Research Progress of the Ion Activity Coefficient of Polyelectrolytes: A Review
Abstract
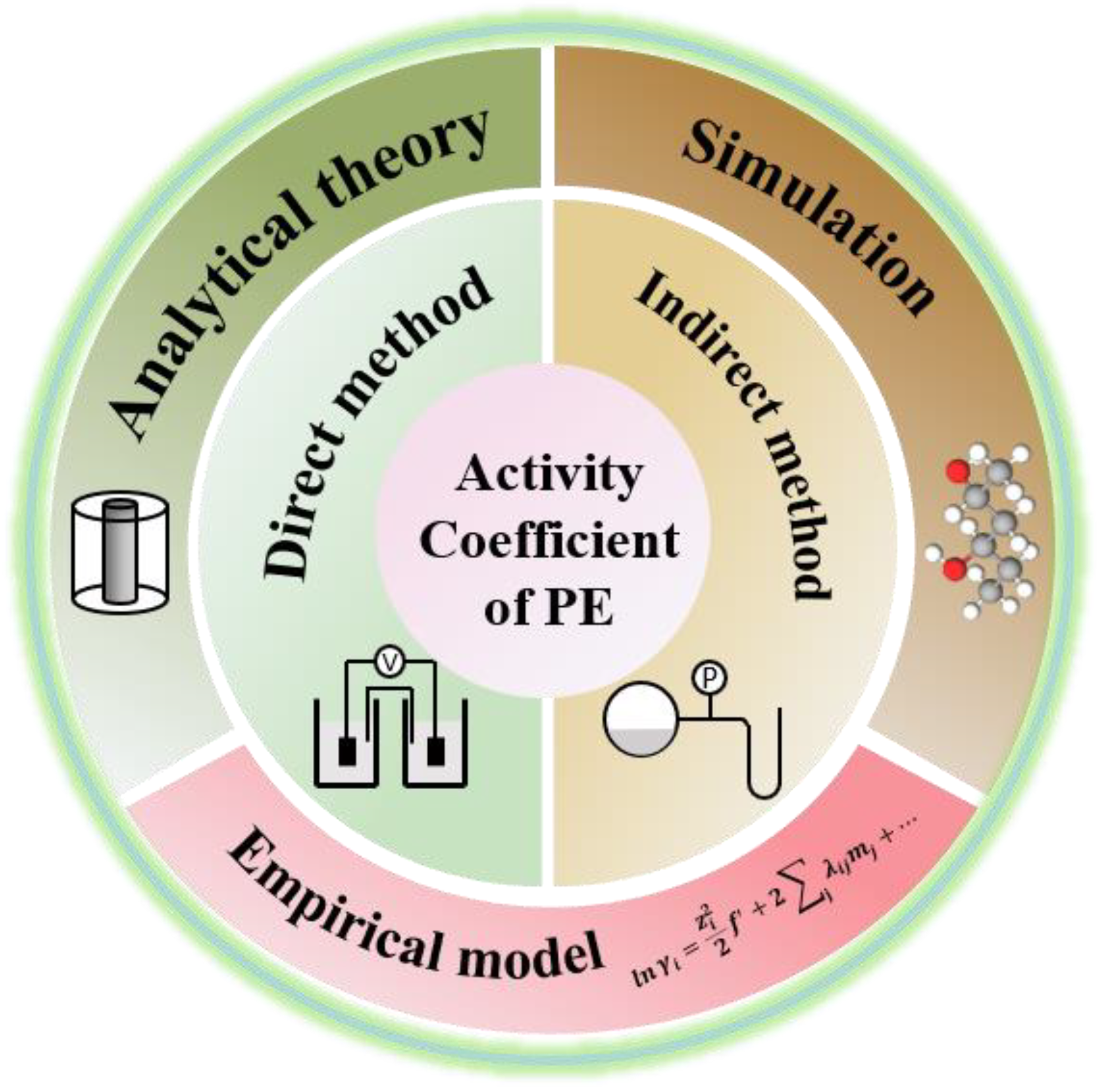
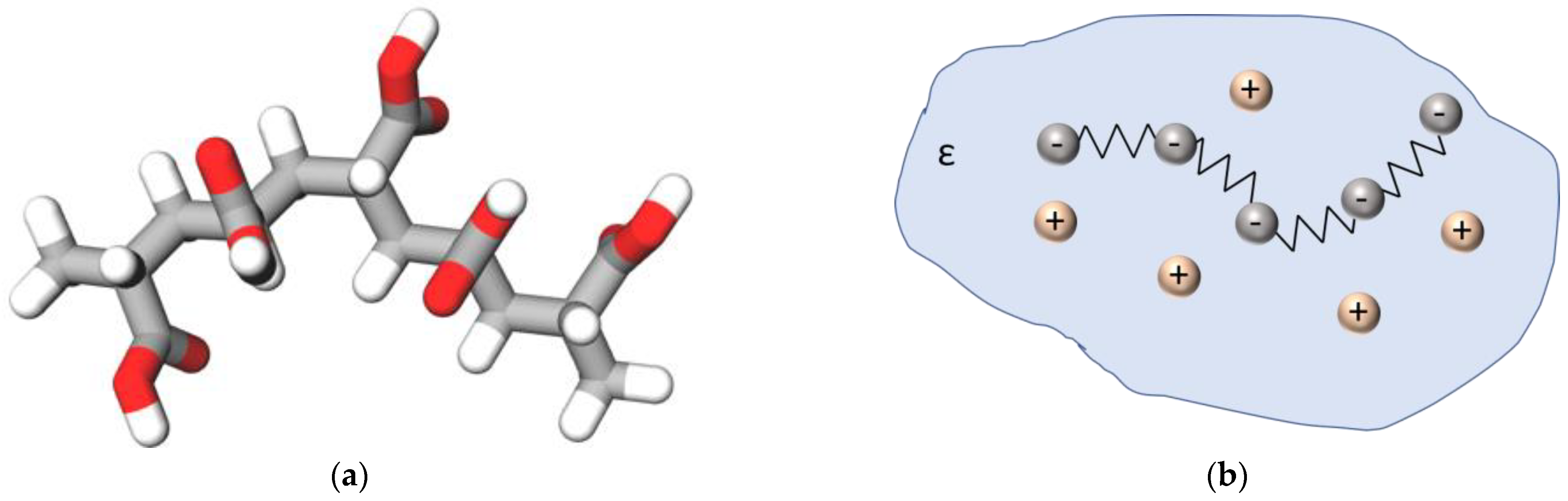
1. Introduction
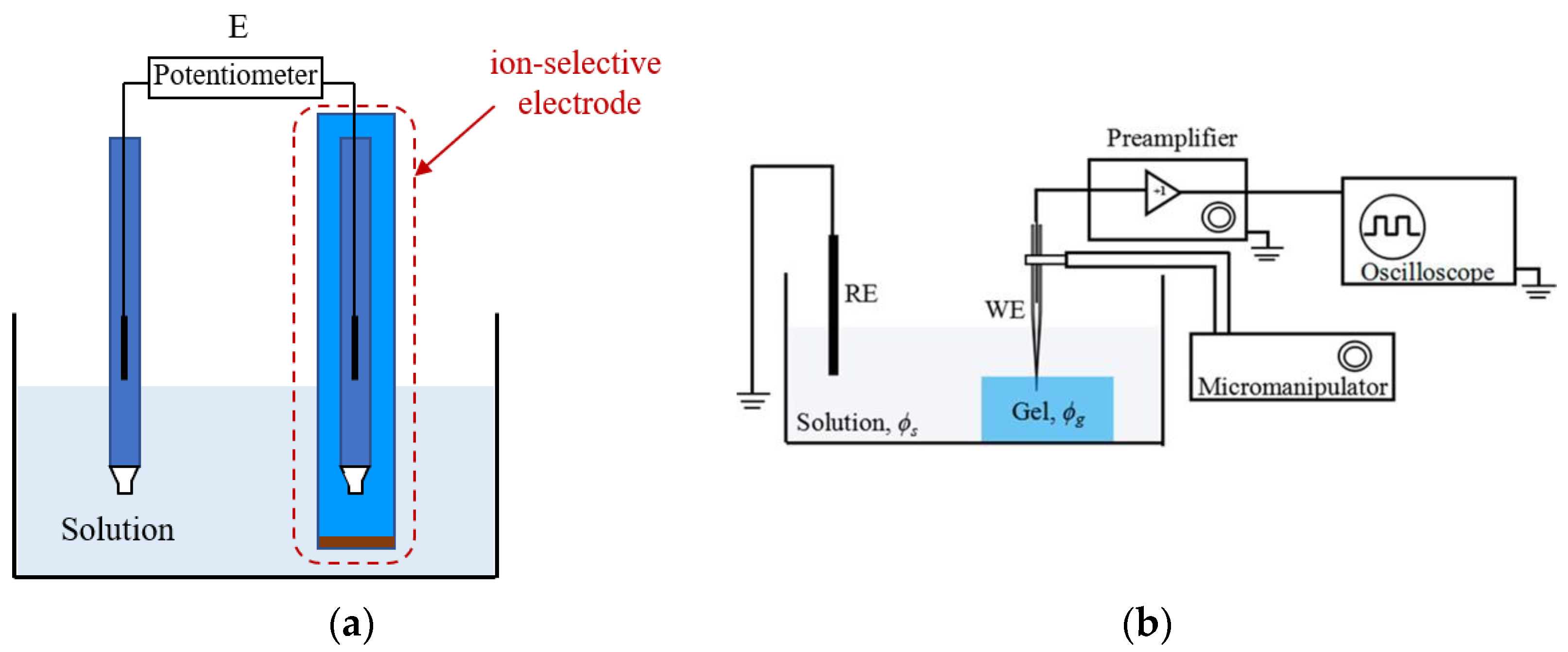
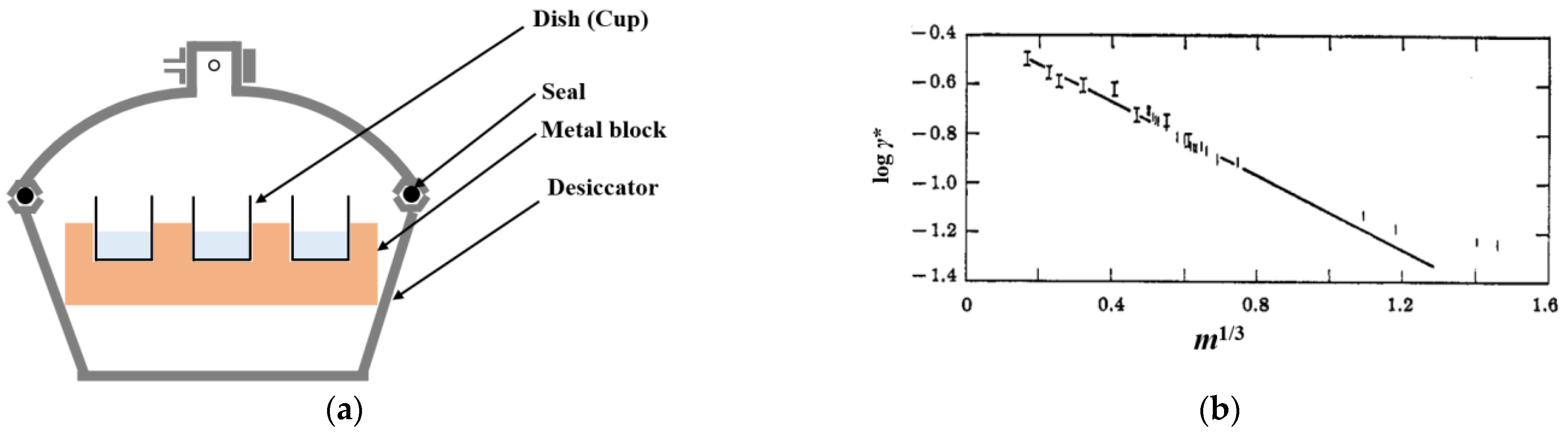
2. Experimental Determination of Ion Activity of Polyelectrolytes
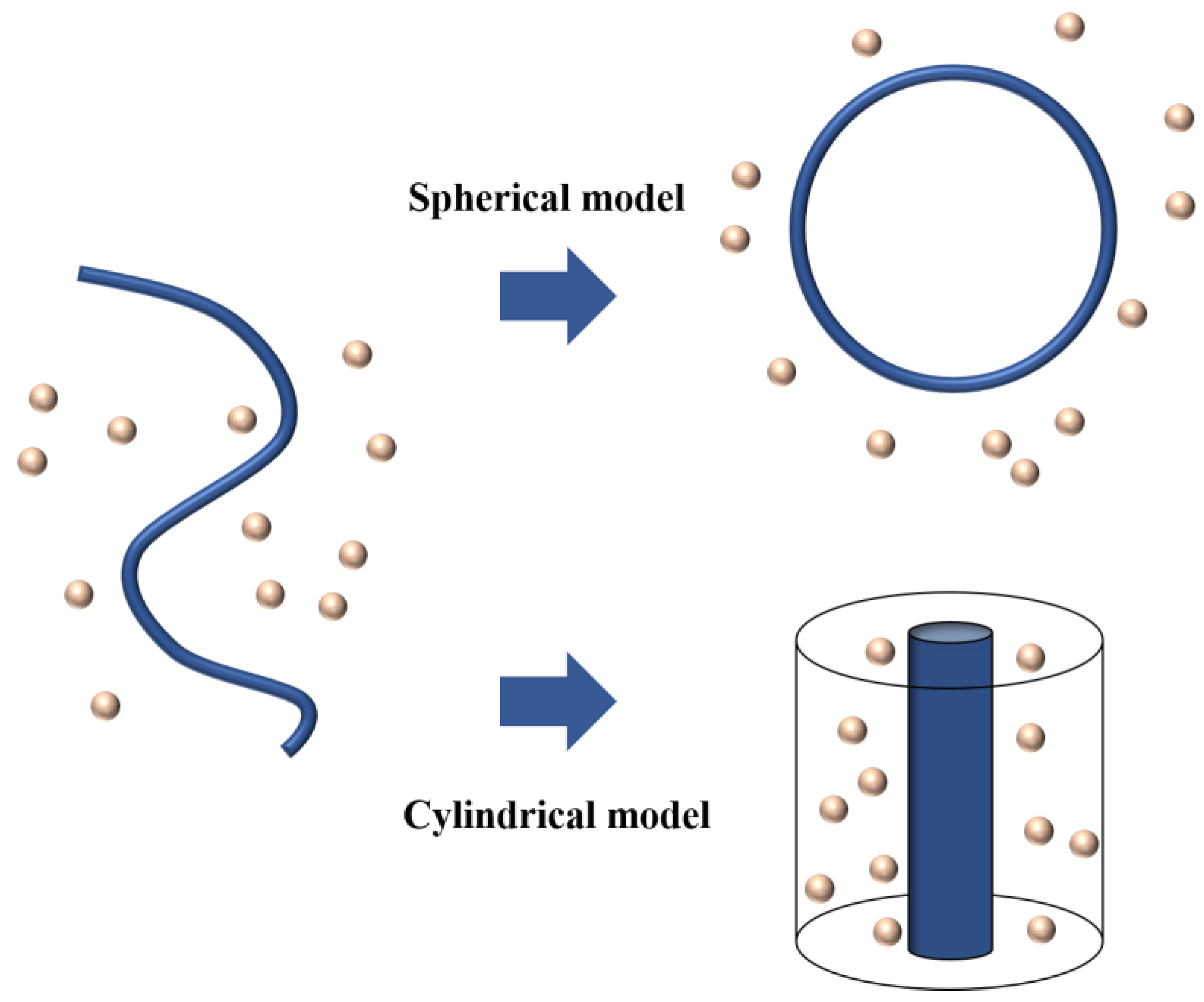
3. Theoretical Models for Ion Activity of Polyelectrolytes
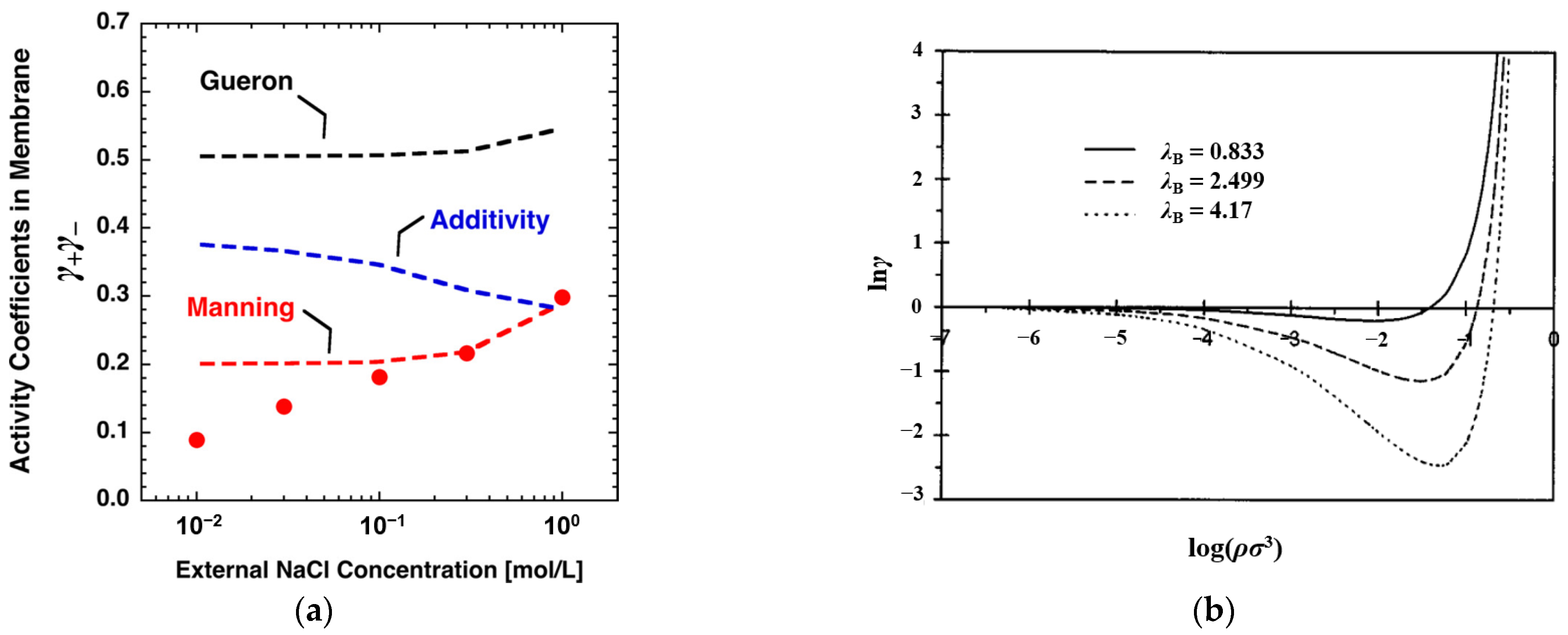
3.1. Analytical Model
3.2. Empirical Model
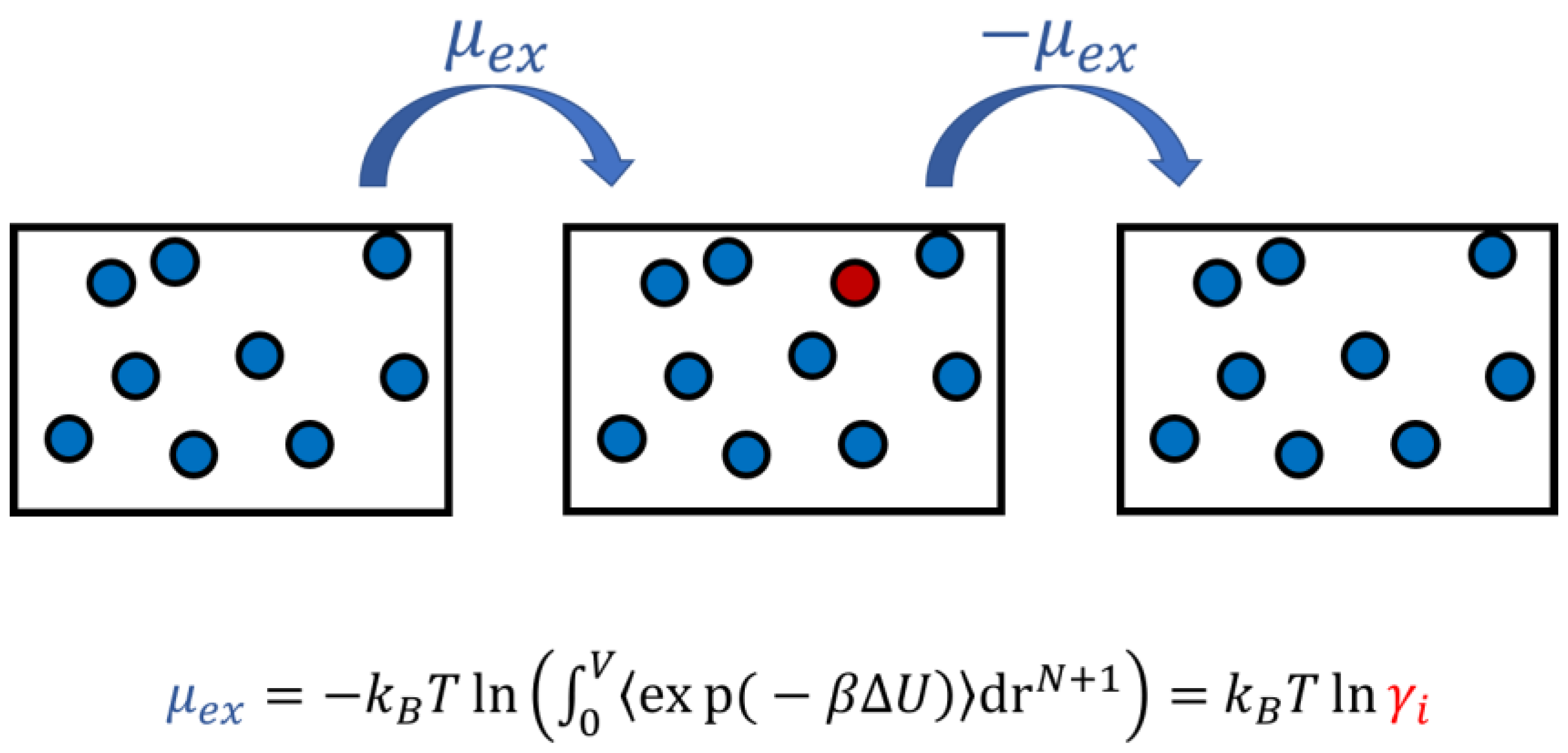
3.3. Monte Carlo Simulation
3.4. Molecular Dynamics Simulation
4. Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, D.; Ren, Q.; Li, Z.; Zhang, L. Chitosan-Based Biomimetically Mineralized Composite Materials in Human Hard Tissue Repair. Molecules 2020, 25, 4785. [Google Scholar] [CrossRef]
- Kalinova, R.; Dimitrov, I. Functional Polyion Complex Micelles for Potential Targeted Hydrophobic Drug Delivery. Molecules 2022, 27, 2178. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Pérez, K.; Toro, N.; Jeldres, M.; Gálvez, E.; Robles, P.; Alvarado, O.; Toledo, P.G.; Jeldres, R.I. Estimating the Shear Resistance of Flocculated Kaolin Aggregates: Effect of Flocculation Time, Flocculant Dose, and Water Quality. Polymers 2022, 14, 1381. [Google Scholar] [CrossRef]
- te Brinke, E.; Achterhuis, I.; Reurink, D.M.; de Grooth, J.; de Vos, W.M. Multiple Approaches to the Buildup of Asymmetric Polyelectrolyte Multilayer Membranes for Efficient Water Purification. ACS Appl. Polym. Mater. 2020, 2, 715–724. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, F.; Chen, L.; Wei, X.; Xue, M.; Yang, F.; Jiang, S. 3D printing hydrogels for actuators: A review. Chin. Chem. Lett. 2021, 32, 2923–2932. [Google Scholar] [CrossRef]
- Li, S.; Miao, Y.; Zhang, M.; Reheman, A.; Huang, L.; Chen, L.; Wu, H. Anti-UV antibacterial conductive lignin-copolymerized hydrogel for pressure sensing. J. For. Eng. 2022, 7, 114–123. [Google Scholar] [CrossRef]
- Kuang, P.; Zhang, Y.; Chen, Y.; Wang, J.; Chu, F. Preparation and properties of conductive hydrogels derived from lignin-graphene oxide composite. J. For. Eng. 2022, 7, 106–112. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Liu, Y.; Li, J.; Chen, L. Salinity gradient power generation using cellulose membrane based on the dissolution-regeneration process. J. For. Eng. 2022, 7, 106–112. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Virya, A.; Lian, K. A review of neutral pH polymer electrolytes for electrochemical capacitors: Transitioning from liquid to solid devices. Mater. Rep. Energy 2021, 1, 100005. [Google Scholar] [CrossRef]
- Gannett, C.N.; Melecio-Zambrano, L.; Theibault, M.J.; Peterson, B.M.; Fors, B.P.; Abruña, H.D. Organic electrode materials for fast-rate, high-power battery applications. Mater. Rep. Energy 2021, 1, 100008. [Google Scholar] [CrossRef]
- Dara, P.K.; Geetha, A.; Mohanty, U.; Raghavankutty, M.; Mathew, S.; Chandragiri Nagarajarao, R.; Rangasamy, A. Extraction and Characterization of Myofibrillar Proteins from Different Meat Sources: A Comparative Study. J. Bioresour. Bioprod. 2021, 6, 367–378. [Google Scholar] [CrossRef]
- Stevens, M.J.; Plimpton, S.J. The effect of added salt on polyelectrolyte structure. Eur. Phys. J. B-Condens. Matter Complex Syst. 1998, 2, 341–345. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Press, C. Activity Coefficients in Electrolyte Solutions, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Panagiotopoulos, A.Z. Simulations of activities, solubilities, transport properties, and nucleation rates for aqueous electrolyte solutions. J. Chem. Phys. 2020, 153, 010903. [Google Scholar] [CrossRef]
- Anderko, A.; Wang, P.; Rafal, M. Electrolyte solutions: From thermodynamic and transport property models to the simulation of industrial processes. Fluid Phase Equilib. 2002, 194–197, 123–142. [Google Scholar] [CrossRef]
- Nagvekar, M.; Tihminlioglu, F.; Danner, R.P. Colligative properties of polyelectrolyte solutions. Fluid Phase Equilib. 1998, 145, 15–41. [Google Scholar] [CrossRef]
- Maurer, G.; Lammertz, S.; Schäfer, L.N. Aqueous Solutions of Polyelectrolytes: Vapor–Liquid Equilibrium and Some Related Properties. In Polymer Thermodynamics: Liquid Polymer-Containing Mixtures; Wolf, B.A., Enders, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 67–136. [Google Scholar]
- Wilcox, K.G.; Kozawa, S.K.; Morozova, S. Fundamentals and mechanics of polyelectrolyte gels: Thermodynamics, swelling, scattering, and elasticity. Chem. Phys. Rev. 2021, 2, 041309. [Google Scholar] [CrossRef]
- Guo, H.; Kurokawa, T.; Takahata, M.; Hong, W.; Katsuyama, Y.; Luo, F.; Ahmed, J.; Nakajima, T.; Nonoyama, T.; Gong, J.P. Quantitative Observation of Electric Potential Distribution of Brittle Polyelectrolyte Hydrogels Using Microelectrode Technique. Macromolecules 2016, 49, 3100–3108. [Google Scholar] [CrossRef]
- Ise, N.; Okubo, T. Mean Activity Coefficient of Polyelectrolytes. I. Measurements of Sodium Polyacrylates1. J. Phys. Chem. 1965, 69, 4102–4109. [Google Scholar] [CrossRef]
- Ise, N.; Okubo, T. Mean Activity Coefficient of Polyelectrolytes. II. Measurements of Sodium Salts of Polyvinyl Alcohols Partially Acetalized with Glyoxylic Acid1. J. Phys. Chem. 1966, 70, 1930–1935. [Google Scholar] [CrossRef]
- Ise, N.; Okubo, T. Mean Activity Coefficient of Polyelectrolytes. III. Measurements of Hydrochlorides of Polyethylenimine and Its Low Molecular Weight Analogs1. J. Phys. Chem. 1966, 70, 2400–2405. [Google Scholar] [CrossRef]
- Kagawa, I.; Katsuura, K. Activity coefficient of byions and ionic strength of polyelectrolyte solutions. J. Polym. Sci. 1952, 9, 405–415. [Google Scholar] [CrossRef]
- Kagawa, I.; Katsuura, K. Activity of counter ion in polyelectrolyte solutions. J. Polym. Sci. 1955, 17, 365–374. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, B.; Liu, H.; Hu, Y. Activity coefficients of NaCl in polyelectrolyte solutions from EMF measurements. Fluid Phase Equilib. 2001, 190, 191–199. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Kitano, T.; Ito, K.; Minakata, A. Sodium ion activity and electrical conductivity of poly(maleic acid) and poly(isobutylene-alt-maleic acid) in aqueous salt-free solution. Macromolecules 1991, 24, 6335–6339. [Google Scholar] [CrossRef]
- Kienzle-Sterzer, C.A.; Bakis, G.; Rodriquez-Sanchez, D.; Rha, C.K. Counter-ion activity in a cationic polyelectrolyte solution. Polym. Bull. 1984, 11, 185–190. [Google Scholar] [CrossRef]
- Kwon, H.J.; Osada, Y.; Gong, J.P. Polyelectrolyte Gels-Fundamentals and Applications. Polym. J. 2006, 38, 1211–1219. [Google Scholar] [CrossRef]
- Katchalsky, A.; Michaeli, I. Polyelectrolyte gels in salt solutions. J. Polym. Sci. 1955, 15, 69–86. [Google Scholar] [CrossRef]
- Safronov, A.P.; Kamalov, I.A.; Shklyar, T.F.; Dinislamova, O.A.; Blyakhman, F.A. Activity of counterions in hydrogels based on poly(acrylic acid) and poly(methacrylic acid): Potentiometric measurements. Polym. Sci. Ser. A 2012, 54, 909–919. [Google Scholar] [CrossRef]
- Nishimura, T.; Guo, H.; Kiyama, R.; Katsuyama, Y.; Gong, J.P.; Kurokawa, T. In Situ Evaluation of the Polymer Concentration Distribution of Microphase-Separated Polyelectrolyte Hydrogels by the Microelectrode Technique. Macromolecules 2021, 54, 10776–10785. [Google Scholar] [CrossRef]
- Muroga, Y.; Suzuki, K.; Kawaguchi, Y.; Nagasawa, M. Potentiometric titration of polyelectrolytes having stiff backbones. Biopolymers 1972, 11, 137–144. [Google Scholar] [CrossRef]
- Jano, I.; Jarvis, T. Activity Coefficients of Individual Ions from Titration Data. J. Solution Chem. 2002, 31, 317–339. [Google Scholar] [CrossRef]
- Dailey, G.W.; Huff, R.B.; Kang, J.; Queen, L.D.; Patterson, C.S. Isopiestic vapor pressure apparatus: A physical chemistry experiment. J. Chem. Educ. 1961, 38, 28. [Google Scholar] [CrossRef]
- Robinson, R.A.; Sinclair, D.A. The Activity Coefficients of the Alkali Chlorides and of Lithium Iodide in Aqueous Solution from Vapor Pressure Measurements. J. Am. Chem. Soc. 1934, 56, 1830–1835. [Google Scholar] [CrossRef]
- Sinclair, D.A. A Simple Method for Accurate Determinations of Vapor Pressures of Solutions. J. Phys. Chem. 1933, 37, 495–504. [Google Scholar] [CrossRef]
- Ise, N.; Okubo, T. Mean activity coefficient of polyelectrolytes. IV. Isopiestic measurements of sodium polyacrylates. J. Phys. Chem. 1967, 71, 1287–1290. [Google Scholar] [CrossRef]
- Ise, N.; Asai, K. Mean activity coefficient of polyelectrolytes. IX. Activity coefficients of poly(ethylenesulfonates) of various gegenions. J. Phys. Chem. 1968, 72, 1366–1369. [Google Scholar] [CrossRef]
- Ise, N.; Okubo, T. Mean activity coefficient of polyelectrolytes. V. Measurements of polyvinyl sulfates of various gegenions. J. Phys. Chem. 1967, 71, 1886–1890. [Google Scholar] [CrossRef]
- Ise, N.; Okubo, T. Mean activity coefficient of polyelectrolytes. VIII. Osmotic and activity coefficients of poly(styrenesulfonates) of various gegenions. J. Phys. Chem. 1968, 72, 1361–1366. [Google Scholar] [CrossRef]
- Ise, N.; Okubo, T. Mean activity coefficient of polyelectrolytes. X. Activity coefficients of polyphosphates of various gegenions. J. Phys. Chem. 1968, 72, 1370–1373. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Gschwend, P.M.; Imboden, D.M. Activity Coefficient and Solubility in Water. In Environmental Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2002; pp. 133–180. [Google Scholar]
- Dolar, D.; Bester, M. Activity Coefficient of a Polyelectrolyte from Solubility Measurements. J. Phys. Chem. 1995, 99, 4763–4767. [Google Scholar] [CrossRef]
- Waxman, M.H.; Sundheim, B.R.; Gregor, H.P. Studies on Ion Exchange Resins. VI. Water Vapor Sorption by Polystyrenesulfonic Acid. J. Phys. Chem. 1953, 57, 969–973. [Google Scholar] [CrossRef]
- Vesnaver, G.; Skerjanc, J. Entropies of dilution of strong polyelectrolyte solutions. J. Phys. Chem. 1986, 90, 4673–4676. [Google Scholar] [CrossRef]
- Reddy, M.; Marinsky, J.A. Further investigation of the osmotic properties of hydrogen and sodium polystyrenesulfonates. J. Phys. Chem. 1970, 74, 3884–3891. [Google Scholar] [CrossRef]
- Oman, S. Osmotic coefficients of aqueous polyelectrolyte solutions at low concentrations, 1. Polystyrenesulfonates with mono- and bivalent counterions. Makromol. Chem. 1974, 175, 2133–2140. [Google Scholar] [CrossRef]
- Oman, S. Osmotic coefficients of aqueous polyelectrolyte solutions at low concentrations, 2. dependence of polystyrenesulfonate osmotic coefficients on macroion molecular weight. Makromol. Chem. 1974, 175, 2141–2148. [Google Scholar] [CrossRef]
- Oman, S. Osmotic coefficients of aqueous polyelectrolyte solutions at low concentrations, 3. Dependence on macroion molecular weight. Makromol. Chem. 1977, 178, 475–484. [Google Scholar] [CrossRef]
- Valiskó, M.; Boda, D. Unraveling the Behavior of the Individual Ionic Activity Coefficients on the Basis of the Balance of Ion–Ion and Ion–Water Interactions. J. Phys. Chem. B 2015, 119, 1546–1557. [Google Scholar] [CrossRef]
- Ochiai, E.-I. Paradox of the activity coefficient. J. Chem. Educ. 1990, 67, 489. [Google Scholar] [CrossRef]
- Molero, M.; Outhwaite, C.W.; Bhuiyan, L.B. Individual ionic activity coefficients from a symmetric Poisson–Boltzmann theory. J. Chem. Soc. Faraday Trans. 1992, 88, 1541–1547. [Google Scholar] [CrossRef]
- Preston, B.N.; Snowden, J.M. Model connective tissue system: The effect of proteoglycan on the diffusional behavior of small non-electrolytes and microions. Biopolymers 1972, 11, 1627–1643. [Google Scholar] [CrossRef]
- Kowblansky, M.; Zema, P. Interactions of sodium ions with the sodium salts of poly(acrylic acid/acrylamide) copolymers of varying charge density. Macromolecules 1981, 14, 166–170. [Google Scholar] [CrossRef]
- Boyd, G.E. Thermodynamic Properties of Strong Electrolyte—Strong Polyelectrolyte Mixtures at 25 °C [1]. In Polyelectrolytes: Papers Initiated by a NATO Advanced Study Institute on Charged and Reactive Polymers held in France, June 1972; Sélégny, E., Mandel, M., Strauss, U.P., Eds.; Springer: Dordrecht, The Netherlands, 1974; pp. 135–155. [Google Scholar]
- Deserno, M.; Holm, C. Cell Model and Poisson-Boltzmann Theory: A Brief Introduction. In Electrostatic Effects in Soft Matter and Biophysics: Proceedings of the NATO Advanced Research Workshop on Electrostatic Effects in Soft Matter and Biophysics Les Houches, France 1–13 October 2000; Springer: Dordrecht, The Netherlands, 2001; pp. 27–52. [Google Scholar]
- Huang, B.; Maset, S.; Bohinc, K. Interaction between Charged Cylinders in Electrolyte Solution; Excluded Volume Effect. J. Phys. Chem. B 2017, 121, 9013–9023. [Google Scholar] [CrossRef]
- Moncho-Jordá, A.; Dzubiella, J. Swelling of ionic microgel particles in the presence of excluded-volume interactions: A density functional approach. PCCP 2016, 18, 5372–5385. [Google Scholar] [CrossRef]
- Adroher-Benítez, I.; Martín-Molina, A.; Ahualli, S.; Quesada-Pérez, M.; Odriozola, G.; Moncho-Jordá, A. Competition between excluded-volume and electrostatic interactions for nanogel swelling: Effects of the counterion valence and nanogel charge. PCCP 2017, 19, 6838–6848. [Google Scholar] [CrossRef]
- Hückel, E.; Debye, P. The theory of electrolytes: I. lowering of freezing point and related phenomena. J. Phys. Z. 1923, 24, 1. [Google Scholar]
- Delville, A. A simple solution to the poisson-boltzmann equation for ion binding to a polyelectrolyte. Chem. Phys. Lett. 1980, 69, 386–388. [Google Scholar] [CrossRef]
- Delville, A.; Gilboa, H.; Laszlo, P. Calculation of activity coefficients from a novel numerical solution of the Poisson–Boltzmann equation and application to 23Na NMR of sodium polystyrene sulfonate. J. Chem. Phys. 1982, 77, 2045–2050. [Google Scholar] [CrossRef]
- Hermans, J.J.; Overbeek, J.T.G. The dimensions of charged long chain molecules in solutions containing electrolytes. Recl. Trav. Chim. Pays-Bas 1948, 67, 761–776. [Google Scholar] [CrossRef]
- Ōsawa, F.; Imai, N.; Kagawa, I. Theory of strong polyelectrolyte solutions. I. Coiled macro ions. J. Polym. Sci. 1954, 13, 93–111. [Google Scholar] [CrossRef]
- Katchalsky, A. Polye1ectrolytes. J. Pure Appl. Chem. 1971, 26, 327–374. [Google Scholar] [CrossRef]
- Kagawa, I.; Nagasawa, M. Statistical thermodynamics of polyelectrolyte solutions. I. Activity coefficient of counter ion in salt-free system. J. Polym. Sci. 1955, 16, 299–310. [Google Scholar] [CrossRef]
- Nagasawa, M.; Kagawa, I. Colligative properties of polyelectrolyte solutions. IV. Activity coefficient of sodium ion. J. Polym. Sci. 1957, 25, 61–76. [Google Scholar] [CrossRef]
- Katchalsky, A.; Lifson, S.; Mazur, J. The electrostatic free energy of polyelectrolyte solutions. I. Randomly kinked macromolecules. J. Polym. Sci. 1953, 11, 409–423. [Google Scholar] [CrossRef]
- Lifson, S.; Katchalsky, A. The electrostatic free energy of polyelectrolyte solutions. II. Fully stretched macromolecules. J. Polym. Sci. 1954, 13, 43–55. [Google Scholar] [CrossRef]
- Oosawa, F. Polyelectrolytes; Dekker (Marcel) Inc.: New York, NY, USA, 1971. [Google Scholar]
- Kamcev, J.; Paul, D.R.; Freeman, B.D. Ion Activity Coefficients in Ion Exchange Polymers: Applicability of Manning’s Counterion Condensation Theory. Macromolecules 2015, 48, 8011–8024. [Google Scholar] [CrossRef]
- Kamcev, J.; Paul, D.R.; Manning, G.S.; Freeman, B.D. Predicting Salt Permeability Coefficients in Highly Swollen, Highly Charged Ion Exchange Membranes. ACS Appl. Mater. Interfaces 2017, 9, 4044–4056. [Google Scholar] [CrossRef]
- von Solms, N.; Chiew, Y.C. Analytical integral equation theory for a restricted primitive model of polyelectrolytes and counterions within the mean spherical approximation. I. Thermodynamic properties. J. Chem. Phys. 1999, 111, 4839–4850. [Google Scholar] [CrossRef]
- Gueron, M.; Weisbuch, G. Polyelectrolyte theory. 2. Activity coefficients in Poisson-Boltzmann and in condensation theory. The polarizability of the counterion sheath. J. Phys. Chem. 1979, 83, 1991–1998. [Google Scholar] [CrossRef]
- Lozada-Cassou, M. Hypernetted chain theory for the distribution of ions around a cylindrical electrode. J. Phys. Chem. 1983, 87, 3729–3732. [Google Scholar] [CrossRef]
- Ho/ye, J.S.; Lomba, E. Mean spherical approximation (MSA) for a simple model of electrolytes. I. Theoretical foundations and thermodynamics. J. Chem. Phys. 1988, 88, 5790–5797. [Google Scholar] [CrossRef]
- Maribo-Mogensen, B.; Kontogeorgis, G.M.; Thomsen, K. Comparison of the Debye–Hückel and the Mean Spherical Approximation Theories for Electrolyte Solutions. Ind. Eng. Chem. Res. 2012, 51, 5353–5363. [Google Scholar] [CrossRef]
- Bernard, O.; Simonin, J.P. Thermodynamic properties of ring polyelectrolytes in the binding mean spherical approximation. J. Mol. Liq. 2017, 228, 121–125. [Google Scholar] [CrossRef][Green Version]
- Vlachy, V.; McQuarrie, D.A. A theory of cylindrical polyelectrolyte solutions. J. Chem. Phys. 1985, 83, 1927–1932. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, H.; Hu, Y.; Prausnitz, J.M. A molecular-thermodynamic model for polyelectrolyte solutions. J. Chem. Phys. 1998, 108, 780–784. [Google Scholar] [CrossRef]
- Pitzer, K.S. Thermodynamics of electrolytes. I. Theoretical basis and general equations. J. Phys. Chem. 1973, 77, 268–277. [Google Scholar] [CrossRef]
- Humphreys, M.P.; Waters, J.F.; Turner, D.R.; Dickson, A.G.; Clegg, S.L. Chemical speciation models based upon the Pitzer activity coefficient equations, including the propagation of uncertainties: Artificial seawater from 0 to 45 °C. Mar. Chem. 2022, 244, 104095. [Google Scholar] [CrossRef]
- Song, K.; Lv, X.; Shi, J.; Yi, Y.; Xin, Y. The solubility behavior of sodium arsenate in NaOH solution based on the Pitzer model. Heliyon 2023, 9, e12849. [Google Scholar] [CrossRef]
- Pessôa Filho, P.d.A.; Maurer, G. An extension of the Pitzer equation for the excess Gibbs energy of aqueous electrolyte systems to aqueous polyelectrolyte solutions. Fluid Phase Equilib. 2008, 269, 25–35. [Google Scholar] [CrossRef]
- Franco, L.F.M.; Mattedi, S.; Pessôa Filho, P.d.A. A new approach for the thermodynamic modeling of the solubility of amino acids and β-lactam compounds as a function of pH. Fluid Phase Equilib. 2013, 354, 38–46. [Google Scholar] [CrossRef]
- Ghalami-Choobar, B.; Fallahkar, T.N. Mean activity coefficients measurement of sodium chloride and thermodynamic modeling of sodium chloride and polysodium acrylate aqueous mixtures at T = (298.2 and 303.2)K. Fluid Phase Equilib. 2014, 378, 51–59. [Google Scholar] [CrossRef]
- Großmann, C.; Tintinger, R.; Zhu, J.; Maurer, G. Aqueous Two-Phase Systems of Poly(ethylene glycol) and Di-Potassium Hydrogen Phosphate with and without partitioning Biomolecules—Experimental Results and Modeling of Thermodynamic Properties. Ber. Bunsenges. Phys. Chem. 1995, 99, 700–712. [Google Scholar] [CrossRef]
- Großmann, C.; Tintinger, R.; Zhu, J.; Maurer, G. Aqueous two-phase systems of poly(ethylene glycol) and dextran—Experimental results and modeling of thermodynamic properties. Fluid Phase Equilib. 1995, 106, 111–138. [Google Scholar] [CrossRef]
- Tintinger, R.; Zhu, J.; Grossmann, C.; Maurer, G. Partitioning of Some Amino Acids and Low Molecular Mass Peptides in Aqueous Two-Phase Systems of Poly(ethylene glycol) and Dextran in the Presence of Small Amounts of K2HPO4/KH2PO4-Buffer at 293 K: Experimental Results and Correlation. J. Chem. Eng. Data 1997, 42, 975–984. [Google Scholar] [CrossRef]
- Lammertz, S.; Grünfelder, T.; Ninni, L.; Maurer, G. A model for the Gibbs energy of aqueous solutions of polyelectrolytes. Fluid Phase Equilib. 2009, 280, 132–143. [Google Scholar] [CrossRef]
- Crea, F.; Giacalone, A.; Gianguzza, A.; Piazzese, D.; Sammartano, S. Modelling of natural and synthetic polyelectrolyte interactions in natural waters by using SIT, Pitzer and Ion Pairing approaches. Mar. Chem. 2006, 99, 93–105. [Google Scholar] [CrossRef]
- Wells, J.D. Thermodynamics of polyelectrolyte solutions. An empirical extension of the manning theory to finite salt concentrations. Biopolymers 1973, 12, 223–227. [Google Scholar] [CrossRef]
- Nagvekar, M.; Danner, R.P. An excess Gibbs free energy model for polyelectrolyte solutions. Fluid Phase Equilib. 1989, 53, 219–227. [Google Scholar] [CrossRef]
- Song, Y.; Chen, C.-C. Symmetric Electrolyte Nonrandom Two-Liquid Activity Coefficient Model. Ind. Eng. Chem. Res. 2009, 48, 7788–7797. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Hossain, N.; Chen, C.-C. Nonrandom two-liquid activity coefficient model for aqueous polyelectrolyte solutions. Fluid Phase Equilib. 2019, 497, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Chen, C.-C. Modeling aqueous multivalent polyelectrolytes systems with polyelectrolyte NRTL model. J. Mol. Liq. 2021, 336, 116237. [Google Scholar] [CrossRef]
- Kremer, K. Computer Simulations for Macromolecular Science. Macromol. Chem. Phys. 2003, 204, 257–264. [Google Scholar] [CrossRef]
- Smith, W.R.; Nezbeda, I.; Kolafa, J.; Moučka, F. Recent progress in the molecular simulation of thermodynamic properties of aqueous electrolyte solutions. Fluid Phase Equilib. 2018, 466, 19–30. [Google Scholar] [CrossRef]
- Metropolis, N.; Rosenbluth, A.W.; Rosenbluth, M.N.; Teller, A.H.; Teller, E. Equation of State Calculations by Fast Computing Machines. J. Chem. Phys. 1953, 21, 1087–1092. [Google Scholar] [CrossRef]
- Hill, T.L. Statistical Mechanics: Principles and Selected Applications; Courier Corporation: North Chelmsford, MA, USA, 2013. [Google Scholar]
- Edgecombe, S.; Linse, P. Monte Carlo Simulations of Cross-Linked Polyelectrolyte Gels with Oppositely Charged Macroions. Langmuir 2006, 22, 3836–3843. [Google Scholar] [CrossRef]
- Edgecombe, S.; Linse, P. Monte Carlo Simulation of Polyelectrolyte Gels: Effects of Polydispersity and Topological Defects. Macromolecules 2007, 40, 3868–3875. [Google Scholar] [CrossRef]
- Yin, D.-W.; Olvera de la Cruz, M.; de Pablo, J.J. Swelling and collapse of polyelectrolyte gels in equilibrium with monovalent and divalent electrolyte solutions. J. Chem. Phys. 2009, 131, 194907. [Google Scholar] [CrossRef]
- Widom, B. Some Topics in the Theory of Fluids. J. Chem. Phys. 1963, 39, 2808–2812. [Google Scholar] [CrossRef]
- Svensson, B.R.; Woodward, C.E. Widom’s method for uniform and non-uniform electrolyte solutions. Mol. Phys. 1988, 64, 247–259. [Google Scholar] [CrossRef]
- Ullner, M.; Staikos, G.; Theodorou, D.N. Monte Carlo Simulations of a Single Polyelectrolyte in Solution: Activity Coefficients of the Simple Ions and Application to Viscosity Measurements. Macromolecules 1998, 31, 7921–7933. [Google Scholar] [CrossRef]
- Ullner, M.; Qamhieh, K.; Cabane, B. Osmotic pressure in polyelectrolyte solutions: Cell-model and bulk simulations. Soft Matter 2018, 14, 5832–5846. [Google Scholar] [CrossRef]
- Sajevic, T.; Reščič, J.; Vlachy, V. Correlation between flexibility of chain-like polyelectrolyte and thermodynamic properties of its solution. J. Condens. Matter Phys. 2011, 14, 33603. [Google Scholar] [CrossRef]
- Bakhshandeh, A.; Levin, Y. Widom insertion method in simulations with Ewald summation. J. Chem. Phys. 2022, 156, 134110. [Google Scholar] [CrossRef]
- Nezbeda, I.; Kolafa, J. A New Version of the Insertion Particle Method for Determining the Chemical Potential by Monte Carlo Simulation. Mol. Simul. 1991, 5, 391–403. [Google Scholar] [CrossRef]
- Bieshaar, R.; Geiger, A.; Medvedev, N.N. Calculation of Chemical Potentials by a Novel Delaunay-Simplex Sampling Technique for Particle Insertion. Mol. Simul. 1995, 15, 189–196. [Google Scholar] [CrossRef]
- Heyes, D.M.; Santos, A. Chemical potential of a test hard sphere of variable size in hard-sphere fluid mixtures. J. Chem. Phys. 2018, 148, 214503. [Google Scholar] [CrossRef]
- Xu, B.; Liu, X.; Zhou, B. Calculation Methods of Solution Chemical Potential and Application in Emulsion Microencapsulation. Molecules 2021, 26, 2991. [Google Scholar] [CrossRef]
- Orkoulas, G.; Panagiotopoulos, A.Z. Chemical potentials in ionic systems from Monte Carlo simulations with distance-biased test particle insertions. Fluid Phase Equilib. 1993, 83, 223–231. [Google Scholar] [CrossRef]
- Bennett, C.H. Efficient estimation of free energy differences from Monte Carlo data. J. Comput. Phys. 1976, 22, 245–268. [Google Scholar] [CrossRef]
- Young, J.M.; Tietz, C.; Panagiotopoulos, A.Z. Activity Coefficients and Solubility of CaCl2 from Molecular Simulations. J. Chem. Eng. Data 2020, 65, 337–348. [Google Scholar] [CrossRef]
- Hempel, S.; Fischer, J.; Paschek, D.; Sadowski, G. Activity Coefficients of Complex Molecules by Molecular Simulation and Gibbs-Duhem Integration. Soft Mater. 2012, 10, 26–41. [Google Scholar] [CrossRef]
- Tironi, I.G.; Van Gunsteren, W.F. A molecular dynamics simulation study of chloroform. Mol. Phys. 1994, 83, 381–403. [Google Scholar] [CrossRef]
- Heidari, M.; Kremer, K.; Cortes-Huerto, R.; Potestio, R. Spatially Resolved Thermodynamic Integration: An Efficient Method To Compute Chemical Potentials of Dense Fluids. J. Chem. Theory Comput. 2018, 14, 3409–3417. [Google Scholar] [CrossRef]
- Bhati, A.P.; Wan, S.; Wright, D.W.; Coveney, P.V. Rapid, Accurate, Precise, and Reliable Relative Free Energy Prediction Using Ensemble Based Thermodynamic Integration. J. Chem. Theory Comput. 2017, 13, 210–222. [Google Scholar] [CrossRef]
- Nishio, T. Monte Carlo simulations on potentiometric titration of cylindrical polyelectrolytes: Introduction of a method and its application to model systems without added salt. Biophys. Chem. 1994, 49, 201–214. [Google Scholar] [CrossRef]
- Wennerström, H.; Jönsson, B.; Linse, P. The cell model for polyelectrolyte systems. Exact statistical mechanical relations, Monte Carlo simulations, and the Poisson–Boltzmann approximation. J. Chem. Phys. 1982, 76, 4665–4670. [Google Scholar] [CrossRef]
- Nishio, T.; Minakata, A. Monte Carlo Simulations of Ion Activities in Rodlike Polyelectrolyte Solutions. Langmuir 1999, 15, 4123–4128. [Google Scholar] [CrossRef]
- Nishio, T.; Minakata, A. Effects of ion size and valence on ion distribution in mixed counterion systems of rodlike polyelectrolyte solution. I. Mixed-size counterion systems with same valence. J. Chem. Phys. 2000, 113, 10784–10792. [Google Scholar] [CrossRef][Green Version]
- Nishio, T.; Minakata, A. Effects of Ion Size and Valence on Ion Distribution in Mixed Counterion Systems of a Rodlike Polyelectrolyte Solution. 2. Mixed-Valence Counterion Systems. J. Phys. Chem. B 2003, 107, 8140–8145. [Google Scholar] [CrossRef]
- Valleau, J.P.; Cohen, L.K. Primitive model electrolytes. I. Grand canonical Monte Carlo computations. J. Chem. Phys. 1980, 72, 5935–5941. [Google Scholar] [CrossRef]
- van Megen, W.; Snook, I.K. The grand canonical ensemble Monte Carlo method applied to electrolyte solutions. Mol. Phys. 1980, 39, 1043–1054. [Google Scholar] [CrossRef]
- Jayaram, B.; Beveridge, D.L. Grand canonical Monte Carlo simulations on aqueous solutions of sodium chloride and sodium DNA: Excess chemical potentials and sources of nonideality in electrolyte and polyelectrolyte solutions. J. Phys. Chem. 1991, 95, 2506–2516. [Google Scholar] [CrossRef]
- Mills, P.; Anderson, C.F.; Record, M.T., Jr. Grand canonical Monte Carlo calculations of thermodynamic coefficients for a primitive model of DNA-salt solutions. J. Phys. Chem. 1986, 90, 6541–6548. [Google Scholar] [CrossRef]
- Vlachy, V.; Haymet, A.D.J. A grand canonical Monte Carlo simulation study of polyelectrolyte solutions. J. Chem. Phys. 1986, 84, 5874–5880. [Google Scholar] [CrossRef]
- Lamperski, S. The individual and mean activity coefficients of an electrolyte from the inverse GCMC simulation. Mol. Simul. 2007, 33, 1193–1198. [Google Scholar] [CrossRef][Green Version]
- Mester, Z.; Panagiotopoulos, A.Z. Mean ionic activity coefficients in aqueous NaCl solutions from molecular dynamics simulations. J. Chem. Phys. 2015, 142, 044507. [Google Scholar] [CrossRef]
- Li, H.; Erbaş, A.; Zwanikken, J.; Olvera de la Cruz, M. Ionic Conductivity in Polyelectrolyte Hydrogels. Macromolecules 2016, 49, 9239–9246. [Google Scholar] [CrossRef]
- Liao, Q.; Dobrynin, A.V.; Rubinstein, M. Molecular Dynamics Simulations of Polyelectrolyte Solutions: Nonuniform Stretching of Chains and Scaling Behavior. Macromolecules 2003, 36, 3386–3398. [Google Scholar] [CrossRef]
- Grest, G.S.; Kremer, K. Molecular dynamics simulation for polymers in the presence of a heat bath. Phys. Rev. A 1986, 33, 3628–3631. [Google Scholar] [CrossRef]
- Kremer, K.; Grest, G.S. Dynamics of entangled linear polymer melts: A molecular-dynamics simulation. J. Chem. Phys. 1990, 92, 5057–5086. [Google Scholar] [CrossRef]
- Yin, D.-W.; Yan, Q.; de Pablo, J.J. Molecular dynamics simulation of discontinuous volume phase transitions in highly-charged crosslinked polyelectrolyte networks with explicit counterions in good solvent. J. Chem. Phys. 2005, 123, 174909. [Google Scholar] [CrossRef]
- Hockney, R.W.; Goel, S.P.; Eastwood, J.W. A 10000 particle molecular dynamics model with long range forces. Chem. Phys. Lett. 1973, 21, 589–591. [Google Scholar] [CrossRef]
- Antypov, D.; Holm, C. The Osmotic Behavior of Short Stiff Polyelectrolytes. Macromol. Symp. 2006, 245–246, 297–306. [Google Scholar] [CrossRef]
- Antypov, D.; Holm, C. Optimal Cell Approach to Osmotic Properties of Finite Stiff-Chain Polyelectrolytes. Phys. Rev. Lett. 2006, 96, 088302. [Google Scholar] [CrossRef]
- Antypov, D.; Holm, C. Osmotic Coefficient Calculations for Dilute Solutions of Short Stiff-Chain Polyelectrolytes. Macromolecules 2007, 40, 731–738. [Google Scholar] [CrossRef]
- Deserno, M.; Holm, C. Theory and simulations of rigid polyelectrolytes. Mol. Phys. 2002, 100, 2941–2956. [Google Scholar] [CrossRef]
- Deserno, M.; Holm, C.; May, S. Fraction of Condensed Counterions around a Charged Rod: Comparison of Poisson−Boltzmann Theory and Computer Simulations. Macromolecules 2000, 33, 199–206. [Google Scholar] [CrossRef]
- Liao, Q.; Dobrynin, A.V.; Rubinstein, M. Molecular Dynamics Simulations of Polyelectrolyte Solutions: Osmotic Coefficient and Counterion Condensation. Macromolecules 2003, 36, 3399–3410. [Google Scholar] [CrossRef]
- Stevens, M.J.; Kremer, K. The nature of flexible linear polyelectrolytes in salt free solution: A molecular dynamics study. J. Chem. Phys. 1995, 103, 1669–1690. [Google Scholar] [CrossRef]
- Košovan, P.; Richter, T.; Holm, C. Modeling of Polyelectrolyte Gels in Equilibrium with Salt Solutions. Macromolecules 2015, 48, 7698–7708. [Google Scholar] [CrossRef]
- Young, J.M.; Panagiotopoulos, A.Z. System-Size Dependence of Electrolyte Activity Coefficients in Molecular Simulations. J. Phys. Chem. B 2018, 122, 3330–3338. [Google Scholar] [CrossRef]
- Fyta, M.; Kalcher, I.; Dzubiella, J.; Vrbka, L.; Netz, R.R. Ionic force field optimization based on single-ion and ion-pair solvation properties. J. Chem. Phys. 2010, 132, 024911. [Google Scholar] [CrossRef]
- Lee, M.-T.; Vishnyakov, A.; Gor, G.Y.; Neimark, A.V. Interactions of Phosphororganic Agents with Water and Components of Polyelectrolyte Membranes. J. Phys. Chem. B 2011, 115, 13617–13623. [Google Scholar] [CrossRef]
- Klamt, A. The COSMO and COSMO-RS solvation models. WIREs Comput. Mol. Sci. 2011, 1, 699–709. [Google Scholar] [CrossRef]
- Bizjak, A.; Reščič, J.; Kalyuzhnyi, Y.V.; Vlachy, V. Theoretical aspects and computer simulations of flexible charged oligomers in salt-free solutions. J. Chem. Phys. 2006, 125, 214907. [Google Scholar] [CrossRef]
- Landsgesell, J.; Holm, C. Cell Model Approaches for Predicting the Swelling and Mechanical Properties of Polyelectrolyte Gels. Macromolecules 2019, 52, 9341–9353. [Google Scholar] [CrossRef]
- Rud, O.; Richter, T.; Borisov, O.; Holm, C.; Košovan, P. A self-consistent mean-field model for polyelectrolyte gels. Soft Matter 2017, 13, 3264–3274. [Google Scholar] [CrossRef]
- Bley, M.; Duvail, M.; Guilbaud, P.; Dufrêche, J.-F. Activity Coefficients of Aqueous Sodium, Calcium, and Europium Nitrate Solutions from Osmotic Equilibrium MD Simulations. J. Phys. Chem. B 2018, 122, 7726–7736. [Google Scholar] [CrossRef]
- Kohns, M.; Schappals, M.; Horsch, M.; Hasse, H. Activities in Aqueous Solutions of the Alkali Halide Salts from Molecular Simulation. J. Chem. Eng. Data 2016, 61, 4068–4076. [Google Scholar] [CrossRef]
- Sastry, N.V.; Dave, P.N.; Valand, M.K. Dilute solution behaviour of polyacrylamides in aqueous media. Eur. Polym. J. 1999, 35, 517–525. [Google Scholar] [CrossRef]
- Sharma, M.; Resta, R.; Car, R. Dipolar Correlations and the Dielectric Permittivity of Water. Phys. Rev. Lett. 2007, 98, 247401. [Google Scholar] [CrossRef]
- Adar, R.M.; Markovich, T.; Levy, A.; Orland, H.; Andelman, D. Dielectric constant of ionic solutions: Combined effects of correlations and excluded volume. J. Chem. Phys. 2018, 149, 054504. [Google Scholar] [CrossRef]
- Arens, L.; Barther, D.; Landsgesell, J.; Holm, C.; Wilhelm, M. Poly(sodium acrylate) hydrogels: Synthesis of various network architectures, local molecular dynamics, salt partitioning, desalination and simulation. Soft Matter 2019, 15, 9949–9964. [Google Scholar] [CrossRef]
- Zhang, A.-k.; Ling, J.; Li, K.; Fu, G.-d.; Nakajima, T.; Nonoyama, T.; Kurokawa, T.; Gong, J.P. Molecular structure and properties of click hydrogels with controlled dangling end defect. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1227–1236. [Google Scholar] [CrossRef]
- Xu, X.; Range, J.; Gygli, G.; Pleiss, J. Analysis of Thermophysical Properties of Deep Eutectic Solvents by Data Integration. J. Chem. Eng. Data 2020, 65, 1172–1179. [Google Scholar] [CrossRef]
| Method | Polyelectrolyte | Counterion | Polyelectrolyte conc. | Salt conc. | Source |
|---|---|---|---|---|---|
| EMF | CMC (Carboxymethyl Cellulose) | K+ | 0.004~0.015 N | 0.005~0.018 N | Kagawa (1952) [26] |
| EMF | PMA (poly(maleic acid)), PIM (poly(isobutylene-alt-maleic acid)) | Na+ | 2.4~60 mN | - | Kawaguchi (1991) [29] |
| EMF | Chitosan (M = 1.3 × 105) | Cl− | 5 × 10−3~11 × 10−3 mM | 0.05~0.5 M | Sterzer (1984) [30] |
| EMF | PDADMA (poly(diallyl dimethyl ammonium)), PASA (poly(anethole sulfonic acid)), PA (polyacrylate) | Na+ | 0.005~0.06 M | 0.01~1 M | Yu (2001) [28] |
| EMF | sulfated proteoglycan | Na+ | 0.01~0.005 M | 0.001~0.1 M | Preston (1972) [56] |
| EMF | PAM (polyacrylamide), PAA (poly(acrylic acid)) | Na+ | 0.018 N, 0.0045 N | 0~0.036 N | Kowblansky (1981) [57] |
| EMF | PAA, PMAA (poly(methacrylic acid)) (Gel) | K+ | - | 0.03~100 mM | Safronov (2012) [33] |
| Isopiestic | PAA | Na+ | 0.12~3 M | 0.008~1 M | Ise (1967) [40] |
| Isopiestic | PSS (polystyrene sulfonate) | Na+ | 0.33~2.69 M | 0.05~0.91 M | Boyd (1974) [58] |
| Solubility | PSS | Ba2+ | 0.006~0.240 M | 0.001~1 M | Dolar (1995) [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Yang, X.; Yang, F.; Zhang, C.; Zhang, Q.; Duan, G.; Jiang, S. Research Progress of the Ion Activity Coefficient of Polyelectrolytes: A Review. Molecules 2023, 28, 2042. https://doi.org/10.3390/molecules28052042
Zhang A, Yang X, Yang F, Zhang C, Zhang Q, Duan G, Jiang S. Research Progress of the Ion Activity Coefficient of Polyelectrolytes: A Review. Molecules. 2023; 28(5):2042. https://doi.org/10.3390/molecules28052042
Chicago/Turabian StyleZhang, Aokai, Xiuling Yang, Feng Yang, Chunmei Zhang, Qixiong Zhang, Gaigai Duan, and Shaohua Jiang. 2023. "Research Progress of the Ion Activity Coefficient of Polyelectrolytes: A Review" Molecules 28, no. 5: 2042. https://doi.org/10.3390/molecules28052042
APA StyleZhang, A., Yang, X., Yang, F., Zhang, C., Zhang, Q., Duan, G., & Jiang, S. (2023). Research Progress of the Ion Activity Coefficient of Polyelectrolytes: A Review. Molecules, 28(5), 2042. https://doi.org/10.3390/molecules28052042









