Effects of Hydroxytyrosol in Endothelial Functioning: A Comprehensive Review
Abstract
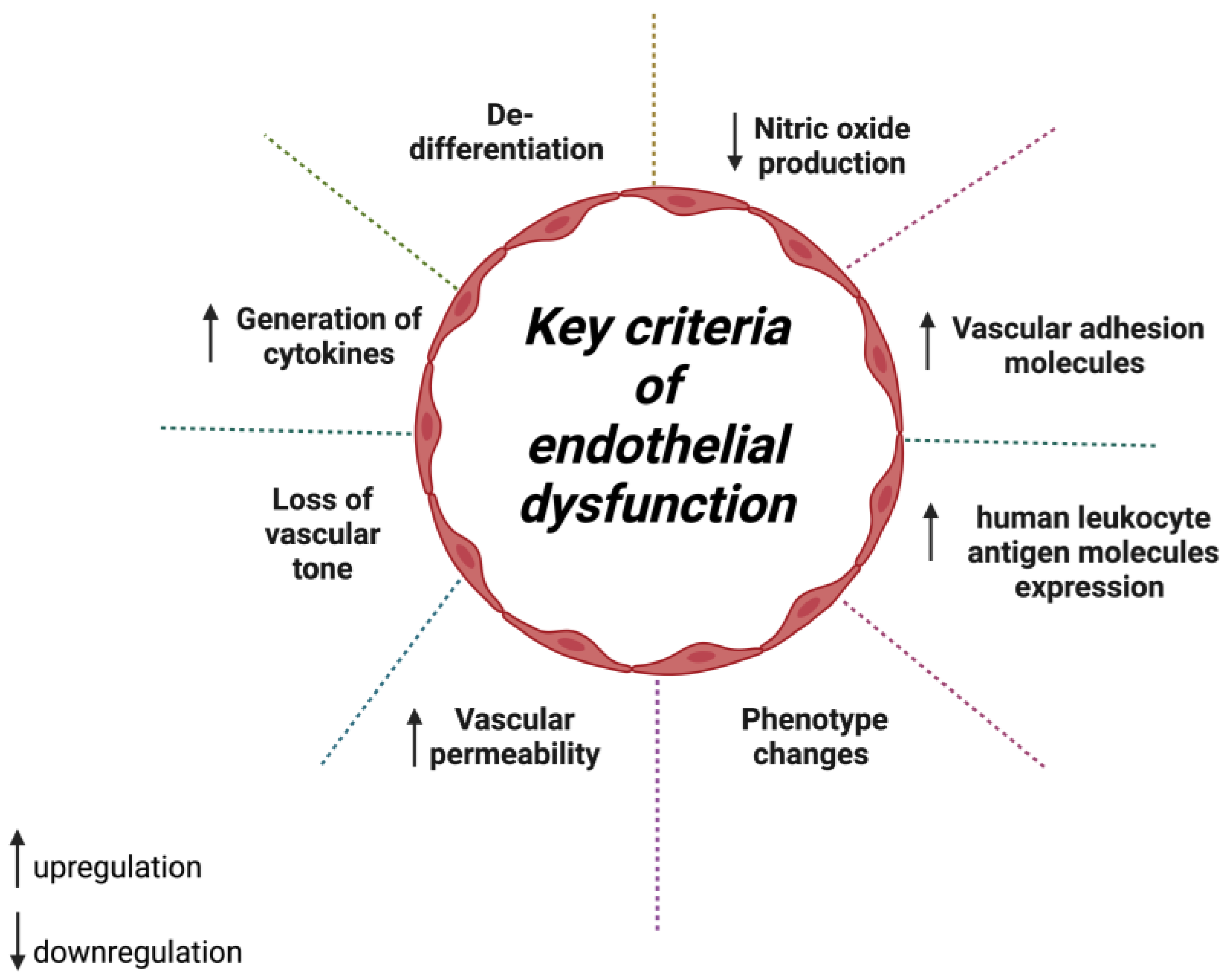
1. Introduction
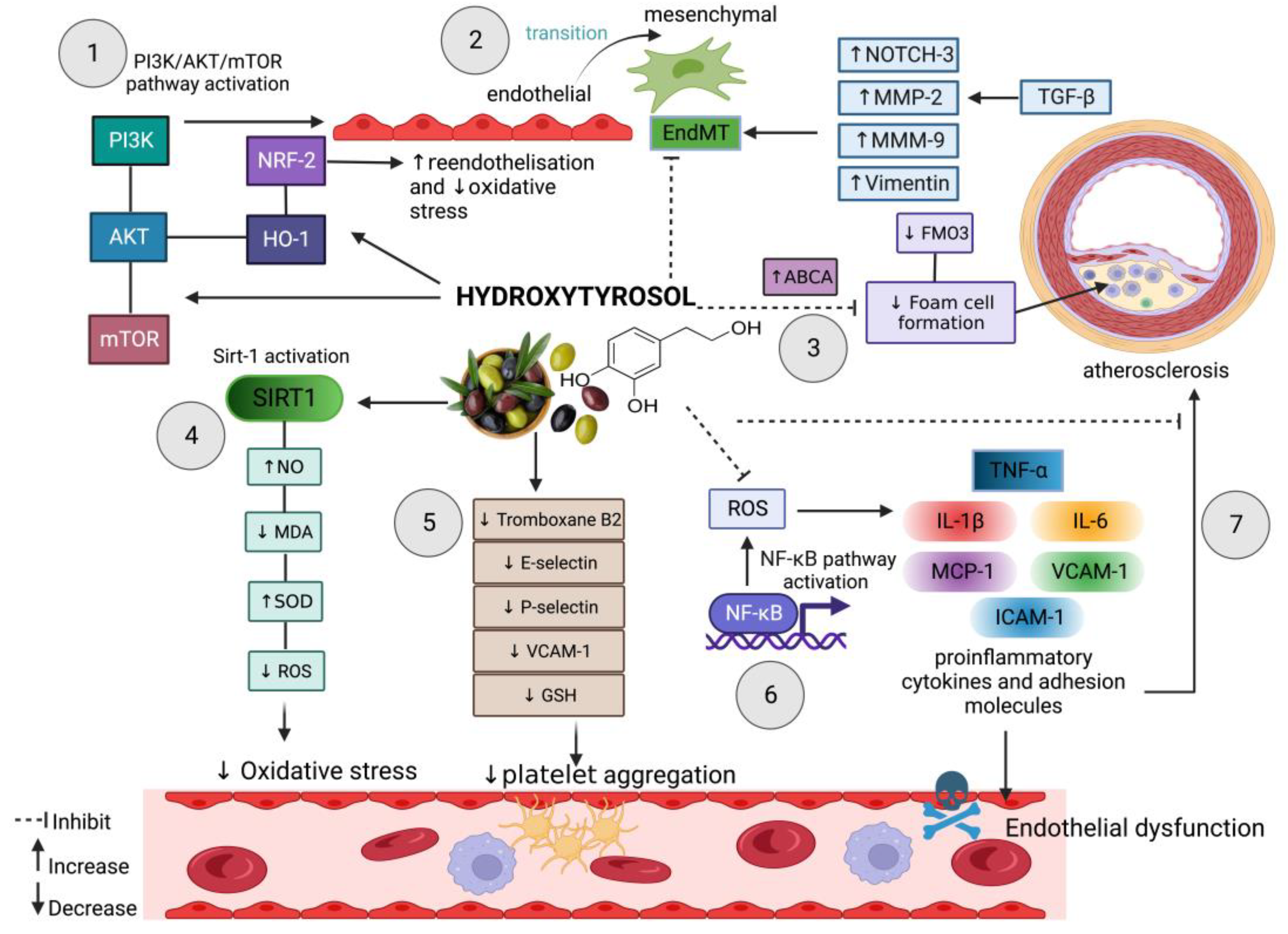
2. Hydroxytyrosol in Oxidative Stress-Induced Endothelial Dysfunction
3. Hydroxytyrosol Defence in Endothelial Dysfunction-Induced Atherosclerosis
4. Hydroxytyrosol Prevents Endothelial-to-Mesenchymal Transition
5. Hydroxytyrosol Ameliorates Angiogenesis and Wound Healing
6. Epigenetic Effect of Hydroxytyrosol in Endothelial Functioning
7. The Effects of Hydroxytyrosol and Its Derivatives on Hypertension
8. Hydroxytyrosol and Vascular Aging-Induced Endothelial Dysfunction
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J. Mechanism of endothelial dysfunction in chronic kidney disease. Clin. Chim. Acta 2010, 411, 1412–1420. [Google Scholar] [CrossRef]
- Garland, C.J.; Dora, K.A. Endothelium-Dependent Hyperpolarization: The Evolution of Myoendothelial Microdomains. J. Cardiovasc. Pharmacol. 2021, 78, S3–S12. [Google Scholar] [CrossRef]
- Neubauer, K.; Zieger, B. Endothelial cells and coagulation. Cell Tissue Res. 2022, 387, 391–398. [Google Scholar] [CrossRef]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta 2013, 1832, 2216–2231. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Kong, W. Endothelial tight junctions and their regulatory signaling pathways in vascular homeostasis and disease. Cell. Signal. 2020, 66, 109485. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Gradisnik, L. Tissue Augmentation in Wound Healing: The Role of Endothelial and Epithelial Cells. Med. Arch. 2018, 72, 444–448. [Google Scholar] [CrossRef]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arter. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Hunt, B.J.; Jurd, K.M. Endothelial cell activation. A central pathophysiological process. BMJ 1998, 316, 1328–1329. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2020, 117, 29–42. [Google Scholar] [CrossRef]
- da Silva, F.C.; de Araújo, B.J.; Cordeiro, C.S.; Arruda, V.M.; Faria, B.Q.; Guerra, J.; Araújo, T.G.; Fürstenau, C.R. Endothelial dysfunction due to the inhibition of the synthesis of nitric oxide: Proposal and characterization of an in vitro cellular model. Front. Physiol. 2022, 13, 978378. [Google Scholar] [CrossRef] [PubMed]
- Bourque, C.; Zhang, Y.; Fu, M.; Racine, M.; Greasley, A.; Pei, Y.; Wu, L.; Wang, R.; Yang, G. H2S protects lipopolysaccharide-induced inflammation by blocking NFκB transactivation in endothelial cells. Toxicol. Appl. Pharmacol. 2018, 338, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pr. 2014, 2014, 291–308. [Google Scholar] [CrossRef]
- Botts, S.R.; Fish, J.E.; Howe, K.L. Dysfunctional Vascular Endothelium as a Driver of Atherosclerosis: Emerging Insights Into Pathogenesis and Treatment. Front. Pharm. 2021, 12, 787541. [Google Scholar] [CrossRef]
- Boulanger, C.M. Endothelium. Arter. Thromb. Vasc. Biol. 2016, 36, e26–e31. [Google Scholar] [CrossRef]
- Peng, J.; Luo, F.; Ruan, G.; Peng, R.; Li, X. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis. 2017, 16, 233. [Google Scholar] [CrossRef]
- Andrés-Jensen, L.; Grell, K.; Rank, C.U.; Albertsen, B.K.; Tuckuviene, R.; Linnemann Nielsen, R.; Lynggaard, L.S.; Jarvis, K.B.; Quist-Paulsen, P.; Trakymiene, S.S.; et al. Endothelial dysfunction and thromboembolism in children, adolescents, and young adults with acute lymphoblastic leukemia. Leukemia 2022, 36, 361–369. [Google Scholar] [CrossRef]
- Castro-Ferreira, R.; Cardoso, R.; Leite-Moreira, A.; Mansilha, A. The Role of Endothelial Dysfunction and Inflammation in Chronic Venous Disease. Ann. Vasc. Surg. 2018, 46, 380–393. [Google Scholar] [CrossRef]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2022, 8, 798958. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Kirkman, D.L.; Edwards, D.G. The Vascular Endothelium in Chronic Kidney Disease: A Novel Target for Aerobic Exercise. Exerc. Sport Sci. Rev. 2016, 44, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Toya, T.; Sara, J.D.; Corban, M.T.; Taher, R.; Godo, S.; Herrmann, J.; Lerman, L.O.; Lerman, A. Assessment of peripheral endothelial function predicts future risk of solid-tumor cancer. Eur. J. Prev. Cardiol. 2020, 27, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Otifi, H.M.; Adiga, B.K. Endothelial Dysfunction in COVID-19 Infection. Am. J. Med. Sci. 2022, 363, 281–287. [Google Scholar] [CrossRef]
- Xing, H.; Zhang, Z.; Shi, G.; He, Y.; Song, Y.; Liu, Y.; Harrington, E.O.; Sellke, F.W.; Feng, J. Chronic Inhibition of mROS Protects Against Coronary Endothelial Dysfunction in Mice With Diabetes. Front. Cell Dev. Biol. 2021, 9, 643810. [Google Scholar] [CrossRef]
- Yimcharoen, M.; Kittikunnathum, S.; Suknikorn, C.; Nak-on, W.; Yeethong, P.; Anthony, T.G.; Bunpo, P. Effects of ascorbic acid supplementation on oxidative stress markers in healthy women following a single bout of exercise. J. Int. Soc. Sports Nutr. 2019, 16, 2. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; González-Acedo, A.; Illescas-Montes, R.; García-Recio, E.; Ramos-Torrecillas, J.; Costela-Ruiz, V.J.; García-Martínez, O. Biological effects of the olive tree and its derivatives on the skin. Food Funct. 2022, 13, 11410–11424. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Peñalver, R.; Ros, G.; Nieto, G. Olive Tree Derivatives and Hydroxytyrosol: Their Potential Effects on Human Health and Its Use as Functional Ingredient in Meat. Foods 2021, 10, 2611. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Reis, J.; Román, A.N.; Toledo, J.B.; Toledo, E. Extra-virgin olive oil for potential prevention of Alzheimer disease. Rev. Neurol. (Paris) 2019, 175, 705–723. [Google Scholar] [CrossRef]
- Abaza, L.; Taamalli, A.; Nsir, H.; Zarrouk, M. Olive Tree (Olea europeae L.) Leaves: Importance and Advances in the Analysis of Phenolic Compounds. Antioxidants 2015, 4, 682–698. [Google Scholar] [CrossRef]
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative Study on Beneficial Effects of Hydroxytyrosol- and Oleuropein-Rich Olive Leaf Extracts on High-Fat Diet-Induced Lipid Metabolism Disturbance and Liver Injury in Rats. Biomed. Res. Int. 2020, 2020, 1315202. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Borjan, D.; Leitgeb, M.; Knez, Ž.; Hrnčič, M.K. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules 2020, 25, 5946. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Abdelgawad, S.M.; Hassab, M.A.E.; Abourehab, M.A.S.; Elkaeed, E.B.; Eldehna, W.M. Olive Leaves as a Potential Phytotherapy in the Treatment of COVID-19 Disease; A Mini-Review. Front. Pharmacol. 2022, 13, 879118. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Sánchez-Carbayo, M.; Fernández-Bolaños, J. Antiproliferative Activity of Olive Extract Rich in Polyphenols and Modified Pectin on Bladder Cancer Cells. J. Med. Food 2020, 23, 719–727. [Google Scholar] [CrossRef]
- Pang, K.L.; Lumintang, J.N.; Chin, K.Y. Thyroid-Modulating Activities of Olive and Its Polyphenols: A Systematic Review. Nutrients 2021, 13, 529. [Google Scholar] [CrossRef]
- Stevens, Y.; Winkens, B.; Jonkers, D.; Masclee, A. The effect of olive leaf extract on cardiovascular health markers: A randomized placebo-controlled clinical trial. Eur. J. Nutr. 2021, 60, 2111–2120. [Google Scholar] [CrossRef]
- Rocha, J.; Borges, N.; Pinho, O. Table olives and health: A review. J. Nutr. Sci. 2020, 9, e57. [Google Scholar] [CrossRef]
- López-López, A.; Cortés-Delgado, A.; Garrido-Fernández, A. Impact of Processing and Extraction on the Minor Components of Green Spanish-Style Gordal Table Olive Fat, as Assessed by Innovative Approaches. Foods 2020, 9, 1907. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, R.; Juan, M.E.; Planas, J.M. Table olive polyphenols: A simultaneous determination by liquid chromatography-mass spectrometry. J. Chromatogr. A 2020, 1609, 460434. [Google Scholar] [CrossRef] [PubMed]
- Foscolou, A.; Critselis, E.; Panagiotakos, D. Olive oil consumption and human health: A narrative review. Maturitas 2018, 118, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D. Olive Oil: Minor Constituents and Health; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of Phenolic Compounds and Their Contribution to Sensory Properties of Olive Oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Wani, T.A.; Masoodi, F.A.; Gani, A.; Baba, W.N.; Rahmanian, N.; Akhter, R.; Wani, I.A.; Ahmad, M. Olive oil and its principal bioactive compound: Hydroxytyrosol—A review of the recent literature. Trends Food Sci. Technol. 2018, 77, 77–90. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Bayram, B.; Esatbeyoglu, T.; Schulze, N.; Ozcelik, B.; Frank, J.; Rimbach, G. Comprehensive analysis of polyphenols in 55 extra virgin olive oils by HPLC-ECD and their correlation with antioxidant activities. Plant Foods Hum. Nutr. 2012, 67, 326–336. [Google Scholar] [CrossRef]
- Romero, C.; Brenes, M.; Yousfi, K.; García, P.; García, A.; Garrido, A. Effect of cultivar and processing method on the contents of polyphenols in table olives. J. Agric. Food Chem. 2004, 52, 479–484. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Auñon-Calles, D.; Canut, L.; Visioli, F. Toxicological evaluation of pure hydroxytyrosol. Food Chem. Toxicol. 2013, 55, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Nikou, T.; Sakavitsi, M.E.; Kalampokis, E.; Halabalaki, M. Metabolism and Bioavailability of Olive Bioactive Constituents Based on In Vitro, In Vivo and Human Studies. Nutrients 2022, 14, 3773. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.; Strassmann, S.; Golz, C. Oral bioavailability and metabolism of hydroxytyrosol from food supplements. Nutrients 2022, 15, 325. [Google Scholar] [CrossRef]
- Kano, S.; Komada, H.; Yonekura, L.; Sato, A.; Nishiwaki, H.; Tamura, H. Absorption, Metabolism, and Excretion by Freely Moving Rats of 3,4-DHPEA-EDA and Related Polyphenols from Olive Fruits (Olea europaea). J. Nutr. Metab. 2016, 2016, 9104208. [Google Scholar] [CrossRef]
- Fernández-Ávila, C.; Montes, R.; Castellote, A.I.; Chisaguano, A.M.; Fitó, M.; Covas, M.I.; Muñoz-Aguallo, D.; Nyyssönen, K.; Zunft, H.J.; López-Sabater, M.C. Fast determination of virgin olive oil phenolic metabolites in human high-density lipoproteins. Biomed. Chromatogr. 2015, 29, 1035–1041. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e04728. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Med. 2018, 5, 13. [Google Scholar] [CrossRef]
- Yao, F.; Jin, Z.; Lv, X.; Zheng, Z.; Gao, H.; Deng, Y.; Liu, Y.; Chen, L.; Wang, W.; He, J.; et al. Hydroxytyrosol Acetate Inhibits Vascular Endothelial Cell Pyroptosis via the HDAC11 Signaling Pathway in Atherosclerosis. Front. Pharm. 2021, 12, 656272. [Google Scholar] [CrossRef] [PubMed]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, C.; Abdullah; Tian, W.; Qiu, Z.; Song, M.; Cao, Y.; Xiao, J. Hydroxytyrosol Alleviates Dextran Sulfate Sodium-Induced Colitis by Modulating Inflammatory Responses, Intestinal Barrier, and Microbiome. J. Agric. Food Chem. 2022, 70, 2241–2252. [Google Scholar] [CrossRef]
- Reyes de la Vega, J.; De La Cruz, J.P.; Muñoz-Marín, J.; Guerrero, A.T.; Lopez-Villodres, J.; Madrona, A.; Espartero, J.L.; Gonzalez-Correa, J. Antiplatelet effect of new lipophilic hydroxytyrosol alkyl ether derivatives in human blood. Eur. J. Nutr. 2012, 52, 591–599. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Franceschelli, S.; Quiles, J.L.; Speranza, L. Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells 2020, 9, 1932. [Google Scholar] [CrossRef]
- Gallardo-Fernández, M.; Hornedo-Ortega, R.; Alonso-Bellido, I.M.; Rodríguez-Gómez, J.A.; Troncoso, A.M.; García-Parrilla, M.C.; Venero, J.L.; Espinosa-Oliva, A.M.; Pablos, R.M. Hydroxytyrosol Decreases LPS- and α-Synuclein-Induced Microglial Activation In Vitro. Antioxidants 2019, 9, 36. [Google Scholar] [CrossRef]
- Kamil, K.; Yazid, M.D.; Idrus, R.B.H.; Kumar, J. Hydroxytyrosol Promotes Proliferation of Human Schwann Cells: An In Vitro Study. Int. J. Environ. Res. Public Health 2020, 17, 4404. [Google Scholar] [CrossRef]
- Costanzo, P.; Oliverio, M.; Maiuolo, J.; Bonacci, S.; De Luca, G.; Masullo, M.; Arcone, R.; Procopio, A. Novel Hydroxytyrosol-Donepezil Hybrids as Potential Antioxidant and Neuroprotective Agents. Front. Chem. 2021, 9, 741444. [Google Scholar] [CrossRef]
- Vilaplana-Pérez, C.; Auñón, D.; García-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front. Nutr. 2014, 1, 18. [Google Scholar] [CrossRef]
- Carluccio, M.A.; Martinelli, R.; Massaro, M.; Calabriso, N.; Scoditti, E.; Maffia, M.; Verri, T.; Gatta, V.; De Caterina, R. Nutrigenomic Effect of Hydroxytyrosol in Vascular Endothelial Cells: A Transcriptomic Profile Analysis. Nutrients 2021, 13, 3990. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of Olive Oil on Markers of Inflammation and Endothelial Function-A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [CrossRef] [PubMed]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharm. 2012, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Popat, K.C.; Reynolds, M.M. Nitric oxide-mediated fibrinogen deposition prevents platelet adhesion and activation. Biointerphases 2018, 13, 06e403. [Google Scholar] [CrossRef]
- Galkina, S.I.; Golenkina, E.A.; Viryasova, G.M.; Romanova, Y.M.; Sud'ina, G.F. Nitric Oxide in Life and Death of Neutrophils. Curr. Med. Chem. 2019, 26, 5764–5780. [Google Scholar] [CrossRef]
- Tabata, K.; Komori, K.; Otsuka, R.; Kajikuri, J.; Itoh, T. Enhancement of Nitric Oxide Production Is Responsible for Minimal Intimal Hyperplasia of Autogenous Rabbit Arterial Grafts. Circ. J. 2017, 81, 1222–1230. [Google Scholar] [CrossRef]
- Somarathna, M.; Hwang, P.T.; Millican, R.C.; Alexander, G.C.; Isayeva-Waldrop, T.; Sherwood, J.A.; Brott, B.C.; Falzon, I.; Northrup, H.; Shiu, Y.T.; et al. Nitric oxide releasing nanomatrix gel treatment inhibits venous intimal hyperplasia and improves vascular remodeling in a rodent arteriovenous fistula. Biomaterials 2022, 280, 121254. [Google Scholar] [CrossRef]
- Incalza, M.A.; D'Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharm. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, J.; Piao, H.; Zhu, Z.; Wei, R.; Liu, K. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front. Immunol. 2022, 13, 1039241. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Pernomian, L.; Bendhack, L. Contribution of oxidative stress to endothelial dysfunction in hypertension. Front. Physiol. 2012, 3, 441. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Maruhashi, T.; Noma, K.; Kihara, Y. Oxidative stress and endothelial dysfunction: Clinical evidence and therapeutic implications. Trends Cardiovasc. Med. 2014, 24, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol Exerts Anti-Inflammatory and Anti-Oxidant Activities in a Mouse Model of Systemic Inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Crudele, A.; Smeriglio, A.; Braghini, M.R.; Panera, N.; Comparcola, D.; Alterio, A.; Sartorelli, M.R.; Tozzi, G.; Raponi, M.; et al. Antioxidant activity of Hydroxytyrosol and Vitamin E reduces systemic inflammation in children with paediatric NAFLD. Dig. Liver Dis. 2021, 53, 1154–1158. [Google Scholar] [CrossRef]
- Zrelli, H.; Wu, C.W.; Zghonda, N.; Shimizu, H.; Miyazaki, H. Combined treatment of hydroxytyrosol with carbon monoxide-releasing molecule-2 prevents TNF α-induced vascular endothelial cell dysfunction through NO production with subsequent NFκB inactivation. Biomed. Res. Int. 2013, 2013, 912431. [Google Scholar] [CrossRef]
- Serreli, G.; Le Sayec, M.; Diotallevi, C.; Teissier, A.; Deiana, M.; Corona, G. Conjugated Metabolites of Hydroxytyrosol and Tyrosol Contribute to the Maintenance of Nitric Oxide Balance in Human Aortic Endothelial Cells at Physiologically Relevant Concentrations. Molecules 2021, 26, 7480. [Google Scholar] [CrossRef]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 2020, 43, 1589–1598. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Koya, D. The protective role of Sirt1 in vascular tissue: Its relationship to vascular aging and atherosclerosis. Aging (Albany NY) 2016, 8, 2290–2307. [Google Scholar] [CrossRef]
- Wang, W.; Shang, C.; Zhang, W.; Jin, Z.; Yao, F.; He, Y.; Wang, B.; Li, Y.; Zhang, J.; Lin, R. Hydroxytyrosol NO regulates oxidative stress and NO production through SIRT1 in diabetic mice and vascular endothelial cells. Phytomedicine 2019, 52, 206–215. [Google Scholar] [CrossRef]
- Bayram, B.; Ozcelik, B.; Grimm, S.; Roeder, T.; Schrader, C.; Ernst, I.M.; Wagner, A.E.; Grune, T.; Frank, J.; Rimbach, G. A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2-dependent gene expression. Rejuvenation Res. 2012, 15, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jing, T.; Yang, X.; He, Y.; Wang, B.; Xiao, Y.; Shang, C.; Zhang, J.; Lin, R. Hydroxytyrosol regulates the autophagy of vascular adventitial fibroblasts through the SIRT1-mediated signaling pathway. Can. J. Physiol Pharm. 2018, 96, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015, 225, R83–R99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Zrelli, H.; Kusunoki, M.; Miyazaki, H. Role of Hydroxytyrosol-dependent Regulation of HO-1 Expression in Promoting Wound Healing of Vascular Endothelial Cells via Nrf2 De Novo Synthesis and Stabilization. Phytother. Res. 2015, 29, 1011–1018. [Google Scholar] [CrossRef]
- Montoya, T.; Aparicio-Soto, M.; Castejón, M.L.; Rosillo, M.; Sánchez-Hidalgo, M.; Begines, P.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Peracetylated hydroxytyrosol, a new hydroxytyrosol derivate, attenuates LPS-induced inflammatory response in murine peritoneal macrophages via regulation of non-canonical inflammasome, Nrf2/HO1 and JAK/STAT signaling pathways. J. Nutr. Biochem. 2018, 57, 110–120. [Google Scholar] [CrossRef]
- Romana-Souza, B.; Saguie, B.O.; Pereira de Almeida Nogueira, N.; Paes, M.; Dos Santos Valença, S.; Atella, G.C.; Monte-Alto-Costa, A. Oleic acid and hydroxytyrosol present in olive oil promote ROS and inflammatory response in normal cultures of murine dermal fibroblasts through the NF-κB and NRF2 pathways. Food Res. Int. 2020, 131, 108984. [Google Scholar] [CrossRef]
- Parzonko, A.; Czerwińska, M.E.; Kiss, A.K.; Naruszewicz, M. Oleuropein and oleacein may restore biological functions of endothelial progenitor cells impaired by angiotensin II via activation of Nrf2/heme oxygenase-1 pathway. Phytomedicine 2013, 20, 1088–1094. [Google Scholar] [CrossRef]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Araki, M.; Kusunoki, M.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol induces proliferation and cytoprotection against oxidative injury in vascular endothelial cells: Role of Nrf2 activation and HO-1 induction. J. Agric. Food Chem. 2011, 59, 4473–4482. [Google Scholar] [CrossRef]
- de S Ribeiro, B.C.; de C Faria, R.V.; de S Nogueira, J.; Valença, S.S.; Chen, L.; Romana-Souza, B. Olive oil promotes the survival and migration of dermal fibroblasts through Nrf2 pathway activation. Lipids 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sindona, G.; Caruso, A.; Cozza, A.; Fiorentini, S.; Lorusso, B.; Marini, E.; Nardi, M.; Procopio, A.; Zicari, S. Anti-inflammatory effect of 3,4-DHPEA-EDA [2-(3,4 -hydroxyphenyl) ethyl (3S, 4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate] on primary human vascular endothelial cells. Curr. Med. Chem. 2012, 19, 4006–4013. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Yang, G.; Xian, Y.; Wang, G.; Zheng, Z.; Jin, Z.; Xie, Y.; Wang, W.; Gu, J.; Lin, R. The protective effect of hydroxytyrosol acetate against inflammation of vascular endothelial cells partly through the SIRT6-mediated PKM2 signaling pathway. Food Funct. 2019, 10, 5789–5803. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Gnoni, A.; Stanca, E.; Cavallo, A.; Damiano, F.; Siculella, L.; Carluccio, M.A. Hydroxytyrosol Ameliorates Endothelial Function under Inflammatory Conditions by Preventing Mitochondrial Dysfunction. Oxidative Med. Cell. Longev. 2018, 2018, 9086947. [Google Scholar] [CrossRef] [PubMed]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, A. Gender differences in plasma and urine metabolites from Sprague–Dawley rats after oral administration of normal and high doses of hydroxytyrosol, hydroxytyrosol acetate, and DOPAC. Eur. J. Nutr. 2017, 56, 215–224. [Google Scholar] [CrossRef]
- Peyrol, J.; Meyer, G.; Obert, P.; Dangles, O.; Pechère, L.; Amiot, M.-J.; Riva, C. Involvement of bilitranslocase and beta-glucuronidase in the vascular protection by hydroxytyrosol and its glucuronide metabolites in oxidative stress conditions. J. Nutr. Biochem. 2018, 51, 8–15. [Google Scholar] [CrossRef]
- Catalán, Ú.; López de Las Hazas, M.C.; Rubió, L.; Fernández-Castillejo, S.; Pedret, A.; de la Torre, R.; Motilva, M.J.; Solà, R. Protective effect of hydroxytyrosol and its predominant plasmatic human metabolites against endothelial dysfunction in human aortic endothelial cells. Mol. Nutr. Food Res. 2015, 59, 2523–2536. [Google Scholar] [CrossRef]
- Catalán, Ú.; López de las Hazas, M.-C.; Piñol, C.; Rubió, L.; Motilva, M.-J.; Fernandez-Castillejo, S.; Solà, R. Hydroxytyrosol and its main plasma circulating metabolites attenuate the initial steps of atherosclerosis through inhibition of the MAPK pathway. J. Funct. Foods 2018, 40, 280–291. [Google Scholar] [CrossRef]
- Wu, X.; Li, C.; Mariyam, Z.; Jiang, P.; Zhou, M.; Zeb, F.; Haq, I.U.; Chen, A.; Feng, Q. Acrolein-induced atherogenesis by stimulation of hepatic flavin containing monooxygenase 3 and a protection from hydroxytyrosol. J. Cell. Physiol. 2018, 234, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Pisanti, S.; Caputo, M.; Citro, M.; Vecchione, C.; Martinelli, R. 3-Hydroxytyrosol Promotes Angiogenesis In Vitro by Stimulating Endothelial Cell Migration. Int. J. Mol. Sci. 2020, 21, 3657. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Marín, J.; De la Cruz, J.P.; Reyes, J.J.; López-Villodres, J.A.; Guerrero, A.; López-Leiva, I.; Espartero, J.L.; Labajos, M.T.; González-Correa, J.A. Hydroxytyrosyl alkyl ether derivatives inhibit platelet activation after oral administration to rats. Food Chem. Toxicol. 2013, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.N.; Zock, P.L.; Katan, M.B. Bioavailability and antioxidant effects of olive oil phenols in humans: A review. Eur. J. Clin. Nutr. 2004, 58, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Montserrat-de la Paz, S.; Lucas, R.; Bermudez, B.; Abia, R.; Morales, J.C.; Muriana, F.J.G. Effect of metabolites of hydroxytyrosol on protection against oxidative stress and inflammation in human endothelial cells. J. Funct. Foods 2017, 29, 238–247. [Google Scholar] [CrossRef]
- Scoditti, E.; Carpi, S.; Massaro, M.; Pellegrino, M.; Polini, B.; Carluccio, M.A.; Wabitsch, M.; Verri, T.; Nieri, P.; De Caterina, R. Hydroxytyrosol Modulates Adipocyte Gene and miRNA Expression under Inflammatory Condition. Nutrients 2019, 11, 2493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wen, K.; Zhang, Z.; Ma, C.; Zheng, N. 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model. Open Life Sci. 2021, 16, 1313–1320. [Google Scholar] [CrossRef]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef]
- Kouka, P.; Priftis, A.; Stagos, D.; Angelis, A.; Stathopoulos, P.; Xinos, N.; Skaltsounis, A.L.; Mamoulakis, C.; Tsatsakis, A.M.; Spandidos, D.A.; et al. Assessment of the antioxidant activity of an olive oil total polyphenolic fraction and hydroxytyrosol from a Greek Olea europea variety in endothelial cells and myoblasts. Int. J. Mol. Med. 2017, 40, 703–712. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Sánchez-Fidalgo, S.; González-Benjumea, A.; Maya, I.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Naturally occurring hydroxytyrosol derivatives: Hydroxytyrosyl acetate and 3,4-dihydroxyphenylglycol modulate inflammatory response in murine peritoneal macrophages. Potential utility as new dietary supplements. J. Agric. Food Chem. 2015, 63, 836–846. [Google Scholar] [CrossRef]
- Kitsati, N.; Mantzaris, M.D.; Galaris, D. Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic signaling via labile iron chelation. Redox Biol. 2016, 10, 233–242. [Google Scholar] [CrossRef]
- D’Angelo, C.; Goldeck, D.; Pawelec, G.; Gaspari, L.; Di Iorio, A.; Paganelli, R. Exploratory study on immune phenotypes in Alzheimer’s disease and vascular dementia. Eur. J. Neurol. 2020, 27, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- López-Villodres, J.A.; Abdel-Karim, M.; De La Cruz, J.P.; Rodríguez-Pérez, M.D.; Reyes, J.J.; Guzmán-Moscoso, R.; Rodriguez-Gutierrez, G.; Fernández-Bolaños, J.; González-Correa, J.A. Effects of hydroxytyrosol on cardiovascular biomarkers in experimental diabetes mellitus. J. Nutr. Biochem. 2016, 37, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Greiten, L.E.; Zhang, B.; Roos, C.M.; Hagler, M.; Jahns, F.-P.; Miller, J.D. Sirtuin 6 Protects Against Oxidative Stress and Vascular Dysfunction in Mice. Front. Physiol. 2021, 12, 753501. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Ji, B.; Gao, Y.-H.; Lin, H.; Ping, F.; Chen, F.; Liu, X.-B. Sirt6 regulates autophagy in AGE-treated endothelial cells via KLF4. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yin, M.; Koroleva, M.; Mastrangelo, M.A.; Zhang, W.; Bai, P.; Little, P.J.; Jin, Z.G. SIRT6 protects against endothelial dysfunction and atherosclerosis in mice. Aging (Albany NY) 2016, 8, 1064–1082. [Google Scholar] [CrossRef]
- Wang, B.; Tang, X.; Yao, L.; Wang, Y.; Chen, Z.; Li, M.; Wu, N.; Wu, D.; Dai, X.; Jiang, H.; et al. Disruption of USP9X in macrophages promotes foam cell formation and atherosclerosis. J. Clin. Investig. 2022, 132, e154217. [Google Scholar] [CrossRef]
- Ren, K.; Li, H.; Zhou, H.F.; Liang, Y.; Tong, M.; Chen, L.; Zheng, X.L.; Zhao, G.J. Mangiferin promotes macrophage cholesterol efflux and protects against atherosclerosis by augmenting the expression of ABCA1 and ABCG1. Aging (Albany NY) 2019, 11, 10992–11009. [Google Scholar] [CrossRef]
- Ogura, M. HDL, cholesterol efflux, and ABCA1: Free from good and evil dualism. J. Pharm. Sci. 2022, 150, 81–89. [Google Scholar] [CrossRef]
- Summerhill, V.I.; Grechko, A.V.; Yet, S.-F.; Sobenin, I.A.; Orekhov, A.N. The Atherogenic Role of Circulating Modified Lipids in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 3561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qin, Y.; Wan, X.; Liu, H.; Iv, C.; Ruan, W.; Lu, L.; He, L.; Guo, X. Hydroxytyrosol Plays Antiatherosclerotic Effects through Regulating Lipid Metabolism via Inhibiting the p38 Signal Pathway. Biomed. Res. Int. 2020, 2020, 5036572. [Google Scholar] [CrossRef] [PubMed]
- Pastor, R.; Bouzas, C.; Tur, J.A. Beneficial effects of dietary supplementation with olive oil, oleic acid, or hydroxytyrosol in metabolic syndrome: Systematic review and meta-analysis. Free Radic. Biol. Med. 2021, 172, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Hirschi, K.K.; Simons, M. The molecular basis of endothelial cell plasticity. Nat. Commun 2017, 8, 14361. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, J.C.; Dimmeler, S.; Harvey, R.P.; Finkel, T.; Aikawa, E.; Krenning, G.; Baker, A.H. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 190–209. [Google Scholar] [CrossRef]
- Li, A.; Peng, W.; Xia, X.; Li, R.; Wang, Y.; Wei, D. Endothelial-to-Mesenchymal Transition: A Potential Mechanism for Atherosclerosis Plaque Progression and Destabilization. DNA Cell Biol. 2017, 36, 883–891. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, C.; Chen, J.; Yang, M.; Afzal, T.A.; An, W.; Maguire, E.M.; He, S.; Luo, J.; Wang, X.; et al. miRNA-200c-3p promotes endothelial to mesenchymal transition and neointimal hyperplasia in artery bypass grafts. J. Pathol. 2021, 253, 209–224. [Google Scholar] [CrossRef]
- Zhong, C.M.; Li, S.; Wang, X.W.; Chen, D.; Jiang, Z.L.; Zhang, C.; He, X.J.; Huang, C.; Jiang, Y.J.; Wu, Q.C. MicroRNA-92a -mediated endothelial to mesenchymal transition controls vein graft neointimal lesion formation. Exp. Cell Res. 2021, 398, 112402. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharm. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Chen, P.Y.; Schwartz, M.A.; Simons, M. Endothelial-to-Mesenchymal Transition, Vascular Inflammation, and Atherosclerosis. Front. Cardiovasc. Med. 2020, 7, 53. [Google Scholar] [CrossRef]
- Chen, P.Y.; Qin, L.; Baeyens, N.; Li, G.; Afolabi, T.; Budatha, M.; Tellides, G.; Schwartz, M.A.; Simons, M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J. Clin. Investig. 2015, 125, 4514–4528. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Nannelli, G.; Giachetti, A.; Morbidelli, L.; Ziche, M.; Donnini, S. Targeting endothelial-to-mesenchymal transition: The protective role of hydroxytyrosol sulfate metabolite. Eur. J. Nutr. 2020, 59, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Razali, R.A.; Lokanathan, Y.; Yazid, M.D.; Ansari, A.S.; Saim, A.B.; Hj Idrus, R.B. Modulation of Epithelial to Mesenchymal Transition Signaling Pathways by Olea Europaea and Its Active Compounds. Int. J. Mol. Sci. 2019, 20, 3492. [Google Scholar] [CrossRef] [PubMed]
- Razali, R.A.; Nik Ahmad Eid, N.A.H.; Jayaraman, T.; Amir Hassan, M.A.; Azlan, N.Q.; Ismail, N.F.; Sainik, N.Q.A.V.; Yazid, M.D.; Lokanathan, Y.; Saim, A.B.; et al. The potential of Olea europaea extracts to prevent TGFβ1-induced epithelial to mesenchymal transition in human nasal respiratory epithelial cells. BMC Complement. Altern. Med. 2018, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Evrard, S.M.; Lecce, L.; Michelis, K.C.; Nomura-Kitabayashi, A.; Pandey, G.; Purushothaman, K.R.; d'Escamard, V.; Li, J.R.; Hadri, L.; Fujitani, K.; et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat. Commun. 2016, 7, 11853. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J. Endothelial-to-Mesenchymal Transition. Circ. Res. 2019, 124, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Vijakumaran, U.; Yazid, M.D.; Hj Idrus, R.B.; Abdul Rahman, M.R.; Sulaiman, N. Molecular Action of Hydroxytyrosol in Attenuation of Intimal Hyperplasia: A Scoping Review. Front. Pharmacol. 2021, 12, 663266. [Google Scholar] [CrossRef]
- Martínez-Lara, E.; Peña, A.; Calahorra, J.; Cañuelo, A.; Siles, E. Hydroxytyrosol decreases the oxidative and nitrosative stress levels and promotes angiogenesis through HIF-1 independent mechanisms in renal hypoxic cells. Food Funct. 2016, 7, 540–548. [Google Scholar] [CrossRef]
- Cheng, Y.; Qu, Z.; Fu, X.; Jiang, Q.; Fei, J. Hydroxytyrosol contributes to cell proliferation and inhibits apoptosis in pulsed electromagnetic fields treated human umbilical vein endothelial cells in vitro. Mol. Med. Rep. 2017, 16, 8826–8832. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Chang, C. Epigenetics in Health and Disease. Adv. Exp. Med. Biol. 2020, 1253, 3–55. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed]
- Peedicayil, J. Epigenetic therapy-a new development in pharmacology. Indian J. Med. Res. 2006, 123, 17. [Google Scholar] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- D'Adamo, S.; Cetrullo, S.; Borzì, R.M.; Flamigni, F. Effect of oxidative stress and 3-hydroxytyrosol on DNA methylation levels of miR-9 promoters. J. Cell Mol. Med. 2019, 23, 7885–7889. [Google Scholar] [CrossRef]
- López de Las Hazas, M.C.; Martin-Hernández, R.; Crespo, M.C.; Tomé-Carneiro, J.; Del Pozo-Acebo, L.; Ruiz-Roso, M.B.; Escola-Gil, J.C.; Osada, J.; Portillo, M.P.; Martinez, J.A.; et al. Identification and validation of common molecular targets of hydroxytyrosol. Food Funct. 2019, 10, 4897–4910. [Google Scholar] [CrossRef]
- Del Saz-Lara, A.; López de Las Hazas, M.C.; Visioli, F.; Dávalos, A. Nutri-Epigenetic Effects of Phenolic Compounds from Extra Virgin Olive Oil: A Systematic Review. Adv. Nutr. 2022, 13, 2039–2060. [Google Scholar] [CrossRef]
- Iqbal, A.M.; Jamal, S.F. Essential Hypertension. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wu, C.Y.; Hu, H.Y.; Chou, Y.J.; Huang, N.; Chou, Y.C.; Li, C.P. High Blood Pressure and All-Cause and Cardiovascular Disease Mortalities in Community-Dwelling Older Adults. Medicine 2015, 94, e2160. [Google Scholar] [CrossRef]
- Quirós-Fernández, R.; López-Plaza, B.; Bermejo, L.M.; Palma-Milla, S.; Gómez-Candela, C. Supplementation with Hydroxytyrosol and Punicalagin Improves Early Atherosclerosis Markers Involved in the Asymptomatic Phase of Atherosclerosis in the Adult Population: A Randomized, Placebo-Controlled, Crossover Trial. Nutrients 2019, 11, 640. [Google Scholar] [CrossRef]
- Hermans, M.P.; Lempereur, P.; Salembier, J.-P.; Maes, N.; Albert, A.; Jansen, O.; Pincemail, J. Supplementation Effect of a Combination of Olive (Olea europea L.) Leaf and Fruit Extracts in the Clinical Management of Hypertension and Metabolic Syndrome. Antioxidants 2020, 9, 872. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef]
- Menichini, D.; Alrais, M.; Liu, C.; Xia, Y.; Blackwell, S.C.; Facchinetti, F.; Sibai, B.M.; Longo, M. Maternal Supplementation of Inositols, Fucoxanthin, and Hydroxytyrosol in Pregnant Murine Models of Hypertension. Am. J. Hypertens. 2020, 33, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Grylls, A.; Seidler, K.; Neil, J. Link between microbiota and hypertension: Focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics. Biomed. Pharm. 2021, 137, 111334. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, Y.; Ramprasath, T.; Zou, M.H. Oxidative Stress, GTPCH1, and Endothelial Nitric Oxide Synthase Uncoupling in Hypertension. Antioxid. Redox Signal. 2021, 34, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Youn, J.-Y.; Cai, H. Mechanisms and consequences of endothelial nitric oxide synthase dysfunction in hypertension. J. Hypertens. 2015, 33, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Ataei Ataabadi, E.; Golshiri, K.; Jüttner, A.; Krenning, G.; Danser, A.H.J.; Roks, A.J.M. Nitric Oxide-cGMP Signaling in Hypertension: Current and Future Options for Pharmacotherapy. Hypertension 2020, 76, 1055–1068. [Google Scholar] [CrossRef]
- Ivanov, M.; Vajic, U.J.; Mihailovic-Stanojevic, N.; Miloradovic, Z.; Jovovic, D.; Grujic-Milanovic, J.; Karanovic, D.; Dekanski, D. Highly potent antioxidant Olea europaea L. leaf extract affects carotid and renal haemodynamics in experimental hypertension: The role of oleuropein. Excli J 2018, 17, 29–44. [Google Scholar] [CrossRef]
- Ilic, S.; Stojiljkovic, N.; Stojanovic, N.; Stoiljkovic, M.; Mitic, K.; Salinger-Martinovic, S.; Randjelovic, P. Effects of oleuropein on rat's atria and thoracic aorta: A study of antihypertensive mechanisms. Can. J. Physiol. Pharm. 2021, 99, 110–114. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Roselló-Catafau, J.; Pintó, X.; Mitjavila, M.T.; Moreno, J.J. Polyphenol fraction of extra virgin olive oil protects against endothelial dysfunction induced by high glucose and free fatty acids through modulation of nitric oxide and endothelin-1. Redox Biol. 2014, 2, 971–977. [Google Scholar] [CrossRef]
- Nicholson, S.K.; Tucker, G.A.; Brameld, J.M. Physiological concentrations of dietary polyphenols regulate vascular endothelial cell expression of genes important in cardiovascular health. Br. J. Nutr. 2010, 103, 1398–1403. [Google Scholar] [CrossRef]
- Lu, C.L.; Liao, M.T.; Hou, Y.C.; Fang, Y.W.; Zheng, C.M.; Liu, W.C.; Chao, C.T.; Lu, K.C.; Ng, Y.Y. Sirtuin-1 and Its Relevance in Vascular Calcification. Int. J. Mol. Sci. 2020, 21, 1593. [Google Scholar] [CrossRef]
- Ren, C.Z.; Wu, Z.T.; Wang, W.; Tan, X.; Yang, Y.H.; Wang, Y.K.; Li, M.L.; Wang, W.Z. SIRT1 exerts anti-hypertensive effect via FOXO1 activation in the rostral ventrolateral medulla. Free Radic. Biol. Med. 2022, 188, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Ma, L.; Wu, G.; Yu, Q.; Lin, H.; Ying, Q.; Wen, D.; Gao, C. G004, a synthetic sulfonylurea compound, exerts anti-atherosclerosis effects by targeting SIRT1 in ApoE(-/-) mice. Vasc. Pharm. 2017, 89, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Khan, A.U.; Shah, A.J.; Connor, J.; Jabeen, Q. Blood pressure lowering effect of olive is mediated through calcium channel blockade. Int. J. Food Sci. Nutr. 2005, 56, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, A.; Rauwald, H.W.; Kampa, B.; Mann, U.; Mohr, F.W.; Dhein, S. Olea europaea leaf extract exerts L-type Ca2+ channel antagonistic effects. J. Ethnopharmacol. 2008, 120, 233–240. [Google Scholar] [CrossRef]
- Liberale, L.; Kraler, S.; Camici, G.G.; Lüscher, T.F. Ageing and longevity genes in cardiovascular diseases. Basic Clin. Pharm. Toxicol. 2020, 127, 120–131. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef]
- Jani, B.; Rajkumar, C. Ageing and vascular ageing. Postgrad. Med. J. 2006, 82, 357–362. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Laina, A.; Stellos, K.; Stamatelopoulos, K. Vascular ageing: Underlying mechanisms and clinical implications. Exp. Gerontol. 2018, 109, 16–30. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Gordon, C.J.; Rowsey, P.J.; Bishop, B.L.; Ward, W.O.; Macphail, R.C. Serum biomarkers of aging in the Brown Norway rat. Exp. Gerontol. 2011, 46, 953–957. [Google Scholar] [CrossRef]
- Bruunsgaard, H.; Ladelund, S.; Pedersen, A.N.; Schroll, M.; Jørgensen, T.; Pedersen, B.K. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin. Exp. Immunol. 2003, 132, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, J.; Jiang, L.; Zhong, L. Suppressive Effects of Hydroxytyrosol on Oxidative Stress and Nuclear Factor-kappaB Activation in THP-1 Cells. Biol. Pharm. Bull. 2009, 32, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, G.; Di Rosa, G.; Scuto, M.; Leri, M.; Stefani, M.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan Maintenance and Prevention of Parkinson’s-like Phenotypes with Hydroxytyrosol and Oleuropein Aglycone in C. elegans. Int. J. Mol. Sci. 2020, 21, 2588. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Hou, C.; Yang, Z.; Li, C.; Jia, L.; Liu, J.; Tang, Y.; Shi, L.; Li, Y.; Long, J.; et al. Hydroxytyrosol mildly improve cognitive function independent of APP processing in APP/PS1 mice. Mol. Nutr. Food Res. 2016, 60, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
| Population Group | Free Hydroxytyrosol Daily Intake from the Consumption of Olive Oils | Free Hydroxytyrosol Daily Intake from the Consumption of Table Olives | ||
|---|---|---|---|---|
| Range of Means (mg/kg bw/day) | Range of 95% (mg/kg bw/day) | Range of Means (mg/kg bw/day) | Range of 95% (mg/kg bw/day) | |
| Children (3–9 years) | 0.00015–0.008 | 0.0003–0.016 | 0.019–0.375 | 0.059–0.270 |
| Adolescents (10–17 years) | 0.00015–0.005 | 0.00046–0.008 | 0.013–0.204 | 0.059–0.382 |
| Adults (18–64 years) | 0.00015–0.004 | 0.00046–0.007 | 0.019–0.185 | 0.079–0.415 |
| Elderly (≥65 years) | 0.00007–0.004 | 0.0016–0.007 | 0.013–0.125 | 0.059 |
| Oxidative Stress | Inflammation |
|---|---|
| HT ↓ PMA stimulated mitochondrial superoxide [105] | HT ↓ PMA stimulated pro-inflammatory cytokines (TNF-α, IL- 1β, VCAM-1, ICAM-1) [105] |
| HT ↑ mitochondrial biogenesis [105] | HT and HT metabolites ↓ inflammatory cytokines (E-selectin, P-selectin, VCAM-1, and ICAM-1) [110,117] |
| HT ↑ NRF-1 gene expression [97,101,105] | HT ↓ cardiac markers (LDH, creatine kinase, and troponin-T) and TNF-α and IL-6 level in in LPS induced cardiac dysfunction [118] |
| HT ↑ NO production [87,88,91,118] | HT-acetate lowers SIRT6 protein and mRNA expression [62,85] |
| HT and HT-AC ↑ SOD and ↓ MDA, ROS [62,118] | Hydroxytyrosol inhibited PMA induced prostaglandin (PG)E2 and COX-2 expression [119] |
| HT ↑ eNOS, PGC1α by counter reacting with TNF-α [117] | HT ↓ TNF-α induced IκBα and NFκBp65 phosphorylation [87] |
| HT derivatives ↑ GSH [110,120] ↑ NO [110] ↓ ROS [121] | HT and HT-AC ↓ TNF-α induced IL-1β, IL-6 and CCL2 [62] |
| HT ↑ HO-1 mRNA, and Nrf2, PI3K/Akt, and ERK1/2 protein expression in [97] | HT metabolites ↓ E-selectin, ICAM-1 and VCAM-1 [111] |
| HT ↓ H2O2 induced oxidative stress, apoptosis, and DNA damage [122] | 3,4-DHPEA-EDA ↓ LPS- and TNF- α induced E-selectin, ICAM- and VCAM-1, CCL2 and NF-kB activation [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijakumaran, U.; Shanmugam, J.; Heng, J.W.; Azman, S.S.; Yazid, M.D.; Haizum Abdullah, N.A.; Sulaiman, N. Effects of Hydroxytyrosol in Endothelial Functioning: A Comprehensive Review. Molecules 2023, 28, 1861. https://doi.org/10.3390/molecules28041861
Vijakumaran U, Shanmugam J, Heng JW, Azman SS, Yazid MD, Haizum Abdullah NA, Sulaiman N. Effects of Hydroxytyrosol in Endothelial Functioning: A Comprehensive Review. Molecules. 2023; 28(4):1861. https://doi.org/10.3390/molecules28041861
Chicago/Turabian StyleVijakumaran, Ubashini, Janushaa Shanmugam, Jun Wei Heng, Siti Sarah Azman, Muhammad Dain Yazid, Nur Atiqah Haizum Abdullah, and Nadiah Sulaiman. 2023. "Effects of Hydroxytyrosol in Endothelial Functioning: A Comprehensive Review" Molecules 28, no. 4: 1861. https://doi.org/10.3390/molecules28041861
APA StyleVijakumaran, U., Shanmugam, J., Heng, J. W., Azman, S. S., Yazid, M. D., Haizum Abdullah, N. A., & Sulaiman, N. (2023). Effects of Hydroxytyrosol in Endothelial Functioning: A Comprehensive Review. Molecules, 28(4), 1861. https://doi.org/10.3390/molecules28041861






