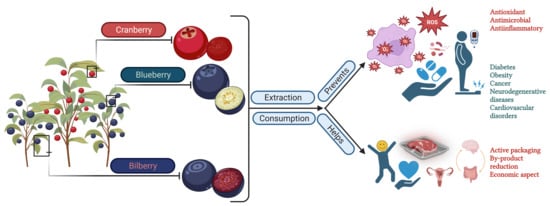
Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants
Abstract
1. Introduction
2. Blueberry/Bilberry (V. myrtillus/V. sect. cyanococcus)
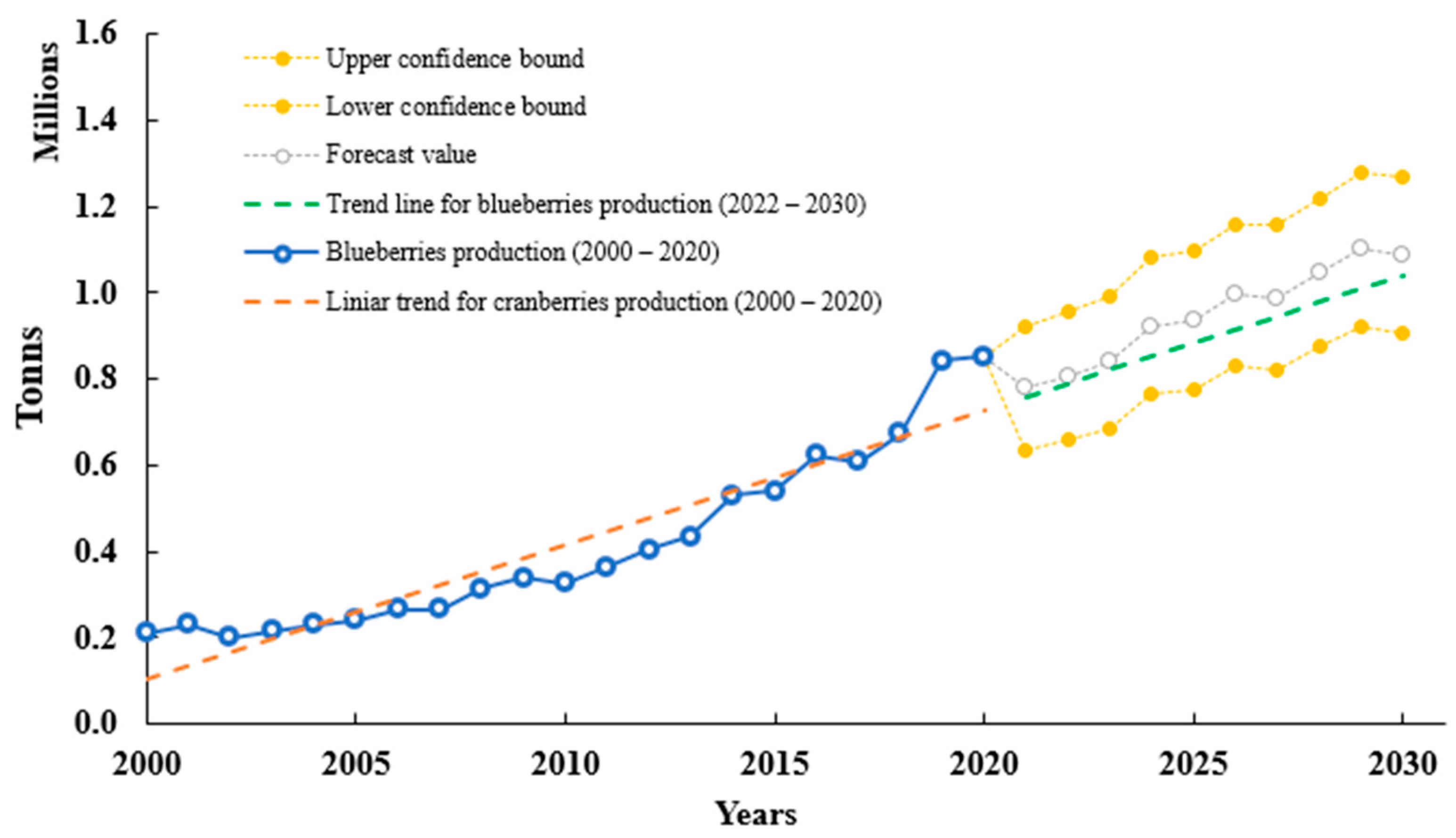
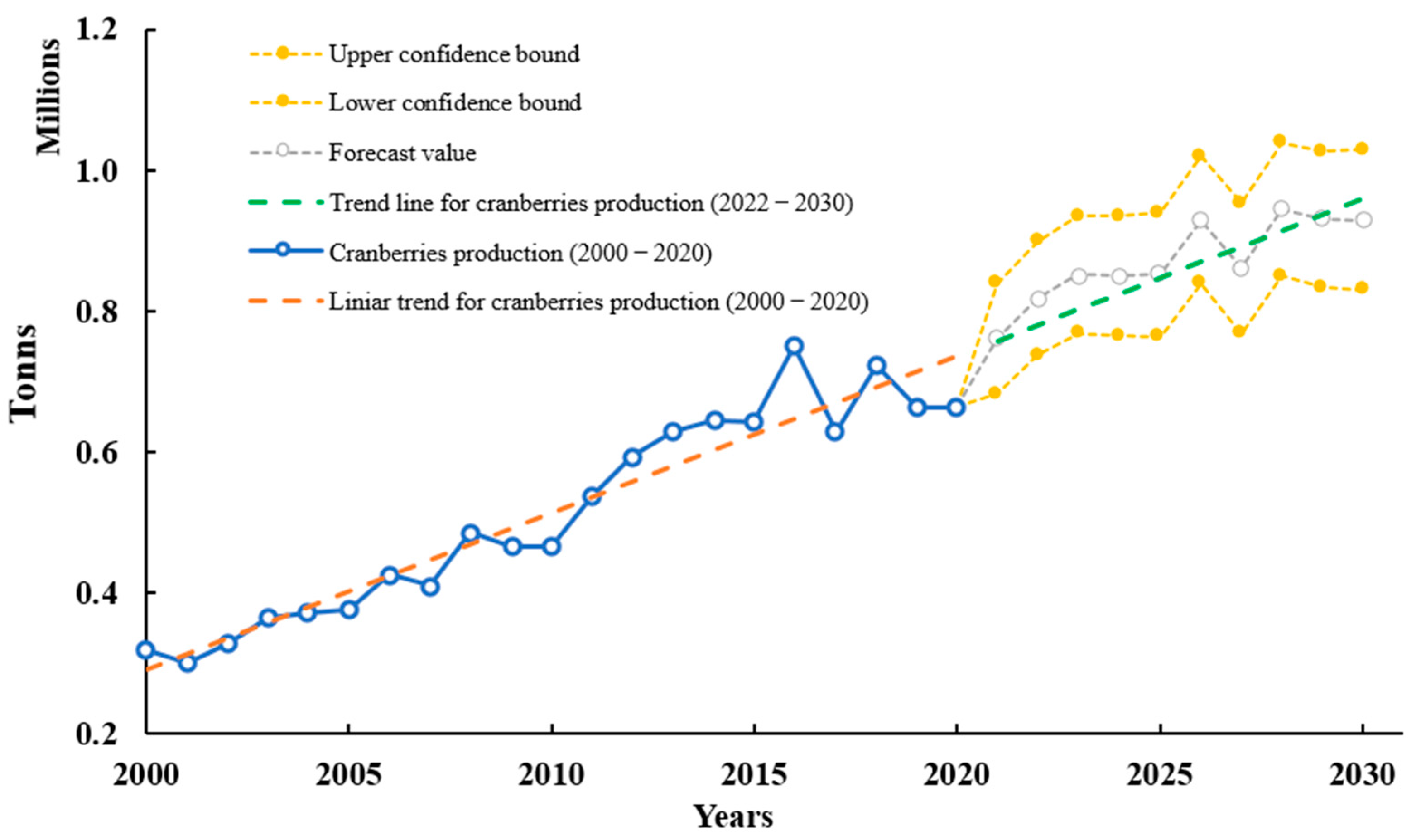
2.1. History, Nomenclature, and Production
2.2. Blueberry Constituents
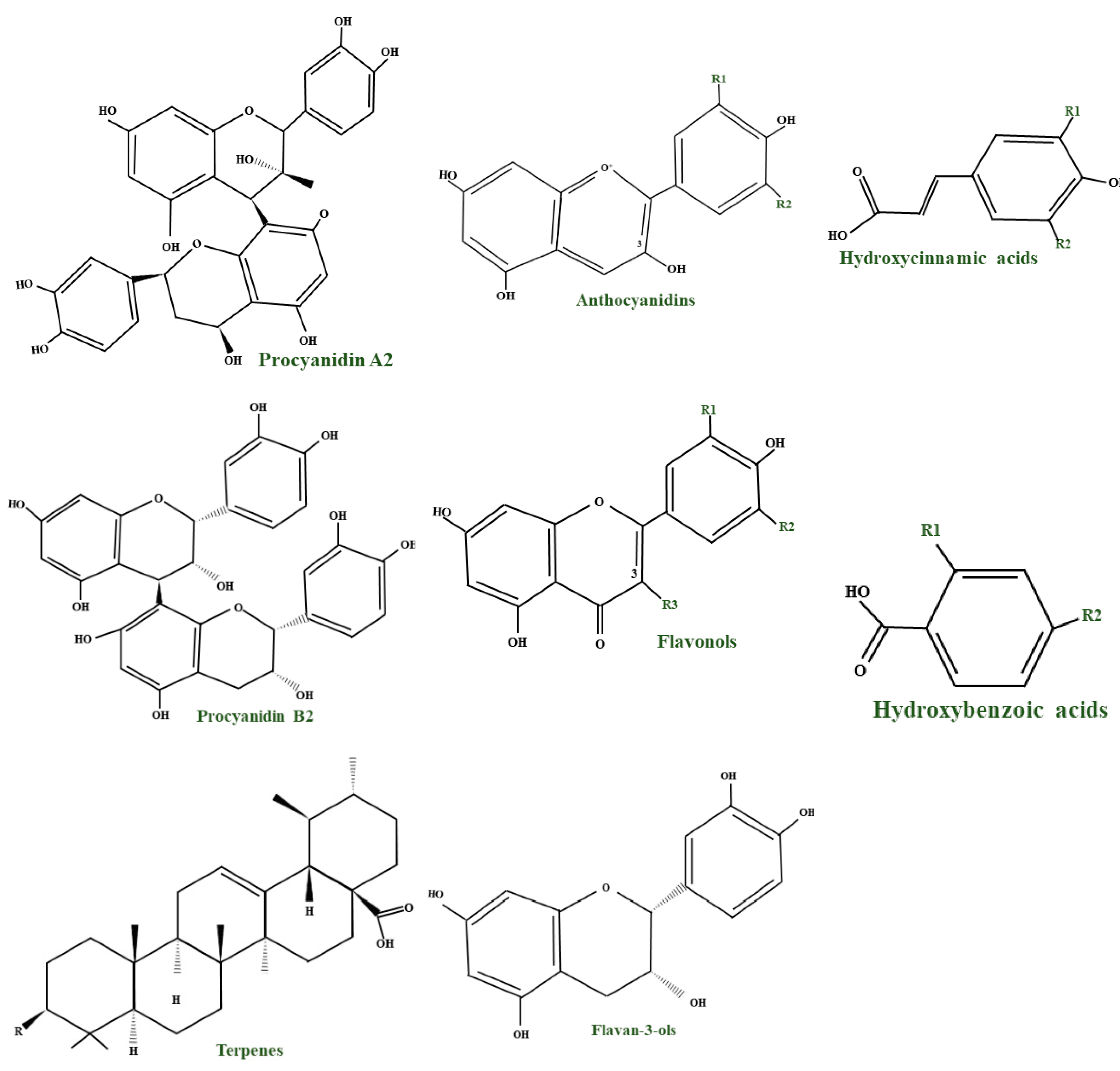
2.2.1. Anthocyanins
2.2.2. Flavonols
2.2.3. Tannins, Procyanidins, and Organic Acids
2.3. Antioxidant Effect
2.4. Antimicrobial Activity
2.5. Health Benefits
2.5.1. Anti-Inflammatory Effect
2.5.2. Anti-Cancer and Anti-Proliferative Activity
2.5.3. Antidiabetic Effect
2.5.4. Anti-Obesity Effect
2.5.5. Cardioprotective Activity
3. Cranberry (V. sect. Oxycoccus)
3.1. History, Nomenclature and Production
3.2. Blueberries Constituents
3.2.1. Phenolic Acids
3.2.2. Anthocyanins and Proanthocyanidins
3.2.3. Flavonoids
| Phytonutrient | Name | Content |
|---|---|---|
| Anthocyanins | Delfinidyn derivatives * | 31.27–43.87 mg/100 g dm |
| Delfinidyn-3-O-glucoside * | 1.1–1.8 mg/100 g dm | |
| Cyanidin derivatives * | 442–967 mg/100 g dm | |
| Cyanidin-3-O-galactoside * | 119.9–180.0 mg/100 g dm | |
| Cyanidin-3-O-glucoside * | 5.5–7.3 mg/100 g dm | |
| Cyanidin-3-O-arabinoside * | 64.5–95.6 mg/100 g dm | |
| Peonidin-3-O-galactoside * | 131.3–310.3 mg/100 g dm | |
| Peonidin-3-O-arabinoside * | 42.9–95.2 mg/100 g dm | |
| Peonidin derivatives * | 192–666 mg/100 g dm | |
| Malvidin derivatives * | 29.85–58.85 mg/100 g dm | |
| Malvidin-3-O-arabinoside * | 1.4–1.9 mg/100 g dm | |
| Total anthocyanins * | 695–1716 mg/100 g dm | |
| Phenolic acid | p–Coumaric acid *** | 2–245 µg/g dw |
| p-Coumaroyl hexose * | 8.6–13.9 mg/100 g dm | |
| p-Coumaroyl hexose isomer * | 3.6–50.0 mg/100 g dm | |
| p-Coumaroyl derivatives * | 210–451 mg/100 g dm | |
| Chlorogenic acid * | 72.00–129.62 mg/100 g dm | |
| Caffeic acid *** | 5–123 µg/g dw | |
| Caffeoyl hexoside * | 92.7–190.2 mg/100 g dm | |
| Caffeoyl hexoside isomer * | 10.9–17.5 mg/100 g dm | |
| Caffeoyl and derivatives * | 39.93–68.28 mg/100 g dm | |
| Ferulic acid *** | 4–39 µg/g dw | |
| Total phenolic acid * | 327–649 mg/100 g dm | |
| Flavonols | Myricetin-3-O-galactoside * | 156.5–348.4 mg/100 g dm |
| Myricetin-3-O-glucoside * | 1.8–6.6 mg/100 g dm | |
| Myricetin-3-O-pentoside * | 6.3–55.6 mg/100 g dm | |
| Myricetin-3-O-glucoronide * | 19.0–38.5 mg/100 g dm | |
| Myricetin-arabinoside *** | 8–273 µg/g dw | |
| Sinapoyl derivatives * | 4.36–5.82 mg/100 g dm | |
| Myricetin derivatives * | 496–926 mg/100 g dm | |
| Quercetin-3-O-galactoside * | 294.6–375.8 mg/100 g dm | |
| Quercetin-3-O-pentoside * | 21.2–122.9 mg/100 g dm | |
| Quercetin-3-O-glucoside * | 4.8–11.5 mg/100 g | |
| Quercetin-p conmaroylhexoside * | 1.3–13.3 mg/100 g dm | |
| Quercetin-3-O-rhamnoside * | 6.2–13.3 mg/100 g dm | |
| Quercetin-rutinoside ***** | 12.0 mg/100 g fw | |
| Quercetin-acetyl-glucosidase ***** | 13.58 mg/100 g fw | |
| Quercetin derivatives * | 107–225 mg/100 g dm | |
| Methoxyquercetin hexoside * | 1.7–25.7 mg/100 g dm | |
| Methoxyquercetin pentoside * | 3.4–61.0 mg/100 g dm | |
| Methoxyquercetin derivatives * | 33.31–43.04 mg/100 g dm | |
| Total flavonols * | 643–1088 mg/100 g dm | |
| Flavan-3-ols and proanthocyanidins | (+)-Catechin * | 2.79–7.53 mg/100 g dm |
| (−)-Epicatechin * | 27.46–56.84 mg/100 g dm | |
| A-type PA-dimer * | 16.94–32.07 mg/100 g dm | |
| A-type PA-trimer * | 27.82–76.94 mg/100 g dm | |
| A-type PA-tetramer * | 41.51–65.61 mg/100 g dm | |
| B-type PA–dimer * | 12.62–36.75 mg/100 g dm | |
| B-type PA–trimer ****** | 0.04–2.93 mg/100 g fw | |
| Polymeric proanthocyanidins * | 651–1109 mg/100 g dm | |
| Sinapyl hexose * | 2.0–3.3 mg/100 g dm | |
| Total flavan–3–ols and proanthocyanidins * | 860–1283 mg/100 g dm | |
| Triterpenoids | Ursolic acid * | 1044–1714 mg/kg dm |
| Oleanolic acid * | 894–1137 mg/100 g dm | |
| Betulinic acid * | 635–824 mg/kg dm | |
| Sum Triterpenoids * | 2892–3671 mg/kg dm | |
| Total Sterols (β–sitosterol and stigmasterol) **** | 107.83 mg/g fw |
3.2.4. Triterpenoids
3.3. Antimicrobial Effect
3.4. Medicinal Effect of Cranberry Consumption
- Gastric cancer: is one of the most frequent types of cancer for all sexes and ages. According to the World Health Organization (WHO) [125], H. pylori are often linked with the evolution of most gastric ulcers. However, eradicating antibiotics is not a strategy because H. pylori colonisation can show some health benefits in reliable situations. Cranberry has many bioactive compounds, including PACs with A–type double linkages. In addition, the presents of the PACs that have an anti-adhesion effect on bacteria are important because they inhibit the initial stage of the infection instead of killing the bacteria [125]. To demonstrate the anti-adhesion effect, Burger et al. (2000) conducted a study on the adhesion of three strains of H. pylori (BZMC-25, EHL-65, and 17874) on gastric mucus acquired from a stomach taken after post-mortem surgeries. Non-dialysable material (NDM) was a cranberry juice dialysed against distilled water in dialysis bags; after six days of dialysis, NDM was lyophilised. NDM at 100 μg mL−1 concentrations inhibited H. pylori BZMC-25 and constrained adhesion to human gastric mucus. The inhibitory effect was dose-dependent, and 50% of the inhibitory concentration relied on BZMC-25, EHL-65, and 17,874 strains [126].
- Obesity: many studies show that cranberry reduces lipid accretion by lowering the mRNA level of some genes associated with fatty-acid-binding protein, lipoprotein lipase, fatty acid synthase, hormone-sensitive lipase, and perilipin 1. However, one of the most important targets for obesity prevention is to decrease leptin and increase adiponectin gene expression and adipocytokines secretion A [127]. In vivo studies on obese diabetic mice have shown that a dose of 5% and 10% of cranberry powder in the diet for six weeks and a cranberry extract (200 mg/kg) for eight weeks increased HDL-cholesterol level and decreased insulin and glucose level. The decreased hepatic, intestinal, and plasma triglyceride accumulation reduced oxidative stress and inflammation [128]. In a study on mice, Kunkel et al. (2012) showed that ursolic acid identified in cranberry reduces obesity, improves glucose tolerance, and decreases hepatic steatosis [129].
- Diabetes: cranberries are rich in phenolic compounds such as quercetin which can inhibit gastric assimilation of glucose in the porcine model [130]. Besides quercetin, they also contain myricetin, which may inhibit glucose transporter type 4 mediated glucose assimilation by rat adipocytes [140]. Patients with type 2 diabetes were involved in two 12-week studies to prove that cranberry bioactive constituents help features of metabolic syndrome and diabetes. Cranberry juice significantly reduces restrained glucose, while cranberry extracts mark down total and LDL–cholesterol. Some studies show that dried low-calorie cranberry-enriched high-fat meal challenge induced postprandial glycaemia, inflammation, and lipid peroxidation in diabetes [131]. Another study shows that daily consumption for 12 weeks of cranberry juice (240 mL) and blueberry extract (9.1–9.8 mg of anthocyanins) for 8 to 12 weeks improved glucose control in type 2 diabetes subjects [141].
- Urinary tract infection (UTI): is a bacterial infection which affects young and sexually active women. Approximately 1 in 3 women will experience an episode of UTI, requiring antibiotics [114]. In vitro studies show that A-type PACs constrain the adhesion of P-fimbriated uropathogenic E. coli to uroepithelial cells [116]. Antibiotics to treat UTIs have been claimed to be very functional, but these antibiotics can damage intestinal microbiota and cause resistance among uropathogens. PACs are usually catabolised by the colon microbiota to give bioactive phenolic metabolites. This phenolic metabolite may be the responsible compound behind the anti-adhesion effect. D–mannose also inhibits adhesion on uroepithelial cells in vitro. Another helpful characteristic of cranberry juice is the pH of 2.5, which causes changes in urine’s physical properties (acidification) [132].
- Periodontitis: is an inflammatory disease which affects tooth-supporting tissues (periodontal ligament and alveolar bone) and is induced by Gram-negative anaerobic bacteria. NDM can inhibit the proliferation of P.gingivalis, T.forsythia, and T.denticola in periodontal pockets. Unusual production of cytokines by host cells caused by periodontopathogens damage tooth-supporting tissues; studies have demonstrated that cranberry was an excellent inhibitor of pro-inflammatory cytokine and chemokine replies caused by lipopolysaccharides [124,133,134].
4. Another Vaccinium sect. V. membranaceum
4.1. History, Nomenclature, and Production
| V. membranaceum | V. parvifolium | V. scoparium | V. ovalifolium | V. ovatum | V. caespitosum | V. deliciosum | |
|---|---|---|---|---|---|---|---|
| Names | bilberries, mountain huckleberry, grouseberries, hortleberries, black huckleberry, hortleberries, and thin-leaf huckleberry | red huckleberry, red bilberry | small-leaved huckleberry, grouseberry, dwarf red whortleberry, and red alpine blueberry | oval-leaf blueberry, oval-leaf bilberry, oval-leaf huckleberry, Alaska blueberry | evergreen huckleberry | dwarf blueberry, dwarf bilberry, dwarf huckleberry, and dwarf whortleberry | cascade huckleberry, cascade bilberry or blue huckleberry |
| Flower | creamy-pink | greenish to pinkish | pink | pink | bright pink | pink | pink |
| Fruit/Berries | large, shining black, dull black, deep purple, rarely red, 9–13 mm d. | red, occasionally faintly glaucous, 7–9 mm diam, more tart than sweet | translucent red, red, or bluish purple, 4 -6 mm d., soft, tart | bright blue, glaucous, dull purplish black or black, 810 mm d. | purplish-black, 6–9 mm d. | bright blue and glaucous, rarely dull black, 59 mm d., great flavour | blue and glaucous, occasionally dull black, maroon, or red, 9–15 mm d., especially flavorful berries |
| Distribution | Rocky Mountains from SW-NW Territories S to N California and N Utah | Pacific coast of N America from Alaska to N California, inland to SE British Columbia | SE British Columbia and adjacent Alberta, E to the Black Hills of South Dakota, and south to SW Colorado | Pacific Rim from Central Japan, Kamchatka, Aleutian Islands, S along the Pacific coast to S central Oregon and inland to N Idaho | Pacific Coast from British Columbia to central California | Alaska to Newfoundland, southward along the Atlantic Seaboard to S Maine and S Vermont; in the W, S to the W highlands of Guatemala | Pacific coastal mountain ranges from S British Columbia to N California, Montana and Idaho |
| Commercial value | food industry: jams, syrups, tea, culinary uses; medicinal uses; cosmetic uses | berries—food industry: jam, jelly, wine; leaves—medicinal use, ornamental use | culinary use | culinary use | the food industry, such as jam; medicinal use; ornamental use | the food industry; ornamental use | food industry |
| * TP | 1.70 mg of GAE/g of FW | 0.81 mg of GAE/g of FW | - | 2.81 mg of GAE/g of FW | 2.84 mg of GAE/g of FW | - | 1.41 mg of GAE/g of FW |
| ** TA | 1.69 mg of C3G/g of FW | 0.11 mg of C3G/g of FW | - | 3.07 mg of C3G/g of FW | 3.64 mg of C3G/g of FW | - | 1.35 mg of C3G/g of FW |
| *** AC | ORAC 21 µmol of TE/g of FW, FRAP 40.5 µmol of TE/g of FW | ORAC 7.3 µmol of TE/g of FW FRAP 10 µmol of TE/g of FW | - | ORAC 37.8 µmol of TE/g of FW FRAP 76.2 µmol of TE/g of FW | ORAC 41.1 µmol of TE/g of FW—FRAP 70.2 µmol of TE/g of FW | - | ORAC 14.6 µmol of TE/g of FW FRAP 30.2 µmol of TE/g of FW |
| **** F3 and F | Catechins > 240 µg/g of FW | Catechins > 154 µg/g of FW | - | Catechins > 104 µg/g of FW | Catechins > 69 µg/g of FW | - | Catechins > 109 µg/g of FW |
| Chlorogenic acid | 62.6 µg/g of FW | 60.2 µg/g of FW | - | 1< µg/g of FW | 466 µg/g of FW | - | 72.4 µg/g of FW |
| Caffeic acid | 17.6 µg/g of FW | 150 µg/g of FW | - | 5.1 µg/g of FW | 5.8 µg/g of FW | - | 41.3 µg/g of FW |
| Ferulic acid | 21.7 µg/g of FW | 38.5 µg/g | - | 17.9 µg/g of FW | 109 µg/g of FW | - | 22.6 µg/g of FW |
| p-hydroxybenzoic acid | 1.5 µg/g of FW | 553 µg/g of FW | - | 1.6 µg/g of FW | 12.1 µg/g of FW | - | 6.9 µg/g of FW |
| p-coumaric acid | 21.1 µg/g of FW | 97.3 µg/g of FW | - | 23.9 µg/g of FW | 32.4 µg/g of FW | - | 16.6 µg/g of FW |
| ***** TPC | 124.5 µg/g of FW | 899 µg/g of FW | - | 48.5 µg/g of FW | 625.3 µg/g of FW | - | 159.8 µg/g of FW |
| ****** Anthocyanidins | 1294 µg/g of FW | 112.9 µg/g of FW | - | 2179 µg/g of FW | 1850 µg/g of FW | - | 996.3 µg/g of FW |
4.2. Constituents
4.3. Ecological Value, Commerce, and Economy
5. Economic Aspects of Using Waste and by-Products of Berries
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Data Food and Agriculture Data (Datenbank). Available online: https://www.fao.org/faostat/en/#data (accessed on 14 December 2022).
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive potential of fruit and egetable wastes. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2019; Volume 91, pp. 157–225. [Google Scholar]
- Precup, G.; Pocol, C.B.; Teleky, B.-E.; Vodnar, D.C. Awareness, Knowledge, and Interest about Prebiotics—A Study among Romanian Consumers. Int. J. Environ. Res. Public Health 2022, 19, 1208. [Google Scholar] [CrossRef]
- Szabo, K.; Mitrea, L.; Călinoiu, L.F.; Teleky, B.-E.; Adria, G.; Plamada, D.; Pascuta, M.S.; Nemeş, S.-A.; Varvara, R.; Vodnar, D.C. Natural Polyphenols Recovery from Apple-, Cereal-, and To- mato- Processing By-Products, and Related Health-Promoting Properties. Molecules 2022, 22, 7977. [Google Scholar] [CrossRef]
- Martau, G.A.; Teleky, B.-E.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Apple Pomace as a Sustainable Substrate in Sourdough Fermentation. Front. Microbiol. 2021, 12, 3850. [Google Scholar] [CrossRef]
- Teleky, B.-E.; Mitrea, L.; Plamada, D.; Nemes, S.A.; Călinoiu, L.-F.; Pascuta, M.S.; Varvara, R.-A.; Szabo, K.; Vajda, P.; Szekely, C.; et al. Development of Pectin and Poly(vinyl alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants 2022, 11, 1729. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aadil, R.M. An update on functional, nutraceutical and industrial applications of watermelon by-products: A comprehensive review. Trends Food Sci. Technol. 2021, 114, 275–291. [Google Scholar] [CrossRef]
- Precup, G.; Teleky, B.-E.; Ranga, F.; Vodnar, D.C. Assessment of Physicochemical and Rheological Properties of Xylo-Oligosaccharides and Glucose-Enriched Doughs Fermented with BB-12. Biology 2022, 11, 10836. [Google Scholar] [CrossRef] [PubMed]
- Teleky, B.E.; Martău, G.A.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Biofunctional soy-based sourdough for improved rheological properties during storage. Sci. Rep. 2022, 12, 17535. [Google Scholar] [CrossRef] [PubMed]
- Precup, G.; Mitrea, L.; Nemes, A.; Călinoiu, L.-F.; Martău, G.-A.; Teleky, B.E.; Coman, V.; Vodnar, D.C. Food processing by-products and molecular gastronomy. In Gastronomy and Food Science; Academic Press: Cambridge, MA, USA, 2021; pp. 137–164. [Google Scholar]
- Martău, G.A.; Coman, V.; Vodnar, D.C. Recent advances in the biotechnological production of erythritol and mannitol. Crit. Rev. Biotechnol. 2020, 40, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Tenuta, M.C.; Loizzo, M.R.; Bonesi, M.; Finetti, F.; Trabalzini, L.; Deguin, B. Vaccinium species (Ericaceae): From chemical composition to bio-functional activities. Appl. Sci. 2021, 11, 5655. [Google Scholar] [CrossRef]
- Wrońska-Pilarek, D.; Szkudlarz, P.; Bocianowski, J. Systematic importance of morphological features of pollen grains of species from Erica (Ericaceae) genus. PLoS ONE 2018, 13, e0204557. [Google Scholar] [CrossRef] [PubMed]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Gailīte, A.; Gaile, A.; Ruņģis, D.E. Genetic diversity and structure of wild vaccinium populations-v. Myrtillus, v. vitis-idaea and v. uliginosum in the Baltic states. Silva Fenn. 2020, 54, 10396. [Google Scholar] [CrossRef]
- Kulkarni, K.P.; Vorsa, N.; Natarajan, P.; Elavarthi, S.; Iorizzo, M.; Reddy, U.K.; Melmaiee, K. Admixture analysis using genotyping-by-sequencing reveals genetic relatedness and parental lineage distribution in highbush blueberry genotypes and cross derivatives. Int. J. Mol. Sci. 2021, 22, 163. [Google Scholar] [CrossRef]
- Role, H.E.; Soybeanfighting, O.F.; Hunger, W.; Thoenes, P. FAO Commodities and Trade Division, Basic Foodstuffs Service 1, 2. Plant Prod 2004, 1–32. [Google Scholar]
- Stefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crisan, G. The Chemical and Biological Profiles of Leaves from Commercial Blueberry Varieties. Plants 2020, 9, 1193. [Google Scholar] [CrossRef]
- Ştefanescu, B.E.; Szabo, K.; Mocan, A.; Crisan, G. Phenolic compounds from five ericaceae species leaves and their related bioavailability and health benefits. Molecules 2019, 24, 2046. [Google Scholar] [CrossRef]
- Clapa, D.; Nemeș, S.-A.; Ranga, F.; Hârța, M.; Vodnar, D.-C.; Călinoiu, L.-F. Micropropagation of Vaccinium corymbosum L.: An Alternative Procedure for the Production of Secondary Metabolites. Horticulturae 2022, 8, 480. [Google Scholar] [CrossRef]
- Mitrea, L.; Nemes, S.-A.; Szabo, K.; Teleky, B.-E.; Vodnar, D.-C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association With Neurological and Psychiatric Disorders. Front. Med. 2022, 9, 813204. [Google Scholar] [CrossRef]
- Aaby, K.; Grimmer, S.; Holtung, L. Extraction of phenolic compounds from bilberry (Vaccinium myrtillus L.) press residue: Effects on phenolic composition and cell proliferation. Lwt 2013, 54, 257–264. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Călinoiu, L.F.; Mitrea, L.; Precup, G.; Bindea, M.; Păcurar, A.M.; Szabo, K.; Ştefănescu, B.E. A.M.; Szabo, K.; Ştefănescu, B.E. A New Generation of Probiotic Functional Beverages Using Bioactive Compounds From Agro-Industrial Waste. In Functional and Medicinal Beverages; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128163979. [Google Scholar]
- Vodnar, D.C.; Calinoiu, L.F.; Mitrea, L. Editorial: Exploiting the effect of dietary fibre on the gut microbiota in patients with pelvic radiotherapy. Br. J. Cancer 2022, 127, 1575–1576. [Google Scholar] [CrossRef]
- Dickerson, F.; Severance, E.; Yolken, R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain. Behav. Immun. 2017, 62, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Pascuta, M.S.; Vodnar, D.C. Nanocarriers for sustainable active packaging: An overview during and post COVID-19. Coatings 2022, 12, 102. [Google Scholar] [CrossRef]
- Martău, G.A.; Călinoiu, L.F.; Vodnar, D.C. Bio-vanillin: Towards a sustainable industrial production. Trends Food Sci. Technol. 2021, 109, 579–592. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Teleky, B.-E. Recent Trends in Antibacterial Coatings and Biofilm. Coatings 2023, 13, 255. [Google Scholar] [CrossRef]
- Pascuta, M.S.; Varvara, R.; Teleky, B.-E.; Szabo, K.; Plamada, D.; Nemes, S.-A.; Mitrea, L.; Mărtau, G.A.; Ciont, C.; Călinoiu, L.-F.; et al. Polysaccharide-Based Edible Gels as Functional Ingredients:Characterization, Applicability, and Human Health Benefit. Gels 2022, 8, 524. [Google Scholar] [CrossRef]
- Braic, R.Ş.; Vari, C.; Imre, S.; Huţanu, A.; Fogarasi, E.; Todea, T.; Groşan, A.; Eşianu, S.; Laczkó-Zöld, E.; Dogaru, M. Vaccinium extracts as modulators in experimental type 1 diabetes. J. Med. Food 2018, 21, 1106–1112. [Google Scholar] [CrossRef]
- Bujor, O.C.; Tanase, C.; Popa, M.E. Phenolic antioxidants in aerial parts of wild Vaccinium species: Towards pharmaceutical and biological properties. Antioxidants 2019, 8, 649. [Google Scholar] [CrossRef]
- Dulf, E.; Vodnar, D.C.; Danku, A.; Martau, G.A.; Teleky, B.-E.; Dulf, F.V.; Ramadan, M.F.; Crisan, O. Mathematical Modeling and Optimization of Lactobacillus Species Single and Co-Culture Fermentation Processes in Wheat and Soy Dough Mixtures. Front. Bioeng. Biotechnol. 2022, 10, 888827. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.-E.; Ranga, F.; Roman, I.; Khaoula, H.; Boudaya, E.; Ltaief, A.B.; Aouani, W.; Thiamrat, M.; Vodnar, D.C. Carotenoid Recovery from Tomato Processing By-Products through Green Chemistry. Molecules 2022, 27, 3771. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H.; Pintea, A. Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem. Cent. J. 2017, 11, 92. [Google Scholar] [CrossRef]
- Antonella, S.; Barreca, D.; Giuseppina, L.; Ersilia, B.; Domenico, T. Bilberry (Vaccinium myrtyllus L.); Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128124918. [Google Scholar]
- Carvalho, M.; Matos, M.; Carnide, V. Identification of cultivated and wild Vaccinium species grown in Portugal. Spanish J. Agric. Res. 2018, 16, e07SC01-01. [Google Scholar] [CrossRef]
- Vaneková, Z.; Rollinger, J.M. Bilberries: Curative and Miraculous—A Review on Bioactive Constituents and Clinical Research. Front. Pharmacol. 2022, 13, 2343. [Google Scholar] [CrossRef] [PubMed]
- Stanoeva, J.P.; Stefova, M.; Andonovska, K.B.; Vankova, A.; Stafilov, T. Phenolics and mineral content in bilberry and bog bilberry from Macedonia. Int. J. Food Prop. 2017, 20, S863–S883. [Google Scholar] [CrossRef]
- Klavins, L.; Maaga, I.; Bertins, M.; Hykkerud, A.L.; Karppinen, K.; Bobinas, Č.; Salo, H.M.; Nguyen, N.; Salminen, H.; Stankevica, K.; et al. Trace element concentration and stable isotope ratio analysis in blueberries and bilberries: A tool for quality and authenticity control. Foods 2021, 10, 567. [Google Scholar] [CrossRef] [PubMed]
- Hera, O.; Teodorescu, R.; Sturzeanu, M. Blueberry(Vaccinium Corymbosum) Breeding Programme in the Main Cultivating Countries. Sci. Pap. Ser. B, Hortic. 2021, LXV, 82–89. [Google Scholar]
- Martin, R.R.; Tzanetakis, I.E. High risk blueberry viruses by region in north america; implications for certification, nurseries, and fruit production. Viruses 2018, 10, 342. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; Benites, S.M.; De La Cruz-Noriega, M.; Cabanillas-Chirinos, L.; Valdiviezo-Dominguez, F.; Álvarez, M.A.Q.; Vega-Ybañez, V.; Angelats-Silva, L. Bioelectricity production from blueberry waste. Processes 2021, 9, 1301. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Vaccinium myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications—A Review. Curr. Pharm. Des. 2020, 26, 1917–1928. [Google Scholar] [CrossRef]
- Michalska, A.; Łysiak, G. Bioactive compounds of blueberries: Post-harvest factors influencing the nutritional value of products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Bandy, B. Protection by Plant Flavonoids Against Myocardial Ischemia-Reperfusion Injury; Elsevier Inc.: Amsterdam, The Netherlands, 2012; ISBN 9780123965400. [Google Scholar]
- Urbonaviciene, D.; Bobinaite, R.; Viskelis, P.; Bobinas, C.; Petruskevicius, A.; Klavins, L.; Viskelis, J. Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.). Antioxidants 2022, 11, 588. [Google Scholar] [CrossRef]
- Gaspar, D.P.; Lechtenberg, M.; Hensel, A. Quality assessment of bilberry fruits (Vaccinium myrtillus) and bilberry-containing dietary supplements. J. Agric. Food Chem. 2021, 69, 2213–2225. [Google Scholar] [CrossRef] [PubMed]
- Gizzi, C.; Belcaro, G.; Gizzi, G.; Feragalli, B.; Dugall, M.; Luzzi, R.; Cornelli, U. Bilberry extracts are not created equal: The role of non anthocyanin fraction. Discovering the “dark side of the force” in a preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2418–2424. [Google Scholar]
- Papanov, S.I.; Petkova, E.G.; Ivanov, I.G. Polyphenols Content and Antioxidant Activity of Bilberry Juice Obtained from Different Altitude Samples. J. Pharm. Res. Int. 2021, 218–223. [Google Scholar] [CrossRef]
- Ștefănescu, B.E.; Nemes, S.A.; Teleky, B.E.; Călinoiu, L.F.; Mitrea, L.; Martău, G.A.; Szabo, K.; Mihai, M.; Vodnar, D.C.; Crișan, G. Microencapsulation and Bioaccessibility of Phenolic Compounds of Vaccinium Leaf Extracts. Antioxidants 2022, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, L.F.; Vodnar, D.C. Whole grains and phenolic acids: A review on bioactivity, functionality, health benefits and bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, M. Antioxidant activity and antimicrobial effect of berry phenolics—A Finnish perspective. Mol. Nutr. Food Res. 2007, 51, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Nohynek, L.; Alakomi, H.L.; Oksman-Caldentey, K.M. Bioactive berry compounds—Novel tools against human pathogens. Appl. Microbiol. Biotechnol. 2005, 67, 8–18. [Google Scholar] [CrossRef]
- Davidson, E.; Zimmermann, B.F.; Jungfer, E.; Chrubasik-Hausmann, S. Prevention of urinary tract infections with vaccinium products. Phyther. Res. 2014, 28, 465–470. [Google Scholar] [CrossRef]
- Toivanen, M.; Huttunen, S.; Lapinjoki, S.; Tikkanen-Kaukanen, C. Inhibition of adhesion of Neisseria meningitidis to human epithelial cells by berry juice polyphenolic fractions. Phyther. Res. 2011, 25, 828–832. [Google Scholar] [CrossRef]
- Huttunen, S.; Toivanen, M.; Arkko, S.; Ruponen, M.; Tikkanen-Kaukanen, C. Inhibition activity of wild berry juice fractions against streptococcus pneumoniae binding to human bronchial cells. Phyther. Res. 2011, 25, 122–127. [Google Scholar] [CrossRef]
- Klavins, L.; Mezulis, M.; Nikolajeva, V.; Klavins, M. Composition, sun protective and antimicrobial activity of lipophilic bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.) extract fractions. Lwt 2021, 138, 110784. [Google Scholar] [CrossRef]
- Colak, N.; Torun, H.; Gruz, J.; Strnad, M.; Hermosín-Gutiérrez, I.; Hayirlioglu-Ayaz, S.; Ayaz, F.A. Bog Bilberry Phenolics, Antioxidant Capacity and Nutrient Profile; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 201, ISBN 9046237737. [Google Scholar]
- de Mello, V.D.F.; Lankinen, M.A.; Lindström, J.; Puupponen-Pimiä, R.; Laaksonen, D.E.; Pihlajamäki, J.; Lehtonen, M.; Uusitupa, M.; Tuomilehto, J.; Kolehmainen, M.; et al. Fasting serum hippuric acid is elevated after bilberry (Vaccinium myrtillus) consumption and associates with improvement of fasting glucose levels and insulin secretion in persons at high risk of developing type 2 diabetes. Mol. Nutr. Food Res. 2017, 61, 1700019. [Google Scholar] [CrossRef] [PubMed]
- Karcheva-Bahchevanska, D.P.; Lukova, P.K.; Nikolova, M.M.; Mladenov, R.D.; Iliev, I.N. Eff ect of Extracts of Bilberries (Vaccinium myrtillus L.) on Amyloglucosidase and α-Glucosidase Activity. Folia Med. (Plovdiv). 2017, 59, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Schink, A.; Neumann, J.; Leifke, A.L.; Ziegler, K.; Fröhlich-Nowoisky, J.; Cremer, C.; Thines, E.; Weber, B.; Pöschl, U.; Schuppan, D.; et al. Screening of herbal extracts for TLR2-and TLR4-dependent anti-inflammatory effects. PLoS ONE 2018, 13, e0203907. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lv, X.D.; Wang, G.E.; Li, Y.F.; Kurihara, H.; He, R.R. Anti-inflammatory effects of anthocyanins-rich extract from bilberry (Vaccinium myrtillus L.) on croton oil-induced ear edema and Propionibacterium acnes plus LPS-induced liver damage in mice. Int. J. Food Sci. Nutr. 2014, 65, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Chehri, A.; Yarani, R.; Yousefi, Z.; Shakouri, S.K.; Ostadrahimi, A.; Mobasseri, M.; Araj-Khodaei, M. Phytochemical and pharmacological anti-diabetic properties of bilberries (Vaccinium myrtillus), recommendations for future studies. Prim. Care Diabetes 2022, 16, 27–33. [Google Scholar] [CrossRef]
- Bljajić, K.; Petlevski, R.; Vujić, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; De Carvalho, I.S.; Končić, M.Z. Chemical composition, antioxidant and α-glucosidase-inhibiting activities of the aqueous and hydroethanolic extracts of Vaccinium myrtillus leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef]
- Ungurianu, A.; Şeremet, O.; Gagniuc, E.; Olaru, O.T.; Guţu, C.; Grǎdinaru, D.; Ionescu-Tȋrgovişte, C.; Marginǎ, D.; Dǎnciulescu-Miulescu, R. Preclinical and clinical results regarding the effects of a plant-based antidiabetic formulation versus well established antidiabetic molecules. Pharmacol. Res. 2019, 150, 104522. [Google Scholar] [CrossRef]
- Roth, S.; Spalinger, M.R.; Müller, I.; Lang, S.; Rogler, G.; Scharl, M. Bilberry-derived anthocyanins prevent IFN-γ-induced pro-inflammatory signalling and cytokine secretion in human THP-1 monocytic cells. Digestion 2014, 90, 179–189. [Google Scholar] [CrossRef]
- Ozdemir, A.; Mercantepe, T.; Erdivanli, B.; Sen, A.; Mercantepe, F.; Tumkaya, L.; Uydu, H.A. Neuroprotective effects of Vaccinium myrtillus on damage-related brain injury. J. Chem. Neuroanat. 2023, 127, 102193. [Google Scholar] [CrossRef]
- Miyake, S.; Takahashi, N.; Sasaki, M.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: Cellular and molecular mechanism. Lab. Investig. 2012, 92, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, L.; Mwinyi, J.; Scharl, M.; Frei, P.; Zeitz, J.; Kullak-Ublick, G.A.; Vavricka, S.R.; Fried, M.; Weber, A.; Humpf, H.U.; et al. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis—An open pilot study. J. Crohn’s Colitis 2013, 7, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Mykkänen, O.T.; Huotari, A.; Herzig, K.H.; Dunlop, T.W.; Mykkänen, H.; Kirjavainen, P.V. Wild blueberries (Vaccinium myrtillus) alleviate inflammation and hypertension associated with developing obesity in mice fed with a high-fat diet. PLoS ONE 2014, 9, e114790. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.S. Vaccinium myrtillus extract prevents or delays the onset of diabetes—Induced blood-retinal barrier breakdown. Int. J. Food Sci. Nutr. 2015, 66, 236–242. [Google Scholar] [CrossRef]
- Del Bubba, M.; Di Serio, C.; Renai, L.; Scordo, C.V.A.; Checchini, L.; Ungar, A.; Tarantini, F.; Bartoletti, R. Vaccinium myrtillus L. extract and its native polyphenol-recombined mixture have anti-proliferative and pro-apoptotic effects on human prostate cancer cell lines. Phyther. Res. 2021, 35, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.A.L.; Persson, K.; Andersson, R.G.G. Effect of Vaccinium myrtillus and its polyphenols on angiotensin-converting enzyme activity in human endothelial cells. J. Agric. Food Chem. 2009, 57, 4626–4629. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Uto, T.; Tanigawa, S.; Kumamoto, T.; Fujii, M.; Hou, D.X. Expression profiling of genes targeted by bilberry (Vaccinium myrtillus) in macrophages through DNA microarray. Nutr. Cancer 2008, 60, 43–50. [Google Scholar] [CrossRef]
- Kolehmainen, M.; Mykkänen, O.; Kirjavainen, P.V.; Leppänen, T.; Moilanen, E.; Adriaens, M.; Laaksonen, D.E.; Hallikainen, M.; Puupponen-Pimiä, R.; Pulkkinen, L.; et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012, 56, 1501–1510. [Google Scholar] [CrossRef]
- Triebel, S.; Trieu, H.L.; Richling, E. Modulation of inflammatory gene expression by a bilberry (Vaccinium myrtillus L.) extract and single anthocyanins considering their limited stability under cell culture conditions. J. Agric. Food Chem. 2012, 60, 8902–8910. [Google Scholar] [CrossRef]
- Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, prebiotics, and synbiotics: Implications and beneficial effects against irritable bowel syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef]
- Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G.; Djilas, S.; Četojević-Simin, D. Dried bilberry (Vaccinium myrtillus L.) extract fractions as antioxidants and cancer cell growth inhibitors. Lwt 2015, 61, 615–621. [Google Scholar] [CrossRef]
- Mauramo, M.; Onali, T.; Wahbi, W.; Vasara, J.; Lampinen, A.; Mauramo, E.; Kivimäki, A.; Martens, S.; Häggman, H.; Sutinen, M.; et al. Bilberry (Vaccinium myrtillus L.) powder has anticarcinogenic effects on oral carcinoma in vitro and in vivo. Antioxidants 2021, 10, 1319. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Morita, R.; Ogawa, T.; Segawa, R.; Takimoto, N.; Suzuki, K.; Hamadate, N.; Hayashi, S.; Odachi, A.; Ogiwara, I.; et al. Tumor suppression effects of bilberry extracts and enzymatically modified isoquercitrin in early preneoplastic liver cell lesions induced by piperonyl butoxide promotion in a two-stage rat hepatocarcinogenesis model. Exp. Toxicol. Pathol. 2014, 66, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Chikaraishi, Y.; Shimazawa, M.; Yokota, S.; Hara, H. Vaccinium myrtillus (bilberry) extracts reduce angiogenesis in vitro and in vivo. Evid.-Based Complement. Altern. Med. 2010, 7, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Sidorova, Y.; Shipelin, V.; Mazo, V.; Zorin, S.; Petrov, N.; Kochetkova, A. Hypoglycemic and hypolipidemic effect of Vaccinium myrtillus L. leaf and Phaseolus vulgaris L. seed coat extracts in diabetic rats. Nutrition 2017, 41, 107–112. [Google Scholar] [CrossRef]
- Varut, R.M.; Gîrd, C.E.; Rotaru, L.T.; Varut, M.C.; Pisoschi, C.G. Evaluation of Polyphenol and Flavonoid Profiles and the Antioxidant Effect of Carduus Acanthoides Hydroalcoholic Extract Compared with Vaccinium myrtillus in an Animal Model of Diabetes Mellitus. Pharm. Chem. J. 2018, 51, 1088–1095. [Google Scholar] [CrossRef]
- Grace, M.H.; Ribnicky, D.M.; Kuhn, P.; Poulev, A.; Logendra, S.; Yousef, G.G.; Raskin, I.; Lila, M.A. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine 2009, 16, 406–415. [Google Scholar] [CrossRef]
- Asgary, S.; Rafieiankopaei, M.; Sahebkar, A.; Shamsi, F.; Goli-malekabadi, N. Anti-hyperglycemic and anti-hyperlipidemic effects of Vaccinium myrtillus fruit in experimentally induced diabetes (antidiabetic effect of Vaccinium myrtillus fruit). J. Sci. Food Agric. 2016, 96, 764–768. [Google Scholar] [CrossRef]
- Chen, K.; Wei, X.; Zhang, J.; Pariyani, R.; Jokioja, J.; Kortesniemi, M.; Linderborg, K.M.; Heinonen, J.; Sainio, T.; Zhang, Y.; et al. Effects of anthocyanin extracts from bilberry (Vaccinium myrtillus L.) and purple potato (Solanum tuberosum L. var. ’Synkeä Sakari’) on the plasma metabolomic profile of Zucker diabetic fatty rats. J. Agric. Food Chem. 2020, 68, 9436–9450. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A.; Szwajgier, D.; Olkowicz, M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS ONE 2017, 12, e0188583. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A.; Rychlik, J.; Grajek, W. Cranberries (Oxycoccus quadripetalus) inhibit lipid metabolism and modulate leptin and adiponectin secretion in 3T3-L1 adipocytes. Food Chem. 2015, 185, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Tanaka, M.; Takanashi, M.; Hussain, A.; Yuan, B.; Toyoda, H.; Kuroda, M. Anthocyanidins-enriched bilberry extracts inhibit 3T3-L1 adipocyte differentiation via the insulin pathway. Nutr. Metab. 2011, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Brader, L.; Overgaard, A.; Christensen, L.P.; Jeppesen, P.B.; Hermansen, K. Polyphenol-rich bilberry ameliorates total cholesterol and LDL-cholesterol when implemented in the diet of Zucker diabetic fatty rats. Rev. Diabet. Stud. 2013, 10, 270–282. [Google Scholar] [CrossRef]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef]
- Mauray, A.; Felgines, C.; Morand, C.; Mazur, A.; Scalbert, A.; Milenkovic, D. Nutrigenomic analysis of the protective effects of bilberry anthocyanin-rich extract in apo E-deficient mice. Genes Nutr. 2010, 5, 343–353. [Google Scholar] [CrossRef]
- Mauray, A.; Felgines, C.; Morand, C.; Mazur, A.; Scalbert, A.; Milenkovic, D. Bilberry anthocyanin-rich extract alters expression of genes related to atherosclerosis development in aorta of apo E-deficient mice. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 72–80. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Lamari, F.N. Berry leaves: An alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef]
- Erlund, I.; Koli, R.; Alfthan, G.; Marniemi, J.; Puukka, P.; Mustonen, P.; Mattila, P.; Jula, A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 2008, 87, 323–331. [Google Scholar] [CrossRef]
- Lankinen, M.; Kolehmainen, M.; Jääskeläinen, T.; Paananen, J.; Joukamo, L.; Kangas, A.J.; Soininen, P.; Poutanen, K.; Mykkänen, H.; Gylling, H.; et al. Effects of whole grain, fish and bilberries on serum metabolic profile and lipid transfer protein activities: A randomized trial (Sysdimet). PLoS ONE 2014, 9, e90352. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Garcia, L.; Schlautman, B.; Covarrubias-Pazaran, G.; Maule, A.; Johnson-Cicalese, J.; Grygleski, E.; Vorsa, N.; Zalapa, J. Massive phenotyping of multiple cranberry populations reveals novel QTLs for fruit anthocyanin content and other important chemical traits. Mol. Genet. Genom. 2018, 293, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Bolivar-Medina, J.L.; Villouta, C.; Workmaster, B.A.; Atucha, A. Floral meristem development in cranberry apical buds during winter rest and its implication on yield prediction. J. Am. Soc. Hortic. Sci. 2019, 144, 314–320. [Google Scholar] [CrossRef]
- Feghali, K.; Feldman, M.; La, V.D.; Santos, J.; Grenier, D. Cranberry proanthocyanidins: Natural weapons against periodontal diseases. J. Agric. Food Chem. 2012, 60, 5728–5735. [Google Scholar] [CrossRef]
- Diaz-Garcia, L.; Rodriguez-Bonilla, L.; Phillips, M.; Lopez-Hernandez, A.; Grygleski, E.; Atucha, A.; Zalapa, J. Comprehensive analysis of the internal structure and firmness in American cranberry (Vaccinium macrocarpon Ait.) fruit. PLoS ONE 2019, 14, e0222451. [Google Scholar] [CrossRef]
- Rodriguez-Bonilla, L.; Williams, K.A.; Rodríguez Bonilla, F.; Matusinec, D.; Maule, A.; Coe, K.; Wiesman, E.; Diaz-Garcia, L.; Zalapa, J. The genetic diversity of cranberry crop wild relatives, Vaccinium macrocarpon aiton and v. Oxycoccos l., in the us, with special emphasis on national forests. Plants 2020, 9, 1446. [Google Scholar] [CrossRef]
- Sandler, H.; DeMoranville, C. Cranberry Production: A Guide for Massachusetts—Summary Edition. UMass Amherst Cranberry Stn. 2008, 1–37. [Google Scholar]
- Neto, C.C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. J. Nutr. 2007, 137, 186S–193S. [Google Scholar] [CrossRef]
- Karlsons, A.; Osvalde, A.; Nollendorfs, V. Research on the mineral composition of american cranberries and wild cranberries in Latvia. Latv. J. Agron. 2009, 12, 65–71. [Google Scholar]
- Česonienė, L.; Daubaras, R. Phytochemical Composition of the Large Cranberry (Vaccinium macrocarpon) and the Small Cranberry (Vaccinium oxycoccos); Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780124081178. [Google Scholar]
- Martinussen, I.; Rohloff, J.; Uleberg, E.; Junttila, O.; Hohtola, A.; Jaakola, L.; Häggman, H. Climatic effects on the production and quality of bilberries (Vaccinium myrtillus). Agron. Vēstis 2009, 71–74. [Google Scholar]
- Kalın, P.; Gülçin, İl.; Gören, A.C. Antioxidant activity and polyphenol content of cranberries (Vaccinium macrocarpon). Rec. Nat. Prod. 2015, 9, 496–502. [Google Scholar]
- Nemzer, B.V.; Al-Taher, F.; Yashin, A.; Revelsky, I.; Yashin, Y. Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health. Overview. Molecules 2022, 27, 1503. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. The effect of different maturity stages on phytochemical composition and antioxidant capacity of cranberry cultivars. Eur. Food Res. Technol. 2018, 244, 705–719. [Google Scholar] [CrossRef]
- Rózańska, D.; Regulska-Ilow, B. The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv. Clin. Exp. Med. 2018, 27, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Babar, A.; Moore, L.; Leblanc, V.; Dudonné, S.; Desjardins, Y.; Lemieux, S.; Bochard, V.; Guyonnet, D.; Dodin, S. Standardised high dose versus low dose cranberry Proanthocyanidin extracts for the prevention of recurrent urinary tract infection in healthy women [PACCANN]: A double blind randomised controlled trial protocol. BMC Urol. 2021, 21, 29. [Google Scholar] [CrossRef]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef]
- Krueger, C.G.; Reed, J.D.; Feliciano, R.P.; Howell, A.B. Quantifying and characterizing proanthocyanidins in cranberries in relation to urinary tract health. Anal. Bioanal. Chem. 2013, 405, 4385–4395. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kolniak-Ostek, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Phytochemical Compounds and Antioxidant Activity in Different Cultivars of Cranberry (Vaccinium macrocarpon L). J. Food Sci. 2017, 82, 2569–2575. [Google Scholar] [CrossRef]
- Wu, X.; Wu, X.; Xue, L.; Tata, A.; Song, M.; Song, M.; Neto, C.C.; Xiao, H. Bioactive Components of Polyphenol-Rich and Non-Polyphenol-Rich Cranberry Fruit Extracts and Their Chemopreventive Effects on Colitis-Associated Colon Cancer. J. Agric. Food Chem. 2020, 68, 6845–6853. [Google Scholar] [CrossRef]
- Narwojsz, A.; Tańska, M.; Mazur, B.; Borowska, E.J. Fruit Physical Features, Phenolic Compounds Profile and Inhibition Activities of Cranberry Cultivars (Vaccinium macrocarpon) Compared to Wild-Grown Cranberry (Vaccinium oxycoccus). Plant Foods Hum. Nutr. 2019, 74, 300–306. [Google Scholar] [CrossRef]
- Noushahi, H.A.; Khan, A.H.; Noushahi, U.F.; Hussain, M.; Javed, T.; Zafar, M.; Batool, M.; Ahmed, U.; Liu, K.; Harrison, M.T.; et al. Biosynthetic pathways of triterpenoids and strategies to improve their Biosynthetic Efficiency. Plant Growth Regul. 2022, 97, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Sedbare, R.; Raudone, L.; Zvikas, V.; Viskelis, J.; Liaudanskas, M.; Janulis, V. Development and Validation of the UPLC-DAD Methodology for the Detection of Triterpenoids and Phytosterols in Fruit Samples of Vaccinium macrocarpon Aiton and Vaccinium oxycoccos L. Molecules 2022, 27, 4403. [Google Scholar] [CrossRef] [PubMed]
- Viskelis, P.; Rubinskiene, M.; Jasutiene, I.; Šarkinas, A.; Daubaras, R.; Česoniene, L. Anthocyanins, antioxidative, and antimicrobial properties of american cranberry (Vaccinium macrocarpon ait.) and their press cakes. J. Food Sci. 2009, 74, C157–C161. [Google Scholar] [CrossRef] [PubMed]
- Daoutidou, M.; Plessas, S.; Alexopoulos, A. Assessment of Antimicrobial Activity of Pomegranate, Cranberry, and Black Chokeberry Extracts against Foodborne Pathogens. Foods 2021, 10, 486. [Google Scholar] [CrossRef]
- Kranz, S.; Guellmar, A.; Olschowsky, P.; Tonndorf-Martini, S.; Heyder, M.; Pfister, W.; Reise, M.; Sigusch, B. Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria. Antibiotics 2019, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B. Potential of cranberry for suppressing Helicobacter pylori, a risk factor for gastric cancer. J. Berry Res. 2020, 10, 11–20. [Google Scholar] [CrossRef]
- Burger, O.; Ofek, I.; Tabak, M.; Weiss, E.I.; Sharon, N.; Neeman, I. A high molecular mass constituent of cranberry juice inhibits Helicobacter pylori adhesion to human gastric mucus. FEMS Immunol. Med. Microbiol. 2000, 29, 295–301. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A. Beneficial effects of cranberry in the prevention of obesity and related complications: Metabolic syndrome and diabetes—A review. J. Funct. Foods 2016, 20, 171–181. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Kunkel, S.D.; Elmore, C.J.; Bongers, K.S.; Ebert, S.M.; Fox, D.K.; Dyle, M.C.; Bullard, S.A.; Adams, C.M. Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver disease. PLoS ONE 2012, 7, e39332. [Google Scholar] [CrossRef]
- Cermak, R.; Landgraf, S.; Wolffram, S. Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br. J. Nutr. 2004, 91, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Schell, J.; Betts, N.M.; Foster, M.; Scofield, R.H.; Basu, A. Cranberries improve postprandial glucose excursions in type 2 diabetes. Food Funct. 2017, 8, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- de Llano, D.G.; Moreno-Arribas, M.V.; Bartolomé, B. Cranberry polyphenols and prevention against urinary tract Infections: Relevant considerations. Molecules 2020, 25, 3523. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B. Oral Health Benefits of Cranberry: A Review. IOSR J. Dent. Med. Sci. 2019, 18, 41–44. [Google Scholar] [CrossRef]
- Nowaczyk, P.M.; Bajerska, J.; Lasik-Kurdyś, M.; Radziejewska-Kubzdela, E.; Szwengiel, A.; Woźniewicz, M. The effect of cranberry juice and a cranberry functional beverage on the growth and metabolic activity of selected oral bacteria. BMC Oral Health 2021, 21, 660. [Google Scholar] [CrossRef]
- Ruel, G.; Couillard, C. Evidences of the cardioprotective potential of fruits: The case of cranberries. Mol. Nutr. Food Res. 2007, 51, 692–701. [Google Scholar] [CrossRef]
- Masnadi Shirazi, K.; Shirinpour, E.; Masnadi Shirazi, A.; Nikniaz, Z. Effect of cranberry supplementation on liver enzymes and cardiometabolic risk factors in patients with NAFLD: A randomized clinical trial. BMC Complement. Med. Ther. 2021, 21, 283. [Google Scholar] [CrossRef]
- Thimóteo, N.S.B.; Iryioda, T.M.V.; Alfieri, D.F.; Rego, B.E.F.; Scavuzzi, B.M.; Fatel, E.; Lozovoy, M.A.B.; Simão, A.N.C.; Dichi, I. Cranberry juice decreases disease activity in women with rheumatoid arthritis. Nutrition 2019, 60, 112–117. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. Cranberries (Vaccinium macrocarpon) and cardiovascular disease risk factors. Nutr. Rev. 2007, 65, 490–502. [Google Scholar] [CrossRef]
- Mantzorou, M.; Zarros, A.; Vasios, G.; Theocharis, S.; Pavlidou, E.; Giaginis, C. Cranberry: A Promising Natural Source of Potential Nutraceuticals with Anticancer Activity. Anticancer. Agents Med. Chem. 2019, 19, 1672–1686. [Google Scholar] [CrossRef]
- Strobel, P.; Allard, C.; Perez-Acle, T.; Calderon, R.; Aldunate, R.; Leighton, F. Myricetin, quercetin and catechin-gallate inhibit glucose uptake in isolated rat adipocytes. Biochem. J. 2005, 386, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.M.U.P.; Caldas, A.P.S.; da Silva, B.P.; Hermsdorff, H.H.M.; Alfenas, R. de C.G. Effects of blueberry and cranberry consumption on type 2 diabetes glycemic control: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.C.; Barney, D.L. Shoot regeneration and plantlet formation by cascade huckleberry, mountain huckleberry, and oval-leaf bilberry on a Zeatin-containing nutrient medium. Horttechnology 2012, 22, 106–113. [Google Scholar] [CrossRef]
- Hummer, K.E. Manna in winter: Indigenous Americans, huckleberries, and blueberries. HortScience 2013, 48, 413–417. [Google Scholar] [CrossRef]
- Lila, M.A.; Kellogg, J.; Grace, M.H.; Yousef, G.G.; Kraft, T.B.; Rogers, R.B. Stressed for success: How the berry’s wild origins result in multifaceted health protections. Acta Hortic. 2014, 1017, 23–44. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E.; Wrolstad, R.E. Anthocyanin pigment and total phenolic content of three Vaccinium species native to the Pacific Northwest of North America. HortScience 2004, 39, 959–964. [Google Scholar] [CrossRef]
- Kerns, B.K.; Alexander, S.J.; Bailey, J.D. Huckleberry abundance, stand conditions, and use in Western Oregon: Evaluating the role of forest management. Econ. Bot. 2004, 58, 668–678. [Google Scholar] [CrossRef]
- Finn, C.; Young, A. Variability for Reproductive and Vegetative Traits in Vaccinium Membranaceum. Acta Hortic. 2002, 181–187. [Google Scholar] [CrossRef]
- Stevens, M.; Darris, D.C. Black Huckleberry Vaccinium membranaceum Dougl. ex Torr.; 2001; USDA NRCS National Plant Data Center & Oregon Plant Materials Center, United States. Available online: http://plant-materials.nrcs.usda.gov (accessed on 22 November 2022).
- Taruscio, T.G.; Barney, D.L.; Exon, J. Content and Profile of Flavanoid and Phenolic Acid Compounds in Conjunction with the Antioxidant Capacity for a Variety of Northwest Vaccinium Berries. J. Agric. Food Chem. 2004, 52, 3169–3176. [Google Scholar] [CrossRef]
- Vander Kloet, S.P.; Dickinson, T.A. The taxonomy of Vaccinium section Myrtillus (Ericaceae). Brittonia 1999, 51, 231–254. [Google Scholar] [CrossRef]
- Barney, D.L. Prospects for domesticating western huckleberries. Small Fruits Rev. 2003, 2, 15–29. [Google Scholar] [CrossRef]
- Barney, D.L.; Lopez, O.A.; King, E. Micropropagation of cascade huckleberry, mountain huckleberry, and oval-leaf bilberry using woody plant medium and Murashige and Skoog medium formulations. Horttechnology 2007, 17, 279–284. [Google Scholar] [CrossRef]
- Gorzelak, M.A.; Hambleton, S.; Massicotte, H.B. Community structure of ericoid mycorrhizas and root-associated fungi of Vaccinium membranaceum across an elevation gradient in the Canadian Rocky Mountains. Fungal Ecol. 2012, 5, 36–45. [Google Scholar] [CrossRef]
- Holden, Z.A.; Kasworm, W.F.; Servheen, C.; Hahn, B.; Dobrowski, S. Sensitivity of berry productivity to climatic variation in the cabinet-yaak grizzly bear recovery zone, Northwest United States, 1989–2010. Wildl. Soc. Bull. 2012, 36, 226–231. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E.; Wrolstad, R.E. Comparison of anthocyanin pigment and other phenolic compounds of Vaccinium membranaceum and Vaccinium ovatum native to the Pacific Northwest of North America. J. Agric. Food Chem. 2004, 52, 7039–7044. [Google Scholar] [CrossRef]
- Kuhnlein, H.V. Nutrient values in indigenous wild plant greens and roots used by the Nuxalk people of Bella Coola, British Columbia. J. Food Compos. Anal. 1990, 3, 38–46. [Google Scholar] [CrossRef]
- Prevéy, J.S.; Parker, L.E.; Harrington, C.A.; Lamb, C.T.; Proctor, M.F. Climate change shifts in habitat suitability and phenology of huckleberry (Vaccinium membranaceum). Agric. For. Meteorol. 2020, 280, 107803. [Google Scholar] [CrossRef]
- Shores, C.R.; Mikle, N.; Graves, T.A. Mapping a keystone shrub species, huckleberry (Vaccinium membranaceum), using seasonal colour change in the Rocky Mountains. Int. J. Remote Sens. 2019, 40, 5695–5715. [Google Scholar] [CrossRef]
- Nemes, S.A.; Szabo, K. Applicability of Agro-Industrial By-Products in Intelligent Food Packaging. Coatings 2020, 10, 550. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Nemeș, S.A.; Pop, O.L.; Coldea, T.E.; Fogarasi, M.; Biriș-Dorhoi, E.S. An Update Regarding the Bioactive Compound of Cereal By-Products: Health Benefits and Potential Applications. Nutrients 2022, 14, 3470. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.-F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Ferenczi, L.-J.; Ştefănescu, B.-E.; VODNAR, D.C. Inhibitory Potential Of Lactobacillus Plantarum on Escherichia Coli. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2017, 74, 99. [Google Scholar] [CrossRef] [PubMed]
- Teleky, B.-E.; Mitrea, L.; Călinoiu, L.-F.; Martău, G.-A.; Vodnar, D.-C. Microbial Processes to Produce Food Ingredients and Products. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–17. ISBN 9780081005965. [Google Scholar]
- Booth, N.L.; Kruger, C.L.; Wallace Hayes, A.; Clemens, R. An innovative approach to the safety evaluation of natural products: Cranberry (Vaccinium macrocarpon Aiton) leaf aqueous extract as a case study. Food Chem. Toxicol. 2012, 50, 3150–3165. [Google Scholar] [CrossRef] [PubMed]
- Stefkov, G.; Hristovski, S.; Petreska Stanoeva, J.; Stefova, M.; Melovski, L.; Kulevanova, S. Resource assessment and economic potential of bilberries (Vaccinium myrtillus and Vaccinium uliginosum) on Osogovo Mtn., R. Macedonia. Ind. Crops Prod. 2014, 61, 145–150. [Google Scholar] [CrossRef]
- Holopainen, M.; Jahodar, L.; Seppanen-Laakso, T.; Laakso, I.; Kauppinen, V. Antimicrobial activity of some Finnish Ericaceous plants. Acta Pharm. Fenn. 1988, 97, 197–202. [Google Scholar]
- Riihinen, K.; Jaakola, L.; Kärenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and “northblue” blueberry (Vaccinium corymbosum x V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef]
- Martz, F.; Jaakola, L.; Julkunen-Tiitto, R.; Stark, S. Phenolic Composition and Antioxidant Capacity of Bilberry (Vaccinium myrtillus) Leaves in Northern Europe Following Foliar Development and Along Environmental Gradients. J. Chem. Ecol. 2010, 36, 1017–1028. [Google Scholar] [CrossRef]
- Rouanet, J.M.; Décordé, K.; Del Rio, D.; Auger, C.; Borges, G.; Cristol, J.P.; Lean, M.E.J.; Crozier, A. Berry juices, teas, antioxidants and the prevention of atherosclerosis in hamsters. Food Chem. 2010, 118, 266–271. [Google Scholar] [CrossRef]
- Wang, S.Y.; HsinShan, L. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Kitrytė, V.; Kavaliauskaitė, A.; Tamkutė, L.; Pukalskienė, M.; Syrpas, M.; Rimantas Venskutonis, P. Zero waste biorefining of lingonberry (Vaccinium vitis-idaea L.) pomace into functional ingredients by consecutive high pressure and enzyme assisted extractions with green solvents. Food Chem. 2020, 322, 126767. [Google Scholar] [CrossRef]
| Class | Phenolic Compounds | Chemical Structures (Main Compound) | |
|---|---|---|---|
| Leaves | Stems | ||
| Catechins | (+)-catechin—R1 = H (–)-epicatechin—R1 = H+ gallocatechin—R1 = OH epigallocatechin—R1 = OH | 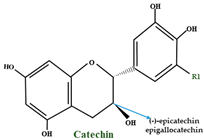 | |
| Cinchonains | cinchonains I cinchonains II | 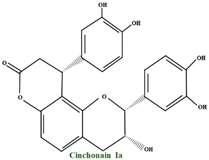 | |
| Phenolic acids | 3,4–dihydroxybenzoic p–coumaroyl quinic acid isomers p–coumaroyl malonic acid p–coumaroyl derivatives p–coumaroyl glucose coumaroyl iridoid | 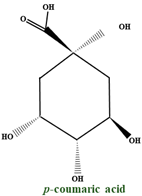 | |
| p–coumaric acid | - | 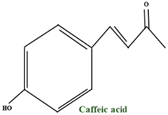 | |
| feruloyl quinic acid isomer | - | ||
| caffeoyl quinic acid isomers | - | ||
| caffeic acid ethyl ester | - | ||
| caffeic acid hexoside | - | ||
| Proantho- cyanidins | B–type dimer B–type trimer B–type tetramer B–type pentamer A–type dimer A–type trimer procyanidin A2 procyanidin B1 procyanidin B2 | 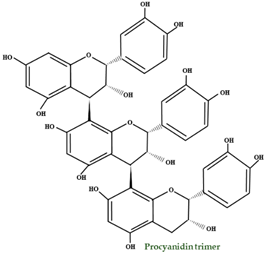 | |
| Flavonols | quercetin–3–O–(4”–HMG)–α–rhamnoside quercetin–3–O–galactoside quercetin–3–O–glucoside quercetin–3–O–rutinoside quercetin–3–O-α–rhamnoside quercetin–3–O–arabinoside quercetin–3–O–glucuronide | 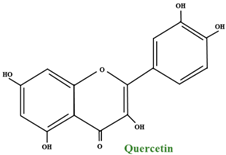 | |
| kaempferol | - | 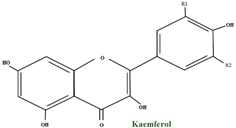 | |
| kaempferol–3–glucuronide | - | ||
| kaempferol–hexoside | - | ||
| kaempferol–O–pentoside | - | ||
| kaempferol–(HMG)–rhamnoside | - | ||
| Lignans | - | Lyoniside (9–O–β–D–xylopyranosyl(+)lyoniresinol) | 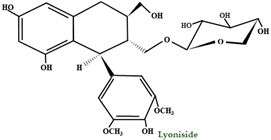 |
| Traditional Uses | Used Parts | Administration | References |
|---|---|---|---|
| Fevers and coughs | Fruits | 50–200 mg/kg | [63] |
| Respiratory inflammations | Leaves/fruits | [58,64] | |
| Antidiabetic | Leaves | 10 mg | [65] |
| Anti-inflammatory | Leaves Fruits | 4 mg/mL 100 ng/mL | [66] [67] |
| α-glucosidase activity | Fruits | 20 μg GAE/mL ME 55 μg GAE/mL AE | [61] |
| Neuroprotective effects | Fruits | 100 mg/kg | [68] |
| Digestive Urinary tract disorders | Fruits | 250 mg GAE/L | [22] |
| Eye inflammation | Fruits | 50 mg/mL | [69] |
| Ulcerative colitis | Fruits | 95 g DW | [70] |
| Hypertension Weight gain reduction | Fruits | 3% (w/w) 10% (w/w) | [71] |
| Sun Protection Factor (SPF) | Fruits | 2.84 g/100 gDW | [58] |
| Diabetic retinopathy | Leaves/fruits | 100 mg/kg | [72] |
| Antiseptic, astringent, tonic | Fruits | 28.3 μg CYA-3-GLU equivalents/mL CM | [73] |
| Angiotensin-Converting Enzyme Activity | Fruits | 0.1 mg/mL | [74] |
| V. macrocarpon | V. oxycoccos | Method | |
|---|---|---|---|
| Macroelements, mg/100 g fw | |||
| Magnesium | 5.2–9.1 | 7.8–9.1 | AAS (Perkin Elmer AAnalyst 700, acetylene-air flame) |
| Sulfur | 5.2–14.3 | 6.5–18.2 | Turbidimetry |
| Potassium | 52.0–98.8 | 78.0–93.6 | FP (Jenwey PFP7, airpropane butane flame) |
| Calcium | 7.8–14.3 | 9.1–18.2 | AAS (Perkin Elmer AAnalyst 700, acetylene-air flame) |
| Phosphorus | 6.5–11.7 | 6.5–7.8 | Colourimetry |
| Nitrogen | 10.4–65.0 | 13.0–78.0 | Colourimetry |
| Microelements, mg/100 g fw | |||
| Iron | 0.22–1.17 | 0.33–0.42 | AAS (Perkin Elmer AAnalyst 700, acetylene-air flame) |
| Manganese | 0.06–0.57 | 2.18–3.95 | AAS (Perkin Elmer AAnalyst 700, acetylene-air flame) |
| Zinc | 0.07–0.17 | 0.14–0.19 | AAS (Perkin Elmer AAnalyst 700, acetylene-air flame) |
| Molybdenum | 0.01–0.02 | 0.01–0.02 | Colourimetry |
| Copper | 0.04–0.08 | 0.06–0.08 | AAS (Perkin Elmer AAnalyst 700, acetylene-air flame) |
| Boron | 0.03–0.12 | 0.10–0.17 | Colourimetry |
| Type of Disease | Mechanism | References |
|---|---|---|
| Gastric cancer | A-type procyanidins in cranberry ↓ the adhesion of H. pylori on human gastric mucus and have an inhibitory effect | [125,126] |
| Obesity | Cranberry ↓ lipid accretion by lowering mRNA level of some genes associated with fatty acid-binding protein, lipoprotein lipase, fatty acid synthase, hormone-sensitive lipase, and perilipin | [127,128,129] |
| Type 2 diabetes | Quercitin ↓ gastric assimilation of glucose and with myricetin can ↓ glucose transporter type 4 mediated glucose assimilation | [130,131] |
| Urinary tract inflammation | A-type PACs ↓ adhesion of P-fimbriated uropathogenic E. coli to uroepithelial cells | [114,116,132] |
| Periodontitis | Cranberry non-dialysable material ↓ proliferation of P.gingivalis, T.forsythia, and T.denticola in periodontal pockets A-type cranberry PACs ↓ production of metalloproteinases | [124,133,134] |
| Anti-inflammatory | Quercetin ↓ of the nuclear factor-kappa B (NF-κB) pathway, NDM lower lipopolysaccharide-induced inflammatory cytokine production | [135] |
| Nonalcoholic fatty liver disease | In vitro study > cranberry diet 3 months > alanine reduction aminotransferase and insulin, + lipid profile effect, insulin resistance and hepatic steatosis in NAFLD patients | [136] |
| Microbial | Cranberry extracts ↓ on pathogenic bacteria: Listeria monocytogenes (ATCC 19117), B. cereus (ATCC 10876), B. subtilis (ATCC 6633), M. luteus (ATCC 9341), E. faecalis (ATCC 29212), S. aureus (ATCC 25923) and Gram-negative E. coli (ATCC25922), Enterobacter aerogenes (ATCC 13048), Slm. typhimurium (ATCC 14028), and Slm. agona bacteria | [122,123] |
| Cardiovascular | The expression of inflammatory genes suited for cardiovascular diseases is ↓ by resveratrol (polyphenol present in cranberry juice) by inflecting the NF-kB and JAK STAT3 pathways in cultured cells | [137,138] |
| Breast and colon cancer | Quercitin and proanthocyanidin inhibited the expansion of MCF-7 human breast adenocarcinoma and HT–29 human colon adenocarcinoma | [106,118] |
| Leukaemia and lung cancer | Ursolic acid can inhibit the growth of some leukaemia cell lines and A-549 human lung carcinoma | [106,139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martău, G.A.; Bernadette-Emőke, T.; Odocheanu, R.; Soporan, D.A.; Bochiș, M.; Simon, E.; Vodnar, D.C. Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants. Molecules 2023, 28, 1533. https://doi.org/10.3390/molecules28041533
Martău GA, Bernadette-Emőke T, Odocheanu R, Soporan DA, Bochiș M, Simon E, Vodnar DC. Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants. Molecules. 2023; 28(4):1533. https://doi.org/10.3390/molecules28041533
Chicago/Turabian StyleMartău, Gheorghe Adrian, Teleky Bernadette-Emőke, Răzvan Odocheanu, Dacian Andrei Soporan, Mihai Bochiș, Elemer Simon, and Dan Cristian Vodnar. 2023. "Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants" Molecules 28, no. 4: 1533. https://doi.org/10.3390/molecules28041533
APA StyleMartău, G. A., Bernadette-Emőke, T., Odocheanu, R., Soporan, D. A., Bochiș, M., Simon, E., & Vodnar, D. C. (2023). Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants. Molecules, 28(4), 1533. https://doi.org/10.3390/molecules28041533