Release Profiles of Carvacrol or Chlorhexidine of PLA/Graphene Nanoplatelets Membranes Prepared Using Electrospinning and Solution Blow Spinning: A Comparative Study
Abstract
1. Introduction
2. Results
3. Materials and Method
3.1. Materials
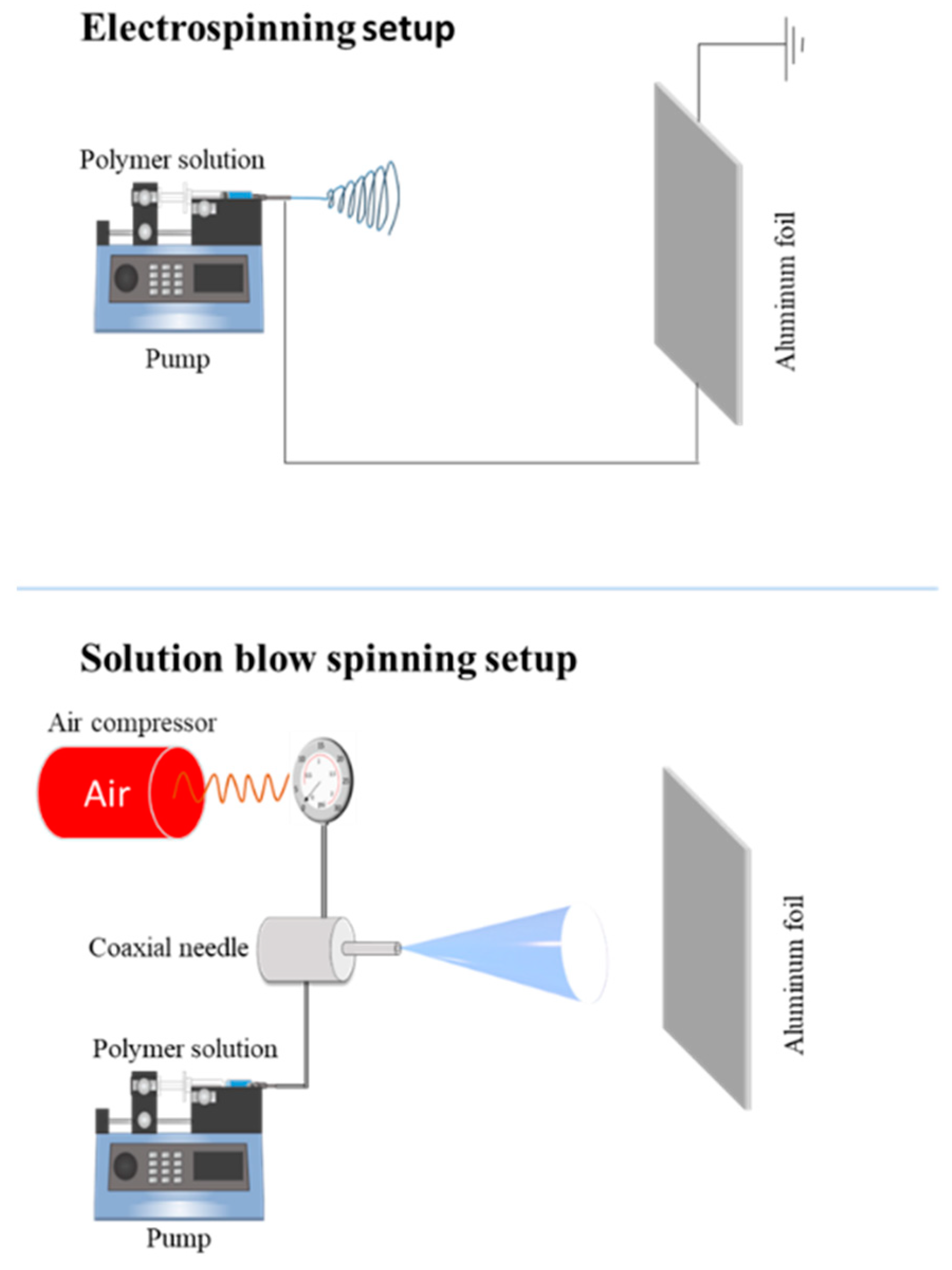
3.2. Preparation
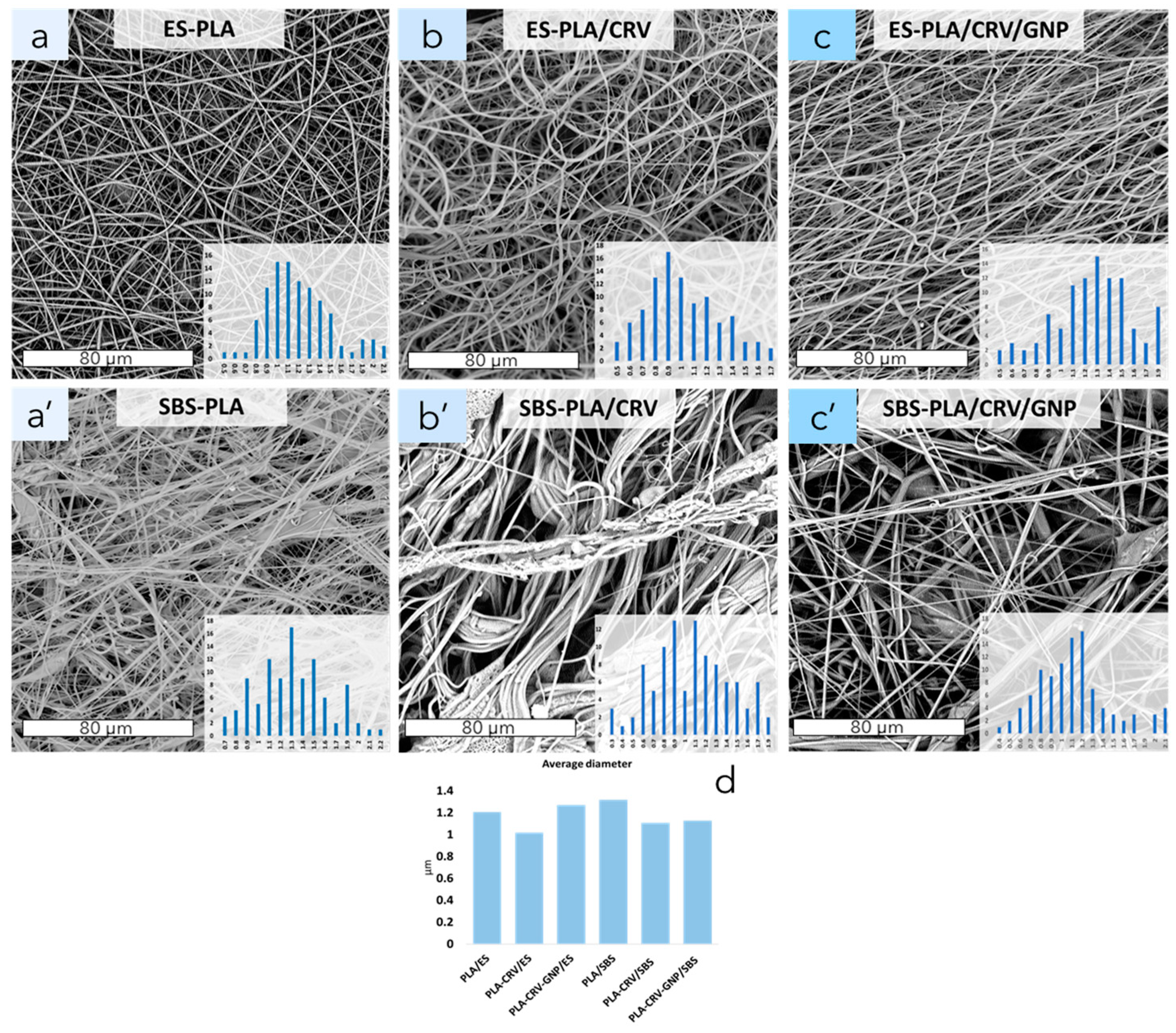
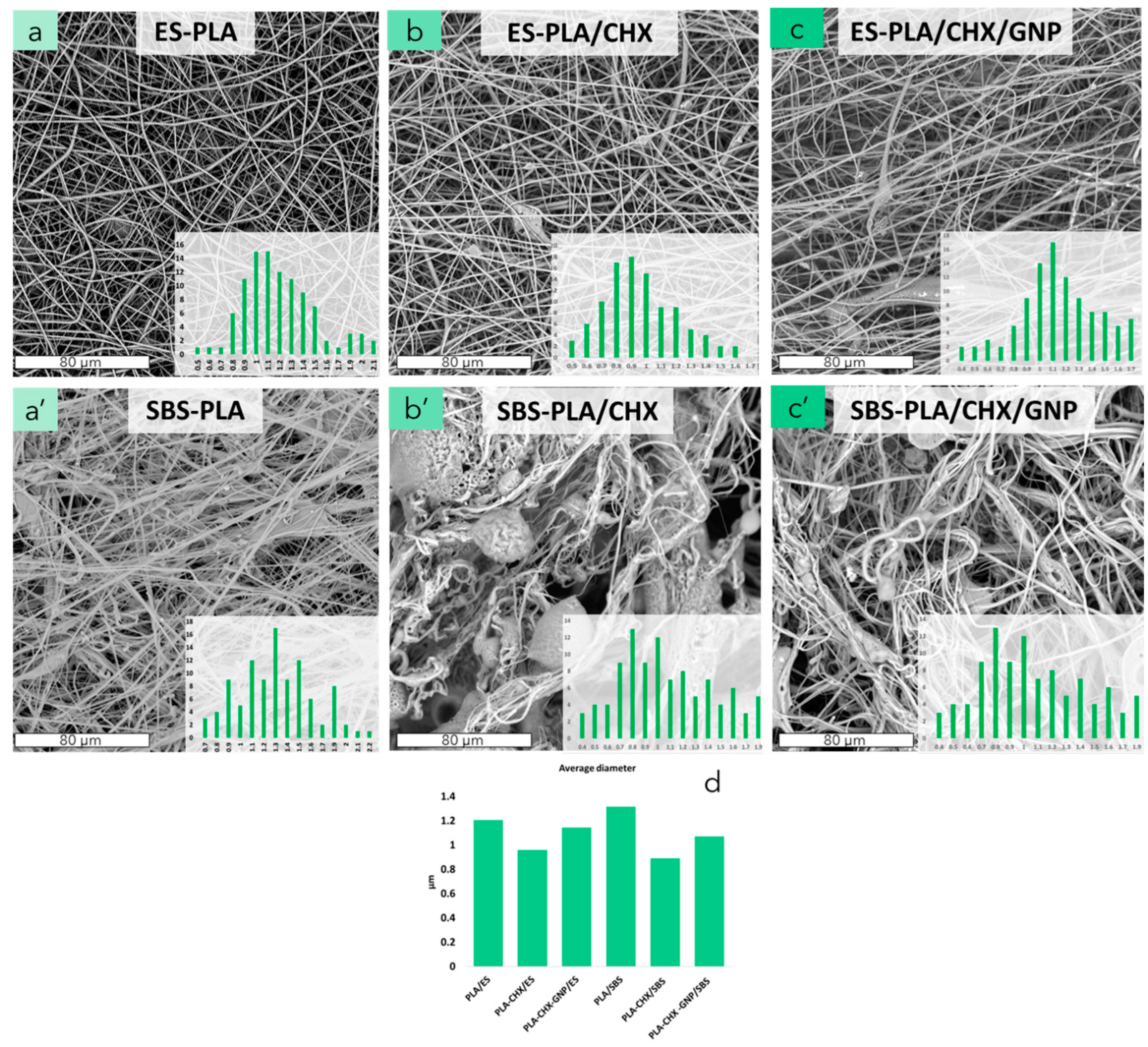
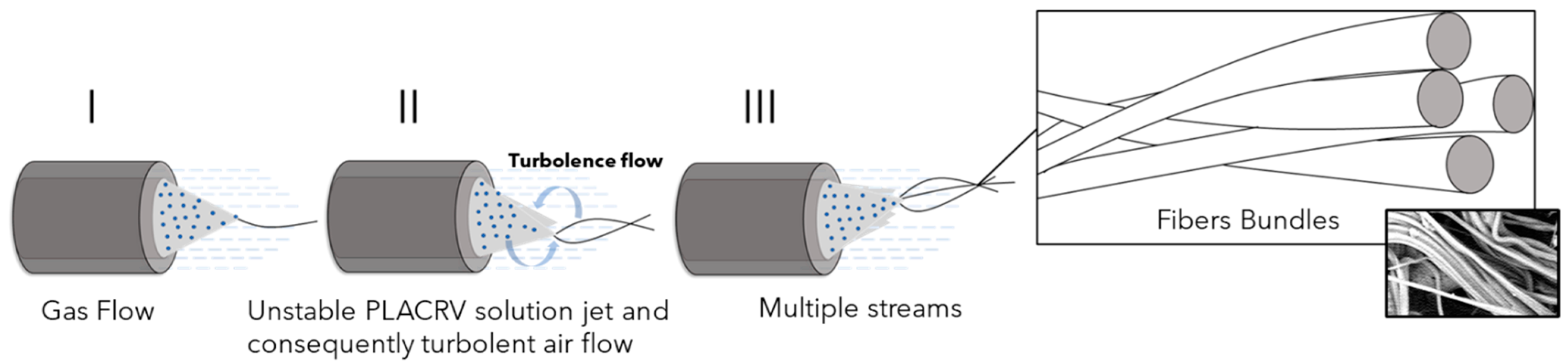
3.3. Morphological Analysis
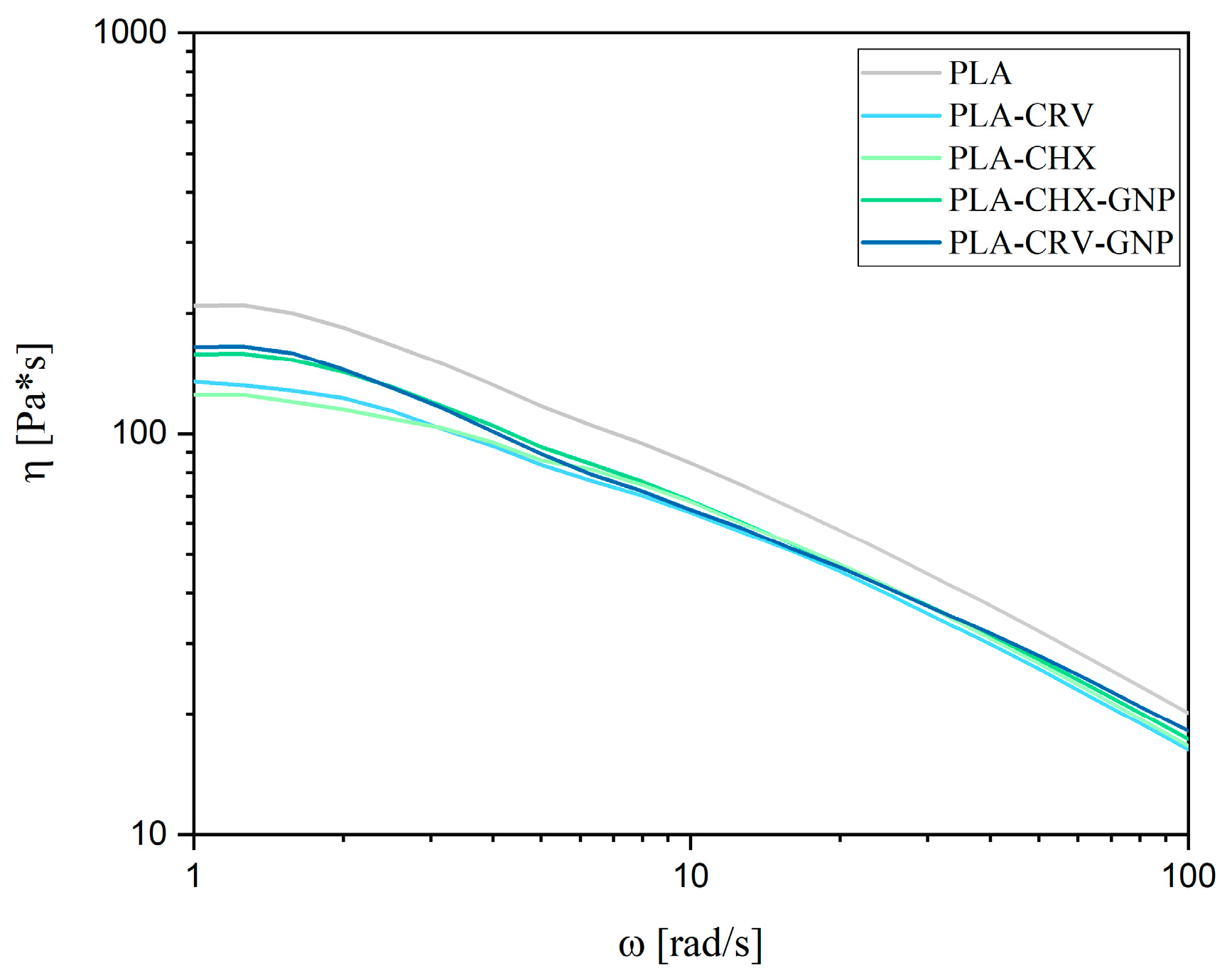
3.4. Rheological Characterization
3.5. FT-IR/ATR Analysis
3.6. Mechanical Properties
3.7. Water Contact Angle (WCA) Measurements
3.8. Release Tests
3.9. Antimicrobial Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gulino, E.F.; Citarrella, M.C.; Maio, A.; Scaffaro, R. An innovative route to prepare in situ graded crosslinked PVA graphene elec-trospun mats for drug release. Compos. Part A Appl. Sci. Manuf. 2022, 155, 106827. [Google Scholar] [CrossRef]
- Subramani, M.; Vekatashwaramurthy, N.; Sambathkumar, R. A Novel Approach on Role of Polymers Used in Sustained Re-lease Drug Delivery System—A Review. Saudi J. Med. Pharm. Sci. 2021, 7, 170–178. [Google Scholar] [CrossRef]
- Morita, R.; Honda, R.; Takahashi, Y. Development of oral controlled release preparations, a PVA swelling controlled release system (SCRS). II. In vitro and in vivo evaluation. J. Control. Release 2000, 68, 115–120. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Botta, L.; Gulino, E.F.; Gulli, D. Tunable release of Chlorhexidine from Polycaprolactone-based filaments containing graphene nanoplatelets. Eur. Polym. J. 2018, 110, 221–232. [Google Scholar] [CrossRef]
- Xuan, H.; Wu, S.; Fei, S.; Li, B.; Yang, Y.; Yuan, H. Injectable nanofiber-polysaccharide self-healing hydrogels for wound healing. Mater. Sci. Eng. C 2021, 128, 112264. [Google Scholar] [CrossRef]
- Scaffaro, R.; Citarrella, M.C.; Gulino, E.F. Opuntia Ficus Indica based green composites for NPK fertilizer controlled release produced by compression molding and fused deposition modeling. Compos. Part A Appl. Sci. Manuf. 2022, 159, 107030. [Google Scholar] [CrossRef]
- Haryńska, A.; Carayon, I.; Kosmela, P.; Szeliski, K.; Łapiński, M.; Pokrywczyńska, M.; Kucińska-Lipka, J.; Janik, H. A comprehensive evaluation of flexible FDM/FFF 3D printing filament as a potential material in medical application. Eur. Polym. J. 2020, 138, 109958. [Google Scholar] [CrossRef]
- Shi, K.; Salvage, J.P.; Maniruzzaman, M.; Nokhodchi, A. Role of release modifiers to modulate drug release from fused deposi-tion modelling (FDM) 3D printed tablets. Int. J. Pharm. 2021, 597, 120315. [Google Scholar] [CrossRef] [PubMed]
- Naebe, M.; Lin, T.; Tian, W.; Dai, L.; Wang, X. Effects of MWNT nanofillers on structures and properties of PVA electrospun nanofibres. Nanotechnology 2007, 18, 225605. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Sun, G.; Ding, B. Electrospun nanofibrous materials: A versatile medium for effective oil/water separation. Mater. Today 2015, 19, 403–414. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Naskar, D.; Kim, H.-W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Non-mulberry silk fibroin grafted PCL nano-fibrous scaffold: Promising ECM for bone tissue engineering. Eur. Polym. J. 2015, 71, 490–509. [Google Scholar] [CrossRef]
- Thakkar, S.; Misra, M. Electrospun polymeric nanofibers: New horizons in drug delivery. Eur. J. Pharm. Sci. 2017, 107, 148–167. [Google Scholar] [CrossRef]
- Scaffaro, R.; Gulino, F.E.; Lopresti, F. Structure–property relationship and controlled drug release from multiphasic electrospun carvacrol-embedded polylactic acid/polyethylene glycol and polylactic acid/polyethylene oxide nanofiber mats. J. Ind. Text. 2018, 49, 943–966. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Micale, G.D. PLA-based functionally graded laminates for tunable controlled release of carvacrol obtained by combining electrospinning with solvent casting. React. Funct. Polym. 2020, 148, 104490. [Google Scholar] [CrossRef]
- Joachim, H. Wendorff, Seema Agarwal, Andreas Greiner, Electrospinning: Materials, Processing, and Applications; Wiley-VCH: Weinheim, Germany, 2012; pp. 40–48. [Google Scholar]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Y.; Xia, J.; Zhou, Z.; Fang, Y.; Zhang, L.; Sun, W. Multi-scale hierarchical scaffolds with aligned micro-fibers for promot-ing cell alignment. Biomed. Mater. 2021, 16, 045047. [Google Scholar] [CrossRef]
- Yu, D.-G.; Li, Q.; Song, W.; Xu, L.; Zhang, K.; Zhou, T. Advanced Technique-Based Combination of Innovation Education and Safety Education in Higher Education. J. Chem. Educ. 2023, 100, 507–516. [Google Scholar] [CrossRef]
- Valero-Valenzuela, A.; Kovacs, K.; Yu, D.-G.; Du, Y.; Chen, J.; Song, W.; Zhou, T. A Correlation Analysis between Undergraduate Students’ Safety Behaviors in the Laboratory and Their Learning Efficiencies. Behav. Sci. 2023, 13, 127. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, G.R. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Hou, Z.; Itagaki, N.; Kobayashi, H.; Tanaka, K.; Takarada, W.; Kikutani, T.; Takasaki, M. Bamboo charcoal/poly(L-lactide) fiber webs pre-pared using laser-heated melt electrospinning. Polymers 2021, 13, 2776. [Google Scholar] [CrossRef]
- Cui, Q.; Bell, D.J.; Rauer, S.B.; Wessling, M. Wet-Spinning of Biocompatible Core–Shell Polyelectrolyte Complex Fibers for Tissue Engineering. Adv. Mater. Interfaces 2020, 7, 2000849. [Google Scholar] [CrossRef]
- Van De Witte, P.; Dijkstra, P.J.; Van Den Berg, J.W.A.; Feijen, J. Phase separation processes in polymer solutions in relation to mem-brane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef]
- Daristotle, J.L.; Behrens, A.M.; Sandler, A.D.; Kofinas, P. A Review of the Fundamental Principles and Applications of Solution Blow Spinning. ACS Appl. Mater. Interfaces 2016, 8, 34951–34963. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Su, Y.; Wang, H.; Wang, X.-X.; Huang, L.-P.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Recent progress and challenges in solution blow spinning. Mater. Horiz. 2020, 8, 426–446. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Brichi, G.S.; Marconcini, J.M.; Mattoso, L.H.C.; Glenn, G.M.; Medeiros, E.S. Effect of Solvent on The Physical and Morphological Properties of Poly(Lactic Acid) Nanofibers Obtained by Solution Blow Spinning. J. Eng. Fibers Fabr. 2014, 9, 117. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, H.; Wang, D.; Ma, Y.; Jia, L. A Facile Strategy for Fabrication Lysozyme-Loaded Mesoporous Silica Nanotubes from Electrospun Silk Fibroin Nanofiber Templates. Molecules 2021, 26, 1073. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zheng, H.; Du, M.; Zhang, Z. Investigation on the Potential Application of Na-Attapulgite as an Excipient in Domperidone Sustained-Release Tablets. Molecules 2022, 27, 8266. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Abdelhakim, H.E. Drug Delivery Applications of Coaxial Electrospun Nanofibres in Cancer Therapy. Molecules 2022, 27, 1803. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.F.; Morreale, M. Degradation and recycling of films based on biodegradable polymers: A short review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.; Day, M.J. Antibacterial activity of chlorhexidine. J. Hosp. Infect. 1993, 25, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Mantripragada, S.; Gbewonyo, S.; Deng, D.; Zhang, L. Oil absorption capability of electrospun carbon nanofibrous membranes having porous and hollow nanostructures. Mater. Lett. 2019, 262, 127069. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Pitarresi, G. Lignocellulosic fillers and graphene nanoplatelets as hybrid reinforcement for polylactic acid: Effect on mechanical properties and degradability. Compos. Sci. Technol. 2020, 190, 108008. [Google Scholar] [CrossRef]
- Berg, M.C.; Zhai, L.; Cohen, R.E.; Rubner, M.F. Controlled Drug Release from Porous Polyelectrolyte Multilayers. Biomacromolecules 2005, 7, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.Q.M. Polyethyelene Glycols and Drug Release. Drug Dev. Ind. Pharm. 1990, 16, 2501–2526. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Nie, J. Preparation of gelatin/PVA nanofibers and their potential application in controlled release of drugs. Carbohydr. Polym. 2007, 69, 538–543. [Google Scholar] [CrossRef]
- He, T.; Wang, J.; Huang, P.; Zeng, B.; Li, H.; Cao, Q.; Zhang, S.; Luo, Z.; Deng, D.Y.; Zhang, H.; et al. Electrospinning polyvinylidene fluoride fibrous membranes containing anti-bacterial drugs used as wound dressing. Colloids Surf. B Biointerfaces 2015, 130, 278–286. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Guo, H.; Lv, R.; Bai, S. Recent advances on 3D printing graphene-based composites. Nano Mater. Sci. 2019, 1, 101–115. [Google Scholar] [CrossRef]
- Li, Y.; Porwal, H.; Huang, Z.; Zhang, H.; Bilotti, E.; Peijs, T. Enhanced Thermal and Electrical Properties of Polystyrene-Graphene Nanofibers via Electrospinning. J. Nanomater. 2016, 2016, 4624976. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Maio, A.; Gallo, G. PLA graphene nanoplatelets nanocomposites: Physical properties and release kinetics of an antimicrobial agent. Compos. Part B Eng. 2016, 109, 138–146. [Google Scholar] [CrossRef]
- Meng, W.; Khayat, K.H. Effect of graphite nanoplatelets and carbon nanofibers on rheology, hydration, shrinkage, mechanical properties, and microstructure of UHPC. Cem. Concr. Res. 2018, 105, 64–71. [Google Scholar] [CrossRef]
- Enayati, M.S.; Behzad, T.; Sajkiewicz, P.; Bagheri, R.; Ghasemi-Mobarakeh, L.; Łojkowski, W.; Pahlevanneshan, Z.; Ahmadi, M. Crystallinity study of electro-spun poly (vinyl alcohol) nanofibers: Effect of electrospinning, filler incorporation, and heat treatment. Iran. Polym. J. 2016, 25, 647–659. [Google Scholar] [CrossRef]
- Sousa, A.M.; Souza, H.K.; Uknalis, J.; Liu, S.-C.; Gonçalves, M.P.; Liu, L. Electrospinning of agar/PVA aqueous solutions and its relation with rheological properties. Carbohydr. Polym. 2015, 115, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Lopresti, F.; D’Arrigo, M.; Marino, A.; Nostro, A. Efficacy of poly(lactic acid)/carvacrol electrospun membranes against Staphylococcus aureus and Candida albicans in single and mixed cultures. Appl. Microbiol. Biotechnol. 2018, 102, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Lopresti, F. Properties-morphology relationships in electrospun mats based on polylactic acid and graphene na-noplatelets. Compos. Part A Appl. Sci. Manuf. 2018, 108, 23–29. [Google Scholar] [CrossRef]
- Shen, J.; Xie, H.; Wang, Q.; Wu, X.; Yang, J.; Chen, C. Evaluation of the interaction of chlorhexidine and MDP and its effects on the durability of dentin bonding. Dent. Mater. 2020, 36, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Maio, A.; Lopresti, F. Effect of graphene and fabrication technique on the release kinetics of carvacrol from polylac-tic acid. Compos. Sci. Technol. 2018, 169, 60–69. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Morreale, M.; La Mantia, F.P. The Effects of Nanoclay on the Mechanical Properties, Carvacrol Release and Degradation of a PLA/PBAT Blend. Materials 2020, 13, 983. [Google Scholar] [CrossRef]
- El Achaby, M.; Arrakhiz, F.-E.; Vaudreuil, S.; Qaiss, A.E.K.; Bousmina, M.; Fassi-Fehri, O. Mechanical, thermal, and rheologi-cal properties of graphene-based polypropylene nanocomposites prepared by melt mixing. Polym. Compos. 2012, 33, 733–744. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Ghorbani, M.; Mohammadi, M.; Pezeshki, A. Improvement of the physico-mechanical properties of antibac-terial electrospun poly lactic acid nanofibers by incorporation of guar gum and thyme essential oil. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126659. [Google Scholar] [CrossRef]
- Ye, B.; Jia, C.; Li, Z.; Li, L.; Zhao, Q.; Wang, J.; Wu, H. Solution-blow spun PLA/SiO2 nanofiber membranes toward high efficiency oil/water separation. J. Appl. Polym. Sci. 2020, 137, 49103. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Chatterjee, P.; Davis, E.; Yu, F.; James, S.; Wildschutte, J.H.; Wiegmann, D.D.; Sherman, D.H.; McKay, R.M.; LiPuma, J.J.; Wildschutte, H. Environmental pseudomonads inhibit cystic fibro-sis patient-derived Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2017, 83, e02701-16. [Google Scholar] [CrossRef]
- Drevets, D.A.; Bronze, M.S. Listeria monocytogenes: Epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol. Med Microbiol. 2008, 53, 151–165. [Google Scholar] [CrossRef]
- Randazzo, W.; Jiménez-Belenguer, A.; Settanni, L.; Perdones, A.; Moschetti, M.; Palazzolo, E.; Guarrasi, V.; Vargas, M.; Germanà, M.A.; Moschetti, G. Antilisterial effect of citrus es-sential oils and their performance in edible film formulations. Food Control. 2016, 59, 750–758. [Google Scholar] [CrossRef]
- Nikaido, H. Prevention of Drug Access to Bacterial Targets: Permeability Barriers and Active Efflux. Science 1994, 264, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Conesa, D.; Cao, J.; Chen, L.; McLandsborough, L.; Weiss, J. Inactivation of Listeria monocytogenes and Escherichia coli O157:H7 Biofilms by Micelle-Encapsulated Eugenol and Carvacrol. J. Food Prot. 2011, 74, 55–62. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Gill, A.; Holley, R. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef]
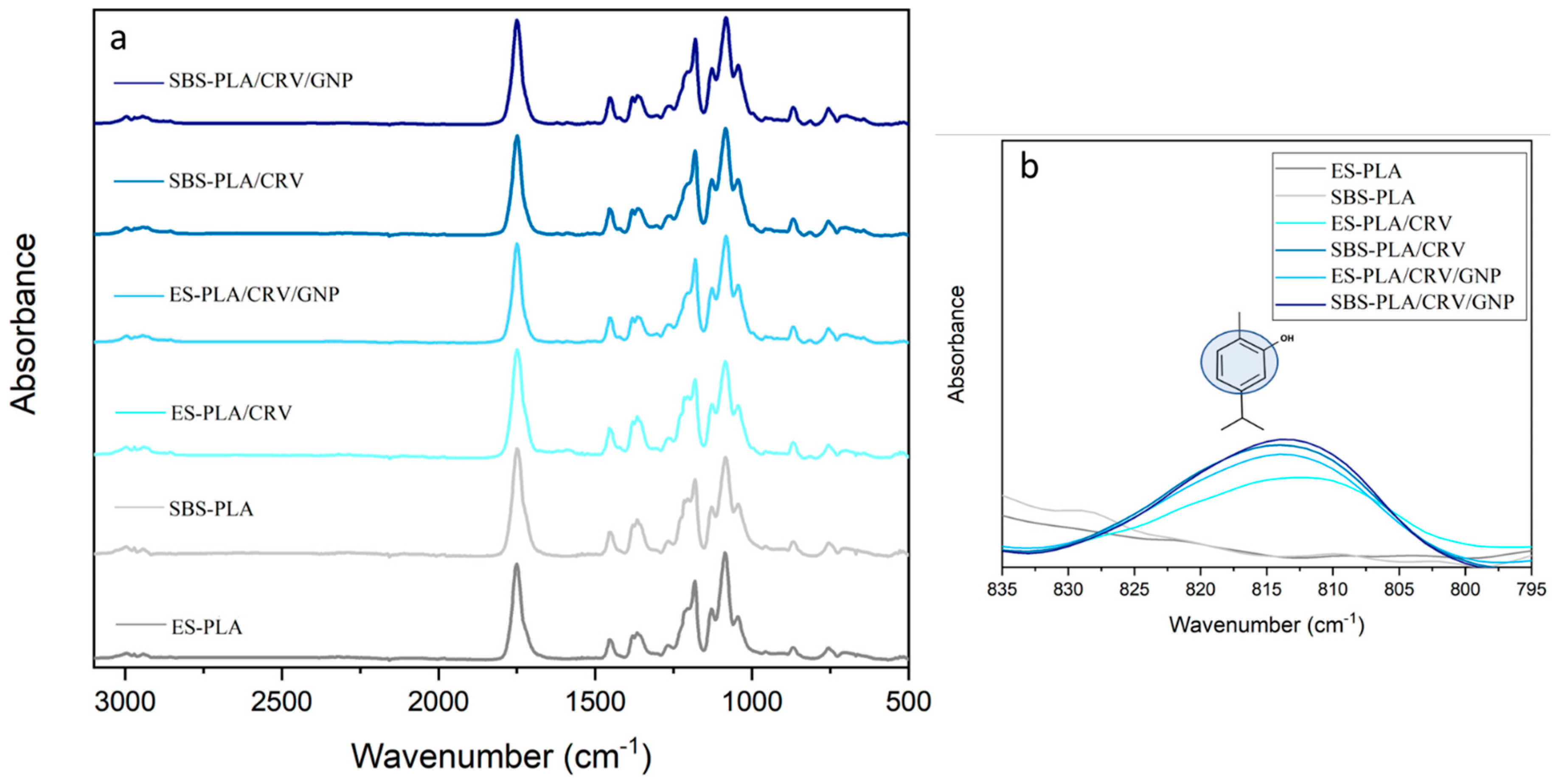
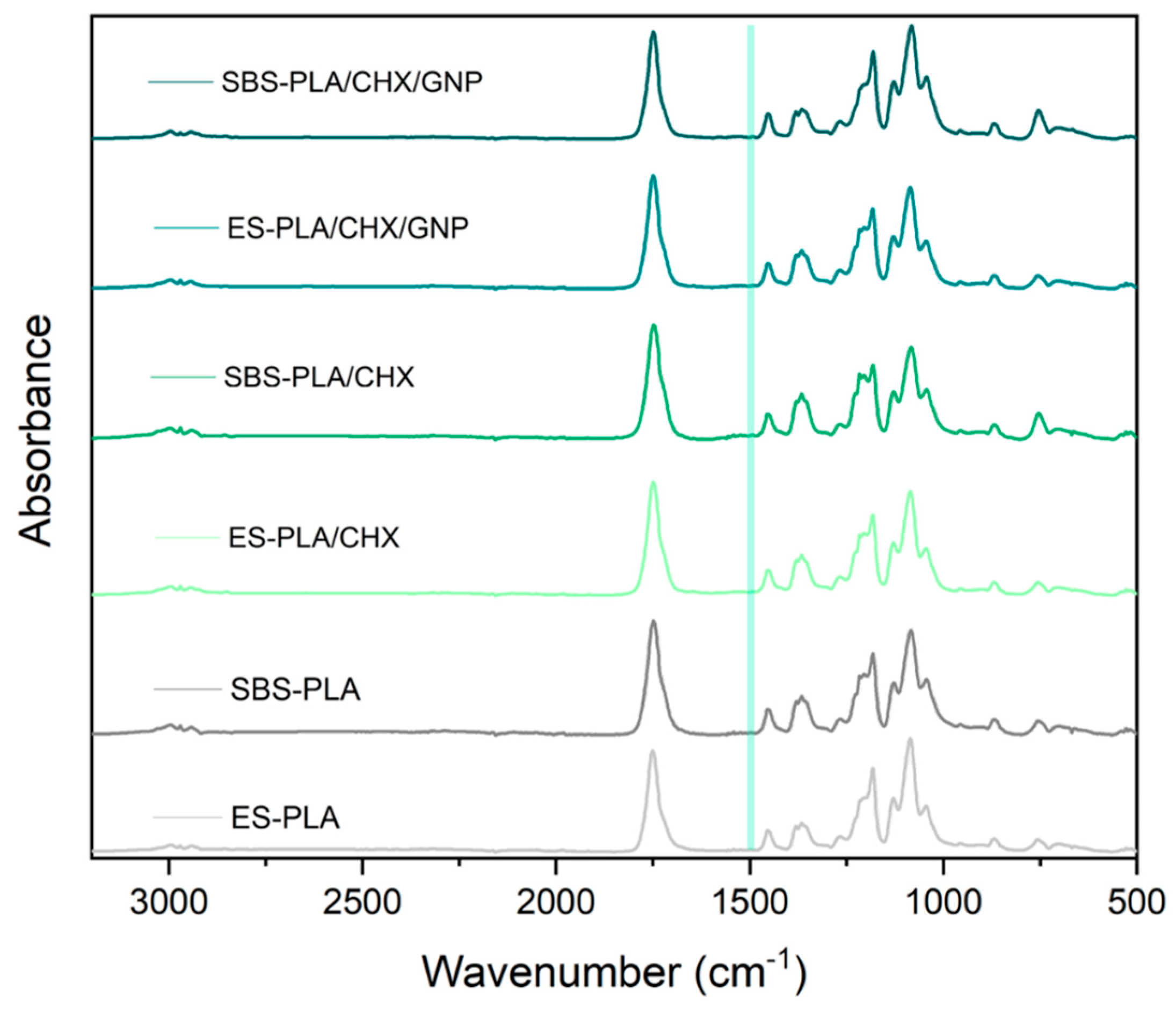
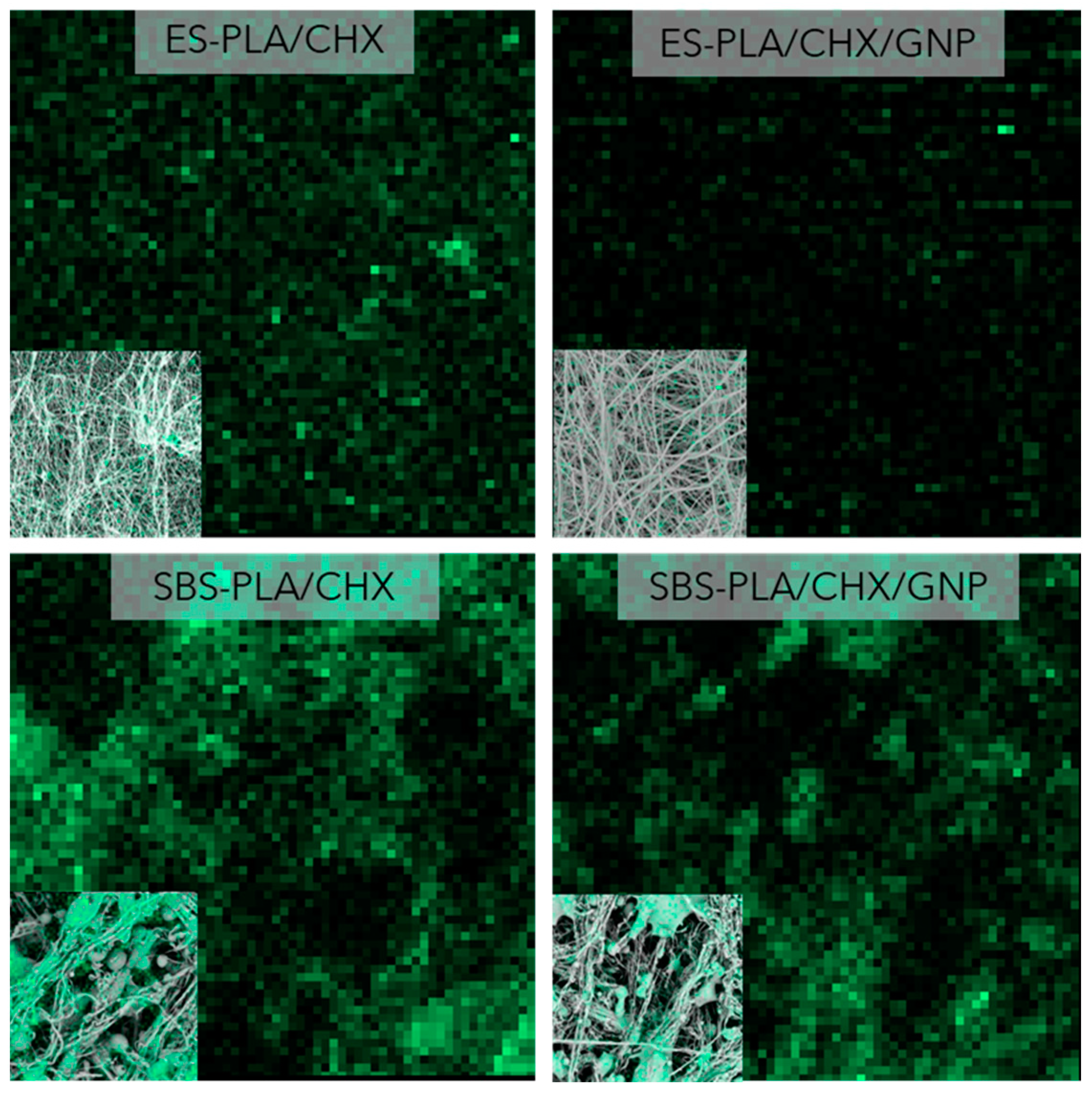


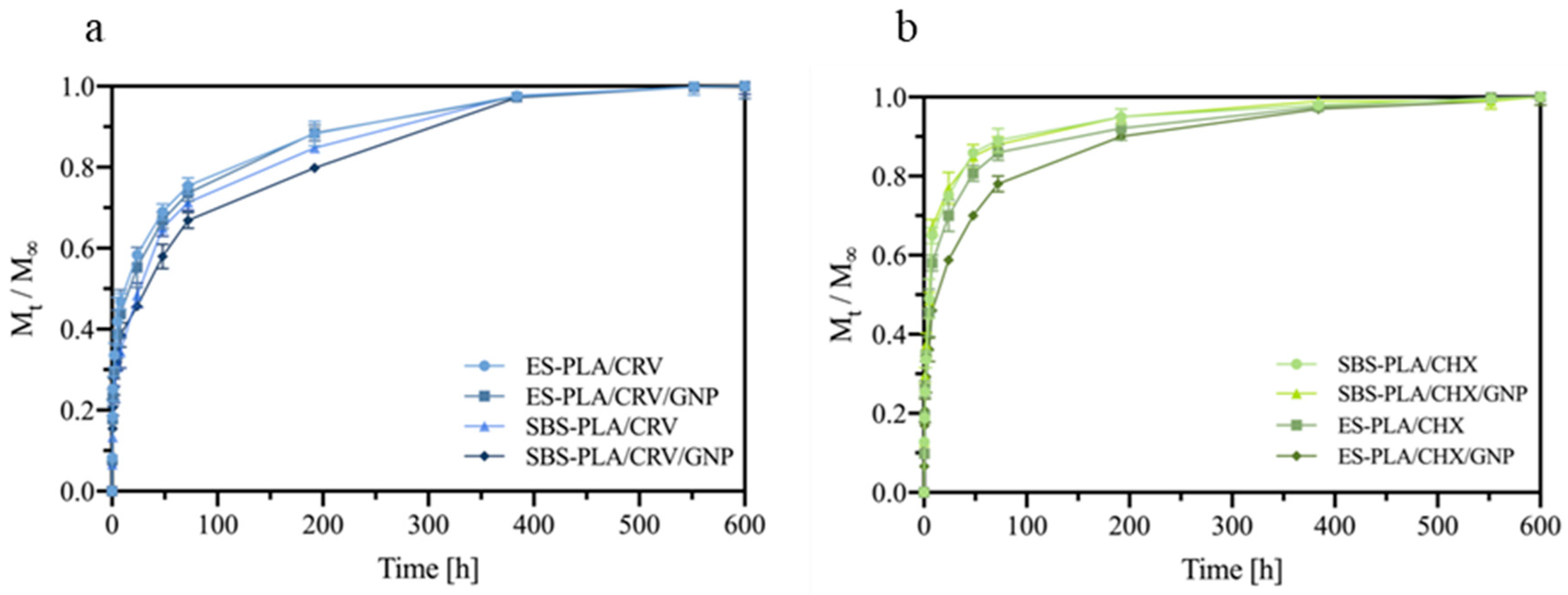
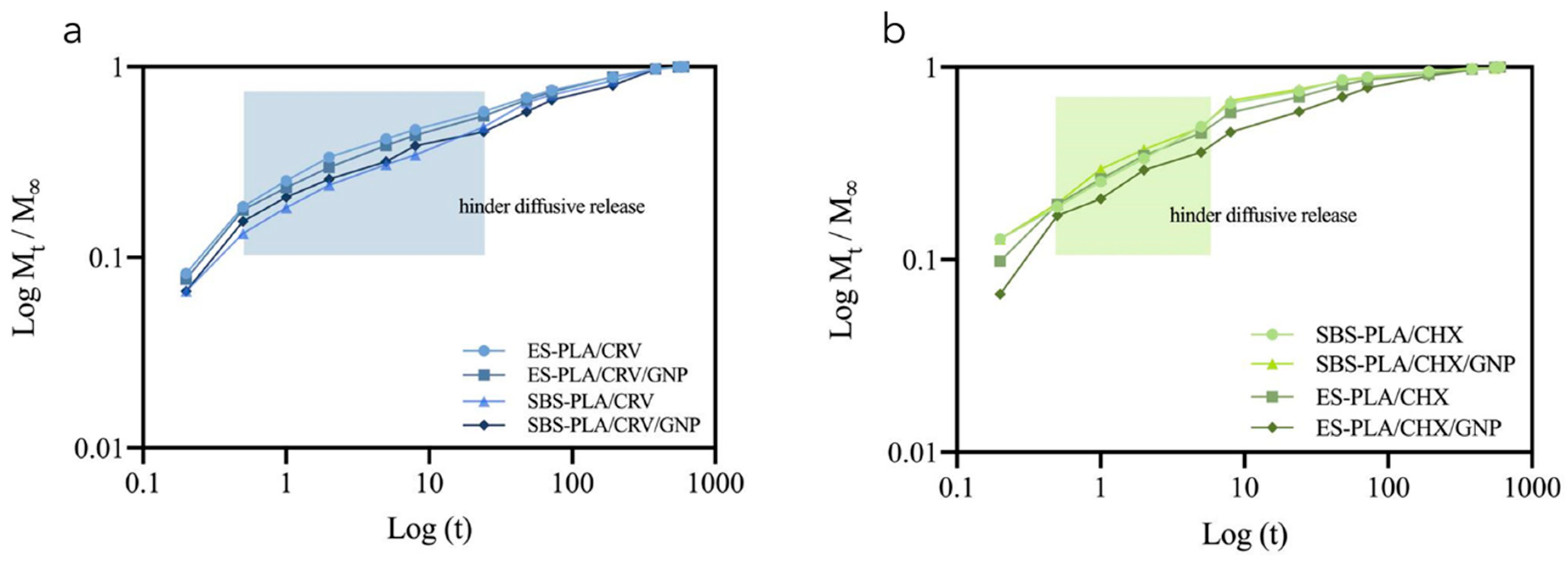
| Sample | E [MPa] | TS [MPa] | EB [%] |
|---|---|---|---|
| ES-PLA | 14.9 ± 1.2 | 2.8 ± 0.2 | 425 ± 0.5 |
| SBS-PLA | 25.8 ± 0.3 | 2.1 ± 0.1 | 21.8 ± 3.1 |
| ES-PLA/CRV | 29.69 ± 0.7 | 2.5 ± 0.3 | 845.9 ± 6 |
| SBS-PLA/CRV | 32.5 ± 0.2 | 1.72 ± 0.2 | 147.8 ± 2.3 |
| ES-PLA/CHX | 16.8 ± 0.6 | 2.3 ± 0 | 428.9 ± 12 |
| SBS-PLA/CHX | 9.7 ± 1.2 | 0.5 ± 0.5 | 35.7 ± 0.8 |
| ES-PLA/CRV/GNP | 95.6 ± 2 | 2.8 ± 0.3 | 273.7 ± 2.5 |
| SBS-PLA/CRV/GNP | 135.2 ± 2.3 | 2.9 ± 0.8 | 154.6 ± 3.1 |
| ES-PLA/CHX/GNP | 87.14 ± 2 | 2.4 ± 1 | 200 ± 12 |
| SBS-PLA/CHX/GNP | 55.8 ± 0.9 | 2 ± 0.2 | 153.63 ± 2.1 |
| Sample Code Name | k | n |
|---|---|---|
| ES-PLA/CRV | 0.25 | 0.31 |
| ES-PLA/CRV/GNP | 0.23 | 0.31 |
| SBS-PLA/CRV | 0.18 | 0.31 |
| SBS-PLA/CRV/GNP | 0.20 | 0.31 |
| ES-PLA/CHX | 0.25 | 0.41 |
| ES-PLA/CHX/GNP | 0.25 | 0.38 |
| SBS-PLA/CHX | 0.27 | 0.46 |
| SBS-PLA/CHX/GNP | 0.19 | 0.41 |
| SAMPLE CODE | TYPE (Strains) | |||
|---|---|---|---|---|
| Escherichia coli (ATCC 25922) | Pseudomonas aeruginosa (ATCC 27853) | Listeria monocytogenes (ATCC 19114) | Staphylococcus aureus (ATCC 33862) | |
| Spot (mm) | ||||
| CP | 28 | 26 | 43 | 30 |
| SBS-PLA | - | - | - | - |
| SBS-PLA/CRV | 15 | - | 12 | 8 |
| SBS-PLA/CRV/GNP | 22 | 7 | 28 | 21 |
| SBS-PLA/CHX | 10 | 7 | 14 | 11 |
| SBS-PLA/CHX/GNP | 12 | - | 10 | 14 |
| ES-PLA | - | - | - | - |
| ES-PLA/CRV | 8 | - | - | - |
| ES-PLA/CRV/GNP | 10 | - | 8 | 10 |
| ES-PLA/CHX | 7 | - | 7 | 10 |
| ES-PLA/CHX/GNP | 7 | - | 8 | 9 |
| Sample Code | Process Method | PLA (wt%) | CHX (wt%) | CRV (wt%) | GNP (wt%) |
|---|---|---|---|---|---|
| ES-PLA | Electrospinning | 10 | - | - | - |
| SBS-PLA | Solution blow spinning | 10 | - | - | - |
| ES-PLA/CRV | Electrospinning | 10 | - | 14 | - |
| SBS-PLA/CRV | Solution blow spinning | 10 | - | 14 | - |
| ES-PLA/CHX | Electrospinning | 10 | 5 | - | - |
| SBS-PLA/CHX | Solution blow spinning | 10 | 5 | - | - |
| ES-PLA/CRV/GNP | Electrospinning | 10 | - | 14 | 1 |
| SBS-PLA/CRV/GNP | Solution blow spinning | 10 | - | 14 | 1 |
| ES-PLA/CHX/GNP | Electrospinning | 10 | 5 | - | 1 |
| SBS-PLA/CHX/GNP | Solution blow spinning | 10 | 5 | - | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scaffaro, R.; Settanni, L.; Gulino, E.F. Release Profiles of Carvacrol or Chlorhexidine of PLA/Graphene Nanoplatelets Membranes Prepared Using Electrospinning and Solution Blow Spinning: A Comparative Study. Molecules 2023, 28, 1967. https://doi.org/10.3390/molecules28041967
Scaffaro R, Settanni L, Gulino EF. Release Profiles of Carvacrol or Chlorhexidine of PLA/Graphene Nanoplatelets Membranes Prepared Using Electrospinning and Solution Blow Spinning: A Comparative Study. Molecules. 2023; 28(4):1967. https://doi.org/10.3390/molecules28041967
Chicago/Turabian StyleScaffaro, Roberto, Luca Settanni, and Emmanuel Fortunato Gulino. 2023. "Release Profiles of Carvacrol or Chlorhexidine of PLA/Graphene Nanoplatelets Membranes Prepared Using Electrospinning and Solution Blow Spinning: A Comparative Study" Molecules 28, no. 4: 1967. https://doi.org/10.3390/molecules28041967
APA StyleScaffaro, R., Settanni, L., & Gulino, E. F. (2023). Release Profiles of Carvacrol or Chlorhexidine of PLA/Graphene Nanoplatelets Membranes Prepared Using Electrospinning and Solution Blow Spinning: A Comparative Study. Molecules, 28(4), 1967. https://doi.org/10.3390/molecules28041967








