Molecular Modes of Action of an Aqueous Nerium oleander Extract in Cancer Cells In Vitro and In Vivo
Abstract
1. Introduction
2. Results
2.1. Phytochemical Profiling of Breastin
2.2. Inhibition of Cell Viability of Cell Lines of Hematopoietic and Solid Tumor Origins
2.3. Role of Classical Drug Resistance Mechanisms for Odoroside H and Neritaloside in the NCI Cell Line Panel
2.4. Cross-Resistance Profile of the NCI Cell Line Panel of Standard Anticancer Agents in Response to Odoroside H and Neritaloside
2.5. COMPARE Analysis of the Oncotest Cell Line Panel of Transcriptome-Wide mRNA Expression in Response to Breastin
2.6. Breastin Affected the Microtubule Network as Detected by Confocal Microscopy
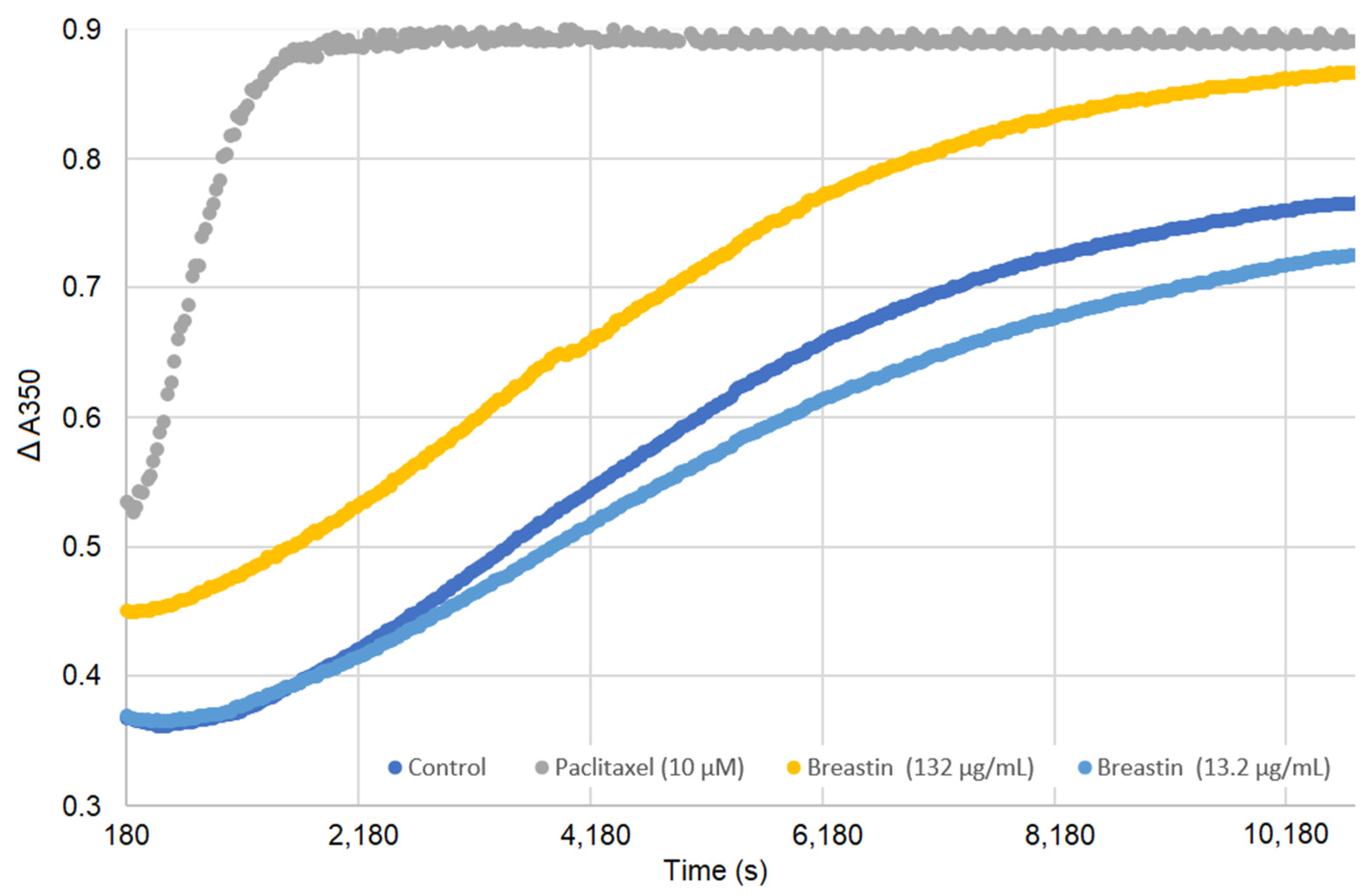
2.7. Breastin Affected Microtubule Polymerization in a Cell-Free Based In Vitro Assay
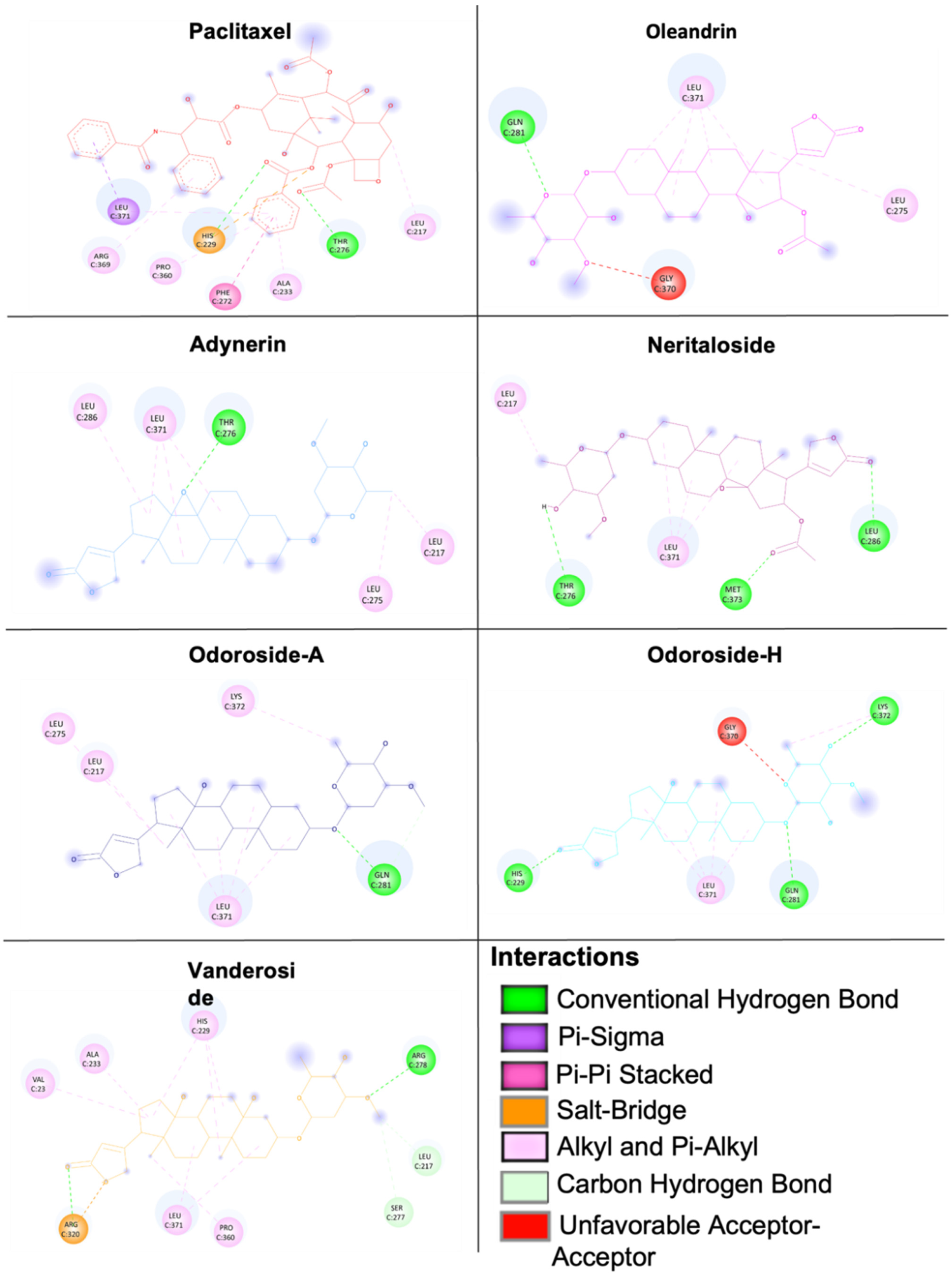
2.8. Molecular Docking of Phytochemicals from Breastin
2.9. Proteome Profiling of Odoroside H and Neritaloside
2.10. Antitumor Activity of Breastin In Vivo
3. Discussion
3.1. Phytochemistry
3.2. Cytotoxicity
3.3. Targets of N. Oleander in Cancer Cells
3.4. Proteomic Expression Profiling
3.5. Activity against Xenograft Tumors
3.6. Acute Toxicity and Side Effects
4. Material and Methods
4.1. Extract Preparation
4.2. Analytical Methods
4.3. Cell Lines
4.4. Cytotoxicity Assays
4.4.1. Resazurin Reduction Assay
4.4.2. Propidium Iodide (PI) Cell Viability Assay
4.4.3. Methyl-Tetrazolium (MTT) Dye Assay
4.5. Imaging of Structure and Dynamics of the Microtubule Cytoskeleton by Fluorescence Microscopy
4.6. Tubulin Polymerization Assay
4.7. COMPARE Analysis
4.8. Molecular Docking
4.9. Hierarchical Cluster Analyses of Proteomic Expression Data
4.10. In Vivo Experiments with Human Xenograft Tumors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Science and Business Media, LLS: New York, NY, USA, 2007. [Google Scholar]
- Singhal, K.G.; Gupta, G.D. Hepatoprotective and antioxidant activity of methanolic extract of flowers of Nerium oleander against CCl4-induced liver injury in rats. Asian Pac. J. Trop. Med. 2012, 5, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.N.; Biswas, K. A concise review on Nerium oleander L.—An important medicinal plant. Trop. Plant Res. 2016, 3, 408–412. [Google Scholar]
- Hase, G.J.; Deshmukh, K.K.; Murade, V.D.; Pokharkar, R.D.; Phatanagre, N.D.; Hase, D.P.; Dichayal, S.; Gosavi, A.B. Phytopharmacology of Nerium oleander L.—A review. Int. J. Phytopharmacol. 2016, 7, 63–67. [Google Scholar]
- Farkhondeh, T.; Kianmehr, M.; Kazemi, T.; Samarghandian, S.; Khazdair, M.R. Toxicity effects of Nerium oleander, basic and clinical evidence: A comprehensive review. Hum. Exp. Toxicol. 2020, 39, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.L.; Zhang, M.H.; Lu, Y.F.; Li, C.Y.; Tang, J.S.; Jiang, M.M. Cardenolides from the leaves of Nerium oleander. Fitoterapia 2018, 127, 293–300. [Google Scholar] [CrossRef]
- Bagrov, A.Y.; Shapiro, J.I.; Fedorova, O.V. Endogenous cardiotonic steroids: Physiology, pharmacology, and novel therapeutic targets. Pharmacol. Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef]
- Whayne, T.F., Jr. Clinical use of digitalis: A state of the art review. Am. J. Cardiovasc. Drugs 2018, 18, 427–440. [Google Scholar] [CrossRef]
- Aronow, W.S.; Frishman, W.H.; Cheng-Lai, A. Cardiovascular drug therapy in the elderly. Cardiol. Rev. 2007, 15, 195–215. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Khalifa, S.A.M.; Taher, E.A.; Farag, M.A.; Saeed, A.; Gamal, M.; Hegazy, M.F.; Youssef, D.; Musharraf, S.G.; Alajlani, M.M.; et al. Cardenolides: Insights from chemical structure and pharmacological utility. Pharmacol. Res. 2019, 141, 123–175. [Google Scholar] [CrossRef]
- Mahringer, A.; Karamustafa, S.; Klotz, D.; Kahl, S.; Konkimalla, V.B.; Wang, Y.; Wang, J.; Liu, H.Y.; Boechzelt, H.; Hao, X.; et al. Inhibition of P-glycoprotein at the blood-brain barrier by phytochemicals derived from traditional Chinese medicine. Cancer Genom. Proteom. 2010, 7, 191–205. [Google Scholar]
- Mohapatra, S.; Biswal, A.K.; Dandapat, J.; Debata, P.R. Leaf extract of Nerium oleander L. inhibits cell proliferation, migration and arrest of cell cycle at G2/M phase in HeLa cervical cancer cell. Anticancer Agents Med. Chem. 2021, 21, 649–657. [Google Scholar] [CrossRef]
- Kumavath, R.; Paul, S.; Pavithran, H.; Paul, M.K.; Ghosh, P.; Barh, D.; Azevedo, V. Emergence of cardiac glycosides as potential drugs: Current and future scope for cancer therapeutics. Biomolecules 2021, 11, 1275. [Google Scholar] [CrossRef]
- Zeino, M.; Paulsen, M.S.; Zehl, M.; Urban, E.; Kopp, B.; Efferth, T. Identification of new P-glycoprotein inhibitors derived from cardiotonic steroids. Biochem. Pharmacol. 2015, 93, 11–24. [Google Scholar] [CrossRef]
- Ayogu, J.I.; Odoh, A.S. Prospects and therapeutic applications of cardiac glycosides in cancer remediation. ACS Comb. Sci. 2020, 22, 543–553. [Google Scholar] [CrossRef]
- Bedane, K.G.; Brieger, L.; Strohmann, C.; Seo, E.J.; Efferth, T.; Spiteller, M. Cytotoxic bufadienolides from the leaves of a medicinal plant Melianthus comosus collected in South Africa. Bioorg. Chem. 2020, 102, 104102. [Google Scholar] [CrossRef]
- Bedane, K.G.; Brieger, L.; Strohmann, C.; Seo, E.J.; Efferth, T.; Spiteller, M. Cytotoxic bufadienolides from the leaves of Melianthus major. J. Nat. Prod. 2020, 83, 2122–2128. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Orta, M.L.; Mateos, S.; López-Lázaro, M. A hydroalcoholic extract from the leaves of Nerium oleander inhibits glycolysis and induces selective killing of lung cancer cells. Planta Med. 2013, 79, 1017–1023. [Google Scholar] [CrossRef]
- Bao, Z.; Tian, B.; Wang, X.; Feng, H.; Liang, Y.; Chen, Z.; Li, W.; Shen, H.; Ying, S. Oleandrin induces DNA damage responses in cancer cells by suppressing the expression of Rad51. Oncotarget 2016, 7, 59572–59579. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, B.; Singh, R. Catharanthus roseus (L.) G. Don: A review of its ethnobotany, phytochemistry, ethnopharmacology and toxicities. J. Ethnopharmacol. 2022, 284, 114647. [Google Scholar] [CrossRef]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A compressive review about Taxol®: History and future challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef]
- Motyka, S.; Jafernik, K.; Ekiert, H.; Sharifi-Rad, J.; Calina, D.; Al-Omari, B.; Szopa, A.; Cho, W.C. Podophyllotoxin and its derivatives: Potential anticancer agents of natural origin in cancer chemotherapy. Biomed. Pharmacother. 2023, 158, 114145. [Google Scholar] [CrossRef] [PubMed]
- Rashan, L.J.; Franke, K.; Khine, M.M.; Kelter, G.; Fiebig, H.H.; Neumann, J.; Wessjohann, L.A. Characterization of the anticancer properties of monoglycosidic cardenolides isolated from Nerium oleander and Streptocaulon tomentosum. J. Ethnopharmacol. 2011, 134, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Hörhammer, L.; Wagner, H.; Luck, R. Zur Kenntnis der Flavonglykoside von Nerium oleander L. Arch. Pharm. 1956, 289, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Balkan, İ.A.; Gören, A.C.; Kırmızıbekmez, H.; Yeşilada, E. Evaluation of the in vitro anti-inflammatory activity of Nerium oleander L. flower extracts and activity-guided isolation of the active constituents. Rec. Nat. Prod. 2018, 12, 128–141. [Google Scholar] [CrossRef]
- Efferth, T.; Konkimalla, V.B.; Wang, Y.F.; Sauerbrey, A.; Meinhardt, S.; Zintl, F.; Mattern, J.; Volm, M. Prediction of broad spectrum resistance of tumors towards anticancer drugs. Clin. Cancer Res. 2008, 14, 2405–2412. [Google Scholar] [CrossRef]
- Pathak, S.; Multani, A.S.; Narayan, S.; Kumar, V.; Newman, R.A. Anvirzel, an extract of Nerium oleander, induces cell death in human but not murine cancer cells. Anticancer Drugs 2000, 11, 455–463. [Google Scholar] [CrossRef]
- Steinhoff, B. Review: Quality of herbal medicinal products: State of the art of purity assessment. Phytomedicine 2019, 60, 153003. [Google Scholar] [CrossRef]
- Wang, X.; Plomley, J.B.; Newman, R.A.; Cisneros, A. LC/MS/MS analyses of an oleander extract for cancer treatment. Anal. Chem. 2000, 72, 3547–3552. [Google Scholar] [CrossRef]
- Begum, S.; Siddiqui, B.S.; Sultana, R.; Zia, A.; Suria, A. Bio-active cardenolides from the leaves of Nerium oleander. Phytochemistry 1999, 50, 435–438. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Khatoon, N.; Begum, S.; Farooq, A.D.; Qamar, K.; Bhatti, H.A.; Ali, S.K. Flavonoid and cardenolide glycosides and a pentacyclic triterpene from the leaves of Nerium oleander and evaluation of cytotoxicity. Phytochemistry 2012, 77, 238–244. [Google Scholar] [CrossRef]
- Arshad Qamar, K.; Dar Farooq, A.; Siddiqui, S.B.; Kabir, N.; Khatoon, N.; Shakil, A.; Erum, S.; Begum, S. Antiproliferative effects of Nerium oleander leaves and their cardiac glycosides odoroside A and oleandrin on MCF-7 cancer cells. Curr. Trad. Med. 2017, 3, 136–145. [Google Scholar]
- Newman, R.A.; Kondo, Y.; Yokoyama, T.; Dixon, S.; Cartwright, C.; Chan, D.; Johansen, M.; Yang, P. Autophagic cell death of human pancreatic tumor cells mediated by oleandrin, a lipid-soluble cardiac glycoside. Integr. Cancer Ther. 2007, 6, 354–364. [Google Scholar] [CrossRef]
- Lin, Y.; Dubinsky, W.P.; Ho, D.H.; Felix, E.; Newman, R.A. Determinants of human and mouse melanoma cell sensitivities to oleandrin. J. Exp. Ther. Oncol. 2008, 7, 195–205. [Google Scholar]
- Yang, P.; Menter, D.G.; Cartwright, C.; Chan, D.; Dixon, S.; Suraokar, M.; Mendoza, G.; Llansa, N.; Newman, R.A. Oleandrin-mediated inhibition of human tumor cell proliferation: Importance of Na,K-ATPase alpha subunits as drug targets. Mol. Cancer Ther. 2009, 8, 2319–2328. [Google Scholar] [CrossRef]
- Yang, P.; Cartwright, C.; Efuet, E.; Hamilton, S.R.; Wistuba, I.I.; Menter, D.; Addington, C.; Shureiqi, I.; Newman, R.A. Cellular location and expression of Na+, K+ -ATPase α subunits affect the anti-proliferative activity of oleandrin. Mol. Carcinog. 2014, 53, 253–263. [Google Scholar] [CrossRef]
- Zeino, M.; Brenk, R.; Gruber, L.; Zehl, M.; Urban, E.; Kopp, B.; Efferth, T. Cytotoxicity of cardiotonic steroids in sensitive and multidrug-resistant leukemia cells and the link with Na+/K+-ATPase. J. Steroid Biochem. Mol. Biol. 2015, 150, 97–111. [Google Scholar] [CrossRef]
- Wen, S.; Chen, Y.; Lu, Y.; Wang, Y.; Ding, L.; Jiang, M. Cardenolides from the Apocynaceae family and their anticancer activity. Fitoterapia 2016, 112, 74–84. [Google Scholar] [CrossRef]
- Arai, M.A.; Akamine, R.; Hayashi, N.; Koyano, T.; Kowithayakorn, T.; Ishibashi, M. The Notch inhibitors isolated from Nerium indicum. J. Nat. Prod. 2018, 81, 1235–1240. [Google Scholar] [CrossRef]
- Smith, J.A.; Madden, T.; Vijjeswarapu, M.; Newman, R.A. Inhibition of export of fibroblast growth factor-2 (FGF-2) from the prostate cancer cell lines PC3 and DU145 by Anvirzel and its cardiac glycoside component, oleandrin. Biochem. Pharmacol. 2001, 62, 469–472. [Google Scholar] [CrossRef]
- Hong, D.S.; Henary, H.; Falchook, G.S.; Naing, A.; Fu, S.; Moulder, S.; Wheler, J.J.; Tsimberidou, A.; Durand, J.B.; Khan, R.; et al. First-in-human study of pbi-05204, an oleander-derived inhibitor of akt, fgf-2, nf-κΒ and p70s6k, in patients with advanced solid tumors. Investig. New Drugs 2014, 32, 1204–1212. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, B.; Liu, X.; Yu, H.; Yong, L.; Liu, X.; Shao, J.; Liu, Z. Inhibition of oleandrin on the proliferation show and invasion of osteosarcoma cells in vitro by suppressing Wnt/β-catenin signaling pathway. J. Exp. Clin. Cancer Res. 2015, 34, 115. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Rhea, P.; Tan, L.; Cartwright, C.; Lee, H.J.; Ravoori, M.K.; Addington, C.; Gagea, M.; Kundra, V.; Kim, S.J.; et al. PBI-05204, a supercritical CO₂ extract of Nerium oleander, inhibits growth of human pancreatic cancer via targeting the PI3K/mTOR pathway. Investig. New Drugs 2015, 33, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.; Akgün-Dar, K.; Kuruca, S.E.; Kiliçaslan-Ayna, T.; Seyhan, V.G.; Atasever, B.; Meriçli, F.; Carin, M. Cytotoxic effects of leaf, stem and root extracts of Nerium oleander on leukemia cell lines and role of the P-glycoprotein in this effect. J. Exp. Ther. Oncol. 2006, 6, 31–38. [Google Scholar]
- Bai, L.; Wang, L.; Zhao, M.; Toki, A.; Hasegawa, T.; Ogura, H.; Kataoka, T.; Hirose, K.; Sakai, J.; Bai, J.; et al. Bioactive pregnanes from Nerium oleander. J. Nat. Prod. 2007, 70, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Bai, L.; Wang, L.; Toki, A.; Hasegawa, T.; Kikuchi, M.; Abe, M.; Sakai, J.; Hasegawa, R.; Bai, Y.; et al. Bioactive cardenolides from the stems and twigs of Nerium oleander. J. Nat. Prod. 2007, 70, 1098–1103. [Google Scholar] [CrossRef]
- Kars, M.D.; Gündüz, U.; Uney, K.; Baş, A.L. Exploring a natural MDR reversal agent: Potential of medicinal food. Asian Pac. J. Trop. Biomed. 2013, 3, 644–649. [Google Scholar] [CrossRef]
- Manna, S.K.; Sah, N.K.; Newman, R.A.; Cisneros, A.; Aggarwal, B.B. Oleandrin suppresses activation of nuclear transcription factor-kappaB, activator protein-1, and c-Jun NH2-terminal kinase. Cancer Res. 2000, 60, 3838–3847. [Google Scholar]
- Kruakaew, S.; Seeka, C.; Lhinhatrakool, T.; Thongnest, S.; Yahuafai, J.; Piyaviriyakul, S.; Siripong, P.; Sutthivaiyakit, S. Cytotoxic cardiac glycoside constituents of Vallaris glabra leaves. J. Nat. Prod. 2017, 80, 2987–2996. [Google Scholar] [CrossRef]
- Ko, Y.S.; Rugira, T.; Jin, H.; Park, S.W.; Kim, H.J. Oleandrin and its derivative odoroside A, both cardiac glycosides, exhibit anticancer effects by inhibiting invasion via suppressing the STAT-3 signaling pathway. Int. J. Mol. Sci. 2018, 19, E3350. [Google Scholar] [CrossRef]
- Wen, S.Y.; Chen, Y.Y.; Deng, C.M.; Zhang, C.Q.; Jiang, M.M. Nerigoside suppresses colorectal cancer cell growth and metastatic potential through inhibition of ERK/GSK3β/β-catenin signaling pathway. Phytomedicine 2018, 57, 352–363. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Krajnović, T.; Kaluđerović, G.N.; Wessjohann, L.A.; Mijatović, S.; Maksimović-Ivanić, D. Versatile antitumor potential of isoxanthohumol: Enhancement of paclitaxel activity in vivo. Pharmacol. Res. 2016, 105, 62–73. [Google Scholar] [CrossRef]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017, 8, 8921–8946. [Google Scholar] [CrossRef]
- Marin, J.J.G.; Briz, O.; Herraez, E.; Lozano, E.; Asensio, M.; Di Giacomo, S.; Romero, M.R.; Osorio-Padilla, L.M.; Santos-Llamas, A.I.; Serrano, M.A.; et al. Molecular bases of the poor response of liver cancer to chemotherapy. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 182–192. [Google Scholar] [CrossRef]
- Erin, N.; Grahovac, J.; Brozovic, A.; Efferth, T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist. Updat. 2020, 53, 100715. [Google Scholar] [CrossRef]
- Efferth, T.; Grassmann, R. Impact of viral oncogenesis on responses to anti-cancer drugs and irradiation. Crit. Rev. Oncog. 2000, 11, 165–187. [Google Scholar]
- Panossian, A.; Efferth, T. Network pharmacology of adaptogens in the assessment of their pleiotropic therapeutic activity. Pharmaceuticals 2022, 15, 1051. [Google Scholar] [CrossRef]
- Scherf, U.; Ross, D.T.; Waltham, M.; Smith, L.H.; Lee, J.K.; Tanabe, L.; Kohn, K.W.; Reinhold, W.C.; Myers, T.G.; Andrews, D.T.; et al. A gene expression database for the molecular pharmacology of cancer. Nat. Genet. 2000, 24, 236–244. [Google Scholar] [CrossRef]
- Staunton, J.E.; Slonim, D.K.; Coller, H.A.; Tamayo, P.; Angelo, M.J.; Park, J.; Scherf, U.; Lee, J.K.; Reinhold, W.O.; Weinstein, J.N.; et al. Chemosensitivity prediction by transcriptional profiling. Proc. Natl. Acad. Sci. USA 2001, 98, 10787–10792. [Google Scholar] [CrossRef]
- Efferth, T.; Oesch, F. Oxidative stress response of tumor cells: Microarray-based comparison between artemisinins and anthracyclines. Biochem. Pharmacol. 2004, 68, 3–10. [Google Scholar] [CrossRef]
- Özenver, N.; Dawood, M.; Fleischer, E.; Klinger, A.; Efferth, T. Chemometric and transcriptomic profiling, microtubule disruption and cell death induction by secalonic acid in tumor cells. Molecules 2020, 25, 3224. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Dawood, M.; Hult, A.K.; Olsson, M.L.; Efferth, T. Network pharmacology of triptolide in cancer cells: Implications for transcription factor binding. Investig. New Drugs 2021, 39, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Kouvonen, P.; Koh, C.C.; Gillet, L.C.; Wolski, W.E.; Röst, H.L.; Rosenberger, G.; Collins, B.C.; Blum, L.C.; Gillessen, S.; et al. Rapid mass spectrometric conversion of tissue biopsy samples into permanent quantitative digital proteome maps. Nat. Med. 2015, 21, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Saleem, M.; Aziz, M.H.; Mukhtar, H. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion markers in CD-1 mouse skin by oleandrin. Toxicol. Appl. Pharmacol. 2004, 195, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Nieto, Y.; Cagnoni, P.J.; Bearman, S.I.; Shpall, E.J.; Matthes, S.; DeBoom, T.; Barón, A.; Jones, R.B. Acute encephalopathy: A new toxicity associated with high-dose paclitaxel. Clin. Cancer Res. 1999, 5, 501–506. [Google Scholar]
- Bielopolski, D.; Evron, E.; Moreh-Rahav, O.; Landes, M.; Stemmer, S.M.; Salamon, F. Paclitaxel-induced pneumonitis in patients with breast cancer: Case series and review of the literature. J. Chemother. 2017, 29, 113–117. [Google Scholar] [CrossRef]
- Garofalo, S.; Grimaldi, A.; Chece, G.; Porzia, A.; Morrone, S.; Mainiero, F.; D’Alessandro, G.; Esposito, V.; Cortese, B.; Di Angelantonio, S.; et al. The glycoside oleandrin reduces glioma growth with direct and indirect effects on tumor cells. J. Neurosci. 2017, 37, 3926–3939. [Google Scholar] [CrossRef]
- Colapietro, A.; Yang, P.; Rossetti, A.; Mancini, A.; Vitale, F.; Chakraborty, S.; Martellucci, S.; Marampon, F.; Mattei, V.; Gravina, G.L.; et al. The botanical drug PBI-05204, a supercritical CO2 extract of Nerium oleander, is synergistic with radiotherapy in models of human glioblastoma. Front. Pharmacol. 2022, 13, 852941. [Google Scholar] [CrossRef]
- Nasu, S.; Milas, L.; Kawabe, S.; Raju, U.; Newman, R. Enhancement of radiotherapy by oleandrin is a caspase-3 dependent process. Cancer Lett. 2002, 185, 145–151. [Google Scholar] [CrossRef]
- Felth, J.; Rickardson, L.; Rosén, J.; Wickström, M.; Fryknäs, M.; Lindskog, M.; Bohlin, L.; Gullbo, J. Cytotoxic effects of cardiac glycosides in colon cancer cells, alone and in combination with standard chemotherapeutic drugs. J. Nat. Prod. 2009, 72, 1969–1974. [Google Scholar] [CrossRef]
- Yong, L.; Ma, Y.; Zhu, B.; Liu, X.; Wang, P.; Liang, C.; He, G.; Zhao, Z.; Liu, Z.; Liu, X. Oleandrin synergizes with cisplatin in human osteosarcoma cells by enhancing cell apoptosis through activation of the p38 MAPK signaling pathway. Cancer Chemother. Pharmacol. 2018, 82, 1009–1020. [Google Scholar] [CrossRef]
- Mekhail, T.; Kaur, H.; Ganapathi, R.; Budd, G.T.; Elson, P.; Bukowski, R.M. Phase 1 trial of Anvirzel in patients with refractory solid tumors. Investig. New Drugs 2006, 24, 423–427. [Google Scholar] [CrossRef]
- Langford, S.D.; Boor, P.J. Oleander toxicity: An examination of human and animal toxic exposures. Toxicology 1996, 109, 1–13. [Google Scholar] [CrossRef]
- Guru, S.; Behera, A.; Barik, S.; Hansdah, U.; Mohanty, C.R.; Sahoo, S. Severe oleander poisoning presenting with hyperkalaemia and unusual electrocardiographic changes. Eur. J. Case Rep. Intern. Med. 2021, 8, 003044. [Google Scholar] [CrossRef]
- Sykes, C.A.; Uzal, F.A.; Mete, A.; Ochoa, J.; Filigenzi, M.; Poppenga, R.H.; Asin, J. Renal lesions in horses with oleander (Nerium oleander) poisoning. Animals 2022, 12, 1443. [Google Scholar] [CrossRef]
- Fiebig, H.H.; Rashan, L.J.; Rashan, F.J. Therapeutic Use of an Extract from the Leaves of Nerium oleander. European Patent Specification EP 1 976 542 B1, 3 March 2010. [Google Scholar]
- Gupta, R.S.; Chopra, A.; Stetsko, D.K. Cellular basis for the species differences in sensitivity to cardiac glycosides (digitalis). J. Cell Physiol. 1986, 127, 197–206. [Google Scholar] [CrossRef]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef]
- Boulos, J.C.; Saeed, M.E.M.; Chatterjee, M.; Bülbül, Y.; Crudo, F.; Marko, D.; Munder, M.; Klauck, S.M.; Efferth, T. Repurposing of the ALK inhibitor crizotinib for acute leukemia and multiple myeloma cells. Pharmaceuticals 2021, 14, 1126. [Google Scholar] [CrossRef]
- Kimmig, A.; Gekeler, V.; Neumann, M.; Frese, G.; Handgretinger, R.; Kardos, G. Susceptibility of multidrug-resistant human leukemia cell lines to human interleukin 2-activated killer cells. Cancer Res. 1990, 50, 6793–6799. [Google Scholar]
- Efferth, T.; Sauerbrey, A.; Olbrich, A.; Gebhart, E.; Rauch, P.; Weber, H.O. Molecular modes of action of artesunate in tumor cell lines. Mol. Pharmacol. 2003, 64, 382–394. [Google Scholar] [CrossRef]
- Kadioglu, O.; Cao, J.; Kosyakova, N.; Mrasek, K.; Liehr, T.; Efferth, T. Genomic and transcriptomic profiling of resistant CEM/ADR-5000 and sensitive CCRF-CEM leukaemia cells for unravelling the full complexity of multi-factorial multidrug resistance. Sci. Rep. 2016, 6, 1–18. [Google Scholar] [CrossRef]
- Fiebig, H.H.; Berger, D.P.; Dengler, W.A.; Wallbrecher, E.; Winterhalter, B.R. Combined in vitro/in vivo test procedure with human tumor xenografts. In Immunodeficient Mice in Oncology; Fiebig, H.H., Berger, D.P., Eds.; Karger Publishers: Basel, Switzerland, 1992; Volume 42, pp. 321–351. [Google Scholar]
- Roth, T.; Burger, A.M.; Dengler, W.; Willmann, H.; Fiebig, H.H. Human tumor cell lines demonstrating the characteristics of patient tumors as useful models for anticancer drug screening. In Relevance of Tumor Models for Anticancer Drug Development; Fiebig, H.H., Burger, A.M., Eds.; Karger Publishers: Basel, Switzerland, 1999; Volume 54, pp. 145–156. [Google Scholar]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- O–Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Adham, A.N.; Abdelfatah, S.; Naqishbandi, A.M.; Mahmoud, N.; Efferth, T. Cytotoxicity of apigenin toward multiple myeloma cell lines and suppression of iNOS and COX-2 expression in STAT1-transfected HEK293 cells. Phytomedicine 2021, 80, 153371. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.F.; Dawood, M.; Mahmoud, N.; Elbadawi, M.; Sugimoto, Y.; Klauck, S.M.; Mohamed, N.; Efferth, T. 2α-Hydroxyalantolactone from Pulicaria undulata: Activity against multidrug-resistant tumor cells and modes of action. Phytomedicine 2021, 81, 153409. [Google Scholar] [CrossRef]
- Dengler, W.A.; Schulte, J.; Berger, D.P.; Mertelsmann, R.; Fiebig, H.H. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assay. Anti-Cancer Drugs 1995, 6, 522–532. [Google Scholar] [CrossRef]
- Davey, J.; Lord, M. Essential Cell Biology: A Practical Approach. Volume 1: Cell Structure; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Paull, K.D.; Shoemaker, R.H.; Hodes, L.; Monks, A.; Scudiero, D.A.; Rubinstein, L.; Plowman, J.; Boyd, M.R. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: Development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst. 1989, 81, 1088–1092. [Google Scholar] [CrossRef]
- Huang, R.; Wallqvist, A.; Covell, D.G. Anticancer metal compounds in NCI’s tumor-screening database: Putative mode of action. Biochem. Pharmacol. 2005, 69, 1009–1039. [Google Scholar] [CrossRef]
- Fang, X.; Shao, L.; Zhang, H.; Wang, S. Web-based tools for mining the NCI databases for anticancer drug discovery. J. Chem. Inf. Comput. Sci. 2004, 44, 249–257. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and autodocktools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Ferreira, L.; Hitchcock, D.B. A comparison of hierarchical methods for clustering functional data. Commun. Stat.—Simul. Comput. 2009, 38, 1925–1949. [Google Scholar] [CrossRef]
- Workman, P.; Twentyman, P.; Balkwill, F.; Balmain, A.; Chaplin, D.; Double, J.; Embleton, J.; Newell, D.; Raymond, R.; Stables, J.; et al. United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) Guidelines for the Welfare of Animals in Experimental Neoplasia (Second Edition). Br. J. Cancer 1998, 77, 1–10. [Google Scholar]
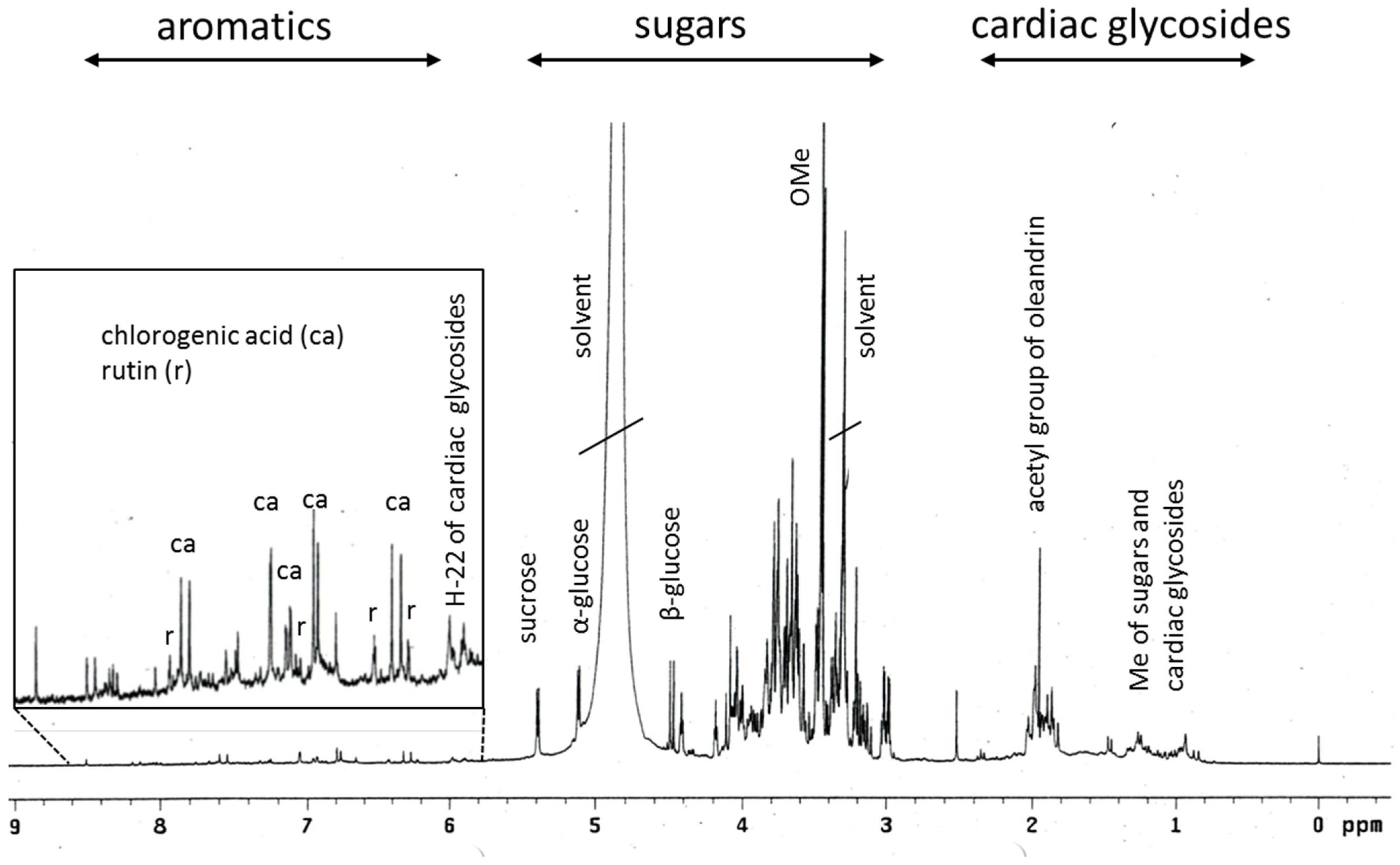
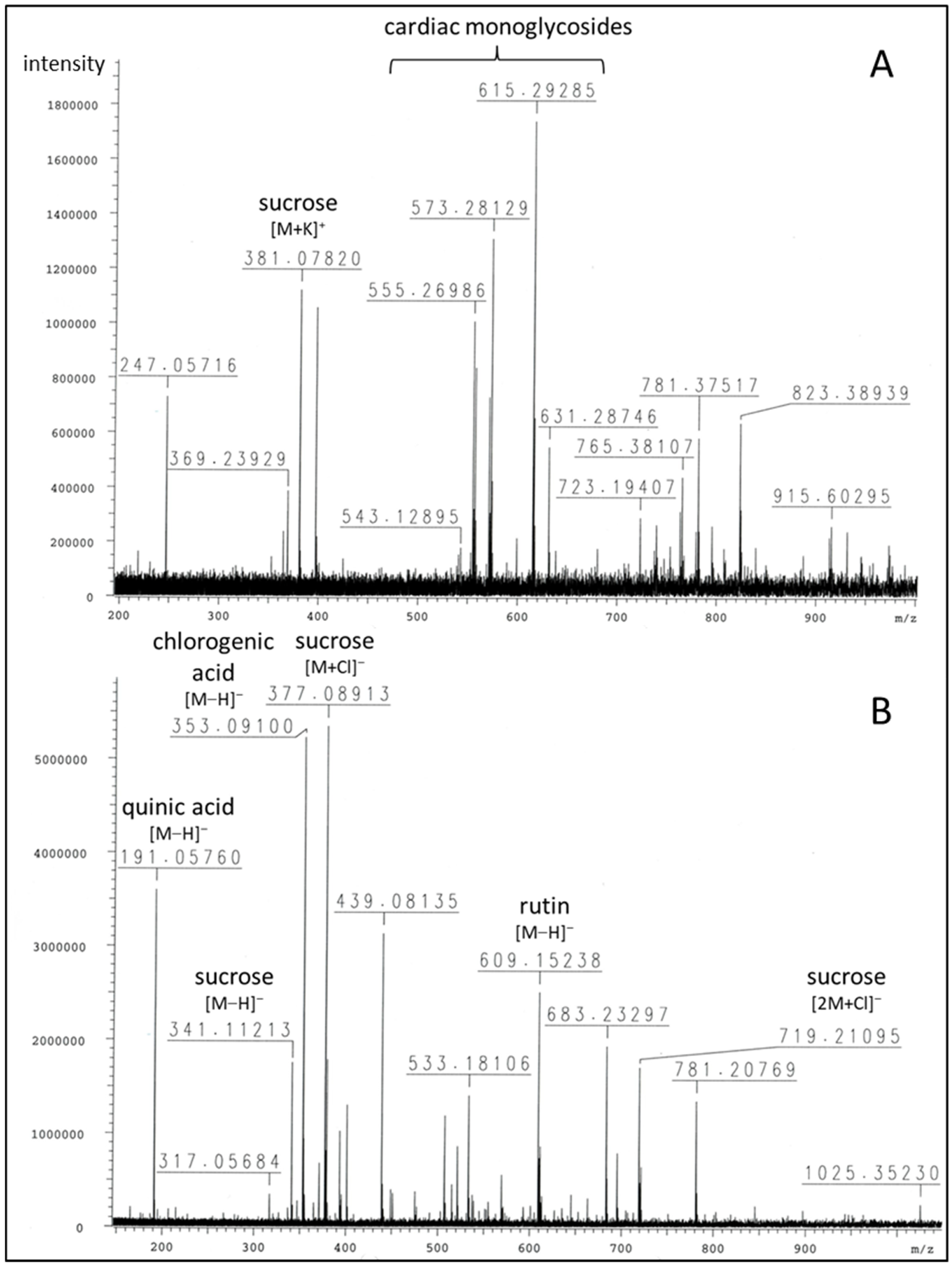

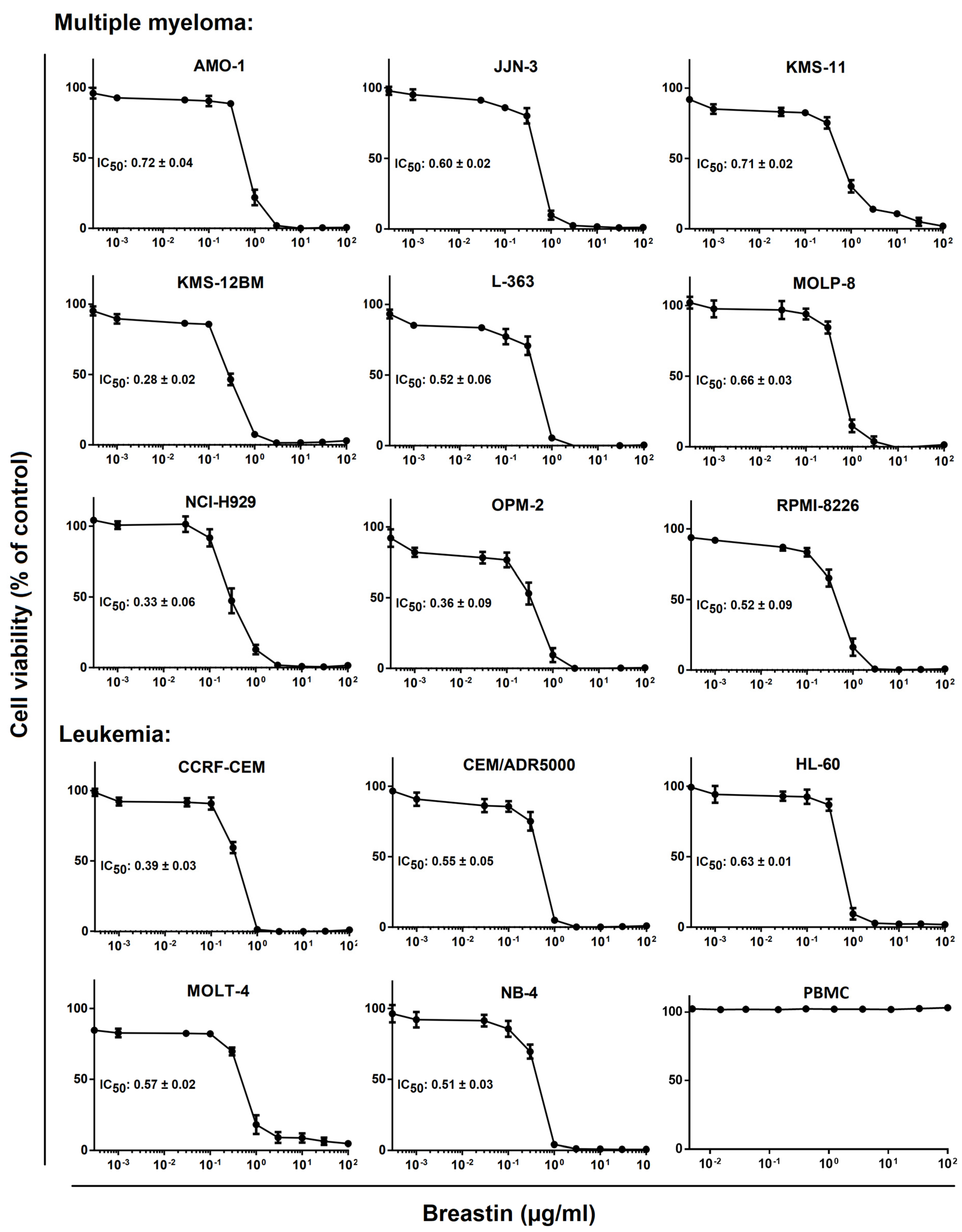

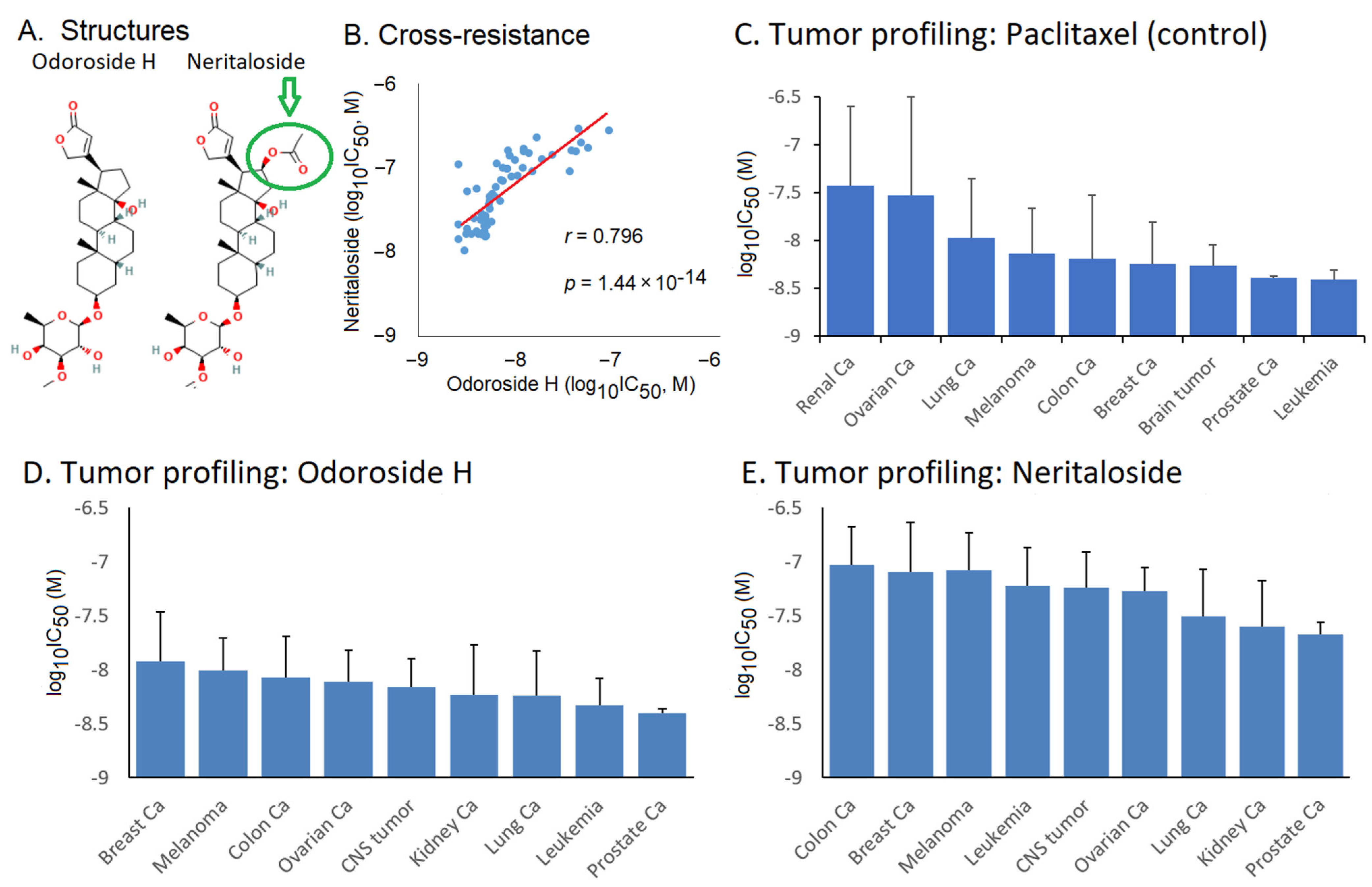





| m/z | Peak Intensity | Formula | Error (ppm) | Ion | Annotation |
|---|---|---|---|---|---|
| Positive mode: | |||||
| 219.02586 | 1.64 × 105 | C6H12O6K+ | 3.1 | [M+K]+ | glucose |
| 247.05716 | 7.30 × 105 | C11H12O5Na+ | 2.2 | unknown | |
| 365.10317 381.07820 723.19407 | 2.39 × 105 1.12 × 106 2.90 × 105 | C12H22O11Na+ C12H22O11K+ C22H44O22K+ | 6.2 3.1 2.1 | [M+Na]+ [M+K]+ [2M+K]+ | sucrose |
| 555.26986 | 1.01 × 106 | C30H44O7K+ | 3.6 | [M+K]+ | 3-O-diginosyl-8,14-epoxy-3-hydroxycard-20(22)-enolide (adynerin), anhydrodigitoxigenin sarmentoside or -diginoside |
| 557.28570 | 8.37 × 105 | C30H46O7K+ | 3.3 | [M+K]+ | digitoxigenindiginoside (odoroside A) |
| 571.26625 | 7.30 × 105 | C30H44O8K+ | 0.9 | [M+K]+ | anhydrodigitoxigenindigitaloside, 3-O-digitalosyl-8,14-epoxy-3-hydroxycard-20(22)-enolide |
| 573.28129 | 1.31 × 106 | C30H46O8K+ | 1.9 | [M+K]+ | digitoxigenindigitaloside (odoroside H), 3-O-diginosyl-3,5,14-trihydroxycard-20(22)-enolide (vanderoside) |
| 599.31794 615.29285 | 2.15 × 105 1.74 × 106 | C32H48O9Na+ C32H48O9K+ | 1.9 0.2 | [M+Na]+ [M+K]+ | oleandrigenin-oleandroside (oleandrin), -sarmentoside or -diginoside |
| 631.28746 | 5.48 × 105 | C32H48O10K+ | 0.7 | [M+K]+ | oleandrigenindigitaloside (neritaloside) |
| Negative mode: | |||||
| 191.05760 | 3.60 × 106 | C7H11O6− | 7.8 | [M−H]− | quinic acid |
| 353.09100 | 5.23 × 106 | C16H17O9− | 9.0 | [M−H]− | chlorogenic acid |
| 341.11213 377.08913 719.21095 | 1.76 × 106 5.35 × 106 1.71 × 106 | C12H21O11− C12H22O11Cl− C24H44O22Cl− | 9.4 9.3 | [M−H]− [M+Cl]− [2M+Cl]− | sucrose |
| 609.15238 | 2.51 × 106 | C27H29O16l− | 10.3 | [M−H]− | rutin |
| Odoroside H | Neritaloside | Control Drug | ||
|---|---|---|---|---|
| (log10IC50, M) | (log10IC50, M) | (log10IC50, M) | ||
| ABCB1 Expression | Epirubicin | |||
| 7q21 (Chromosomal | r-value | −0.039 | 0.004 | * 0.447 |
| Locus of ABCB1 Gene) | p-value | 0.390 | 0.489 | * 3.55 × 10−4 |
| ABCB1 Expression | r-value | −0.051 | −0.022 | * 0.533 |
| (Microarray) | p-value | 0.350 | 0.436 | * 6.82 × 10−6 |
| ABCB1 Expression | r-value | −0.036 | −0.104 | * 0.410 |
| (RT-PCR) | p-value | 0.401 | 0.233 | * 1.54 × 10−3 |
| Rhodamine 123 | r-value | −0.023 | 0.022 | * 0.526 |
| Accumulation | p-value | 0.433 | 0.435 | * 1.12 × 10−5 |
| ABCB5 Expression | Maytansine | |||
| ABCB5 Expression | r-value | 0.107 | 0.140 | * 0.454 |
| (Microarray) | p-value | 0.209 | 0.143 | * 6.67 × 10−4 |
| ABCB5 Expression | r-value | 0.140 | 0.227 | * 0.402 |
| (RT-PCR) | p-value | 0.143 | * 0.040 | * 0.0026 |
| ABCC1 Expression | Vinblastine | |||
| DNA Gene | r-value | 0.008 | 0.010 | * 0.429 |
| Copy Number | p-value | 0.477 | 0.233 | * 0.001 |
| ABCC1 Expression | r-value | −0.141 | −0.254 | * 0.398 |
| (Microarray) | p-value | 0.145 | * 0.027 | * 0.003 |
| ABCC1 Expression | r-value | −0.151 | −0.265 | 0.299 |
| (RT-PCR) | p-value | 0.156 | 0.0357 | * 0.036 |
| ABCG2 Expression | Pancratistatin | |||
| ABCG2 Expression | r-value | −0.099 | −0.150 | * 0.329 |
| (Microarray) | p-value | 0.230 | 0.131 | * 0.006 |
| ABCG2 Expression | r-value | −0.049 | −0.058 | * 0.346 |
| (Western Blot) | p-value | 0.356 | 0.332 | * 0.004 |
| EGFR Expression | Erlotinib | |||
| EGFR Gene | r-value | 0.013 | −0.194 | −0.245 |
| Copy Number | p-value | 0.459 | 0.069 | * 0.029 |
| EGFR Expression | r-value | 0.090 | −0.275 | * −0.458 |
| (Microarray) | p-value | 0.248 | * 0.017 | * 1.15 × 10−4 |
| EGFR Expression | r-value | 0.143 | −0.111 | * −0.379 |
| (PCR Slot Blot) | p-value | 0.143 | 0.203 | * 0.002 |
| EGFR Expression | r-value | −0.052 | −0.293 | * −0.376 |
| (Protein Array) | p-value | 0.349 | * 0.012 | * 0.001 |
| N-/K-/H-RAS Mutations | Melphalan | |||
| TP53 Mutation | r-value | 0.131 | 0.182 | * 0.367 |
| (cDNA Sequencing) | p-value | 0.161 | 0.084 | * 0002 |
| TP53 Mutation | 5-Fluorouracil | |||
| TP53 Mutation | r-value | −0.222 | −0.218 | * −0.502 |
| (cDNA Sequencing) | p-value | 0.047 | 0.050 | * 3.50 × 10−5 |
| TP53 Function | r-value | −0.119 | 0.115 | * −0.436 |
| (Yeast Functional Assay) | p-value | 0.195 | 0.204 | * 5.49 × 10−4 |
| WT1 Expression | Ifosfamide | |||
| WT1 Expression | r-value | −0.019 | 0.010 | * −0.316 |
| (Microarray) | p-value | 0.442 | 0.469 | * 0.007 |
| GSTP1 Expression | Etoposide | |||
| GSTP1 Expression | r-value | −0.124 | −0.071 | 0.399 |
| (Microarray) | p-value | −0.0173 | 0.296 | * 9.58 × 10−4 |
| GST Expression | r-value | 0.008 | −0.064 | 0.509 |
| (Northern Blot) | p-value | 0.474 | 0.315 | * 2.24 × 10−5 |
| HSP90 Expression | Geldanamycin | |||
| HSP90 Expression | r-value | −0.011 | 0.466 | * −0.392 |
| (Microarray) | p-value | 0.076 | 0.283 | * 0.001 |
| Proliferation | 5-Fluorouracil | |||
| Cell Doubling | r-value | 0.079 | −0.008 | * 0.627 |
| p-value | 0.279 | 0.477 | * 7–14 × 10−6 |
| No. | R-Value | p-Value | Standard Agent | Mode of Action | Mode of Action |
|---|---|---|---|---|---|
| IC50-Based Spearman Rank Correlation | |||||
| 1 | 0.576 | 0.0014 | VER-49009 | Heat shock protein 90 inhibitor | |
| 2 | 0.492 | 0.0081 | 4-Hydroperoxy-ifosphamide | Alkylating agent | |
| 3 | 0.429 | 0.0230 | GSK461364A | PLK1 inhibitor | Mitosis-related |
| 4 | 0.390 | 0.0403 | MST-312 | Telomerase inhibitor | |
| 5 | 0.381 | 0.0453 | BI2536 | PLK1 inhibitor | Mitosis-related |
| 6 | 0.378 | 0.0470 | Thiotepa | Alkylating agent | |
| 7 | 0.376 | 0.0488 | BI 6727 3 HCl | PLK1 inhibitor | Mitosis-related |
| IC70-Based Spearman Rank Correlation | |||||
| 1 | 0.487 | 0.0087 | Vinorelbine bistartrate | Tubulin inhibitor | Mitosis-related |
| 2 | 0.470 | 0.0112 | 4-Hydroperoxy-ifosphamide | Alkylating agent | |
| 3 | 0.455 | 0.0151 | VER-49009 | Heat shock protein 90 inhibitor | |
| 4 | 0.446 | 0.0176 | Ispinesib, mesylate | Eg5 inhibitor | Mitosis-related |
| 5 | 0.428 | 0.0230 | Methotrexate hydrate | Antimetabolite | |
| 6 | 0.424 | 0.0246 | Vindesine sulfate | Tubulin inhibitor | Mitosis-related |
| 7 | 0.415 | 0.0282 | BI6727 3HCl | PLK1 inhibitor | Mitosis-related |
| 8 | 0.404 | 0.0333 | BI2536 | PLK1 inhibitor | Mitosis-related |
| 9 | 0.395 | 0.0374 | Vincristine sulfate | Tubulin inhibitor | Mitosis-related |
| 10 | 0.395 | 0.0377 | Vinflunine di-tartrate | Tubulin inhibitor | Mitosis-related |
| 11 | 0.391 | 0.0394 | Purvalanol A | CDK inhibitor | |
| 12 | 0.389 | 0.0409 | Suberic bis-hydroxamic acid | HDAC inhibitor | |
| 13 | 0.380 | 0.0463 | Bleomycin sulfate | DNA synthesis inhibitor | |
| 14 | 0.380 | 0.0460 | GSK461364A | PLK1 inhibitor | Mitosis-related |
| Tubulin Binding Sites | Compounds | LBE (kcal/mol) | pKi (µM) | Amino Acids Involved in Ligand Interaction |
|---|---|---|---|---|
| Blind docking | Vinorelbine | −10.28 ± 0.46 | 0.04 ± 0.03 | HIS197, SER198, ASP199, VAL260, PRO263 |
| Paclitaxel | −9.45 ± 0.08 | 0.12 ± 0.02 | GLN256, THR257, VAL260, PRO261, ALA314, CYS347 | |
| Colchicine | −6.82 ± 1.01 | 22.58 ± 15.61 | ASP199, PRO263, HIS406, TRP407 | |
| Nocodazole | −6.31 ± 0.11 | 23.65 ± 4.43 | THR382, ALA385, ALA426, GLU429, GLU433 | |
| Adynerin | −8.52 ± 0.16 | 0.59 ± 0.16 | CYS12, THR145, VAL171, SER174, GLU183, ASP205, ASN206 | |
| Neritaloside | −8.80 ± 0.28 | 0.40 ± 0.19 | LYS163, LEU195, GLN256, VAL260, PRO263, HIS266 | |
| Oleandrin | −8.42 ± 0.04 | 0.67 ± 0.04 | LYS163, GLU196, ASP199, THR257, VAL260, PRO263, HIS266 | |
| Vanderoside | −8.66 ± 0.02 | 0.45 ± 0.01 | GLN11, CYS12, GLN15, SER140, VAL171, SER174, GLU183, ASP205, ASN206, TYR 224 | |
| Odoroside A | −8.15 ± 0.02 | 1.06 ± 0.04 | VAL23, LEU217, HIS229, ALA233, SER236, THR276, ARG320, PRO360, LEU371 | |
| Odoroside H | −8.06 ± 0.18 | 1.29 ± 0.36 | GLN11, CYS12, GLU71, ASP205 | |
| Vinca alkaloid-binding site (defined docking) | Vinorelbine | −10.86 ± 0.39 | 0.01 ± 0.00 | CYS12, SER140, LEU141, GLY142, VAL171, SER174, VAL177, SER178, ASP179, GLU183, ILE204, ASN206, TYR224 |
| Adynerin | −8.91 ± 0.15 | 0.37 ± 0.09 | CYS12, ALA99, SER140, VAL171, SER178, ASP179 | |
| Neritaloside | −9.75 ± 0.23 | 0.05 ± 0.03 | GLN11, CYS12, PRO173, SER174, GLU183, ASN206, TYR210, TYR224 | |
| Oleandrin | −8.73 ± 0.53 | 1.10 ± 0.79 | LYS176, VAL177, TYR210, ASP211, PHE214, PRO222, TYR224 | |
| Vanderoside | −9.87 ± <0.01 | 0.04 ± 0.00 | CYS12, GLN15, SER140, VAL171, PRO173, SER174, GLU183, ASP205, ASN206, TYR224, GLY225 | |
| Odoroside A | −9.15 ± 0.05 | 0.17 ± 0.02 | CYS12, ALA99, ASN101, THR145, VAL171, SER174, ASN206, GLU207 | |
| Odoroside H | −9.08 ± 0.04 | 0.29 ± 0.02 | CYS12, GLU71, ASN101, THR145, VAL171, SER174 | |
| Taxane-binding site (defined docking) | Paclitaxel | −9.93 ± 0.29 | 0.14 ± 0.11 | LEU217, HIS229, ALA233, PHE272, THR276, PRO360, ARG369, LEU371 |
| Adynerin | −8.02 ± 0.03 | 1.93 ± 0.03 | LEU217, LEU275, THR276, LEU286, LEU371 | |
| Neritaloside | −8.19 ± 0.11 | 1.02 ± 0.17 | LEU275, GLN281, GLY370, LEU371 | |
| Oleandrin | −8.50 ± 0.21 | 0.63 ± 0.21 | LEU217, THR276, LEU286, LEU 371, MET373 | |
| Vanderoside | −7.92 ± 0.07 | 1.61 ± 0.19 | VAL23, LEU217, HIS229, ALA233, SER277, ARG278, ARG320, PRO360, LEU371 | |
| Odoroside A | −8.14 ± 0.08 | 2.91 ± 0.69 | LEU217, LEU275, GLN281, LEU371, LYS372 | |
| Odoroside H | −8.36 ± 0.11 | 1.47 ± 0.84 | HIS229, GLN281, GLY370, LEU371, LYS372 | |
| Colchicine-binding site (blind docking) | Colchicine | −7.57 ± 0.01 | 2.83 ± 0.04 | ASP69, THR145, ALA180, TRY224, LEU248, LYS254 |
| Adynerin | −6.77 ± 0.05 | 10.91 ± 0.91 | GLY10, ALA99, SER178, ALA180, LEU248, LYS254 | |
| Neritaloside | −5.80 ± 0.14 | 57.77 ± 13.31 | LEU70, GLU71, ALA99, VAL177, THR179, ALA180, GLU183, ASN206, TYR224, LEU248 | |
| Oleandrin | −5.26 ± 0.14 | 144.33 ± 31.57 | ALA12, GLU71, ALA99, PRO173, VAL177, ALA180, GLU183, ASN206, TYR210, TYR224, LEU248, LYS254, ASP329 | |
| Vanderoside | −5.51 ± 0.22 | 98.22 ± 33.98 | GLN11, ASP69, LEU70, GLU71, VAL74, ASP98, TYR210, LEU248, LYS254, ASP329 | |
| Odoroside A | −8.19 ± 0.12 | 1.02 ± 0.20 | GLN11, LEU70, GLU71, ALA99, THR145, ALA180, GLU183, TYR224, LEU248 | |
| Odoroside H | −6.68 ± 0.08 | 12.90 ± 1.60 | GLN11, LEU70, GLU71, ALA99, THR179, ALA180, TYR210, TYR224, LEU248, LYS254, ASP329 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashan, L.J.; Özenver, N.; Boulos, J.C.; Dawood, M.; Roos, W.P.; Franke, K.; Papasotiriou, I.; Wessjohann, L.A.; Fiebig, H.-H.; Efferth, T. Molecular Modes of Action of an Aqueous Nerium oleander Extract in Cancer Cells In Vitro and In Vivo. Molecules 2023, 28, 1871. https://doi.org/10.3390/molecules28041871
Rashan LJ, Özenver N, Boulos JC, Dawood M, Roos WP, Franke K, Papasotiriou I, Wessjohann LA, Fiebig H-H, Efferth T. Molecular Modes of Action of an Aqueous Nerium oleander Extract in Cancer Cells In Vitro and In Vivo. Molecules. 2023; 28(4):1871. https://doi.org/10.3390/molecules28041871
Chicago/Turabian StyleRashan, Luay J., Nadire Özenver, Joelle C. Boulos, Mona Dawood, Wynand P. Roos, Katrin Franke, Ioannis Papasotiriou, Ludger A. Wessjohann, Heinz-Herbert Fiebig, and Thomas Efferth. 2023. "Molecular Modes of Action of an Aqueous Nerium oleander Extract in Cancer Cells In Vitro and In Vivo" Molecules 28, no. 4: 1871. https://doi.org/10.3390/molecules28041871
APA StyleRashan, L. J., Özenver, N., Boulos, J. C., Dawood, M., Roos, W. P., Franke, K., Papasotiriou, I., Wessjohann, L. A., Fiebig, H.-H., & Efferth, T. (2023). Molecular Modes of Action of an Aqueous Nerium oleander Extract in Cancer Cells In Vitro and In Vivo. Molecules, 28(4), 1871. https://doi.org/10.3390/molecules28041871







