Recent Progress in Vacuum Engineering of Ionic Liquids
Abstract
1. Introduction
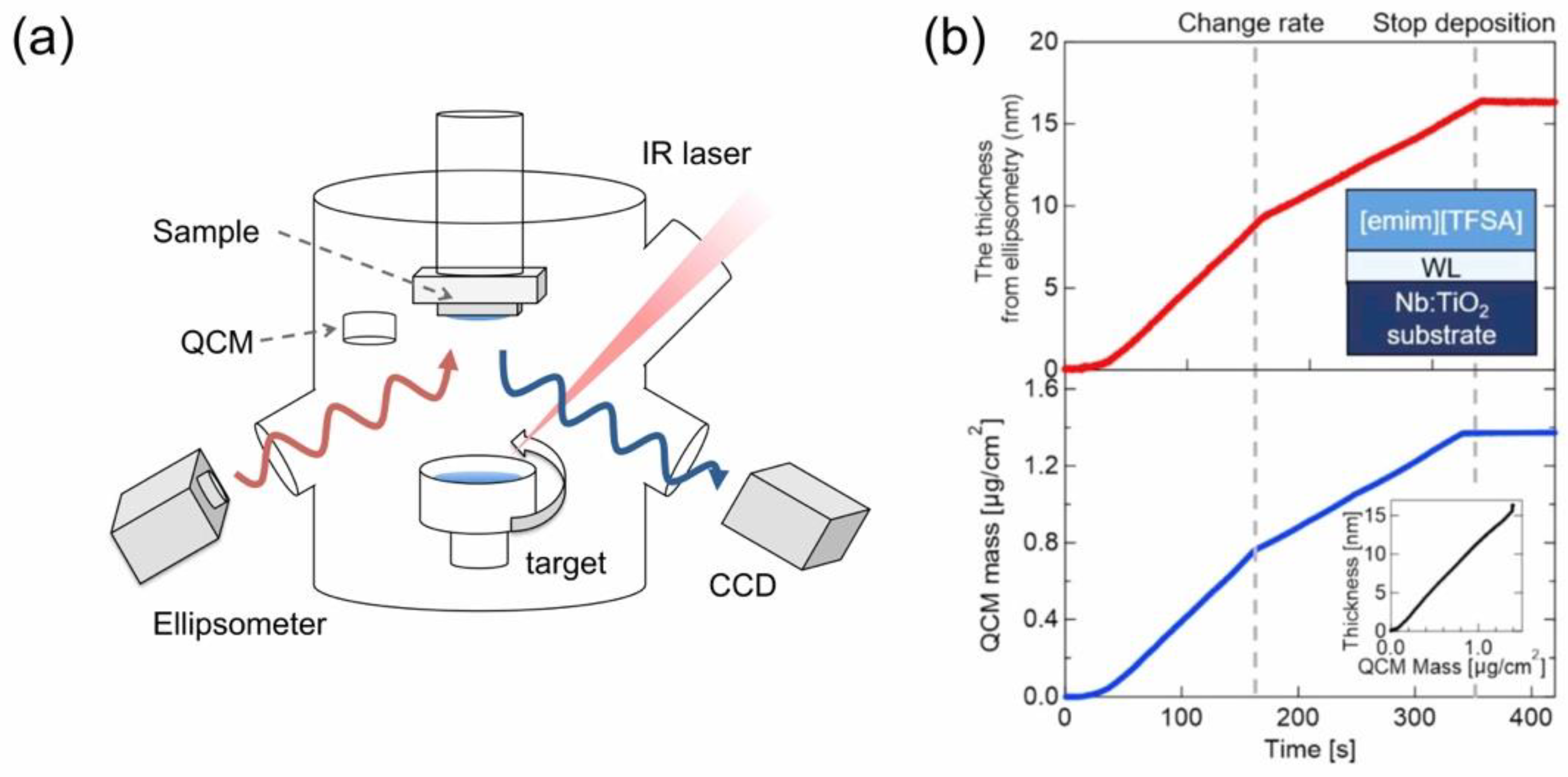
2. New Vacuum Deposition Process of ILs as an Art of Engineering
3. Unique Properties of Nano IL Films
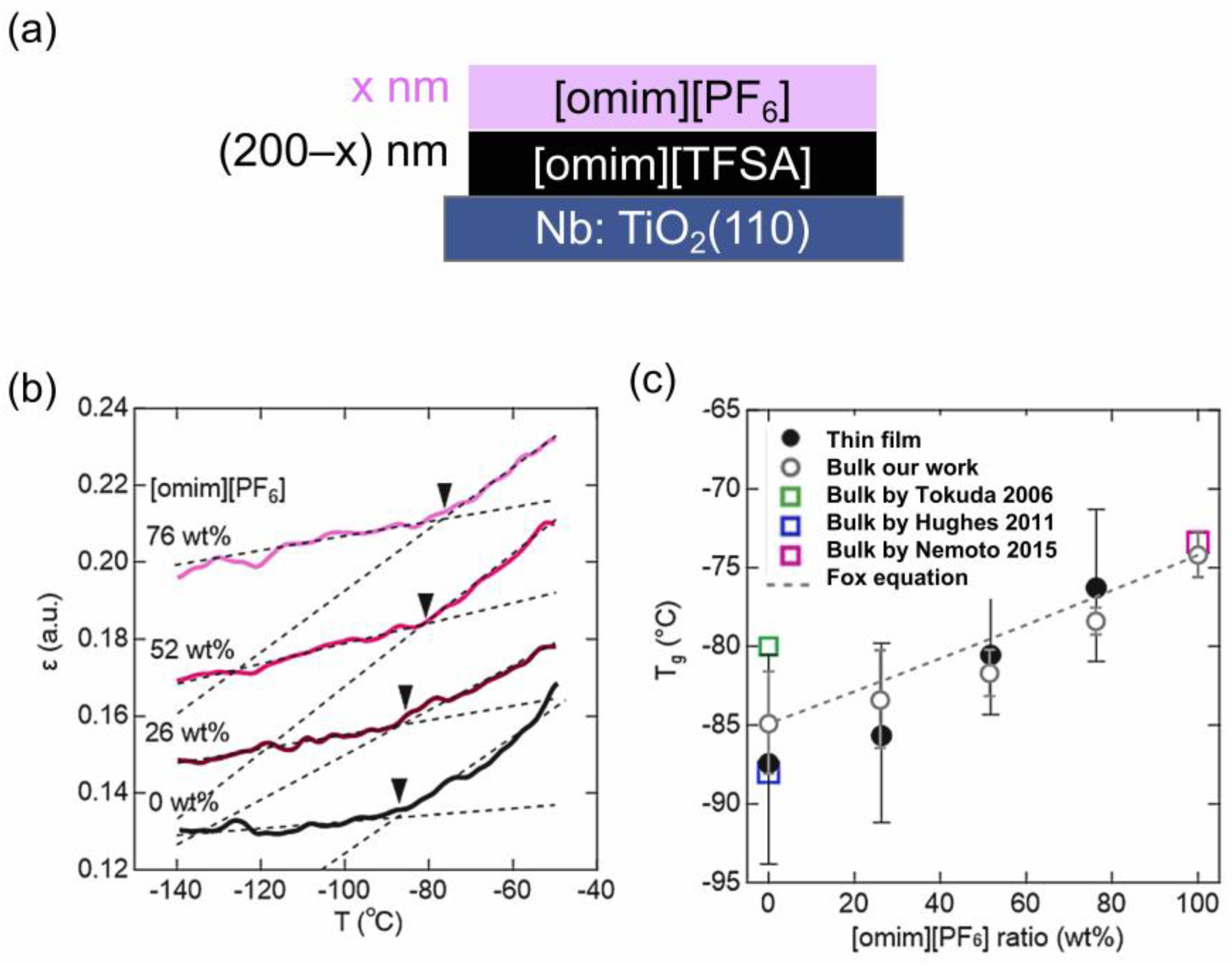
3.1. Glass Transition [23]
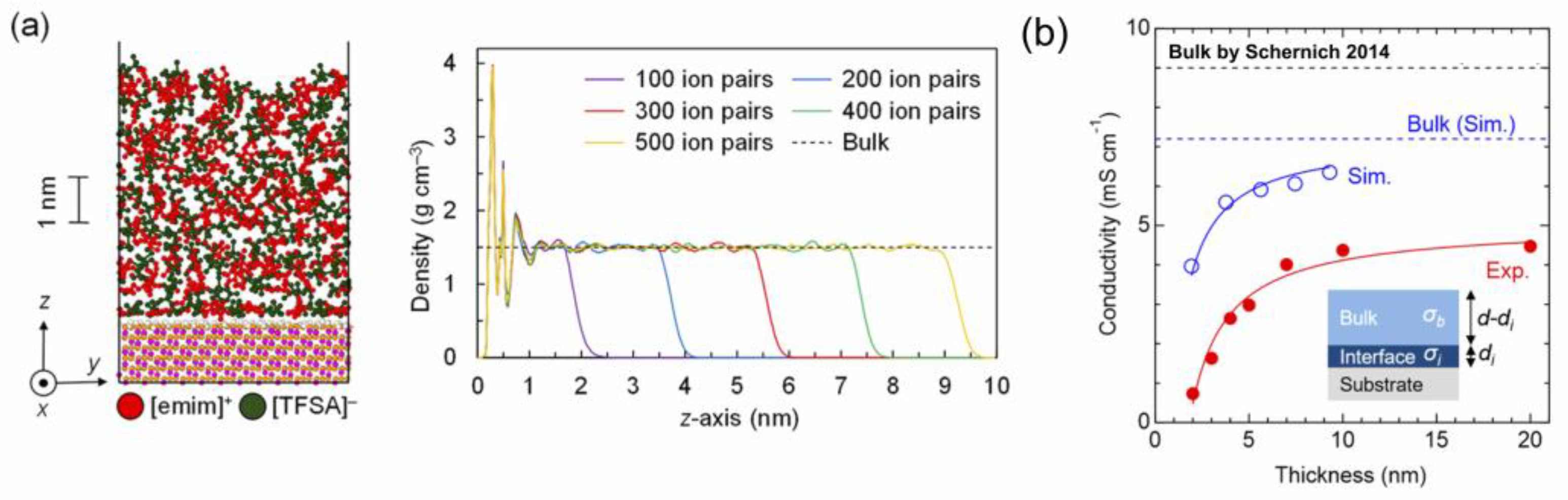
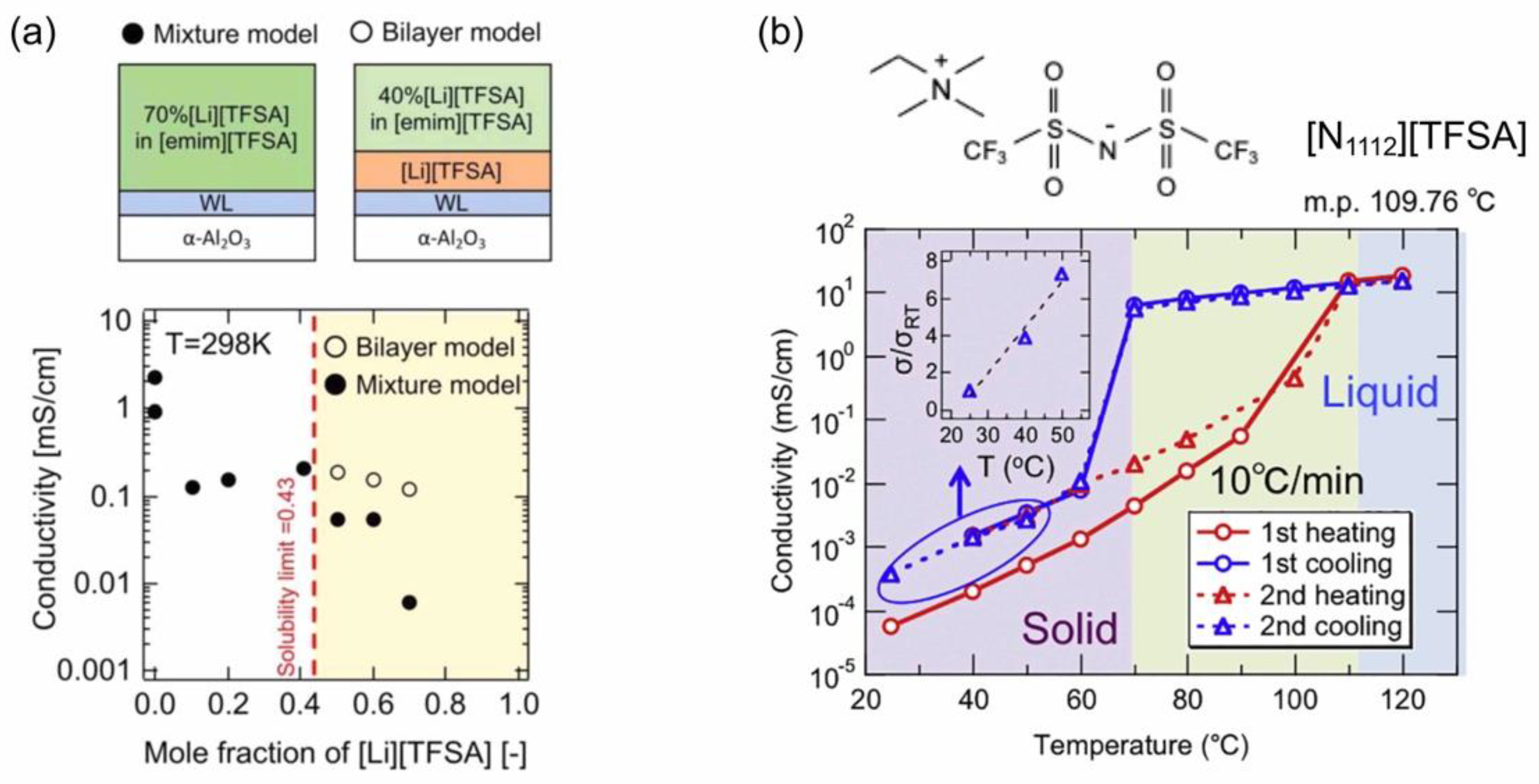
3.2. Ionic Conductivity
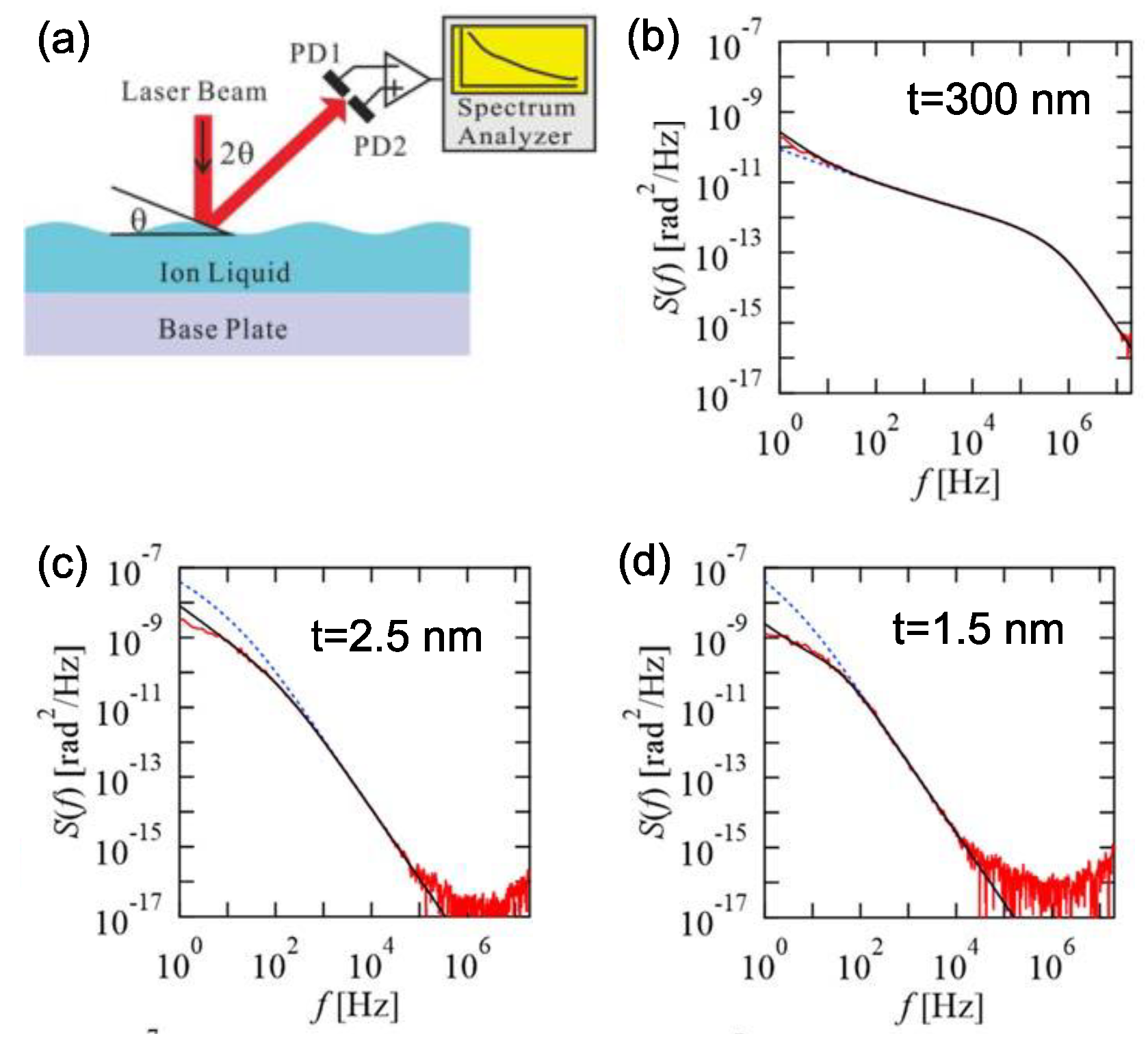
3.3. Viscoelasitcity
4. Applications of Vacuum-Deposited ILs
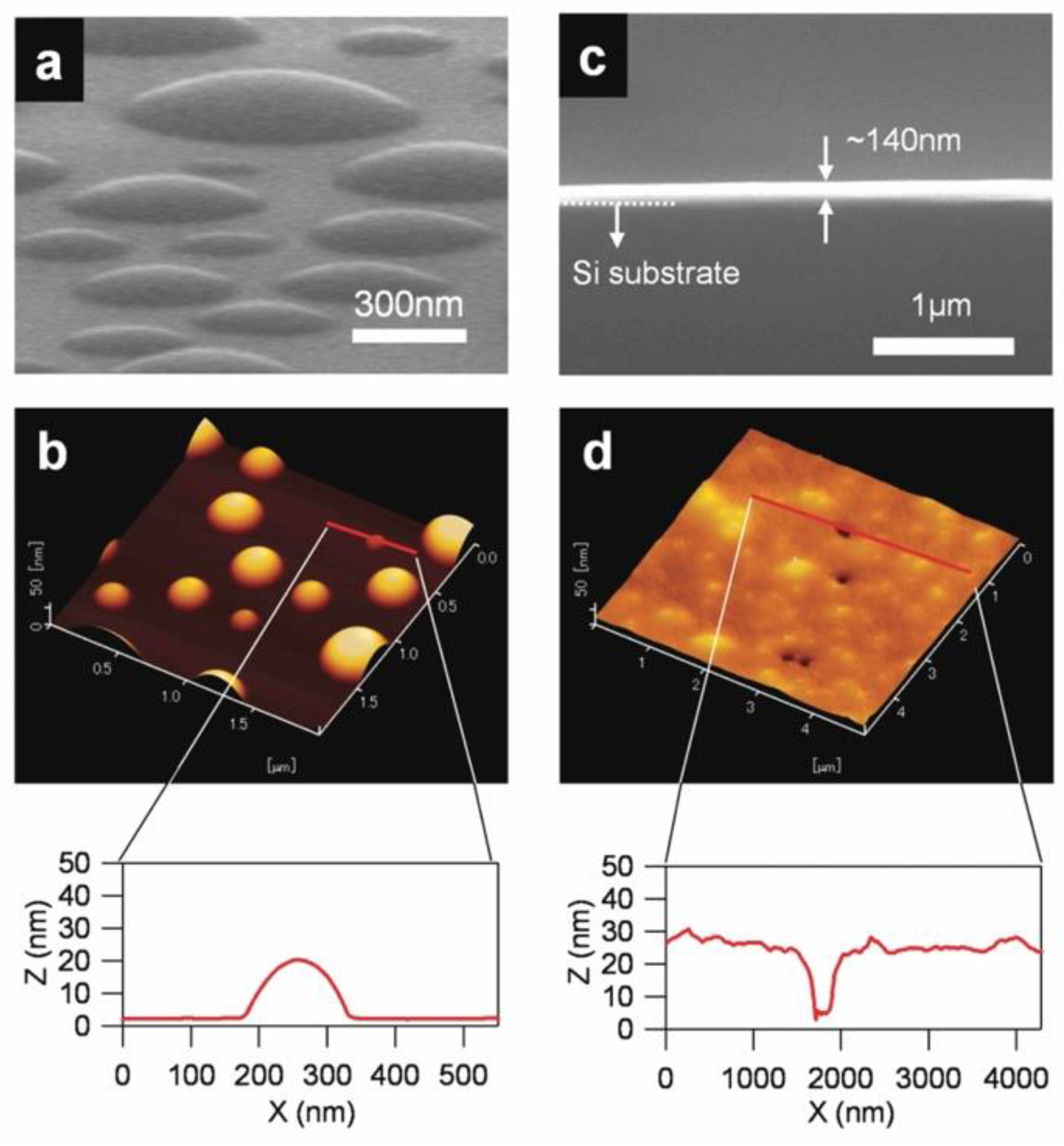
4.1. Vaccum Deposition via Thin Film IL
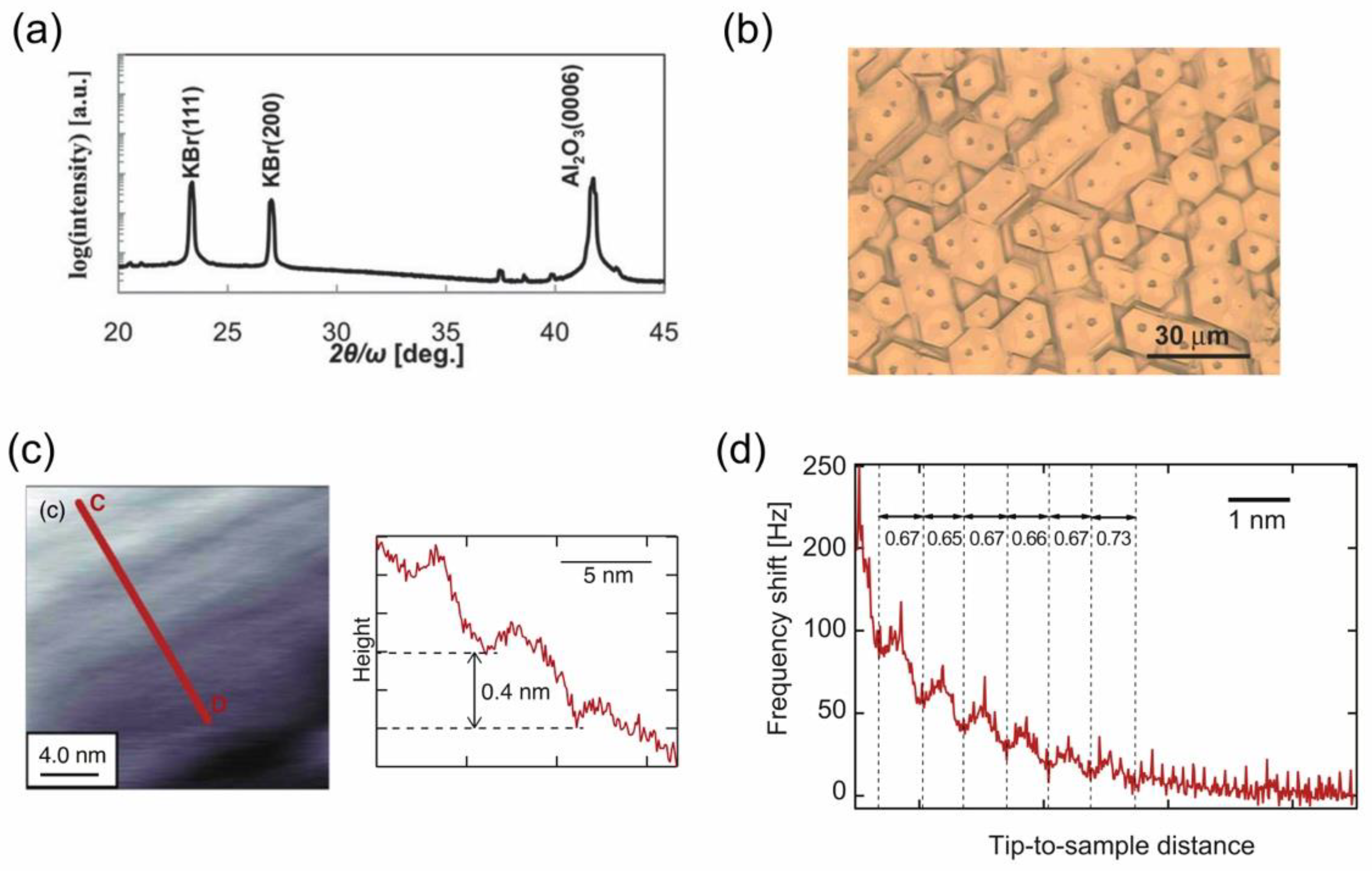
4.1.1. Preparation of a KBr(111) Film and Its Solvation Structure of IL on the (111) Surface
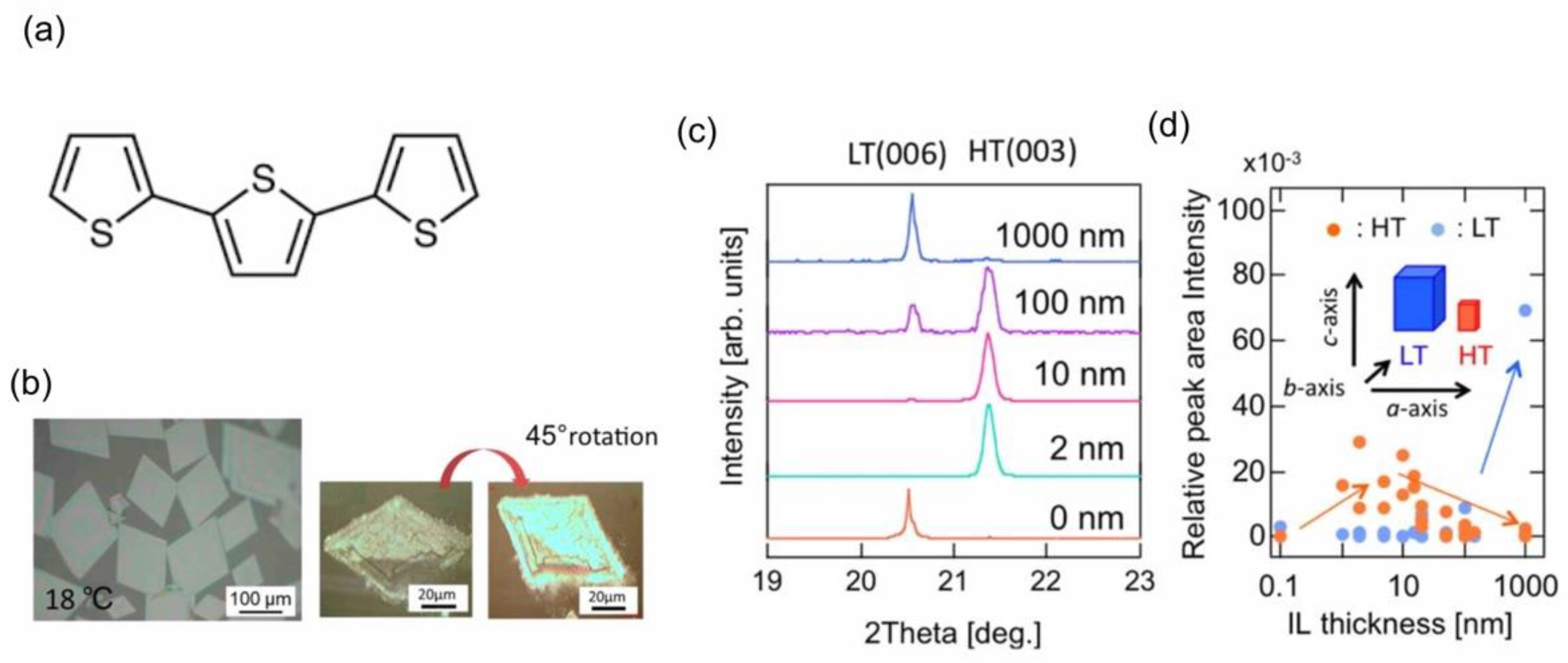
4.1.2. Polymorphs Control of 2,2′:5′,2″-Terthiophene
4.2. Recent Applications
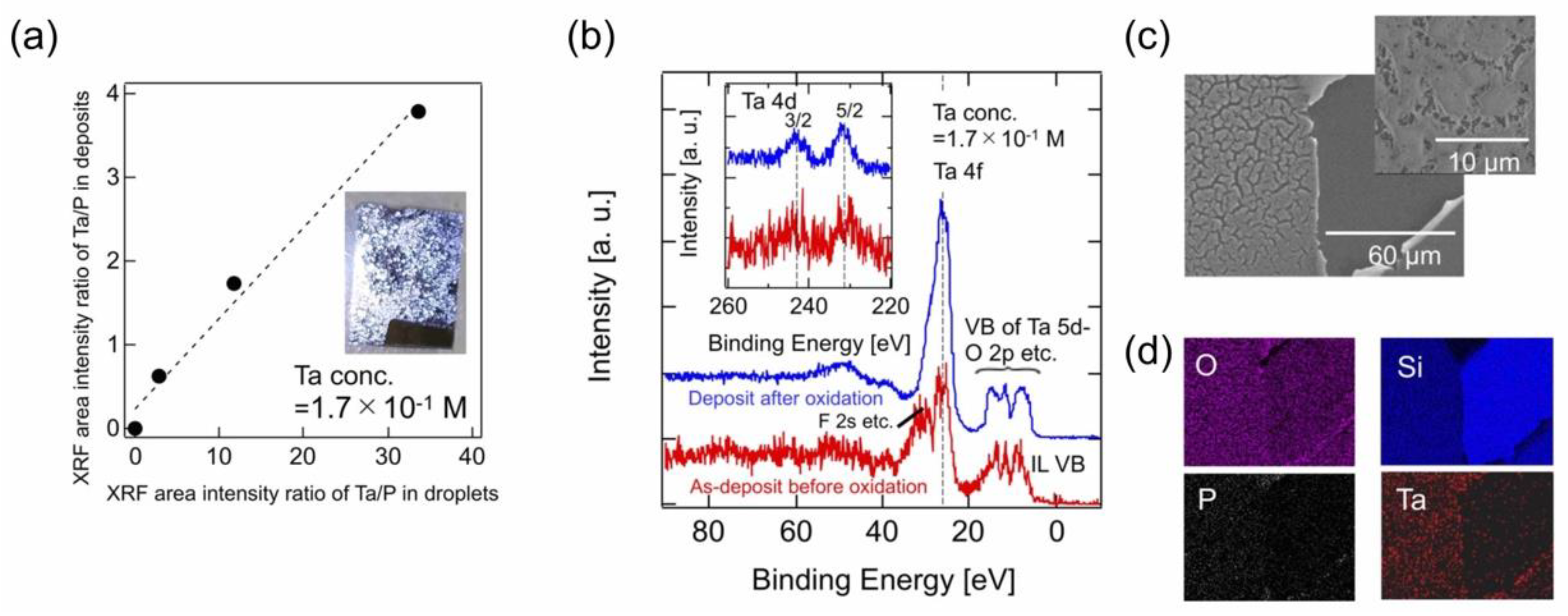
4.2.1. Vacuum Deposition of Metal Ion-Containing IL
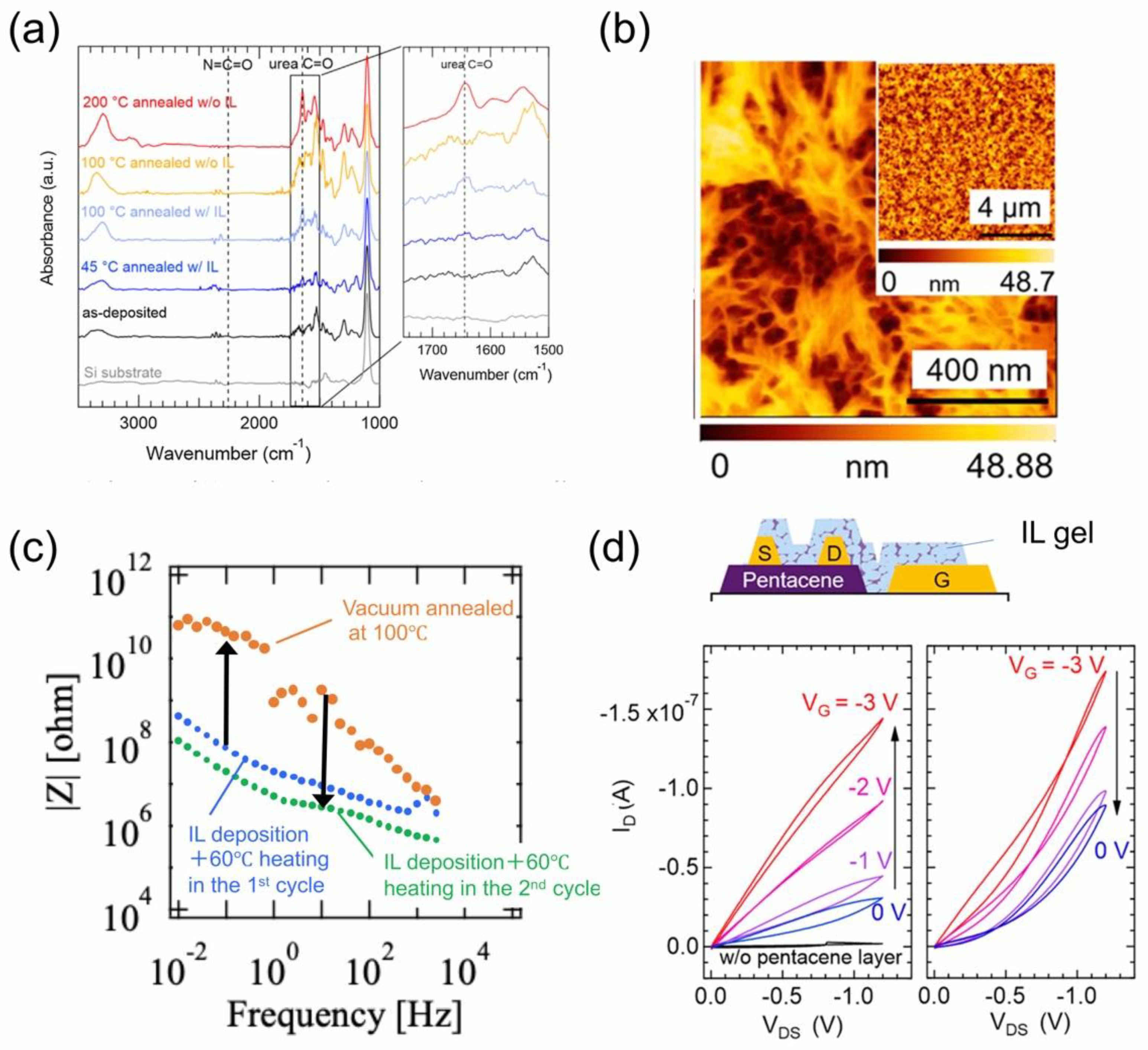
4.2.2. IL Gel Films as an Electrolyte Nanosheet
5. Summary and Future Perspectives
Funding
Conflicts of Interest
References
- Ishii, Y.; Shimada, T.; Okazaki, N.; Hasegawa, T. Wetting-Dewetting Oscillations of Liquid Films during Solution-Mediated Vacuum Deposition of Rubrene. Langmuir 2007, 23, 6864–6869. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed]
- Micaelo, N.M.; Baptista, A.M.; Soares, C.M. Parametrization of 1-Butyl-3-methylimidazolium Hexafluorophosphate/Nitrate Ionic Liquid for the GROMOS Force Field. J. Phys. Chem. B 2006, 110, 14444–14451. [Google Scholar] [CrossRef] [PubMed]
- Höfft, O.; Bahr, S.; Himmerlich, M.; Krischok, S.; Schaefer, J.A.; Kempter, V. Electronic Structure of the Surface of the Ionic Liquid [EMIM][Tf2N] Studied by Metastable Impact Electron Spectroscopy (MIES), UPS, and XPS. Langmuir 2006, 22, 7120–7123. [Google Scholar] [CrossRef] [PubMed]
- Kuwabata, S.; Kongkanand, A.; Oyamatsu, D.; Torimoto, T. Observation of Ionic Liquid by Scanning Electron Microscope. Chem. Lett. 2006, 35, 600–601. [Google Scholar] [CrossRef]
- Torimoto, T.; Okazaki, K.; Kiyama, T.; Hirahara, K.; Tanaka, N.; Kuwabata, S. Sputter deposition onto ionic liquids: Simple and clean synthesis of highly dispersed ultrafine metal nanoparticles. Appl. Phys. Lett. 2006, 89, 243117. [Google Scholar] [CrossRef]
- Kaneko, T.; Baba, K.; Hatakeyama, R. Static gas-liquid interfacial direct current discharge plasmas using ionic liquid cathode. J. Appl. Phys. 2009, 105, 103306. [Google Scholar] [CrossRef]
- Fukui, K.; Yokota, Y.; Imanishi, A. Local Analyses of Ionic Liquid/Solid Interfaces by Frequency Modulation Atomic Force Microscopy and Photoemission Spectroscopy. Chem. Rec. 2014, 14, 964–973. [Google Scholar] [CrossRef]
- Brettholle, M.; Höfft, O.; Klarhöfer, L.; Mathes, S.; Macus-Friedrichs, W.; Abedin, S.Z.E.; Krischok, S.; Janek, J.; Endres, F. Plasma Electrochemistry in Ionic Liquids: Deposition of Copper Nanoparticles. Phys. Chem. Chem. Phys. 2010, 12, 1750–1755. [Google Scholar] [CrossRef]
- Earle, M.J.; Esperanca, M.S.S.; Gilea, M.A.; Canongia Lopes, J.N.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef]
- Paulechka, Y.U.; Zaitsau, D.H.; Kaboand, G.J.; Strechan, A.A. Vapor pressure and thermal stability of ionic liquid 1-butyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)amide. Thermochim. Acta 2005, 439, 158–160. [Google Scholar] [CrossRef]
- Armstrong, J.P.; Hurst, C.; Jones, R.G.; Licence, P.; Lovelock, K.R.J.; Satterley, C.J.; Villar-Garcia, I.J. Vapourisation of ionic liquids. Phys. Chem. Chem. Phys. 2007, 9, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Cremer, T.; Killian, M.; Gottfried, J.M.; Paape, N.; Wasserscheid, P.; Maier, F.; Steinrück, H.-P. Physical Vapor Deposition of [EMIM][Tf2N]: A New Approach to the Modification of Surface Properties with Ultrathin Ionic Liquid Films. ChemPhysChem 2008, 9, 2185–2190. [Google Scholar] [CrossRef] [PubMed]
- Buchner, F.; Forster-Tonigold, K.; Uhl, B.; Alwast, D.; Wagner, N.; Frank-hondeh, H.; Groß, A.; Behm, R.J. Toward the Microscopic Identification of Anions and Cations at the Ionic Liquid|Ag(111) Interface: A Combined Experimental and Theoretical Investigation. ACS Nano 2013, 9, 7773–7784. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kobayashi, K.; Kitaura, R.; Miyata, Y.; Shinohara, H. Direct HRTEM Observation of Ultrathin Freestanding Ionic Liquid Film on Carbon Nanotube Grid. ACS Nano 2011, 5, 4902–4908. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.C.S.; Rocha, R.M.; Vaz, I.C.M.; Torres, M.C.; Mendes, A.; Santos, L.M.N.B.F. Description and Test of a New Multilayer Thin Film Vapor Deposition Apparatus for Organic Semiconductor Materials. J. Chem. Eng. Data 2015, 60, 3776–3791. [Google Scholar] [CrossRef]
- Yaginuma, S.; Itaka, K.; Haemori, M.; Katayama, M.; Ueno, K.; Ohnishi, T.; Lippmaa, M.; Matsumoto, Y.; Koinuma, H. Molecular Layer-by-Layer Growth of C 60 Thin Films by Continuous-Wave Infrared Laser Deposition. Appl. Phys. Express 2008, 1, 015005. [Google Scholar] [CrossRef]
- Maruyama, S.; Takeyama, Y.; Taniguchi, H.; Fukumoto, H.; Itoh, M.; Kumigashira, H.; Oshima, M.; Yamamoto, T.; Matsumoto, Y. Molecular Beam Deposition of Nanoscale Ionic Liquids in Ultrahigh Vacuum. ACS Nano 2010, 4, 5946–5952. [Google Scholar] [CrossRef]
- Takazawa, R.; Toyabe, K.; Maruyama, S.; Matsumoto, Y. In situ diagnosis of vacuum-deposited nanoscale ionic liquid [emim][TFSA], Li salt [Li][TFSA] and their solution films through a combination of ellipsometry and impedance spectroscopy. Jpn. J. Appl. Phys. 2020, 59, 085001. [Google Scholar] [CrossRef]
- Takahashi, R.; Maruyama, S.; Ohsawa, Y.; Matsumoto, Y. Vapor Deposition Polymerization and Porous Structural Formation of Polyurea Thin Films via Nano-ionic Liquid Films. Chem. Lett. 2018, 47, 1460–1463. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Coelhoa, A.F.S.M.G.; Mendesb, A.; Santos, L.M.N.B.F. Nucleation and growth of microdroplets of ionic liquids deposited by physical vapor method onto different surfaces. Appl. Surf. Sci. 2018, 428, 242–249. [Google Scholar] [CrossRef]
- Teixeira, M.S.M.; Santos, L.M.N.B.F.; Costa, J.C.S. Nucleation, Coalescence, and Thin-Film Growth of Triflate-Based Ionic Liquids on ITO, Ag, and Au Surfaces. Colloids Interfaces 2022, 6, 46. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Maruyama, S.; Matsumoto, Y. In situ vacuum ellipsometry approach to investigation of glass transition behavior in ionic liquid thin films. Chem. Phys. Lett. 2020, 754, 137691. [Google Scholar]
- Tokuda, H.; Tsuzuki, S.; Susan, M.A.B.H.; Hayamizu, K.; Watanabe, M. How ionic are room-temperature ionic liquids? An indicator of the physicochemical properties. J. Phys. Chem. B 2006, 110, 19593–19600. [Google Scholar] [CrossRef]
- Hughes, T.J.; Syed, T.; Graham, B.F.; Marsh, K.N.; May, E.F. Heat Capacities and Low Temperature Thermal Transitions of 1-Hexyl and 1-Octyl-3-methylimidazolium bis (trifluoromethylsulfonyl)amide. J. Chem. Eng. Data 2011, 56, 2153–2159. [Google Scholar] [CrossRef]
- Nemoto, F.; Kofu, M.; Yamamuro, O. Thermal and Structural Studies of Imidazolium- Based Ionic Liquids with and without Liquid-Crystalline Phases: The Origin of Nanostructure. J. Phys. Chem. B 2015, 119, 5028–5034. [Google Scholar] [CrossRef]
- Tress, M.; Erber, M.; Mapesa, E.U.; Huth, H.; Müller, J.; Serghei, A.; Schick, C.; Eichhorn, K.-J.; Voit, B.; Kremer, F. Glassy Dynamics and Glass Transition in Nanometric Thin Layers of Polystyrene. Macromolecules 2010, 43, 9937–9944. [Google Scholar] [CrossRef]
- Fox, T.G. Influence of diluent and of copolymer composition on the glass temperature of a polymer system. Bull. Am. Phys. Soc. 1956, 1, 123–313. [Google Scholar]
- Maruyama, S.; Prastiawan, I.B.H.; Toyabe, K.; Higuchi, Y.; Koganezawa, T.; Kubo, M.; Matsumoto, Y. Ionic Conductivity in Ionic Liquid Nano Thin Films. ACS Nano 2018, 12, 10509–10517. [Google Scholar] [CrossRef]
- Yamauchi, M.; Maruyama, S.; Ohashi, N.; Toyabe, K.; Matsumoto, Y. Epitaxial growth of atomically flat KBr(111) films via thin film ionic liquid in a vacuum. CrystEngComm 2016, 18, 3399–3403. [Google Scholar] [CrossRef]
- Schernich, S.; Kostyshyn, D.; Wagner, V.; Taccardi, N.; Laurin, M.; Wasserscheid, P.; Libuda, J. Interactions Between the Room- Temperature Ionic Liquid [C2C1Im][OTf] and Pd(111), Well- Ordered Al2O3, and Supported Pd Model Catalysts from IR Spectroscopy. J. Phys. Chem. C 2014, 118, 3188–3193. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Araújo, J.M.M.; Oliveira, F.S.; Esperança, J.M.S.S.; Lopes, J.N.C.; Marrucho, I.M.; Rebelo, L.P.N. Solubility of inorganic salts in pure ionic liquids. J. Chem. Thermodyn. 2012, 55, 29–36. [Google Scholar] [CrossRef]
- Yoshii, A.; Maruyama, S.; Toyabe, K.; Takazawa, R.; Koganezawa, T.; Matsumoto, Y. Fabrication of ionic liquid polycrystalline nano thin films and their ion conducting properties accompanied by solid-liquid phase transition. Thin Solid Films 2019, 677, 77–82. [Google Scholar] [CrossRef]
- Chen, H.; He, Y.; Zhu, J.; Alias, H.; Ding, Y.; Nancarrow, P.; Hardacre, C.; Rooney, D.; Tan, C. Rheological and heat transfer behaviour of the ionic liquid, [C4mim][NTf2]. Int. J. Heat Fluid Flow 2008, 29, 149–155. [Google Scholar] [CrossRef]
- Shakeel, A.; Mahmood, H.; Farooq, U.; Ullah, Z.; Yasin, S.; Iqbal, T.; Chassagne, C.; Moniruzzaman, M. Rheology of Pure Ionic Liquids and Their Complex Fluids: A Review. ACS Sustain. Chem. Eng. 2019, 7, 13586–13626. [Google Scholar] [CrossRef]
- Maruyama, S.; Ishikawa, Y.; Mitsui, T.; Aoki, K.; Matsumoto, Y. Surface thermal fluctuation spectroscopy study of ultra-thin ionic liquid films on quartz. Appl. Phys. Express 2021, 14, 075503. [Google Scholar] [CrossRef]
- Mitsui, T.; Aoki, K. Fluctuation spectroscopy of surface melting of ice with and without impurities. Phys. Rev. E 2019, 99, 010801. [Google Scholar] [CrossRef]
- Tay, A.; Thibierge, C.; Fournier, D.; Fretigny, C.; Lequeux, F.; Monteux, C.; Roger, J.P.; Talini, L. Probing thermal waves on the free surface of various media: Surface fluctuation specular reflection spectroscopy. Rev. Sci. Instrum. 2008, 79, 103107. [Google Scholar] [CrossRef]
- Wagner, R.S.; Ellis, W.C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89–91. [Google Scholar] [CrossRef]
- Deshmukh, P.; Sharma, M.; Nalamati, S.; Reynoids, C.L., Jr.; Liu, Y.; Iyer, S. Molecular beam epitaxial growth of high quality Ga-catalyzed GaAs1–xSbx(x > 0.8) nanowires on Si (111) with photoluminescence emission reaching 1.7 μm. Semicond. Sci. Technol. 2018, 33, 125007. [Google Scholar] [CrossRef]
- Hu, J.; Odom, T.W.; Lieber, C.M. Chemistry and Physics in One Dimension: Synthesis and Properties of Nanowires and Nanotubes. Acc. Chem. Res. 1999, 32, 435–445. [Google Scholar] [CrossRef]
- Purushothaman, V.; Ramakrishnan, V.; Jeganathan, K. Interplay of VLS and VS growth mechanism for GaN nanowires by a self- catalytic approach. RSC Adv. 2012, 2, 4802–4806. [Google Scholar] [CrossRef]
- Ferro, G.; Chaussende, D.; Cauwet, F.; Monteil, Y. Effect of the Si Droplet Size on the VLS Growth Mechanism of SiC Homoepitaxial Layers. Mater. Sci. Forum 2002, 389–393, 287–290. [Google Scholar] [CrossRef]
- Osumi, A.; Nakano, K.; Sannodo, N.; Maruyama, S.; Matsumoto, Y.; Mitani, T.; Kato, T.; Yonezawa, Y.; Okumura, H. Platinum additive impacts on vapor-liquid-solid growth chemical interface for high-quality SiC single crystal films. Mater. Today Chem. 2020, 16, 100266. [Google Scholar] [CrossRef]
- Yun, K.S.; Choi, B.D.; Matsumoto, Y.; Song, J.H.; Kanda, N.; Itoh, T.; Kawasaki, M.; Chikyow, T.; Ahmet, A.P.; Koinuma, H. Vapor–liquid–solid tri-phase pulsed-laser epitaxy of RBa2Cu3O7-y single-crystal films. Appl. Phys. Lett. 2002, 80, 61. [Google Scholar] [CrossRef]
- Takahashi, R.; Yonezawa, Y.; Ohtani, M.; Kawasaki, M.; Nakajima, K.; Chikyow, T.; Koinuma, H.; Matsumoot, Y. Perfect Bi4Ti3O12 Single-Crystal Films via Flux-Mediated Epitaxy. Adv. Funct. Mater. 2006, 16, 485–491. [Google Scholar] [CrossRef]
- Voigt, M.; Dorsfeld, S.; Volz, A.; Sokolowski, M. Nucleation and Growth of Molecular Organic Crystals in a Liquid Film under Vapor Deposition. Phys. Rev. Lett. 2002, 91, 026103. [Google Scholar] [CrossRef]
- Takeyama, Y.; Maruyama, S.; Matsumoto, Y. Growth of Single-Crystal Phase Pentacene in Ionic Liquids by Vacuum Deposition. Cryst. Growth Des. 2011, 11, 2273–2278. [Google Scholar] [CrossRef]
- Takeyama, Y.; Ono, S.; Matsumoto, Y. Organic single crystal transistor characteristics of single-crystal phase pentacene grown by ionic liquid-assisted vacuum deposition. Appl. Phys. Lett. 2012, 101, 083303. [Google Scholar] [CrossRef]
- Takeyama, Y.; Maruyama, S.; Taniguchi, H.; Itoh, M.; Ueno, K.; Matsumoto, Y. Ionic liquid-mediated epitaxy of high-quality C60 crystallites in a vacuum. CrystEngComm 2012, 14, 4939–4945. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Campos, R.M.; Castro, A.C.M.; Farinha, A.F.M.; Olivelria, G.N.P.; Araüjo, J.P.; Santos, L.M.N.B.F. The effect of onic liquids on the nucleation and growth of perylene films obtained by vapor deposition. CrystEngComm 2023, 25, 913–924. [Google Scholar] [CrossRef]
- Horike, S.; Koshiba, Y.; Misaki, M.; Ishida, K. Crystal growth of rubrene in ionic liquids by vacuum vapor deposition. Jpn. J. Appl. Phys. 2014, 53, 05FT03. [Google Scholar] [CrossRef]
- Horike, S.; Misaki, M.; Koshiba, Y.; Morimoto, M.; Ishida, K. Unique Morphology and Optical Properties of Tris(8-hydroxyquinoline)aluminum Crystal Grown by Ionic Liquid-assisted Vacuum Vapor Deposition. Chem. Lett. 2016, 45, 1156–1158. [Google Scholar] [CrossRef]
- Kumada, S.; Matsuzaki, K.; Hosono, H.; Susaki, T. Tuning of Surface Roughness and Lattice Constant in MgO(111)/Al2O3(0001) Grown by Laser Energy Controlled Pulsed Laser Deposition. Jpn. J. Appl. Phys. 2011, 50, 085503. [Google Scholar] [CrossRef]
- Kato, S.; Takeyama, Y.; Maruyama, S.; Matsumoto, Y. Nonfaceted Growth of (111)-Oriented Epitaxial Alkali-Halide Crystals via an Ionin Liquid Flux in a Vacuum. Cryst. Growth Des. 2010, 10, 3608–3611. [Google Scholar] [CrossRef]
- Mungse, H.P.; Okudaira, S.; Yamauchi, M.; Ichii, T.; Utsunomiya, T.; Maruyama, S.; Matsumoto, Y.; Sugimura, H. Surface charge dependent structure of ionic liquid/alkali halide interfaces investigated by atomic force microscopy. Jpn. J. Appl. Phys. 2022, 61, SL1009. [Google Scholar] [CrossRef]
- Mattheus, C.C.; Dros, A.B.; Baas, J.; Meetsma, A.; de Boer, J.L.; Palstra, T.T.M. Polymorphism in pentacene. Acta Cryst. C 2001, 57, 939–941. [Google Scholar] [CrossRef]
- Soeda, J.; Okamoto, T.; Hamaguchi, A.; Ikeda, Y.; Sato, H.; Yamano, A.; Takeya, J. Two-dimensional crystal growth of thermally converted organic semiconductors at the surface of ionic liquid and high-mobility organic field-effect transistors. J. Org. Electron. 2013, 14, 1211–1217. [Google Scholar] [CrossRef]
- Bischof, D.; Tripp, M.W.; Ivlev, S.I.; Koert, U.; Witte, G. Solvent Polarity Controlled Polymorphs of Polar Aromatic Molecules. Cryst. Growth Des. 2022, 22, 6857–6862. [Google Scholar] [CrossRef]
- Okawara, K.; Maruyama, S.; Matsumoto, Y. Ionic liquid-assisted vapor deposition and polymorphs control of 2,2′:5′,2′′-terthiophene crystals. Jpn. J. Appl. Phys. 2019, 58, 085503. [Google Scholar] [CrossRef]
- Schweicher, G.; Paquay, N.; Amato, C.; Resel, R.; Koni, M.; Talvy, S.; Lemaur, V.; Cornil, J.; Greets, Y.; Gbabode, G. Toward Single Crystal Thin Films of Terthiophene by Directional Crystallization Using a Thermal Gradient. Cryst. Growth Des. 2011, 11, 3663–3672. [Google Scholar] [CrossRef]
- Endres, F. Ionic Liquids: Solvents for the Electrodeposition of Metals and Semiconductors. ChemPhysChem 2002, 3, 144–154. [Google Scholar] [CrossRef]
- Seki, S.; Kobayashi, Y.; Miyashiro, H.; Ohno, Y.; Usami, A.; Mita, Y.; Watanabe, M.; Terada, N. Highly reversible lithium metal secondary battery using a room temperature ionic liquid/lithium salt mixture and a surface-coated cathode active material. Chem. Commun. 2006, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Krischok, S.; Ispas, A.; Zühlsdorff, A.; Ulbrich, A.; Bund, A.; Endres, F. Ta and Nb electrodeposition from ionic liquids. ECS Trans. 2012, 50, 229–237. [Google Scholar] [CrossRef]
- Chaneliere, C.; Autran, J.L.; Devine, R.A.B.; Balland, B. Tantalum pentoxide (Ta2O5) thin films for advanced dielectric applications. Mater. Sci. Eng. R 1998, 22, 269–322. [Google Scholar] [CrossRef]
- Hozuki, N.; Kaminaga, K.; Maruyama, S.; Shiga, D.; Kumigashira, H.; Takato, H.; Kondo, M.; Matsumoto, Y. Room-Temperature Preparation of Ta Ions-Containing Ionic Liquid and its Vapor Deposition toward Ta-Oxide Film Coating. J. Electrochem. Soc. 2022, 169, 013504. [Google Scholar] [CrossRef]
- Borisenko, N.; Ispas, A.; Zschippang, E.; Liu, Q.; Abedin, S.Z.E.; Bund, A.; Endres, F. In situ STM and EQCM studies of tantalum electrodeposition from TaF5 in the air- and water-stable ionic liquid 1-butyl-1-methylpyrrolidinium bis (trifluoromethylsulfonyl)amide. Electrochim. Acta 2009, 54, 1519–1528. [Google Scholar] [CrossRef]
- Krebs, F.; Höfft, O.; Endres, F. Investigations on the electrochemistry and reactivity of tantalum species in 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide using X-ray photoelectron spectroscopy (in situ and ex-situ XPS). Appl. Surf. Sci. 2023, 608, 155130. [Google Scholar] [CrossRef]
- Maruyama, S.; Ohsawa, Y.; Takahashi, R.; Matsumoto, Y. In situ AFM study of low-temperature polymerization and network formation of thin film polyurea in ionic liquid. Eur. Polym. J. 2018, 105, 421–425. [Google Scholar] [CrossRef]
- Watanabe, H.; Takazawa, R.; Takahashi, R.; Maruyama, S.; Matsumoto, Y. Nanogels Constituted of Polyurea Filled with an Ionic Liquid as an Electrolyte for Electric Double-Layer Transistors. ACS Appl. Nano Mater. 2020, 3, 9610–9615. [Google Scholar] [CrossRef]
- Kanai, M.; Watanabe, K.; Maruyama, S.; Matsumoto, Y. Ionic liquid/ZnO(000-1) single crystal and epitaxial film interfaces studied through a combination of electrochemical measurements and a pulsed laser deposition process under vacuum. Phys. Chem. Chem. Phys. 2019, 21, 25506–25512. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Kanai, M.; Maruyama, S.; Matsumoto, Y. Vacuum Electrochemistry Approach to Investigate Electrical Double-Layer Capacitances of Ionic Liquid for Epitaxial Thin-Film Electrodes of TiO2 and SrO on Niobium-Doped (001)SrTiO3. ChemElectroChem 2020, 7, 3253–3259. [Google Scholar] [CrossRef]
- Okawara, K.; Nishimura, T.; Maruyama, S.; Kubo, M.; Matsumoto, Y. In-vacuum electropolymerization of vapor-deposited source molecules into polymer films in ionic liquid. React. Chem. Eng. 2020, 5, 33–38. [Google Scholar] [CrossRef]
- Komatsu, H.; Tanaka, M.; Kaminaga, K.; Maruyama, S.; Matsumoto, Y. Electric Double Layer Action of High-quality Ionic Liquid Crystal Thin Films. Chem. Lett. 2022, 51, 162–165. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, Y. Recent Progress in Vacuum Engineering of Ionic Liquids. Molecules 2023, 28, 1991. https://doi.org/10.3390/molecules28041991
Matsumoto Y. Recent Progress in Vacuum Engineering of Ionic Liquids. Molecules. 2023; 28(4):1991. https://doi.org/10.3390/molecules28041991
Chicago/Turabian StyleMatsumoto, Yuji. 2023. "Recent Progress in Vacuum Engineering of Ionic Liquids" Molecules 28, no. 4: 1991. https://doi.org/10.3390/molecules28041991
APA StyleMatsumoto, Y. (2023). Recent Progress in Vacuum Engineering of Ionic Liquids. Molecules, 28(4), 1991. https://doi.org/10.3390/molecules28041991





