Oxidant-Free Electrochemical Direct Oxidative Benzyl Alcohols to Benzyl Aldehydes Using Three-Dimensional Printing PPAR Polyoxometalate
Abstract
:1. Introduction
2. Results and Discussion
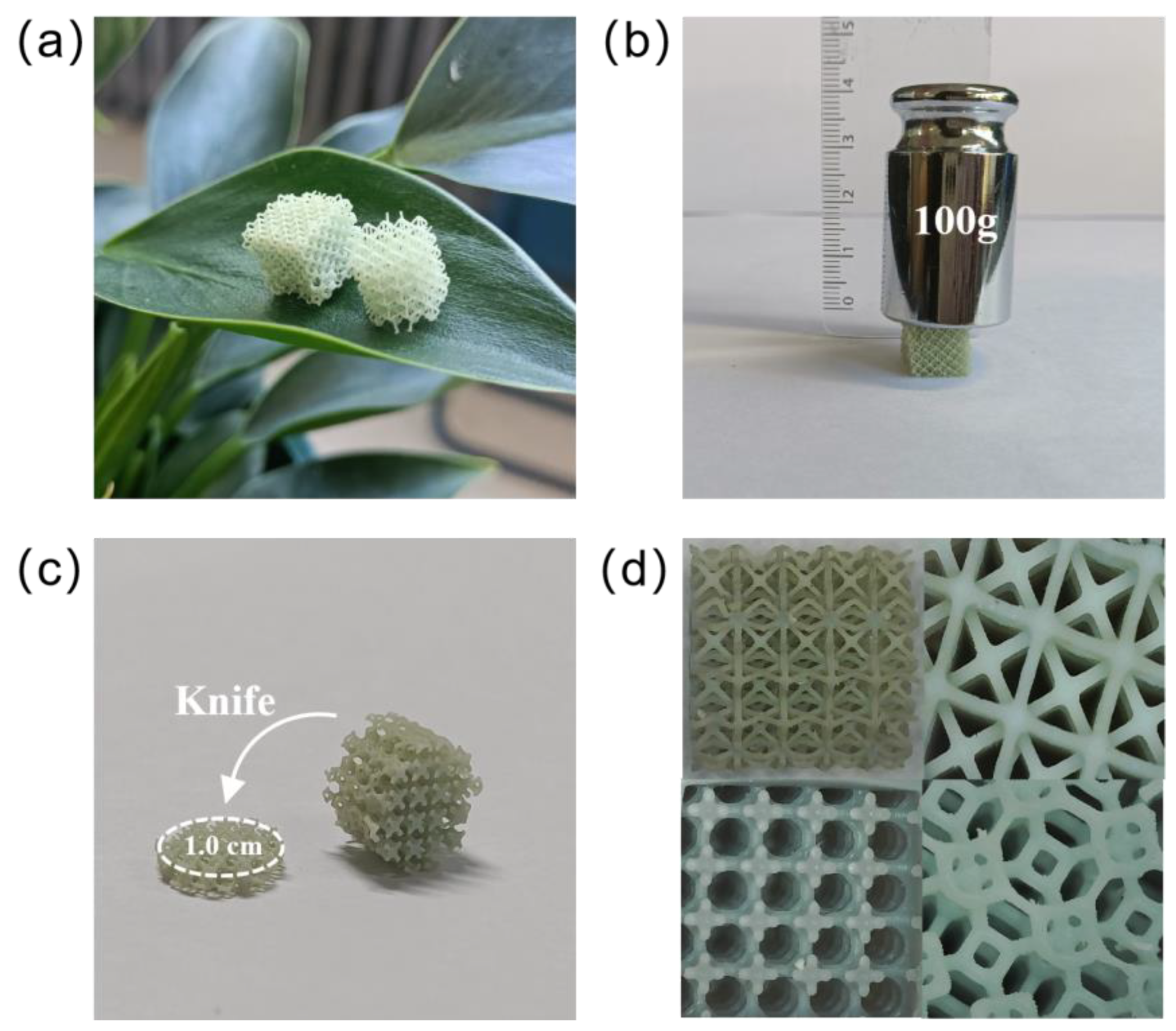
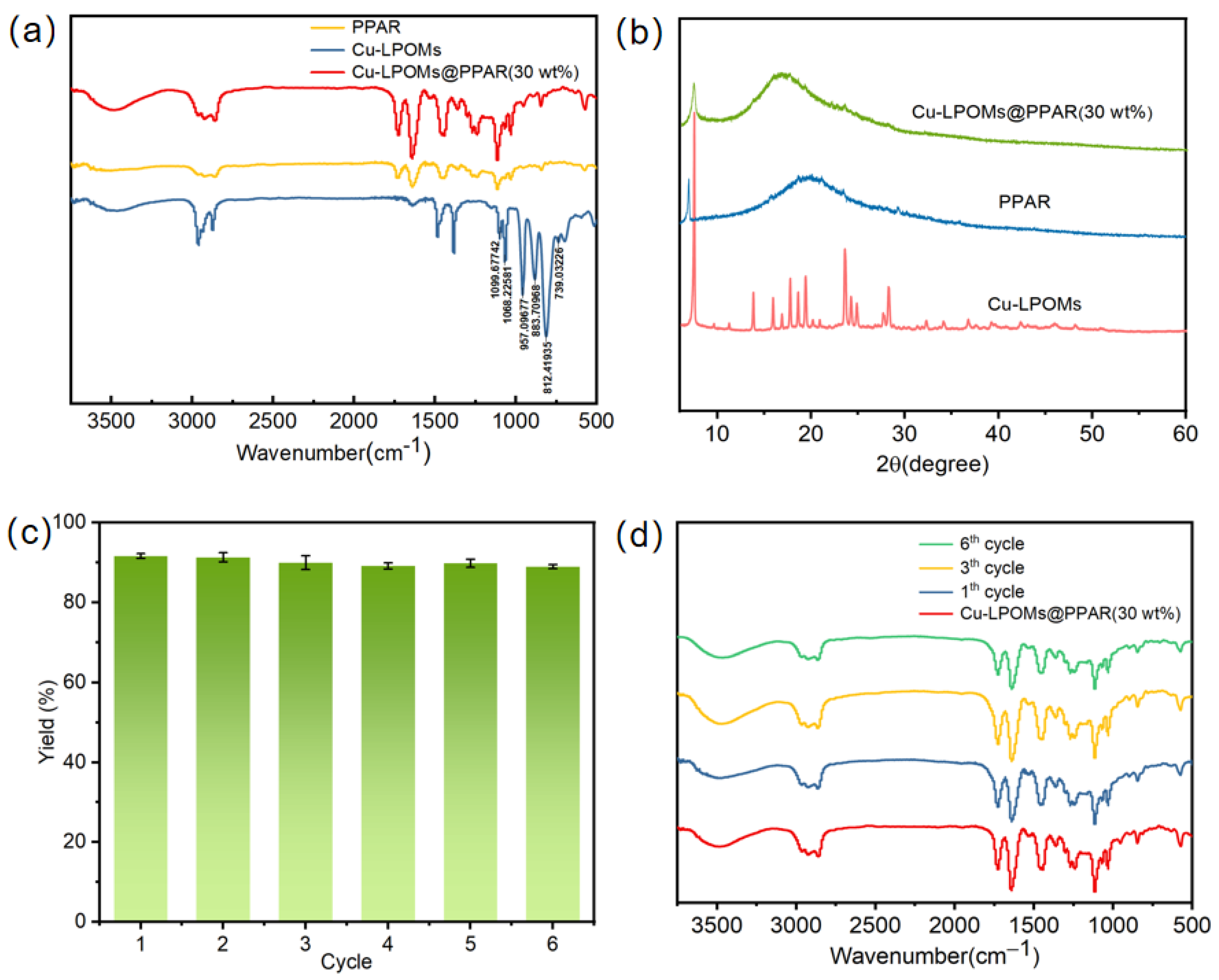
2.1. Performance Test of Cu-LPOMs@PPAR
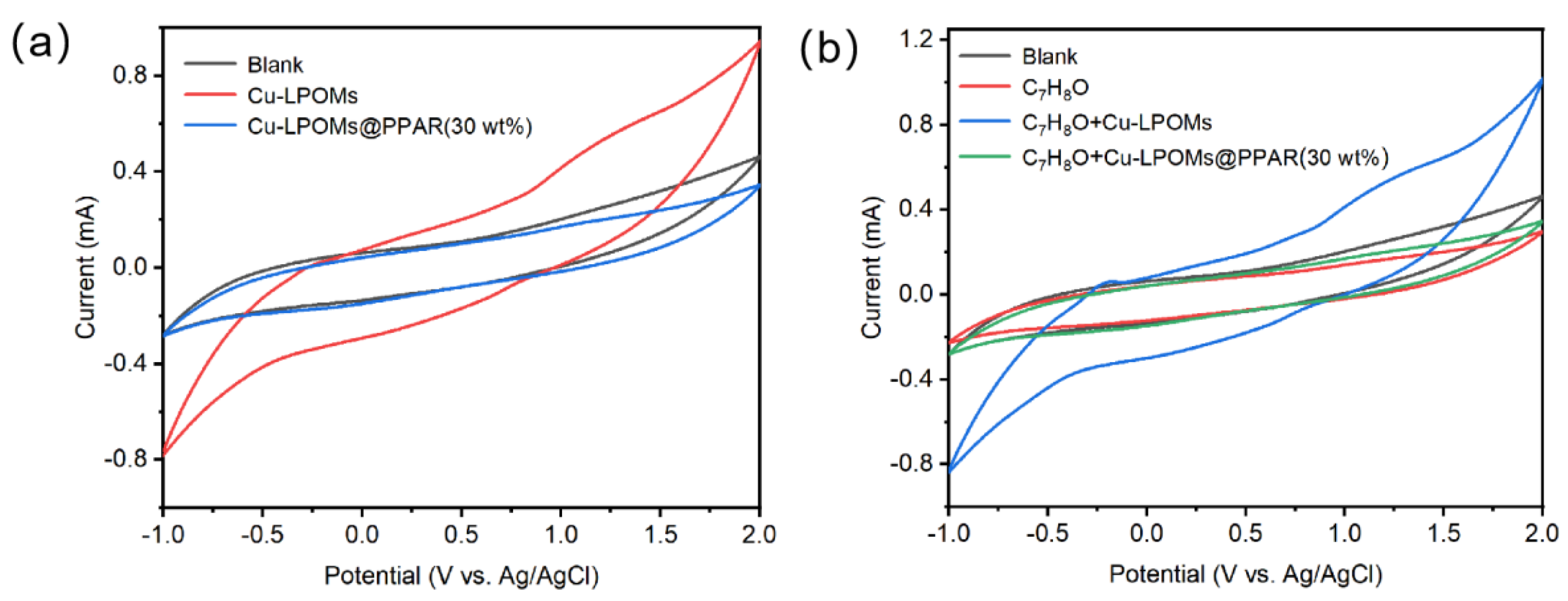
2.2. Electrocatalytic Property of Cu-LPOMs@PPAR
2.3. Selection of Experimental Conditions
2.4. Oxidant-Free Electrochemical Oxidative Benzyl Alcohols
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Synthesis of (TBA)4H[PW11CuO39](Cu-LPOMs)
3.4. Synthesis of Cu-LPOMs@PPAR
3.5. Procedure for Electrocatalytic Oxidation of Benzyl Alcohols by Cu-LPOMs@PPAR
3.6. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dell’Anna, M.M.; Mali, M.; Mastrorilli, P. Oxidation of benzyl alcohols to aldehydes and ketones under air in water using a polymer supported palladium catalyst. J. Mol. Catal. A Chem. 2014, 386, 114–119. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, J.; Lei, A. Recent advances in electrochemical oxidative cross-coupling with hydrogen evolution involving radicals. Chem. Soc. Rev. 2021, 50, 10058–10086. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, X.; Huang, P.; Lei, A. Recent Advances in Electrochemical Oxidative Cross-Coupling of Alkenes with H2 Evolution. ChemCatChem 2020, 12, 27–40. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, D.; Yang, Z.; Zhang, H.; Wang, S.; Lei, A. Electrochemical oxidative C (sp3)–H azolation of lactams under mild conditions. Green Chem. 2020, 22, 3742–3747. [Google Scholar] [CrossRef]
- Zeng, L.; Li, H.; Hu, J.; Zhang, D.; Hu, J.; Peng, P.; Lei, A. Electrochemical oxidative aminocarbonylation of terminal alkynes. Nat. Catal. 2020, 3, 438–445. [Google Scholar] [CrossRef]
- Babu, S.G.; Priyadarsini, P.A.; Karvembu, R. Copper on boehmite: A simple, selective, efficient and reusable heterogeneous catalyst for oxidation of alcohols with periodic acid in water at room temperature. Appl. Catal. A Gen. 2011, 392, 218–224. [Google Scholar] [CrossRef]
- Ahmad, J.U.; Räisänen, M.T.; Kemell, M. Facile open air oxidation of benzylic alcohols in distilled water by in situ made copper(II) complexes. Appl. Catal. A Gen. 2012, 449, 153–162. [Google Scholar] [CrossRef]
- Karimi, B.; Behzadnia, H.; Bostina, M. A Nano-Fibrillated Mesoporous Carbon as an Effective Support for Palladium Nanoparticles in the Aerobic Oxidation of Alcohols “on Pure Water”. Chem. Eur. J 2012, 18, 8634–8640. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Y.; Luo, J.; Wang, F.; Cao, X.; Huang, S. Electrochemical Oxidative Oxydihalogenation of Alkynes for the Synthesis of α, α-Dihaloketones. Org. Lett. 2020, 22, 1169–1174. [Google Scholar] [CrossRef]
- Heravi, M.M.; Ghalavand, N.; Hashemi, E. Hydrogen peroxide as a green oxidant for the selective catalytic oxidation of benzylic and heterocyclic alcohols in different media: An overview. Chemistry 2020, 2, 101–178. [Google Scholar] [CrossRef]
- Varsha, M.V.; Nageswaran, G. Direct electrochemical synthesis of metal organic frameworks. J. Electrochem. Soc. 2020, 167, 155527. [Google Scholar]
- Blanco, D.E.; Dookhith, A.Z.; Modestino, M.A. Enhancing selectivity and efficiency in the electrochemical synthesis of adiponitrile. React. Chem. Eng. 2019, 4, 8–16. [Google Scholar] [CrossRef]
- Kyriakou, V.; Garagounis, I.; Vasileiou, E. Progress in the electrochemical synthesis of ammonia. Catal. Today 2017, 286, 2–13. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, X.; Jing, Y. Electrochemical synthesis of urea on MBenes. Nat. Commun. 2021, 12, 4080. [Google Scholar] [CrossRef]
- Soloveichik, G. Electrochemical synthesis of ammonia as a potential alternative to the Haber–Bosch process. Nat. Catal. 2019, 2, 377–380. [Google Scholar] [CrossRef]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, K.; Zeng, C. Use of Electrochemistry in the Synthesis of Heterocyclic Structures. Chem. Rev. 2018, 118, 4485–4540. [Google Scholar] [CrossRef]
- Tang, S.; Liu, Y.; Lei, A. Electrochemical Oxidative Cross-coupling with Hydrogen Evolution: A Green and Sustainable Way for Bond Formation. Curr. Pollut. Rep. 2018, 4, 27–45. [Google Scholar] [CrossRef]
- Wiebe, A.; Gieshoff, T.; Mhle, S. Electrifying Organic Synthesis. Angew. Chem. Int. Ed. 2018, 57, 5594–5619. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, S.; Li, Z. Metal-and oxidant-free electrochemically promoted oxidative coupling of amines. RSC Adv. 2022, 12, 118–122. [Google Scholar] [CrossRef]
- Yang, B.; Ma, S.; Cui, R. Simultaneous removal of NOx and SO2 with H2O2 catalyzed by alkali/magnetism-modified fly ash: High efficiency, low cost and catalytic mechanism. Chem. Eng. J. 2019, 359, 233–243. [Google Scholar] [CrossRef]
- Yang, B.; Ma, S.; Cui, R. Novel low-cost simultaneous removal of NO and SO2 with ·OH from decomposition of H2O2 catalyzed by alkali-magnetic modified fly ash. Ind. Eng. Chem. Res. 2019, 58, 5339–5347. [Google Scholar] [CrossRef]
- Ren, C.; Li, H.; Li, R. Electrocatalytic study of a 1, 10-phenanthroline–cobalt (II) metal complex catalyst supported on reduced graphene oxide towards oxygen reduction reaction. RSC Adv. 2016, 6, 33302–33307. [Google Scholar] [CrossRef]
- Chu, X.; Wu, Y.; Lu, H. Copper-Catalyzed Direct Carbamoylation of Quinoxalin-2 (1H)-ones with Hydrazinecarboxamides Under Mild Conditions. Eur. J. Org. Chem. 2020, 2020, 1141–1144. [Google Scholar] [CrossRef]
- Liu, C.K.; Chen, M.Y.; Lin, X.X. Catalyst-and oxidant-free electrochemical para-selective hydroxylation of N-arylamides in batch and continuous-flow. Green Chem. 2020, 22, 6437–6443. [Google Scholar] [CrossRef]
- Liu, G.; Qi, Y.; Li, J. Flowing scalable production of sulfenamides by active site-tuned lacunary polyoxometalate foams. J. Mater. Chem. A 2023, 11, 12258–12265. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Y.; Chen, Y. Indirect Electrocatalysis S-N/S-S Bond Construction by Robust Polyoxometalate Based Foams. Adv. Mater. 2023, 2304716. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Synthesis, structures and applications of electron-rich polyoxometalates. Nat. Rev. Chem. 2018, 2, 112. [Google Scholar] [CrossRef]
- López, X.; Carbó, J.J.; Bo, C. Structure, properties and reactivity of polyoxometalates: A theoretical perspective. Chem. Soc. Rev. 2012, 41, 7537–7571. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Polyoxometalates in solution: Speciation under spotlight. Chem. Soc. Rev. 2020, 49, 7568–7601. [Google Scholar] [CrossRef]
- Stuckart, M.; Monakhov, K.Y. Polyoxometalate encapsulation into metal–organic frameworks: The way towards functional nanomaterials for water splitting. J. Mater. Chem. A 2018, 6, 17849–17853. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Liu, G.; Sun, Z.; Shi, X. 2D-Layer-Structure Bi To Quasi-1D-Structure NiBi3: Structural Dimensionality Reduction to Superior Sodium and Potassium Ion Storage. Adv. Mater. 2023, 2305551. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Caspers, M. An overview of additive manufacturing (3D printing) for microfabrication. Microsyst. Technol. 2017, 23, 1117–1124. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An overview on 3D printing technology: Technological, materials, and applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, P.; Chao, Y. Recent advances in 3D printing for catalytic applications. Chem. Eng. J. 2022, 433, 134341. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, P.; Li, X. 3D printing of metal-organic frameworks decorated hierarchical porous ceramics for high-efficiency catalytic degradation. Chem. Eng. J. 2020, 397, 125392. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. MOF/COF-based materials using 3D printing technology: Applications in water treatment, gas removal, biomedical, and electronic industries. New J. Chem. 2021, 45, 13247–13257. [Google Scholar] [CrossRef]
- Bu, X.; Mao, Z.; Bu, Y. Remarkable gas bubble transport driven by capillary pressure in 3D printing-enabled anisotropic structures for efficient hydrogen evolution electrocatalysts. Appl. Catal. B Environ. 2023, 320, 121995. [Google Scholar] [CrossRef]
- Wang, H.; Wang, P.; Wang, Q. Preparation of a high-precision gama-Al2O3 structured catalyst by DLP 3D direct printing for hydrogen production from methanol. Ind. Eng. Chem. Res. 2021, 60, 13107–13114. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. 3D-printing technologies for electrochemical applications. Chem. Soc. Rev. 2016, 45, 2740–2755. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, Y.; Yang, L. Capillary-based fluorescence microsensor with polyoxometalates as nanozyme for rapid and ultrasensitive detection of artemisinin. Microchim. Acta 2022, 189, 40. [Google Scholar] [CrossRef] [PubMed]
- San Felices, L.; Vitoria, P.; Gutiérrez-Zorrilla, J.M. Hybrid Inorganic−Metalorganic Compounds Containing Copper (II)-Monosubstituted Keggin Polyanions and Polymeric Copper (I) Complexes. Inorg. Chem. 2006, 45, 7748–7757. [Google Scholar] [CrossRef]


 | |||
| 1a | 1b | ||
| Entry | Variation(s) from the Standard Conditions | Yield b (%) | |
| 1 | None | 95 | |
| 2 | No catalyst | 50 | |
| 3 | Without electricity | n.d. | |
| 4 | 3 V, 8 h | 75 | |
| 5 | 5 V, 8 h | 90 | |
| 6 | 7 V, 8 h | 83 | |
| 7 | H2O as solvent | n.d. | |
| 8 | DMF as solvent | n.d. | |
| 9 | n-Bu4NI instead of n-Bu4NBF4 | 58 | |
| 10 | Platinum plate as the cathode | 65 | |
| Entry | Benzyl Alcohol (a) | Aldehyde (b) | Yield b (%) |
|---|---|---|---|
| 0 | 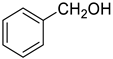 | 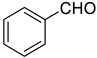 | 75 |
| 1 | 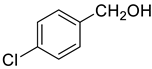 |  | 85 |
| 2 | 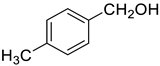 | 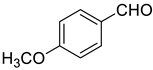 | 91 |
| 3 | 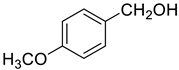 | 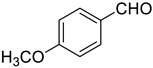 | 90 |
| 4 | 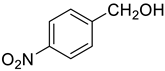 | 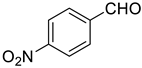 | 80 |
| 5 | 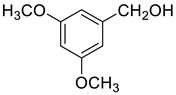 | 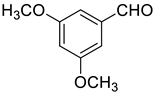 | 75 |
| 6 | 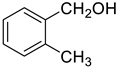 | 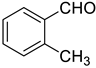 | 92 |
| 7 | 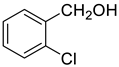 | 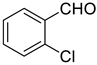 | 80 |
| 8 |  | 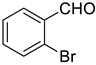 | 86 |
| 9 | 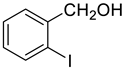 | 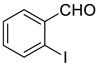 | 90 |
| 10 |  | 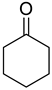 | 87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Liu, R.; Lv, X.; Jiang, L.; Tang, S.; Liu, G.; Shen, G.; Huang, X.; Ma, C.; Yang, B. Oxidant-Free Electrochemical Direct Oxidative Benzyl Alcohols to Benzyl Aldehydes Using Three-Dimensional Printing PPAR Polyoxometalate. Molecules 2023, 28, 6460. https://doi.org/10.3390/molecules28186460
Zhang W, Liu R, Lv X, Jiang L, Tang S, Liu G, Shen G, Huang X, Ma C, Yang B. Oxidant-Free Electrochemical Direct Oxidative Benzyl Alcohols to Benzyl Aldehydes Using Three-Dimensional Printing PPAR Polyoxometalate. Molecules. 2023; 28(18):6460. https://doi.org/10.3390/molecules28186460
Chicago/Turabian StyleZhang, Wenhui, Ran Liu, Xueyan Lv, Lirong Jiang, Silu Tang, Gang Liu, Guodong Shen, Xianqiang Huang, Chen Ma, and Bingchuan Yang. 2023. "Oxidant-Free Electrochemical Direct Oxidative Benzyl Alcohols to Benzyl Aldehydes Using Three-Dimensional Printing PPAR Polyoxometalate" Molecules 28, no. 18: 6460. https://doi.org/10.3390/molecules28186460
APA StyleZhang, W., Liu, R., Lv, X., Jiang, L., Tang, S., Liu, G., Shen, G., Huang, X., Ma, C., & Yang, B. (2023). Oxidant-Free Electrochemical Direct Oxidative Benzyl Alcohols to Benzyl Aldehydes Using Three-Dimensional Printing PPAR Polyoxometalate. Molecules, 28(18), 6460. https://doi.org/10.3390/molecules28186460






