mTOR Signaling Disruption and Its Association with the Development of Autism Spectrum Disorder
Abstract
1. Introduction
2. Aims and Methods
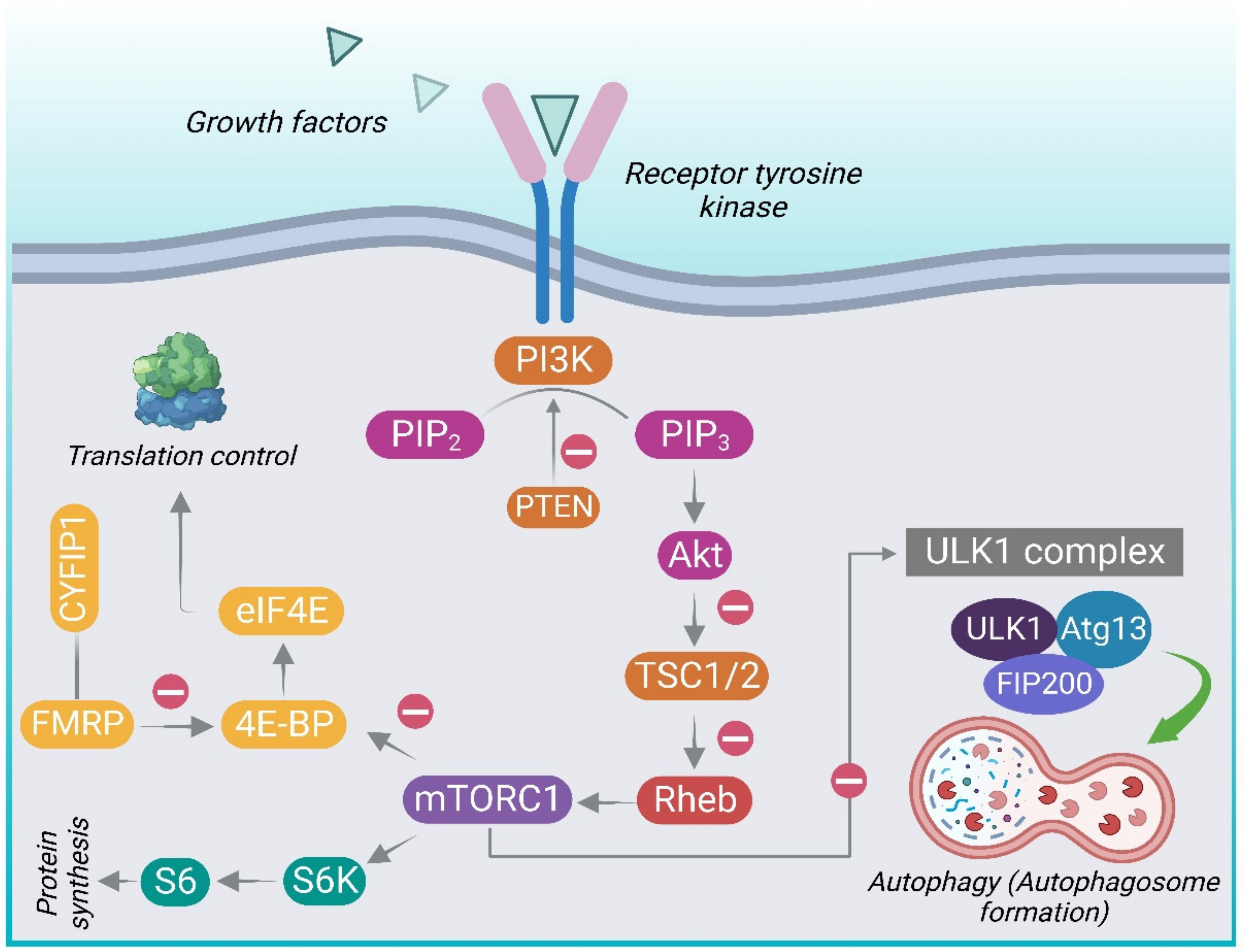
3. P13K/Akt/mTOR Pathway
3.1. The Structure and Components of mTOR
3.2. Activation of mTOR Signaling
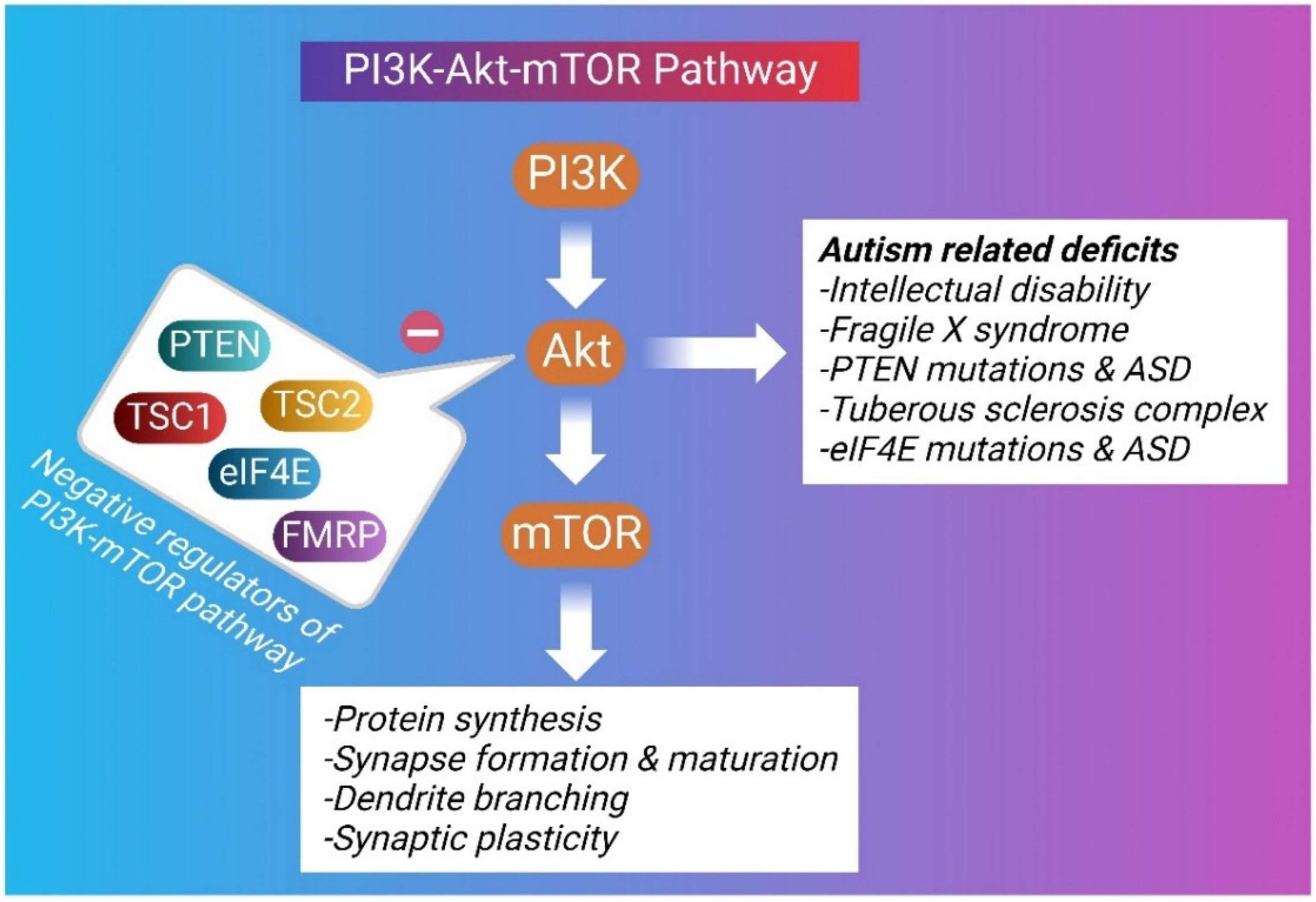
4. Role of PI3K/Akt/mTOR in Autism Spectrum Disorder
4.1. Fragile X Syndrome
4.2. PTEN in ASD
4.3. TSC in ASD
4.4. The Excitatory/Inhibitory Imbalance
4.5. Inflammatory Mechanisms in ASD
4.6. Regulation of Translational Machinery in ASD
5. Phytoconstituents in ASD
6. Discussion
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism Spectrum Disorder |
| CYFIP1 | Cytoplasmic FMRP-Interacting Protein 1 |
| eIF4E | Eukaryotic Initiation Factor 4E |
| 4E-BP | Eukaryotic Initiation Factor 4E-Binding Protein |
| FMRP | Fragile X Mental Retardation Protein |
| FXS | Fragile X Syndrome |
| KO | Knockout |
| LTD | Long-Term Depression |
| LTP | Long-Term Potentiation |
| mGluR | Metabotropic Glutamate Receptor |
| mTOR | Mammalian Target of Rapamycin |
| mTORC1 | Mammalian Target of Rapamycin Complex 1 |
| PTEN | Phosphatase and Tensin Homolog |
| Rheb | Ras Homolog Enriched in Brain |
| S6K | Ribosomal Protein S6 Kinase |
| TSC | Tuberous Sclerosis Complex |
References
- Santini, E.; Huynh, T.N.; MacAskill, A.F.; Carter, A.G.; Pierre, P.; Ruggero, D.; Kaphzan, H.; Klann, E. Exaggerated Translation Causes Synaptic and Behavioural Aberrations Associated with Autism. Nature 2013, 493, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Schmunk, G.; Gargus, J.J. Channelopathy Pathogenesis in Autism Spectrum Disorders. Front. Genet. 2013, 4, 222. [Google Scholar] [CrossRef] [PubMed]
- Ellena, G.; Battaglia, S.; Làdavas, E. The Spatial Effect of Fearful Faces in the Autonomic Response. Exp. Brain Res. 2020, 238, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial Impairment: A Common Motif in Neuropsychiatric Presentation? The Link to the Tryptophan–Kynurenine Metabolic System. Cells 2022, 11, 2607. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Vitale, F.; Battaglia, S.; Avenanti, A. Early Right Motor Cortex Response to Happy and Fearful Facial Expressions: A TMS Motor-Evoked Potential Study. Brain Sci. 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; De Rubeis, S.; Drapeau, E.; Buxbaum, J.D.; et al. Autism Spectrum Disorder: Neuropathology and Animal Models. Acta Neuropathol. 2017, 134, 537–566. [Google Scholar] [CrossRef]
- Khadem-Reza, Z.K.; Zare, H. Evaluation of Brain Structure Abnormalities in Children with Autism Spectrum Disorder (ASD) Using Structural Magnetic Resonance Imaging. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 135. [Google Scholar] [CrossRef]
- Tanaka, M.; Spekker, E.; Szabó, Á.; Polyák, H.; Vécsei, L. Modelling the Neurodevelopmental Pathogenesis in Neuropsychiatric Disorders. Bioactive Kynurenines and Their Analogues as Neuroprotective Agents—In Celebration of 80th Birthday of Professor Peter Riederer. J. Neural. Transm. 2022, 129, 627–642. [Google Scholar] [CrossRef]
- Li, Y.-J.; Zhang, X.; Li, Y.-M. Antineuroinflammatory Therapy: Potential Treatment for Autism Spectrum Disorder by Inhibiting Glial Activation and Restoring Synaptic Function. CNS Spectr. 2020, 25, 493–501. [Google Scholar] [CrossRef]
- Sharma, A.; Hoeffer, C.A.; Takayasu, Y.; Miyawaki, T.; McBride, S.M.; Klann, E.; Zukin, R.S. Dysregulation of MTOR Signaling in Fragile X Syndrome. J. Neurosci. 2010, 30, 694–702. [Google Scholar] [CrossRef]
- Luo, C.; Ye, W.-R.; Shi, W.; Yin, P.; Chen, C.; He, Y.-B.; Chen, M.-F.; Zu, X.-B.; Cai, Y. Perfect Match: MTOR Inhibitors and Tuberous Sclerosis Complex. Orphanet. J. Rare Dis. 2022, 17, 106. [Google Scholar] [CrossRef] [PubMed]
- Winden, K.D.; Ebrahimi-Fakhari, D.; Sahin, M. Abnormal MTOR Activation in Autism. Annu. Rev. Neurosci. 2018, 41, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Ikeda, K. Genetic and Environmental Contributions to Autism Spectrum Disorder Through Mechanistic Target of Rapamycin. Biol. Psychiatry Glob. Open Sci. 2022, 2, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Hashimoto, T.; Tsuda, Y.; Nakatsu, T.; Kitaoka, T.; Kyotani, S. Assessment of Oxidative Stress in Autism Spectrum Disorder Using Reactive Oxygen Metabolites and Biological Antioxidant Potential. PLoS ONE 2020, 15, e0233550. [Google Scholar] [CrossRef]
- Shuid, A.N.; Jayusman, P.A.; Shuid, N.; Ismail, J.; Kamal Nor, N.; Naina Mohamed, I. Update on Atypicalities of Central Nervous System in Autism Spectrum Disorder. Brain Sci. 2020, 10, 309. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Kim, G.H.; Tan, J.-W.; Riso, A.E.; Sun, Y.; Xu, E.Y.; Liao, G.-Y.; Xu, H.; Lee, S.-H.; Do, N.-Y.; et al. Elevated Protein Synthesis in Microglia Causes Autism-like Synaptic and Behavioral Aberrations. Nat. Commun. 2020, 11, 1797. [Google Scholar] [CrossRef]
- Zapata-Muñoz, J.; Villarejo-Zori, B.; Largo-Barrientos, P.; Boya, P. Towards a Better Understanding of the Neuro-Developmental Role of Autophagy in Sickness and in Health. Cell Stress 2021, 5, 99–118. [Google Scholar] [CrossRef]
- Marotta, R.; Risoleo, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The Neurochemistry of Autism. Brain Sci. 2020, 10, 163. [Google Scholar] [CrossRef]
- Garro-Martínez, E.; Fullana, M.N.; Florensa-Zanuy, E.; Senserrich, J.; Paz, V.; Ruiz-Bronchal, E.; Adell, A.; Castro, E.; Díaz, Á.; Pazos, Á.; et al. MTOR Knockdown in the Infralimbic Cortex Evokes A Depressive-like State in Mouse. Int. J. Mol. Sci. 2021, 22, 8671. [Google Scholar] [CrossRef]
- Singla, R.; Mishra, A.; Cao, R. The Trilateral Interactions between Mammalian Target of Rapamycin (MTOR) Signaling, the Circadian Clock, and Psychiatric Disorders: An Emerging Model. Transl. Psychiatry 2022, 12, 355. [Google Scholar] [CrossRef]
- Birdsall, V.; Waites, C.L. Autophagy at the Synapse. Neurosci. Lett. 2019, 697, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Crino, P.B. The MTOR Signalling Cascade: Paving New Roads to Cure Neurological Disease. Nat. Rev. Neurol. 2016, 12, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhou, X.; Lu, J.-H.; Yue, Z. Autophagy Deficiency in Neurodevelopmental Disorders. Cell Biosci. 2021, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Rapaka, D.; Bitra, V.R.; Challa, S.R.; Adiukwu, P.C. MTOR Signaling as a Molecular Target for the Alleviation of Alzheimer’s Disease Pathogenesis. Neurochem. Int. 2022, 155, 105311. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, C.; Iyaswamy, A.; Krishnamoorthi, S.; Sreenivasmurthy, S.G.; Liu, J.; Wang, Z.; Tong, B.C.-K.; Song, J.; Lu, J.; et al. Balancing MTOR Signaling and Autophagy in the Treatment of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 728. [Google Scholar] [CrossRef]
- Sadowski, K.; Kotulska-Jóźwiak, K.; Jóźwiak, S. Role of MTOR Inhibitors in Epilepsy Treatment. Pharmacol. Rep. 2015, 67, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Athira, K.V.; Mohan, A.S.; Chakravarty, S. Rapid Acting Antidepressants in the MTOR Pathway: Current Evidence. Brain Res. Bull. 2020, 163, 170–177. [Google Scholar] [CrossRef]
- Gao, R.; Penzes, P. Common Mechanisms of Excitatory and Inhibitory Imbalance in Schizophrenia and Autism Spectrum Disorders. Curr. Mol. Med. 2015, 15, 146–167. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Zanetti, M.V.; Teixeira, A.L.; Uno, M.; Valiengo, L.L.; Soeiro-de-Souza, M.G.; Oba-Shinjo, S.M.; de Sousa, R.T.; Zarate, C.A.; Gattaz, W.F.; et al. Decreased AKT1/MTOR Pathway MRNA Expression in Short-Term Bipolar Disorder. Eur. Neuropsychopharmacol. 2015, 25, 468–473. [Google Scholar] [CrossRef]
- Sato, A.; Kotajima-Murakami, H.; Tanaka, M.; Katoh, Y.; Ikeda, K. Influence of Prenatal Drug Exposure, Maternal Inflammation, and Parental Aging on the Development of Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 821455. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Microbiome Disturbances and Autism Spectrum Disorders. Drug Metab. Dispos. 2015, 43, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Lin, Y.-K.; Lai, J.-H.; Lo, Y.-C.; Yang, Y.-C.S.H.; Ye, S.-Y.; Lee, C.-J.; Wang, C.-C.; Chiang, Y.-H.; Tseng, S.-H. Maternal Immune Activation Causes Social Behavior Deficits and Hypomyelination in Male Rat Offspring with an Autism-Like Microbiota Profile. Brain Sci. 2021, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Abuaish, S.; Al-Otaibi, N.M.; Abujamel, T.S.; Alzahrani, S.A.; Alotaibi, S.M.; AlShawakir, Y.A.; Aabed, K.; El-Ansary, A. Fecal Transplant and Bifidobacterium Treatments Modulate Gut Clostridium Bacteria and Rescue Social Impairment and Hippocampal BDNF Expression in a Rodent Model of Autism. Brain Sci. 2021, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Liu, D.; Pathak, S.S.; Yang, B.; Li, J.; Karthikeyan, R.; Chao, O.Y.; Yang, Y.-M.; Jin, V.X.; Cao, R. Disruption of Circadian Rhythms by Ambient Light during Neurodevelopment Leads to Autistic-like Molecular and Behavioral Alterations in Adult Mice. Cells 2021, 10, 3314. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Almehmadi, M.; Abdulaziz, O.; Allahyani, M.; Alsaiari, A.A.; Halawi, M.; Alamer, E. Investigation of Antistress and Antidepressant Activities of Synthetic Curcumin Analogues: Behavioral and Biomarker Approach. Biomedicines 2022, 10, 2385. [Google Scholar] [CrossRef]
- Cruz-Martins, N.; Quispe, C.; Kırkın, C.; Şenol, E.; Zuluğ, A.; Özçelik, B.; Ademiluyi, A.O.; Oyeniran, O.H.; Semwal, P.; Kumar, M.; et al. Paving Plant-Food-Derived Bioactives as Effective Therapeutic Agents in Autism Spectrum Disorder. Oxid Med. Cell Longev. 2021, 2021, 1131280. [Google Scholar] [CrossRef]
- Latacz, A.; Russell, J.A.; Oc, E.; Zubel, J.; Pierzcha, K. Review MTOR Pathway–Novel Modulator of Astrocyte Activity. Folia Biol. 2015, 63, 95–105. [Google Scholar] [CrossRef]
- Ryskalin, L.; Limanaqi, F.; Frati, A.; Busceti, C.; Fornai, F. MTOR-Related Brain Dysfunctions in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2018, 19, 2226. [Google Scholar] [CrossRef]
- Cai, Z.; Yan, L.-J. Rapamycin, Autophagy, and Alzheimer’s Disease. J. Biochem. Pharmacol. Res. 2013, 12, 84. [Google Scholar]
- Cai, Z.; Zhao, B.; Li, K.; Zhang, L.; Li, C.; Quazi, S.H.; Tan, Y. Mammalian Target of Rapamycin: A Valid Therapeutic Target through the Autophagy Pathway for Alzheimer’s Disease? J. Neurosci. Res. 2012, 90, 1105–1118. [Google Scholar] [CrossRef]
- Friedman, L.G.; Qureshi, Y.H.; Yu, W.H. Promoting Autophagic Clearance: Viable Therapeutic Targets in Alzheimer’s Disease. Neurotherapeutics 2015, 12, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Moving to the Rhythm with Clock (Circadian) Genes, Autophagy, MTOR, and SIRT1 in Degenerative Disease and Cancer. Curr. Neurovascular Res. 2017, 14, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Taking Aim at Alzheimer’s Disease through the Mammalian Target of Rapamycin. Ann. Med. 2014, 46, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Bockaert, J.; Marin, P. MTOR in Brain Physiology and Pathologies. Physiol. Rev. 2015, 95, 1157–1187. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Guan, K.-L. MTOR: A Pharmacologic Target for Autophagy Regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Lee, H.-K.; Kwon, B.; Lemere, C.A.; de la Monte, S.; Itamura, K.; Ha, A.Y.; Querfurth, H.W. MTORC2 (Rictor) in Alzheimer’s Disease and Reversal of Amyloid-β Expression-Induced Insulin Resistance and Toxicity in Rat Primary Cortical Neurons. J. Alzheimer’s Dis. 2017, 56, 1015–1036. [Google Scholar] [CrossRef]
- Kuang, H.; Tan, C.; Tian, H.; Liu, L.; Yang, M.; Hong, F.; Yang, S. Exploring the Bi-directional Relationship between Autophagy and Alzheimer’s Disease. CNS Neurosci. Ther. 2019, 26, 155–166. [Google Scholar] [CrossRef]
- Cai, Z.; Zhou, Y.; Xiao, M.; Yan, L.-J.; He, W. Activation of MTOR: A Culprit of Alzheimer’s Disease? Neuropsychiatr. Dis. Treat. 2015, 2015, 1015–1030. [Google Scholar] [CrossRef]
- Yang, H.; Rudge, D.G.; Koos, J.D.; Vaidialingam, B.; Yang, H.J.; Pavletich, N.P. MTOR Kinase Structure, Mechanism and Regulation. Nature 2013, 497, 217–223. [Google Scholar] [CrossRef]
- Galvan, V.; Hart, M.J. Vascular MTOR-Dependent Mechanisms Linking the Control of Aging to Alzheimer’s Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 992–1007. [Google Scholar] [CrossRef]
- Singh, A.K.; Kashyap, M.P.; Tripathi, V.K.; Singh, S.; Garg, G.; Rizvi, S.I. Neuroprotection Through Rapamycin-Induced Activation of Autophagy and PI3K/Akt1/MTOR/CREB Signaling Against Amyloid-β-Induced Oxidative Stress, Synaptic/Neurotransmission Dysfunction, and Neurodegeneration in Adult Rats. Mol. Neurobiol. 2017, 54, 5815–5828. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cheng, J.; North, B.J.; Wei, W. Functional Analyses of Major Cancer-Related Signaling Pathways in Alzheimer’s Disease Etiology. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Di Domenico, F.; Barone, E.; Butterfield, D.A. MTOR in Alzheimer Disease and Its Earlier Stages: Links to Oxidative Damage in the Progression of This Dementing Disorder. Free. Radic. Biol. Med. 2021, 169, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Chen, D.; Chen, N. Physical Activity Alleviates Cognitive Dysfunction of Alzheimer’s Disease through Regulating the MTOR Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 1591. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yan, L.-J.; Li, K.; Quazi, S.H.; Zhao, B. Roles of AMP-Activated Protein Kinase in Alzheimer’s Disease. Neuromol. Med. 2012, 14, 1–14. [Google Scholar] [CrossRef]
- Maiese, K. Driving Neural Regeneration through the Mammalian Target of Rapamycin. Neural. Regen Res. 2014, 9, 1413. [Google Scholar] [CrossRef]
- Weinberg, M.A. RES-529: A PI3K/AKT/MTOR Pathway Inhibitor That Dissociates the MTORC1 and MTORC2 Complexes. Anti-Cancer Drugs 2016, 27, 475–487. [Google Scholar] [CrossRef]
- Pourtalebi Jahromi, L.; Sasanipour, Z.; Azadi, A. Promising Horizon to Alleviate Alzeheimer’s Disease Pathological Hallmarks via Inhibiting MTOR Signaling Pathway: A New Application for a Commonplace Analgesic. Med. Hypotheses 2018, 110, 120–124. [Google Scholar] [CrossRef]
- Popova, N.V.; Jücker, M. The Role of MTOR Signaling as a Therapeutic Target in Cancer. Int. J. Mol. Sci. 2021, 22, 1743. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative Stress and Autophagy: The Clash between Damage and Metabolic Needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Chandran, A.; Iyo, A.H.; Jernigan, C.S.; Legutko, B.; Austin, M.C.; Karolewicz, B. Reduced Phosphorylation of the MTOR Signaling Pathway Components in the Amygdala of Rats Exposed to Chronic Stress. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 40, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Cordero, J.G.; García-Escudero, R.; Avila, J.; Gargini, R.; García-Escudero, V. Benefit of Oleuropein Aglycone for Alzheimer’s Disease by Promoting Autophagy. Oxidative Med. Cell. Longev. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, M.; Ahmadi, M.; Afshar, S.; Lorzadeh, S.; Adlimoghaddam, A.; Rezvani Jalal, N.; West, R.; Dastghaib, S.; Igder, S.; Torshizi, S.R.N.; et al. Enhancing Autophagy in Alzheimer’s Disease through Drug Repositioning. Pharmacol. Ther. 2022, 237, 108171. [Google Scholar] [CrossRef] [PubMed]
- Festa, B.P.; Barbosa, A.D.; Rob, M.; Rubinsztein, D.C. The Pleiotropic Roles of Autophagy in Alzheimer’s Disease: From Pathophysiology to Therapy. Curr. Opin. Pharmacol. 2021, 60, 149–157. [Google Scholar] [CrossRef]
- Mitjans, M.; Begemann, M.; Ju, A.; Dere, E.; Wüstefeld, L.; Hofer, S.; Hassouna, I.; Balkenhol, J.; Oliveira, B.; van der Auwera, S.; et al. Sexual Dimorphism of AMBRA1-Related Autistic Features in Human and Mouse. Transl. Psychiatry 2017, 7, e1247. [Google Scholar] [CrossRef]
- Chen, J. Dysregulation of the IGF-I/PI3K/AKT/MTOR Signaling Pathway in Autism Spectrum Disorders. Int. J. Dev. Neurosci. 2014, 35, 35–41. [Google Scholar] [CrossRef]
- Chaudry, S.; Vasudevan, N. MTOR-Dependent Spine Dynamics in Autism. Front. Mol. Neurosci. 2022, 15, 877609. [Google Scholar] [CrossRef]
- Kassai, H.; Sugaya, Y.; Noda, S.; Nakao, K.; Maeda, T.; Kano, M.; Aiba, A. Selective Activation of MTORC1 Signaling Recapitulates Microcephaly, Tuberous Sclerosis, and Neurodegenerative Diseases. Cell Rep. 2014, 7, 1626–1639. [Google Scholar] [CrossRef]
- Opazo, P.; Watabe, A.M.; Grant, S.G.N.; O’Dell, T.J. Phosphatidylinositol 3-Kinase Regulates the Induction of Long-Term Potentiation through Extracellular Signal-Related Kinase-Independent Mechanisms. J. Neurosci. 2003, 23, 3679–3688. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.-X.; Zhang, Q.-L. PI3K/AKT/MTOR-Mediated Autophagy in the Development of Autism Spectrum Disorder. Brain Res. Bull. 2016, 125, 152–158. [Google Scholar] [CrossRef]
- Lieberman, O.J.; Cartocci, V.; Pigulevskiy, I.; Molinari, M.; Carbonell, J.; Broseta, M.B.; Post, M.R.; Sulzer, D.; Borgkvist, A.; Santini, E. MTOR Suppresses Macroautophagy During Striatal Postnatal Development and Is Hyperactive in Mouse Models of Autism Spectrum Disorders. Front. Cell Neurosci. 2020, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Onore, C.; Yang, H.; Van de Water, J.; Ashwood, P. Dynamic Akt/MTOR Signaling in Children with Autism Spectrum Disorder. Front. Pediatr. 2017, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Rosina, E.; Battan, B.; Siracusano, M.; Di Criscio, L.; Hollis, F.; Pacini, L.; Curatolo, P.; Bagni, C. Disruption of MTOR and MAPK Pathways Correlates with Severity in Idiopathic Autism. Transl. Psychiatry 2019, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.; Xiong, Y.; Deng, J.; Lü, M.; Tang, B.; Zhang, X.; Li, Y. [Mechanism of valproic acid-induced dendritic spine and synaptic impairment in the prefrontal cortex for causing core autistic symptoms in mice. Nan Fang Yi Ke Da Xue Xue Bao 2022, 42, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L.; et al. Fragile X Syndrome. Nat. Rev. Dis. Primers 2017, 3, 17065. [Google Scholar] [CrossRef]
- Casingal, C.R.; Kikkawa, T.; Inada, H.; Sasaki, Y.; Osumi, N. Identification of FMRP Target MRNAs in the Developmental Brain: FMRP Might Coordinate Ras/MAPK, Wnt/β-Catenin, and MTOR Signaling during Corticogenesis. Mol. Brain 2020, 13, 167. [Google Scholar] [CrossRef]
- Sato, A. MTOR, a Potential Target to Treat Autism Spectrum Disorder. CNS Neurol. Disord. Drug Targets 2016, 15, 533–543. [Google Scholar] [CrossRef]
- Kazdoba, T.M.; Leach, P.T.; Silverman, J.L.; Crawley, J.N. Modeling Fragile X Syndrome in the Fmr1 Knockout Mouse. Intractable Rare Dis. Res. 2014, 3, 118–133. [Google Scholar] [CrossRef]
- Grossman, A.W.; Elisseou, N.M.; McKinney, B.C.; Greenough, W.T. Hippocampal Pyramidal Cells in Adult Fmr1 Knockout Mice Exhibit an Immature-Appearing Profile of Dendritic Spines. Brain Res. 2006, 1084, 158–164. [Google Scholar] [CrossRef]
- Nisar, S.; Bhat, A.A.; Masoodi, T.; Hashem, S.; Akhtar, S.; Ali, T.A.; Amjad, S.; Chawla, S.; Bagga, P.; Frenneaux, M.P.; et al. Genetics of Glutamate and Its Receptors in Autism Spectrum Disorder. Mol. Psychiatry 2022, 27, 2380–2392. [Google Scholar] [CrossRef]
- Huber, K.M.; Gallagher, S.M.; Warren, S.T.; Bear, M.F. Altered Synaptic Plasticity in a Mouse Model of Fragile X Mental Retardation. Proc. Natl. Acad. Sci. USA 2002, 99, 7746–7750. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Bui, N.; Perkins, J.R.; Yuva-Paylor, L.A.; Paylor, R. Group I Metabotropic Glutamate Receptor Antagonists Alter Select Behaviors in a Mouse Model for Fragile X Syndrome. Psychopharmacology 2012, 219, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Saré, R.M.; Song, A.; Loutaev, I.; Cook, A.; Maita, I.; Lemons, A.; Sheeler, C.; Smith, C.B. Negative Effects of Chronic Rapamycin Treatment on Behavior in a Mouse Model of Fragile X Syndrome. Front. Mol. Neurosci. 2018, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Hoeffer, C.A.; Sanchez, E.; Hagerman, R.J.; Mu, Y.; Nguyen, D.V.; Wong, H.; Whelan, A.M.; Zukin, R.S.; Klann, E.; Tassone, F. Altered MTOR Signaling and Enhanced CYFIP2 Expression Levels in Subjects with Fragile X Syndrome. Genes Brain Behav. 2012, 11, 332–341. [Google Scholar] [CrossRef]
- Gross, C.; Nakamoto, M.; Yao, X.; Chan, C.-B.; Yim, S.Y.; Ye, K.; Warren, S.T.; Bassell, G.J. Excess Phosphoinositide 3-Kinase Subunit Synthesis and Activity as a Novel Therapeutic Target in Fragile X Syndrome. J. Neurosci. 2010, 30, 10624–10638. [Google Scholar] [CrossRef] [PubMed]
- Gantois, I.; Khoutorsky, A.; Popic, J.; Aguilar-Valles, A.; Freemantle, E.; Cao, R.; Sharma, V.; Pooters, T.; Nagpal, A.; Skalecka, A.; et al. Metformin Ameliorates Core Deficits in a Mouse Model of Fragile X Syndrome. Nat. Med. 2017, 23, 674–677. [Google Scholar] [CrossRef]
- Qin, M.; Kang, J.; Burlin, T.V.; Jiang, C.; Smith, C.B. Postadolescent Changes in Regional Cerebral Protein Synthesis: An In Vivo Study in the Fmr1 Null Mouse. J. Neurosci. 2005, 25, 5087–5095. [Google Scholar] [CrossRef]
- Napoli, I.; Mercaldo, V.; Boyl, P.P.; Eleuteri, B.; Zalfa, F.; De Rubeis, S.; Di Marino, D.; Mohr, E.; Massimi, M.; Falconi, M.; et al. The Fragile X Syndrome Protein Represses Activity-Dependent Translation through CYFIP1, a New 4E-BP. Cell 2008, 134, 1042–1054. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Kaphzan, H.; Alvarez-Dieppa, A.C.; Murphy, J.P.; Pierre, P.; Klann, E. Genetic Removal of P70 S6 Kinase 1 Corrects Molecular, Synaptic, and Behavioral Phenotypes in Fragile X Syndrome Mice. Neuron 2012, 76, 325–337. [Google Scholar] [CrossRef]
- Yan, J.; Porch, M.W.; Court-Vazquez, B.; Bennett, M.V.L.; Zukin, R.S. Activation of Autophagy Rescues Synaptic and Cognitive Deficits in Fragile X Mice. Proc. Natl. Acad. Sci. USA 2018, 115, E9707–E9716. [Google Scholar] [CrossRef]
- Lugo, J.N.; Smith, G.D.; Arbuckle, E.P.; White, J.; Holley, A.J.; Floruta, C.M.; Ahmed, N.; Gomez, M.C.; Okonkwo, O. Deletion of PTEN Produces Autism-like Behavioral Deficits and Alterations in Synaptic Proteins. Front. Mol. Neurosci. 2014, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Parada, L.F. PTEN Signaling in Autism Spectrum Disorders. Curr. Opin. Neurobiol. 2012, 22, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.-H.; Luikart, B.W.; Powell, C.M.; Zhou, J.; Matheny, S.A.; Zhang, W.; Li, Y.; Baker, S.J.; Parada, L.F. Pten Regulates Neuronal Arborization and Social Interaction in Mice. Neuron 2006, 50, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.S.; Tso, W.W.Y.; Ip, J.J.K.; Mak, C.C.Y.; Leung, G.K.C.; Tsang, M.H.Y.; Ying, D.; Pei, S.L.C.; Lee, S.L.; Yang, W.; et al. Identification of Mutations in the PI3K-AKT-MTOR Signalling Pathway in Patients with Macrocephaly and Developmental Delay and/or Autism. Mol. Autism. 2017, 8, 66. [Google Scholar] [CrossRef]
- Busch, R.M.; Srivastava, S.; Hogue, O.; Frazier, T.W.; Klaas, P.; Hardan, A.; Martinez-Agosto, J.A.; Sahin, M.; Eng, C. Neurobehavioral Phenotype of Autism Spectrum Disorder Associated with Germline Heterozygous Mutations in PTEN. Transl. Psychiatry 2019, 9, 253. [Google Scholar] [CrossRef]
- Clipperton-Allen, A.E.; Page, D.T. Pten Haploinsufficient Mice Show Broad Brain Overgrowth but Selective Impairments in Autism-Relevant Behavioral Tests. Hum. Mol. Genet. 2014, 23, 3490–3505. [Google Scholar] [CrossRef]
- Tai, C.; Chang, C.-W.; Yu, G.-Q.; Lopez, I.; Yu, X.; Wang, X.; Guo, W.; Mucke, L. Tau Reduction Prevents Key Features of Autism in Mouse Models. Neuron 2020, 106, 421–437.e11. [Google Scholar] [CrossRef]
- Zhou, J.; Blundell, J.; Ogawa, S.; Kwon, C.-H.; Zhang, W.; Sinton, C.; Powell, C.M.; Parada, L.F. Pharmacological Inhibition of MTORC1 Suppresses Anatomical, Cellular, and Behavioral Abnormalities in Neural-Specific Pten Knock-Out Mice. J. Neurosci. 2009, 29, 1773–1783. [Google Scholar] [CrossRef]
- Varga, E.A.; Pastore, M.; Prior, T.; Herman, G.E.; McBride, K.L. The Prevalence of PTEN Mutations in a Clinical Pediatric Cohort with Autism Spectrum Disorders, Developmental Delay, and Macrocephaly. Genet. Med. 2009, 11, 111–117. [Google Scholar] [CrossRef]
- Skelton, P.D.; Stan, R.V.; Luikart, B.W. The Role of PTEN in Neurodevelopment. Complex Psychiatry 2019, 5, 60–71. [Google Scholar] [CrossRef]
- Frazier, T.W.; Embacher, R.; Tilot, A.K.; Koenig, K.; Mester, J.; Eng, C. Molecular and Phenotypic Abnormalities in Individuals with Germline Heterozygous PTEN Mutations and Autism. Mol. Psychiatry 2015, 20, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-Y.; Liu, F.-C. Molecular Pathology and Pharmacological Treatment of Autism Spectrum Disorder-Like Phenotypes Using Rodent Models. Front. Cell Neurosci. 2018, 12, 422. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, Y.; Xu, J.; Hu, X.; Gu, L.; Li, X.; Hu, P.; Liao, T.; Xia, Q.; Sun, Q.; et al. Neuroligin 3 Regulates Dendritic Outgrowth by Modulating Akt/MTOR Signaling. Front. Cell Neurosci. 2019, 13, 518. [Google Scholar] [CrossRef] [PubMed]
- Potter, W.B.; Basu, T.; O’Riordan, K.J.; Kirchner, A.; Rutecki, P.; Burger, C.; Roopra, A. Reduced Juvenile Long-Term Depression in Tuberous Sclerosis Complex Is Mitigated in Adults by Compensatory Recruitment of MGluR5 and Erk Signaling. PLoS Biol. 2013, 11, e1001627. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, P.; Moavero, R. MTOR Inhibitors in Tuberous Sclerosis Complex. Curr. Neuropharmacol. 2012, 10, 404–415. [Google Scholar] [CrossRef]
- Lee, K.-M.; Hwang, S.-K.; Lee, J.-A. Neuronal Autophagy and Neurodevelopmental Disorders. Exp. Neurobiol. 2013, 22, 133–142. [Google Scholar] [CrossRef]
- Caglayan, A.O. Genetic Causes of Syndromic and Non-Syndromic Autism. Dev. Med. Child Neurol. 2010, 52, 130–138. [Google Scholar] [CrossRef]
- Goorden, S.M.I.; van Woerden, G.M.; van der Weerd, L.; Cheadle, J.P.; Elgersma, Y. Cognitive Deficits in Tsc1+/− Mice in the Absence of Cerebral Lesions and Seizures. Ann. Neurol. 2007, 62, 648–655. [Google Scholar] [CrossRef]
- Tsai, P.T.; Hull, C.; Chu, Y.; Greene-Colozzi, E.; Sadowski, A.R.; Leech, J.M.; Steinberg, J.; Crawley, J.N.; Regehr, W.G.; Sahin, M. Autistic-like Behavior and Cerebellar Dysfunction in Purkinje Cell Tsc1 Mutant Mice. Nature 2012, 488, 647–651. [Google Scholar] [CrossRef]
- Sato, A.; Kasai, S.; Kobayashi, T.; Takamatsu, Y.; Hino, O.; Ikeda, K.; Mizuguchi, M. Rapamycin Reverses Impaired Social Interaction in Mouse Models of Tuberous Sclerosis Complex. Nat. Commun. 2012, 3, 1292. [Google Scholar] [CrossRef]
- Tang, G.; Gudsnuk, K.; Kuo, S.-H.; Cotrina, M.L.; Rosoklija, G.; Sosunov, A.; Sonders, M.S.; Kanter, E.; Castagna, C.; Yamamoto, A.; et al. Loss of MTOR-Dependent Macroautophagy Causes Autistic-like Synaptic Pruning Deficits. Neuron 2014, 83, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Reith, R.M.; McKenna, J.; Wu, H.; Hashmi, S.S.; Cho, S.-H.; Dash, P.K.; Gambello, M.J. Loss of Tsc2 in Purkinje Cells Is Associated with Autistic-like Behavior in a Mouse Model of Tuberous Sclerosis Complex. Neurobiol. Dis. 2013, 51, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.; Barsotti, N.; Bertero, A.; Trakoshis, S.; Ulysse, L.; Locarno, A.; Miseviciute, I.; De Felice, A.; Canella, C.; Supekar, K.; et al. MTOR-Related Synaptic Pathology Causes Autism Spectrum Disorder-Associated Functional Hyperconnectivity. Nat. Commun. 2021, 12, 6084. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Wagh, V.; Reis, S.A.; Erdin, S.; Beauchamp, R.L.; Shaikh, G.; Talkowski, M.; Thiele, E.; Sheridan, S.D.; Haggarty, S.J.; et al. TSC Patient-Derived Isogenic Neural Progenitor Cells Reveal Altered Early Neurodevelopmental Phenotypes and Rapamycin-Induced MNK-EIF4E Signaling. Mol. Autism. 2020, 11, 2. [Google Scholar] [CrossRef]
- Ehninger, D.; Silva, A.J. Rapamycin for Treating Tuberous Sclerosis and Autism Spectrum Disorders. Trends Mol. Med. 2011, 17, 78–87. [Google Scholar] [CrossRef]
- Hui, K.K.; Tanaka, M. Autophagy Links MTOR and GABA Signaling in the Brain. Autophagy 2019, 15, 1848–1849. [Google Scholar] [CrossRef]
- Antoine, M.W.; Langberg, T.; Schnepel, P.; Feldman, D.E. Increased Excitation-Inhibition Ratio Stabilizes Synapse and Circuit Excitability in Four Autism Mouse Models. Neuron 2019, 101, 648–661.e4. [Google Scholar] [CrossRef]
- Nelson, S.B.; Valakh, V. Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron 2015, 87, 684–698. [Google Scholar] [CrossRef]
- Jiang, C.-C.; Lin, L.-S.; Long, S.; Ke, X.-Y.; Fukunaga, K.; Lu, Y.-M.; Han, F. Signalling Pathways in Autism Spectrum Disorder: Mechanisms and Therapeutic Implications. Sig. Transduct. Target Ther. 2022, 7, 229. [Google Scholar] [CrossRef]
- Reichelt, A.C.; Rodgers, R.J.; Clapcote, S.J. The Role of Neurexins in Schizophrenia and Autistic Spectrum Disorder. Neuropharmacology 2012, 62, 1519–1526. [Google Scholar] [CrossRef]
- Mehta, M.V.; Gandal, M.J.; Siegel, S.J. MGluR5-Antagonist Mediated Reversal of Elevated Stereotyped, Repetitive Behaviors in the VPA Model of Autism. PLoS ONE 2011, 6, e26077. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Y.; Chen, W.-J.; Huang, Z.-P.; Yang, G.; Wu, M.-L.; Xu, D.-E.; Yang, W.-L.; Luo, Y.-C.; Xiao, Z.-C.; Xu, R.-X.; et al. TRIM32 Deficiency Impairs the Generation of Pyramidal Neurons in Developing Cerebral Cortex. Cells 2022, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Lionel, A.C.; Tammimies, K.; Vaags, A.K.; Rosenfeld, J.A.; Ahn, J.W.; Merico, D.; Noor, A.; Runke, C.K.; Pillalamarri, V.K.; Carter, M.T.; et al. Disruption of the ASTN2/TRIM32 Locus at 9q33.1 Is a Risk Factor in Males for Autism Spectrum Disorders, ADHD and Other Neurodevelopmental Phenotypes. Hum. Mol. Genet. 2014, 23, 2752–2768. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-W.; Zou, M.-M.; Li, Y.-F.; Chen, W.-J.; Liu, J.-C.; Chen, H.; Fang, L.-P.; Zhang, Y.; Wang, Z.-T.; Chen, J.-B.; et al. Absence of TRIM32 Leads to Reduced GABAergic Interneuron Generation and Autism-like Behaviors in Mice via Suppressing MTOR Signaling. Cerebral. Cortex 2020, 30, 3240–3258. [Google Scholar] [CrossRef]
- Hui, K.; Takashima, N.; Watanabe, A.; Chater, T.; Matsukawa, H.; Nekooki-Machida, Y.; Nilsson, P.; Endo, R.; Goda, Y.; Saido, T.; et al. GABARAPs Dysfunction by Autophagy Deficiency in Adolescent Brain Impairs GABA A Receptor Trafficking and Social Behavior. Sci. Adv. 2019, 5, eaau8237. [Google Scholar] [CrossRef]
- Bjorklund, G.; Saad, K.; Chirumbolo, S.; Kern, J.K.; Geier, D.A.; Geier, M.R.; Urbina, M.A. Immune Dysfunction and Neuroinflammation in Autism Spectrum Disorder. Acta Neurobiol. Exp. 2016, 76, 257–268. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Patel, A.B.; Doyle, R. Atopic Diseases and Inflammation of the Brain in the Pathogenesis of Autism Spectrum Disorders. Transl. Psychiatry 2016, 6, e844. [Google Scholar] [CrossRef]
- Cianciulli, A.; Porro, C.; Calvello, R.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Microglia Mediated Neuroinflammation: Focus on PI3K Modulation. Biomolecules 2020, 10, 137. [Google Scholar] [CrossRef]
- London, A.; Cohen, M.; Schwartz, M. Microglia and Monocyte-Derived Macrophages: Functionally Distinct Populations That Act in Concert in CNS Plasticity and Repair. Front. Cell Neurosci. 2013, 7, 34. [Google Scholar] [CrossRef]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The Influence of Neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef]
- Wang, B.; Qin, Y.; Wu, Q.; Li, X.; Xie, D.; Zhao, Z.; Duan, S. MTOR Signaling Pathway Regulates the Release of Proinflammatory Molecule CCL5 Implicated in the Pathogenesis of Autism Spectrum Disorder. Front. Immunol. 2022, 13, 818518. [Google Scholar] [CrossRef] [PubMed]
- Onore, C.; Careaga, M.; Ashwood, P. The Role of Immune Dysfunction in the Pathophysiology of Autism. Brain Behav. Immun. 2012, 26, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Gkogkas, C.G.; Khoutorsky, A.; Ran, I.; Rampakakis, E.; Nevarko, T.; Weatherill, D.B.; Vasuta, C.; Yee, S.; Truitt, M.; Dallaire, P.; et al. Autism-Related Deficits via Dysregulated EIF4E-Dependent Translational Control. Nature 2013, 493, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Neves-Pereira, M.; Müller, B.; Massie, D.; Williams, J.H.G.; O’Brien, P.C.M.; Hughes, A.; Shen, S.-B.; Clair, D.S.; Miedzybrodzka, Z. Deregulation of EIF4E: A Novel Mechanism for Autism. J. Med. Genet. 2009, 46, 759–765. [Google Scholar] [CrossRef]
- Santini, E.; Huynh, T.N.; Longo, F.; Koo, S.Y.; Mojica, E.; D’Andrea, L.; Bagni, C.; Klann, E. Reducing EIF4E-EIF4G Interactions Restores the Balance between Protein Synthesis and Actin Dynamics in Fragile X Syndrome Model Mice. Sci. Signal. 2017, 10, eaan0665. [Google Scholar] [CrossRef]
- Fakhri, S. Natural Products Attenuate PI3K/Akt/MTOR Signaling Pathway: A Promising Strategy in Regulating Neurodegeneration. Phytomedicine 2021, 23, 153664. [Google Scholar] [CrossRef]
- Sharma, A.; Bhalla, S.; Mehan, S. PI3K/AKT/MTOR Signalling Inhibitor Chrysophanol Ameliorates Neurobehavioural and Neurochemical Defects in Propionic Acid-Induced Experimental Model of Autism in Adult Rats. Metab. Brain Dis. 2022, 37, 1909–1929. [Google Scholar] [CrossRef]
- Bhandari, R.; Kuhad, A. Resveratrol Suppresses Neuroinflammation in the Experimental Paradigm of Autism Spectrum Disorders. Neurochem. Int. 2017, 103, 8–23. [Google Scholar] [CrossRef]
- Bambini-Junior, V.; Zanatta, G.; Della Flora Nunes, G.; Mueller de Melo, G.; Michels, M.; Fontes-Dutra, M.; Nogueira Freire, V.; Riesgo, R.; Gottfried, C. Resveratrol Prevents Social Deficits in Animal Model of Autism Induced by Valproic Acid. Neurosci. Lett. 2014, 583, 176–181. [Google Scholar] [CrossRef]
- Pragnya, B.; Kameshwari, J.S.L.; Veeresh, B. Ameliorating Effect of Piperine on Behavioral Abnormalities and Oxidative Markers in Sodium Valproate Induced Autism in BALB/C Mice. Behav. Brain Res. 2014, 270, 86–94. [Google Scholar] [CrossRef]
- Bhandari, R.; Kuhad, A. Neuropsychopharmacotherapeutic Efficacy of Curcumin in Experimental Paradigm of Autism Spectrum Disorders. Life Sci. 2015, 141, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Al-Askar, M.; Bhat, R.S.; Selim, M.; Al-Ayadhi, L.; El-Ansary, A. Postnatal Treatment Using Curcumin Supplements to Amend the Damage in VPA-Induced Rodent Models of Autism. BMC Complement. Altern. Med. 2017, 17, 259. [Google Scholar] [CrossRef] [PubMed]
- de Mattos, B.D.S.; Soares, M.S.P.; Spohr, L.; Pedra, N.S.; Teixeira, F.C.; de Souza, A.A.; Stefanello, F.M.; Baldissarelli, J.; Gamaro, G.D.; Spanevello, R.M. Quercetin Prevents Alterations of Behavioral Parameters, Delta-Aminolevulinic Dehydratase Activity, and Oxidative Damage in Brain of Rats in a Prenatal Model of Autism. Int. J. Dev. Neurosci. 2020, 80, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Asadi, S.; Panagiotidou, S. A Case Series of a Luteolin Formulation (NeuroProtek®) in Children with Autism Spectrum Disorders. Int. J. Immunopathol. Pharmacol. 2012, 25, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Tsilioni, I.; Taliou, A.; Francis, K.; Theoharides, T.C. Children with Autism Spectrum Disorders, Who Improved with a Luteolin-Containing Dietary Formulation, Show Reduced Serum Levels of TNF and IL-6. Transl. Psychiatry 2015, 5, e647. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.W.; Singh, K.; Connors, S.L.; Liu, H.; Panjwani, A.A.; Lee, L.-C.; Diggins, E.; Foley, A.; Melnyk, S.; Singh, I.N.; et al. Randomized Controlled Trial of Sulforaphane and Metabolite Discovery in Children with Autism Spectrum Disorder. Mol. Autism. 2021, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Bent, S.; Lawton, B.; Warren, T.; Widjaja, F.; Dang, K.; Fahey, J.W.; Cornblatt, B.; Kinchen, J.M.; Delucchi, K.; Hendren, R.L. Identification of Urinary Metabolites That Correlate with Clinical Improvements in Children with Autism Treated with Sulforaphane from Broccoli. Mol. Autism. 2018, 9, 35. [Google Scholar] [CrossRef]
- Van Aller, G.S.; Carson, J.D.; Tang, W.; Peng, H.; Zhao, L.; Copeland, R.A.; Tummino, P.J.; Luo, L. Epigallocatechin Gallate (EGCG), a Major Component of Green Tea, Is a Dual Phosphoinositide-3-Kinase/MTOR Inhibitor. Biochem. Biophys. Res. Commun. 2011, 406, 194–199. [Google Scholar] [CrossRef]
- Pawlik, A.; Wiczk, A.; Kaczyńska, A.; Antosiewicz, J.; Herman-Antosiewicz, A. Sulforaphane Inhibits Growth of Phenotypically Different Breast Cancer Cells. Eur. J. Nutr. 2013, 52, 1949–1958. [Google Scholar] [CrossRef]
- Zhang, Y.; Gilmour, A.; Ahn, Y.-H.; de la Vega, L.; Dinkova-Kostova, A.T. The Isothiocyanate Sulforaphane Inhibits MTOR in an NRF2-Independent Manner. Phytomedicine 2021, 86, 153062. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Connors, S.L.; Macklin, E.A.; Smith, K.D.; Fahey, J.W.; Talalay, P.; Zimmerman, A.W. Sulforaphane Treatment of Autism Spectrum Disorder (ASD). Proc. Natl. Acad. Sci. USA 2014, 111, 15550–15555. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Jeong, H.; Lee, M.N.; Koh, A.; Kwon, O.; Yang, Y.R.; Noh, J.; Suh, P.-G.; Park, H.; Ryu, S.H. Resveratrol Induces Autophagy by Directly Inhibiting MTOR through ATP Competition. Sci. Rep. 2016, 6, 21772. [Google Scholar] [CrossRef]
- Tian, Y.; Song, W.; Li, D.; Cai, L.; Zhao, Y. Resveratrol As A Natural Regulator Of Autophagy For Prevention And Treatment Of Cancer. Onco. Targets Ther. 2019, 12, 8601–8609. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, J.; Zheng, X.; Yang, X.; Ding, Y.; Fang, T.; Zhang, Y.; Wang, S.; Zhang, X.; Luo, X.; et al. Luteolin, a Natural Flavonoid, Inhibits Methylglyoxal Induced Apoptosis via the MTOR/4E-BP1 Signaling Pathway. Sci. Rep. 2017, 7, 7877. [Google Scholar] [CrossRef]
- Patel, A.B.; Tsilioni, I.; Leeman, S.E.; Theoharides, T.C. Neurotensin Stimulates Sortilin and MTOR in Human Microglia Inhibitable by Methoxyluteolin, a Potential Therapeutic Target for Autism. Proc. Natl. Acad. Sci. USA 2016, 113, E7049–E7058. [Google Scholar] [CrossRef] [PubMed]
- Bruning, A. Inhibition of MTOR Signaling by Quercetin in Cancer Treatment and Prevention. Anti-Cancer Agents Med. Chem. Anti-Cancer Agents 2013, 13, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, D.; Popic, J.; Enns, J.P.; Inserra, A.; Skalecka, A.; Markopoulos, A.; Posa, L.; Lopez-Canul, M.; Qianzi, H.; Lafferty, C.K.; et al. Lysergic Acid Diethylamide (LSD) Promotes Social Behavior through MTORC1 in the Excitatory Neurotransmission. Proc. Natl. Acad. Sci. USA 2021, 118, e2020705118. [Google Scholar] [CrossRef]
- Yu, S.; Shen, G.; Khor, T.O.; Kim, J.-H.; Kong, A.-N.T. Curcumin Inhibits Akt/Mammalian Target of Rapamycin Signaling through Protein Phosphatase-Dependent Mechanism. Mol. Cancer Ther. 2008, 7, 2609–2620. [Google Scholar] [CrossRef]
- Johnson, S.M.; Gulhati, P.; Arrieta, I.; Wang, X.; Uchida, T.; Gao, T.; Evers, B.M. Curcumin Inhibits Proliferation of Colorectal Carcinoma by Modulating Akt/MTOR Signaling. Anticancer. Res. 2009, 29, 3185–3190. [Google Scholar]
- Kuo, C.-J. Potential Therapeutic Effect of Curcumin, a Natural MTOR Inhibitor, in Tuberous Sclerosis Complex. Phytomedicine 2019, 8, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Agramonte, M.D.L.A.; Michalski, B.; Vidal-Martinez, B.; Hernández, L.R.; Santiesteban, M.W.; Fahnestock, M. BDNF, ProBDNF and IGF-1 Serum Levels in Naïve and Medicated Subjects with Autism. Sci. Rep. 2022, 12, 13768. [Google Scholar] [CrossRef] [PubMed]
- DeSpenza, T.; Carlson, M.; Panchagnula, S.; Robert, S.; Duy, P.Q.; Mermin-Bunnell, N.; Reeves, B.C.; Kundishora, A.; Elsamadicy, A.A.; Smith, H.; et al. PTEN Mutations in Autism Spectrum Disorder and Congenital Hydrocephalus: Developmental Pleiotropy and Therapeutic Targets. Trends Neurosci. 2021, 44, 961–976. [Google Scholar] [CrossRef]
- Srivastava, S.; Jo, B.; Zhang, B.; Frazier, T.; Gallagher, A.S.; Peck, F.; Levin, A.R.; Mondal, S.; Li, Z.; Filip-Dhima, R.; et al. A Randomized Controlled Trial of Everolimus for Neurocognitive Symptoms in PTEN Hamartoma Tumor Syndrome. Hum. Mol. Genet. 2022, 31, ddac111. [Google Scholar] [CrossRef] [PubMed]
- Overwater, I.E.; Rietman, A.B.; Mous, S.E.; Bindels-de Heus, K.; Rizopoulos, D.; Ten Hoopen, L.W.; van der Vaart, T.; Jansen, F.E.; Elgersma, Y.; Moll, H.A.; et al. A Randomized Controlled Trial with Everolimus for IQ and Autism in Tuberous Sclerosis Complex. Neurology 2019, 93, e200–e209. [Google Scholar] [CrossRef]

| Human Gene | Protein | Function | Disorder |
|---|---|---|---|
| FMR1 (fragile X messenger ribonucleoprotein 1) | FMRP (fragile X mental retardation protein) | Negative regulator of protein translation | Fragile X syndrome |
| EIF4E | eIF4E (eukaryotic translation initiation factor 4E) | Translation initiation factor complex | ASD |
| PTEN | PTEN (phosphatase and tensin homolog) | Phosphatase | PHTS |
| TSC1/TSC2 | Hamartin/tuberin | GTPase-activating protein | Tuberous sclerosis |
| Preclinical Investigations for Phytochemicals Effective in ASD | ||||
|---|---|---|---|---|
| Compound (s) | Method | Dose | Mechanisms and Outcomes | References |
| Chrysophanol | Propanoic acid-induced model of autism | (10, 20 mg/kg) | ↓Akt, ↓mTOR, ↓ caspase-3, Bax, and ↑Bcl-2, ↓TNF and IL-1β, ↓AchE, ↓ LDH ↑SOD ↑GSH ↑dopamine, ↑serotonin ↑acetylcholine | [138] |
| Resveratrol | Propanoic acid-induced model of autism | 5, 10, and 15 mg/kg | Improved behavioral, biochemical changes; reduced neuroinflammation, mitochondrial dysfunction, and oxidative/nitrosative stress; decreased inflammatory cytokines (TNF-α and IL-6) | [139] |
| VPA-induced model of autism | 3.6 mg/kg | Activated sirtuins and ↓ inflammation | [140] | |
| Piperine | VPA-induced model of autism | 20 mg/kg | Lowered oxidative stress; improved social behavior; ↓anxiety | [141] |
| Curcumin | Propanoic acid-induced model of autism | 50, 100, and 200 mg/kg | Lowered oxidative-nitrosative stress, mitochondrial dysfunction, ↓TNF-α, ↓MMP-9; improved behavioral outcomes | [142] |
| VPA-induced model of autism | 1 g/kg | Reduced oxidative stress, ↓IL-6 levels | [143] | |
| Quercetin | VPA-induced model of autism | 50 mg/kg | Improved behavioral changes; reduced oxidative stress | [144] |
| Clinical studies for the therapeutic action of phytoconstituents in the management of ASD. | ||||
| Luteolin | 4–14 year-old children | NeuroProtek®, (Luteolin: 100 mg/capsule Rutin: 30 mg/capsule Quercetin: 70 mg/capsule) | Improved behavioral outcomes; reduced gut and brain inflammation; reduced serum interleukin-6 and tumor necrosis factor | [145,146] |
| Sulforaphane | 3–12 year-old children with ASD. | 125 mg broccoli seed powder | Improvements in social responsiveness and interaction (Clinical trial identifier: NCT02561481) | [147] |
| 5 years to 22 years (children and young adults) | 125 mg broccoli seed extract +50 mg dried broccoli sprouts | Improvement in social responsiveness (Clinical trial identifier: NCT02654743) | [148] | |
| Epigallocatechin Gallate | 18 to 55 years | 400 mg/day | Improvement in memory and cognition (Clinical trial identifier: NCT01855971) | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.D.; Jha, N.K.; Ojha, S.; Sadek, B. mTOR Signaling Disruption and Its Association with the Development of Autism Spectrum Disorder. Molecules 2023, 28, 1889. https://doi.org/10.3390/molecules28041889
Thomas SD, Jha NK, Ojha S, Sadek B. mTOR Signaling Disruption and Its Association with the Development of Autism Spectrum Disorder. Molecules. 2023; 28(4):1889. https://doi.org/10.3390/molecules28041889
Chicago/Turabian StyleThomas, Shilu Deepa, Niraj Kumar Jha, Shreesh Ojha, and Bassem Sadek. 2023. "mTOR Signaling Disruption and Its Association with the Development of Autism Spectrum Disorder" Molecules 28, no. 4: 1889. https://doi.org/10.3390/molecules28041889
APA StyleThomas, S. D., Jha, N. K., Ojha, S., & Sadek, B. (2023). mTOR Signaling Disruption and Its Association with the Development of Autism Spectrum Disorder. Molecules, 28(4), 1889. https://doi.org/10.3390/molecules28041889










