Siloxene Nanosheets and Their Hybrid Gel Glasses for Broad-Band Optical Limiting
(This article belongs to the Section Materials Chemistry)
Abstract
1. Introduction
2. Results and Discussion
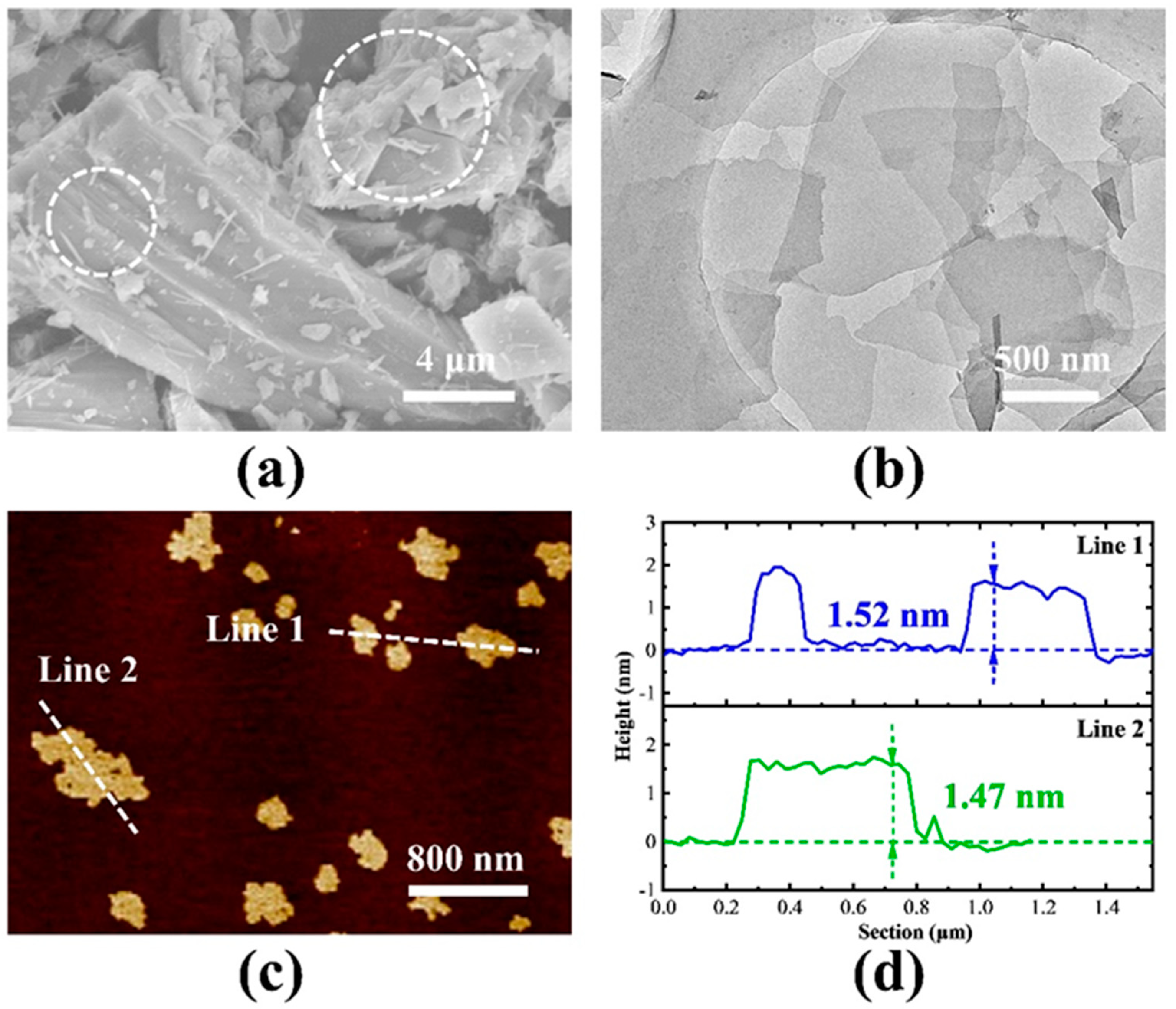
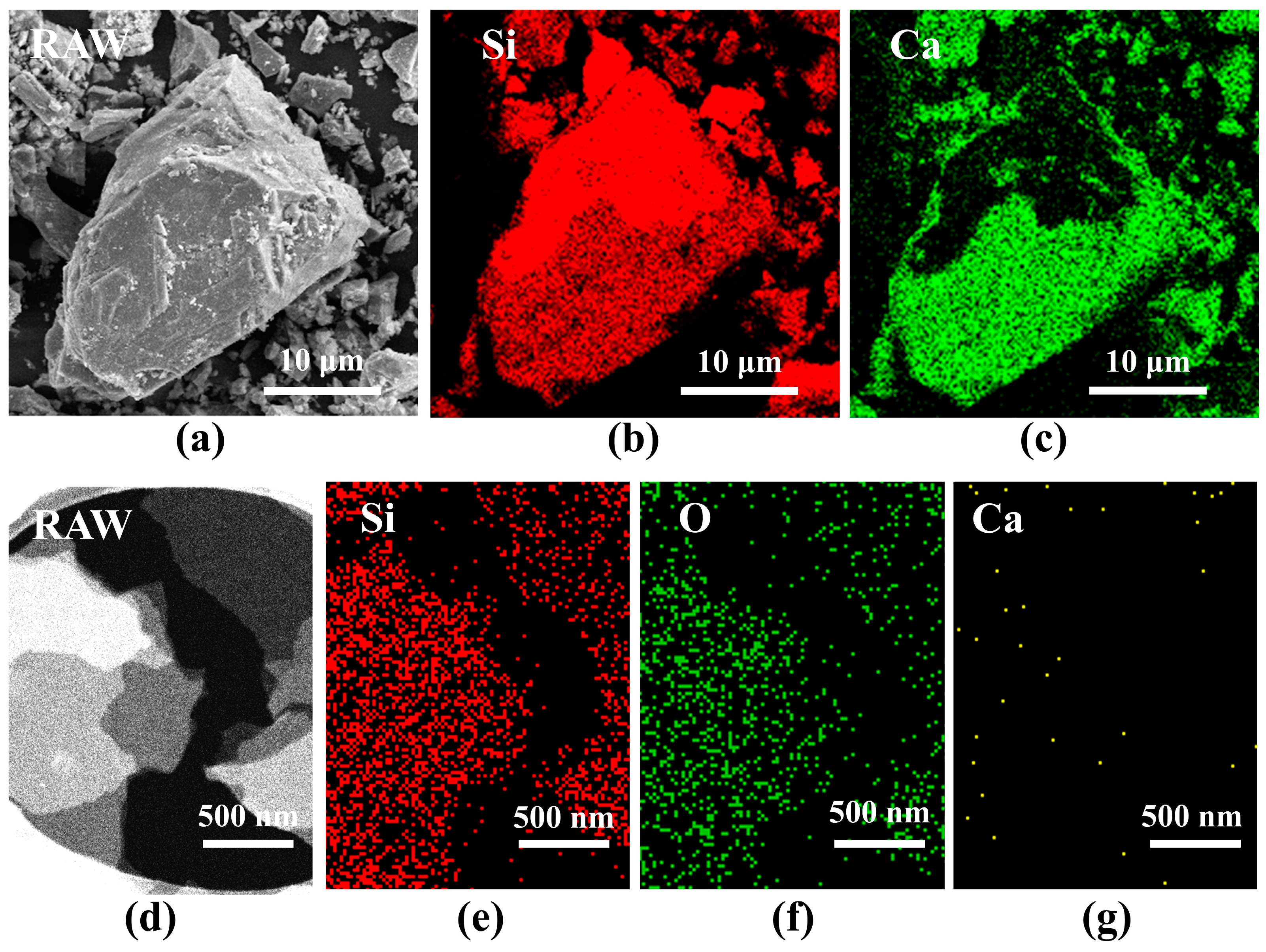
2.1. Morphological and Elemental Characterization of Siloxene Nanosheets
2.2. Structure and Elemental Characterization of Siloxene Nanosheets
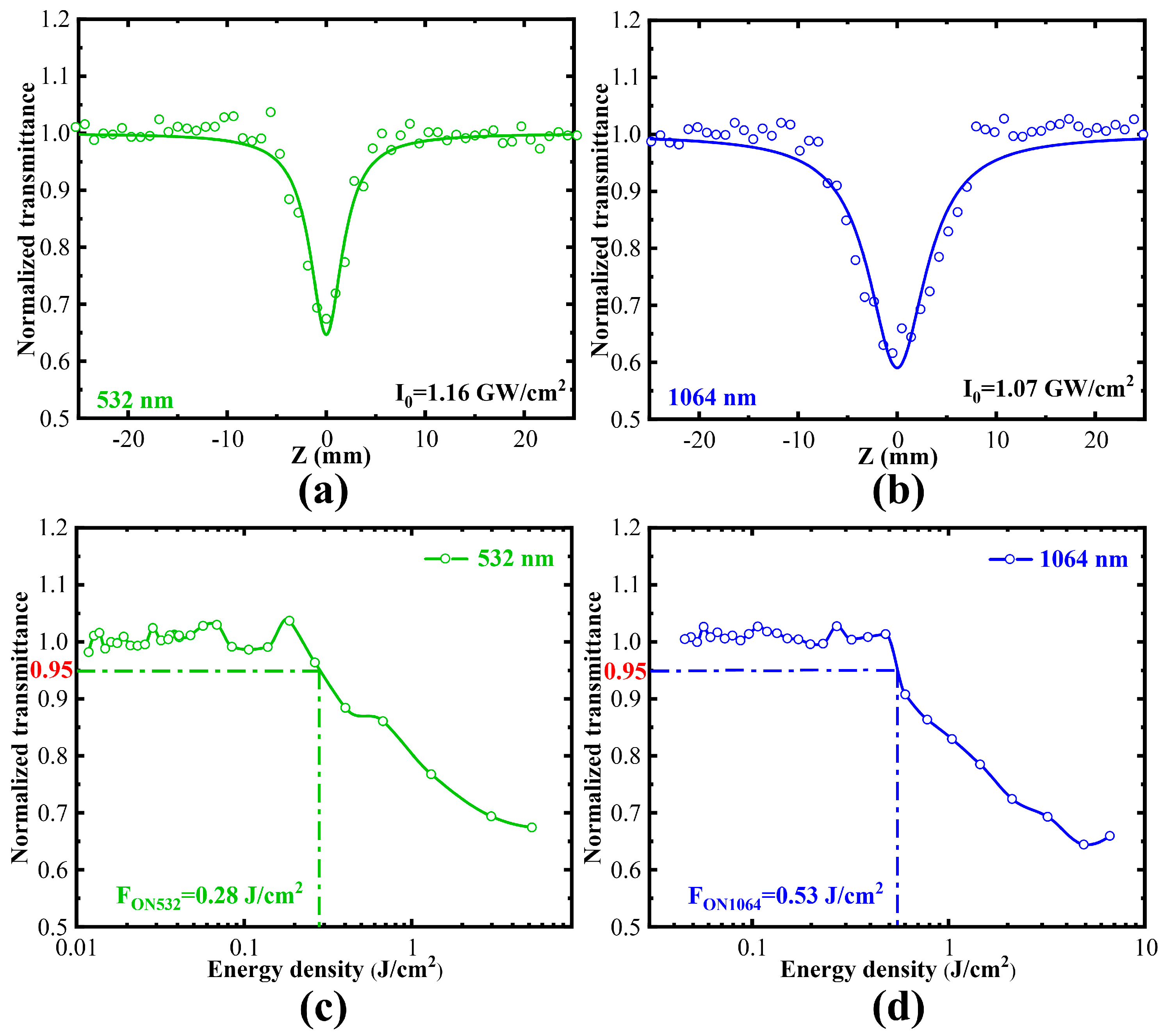
2.3. The Nonlinear Optical Properties of Siloxene Nanosheets
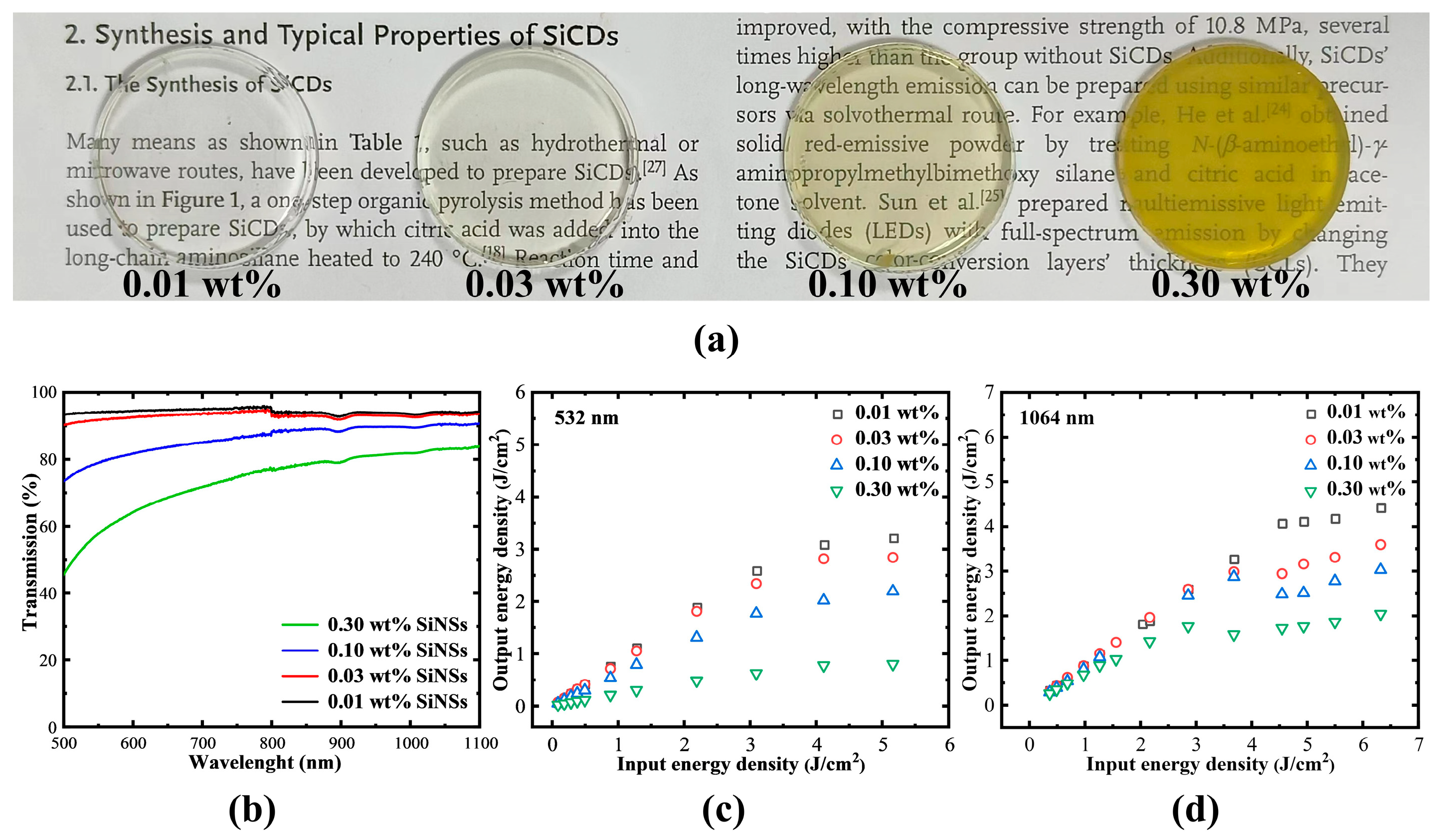
2.4. The Nonlinear Optical Properties of Siloxene Nanosheets Hybrid Gel Glasses
3. Materials and Methods
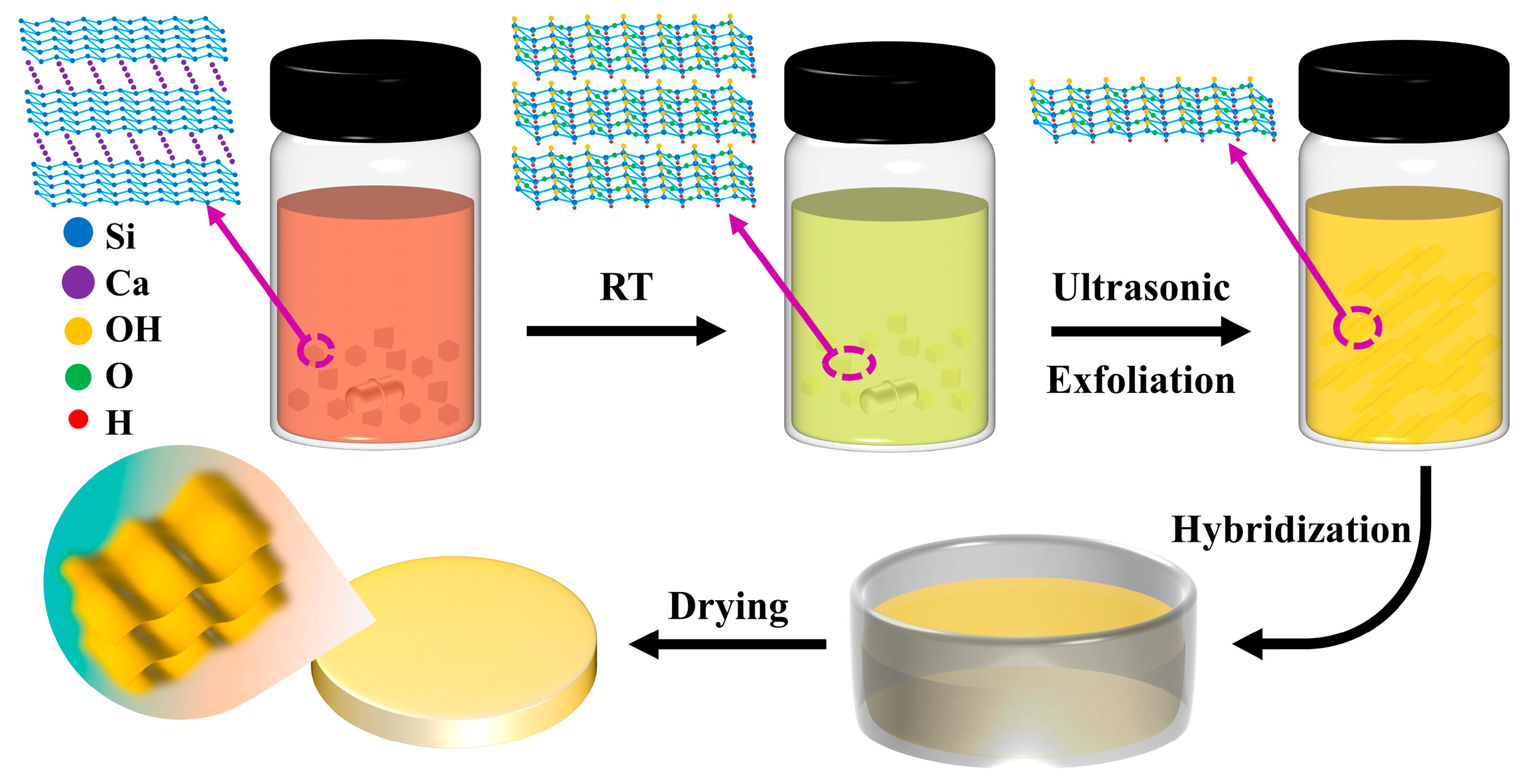
3.1. Material Preparation
3.2. Preparation of Siloxene Nanosheets
3.3. Preparation of Siloxene Nanosheets Hybrid Gel Glasses
3.4. Characterization Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Di Piazza, A.; Mueller, C.; Hatsagortsyan, K.Z.; Keitel, C.H. Extremely high-intensity laser interactions with fundamental quantum systems. Rev. Mod. Phys. 2012, 84, 1177–1228. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, T.; Dong, N.; Fan, F.; Zhang, S.; Zhuang, X.; Sun, J.; Zhang, B.; Zhang, X.; Wang, J.; et al. Graphene and its derivatives for laser protection. Prog. Mater. Sci. 2016, 84, 118–157. [Google Scholar] [CrossRef]
- Zhou, G.J.; Wong, W.Y. Organometallic acetylides of PtII, AuI and HgII as new generation optical power limiting materials. Chem. Soc. Rev. 2011, 40, 2541–2566. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z. Graphene-analogous low-dimensional materials. Prog. Mater. Sci. 2013, 58, 1244–1315. [Google Scholar] [CrossRef]
- Xu, L.; Sun, J.; Tang, T.; Zhang, H.; Sun, M.; Zhang, J.; Li, J.; Huang, B.; Wang, Z.; Xie, Z.; et al. Metallated Graphynes as a New Class of Photofunctional 2D Organometallic Nanosheets. Angew. Chem. 2021, 60, 11326–11334. [Google Scholar] [CrossRef]
- Jose, D.; Datta, A. Structures and Chemical Properties of Silicene: Unlike Graphene. Acc. Chem. Res. 2014, 47, 593–602. [Google Scholar] [CrossRef]
- Kara, A.; Enriquez, H.; Seitsonen, A.P.; Lew Yan Voon, L.C.; Vizzini, S.; Aufray, B.; Oughaddou, H. A review on silicene—New candidate for electronics. Surf. Sci. Rep. 2012, 67, 1–18. [Google Scholar] [CrossRef]
- Guzmán-Verri, G.G.; Voon, L.C.L.Y. Electronic structure of silicon-based nanostructures. Phys. Rev. B 2007, 76, 075131. [Google Scholar] [CrossRef]
- Cahangirov, S.; Topsakal, M.; Akturk, E.; Sahin, H.; Ciraci, S. Two- and one-dimensional honeycomb structures of silicon and germanium. Phys. Rev. Lett. 2009, 102, 236804. [Google Scholar] [CrossRef]
- Houssa, M.; Pourtois, G.; Afanas’ev, V.V.; Stesmans, A. Can silicon behave like graphene? A first-principles study. Appl. Phys. Lett. 2010, 97, 112106. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Lyu, P.; Nachtigall, P.; Xu, Y. Few-Layer Silicene Nanosheets with Superior Lithium-Storage Properties. Adv. Mater. 2018, 30, 1800838. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, H.; Yu, Z.; Quhe, R.; Zhou, S.; Wang, Y.; Liu, C.; Zhong, H.; Han, N.; Lu, J.; et al. Rise of Silicene: A Competitive 2D Material. Prog. Mater. Sci. 2016, 83, 24–151. [Google Scholar] [CrossRef]
- Borlido, P.; Rödl, C.; Marqes, M.A.L.; Botti, S. The ground state of two-dimensional silicon. 2D Mater. 2018, 5, 035010. [Google Scholar] [CrossRef]
- Grazianetti, C.; De Rosa, S.; Martella, C.; Targa, P.; Codegoni, D.; Gori, P.; Pulci, O.; Molle, A.; Lupi, S. Optical Conductivity of Two-Dimensional Silicon: Evidence of Dirac Electrodynamics. Nano Lett. 2018, 18, 7124–7132. [Google Scholar] [CrossRef]
- Yamanaka, S.; Matsu-ura, H.; Ishikawa, M. New deintercalation rection of calcium from calcium disilicide. Synthesis of layered polysilane. Mater. Res. Bull. 1996, 31, 307–316. [Google Scholar] [CrossRef]
- Dettlaff-Weglikowska, U.; Hönle, W.; Molassioti-Dohms, A.; Finkbeiner, S.; Weber, J. Structure and optical properties of the planar silicon compounds polysilane and Wöhler siloxene. Phys. Rev. B 1997, 56, 1132–1140. [Google Scholar] [CrossRef]
- Nakano, H.; Ishii, M.; Nakamura, H. Preparation and structure of novel siloxene nanosheets. Chem. Commun. 2005, 23, 2945–2947. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, K.; Zaccaria, R.; Huang, H.; Xia, Y.; Liu, Z. Two-dimensional silicon suboxides nanostructures with Si nanodomains confined in amorphous SiO2 derived from siloxene as high performance anode for Li-ion batteries. Nano Energy 2017, 39, 546–553. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Kim, S. Two-dimensional siloxene nanosheets: Novel high-performance supercapacitor electrode materials. Energy Environ. Sci. 2018, 11, 1595–1602. [Google Scholar] [CrossRef]
- Xu, K.; Ben, L.; Li, H.; Huang, X. Silicon-based nanosheets synthesized by a topochemical reaction for use as anodes for lithium ion batteries. Nano Res. 2015, 8, 2654–2662. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Sahoo, S.; Mariappan, V.K.; Kim, S.J. Understanding the Thermal Treatment Effect of Two-Dimensional Siloxene Sheets and the Origin of Superior Electrochemical Energy Storage Performances. ACS Appl. Mater. Interfaces 2019, 11, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Baldwin, R.K.; Pettigrew, K.A.; Kauzlarich, S.M. Solution Synthesis of Ultrastable Luminescent Siloxane-Coated Silicon Nanoparticles. Nano Lett. 2004, 4, 1181–1186. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, J.; Bai, C.; Chen, N.; Qu, L. 2D Silicene Nanosheets for High-Performance Zinc-Ion Hybrid Capacitor Application. ACS Nano 2021, 15, 16533–16541. [Google Scholar] [CrossRef]
- Guo, Q.; Bai, C.; Gao, C.; Chen, N.; Qu, L. Two Dimensional Silicene Nanosheets: A New Choice of Electrode Material for High-Performance Supercapacitor. ACS Appl. Mater. Interfaces 2022, 14, 39014–39021. [Google Scholar] [CrossRef]
- You, J.W.; Bongu, S.R.; Bao, Q.; Panoiu, N.C. Nonlinear optical properties and applications of 2D materials: Theoretical and experimental aspects. Nanophotonics 2018, 8, 63–97. [Google Scholar] [CrossRef]
- Papadakis, I.; Bouza, Z.; Couris, S.; Bourlinos, A.B.; Mouselimis, V.; Kouloumpis, A.; Gournis, D.; Bakandritsos, A.; Ugolotti, J.; Zboril, R. Hydrogenated Fluorographene: A 2D Counterpart of Graphane with Enhanced Nonlinear Optical Properties. J. Phys. Chem. C 2017, 121, 22567–22575. [Google Scholar] [CrossRef]
- Agrawal, A.; Yi, G.C. Database on the nonlinear optical properties of graphene based materials. Data Brief 2020, 28, 105049. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, M.; Papadakis, I.; Stathis, A.; Kloberg, M.J.; Mock, J.; Kratky, T.; Gunther, S.; Rieger, B.; Becherer, M.; Lyuleeva-Husemann, A.; et al. Silicon Nanosheets versus Graphene Nanosheets: A Comparison of Their Nonlinear Optical Response. J. Phys. Chem. Lett. 2021, 12, 815–821. [Google Scholar] [CrossRef]
- Stavrou, M.; Stathis, A.; Papadakis, I.; Lyuleeva-Husemann, A.; Koudoumas, E.; Couris, S. Silicon Nanosheets: An Emerging 2D Photonic Material with a Large Transient Nonlinear Optical Response beyond Graphene. Nanomaterials 2021, 12, 90. [Google Scholar] [CrossRef]
- Stathis, A.; Stavrou, M.; Papadakis, I.; Mock, J.; Kloberg, M.J.; Becherer, M.; Lyuleeva-Husemann, A.; Couris, S. Silicon Nanosheets: A Promising 2D Material with Strong Ultrafast Nonlinear Optical Response. J. Phys. Chem. C 2021, 125, 18510–18516. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, M.; Wang, Z.; Han, K.; Liu, X.; Xu, X. Nonlinear absorption properties of silicene nanosheets. Nanotechnology 2018, 29, 225701. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, L.; Ding, D.; Wang, Y.; Huang, J.; He, H.; Ye, Z. Silicene Quantum Dots Confined in Few-Layer Siloxene Nanosheets for Blue-Light-Emitting Diodes. ACS Appl. Nano Mater. 2019, 3, 538–546. [Google Scholar] [CrossRef]
- Meier, C.; Lüttjohann, S.; Kravets, G.; Nienhaus, H.; Lorke, A.; Wiggers, H. Raman properties of silicon nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2006, 32, 155–158. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Y.; Guan, R.; Ren, J.; Xie, Z. Silane-Functionalized Carbon Dots and Their Polymerized Hybrids: From Optoelectronics to Biotherapy. Small 2021, 17, 2105273. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Wang, E.; Zhong, Q.; Zhou, T.; Chen, H.; Chen, S.; Lu, G.; Liang, C.; Peng, X. Exceptional size-dependent property of TiS2 nanosheets for optical limiting. Appl. Surf. Sci. 2021, 541, 148371. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Y.; Shen, W.; Xiong, Y.; Lin, C.; Gao, Y.; Al-Ammari, A.; Liu, K.; Ma, T.; Chen, J.; et al. Antimonene nanosheets fabricated by laser irradiation technique with outstanding nonlinear absorption responses. Appl. Phys. Lett. 2020, 116, 261903. [Google Scholar] [CrossRef]
- Dong, N.; Li, Y.; Feng, Y.; Zhang, S.; Zhang, X.; Chang, C.; Fan, J.; Zhang, L.; Wang, J. Optical Limiting and Theoretical Modelling of Layered Transition Metal Dichalcogenide Nanosheets. Sci. Rep. 2015, 5, 14646. [Google Scholar] [CrossRef]
- Zheng, X.; Feng, M.; Zhan, H. Giant optical limiting effect in Ormosil gel glasses doped with graphene oxide materials. J. Mater. Chem. C 2013, 1, 6759–6766. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, F.; Liu, C. Organic-inorganic hybrid functional carbon dot gel glasses. Adv. Mater. 2012, 24, 1716–1721. [Google Scholar] [CrossRef]
- Xing, F.; Wang, J.; Wang, Z.; Li, Y.; Gou, X.; Zhang, H.; Zhou, S.; Zhao, J.; Xie, Z. Covalently Silane-Functionalized Antimonene Nanosheets and Their Copolymerized Gel Glasses for Broadband Vis-NIR Optical Limiting. ACS Appl. Mater. Interfaces 2021, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]

| Element | CaSi2 Powders | Element | Siloxene Nanosheets | ||
|---|---|---|---|---|---|
| Weight% | Atomic% | Weight% | Atomic% | ||
| Si K | 72.24 | 78.78 | Si K | 48.76 | 35.25 |
| Ca K | 27.76 | 21.22 | Ca K | 0.36 | 0.18 |
| O K | 50.88 | 64.57 | |||
| Total | 100.00 | 100.00 | Total | 100.00 | 100.00 |
| Element | Weight% | Atomic% |
|---|---|---|
| O 1s | 40.41 | 48.37 |
| Si 2p | 48.54 | 33.1 |
| C 1s | 11.05 | 17.63 |
| Totals | 100.00 | 100.00 |
| Samples Concentration | 532 nm | 1064 nm | ||||
|---|---|---|---|---|---|---|
| (wt%) | FON (J/cm2) | ROL (out/in) | Tlinear (out/in) | FON (J/cm2) | ROL (out/in) | Tlinear (out/in) |
| 0.30 | 0.45 | 0.15 | 0.54 | 1.45 | 0.32 | 0.83 |
| 0.10 | 1.60 | 0.42 | 0.77 | 3.32 | 0.48 | 0.90 |
| 0.03 | 2.38 | 0.55 | 0.91 | 3.19 | 0.57 | 0.93 |
| 0.01 | 2.70 | 0.62 | 0.94 | 4.75 | 0.70 | 0.94 |
| Material | Substrate | Laser Parameters | FON (J/cm2) | Literature |
|---|---|---|---|---|
| TiS2 nanosheets | PMMA glasses | 532 nm 7 ns | 0.067 | [35] |
| Sb nanosheets | isopropyl alcohol | 532 nm 1.8 ns | 0.162 | [36] |
| Graphene | NMP | 532 nm 6 ns | 0.44 | [37] |
| 1064 nm 6 ns | 0.64 | |||
| Graphene-Ormosil | gel glasses | 532 nm 8 ns | 0.03 | [38] |
| SiNSs | ethanol | 532 nm 4.5 ns | 0.28 | This work |
| 1064 nm 6.5 ns | 0.53 | |||
| gel glasses | 532 nm 4.5 ns | 0.45 | ||
| 1064 nm 6.5 ns | 1.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Li, N.; Li, Y.; Ma, Q.; Xie, Z.; Zhou, S. Siloxene Nanosheets and Their Hybrid Gel Glasses for Broad-Band Optical Limiting. Molecules 2023, 28, 2143. https://doi.org/10.3390/molecules28052143
Lv X, Li N, Li Y, Ma Q, Xie Z, Zhou S. Siloxene Nanosheets and Their Hybrid Gel Glasses for Broad-Band Optical Limiting. Molecules. 2023; 28(5):2143. https://doi.org/10.3390/molecules28052143
Chicago/Turabian StyleLv, Xugui, Nan Li, Yunfei Li, Qingyu Ma, Zheng Xie, and Shuyun Zhou. 2023. "Siloxene Nanosheets and Their Hybrid Gel Glasses for Broad-Band Optical Limiting" Molecules 28, no. 5: 2143. https://doi.org/10.3390/molecules28052143
APA StyleLv, X., Li, N., Li, Y., Ma, Q., Xie, Z., & Zhou, S. (2023). Siloxene Nanosheets and Their Hybrid Gel Glasses for Broad-Band Optical Limiting. Molecules, 28(5), 2143. https://doi.org/10.3390/molecules28052143






