Biosorption of Cadmium and Lead by Dry Biomass of Nostoc sp. MK-11: Kinetic and Isotherm Study
Abstract
1. Introduction
2. Results and Discussion
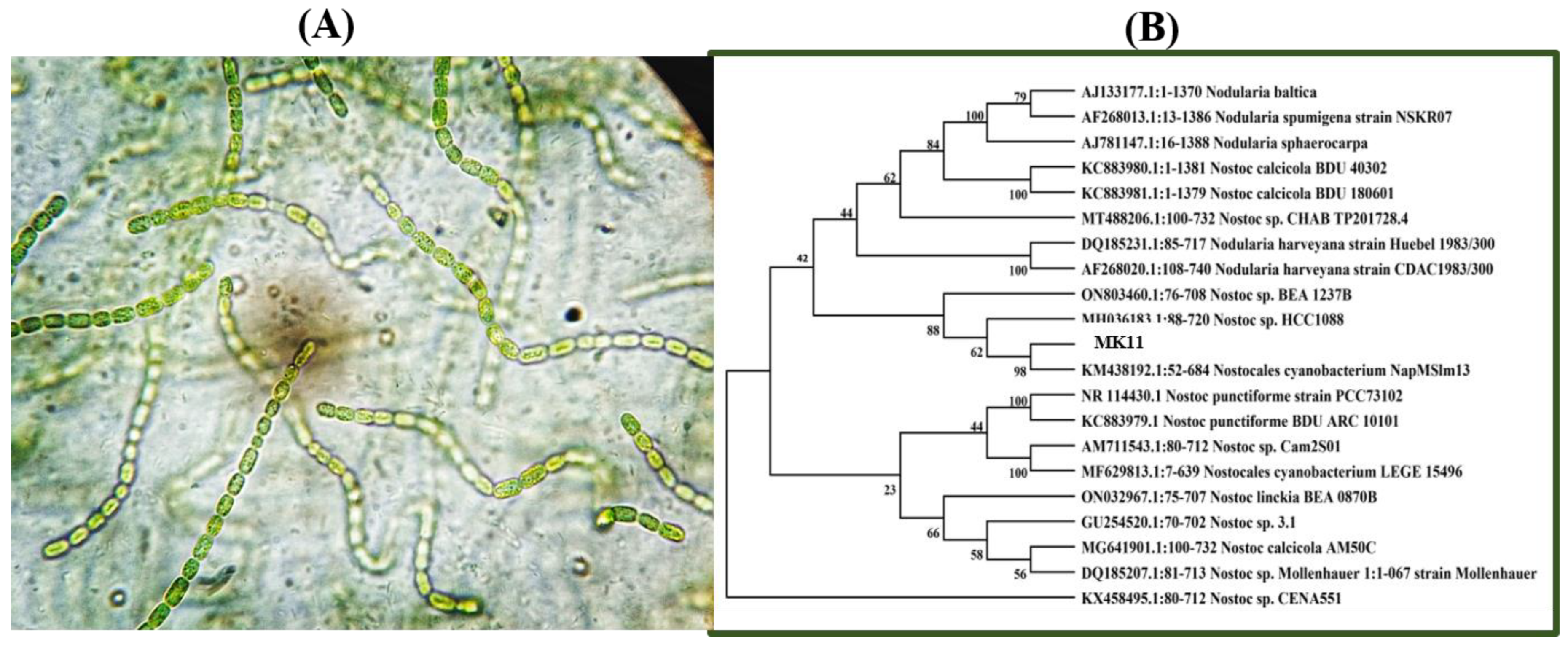
2.1. Identification of the Nostoc sp. MK-11
2.2. Characterization
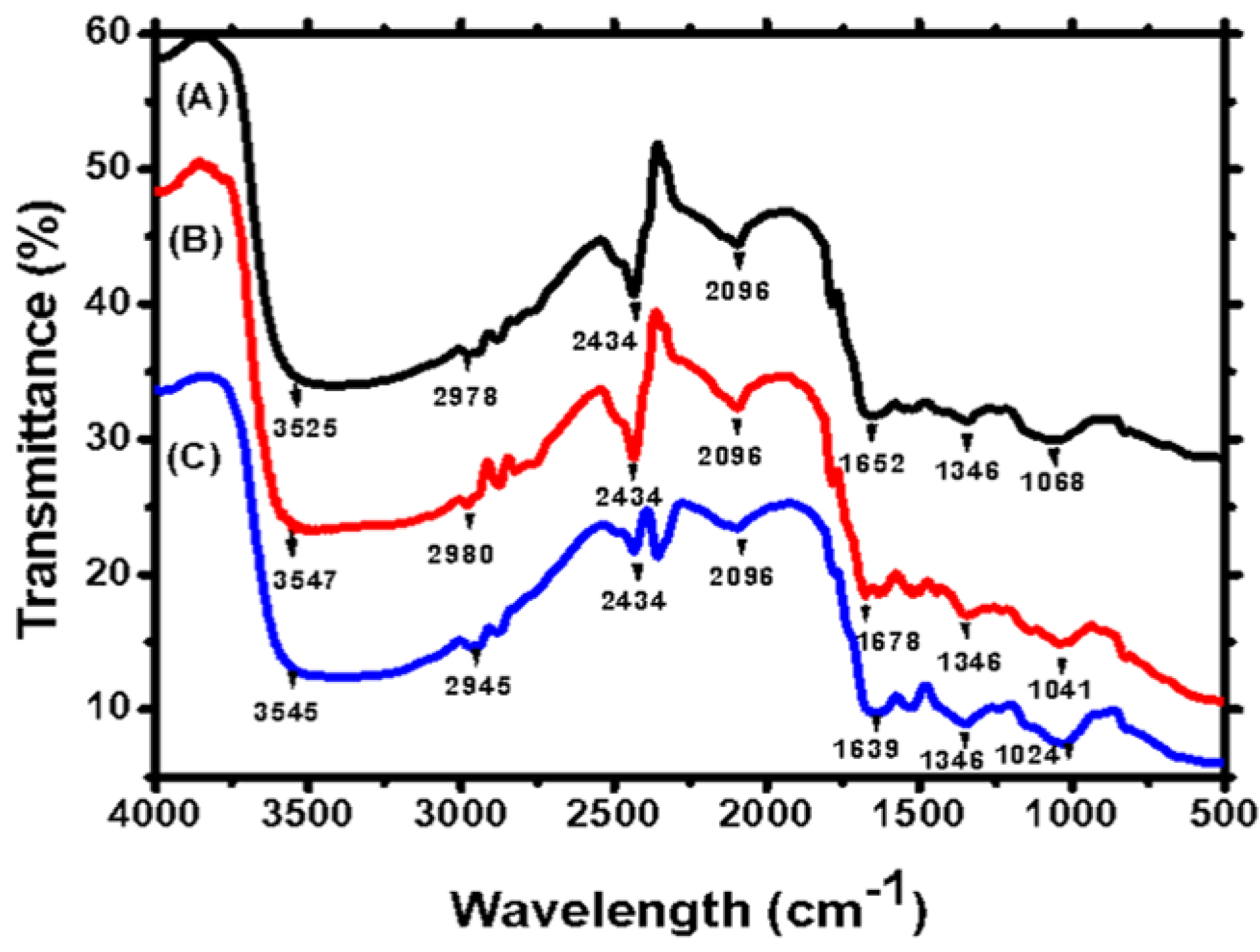
2.2.1. FTIR
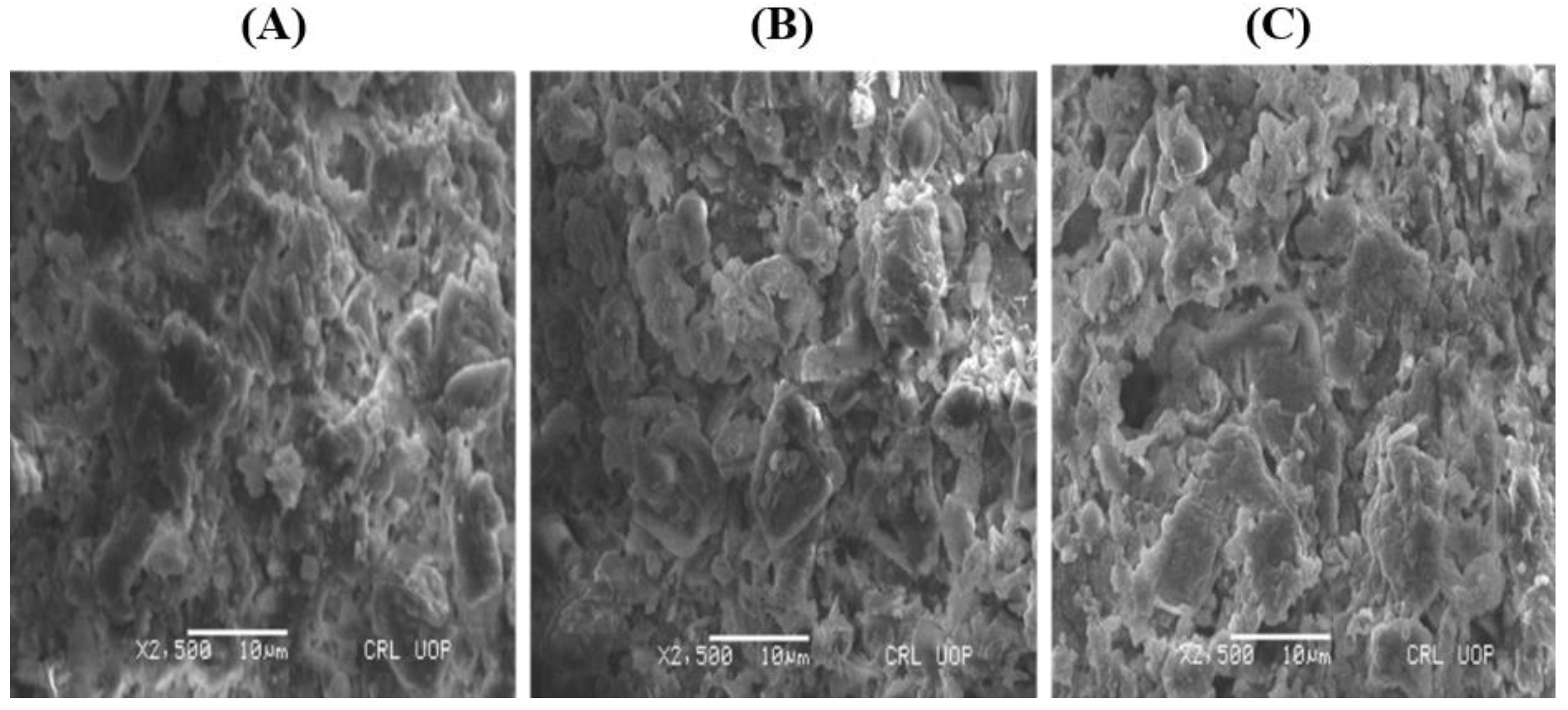
2.2.2. SEM
2.3. Effect of pH
2.4. Effect of Contact Time
2.5. Effect of Various Initial Metal Concentrations
2.6. Effect of Biomass Dosage
2.7. Kinetic Study
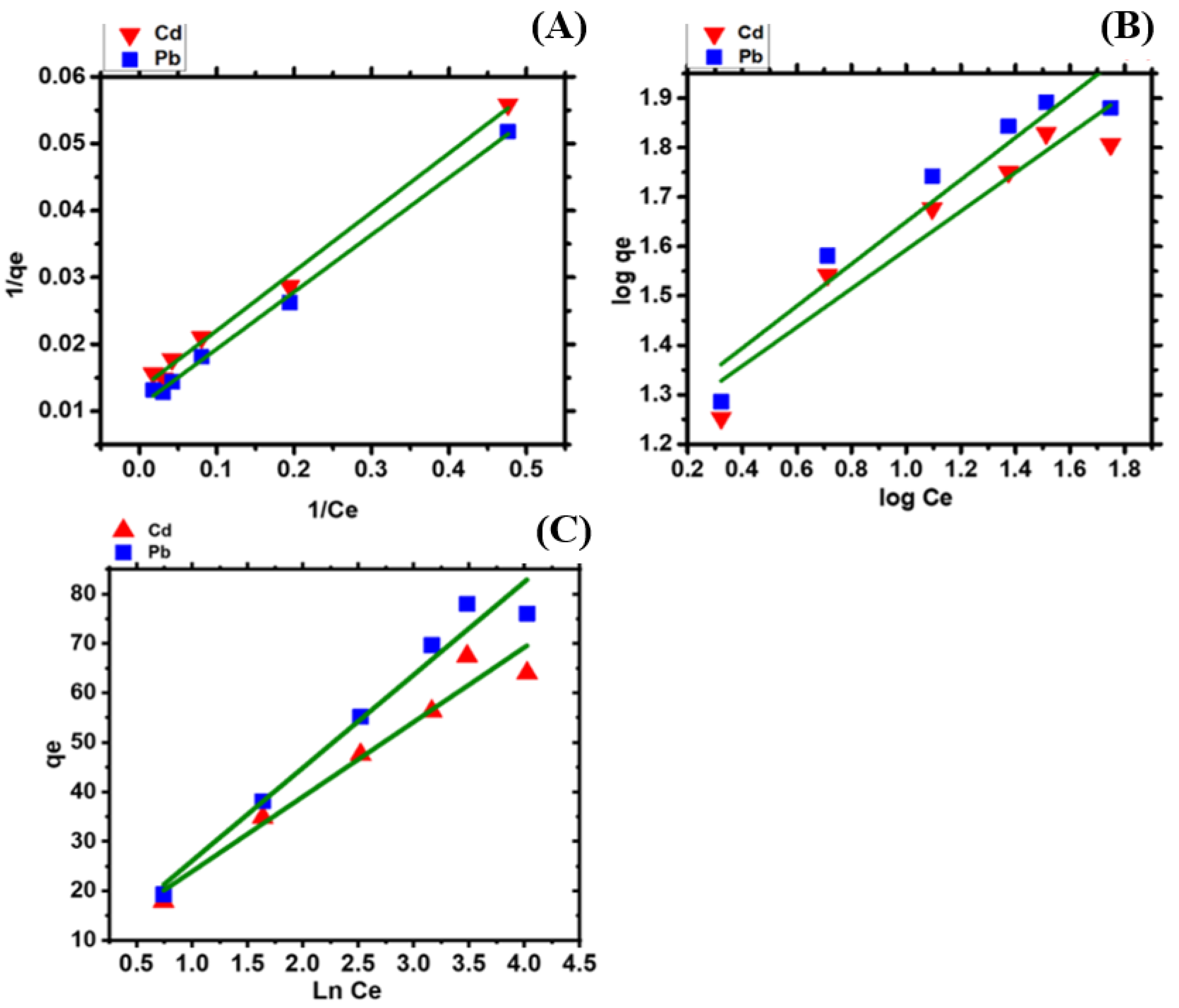
2.8. Isotherm Study
2.9. Desorption
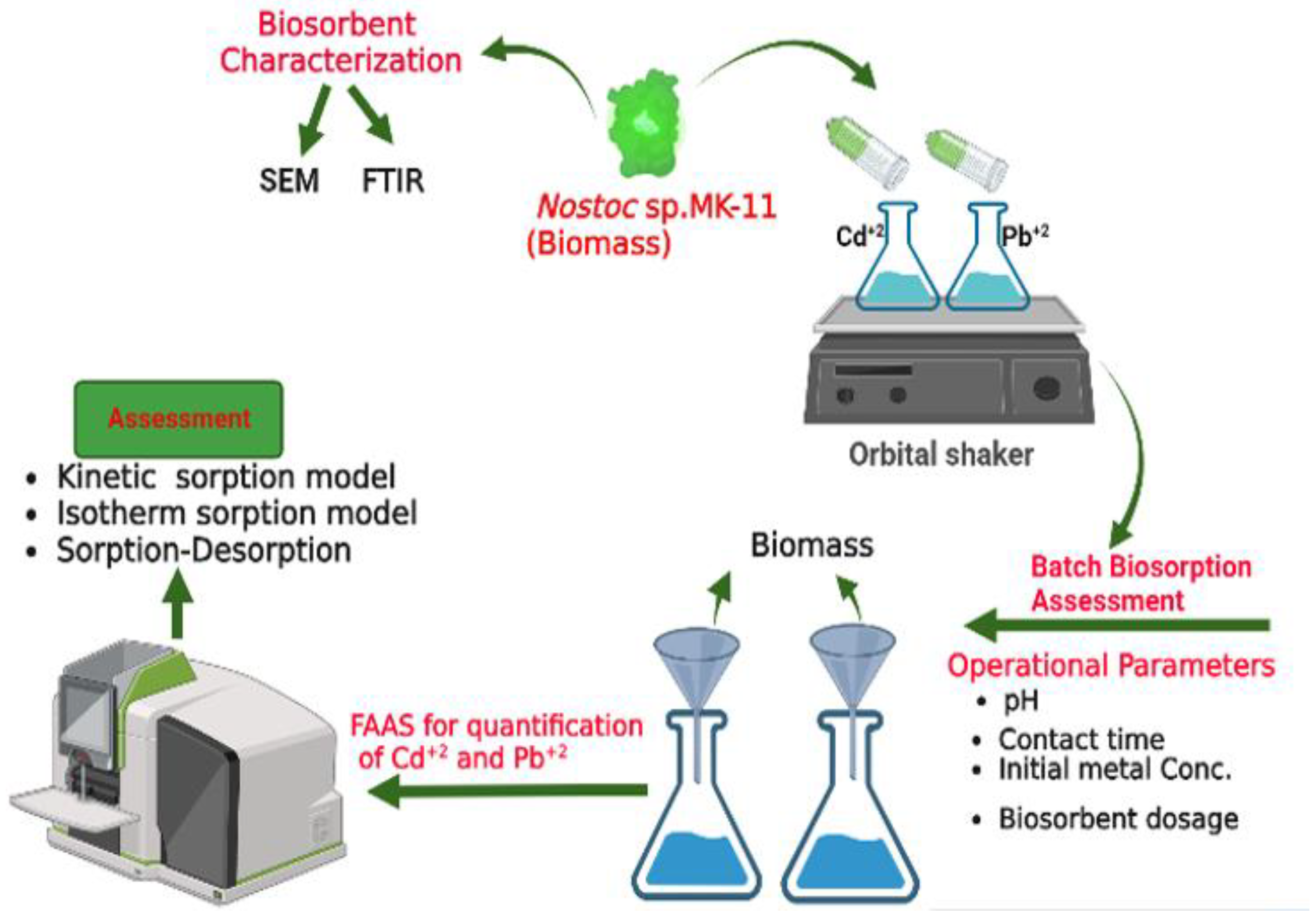
3. Materials and Methods
3.1. Cyanobacterial Strain and Biomass
3.2. Batch Experiments
3.3. Biosorbent Characterization
3.4. Kinetics Study
3.5. Isotherm Study
3.6. Desorption Study
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, Q.; Wang, L.; Xu, R.; Yang, Y.; Yin, H.; Jin, S.; Jiang, T. Potentiality of phosphorus−accumulating organisms biomasses in biosorption of Cd (II), Pb (II), Cu (II) and Zn (II) from aqueous solutions: Behaviors and mechanisms. Chemosphere 2022, 303, 135095. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhang, Q.; Ma, Z.B.; Wang, X.M.; Chen, H.; Wang, J.J. Fractionation and mobility risks of heavy metals and metalloids in wastewater-irrigated agricultural soils from greenhouses and fields in Gansu, China. Geoderma 2018, 328, 1–9. [Google Scholar] [CrossRef]
- Yu, Q.; Fein, J.B. Enhanced removal of dissolved Hg (II), Cd (II), and Au (III) from water by Bacillus subtilis bacterial biomass containing an elevated concentration of sulfhydryl sites. Environ. Sci. Technol. 2017, 51, 14360–14367. [Google Scholar] [CrossRef] [PubMed]
- Arancibia-Miranda, N.; Manquián-Cerda, K.; Pizarro, C.; Maldonado, T.; Suazo-Hernández, J.; Escudey, M.; Bolan, N.; Sarkar, B. Mechanistic insights into simultaneous removal of copper, cadmium and arsenic from water by iron oxide-functionalized magnetic imogolite nanocomposites. J. Hazard. Mater. 2020, 398, 122940. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Y.; Chen, J.P. Treatment of lead contaminated water by a PVDF membrane that is modified by zirconium, phosphate and PVA. Water Res. 2016, 101, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Luo, H.; Hu, T.; Li, H.; Fu, J. Toxic effects, uptake, and translocation of Cd and Pb in perennial ryegrass. Ecotoxicology 2013, 22, 207–214. [Google Scholar] [CrossRef]
- Liu, T.; Lawluvy, Y.; Shi, Y.; Ighalo, J.O.; He, Y.; Zhang, Y.; Yap, P.S. Adsorption of cadmium and lead from aqueous solution using modified biochar: A review. J. Environ. Chem. Eng. 2022, 10, 106502. [Google Scholar] [CrossRef]
- Padmaja, M.; Bhavani, R.; Pamila, R. Adsorption of cadmium from aqueous solutions using low cost materials-a review. Int. J. Eng. Technol. 2018, 7, 26–29. [Google Scholar] [CrossRef]
- Hajialigol, S.; Taher, M.; Malekpour, A. A new method for the selective removal of cadmium and zinc ions from aqueous solution by modified clinoptilolite. Adsorpt. Sci. Technol. 2006, 24, 487–496. [Google Scholar] [CrossRef]
- Selatnia, A.; Boukazoula, A.; Kechid, N.; Bakhti, M.Z.; Chergui, A.; Kerchich, Y. Biosorption of lead (II) from aqueous solution by a bacterial dead Streptomyces rimosus biomass. Biochem. Eng. J. 2004, 19, 127–135. [Google Scholar] [CrossRef]
- Gu, S.H.; Nicolas, V.; Lalis, A.; Sathirapongsasuti, N.; Yanagihara, R. Factorial experimental design for the optimization of highly selective adsorption removal of lead and copper ions using metal organic framework MOF-2 (Cd). J. Mol. Liq. 2018, 272, 15–26. [Google Scholar] [CrossRef]
- Yu, C.; Shao, J.; Sun, W.; Yu, X. Treatment of lead contaminated water using synthesized nano-iron supported with bentonite/graphene oxide. Arab. J. Chem. 2018, 13, 3474–3483. [Google Scholar] [CrossRef]
- Zhang, H.; Song, Y.; Nengzi, L.C.; Gou, J.; Li, B.; Cheng, X. An electron-scale comparative study on the adsorption of six divalent heavy metal cations on MnFe2O4@ CAC hybrid: Experimental and DFT investigations. Chem. Eng. J. 2020, 381, 122656. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, B.; Lin, L.; Qiu, W.; Song, Z. Adsorption of Cu (II) and Cd (II) from aqueous solutions by ferromanganese binary oxide–biochar composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Kajeiou, M.; Alem, A.; Mezghich, S.; Ahfir, N.D.; Mignot, M.; Devouge-Boyer, C.; Pantet, A. Competitive and non-competitive zinc, copper and lead biosorption from aqueous solutions onto flax fibers. Chemosphere 2020, 260, 127505. [Google Scholar] [CrossRef]
- Saqib, S.; Faryad, S.; Afridi, M.I.; Arshad, B.; Younas, M.; Naeem, M.; Zaman, W.; Ullah, F.; Nisar, M.; Ali, S. Bimetallic assembled silver nanoparticles impregnated in Aspergillus fumigatus extract damage the bacterial membrane surface and release cellular contents. Coatings 2022, 12, 1505. [Google Scholar] [CrossRef]
- Saqib, S.; Zaman, W.; Ayaz, A.; Habib, S.; Bahadur, S.; Hussain, S.; Muhammad, S.; Ullah, F. Postharvest disease inhibition in fruit by synthesis and characterization of chitosan iron oxide nanoparticles. Biocatal. Agric. Biotechnol. 2020, 28, 101729. [Google Scholar] [CrossRef]
- Inthorn, D.; Silapanuntakul, S.; Incharoensakdi, A. Filamentous cyanobacteria can efficiently remove cadmium present in aqueous solution at low concentration. Asian J. Microbiol. Biotechnol. Environ. Sci. 2002, 4, 1–6. [Google Scholar]
- Dönmez, G.; Aksu, Z. Removal of chromium (VI) from saline wastewaters by Dunaliella species. Process Biochem. 2002, 38, 751–762. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Cepoi, L.; Povar, I.; Chiriac, T.; Rodlovskaya, E.; Culicov, O.A. Metal uptake from complex industrial effluent by cyanobacteria Arthrospira platensis. Water Air Soil Pollut. 2018, 229, 220. [Google Scholar] [CrossRef]
- Al-Homaidan, A.A.; Alabdullatif, J.A.; Al-Hazzani, A.A.; Al-Ghanayem, A.A.; Alabbad, A.F. Adsorptive removal of cadmium ions by Spirulina platensis dry biomass. Saudi J. Biol. Sci. 2015, 22, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Greenlaw, P.N.; Shane, B.S. Adsorption of heavy metals by green algae and ground rice hulls. J. Environ. Sci. Health Part A 1993, 28, 37–50. [Google Scholar] [CrossRef]
- Shen, L.; Li, Z.; Wang, J.; Liu, A.; Li, Z.; Yu, R.; Wu, X.; Liu, Y.; Li, J.; Zeng, W. Characterization of extracellular polysaccharide/protein contents during the adsorption of Cd (II) by Synechocystis sp. PCC6803. Environ. Sci. Pollut. Res. 2018, 25, 20713–20722. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, N.; Yang, X.; Song, L.; Yang, S. Toxic metal biosorption by macrocolonies of cyanobacterium Nostoc sphaeroides Kützing. J. Appl. Phycol. 2016, 28, 2265–2277. [Google Scholar] [CrossRef]
- Arief, V.O.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Recent progress on biosorption of heavy metals from liquids using low cost biosorbents: Characterization, biosorption parameters and mechanism studies. CLEAN Soil Air Water 2008, 36, 937–962. [Google Scholar] [CrossRef]
- Murphy, V.; Hughes, H.; McLoughlin, P. Cu (II) binding by dried biomass of red, green and brown macroalgae. Water Res. 2007, 41, 731–740. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Rodrigues, M.S.; De Carvalho, J.C.M.; Lodi, A.; Finocchio, E.; Perego, P.; Converti, A. Adsorption of Ni2+, Zn2+ and Pb2+ onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris. I. Single metal systems. Chem. Eng. J. 2011, 173, 326–333. [Google Scholar] [CrossRef]
- Tuzen, M.; Sarı, A.; Mendil, D.; Uluozlu, O.D.; Soylak, M.; Dogan, M. Characterization of biosorption process of As (III) on green algae Ulothrix cylindricum. J. Hazard. Mater. 2009, 165, 566–572. [Google Scholar] [CrossRef]
- Satya, A.; Harimawan, A.; Haryani, G.S.; Johir, M.A.H.; Vigneswaran, S.; Ngo, H.H.; Setiadi, T. Batch study of cadmium biosorption by carbon dioxide enriched Aphanothece sp. dried biomass. Water 2020, 12, 264. [Google Scholar] [CrossRef]
- Dirbaz, M.; Roosta, A. Adsorption, kinetic and thermodynamic studies for the biosorption of cadmium onto microalgae Parachlorella sp. J. Environ. Chem. Eng. 2018, 6, 2302–2309. [Google Scholar] [CrossRef]
- Matheickal, J.T.; Yu, Q. Biosorption of lead (II) and copper (II) from aqueous solutions by pre-treated biomass of Australian marine algae. Bioresour. Technol. 1999, 69, 223–229. [Google Scholar] [CrossRef]
- Singh, A.; Mehta, S.; Gaur, J. Removal of heavy metals from aqueous solution by common freshwater filamentous algae. World J. Microbiol. Biotechnol. 2007, 23, 1115–1120. [Google Scholar] [CrossRef]
- Saif, M.M.S.; Kumar, N.S.; Prasad, M. Binding of cadmium to Strychnos potatorum seed proteins in aqueous solution: Adsorption kinetics and relevance to water purification. Colloids Surf. B Biointerfaces 2012, 94, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Sarı, A.; Tuzen, M. Biosorption of Pb (II) and Cd (II) from aqueous solution using green alga (Ulva lactuca) biomass. J. Hazard. Mater. 2008, 152, 302–308. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.; El Sabagh, S.; Abou El-Souod, G.; Elbeltagy, A. Biosorption of cadmium from aqueous solution by free and immobilized dry biomass of Chlorella vulgaris. Int. J. Environ. Res. 2019, 13, 511–521. [Google Scholar] [CrossRef]
- Lofrano, G.; Brown, J.; Feo, G.D. Heavy metal removal through biosorptive pathways. In Advances in Water Treatment and Pollution Prevention; Springer: Berlin/Heidelberg, Germany, 2012; pp. 95–145. [Google Scholar] [CrossRef]
- Romera, E.; Gonzalez, F.; Ballester, A.; Blazquez, M.L.; Munoz, J.A. Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Balasubramanian, R.; Iyer, C. Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu (II) from aqueous solutions. Bioresour. Technol. 2007, 98, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Oliveira, W.E.; Franca, A.S.; Oliveira, L.S.; Rocha, S.D. Untreated coffee husks as biosorbents for the removal of heavy metals from aqueous solutions. J. Hazard. Mater. 2008, 152, 1073–1081. [Google Scholar] [CrossRef]
- Bayo, J. Kinetic studies for Cd (II) biosorption from treated urban effluents by native grapefruit biomass (Citrus paradisi L.): The competitive effect of Pb (II), Cu (II) and Ni (II). Chem. Eng. J. 2012, 15, 278–287. [Google Scholar] [CrossRef]
- Gupta, V.; Rastogi, A. Equilibrium and kinetic modelling of cadmium (II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J. Hazard. Mater. 2008, 153, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Padmesh, T.V.N.; Palanivelu, K.; Velan, M. Biosorption of nickel (II) ions onto Sargassum wightii: Application of two-parameter and three-parameter isotherm models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Trejo, P.V.; Guibal, E.; Louvier-Hernández, J.F. Arsenic sorption on chitosan-based sorbents: Comparison of the effect of molybdate and tungstate loading on As (V) sorption properties. J. Polym. Environ. 2020, 28, 934–947. [Google Scholar] [CrossRef]
- Akar, T.; Celik, S.; Ari, A.G.; Akar, S.T. Removal of Pb2+ ions from contaminated solutions by microbial composite: Combined action of a soilborne fungus Mucor plumbeus and alunite matrix. Chem. Eng. J. 2013, 215, 626–634. [Google Scholar] [CrossRef]
- Luo, J.-M.; Xiao, X. Biosorption of cadmium (II) from aqueous solutions by industrial fungus Rhizopus cohnii. Trans. Nonferrous Met. Soc. China 2010, 20, 1104–1111. [Google Scholar] [CrossRef]
- Jalali, R.; Ghafourian, H.; Asef, Y.; Davarpanah, S.J.; Sepehr, S. Removal and recovery of lead using nonliving biomass of marine algae. J. Hazard. Mater. 2002, 92, 253–262. [Google Scholar] [CrossRef]
- Perez-Rama, M.; Torres, E.; Suarez, C.; Herrero, C.; Abalde, J. Sorption isotherm studies of Cd (II) ions using living cells of the marine microalga Tetraselmis suecica (Kylin) Butch. J. Environ. Manag. 2010, 91, 2045–2050. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Ferreira, L.S.; de Carvalho, J.C.M.; Lodi, A.; Finocchio, E.; Converti, A. Metal biosorption onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris: Multi-metal systems. J. Hazard. Mater. 2012, 217, 246–255. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 1997, 63, 3327–3332. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Singh, A.K.; Sikandar, M. Study of sorption and desorption of Cd (II) from aqueous solution using isolated green algae Chlorella vulgaris. Appl. Water Sci. 2018, 8, 225. [Google Scholar] [CrossRef]
- Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 1985, 125, 1–15. [Google Scholar] [CrossRef]
- Raize, O.; Argaman, Y.; Yannai, S. Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnol. Bioeng. 2004, 87, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Tuzun, I.; Bayramoglu, G.; Yalcın, E.; Basaran, G.; Celik, G.; Arıca, M.Y. Equilibrium and kinetic studies on biosorption of Hg (II), Cd (II) and Pb (II) ions onto microalgae Chlamydomonas reinhardtii. J. Environ. Manag. 2005, 77, 85–92. [Google Scholar] [CrossRef]

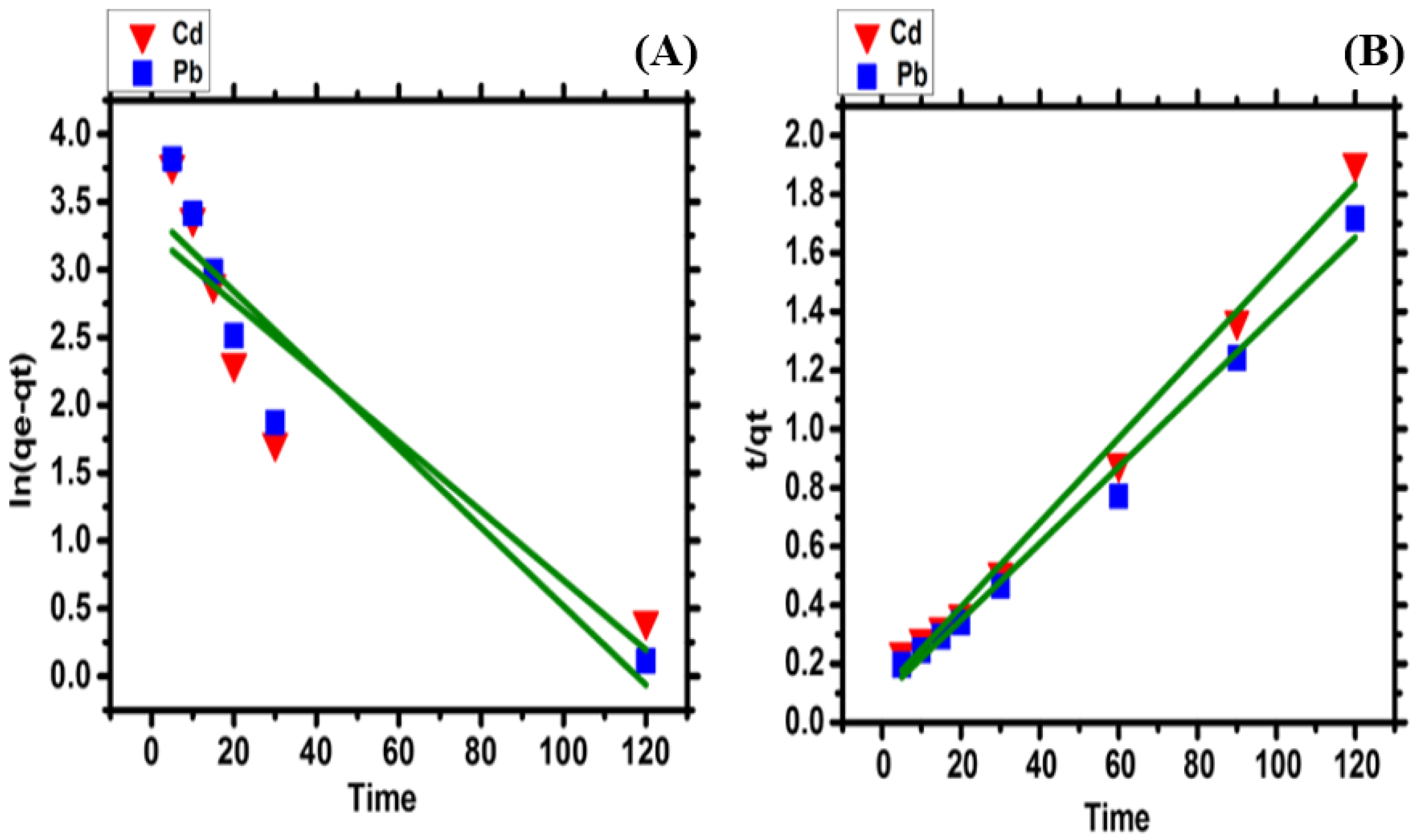
| qe (Experiment) | Pseudo-First Order | Pseudo-Second Order | |||||
|---|---|---|---|---|---|---|---|
| Metals | (mg/g) | qe (cal) (mg/g) | K1 (min−1) | R2 | qe(cal) (mg/g) | K2 (gmg−1 min−1) | R2 |
| Cd | 67.9 | 26.206 | −0.0004 | 0.770 | 69.492 | 0.00196 | 0.991 |
| Pb | 77.75 | 30.569 | −0.0003 | 0.863 | 76.923 | 0.00192 | 0.990 |
| Langmuir | Freundlich | Temkin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metals | qmax (mg/g) | KL (L/mg) | RL | R2 | KF (mg/g) | 1/n | R2 | KT | BT | R2 |
| Cd | 75.757 | 0.149 | 0.0627 | 0.993 | 15.915 | 0.391 | 0.897 | 1.752 | 15.796 | 0.947 |
| Pb | 83.963 | 0.428 | 0.0098 | 0.998 | 27.664 | 0.328 | 0.844 | 7.228 | 14.68 | 0.928 |
| Metals | Cycles | Biosorption (S) (mg/g) | Desorption (D) (mg/g) | Ratio of D/B (mg/g) | % Desorption |
|---|---|---|---|---|---|
| Cd | 1 | 65.65 | 61.22 | 0.932 | 93.252 |
| 2 | 62 | 57.5 | 0.927 | 92.741 | |
| 3 | 57.02 | 52.11 | 0.913 | 91.388 | |
| Pb | 1 | 74.49 | 71.4 | 0.958 | 95.851 |
| 2 | 69.22 | 64.45 | 0.931 | 93.108 | |
| 3 | 60 | 56 | 0.933 | 93.333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaleem, M.; Minhas, L.A.; Hashmi, M.Z.; Ali, M.A.; Mahmoud, R.M.; Saqib, S.; Nazish, M.; Zaman, W.; Samad Mumtaz, A. Biosorption of Cadmium and Lead by Dry Biomass of Nostoc sp. MK-11: Kinetic and Isotherm Study. Molecules 2023, 28, 2292. https://doi.org/10.3390/molecules28052292
Kaleem M, Minhas LA, Hashmi MZ, Ali MA, Mahmoud RM, Saqib S, Nazish M, Zaman W, Samad Mumtaz A. Biosorption of Cadmium and Lead by Dry Biomass of Nostoc sp. MK-11: Kinetic and Isotherm Study. Molecules. 2023; 28(5):2292. https://doi.org/10.3390/molecules28052292
Chicago/Turabian StyleKaleem, Muhammad, Lubna Anjum Minhas, Muhammad Zafar Hashmi, Mohammad Ajmal Ali, Rania M. Mahmoud, Saddam Saqib, Moona Nazish, Wajid Zaman, and Abdul Samad Mumtaz. 2023. "Biosorption of Cadmium and Lead by Dry Biomass of Nostoc sp. MK-11: Kinetic and Isotherm Study" Molecules 28, no. 5: 2292. https://doi.org/10.3390/molecules28052292
APA StyleKaleem, M., Minhas, L. A., Hashmi, M. Z., Ali, M. A., Mahmoud, R. M., Saqib, S., Nazish, M., Zaman, W., & Samad Mumtaz, A. (2023). Biosorption of Cadmium and Lead by Dry Biomass of Nostoc sp. MK-11: Kinetic and Isotherm Study. Molecules, 28(5), 2292. https://doi.org/10.3390/molecules28052292










