Effects and Mechanism of the Leontopodium alpinum Callus Culture Extract on Blue Light Damage in Human Foreskin Fibroblasts
Abstract
1. Introduction
2. Results
2.1. Analysis of the LACCE Chemical Composition
2.2. High-Energy Visible-Induced Photodamage Model in HFF Cells
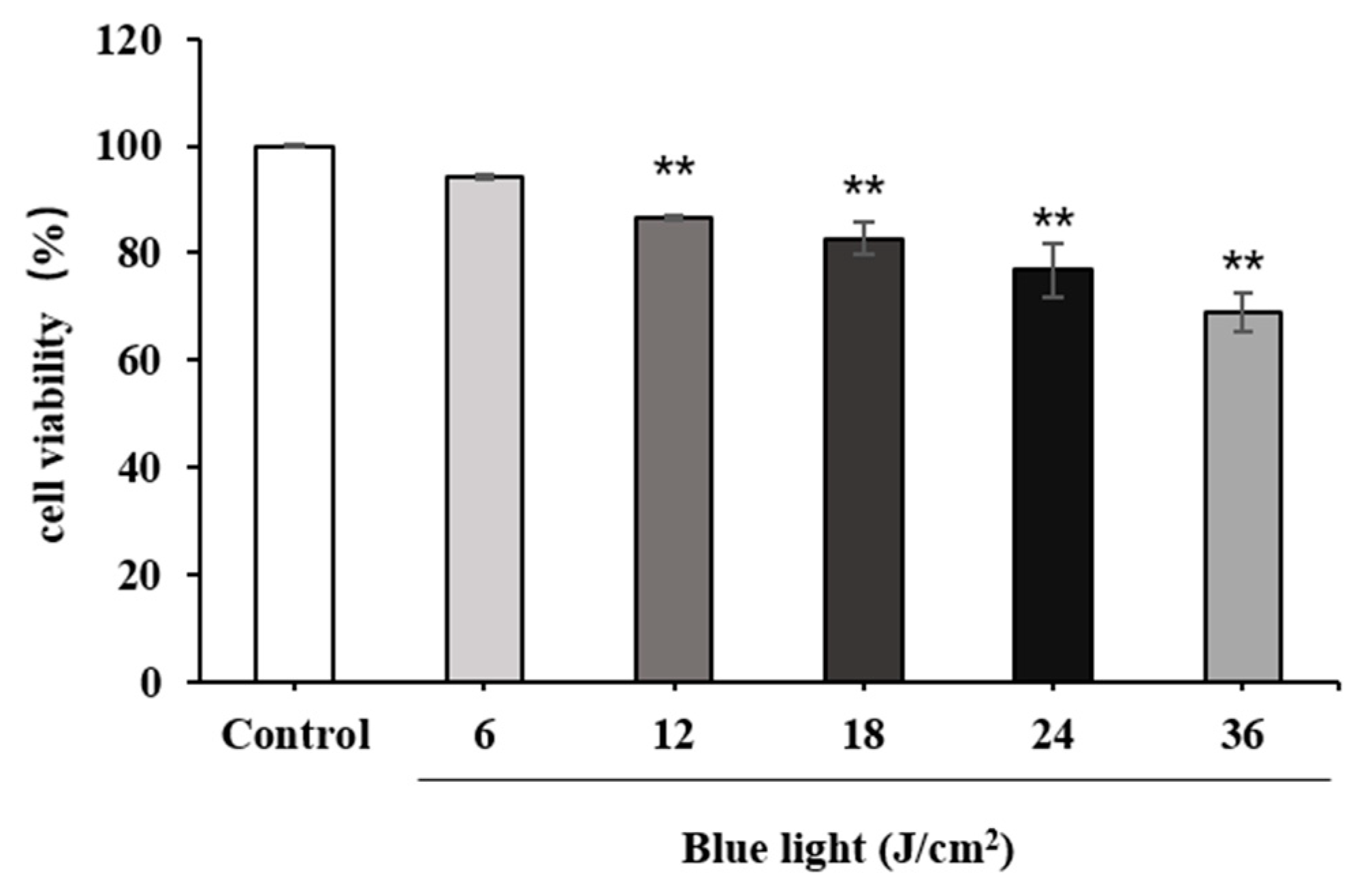
2.2.1. Effect of Blue Light on HFF Cell Viability
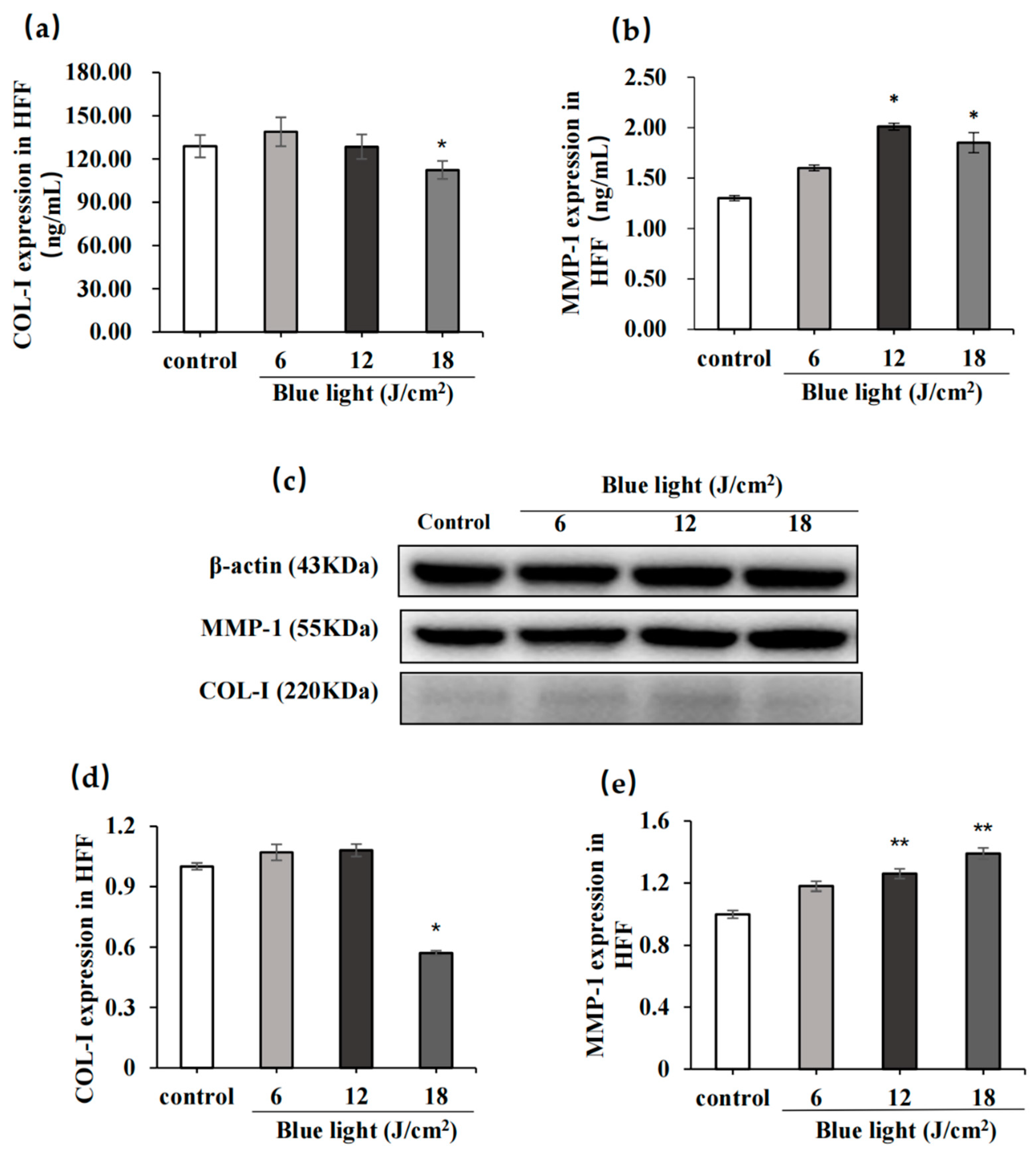
2.2.2. Effect of Blue Light on COL-I and MMP-1
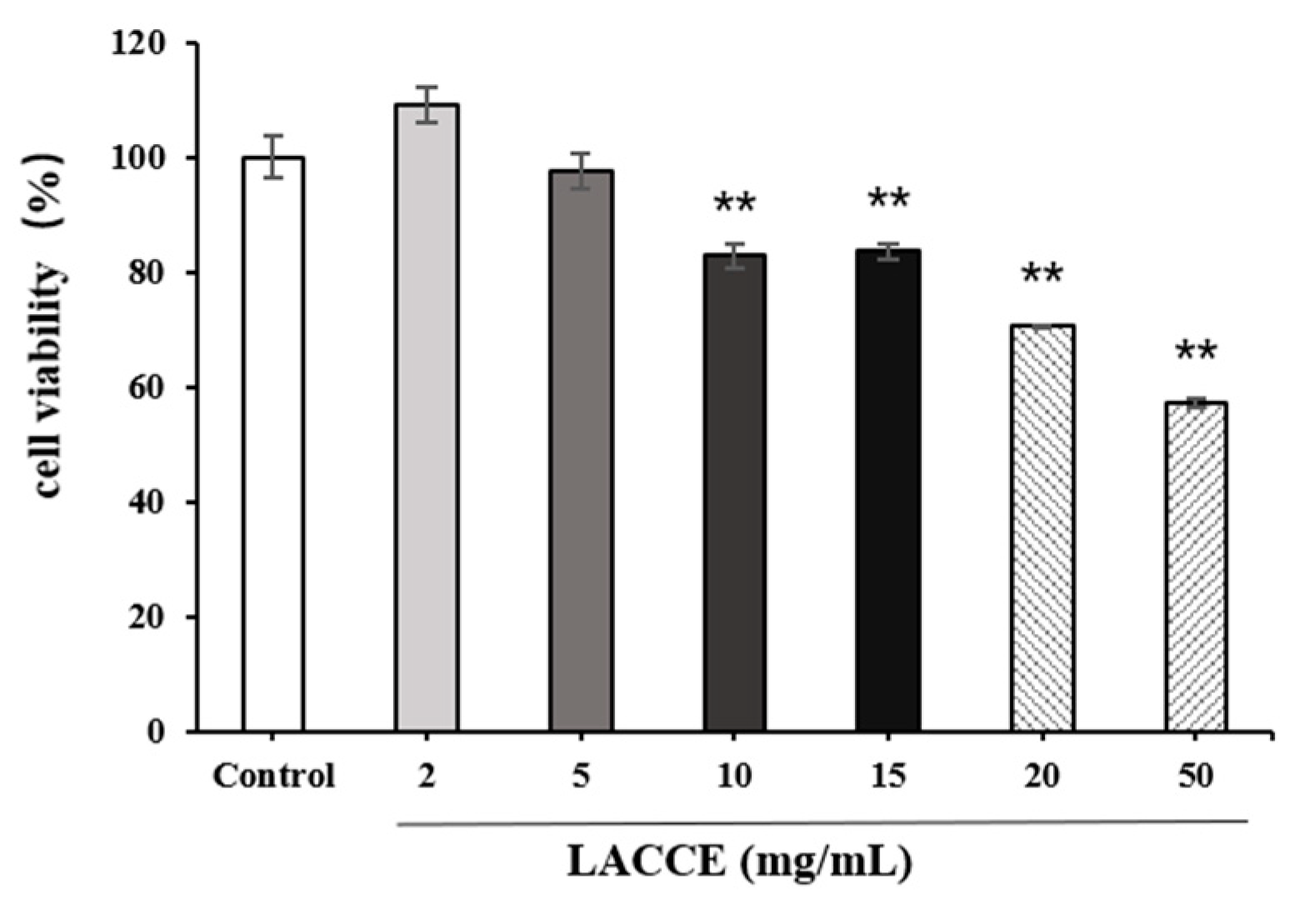
2.3. HFF Cell Viability after LACCE Treatment
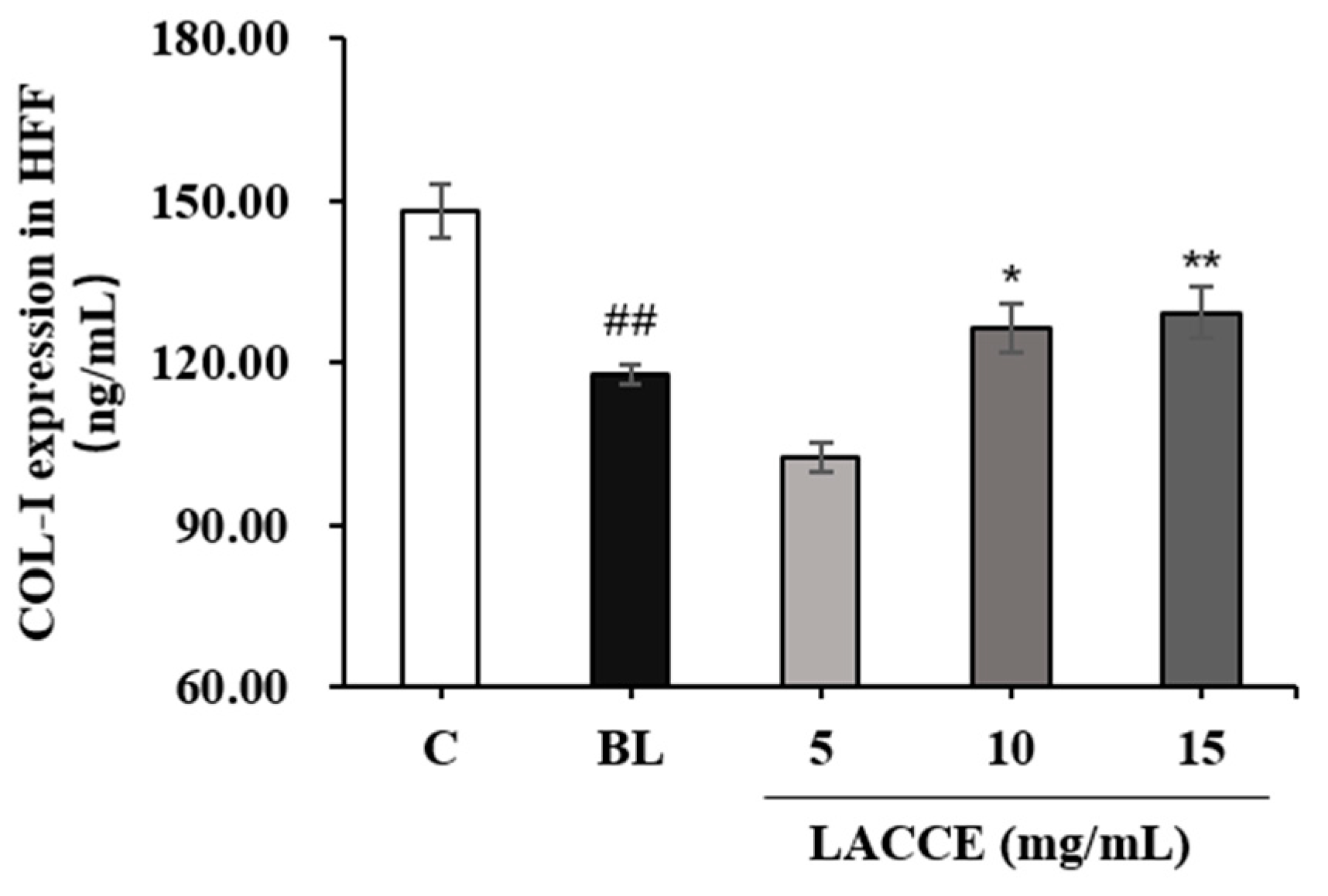
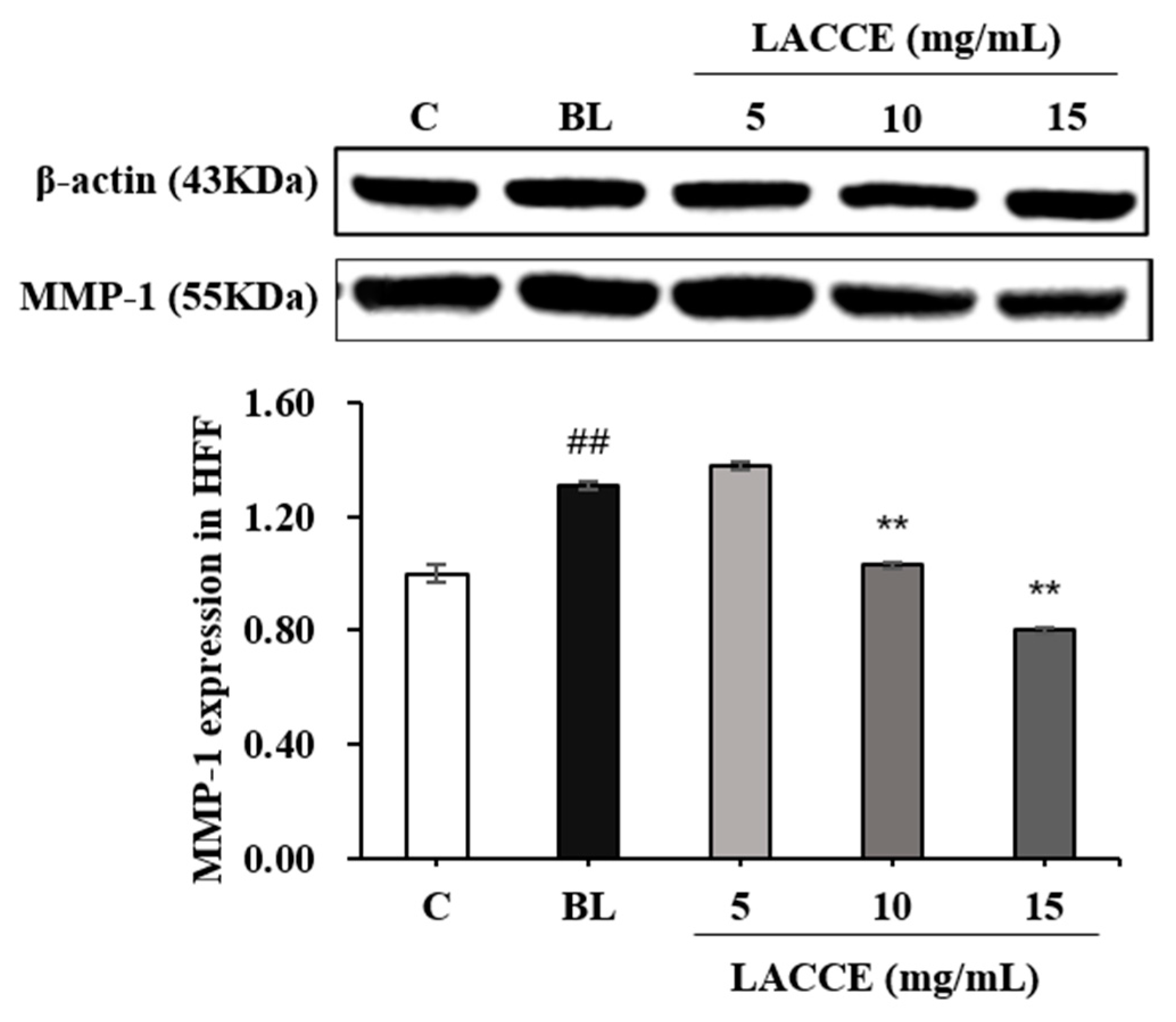
2.4. Effects of the LACCE on COL-I and MMP-1 Levels
2.5. Effects of the LACCE on the OPN3-Calcium-Dependent Signal Transduction Pathways
2.5.1. Effect of the LACCE on OPN3 Levels
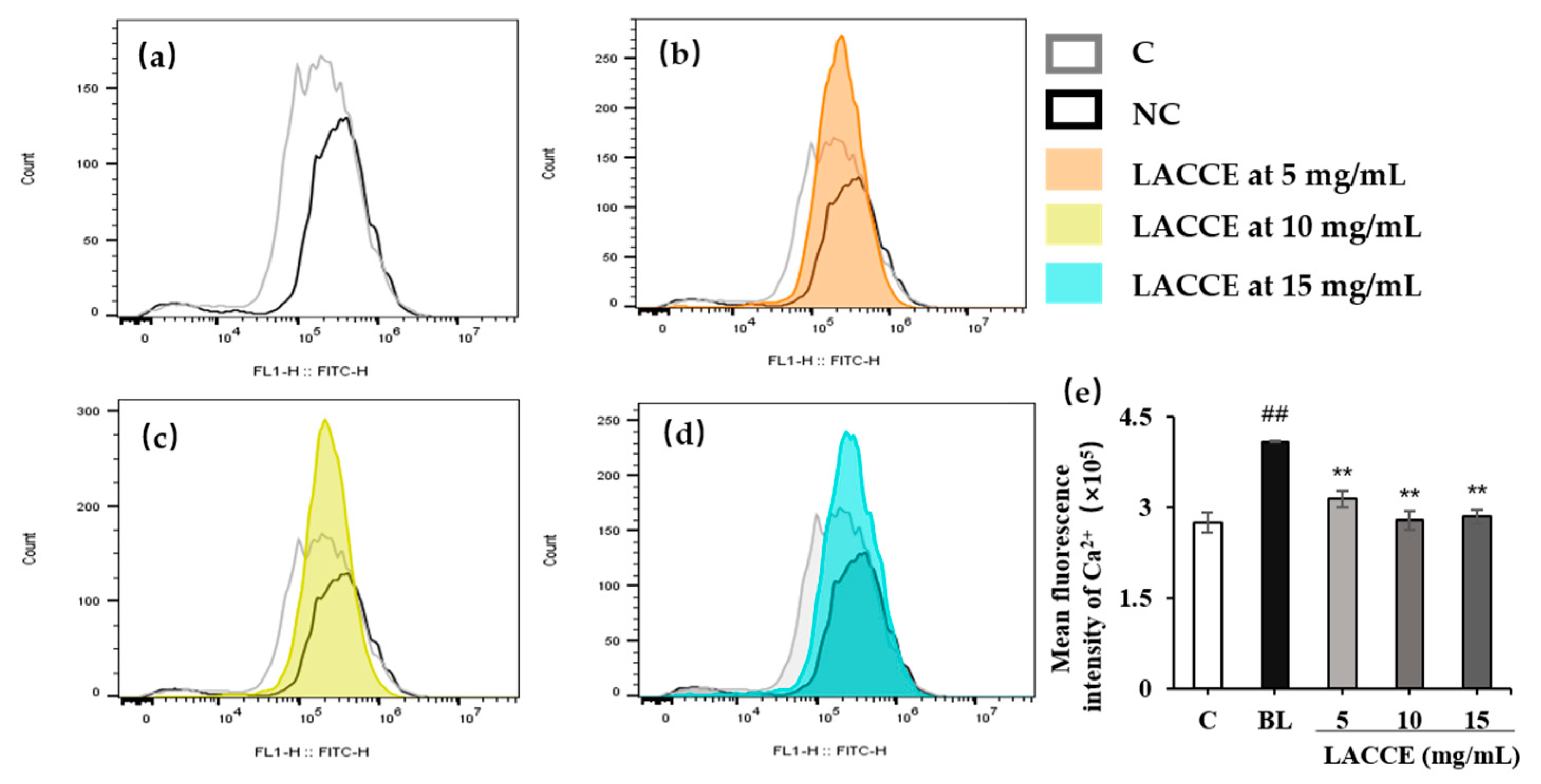
2.5.2. Effect of the LACCE on Ca2+ Flow into the HFF Cells
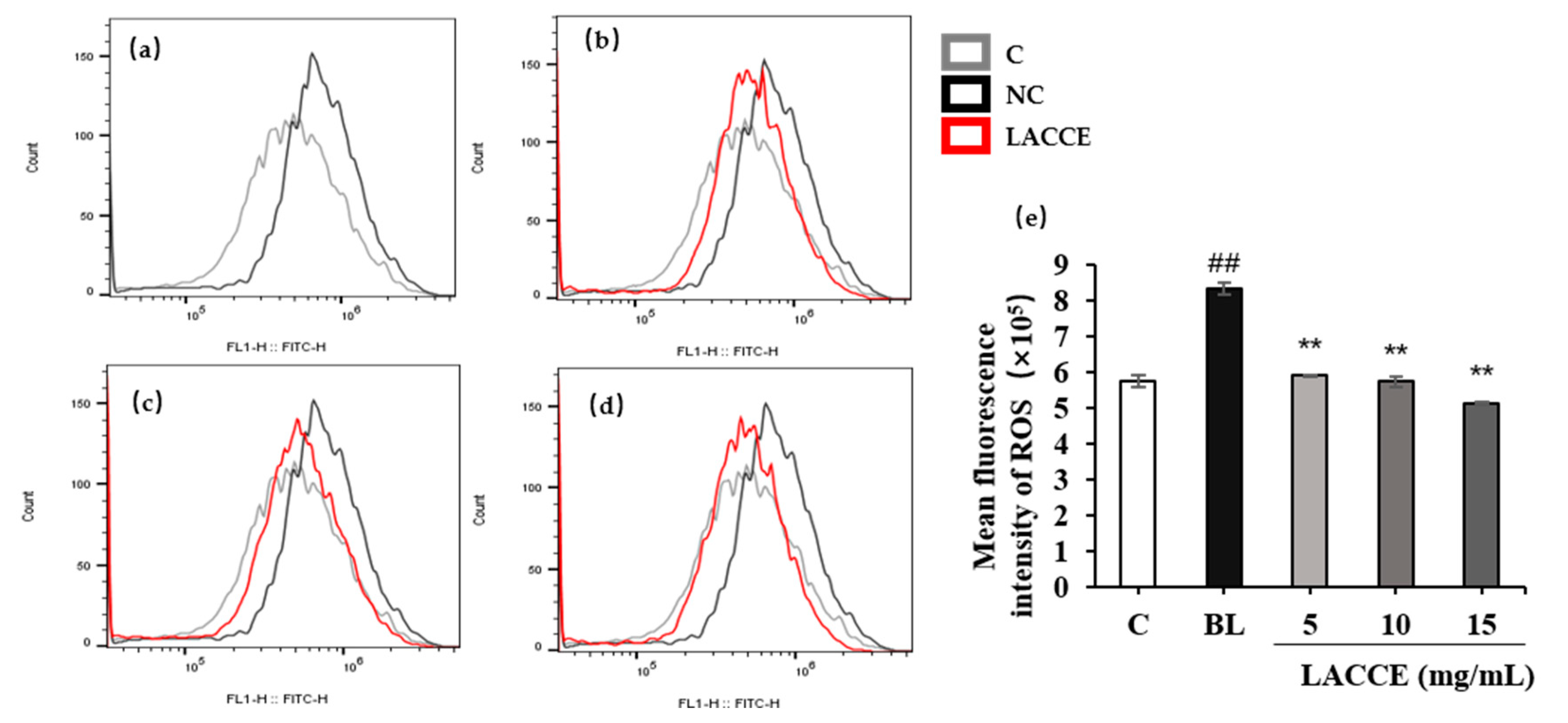
2.5.3. Effect of the LACCE on ROS Levels in HFF Cells
3. Discussion
4. Materials and Methods
4.1. Analysis of the Compounds in LACCE Using UPLC-MS/MS
4.2. Analysis of the Compounds in the LACCE Using HPLC
4.3. Cell Culture and Materials
4.4. LACCE Preparation
4.5. Cell Culture
4.6. Cell Viability Assay
4.7. Measurement of COL-I and MMP-1 Levels with ELISA
4.8. Measurement of COL-I and MMP-1 Levels with WB
4.9. Using Flow Cytometry for ROS Production Analysis
4.10. Using Flow Cytometry for Ca2+ Production Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campiche, R.; Curpen, S.J.; Lutchmanen-Kolanthan, V.; Gougeon, S.; Cherel, M.; Laurent, G.; Gempeler, M.; Schuetz, R. Pigmentation effects of blue light irradiation on skin and how to protect against them. Int. J. Cosmet. Sci. 2020, 42, 399–406. [Google Scholar] [CrossRef]
- Algvere, P.V.; Marshall, J.; Seregard, S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol. Scand. 2006, 84, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, N.; Mortazavi, G.; Zarei, S.; Faraz, M.; Mortazavi, S.A.R. Can Light Emitted from Smartphone Screens and Taking Selfies Cause Premature Aging and Wrinkles? J. Biomed. Phys. Eng. 2018, 8, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Bonina, F.; Cardile, V. Hydroxytyrosol from olive fruits prevents blue-light-induced damage in human keratinocytes and fibroblasts. J. Cell. Physiol. 2019, 234, 9065–9076. [Google Scholar] [CrossRef]
- Mann, T.; Eggers, K.; Rippke, F.; Tesch, M.; Buerger, A.; Darvin, M.E.; Schanzer, S.; Meinke, M.C.; Lademann, J.; Kolbe, L. High-energy visible light at ambient doses and intensities induces oxidative stress of skin-Protective effects of the antioxidant and Nrf2 inducer Licochalcone A in vitro and in vivo. Photodermatol. Photoimmunol. Photomed. 2020, 36, 135–144. [Google Scholar] [CrossRef]
- Austin, E.; Huang, A.; Adar, T.; Wang, E.; Jagdeo, J. Electronic device generated light increases reactive oxygen species in human fibroblasts. Lasers Surg. Med. 2018, 50, 689–695. [Google Scholar] [CrossRef] [PubMed]
- de Gálvez, E.N.; Aguilera, J.; Solis, A.; de Gálvez, M.V.; de Andrés, J.R.; Herrera-Ceballos, E.; Gago-Calderon, A. The potential role of UV and blue light from the sun, artificial lighting, and electronic devices in melanogenesis and oxidative stress. J. Photochem. Photobiol. B Biol. 2022, 228, 112405. [Google Scholar] [CrossRef] [PubMed]
- Opländer, C.; Hidding, S.; Werners, F.B.; Born, M.; Pallua, N.; Suschek, C.V. Effects of blue light irradiation on human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2011, 103, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Ko, H.J.; Kim, K.; Sohn, Y.; Min, S.Y.; Kim, J.A.; Na, D.; Yeon, J.H. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J. Extracell. Vesicles 2020, 9, 1703480. [Google Scholar] [CrossRef] [PubMed]
- Olinski, L.E.; Lin, E.M.; Oancea, E. Illuminating insights into opsin 3 function in the skin. Adv. Biol. Regul. 2020, 75, 100668. [Google Scholar] [CrossRef]
- Haltaufderhyde, K.; Ozdeslik, R.N.; Wicks, N.L.; Najera, J.A.; Oancea, E. Opsin Expression in Human Epidermal Skin. Photochem. Photobiol. 2015, 91, 117–123. [Google Scholar] [CrossRef]
- Austin, E.; Geisler, A.N.; Nguyen, J.; Kohli, I.; Hamzavi, I.; Lim, H.W.; Jagdeo, J. Visible light. Part I: Properties and cutaneous effects of visible light. J. Am. Acad. Derm. 2021, 84, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Choi, E.H.; Mesinkovska, N.A. The expression of opsins in the human skin and its implications for photobiomodulation: A Systematic Review. Photodermatol. Photoimmunol. Photomed. 2020, 36, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.K.; De Donatis, G.M.; Chignon-Sicard, B.; Rocchi, S.; Passeron, T. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. J. Investig. Dermatol. 2018, 138, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Pellicena, I.; Uzunbajakava, N.E.; Mignon, C.; Raafs, B.; Botchkarev, V.A.; Thornton, M.J. Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing? Lasers Surg. Med. 2019, 51, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wang, Y.; Lu, H. Opsin 3 is a key regulator of ultraviolet A-induced photoageing in human dermal fibroblast cells. Br. J. Dermatol. 2020, 182, 1228–1244. [Google Scholar] [CrossRef] [PubMed]
- GE, G. The Effects of Blue Light on Skin Aging and Its Molecular Mechanism. Ph.D. Thesis, China Medical University, Taichung, Taiwan, 2021. [Google Scholar]
- Tauchen, J.; Kokoska, L. The chemistry and pharmacology of Edelweiss: A review. Phytochem. Rev. 2017, 16, 295–308. [Google Scholar] [CrossRef]
- Costa, S.; Schwaiger, S.; Cervellati, R.; Stuppner, H.; Speroni, E.; Guerra, M.C. In vitro evaluation of the chemoprotective action mechanisms of leontopodic acid against aflatoxin B1 and deoxynivalenol-induced cell damage. J. Appl. Toxicol. JAT 2009, 29, 7–14. [Google Scholar] [CrossRef]
- Pralea, I.E.; Moldovan, R.C.; Țigu, A.B.; Petrache, A.M.; Hegheș, S.C.; Mitoi, M.; Cogălniceanu, G.; Iuga, C.A. Profiling of Polyphenolic Compounds of Leontopodium alpinum Cass Callus Cultures Using UPLC/IM-HRMS and Screening of In Vitro Effects. Plants 2022, 11, 100. [Google Scholar] [CrossRef]
- Reisinger, U.; Schwaiger, S.; Zeller, I.; Messner, B.; Stigler, R.; Wiedemann, D.; Mayr, T.; Seger, C.; Schachner, T.; Dirsch, V.M.; et al. Leoligin, the major lignan from Edelweiss, inhibits intimal hyperplasia of venous bypass grafts. Cardiovasc. Res. 2009, 82, 542–549. [Google Scholar] [CrossRef]
- Dobner, M.J.; Sosa, S.; Schwaiger, S.; Altinier, G.; Della Loggia, R.; Kaneider, N.C.; Stuppner, H. Anti-inflammatory activity of Leontopodium alpinum and its constituents. Planta Med. 2004, 70, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Speroni, E.; Schwaiger, S.; Egger, P.; Berger, A.T.; Cervellati, R.; Govoni, P.; Guerra, M.C.; Stuppner, H. In vivo efficacy of different extracts of Edelweiss (Leontopodium alpinum Cass.) in animal models. J. Ethnopharmacol. 2006, 105, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Daniela, L.; Alla, P.; Maurelli, R.; Elena, D.; Giovanna, P.; Vladimir, K.; Roberto, D.T.; Chiara, D.L.; Saveria, P.; Liudmila, K. Anti-inflammatory effects of concentrated ethanol extracts of Edelweiss (Leontopodium alpinum Cass.) callus cultures towards human keratinocytes and endothelial cells. Mediat. Inflamm. 2012, 2012, 498373. [Google Scholar] [CrossRef]
- Cho, W.K.; Kim, H.I.; Kim, S.Y.; Seo, H.H.; Song, J.; Kim, J.; Shin, D.S.; Jo, Y.; Choi, H.; Lee, J.H.; et al. Anti-Aging Effects of Leontopodium alpinum (Edelweiss) Callus Culture Extract Through Transcriptome Profiling. Genes 2020, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Marlot, L.; Batteau, M.; Escofet, M.C.; Nuccio, S.; Coquoin, V.; De Vaumas, R.; Faure, K. Two-dimensional multi-heart cutting centrifugal partition chromatography-liquid chromatography for the preparative isolation of antioxidants from Edelweiss plant. J. Chromatogr. A 2017, 1504, 55–63. [Google Scholar] [CrossRef] [PubMed]
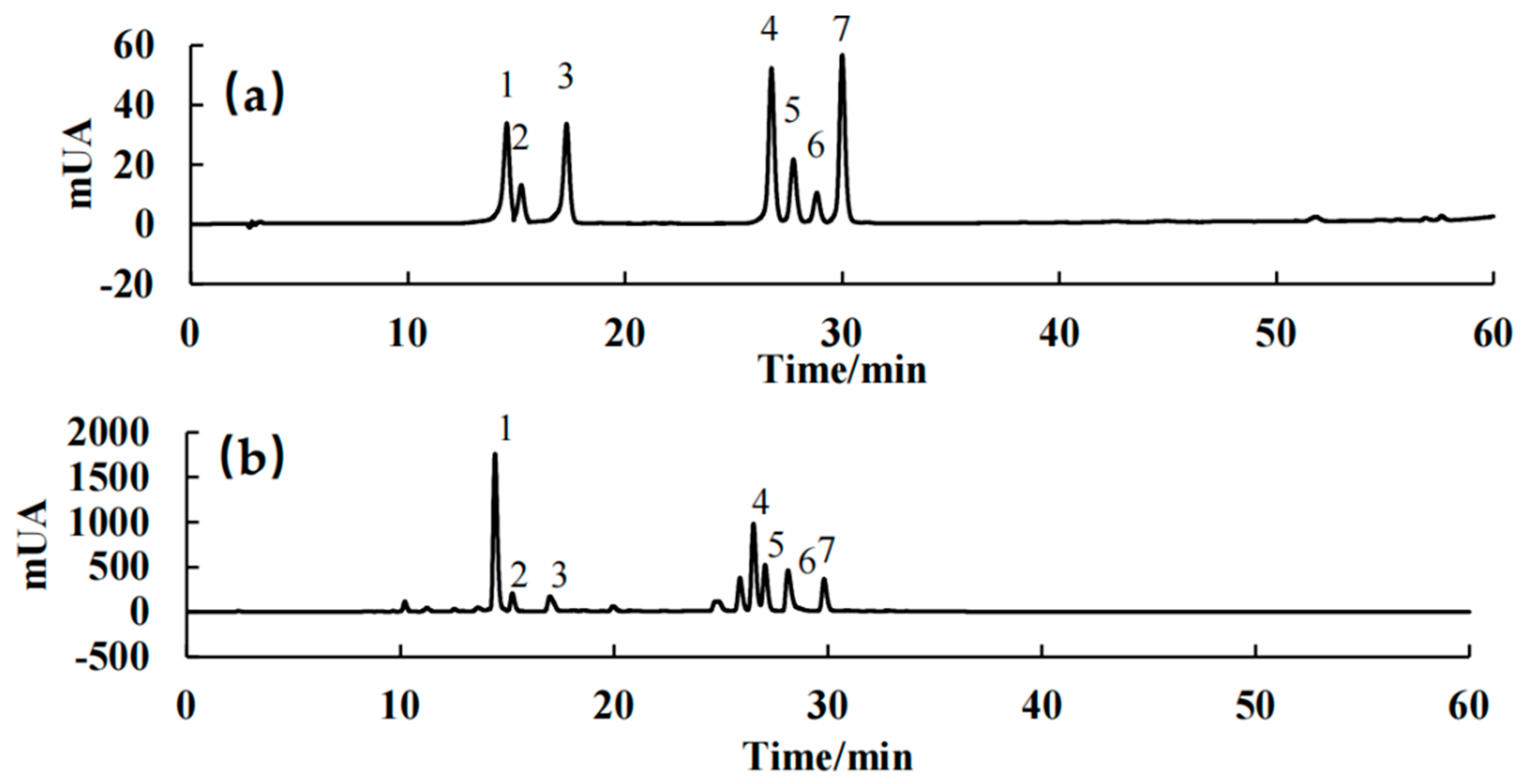


| No. | Components | Regression Equation | R2 | Range (μg/mL) | Rt (Retention Times) | Composition (μg/mL) |
|---|---|---|---|---|---|---|
| 1 | Syringin | y = 18.73x − 9.335 | 0.9996 | 5–80 | 14.58 | 40.49 ± 2.43 |
| 2 | Chlorogenic acid | y = 31.22x − 29.281 | 0.9999 | 5–80 | 15.28 | 125.96 ± 8.21 |
| 3 | Cynarin | y = 25.48x − 16.612 | 0.9996 | 2–50 | 17.34 | 30.58 ± 3.40 |
| 4 | Isochlorogenic acid | y = 30.74x − 41.030 | 0.9998 | 2–80 | 26.76 | 60.97 ± 5.85 |
| 5 | Asiatica | y = 21.21x − 19.960 | 0.9999 | 2–80 | 27.68 | 52.33 ± 2.37 |
| 6 | Isoquercitrin | y = 18.73x − 9.335 | 0.9997 | 5–80 | 28.89 | 128.27 ± 6.39 |
| 7 | Isochlorogenic acid C | y = 18.73x − 9.335 | 0.9997 | 0.8–80 | 29.97 | 72.04 ± 2.96 |
| Parameter | Leontopodic acid A | Leontopodic acid B |
|---|---|---|
| Regression equation | y = 0.8106x − 5.2966 | y = 1.5604x + 13.3110 |
| Correlation coefficient (R2) | 0.9962 | 0.9990 |
| Range (ng/mL) | 10–100 | 10–100 |
| LOD (μg/mL) | 3 | 0.4 |
| LOQ (μg/mL) | 10 | 5 |
| Rt (retention times) | 2.06 | 1.73 |
| Composition (μg/mL) | 184.13 ± 6.21 | 161.10 ± 3.85 |
| Time/min | A % | B % | Flow Rate (mL/min) |
|---|---|---|---|
| Initial | 0 | 100 | 0.3 |
| 1.00 | 0 | 100 | 0.3 |
| 1.50 | 70 | 30 | 0.3 |
| 4.00 | 70 | 30 | 0.3 |
| 4.01 | 0 | 100 | 0.3 |
| 5.00 | 0 | 100 | 0.3 |
| Component | Parent Ion | Daughter Ion | Ion Source Mode | Capillary (kV) | Cone (V) | Collision (V) |
|---|---|---|---|---|---|---|
| Leontopodic Acid A | 781.10 | 190.99 | ES- | 3.5 | 50 | 24 |
| 295.01 | 50 | 24 | ||||
| 457.01 | 50 | 24 | ||||
| 617.01 | 50 | 24 | ||||
| Leontopodic Acid B | 695.12 | 84.91 | ES- | 3.5 | 46 | 36 |
| 208.99 | 46 | 36 | ||||
| 370.98 | 46 | 36 |
| Time/min | A % | B % | Flow Rate (mL/min) |
|---|---|---|---|
| 1 | 90 | 10 | 1 |
| 5 | 80 | 20 | 1 |
| 55 | 20 | 80 | 1 |
| 60 | 0 | 100 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Guo, M.; Geng, Z.; Wang, Z.; Zhang, H.; Li, S.; Ling, X.; Li, L. Effects and Mechanism of the Leontopodium alpinum Callus Culture Extract on Blue Light Damage in Human Foreskin Fibroblasts. Molecules 2023, 28, 2172. https://doi.org/10.3390/molecules28052172
Meng X, Guo M, Geng Z, Wang Z, Zhang H, Li S, Ling X, Li L. Effects and Mechanism of the Leontopodium alpinum Callus Culture Extract on Blue Light Damage in Human Foreskin Fibroblasts. Molecules. 2023; 28(5):2172. https://doi.org/10.3390/molecules28052172
Chicago/Turabian StyleMeng, Xianyao, Miaomiao Guo, Zaijun Geng, Ziqiang Wang, Huirong Zhang, Sunhua Li, Xiao Ling, and Li Li. 2023. "Effects and Mechanism of the Leontopodium alpinum Callus Culture Extract on Blue Light Damage in Human Foreskin Fibroblasts" Molecules 28, no. 5: 2172. https://doi.org/10.3390/molecules28052172
APA StyleMeng, X., Guo, M., Geng, Z., Wang, Z., Zhang, H., Li, S., Ling, X., & Li, L. (2023). Effects and Mechanism of the Leontopodium alpinum Callus Culture Extract on Blue Light Damage in Human Foreskin Fibroblasts. Molecules, 28(5), 2172. https://doi.org/10.3390/molecules28052172





