Bio-Template Synthesis of V2O3@Carbonized Dictyophora Composites for Advanced Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
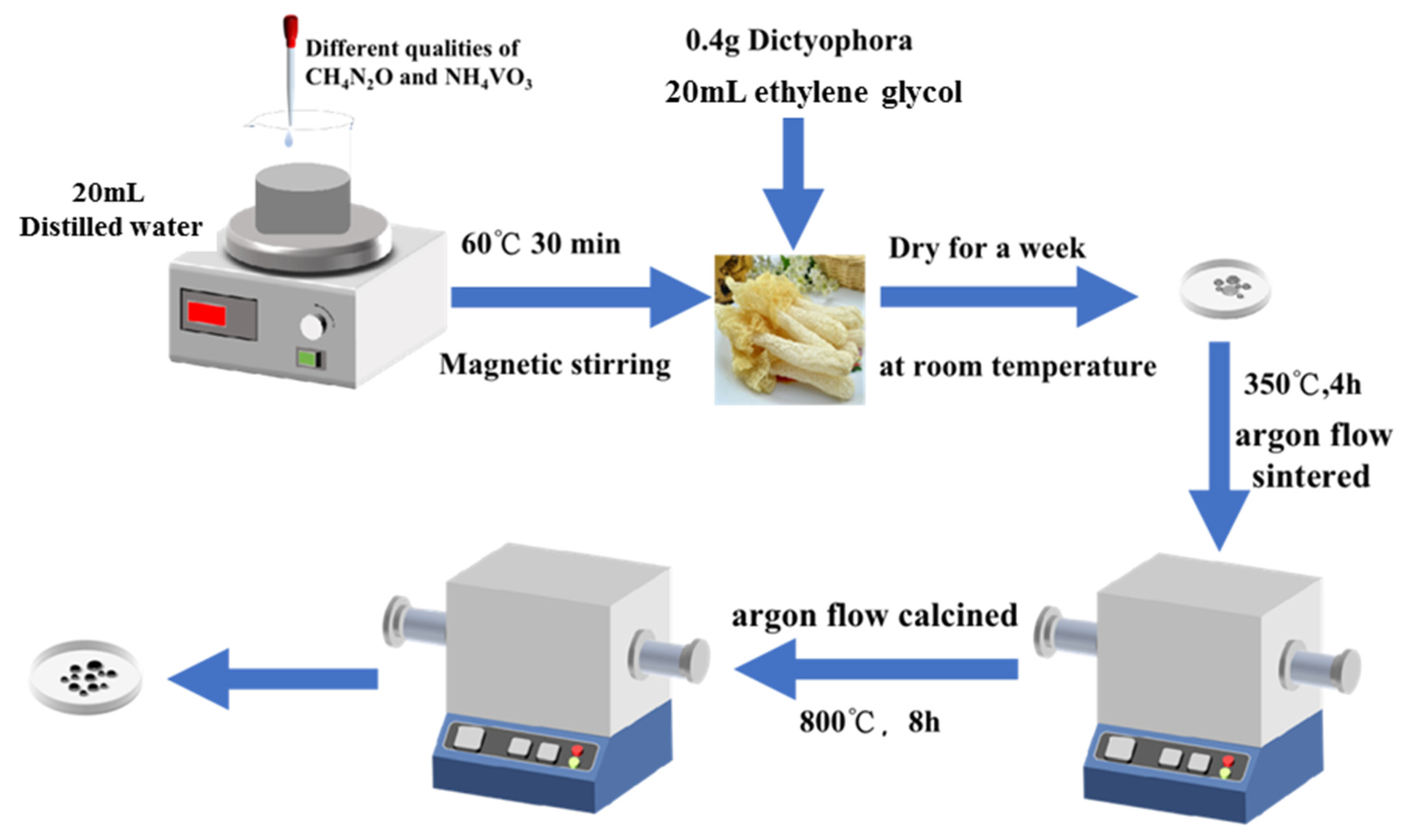
4.1. Materials Synthesis
4.2. Materials Characterization
4.3. Electrochemical Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pang, Q.; Sun, C.; Yu, Y.; Zhao, K.; Zhang, Z.; Voyles, P.M.; Chen, G.; Wei, Y.; Wang, X. H2V3O8 Nanowire/Graphene Electrodes for Aqueous Rechargeable Zinc Ion Batteries with High Rate Capability and Large Capacity. Adv. Energy Mater. 2018, 8, 1800144. [Google Scholar] [CrossRef]
- Tamilselvan, M.; Sreekanth, T.; Yoo, K.; Kim, J. Binder-Free Coaxially Grown V6O13 Nanobelts on Carbon Cloth as Cathodes for Highly Reversible Aqueous Zinc Ion Batteries. Appl. Surf. Sci. 2020, 529, 147077. [Google Scholar] [CrossRef]
- Yu, J.; Liu, S.; Duan, G.; Fang, H.; Hou, H. Dense and thin coating of gel polymer electrolyte on sulfur cathode toward high performance Li-sulfur battery. Compos. Commun. 2020, 19, 239–245. [Google Scholar] [CrossRef]
- Chen, S.; Jeong, S.R.; Tao, S. Key materials and future perspective for aqueous rechargeable lithium-ion batteries. Mater. Rep. Energy 2022, 2, 100096. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, Z.; Lan, D.; Liu, Y.; Cui, J. N-doped carbon/V2O3 microfibers as high-rate and ultralong-life cathode for rechargeable aqueous zinc-ion batteries. J. Alloys Compd. 2020, 861, 158560. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Lv, T.T.; Wang, H.; Guo, X.T.; Pang, H. Nsutite-Type VO2 Microcrystals as Highly Durable Cathode Materials for Aqueous Zinc-Ion Batteries. Chem. Eng. J. 2021, 417, 128408. [Google Scholar] [CrossRef]
- Chen, X.; Hu, X.; Chen, Y.; Cao, X.; Huang, Y.; Zhang, H.; Liu, J.-H.; Wang, Y.; Chou, S.-L.; Cao, D. Ultrastable hydrated vanadium dioxide cathodes for high-performance aqueous zinc ion batteries with H+/Zn2+ Co-insertion mechanism. J. Mater. Chem. A 2022, 10, 22194–22204. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Xu, L.; Jiang, H.M.; Liu, Y.Y.; Meng, C.G. Polyaniline-expanded the interlayer spacing of hydrated vanadium pentoxide by the interface-intercalation for aqueous rechargeable Zn-ion batteries. J. Colloid Interface Sci. 2021, 603, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Rong, Y.; Yang, Z.; Deng, L.; Wu, J. V2O3@Amorphous Carbon as a Cathode of Zinc Ion Batteries with High Stability and Long Cycling Life. Ind. Eng. Chem. Res. 2021, 60, 1517–1525. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Waqar, M.; Yang, J.; Liu, Y.; Sun, J.; Feng, Z.; Sun, J.; Pan, Z.; Meng, C.; et al. Anomalous Zn2+ Storage Behavior in Dual-Ion-In-Sequence Reconstructed Vanadium Oxides. Adv. Funct. Mater. 2023, 33, 2213127. [Google Scholar] [CrossRef]
- Li, J.; Xue, H.; Xu, N.; Zhang, X.; Wang, Y.; He, R.; Huang, H.; Qiao, J. Co/Ni dual-metal embedded in heteroatom doped porous carbon core-shell bifunctional electrocatalyst for rechargeable Zn-air batteries. Mater. Rep. Energy 2022, 2, 100090. [Google Scholar] [CrossRef]
- Guo, W.; Guo, X.; Yang, L.; Wang, T.; Zhang, M.; Duan, G.; Liu, X.; Li, Y. Synthetic melanin facilitates MnO supercapacitors with high specific capacitance and wide operation potential window. Polymer 2021, 235, 124276. [Google Scholar] [CrossRef]
- Ding, Y.C.; Peng, Y.Q.; Chen, S.H.; Zhang, X.X.; Li, Z.Q.; Zhu, L.; Mo, L.E.; Hu, L.H. Hierarchical Porous Metallic V2O3@C for Advanced Aqueous Zinc-Ion Batteries. Acs Appl. Mater. Interfaces 2019, 11, 44109–44117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Liu, Y.; Shi, X.; Zhang, Y.; Bai, L.; Wang, Q.; Sun, L. Oxygen-Deficient α-MnO2 Nanotube/Graphene/N, P Codoped Porous Carbon Composite Cathode To Achieve High-Performing Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 36668–36678. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-L.; Li, D.-S.; Liu, X.-M.; Wang, Y.-F.; Liu, W.-L.; Ren, M.-M.; Kong, F.; Wang, S.-J.; Zhou, R.-C. Biomass-derived mesoporous carbons materials coated by α-Mn3O4 with ultrafast zinc-ion diffusion ability as cathode for aqueous zinc ion batteries. Electrochim. Acta 2020, 335, 135642. [Google Scholar] [CrossRef]
- Zhu, X.D.; Cao, Z.Y.; Li, X.L.; Pei, L.Y.; Jones, J.; Zhou, Y.N.; Dong, P.; Wang, L.P.; Xe, M.X.; Shen, J.F. Ion-intercalation regulation of MXene-derived hydrated vanadates for high-rate and long-life Zn-Ion batteries. Energy Storage Mater. 2022, 45, 568–577. [Google Scholar] [CrossRef]
- Lv, T.T.; Luo, X.; Yuan, G.Q.; Yang, S.Y.; Pang, H. Layered VO2@N-doped carbon composites for high-performance rechargeable aqueous zinc-ion batteries. Chem. Eng. J. 2022, 428, 131211. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, D.H.; Ma, Y.J.; Zhi, L.J. Cascading V2O3/N-doped carbon hybrid nanosheets as high-performance cathode materials for aqueous zinc-ion batteries. Chin. Chem. Lett. 2022, 33, 1430–1434. [Google Scholar] [CrossRef]
- Zheng, J.; Zhan, C.Y.; Zhang, K.; Fu, W.W.; Nie, Q.J.; Zhang, M.; Shen, Z.R. Rapid Electrochemical Activation of V2O3@C Cathode for High-Performance Zinc-Ion Batteries in Water-in-Salt Electrolyte. Chemsuschem 2022, 15, e202200075. [Google Scholar] [CrossRef]
- Ding, J.W.; Zheng, H.Y.; Gao, H.G.; Liu, Q.N.; Hu, Z.; Han, L.F.; Wang, S.W.; Wu, S.D.; Fang, S.M.; Chou, S.L. In Situ Lattice Tunnel Distortion of Vanadium Trioxide for Enhancing Zinc Ion Storage. Adv. Energy Mater. 2021, 11, 2100973. [Google Scholar] [CrossRef]
- Du, Y.H.; Wang, X.Y.; Zhang, Y.; Zhang, H.B.; Man, J.Z.; Liu, K.; Sun, J.C. High mass loading CaV4O9 microflowers with amorphous phase transformation as cathode for aqueous zinc-ion battery. Chem. Eng. J. 2022, 434, 134642. [Google Scholar] [CrossRef]
- Chen, L.N.; Ruan, Y.S.; Zhang, G.B.; Wei, Q.L.; Jiang, Y.L.; Xiong, T.F.; He, P.; Yang, W.; Yan, M.Y.; An, Q.Y.; et al. Ultrastable and High-Performance Zn/VO2 Battery Based on a Reversible Single-Phase Reaction (vol 31, pg 699, 2019). Chem. Mater. 2019, 31, 5342. [Google Scholar] [CrossRef]
- Kulkarni, P.; Beere, H.K.; Jalalah, M.; Alsaiari, M.; Balakrishna, R.G.; Harraz, F.A.; Ghosh, D. Developing a high-performance aqueous zinc battery with Zn2+ pre-intercalated V3O7middotH2O cathode coupled with surface engineered metallic zinc anode. J. Electroanal. Chem. 2022, 924, 116851. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Lin, X.R.; Jin, X.; Han, T.L.; Zhang, H.G.; Liu, J.Y. A flexible self-healing Zn3V2O7(OH)(2)center dot 2H(2)O-based Zn-ion battery under continuous folding and twisting. Chem. Commun. 2022, 58, 8117–8120. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Q.; Chi, M.; Guo, C.; Wang, S.; Min, D. Preparation and performance of different carbonized wood electrodes. J. For. Eng. 2022, 7, 127–135. [Google Scholar]
- Wang, R.; Xuelian, Z.; Xu, T.; Bian, H.; Dai, H. Research progress on the preparation of lignin-derived carbon dots and graphene quantum dots. J. For. Eng. 2021, 6, 29–37. [Google Scholar]
- Hu, J.X.; Xie, Y.Y.; Zheng, J.Q.; Li, H.Z.; Wang, T.S.; Lai, Y.Q.; Zhang, Z.A. Encapsulating V2O3 Nanoparticles in Hierarchical Porous Carbon Nanosheets via C-O-V Bonds for Fast and Durable Potassium-Ion Storage. Acs Appl. Mater. Interfaces 2021, 13, 12149–12158. [Google Scholar] [CrossRef]
- Du, Y.H.; Wang, X.Y.; Man, J.Z.; Sun, J.C. A novel organic-inorganic hybrid V2O5@polyaniline as high-performance cathode for aqueous zinc-ion batteries. Mater. Lett. 2020, 272, 127813. [Google Scholar] [CrossRef]
- Jiang, L.; Qu, Y.; Ren, Z.Y.; Yu, P.; Zhao, D.D.; Zhou, W.; Wang, L.; Fu, H.G. In Situ Carbon-Coated Yolk-Shell V2O3 Microspheres for Lithium-Ion Batteries. Acs Appl. Mater. Interfaces 2015, 7, 1595–1601. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Wang, F.; Zhao, L.; Zhang, Q.; Xu, W.; He, S. Review on porous carbon materials engineered by ZnO templates: Design, synthesis and capacitance performance. Mater. Des. 2021, 201, 109518. [Google Scholar] [CrossRef]
- He, Z.X.; Cheng, G.; Jiang, Y.Q.; Li, Y.H.; Zhu, J.; Meng, W.; Zhou, H.Z.; Dai, L.; Wang, L. Novel 2D porous carbon nanosheet derived from biomass: Ultrahigh porosity and excellent performances toward V2+/V3+ redox reaction for vanadium redox flow battery. Int. J. Hydrogen Energy 2020, 45, 3959–3970. [Google Scholar] [CrossRef]
- Li, R.; Lou, Z.; Gu, S.; Wang, Q.; Liu, J.; Yanjun, L. Preparation of magnetic carbon with microwave absorption property using bamboo powder. J. For. Eng. 2021, 6, 112–120. [Google Scholar]
- Li, Y.; Lin, W.; Xue, L.; Xie, J.; Wei, B.; Chen, G.; Chen, D. Facile preparation of V2O3/black fungus-derived carbon composite with hierarchical porosity as a promising electrode for lithium/sodium ion batteries. J. Alloys Compd. 2022, 905, 164258. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar] [CrossRef]
- Duan, G.; Zhao, L.; Chen, L.; Wang, F.; He, S.; Jiang, S.; Zhang, Q. ZnCl2 regulated flax-based porous carbon fibers for supercapacitors with good cycling stability. New J. Chem. 2021, 45, 22602–22609. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, L.; Zhao, L.N.; Huang, X.X.; Li, W.; Li, T.; Shen, T.; Sun, S.N.; Hou, Y.L. Free-Standing, Foldable V2O3/Multichannel Carbon Nanofibers Electrode for Flexible Li-Ion Batteries with Ultralong Lifespan. Small 2020, 16, 2005302. [Google Scholar] [CrossRef]
- Zakharova, G.S.; Thauer, E.; Enyashin, A.N.; Deeg, L.F.; Zhu, Q.; Klingeler, R. V2O3/C composite fabricated by carboxylic acid-assisted sol-gel synthesis as anode material for lithium-ion batteries. J. Sol-Gel Sci. Technol. 2021, 98, 549–558. [Google Scholar] [CrossRef]
- Bai, Y.C.; Tang, Y.K.; Liu, L.; Li, X.H.; Gao, Y. Peapod-like CNT@V2O3 with Superior Electrochemical Performance as an Anode for Lithium-Ion Batteries. Acs Sustain. Chem. Eng. 2018, 6, 14614–14620. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Zhang, Y.F.; Yu, X.M.; Yu, Y.T.; Huang, C.; Meng, C.G. Aluminum-ion intercalation and reduced graphene oxide wrapping enable the electrochemical properties of hydrated V2O5 for Zn-ion storage. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 641, 128473. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Pan, Z.H.; Tian, D.; Hu, T.; Jiang, H.M.; Yang, J.; Sun, J.J.; Zheng, J.Q.; Meng, C.G.; Zhang, Y.F. Employing “one for two” strategy to design polyaniline-intercalated hydrated vanadium oxide with expanded interlayer spacing for high-performance aqueous zinc-ion batteries. Chem. Eng. J. 2020, 399, 125842. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.S.; Moon, S.H.; Park, D.H.; Kim, M.C.; Choi, J.H.; Shin, J.H.; Park, K.W. Enhanced electrochemical performance of a selectively formed V2O3/C composite structure for Li-ion batteries. Electrochim. Acta 2021, 389, 138685. [Google Scholar] [CrossRef]
- Henry, A.; Hesemann, P.; Alauzun, J.G.; Boury, B. Reductive mineralization of cellulose with vanadium, iron and tungsten chlorides and access to MxOy metal oxides and MxOy/C metal oxide/carbon composites. Carbohydr. Polym. 2017, 174, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Fan, M.J.; Liu, X.H.; Huang, C.; Li, H.B. Beltlike V2O3@C Core-Shell-Structured Composite: Design, Preparation, Characterization, Phase Transition, and Improvement of Electrochemical Properties of V2O3. Eur. J. Inorg. Chem. 2012, 10, 1650–1659. [Google Scholar] [CrossRef]
- Zheng, J.Q.; Zhang, Y.F.; Jing, X.Y.; Liu, X.Y.; Hu, T.; Lv, T.M.; Zhang, S.Q.; Meng, C.G. Synthesis of amorphous carbon coated on V2O3 core-shell composites for enhancing the electrochemical properties of V2O3 as supercapacitor electrode. Colloids Surf. A-Physicochem. Eng. Asp. 2017, 518, 188–196. [Google Scholar] [CrossRef]
- Liu, X.D.; Wang, Z.Q.; Niu, Y.L.; Liu, C.Y.; Chen, H.M.; Ren, X.Z.; Wang, M.S.; Lau, W.M.; Zhou, D. Scalable synthesis of novel V2O3/carbon composite as advanced cathode material for aqueous zinc-ion batteries. Ceram. Int. 2022, 48, 15594–15602. [Google Scholar] [CrossRef]
- Cui, T.; Wang, Y.P.; Ye, T.; Wu, J.; Chen, Z.; Li, J.; Lei, Y.; Wang, D.; Li, Y. Engineering Dual Single-Atom Sites on 2D Ultrathin N-doped Carbon Nanosheets Attaining Ultra-Low-Temperature Zinc-Air Battery. Angew. Chem. Int. Ed. Engl. 2022, 61, e202115219. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Zhan, L.; Yang, P.; Tang, S.; Liu, M.; Zhao, X.; Xiong, Y.; Chen, Z.; Lei, Y. Electron accumulation enables Bi efficient CO2 reduction for formate production to boost clean Zn-CO2 batteries. Nano Energy 2022, 92, 106780. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, Q.; Lei, Y.; Tang, S.; Xu, L.; Xiong, Y.; Fang, G.; Wang, Y.; Yang, P.; Liu, J.; et al. Quasi-solid-state Zn-air batteries with an atomically dispersed cobalt electrocatalyst and organohydrogel electrolyte. Nat. Commun. 2022, 13, 3689. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.Y.; Yue, W.C.; Li, X.; Gao, N.; Zhang, Y.J.; Hu, C.Q. V2O3/VN Catalysts Decorated Free-Standing Multifunctional Interlayer for High-Performance Li-S Battery. Chem. Eng. J. 2022, 441, 136082. [Google Scholar] [CrossRef]
- Saroha, R.; Heo, J.; Liu, Y.; Angulakshmi, N.; Lee, Y.; Cho, K.-K.; Ahn, H.-J.; Ahn, J.-H. V2O3-decorated carbon nanofibers as a robust interlayer for long-lived, high-performance, room-temperature sodium–sulfur batteries. Chem. Eng. J. 2022, 431, 134205. [Google Scholar] [CrossRef]
- Won, J.M.; Ko, Y.N.; Lee, J.K.; Kang, Y.C. Superior electrochemical properties of rutile VO2-carbon composite microspheres as a promising anode material for lithium ion batteries. Electrochim. Acta 2015, 156, 179–187. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, B.; Tang, L.B.; An, C.S.; He, Z.J.; Tong, H.; Yu, W.J.; Zheng, J.C. V2O3/rGO composite as a potential anode material for lithium ion batteries. Ceram. Int. 2018, 44, 15044–15049. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X.; Cho, Y.R. Enhanced Electrochemical Performance of Zn/VOx Batteries by a Carbon-Encapsulation Strategy. Acs Appl. Mater. Interfaces 2022, 14, 11654–11662. [Google Scholar] [CrossRef]
- Ren, H.-Z.; Zhang, J.; Wang, B.; Luo, H.; Jin, F.; Zhang, T.-R.; Ding, A.; Cong, B.-W.; Wang, D.-L. A V2O3@N–C cathode material for aqueous zinc-ion batteries with boosted zinc-ion storage performance. Rare Met. 2022, 41, 1605–1615. [Google Scholar] [CrossRef]
- Dai, X.; Wan, F.; Zhang, L.L.; Cao, H.M.; Niu, Z.Q. Freestanding graphene/VO2 composite films for highly stable aqueous Zn-ion batteries with superior rate performance. Energy Storage Mater. 2019, 17, 143–150. [Google Scholar] [CrossRef]
- Deng, L.; Chen, H.; Wu, J.; Yang, Z.; Rong, Y.; Fu, Z. V2O3 as cathode of zinc ion battery with high stability and long cycling life. Ionics 2021, 27, 3393–3402. [Google Scholar] [CrossRef]
- Bin, D.; Wang, Y.; Tamirat, A.G.; Zhu, P.; Yang, B.; Wang, J.; Huang, J.; Xia, Y. Stable High-Voltage Aqueous Zinc Battery Based on Carbon-Coated NaVPO4F Cathode. ACS Sustain. Chem. Eng. 2021, 9, 3223–3231. [Google Scholar] [CrossRef]
- Venkatkarthick, R.; Rodthongkum, N.; Zhang, X.; Wang, S.; Pattananuwat, P.; Zhao, Y.; Liu, R.; Qin, J. Vanadium-Based Oxide on Two-Dimensional Vanadium Carbide MXene (V2Ox@V2CTx) as Cathode for Rechargeable Aqueous Zinc-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 4677–4689. [Google Scholar] [CrossRef]
- Li, Y.; Xu, P.; Jiang, J.; Yao, J.; Huang, B.; Yang, J. Facile synthesis of ultra-large V2O5 xerogel flakes and its application as a cathode material for aqueous Zn-ion batteries. Mater. Today Commun. 2021, 26, 101849. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Niu, Y.; Liu, C.; Chen, H.; Ren, X.; Liu, Z.; Lau, W.-M.; Zhou, D. Electrospun V2O3@Carbon Nanofibers as a Flexible and Binder-Free Cathode for Highly Stable Aqueous Zn-Ion Full Batteries. ACS Appl. Energy Mater. 2022, 5, 3525–3535. [Google Scholar] [CrossRef]
- Lan, B.; Tang, C.; Chen, L.; Zhang, W.; Tang, W.; Zuo, C.; Fu, X.; Dong, S.; An, Q.; Luo, P. FeVO4⋅nH2O@rGO nanocomposite as high performance cathode materials for aqueous Zn-ion batteries. J. Alloys Compd. 2020, 818, 153372. [Google Scholar] [CrossRef]

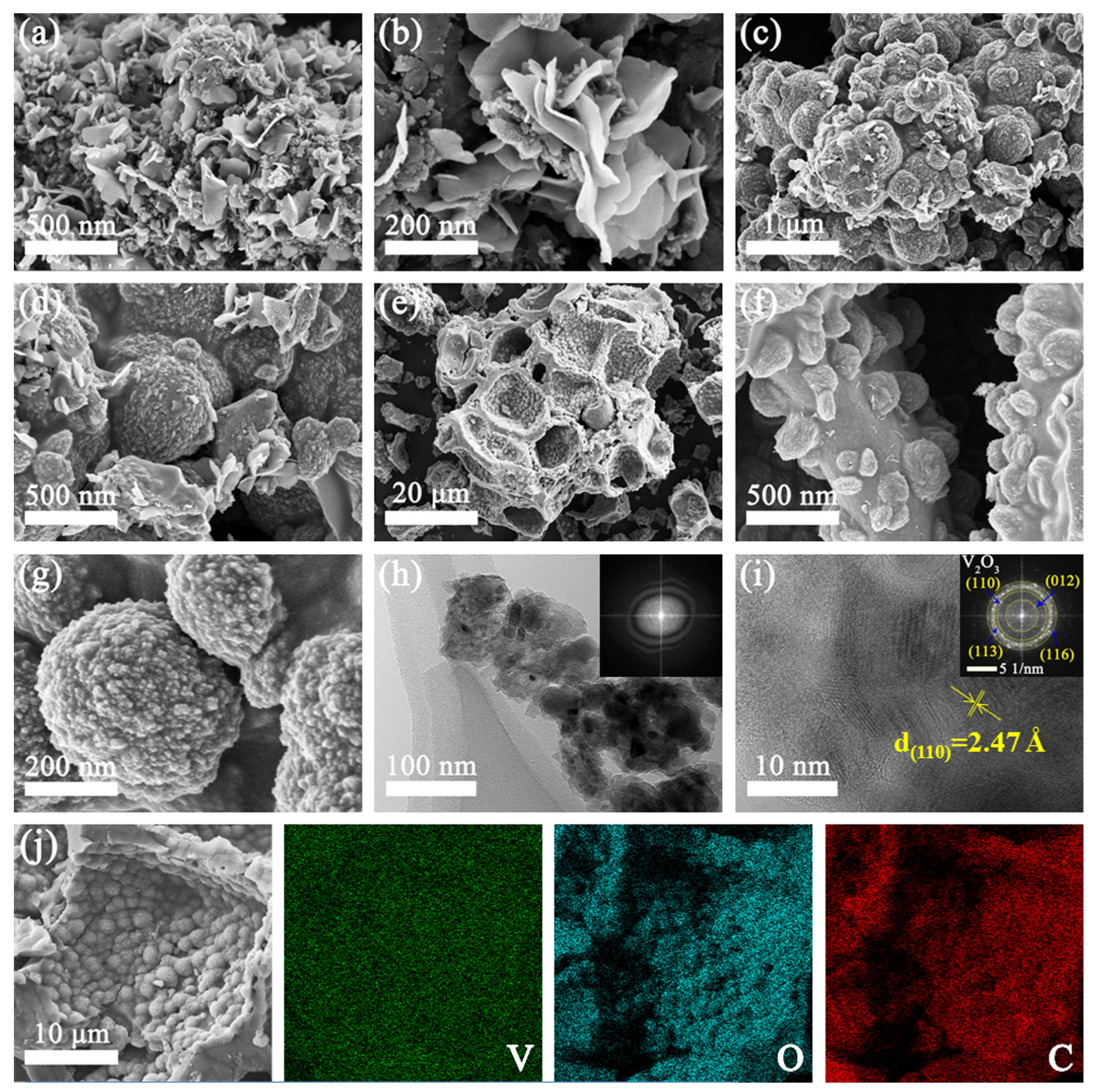

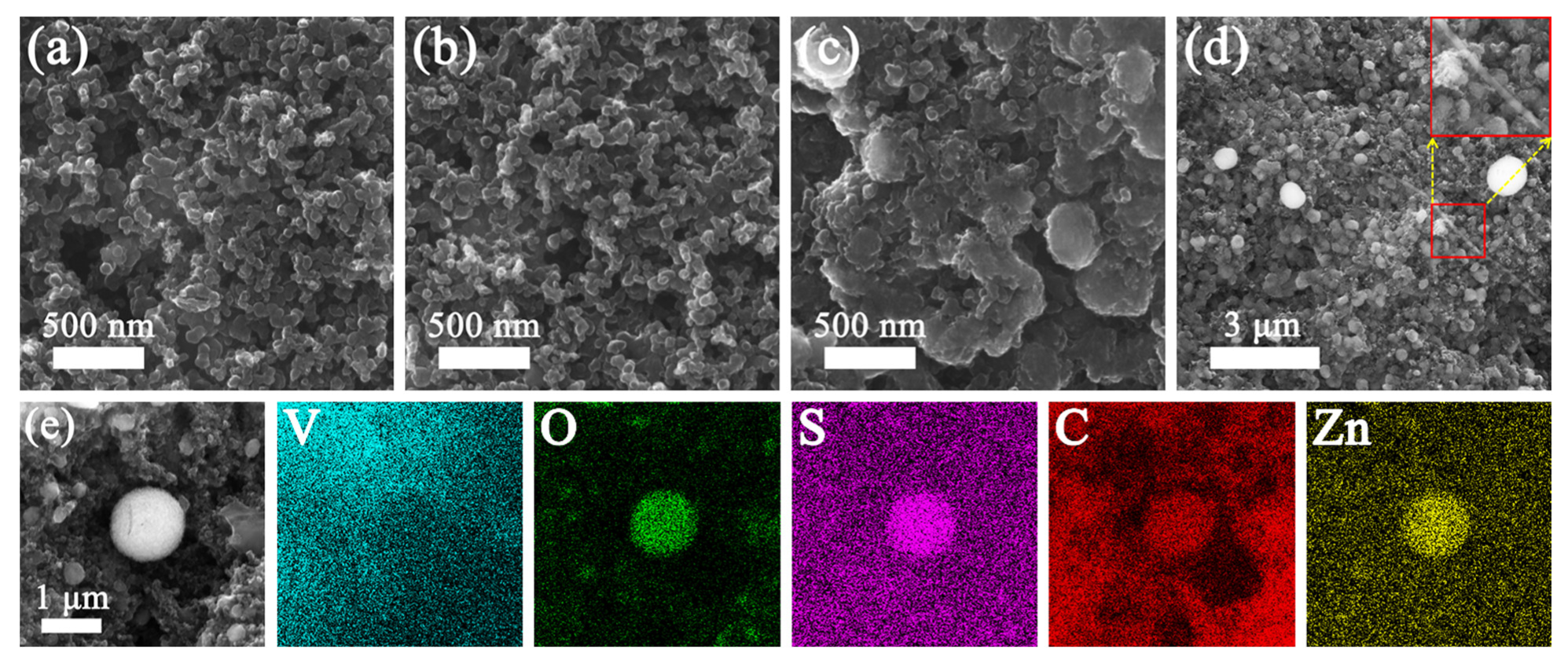
| Materials | Method of Synthesis | Current Density (A g−1) | Specific Capacity (mAh g−1) | Capacity Retention | Cycle Number | Ref./Year |
|---|---|---|---|---|---|---|
| V2O3@carbonized dictyophora | evaporation-induced self-assembly technique | 1 | 151.9 | 89.24% | 1000 | This work |
| V2O3@amorphous carbon | Calcination | 1 | 116 | 90.7% | 1600 | [9]/2021 |
| V2O3 | Reduction method of boron | 0.1 | 161 | 76.9% | 100 | [57]/2021 |
| Carbon-coated NaVPO4F | CVD | 0.1 | 87.4 | 94.5% | 400 | [58]/2021 |
| V2Ox@V2CTx | High-temperature etching and electrochemical active | 1 | 87.3 | 81.6% | 200 | [59]/2020 |
| V2O5 xerogel flakes | Hydrothermal | 1 | 135 | 64% | 200 | [60]/2021 |
| V2O3@Carbon Nanofibers | Electrospinning | 0.2 | 120 | 80% | 1000 | [61]/2022 |
| FeVO4•nH2O@rGO | Hydrothermal | 1 | 92 | 43.8% a | 1000 | [62]/2020 |
| Samples | Dictyophora | C2H6O2 | CH4N2O | NH4VO3 |
|---|---|---|---|---|
| VOCD-1 | 0.40 g | 20 mL | 0.75 g | 0.55 g |
| VOCD-2 | 0.40 g | 20 mL | 1.50 g | 1.10 g |
| VOCD-3 | 0.40 g | 20 mL | 3.00 g | 2.20 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Zeng, G.; Jin, H.; Jiang, S.; Huang, M.; Zhang, C.; Chen, H. Bio-Template Synthesis of V2O3@Carbonized Dictyophora Composites for Advanced Aqueous Zinc-Ion Batteries. Molecules 2023, 28, 2147. https://doi.org/10.3390/molecules28052147
Zhou W, Zeng G, Jin H, Jiang S, Huang M, Zhang C, Chen H. Bio-Template Synthesis of V2O3@Carbonized Dictyophora Composites for Advanced Aqueous Zinc-Ion Batteries. Molecules. 2023; 28(5):2147. https://doi.org/10.3390/molecules28052147
Chicago/Turabian StyleZhou, Wei, Guilin Zeng, Haotian Jin, Shaohua Jiang, Minjie Huang, Chunmei Zhang, and Han Chen. 2023. "Bio-Template Synthesis of V2O3@Carbonized Dictyophora Composites for Advanced Aqueous Zinc-Ion Batteries" Molecules 28, no. 5: 2147. https://doi.org/10.3390/molecules28052147
APA StyleZhou, W., Zeng, G., Jin, H., Jiang, S., Huang, M., Zhang, C., & Chen, H. (2023). Bio-Template Synthesis of V2O3@Carbonized Dictyophora Composites for Advanced Aqueous Zinc-Ion Batteries. Molecules, 28(5), 2147. https://doi.org/10.3390/molecules28052147







