Baicalin Relieves LPS-Induced Lung Inflammation via the NF-κB and MAPK Pathways
Abstract
1. Introduction
2. Results
2.1. In Vitro Study
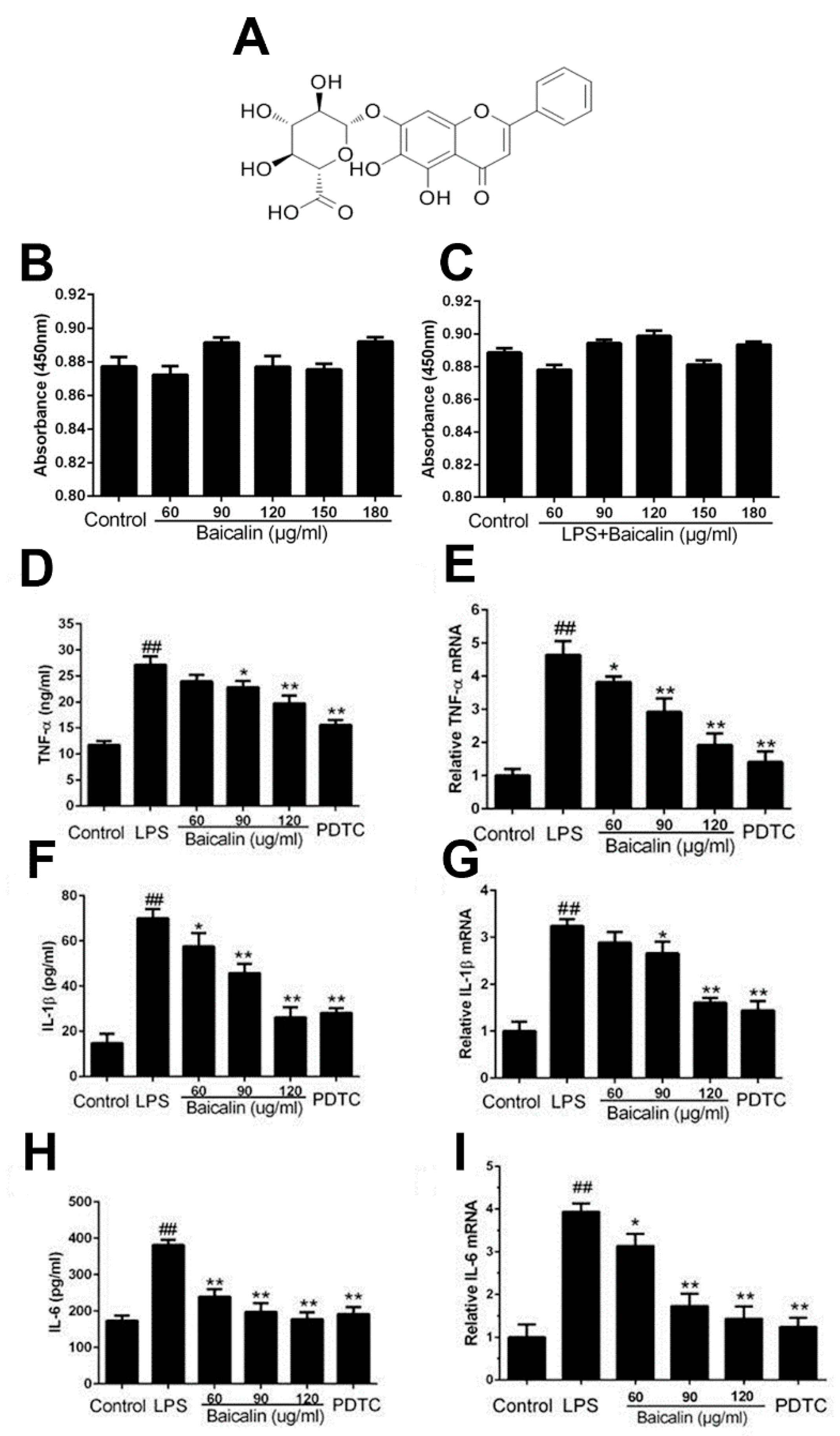
2.1.1. Baicalin Had No Effect on Cell Viability
2.1.2. Baicalin Inhibits Inflammatory Cytokine Production in RAW264.7 Cells
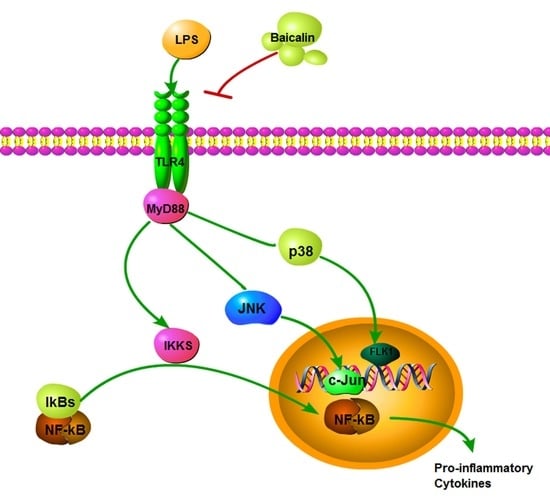
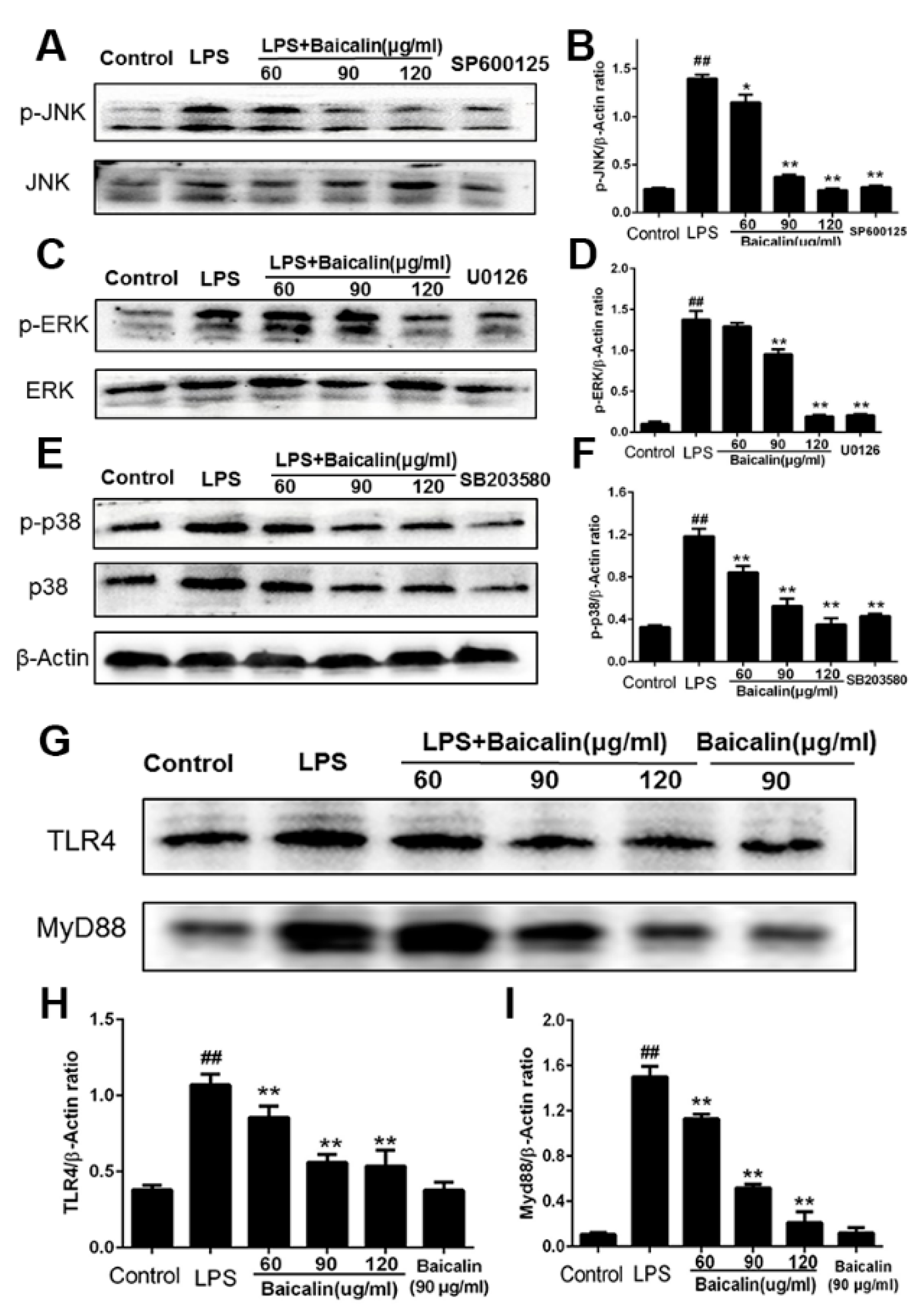
2.1.3. Baicalin Inhibits NF-κB and MAPK Activation of RAW264.7 Cells
2.2. In Vivo Study
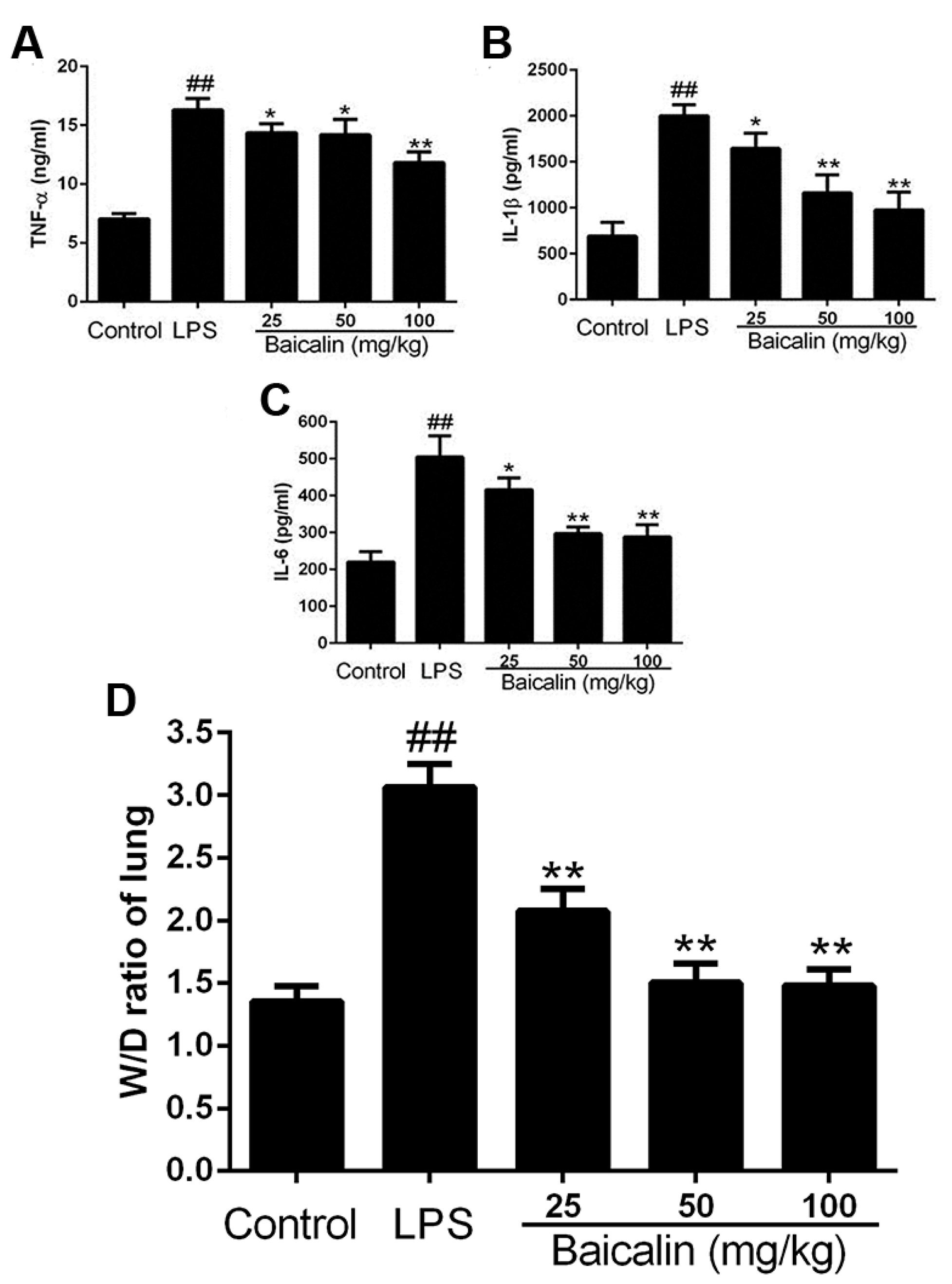
2.2.1. Baicalin Decreases Cytokine Levels in LPS-Induced ALI Mice
2.2.2. Baicalin Reduced the Increase Inflammatory Cells Induced by LPS
2.2.3. Baicalin Reduced Lung Tissue W/D Ratio
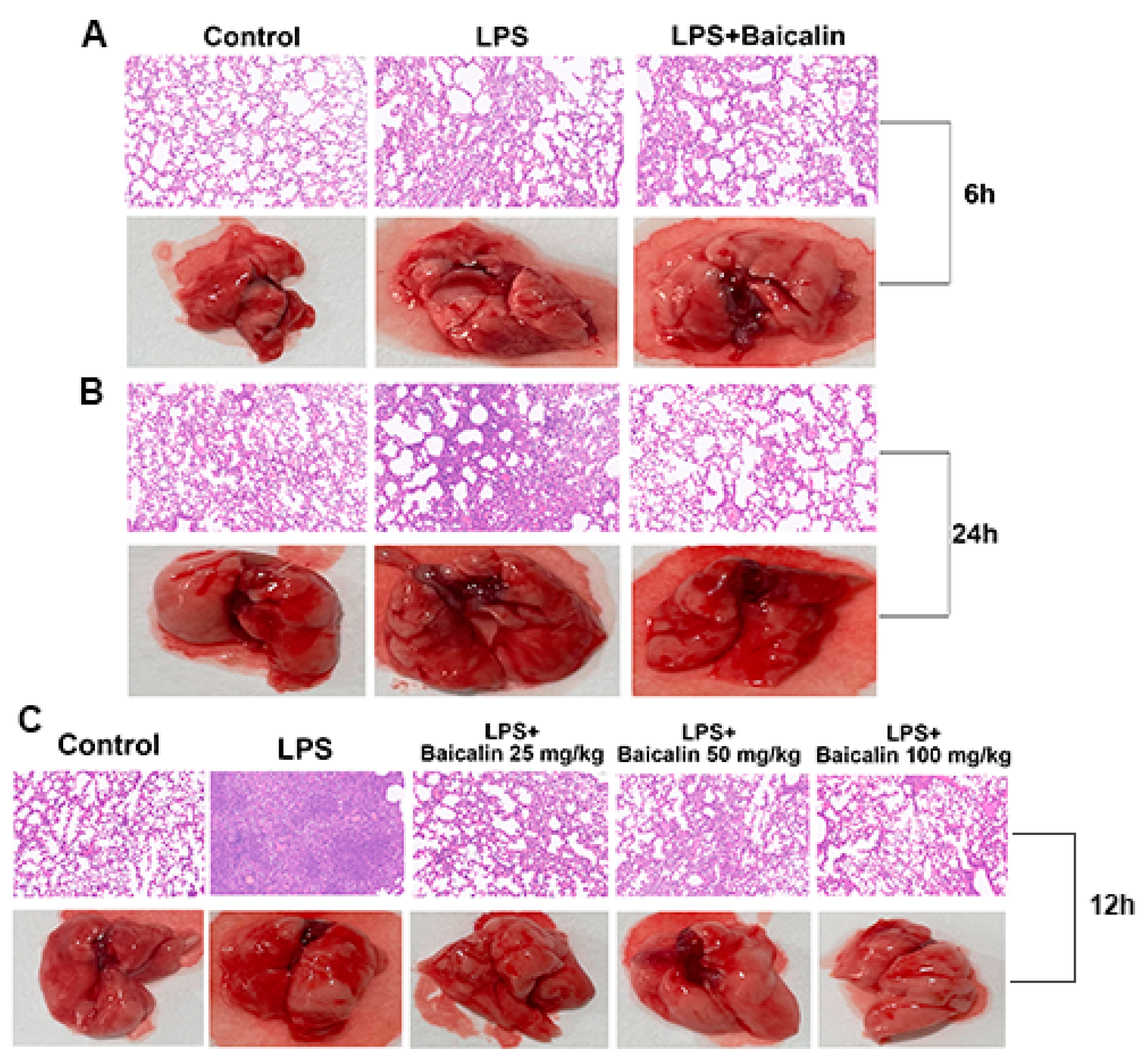
2.2.4. Baicalin Alleviates Lung Tissue Histopathological Changes in Mice Caused by LPS
2.2.5. Baicalin Reduces α-1 AT, IgE, and MPO and Increases IgG in LPS-Induced ALI Mice
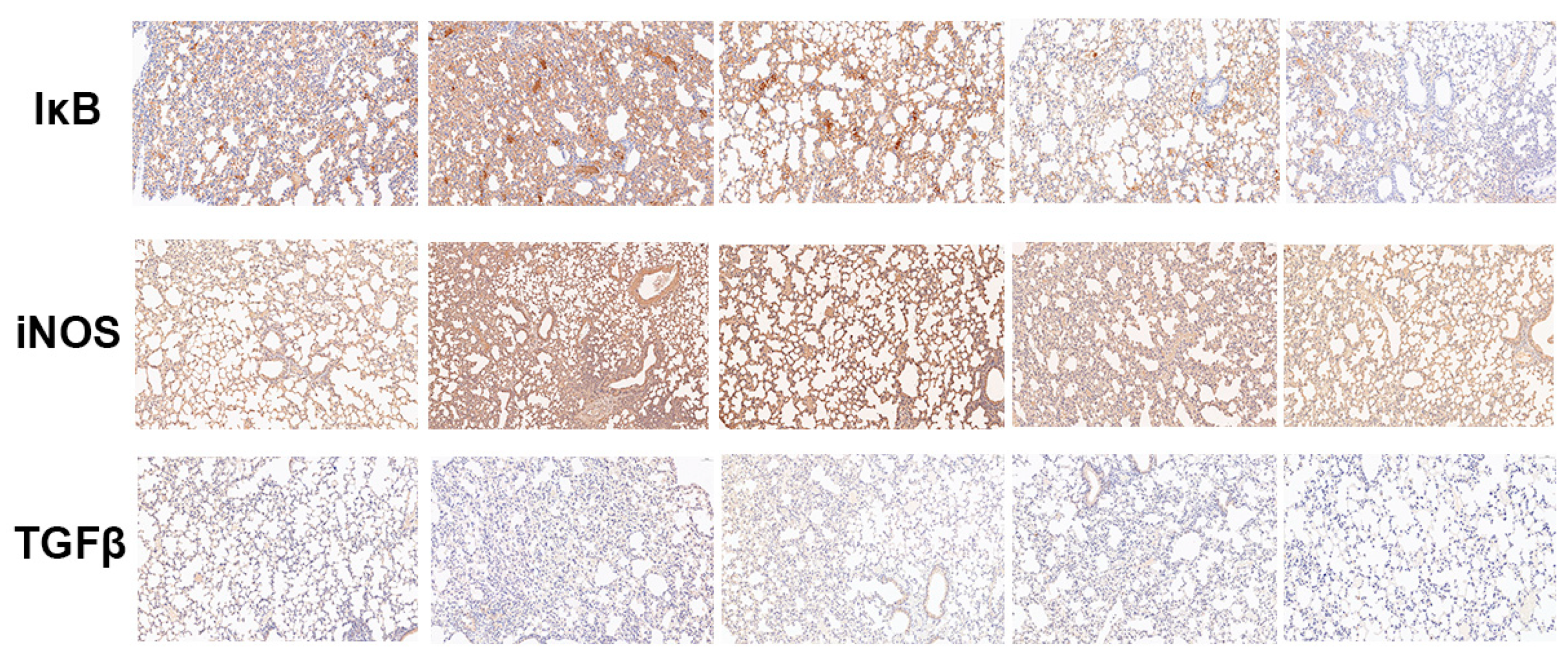
2.2.6. Baicalin Decreased the Protein Expression of IκB, iNOS, and TGF-β in LPS-Induced ALI Mice
3. Experimental Section
3.1. Cell Culture and Animal Feeding
3.2. Chemicals and Reagents
3.3. In Vitro Study
3.3.1. Cell Ability Assay
3.3.2. Cytokines’ Assay
3.3.3. Western Blot Analysis
3.3.4. Quantitative Real-Time Polymerase Chain Reaction
3.3.5. Immunofluorescence
3.4. In Vivo Study
3.4.1. Mice Model
3.4.2. Blood Routine Examination
3.4.3. BALF Collection and Cytokine Assay
3.4.4. Lung W/D Ratio
3.4.5. Histopathologic Evaluation of the Lung Tissue
3.4.6. Alpha-1 Antitrypsin (α-1 AT), IgE, IgG, and Myeloperoxidase (MPO) Activity Assay
3.4.7. Immunohistochemical of Lung Tissue
3.4.8. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviation List
References
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J.; Huang, Y.; Wynne, A.; Hanke, M.; Himler, J.; Bailey, M.T.; Sheridan, J.F.; Godbout, J.P. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflammation 2008, 5, 15. [Google Scholar] [CrossRef]
- Hou, Y.; Xie, G.; Liu, X.; Li, G.; Jia, C.; Xu, J.; Wang, B. Minocycline protects against lipopolysaccharide-induced cognitive impairment in mice. Psychopharmacology 2016, 233, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Zemans, R.L.; Colgan, S.P.; Downey, G.P. Transepithelial migration of neutrophils: Mechanisms and implications for acute lung injury. Am. J. Respir. Cell Mol. Biol. 2009, 40, 519–535. [Google Scholar] [CrossRef]
- Knapp, S.; Florquin, S.; Golenbock, D.T.; Van, d.P.T. Pulmonary lipopolysaccharide (LPS)-binding protein inhibits the LPS-induced lung inflammation in vivo. J. Immunol. 2006, 176, 3189. [Google Scholar] [CrossRef] [PubMed]
- Tobias, P.S.; Mathison, J.C.; Ulevitch, R.J. A family of lipopolysaccharide binding proteins involved in responses to gram-negative sepsis. J. Biol. Chem. 1988, 263, 13479–13481. [Google Scholar] [CrossRef]
- Tomlinson, J.E.; Blikslager, A.T. Interactions between lipopolysaccharide and the intestinal epithelium. J. Am. Vet. Med. Assoc. 2004, 224, 1446–1452. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85. [Google Scholar] [CrossRef]
- Sweet, M.J.; Hume, D.A. Endotoxin signal transduction in macrophages. J. Leukoc. Biol. 1996, 60, 8. [Google Scholar] [CrossRef]
- Stridh, L.; Ek, C.J.; Wang, X.; Nilsson, H.; Mallard, C. Regulation of toll-like receptors in the choroid plexus in the immature brain after systemic inflammatory stimuli. Transl. Stroke Res. 2013, 4, 220. [Google Scholar] [CrossRef] [PubMed]
- Panday, A.; Inda, M.E.; Bagam, P.; Sahoo, M.K.; Osorio, D.; Batra, S. Transcription factor NF-κB: An update on intervention strategies. Arch. Immunol. Ther. Exp. 2016, 64, 463–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, X.; Chu, X.; Zhang, X.; Song, K.; Jiang, Y.; Yu, L.; Deng, X. Preventive effects of valnemulin on lipopolysaccharide-induced acute lung injury in mice. Inflammation 2010, 33, 306–314. [Google Scholar] [CrossRef]
- Berlier, J.L.; Rigutto, S.; Dalla Valle, A.; Lechanteur, J.; Soyfoo, M.S.; Gangji, V.; Rasschaert, J. Adenosine triphosphate prevents serum deprivation-induced apoptosis in human mesenchymal stem cells via activation of the MAPK signaling pathways. Stem Cells 2015, 33, 211. [Google Scholar] [CrossRef] [PubMed]
- Olayanju, A.; Copple, I.M.; Bryan, H.K.; Edge, G.T.; Sison, R.L.; Wong, M.W.; Lai, Z.Q.; Lin, Z.X.; Dunn, K.; Sanderson, C.M. Brusatol provokes a rapid and transient inhibition of Nrf2 signaling and sensitizes mammalian cells to chemical toxicity-implications for therapeutic targeting of Nrf2. Free Radic. Biol. Med. 2015, 78, 202–212. [Google Scholar] [CrossRef]
- Sakata, S.; Hayashi, S.; Fujishiro, T.; Kawakita, K.; Kanzaki, N.; Hashimoto, S.; Iwasa, K.; Chinzei, N.; Kihara, S.; Haneda, M. Oxidative stress-induced apoptosis and matrix loss of chondrocytes is inhibited by eicosapentaenoic acid. J. Orthop. Res. 2015, 33, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Shi, Z.; Peng, Z.; Zhao, C.; Zhang, Y.; Wang, Z.; Li, X.; Liu, G.; Li, X. Acetoacetate induces hepatocytes apoptosis by the ROS-mediated MAPKs pathway in ketotic cows. J. Cell. Physiol. 2017, 232, 3296–3308. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Q. Activation of PPARγ by baicalin attenuates pulmonary hypertension in an infant rat model by suppressing HMGB1/RAGE signaling. FEBS Open Bio 2017, 7, 477. [Google Scholar] [CrossRef]
- Huang, Y.; Tsang, S.Y.; Yao, X.; Chen, Z.Y. Biological properties of baicalein in cardiovascular system. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 177. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wei, Z.; Zhou, E.; Zhang, N.; Yang, Z. Cyanidin-3-O-β-glucoside inhibits lipopolysaccharide-induced inflammatory response in mouse mastitis model. J. Lipid Res. 2014, 55, 1111–1119. [Google Scholar] [CrossRef]
- Płóciennikowska, A.; Hromadajudycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. Cmls 2015, 72, 557. [Google Scholar] [CrossRef]
- Reiss, L.K.; Uhlig, U.; Uhlig, S. Models and mechanisms of acute lung injury caused by direct insults. Eur. J. Cell Biol. 2012, 91, 590. [Google Scholar] [CrossRef] [PubMed]
- Barabutis, N.; Dimitropoulou, C.; Birmpas, C.; Joshi, A.; Thangjam, G.; Catravas, J.D. p53 protects against LPS-Induced lung endothelial barrier dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L776. [Google Scholar] [CrossRef] [PubMed]
- Birukov, K.G.; Zebda, N.; Birukova, A.A. Barrier enhancing signals in pulmonary edema. Compr. Physiol. 2013, 3, 429–484. [Google Scholar]
- Antonov, A.; Snead, C.; Gorshkov, B.; Antonova, G.N.; Verin, A.D.; Catravas, J.D. Heat shock protein 90 inhibitors protect and restore pulmonary endothelial barrier function. Am. J. Respir. Cell Mol. Biol. 2008, 39, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, X.; Yu, S.; Kou, J. Molecular mechanisms in lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Int. Immunopharmacol. 2015, 29, 937–946. [Google Scholar] [CrossRef]
- Tasaka, S.; Amaya, F.; Hashimoto, S.; Ishizaka, A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid. Redox Signal. 2008, 10, 739–753. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zimmerman, G.A. Acute Lung Injury and the Acute Respiratory Distress Syndrome; Springer: London, UK, 2009; pp. 369–376. [Google Scholar]
- Aggarwal, N.R.; D’Alessio, F.R.; Tsushima, K.; Files, D.C.; Damarla, M.; Sidhaye, V.K.; Fraig, M.M.; Polotsky, V.Y.; King, L.S. Moderate oxygen augments lipopolysaccharide-induced lung injury in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 298, L371–L381. [Google Scholar] [CrossRef]
- Baltimore, D. Discovering NF-kappaB. Cold Spring Harb. Perspect. Biol. 2009, 1, a000026. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725. [Google Scholar] [CrossRef]
- Rico-Rosillo, G.; Vega-Robledo, G.B. The involvement of NF-κB transcription factor in asthma. Alergia 2011, 58, 107–111. [Google Scholar]
- Sun, X.; Chen, X.; Wang, T.; Wang, Z.; Sun, G.; Li, X.; Li, X.; Liu, G. Histamine induces bovine rumen epithelial cell inflammatory response via NF-κB pathway. Cell. Physiol. Biochem. 2017, 42, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Yu, L.; Zhang, X.; Wu, Q.; Wang, D.; Wang, X.; Xia, C.; Feng, H. Asiaticoside attenuates lipopolysaccharide-induced acute lung injury via down-regulation of NF-κB signaling pathway. Int. Immunopharmacol. 2015, 26, 181. [Google Scholar] [CrossRef] [PubMed]


| Treatment 1 | |||||||
|---|---|---|---|---|---|---|---|
| Items | CON | LPS (5 mg/kg) | LPS + BA (25 mg/kg) | LPS + BA (50 mg/kg) | LPS + BA (100 mg/kg) | SEM | p-Value |
| WBC 2 (109/L) | 6.1 b | 13.1 a | 8.6 b | 8.0 b | 6.0 b | 0.77 | 0.002 |
| Lymph 3 (109/L) | 4.1 bc | 8.4 a | 5.1 b | 4.3 bc | 3.1 c | 0.52 | 0.000 |
| Mon 4 (109/L) | 0.3 b | 1.3 a | 0.8 ab | 0.4 b | 0.3 b | 0.13 | 0.033 |
| Gran 5 (109/L) | 1.7 b | 3.4 a | 2.7 a | 3.3 a | 2.6 ab | 0.20 | 0.023 |
| Lymph% | 67.73 a | 64.50 a | 59.73 ab | 53.77 b | 51.73 b | 1.93 | 0.009 |
| Mon% | 4.59 | 9.67 | 8.77 | 4.90 | 4.80 | 0.89 | 0.180 |
| Gran% | 27.63 bc | 25.87 c | 31.50 b | 41.33 a | 43.43 a | 1.99 | 0.000 |
| RBC 6 (1012/L) | 12.02 | 11.43 | 12.09 | 11.57 | 11.38 | 0.73 | 0.998 |
| HGB 7 (g/L) | 183 | 172 | 190 | 175 | 174 | 7.18 | 0.950 |
| HCT 8% | 58.0 | 54.0 | 58.3 | 57.1 | 55.9 | 1.79 | 0.961 |
| MCV 9 (fL) | 48.3 | 47.3 | 48.3 | 49.4 | 49.2 | 1.37 | 0.993 |
| MCH 10 (pg) | 15.2 | 15.0 | 14.8 | 15.1 | 15.2 | 0.73 | 1.000 |
| MCHC 11 (g/L) | 315 | 318 | 308 | 306 | 311 | 3.73 | 0.886 |
| RDW 12% | 14.3 | 13.5 | 14.3 | 13.3 | 14.0 | 0.56 | 0.980 |
| PLT 13 (109/L) | 1143 | 1017 | 874 | 1072 | 1039 | 58.06 | 0.739 |
| MPV 14 (fL) | 7.2 | 6.7 | 6.9 | 6.8 | 6.6 | 0.41 | 0.995 |
| PDW 15 | 16.7 | 16.9 | 16.8 | 16.7 | 16.6 | 0.66 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, B.; Zhang, H.; Zhu, Z.; Ling, Z.; Zeng, F.; Wang, Y.; Wang, J. Baicalin Relieves LPS-Induced Lung Inflammation via the NF-κB and MAPK Pathways. Molecules 2023, 28, 1873. https://doi.org/10.3390/molecules28041873
Shen B, Zhang H, Zhu Z, Ling Z, Zeng F, Wang Y, Wang J. Baicalin Relieves LPS-Induced Lung Inflammation via the NF-κB and MAPK Pathways. Molecules. 2023; 28(4):1873. https://doi.org/10.3390/molecules28041873
Chicago/Turabian StyleShen, Bingyu, Haoqing Zhang, Zhengjin Zhu, Zixi Ling, Fangyuan Zeng, Yazhou Wang, and Jianguo Wang. 2023. "Baicalin Relieves LPS-Induced Lung Inflammation via the NF-κB and MAPK Pathways" Molecules 28, no. 4: 1873. https://doi.org/10.3390/molecules28041873
APA StyleShen, B., Zhang, H., Zhu, Z., Ling, Z., Zeng, F., Wang, Y., & Wang, J. (2023). Baicalin Relieves LPS-Induced Lung Inflammation via the NF-κB and MAPK Pathways. Molecules, 28(4), 1873. https://doi.org/10.3390/molecules28041873