Neuroprotection by Skimmianine in Lipopolysaccharide-Activated BV-2 Microglia
Abstract
1. Introduction
2. Results
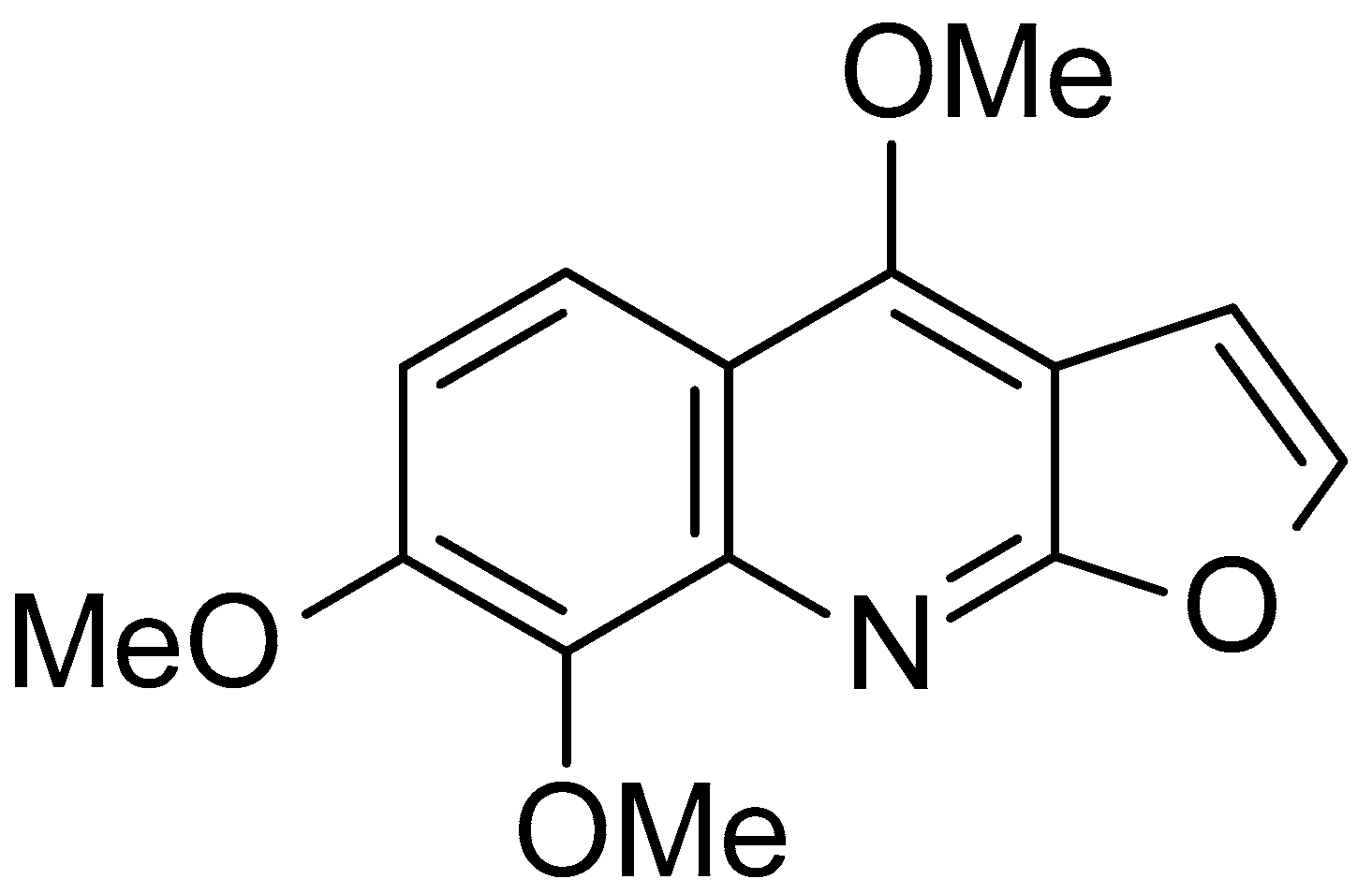
2.1. Skimmianine Did Not Affect the Viability of BV-2 Microglia
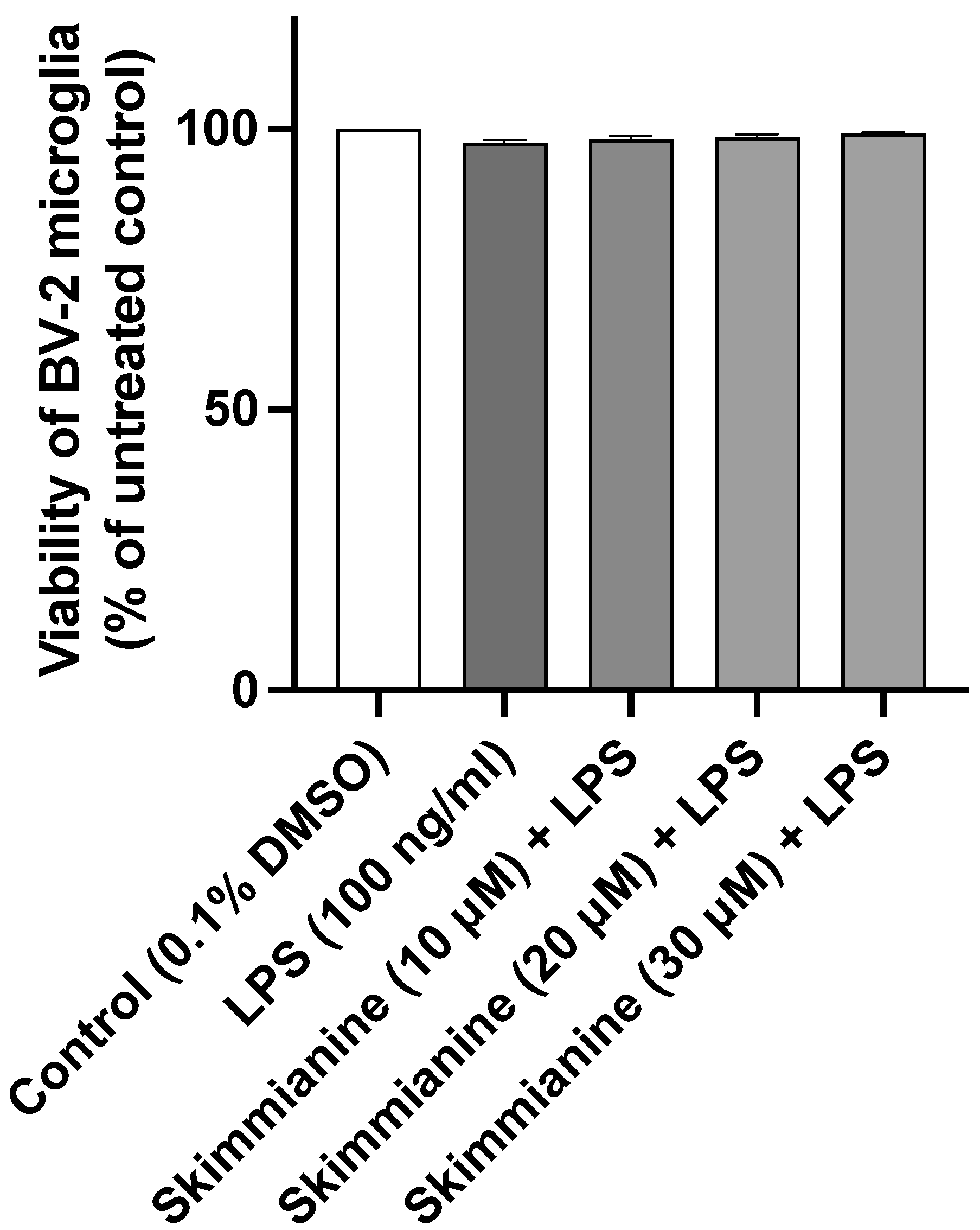
2.2. Skimmianine Reduced Elevated Levels of TNFα and IL-6 in LPS-Activated BV-2 Microglia
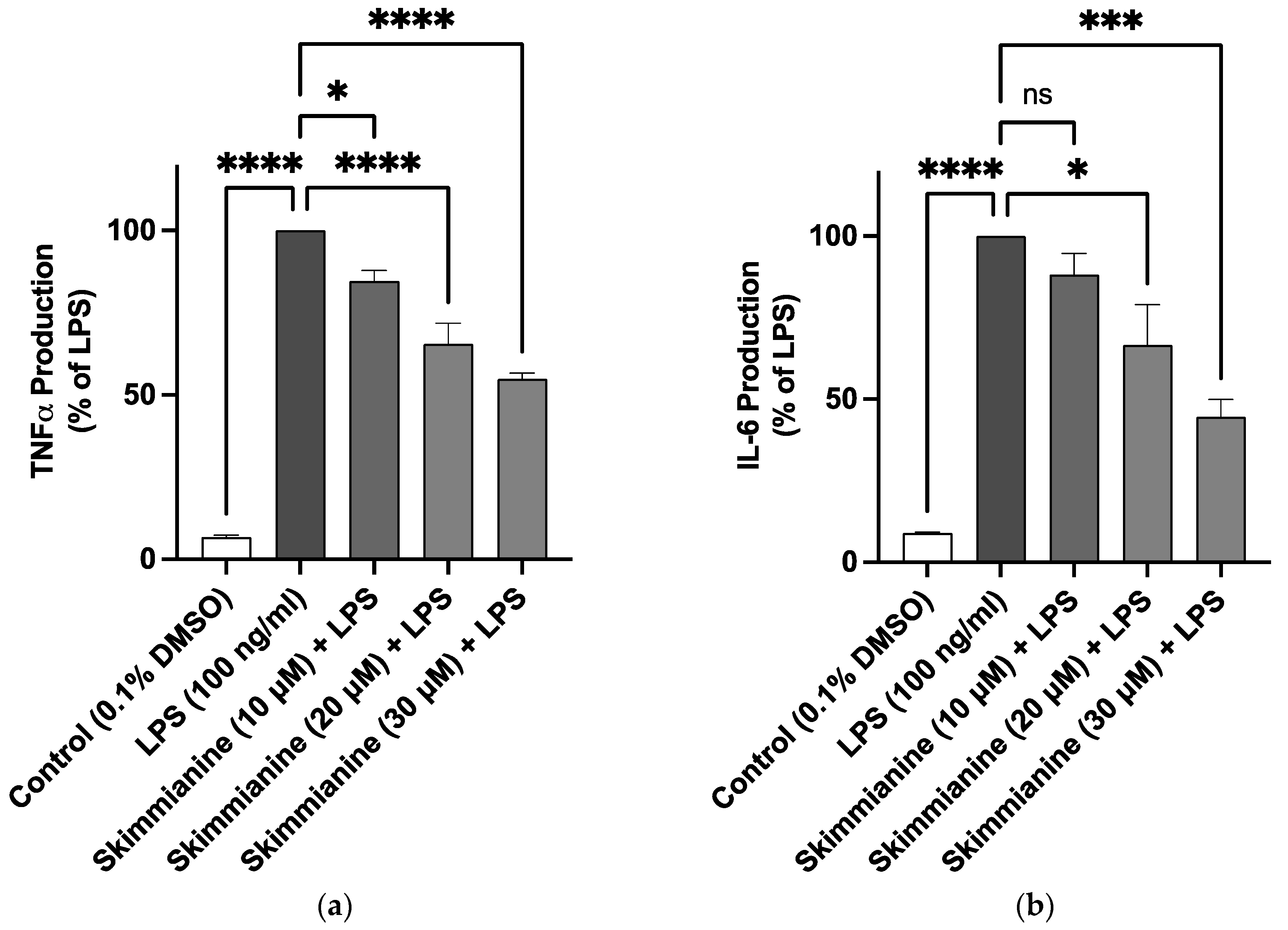
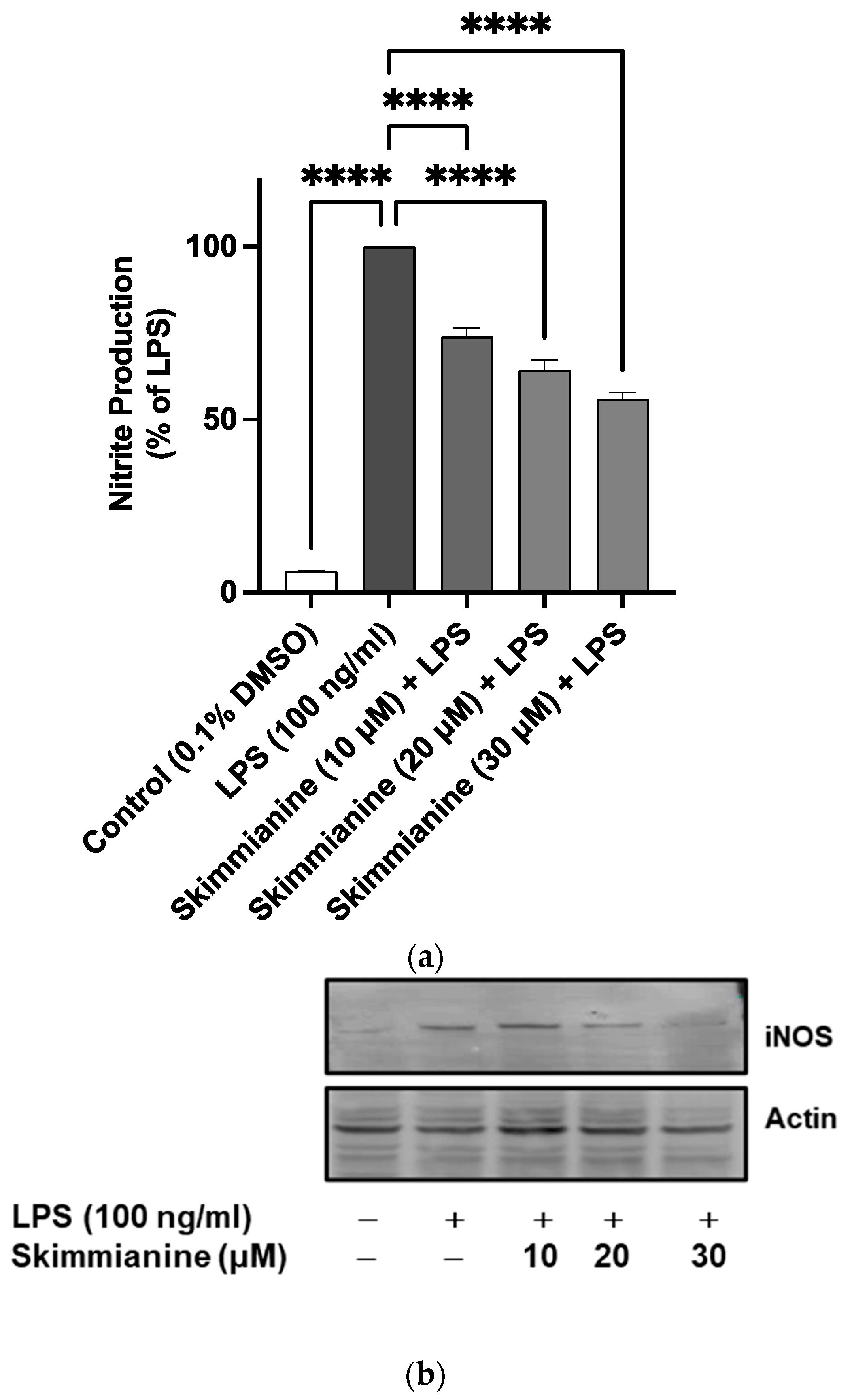
2.3. Effects of Skimmianine on iNOS-Mediated NO Production
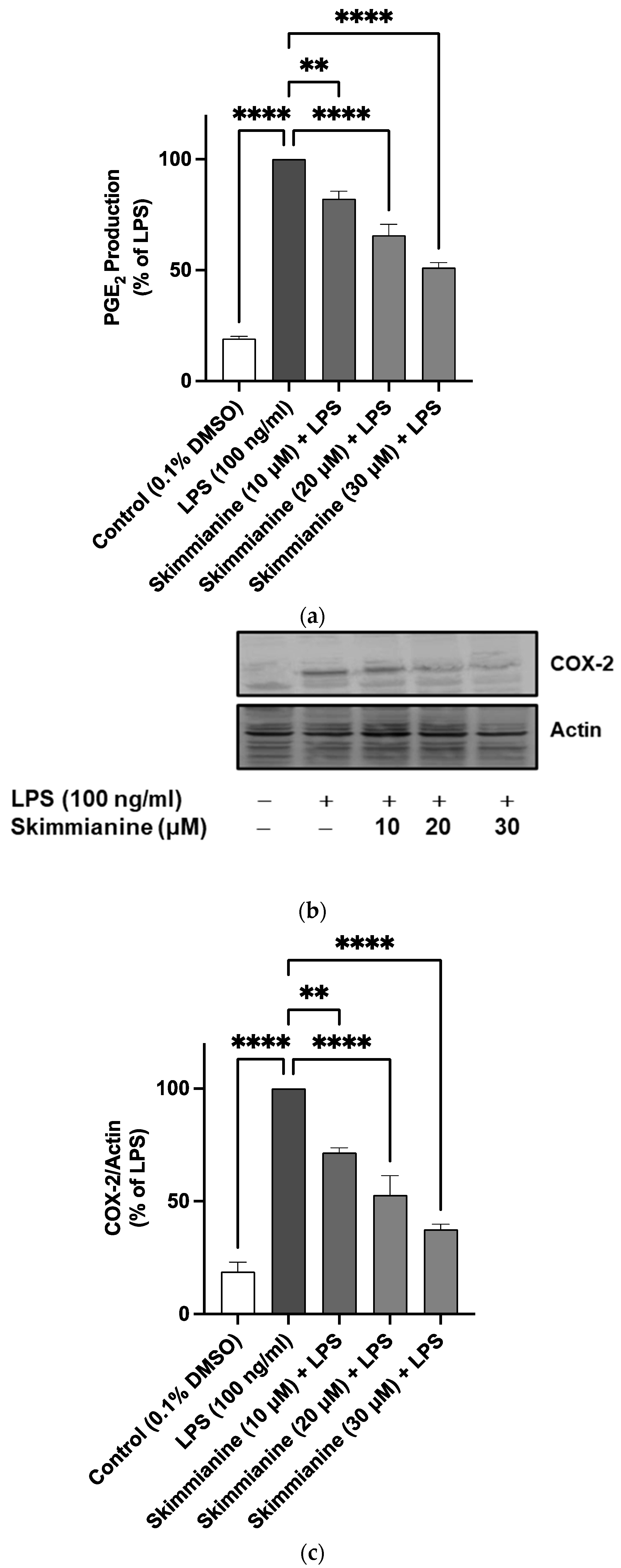
2.4. Skimmianine Reduced PGE2 Production and COX-2 Protein Expression in LPS-Activated BV-2 Microglia
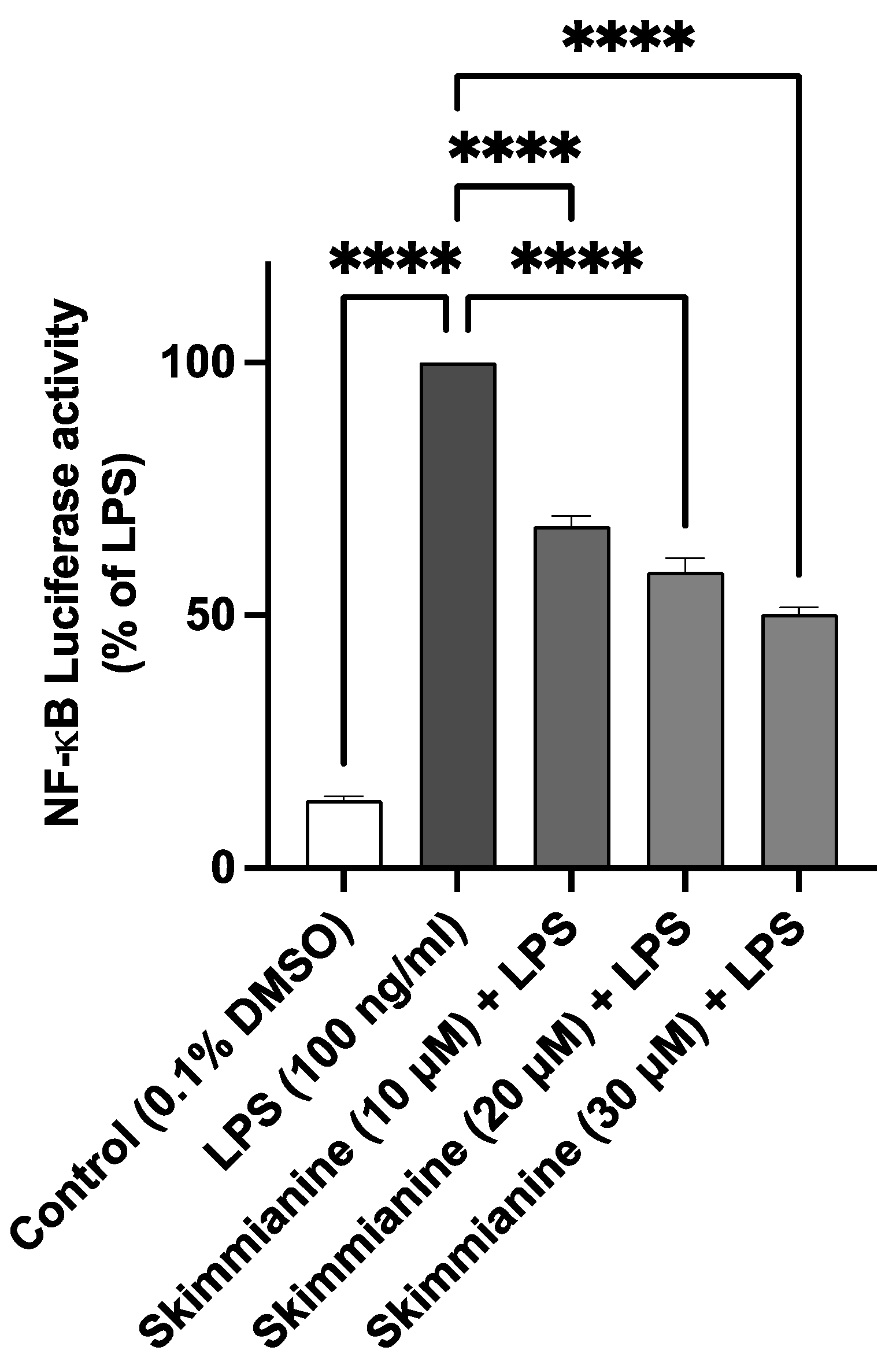
2.5. Skimmianine Reduced LPS-Induced NF-κB-Mediated Gene Transcription in BV-2 Microglia
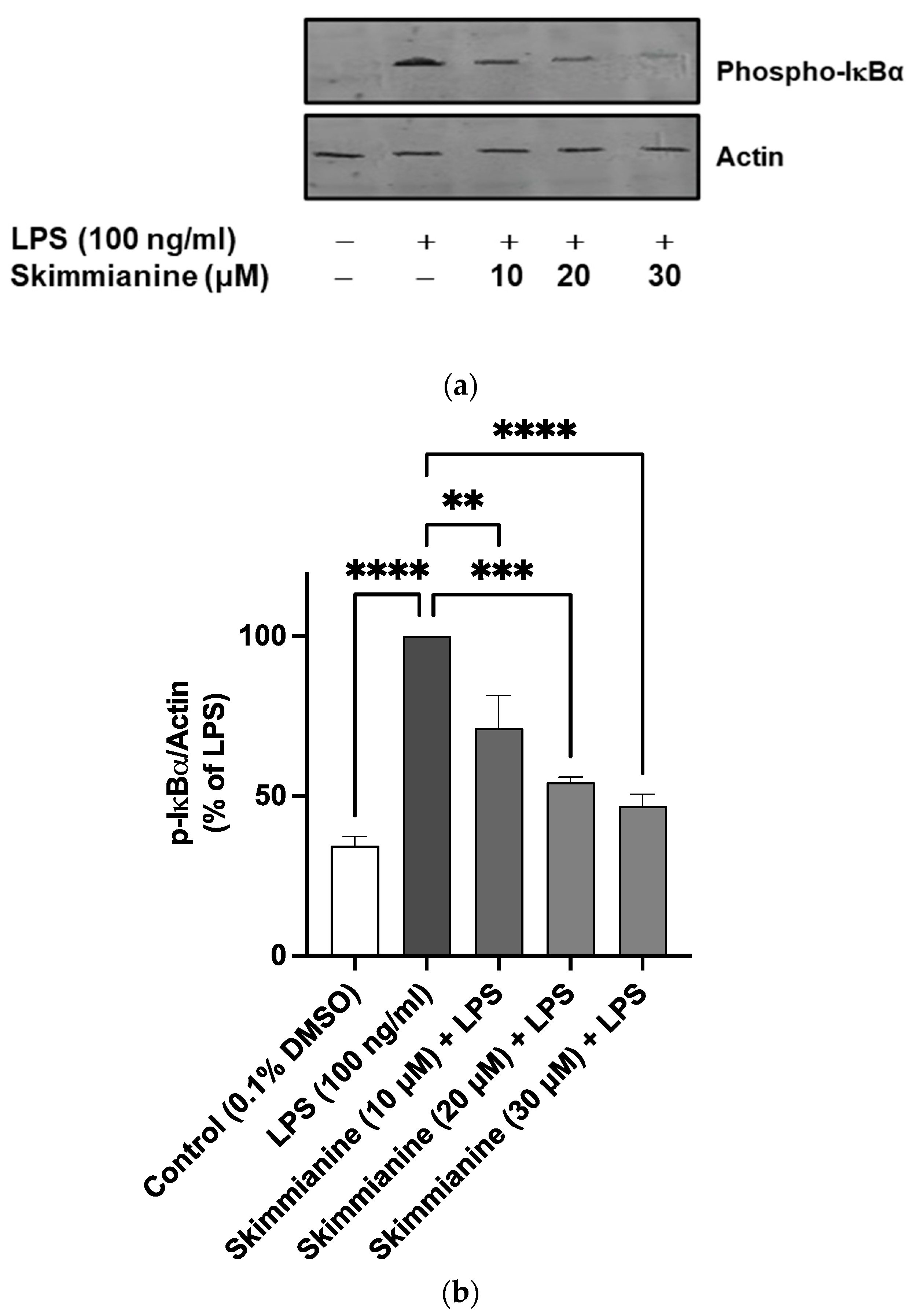
2.6. Skimmianine Inhibited Phosphorylation of IκB
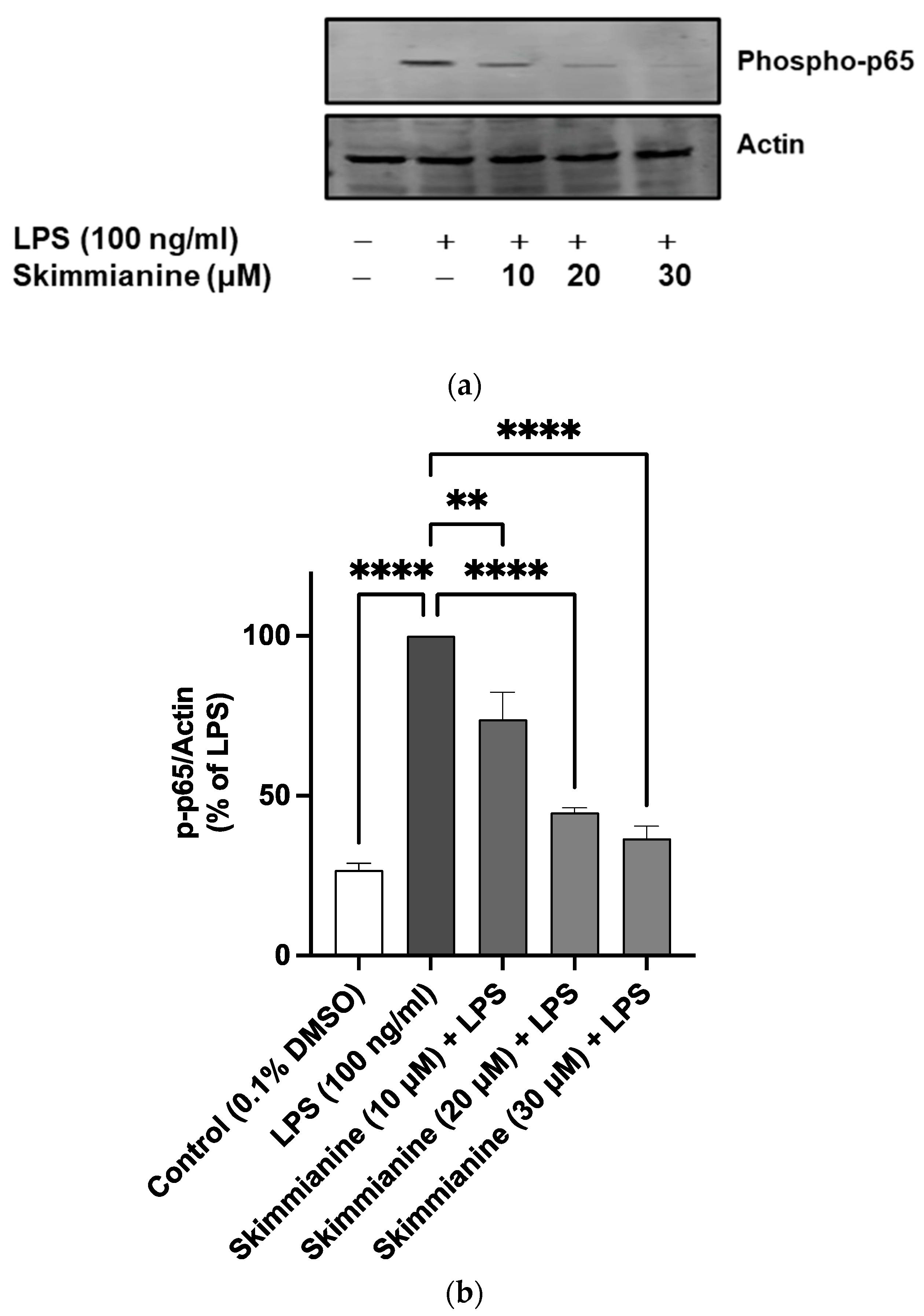
2.7. Skimmianine Interferes with the Nuclear Translocation of p65/NF-κB
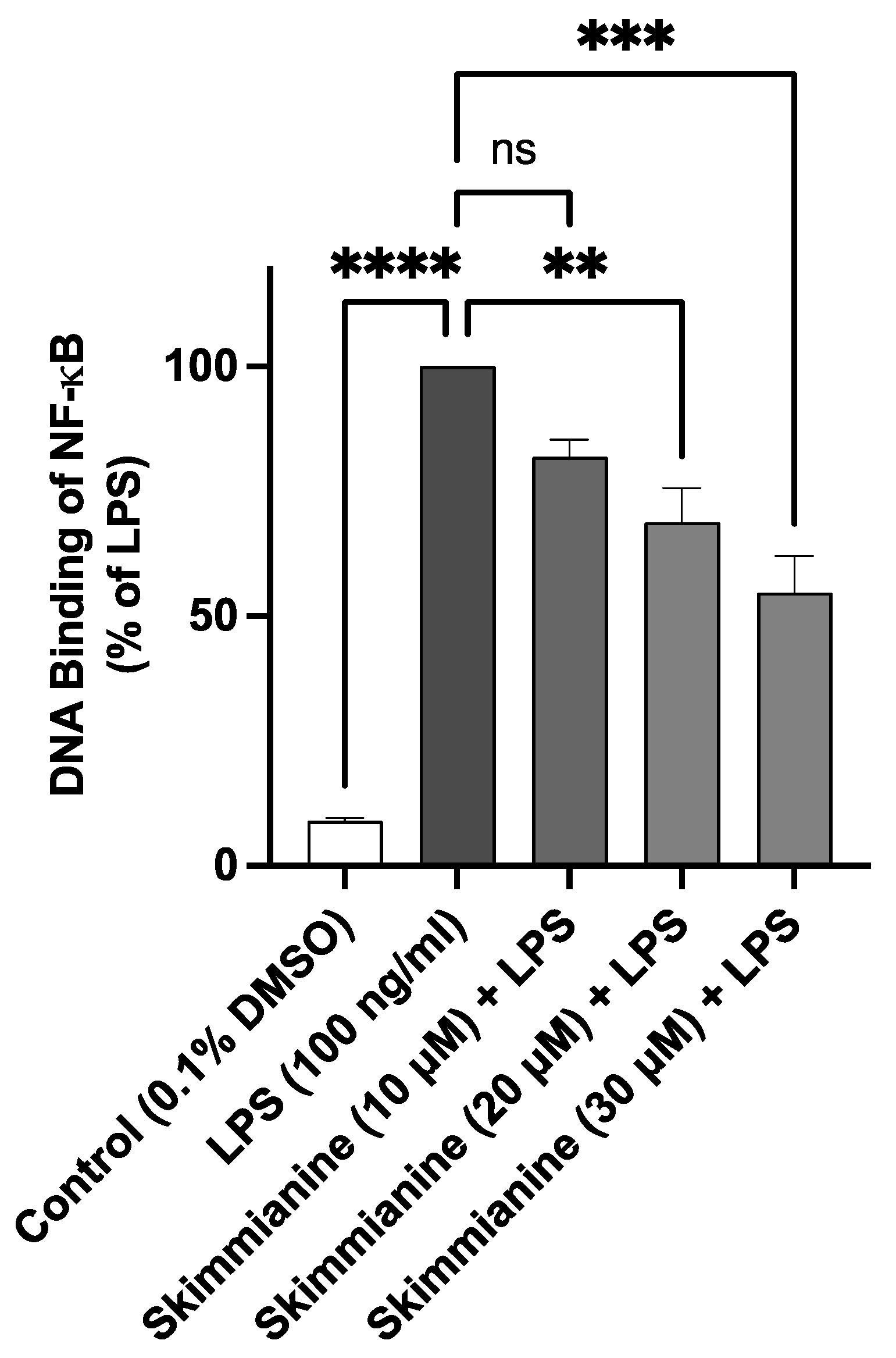
2.8. Skimmianine Interferes with the DNA Binding Capacity of NF-κB
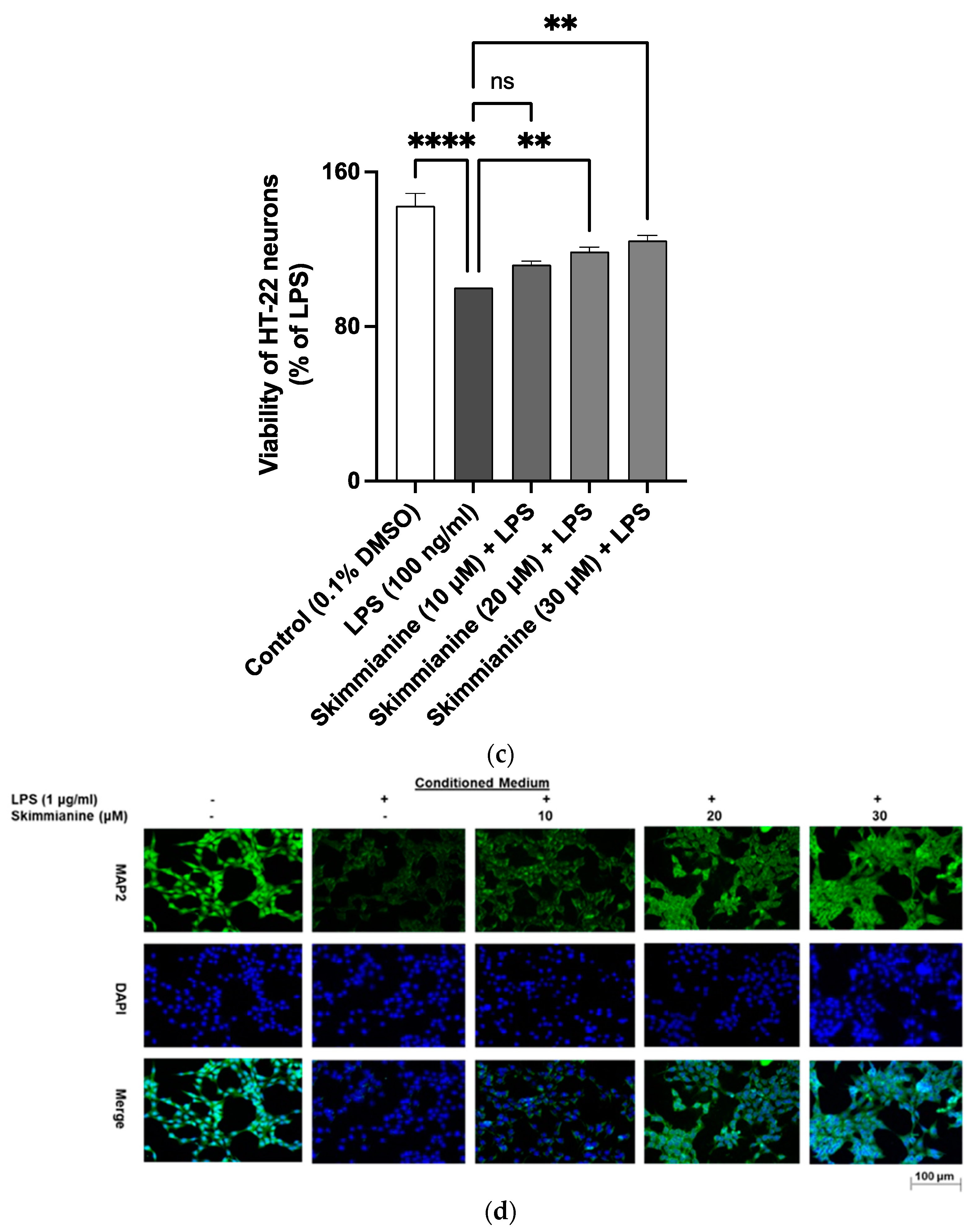
2.9. Skimmianine Prevented LPS Neuroinflammation-Mediated Neurotoxicity in HT-22 Neurons
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Determination of BV-2 Cell Viability
4.4. ELISA for TNFα, IL-6 and IL-10
4.5. Griess Assay
4.6. Enzyme Immunoassay for PGE2
4.7. Western Blotting
4.8. Transient Transfection and NF-κB Reporter Gene Assay
4.9. NF-κB Transcription Factor Assay
4.10. BV-2 Microglia Conditioned Medium-Mediated Neurotoxicity
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Devadoss, T.; Jha, N.K.; Baidya, M.; Gupta, G.; Chellappan, D.K.; Singh, S.K.; Dua, K. Targeting inflammation: A potential approach for the treatment of depression. Metab. Brain Dis. 2022, 38, 45–59. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, L.; Teng, J.; Miao, W. HMGB1 mediates microglia activation via the TLR4/NF-κB pathway in coriaria lactone induced epilepsy. Mol. Med. Rep. 2018, 17, 5125–5131. [Google Scholar] [CrossRef]
- Strangward, P.; Haley, M.J.; Albornoz, M.G.; Barrington, J.; Shaw, T.; Dookie, R.; Zeef, L.; Baker, S.M.; Winter, E.; Tzeng, T.-C.; et al. Targeting the IL33–NLRP3 axis improves therapy for experimental cerebral malaria. Proc. Natl. Acad. Sci. USA 2018, 115, 7404–7409. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, R.; Kosoko, A.M.; Olajide, O.A. Induction of Neuroinflammation and Neurotoxicity by Synthetic Hemozoin. Cell. Mol. Neurobiol. 2019, 39, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, S.; Tao, J.; Gao, Y.; Meng, G.; Cao, D.; Gao, L. HIV-1 Tat drives the Fabp4/NF-κB feedback loop in microglia to mediate inflammatory response and neuronal apoptosis. J. NeuroVirol. 2022, 28, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Rakshasa-Loots, A.M.; Whalley, H.C.; Vera, J.H.; Cox, S.R. Neuroinflammation in HIV-associated depression: Evidence and future perspectives. Mol. Psychiatry 2022, 27, 3619–3632. [Google Scholar] [CrossRef]
- Beckman, D.; Bonillas, A.; Diniz, G.B.; Ott, S.; Roh, J.W.; Elizaldi, S.R.; Schmidt, B.A.; Sammak, R.L.; Van Rompay, K.K.; Iyer, S.S.; et al. SARS-CoV-2 infects neurons and induces neuroinflammation in a non-human primate model of COVID-19. Cell Rep. 2022, 41, 111573. [Google Scholar] [CrossRef]
- Olajide, O.A.; Iwuanyanwu, V.U.; Adegbola, O.D.; Al-Hindawi, A.A. SARS-CoV-2 Spike Glycoprotein S1 Induces Neuroinflammation in BV-2 Microglia. Mol. Neurobiol. 2021, 59, 445–458. [Google Scholar] [CrossRef]
- Olajide, O.A.; Sarker, S.D. Alzheimer’s disease: Natural products as inhibitors of neuroinflammation. Inflammopharmacology 2020, 28, 1439–1455. [Google Scholar] [CrossRef]
- Queiroz, E.F.; Hay, A.-E.; Chaaib, F.; van Diemen, D.; Diallo, D.; Hostettmann, K. New and Bioactive Aromatic Compounds from Zanthoxylum zanthoxyloides. Planta Med. 2006, 72, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-D.; Zhang, D.-B.; Ren, J.; Yang, M.-J. Skimmianine, a furoquinoline alkaloid from Zanthoxylum nitidum as a potential acetylcholinesterase inhibitor. Med. Chem. Res. 2011, 21, 722–725. [Google Scholar] [CrossRef]
- Phuyal, N.; Jha, P.K.; Raturi, P.P.; Rajbhandary, S. Zanthoxylum armatum DC.: Current knowledge, gaps and opportunities in Nepal. J. Ethnopharmacol. 2018, 229, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Ogunrinade, F.A.; Guetchueng, S.T.; Katola, F.O.; Aderogba, M.A.; Akande, I.S.; Sarker, S.D.; Olajide, O.A. Zanthoxylum zanthoxyloides inhibits lipopolysaccharide- and synthetic hemozoin-induced neuroinflammation in BV-2 microglia: Roles of NF-κB transcription factor and NLRP3 inflammasome activation. J. Pharm. Pharmacol. 2020, 73, 118–134. [Google Scholar] [CrossRef]
- Cardoso-Lopes, E.M.; Maier, J.A.; Da Silva, M.R.; Regasini, L.O.; Simote, S.Y.; Lopes, N.P.; Pirani, J.R.; Bolzani, V.D.S.; Young, M.C.M. Alkaloids from Stems of Esenbeckia leiocarpa Engl. (Rutaceae) as Potential Treatment for Alzheimer Disease. Molecules 2010, 15, 9205–9213. [Google Scholar] [CrossRef]
- Ratheesh, M.; Sindhu, G.; Helen, A. Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm. Res. 2013, 62, 367–376. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Wu, J.-B.; Hwang, T.-L.; Kuo, Y.-H.; Chen, J.-J. A New Quinolone and Other Constituents from the Fruits of Tetradium ruticarpum: Effects on Neutrophil Pro-Inflammatory Responses. Chem. Biodivers. 2010, 7, 1828–1834. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Hwang, T.-L.; Chang, T.-H.; Lim, Y.-P.; Sung, P.-J.; Lee, T.-H.; Chen, J.-J. New coumarins and anti-inflammatory constituents from Zanthoxylum avicennae. Food Chem. 2012, 135, 17–23. [Google Scholar] [CrossRef]
- Yoon, J.S.; Jeong, E.J.; Yang, H.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Inhibitory alkaloids from Dictamnus dasycarpus root barks on lipopolysaccharide-induced nitric oxide production in BV2 cells. J. Enzym. Inhib. Med. Chem. 2011, 27, 490–494. [Google Scholar] [CrossRef]
- Schütze, S.; Loleit, T.; Zeretzke, M.; Bunkowski, S.; Brück, W.; Ribes, S.; Nau, R. Additive Microglia-Mediated Neuronal Injury Caused by Amyloid-β and Bacterial TLR Agonists in Murine Neuron-Microglia Co-Cultures Quantified by an Automated Image Analysis using Cognition Network Technology. J. Alzheimer’s Dis. 2012, 31, 651–657. [Google Scholar] [CrossRef]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef]
- Asiimwe, N.; Yeo, S.G.; Kim, M.-S.; Jung, J.; Jeong, N.Y. Nitric Oxide: Exploring the Contextual Link with Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2016, 2016, 7205747. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M.G. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Dickson, D.W.; Liu, W.; Brosnan, C.F. Induction of nitric oxide synthase activity in human astrocytes by interleukin-1β and interferon-γ. J. Neuroimmunol. 1993, 46, 19–24. [Google Scholar] [CrossRef]
- Yuste, J.E.; Tarragon, E.; Campuzano, C.M.; Cros, E.T. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Oriowo, M.A. Anti-Inflammatory Activity of Piperonyl-4-Acrylic Isobutyl Amide, an Extractive from Zanthoxylum zanthoxyloides. Planta Med. 1982, 44, 54–56. [Google Scholar] [CrossRef]
- Hong, M.; Xiao, K.; Lin, P.; Lin, J. Five Rutaceae family ethanol extracts alleviate H2O2 and LPS-induced inflammation via NF-κB and JAK-STAT3 pathway in HaCaT cells. Chin. J. Nat. Med. 2022, 20, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, X.; Hu, H.; Zhong, W.; Cao, R.; Xu, Y.; Li, R. Chemical Composition and Antifungal, Anti-Inflammatory, Antiviral, and Larvicidal Activities of the Essential Oils of Zanthoxylum acanthopodium DC. from China and Myanmar. Molecules 2022, 27, 5243. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Youcefi, F.; Keddari, S.; Saimi, Y.; Elhao, S.O.; Cacciola, F. Phenolic content and in vitro antioxidant and anti-inflammatory evaluation of Algerian Ruta graveolens L. Chem. Biodivers. 2022, 19, e202200545. [Google Scholar] [CrossRef]
- Loonat, F.; Amabeoku, G. Antinociceptive, Anti-Inflammatory and Antipyretic Activities of the Leaf Methanol Extract of Ruta graveolens L. (Rutaceae) in Mice and Rats. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kacem, M.; Simon, G.; Leschiera, R.; Misery, L.; Elfeki, A.; Lebonvallet, N. Antioxidant and anti-inflammatory effects of Ruta chalepensis L. extracts on LPS-stimulated RAW 264.7 cells. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Ratheesh, M.; Shyni, G.L.; Sindhu, G.; Helen, A. Protective Effects of Isolated Polyphenolic and Alkaloid Fractions of Ruta graveolens L. on Acute and Chronic Models of Inflammation. Inflammation 2009, 33, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, J.; Matsumoto, Y.; Kreutzberg, G.W. Microglia: Intrinsic immuneffector cell of the brain. Brain Res. Rev. 1995, 20, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Q.; Johansson, J.U.; Liang, L.X.; Woodling, N.S.; Priyam, P.; Loui, T.M.; Merchant, M.; Breyer, R.M.; Montine, T.J.; et al. Inflammatory prostaglandin E2signaling in a mouse model of Alzheimer disease. Ann. Neurol. 2012, 72, 788–798. [Google Scholar] [CrossRef]
- Aloisi, F. Immune function of microglia. Glia 2001, 36, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef]
- Hoozemans, J.; Rozemuller, J.; van Haastert, E.; Veerhuis, R.; Eikelenboom, P. Cyclooxygenase-1 and -2 in the Different Stages of Alzheimers Disease Pathology. Curr. Pharm. Des. 2008, 14, 1419–1427. [Google Scholar] [CrossRef]
- Combrinck, M.; Williams, J.; De Berardinis, M.A.; Warden, D.; Puopolo, M.; Smith, D.; Minghetti, L. Levels of CSF prostaglandin E2, cognitive decline, and survival in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Kučić, N.; Rački, V.; Šverko, R.; Vidović, T.; Grahovac, I.; Mršić-Pelčić, J. Immunometabolic Modulatory Role of Naltrexone in BV-2 Microglia Cells. Int. J. Mol. Sci. 2021, 22, 8429. [Google Scholar] [CrossRef]
- Li, C.-H.; Zhou, Y.; Tu, P.-F.; Zeng, K.-W.; Jiang, Y. Natural carbazole alkaloid murrayafoline A displays potent anti-neuroinflammatory effect by directly targeting transcription factor Sp1 in LPS-induced microglial cells. Bioorg. Chem. 2022, 129, 106178. [Google Scholar] [CrossRef]
- Kalyankumarraju, M.; Puppala, E.R.; Ahmed, S.; Kumar, G.J.; Tene, K.; Syamprasad, N.P.; Sahu, B.D.; Barua, C.C.; Naidu, V. Zanthoxylum alatum Roxb. seed extract ameliorates stress aggravated DSS-induced ulcerative colitis in mice: Plausible role on NF-κB signaling axis. J. Ethnopharmacol. 2021, 279, 114385. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Choi, E.; Hong, Y.H.; Kim, H.; Jang, Y.-J.; Lee, J.S.; Choung, E.S.; Woo, B.Y.; Hong, Y.D.; Lee, S.; et al. Syk/NF-κB-targeted anti-inflammatory activity of Melicope accedens (Blume) T.G. Hartley methanol extract. J. Ethnopharmacol. 2021, 271, 113887. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, H.; Ha, I.J.; Yang, W.M. Zanthoxylum piperitum alleviates the bone loss in osteoporosis via inhibition of RANKL-induced c-fos/NFATc1/NF-κB pathway. Phytomedicine 2020, 80, 153397. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, M.; Peng, Y.; Gao, M.; Yang, B. Rutaecarpine ameliorated sepsis-induced peritoneal resident macrophages apoptosis and inflammation responses. Life Sci. 2019, 228, 11–20. [Google Scholar] [CrossRef]
- Santhanam, R.K.; Fakurazi, S.; Ahmad, S.; Abas, F.; Ismail, I.S.; Rukayadi, Y.; Akhtar, M.T.; Shaari, K. Inhibition of UVB-induced pro-inflammatory cytokines and MMP expression by Zanthoxylum rhetsa bark extract and its active constituent hesperidin. Phytother. Res. 2018, 32, 1608–1616. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogunrinade, F.A.; Iwuanyanwu, V.U.; Sarker, S.D.; Olajide, O.A. Neuroprotection by Skimmianine in Lipopolysaccharide-Activated BV-2 Microglia. Molecules 2023, 28, 1317. https://doi.org/10.3390/molecules28031317
Ogunrinade FA, Iwuanyanwu VU, Sarker SD, Olajide OA. Neuroprotection by Skimmianine in Lipopolysaccharide-Activated BV-2 Microglia. Molecules. 2023; 28(3):1317. https://doi.org/10.3390/molecules28031317
Chicago/Turabian StyleOgunrinade, Folashade A., Victoria U. Iwuanyanwu, Satyajit D. Sarker, and Olumayokun A. Olajide. 2023. "Neuroprotection by Skimmianine in Lipopolysaccharide-Activated BV-2 Microglia" Molecules 28, no. 3: 1317. https://doi.org/10.3390/molecules28031317
APA StyleOgunrinade, F. A., Iwuanyanwu, V. U., Sarker, S. D., & Olajide, O. A. (2023). Neuroprotection by Skimmianine in Lipopolysaccharide-Activated BV-2 Microglia. Molecules, 28(3), 1317. https://doi.org/10.3390/molecules28031317






