Effect of Liquefaction of Honey on the Content of Phenolic Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Development of HPLC-CoulArray Method
2.2. Temperature and Duration of Individual Types of Honey Treatment
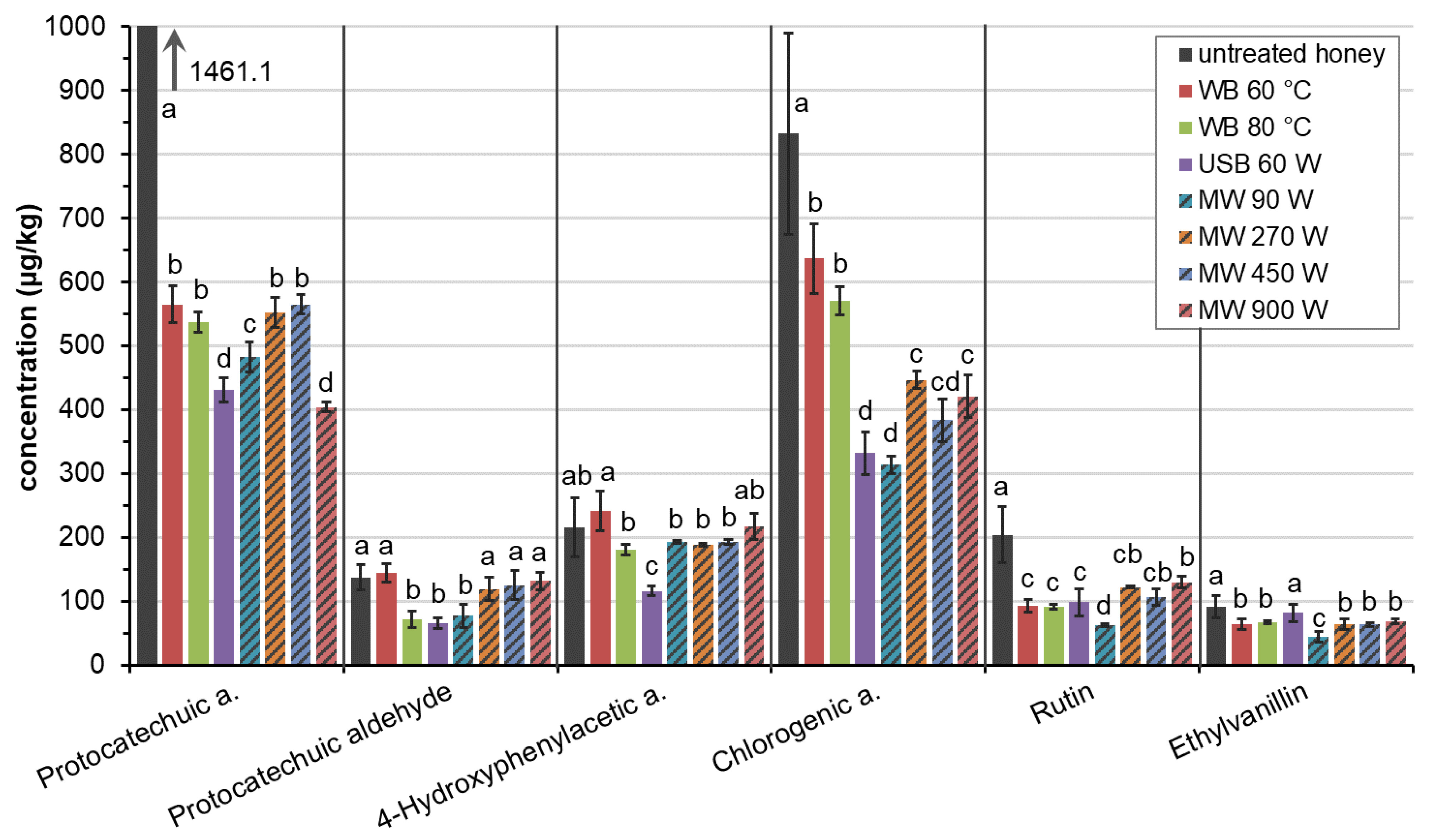
2.3. The Effect of Type of Liquefaction on Antioxidant Content
3. Materials and Methods
3.1. Chemical and Standards
3.2. Equipment
3.3. Methods
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Nagai, T.; Inoue, R.; Kanamori, N.; Suzuki, N.; Nagashima, T. Characterization of honey from different floral sources. Its functional properties and effects of honey species on storage of meat. Food Chem. 2006, 97, 256–262. [Google Scholar] [CrossRef]
- Inoue, K.; Murayarna, S.; Seshimo, F.; Takeba, K.; Yoshimura, Y.; Nakazawa, H. Identification of phenolic compound in manuka honey as specific superoxide anion radical scavenger using electron spin resonance (ESR) and liquid chromatography with coulometric array detection. J. Sci. Food Agric. 2005, 85, 872–878. [Google Scholar] [CrossRef]
- Ohmenhaeuser, M.; Monakhova, Y.B.; Kuballa, T.; Lachenmeier, D.W. Qualitative and Quantitative Control of Honeys Using NMR Spectroscopy and Chemometrics. ISRN Anal. Chem. 2013, 2013, 825318. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Gheldof, N.; Engeseth, N.J. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef]
- Amiot, M.J.; Aubert, S.; Gonnet, M.; Tacchini, M. The phenolic-compounds in honey—Premilinary-study upon identification and family quantification. Apidologie 1989, 20, 115–125. [Google Scholar] [CrossRef]
- Gasic, U.; Keckes, S.; Dabic, D.; Trifkovic, J.; Milojkovic-Opsenica, D.; Natic, M.; Tesic, Z. Phenolic profile and antioxidant activity of Serbian polyfloral honeys. Food Chem. 2014, 145, 599–607. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Gomez-Romero, M.; Aboud, F.; Giuseppe, A.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Characterisation of phenolic compounds in Algerian honeys by RP-HPLC coupled to electrospray time-of-flight mass spectrometry. Lwt-Food Sci. Technol. 2017, 85, 460–469. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Gonzalez-Paramas, A.M.; Damiani, E.; Astolfi, P.; Martinez-Sanchez, G.; Bompadre, S.; Quiles, J.L.; Santos-Buelga, C.; Battino, M. Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chem. Toxicol. 2012, 50, 1508–1516. [Google Scholar] [CrossRef]
- Can, Z.; Yildiz, O.; Sahin, H.; Turumtay, E.A.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Escriche, I.; Kadar, M.; Juan-Borras, M.; Domenech, E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014, 142, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Escuredo, O.; Miguez, M.; Fernandez-Gonzalez, M.; Seijo, M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef]
- Soler, C.; Gil, M.; Garciaviguera, C.; Tomasbarberan, F.A. Flavonoid patterns of french honeys with different floral origin. Apidologie 1995, 26, 53–60. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P.; Di Lorenzo, A.; Vista, S.; Tenore, G.C.; Daglia, M. Chemical Composition of Different Botanical Origin Honeys Produced by Sicilian Black Honeybees (Apis mellifera ssp sicula). J. Agric. Food Chem. 2015, 63, 5864–5874. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gou, X.; Yue, T.; Ren, R.; Zhao, H.; He, L.; Liu, C.; Cao, W. Evaluation of physicochemical properties of Qinling Apis cerana honey and the antimicrobial activity of the extract against Salmonella Typhimurium LT2 in vitro and in vivo. Food Chem. 2021, 337, 127774. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Wang, J.; Li, X.; Wang, W.; Huang, Z. Sugaring-out assisted liquid-liquid extraction coupled with high performance liquid chromatography-electrochemical detection for the determination of 17 phenolic compounds in honey. J. Chromatogr. A 2019, 1601, 104–114. [Google Scholar] [CrossRef]
- Rusko, J.; Vainovska, P.; Vilne, B.; Bartkevics, V. Phenolic profiles of raw mono- and polyfloral honeys from Latvia. J. Food Compos. Anal. 2021, 98, 103813. [Google Scholar] [CrossRef]
- Michalkiewicz, A.; Biesaga, M.; Pyrzynska, K. Solid-phase extraction procedure for determination of phenolic acids and some flavonols in honey. J. Chromatogr. A 2008, 1187, 18–24. [Google Scholar] [CrossRef]
- Oroian, M.; Sorina, R. Honey authentication based on physicochemical parameters and phenolic compounds. Comput. Electron. Agric. 2017, 138, 148–156. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, W.; Chen, W.J.; Xiao, X.H.; Zheng, J.B. Simultaneous determination of four phenolic components in citrus honey by high performance liquid chromatography using electrochemical detection. Food Chem. 2009, 114, 1537–1541. [Google Scholar] [CrossRef]
- Zhao, J.; Du, X.J.; Cheng, N.; Chen, L.Z.; Xue, X.F.; Wu, L.M.; Cao, W. Identification of monofloral honeys using HPLC-ECD and chemometrics. Food Chem. 2016, 194, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Jandera, P.; Skerikova, V.; Rehova, L.; Hajek, T.; Baldrianova, L.; Skopova, G.; Kellner, V.; Horna, A. RP-HPLC analysis of phenolic compounds and flavonoids in beverages and plant extracts using a CoulArray detector. J. Sep. Sci. 2005, 28, 1005–1022. [Google Scholar] [CrossRef] [PubMed]
- Mattonai, M.; Parri, E.; Querci, D.; Degano, I.; Ribechini, E. Development and validation of an HPLC-DAD and HPLC/ESI-MS2 method for the determination of polyphenols in monofloral honeys from Tuscany (Italy). Microchem. J. 2016, 126, 220–229. [Google Scholar] [CrossRef]
- Sousa, J.M.; de Souza, E.L.; Marques, G.; Meireles, B.; Cordeiro, A.T.D.; Gullon, B.; Pintado, M.M.; Magnani, M. Polyphenolic profile and antioxidant and antibacterial activities of monofloral honeys produced by Meliponini in the Brazilian semiarid region. Food Res. Int. 2016, 84, 61–68. [Google Scholar] [CrossRef]
- Paramas, A.M.G.; Barez, J.A.G.; Marcos, C.C.; Garcia-Villanova, R.J.; Sanchez, J.S. HPLC-fluorimetric method for analysis of amino acids in products of the hive (honey and bee-pollen). Food Chem. 2006, 95, 148–156. [Google Scholar] [CrossRef]
- El Sohaimy, S.A.; Masry, S.H.D.; Shehata, M.G. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Valverde, S.; Ares, A.M.; Elmore, J.S.; Bernal, J. Recent trends in the analysis of honey constituents. Food Chem. 2022, 387. [Google Scholar] [CrossRef]
- Bulut, L.; Kilic, M. Kinetics of hydroxymethylfurfural accumulation and color change in honey during storage in relation to moisture content. J. Food Process. Preserv. 2009, 33, 22–32. [Google Scholar] [CrossRef]
- Kowalski, S. Changes of antioxidant activity and formation of 5-hydroxymethylfurfural in honey during thermal and microwave processing. Food Chem. 2013, 141, 1378–1382. [Google Scholar] [CrossRef]
- Janghu, S.; Bera, M.B.; Nanda, V.; Rawson, A. Study on Power Ultrasound Optimization and Its Comparison with Conventional Thermal Processing for Treatment of Raw Honey. Food Technol. Biotechnol. 2017, 55, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Villacres-Granda, I.; Proano, A.; Coello, D.; Debut, A.; Vizuete, K.; Ballesteros, I.; Granda-Albuja, G.; Rosero-Mayanquer, H.; Battino, M.; Giampieri, F.; et al. Effect of thermal liquefaction on quality, chemical composition and antibiofilm activity against multiresistant human pathogens of crystallized eucalyptus honey. Food Chem. 2021, 365, 130519. [Google Scholar] [CrossRef]
- Bucekova, M.; Juricova, V.; Monton, E.; Martinotti, S.; Ranzato, E.; Majtan, J. Microwave processing of honey negatively affects honey antibacterial activity by inactivation of bee-derived glucose oxidase and defensin-1. Food Chem. 2018, 240, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Lukasiewicz, M.; Bednarz, S.; Panus, M. Diastase Number Changes During Thermal and Microwave Processing of Honey. Czech J. Food Sci. 2012, 30, 21–26. [Google Scholar] [CrossRef]
- Hebbar, H.U.; Nandini, K.E.; Lakshmi, M.C.; Subramanian, R. Microwave and infrared heat processing of honey and its quality. Food Sci. Technol. Res. 2003, 9, 49–53. [Google Scholar] [CrossRef]
- Bartakova, K.; Drackova, M.; Borkovcova, I.; Vorlova, L. Impact of Microwave Heating on Hydroxymethylfurfural Content in Czech Honeys. Czech J. Food Sci. 2011, 29, 328–336. [Google Scholar] [CrossRef]
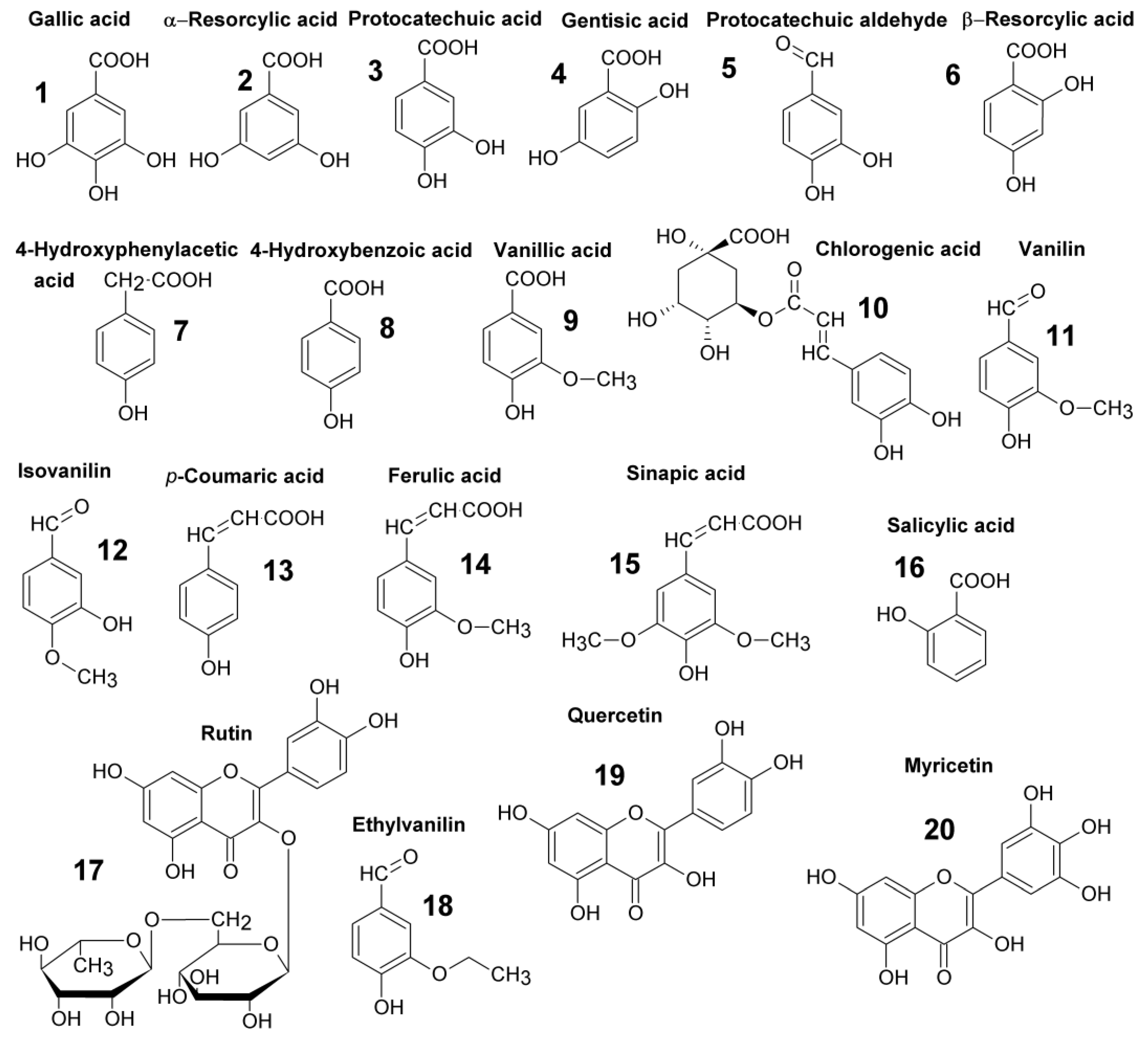

| No. | Compounds | tr (min) | PDP (mV) | SPE Recovery (%) | SPE RSD (%) | LOD (µg/kg) | LOQ (µg/kg) |
|---|---|---|---|---|---|---|---|
| 1 | Gallic acid | 2.72 | 400 | 79.42 | 1.84 | 15.1 | 50.1 |
| 2 | α-Resorcylic acid | 4.82 | 900 | 83.94 | 1.40 | 5.7 | 12.3 |
| 3 | Protocatechic acid | 5.34 | 900 | 71.98 | 4.74 | 14.3 | 54.8 |
| 4 | Gentisic acid | 6.44 | 900 | 62.33 | 3.65 | 10.0 | 25.4 |
| 5 | Protocatechuic aldehyde | 9.03 | 500 | 99.86 | 4.47 | 16.0 | 53.4 |
| 6 | β-Resorcylic acid | 9.49 | 900 | 68.24 | 2.63 | 5.0 | 11.6 |
| 7 | 4-Hydroxyphenylacetic acid | 14.06 | 900 | 95.64 | 2.73 | 6.8 | 22.9 |
| 8 | 4-Hydroxybenzoic acid | 15.11 | 900 | 91.34 | 2.17 | 8.9 | 30.8 |
| 9 | Vanillic acid | 15.85 | 900 | 86.77 | 1.42 | 6.2 | 23.3 |
| 10 | Chlorogenic acid | 16.81 | 900 | 95.78 | 3.26 | 4.3 | 14.4 |
| 11 | Vanillin | 22.03 | 900 | 88.20 | 1.14 | 16.3 | 45.1 |
| 12 | Isovanillin | 22.48 | 900 | 83.17 | 3.13 | 7.6 | 18.7 |
| 13 | p-Coumaric acid | 23.71 | 900 | 85.76 | 2.38 | 1.0 | 6.4 |
| 14 | Ferulic acid | 27.97 | 600 | 93.39 | 4.00 | 8.3 | 25.6 |
| 15 | Sinapic acid | 29.31 | 500 | 70.92 | 3.99 | 10.3 | 32.5 |
| 16 | Salicylic acid | 29.87 | 900 | 56.40 | 3.01 | 9.8 | 22.6 |
| 17 | Rutin | 31.12 | 500 | 90.67 | 4.78 | 2.9 | 9.8 |
| 18 | Ethylvanilline | 31.66 | 800 | 82.52 | 3.54 | 10.0 | 33.2 |
| 19 | Myricetin | 34.92 | 300 | 88.34 | 3.79 | 1.6 | 7.4 |
| 20 | Quercetin | 37.41 | 300 | 85.46 | 3.78 | 15.1 | 50.1 |
| Parameter | WB 60 °C | WB 80 °C | USB 60 W | MW 90 W | MW 270 W | MW 450 W | MW 900 W |
|---|---|---|---|---|---|---|---|
| time (min) | 65 | 20 | 90 | 11 | 1 | 0.50 | 0.33 |
| temperature (°C) | 60 | 80 | 45 | 79 | 82 | 76 | 89 |
| Compound | WB 60 °C (%) | WB 80 °C (%) | USB 60 W (%) | MW 90 W (%) | MW 270 W (%) | MW 450 W (%) | MW 900 W (%) |
|---|---|---|---|---|---|---|---|
| Protocatechuic a. | −61.3 | −63.2 | −70.5 | −67.0 | −62.2 | −61.4 | −72.4 |
| Protocatechuic aldehyde | 5.5 | −47.5 | −52.5 | −43.9 | −13.3 | −9.0 | −3.9 |
| 4-Hydroxyphenylacetic a. | 11.7 | −16.2 | −46.3 | −10.3 | −12.7 | −10.8 | 0.6 |
| Chlorogenic a. | −23.5 | −31.4 | −60.1 | −62.3 | −46.4 | −53.9 | −49.5 |
| Rutin | −54.7 | −55.2 | −51.8 | −69.5 | −40.2 | −47.6 | −36.4 |
| Ethylvanillin | −29.3 | −25.9 | −10.1 | −50.8 | −29.9 | −30.4 | −25.0 |
| average changes | −25.3 | −39.9 | −48.5 | −50.6 | −34.1 | −35.5 | −31.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hájek, T. Effect of Liquefaction of Honey on the Content of Phenolic Compounds. Molecules 2023, 28, 714. https://doi.org/10.3390/molecules28020714
Hájek T. Effect of Liquefaction of Honey on the Content of Phenolic Compounds. Molecules. 2023; 28(2):714. https://doi.org/10.3390/molecules28020714
Chicago/Turabian StyleHájek, Tomáš. 2023. "Effect of Liquefaction of Honey on the Content of Phenolic Compounds" Molecules 28, no. 2: 714. https://doi.org/10.3390/molecules28020714
APA StyleHájek, T. (2023). Effect of Liquefaction of Honey on the Content of Phenolic Compounds. Molecules, 28(2), 714. https://doi.org/10.3390/molecules28020714