Effects of Dietary Ferulic Acid on Intestinal Health and Ileal Microbiota of Tianfu Broilers Challenged with Lipopolysaccharide
Abstract
1. Introduction
2. Results
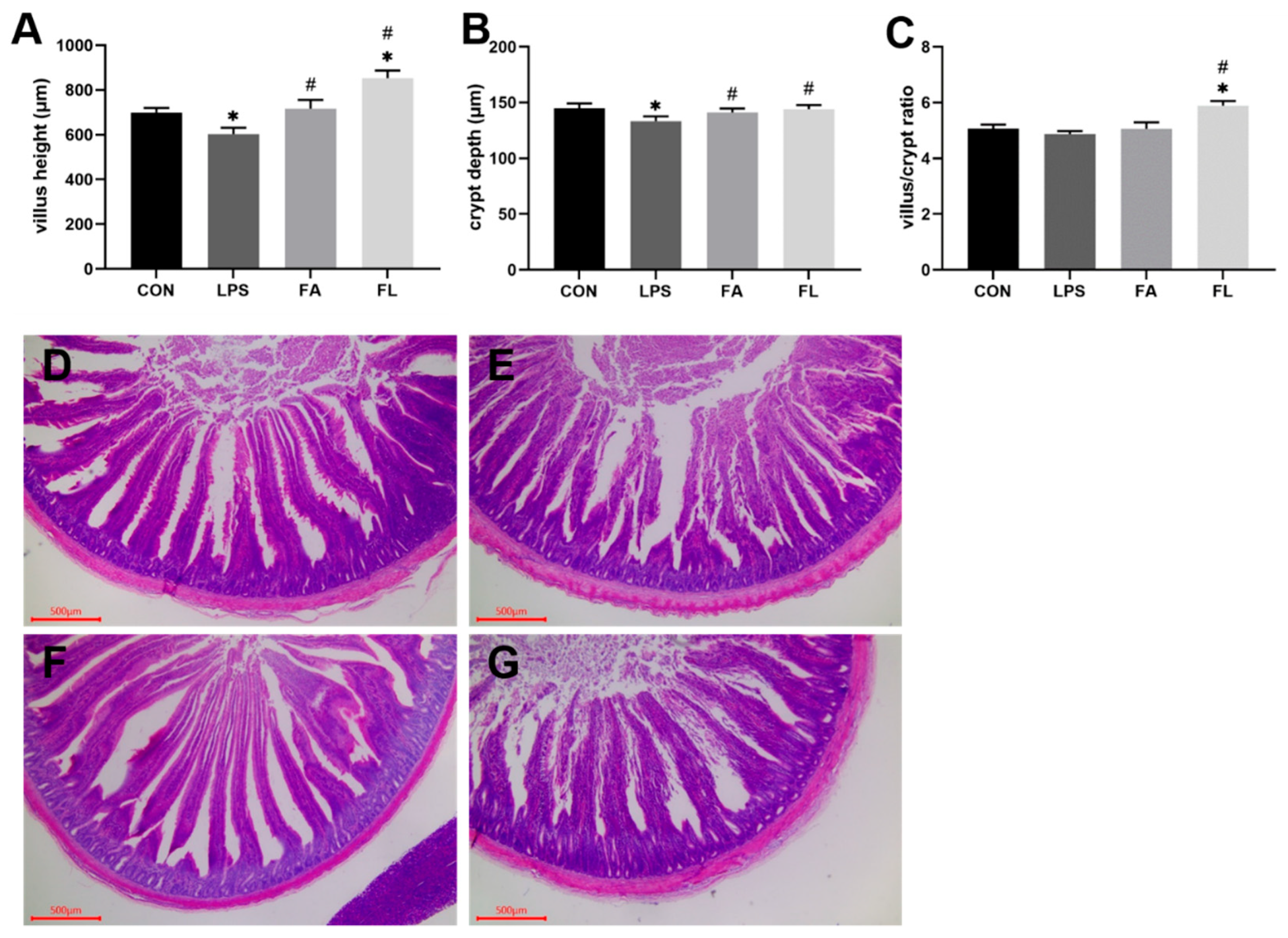
2.1. Intestinal Morphological Analysis
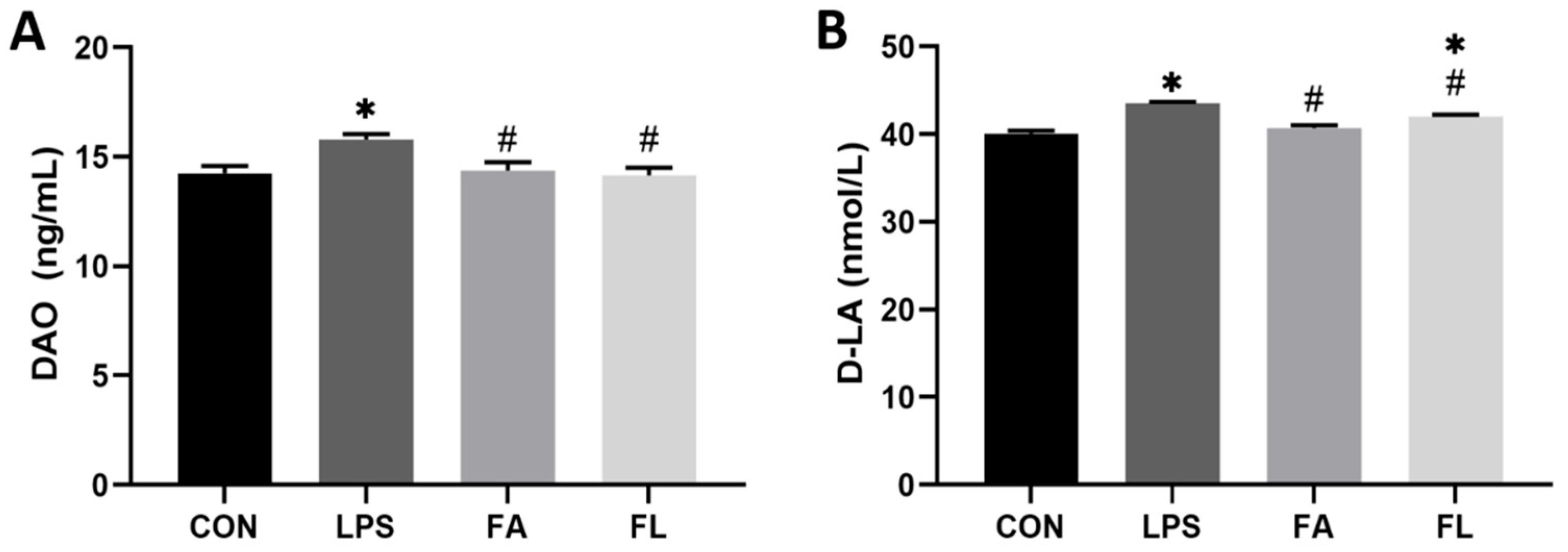
2.2. Intestinal Permeability Biochemical Analysis
2.3. Antioxidant Parameters of Intestinal Mucosa
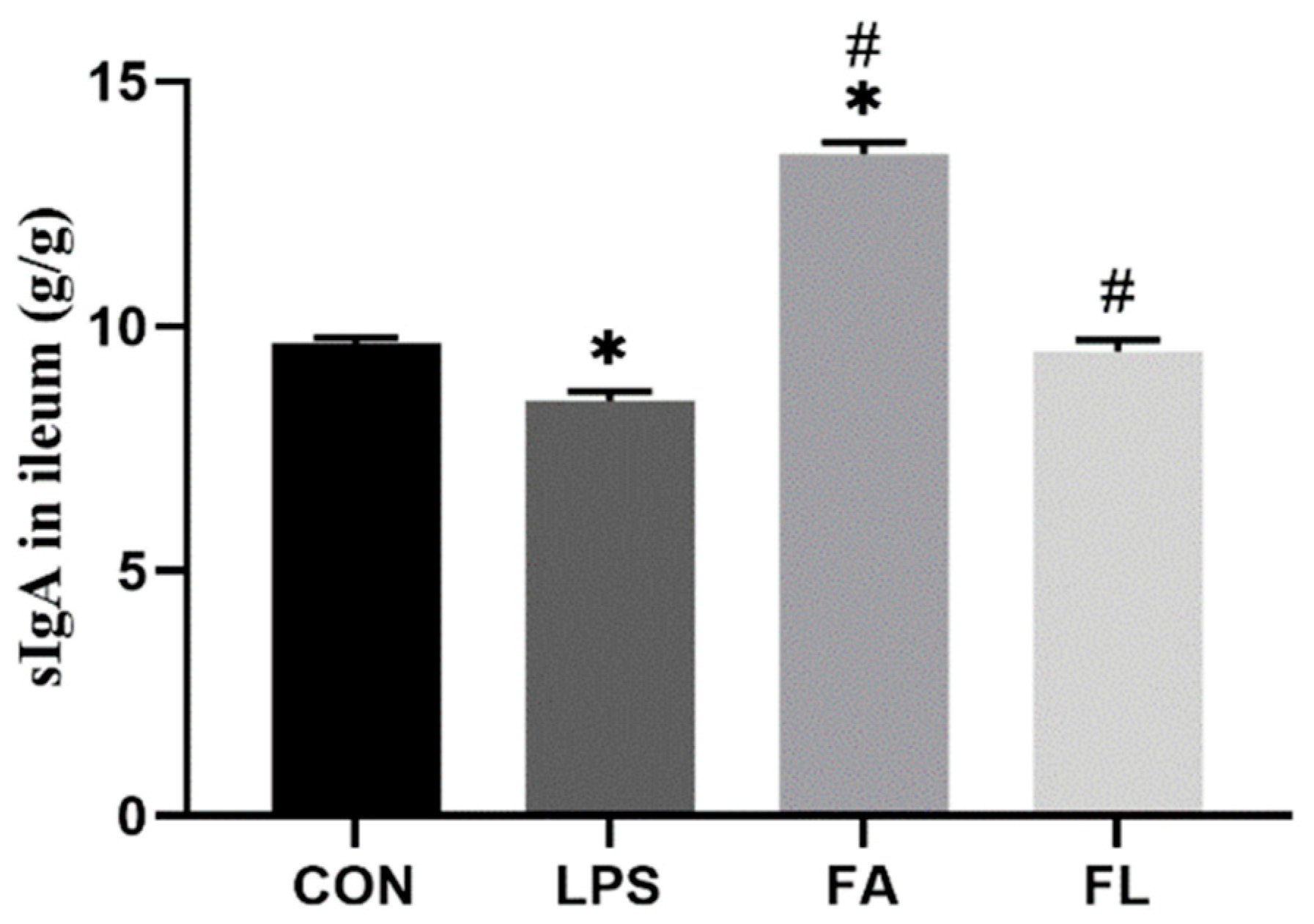
2.4. sIgA Content in Ileal Mucosa
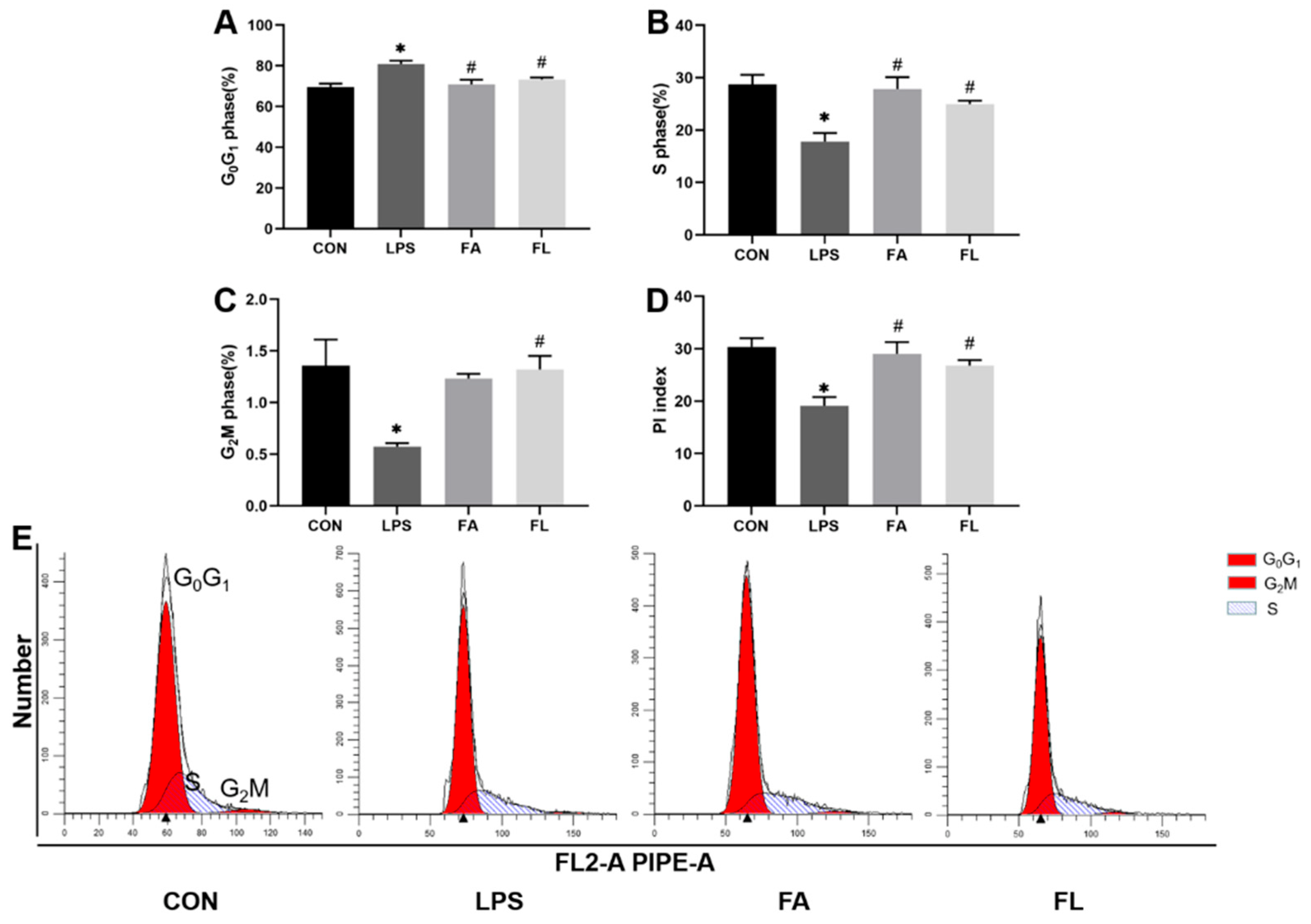
2.5. Life Cycle of the Ileal Epithelium
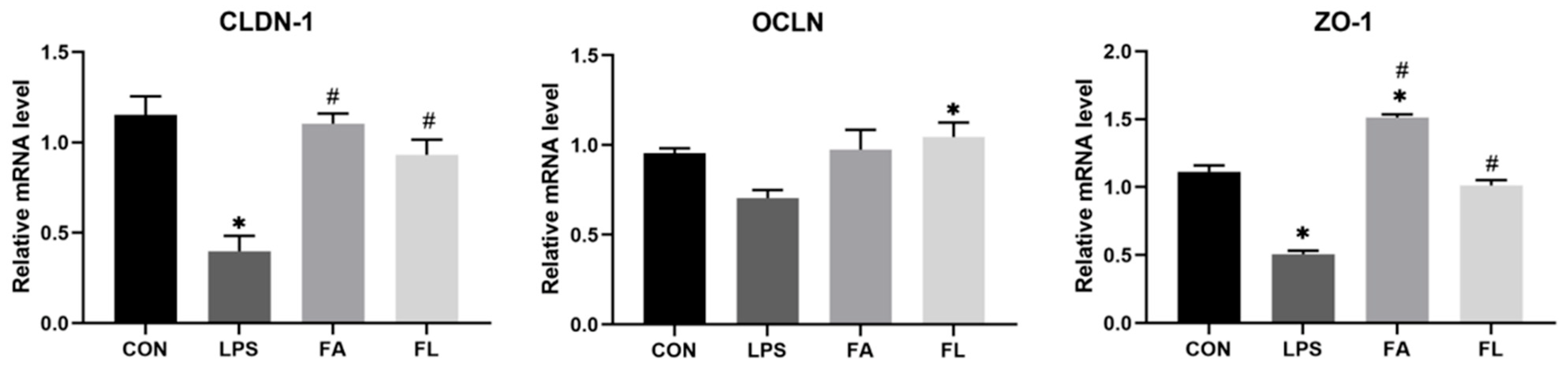
2.6. Relative mRNA Expressions of Intestinal Tight Junction Proteins
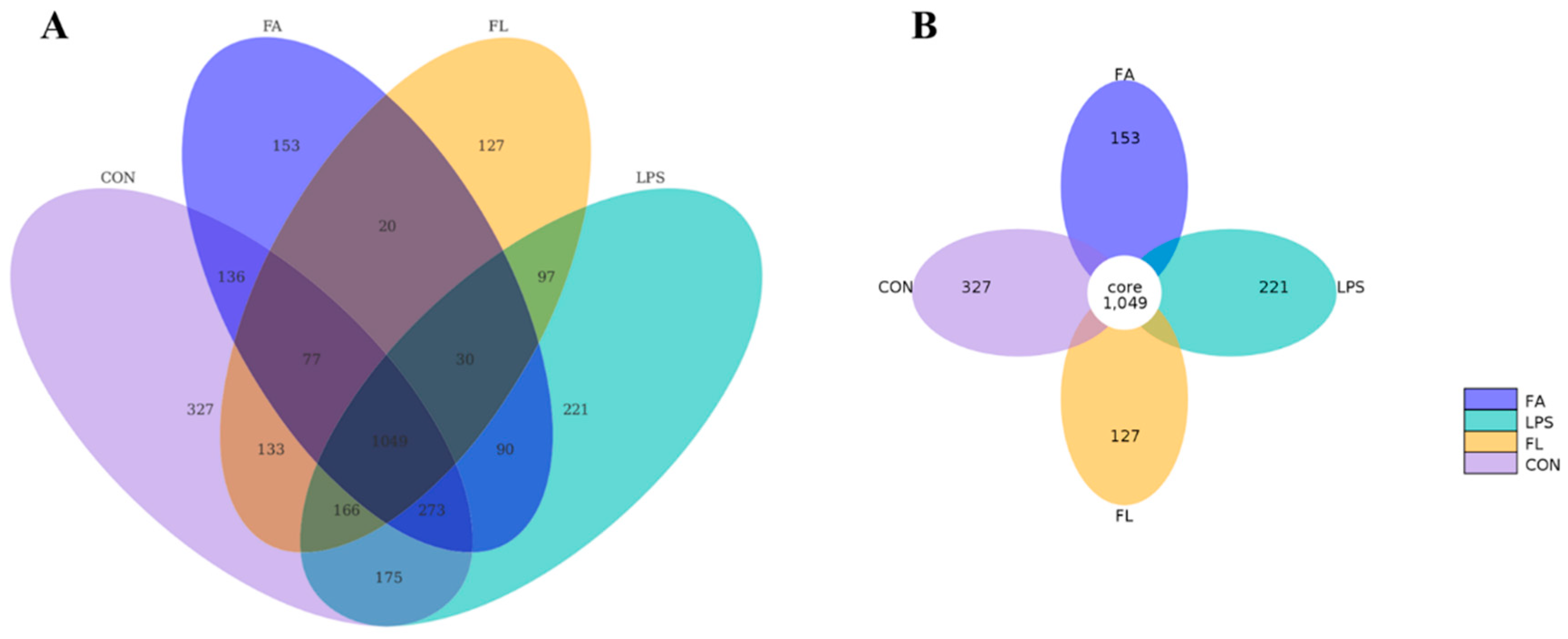
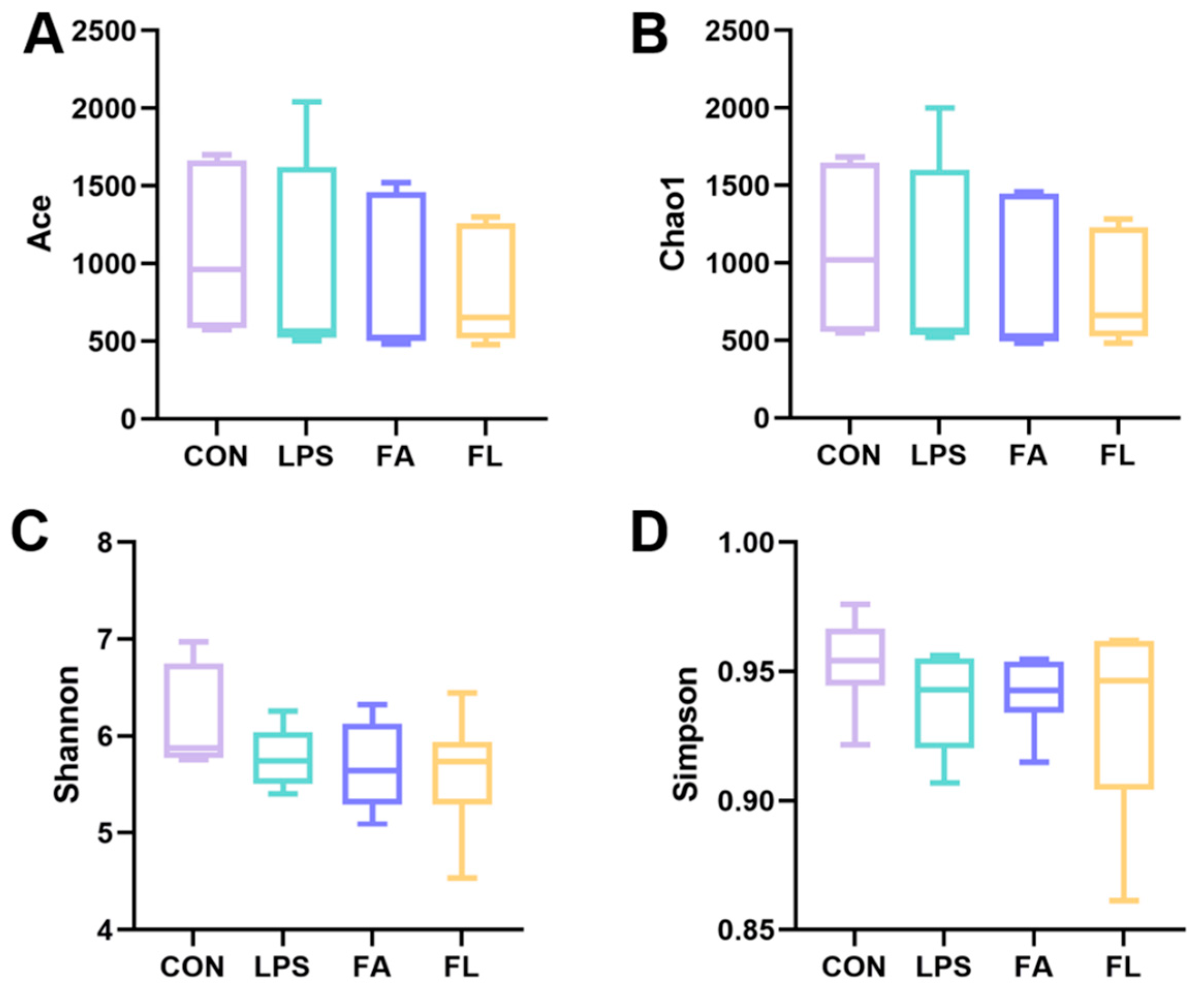
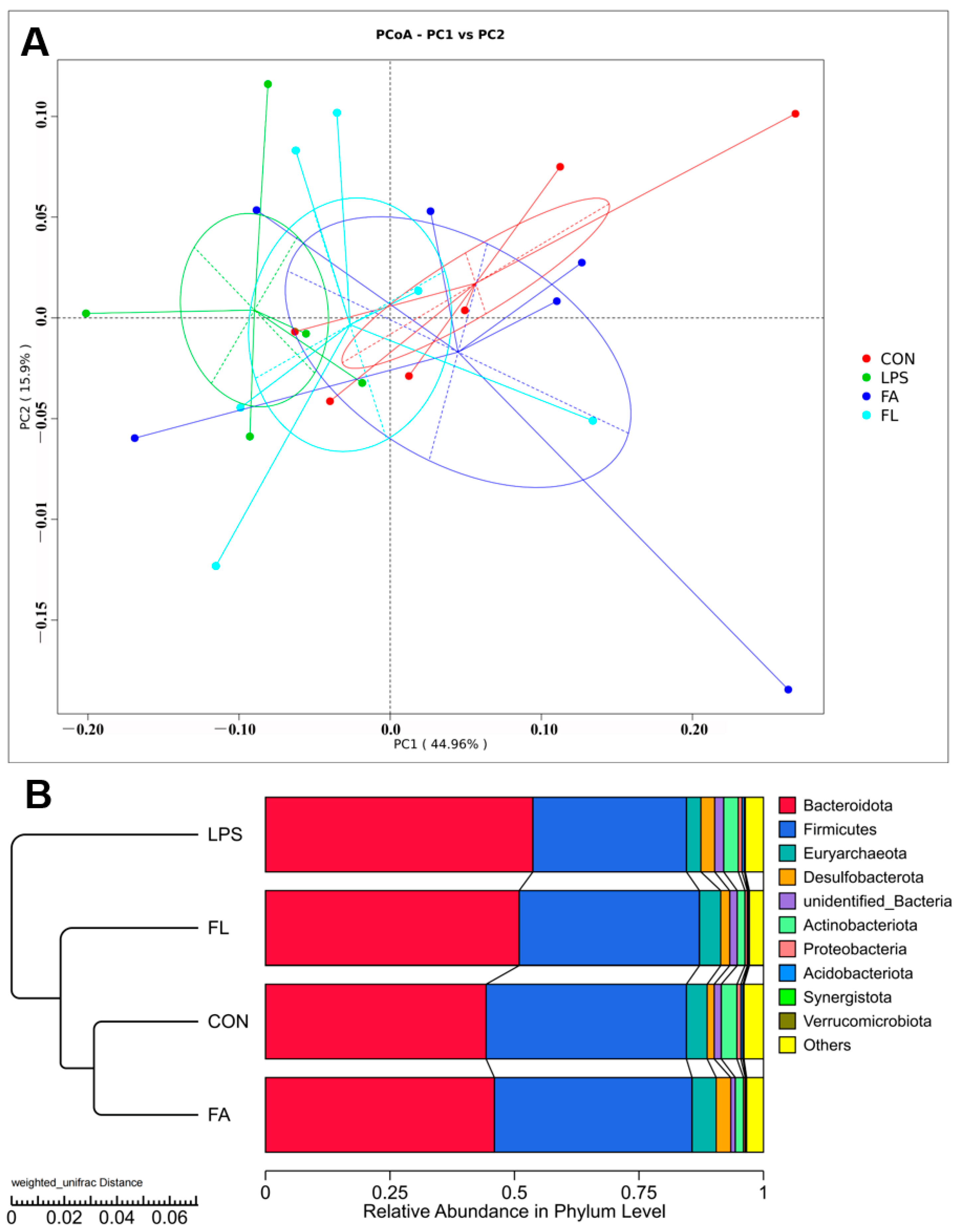
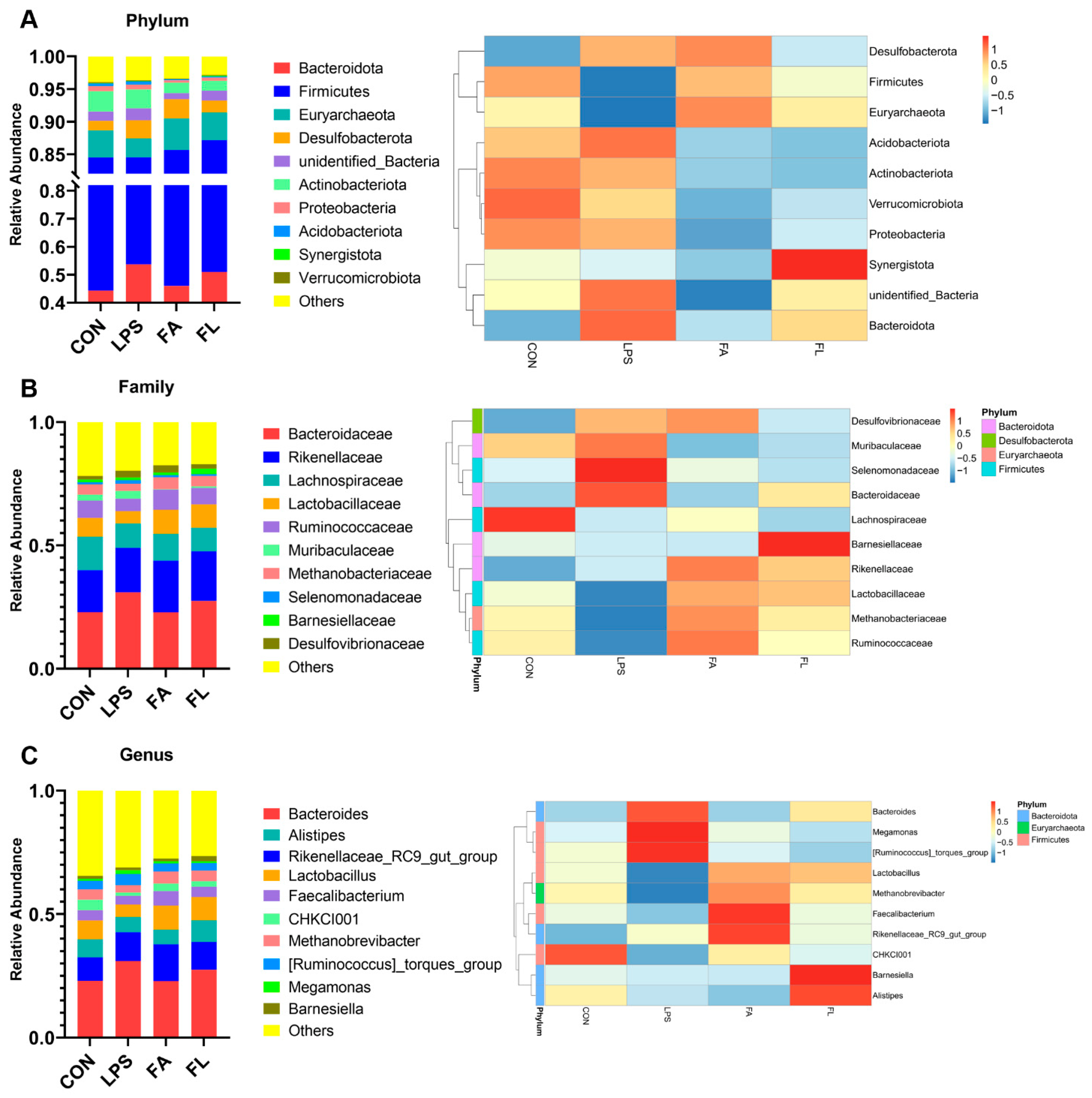
2.7. Ileal Microbiota Composition
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Experimental Design
4.3. Sample Collection and Measurement
4.4. Morphological Analysis
4.5. Permeability and sIgA Content Analysis
4.6. Antioxidant Parameters
4.7. Life Cycle of Ileum Epithelium
4.8. Real-Time Quantificative PCR (RT-qPCR)
4.9. 16S rRNA Sequencing and Data Analysis
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Di Lorenzo, F.; Duda, K.A.; Lanzetta, R.; Silipo, A.; De Castro, C.; Molinaro, A. A journey from structure to function of bacterial lipopolysaccharides. Chem. Rev. 2021, 122, 15767–15821. [Google Scholar] [CrossRef]
- Geng, Y.; Ma, Q.; Wang, Z.; Guo, Y. Dietary vitamin d3 supplementation protects laying hens against lipopolysaccharide-induced immunological stress. Nutr. Metab. 2018, 15, 58. [Google Scholar] [CrossRef]
- Anders, L.C.; Lang, A.L.; Anwar-Mohamed, A.; Douglas, A.N.; Bushau, A.M.; Falkner, K.C.; Hill, B.G.; Warner, N.L.; Arteel, G.E.; Cave, M.; et al. Vinyl chloride metabolites potentiate inflammatory liver injury caused by lps in mice. Toxicol. Sci. 2016, 151, 312–323. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Peng, Y.L.; Liu, Y.Z.; Wu, T.Y.; Shen, X.L.; Zhou, J.R.; Sun, D.Y.; Huang, A.J.; Wang, X.; et al. Involvement of inflammasome activation in lipopolysaccharide–induced mice depressive–like behaviors. CNS Neurosci. Ther. 2014, 20, 119–124. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, H.; Zhang, T.; Zhang, C.; He, Y.; Du, L.; Zuo, F.; Wang, W. Lung-protective effect of punicalagin on lps-induced acute lung injury in mice. Biosci. Rep. 2022, 42, BSR20212196. [Google Scholar] [CrossRef]
- Tgkagi, T.; Taguchi, O.; Aoki, S.; Toda, M.; Yamaguchi, A.; Fujimoto, H.; Bobeda-Ruiz, D.; Gil-Bernabe, P.; Ramirez, A.Y.; Naito, M.; et al. Direct effects of protein s in ameliorating acute lung injury. J. Thromb. Haemost. 2009, 7, 2053–2063. [Google Scholar] [CrossRef]
- Plotnikov, E.; Pevzner, I.; Zorova, L.; Chernikov, V.; Prusov, A.; Kireev, I.; Silachev, D.; Skulachev, V.; Zorov, D. Mitochondrial damage and mitochondria-targeted antioxidant protection in lps-induced acute kidney injury. Antioxidants 2019, 8, 176. [Google Scholar] [CrossRef]
- Bhattacharyya, J.; Biswas, S.; Datta, A.G. Mode of action of endotoxin: Role of free radicals and antioxidants. Curr. Med. Chem. 2004, 11, 359–368. [Google Scholar] [CrossRef]
- Jiang, J.; Qi, L.; Wei, Q.; Shi, F. Maternal stevioside supplementation ameliorates intestinal mucosal damage and modulates gut microbiota in chicken offspring challenged with lipopolysaccharide. Food Funct. 2021, 12, 6014–6028. [Google Scholar] [CrossRef]
- Yan, L.; Lv, Z.Z.; An, S.; Xing, K.; Wang, Z.G.; Lv, M.B.; Choct, M.; Guo, Y.M.; Zhou, G.L. Effects of rearing system and narasin on growth performance, gastrointestinal development, and gut microbiota of broilers. Poult. Sci. 2021, 100, 100840. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; He, L.; Wan, D.; Liu, G.; Wu, X.; Yin, Y. Serine prevents lps-induced intestinal inflammation and barrier damage via p53-dependent glutathione synthesis and ampk activation. J. Funct. Food 2017, 39, 225–232. [Google Scholar] [CrossRef]
- Ozdemir, D.; Uysal, N.; Tugyan, K.; Gonenc, S.; Acikgoz, O.; Aksu, I.; Ozkan, H. The effect of melatonin on endotoxemia-induced intestinal apoptosis and oxidative stress in infant rats. Intensive Care Med. 2007, 33, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.F.; Chen, Y.P.; Jin, R.; Wang, C.; Wen, C.; Zhou, Y.M. Dietary chitooligosaccharide supplementation alleviates intestinal barrier damage, and oxidative and immunological stress in lipopolysaccharide-challenged laying hens. Poult. Sci. 2022, 101, 101701. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, Q.; Zeng, X.; Xu, Y.; Jin, K.; Liu, J.; Cao, G. Effects of probiotics on the growth performance, antioxidant functions, immune responses, and caecal microbiota of broilers challenged by lipopolysaccharide. Front. Vet. Sci. 2022, 9, 846649. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Yu, Y.H. Bacillus subtilis-fermented products ameliorate the growth performance and alter cecal microbiota community in broilers under lipopolysaccharide challenge. Poult. Sci. 2021, 100, 875–886. [Google Scholar] [CrossRef]
- Ullah, A.; Anjum, A.A.; Rabbani, M.; Nawaz, M.; Ashraf, M.; Ijaz, M.; Ali, A.; Rashid, A.; Najeeb, I.; Pervez, A. Phytochemical composition and in-vitro activity of ethanolic extract of eucalyptus globulus leaves against multidrug resistant poultry pathogens. Cell. Mol. Biol. 2021, 67, 159–164. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, D.; Shi, B.; Faucitano, L.; Guo, X.; Li, T.; Xu, Y.; Yan, S. Dietary supplementation with artemisia argyi extract on inflammatory mediators and antioxidant capacity in broilers challenged with lipopolysaccharide. Ital. J. Anim. Sci. 2020, 19, 1091–1098. [Google Scholar] [CrossRef]
- Konieczka, P.; Szkopek, D.; Kinsner, M.; Fotschki, B.; Juskiewicz, J.; Banach, J. Cannabis-derived cannabidiol and nanoselenium improve gut barrier function and affect bacterial enzyme activity in chickens subjected to C. Perfringens challenge. Vet. Res. 2020, 51, 141. [Google Scholar] [CrossRef]
- Csernus, B.; Biro, S.; Babinszky, L.; Komlosi, I.; Javor, A.; Stundl, L.; Remenyik, J.; Bai, P.; Olah, J.; Pesti-Asboth, G.; et al. Effect of carotenoids, oligosaccharides and anthocyanins on growth performance, immunological parameters and intestinal morphology in broiler chickens challenged with escherichia coli lipopolysaccharide. Animals 2020, 10, 347. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, J.K.; Kim, K.M.; Lee, H.J.; Kim, S. In vitro and in vivo antithrombotic and cytotoxicity effects of ferulic acid. J. Biochem. Mol. Toxicol. 2018, 32, e22004. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Sun, Y.; Yang, M.; Song, K.; Zheng, Z.; Chen, Y.; Liu, X.; Jia, Z.; Dong, R.; et al. Antimicrobial activity of ferulic acid against cronobacter sakazakii and possible mechanism of action. Foodborne Pathog. Dis. 2016, 13, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Ghosh, S.; Das, A.K.; Sil, P.C. Ferulic acid protects hyperglycemia-induced kidney damage by regulating oxidative insult, inflammation and autophagy. Front. Pharmacol. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Ali, T.; Alam, S.I.; Ullah, R.; Zeb, A.; Lee, K.W.; Rutten, B.; Kim, M.O. Ferulic acid rescues lps-induced neurotoxicity via modulation of the tlr4 receptor in the mouse hippocampus. Mol. Neurobiol. 2019, 56, 2774–2790. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Z.; Shen, L.; Guo, X.; Ji, D.; Sun, S.; Edwards, M.G.; Frank, F.; Li, J.F.; Salama, A.; et al. Well modelling methods in thermal reservoir simulation. Oil Gas Sci. Technol. 2020, 75, 63. [Google Scholar] [CrossRef]
- Zheng, Y.; You, X.; Guan, S.; Huang, J.; Wang, L.; Zhang, J.; Wu, J. Poly(ferulic acid) with an anticancer effect as a drug nanocarrier for enhanced colon cancer therapy. Adv. Funct. Mater. 2019, 29, 1808646. [Google Scholar] [CrossRef]
- Yin, X.; Liu, W.; Chen, H.; Qi, C.; Chen, H.; Niu, H.; Yang, J.; Kwok, K.W.H.; Dong, W. Effects of ferulic acid on muscle development and intestinal microbiota of zebrafish. J. Anim. Physiol. Anim. Nutr. 2022, 106, 429–440. [Google Scholar] [CrossRef]
- Hu, R.; Wu, S.; Li, B.; Tan, J.; Yan, J.; Wang, Y.; Tang, Z.; Liu, M.; Fu, C.; Zhang, H.; et al. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim. Nutr. 2022, 8, 144–152. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Li, M.; Huang, Z.; Jiang, J.; Chen, Y.; Chen, J.; Jia, Y.; Zhang, L.; Zhou, F. Ferulic acid ameliorates atherosclerotic injury by modulating gut microbiota and lipid metabolism. Front. Pharmacol. 2021, 12, 621339. [Google Scholar] [CrossRef]
- Furness, J.B.; Kunze, W.A.; Clerc, N. Nutrient tasting and signaling mechanisms in the gut. Ii. The intestine as a sensory organ: Neural, endocrine, and immune responses. Am. J. Physiol. 1999, 277, G922–G928. [Google Scholar]
- Gautam, R.; Heo, Y.; Lim, G.; Song, E.; Roque, K.; Lee, J.; Kim, Y.; Cho, A.; Shin, S.; Kim, C.; et al. Altered immune responses in broiler chicken husbandry workers and their association with endotoxin exposure. Ind. Health 2018, 56, 10–19. [Google Scholar] [CrossRef]
- Tan, J.; Liu, S.; Guo, Y.; Applegate, T.J.; Eicher, S.D. Dietary l-arginine supplementation attenuates lipopolysaccharide-induced inflammatory response in broiler chickens. Br. J. Nutr. 2014, 111, 1394–1404. [Google Scholar] [CrossRef]
- Xiong, W.; Ma, H.; Zhang, Z.; Jin, M.; Wang, J.; Xu, Y.; Wang, Z. The protective effect of icariin and phosphorylated icariin against lps-induced intestinal epithelial cells injury. Biomed. Pharmacother. 2019, 118, 109246. [Google Scholar] [CrossRef]
- Rizzo, V.; Ferlazzo, N.; Currò, M.; Isola, G.; Matarese, M.; Bertuccio, M.P.; Caccamo, D.; Matarese, G.; Ientile, R. Baicalin-induced autophagy preserved lps-stimulated intestinal cells from inflammation and alterations of paracellular permeability. Int. J. Mol. Sci. 2021, 22, 2315. [Google Scholar] [CrossRef]
- Yan, J.; Gong, Z.; Zhang, T.; Cai, W. Sodium butyrate attenuates soybean oil-based lipid emulsion-induced increase in intestinal permeability of lipopolysaccharide by modulation of p-glycoprotein in caco-2 cells. Biochem. Biophys. Res. Commun. 2017, 482, 791–795. [Google Scholar] [CrossRef]
- Kong, Y.; Yan, T.; Tong, Y.; Deng, H.; Tan, C.; Wan, M.; Wang, M.; Meng, X.; Wang, Y. Gut microbiota modulation by polyphenols fromaronia melanocarpa of lps-induced liver diseases in rats. J. Agric. Food Chem. 2021, 69, 3312–3325. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.; Guo, S.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K.; Abdel-Raheem, S.; Böhm, J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009, 88, 49–56. [Google Scholar] [CrossRef]
- Jia, G.; Yan, J.Y.; Cai, J.Y.; Wang, K.N. Effects of encapsulated and non-encapsulatedcompound acidifiers on gastrointestinal ph andintestinal morphology and function in weaningpiglets. J. Anim. Feed Sci. 2010, 19, 81–92. [Google Scholar] [CrossRef]
- Shu, G.; Tang, Z.; Du, H.; Zheng, Y.; Chang, L.; Li, H.; Xu, F.; Fu, H.; Zhang, W.; Lin, J. Effects of dietary ferulic acid supplementation on hepatic injuries in tianfu broilers challenged with lipopolysaccharide. Toxins 2022, 14, 227. [Google Scholar] [CrossRef]
- Gadde, U.D.; Oh, S.; Lee, Y.; Davis, E.; Zimmerman, N.; Rehberger, T.; Lillehoj, H.S. Retracted: Dietary bacillus subtilis-based direct-fed microbials alleviate lps-induced intestinal immunological stress and improve intestinal barrier gene expression in commercial broiler chickens. Res. Vet. Sci. 2017, 114, 236–243. [Google Scholar] [CrossRef]
- An, J.; Shi, J.; Liu, K.; Li, A.; He, B.; Wang, Y.; Duan, T.; Wang, Y.; He, J. Effects of solid-state fermented wheat bran on growth performance, immune function, intestinal morphology and microflora in lipopolysaccharide-challenged broiler chickens. Animals 2022, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, F.; Xu, L.; Yin, P.; Li, D.; Mei, C.; Jiang, L.; Ma, Y.; Xu, J. Protective effects of ferulic acid against heat stress-induced intestinal epithelial barrier dysfunction in vitro and in vivo. PLoS ONE 2016, 11, e145236. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Rong, H.; Zhang, X.; Dong, M. Ferulic acid ameliorates mpp+/mptp-induced oxidative stress via erk1/2-dependent nrf2 activation: Translational implications for parkinson disease treatment. Mol. Neurobiol. 2020, 57, 2981–2995. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, J.; Hao, Y.; Liu, Y. Identification of the dpph radical scavenging reaction adducts of ferulic acid and sinapic acid and their structure-antioxidant activity relationship. LWT 2021, 146, 111411. [Google Scholar] [CrossRef]
- Thompson, J.S.; Vaughan, W.P.; Forst, C.F.; Jacobs, D.L.; Weekly, J.S.; Rikkers, L.F. The effect of the route of nutrient delivery on gut structure and diamine oxidase levels. J. Parenter. Enter. Nutr. 1987, 11, 28–32. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Z.; Zhan, W.; Jiang, R.; Yang, C.; Zhan, H.; Xiong, Y. Recombinant tsp53 modulates intestinal epithelial barrier integrity via upregulation of zo1 in lpsinduced septic mice. Mol. Med. Rep. 2018, 17, 1212–1218. [Google Scholar]
- Yang, L.; Liu, G.; Lian, K.; Qiao, Y.; Zhang, B.; Zhu, X.; Luo, Y.; Shang, Y.; Gu, X. Dietary leonurine hydrochloride supplementation attenuates lipopolysaccharide challenge-induced intestinal inflammation and barrier dysfunction by inhibiting the nf-κb/mapk signaling pathway in broilers. J. Anim. Sci. 2019, 97, 1679–1692. [Google Scholar] [CrossRef]
- Yu, L.; Wen, H.; Jiang, M.; Wu, F.; Tian, J.; Lu, X.; Xiao, J.; Liu, W. Effects of ferulic acid on intestinal enzyme activities, morphology, microbiome composition of genetically improved farmed tilapia (oreochromis niloticus) fed oxidized fish oil. Aquaculture 2020, 528, 735543. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Mestecky, J.; Russell, M.W.; Elson, C.O. Intestinal iga: Novel views on its function in the defence of the largest mucosal surface. Gut 1999, 44, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Kaetzel, C.S. Cooperativity among secretory iga, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunol. Lett. 2014, 162, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Dai, H.; Wei, Q.; Jin, S.; Wang, J.; Wei, X.; Yuan, Y.; Yu, D.; Shi, F. Dietary genistein supplementation protects against lipopolysaccharide-induced intestinal injury through altering transcriptomic profile. Poult. Sci. 2020, 99, 3411–3427. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Jia, A.; Li, Y.; Bi, Y.; Liu, G. Microbiota regulate the development and function of the immune cells. Int. Rev. Immunol. 2018, 37, 79–89. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary protein and gut microbiota composition and function. Curr. Protein Pept. Sci. 2018, 20, 145–154. [Google Scholar] [CrossRef]
- Schmidt, F.; Dahlke, K.; Batra, A.; Ring, C.; Keye, J.; Loh, G.; Schumann, M.; Kühl, A.A.; Blaut, M.; Siegmund, B. Impact of microbiota on intestinal immune cell composition and intestinal barrier function. Gastroenterology 2017, 152, S1000. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from europe and rural africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Sonalio, K.; Almeida, H.M.S.; Mechler-Dreibi, M.L.; Storino, G.Y.; Haesebrouck, F.; Maes, D.; de Oliveira, L.G. Infuence of mycoplasma hyopneumoniae natural infection on the respiratory microbiome diversity of fnishing pigs. Vet. Res. 2022, 53, 20. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and firmicutes/bacteroidetes ratio in an adult ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Hernandez, A.; Martinez-Martinez, E.; Gomez-Gordo, R.; Lopez-Andres, N.; Fernandez-Celis, A.; Gutierrrez-Miranda, B.; Nieto, M.L.; Alarcon, T.; Alba, C.; Gomez-Garre, D.; et al. The interaction between mitochondrial oxidative stress and gut microbiota in the cardiometabolic consequences in diet-induced obese rats. Antioxidants 2020, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Chang, C.; Liang, C.; Hung, K.; Hsieh, C. In vitro antioxidant activities, free radical scavenging capacity, and tyrosinase inhibitory of flavonoid compounds and ferulic acid from spiranthes sinensis (pers.) Ames. Molecules 2014, 19, 4681–4694. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Buton, A.; Bobay, L. Evolution of chi motifs in proteobacteria. G3 Genes Genom Genet 2021, 11, jkaa054. [Google Scholar] [CrossRef]
- Yang, K.M.; Jiang, Z.Y.; Zheng, C.T.; Wang, L.; Yang, X.F. Effect of lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic escherichia coli k88. J. Anim. Sci. 2014, 92, 1496–1503. [Google Scholar] [CrossRef]
- Gangadoo, S.; Dinev, I.; Chapman, J.; Hughes, R.J.; Van, T.T.H.; Moore, R.J.; Stanley, D. Selenium nanoparticles in poultry feed modify gut microbiota and increase abundance of faecalibacterium prausnitzii. Appl. Microbiol. Biotechnol. 2018, 102, 1455–1466. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Q.; Huang, X.; Jiang, G.; Li, C.; Zhang, X.; Liu, S.; He, L.; Liu, Y.; Dai, Q.; et al. Effects of dietary ferulic acid on the intestinal microbiota and the associated changes on the growth performance, serum cytokine profile, and intestinal morphology in ducks. Front. Microbiol. 2021, 12, 698213. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Liang, X.; Wang, M.; Zhu, X.; Luo, Y.; Shang, Y.; Yang, J.Q.; Zhou, P.; Gu, X.L. Effects of berberine on the growth performance, antioxidative capacity and immune response to lipopolysaccharide challenge in broilers. Anim. Sci. J. 2019, 90, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Ingredient | % | Calculated Nutrients | % |
|---|---|---|---|
| Corn | 59.50 | Metabolizable energy (MJ kg−1) | 12.80 |
| Soybean meal | 32.90 | Crude protein | 19.70 |
| Vegetable oil | 4.65 | Lysine | 1.08 |
| CaCO3 | 0.50 | Methionine | 0.40 |
| CaHPO4 | 1.60 | Methionine + Cystine | 0.74 |
| NaCl | 0.30 | Calcium | 0.77 |
| Choline | 0.10 | Nonphytate P | 0.40 |
| DL-Met | 0.12 | ||
| Premix 1 | 0.33 | ||
| Total | 100 |
| Gene | Accession Number | Primer Sequence (5′–3′) | Product Length (bp) |
|---|---|---|---|
| CLDN-1 | XM_001013611.2 | F: CATACTCCTGGGTCTGGTTGGT R: GACAGCCATCCGCATCTTCT | 100 |
| OCLN | NM_205128.1 | F: CTCAATCAGCTCAGCCGAC R: TCTCCTGCTTCTTGCTTTGGTA | 130 |
| ZO-1 | NM_040706827.1 | F: GTAAACCACTGCCTACACC R: ATATCTTAACTCTACTTCGCACA | 90 |
| β-actin | NM_205518.1 | F: AAGGATCTGTATGCCAACACA R: AGACAGAGTACTTGCGCTCA | 148 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Shu, G.; Du, H.; Zheng, Y.; Fu, H.; Zhang, W.; Lv, C.; Xu, F.; Li, H.; Ouyang, P.; et al. Effects of Dietary Ferulic Acid on Intestinal Health and Ileal Microbiota of Tianfu Broilers Challenged with Lipopolysaccharide. Molecules 2023, 28, 1720. https://doi.org/10.3390/molecules28041720
Tang Z, Shu G, Du H, Zheng Y, Fu H, Zhang W, Lv C, Xu F, Li H, Ouyang P, et al. Effects of Dietary Ferulic Acid on Intestinal Health and Ileal Microbiota of Tianfu Broilers Challenged with Lipopolysaccharide. Molecules. 2023; 28(4):1720. https://doi.org/10.3390/molecules28041720
Chicago/Turabian StyleTang, Ziting, Gang Shu, Hong Du, Yilei Zheng, Hualin Fu, Wei Zhang, Cheng Lv, Funeng Xu, Haohuan Li, Ping Ouyang, and et al. 2023. "Effects of Dietary Ferulic Acid on Intestinal Health and Ileal Microbiota of Tianfu Broilers Challenged with Lipopolysaccharide" Molecules 28, no. 4: 1720. https://doi.org/10.3390/molecules28041720
APA StyleTang, Z., Shu, G., Du, H., Zheng, Y., Fu, H., Zhang, W., Lv, C., Xu, F., Li, H., Ouyang, P., Lin, J., Chang, L.-J., Amevor, F. K., & Zhao, X. (2023). Effects of Dietary Ferulic Acid on Intestinal Health and Ileal Microbiota of Tianfu Broilers Challenged with Lipopolysaccharide. Molecules, 28(4), 1720. https://doi.org/10.3390/molecules28041720







