Green Synthesis of Silver Nanoparticles from Allium cepa L. Peel Extract, Their Antioxidant, Antipathogenic, and Anticholinesterase Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of UV-Vis Spectroscopy
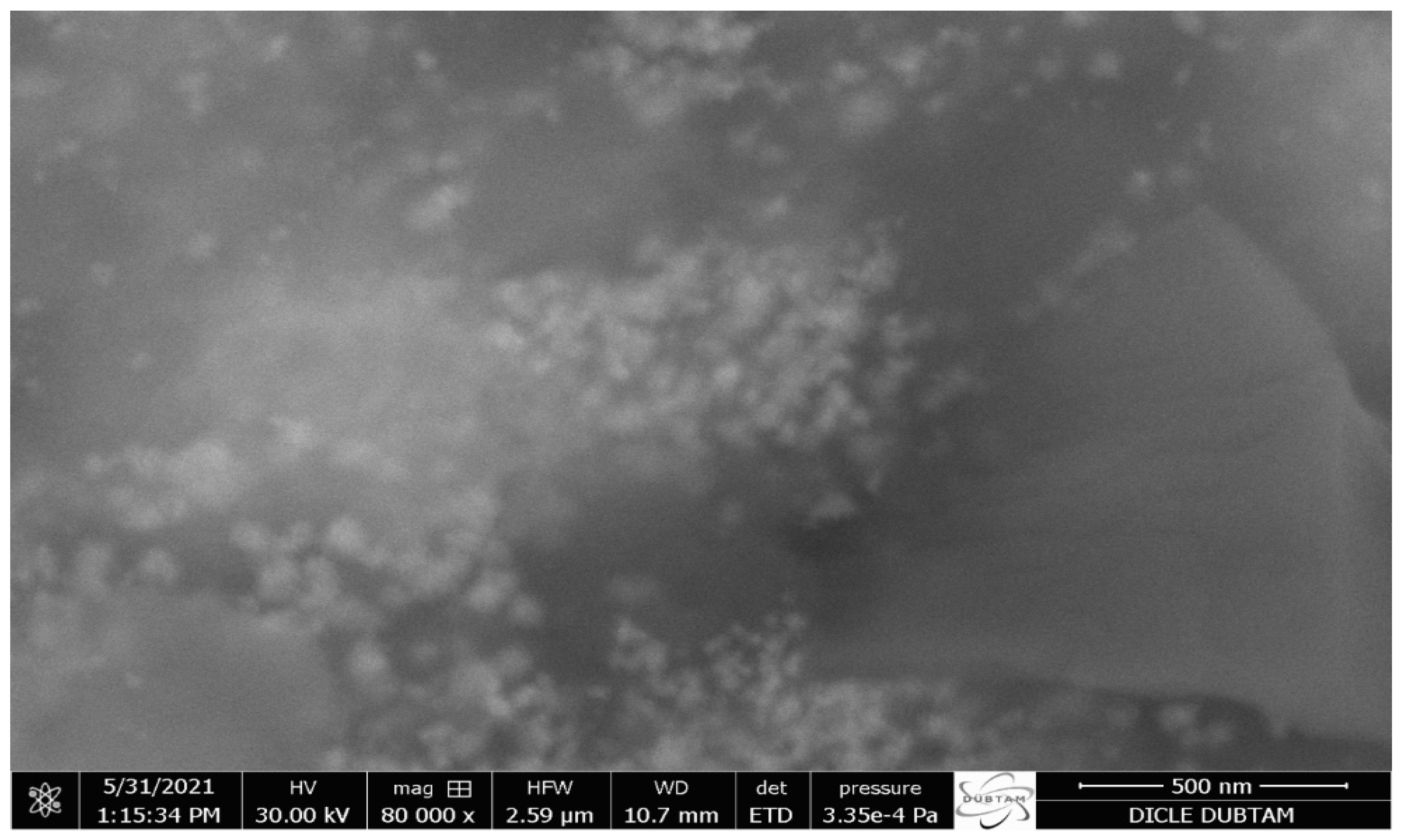
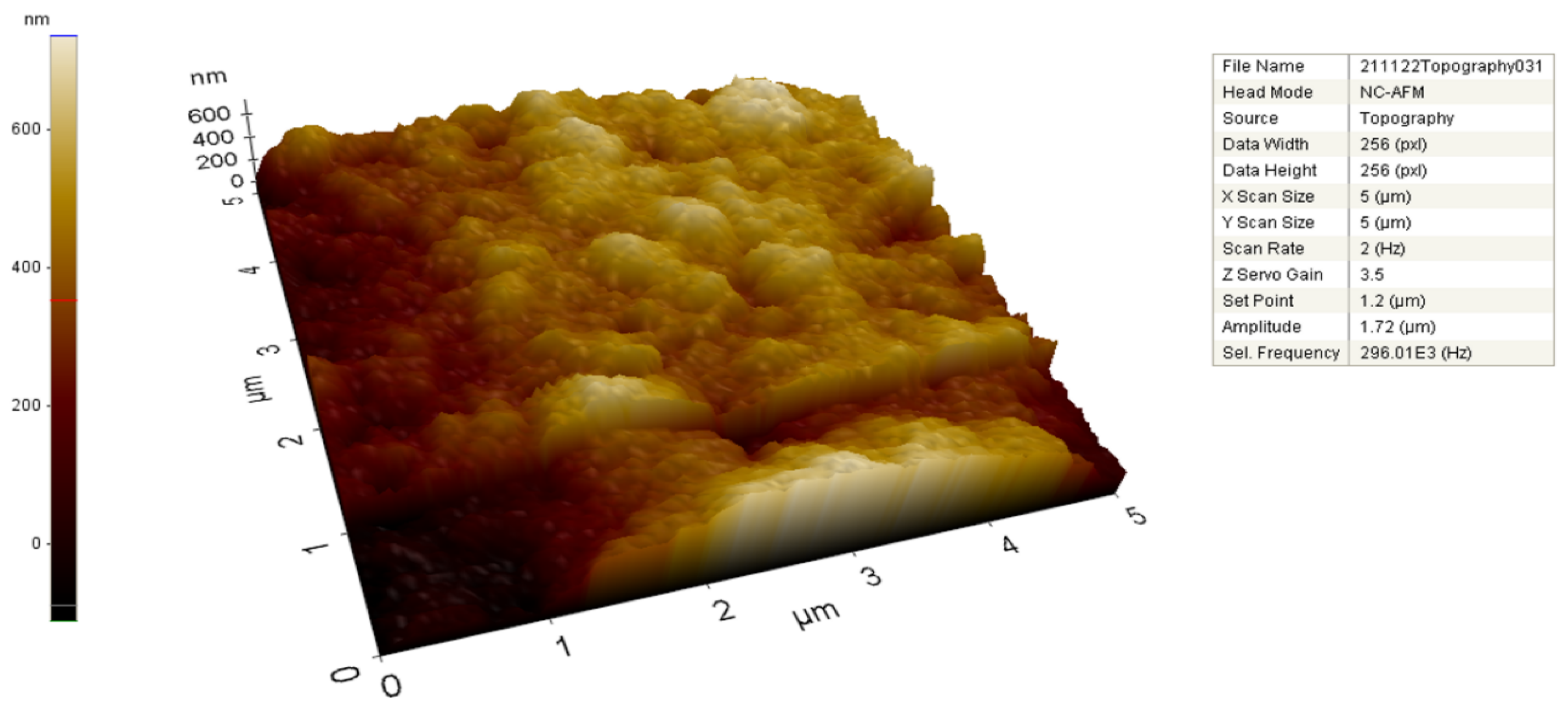
2.2. FE-SEM, TEM, and AFM Analysis
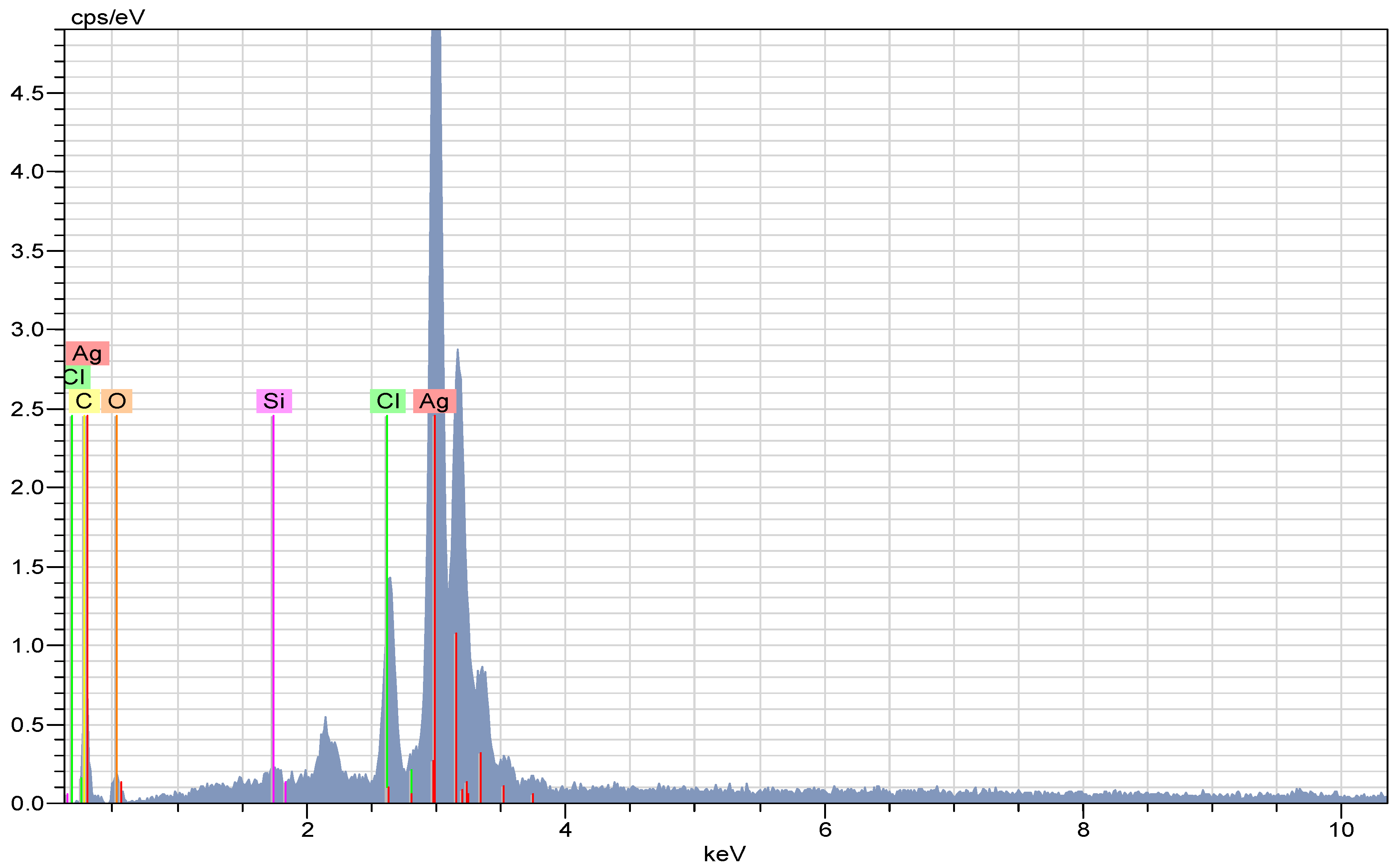
2.3. EDX Analysis of the AC-AgNPs
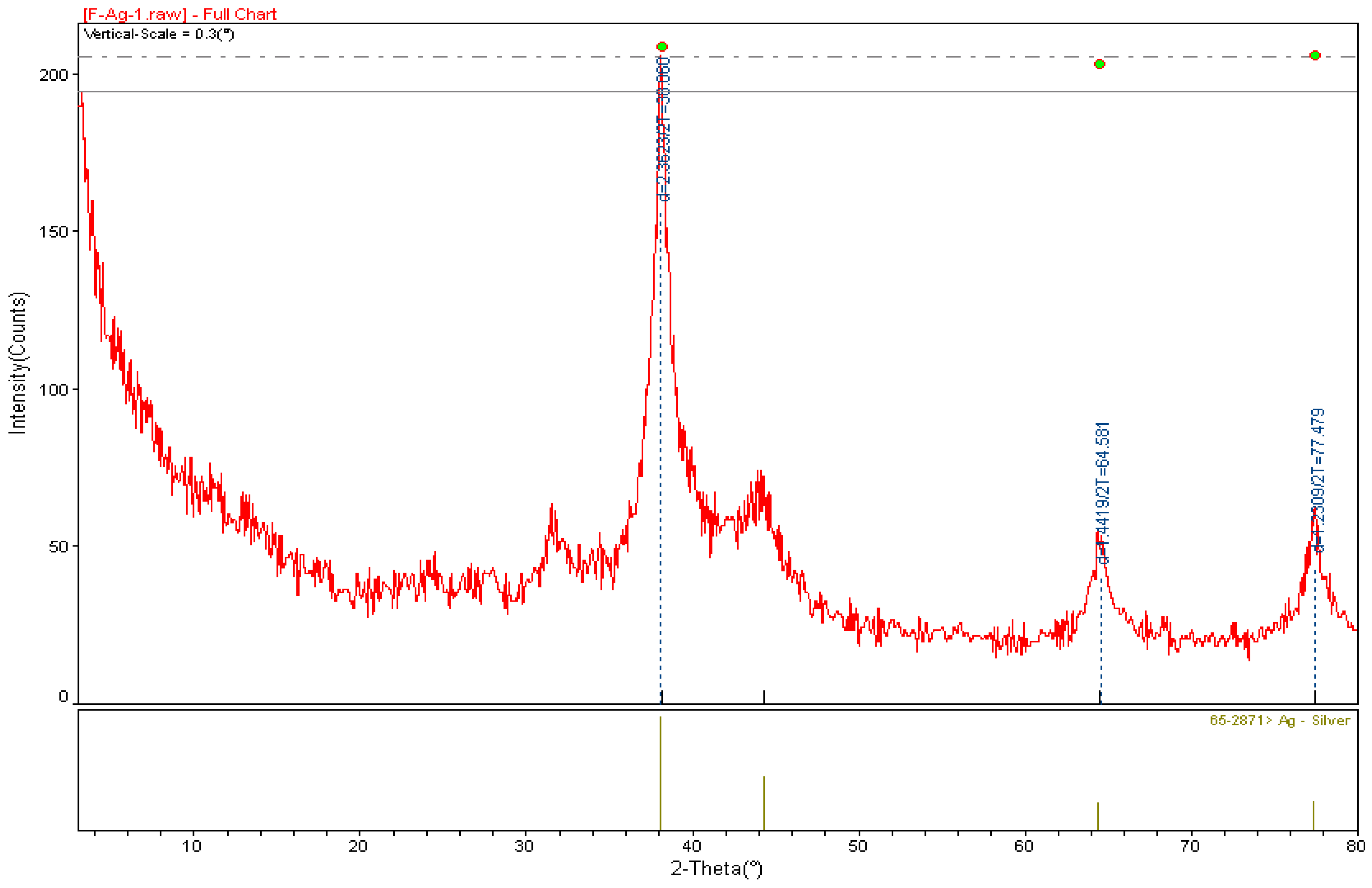
2.4. X-ray Diffraction (XRD) Analysis of A. cepa-AgNPs
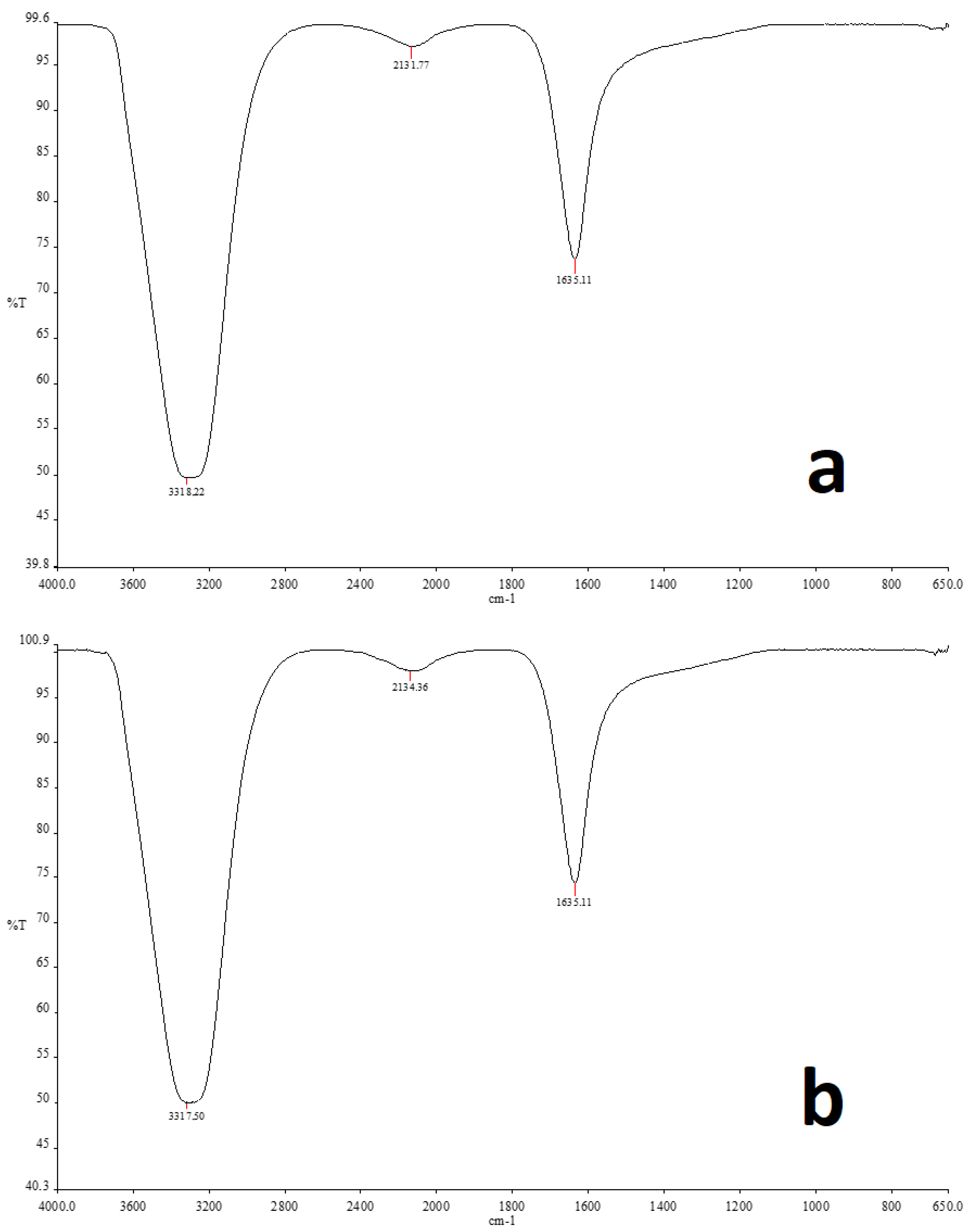
2.5. FT-IR Analysis
2.6. TG and DT Analyze
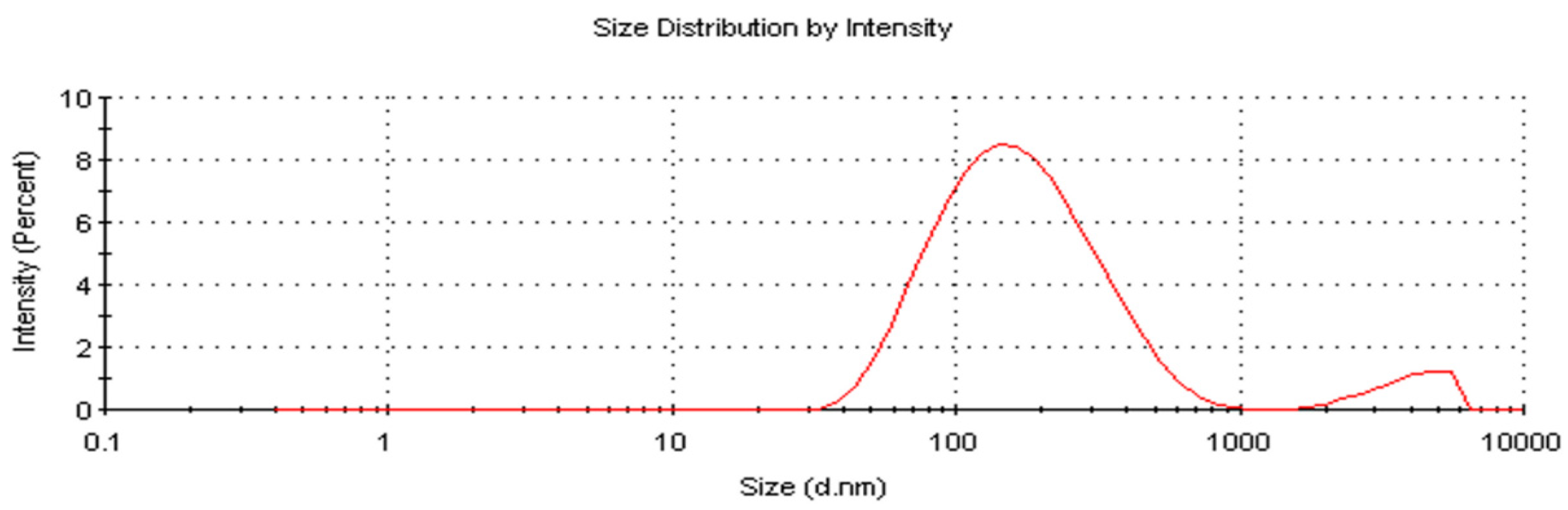
2.7. Zeta Size and Potential of AC-AgNPs
2.8. Antimicrobial Activity
2.9. Antioxidant Activity of AC-AgNPs
2.10. Anticholinesterase Activity of Biosynthesized AgNPs
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction Process
3.3. Biosynthesis of AC-AgNPs
3.4. Structural and Thermal Characterization of AC-AgNPs
3.5. Antimicrobial Activity or Antibacterial and Antfungal Activity of AC-AgNPs
3.6. Anticholinesterase Activities of AC-AgNPs
3.7. Antioxidant Activities of AC-AgNps
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nahar, K.; Aziz, S.; Bashar, M.S.; Haque, M.A.; Al-Reza, S.M. Synthesis and characterization of Silver nanoparticles from Cinnamomum tamala leaf extract and its antibacterial potential. Int. J. Nano Dimens. 2020, 11, 88–98. [Google Scholar]
- Muhammad, I.; Abbas, S.Q.; Hassan, M.; Majid, M.; Jin, H.Z.; Bungau, S. Stress driven discovery of natural products from actinobacteria with anti-oxidant and cytotoxic activities including docking and admet properties. Int. J. Mol. Sci. 2021, 22, 11432. [Google Scholar]
- Atalar, M.N.; Baran, A.; Baran, M.F.; Keskin, C.; Aktepe, N.; Yavuz, Ö.; İrtegun Kandemir, S. Economic fast synthesis of olive leaf extract and silver nanoparticles and biomedical applications. Part. Sci. Technol. 2022, 40, 589–597. [Google Scholar] [CrossRef]
- Baran, A.; Keskin, C.; Baran, M.F.; Huseynova, I.; Khalilov, R.; Eftekhari, A.; Irtegun-Kandemir, S.; Kavak, D.E. Ecofriendly Synthesis of Silver Nanoparticles Using Ananas comosus Fruit Peels: Anticancer and Antimicrobial Activities. Bioinorg. Chem. Appl. 2021, 2021, 2058149. [Google Scholar] [CrossRef]
- Hatipoğlu, A. Rapid green synthesis of gold nanoparticles: Synthesis, characterization, and antimicrobial activities. Prog. Nutr. 2021, 23, e2021242. [Google Scholar] [CrossRef]
- Majid, M.; Farhan, A.; Asad, M.I.; Khan, M.R.; Hassan, S.S.; Haq, I.U.; Bungau, S. An extensive pharmacological evaluation of new anti-cancer triterpenoid (nummularic acid) from Ipomoea batatas through in vitro, in silico, and in vivo studies. Molecules 2022, 27, 2474. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.M. Preparation of antimicrobial metallic nanoparticles with bioactive compounds. Mater. Sci. Eng. C 2019, 103, 109809. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Baran, M.F.; Keskin, C.; Irtegun-Kandemir, S.; Valiyeva, M.; Mehraliyeva, S.; Khalilov, R.; Eftekhari, A. Ecofriendly/Rapid Synthesis of Silver Nanoparticles Using Extract of Waste Parts of Artichoke (Cynara scolymus L.) and Evaluation of their Cytotoxic and Antibacterial Activities. J. Nanomater. 2021, 2021, 2270472. [Google Scholar] [CrossRef]
- Zulfiqar, H.; Amjad, M.S.; Mehmood, A.; Mustafa, G.; Binish, Z.; Khan, S.; Arshad, H.; Proćków, J.; Pérez de la Lastra, J.M. Antibacterial, Antioxidant, and Phytotoxic Potential of Phytosynthesized Silver Nanoparticles Using Elaeagnus umbellata Fruit Extract. Molecules 2022, 27, 5847. [Google Scholar] [CrossRef]
- Alwhibi, M.; Soliman, D.; Awad, M.; Alangery, A.; Al Dehaish, H.; Alwasel, Y. Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications. Green Process. Synth. 2021, 10, 412–420. [Google Scholar] [CrossRef]
- Hassan, S.S.; Abdel-Daim, M.M.; Behl, T.; Bungau, S. Natural products for chronic diseases: A ray of hope. Molecules 2022, 27, 5573. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, B.; Chang, X.; Gan, J.; Li, W.; Niu, S.; Kong, L.; Wu, T.; Zhang, T.; Tang, M.; et al. Silver nanoparticles modulate mitochondrial dynamics and biogenesis in HepG2 cells. Environ. Pollut. 2020, 256, 113430. [Google Scholar] [CrossRef]
- WHO. Cancer. World Health Organization. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 6 May 2022).
- Aktepe, N.; Baran, A. Fast and Low-Cost Biosynthesis of AgNPs with Almond Leaves: Medical Applications with Biocompatible Structures. Prog. Nutr. 2021, 23, e2021271. [Google Scholar] [CrossRef]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Shah, S.A.A.; Tripathy, M.; Paliwal, N. Green synthesis, characterization, antibacterial, antioxidant and photocatalytic activity of Parkia speciosa leaves extract mediated silver nanoparticles. Results Phys. 2019, 15, 102565. [Google Scholar] [CrossRef]
- Almalah, H.; Alzahrani, H.A.; Abdelkader, H.S. Green Synthesis of Silver Nanoparticles using Cinnamomum Zylinicum and their Synergistic Effect against Multi-Drug Resistance Bacteria. J. Nanotechnol. Res. 2019, 1, 95–107. [Google Scholar] [CrossRef]
- Abduljawad, A.A.; Elawad, M.A.; Elkhalifa, M.E.; Ahmed, A.; Hamdoon, A.A.; Salim, L.H.; Ashraf, M.; Ayaz, M.; Hassan, S.S.; Bungau, S. Alzheimer’s disease as a major public health concern: Role of dietary saponins in mitigating neurodegenerative disorders and their underlying mechanisms. Molecules 2022, 27, 6804. [Google Scholar] [CrossRef] [PubMed]
- Miculas, D.C.; Negru, P.A.; Bungau, S.G.; Behl, T.; Tit, D.M. Pharmacotherapy Evolution in Alzheimer’s Disease: Current Framework and Relevant Directions. Cells 2023, 12, 131. [Google Scholar] [CrossRef]
- Suganthy, N.; Sri Ramkumar, V.; Pugazhendhi, A.; Benelli, G.; Archunan, G. Biogenic synthesis of gold nanoparticles from Terminalia arjuna bark extract: Assessment of safety aspects and neuroprotective potential via antioxidant, anticholinesterase, and antiamyloidogenic effects. Environ. Sci. Pollut. Res. 2018, 25, 10418–10433. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ayaz, M.; Ovais, M.; Wadood, A.; Ali, M.; Shinwari, Z.K.; Maaza, M. In vitro cholinesterase enzymes inhibitory potential and in silico molecular docking studies of biogenic metal oxides nanoparticles. Inorg. Nano-Met. Chem. 2018, 48, 441–448. [Google Scholar] [CrossRef]
- Gul, R.; Jan, H.; Lalay, G.; Andleeb, A.; Usman, H.; Zainab, R.; Abbasi, B.H. Medicinal Plants and Biogenic Metal Oxide Nanoparticles: A Paradigm Shift to Treat Alzheimer’s Disease. Coating 2021, 11, 717. [Google Scholar] [CrossRef]
- Teshika, J.D.; Zakariyyah, A.M.; Toorabally, Z.; Zengin, G.; Rengasamy, K.R.; Pandian, S.K.; Mahomoodally, F.M. Traditional and modern uses of onion bulb (Allium cepa L.): A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 59, 39–70. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Nutraceutical, pharmaceutical and therapeutic uses of Allium cepa: A review. Int. J. Green Pharm. 2016, 10, 46–64. [Google Scholar] [CrossRef]
- Bystrická, J.; Musilová, J.; Vollmannová, A.; Timoracká, M.; Kavalcová, P. Bioactive components of onion (Allium cepa L.)—A Review. Acta Aliment. 2013, 42, 11–22. [Google Scholar] [CrossRef]
- Zhao, X.X.; Lin, F.J.; Li, H.; Li, H.B.; Wu, D.T.; Geng, F.; Ma, W.; Wang, Y.; Miao, B.H.; Gan, R.Y. Recent Advances in Bioactive Compounds, Health Functions, and Safety Concerns of Onion (Allium cepa L.). Front. Nutr. 2021, 8, 669805. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.R.; Seyedabadi, M.; Montazeri, M.; Khan, B.A.; Ebrahimzadeh, M.A. Allium paradoxum extract mediated green synthesis of SeNPs: Assessment of their anticancer, antioxidant, iron chelating activities, and antimicrobial activities against fungi, ATCC bacterial strains, Leishmania parasite, and catalytic reduction of methylene blue. Mater. Chem. Phys. 2023, 296, 127240. [Google Scholar]
- Abdellatif, A.A.; Mahmood, A.; Alsharidah, M.; Mohammed, H.A.; Alenize, S.K.; Bouazzaoui, A.; Al Rugaie, O.; Alnuqaydan, A.M.; Ahmad, R.; Vaali-Mohammad, M.A.; et al. Bioactivities of the green synthesized silver nanoparticles reduced using Allium cepa L aqueous extracts induced apoptosis in colorectal cancer cell lines. J. Nanomater. 2022, 2022, 1746817. [Google Scholar] [CrossRef]
- Ahmed Naseer, M.I.; Ali, S.; Nazir, A.; Abbas, M.; Ahmad, N. Green synthesis of silver nanoparticles using Allium cepa extract and their antimicrobial activity evaluation. Chem. Int. 2022, 8, 89–94. [Google Scholar]
- Bale, V.K.; Katreddi, H.R. Green synthesis, characterization and antimicrobial activity of nanosized Cuprous Oxide fabricated using aqueous extracts of Allium cepa and Raphanus sativus. Int. J. Nano Dimens. 2022, 13, 214–226. [Google Scholar]
- Sahila, S.; Prabhu, N.; Simiyon, G.G.; Jayakumari, L.S. A novel green and eco-friendly synthesis of nickel oxide nanoparticles by auto combustion technique using Allium cepa bulb extract and their dielectric behaviour. Chem. Data Collect. 2022, 38, 100837. [Google Scholar] [CrossRef]
- Shanmugam, J.; Dhayalan, M.; Savaas Umar, M.R.; Gopal, M.; Ali Khan, M.; Simal-Gandara, J.; Cid-Samamed, A. Green Synthesis of Silver Nanoparticles Using Allium cepa var. aggregatum Natural Extract: Antibacterial and Cytotoxic Properties. Nanomaterials 2022, 12, 1725. [Google Scholar] [CrossRef]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.K.; Ravishankar Rai, V.; Ramachandra, Y.L. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Gomathi, M.; Prakasam, A.; Rajkumar, P.V.; Rajeshkumar, S.; Chandrasekaran, R.; Anbarasan, P.M. Green synthesis of silver nanoparticles using Gymnema sylvestre leaf extract and evaluation of its antibacterial activity. S. Afr. J. Chem. Eng. 2020, 32, 1–4. [Google Scholar] [CrossRef]
- Uddin, A.K.M.R.; Siddique, M.A.B.; Rahman, F.; Ullah, A.K.M.A.; Khan, R. Cocos nucifera Leaf Extract Mediated Green Synthesis of Silver Nanoparticles for Enhanced Antibacterial Activity. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3305–3316. [Google Scholar] [CrossRef]
- Gecer, E.N.; Erenler, R.; Temiz, C.; Genc, N.; Yildiz, I. Green synthesis of silver nanoparticles from Echinacea purpurea (L.) Moench with the antioxidant profile. Part. Sci. Technol. 2022, 40, 50–57. [Google Scholar] [CrossRef]
- Alsammarraie, F.K.; Wang, W.; Zhou, P.; Mustapha, A.; Lin, M. Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities. Colloids Surf. B Biointerfaces 2018, 171, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Alzohairy, M.A. One-Pot Facile Green Synthesis of Silver Nanoparticles Using Seed Extract of Phoenix dactylifera and Their Bactericidal Potential against MRSA. Evid.-Based Complement. Altern. Med. 2018, 2018, 1860280. [Google Scholar] [CrossRef] [PubMed]
- Venkatadri, B.; Shanparvish, E.; Rameshkumar, M.R.; Arasu, M.V.; Al-Dhabi, N.A.; Ponnusamy, V.K.; Agastian, P. Green synthesis of silver nanoparticles using aqueous rhizome extract of Zingiber officinale and Curcuma longa: In-vitro anti-cancer potential on human colon carcinoma HT-29 cells. Saudi J. Biol. Sci. 2020, 27, 2980–2986. [Google Scholar] [CrossRef]
- Jalilian, F.; Chahardoli, A.; Sadrjavadi, K.; Fattahi, A.; Shokoohinia, Y. Green synthesized silver nanoparticle from Allium ampeloprasum aqueous extract: Characterization, antioxidant activities, antibacterial and cytotoxicity effects. Adv. Powder Technol. 2020, 31, 1323–1332. [Google Scholar] [CrossRef]
- Singh, V.; Shrivastava, A.; Wahi, N. Biosynthesis of silver nanoparticles by plants crude extracts and their characterization using UV, XRD, TEM and EDX. Afr. J. Biotechnol. 2015, 14, 2554–2567. [Google Scholar]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile green synthesis of silver nanoparticles using aqueous leaf extract of Origanum majorana with potential bioactivity against multidrug resistant bacterial strains. Crystals 2022, 12, 603. [Google Scholar] [CrossRef]
- Aref, M.S.; Salem, S.S. Bio-callus synthesis of silver nanoparticles, characterization, and antibacterial activities via Cinnamomum camphora callus culture. Biocatal. Agric. Biotechnol. 2020, 27, 101689. [Google Scholar] [CrossRef]
- Kambale, E.K.; Nkanga, C.I.; Mutonkole, B.-P.I.; Bapolisi, A.M.; Tassa, D.O.; Liesse, J.-M.I.; Krause, R.W.M.; Memvanga, P.B. Green synthesis of antimicrobial silver nanoparticles using aqueous leaf extracts from three Congolese plant species (Brillantaisia patula, Crossopteryx febrifuga and Senna siamea). Heliyon 2020, 6, e04493. [Google Scholar] [CrossRef]
- Baran, A.; Fırat Baran, M.; Keskin, C.; Hatipoğlu, A.; Yavuz, Ö.; İrtegün Kandemir, S.; Adican, M.T.; Khalilov, R.; Mammadova, A.; Ahmadian, E.; et al. Investigation of antimicrobial and cytotoxic properties and specification of silver nanoparticles (AgNPs) derived from Cicer arietinum L. green leaf extract. Front. Bioeng. Biotechnol. 2022, 10, 855136. [Google Scholar] [CrossRef] [PubMed]
- Aktepe, N.; Baran, A.; Atalar, M.N.; Baran, M.F.; Keskin, C.; Düz, M.Z.; Yavuz, Ö.; İrtegun Kandemir, S.; Kavak, D.E. Biosynthesis of Black Mulberry Leaf Extract and Silver NanoParticles (AgNPs): Characterization, Antimicrobial and Cytotoxic Activity Applications. MAS J. Appl. Sci. 2021, 6, 685–700. [Google Scholar] [CrossRef]
- Hatipoğlu, A. Green Synthesis of Silver Nanoparticles and Their Antimicrobial Effects on Some Food Pathogens. SDU J. Nat. Appl. Sci. 2022, 26, 106–114. [Google Scholar] [CrossRef]
- Wanjari, A.K.; Patil, M.P.; Chaudhari, U.E.; Gulhane, V.N.; Kim, G.D.; Kiddane, A.T. Bactericidal and photocatalytic degradation of methyl orange of silver-silver chloride nanoparticles synthesized using aqueous phyto-extract. Part. Sci. Technol. 2022, 40, 1033–1040. [Google Scholar] [CrossRef]
- Hamelian, M.; Varmira, K.; Veisi, H. Green synthesis and characterizations of gold nanoparticles using Thyme and survey cytotoxic effect, antibacterial and antioxidant potential. J. Photochem. Photobiol. B 2018, 184, 71–79. [Google Scholar] [CrossRef]
- Aljabali, A.; Akkam, Y.; Al Zoubi, M.; Al-Batayneh, K.; Al-Trad, B.; Abo Alrob, O.; Alkilany, A.M.; Benamara, M.; Evans, D. Synthesis of Gold Nanoparticles Using Leaf Extract of Ziziphus zizyphus and their Antimicrobial Activity. Nanomaterials 2018, 8, 174. [Google Scholar] [CrossRef]
- Jebril, S.; Khanfir ben Jenana, R.; Dridi, C. Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: In vitro and in vivo. Mater. Chem. Phys. 2020, 248, 122898. [Google Scholar] [CrossRef]
- Sankar, R.; Karthik, A.; Prabu, A.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf. B Biointerfaces 2013, 108, 80–84. [Google Scholar] [CrossRef]
- Sharma, V.; Kaushik, S.; Pandit, P.; Dhull, D.; Yadav, J.P.; Kaushik, S. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl. Microbiol. Biotechnol. 2018, 103, 881–891. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Li, C.; Nabeel, F.; Khalid, M.; Iqbal, H.M.N. Catalytic potential of bio-synthesized silver nanoparticles using Convolvulus arvensis extract for the degradation of environmental pollutants. J. Photochem. Photobiol. B 2018, 181, 44–52. [Google Scholar] [CrossRef]
- Dadashpour, M.; Firouzi-Amandi, A.; Pourhassan-Moghaddam, M.; Maleki, M.J.; Soozangar, N.; Jeddi, F.; Nouri, M.; Zarghami, M.; Pilehvar-Soltanahmadi, Y. Biomimetic synthesis of silver nanoparticles using Matricaria chamomilla extract and their potential anticancer activity against human lung cancer cells. Mater. Sci. Eng. C 2018, 92, 902–912. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Salomoni, R.; Léo, P.; Montemor, A.; Rinaldi, B.; Rodrigues, M. Antibacterial effect of silver nanoparticles in Pseudomonas aeruginosa. Nanotechnol. Sci. Appl. 2017, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Arsène, M.; Podoprigora, I.V.; Davares, A.; Razan, M.; Das, M.S.; Senyagin, A.N. Antibacterial activity of grapefruit peel extracts and green-synthesized silver nanoparticles. Vet. World 2021, 14, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Nagaich, U.; Gulati, N.; Chauhan, S. Antioxidant and Antibacterial Potential of Silver Nanoparticles: Biogenic Synthesis Utilizing Apple Extract. J. Pharm. 2016, 2016, 7141523. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, H.; Mehmood, A.; Ahmad, K.S.; Raffi, M. Green synthesis of silver nanoparticles and their applications as an alternative antibacterial and antioxidant agents. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110901. [Google Scholar] [CrossRef]
- Adu, O.T.; Mohamed, F.; Naidoo, Y.; Adu, T.S.; Chenia, H.; Dewir, Y.H.; Rihan, H. Green Synthesis of Silver Nanoparticles from Diospyros villosa Extracts and Evaluation of Antioxidant, Antimicrobial and Anti-Quorum Sensing Potential. Plants 2022, 11, 2514. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. A comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Kucukaydin, S.; Yeskaliyeva, B.; Ozturk, M.; Dinica, R.M. Non-Alkaloid Cholinesterase Inhibitory Compounds from Natural Sources. Molecules 2021, 26, 5582. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colourimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bousetla, A.; Keskinkaya, H.B.; Bensouici, C.; Lefahal, M.; Atalar, M.N.; Akkal, S. LC-ESI/MS-phytochemical profiling with antioxidant and antiacetylcholinesterase activities of Algerian Senecio angulatus L. f extracts. Nat. Prod. Res. 2021, 37, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Blois, M. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Varadharaj, V.; Ramaswamy, A.; Sakthivel, R.; Subbaiya, R.; Barabadi, H.; Chandrasekaran, M.; Saravanan, M. Antidiabetic and antioxidant activity of green synthesized starch nanoparticles: An in vitro study. J. Clust. Sci. 2020, 31, 1257–1266. [Google Scholar] [CrossRef]




| Pathogenes | AC-AgNPs µg mL−1 | AgNO3 Solution µg mL−1 | Control Antibiotics µg mL−1 * |
|---|---|---|---|
| Staphylococcus aureus ATCC 29213 | 0.125 ± 0.1 | 2.65 ± 0.2 | 2.00 ± 0.2 |
| Bacillus subtilis ATCC 11774 | 0.0625 ± 0.2 | 1.32 ± 0.1 | 1.00 ± 0.1 |
| Escherichia coli ATCC 25922 | 1.00 ± 0.1 | 0.66 ± 0.2 | 2.00 ± 0.2 |
| Pseudomonas aeruginosa ATCC 27853 | 0.25 ± 0.2 | 1.32 ± 0.2 | 4.00 ± 0.2 |
| Candida albicans ATCC 10231 | 0.50 ± 0.1 | 0.66 ± 0.1 | 2.00 ± 0.1 |
| Sample/Standard | β-Carotene/Linoleic Acid Assay | DPPH• Assay | ABTS•+ Assay | CUPRAC Assay | Metal-Chelating Assay a | |
|---|---|---|---|---|---|---|
| IC50 | IC50 | IC50 | A0.50 | IC50 | ||
| Sample | AC-AgNPs | 116.9 ± 1.20 | 151.3 ± 1.65 | 128.5 ± 1.17 | 143.8 ± 1.67 | 120.4 ± 0.98 |
| Standards | α-Tocopherol | 2.10 ± 0.05 | 38.15 ± 0.45 | 35.50 ± 0.56 | 61.40 ± 0.75 | NT b |
| BHA | 1.45 ± 0.03 | 19.82 ± 0.33 | 12.80 ± 0.08 | 25.50 ± 0.43 | NT | |
| EDTA | NT | NT | NT | NT | 5.50 ± 0.45 | |
| AChE | BChE | |||
|---|---|---|---|---|
| Extracts/Standards | Inhibition (%) (at 200 µg/mL) | IC50 (µg/mL) | Inhibition (%) (at 200 µg/mL) | IC50 (µg/mL) |
| A. cepa-AgNPs | 62.20 ± 0.47 | 87.25 ± 0.56 | 66.27 ± 0.44 | 71.33 ± 0.98 |
| Galantamine | 85.50 ± 0.60 | 5.50 ± 0.20 | 74.65 ± 0.25 | 42.20 ± 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, M.F.; Keskin, C.; Baran, A.; Hatipoğlu, A.; Yildiztekin, M.; Küçükaydin, S.; Kurt, K.; Hoşgören, H.; Sarker, M.M.R.; Sufianov, A.; et al. Green Synthesis of Silver Nanoparticles from Allium cepa L. Peel Extract, Their Antioxidant, Antipathogenic, and Anticholinesterase Activity. Molecules 2023, 28, 2310. https://doi.org/10.3390/molecules28052310
Baran MF, Keskin C, Baran A, Hatipoğlu A, Yildiztekin M, Küçükaydin S, Kurt K, Hoşgören H, Sarker MMR, Sufianov A, et al. Green Synthesis of Silver Nanoparticles from Allium cepa L. Peel Extract, Their Antioxidant, Antipathogenic, and Anticholinesterase Activity. Molecules. 2023; 28(5):2310. https://doi.org/10.3390/molecules28052310
Chicago/Turabian StyleBaran, Mehmet Fırat, Cumali Keskin, Ayşe Baran, Abdulkerim Hatipoğlu, Mahmut Yildiztekin, Selçuk Küçükaydin, Kadri Kurt, Hülya Hoşgören, Md. Moklesur Rahman Sarker, Albert Sufianov, and et al. 2023. "Green Synthesis of Silver Nanoparticles from Allium cepa L. Peel Extract, Their Antioxidant, Antipathogenic, and Anticholinesterase Activity" Molecules 28, no. 5: 2310. https://doi.org/10.3390/molecules28052310
APA StyleBaran, M. F., Keskin, C., Baran, A., Hatipoğlu, A., Yildiztekin, M., Küçükaydin, S., Kurt, K., Hoşgören, H., Sarker, M. M. R., Sufianov, A., Beylerli, O., Khalilov, R., & Eftekhari, A. (2023). Green Synthesis of Silver Nanoparticles from Allium cepa L. Peel Extract, Their Antioxidant, Antipathogenic, and Anticholinesterase Activity. Molecules, 28(5), 2310. https://doi.org/10.3390/molecules28052310







