A Near-Infrared Fluorescent and Photoacoustic Probe for Visualizing Biothiols Dynamics in Tumor and Liver
Abstract
1. Introduction
2. Results and Discussion
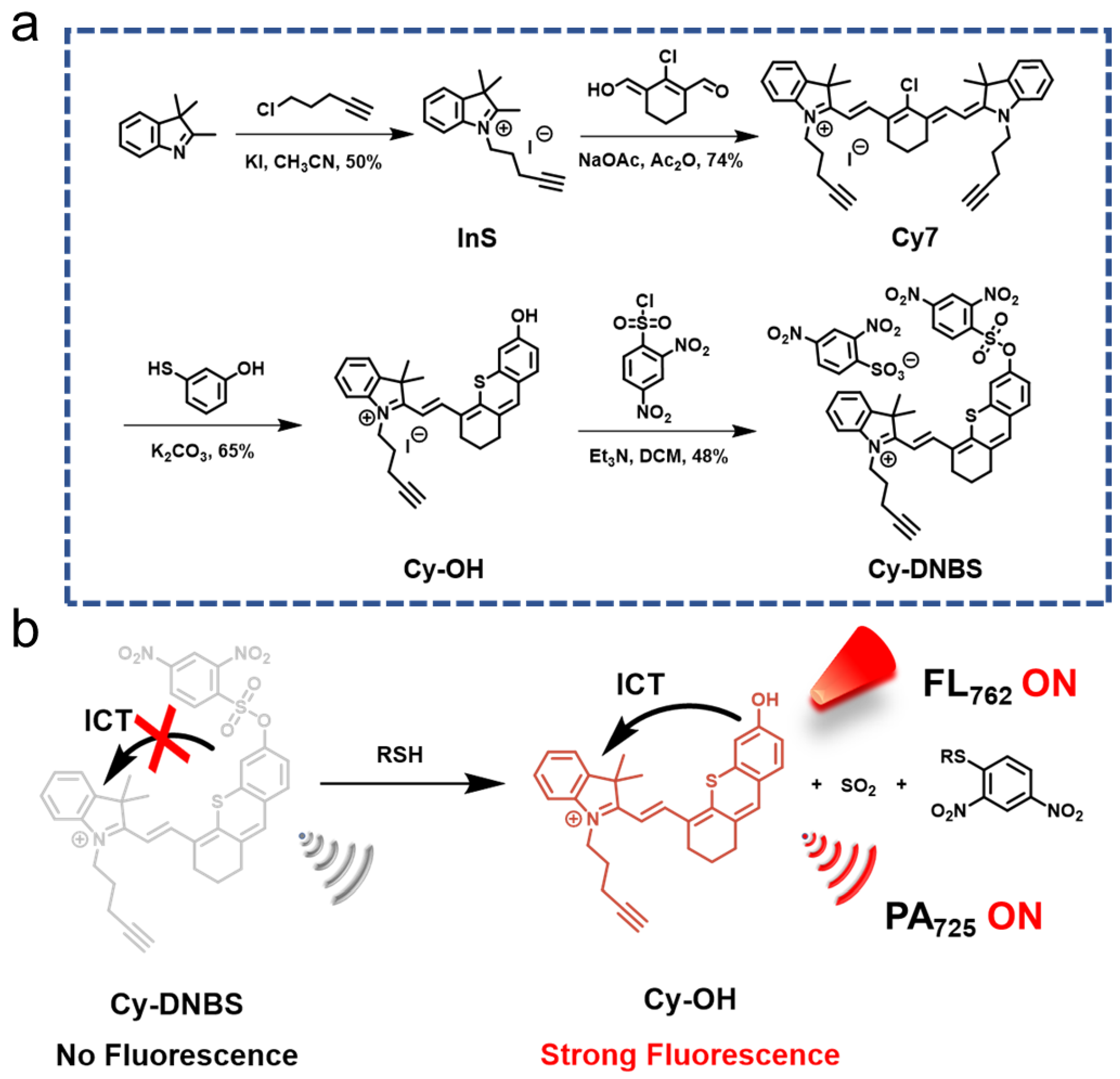
2.1. Design and Synthesis of Cy-DNBS
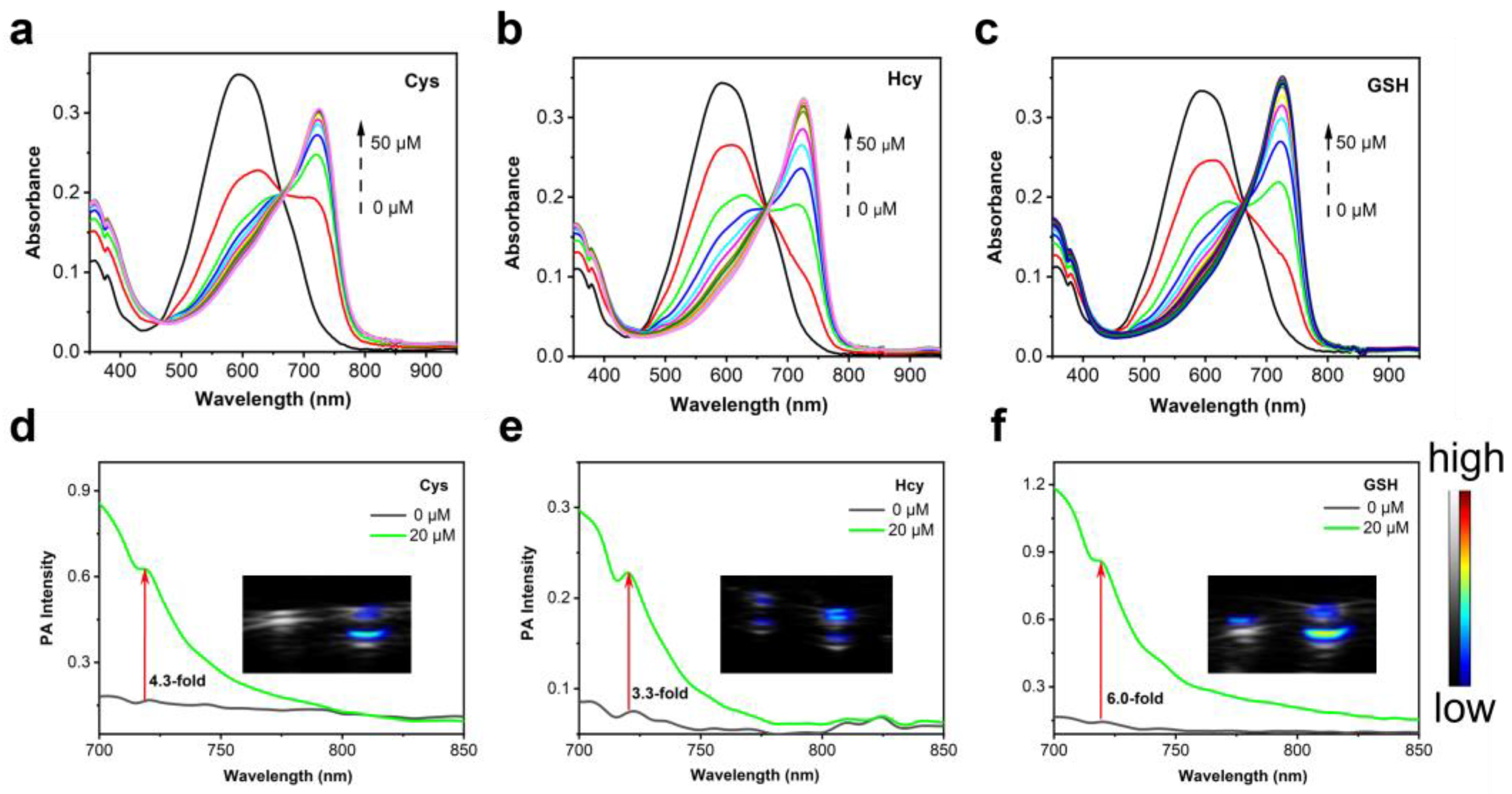
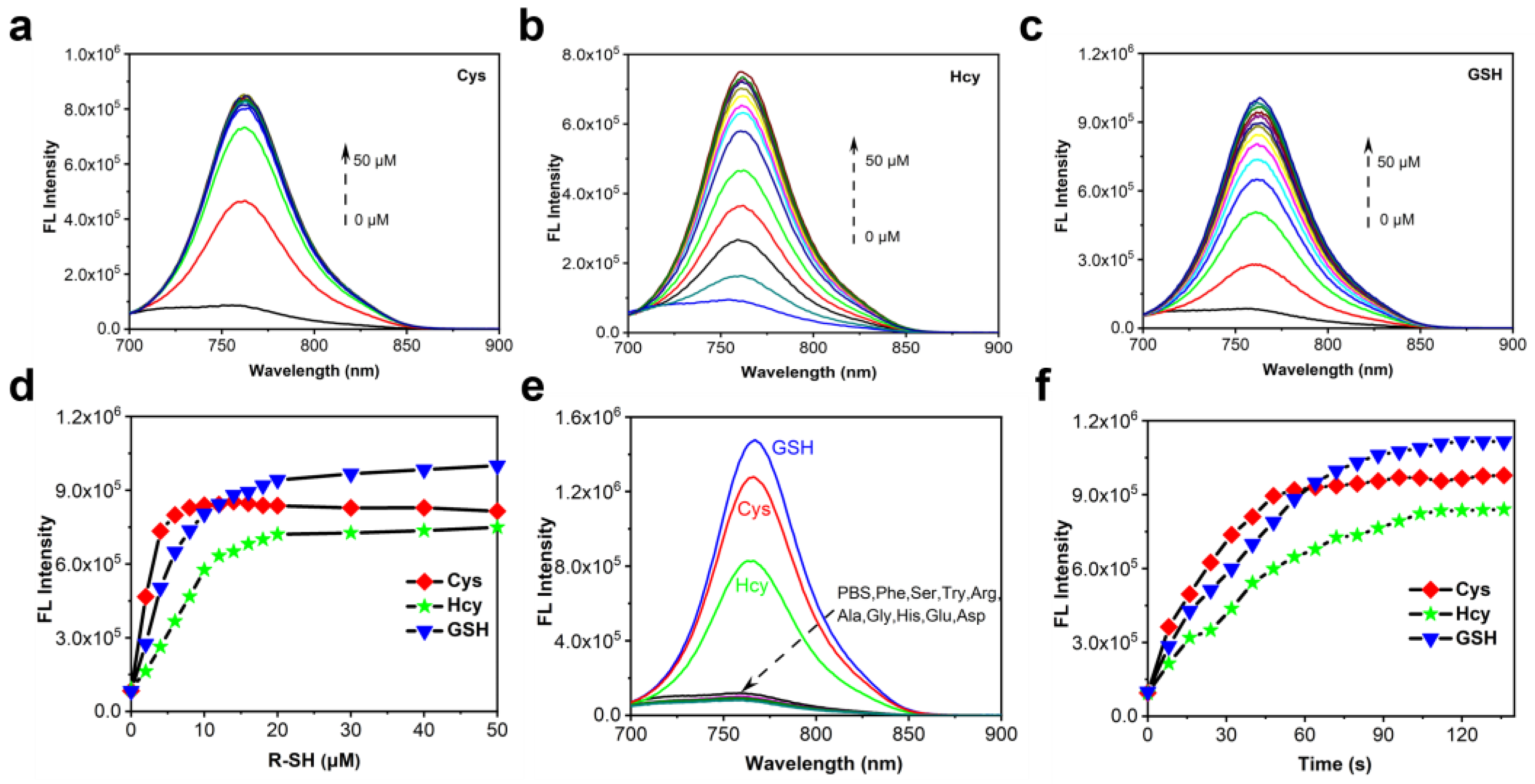
2.2. Optical Properties of Cy-DNBS towards Biothiols
2.3. Intracellular Biothiols Imaging with Cy-DNBS
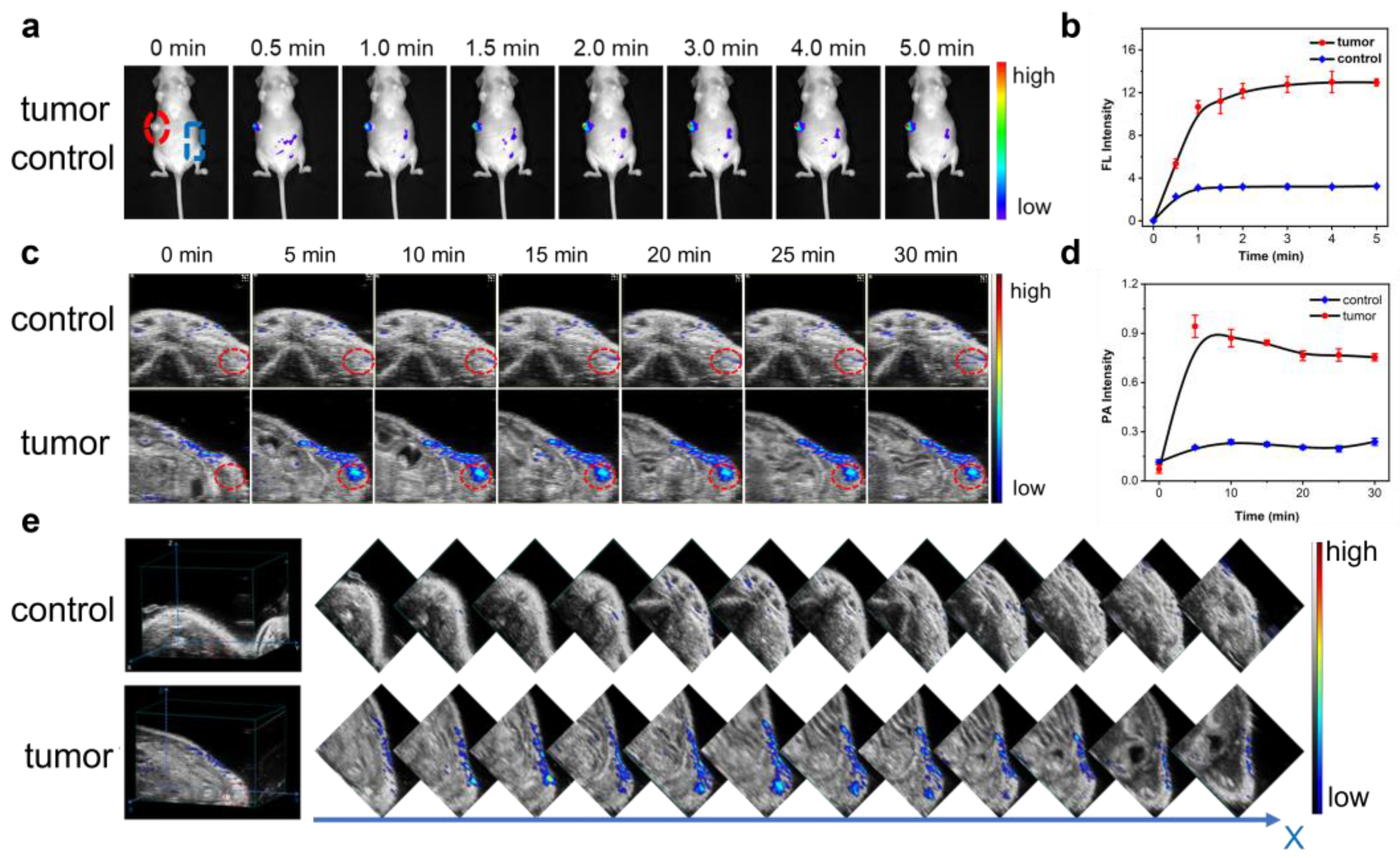
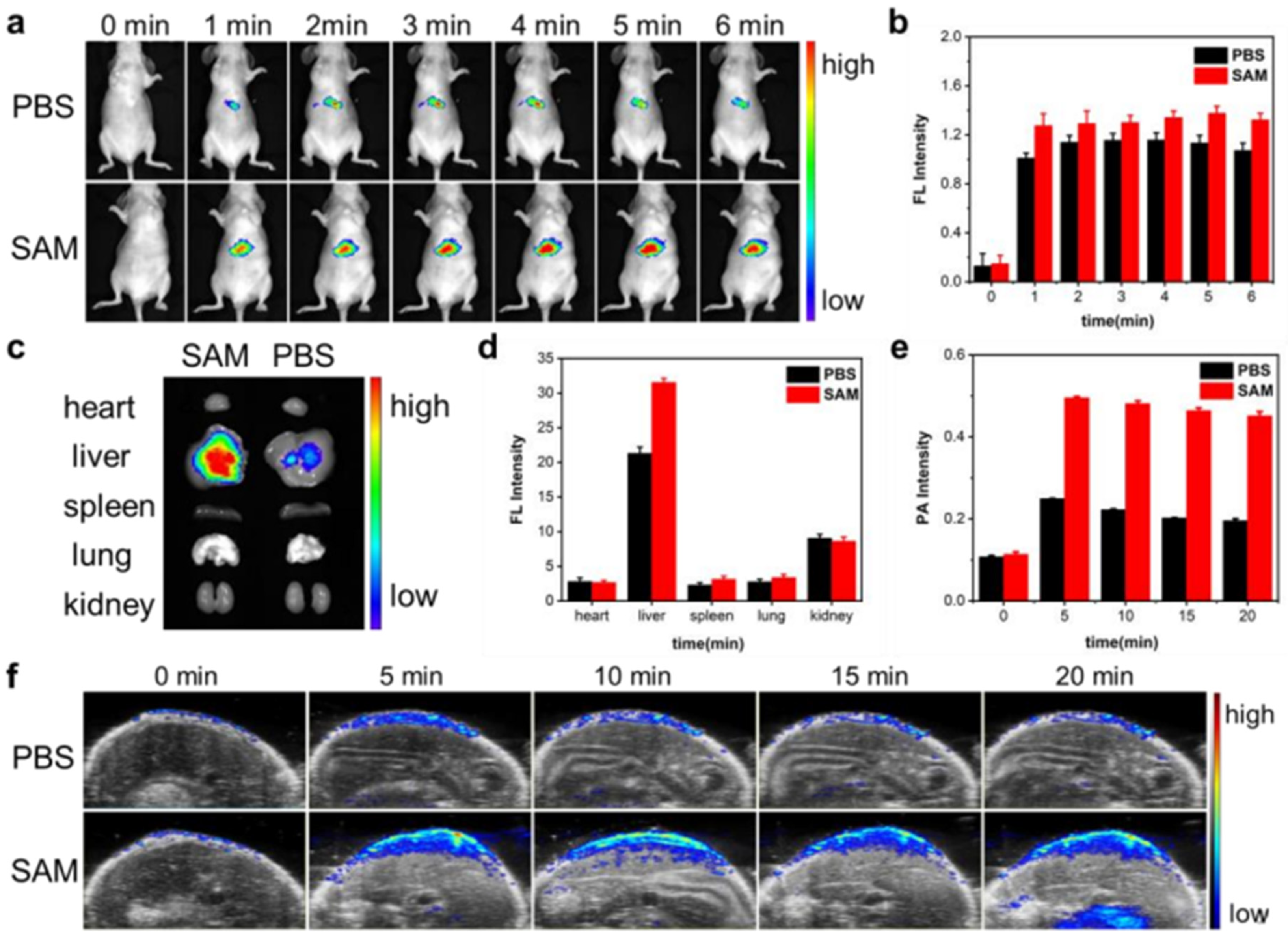
2.4. Dynamically Tracking Biothiols in Tumor by NIRFI and PAI
2.5. Real-Time Visualizing Biothiols in Liver of Drug-Treated Mouse Model
3. Conclusions
4. Materials and Methods
4.1. Synthesis and Characterization
4.1.1. Synthesis of Indolenine Salt (InS)
4.1.2. Synthesis of Cy7
4.1.3. Synthesis of Cy-OH
4.1.4. Synthesis of Cy-DNBS
4.2. Detection Methods
4.2.1. Optical Study
4.2.2. Quantum Yield Calculation
4.2.3. MTT Assay
4.2.4. Cell Imaging
4.2.5. Imaging of Cy-DNBS towards Endogenous and Exogenous Biothiols
4.2.6. In Vivo Fluorescence Imaging
4.2.7. In Vivo Photoacoustic Imaging
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balendiran, G.K.; Dabur, R.; Fraser, D. The role of glutathione in cancer. Cell Biochem. Funct. 2004, 22, 343–352. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Zhang, S.; Ong, C.N.; Shen, H.M. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004, 208, 143–153. [Google Scholar] [CrossRef]
- Ball, R.O.; Courtney-Martin, G.; Pencharz, P.B. The in vivo sparing of methionine by cysteine in sulfur amino acid requirements in animal models and adult humans. J. Nutr. 2006, 136, 1682–1693. [Google Scholar] [CrossRef]
- Jouandin, P.; Marelja, Z.; Shih, Y.H.; Parkhitko, A.A.; Dambowsky, M.; Asara, J.M.; Nemazanyy, I.; Dibble, C.C.; Simons, M.; Perrimon, N. Lysosomal cystine mobilization shapes the response of TORC1 and tissue growth to fasting. Science 2022, 375, eabc4203. [Google Scholar] [CrossRef]
- Ozkan, Y.; Ozkan, E.; Simşek, B. Plasma total homocysteine and cysteine levels as cardiovascular risk factors in coronary heart disease. Int. J. Cardiol. 2002, 82, 269–277. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007, 113, 234–258. [Google Scholar] [CrossRef]
- Hwang, C.; Sinskey, A.J.; Lodish, H.F. Oxidized Redox State of Glutathione in the Endoplasmic Reticulum. Science 1992, 257, 1496–1502. [Google Scholar] [CrossRef]
- Armstrong, J.S.; Steinauer, K.K.; Hornung, B.; Irish, J.M.; Lecane, P.; Birrell, G.W.; Peehl, D.M.; Knox, S.J. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002, 9, 252–263. [Google Scholar] [CrossRef]
- Desideri, E.; Ciccarone, F.; Ciriolo, M.R. Targeting Glutathione Metabolism: Partner in Crime in Anticancer Therapy. Nutrients 2019, 11, 1926. [Google Scholar] [CrossRef]
- Ogiwara, H.; Takahashi, K.; Sasaki, M.; Kuroda, T.; Yoshida, H.; Watanabe, R.; Maruyama, A.; Makinoshima, H.; Chiwaki, F.; Sasaki, H.; et al. Targeting the Vulnerability of Glutathione Metabolism in ARID1A-Deficient Cancers. Cancer Cell 2019, 35, 177–190. [Google Scholar] [CrossRef]
- Sarris, J.; Murphy, J.; Stough, C.; Mischoulon, D.; Bousman, C.; MacDonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; Cribb, L.; et al. S-Adenosylmethionine (SAMe) monotherapy for depression: An 8-week double-blind, randomised, controlled trial. Psychopharmacology 2020, 237, 209–218. [Google Scholar] [CrossRef]
- Williams, A.L.; Girard, C.; Jui, D.; Sabina, A.; Katz, D.L. S-adenosylmethionine (SAMe) as treatment for depression: A systematic review. Clin. Investig. Med. 2005, 28, 132–139. [Google Scholar]
- Sharma, A.; Gerbarg, P.; Bottiglieri, T.; Massoumi, L.; Carpenter, L.L.; Lavretsky, H.; Muskin, P.R.; Brown, R.P.; Mischoulon, D. S-Adenosylmethionine (SAMe) for Neuropsychiatric Disorders: A Clinician-Oriented Review of Research. J. Clin. Psychiatry 2017, 78, e656–e667. [Google Scholar] [CrossRef]
- Di Padova, C. S-adenosylmethionine in the treatment of osteoarthritis. Review of the clinical studies. Am. J. Med. 1987, 83, 60–65. [Google Scholar] [CrossRef]
- Hosea Blewett, H.J. Exploring the mechanisms behind S-adenosylmethionine (SAMe) in the treatment of osteoarthritis. Crit. Rev. Food Sci. Nutr. 2008, 48, 458–463. [Google Scholar] [CrossRef]
- Lu, S.C.; Mato, J.M. S-adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 2012, 92, 1515–1542. [Google Scholar] [CrossRef]
- Mato, J.M.; Lu, S.C. Role of S-adenosyl-L-methionine in liver health and injury. Hepatology 2007, 45, 1306–1312. [Google Scholar] [CrossRef]
- Mato, J.M.; Corrales, F.J.; Lu, S.C.; Avila, M.A. S-Adenosylmethionine: A control switch that regulates liver function. FASEB J. 2002, 16, 15–26. [Google Scholar] [CrossRef]
- Avila, M.A.; Garcia-Trevijano, E.R.; Martinez-Chantar, M.L.; Latasa, M.U.; Perez-Mato, I.; Martinez-Cruz, L.A.; del Pino, M.M.; Corrales, F.J.; Mato, J.M. S-Adenosylmethionine revisited: Its essential role in the regulation of liver function. Alcohol 2002, 27, 163–167. [Google Scholar] [CrossRef]
- Yan, Y.; Yan, Q.; Qian, L.; Jiang, Y.; Chen, X.; Zeng, S.; Xu, Z.; Gong, Z. S-adenosylmethionine administration inhibits levodopa-induced vascular endothelial growth factor-A expression. Aging 2020, 12, 21290–21307. [Google Scholar] [CrossRef]
- Mora, S.I.; Garcia-Roman, J.; Gomez-Nanez, I.; Garcia-Roman, R. Chronic liver diseases and the potential use of S-adenosyl-L-methionine as a hepatoprotector. Eur. J. Gastroen. Hepat. 2018, 30, 893–900. [Google Scholar] [CrossRef]
- Czarnecka, A.; Milewski, K.; Jaźwiec, R.; Zielińska, M. Intracerebral Adminis tration of S-Adenosylhomocysteine or S-Adenosylmethionine Attenuates the Increases in the Cortical Extracellular Levels of Dimethylarginines Without Affecting cGMP Level in Rats with Acute Liver Failure. Neurotox. Res. 2017, 31, 99–108. [Google Scholar] [CrossRef]
- Zeng, Q.; Guo, Q.; Yuan, Y.; Wang, B.; Sui, M.; Lou, X.; Bouchard, L.S.; Zhou, X. Ultrasensitive molecular building block for biothiol NMR detection at picomolar concentrations. iScience 2021, 24, 103515–103531. [Google Scholar] [CrossRef]
- Dega, N.K.; Ganganboina, A.B.; Tran, H.L.; Kuncoro, E.P.; Doong, R.A. BSA-stabilized manganese phosphate nanoflower with enhanced nanozyme activity for highly sensitive and rapid detection of glutathione. Talanta 2022, 237, 122957–122966. [Google Scholar] [CrossRef]
- Li, Y.L.; Jiang, L.; Zou, Y.Q.; Song, Z.B.; Jin, S.Z. Highly reproducible SERS sensor based on self-assembled Au nanocubic monolayer film for sensitive and quantitative detection of glutathione. Appl. Surf. Sci. 2021, 540, 148381–148388. [Google Scholar] [CrossRef]
- Liu, T.; Chen, S.; Ruan, K.; Zhang, S.; He, K.; Li, J.; Chen, M.; Yin, J.; Sun, M.; Wang, X.; et al. A handheld multifunctional smartphone platform integrated with 3D printing portable device: On-site evaluation for glutathione and azodicarbonamide with machine learning. J. Hazard. Mater. 2022, 426, 128091–128102. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, L.; Tian, Y. Highly Stable Electrochemical Probe with Bidentate Thiols for Ratiometric Monitoring of Endogenous Polysulfide in Living Mouse Brains. Anal. Chem. 2022, 94, 1447–1455. [Google Scholar] [CrossRef]
- Yan, D.; Liu, L.; Liu, X.; Liu, Q.; Hou, P.; Wang, H.; Xia, C.; Li, G.; Ma, C.; Chen, S. Simultaneous Discrimination of Cys/Hcy and GSH With Simple Fluorescent Probe Under a Single-Wavelength Excitation and its Application in Living Cells, Tumor Tissues, and Zebrafish. Front. Chem. 2022, 10, 856994–857004. [Google Scholar] [CrossRef]
- Umezawa, K.; Yoshida, M.; Kamiya, M.; Yamasoba, T.; Urano, Y. Rational design of reversible fluorescent probes for live-cell imaging and quantification of fast glutathione dynamics. Nat. Chem. 2017, 9, 279–286. [Google Scholar] [CrossRef]
- Fang, H.; Chen, Y.; Wang, Y.; Geng, S.; Yao, S.; Song, D.; He, W.; Guo, Z. A dual-modal probe for NIR fluorogenic and ratiometric photoacoustic imaging of Cys/Hcy in vivo. Sci. China Chem. 2020, 63, 699–706. [Google Scholar] [CrossRef]
- Guo, R.; Huang, F.; Zhang, B.; Yan, Y.; Che, J.; Jin, Y.; Zhuang, Y.; Dong, R.; Li, Y.; Tan, B.; et al. GSH Activated Biotin-tagged Near-Infrared Probe for Efficient Cancer Imaging. Theranostics 2019, 9, 3515–3525. [Google Scholar] [CrossRef]
- Lim, S.Y.; Hong, K.H.; Kim, D.I.; Kwon, H.; Kim, H.J. Tunable heptamethine-azo dye conjugate as an NIR fluorescent probe for the selective detection of mitochondrial glutathione over cysteine and homocysteine. J. Am. Chem. Soc. 2014, 136, 7018–7025. [Google Scholar] [CrossRef]
- Morozumi, A.; Kamiya, M.; Uno, S.N.; Umezawa, K.; Kojima, R.; Yoshihara, T.; Tobita, S.; Urano, Y. Spontaneously Blinking Fluorophores Based on Nucleophilic Addition/Dissociation of Intracellular Glutathione for Live-Cell Super-resolution Imaging. J. Am. Chem. Soc. 2020, 142, 9625–9633. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yu, F.; Tang, B.; Yang, Z.; Fan, W.; Zhang, M.; Wang, Z.; Jacobson, O.; Zhou, Z.; Li, L.; et al. Tumor Microenvironment-Activated Ultrasensitive Nanoprobes for Specific Detection of Intratumoral Glutathione by Ratiometric Photoacoustic Imaging. ACS Appl. Mater. Interfaces 2019, 11, 27558–27567. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Ji, N.; Zhan, Y.; Tian, Q.Q.; Cai, Z.D.; Lu, X.L.; He, W. Rational design of a new near-infrared fluorophore and apply to the detection and imaging study of cysteine and thiophenol. Anal. Chim. Acta 2021, 1186, 339116–339126. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pu, K. Activatable Molecular Probes for Second Near-Infrared Fluorescence, Chemiluminescence, and Photoacoustic Imaging. Angew. Chem. Int. Ed. 2020, 59, 11717–11731. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, X.; Dai, T.; Wang, H.; Chen, X.; Chen, B.; Sun, T.; Wang, F.; Chu, S.; Rao, J. Reversibly Photoswitching Upconversion Nanoparticles for Super-Sensitive Photoacoustic Molecular Imaging. Angew. Chem. Int. Ed. 2022, 61, e202116802. [Google Scholar]
- Wang, C.; Du, W.; Wu, C.; Dan, S.; Sun, M.; Zhang, T.; Wang, B.; Yuan, Y.; Liang, G. Cathespin B-Initiated Cypate Nanoparticle Formation for Tumor Photoacoustic Imaging. Angew. Chem. Int. Ed. 2022, 61, e202114766. [Google Scholar]
- Qi, J.; Li, J.; Liu, R.; Li, Q.; Zhang, H.; Lam, J.W.Y.; Kwok, R.T.K.; Liu, D.; Ding, D.; Tang, B.Z. Boosting Fluorescence-Photoacoustic-Raman Properties in One Fluorophore for Precise Cancer Surgery. Chem 2019, 5, 2657–2677. [Google Scholar] [CrossRef]
- Ong, S.Y.; Zhang, C.; Dong, X.; Yao, S.Q. Recent Advances in Polymeric Nanoparticles for Enhanced Fluorescence and Photoacoustic Imaging. Angew. Chem. Int. Ed. 2021, 60, 17797–17809. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lai, H.; Guo, H.; Peng, D.; Han, L.; Gu, Y.; Wei, Z.; Zhao, D.; Zheng, N.; Hu, D.; et al. Side-Chain-Tuned Molecular Packing Allows Concurrently Boosted Photoacoustic Imaging and NIR-II Fluorescence. Angew. Chem. Int. Ed. 2022, 61, e202117433. [Google Scholar]
- Zhao, Z.; Swartchick, C.B.; Chan, J. Targeted contrast agents and activatable probes for photoacoustic imaging of cancer. Chem. Soc. Rev. 2022, 51, 829–868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, H.; Wang, L.; Qin, X.; Jiang, B.P.; Ji, S.C.; Shen, X.C.; Liang, H. A General Approach to Design Dual Ratiometric Fluorescent and Photoacoustic Probes for Quantitatively Visualizing Tumor Hypoxia Levels In Vivo. Angew. Chem. Int. Ed. 2022, 61, e202107076. [Google Scholar]
- Li, H.; Kim, H.; Xu, F.; Han, J.; Yao, Q.; Wang, J.; Pu, K.; Peng, X.; Yoon, J. Activity-based NIR fluorescent probes based on the versatile hemicyanine scaffold: Design strategy, biomedical applications, and outlook. Chem. Soc. Rev. 2022, 51, 1795–1835. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liew, S.; Wei, X.; Pu, K. Hemicyanine-Based Near-Infrared Activatable Probes for Imaging and Diagnosis of Diseases. Angew. Chem. Int. Ed. 2021, 60, 26454–26475. [Google Scholar] [CrossRef]
- Qin, Z.; Ren, T.B.; Zhou, H.; Zhang, X.; He, L.; Li, Z.; Zhang, X.B.; Yuan, L. NIRII-HDs: A Versatile Platform for Developing Activatable NIR-II Fluorogenic Probes for Reliable In Vivo Analyte Sensing. Angew. Chem. Int. Ed. 2022, 61, e202201541. [Google Scholar] [CrossRef]
- Chen, D.; Long, Z.; Sun, Y.; Luo, Z.; Lou, X. A red-emission probe for intracellular biothiols imaging with a large Stokes shift. J. Photochem. Photobiol. A Chem. 2019, 368, 90–96. [Google Scholar] [CrossRef]
- Li, B.; Zhang, D.; An, R.; Zhu, Y. A 7-Hydroxybenzoxazinone-Containing Fluorescence Turn-On Probe for Biothiols and Its Bioimaging Applications. Molecules 2019, 24, 3102. [Google Scholar] [CrossRef]
- Lin, X.; Hu, Y.; Yang, D.; Chen, B. Cyanine-coumarin composite NIR dye based instantaneous -response probe for biothiols detection and oxidative stress assessment of mitochondria. Dye. Pigment. 2020, 174, 107956–107963. [Google Scholar] [CrossRef]
- Maeda, H.; Matsuno, H.; Ushida, M.; Katayama, K.; Saeki, K.; Itoh, N. 2,4-Dinitrobenzenesulfonyl fluoresceins as fluorescent alternatives to Ellman’s reagent in thiol-quantification enzyme assays. Angew. Chem. Int. Ed. 2005, 44, 2922–2925. [Google Scholar] [CrossRef]
- Calabrese, G.; Morgan, B.; Riemer, J. Mitochondrial Glutathione: Regulation and Functions. Antioxid. Redox Signal. 2017, 27, 1162–1177. [Google Scholar] [CrossRef]
- Hastings, K.T.; Cresswell, P. Disulfide reduction in the endocytic pathway: Immunological functions of gamma-interferon-inducible lysosomal thiol reductase. Antioxid. Redox Signal. 2011, 15, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mu, X.; Han, Z.; Yang, S.; Zhang, C.; Guo, Z.; Bai, Y.; He, W. An Optical/Photoacoustic Dual-Modality Probe: Ratiometric in/ex Vivo Imaging for Stimulated H2S Upregulation in Mice. J. Am. Chem. Soc. 2019, 141, 17973–17977. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Yao, S.; Chen, Y.; Wu, Y.; Li, Y.; He, W.; Guo, Z. A Near-Infrared Fluorescent and Photoacoustic Probe for Visualizing Biothiols Dynamics in Tumor and Liver. Molecules 2023, 28, 2229. https://doi.org/10.3390/molecules28052229
Ding W, Yao S, Chen Y, Wu Y, Li Y, He W, Guo Z. A Near-Infrared Fluorescent and Photoacoustic Probe for Visualizing Biothiols Dynamics in Tumor and Liver. Molecules. 2023; 28(5):2229. https://doi.org/10.3390/molecules28052229
Chicago/Turabian StyleDing, Weizhong, Shankun Yao, Yuncong Chen, Yanping Wu, Yaheng Li, Weijiang He, and Zijian Guo. 2023. "A Near-Infrared Fluorescent and Photoacoustic Probe for Visualizing Biothiols Dynamics in Tumor and Liver" Molecules 28, no. 5: 2229. https://doi.org/10.3390/molecules28052229
APA StyleDing, W., Yao, S., Chen, Y., Wu, Y., Li, Y., He, W., & Guo, Z. (2023). A Near-Infrared Fluorescent and Photoacoustic Probe for Visualizing Biothiols Dynamics in Tumor and Liver. Molecules, 28(5), 2229. https://doi.org/10.3390/molecules28052229






