Molecular Engineering of Near-Infrared Fluorescent Probes for Cell Membrane Imaging
Abstract
1. Introduction
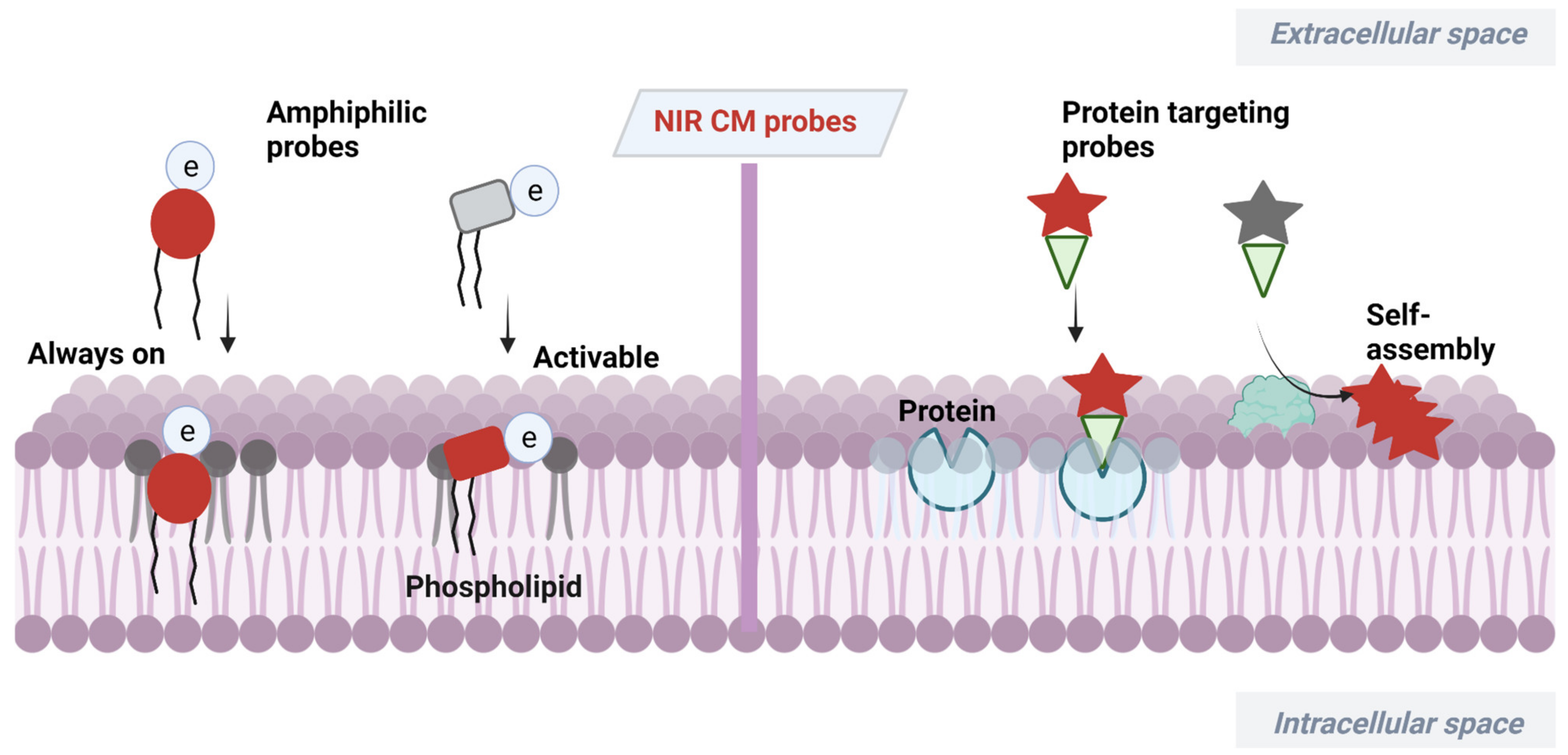
2. NIR Molecular Probes for CM Imaging
2.1. Amphiphilic NIR Probes for CM Imaging
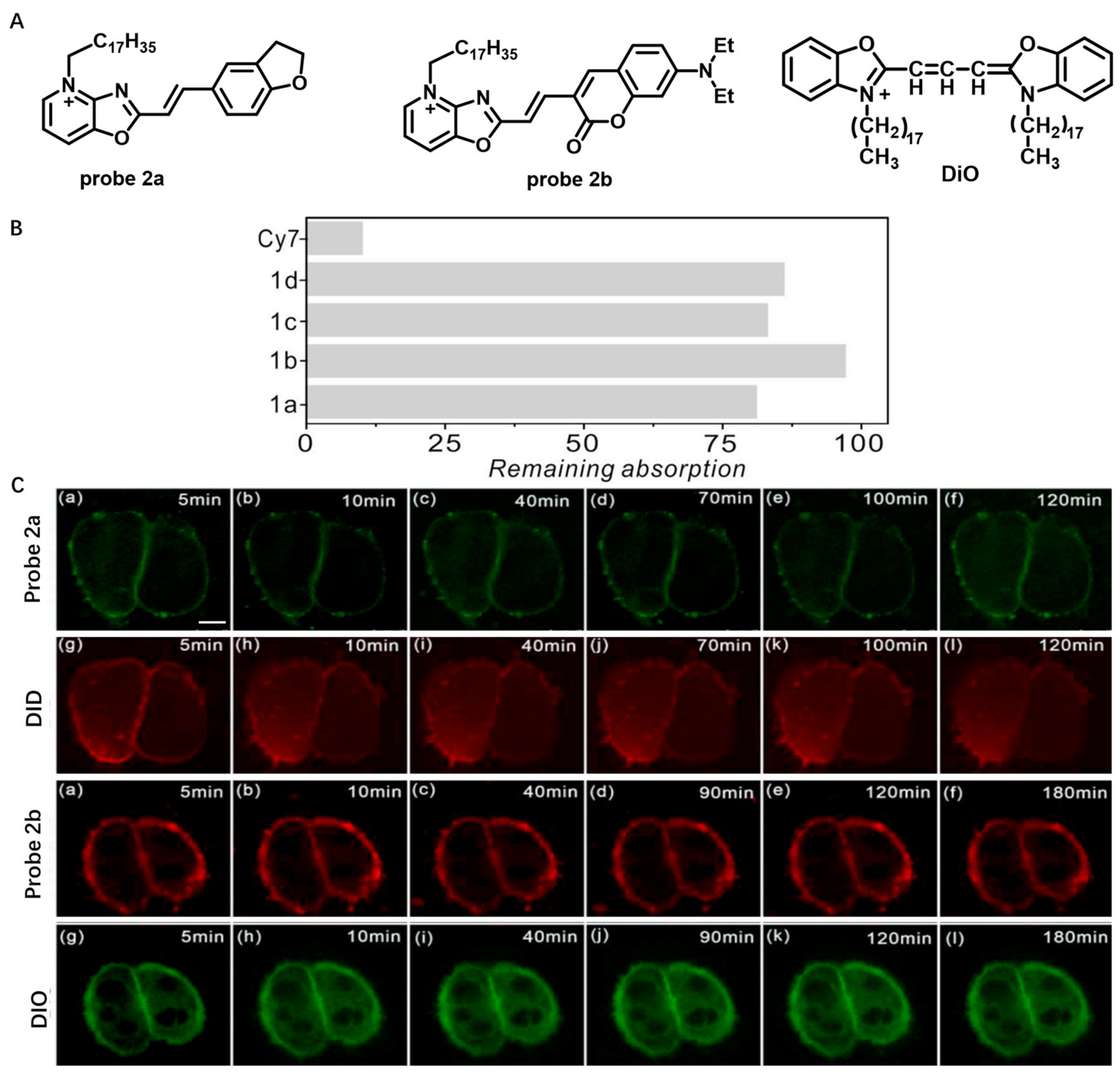
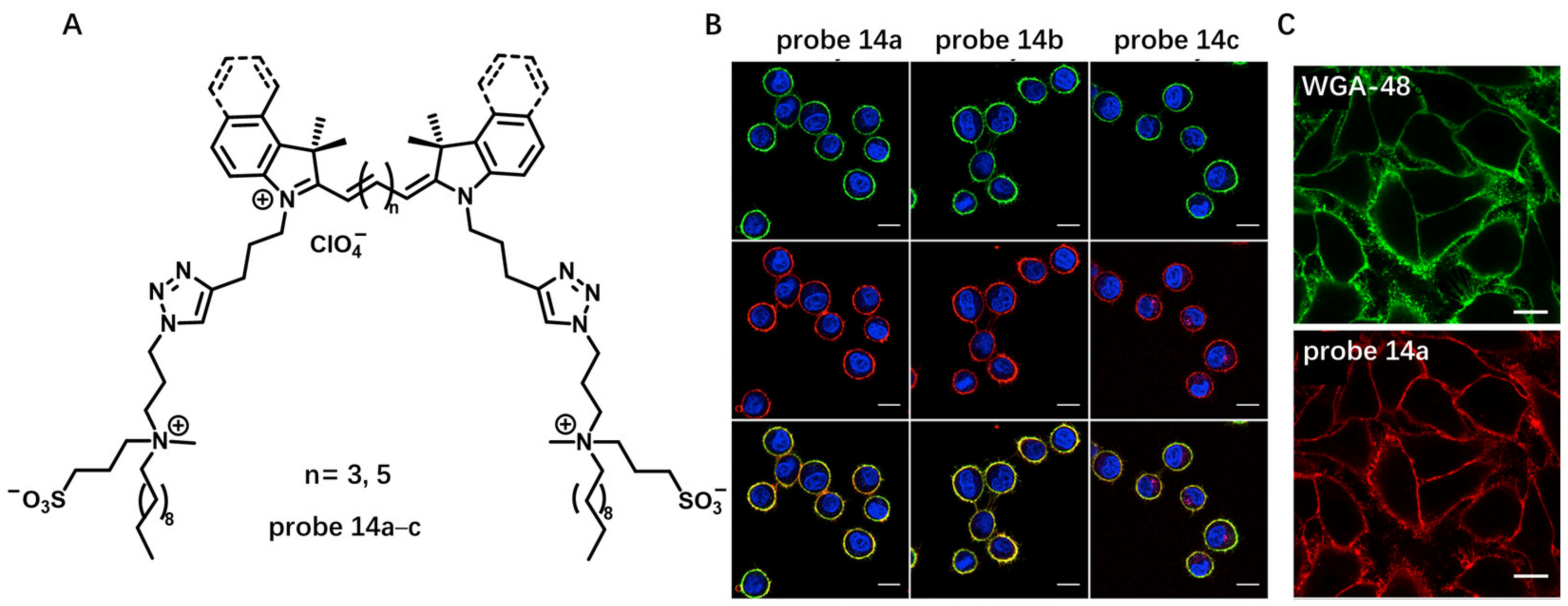
2.1.1. Always-On Amphiphilic Probes
2.1.2. Activatable Amphiphilic Probes
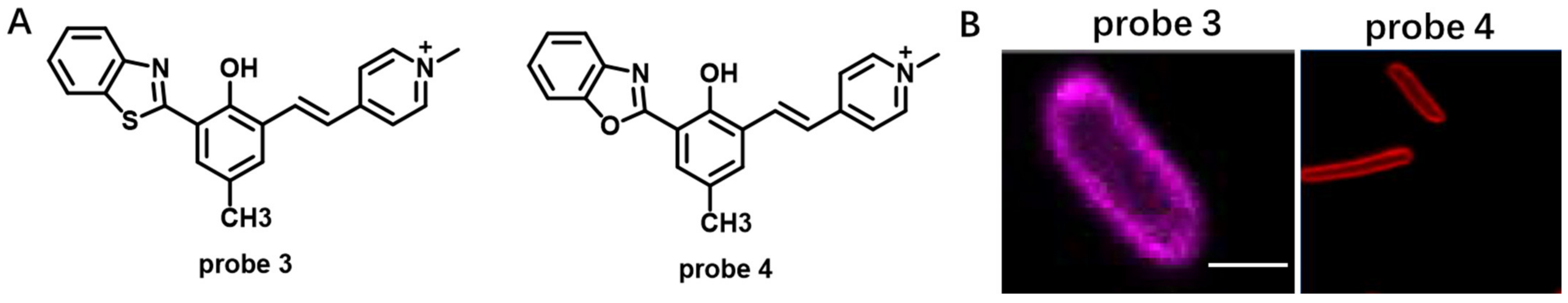
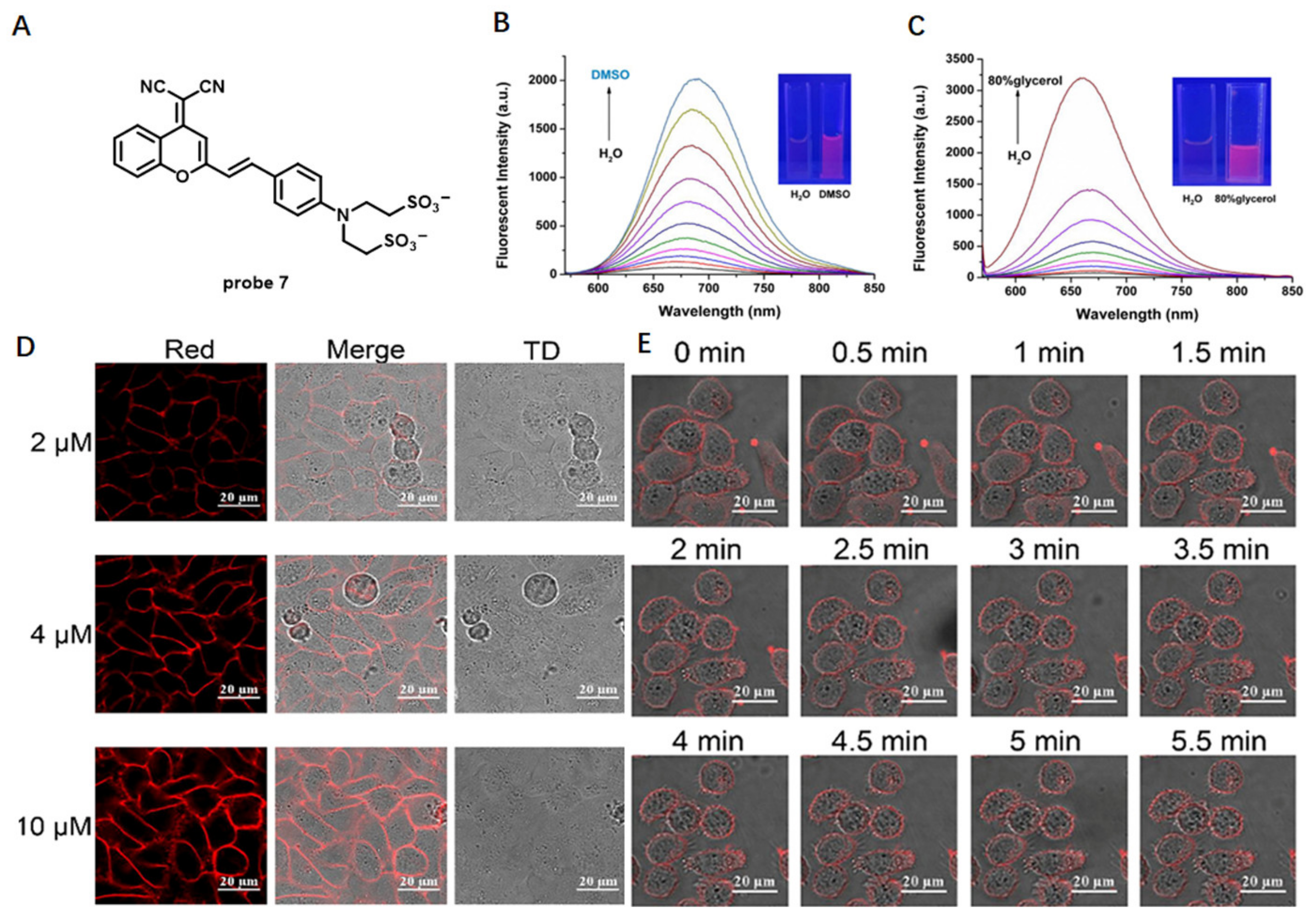
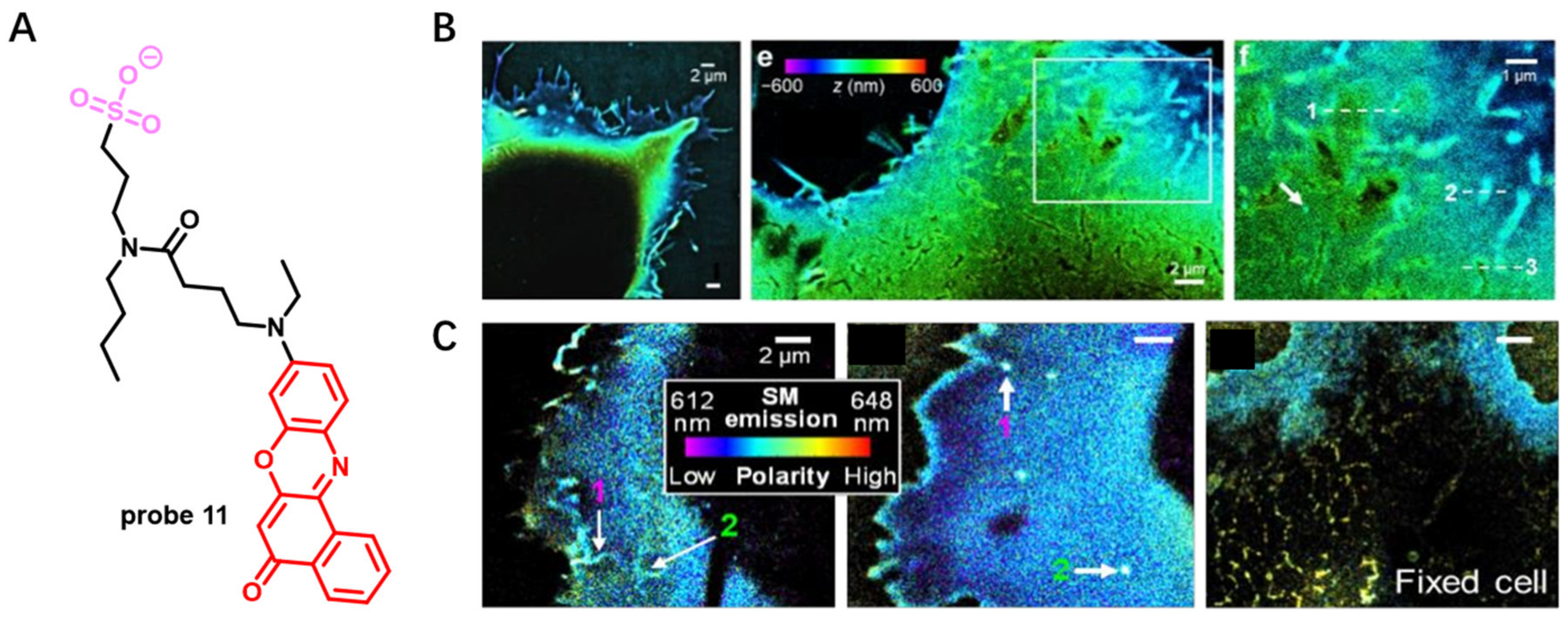
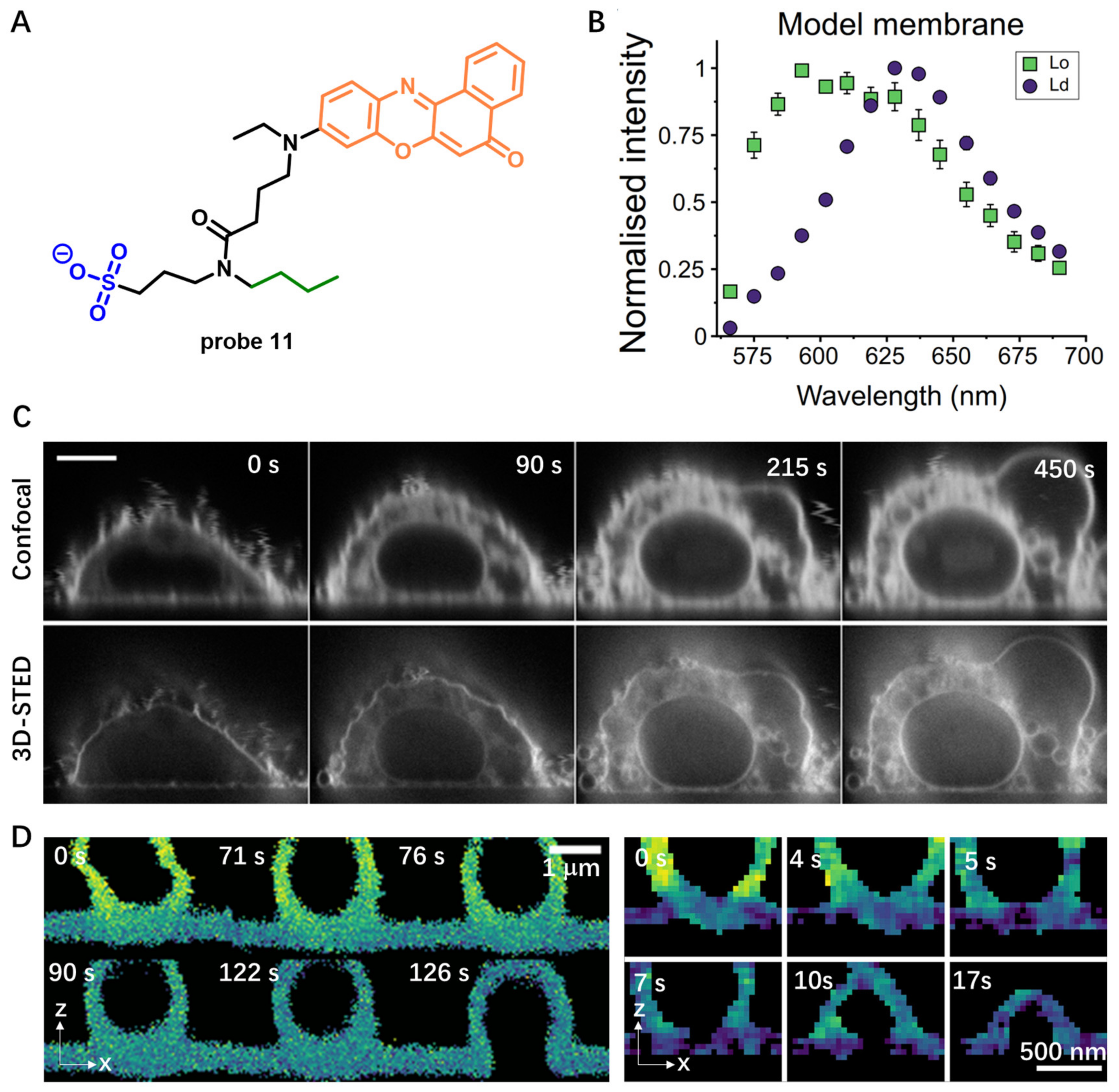
Solvatochromic- or Fluorogenic-Dyes-Based Probes
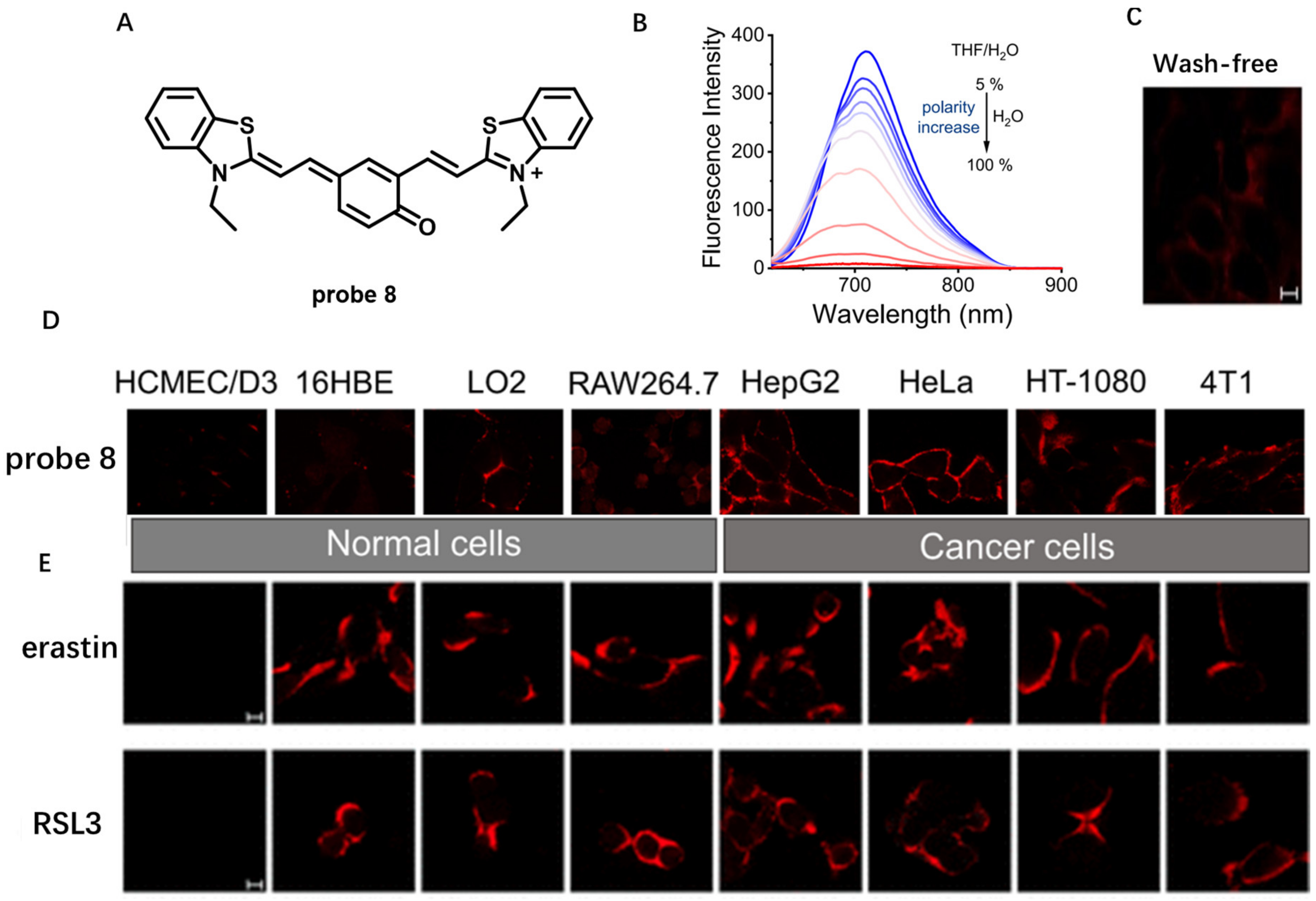
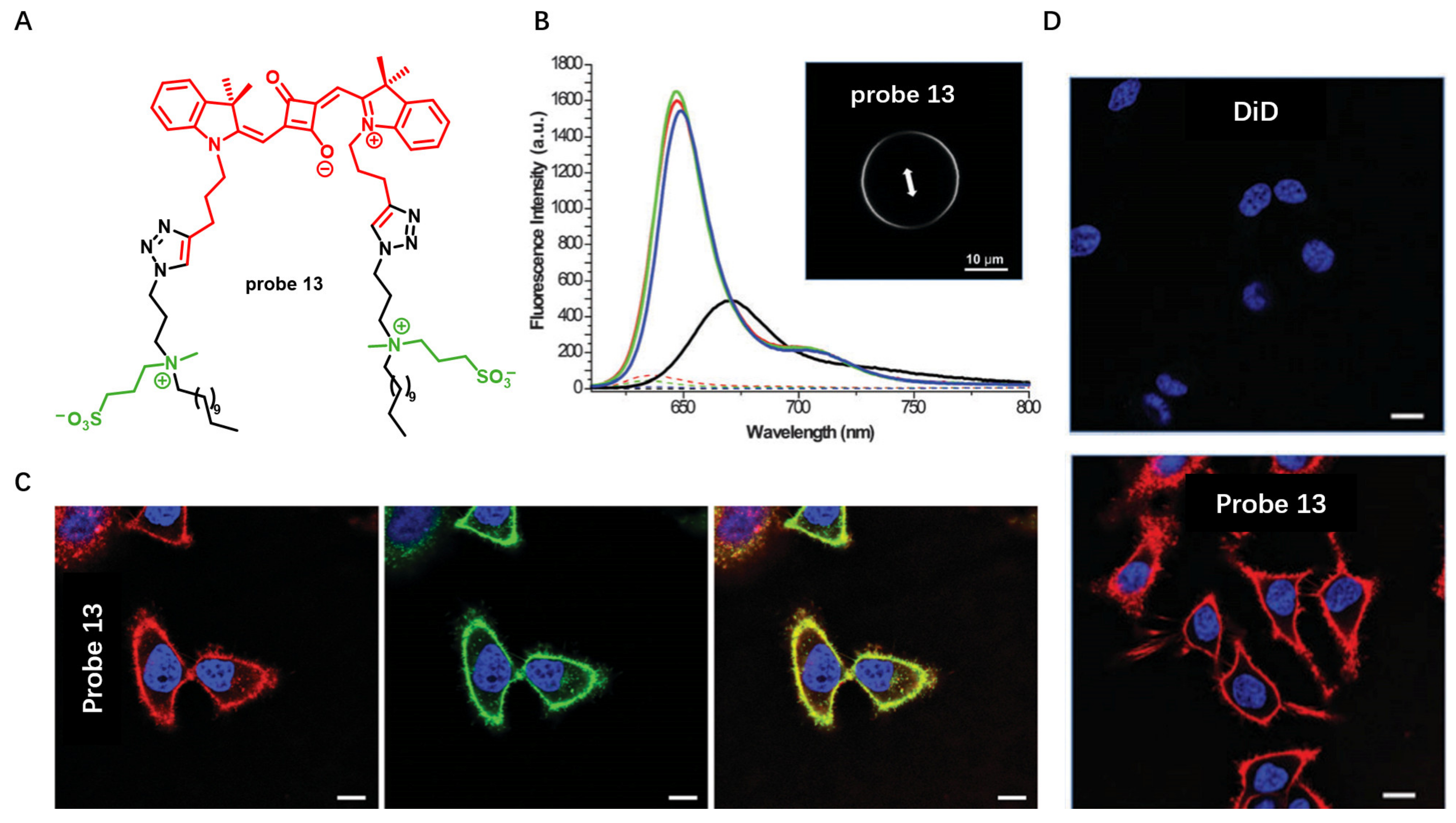
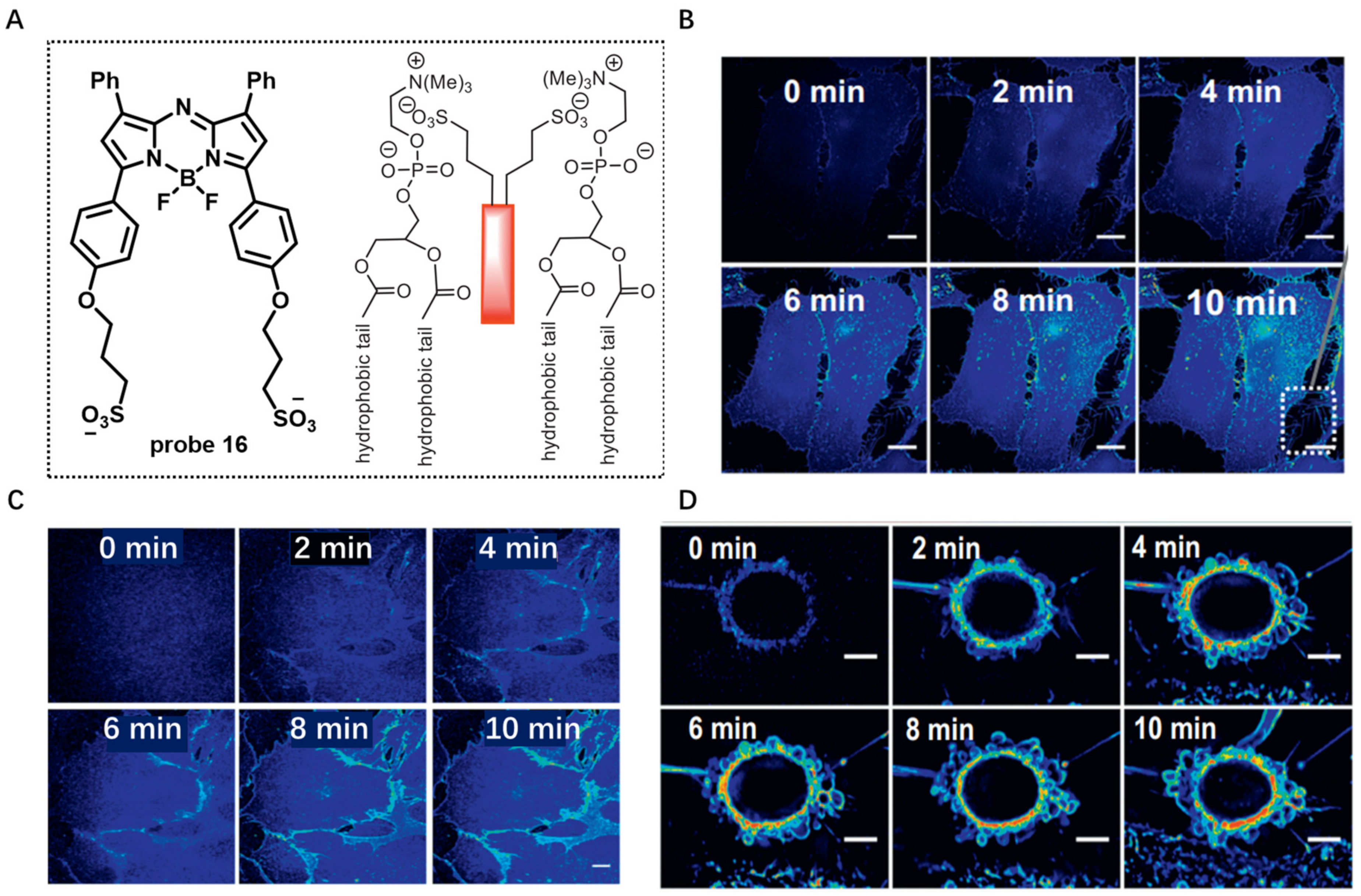
Probes Based on Disassembly Light-Up Fluorescence Strategy
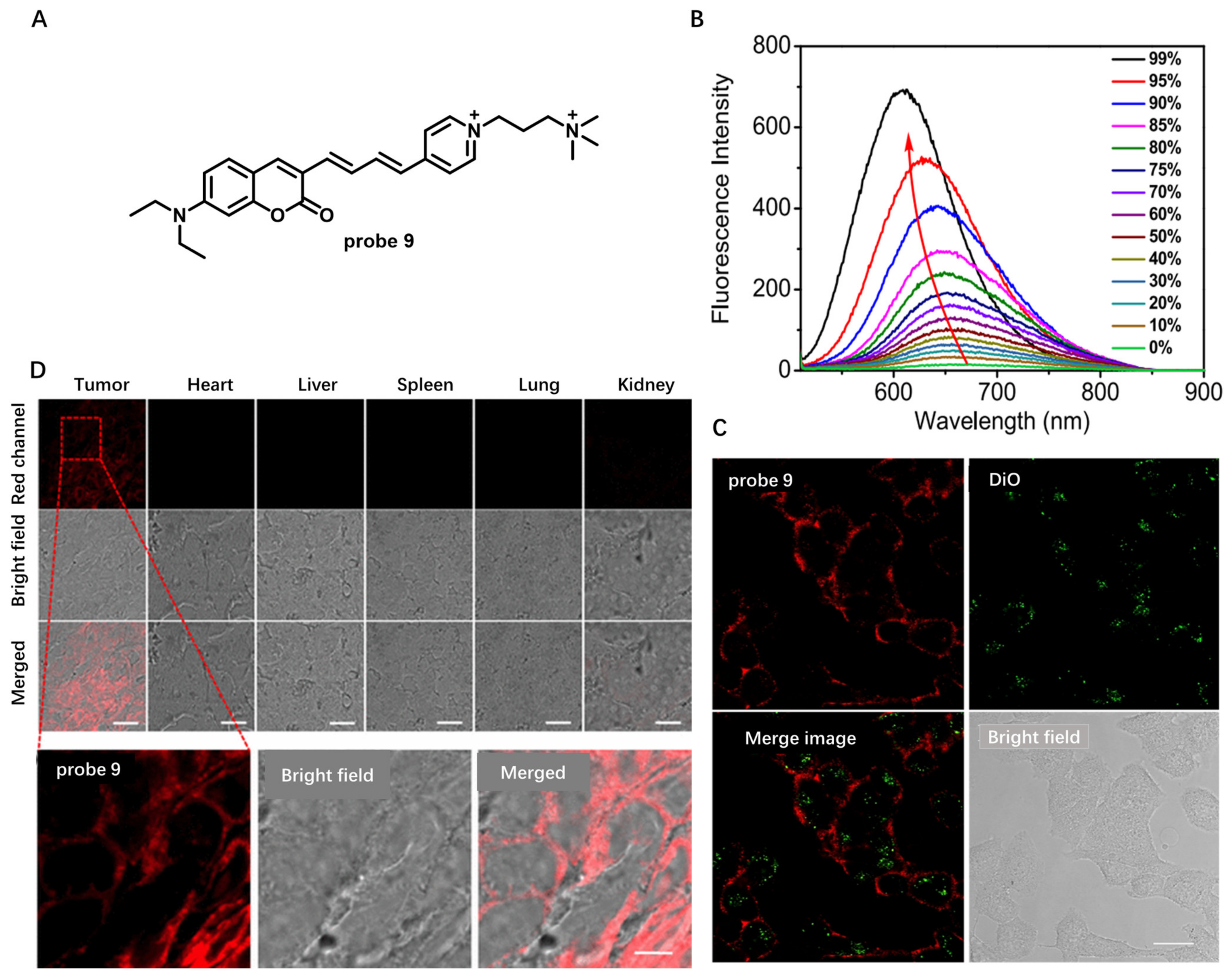
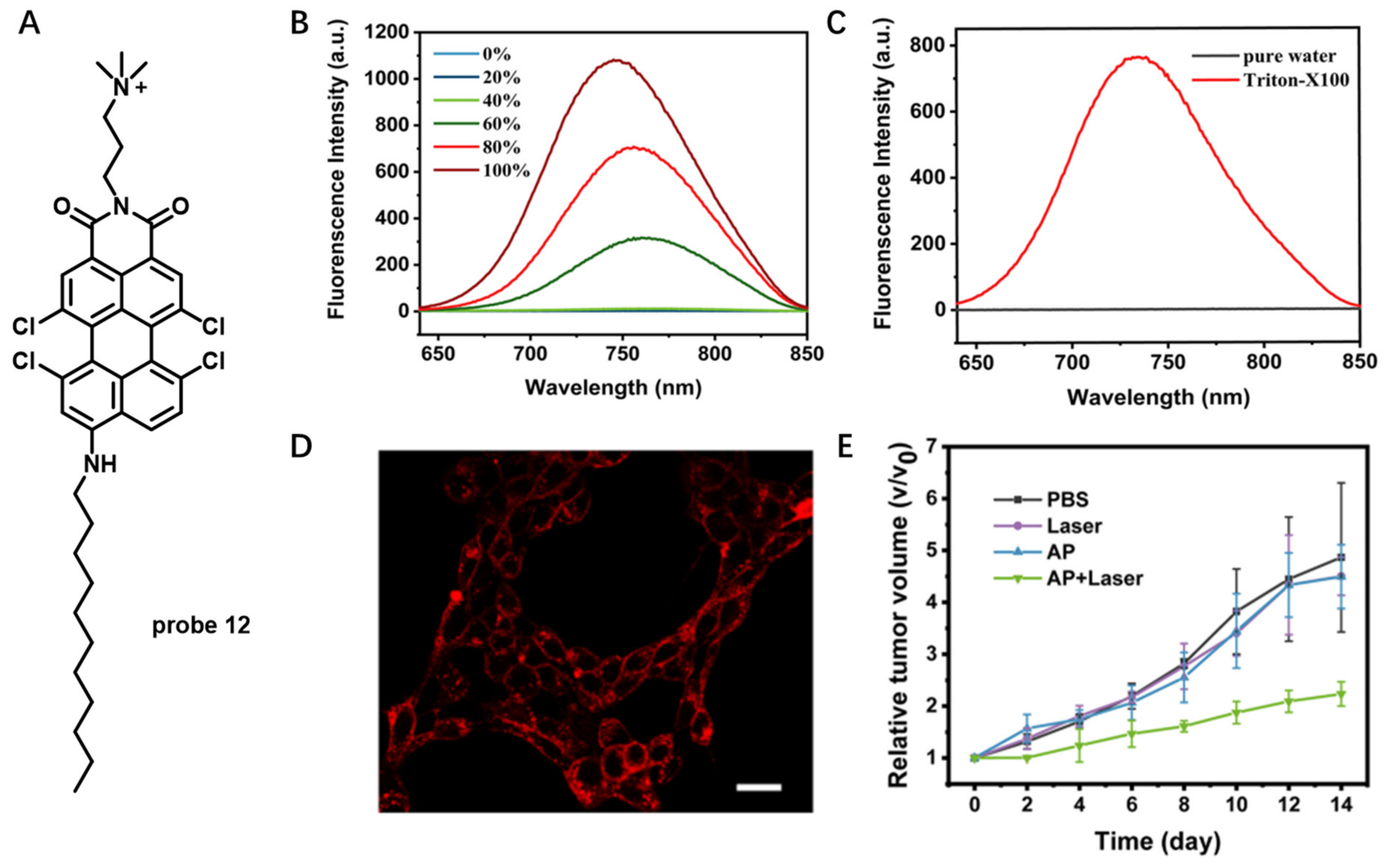
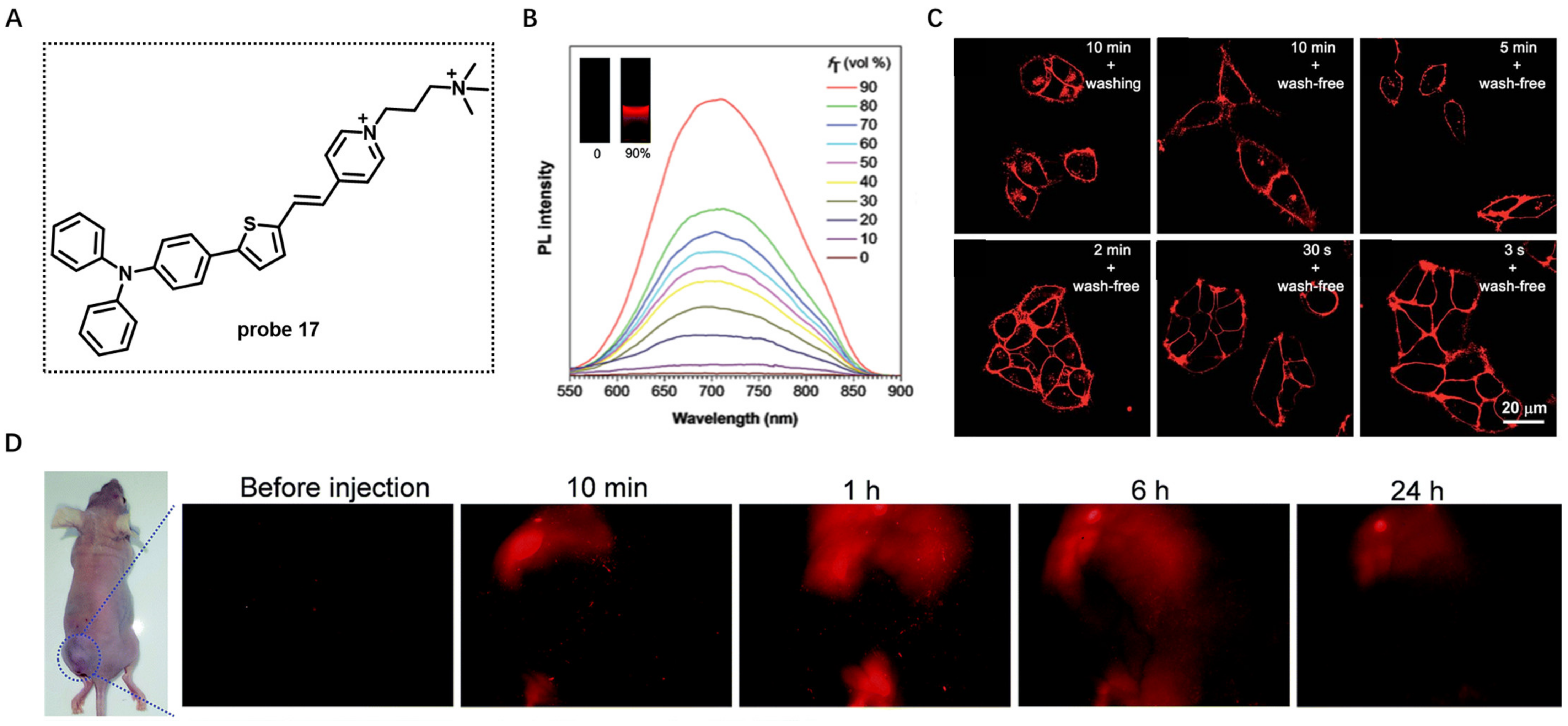
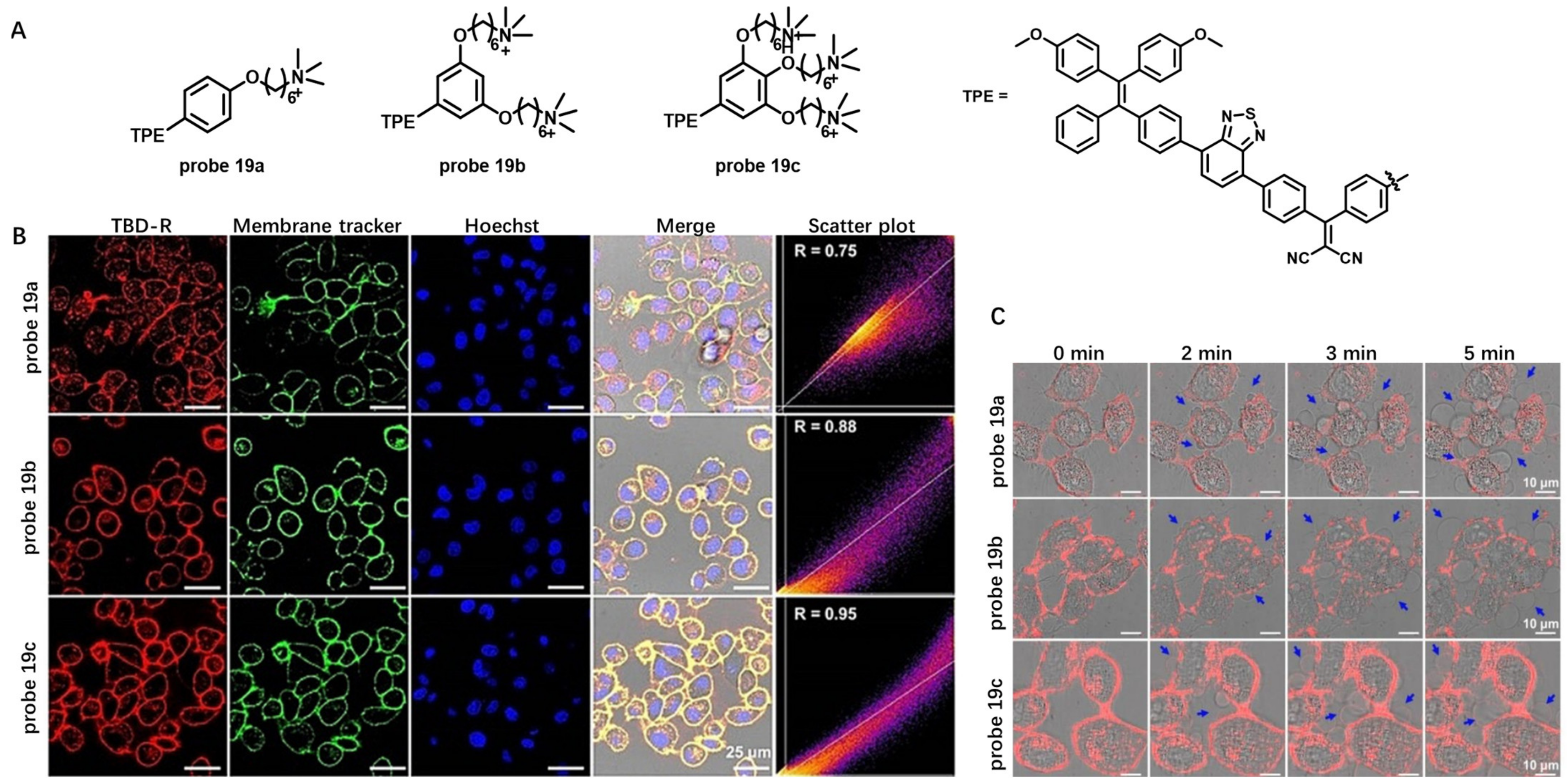
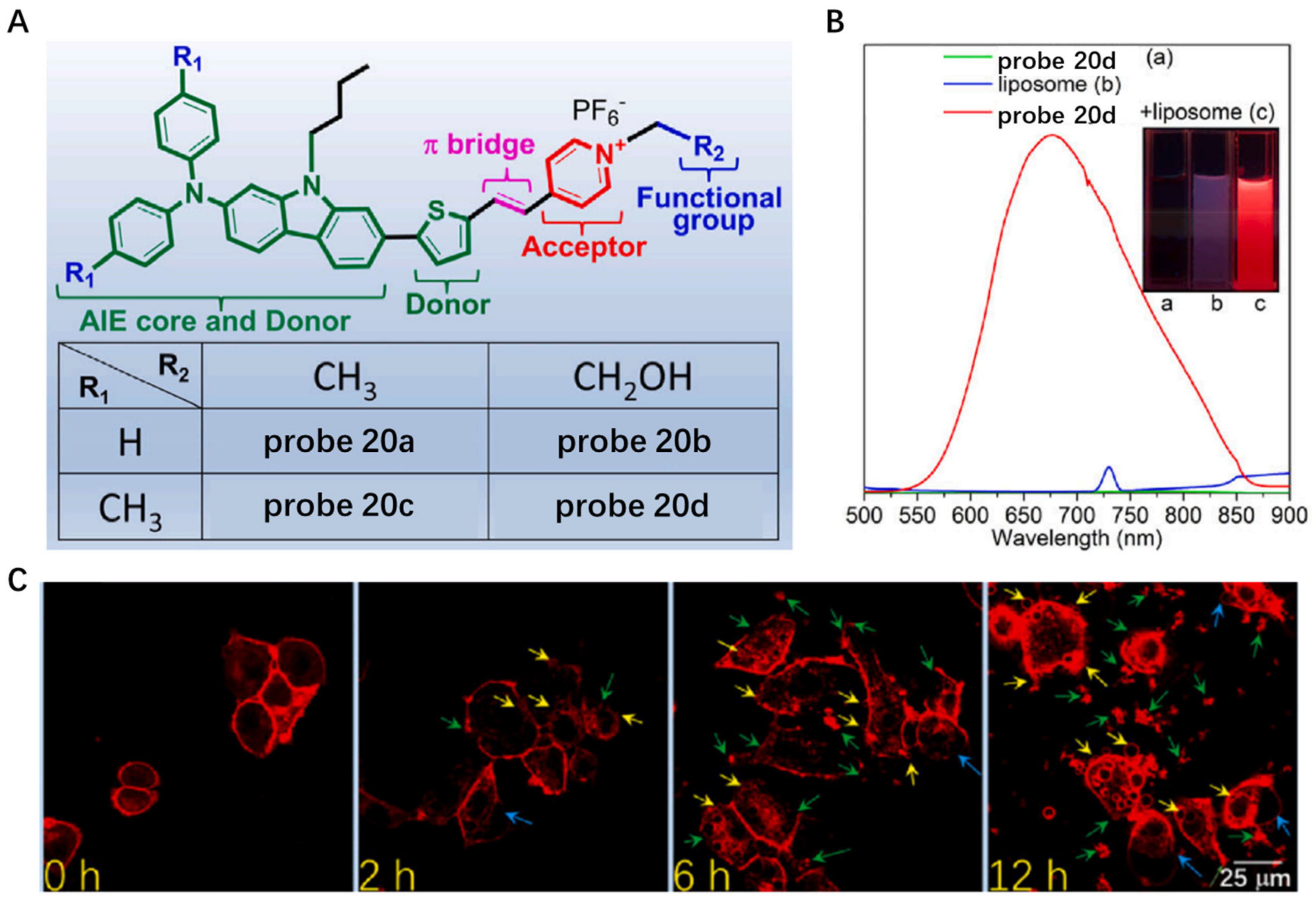
Aggregation-Induced-Emission (AIE)-Based Probes
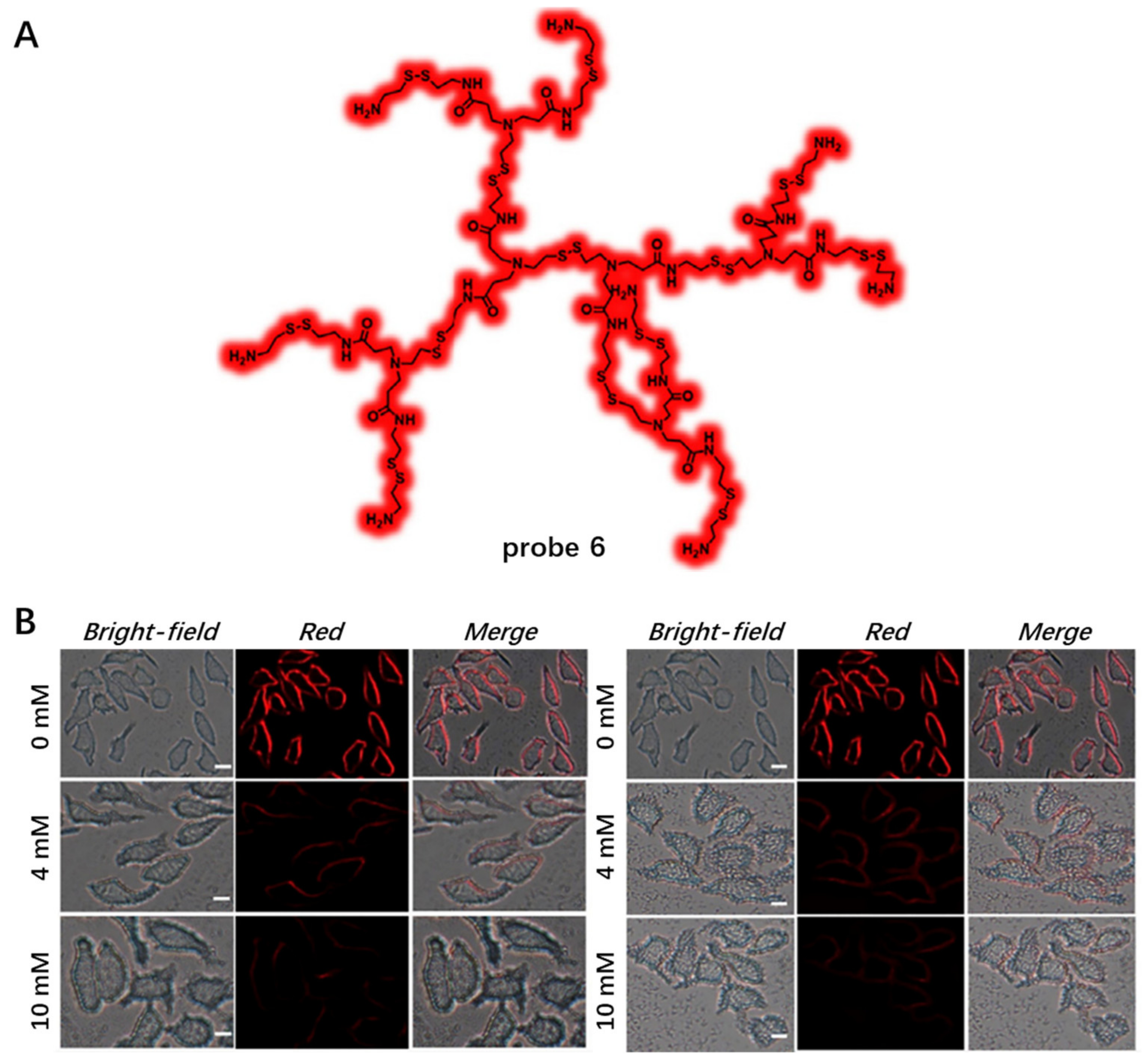
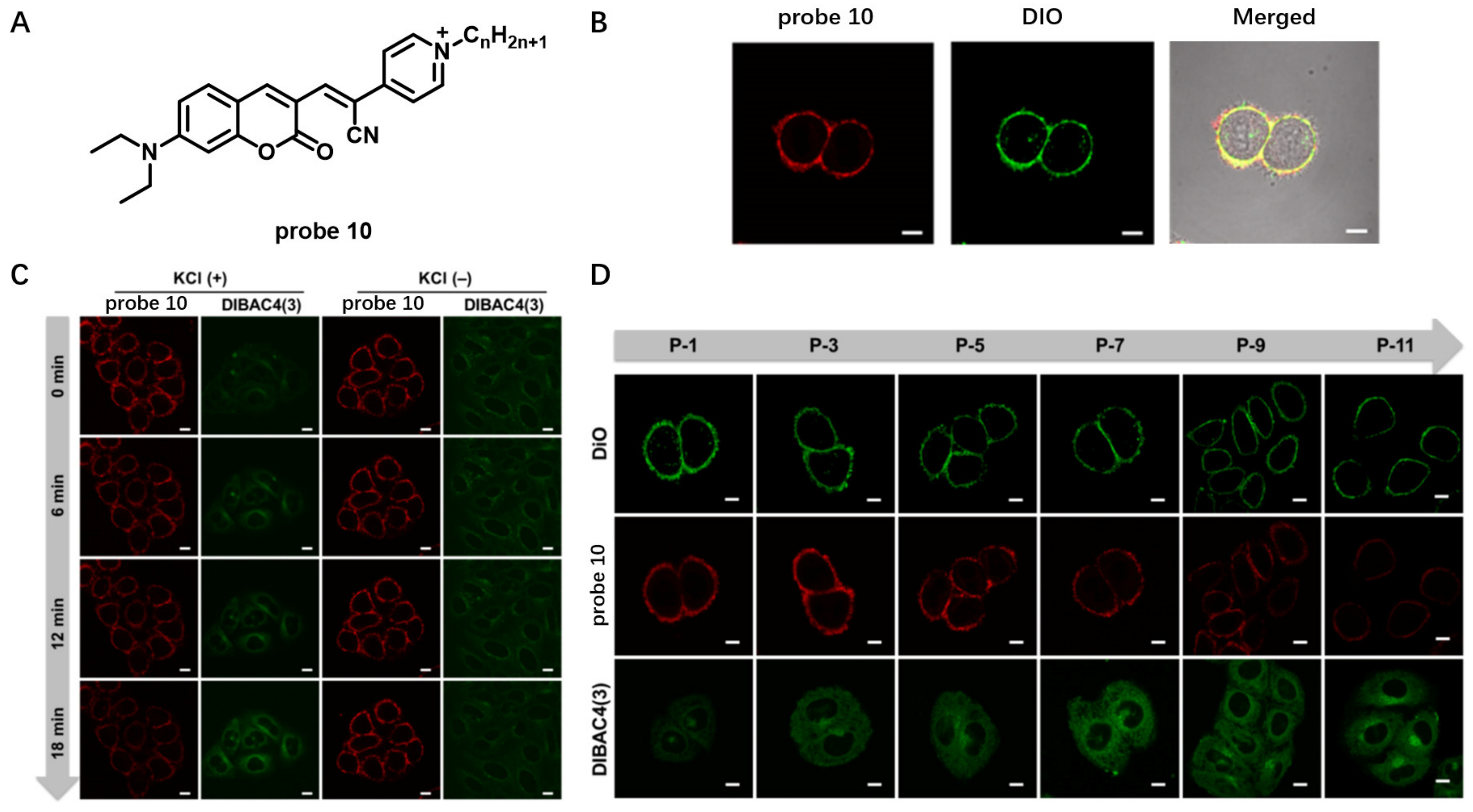
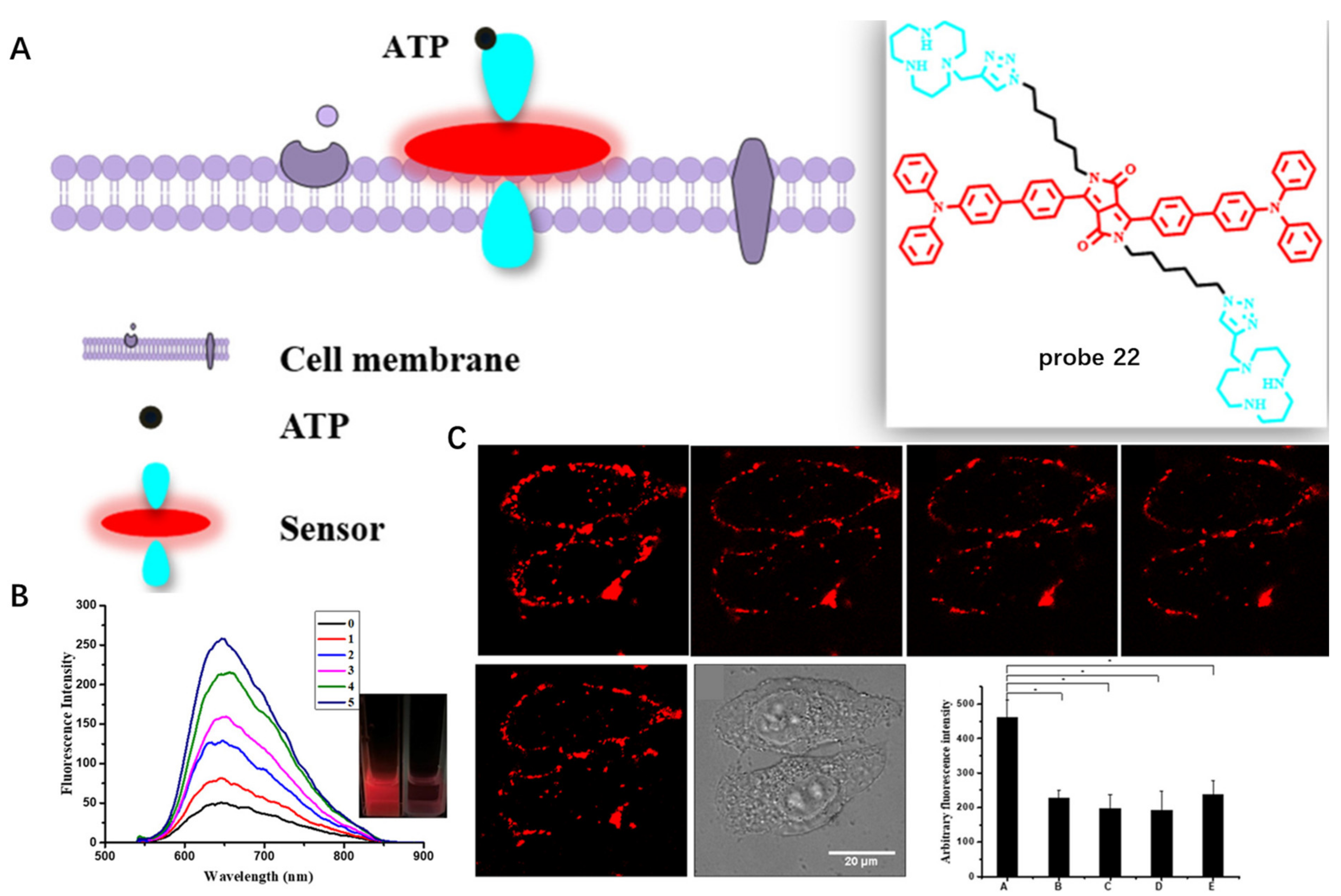
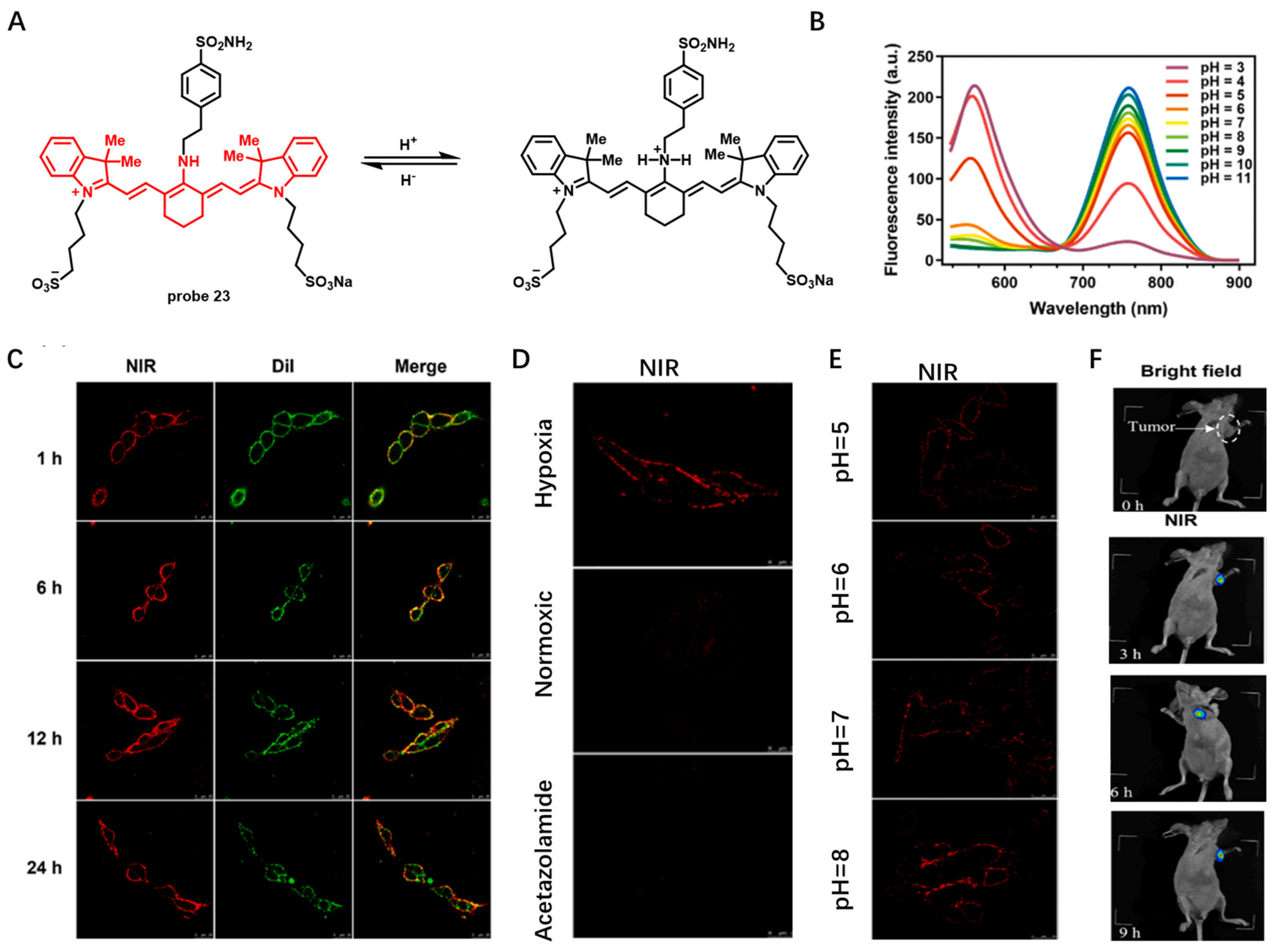
Other
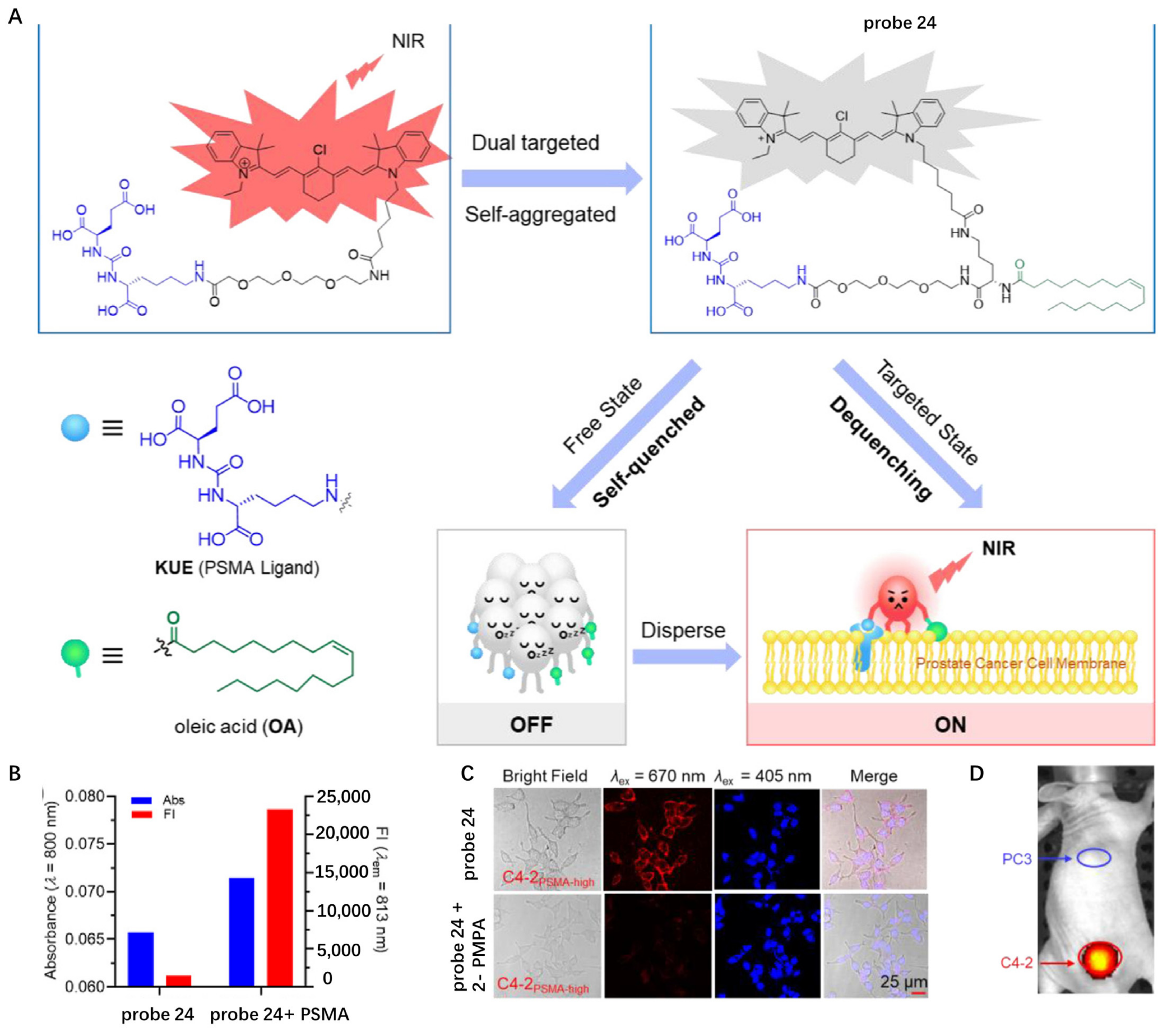
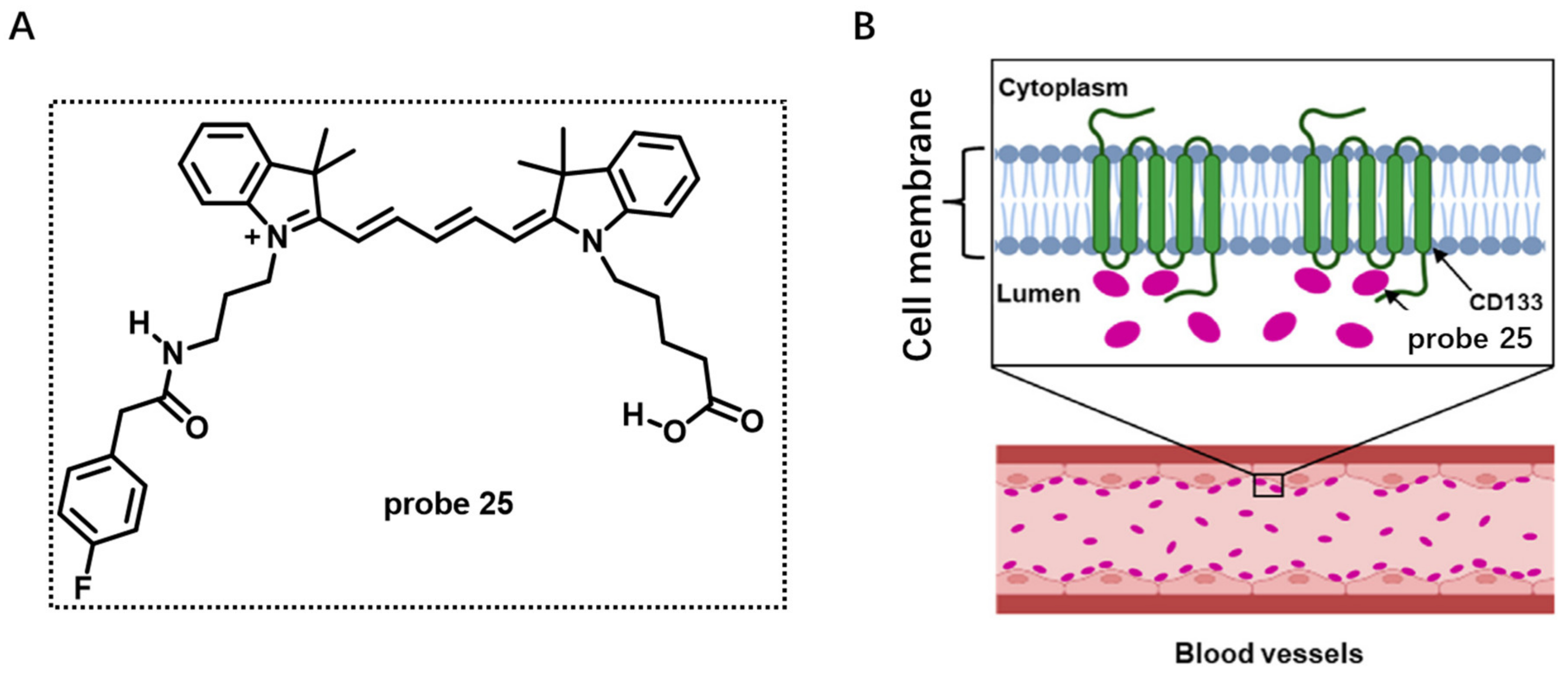
2.2. Proteins Targeting Probes
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kreder, R.; Oncul, S.; Kucherak, O.A.; Pyrshev, K.A.; Real, E.; Mély, Y.; Klymchenko, A.S. Blue fluorogenic probes for cell plasma membranes fill the gap in multicolour imaging. RSC Adv. 2015, 5, 22899–22905. [Google Scholar] [CrossRef]
- Sakai, N.; Matile, S. Conjugated polyimine dynamers as phase-sensitive membrane probes. J. Am. Chem. Soc. 2018, 140, 11438–11443. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.G. Lipid rafts generate digital-like signal transduction in cell plasma membranes. Biotechnol. J. 2012, 7, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.M.; Campelo, F.; Liska, N.; Chernomordik, L.V.; Marrink, S.J.; McMahon, H.T. Mechanisms shaping cell membranes. Curr. Opin. Cell Biol. 2014, 29, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Masters, T.A.; Pontes, B.; Viasnoff, V.; Li, Y.; Gauthier, N.C. Plasma membrane tension orchestrates membrane trafficking, cytoskeletal remodeling, and biochemical signaling during phagocytosis. Proc. Natl. Acad. Sci. USA 2013, 110, 11875–11880. [Google Scholar] [CrossRef]
- Surrey, T.; Jähnig, F. Refolding and oriented insertion of a membrane protein into a lipid bilayer. Proc. Natl. Acad. Sci. USA 1992, 89, 7457–7461. [Google Scholar] [CrossRef]
- Cardone, A.; Lopez, F.; Affortunato, F.; Busco, G.; Hofer, A.M.; Mallamaci, R.; Martinelli, C.; Colella, M.; Farinola, G.M. An aryleneethynylene fluorophore for cell membrane staining. Biochim. Biophys. Acta 2012, 1818, 2808–2817. [Google Scholar] [CrossRef]
- Kahya, N.; Scherfeld, D.; Bacia, K.; Poolman, B.; Schwille, P. Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J. Biol. Chem. 2003, 278, 28109–28115. [Google Scholar] [CrossRef]
- Koivusalo, M.; Welch, C.; Hayashi, H.; Scott, C.C.; Kim, M.; Alexander, T.; Touret, N.; Hahn, K.M.; Grinstein, S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010, 188, 547–563. [Google Scholar] [CrossRef]
- Zhang, C.; Jin, S.; Yang, K.; Xue, X.; Li, Z.; Jiang, Y.; Chen, W.Q.; Dai, L.; Zou, G.; Liang, X.J. Cell membrane tracker based on restriction of intramolecular rotation. ACS Appl. Mater. Interfaces 2014, 6, 8971–8975. [Google Scholar] [CrossRef]
- Dal Molin, M.; Verolet, Q.; Colom, A.; Letrun, R.; Derivery, E.; Gonzalez-Gaitan, M.; Vauthey, E.; Roux, A.; Sakai, N.; Matile, S. Fluorescent flippers for mechanosensitive membrane probes. J. Am. Chem. Soc. 2015, 137, 568–571. [Google Scholar] [CrossRef]
- Wang, K.-N.; Qi, G.; Chu, H.; Chao, X.-J.; Liu, L.-Y.; Li, G.; Cao, Q.; Mao, Z.-W.; Liu, B. Probing cell membrane damage using a molecular rotor probe with membrane-to-nucleus translocation. Mater. Horiz. 2020, 7, 3226–3233. [Google Scholar] [CrossRef]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef]
- Jain, S.K.; Shohet, S.B. A novel phospholipid in irreversibly sickled cells: Evidence for in vivo peroxidative membrane damage in sickle cell disease. Blood 1984, 63, 362–367. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, R.; Wang, Y.; Guo, P.; Liu, X.; Ji, F.; Hu, J.; Yan, X.; Wang, G.P.; Zhang, L.; et al. Carbon helical nanorobots capable of cell membrane penetration for single cell targeted SERS bio-sensing and photothermal cancer therapy. Adv. Funct. Mater. 2022, 32, 2200600. [Google Scholar] [CrossRef]
- Bu, Y.; Xu, T.; Zhu, X.; Zhang, J.; Wang, L.; Yu, Z.; Yu, J.; Wang, A.; Tian, Y.; Zhou, H.; et al. A NIR-I light-responsive superoxide radical generator with cancer cell membrane targeting ability for enhanced imaging-guided photodynamic therapy. Chem. Sci. 2020, 11, 10279–10286. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, Y.; Jiang, J.H.; Wu, H.L.; Shen, G.L.; Yu, R.Q. An ultrasensitive chemiluminescence biosensor for cholera toxin based on ganglioside-functionalized supported lipid membrane and liposome. Biosens. Bioelectron. 2008, 24, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Saez, A.J.; Schwille, P. Surface analysis of membrane dynamics. Biochim. Biophys. Acta 2010, 1798, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, H.; Taniguchi, M.; Okazaki, T. Analysis of lipid-composition changes in plasma membrane microdomains. J. Lipid Res. 2015, 56, 1594–1605. [Google Scholar] [CrossRef]
- Zhao, W.; Tian, Y.; Cai, M.; Wang, F.; Wu, J.; Gao, J.; Liu, S.; Jiang, J.; Jiang, S.; Wang, H. Studying the nucleated mammalian cell membrane by single molecule approaches. PLoS ONE 2014, 9, e91595. [Google Scholar] [CrossRef]
- Li, K.; Ren, T.B.; Huan, S.; Yuan, L.; Zhang, X.B. Progress and perspective of solid-state organic fluorophores for biomedical applications. J. Am. Chem. Soc. 2021, 143, 21143–21160. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Liu, T.; Dong, P.; Wang, W.; Ge, G.; Wang, B.; Yu, Z.; Shi, L.; Tian, X.; Huo, X.; et al. Molecular design strategy to construct the near-infrared fluorescent probe for selectively sensing human cytochrome P450 2J2. J. Am. Chem. Soc. 2019, 141, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.B.; Xu, W.; Zhang, W.; Zhang, X.X.; Wang, Z.Y.; Xiang, Z.; Yuan, L.; Zhang, X.B. A general method to increase stokes shift by introducing alternating vibronic structures. J. Am. Chem. Soc. 2018, 140, 7716–7722. [Google Scholar] [CrossRef]
- Schaferling, M. The art of fluorescence imaging with chemical sensors. Angew. Chem. Int. Ed. 2012, 51, 3532–3554. [Google Scholar] [CrossRef] [PubMed]
- Paige, J.; Nguyen-Duc, T.; Song, W.; Jaffrey, S. Fluorescence imaging of cellular metabolites with RNA. Science 2012, 335, 1194. [Google Scholar] [CrossRef]
- Chazotte, B. Labeling membrane glycoproteins or glycolipids with fluorescent wheat germ agglutinin. Cold Spring Harb. Protoc. 2011, 2011, 570–572. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Kreder, R. Fluorescent probes for lipid rafts: From model membranes to living cells. Chem. Biol. 2014, 21, 97–113. [Google Scholar] [CrossRef]
- Ben-Bassat, H.; Goldblum, N. Concanavalin a receptors on the surface membrane of lymphocytes from patient’s with Hodgkin’s disease and other malignant lymphomas. Proc. Natl. Acad. Sci. USA 1975, 72, 1046–1049. [Google Scholar] [CrossRef]
- Cheng, D.; Peng, J.; Lv, Y.; Su, D.; Liu, D.; Chen, M.; Yuan, L.; Zhang, X. De Novo Design of Chemical Stability Near-Infrared Molecular Probes for High-Fidelity Hepatotoxicity Evaluation In Vivo. J. Am. Chem. Soc. 2019, 141, 6352–6361. [Google Scholar] [CrossRef]
- Li, K.; Xu, S.; Xiong, M.; Huan, S.Y.; Yuan, L.; Zhang, X.B. Molecular engineering of organic-based agents for in situ bioimaging and phototherapeutics. Chem. Soc. Rev. 2021, 50, 11766–11784. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef]
- Liu, H.W.; Chen, L.; Xu, C.; Li, Z.; Zhang, H.; Zhang, X.B.; Tan, W. Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging. Chem. Soc. Rev. 2018, 47, 7140–7180. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, L.; Xu, C.; Ren, T.-B.; Xu, S.; Lou, X.; Yuan, L.; Zhang, X.-B. An integration strategy to develop dual-state luminophores with tunable spectra, large stokes shift, and activatable fluorescence for high-contrast imaging. CCS Chem. 2022, 4, 2153–2164. [Google Scholar] [CrossRef]
- Cheng, D.; Pan, Y.; Wang, L.; Zeng, Z.; Yuan, L.; Zhang, X.; Chang, Y.-T. Selective visualization of the endogenous peroxynitrite in an inflamed mouse model by a mitochondria-targetable two-photon ratiometric fluorescent probe. J. Am. Chem. Soc. 2016, 139, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Karton-Lifshin, N.; Albertazzi, L.; Bendikov, M.; Baran, P.S.; Shabat, D. “Donor-two-acceptor” dye design: A distinct gateway to NIR fluorescence. J. Am. Chem. Soc. 2012, 134, 20412–20420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Teng, L.; Xu, C.; Liu, H.W.; Xu, S.; Guo, H.; Yuan, L.; Zhang, X.B. A “Double-Locked” and enzyme-activated molecular probe for accurate bioimaging and hepatopathy differentiation. Chem. Sci. 2019, 10, 10931–10936. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Dan, C.; Dongdong, S.; Chen, M.; Yin, B.C.; Yuan, L.; Zhang, X.B. Visualization of oxidative injury in the mouse kidney using selective superoxide anion fluorescent probes. Chem. Sci. 2018, 9, 7606–7613. [Google Scholar] [CrossRef]
- Koo, C.K.; Wong, K.L.; Man, C.W.; Tam, H.L.; Tsao, S.W.; Cheah, K.W.; Lam, M.H. Two-photon plasma membrane imaging in live cells by an amphiphilic, water-soluble cyctometalated platinum(II) complex. Inorg. Chem. 2009, 48, 7501–7503. [Google Scholar] [CrossRef]
- Liu, H.; Kwong, B.; Irvine, D.J. Membrane anchored immunostimulatory oligonucleotides for in vivo cell modification and localized immunotherapy. Angew. Chem. Int. Ed. 2011, 50, 7052–7055. [Google Scholar] [CrossRef]
- Kalchenko, V.; Shivtiel, S.; Malina, V.; Lapid, K.; Haramati, S.; Lapidot, T.; Brill, A.; Harmelin, A. Use of lipophilic near-infrared dye in whole-body optical imaging of hematopoietic cell homing. J. Biomed. Opt. 2006, 11, 050507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, T.; Su, Y.; Luo, S.; Zhu, Y.; Tan, X.; Fan, S.; Zhang, L.; Zhou, Y.; Cheng, T.; et al. A near-infrared fluorescent heptamethine indocyanine dye with preferential tumor accumulation for in vivo imaging. Biomaterials 2010, 31, 6612–6617. [Google Scholar] [CrossRef]
- Schmidt, T.; Schütz, G.; Baumgartner, W.; Gruber, H.; Schindler, H. Characterization of photophysics and mobility of single molecules in a fluid lipid membrane. J. Phys. Chem. 1995, 99, 17662–17668. [Google Scholar] [CrossRef]
- Huang, Y.; Xing, J.; Gong, Q.; Chen, L.C.; Liu, G.; Yao, C.; Wang, Z.; Zhang, H.L.; Chen, Z.; Zhang, Q. Reducing aggregation caused quenching effect through co-assembly of PAH chromophores and molecular barriers. Nat. Commun. 2019, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Y.; Chen, Y.; Zhao, E.; Hong, Y.; Chen, S.; Lam, J.W.Y.; Chen, Y.; Hou, J.; Tang, B.Z. Amphiphilic tetraphenylethene-based pyridinium salt for selective cell-membrane imaging and room-light-induced special reactive oxygen species generation. ACS Appl. Mater. Interfaces 2019, 11, 10567–10577. [Google Scholar] [CrossRef] [PubMed]
- Mustroph, H. Cyanine dyes. Phys. Sci. Rev. 2020, 5, 20190145. [Google Scholar] [CrossRef]
- Shindy, H.A. Fundamentals in the chemistry of cyanine dyes: A review. Dyes Pigments 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Heinrich, L.; Freyria, A.M.; Melin, M.; Tourneur, Y.; Maksoud, R.; Bernengo, J.C.; Hartmann, D.J. Confocal laser scanning microscopy using dialkylcarbocyanine dyes for cell tracing in hard and soft biomaterials. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 153–161. [Google Scholar] [CrossRef]
- Honig, M.; Hume, R. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J. Cell Biol. 1986, 103, 171–187. [Google Scholar] [CrossRef]
- Chen, P.-L.; Shi, Q.-Y.; Chen, T.; Wang, P.; Liu, Y.; Liu, L.-H. A dual-usage near-infrared (NIR) cell membrane targeting chimeric peptide for cancer cell membrane imaging and photothermal ablation. J. Mater. Sci. 2020, 55, 7843–7856. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliver. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, L.H.; Qiu, W.X.; Zhang, Y.H.; Song, W.; Zhang, L.; Wang, S.B.; Zhang, X.Z. A transformable chimeric peptide for cell encapsulation to overcome multidrug resistance. Small 2018, 14, e1703321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-N.; Xu, B.; Qiu, L.-H.; Xu, Y.-J.; Sun, R.; Ge, J.-F. A series of novel cell membrane fluorescent probes based on oxazolopyridine unit. Dyes Pigments 2021, 185, 108883. [Google Scholar] [CrossRef]
- Dahal, D.; Ojha, K.R.; Alexander, N.; Konopka, M.; Pang, Y. An NIR-emitting ESIPT dye with large stokes shift for plasma membrane of prokaryotic (E. coli) cells. Sens. Actuators B Chem. 2018, 259, 44–49. [Google Scholar] [CrossRef]
- Dahal, D.; Ojha, K.; Pokhrel, S.; Paruchuri, S.; Konopka, M.; Liu, Q.; Pang, Y. NIR-emitting styryl dyes with large stokes’ shifts for imaging application: From cellular plasma membrane, mitochondria to zebrafish neuromast. Dyes Pigments 2021, 194, 109629. [Google Scholar] [CrossRef]
- Thompson, A.D.; Omar, M.H.; Rivera-Molina, F.; Xi, Z.; Koleske, A.J.; Toomre, D.K.; Schepartz, A. Long-term live-cell STED nanoscopy of primary and cultured cells with the plasma membrane HIDE probe DiI-SiR. Angew. Chem. Int. Ed. 2017, 56, 10408–10412. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lü, S.; Gao, C.; Bai, X.; Feng, C.; Gao, N.; Liu, M. Multifunctional drug carriers comprised of mesoporous silica nanoparticles and polyamidoamine dendrimers based on layer-by-layer assembly. Mater. Des. 2015, 88, 1127–1133. [Google Scholar] [CrossRef]
- Guan, X.-L.; Yang, X.-Q.; Lai, S.-J.; Ding, Y.-Y.; Wei, J.-Y.; Zhang, J.-M.; Zhang, L.-Y.; Li, C.-H.; Tong, J.-H.; Lei, Z.-Q. Design and synthesis of biodegradable nonconjugated S-S-PAMAM dendrimers with unexpected deep-red/NIR emission and cell membrane targeting ability for biological imaging. Mater. Des. 2022, 221, 110982. [Google Scholar] [CrossRef]
- Klymchenko, N.S. Solvatochromic and fluorogenic dyes as environment-sensitive probes: Design and biological applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Luo, J.; Peng, J.; An, B.; Qiao, Z.; Wei, N.; Zhang, Y.; Zhu, W. Highly efficient cell membrane tracker based on a solvatochromic dye with near-infrared emission. ACS Omega 2020, 5, 11829–11835. [Google Scholar] [CrossRef]
- Feng, S.; Liu, Y.; Li, Q.; Gui, Z.; Feng, G. Two water-soluble and wash-free fluorogenic probes for specific lighting up cancer cell membranes and tumors. Anal. Chem. 2022, 94, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, M.; Meng, F.; Lv, J.; Yang, M.; Gao, J.; Wei, G.; Yuan, Z.; Li, H. Monitoring cell plasma membrane polarity by a NIR fluorescence probe with unexpected cell plasma membrane-targeting ability. ACS Omega 2022, 7, 46891–46899. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hong, J.; Feng, S.; Gong, S.; Feng, G. Polarity-sensitive cell membrane probe reveals lower polarity of tumor cell membrane and its application for tumor diagnosis. Anal. Chem. 2022, 94, 11089–11095. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Poole, K.; Goyette, J.; Gaus, K. Introducing membrane charge and membrane potential to T cell signaling. Front. Immunol. 2017, 8, 1513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Li, Y.; Yin, W.; Zhuang, J.; Jia, Q.; Wang, Z.; Li, N. Controllable coumarin-based NIR fluorophores: Selective subcellular imaging, cell membrane potential indication, and enhanced photodynamic therapy. ACS Appl. Mater. Interfaces 2020, 12, 2076–2086. [Google Scholar] [CrossRef]
- Danylchuk, D.I.; Moon, S.; Xu, K.; Klymchenko, A.S. Switchable solvatochromic probes for live-cell super-resolution imaging of plasma membrane organization. Angew. Chem. Int. Ed. 2019, 58, 14920–14924. [Google Scholar] [CrossRef]
- Carravilla, P.; Dasgupta, A.; Zhurgenbayeva, G.; Danylchuk, D.I.; Klymchenko, A.S.; Sezgin, E.; Eggeling, C. Long-term STED imaging of membrane packing and dynamics by exchangeable polarity-sensitive dyes. Biophys. Rep. 2021, 1, 100023. [Google Scholar] [CrossRef]
- Cai, Y.; Ni, D.; Cheng, W.; Ji, C.; Wang, Y.; Mullen, K.; Su, Z.; Liu, Y.; Chen, C.; Yin, M. Enzyme-triggered disassembly of perylene monoimide-based nanoclusters for activatable and deep photodynamic therapy. Angew. Chem. Int. Ed. 2020, 59, 14014–14018. [Google Scholar] [CrossRef]
- Yang, N.; Song, S.; Ren, J.; Liu, C.; Li, Z.; Qi, H.; Yu, C. Controlled aggregation of a perylene-derived probe for near-infrared fluorescence imaging and phototherapy. ACS Appl. Biol. Mater. 2021, 4, 5008–5015. [Google Scholar] [CrossRef]
- Collot, M.; Kreder, R.; Tatarets, A.L.; Patsenker, L.D.; Mely, Y.; Klymchenko, A.S. Bright fluorogenic squaraines with tuned cell entry for selective imaging of plasma membrane vs. endoplasmic reticulum. Chem. Commun. 2015, 51, 17136–17139. [Google Scholar] [CrossRef]
- Collot, M.; Ashokkumar, P.; Anton, H.; Boutant, E.; Faklaris, O.; Galli, T.; Mely, Y.; Danglot, L.; Klymchenko, A.S. MemBright: A family of fluorescent membrane probes for advanced cellular imaging and neuroscience. Cell Chem. Biol. 2019, 26, 600–614. [Google Scholar] [CrossRef]
- Collot, M.; Boutant, E.; Fam, K.T.; Danglot, L.; Klymchenko, A.S. Molecular tuning of styryl dyes leads to versatile and efficient plasma membrane probes for cell and tissue imaging. Bioconjug. Chem. 2020, 31, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; O’Shea, D.F. Azadipyrromethenes: From traditional dye chemistry to leading edge applications. Chem. Soc. Rev. 2016, 45, 3846–3864. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Cheung, S.; Sampedro, G.; Chen, Z.L.; Cahill, R.A.; O’Shea, D.F. A DIE responsive NIR-fluorescent cell membrane probe. BBA Biomembr. 2018, 1860, 2272–2280. [Google Scholar] [CrossRef]
- Wang, D.; Su, H.; Kwok, R.T.K.; Hu, X.; Zou, H.; Luo, Q.; Lee, M.M.S.; Xu, W.; Lam, J.W.Y.; Tang, B.Z. Rational design of a water-soluble NIR AIEgen, and its application in ultrafast wash-free cellular imaging and photodynamic cancer cell ablation. Chem. Sci. 2018, 9, 3685–3693. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, C.Y.Y.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. A photostable AIE luminogen with near infrared emission for monitoring morphological change of plasma membrane. J. Mater. Chem. B 2018, 6, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Wu, M.; Liu, X.; Chen, H.; Duan, Y.; Liu, J.; Pan, Y.; Liu, B. Activation of pyroptosis by membrane-anchoring AIE photosensitizer design: New prospect for photodynamic cancer cell ablation. Angew. Chem. Int. Ed. 2021, 60, 9093–9098. [Google Scholar] [CrossRef]
- Dai, Y.; Xue, K.; Zhao, X.; Zhang, P.; Zhang, D.; Qi, Z. Rationally designed near-infrared AIEgens photosensitizer for cell membrane-targeted photo-driven theranostics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 286, 122013. [Google Scholar] [CrossRef]
- Farrugia, G.; Szurszewski, J.H. Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract. Gastroenterology 2014, 147, 303–313. [Google Scholar] [CrossRef]
- Yusufzai, T.; Kadonaga, J. HARP is an ATP-driven annealing helicase. Science 2008, 322, 748–750. [Google Scholar] [CrossRef]
- Xu, S.; Liu, H.W.; Yin, X.; Yuan, L.; Huan, S.Y.; Zhang, X.B. A cell membrane-anchored fluorescent probe for monitoring carbon monoxide release from living cells. Chem. Sci. 2019, 10, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gu, X.; Dong, P.; Chu, L.; Zhang, Z.; Cheng, Z.; Yang, F. Cell-membrane-targeted near-infrared fluorescent probe for detecting extracellular ATP. Analyst 2022, 147, 4167–4173. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, R.; Cui, X.; Hu, C.; Chen, Z. A pH-sensitive carbonic anhydrase IX-targeted near-infrared probe for fluorescent sensing and imaging of hypoxic osteosarcoma. Sens. Actuators B Chem. 2023, 379, 133171. [Google Scholar] [CrossRef]
- Wu, L.-L.; Zhao, Q.; Wang, Q.; Zhang, Q.; Yang, F.; Zheng, B.; Hu, H.-Y.; Xing, N. Membrane dual-targeting probes: A promising strategy for fluorescence-guided prostate cancer surgery and lymph node metastases detection. Acta Pharm. Sin. B, 2022; in press. [Google Scholar] [CrossRef]
- Abdul Sisak, M.A.; Louis, F.; Miyao, T.; Lee, S.H.; Chang, Y.T.; Matsusaki, M. Mechanism assay of interaction between blood vessels-near infrared probe and cell surface marker proteins of endothelial cells. Mater. Today Bio 2022, 15, 100332. [Google Scholar] [CrossRef]
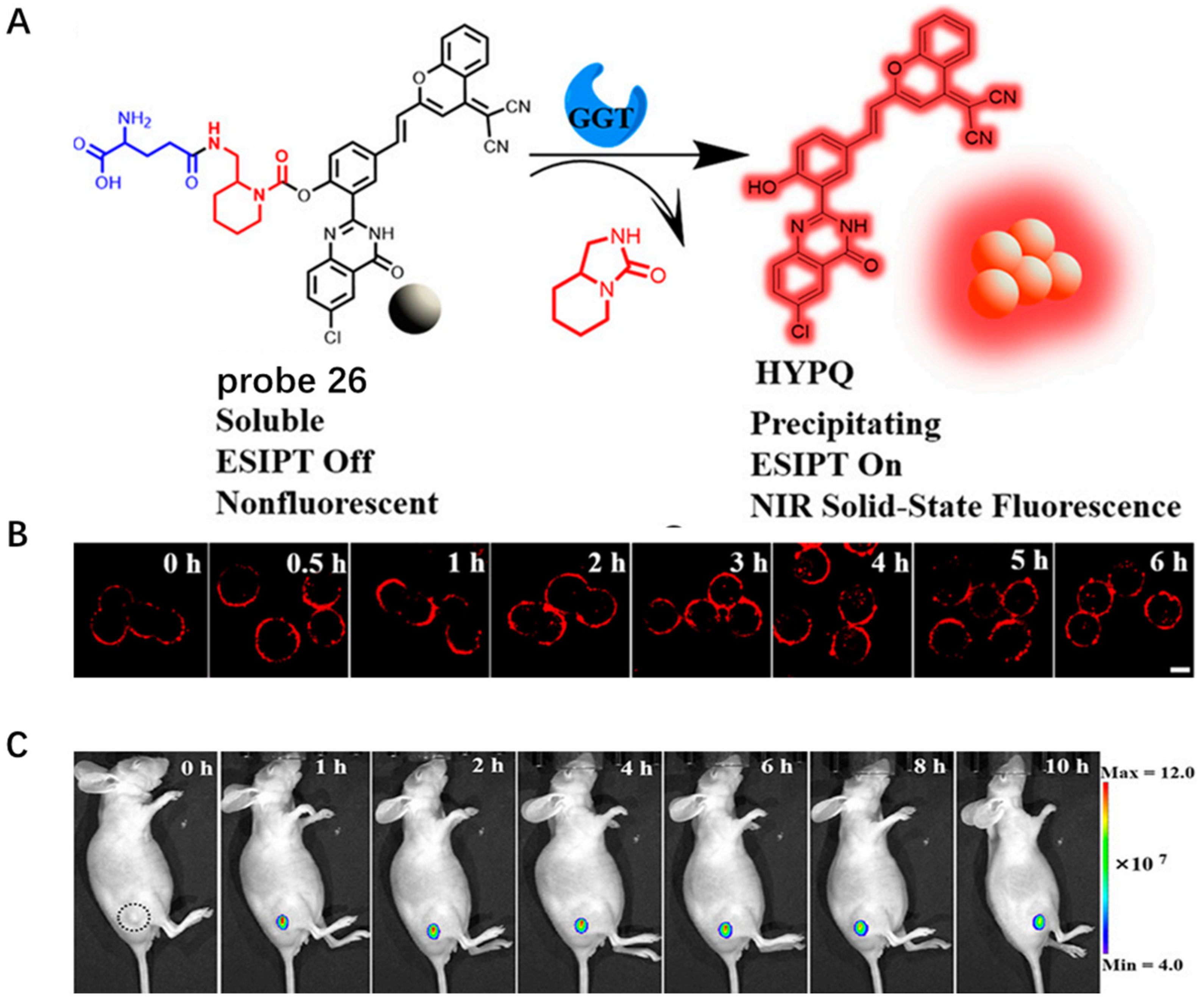
- Li, K.; Lyu, Y.; Huang, Y.; Xu, S.; Liu, H.W.; Chen, L.; Ren, T.B.; Xiong, M.; Huan, S.; Yuan, L.; et al. A de novo strategy to develop NIR precipitating fluorochrome for long-term in situ cell membrane bioimaging. Proc. Natl. Acad. Sci. USA 2021, 118, e2018033118. [Google Scholar] [CrossRef]
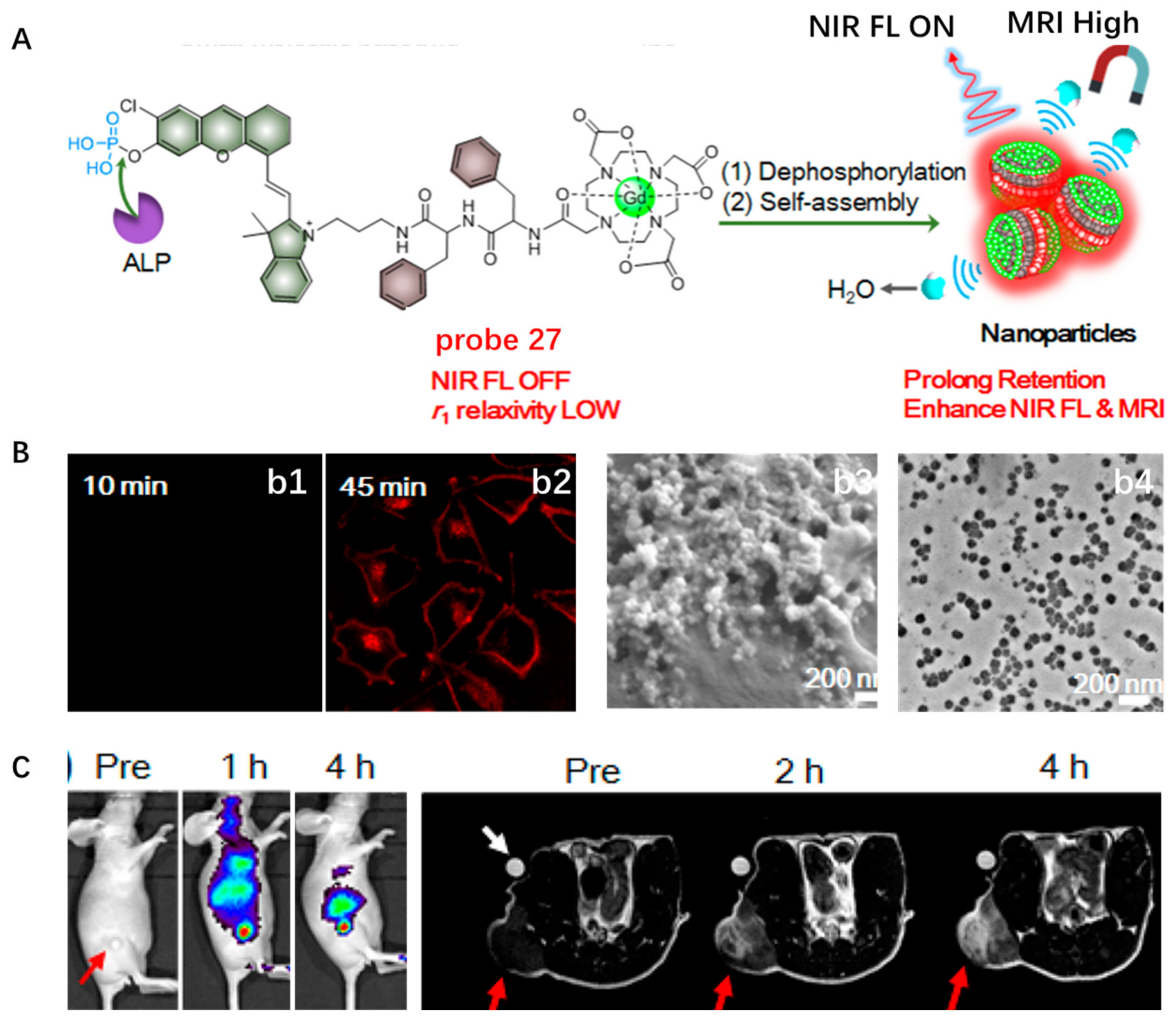
- Yan, R.; Hu, Y.; Liu, F.; Wei, S.; Fang, D.; Shuhendler, A.J.; Liu, H.; Chen, H.Y.; Ye, D. Activatable NIR fluorescence/MRI bimodal probes for in vivo imaging by enzyme-mediated fluorogenic reaction and self-assembly. J. Am. Chem. Soc. 2019, 141, 10331–10341. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Pan, W.; Song, Z.-L.; Yuan, L. Molecular Engineering of Near-Infrared Fluorescent Probes for Cell Membrane Imaging. Molecules 2023, 28, 1906. https://doi.org/10.3390/molecules28041906
Xu S, Pan W, Song Z-L, Yuan L. Molecular Engineering of Near-Infrared Fluorescent Probes for Cell Membrane Imaging. Molecules. 2023; 28(4):1906. https://doi.org/10.3390/molecules28041906
Chicago/Turabian StyleXu, Shuai, Wenjing Pan, Zhi-Ling Song, and Lin Yuan. 2023. "Molecular Engineering of Near-Infrared Fluorescent Probes for Cell Membrane Imaging" Molecules 28, no. 4: 1906. https://doi.org/10.3390/molecules28041906
APA StyleXu, S., Pan, W., Song, Z.-L., & Yuan, L. (2023). Molecular Engineering of Near-Infrared Fluorescent Probes for Cell Membrane Imaging. Molecules, 28(4), 1906. https://doi.org/10.3390/molecules28041906






