Potential Anticancer Activity of Juniperus procera and Molecular Docking Models of Active Proteins in Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Extraction of Bioactive Compounds
2.2. Effect of Methanolic Extract of J. procera on the Proliferation of Several Cancer Cell Lines
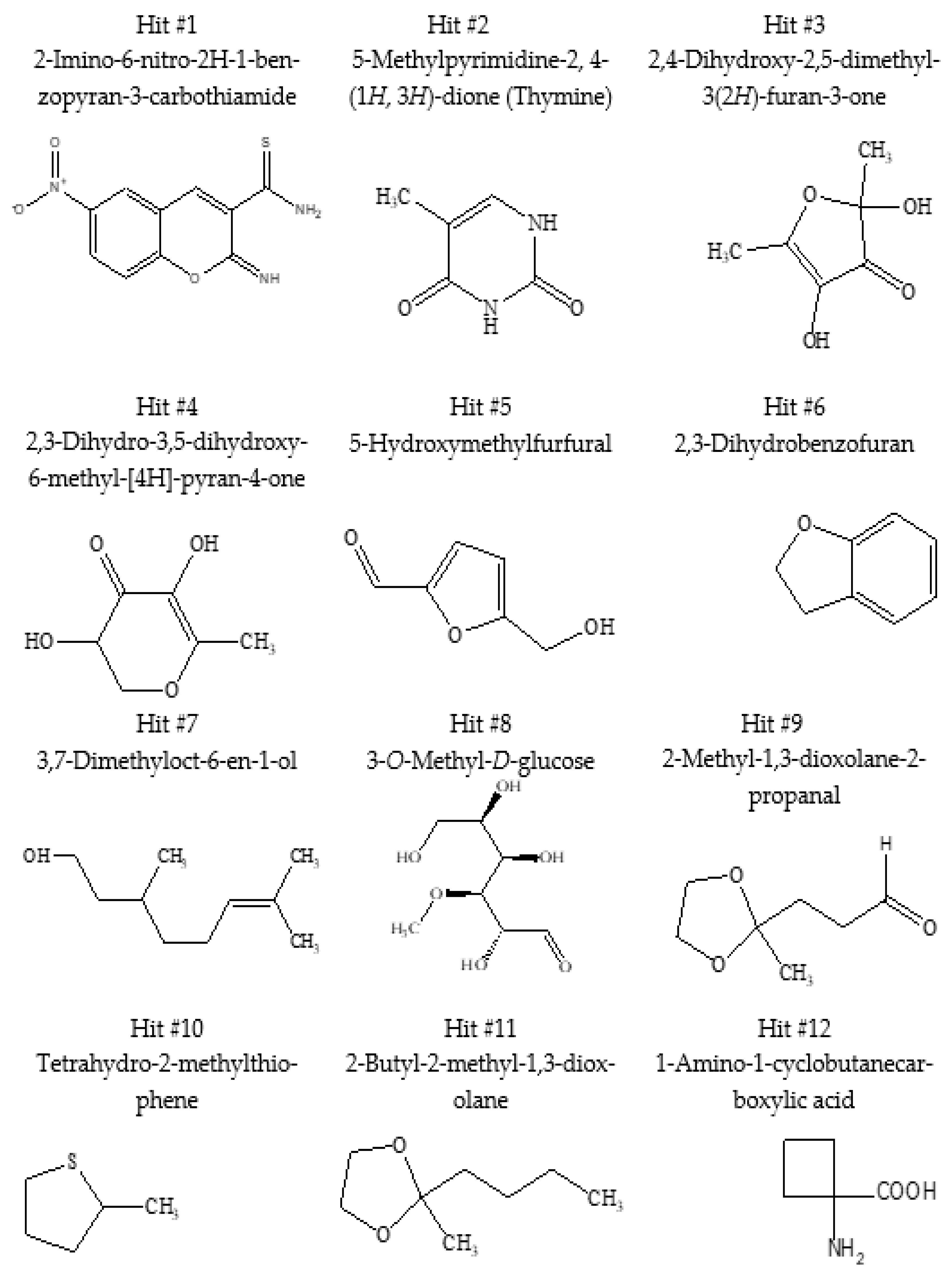
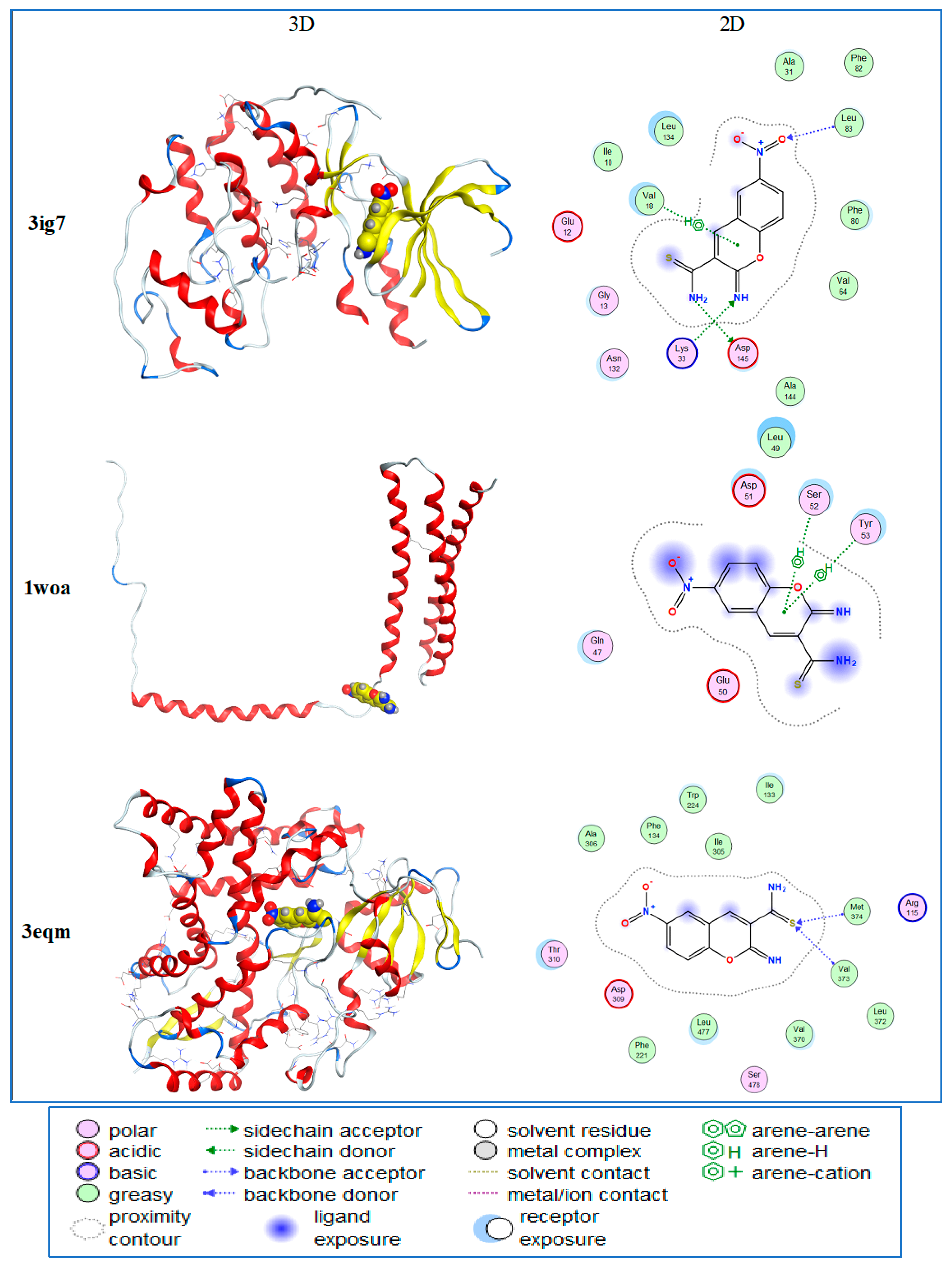
2.3. GC/MS Analysis of Juniperus procera Leaf Extract and Molecular Docking Study
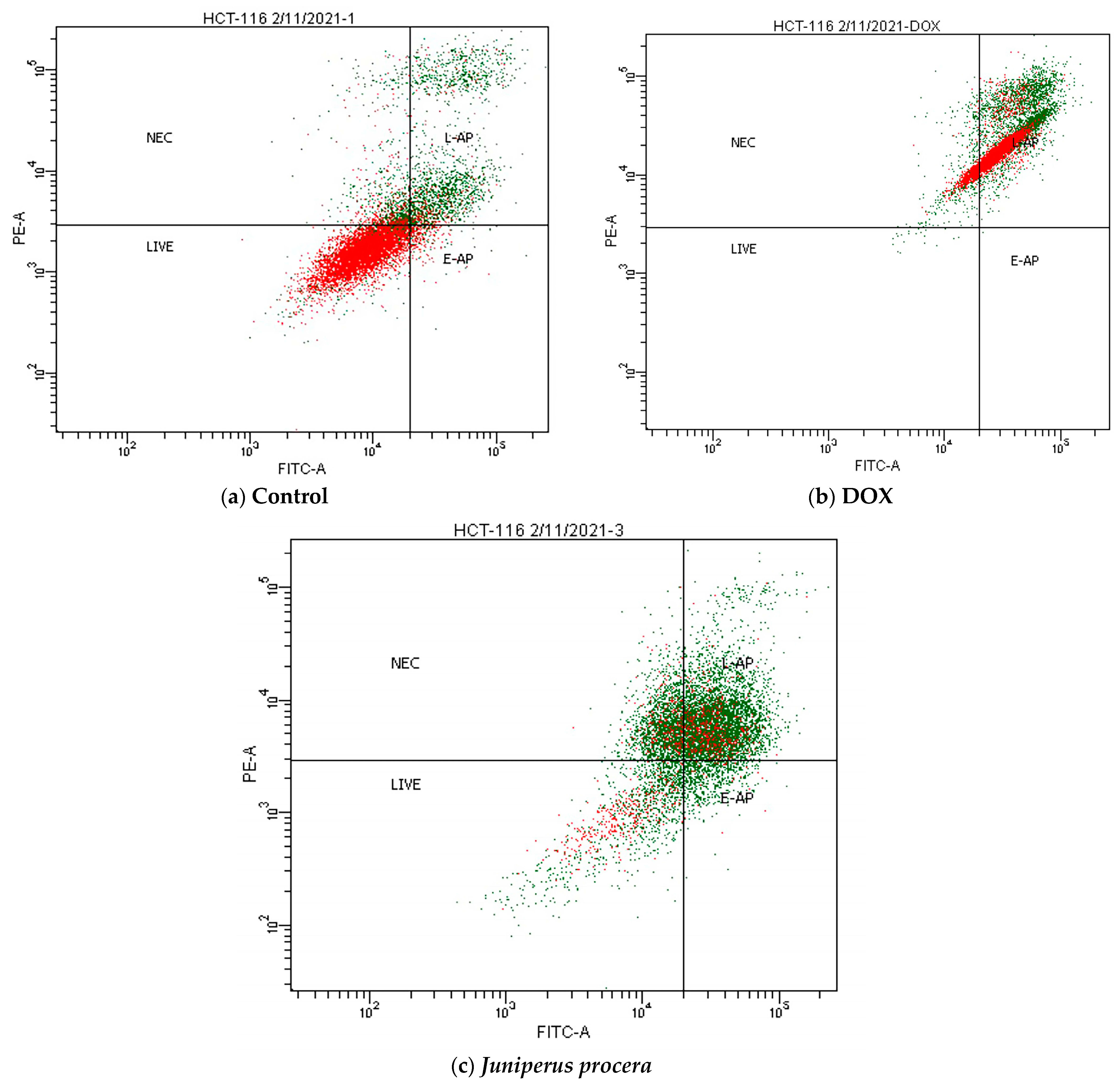
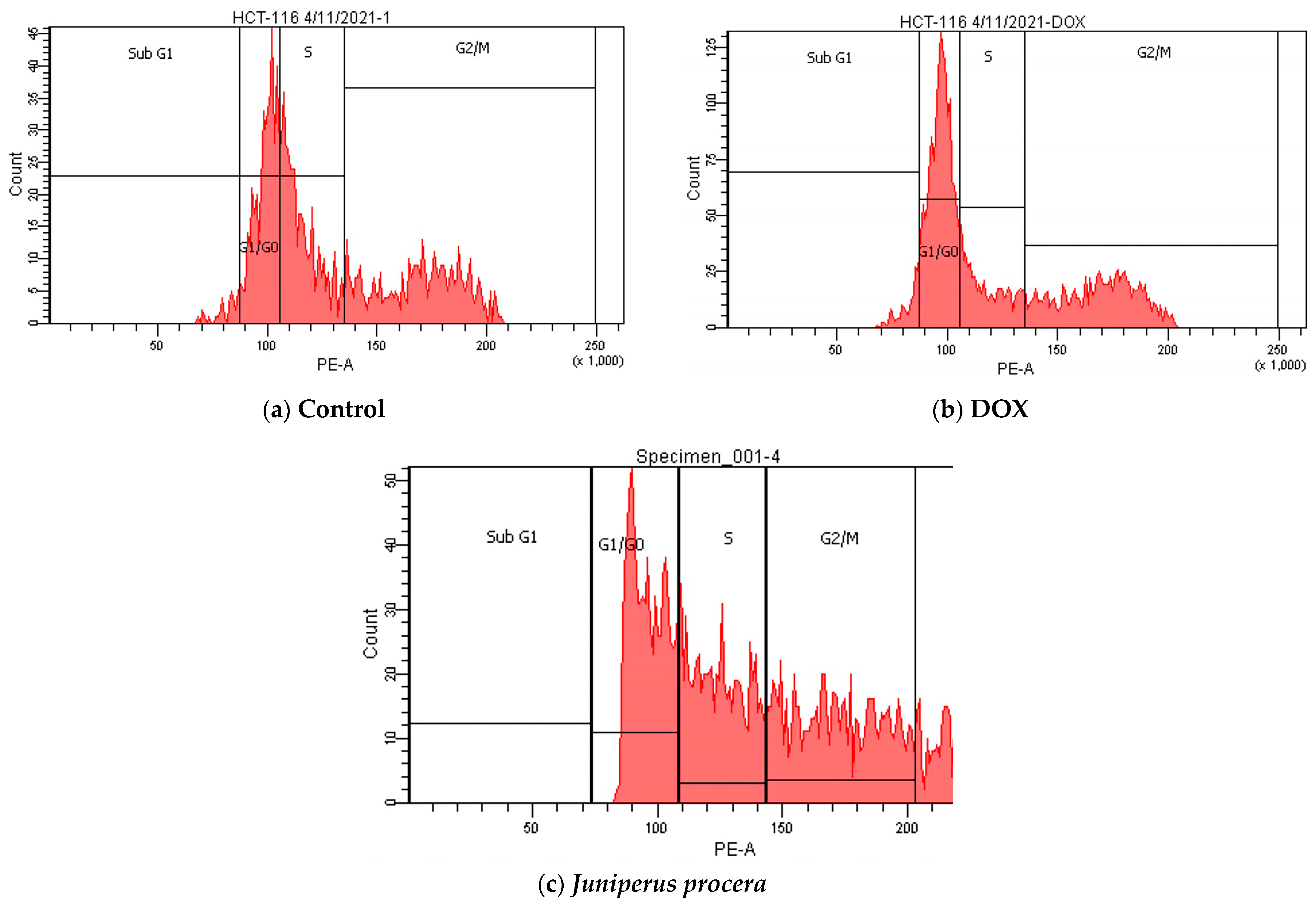
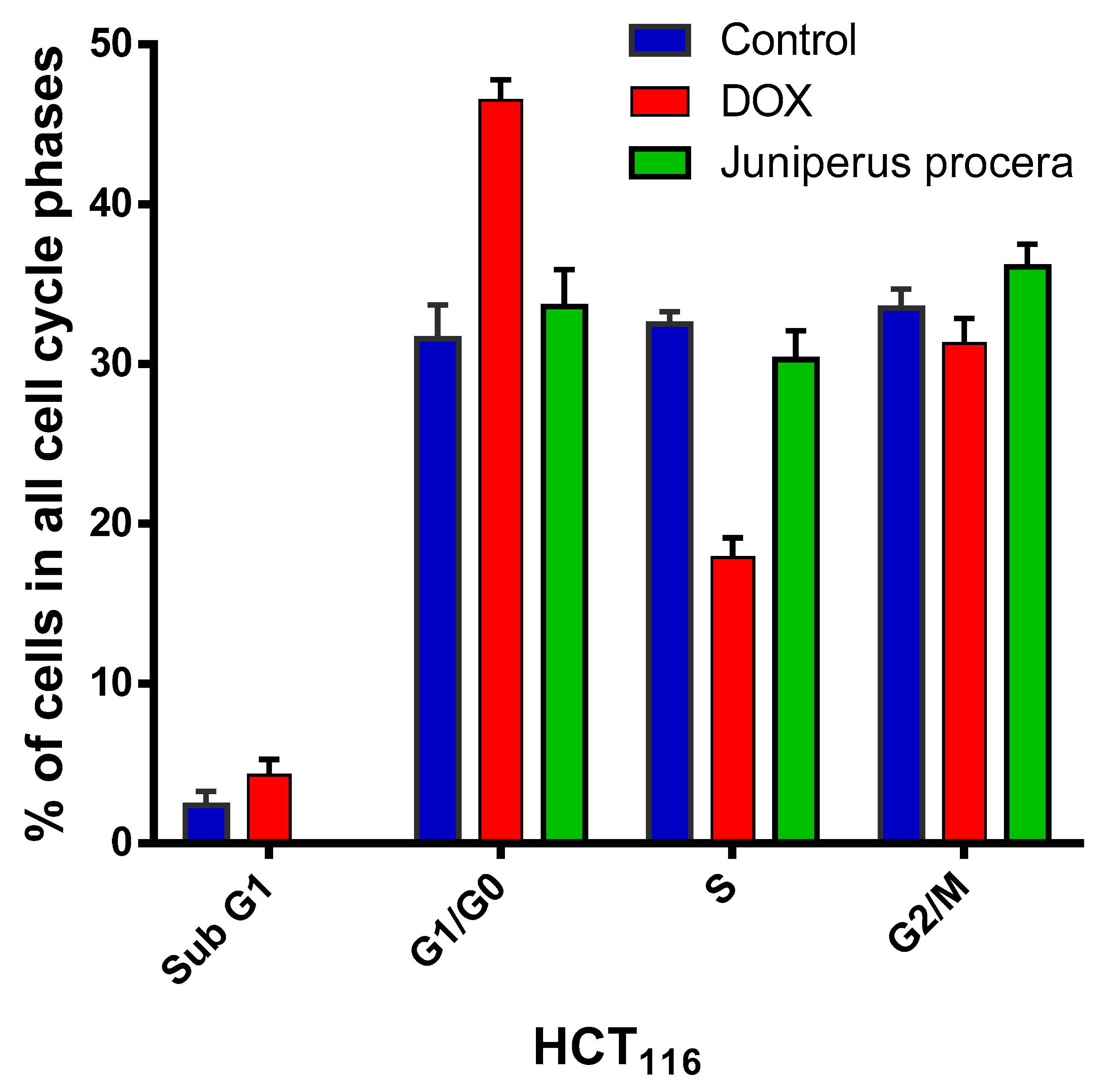
2.4. Flow-Cytometry Assessment of Apoptosis and Cell-Cycle Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction of Plant Material
4.3. Human Cell Line and Culture Conditions
4.4. Evaluations of the IC50 of J. procera in Human Cell Lines Using the MTT Assay
4.5. Assessment of Apoptosis in HCT116 Cells Treated with J. procera
4.6. Evaluation of the Cell Cycle in HCT116 Treated with J. procera
4.7. GS/MS Examination of Methanolic Extract of J. procera Leaves
4.8. Molecular Docking Study of J. procera Extract with Four Different Cancer Proteins
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Dong, J.; Chen, H. Cardiotoxicity of anticancer therapeutics. Front. Cardiovasc. Med. 2018, 5, 9. [Google Scholar] [CrossRef]
- Kilickap, S.; Akgul, E.; Aksoy, S.; Aytemir, K.; Barista, I. Doxorubicin-induced second degree and complete atrioventricular block. EP Eur. 2005, 7, 227–230. [Google Scholar] [CrossRef]
- Al-Zahrani, A.; Ibraheem, F.; El-Senduny, F.F. Anticancer activities of Saudi Juniperus procera extracts and their effects on the regulatory mechanisms in controlling growth of human cancerous cells. Res. Sq. 2022, 1–25. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The application of medicinal plants in traditional and modern medicine: A review of Thymus vulgaris. Int. J. Clin. Med. 2015, 6, 635. [Google Scholar] [CrossRef]
- Mintah, S.O.; Asafo-Agyei, T.; Archer, M.-A.; Junior, P.A.-A.; Boamah, D.; Kumadoh, D.; Appiah, A.; Ocloo, A.; Boakye, Y.D.; Agyare, C. Medicinal plants for treatment of prevalent diseases. Pharmacogn. Med. Plants 2019, 9, 1–19. [Google Scholar]
- Al-Attar, A.M.; Alrobai, A.A.; Almalki, D.A. Effect of Olea oleaster and Juniperus procera leaves extracts on thioacetamide induced hepatic cirrhosis in male albino mice. Saudi J. Biol. Sci. 2016, 23, 363–371. [Google Scholar] [CrossRef]
- Dailah, H.G. The ethnomedicinal evidences pertaining to traditional medicinal herbs used in the treatment of respiratory illnesses and disorders in Saudi Arabia: A review. Saudi J. Biol. Sci. 2022, 29, 103386. [Google Scholar] [CrossRef]
- Jansen, P.C.M. Spices, Condiments and Medicinal Plants in Ethiopia, Their Taxonomy and Agricultural Significance; Wageningen University and Research: Wageningen, The Netherlands, 1981. [Google Scholar]
- Topçu, G.; Erenler, R.; Çakmak, O.; Johansson, C.B.; Çelik, C.; Chai, H.-B.; Pezzuto, J.M. Diterpenes from the berries of Juniperus excelsa. Phytochemistry 1999, 50, 1195–1199. [Google Scholar] [CrossRef]
- Alkhedaide, A.; Nassan, M.A.; Ismail, T.A.; Soliman, M.M.; Mohamed, E.H.; Amer, H.H.; Aldhahrani, A. Hypoglycemic and antioxidant effect of Juniperus procera extract on rats with streptozotocin-induced diabetes. Pathophysiology 2019, 26, 361–368. [Google Scholar] [CrossRef]
- Alkhedaide, A.Q. Anti-inflammatory effect of Juniperus procera extract in rats exposed to streptozotocin toxicity. Anti-Inflamm. Anti-Allergy Agents Med. Chem. Former. Curr. Med. Chem. Anti-Inflamm. Anti-Allergy Agents 2019, 18, 71–79. [Google Scholar] [CrossRef]
- AlShahrani, A.; AlShahrani, I.; Hosmani, J.; Togoo, R.A.; Sakinatulain, T.; Alam, T.; Hameed, M.S. Anticancer activity of Juniperus procera grown in southwestern region of Saudi Arabia on human oral squamous cell carcinoma cell lines. Pharmacogn. Mag. 2020, 16, 499. [Google Scholar]
- El-Said, H.; Ashgar, S.S.; Bader, A.; AlQathama, A.; Halwani, M.; Ascrizzi, R.; Flamini, G. Essential oil analysis and antimicrobial evaluation of three aromatic plant species growing in Saudi Arabia. Molecules 2021, 26, 959. [Google Scholar] [CrossRef]
- Karunamoorthi, K.; Girmay, A.; Fekadu, S. Larvicidal efficacy of Ethiopian ethnomedicinal plant Juniperus procera essential oil against Afrotropical malaria vector Anopheles arabiensis (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2014, 4, S99–S106. [Google Scholar] [CrossRef]
- Almadiy, A.A. Chemical composition, insecticidal and biochemical effects of two plant oils and their major fractions against Aedes aegypti, the common vector of dengue fever. Heliyon 2020, 6, e04915. [Google Scholar] [CrossRef]
- Kampa, M.; Nifli, A.-P.; Notas, G.; Castanas, E. Polyphenols and cancer cell growth. Rev. Physiol. Biochem. Pharmacol. 2007, 159, 79–113. [Google Scholar]
- Balan, K.V.; Demetzos, C.; Prince, J.; Dimas, K.; Cladaras, M.; Han, Z.; Wyche, J.H.; Pantazis, P. Induction of apoptosis in human colon cancer HCT116 cells treated with an extract of the plant product, Chios mastic gum. Vivo 2005, 19, 93–102. [Google Scholar]
- Thuwaini, M.M. Natural sources as promising future anticancer therapies-A review. GSC Biol. Pharm. Sci. 2022, 19, 084–113. [Google Scholar] [CrossRef]
- Magdah, G. Cytogenetic toxicity of Juniperus procera extract with silver nanoparticles against carcinoma colon (Caco2) cell line in vitro. Int. J. Pharmacol. 2019, 15, 576–585. [Google Scholar]
- Galati, G.; Teng, S.; Moridani, M.Y.; Chan, T.S.; O’Brien, P.J. Cancer chemoprevention and apoptosis mechanisms induced by dietary polyphenolics. Drug Metab. Drug Interact. 2000, 17, 311–350. [Google Scholar] [CrossRef]
- Adnan, M.; Oh, K.K.; Azad, M.O.K.; Shin, M.H.; Wang, M.-H.; Cho, D.H. Kenaf (Hibiscus cannabinus L.) leaves and seed as a potential source of the bioactive compounds: Effects of various extraction solvents on biological properties. Life 2020, 10, 223. [Google Scholar] [CrossRef]
- Salih, A.M.; Al-Qurainy, F.; Nadeem, M.; Tarroum, M.; Khan, S.; Shaikhaldein, H.O.; Al-Hashimi, A.; Alfagham, A.; Alkahtani, J. Optimization method for phenolic compounds extraction from medicinal plant (Juniperus procera) and phytochemicals screening. Molecules 2021, 26, 7454. [Google Scholar] [CrossRef]
- Alghamdi, M.D.; Nazreen, S.; Ali, N.M.; Amna, T. ZnO Nanocomposites of Juniperus procera and Dodonaea viscosa Extracts as Antiproliferative and Antimicrobial Agents. Nanomaterials 2022, 12, 664. [Google Scholar] [CrossRef]
- Fernandez, A.; Cock, I.E. The therapeutic properties of Juniperus communis L.: Antioxidant capacity, bacterial growth inhibition, anticancer activity and toxicity. Pharmacogn. J. 2016, 8, 273–280. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Curcic, M.G.; Zizic, J.B.; Topuzovic, M.D.; Solujic, S.R.; Markovic, S.D. Teucrium plant species as natural sources of novel anticancer compounds: Antiproliferative, proapoptotic and antioxidant properties. Int. J. Mol. Sci. 2011, 12, 4190–4205. [Google Scholar] [CrossRef]
- Alamri, M.A.; Al-Jahdali, M.; Al-Radadi, N.S.; Hussien, M.A. Characterization, theoretical investigation, and biological applications of Mn (II), Co (II), Ni (II), Cu (II), and Zn (II) complexes of a triazene ligand containing a benzothiazole ring. Appl. Organomet. Chem. 2022, 36, e6466. [Google Scholar] [CrossRef]
- Pozo, K.; Bibb, J.A. The emerging role of Cdk5 in cancer. Trends Cancer 2016, 2, 606–618. [Google Scholar] [CrossRef]
- Sharfalddin, A.A.; Emwas, A.-H.; Jaremko, M.; Hussien, M.A. Transition metal complexes of 6-mercaptopurine: Characterization, Theoretical calculation, DNA-Binding, molecular docking, and anticancer activity. Appl. Organomet. Chem. 2021, 35, e6041. [Google Scholar] [CrossRef]
- Das, D.; Patra, M.; Chakrabarti, A. Binding of hemin, hematoporphyrin, and protoporphyrin with erythroid spectrin: Fluorescence and molecular docking studies. Eur. Biophys. J. 2015, 44, 171–182. [Google Scholar] [CrossRef]
- Eweas, A.F.; Khalifa, N.M.; Ismail, N.S.; Al-Omar, M.A.; Soliman, A.M.M. Synthesis, molecular docking of novel 1, 8-naphthyridine derivatives and their cytotoxic activity against HepG2 cell lines. Med. Chem. Res. 2014, 23, 76–86. [Google Scholar] [CrossRef]
- Denny, W.A. Nitroaromatic Hypoxia-Activated Prodrugs for Cancer Therapy. Pharmaceuticals 2022, 15, 187. [Google Scholar] [CrossRef]
- Demarcq, C.; Bunch, R.T.; Creswell, D.; Eastman, A. The role of cell cycle progression in cisplatin-induced apoptosis in Chinese hamster ovary cells. Cell Growth Differ.-Publ. Am. Assoc. Cancer Res. 1994, 5, 983–994. [Google Scholar]
- Smith, E.; Palethorpe, H.M.; Tomita, Y.; Pei, J.V.; Townsend, A.R.; Price, T.J.; Young, J.P.; Yool, A.J.; Hardingham, J.E. The purified extract from the medicinal plant Bacopa monnieri, bacopaside II, inhibits growth of colon cancer cells in vitro by inducing cell cycle arrest and apoptosis. Cells 2018, 7, 81. [Google Scholar] [CrossRef]
- Ali, E.M.; Elashkar, A.A.; El-Kassas, H.Y.; Salim, E.I. Methotrexate loaded on magnetite iron nanoparticles coated with chitosan: Biosynthesis, characterization, and impact on human breast cancer MCF-7 cell line. Int. J. Biol. Macromol. 2018, 120, 1170–1180. [Google Scholar] [CrossRef]
- Plumb, J.A. Cell sensitivity assays: The MTT assay. In Cancer Cell Culture; Springer: Berlin/Heidelberg, Germany, 2004; pp. 165–169. [Google Scholar]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. JoVE J. Vis. Exp. 2011, 50, e2597. [Google Scholar]
- Fried, J.; Perez, A.G.; Clarkson, B.D. Flow cytofluorometric analysis of cell cycle distributions using propidium iodide. Properties of the method and mathematical analysis of the data. J. Cell Biol. 1976, 71, 172–181. [Google Scholar] [CrossRef]
- Gurung, A.B.; Bhattacharjee, A.; Ali, M.A. Exploring the physicochemical profile and the binding patterns of selected novel anticancer Himalayan plant derived active compounds with macromolecular targets. Inform. Med. Unlocked 2016, 5, 1–14. [Google Scholar] [CrossRef]

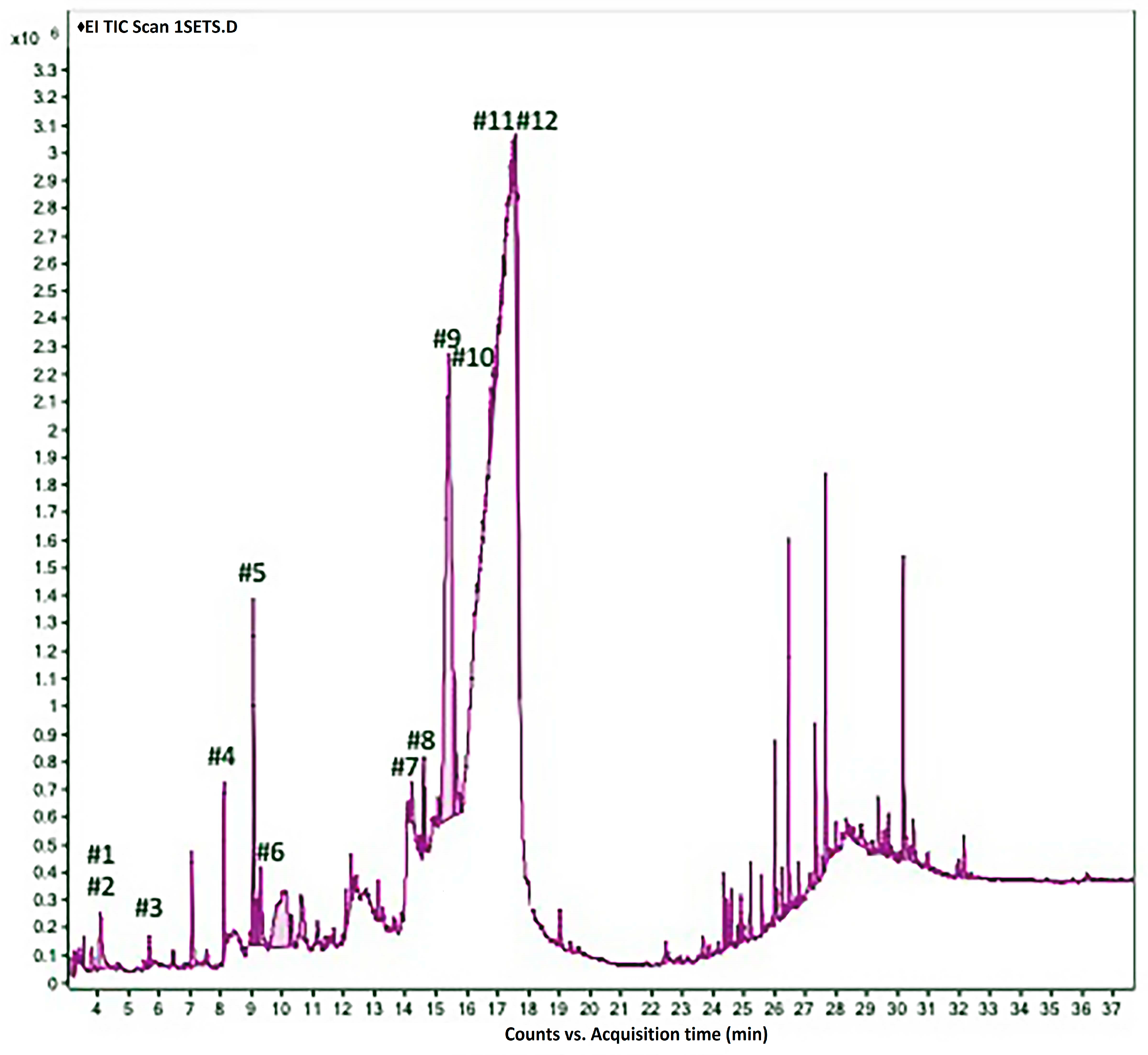
| IC50 (μg/mL) | HCT116 | HepG2 | MCF-7 | JK |
|---|---|---|---|---|
| Range of IC50 (μg/mL) | ||||
| 93–142 | 60–96 | 96–130 | 104–150 | |
| Average Value of IC50 (μg/mL) | 115 | 75 | 112 | 124 |
| No. | Compound Name | Molecular Formula | RT (Min) | RC (%) Rel. % |
|---|---|---|---|---|
| 1 | 2-Imino-6-nitro-2H-1-benzopyran-3-carbothiamide | C10H7N3O3S | 4.079 | 0.49 |
| 2 | 5-Methylpyrimidine-2,4-(1H,3H)-dione (Thymine) | C5H6N2O2 | 4.079 | 0.47 |
| 3 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | C6H8O4 | 5.666 | 0.10 |
| 4 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-[4H]-pyran-4-one | C6H8O4 | 8.09 | 0.40 |
| 5 | 5-Hydroxymethylfurfural | C6H6O3 | 9.051 | 1.24 |
| 6 | 2,3-Dihydrobenzofuran | C8H8O | 9.223 | 0.12 |
| 7 | 3,7-Dimethyloct-6-en-1-ol | C10H20O | 14.206 | 1.91 |
| 8 | 3-O-Methyl-D-glucose (3-Methylglucose) | C7H14O6 | 14.895 | 1.03 |
| 9 | 2-Methyl-1,3-dioxolane-2-propanal | C7H12O3 | 15.413 | 11.40 |
| 10 | Tetrahydro-2-methylthiophene | C5H10S | 15.601 | 2.00 |
| 11 | 2-Butyl-2-methyl-1,3-dioxolane | C8H10O2 | 16.253 | 5.94 |
| 12 | 1-Amino-1-cyclobutanecarboxylic acid | C5H9O2N | 16.339 | 2.10 |
| Hit | Colon | Erythroid | Breast | Liver | ||||
|---|---|---|---|---|---|---|---|---|
| 3ig7 | 1owa | 3eqm | 4fm9 | |||||
| S | RMSD | S | RMSD | S | RMSD | S | RMSD | |
| Hit 1 | −5.56 | 0.81 | −6.71 | 2.03 | −5.88 | 1.31 | −5.66 | 0.90 |
| Hit 2 | −4.28 | 1.75 | −3.74 | 0.53 | −4.47 | 0.53 | −4.62 | 1.07 |
| Hit 3 | −4.51 | 0.69 | −4.05 | 0.94 | −5.07 | 3.52 | −5.02 | 1.65 |
| Hit 4 | −4.50 | 0.65 | −3.99 | 0.72 | −4.76 | 2.89 | −4.99 | 1.55 |
| Hit 5 | −4.22 | 0.79 | −3.91 | 1.76 | −4.67 | 2.09 | −4.55 | 1.19 |
| Hit 6 | −4.43 | 1.43 | −4.03 | 1.11 | −4.53 | 1.10 | −4.69 | 0.91 |
| Hit 7 | −5.34 | 1.28 | −4.39 | 2.00 | −5.69 | 0.82 | −5.43 | 0.80 |
| Hit 8 | −5.07 | 1.11 | −4.23 | 1.55 | −5.45 | 0.96 | −5.28 | 2.16 |
| Hit 9 | −4.86 | 2.09 | −4.20 | 1.53 | −5.10 | 0.98 | −5.07 | 1.04 |
| Hit 10 | −4.15 | 2.66 | −3.70 | 0.94 | −4.38 | 2.56 | −4.13 | 1.76 |
| Hit 11 | −5.17 | 0.58 | −4.31 | 0.81 | −5.43 | 1.66 | −5.33 | 1.23 |
| Hit 12 | −4.22 | 1.15 | −3.56 | 3.80 | −4.45 | 4.00 | −4.29 | 0.95 |
| Ligand | Receptor | Interaction | Distance | E (kcal/mol) | |
|---|---|---|---|---|---|
| 3ig7 | N 16 | OD1 ASP 145(A) | H-donor | 2.85 | −2.0 |
| O 21 | N LEU 83 (A) | H-acceptor | 3.03 | −1.3 | |
| N 23 | NZ LYS 33 (A) | H-acceptor | 3.02 | −9.7 | |
| 6-ring | CG2 VAL 18 (A) | pi-H | 4.40 | −0.5 | |
| 1woa | 6-ring | CA SER 52 (A) | pi-H | 3.59 | −1.1 |
| 6-ring | N TYR 53 (A) | pi-H | 4.43 | −1.2 | |
| 3eqm | S 19 | CA VAL 373 | H-acceptor | 3.68 | −1.1 |
| S 19 | N MET 374 | H-acceptor | 3.28 | −2.7 | |
| 4fm9 | S 19 | NE ARG 673 (A) | H-acceptor | 2.98 | −2.5 |
| S 19 | NH1 ARG 673 (A) | H-acceptor | 2.36 | −1.8 |
| Treatment | % of Viable Cells(Lower Left) | % of Necrosis(Upper Left) | % of Early Apoptosis(Lower Right) | % of Late Apoptosis(Upper Right) | Total % of Apoptosis |
|---|---|---|---|---|---|
| Control | 93.7 | 3.2 | 1.1 | 2 | 3.2 |
| DOX 3 | 0 | 10.7 | 0 | 89.3 | 89.3 |
| J. procera | 31 | 20 | 3.8 | 44.8 | 48.6 |
| Drugs | Control | DOX | J. procera Extract | |
|---|---|---|---|---|
| Phase | ||||
| Sub G1 | 2.4 | 4.3 | 0 | |
| G1/G0 | 31.6 | 46.5 | 33.6 | |
| S | 32.5 | 17.9 | 30.3 | |
| G2/M | 33.5 | 31.3 | 36.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhayyani, S.; Akhdhar, A.; Asseri, A.H.; Mohammed, A.M.A.; Hussien, M.A.; Roselin, L.S.; Hosawi, S.; AlAbbasi, F.; Alharbi, K.H.; Baty, R.S.; et al. Potential Anticancer Activity of Juniperus procera and Molecular Docking Models of Active Proteins in Cancer Cells. Molecules 2023, 28, 2041. https://doi.org/10.3390/molecules28052041
Alhayyani S, Akhdhar A, Asseri AH, Mohammed AMA, Hussien MA, Roselin LS, Hosawi S, AlAbbasi F, Alharbi KH, Baty RS, et al. Potential Anticancer Activity of Juniperus procera and Molecular Docking Models of Active Proteins in Cancer Cells. Molecules. 2023; 28(5):2041. https://doi.org/10.3390/molecules28052041
Chicago/Turabian StyleAlhayyani, Sultan, Abdullah Akhdhar, Amer H. Asseri, Abdelhafeez M. A. Mohammed, Mostafa A. Hussien, L. Selva Roselin, Salman Hosawi, Fahad AlAbbasi, Khadijah H. Alharbi, Roua S. Baty, and et al. 2023. "Potential Anticancer Activity of Juniperus procera and Molecular Docking Models of Active Proteins in Cancer Cells" Molecules 28, no. 5: 2041. https://doi.org/10.3390/molecules28052041
APA StyleAlhayyani, S., Akhdhar, A., Asseri, A. H., Mohammed, A. M. A., Hussien, M. A., Roselin, L. S., Hosawi, S., AlAbbasi, F., Alharbi, K. H., Baty, R. S., Kalantan, A. A., & Ali, E. M. M. (2023). Potential Anticancer Activity of Juniperus procera and Molecular Docking Models of Active Proteins in Cancer Cells. Molecules, 28(5), 2041. https://doi.org/10.3390/molecules28052041









