Phytomediated Silver Nanoparticles (AgNPs) Embellish Antioxidant Defense System, Ameliorating HLB-Diseased ‘Kinnow’ Mandarin Plants
Abstract
1. Introduction
2. Results and Discussion
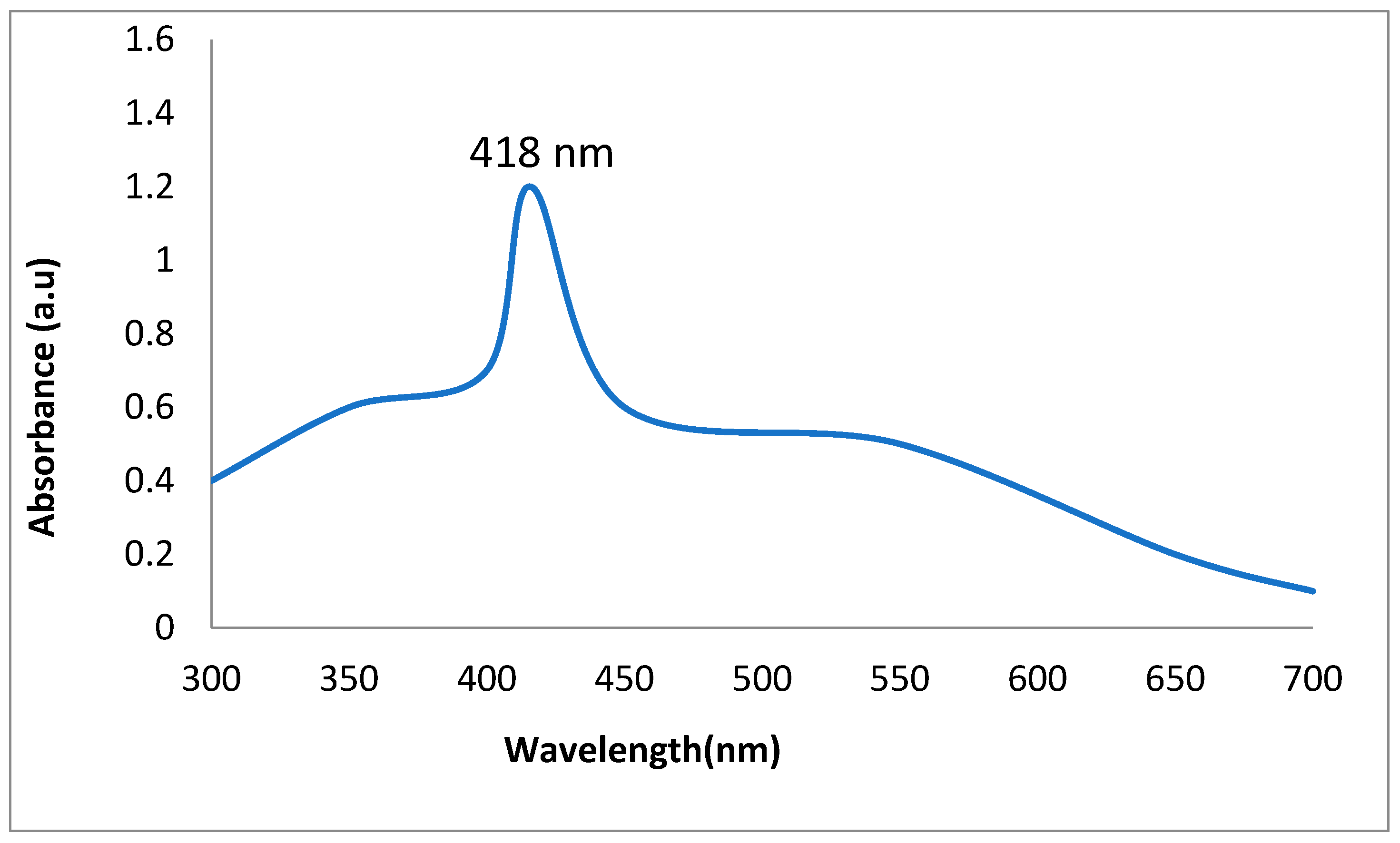
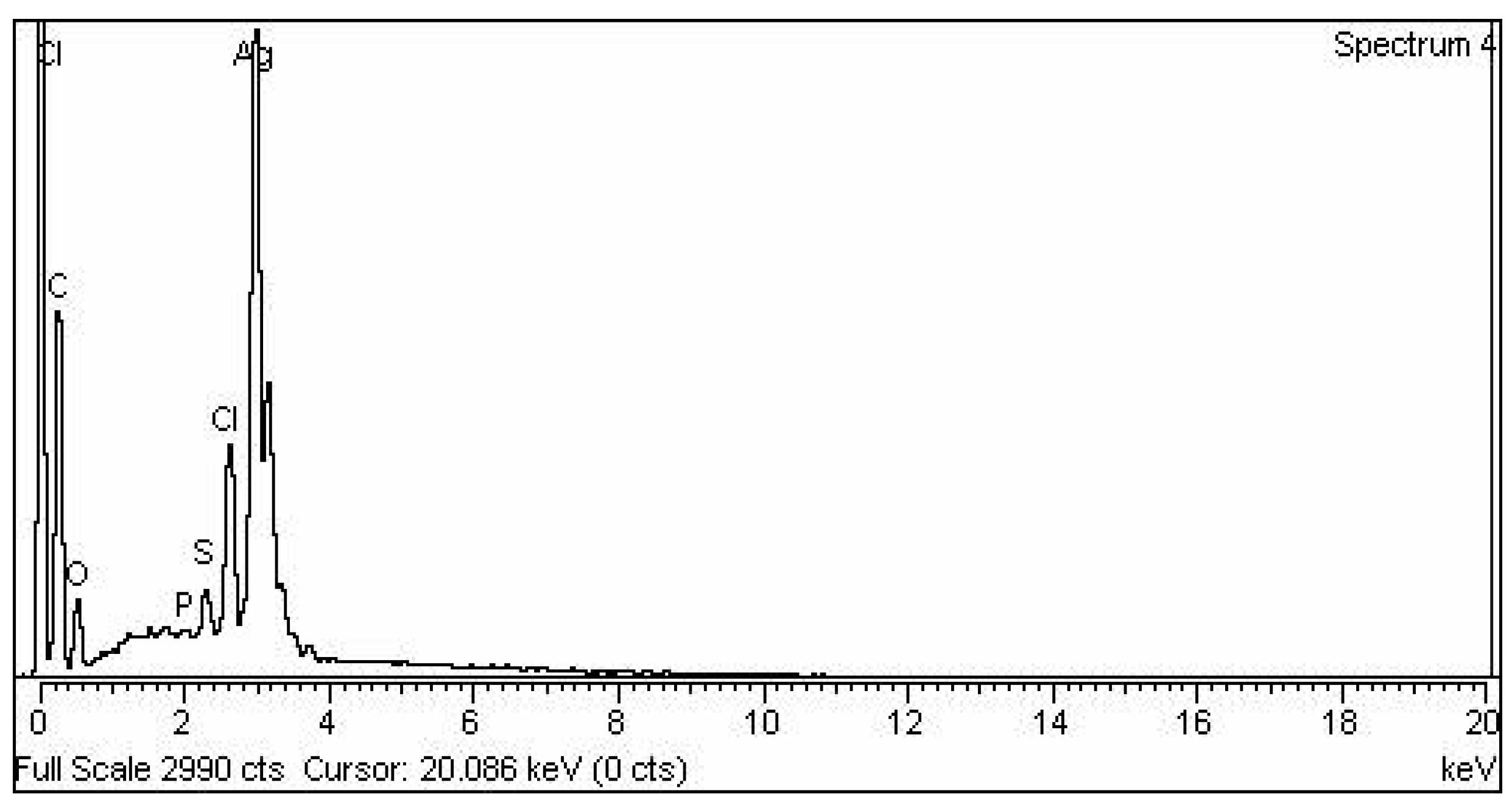
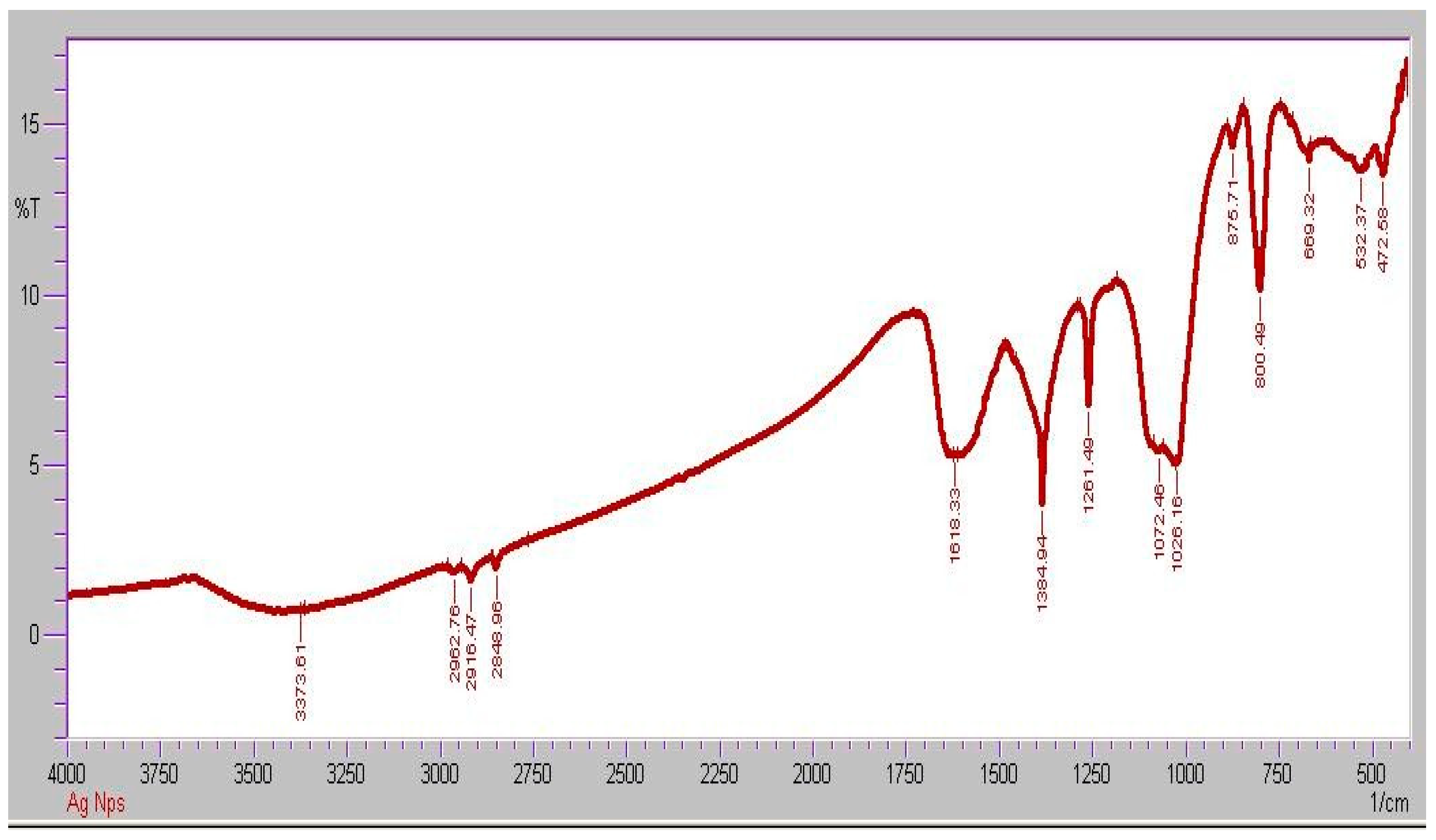
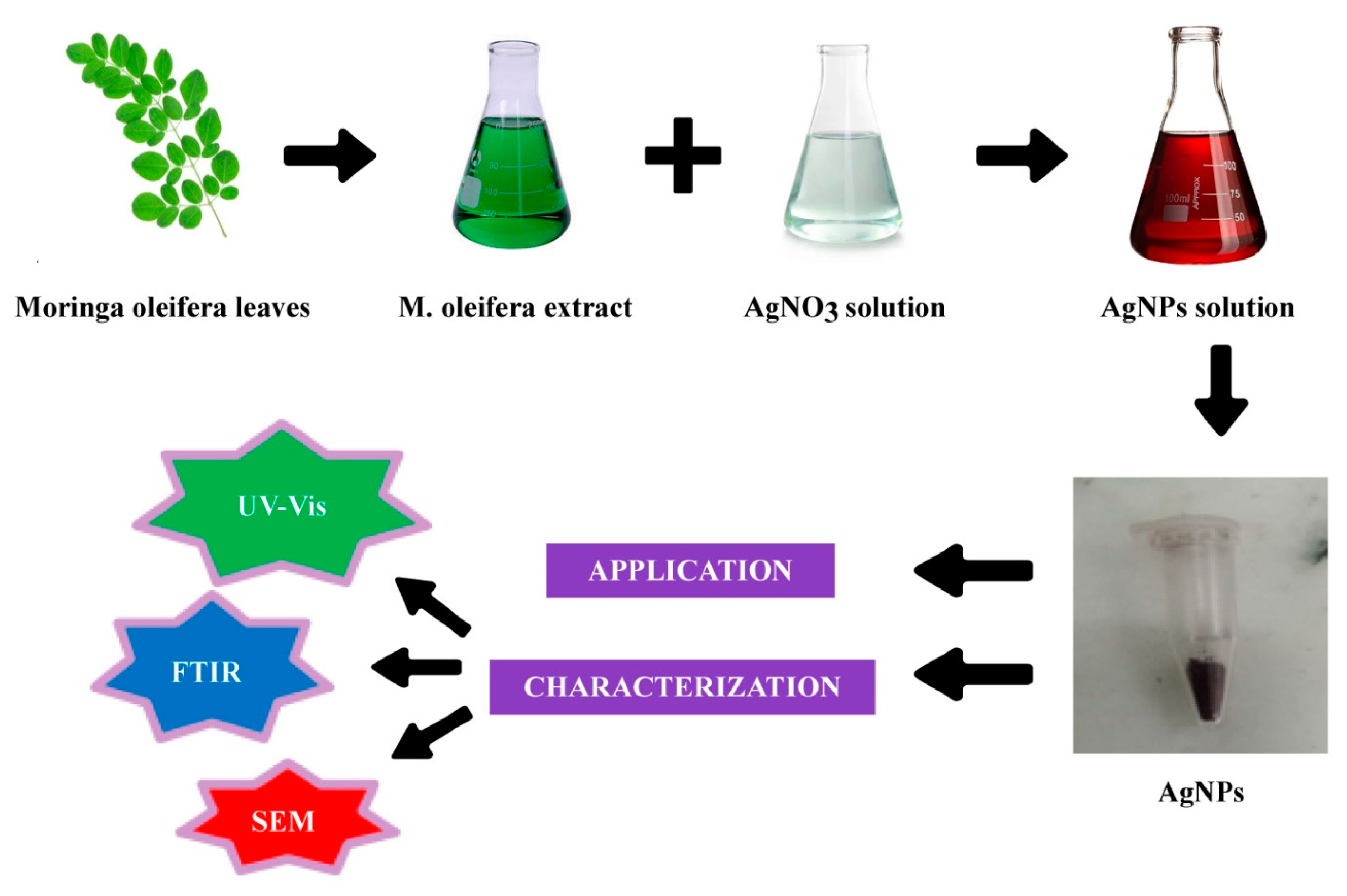
2.1. Synthesis and Characterization of AgNPs
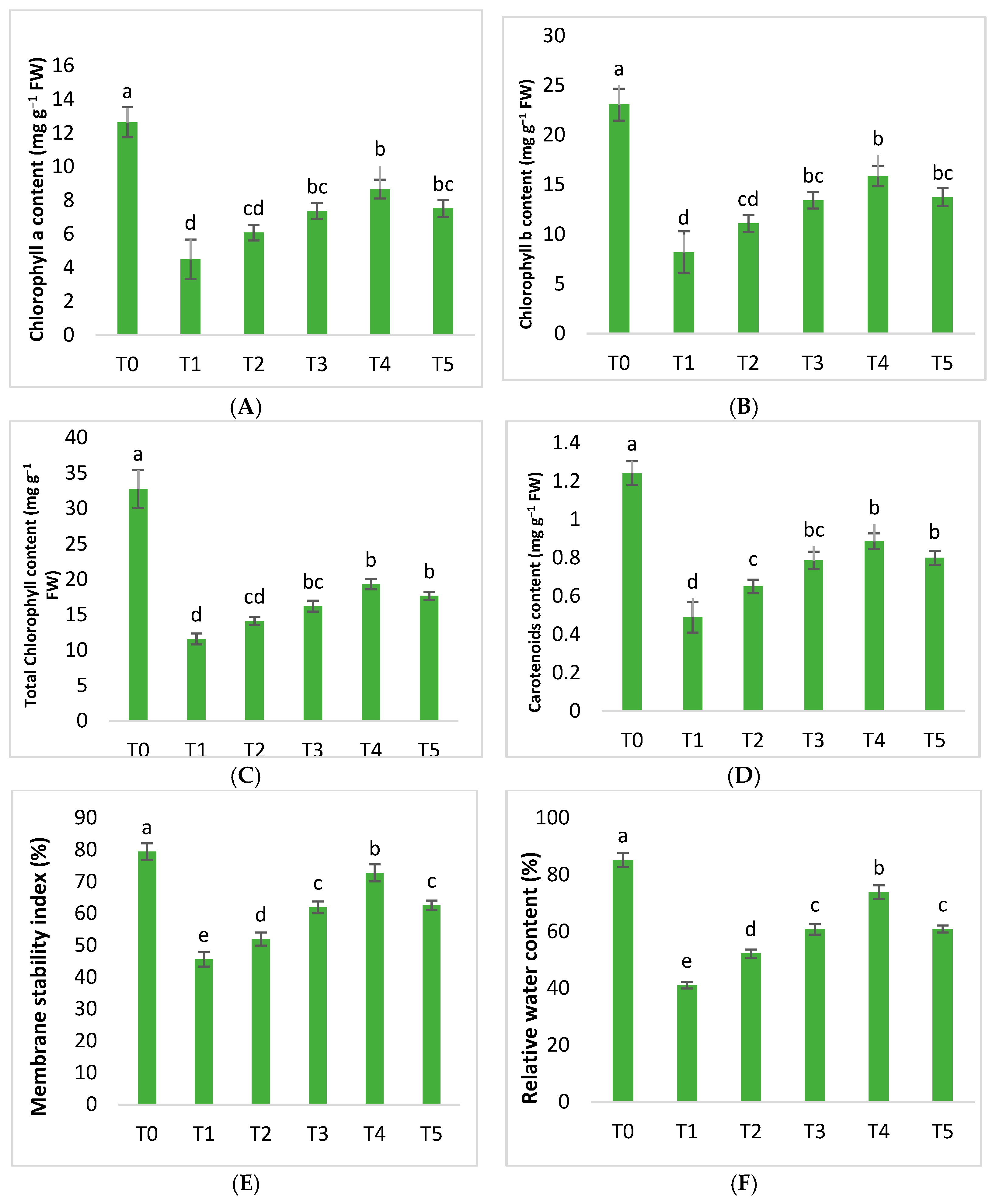
2.2. Physiological Parameters
2.3. Biochemical Parameters
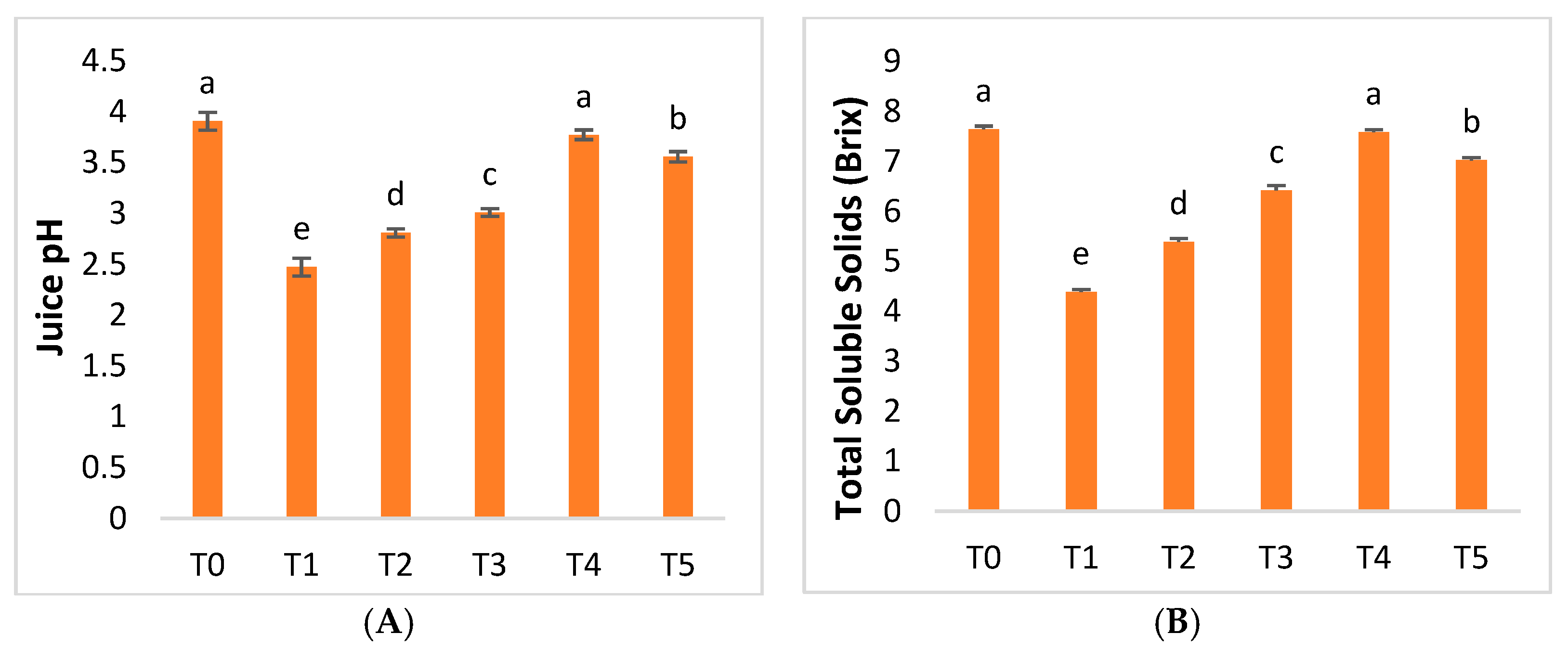
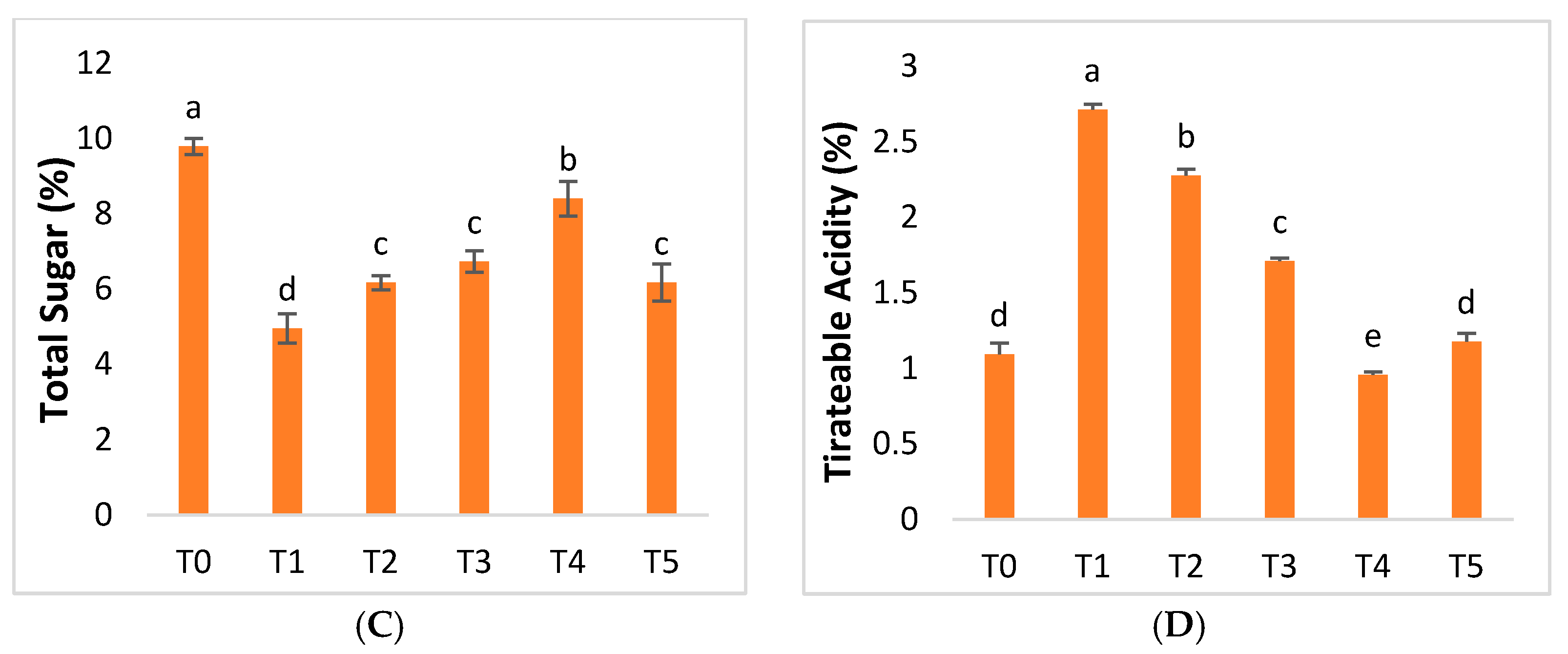
2.4. Fruit Quality Parameters
3. Materials and Methods
3.1. AgNPs Synthesis
3.1.1. Preparation of Plant Extract
3.1.2. Biofabrication of AgNPs
3.2. Characterization of Nanoparticles
3.3. Experimental Plan and Application of Biogenic AgNPs
3.4. Physiological Parameters
3.4.1. Chlorophyll and Carotenoid Content
3.4.2. Relative Water Content
3.4.3. Membrane Stability Index
3.4.4. Peroxidase, Superoxide Dismutase, and Catalase Activity
3.4.5. Total Phenolic Content (TPC)
3.4.6. Total Flavonoid Content (TFC)
3.4.7. Proline Content and Sugar Content
3.4.8. Fruit Weight, Fruit Diameter, Peel Diameter, and Peel Weight
3.4.9. Juice Weight, Rag Weight, and Juice pH
3.4.10. Total Soluble Solids and Titratable Acidity
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shakour, Z.T.A.; Fayek, N.M.; Farag, M.A. How do biocatalysis and biotransformation affect Citrus dietary flavonoids chemistry and bioactivity? A review. Crit. Rev. Biotechnol. 2020, 40, 689–714. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Heying, E. Tanumihardjo SA History, Global Distribution, and Nutritional Importance of Citrus Fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Patsalou, M.; Menikea, K.K.; Makri, E.; Vasquez, M.I.; Drouza, C.; Koutinas, M. Development of a citrus peel-based biorefinery strategy for the production of succinic acid. J. Clean. Prod. 2017, 166, 706–716. [Google Scholar] [CrossRef]
- Karn, A.; Zhao, C.; Yang, F.; Cui, J.; Gao, Z.; Wang, M.; Zheng, J. In-vivo biotransformation of citrus functional components and their effects on health. Crit. Rev. Food Sci. Nutr. 2021, 61, 756–776. [Google Scholar] [CrossRef]
- Ledesma-Escobar, C.A.; de Castro, M.D.L. Towards a comprehensive exploitation of citrus. Trends Food Sci. Technol. 2014, 39, 63–75. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C.; Shi, H.; Liao, Y.; Xu, F.; Du, H.; Zheng, J. Nutrients and bioactives in citrus fruits: Different citrus varieties, fruit parts, and growth stages. Crit. Rev. Food Sci. Nutr. 2021, 1–24. [Google Scholar] [CrossRef]
- Nawaz, R.; Abbasi, N.A.; Hafiz, I.A.; Khalid, A.; Ahmad, T.; Aftab, M. Impact of climate change on kinnow fruit industry of Pakistan. Agrotechnology 2019, 8, 2. [Google Scholar] [CrossRef]
- Razi, M.F.; Keremane, M.L.; Ramadugu, C.; Roose, M.; Khan, I.A.; Lee, R.F. Detection of citrus huanglongbing-associated ‘CandidatusLiberibacter asiaticus’ in citrus and Diaphorinacitri in Pakistan, seasonal variability, and implications for disease management. Phytopathology 2014, 104, 257–268. [Google Scholar] [CrossRef]
- Khalil, S.A.; Sattar, A.; Zamir, R. Development of sparse-seeded mutant kinnow (Citrus reticulata Blanco) through budwood irradiation. Afr. J. Biotechnol. 2011, 10, 14562–14565. [Google Scholar]
- Jaouad, M.; Moinina, A.; Ezrari, S.; Lahlali, R. Key pests and diseases of citrus trees with emphasis on root rot diseases: An overview. Moroc. J. Agric. Sci. 2020, 1. [Google Scholar]
- Tipu, M.M.H.; Rahman, M.M.; Islam, M.M.; Elahi, F.E.; Jahan, R.; Islam, M.R. Citrus greening disease (HLB) on Citrus reticulata (Mandarin) caused by Candidatus Liberibacter asiaticus in Bangladesh. Physiol. Mol. Plant Pathol. 2020, 112, 101558. [Google Scholar] [CrossRef]
- Brlansky, R. Citrus Greening or Huanglonbing Disease; NPDN: Corvallis, OR, USA, 2007. [Google Scholar]
- Zheng, Z.; Chen, J.; Deng, X. Historical perspectives, management, and current research of citrus HLB in Guangdong Province of China, where the disease has been endemic for over a hundred years. Phytopathology 2018, 108, 1224–1236. [Google Scholar] [CrossRef]
- Pitino, M.; Sturgeon, K.; Dorado, C.; Cano, L.M.; Manthey, J.A.; Shatters, R.G., Jr.; Rossi, L. Quercus leaf extracts display curative effects against Candidatus Liberibacter asiaticus that restore leaf physiological parameters in HLB-affected citrus trees. Plant Physiol. Biochem. 2020, 148, 70–79. [Google Scholar] [CrossRef]
- Devi, E.J.; Labala, R.K.; Modak, R.; Singh, N.S.; Devi, H.S. Molecular detection of “Candidatus Liberibacter asiaticus” causing HLB in Manipur and correlation of its incidence with elevation. Trop. Plant Pathol. 2020, 45, 658–667. [Google Scholar] [CrossRef]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Early root infection and damage in Huanglongbing disease development. J Citrus Pathol. 2014, 1, 232. [Google Scholar] [CrossRef]
- Gopal, K.; Gopi, V.; Kalyani, L.; Sreelatha, M.; Sreenivasulu, B. Symptom-based diagnosis of Huanglongbing (citrus greening) disease by PCR in sweet orange (Citrus sinensis Osbeck) and acid lime (Citrus aurantifolia Swingle). Arch. Phytopathol. Plant Prot. 2010, 43, 863–870. [Google Scholar] [CrossRef]
- Mira, A.; Yu, S.; Mohamed, E.; El Aiedy, A.; Elabasy, U.; Gmitter, F. Evaluation of Huanglongbing tolerance in citrus breeding populations. J. Product. Dev. 2019, 24, 371–390. [Google Scholar] [CrossRef]
- Sajid, A.; Ghazanfar, M.U.; Rauf, S.; Hussain, Z.; Ahmad, S.; Iftikhar, Y. Incidence and molecular detection of greening disease in two citrus cultivars in Sargodha, Pakistan. Sarhad J. Agric. 2021, 37, 296–301. [Google Scholar] [CrossRef]
- Naqvi, S.A.H.; Atta, S.; Liu, H.; Rehman, A.U.; Khan, A.A. Serological and molecular based detection of graft-transmissible pathogens associated with citrus from non-core areas of pakistan. Pak. J. Agric. Sci. 2017, 54, 793–799. [Google Scholar]
- Canales, E.; Coll, Y.; Hernández, I.; Portieles, R.; Rodríguez García, M.; López, Y.; Borrás-Hidalgo, O. ‘CandidatusLiberibacter asiaticus’, causal agent of citrus Huanglongbing, is reduced by treatment with Brassinosteroids. PLoS ONE 2016, 11, e0146223. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Tolerance of the trifoliate citrus hybrid US-897 (Citrus reticulata Blanco × Poncirus trifoliata L. Raf.) to Huanglongbing. HortScience 2011, 46, 16–22. [Google Scholar] [CrossRef]
- Wang, N.; Pierson, E.A.; Setubal, J.C.; Xu, J.; Levy, J.G.; Zhang, Y.; Li, J.; Rangel, L.T.; Martins, J., Jr. The Candidatus Liberibacter–host interface: Insights into pathogenesis mechanisms and disease control. Annu. Rev. Phytopathol. 2017, 55, 451–482. [Google Scholar] [CrossRef]
- Chen, X.D.; Gill, T.A.; Pelz-Stelinski, K.S.; Stelinski, L.L. Risk assessment of various insecticides used for management of Asian citrus psyllid, Diaphorinacitri in Florida citrus, against honey bee, Apismellifera. Ecotoxicology 2017, 26, 351–359. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, Y.; Powell, C.A.; Doud, M.S.; Yang, C.; Duan, Y. Effective antibiotics against ‘Candidatus Liberibacter asiaticus’ in HLB-affected citrus plants identified via the graft-based evaluation. PLoS ONE 2014, 9, e111032. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, J.; Wang, N. Control of citrus Huanglongbing via trunk injection of plant defense activators and antibiotics. Phytopathology 2018, 108, 186–195. [Google Scholar] [CrossRef]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef]
- Shahbaz, M.; Akram, A.; Raja, N.I.; Mukhtar, T.; Mashwani, Z.U.; Mehak, A.; Fatima, N.; Sarwar, S.; Haq, E.U.; Yousaf, T. Green synthesis and characterization of selenium nanoparticles and its application in plant disease management: A review. Pak. J. Phytopathol. 2022, 34, 189-102. [Google Scholar] [CrossRef]
- Hassan, H.U.; Raja, N.I.; Abasi, F.; Mehmood, A.; Qureshi, R.; Manzoor, Z.; Shahbaz, M.; Proćków, J. Comparative study of antimicrobial and antioxidant potential of olea ferruginea fruit extract and its mediated selenium nanoparticles. Molecules 2022, 27, 5194. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Balciunaitiene, A.; Viskelis, P.; Viskelis, J.; Streimikyte, P.; Liaudanskas, M.; Bartkiene, E.; Lele, V. Green synthesis of silver nanoparticles using extract of Artemisia absinthium L.; Humuluslupulus L. and Thymus vulgaris L.; physico-chemical characterization, antimicrobial and antioxidant activity. Processes 2021, 9, 1304. [Google Scholar] [CrossRef]
- Gopinath, V.; Kamath, S.M.; Priyadarshini, S.; Chik, Z.; Alarfaj, A.A.; Hirad, A.H. Multifunctional applications of natural polysaccharide starch and cellulose: An update on recent advances. Biomed. Pharmacother. 2022, 146, 112492. [Google Scholar] [CrossRef]
- Abasi, F.; Raja, N.I.; Mashwani, Z.U.R.; Amjad, M.S.; Ehsan, M.; Mustafa, N.; Proćków, J. Biogenic Silver Nanoparticles as a Stress Alleviator in Plants: A Mechanistic Overview. Molecules 2022, 27, 3378. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Mathew, G.M.; Pancrecious, J.K.; Nair, J.B.; Rajan, T.P.D.; Maiti, K.K.; Pai, B.C. Biogenic Ag nanoparticles from neem extract: Their structural evaluation and antimicrobial effects against Pseudomonas nitroreducens and Aspergillus unguis (NII 08123). ACS Biomater. Sci. Eng. 2019, 6, 235–245. [Google Scholar] [CrossRef]
- Javed, B.; Ikram, M.; Farooq, F.; Sultana, T.; Mashwani, Z.U.R.; Raja, N.I. Biogenesis of silver nanoparticles to treat cancer, diabetes, and microbial infections: A mechanistic overview. Appl. Microbiol. Biotechnol. 2021, 105, 2261–2275. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.U.; Bogdanchikova, N.; Garibo, D. Antibacterial and antifungal studies of biosynthesized silver nanoparticles against plant parasitic nematode Meloidogyne incognita, plant pathogens Ralstonia solanacearum and Fusarium oxysporum. Molecules 2021, 26, 2462. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Elshahawy, I.E. Silver nanoparticles inactivate sclerotial formation in controlling white rot disease in onion and garlic caused by the soil borne fungus Stromatinia cepivora. Eur. J. Plant Pathol. 2021, 160, 917–934. [Google Scholar] [CrossRef]
- Popova, T.P.; Ignatov, I.; Huether, F.; Petrova, T. Antimicrobial activity of colloidal nanosilver 24 ppm in vitro. Bulg. Chem. Commun. 2021, 53, 365–370. [Google Scholar]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Ghojavand, S.; Madani, M.; Karimi, J. Green synthesis, characterization and antifungal activity of silver nanoparticles using stems and flowers of felty germander. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2987–2997. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci. Rep. 2020, 10, 1–19. [Google Scholar] [CrossRef]
- Bao, V.V.Q.; Vuong, L.D.; Luan, L.V. Biomimetic synthesis of silver nanoparticles for preparing preservative solutions for mandarins (Citrus deliciosa Tenore). Nano LIFE 2018, 8, 1850003. [Google Scholar] [CrossRef]
- Osonga, F.J.; Akgul, A.; Yazgan, I.; Akgul, A.; Eshun, G.B.; Sakhaee, L.; Sadik, O.A. Size and shape-dependent antimicrobial activities of silver and gold nanoparticles: A model study as potential fungicides. Molecules 2020, 25, 2682. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.H. Biocompatible fungal chitosan encapsulated phytogenic silver nanoparticles enhanced antidiabetic, antioxidant and antibacterial activity. Int. J. Biol. Macromol. 2020, 153, 63–71. [Google Scholar] [CrossRef]
- Nurul Aini, A.; Al Farraj, D.A.; Endarko, E.; Rubiyanto, A.; Nur, H.; Al Khulaifi, M.M.; Syafiuddin, A. A new green method for the synthesis of silver nanoparticles and their antibacterial activities against gram-positive and gram-negative bacteria. J. Chin. Chem. Soc. 2019, 66, 705–712. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Bai, Y.; Li, W.; Li, X.; Xing, X.; Fu, J. Anticancer and Antibacterial Activities of Silver Nanoparticles (AgNPs) Synthesized from Cucumis melo L. J. Nanosci. Nanotechnol. 2020, 20, 4143–4151. [Google Scholar] [CrossRef]
- Jain, N.; Jain, P.; Rajput, D.; Patil, U.K. Green synthesized plant-based silver nanoparticles: Therapeutic prospective for anticancer and antiviral activity. Micro Nano Syst. Lett. 2021, 9, 1–24. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.D.; Shahriar, S.M.; Ahammad, A.J.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef]
- Stephano-Hornedo, J.L.; Torres-Gutiérrez, O.; Toledano-Magaña, Y.; Gradilla-Martínez, I.; Pestryakov, A.; Sánchez-González, A.; Bogdanchikova, N. Argovit™ silver nanoparticles to fight Huanglongbing disease in Mexican limes (Citrus aurantifolia Swingle). RSC Adv. 2020, 10, 6146–6155. [Google Scholar] [CrossRef]
- Dzuvor, C.K.; Pan, S.; Amanze, C.; Amuzu, P.; Asakiya, C.; Kubi, F. Bioactive components from Moringa oleifera seeds: Production, functionalities and applications–a critical review. Crit. Rev. Biotechnol. 2022, 42, 271–293. [Google Scholar] [CrossRef]
- Shahbaz, M.; Akram, A.; Raja, N.I.; Mukhtar, T.; Mehak, A.; Fatima, N.; Ajmal, M.; Ali, K.; Mustafa, N.; Abasi, F. Antifungal activity of green synthesized selenium nanoparticles and their effect on physiological, biochemical, and antioxidant defense system of mango under mango malformation disease. PLoS ONE 2023, 18, e0274679. [Google Scholar] [CrossRef]
- Huang, D.; Dang, F.; Huang, Y.; Chen, N.; Zhou, D. Uptake, translocation, and transformation of silver nanoparticles in plants. Environ. Sci. Nano 2022, 9, 12–39. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Chen, L. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469. [Google Scholar] [CrossRef]
- Arora, S.; Arora, S. Nutritional significance and therapeutic potential of Moringa oleifera: The wonder plant. J. Food Biochem. 2021, 45, e13933. [Google Scholar] [CrossRef]
- Shahbaz, M.; Fatima, N.; Mashwani, Z.U.R.; Akram, A.; Haq, E.U.; Mehak, A.; Abasi, F.; Ajmal, M.; Yousaf, T.; Raja, N.I.; et al. Effect of Phytosynthesized Selenium and Cerium Oxide Nanoparticles on Wheat (Triticum aestivum L.) against Stripe Rust Disease. Molecules 2022, 27, 8149. [Google Scholar] [CrossRef]
- Moodley, J.S.; Krishna, S.B.N.; Pillay, K.; Govender, P. Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015011. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Hoppe, H.; Okoh, A.I. Plant-based synthesis of silver nanoparticles using aqueous leaf extract of Salvia officinalis: Characterization and its antiplasmodial activity. J. Clust. Sci. 2021, 32, 101–109. [Google Scholar] [CrossRef]
- Avirdi, E.; Hooshmand, S.E.; Sepahvand, H.; Vishwanathan, V.; Bahadur, I.; Katata-Seru, L.M.; Varma, R.S. Ionic liquids-assisted greener preparation of silver nanoparticles. Curr. Opin. Green Sustain. Chem. 2021, 33, 100581. [Google Scholar] [CrossRef]
- Biba, R.; Košpić, K.; Komazec, B.; Markulin, D.; Cvjetko, P.; Pavoković, D.; Balen, B. Surface coating-modulated phytotoxic responses of silver nanoparticles in plants and freshwater green algae. Nanomaterials 2022, 12, 24. [Google Scholar] [CrossRef]
- Sadak, M.S. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonellafoenum-graecum). Bull. Natl. Res. Cent. 2019, 43, 38. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A.; Pradhan, S. Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol. Environ. Saf. 2018, 161, 624–633. [Google Scholar] [CrossRef]
- Khalofah, A.; Kilany, M.; Migdadi, H. Phytostimulatory influence of Comamonas testosteroni and silver nanoparticles on Linum usitatissimum L. under salinity stress. Plants 2021, 10, 790. [Google Scholar] [CrossRef]
- Kumari, R.; Ashraf, S.; Bagri, G.K.; Khatik, S.K.; Bagri, D.K.; Bagdi, D.L. Extraction and estimation of chlorophyll content of seed treated lentil crop using DMSO and acetone. J. Pharmacogn. Phytochem. 2018, 7, 249–250. [Google Scholar]
- Amin, B.; Atif, M.J.; Meng, H.; Ghani, M.I.; Ali, M.; Wang, X.; Cheng, Z. Biochemical and Physiological Responses of Cucumis sativus Cultivars to Different Combinations of Low-Temperature and High Humidity. J. Plant Growth Regul. 2022, 42, 390–406. [Google Scholar] [CrossRef]
- Kusumiyati, K.; Hadiwijaya, Y.; Suhandy, D.; Munawar, A.A. Prediction of water content and soluble solids content of ‘manalagi’apples using near infrared spectroscopy. IOP Conf. Ser. Earth Environ. Sci. 2021, 922, 012062. [Google Scholar] [CrossRef]
- Shahbaz, M.; Akram, A.; Mehak, A.; Haq, E.U.; Fatima, N.; Wareen, G.; Fitriatin, B.N.; Sayyed, R.Z.; Ilyas, N.; Sabullah, M.K. Evaluation of Selenium Nanoparticles in Inducing Disease Resistance against Spot Blotch Disease and Promoting Growth in Wheat under Biotic Stress. Plants 2023, 12, 761. [Google Scholar] [CrossRef]
- Kojonna, T.; Suttiyut, T.; Khunpolwattana, N.; Pongpanich, M.; Suriya-Arunroj, D.; Comai, L.; Chadchawan, S. Identification of a Negative Regulator for Salt Tolerance at Seedling Stage via a Genome-Wide Association Study of Thai Rice Populations. Int. J. Mol. Sci. 2022, 23, 1842. [Google Scholar] [CrossRef]
- Denaxa, N.K.; Damvakaris, T.; Roussos, P.A. Antioxidant defense system in young olive plants against drought stress and mitigation of adverse effects through external application of alleviating products. Sci. Hortic. 2020, 259, 108812. [Google Scholar] [CrossRef]
- Fimognari, L.; Dölker, R.; Kaselyte, G.; Jensen, C.N.; Akhtar, S.S.; Großkinsky, D.K.; Roitsch, T. Simple semi-high throughput determination of activity signatures of key antioxidant enzymes for physiological phenotyping. Plant Methods 2020, 16, 1–19. [Google Scholar] [CrossRef]
- Hadwan, M.H.; Abed, H.N. Data supporting the spectrophotometric method for the estimation of catalase activity. Data Brief 2016, 6, 194–199. [Google Scholar] [CrossRef]
- Salih, A.M.; Qahtan, A.A.; Al-Qurainy, F.; Al-Munqedhi, B.M. Impact of Biogenic Ag-Containing Nanoparticles on Germination Rate, Growth, Physiological, Biochemical Parameters, and Antioxidants System of Tomato (Solanum tuberosum L.) In Vitro. Processes 2022, 10, 825. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef]
- Bhagyawant, S.S.; Narvekar, D.T.; Gupta, N.; Bhadkaria, A.; Koul, K.K.; Srivastava, N. Variations in the antioxidant and free radical scavenging under induced heavy metal stress expressed as proline content in chickpea. Physiol. Mol. Biol. Plants 2019, 25, 683–696. [Google Scholar] [CrossRef]
- Fang, W.; Chi, Z.; Li, W.; Zhang, X.; Zhang, Q. Comparative study on the toxic mechanisms of medical nanosilver and silver ions on the antioxidant system of erythrocytes: From the aspects of antioxidant enzyme activities and molecular interaction mechanisms. J. Nanobiotechnol. 2019, 17, 66. [Google Scholar] [CrossRef]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of drought on soluble sugars and free proline content in selected Arabidopsis mutants. Biology 2020, 9, 367. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F.; Álvarez-Suarez, J.M. Green synthesis of silver nanoparticles using Astragalustribuloidesdelile. root extract: Characterization, antioxidant, antibacterial, and anti-inflammatory activities. Nanomaterials 2020, 10, 2383. [Google Scholar] [CrossRef]
- Mirmoeini, T.; Pishkar, L.; Kahrizi, D.; Barzin, G.; Karimi, N. Phytotoxicity of green synthesized silver nanoparticles on Camelina sativa L. Physiol. Mol. Biol. Plants 2021, 27, 417–427. [Google Scholar] [CrossRef]
- Qian, H.; Peng, X.; Han, X.; Ren, J.; Sun, L.; Fu, Z. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J. Environ. Sci. 2013, 25, 1947–1956. [Google Scholar] [CrossRef]
- Quiterio-Gutiérrez, T.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Hernández-Fuentes, A.D.; Sandoval-Rangel, A.; Benavides-Mendoza, A.; Juárez-Maldonado, A. The application of selenium and copper nanoparticles modifies the biochemical responses of tomato plants under stress by Alternariasolani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Jaskani, M.J.; Khan, A.S.; Haider, M.S.; Shafqat, W.; Asif, M.; Mehmood, A. Influence of different rootstocks on physico-chemical quality attributes of Kinnow mandarin. Pak. J. Agric. Sci 2021, 58, 929–935. [Google Scholar]
- Saleem, B.A.; Malik, A.U.; Pervez, M.A.; Khan, A.S.; Khan, M.N. Spring application of growth regulators affects fruit quality of ‘Blood Red’sweet orange. Pak. J. Bot 2008, 40, 1013–1023. [Google Scholar]
- Adumanya, O.C.U.; Onyeme, A.C.; Umensofor, G.A.; Nwinee, S.A. Ascorbic acid and pH levels of matured indigenous fruits in south-east Nigeria. Transatl. J. Multidiscip. Res. 2021, 3, 82–100. [Google Scholar]
- Chung, I.M.; Rajakumar, G.; Thiruvengadam, M. Effect of silver nanoparticles on phenolic compounds production and biological activities in hairy root cultures of Cucumis anguria. Acta Biol. Hung. 2018, 69, 97–109. [Google Scholar] [CrossRef]
- Raigond, P.; Raigond, B.; Kaundal, B.; Singh, B.; Joshi, A.; Dutt, S. Effect of zinc nanoparticles on antioxidative system of potato plants. J. Environ. Biol. 2017, 38, 435. [Google Scholar] [CrossRef]
- Marchan, S.; Hector, T.; Bascombe, K. The pH and Titratable Acidity of Still and Sparkling Flavored Waters: The Effects of Temperature and Storage Time. Open J. Stomatol. 2021, 11, 148. [Google Scholar] [CrossRef]
- Dada, A.O.; Adekola, F.A.; Odebunmi, E.O. Liquid phase scavenging of Cd (II) and Cu (II) ions onto novel nanoscale zerovalent manganese (nZVMn): Equilibrium, kinetic and thermodynamic studies. Environ. Nanotechnol. Monit. Manag. 2017, 8, 63–72. [Google Scholar] [CrossRef]
- Nejatzadeh, F. Effect of silver nanoparticles on salt tolerance of Saturejahortensis L. during in vitro and in vivo germination tests. Heliyon 2021, 7, e05981. [Google Scholar] [CrossRef]
- Femi-Adepoju, A.G.; Dada, A.O.; Otun, K.O.; Adepoju, A.O.; Fatoba, O.P. Green synthesis of silver nanoparticles using terrestrial fern (Gleichenia pectinata (Willd.) C. Presl.): Characterization and antimicrobial studies. Heliyon 2019, 5, e01543. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Fiol, N.; Olivella, M.À.; Gominho, J.; Villaescusa, I.; Pereira, H. Chemical characterization of different granulometric fractions of grape stalks waste. Ind. Crops Prod. 2013, 50, 494–500. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Lee, C.S. Green synthesis of gold and silver nanoparticles using leaf extract of clerodendruminerme; characterization, antimicrobial, and antioxidant activities. Biomolecules 2020, 10, 835. [Google Scholar] [CrossRef]
- Kavaz, D.; Umar, H.; Shehu, S. Synthesis, characterization, antimicrobial and antimetastatic activity of silver nanoparticles synthesized from Ficusingens leaf. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S1193–S1203. [Google Scholar] [CrossRef]
- Yu, C.; Tang, J.; Liu, X.; Ren, X.; Zhen, M.; Wang, L. Green biosynthesis of silver nanoparticles using Eriobotrya japonica (Thunb.) leaf extract for reductive catalysis. Materials 2019, 12, 189. [Google Scholar] [CrossRef]
- Srivastava, V.; Choubey, A.K. Synthesis of nanostructured silver particles using Citrus limetta peel extract for catalytic degradation of azo dyes through electron relay effect. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 045015. [Google Scholar] [CrossRef]
- Xu, H.; Wang, L.; Su, H.; Gu, L.; Han, T.; Meng, F.; Liu, C. Making good use of food wastes: Green synthesis of highly stabilized silver nanoparticles from grape seed extract and their antimicrobial activity. Food Biophys. 2015, 10, 12–18. [Google Scholar] [CrossRef]
- Alidoust, D.; Isoda, A. Phytotoxicity assessment of γ-Fe2O3 nanoparticles on root elongation and growth of rice plant. Environ. Earth Sci. 2014, 71, 5173–5182. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.U.; Hasan, M.A.; Yadav, K.K.; Pinto, M.; Malik, N.; Sharma, G.K. Agro-nanotechnology as an emerging field: A novel sustainable approach for improving plant growth by reducing biotic stress. Appl. Sci. 2021, 11, 2282. [Google Scholar] [CrossRef]
- Faghihi, R.; Larijani, K.; Abdossi, V.; Moradi, P. Evaluation of the effect of plastics bags containing silver nanocomposite of grapefruit’s peel on cucumber postharvest nutritional value and their possible penetration in tissue. Food Health J. 2020, 3, 35–39. [Google Scholar]
- Mehta, C.M.; Srivastava, R.; Arora, S.; Sharma, A.K. Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech 2016, 6, 254. [Google Scholar]
- Satti, S.H.; Raja, N.I.; Javed, B.; Akram, A.; Mashwani, Z.U.R.; Ahmad, M.S.; Ikram, M. Titanium dioxide nanoparticles elicited agro-morphological and physicochemical modifications in wheat plants to control Bipolarissorokiniana. PLoS ONE 2021, 16, e0246880. [Google Scholar] [CrossRef]
- Iqbal, M.; Raja, N.I.; Mashwani, Z.U.R.; Wattoo, F.H.; Hussain, M.; Ejaz, M.; Saira, H. Assessment of AgNPs exposure on physiological and biochemical changes and antioxidative defence system in wheat (Triticum aestivum L.) under heat stress. IET Nanobiotechnol. 2019, 13, 230–236. [Google Scholar] [CrossRef]
- Yashveer, S.; Redhu, N.S.; Singh, V.; Sangwan, S.; Laxman, H.V.; Tokas, J.; Sindhu, A. Nanoparticles in Agriculture: Characterization, Uptake and Role in Mitigating Heat Stress. Nat. Resour. Hum. Health 2022, 1–22. [Google Scholar] [CrossRef]
- Hajian, M.H.; Ghorbanpour, M.; Abtahi, F.; Hadian, J. Differential effects of biogenic and chemically synthesized silver-nanoparticles application on physiological traits, antioxidative status and californidine content in California poppy (Eschscholzia californica Cham). Environ. Pollut. 2022, 292, 118300. [Google Scholar] [CrossRef]
- Monreal, J.A.; Jimenez, E.T.; Remesal, E.; Morillo-Velarde, R.; García-Mauriño, S.; Echevarría, C. Proline content of sugar beet storage roots: Response to water deficit and nitrogen fertilization at field conditions. Environ. Exp. Bot. 2007, 60, 257–267. [Google Scholar] [CrossRef]
- Shahri, W.; Ahmad, S.S.; Tahir, I. Sugar signaling in plant growth and development. In Plant Signaling: Understanding the Molecular Crosstalk; Springer: New Delhi, India, 2014; pp. 93–116. [Google Scholar]
- Gupta, N.K.; Agarwal, S.; Agarwal, V.P.; Nathawat, N.S.; Gupta, S.; Singh, G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol. Plant. 2013, 35, 1837–1842. [Google Scholar] [CrossRef]
- Ma, W.; Pang, Z.; Huang, X.; Pandey, S.S.; Li, J.; Xu, J.; Wang, N. Citrus Huanglongbing is an immune-mediated disease that can be treated by mitigating reactive oxygen species triggered cell death of the phloem tissues caused by Candidatus Liberibacter asiaticus. bioRxiv 2021. [Google Scholar] [CrossRef]
- Hussain, S.; Rao, M.J.; Anjum, M.A.; Ejaz, S.; Umar, U.U.D.; Ali, M.A.; Naqvi, S.A.H. Effect of different combinations of antibiotics on fruit quality and antioxidant defense system in Huanglongbing infected Kinnow orchards. AMB Express 2019, 9, 147. [Google Scholar] [CrossRef]
- KaramiMehrian, S.; Heidari, R.; Rahmani, F. Effect of silver nanoparticles on free amino acids content and antioxidant defense system of tomato plants. Indian J. Plant Physiol. 2015, 20, 257–263. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Dasgupta, A.; Sarkar, J.; Panda, K.; Acharya, K. Chitosan nanoparticles: A positive modulator of innate immune responses in plants. Sci. Rep. 2015, 5, 15195. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982. [Google Scholar] [CrossRef]
- Vennila, K.; Chitra, L.; Balagurunathan, R.; Palvannan, T. Comparison of biological activities of selenium and silver nanoparticles attached with bioactive phytoconstituents: Green synthesized using Spermacoce hispida extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015005. [Google Scholar] [CrossRef]
- Mattos, D., Jr.; Kadyampakeni, D.M.; da Silva, J.R.; Vashisth, T.; Boaretto, R.M. Reciprocal effects of huanglongbing infection and nutritional status of citrus trees: A review. Trop. Plant Pathol. 2020, 45, 586–596. [Google Scholar] [CrossRef]
- Kunwar, S.; Grosser, J.; Gmitter, F.G.; Castle, W.S.; Albrecht, U. Field performance of ‘Hamlin’orange trees grown on various rootstocks in huanglongbing-endemic conditions. HortScience 2021, 56, 244–253. [Google Scholar] [CrossRef]
- Thompson, A.K. Harvest maturity and methods (Chapter 2 and postharvest treatments (Chapter 4). In Postharvest Technology of Fruits and Vegetables; Blackwell Science: Hoboken, NJ, USA, 2014; Volume 95, pp. 35–37. [Google Scholar]
- Silva, S.R.D.; Girardi, E.A.; Santos, M.G.; Cantuarias-Avilés, T.E.; Stuchi, E.S. Plant growth, yield and fruit quality of Clementine mandarin selections under subtropical climate in Brazil. Rev. Frutic. 2018, 40. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; Zacarías, L. Maturity indicators and citrus fruit quality. Stewart Postharvest Rev. 2014, 10, 1–6. [Google Scholar]
- Koh, J.; Morales-Contreras, B.E.; Guerra-Rosas, M.I.; Osorio-Hernández, E.; Culver, C.A.; Morales-Castro, J.; Wicker, L. Huanglongbing disease and quality of pectin and fruit juice extracted from Valencia oranges. LWT 2020, 131, 109692. [Google Scholar] [CrossRef]
- Tyl, C.; Sadler, G.D. pH and titratable acidity. In Food Analysis; Springer: Cham, Switzerland, 2017; pp. 389–406. [Google Scholar]


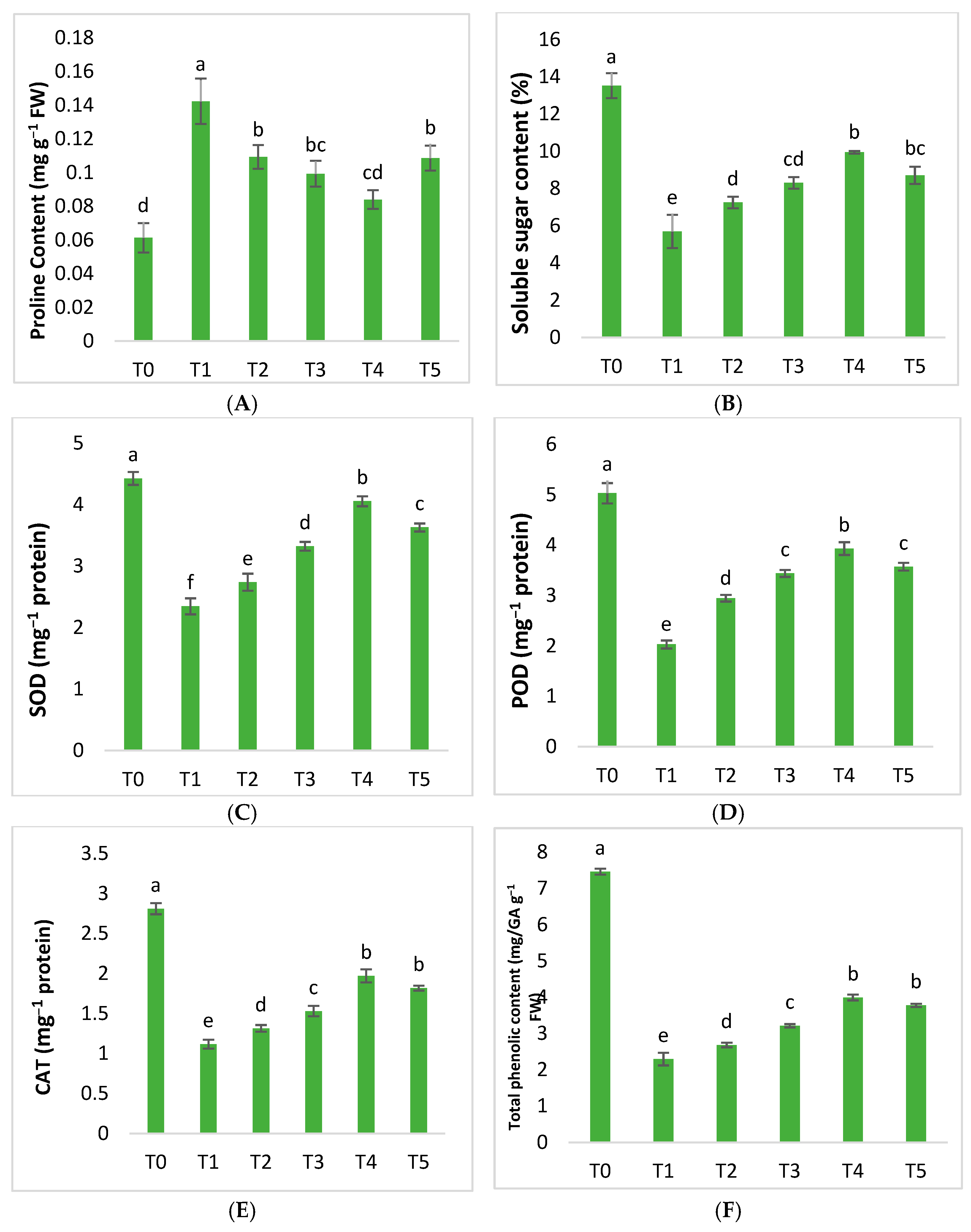
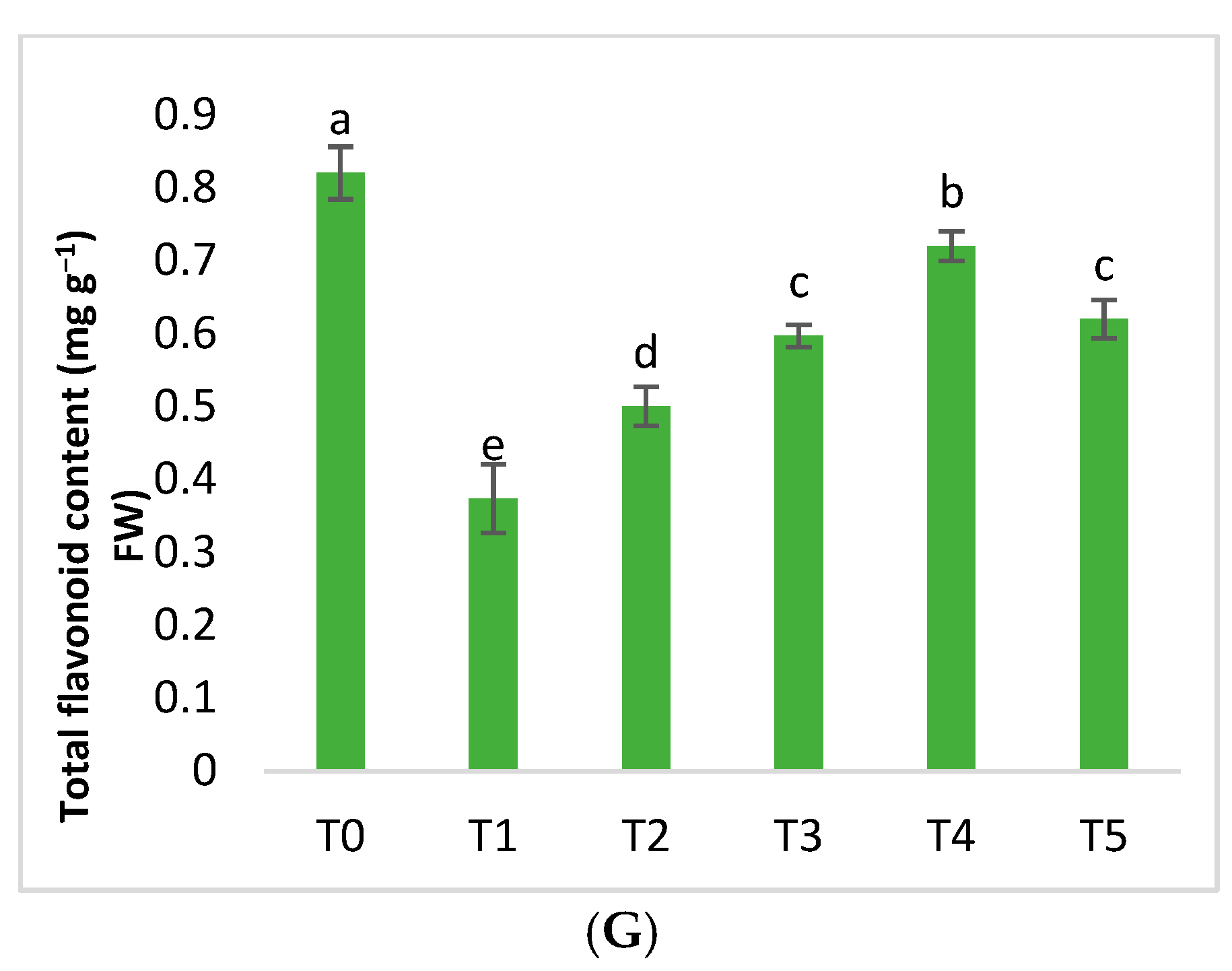
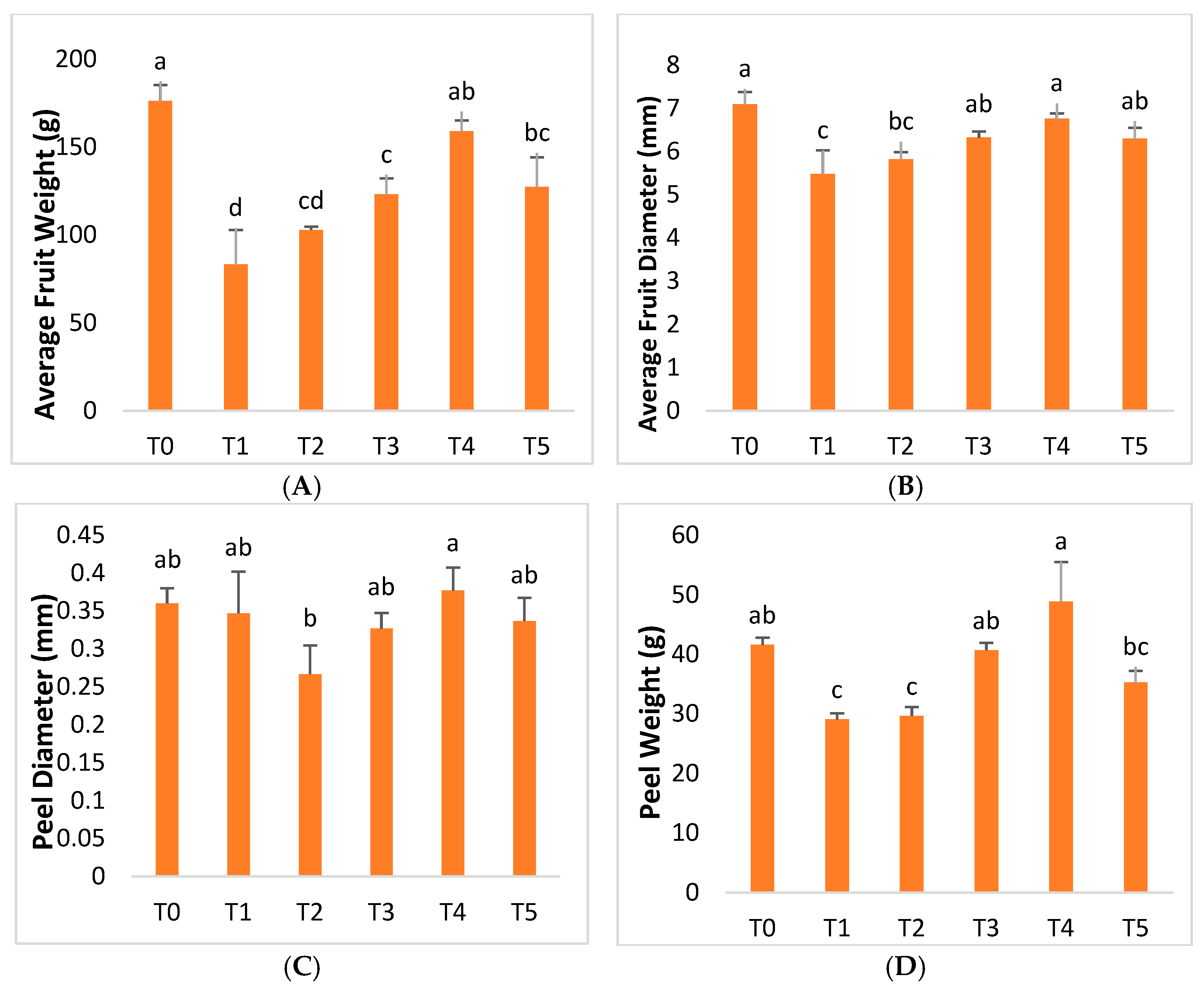
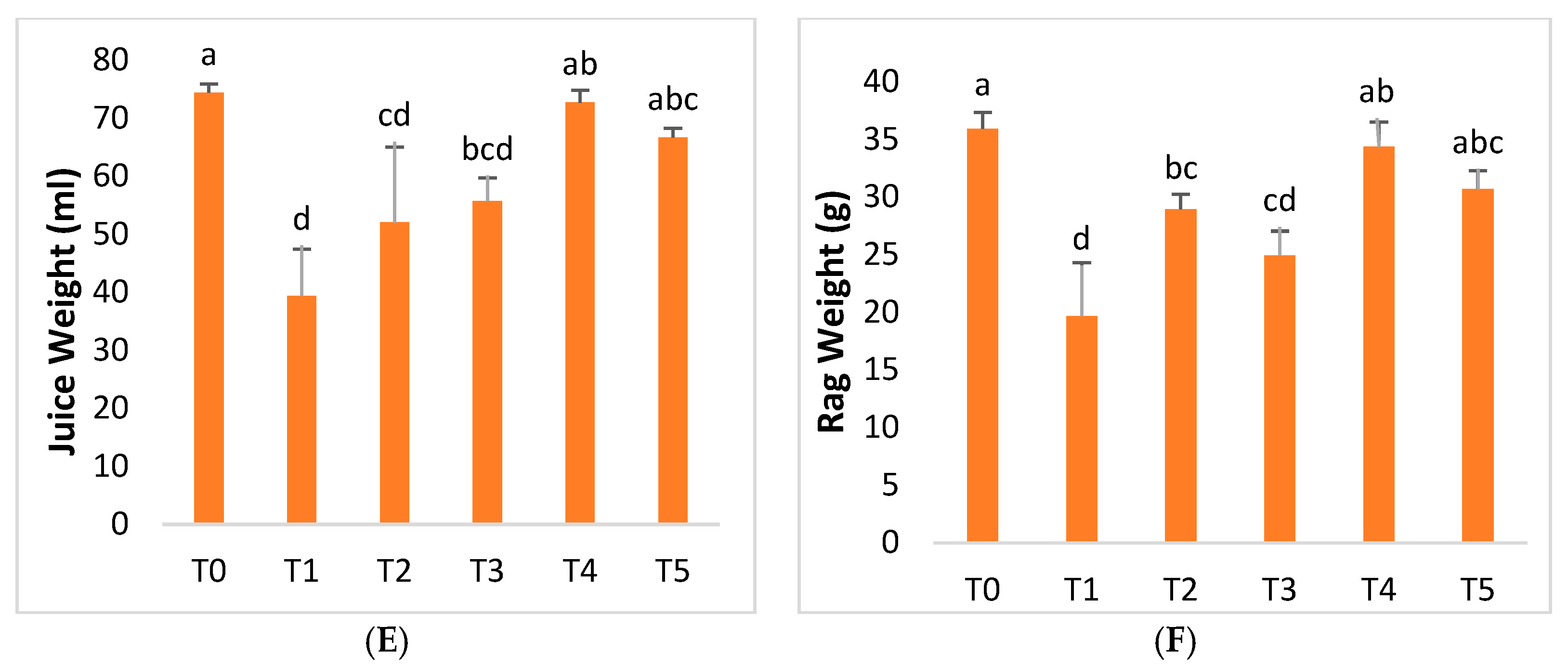

| Treatment | Conditions |
|---|---|
| T0 | Control (healthy plants) |
| T1 | Diseased (without AgNPs) |
| T2 | Diseased + 25 mgL−1 AgNPs |
| T3 | Diseased + 50 mgL−1 AgNPs |
| T4 | Diseased + 75 mgL−1 AgNPs |
| T5 | Diseased + 100 mgL−1 AgNPs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umair Raza, M.; Abasi, F.; Shahbaz, M.; Ehsan, M.; Seerat, W.; Akram, A.; Raja, N.I.; Mashwani, Z.u.-R.; Hassan, H.U.; Proćków, J. Phytomediated Silver Nanoparticles (AgNPs) Embellish Antioxidant Defense System, Ameliorating HLB-Diseased ‘Kinnow’ Mandarin Plants. Molecules 2023, 28, 2044. https://doi.org/10.3390/molecules28052044
Umair Raza M, Abasi F, Shahbaz M, Ehsan M, Seerat W, Akram A, Raja NI, Mashwani Zu-R, Hassan HU, Proćków J. Phytomediated Silver Nanoparticles (AgNPs) Embellish Antioxidant Defense System, Ameliorating HLB-Diseased ‘Kinnow’ Mandarin Plants. Molecules. 2023; 28(5):2044. https://doi.org/10.3390/molecules28052044
Chicago/Turabian StyleUmair Raza, Muhammad, Fozia Abasi, Muhammad Shahbaz, Maria Ehsan, Wajiha Seerat, Abida Akram, Naveed Iqbal Raja, Zia ur-Rehman Mashwani, Hammad Ul Hassan, and Jarosław Proćków. 2023. "Phytomediated Silver Nanoparticles (AgNPs) Embellish Antioxidant Defense System, Ameliorating HLB-Diseased ‘Kinnow’ Mandarin Plants" Molecules 28, no. 5: 2044. https://doi.org/10.3390/molecules28052044
APA StyleUmair Raza, M., Abasi, F., Shahbaz, M., Ehsan, M., Seerat, W., Akram, A., Raja, N. I., Mashwani, Z. u.-R., Hassan, H. U., & Proćków, J. (2023). Phytomediated Silver Nanoparticles (AgNPs) Embellish Antioxidant Defense System, Ameliorating HLB-Diseased ‘Kinnow’ Mandarin Plants. Molecules, 28(5), 2044. https://doi.org/10.3390/molecules28052044







