on Catalytic Fast Co-Pyrolysis Using Analytical Py-GC/MS. Molecules. 2023; 28(5):2313. https://doi.org/10.3390/molecules28052313
Chicago/Turabian StyleMariyam, Sabah, Shifa Zuhara, Prakash Parthasarathy, and Gordon McKay. 2023. "A Review on Catalytic Fast Co-Pyrolysis Using Analytical Py-GC/MS" Molecules 28, no. 5: 2313. https://doi.org/10.3390/molecules28052313
APA StyleMariyam, S., Zuhara, S., Parthasarathy, P., & McKay, G. (2023). A Review on Catalytic Fast Co-Pyrolysis Using Analytical Py-GC/MS. Molecules, 28(5), 2313. https://doi.org/10.3390/molecules28052313








