Anti-Alzheimer’s Natural Products Derived from Plant Endophytic Fungi
Abstract
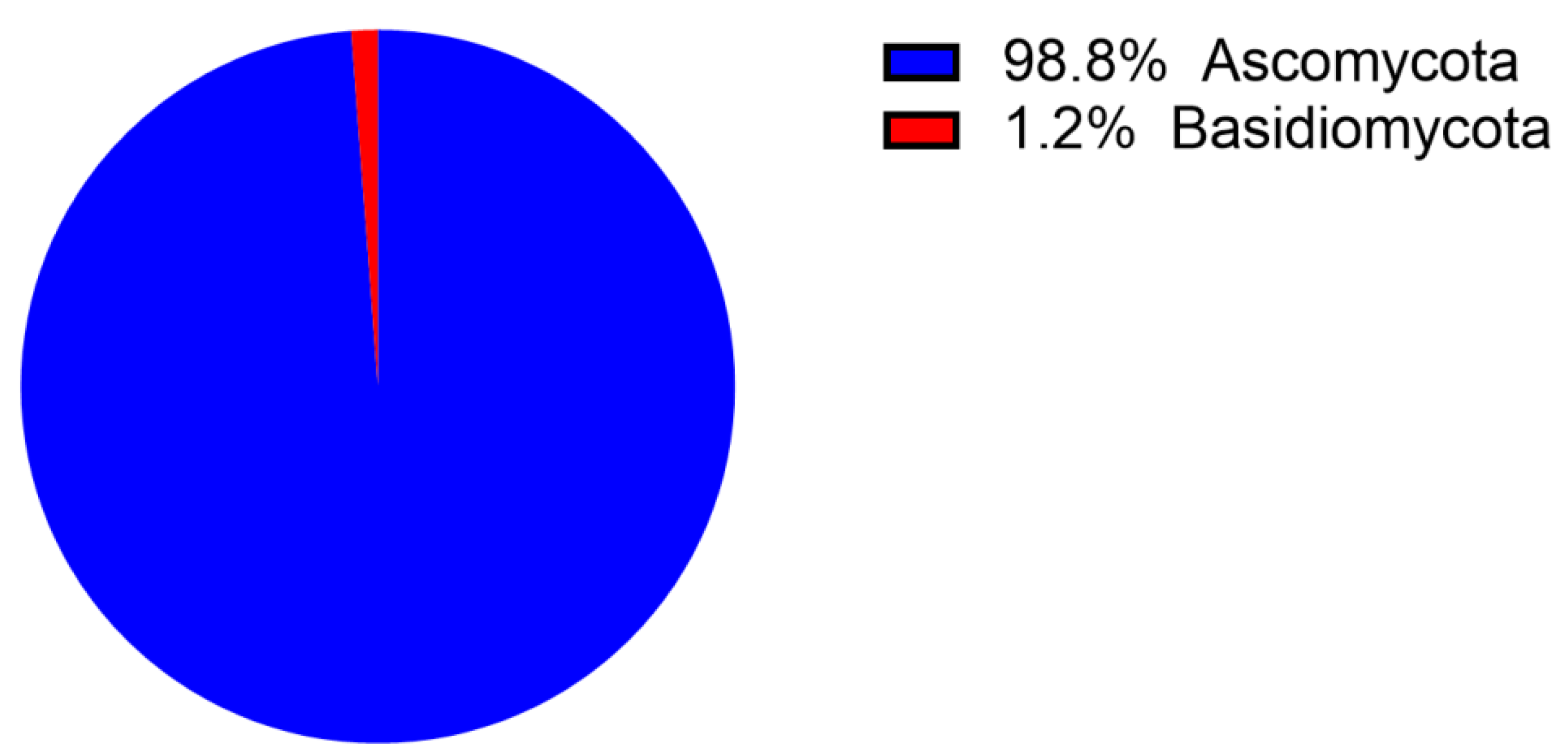
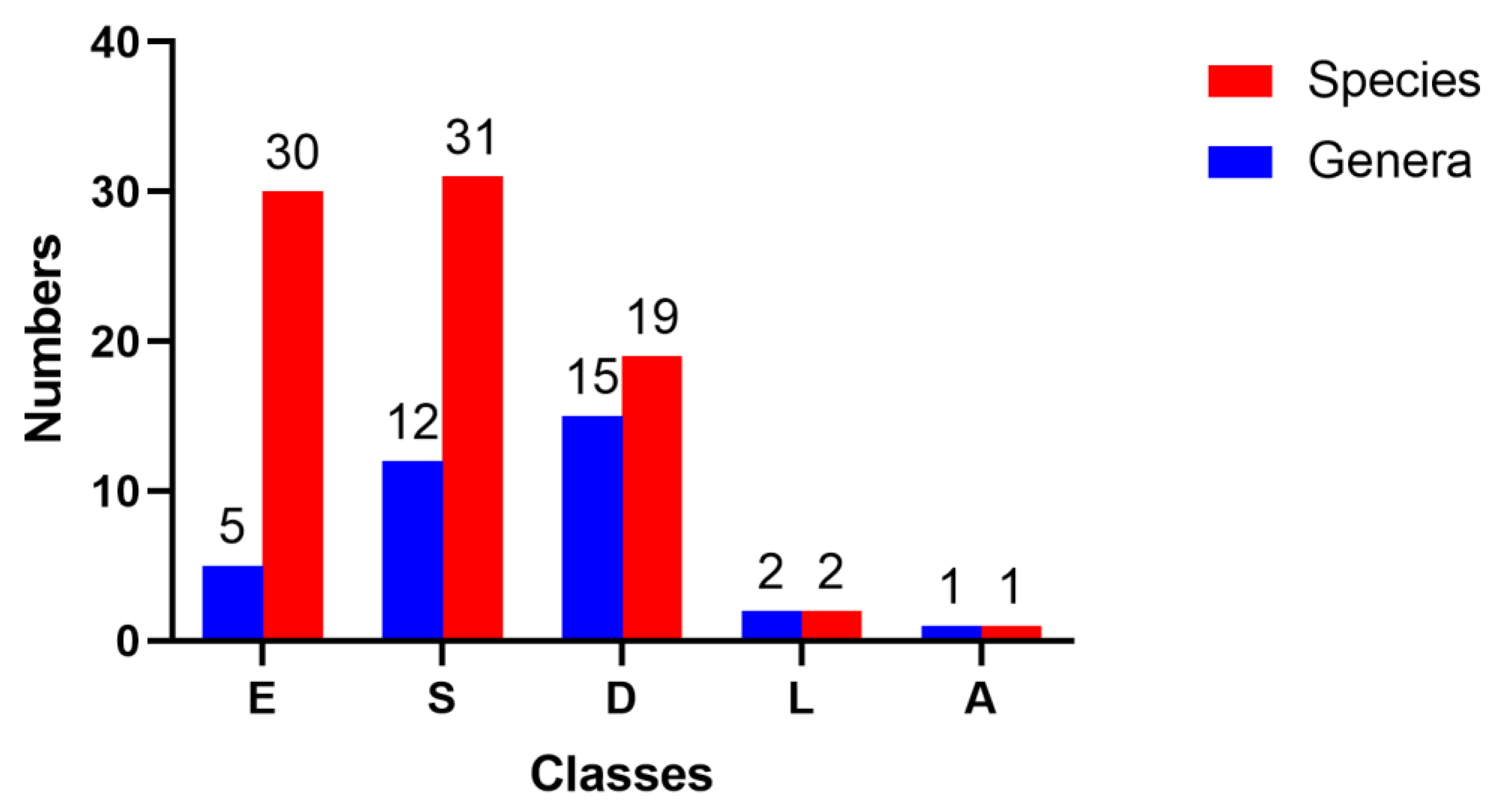
1. Introduction
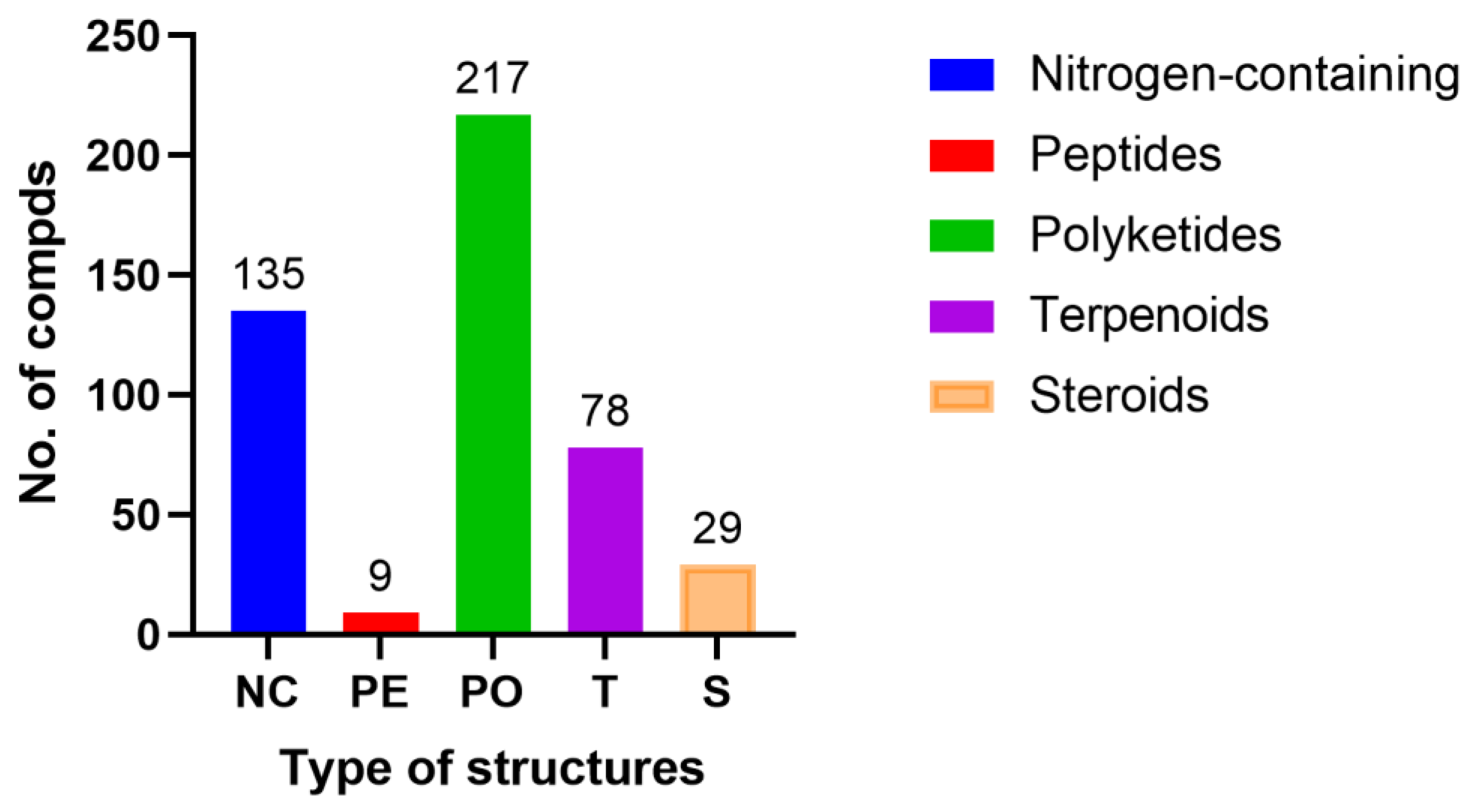
2. Bioactive Compounds from Plant Endophytic Fungi
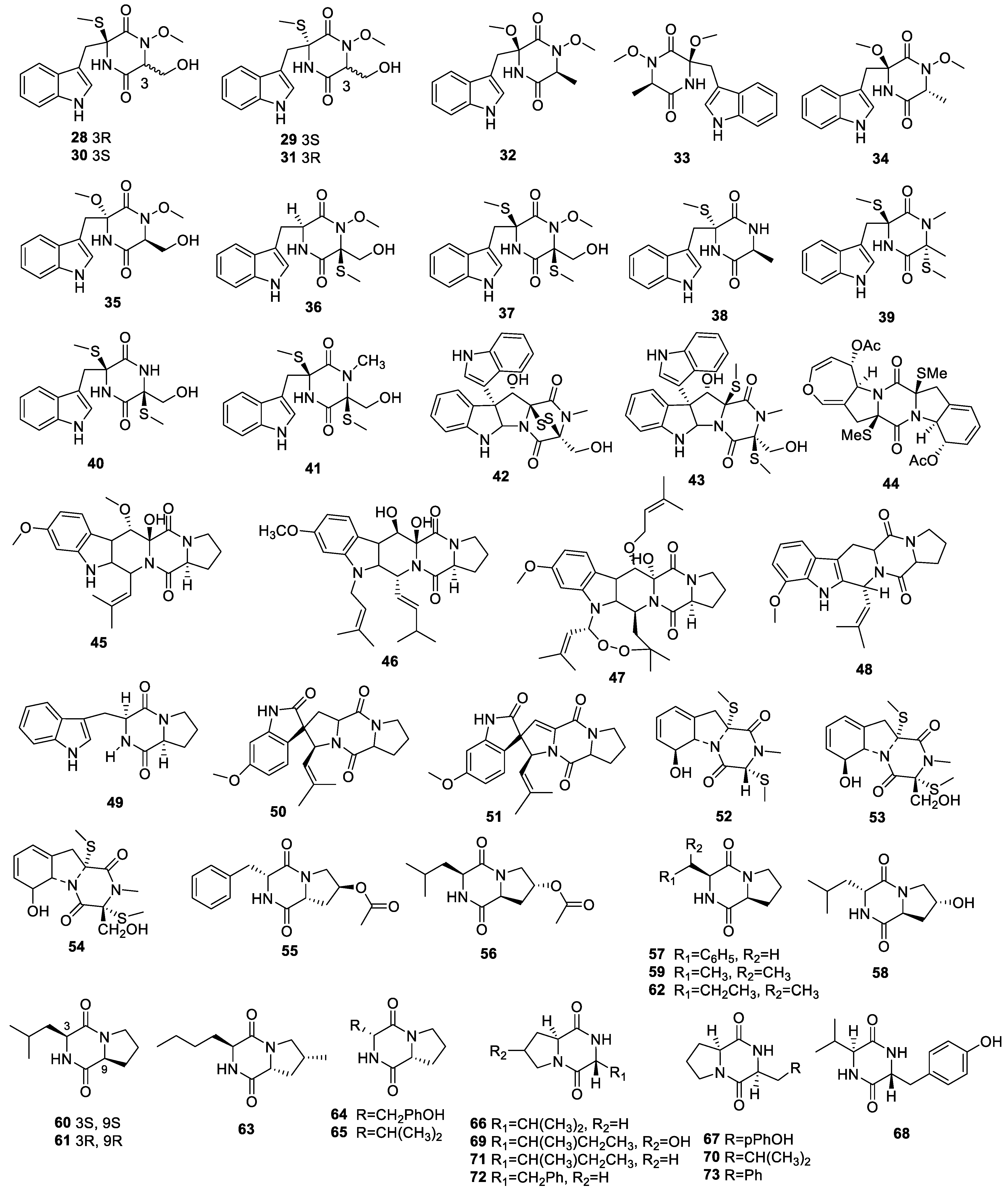
2.1. Alkaloids
2.1.1. Cytochalasans
2.1.2. Diketopiperazine Derivatives
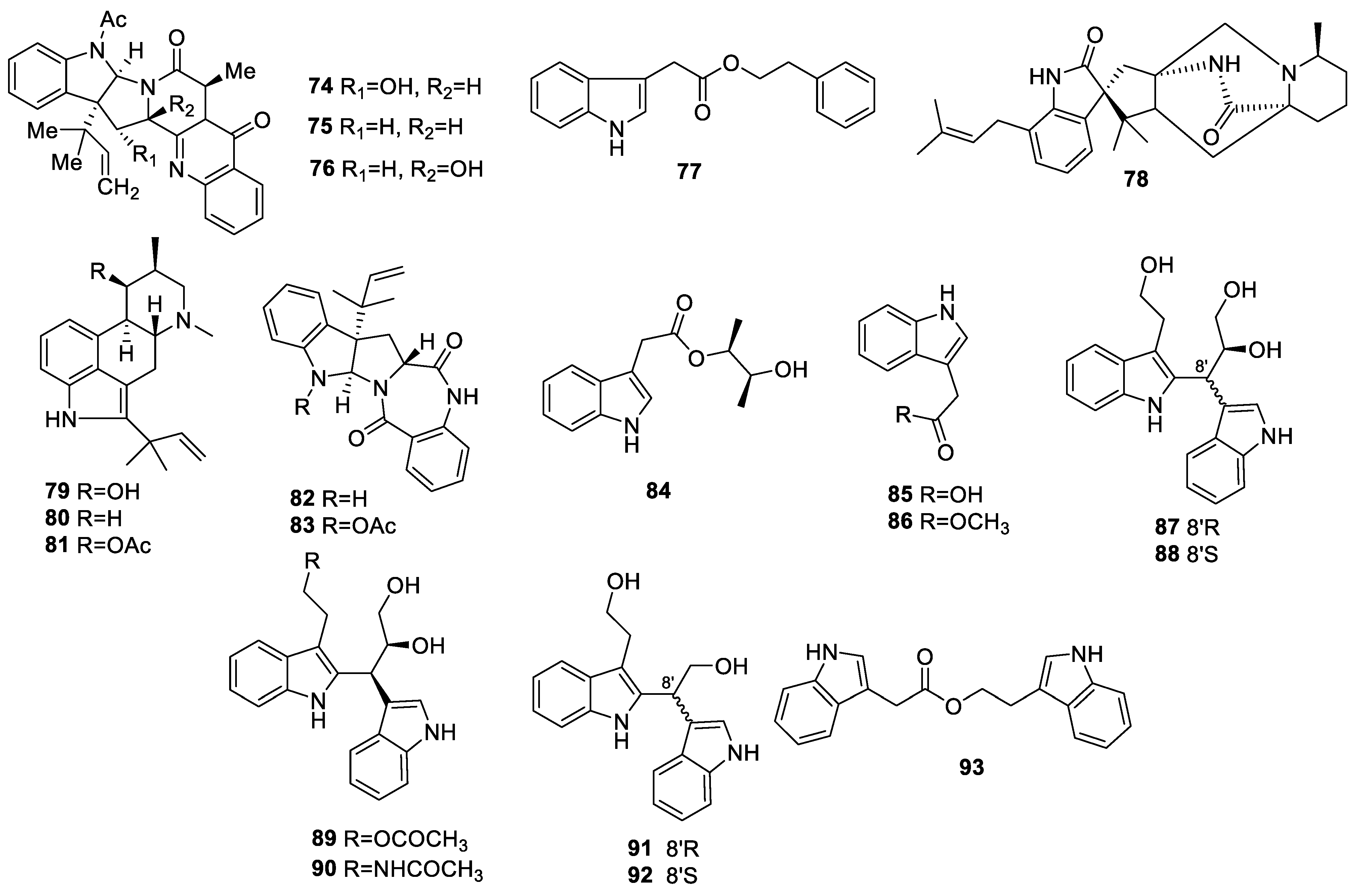
2.1.3. Indole Alkaloids
2.1.4. Other Alkaloids
2.2. Peptides
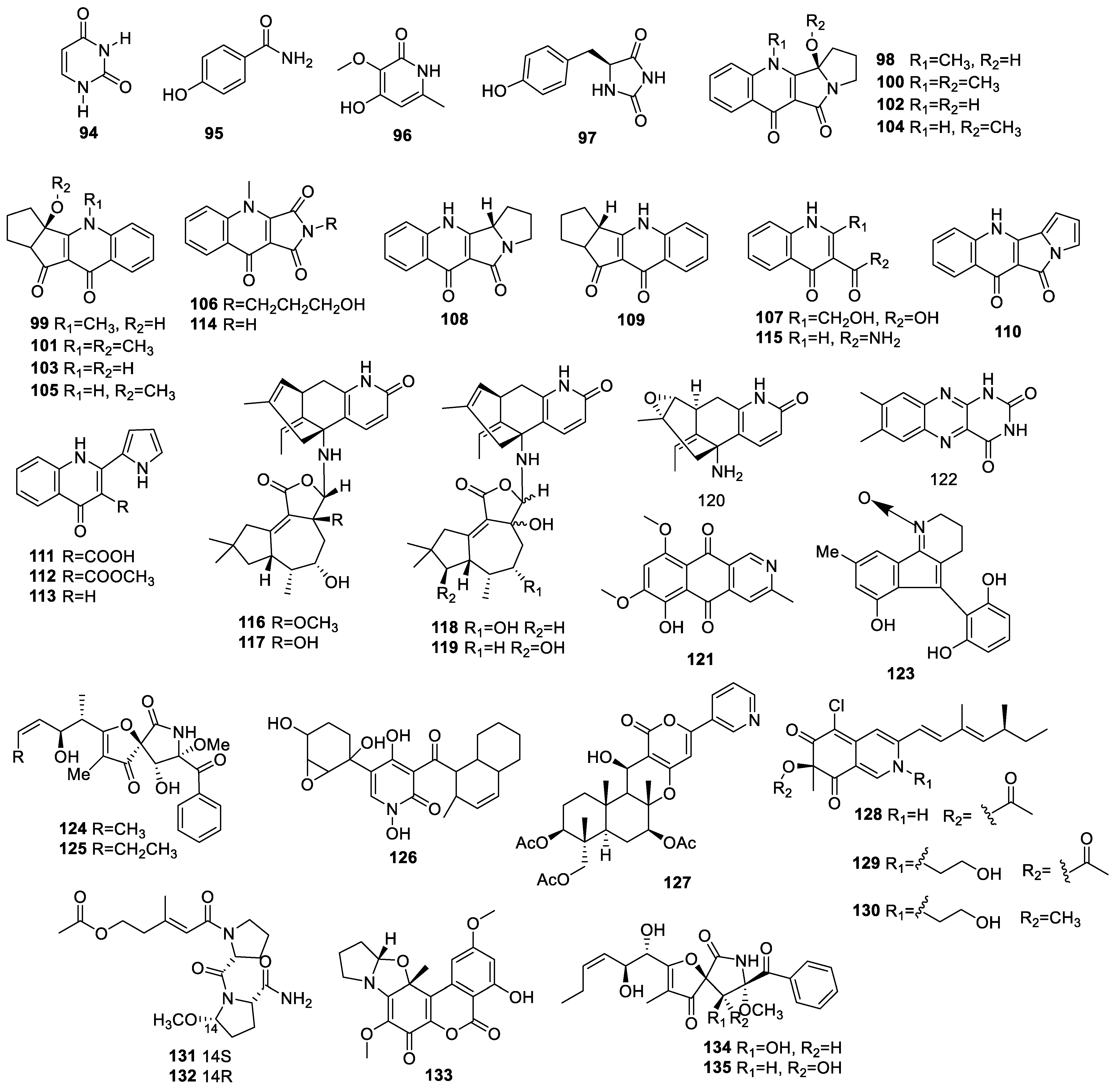
2.3. Polyketides
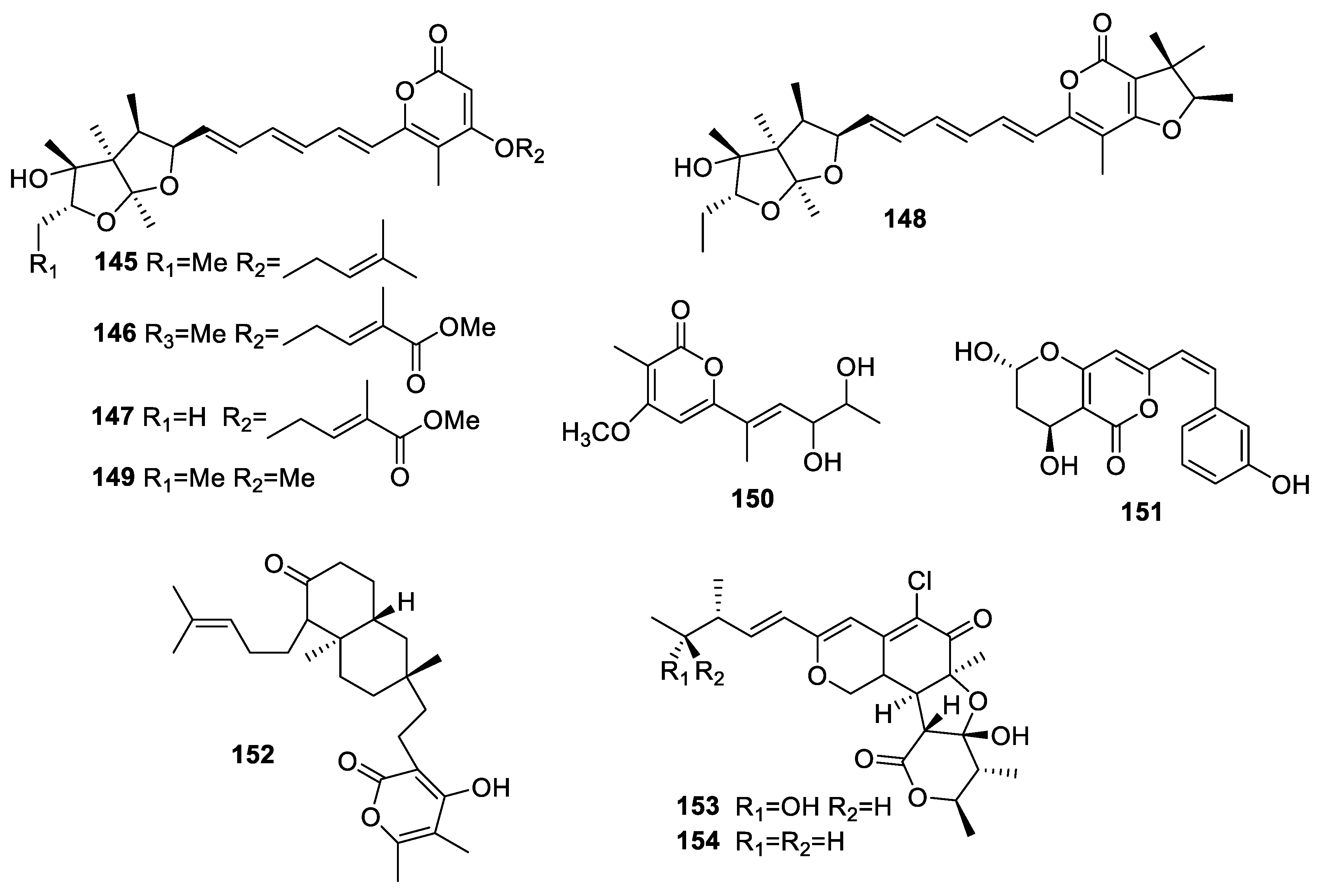
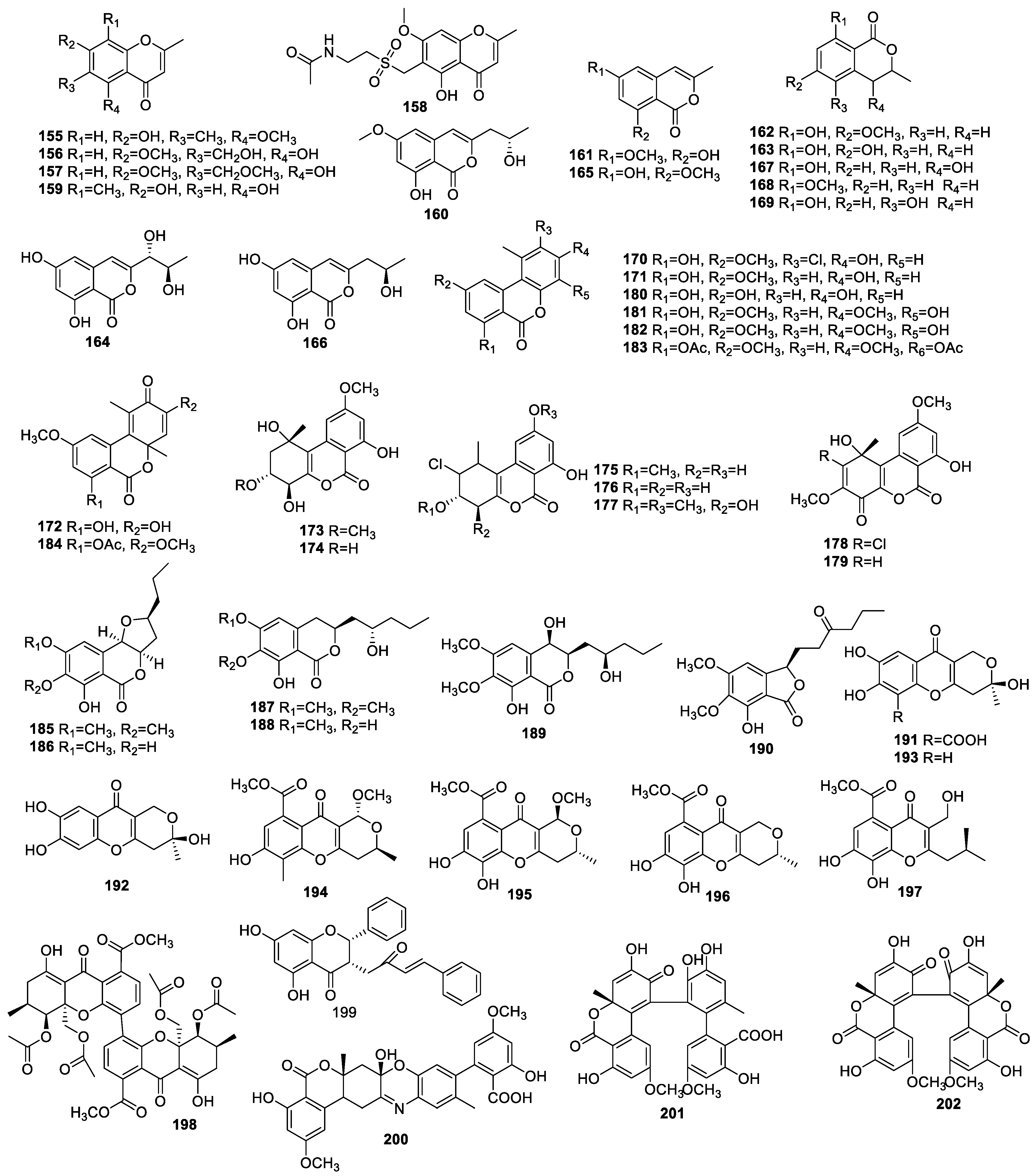
2.3.1. Pyranones and Pyranyl Derivatives
Simple Pyranones
Benzopyrones
Pyranyl Derivatives
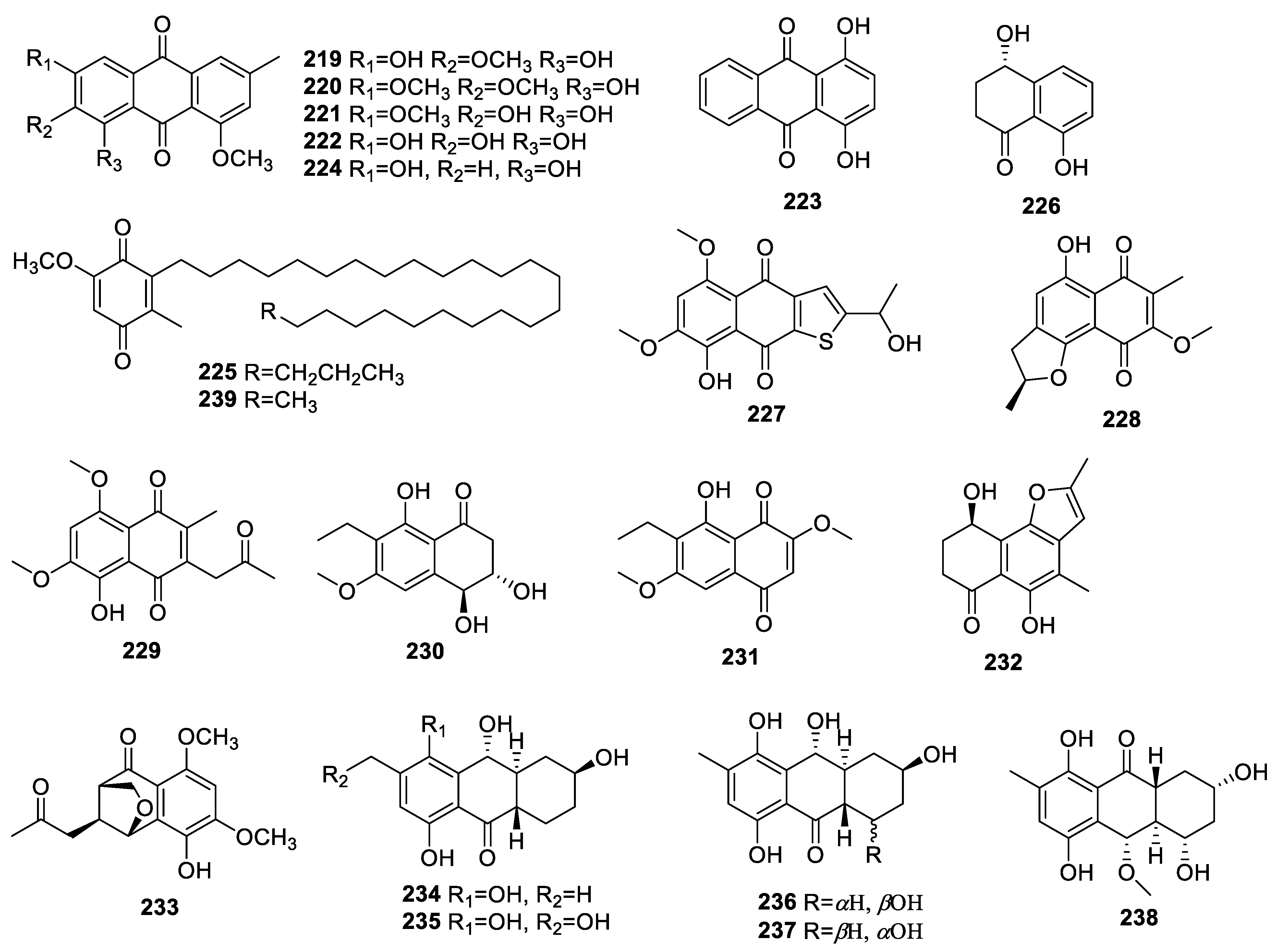
2.3.2. Quinones
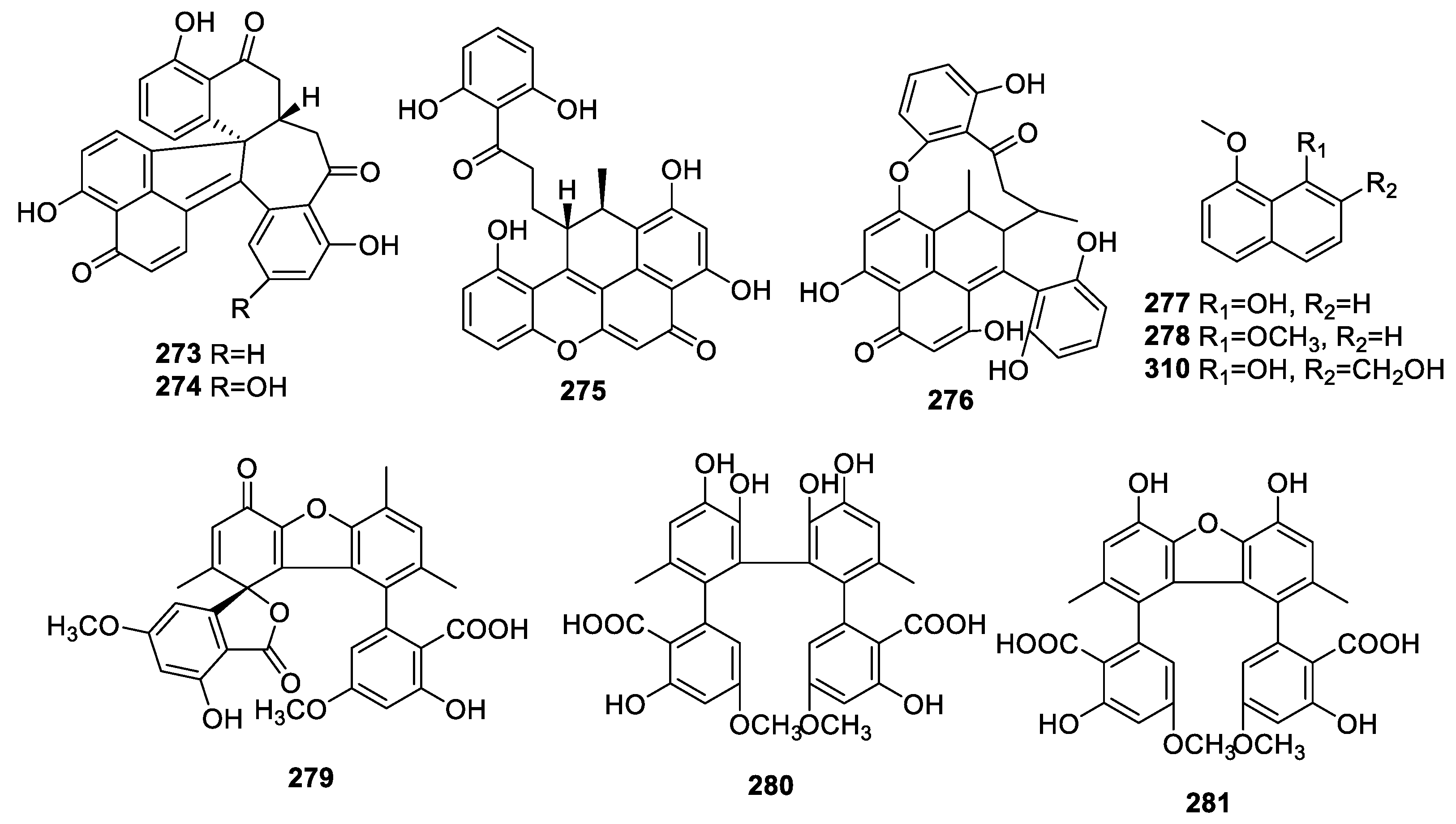
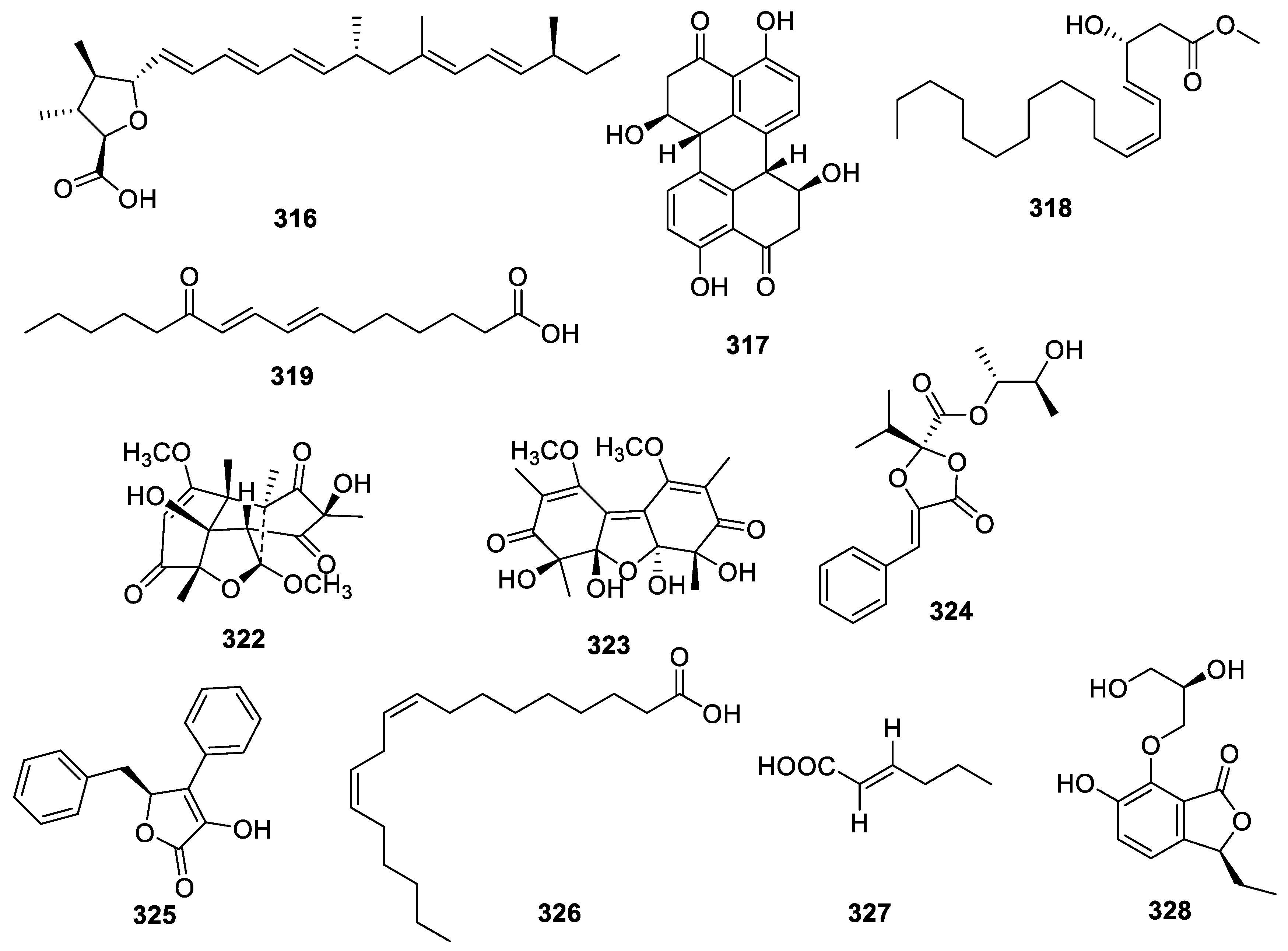
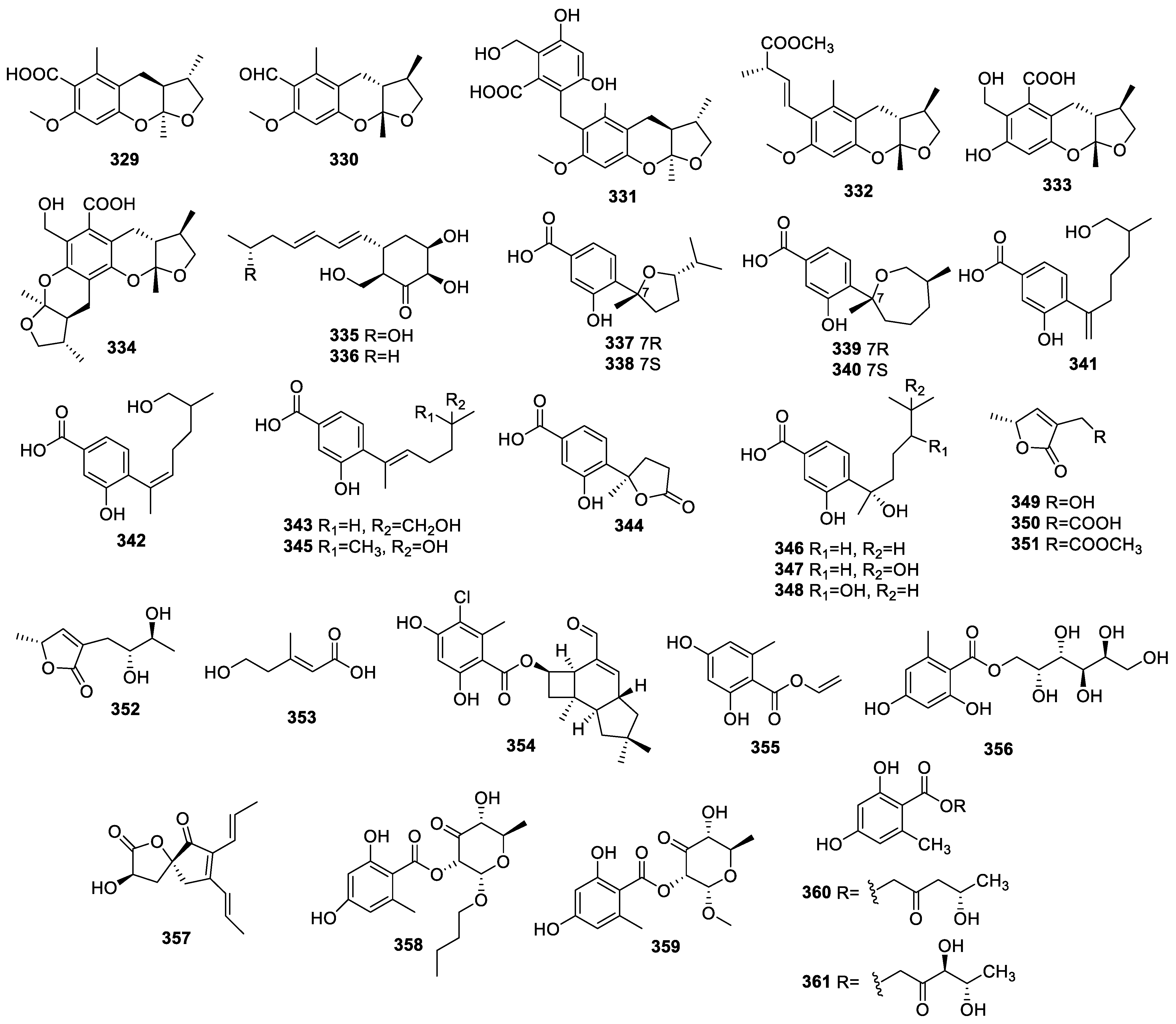
2.3.3. Other Polyketides
2.4. Terpenoids
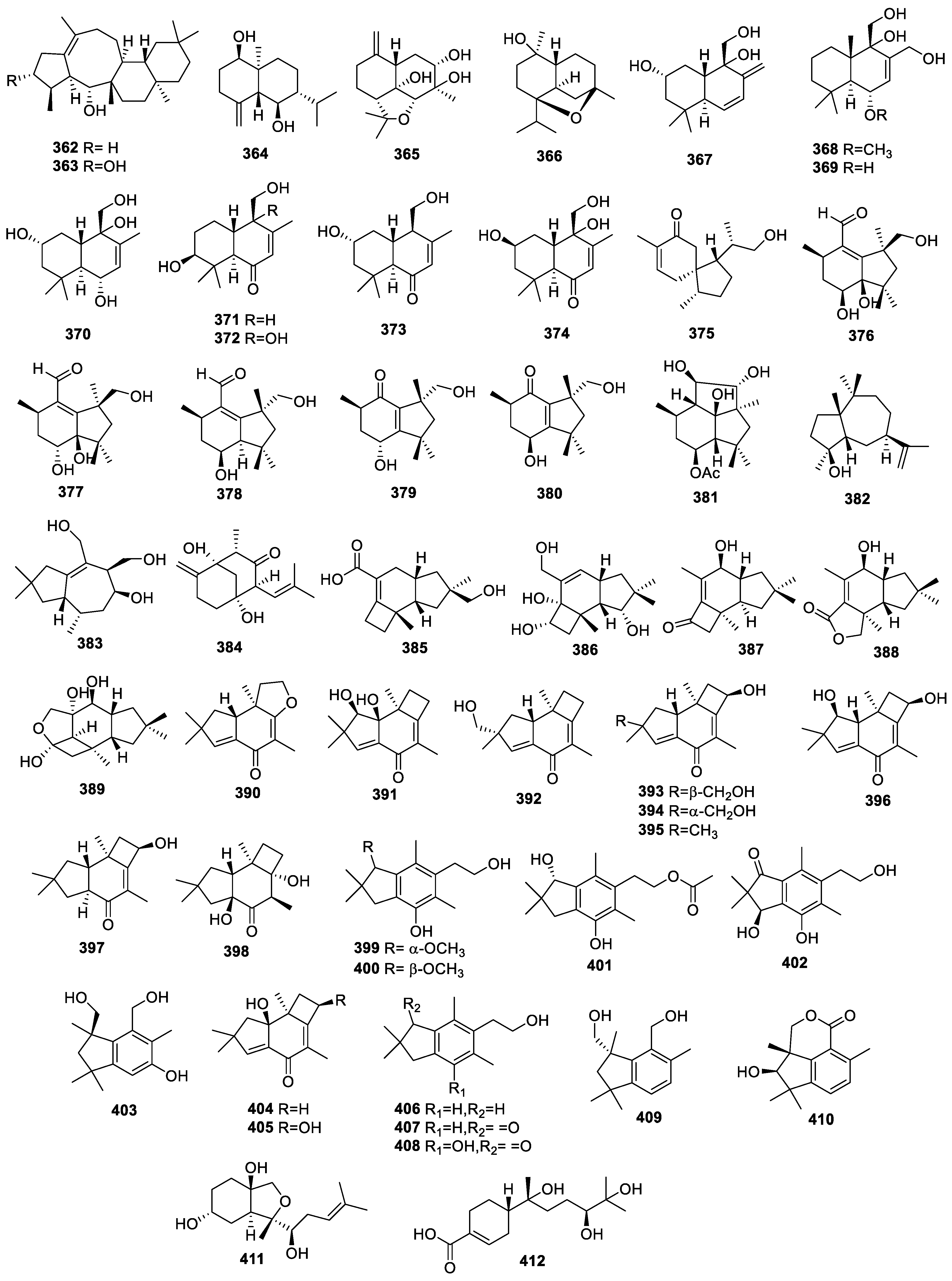
2.4.1. Sesquiterpenoids
2.4.2. Meroterpenoids
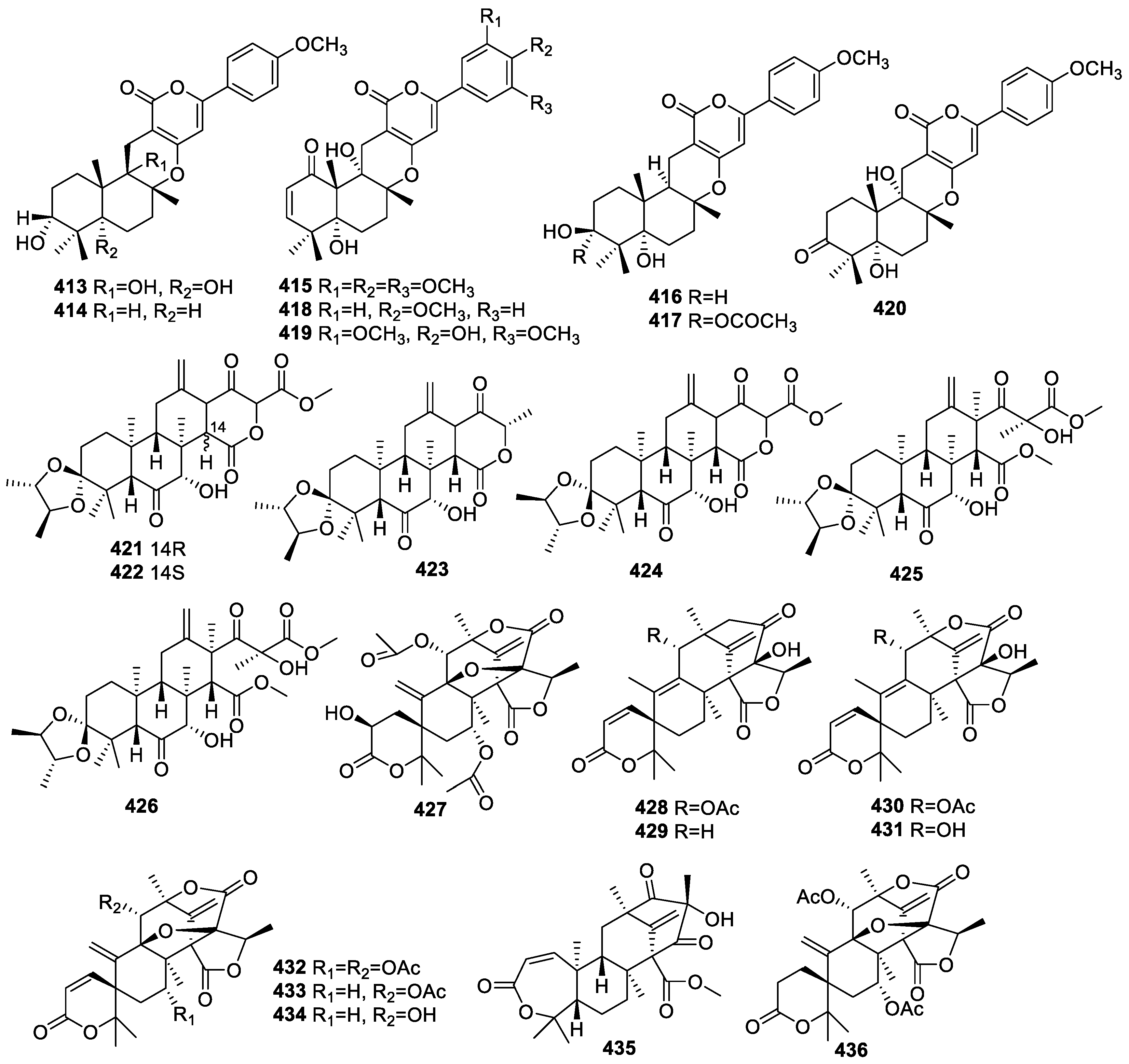
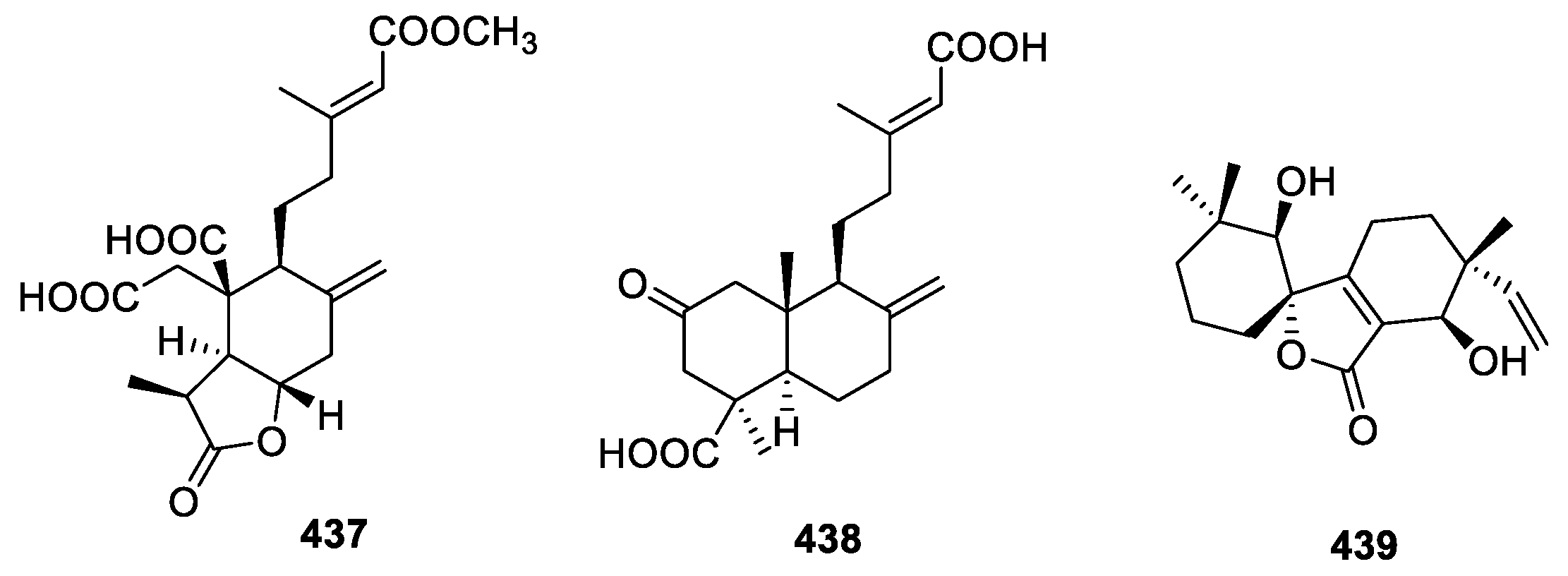
2.4.3. Diterpenoids
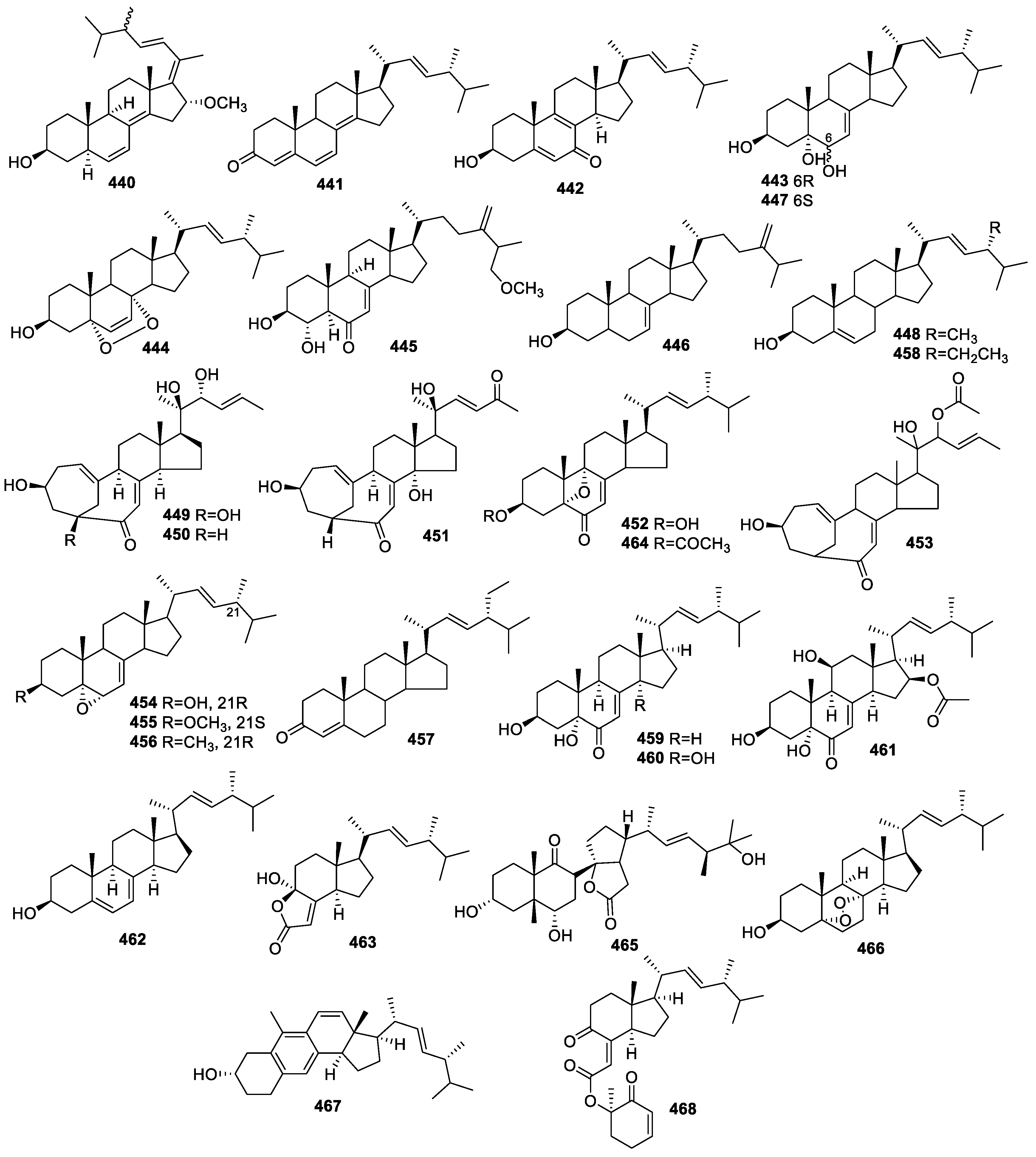
2.5. Steroids
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AChE | Acetylcholinesterase |
| BChE | Butyrylcholinesterase |
| BACE1 | β-site amyloid precursor protein-cleaving enzyme 1 |
| PC12 | Rat pheochromocytoma cells |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | 2,2-Azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) |
| EC50 | Half effective concentration |
| IC50 | Half maximal inhibitory concentration |
| H2O2 | Hydrogen peroxide |
| HT22 | Mouse hippocampal cells |
| JNK | c-Jun N-terminal kinase |
| ERK | Extracellular signal-regulated kinase |
| huAChE-ICER | Immobilized capillary enzyme reactors |
| ROS | Reactive oxygen species |
| MAPK | Mitogen-activated protein kinases |
| ORAC | Oxygen radial absorbance capacity against ROO∙ |
| MPP+ | 1-Methy-4-phenylpyridinium |
References
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [PubMed]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.C. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022, 18, 700–789. [Google Scholar]
- Piemontese, L. New approaches for prevention and treatment of Alzheimer’s disease: A fascinating challenge. Neural Regen. Res. 2017, 12, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer Disease: An Update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, M.N.; Hendrix, S.; Harrison, J.E. FDA position statement Early Alzheimer’s disease: Developing drugs for treatment, Guidance for Industry. Alzheimers Dement. 2019, 5, 13–19. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement. 2019, 5, 272–293. [Google Scholar] [CrossRef] [PubMed]
- Petrini, O.; Fisher, P.J. Occurrence of fungal endophytes in twigs of Salix fragilis and Quercus robur. Mycol. Res. 1990, 94, 1077–1080. [Google Scholar]
- Wen, J.; Okyere, S.K.; Wang, S.; Wang, J.; Xie, L.; Ran, Y.; Hu, Y. Endophytic Fungi: An Effective Alternative Source of Plant-Derived Bioactive Compounds for Pharmacological Studies. J. Fungi 2022, 8, 205. [Google Scholar] [CrossRef]
- Zheng, R.; Li, S.; Zhang, X.; Zhao, C. Biological Activities of Some New Secondary Metabolites Isolated from Endophytic Fungi: A Review Study. Int. J. Mol. Sci. 2021, 22, 959. [Google Scholar] [CrossRef]
- Gakuubi, M.M.; Munusamy, M.; Liang, Z.X.; Ng, S.B. Fungal Endophytes: A Promising Frontier for Discovery of Novel Bioactive Compounds. J. Fungi 2021, 7, 786. [Google Scholar] [CrossRef] [PubMed]
- Ancheeva, E.; Daletos, G.; Proksch, P. Bioactive Secondary Metabolites from Endophytic Fungi. Curr. Med. Chem. 2020, 27, 1836–1854. [Google Scholar] [CrossRef] [PubMed]
- Chapla, V.; Zeraik, M.; Cafeu, M.; Silva, G.; Cavalheiro, A.; Bolzani, V.; Young, M.; Pfenning, L.; Araujo, A. Griseofulvin, Diketopiperazines and Cytochalasins from Endophytic Fungi Colletotrichum crassipes and Xylaria sp., and Their Antifungal, Antioxidant and Anticholinesterase Activities. J. Braz. Chem. Soc. 2018, 29, 1707–1713. [Google Scholar] [CrossRef]
- Ge, H.M.; Peng, H.; Guo, Z.K.; Cui, J.T.; Tan, R.X. Bioactive Alkaloids from the Plant Endophytic Fungus Aspergillus terreus. Planta Med. 2010, 76, 822–824. [Google Scholar] [CrossRef] [PubMed]
- Chapla, V.M.; Zeraik, M.L.; Ximenes, V.F.; Zanardi, L.M.; Lopes, M.N.; Cavalheiro, A.J.; Silva, D.H.; Young, M.C.; Fonseca, L.M.; Bolzani, V.S.; et al. Bioactive secondary metabolites from Phomopsis sp., an endophytic fungus from Senna spectabilis. Molecules 2014, 19, 6597–6608. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Z.; Guo, X.; Huang, J.P.; Yan, Y.; Huang, S.X.; Yang, J. Bisaspochalasins D and E: Two Heterocycle-Fused Cytochalasan Homodimers from an Endophytic Aspergillus flavipes. J. Org. Chem. 2021, 86, 11198–11205. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.; Yang, Y.; Duang, R.; Chen, G.; Li, X.; Li, Q.; Qin, S.; Li, S.; Zhao, L.; et al. Anti-phytopathogen, multi-target acetylcholinesterase inhibitory and antioxidant activities of metabolites from endophytic Chaetomium globosum. Nat. Prod. Res. 2016, 30, 2616–2619. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ju, J.-J.; Liu, Q.; Wang, S.-S.; Meng, H.; Ge, X.-Q.; Huang, W.-Y. Antioxidative and Neuroprotective Effects of the Cytochalasans from Endophytes. Nat. Prod. Commun. 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Sallam, A.; Sabry, M.A.; Galala, A.A. Westalsan: A New Acetylcholine Esterase Inhibitor from the Endophytic Fungus Westerdykella nigra. Chem. Biodivers. 2021, 18, e2000957. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.M.; Meng, L.H.; Konuklugil, B.; Li, X.; Li, H.L.; Wang, B.G. Isolation and characterization of three pairs of indolediketopiperazine enantiomers containing infrequent N-methoxy substitution from the marine algal-derived endophytic fungus Acrostalagmus luteoalbus TK-43. Bioorg. Chem. 2019, 90, 103030. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.M.; Li, X.; Li, H.L.; Konuklugil, B.; Wang, B.G. Uncommon N-Methoxyindolediketopiperazines from Acrostalagmus luteoalbus, a Marine Algal Isolate of Endophytic Fungus. Chin. J. Chem. 2021, 39, 2808–2814. [Google Scholar] [CrossRef]
- Bang, S.; Song, J.H.; Lee, D.; Lee, C.; Kim, S.; Kang, K.S.; Lee, J.H.; Shim, S.H. Neuroprotective Secondary Metabolite Produced by an Endophytic Fungus, Neosartorya fischeri JS0553, Isolated from Glehnia littoralis. J. Agric. Food Chem. 2019, 67, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-H.; Yang, Z.-D.; Shu, Z.-M.; Wang, Y.-G.; Wang, M.-G. Secondary Metabolites and Biological Activities of Talaromyces sp. LGT-2, an Endophytic Fungus from Tripterygium Wilfordii. Iran. J. Pharm. Res. 2016, 15, 453–457. [Google Scholar]
- Huang, D.Y.; Nong, X.H.; Zhang, Y.Q.; Xu, W.; Sun, L.Y.; Zhang, T.; Chen, G.Y.; Han, C.R. Two new 2,5-diketopiperazine derivatives from mangrove-derived endophytic fungus Nigrospora camelliae-sinensis S30. Nat. Prod. Res. 2021, 36, 3651–3656. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Silva, G.H.; Regasini, L.O.; Evangelista, A.H.; Young, M.C.M.; Bolzani, V.S.; Araujo, A.R. Bioactive Metabolites Produced by Penicillium sp.1 and sp.2, Two Endophytes Associated with Alibertia macrophylla (Rubiaceae). Z. Naturforsch. C J. Biosci. 2009, 64, 824–830. [Google Scholar] [CrossRef]
- Chapla, V.M.; Zeraik, M.L.; Leptokarydis, I.H.; Silva, G.H.; Bolzani, V.S.; Young, M.C.; Pfenning, L.H.; Araujo, A.R. Antifungal compounds produced by Colletotrichum gloeosporioides, an endophytic fungus from Michelia champaca. Molecules 2014, 19, 19243–19252. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wen, J.; Zhu, T.; Fang, Y.; Gu, Q.; Zhu, W. Chrysogenamide A from an Endophytic Fungus Associated with Cistanche deserticola and Its Neuroprotective Effect on SH-SY5Y Cells. J. Antibiot. 2008, 61, 81–85. [Google Scholar] [CrossRef]
- Ge, H.M.; Yu, Z.G.; Zhang, J.; Wu, J.H.; Tan, R.X. Bioactive Alkaloids from Endophytic Aspergillus fumigatus. J. Nat. Prod. 2009, 72, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hu, Y.W.; Qu, W.; Chen, M.H.; Zhou, L.S.; Bi, Q.R.; Luo, J.G.; Liu, W.Y.; Feng, F.; Zhang, J. Cytotoxic and neuroprotective activities of constituents from Alternaria alternate, a fungal endophyte of Psidium littorale. Bioorg. Chem. 2019, 90, 103046. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Melong, R.; Tsamo, A.T.; Maffo, T.; Kapche, D.G.W.F.; Ngadjui, B.T.; McGaw, L.J.; Eloff, J.N. Cytotoxicity, antioxidant and antibacterial activity of four compounds produced by an endophytic fungus Epicoccum nigrum associated with Entada abyssinica. Rev. Bras. Farmacogn. 2017, 27, 251–253. [Google Scholar] [CrossRef]
- Bai, Y.; Yi, P.; Zhang, S.; Hu, J.; Pan, H. Novel Antioxidants and alpha-Glycosidase and Protein Tyrosine Phosphatase 1B Inhibitors from an Endophytic Fungus Penicillium brefeldianum F4a. J. Fungi 2021, 7, 913. [Google Scholar] [CrossRef]
- Shao, L.; Wu, P.; Xu, L.; Xue, J.; Li, H.; Wei, X. Colletotryptins A-F, new dimeric tryptophol derivatives from the endophytic fungus Colletotrichum sp. SC1355. Fitoterapia 2020, 141, 104465. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Q.; Gao, Y.Q.; Tang, J.J.; Zhang, A.L.; Gao, J.M. Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J. Agric. Food Chem. 2014, 62, 3584–3590. [Google Scholar] [CrossRef]
- Chen, C.M.; Chen, W.H.; Pang, X.Y.; Liao, S.R.; Wang, J.F.; Lin, X.P.; Yang, B.; Zhou, X.F.; Luo, X.W.; Liu, Y.H. Pyrrolyl 4-quinolone alkaloids from the mangrove endophytic fungus Penicillium steckii SCSIO 41025: Chiral resolution, configurational assignment, and enzyme inhibitory activities. Phytochemistry 2021, 186, 112730. [Google Scholar] [CrossRef]
- Ying, Y.M.; Shan, W.G.; Zhan, Z.J. Biotransformation of huperzine A by a fungal endophyte of Huperzia serrata furnished sesquiterpenoid-alkaloid hybrids. J. Nat. Prod. 2014, 77, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.M.; Liu, S.X.; Huang, C.H.; Pang, J.Y.; Lin, Y.C. Secondary metabolites of a mangrove endophytic fungus Aspergillus terreus (No. GX7-3B) from the South China Sea. Mar. Drugs 2013, 11, 2616–2624. [Google Scholar] [CrossRef]
- Xiao, W.J.; Chen, H.Q.; Wang, H.; Cai, C.H.; Mei, W.L.; Dai, H.F. New secondary metabolites from the endophytic fungus Fusarium sp. HP-2 isolated from “Qi-Nan” agarwood. Fitoterapia 2018, 130, 180–183. [Google Scholar] [CrossRef]
- Li, W.S.; Hu, H.B.; Huang, Z.H.; Yan, R.J.; Tian, L.W.; Wu, J. Phomopsols A and B from the Mangrove Endophytic Fungus Phomopsis sp. xy21: Structures, Neuroprotective Effects, and Biogenetic Relationships. Org. Lett. 2019, 21, 7919–7922. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Baek, J.Y.; Kim, G.J.; Kim, J.; Kim, S.; Deyrup, S.T.; Choi, H.; Kang, K.S.; Shim, S.H. Azaphilones from an Endophytic Penicillium sp. Prevent Neuronal Cell Death via Inhibition of MAPKs and Reduction of Bax/Bcl-2 Ratio. J. Nat. Prod. 2021, 84, 2226–2237. [Google Scholar] [CrossRef]
- Dai, Y.; Li, K.; She, J.; Zeng, Y.; Wang, H.; Liao, S.; Lin, X.; Yang, B.; Wang, J.; Tao, H.; et al. Lipopeptide Epimers and a Phthalide Glycerol Ether with AChE Inhibitory Activities from the Marine-Derived Fungus Cochliobolus Lunatus SCSIO41401. Mar. Drugs 2020, 18, 547. [Google Scholar] [PubMed]
- Wang, A.; Zhao, S.; Gu, G.; Xu, D.; Zhang, X.; Lai, D.; Zhou, L. Rhizovagine A, an unusual dibenzo-alpha-pyrone alkaloid from the endophytic fungus Rhizopycnis vagum Nitaf22. RSC Adv. 2020, 10, 27894–27898. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Lee, C.; Kim, S.; Song, J.H.; Kang, K.S.; Deyrup, S.T.; Nam, S.J.; Xia, X.; Shim, S.H. Neuroprotective Glycosylated Cyclic Lipodepsipeptides, Colletotrichamides A-E, from a Halophyte-Associated Fungus, Colletotrichum gloeosporioides JS419. J. Org. Chem. 2019, 84, 10999–11006. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Waqas, M.; Kang, S.M.; Al-Rawahi, A.; Lee, I.J. Sorokiniol: A new enzymes inhibitory metabolite from fungal endophyte Bipolaris sorokiniana LK12. BMC Microbiol. 2016, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Tousif, M.I.; Riaz, N.; Ahmed, I.; Schulz, B.; Ashraf, M.; Nasar, R.; Pescitelli, G.; Hussain, H.; Jabbar, A.; et al. Cryptosporioptide: A bioactive polyketide produced by an endophytic fungus Cryptosporiopsis sp. Phytochemistry 2013, 93, 199–202. [Google Scholar] [CrossRef]
- Wang, M.; Sun, M.; Hao, H.; Lu, C. Avertoxins A-D, Prenyl Asteltoxin Derivatives from Aspergillus versicolor Y10, an Endophytic Fungus of Huperzia serrata. J. Nat. Prod. 2015, 78, 3067–3070. [Google Scholar] [CrossRef]
- Wang, P.; Cui, Y.; Cai, C.H.; Kong, F.D.; Chen, H.Q.; Zhou, L.M.; Song, X.M.; Mei, W.L.; Dai, H.F. A new cytochalasin derivative from the mangrove-derived endophytic fungus Xylaria sp. HNWSW-2. J. Asian Nat. Prod. Res. 2018, 20, 1002–1007. [Google Scholar]
- Khan, A.L.; Ali, L.; Hussain, J.; Rizvi, T.S.; Al-Harrasi, A.; Lee, I.J. Enzyme Inhibitory Radicinol Derivative from Endophytic fungus Bipolaris sorokiniana LK12, Associated with Rhazya stricta. Molecules 2015, 20, 12198–12208. [Google Scholar] [CrossRef]
- Lai, D.; Li, J.; Zhao, S.; Gu, G.; Gong, X.; Proksch, P.; Zhou, L. Chromone and isocoumarin derivatives from the endophytic fungus Xylomelasma sp. Samif07, and their antibacterial and antioxidant activities. Nat. Prod. Res. 2021, 35, 4616–4620. [Google Scholar] [CrossRef]
- Xiao, Y.; Liang, W.; Zhang, Z.; Wang, Y.; Zhang, S.; Liu, J.; Chang, J.; Ji, C.; Zhu, D. Polyketide Derivatives from the Endophytic Fungus Phaeosphaeria sp. LF5 Isolated from Huperzia serrata and Their Acetylcholinesterase Inhibitory Activities. J. Fungi 2022, 8, 232. [Google Scholar] [CrossRef]
- Meng, X.; Mao, Z.; Lou, J.; Xu, L.; Zhong, L.; Peng, Y.; Zhou, L.; Wang, M. Benzopyranones from the endophytic fungus Hyalodendriella sp. Ponipodef12 and their bioactivities. Molecules 2012, 17, 11303–11314. [Google Scholar] [CrossRef]
- Mao, Z.; Lai, D.; Liu, X.; Fu, X.; Meng, J.; Wang, A.; Wang, X.; Sun, W.; Liu, Z.L.; Zhou, L.; et al. Dibenzo-alpha-pyrones: A new class of larvicidal metabolites against Aedes aegypti from the endophytic fungus Hyalodendriella sp. Ponipodef12. Pest Manag. Sci. 2017, 73, 1478–1485. [Google Scholar] [CrossRef]
- Hormazabala, E.; Schmeda-Hirschmann, G.; Astudilloa, L.; Rodrı’guezb, J.; Theodulozb, C. Metabolites from Microsphaeropsis olivacea, an Endophytic Fungus of Pilgerodendron uviferum. Z. Naturforsch. C. J. Biosci. 2005, 60, 11–21. [Google Scholar] [CrossRef]
- Tianpanich, K.; Prachya, S.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Radical Scavenging and Antioxidant Activities of Isocoumarins and a Phthalide from the Endophytic Fungus Colletotrichum sp. J. Nat. Prod. 2011, 74, 79–81. [Google Scholar] [CrossRef]
- Huang, M.; Li, J.; Liu, L.; Yin, S.; Wang, J.; Lin, Y. Phomopsichin A–D; Four New Chromone Derivatives from Mangrove Endophytic Fungus Phomopsis sp. 33. Mar. Drugs 2016, 14, 215. [Google Scholar] [CrossRef]
- Liua, J.-F.; Chen, W.-J.; Xin, B.-R.; Lu, J. Metabolites of the Endophytic Fungus Penicillium sp. FJ-1 of Acanthus ilicifolius. Nat. Prod. Commun. 2014, 9, 799–801. [Google Scholar] [CrossRef]
- Yang, C.L.; Wu, H.M.; Liu, C.L.; Zhang, X.; Guo, Z.K.; Chen, Y.; Liu, F.; Liang, Y.; Jiao, R.H.; Tan, R.X.; et al. Bialternacins A–F, Aromatic Polyketide Dimers from an Endophytic Alternaria sp. J. Nat. Prod. 2019, 82, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Feng, X.; Xiao, Z.; Liu, L.; Li, H.; Ma, L.; Lu, Y.; Ju, J.; She, Z.; Lin, Y. Azaphilones and p-terphenyls from the mangrove endophytic fungus Penicillium chermesinum (ZH4-E2) isolated from the South China Sea. J. Nat. Prod. 2011, 74, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Chapla, V.M.; Honório, A.E.; Gubiani, J.R.; Vilela, A.F.L.; Young, M.C.M.; Cardoso, C.L.; Pavan, F.R.; Cicarelli, R.M.; Michel Pinheiro Ferreira, P.; Bolzani, V.d.S.; et al. Acetylcholinesterase inhibition and antifungal activity of cyclohexanoids from the endophytic fungus Saccharicola sp. Phytochem. Lett. 2020, 39, 116–123. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, C.; Lee, D.; Kim, S.; Bang, S.; Shin, M.S.; Lee, J.; Kang, K.S.; Shim, S.H. Neuroprotective Compound from an Endophytic Fungus, Colletotrichum sp. JS-0367. J. Nat. Prod. 2018, 81, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, N.; Zhao, P.J. Acetylcholinesterase inhibitory active metabolites from the endophytic fungus Colletotrichum sp. YMF432. Nat. Prod. Res. 2019, 33, 1794–1797. [Google Scholar] [CrossRef]
- Li, H.L.; Li, X.M.; Li, X.; Wang, C.Y.; Liu, H.; Kassack, M.U.; Meng, L.H.; Wang, B.G. Antioxidant Hydroanthraquinones from the Marine Algal-Derived Endophytic Fungus Talaromyces islandicus EN-501. J. Nat. Prod. 2017, 80, 162–168. [Google Scholar] [CrossRef]
- Lei, H.M.; Ma, N.; Wang, T.; Zhao, P.J. Metabolites from the endophytic fungus Colletotrichum sp. F168. Nat. Prod. Res. 2021, 35, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Chomcheon, P.; Wiyakrutta, S.; Sriubolmas, N.; Ngamrojanavanich, N.; Kengtong, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Aromatase inhibitory, radical scavenging, and antioxidant activities of depsidones and diaryl ethers from the endophytic fungus Corynespora cassiicola L36. Phytochemistry 2009, 70, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Z.; Qiao, F.; Xu, G.-W.; Zhao, J.; Teng, J.-F.; Li, C.; Deng, W.-J. Neuroprotective metabolites from the endophytic fungus Penicillium citrinum of the mangrove Bruguiera gymnorrhiza. Phytochem. Lett. 2015, 12, 148–152. [Google Scholar] [CrossRef]
- Strobela, G.; Forda, E.; Woraponga, J.; Harperb, J.K.; Arifb, A.M.; Grantb, D.M.; Fungc, P.C.W.; Chaud, R.M.W. Isopestacin, an isobenzofuranone from Pestalotiopsis microspora, possessing antifungal and antioxidant activities. Phytochemistry 2002, 60, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Alurappa, R.; Bojegowda, M.R.; Kumar, V.; Mallesh, N.K.; Chowdappa, S. Characterisation and bioactivity of oosporein produced by endophytic fungus Cochliobolus kusanoi isolated from Nerium oleander L. Nat. Prod. Res. 2014, 28, 2217–2220. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Wei, Q.; Chen, G.; Cai, J.; She, Z. Chemical Constituents from the Mangrove Endophytic Fungus Sporothrix sp. Chem. Nat. Compd. 2013, 49, 137–140. [Google Scholar] [CrossRef]
- Wen, L.; Cai, X.; Xu, F.; She, Z.; Chan, W.L.; Vrijmoed, L.L.P.; Jones, E.B.G.; Lin, Y. Three Metabolites from the Mangrove Endophytic Fungus Sporothrix sp. (#4335) from the South China Sea. J. Org. Chem 2009, 74, 1093–1098. [Google Scholar]
- Tan, Q.; Yan, X.; Lin, X.; Huang, Y.; Zheng, Z.; Song, S.; Lu, C.; Shen, Y. Chemical Constituents of the Endophytic Fungal Strain Phomopsis sp. NXZ-05 of Camptotheca acuminata. Helv. Chim. Acta 2007, 90, 1811–1817. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Li, X.M.; Li, X.; Yang, S.Q.; Meng, L.H.; Wang, B.G. Polyketides from the Mangrove-Derived Endophytic Fungus Cladosporium cladosporioides. Mar. Drugs 2019, 17, 296. [Google Scholar] [CrossRef]
- Qiao, M.F.; Ji, N.Y.; Miao, F.P.; Yin, X.L. Steroids and an oxylipin from an algicolous isolate of Aspergillus flavus. Magn. Reson. Chem. 2011, 49, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Kaaniche, F.; Hamed, A.; Abdel-Razek, A.S.; Wibberg, D.; Abdissa, N.; El Euch, I.Z.; Allouche, N.; Mellouli, L.; Shaaban, M.; Sewald, N. Bioactive secondary metabolites from new endophytic fungus Curvularia sp. isolated from Rauwolfia macrophylla. PLoS ONE 2019, 14, e0217627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Yang, Z.D.; Yang, X.; Yang, L.J.; Yao, X.J.; Shu, Z.M. Two new compounds, Talaromycin A and B, isolated from an endophytic fungus, Talaromyces aurantiacus. Nat. Prod. Res. 2020, 34, 2802–2808. [Google Scholar] [CrossRef]
- Polli, A.D.; Ribeiro, M.; Garcia, A.; Polonio, J.C.; Santos, C.M.; Silva, A.A.; Orlandelli, R.C.; Castro, J.C.; Abreu-Filho, B.A.; Cabral, M.R.P.; et al. Secondary metabolites of Curvularia sp. G6-32, an endophyte of Sapindus saponaria, with antioxidant and anticholinesterasic properties. Nat. Prod. Res. 2021, 35, 4148–4153. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yan, S.; Liu, Y.; Zhang, X.; Cao, F.; He, Y.; Li, F.; Liu, J.; Wang, J.; Hu, Z.; et al. New secondary metabolites with immunosuppressive and BChE inhibitory activities from an endophytic fungus Daldinia sp. TJ403-LS1. Bioorg. Chem. 2021, 114, 105091. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Li, L.Y.; Shang, H.; Liu, Y.; Yu, M.; Ding, G.; Zou, Z.M. Trematosphones A and B, Two Unique Dimeric Structures from the Desert Plant Endophytic Fungus Trematosphaeria terricola. Org. Lett. 2019, 21, 2139–2142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, Y.; Hu, Y.; Lv, X.; Shi, Z.; Yu, Y.; Jiang, X.; Feng, F.; Xu, J. Neuroprotective Activities of Constituents from Phyllosticta capitalensis, an Endophyte Fungus of Loropetalum chinense var. rubrum. Chem. Biodivers. 2021, 18, e2100314. [Google Scholar] [CrossRef]
- Sang, X.-N.; Chen, S.-F.; Chen, G.; An, X.; Li, S.-G.; Li, X.-N.; Lin, B.; Bai, J.; Wang, H.-F.; Pei, Y.-H. Phomeketales A–F, six unique metabolites from the endophytic fungus Phoma sp. YN02-P-3. RSC Adv. 2016, 6, 64890–64894. [Google Scholar] [CrossRef]
- Huang, X.-S.; Yang, B.; Sun, X.-F.; Xia, G.-P.; Liu, Y.-Y.; Ma, L.; She, Z.-G. A New Polyketide from the Mangrove Endophytic Fungus Penicillium sp. sk14JW2P. Helv. Chim. Acta 2014, 97, 664–668. [Google Scholar] [CrossRef]
- Wang, P.; Yu, J.H.; Zhu, K.; Wang, Y.; Cheng, Z.Q.; Jiang, C.S.; Dai, J.G.; Wu, J.; Zhang, H. Phenolic bisabolane sesquiterpenoids from a Thai mangrove endophytic fungus, Aspergillus sp. xy02. Fitoterapia 2018, 127, 322–327. [Google Scholar] [CrossRef]
- Li, H.T.; Tang, L.H.; Liu, T.; Yang, R.N.; Yang, Y.B.; Zhou, H.; Ding, Z.T. Protoilludane-type sesquiterpenoids from Armillaria sp. by co-culture with the endophytic fungus Epicoccum sp. associated with Gastrodia elata. Bioorg. Chem. 2020, 95, 103503. [Google Scholar] [CrossRef]
- Xu, Q.L.; Xiao, Y.S.; Shen, Y.; Wu, H.M.; Zhang, X.; Deng, X.Z.; Wang, T.T.; Li, W.; Tan, R.X.; Jiao, R.H.; et al. Novel chaetospirolactone and orsellide F from an endophytic fungus Chaetomium sp. J. Asian Nat. Prod. Res. 2018, 20, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Huang, H.; Shao, C.; Xia, X.; Ma, L.; Huang, X.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenols A and B, New Sesterterpenoids Isolated from a Mangrove Endophytic Fungus Aspergillus sp. 085242. Org. Lett. 2013, 15, 2522–2525. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhou, Q.; Li, X.Q.; Luo, T.; Yuan, X.L.; Zhang, Z.F.; Zhang, P. Cadinane- and drimane-type sesquiterpenoids produced by Paecilomyces sp. TE-540, an endophyte from Nicotiana tabacum L., are acetylcholinesterase inhibitors. Bioorg. Chem. 2020, 104, 104252. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Su, X.; Yan, W.; Wu, M.; Wu, Y.; Lu, J.; He, X.; Ding, X.; Xue, Y. Acorenone C: A New Spiro-Sesquiterpene from a Mangrove-Associated Fungus, Pseudofusicoccum sp. J003. Front. Chem. 2021, 9, 780304. [Google Scholar] [CrossRef]
- Medina, R.P.; Araujo, A.R.; Batista, J.M., Jr.; Cardoso, C.L.; Seidl, C.; Vilela, A.F.L.; Domingos, H.V.; Costa-Lotufo, L.V.; Andersen, R.J.; Silva, D.H.S. Botryane terpenoids produced by Nemania bipapillata, an endophytic fungus isolated from red alga Asparagopsis taxiformis—Falkenbergia stage. Sci. Rep. 2019, 9, 12318. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Yang, X.Q.; Zhang, Z.X.; Wang, B.Y.; Hu, M.; Yang, Y.B.; Zhou, H.; Ding, Z.T. New azaphilones and tremulane sesquiterpene from endophytic Nigrospora oryzae cocultured with Irpex lacteus. Fitoterapia 2018, 130, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Yang, Z.D.; Sun, J.H.; Song, T.T.; Zhu, B.Y.; Zhao, J.W. Colletotrichine A, a new sesquiterpenoid from Colletotrichum gloeosporioides GT-7, a fungal endophyte of Uncaria rhynchophylla. Nat. Prod. Res. 2018, 32, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wu, Y.; Qiao, Y.; Guo, Y.; Wang, J.; Hu, Z.; Zhang, Q.; Li, X.; Huang, J.; Zhou, Q.; et al. Protoilludane, Illudalane, and Botryane Sesquiterpenoids from the Endophytic Fungus Phomopsis sp. TJ507A. J. Nat. Prod. 2018, 81, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Yang, Z.D.; Li, X.F.; Sun, J.H.; Yang, L.J.; Zhang, X.G. Colletotrichine B, a new sesquiterpenoid from Colletotrichum gloeosporioides GT-7, a fungal endophyte of Uncaria rhynchophylla. Nat. Prod. Res. 2019, 33, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-L.; Dai, Y.; She, J.-L.; Zeng, Y.-B.; Dai, H.-F.; Ou, S.-L.; Zhou, X.-F.; Liu, Y.-H. Bisabolanoic acid A, a new polychiral sesquiterpene with AChE inhibitory activity from a mangrove-derived fungus Colletotrichum sp. J. Asian Nat. Prod. Res. 2021, 24, 88–95. [Google Scholar] [CrossRef]
- Huang, X.; Sun, X.; Ding, B.; Lin, M.; Liu, L.; Huang, H.; She, Z. A New Anti-acetylcholinesterase α-Pyrone Meroterpene, Arigsugacin I, from Mangrove Endophytic Fungus Penicillium sp. sk5GW1L of Kandelia candel. Planta Med. 2013, 79, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wang, Z.; Huang, X.; Liu, Y.; Chen, W.; She, Z. Bioactive alpha-pyrone meroterpenoids from mangrove endophytic fungus Penicillium sp. Nat. Prod. Res. 2016, 30, 2805–2812. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Zhou, Q.; Gao, W.; Liu, M.; Chen, C.; Li, X.N.; Lai, Y.; Zhou, Y.; Li, D.; Hu, Z.; et al. Anti-BACE1 and anti-AchE activities of undescribed spiro-dioxolane-containing meroterpenoids from the endophytic fungus Aspergillus terreus Thom. Phytochemistry 2019, 165, 112041. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Cui, H.; Liu, X.; Xiao, Z.; Wen, S.; She, Z.; Huang, X. Acetylcholinesterase Inhibitory Meroterpenoid from a Mangrove Endophytic Fungus Aspergillus sp. 16-5c. Molecules 2017, 22, 727. [Google Scholar] [CrossRef]
- Qi, B.; Jia, F.; Luo, Y.; Ding, N.; Li, S.; Shi, F.; Hai, Y.; Wang, L.; Zhu, Z.X.; Liu, X.; et al. Two new diterpenoids from Penicillium chrysogenum MT-12, an endophytic fungus isolated from Huperzia serrata. Nat. Prod. Res. 2022, 36, 814–821. [Google Scholar] [CrossRef]
- Guo, Z.K.; Wang, R.; Huang, W.; Li, X.N.; Jiang, R.; Tan, R.X.; Ge, H.M. Aspergiloid I, an unprecedented spirolactone norditerpenoid from the plant-derived endophytic fungus Aspergillus sp. YXf3. Beilstein J. Org. Chem. 2014, 10, 2677–2682. [Google Scholar] [CrossRef]
- Qiao, M.-F.; Ji, N.-Y.; Liu, X.-H.; Li, F.; Xue, Q.-Z. Asporyergosterol, A New Steroid from an Algicolous Isolate of Aspergillus oryzae. Nat. Prod. Commun. 2010, 5, 1575–1578. [Google Scholar] [CrossRef]
- Yu, F.X.; Li, Z.; Chen, Y.; Yang, Y.H.; Li, G.H.; Zhao, P.J. Four new steroids from the endophytic fungus Chaetomium sp. M453 derived of Chinese herbal medicine Huperzia serrata. Fitoterapia 2017, 117, 41–46. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Fang, T.; Wu, L.; Liu, W.; Tang, J.; Long, Y. New Steroid and Isocoumarin from the Mangrove Endophytic Fungus Talaromyces sp. SCNU-F0041. Molecules 2022, 27, 5766. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Wang, Z.; Song, L.; Fu, W.; Liu, L. Anti-Alzheimer’s Natural Products Derived from Plant Endophytic Fungi. Molecules 2023, 28, 2259. https://doi.org/10.3390/molecules28052259
Zhu J, Wang Z, Song L, Fu W, Liu L. Anti-Alzheimer’s Natural Products Derived from Plant Endophytic Fungi. Molecules. 2023; 28(5):2259. https://doi.org/10.3390/molecules28052259
Chicago/Turabian StyleZhu, Juntai, Zimo Wang, Lixia Song, Wanxin Fu, and Li Liu. 2023. "Anti-Alzheimer’s Natural Products Derived from Plant Endophytic Fungi" Molecules 28, no. 5: 2259. https://doi.org/10.3390/molecules28052259
APA StyleZhu, J., Wang, Z., Song, L., Fu, W., & Liu, L. (2023). Anti-Alzheimer’s Natural Products Derived from Plant Endophytic Fungi. Molecules, 28(5), 2259. https://doi.org/10.3390/molecules28052259





