Structural, Binding and Functional Properties of Milk Protein-Polyphenol Systems: A Review
Abstract
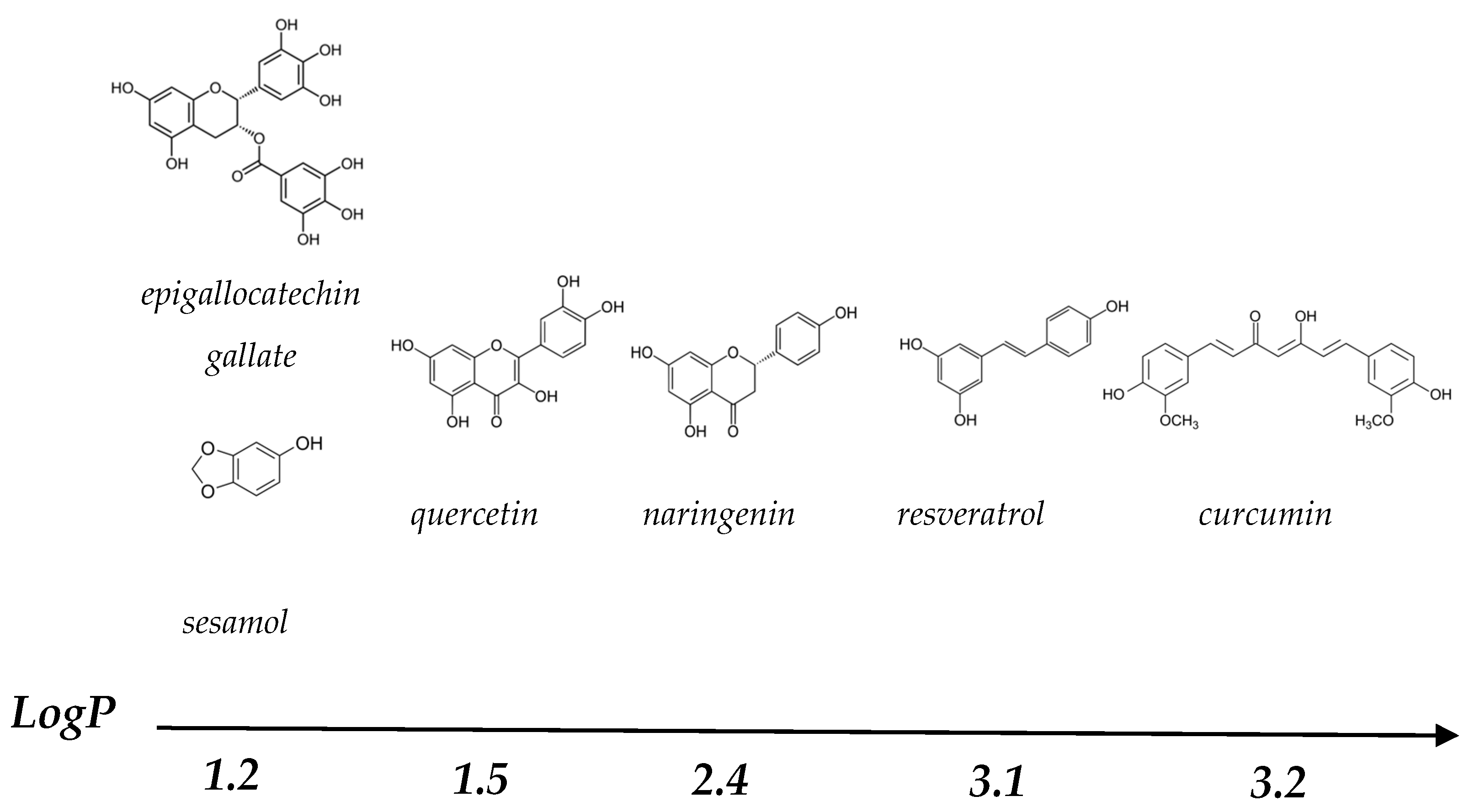
1. Introduction
- Hydrophobic interactions occur between the aromatic benzenoid rings of PP and the hydrophobic regions of proteins. These interactions are temperature-dependent and a reduction in temperature decreases the strength of the hydrophobic interaction between proteins and PP.
- A hydrogen bond is a dipole-dipole interaction, involving hydrogen and electronegative ions, e.g., Cl, F, N, O and S. The hydrogen bonds between proteins and PP are mainly between the hydroxyl groups of the PP and the polar amino acids of the proteins, and an increase in temperature will lead to a decrease in the amount of hydrogen bonds.
- Van der Waals interactions are the attractive forces between dipoles of non-charged atoms and molecules and the distance between the dipoles determines the strength of the van der Waals interactions. Temperature has a small influence on the strength of van der Waals interactions.
- Electrostatic interactions are the interactions between two charged atoms or molecules. Depending on pH, the amino acid residues of proteins can be positively charged (arginine, histidine and lysine) or negatively charged (aspartic acid and glutamic acid). These amino acids can interact with PP of an opposite charge through electrostatic interactions. In addition, salt ions can screen the charges present on the protein and PP, which will lead to decreased electrostatic interactions.
2. Casein-Based Systems
2.1. Native Casein Micelles
| Protein | Polyphenol | Particle Diameter (nm) | EE (%) | Antioxidant Activity Assay | Antitumor Recorded Cells | Ref. |
|---|---|---|---|---|---|---|
| Casein micelles | curcumin | 138–150 | n.r. | n.r. | n.a. | [38] |
| <200 | n.r. | n.r. | cervical | [39] | ||
| n.r. | n.r. | n.r. | n.a. | [40] | ||
| 176–187 | n.r. | n.r. | n.a. | [41] | ||
| n.r. | n.r. | n.r. | n.a. | [42] | ||
| n.r. | 97 | FRAP and ABTS | n.a. | [43] | ||
| epigallocatechin gallate | n.r. | 18–95 | n.r. | n.a. | [44] | |
| green tea flavonoids | n.r. | n.r. | n.r. | n.a. | [45] | |
| resveratrol | 138–150 | n.r. | n.r. | n.a. | [38] | |
| tea catechins | n.r. | n.r. | n.r. | adenocarcinoma | [46] | |
| tea polyphenols | n.r. | n.r. | n.r. | n.a. | [47] | |
| Re-assembled casein micelles | curcumin and quercetin | 73–187 | 93–97 | n.r. | breast | [12] |
| epigallocatechin gallate | 68 | 85 | n.r. | n.a. | [48] | |
| sesamol | 158–166 | 28–35 | n.r. | n.a. | [49] | |
| quercetin | 182–334 | 26–97 | n.r. | n.a. | [50] | |
| Casein nano particles | curcumin | 169 | 83 | ABTS | n.a. | [10] |
| 104–213 | 70–100 | n.r. | colon | [51] | ||
| curcumin and quercetin | 187 | >99 | n.r. | breast | [12] | |
| epigallocatechin gallate | 162–246 | n.r. | n.r. | n.a. | [48] | |
| with (2-hydroxypropyl-β-cyclodextrin) | quercetin | 171–251 | 75–83 | n.r. | n.a. | [52] |
| β-casein micelles | curcumin | n.r. | n.r. | ABTS | n.a. | [11] |
| naringenin | ~30 | n.r. | n.r. | n.a. | [26] | |
| ~20 | n.r. | n.r. | n.a. | [53] | ||
| 9–12 and 79–359 | n.r. | n.r. | n.a. | [54] | ||
| resveratrol | n.r. | n.r. | n.r. | n.a. | [55] | |
| 6–13 | 59 or 69 | n.r. | n.a. | [56] |

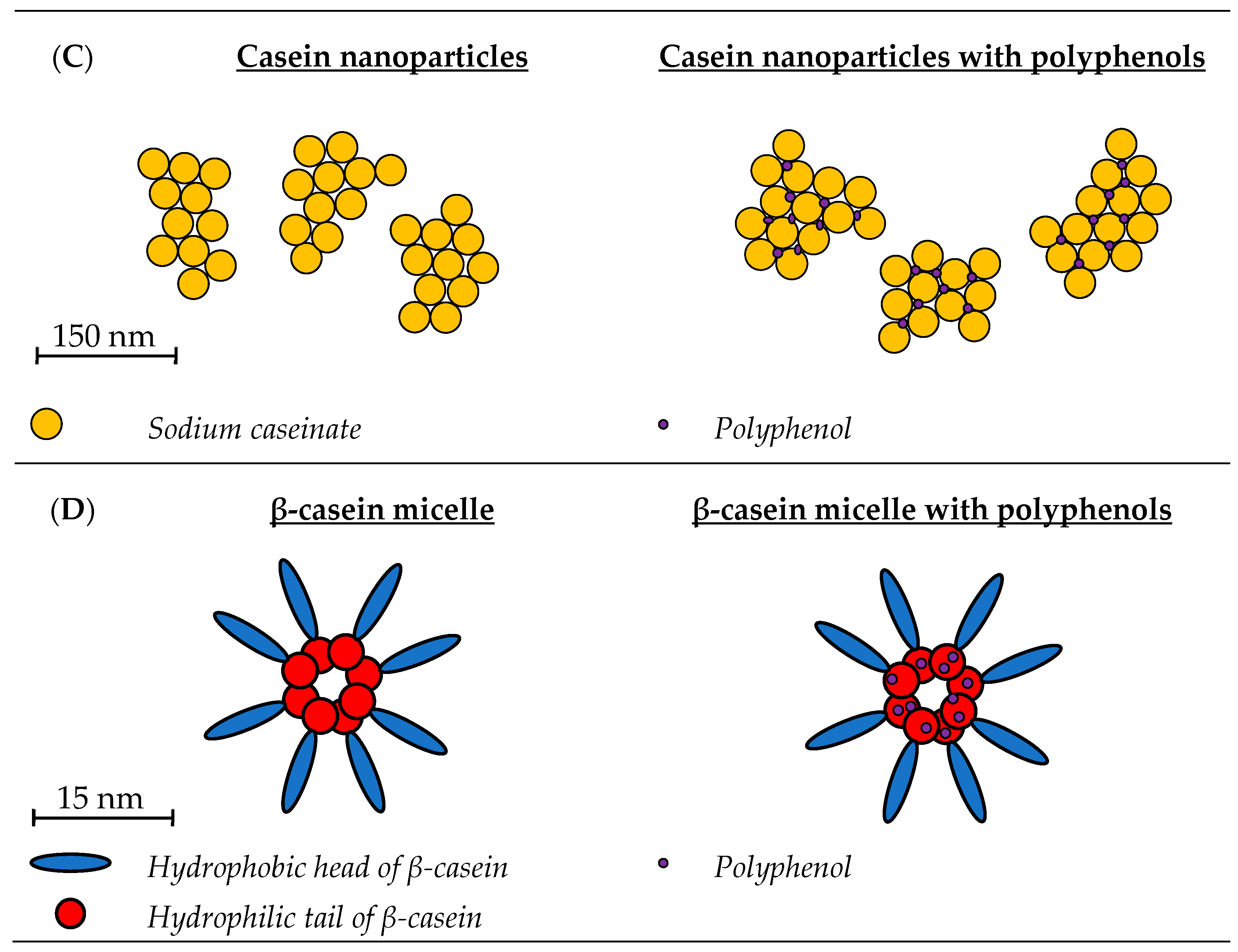
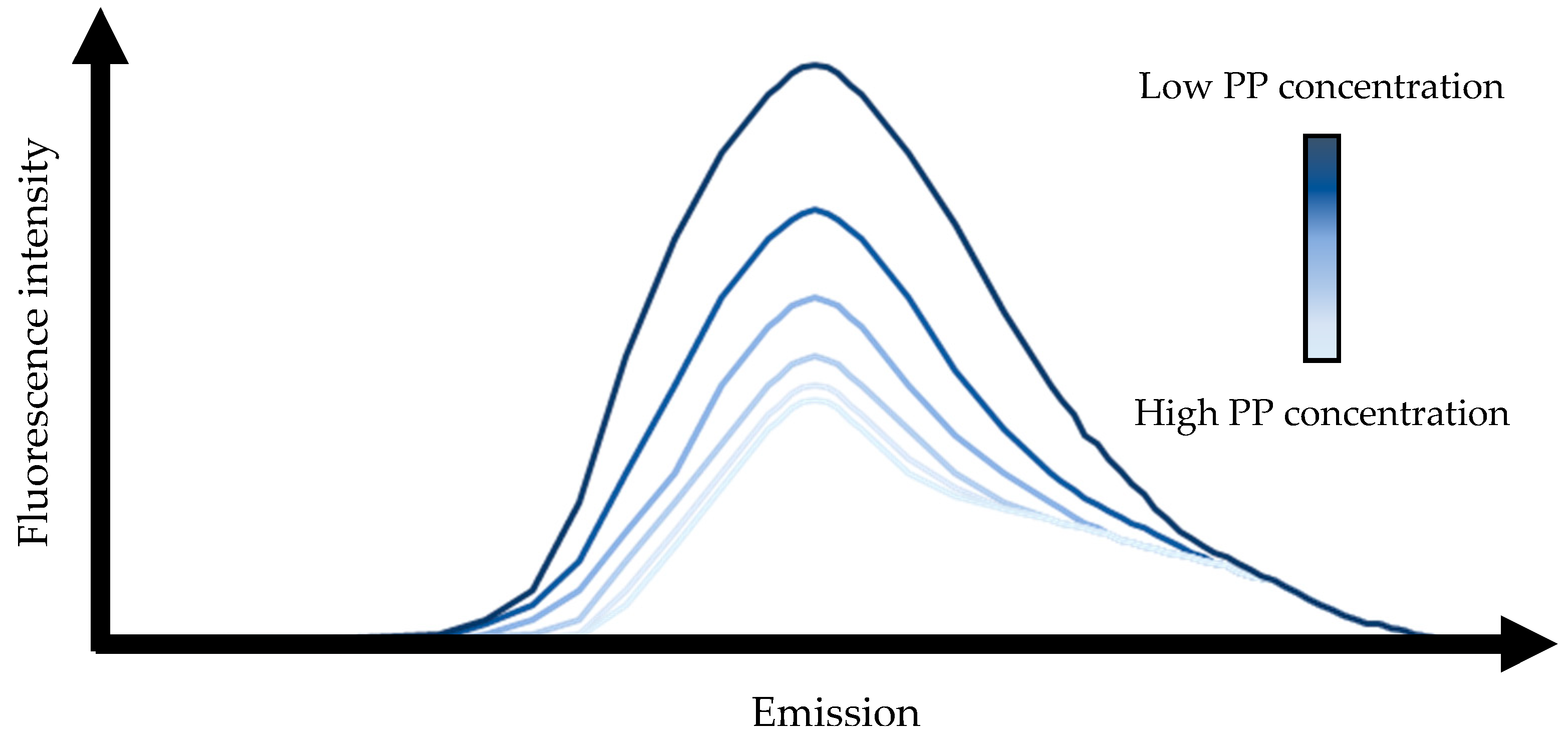
2.1.1. Physicochemical Properties of Casein Micelle-Polyphenol Complexes
2.1.2. Effect of Casein Micelles on the Antitumor Activity of Polyphenols
2.1.3. Influence of Spray Drying and Rehydration on the Structure and Antioxidant Activity of CM-Curcumin Complexes
2.2. Sodium Caseinate
2.2.1. Re-Assembled Casein Micelles
Physicochemical Properties of Re-Assembled Casein Micelle-Polyphenol Complexes
Influence of Re-Assembled Casein Micelles on the Antitumor Activity of Polyphenols
2.2.2. Casein Nanoparticles
Physicochemical Properties of Casein Nanoparticle-Polyphenol Complexes
Functional Properties of Casein-Polyphenol Nanoparticles
Influence of Casein Nanoparticles on the Bioavailability of Polyphenols
Effect of Casein Nanoparticles on the Antitumor Activity of Polyphenols
2.3. β-Casein Micelles
2.3.1. Physicochemical Properties of β-Casein Micelle-PP Complexes
2.3.2. Functional Properties of β-Casein Micelle-PP Complexes
3. Whey Protein-Based Systems
| Protein | Polyphenol | Particle Diameter (nm) | EE (%) | Antioxidant Activity Assay | Antitumor Recorded Cells | Ref. |
|---|---|---|---|---|---|---|
| Whey protein isolate | blackcurrant juice, cran-berry juice and musca-dine grape juice | 5000, 30,000 and 100,000 | 28–84 | n.r. | n.a. | [33] |
| blueberry juice and cranberry juice | 1000–100,000 | 21–46 | n.r. | n.a. | [34] | |
| with pectin | Anthocyanin-rich extract Grape seed extract, hibiscus extract, tannic acid and chatechin | 175–204 | 35 or 55 | n.r. | n.a. | [77] |
| 2000–17,000 | n.r. | n.r. | n.a. | [78] | ||
| β-lactoglobulin (unheated) | (+) catechin | <458 | n.r. | ABTS and ORAC | n.a. | [79] |
| green tea extract | 33 | n.r. | n.r. | n.a. | [80] | |
| 33–955 | n.r. | n.r. | breast, colon, glioma, kidney, lung, melanoma, ovarian and prostate | [81] | ||
| 15–200 | 62–85 | n.r. | n.a. | [82] | ||
| tea catechins | n.r. | n.r. | n.r. | n.a. | [83] | |
| β-lactoglobulin (heated) | epigallocatechin gallate | <50 | n.r. | n.r. | n.a. | [84] |
| <50 | 60–70 | n.r. | n.a. | [85] | ||
| 43 | 40 | FRAP and ORAC | n.a. | [86] | ||
| 2–50 | 74 | n.r. | n.a. | [87] | ||
| 31 | 59 | n.r. | adenocarcinoma, cervical, esophageal carcinoma, gastric, lung, melanoma ovarian and prostate | [88] | ||
| Blood serum albumin | quercetin | 9–12 | n.r. | ABTS and DPPH | n.a. | [35] |
| quercetin | ~10 nm | n.r. | ABTS and DPPH | n.a. | [36] | |
| tea catechins | n.r. | 45–65 | n.r. | n.a. | [89] |

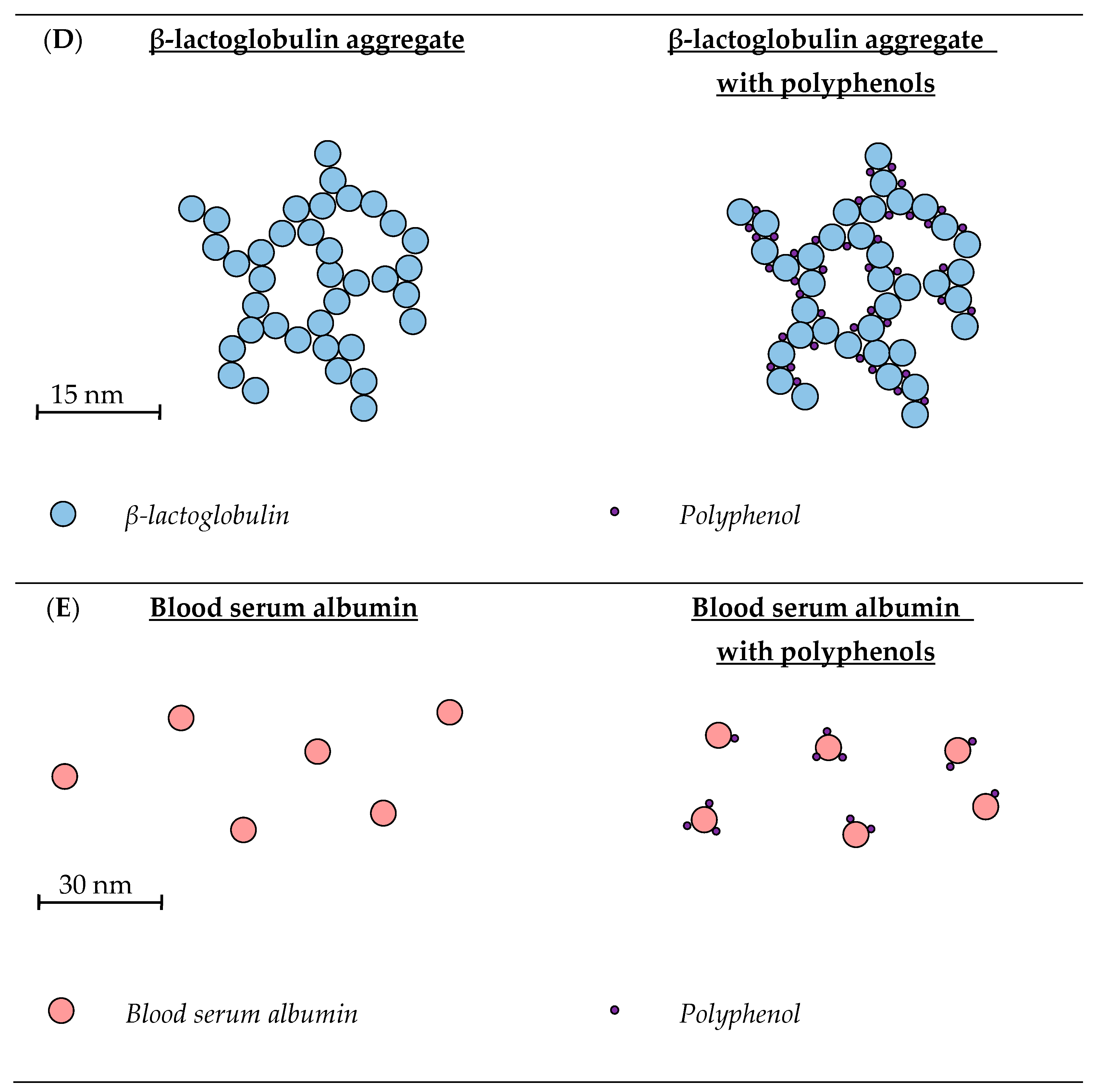
3.1. Whey Protein Isolate
3.1.1. Whey Protein Isolate Aggregates
3.1.2. Whey Protein Isolate-Pectin Aggregates
3.2. β-Lactoglobulin
3.2.1. Unheated β-Lactoglobulin
Physicochemical Properties of Unheated β-Lactoglobulin
Effect of Unheated β-Lactoglobulin on the Antitumor Activity of EGCG
3.2.2. Heated β-Lactoglobulin
Physicochemical Properties of Heated β-Lactoglobulin
Influence of Heated β-Lactoglobulin on Bioaccessibility and Bioavailability of EGCG
Effect of Heated β-Lactoglobulin on Health Benefits of EGCG
3.3. Blood Serum Albumin
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of Polyphenols and Their Properties. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–44. [Google Scholar]
- el Gharras, H. Polyphenols: Food sources, properties and applications-A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Eran Nagar, E.; Okun, Z.; Shpigelman, A. Digestive fate of polyphenols: Updated view of the influence of chemical structure and the presence of cell wall material. Curr. Opin. Food Sci. 2020, 31, 38–46. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects; Research Signpost: Trivandrum, India, 2006; pp. 23–67. [Google Scholar]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols-A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, 21–32. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced dispersibility and bioactivity of curcumin by encapsulation in casein nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef]
- Esmaili, M.; Ghaffari, S.M.; Moosavi-Movahedi, Z.; Atri, M.S.; Sharifizadeh, A.; Farhadi, M.; Yousefi, R.; Chobert, J.M.; Haertlé, T.; Moosavi-Movahedi, A.A. Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. LWT-Food Sci. Technol. 2011, 44, 2166–2172. [Google Scholar] [CrossRef]
- Ghayour, N.; Hosseini, S.M.H.; Eskandari, M.H.; Esteghlal, S.; Nekoei, A.R.; Hashemi Gahruie, H.; Tatar, M.; Naghibalhossaini, F. Nanoencapsulation of quercetin and curcumin in casein-based delivery systems. Food Hydrocoll. 2019, 87, 394–403. [Google Scholar] [CrossRef]
- DePeters, E.J.; Cant, J.P. Nutritional Factors Influencing the Nitrogen Composition of Bovine Milk: A Review. J. Dairy Sci. 1992, 75, 2043–2070. [Google Scholar] [CrossRef]
- Goulding, D.A.; Fox, P.F.; O’Mahony, J.A. Milk Proteins: An Overview. In Milk Proteins. From Expression to Food; Boland, M., Singh, H., Eds.; Academic Press: London, UK, 2020; pp. 21–98. ISBN 978-0-12-815251-5. [Google Scholar]
- Horne, D.S. Milk Proteins: Casein, Micellar Structure. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2011; pp. 772–779. [Google Scholar]
- O’Mahony, J.A.; Fox, P.F. Milk Proteins: Introduction and Historical Aspects. In Advanced Dairy Chemistry, Volume 1A: Proteins: Basic Aspects; McSweeney, P.L.H., Fox, P.F., Eds.; Springer: Boston, MA, USA, 2013; pp. 43–85. [Google Scholar]
- Swaisgood, H.E. Chemistry of the Caseins. In Advanced Dairy Chemistry—1 Proteins Part A; Fox, P.F., McSweeney, P.L.H., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003; pp. 139–201. [Google Scholar]
- Holt, C. Structure and Stability of Bovine Casein Micelles. In Advances in Protein Chemistry; Elsevier: Amsterdam, The Netherlands, 1992; Volume 43, pp. 63–151. ISBN 0065-3233. [Google Scholar]
- Horne, D.S. Casein interactions: Casting light on the black boxes, the structure in dairy products. Int. Dairy J. 1998, 8, 171–177. [Google Scholar] [CrossRef]
- Dalgleish, D.G. On the structural models of bovine casein micelles-Review and possible improvements. Soft Matter 2011, 7, 2265–2272. [Google Scholar] [CrossRef]
- Yazdi, S.R.; Corredig, M. Heating of milk alters the binding of curcumin to casein micelles. A fluorescence spectroscopy study. Food Chem. 2012, 132, 1143–1149. [Google Scholar] [CrossRef]
- Brodkorb, A.; Croguennec, T.; Bouhallab, S.; Kehoe, J.J. Heat-Induced Denaturation, Aggregation and Gelation of Whey Proteins. In Advanced Dairy Chemistry; Springer: New York, NY, USA, 2016; pp. 155–178. [Google Scholar]
- Nicolai, T.; Britten, M.; Schmitt, C. β-Lactoglobulin and WPI aggregates: Formation, structure and applications. Food Hydrocoll. 2011, 25, 1945–1962. [Google Scholar] [CrossRef]
- Chanphai, P.; Bourassa, P.; Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.A. Review on the loading efficacy of dietary tea polyphenols with milk proteins. Food Hydrocoll. 2018, 77, 322–328. [Google Scholar] [CrossRef]
- Chen, H.; Wooten, H.; Thompson, L.; Pan, K. Nanoparticles of Casein Micelles for Encapsulation of Food Ingredients. In Biopolymer Nanostructures for Food Encapsulation Purposes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 39–68. [Google Scholar]
- Moeiniafshari, A.A.; Zarrabi, A.; Bordbar, A.K. Exploring the interaction of naringenin with bovine beta-casein nanoparticles using spectroscopy. Food Hydrocoll. 2015, 51, 1–6. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Liu, M.; Liu, Z.; Xu, H.; Lai, F. Analysis of binding interaction between (-)-epigallocatechin (EGC) and β-lactoglobulin by multi-spectroscopic method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 82, 164–168. [Google Scholar] [CrossRef]
- Sousa, M.; Teixeira, V.H.; Soares, J. Dietary Strategies to Recover from Exercise-Induced Muscle Damage. Int. J. Food Sci. Nutr. 2014, 65, 151–163. [Google Scholar] [CrossRef]
- Dumpler, J.; Huppertz, T.; Kulozik, U. Invited Review: Heat Stability of Milk and Concentrated Milk: Past, Present, and Future Research Objectives. J. Dairy Sci. 2020, 103, 10986–11007. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Abo El-Fotoh, W.S.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release 2011, 153, 206–216. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Soop, M.; Nehra, V.; Henderson, G.C.; Boirie, Y.; Ford, G.C.; Sreekumaran Nair, K.; Nair, K.S. Coingestion of whey protein and casein in a mixed meal: Demonstration of a more sustained anabolic effect of casein. Am. J. Physiol. Endocrinol. Metab. 2012, 303, 152–162. [Google Scholar] [CrossRef]
- Schneider, M.; Esposito, D.; Lila, M.A.; Foegeding, E.A. Formation of whey protein-polyphenol meso-structures as a natural means of creating functional particles. Food Funct. 2016, 7, 1306–1318. [Google Scholar] [CrossRef]
- Diaz, J.T.; Foegeding, E.A.; Lila, M.A. Formulation of protein-polyphenol particles for applications in food systems. Food Funct. 2020, 11, 5091–5104. [Google Scholar] [CrossRef]
- Fang, R.; Jing, H.; Chai, Z.; Zhao, G.; Stoll, S.; Ren, F.; Liu, F.; Leng, X. Design and characterization of protein-quercetin bioactive nanoparticles. J. Nanobiotechnol. 2011, 9, 19. [Google Scholar] [CrossRef]
- Fang, R.; Hao, R.; Wu, X.; Li, Q.; Leng, X.; Jing, H. Bovine serum albumin nanoparticle promotes the stability of quercetin in simulated intestinal fluid. J. Agric. Food Chem. 2011, 59, 6292–6298. [Google Scholar] [CrossRef]
- Zhang, S.; Vardhanabhuti, B. Effect of initial protein concentration and pH on in vitro gastric digestion of heated whey proteins. Food Chem. 2014, 145, 473–480. [Google Scholar] [CrossRef]
- Yazdi, S.Y.; Corredig, M.; Dalgleish, D.G. Studying the structure of β-casein-depleted bovine casein micelles using electron microscopy and fluorescent polyphenols. Food Hydrocoll. 2014, 42, 171–177. [Google Scholar] [CrossRef]
- Sahu, A.; Kasoju, N.; Bora, U. Fluorescence study of the curcumin-casein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules 2008, 9, 2905–2912. [Google Scholar] [CrossRef]
- Nadi, M.M.; Ashrafi Kooshk, M.R.; Mansouri, K.; Ghadami, S.A.; Amani, M.; Ghobadi, S.; Khodarahmi, R. Comparative spectroscopic studies on curcumin stabilization by association to bovine serum albumin and casein: A Perspective on drug-delivery application. Int. J. Food Prop. 2015, 18, 638–659. [Google Scholar] [CrossRef]
- Khanji, A.N.; Michaux, F.; Jasniewski, J.; Petit, J.; Lahimer, E.; Cherif, M.; Salameh, D.; Rizk, T.; Banon, S. Structure and gelation properties of casein micelles doped with curcumin under acidic conditions. Food Funct. 2015, 6, 3624–3633. [Google Scholar] [CrossRef]
- Hudson, E.A.; de Paula, H.M.C.; da Silva, R.M.; Pires, A.C.d.S.; da Silva, L.H.M. Curcumin-micellar casein multisite interactions elucidated by surface plasmon resonance. Int. J. Biol. Macromol. 2019, 133, 860–866. [Google Scholar] [CrossRef]
- Khanji, A.N.; Michaux, F.; Petit, J.; Salameh, D.; Rizk, T.; Jasniewski, J.; Banon, S. Structure, gelation, and antioxidant properties of curcumin-doped casein micelle powder produced by spray-drying. Food Funct. 2018, 9, 971–981. [Google Scholar] [CrossRef]
- Haratifar, S.; Corredig, M. Interactions between tea catechins and casein micelles and their impact on renneting functionality. Food Chem. 2014, 143, 27–32. [Google Scholar] [CrossRef]
- Yuksel, Z.; Avci, E.; Erdem, Y.K. Characterization of binding interactions between green tea flavonoids and milk proteins. Food Chem. 2010, 121, 450–456. [Google Scholar] [CrossRef]
- Guri, A.; Haratifar, S.; Corredig, M. Bioefficacy of tea catechins associated with milk caseins tested using different In Vitro digestion models. Food Dig. 2014, 5, 8–18. [Google Scholar] [CrossRef]
- van de Langerijt, T.M.; O’Mahony, J.A.; Crowley, S.V. The influence of sodium caseinate and β-casein concentrate on the physicochemical properties of casein micelles and the role of tea polyphenols in mediating these interactions. LWT-Food Sci. Technol. 2021, 154, 112775. [Google Scholar]
- Malekhosseini, P.; Alami, M.; Khomeiri, M.; Esteghlal, S.; Nekoei, A.R.; Hosseini, S.M.H. Development of casein-based nanoencapsulation systems for delivery of epigallocatechin gallate and folic acid. Food Sci. Nutr. 2019, 7, 519–527. [Google Scholar]
- Santos Basurto, M.A.; Cardador Martínez, A.; Castaño Tostado, E.; Bah, M.; Reynoso Camacho, R.; Amaya Llano, S.L. Study of the Interactions Occurring During the Encapsulation of Sesamol within Casein Micelles Reformed from Sodium Caseinate Solutions. J. Food Sci. 2018, 83, 2295–2304. [Google Scholar] [CrossRef]
- Ghatak, D.; Iyyaswami, R. Selective encapsulation of quercetin from dry onion peel crude extract in reassembled casein particles. Food Bioprod. Process. 2019, 115, 100–109. [Google Scholar] [CrossRef]
- Pan, K.; Luo, Y.; Gan, Y.; Baek, S.J.; Zhong, Q. PH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter 2014, 10, 6820–6830. [Google Scholar] [CrossRef]
- Peñalva, R.; Esparza, I.; Morales-Gracia, J.; González-Navarro, C.J.; Larrañeta, E.; Irache, J.M. Casein nanoparticles in combination with 2-hydroxypropyl-β-cyclodextrin improves the oral bioavailability of quercetin. Int. J. Pharm. 2019, 570, 118652. [Google Scholar] [CrossRef]
- Li, M.; Fokkink, R.; Ni, Y.; Kleijn, J.M. Bovine beta-casein micelles as delivery systems for hydrophobic flavonoids. Food Hydrocoll. 2019, 96, 653–662. [Google Scholar] [CrossRef]
- Li, M.; Wang, K.; Wang, Y.; Han, Q.; Ni, Y.; Wen, X. Effects of genipin concentration on cross-linked β-casein micelles as nanocarrier of naringenin: Colloidal properties, structural characterization and controlled release. Food Hydrocoll. 2020, 108, 105989. [Google Scholar] [CrossRef]
- Ghorbani Gorji, E.; Rocchi, E.; Schleining, G.; Bender-Bojalil, D.; Furtmüller, P.G.; Piazza, L.; Iturri, J.J.; Toca-Herrera, J.L. Characterization of resveratrol-milk protein interaction. J. Food Eng. 2015, 167, 217–225. [Google Scholar] [CrossRef]
- Cheng, H.; Dong, H.; Wusigale; Liang, L. A comparison of β-casein complexes and micelles as vehicles for trans-/cis-resveratrol. Food Chem. 2020, 330, 127209. [Google Scholar] [CrossRef]
- Griffin, M.C.A.; Lyster, R.L.J.; Price, J.C. The disaggregation of calcium-depleted casein micelles. Eur. J. Biochem. 1988, 174, 339–343. [Google Scholar] [CrossRef]
- Semo, E.; Kesselman, E.; Danino, D.; Livney, Y.D. Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocoll. 2007, 21, 936–942. [Google Scholar] [CrossRef]
- Acharya, D.P.; Sanguansri, L.; Augustin, M.A. Binding of resveratrol with sodium caseinate in aqueous solutions. Food Chem. 2013, 141, 1050–1054. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Grinberg, V.Y.; de Kruif, C.G. Association behavior of β-casein. J. Colloid Interface Sci. 2003, 258, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Schuck, P. Spray Drying of Dairy Products: State of the Art. Lait 2002, 82, 375–382. [Google Scholar] [CrossRef]
- Barraquio, V.L.; van de Voort, F.R. Sodium caseinate from skim milk powder by extrusion processing: Physicochemical and functional properties. J. Food Sci. 1991, 56, 1552–1556. [Google Scholar] [CrossRef]
- O’Regan, J.; Mulvihill, D.M. Milk Protein Products|Caseins and Caseinates, Industrial Production, Compositional Standards, Specifications, and Regulatory Aspects. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2011; pp. 855–863. [Google Scholar]
- HadjSadok, A.; Pitkowski, A.; Nicolai, T.; Benyahia, L.; Moulai-Mostefa, N. Characterisation of sodium caseinate as a function of ionic strength, pH and temperature using static and dynamic light scattering. Food Hydrocoll. 2008, 22, 1460–1466. [Google Scholar] [CrossRef]
- Sáiz-Abajo, M.J.; González-Ferrero, C.; Moreno-Ruiz, A.; Romo-Hualde, A.; González-Navarro, C.J. Thermal protection of β-carotene in re-assembled casein micelles during different processing technologies applied in food industry. Food Chem. 2013, 138, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Zimet, P.; Rosenberg, D.; Livney, Y.D. Re-assembled casein micelles and casein nanoparticles as nano-vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocoll. 2011, 25, 1270–1276. [Google Scholar] [CrossRef]
- Hindmarsh, J.P.; Watkinson, P. Experimental evidence for previously unclassified calcium phosphate structures in the casein micelle. J. Dairy Sci. 2017, 100, 6938–6948. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein-phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- McMahon, D.J.; Oommen, B.S. Supramolecular structure of the casein micelle. J. Dairy Sci. 2008, 91, 1709–1721. [Google Scholar] [CrossRef]
- Loewen, A.; Chan, B.; Li-Chan, E.C.Y. Optimization of vitamins A and D3 loading in re-assembled casein micelles and effect of loading on stability of vitamin D3 during storage. Food Chem. 2018, 240, 472–481. [Google Scholar] [CrossRef]
- Pfaff, M.; Anderer, F.A. Casein kinase II accumulation in the nucleolus and its role in nucleolar phosphorylation. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 1988, 969, 100–109. [Google Scholar] [CrossRef]
- Huppertz, T. Chemistry of the Caseins. In Advanced Dairy Chemistry; Springer: New York, NY, USA, 2013; pp. 135–160. [Google Scholar]
- Shapira, A.; Markman, G.; Assaraf, Y.G.; Livney, Y.D. β-casein-based nanovehicles for oral delivery of chemotherapeutic drugs: Drug-protein interactions and mitoxantrone loading capacity. Nanomedicine 2010, 6, 547–555. [Google Scholar] [CrossRef] [PubMed]
- de Kruif, C.G.; Grinberg, V.Y. Micellisation of β-casein. Colloids Surf. A Physicochem. Eng. Asp. 2002, 210, 183–190. [Google Scholar] [CrossRef]
- Deeth, H.; Bansal, N. Whey Proteins: An Overview. In Whey Proteins: From Milk to Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–50. [Google Scholar]
- Schmitt, C.; Bovay, C.; Rouvet, M.; Shojaei-Rami, S.; Kolodziejczyk, E. Whey protein soluble aggregates from heating with NaCl: Physicochemical, interfacial, and foaming properties. Langmuir 2007, 23, 4155–4166. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Maya, I.J.; McClements, D.J. Biopolymer nanoparticles as potential delivery systems for anthocyanins: Fabrication and properties. Food Res. Int. 2015, 69, 1–8. [Google Scholar] [CrossRef]
- Thongkaew, C.; Gibis, M.; Hinrichs, J.; Weiss, J. Polyphenol interactions with whey protein isolate and whey protein isolate-pectin coacervates. Food Hydrocoll. 2014, 41, 103–112. [Google Scholar] [CrossRef]
- Oliveira, A.L.; von Staszewski, M.; Pizones Ruiz-Henestrosa, V.M.; Pintado, M.; Pilosof, A.M.R. Impact of pectin or chitosan on bulk, interfacial and antioxidant properties of (+)-catechin and β-lactoglobulin ternary mixtures. Food Hydrocoll. 2016, 55, 119–127. [Google Scholar] [CrossRef]
- von Staszewski, M.; Pizones Ruiz-Henestrosa, V.M.; Pilosof, A.M.R. Green tea polyphenols-β-lactoglobulin nanocomplexes: Interfacial behavior, emulsification and oxidation stability of fish oil. Food Hydrocoll. 2014, 35, 505–511. [Google Scholar] [CrossRef]
- von Staszewski, M.; Jara, F.L.; Ruiz, A.L.T.G.; Jagus, R.J.; Carvalho, J.E.; Pilosof, A.M.R. Nanocomplex formation between β-lactoglobulin or caseinomacropeptide and green tea polyphenols: Impact on protein gelation and polyphenols antiproliferative activity. J. Funct. Foods 2012, 4, 800–809. [Google Scholar] [CrossRef]
- Rodríguez, S.D.; von Staszewski, M.; Pilosof, A.M.R. Green tea polyphenols-whey proteins nanoparticles: Bulk, interfacial and foaming behavior. Food Hydrocoll. 2015, 50, 108–115. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Hasni, I.; Bourassa, P.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.-A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef]
- Shpigelman, A.; Israeli, G.; Livney, Y.D. Thermally-induced protein-polyphenol co-assemblies: Beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG. Food Hydrocoll. 2010, 24, 735–743. [Google Scholar] [CrossRef]
- Shpigelman, A.; Cohen, Y.; Livney, Y.D. Thermally-induced β-lactoglobulin-EGCG nanovehicles: Loading, stability, sensory and digestive-release study. Food Hydrocoll. 2012, 29, 57–67. [Google Scholar] [CrossRef]
- Zagury, Y.; Kazir, M.; Livney, Y.D. Improved antioxidant activity, bioaccessibility and bioavailability of EGCG by delivery in β-lactoglobulin particles. J. Funct. Foods 2019, 52, 121–130. [Google Scholar] [CrossRef]
- Zagury, Y.; Chen, S.; Edelman, R.; Karnieli, E.; Livney, Y.D. β-Lactoglobulin delivery system for enhancing EGCG biological efficacy in HFD obesity mice model. J. Funct. Foods 2019, 59, 362–370. [Google Scholar] [CrossRef]
- Wu, M.; Jin, J.; Jin, P.; Xu, Y.; Yin, J.; Qin, D.; Wang, K.; Du, Q. Epigallocatechin gallate-β-lactoglobulin nanoparticles improve the antitumor activity of EGCG for inducing cancer cell apoptosis. J. Funct. Foods 2017, 39, 257–263. [Google Scholar] [CrossRef]
- Chanphai, P.; Tajmir-Riahi, H.A. Tea polyphenols bind serum albumins: A potential application for polyphenol delivery. Food Hydrocoll. 2019, 89, 461–467. [Google Scholar] [CrossRef]
- Krebs, M.R.H.; Devlin, G.L.; Donald, A.M. Protein particulates: Another generic form of protein aggregation? Biophys. J. 2007, 92, 1336–1342. [Google Scholar] [CrossRef]
- Boland, M. Whey Proteins. In Handbook of Food Proteins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 30–55. [Google Scholar]
- Morr, C.V.; Ha, E.Y.W. Whey Protein Concentrates and Isolates: Processing and Functional Properties. Crit. Rev. Food Sci. Nutr. 1993, 33, 431–476. [Google Scholar] [CrossRef]
- O’Regan, J.; Ennis, M.P.; Mulvihill, D.M. Milk Proteins. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009; pp. 298–358. [Google Scholar]
- Sumpio, B.E.; Cordova, A.C.; Berke-Schlessel, D.W.; Qin, F.; Chen, Q.H. Green Tea, the “Asian Paradox”, and Cardiovascular Disease. J. Am. Coll. Surg. 2006, 202, 813–825. [Google Scholar] [CrossRef]
- Kao, Y.H.; Chang, H.H.; Lee, M.J.; Chen, C.L. Tea, obesity, and diabetes. Mol. Nutr. Food Res. 2006, 50, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Williamson, G. A review of the health effects of green tea catechins in in vivo animal models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van de Langerijt, T.M.; O’Mahony, J.A.; Crowley, S.V. Structural, Binding and Functional Properties of Milk Protein-Polyphenol Systems: A Review. Molecules 2023, 28, 2288. https://doi.org/10.3390/molecules28052288
van de Langerijt TM, O’Mahony JA, Crowley SV. Structural, Binding and Functional Properties of Milk Protein-Polyphenol Systems: A Review. Molecules. 2023; 28(5):2288. https://doi.org/10.3390/molecules28052288
Chicago/Turabian Stylevan de Langerijt, Tessa M., James A. O’Mahony, and Shane V. Crowley. 2023. "Structural, Binding and Functional Properties of Milk Protein-Polyphenol Systems: A Review" Molecules 28, no. 5: 2288. https://doi.org/10.3390/molecules28052288
APA Stylevan de Langerijt, T. M., O’Mahony, J. A., & Crowley, S. V. (2023). Structural, Binding and Functional Properties of Milk Protein-Polyphenol Systems: A Review. Molecules, 28(5), 2288. https://doi.org/10.3390/molecules28052288






