Chemotaxonomic Variation in Volatile Component Contents in Ancient Platycladus orientalis Leaves with Different Tree Ages in Huangdi Mausoleum
Abstract
1. Introduction
2. Results
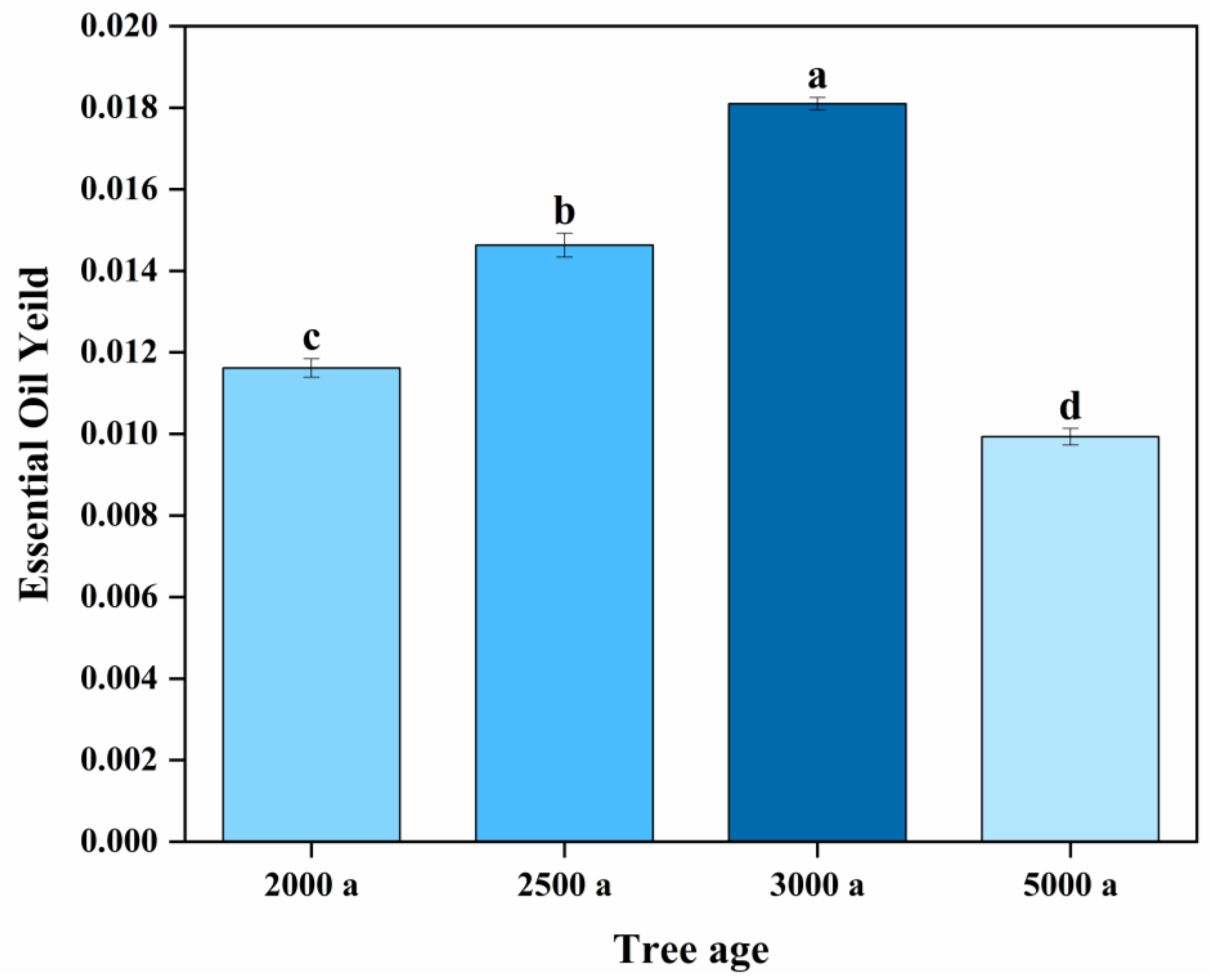
2.1. Essential Oil Yield of Ancient Platycladus orientalis Leaves with Different Tree Ages
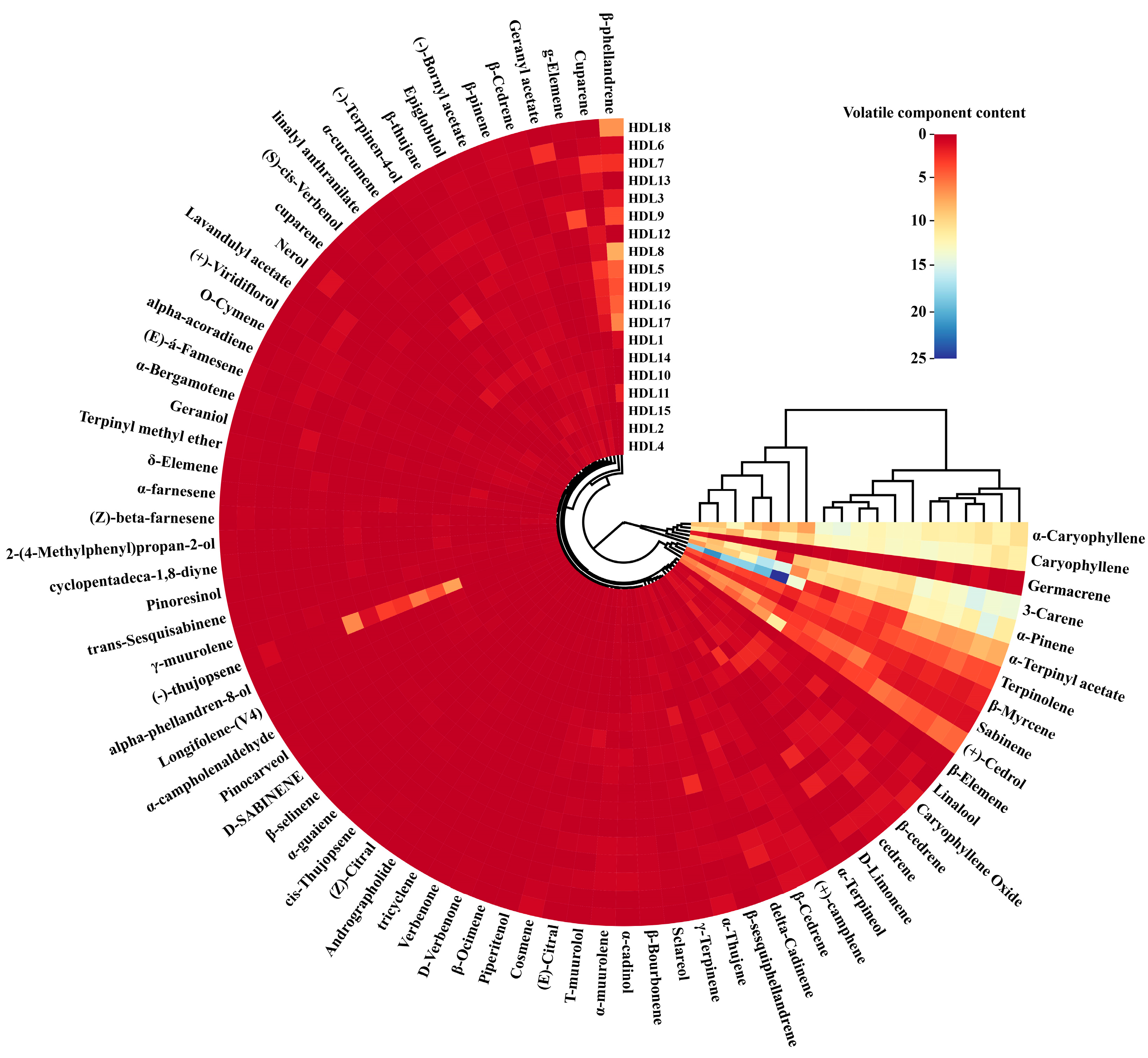
2.2. Analysis of Volatile Components in Ancient Platycladus orientalis Leaves with Different Tree Ages
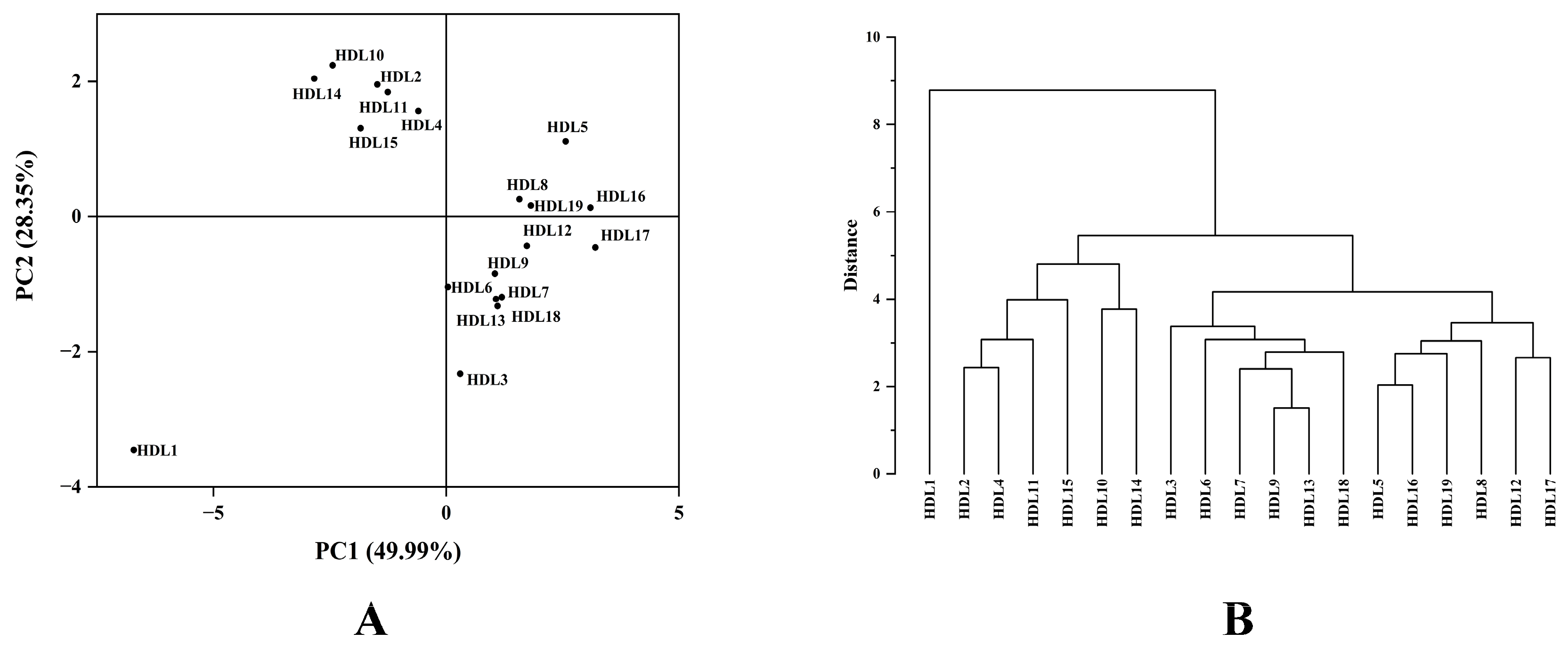
2.3. HCA and PCA
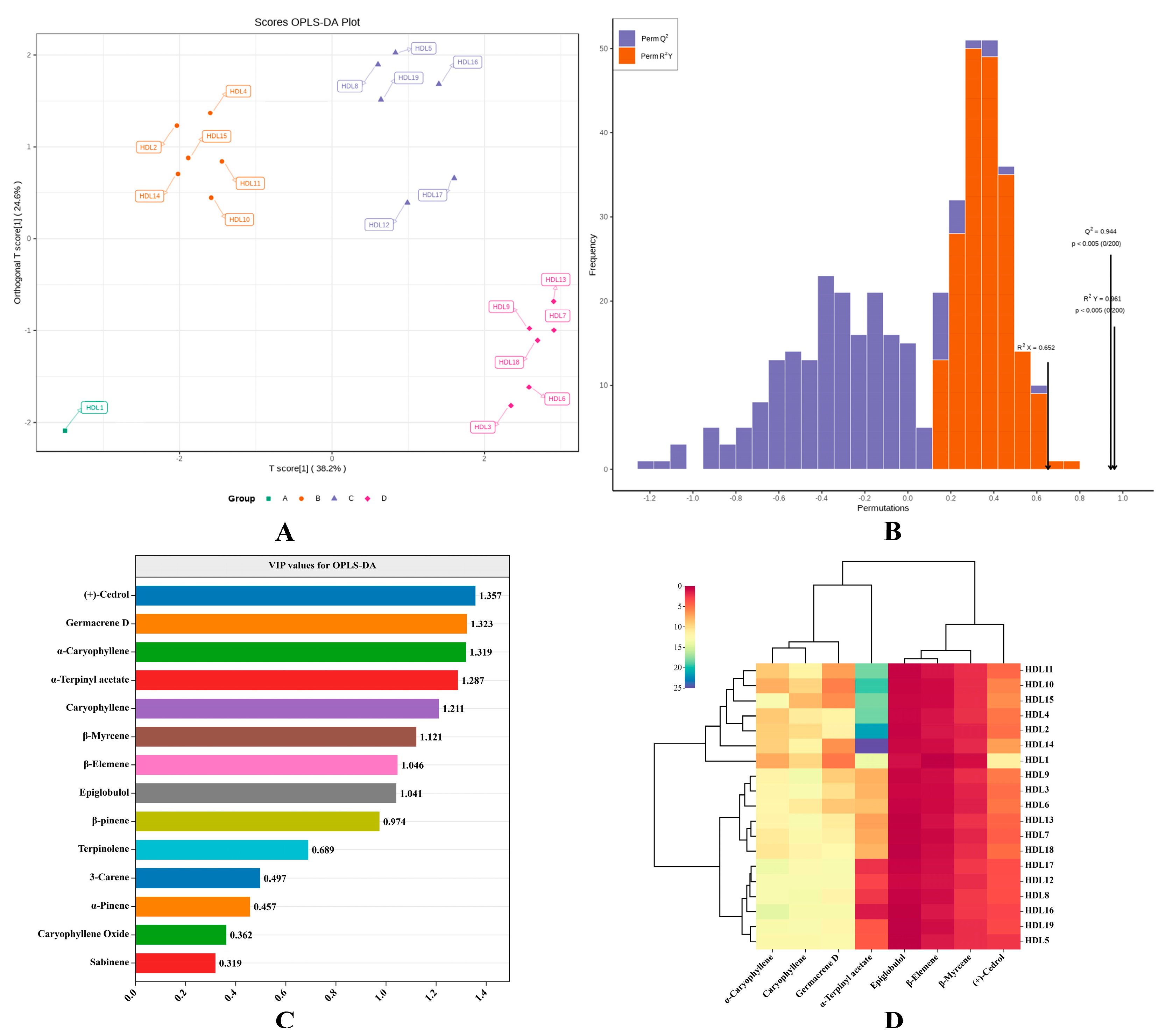
2.4. OPLS–DA Analysis
2.5. Analysis of Differential Volatile Components in 19 Ancient Platycladus orientalis Leaves with Different Tree Ages
3. Discussion
4. Materials and Methods
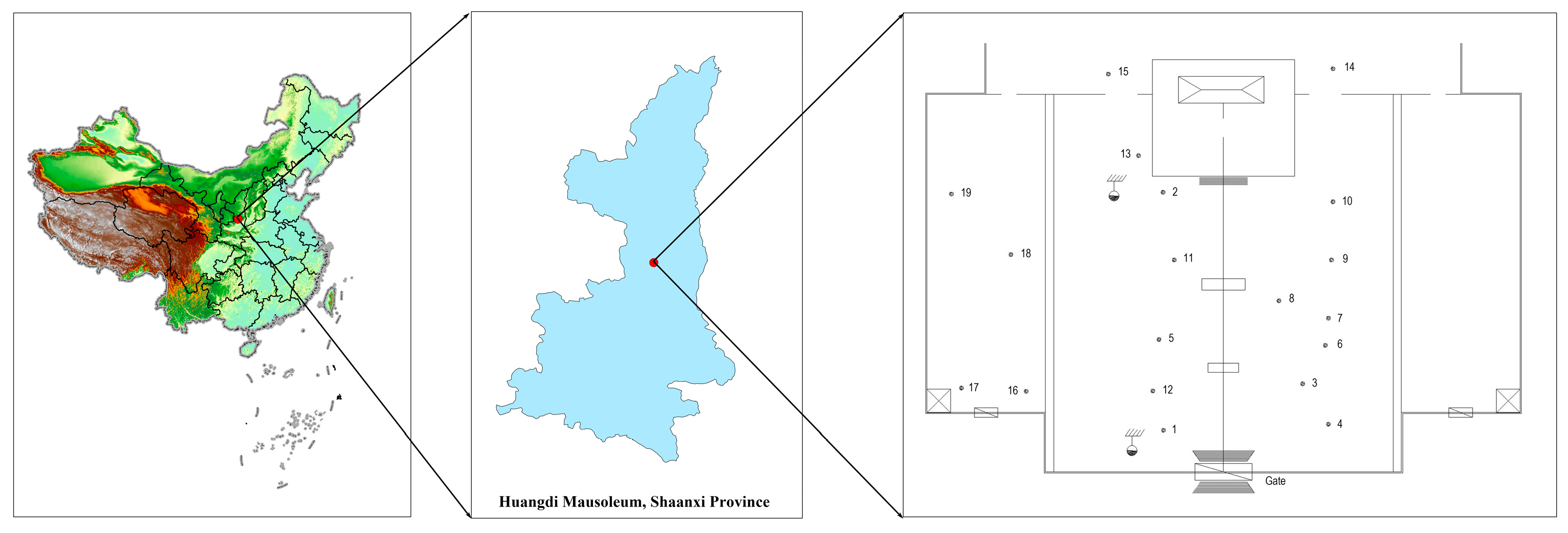
4.1. Plant Materials
4.2. Essential Oil Extraction
4.3. HS–SPME–GC–O–MS Analysis
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cui, B.; Deng, P.; Zhang, S.; Zhao, Z. Genetic diversity and population genetic structure of ancient Platycladus orientalis L. (Cupressaceae) in the middle reaches of the Yellow River by chloroplast microsatellite markers. Forests 2021, 12, 592. [Google Scholar] [CrossRef]
- Jia, K.H.; Zhao, W.; Maier, P.A.; Hu, X.G.; Jin, Y.Q.; Zhou, S.S.; Jiao, S.Q.; El-Kassaby, Y.A.; Wang, T.L.; Wang, X.R.; et al. Landscape genomics predicts climate change-related genetic offset for the widespread Platycladus orientalis (Cupressaceae). Evol. Appl. 2020, 13, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.Y.; Jiang, Z.H.; Zhang, X.; Zhang, T.; Zhu, H.L.; Cui, B.; Li, Y.M.; Zhao, F.; Zhao, Z. Leaf anatomy and ultrastructure in senescing ancient tree, Platycladus orientalis L. (Cupressaceae). PeerJ 2019, 7, e6766. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.G.; Mao, J.F.; El-Kassaby, Y.A.; Jia, K.H.; Jiao, S.Q.; Zhou, S.S.; Li, Y.; Wang, T. Local adaptation and response of Platycladus orientalis (L.) Franco populations to climate change. Forests 2019, 10, 622. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Jiang, Z.H.; Zhang, X.; Lai, Q.; Zhao, Z. Tree age did not affect the leaf anatomical structure or ultrastructure of Platycladus orientalis. PeerJ 2019, 7, e7938. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.L.; Chai, Y.Y.; Wang, F.; Li, Y.M.; Su, L.; Zhao, Z. Physiology and proteomics research on the leaves of ancient Platycladus orientalis (L.) during winter. J. Proteom. 2015, 126, 263–278. [Google Scholar] [CrossRef]
- Cui, B.; Deng, P.; Tian, L.H.; Wang, Q.Q.; Zhang, S.; Zhao, Z. Genetic evaluation of ancient Platycladus orientalis L. (Cupressaceae) in the middle reaches of the Yellow River using nuclear microsatellite markers. Forests 2021, 12, 1616. [Google Scholar] [CrossRef]
- Wei, L.; Pu, D.W.; Mi, S.C.; Yang, H.Y.; Wei, L.G.; Lu, Q.R.; Zu, Y.A. Essential oil extraction and evaluation from the fresh Platycladus orientalis (L.) Franco seed peel waste by an environment-friendly method. Sustain. Chem. Pharm. 2022, 29, 100771. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Wang, Z.H.; Zhang, J.; Wang, J.; Zhong, J.L.; Wang, Y. Discrimination of wood-boring beetles infested Platycladus orientalis plants by using gas chromatography-ion mobility spectrometry. Comput. Electron. Agr. 2021, 180, 105896. [Google Scholar] [CrossRef]
- Menggen, Q.Q.G.; Liu, J.J. Study on extraction process and composition analysis of essential oil in leaf of Platycladus orientalis. Food Ind. 2013, 34, 89–93. [Google Scholar]
- Zheng, C.; Zhou, Q.; Wang, Z.; Wang, J. Behavioral responses of Platycladus orientalis plant volatiles to Phloeosinus aubei by GC-MS and HS-GC-IMS for discrimination of different invasive severity. Anal. Bioanal. Chem. 2021, 413, 5789–5798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Yang, J.G.; Du, G.F.; Zhang, D.H.; Xiong, J.; Ning, Z.X.; Guo, X.D. Differences in the essential oils from different ages of Platycladus orientalis. Mod. Food Sci. Technol. 2017, 33, 96–100,107. [Google Scholar]
- Ho, K.L.; Ng, Z.X.; Wang, C.W.; Mat Junit, S.; Huah Lim, S.; Ngo, C.T.; Yong, A.C.H.; Yong, P.H. Comparative analysis of in vitro enzyme inhibitory activities and phytochemicals from Platycladus orientalis (L.) Franco via solvent partitioning method. Appl. Biochem. Biotechnol. 2022, 194, 3621–3644. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.H.; Hou, D.Y.; Liu, X.Y.; Li, X.C.; Geng, H.L. Analysis of volatile components from leaf twigs in biota orientalis with different extraction methods by gas chromatography-mass spectrometry. J. Chin. Mass Spectrom. Soc. 2006, 27, 226–231. [Google Scholar]
- Selim, Y.; El-Sharkawy, E.; Abd El-Azim, M.H.M. New cytotoxic flavonoids from aerial parts of Platycladus orientalis L. Nat. Prod. Res. 2020, 34, 1763–1771. [Google Scholar] [CrossRef]
- Jiang, J.H.; Li, X.C.; Gao, X.Q.; Gao, T.H.; Chen, F.M.; Feng, Y.J.; Huang, L.B. Volatile constituents from Platycladus orentalis and their antitumor activities. For. Res. 2006, 19, 311–315. [Google Scholar]
- Darwish, R.S.; Hammoda, H.M.; Ghareeb, D.A.; Abdelhamid, A.S.; Harraz, F.M.; Shawky, E. Chemical profiling and identification of anti-inflammatory biomarkers of oriental Thuja (Platycladus orientalis) using UPLC/MS/MS and network pharmacology-based analyses. Nat. Prod. Res. 2021, 36, 1–5. [Google Scholar] [CrossRef]
- Lin, L.; Cen, J.L.; Jin, H.J.; Du, Y.Q. GC-MS analysis of essential oil from five Cupressaceae plants. Guihaia 2015, 35, 580–585. [Google Scholar]
- Sanei-Dehkordi, A.; Gholami, S.; Abai, M.R.; Sedaghat, M.M. Essential oil composition and larvicidal evaluation of Platycladus orientalis against two mosquito vectors, anopheles stephensi and culex pipiens. J. Arthropod. Borne. Dis. 2018, 12, 101–107. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Akbarzadeh, A.; Hashemi-Moghaddam, H.; Bahmanpour, H.; Shafaghat, A.; Lotfi, S. The relationship between chemical composition of the essential oils of Platycladus orientalis (L.) Franco and soils contamination in national oil company of Shahrood, Iran. J. Essent. Oil Bear. Plant. 2017, 20, 1209–1225. [Google Scholar] [CrossRef]
- Dong, M.Y.; Wang, B.Q.; Jiang, Y.; Ding, X.Y. Environmental controls of diurnal and seasonal variations in the stem radius of Platycladus orientalis in Northern China. Forests 2019, 10, 784. [Google Scholar] [CrossRef]
- Kim, M.G.; Lee, N.H.; Kim, J.M.; Lee, S.G.; Lee, H.S. Chemical composition of essential oils extracted from five Juniperus chinensis varieties in Korea. J. Essent. Oil Bear. Plant. 2015, 18, 852–856. [Google Scholar] [CrossRef]
- Kagawa, D.; Jokura, H.; Ochiai, R.; Tokimitsu, I.; Tsubone, H. The sedative effects and mechanism of action of cedrol inhalation with behavioral pharmacological evaluation. Planta Med. 2003, 69, 637–641. [Google Scholar]
- Zheng, T.; Su, K.X.; Gao, M.S.; Zhang, D.L.; Chen, X.Y.; Liu, S.M. Chemotaxonomic variation in volatile component contents and their correlation between climate factors in Chinese prickly ash peels (Zanthoxylum bungeanum Maxim.). Food Chem. X 2021, 12, 100176. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Lim, J.P.; Jeon, H. Chemical composition and antibacterial activities of the essential oil from Abies koreana. Phytother. Res. 2007, 21, 1246–1250. [Google Scholar] [CrossRef]
- Gong, S.S.; Feng, Z.P.; Qu, A.R.; Sun, J.H.; Xu, X.K.; Lai, Y.; Kong, Y.H. Effects of land-use types on the temporal dynamics of soil active carbon and nitrogen in the rocky mountainous of North China. Soil Sci. Plant. Nutr. 2022, 68, 72–80. [Google Scholar] [CrossRef]
- Di Donato, F.; Biancolillo, A.; Mazzulli, D.; Rossi, L.; D’Archivio, A.A. HS-SPME/GC–MS volatile fraction determination and chemometrics for the discrimination of typical Italian Pecorino cheeses. Microchem. J. 2021, 165, 106133. [Google Scholar] [CrossRef]
- Qin, G.H.; Tao, S.T.; Cao, Y.F.; Wu, J.Y.; Zhang, H.P.; Huang, W.J.; Zhang, S.L. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC–MS. Food Chem. 2012, 134, 2367–2382. [Google Scholar] [CrossRef]
- Xu, S.R.; Li, H.M.; Dong, P.L.; Wang, M.M.; Chen, C.P.; Feng, S.L.; Fan, J. High-throughput profiling volatiles in edible oils by cooling assisted solid-phase microextraction technique for sensitive discrimination of edible oils adulteration. Anal. Chim. Acta. 2022, 1221, 340159. [Google Scholar] [CrossRef]
- Cui, B.; Liu, S.M.; Zheng, T. Chemotaxonomic identification of key taste and nutritional components in ‘Shushanggan apricot’ fruits by widely targeted metabolomics. Molecules 2022, 27, 3870. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, D.L.; Sun, B.Y.; Liu, S.M. Evaluating the impacts of climate factors and flavonoids content on Chinese prickly ash peel color based on HPLC-MS and structural equation model. Foods 2022, 11, 2539. [Google Scholar] [CrossRef] [PubMed]
- Ignea, C.; Raadam, M.H.; Koutsaviti, A.; Zhao, Y.; Duan, Y.T.; Harizani, M.; Miettinen, K.; Georgantea, P.; Rosenfeldt, M.; Viejo-Ledesma, S.E.; et al. Expanding the terpene biosynthetic code with non-canonical 16 carbon atom building blocks. Nat. Commun. 2022, 13, 5188. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.W.; Tang, R.H.; Zhang, A.L.; Du, Z.Z.; Yang, L.P.; Xu, Y.Q.; Zhong, Y.L.; Yang, R.; Chen, W.Y.; Pu, C.X. Mining, expression, and phylogenetic analysis of volatile terpenoid biosynthesis-related genes in different tissues of ten Elsholtzia species based on transcriptomic analysis. Phytochemistry 2022, 203, 113419. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.Y.; Jin, B.L.; Chen, L.L.; Tian, M.; Ma, R.; Yin, B.W.; Zhang, H.Y.; Guo, J.; Tang, J.F.; Chen, T.; et al. Functional identification of the terpene synthase family involved in diterpenoid alkaloids biosynthesis in Aconitum carmichaelii. Acta Pharm. Sin. B 2021, 11, 3310–3321. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.Y.; Chen, X.Y.; Guo, S.X.; Wang, B.C.; Li, B. Recent advances and new insights in biosynthesis of dendrobine and sesquiterpenes. Appl. Microbiol. Biotechnol. 2021, 105, 6597–6606. [Google Scholar] [CrossRef]
- Chen, X.N.; Kollner, T.G.; Xiong, W.G.; Wei, G.; Chen, F. Emission and biosynthesis of volatile terpenoids from the plasmodial slime mold Physarum polycephalum. Beilstein. J. Org. Chem. 2019, 15, 2872–2880. [Google Scholar] [CrossRef]
- Courdavault, V.; Papon, N. A new path for terpenoid biosynthesis. Trends Biochem. Sci. 2022, 47, 906–908. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Huang, X.W.; Zhao, L.H.; Pang, S.; Liu, Y.J.; Liu, J.R.; Zhang, M.Q. Effects of varieties, cultivation methods, and origins of citrus sinensis ‘hongjiang’ on volatile organic compounds: HS-SPME-GC/MS Analysis coupled with OPLS-DA. Agriculture 2022, 12, 1725. [Google Scholar] [CrossRef]
- Tu, X.H.; Liu, Y.J.; Yan, L.Y.; Li, W.X.; Luo, P.; Du, L.Q.; Lu, J.N. Effects of four drying methods on Amomum villosum Lour. ‘Guiyan1’ volatile organic compounds analyzed via headspace solid phase microextraction and gas chromatography-mass spectrometry coupled with OPLS-DA. RSC Adv. 2022, 12, 26485–26496. [Google Scholar] [CrossRef]
- Liu, B.Q.; Chen, X.Q.; Wu, X.G.; Zhang, W.; Wang, Z.H. Study of Pu’er raw materials grade classification by PCA and PLS-DA. J. Tea Sci. 2015, 35, 179–184. [Google Scholar]
- Lota, M.; de Rocca Serra, D.; Tomi, F.; Casanova, J. Chemical variability of peel and leaf essential oils of 15 species of mandarins. Biochem. Syst. Ecol. 2001, 29, 77–104. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Li, H.; Wang, Y.Z.; Li, W.Y. Characteristic fingerprinting based on macamides for discrimination of maca (Lepidium meyenii) by LC/MS/MS and multivariate statistical analysis. J. Sci. Food Agric. 2016, 96, 4475–4483. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, Y.; Zhan, Y.; Yang, Y.; Wu, Z.; Tan, H.J. Difference analysis of volatile components of essential oil of Liangping pomelo peel with different tree ages. Sci. Technol. Food Ind. 2020, 41, 17–24. [Google Scholar]

| Samples | α-Pi | Sa | Ca | Ter | Cary | α-Cary | Germ | Ele | Myr | β-Pi | Cary Oxide | Cedr | Epi | α-Ter |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HDL1 | 6.40 | 3.02 | 8.90 | 2.17 | 9.61 | 7.34 | 5.27 | 0.17 | 0.90 | 0.23 | 2.28 | 11.30 | 0.98 | 13.88 |
| HDL2 | 9.02 | 6.69 | 8.70 | 3.25 | 9.90 | 9.15 | 11.40 | 1.14 | 1.57 | 0.32 | 0.39 | 4.92 | 0.44 | 21.42 |
| HDL4 | 6.88 | 5.97 | 10.17 | 4.12 | 10.93 | 8.81 | 11.53 | 1.11 | 2.13 | 0.34 | 0.27 | 5.17 | 0.56 | 18.29 |
| HDL10 | 16.76 | 7.29 | 8.92 | 3.21 | 9.55 | 7.54 | 5.57 | 0.75 | 2 | 0.57 | 0.63 | 5.77 | 0.54 | 19.42 |
| HDL11 | 12.48 | 6.36 | 10.41 | 3.35 | 11.52 | 8.81 | 6.94 | 1.04 | 2.05 | 0.24 | 0.51 | 4.57 | 0.39 | 18.18 |
| HDL14 | 14.98 | 5.04 | 1.14 | 3.74 | 11.47 | 9.23 | 6.36 | 0.86 | 1.82 | 0.43 | 0.69 | 6.89 | 0.6 | 25.68 |
| HDL15 | 9.43 | 6.61 | 11.51 | 4.25 | 8.04 | 12.86 | 6.17 | 0.80 | 2.05 | 0.37 | 0.35 | 6.17 | 0.64 | 18.12 |
| HDL3 | 12.20 | 1.16 | 12.56 | 4.09 | 12.99 | 11.91 | 10.18 | 0.78 | 1.62 | 0.29 | 1.50 | 4.88 | 0.62 | 7.82 |
| HDL6 | 14.92 | 1.24 | 13.86 | 3.83 | 10.88 | 12.04 | 8.75 | 0.75 | 1.44 | 0.58 | 0.63 | 5.12 | 0.46 | 8.42 |
| HDL7 | 11.56 | 1.29 | 15.12 | 4.95 | 12.34 | 10.89 | 11.46 | 0.64 | 1.69 | 0.49 | 0.27 | 4.09 | 0.37 | 7.27 |
| HDL9 | 12.36 | 1.54 | 13.67 | 4.28 | 13.53 | 11.64 | 9.03 | 0.87 | 2.07 | 0.49 | 0.51 | 5.36 | 0.48 | 7.69 |
| HDL13 | 12.89 | 1.31 | 13.41 | 4.17 | 12.91 | 11.69 | 11.02 | 0.95 | 2.16 | 0.44 | 0.47 | 4.42 | 0.29 | 7.01 |
| HDL18 | 11.4 | 1.11 | 14.03 | 3.73 | 11.74 | 10.59 | 12.45 | 0.87 | 2.02 | 0.49 | 1.42 | 4.83 | 0.23 | 7.78 |
| HDL5 | 10.32 | 4.91 | 10.99 | 3.35 | 12.34 | 12.29 | 12.62 | 1.23 | 2.07 | 0.55 | 0.33 | 2.34 | 0.19 | 3.93 |
| HDL8 | 9.49 | 5.68 | 10.80 | 2.31 | 13.18 | 13.00 | 11.84 | 0.88 | 2.52 | 0.46 | 0.61 | 3.38 | 0.47 | 2.47 |
| HDL12 | 9.53 | 3.19 | 10.20 | 3.87 | 12.93 | 13 | 12.82 | 1.08 | 1.99 | 0.37 | 1.32 | 3.36 | 0.79 | 2.93 |
| HDL16 | 9.62 | 3.98 | 10.68 | 3.26 | 13.1 | 14.41 | 13.13 | 1.22 | 2.42 | 0.5 | 0.98 | 2.94 | 0.21 | 1.29 |
| HDL17 | 10.23 | 3.02 | 9.33 | 3.84 | 12.55 | 13.79 | 12.97 | 0.96 | 2.44 | 0.55 | 1.63 | 3.34 | 0.5 | 2.24 |
| HDL19 | 9.17 | 4.89 | 10.55 | 4.07 | 12.92 | 13.12 | 12.11 | 0.86 | 2.05 | 0.37 | 0.38 | 3.14 | 0.25 | 3.84 |
| Mean | 11.03 | 3.91 | 10.79 | 3.68 | 11.71 | 11.16 | 10.09 | 0.89 | 1.95 | 0.43 | 0.80 | 4.84 | 0.47 | 10.40 |
| CV/% | 23.63% | 54.70% | 27.33% | 17.76% | 12.72% | 18.50% | 26.57% | 26.28% | 19.10% | 24.95% | 69.02% | 39.41% | 41.79% | 70.77% |
| Samples No. | Diameter at Breast Height (cm) | Height (m) | Crown Width (m) | Tree Age (a) | |
|---|---|---|---|---|---|
| East-West Crown | South-North Crown | ||||
| HDL1 | 760 | 19.3 | 14.4 | 15.3 | 5000 |
| HDL2 | 518 | 14.8 | 12.6 | 12.7 | 3000 |
| HDL3 | 334 | 14.0 | 10.1 | 13.5 | 2500 |
| HDL4 | 510 | 19.0 | 9.8 | 11.2 | 3000 |
| HDL5 | 346 | 18.5 | 9.3 | 10.9 | 2000 |
| HDL6 | 470 | 20.5 | 12.1 | 15.8 | 2500 |
| HDL7 | 502 | 17.0 | 13.9 | 11.8 | 2500 |
| HDL8 | 368 | 18.0 | 10.9 | 9.9 | 2000 |
| HDL9 | 500 | 17.0 | 18.5 | 11.8 | 2500 |
| HDL10 | 516 | 18.0 | 12.9 | 18.5 | 3000 |
| HDL11 | 470 | 21.5 | 20.2 | 19.0 | 3000 |
| HDL12 | 440 | 9.5 | 8.8 | 13.8 | 2500 |
| HDL13 | 579 | 15.0 | 16.8 | 10.9 | 2000 |
| HDL14 | 430 | 11.4 | 5.4 | 12.5 | 3000 |
| HDL15 | 500 | 12.8 | 10.1 | 12.3 | 3000 |
| HDL16 | 525 | 12.0 | 14.6 | 9.2 | 2000 |
| HDL17 | 388 | 16.0 | 13.1 | 13.2 | 2000 |
| HDL18 | 430 | 15.0 | 11.7 | 9.8 | 2500 |
| HDL19 | 540 | 20.0 | 15.0 | 10.0 | 2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, B.; Zheng, T.; Deng, P.; Zhang, S.; Zhao, Z. Chemotaxonomic Variation in Volatile Component Contents in Ancient Platycladus orientalis Leaves with Different Tree Ages in Huangdi Mausoleum. Molecules 2023, 28, 2043. https://doi.org/10.3390/molecules28052043
Cui B, Zheng T, Deng P, Zhang S, Zhao Z. Chemotaxonomic Variation in Volatile Component Contents in Ancient Platycladus orientalis Leaves with Different Tree Ages in Huangdi Mausoleum. Molecules. 2023; 28(5):2043. https://doi.org/10.3390/molecules28052043
Chicago/Turabian StyleCui, Bei, Tao Zheng, Ping Deng, Sheng Zhang, and Zhong Zhao. 2023. "Chemotaxonomic Variation in Volatile Component Contents in Ancient Platycladus orientalis Leaves with Different Tree Ages in Huangdi Mausoleum" Molecules 28, no. 5: 2043. https://doi.org/10.3390/molecules28052043
APA StyleCui, B., Zheng, T., Deng, P., Zhang, S., & Zhao, Z. (2023). Chemotaxonomic Variation in Volatile Component Contents in Ancient Platycladus orientalis Leaves with Different Tree Ages in Huangdi Mausoleum. Molecules, 28(5), 2043. https://doi.org/10.3390/molecules28052043






